Abstract
The inhibitory receptor interleukin-1 receptor 8 (IL-1R8) has been recently recognized to be expressed also by human natural killer (NK) cells. This study was aimed to design and optimize IL-1R8 silencing conditions in human NK cells to precisely establish the activity of such receptor in these cells. Electroporation of freshly isolated or IL-2-cultured NK cells with small interfering RNA (siRNA), resulted in a marked, even though variable, IL-1R8-silencing. Although the expression profile revealed downregulation of most genes involved in several intracellular pathways, some genes related to proliferation, expression of some chemokine receptors, antibody-dependent cell cytotoxicity and cytotoxic activity were upregulated in IL-1R8-silenced NK cells. Furthermore, upon IL-15 activation, the majority of genes involved in NK cell function were upregulated in IL-1R8-siRNA—compared with control—siRNA-transfected NK cells. More importantly, in agreement with these findings, the reduction of IL-1R8 gene expression levels resulted in enhanced expression of NK cell activation markers, production of cytokines and chemokines, and cytotoxic activity against several NK cell targets with different susceptibility to NK-mediated lysis. Similar results were obtained following stimulation with IL-18. All together these data, deeply impacting on the main effector functions of human NK cells, can lead to a better understanding of IL-1R8-mediated regulation on these cells and to the design of new strategies for improving NK cell-mediated anti-tumor responses.
Keywords: immunomodulation, immunotherapy, natural killer cells
Introduction
Interleukin-1 receptor 8 (IL-1R8), also known as single-immunoglobulin interleukin-1 receptor related or Toll-interleukin-1 receptor 8 (TIR-8), is a member of the interleukin-1 receptor family. IL-1R8 displays a single extracellular Ig domain, a transmembrane portion, a cytoplasmic TIR domain and a 95 amino acids long tail which is not present in other TIR domain-containing receptors.1 IL-1R8 is broadly expressed in epithelial tissues (kidney, gut, liver, lung and lymphoid organs) as well as in human blood and immune cells, including platelets,2 neutrophils, monocytes, dendritic-, T, B, and natural killer (NK) cells (reviewed in Molgora et al3). In human peripheral blood mononuclear cells (PBMC), IL-1R8 has been shown to interact with the IL-18Rα chain forming an inhibitory receptor triggered by the anti-inflammatory cytokine IL-37.4 IL-1R8 functions as a negative regulator of either Toll-like receptors (TLR) or IL-1R-signaling pathways as shown by the enhanced cell activation in IL-1R8-deficient mice following stimulation with either IL-1 or certain TLR ligands.5 At the functional level, the loss of IL-1R8 results in the inhibition of murine macrophage migration while enhancing their proliferation.6 In addition, it correlates with a higher frequency of mature NK cells in different tissues and to an increased expression of activating receptors, interferon γ (IFN-γ), granzyme B (GrzB) and CD107a.7 The enhanced NK cells functional profile of both IL-1R8−/− mice and human NK cells, observed 7 days after transfection, was mainly correlated to treatment with IL-18,7 a cytokine playing a key role in NK cell activation and differentiation.
This study was aimed to optimize IL-1R8 silencing and to establish whether such inhibition could be relevant for human NK cells under basal conditions or upon stimulation either with IL-15, a cytokine which promotes NK cells activation, proliferation, and cytotoxic activity8 or with IL-18. The present data could lead to a better understanding of IL-1R8-mediated regulation on human NK cells and to shed light on possible modulatory mechanisms on IL-15 signaling cascade.
Methods
Human samples
Buffy coats were collected from volunteer blood donors (HD) admitted to the blood transfusion service of IRCCS Bambino Gesù Children’s Hospital after obtaining informed consent. The Ethical Committee of IRCCS Bambino Gesù Children’s Hospital approved (2058_OPBG_2020) and conducted the study in accordance with the tenants of the Declaration of Helsinki.
Cells lines and cell culture
K562 (chronic myelogenous leukemia), Karpas 299 (human non-Hodgkin’s Ki-positive large cell lymphoma, CD19−), Nalm-18 (childhood B acute lymphoblastic leukemia, CD19+) and IMR-32 (human neuroblastoma) cell lines were purchased from American Type Culture Collection (Rockville, MDA). K562, Karpas 299, Nalm-18 and freshly isolated NK cells were cultured in RPMI 1640 (Euroclone, MI, IT) while IMR-32 in Dulbecco’s Modified Eagle Medium high glucose (Euroclone). Both culture media were supplemented with 2 mM l-glutamine (Euroclone), 1% penicillin–streptomycin–neomycin mixture (Euroclone) and 10% heat-inactivated fetal bovine serum (Euroclone).
Highly purified (≥90%) CD56+, CD3− peripheral blood NK (PB-NK) cells were isolated from HDs’ PBMCs as described in Quatrini et al.9 After electroporation, NK cells were cultured with 2 ng/mL IL-15 (Miltenyi Biotec, Bergisch Gladbach, Germany) or 10 ng/mL IL-18 (R&D Systems, Bio-Techne, Minneapolis, Minn) for 48 hours at 37°C. Thereafter, NK cells were either harvested (basal conditions) or activated either with 20 ng/mL IL-15 (Miltenyi Biotec) or 100 ng/mL IL-18 (R&D Systems) for 18 hours as specified in the text.
Polyclonal activated NK cells were obtained from freshly isolated NK cells and cultured as described in Ingegnere et al. 10.
