Abstract
The search for the best wellness practice has promoted the development of devices integrating different technologies and guided meditation. However, the final effects on the electrical activity of the brain remain relatively sparse. Here, we have analyzed of the alpha and theta electroencephalographic oscillations during the realization of the arrest reaction (AR; eyes close/eyes open transition) when a chromotherapy session performed in a dedicated room [Rebalance (RB) device], with an ergonomic bed integrating pulsed-wave light (PWL) stimulation, guided breathing, and body scan exercises. We demonstrated that the PWL induced an evoked-related potential characterized by the N2-P3 components maximally recorded on the fronto-central areas and accompanied by an event-related synchronization (ERS) of the delta–theta–alpha oscillations. The power of the alpha and theta oscillations was analyzed during repeated ARs testing realized along with the whole RB session. We showed that the power of the alpha and theta oscillations was significantly increased during the session in comparison to their values recorded before. Of the 14 participants, 11 and 6 showed a significant power increase of the alpha and theta oscillations, respectively. These increased powers were not observed in two different control groups (n = 28) who stayed passively outside or inside the RB room but without any type of stimulation. These preliminary results suggest that PWL chromotherapy and guided relaxation induce measurable electrical brain changes that could be beneficial under neuropsychiatric perspectives.
Keywords: chromotherapy, EEG, visual evoked potentials, alpha-theta oscillations, wellness
Introduction
The development of different types of physical and mental exercises, such as relaxation, guided breathing (Doll et al., 2016), mental imagery (Cebolla et al., 2017), and meditation (Zorn et al., 2021), has acquired a paramount position in the Western world because of the increasing popular research of a singular serenity sustained by wellness practice (Cahn and Polich, 2006; Ryznar and Levine, 2021).
As the mindfulness-based stress reduction method proposed by Kabat-Zinn (1982), the scientific interest for mindfulness intervention has augmented exponentially (Creswell, 2017). Differently operationalized, mindfulness intervention consists of helping subjects to pay attention to their ongoing moment experience and to reach a self-regulated attentional state, emphasizing curiosity and openness, and relying on attention stability and meta-awareness (Quaglia et al., 2015, 2019). The effects of meditation practice on morphological plasticity of the brain have been reviewed by the meta-analysis of Fox et al. (2014), concluding its existence in meditators of consistent and medium-sized brain structure differences in the gray and white matter of the prefrontal cortex, sensory cortices, insula, and hippocampus. In parallel, EEG studies on the meditation effects have demonstrated the potentiation of the theta and alpha oscillations during these practices (Aftanas and Golocheikine, 2001; Lagopoulos et al., 2009; Henz and Schöllhorn, 2017).
Because of the positive outcomes of mindfulness practice on stress (Gotink et al., 2016), chronic pain (Hilton et al., 2017; Poletti et al., 2021), obesity (Lattimore, 2020), sleep (Kwekkeboom and Bratzke, 2016), emotion (Lutz et al., 2016; Kral et al., 2018; Makowski et al., 2019), and cognition (Tang et al., 2015), the number of practitioners has significantly increased in recent years. However, it is well known that the practice of meditation and even more mindfulness is not given to everyone. This is one reason environments like chromotherapy rooms (Azeemi and Raza, 2005; Azeemi et al., 2019) suitable for meditation, such as the present Rebalance (RB) device, have been developed. In this technological setup, the search for optimal comfort was carried out within an enclosure whose ceiling includes a continuous series of LEDs arranged in bars radiating from the center. The successive lighting of these LEDs produces a visual sensation of waves in movement that we have qualified for pulsed-wave light (PWL) stimulation. These eccentric and concentric visual stimulations at the theta–alpha rhythm may induce oscillatory entrainment. This brainwave entrainment is a new non-invasive technique able to reconfigure the oscillatory dynamics of the brain for a better mental health and cognitive performance (Lakatos et al., 2019; Addante et al., 2021; Poltorak, 2021; Quirk et al., 2021; Yadav et al., 2021).
In this context, the two main types of EEG oscillation, theta and alpha, represent appropriate rhythmic activities to be investigated during an RB session. The cortical theta is mainly influenced by the hippocampal theta oscillation, which is in turn modulated by the medial septum-diagonal band of Broca and the supramamillary nucleus playing the role of a subcortical theta pacemaker (Vertes and Kocsis, 1997; Vertes et al., 2004). A 6-Hz theta peak has been recorded in the human hippocampus (Kahana et al., 1999). A “slow-theta” (2.5–5 Hz) related to successful associative memory (Pastötter and Bäuml, 2014; Lega et al., 2016) and a “fast-theta” (5–9 Hz) related to phase reset and reinstatement of oscillatory patterns for successful recollection were well defined (Kota et al., 2020; Ter Wal et al., 2021).
Likewise, for the theta rhythm, the cortical alpha is influenced by a complex interplay between cortical and subcortical generators. Considered as the major alpha pacemaker, the thalamus (Lorincz et al., 2009) is additionally modulated by the reticular formation and the pulvinar nucleus (Saalmann and Kastner, 2009, 2011). Peaking at about 10 Hz, the alpha rhythm dominates the EEG and governs different sensorimotor modalities. It is thus well suited for studying the effect of the mixing of the different stimulations offered by the RB device. In addition, the alpha oscillation is considered as a global rhythmic coordinator of the brain activity providing top-down signals from higher hierarchical cortical areas to lower cortical and subcortical regions (e.g., thalamus and pulvinar) (Halgren et al., 2019). In this way, fluctuations in the top-down modulated alpha oscillation influence how visual information is treated during attention processes (Sauseng et al., 2005; Kelly et al., 2006).
The combination of different wellness practices (Zgierska et al., 2016; Hanley et al., 2021; Liu et al., 2021; Polaski et al., 2021) has demonstrated an increased efficiency promoting the development of devices, such as chromotherapy room. Chromotherapy is an ancient alternative medicine, often considered as a pseudoscience, planning to use the energy of electromagnetic radiations in the visible light spectrum to produce physiological and or psychological effects in humans.
Different commercially available systems have been developed for facilitating wellness. It has been advanced that the entrance into such chromotherapy rooms facilitated mindfulness and related practice. In addition, the exposition to oscillating red, green, and blue lights produced significant effects on the cardio-autonomic control by increasing the heart rate variability (Grote et al., 2013). By using the similar alternation of red, green, and blue light at 10 s corresponding to the blood pressure oscillation (as used in the present RB device), a resonance effect between the internal blood pressure rhythms and the external light oscillation may induce positive effects on cardio-autonomic regulation and allow subjective wellbeing (Grote et al., 2013). In the same vein, Tallon-Baudry et al. (2018) suggest that the conscious perception of visual input is the consequence of the integration of the visual content with the neural monitoring of bodily state in which the visceral inputs as the heart rate signaling are the important elements (Park et al., 2014, 2016). In this context, it was shown that the alpha peak frequency was correlated with the heart rate during wakefulness (Lechinger et al., 2015), and the alpha power was higher when attention was directed to the heart instead of a visual task (Villena-González et al., 2017).
The motivation to use repeated transition from the eye-closed (EC) to the eye-opened (EO), called the arrest reaction (AR) by Berger (1929) was 2-fold: (1) it is a convenient and reproducible maneuver for the quantification of the dynamics of the alpha rhythm (8–13 Hz), which is higher during the EC than during the EO condition (Cheron et al., 2006); (2) it follows the practice of mindfulness, which often requires the closing of the eyes to facilitate both exteroceptions- and interoceptions, (Jao et al., 2013; Bastarrika-Iriarte and Caballero-Gaudes, 2019). This EC state is often used as a physiological baseline state for the default mode network (DMN) of the human brain, upon which the performance of goal-directed behaviors can be realized (Raichle et al., 2001).
Based on the main characteristic of the RB device offering a combination of (1) meditation facilities (guided breathing and body scan), (2) chromotherapy, and (3) PWL entrainment, we hypothesize that the RB sessions can induce a power increase of the alpha and theta oscillations recorded at rest in the EC and EO state. For this, after studying the effect of PWL on the EEG dynamics, we have quantified the power spectrum of the alpha and theta EEG oscillations during repeated AR tests performed after each of the RB sessions.
Materials and Methods
Participants
The theta–alpha oscillation power spectrum variations were analyzed during AR in a total of 42 participants. These were separated into three age-matched groups (ranging from 22 to 65 years); the action in the RB (AIR) group (testing of the RB effect) (n = 14, female n = 5, male n = 9) and two control groups, the passive in the RB (PIR) group staying passively in the RB bed [n = 10, (female n = 4, male n = 6)] and the passive outside RB (POR) where participants were comfortably seated outside of the RB bed (n = 18, female n = 10, male n = 8). All participants were right-handed and had no neurological condition. The two control groups (non-athletes) were studied to verify the absence of modifications in the theta–alpha power along with the same experimental procedure but without the RB stimulation.
The effects of the combined stimulation (chromotherapy PWL + breathing and body scan exercises) provided by the RB system on the EEG oscillations were collected in the AIR group. Among the 14 participants, 10 of them were elite Belgian athletes, the older participant was a former elite athlete and present coach. The four remaining participants were non-elite athletes. Three of these participants have already experienced the RB system 1 month before the present study. In the PIR control group, participants have no previous practice of the RB system and were asked to lie in the RB bed and to stay calm, to keep their eyes open, and just to follow the audio orders. In the POR control group, the participants were asked to stay relaxed comfortably (Supplementary Table 1) and to eat, with their eyes continuously closed while their EEG was recorded for 34 min.
Each participant gave informed consent to the experimental procedures. All experimental protocols were approved by the Ethic comity of Université Libre de Bruxelles, CHU Brugmann, and were conducted in conformity with the European Union directive 2001/20/EC of the European Parliament.
The Rebalance Device
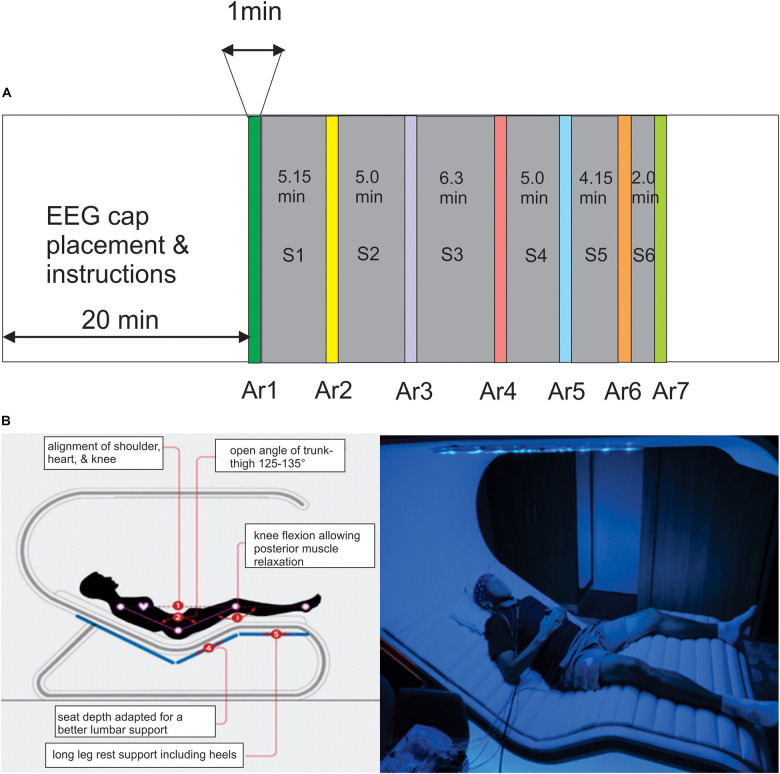
This device consists of an ergonomic bed integrating chromotherapy system (Figure 1B1, France). The covering top includes a circle of 16 radial arms each one including an array of LEDs providing a continuous or intermittent rhythmic wave (pulsed-wave light, PWL) presenting 8 different color lightings ranging from purple (415 nm) to red (720 nm) with different levels of illuminance ranging from 7 to 120 lx. The whole RB session consisted of 27.6 min during which chromotherapy stimulations and breathing exercises are proposed in six successive sub-sessions (S1–S6) (Figure 1A and Supplementary Table 1). The breathing exercises are voice-guided and composed of a cycle of five counts for inspiration, five counts for keeping the air at the end of inspiration followed by five counts for expiration, and five counts for keeping the expiration volume before reinitiating the cycle. These breathing exercises only occurred during S1, S3, and S4. A guided body scan exercise was performed only during S2.
