Abstract
Background
Previous research in animals and humans has demonstrated a potential role of stress regulatory systems, such as the hypothalamic-pituitary-adrenal (HPA) axis and the endocannabinoid (eCB) system, in the development of substance use disorders. We thus investigated alterations of HPA and eCB markers in individuals with chronic cocaine use disorder by using an advanced hair analysis technique.
Methods
We compared hair concentrations of glucocorticoids (cortisone, cortisol) and the eCBs 2-arachidonylglycerol, anandamide (AEA), oleoylethanolamide (OEA), and palmitoylethanolamide (PEA) between 48 recreational cocaine users (RCU), 25 dependent cocaine users (DCU), and 67 stimulant-naïve controls. Self-reported substance use and hair concentrations of substances were also assessed.
Results
Significantly higher concentrations of hair cortisone were found in RCU and DCU compared with controls. Hair concentrations of OEA and PEA were significantly lower in DCU compared with RCU and controls. Additionally, within cocaine users, elevated cocaine hair concentration was a significant predictor for increased glucocorticoid and decreased OEA hair levels. Moreover, higher 3,4-methylenedioxymethamphetamine hair concentration was correlated with elevated cortisone and AEA, OEA, and PEA levels in hair within cocaine users, whereas more self-reported cannabis use was associated with lower eCBs levels in hair across the total sample.
Conclusion
Our findings support the hypothesis that the HPA axis and eCB system might be important regulators for substance use disorders. The mechanistic understanding of changes in glucocorticoid and eCB levels in future research might be a promising pharmacological target to reduce stress-induced craving and relapse specifically in cocaine use disorder.
Keywords: Addiction, cortisol, endocannabinoids, hair, stimulants, substance use disorder
Significance Statement.
Stress-related glucocorticoids and endocannabinoids (eCBs) are suggested to be important regulators in the development of substance use disorders. An advanced hair analysis technique was used for the determination of alterations of long-term cumulative stress markers of chronic cocaine users compared with a control group. Higher glucocorticoid concentrations were found in hair of chronic cocaine users, whereas their oleoylethanolamide and palmitoylethanolamide concentrations were lower compared with the control group. Furthermore, 3,4-methylenedioxymethamphetamine (MDMA) hair concentrations and self-reported cannabis use were correlated with alterations in glucocorticoid and eCB levels in hair. The here shown long-term effect of cocaine, MDMA, and cannabis use on various stress markers in hair suggests that alterations of the HPA axis and eCB system might have a crucial impact on the development of substance use disorders in general.
Introduction
Stress has been proposed to be a crucial risk factor for developing and maintaining substance use disorders and thus being a principal contributor to the vicious circle of addiction (Sinha, 2008; Volkow et al., 2011; G. F. Koob et al., 2014). A number of preclinical and human studies have additionally shown that various addictive substances induce physiological stress responses in the hypothalamic-pituitary adrenal (HPA) axis (Armario, 2010; Wemm and Sinha, 2019). Recently, the endocannabinoid (eCB) system also attracted increased attention in stress and addiction research due to its stress-buffering effects in animals and humans (Moreira et al., 2015; deRoon-Cassini et al., 2020). The eCBs anandamide (AEA) and 2-arachidonoylglycerol (2-AG) were especially associated with stress-induced activation of the HPA axis (Moreira et al., 2015). Accumulating evidence from preclinical and clinical studies now suggests that direct activation of the cannabinoid type-1 (CB1) receptor by AEA, 2-AG, and its indirect activation by palmitoylethanolamide (PEA), and oleoylethanolamide (OEA) plays a key modulatory role in stress vulnerability and resilience (Hill et al., 2013; Bluett et al., 2014; Mayo et al., 2019).
Cocaine is highly addictive: 5%–6% of cocaine users develop dependence in the first year of use, and 15%–21% develop dependence during lifetime (Wagner and Anthony, 2002; Lopez-Quintero et al., 2011). Once cocaine dependence develops, it is associated with high relapse rates of 40%–60% during or after specialized treatment (Simpson et al., 1999; McLellan et al., 2000). Specifically, inadequate coping of stress responses has been associated with both the initiation and relapse of cocaine use (Milivojevic and Sinha, 2018). However, physiological stress responses can also be modulated by cocaine itself, as described in the following.
Acute cocaine administration in animals and humans has been reported to increase glucocorticoids (G. Koob and Kreek, 2007; Sinha, 2008; Wemm and Sinha, 2019). Animal studies have furthermore shown that chronic administration of cocaine increases the physiological stress load and thus sustainably changes the responsiveness of the HPA axis (J. R. McReynolds et al., 2014; Wemm and Sinha, 2019). In line with that, chronic cocaine users have been shown to display chronically elevated basal levels of cortisol in blood and saliva (Contoreggi et al., 2003; Fox et al., 2009; Wemm and Sinha, 2019) and a downregulation of the glucocorticoid receptor gene (NR3C1) in blood (Schote et al., 2019). Furthermore, elevated activity of the HPA axis in response to psychosocial stress has been associated with increased cocaine craving and relapse (Sinha et al., 2006). In summary, enhanced activity of the HPA axis seems to play an important role in development and maintenance of cocaine use disorder.
Furthermore, preclinical studies in rats indicate that acute cocaine administration affects the eCB system by increasing AEA, whereas chronic cocaine administration for 10 days decreased 2-AG in the limbic forebrain but did not change AEA levels (Gonzalez et al., 2002a, 2002b). However, recently, alterations of eCB levels, including AEA, 2-AG, OEA, and PEA, in different rat brain structures have been reported after 14 days of chronic active and passive cocaine administration as well as in cocaine-induced reinstatement after 10 days of extinction training (Bystrowska et al., 2014, 2019). Accordingly, eCB signaling, specifically via the CB1 receptor, has been postulated to be critically involved in cocaine-seeking and relapse behavior (resulting in maintenance of cocaine use) rather than being associated with cocaine reward (entailing initiation of cocaine use and dependence) (Wiskerke et al., 2008; Bystrowska et al., 2019; Higginbotham et al., 2021). Recent findings suggest that stress-induced corticosterone release in rats promotes cocaine seeking by endocannabinoid mobilization (i.e., 2-AG) in the prelimbic cortex (Jayme R. McReynolds et al., 2018). Additional evidence corroborating the involvement of the eCB system in cocaine use disorder comes from animal studies investigating the degrading enzyme fatty acid amide hydrolase (FAAH), which terminates the eCB signaling in the brain (Cravatt et al., 1996; Mulder and Cravatt, 2006). Chronic administration of the FAAH inhibitor in rats, resulting in increased levels of AEA, significantly decreased cocaine-seeking behavior as well as cue- and stress-induced relapse (Chauvet et al., 2014). In humans, a single study has investigated eCB concentrations in biological samples of individuals with cocaine use disorder, reporting increased blood plasma levels of AEA, PEA, and OEA accompanied by decreased 2-AG levels in recently abstinent cocaine users compared with healthy controls (Pavón et al., 2013).
The measurement of neuroendocrine indicators of the physiological stress response can be done in blood, urine, and saliva but show high intra-individual fluctuations because of the underlying circadian rhythm and reflect only momentary concentrations at the time of sampling (Stalder and Kirschbaum, 2012). In contrast, measurement of neuroendocrine markers by hair analysis can reflect cumulative or chronic stress exposure across extended time periods up to several months (Stalder and Kirschbaum, 2012). Therefore, the long-term determination of hormones in the keratinized matrix of hair has become an important tool in stress research in recent years (for review, see Staufenbiel et al., 2013) and has been shown to be a biomarker for sustained alterations in neuroendocrine stress responses. Several studies have investigated hair cortisol across a range of stress-related conditions in humans. Increased hair cortisol has been shown to be associated with stressful conditions such as chronic pain (Van Uum et al., 2008), alcohol use disorder (Stalder et al., 2010), major depression (Dettenborn et al., 2012), and post-traumatic stress disorder (Steudte et al., 2011a). Moreover, eCB levels in hair have been correlated with post-traumatic stress disorder symptoms (Wilker et al., 2016) and traumatic stress (Behnke et al., 2020), borderline personality disorder (Wingenfeld et al., 2018), burnout and anxiety disorders (Gao et al., 2020), anorexia nervosa (Tam et al., 2021), and depressive symptoms (Croissant et al., 2020).
The recently established combined measurement of glucocorticoids and endocannabinoids in hair enables a retrospective monitoring of the HPA axis and the eCB system (Voegel et al., 2021). In combination with toxicological hair analysis, it provides a promising new methodology for investigating the relationship between the exposure to psychoactive substances and psychoneuroendocrinological biomarkers.
