Significance
In species with internal fertilization, sperm spend an important part of their lives within the female. To examine the life history of the sperm during this time, we used semiquantitative proteomics and sex-specific isotopic labeling in fruit flies to determine the extent of molecular continuity between male and female reproductive tracts and provide a global catalog of sperm-associated proteins. Multiple seminal fluid proteins and female proteins associate with sperm immediately after mating. Few seminal fluid proteins remain after long-term sperm storage, whereas female-derived proteins constitute one-fifth of the postmating sperm proteome by then. Our data reveal a molecular “hand-off” from males to females, which we postulate to be an important component of sperm–female interactions.
Keywords: sperm, seminal proteins, female reproductive tract, proteome, fertility
Abstract
Interactions between sperm and the female reproductive tract (FRT) are critical to reproductive success and yet are poorly understood. Because sperm complete their functional maturation within the FRT, the life history of sperm is likely to include a molecular “hand-off” from males to females. Although such intersexual molecular continuity is likely to be widespread among all internally fertilizing species, the identity and extent of female contributions are largely unknown. We combined semiquantitative proteomics with sex-specific isotopic labeling to catalog the posttesticular life history of the sperm proteome and determine the extent of molecular continuity between male and FRTs. We show that the Drosophila melanogaster sperm proteome undergoes substantial compositional changes after being transferred to the FRT. Multiple seminal fluid proteins initially associate with sperm, but most become undetectable after sperm are stored. Female-derived proteins also begin to associate with sperm immediately after mating, and they comprise nearly 20% of the postmating sperm proteome following 4 d of storage in the FRT. Female-derived proteins that associate with sperm are enriched for processes associated with energy metabolism, suggesting that female contributions support sperm viability during the prolonged period between copulation and fertilization. Our research provides a comprehensive characterization of sperm proteome dynamics and expands our understanding of the critical process of sperm–FRT interactions.
In 1951, Colin Russel Austin and Min Chue Chang independently reported that mammalian sperm must spend a minimum period of time within the female reproductive tract (FRT) to achieve the capacity to fertilize oocytes, thus unequivocally demonstrating the importance of sperm–FRT interactions for reproductive success (1, 2). We now know that such interactions are widespread across animal taxa (3) and that they influence sperm migration and storage, help maintain sperm viability, coordinate the physiological and molecular modifications to sperm needed for fertilization (3–9), and critically impact competitive fertilization success (10–13). Nevertheless, the molecular components of the FRT that interact with sperm are still poorly understood (3).
Sperm cells are unique in that they spend the most critical part of their life history in a foreign environment (i.e., the FRT in the case of internal fertilizers). During their transit through the male and female reproductive tracts, sperm encounter different extracellular environments that alter their molecular composition. For example, in mammals, extracellular vesicles of the epididymis and oviduct fuse with the sperm membrane, enabling the transfer of important cargo including glycosylphosphatidylinositol-anchored proteins, heat shock proteins, oviductins, apolipoproteins, and proteins related to energy metabolism (7, 14–17). In Drosophila melanogaster, the composition of sperm in the male and FRTs also changes due to contributions from seminal fluid. For example, the seminal fluid protein (SFP) “Sex Peptide” binds to sperm, which enhances its stability and facilitates the peptide’s movement into storage with sperm (18). Subsequent proteolytic cleavage releases the active region of Sex Peptide from sperm so that it can bind to its female receptor and, in turn, induce long-term postmating changes in female behavior and physiology (19–21). At least eight additional SFPs act transiently in this “long-term response network” to facilitate Sex Peptide–sperm binding (18, 22, 23), perhaps by modifying the sperm surface (24). However, the association of seminal proteins with sperm has not yet been globally examined, and the existence and nature of any female contributions to sperm have yet to be characterized.
Sperm are streamlined cells with a limited capacity for transcription, so their survival and fertilization success depend on support and protection during the period between ejaculation and fertilization (25). This is particularly important in species with long periods of sperm storage in the FRT, as occurs in diverse invertebrate and vertebrate taxa (8, 25, 26). Carbohydrates and proteins from seminal fluid support energy production and enhance viability during ejaculation and immediately after sperm transfer (27–29), but most seminal components do not persist throughout the duration of sperm storage (25). Consequently, meeting the physiological requirements of sperm may necessitate a molecular “hand-off” from males to females (4, 8, 9, 30, 31). Even in species with relatively short durations of female sperm storage (e.g., most mammals), molecular contributions from females are necessary for sperm motility, metabolism, and capacitation (3, 6, 7). For example, in humans female-derived pyruvate enhances hyperactivated sperm motility and promotes capacitation (32), and female-derived chaperone proteins from the FRT bind to sperm and facilitate the acquisition of fertilization competence (6, 33). Molecular continuity between the sexes is likely widespread among all internally fertilizing species (3) and may offer a unifying framework for understanding the unique biology of sperm in the FRT.
Although previous studies have identified individual proteins that bind to sperm (e.g., refs. 6, 7, 14, 15, 17, and 20) and secreted proteins that are present or produced in the reproductive tracts of D. melanogaster females (34–38) and males (reviewed in ref. 39), there has been no comprehensive determination, in any organism, of the number, nature, and source of proteins that associate with sperm in the FRT. To provide such a global view, we used whole-cell semiquantitative proteomics and sex-specific isotopic labeling to track the composition of the D. melanogaster sperm proteome across the major “stages” of posttesticular maturation (Fig. 1), namely, from storage in the male seminal vesicle, through ejaculation (when spermatozoa are mixed with SFPs), and following prolonged storage in females. In addition to identifying additional SFPs that transiently associate with sperm in the FRT, we made the intriguing discovery that numerous female proteins become associated with sperm, beginning immediately after mating, indicating substantial intersexual molecular continuity during the functional maturation of sperm. Our results provide a systematic and quantitative evaluation of sperm proteome dynamics and demonstrate the prevalence of female-mediated, postejaculatory modifications to sperm (3).
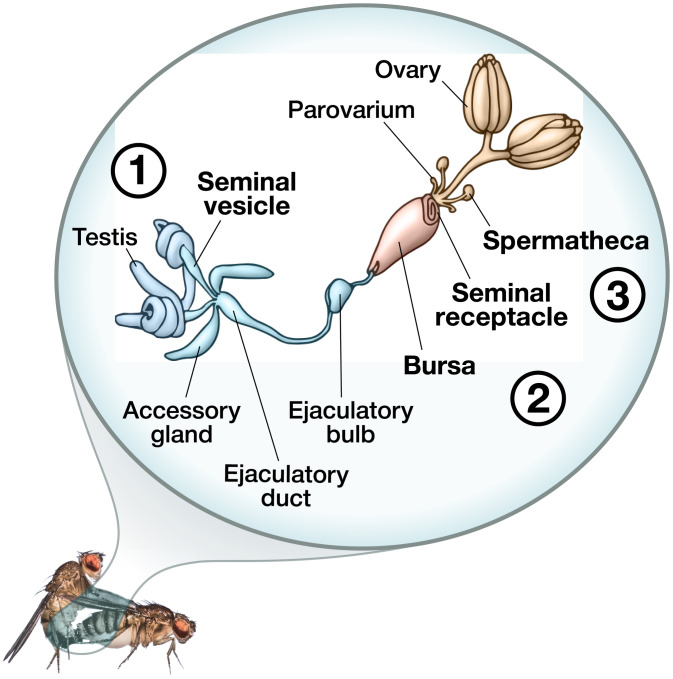
Fig. 1.
During mating in Drosophila, sperm leave the male’s seminal vesicles and descend through the ejaculatory duct, where they mix with SFPs from the accessory glands, ejaculatory duct, and ejaculatory bulb. This mixing continues after insemination within the female’s bursa, where sperm also mix with FRT-derived proteins. Sperm are then stored in the seminal receptacle or paired spermathecae or they are ejected from the female along with the mating plug. Sperm therefore encounter many environments during their movement through the male and FRTs. Reproductive organs highlighted in bold indicate where sperm samples in this study were collected to characterize the sperm proteome across the major stages of posttesticular maturation, namely, the 1) seminal vesicles, 2) bursa, and 3) sperm-storage organs. Image credit: illustration by Siyuan Cong (Syracuse University, Syracuse, NY) and Ben Woolsey (B+Artworks, Philadelphia, PA).