FIGURE 1.
(A) The timing of the Rebalance (RB) session consisted of the succession of six modules interrupted by the arrest reaction (AR) testing (AR2–AR7) during which the power of the alpha oscillation was measured, AR1 is the control testing realized before the session. (B) Illustration of the RB impulse system in which the participants are comfortably placed in supine position and equipped with the EEG cap recording and earphones.
EEG Data Treatment
Cleaning of the Data
EEG data were recorded with a POR (Supplementary Table 1) system eego™ sports (EEG system LE-200, ANT Neuro b.v., Enschede, Netherlands) comprising an active-shield cap comfortably adjusted to the participant’s head using 64 Ag/AgCl sintered ring electrodes, the shielded co-axial cables (WaveGuard™ original cap; 10/10 electrode system placements) connected to an amplifier (ANT DC-eego amplifiers 2 kHz; CE Class IIa medical device), and a tablet [TRAVELline T10-B5 Pro Tablet, Atom (TM) x5-Z8350 1.44 GHz] placed close to the participant. The signals were recorded and displayed online via a Wi-Fi connection with an off-field computer (Dell Inc., Intel(R) Core™ i7-10510U). All recordings were initially referenced to the frontal CPz electrode. Vertical and horizontal eye movements (EOG) were recorded bipolarly. All electrode impedances were maintained below 5 kΩ. Scalp potentials were amplified and digitized at a rate of 2.048 Hz using a resolution of 16 bit (range 11 mV). To correct the dampening effect of the oscillation amplitude close to the reference point, we transformed the data by subtracting the average activity across all electrodes (i.e., average referencing). After downsampling to 512 Hz, a zero-phase IIR band-pass filter from 1 to 40 Hz was applied. The artifactual portions of the EEG data were rejected after an appropriate independent component analysis (ICA) performed with the EEGLAB software (v2021.0) (Delorme and Makeig, 2004).
Event-Related Potentials
For the time domain analysis, the event-related potentials (ERPs) were calculated by averaging baseline-corrected epochs extracted from − 1 to 1 s around the onset of the PWL stimulation occurring at a variable frequency of 3.4–5.6–8.2–10.8 Hz during the 6 RB stimulation sessions (S1–S6). A total of 214 trials per subject, including these different PWLs, were used for averaging. A pulse was produced by the system generating the PWL at the onset of each stimulation and that without any jitter. The ERP and the event-related spectral perturbation (ERSP) were thus triggered by these external pulses precisely synchronized with the onset of the PWL stimulation. This grand average analysis was only performed on seven participants’ data for which the electrical artifacts due to the PWL were easily identified and deleted by the ICA procedure and for which EMG and movement artifacts were easily removed. We used the scalp topography, temporal activity localization, and spectral magnitude criteria to identify the ICA related to artifacts (a maximum of four ICA components per participant were deleted).
Event-Related Spectral Perturbation and Intertrial Coherency
For the time-frequency analysis of EEG oscillations, we first calculated the baseline-normalized ERSP (Pfurtscheller and Neuper, 1994; Makeig et al., 2002). ERSP measures variations in the power spectrum of ongoing rhythms at specific periods and frequency ranges. In ERSP measurements, event-related desynchronization (ERD) indicates a power spectrum reduction while event-related synchronization (ERS) indicates a power spectrum increase. In parallel, the phase-locking of the ongoing oscillations was calculated by the intertrial coherency (ITC) analysis. FFT was used as a spectral decomposition technique from 2 to 40 Hz frequency range and performed on 2,048 time points with a time window of 200 points. A divisive baseline ranging from −1 s to 0 s was used.
Power and Frequency Peak Analysis of the Alpha and Theta Oscillation During the Arrest Reaction Testing
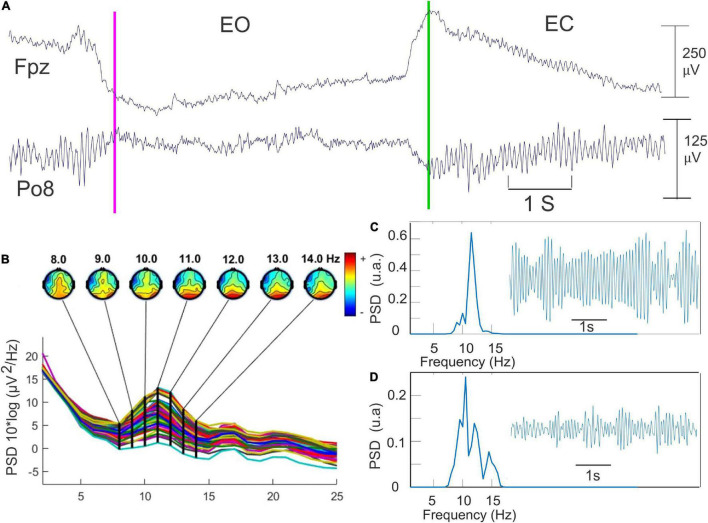
The occurrence of the EEG signals corresponding to the eyes opening and eyes closing was initially detected by visual inspection of the raw signals of the frontal electrodes (Figure 2A). The AR testing was performed before (AR1), during (AR2–AR6), and after (AR7) the whole RB session. Given the time constraints linked to the optimal use of the RB stimulations, each AR testing consisted of only five repetitions of 6 s eyes-closed and 6 s eyes-opened representing 1 min of recording. The duration of the six modules of RB stimulation ranged from 2 to 6.3 min. The total duration of the RB session was 34.6 min (27.6 min of stimulation and 7 min for the AR testing). About 20 min was needed for the placement of the EEG cap (Figure 2). The quantification of the alpha oscillation was repeatedly performed on the filtered signals (8–14 Hz) of each of these 6 s periods recorded by the parieto-occipital electrodes showing the bigger alpha amplitude (Figures 2B,C). We standardized the amplitude of these alpha oscillations by subtracting the mean amplitude from the original signals and dividing the result by the SD. The power spectral density (PSD) was then calculated using Welch’s averaged periodograms with a one-second Hamming window and a frequency resolution of 0.45 Hz with an overlapping of 50%. The maximal power and the frequency of the alpha were automatically extracted from the PSD data.
FIGURE 2.
The methodology used for the quantification of the alpha oscillation. (A) Detection of opening (pink line) and closing of the eyes (green line) in Fpz recording. The topographical map provided by the EEGLab toolbox for detecting the scalp electrode presenting the higher alpha power. (B) An example of 6 s of the filtered EEG data (8–14 Hz) recorded during the eye-closed (EC) state. (C) Fast Fourier transform of this data set corresponding of the EC state. (D) the same procedure as in C but during the EO state.
The individual frequency peak of the alpha oscillation has been used as a biomarker of stress and arousal during sports exercise but also as a stable neurophysiological marker (Christie et al., 2017) and a useful measurement for the evaluation of stress and fatigue recovery (Bertollo et al., 2017).
The same procedure was applied for the analysis of low and high theta oscillations. In this case, the EC and EO periods were pooled together, and appropriate filtering was used, 3.0–5.5 Hz and 5.5–8 Hz for the low and the high theta oscillations, respectively. The theta power quantifications were performed on the Cz electrode, which showed a greater amplitude and may thus correspond to the frontal midline theta (FM Theta), which is known to be extended from the anterior part of the frontal to the centro-parietal cortex (Piarulli et al., 2018).
In addition to the power analysis, the individual alpha and theta (low and high) peak frequencies (iPF) were measured during different AR testing methods (AR1–AR7).
Statistical Analysis
The independent variables are the different RB sessions, the alpha and theta powers while the dependent variables are the related frequency peaks measured during AR1–AR7. As these latter variables could be influenced by the RB active session, the RB environment by itself (without any stimulation), and the time-lapse, we also included two control groups one being inside the passive RB system (the PIR group) and the other one being outside the RB system (the POR group). Statistical analysis within each subject was followed by group statistics. For each subject of the AIR group, we tested the null hypothesis that the alpha and theta powers measured during the different AR periods (AR2–AR7) were not modified by the RB session (AR1 = AR2, AR1 = AR3, AR1 = AR4, AR1 = AR5, AR1 = AR6, AR1 = AR7). For this, the homogeneity and normality of the variances were first checked by the Bartlett and Shapiro–Wilk test. Then, parametric (t-test and ANOVA) or non-parametric (Kruskal–Wallis test) and the nparcomp function (Konietschke et al., 2015) were applied for the comparative analysis between the AR1 (control) and AR2–AR7 (RB) using the software R version 4.0.5. The same procedure was applied for the group statistics.
The results are reported as mean ± SD and illustrated in box plots. The level of significance was set at p < 0.05. Off-line data treatment and statistics were performed using MATLAB® (R2021a; MathWorks, Natick, MA, United States).
Results
Analysis of the Event-Related Potential and Event-Related Spectral Perturbation Produced by the Pulsed-Wave Light
Before undertaking the quantification of the alpha oscillation induced by the RB session during the AR testing, we have first examined the effect produced by the PWL stimulation on the EEG dynamics. For this, 214 PWLs per subject were used for the ERP and ERSP analysis. Figure 3 illustrated the ERP configuration on the whole scalp showing that the most significant ERP occurred in the fronto-central region with a first negative peaking at 331.6 ± 48.7 ms with an amplitude of 1.60 ± 0.40 μV (n = 6) followed by a positive component peaking at 476.0 ± 58.7 ms with an amplitude of 1.68 ± 0.68 μV (n = 6). These N331-P476 components were accompanied by an ERS of the delta–theta–alpha frequency band peaking at the latency of 354.4 ± 69.1 ms and an ITC in the same frequency band of 20–40% of the trials occurring at the transition between the N331 and the P476 component (Figures 4A–C). Despite the absence of a reliable ERP in the parieto-occipital region (Figures 3, 4D), the ERSP analysis showed the presence of long duration (from 200 to 1,000 ms) delta-theta–alpha ERD (Figure 4D). Even though our working hypothesis was focused on the alpha oscillation, the presence of a significant ERS in the theta band induced by the PWL stimulation has motivated the analysis of the power of the low and high theta oscillation during the AR testing.
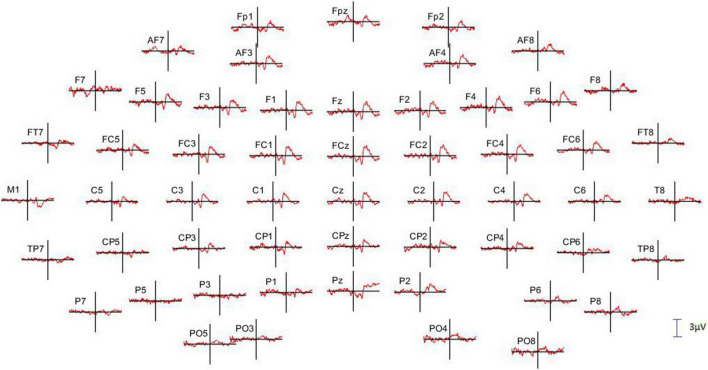
FIGURE 3.