Cell electroporation
Resting NK cells were electroporated with the Neon Transfection System (Thermo Fisher Scientific, Waltham, Massachusetts, USA) using the following conditions: 1600 V, 20 ms, 1 pulse. IL-2-cultured NK cells were electroporated as previously described.10 The FITC-labeled siRNA (Block-It fluorescent control, Thermo Fisher Scientific) transfection efficiency was determined by FACS analysis (Cytoflex S flow cytometer, Beckman Coulter). The effectiveness of IL-1R8 silencing was assessed by RT-PCR before each experiment.
Analysis of cytotoxic activity
NK-cell cytotoxicity was analyzed by incubating IL-1R8 siRNA or Control siRNA NK cells (mock) with K562, Karpas 299, Nalm-18 or IMR-32 cell lines at an effector-to-target (E:T) ratio ranging from 40:1 to 0.6:1. Cytotoxicity was assessed using a flow cytometric assay for NK-cell killing developed by McGinnes11 and modified as described.10 Specific lysis was calculated as dead target cells (Td) of target cells cultured with effector cells minus Td of target cells cultured alone.
Flow cytometry analysis
To verify the purity of NK separation, NK cells were stained with CD56-BV650 (BD, Clone NCAM 16.2, Erembodegem, Belgium) and CD3-FITC (Miltenyi Biotec, Clone REA613) antibodies. To analyze IL-1R8 surface expression, NK cells were incubated with FcR Blocking Reagent (Miltenyi Biotec) for 10 min at 4°C, stained with anti-IL-1R8 antibody or normal goat IgG control (R&D Systems) for 30 min at 4°C followed by donkey anti-goat IgG (PE, Abcam, Cambridge, UK)) for 25 min at 4°C. Dead cells were excluded as Zombie Aqua (Biolegend, San Diego, CA) positive. To evaluate NK cells activation, samples were stained with CD69 FITC (Miltenyi Biotec, Clone FN50) for 30 min at 4°C. NK cell samples were acquired using the Cytoflex S flow cytometer and analyzed with the Kaluza software 2.1 (Beckman Coulter, Brea, California, USA).
Determination of released cytokines and chemokines
Released levels of granulocyte-macrophage colony-stimulating factor(GM-CSF), tumor necrosis factor α (TNFα), chemokine (C-C motif) ligand 3 (CCL3) (MIP-1α), chemokine (C-X-C motif) ligand 8 (CXCL8) (IL-8) and IFN-γ were measured in cell supernatants (following 18 hours IL-15 or IL-18 activation) using commercially available ELISA kits according to the manufacturer instructions (DuoSet ELISA, R&D systems).
Migration assay
Supernatants were collected from IL-1R8 siRNA or mock NK cells following 18 hours activation with IL-15. Cell migration assay was performed using 105 whole blood cells in a final volume of 200 µL in a 96-well plate containing filters (pore-size: 8 µm) (Abcam). The assay was performed and analyzed by flow cytometry as previously described.12 Anti-CXCL8 monoclonal antibody (MA5-23697, Thermo Fisher Scientific) was kindly provided by Dr. Fionda (Sapienza—University of Rome, Italy) and used at the concentration of 2.5 µg/mL. Results are plotted as absolute number of migrated cells and analyzed using one-way analysis of variance statistical comparison.
Protein extract and western blot analysis
NK cells protein extraction and western blot analysis have been performed as described in Mariotti et al.13 The following antibodies were used: anti-IL-1R8 1:500 (Abcam), anti-β-Actin 1:5000 (Sigma-Aldrich), goat-anti-mouse-HRP and goat-anti-rabbit-HRP (Cell Signaling Technology).
RNA isolation and analysis
Total RNA extraction from purified NK cells was performed with RNeasy Plus micro kit following the manufacturer’s protocol (Qiagen GmbH, Hilden, Germany). RNA concentration and purity were evaluated by spectrophotometric analysis (Nanodrop 2000; Thermo Fisher Scientific). For gene expression analysis, total RNA was reverse transcribed using High-Capacity cDNA reverse transcription kit (Applied Biosystems, Waltham, Massachusetts, USA) according to manufacturer’s instructions. Real-time PCR were carried out in 20 µL of total volume with SyberGreen Fast Advanced Master Mix (Applied Biosystems). The following primers were used: ActB Fwd 5’-ACCGCGAGAAGATGACCCAGA-3’; ActB-Rev 5’-GGATAGCACAGCCTGGATAGCAA-3’; IL-1R8 Fwd 5’-TCAGTGGCTCTGAACTGCAC-3’ IL-1R8 Rev 5’-GTACCAGAGCAGCACGTTGA-3’. Expression values were calculated by ΔΔCt method, using QuantStudio Real-Time PCR system software V.1.3 (Applied Biosystems).
For gene profile analysis, RT-PCR on either basal or IL-15 activated NK cells (2 hours) was performed by 384-wells custom TaqMan microfluidic array card for the detection of selected critical genes in NK cell biology (Thermo Fisher Scientific) as previously described.14
Data were analyzed using Thermo Fisher Cloud with Design and Analysis New qPCR application (Thermo Fisher Scientific). ActB was used as reference gene in all the experiments.
Statistical analysis
Statistical analyses were performed using the GraphPad Prism V.6.0 software (La Jolla, California, USA).
Results and discussion
Although a high expression of IL-1R8 at mRNA and protein levels in human circulating NK cells has been reported,7 the precise activity of such inhibitory receptor in these cells has not been clarified. The aim of this study is to investigate how IL-1R8 silencing can affect human PB-NK cell function.