Results and Discussion
This study paints a detailed picture of the life history of the Drosophila melanogaster sperm proteome. To catalog compositional changes to the sperm proteome as sperm move through the male and FRTs, we purified sperm samples from the seminal vesicles and the following four spatiotemporal points within the FRT: the bursa (uterus) 30 min after mating, the seminal receptacle 2 h after mating, the seminal receptacle 4 d after mating, and the spermathecae 4 d after mating. Our proteomic analysis of sperm composition across multiple reproductive tissues and multiple postmating timepoints provides a comprehensive inventory of the compositional changes to sperm over time within the FRT and of the male- and female-derived proteins that closely associate with them.
Multiple SFPs Are Associated with Sperm within the Female.
As a first step in characterizing the proteins that associate with sperm after mating, we compared tissue- and sex-biased expression for all proteins identified in sperm dissected from the bursa 30 min after mating using expression data from FlyAtlas (ref. 40; Materials and Methods for details). Sperm samples were composed of three protein categories, as follows: 1) canonical sperm proteins, 2) SFPs, and 3) female-derived proteins. We define canonical sperm proteins as those present in postspermatogenic sperm cells in the seminal vesicles, where they are stored before mixing with SFPs to form the ejaculate (41, 42). SFPs are defined as the nonspermatozoal proteins in the ejaculate that are transferred to females during mating (39). Female-derived proteins are defined as those produced by females, identified via our sex-specific isotopic labeling (see below), that associate with sperm after mating. Our analyses were primarily aimed at characterizing the composition of SFPs and female-derived proteins that associate with sperm after mating. We collectively refer to sperm samples dissected from the FRT (regardless of postmating timepoint) as the “postmating sperm proteome.” For clarity, we specifically refer to the sperm samples dissected at 30 min after mating as the “transferred sperm proteome” and samples dissected at 2 h or 4 d after mating as the “stored sperm proteome.”
Our analysis of the transferred sperm proteome identified 1,212 canonical sperm proteins and 182 SFPs (Fig. 2A), including all 8 SFPs in the long-term response network (18, 22, 23). Identification of these network proteins provided an internal control, validating our approach. We also identified 22 previously uncharacterized SFPs based on their enriched expression in the male accessory glands (Materials and Methods for criteria), thus further expanding the seminal fluid proteome (Dataset S1). Because sperm samples were washed prior to proteomic analysis, SFPs that are transferred during mating but do not physically associate with sperm should have either been absent or at low abundance. Consistent with this prediction, numerous SFPs that are highly expressed in the male accessory glands were not identified in our full dataset (SI Appendix, Fig. S1), indicating that our protocol was effective in excluding nonassociated proteins or those with nonspecific associations.
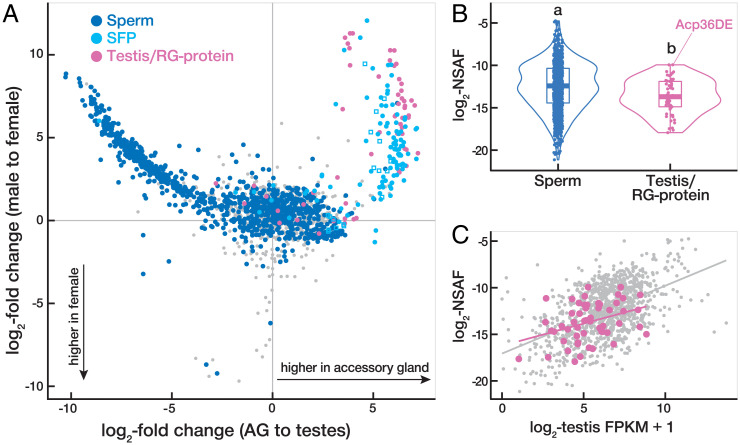
Fig. 2.
Multiple SFPs are associated with sperm in the FRT. (A) Tissue-biased expression versus sex-biased expression for all 1,969 proteins identified in sperm dissected from the bursa at 30 min after mating. Canonical sperm proteins (n = 1,152) are plotted in dark blue, SFPs (n = 122) in light blue (including previously uncharacerized SFPs as squares), testis/RG-proteins (n = 60) in pink, and unannotated proteins (n = 635) in gray. Tissue- and sex-biased expression are based on data from FlyAtlas2 (40). (B) Violin plot comparing the distributions among sperm and testis/RG-proteins in their abundance in the canonical sperm proteome. Letters denote distributions that are significantly different from each other (Kruskal–Wallis and post hoc Dunn’s tests, P < 0.05). (C) Correlations between testis expression (data from 40) and abundance in the canonical sperm proteome (this study) are not significantly different between testis/RG-proteins (pink; Pearson’s r = 0.37) and canonical sperm proteins (gray; Pearson’s r = 0.51; F = 1.91, P = 0.15).
Proteins that directly interact with one another, or are parts of larger multiprotein complexes or networks, often exhibit correlated evolutionary rates. We compared the evolutionary rates of the sperm-associated SFPs identified here with those of nonassociated SFPs to determine the degree to which they exhibit a pattern of correlated molecular evolution with canonical sperm proteins (43). This analysis revealed that sperm-associated SFPs, but not nonassociated SFPs, exhibit enhanced evolutionary rate covariation with sperm proteins relative to background genome-wide expectations (P < 0.0001), consistent with the occurrence of molecular interactions between sperm and the sperm-associated SFPs.
Although SFPs have been defined as nonsperm components of the ejaculate that are produced by male reproductive glands (34, 39), some SFPs have also been previously identified as components of the canonical sperm proteome (42). Our analysis of the transferred sperm proteome similarly identified 60 proteins that have been categorized as both secreted SFPs and canonical sperm proteins (Fig. 2A). To explore this observation further, we conducted additional mass spectrometry analyses on sperm dissected from the seminal vesicles (SI Appendix, Supplemental Methods) to expand upon the previous characterization of the canonical sperm proteome (41, 42). In total, we identified 67 proteins in the updated canonical sperm proteome that are also reported to be SFPs (Dataset S2). These SFPs are expressed at high levels in male reproductive glands (SI Appendix, Fig. S2), the defining characteristic of SFPs, but are also expressed in the testes (SI Appendix, Fig. S2), are abundant in the canonical sperm proteome (Fig. 2B), and appear to be incorporated into sperm in a manner indistinguishable (based on our proteomic analysis) from canonical sperm proteins (Fig. 2C). Additionally, 65.7% of these SFPs exhibit evidence of testis expression compared to only 38.0% among the remaining SFPs (χ2 = 15.2, P < 0.0001) based on single-cell testis transcriptomic data from the Fly Cell Atlas Consortium (44). Given multiple lines of evidence that this subset of SFPs is produced in both the testes and the reproductive glands and were identified both as canonical sperm proteins and as secreted SFPs, we refer to them as testis/reproductive gland proteins (testis/RG-proteins). We highlight that Acp36DE is one of these testis/RG-proteins and hypothesize that the synthesis of Acp36DE in the testes explains its tight association with sperm (45) and high abundance in the canonical sperm proteome (Fig. 2B). Collectively, our results indicate molecular continuity between male reproductive tissues, as proteins produced in the testis during spermatogenesis and those derived from the reproductive glands are not entirely distinct.
Female Molecules Contribute to the Postmating Sperm Proteome.
Although most proteins detected in our sperm samples dissected from the bursa 30 min after mating exhibited the expected pattern of high male-biased expression, we also identified numerous proteins with female-biased expression (Fig. 3A), based on expression data from FlyAtlas2 (40). As FRT proteins are known to closely interact with sperm in mammals (7, 9, 46–48), we hypothesized that these female-biased proteins are produced by females and associate with sperm. Although no such female-derived proteins have been characterized in Drosophila (but see refs. 21, 49, and 50), interactions between sperm and female proteins are expected to be extensive given the protracted duration of sperm storage. Fertilizing sperm are typically stored for ∼6 h to 4 d but are capable of surviving in the FRT for up to 14 d (4, 51).
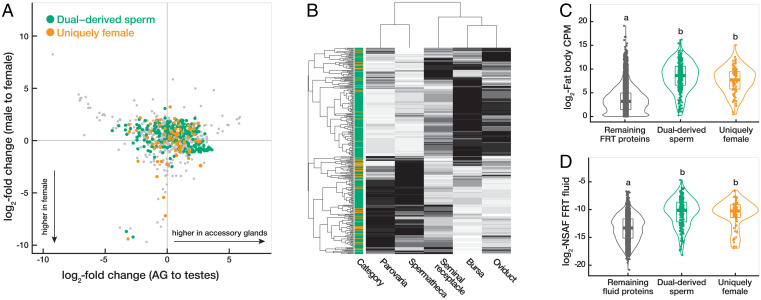
Fig. 3.
