Abstract
Opioids are among the most effective analgesics and the mainstay of pain management. However, concerns about safety and abuse liability have challenged their widespread use by the medical community. Opioid-sparing therapies include drugs that in combination with opioids have the ability to enhance analgesia while decreasing opioid requirement as well as their side effects. Sex differences in antinociceptive responses to opioids have received increasing attention in recent years. However, the molecular mechanisms underlying sex differences related to opioid-sparing adjuncts remain largely unexplored. Using warm water tail-withdrawal as a mouse model of acute thermal nociception, our data suggest that adjunctive administration of the serotonin 5-HT2A receptor (5-HT2AR) antagonist volinanserin dose-dependently enhanced potency of the opioid analgesic oxycodone in male, but not female, mice. This antinociceptivelike response induced by oxycodone was also augmented in 5-HT2AR knockout (5-HT2AR−/−) male, but not female mice; an effect that was reversed by Cre-loxP-mediated selective expression of 5-HT2AR in dorsal root ganglion (DRG) neurons of 5-HT2AR−/− littermates. Pharmacological inhibition with volinanserin or genetic deletion in 5-HT2AR−/− animals potentiated the ability of oxycodone to reduce DRG excitability in male mice. Adjunctive volinanserin did not affect oxycodone-induced conditioned place preference (CPP), whereas it reduced oxycodone-induced locomotor sensitization in male and female mice. Together, these results suggest that adjunctive volinanserin augments opioid-induced antinociception, but not abuse-related behavior, through a sex-specific signaling crosstalk mechanism that requires 5-HT2AR expression in mouse DRG neurons. Ultimately, our results may pave the way for the clinical evaluation of volinanserin as a potential sex-specific opioid adjuvant.
Keywords: Oxycodone, serotonin 5-HT2A receptor, opioid receptor, G protein-coupled receptor (GPCR), analgesia, pain, antinociception, non-opioid adjuvant, substance use disorder
Graphical Abstract
1. Introduction
Over the past 25 years the United States has experienced a dramatic increase in deaths from opioid overdose, opioid use disorder, and other harms in parallel with increases in the prescribing of opioid medications for acute and chronic pain management (Volkow and Collins, 2017; Jalal et al., 2018; Townsend et al., 2020). However, opioids are still a mainstay of pain treatment despite their abuse concerns (Twycross and Lickiss, 1996; Christie, 2008). Nonopioid adjuvants, defined as drugs that enhance the antinociceptive efficacy when used in conjunction with opioid analgesics, are a promising strategy to mitigate risks associated with opioid use. The rationale behind the combination of opioids and non-opioid adjuvants lies in their capacity to modulate pain-related pathways, thereby increasing analgesia and reducing opioid doses as well as their side effects, including misuse rate. Commonly used non-opioid adjuvants include glucocorticoids, non-steroidal anti-inflammatory drugs (NSAIDS), and cannabinoids (Ghosh and Berger, 2014). However, these adjunctive effects are mostly additive, since current non-opioid adjuvants are generally analgesics per se, albeit weaker than opioids (Goldstein, 2002).
Pharmacological or genetic tools that directly or indirectly modulate opioid-induced signaling pathways downstream of the μ-opioid receptor (μ-OR) lead to a positive augmentation of opioid-dependent antinociception, yet they also usually increase opioid-induced negative effects, such as abuse potential, constipation, and respiratory depression (Bohn et al., 1999; Bohn et al., 2000; Raehal et al., 2005; Nelson and Camilleri, 2016; Porter-Stransky and Weinshenker, 2017; Zebala et al., 2019; Kliewer et al., 2020). It would therefore be important from a translational perspective to evaluate the adjunctive effects of pharmacological tools that directly target molecular mechanisms and circuits related to opioid-induced antinociceptive outcomes, but not to opioid-induced negative side effects.
Previous findings have shown a functional crosstalk between the serotonin (5-hydroxytryptamine, 5-HT) and the opioid receptor systems. As an example, the relatively selective serotonin 2C receptor (5-HT2CR) agonist lorcaserin was reported to reduce abuse-related properties of several drugs, including opioids, in rodent models (Neelakantan et al., 2017; Anastasio et al., 2020). Interestingly, we recently showed that adjunctive administration of lorcaserin augments opioid-induced antinociception in mice. This was tested using the warm water tail-withdrawal test (or tail-flick response, TFR) as a mouse model of acute thermal nociception (Sierra et al., 2020). Lorcaserin, however, has already been placed into Schedule IV because of its psychedelic properties (Iqbal et al., 2019), probably mediated via agonist effects at the closely related serotonin 2A receptor (5-HT2AR) (Lopez-Gimenez and Gonzalez-Maeso, 2018). More recently, the FDA requested that the manufacturer voluntarily withdraw lorcaserin from the market because of increased occurrence of cancer (Sharretts et al., 2020). These cancer concerns and drug abuse potential will most likely limit the use of lorcaserin as an adjuvant to augment opioid-induced analgesia.
Serotonin 5-HT2CR and 5-HT2AR are class A G protein-coupled receptors (GPCRs) that modulate the function of similar canonical pathways downstream – these include stimulation of heterotrimeric Gq/11 proteins followed by activation of phospholipase C (PLC), which promotes the generation of intracellular inositol phosphates (IP) and hence release of Ca2+ from the endoplasmic reticulum (McCorvy and Roth, 2015; Sharp and Barnes, 2020). Activation of 5-HT2CR and 5-HT2AR usually exerts opposing behavioral effects in rodent models (Filip et al., 2004; Gonzalez-Maeso et al., 2007; Halberstadt et al., 2009; Canal et al., 2010; Halberstadt et al., 2016). As an example, activation of 5-HT2AR by the agonist DOI increases locomotor activity whereas activation of 5-HT2CR by the agonist WAY161503 produces reduction in locomotor activity (Halberstadt et al., 2009). Interestingly, previous results suggested that 5-HT2AR antagonists, such as volinanserin (also known as M100907) and MDL11,939, induce antinociceptive effects in experimental models of neuropathic pain (Kayser et al., 2007; Thibault et al., 2008; Van Steenwinckel et al., 2008; Aira et al., 2012). These previous studies, however, tested the outcomes of relatively high doses of volinanserin by itself, and not as an adjuvant (Kayser et al., 2007; Thibault et al., 2008; Van Steenwinckel et al., 2008; Aira et al., 2012), which may also negatively affect locomotor activity and motor coordination in rodents (Fribourg et al., 2011; Hideshima et al., 2018).
Sex differences in opioid analgesia have been demonstrated in multiple preclinical studies using different rodent models of acute and chronic pain (Craft, 2003; Loyd et al., 2008; Diester et al., 2019; Pisanu et al., 2019). These include sex differences in opiate sensitivity due to sex-related pharmacokinetic variations with higher brain levels of morphine in male rats (Fillingim and Gear, 2004). This however may not fully explain sex differences in opioid antinociception since they are also observed after central administration of morphine and other opioid analgesics (Kepler et al., 1989; Krzanowska and Bodnar, 1999; Krzanowska et al., 2002). Further investigation is required to determine whether sex differences in opioid analgesia are due to changes in opiate receptor density, binding and localization, as well as sex-related phenotypes in the anatomy and physiology of opiate-responsive neural circuits.
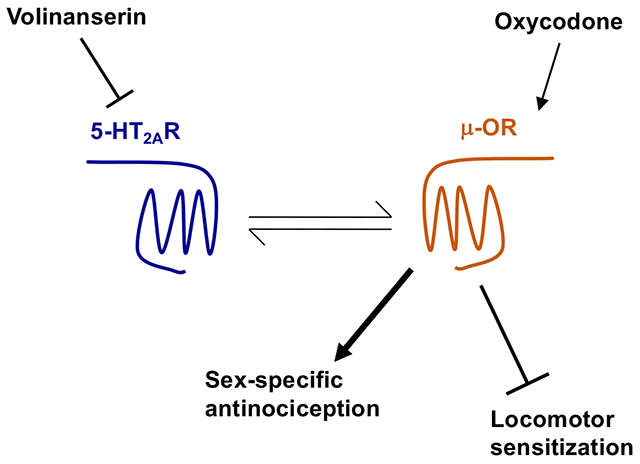
Here we tested whether volinanserin enhanced the antinociceptive effect induced by the opioid analgesic drug oxycodone in male and female mice. Using a Cre-loxP approach to restore 5-HT2AR expression in cell types and brain regions of 5-HT2AR−/− mice that included dorsal root ganglion (DRG) neurons, frontal cortex glutamatergic neurons and dopaminergic neurons, we also evaluated the cell target responsible for the adjunctive effects of volinanserin on mouse models of antinociception and reward-related behaviors. Our data suggest that adjunctive volinanserin augments oxycodone-induced antinociception through a sex-specific signaling crosstalk mechanism that requires 5-HT2AR expression in DRG neurons, whereas 5-HTA2R-dependent signaling either augmented or reduced oxycodone-induced locomotor sensitization based upon the neuronal target.
2. Materials and methods
2.1. Drugs
Oxycodone and 2-((1S,2S,5S)-5-hydroxy-2-(3-hydroxypropyl)cyclohexyl)-5-(2-methyloctan-2-yl)phenol (CP55,490) were generously supplied by the National Institute on Drug Abuse Drug Supply Program (Washington, DC). (R)-(2,3-Dimethoxyphenyl)-[1-[2-(4-fluorophenyl)ethyl]piperidin-4-yl]methanol (volinanserin, M100907) was purchased from Tocris (Minneapolis, MN). Volinanserin was dissolved in 1 eq HCl and water, brought to a pH ~7.4 using NaOH 1M (1 mg/ml stock solutions), and diluted in sterile saline (0.9%). Oxycodone and CP55,490 were dissolved in sterile saline (0.9%). Oxycodone was administered via oral gavage (o.g.) for tail-flick and locomotion studies or subcutaneously (s.c.) for CPP assays. CP55,490 and volinanserin were administered intraperitoneally (i.p.). Saline (0.9%) was used as a vehicle. All other chemicals were obtained from standard sources.
2.2. Animals
All assays were performed on adult (10-20 weeks old) mice. 5-HT2AR knockout (loxP-STOP-loxP; 5-HT2AR−/−) on a 129S6/SvEv background have been previously described (Gonzalez-Maeso et al., 2007), and were backcrossed onto the inbred C57BL/6J background obtained from The Jackson Laboratory (Bar Harbor, ME). Pirt-Cre mice on a C57BL/6J background were kindly donated by Dr. Xinzhong Dong at Johns Hopkins University (Kim et al., 2008; Kim et al., 2016). Mice expressing a monomeric red fluorescent protein (mCherry) knocked into the last exon of μ-OR on a C57BL/6J background (μ-OR-mCherry) (Stock No. 029013), Emx1-Cre mice (Stock No. 005628) and DAT-Cre mice (Stock No. 006660) on a C57BL/6J background were obtained from The Jackson Laboratory (Bar Harbor, ME). Mice were housed in groups of three to five in 500 cm2 Plexiglas cages (Allentown, NJ) in the animal care facilities (22 °C, 12-h light-dark cycle) with ad libitum access to food (Teklad LM-485 mouse diet, Envigo) and water. The cage contained bedding (Teklad L/4” Corncob bedding, Envigo) and one Nestlests™ (Ancare, Bellmore, NY, USA). All procedures were conducted in accordance with the National Institutes of Health (NIH) guidelines and were approved by the Virginia Commonwealth University Animal Care and Use Committee. Behavioral testing took place between 9:00 a.m. and 6:00 p.m. Behavioral tests were separated by at least one week wash-out period, and separate cohorts of mice were used for each behavioral condition.
2.3. Warm water tail-withdrawal test
The warm water tail-withdrawal test (hereinafter referred to as TFR test) was carried out as previously reported (Sierra et al., 2020) with minor modifications. Briefly, mice were moved to the laboratory and allowed to acclimate for approximately one-week prior experimentation. The mouse’s tail was then immersed in a 52 °C water bath, and the latency to withdraw the tail from the bath was measured with a maximum cut-off value of 10 s to prevent tissue damage to the tail. Before drug or vehicle administration, the baseline (control) latency for each mouse was determined and only mice with a control reaction time from 2 to 4 s were tested. Experimenters were blind to experimental conditions throughout. The test latency was assessed 20 min after drug or vehicle administration (cumulative drug studies) or every 20 min after drug or vehicle administration (time-course assays). Antinociception was quantified according to the method of Harris & Pierson (Harris and Pierson, 1964) as the percentage of maximum possible effect (%MPE) which was calculated as: %MPE = [(test – baseline)/(10 – baseline)] × 100.
