Abstract
Background/Aim: Despite evidence of an association between pulmonary diseases and periodontopathic bacteria, the molecular mechanisms remain unknown. Matrix metalloproteinase-9 (MMP9) plays important roles in pneumonia, chronic obstructive pulmonary disease, and asthma; therefore, we assessed the effects of Fusobacterium nucleatum on MMP9 expression in mouse lung and A549 human alveolar epithelial cells.
Materials and Methods: Heat-killed F. nucleatum was administered to the trachea of mice or added to A549 cell cultures. MMP9 expression was determined using real-time PCR and western blotting. The involvement of mitogen-activated protein kinases (MAPKs) and nuclear factor-ĸB (NF-ĸB) in MMP9 expression was examined.
Results: F. nucleatum induced expression of MMP9 in mouse lung and bronchoalveolar lavage fluid. In A549 cells, F. nucleatum induced production of MMP9 protein and mRNA in a density-dependent manner; this was inhibited by inhibitors of extracellular-regulated kinase 1/2 and NF-ĸB, but not of p38 and Jun N-terminal protein kinase.
Conclusion: F. nucleatum may contribute to the onset of pulmonary diseases via MMP9 expression through extracellular-regulated kinase 1/2 and NF-ĸB activation.
Keywords: Fusobacterium nucleatum, periodontal disease, matrix metalloproteinase-9, pulmonary disease
Pulmonary diseases, including pneumonia and chronic obstructive pulmonary disease (COPD), are among the most common diseases that lead to increased morbidity and mortality worldwide (1). Pneumonia, an inflammatory condition of the lung parenchyma, is usually initiated by the introduction of bacteria into the lower airway (2). The aspiration of oropharyngeal secretions colonized with pathogenic bacteria is thought to be a major cause of pneumonia in the elderly. COPD, the third-leading cause of death worldwide (3), is characterized by emphysema and airflow limitation, symptoms that likely result from chronic inflammation in the lung periphery. The effective prevention of pulmonary diseases has important implications for clinical management and public health.
Periodontal disease, which is highly prevalent worldwide, is an inflammatory reaction induced by bacteria, including Porphyromonas gingivalis and Fusobacterium nucleatum. This disease leads to the destruction of the periodontium, including the periodontal bone and connective tissue attachment. Recently, numerous studies have found that periodontal disease is associated with pulmonary diseases, including pneumonia, COPD, and asthma (4-7). Previous studies demonstrated that periodontopathic bacteria induced the production of proinflammatory cytokines in respiratory organs and by cell lines from respiratory tissue (8-10). However, the details of the relationship between periodontal disease and pulmonary diseases are not fully understood.
F. nucleatum, a Gram-negative anaerobic bacterium, is one of the most abundant species in the oral cavity; moreover, it has been implicated in various periodontal diseases, including gingivitis and periodontitis (11). Recent studies have implicated F. nucleatum in several systemic diseases, including pulmonary diseases, colorectal cancer, and preterm birth (11). F. nucleatum is reportedly frequently detected in the lower airway tract of patients with pneumonia (12-14). In addition, in patients with severe COPD, the genus Fusobacterium was found to be increased in bronchoalveolar lavage fluid (BALF) (15) and sputum (16). Our previous studies demonstrated that heat-killed F. nucleatum strongly induced inflammatory cytokines in mouse lung and in respiratory epithelial cells (8,9). Although it is possible that the tracheal aspiration of F. nucleatum plays a part in the pathogenesis of pulmonary diseases, little is known about the molecular mechanisms through which F. nucleatum affects the respiratory tract.
Matrix metalloproteinases (MMPs) are involved in extracellular matrix turnover and tissue repair (17). The dysregulation of MMPs leads to pathological conditions through tissue degradation (18). In particular, the dysregulation of MMP9 plays an important role in pulmonary diseases such as pneumonia (17), COPD, and asthma (19). MMP9 expression is regulated by mitogen-activated protein kinases [MAPKs, including p38-MAPK, c-Jun N-terminal protein kinase (JNK), and extracellular signal-regulated kinase (ERK)], and nuclear factor-kappa B (NF-ĸB) in various cell types (20,21). MMP9 is one of the key executors of inflammatory reactions, which are capable of causing local tissue damage (17), processing cytokines (18), and promoting the infiltration of inflammatory cells (22). Thus, it is important to understand what causes the dysregulation of MMP9 expression in the lung. It is not known whether periodontopathic bacteria can induce the expression of MMP9 in the respiratory tract.
In the present study, we examined the effect of F. nucleatum on the expression of MMP9 in the mouse lung and human respiratory epithelial cells in order to evaluate the possible molecular basis linking periodontopathic bacteria to pulmonary diseases. Therefore, in this study, we assessed the effects of heat-killed F. nucleatum on the expression of MMP9 in mouse lung and respiratory epithelial cells. As F. nucleatum, an anaerobic bacterium, is considered to be incapable of surviving in the respiratory tract, we used heat-killed F. nucleatum.
Materials and Methods
Reagents. p38-MAPK inhibitor (SB239063; 10 μM), JNK inhibitor (SP600125; 10 μM), ERK1/2 inhibitor (U0126; 10 μM) (Merck, Darmstadt, Germany), and NF-ĸB inhibitor (BAY11-7082; 10 μM) (Wako, Osaka, Japan) were added 60 min prior to the addition of F. nucleatum. All inhibitor stocks were dissolved in dimethyl sulfoxide (DMSO) at a concentration of 10 mM.