Female molecules contribute to the postmating sperm proteome. (A) Tissue-biased expression versus sex-biased expression for all female-derived proteins associated with sperm at 30 min after mating. Dual-derived sperm proteins are plotted in green, uniquely female-derived proteins in orange, and unannotated proteins in gray. Exclusively male-derived sperm proteins and SFPs are not shown. Tissue- and sex-biased expression are based on data from FlyAtlas2 (40). (B) Heat map plotting the gene expression of female-derived sperm-associated proteins in each FRT tissue based on data from McDonough-Goldstein et al. (37). Hierarchical clustering of tissues (columns) and proteins (rows) is based on Euclidean distance. Each protein is categorized as dual-derived sperm (green) or uniquely female-derived (orange). Shading corresponds to relative expression levels for each protein (black = high expression, white = low expression). (C, D) Violin plots comparing the distributions among dual-derived sperm and uniquely female-derived proteins in absolute expression in the reproductive fat body (37) (C) and protein abundance in the FRT fluid using data from McDonough-Goldstein et al. (38) (D). The remaining proteins in the fluid or FRT are plotted in gray for comparison. Letters above the violin plots denote distributions that are significantly different from each other (Kruskal–Wallis and post hoc Dunn’s tests, P < 0.05).
To identify female-derived proteins that interact with sperm, we used sex-specific isotopic labeling (heavy lysine and arginine; Materials and Methods) to determine the sex of origin for proteins in the postmating sperm proteome. Results from this experiment confirmed that many of the proteins with high female-biased expression were derived from females (Fig. 3A). In total, we identified 328 female-derived proteins (Dataset S3). We note that high proteomic coverage of female-derived proteins was challenging because most of the samples were comprised of male-derived sperm proteins. We therefore anticipate that the extent of female contributions is likely even more extensive than currently estimated from our labeling results.
Most of the female-derived proteins that were found to associate with sperm were also previously identified as male-derived components of the canonical sperm proteome. Of the 328 female-derived proteins identified in our sperm samples, 245 are canonical sperm proteins, which is a significantly greater degree of overlap (75%) than expected by chance (Z = 30.2, P < 0.0001). Because of the evidence that these overlapping proteins are produced by both males and females, we refer to them as “dual-derived” sperm proteins and the remaining female-derived proteins as “uniquely female.” This evidence for molecular continuity between the sexes in the production of sperm and sperm-associated proteins is unlikely to be unique to Drosophila. In cattle, over one-third of the proteins in the oviductal fluid that associate with sperm are also known to be canonical sperm proteins (47), and in honey bees, 8% of the proteins in the spermathecal fluid are also canonical sperm proteins (30). We predict that such intersexual molecular continuity is probably widespread (perhaps even universal) among internally fertilizing species, given the need for some degree of male-female cooperation in maintaining sperm viability during the period between ejaculation and fertilization. An example of such cooperation, although not involving sperm-association per se, is seen with glucose dehydrogenase (GLD), an enzyme needed for sperm storage/utilization in mated D. melanogaster females (52). GLD is synthesized in and secreted by the male ejaculatory duct and the female spermathecal ducts. The absence of GLD from both the male and female results in a much stronger phenotype than occurs when only the female or only the male lacks GLD. Thus, both sexes must contribute this dual-derived molecule for optimal fertility.
Female-Derived Sperm-Associated Proteins Originate from the FRT Fluid.
We hypothesized that female-derived proteins that associate with sperm originate from the luminal fluid of the FRT, such that they are present in the extracellular environment and poised to interact with sperm upon receipt of the ejaculate. To explore the origin of female-derived sperm-associated proteins (i.e., both dual-derived sperm proteins and uniquely female proteins), we compared their gene expression patterns across FRT tissues (37) and protein abundances in FRT fluid (38). We found that female-derived sperm-associated proteins are expressed broadly across all FRT tissues (Fig. 3B) and 25% (83/328 proteins) are expressed at significantly higher levels in every FRT tissue compared to the whole fly (37). Hierarchical clustering analysis of their expression identified two distinct clades (Fig. 3B)—one comprised the epithelial tissues (i.e., bursa, oviduct, and seminal receptacle) that exhibit particularly high expression in the bursa, and the other comprised the glandular tissues (i.e., spermatheca and parovaria). These patterns are consistent with a scenario in which proteins from the spermatheca and parovaria contribute to the extracellular environment of the FRT fluid, and proteins from the bursa are poised to interact with sperm immediately after ejaculate transfer (see below). Female-derived sperm-associated proteins also exhibited high expression in the reproductive fat body surrounding the parovaria and spermatheca (Fig. 3C). The reproductive fat body was recently identified as the primary contributor of proteins in the FRT fluid (38), so these results provide further evidence that female-derived sperm-associated proteins originate from the FRT fluid.
Because proteins in the FRT fluid are more likely to be involved in dynamic sperm–FRT interactions than are intracellular proteins in FRT tissues (38), we hypothesized that female-derived sperm-associated proteins would be enriched among the contents of the FRT fluid. In support of this hypothesis, we found that 75% of female-derived sperm-associated proteins were also identified in the FRT fluid, and female-derived sperm-associated proteins exhibited a significantly greater overlap with the FRT fluid (247/756 fluid proteins [33%]) than proteins restricted to FRT tissues (76/1,084 tissue proteins [7%]; Fisher’s exact test, P < 0.001). Female-derived sperm-associated proteins were also significantly more abundant in the FRT fluid than the remaining FRT fluid proteins (Student’s t test: t = −18.2; P < 0.001; Fig. 3D), which provides further evidence that the FRT is poised to interact with sperm upon receipt of the ejaculate and suggests that a primary function of FRT fluid is to contribute to the postmating sperm proteome. Lastly, female-derived sperm-associated proteins exhibit a significantly greater overlap with canonical sperm proteins than the protein constituents of FRT fluid (75% versus 34%, respectively; Fisher’s exact test, P < 0.001). This indicates that female-derived sperm-associated proteins are a nonrandom, highly specific subset of the proteins in FRT fluid, as observed in mammals (46, 47).
Female-derived sperm-associated proteins are significantly enriched for biological processes associated with energy metabolism (SI Appendix, Fig. S3), including organic acid metabolic process, generation of precursor metabolites and energy, carbohydrate metabolic process, and adenosine triphosphate metabolic process. These functional enrichments suggest that females actively contribute metabolic proteins to the postmating sperm proteome, which may support energy production and sperm viability over prolonged periods of storage. Extracellular vesicles within mammalian oviducts are enriched in proteins related to energy metabolism and improve sperm motility and fertilization capacity by binding to sperm membranes (17). In honeybees, glycolytic enzymes are enriched in the females’ spermathecal fluid, which may provide stored sperm with energy to support their long-term survival (30, 53, 54). Thus, the molecular life history of sperm may involve metabolic provisioning by females, perhaps via fusion of extracellular vesicles (17), to meet essential physiological requirements during storage (30, 38).
Female-derived sperm-associated proteins also included a significant overrepresentation of molecular chaperones (Gene Ontology, protein folding; adjusted P < 0.001). This observation is intriguing because molecular chaperones have been identified in FRT fluid across diverse taxa (30, 33, 46, 47, 55, 56) and are known to coordinate the final stages of sperm maturation within the FRT in mammals (6). In total, we identified 18 female-derived molecular chaperones in our sperm samples (Dataset S3), many of which belong to molecular chaperone families involved in sperm surface remodeling critical for sperm–oocyte recognition. These included the chaperonin-containing TCP1 complex (57), heat shock proteins (58), and protein disulfide isomerases (59). Notably, all the chaperonin-containing TCP1 complex proteins were derived solely from females and constitute a primary hub within a highly connected and significant protein network observed among uniquely female sperm proteins (3.9-fold enrichment in edges; protein–protein interaction enrichment P = 7.74e-14; SI Appendix, Fig. S4). Additionally, female-derived proteins exhibited significantly enhanced evolutionary rate covariation (P = 0.012) relative to the background genome-wide null distribution, an observation that further supports molecular interactions among this group of proteins (43). We hypothesize that molecular chaperones in the FRT fluid mediate remodeling of the sperm surface, as they do in mammals (6), which may facilitate the final maturation steps prior to interacting with and fertilizing the oocyte.
Compositional Changes to the Postmating Sperm Proteome.
To evaluate compositional changes to the postmating sperm proteome as it moves through and is stored in the FRT, we compared the number and relative abundance of canonical sperm proteins, SFPs, and female-derived proteins in sperm samples dissected from the FRT at 30 min, 2 h, and 4 d after mating (Fig. 4). Relative protein abundances were calculated from the summed normalized spectral abundance factors (NSAFs; ref. 60) for each protein category at a given time point. These analyses were conducted on a conservative dataset to ensure robust quantitative results (Materials and Methods for inclusion criteria). Below, we highlight the two key compositional changes observed across the life history of the postmating sperm proteome.