2.4. Complete Freund’s adjuvant (CFA)-induced inflammatory model
CFA-induced paw inflammation was induced as previously reported with minor modifications (Alsharari et al., 2013). Briefly, mice were habituated on an elevated mesh stand with 15 cm diameter acrylic enclosures for 3 consecutive days (40 min/session). On the fourth day, mice were placed on the elevated mesh after which mechanical threshold (baseline) was assessed by applying calibrated von Frey filaments (Stoelting Co., Wood Dale, IL) to the midplantar region of the right hindpaw for approximately 2 s per stimulus, as previously reported (Sierra et al., 2019). A response (sudden paw withdrawal or licking) in two out of the three trials was considered positive. The next day, mice were injected with Complete Freund’s Adjuvant (Sigma-Aldrich) in the intraplantar region of the right hindpaw and, 24 h later, mice were tested again. After this, mice received volinanserin (0.125 mg/kg, i.p.) or vehicle and 20 min later an oral dose of oxycodone (4 mg/kg, o.g.) or vehicle, and were tested 20 min later. The mechanical threshold (paw withdrawal threshold) is expressed in grams, indicating the force of the von Frey hair to which the animal reacted (paw withdrawal or licking), or as threshold difference over the baseline.
2.5. Locomotor activity
Locomotor activity assays were carried out as previously reported (Sierra et al., 2020) with minor modifications. Briefly, mice were moved to the laboratory and allowed to acclimate for 3 days prior to the experiments. Spontaneous locomotor activity was measured in enclosed, sound attenuating activity monitors (Med Associates, St. Albans, VT) that record “ambulatory counts” via photo beam breaks. Numbers of photo beam breaks were recorded in 5-min time blocks. For acute locomotor activity upon volinanserin treatment, mice were administered volinanserin (i.p.) or vehicle (i.p.) and placed in the locomotor chamber. After 20 min, mice were administered vehicle (o.g.) and immediately placed in the locomotor chamber for another 30 min. For acute locomotor activity upon volinanserin/oxycodone treatment, mice were administered volinanserin (i.p.) or vehicle (i.p.) and placed in the locomotor chamber. After 20 min, mice were administered oxycodone (o.g.) or vehicle and immediately placed in the locomotor chamber for another 30 min. For locomotion sensitization assays, mice received volinanserin (i.p.) or vehicle, and/or oxycodone (o.g.) or vehicle (o.g.) for 5 days (days 1-5), through which locomotor activity was tested daily. On day 8, all mice received a saline (i.p.) injection followed by oxycodone (o.g.) after 20 min and immediately placed in the locomotor chamber for 30 min.
2.6. Conditioned place preference (CPP)
CPP was tested using an unbiased design as previously described (Walters et al., 2006). Briefly, the three-compartment place preference apparatus (Med Associates, St Albans, VT) contained two pairing compartments (20 × 20 × 20 cm each) with unique visual and tactile cues. That is, one compartment has black walls with a stainless-steel grid floor and the other side has white walls with a stainless-steel mesh floor. A third smaller grey chamber with a smooth PVC floor separates both compartments through two guillotine doors. Mice were handled for 1 week followed by a 5-day protocol in three phases. The first day (preconditioning phase), mice were placed in the grey chamber for 5 min (habituation) followed by a 15 min test period with both guillotine doors opened to determine the baseline time spent in the pairing sides of the apparatus. An automated video tracking system was used to record the preference score, and mice were divided into four groups (saline/saline, saline/oxycodone, volinanserin/saline, volinanserin/oxycodone) with equal bias. After this, mice were randomly assigned to receive the experimental treatment on the black or white compartments. The next three days (conditioning phase), mice received vehicle or experimental treatment in two sessions more than 4 h apart. During conditioning phase, mice received volinanserin (i.p.) or vehicle (i.p.) and were placed in the cage for 20 min. Next, mice received oxycodone (s.c.) or vehicle (s.c.) and placed in the cage for 15 min. After this, mice were placed into either the black or the white compartment with the guillotine doors closed for 20 min. On day 5 (postconditioning phase), mice were drug-free and allowed free access to the pairing compartments after the 5 min habituation period and the time spent in each compartment was recorded. Results are expressed as preference score and was calculated as the difference in time(s) spent in the experimental treatment or saline (control group) paired compartment on day 5 and the time spent in that compartment on day 1.
2.7. Primary culture of dorsal root ganglia neurons
Primary dorsal root ganglia (DRG) cultures were prepared from adult mice as previously reported (Ross et al., 2012). Briefly, lumbar L5 – sacral S1 DRGs were immediately harvested using a dissecting microscope and transferred to a 35 mm petri dish containing Hank’s balanced salt solution (HBSS). Intact DRGs were first enzymatically digested with papain (15 U/ml) (Worthington Biochemical Corporation, Lakewood, NJ) at 37 °C for 18 minutes. After the initial digestion step, DRGs were transferred to a new 35 mm petri dish containing 1.5 mg/ml collagenase from Clostridium histolyticum (MilliporeSigma, Burlington, MA) in HBSS and incubated for 1 hour at 37 °C. After this, DRGs were transferred to a sterile 15 ml conical tube containing ice-cold (4°C) DMEM/F12 (Gibco, Thermo Fisher Scientific, Waltham, MA) supplemented with 10% fetal bovine serum (FBS) (Neuromics, Edina, MN) for trituration. Dissociated DRG cells were then centrifuged at 350 ×g for 5 minutes. The supernatant was decanted and the pellet was re-suspended in complete neuron media (Neurobasal A media supplemented with 1% FBS, 1× B-27, 10 ng/ml glial line-derived neurotrophic factor (GDNF), 2 mM L-glutamine, and 100 U/ml penicillin/streptomycin/amphotericin B. Neurobasal-A media and B27 were purchased from Gibco, Thermo Fisher Scientific (Waltham, MA), GDNF was purchased from Neuromics (Edina, MN), and penicillin/streptomycin/amphotericin B was purchased from Corning (Corning, NY). Isolated cells were then plated on glass cover slips (1 DRG/well) coated with laminin and poly-D-lysine (MilliporeSigma, Burlington, MA) and incubated overnight (15-18 hours) at 37°C in a humidified 5% CO2/air stabilized incubator.
2.8. Whole-cell patch clamp electrophysiology
Patch clamp experiments were performed as previously reported (Ross et al, 2012). Briefly, micropipettes (2-4 MΩ resistance) were pulled (Model P-97 Flaming-Brown Micropipette Puller, Sutter Instruments, Novato, CA) and fire-polished 1.5/0.84 o.d./i.d. (mm) borosilicate glass capillaries (World Precision Instruments, Sarasota, FL). The internal physiological solution constituted 100 mM L-aspartic acid (K salt), 30 mM KCl, 4.5 mM Na2ATP, 1 mM MgCl2, 10 mM HEPES, 0.1 mM EGTA and 0.5 mM NaGTP and was pH adjusted to 7.2 with 3 M KOH. Coverslips with adherent cells were transferred to a microscope stage plate constantly perfused with external physiological salt solution composed of 135 mM NaCl, 5.4 mM KCl, 0.33 mM NaH2PO4, 5 mM HEPES, 5 mM glucose, 2 mM CaCl2 and 1 mM MgCl2, and adjusted to a pH of 7.4 with 1 M NaOH. Whole-cell current-clamp recordings were made at room temperature using HEKA EPC 10 (HEKA, Bellmore, NY) or Axopatch 200B (Molecular Devices, Sunnyvale, CA) amplifiers at a 10 kHz sampling frequency and 2.9 kHz low-pass Bessel filtering. Pulse generation and data acquisition were achieved with PatchMaster v2x60 (HEKA) or pClamp 10.2 software (Molecular Devices). The current-clamp protocol consisted of 10-15 pA steps, beginning at −30 pA to assess both active and passive cell properties. All current clamp recordings were performed on mCherry-positive cells three-five minutes after achieving whole-cell mode to allow dialysis of internal solution. Pre- “baseline” recordings were taken to ensure cell stability before beginning drug perfusion. Once the patch stabilized, external solution containing 0.3 μM oxycodone was perfused. A recording was taken immediately before this exchange, and served as the “baseline” recording in all studies. A recording was then taken for 10 minutes to capture neuronal responses to oxycodone. Electrical properties such as threshold potential, rheobase, resting membrane potential and capacitance were extrapolated from each recording, and the difference between baseline and following drug exposure was calculated for each cell. Action potential (AP) derivatives were determined using the differential function in the PatchMaster or Clampfit software, where the derivative of the voltage with respect to time (dV/dt) was calculated in order to estimate threshold potential. Threshold potential was defined as the voltage at which dV/dt significantly deviated from zero during the course of the action potential uprise. It was used as the primary measure of neuronal excitability in our experiments. Each coverslip was discarded following drug exposure and the same process was repeated on a freshly-mounted coverslip. For experiments with volinanserin, isolated cells were incubated for 30 minutes in 1 μM volinanserin and neuronal excitability was recorded as described above. Values reported were not corrected for junction potentials (~12 mV). Experiments were performed only on cells with healthy morphology and stable patch. Measures from individual neurons were considered as independent values and not replicates for data analysis. In all electrophysiology experiments ‘n’ represents the number of neurons per group and ‘N’ denotes number of animals per group.
2.9. Radioligand binding assays
Radioligand binding studies were performed in male and female mouse frontal cortex (bregma 2.00 to 1.40 mm), striatum (bregma 1.30 to 0.70 mm) and the DRG (lumbar L5 – sacral S1), as previously described (Shah et al., 2019; de la Fuente Revenga et al., 2021). Briefly, samples were dissected and immediately stored at −80 °C until the day of the assay. Tissue samples were homogenized using a Teflon-glass grinder (50 up and-down strokes) in 1 mL of binding buffer (50 mM Tris-HCl; pH 7.4) supplemented with 0.25 M sucrose and protease inhibitors. The volume was made up to 10 ml with binding buffer, and the crude homogenate was centrifuged at 3000 rpm for 5 min at 4 °C. The supernatant was centrifuged at 18000 rpm for 10 min at 4 °C, and the resultant pellet (P2) was washed with 10 ml of binding buffer and recentrifuged at 18000 rpm for 15 min. Protein concentration was determined using the BioRad protein estimation assay. Samples suspended in binding buffer were incubated with 5 nM [3H]ketanserin (PerkinElmer Life and Analytical Sciences). The final volume in each well was 200 μL. Methysergide (10 μM, Tocris Bioscience) was used to determine nonspecific binding. We previously reported that under these experimental conditions specific [3H]ketanserin binding is almost absent in frontal cortex membrane preparations of 5-HT2AR−/− mice (Gonzalez-Maeso et al., 2007). The free ligand was separated from bound ligand by rapid filtration under vacuum through GF/C glass fiber filters using a MicroBeta Filtermat-96 harvester (PerkinElmer). These filters were then rinsed with ice-cold incubation buffer, dried at 65 °C for 1 h, and counted for radioactivity using a MicroBeta2 detector (PerkinElmer).
2.10. Volinanserin and oxycodone distribution
Seven-point calibration curves for oxycodone at 10-1000 ng/ml or ng/kg for blood or brain/nerve tissue homogenate and for volinanserin at 0.1-10ng/ml or ng/kg for blood or brain/nerve tissue homogenate along with positive and negative controls with or without internal standard (ISTD) were prepared in drug-free mouse blood or brain tissue with each analytical run. Oxycodone and volinanserin were extracted from all calibrators, controls and samples using a liquid/liquid method. In brief, 10 ng/mL oxycodone-d6 and 25H-NBOMe (2-(2,5-dimethoxyphenyl)-N-(2-methoxybenzyl)ethanamine), the Internal standards (ISTDs), were added to each sample along with 200 μl of 0.2 M sodium hydroxide solution followed by 1 ml of hexane/ethyl acetate (9:1).
Samples were mixed and centrifuged. The hexane/ethyl acetate was removed and dried down. The samples were then reconstituted in mobile phase and placed in auto-sampler vials for analysis. The analysis took place on a Shimadzu UPLC system (Kyoto, Japan) attached to a Sciex 6500+ QTRAP system with an IonDrive Turbo V source for TurbolonSpray® (Sciex, Ontario, Canada) controlled by Analyst software (Sciex, Ontario, Canada). Chromatographic separation was performed using a Thermo Hypersil Gold column, 100 × 2.1 mm, 3 micron (Thermofisher Scientific, Waltham, MA). The mobile phase consisted of A: 0.1 mM ammonium formate and B: methanol. The initial mobile phase conditions were 10% B increased to 50% B for 1 min followed by an increased for 2 min to 80% B. The 80% B was held for 0.1 min before returning to 10% B. The flow rate of 1 mL/min was used. The source temperature was set at 650°C, and curtain gas had a flow rate of 30 mL/min. The ionspray voltage was 5500 V, with the ion source gases 1 and 2 having flow rates of 40 mL/min. The declustering potential for oxycodone, volinanserin, and 25H-NBOMe were 75, 100 and 35 V, respectively. The acquisition mode used was multiple reaction monitoring (MRM). The following transition ions (m/z) with their corresponding collision energy (eV) in parentheses were monitored in positive mode: oxycodone 316>256 (35) & 316>298 (25); oxycodone-d6 322>262 (35) & 322>304 (25); volinanserin 374>356 (25) & 374>152 (31); 25H-NBOMe 302>121 (24) & 302>91 (49). The total run time for the analytical method was 4.0 minutes. Linear regression of the peak area of ratios of the quantification transition ions of oxycodone or volinanserin and their ISTDs were used to construct the calibration curves.