The aim of the present project was to investigate the relationship between both neuroendocrine stress systems and recreational and dependent cocaine users with an advanced hair analysis technique (Voegel et al., 2021). We thus investigated hair concentrations of glucocorticoids (cortisone, cortisol) and several eCBs (2-AG, AEA, OEA, and PEA) between chronic recreational cocaine users (RCU) and dependent cocaine users (DCU) and stimulant-naïve controls. Additionally, hair concentrations of various drugs have been determined and related to glucocorticoid and eCBs hair concentrations. Specifically, 3,4-methylenedioxymethamphetamine (MDMA, “ecstasy”) and cannabis were considered as potential confounders in the analyses given that MDMA has a strong impact on the HPA axis (Parrott et al., 2008, 2014), while exogenous cannabinoids have—per definition—a direct effect on the eCB system (Di Marzo et al., 2000). Moreover, both substances are known to be highly co-used by urban Swiss cocaine users, the setting where we recruited our study participants (Quednow et al., in press). Based on previous findings, we expected to find increased glucocorticoid hair concentrations and altered eCB hair concentrations in chronic cocaine users compared to controls (Baumann et al., 1995; Chauvet et al., 2014; Hamilton et al., 2018). We hypothesized that these effects would be more pronounced in the group of DCU. Moreover, we expected a positive correlation between glucocorticoid and cocaine hair concentrations and a correlation between cocaine and eCB hair concentrations.
MATERIALS AND METHODS
Participants
Hair samples were collected as part of the longitudinal Zurich Cocaine Cognition Study. From the previously included 166 Zurich Cocaine Cognition Study participants (Vonmoos et al., 2013b), hair samples of 140 participants were available for the additional neuroendocrinological analyses reported here. These participants were divided into the cocaine-naïve control group (n = 67) and chronic cocaine users (n = 73). The cocaine users were further subdivided into RCU (n = 48) and DCU (n = 25). All inclusion criteria and procedures are described in the supplementary Materials. The study was approved by the Cantonal Ethics Committee of Zurich (KEK-Nr: E-14/2009). All participants provided written informed consent and were financially compensated for their participation.
Hair Analysis
For the analysis, 2 hair strands were collected from the posterior vertex region and cut as close to the scalp as possible. The hair samples were stored in aluminum foil at room temperature. The 2 hair strands were cut into a 3-cm hair segment proximal to the scalp. This segment represents approximately the last 3 months prior to the sampling, based on an average growth rate of 1 cm/month. Hair analysis for drugs was performed with 1 of the hair strands according to the protocol described in Vonmoos et al. (2013a). The following compounds were analyzed with liquid chromatography-tandem mass spectrometry (LC-MS/MS) for this study: cocaine and its metabolites benzoylecgonine and norcocaine, as well as MDMA. For further calculations, the sum of cocaine, benzoylecgonine, and norcocaine was used as a variable (cocaine total hair concentration). Steroid hormone and eCB analysis was carried out with LC-MS/MS following a protocol by Voegel et al. (2021) with the second hair strand. Two steroid hormones (cortisone, cortisol) and 4 eCBs (2-AG, AEA, OEA, PEA) were quantified. For further analysis, the sum of cortisol and cortisone (cortisol/cortisone sum) was calculated.
Data Analysis
Statistical analyses were conducted using R version 3.6.1. (R Core Team, 2018). The supplementary Table A1 summarizes all variables included in the statistical analyses, indicating whether they were numerical, factorial, or binary as well as log-transformed for non-normal distributed variables. A schematic process of the analytical steps is included in the supplementary Materials (Figure A1).
Testing for Age and Sex as Covariates
Previous studies have reported influences of sex and age on the concentration of glucocorticoids and eCBs in hair (Dettenborn et al., 2012; Mwanza et al., 2016; Binz et al., 2018; Gao et al., 2020; Lanfear et al., 2020). Therefore, linear regression analyses were conducted with glucocorticoid and eCB hair concentrations as dependent variables and sex and age as regressors within the control group to determine whether they should be included as covariates in the other analyses. Within the control group, sex had a significant association with the endocannabinoids 2-AG and AEA (P < .05; see Table A2 in supplementary materials). Hence, all models with 2-AG or AEA as dependent variables were controlled for sex.
Between-Group Analyses
We used standard linear models in R [lm() and ANOVA() with type I tests] to test for differences in glucocorticoid and eCB hair concentrations between groups. To compare between controls and chronic cocaine users, controls and DCU, controls and RCU, and RCU with DCU, the 3-level factor was split repeatedly into 2 contrasts. For example, we split groups into controls vs chronic cocaine users and RCU vs DCU and then fitted the model as controls vs chronic cocaine users + RCU vs DCU (note that with type I tests, the terms are added sequentially).
A sensitivity analysis for group comparisons (3 groups) of glucocorticoid and eCB hair concentrations was conducted using G*Power 3.1 (Faul et al., 2009). With the available 140 hair samples and assuming an α-error probability of .05 and a power of 80%, we are able to reliably detect at least effects with a medium effect size of Cohen’s f = 0.265 (d = 0.530).
Predictors of Neuroendocrine Hair Concentration
To test whether additional variables such as self-reports of stress-related psychiatric symptoms and cannabis use had an association with glucocorticoid and eCB hair concentrations, we tested each variable combination with a linear model. Given that controls rarely had non-zero values for cocaine-/MDMA-related variables, linear relationships between cocaine-/MDMA-related variables and glucocorticoids were tested within chronic cocaine users only.
If a variable was significantly associated with 1 measurement, this variable was later taken into account in all models with measurements of the same class. For example, if a variable was significantly associated with cortisone concentration, it was used in models including cortisol concentration or cortisone/cortisol sum. The covariates identified in these analyses were then used to test whether the association between cocaine or MDMA hair concentrations and glucocorticoid/eCB concentrations were direct or potentially indirect via the covariates. We did this by including the covariates and the cocaine or MDMA hair concentrations in linear regression models with type II tests, each term was tested after correcting for all other terms (interactions were not included). These analyses were done only within chronic cocaine users. For the glucocorticoids, the following covariates were included: MDMA concentration in hair, cannabis concentration in urine, cigarettes per week, years of school education, SCL-90R anxiety scale, and SCL-90R positive symptom distress index. For the eCBs, MDMA concentration in hair, years of cocaine use, years of cannabis use, ADHD sum score, and SCL-90R positive symptom distress index were included (for details, see Table A3, supplementary Materials). The statistical comparisons were carried out with a significance level of P < .05 (2-tailed).
RESULTS
Demographic data and drug use variables of chronic cocaine users and controls are displayed in Table 1. The groups did not differ in terms of sex and age. However, as shown previously in this cohort (Preller et al., 2013; Vonmoos et al., 2013b), cocaine users had fewer years of school education, smoked a higher number of cigarettes per week, and drank more alcohol per week. Moreover, cocaine users showed a higher level of symptoms of depression (BDI) and ADHD as well as elevated SCL-90-R Anxiety, Psychoticism, and PSDI scores.
Table 1.
Demographic Data and Drug Use Variables
| Stimulant-naïve controls | Chronic cocaine users | ||||
|---|---|---|---|---|---|
| n = 67 | n = 73 | Value | df | P | |
| Female/malea | 20/47 | 24/49 | χ 2 = 0.15 | 1 | .700 |
| Ageb | 29.1 (7.6) | 31.3 (9.5) | t = −1.50 | 138 | .135 |
| Years of educationb | 11.0 (1.8) | 9.9 (1.4) | t = 4.09 | 124.7 | <.001 |
| Verbal IQb | 108 (12.3) | 103 (11.1) | t = 2.45 | 138 | .015 |
| BDI sum scoreb | 4.3 (4.5) | 8.8 (7.1) | t = −4.48 | 122.8 | <.001 |
| ADHD-SRb | 7.9 (4.9) | 13.6 (8.9) | t = −4.74 | 113.8 | <.001 |
| SCL-90-R Anxietyb | 0.23 (0.3) | 0.57 (0.6) | t = −4.35 | 104.6 | <.001 |
| SCL-90-R Psychoticismb | 0.13 (0.2) | 0.36 (0.4) | t = −4.47 | 105.3 | <.001 |
| SCL-90-R PSDIb | 1.1 (0.3) | 1.5 (0.5) | t = −5.60 | 131.7 | <.001 |
| Smoker/non-smokera | 50/17 | 69/4 | χ 2 = 10.84 | 1 | .001 |
| Cigarettes per weekb,c | 70.7 (57.7) | 108 (60) | t = −3.44 | 117 | .001 |
| Alcohol grams/weekb | 124 (127) | 195 (231) | t = −2.30 | 113.7 | .023 |
| Cocaine | |||||
| Grams per weekb | — | 2.6 (4.5) | |||
| Lifetime gramsb | 0.12 (0.7) | 2625 (6999) | |||
| Years of used | — | 6.0 (1.0–30.0) | |||
| Cocaine total hair concentratione pg/mg | 5.4 (26.5); 0 (0–170) |
9674 (16964); 2465 (45.0–82700) |
|||
| Cannabis | |||||
| Cannabis consumptiona (y/n) | 31/36 | 47/26 | χ 2 = 4.65 | 1 | .031 |
| Grams per weekb | 0.58 (1.5) | 1.0 (2.7) | t = −0.49 | 76 | .625 |
| Lifetime gramsd | 55.1 (0–54750) |
548 (0–27740) |
t = −2.65 | 48.6 | .011 |
| Years of used | 4.0 (0–28.0) | 7.0 (0–34.0) | t = −2.11 | 76 | .038 |
| MDMA | |||||
| Pills per weekb | — | 0.07 (0.2) | |||
| Lifetime pillsb | 1.0 (3.5) | 42.3 (105) | |||
| Years of used | 0 (0–13.0) | 0 (0–12.0) | |||
| Positive hair testa (y/n) | 2/65 | 32/41 | |||
| Hair concentratione pg/mg | 8.3 (45.3); 0 (0–310) |
746 (2014); 0 (0–12000) |
Abbreviations: ADHD, attention-deficit/hyperactivity disorder; BDI, Beck’s Depression Inventory; MDMA, 3,4-Methylenedioxymethamphetamine; PSDI, Positive Symptom Distress Index.
