Abstract
Introduction: In Chile, 1 in 8 pregnant women of middle socioeconomic level has gestational diabetes mellitus (GDM), and in general, 5–10% of women with GDM develop type 2 diabetes after giving birth. Recently, various technological tools have emerged to assist patients with GDM to meet glycemic goals and facilitate constant glucose monitoring, making these tasks more straightforward and comfortable.
Objective: To evaluate the impact of remote monitoring technologies in assisting patients with GDM to achieve glycemic goals, and know the respective advantages and disadvantages when it comes to reducing risk during pregnancy, both for the mother and her child.
Methods: A total of 188 articles were obtained with the keywords “gestational diabetes mellitus,” “GDM,” “gestational diabetes,” added to the evaluation levels associated with “glucose level,” “glycemia,” “glycemic index,” “blood sugar,” and the technological proposal to evaluate with “glucometerm” “mobile application,” “mobile applications,” “technological tools,” “telemedicine,” “technovigilance,” “wearable” published during the period 2016–2021, excluding postpartum studies, from three scientific databases: PUBMED, Scopus and Web of Science. These were managed in the Mendeley platform and classified using the PRISMA method.
Results: A total of 28 articles were selected after elimination according to inclusion and exclusion criteria. The main measurement was glycemia and 4 medical devices were found (glucometer: conventional, with an infrared port, with Bluetooth, Smart type and continuous glucose monitor), which together with digital technology allow specific functions through 2 identified digital platforms (mobile applications and online systems). In four articles, the postprandial glucose was lower in the Tele-GDM groups than in the control group. Benefits such as improved glycemic control, increased satisfaction and acceptability, maternal confidence, decreased gestational weight gain, knowledge of GDM, and other relevant aspects were observed. There were also positive comments regarding the optimization of the medical team’s time.
Conclusion: The present review offers the opportunity to know about the respective advantages and disadvantages of remote monitoring technologies when it comes to reducing risk during pregnancy. GDM centered technology may help to evaluate outcomes and tailor personalized solutions to contribute to women’s health. More studies are needed to know the impact on a healthcare system.
Keywords: remote monitoring, telemedicine, gestational diabetes (GDM), technovigilance, mobile applications
Introduction
Gestational diabetes mellitus (GDM) is diagnosed when glycemia increases during pregnancy without any previous history (American Diabetes Association, 2020). GDM is currently the most common medical complication of pregnancy and the prevalence of undiagnosed hyperglycemia and even overt diabetes in young women is increasing (McIntyre et al., 2019). In Chile, 1 in 8 pregnant women of middle socioeconomic status has GDM (Garmendia et al., 2020), and 5–10% of women with GDM develop type 2 diabetes after delivery, maintaining a linear growth (Auvinen et al., 2020).
Maternal overweight and obesity (Shin and Sond, 2014), late age at childbearing (Anna et al., 2008), previous history of GDM, family history of type 2 diabetes mellitus, and ethnicity are the main risk factors for GDM (McIntyre et al., 2019). Diagnosis is usually made by an oral glucose tolerance test (OGTT), although in some parts of the world a non-fasting glucose challenge test (GCT) is used to screen for those women who require a full OGTT (McIntyre and Moses, 2020).
In Chile, the diagnosis of diabetes during the first trimester of pregnancy is based on the same criteria used for the general population (World Health Organization, 2006; ADA, 2014). 1) Common symptoms related to diabetes (polydipsia, polyuria, polyphagia and low weight) and a glycemia at any time of the day greater or equal than 200 mg/dl, unrelated to the time elapsed since last meal. 2) Fasting glycemia greater than or equal to 126 mg/dl. Confirm with a second glycemia ≥126 mg/dl, on a different day. 3) Glycemia greater than or equal to 200 mg/dl 2 h after a 75 g glucose load during an OGTT (Ministerio de Salud, 2014).
GDM in the health care system has tripled over a 14-years period, which could be explained by changes in the trend of risk factors during this period (Garmendia et al., 2020), such as the increased prevalence of obesity, along with later fertility which has been associated with higher risk gestation. All this results in an increased burden of care and demand for specialized services (Ministerio de Salud, 2014). For proper management of GDM during pregnancy, it is important to meet the glycemic goals, so patients must achieve constant monitoring, at least once a day, and maybe more, depending on the severity of the glycemia alteration in pregnancy, and they must also comply with dietary treatment and an exercise plan suggested by a specialist (Ministerio de Salud, 2014; McIntyre and Moses, 2020).
The goal of treatment of a woman with GDM is to achieve optimal metabolic control from the time of conception and throughout pregnancy, with fasting and postprandial euglycemia levels, and to screen for and treat any intercurrent pathology (Jovanovic et al., 2005). In Chile, the glycemic target is to maintain fasting glycemia levels between 60 and 90 mg/dl and <140 mg/dl, 1 h postprandial and <120 mg/dl, 2 h postprandial and HbA1c <6% (Ministerio de Salud, 2014).
Keeping a constant and orderly manual record can be complex and cumbersome, considering current lifestyles: women with more than one child often work full or part-time in addition to being homemakers. Thus, to facilitate the constant monitoring of glucose levels or to keep a caloric record of the diet suggested by a specialist, several technological tools have emerged that will make this type of task simpler and more comfortable. This is where the term Telemonitoring or E-Health comes into play, which has made it possible to simplify self-care by empowering the patients themselves to manage their health, while keeping health personnel informed and facilitating access to timely medical care (Lemelin et al., 2020).
The main objective of the review is to evaluate the impact of current technologies and methods of assisting patients with GDM to achieve glycemic goals, and know the respective advantages and disadvantages of these technologies when it comes to reducing risk during pregnancy, both for the mother and her child.
Methodology
This systematic review was carried out following the guidelines for systematic reviews and meta-analysis (PRISMA) (Urrútia and Bonfill, 2010). To access the literature of interest in a database, in this case, Web of Science, Scopus and Pubmed, it was necessary to identify the search criteria. The following were established as inclusion criteria: Articles related to current technologies for remote monitoring of GDM and parameters and variables related to blood glucose control and treatment compliance monitoring, articles published between 2016 and 2021. As exclusion criteria were established: publications referring to the post-natal/postpartum period, articles related to other types of diabetes, articles related to the diagnosis of GDM, articles related to Pregestational Diabetes Mellitus (PGD), articles from Systematic Reviews and Meta-analyses, articles involving technologies that have not been tested in patients with GDM and articles related to technologies for the use of clinical staff. It should be noted that articles related to GDM treatments and therapies were not excluded from the selection, since a topic of interest in the review is remote monitoring of compliance with these. In addition, there was no exclusion of articles according to the number of study cases, nor will there be exclusion according to the age range of the subjects. After establishing the databases and search criteria, it was important to consider the keywords we used to perform the Boolean expression that gave us the related articles in the databases. To perform the search in the databases, it was necessary to form the optimal search expression that completely covers the topics of interest of the subject to be investigated. Synonyms for gestational diabetes, glycemic levels, and technology or monitoring were chosen among the keywords.
Once the articles that were considered for the review were determined (according to the inclusion/exclusion criteria), the articles were categorized by the type of technology used for remote monitoring of GDM, monitoring parameters, accuracy according to the studies, and by the advantages and disadvantages of the different methods, to estimate which ones might be more practical for patients with GDM.