2.11. Immunostaining
Immunostaining was carried out in mouse brain and L5-L6 DRG samples. For immunohistofluorescence, sections were incubated with two polyclonal primary antibodies against mCherry (1:200, Origene, MD, USA) and 5-HT2AR (1:50, Neuromics, MN, USA) overnight at 4°C followed by a secondary donkey anti-goat Alexa 594 or anti-rabbit Alexa 488-conjugated antibody (1:100). Sections were counterstained with a mounting medium with DAPI. For immunohistochemistry, sections were incubated with the polyclonal anti-5-HT2AR antibody (1:50) overnight at 4°C followed by a secondary donkey antirabbit-HRP (1:5000, Abcam) 90 min at RT and finally visualized in brown with DAB (Sigma). The specificity of the primary antibody against 5-HT2AR was previously confirmed in experiments with knockout mice (Moreno et al., 2016). Stained samples were inspected under a Zeiss 710 confocal laser-scanning microscope (CLSM). To ensure appropriate visualization of the labeled elements and to avoid false positive results, the emission from the argon laser at 488 nm was filtered through a band pass filter of 505–530 nm and color-coded in green. The emission following excitation with the helium laser at 543 nm was filtered through a band-pass filter of 560–615 nm and color coded in red.
2.12. Data Analysis
Statistical analyses were performed with GraphPad Prism software version 9. No statistical methods were used to predetermine sample sizes, but our sample sizes are similar to those reported in our previous publications. Animals were randomly allocated into the different experimental groups. Datapoints were excluded based on previously established criterion and were set to ± 2 s.d. from the group mean. Nonlinear regression analysis was performed on individual oxycodone dose-response assays. The doses of oxycodone that elicited half-maximal effects (EC50) were obtained and normalized as log EC50 values to be statistically compared (Table S1). Statistical significance of experiments involving three or more groups and two or more treatments was assessed by two-way or three-way ANOVA followed by Bonferroni’s multiple comparison test. Statistical significance of experiments involving time courses was assessed by two-way repeated measures ANOVA followed by Bonferroni’s multiple comparison test. Statistical significance of experiments involving three or more groups was assessed by one-way ANOVA followed by Bonferroni’s multiple comparison test. Statistical significance of experiments involving two groups was assessed by Student’s t test. The level of significance was chosen at p = 0.05. All values included in the figures represent mean ± S.E.M.
3. Results
3.1. Sex-specific adjunctive effect of volinanserin on oxycodone-induced antinociception
To assess antinociception, we tested the effect of adjunctive low non-antinociceptive doses of volinanserin with cumulative doses of oxycodone in male and female mice. Doses of volinanserin were selected based on their lack of suppressor effect on exploratory behavior (Fig. S1A), whereas similar doses of volinanserin (0.125 mg/kg) reduced oxycodone (8 mg/kg)-induced locomotor hyperactivity in male and female mice (Fig. S1B). We also found that exploratory behavior (Fig. S1A) and oxycodone-induced locomotor hyperactivity (Fig. S1B) were both significantly increased in female mice.
Interestingly, volinanserin induced a dose-dependent increase of the antinociceptive effect achieved by cumulative oxycodone in male mice (Fig. 1A and Table S1). A similar effect was observed in time-course assays testing the effect of adjunctive volinanserin (0.125 mg/kg) on the antinociceptive response induced by oxycodone (4 mg/kg) (Fig. 1B). The effect of volinanserin (0.125 mg/kg) by itself on the TFR was comparable to vehicle (Fig. 1B).
Fig. 1.
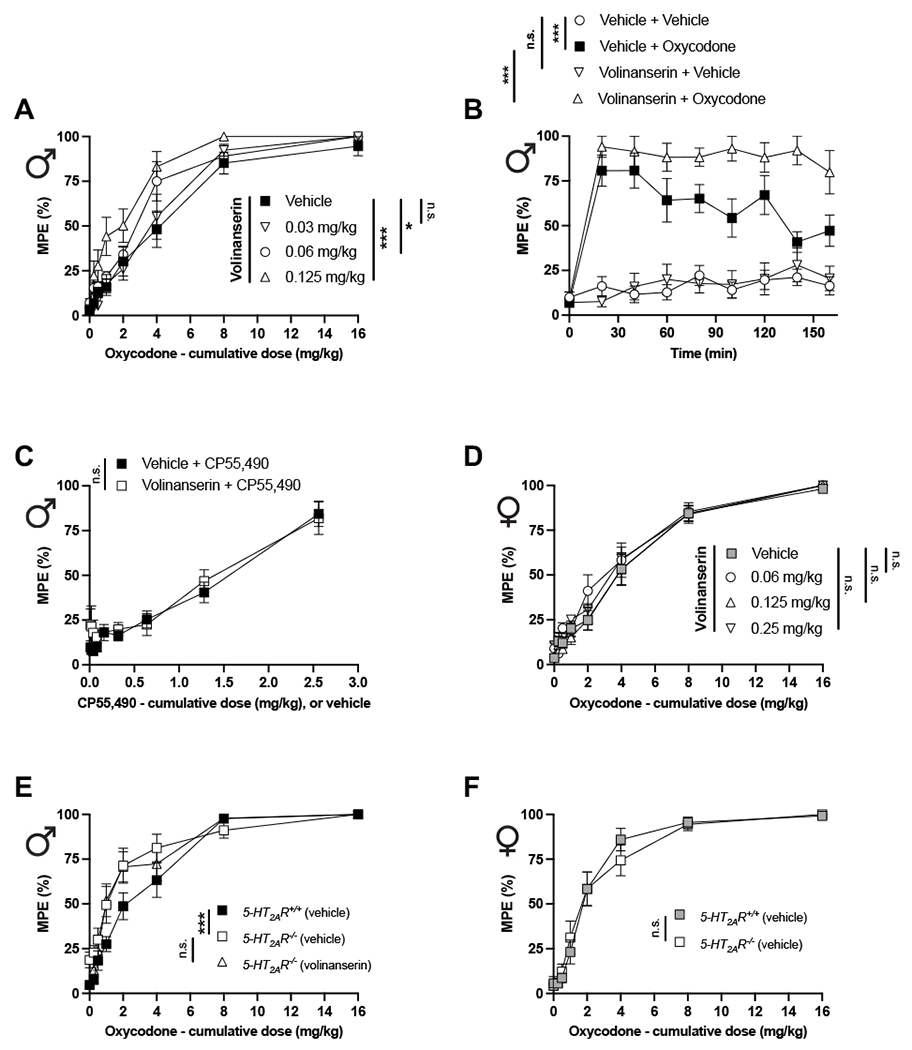
Pharmacological or genetic blockade of 5-HT2AR augments oxycodone-induced antinociception in male but not female mice. (A) Adjunctive effect of different doses of the 5-HT2AR antagonist volinanserin or vehicle on the cumulative dose-response induced by oxycodone in the TFR test in male 5-HT2AR+/+ mice (n = 10 mice per group) (Oxycodone effect F[7,288] = 150.8, p < 0.001; Volinanserin effect F[3,288] = 14.04, p < 0.001). (B) Time-course of the effect of volinanserin (0.12 mg/kg) on oxycodone (4 mg/kg)-induced antinociception in male 5-HT2AR+/+ mice (n = 9-10 mice per group) (Time effect F[8,272] = 159.5, p < 0.001; Volinanserin/Oxycodone effect F[3,34] = 32.23, p < 0.001). (C) Adjunctive effect of volinanserin (0.125 mg/kg) or vehicle on the cumulative dose-response induced by the cannabinoid receptor agonist CP55,490 in the TFR test in male 5-HT2AR+/+ mice (n = 9-10 mice per group) (Oxycodone effect F[8,135] = 33.23, p < 0.001; Volinanserin effect F[1,135] = 3.61, p < 0.05). (D) Adjunctive effect of different doses of volinanserin or vehicle on the cumulative dose-response induced by oxycodone in the TFR test in female 5-HT2AR+/+ mice (n = 15 mice per group) (Oxycodone effect F[7,448] = 239.1, p < 0.001; Volinanserin effect F[3,488] = 2.26, p > 0.05). (E) Adjunctive effect of volinanserin (0.125 mg/kg) or vehicle on the cumulative dose-response induced by oxycodone in the TFR test in male 5-HT2AR+/+ and 5-HT2AR−/− mice (n = 10 mice per group) (Oxycodone effect F[7,216] = 105.9, p < 0.001; Genotype effect F[2,216] = 8.619, p < 0.001). (F) Cumulative dose-response induced by oxycodone in the TFR test in female 5-HT2AR+/+ and 5-HT2AR−/− mice (n = 14 mice per group) (Oxycodone effect F[7,208] = 106.0, p < 0.001; Genotype effect F[1,208] = 0.003, p > 0.05). Two-way ANOVA (A, C, D, E, F) or two-way repeated measures ANOVA (B) followed by Bonferroni’s multiple comparison test. *p < 0.05, **p < 0.01, ***p < 0.001, n.s., not significant. Data are mean ± S.E.M.
Additionally, adjunctive volinanserin administration did not affect the antinociceptive effect induced by the cannabinoid receptor agonist CP55,490 in male mice (Fig. 1C). Importantly, a similar dose-response paradigm of volinanserin on cumulative oxycodone did not induce adjunctive antinociceptive effect in female mice (Fig. 1D and Table S1).
We next tested the effect of cumulative oxycodone on the TFR assay in male and female 5-HT2A−/−R mice as compared to 5-HT2A+/+R littermate controls. Interestingly, the antinociceptive effect of cumulative oxycodone was significantly increased in male (Fig. 1E and Table S1), but not female 5-HT2A−/−R (Fig. 1F and Table S1) mice, as compared to male and female 5-HT2A+/+R controls, respectively. Additionally, adjunctive volinanserin (0.125 mg/kg) did not affect the antinociceptive response induced by cumulative oxycodone in male 5-HT2A−/−R mice (Fig. 1E). Our data also showed that cumulative oxycodone induced a similar antinociceptive TFR effect in male and female 5-HT2A+/+R animals (Oxycodone effect F[7,464] = 217.0, p < 0.001; Sex effect F[1,464] = 0.26, p > 0.05, n = 30 mice per group) (Table S1).
We also applied the CFA model of inflammatory pain to test whether adjunctive volinanserin affects the antinociceptive action of oxycodone under inflammatory pain states. After assessing baseline mechanical sensitivity, mice were injected with CFA on the right hindpaw and the next day mechanical hypersensitibity was verified in the von Frey filament test. Mice were then pretreated with volinanserin (0.125 mg/kg) or vehicle, and 20 min later they received oxycodone (4 mg/kg) or vehicle (Fig. S2A). Among male mice, there was a significant difference among the mechanical thresholds between the four experimental groups (vehicle + vehicle, vehicle + oxycodone, volinanserin + vehicle, and volinanserin + oxycodone) (Fig. S2B). Additionally, oxycodone reduced CFA-induced mechanical hypersensitivity, an antinociceptive effect that was potentiated upon volinanserin treatment (Fig. S2C). As above with the TFR test (Fig. 1B), volinanserin by itself did not affect CFA-induced mechanical hypersensitivity (Fig. S2C).
3.2. Adjunctive volinanserin affects oxycodone-induced locomotor sensitization
Opioids are powerful pain relievers, but also potent inducers of abuse-related behaviors in rodent models. We tested the effect of adjunctive volinanserin on two models related to reward-related behaviors: oxycodone-induced conditioned place preference (CPP) and oxycodone-induced locomotor sensitization. As expected (Niikura et al., 2013), oxycodone alone (3 mg/kg) induced CPP, whereas CPP was unaffected by volinanserin alone (0.125 mg/kg) in male (Fig. 2A) and female (Fig. 2B) mice. We also found that adjunctive volinanserin did not affect oxycodone-induced CPP in either male (Fig. 2A) of female (Fig. 2B) mice. Additionally, CPP behavior was comparable among both sexes (Volinanserin/Oxycodone effect F[3,99] = 25.13, p < 0.001; Sex effect F[1,99] = 1.96, p > 0.05, n = 13-14 mice per group).
