Abstract
Background
The genetic engineering of crops has enhanced productivity in the face of climate change and a growing global population by conferring desirable genetic traits, including the enhancement of biotic and abiotic stress tolerance, to improve agriculture. The clustered regularly interspaced short palindromic repeats (CRISPR/Cas9) system has been found to be a promising technology for genomic editing. Protoplasts are often utilized for the development of genetically modified plants through in vitro integration of a recombinant DNA fragment into the plant genome. We targeted the citrus Nonexpressor of Pathogenesis-Related 3 (CsNPR3) gene, a negative regulator of systemic acquired resistance (SAR) that governs the proteasome-mediated degradation of NPR1 and developed a genome editing technique targeting citrus protoplast DNA to produce stable genome-edited citrus plants.
Results
Here, we determined the best cationic lipid nanoparticles to deliver donor DNA and described a protocol using Lipofectamine™ LTX Reagent with PLUS Reagent to mediate DNA delivery into citrus protoplasts. A Cas9 construct containing a gRNA targeting the CsNPR3 gene was transfected into citrus protoplasts using the cationic lipid transfection agent Lipofectamine with or without polyethylene glycol (PEG, MW 6000). The optimal transfection efficiency for the encapsulation was 30% in Lipofectamine, 51% in Lipofectamine with PEG, and 2% with PEG only. Additionally, plasmid encapsulation in Lipofectamine resulted in the highest cell viability percentage (45%) compared with PEG. Nine edited plants were obtained and identified based on the T7EI assay and Sanger sequencing. The developed edited lines exhibited downregulation of CsNPR3 expression and upregulation of CsPR1.
Conclusions
Our results demonstrate that utilization of the cationic lipid-based transfection agent Lipofectamine is a viable option for the successful delivery of donor DNA and subsequent successful genome editing in citrus.
Supplementary Information
The online version contains supplementary material available at 10.1186/s13007-022-00870-6.
Keywords: Citrus, Protoplast, CRISPR/Cas9, Genome editing, Lipofection, Systemic acquired resistance (SAR), NPR3
Background
Citrus is an important fruit crop grown globally with many health benefits. However, it is also among the most difficult plants to improve through traditional breeding approaches due to its complex reproductive biology [1]. Because they lack pathogen resistance, commercial sweet orange cultivars are vulnerable to a plethora of diseases, including citrus canker resulting from Xanthomonas axonopodis infection and Huanglongbing (HLB) from the suspected causal agent Candidatus Liberibacter asiaticus (CaLas), [2]. Genetic improvement of citrus using pathogen resistance genes that enhance plant defense mechanisms utilizing systemic acquired resistance (SAR) has resulted in the production of transgenic canker-resistant trees [3]. SAR employs the plant’s inherent pathogen defense system expressing specific defense genes to attenuate disease development and reduce pathogen spread [4]. The most well-studied regulator of SAR, Nonexpressor of PR1 (NPR1), is also one of the most heavily researched transcriptional coactivators in the SA-dependent signaling pathway [4, 5]. NPR3 represses NPR1 function by enhancing NPR1 degradation and decreasing defense pathway activation [6].
Genome editing by CRISPR/Cas9 is an emerging technology in plant improvement genetics. Using the Cas9 protein, we can precisely modify a targeted location by deletion, replacement, or insertion to develop novel traits. The application of genome editing requires synthetic nucleases that introduce double-strand breaks (DBSs) into a specified region of the genome and the natural DNA repair machinery that causes modifications [7]. Although there has been tremendous progress in plant development using CRISPR/Cas9, substantial efforts are still needed in this area to develop new methodologies that enhance transformation efficiency. CRISPR plasmid delivery into plant cells has also been limited to Agrobacterium-mediated delivery or particle bombardment [8]. However, challenges remain, such as the cell’s recalcitrance to gene editing, its inefficiency, or the difficulty of cell regeneration. CRISPR/Cas9-modified protoplasts are a viable option to modify citrus plants.
Protoplasts are often utilized for the development of genetically modified plants by the in vitro integration of recombinant DNA (either plasmid-based or linear) into the plant genome. The polyethylene glycol (PEG)-mediated method is widely used for protoplasts; however, it has a low transformation efficiency [9]. This low transformation efficiency occurs for many reasons, including the endosomal entrapment of donor DNA [10–12], degradation of donor DNA by cytosolic nucleases [13], or limitation of nuclear translocation due to the charge or size of the donor DNA [14, 15]. Other factors affecting the transformation of citrus protoplasts include the sensitivity of protoplasts to osmotic stress, PEG cytotoxicity, the type of plasmid construct, the physiological conditions of the cultivar, the low yield of protoplasts, and enzymatic degradation of the DNA construct prior to nuclear translocation due to prolonged exposure of plasmid DNA to the cytoplasm [16].
Novel methods to overcome cellular challenges can increase protoplast transformation efficiency and result in more viable cells with integrated donor DNA in their genomes. Donor DNA, siRNA, and proteins have been successfully delivered into animal and plant cells using viral vectors, lipofections and nanoparticles [17–21]. Lipofection (or liposome transfection) is a technique for delivering genetic material into a cell via liposomes, which can easily merge with the cell membrane since they are both made of a phospholipid bilayer. Nanostructured lipid carriers (NLCs), with a size ≤ 100 nm, are a potential delivery system that may have some advantages in certain circumstances when compared with other colloidal carriers [22]. Nanostructured lipid carriers have been utilized to encapsulate and deliver DNA to protect it from cellular nucleases, thus enhancing translocation of the DNA into the nucleus [23, 24]. The use of these chemicals depends on their ability to form lipoplexes with negatively charged donor DNA molecules [25, 26]. Lipofection uses cationic lipids to completely neutralize the charge of the CRISPR reagent. This results in a net positive charge that can relatively easily associate with the negatively charged surface of cells, resulting in uptake and intracellular distribution of the complex [27, 28]. However, this approach still needs optimization and is likely plant- and tissue type-dependent.
In addition to nanoparticles, cell penetrating peptides (CPPs) can also aid in the delivery of donor DNA into cells [29, 30]. CPPs are short peptides of no more than 30 hydrophobic amino acids [31]. Arginine-rich peptides and amphipathic peptides are the two most widely used peptides that aid in efficient DNA contract translocation into the nucleus [32–34]. These arginine-rich peptides have also been used in plant systems for protein delivery into onion (Allium cepa L.) and tomato (Solanum lycopersicum L.) [35] and for delivery of plasmid DNA into the nucleus of intact mung bean roots (Vigna radiata L.) and soybean (Glycine max L.) [36]. CPPs can be used on their own or in conjunction with polymer-based or lipid-based nanoparticles [37, 38]. CRISPR/Cas9 editing technology has been successfully utilized in citrus cells [39]. However, genome editing of citrus protoplasts is challenging, and stable genome-edited citrus using protoplasts has not been previously produced. Previous methods using PEG have proven to be cytotoxic to protoplasts [40]; therefore, new methods of transfection are needed to improve transfection efficiency without sacrificing cell health.
In this study we outline a new protocol for protoplast transfection using Lipofectamine™ LTX Reagent with PLUS Reagent to ensure donor DNA delivery in sweet orange protoplasts with low cytotoxic effects on the cells. We utilized this newly developed protocol to successfully edit the CsNPR3 gene using a CRISPR/Cas9 construct and regenerate stable genome edited sweet orange citrus plants.
Methods
Cell culture and protoplast isolation
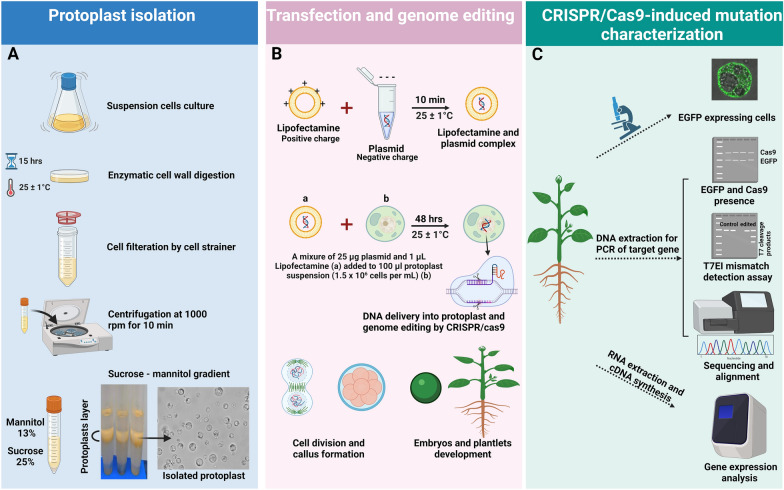
All the experiments in this study were conducted using the sweet orange (Citrus sinensis L. Osbeck) cultivar ‘N7-3’. ‘N7-3’ is a seedless sweet orange, which restricts its use in other forms of juvenile transformation systems [41]. Embryogenic calli and suspension cultures were established and maintained as previously described [42, 43]. Suspended cells were subcultured at 14 day intervals in modified H + H medium [44]. Cells were digested in protoplast incubation medium containing 0.6 M mannitol, 10 mM CaCl2, 10 mM MES buffer, 0.75% (w/v) Cellulase Onozuka RS (Yakult Honsha, Tokyo, Japan), 0.75% (w/v) Macerozyme R-10 (Yakult Honsha, Tokyo, Japan), pH 5.6, at 25 ± 1 °C for 15 h. The digested cells were filtered by a sterile cell strainer with 100 µM nylon mesh (Thermo Fisher Scientific, Waltham, MA). Protoplasts were collected by centrifugation on a sucrose-mannitol gradient and maintained as outlined in Fig. 1. The protoplasts were counted with a hemocytometer and then diluted to the desired concentration with BH3 media [42].