For an efficient gene silencing, the synthesis of siRNA must take into consideration few features including appropriate length, specificity and nucleotide content.15 Through an in-silico approach, we selected six different siRNA targeting the IL-1R8 mRNA (online supplemental figure S1A). To silence the IL-1R8 gene in primary human NK cells, we took advantage of the electroporation system previously set up in our lab,10 optimizing the conditions for an efficient transfection of IL-2-cultured NK cells using control FITC-labeled-siRNA (online supplemental figure S1B). Considering that the highest IL-1R8 silencing (up to 80%) occurred on transfection of NK cells with siRNA6, this molecule was used for all the subsequent experiments (online supplemental figure S1C).
jitc-2021-003858supp001.pdf (2.3MB, pdf)
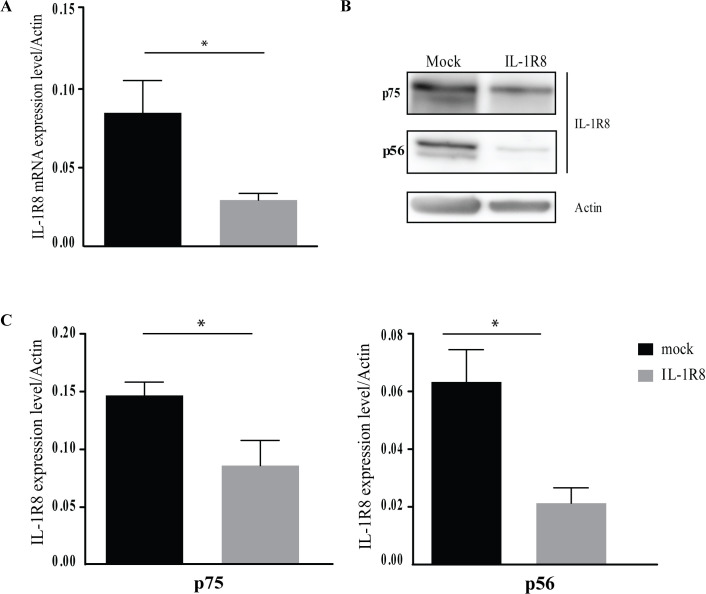
To better characterize IL-1R8 function, we induced its silencing also on freshly isolated (resting) NK cells from HDs. In a time course assay, the highest IL-1R8 mRNA silencing occurred 48 hours after electroporation whereas a recovery of its expression was observed at longer intervals (online supplemental figure S2A). Indeed, approximately 70% reduction of IL-1R8 mRNA level was detected in siRNA- versus mock NK cells (figure 1A), demonstrating that we could efficiently silence the target gene also in resting NK cells. Then, to confirm the mRNA reduction, we also assayed the amount of IL-1R8 protein (figure 1B, C and online supplemental figure S2B). Recent reports on different human cells indicate that IL-1R8 protein can be detected in two isoforms at 56 and 75 KDa.2 16 17 In IL-1R8-siRNA-NK cells, IL-1R8 protein levels were reduced approximately by 60% and 40% for p56 and p75 isoforms, respectively (figure 1B, C) and approximately by 20% at the cell surface level (online supplemental figure S2B) compared with mock NK cells.
Figure 1.
Interleukin-1 receptor 8 (IL-1R8) silencing in resting natural killer (NK) cells. (A–C) Validation of IL-1R8 silencing with siRNA6. Samples of resting NK cells were collected after 48 hours of transfection and analyzed for mRNA and protein expression. (A) RT-PCR assessing IL-1R8 mRNA expression in mock-transfected or siRNA-transfected resting NK cells from eight healthy donors. IL-1R8 expression was normalized over Actin. Values are mean±SEM. Statistical significance has been determined by paired t test. P value, *p<0.05. (B) Western blot analysis of IL-1R8 protein in mock-silenced and IL-1R8-silenced resting NK cells. A representative image from three independent experiments has been reported. (C) IL-1R8 protein quantification in mock-transfected and IL-1R8-siRNA-transfected resting NK cells from three healthy donors. Expression of IL-1R8 p75 (left panel) and p56 (right panel) isoforms has been normalized over Actin. Reduction of both IL-1R8 isoforms was detected in IL-1R8-siRNA transfected NK cells. Values are mean±SEM. Statistical significance has been determined by paired t test. P value, *p<0.05.