Fig. 2.
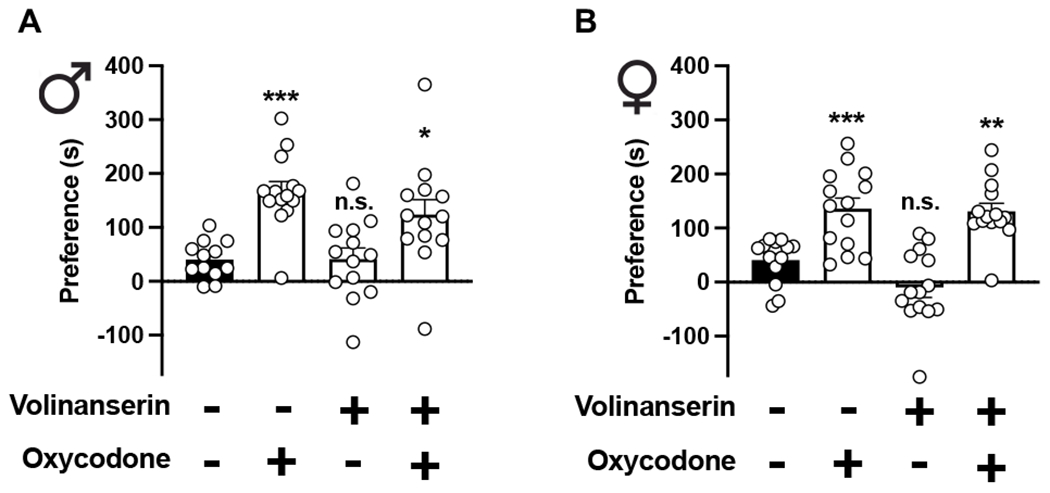
Adjunctive volinanserin does not affect oxycodone-induced CPP. Male (A) and female (B) 5-HT2AR+/+ mice were conditioned with oxycodone (3 mg/kg), volinanserin (0.125 mg/kg), oxycodone and volinanserin, or vehicle for 3 days (n = 13-15 mice per group). CPP was observed in oxycodone-conditioned mice, but adjunctive oxycodone does not affect oxycodone reward as measured by CPP (Males F[3,48] = 9.45, p < 0.001; Females F[3,51] = 18.42, p < 0.001). One-way ANOVA followed by Bonferroni’s multiple comparison test. *p < 0.05, **p <0.01, ***p < 0.001, n.s., not significant. Data are mean ± S.E.M.
To test the effect of adjunctive volinanserin on the locomotor sensitization phenotype induced by repeated oxycodone, male and female mice received a combination of volinanserin (0.125 mg/kg) and oxycodone (8 mg/kg) alone or together, or vehicle alone once a day for five days – locomotor activity was evaluated thereafter. On day eight (i.e., three days after the last volinanserin/oxycodone/vehicle administration), all mice received a single dose of vehicle followed by oxycodone (8 mg/kg) and after this, locomotor activity was evaluated (Fig. 3A). As previously reported with other opioids (Kennedy et al., 2013), repeated oxycodone induced locomotor sensitization in male (Fig. 3B) and female (Fig. 3C) 5-HT2A+/+R mice, whereas this oxycodone-induced locomotor sensitization phenotype was significantly increased in female 5-HT2A+/+R mice as compared to male 5-HT2A+/+R animals (Locomotor sensitization: Time (days) effect F[5,130] = 35.71, p < 0.001; Sex effect F[1,26] = 4.23, p < 0.05 – Oxycodone effect on day 8: t27 = 2.75, p < 0.05, n = 14 mice per group). Volinanserin (0.125 mg/kg) by itself did not affect locomotor behavior in either male (Fig. 3B) or female (Fig. 3C) 5-HT2A+/+R mice. Notably, adjunctive volinanserin significantly reduced oxycodone-induced locomotor sensitization in both male (Figs. 3B,D) and female (Figs. 3C,E) 5-HT2A+/+R mice. Additionally, we found that hyperlocomotor activity induced by oxycodone was significantly increased during the locomotor sensitization period (days 1-5) in male 5-HT2A−/−R mice as compared to male 5-HT2A+/+R mice receiving repeated oxycodone (Fig. 3B), a genotype effect that was not observed in female animals (Fig. 3C). However, when oxycodone effect on locomotor sensitization was tested on day 8, this phenotype was comparable between 5-HT2A−/−R and 5-HT2A+/+R mice of both sexes (Figs. 3D,E).
Fig. 3.
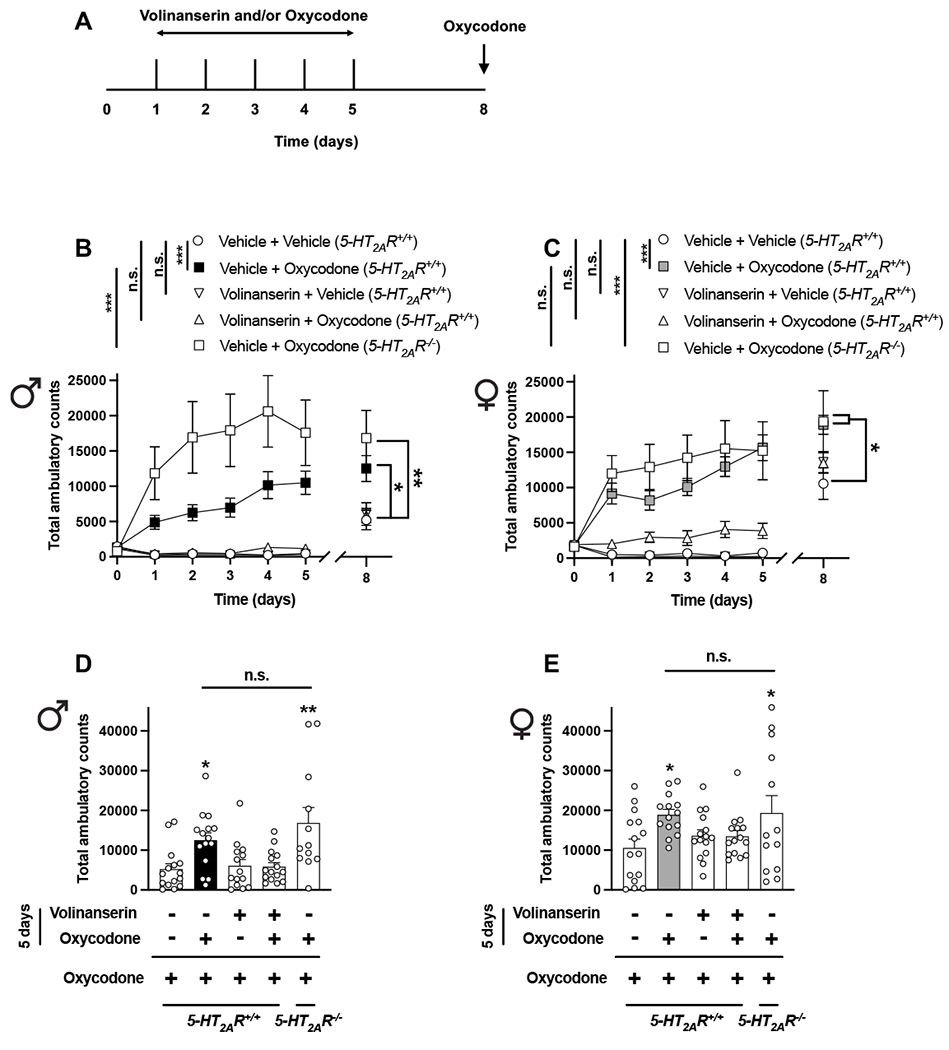
Adjunctive volinanserin prevents oxycodone-induced locomotor sensitization in male and female mice. (A) Timeline of the experimental design. (B-E) Male (B and D) and female (C and E) mice were given oxycodone (8 mg/kg), volinanserin (0.125 mg/kg), volinanserin and oxycodone, or vehicle once a day for 5 days, after which locomotor activity was evaluated. On day 8 (3 days after the last injection), all mice received a single dose of oxycodone (8 mg/kg), and then tested for locomotor activity (n = 12-15 mice per group). Two-way ANOVA (B – days 0-5, Time effect F[5,390] = 5.96, p < 0.001, Volinanserin/Oxycodone/Genotype effect F[4,390] = 64.43, p < 0.001; C – days 0-5, Time effect F[5,402] = 8.32, p < 0.001, Volinanserin/Oxycodone/Genotype effect F[4,402] = 76.31, p < 0.001) or one-way ANOVA (B – day 8 and D, F[4,66] = 6.02, p < 0.001; C – day 8 and E, F[4,67] = 2.59, p < 0.05) followed by Bonferroni’s multiple comparison test. *p < 0.05, **p < 0.01, ***p < 0.001, n.s., not significant. Data are mean ± S.E.M.
We next evaluated whether these sex-specific differences were related to pharmacokinetic differences between male and female mice by testing the distribution of volinanserin and its effects on the concentrations of oxycodone in plasma and the CNS. Using UPLC-MS/MS assays, we found that concentrations of volinanserin (Fig. S3) and oxycodone (Fig. S4) were lower 120-min after drug administration in male and female mice. The pharmacokinetic properties of volinanserin were similar in male and female mice (Fig. S3). Similar to previous findings with morphine in rats (Fillingim and Gear, 2004), concentrations of oxycodone were higher in brain samples, but not in plasma samples, of male as compared to female mice (Fig. S4 and Table S2). However, the concentrations of oxycodone in plasma and brain samples were unaffected by adjunctive volinanserin (0.125 mg/kg) (Fig. S4 and Table S2).
3.3. Volinanserin potentiates the ability of oxycodone to reduce DRG excitability
Based on these sex-related differences on the adjunctive effect of volinanserin, particularly in the TFR as a model of antinociception, we tested the level of expression of 5-HT2AR in three different regions in male and female mice: frontal cortex, striatum and the DRG. Density of 5-HT2AR as evaluated by [3H]ketanserin binding assays was slightly but significantly reduced in the frontal cortex of female mice (Fig. 4A), whereas density of the same serotonin receptor was comparable in striatal membrane preparations of male and female mice (Fig. 4A). Interestingly, this radioligand binding assay showed undetectable levels of specific [3H]ketanserin binding in the DRG of female mice (Fig. 4A). To corroborate this intriguing phenotype using an independent experimental approach, we also tested immunoreactivity with the anti-5-HT2AR antibody in DRG sections of male and female mice. As above with [3H]ketanserin binding assays, the immunoreactivity against 5-HT2AR was reduced in the DRG of female mice (Fig. 4B).
Fig. 4.