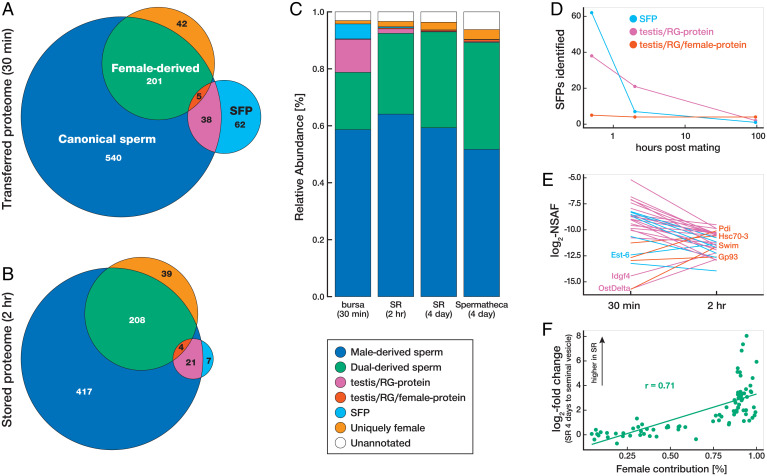
Fig. 4.
Compositional changes to the postmating sperm proteome. (A and B) Venn diagrams indicating the overlap and number of canonical sperm proteins, SFPs, and female-derived proteins in the transferred sperm proteome (bursa 30 min after mating) (A) and stored sperm proteome (seminal receptacle [SR] 2 h after mating) (B). (C) Relative abundance of the nonoverlapping protein categories at each collection point. Relative abundances were calculated from the summed NSAFs for each protein category at the respective collection point. (D) Number of reproductive gland proteins identified at 30 min, 2 h, and 4 d after mating. (E) Abundance comparisons of reproductive gland proteins at 30 min and 2 h after mating. (F) Correlation between female contribution and the change in abundance after storage among dual-derived sperm proteins (Pearson’s r = 0.71; P = 0.001).
The first major compositional change was a decrease in the number and abundance of SFPs over time. SFPs are expected to be rapidly cleaved or degraded after being transferred to the FRT (22, 61, 62), and our results confirm a decrease in the number of SFPs that associate with sperm in the FRT. Specifically, we identified 61 SFPs from our conservative dataset that are associated with sperm in the bursa at 30 min after mating, 7 in the seminal receptacle at 2 h after mating, and only 1 in the seminal receptacle at 4 d after mating (Fig. 4D). We note that Sex Peptide was also associated with sperm in the seminal receptacle at 4 d after mating (Dataset S4), although it did not meet the strictest criteria for inclusion in our conservative dataset. These results indicate that numerous SFPs are transferred to females and associate with sperm, but only a few accompany sperm into storage. The high representation of previously known sperm-bound proteins (the network proteins) among stored SFPs suggests that SFPs can only enter storage if they are physically bound to sperm.
Previously identified sperm-bound SFPs are Sex Peptide, which binds to sperm long term, and several SFPs in the long-term response network, which bind to sperm more transiently and facilitate the binding of Sex Peptide to sperm (18, 22–24). We detected several network proteins in our samples of stored sperm. Specifically, of the seven SFPs that were identified on sperm 2 h after mating, three are part of the long-term response network, namely, Aqrs, CG9997, and lectin-46Cb (formerly CG1652). The detection of network proteins in our datasets validates our approach and suggests that other SFPs detected here as being sperm associated might play important roles in fertility. This possibility can be tested by future genetic studies.
In contrast to the decrease observed for SFPs, the spatiotemporal decline in testis/RG-proteins was much less substantial (Fig. 4D). Testis/RG-proteins were associated with sperm in the sperm-storage organs and persisted in the FRT for longer periods than the majority of SFPs (Fisher’s exact test, P < 0.001). This pattern of retention is consistent with a scenario in which some of each testis/RG-protein is derived from the testis and thus is integrated into sperm and more refractory to degradation.
In addition to identifying testis/RG-proteins as a subset of proteins that are derived from both the testes and the male reproductive glands, we identified six proteins (Gp93, Hsc70-3, Pdi, regucalcin, Swim, and Tsf1) in the postmating sperm proteome that are derived from the testes, the male reproductive glands, and the FRT. Unlike SFPs and testis/RG-proteins described above, we detected virtually no decrease in the number of these proteins (Fig. 4D), and they increased in abundance over time (Fig. 4E). Thus, contributions by females to the production of these proteins alters their collective fate within the FRT, with testis/RG/female-proteins being significantly more spatiotemporally stable than the sperm-associated proteins derived exclusively from males. We note that one SFP (Est-6) and two testis/RG-proteins (Idgf4 and OstDelta) were similarly observed to increase in abundance after storage (Fig. 4E). All three of these proteins have moderate-to-high enrichment in the spermathecae (40), which indicates that they are also produced by females but simply not detected in our labeling experiment.
The second major compositional change we observed was an increase in the number and abundance of female-derived sperm-associated proteins. At just 30 min after mating, there were over 40 uniquely female proteins associated with sperm (Fig. 4A), and they comprised 1.2% of the total protein in our sperm sample (Fig. 4C). To explore whether this might be mediated by direct molecular interactions, we evaluated the extent of evolutionary rate covariation between canonical sperm and uniquely female-derived proteins and observed a significant enhancement (P < 0.0001). These results indicate that female contributions begin to influence the composition of the sperm proteome soon after ejaculate transfer to the bursa, potentially through direct interaction with canonical sperm proteins, which is consistent with the high levels of bursa expression of many female-derived sperm-associated proteins (Fig. 3B).
We estimate that 19.1% of the proteins in long-term stored sperm (4 d after mating) are derived from females (based on summed signal intensities of heavy-labeled peptides from our isotopic labeling experiment; Materials and Methods). We also estimated the extent of female contribution among dual-derived sperm proteins on a protein-by-protein basis, revealing a positive correlation between the degree of female contribution and the increase in protein abundance between the transferred and long-term stored samples (Pearson’s r = 0.50, P < 0.001). This relationship was even stronger, as might be expected, when the analysis compared protein abundances between the canonical and long-term-stored sperm proteomes (Pearson’s r = 0.71, P < 0.001; Fig. 4F). Because female-derived sperm-associated proteins increase in abundance over time and are enriched for biological processes associated with energy metabolism (SI Appendix, Fig. S4), our findings suggest that females contribute to metabolic requirements of sperm throughout extended periods of storage. We suggest this previously unrecognized phenomenon of females adding (and potentially replacing) sperm proteins to be a biological equivalent of the “Ship of Theseus,” a thought experiment on the metaphysics of identity that was recorded by the Greek philosopher Plutarch (63). Many biologists have an ingrained perception of spermatozoa as intrinsically and unequivocally male cells. Our data, along with other advances in the understanding of ejaculate–female interactions (reviewed in ref. 3), are revealing this view to be inaccurate and perhaps an impediment to our understanding of fundamental reproductive processes and of postcopulatory sexual selection. For many species with internal fertilization, sperm spend the majority of their lifespan within the FRT, and as the present study demonstrates, they become the products of this environment as well as that of the male reproductive tract. The genetic tools available for D. melanogaster will facilitate future studies to visualize the nature of the association of these female-derived proteins with sperm, determine their role in fertility, and discern their contribution to postcopulatory sexual selection.
Conclusions
Ever since Austin’s and Chang’s independent discoveries that mammalian sperm must spend a minimum amount of time in the FRT to acquire their fertilizing capacity (1, 2), sperm–female interactions have been recognized as essential to fertility. A robust understanding of the in vivo molecular biology of sperm therefore has important biomedical implications for advancing assisted reproductive technologies in humans as well as endangered species, as defective male–female interactions are important contributors of idiopathic infertility (64, 65). To this end, our study provides a comprehensive, quantitative analysis of the molecular life history of the D. melanogaster sperm proteome. We demonstrate that the postmating sperm proteome undergoes substantial compositional changes as it moves through the male and FRTs, with male-derived sperm-associated proteins progressively lost and female-derived sperm-associated proteins progressively gained throughout prolonged storage in the FRT (Fig. 5). Most proteins contributed to sperm by females overlap with male-derived canonical sperm proteins, with female-derived proteins primarily being involved in processes associated with energy metabolism. Our results therefore support the hypothesis that molecular continuity exists between male and FRTs, with females providing proteins to replace those transferred by males in the ejaculate but not retained in the FRT. This female provisioning is presumed to maintain sperm viability and provide a supportive environment during the prolonged period between copulation and fertilization. We also note that the sperm-associated proteins identified here as part of the postmating sperm proteome are likely to include proteins that affect female physiology and/or sperm function. Indeed, genetic analyses have already confirmed an important role in fertility for eight of the sperm-associated proteins identified in this study, namely, Sex Peptide, Aqrs, Antr, Sems, CG9997, CG17575, lectin-46Ca, and lectin-46Cb (18, 19, 23, 24). Functional analyses of additional sperm-associated proteins are expected to uncover other important contributors of reproductive outcomes.