Significant P-values are marked in bold.
a Number
b Mean (standard deviation)
c Only within smokers
d Median (range)
e Mean (standard deviation); median (range)
Group Comparisons: Chronic Cocaine Users and Control Group
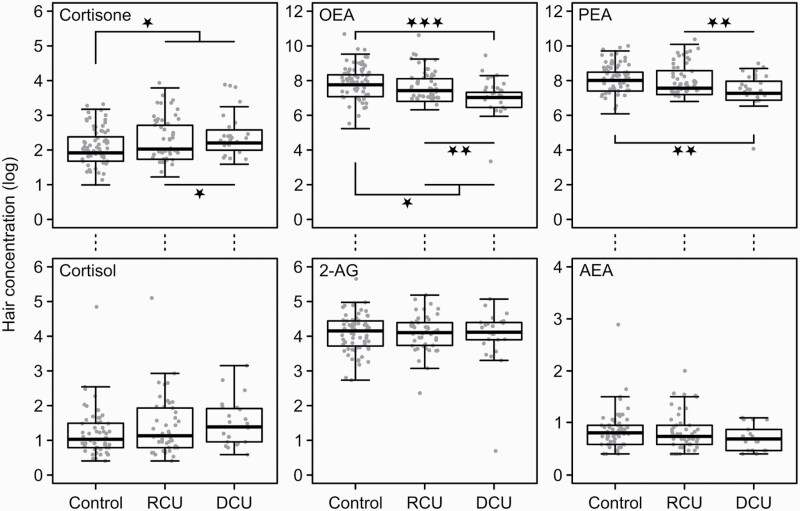
Table 2 and Figure 1 display glucocorticoid and eCB hair concentrations in cocaine user groups and controls. Chronic cocaine users showed elevated cortisone hair concentrations compared with controls (F(1,136) = 6.69, P = .011, η 2 = 0.047), which was mainly driven by the DCU group (controls vs DCU: F(1,136) = 6.50, P = .012, η 2 = 0.045). Variance of cortisone was not significantly explained by the group contrasts RCU vs DCU (F(1,136) = 1.01, P = .317, η 2 = 0.007), but a marginally significant difference was found for the contrast controls vs RCU (F(1,136) = 3.53, P = .062, η 2 = 0.025). None of the group contrasts was a significant predictor for cortisol and cortisol/cortisone sum. However, for cortisol, the comparison between chronic cocaine users and controls was marginally significant (F(1,125) = 2.93, P = .090, η 2 = 0.023). For cortisol/cortisone sum, the differences in hair concentration between chronic cocaine users and controls (F(1,125) = 3.77, P = .054, η 2 = 0.029) and between controls and DCU (F(1,125) = 3.72, P = .056, η 2 = 0.029) were not significant. All other contrasts for cortisol and cortisone sum were clearly insignificant (P > .11).
Table 2.
Group Comparisons: Glucocorticoid and eCB Hair Concentrations in Chronic Cocaine Users and Healthy Controls
| Stimulant-naïve controls | Chronic cocaine users | |||||
|---|---|---|---|---|---|---|
| n = 67 | n = 73 | F | df | P | η2 | |
| Glucocorticoid hair concentrations | ||||||
| Cortisol pg/mg | 4.3 (15.0); 1.5 (0.5–126) |
6.2 (18.8); 2.2(0.5–163) |
2.93 | 1, 125 | .090 | 0.02 |
| Cortisone pg/mg | 7.4 (4.7); 5.8 (1.7–22.9) |
10.8 (9.4); 7.7 (0.19–43.2) |
6.69 | 1, 136 | .011 | 0.05 |
| Cortisol/cortisone sum | 11.7 (16.6); 7.5 (2.7–138) |
16.9 (23.9); 9.6 (0.32–189) |
3.77 | 1, 125 | .054 | 0.03 |
| eCB hair concentrations | ||||||
| 2-AG pg/mg | 67.4 (40.2); 62.6 (14.4–284) |
66.0 (33.4); 60.3 (1.0–178) |
0.08 | 1, 137 | .784 | <0.01 |
| AEA pg/mg | 1.6 (2.0); 1.2 (0.3–17.0) |
1.3 (1.0); 1.0 (0.3–6.4) |
0.68 | 1, 130 | .411 | <0.01 |
| OEA pg/mg | 3524 (4429); 2378 (186–32598) |
2654 (3940); 1372 (20.1–30456) |
4.62 | 1, 137 | .033 | 0.03 |
| PEA pg/mg | 3916 (3273); 3010 (438–16457) |
3385 (3842); 1834 (42.7–24077) |
3.76 | 1, 137 | .054 | 0.03 |
Abbreviations: AEA, anandamide; eCB, endocannabiniod; OEA, oleoylethanolamide; PEA, palmitoylethanolamide; 2-AG, 2-arachidonylglycerol.
Mean (standard deviation); median (range) are shown with significant P-values marked in bold.
Figure 1.
Group differences in glucocorticoids and eCB hair concentrations displayed by boxplots with individual data points. Boxplots show the median with the lower and upper quartiles ±1.5 inner quartile range.
Significant P-values marked with P < .05*, P < .01**, P < .001***
Abbreviations: 2-AG, 2-arachidonylglycerol; AEA, anandamide; DCU, dependent cocaine user, OEA, oleoylethanolamide; PEA, palmitoylethanolamide; RCU, recreational cocaine users.
For the eCBs, significant group effects were found for OEA with the group contrasts controls vs chronic cocaine users (F(1,137) = 4.62, P = .033, η 2 = 0.031), controls vs DCU (F(1,137) = 12.23, P < .001, η 2 = 0.082), and RCU vs DCU (F(1,137) = 7.91, P = .006, η 2 = 0.053). Chronic cocaine users showed reduced OEA hair concentrations compared with controls, which was again mainly driven by the DCU group. Controls and RCU did not differ in OEA (F(1,137) = 0.44, P = .506, η 2 = 0.003). Furthermore, we found significant predictors of group for PEA with the group contrasts controls vs DCU (F(1,137) = 11.09, P = .001, η 2 = 0.075) and RCU vs DCU (F(1,137) = 7.77, P = .006, η 2 = 0.052). Variance of PEA was not significantly explained by the group contrasts controls vs RCU (F(1,137) = 0.24, P = .625, η 2 = 0.002) and controls vs chronic cocaine users (F(1,137) = 3.76, P = .054, η 2 = 0.025), indicating that lower PEA hair concentration was mainly driven by dependency of cocaine use. None of the group contrasts were a significant predictor for AEA and 2-AG (P > .4).
Within Chronic Cocaine User: Predictors of Neuroendocrine Hair Concentration
Glucocorticoids and Cocaine Use
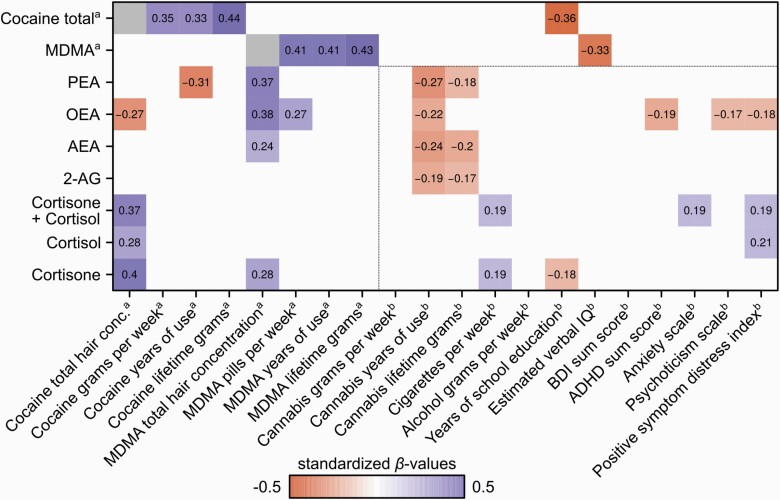
Using within-group regressions, increased cocaine total hair concentration was found to be a significant predictor for elevated cortisol (F(1,67) = 5.31, P = .024, η 2 = 0.073), cortisone (F(1,70) = 11.06, P = .001, η 2 = 0.136), and cortisol/cortisone sum hair concentrations (F(1,67) = 8.79, P = .004, η 2 = 0.116) (see Table A3 in supplementary Materials). Significant regression coefficients (standardized βvalues) for glucocorticoids are shown in Figure 2. To test whether the effect of cocaine total hair concentration on glucocorticoids was a direct effect, we controlled for previous identified variables associated with glucocorticoid hair concentration (i.e., MDMA hair concentration, cigarettes per week, years of school education, SCL-90-R anxiety scale, and SCL-90-R positive symptom distress). The effect of cocaine total hair concentration as a significant predictor for cortisone (F(1,65) = 7.10, P = .010, η 2 = 0.087) and cortisol/cortisone sum (F(1,62) = 5.00, P = .029, η 2 = 0.067) remained significant after controlling for the confounding variables, whereas the effect of cocaine hair concentration on cortisol was no longer significant (F(1,62) = 3.06, P = .085, η 2 = 0.067).