Full scalp array of grand average event-related potential (ERP; n = 6 subjects) showing the fronto-central distribution of the N331-P476 components triggered by the onset of the PWL. The vertical lines indicate the onset of the PWL. Only 55 of the 64 electrodes are illustrated, and the missing ones present too many artifacts. The calibration bar corresponds to 3 μV, positivity up.
FIGURE 4.
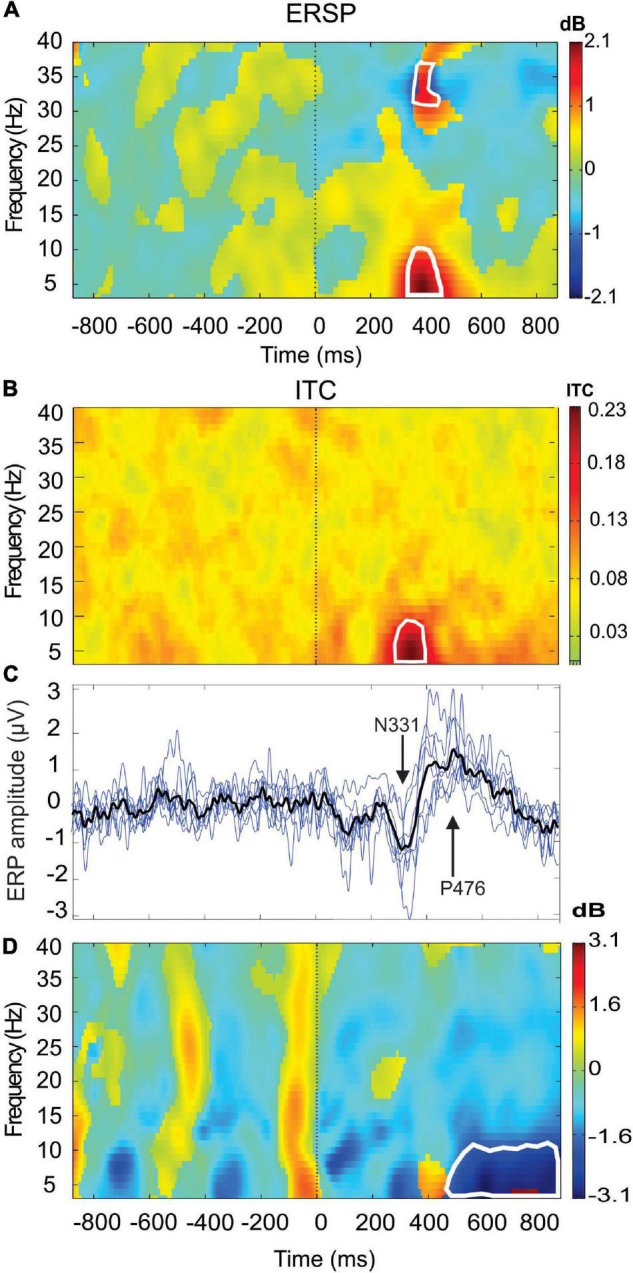
Event-related spectral perturbation (ERSP) (A) and intertrial coherency (ITC) (B) (grand average n = 6 subjects) are recorded at the Fz electrode showing the event-related synchronization (ERS) in the delta-theta–alpha frequency bands concomitant of a theta phase-locking (red area). Also note the presence of beta-gamma ERS. In (C), superimposition of the ERPs of the six single subjects (blue lines) (n = 214 PWL stimulation) and the grand average ERP (black line, n = 1,284). In (D), ERSP is recorded in PO8 electrodes showing a theta–alpha event-related desynchronization (ERD) (blue area). The areas of statistical significance at p < 0.01 are marked by white squares.
Analysis of the Alpha Power During the Arrest Reaction Testing
For each subject, the alpha analysis performed on the EEG data filtered from 8 to 13 Hz was therefore carried out 35 times during the 34.6 min of the RB session distributed according to the six different types of PWL stimulation and breathing exercises.
For the majority of participants (11 of 14), a significant increase in the alpha oscillation between at least the AR1 and one of the other AR tests (AR2–AR7) was reported in the E0 (n = 8 participants) and the EC (n = 5 participants). Two participants increased the alpha power in both EO and EC conditions, and in three participants the alpha power was not significantly modified by the RB sessions.
Figure 5A illustrates the changes in the spectral power of the alpha rhythm measured in the EO state between all RB sessions (S1–S6) in the eight subjects who showed a significant increase during at least one of the six AR tests (AR2–AR7). In the control situation (AR1), i.e., before the session, the mean power of the alpha was 0.19 ± 0.08 and a significant increase was observed in AR5 for which a value of 0.39 ± 0.15 was reached (p = 0.00002, Kruskal–Wallis test) after the session S4. It is interesting to note that the S4 session consisted of the Generic Square breathing maneuver in addition to PWL stimulation. For the other measurements (AR2, AR3, AR4, AR6, and AR7), the observed increases were not statistically significant and a break in the progressive increase from AR1 to AR5 was observed. When these alpha power values were normalized, they reached 224% and 144% during AR5 and AR4, respectively, of the control value (p < 0.00007 and p < 0.0009, Kruskal–Wallis test). The stability of the alpha rhythm individual frequency peak (ranging from 10.48 ± 1.18 to 10.88 ± 1.03 Hz) throughout the RB sessions was observed for this group of participants (Figure 5B). When all the participants (n = 14) (responders and non-responders) were taken into account, the power increase of the alpha oscillation between AR1 and AR5 in the EO state remained significant (p < 0.0009, Kruskal–Wallis test) reaching 169% of the control value.
FIGURE 5.
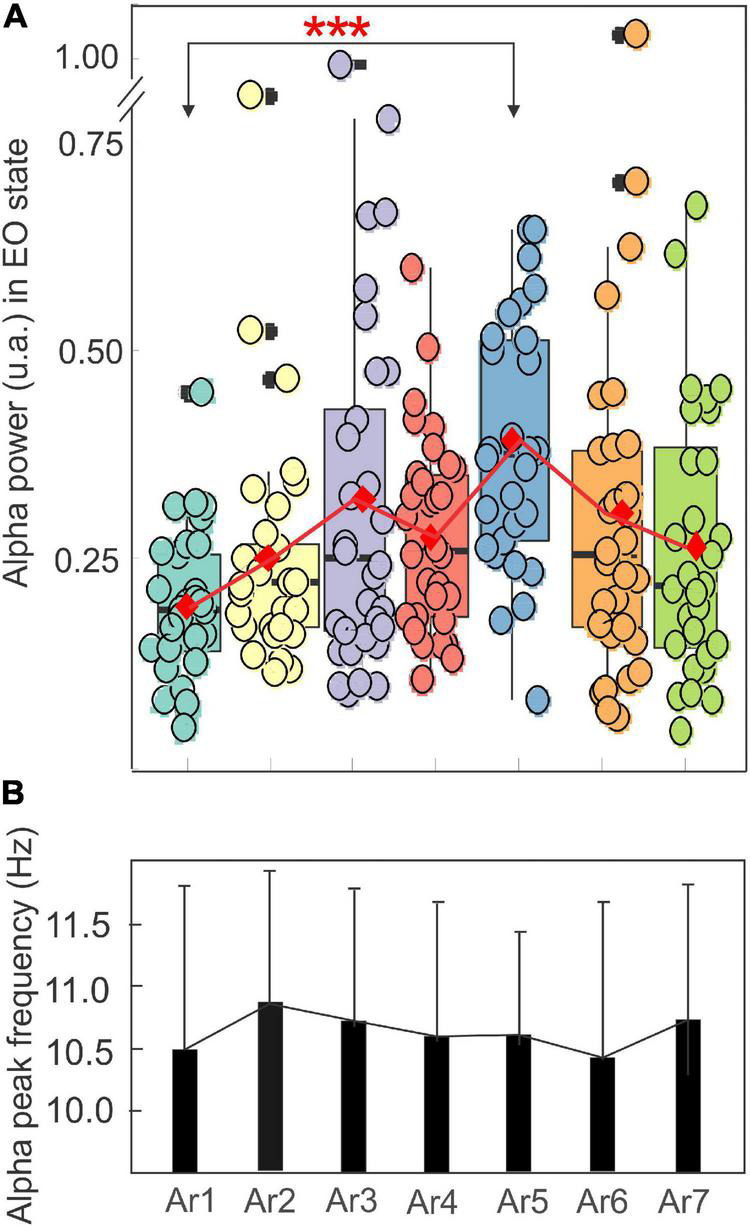
Evolution of the mean ± SD of the power in a box plot configuration (A) and of the frequency peak (B) of the alpha rhythm with the eyes open from AR1 (control before the RB session) to AR7, in the eight subjects who showed an increase of the alpha oscillation for at least one AR testing (AR2–AR7).
By contrast, the alpha power remained constant throughout the control situation performed by the first control group in which the participants stay passively in the RB system without any type of stimulation. In this situation, the alpha power was 0.13 ± 0.13 before the session (AR1) and never significantly increased higher than 0.14 ± 0.13 (AR4) (Figure 6A).
FIGURE 6.
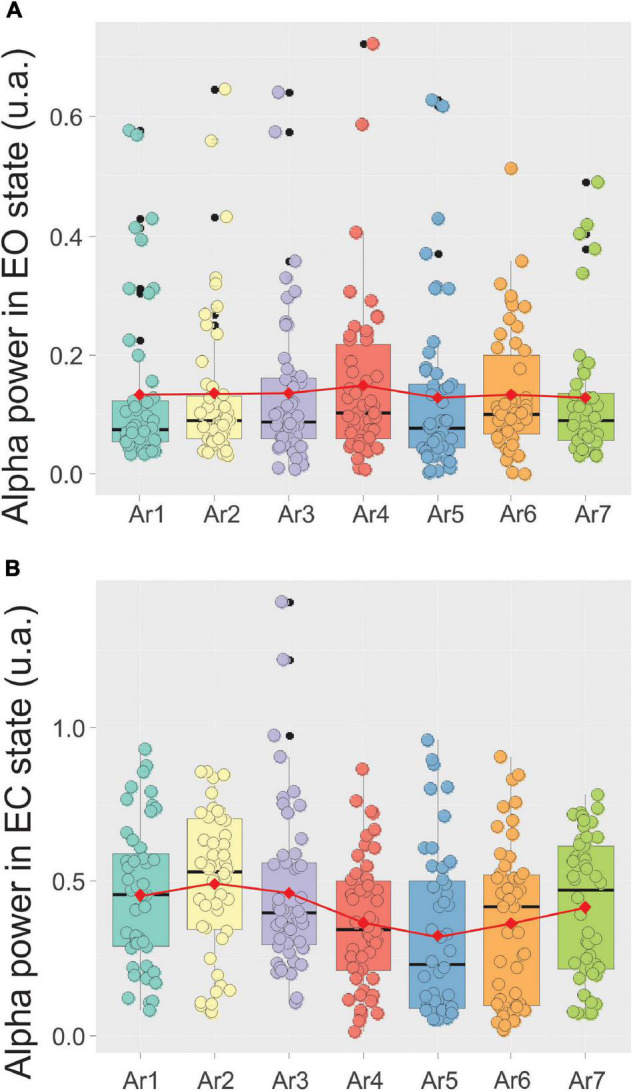
Evolution of the mean ± SD of the alpha power in a box plot configuration in the EC state (A) and the eye opened (EO) state (B) from AR1 (control before the RB session) to AR7, in the 10 subjects of the first control group.