Results
Result Selection
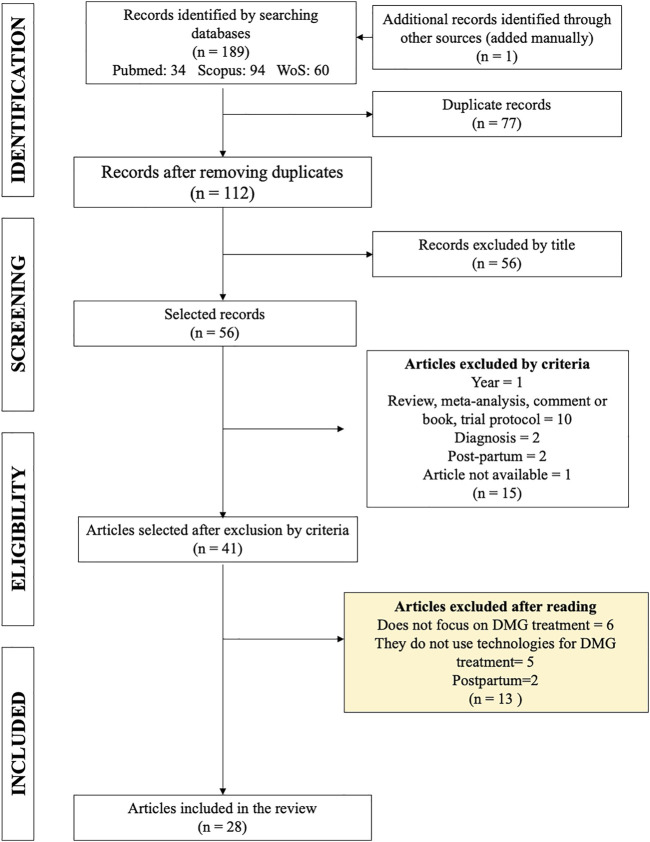
The search in the aforementioned databases resulted in a total of 188 articles, and one article was added manually as it met the inclusion criteria (Figure 1), of which 77 were eliminated as duplicates, leaving 112 publications for analysis. After eliminating duplicate entries, the titles were read. From this reading, it was deemed necessary to exclude a further 56 articles with titles too far from the topic of interest. Among the reasons for exclusion of articles by title were: meta-analysis, not using technology, focused on prediction or diagnosis of GDM, studies on mice, not focused on GDM or including the word “postpartum.”
FIGURE 1.
PRISMA diagram for the articles selection process.
The articles (abstracts) were then analyzed using the list of inclusion and exclusion criteria defined in the Methodology section. As a result, 15 articles that did not meet the guidelines were eliminated: 1 for being outside the stipulated time range; 2 for being concerned with the postpartum period; 2 for focusing on the diagnosis of GDM; 10 articles for being systematic reviews, meta-analyses, commentaries, chapters of books or trial protocols. One article was eliminated because it was not available on any platform. At this point, 41 articles were available for further reading.
Finally, the articles were read, and the decision was made to exclude a further 13 articles. Six of these did not focus on GDM, five did not use technologies for the treatment of GDM and two articles focused on postpartum measurements. In the end, 28 articles were selected for the systematic review (Supplementary Table S1).
Characteristics of the Selected Studies
Based on the results presented above, we proceeded to analyze each article individually to gather as much information as possible (Table 1).
TABLE 1.
Main characteristics of selected articles.
| Approach of study | Temporality | Geographic location of the study group | Year of publication |
|---|---|---|---|
| Quantitative (85.7%) | Retrospective (21.4%) | Asia (32.1%) | 2016 (7.2%) |
| Qualitative (10.7%) | Prospective (75%) | Europe (46.4%) | 2017 (14.3%) |
| Mixed (3.6%) | Mixed (3.6%) | North America (14.3%) | 2018 (35.6%) |
| Oceania (7.2%)s | 2019 (14.3%) | ||
| 2020 (14.3%) | |||
| 2021 (14.3%) |
Most of the studies were quantitative (24 studies) and prospective (9 studies). Regarding the location of the study group of pregnant women, they were mainly concentrated in Europe (13 studies) and Asia (9 studies). It is important to note that there were no studies that focused on pregnant women in Latin America, the Caribbean, or in Africa. As for the year of publication, the largest number of studies that met the inclusion criteria were published in 2018. Only two studies that met the inclusion criteria were published in 2016.
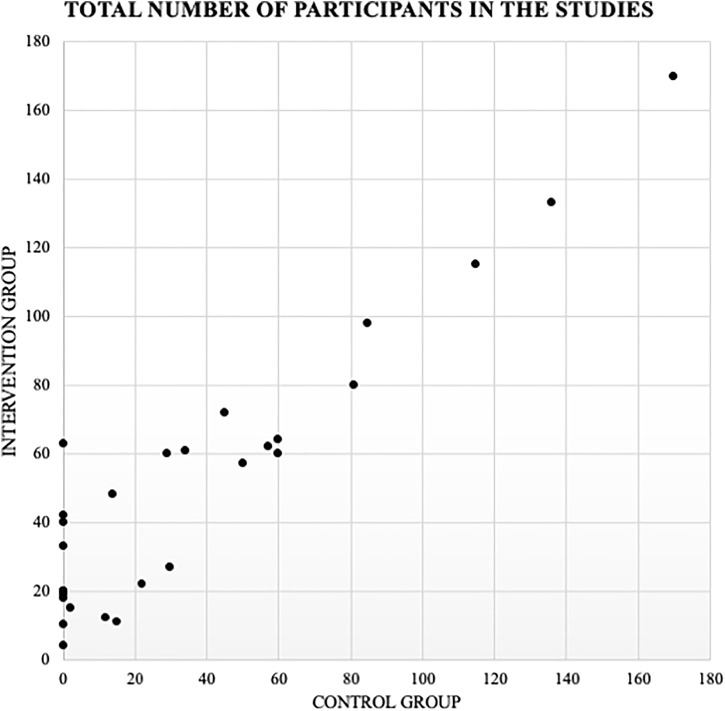
Of the total number of articles, 18 of these conducted two study groups or “case controls” where one group did not have the technology intervention for control of GDM and the other did. A total of 9 studies chose only to evaluate the technology tool prospectively, where there is only one study group of intervened pregnant women. One study divides the patients into 3 study groups, separating patients without GDM and with GDM, and separating the latter into those intervened using the technology tool and those not intervened (Figure 2).
FIGURE 2.
Graphical representation of the distribution of the number of participants. Intervention (with technological intervention for the treatment of GDM) v/s Control (without technological intervention for the treatment of GDM).
Measurement Parameters
The measurements included in the technology (application) used in the studies include the following characteristics: glycemia (26 articles), bodyweight measurement (7 articles), blood pressure (4 articles), insulin dose (3 articles), number of measurements (2 articles), meals (8 articles), ketonuria (2 articles), physical activity level (7 articles), anxiety and/or depression levels (1 article), medications (1 article), satisfaction levels and quality of life (2 articles). The article that included the technology with the most evaluations included 6 measures: glycemia, weight, blood pressure, ketonuria, physical activity, and satisfaction and quality of life levels (Peleg et al., 2017).
Medical Devices
Regarding the medical equipment used for glucose monitoring, 11 articles did not specify which one they used or focused on blood results at the beginning and end of the evaluations (Harrison et al., 2017; Johnson and Berry, 2018; Miremberg et al., 2018; Rasekaba et al., 2018; Skar et al., 2018; Triberti et al., 2018; Yang et al., 2018; Kim et al., 2019; Kim et al., 2021; Tian et al., 2021; Varnfield et al., 2021) The most commonly used glucometer was with BlueTooth: This type of glucometer transfers information to the technological device using a Bluetooth integrated into the device. It is necessary that the technological device also have BlueTooth (in this case the most used was a smartphone). It is important to note that, at the time of transferring information, the glucometer should be at a recommended safe distance of 10 m from the technological device. This medical device was used in 6 articles (22.2%) of the total number of articles chosen (Borgen et al., 2017; Peleg et al., 2017; Rigla et al., 2018; Albert et al., 2020; Lemelin et al., 2020; Seo et al., 2020). As for the Smart glucometer, this device measures glycemia just like a conventional glucometer, but must be connected directly by inserting its 3.5 mm jack into the headphone jack of the smartphone. Using the corresponding mobile application, it is possible to perform the measurement and transfer the data to the clinical staff for later review. Three articles (11%) used this medical device (Al-ofi et al., 2019; Guo et al., 2019; Yew et al., 2021). Two articles used a glucometer with an infrared port (Caballero-Ruiz et al., 2016; Caballero-Ruiz et al., 2017), which emits the information to a device reader, which is connected to a computer that will automatically receive the information. This information, after being received by the computer, is uploaded to the platform. It should be noted that the glucometer should be at a distance of 10 cm and the infrared ports facing the front. The continuous glucose monitor requires the insertion of a glucose sensor under the skin, which will receive information that will be continuously transmitted from the patient to the digital platform through the technological device. This medical device was used in two articles (Pustozerov et al., 2018; Pustozerov and Popova, 2018). All studies had moderately invasive methods to achieve glycemic control. Conventional glucometers, smart, Bluetooth and infrared, require blood sampling by the user at certain times depending on each study. The continuous glucose monitor, which goes through the skin, however, does not require the user to be aware of the schedules; she only has to wear it. As for the studies that did not use a glucose monitor, they measured blood glycemia and the other parameters, which implies at least two blood samples per study.
