Abstract
Context:
Rational drug use has a great role of influence in health care. The fact sheet given by the World Health Organization (WHO) shows that around 50% of the drugs are prescribed, dispensed, and sold inappropriately. One of the major consequences of irrational drug use in infections is antibiotic resistance.
Aim:
The present study aims to assess the antibiotic-prescribing pattern by auditing the prescriptions in a teaching hospital.
Settings and Design:
A prospective cross-sectional study was conducted in the pharmacy of a teaching hospital to evaluate the prescriptions of the outpatient department.
Materials and Methods:
The prescriptions used to treat symptoms suggestive of infections were taken into consideration. A total of 1,000 prescriptions were analyzed.
Data Analysis:
The data was analysed using Microsoft Excel.
Results:
A total of 2,536 drugs were prescribed. The average number of drugs per prescription was 2.5. The percentage of encounters with antibiotics prescribed was 17.5%. The percentage of encounters prescribed with a generic name and with drugs from the essential drug list was 87.5% and 65%, respectively. There were no injections prescribed. Amoxicillin and ciprofloxacin were the most common antibiotics prescribed. The duration of the treatment was mentioned in all the prescriptions.
Conclusions:
Our study shows that the percentage of antibiotic usage is within the WHO standard value. The average number of drugs per prescription was slightly higher than the WHO value. Steps should be taken to improve the generic prescribing by the physicians.
Keywords: Antibiotic, prescribing indicators, prescriptions, rational drug use, resistance, WHO
Introduction
As per the World Health Organization (WHO), rational use of drugs is described “as a state in which medications are received by patients appropriately according to their clinical needs and individual requirements, for an adequate period at the lowest cost.”[1] The consequences of irrational drug use include polypharmacy, increased use of branded drugs, increased drug costs per person, drug interactions, adverse effects, irrational antibiotic prescribing, and antibiotic resistance.[2,3] Antibiotic resistance is rising tremendously, and it is a major threat to health care worldwide, food safety, and development.[4] Although antibiotic resistance is a natural process on its own, there are several other causes that increase the resistance of the bacteria to antibiotics. The causes include inappropriate antibiotic prescribing, self-medication, and wide use of antibiotics in agriculture and animal husbandry.[5,6] The irrational and persistent use of antibiotics may lead to a postantibiotic era in which even mild infections can kill patients.[7] There is a need for an antibiotic policy that comprises the microbiological data and prescription auditing in any geographical area.[8] The WHO core drug use indicators include prescribing indicators, facility specific indicators, and patient care indicators. A major application of the WHO prescribing indicators is to identify drug use problem areas and alerting doctors regarding the judicious use of medicines.[9] In 2019, the WHO introduced the AWaRe (Access, Watch, Reserve) classification tool as a part of antibiotic stewardship to enhance the optimal use of antibiotics.[10] The highest burden of infections such as upper respiratory infection, gastrointestinal infections, and ear infections is treated by primary-care physicians. The AWaRe classification tool serves a good guidance while prescribing antibiotics. The aim and objective of the present study are to evaluate the antibiotic-prescribing pattern by auditing the prescriptions in a teaching hospital and to use the AWaRe classification tool to identify the group of prescribed antibiotics.
Materials and Methods
A prospective cross-sectional study was conducted in one of the pharmacies of teaching hospital in Puducherry. The study was conducted after obtaining the institute scientific committee and ethics committee approval, JIPMER, Puducherry. The main pharmacy dispenses most of the medicines for adult patients with common diseases such as infections, diabetes, and hypertension from the departments of medicine and surgery. Departments such as pediatrics, obstetrics and gynecology, and other superspecialties have their respective pharmacy that covers comparatively less population compared with the main pharmacy. The prescriptions were collected from the main pharmacy 3 days a week. The prescriptions were retained in the pharmacy after dispensing the medications. The prescriptions used to treat symptoms suggestive of infection (cough, fever, chills, wheeze, diarrhea, sore throat, running nose, abdominal pain, burning micturition, chest pain, generalized body pain, skin infections, and bone infections) were included. The investigator visited the pharmacy and collected the digital photographs of the prescriptions. The prescriptions were collected in the morning from 9 a.m to 12 p.m, and they were collected from May 2019 to August 2019. A total of 1,000 prescriptions were collected. The prescribers were physicians in the outpatient departments. The digital photographs of the prescriptions were preserved for data analysis. The collected softcopies of the prescriptions were assessed for the patient demographic characteristics, symptoms, signs, diagnosis, drugs prescribed, duration of the treatment, and rational use.