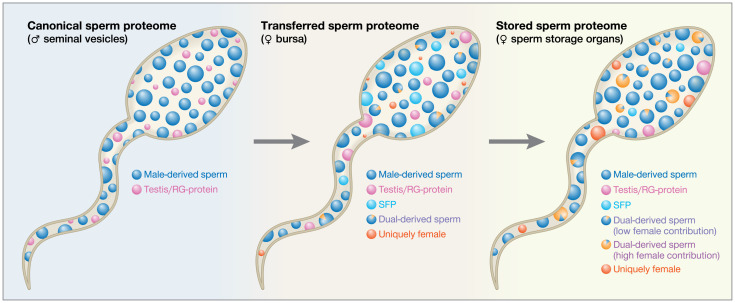
Fig. 5.
Schematic of the life history of the sperm proteome. Each circle depicts a representative protein. The circle’s size indicates that protein’s abundance and its color represent protein origin. The sperm proteome transitions through the following three major stages during its posttesticular maturation: the canonical, transferred, and stored sperm proteomes. After spermatogenesis, male-derived sperm proteins and testis/RG-proteins are stored in the seminal vesicles and comprise the canonical sperm proteome. Upon mating, the canonical sperm proteome mixes with contents from the male reproductive glands, and the resulting ejaculate immediately interacts with the FRT. The transferred sperm proteome is transiently stored in the bursa and contains male-derived sperm proteins, testis/RG-proteins, and SFPs, as well as female contributions to the dual-derived sperm proteins and a small amount of uniquely female proteins. Finally, the transferred sperm proteome is transported to the female’s sperm-storage organs (seminal receptacle and spermathecae). SFPs are progressively lost and/or degraded, and female-derived proteins are progressively gained as sperm continue to interact with the FRT. The stored sperm proteome therefore contains fewer SFPs and testis/RG-proteins, greater female contributions to the dual-derived sperm proteins, and higher abundances of uniquely female proteins.
We propose that the complementary concepts of the life history of the sperm proteome and intersexual molecular continuity offer a unifying and predictive framework for studying the diversity in reproductive biology across taxa. For example, variation in the duration of sperm storage by females is predicted to be correlated with the extent of sperm–female interactions and patterns of sex-biased gene expression. As the life history of sperm in the FRT evolutionarily expands, genes contributing to spermiogenesis, sperm modification, and/or the maintenance of sperm metabolism are expected to shift from exclusively male-biased to increasingly shared between the sexes (3, 66–68). Intersexual molecular continuity also provides a conceptual framework for understanding postmating sexual selection and reproductive isolation. As postulated by Pitnick et al. (3), the selective failure by females to modify the sperm of some males but not others, including both the addition of uniquely female proteins and the potential replacement of canonical sperm proteins by female-derived proteins, may represent a general and widespread mechanism by which females could bias fertilization against less-preferred males (3, 69) and may contribute to reproductive isolation after matings between different species (3, 70–72). Future investigations are necessary to resolve the adaptive value of the molecular continuity mechanisms identified here. We note, however, that this process may be the target of selection for sexual cooperation and/or conflict (73, 74). Lastly, although our proteomics approach is an important first step in understanding the molecular bases of sperm-FRT interactions, intersexual molecular continuity is expected to be a multifaceted phenomenon involving not only proteins, but also carbohydrates, metabolites, other small molecules, and vesicles. By studying the intimate ways in which sperm interact with the FRT during the final stages of functional maturation, our research advances understanding of animal fertility and the contributions of each sex to reproductive success.
Materials and Methods
Sample Collection and Preparation.
A Drosophila melanogaster wild-type LHM stock (75) was maintained at ambient light and room temperature in half-pint milk bottles on standard cornmeal or yeast-glucose media supplemented with live yeast grains. Flies were collected within 12 h of eclosion and maintained as virgins in vials for 4 to 5 d before being used in experiments.
Sperm were purified from the following four distinct spatiotemporal conditions within the FRT: the bursa 30 min after mating, the seminal receptacle 2 h after mating, the seminal receptacle 4 d after mating, and the spermathecae 4 d after mating. Our collections span the major transitions in female postmating responses (76) and allow us to characterize compositional changes in the sperm proteome after prolonged periods of storage. Note that in D. melanogaster, the seminal receptacle has been shown to be the primary source of sperm for fertilization, with the spermathecae postulated to function in longer-term sperm storage. Although there is no direct evidence for sperm moving between storage organs, it has been postulated that sperm migrate from the spermathecae to the seminal receptacle, but not vice versa (51, 77).
To collect the bursa samples, we aspirated males and females into individual food vials and flash froze the females in liquid nitrogen 30 min after mating. The lower reproductive tracts of ∼180 females were dissected in phosphate-buffered saline (PBS), and the sperm mass in the bursa (excluding the mating plug) was isolated and pooled in PBS per replicate. To collect the seminal receptacle and spermathecae samples, we paired 20 males with 10 females in food vials for 90 min, transferred the females to clean food vials, and flash froze the females in liquid nitrogen 2 h or 4 d after mating. The lower reproductive tract of ∼480 females (2-h samples) or 600 females (4-d samples) were dissected in PBS, and the sperm in the seminal receptacle or spermatheca were isolated and pooled in PBS per replicate. Sperm samples were washed in PBS and pelleted by centrifugation. Two replicates were collected for each spatiotemporal condition for a total of 8 purified sperm samples isolated from over 3,700 FRT dissections.
To ensure robust results, replicate sperm samples were isolated and purified in two different laboratories (Syracuse and Cornell). Sample preparation was standardized across the two laboratories except that samples were subjected to a different number of PBS washes (three at Syracuse versus two at Cornell) to determine if additional washing minimized nonspecific protein interactions. The extra wash did not reduce the number or abundance of identified of sperm-associated (i.e., SFP or female-derived) proteins. We cannot rule out the possibility that washing samples reduced our ability to detect low affinity, but biologically meaningful, interactions.
Heavy Isotope Labeling.
To identify female-derived proteins, we heavy labeled whole flies using a modified version of the stable isotope labeling by amino acids in culture method (78, 79). Male and female flies were raised from embryos on media containing lysine/arginine double auxotropic, labeled yeast, which was cultured on heavy lysine/arginine (13C6 15N2 L-lysine and 13C6 15N4 L-arginine) media (see 38 for details). This approach ensured that all tryptic peptides were heavy labeled. The labeling efficiency of whole males and females was estimated to be >90% (38). We conducted a reciprocal cross in which heavy-labeled males were mated to unlabeled females and heavy-labeled females were mated to unlabeled males. Heavy-labeled flies were collected and maintained as virgins in vials containing heavy yeast media for 4 to 5 d prior to experiments. Sperm samples were collected 4 d after mating from the seminal receptacle as described above, except that heavy-labeled and unlabeled females were maintained on their respective media throughout the experiment.
Liquid Chromatography–Mass Spectrometry (LC-MS/MS) Analysis.
Washed sperm samples were solubilized in 2× Laemmli buffer. Solubilized samples were separated by 1D sodium dodecyl–sulfate polyacrylamide gel electrophoresis, digested with trypsin, and analyzed by LC-MS/MS (SI Appendix, Supplemental Methods for details). Raw spectral data were searched against the D. melanogaster protein database (dmel-all-translation-r6.32; www.FlyBase.org) and appended with the cRAP v1.0 contaminant database (www.thegpm.org), using the standard workflow in PEAKS X+ (de novo + PEAKS DB + PEAKS PTM + SPIDER; Bioinformatics Solutions Inc.).
Spectral data from all eight unlabeled samples were run together in a combined analysis using the following search parameters: mass tolerance of 15 ppm for parent ions and 0.5 Da for fragment ions; carbamidomethylation (C) as a fixed modification, oxidation (M), deamidation (NQ), acetylation (K), phosphorylation (STY), and ubiquitination (GG and LRGG) as variable modifications; and up to three missed tryptic cleavages. Peptide identifications were filtered to a false discovery rate (FDR) of <1% based on the decoy-fusion approach (80). Protein identifications were filtered to a −10lgP score of ≥20, at least 2 unique peptide-spectrum matches (PSMs), and 1 identification per protein group. Our analysis identified 354,532 PSMs and 2,339 proteins (Dataset S4).