Figure 2.
Heat matrix of regression coefficients with significant standardized β-values are shown (P < .05). Dependent variables are listed on the y-axis and regressors on the x-axis.aWithin chronic cocaine users.
b Overall participants (chronic cocaine users and controls).
Abbreviations: 2-AG, 2-arachidonylglycerol; ADHD, attention deficit hyperactivity disorder; AEA, anandamide; BDI, Beck Depression Inventory; MDMA, 3,4-methylenedioxymethamphetamine; OEA, oleoylethanolamide; PEA, palmitoylethanolamide.
eCBs and Cocaine Use
We identified increased cocaine total hair concentration as a significant predictor for reduced OEA (F(1,71) = 5.21, P = .025, η 2 = 0.068), whereas the other eCB hair concentrations of PEA (F(1,71) = 3.11, P = .082, η 2 = 0.042), AEA (F(1,66) = 0.02, P = .898, η 2 = 0.0002), and 2-AG (F(1,71 = 1.66, P = .202, η 2 = 0.023) did not reach the significance threshold within chronic cocaine users (see Table A3 in supplementary Materials). Moreover, we found that increased years of cocaine use in self-reports significantly predicted lower PEA levels (F(1,71) = 6.70, P = .012, η 2 = 0.086). Significant regression coefficients (standardized β values) for eCBs are shown in Figure 2. To test whether the effect of cocaine total hair concentration on eCBs was direct, we used multiple linear regression models type II to control for previously identified variables associated with eCB hair concentration (i.e., MDMA hair concentration, cocaine years of use, cannabis years of use, ADHD sum score, and SCL-90-R positive symptom distress). The effect of cocaine total hair concentration as a significant predictor for OEA remained significant after controlling for the confounding variables (F(1,66) = 4.21, P = .044, η 2 = 0.048).
MDMA Use: Glucocorticoids and eCBs
As an exploratory analysis, we found increased MDMA hair concentration to be a significant predictor for elevated cortisone (F(1,70) = 5.11, P = .027, η 2 = 0.068) as well as for the eCBs AEA (F(1,66) = 4.96, P = .029, η 2 = 0.064), OEA (F(1,71) = 11.91, P = .001, η 2 = 0.144), and PEA (F(1,71) = 9.48, P = .003, η 2 = 0.118) but not for 2-AG (F(1,70) = 2.69, P = .106, η 2 = 0.037) (see Figure 2). Furthermore, we found that more recent MDMA use (pills per week) significantly predicted elevated OEA (F(1,71) = 5.58, P = .021, η 2 = 0.073), whereas years of MDMA use and cumulative MDMA pills use—as indicated by the questionnaire—were not associated with glucocorticoids and eCBs (P > .113). This indicates that recent exposure to MDMA rather than chronic MDMA use might be associated with alterations of the eCB system. To test whether the effect of MDMA hair concentration on glucocorticoids and eCBs was direct, we controlled for the identified confounding variables for glucocorticoids and eCBs mentioned before. The effect of MDMA hair concentration as a significant predictor for the eCBs AEA (F(1,61) = 5.03, P = .029, η 2 = 0.068), OEA (F(1,66) = 13.48, P < .001, η 2 = 0.152), and PEA (F(1,66) = 9.56, P = .003, η 2 = 0.113) remained significant after controlling for the confounding variables. Furthermore, the MDMA hair concentration effect for cortisone also remained significant after controlling for the confounding variables (F(1,65) = 4.47, P = .038, η 2 = 0.055). No interactions between cocaine total hair concentration and MDMA substance variables on eCBs and cortisone were significant (P > .315).
We found strong correlations of subjective reported cocaine and MDMA use with cocaine and MDMA hair concentration (P < .005), respectively (see Figure 2), indicating a high reliability of the substance self-reports in our chronic cocaine user sample.
Overall Predictors of Neuroendocrine Hair Concentration
Cannabis
Additional exploratory analyses were conducted over all participants (see Figure 2). Most interestingly, we found years of cannabis use and lifetime cannabis use as significant regressors for eCB hair concentrations overall. In particular, a longer period of cannabis use (years of use) significantly predicted decreased AEA (F(1,66) = 9.02, P = .003, η 2 = 0.059), 2-AG (F(1,70) = 5.30, P = .023, η 2 = 0.036), OEA (F(1,71) = 6.74, P = .010, η 2 = 0.047), and PEA (F(1,71) = 11.02, P = .001, η 2 = 7.39). Higher cumulative grams of cannabis used (lifetime use) was a significant predictor for AEA (F(1,66) = 6.04, P = .015, η 2 = 0.040), 2-AG (F(1,70) = 4.01, P = .047, η 2 = 0.028), and OEA (F(1,71) = 3.60, P = .060, η 2 = 0.0254), whereas more recent cannabis use (grams per week) was not significantly associated with eCBs (P > .221). This indicates that chronic exposure to cannabis rather than recent use of cannabis might be associated with changes in the eCB system.
Psychiatric Symptoms
Furthermore, psychiatric symptoms were studied in all participants (see Figure 2). We found ADHD as a significant regressor for OEA hair concentrations (F(1,138) = 5.00, P = .027, η 2 = 0.035). Higher SCL-90-R anxiety scores significantly predicted higher cortisol/cortisone sum concentrations in hair (F(1,126) = 3.94, p = .049, η 2 = 0.030) and a higher SCL-90-R positive symptom distress index was significantly associated with cortisol (F(1,126) = 5.48, P = .021, η 2 = 0.042) and cortisol/cortisone sum (F(1,126) = 4.46, P = .037, η 2 = 0.034). Lower OEA levels in hair were significantly associated with higher SCL-90-R psychoticism scores (F(1,138) = 4.12, P = .044, η 2 = 0.029) and a higher SCL-90-R positive symptom distress index (F(1,138) = 4.51, P = .035, η 2 = 0.032).
Discussion
The aim of our study was to investigate neuroendocrine markers of sustained stress exposure in chronic cocaine users by using a novel hair analysis approach. Furthermore, concurrent use of MDMA and cannabis as well as psychiatric symptoms were investigated as important potential confounders. As expected, chronic cocaine users showed increased cortisone and altered OEA and PEA hair concentrations compared with controls, which was specifically driven by the cocaine-dependent group. Within cocaine users, elevated cortisone and decreased OEA levels were associated with higher cocaine hair concentrations indicating a dose-dependent effect. Moreover, decreased PEA levels were predicted by duration of cocaine use. Findings support the notion of a dysfunctional physiological stress system in cocaine users and suggest that alterations of the HPA axis and eCB system might have crucial impact on cocaine use disorder.
Cocaine
Based on a novel hair analysis technique (Voegel et al., 2021), we reliably assessed eCB hair concentrations in chronic cocaine users and substance-naïve controls. We found decreased levels of OEA and PEA specifically in cocaine dependent users compared with recreational cocaine users and the control group. Reduced PEA and OEA levels in cocaine users were further predicted by elevated cocaine hair concentrations and years of cocaine use indicating that a reduced eCB signaling in cocaine users might be related to the severity of cocaine use. Endogenous up-regulation as well as exogenous administration of PEA and OEA in rodents were reported to show neuroprotective and anti-inflammatory effects in models of neuropsychiatric and neurodegenerative diseases such as Alzheimer disease, Parkinson disease, and stroke (for review see Herrera et al., 2016). Human and non-human primate studies have shown that chronic cocaine exposure can cause a decrease in gray matter volume in orbitofrontal, insular, parahippocampal, and anterior cingulate cortices, which has been shown to relate to the duration of cocaine use in humans (Ersche et al., 2011; Hirsiger et al., 2019; Rabin et al., 2020; Jedema et al., 2021). Given the reported neuroprotective effects of PEA and OEA, our results indicate that depending on cocaine use severity (duration and dose of cocaine use in the last 3 months), the cortical effects of cocaine might be caused by its dampening effects on the neuroprotective eCB signaling, which may in turn result in reduced gray matter volumes and subsequently in impaired cognitive functioning. Therefore, exogenous administration (or pharmacologically-induced increase) of OEA or PEA might be a potential therapeutic target for cocaine use disorder to prevent neural damage and cognitive deficits as it was recently proposed for alcohol use disorder (Bilbao et al., 2016; Orio et al., 2018).