The prescriptions were analyzed for the following prescribing indicators of drug use:
Average number of medicines per encounter
Percentage of prescriptions with a generic name
Percentage of medicines prescribed from essential medicines list (EML)
Percentage of prescriptions with an antibiotic prescribed
Percentage of encounters with an injection prescribed.
Results
All the values were analyzed using Microsoft Excel and expressed as a percentage. The patient encounters assessed were 1,000, and among them were 434 males and 566 females. The total number of drugs prescribed was 2,536, out of which 175 were antibiotics.
The basic demographic characteristics of the patient population is shown in Figure 1 and Table 1. Most of the patients were in the age group 40-60 years and 57% of the population were males [Table 1]. The WHO prescribing indicators of all the prescriptions were given in Table 2. The data on the prescription pattern of antibiotics is listed in Table 3.The major groups of drugs prescribed were proton pump inhibitors (40.7%) followed by analgesics (25.5%) as shown in Table 4. The WHO prescribing indicators of the present study was compared with other Indian studies in Table 5.
Figure 1.
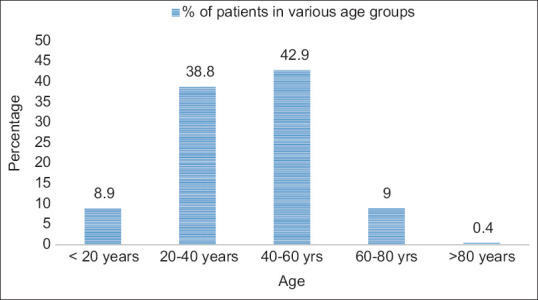
Agewise distribution of the patient population
Table 1.
Gender distribution of the patient population
| Gender | Percentage |
|---|---|
| Male | 57 |
| Female | 43 |
Table 2.
WHO prescribing indicators
| Indicators of drug use | Value | WHO optimal values |
|---|---|---|
| Average number of medicines per encounter | 2.5 | 1.6-1.8 |
| Percentage of prescriptions with generic name | 87.5% | 100% |
| Percentage of encounters with drugs prescribed from EML | 62.5% | 100% |
| Number of encounters with an injection prescribed | 0 | 13.4%-24.1% |
| Percentage of encounters with one or more antibiotics | 17.5% | 20%-26.8% |
WHO=World Health Organization, EML=essential medicines list
Table 3.
Prescription pattern of antibiotics
| Indicator | Number of prescriptions |
|---|---|
| Total number of prescriptions | 1,000 |
| Total number of drugs prescribed | 2,536 |
| Number of prescriptions with treatment schedule for all drugs | 990 (99%) |
| Number of prescriptions with average duration of antibiotic therapy | 175 (100%) |
| Number of prescriptions with antibiotics | |
| One | 162 |
| Two | 13 |
Table 4.
Distribution of the drugs among the prescriptions
| Distribution of drugs | Percentage of encounters |
|---|---|
| Antibiotics | 18.6 |
| Antihistamines | 28.8 |
| Antipyretics | 23.3 |
| Analgesics | 25.5 |
| Proton pump inhibitors | 40.7 |
| Vitamins | 12.7 |
| Minerals | 12 |
Table 5.