Technology and Digital Platform
The digital technologies used for GDM monitoring were smartphones, computers and tablets. A total of 14 articles used only a smartphone, 4 articles used a smartphone and computer, 5 articles used a smartphone, computer and tablet, 4 articles used only a computer and one article used a basic phone. The use of these technologies depends on the digital platform used for the telemonitoring of MGD. The articles that used mobile applications relied on smartphones for data collection (Bromuri et al., 2016; Borgen et al., 2017; Harrison et al., 2017; Mackillop et al., 2018; Miremberg et al., 2018; Rigla et al., 2018; Skar et al., 2018; Triberti et al., 2018; Yang et al., 2018; Al-ofi et al., 2019; Guo et al., 2019; Seo et al., 2020; Tian et al., 2021; Varnfield et al., 2021), the articles that used online systems used computers for data collection (Caballero-Ruiz et al., 2016; Caballero-Ruiz et al., 2017; Kim et al., 2019; Wernimont et al., 2020), and the articles that used multiplatform systems used smartphones and computers (Peleg et al., 2017; Albert et al., 2020; Kim et al., 2021; Yew et al., 2021), and four of these also used tablets (Pustozerov et al., 2018; Pustozerov and Popova, 2018; Rasekaba et al., 2018; Alqudah et al., 2019; Lemelin et al., 2020). Only one article did not specify its system used, however, they relied on smartphone data collection (Johnson and Berry, 2018).
Functions of the Technology for Monitoring GMD
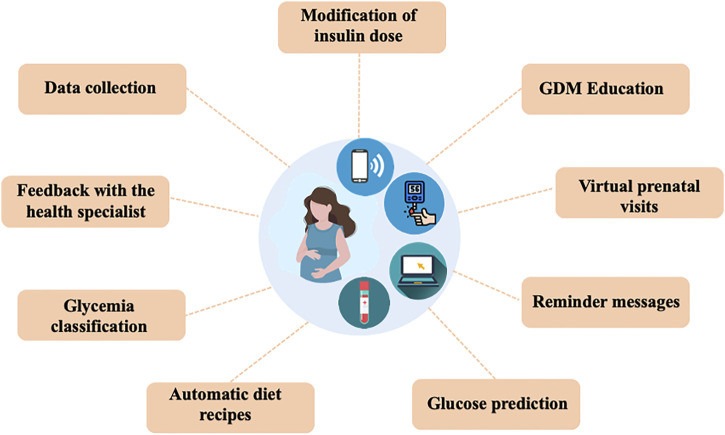
In the selected articles, the technological applications were mainly focused on the objective of improving glycemic control and supporting pregnant women in understanding GDM. Nine functions were selected that were common in the applied technologies: data collection, feedback with the specialist, glycemia classification, automatic feeding recipes, education in GDM, virtual prenatal visits, reminder messages, glycemia prediction and live modification of insulin doses (Figure 3).
FIGURE 3.
The technological applications were mainly focused on the objective of improving glycemic control and supporting pregnant women in understanding GDM. Nine functions were selected that were common in the applied technologies.
Data Collection
This function allows the patient to record all values that are requested by the clinical staff. Some are downloaded automatically and others need to be recorded manually. This function is enabled in 81.5% (22 articles) of the total, which is the most repeated (Bromuri et al., 2016; Borgen et al., 2017; Harrison et al., 2017; Peleg et al., 2017; Mackillop et al., 2018; Miremberg et al., 2018; Pustozerov et al., 2018; Pustozerov and Popova, 2018; Rasekaba et al., 2018; Rigla et al., 2018; Triberti et al., 2018; Yang et al., 2018; Al-ofi et al., 2019; Guo et al., 2019; Kim et al., 2019; Albert et al., 2020; Lemelin et al., 2020; Seo et al., 2020; Wernimont et al., 2020; Kim et al., 2021; Varnfield et al., 2021; Yew et al., 2021).
Feedback With the Health Specialist
Feedback refers to the communication that exists between the patient and the clinical staff, either through telephone calls, video calls, or text messages. Thirty-seven percent of the articles (10 articles) have this function (Caballero-Ruiz et al., 2017; Peleg et al., 2017; Miremberg et al., 2018; Pustozerov and Popova, 2018; Rasekaba et al., 2018; Triberti et al., 2018; Al-ofi et al., 2019; Albert et al., 2020; Lemelin et al., 2020; Varnfield et al., 2021). In most cases, it goes along with data collection.
Glycemia Classification
This type of function allows the patient to automatically associate the appropriate meal and time of measurement (preprandial or postprandial) to each incomplete glucose data downloaded from a glucometer. Five articles (19%) presented this function (Caballero-Ruiz et al., 2016; Borgen et al., 2017; Skar et al., 2018; Seo et al., 2020; Yew et al., 2021).
Automatic Diet Recipes
This function delivers automatically personalized diets based on the data recorded by the patients. Like the glycemia classification, this function is present in five articles (19%) (Borgen et al., 2017; Caballero-Ruiz et al., 2017; Skar et al., 2018; Seo et al., 2020; Yew et al., 2021).
GDM Education
Through messages or information uploaded to the platform, additional data on diets, exercises, medications, etc. are delivered to treat GDM. Educational information on GDM is presented in 44.4% (12 articles) (Borgen et al., 2017; Johnson and Berry, 2018; Mackillop et al., 2018; Skar et al., 2018; Triberti et al., 2018; Yang et al., 2018; Guo et al., 2019; Albert et al., 2020; Seo et al., 2020; Kim et al., 2021; Tian et al., 2021; Yew et al., 2021).
Virtual Prenatal Visits
This intervention makes it possible to alternate the usual clinical visits to the clinic with virtual visits from the patient’s home. This function is enabled in three articles (Harrison et al., 2017; Mackillop et al., 2018; Kim et al., 2019).
Reminder Messages
Patients are reminded to perform the corresponding glucose measurements using text messages. This function is found in 7 articles (Borgen et al., 2017; Johnson and Berry, 2018; Skar et al., 2018; Seo et al., 2020; Kim et al., 2021; Tian et al., 2021; Yew et al., 2021).
Glucose Prediction
This function allows the prediction of the glucose value without the patient having to measure with the glucometer, all this thanks to the nutritional information recorded by the patient. This function can be found in one article (Pustozerov et al., 2018).
Modification of Insulin Dose
The system has automatic responses or responses mediated by health professionals every time the patient needs to modify her insulin doses according to the glycemia measurement. This function is found in two articles (Albert et al., 2020; Yew et al., 2021).
Glycemic Results
Regarding glycemic results as such, in general, the technological interventions had positive results in the control of GDM. The articles that measured fasting glycemia decreased in the intervention group, in contrast to the control group in four articles (Yang et al., 2018; Guo et al., 2019; Seo et al., 2020; Kim et al., 2021). As for the articles that measured postprandial glycemia, this decreased significantly in four articles that evaluated this measurement (Miremberg et al., 2018; Yang et al., 2018; Al-ofi et al., 2019; Seo et al., 2020). Other studies evaluated glycosylated hemoglobin levels, all of which showed a decrease in this parameter in the experimental group (with technological intervention) versus the non-experimental group (Guo et al., 2019; Kim et al., 2019; Wernimont et al., 2020; Kim et al., 2021) (Table 2).
TABLE 2.
