Summary
Background
Although the current consensus recommends a standard treatment of high-dose intravenous immunoglobulin with high-dose aspirin to manage Kawasaki disease (KD), the use of different adjunctive therapies remains controversial. The aim of the current network meta-analysis (NMA) was to compare the efficacy and tolerability of different existing interventions for the initial and refractory stages of KD.
Methods
An NMA of randomised controlled trials (RCTs) was conducted using the frequentist model applied after electronic searches in PubMed, Embase, ScienceDirect, ProQuest, ClinicalTrials.gov, ClinicalKey, Cochrane CENTRAL, and Web of Science. The main outcomes were reduced fever duration/diminished severity of fever subsided. The initial stage of KD was defined as the first stage to treat patients with KD; the refractory stage of KD represents KD patients who failed to respond to standard KD treatment. The cut-off points for intravenous immunoglobulin (IVIG) were low (100–400 mg), medium (1 g), and high (at least 2 g).
Findings
A total of fifty-six RCTs with 6486 participants were included. NMA demonstrated that the medium-dosage IVIG + aspirin + infliximab [mean difference=−1.76 days (95% confidence intervals (95% CIs): −3.65 to 0.13 days) compared to high-dosage IVIG + aspirin] exhibited the shortest fever duration; likewise, the medium-dosage IVIG + aspirin + infliximab [odds ratio (OR)=0.50, 95% CIs: 0.18–1.37 compared to high-dosage IVIG + aspirin] exhibited the smallest incidence of coronary artery lesion (CAL) in the initial-stage KD. In the refractory-stage KD, the high-dosage IVIG + pulse steroid therapy (OR=0.04, 95% CIs: 0.00–0.43 compared to the high-dosage IVIG only) had the best rate of decline of fever; likewise, the high-dosage IVIG + ciclosporin [OR=0.05 (95% CIs: 0.00–1.21) compared to the high-dosage IVIG only] exhibited the smallest incidence of CAL. Infliximab significantly improved resolution compared to the high-dosage IVIG only group (OR=0.20, 95%CIs: 0.07–0.62) in refractory-stage KD.
Interpretation
The NMA demonstrated that the combination therapy with the standard therapy of IVIG and aspirin might have an additional effect on shortening the duration of fever and lowering the CAL incidence rate in patients with acute KD. Moreover, the combination therapy with high-dose IVIG and pulse steroid therapy or cyclosporine therapy might have an additional effect on improving the rate of decline of fever and lowering the incidence rate of CAL in children with refractory KD. Because some of the findings of this NMA should be considered hypothesis-generating rather than confirmatory, further evidence from de novo randomised trials is needed to support our results.
Funding
None.
Keywords: Coronary artery lesion, Cardiovascular, Pediatrics, Kawasaki disease, Coronary artery disease
Abbreviations: CI, confidence interval; NMA, network meta-analysis; SMD, standardized mean difference; IVIG, intravenous immune globulin; ACONT, aspirin only; ILA, low dose IVIG + aspirin; IMA, medium dose IVIG + aspirin; FIMA, medium dose IVIG + aspirin + infliximab; IHA, high dose IVIG + aspirin; CIHA, high dose IVIG + aspirin + clarithromycin; VMIHA, high dose IVIG + aspirin + pulsed-dose intravenous methylprednisolone; FIHA, high dose IVIG + aspirin + infliximab; OR, odds ratio; IHonly, high dose IVIG only; InF, infliximab; PulSte, intravenous pulse steroid therapy; IHSte, high dose IVIG + steroid therapy; IHPulSte, high dose IVIG + intravenous pulse steroid therapy
Research in context.
Evidence before this study
Previous guidelines recommend a standard treatment of high-dose intravenous immunoglobulin with high-dose aspirin to manage Kawasaki disease (KD). However, the effectiveness and risk of coronary artery lesion (CAL) associated with the use of different adjunctive therapies, remains unknown.
Added value of this study
The current network meta-analysis (NMA) revealed that medium intravenous immunoglobulin (IVIG) + aspirin + infliximab resulted in the shortest fever duration and the lowest incidence of CAL in the initial stage of KD. The high IVIG + pulse steroid and high IVIG + cyclosporine group exhibited the best defervescence rate and the lowest CAL incidence in refractory-stage KD.
Implications of all the available evidence
The infliximab plus standard therapy might be the best treatment for acute KD. Pulse methylprednisolone plus IVIG is the best choice for refractory KD.
Alt-text: Unlabelled box
Introduction
Kawasaki disease (KD) is an acute inflammatory vasculitis primarily affecting infants and is the leading cause of acquired cardiovascular disease in children.1 First reported in 1967 in Japan, the prevalence of KD has increased worldwide, increasing from 3.4–218.6 per 100,000 children.2 Without timely treatment, up to 25% of children with KD develop sustained coronary artery lesions, including coronary artery aneurysms.3 These children may progress to developing coronary thrombus, stenosis, myocardial ischaemia, ischaemia-induced arrhythmia, and even sudden death.4 In a previous limited study (n=261), among adults younger than 40 years who underwent coronary angiography for suspected myocardial ischaemia, 5% had aneurysms secondary to KD as the cause of coronary disease.3
The management of KD to reduce serious cardiac sequelae has raised major concerns regarding child health. Although most patients with KD benefit from standard therapy with intravenous immunoglobulin (IVIG) combined with acetylsalicylic acid (ASA), 20% of children still develop coronary artery dilatation, and 5% progress to coronary artery aneurysms.5 In addition, approximately 10%–20% of patients with KD are resistant to conventional therapy, presenting with persistent or recurrent fever, and are at a higher risk of subsequent coronary artery diseases.6
Several treatment strategies have been proposed to address the issue of the association between IVIG resistance, recrudescent fever, and the accompanying higher risk for coronary artery lesions (CALs),7 including adjuvant corticosteroid therapy,8 anti-tumour necrosis factor (TNF-α) monoclonal antibody,9 and adjunctive clarithromycin therapy.10 Furthermore, previous pairwise meta-analyses have mainly focused on the efficacy of corticosteroids as either adjunctive or rescue therapy.11,12 However, safety concerns remain, concerning the insufficiency of data available for the incidence of adverse effects of corticosteroids. Few head-to-head studies have compared the efficacy of different protocols for the treatment of KD other than conventional therapy.
To clarify these issues, we conducted a systematic review and network meta-analysis (NMA) of RCTs that investigated the efficacy and safety of various pharmacological regimens used as initial or rescue adjunctive treatment for the prevention of the occurrence of coronary artery lesions (CAL) and their ability to shorten the duration of fever.
Methods
General guidelines for the current study
The current network meta-analysis (NMA) followed the preferred reporting items for systematic reviews and meta-analyses (PRISMA) 2020 guidelines13 and AMSTAR2 (assessing the methodological quality of systematic review) guidelines.14 The current NMA is registered in PROSPERO: CRD42021220936. The current study complied with the Institutional Review Board of the Tri-Service General Hospital (TSGHIRB: B-109-29).