Comparison of the WHO prescribing indicators with other Indian studies
| Indicators of drug use | Present study | Pradheepkumar[15] (Private pediatric specialty hospitals, Anantapur District) | Atif et al.[18] (Two tertiary hospitals at Bahawalpur, Punjab) | Prasad et al.[23] (Secondary- care referral hospital, South India) | Mani and Hariharan[25] (Suburban hospital, Central Kerala) | Aravamuthan et al.[22] (Rural Community pharmacies, Tamil Nadu) |
|---|---|---|---|---|---|---|
| Average number of medicines per encounter | 2.5 | 3.53 | 2.82 | 2.7 | — | 3.7 |
| Percentage of prescriptions with generic name | 87.5% | 23.43% | 56.6% | 42.9% | 31% | 2.5% |
| Percentage of encounters with drugs prescribed from EML | 62.5% | 91.48% | 98.8% | 95.6% | 69% | 99.8% |
| Number of encounters with an injection prescribed | 0 | — | 0 | 1.6% | 48% | 7.2% |
| Percentage of encounters with one or more antibiotics | 17.5% | 50.05% | 51.5% | 9.6% | 29% | 22% |
Discussion
The present study reveals that the percentage of encounters with antibiotics prescribed is 17.5%, which is less when compared with the WHO optimal reference value (<30%), and it indicates that the usage of antibiotics is not high. The value is less compared with many studies as shown in Table 2. It is less when compared with a study conducted in the outpatient department of a hospital in Nepal.[11] A study conducted by Bianco et al.[12] in Italy showed that the antibiotics were prescribed irrationally for 66.5% of the patients with respiratory tract infections. Most of the prescription pattern monitoring studies conducted in India showed an inappropriate usage of antibiotics and a lack of prescribing as per standard treatment guidelines.[13] A study conducted in the United States identified that the overprescription of antibiotics for viral infections can be reduced by increasing the time spent during patient consultation.[14] The rational antibiotic usage can be enhanced by increasing the availability of diagnostic tools, prescribing as per the evidence-based guidelines, and increasing the patient consultation time. In this study, the prescriptions with antibiotics fall within the WHO value, which is a good indication that the prescribers were cautious while prescribing antibiotics in treating infections.
Amoxicillin is the most commonly prescribed antibiotic among the drugs in our study. The major antibiotic groups used were the penicillins and quinolones [Figure 2]. This differs from the study reported by Pradheepkumar[15] in which cephalosporins were the most commonly prescribed antibiotics followed by the penicillins. The duration of the therapy was also mentioned in all the antibiotic prescriptions [Table 3]. The study shows that 93% (162 out of 175) of the prescriptions had only one antibiotic prescribed, and 13 prescriptions had two antibiotics. The WHO introduced a database in 2019 called the AWaRe classification database that categorized the 180 antibiotics into access, watch, and reserve groups of antibiotics. It was a tool to check the optimal and appropriate use of antibiotics. The present study is an outpatient study, and the commonly used antibiotics belong to the access group of antibiotics except for ciprofloxacin [Table 6]. The study differs from the study that reported the antibiotic-prescribing pattern from 69 countries, and the data suggested that the use of watch antibiotics were higher in the middle- and lower-income countries.[16] The results also vary from the study on antibiotic-prescribing pattern reported in Kazakhstan.[17] Among the prescribers, primary-care physicians contribute to a major role in prescribing antibiotics for mild to moderate infections, and it is highly recommended that the AWaRe classification of drugs may be a valuable tool to choose antibiotics. At a primary-care level, access group antibiotics are easily accessible, whereas watch group antibiotics need supervision, and hence access antibiotics may be preferred more than watch antibiotics. AWaRe tool is used not only to monitor the antibiotic prescribing but also to guide the policymakers to categorize the essential medicine list and update the national treatment guidelines.
Figure 2.
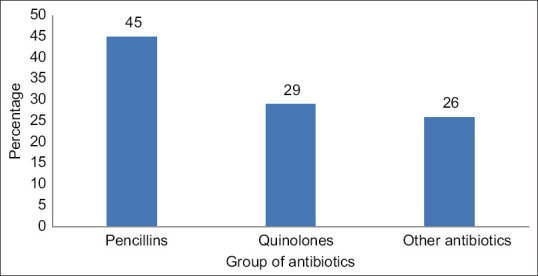
Percentage of various groups of antibiotics prescribed
Table 6.