Results of glycemic control in pregnant women with GDM.
| Autor | Control group (n) | Intervetion group (n) | Technology used | Intervention Time | Glycemic control results | Other results |
|---|---|---|---|---|---|---|
| Al-Ofi et al. (2019) | 30 | 27 | Smart glucometer + Smartphone + Mobile Application | From week 24 to week 28 the pregnant women began to use the technology (4 weeks). The comparison tests were measured between gestational weeks 38 to 40 (2 weeks) | PPG 2 h is significantly lower in the intervention group (p = 0.002) | Most of the pregnant women in the intervention group had adequate gestational weight gain. The weight at the end of pregnancy in the intervention group was significantly lower (p = 0.03) |
| Fasting glycemia and HbA1c were not significantly different | ||||||
| Guo, et al. (2019) | 60 | 64 | Smart glucometer + Smartphone + Mobile Application | 13 weeks | HbA1c before delivery (%) 5.3 ± 0.3 (C) v/s 4.7 ± 0.2 (I) (p < 0.001) Off-target fasting glucose measurement (%) 8.3 ± 0.6 (C) v/s 4.6 ± 0.4 (I) (p < 0.001) Off-target 2 h post-prandial glucose measurement (%) 14.7 ± 0.8 (C) v/s 7.9 ± 0.7 (I) (p < 0.001) | Weight gain after treatment (kg) |
| 4.8 ± 0.7 v/s 3.2 ± 0.8 (p < 0.001) | ||||||
| Yang et al. (2018) | 50 | 57 | Smartphone + Mobile Application | From week 24–28 until term (approximately 13 weeks) | FBG (mmol/l) 5.31 ± 1.29 (C) v/s 4.31 ± 0.75 (I) (p = 0.000) 1 h PBG | Preterm delivery was significantly less likely in group A than in group B (p < 0.05) |
| 7.75 ± 2.08 (C) v/s 7.71 ± 0.73 (I) (p = 0.780) 2 h PBG 6.94 ± 2.47 (C) v/s 5.76 ± 0.67 (I) (p = 0.000) | ||||||
| Miremberg et al. (2018) | 60 | 60 | Smartphone + Mobile Application | From week 24–28 until term (approximately 13 weeks) | Mean blood glucose (mg/dl) vs. 112.6 ± 7.4 (C) v/s 105.1 ± 8.6 (I)(p < 0.001)Off-target fasting glucose measurement (%) 8.4 ± 0.6 (C) v/s 4.7 ± 0.4 (I) (p < 0.001)Off-target 1 h post-prandial glucose measurement (%) 14.3 ± 0.8 (C) v/s 7.7 ± 0.8 (I) (p < 0.001) Rate of pregnancies requiring insulin treatment 30.0 (C) v/s 13.3 (I) (p = 0.044) | — |
| Kim et al. (2021) | 62 | 57 | Computer/Smartphone + Multiplatform application | 12 weeks | Fasting flucose (mg/L) 103 ± 15.6 (C) v/s 92 ± 6.8 (p = 0.031) HbA1c (%)5.6 ± 0.3 (C) v/s 5.4 ± 0.3 (I) (p = 0.019) | Body weight (kg) |
| 68.2 ± 17.1 kg (C) v/s 61.5 ± 8.6 (I) | ||||||
| (p = 0.007) | ||||||
| Body fat (%) | ||||||
| 37.4 ± 5.9 (C) ± 32 ± 5.1 (I) | ||||||
| (p=<0.001) | ||||||
| Diabetes knowledge | ||||||
| 0.62 ± 0.9 (C) v/s 0.64 ± 0.9 (I) | ||||||
| (p = 0.558) | ||||||
| Dietary habits | ||||||
| 3.8 ± 0.4 (C) v/s 4 ± 0.3 (I) | ||||||
| (p < 0.001) | ||||||
| Health Promoting Lifestyle Profile Total Score | ||||||
| 2.64 ± 0.38 (C) v/s 2.82 ± 0.3 (I) | ||||||
| (p < 0.001) | ||||||
| Kim et al. (2019) | 22 | 22 | Computer + Web system | 12 weeks | HbA1c (%) | Anxiety in the experimental group decreased by 5.1 points but increased by 1.0 points in the control group (p = 0.048). Depression increased in both groups |
| 5.3 ± 0.2 (C) v/s 5.0 ± 0.2 (I) | ||||||
| (p = 0.001) | ||||||
| Glycated albumin (%) | ||||||
| 11.0 ± 1.4 (C) v/s 10.8 ± 1.2 (I) | ||||||
| (p = 0.776) | ||||||
| Fasting glucose (mg/dl) | ||||||
| 80.9 ± 8.4 (C) v/s 78.8 ± 8.4 (I) | ||||||
| (p = 0.075) | ||||||
| 1 h PBG (mg/dl) | ||||||
| 117.2 ± 22.2 (C) v/s 129.5 ± 19.9 (I) | ||||||
| (p = 0.489) | ||||||
| Seo et al. (2020) | 0 | 4 | Glucometer Bluetooth + Smartphone + Mobile application | From week 24–28 until 32—36 weeks (approximately 8 weeks) | Fasting glucose (mg/dl) 104.5 (C) v/s 94.75 (I) 2 h PBG (mg/dl) 223.25 (C) v/s 150.5 (I) | Alteration in the intake of some nutrients: |
| 1. Reduced consumption of: Calories, proteins, fats, Vitamin A, Vitamin C, Thiamine, Riboflavin, Calcium, animal and vegetable iron | ||||||
| 2. Increased consumption of: Carbohydrates | ||||||
| Wernimont et al. (2020) | 45 | 72 | Glucometer smart and standard glucometer (control) + Computer + Web system | From the first prenatal visit until delivery (approximately 34 weeks) | At delivery, women using the cellular glucometer had an average HbA1c of 6.0% compared with an average HbA1c of 6.8% for those women using a standard glucometer. The average decrease in HbA1c from baseline visit to delivery was significantly greater for women using the cellular glucometer (-2.6 ± 1.7%) compared with those using a standard glucometer (-1.4 ± 1.4%) | — |
All glycemic outcomes had significant improvements in the intervention groups with the use of GDM monitoring technologies. In addition to glycemic outcomes, there were positive results in terms of lower weight gain during pregnancy (Al-ofi et al., 2019; Guo et al., 2019; Kim et al., 2021), decreased complications at delivery, body fat, dietary habits and Health Promoting Lifestyle Profile Total Score (Kim et al., 2021). Anxiety decreased in the experimental group, but depression increased in both groups (Kim et al., 2019). The consumption of certain nutrients was affected in one of the studies that sought glycemic control through a mobile application (Seo et al., 2020) (Table 2).
Other Results Associated With the Maternal Perception of the Technologies Used for the Control of GDM
User satisfaction and Acceptability
Some of the conclusions of the articles were: Multi-platform system with Bluetooth glucometer monitoring improved user satisfaction (Peleg et al., 2017). In another study, many participants appreciated the ease of access (not having to keep a paper diary), ease of use, and convenience of the mobile application. They liked being able to monitor their blood glycemia values, the application’s ability to connect them quickly, and being able to get answers from their physician (Varnfield et al., 2021). In another study they state that as telemedicine becomes increasingly common in healthcare, user feedback will be essential to tailoring, communicating, and supporting the acceptance and success of these programs (Harrison et al., 2017). In another study, remote glycemia monitoring in women with GDM was shown to be safe. Although glycemic control and maternal and neonatal outcomes were similar, women preferred this model of care (Mackillop et al., 2018). In another study, more than a third of patients gave a cross-platform app with continuous glucose monitoring the maximum score of 10 points for its usefulness in monitoring the disease. At the same time, the convenience of the app received high ratings, with an average value of 8 (Pustozerov and Popova, 2018). Patients who used the system based on a mobile app and Bluetooth glucometer monitoring, according to a survey at the end of the study, reflect a high degree of satisfaction (Rigla et al., 2018). In another study where patients with DM2 and GDM used an app to record glycemic values, they generally adhered satisfactorily to the use of the application (Triberti et al., 2018). In a qualitative study reviewing the perception of mHealth solutions for diabetes in pregnancy, most of these women are willing to self-manage their condition from home and be monitored remotely by a healthcare team (Alqudah et al., 2019).
Increased Awareness and Motivation
For many of the women in the study where a mobile app was used, self-monitoring of blood glucose values, including an overview and real-time feedback, was the most important aspect of the app for increasing self-awareness and motivation (Skar et al., 2018). As a conclusion of the study, it is mentioned that there is still much room for improvement in the usability of the multiplatform application associated with continuous glucose monitoring, especially when it comes to perceiving patient motivation (Pustozerov and Popova, 2018).
Other Psychological Variables
Patients’ spiritual growth, level of interpersonal relationships and stress management improved significantly (p < 0.001) with the use of the cross-platform application-based system (Kim et al., 2021). In another study, anxiety in the experimental group, which used the web system, decreased by 5.1 points and increased by 1.0 points in the control group (p = 0.048). Depression increased in both groups (Kim et al., 2019). However, contrary to this result, in another randomized controlled trial, the intervention (multiplatform application) did not increase anxiety or depression (Yew et al., 2021). In another study, patients rated the sense of security provided by the system based on a multiplatform application with Bluetooth glucometer monitoring (Peleg et al., 2017).