Figure 7 illustrates, using the same arrangement as in Figure 5, the evolution of the spectral power of the alpha rhythm measured in the EC state between all RB sessions in the five subjects exhibiting a significant increase during at least one of the six AR tests. The stability of the alpha rhythm frequency peak for this group of participants (ranging from 10.79 ± 0.73 to 11.51 ± 0.9 Hz) was also observed throughout the RB session (Figure 7B). In the control situation (AR1), the mean alpha power was 0.31 ± 0.18 and a significant increase was observed during AR4 where it reached a value of 0.52 ± 0.23 (p < 0.03, Kruskal–Wallis test) and maintained a value of 0.56 ± 0.22 (p < 0.005) during the AR5 test. For the other measurements (AR2, AR3, AR6, and AR7), the observed increases were not statistically significant. When these alpha power values were normalized, they reached a percentage higher than 152% in each AR testing with the highest score in AR5 of 219%. When all the participants were taken into account, a value of 139% was obtained in AR5 but did not reach a significant level. By contrast, the alpha power recorded in the EC state in the first group of control remained unchanged and was 0.45 ± 0.23 before the session (AR1) and never increased to more than 0.49 ± 0.23 recorded in AR2 (Figure 6B). The stability of the alpha power in the EC state along with a comparable period than the RB session was checked in the second group of control for which a value of 0.62 ± 0.05 was obtained before the session (AR1) and never increased to more than 0.63 ± 0.05 during the 34 min of recording.
FIGURE 7.
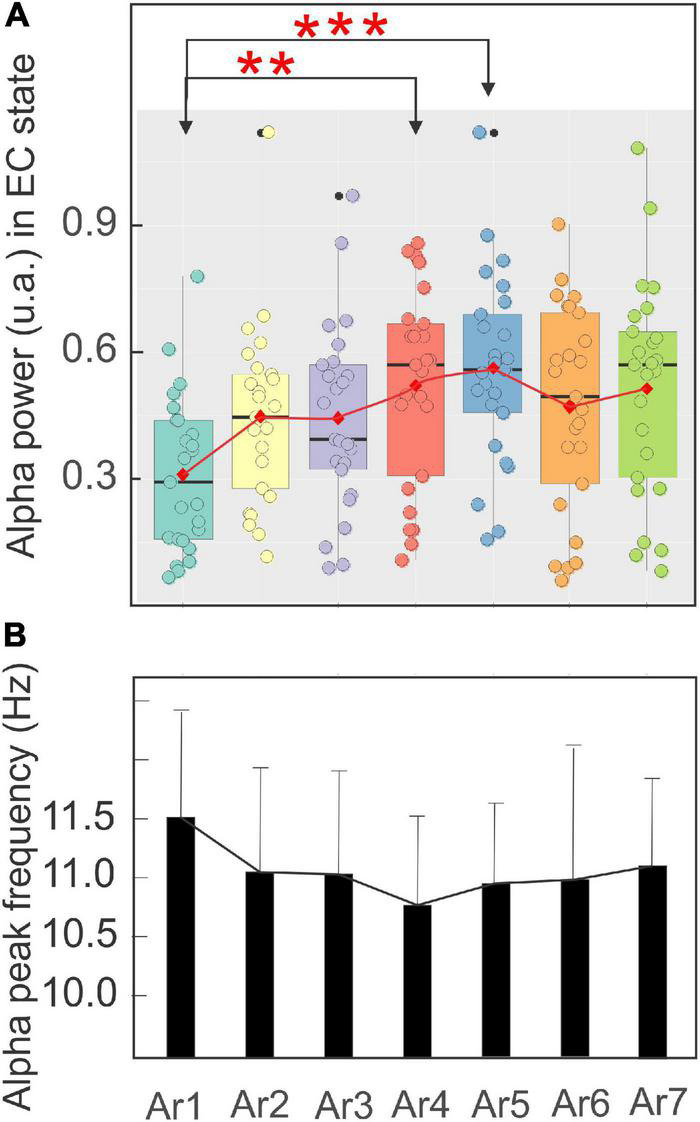
Evolution of the mean ± SD of the power in a boxplot configuration (A) and of the frequency peak (B) of the alpha rhythm with the eyes closed from AR1 (control before the RB session) to AR7, in the five subjects who showed an increase of the alpha oscillation for at least one AR testing (AR2–AR7). The asterisk indicated the level of significance *p < 0.05 and **p < 0.005.
The analysis of the difference between the alpha power peaks measured during the EC and EO state showed that in 10 participants (of the 14) this value increased during the active RB session for at least one AR but reached a statistical significance for only three participants. For the other three participants, this value decreased (with no significance) and in one participant the difference remained unchanged during the RB session.
We also analyzed the relation between the mean initial control value (AR1) and the mean alpha score reached during the RB session (AR2–AR7). We obtained a significant correlation for the EC state (Figure 8A) (r = − 0.59, p = 0.0005) but not for the EO state (Figure 8B).
FIGURE 8.
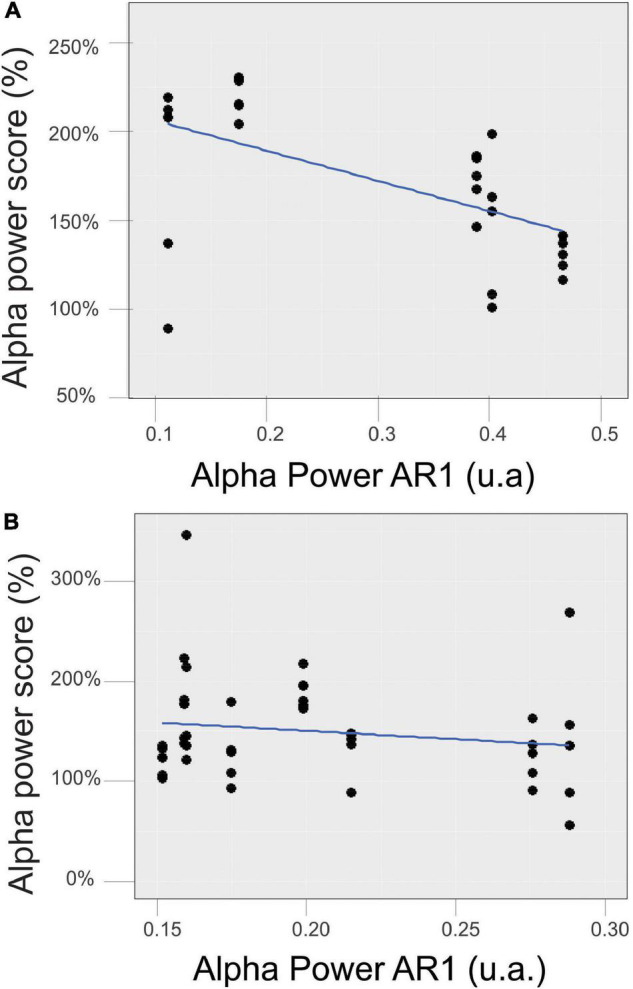
Relationship between the mean initial control value of the alpha power in the EC (A) and EO (B) states measured during AR1 and the mean score reached during the RB session (AR2–AR7).
Analysis of the Low Theta and High Theta Power During the Arrest Reaction Testing
The same analysis procedure was performed for the low-frequency (3–5.5 Hz) and high-frequency (5.5–8 Hz) theta rhythm. Figure 9A illustrates, according to the same arrangement as in Figure 5, the evolution of the spectral power of the low-frequency theta rhythm (EC and EO pooled) during the RB session in six subjects exhibiting a significant increase of these rhythms during at least one of the six AR testing.
FIGURE 9.
Evolution of the mean ± SD of the low theta (A) and high theta (C) (EC and EO pooled) power in a boxplot configuration and of the respective frequency peaks (B,D) (control before the RB session) to AR7, in the six subjects who showed an increase of these theta oscillations for at least one AR testing (AR2–AR7). The asterisk indicated the level of significance *p < 0.05 and **p < 0.005.
In the control situation (AR1), the mean power of the low theta was 0.27 ± 0.07 and a significant increase is observed from AR3 where it reached a value of 0.39 ± 0.11 (p < 0.001, Kruskal–Wallis test) and 0.41 ± 0.11 (p < 0.0001) during the session AR4, 0.40 ± 0.10 during AR6 (p < 0.0007) and 0.39 ± 0.12 during AR7 (p < 0.0015) (Figure 9A). Expressed in percentage, these increased powers were about 50%. For the other measurements (AR2 and AR5), the observed increases were not statistically significant. Figure 9B illustrates the frequency stability of the low theta rhythm (ranging from 3.67 ± 0.97 to 4.04 ± 0.98 Hz) without a significant variation throughout the RB session.
In AR1, the mean power of the high theta was 0.22 ± 0.09 and a significant increase is observed only from AR6 where it is 0.31 ± 0.13 (p < 0.002, Kruskal–Wallis test) and maintaining a value of 0.30 ± 0.13 (p < 0.02) during the AR7 test. It should be noted that despite a larger average value from AR2 (0.35 ± 0.19), the statistical threshold was not reached because the dispersion of the data was too excessive (Figure 9C). Figure 9D illustrates the stability of the frequency of the high theta rhythm (ranging from 6.54 ± 1.2 to 6.82 ± 1.5 Hz) without a significant variation throughout the RB session.
Discussion
Event-Related Potential During the Pulsed-Wave Light Stimulation
Our findings first demonstrated that the PWL stimulation RB was able to produce an ERP in the fronto-central region characterized by an N331-P476 component accompanied by an ERS of the delta–theta–alpha frequency band. These ERP components can be viewed as the N2-P3 commonly described in the literature (Greimel et al., 2013; Decroix et al., 2020) relative to the involvement of the visual dorsal pathway considered as the “where” pathway originating in the magnocellular layer of the lateral geniculate nucleus (LGN) to the primary visual cortex (V1), forward to the middle temporal area (MT/V5) and then to the posterior parietal cortex. From this important node, the dorsal pathway reaches the prefrontal, premotor, and medial temporal cortex (Kravitz et al., 2011). The parieto–prefrontal cortex is implicated in spatial working memory while the premotor cortex supports visually-guided action and the medial temporal cortex is implicated in navigation. The distribution on the scalp of the present N2-P3 ERP and the presence of theta ERS may indicate the contribution of the visual dorsal pathway during the PWL stimulation.
Because the present PWL is composed of different colors ranging from purple (415 nm) to red (720 nm), the activation of the short-wavelength (S) and long medium-wavelength (L/M) cones may logically contribute to some extent to the evoked response by the way of the parvocellular ventral pathway (Horwitz, 2020). Besides, it has been also proposed that some color signaling may reach the MT area of the dorsal pathway assuming a functional grouping of the parvocellular and magnocellular inputs (Conway, 2014).
It is important to note that the mixing nature of this PWL stimulation, including different colors and luminance motion, provides a definitive conclusion about a comparative analysis of the classical N2 related to the onset motion of simple visual item peaking at about 200 ms (Hirai et al., 2009) with the present N2. However, the longer latency of the present N2 (331 ms) could correspond to the second negativity related to the biological motion described by Krakowski et al. (2011) presenting a latency between 200 and 350 ms and a source bilaterally located in the posterior temporal cortex within the human homolog of the MT gyrus in monkeys and the posterior superior temporal sulcus. In the same trend, our N2 activity approximatively corresponds to the second component described by Hirai et al. (2003), the N2c of Inuggi et al. (2018), and “N300” named by Jokisch et al. (2005). Using coherent moving dots, Lange-Malecki et al. (2018) have also recorded similar fronto-central negativity (at FCz), peaking around 200–400 ms after the onset of the motion stimulus and called this component N2. Previously, we have shown that a similar ERSP during a video of human walking 3D-animation was also characterized by a delta–theta ERS of long duration accompanied by an alpha ERD (Zarka et al., 2014). Decreases in alpha power have been related to anticipative modulation of attention (Capotosto et al., 2009, 2012), visual perception, and encoding (Foxe and Snyder, 2011; Van Diepen et al., 2019). A significant ERD of the mu rhythms (8–13 Hz) in response to the biological motion was also described by Ulloa and Pineda (2007). After the N331 component, the P476 may correspond to the positivity recorded at centro-parietal electrodes starting after about 400 ms of biological motion when it was attended (Krakowski et al., 2011) and interpreted by these authors as associated with the deciphering of the meaning of the motion stimulus.