jitc-2021-003858supp002.pdf (2.1MB, pdf)
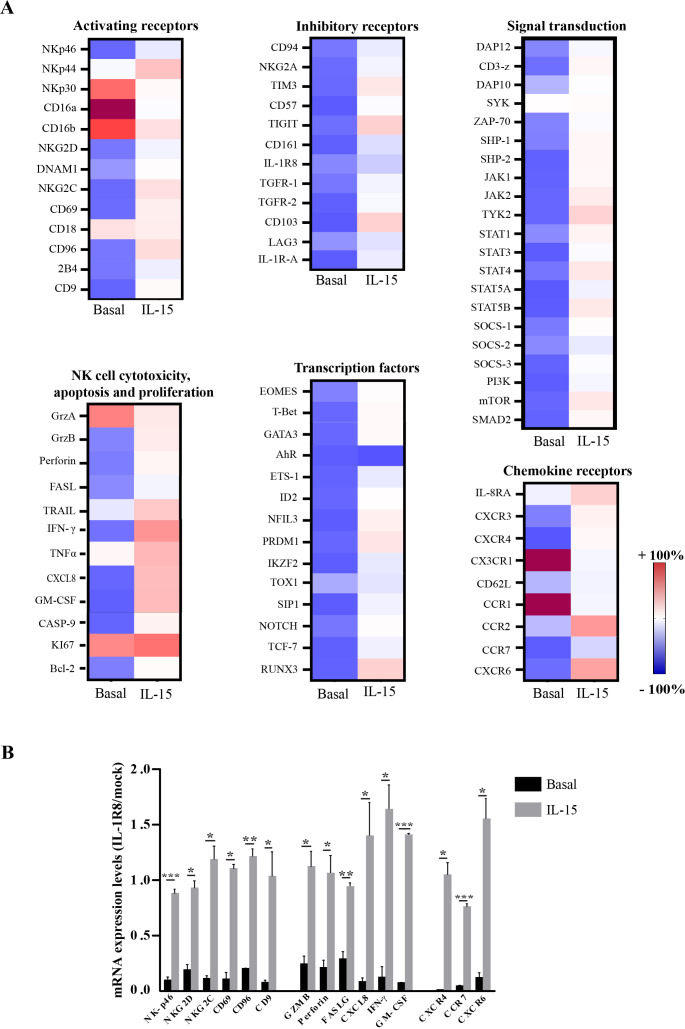
In order to achieve a comprehensive view of the effects of IL-1R8 silencing on NK cells, we evaluated the gene expression profiles of IL-1R8-siRNA (compared with mock) NK cells by using a CARD array (a 384-wells custom TaqMan microfluidic array card), including the most relevant receptors, cytokines, signaling and transcription factors of NK cells. This analysis was performed on unstimulated- (basal condition, figure 2A, left column) or IL-15-stimulated (figure 2A, right column) mock-transfected and IL-1R8-transfected NK cells.
Figure 2.
Interleukin-1 receptor 8 (IL-1R8) silencing under basal conditions and on IL-15 activation impacts on NK cell gene expression profile. (A) Gene-expression heatmaps represented as a ratio between IL-1R8-transfected and mock-transfected resting (left column, basal,) or IL-15 activated (right column, 2 hours) NK cells. Gene expression profiles were analyzed using 384-wells TaqMan array microfluidic cards. mRNA intensities are displayed as colors ranging from red to blue as shown in the key. Genes were divided into categories according to their function. The heatmap for basal and IL-15 treatment conditions is relative to independent experiments performed on three healthy donors. (B) Expression of the most upregulated genes, involved in different NK cell functions. mRNA levels have been reported as a ratio between IL-1R8 siRNA-transfected and mock-transfected NK cells in basal conditions (black columns) or upon IL-15 activation (gray columns).
In basal conditions, the genes upregulated were limited in number and included some NK receptors (NCR3/NKp30 and CD16 a/b), chemokine receptors (CCR1, CCR5 and CX3CR1), cytokines (TNFα), granzyme A (GrzA) and the proliferation marker Ki67 (figure 2A). In contrast, a large number of genes encoding for NK receptors (KLRK1, CD226, 2B4, TIGIT, TIM3, KLRD1/2), cytokine and chemokine receptors (TGF-βR1/2, CXCR3, CXCR4, CXCR6, CCR7), signal transduction and transcription factors appeared to be strongly downregulated in IL-1R8-siRNA-NK compared with mock-NK cells. These data highlight the IL-1R8 involvement, at various levels, in the regulation of multiple cellular pathways (figure 2A and online supplemental figure S3).
jitc-2021-003858supp003.pdf (3.4MB, pdf)
In contrast, a different scenario was detected upon 2 hours culture with IL-15 (figure 2A). The activation marker CD69, as well as different genes belonging to the IL-15 signaling pathway, such as JAK1/2, STATs (STAT1, STAT3, STAT4, STAT5A/5B) and mTOR, associated with their downstream antiapoptotic factor BCL2 (S3),18 were found to be upregulated (figure 2A) in IL-1R8-silenced NK cells. In agreement with the well-known effects of IL-15 on NK cell maturation, IL-1R8-siRNA-activated NK cells showed a significant increased expression of the transcription factors ETS1, NFIL3, T-bet, Eomes as well as other proteins controlling NK cell development19 and maturation20 21 (online supplemental figure S3). The latter results could suggest a possible interplay between IL-15-dependent signaling pathway and IL-1R8 function.
The increased expression of the genes belonging to NK cell development and maturation, detected in IL-1R8-silenced NK cells on stimulation with IL-15, could be the result of compensatory mechanisms. Indeed, IL-1R8 is associated with a more mature (exhausted) NK cell stage.7 Therefore, the loss of this receptor would result in overexpression of genes involved in maturation and development. A similar genetic compensatory mechanism has been reported for different genes in different phyla.22
In addition, we detected an upregulation of genes encoding chemokine receptors (IL-8RA, CXCR3, CXCR4, CXCR6, CCR2, CCR7) which may play a role in NK cell-homing into tumor microenvironment, and secretory molecules of the killing machinery, such as GrzB and Perforin 1 (figure 2A, B). These data are in line with the increased expression of mTOR (known to control GrzB and IFN-ϒ production) in IL-1R8-silenced IL-15-activated NK cells (figure 2A and online supplemental figure S3). Interestingly, the CD161 gene, known to mark a functionally distinct subset of proinflammatory NK cells,23 was found significantly upregulated in IL-1R8-silenced-NK cells. Consistent with the findings of another group reporting that CD161 expression on NK cells is directly related to CXCL8 release,24 activated IL-1R8 siRNA NK cells exhibited enhanced levels of both CXCL8 gene and protein as compared with mock NK cells.