Search strategy and selection criteria
We conducted a systematic publication review with the keywords (mucocutaneous lymph node syndrome OR Kawasaki disease) AND (randomized controlled trial) from inception until November 15th, 2020 in the PubMed, Embase, ScienceDirect, ProQuest, ClinicalTrials.gov, ClinicalKey, Cochrane CENTRAL, and Web of Science databases. To include as many types of pharmacologic treatments as possible, we did not include keywords for specific treatments. No language restrictions were included. We also conducted manual searches for potentially eligible articles from the reference lists of review articles or pairwise meta-analyses.11,12,15
Inclusion and exclusion criteria
The PICO included: (1) patient or problem: participants with KD; (2) intervention: pharmacological regimens used as initial or rescue adjunctive treatment to KD; (3) comparator: placebo-controlled or active-controlled; and (4) outcome: in the initial stage of KD, it was the fever duration and CAL incidence rate and in the refractory KD stage, it was the rate of decrease of fever and CAL incidence rate (detailed definition of outcome, please see the statement below). The definition of initial stage treatment of KD indicated the initial stage regimen to treat patients with KD; the refractory stage treatment of KD represents the rescue therapy which was applied to patients with KD who failed to respond to standard KD treatment.
We only included RCTs, either placebo-controlled or active-controlled design, in humans. The intervention arms for comparison were the pharmacologic interventions applied to patients with KD, which were defined according to the diagnostic criteria.1 We categorised the interventions into three dosage groups, namely, the IVIG low group received 100–400 mg, the medium group received 1 g, and the high group received 2 g.16,17
The exclusion criteria were as follows: (1) not a clinical trial; (2) not an RCT; (3) did not report the target outcomes of interest; or (4) was not related to pharmacologic interventions. In cases of duplicated usage of data (i.e. different articles based on the same sample sources), we only included the report with the most informative and largest sample sources.
Data extraction
Two authors (Lei and Tseng) independently screened the studies, extracted the relevant data from the manuscripts, and assessed the risk of bias. In situations of discrepancy, the corresponding author (Kao) was involved. If there was a lack of available data from the manuscripts, we contacted the corresponding authors or coauthors to obtain the original data.
Outcomes
The current NMA consisted of two parts to the analyses, including the treatment of initial-stage patients with KD and treatment of refractory stage patients with KD. The outcome in the first part was fever duration and the incidence of CAL. The outcome in the second part was the rate of decline of fever (based on failure to reduce the fever) and the incidence of CAL. The safety profile was a serious rate of adverse events in both stages (i.e., initial and refractory stages). The definition of serious adverse events varied across the included RCTs. In brief, we considered the following (but not limited to) serious adverse events, including liver function abnormalities, anaemia, neutropenia, intussusception, congestive heart failure, sepsis, lymphadenopathy, splenomegaly, generalised oedema, shock, or anaphylaxis.
Cochrane risk of bias tool
Two independent authors (Lei and Tseng) evaluated the risk of bias (inter-rater reliability, 0.87) for each domain described in the Cochrane risk of bias tool.18
Statistical analysis
The NMA was performed using STATA (version 16.0; StataCorp LLC, College Station, TX, USA). For continuous data, we estimated the summary mean difference (MD) with 95% confidence intervals (95% CIs). For categorical data, we estimated summary odds ratio (OR) with 95% CIs. For categorical data, we applied a 0.5 zero-cell correction during the meta-analysis. However, if the scores were zero in both the intervention and control arms in a study, we did not apply this correction because of the risk of increasing bias.19,20 We used the frequentist models of the NMA to compare effect sizes (ES) between studies with the same interventions. All comparisons used a two-tailed test. Heterogeneity among the studies was evaluated by the tau value, which was the estimated standard deviation of the effect across the included studies.
The meta-analysis applied in the current study used a mixed comparison with generalised linear mixed models to analyse the direct and indirect comparisons among the NMA.21 Specifically, indirect comparisons were calculated by the transitivity; thus, the differences between treatments A and B can be calculated from their comparisons with a third treatment, C. To compare multiple treatment arms, we combined direct and indirect evidence from the included studies.22 Direct evidence between two treatment arms (i.e. treatments A and B) indicated that there was a direct comparison between treatments A and B in at least one of the included studies. Conversely, indirect evidence between the two treatment arms (i.e. treatments A and C) indicated that we obtained the effect size (ES) comparison between treatments A and C by combining the ES between comparing pairs of treatments A and B and the ES between comparing pairs of treatments B and C. For example, eFigure 2a shows that there was no direct comparison between the high-dose IVIG + aspirin + infliximab (FIHA) and high-dose IVIG + aspirin + clarithromycin (CIHA) groups in the included studies. Thus, we obtained indirect evidence between the FIHA and CIHA groups via comparisons with the high-dose IVIG + aspirin (IHA) group. The STATA program used in our NMA was the mvmeta command.23 We used restricted maximum likelihood methods to evaluate between-study variance.24
Figure 2.
The network structure of (2a) shortening fever duration in initial stage and (2b) rate of failure to subside fever in refractory stage.
Depicts the overall network structure of the current network meta-analysis of (2a) shortening fever duration by different interventions in the initial treatment of Kawasaki disease (n=36 articles with 8 individual treatment arms) and (2b) rate of failure to subside fever by different interventions in refractory treatment for Kawasaki disease (n=7 articles with six individual treatment arms). The lines between nodes represent direct comparisons in various trials, and the size of each circle is proportional to the size of the population involved in each specific treatment. The thickness of the lines is proportional to the number of trials connected to the network.
To provide more clinical applications, we calculated the relative ranking probabilities between the treatment effects of all treatments for the target outcomes. Briefly, the surface under the cumulative ranking curve (SUCRA) is the percentage of the mean rank of each medication relative to an imaginary intervention that is the worst without uncertainty.25 When the area under the curve was less, the treatment had a higher rank of fever relief.
To reduce the potential source of heterogeneity, we conducted further subgroup analysis. To be specific, in the refractory stage, some of the included RCTs recruited participants who had failed to receive standard therapy (i.e. IVIG 2 g/kg or IVIG 2 g/kg plus aspirin) before enrolment, while others applied a predicted high-risk of refractoriness without actual initial treatment before enrolment. Therefore, in the part of refractory KD, we arranged subgroup analysis focusing on RCTs recruiting participants who had failed to receive standard therapy (i.e. IVIG 2g/kg or IVIG 2 g/kg plus aspirin) before enrolment. Finally, to detect the potential evidence conflict between the direct and indirect comparisons, we evaluated the potential inconsistencies between the direct and indirect evidence within the network using the loop-specific approach. Local inconsistencies were evaluated using the node-splitting method. Furthermore, we used a design-by-treatment model to evaluate global inconsistencies among the entire NMA.26
Ethics: This was an observational study. The Institutional Review Board of the Tri-Service General Hospital confirmed that no ethical approval was required (TSGHIRB: B-109-29).