Although the present PWL motion was entirely different from biological motion, it could be possible that this late positivity represents a perceptual closure task (Krakowski et al., 2011) assumed by a top-down mechanism (Kröger et al., 2014) integrating a global moving wave effect produced by colored and luminance associated to breathing exercise. According to “embodied cognition” principles, the present active RB system associating PWL stimulation alternating eccentric and concentric motion with breathing maneuvers probably induces interoceptive signals about bodily states, which can modify the significance of the visual inputs (Belli et al., 2021) contributing to a peculiar abstract shaping of the brain states during the RB sessions.
The pulsed light effect mimicking concentric and eccentric waves may induce steady-state visual evoked potential-like (SSVEP-like) response and the possible entrainment of alpha neural generators during the RB sessions. However, the presence of numerous variations of color, luminance, and PWL frequency during the same session precluded the possibility to characterize such SSVEP-like in our participants. The possibility of the existence of a direct post-SSVEP effect during the AR testing must be taken into account. However, the recent study of Otero et al. (2020) demonstrated that the duration of the persistence effect of sinusoidally varying light stimulation inducing the SSVEP response was 241.30 ± 119.96 ms, which was too short for inducing a significant interference in the alpha power values obtained during the repeated AR measurements. In addition, the relatively low ITC values peaking at the same latency of the theta–alpha ERS could be explained by the fact that the entrainment effect was disturbed by the averaging of the different PWL frequencies.
The Effects of Rebalance Session on the Alpha and Theta Oscillation
The RB session was able to significantly increase the power of alpha and the theta oscillations during the AR testing in all 14 subjects. Seven subjects have increased the alpha and theta power, three subjects only the theta power (they were alpha “non-responders”), and four subjects only the alpha power. We also found that a lower alpha power preceding the RB session was linked to an effective increase of the alpha power during the RB session in the EC state. Neither the PIR nor the POR control groups show any significant variation.
Even though the alpha oscillation (peaking around 10 Hz) is the dominant EEG rhythm in awake relaxed subjects, their basic physiological understanding and related functions remind largely open to discussion (Cheron et al., 2006, 2016; Foxe and Snyder, 2011; Sadaghiani and Kleinschmidt, 2016; Cebolla and Cheron, 2019; Van Diepen et al., 2019; Samaha et al., 2020; Wilson and Foxe, 2020). In an earlier reported by Berger (1929), the dominant alpha rhythm significantly increases when the eyes are closed and decreases when the eyes are opened, which is called the AR achieved in numerous studies (Adrian and Matthews, 1934; Klimesch et al., 1997; Klimesch, 1999; Cheron et al., 2006; Bazanova and Vernon, 2014). Interestingly, alpha can also increase when the eyes are open if an opal glass bowl is placed in front of the participant’s face producing a uniform visual field (Figure 9 of Adrian and Matthews, 1934) and once it reaches a certain level it potentially remain increased even if the illumination of the bowl is modified Adrian and Matthews (1934).
In our study, the alpha oscillation in the EO was performed in darkness in the passive RB environment, which might help the alpha occurrence. This corroborates more recent studies showing that alpha oscillation with eyes open was reinforced in a dark quiet room during the multimodal Ganzfeld, which consisted of an audio-visual environment with an unfilled homogenous color visual field and waterfall noise (Pütz et al., 2006; Wackermann et al., 2008; Miskovic et al., 2019). A recent MEG study by Popov et al. (2019) demonstrated that the alpha power modulation of the visual cortex is retinotopically organized and that alpha ERD reflects a gain increase or engagement in the attended visual field while alpha ERS reflects a gain decrease or disengagement in the unattended field. Popov et al. (2019) insisted that these modulations are due to internal attention rather than an external stimulus input. We did not find such hemispherical alpha modulation in our study which could be explained by the type of visual stimulation used here which covered the entire visual field and the fact that there was not any spatial attentional demand. Considering that alpha showed ERD during the RB sessions, we speculate that the alpha power increase during the AR testing could be due to a rebound effect as is explained by the inhibition hypothesis of Klimesch et al. (2007).
It has been reported that the alpha oscillation power increase on the right frontal area is correlated with spiritual experience during a sensory deprivation environment (Glicksohn and Ben-Soussan, 2020). We did not find the alpha but theta power increase in frontal regions; no participant of our study reported any spiritual or mystic experience during the RB sessions.
The Individual Alpha Peak Frequency Was Not Modified by Rebalance Sessions
Participants’ mean individual alpha peak frequency (IAPF) corresponds to the higher IAPF values reported by Bazanova and Vernon (2014) in 96 healthy subjects, which could be explained by the fact that the majority of our participants belonged to sports elites and that the IAPF was known to be higher in good performers [cognitive composite performance (Bazanova et al., 2009; Grandy et al., 2013a,b)]. On the contrary, IAPF decrease is related to the occurrence of fatigue, bad performance (Klimesch et al., 1993), and cognitive preparedness (Grandy et al., 2013a,b). The IAPF remained stable in our study.
Theta rhythm is considered as a basic physiological element of the global oscillatory synchronization processes connecting multiple brain regions (Buzsáki and Draguhn, 2004; Buzsáki, 2005; Fries, 2005). A clear theta peak at ∼6 Hz has been recorded in the human hippocampus (Kahana et al., 1999) during the navigation task (Kaplan and Friston, 2018). Interestingly, the duration of the theta ERS produced by the onset of navigational movement was about 300 ms, a value very close to the present ERS evoked by the PWL onset. In human intracranial EEG recordings, Raghavachari et al. (2006) have also reported the theta oscillation during a working memory task in the middle frontal gyrus. In addition, the theta oscillation is in close connection with the activities of the DMN (Raichle et al., 2001; Hsieh and Ranganath, 2014; Raichle, 2015), and the theta in the frontal midline (FMT) is negatively correlated with blood oxygenation level-dependent (BOLD) signal of the DMN (Scheeringa et al., 2008). As the same correlation was reported between a high power of alpha oscillation and a low BOLD signal, we may speculate that the RB session could favor a cerebral low energetic demand.
The multiplicity of functional roles played by this oscillation concern also the memory function. It has been demonstrated that the theta power increase occurred during successful encoding and promoted the emergence of gamma oscillation being linked to the formation of new episodic memories (Sederberg et al., 2003). Still, the relation between memory formation and theta oscillation is not simple and it depends on context matching. Staudigl and Hanslmayr (2013) demonstrated that high theta power increased when the memory encoding was associated with a successful recognition, whereas it decreased in the opposite case. During working memory retrieval, the number of to-be-distinguished items is correlated with an increase of the theta power (Meyer et al., 2015). The theta oscillation contributes to setting the dynamics of memory encoding and retrieval inside the cortical circuits (Hasselmo and Stern, 2014) engaging the spatio-temporal coding realized into the hippocampus and entorhinal cortex. In addition and considering that spatial attention is a discontinuous process, intrinsic frontoparietal theta, assumes such rhythmic sampling formed by two attentional states alternating between engagement (enhanced perception) and disengagement (reduced perception) (Fiebelkorn and Kastner, 2018, 2020). It was demonstrated in the macaque that these theta-dependent states are coordinated by the pulvinar nucleus (Fiebelkorn et al., 2019).
We may speculate that in the present situation, the PWL induced a visual stimulus-driven condition without a visual goal-directed condition while the breathing demands created a mixing situation where the theta could play an integrative rule.
The modulation of ongoing theta oscillations implicating long-range theta coherence across the neocortex has also been found during the decision process (Cohen and Donner, 2013). In this context, it has been recently proposed that theta coherence between the medial prefrontal cortex (mPFC) and the anterior cingulate cortex (ACC) predicts the speed and the task performance and represents a crucial mechanism for a performing cognitive control (Myers et al., 2021). In the same way, numerous studies (Helfrich et al., 2019; Kaiser and Schütz-Bosbach, 2021) have suggested that the theta oscillation assumed a general control mechanism, exerting a top-down control during both sensorimotor interference and goal behavior. Gärtner et al. (2014) demonstrated the existence of a close link between working memory performance and the theta oscillation (4–8 Hz) which were both reduced under stress conditions. Furthermore, this FM theta helps to maintain the working memory process and is significantly vulnerable to aging (Tóth et al., 2014).
A recent study dedicated to the detection of Alzheimer’s disease using EEG (Özbek et al., 2021) showed that alpha and theta powers were higher in young than in the older healthy subject. All these might suggest that the oscillatory power increases observed after the RB session might reflect a positive outcome.
The theta and alpha oscillations shared similar properties (Bruegger and Abegg, 2021; Liang et al., 2021). For example, their frequency and power have been linked to attention and perception. The fact that the power of both oscillations was significantly increased but with the maintenance of their frequency peak during the RB session points to a common gain effect exerted by the combination of PWL and breathing exercises. For Aftanas and Golocheikine (2001), the selective increase of theta and alpha oscillating contribution during meditative practice may be viewed with the states of internalized attention and emotionally positive “blissful” experience as those often reported by the practitioners of RB devices.
Despite the heterogeneity of meditative methods (Deolindo et al., 2020), the enhancement of the theta oscillation (Tanaka et al., 2014; DeLosAngeles et al., 2016; Tang et al., 2019) and its synchronization throughout multiple areas of the brain (Wong et al., 2015; Lardone et al., 2018) represent consistent results, which might be linked to the power theta increase reproduced in this study. Because PWL stimulation and breathing exercises are potentially able to induce oscillatory modulation of the brain rhythms, it is at first sight difficult to dissociate in the present effects those due to guided breathing from those due to the PWL stimulation. The absence of breathing during S2, S5, and S6 indicates that breathing was not the raison of the alpha and theta power behavior, which was maintained at higher levels during AR3, AR6, and AR7. This suggests that the effect of the PWL chromotherapy alone (in the absence of the guided breathing) was able to induce theta–alpha oscillation potentiation.
It is important to note that the present results cannot dissociate the effects of the stimulation from the athletic level on the participants and that other non-stimulation-related factors might have contributed to the observed group differences. Further studies will need to integrate control groups composed of athletes distributed in AIR, PIR, and POR conditions. Based on these preliminary results, we may advance that the present chromotherapy PWL stimulation and guided relaxation, inducing oscillatory entrainment can rapidly increase the power of theta–alpha brain oscillations measured during EC–EO wakefulness states. This could be further explored as complementary neuropsychiatric perspectives.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
The studies involving human participants were reviewed and approved by Ethics committee of Université Libre de Bruxelles, CHU Brugmann, and conducted in conformity with the European Union directive 2001/20/EC of the European Parliament. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
A-MC and GC conceived the original idea. GC, MP, and CS designed the experiment. MP, DR, A-MC, DZ, and GC performed the experiments. DR and MP performed the data analysis. GC wrote the manuscript. A-MC contributed to the writing of this manuscript. All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We would like to thank the research fund of the Université Libre de Bruxelles, the Leibu fund, and the Brain & Society Foundation, Belgium. We would also like to thank J. Borlée and S. Dan for their valuable discussions and constructive suggestions throughout this work, as well as T. D’Angelo, E. Toussaint, and E. Hortmanns, for their expert technical assistance, E. Pecoraro for secretarial assistance, and S. Mongold for revising the English text.