Notably, the increase of the Shp-2 gene, found in activated IL-1R8 siRNA NK cells (figure 2A, right panel), is in agreement with a recent report showing its involvement in NK cell responses upon IL-15 and IL-2 activation.25 Of note, both RORC and AHR mRNA did not show any variation, remaining in a repressed state also after IL-15 treatment. Similarly, Ki67 and ITGB2, which were upregulated in basal conditions, remained in an active state with only a slight increase of their expression level.
The genes encoding the main activating and inhibitory receptors, as well as the critical cytokines and molecules associated with NK cell cytotoxicity, whose expression was significantly increased between basal and IL-15-treated conditions, are summarized in figure 2B. Results highlight a general upregulation of the genes involved in NK cell effector functions, thus indicating that IL-1R8 impacts on the NK cell profile and that its reduction may unleash NK cell anti-tumor function (figure 2B).
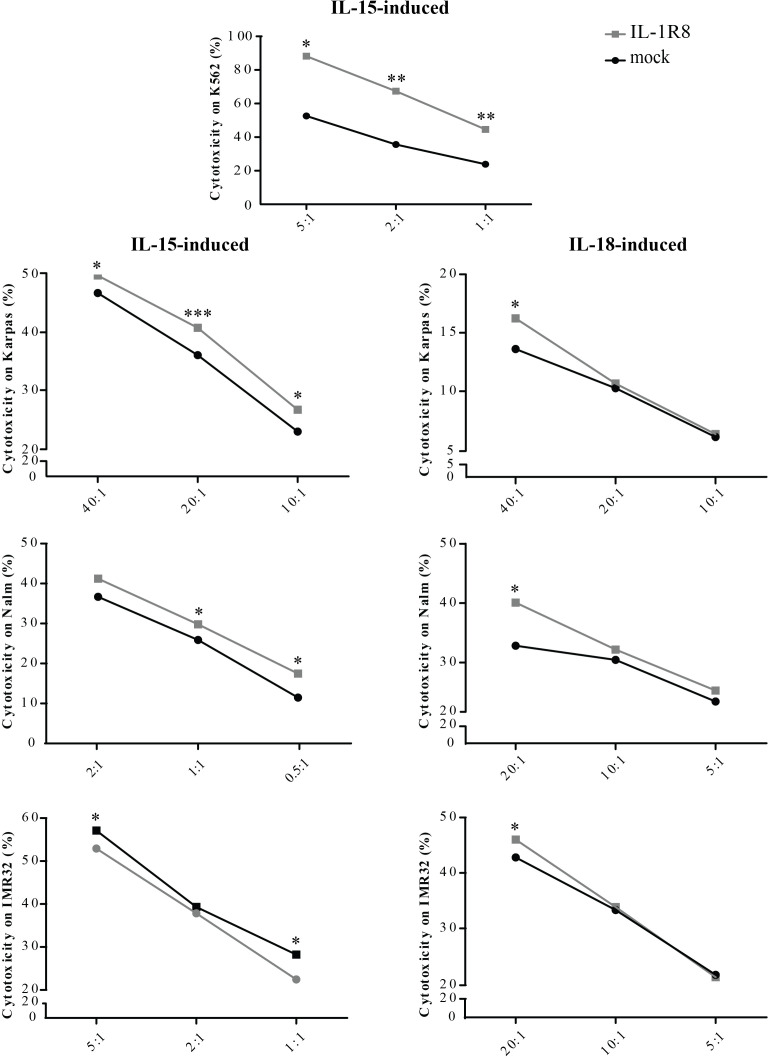
We further investigated whether the observed upregulation of the genes linked to NK cell activation could reflect an increase in NK cell function. For this purpose, functional analyses were performed on both mock-and IL-1R8 siRNA-transfected resting NK cells stimulated for 18 hours with IL-15. To investigate the effect of IL-1R8 silencing on NK cell effector function, we analyzed the NK cell activity against different targets known to be highly (K562, Nalm-18 and IMR-32) or poorly (Karpas 299) susceptible to NK cell-mediated killing.10 26 As shown in figure 3, the reduction of IL-1R8 expression levels resulted in increased NK cell cytotoxic activity against all target cells at different E:T ratios (figure 3, left column). The effect of IL-1R8 silencing on NK cell cytotoxicity was also tested following stimulation with IL-18, resulting in a similar, although, less pronounced, increase in the NK cell mediated killing (figure 3, right column). Moreover, IL-1R8 silenced NK cells were able to release higher amount of IFN-γ, further confirming the role played by IL-1R8 in regulating NK cell effector function (online supplemental figure S4).
Figure 3.