Role of funding source
None.
Results
After the initial screening, 100 articles underwent a full-text review (Figure 1). However, 44 patients were excluded for various reasons (Figure 1 and eTable 3). Finally, 56 articles were included in the NMA. Specifically, 48 articles were included to examine the initial treatment, and eight were included to examine refractory KD (eTable 4). The entire network structure of the treatment arms is shown in Figure 2a and 2b.
Figure 1.
Flowchart of the current network meta-analysis.
Depicts the entire flowchart of the current network meta-analysis
Characteristics of the included studies
A total of 6,486 participants were included in the 56 RCTs. Publication dates ranged from 1984 to 2019. The reported range of patient age was 1.54–5.73 years old with an average of 2.62 years. The included trials comprised 42.3% females (eTable 4). In the initial stage of KD, there were 5,904 children (mean age=2.5 years, range 1.5–5.7 years; female: 42.3%, range 10.0–86.0%) and mean follow-up duration was 13.3 weeks (range 1.6–78 weeks). In the refractory stage of KD, there were 582 children (mean age=2.8 years, range 2.5–3.5 years; female: 42.4%, range 25.0–50.0%) and mean follow-up duration was 7.2 weeks (range 1.0–20.0 weeks).
Initial-stage treatment in patients with KD: fever duration and CAL incidence rate
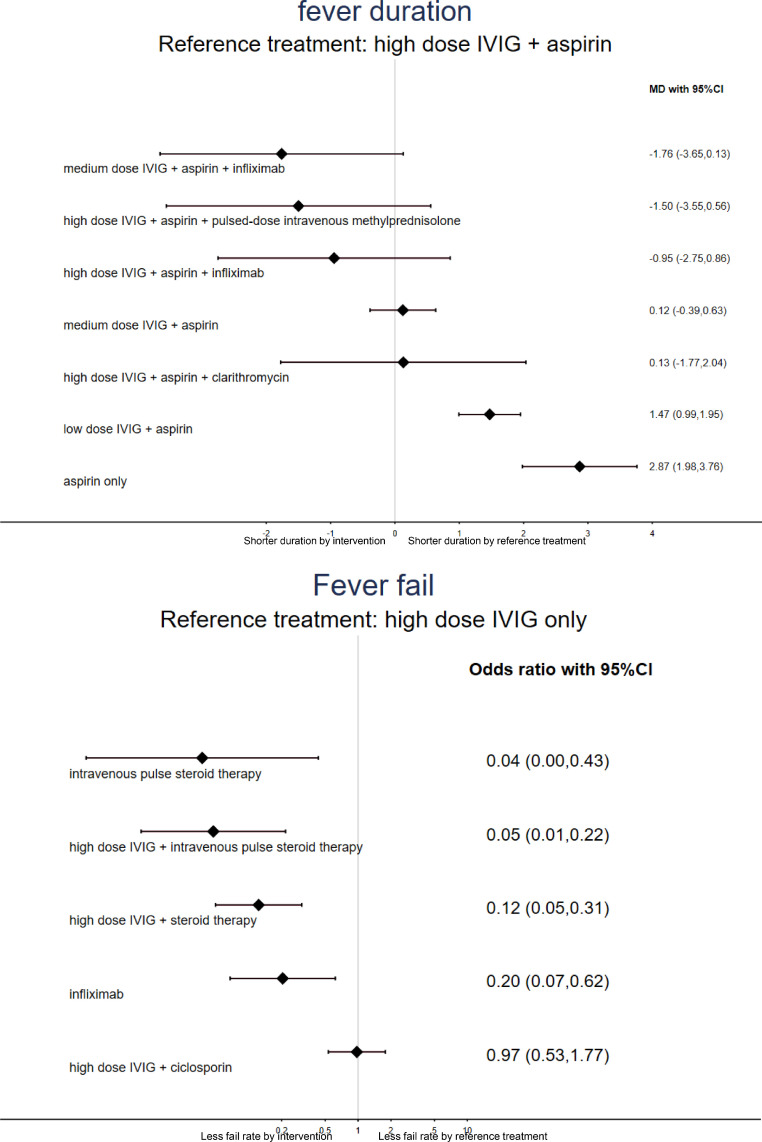
Thirty-six articles with eight individual treatment arms investigated fever duration in the treatment of initial-stage KD. In the NMA, only the low IVIG + aspirin and aspirin-only groups had a significantly longer fever duration than the high IVIG +aspirin group. None of the other investigated treatments had a significantly shorter fever duration than the high IVIG + aspirin group (Table 1a, Figures 2a and 3a). The medium IVIG + aspirin + infliximab treatment had the shortest fever duration among all pharmacologic interventions (eTable 5a).
Table 1a.
League table of association between individual intervention and fever duration in initial stage treatment to Kawasaki disease
| medium dose IVIG + aspirin + infliximab | high dose IVIG + aspirin + pulsed-dose intravenous methylprednisolone | high dose IVIG + aspirin + infliximab | high dose IVIG + aspirin | high dose IVIG + aspirin + clarithromycin | medium dose IVIG + aspirin | low dose IVIG +aspirin | aspirin only | |
|---|---|---|---|---|---|---|---|---|
| medium dose IVIG + aspirin + infliximab | *-1.89 (-2.07,-1.71) | |||||||
| high dose IVIG + aspirin + pulsed-dose intravenous methylprednisolone | -0.26 (-3.05,2.53) | *-1.50 (-2.47,-0.53) | ||||||
| high dose IVIG + aspirin + infliximab | -0.82 (-3.43,1.80) | -0.55 (-3.29,2.18) | *-1.00 (-1.05,-0.96) | |||||
| high dose IVIG + aspirin | -1.76 (-3.65,0.13) | -1.50 (-3.55,0.56) | -0.95 (-2.75,0.86) | -0.13 (-0.72,0.46) | -0.06 (-0.21,0.09) | *-1.46 (-1.87,-1.05) | *-3.20 (-4.46,-1.94) | |
| high dose IVIG + aspirin + clarithromycin | -1.89 (-4.58,0.79) | -1.63 (-4.43,1.17) | -1.08 (-3.70,1.55) | -0.13 (-2.04,1.77) | ||||
| medium dose IVIG + aspirin | *-1.88 (-3.70,-0.06) | -1.62 (-3.74,0.50) | -1.07 (-2.94,0.81) | -0.12 (-0.63,0.39) | 0.01 (-1.96,1.98) | *-1.50 (-1.85,-1.14) | -1.00 (-2.18,0.18) | |
| low dose IVIG + aspirin | *-3.23 (-5.12,-1.35) | *-2.97 (-5.08,-0.86) | *-2.42 (-4.28,-0.55) | *-1.47 (-1.95,-0.99) | -1.34 (-3.31,0.62) | *-1.35 (-1.84,-0.86) | *-1.66 (-3.03,-0.29) | |
| aspirin only | *-4.63 (-6.67,-2.60) | *-4.37 (-6.61,-2.13) | *-3.82 (-5.82,-1.81) | *-2.87 (-3.76,-1.98) | *-2.74 (-4.84,-0.64) | *-2.75 (-3.66,-1.84) | *-1.40 (-2.23,-0.57) |
Pairwise (upper-right portion) and network (lower-left portion) meta-analysis results are presented as estimate effect sizes for the outcome of fever duration. Interventions are reported in order of mean ranking of shortening fever duration, and outcomes are expressed as standardized mean difference (SMD) (95% confidence intervals). For the pairwise meta-analyses, SMD of less than 0 indicate that the treatment specified in the row got shorter fever duration than that specified in the column. For the network meta-analysis (NMA), SMD of less than 0 indicate that the treatment specified in the column got shorter fever duration than that specified in the row. Bold results marked with * indicate statistical significance.