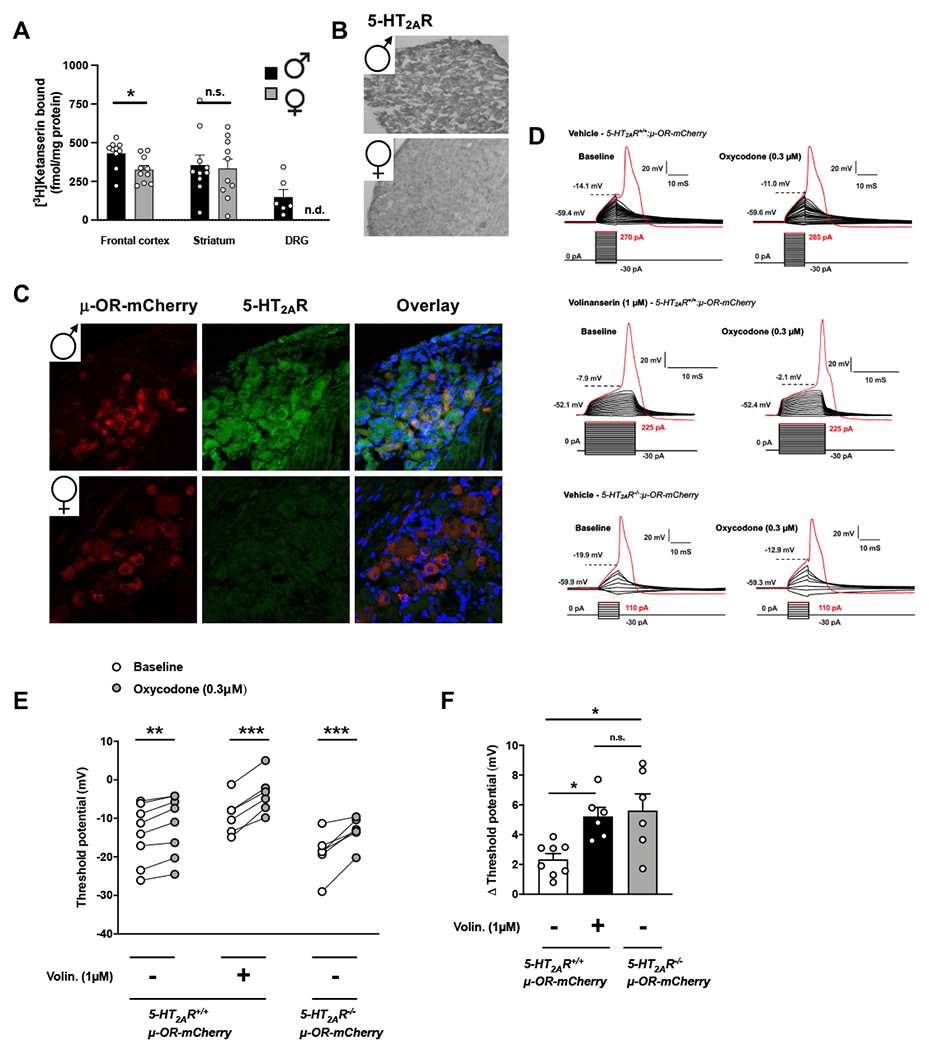
Co-localization and functional crosstalk of 5-HT2AR and μ-OR in the DRG of male mice. (A) Serotonin 5-HT2AR density assessed by [3H]ketanserin binding in membrane preparations of frontal cortex, striatum and DRG of male and female mice (n = 6-10 mice per group). (B) Representative immunohistochemical images with anti-5-HT2AR and HRP-conjugated secondary antibody in DRG tissue samples of male and female mice. (C) Representative immunofluorescent images with anti-5-HT2AR antibody (green) in DRG tissue samples of μ-OR-mCherry (red) male and female mice. Nuclei were stained with DAPI (blue). (D-F) Pharmacological inhibition with volinanserin or genetic deletion in 5-HT2AR−/− mice potentiates the ability of oxycodone to reduce DRG neuron excitability. Representative whole-cell current-clamp traces of mCherry-positive DRG neurons from 5-HT2AR+/+:μ-OR-mCherry (top and middle) or 5-HT2AR−/−:μ-OR-mCherry (bottom) male mice pre-treated (30 min) with volinanserin (1 μM) or vehicle indicating action potentials at baseline (left) and after acute oxycodone (0.3 μM) challenge (right). Action potential (trace in red) is generated by a step-wise current stimulation protocol with the first stimulus to elicit action potential marked in red (D). Effect of volinanserin on threshold potentials (mV) to oxycodone in individual mCherry-positive neurons of 5-HT2AR+/+μ-OR-mCherry and 5-HT2AR−/−:μ-OR-mCherry male mice (n = 6-8 cells per group) (E). Change (Δ) in threshold potential after oxycodone in individual mCherry-positive DRG neurons pre-treated with volinanserin or vehicle. Student’s t-test (A – Frontal cortex t18 = 2.69, Striatum t18 = 0.25) and two-way ANOVA (E – F[2,17] = 3.83, p < 0.05) or one-way ANOVA (F – F[2,17] = 4.27, p < 0.05) followed by Bonferroni’s multiple comparison test. *p < 0.05, **p < 0.01, ***p < 0.001, n.s., not significant, n.d., not specific binding detected. Data are mean ± S.E.M.
Previous findings have suggested that 5-HT2AR is expressed in pain-related circuits including the dorsal horn of the spinal cord (Thibault et al., 2008; Van Steenwinckel et al., 2008; Aira et al., 2012). By testing immunoreactivity with the anti-5-HT2AR antibody in DRG and spinal cord sections of transgenic μ-OR-mCherry mice, our data show that mCherry co-localized with many 5-HT2AR-positive neurons in the DRG (Fig. 4C), whereas very few or no cells showed co-localization of 5-HT2AR and mCherry in the dorsal horn of the spinal cord of male mice (Fig. S5). Additionally, anti-5-HT2AR immunoreactivity was also reduced in the DRG of female mice as compared to males (Fig. 4C).
Next, we tested the allosteric effect of volinanserin on oxycodone-induced signaling events in DRG cells of male mice. To do so, we characterized the action of the 5-HT2AR antagonist volinanserin on μ-OR-mediated inhibition of neuronal excitability in mCherry-positive DRG cells of μ-OR-mCherry male animals. Our data show that, as expected (Ross et al., 2012), oxycodone (0.3 μM) augmented the threshold potential in mCherry-positive DRG cells of μ-OR-mCherry male mice (Figs. 4D,E). Notably, this effect induced by the opioid agonist oxycodone was allosterically augmented in mCherry-positive DRG cells of μ-OR-mCherry male mice exposed to volinanserin (Figs. 4D–F). A similar augmentation in threshold potential was observed in mCherry-positive DRG cells of 5-HT2AR−/− μ-OR-mCherry male mice as compared to 5-HT2AR+/+ μ-OR-mCherry male controls (Figs. 4D–F).
Our next question was related to the cell target responsible for the adjunctive effects of volinanserin on oxycodone-induced antinociception and locomotor sensitization. Related to antinociception, it has been suggested that the 5-HT2AR in the dorsal horn of the spinal cord might be responsible for the effects of volinanserin and other 5-HT2AR antagonists on different animal models of antinociception (Aira et al., 2012). Based on our findings suggesting co-localization and allosteric crosstalk between 5-HT2AR and μ-OR in DRG cells, we tested whether 5-HT2ARs expressed in the DRG are sufficient to mediate the adjunctive effect of volinanserin on opioid-induced antinociception.
3.4. Adjunctive effect of volinanserin on opioid-induced antinociception requires 5-HT2AR expression in the DRG
Previous findings reported that a membrane protein, called posphoinositide interacting regulator of TRP (Pirt), is expressed specifically in the peripheral nervous system, predominantly in almost all primary sensory neurons, but not in the CNS (including spinal cord), glia, or other peripheral tissue (Kim et al., 2008; Kim et al., 2016). The 5-HT2AR−/− mice contain a “neo-stop” sequence inserted into the 5’ untranslated region of the 5-HT2AR (Htr2a) gene (Gonzalez-Maeso et al., 2007). This neo-stop cassette is flanked by loxP sequences (loxP-STOP-loxP), allowing it to be excised by Cre recombinase. Because the 5-HT2AR coding region remains under the control of its endogenous promoter, breeding 5-HT2AR−/− mice with Pirt-Cre mice was expected to rescue expression of 5-HT2AR only in cell types where the expression patterns of the 5-HT2AR and Pirt promoters overlap (Fig. 5A). Accordingly, our data demonstrate that Pirt-Cre restored 5-HT2AR expression to DRG neurons of 5-HT2AR−/−:Pirt-Cre mice (one of the only structures where 5-HT2AR and Pirt are co-expressed) (Fig. 5B).
Fig. 5.
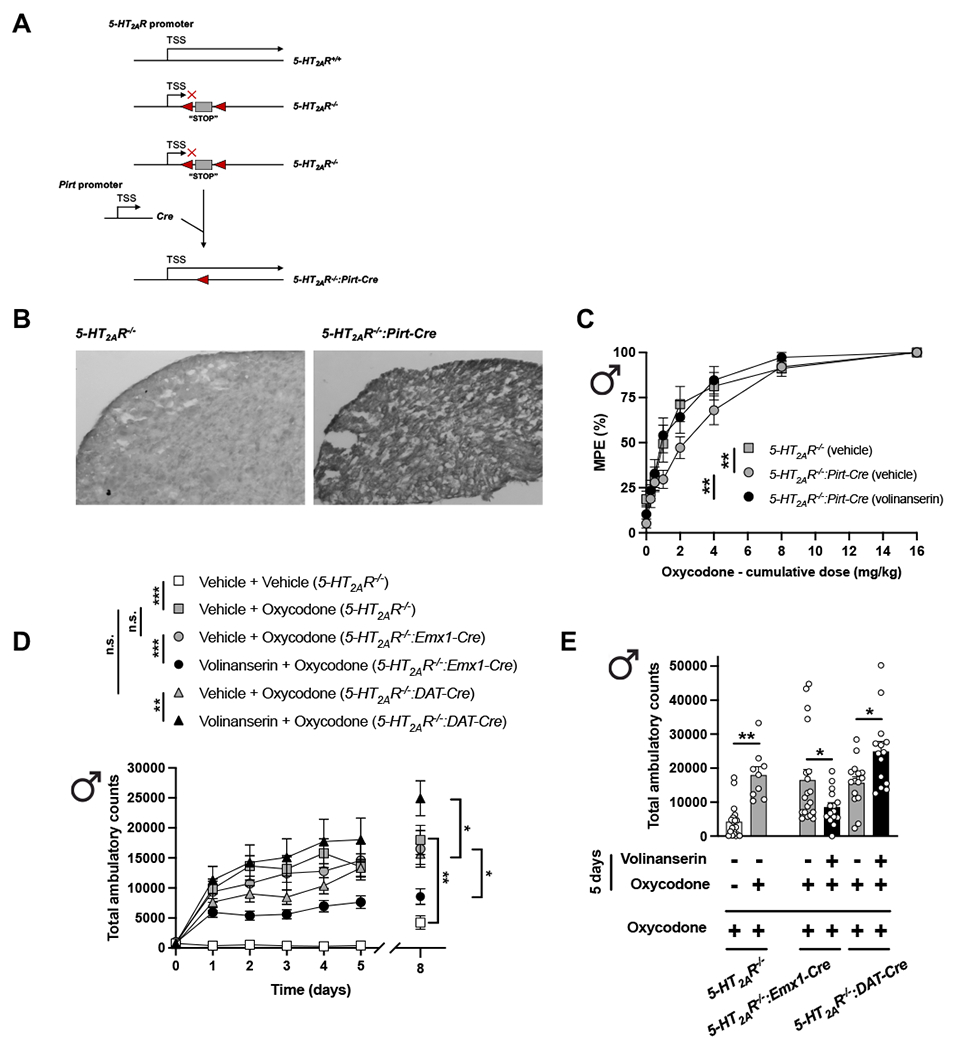
Serotonin 5-HT2AR in DRG neurons represses oxycodone-induced antinociception in male mice. (A) Restoration of 5-HT2AR expression in DRG neurons of 5-HT2AR−/− male mice. By insertion of a “stop” cassette 5’ upstream of exon 1 (grey box) flanked by loxP sites (red arrows), we interrupted the translation of the 5-HT2AR gene. Pirt-Cre excises the stop cassette and restores expression of 5-HT2AR to areas where their promoter activities overlap. (B) Representative immunohistochemical images with anti-5-HT2AR antibody and HRP-conjugated secondary antibody in DRG tissue samples of 5-HT2AR−/− and 5-HT2AR−/−:Pirt-Cre mice. Note that 5-HT2AR immunoreactivity is rescued in the DRG of 5-HT2AR−/−:Pirt-Cre male mice. (C) Adjunctive effect of volinanserin (0.125 mg/kg) or vehicle on the cumulative dose-response induced by oxycodone in the TFR test in male 5-HT2AR−/− and 5-HT2AR−/−:Pirt-Cre mice (n = 10-12 mice per group) (Oxycodone effect F[7,244] = 90.34, p < 0.001, Volinanserin/Genotype effect F[4,244] = 6.90, p < 0.01). (D and E) Male 5-H2AR−/−:Emx1-Cre, 5-HT2AR−/−:DAT-Cre and 5-HT2AR−/− mice were given oxycodone (8 mg/kg), volinanserin (0.125 mg/kg) and oxycodone, or vehicle once a day for 5 days, after which locomotor activity was evaluated. On day 8 (3 days after the last injection), all mice received a single dose of oxycodone (8 mg/kg), and then tested for locomotor activity (n = 9-20 mice per group). Two-way ANOVA (D – days 0-5, Time effect F[5,522] = 24.44, p < 0.001, Volinanserin/Oxycodone/Genotype effect F[5,522] = 39.77, p < 0.001) or one-way ANOVA (D – day 8 and E, F[6,85] = 9.45, p < 0.001) followed by Bonferroni’s multiple comparison test. *p < 0.05, **p < 0.01, ***p < 0.001, n.s., not significant. Data are mean ± S.E.M.
We next tested the antinociceptive effect of cumulative oxycodone in 5-HT2AR−/−:Pirt-Cre and 5-HT2AR−/− littermates. Importantly, our data showed that Cre-mediated restoration of 5-HT2AR expression exclusively in DRG neurons of 5-HT2AR−/−:Pirt-Cre mice is sufficient to observe the adjunctive effect of volinanserin on oxycodone-induced antinociception (Fig. 5C and Table S1). Additionally, Cre-mediated restoration of 5-HT2AR expression in DRG cells was sufficient to reverse the augmentation of oxycodone-induced antinociception observed in 5-HT2AR−/− mice (Fig. 5C and Table S1).