Surprisingly, we did not find differences of AEA and 2-AG hair concentrations between groups. This might be explained by the findings that concentrations of PEA and OEA are generally higher than AEA and 2-AG in biological samples such as plasma and saliva (Hillard, 2018). Therefore, changes in AEA and 2-AG in low concentration levels might be difficult to detect in the hair matrix. Another explanation might be related to phasic vs tonic eCB release in the brain. PEA and OEA have been reported to potentiate AEA and 2-AG levels in humans through the so-called “entourage effect” by competing for or inhibiting the degradative FAAH enzyme (Di Marzo et al., 2001; Petrosino et al., 2016). Therefore, our findings suggest that AEA and 2-AG levels might also be reduced in dependent cocaine users. However, reduced levels might occur in a more phasic and event-related manner, whereas PEA and OEA levels may underlie a tonic reduction in chronic cocaine users over time. The findings by Pavón et al. (2013) indicate a phasic increase of AEA, OEA, and PEA as well as phasic decrease of 2-AG plasma levels in individuals with cocaine use disorder compared with healthy controls. However, cocaine users were recruited from an outpatient treatment program showing 2 weeks of cocaine abstinence, current psychological and pharmacological treatment interventions, and a high burden of psychiatric co-morbidities. Future studies should therefore address these issues by analyzing plasma samples of a less confounded cocaine user group with various abstinence periods to investigate the stability of differences in eCB levels between chronic cocaine and stimulant-naïve controls.
In line with previous findings, our results showed that individuals with chronic cocaine use had increased glucocorticoid levels in hair. Moreover, cortisone and cortisol levels were significantly predicted by cocaine hair concentration in chronic cocaine users, indicating a dose-dependent effect of cocaine use on the HPA axis response to stress. However, studies to date have mainly investigated the acute changes of the HPA axis response to stress between cocaine users and control groups, but not sustained neuroendocrine response over months in hair (Heesch et al., 1995; Wemm and Sinha, 2019). The investigation of the last 3 months (3 cm hair strand) enabled a new perspective on the long-term changes in cocaine users of cortisol and cortisone, respectively. Interestingly, cortisone seemed to be a stronger biomarker for stress in the hair matrix compared with cortisol. The pattern for cortisol was similar and a trend for higher cortisol levels in hair of cocaine users was found but the relationship for cortisone was stronger. As previously described, hair cortisol seems to be prone to outliers whereas hair cortisone seems to be a more reliable marker for sustained stress (Feeney et al., 2018). Cortisol concentrations in sweat and sebum might contribute to the incorporation into the hair matrix leading to higher variations in concentration. Another explanation could be that cortisone is less hydrophilic than cortisol and thus can be incorporated at higher levels into the hair matrix.
MDMA
Moreover, the toxicological hair analysis included measurements of MDMA concentration in hair, which had significant associations with cortisone. In line with our study results, MDMA administration has been shown to increase acute cortisol levels in healthy volunteers in saliva (Parrott et al., 2008) and in plasma samples (Dumont and Verkes, 2006; Hysek et al., 2014; Seibert et al., 2014). Higher cortisol levels of recreational MDMA users have been previously found in urine (Wolff et al., 2012) as well as in hair samples (Parrott et al., 2014). For the eCBs, MDMA concentration in hair was also significantly associated with AEA, OEA, and PEA. This effect remained after controlling for several covariates including cocaine hair concentration. MDMA activates indirectly (via excessive serotonin release) and directly the serotonin-2A (5-HT2A) receptor, which has been reported to be linked to the eCB signaling system in humans and animals (Haj-Dahmane and Shen, 2011; Valverde and Rodriguez-Arias, 2013). Specifically, activation of 5-HT neurons has been reported to induce phasic release of eCBs (Peters et al., 2021). Our results are in line with these findings showing that increased MDMA use over the last 3 months was accompanied by elevated hair levels of eCBs. However, a recent study has reported no differences of AEA and 2-AG in blood after a single dose of MDMA in polysubstance users (Haijen et al., 2018). In comparison to our study, Haijen et al. only used a single dose of MDMA (75 mg) and analyzed 3 blood samples on the same day. No direct effect of MDMA on blood eCB concentrations was observed, but eCB concentrations changed over the time of day (Haijen et al., 2018). The possibility to measure long-term effects of MDMA on the eCB levels in hair over a 3 months period enabled us to strengthen the hypothesis that the eCB system and the 5-HT system interact with each other. Furthermore, we found a positive association between OEA and the number of Ecstasy pills used per week, indicating that more recent exposure to MDMA but not chronic MDMA use (MDMA years of use, MDMA lifetime grams) might be associated with alterations of the eCB system. Several studies showed that the eCB system might be involved in the rewarding and reinforcement processes of MDMA (for review see Robledo, 2010). Our results propose that repeated recent MDMA use may lead to a higher endocannabinoid production, resulting in higher deposits in the hair matrix, which was independent of cocaine use (no interaction with cocaine hair concentration). However, the underlying mechanism of the role of the eCB system in MDMA use disorder still has to be investigated.
Cannabis
The effect of cannabis on the eCB system over the whole sample was particularly interesting. Increased years of cannabis use and lifetime grams of cannabis consumed significantly predicted lower 2-AG, AEA, OEA, and PEA levels in hair, whereas more recent cannabis use (grams per week) did not show any associations with eCB hair concentrations. This indicates that chronic exposure to cannabis rather than recent cannabis use might be associated with changes in the eCB system. This was in line with a previous study in humans reporting lower AEA levels in cerebrospinal fluid in frequent cannabis users compared with infrequent users (Morgan et al., 2013). Preclinical studies have demonstrated reduced eCB levels and CB1 receptor signaling depending on brain-structures in rats after treatment with THC (Di Marzo et al., 2000). Moreover, cannabis users showed strongly downregulated CB1 receptors in a positron emission tomography study using the CB1 receptor inverse agonist radioligand [18F]FMPEP-d2 (Hirvonen et al., 2012). Thus, our findings corroborate the reported previous findings that repeated cannabis use might down-regulate the eCB signaling system over time.
Psychiatric Symptoms
For hair cortisol and cortisol/cortisone sum, we found positive associations with SCL-90-R anxiety and PSDI ratings in the total sample. Previous studies have shown controversial findings with either lower (Steudte et al., 2011b) or normal (Steudte-Schmiedgen et al., 2017) hair cortisol levels in individuals with generalized anxiety disorder as well as higher hair cortisol levels in patients with single-episode anxiety disorders (Elnazer et al., 2021). Moreover, a study investigating a very large sample of 1166 patients showed that hair cortisol was increased in patients with comorbid depression and anxiety but normal in patients suffering from only 1 of both disorders (Gerritsen et al., 2019). The association between hair cortisol and PSDI ratings is in accordance with the meta-analytic finding that hair cortisol is elevated in individuals exposed to chronic or ongoing stress (Stalder et al., 2017). Furthermore, we found a negative association of OEA and ADHD sum score, SCL-90-R psychoticism scale and PSDI. In fact, it has been previously suggested that the eCB system is critically involved in the etiopathogenesis of psychosis (Leweke, 2012; Minichino et al., 2019) and ADHD (for review see Navarrete et al., 2020). Overall, psychiatric symptoms are often associated with reduced hair eCB levels making them candidates for the investigation of potential biomarkers for psychiatric diseases and providing new possibilities in the pharmacotherapy of psychiatric disorders.
Strengths and Limitations
The main strength of this study was the use of hair analysis as a reliable tool to investigate cumulative stress marker alterations in chronic cocaine users. Firstly, we used 2 highly specific analytical methods for the determination of neuroendocrine stress markers (glucocorticoids, eCBs) and substances (cocaine and its metabolites, MDMA) in hair. The combination with additional subjective self-reports of substance use over time (cocaine, MDMA, and cannabis use) provided novel insights into substance-specific interactions of chronic substance use and the HPA axis as well as eCB system. Secondly, our analysis tested several covariates to ensure the robustness of the results. Although previous studies have reported that sex and age had an influence on hair cortisol and cortisone levels (Dettenborn et al., 2012; Stalder et al., 2017; Binz et al., 2018; Lanfear et al., 2020), we did not find any effects of these covariates for glucocorticoids in the present sample. This might be explained by the fact that this study did not include infants, young children, and older adults as in other previous studies (Binz et al., 2018; Lanfear et al., 2020). Thirdly, we determined the direct effect of cocaine hair concentration on glucocorticoid and eCB hair levels by controlling for associated confounding variables based on the hair toxicology and self-reports. This allowed us to infer cocaine-specific interactions with the HPA axis and eCB system.
It is important to note that this study also had limitations. The complexity of the endocannabinoid system with several ligands and receptors is inevitably difficult to analyze in the hair matrix. It cannot replace the measurement of acute changes of eCBs and further studies are needed to investigate the correlation of acute plasma and sustained hair eCB levels. Moreover, previous studies have reported that alterations of the eCB signaling system in animals and humans were related to specific brain-structures showing either increased, decreased, or no changes in eCB levels (Gonzalez et al., 2002a; Bystrowska et al., 2014, 2019). Our findings of decreased PEA and OEA hair levels cannot address the question of regional brain-specific differences in eCB concentration but give information of circulating eCB levels overall.