Spectral data from the reciprocal heavy-labeled crosses were analyzed separately in PEAKS X+ as described above, except that the mass error was set at 20 ppm for parent ions and 0.1 Da for fragment ions. For the heavy-labeled female cross, heavy lysine (13C6 15N2: 8.01), heavy arginine (13C6 15N4: 10.01), and the metabolic conversion of arginine to proline (R to P: 6.01) were added as variable modifications to identify heavy-labeled female proteins. Heavy labeling of both lysine and arginine ensured that all tryptic peptides were labeled, but we also applied a stringent peptide filter of −10lgP score of ≥35 (equivalent to FDR of <0.01%) to minimize false-positive identifications. Protein identifications were filtered further to a −10lgP score of ≥20 and at least 2 unique PSMs.
The final identification of female-derived proteins was based solely on the search for unlabeled proteins from the heavy male dataset because MS resolution is highest for unlabeled peptides (61). To be conservative, we also excluded 17 proteins (5%) for which FRT expression has yet to be confirmed (37) to remove any potentially spurious identifications. Our final list contains 328 female-derived proteins (Dataset S3).
Protein Annotations.
To compare compositional changes to the postmating sperm proteome, we categorized proteins as canonical sperm proteins, female-derived proteins, and/or SFPs. Canonical sperm proteins were identified based on previous annotation to the D. melanogaster sperm proteome (41, 42) or to an expanded canonical sperm proteome based on newly analyzed seminal vesicle samples as part of this study (SI Appendix, Supplemental Methods and Dataset S2 for details). Female-derived proteins were based upon unlabeled proteins identified in the heavy-labeled male cross that also exhibit expression in the FRT (Dataset S3). SFPs were identified from a revised version of the list of “high confidence” SFPs by Wigby et al. (39). Our list also includes 22 previously uncharacterized SFPs that were classified as SFPs because they 1) are not canonical sperm proteins or female-derived proteins, 2) were identified in our combined analysis of sperm samples purified from the FRT, and 3) exhibited expression patterns that are characteristic of most SFPs, namely, they have high expression in male accessory glands (i.e., log2(accessory gland fragments per kilobase per million mapped reads [FPKM]+1/male whole body FPKM+1) > 1), low expression in testes (i.e., log2 (testis FPKM+1/male whole body FPKM+1) < −1), and higher expression in male accessory glands than other male tissues in FlyAtlas2 (40). Our revised list includes 309 SFPs (Dataset S1).
Protein Quantitation.
To compare compositional changes in the postmating sperm proteome, we filtered our dataset to exclude proteins that were not confidently identified as present in at least one spatiotemporal condition (i.e., at least 1 PSM in each replicate and at least 10 PSMs across both replicates). All quantitative analyses were conducted using this filtered dataset of 1,116 proteins. Of these high-confidence identifications, 877 (79%) were canonical sperm proteins, 109 (10%) were SFPs, and 297 (27%) were female-derived proteins. In total, we were able to classify 90% of the high-confidence proteins into one of these three categories. Protein abundances were quantified as NSAF, which are the number of spectral counts per protein corrected for protein length and normalized for the sum of all protein abundances in the sample (60). NSAFs were calculated using the average spectral count for a given protein across replicates. For comparisons, we also calculated NSAFs of sperm proteins stored in the seminal vesicles (based on data from our newly analyzed seminal vesicle samples; SI Appendix, Supplemental Methods) and female proteins in virgin FRT fluid (based on data from 38).
We also estimated the abundance of female-derived proteins relative to male-derived proteins after long-term sperm storage by comparing the summed signal intensities of heavy-labeled peptides to total peptides in the heavy-labeled female cross (78). This estimate was based on the heavy-labeled female cross to provide a conservative estimate due to the potential impact of incomplete labeling. This analysis was also done on a protein-by-protein basis by comparing the signal intensities of heavy-labeled peptides to total peptides for each protein. Female contribution ranged from 0 to 1, with 0 indicating the protein of interest is produced exclusively by males and 1 indicating the protein is produced exclusively by females. Estimates of female contribution are based on a filtered heavy female dataset that excluded proteins with fewer than five PSMs.
Gene Expression and GO Analyses.
Sex-biased and tissue-biased expression patterns were based on FPKM values from FlyAtlas2 (40). Sex-biased expression was calculated as log2(male whole body FPKM+1/female whole body FPKM+1). Tissue-biased expression was calculated as log2(male accessory glands FPKM+1/testis FPKM+1). Absolute expression in the accessory glands and testis was calculated as log2(tissue FPKM+1). We analyzed the expression patterns of female-derived proteins in each FRT tissue (i.e., bursa, oviduct, seminal receptacle, spermathecae, parovaria, and the reproductive fat body surrounding the spermathecae and parovaria) using log2-scaled, normalized counts per million values of unmated females from McDonough-Goldstein et al. (37).
Functional enrichment was examined using clusterProfiler version 4.0 (81). Functional coherence of female-derived proteins in the FRT fluid was assessed using GO enrichment analysis with the FRT transcriptome (37) as the background dataset. GO terms were considered significantly enriched if the Benjamini–Hochberg-adjusted P value was <0.01.
We used FlyMine (82) to identify the GO annotations for female-derived proteins. Protein network analysis was conducted using STRING version 11.5 (83) using default parameters with the exception of requiring the highest confidence interaction scores (0.90). Evolutionary rate covariation among female-derived proteins was assessed using a group analysis as implemented by Clark et al. (43). Evolutionary rate covariation between 1) female-derived and canonical sperm proteins and 2) sperm-associated and non-sperm-associated SFPs and canonical sperm proteins was calculated using the data of Findlay et al. (23) and statistically assessed using a permutation-based approach using background, genome-wide evolutionary rate covariation as the null distribution (10,000 iterations).
Statistical Analyses.
To evaluate the extent of overlap between female-derived proteins and canonical sperm proteins, we used the normal approximation to calculate the probability of observing the sample proportion given a population proportion of 15% (2,105 sperm proteins out of 13,963 total proteins, after excluding multiple isoforms). We examined the effect of protein category (SFP or testis/RG-protein) on the relationship between testis expression (data from ref. 40) and abundance in the canonical sperm proteome (this study) by comparing the goodness-of-fit of linear models with and without protein category as an explanatory variable.
Supplementary Material
Acknowledgments
We are very grateful to the late Dr. Stuart Moss, the National Institute of Child Health and Human Development (NICHD) program officer for the R21 grant that funded this work. It was Dr. Moss’s continual and generous advice, encouragement, and support that made this study possible. We thank Tim Karr for being a wellspring of ideas and inspiration and Caitlin McDonough-Goldstein for access to unpublished data, critical input, and technical assistance. We thank Shelley Stevens and Nicholas Palmateer for preparing seminal vesicle sperm samples; Peter Wengert, Yasir Ahmed-Braimah, and Kirill Borziak for analytical support; Sharleen Buel for assistance generating labeled flies; Wyn Pitnick for enlightening us on the “Ship of Theseus” thought experiment; and Ben Woolsey for his artistic contributions to the figures. This research was funded by the National Science Foundation (DEB 1655840 to S.D., S.P., and M.F.W.), the National Institutes of Health (NICHD R21HD088910 to S.D., S.P. and M.F.W. and R37/R01HD038921 to M.F.W.), and a generous gift from Mike and Jane Weeden to Syracuse University.
Footnotes
Reviewers: M.D., University of Southern California; M.S., New York University; and W.S., University of Washington.
The authors declare no competing interest.
Data Availability
Mass spectrometry data have been deposited to the ProteomeXchange Consortium via the PRIDE partner repository (84) with the dataset identifiers PXD027807 (complete unlabeled dataset), PXD027802 (heavy male dataset), PXD027806 (heavy female dataset), and PXD027808 (seminal vesicle sperm proteome). The complete, unfiltered list of protein identifications from our unlabeled sperm samples is reported in Dataset S5.