Classification of the most commonly prescribed antibiotics as per WHO AWaRe classification
| Antibiotic | AWaRe classification |
|---|---|
| Phenoxymethylpenicillin | Access group |
| Amoxicillin | Access group |
| Cloxacillin | Access group |
| Ciprofloxacin | Watch group |
| Doxycycline | Access group |
WHO=World Health Organization, AWaRe=Access, Watch, Reserve
The average number of drugs per prescription was 2.5, which is slightly higher when compared to the WHO standard value (WHO value = 2) [Table 2]. The value was less than those obtained in the studies conducted by Atif et al.[18] (2.8 per prescription) and Mashalla et al.[19] in Botsawna (2.8 drugs per prescription), and similar to the one in the study reported by Mamo and Alemu[20] (2.5 per prescription). Gopalakrishnan et al.[21] reported that the average number of drugs per prescription in the prescriptions of rural practitioners and urban practitioners was 4.03 and 5.05, respectively. The value was also less than the other study conducted in the community pharmacies of southern India [Table 5].[22]
The percentage of encounters with the generic name is 87.5%, which is lower when compared with the WHO standard value (100%). But it is a reasonable value when compared with another study conducted in South India(42.9%).[23] The concept of generic prescribing should be motivated among the prescribers as it reduces the drug costs and improves the interphysician communication. The WHO has given the standards of writing a good prescription in which generic prescribing is the strongest recommendation.[24] As per our study, the percentage of encounters with drugs prescribed from EML is 62.5%, which is lower than the WHO standard value (100%). A study conducted in a hospital in southern India revealed that 69% of drugs were from the essential drug list.[25] The prescriptions had no injection prescribed since the prescriptions were collected from a pharmacy dispensing outpatient department prescriptions.
Limitations
The data were collected from the pharmacy and not from the outpatient departments as any missing elements in the prescription could not be clarified. Another limitation of the study is that the prescriptions were collected in a major pharmacy of the teaching hospital and the other specialty pharmacies were not considered.
Conclusion
The antibiotic usage was within the limits, whereas the other prescribing indicators were slightly higher or lower than the WHO standard values. Generic drug prescribing and essential drugs prescribing should be encouraged among the prescribers. The antibiotics mostly prescribed in the outpatient departments belong to the access group as per the AWaRe classification of antibiotics.
Key points
Every hospital should monitor drug utilization based on the WHO prescribing indicators.
Generic name prescribing and essential drug prescribing are highly recommended among primary-care physicians.
Prescription of antibiotics should be better avoided for viral infections.
When prescribed, the antibiotic should be prescribed based on the AWaRe assessment tool, and access antibiotics are highly recommended at the primary-care level.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.WHO |Rational use of medicines [Internet] WHO. [Last accessed on 2019 Aug 01]. Available from: http://www.who.int/medicines/areas/rational_use/en/
- 2.Ofori-Asenso R, Agyeman AA. Irrational use of medicines—A summary of key concepts. Pharmacy. 2016;4:35. doi: 10.3390/pharmacy4040035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Irrational use of antibiotics:An understanding about antibiotic resistance. 2019. [Last accessed on 2019 Jul 8]. Available from: https://www.bmj.com/content/352/bmj.i1202/rr .
- 4.Antibiotic resistance [Internet] 2020. [cited 2019 Jul 8]. Available from: https://www.who.int/news-room/fact-sheets/detail/antibiotic-resistance .
- 5.Ventola CL. The antibiotic resistance crisis. Pharm Ther. 2015;40:277–83. [PMC free article] [PubMed] [Google Scholar]
- 6.Prestinaci F, Pezzotti P, Pantosti A. Antimicrobial resistance:A global multifaceted phenomenon. Pathog Glob Health. 2015;109:309–18. doi: 10.1179/2047773215Y.0000000030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zaman SB, Hussain MA, Nye R, Mehta V, Mamun KT, Hossain N. A review on antibiotic resistance:Alarm bells are ringing. Cureus. 2017;9:e1403. doi: 10.7759/cureus.1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wattal C. Development of antibiotic resistance and its audit in our country:How to develop an antibiotic policy. Indian J Med Microbiol. 2012;30:381–3. doi: 10.4103/0255-0857.103755. [DOI] [PubMed] [Google Scholar]
- 9.Ofori-Asenso R. A closer look at the World Health Organization's prescribing indicators. J Pharmacol Pharmacother. 2016;7:51–4. doi: 10.4103/0976-500X.179352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.WHO |WHO releases the 2019 AWaRe Classification Antibiotics [Internet] WHO. [Last accessed on 2020 Mar 04]. Available from: http://www.who.int/medicines/news/2019/WHO_releases2019AWaRe_classification_antibiotics/en/
- 11.Shrestha R, Prajapati S. Assessment of prescription pattern and prescription error in outpatient Department at Tertiary Care District Hospital, Central Nepal. J Pharm Policy Pract. 2019;12:16. doi: 10.1186/s40545-019-0177-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bianco A, Papadopoli R, Mascaro V, Pileggi C, Pavia M. Antibiotic prescriptions to adults with acute respiratory tract infections by Italian general practitioners. Infect Drug Resist. 2018;11:2199–205. doi: 10.2147/IDR.S170349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jain S, Upadhyaya P, Goyal J, Kumar A, Jain P, Seth V, et al. A systematic review of prescription pattern monitoring studies and their effectiveness in promoting rational use of medicines. Perspect Clin Res. 2015;6:86–90. doi: 10.4103/2229-3485.154005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Imanpour S, Nwaiwu O, McMaughan DK, DeSalvo B, Bashir A. Factors associated with antibiotic prescriptions for the viral origin diseases in office-based practices, 2006-2012. JRSM Open. 2017;8:2054270417717668. doi: 10.1177/2054270417717668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pradeepkumar Assessment of antibiotic prescribing pattern in pediatric patients:A cross-sectional hospital-based survey [Internet] [Last accessed on 2019 Jul 25]. Available from: http://www.cjhr.org/article.asp?issn=2348-3334;year=2017;volume=4;issue=4;spage=235;epage=237;aulast=Pradeepkumar .