Effects of Interleukin-1 receptor 8 (IL-1R8) silencing on natural killer (NK) cell-cytotoxicity. Cytotoxicity of mock-transfected (black circles) or IL-1R8-transfected (gray squares) NK cells, against different tumor cell lines at the indicated Effector (E):Target (T) ratios, upon IL-15 (left column) or IL-18 (right column) stimulation. Values (% cytotoxicity) represent the mean of independent experiments performed on at least five healthy donors. Data have been compared using paired t test. *p<0.05, **p<0.01, ***p<0.005.
jitc-2021-003858supp004.pdf (1.5MB, pdf)
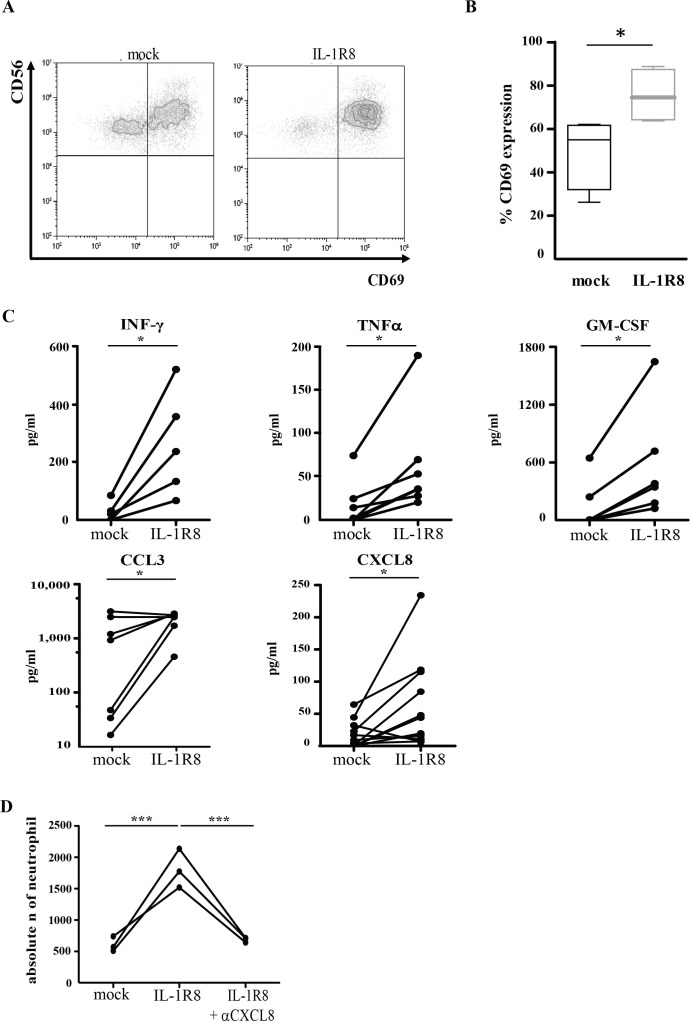
In agreement with the results of the gene array and as a further confirmation of the activated phenotype observed in IL-1R8-silenced NK cells, a significant increase of surface CD69 (figure 4A, B) was detected upon IL-15 stimulation. In addition, the release of significantly higher concentrations of IFN-γ, TNFα, GM-CSF, CCL3 and CXCL8 were observed in IL-1R8-silenced NK cells as compared with mock NK cells (figure 4C). Since CXCL8 and GM-CSF are both known to be potent neutrophil chemoattractants, we assayed whether supernatants from IL-15-activated IL-1R8-silenced NK cells could induce migration of these cells. Indeed, compared with supernatants from mock NK cells, a significant increase in the migration of neutrophils was reported in samples treated with medium derived from IL-1R8-silenced NK cells (figure 4D). The addition of anti-CXCL8 monoclonal antibody abrogated this effect, confirming that neutrophil migration was CXCL8 dependent (figure 4D). These data suggest a dynamic cellular crosstalk that might be crucial for the potentiation of the anti-tumor response.
Figure 4.
IL-1R8 silencing increases natural killer (NK) cell effector functions. (A, B) CD69 surface expression on mock-transfected and IL-1R8-siRNA-transfected NK cells following 18 hours activation with IL-15. The dot plot of a representative donor is shown in panel A. Percentage of CD69 expression from independent experiments performed on four healthy donors (panel B). Statistical significance has been determined by unpaired t test. *P<0.05. (C) Interferon γ (IFN-γ), tumor necrosis factor α (TNFα), GM-CSF, CCL3 and CXCL8 production (pg/mL) of mock-silenced and IL-1R8-silenced NK cells following 18 hours activation with IL-15. Each dot represents one donor. Statistical significance has been determined by paired t test. *P<0.05. (D) Transwell migration assay of human neutrophils with supernatants derived from mock-transfected or IL-1R8-transfected NK cells in the absence or in the presence of anti-CXCL8 monoclonal antibody. Independent experiments performed in three healthy donors have been reported. Statistical significance has been determined by one way analysis of variance. ***P<0.005.
In line with these findings, other authors have shown an inverse correlation between the IL-1R8 level and CD69 expression and/or IFN-γ production in human primary NK cells cultured with IL-18 and IL-12.7
Taken together, our results show that, upon IL-1R8 silencing, a general modification of gene expression occurs in resting human NK cells. In particular, while some crucial genes (such as GrzA, NKp30, CD16 a/b, Ki67, CCR1, CX3CR1) were upregulated, the expression of several genes regulating different NK cell pathways (ie, activating or chemokine receptors and cytokine release) resulted to be downregulated. Interestingly, the expression of this latter group of genes was significantly increased upon IL-15 treatment in the absence of IL-1R8. The increased profile of activating genes induced by IL-1R8 silencing after IL-15 stimulation was mirrored by a significant increase of both cytotoxicity and cytokine production. Of notice, similar results, although to a less extent, were also observed in IL-1R8- silenced NK cells upon IL-18 stimulation. Accordingly, the functional/phenotypic signature of IL-1R8-deficient murine NK cells has been related to the stimulating effects of IL-18 alone or in combination with other cytokines.7
Although IL-1R8-silenced NK cells showed a limited decrease of IL-1R8 on the cell surface, this causes a major increase of the IL-1R8-silenced NK cell function upon IL-15 activation, underscoring the relevance of our results. In addition, these data suggest the existence of some unknown interactions between IL-15 and IL-1R8 signatures. Despite the fact that IL-1R8 is known to negatively modulate IL1R and TLR downstream signaling events, it remains unclear how its deficiency might impact on IL-15-activated NK cells functions.