Figure 3.
Forest plot of NMA of (3a) shortening fever duration in initial stage and (3b) rate of failure to subside fever in refractory stage.
a: when MD < 0, this indicates shorter duration by intervention than the reference treatment (reference treatment: high dose IVIG + aspirin) in the initial treatment of Kawasaki disease (n=36 articles with 8 individual treatment arms); b: when the odds ratio < 1, this indicates a better response rate (less rate of failure to subside fever) by intervention than the reference treatment (reference treatment: high dose IVIG only) in refractory treatment to Kawasaki disease (n=7 articles with 6 individual treatment arms).
Abbreviation: CI: confidence interval; IVIG: intravenous immunoglobulin; NMA: network meta-analysis; SMD: standardized mean difference; IVIG: intravenous immune globulin; OR: odds ratio.
Forty-nine articles with ten individual treatment arms mentioned the rate of incidence of CAL in the treatment of initial-stage KD. Only the low IVIG + aspirin and aspirin-only groups had significantly higher CAL incidence rates than the high IVIG + aspirin group (eTable 6a, eFigures 1a and 2a). The medium IVIG + aspirin + infliximab group had the lowest rate of incidence of CAL among all the pharmacologic interventions (eTable 5b).
Refractory-stage treatment in patients with KD: rate of decline of fever and rate of incidence of CAL
Seven articles with six individual treatment arms investigated the rate of decline of fever in the treatment of refractory KD. Almost all the interventions had a significantly better rate of decline of fever than the high IVIG only groups, except for the high IVIG + cyclosporin group (Table 1b, Figures 2b and 3b). The high IVIG + pulse steroid therapy [OR=0.04 (95% CIs: 0.00 to 0.43)] had the fastest rate of decline of fever among all the pharmacologic interventions (eTable 5c). In the subgroup analysis, there were four RCTs recruiting participants who had definitely failed to receive standard therapy (i.e. IVIG 2 g/kg or IVIG 2 g/kg plus aspirin) before enrolment.27–30 The main result of the current NMA revealed that the high IVIG + pulse steroid therapy [OR=0.04 (95% CIs: 0.00 to 0.43) compared to the high IVIG only groups] exhibited the fastest rate of decline of fever among all pharmacologic interventions (eTables 5d, 6b, eFigures 1b, and 2b).
Table 1b.
League table of association between individual intervention and rate of failure to subside fever in refractory stage treatment to Kawasaki disease.
| high dose IVIG + intravenous pulse steroid therapy | intravenous pulse steroid therapy | high dose IVIG + steroid therapy | infliximab | high dose IVIG + ciclosporin | high dose IVIG only | |
|---|---|---|---|---|---|---|
| high dose IVIG + intravenous pulse steroid therapy | *0.05 (0.01,0.22) | |||||
| intravenous pulse steroid therapy | 1.26 (0.07,22.53) | *0.04 (0.00,0.43) | ||||
| high dose IVIG + steroid therapy | 0.38 (0.07,2.26) | 0.30 (0.02,4.14) | *0.12 (0.05,0.31) | |||
| infliximab | 0.23 (0.04,1.52) | 0.18 (0.01,2.69) | 0.60 (0.14,2.53) | *0.21 (0.07,0.62) | ||
| high dose IVIG + ciclosporin | *0.05 (0.01,0.25) | *0.04 (0.00,0.48) | *0.13 (0.04,0.38) | *0.21 (0.06,0.74) | 0.97 (0.54,1.78) | |
| high dose IVIG only | *0.05 (0.01,0.22) | *0.04 (0.00,0.43) | *0.12 (0.05,0.31) | *0.20 (0.07,0.62) | 0.97 (0.53,1.77) |
Pairwise (upper-right portion) and network (lower-left portion) meta-analysis results are presented as estimate effect sizes for the outcome of rate of failure to subside fever. Interventions are reported in order of mean ranking of efficacy of fever subside, and outcomes are expressed as odds ratio (OR) (95% confidence intervals). For the pairwise meta-analyses, OR of less than 1 indicate that the treatment specified in the row got less rate of failure to subside fever (i.e. better efficacy in subsiding fever) than that specified in the column. For the network meta-analysis (NMA), OR of less than 1 indicate that the treatment specified in the column got less rate of failure to subside fever (i.e. better efficacy in subsiding fever) than that specified in the row. Bold results marked with * indicate statistical significance.
Abbreviation: CI: confidence interval; NMA: network meta-analysis; SMD: standardized mean difference; IVIG: intravenous immune globulin; OR: odds ratio.
Seven articles with seven individual treatment arms mentioned the rate of incidence of CAL in the treatment of refractory KD. Only the high IVIG + steroid and high IVIG + pulse steroid therapy groups had significantly lower rates of incidence of CAL than the high IVIG only groups (eTable 6c, eFigures 1c and 2c). The high IVIG + cyclosporin group had the lowest incidence of CAL among all pharmacologic interventions (eTable 5e). In the subgroup analysis, there were four RCTs recruiting participants who had definitely failed to receive standard therapy (i.e. IVIG 2 g/kg or IVIG 2 g/kg plus aspirin) before enrolment.28, 29, 30, 31 The main result of the current NMA revealed that none of the investigated treatments was associated with significantly different CAL incidence rates compared to the other treatments (eTables 5f and 6d, and eFigures 1d and 2d).
Safety profile: serious adverse event rates
For the initial treatments, 20 articles with nine individual treatment arms were investigated in the current NMA. None of the investigated pharmacological interventions had significantly different serious adverse event rates than the high IVIG + aspirin group (eTable 6e, eFigures 1e and 2e). The low IVIG + aspirin group, the high IVIG + aspirin + pulse steroid therapy group, and the high IVIG + aspirin + infliximab group had the three lowest serious adverse event rates among all pharmacologic interventions (eTable 5g).