Bacterial culture. Fusobacteria nucleatum ATCC 25586 (American Type Culture Collection, Manassas, VA, USA) was grown in brain-heart infusion broth (Becton Dickinson, Franklin Lakes, NJ, USA). Bacterial cell cultures were grown under anaerobic conditions (80% N2, 10% H2, and 10% CO2) at 37˚C using an anaerobic chamber (ANX-3; Hirasawa, Tokyo, Japan) for 24 h. The bacterial cell density was adjusted to 1.0×1010 colony-forming units (CFU)/ml and then heat-killed at 60˚C for 1 h.
Mice and inoculation with F. nucleatum. Male C57BL/6J mice were purchased from CLEA Japan (Tokyo, Japan). All experimental procedures were approved by the Nihon University Animal Care and Use Committee (AP18DEN031). At 13 weeks of age, the mice were anesthetized with isoflurane and intratracheally inoculated with 50 μl with 1.0×108 CFU of F. nucleatum once per day for 7 days. At 1 day after the final inoculation of F. nucleatum, BALF was recovered, and lung tissues were collected.
Cell culture. Human alveolar epithelial (A549) cells were maintained in Dulbecco’s modified Eagle’s medium (DMEM; Sigma-Aldrich, St. Louis, MO, USA) supplemented with 10% fetal bovine serum, 100 U/ml penicillin, and 100 μg/ml streptomycin at 37˚C in an atmosphere of 5% CO2. For experiments, confluent A549 cells were prepared by seeding 24-well plates (2.5×105 cells/well) overnight. Before the addition of F. nucleatum, the cells were washed with serum-free DMEM, and then the medium was replaced with serum-free DMEM. Subsequently, A549 cells were treated with different numbers of F. nucleatum (1.0×107, 5.0×107, 1.0×108, CFU/ml) for 1 or 24 h. Phosphate-buffered saline (mock) was used as control.
Real-time quantitative polymerase chain reaction. Total RNA from mouse lung tissues or cultured cells were isolated using RNeasy Plus Mini Kit (Qiagen, Hilden, Germany). The RNA samples were reverse-transcribed using PrimeScript RT Master Mix (Takara Bio, Shiga, Japan). The primer sequences used for the amplification of each gene were as follows: Mouse Mmp9, forward 5’-GCCCTGGAACTCACAC GACA-3’ and reverse 5’-TTGGAAACTCACACGCCAGAAG-3’; mouse Actb, forward 5’-GGTCAGAAGGACTCCTATGTGG-3’ and reverse 5’-TGTCGTCCCAGTTGGTAACA-3’; human MMP9, forward 5’-ACTTTGACAGCGACAAGAAGTG-3’ and reverse 5’-GGCACTGAGGAATGATCTAAGC-3’; human glyceraldehyde 3-phosphate dehydrogenase (GAPDH), forward 5’-ACCAGCC CCAGCAAGAGCACAAG-3’ and reverse 5’-TTCAAGGGGTC TACATGGCAACTG-3’. The amplification and detection of the cDNA were accomplished using a TP-800 Thermal Cycler Dice Real-Time System (Takara Bio) with TB Grenn Premix Ex Taq (Takara Bio). Relative quantification of gene expression was determined by using the ΔΔCt method.
Western blotting analysis. BALF or A549 cell-culture supernatants were prepared, resolved by 5-20% sodium dodecyl sulphate-polyacrylamide gel electrophoresis, and transferred to polyvinylidene difluoride membranes (Millipore, Billerica, MA, USA). After non-specific binding was blocked by incubation of the membrane in 2% bovine serum albumin, the membrane was incubated with appropriate primary and secondary antibodies, washed thoroughly, and examined using ECL Prime (Cytiva, Tokyo, Japan). The primary antibodies were anti-MMP9 (Proteintech, Rosemont, IL, USA), anti-phospho-p38-MAPK, anti-p38-MAPK, anti-phospho-JNK, anti-JNK, anti-phospho-ERK1/2, anti-ERK1/2, anti-phospho-NF-ĸB p65, anti-NF-ĸB p65, anti-IĸBα (Cell Signaling Technology, Danvers, MA, USA), and anti-b-actin antibody (Santa Cruz Biotechnology, Santa Cruz, CA, USA). Horseradish peroxidase-linked anti-rabbit IgG and horseradish peroxidase-linked anti-mouse IgG (Thermo Fisher Scientific, Rockford, IL, USA) were used as the secondary antibodies. The bands were visualized using a ChemiDoc XRS System (Bio-Rad, Hercules, CA, USA).
Gelatin zymography. The experimental procedures for gelatin zymography were performed as described in a previous study (23). Briefly, BALF or the conditioned medium were mixed with non-reducing sample buffer and resolved by electrophoresis on a 7.5% sodium dodecyl sulfate polyacrylamide gel containing 0.4% gelatin as an MMP substrate. The gels were incubated with reaction buffer (50 mM Tris–HCl, 5 mM CaCl2, 1 μM ZnCl2, and 1% Triton X-100) at 37˚C for 48 h, gelatinolytic activities were visualized as clear bands against a blue background. Gelatinolytic activity was evaluated qualitatively.
Statistical analysis. All experiments were repeated at least three times. Controls used were either phosphate-buffered saline (mock)-treated samples or a combination of DMSO and mock-treated samples. Statistical analyses were performed using KaleidaGraph (Synergy Software, Reading, PA, USA). Student’s t-test was used for comparing the means of two groups. For comparisons of more than two groups, one-way analysis of variance with Tukey’s multiple comparison test was used. Differences were regarded as significant at p<0.05. The data are expressed as the mean±standard error of the mean.