Other Results Associated With the Medical Team Perception of the Technologies Used for the Control of GDM
Medical Team Satisfaction and Acceptability
The Bluetooth glucometer-based multiplatform system improved medical team satisfaction (Peleg et al., 2017). Physicians showed high levels of satisfaction with the mobile application (Varnfield et al., 2021).
Optimization of Patient Management
Some conclusions from the articles were: The medical team agreed that the multi-platform application and Bluetooth glucometer monitoring facilitated the glycemic management of patients (Peleg et al., 2017). The cross-platform glucometer app can be an excellent tool to avoid unnecessary hospital visits while maintaining better quality medical care and reducing physician workload in the management of GDM (Albert et al., 2020). In another study, all physicians either strongly agreed or agreed that the mobile application improved their efficiency in caring for their patients (Varnfield et al., 2021). In another study, the telemedicine system for GDM intervention did not change health care utilization or clinical outcomes compared to usual care (Rasekaba et al., 2018).
Improved Glycemic Control Remotely From the Patient
Some conclusions from the articles were: The use of the multiplatform application and Bluetooth glucometer monitoring resulted in high patient compliance with self-measurement. And physicians agreed that it facilitated patient management (Peleg et al., 2017). In the Smartphone group, there were more glucose measurements, and these measurements were significantly lower compared to the control group (Miremberg et al., 2018). The time needed to achieve optimal glycemic control was significantly shorter for participants in the intervention group using the web-based system than for those using usual care alone. Along with this, women in the intervention group required fewer insulin titrations than controls (Rasekaba et al., 2018). In another study where patients with DM2 and GDM used a mobile app to record glycemic values, there were no significant differences in the recording of glycemia between the two groups (Triberti et al., 2018). With the use of a mobile application, patients in the study showed greater compliance in glucose measurements (Rigla et al., 2018).
Time Spent by Clinicians in Patient Assessment and Evaluation
One article showed an 88.6% reduction in face-to-face visits and a 27.4% reduction in time spent by clinicians evaluating patients in the intervention group that used a multiplatform application with Bluetooth glucometer monitoring. The system detected all situations requiring therapeutic adjustment, generating safe recommendations (Albert et al., 2020). The use of a web System reduced personal visits, as well as the time physicians spend evaluating patients, this improves physicians’ efficiency in overcoming their increasing workload (Caballero-Ruiz et al., 2017). It was seen in another study that the telehomecare intervention group (THC) had an average of 1.5 versus 3.3 more medical visits than the control group. It increased 10 times more group nursing interventions compared to the control group, promoting greater GDM education. The results of this study show a significant decrease in medical visits and total health care costs for women in the THC group (Lemelin et al., 2020).
Some Problems or Adverse Study Results
Glucose Measurement
Some patients experienced discharge problems with the glucose meter (Albert et al., 2020). In another study, many women experienced technical problems in using the application. Several had problems with the automatic transfer of blood glucose values to the application, and many stopped using the application to record blood glucose values because of this (Skar et al., 2018).
Lack of Commitment of Medical Personnel to the Application
The pregnant women stated that the health professionals had little knowledge about the application and that they could not help them when they had problems with the application. Women also reported that their health professionals seemed to have little interest in the application and that they seemed more comfortable looking at blood glucose values on paper, which is standard procedure in the treatment of GDM. Some women stopped using the application to record blood glucose values because their health professionals only looked at their books with the recorded levels and not the application. The lack of support from their health professionals generated some frustration (Skar et al., 2018).
Frustration and Misinformation From the Patient
In a qualitative study of system use, some patients admitted that they sometimes “cheated” to get better values and feedback comments. One patient reported waiting 10 min to take her blood sugar to see if the value was lower. In addition, this same app generated some frustration and stress in the users. They stated that it is stressful to think about blood glucose values all the time, and it is frustrating to think if they are out of range. These negative feelings were associated with women who had problems controlling their blood glucose values. Of the women who had to use insulin, none used the application to monitor their blood glucose values, as they saw it as a burden (Skar et al., 2018).
Discussion
The systematic review conducted provides valuable information to be able to apply telemedicine to pregnant women with GDM. Moreover, it may lay the groundwork for revising technological monitoring of other diseases in pregnancy. One of the strengths of this work was the robust and rigorous search strategy used to find the selected articles since we searched for content in 3 popular repositories that store a large number of current scientific papers, and the methodology explicates in detail what we want to find. This work also allows us to identify which devices and technologies are currently being used and how they are being used (concerning measurements and functions), in addition to describing in detail the main conclusions of the studies; not only for glycemic control and treatment of GDM, but also other characteristics of the use of technological systems, such as user satisfaction, measurements of psychological variables in pregnant women, motivation for using said systems, perceptions of the medical team, optimization of care times, and others. It also includes the possible errors that may be involved in the use of these systems.
Most of the studies were prospective and case-control studies, which reflects good robustness in terms of the quality of the results. Ideally, this type of study should be multicenter to further support the results; however, its application can be difficult because they are systems or studies that require large financial resources.
An important detail to consider is that there are no records in Latin America, so a parallel search was performed and a Brazilian author was found with several publications, mainly on intelligent mobile systems for pregnancy care and prediction, and decision making systems. Both systems presented in the articles found showed high accuracy of 80%, in predicting hypertensive disorders, so they are good predictive methods, but in both articles concluded that more studies were needed [Moreira et al., 2016a; Moreira et al., 2016b (1)]. It is important to encourage this type of study in the population of pregnant women in Latin America because overweight and obesity is a major problem among women of reproductive age in Latin America and the Caribbean, where it is estimated that 70% of women between 20 and 49 years of age are overweight or obese, which are risk factors for GDM. According to the International Diabetes Federation, approximately 12% of live births in Latin America and the Caribbean may be affected by hyperglycemia during pregnancy (Organización Panamericana de la Salud. Hiperglucemia y embarazo en las Américas, 2016; WHO et al., 2019) which is why it is relevant to invite the authors to continue searching for technological strategies for monitoring GDM, based on the results observed in the present review.
It is important to consider that inadequate management of gestational diabetes can lead to various complications, including increased likelihood of a large-for-gestational-age newborn, cesarean section, increased fetal insulin levels, and neonatal fat levels (Coustan et al., 2010). Elevated glycosylated hemoglobin levels were also associated with preeclampsia and preterm delivery (Lowe et al., 2012). On the other hand, a higher maternal BMI, independent of maternal glycemia, is strongly associated with a higher frequency of pregnancy complications, particularly those related to excess fetal growth, adiposity and preeclampsia (Metzger, 2010). Therefore, it is relevant that technologies for the management of GDM should include intake control, weight control and energy and nutrient adequacy to control weight gain in pregnancy.
The categories used in the monitoring of GDM using a technological system coincided precisely with the models of prevention and treatment of GDM: healthy eating and carbohydrate counting (Yuen, 2015; Awuchi et al., 2020), exercise (Mottola, 2008; Bianchi et al., 2017) and lifestyle changes (Moholdt et al., 2020).
In the case of manual transfer of information with standard glucometers, a margin of error may occur at the time of transcribing the value to the digital platform. On the other hand, glucometers with an infrared port must have an additional device (a device reader) for direct transfer to occur. Continuous glucose monitoring (CGM) is becoming increasingly reliable and has demonstrated efficacy in terms of improving glycosylated hemoglobin, reducing hypoglycemia and improving time in the target glucose range. This system that provides immediate feedback to patients and decision support tools for patients and providers have demonstrated superior results compared to intermittent self-monitored blood glucose monitoring (Reddy et al., 2020). More information is needed to know whether the Smart glucometer offers the same reliability as a glucometer with Bluetooth. Just by chance, it is estimated that the most convenient glucometer for pregnant women would be the Bluetooth glucometer since the download is automatic and direct to the technology (as long as this technology has a BlueTooth connection).
There are several factors associated with which platform would be the most convenient. One of them is the need for an internet connection, for example, to access a web system it is necessary to be connected to the internet, while a mobile application may require a connection for a limited time. On the other hand, to be able to download a mobile application, it is necessary to have enough storage space in the digital technology, so it must be a lightweight application. Ideally, the platform should be available in both forms (web page and mobile application), many of the studies reviewed used multiplatform applications, which is the most advantageous concerning the usability of the technology (Delia et al., 2015).