Conclusions
To our knowledge, this is the first study to identify a variety of cumulative neuroendocrine markers of sustained stress in chronic cocaine users by hair analysis. Our results corroborated previous findings of a dysfunctional HPA stress response and established novel findings of a reduced eCB signaling system in cocaine users. Specifically, PEA and OEA were reduced in cocaine users, which might also indirectly affect the stress buffering effects of AEA and 2-AG. We found that cannabis and MDMA are important co-contributors for glucocorticoid and eCBs alterations in hair of chronic cocaine users. Given that stress has been proposed as a key risk factor for developing substance use disorders and increased relapse, our findings contribute to the search for novel and improved treatments for cocaine relapse and abstinence for instance by targeting the eCB system in a pharmaco-therapeutic setting.
Supplementary Material
Acknowledgments
The authors express their gratitude to Emma Louise Kessler, MD for the generous legacy she donated to the Institute of Forensic Medicine at the University of Zurich, Switzerland for research purposes. The authors thank Lydia Johnson-Ferguson for proofreading the manuscript.
This work was supported by the Forschungskredit of the University of Zurich (Grant no. FK-20-041) to Clarissa D. Voegel. The work was supported by grants from the Swiss National Science Foundation (SNSF; grant No. PP00P1-123516/1 and PP00P1-146326/1) and the Olga Mayenfisch Foundation to B. B. Quednow. The funders of the study did not influence the design and conduct of the study; the collection, management, analysis, and interpretation of the data; the preparation, review, and approval of the manuscript; and the decision to submit the manuscript for publication.
Interest Statement
The authors declare no conflict of interest.
References
- Armario A (2010) Activation of the hypothalamic-pituitary-adrenal axis by addictive drugs: different pathways, common outcome. Trends Pharmacol Sci 31:318–325. [DOI] [PubMed] [Google Scholar]
- Baumann MH, Gendron TM, Becketts KM, Henningfield JE, Gorelick DA, Rothman RB (1995) Effects of intravenous cocaine on plasma cortisol and prolactin in human cocaine abusers. Biol Psychiatry 38:751–755. [DOI] [PubMed] [Google Scholar]
- Behnke A, Karabatsiakis A, Krumbholz A, Karrasch S, Schelling G, Kolassa IT, Rojas R (2020) Associating Emergency Medical Services personnel’s workload, trauma exposure, and health with the cortisol, endocannabinoid, and N-acylethanolamine concentrations in their hair. Sci Rep 10:22403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilbao A, Serrano A, Cippitelli A, Pavón FJ, Giuffrida A, Suárez J, García-Marchena N, Baixeras E, Gómez de Heras R, Orio L, Alén F, Ciccocioppo R, Cravatt BF, Parsons LH, Piomelli D, Rodríguez de Fonseca F (2016) Role of the satiety factor oleoylethanolamide in alcoholism. Addict Biol 21:859–872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binz TM, Rietschel L, Streit F, Hofmann M, Gehrke J, Herdener M, Quednow BB, Martin NG, Rietschel M, Kraemer T, Baumgartner MR (2018) Endogenous cortisol in keratinized matrices: systematic determination of baseline cortisol levels in hair and the influence of sex, age and hair color. Forensic Sci Int 284:33–38. [DOI] [PubMed] [Google Scholar]
- Bluett RJ, Gamble-George JC, Hermanson DJ, Hartley ND, Marnett LJ, Patel S (2014) Central anandamide deficiency predicts stress-induced anxiety: behavioral reversal through endocannabinoid augmentation. Transl Psychiatry 4:e408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bystrowska B, Smaga I, Frankowska M, Filip M (2014) Changes in endocannabinoid and N-acylethanolamine levels in rat brain structures following cocaine self-administration and extinction training. Prog Neuropsychopharmacol Biol Psychiatry 50:1–10. [DOI] [PubMed] [Google Scholar]
- Bystrowska B, Frankowska M, Smaga I, Niedzielska-Andres E, Pomierny-Chamiolo L, Filip M (2019) Cocaine-induced reinstatement of cocaine seeking provokes changes in the endocannabinoid and N-acylethanolamine levels in rat brain structures. Molecules 24:1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chauvet C, Nicolas C, Thiriet N, Lardeux MV, Duranti A, Solinas M (2014) Chronic stimulation of the tone of endogenous anandamide reduces cue- and stress-induced relapse in rats. Int J Neuropsychopharmacol 18:pyu025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Contoreggi C, Herning RI, Koeppl B, Simpson PM, Negro PJ Jr, Fortner-Burton C, Hess J (2003) Treatment-seeking inpatient cocaine abusers show hypothalamic dysregulation of both basal prolactin and cortisol secretion. Neuroendocrinology 78:154–162. [DOI] [PubMed] [Google Scholar]
- Cravatt BF, Giang DK, Mayfield SP, Boger DL, Lerner RA, Gilula NB (1996) Molecular characterization of an enzyme that degrades neuromodulatory fatty-acid amides. Nature 384:83–87. [DOI] [PubMed] [Google Scholar]
- Croissant M, Glaesmer H, Klucken T, Kirschbaum C, Gao W, Stalder T, Sierau S (2020) Endocannabinoid concentrations in hair and mental health of unaccompanied refugee minors. Psychoneuroendocrinology 116:104683. [DOI] [PubMed] [Google Scholar]
- deRoon-Cassini TA, Stollenwerk TM, Beatka M, Hillard CJ (2020) Meet your stress management professionals: the endocannabinoids. Trends Mol Med 26:953–968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dettenborn L, Tietze A, Kirschbaum C, Stalder T (2012) The assessment of cortisol in human hair: associations with sociodemographic variables and potential confounders. Stress 15:578–588. [DOI] [PubMed] [Google Scholar]
- Di Marzo V, Berrendero F, Bisogno T, González S, Cavaliere P, Romero J, Cebeira M, Ramos JA, Fernández-Ruiz JJ (2000) Enhancement of anandamide formation in the limbic forebrain and reduction of endocannabinoid contents in the striatum of delta9-tetrahydrocannabinol-tolerant rats. J Neurochem 74:1627–1635. [DOI] [PubMed] [Google Scholar]
- Di Marzo V, Melck D, Orlando P, Bisogno T, Zagoory O, Bifulco M, Vogel Z, De Petrocellis L (2001) Palmitoylethanolamide inhibits the expression of fatty acid amide hydrolase and enhances the anti-proliferative effect of anandamide in human breast cancer cells. Biochem J 358:249–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumont GJ, Verkes RJ (2006) A review of acute effects of 3,4-methylenedioxymethamphetamine in healthy volunteers. J Psychopharmacol 20:176–187. [DOI] [PubMed] [Google Scholar]
- Elnazer HY, Lau LCK, Amaro H, Baldwin DS (2021) Hair cortisol concentration in anxiety disorders: exploration of relationships with symptom severity and inflammatory markers. Acta Neuropsychiatr 33:104–110. [DOI] [PubMed] [Google Scholar]
- Ersche KD, Barnes A, Jones PS, Morein-Zamir S, Robbins TW, Bullmore ET (2011) Abnormal structure of frontostriatal brain systems is associated with aspects of impulsivity and compulsivity in cocaine dependence. Brain 134:2013–2024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faul F, Erdfelder E, Buchner A, Lang AG (2009) Statistical power analyses using G*Power 3.1: tests for correlation and regression analyses. Behav Res Methods 41:1149–1160. [DOI] [PubMed] [Google Scholar]
- Feeney JC, O’Halloran AM, Kenny RA (2018) The association between hair cortisol, hair cortisone, and cognitive function in a population-based cohort of older adults: results from the Irish longitudinal study on ageing. J Gerontol A Biol Sci Med Sci 75:257–265. [DOI] [PubMed] [Google Scholar]
- Fox HC, Jackson ED, Sinha R (2009) Elevated cortisol and learning and memory deficits in cocaine dependent individuals: relationship to relapse outcomes. Psychoneuroendocrinology 34:1198–1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao W, Walther A, Wekenborg M, Penz M, Kirschbaum C (2020) Determination of endocannabinoids and N-acylethanolamines in human hair with LC-MS/MS and their relation to symptoms of depression, burnout, and anxiety. Talanta 217:121006. [DOI] [PubMed] [Google Scholar]
- Gerritsen L, Staufenbiel SM, Penninx BWJH, van Hemert AM, Noppe G, de Rijke YB, van Rossum EFC (2019) Long-term glucocorticoid levels measured in hair in patients with depressive and anxiety disorders. Psychoneuroendocrinology 101:246–252. [DOI] [PubMed] [Google Scholar]