Footnotes
Funding
This study received funding from the Research fund of the Université Libre de Bruxelles, the Leibu fund and, the Brain & Society Foundation.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpsyg.2022.792872/full#supplementary-material
References
- Addante R. J., Yousif M., Valencia R., Greenwood C., Marino R. (2021). Boosting brain waves improves memory. Front. Young Minds 9:605677. 10.3389/frym.2021.605677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adrian E. D., Matthews B. H. C. (1934). The Berger rhythm: potential changes from the occipital lobes in man. Brain 57 355–385. 10.1093/brain/awp324 [DOI] [PubMed] [Google Scholar]
- Aftanas L. I., Golocheikine S. A. (2001). Human anterior and frontal midline theta and lower alpha reflect emotionally positive state and internalized attention: high-resolution EEG investigation of meditation. Neurosci. Lett. 310 57–60. 10.1016/s0304-3940(01)02094-8 [DOI] [PubMed] [Google Scholar]
- Azeemi S. T. Y., Rafiq H. M., Ismail I., Kazmi S. R., Azeemi A. (2019). The mechanistic basis of chromotherapy: current knowledge and future perspectives. Complement. Ther. Med. 46 217–222. 10.1016/j.ctim.2019.08.025 [DOI] [PubMed] [Google Scholar]
- Azeemi S. T. Y., Raza M. (2005). A critical analysis of chromotherapy and its scientific evolution. Evid. Based Complement. Alternat. Med. 2 481–488. 10.1093/ecam/neh137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bastarrika-Iriarte A., Caballero-Gaudes C. (2019). Closing eyes during auditory memory retrieval modulates alpha rhythm but does not alter tau rhythm. Neuroimage 197 60–68. 10.1016/j.neuroimage.2019.04.053 [DOI] [PubMed] [Google Scholar]
- Bazanova O. M., Mernaya E. M., Shtark M. B. (2009). Biofeedback in psychomotor training. Electrophysiological basis. Neurosci. Behav. Physiol. 39 437–447. 10.1007/s11055-009-9157-z [DOI] [PubMed] [Google Scholar]
- Bazanova O. M., Vernon D. (2014). Interpreting EEG alpha activity. Neurosci. Biobehav. Rev. 44 94–110. 10.1016/j.neubiorev.2013.05.007 [DOI] [PubMed] [Google Scholar]
- Belli F., Felisatti A., Fischer M. H. (2021). “BreaThink”: breathing affects production and perception of quantities. Exp. Brain Res. 239 2489–2499. 10.1007/s00221-021-06147-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger H. (1929). Über das elektrenkephalogramm des menschen. Arch. Für Psychiatr. Nervenkrankh. 87 527–570. [Google Scholar]
- Bertollo M., Nakamura F., Bortoli L., Robazza C. (2017). “Psychophysiological features of soccer players’ recovery-stress balance during the inseason competitive phase,” in Sport, Recovery and Performance: Interdisciplinary Insights, eds Kellmann M., Beckmann J. (Abingdon: Routledge; ), 75–86. [Google Scholar]
- Bruegger D., Abegg M. (2021). Prediction of cortical theta oscillations in humans for phase-locked visual stimulation. J. Neurosci. Methods 361:109288. 10.1016/j.jneumeth.2021.109288 [DOI] [PubMed] [Google Scholar]
- Buzsáki G. (2005). Theta rhythm of navigation: link between path integration and landmark navigation, episodic and semantic memory. Hippocampus 15 827–840. 10.1002/hipo.20113 [DOI] [PubMed] [Google Scholar]
- Buzsáki G., Draguhn A. (2004). Neuronal oscillations in cortical networks. Science 304 1926–1929. 10.1126/science.1099745 [DOI] [PubMed] [Google Scholar]
- Cahn B. R., Polich J. (2006). Meditation states and traits: EEG, ERP, and neuroimaging studies. Psychol. Bull. 132 180–211. 10.1037/0033-2909.132.2.180 [DOI] [PubMed] [Google Scholar]
- Capotosto P., Babiloni C., Romani G. L., Corbetta M. (2009). Frontoparietal cortex controls spatial attention through modulation of anticipatory alpha rhythms. J. Neurosci. 29 5863–5872. 10.1523/JNEUROSCI.0539-09.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capotosto P., Babiloni C., Romani G. L., Corbetta M. (2012). Differential contribution of right and left parietal cortex to the control of spatial attention: a simultaneous EEG-rTMS study. Cereb. Cortex 2012 446–454. 10.1093/cercor/bhr127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cebolla A. M., Cheron G. (2019). Understanding neural oscillations in the human brain: from movement to consciousness and vice versa. Front. Psychol. 10:1930. 10.3389/fpsyg.2019.01930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cebolla A.-M., Palmero-Soler E., Leroy A., Cheron G. (2017). EEG spectral generators involved in motor imagery: a swLORETA study. Front. Psychol. 8:2133. 10.3389/fpsyg.2017.02133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheron G., Leroy A., De Saedeleer C., Bengoetxea A., Lipshits M., Cebolla A., et al. (2006). Effect of gravity on human spontaneous 10-Hz electroencephalographic oscillations during the arrest reaction. Brain Res. 1121 104–116. 10.1016/j.brainres.2006.08.098 [DOI] [PubMed] [Google Scholar]
- Cheron G., Petit G., Cheron J., Leroy A., Cebolla A., Cevallos C., et al. (2016). Brain oscillations in sport: toward EEG biomarkers of performance. Front. Psychol. 7:246. 10.3389/fpsyg.2016.00246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christie S., di Fronso S., Bertollo M., Werthner P. (2017). Individual alpha peak frequency in ice hockey shooting performance. Front. Psychol. 8:762. 10.3389/fpsyg.2017.00762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen M. X., Donner T. H. (2013). Midfrontal conflict-related theta-band power reflects neural oscillations that predict behavior. J. Neurophysiol. 110 2752–2763. 10.1152/jn.00479.2013 [DOI] [PubMed] [Google Scholar]
- Conway B. R. (2014). Color signals through dorsal and ventral visual pathways. Vis. Neurosci. 31 197–209. 10.1017/S0952523813000382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creswell J. D. (2017). Mindfulness interventions. Annu. Rev. Psychol. 68 491–516. [DOI] [PubMed] [Google Scholar]
- Decroix J., Roger C., Kalénine S. (2020). Neural dynamics of grip and goal integration during the processing of others’ actions with objects: an ERP study. Sci. Rep. 10:5065. 10.1038/s41598-020-61963-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delorme A., Makeig S. (2004). EEGLAB: an open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. J. Neurosci. Methods 134 9–21. 10.1016/j.jneumeth.2003.10.009 [DOI] [PubMed] [Google Scholar]
- DeLosAngeles D., Williams G., Burston J., Fitzgibbon S. P., Lewis T. W., Grummett T. S., et al. (2016). Electroencephalographic correlates of states of concentrative meditation. Int. J. Psychophysiol. 110 27–39. 10.1016/j.ijpsycho.2016.09.020 [DOI] [PubMed] [Google Scholar]
- Deolindo C. S., Ribeiro M. W., Aratanha M. A., Afonso R. F., Irrmischer M., Kozasa E. H. (2020). A critical analysis on characterizing the meditation experience through the electroencephalogram. Front. Syst. Neurosci. 14:53. 10.3389/fnsys.2020.00053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doll A., Hölzel B. K., Mulej Bratec S., Boucard C. C., Xie X., Wohlschläger A. M., et al. (2016). Mindful attention to breath regulates emotions via increased amygdala-prefrontal cortex connectivity. Neuroimage 134 305–313. 10.1016/j.neuroimage.2016.03.041 [DOI] [PubMed] [Google Scholar]
- Fiebelkorn I. C., Kastner S. (2018). A rhythmic theory of attention. Trends Cogn. Sci. 23 87–101. 10.1016/j.tics.2018.11.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiebelkorn I. C., Kastner S. (2020). Functional specialization in the attention network. Annu. Rev. Psychol. 71 221–249. 10.1146/annurev-psych-010418-103429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiebelkorn I. C., Pinsk M. A., Kastner S. (2019). The mediodorsal pulvinar coordinates the macaque fronto-parietal network during rhythmic spatial attention. Nat. Commun. 10:215. 10.1038/s41467-018-08151-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox K. C. R., Nijeboer S., Dixon M. L., Floman J. L., Ellamil M., Rumak S. P., et al. (2014). Is meditation associated with altered brain structure? A systematic review and meta-analysis of morphometric neuroimaging in meditation practitioners. Neurosci. Biobehav. Rev. 43 48–73. 10.1016/j.neubiorev.2014.03.016 [DOI] [PubMed] [Google Scholar]
- Foxe J. J., Snyder A. C. (2011). The role of alpha-band brain oscillations as a sensory suppression mechanism during selective attention. Front. Psychol. 2:154. 10.3389/fpsyg.2011.00154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fries P. (2005). A mechanism for cognitive dynamics: neuronal communication through neuronal coherence. Trends Cogn. Sci. 9 474–480. 10.1016/j.tics.2005.08.011 [DOI] [PubMed] [Google Scholar]
- Gärtner M., Rohde-Liebenau L., Grimm S., Bajbouj M. (2014). Working memory-related frontal theta activity is decreased under acute stress. Psychoneuroendocrinology 43 105–113. 10.1016/j.psyneuen.2014.02.009 [DOI] [PubMed] [Google Scholar]
- Glicksohn J., Ben-Soussan T. D. (2020). Immersion, absorption, and spiritual experience: some preliminary findings. Front. Psychol. 11:2118. 10.3389/fpsyg.2020.02118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotink R. A., Meijboom R., Vernooij M. W., Smits M., Hunink M. G. M. (2016). 8-week mindfulness based stress reduction induces brain changes similar to traditional long-term meditation practice – a systematic review. Brain Cogn. 108 32–41. 10.1016/j.bandc.2016.07.001 [DOI] [PubMed] [Google Scholar]
- Grandy T. H., Werkle-Bergner M., Chicherio C., Lövdén M., Schmiedek F., Lindenberger U. (2013a). Individual alpha peak frequency is related to latent factors of general cognitive abilities. Neuroimage 79 10–18. 10.1016/j.neuroimage.2013.04.059 [DOI] [PubMed] [Google Scholar]
- Grandy T. H., Werkle-Bergner M., Chicherio C., Schmiedek F., Lövdén M., Lindenberger U. (2013b). Peak individual alpha frequency qualifies as a stable neurophysiological trait marker in healthy younger and older adults. Psychophysiology 50 570–582. 10.1111/psyp.12043 [DOI] [PubMed] [Google Scholar]
- Greimel E., Bartling J., Dunkel J., Brückl M., Deimel W., Remschmidt H., et al. (2013). The temporal dynamics of coherent motion processing in autism spectrum disorder: evidence for a deficit in the dorsal pathway. Behav. Brain Res. 251 168–175. 10.1016/j.bbr.2013.05.055 [DOI] [PubMed] [Google Scholar]
- Grote V., Kelz C., Goswami N., Stossier H., Tafeit E., Moser M. (2013). Cardio-autonomic control and wellbeing due to oscillating color light exposure. Physiol. Behav. 114–115 55–64. 10.1016/j.physbeh.2013.03.007 [DOI] [PubMed] [Google Scholar]
- Halgren M., Ulbert I., Bastuji H., Fabó D., Erõss L., Rey M., et al. (2019). The generation and propagation of the human alpha rhythm. Proc. Natl. Acad. Sci. U.S.A. 116 23772–23782. 10.1073/pnas.1913092116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanley A. W., Garland E. L., Zingg R. W. (2021). Mindfulness-based waiting room intervention for osteopathic manipulation patients: a pilot randomized controlled trial. J. Osteopath. Med. 121 337–348. 10.1515/jom-2020-0186 [DOI] [PubMed] [Google Scholar]