For refractory treatment, only five articles provided information on serious adverse events. Therefore, NMA was not performed.
Risk of bias, inconsistency, and publication bias
We found that 52.3% (205/392), 41.3% (162/392), and 6.38% (25/392) of the included studies had an overall low, unclear, and high risk of bias, respectively (eFigure 3a and b). Funnel plots of publication bias across the included studies (eFigure 4a–j) revealed general symmetry, and the results of Egger's test indicated no significant publication bias among the articles included in the NMA. In general, the NMA did not demonstrate local or global inconsistencies, as assessed using the loop-specific approach, node-splitting method, or the design-by-treatment method, except for global inconsistency in the incidence of CAL in the refractory stage (eTables 7 and 8). However, there was no local inconsistency detected in the NMA of the incidence of CAL in the refractory stage by the node-splitting method.
Discussion
To the best of our knowledge, the present study is the first to address the effect of various treatments for preventing CALs and shortening the duration of fever of KD. The current NMA found that the combination therapy with the standard therapy of IVIG and aspirin might have an additional effect on shortening the duration of fever and lowering the incidence of CAL. For refractory KD, the combination therapy of high-dose IVIG with pulse steroid or cyclosporine might have an additional effect on improving the rate of decline of fever and lowering the incidence of CAL. Further, the main results remained the same when we focused on RCTs recruiting participants who had definitely failed to receive a standard therapy (i.e. IVIG 2 g/kg or IVIG 2 g/kg plus aspirin) before enrolment. Finally, the high IVIG + aspirin + infliximab group had the lowest serious adverse event rate among all pharmacologic interventions.
A key finding of the current NMA was that patients that received medium IVIG + aspirin + infliximab experienced the shortest duration of and the lowest incidence of CAL when used as the initial treatment. Previous meta-analyses have mainly demonstrated the benefits of adjuvant corticosteroid therapy on the defervescence time and prevention of CAL.32, 33, 34 Nevertheless, our NMA offered further evidence of better pharmacological interventions to achieve a shorter duration of fever and a lower incidence of CAL. Specifically, the current NMA demonstrated that additional infliximab had the best effect among the other combinations or conventional therapies. The serum concentrations of TNF-α, a potent proinflammatory cytokine, are elevated in patients with acute KD and this can initiate a series of downstream inflammatory signalling cascades along with other cytokines, leading to intense activation of the immune response, including fever.9,35 This has also been consistently reported as a powerful risk factor for CAL.36 Thus, it is reasonable that preventing the elevation of TNF-α and blocking its receptor could mitigate the intense inflammatory response. Moreover, during the sub-acute stage of KD, CD4+CD25+ regulatory T cells are more abundant, implicating the contribution of regulatory T cells in the alleviation of disease during the acute phase.37 Infliximab has been shown to increase both the expression and survival of regulatory T cells in patients with rheumatoid arthritis and inflammatory bowel disease.38,39 Although corticosteroids have strong anti-inflammatory effects and can thus benefit patients with KD in the acute stage, they do not induce beneficial regulatory T cells. Therefore, the dual effects of mitigating specific inflammatory cytokines and expanding regulatory T cells could be responsible for the better effect of additional infliximab as the initial treatment in acute KD, compared with corticosteroids on the duration of fever and incidence of CAL. Interestingly, the combination therapy of high-dose IVIG + infliximab did not induce a greater benefit than the medium IVIG + infliximab. This is important because the cost of IVIG therapy for KD is high and may be a burden for the health care system. Further controlled trials and cost-benefit analyses are warranted to validate these results.
Another finding of the current NMA was that the high IVIG + pulse steroid therapy and the high IVIG + cyclosporin therapy exhibited the best rate of decline of fever and incidence of CAL when used as the refractory treatment, respectively. Currently, only one previous pairwise meta-analysis addressed the benefits of a single corticosteroid therapy for refractory disease.40 Our results further addressed two new findings regarding the choice of drugs in such difficult conditions. Although all regimens except IVIG + cyclosporine showed significantly better efficacy for defervescence than single IVIG therapy, the current NMA demonstrated that the combination therapy using IVIG + pulse steroid was even better than the other regimens. It is well known that either a single dose of pulse methylprednisolone or a general high-dose steroid therapy can have profound and universal anti-inflammatory effects.41 Previous studies have also shown that the levels of inflammatory cytokines, including C-reactive protein (CRP), monocyte chemoattractant protein-1 (MCP-1), NF-κB cascade, and TNF-α, were suppressed more rapidly by intravenous methylprednisolone (IVMP) than by additional IVIG in KD patients and may thus be related to the course of the fever.42 In addition, high-dose IVIG inhibits only parts of the inflammation-related cells, including monocytes/macrophages and coronary arterial endothelial cells, whereas IVMP suppresses not only these two cell types, but also T cells that participate in the inflammatory response and fibroblast proliferation, which is observed in the subsequent stage after the formation of coronary artery aneurysm.1,40,43 Notably, IVMP can increase neutrophils in the peripheral blood, and neutrophilia is a poor prognosis factor for KD; conversely, IVIG therapy induces neutrophil apoptosis in KD.44 Simultaneously, the serum levels of TNF-α, CRP, and MCP-1 recover after completion of IVMP single therapy in patients with KD compared with levels in patients receiving IVIG plus IVMP therapy.42 Therefore, the synergistic effect of IVIG + IVMP may be why it can outweigh either single therapy. Second, the effect of additional ciclosporin therapy with conventional IVIG exceeds that of the additional steroid treatment in lowering the incidence of CAL in recalcitrant KD. Cyclosporin inhibits excess inflammation by specifically suppressing the calcium-nuclear factor of activated T cells (NFAT) pathway. Children carrying genetic variants of the ITPKC and CASP3 genes, which lead to increased signal transduction in NFAT, had higher susceptibility to KD.45 A relationship between the upregulation of the NFAT pathway with unresponsiveness to IVIG and the development of CAL has also been demonstrated.46 Moreover, the concentration of CRP decreased more rapidly in KD children who received additional cyclosporin treatment, indicating better efficacy for the suppression of the acute inflammatory response.47 Therefore, the simultaneous suppression by cyclosporin of nonspecific acute-phase inflammation and the specific NFAT pathway may prevent subsequent coronary artery abnormalities. It is worth noting that the additional target therapy, aside from steroids as initial or refractory treatment, works well or even better than the additional steroids in reducing the incidence of CAL. It is also highly anticipated that more controlled trials will be conducted using different non-steroid target therapies with IVIG combination regimens with the aim of lowering the incidence of CAL in the future.