Fig. 1.
Schematic illustration of protoplast isolation and CRISPR/Cas9-mediated genome-editing steps via Lipofectamine. Protoplasts isolated from in vitro grown cell suspension (A). Protoplast layer represents protoplasts between solutions of sucrose 25% and mannitol 13%. Bright field of protoplast 1 h after isolation. Mixing of lipofectamine and plasmid constructs targeting NPR3 and protoplast transfection (B). Characterization steps by monitoring EGFP expression and cas9 presence in the genomic DNA, T7EI assay and sanger sequencing and gene expression analysis (C). GFP-expressed cell was monitored by a confocal laser scanning microscope. The figure was created in BioRender.com
Plasmid construction
The CsNPR3 DNA sequence (orange 1.1g007849m) was identified from the Citrus sinensis genome assembly maintained in Phytozome (https://phytozome-next.jgi.doe.gov) by blasting AtNPR3 (AT5G45110) against the genome. A gRNA specific to CsNPR3 (TGATGAGAACACTGCAGTTGAGG) that was predicted to have low off-target effects was obtained using the online tool found at CRISPR-direct (http://crispr.dbcls.jp/ [45]). The chemically synthesized gRNA was placed under the control of the Arabidopsis U6–26 promoter in a transformation construct based on the pCAMBIA2300-EGFP vector [46] and containing the 35S—Cas9 transgene that had been codon optimized for Arabidopsis and contained two nuclear localization sequences—one at the 5ʹ end and the other at the 3ʹ end. Sanger sequencing was used to verify the resulting binary vector construct (pEN-NPR3) (Additional file 1: Fig. S1). NEB 10-beta competent E. coli (New England Biolabs, Ipswich, MA) was transformed with the binary vector and plated overnight to allow the bacteria to grow. Pure plasmid DNA was extracted using a Qiagen EndoFree Plasmid Maxi Kit (QIAGEN Sciences Inc., Germantown, MD) for all subsequent experiments.
Evaluation of cationic lipid transfection and cell-penetrating peptides (C3POs)
Cationic lipid transfection reagents were evaluated for their efficacy in protoplast transformation by observing enhanced green fluorescent protein (EGFP) expression and cell morphology. Protoplasts (1.5 × 106 cells per mL) were resuspended in a 1:1 (v:v) mixture of 0.6 M BH3 and 0.6 M EME sucrose in a Thermo Scientific™ BioLite 6-well plate (Thermo Fisher Scientific, Waltham, MA).
Several commercially available DNA transfection reagents were tested for DNA delivery into citrus protoplasts, including Chariot™ Protein Delivery Reagent (Active motif, Carlsbad, California), Escort™ (Sigma–Aldrich, St. Louis, MO), FuGENE® (Promega, Madison, Wisconsin, USA), Lipofectamine® 3000 (Thermo Fisher Scientific, Waltham, MA), Lipofectamine™ LTX with PLUS (Thermo Fisher Scientific, Waltham, MA), TransIT®-2020 (Mirus, Madison, WI), and Xfect™ (Takara Bio USA Inc., Mountain View, CA). All transfection reagents were initially evaluated according to the manufacturer’s standard protocol to determine the best cationic lipid for citrus protoplast transformation (Table 1). Lipofectamine LTX with PLUS reagent was subsequently chosen for the remainder of the study.
Table 1.
Different transfection reagents were assessed for transfection efficiency in protoplasts indicated by EGFP presence
| Transfection reagent | Concentrationa | EGFP presence | Cell health |
|---|---|---|---|
| Chariot™ | 0.25 µl | Yes | + b |
| Escort™ | 25 µl | No | + |
| FuGENE® | 5.0 µl | No | + + |
| Lipofectamine® 3000 | 0.5 µl | Yes | + + |
| Lipofectamine® LTX with plus™ reagent | 0.5 µl | Yes | + + + |
| TransIT®-2020 | 1.0 µl | No | + + |
| X-fect™ | 7.5 µl | No | + |
aRepresents the concentration of transfection reagents mixed with 25 µg of plasmid DNA/ 100 protoplast (1.5 × 106 cells per mL)
bThe effects of the transfection reagents on protoplast cell health and division were assessed. Poor (less than 20% intact protoplasts; +), good (over 50% intact protoplasts; + +), excellent (over 90% intact protoplasts; + + +)
Small cell-penetrating peptides (CPPs) have been shown to aid in transfection. Arginine9 conjugated with TAMARA (Arg9-TAMRA) was used to evaluate whether CPPs improved the transformation efficiency. A 1 mg/ml stock solution was made by dissolving Arg9-TAMRA (Anaspec, Fremont, CA) in deionized water and stored at − 20 °C. In a preliminary study, Lipofectamine was tested with or without Arg9-TAMRA. Lipofectamine LTX and PLUS (0.5%) were mixed with 25 µg of plasmid DNA. The mixture was incubated for 10 min at room temperature to allow for DNA-lipid complex (lipoplex) formation. A total of 90 μl of Arg9 was added to each well. Cells were maintained in the dark at 25 ± 1 °C for 24 h before evaluation.
Polyethylene glycol and lipofectamine-mediated CRISPR/Cas9 plasmid delivery
CRISPR/Cas9 plasmid delivery was tested with the use of Lipofectamine LTX Reagent with PLUS reagent (Thermo Fisher Scientific, Waltham, MA) alone or combined with polyethylene glycol (PEG). Lipofectamine LTX and PLUS (0.5%, 1%, 2%, or 4%) were mixed briefly with 25, 50, 75 and 100 µg of plasmid DNA, added to 100 µl of protoplasts at 1.5 × 106 cells per mL, and incubated for 10 min. When Lipofectamine was applied alone, the diluted transfection reagents were added to the tube containing the CRISPR/Cas9 plasmid, followed by incubation at room temperature for 10 min. Then, the mixture was applied to 100 µl of protoplasts, mixed gently and incubated for 48 h. After 48 h, the transfection reagents and plasmid were removed by centrifugation at 1000 rpm for 2 min. For PEG treatments, 100 µl of protoplasts was added to the transfection mixture and incubated at room temperature for 15 min. A total of 100 µl of freshly prepared PEG solution (60% (W/V) PEG 6000, 0.4 M mannitol, 0.1 M CaCl2) was added to the protoplasts in a 2.0 ml Eppendorf tube and incubated at room temperature for 25 min in the dark. After incubation, the protoplasts were washed twice with 500 µl of BH3 liquid media. The protoplasts were pelleted by centrifugation at 1000 rpm for 5 min. The protoplasts were gently resuspended in 700 µl of 0.6 M BH3 and 0.6 M EME sucrose at a 1:1 ratio in a small 60 × 15 mm Petri dish, and the dishes were kept in the dark at 25 ± 1 °C for 48 h before EGFP was observed to evaluate the transfection efficiency. Each experiment was repeated six times.
Protoplast regeneration and embryo initialization
Transfected protoplasts were resuspended in liquid media containing 0.6 M BH3:EME maltose at a 1:2 ratio and cultured for 30 days. The protoplasts were embedded in EME maltose semisolid medium containing 50 g/l maltose and 0.5 g/l malt extract and cultured in 25 ml petri dishes at 25 ± 1 °C in the dark. Subsequently, the cells were fed a few drops of the same media mixture (1:2) at three-week intervals. After 4–8 weeks, the cultures were transferred to the light (16 h light [30 μmol/m2/s] and 8 h darkness) and cultured at 25 ± 1 °C until microcallus and globular embryo formation. Induction of globular embryos was usually observed after approximately 1–2 months on EME-maltose medium.
Globular embryos visually observed to express EGFP were transferred to fresh semisolid EME-maltose medium and cultured over 0.2 µM sterilized cellulose acetate membrane filters to allow for embryo growth. Small torpedo stage transgenic embryos were subsequently enlarged on EME-1500 semisolid medium supplemented with 50 g/l sucrose and 1.5 g/l malt extract. For further embryo maturation and germination, the embryos were moved to B + medium containing 25 g/l sucrose, 0.10 µM NAA and 2.89 µM GA3. Shoots that formed were transferred for further root development and growth into RMAN rooting medium supplemented with 0.10 µM NAA. All plant growth regulator stocks were purchased from PhytoTech Labs, Lenexa, KS. Plantlets were subsequently micro grafted onto UFR15 rootstock [47] according to Dutt and Grosser [46]. Plants were acclimated following transfer to the greenhouse for further morphological, biochemical, and molecular analyses. All media formulations were prepared as previously outlined [42].