Results
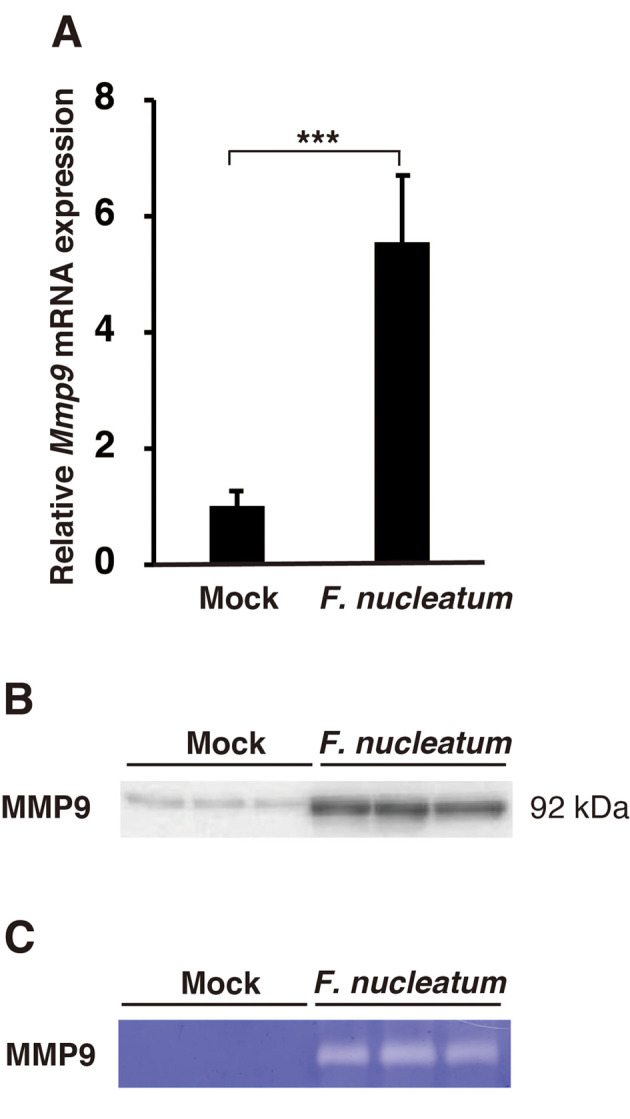
Effects of F. nucleatum on MMP9 expression and activity in mice. We investigated whether the intratracheal administration of F. nucleatum affected MMP9 mRNA expression in the mouse lung from one day after the final administration of F. nucleatum. As shown in Figure 1A, F. nucleatum-induced the expression of Mmp9 mRNA (5.54 ± 1.15-fold increase). Next, MMP9 protein expression in BALF was confirmed by western blotting (Figure 1B). The MMP9 protein level was increased by the administration of F. nucleatum. In addition, using gelatin zymography, we observed that MMP9 activity in BALF was increased by the administration of F. nucleatum (Figure 1C).
Figure 1. Effects of Fusobacterium nucleatum on matrix metalloproteinase 9 (MMP9) expression in vivo. The mice were intratracheally administered either phosphate-buffered saline or F. nucleatum (1.0×108 CFU/mouse) once per day for 7 days. A: The lungs were harvested at 1 day after the final administration of F. nucleatum. Mmp9 mRNA expression in the lung was determined by real-time polymerase chain reaction. The dats are presented as the mean±standard error of the mean; n=6. ***Significantly different at p<0.001. Bronchoalveolar lavage fluid (BALF) was collected at 1 day after phosphate-buffered saline or the final inoculation of F. nucleatum and analyzed by western blotting for MMP9 expression (B) and by gelatin zymography for MMP9 activity (C). Mock-treated samples were used as a control.
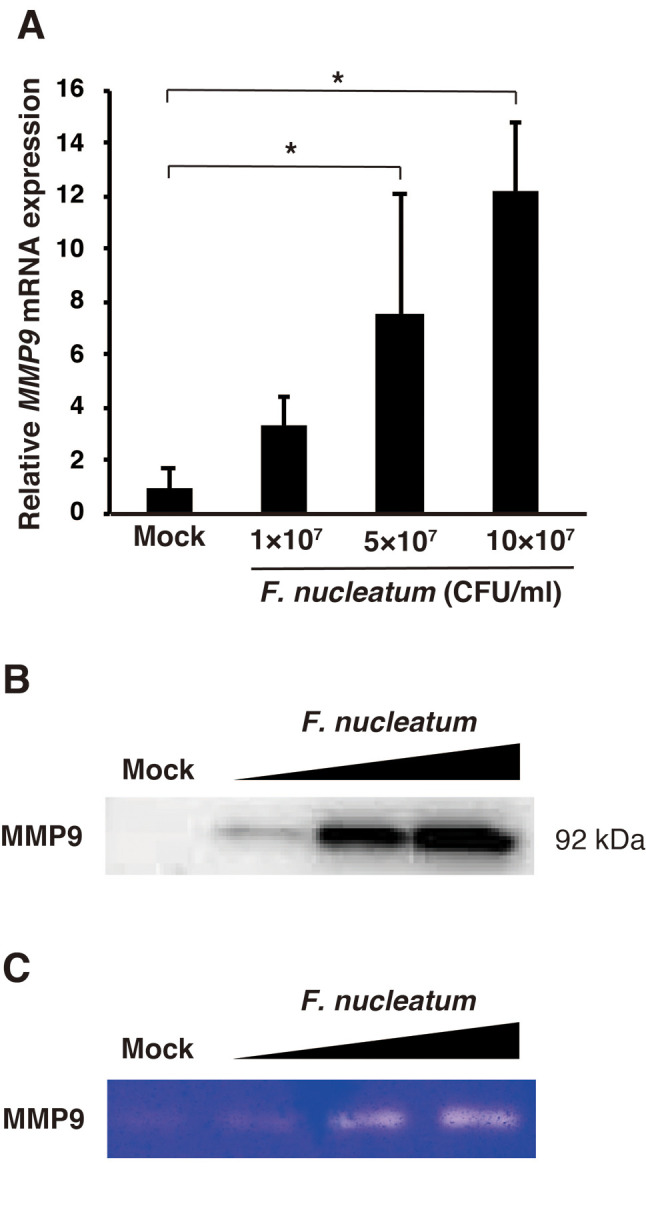
Effects of F. nucleatum on MMP9 expression and activity in A549 cells. Pulmonary epithelial cells are a major source of MMP9 (24). To investigate whether F. nucleatum up-regulated MMP9 mRNA in A549 cells, we quantified the expression of MMP9 mRNA using real-time polymerase chain reaction. A dose-dependent increase in MMP9 mRNA expression was observed, with the mRNA expression increased by 12.27±2.54-fold at 10×107 CFU/ml (Figure 2A). Western blotting analysis showed that the protein level of MMP9 in the conditioned medium from F. nucleatum-treated A549 cells was increased (Figure 2B). In addition, gelatin zymographic analysis revealed that F. nucleatum-induced MMP9 activity (Figure 2C).