Finally, both the high IVIG + aspirin + infliximab and the high IVIG + aspirin + IVMP groups exhibited the lowest serious adverse event rates among all the pharmacologic interventions when used as the initial treatment. Nevertheless, although the adverse events related to a single methylprednisolone pulse steroid therapy were almost transient and recovered spontaneously, hypothermia, hypokalaemia, shock, respiratory failure, and hypercoagulopathy have been reported.48,49 In addition, various pathogens possibly trigger KD, including Staphylococcus, haemolytic Streptococcus,50 and Yersinia, and these may not be cleared thoroughly under the negative effects of high-dose steroids on the immune system, resulting in persistent infectious status and the risk of shock. Furthermore, the complications associated with chronic infliximab therapy have been clearly documented in children with juvenile arthritis and Crohn's disease by increasing the risk of mycobacterial infection and the development of anti-drug antibodies. Although single infusions of infliximab for KD were tolerated by children and infants, with only local reactions over the injection site, and there are only 2.5%–5% of adverse events per infusion among paediatric Crohn's disease,51,52 patients should be cautious and closely monitored when using either the additional infliximab or IVMP therapy in clinical practice.
Although the overall risk of bias of the current NMA was relatively low, this study has several limitations that need to be addressed. First, some analyses in the current study were potentially underpowered by small sample sizes, heterogeneity in the characteristics of the participants (e.g. initial disease severity of KD, atypical or typical presentation of KD, and trial duration), the small numbers of trials for some treatment arms, heterogeneous aspirin dosage, definition of defervescence, and standardisation of CAL assessment. The diversity of diagnostic criteria across the areas (i.e. Asia, the US, and other countries) or publication years might have contributed to potential bias in the main results. The wide variety of diagnostic criteria for KD across different areas and years could also potentially increase the heterogeneity of recruited participants. We attempted to resolve this issue of heterogeneity using a statistical method. However, although there has been no significant inconsistency in most NMA cases, clinicians should still need to pay special attention when applying our results in clinical practice. Second, the methodological quality of the included trials may have contributed to the outcomes, which may then have limited the interpretation. Third, severe presentations such as Kawasaki shock syndrome were not shown in the studies pooled in the current analysis; thus, our results may not be applicable for the treatment of Kawasaki shock syndrome. Fourth, there have been some RCTs investigating the treatment effect of additive dipyridamole in Kawasaki disease which were excluded from the current NMA because of disconnection from the network structure. Fifth, we chose the incidence of CAL as one of the outcomes in the current NMA because this was what most RCTs provided. Although information on the severity of CAL is clinically important, these data were limited among the included RCTs. Finally, global inconsistency was detected in the NMA of the incidence of CAL in the refractory stage. However, no local inconsistency was detected in the NMA of the incidence of CAL in the refractory stage by the node-splitting method. The limited information on available indirect evidence limits the power of the inconsistency test. Therefore, the non-statistically significant P-value for these tests is not considered reassuring.
In summary, under the currently limited evidence in the current NMA, the present data suggest that combination therapy with the standard therapy of IVIG and aspirin on decreasing fever duration might have additional effects. However, more clinical trials with larger sample sizes are required to confirm this finding. In addition, for children with refractory KD, the combination therapy of high-dose IVIG with pulse steroid therapy or cyclosporine therapy might have an additional effect on improving the decline in fever and lowering the incidence of CAL. Further, the main results remained the same when we focused on RCTs recruiting participants who had definitely failed to receive standard therapy (i.e. IVIG 2 g/kg or IVIG 2 g/kg plus aspirin) before enrolment.
Contributors
WT Lei, LS Chang, and BY Zeng, who contributed equally as first authors, took the whole responsibility of literature search, data extraction, and manuscript drafting.
YK Tu and YC Wu contributed in study design, concept formation, statistical revision, and major revision of the manuscript.
R Uehara, YJ Matsuoka, KP Su, PC Lee, JL Cavalcante, B Stubbs, PY Lin, CW Hsu, TY Chen, YW Chen, PY Yeh, and CK Sun contributed in study design, concept formation, and major revision of the manuscript.
PT Tseng and YH Kao, who contributed equally as corresponding authors, took the responsibility of collection of all information from the other co-authors, major revision of the manuscript, and full access to the data.
All authors read and approved the final version of the manuscript
Data sharing statement
All the data and materials of the current study are available upon reasonable request.
Declaration of interests
The authors report no financial interests or potential conflicts of interest.
Acknowledgment
The current study did not receive any funding or financial supports.
Brendon Stubbs is supported by a Clinical Lectureship (ICA-CL-2017-03-001) jointly funded by Health Education England (HEE) and the National Institute for Health Research (NIHR). Brendon Stubbs is part funded by the NIHR Biomedical Research Centre at South London and Maudsley NHS Foundation Trust. Brendon Stubbs is also supported by the Maudsley Charity, King's College London and the NIHR South London Collaboration for Leadership in Applied Health Research and Care (CLAHRC) funding. This paper presents independent research. The views expressed in this publication are those of the authors and not necessarily those of the acknowledged institutions.
Footnotes
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.ebiom.2022.103946.
Contributor Information
Ping-Tao Tseng, Email: ducktseng@gmail.com.
Yu-Hsuan Kao, Email: evakao65@gmail.com.