Protoplast viability evaluation and transformation efficiency
Protoplasts were observed for cell morphology and putative pEN-NPR3 plasmid transformation 48 h after transfection as indicated by EGFP expression under brightfield microscopy and EGFP fluorescence using a Zeiss Scope A1 fluorescence microscope (Carl Zeiss Microscopy, Gottingen, Germany) equipped with a FITC filter. Cell health was reported based on morphological observations in response to transfection reagents and was expressed qualitatively using the following symbols: + (poor; less than 20% intact protoplasts), + + (good, over 50% intact protoplasts) and + + + (excellent; over 90% intact protoplasts). A Leica SP8 confocal microscope (Leica Microsystems, Wetzlar, Germany) was used to visualize the penetration and localization of the ARG9-TAMRA CPP. Protoplast nuclei were counterstained with DAPI (0.5 mg/ml) diluted 1:250 in protoplast media. DAPI was visualized using a 405-diode laser, and the TAMRA signal was visualized using a HeNe 561 laser. Images were captured with Leica’s LASX software (Leica Microsystems, Wetzlar, Germany).
Viability was monitored, and images were recorded 48 h after transfection. The number of viable cells was counted using a Countess II automated cell counter (Thermo Fisher Scientific, Waltham, MA) to determine the cell viability percentage [Cell viability % = (Number of viable cells/Total number of cells) × 100]. The transformation efficiency was calculated as the percentage of EGFP-expressing cells out of the total number of viable cells within 48 h of each treatment. [Transformation efficiency % = (Number of EGFP-expressing cells/Total number of viable cells) × 100].
Detection of CRISPR/Cas9-induced mutations
Genomic DNA was isolated from the regenerated plants in three biological replicates using the GeneJET Plant Genomic DNA Purification Mini Kit (Thermo Fisher Scientific, Waltham, MA) following the manufacturer’s protocol. To confirm the presence of the Cas9 and EGFP transgenes in the putative edited embryos, duplex PCR was carried out in a thermal cycler (C1000; Bio–Rad Laboratories, Hercules, CA) using GoTaq Green Master PCR Mix (Promega Corp, Madison WI) and primers that amplified the Cas9 and EGFP genes.
Genomic DNA was used as a template for T7 endonuclease I (T7EI) mismatch detection assays. Primers were designed to amplify around the target region of CsNPR3 using 10 ng of plant genomic DNA by Q5 High-Fidelity DNA polymerase (Thermo Fisher Scientific, Waltham, MA). The PCR products were purified using a PureLink Quick PCR Purification Kit (Invitrogen, Thermo Fisher Scientific) and quantified using a NanoDrop 2000c spectrophotometer (Thermo Fisher Scientific, Waltham, MA). A T7EI assay was used to evaluate 1 μg of the purified PCR product. The T7EI assay was performed according to the manufacturer’s instructions (New England Biolabs, Ipswich, MA). Briefly, the PCR products were denatured at 95 °C, and reannealing was carried out by ramp PCR from 95 to 85 °C at − 2 °C/s and 85 to 25 °C at − 0.1 °C/s. These annealed PCR products were incubated with T7 endonuclease I (New England Biolabs, Ipswich, MA) at 37 °C for 15 min and analyzed via 2% (w/v) agarose gel electrophoresis. PCR products were cloned into the pJET plasmid vector using a CloneJET PCR Cloning Kit (Thermo Fisher Scientific, Waltham, MA) and sequenced by the Sanger method. Ten colonies for each sample were used for colony sequencing. The sequencing results were compared with the sequence of the CsNPR3 gene by alignment using the SnapGene program version 5.3.2 (SnapGene, San Diego, CA). All primer sequences are outlined in Additional file 1: Table S1.
Gene expression assessment
Total RNA was isolated from 100 mg of leaf tissues using the Direct-zol™ RNA Miniprep Plus Kit (Zymo Research, Tustin, CA) according to the manufacturer’s protocol. cDNA was synthesized at 42 °C for 60 min by the RevertAid First Strand cDNA Synthesis Kit (Thermo Fisher Scientific, Waltham, MA) and quantified by a Nanodrop 2000c spectrophotometer. For RT–qPCR, 40 ng cDNA was added to PowerUp SYBR Green Master Mix for a final volume of 20 μl (Applied Biosystems, Foster City, CA) according to the manufacturer’s instructions. Each sample was tested twice in three replicates, and the data were analyzed using Applied Biosystems software version 3.0.1 (Thermo Fisher Scientific, Applied Biosystems, Foster City, CA). The Ct value of the PCR curve was analyzed and compared with that of the wild type. The relative expression of the selected gene was calculated by the 2−ΔΔCT method [48]. Actin was used as a housekeeping gene to provide an endogenous control. Transcript levels of the CsNPR3 and CsPR1 genes were evaluated.
Statistical analysis
Analysis of variance (ANOVA) was conducted in JMP Pro version 15 (SAS Institute, Cary, NC, USA). The combinations of Lipofectamine and DNA concentrations were designed as a factorial experiment with two factors (Lipofectamine concentrations (four levels) and DNA concentrations (four levels)). The effect of transfection agents on the number of EGFP-positive cells and cell viability and the relative expression data were analyzed using one-way ANOVA. Each experiment was repeated six times with three replicates, and each replicate represented four plates. Tukey’s method was used to compare means among the samples. Differences were significant when p values were less than 0.05%.
Results
Lipoplex-mediated DNA delivery into citrus protoplasts
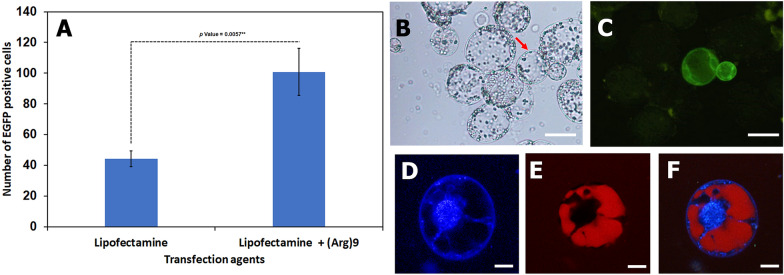
Several transfection reagents were evaluated for their transformation efficiency and their effects on cell health at different concentrations (Table 1). Based on these results, only 0.25 µl Chariot, 0.5 µl Lipofectamine 3000 and 0.5 µl Lipofectamine LTX mixed with 25 µg of plasmid DNA/100 protoplast (1.5 × 106 cells per mL) produced EGFP-expressing cells. Lipofectamine LTX outperformed the other transfection reagents, resulting in a high transformation efficiency without compromising cell health and producing over 90% intact protoplasts (Fig. 2). Cell-penetrating peptides have been shown to aid in donor DNA delivery into the cell; therefore, the transfection efficiency of Lipofectamine LTX was compared with and without the Arg9 CPP. Lipofectamine LTX coupled to CPP Arg9 resulted in more EGFP-positive cells, indicating a higher transfection efficiency than Lipofectamine LTX alone (Fig. 2A).
Fig. 2.
Transient gene expression in Citrus sinensis protoplasts. The average number of EGFP positive cells in ‘N7-3’ protoplast cultures were greater in those treated with Lipofectamine LTX + the Arg9 CCP compared to those treated with Lipofectamine LTX only (A). Error bars represent standard error. Brightfield (B) and fluorescent (C) images showed that some of the protoplasts were EGFP positive 72 h after transfection. Confocal image of a protoplast cell indicating that the Arg9 CPP conjugated with TAMRA penetrated the cell membrane but not the nuclear membrane. There was no colocalization between the nucleus stained with DAPI (D) and the TAMARA signal (E) indicated by the merge (F). Scale bar indicates 100 µM in length
While lipoplexes conjugated to Arg9 CPP increase the transfection efficiency, less is known about their cellular mechanisms. Accordingly, we examined the behavior of lipoplexes and CPPs inside the cell. The CPP did not enter the nucleus when Arg9 CPP conjugated to a TAMRA reporter was transfected into citrus protoplasts (Fig. 2D–F). Additionally, plants were not generated from these transfection attempts, as the cells failed to divide and produce microcalli.
Optimization of lipofectamine-mediated DNA delivery into citrus protoplasts
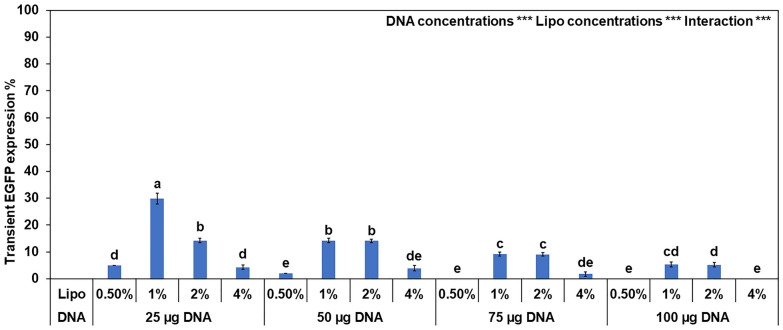
Lipofectamine LTX improved the transformation in our previous experiment and had few negative effects on cell health. Further optimization of the citrus protoplast transfection system was performed to target the CsNPR3 gene and produce a population of genome-edited plants. The pEN-NPR3 construct was encapsulated in cationic lipid nanoparticles using Lipofectamine™ LTX Reagent supplemented with the manufacturer-provided PLUS Reagent, which has been reported to further enhance the transfection efficiency of Lipofectamine LTX [49]. We tested whether different DNA concentrations (25, 50, 75 and 100 µg) and different amounts of Lipofectamine LTX with PLUS (0.5, 1, 2, and 4%) would affect the transfection efficiency of CRISPR/Cas9 construct delivery into sweet orange protoplasts. It was observed that the lowest DNA concentration (25 µg) produced the best transformation efficiency (32%) when combined with 1% transfection reagent (Fig. 3).