Figure 2. Effect of Fusobacterium nucleatum on MMP9 expression in vitro. A549 cells were treated with 1.0×107, 5.0×107, or 1.0×108 CFU/ml F. nucleatum for 24 h. A: MMP9 mRNA expression was determined by real-time polymerase chain reaction. The data are presented as the mean±standard error of the mean; n=3. *Significantly different at p<0.05. The conditioned medium was analyzed by western blotting for MMP9 expression (B) and by gelatin zymography for MMP9 activity (C). Mock-treated samples were used as a control.
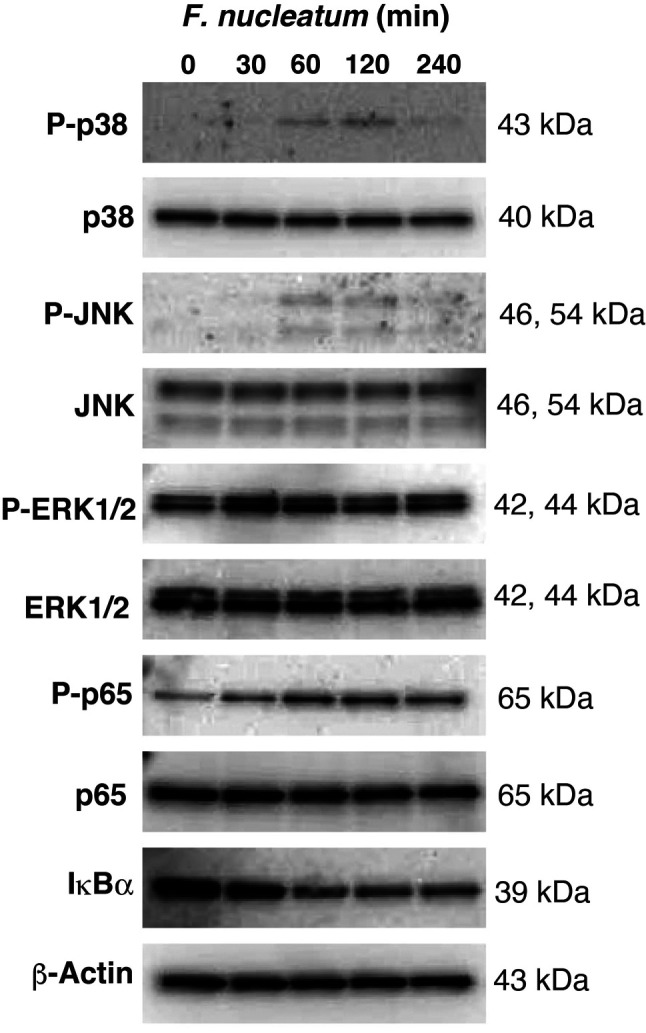
F. nucleatum induces activation of MAPKs and NF-ĸB. Several studies have indicated that F. nucleatum induces the expression of many genes via the activation of MAPKs and NF-ĸB in gingival fibroblasts and macrophages (25,26). However, nothing is known about whether F. nucleatum activates MAPKs and NF-ĸB in respiratory cells. We therefore examined whether F. nucleatum could activate MAPKs and NF-ĸB in A549 cells. As shown in Figure 3, F. nucleatum increased the phosphorylation of p38-MAPK, JNK, and ERK1/2. NF-ĸB is usually sequestered in the cytoplasm by inhibitor of ĸB α (IĸBα). NF-ĸB activation requires the degradation of IĸBα; this results in the transport of NF-ĸB p65/50 into the nucleus. In addition to nuclear translocation, the phosphorylation of NF-ĸB p65 is also essential for its maximal transcriptional activity (27). As shown in the lower panel of Figure 3, F. nucleatum caused the phosphorylation of p65 and the degradation of IĸBα after 60 min of stimulation. Our data suggest that F. nucleatum activated MAPKs and NF-ĸB in A549 cells.
Figure 3. Effects of Fusobacterium nucleatum on the activation of mitogen-activated protein kinases (MAPKs) and nuclear factor-kappa B (NF-ĸB). A549 cells were treated with 1.0×108 CFU/ml F. nucleatum for the indicated time. Whole cell extracts were prepared and subjected to western blotting with p38-MAPK, phosphorylated p38-MAPK, c-Jun N-terminal protein kinase (JNK), phosphorylated JNK, extracellular signal-regulated kinase 1/2 (ERK1/2), phosphorylated ERK1/2, NF-ĸB p65, phosphorylated NF-ĸB p65, and NF-ĸB inhibitor IĸBα.