Appendix. Supplementary materials
References
- 1.McCrindle B.W., Rowley A.H., Newburger J.W., et al. Diagnosis, treatment, and long-term management of Kawasaki disease: a scientific statement for health professionals from the American Heart Association. Circulation. 2017;135(17):e927–ee99. doi: 10.1161/CIR.0000000000000484. [DOI] [PubMed] [Google Scholar]
- 2.Uehara R., Belay E.D. Epidemiology of Kawasaki disease in Asia, Europe, and the United States. J Epidemiol. 2012;22(2):79–85. doi: 10.2188/jea.JE20110131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Daniels L.B., Tjajadi M.S., Walford H.H., et al. Prevalence of awasaki disease in young adults with suspected myocardial ischemia. Circulation. 2012;125(20):2447–2453. doi: 10.1161/CIRCULATIONAHA.111.082107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gordon J.B., Kahn A.M., Burns J.C. When children with Kawasaki disease grow up: myocardial and vascular complications in adulthood. J Am Coll Cardiol. 2009;54(21):1911–1920. doi: 10.1016/j.jacc.2009.04.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dajani A.S., Taubert K.A., Takahashi M., et al. Guidelines for long-term management of patients with Kawasaki disease. Report from the committee on rheumatic fever, endocarditis, and Kawasaki disease, council on cardiovascular disease in the young, American Heart Association. Circulation. 1994;89(2):916–922. doi: 10.1161/01.cir.89.2.916. [DOI] [PubMed] [Google Scholar]
- 6.Uehara R., Belay E.D., Maddox R.A., et al. Analysis of potential risk factors associated with nonresponse to initial intravenous immunoglobulin treatment among Kawasaki disease patients in Japan. Pediatr Infect Dis J. 2008;27(2):155–160. doi: 10.1097/INF.0b013e31815922b5. [DOI] [PubMed] [Google Scholar]
- 7.Miura M., Kobayashi T., Kaneko T., et al. Association of severity of coronary artery aneurysms in patients with Kawasaki disease and risk of later coronary events. JAMA Pediatr. 2018;172(5) doi: 10.1001/jamapediatrics.2018.0030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ogata S., Ogihara Y., Honda T., Kon S., Akiyama K., Ishii M. Corticosteroid pulse combination therapy for refractory Kawasaki disease: a randomized trial. Pediatrics. 2012;129(1):e17–e23. doi: 10.1542/peds.2011-0148. [DOI] [PubMed] [Google Scholar]
- 9.Hirono K., Kemmotsu Y., Wittkowski H., et al. Infliximab reduces the cytokine-mediated inflammation but does not suppress cellular infiltration of the vessel wall in refractory Kawasaki disease. Pediatr Res. 2009;65(6):696–701. doi: 10.1203/PDR.0b013e31819ed68d. [DOI] [PubMed] [Google Scholar]
- 10.Nanishi E., Nishio H., Takada H., et al. Clarithromycin plus intravenous immunoglobulin therapy can reduce the relapse rate of Kawasaki disease: a Phase 2, open-label, randomized control study. J Am Heart Assoc. 2017;6(7) doi: 10.1161/JAHA.116.005370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen S., Dong Y., Kiuchi M.G., et al. Coronary artery complication in Kawasaki disease and the importance of early intervention : a systematic review and meta-analysis. JAMA Pediatr. 2016;170(12):1156–1163. doi: 10.1001/jamapediatrics.2016.2055. [DOI] [PubMed] [Google Scholar]
- 12.Wardle A.J., Connolly G.M., Seager M.J., Tulloh RM. Corticosteroids for the treatment of Kawasaki disease in children. Cochrane Database Syst Rev. 2017;1 doi: 10.1002/14651858.CD011188.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Page M.J., McKenzie J.E., Bossuyt P.M., et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. doi: 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shea B.J., Reeves B.C., Wells G., et al. AMSTAR 2: a critical appraisal tool for systematic reviews that include randomised or non-randomised studies of healthcare interventions, or both. BMJ. 2017;358:j4008. doi: 10.1136/bmj.j4008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yang T.J., Lin M.T., Lu C.Y., et al. The prevention of coronary arterial abnormalities in Kawasaki disease: a meta-analysis of the corticosteroid effectiveness. J Microbiol Immunol Infect. 2018;51(3):321–331. doi: 10.1016/j.jmii.2017.08.012. [DOI] [PubMed] [Google Scholar]
- 16.Eleftheriou D., Levin M., Shingadia D., Tulloh R., Klein N.J., Brogan PA. Management of Kawasaki disease. Arch Dis Child. 2014;99(1):74–83. doi: 10.1136/archdischild-2012-302841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yeo J.S., Choi J.W. Effectiveness of medium-dose intravenous immunoglobulin (1 g/kg) in the treatment of Kawasaki disease. Korean Circ J. 2010;40(2):81–85. doi: 10.4070/kcj.2010.40.2.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Higgins J.G.S. The Cochrane Collaboration; 2009. Cochrane Handbook for Systematic Reviews of Interventions. Version 5.0.2. [Google Scholar]
- 19.Cheng J., Pullenayegum E., Marshall J.K., Iorio A., Thabane L. Impact of including or excluding both-armed zero-event studies on using standard meta-analysis methods for rare event outcome: a simulation study. BMJ Open. 2016;6(8) doi: 10.1136/bmjopen-2015-010983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brockhaus A.C., Bender R., Skipka G. The Peto odds ratio viewed as a new effect measure. Stat Med. 2014;33(28):4861–4874. doi: 10.1002/sim.6301. [DOI] [PubMed] [Google Scholar]
- 21.Tu Y.K. Use of generalized linear mixed models for network meta-analysis. Med Decis Mak Int J Soc Med Decis Mak. 2014;34(7):911–918. doi: 10.1177/0272989X14545789. [DOI] [PubMed] [Google Scholar]
- 22.Lu G., Ades A.E. Combination of direct and indirect evidence in mixed treatment comparisons. Stat Med. 2004;23(20):3105–3124. doi: 10.1002/sim.1875. [DOI] [PubMed] [Google Scholar]
- 23.White I.R. Network meta-analysis. Stata J. 2015;15(4):951–985. [Google Scholar]
- 24.Kontopantelis E., Springate D.A., Reeves D. A re-analysis of the Cochrane library data: the dangers of unobserved heterogeneity in meta-analyses. PLoS One. 2013;8(7):e69930. doi: 10.1371/journal.pone.0069930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Salanti G., Ades A.E., Ioannidis J.P. Graphical methods and numerical summaries for presenting results from multiple-treatment meta-analysis: an overview and tutorial. J Clin Epidemiol. 2011;64(2):163–171. doi: 10.1016/j.jclinepi.2010.03.016. [DOI] [PubMed] [Google Scholar]
- 26.Higgins J.P., Del Giovane C., Chaimani A., Caldwell D.M., Salanti G. Evaluating the quality of evidence from a network meta-analysis. Value Health. 2014;17(7):A324. doi: 10.1016/j.jval.2014.08.572. [DOI] [PubMed] [Google Scholar]