Fig. 3.
Effect of different concentrations of CRISPR/Cas9 plasmid encapsulated in different amounts of Lipofectamine (Lipo) mediated DNA delivery into citrus protoplasts. Four concentrations of DNA were used (25, 50, 75 and 100 µg) and four amounts of Lipo (0.5, 1, 2 and 4%). ***Expressed p value < 0.0001. Transfection efficiency was calculated as the percentage of GFP-expressed cells by the total number of the viable cells within 48 h of each treatment ± standard errors (vertical bars). Different letters represent significant differences by Tukey’s honestly test (p ≤ 0.05)
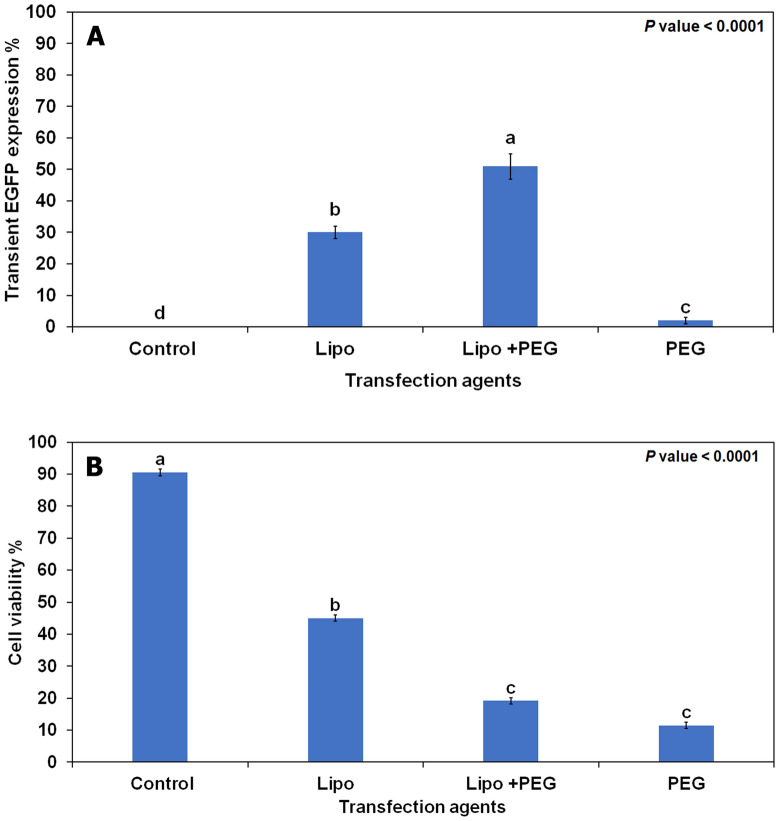
Protoplast transfection methods using PEG usually result in a low transfection efficiency, primarily due to PEG cytotoxicity. Accordingly, we tested whether using Lipofectamine in addition to polyethylene glycol (PEG) or PEG alone would affect DNA delivery and cell viability in citrus protoplasts. The transfection efficiency was approximately 30% using Lipofectamine alone and 51% when Lipofectamine was combined with PEG (Fig. 4A). Although using Lipofectamine combined with PEG increased transfection efficiency, low levels of cell viability were recorded when PEG was used alone or combined with Lipofectamine (11.50 and 19.16%, respectively) (Fig. 4B). Additionally, severe damage was observed in the transfected protoplasts using PEG (Additional file 1: Figure S2). In this study, we generated approximately 100 EGFP + embryos using our optimized protocol. After 6 weeks in B + germination medium, the germinating plants with a well-developed apical meristem were transferred to rooting medium (RMAN) for root production and growth (Fig. 5).
Fig. 4.
Evaluation of transfection agents on the transient transformation efficiency (A), and cell viability (B) of citrus protoplasts
Fig. 5.
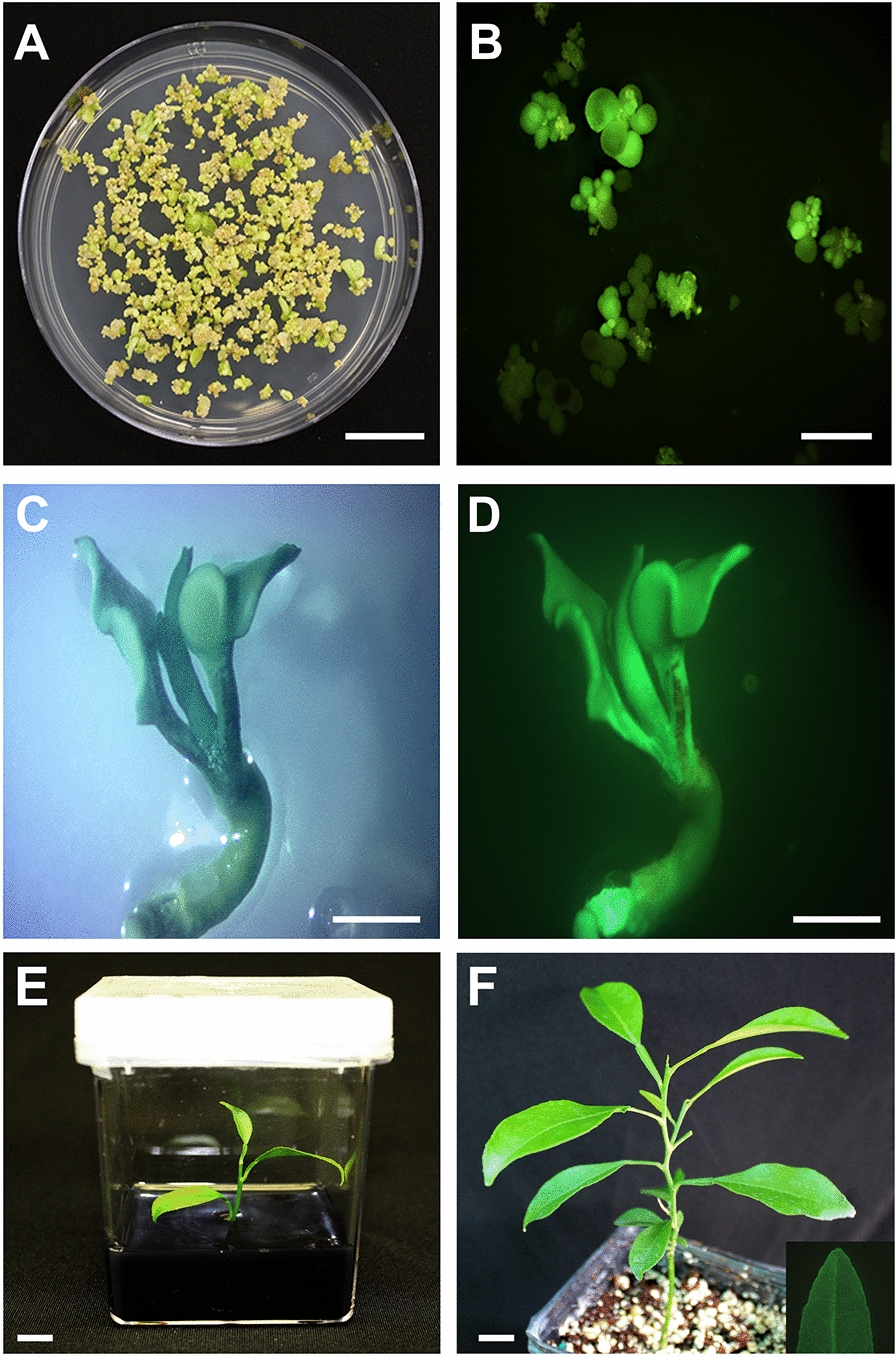
Development of genome edited transgenic plants following lipofectamine-mediated plasmid delivery into protoplast. A Colonies of developed embryos, (B) EGFP-expressing embryos. A germinating EGFP expressing seedling visualized under white light (C) and the same seedling exhibiting EGFP expression under an epi-fluorescence stereomicroscope (D). E Regenerated plants in tissue culture medium before micrografting and (F) Micrografted plant well acclimated in the greenhouse. Inset shows a section of the leaf exhibiting EGFP fluorescence as visualized under the dissecting microscope fitted with a NIGHTSEA fluorescence adapter. Scale bar indicates 1 cm in length
Verification of gene editing in plant tissues using T7EI and sanger sequencing
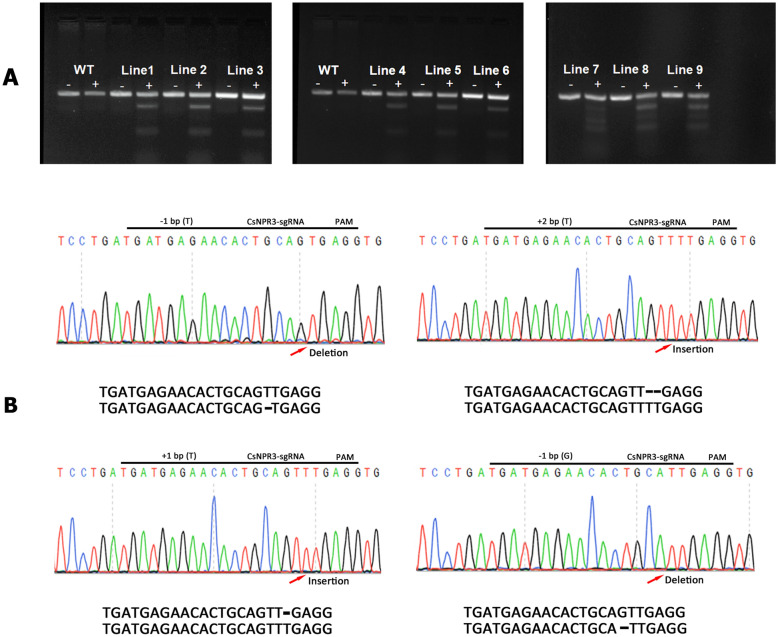
Following transfection and successful regeneration, genome editing was explored in citrus tissue. Twelve EGFP-positive plantlets were randomly selected for analysis, gDNA was isolated, and the presence of both the Cas9 and EGFP genes was tested. PCR analysis of the genomic DNA determined that nine lines tested positive for the presence of both the Cas9 and EGFP genes (Additional file 1: Figure S3). Mutations from 9 independent transgenic lines containing NPR3-sgRNA were also detected using the T7 endonuclease I (T7EI) assay (Fig. 6A). In the T7EI assay, DNA fragments with mutations were digested by the T7EI enzyme, whereas DNA fragments were not digested in the wild-type and untreated controls. The results were further verified by Sanger sequencing (Fig. 6B). The Sanger sequencing results of the PCR-amplified NPR3-gRNA sequence revealed that all nine transgenic plants carried at least one mutation in the CsNPR3 gene. We commonly observed single or double base changes, such as addition of a T or deletions, such as T or G deletions, in the CsNPR3 gene.