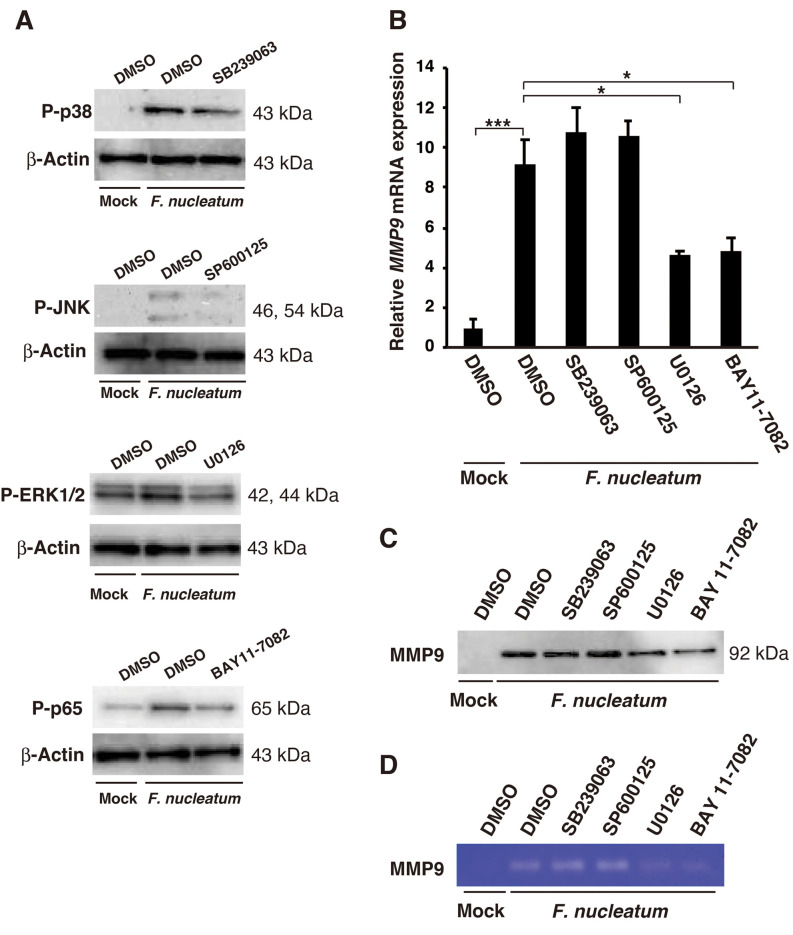
Involvement of ERK and NF-ĸB pathways in F. nucleatum-induced MMP9 expression. The involvement of the signaling pathways in F. nucleatum-induced MMP9 expression was explored by using MAPKs and NF-ĸB inhibitors, including SB239063 (p38-MAPK inhibitor), SP600125 (JNK inhibitor), U0126 (ERK1/2 inhibitor), and BAY11-7082 (NF-ĸB inhibitor). The F. nucleatum-induced phosphorylation of p38-MAPK, JNK, ERK, and NF-ĸB p65 was reduced by SB239063, SP600125, U0126, and BAY11-7082, respectively (Figure 4A). As shown in Figure 4B, U0126 and BAY11-7082 reduced F. nucleatum-induced MMP9 mRNA expression. In addition, U0126 and BAY11-7082 reduced F nucleatum-induced MMP9 expression and activity, as determined by western blotting (Figure 4C) and gelatin zymographic analysis (Figure 4D). In comparison, SB239063 and SP600125 had a negligible effect on F. nucleatum-induced MMP9 expression.
Figure 4. Effects of various inhibitors on Fusobacterium nucleatum-induced matrix metalloproteinase 9 (MMP9) expression. A: A549 cells were pre-treated with 10 μM SB239063 (p38-MAPK inhibitor), 10 μM SP600125 (JNK inhibitor), 10 μM U0126 (ERK1/2), and 10 μM BAY11-7082 (NF-ĸB inhibitor) for 1 h, and then treated with 1.0×108 CFU/ml F. nucleatum for 1 h. p38-MAPK, c-Jun N-terminal protein kinase (JNK), extracellular signaling-regulated kinase 1/2 (ERK1/2), and NF-ĸB p65 phosphorylation was examined via western blotting. B: A549 cells were pre-treated with 10 μM SB239063, 10 μM SP600125, 10 μM U0126, and 10 μM BAY11-7082 for 1 h, and then treated with 1.0×108 CFU/ml F. nucleatum for 24 h. MMP9 mRNA expression was determined by real-time polymerase chain reaction. The data are presented as the mean±standard error of the mean; n=3. Significantly different at *p<0.05 and ***p<0.001. The conditioned medium was analyzed by western blotting for MMP9 expression (C) and by gelatin zymography for MMP9 activity (D). DMSO and mock-treated samples were used as controls.
Discussion
Periodontal pathogens, including F. nucleatum, are probably associated with pulmonary diseases, such as pneumonia and COPD exacerbation (11,28,29). However, little is known about the mechanism through which periodontal bacteria are involved in the pathogenesis of pulmonary diseases. In this study, we attempted to elucidate the effect of F. nucleatum on MMP9 expression in mouse lung and A549 cells. The intratracheal administration of F. nucleatum to mice induced MMP9 expression. In A549 cells, F. nucleatum increased MMP9 expression and intracellular signaling via the activation of ERK and NF-ĸB were involved in F. nucleatum-induced MMP9 expression. These observations suggest that F. nucleatum may play a role in the development of pulmonary diseases through MMP9 expression.