Using a similar loxP-Cre approach, we also rescued 5-HT2AR expression in cortical glutamatergic (5-HT2AR−/−:Emx1-Cre mice) and dopaminergic (5-HT2AR−/−:DAT-Cre) cells and tested the effect of adjunctive volinanserin or vehicle on oxycodone-induced locomotor sensitization in male mice. As expected (see above), repeated oxycodone induced locomotor sensitization in 5-HT2AR−/−’ animals compared to vehicle (Fig. 5D). Notably, this oxycodone-induced locomotor sensitization was similar in 5-HT2AR−/−:Emx1-Cre mice and 5-HT2AR−/−:DAT-Cre as compared to full knockout 5-HT2AR−/− animals (Fig. 5D). Importantly, the effect of adjunctive volinanserin led to opposite effects on this phenotype with reduction and augmentation of oxycodone-induced locomotor sensitization in 5-HT2AR−/−:Emx1-Cre versus 5-HT2AR−/−:DAT-Cre mice, respectively (Figs. 5D,E).
4. Discussion
Here we report that adjunctive administration of the highly selective serotonin 5-HT2AR antagonist volinanserin augments the antinociceptive effect of the μ-OR agonist oxycodone in male mice. A similar phenotype was observed upon genetic deletion of 5-HT2AR in male mice. However, the effects of either pharmacological blockade or genetic deletion of 5-HT2AR on oxycodone-induced antinociceptive effects were absent in female mice. We also show that sex differences related to the effect of 5-HT2AR blockade on oxycodone-induced antinociception correlated with the lower density of 5-HT2AR in the DRG of female mice. Adjunctive volinanserin did not affect oxycodone-induced CPP but prevented oxycodone-induced locomotor sensitization in male and female mice. Together, these data suggest that volinanserin may serve as a new approach to potentiate opioids’ antinociceptive effects thus reducing the dose of the analgesic drug needed to meet a therapeutic goal in pain management. Additionally, the lack of potentiation of side effects of opioids suggests a degree of specificity in the action of adjunctive volinanserin that could aid in reducing opioid’s abuse potential. Such specificity becomes apparent in the sex-related nature of these adjunctive effects. Although our findings provide intriguing observations related to the absence of 5-HT2AR density and immunoreactivity in the DRG of female C57BL/6J mice, further investigation will be needed to unravel whether this sex-related difference is replicated in additional strains of mice as well as postmortem human samples, which would strengthen the translational potential of this preclinical phenotype.
An important finding of this study is the critical role of DRG cells in the effect of 5-HT2AR blockade on the signaling and antinociceptive responses induced by oxycodone. Our current data, as well as previous findings by other groups using Pirt-Cre mice (Kim et al., 2008; Kim et al., 2016), indicate that Cre-mediated restoration of 5-HT2AR expression occurs exclusively in most of the nociceptive neurons in the DRG of 5-HT2AR−/−:Pirt-Cre mice. This suggests that 5-HT2AR in DRG nociceptive neurons is sufficient to mediate the adjunctive effect of volinanserin on oxycodone-induced thermal antinociception in male mice. Although 5-HT2AR is also expressed in additional circuits related to nociception, such as the dorsal horn in the spinal cord, according to our findings it seems possible that cellular co-localization of 5-HT2AR and μ-OR in the DRG is necessary for their crosstalk to occur. A tentative explanation for the molecular mechanisms underlying this crosstalk between 5-HT2AR and μ-OR might be related to processes related to the effect of 5-HT2AR on desensitization, internalization and trafficking of μ-OR (Lopez-Gimenez et al., 2008). Most of our pain-related findings were obtained using TFR as a mouse model of acute thermal nociception. These results suggest that the crosstalk between 5-HT2AR and μ-OR in the DRG may represent a potential molecular target to augment opioid-induced antinociception in a model of acute pain, and open a new line of research for alternative behavior models of antinociception and pain-related paradigms such as chronic inflammation or chemotherapy-induced neuropathic pain.
Our current findings suggest that the adjunctive effects of volinanserin on oxycodone-induced thermal antinociception are not a consequence of nonspecific responses to acute 5-HT2AR blockade such as sedation, since doses up to 0.5 mg/kg did not affect spontaneous exploratory behavior. We also report that a lower dose of volinanserin (0.125 mg/kg) reduced oxycodone-induced hyperlocomotion. This corroborates earlier results that only higher doses (>1.0 mg/kg) of volinanserin reduced spontaneous locomotor activity, whereas lowers doses (<0.3 mg/kg) of this serotonin receptor antagonist were sufficient to reduce MK801-induced hyperlocomotor activity in mice (Martin et al., 1997). Both spontaneous exploratory behavior and oxycodone-induced hyperlocomotor activity were increased in female mice as compared to males. However, locomotor sensitization upon repeated oxycodone treatment was significantly increased in male, but not female, 5-HT2A−/−R mice as compared to 5-HT2A+/+R controls. Since coadministration of volinanserin significantly reduced oxycodone-induced locomotor sensitization in both male and female mice, together, these findings indicate that locomotor activity is differentially affected by sex as an independent variable depending upon the paradigm of oxycodone administration (acute vs repeated) and the experimental method employed to achieve blockade of 5-HT2AR-dependent signaling (pharmacological with volinanserin or genetic in 5-HT2A−/−R mice).
Although the density of 5-HT2AR in the DRG of naïve female mice was significantly reduced as compared to naïve male mice, which may serve as an explanation of why the adjunctive effect of volinanserin on oxycodone-induced antinociception was unobservable in female mice, it remains unclear why there was an absence of sex-related differences in the oxycodone-induced antinociception in male and female mice. It could also be explained by compensatory mechanisms that appear in the DRG of female mice or alternatively sex-related differences in the pharmacokinetics of opioids, including oxycodone, as suggested by our data with lower concentrations of oxycodone in brain samples of female mice.
As mentioned above, previous reports suggested that chronic 5-HT2AR blockade with volinanserin progressively decreased spinal nerve ligation-induced mechanical and thermal allodynia over the days following injury in rats (Aira et al., 2012). Based on our findings in 5-HT2AR+/+ and 5-HT2AR−/− mice, and considering that repeated administration of 5-HT2AR antagonists leads to a profound downregulation of the target receptor (Kurita et al., 2012; Moreno et al., 2013; Ibi et al., 2017), this particular phenotype could be explained by a potential reduction of 5-HT2AR density upon chronic volinanserin treatment. Acute epidural administration of the 5-HT2AR antagonist MDL11,939 reduced mechanical sensitization induced by vincristine (Thibault et al., 2008) or 2′,3′-dideoxycytidine (ddC) (Van Steenwinckel et al., 2008) treatment in rats. Our previous data with the 5-HT2CR agonist lorcaserin showed antinociceptive effects on the TFR model using intrathecal, but not subcutaneous, as a route of administration (Sierra et al., 2020). Although our data presented here suggest that 5-HT2AR in DRG neurons is necessary and sufficient for the adjunctive effect of volinanserin on oxycodone-induced antinociception, whether different routes of administration lead to antinociceptive effects upon volinanserin alone remains to be investigated. Previous findings also suggested similar sensitivity to mechanical and thermal stimuli in 5-HT2AR−/− and paired 5-HT2AR+/+ on 129S6/SvEv background mice (Kayser et al., 2007). A potential explanation behind differences between these results and our data suggesting a profound effect of the 5-HT2AR−/− genotype in C57BL/6J mice might be related to mouse strain-related variations. As an example, our pilot data in 129S6/SvEv wild-type mice suggested a much lower sensitivity to both mechanical and thermal stimuli as compared to C57BL/6J wild-type mice (data not shown).
Related to the divergent effects of adjunctive volinanserin on opioid-induced antinociception (i.e., augmentation in male but not female mice) versus abuse-related behaviors (i.e., lack of effect on oxycodone-induced CPP and reduction of oxycodone-induced locomotor sensitization in male and female mice), it is tempting to speculate that different patterns of expression and co-localization between 5-HT2AR and μ-OR in brain reward pathways may account for these specific measurements from behavioral tests. Thus, previous results by other groups suggested that the 5-HT2AR is present in dopaminergic neurons within (VTA) as well as highly expressed in the postsynaptic density of frontal cortex pyramidal neurons (Lopez-Gimenez et al., 1997; Jakab and Goldman-Rakic, 1998; Lopez-Gimenez et al., 1998; Lopez-Gimenez et al., 2001), but is virtually absent in the nucleus accumbens (NAc) (Jakab and Goldman-Rakic, 1998; Raote et al, 2007). The μ-OR, on the contrary, is highly expressed in inhibitory GABAergic interneurons in the VTA, as well as postsynaptically in the NAc and presynaptically in the frontal cortex (Bonci and Williams, 1997; Nestler, 2005). Our data show that selective expression of 5-HT2AR in cell targets that included forebrain glutamatergic neurons and dopaminergic neurons – mostly midbrain presynaptic dopamine D2 receptor-expressing neurons, characterized by dopamine transporter expression (Slosky et al., 2020) – differentially affects the outcome of adjunctive volinanserin on oxycodone-induced locomotor sensitization with a reduction in 5-HT2AR−/−:Emx1-Cre mice and an augmentation in 5-HT2AR−/−:DAT-Cre mice as compared to 5-HT2AR−/− controls. Further investigation will be necessary to unravel the molecular and neural circuit mechanisms underlying these differences, as well as sex-related phenotypes using this Cre-loxP experimental system.
We selected volinanserin because of its relatively high affinity targeting 5-HT2AR as an antagonist compared to other 5-HT2R receptors (Barnes et al., 2021). However, our previous in vitro data suggested that some of the effects induced on cellular processes such as 5-HT2AR subcellular localization, protein expression, trafficking and crosstalk with additional GPCRs depended on the structural properties and functional antagonist activities of the tested ligand (Fribourg et al, 2011; Toneatti et al., 2020). Whether additional 5-HT2AR antagonists such as pimavanserin, altanserin or methysergide behave as non-opioid adjuvants will be the goal of future studies.
5. Conclusion
These data suggest a new cell-type signaling crosstalk mechanism that distinguishes the adjunctive effects of 5-HT2AR pharmacological blockade on a mouse model of thermal acute nociception, and propose an explanation of how sex-related differences modulate these adjunctive effects related to antinociception and opioid use disorder behavior models in mice. From a translational perspective, our findings enable the exploration of potential opioid-sparing strategies exploiting the intertwining of the opioid and serotonergic receptor systems.
Supplementary Material
Fig. S1. (A) Effect of volinanserin (mg/kg) or vehicle on locomotor activity in male and female mice (10 mice per group) (Volinanserin effect F[2,54] = 2.99, p > 0.05; Sex effect F[1,54] = 4.49, p < 0.05). (B) Effect of volinanserin (0.125 mg/kg) or vehicle on oxycodone (8 mg/kg)-induced locomotor hyperactivity in male and female mice (n = 10 mice per group) (Volinanserin/Oxycodone effect F[3,72] = 41.70, p < 0.001; Sex effect F[1,72] = 18.90, p < 0.001) were both increased in female mice. Two-way ANOVA followed by Bonferroni’s multiple comparison test. *p < 0.05, **p < 0.01, ***p < 0.001, n.s., not significant. Data are mean ± S.E.M.
Fig. S2. Adjunctive volinanserin augments oxycodone-induced antinociceptive effect in a model of inflammatory pain. (A) Timeline of the experimental design. Baseline (BL) was assessed by applying calibrated von Frey filaments to the hindpaw. The day after, mice were injected with CFA and baseline CFA (CFA BL) measurement was taken 24 h after CFA treatment. After this, mice received volinanserin (0.125 mg/kg, i.p.) or vehicle and 20 min later an oral dose of oxycodone (4 mg/kg) or vehicle, and were tested 20 min later. (B) Adjunctive effect of volinanserin on the antinociceptive effect of oxycodone in the CFA-induced inflammatory pain model in male mice (n = 9-10 mice per group). (C) Adjunctive volinanserin augmented oxycodone-induced paw withdrawal threshold (PWT) (n = 9-10 mice per group). Two-way ANOVA (C – Drug effect F[3,103] = 4.97, p < 0.01; Time effect F[2,103] = 66.22 p < 0.001) or one-way ANOVA (D – F[3,33] = 7.05, p < 0.001 followed by Bonferroni’s multiple comparison test. *p < 0.05, **p < 0.01. Data are mean ± S.E.M.