While our study has supplied interesting information on the effects of IL-1R8 silencing of resting NK cells, some limitations must be acknowledged. Indeed, gene expression analysis was performed following 2-hour stimulation with IL-15, raising the question of whether a different time point would have led to a further increase or even changes in the transcript levels. Moreover, even though we observed modulation of mRNA expression level of several genes, we prevalently focused our attention on the genes mainly involved in regulating NK cells anti-tumor activity. A further limitation to be considered is that IL-1R8 silencing through siRNA led to a transient modification of gene expression thus, long-term effects of such modification cannot be evaluated.
To exclude any bias of the system, such as modification in receptors expression and activation of signaling cascade due to release of molecules after electroporation,27 we analyzed IL-15 receptor expression and NFkB phosphorylation in not electroporated, mock-silenced and IL-1R8-silenced NK cells. No differences were observed in IL-15 receptors subunits expression and NFkB phosphorylation among all samples analyzed (data not shown).
In conclusion, compared with previous results on human NK cells,7 the novelties of the present study include: (1) the IL-1R8 silencing of human resting NK cells; (2) the upregulated expression of several genes on both resting- and IL-15-activated IL-1R8-silenced NK cells; (3) the increased function (activation markers, cytokine and chemokine production, and cytotoxicity against different tumor cell targets) of IL-1R8 silenced-NK cells at basal level and upon IL-15 and IL-18 activation.
Overall, this study provides a deeper insight into IL-1R8 regulatory role on human NK cells, confirming the data obtained in mice that IL-1R8 may be a detrimental player for NK cell-mediated anti-tumor responses. Thus, IL-1R8 could be considered a candidate target for the development of novel efficient NK cell-based therapies for the treatment of both adult and pediatric tumors.
Footnotes
NL, FRM and TI contributed equally.
Contributors: NL, FRM and TI designed and performed experiments, interpreted data and wrote the manuscript. CA and BR performed the experiments. AP and IV reviewed the manuscript. BA provided reagents. CG and AM discussed results and reviewed the manuscript. EM and LM designed experiments, discussed results and reviewed the manuscript.
Funding: This work was supported by grants from the Ministero della Salute (grant no. RC-2020 OPBG to LM and EM; GR-2018-12365485 to AP), Associazione Italiana per la Ricerca sul Cancro (project no. 5x1000 2018 Id 21147, projects no. IG 2017 Id 19920 to LM and IG 2019 Id 23465 to AM) and Ministero dell'Istruzione dell'Università e della Ricerca (PRIN, 20174T7NXL to CG). The project leading to these results was possible thanks to the i-CARE fellowship to NL awarded by AIRC and from the European Union’s Horizon 2020 research and innovation programme under the Marie Skłodowska-Curie grant agreement No. 800924NL. FRM, BR and CA are recipients of a grant awarded by Fondazione Veronesi.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
Data are available upon reasonable request. All data relevant to the study are included in the article or uploaded as supplementary information.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
This study involves human participants and was approved by Bambino Gesù Children’s Hospital 2058_OPBG_2020 and conducted the study in accordance with the tenants of the Declaration of Helsinki. Participants gave informed consent to participate in the study before taking part.
References
- 1.Thomassen E, Renshaw BR, Sims JE. Identification and characterization of SIGIRR, a molecule representing a novel subtype of the IL-1R superfamily. Cytokine 1999;11:389–99. 10.1006/cyto.1998.0452 [DOI] [PubMed] [Google Scholar]
- 2.Anselmo A, Riva F, Gentile S, et al. Expression and function of IL-1R8 (TIR8/SIGIRR): a regulatory member of the IL-1 receptor family in platelets. Cardiovasc Res 2016;111:373–84. 10.1093/cvr/cvw162 [DOI] [PubMed] [Google Scholar]
- 3.Molgora M, Supino D, Mantovani A, et al. Tuning inflammation and immunity by the negative regulators IL-1R2 and IL-1R8. Immunol Rev 2018;281:233–47. 10.1111/imr.12609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nold-Petry CA, Lo CY, Rudloff I, et al. Il-37 requires the receptors IL-18Rα and IL-1R8 (SIGIRR) to carry out its multifaceted anti-inflammatory program upon innate signal transduction. Nat Immunol 2015;16:354–65. 10.1038/ni.3103 [DOI] [PubMed] [Google Scholar]
- 5.Wald D, Qin J, Zhao Z, et al. SIGIRR, a negative regulator of Toll-like receptor-interleukin 1 receptor signaling. Nat Immunol 2003;4:920–7. 10.1038/ni968 [DOI] [PubMed] [Google Scholar]
- 6.Guo J, Zhan X, Xu G, et al. Transcriptomic analysis reveals that IL-1R8/Sigirr is a novel macrophage migration regulator and suppresses macrophage proliferation through p38 MAPK signaling pathway. Biomed Pharmacother 2020;124:109846. 10.1016/j.biopha.2020.109846 [DOI] [PubMed] [Google Scholar]