Fig. 6.
Targeted mutagenesis of NPR3. T7 Endonuclease I (T7EI) assay targeting CsNPR3 indicating cleavage products from targeted mutagenesis (A). Positive sign ( +) is used to indicate T7EI reaction by incubate the annealed PCR product at 37 ºC for 15 min. The untreated samples are marked with negative sign (−). Sanger sequencing chromatograms of four selected lines. Arrows point to the editing site and the modifications are indicated alongside (B)
Differential gene expression in the edited plants
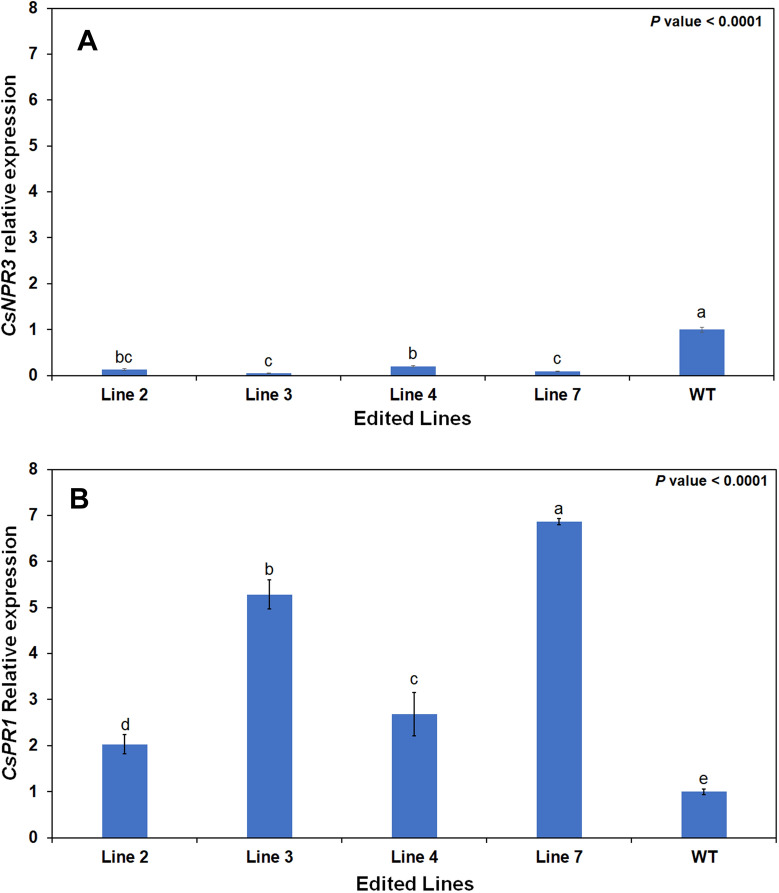
The stable edited lines were micro grafted onto UFR15 [47] rootstocks for faster growth and subsequently transferred to the greenhouse for acclimatization. The transcript levels of CsNPR3 and CsPR1 were analyzed using RNA isolated from fully expanded leaf from four randomly selected edited plants. There was none to very little CsNPR3 expression in all edited lines while CsPR1 expression was significantly upregulated. Edited lines 3 and 7 had the highest expression, while the gene expression in lines 2 and 4 were lower but still statistically significant compared to control (Fig. 7).
Fig. 7.
Changes in relative expression of CsNPR3 (A), and CsPR1 (B) of four selected and edited sweet orange ‘N7-3’ plants. Means compared using Tukey–Kramer HSD test, means followed by the same letter were not different at (p < 0.05)
Discussion
The CRISPR/Cas9 system has now been applied widely as a new approach to genome editing in many plant species, including citrus [50]. It can be challenging to compare the different transfection methods because of the many variables that control successful transfection and editing. The cell’s recalcitrance of gene editing and the difficulty of cell regeneration are the main factors that affect which approach is utilized. CRISPR plasmid delivery into citrus cells has been limited to Agrobacterium-mediated delivery [39, 50]. Most DNA insertion techniques rely on juvenile tissues, which can give rise to homozygous, heterozygous, biallelic, or chimeric edited plants [51]. Conversely, some factors could cause damage to the plasmid DNA, thus inducing cleavage under inappropriate environmental conditions or by DNAses [52]. In plant cells, the cell wall restricts the direct entry of DNA into the cytoplasm. However, protoplasts with their cell wall removed enzymatically are more amenable to the entry of DNA [53]. Since protoplasts give rise to plants derived from a single cell, there is a high possibility of producing homozygous edited plants, which are desirable in vegetatively propagated cultivars. However, due to the difficulty of achieving protoplast transfection in woody fruit trees, which are mainly limited by the cytotoxicity of PEG used in this process, there have been very few successful studies reporting the integration of donor DNA into woody tree protoplast genomes using CRISPR/Cas9 [54]. In citrus, even when using highly viable protoplasts from young embryogenic cultures, the transformation rate and production of stable transgenic plants remain low [55, 56]. If the cells are also negatively affected due to the cytotoxicity of the transfection reagent, then there will be fewer recombination events, thus lowering the transformation efficiency. We suggest herein a simplified method for the insertion of foreign DNA into the citrus cell and stable integration of the DNA into the citrus genome.
Accordingly, a protocol was developed to deliver DNA using cationic lipid nanoparticles into citrus protoplasts for successful gene editing. Polymeric nanoparticles and cationic lipid formulations work to encapsulate DNA, allowing them to escape cellular endosomal barriers and protecting them from enzymatic degradation [57–60]. Comparative studies have been conducted to further evaluate the performance of Lipofectamine and have demonstrated that the activity of lipid formulations is enhanced when coupled with cell penetrating peptides [38, 61]. Lipofectamine LTX was best suited for protoplast transfection and resulted in good EGFP expression. Additionally, when used at an optimum concentration, it had a negligible cytotoxic effect on the cells.
Previous studies have demonstrated that the activity of lipid formulations is enhanced when combined with cell penetrating peptides (CPPs) [37, 38]. These CPPs are a short group of amino acids designed to deliver nucleic acids [31, 62] and lipoplexes (lipid + DNA complexes) into the cell [63, 64]. Treatment with Lipofectamine LTX coupled to Arg9 resulted in more EGFP-positive cells than treatment with Lipofectamine LTX alone. These data are consistent with previous reports that adding arginine-rich CPPs to lipoplexes enhanced the transformation efficiency [63, 65–67]. CPPs not only enhance the transformation efficiency but also improve the stability of lipoplexes [68] and promote translocation, which results in higher levels of gene expression [63].