Fig. S3. (A) Plasma concentrations of volinanserin 40 and 120 min after a single administration (0.125 mg/kg) (n = 4 mice per group) (Time effect F[1,13] = 13.59, p < 0.01; Sex effect F[1,13] = 1.80, p > 0.05). (B) Brain concentrations of volinanserin 40 and 120 min after a single administration (0.125 mg/kg) (n = 3-4 mice per group) (Time effect F[1,12] = 3.20, p = 0.09; Sex effect F[1,12] = 0.007, p > 0.05). Two-way ANOVA followed by Bonferroni’s multiple comparison test. *p < 0.05, **p < 0.01, ***p < 0.001, n.s., not significant. Data are mean ± S.E.M.
Fig. S4. Plasma (A) and brain (B) concentrations of oxycodone 40 and 120 min after a single administration of oxycodone (8 mg/kg) alone, or together with volinanserin (0.125 mg/kg) in male and female mice (n = 3-4 mice per group). Two-way ANOVA (A – Time effect males F[1,13] = 8.01, p < 0.05; Volinanserin effect males F[1,13] = 0.05, p > 0.05; Time effect females F[1,12] = 14.45, p < 0.01; Volinanserin effect females F[1,12] = 2.21, p > 0.05; B – Time effect males F[1,13] = 6.07, p < 0.05; Volinanserin effect males F[1,13] = 0.62, p > 0.05; Time effect females F[1,12] = 70.86, p < 0.001; Volinanserin effect females F[1,12] = 2.03, p > 0.05) followed by Bonferroni’s multiple comparison test. *p < 0.05, **p < 0.01, ***p < 0.001, n.s., not significant. Data are mean ± S.E.M. For sex-related differences (three-way ANOVA), see also Table S2.
Fig. S5. Lack of co-localization of 5-HT2AR and μ-OR in the dorsal horn of the spinal cord of male mice. (A) Representative immunofluorescent images with anti-5-HT2AR antibody (green) in tissue samples of μ-OR-mCherry (red) male mice. Lower panels (B) show higher magnification images. Red and white arrowheads indicate μ-OR-mCherry and 5-HT2AR immunoreactivity, respectively.
HIGHLIGHTS.
Adjunctive volinanserin augments oxycodone-induced antinociception in male but not female mice
Adjunctive volinanserin does not affect oxycodone-induced conditioned place preference in male or female mice
Adjunctive volinanserin reduces oxycodone-induced locomotor sensitization in male and female mice
Serotonin 5-HT2A receptor in dorsal root ganglion neurons is necessary for the adjunctive antinociceptive effect of volinanserin
Volinanserin may serve as a new approach to augment opioid-induced analgesia
Funding and disclosure
The authors thank Dr. Xinzhong Dong at Johns Hopkins University for the donation of Pirt-Cre mice, and Dr. Juan López-Giménez at CSIC for his insightful discussions. NIH R01MH084894 (J.G.-M.), R01MH111940 (J.G.-M.), P30DA033934 (J.L.P., W.L.D., H.I.A., J.G.-M.), R01DA036975 (W.L.D.), T32DA007027 (W.L.D), R01DK118137 (L.Y.Q.) and T32MH020030 (M.d.l.F.R.) participated in the funding of this study.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
CRediT authors contribution statement
Salvador Sierra: Conceptualization, Methodology, Formal analysis, Investigation, Writing – original draft, Visualization. Karan H. Muchhala: Investigation, Writing – review and editing. Donald K. Jessup: Investigation, Writing – review and editing. Katherine M. Contreras: Investigation, Writing – review and editing. Urjita H. Shah: Investigation, Writing – review and editing. David L. Stevens: Investigation, Writing – review and editing. Jennifer Jimenez: Investigation, Writing – review and editing. Xiomara K. Cuno Lavilla: Investigation, Writing – review and editing. Mario de la Fuente Revenga: Methodology, Investigation, Writing – review and editing. Kumiko M. Lippold: Investigation, Writing – review and editing. Shanwei Shen: Investigation, Writing – review and editing. Justin L. Poklis: Investigation, Resources, Writing – review and editing. Liya Y. Qiao: Resources, Supervision, Writing – review and editing. William L. Dewey: Resources, Supervision, Writing – review and editing. Hamid I. Akbarali: Resources, Supervision, Writing – review and editing. M. Imad Damaj: Resources, Supervision, Writing – review and editing. Javier González-Maeso: Conceptualization, Formal analysis, Writing – original draft, Visualization, Supervision, Funding acquisition.
Declaration of competing interest
J.G.-M. has a sponsored research contract with NeuRistic, and M.d.l.F.R. has a consulting agreement with Noetic Fund. The remaining authors declare that they have no conflict of interest.
References
- Aira Z, Buesa I, Gallego M, Garcia del Cano G, Mendiable N, Mingo J et al. (2012). Time-dependent cross talk between spinal serotonin 5-HT2A receptor and mGluR1 subserves spinal hyperexcitability and neuropathic pain after nerve injury. J Neurosci 32: 13568–13581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alsharari SD, Freitas K, Damaj MI (2013). Functional role of alpha7 nicotinic receptor in chronic neuropathic and inflammatory pain: studies in transgenic mice. Biochem Pharmacol 86: 1201–1207. [DOI] [PubMed] [Google Scholar]
- Anastasio NC, Sholler DJ, Fox RG, Stutz SJ, Merritt CR, Bjork JM et al. (2020). Suppression of cocaine relapse-like behaviors upon pimavanserin and lorcaserin co-administration. Neuropharmacology 168: 108009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes NM, Ahern GP, Becamel C, Bockaert J, Camilleri M, Chaumont-Dubel S et al. (2021). International Union of Basic and Clinical Pharmacology. CX. Classification of Receptors for 5-hydroxytryptamine; Pharmacology and Function. Pharmacol Rev 73: 310–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohn LM, Gainetdinov RR, Lin FT, Lefkowitz RJ, Caron MG (2000). Mu-opioid receptor desensitization by beta-arrestin-2 determines morphine tolerance but not dependence. Nature 408: 720–723. [DOI] [PubMed] [Google Scholar]
- Bohn LM, Lefkowitz RJ, Gainetdinov RR, Peppel K, Caron MG, Lin FT (1999). Enhanced morphine analgesia in mice lacking beta-arrestin 2. Science 286: 2495–2498. [DOI] [PubMed] [Google Scholar]
- Bonci A, Williams JT (1997). Increased probability of GABA release during withdrawal from morphine. J Neurosci 17: 796–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canal CE, Olaghere da Silva UB, Gresch PJ, Watt EE, Sanders-Bush E, Airey DC (2010). The serotonin 2C receptor potently modulates the head-twitch response in mice induced by a phenethylamine hallucinogen. Psychopharmacology (Berl) 209: 163–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christie MJ (2008). Cellular neuroadaptations to chronic opioids: tolerance, withdrawal and addiction. Br J Pharmacol 154: 384–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craft RM (2003). Sex differences in opioid analgesia: “from mouse to man”. Clin J Pain 19: 175–186. [DOI] [PubMed] [Google Scholar]
- de la Fuente Revenga M, Shah UH, Nassehi N, Jaster AM, Hemanth P, Sierra S et al. (2021). Psychedelic-like Properties of Quipazine and Its Structural Analogues in Mice. ACS Chem Neurosci [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diester CM, Banks ML, Neigh GN, Negus SS (2019). Experimental design and analysis for consideration of sex as a biological variable. Neuropsychopharmacology 44: 2159–2162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filip M, Bubar MJ, Cunningham KA (2004). Contribution of serotonin (5-hydroxytryptamine; 5-HT) 5-HT2 receptor subtypes to the hyperlocomotor effects of cocaine: acute and chronic pharmacological analyses. J Pharmacol Exp Ther 310: 1246–1254. [DOI] [PubMed] [Google Scholar]
- Fillingim RB, Gear RW (2004). Sex differences in opioid analgesia: clinical and experimental findings. Eur J Pain 8: 413–425. [DOI] [PubMed] [Google Scholar]
- Fribourg M, Moreno JL, Holloway T, Provasi D, Baki L, Mahajan R et al. (2011). Decoding the Signaling of a GPCR Heteromeric Complex Reveals a Unifying Mechanism of Action of Antipsychotic Drugs. Cell 147: 1011–1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh A, Berger A (2014). Opioids, adjuvants, and interventional options for pain management of symptomatic metastases. Ann Palliat Med 3: 172–191. [DOI] [PubMed] [Google Scholar]
- Goldstein FJ (2002). Adjuncts to opioid therapy. J Am Osteopath Assoc 102: S15–21. [PubMed] [Google Scholar]
- Gonzalez-Maeso J, Weisstaub NV, Zhou M, Chan P, Ivic L, Ang R et al. (2007). Hallucinogens Recruit Specific Cortical 5-HT(2A) Receptor-Mediated Signaling Pathways to Affect Behavior. Neuron 53: 439–452. [DOI] [PubMed] [Google Scholar]
- Halberstadt AL, Sindhunata IS, Scheffers K, Flynn AD, Sharp RF, Geyer MA et al. (2016). Effect of 5-HT2A and 5-HT2C receptors on temporal discrimination by mice. Neuropharmacology 107: 364–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halberstadt AL, van der Heijden I, Ruderman MA, Risbrough VB, Gingrich JA, Geyer MA et al. (2009). 5-HT(2A) and 5-HT(2C) receptors exert opposing effects on locomotor activity in mice. Neuropsychopharmacology 34: 1958–1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris LS, Pierson AK (1964). Some Narcotic Antagonists in the Benzomorphan Series. J Pharmacol Exp Ther 143: 141–148. [PubMed] [Google Scholar]
- Hideshima KS, Hojati A, Saunders JM, On DM, de la Fuente Revenga M, Shin JM et al. (2018). Role of mGlu2 in the 5-HT2A receptor-dependent antipsychotic activity of clozapine in mice. Psychopharmacology (Berl) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibi D, de la Fuente Revenga M, Kezunovic N, Muguruza C, Saunders JM, Gaitonde SA et al. (2017). Antipsychotic-induced Hdac2 transcription via NF-kappaB leads to synaptic and cognitive side effects. Nat Neurosci 20: 1247–1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iqbal M, Khalil NY, Ezzeldin E, Al-Rashood KA (2019). Simultaneous Detection and Quantification of Three Novel Prescription Drugs of Abuse (Suvorexant, Lorcaserin and Brivaracetam) in Human Plasma by UPLC-MS-MS. J Anal Toxicol 43: 203–211. [DOI] [PubMed] [Google Scholar]
- Jakab RL, Goldman-Rakic PS (1998). 5-Hydroxytryptamine2A serotonin receptors in the primate cerebral cortex: possible site of action of hallucinogenic and antipsychotic drugs in pyramidal cell apical dendrites. Proc Natl Acad Sci U S A 95: 735–740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jalal H, Buchanich JM, Roberts MS, Balmert LC, Zhang K, Burke DS (2018). Changing dynamics of the drug overdose epidemic in the United States from 1979 through 2016. Science 361: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kayser V, Elfassi IE, Aubel B, Melfort M, Julius D, Gingrich JA et al. (2007). Mechanical, thermal and formalin-induced nociception is differentially altered in 5-HT(1A)−/−, 5-HT(1B)−/−, 5-HT(2A)−/−, 5-HT(3A)−/− and 5-HTT−/− knock-out male mice. Pain 130: 235–248. [DOI] [PubMed] [Google Scholar]
- Kennedy PJ, Feng J, Robison AJ, Maze I, Badimon A, Mouzon E et al. (2013). Class I HDAC inhibition blocks cocaine-induced plasticity by targeted changes in histone methylation. Nat Neurosci 16: 434–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kepler KL, Kest B, Kiefel JM, Cooper ML, Bodnar RJ (1989). Roles of gender, gonadectomy and estrous phase in the analgesic effects of intracerebroventricular morphine in rats. Pharmacol Biochem Behav 34: 119–127. [DOI] [PubMed] [Google Scholar]
- Kim AY, Tang Z, Liu Q, Patel KN, Maag D, Geng Y et al. (2008). Pirt, a phosphoinositide-binding protein, functions as a regulatory subunit of TRPV1. Cell 133: 475–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim YS, Anderson M, Park K, Zheng Q, Agarwal A, Gong C et al. (2016). Coupled Activation of Primary Sensory Neurons Contributes to Chronic Pain. Neuron 91: 1085–1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kliewer A, Gillis A, Hill R, Schmiedel F, Bailey C, Kelly E et al. (2020). Morphine-induced respiratory depression is independent of beta-arrestin2 signalling. Br J Pharmacol 177: 2923–2931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krzanowska EK, Bodnar RJ (1999). Morphine antinociception elicited from the ventrolateral periaqueductal gray is sensitive to sex and gonadectomy differences in rats. Brain Res 821: 224–230. [DOI] [PubMed] [Google Scholar]