- Hasselmo M. E., Stern C. E. (2014). Theta rhythm and the encoding and retrieval of space and time. Neuroimage 85 Pt 2 656–666. 10.1016/j.neuroimage.2013.06.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helfrich R. F., Breska A., Knight R. T. (2019). Neural entrainment and network resonance in support of top-down guided attention. Curr. Opin. Psychol. 29 82–89. 10.1016/j.copsyc.2018.12.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henz D., Schöllhorn W. I. (2017). EEG brain activity in dynamic health qigong training: same effects for mental practice and physical training? Front. Psychol. 8:154. 10.3389/fpsyg.2017.00154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilton L., Hempel S., Ewing B. A., Apaydin E., Xenakis L., Newberry S., et al. (2017). Mindfulness meditation for chronic pain: systematic review and meta-analysis. Ann. Behav. Med. Publ. Soc. Behav. Med. 51 199–213. 10.1007/s12160-016-9844-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirai M., Fukushima H., Hiraki K. (2003). An event-related potentials study of biological motion perception in humans. Neurosci. Lett. 344 41–44. 10.1016/s0304-3940(03)00413-0 [DOI] [PubMed] [Google Scholar]
- Hirai M., Watanabe S., Honda Y., Kakigi R. (2009). Developmental changes in point-light walker processing during childhood and adolescence: an event-related potential study. Neuroscience 161 311–325. 10.1016/j.neuroscience.2009.03.026 [DOI] [PubMed] [Google Scholar]
- Horwitz G. D. (2020). Signals related to color in the early visual cortex. Annu. Rev. Vis. Sci. 6 287–311. 10.1146/annurev-vision-121219-081801 [DOI] [PubMed] [Google Scholar]
- Hsieh L.-T., Ranganath C. (2014). Frontal midline theta oscillations during working memory maintenance and episodic encoding and retrieval. Neuroimage 85 721–729. 10.1016/j.neuroimage.2013.08.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inuggi A., Campus C., Vastano R., Saunier G., Keuroghlanian A., Pozzo T. (2018). Observation of point-light-walker locomotion induces motor resonance when explicitly represented; an eeg source analysis study. Front. Psychol. 9:303. 10.3389/fpsyg.2018.00303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jao T., Vértes P. E., Alexander-Bloch A. F., Tang I. N., Yu Y. C., Chen J. H., et al. (2013). Volitional eyes opening perturbs brain dynamics and functional connectivity regardless of light input. Neuroimage 69 21–34. 10.1016/j.neuroimage.2012.12.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jokisch D., Daum I., Suchan B., Troje N. F. (2005). Structural encoding and recognition of biological motion: evidence from event-related potentials and source analysis. Behav. Brain Res. 157 195–204. 10.1016/j.bbr.2004.06.025 [DOI] [PubMed] [Google Scholar]
- Kabat-Zinn J. (1982). An outpatient program in behavioral medicine for chronic pain patients based on the practice of mindfulness meditation: theoretical considerations and preliminary results. Gen. Hosp. Psychiatry 4 33–47. 10.1016/0163-8343(82)90026-3 [DOI] [PubMed] [Google Scholar]
- Kahana M. J., Sekuler R., Caplan J. B., Kirschen M., Madsen J. R. (1999). Human theta oscillations exhibit task dependence during virtual maze navigation. Nature 399 781–784. 10.1038/21645 [DOI] [PubMed] [Google Scholar]
- Kaiser J., Schütz-Bosbach S. (2021). Motor interference, but not sensory interference, increases midfrontal theta activity and brain synchronization during reactive control. J. Neurosci. 41 1788–1801. 10.1523/JNEUROSCI.1682-20.2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan R., Friston K. J. (2018). Planning and navigation as active inference. Biol. Cybern. 112 323–343. 10.1007/s00422-018-0753-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly S. P., Lalor E. C., Reilly R. B., Foxe J. J. (2006). Increases in alpha oscillatory power reflect an active retinotopic mechanism for distracter suppression during sustained visuospatial attention. J. Neurophysiol. 95 3844–3851. 10.1152/jn.01234.2005 [DOI] [PubMed] [Google Scholar]
- Klimesch W. (1999). EEG alpha and theta oscillations reflect cognitive and memory performance: a review and analysis. Brain Res. Brain Res. Rev. 29 169–195. 10.1016/s0165-0173(98)00056-3 [DOI] [PubMed] [Google Scholar]
- Klimesch W., Doppelmayr M., Pachinger T., Ripper B. (1997). Brain oscillations and human memory: EEG correlates in the upper alpha and theta band. Neurosci. Lett. 238 9–12. 10.1016/s0304-3940(97)00771-4 [DOI] [PubMed] [Google Scholar]
- Klimesch W., Sauseng P., Hanslmayr S. (2007). EEG alpha oscillations: the inhibition-timing hypothesis. Brain Res. Rev. 53 63–88. 10.1016/j.brainresrev.2006.06.003 [DOI] [PubMed] [Google Scholar]
- Klimesch W., Schimke H., Pfurtscheller G. (1993). Alpha frequency, cognitive load and memory performance. Brain Topogr. 5 241–251. 10.1007/BF01128991 [DOI] [PubMed] [Google Scholar]
- Konietschke F., Placzek M., Schaarschmidt F., Hothorn L. A. (2015). nparcomp: An R Software package forNonparametric multiple comparisons and simultaneous CIs. J. Stat. Softw. 64 1–16. 10.1007/978-1-4899-7180-7_1 [DOI] [Google Scholar]
- Kota S., Rugg M. D., Lega B. C. (2020). Hippocampal theta oscillations support successful associative memory formation. J. Neurosci. 40 9507–9518. 10.1523/jneurosci.0767-20.2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krakowski A. I., Ross L. A., Snyder A. C., Sehatpour P., Kelly S. P., Foxe J. J. (2011). The neurophysiology of human biological motion processing: a high-density electrical mapping study. Neuroimage 56 373–383. 10.1016/j.neuroimage.2011.01.058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kral T. R. A., Schuyler B. S., Mumford J. A., Rosenkranz M. A., Lutz A., Davidson R. J. (2018). Impact of short- and long-term mindfulness meditation training on amygdala reactivity to emotional stimuli. Neuroimage 181 301–313. 10.1016/j.neuroimage.2018.07.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kravitz D. J., Saleem K. S., Baker C. I., Mishkin M. (2011). A new neural framework for visuospatial processing. Nat. Rev. Neurosci. 12 217–230. 10.1038/nrn3008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kröger A., Bletsch A., Krick C., Siniatchkin M., Jarczok T. A., Freitag C. M., et al. (2014). Visual event-related potentials to biological motion stimuli in autism spectrum disorders. Soc. Cogn. Affect. Neurosci. 9 1214–1222. 10.1093/scan/nst103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwekkeboom K. L., Bratzke L. C. (2016). A systematic review of relaxation, meditation, and guided imagery strategies for symptom management in heart failure. J. Cardiovasc. Nurs. 31 457–468. 10.1097/JCN.0000000000000274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagopoulos J., Xu J., Rasmussen I., Vik A., Malhi G. S., Eliassen C. F., et al. (2009). Increased theta and alpha EEG activity during nondirective meditation. J. Altern. Complement. Med. 15 1187–1192. 10.1089/acm.2009.0113 [DOI] [PubMed] [Google Scholar]
- Lakatos P., Gross J., Thut G. (2019). A new unifying account of the roles of neuronal entrainment. Curr. Biol. 29 R890–R905. 10.1016/j.cub.2019.07.075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lange-Malecki B., Treue S., Rothenberger A., Albrecht B. (2018). Cognitive control over visual motion processing – are children with ADHD especially compromised? a pilot study of flanker task event-related potentials. Front. Hum. Neurosci. 12:491. 10.3389/fnhum.2018.00491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lardone A., Liparoti M., Sorrentino P., Rucco R., Jacini F., Polverino A., et al. (2018). Mindfulness meditation is related to long-lasting changes in hippocampal functional topology during resting state: a magnetoencephalography study. Neural Plast. 2018:5340717. 10.1155/2018/5340717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lattimore P. (2020). Mindfulness-based emotional eating awareness training: taking the emotional out of eating. Eat. Weight Disord. 25 649–657. 10.1007/s40519-019-00667-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lechinger J., Heib D. P. J., Gruber W., Schabus M., Klimesch W. (2015). Heartbeat-related EEG amplitude and phase modulations from wakefulness to deep sleep: interactions with sleep spindles and slow oscillations. Psychophysiology 52 1441–1450. 10.1111/psyp.12508 [DOI] [PubMed] [Google Scholar]
- Lega B., Burke J., Jacobs J., Kahana M. J. (2016). Slow-Theta-to-Gamma Phase-amplitude coupling in human hippocampus supports the formation of new episodic memories. Cereb. Cortex 26 268–278. 10.1093/cercor/bhu232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang M., Zheng J., Isham E., Ekstrom A. (2021). Common and distinct roles of frontal midline theta and occipital alpha oscillations in coding temporal intervals and spatial distances. J. Cogn. Neurosci. 33 2311–2327. 10.1162/jocn_a_01765 [DOI] [PubMed] [Google Scholar]
- Liu Y.-W., Chen Z.-H., Luo J., Yin M.-Y., Li L.-L., Yang Y.-D., et al. (2021). Explore combined use of transcranial direct current stimulation and cognitive training on executive function after stroke. J. Rehabil. Med. 53:jrm00162. 10.2340/16501977-2807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorincz M. L., Kékesi K. A., Juhász G., Crunelli V., Hughes S. W. (2009). Temporal framing of thalamic relay-mode firing by phasic inhibition during the alpha rhythm. Neuron 63 683–696. 10.1016/j.neuron.2009.08.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutz J., Brühl A. B., Doerig N., Scheerer H., Achermann R., Weibel A., et al. (2016). Altered processing of self-related emotional stimuli in mindfulness meditators. Neuroimage 124 958–967. 10.1016/j.neuroimage.2015.09.057 [DOI] [PubMed] [Google Scholar]
- Makeig S., Westerfield M., Jung T. P., Enghoff S., Townsend J., Courchesne E., et al. (2002). Dynamic brain sources of visual evoked responses. Science 295 690–694. [DOI] [PubMed] [Google Scholar]
- Makowski D., Sperduti M., Lavallée S., Nicolas S., Piolino P. (2019). Dispositional mindfulness attenuates the emotional attentional blink. Conscious. Cogn. 67 16–25. 10.1016/j.concog.2018.11.004 [DOI] [PubMed] [Google Scholar]
- Meyer L., Grigutsch M., Schmuck N., Gaston P., Friederici A. D. (2015). Frontal-posterior theta oscillations reflect memory retrieval during sentence comprehension. Cortex 71 205–218. 10.1016/j.cortex.2015.06.027 [DOI] [PubMed] [Google Scholar]
- Miskovic V., Bagg J. O., Ríos M., Pouliot J. J. (2019). Electrophysiological and phenomenological effects of short-term immersion in an altered sensory environment. Conscious. Cogn. 70 39–49. 10.1016/j.concog.2019.02.003 [DOI] [PubMed] [Google Scholar]
- Myers J. C., Chinn L. K., Sur S., Golob E. J. (2021). Widespread theta coherence during spatial cognitive control. Neuropsychologia 160:107979. 10.1016/j.neuropsychologia.2021.107979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otero M., Prado-Gutiérrez P., Weinstein A., Escobar M.-J., El-Deredy W. (2020). Persistence of EEG alpha entrainment depends on stimulus phase at offset. Front. Hum. Neurosci. 14:139. 10.3389/fnhum.2020.00139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Özbek Y., Fide E., Yener G. G. (2021). Resting-state EEG alpha/theta power ratio discriminates early-onset Alzheimer’s disease from healthy controls. Clin. Neurophysiol. 132 2019–2031. 10.1016/j.clinph.2021.05.012 [DOI] [PubMed] [Google Scholar]