- 27.Burns J.C., Best B.M., Mejias A., et al. Infliximab treatment of intravenous immunoglobulin-resistant Kawasaki disease. J Pediatr. 2008;153(6):833–838. doi: 10.1016/j.jpeds.2008.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mori M., Hara T., Kikuchi M., et al. Infliximab versus intravenous immunoglobulin for refractory Kawasaki disease: a phase 3, randomized, open-label, active-controlled, parallel-group, multicenter trial. Sci Rep. 2018;8(1):1994. doi: 10.1038/s41598-017-18387-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Youn Y., Kim J., Hong Y.M., Sohn S. Infliximab as the first retreatment in patients with Kawasaki disease resistant to initial intravenous immunoglobulin. Pediatr Infect Dis J. 2016;35(4):457–459. doi: 10.1097/INF.0000000000001039. [DOI] [PubMed] [Google Scholar]
- 30.Miura M., Ohki H., Yoshiba S., et al. Adverse effects of methylprednisolone pulse therapy in refractory Kawasaki disease. Arch Dis Child. 2005;90(10):1096–1097. doi: 10.1136/adc.2004.062299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hashino K., Ishii M., Iemura M., Akagi T., Kato H. Re-treatment for immune globulin-resistant Kawasaki disease: a comparative study of additional immune globulin and steroid pulse therapy. Pediatr Int. 2001;43(3):211–217. doi: 10.1046/j.1442-200x.2001.01373.x. [DOI] [PubMed] [Google Scholar]
- 32.Chen S., Dong Y., Yin Y., Krucoff MW. Intravenous immunoglobulin plus corticosteroid to prevent coronary artery abnormalities in Kawasaki disease: a meta-analysis. Heart. 2013;99(2):76–82. doi: 10.1136/heartjnl-2012-302126. [DOI] [PubMed] [Google Scholar]
- 33.Yang T.J., Lin M.T., Lu C.Y., et al. The prevention of coronary arterial abnormalities in Kawasaki disease: a meta-analysis of the corticosteroid effectiveness. J Microbiol Immunol Infect. 2018;51(3):321–331. doi: 10.1016/j.jmii.2017.08.012. [DOI] [PubMed] [Google Scholar]
- 34.Zhu B.H., Lv H.T., Sun L., et al. A meta-analysis on the effect of corticosteroid therapy in Kawasaki disease. Eur J Pediatr. 2012;171(3):571–578. doi: 10.1007/s00431-011-1585-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Singh S., Sharma D., Suri D., Gupta A., Rawat A., Rohit MK. Infliximab is the new kid on the block in Kawasaki disease: a single-centre study over 8 years from North India. Clin Exp Rheumatol. 2016;34(3):S134–S138. Suppl 97. [PubMed] [Google Scholar]
- 36.Newburger J.W., Takahashi M., Gerber M.A., et al. Diagnosis, treatment, and long-term management of Kawasaki disease: a statement for health professionals from the committee on rheumatic fever, endocarditis and Kawasaki disease, council on cardiovascular disease in the young, American Heart Association. Circulation. 2004;110(17):2747–2771. doi: 10.1161/01.CIR.0000145143.19711.78. [DOI] [PubMed] [Google Scholar]
- 37.Franco A., Shimizu C., Tremoulet A.H., Burns JC. Memory T-cells and characterization of peripheral T-cell clones in acute Kawasaki disease. Autoimmunity. 2010;43(4):317–324. doi: 10.3109/08916930903405891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Boschetti G., Nancey S., Sardi F., Roblin X., Flourie B., Kaiserlian D. Therapy with anti-TNFalpha antibody enhances number and function of Foxp3(+) regulatory T cells in inflammatory bowel diseases. Inflamm Bowel Dis. 2011;17(1):160–170. doi: 10.1002/ibd.21308. [DOI] [PubMed] [Google Scholar]
- 39.Nadkarni S., Mauri C., Ehrenstein MR. Anti-TNF-alpha therapy induces a distinct regulatory T cell population in patients with rheumatoid arthritis via TGF-beta. J Exp Med. 2007;204(1):33–39. doi: 10.1084/jem.20061531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ogata S., Ogihara Y., Nomoto K., et al. Clinical score and transcript abundance patterns identify Kawasaki disease patients who may benefit from addition of methylprednisolone. Pediatr Res. 2009;66(5):577–584. doi: 10.1203/PDR.0b013e3181baa3c2. [DOI] [PubMed] [Google Scholar]
- 41.Sundel R.P., Baker A.L., Fulton D.R., Newburger JW. Corticosteroids in the initial treatment of Kawasaki disease: report of a randomized trial. J Pediatr. 2003;142(6):611–616. doi: 10.1067/mpd.2003.191. [DOI] [PubMed] [Google Scholar]
- 42.Miura M., Kohno K., Ohki H., Yoshiba S., Sugaya A., Satoh M. Effects of methylprednisolone pulse on cytokine levels in Kawasaki disease patients unresponsive to intravenous immunoglobulin. Eur J Pediatr. 2008;167(10):1119–1123. doi: 10.1007/s00431-007-0642-5. [DOI] [PubMed] [Google Scholar]
- 43.Stahn C., Lowenberg M., Hommes D.W., Buttgereit F. Molecular mechanisms of glucocorticoid action and selective glucocorticoid receptor agonists. Mol Cell Endocrinol. 2007;275(1-2):71–78. doi: 10.1016/j.mce.2007.05.019. [DOI] [PubMed] [Google Scholar]
- 44.Tsujimoto H., Takeshita S., Nakatani K., Kawamura Y., Tokutomi T., Sekine I. Intravenous immunoglobulin therapy induces neutrophil apoptosis in Kawasaki disease. Clin Immunol (Orlando, Fla) 2002;103(2):161–168. doi: 10.1006/clim.2002.5209. [DOI] [PubMed] [Google Scholar]
- 45.Onouchi Y., Gunji T., Burns J.C., et al. ITPKC functional polymorphism associated with Kawasaki disease susceptibility and formation of coronary artery aneurysms. Nat Genet. 2008;40(1):35–42. doi: 10.1038/ng.2007.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Onouchi Y., Suzuki Y., Suzuki H., et al. ITPKC and CASP3 polymorphisms and risks for IVIG unresponsiveness and coronary artery lesion formation in Kawasaki disease. Pharmacogenomics J. 2013;13(1):52–59. doi: 10.1038/tpj.2011.45. [DOI] [PubMed] [Google Scholar]
- 47.Hamada H., Suzuki H., Onouchi Y., et al. Efficacy of primary treatment with immunoglobulin plus ciclosporin for prevention of coronary artery abnormalities in patients with Kawasaki disease predicted to be at increased risk of non-response to intravenous immunoglobulin (KAICA): a randomised controlled, open-label, blinded-endpoints, phase 3 trial. Lancet. 2019;393(10176):1128–1137. doi: 10.1016/S0140-6736(18)32003-8. [DOI] [PubMed] [Google Scholar]
- 48.Newburger J.W., Sleeper L.A., McCrindle B.W., et al. Randomized trial of pulsed corticosteroid therapy for primary treatment of Kawasaki disease. N Engl J Med. 2007;356(7):663–675. doi: 10.1056/NEJMoa061235. [DOI] [PubMed] [Google Scholar]
- 49.Okada K., Hara J., Maki I., et al. Pulse methylprednisolone with gammaglobulin as an initial treatment for acute Kawasaki disease. Eur J Pediatr. 2009;168(2):181–185. doi: 10.1007/s00431-008-0727-9. [DOI] [PubMed] [Google Scholar]
- 50.Nagata S. Causes of Kawasaki disease-from past to present. Front Pediatr. 2019;7:18. doi: 10.3389/fped.2019.00018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Baldassano R., Braegger C.P., Escher J.C., et al. Infliximab (REMICADE) therapy in the treatment of pediatric Crohn's disease. Am J Gastroenterol. 2003;98(4):833–838. doi: 10.1111/j.1572-0241.2003.07343.x. [DOI] [PubMed] [Google Scholar]
- 52.Parashette K.R., Makam R.C., Cuffari C. Infliximab therapy in pediatric Crohn's disease: a review. Clin Exp Gastroenterol. 2010;3:57–63. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.