To investigate the behavior of CPPs and lipoplexes in citrus protoplasts, an Arg9 CPP conjugated with TAMARA was used to track the movement of the CPP inside the cell. We found that the CPP did not cross the nuclear envelope but remained in the cytoplasm. It is not known whether the CPP was cleaved and dissociated in the cytoplasm or if the complex was intact. However, our data indicate that CPPs result in increased transfection. It is possible that the Arg9 CPP dissociated from the DNA complex in the cytoplasm and that the donor DNA crossed the nuclear envelope to be integrated into the citrus genome. Even though CPPs improve the transfection efficiency, they may only increase the probability of genetic recombination events simply because donor DNA plasmids can cross the cell membrane and enter the cell. However, the presence of the Arg9 CPP may affect cell division given that it was difficult to generate stably edited plants from these transfected cells. Thus, the PLUS reagent, which is also widely used in mammalian transfection studies [69], was used in conjunction with Lipofectamine LTX for the remainder of the study, as it has been reported by the manufacturer to further improve Lipofectamine LTX transfection.
Integration of CRISPR/Cas9 construct and expression was validated and several mutations targeting the CsNPR3 gene was obtained (Fig. 6). Successful DNA delivery and genome editing depend on many factors, including the efficiency of DNA delivery, cell viability maintenance and cellular toxicity. Polymeric nanoparticles and cationic lipid formulations work by encapsulating DNA [70]. An optimized Lipofectamine LTX Reagent with PLUS reagent protocol allowed for the stable transfection and gene editing of citrus protoplasts, which generated several stably edited cell lines. Additionally, the use of a lower plasmid DNA concentration (25 µg) resulted in the highest transfection efficiency. Once nanoparticles are introduced into the cell, they allow DNA to escape endosomal barriers and protect it from enzymatic degradation [57, 71]. DNA release from the DNA-polymer complex is essential for translocation and subsequently highlights a drawback of polyplex-mediated DNA delivery [72]. Thomas and Klibanov [73] have also demonstrated that the proper compaction of DNA into the polymer matrix is a prerequisite for the high transfection efficiency of polymer-based nanoparticles. Interestingly, encapsulation of the plasmid DNA in the lower concentration of Lipofectamine used in this study led to higher transfection efficiency. This result is most likely due to a reduction in reagent cytotoxicity to the cells, allowing for more healthy cells to take up and integrate the donor DNA. In some cases, the low transformation efficiency is caused by the endosomal entrapment of donor DNA [10–12], degradation of donor DNA by cytosolic nucleases [13], or limitation of nuclear translocation due to the charge or size of the donor DNA [14, 15]. The introduction of DNA encapsulated in Lipofectamine enhances the transfection efficiency because of the positive charge and maintains cell viability. An increase in the transfection efficiency was recorded when Lipofectamine was used with the addition of PEG. However, the addition of PEG resulted in decreased cell viability and cellular damage. If cellular health is poor following transfection, the downstream establishment of rapidly proliferating embryogenic masses and the subsequent production of plants will be difficult. Pathirana et al. [74] illustrated the effect of PEG in inducing osmotic stress conditions that resulted in changes in cellular and nuclear morphology, DNA fragmentation, cell plasma membrane permeabilization, reactive oxygen species (ROS) overproduction and severe oxidative stress. Additionally, electroporation and DNA carriers activate cellular DNA damage signaling pathways. The low toxicity, simplicity, and high efficiency of using Lipofectamine make it a viable alternative method to deliver DNA for the stable transformation of plant protoplasts. Using the protocol developed in this study, frame shift mutations were obtained through the insertion of 1 or 2 nucleotides or the deletion of 1 nucleotide as previously observed in a callus transformation study [39]. The CsPR1 gene was upregulated in all CRISPR-edited lines. PR1 is used as a marker for the SAR induction process and is naturally upregulated in response to stress [75]. It is indeed possible that enhanced basal upregulation of the CsPR1 gene in our edited lines could result in a robust defense response when the edited lines are challenged by different plant pathogens [76].
Conclusion
We have developed a genome editing system that can effectively improve the efficiency of citrus protoplast transfection and cell viability by using lipid-encapsulated DNA compared to naked plasmid DNA and traditional PEG-mediated transformation. Genomic analysis confirmed the presence of mutations in the CsNPR3 gene, and DNA fragments with mutations were successfully digested by the T7EI enzyme. The edited citrus plants for the SAR-related gene CsNPR3 had enhanced CsPR1 gene expression. This developed protocol can be used in further applications using CRISPR/Cas9 genome editing to improve plant growth and increase tolerance to biotic and abiotic stress.
Supplementary Information
Additional file 1: Table S1. Primer sequences used in this study.
Additional file 2: Figure S1. A diagrammatic representation of the DNA construct used in this study targeting the CsNPR3 gene.
Additional file 3: Figure S2. Protoplast damage following transfection using polyethylene glycol (PEG). Red Arrow indicates intact protoplasts.
Additional file 4: Figure S3. Amplification products obtained from duplex PCR of transgenic ‘N7-3’ genomic DNA with gene-specific oligonucleotide primers. A 800 bp fragment of the Cas9 gene was amplified along with a 490 bp fragment of the EGFP gene. M, 1 kb marker.
Abbreviations
- CPP
Cell penetrating peptides
- CRISPR
Clustered regularly interspaced short palindromic repeats
- EGFP
Enhanced green fluorescent protein
- FITC
Fluorescein Isothiocyanate
- gRNA
Guide RNA
- MES
2-(N-Morpholino) ethanesulfonic acid
- PCR
Polymerase chain reaction
- PEG
Polyethylene glycol
- ROS
Reactive oxygen species
- RNA
Ribonucleic acid
- SAR
Systemic acquired resistance
Authors’ contributions
LM optimized the protocol, generated the plants, and analyzed the data. PK performed the preliminary experimental work. DS conducted the microscopy work. JWG provided resources. MD designed the study and supervised the research. LM, PK, DS and MD wrote the manuscript. All authors read and approved the final manuscript.
Funding
This research was funded in part by a grant of the Specialty Crop Research Initiative (SCRI) Citrus Disease Research and Extension Program (CDRE), grant number FLA-MCS-005676. The funding body played no role in the design of the study and collection, analysis, and interpretation of data and in writing the manuscript.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Talon M, Gmitter FG. Citrus genomics. Int J Plant Genomics. 2008 doi: 10.1155/2008/528361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Duan YX, Liu X, Fan J, Li DL, Wu RC, Guo WW. Multiple shoot induction from seedling epicotyls and transgenic citrus plant regeneration containing the green fluorescent protein gene. Bot Stud. 2007;48:165–171. [Google Scholar]
- 3.Zhang X, Francis MI, Dawson WO, Graham JH, Orbović V, Triplett EW, Mou Z. Over-expression of the Arabidopsis NPR1 gene in citrus increases resistance to citrus canker. Eur J Plant Pathol. 2010;128:91–100. [Google Scholar]
- 4.Dutt M, Barthe G, Irey M, Grosser J. Transgenic citrus expressing an Arabidopsis NPR1 gene exhibit enhanced resistance against Huanglongbing (HLB; Citrus Greening) PLoS ONE. 2016;11:e0147657. doi: 10.1371/journal.pone.0147657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang H-Z, Cai XZ. Nonexpressor of pathogenesis-related genes 1 (NPR1): a key node of plant disease resistance signalling network. Chin J Biotechnol. 2005;21:511–515. [PubMed] [Google Scholar]
- 6.Shi Z, Zhang Y, Maximova SN, Guiltinan MJ. TcNPR3 from Theobroma cacao functions as a repressor of the pathogen defense response. BMC Plant Biol. 2013;13:1–12. doi: 10.1186/1471-2229-13-204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Corte ED, Mahmoud ML, Moraes ST, Mou Z, Grosser WJ, Dutt M. Development of improved fruit, vegetable, and ornamental crops using the CRISPR/Cas9 genome editing technique. Plants. 2019;8:601. doi: 10.3390/plants8120601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ghogare R, Williamson-Benavides B, Ramírez-Torres F, Dhingra A. CRISPR-associated nucleases: the dawn of a new age of efficient crop improvement. Transgenic Res. 2020;29:1–35. doi: 10.1007/s11248-020-00217-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Guo W, Cai X, Cheng Y, Grosser J, Deng X. Protoplast technology and citrus improvement. In: Xu Z, Li J, Xue Y, Yang W, editors. Biotechnology and sustainable agriculture 2006 and beyond. Dordrecht: Springer; 2007. pp. 461–464. [Google Scholar]