- Gonzalez S, Fernandez-Ruiz J, Sparpaglione V, Parolaro D, Ramos JA (2002a) Chronic exposure to morphine, cocaine or ethanol in rats produced different effects in brain cannabinoid CB(1) receptor binding and mRNA levels. Drug Alcohol Depend 66:77–84. [DOI] [PubMed] [Google Scholar]
- Gonzalez S, Cascio MG, Fernández-Ruiz J, Fezza F, Di Marzo V, Ramos JA (2002b) Changes in endocannabinoid contents in the brain of rats chronically exposed to nicotine, ethanol or cocaine. Brain Res 954:73–81. [DOI] [PubMed] [Google Scholar]
- Haijen E, Farre M, de la Torre R, Pastor A, Olesti E, Pizarro N, Ramaekers JG, Kuypers KPC (2018) Peripheral endocannabinoid concentrations are not associated with verbal memory impairment during MDMA intoxication. Psychopharmacology 235:709–717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haj-Dahmane S, Shen RY (2011) Modulation of the serotonin system by endocannabinoid signaling. Neuropharmacology 61:414–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton J, Marion M, Figueiredo A, Clavin BH, Deutsch D, Kaczocha M, Haj-Dahmane S, Thanos PK (2018) Fatty acid binding protein deletion prevents stress-induced preference for cocaine and dampens stress-induced corticosterone levels. Synapse 72:e22031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heesch CM, Negus BH, Keffer JH, Snyder RW 2nd, Risser RC, Eichhorn EJ (1995) Effects of cocaine on cortisol secretion in humans. Am J Med Sci 310:61–64. [DOI] [PubMed] [Google Scholar]
- Herrera MI, Kölliker-Frers R, Barreto G, Blanco E, Capani F (2016) Glial modulation by N-acylethanolamides in brain injury and neurodegeneration. Front Aging Neurosci 8:81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higginbotham JA, Jones NM, Wang R, Christian RJ, Ritchie JL, McLaughlin RJ, Fuchs RA (2021) Basolateral amygdala CB1 receptors gate HPA axis activation and context-cocaine memory strength during reconsolidation. Neuropsychopharmacology 46:1554–1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill MN, Kumar SA, Filipski SB, Iverson M, Stuhr KL, Keith JM, Cravatt BF, Hillard CJ, Chattarji S, McEwen BS (2013) Disruption of fatty acid amide hydrolase activity prevents the effects of chronic stress on anxiety and amygdalar microstructure. Mol Psychiatry 18:1125–1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillard CJ (2018) Circulating endocannabinoids: from whence do they come and where are they going? Neuropsychopharmacology 43:155–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirsiger S, Hänggi J, Germann J, Vonmoos M, Preller KH, Engeli EJE, Kirschner M, Reinhard C, Hulka LM, Baumgartner MR, Chakravarty MM, Seifritz E, Herdener M, Quednow BB (2019) Longitudinal changes in cocaine intake and cognition are linked to cortical thickness adaptations in cocaine users. Neuroimage Clin 21:101652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirvonen J, Goodwin RS, Li CT, Terry GE, Zoghbi SS, Morse C, Pike VW, Volkow ND, Huestis MA, Innis RB (2012) Reversible and regionally selective downregulation of brain cannabinoid CB1 receptors in chronic daily cannabis smokers. Mol Psychiatry 17:642–649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hysek CM, Simmler LD, Schillinger N, Meyer N, Schmid Y, Donzelli M, Grouzmann E, Liechti ME (2014) Pharmacokinetic and pharmacodynamic effects of methylphenidate and MDMA administered alone or in combination. Int J Neuropsychopharmacol 17:371–381. [DOI] [PubMed] [Google Scholar]
- Jedema HP, Song X, Aizenstein HJ, Bonner AR, Stein EA, Yang Y, Bradberry CW (2021) Long-term cocaine self-administration produces structural brain changes that correlate with altered cognition. Biol Psychiatry 89:376–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob G, Kreek MJ (2007) Stress, dysregulation of drug reward pathways, and the transition to drug dependence. Am J Psychiatry 164:1149–1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF, Buck CL, Cohen A, Edwards S, Park PE, Schlosburg JE, Schmeichel B, Vendruscolo LF, Wade CL, Whitfield TW Jr, George O (2014) Addiction as a stress surfeit disorder. Neuropharmacology 76 Pt B:370–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanfear JH, Voegel CD, Binz TM, Paul RA (2020) Hair cortisol measurement in older adults: influence of demographic and physiological factors and correlation with perceived stress. Steroids 163:108712. [DOI] [PubMed] [Google Scholar]
- Leweke FM (2012) Anandamide dysfunction in prodromal and established psychosis. Curr Pharm Des 18:5188–5193. [DOI] [PubMed] [Google Scholar]
- Lopez-Quintero C, Pérez de los Cobos J, Hasin DS, Okuda M, Wang S, Grant BF, Blanco C (2011) Probability and predictors of transition from first use to dependence on nicotine, alcohol, cannabis, and cocaine: results of the National Epidemiologic Survey on Alcohol and Related Conditions (NESARC). Drug Alcohol Depend 115:120–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayo LM, Asratian A, Lindé J, Morena M, Haataja R, Hammar V, Augier G, Hill MN, Heilig M (2019) Elevated anandamide, enhanced recall of fear extinction, and attenuated stress responses following inhibition of fatty acid amide hydrolase: a randomized, controlled experimental medicine trial. Biol Psychiatry 87:538–547. [DOI] [PubMed] [Google Scholar]
- McLellan AT, Lewis DC, O’Brien CP, Kleber HD (2000) Drug dependence, a chronic medical illness: implications for treatment, insurance, and outcomes evaluation. JAMA 284:1689–1695. [DOI] [PubMed] [Google Scholar]
- McReynolds JR, Peña DF, Blacktop JM, Mantsch JR (2014) Neurobiological mechanisms underlying relapse to cocaine use: contributions of CRF and noradrenergic systems and regulation by glucocorticoids. Stress 17:22–38. [DOI] [PubMed] [Google Scholar]
- McReynolds JR, Doncheck EM, Li Y, Vranjkovic O, Graf EN, Ogasawara D, Cravatt BF, Baker DA, Liu QS, Hillard CJ, Mantsch JR (2018) Stress promotes drug seeking through glucocorticoid-dependent endocannabinoid mobilization in the prelimbic cortex. Biol Psychiatry 84:85–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milivojevic V, Sinha R (2018) Central and peripheral biomarkers of stress response for addiction risk and relapse vulnerability. Trends Mol Med 24:173–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minichino A, Senior M, Brondino N, Zhang SH, Godwlewska BR, Burnet PWJ, Cipriani A, Lennox BR (2019) Measuring disturbance of the endocannabinoid system in psychosis: a systematic review and meta-analysis. JAMA Psychiatry 76:914–923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreira FA, Jupp B, Belin D, Dalley JW (2015) Endocannabinoids and striatal function: implications for addiction-related behaviours. Behav Pharmacol 26:59–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan CJ, Page E, Schaefer C, Chatten K, Manocha A, Gulati S, Curran HV, Brandner B, Leweke FM (2013) Cerebrospinal fluid anandamide levels, cannabis use and psychotic-like symptoms. Br J Psychiatry 202:381–382. [DOI] [PubMed] [Google Scholar]
- Mulder AM, Cravatt BF (2006) Endocannabinoid metabolism in the absence of fatty acid amide hydrolase (FAAH): discovery of phosphorylcholine derivatives of N-acyl ethanolamines. Biochemistry 45:11267–11277. [DOI] [PubMed] [Google Scholar]
- Mwanza C, Chen Z, Zhang Q, Chen S, Wang W, Deng H (2016) Simultaneous HPLC-APCI-MS/MS quantification of endogenous cannabinoids and glucocorticoids in hair. J Chromatogr B Analyt Technol Biomed Life Sci 1028:1–10. [DOI] [PubMed] [Google Scholar]
- Navarrete F, García-Gutiérrez MS, Gasparyan A, Austrich-Olivares A, Femenía T, Manzanares J (2020) Cannabis use in pregnant and breastfeeding women: behavioral and neurobiological consequences. Front Psychiatry 11:586447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orio L, Alen F, Pavón FJ, Serrano A, García-Bueno B (2018) Oleoylethanolamide, neuroinflammation, and alcohol abuse. Front Mol Neurosci 11:490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parrott AC, Lock J, Conner AC, Kissling C, Thome J (2008) Dance clubbing on MDMA and during abstinence from Ecstasy/MDMA: prospective neuroendocrine and psychobiological changes. Neuropsychobiology 57:165–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parrott AC, Sands HR, Jones L, Clow A, Evans P, Downey LA, Stalder T (2014) Increased cortisol levels in hair of recent Ecstasy/MDMA users. Eur Neuropsychopharmacol 24:369–374. [DOI] [PubMed] [Google Scholar]