MMP9 expression is up-regulated in patients with pneumonia (1,30), COPD, and asthma (19). The augmentation of MMP9 expression leads to the degradation of components of the lung extracellular matrix, such as elastin and collagen (1). In addition, MMP9 cleaves interleukin-8 (IL8) and IL1β into their active forms, affecting pathological processes (31). Major respiratory pathogens, including Streptococcus pneumoniae, Staphylococcus aureus, and Haemophilus influenzae, have the capacity to induce MMP9 production (32,33). These observations suggest that F. nucleatum-induced MMP9 expression is associated with the development of pulmonary diseases.
F. nucleatum has been shown to activate MAPKs (p38-MAPK, JNK, and ERK) and the NF-ĸB pathway in gingival fibroblasts and macrophages (25,26); in this study, the same findings were shown in lung epithelial cells. Although MMP9 expression appears to be regulated by all three of these MAPKs and NF-ĸB, nothing is known about the signaling pathways that mediate MMP9 expression induced by F. nucleatum in epithelial lung cells. In the current study, the specific inhibitors of ERK1/2 (U0126) and NF-ĸB (BAY 11-7082) effectively reduced F. nucleatum-induced MMP9 expression. In contrast, specific inhibitors of p38-MAPK (SB239063) and JNK (SP600125) did not alter F. nucleatum-induced MMP9 expression. These data suggest that ERK1/2 and NF-ĸB signaling are involved in F. nucleatum-induced MMP9 expression in A549 cells. IL1β-induced MMP9 expression was shown to be mediated through the activation of all three MAPKs and NF-ĸB in A549 cells (20). In contrast, although tumor necrosis factor-α can activate all these three MAPKs and NF-ĸB in A549 cells, it induced MMP9 expression via JNK, ERK1/2, and NF-ĸB signaling, but not via p38-MAPK signaling (34). Therefore, the signaling pathway inducing MMP9 expression may be different depending on the stimulus. Further studies are required to elucidate the signaling pathway for F. nucleatum-induced MMP9 expression in the lung in vivo.
The most recent evidence supports the idea that periodontal disease and poor oral hygiene are risk factors for the development of pneumonia and contribute to COPD exacerbation (2,5). Periodontal treatment resulted in a significantly lower risk of pneumonia and frequency of COPD exacerbation (35,36). Periodontopathic bacteria have been reported as etiological pathogens of pulmonary diseases. In particular, F. nucleatum was frequently detected in the lower airway tract from patients with pneumonia patients and in those with lung abscesses (13,14,37). In addition, the level of sputum antibodies against F. nucleatum were markedly elevated in patients with COPD exacerbation (29). Thus, F. nucleatum may be associated with the risk of pulmonary disease development. The salivary levels of F. nucleatum were increased in patients with periodontal diseases (38). Hence, it is quite possible that in patients with periodontal disease, aspiration of saliva, including F. nucleatum, may play an important role in the pathogenesis of pulmonary diseases. It appears that good oral hygiene and healthy periodontal conditions may prevent or reduce pulmonary diseases.
A major limitation of our study was that we used only heat-killed whole bacterial cells to investigate the effects of F. nucleatum on MMP9 expression. F. nucleatum expresses several virulence factors, including adhesins, lipopoly-saccharide, and butyrate (39). In particular, fusobacterial adhesin FadA has been identified to bind host cells and to trigger the host immune responses (11,39). Although we were able to verify the ability of F. nucleatum to induce MMP9 expression, further study is needed to identify, in detail, the mechanism of F. nucleatum-induced MMP9 expression that focuses on F. nucleatum virulence factors.
Although further studies are needed, this study has provided evidence suggesting that F. nucleatum, which can be aspirated into the lung, may cause the development and progression of pulmonary diseases through MMP9 expression. Pulmonary diseases can be prevented by periodontal treatment and professional oral care (10,35,36); therefore, it is considered that practicing good oral health to reduce the aspiration of oral bacteria into the lung can potentially prevent the development or progression of pulmonary diseases.
Conflicts of Interest
The Authors declare no conflicts of interest.
Authors’ Contributions
RS, KS and NK designed and performed the experiments, analyzed the data, and wrote the article. SM, YG, TK, and YY contributed to the discussion, analyzed the data, and reviewed drafts of the article. KI was responsible for the study concept and article writing.
Acknowledgements
This work was supported by JSPS KAKENHI Grant Number JP21K10265, Sato Fund of Nihon University School of Dentistry, Uemura Fund of Nihon University School of Dentistry, Grant from Dental Research Center, Nihon University School of Dentistry, and Nihon University Multidisciplinary Research Grant for 2021-2022.