References
- 1.Austin C. R., Observations on the penetration of the sperm in the mammalian egg. Aust. J. Sci. Res. B 4, 581–596 (1951). [DOI] [PubMed] [Google Scholar]
- 2.Chang M. C., Fertilizing capacity of spermatozoa deposited into the fallopian tubes. Nature 168, 697–698 (1951). [DOI] [PubMed] [Google Scholar]
- 3.Pitnick S., Wolfner M. F., Dorus S., Post-ejaculatory modifications to sperm (PEMS). Biol. Rev. Camb. Philos. Soc. 95, 365–392 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bloch Qazi M. C., Heifetz Y., Wolfner M. F., The developments between gametogenesis and fertilization: Ovulation and female sperm storage in Drosophila melanogaster. Dev. Biol. 256, 195–211 (2003). [DOI] [PubMed] [Google Scholar]
- 5.Suarez S. S., Pacey A. A., Sperm transport in the female reproductive tract. Hum. Reprod. Update 12, 23–37 (2006). [DOI] [PubMed] [Google Scholar]
- 6.Dun M. D., Aitken R. J., Nixon B., The role of molecular chaperones in spermatogenesis and the post-testicular maturation of mammalian spermatozoa. Hum. Reprod. Update 18, 420–435 (2012). [DOI] [PubMed] [Google Scholar]
- 7.Ghersevich S., Massa E., Zumoffen C., Oviductal secretion and gamete interaction. Reproduction 149, R1–R14 (2015). [DOI] [PubMed] [Google Scholar]
- 8.Orr T. J., Brennan P. L. R., Sperm storage: Distinguishing selective processes and evaluating criteria. Trends Ecol. Evol. 30, 261–272 (2015). [DOI] [PubMed] [Google Scholar]
- 9.Suarez S. S., Mammalian sperm interactions with the female reproductive tract. Cell Tissue Res. 363, 185–194 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nilsson T., Fricke C., Arnqvist G., The effects of male and female genotype on variance in male fertilization success in the red flour beetle (Tribolium castaneum). Behav. Ecol. Sociobiol. 53, 227–233 (2003). [Google Scholar]
- 11.Birkhead T. R., Chaline N., Biggins J. D., Burke T., Pizzari T., Nontransitivity of paternity in a bird. Evolution 58, 416–420 (2004). [PubMed] [Google Scholar]
- 12.Chow C. Y., Wolfner M. F., Clark A. G., The genetic basis for male x female interactions underlying variation in reproductive phenotypes of Drosophila. Genetics 186, 1355–1365 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lüpold S., et al. , How female × male and male × male interactions influence competitive fertilization in Drosophila melanogaster. Evol. Lett. 4, 416–429 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gatti J.-L., et al. , Post-testicular sperm environment and fertility. Anim. Reprod. Sci. 82-83, 321–339 (2004). [DOI] [PubMed] [Google Scholar]
- 15.Sullivan R., Saez F., Epididymosomes, prostasomes, and liposomes: Their roles in mammalian male reproductive physiology. Reproduction 146, R21–R35 (2013). [DOI] [PubMed] [Google Scholar]
- 16.Al-Dossary A. A., Bathala P., Caplan J. L., Martin-DeLeon P. A., Oviductosome-sperm membrane interaction in cargo delivery. J. Biol. Chem. 290, 17710–17723 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ferraz M. A. M. M., Carothers A., Dahal R., Noonan M. J., Songsasen N., Oviductal extracellular vesicles interact with the spermatozoon’s head and mid-piece and improves its motility and fertilizing ability in the domestic cat. Sci. Rep. 9, 9484 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ram K. Ravi, Wolfner M. F., A network of interactions among seminal proteins underlies the long-term postmating response in Drosophila. Proc. Natl. Acad. Sci. U.S.A. 106, 15384–15389 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Peng J., et al. , Gradual release of sperm bound sex-peptide controls female postmating behavior in Drosophila. Curr. Biol. 15, 207–213 (2005). [DOI] [PubMed] [Google Scholar]
- 20.Ravi Ram K., Wolfner M. F., Seminal influences: Drosophila Acps and the molecular interplay between males and females during reproduction. Integr. Comp. Biol. 47, 427–445 (2007). [DOI] [PubMed] [Google Scholar]
- 21.Yapici N., Kim Y.-J., Ribeiro C., Dickson B. J., A receptor that mediates the post-mating switch in Drosophila reproductive behaviour. Nature 451, 33–37 (2008). [DOI] [PubMed] [Google Scholar]
- 22.LaFlamme B. A., Ram K. Ravi, Wolfner M. F., The Drosophila melanogaster seminal fluid protease “seminase” regulates proteolytic and post-mating reproductive processes. PLoS Genet. 8, e1002435 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Findlay G. D., et al. , Evolutionary rate covariation identifies new members of a protein network required for Drosophila melanogaster female post-mating responses. PLoS Genet. 10, e1004108 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Singh A., et al. , Long-term interaction between Drosophila sperm and sex peptide is mediated by other seminal proteins that bind only transiently to sperm. Insect Biochem. Mol. Biol. 102, 43–51 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wolfner M. F., Precious essences: Female secretions promote sperm storage in Drosophila. PLoS Biol. 9, e1001191 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Levine B. A., Schuett G. W., Booth W., Exceptional long-term sperm storage by a female vertebrate. PLoS One 16, e0252049 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Poiani A., Complexity of seminal fluid: A review. Behav. Ecol. Sociobiol. 60, 289–310 (2006). [Google Scholar]
- 28.den Boer S. P. A., Boomsma J. J., Baer B., Seminal fluid enhances sperm viability in the leafcutter ant Atta colombica. Behav. Ecol. Sociobiol. 62, 1843–1849 (2008). [Google Scholar]
- 29.King M., Eubel H., Millar A. H., Baer B., Proteins within the seminal fluid are crucial to keep sperm viable in the honeybee Apis mellifera. J. Insect Physiol. 57, 409–414 (2011). [DOI] [PubMed] [Google Scholar]
- 30.Baer B., Eubel H., Taylor N. L., O’Toole N., Millar A. H., Insights into female sperm storage from the spermathecal fluid proteome of the honeybee Apis mellifera. Genome Biol. 10, R67 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.den Boer S. P. A., Boomsma J. J., Baer B., Honey bee males and queens use glandular secretions to enhance sperm viability before and after storage. J. Insect Physiol. 55, 538–543 (2009). [DOI] [PubMed] [Google Scholar]
- 32.Hereng T. H., et al. , Exogenous pyruvate accelerates glycolysis and promotes capacitation in human spermatozoa. Hum. Reprod. 26, 3249–3263 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lachance C., Bailey J. L., Leclerc P., Expression of Hsp60 and Grp78 in the human endometrium and oviduct, and their effect on sperm functions. Hum. Reprod. 22, 2606–2614 (2007). [DOI] [PubMed] [Google Scholar]
- 34.Swanson W. J., Clark A. G., Waldrip-Dail H. M., Wolfner M. F., Aquadro C. F., Evolutionary EST analysis identifies rapidly evolving male reproductive proteins in Drosophila. Proc. Natl. Acad. Sci. U.S.A. 98, 7375–7379 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mack P. D., Kapelnikov A., Heifetz Y., Bender M., Mating-responsive genes in reproductive tissues of female Drosophila melanogaster. Proc. Natl. Acad. Sci. U.S.A. 103, 10358–10363 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kapelnikov A., et al. , Mating induces an immune response and developmental switch in the Drosophila oviduct. Proc. Natl. Acad. Sci. U.S.A. 105, 13912–13917 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McDonough-Goldstein C. E., Borziak K., Pitnick S., Dorus S., Drosophila female reproductive tract gene expression reveals coordinated mating responses and rapidly evolving tissue-specific genes. G3 (Bethesda) 11, jkab020 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McDonough-Goldstein C. E., et al. , Pronounced postmating response in the Drosophila female reproductive tract fluid proteome. Mol. Cell. Proteomics 20, 100156 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wigby S., et al. , The Drosophila seminal proteome and its role in postcopulatory sexual selection. Philos. Trans. R. Soc. Lond. B Biol. Sci. 375, 20200072 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Leader D. P., Krause S. A., Pandit A., Davies S. A., Dow J. A. T., FlyAtlas 2: A new version of the Drosophila melanogaster expression atlas with RNA-Seq, miRNA-Seq and sex-specific data. Nucleic Acids Res. 46 (D1), D809–D815 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dorus S., et al. , Genomic and functional evolution of the Drosophila melanogaster sperm proteome. Nat. Genet. 38, 1440–1445 (2006). [DOI] [PubMed] [Google Scholar]
- 42.Wasbrough E. R., et al. , The Drosophila melanogaster sperm proteome-II (DmSP-II). J. Proteomics 73, 2171–2185 (2010). [DOI] [PubMed] [Google Scholar]