- Krzanowska EK, Ogawa S, Pfaff DW, Bodnar RJ (2002). Reversal of sex differences in morphine analgesia elicited from the ventrolateral periaqueductal gray in rats by neonatal hormone manipulations. Brain Res 929: 1–9. [DOI] [PubMed] [Google Scholar]
- Kurita M, Holloway T, Garcia-Bea A, Kozlenkov A, Friedman AK, Moreno JL et al. (2012). HDAC2 regulates atypical antipsychotic responses through the modulation of mGlu2 promoter activity. Nat Neurosci 15: 1245–1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Gimenez JF, Gonzalez-Maeso J (2018). Hallucinogens and Serotonin 5-HT2A Receptor-Mediated Signaling Pathways. Curr Top Behav Neurosci 36: 45–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Gimenez JF, Mengod G, Palacios JM, Vilaro MT (1997). Selective visualization of rat brain 5-HT2A receptors by autoradiography with [3H]MDL 100,907. Naunyn Schmiedebergs Arch Pharmacol 356: 446–454. [DOI] [PubMed] [Google Scholar]
- Lopez-Gimenez JF, Vilaro MT, Milligan G (2008). Morphine desensitization, internalization, and down-regulation of the mu opioid receptor is facilitated by serotonin 5-hydroxytryptamine2A receptor coactivation. Mol Pharmacol 74: 1278–1291. [DOI] [PubMed] [Google Scholar]
- Lopez-Gimenez JF, Vilaro MT, Palacios JM, Mengod G (1998). [3H]MDL 100,907 labels 5-HT2A serotonin receptors selectively in primate brain. Neuropharmacology 37: 1147–1158. [DOI] [PubMed] [Google Scholar]
- Lopez-Gimenez JF, Vilaro MT, Palacios JM, Mengod G (2001). Mapping of 5-HT2A receptors and their mRNA in monkey brain: [3H]MDL100,907 autoradiography and in situ hybridization studies. J Comp Neurol 429: 571–589. [DOI] [PubMed] [Google Scholar]
- Loyd DR, Wang X, Murphy AZ (2008). Sex differences in micro-opioid receptor expression in the rat midbrain periaqueductal gray are essential for eliciting sex differences in morphine analgesia. J Neurosci 28: 14007–14017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin P, Waters N, Waters S, Carlsson A, Carlsson ML (1997). MK-801-induced hyperlocomotion: differential effects of M100907, SDZ PSD 958 and raclopride. Eur J Pharmacol 335: 107–116. [DOI] [PubMed] [Google Scholar]
- McCorvy JD, Roth BL (2015). Structure and function of serotonin G protein-coupled receptors. Pharmacol Ther 150: 129–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno JL, Holloway T, Umali A, Rayannavar V, Sealfon SC, Gonzalez-Maeso J (2013). Persistent effects of chronic clozapine on the cellular and behavioral responses to LSD in mice. Psychopharmacology (Berl) 225: 217–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno JL, Miranda-Azpiazu P, Garcia-Bea A, Younkin J, Cui M, Kozlenkov A et al. (2016). Allosteric signaling through an mGlu2 and 5-HT2A heteromeric receptor complex and its potential contribution to schizophrenia. Sci Signal 9: ra5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neelakantan H, Holliday ED, Fox RG, Stutz SJ, Comer SD, Haney M et al. (2017). Lorcaserin Suppresses Oxycodone Self-Administration and Relapse Vulnerability in Rats. ACS Chem Neurosci 8: 1065–1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson AD, Camilleri M (2016). Opioid-induced constipation: advances and clinical guidance. Ther Adv Chronic Dis 7: 121–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nestler EJ (2005). Is there a common molecular pathway for addiction? Nat Neurosci 8: 1445–1449. [DOI] [PubMed] [Google Scholar]
- Niikura K, Ho A, Kreek MJ, Zhang Y (2013). Oxycodone-induced conditioned place preference and sensitization of locomotor activity in adolescent and adult mice. Pharmacol Biochem Behav 110: 112–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pisanu C, Franconi F, Gessa GL, Mameli S, Pisanu GM, Campesi I et al. (2019). Sex differences in the response to opioids for pain relief: A systematic review and meta-analysis. Pharmacol Res 148: 104447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter-Stransky KA, Weinshenker D (2017). Arresting the Development of Addiction: The Role of beta-Arrestin 2 in Drug Abuse. J Pharmacol Exp Ther 361: 341–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raehal KM, Walker JK, Bohn LM (2005). Morphine side effects in beta-arrestin 2 knockout mice. J Pharmacol Exp Ther 314: 1195–1201. [DOI] [PubMed] [Google Scholar]
- Raote I, Bhattacharya A, Panicker MM, 2007. Serotonin 2A (5-HT2A) Receptor Function: Ligand-Dependent Mechanisms and Pathways. In: Chattopadhyay A, (Ed), Serotonin Receptors in Neurobiology, Boca Raton (FL). [PubMed] [Google Scholar]
- Ross GR, Gade AR, Dewey WL, Akbarali HI (2012). Opioid-induced hypernociception is associated with hyperexcitability and altered tetrodotoxin-resistant Na+ channel function of dorsal root ganglia. Am J Physiol Cell Physiol 302: C1152–1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah UH, Gaitonde SA, Moreno JL, Glennon RA, Dukat M, Gonzalez-Maeso J (2019). A revised pharmacophore model for 5-HT2A receptor antagonists derived from the atypical antipsychotic agent risperidone. ACS Chem Neurosci [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp T, Barnes NM (2020). Central 5-HT receptors and their function; present and future. Neuropharmacology 177: 108155. [DOI] [PubMed] [Google Scholar]
- Sharretts J, Galescu O, Gomatam S, Andraca-Carrera E, Hampp C, Yanoff L (2020). Cancer Risk Associated with Lorcaserin - The FDA’s Review of the CAMELLIA-TIMI 61 Trial. N Engl J Med 383: 1000–1002. [DOI] [PubMed] [Google Scholar]
- Sierra S, Gupta A, Gomes I, Fowkes M, Ram A, Bobeck EN et al. (2019). Targeting Cannabinoid 1 and Delta Opioid Receptor Heteromers Alleviates Chemotherapy-Induced Neuropathic Pain. ACS Pharmacol Transl Sci 2: 219–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sierra S, Lippold KM, Stevens DL, Poklis JL, Dewey WL, Gonzalez-Maeso J (2020). Adjunctive effect of the serotonin 5-HT2C receptor agonist lorcaserin on opioid-induced antinociception in mice. Neuropharmacology 167: 107949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slosky LM, Bai Y, Toth K, Ray C, Rochelle LK, Badea A et al. (2020). beta-Arrestin-Biased Allosteric Modulator of NTSR1 Selectively Attenuates Addictive Behaviors. Cell 181: 1364–1379 e1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thibault K, Van Steenwinckel J, Brisorgueil MJ, Fischer J, Hamon M, Calvino B et al. (2008). Serotonin 5-HT2A receptor involvement and Fos expression at the spinal level in vincristine-induced neuropathy in the rat. Pain 140: 305–322. [DOI] [PubMed] [Google Scholar]
- Toneatti R, Shin JM, Shah UH, Mayer CR, Saunders JM, Fribourg M et al. (2020). Interclass GPCR heteromerization affects localization and trafficking. Sci Signal 13: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Townsend EA, Negus SS, Banks ML (2020). Medications Development for Treatment of Opioid Use Disorder. Cold Spring Harb Perspect Med [DOI] [PMC free article] [PubMed] [Google Scholar]
- Twycross R, Lickiss N (1996). Pain control and the World Health Organization analgesic ladder. JAMA 275: 835; author reply 836. [DOI] [PubMed] [Google Scholar]
- Van Steenwinckel J, Brisorgueil MJ, Fischer J, Verge D, Gingrich JA, Bourgoin S et al. (2008). Role of spinal serotonin 5-HT2A receptor in 2′,3′-dideoxycytidine-induced neuropathic pain in the rat and the mouse. Pain 137: 66–80. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Collins FS (2017). The Role of Science in Addressing the Opioid Crisis. N Engl J Med 377: 391–394. [DOI] [PubMed] [Google Scholar]
- Walters CL, Brown S, Changeux JP, Martin B, Damaj MI (2006). The beta2 but not alpha7 subunit of the nicotinic acetylcholine receptor is required for nicotine-conditioned place preference in mice. Psychopharmacology (Berl) 184: 339–344. [DOI] [PubMed] [Google Scholar]
- Zebala JA, Schuler AD, Kahn SJ, Maeda DY (2019). Desmetramadol Is Identified as a G-Protein Biased micro Opioid Receptor Agonist. Front Pharmacol 10: 1680. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1. (A) Effect of volinanserin (mg/kg) or vehicle on locomotor activity in male and female mice (10 mice per group) (Volinanserin effect F[2,54] = 2.99, p > 0.05; Sex effect F[1,54] = 4.49, p < 0.05). (B) Effect of volinanserin (0.125 mg/kg) or vehicle on oxycodone (8 mg/kg)-induced locomotor hyperactivity in male and female mice (n = 10 mice per group) (Volinanserin/Oxycodone effect F[3,72] = 41.70, p < 0.001; Sex effect F[1,72] = 18.90, p < 0.001) were both increased in female mice. Two-way ANOVA followed by Bonferroni’s multiple comparison test. *p < 0.05, **p < 0.01, ***p < 0.001, n.s., not significant. Data are mean ± S.E.M.
Fig. S2. Adjunctive volinanserin augments oxycodone-induced antinociceptive effect in a model of inflammatory pain. (A) Timeline of the experimental design. Baseline (BL) was assessed by applying calibrated von Frey filaments to the hindpaw. The day after, mice were injected with CFA and baseline CFA (CFA BL) measurement was taken 24 h after CFA treatment. After this, mice received volinanserin (0.125 mg/kg, i.p.) or vehicle and 20 min later an oral dose of oxycodone (4 mg/kg) or vehicle, and were tested 20 min later. (B) Adjunctive effect of volinanserin on the antinociceptive effect of oxycodone in the CFA-induced inflammatory pain model in male mice (n = 9-10 mice per group). (C) Adjunctive volinanserin augmented oxycodone-induced paw withdrawal threshold (PWT) (n = 9-10 mice per group). Two-way ANOVA (C – Drug effect F[3,103] = 4.97, p < 0.01; Time effect F[2,103] = 66.22 p < 0.001) or one-way ANOVA (D – F[3,33] = 7.05, p < 0.001 followed by Bonferroni’s multiple comparison test. *p < 0.05, **p < 0.01. Data are mean ± S.E.M.
Fig. S3. (A) Plasma concentrations of volinanserin 40 and 120 min after a single administration (0.125 mg/kg) (n = 4 mice per group) (Time effect F[1,13] = 13.59, p < 0.01; Sex effect F[1,13] = 1.80, p > 0.05). (B) Brain concentrations of volinanserin 40 and 120 min after a single administration (0.125 mg/kg) (n = 3-4 mice per group) (Time effect F[1,12] = 3.20, p = 0.09; Sex effect F[1,12] = 0.007, p > 0.05). Two-way ANOVA followed by Bonferroni’s multiple comparison test. *p < 0.05, **p < 0.01, ***p < 0.001, n.s., not significant. Data are mean ± S.E.M.
Fig. S4. Plasma (A) and brain (B) concentrations of oxycodone 40 and 120 min after a single administration of oxycodone (8 mg/kg) alone, or together with volinanserin (0.125 mg/kg) in male and female mice (n = 3-4 mice per group). Two-way ANOVA (A – Time effect males F[1,13] = 8.01, p < 0.05; Volinanserin effect males F[1,13] = 0.05, p > 0.05; Time effect females F[1,12] = 14.45, p < 0.01; Volinanserin effect females F[1,12] = 2.21, p > 0.05; B – Time effect males F[1,13] = 6.07, p < 0.05; Volinanserin effect males F[1,13] = 0.62, p > 0.05; Time effect females F[1,12] = 70.86, p < 0.001; Volinanserin effect females F[1,12] = 2.03, p > 0.05) followed by Bonferroni’s multiple comparison test. *p < 0.05, **p < 0.01, ***p < 0.001, n.s., not significant. Data are mean ± S.E.M. For sex-related differences (three-way ANOVA), see also Table S2.
Fig. S5. Lack of co-localization of 5-HT2AR and μ-OR in the dorsal horn of the spinal cord of male mice. (A) Representative immunofluorescent images with anti-5-HT2AR antibody (green) in tissue samples of μ-OR-mCherry (red) male mice. Lower panels (B) show higher magnification images. Red and white arrowheads indicate μ-OR-mCherry and 5-HT2AR immunoreactivity, respectively.