References
- 1.Hendrix AY, Kheradmand F. The role of matrix metalloproteinases in development, repair, and destruction of the lungs. Prog Mol Biol Transl Sci. 2017;148:1–29. doi: 10.1016/bs.pmbts.2017.04.004. [DOI] [PubMed] [Google Scholar]
- 2.Scannapieco FA, Cantos A. Oral inflammation and infection, and chronic medical diseases: implications for the elderly. Periodontol 2000. 2016;72(1):153–175. doi: 10.1111/prd.12129. [DOI] [PubMed] [Google Scholar]
- 3.Celli BR, Wedzicha JA. Update on clinical aspects of chronic obstructive pulmonary disease. N Engl J Med. 2019;381(13):1257–1266. doi: 10.1056/NEJMra1900500. [DOI] [PubMed] [Google Scholar]
- 4.Pihlstrom BL, Michalowicz BS, Johnson NW. Periodontal diseases. Lancet. 2005;366(9499):1809–1820. doi: 10.1016/S0140-6736(05)67728-8. [DOI] [PubMed] [Google Scholar]
- 5.Kelly N, El Karim I. Periodontitis may be associated with respiratory diseases such as asthma, COPD, and pneumonia. J Evid Based Dent Pract. 2020;20(4):101498. doi: 10.1016/j.jebdp.2020.101498. [DOI] [PubMed] [Google Scholar]
- 6.Zeng XT, Tu ML, Liu DY, Zheng D, Zhang J, Leng W. Periodontal disease and risk of chronic obstructive pulmonary disease: a meta-analysis of observational studies. PLoS One. 2012;7(10):e46508. doi: 10.1371/journal.pone.0046508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu Z, Zhang W, Zhang J, Zhou X, Zhang L, Song Y, Wang Z. Oral hygiene, periodontal health and chronic obstructive pulmonary disease exacerbations. J Clin Periodontol. 2012;39(1):45–52. doi: 10.1111/j.1600-051X.2011.01808.x. [DOI] [PubMed] [Google Scholar]
- 8.Hayata M, Watanabe N, Tamura M, Kamio N, Tanaka H, Nodomi K, Miya C, Nakayama E, Ueda K, Ogata Y, Imai K. The periodontopathic bacterium fusobacterium nucleatum induced proinflammatory cytokine production by human respiratory epithelial cell lines and in the lower respiratory organs in mice. Cell Physiol Biochem. 2019;53(1):49–61. doi: 10.33594/000000120. [DOI] [PubMed] [Google Scholar]
- 9.Koike R, Cueno ME, Nodomi K, Tamura M, Kamio N, Tanaka H, Kotani A, Imai K. Heat-killed Fusobacterium nucleatum triggers varying heme-related inflammatory and stress responses depending on primary human respiratory epithelial cell type. Molecules. 2020;25(17):3839. doi: 10.3390/molecules25173839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Takahashi Y, Watanabe N, Kamio N, Kobayashi R, Iinuma T, Imai K. Aspiration of periodontopathic bacteria due to poor oral hygiene potentially contributes to the aggravation of COVID-19. J Oral Sci. 2020;63(1):1–3. doi: 10.2334/josnusd.20-0388. [DOI] [PubMed] [Google Scholar]
- 11.Han YW. Fusobacterium nucleatum: a commensal-turned pathogen. Curr Opin Microbiol. 2015;23:141–147. doi: 10.1016/j.mib.2014.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bartlett JG. How important are anaerobic bacteria in aspiration pneumonia: when should they be treated and what is optimal therapy. Infect Dis Clin North Am. 2013;27(1):149–155. doi: 10.1016/j.idc.2012.11.016. [DOI] [PubMed] [Google Scholar]
- 13.Yamasaki K, Kawanami T, Yatera K, Fukuda K, Noguchi S, Nagata S, Nishida C, Kido T, Ishimoto H, Taniguchi H, Mukae H. Significance of anaerobes and oral bacteria in community-acquired pneumonia. PLoS One. 2013;8(5):e63103. doi: 10.1371/journal.pone.0063103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nagaoka K, Yanagihara K, Harada Y, Yamada K, Migiyama Y, Morinaga Y, Izumikawa K, Kohno S. Quantitative detection of periodontopathic bacteria in lower respiratory tract specimens by real-time PCR. J Infect Chemother. 2017;23(2):69–73. doi: 10.1016/j.jiac.2016.09.013. [DOI] [PubMed] [Google Scholar]
- 15.Pragman AA, Kim HB, Reilly CS, Wendt C, Isaacson RE. The lung microbiome in moderate and severe chronic obstructive pulmonary disease. PLoS One. 2012;7(10):e47305. doi: 10.1371/journal.pone.0047305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee SW, Kuan CS, Wu LS, Weng JT. Metagenome and metatranscriptome profiling of moderate and severe COPD sputum in Taiwanese Han males. PLoS One. 2016;11(7):e0159066. doi: 10.1371/journal.pone.0159066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chiang TY, Tsao SM, Yeh CB, Yang SF. Matrix metalloproteinases in pneumonia. Clin Chim Acta. 2014;433:272–277. doi: 10.1016/j.cca.2014.03.031. [DOI] [PubMed] [Google Scholar]
- 18.Klein T, Bischoff R. Physiology and pathophysiology of matrix metalloproteases. Amino Acids. 2011;41(2):271–290. doi: 10.1007/s00726-010-0689-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Grzela K, Litwiniuk M, Zagorska W, Grzela T. Airway remodeling in chronic obstructive pulmonary disease and asthma: the role of matrix metalloproteinase-9. Arch Immunol Ther Exp (Warsz) 2016;64(1):47–55. doi: 10.1007/s00005-015-0345-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lin CC, Kuo CT, Cheng CY, Wu CY, Lee CW, Hsieh HL, Lee IT, Yang CM. IL-1 beta promotes A549 cell migration via MAPKs/AP-1- and NF-kappaB-dependent matrix metalloproteinase-9 expression. Cell Signal. 2009;21(11):1652–1662. doi: 10.1016/j.cellsig.2009.07.002. [DOI] [PubMed] [Google Scholar]