- 10.Contento AL, Bassham DC. Structure and function of endosomes in plant cells. J Cell Sci. 2012;125:3511–3518. doi: 10.1242/jcs.093559. [DOI] [PubMed] [Google Scholar]
- 11.Marty F. Plant vacuoles. Plant Cell. 1999;11:587–599. doi: 10.1105/tpc.11.4.587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Varkouhi AK, Scholte M, Storm G, Haisma HJ. Endosomal escape pathways for delivery of biologicals. J Control Release. 2011;151:220–228. doi: 10.1016/j.jconrel.2010.11.004. [DOI] [PubMed] [Google Scholar]
- 13.Lechardeur D, Sohn K, Haardt M, Joshi P, Monck M, Graham R, Beatty B, Squire J, O’brodovich H, Lukacs G. Metabolic instability of plasmid DNA in the cytosol: a potential barrier to gene transfer. Gene Ther. 1999;6:482–497. doi: 10.1038/sj.gt.3300867. [DOI] [PubMed] [Google Scholar]
- 14.Tachibana R, Harashima H, Shinohara Y, Kiwada H. Quantitative studies on the nuclear transport of plasmid DNA and gene expression employing nonviral vectors. Adv Drug Deliv Rev. 2001;52:219–226. doi: 10.1016/s0169-409x(01)00211-3. [DOI] [PubMed] [Google Scholar]
- 15.Zanta MA, Belguise-Valladier P, Behr JP. Gene delivery: a single nuclear localization signal peptide is sufficient to carry DNA to the cell nucleus. Proc Natl Acad Sci USA. 1999;96:91–96. doi: 10.1073/pnas.96.1.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Armstrong CL, Petersen WL, Buchholz WG, Bowen BA, Sulc SL. Factors affecting PEG-mediated stable transformation of maize protoplasts. Plant Cell Rep. 1990;9:335–339. doi: 10.1007/BF00232864. [DOI] [PubMed] [Google Scholar]
- 17.Zhang L, Gu F, Chan J, Wang A, Langer R, Farokhzad O. Nanoparticles in medicine: therapeutic applications and developments. Clin Pharmacol Ther. 2008;83:761–769. doi: 10.1038/sj.clpt.6100400. [DOI] [PubMed] [Google Scholar]
- 18.Salata OV. Applications of nanoparticles in biology and medicine. J Nanobiotechnology. 2004;2:1–6. doi: 10.1186/1477-3155-2-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Martin-Ortigosa S, Valenstein JS, Lin VSY, Trewyn BG, Wang K. Gold functionalized mesoporous silica nanoparticle mediated protein and DNA codelivery to plant cells via the biolistic method. Adv Funct Mater. 2012;22:3576–3582. [Google Scholar]
- 20.Demirer GS, Zhang H, Goh NS, González-Grandío E, Landry MP. Carbon nanotube–mediated DNA delivery without transgene integration in intact plants. Nat Protoc. 2019;14:2954–2971. doi: 10.1038/s41596-019-0208-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu W, Rudis MR, Cheplick MH, Millwood RJ, Yang J-P, Ondzighi-Assoume CA, Montgomery GA, Burris KP, Mazarei M, Chesnut JD. Lipofection-mediated genome editing using DNA-free delivery of the Cas9/gRNA ribonucleoprotein into plant cells. Plant Cell Rep. 2020;39:245–257. doi: 10.1007/s00299-019-02488-w. [DOI] [PubMed] [Google Scholar]
- 22.Tamjidi F, Shahedi M, Varshosaz J, Nasirpour A. Nanostructured lipid carriers (NLC): A potential delivery system for bioactive food molecules. Innov Food Sci Emerg Technol. 2013;19:29–43. [Google Scholar]
- 23.Godbey W, Mikos A. Recent progress in gene delivery using non-viral transfer complexes. J Control Release. 2001;72:115–125. doi: 10.1016/s0168-3659(01)00267-x. [DOI] [PubMed] [Google Scholar]
- 24.Lenaghan SC, Stewart CN. An automated protoplast transformation system. In: Qi Y, editor. Plant Genome Editing with CRISPR Systems. NY: Springer; 2019. pp. 355–363. [DOI] [PubMed] [Google Scholar]
- 25.Cohen H, Levy R, Gao J, Fishbein I, Kousaev V, Sosnowski S, Slomkowski S, Golomb G. Sustained delivery and expression of DNA encapsulated in polymeric nanoparticles. Gene Ther. 2000;7:1896–1905. doi: 10.1038/sj.gt.3301318. [DOI] [PubMed] [Google Scholar]
- 26.Mansouri S, Lavigne P, Corsi K, Benderdour M, Beaumont E, Fernandes JC. Chitosan-DNA nanoparticles as non-viral vectors in gene therapy: strategies to improve transfection efficacy. Eur J Pharm Biopharm. 2004;57:1–8. doi: 10.1016/s0939-6411(03)00155-3. [DOI] [PubMed] [Google Scholar]
- 27.Liu Z, Zhang C, Liu H, Feng L. Efficient synergism of NiSe2 nanoparticle/NiO nanosheet for energy-relevant water and urea electrocatalysis. Appl Catal B. 2020;276:119165. [Google Scholar]
- 28.Almofti MR, Ichikawa T, Yamashita K, Terada H, Shinohara Y. Silver ion induces a cyclosporine a-insensitive permeability transition in rat liver mitochondria and release of apoptogenic cytochrome C. J Biochem. 2003;134:43–49. doi: 10.1093/jb/mvg111. [DOI] [PubMed] [Google Scholar]
- 29.Deshayes S, Morris M, Divita G, Heitz F. Cell-penetrating peptides: tools for intracellular delivery of therapeutics. Cell Mol Life Sci. 2005;62:1839–1849. doi: 10.1007/s00018-005-5109-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hällbrink M, Florén A, Elmquist A, Pooga M, Bartfai T, Langel Ü. Cargo delivery kinetics of cell-penetrating peptides. Biochim Biophys Acta Biomembr BBA. 2001;1515:101–109. doi: 10.1016/s0005-2736(01)00398-4. [DOI] [PubMed] [Google Scholar]
- 31.Kato T, Yamashita H, Misawa T, Nishida K, Kurihara M, Tanaka M, Demizu Y, Oba M. Plasmid DNA delivery by arginine-rich cell-penetrating peptides containing unnatural amino acids. Bioorg Med Chem. 2016;24:2681–2687. doi: 10.1016/j.bmc.2016.04.031. [DOI] [PubMed] [Google Scholar]
- 32.Lindgren M, Hällbrink M, Prochiantz A, Langel Ü. Cell-penetrating peptides. Trends Pharmacol Sci. 2000;21:99–103. doi: 10.1016/s0165-6147(00)01447-4. [DOI] [PubMed] [Google Scholar]
- 33.Milletti F. Cell-penetrating peptides: classes, origin, and current landscape. Drug Discov Today. 2012;17:850–860. doi: 10.1016/j.drudis.2012.03.002. [DOI] [PubMed] [Google Scholar]
- 34.Pooga M, Langel Ü. Classes of cell-penetrating peptides. In: Langel Ü, editor. cell-penetrating peptides. NY: Springer; 2015. pp. 3–28. [DOI] [PubMed] [Google Scholar]
- 35.Chang M, Chou JC, Lee HJ. Cellular internalization of fluorescent proteins via arginine-rich intracellular delivery peptide in plant cells. Plant Cell Physiol. 2005;46:482–488. doi: 10.1093/pcp/pci046. [DOI] [PubMed] [Google Scholar]
- 36.Chen CP, Chou JC, Liu BR, Chang M, Lee HJ. Transfection and expression of plasmid DNA in plant cells by an arginine-rich intracellular delivery peptide without protoplast preparation. FEBS Lett. 2007;581:1891–1897. doi: 10.1016/j.febslet.2007.03.076. [DOI] [PubMed] [Google Scholar]
- 37.Vandenbroucke RE, De Smedt SC, Demeester J, Sanders NN. Cellular entry pathway and gene transfer capacity of TAT-modified lipoplexes. Biochim Biophys Acta Biomembr. 2007;68:571–579. doi: 10.1016/j.bbamem.2006.11.006. [DOI] [PubMed] [Google Scholar]
- 38.El-Sayed A, Futaki S, Harashima H. Delivery of macromolecules using arginine-rich cell-penetrating peptides: ways to overcome endosomal entrapment. AAPS J. 2009;11:13–22. doi: 10.1208/s12248-008-9071-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dutt M, Mou Z, Zhang X, Tanwir SE, Grosser JW. Efficient CRISPR/Cas9 genome editing with Citrus embryogenic cell cultures. BMC Biotechnol. 2020;20:1–7. doi: 10.1186/s12896-020-00652-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Marchant R, Davey M, Power J. Protoplast fusion. In: Methods in Plant Biochemistry. vol. 10: Elsevier; 1993:187–205.
- 41.Grosser JW, Gmitter Jr FG, Castle WS. Sweet orange tree named ‘N7–3’. In.: U.S. Patent Application No. 12/454,988.; 2010.
- 42.Grosser JW, Gmitter FG. Protoplast fusion and citrus improvement. Plant Breed Rev. 1990;8:339–374. [Google Scholar]
- 43.Albiach-Marti MR, Grosser JW, Gowda S, Mawassi M, Satyanarayana T, Garnsey SM, Dawson WO. Citrus tristeza virus replicates and forms infectious virions in protoplasts of resistant citrus relatives. Mol Breed. 2004;14:117–128. [Google Scholar]
- 44.Kaur P, Stanton D, Grosser JW, Dutt M. Yield and transformation ability of citrus protoplasts derived from either cell suspension cultures or embryogenic callus. Proc Fla State Hort Soc. 2018;131:65–69. [Google Scholar]
- 45.Naito Y, Hino K, Bono H, Ui-Tei K. CRISPRdirect: software for designing CRISPR/Cas guide RNA with reduced off-target sites. Bioinform. 2015;31:1120–1123. doi: 10.1093/bioinformatics/btu743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dutt M, Grosser JW. An embryogenic suspension cell culture system for Agrobacterium-mediated transformation of citrus. Plant Cell Rep. 2010;29:1251–1260. doi: 10.1007/s00299-010-0910-0. [DOI] [PubMed] [Google Scholar]
- 47.Grosser JW. Citrus rootstock named ‘UFR-15’. In: U.S. Patent Application No. 14/544,571; 2016.