- Park H.-D., Bernasconi F., Bello-Ruiz J., Pfeiffer C., Salomon R., Blanke O. (2016). Transient modulations of neural responses to heartbeats covary with bodily self-consciousness. J. Neurosci. 36 8453–8460. 10.1523/JNEUROSCI.0311-16.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park H.-D., Correia S., Ducorps A., Tallon-Baudry C. (2014). Spontaneous fluctuations in neural responses to heartbeats predict visual detection. Nat. Neurosci. 17 612–618. 10.1038/nn.3671 [DOI] [PubMed] [Google Scholar]
- Pastötter B., Bäuml K.-H. T. (2014). Distinct slow and fast cortical theta dynamics in episodic memory retrieval. Neuroimage 94 155–161. 10.1016/j.neuroimage.2014.03.002 [DOI] [PubMed] [Google Scholar]
- Pfurtscheller G., Neuper C. (1994). Event-related synchronization of mu rhythm in the EEG over the cortical hand area in man. Neurosci. Lett. 174 93–96. 10.1016/0304-3940(94)90127-9 [DOI] [PubMed] [Google Scholar]
- Piarulli A., Zaccaro A., Laurino M., Menicucci D., De Vito A., Bruschini L., et al. (2018). Ultra-slow mechanical stimulation of olfactory epithelium modulates consciousness by slowing cerebral rhythms in humans. Sci. Rep. 8:6581. 10.1038/s41598-018-24924-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polaski A. M., Phelps A. L., Smith T. J., Helm E. R., Morone N. E., Szucs K. A., et al. (2021). Integrated meditation and exercise therapy: a randomized controlled pilot of a combined nonpharmacological intervention focused on reducing disability and pain in patients with chronic low back pain. Pain Med. 22 444–458. 10.1093/pm/pnaa403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poletti S., Abdoun O., Zorn J., Lutz A. (2021). Pain regulation during mindfulness meditation: phenomenological fingerprints in novices and experts practitioners. Eur. J. Pain 25 1583–1602. 10.1002/ejp.1774 [DOI] [PubMed] [Google Scholar]
- Poltorak A. (2021). Replicating cortical signatures may open the possibility for “Transplanting” brain states via brain entrainment. Front. Hum. Neurosci. 15:710003. 10.3389/fnhum.2021.710003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popov T., Gips B., Kastner S., Jensen O. (2019). Spatial specificity of alpha oscillations in the human visual system. Hum. Brain Mapp. 40 4432–4440. 10.1002/hbm.24712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pütz P., Braeunig M., Wackermann J. (2006). EEG correlates of multimodal ganzfeld induced hallucinatory imagery. Int. J. Psychophysiol. 61 167–178. 10.1016/j.ijpsycho.2005.09.002 [DOI] [PubMed] [Google Scholar]
- Quaglia J. T., Goodman R. J., Brown K. W. (2015). From mindful attention to social connection: the key role of emotion regulation. Cogn. Emot. 29 1466–1474. 10.1080/02699931.2014.988124 [DOI] [PubMed] [Google Scholar]
- Quaglia J. T., Zeidan F., Grossenbacher P. G., Freeman S. P., Braun S. E., Martelli A., et al. (2019). Brief mindfulness training enhances cognitive control in socioemotional contexts: behavioral and neural evidence. PLoS One 14:e0219862. 10.1371/journal.pone.0219862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quirk C. R., Zutshi I., Srikanth S., Fu M. L., Devico Marciano N., Wright M. K., et al. (2021). Precisely timed theta oscillations are selectively required during the encoding phase of memory. Nat. Neurosci. 24 1614–1627. 10.1038/s41593-021-00919-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raghavachari S., Lisman J. E., Tully M., Madsen J. R., Bromfield E. B., Kahana M. J. (2006). Theta oscillations in human cortex during a working-memory task: evidence for local generators. J. Neurophysiol. 95 1630–1638. 10.1152/jn.00409.2005 [DOI] [PubMed] [Google Scholar]
- Raichle M. E. (2015). The brain’s default mode network. Annu. Rev. Neurosci. 38 433–447. [DOI] [PubMed] [Google Scholar]
- Raichle M. E., MacLeod A. M., Snyder A. Z., Powers W. J., Gusnard D. A., Shulman G. L. (2001). A default mode of brain function. Proc. Natl. Acad. Sci. U. S. A. 98 676–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryznar E., Levine R. B. (2021). Twelve tips for mindful teaching and learning in medical education. Med. Teach. 1–8. [Epub ahead of print] 10.1080/0142159X.2021.1901869 [DOI] [PubMed] [Google Scholar]
- Saalmann Y. B., Kastner S. (2009). Gain control in the visual thalamus during perception and cognition. Curr. Opin. Neurobiol. 19 408–414. 10.1016/j.conb.2009.05.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saalmann Y. B., Kastner S. (2011). Cognitive and perceptual functions of the visual thalamus. Neuron 71 209–223. 10.1016/j.neuron.2011.06.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadaghiani S., Kleinschmidt A. (2016). Brain networks and α-oscillations: structural and functional foundations of cognitive control. Trends Cogn. Sci. 20 805–817. 10.1016/j.tics.2016.09.004 [DOI] [PubMed] [Google Scholar]
- Samaha J., Iemi L., Haegens S., Busch N. A. (2020). Spontaneous brain oscillations and perceptual decision-making. Trends Cogn. Sci. 24 639–653. 10.1016/j.tics.2020.05.004 [DOI] [PubMed] [Google Scholar]
- Sauseng P., Klimesch W., Stadler W., Schabus M., Doppelmayr M., Hanslmayr S., et al. (2005). A shift of visual spatial attention is selectively associated with human EEG alpha activity. Eur. J. Neurosci. 22 2917–2926. 10.1111/j.1460-9568.2005.04482.x [DOI] [PubMed] [Google Scholar]
- Scheeringa R., Bastiaansen M. C. M., Petersson K. M., Oostenveld R., Norris D. G., Hagoort P. (2008). Frontal theta EEG activity correlates negatively with the default mode network in resting state. Int. J. Psychophysiol. 67 242–251. 10.1016/j.ijpsycho.2007.05.017 [DOI] [PubMed] [Google Scholar]
- Sederberg P. B., Kahana M. J., Howard M. W., Donner E. J., Madsen J. R. (2003). Theta and gamma oscillations during encoding predict subsequent recall. J. Neurosci. 23 10809–10814. 10.1523/jneurosci.23-34-10809.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staudigl T., Hanslmayr S. (2013). Theta oscillations at encoding mediate the context-dependent nature of human episodic memory. Curr. Biol. 23 1101–1106. 10.1016/j.cub.2013.04.074 [DOI] [PubMed] [Google Scholar]
- Tallon-Baudry C., Campana F., Park H.-D., Babo-Rebelo M. (2018). The neural monitoring of visceral inputs, rather than attention, accounts for first-person perspective in conscious vision. Cortex 102 139–149. 10.1016/j.cortex.2017.05.019 [DOI] [PubMed] [Google Scholar]
- Tanaka G. K., Peressutti C., Teixeira S., Cagy M., Piedade R., Nardi A. E., et al. (2014). Lower trait frontal theta activity in mindfulness meditators. Arq. Neuropsiquiatr. 72 687–693. 10.1590/0004-282x20140133 [DOI] [PubMed] [Google Scholar]
- Tang Y.-Y., Hölzel B. K., Posner M. I. (2015). The neuroscience of mindfulness meditation. Nat. Rev. Neurosci. 16 213–225. [DOI] [PubMed] [Google Scholar]
- Tang Y.-Y., Tang R., Rothbart M. K., Posner M. I. (2019). Frontal theta activity and white matter plasticity following mindfulness meditation. Curr. Opin. Psychol. 28 294–297. 10.1016/j.copsyc.2019.04.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ter Wal M., Linde-Domingo J., Lifanov J., Roux F., Kolibius L. D., Gollwitzer S., et al. (2021). Theta rhythmicity governs human behavior and hippocampal signals during memory-dependent tasks. Nat. Commun. 12:7048. 10.1038/s41467-021-27323-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tóth B., Kardos Z., File B., Boha R., Stam C. J., Molnár M. (2014). Frontal midline theta connectivity is related to efficiency of WM maintenance and is affected by aging. Neurobiol. Learn. Mem. 114 58–69. 10.1016/j.nlm.2014.04.009 [DOI] [PubMed] [Google Scholar]
- Ulloa E. R., Pineda J. A. (2007). Recognition of point-light biological motion: mu rhythms and mirror neuron activity. Behav. Brain Res. 183 188–194. 10.1016/j.bbr.2007.06.007 [DOI] [PubMed] [Google Scholar]
- Van Diepen R. M., Foxe J. J., Mazaheri A. (2019). The functional role of alpha-band activity in attentional processing: the current zeitgeist and future outlook. Curr. Opin. Psychol. 29 229–238. 10.1016/j.copsyc.2019.03.015 [DOI] [PubMed] [Google Scholar]
- Vertes R. P., Hoover W. B., Viana Di Prisco G. (2004). Theta rhythm of the hippocampus: subcortical control and functional significance. Behav. Cogn. Neurosci. Rev. 3 173–200. 10.1177/1534582304273594 [DOI] [PubMed] [Google Scholar]
- Vertes R. P., Kocsis B. (1997). Brainstem-diencephalo-septohippocampal systems controlling the theta rhythm of the hippocampus. Neuroscience 81 893–926. 10.1016/s0306-4522(97)00239-x [DOI] [PubMed] [Google Scholar]
- Villena-González M., Moënne-Loccoz C., Lagos R. A., Alliende L. M., Billeke P., Aboitiz F., et al. (2017). Attending to the heart is associated with posterior alpha band increase and a reduction in sensitivity to concurrent visual stimuli. Psychophysiology 54 1483–1497. 10.1111/psyp.12894 [DOI] [PubMed] [Google Scholar]
- Wackermann J., Pütz P., Allefeld C. (2008). Ganzfeld-induced hallucinatory experience, its phenomenology and cerebral electrophysiology. Cortex 44 1364–1378. 10.1016/j.cortex.2007.05.003 [DOI] [PubMed] [Google Scholar]
- Wilson T. J., Foxe J. J. (2020). Cross-frequency coupling of alpha oscillatory power to the entrainment rhythm of a spatially attended input stream. Cogn. Neurosci. 11 71–91. 10.1080/17588928.2019.1627303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong W. P., Camfield D. A., Woods W., Sarris J., Pipingas A. (2015). Spectral power and functional connectivity changes during mindfulness meditation with eyes open: a magnetoencephalography (MEG). study in long-term meditators. Int. J. Psychophysiol. 98 95–111. 10.1016/j.ijpsycho.2015.07.006 [DOI] [PubMed] [Google Scholar]
- Yadav G. S., Cidral-Filho F. J., Iyer R. B. (2021). Using heartfulness meditation and brainwave entrainment to improve teenage mental wellbeing. Front. Psychol. 12:742892. 10.3389/fpsyg.2021.742892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarka D., Cevallos C., Petieau M., Hoellinger T., Dan B., Cheron G. (2014). Neural rhythmic symphony of human walking observation: upside-down and uncoordinated condition on cortical theta, alpha, beta and gamma oscillations. Front. Syst. Neurosci. 8:169. 10.3389/fnsys.2014.00169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zgierska A. E., Burzinski C. A., Cox J., Kloke J., Stegner A., Cook D. B., et al. (2016). Mindfulness meditation and cognitive behavioral therapy intervention reduces pain severity and sensitivity in opioid-treated chronic low back pain: pilot findings from a randomized controlled trial. Pain Med. 17 1865–1881. 10.1093/pm/pnw006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zorn J., Abdoun O., Sonié S., Lutz A. (2021). Cognitive defusion is a core cognitive mechanism for the sensory-affective uncoupling of pain during mindfulness meditation. Psychosom. Med. 83 566–578. 10.1097/PSY.0000000000000938 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.