- 21.Inaba H, Sugita H, Kuboniwa M, Iwai S, Hamada M, Noda T, Morisaki I, Lamont RJ, Amano A. Porphyromonas gingivalis promotes invasion of oral squamous cell carcinoma through induction of proMMP9 and its activation. Cell Microbiol. 2014;16(1):131–145. doi: 10.1111/cmi.12211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kumagai K, Ohno I, Okada S, Ohkawara Y, Suzuki K, Shinya T, Nagase H, Iwata K, Shirato K. Inhibition of matrix metalloproteinases prevents allergen-induced airway inflammation in a murine model of asthma. J Immunol. 1999;162(7):4212–4219. [PubMed] [Google Scholar]
- 23.Nakai K, Kawato T, Morita T, Iinuma T, Kamio N, Zhao N, Maeno M. Angiotensin II induces the production of MMP-3 and MMP-13 through the MAPK signaling pathways via the AT(1) receptor in osteoblasts. Biochimie. 2013;95(4):922–933. doi: 10.1016/j.biochi.2012.12.016. [DOI] [PubMed] [Google Scholar]
- 24.Hozumi A, Nishimura Y, Nishiuma T, Kotani Y, Yokoyama M. Induction of MMP-9 in normal human bronchial epithelial cells by TNF-alpha via NF-kappa B-mediated pathway. Am J Physiol Lung Cell Mol Physiol. 2001;281(6):L1444–L1452. doi: 10.1152/ajplung.2001.281.6.L1444. [DOI] [PubMed] [Google Scholar]
- 25.Kang W, Jia Z, Tang D, Zhang Z, Gao H, He K, Feng Q. Fusobacterium nucleatum facilitates apoptosis, ROS generation, and inflammatory cytokine production by activating AKT/MAPK and NF-ĸB signaling pathways in human gingival fibroblasts. Oxid Med Cell Longev. 2019;2019:1681972. doi: 10.1155/2019/1681972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Park SR, Kim DJ, Han SH, Kang MJ, Lee JY, Jeong YJ, Lee SJ, Kim TH, Ahn SG, Yoon JH, Park JH. Diverse Toll-like receptors mediate cytokine production by Fusobacterium nucleatum and Aggregatibacter actinomycetemcomitans in macrophages. Infect Immun. 2014;82(5):1914–1920. doi: 10.1128/IAI.01226-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hayden MS, Ghosh S. Signaling to NF-kappaB. Genes Dev. 2004;18(18):2195–2224. doi: 10.1101/gad.1228704. [DOI] [PubMed] [Google Scholar]
- 28.Scannapieco FA. Role of oral bacteria in respiratory infection. J Periodontol. 1999;70(7):793–802. doi: 10.1902/jop.1999.70.7.793. [DOI] [PubMed] [Google Scholar]
- 29.Brook I, Frazier EH. Immune response to Fusobacterium nucleatum and Prevotella intermedia in the sputum of patients with acute exacerbation of chronic bronchitis. Chest. 2003;124(3):832–833. doi: 10.1378/chest.124.3.832. [DOI] [PubMed] [Google Scholar]
- 30.Yang SF, Chu SC, Chiang IC, Kuo WF, Chiou HL, Chou FP, Kuo WH, Hsieh YS. Excessive matrix metalloproteinase-9 in the plasma of community-acquired pneumonia. Clin Chim Acta. 2005;352(1-2):209–215. doi: 10.1016/j.cccn.2004.09.025. [DOI] [PubMed] [Google Scholar]
- 31.Chakrabarti S, Patel KD. Matrix metalloproteinase-2 (MMP-2) and MMP-9 in pulmonary pathology. Exp Lung Res. 2005;31(6):599–621. doi: 10.1080/019021490944232. [DOI] [PubMed] [Google Scholar]
- 32.Vissers M, Hartman Y, Groh L, de Jong DJ, de Jonge MI, Ferwerda G. Recognition of Streptococcus pneumoniae and muramyl dipeptide by NOD2 results in potent induction of MMP-9, which can be controlled by lipopolysaccharide stimulation. Infect Immun. 2014;82(12):4952–4958. doi: 10.1128/IAI.02150-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Saliu F, Rizzo G, Bragonzi A, Cariani L, Cirillo DM, Colombo C, Daccò V, Girelli D, Rizzetto S, Sipione B, Cigana C, Lorè NI. Chronic infection by nontypeable Haemophilus influenzae fuels airway inflammation. ERJ Open Res. 2021;7(1):00614–2020. doi: 10.1183/23120541.00614-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lin CC, Tseng HW, Hsieh HL, Lee CW, Wu CY, Cheng CY, Yang CM. Tumor necrosis factor-alpha induces MMP-9 expression via p42/p44 MAPK, JNK, and nuclear factor-kappaB in A549 cells. Toxicol Appl Pharmacol. 2008;229(3):386–398. doi: 10.1016/j.taap.2008.01.032. [DOI] [PubMed] [Google Scholar]
- 35.Yang LC, Suen YJ, Wang YH, Lin TC, Yu HC, Chang YC. The association of periodontal treatment and decreased pneumonia: a nationwide population-based cohort study. Int J Environ Res Public Health. 2020;17(1):356. doi: 10.3390/ijerph17010356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhou X, Han J, Liu Z, Song Y, Wang Z, Sun Z. Effects of periodontal treatment on lung function and exacerbation frequency in patients with chronic obstructive pulmonary disease and chronic periodontitis: a 2-year pilot randomized controlled trial. J Clin Periodontol. 2014;41(6):564–572. doi: 10.1111/jcpe.12247. [DOI] [PubMed] [Google Scholar]
- 37.Ikegami H, Noguchi S, Fukuda K, Akata K, Yamasaki K, Kawanami T, Mukae H, Yatera K. Refinement of microbiota analysis of specimens from patients with respiratory infections using next-generation sequencing. Sci Rep. 2021;11(1):19534. doi: 10.1038/s41598-021-98985-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Saygun I, Nizam N, Keskiner I, Bal V, Kubar A, Açıkel C, Serdar M, Slots J. Salivary infectious agents and periodontal disease status. J Periodontal Res. 2011;46(2):235–239. doi: 10.1111/j.1600-0765.2010.01335.x. [DOI] [PubMed] [Google Scholar]
- 39.de Andrade KQ, Almeida-da-Silva CLC, Coutinho-Silva R. Immunological pathways triggered by Porphyromonas gingivalis and Fusobacterium nucleatum: Therapeutic possibilities. Mediators Inflamm. 2019;2019:7241312. doi: 10.1155/2019/7241312. [DOI] [PMC free article] [PubMed] [Google Scholar]