- 7.Molgora M, Bonavita E, Ponzetta A, et al. IL-1R8 is a checkpoint in NK cells regulating anti-tumour and anti-viral activity. Nature 2017;551:110–4. 10.1038/nature24293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Santana Carrero RM, Beceren-Braun F, Rivas SC, et al. IL-15 is a component of the inflammatory milieu in the tumor microenvironment promoting antitumor responses. Proc Natl Acad Sci U S A 2019;116:599–608. 10.1073/pnas.1814642116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Quatrini L, Vacca P, Tumino N, et al. Glucocorticoids and the cytokines IL-12, IL-15, and IL-18 present in the tumor microenvironment induce PD-1 expression on human natural killer cells. J Allergy Clin Immunol 2021;147:349–60. 10.1016/j.jaci.2020.04.044 [DOI] [PubMed] [Google Scholar]
- 10.Ingegnere T, Mariotti FR, Pelosi A, et al. Human CAR NK cells: a new non-viral method allowing high efficient transfection and strong tumor cell killing. Front Immunol 2019;10:957. 10.3389/fimmu.2019.00957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McGinnes K, Chapman G, Marks R, et al. A fluorescence NK assay using flow cytometry. J Immunol Methods 1986;86:7–15. 10.1016/0022-1759(86)90258-9 [DOI] [PubMed] [Google Scholar]
- 12.Veneziani I, Alicata C, Pelosi A, et al. Toll-like receptor 8 agonists improve NK-cell function primarily targeting CD56brightCD16- subset. J Immunother Cancer 2022;10:e003385. 10.1136/jitc-2021-003385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mariotti FR, Petrini S, Ingegnere T, et al. PD-1 in human NK cells: evidence of cytoplasmic mRNA and protein expression. Oncoimmunology 2019;8:1557030. 10.1080/2162402X.2018.1557030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pelosi A, Besi F, Tumino N, et al. NK cells and PMN-MDSCs in the graft from G-CSF mobilized haploidentical donors display distinct gene expression profiles from those of the Non-Mobilized counterpart. Front Immunol 2021;12:657329. 10.3389/fimmu.2021.657329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fakhr E, Zare F, Teimoori-Toolabi L. Precise and efficient siRNA design: a key point in competent gene silencing. Cancer Gene Ther 2016;23:73–82. 10.1038/cgt.2016.4 [DOI] [PubMed] [Google Scholar]
- 16.Jia C, Zhuge Y, Zhang S, et al. IL-37b alleviates endothelial cell apoptosis and inflammation in Kawasaki disease through IL-1R8 pathway. Cell Death Dis 2021;12:575. 10.1038/s41419-021-03852-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhao M, Li Y, Guo C, et al. Il-37 isoform D downregulates pro-inflammatory cytokines expression in a Smad3-dependent manner. Cell Death Dis 2018;9:582. 10.1038/s41419-018-0664-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mishra A, Sullivan L, Caligiuri MA. Molecular pathways: interleukin-15 signaling in health and in cancer. Clin Cancer Res 2014;20:2044–50. 10.1158/1078-0432.CCR-12-3603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Barton K, Muthusamy N, Fischer C, et al. The Ets-1 transcription factor is required for the development of natural killer cells in mice. Immunity 1998;9:555–63. 10.1016/S1074-7613(00)80638-X [DOI] [PubMed] [Google Scholar]
- 20.Gordon SM, Chaix J, Rupp LJ, et al. The transcription factors T-bet and Eomes control key checkpoints of natural killer cell maturation. Immunity 2012;36:55–67. 10.1016/j.immuni.2011.11.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kamizono S, Duncan GS, Seidel MG, et al. Nfil3/E4bp4 is required for the development and maturation of NK cells in vivo. J Exp Med 2009;206:2977–86. 10.1084/jem.20092176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.El-Brolosy MA, Stainier DYR. Genetic compensation: a phenomenon in search of mechanisms. PLoS Genet 2017;13:e1006780. 10.1371/journal.pgen.1006780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kurioka A, Cosgrove C, Simoni Y, et al. Cd161 defines a functionally distinct subset of pro-inflammatory natural killer cells. Front Immunol 2018;9:486. 10.3389/fimmu.2018.00486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Montaldo E, Vitale C, Cottalasso F, et al. Human NK cells at early stages of differentiation produce CXCL8 and express CD161 molecule that functions as an activating receptor. Blood 2012;119:3987–96. 10.1182/blood-2011-09-379693 [DOI] [PubMed] [Google Scholar]
- 25.Niogret C, Miah SMS, Rota G, et al. Shp-2 is critical for ERK and metabolic engagement downstream of IL-15 receptor in NK cells. Nat Commun 2019;10:1444. 10.1038/s41467-019-09431-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Canzonetta C, Pelosi A, Di Matteo S, et al. Identification of neuroblastoma cell lines with uncommon TAZ+/mesenchymal stromal cell phenotype with strong suppressive activity on natural killer cells. J Immunother Cancer 2021;9:e001313. 10.1136/jitc-2020-001313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Polajzer T, Jarm T, Miklavcic D. Analysis of damage-associated molecular pattern molecules due to electroporation of cells in vitro. Radiol Oncol 2020;54:317–28. 10.2478/raon-2020-0047 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
jitc-2021-003858supp001.pdf (2.3MB, pdf)
jitc-2021-003858supp002.pdf (2.1MB, pdf)
jitc-2021-003858supp003.pdf (3.4MB, pdf)
jitc-2021-003858supp004.pdf (1.5MB, pdf)
Data Availability Statement
Data are available upon reasonable request. All data relevant to the study are included in the article or uploaded as supplementary information.