- 16.Pauwels I, Versporten A, Drapier N, Vlieghe E, Goossens H Global-PPS Network. Hospital antibiotic prescribing patterns in adult patients according to the WHO Access, Watch and Reserve classification (AWaRe):Results from a worldwide point prevalence survey in 69 countries. J Antimicrob Chemother. 2021;76:1614–24. doi: 10.1093/jac/dkab050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhussupova G, Utepova D, Orazova G, Zhaldybayeva S, Skvirskaya G, Tossekbayev K. Evaluation of antibiotic use in Kazakhstan for the period 2017-2019 based on WHO access, watch and reserve classification (AWaRe 2019) Antibiotics (Basel) 2021;10:58. doi: 10.3390/antibiotics10010058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Atif M, Sarwar MR, Azeem M, Umer D, Rauf A, Rasool A, et al. Assessment of WHO/INRUD core drug use indicators in two tertiary care hospitals of Bahawalpur, Punjab, Pakistan. J Pharm Policy Pract. 2016;9:27. doi: 10.1186/s40545-016-0076-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mashalla Y, Setlhare V, Massele A, Sepako E, Tiroyakgosi C, Kgatlwane J, et al. Assessment of prescribing practices at the primary healthcare facilities in Botswana with an emphasis on antibiotics:Findings and implications. Int J Clin Pract. 2017:71. doi: 10.1111/ijcp.13042. doi:10.1111/ijcp. 13042. [DOI] [PubMed] [Google Scholar]
- 20.Mamo DB, Alemu BK. Rational drug-use evaluation based on World Health Organization core drug-use indicators in a tertiary referral hospital, Northeast Ethiopia:A cross-sectional study. Drug Healthc Patient Saf. 2020;12:15–21. doi: 10.2147/DHPS.S237021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gopalakrishnan S, Ganeshkumar P, Katta A. Assessment of prescribing practices among urban and rural general practitioners in Tamil Nadu. Indian J Pharmacol. 2013;45:252–7. doi: 10.4103/0253-7613.111931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Aravamuthan A, Arputhavanan M, Subramaniam K, Udaya Chander J SJ. Assessment of current prescribing practices using World Health Organization core drug use and complementary indicators in selected rural community pharmacies in Southern India. J Pharm Policy Pract. 2016;10:1. doi: 10.1186/s40545-016-0074-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Prasad PS, Rudra JT, Vasanthi P, Sushitha U, Sadiq MJ, Narayana G. Assessment of drug use pattern using World Health Organization core drug use indicators at Secondary Care Referral Hospital of South India. CHRISMED J Health Res. 2015;2:223–8. [Google Scholar]
- 24.Guide to Good Prescribing-A Practical Manual:Part 3:Treating your patients:Chapter 9. STEP 4:Write a prescription [Internet] 1994. [Last accessed on 2019 Aug 27]. Available from: https://apps.who.int/medicinedocs/en/d/Jwhozip23e/5.4.html .
- 25.Mani S, Hariharan TS. A prospective study on the pattern of antibiotic use in a tertiary care hospital. Int J Basic Clin Pharmacol. 2017;6:2237–43. [Google Scholar]