In the selected articles, the technological applications were mainly focused on the objective of improving glycemic control and supporting pregnant women in understanding GDM. These functions are relevant and it is good that they are included in the monitoring of the GDM with the use of technologies. According to the literature, GDM can have serious effects if not adequately treated. An important part of the management of GDM involves patient education on diet, exercise, self-monitoring of blood glucose and self-administration of insulin (Evans and Patry, 2004). It is precisely these functions that the authors of the reviewed articles seek to implement in their systems. It is important to note that none of the selected articles has focused on evaluating the impact of a specific diet on gestational diabetes using these technologies. As future work, it will be of great relevance to analyze various types of diet, such as the effect of a very low-calorie diet through remote monitoring of gestational diabetes.
According to the results found, only 30% of the studies showed glycemic monitoring figures; of these, 100% showed improvements in the measurement parameters: fasting glycemia, postprandial 1 or 2 h, HbA1c, % of measurements outside the recommended range, etc. Even so, it can be concluded that the use of technologies in pregnant women with diabetes is promising; however, studies that continue to support their efficacy in a multicenter, randomized and controlled manner are lacking. It has been previously demonstrated in 11 systematic reviews and 15 meta-analyses, most focused on patients with type 1 diabetes (10 and 6, respectively), reported a reduction in glycosylated hemoglobin (HbA1c) levels from 0.17 to 0.70% after the use of diabetes monitoring systems. Among the control systems is conventional monitoring with traditional glucometer, continuous glucose monitoring, noninvasive glucose monitor, artificial pancreas, insulin pump with sensors and mobile technology or telemedicine (Kamusheva et al., 2021). In this review, we have focused mainly on mobile technology or telemedicine for diabetes monitoring in pregnancy, so the results contribute to this area.
The satisfaction of the patients and the medical team was demonstrated, which provides positive aspects to the use of technology in GDM. What patients highlighted the most was the short time required to use this type of service, in addition to the comfort they provide at the time of monitoring. This empowers patients for their health, increasing their security and confidence concerning their pregnancy condition since they know how they are currently and how to improve their quality of life for the benefit of themselves and their children. On the part of the medical team, what was most valued was the optimization of the time spent on education and monitoring, as well as the greater amount of data associated with glycemic control that they were able to obtain with the applications used.
Some limitations or negative aspects of the use of the technology were concerning glucose measurement, mainly associated with technical problems, though also with the users’ feeling that the clinical staff was not sufficiently committed to the application, which caused them some frustration. Others were associated with the fact that the information using standard glucometers could be modified or altered, or that the pregnant women did not take their glycemia as specified according to the instructions, which could lead to untruthful information at the time of analysis. It was also observed that the use of technology could generate stress in pregnant women due to the excess of information to be processed (both in terms of content and measurement taking).
Limitations and Future Projections
As for weaknesses of this work, because it details in such a specific way what we want to find, with the inclusion criteria limiting the articles to those published in the last 6 years, there is a probability that texts were left out which could have provided more information regarding remote monitoring of GDM. Also, in the exclusion criteria, the post-natal period is left out, so it cannot be deduced whether the use of technologies decreases maternal or neonatal risks associated with this period in this work.
Regarding the limitations of this review, we recognize that there is no agreement on which is the most appropriate methodology for screening and diagnosis of GDM or standard treatment protocols, so patients may not be precisely comparable among all trials. On the other hand, although values associated with glycemia obtained in patients were found, there was no single or exclusive measurement parameter, which makes it impossible to compare quantitatively the technologies used in the selected articles. There is also no specific information on the digital technology used; only one article specifies the brand and model of the technology, so we do not know what storage space is required for use of the platform, or what operating system it is compatible with if it is a mobile application.
It is important to consider how all the information that can be collected by the digital platforms discussed above can help us. From this arises the definition of Big Data: data sets whose size is beyond the capacity of typical database software to capture, store, manage and analyze (Bahri et al., 2019). During the search of databases, an article emerged about a prototype data integration system from mobile apps and glucometers that aims to standardize the data and have it stored to assist clinicians in the diagnosis and treatment of GDM, but since it is a prototype, there is no information about the results (Pais et al., 2016). It is also necessary to make way for Artificial Intelligence technologies that help to predict glucose, such as the glucose prediction system mentioned in the article by (Pustozerov et al., 2018), as it would allow minimization of invasiveness that can result from the constant use of a glucometer several times a day, and thus improve the quality of life of patients. It would be interesting to optimize resources in medical care by using predictive models for insulin adjustment based on glycemia monitoring and to take it to a clinical context where its use can be validated and prototyped.
Conclusion
The objective of this systematic review was to determine the impact of current technologies, and recognize types and methods that assist patients with GDM. At the end of this work, it was concluded that monitoring technologies are safe and at no time did they worsen the glycemic status of pregnant women. However, a greater number of randomized controlled multi-center studies and clinical trials are needed, as well as a standardization of the measurements to be established to consider a given measurement “an improvement in glycemic control”. There were benefits such as increased satisfaction and acceptability, maternal confidence, and knowledge of GDM and thus improvements in the quality of the health service delivered. There were also positive comments in terms of optimizing the time of the medical team. GDM centered technology may help to evaluate outcomes and tailor personalize solutions. Further studies are still needed to understand the efficacy and economic impact that could arise from the use of this type of intervention. The present review provides an opportunity to learn about the use of technology in GDM and contribute to women’s health.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Author Contributions
AB and BG provide the principal idea, search for information, and write the manuscript. FP was full the support on the clinical approach and Gestational Diabetes Mellitus (GDM). RT and SC were the full support for the Remote Technology for Health (search and discussion). SC was the support on PRISMA technique. LS and JP were the support on the discussion on clinical approach on GDM. RS was the organizer of the manuscript, support the discussion on technology for remote monitoring of GDM.