- 43.Clark N. L., Alani E., Aquadro C. F., Evolutionary rate covariation reveals shared functionality and coexpression of genes. Genome Res. 22, 714–720 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Li H., et al. , Fly Cell Atlas: A single-cell transcriptomic atlas of the adult fruit fly. Science, in press. [DOI] [PMC free article] [PubMed]
- 45.Neubaum D. M., Wolfner M. F., Mated Drosophila melanogaster females require a seminal fluid protein, Acp36DE, to store sperm efficiently. Genetics 153, 845–857 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Killian G., Physiology and endocrinology symposium: Evidence that oviduct secretions influence sperm function: A retrospective view for livestock. J. Anim. Sci. 89, 1315–1322 (2011). [DOI] [PubMed] [Google Scholar]
- 47.Lamy J., et al. , Identification by proteomics of oviductal sperm-interacting proteins. Reproduction 155, 457–466 (2018). [DOI] [PubMed] [Google Scholar]
- 48.Luongo C., et al. , Sperm proteome after interaction with reproductive fluids in porcine: From the ejaculation to the fertilization site. Int. J. Mol. Sci. 21, 6060 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Delbare S. Y. N., Chow C. Y., Wolfner M. F., Clark A. G., Roles of female and male genotype in post-mating responses in Drosophila melanogaster. J. Hered. 108, 740–753 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.McCullough E. L., McDonough C. E., Pitnick S., Dorus S., Quantitative proteomics reveals rapid divergence in the postmating response of female reproductive tracts among sibling species. Proc. Biol. Sci. 287, 20201030 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Manier M. K., et al. , Resolving mechanisms of competitive fertilization success in Drosophila melanogaster. Science 328, 354–357 (2010). [DOI] [PubMed] [Google Scholar]
- 52.Iida K., Cavener D. R., Glucose dehydrogenase is required for normal sperm storage and utilization in female Drosophila melanogaster. J. Exp. Biol. 207, 675–681 (2004). [DOI] [PubMed] [Google Scholar]
- 53.Poland V., et al. , Stored sperm differs from ejaculated sperm by proteome alterations associated with energy metabolism in the honeybee Apis mellifera. Mol. Ecol. 20, 2643–2654 (2011). [DOI] [PubMed] [Google Scholar]
- 54.Paynter E., et al. , Insights into the molecular basis of long-term storage and survival of sperm in the honeybee (Apis mellifera). Sci. Rep. 7, 40236 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gotoh A., et al. , Transcriptome profiling of the spermatheca identifies genes potentially involved in the long-term sperm storage of ant queens. Sci. Rep. 7, 5972 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yu H., et al. , Identification of rabbit oviductal fluid proteins involved in pre-fertilization processes by quantitative proteomics. Proteomics 19, e1800319 (2019). [DOI] [PubMed] [Google Scholar]
- 57.Dun M. D., et al. , The chaperonin containing TCP1 complex (CCT/TRiC) is involved in mediating sperm-oocyte interaction. J. Biol. Chem. 286, 36875–36887 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Asquith K. L., Baleato R. M., McLaughlin E. A., Nixon B., Aitken R. J., Tyrosine phosphorylation activates surface chaperones facilitating sperm-zona recognition. J. Cell Sci. 117, 3645–3657 (2004). [DOI] [PubMed] [Google Scholar]
- 59.Ellerman D. A., Myles D. G., Primakoff P., A role for sperm surface protein disulfide isomerase activity in gamete fusion: Evidence for the participation of ERp57. Dev. Cell 10, 831–837 (2006). [DOI] [PubMed] [Google Scholar]
- 60.Bubis J. A., Levitsky L. I., Ivanov M. V., Tarasova I. A., Gorshkov M. V., Comparative evaluation of label-free quantification methods for shotgun proteomics. Rapid Commun. Mass Spectrom. 31, 606–612 (2017). [DOI] [PubMed] [Google Scholar]
- 61.Findlay G. D., Yi X., Maccoss M. J., Swanson W. J., Proteomics reveals novel Drosophila seminal fluid proteins transferred at mating. PLoS Biol. 6, e178 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sepil I., et al. , Quantitative proteomics identification of seminal fluid proteins in male Drosophila melanogaster. Mol. Cell. Proteomics 18 (suppl. 1), S46–S58 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Rose D., et al. , The Ship of Theseus puzzle. Oxford Stud. Exp. Philos. 3, 158–164 (2020). [Google Scholar]
- 64.Liu D. Y., Garrett C., Baker H. W. G., Clinical application of sperm-oocyte interaction tests in in vitro fertilization—Embryo transfer and intracytoplasmic sperm injection programs. Fertil. Steril. 82, 1251–1263 (2004). [DOI] [PubMed] [Google Scholar]
- 65.Herrick J. R., Assisted reproductive technologies for endangered species conservation: Developing sophisticated protocols with limited access to animals with unique reproductive mechanisms. Biol. Reprod. 100, 1158–1170 (2019). [DOI] [PubMed] [Google Scholar]
- 66.Haerty W., et al. , Evolution in the fast lane: Rapidly evolving sex-related genes in Drosophila. Genetics 177, 1321–1335 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zhang Y., Sturgill D., Parisi M., Kumar S., Oliver B., Constraint and turnover in sex-biased gene expression in the genus Drosophila. Nature 450, 233–237 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sirot L. K., et al. , Molecular characterization and evolution of a gene family encoding both female- and male-specific reproductive proteins in Drosophila. Mol. Biol. Evol. 31, 1554–1567 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Firman R. C., Gasparini C., Manier M. K., Pizzari T., Postmating female control: 20 years of cryptic female choice. Trends Ecol. Evol. 32, 368–382 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Howard D. J., Palumbi S. R., Birge L. M., Manier M. K., “Sperm and speciation” in Sperm Biology, Birkhead T. R., Hosken D. J., Pitnick S., Eds. (Academic Press, 2009), pp. 367–403. [Google Scholar]
- 71.Manier M. K., et al. , Postcopulatory sexual selection generates speciation phenotypes in Drosophila. Curr. Biol. 23, 1853–1862 (2013). [DOI] [PubMed] [Google Scholar]
- 72.McDonough C. E., Whittington E., Pitnick S., Dorus S., Proteomics of reproductive systems: Towards a molecular understanding of postmating, prezygotic reproductive barriers. J. Proteomics 135, 26–37 (2016). [DOI] [PubMed] [Google Scholar]
- 73.Andersson M., Sexual Selection (Princeton University Press, 1994). [Google Scholar]
- 74.Arnqvist G., Rowe L., Sexual Conflict (Princeton University Press, 2005). [Google Scholar]
- 75.Chippindale A. K., Gibson J. R., Rice W. R., Negative genetic correlation for adult fitness between sexes reveals ontogenetic conflict in Drosophila. Proc. Natl. Acad. Sci. U.S.A. 98, 1671–1675 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Carmel I., Tram U., Heifetz Y., Mating induces developmental changes in the insect female reproductive tract. Curr. Opin. Insect Sci. 13, 106–113 (2016). [DOI] [PubMed] [Google Scholar]
- 77.Manier M. K., Lüpold S., Pitnick S., Starmer W. T., An analytical framework for estimating fertilization bias and the fertilization set from multiple sperm-storage organs. Am. Nat. 182, 552–561 (2013). [DOI] [PubMed] [Google Scholar]
- 78.Ong S.-E., Mann M., A practical recipe for stable isotope labeling by amino acids in cell culture (SILAC). Nat. Protoc. 1, 2650–2660 (2006). [DOI] [PubMed] [Google Scholar]
- 79.Sury M. D., Chen J.-X., Selbach M., The SILAC fly allows for accurate protein quantification in vivo. Mol. Cell. Proteomics 9, 2173–2183 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zhang J., et al. , PEAKS DB: De novo sequencing assisted database search for sensitive and accurate peptide identification. Mol. Cell. Proteomics 11, M111.010587 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Wu T., et al. , clusterProfiler 4.0: A universal enrichment tool for interpreting omics data. Innovation (N Y) 2, 100141 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Lyne R., et al. , FlyMine: An integrated database for Drosophila and Anopheles genomics. Genome Biol. 8, R129 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Szklarczyk D., et al. , The STRING database in 2021: Customizable protein-protein networks, and functional characterization of user-uploaded gene/measurement sets. Nucleic Acids Res. 49 (D1), D605–D612 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Vizcaíno J. A., et al. , 2016 update of the PRIDE database and its related tools. Nucleic Acids Res. 44 (D1), D447–D456 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Mass spectrometry data have been deposited to the ProteomeXchange Consortium via the PRIDE partner repository (84) with the dataset identifiers PXD027807 (complete unlabeled dataset), PXD027802 (heavy male dataset), PXD027806 (heavy female dataset), and PXD027808 (seminal vesicle sperm proteome). The complete, unfiltered list of protein identifications from our unlabeled sperm samples is reported in Dataset S5.