- Pavón FJ, Araos P, Pastor A, Calado M, Pedraz M, Campos-Cloute R, Ruiz JJ, Serrano A, Blanco E, Rivera P, Suárez J, Romero-Cuevas M, Pujadas M, Vergara-Moragues E, Gornemann I, Torrens M, de la Torre R, Rodríguez de Fonseca F (2013) Evaluation of plasma-free endocannabinoids and their congeners in abstinent cocaine addicts seeking outpatient treatment: impact of psychiatric co-morbidity. Addict Biol 18:955–969. [DOI] [PubMed] [Google Scholar]
- Peters KZ, Cheer JF, Tonini R (2021) Modulating the neuromodulators: dopamine, serotonin, and the endocannabinoid system. Trends Neurosci 44:464–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrosino S, Schiano Moriello A, Cerrato S, Fusco M, Puigdemont A, De Petrocellis L, Di Marzo V (2016) The anti-inflammatory mediator palmitoylethanolamide enhances the levels of 2-arachidonoyl-glycerol and potentiates its actions at TRPV1 cation channels. Br J Pharmacol 173:1154–1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preller KH, Ingold N, Hulka LM, Vonmoos M, Jenni D, Baumgartner MR, Vollenweider FX, Quednow BB (2013) Increased sensorimotor gating in recreational and dependent cocaine users is modulated by craving and attention-deficit/hyperactivity disorder symptoms. Biol Psychiatry 73:225–234. [DOI] [PubMed] [Google Scholar]
- Quednow BB, Steinhoff A, Bechtiger L, Ribeaud D, Eisner M, Shanahan L. High prevalence and early onsets: legal and illegal substance use in an urban cohort of young adults in switzerland. Eur Addict Res. In Press. [DOI] [PubMed] [Google Scholar]
- R Core Team (2018) R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing. [Google Scholar]
- Rabin RA, Mackey S, Parvaz MA, Cousijn J, Li CS, Pearlson G, Schmaal L, Sinha R, Stein E, Veltman D, Thompson PM, Conrod P, Garavan H, Alia-Klein N, Goldstein RZ (2020) Common and gender-specific associations with cocaine use on gray matter volume: data from the ENIGMA addiction working group. Hum Brain Mapp. doi: 10.1002/hbm.25141. Online ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robledo P (2010) Cannabinoids, opioids and MDMA: neuropsychological interactions related to addiction. Curr Drug Targets 11:429–439. [DOI] [PubMed] [Google Scholar]
- Schote AB, Jäger K, Kroll SL, Vonmoos M, Hulka LM, Preller KH, Meyer J, Grünblatt E, Quednow BB (2019) Glucocorticoid receptor gene variants and lower expression of NR3C1 are associated with cocaine use. Addict Biol 24:730–742. [DOI] [PubMed] [Google Scholar]
- Seibert J, Hysek CM, Penno CA, Schmid Y, Kratschmar DV, Liechti ME, Odermatt A (2014) Acute effects of 3,4-methylenedioxymethamphetamine and methylphenidate on circulating steroid levels in healthy subjects. Neuroendocrinology 100:17–25. [DOI] [PubMed] [Google Scholar]
- Simpson DD, Joe GW, Fletcher BW, Hubbard RL, Anglin MD (1999) A national evaluation of treatment outcomes for cocaine dependence. Arch Gen Psychiatry 56:507–514. [DOI] [PubMed] [Google Scholar]
- Sinha R (2008) Chronic stress, drug use, and vulnerability to addiction. Ann N Y Acad Sci 1141:105–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha R, Garcia M, Paliwal P, Kreek MJ, Rounsaville BJ (2006) Stress-induced cocaine craving and hypothalamic-pituitary-adrenal responses are predictive of cocaine relapse outcomes. Arch Gen Psychiatry 63:324–331. [DOI] [PubMed] [Google Scholar]
- Stalder T, Kirschbaum C (2012) Analysis of cortisol in hair–state of the art and future directions. Brain Behav Immun 26:1019–1029. [DOI] [PubMed] [Google Scholar]
- Stalder T, Kirschbaum C, Heinze K, Steudte S, Foley P, Tietze A, Dettenborn L (2010) Use of hair cortisol analysis to detect hypercortisolism during active drinking phases in alcohol-dependent individuals. Biol Psychol 85:357–360. [DOI] [PubMed] [Google Scholar]
- Stalder T, Steudte-Schmiedgen S, Alexander N, Klucken T, Vater A, Wichmann S, Kirschbaum C, Miller R (2017) Stress-related and basic determinants of hair cortisol in humans: a meta-analysis. Psychoneuroendocrinology 77:261–274. [DOI] [PubMed] [Google Scholar]
- Staufenbiel SM, Penninx BW, Spijker AT, Elzinga BM, van Rossum EF (2013) Hair cortisol, stress exposure, and mental health in humans: a systematic review. Psychoneuroendocrinology 38:1220–1235. [DOI] [PubMed] [Google Scholar]
- Steudte S, Kolassa IT, Stalder T, Pfeiffer A, Kirschbaum C, Elbert T (2011a) Increased cortisol concentrations in hair of severely traumatized Ugandan individuals with PTSD. Psychoneuroendocrinology 36:1193–1200. [DOI] [PubMed] [Google Scholar]
- Steudte S, Stalder T, Dettenborn L, Klumbies E, Foley P, Beesdo-Baum K, Kirschbaum C (2011b) Decreased hair cortisol concentrations in generalised anxiety disorder. Psychiatry Res 186:310–314. [DOI] [PubMed] [Google Scholar]
- Steudte-Schmiedgen S, Wichmann S, Stalder T, Hilbert K, Muehlhan M, Lueken U, Beesdo-Baum K (2017) Hair cortisol concentrations and cortisol stress reactivity in generalized anxiety disorder, major depression and their comorbidity. J Psychiatr Res 84:184–190. [DOI] [PubMed] [Google Scholar]
- Tam FI, Steding J, Steinhäuser JL, Ritschel F, Gao W, Weidner K, Roessner V, Kirschbaum C, Ehrlich S (2021) Hair endocannabinoid concentrations in individuals with acute and weight-recovered anorexia nervosa. Prog Neuropsychopharmacol Biol Psychiatry 107:110243. [DOI] [PubMed] [Google Scholar]
- Valverde O, Rodríguez-Árias M (2013) Modulation of 3,4-methylenedioxymethamphetamine effects by endocannabinoid system. Curr Pharm Des 19:7081–7091. [DOI] [PubMed] [Google Scholar]
- Van Uum SH, Sauvé B, Fraser LA, Morley-Forster P, Paul TL, Koren G (2008) Elevated content of cortisol in hair of patients with severe chronic pain: a novel biomarker for stress. Stress 11:483–488. [DOI] [PubMed] [Google Scholar]
- Voegel CD, Baumgartner MR, Kraemer T, Wüst S, Binz TM (2021) Simultaneous quantification of steroid hormones and endocannabinoids (ECs) in human hair using an automated supported liquid extraction (SLE) and LC-MS/MS - Insights into EC baseline values and correlation to steroid concentrations. Talanta 222:121499. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Baler RD, Goldstein RZ (2011) Addiction: pulling at the neural threads of social behaviors. Neuron 69:599–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vonmoos M, Hulka LM, Preller KH, Jenni D, Schulz C, Baumgartner MR, Quednow BB (2013a) Differences in self-reported and behavioral measures of impulsivity in recreational and dependent cocaine users. Drug Alcohol Depend 133:61–70. [DOI] [PubMed] [Google Scholar]
- Vonmoos M, Hulka LM, Preller KH, Jenni D, Baumgartner MR, Stohler R, Bolla KI, Quednow BB (2013b) Cognitive dysfunctions in recreational and dependent cocaine users: role of attention-deficit hyperactivity disorder, craving and early age at onset. Br J Psychiatry 203:35–43. [DOI] [PubMed] [Google Scholar]
- Wagner FA, Anthony JC (2002) From first drug use to drug dependence; developmental periods of risk for dependence upon marijuana, cocaine, and alcohol. Neuropsychopharmacology 26:479–488. [DOI] [PubMed] [Google Scholar]
- Wemm SE, Sinha R (2019) Drug-induced stress responses and addiction risk and relapse. Neurobiol Stress 10:100148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilker S, Pfeiffer A, Elbert T, Ovuga E, Karabatsiakis A, Krumbholz A, Thieme D, Schelling G, Kolassa IT (2016) Endocannabinoid concentrations in hair are associated with PTSD symptom severity. Psychoneuroendocrinology 67:198–206. [DOI] [PubMed] [Google Scholar]
- Wingenfeld K, Dettenborn L, Kirschbaum C, Gao W, Otte C, Roepke S (2018) Reduced levels of the endocannabinoid arachidonylethanolamide (AEA) in hair in patients with borderline personality disorder - a pilot study. Stress 21:366–369. [DOI] [PubMed] [Google Scholar]
- Wiskerke J, Pattij T, Schoffelmeer AN, De Vries TJ (2008) The role of CB1 receptors in psychostimulant addiction. Addict Biol 13:225–238. [DOI] [PubMed] [Google Scholar]
- Wolff K, Tsapakis EM, Pariante CM, Kerwin RW, Forsling ML, Aitchison KJ (2012) Pharmacogenetic studies of change in cortisol on ecstasy (MDMA) consumption. J Psychopharmacol 26:419–428. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.