Funding
This work was partially founded by the following grants: Centro de Investigación y Desarrollo en Ingeniería en Salud, Universidad de Valparaíso, Chile. CIDIS-UV 14; ANID – Millennium Science Initiative Program ICN2021-004; Proyecto PUENTE, UVA20993; Fondo Nacional de Desarrollo Científico y Tecnológico (FONDECYT) (grant number 1190316 and 1221938), Chile; and International Sabbaticals (University Medical Centre Groningen, University of Groningen, The Netherlands) from the Vicerectorate of Academic Affairs, Academic Development Office of the Pontificia Universidad Católica de Chile.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fbioe.2022.819697/full#supplementary-material
References
- Al-ofi E. A., Mosli H. H., Ghamri K. A., Ghazali S. M. (2019). Management of Postprandial Hyperglycaemia and Weight Gain in Women with Gestational Diabetes Mellitus Using a Novel Telemonitoring System. J. Int. Med. Res. 47, 754–764. 10.1177/0300060518809872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albert L., Capel I., García-Sáez G., Martín-Redondo P., Hernando M. E., Rigla M. (2020). Managing Gestational Diabetes Mellitus Using a Smartphone Application with Artificial Intelligence (SineDie) during the COVID-19 Pandemic: Much More Than Just Telemedicine. Diabetes Res. Clin. Pract. 169, 108396. 10.1016/j.diabres.2020.108396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alqudah A., McMullan P., Todd A., O’Doherty C., McVey A., McConnell M., et al. (2019). Service Evaluation of Diabetes Management during Pregnancy in a Regional Maternity Hospital: Potential Scope for Increased Self-Management and Remote Patient Monitoring through mHealth Solutions. BMC Health Serv. Res. 19, 662. 10.1186/s12913-019-4471-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Diabetes Association (2020). Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes-2020. Diabetes Care 43, S14–S31. 10.2337/dc20-S002 [DOI] [PubMed] [Google Scholar]
- American Diabetes Association (2014). Standards of Medical Care in Diabetes–2014. Diabetes care 37 Suppl 1, S14–S80. 10.2337/dc14-S014 [DOI] [PubMed] [Google Scholar]
- Anna V., van der Ploeg H. P., Cheung N. W., Huxley R. R., Bauman A. E. (2008). Sociodemographic Correlates of the Increasing Trend in Prevalence of Gestational Diabetes Mellitus in a Large Population of Women between 1995 and 2005. Diabetes Care 31, 2288–2293. 10.2337/dc08-1038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auvinen A.-M., Luiro K., Jokelainen J., Järvelä I., Knip M., Auvinen J., et al. (2020). Type 1 and Type 2 Diabetes after Gestational Diabetes: a 23 Year Cohort Study. Diabetologia 63, 2123–2128. 10.1007/s00125-020-05215-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Awuchi C. G., Echeta C. K., Igwe V. S. (2020). Diabetes and the Nutrition and Diets for its Prevention and Treatment: A Systematic Review and Dietetic Perspective. Health Sci. Res. 6, 5–19. [Google Scholar]
- Bahri S., Zoghlami N., Abed M., Tavares J. M. R. S. (2019). BIG DATA for Healthcare: A Survey. IEEE Access 7, 7397–7408. 10.1109/access.2018.2889180 [DOI] [Google Scholar]
- Bianchi C., Battini L., Aragona M., Lencioni C., Ottanelli S., Romano M., et al. (2017). Prescribing Exercise for Prevention and Treatment of Gestational Diabetes: Review of Suggested Recommendations. Gynecol. Endocrinol. 33, 254–260. 10.1080/09513590.2016.1266474 [DOI] [PubMed] [Google Scholar]
- Borgen I., Garnweidner-Holme L. M., Jacobsen A. F., Bjerkan K., Fayyad S., Joranger P., et al. (2017). Smartphone Application for Women with Gestational Diabetes Mellitus: A Study Protocol for a Multicentre Randomised Controlled Trial. BMJ Open 7, e013117. 10.1136/bmjopen-2016-013117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bromuri S., Puricel S., Schumann R., Krampf J., Ruiz J., Schumacher M. (2016). An Expert Personal Health System to Monitor Patients Affected by Gestational Diabetes Mellitus: A Feasibility Study. Ais 8, 219–237. 10.3233/AIS-160365 [DOI] [Google Scholar]
- Caballero-Ruiz E., García-Sáez G., Rigla M., Villaplana M., Pons B., Hernando M. E. (2017). A Web-Based Clinical Decision Support System for Gestational Diabetes: Automatic Diet Prescription and Detection of Insulin Needs. Int. J. Med. Inform. 102, 35–49. 10.1016/j.ijmedinf.2017.02.014 [DOI] [PubMed] [Google Scholar]
- Caballero-Ruiz E., García-Sáez G., Rigla M., Villaplana M., Pons B., Hernando M. E. (2016). Automatic Classification of Glycaemia Measurements to Enhance Data Interpretation in an Expert System for Gestational Diabetes. Exp. Sys. App. 63, 386–396. 10.1016/j.eswa.2016.07.019 [DOI] [Google Scholar]
- Coustan D. R., Lowe L. P., Metzger B. E., Dyer A. R. (2010). The Hyperglycemia and Adverse Pregnancy Outcome (HAPO) Study: Paving the Way for New Diagnostic Criteria for Gestational Diabetes Mellitus. Am. J. Obstet. Gynecol. 202, 654–656. 10.1016/j.ajog.2010.04.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delia L., Galdamez N., Thomas P., Corbalan L., Pesado P. (2015). “Multi-platform mobile Application Development Análisis,” in IEEE RCIS. Athens, Greece, May 13-15, 2015. 10.1109/RCIS.2015.7128878 [DOI] [Google Scholar]
- Evans E., Patry R. (2004). Management of Gestational Diabetes Mellitus and Pharmacists' Role in Patient Education. Pharm 61, 1460–1465. 10.1093/ajhp/61.14.1460 [DOI] [PubMed] [Google Scholar]
- Garmendia M. L., Mondschein S., Montiel B., Kusanovic J. P. (2020). Trends and Predictors of Gestational Diabetes Mellitus in Chile. Int. J. Gynecol. Obstet. 148, 210–218. 10.1002/ijgo.13023 [DOI] [PubMed] [Google Scholar]
- Guo H., Zhang Y., Li P., Zhou P., Chen L.-M., Li S.-Y. (2019). Evaluating the Effects of mobile Health Intervention on Weight Management, Glycemic Control and Pregnancy Outcomes in Patients with Gestational Diabetes Mellitus. J. Endocrinol. Invest. 42, 709–714. 10.1007/s40618-018-0975-0 [DOI] [PubMed] [Google Scholar]
- Harrison T. N., Sacks D. A., Parry C., Macias M., Ling Grant D. S., Lawrence J. M. (2017). Acceptability of Virtual Prenatal Visits for Women with Gestational Diabetes. Women's Health Issues 27, 351–355. 10.1016/j.whi.2016.12.009 [DOI] [PubMed] [Google Scholar]
- Johnson Q. B., Berry D. C. (2018). Impacting Diabetes Self-Management in Women with Gestational Diabetes Mellitus Using Short Messaging Reminders. J. Am. Assoc. Nurse Pract. 30, 320–326. 10.1097/JXX.0000000000000059 [DOI] [PubMed] [Google Scholar]
- Jovanovic L., Knopp R. H., Kim H., Cefalu W. T., Zhu X.-D., Lee Y. J., et al. (2005). Elevated Pregnancy Losses at High and Low Extremes of Maternal Glucose in Early Normal and Diabetic Pregnancy. Diabetes Care 28, 1113–1117. 10.2337/diacare.28.5.1113 [DOI] [PubMed] [Google Scholar]
- Kamusheva M., Tachkov K., Dimitrova M., Mitkova Z., García-Sáez G., Hernando M. E., et al. (2021). A Systematic Review of Collective Evidences Investigating the Effect of Diabetes Monitoring Systems and Their Application in Health Care. Front. Endocrinol. 12, 636959. 10.3389/fendo.2021.636959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S.-H., Kim H. J., Shin G. (2021). Self-management mobile Virtual Reality Program for Women with Gestational Diabetes. Int. J. Environ. Res. Public Health 18, 1–12. 10.3390/ijerph18041539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim Y.-S., Kim H.-S., Kim Y.-L. (2019). Effects of a Web-Based Self-Management Program on the Behavior and Blood Glucose Levels of Women with Gestational Diabetes Mellitus. Telemed. e-Health 25, 407–414. 10.1089/tmj.2017.0332 [DOI] [PubMed] [Google Scholar]
- Lemelin A., Paré G., Bernard S., Godbout A. (2020). Demonstrated Cost-Effectiveness of a Telehomecare Program for Gestational Diabetes Mellitus Management. Diabetes Tech. Ther. 22, 195–202. 10.1089/dia.2019.0259 [DOI] [PubMed] [Google Scholar]