- 48.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 49.Vozza-Brown L, Fan J, Vasu S, Yu X, Wang B, Lakshmipathy U, Macemon J, Chiou H, Frimpong K. Lipofectamine™ LTX: a new transfection reagent for effective transfection of primary cells, hard-to-transfect cells and sensitive established cell lines. Invitrogen Corp Bulletin.
- 50.Jia H, Zou X, Orbovic V, Wang N. Genome editing in citrus tree with CRISPR/Cas9. In: Plant genome editing with CRISPR systems. NY: Springer; 2019. pp 235–241. [DOI] [PubMed]
- 51.Zhang N, Roberts HM, Van Eck J, Martin GB. Generation and molecular characterization of CRISPR/Cas9-induced mutations in 63 immunity-associated genes in tomato reveals specificity and a range of gene modifications. Front Plant Sci. 2020;11:10. doi: 10.3389/fpls.2020.00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Quick DJ, Anseth KS. Gene delivery in tissue engineering: a photopolymer platform to coencapsulate cells and plasmid DNA. Pharm Res. 2003;20:1730–1737. doi: 10.1023/b:pham.0000003368.66471.6a. [DOI] [PubMed] [Google Scholar]
- 53.Liu K, Xu C, Liu J. Regulation of cell binding and entry by DNA origami mediated spatial distribution of aptamers. J Mater Chem B. 2020;8:6802–6809. doi: 10.1039/d0tb00663g. [DOI] [PubMed] [Google Scholar]
- 54.Huang X, Wang Y, Xu J, Wang N. Development of multiplex genome editing toolkits for citrus with high efficacy in biallelic and homozygous mutations. Plant Mol Biol. 2020;104:297–307. doi: 10.1007/s11103-020-01043-6. [DOI] [PubMed] [Google Scholar]
- 55.Niedz RP, McKendree WL, Shatters RG. Electroporation of embryogenic protoplasts of sweet orange (Citrus sinensis (L.) Osbeck) and regeneration of transformed plants. In Vitro Cell Dev Biol Plant. 2003;39:586–594. [Google Scholar]
- 56.Guo W, Duan Y, Olivares-Fuster O, Wu Z, Arias CR, Burns JK, Grosser JW. Protoplast transformation and regeneration of transgenic Valencia sweet orange plants containing a juice quality-related pectin methylesterase gene. Plant Cell Rep. 2005;24:482–486. doi: 10.1007/s00299-005-0952-x. [DOI] [PubMed] [Google Scholar]
- 57.Panyam J, Labhasetwar V. Biodegradable nanoparticles for drug and gene delivery to cells and tissue. Adv Drug Deliv Rev. 2003;55:329–347. doi: 10.1016/s0169-409x(02)00228-4. [DOI] [PubMed] [Google Scholar]
- 58.Panyam J, Zhou WZ, Prabha S, Sahoo SK, Labhasetwar V. Rapid endo-lysosomal escape of poly (dl-lactide-coglycolide) nanoparticles: implications for drug and gene delivery. FASEB J. 2002;16:1217–1226. doi: 10.1096/fj.02-0088com. [DOI] [PubMed] [Google Scholar]
- 59.Zabner J, Fasbender AJ, Moninger T, Poellinger KA, Welsh MJ. Cellular and molecular barriers to gene transfer by a cationic lipid. J Biol Chem. 1995;270:18997–19007. doi: 10.1074/jbc.270.32.18997. [DOI] [PubMed] [Google Scholar]
- 60.de Lima MCP, Simoes S, Pires P, Faneca H, Düzgüneş N. Cationic lipid–DNA complexes in gene delivery: from biophysics to biological applications. Adv Drug Deliv Rev. 2001;47:277–294. doi: 10.1016/s0169-409x(01)00110-7. [DOI] [PubMed] [Google Scholar]
- 61.Vandenbroucke JP, Von Elm E, Altman DG, Gøtzsche PC, Mulrow CD, Pocock SJ, Poole C, Schlesselman JJ, Egger M, Initiative S. Strengthening the reporting of observational studies in epidemiology (STROBE) explanation and elaboration. PLoS Med. 2007;4:e297. doi: 10.1371/journal.pmed.0040297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zorko M, Langel Ü. Cell-penetrating peptides: mechanism and kinetics of cargo delivery. Adv Drug Deliv Rev. 2005;57:529–545. doi: 10.1016/j.addr.2004.10.010. [DOI] [PubMed] [Google Scholar]
- 63.Koren E, Torchilin VP. Cell-penetrating peptides: breaking through to the other side. Trends Mol Med. 2012;18:385–393. doi: 10.1016/j.molmed.2012.04.012. [DOI] [PubMed] [Google Scholar]
- 64.Khalil IA, Kogure K, Futaki S, Harashima H. High density of octaarginine stimulates macropinocytosis leading to efficient intracellular trafficking for gene expression. J Biol Chem. 2006;281:3544–3551. doi: 10.1074/jbc.M503202200. [DOI] [PubMed] [Google Scholar]
- 65.Gupta B, Levchenko TS, Torchilin VP. TAT peptide-modified liposomes provide enhanced gene delivery to intracranial human brain tumor xenografts in nude mice. Oncol Res. 2007;16:351–359. doi: 10.3727/000000006783980946. [DOI] [PubMed] [Google Scholar]
- 66.Torchilin VP, Levchenko TS, Rammohan R, Volodina N, Papahadjopoulos-Sternberg B, D'Souza GG. Cell transfection in vitro and in vivo with nontoxic TAT peptide-liposome–DNA complexes. Proc Natl Acad Sci USA. 2003;100:1972–1977. doi: 10.1073/pnas.0435906100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kale AA, Torchilin VP. “Smart” drug carriers: PEGylated TATp-modified pH-sensitive liposomes. J Liposome Res. 2007;17:197–203. doi: 10.1080/08982100701525035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Morris MC, Chaloin L, Heitz F, Divita G. Translocating peptides and proteins and their use for gene delivery. Curr Opin Biotechnol. 2000;11:461–466. doi: 10.1016/s0958-1669(00)00128-2. [DOI] [PubMed] [Google Scholar]
- 69.Baker EA, Vaughn MW, Haviland D. Choices in transfection methodologies. Focus. 2000;22:31–33. [Google Scholar]
- 70.Li W, Szoka FC. Lipid-based nanoparticles for nucleic acid delivery. Pharm Res. 2007;24:438–449. doi: 10.1007/s11095-006-9180-5. [DOI] [PubMed] [Google Scholar]
- 71.Prabha S, Zhou WZ, Panyam J, Labhasetwar V. Size-dependency of nanoparticle-mediated gene transfection: studies with fractionated nanoparticles. Int J Pharm. 2002;244:105–115. doi: 10.1016/s0378-5173(02)00315-0. [DOI] [PubMed] [Google Scholar]
- 72.Schaffer DV, Fidelman NA, Dan N, Lauffenburger DA. Vector unpacking as a potential barrier for receptor-mediated polyplex gene delivery. Biotechnol Bioeng. 2000;67:598–606. doi: 10.1002/(sici)1097-0290(20000305)67:5<598::aid-bit10>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 73.Thomas M, Klibanov A. Non-viral gene therapy: polycation-mediated DNA delivery. Appl Microbiol Biotechnol. 2003;62:27–34. doi: 10.1007/s00253-003-1321-8. [DOI] [PubMed] [Google Scholar]
- 74.Pathirana R, West P, Hedderley D, Eason J. Cell death patterns in Arabidopsis cells subjected to four physiological stressors indicate multiple signalling pathways and cell cycle phase specificity. Protoplasma. 2017;254:635–647. doi: 10.1007/s00709-016-0977-8. [DOI] [PubMed] [Google Scholar]
- 75.Mitsuhara I, Iwai T, Seo S, Yanagawa Y, Kawahigasi H, Hirose S, Ohkawa Y, Ohashi Y. Characteristic expression of twelve rice PR1 family genes in response to pathogen infection, wounding, and defense-related signal compounds (121/180) Mol Genet Genomics. 2008;279:415–427. doi: 10.1007/s00438-008-0322-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Agrawal GK, Rakwal R, Jwa NS, Agrawal VP. Signalling molecules and blast pathogen attack activates rice OsPR1a and OsPR1b genes: a model illustrating components participating during defence/stress response. Plant Physiol Biochem. 2001;39:1095–1103. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Table S1. Primer sequences used in this study.
Additional file 2: Figure S1. A diagrammatic representation of the DNA construct used in this study targeting the CsNPR3 gene.
Additional file 3: Figure S2. Protoplast damage following transfection using polyethylene glycol (PEG). Red Arrow indicates intact protoplasts.
Additional file 4: Figure S3. Amplification products obtained from duplex PCR of transgenic ‘N7-3’ genomic DNA with gene-specific oligonucleotide primers. A 800 bp fragment of the Cas9 gene was amplified along with a 490 bp fragment of the EGFP gene. M, 1 kb marker.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.