- Lowe L. P., Metzger B. E., Dyer A. R., Lowe J., McCance D. R., Lappin T. R. J., et al. (2012). Hyperglycemia and Adverse Pregnancy Outcome (HAPO) Study: Associations of Maternal A1C and Glucose with Pregnancy Outcomes. Diabetes Care 35, 574–580. 10.2337/dc11-1687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackillop L., Hirst J. E., Bartlett K. J., Birks J. S., Clifton L., Farmer A. J., et al. (2018). Comparing the Efficacy of a Mobile Phone-Based Blood Glucose Management System with Standard Clinic Care in Women with Gestational Diabetes: Randomized Controlled Trial. JMIR Mhealth Uhealth 6, e71. 10.2196/mhealth.9512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntyre H. D., Catalano P., Zhang C., Desoye G., Mathiesen E. R., Damm P. (2019). Gestational Diabetes Mellitus. Nat. Rev. Dis. Primers 5, 47–19. 10.1038/s41572-019-0098-8 [DOI] [PubMed] [Google Scholar]
- McIntyre H. D., Moses R. G. (2020). The Diagnosis and Management of Gestational Diabetes Mellitus in the Context of the COVID-19 Pandemic. Diabetes Care 43, 1433–1434. 10.2337/dci20-0026 [DOI] [PubMed] [Google Scholar]
- Metzger B. E. (2010). Hyperglycaemia and Adverse Pregnancy Outcome (HAPO) Study: Associations with Maternal Body Mass index. BJOG 117, 575–584. 10.1111/j.1471-0528.2009.02486.x [DOI] [PubMed] [Google Scholar]
- Ministerio de Salud (2014). Guía Diabetes Y Embarazo. Chile: Ministerio de Salud. [Google Scholar]
- Miremberg H., Ben-Ari T., Betzer T., Raphaeli H., Gasnier R., Barda G., et al. (2018). The Impact of a Daily Smartphone-Based Feedback System Among Women with Gestational Diabetes on Compliance, Glycemic Control, Satisfaction, and Pregnancy Outcome: a Randomized Controlled Trial. Am. J. Obstet. Gynecol. 218, 453. 10.1016/j.ajog.2018.01.044 [DOI] [PubMed] [Google Scholar]
- Moholdt T., Hayman M., Shorakae S., Brown W. J., Harrison C. L. (2020). The Role of Lifestyle Intervention in the Prevention and Treatment of Gestational Diabetes. Semin. Reprod. Med. 38, 398–406. 10.1055/s-0040-1722208 [DOI] [PubMed] [Google Scholar]
- Moreira M. W. L., Rodrigues J. J. P. C., Oliveira A. M. B., Saleem K. (2016b). “Smart mobile System for Pregnancy Care Using Body Sensors,” in IEEE. MoWNeT, Cairo, Egypt, April 11-13, 2016. 10.1109/MoWNet.2016.7496609 [DOI] [Google Scholar]
- Moreira M. W. L., Rodrigues J. J. P. C., Oliveira A. M. B., Saleem K., Neto A. (2016a). “Performance Evaluation of Predictive Classifiers for Pregnancy Care,” in IEEE GLOBECOM, Washington, DC, USA, December 04-08, 2016. 10.1109/GLOCOM.2016.7842136 [DOI] [Google Scholar]
- Mottola M. F. (2008). The Role of Exercise in the Prevention and Treatment of Gestational Diabetes Mellitus. Curr. Sports Med. Rep. 6, 381–386. 10.1007/s11892-008-0053-7 [DOI] [PubMed] [Google Scholar]
- Organización Panamericana de la Salud. Hiperglucemia y embarazo en las Américas (2016). Informe final de la Conferencia Panamericana sobre Diabetes y Embarazo. Lima, Perú: 8–10 de septiembre de 2015). Washington, DC: Institutional Repository for Information Sharing. [Google Scholar]
- Pais S., Parry D., Rush E., Rowan J. (2016). Data Integration for mobile Wellness Apps to Support Treatment of GDM. ACSW 64, 1–7. 10.1145/2843043.2843382 [DOI] [Google Scholar]
- Peleg M., Shahar Y., Quaglini S., Broens T., Budasu R., Fung N., et al. (2017). Assessment of a Personalized and Distributed Patient Guidance System. Int. J. Med. Inform. 101, 108–130. 10.1016/j.ijmedinf.2017.02.010 [DOI] [PubMed] [Google Scholar]
- Pustozerov E., Popova P. (2018). Mobile-based Decision Support System for Gestational Diabetes Mellitus. USBEREIT 2018, 45–48. 10.1109/USBEREIT.2018.8384546 [DOI] [Google Scholar]
- Pustozerov E., Popova P., Tkachuk A., Bolotko Y., Yuldashev Z., Grineva E. (2018). Development and Evaluation of a Mobile Personalized Blood Glucose Prediction System for Patients with Gestational Diabetes Mellitus. JMIR. Mhealth Uhealth 6, e6. 10.2196/mhealth.9236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasekaba T. M., Furler J., Young D., Liew D., Gray K., Blackberry I., et al. (2018). Using Technology to Support Care in Gestational Diabetes Mellitus: Quantitative Outcomes of an Exploratory Randomised Control Trial of Adjunct Telemedicine for Gestational Diabetes Mellitus (TeleGDM). Diabetes Res. Clin. Pract. 142, 276–285. 10.1016/j.diabres.2018.05.049 [DOI] [PubMed] [Google Scholar]
- Reddy N., Verma N., Dungan K., Feingold K. R., Anawalt B., Boyce A., et al. (2020). Monitoring Technologies – Continuous Glucose Monitoring, Mobile Technology, Biomarkers of Glycemic Control. San Francisco, USA: Endotext. [Google Scholar]
- Rigla M., Martínez-Sarriegui I., García-Sáez G., Pons B., Hernando M. E. (2018). Gestational Diabetes Management Using Smart Mobile Telemedicine. J. Diabetes Sci. Technol. 12 (12), 260–264. 10.1177/1932296817704442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo Y., Kim E. M., Choi J. S., Park C.-Y. (2020). Using a Mobile-based Nutritional Intervention Application Improves Glycemic Control but Reduces the Intake of Some Nutrients in Patients with Gestational Diabetes Mellitus: A Case Series Study. Clin. Nutr. Res. 9, 73–79. 10.7762/cnr.2020.9.1.73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin D., Song W. O. (2014). Prepregnancy Body Mass index Is an Independent Risk Factor for Gestational Hypertension, Gestational Diabetes, Preterm Labor, and Small- and Large-For-Gestational-Age Infants. J. Maternal-Fetal Neonatal Med. 28, 1679–1686. 10.3109/14767058.2014.964675 [DOI] [PubMed] [Google Scholar]
- Skar J. B., Garnweidner-Holme L. M., Lukasse M., Terragni L. (2018). Women's Experiences with Using a Smartphone App (The Pregnant+ App) to Manage Gestational Diabetes Mellitus in a Randomised Controlled Trial. Midwifery 58, 102–108. 10.1016/j.midw.2017.12.021 [DOI] [PubMed] [Google Scholar]
- Tian Y., Zhang S., Huang F., Ma L. (2021). Comparing the Efficacies of Telemedicine and Standard Prenatal Care on Blood Glucose Control in Women with Gestational Diabetes Mellitus: Randomized Controlled Trial. JMIR Mhealth Uhealth 9, e22881. 10.2196/22881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Triberti S., Bigi S., Rossi M. G., Caretto A., Laurenzi A., Dozio N., et al. (2018). The ActiveAgeing Mobile App for Diabetes Self-Management: First Adherence Data and Analysis of Patients' In-App Notes. LNICST 253, 129–138. 10.1007/978-3-030-01093-5_17 [DOI] [Google Scholar]
- Urrútia G., Bonfill X. (2010). Declaración PRISMA: una propuesta para mejorar la publicación de revisiones sistemáticas y metaanálisis. Medicina Clínica 135, 507–511. 10.1016/j.medcli.2010.01.015 [DOI] [PubMed] [Google Scholar]
- Varnfield M., Redd C., Stoney R. M., Higgins L., Scolari N., Warwick R., et al. (2021). M♡THer, an mHealth System to Support Women with Gestational Diabetes Mellitus: Feasibility and Acceptability Study. Diabetes Tech. Ther. 23, 358–366. 10.1089/dia.2020.0509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wernimont S. A., Sheng J. S., Fleener D., Summers K. M., Syrop C., Andrews J. I. (2020). Cellular-Enabled Glucometers and Maternal Glucose Control: A Quality Improvement Initiative. J. Diabetes Sci. Technol. 14, 77–82. 10.1177/1932296819856360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO UNICEF UNFPA World Bank United nations population division (2019). Trends in Maternal Mortality: 2000 to 2017: Estimates by WHO, UNICEF, UNFPA, World Bank Group and the United Nations Population Division. Geneva: World Health Organization, UNICEF, UNFPA, World Bank, Group and the United Nations Population Division. [Google Scholar]
- World Health Organization (2006). Definition and Diagnosis of Diabetes Mellitus and Intermediate Hyperglycaemia. Geneva: WHO. [Google Scholar]
- Yang P., Lo W., He Z.-l., Xiao X.-m. (2018). Medical Nutrition Treatment of Women with Gestational Diabetes Mellitus by a Telemedicine System Based on Smartphones. J. Obstet. Gynaecol. Res. 44, 1228–1234. 10.1111/jog.13669 [DOI] [PubMed] [Google Scholar]
- Yew T. W., Chi C., Chan S.-Y., van Dam R. M., Lim C. S., Foong P. S., et al. (2021). A Randomized Controlled Trial to Evaluate the Effects of a Smartphone Application-Based Lifestyle Coaching Program on Gestational Weight Gain, Glycemic Control, and Maternal and Neonatal Outcomes in Women with Gestational Diabetes Mellitus: The SMART-GDM Study. The SMART-GDM Diabetes Care 44, 456–463. 10.2337/dc20-1216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuen L. (2015). Gestational Diabetes Mellitus: Challenges for Different Ethnic Groups. Wjd 6, 1024–1032. 10.4239/wjd.v6.i8.1024 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.