Abstract
Purpose of Review
Despite decades of research, knowledge of the mechanisms maintaining anorexia nervosa (AN) remains incomplete and clearly effective treatments elusive. Novel theoretical frameworks are needed to advance mechanistic and treatment research for this disorder. Here, we argue the utility of engaging a novel lens that differs from existing perspectives in psychiatry. Specifically, we argue the necessity of expanding beyond two historically common perspectives: (1) the descriptive perspective: the tendency to define mechanisms on the basis of surface characteristics and (2) the deficit perspective: the tendency to search for mechanisms associated with under-functioning of decision-making abilities and related circuity, rather than problems of over-functioning, in psychiatric disorders.
Recent Findings
Computational psychiatry can provide a novel framework for understanding AN because this approach emphasizes the role of computational misalignments (rather than absolute deficits or excesses) between decision-making strategies and environmental demands as the key factors promoting psychiatric illnesses. Informed by this approach, we argue that AN can be understood as a disorder of excess goal pursuit, maintained by over-engagement, rather than disengagement, of executive functioning strategies and circuits. Emerging evidence suggests that this same computational imbalance may constitute an under-investigated phenotype presenting transdiagnostically across psychiatric disorders.
Summary
A variety of computational models can be used to further elucidate excess goal pursuit in AN. Most traditional psychiatric treatments do not target excess goal pursuit or associated neurocognitive mechanisms. Thus, targeting at the level of computational dysfunction may provide a new avenue for enhancing treatment for AN and related disorders.
Keywords: Computational psychiatry, Eating disorder, Anorexia nervosa, Goal pursuit, Executive functioning, Decision-making
Introduction
Anorexia nervosa (AN) is a poorly understood illness that is associated with a host of serious physiological and psychological outcomes [1, 2]. AN has one of the highest mortality rates of any psychiatric illness [3]. Despite a proliferation of mechanistic models [4•], treatment for AN is extremely limited [5] and has not improved in the last 50 years [6].
A major limitation to developing effective treatments for AN is an incomplete comprehension about the basic mechanisms underlying this disorder [7••]. Especially puzzling is how individuals with AN engage in such unrelenting pursuit of weight loss. Most individuals at some point attempt to alter their diet and/or activity levels to impact their weight [8]. In many cases, weight management attempts for individuals without an eating disorder involve healthy behaviors (e.g., opting for vegetables over less nutritive foods, increasing moderate exercise) [9]. Even when more extreme forms of dietary restriction (e.g., fasting) are used, most people do not sustain these behaviors long-term, apply them flexibly based on context (e.g., daily life vs. vacation), or abandon them once a weight goal is met [10]. In contrast, the drive towards weight loss in AN is rigid and repetitive, persisting well beyond severe negative consequences [7••]. Individuals with AN engage in weight control even after intensive treatment [11] and while recognizing the potential for negative outcomes, including death [12]. This inability to abandon weight loss goals may reflect an altered decision-making process in AN.
In this paper, we posit that AN may be conceptualized as a disorder of excess goal pursuit. Individuals with this disorder may extend too far a set of behaviors that are socially condoned and that can be adaptive in some contexts (e.g., exercise). We further propose that progress in understanding excess goal pursuit in AN may be enhanced by employing new frameworks for approaching mechanistic questions. Finally, we suggest that computational psychiatry encompasses a novel set of scientific tools that can be used to provide fresh perspectives on psychiatric mechanisms. Not only can this approach provide further insight into AN, but it may also help us better understand an under-studied subset of other psychiatric disorders in which potentially useful actions (e.g., work, saving money) are extended too far.
The Descriptive Perspective of Psychiatry
Currently, a number of broad theoretical frameworks have been employed to identify psychiatric mechanisms. One such framework is the descriptive perspective, which involves developing theories and treatments based on symptoms or diagnoses that are presumed to reflect underlying mechanisms on the basis of face validity [13•]. Descriptive models have often been helpful in developing a foundational understanding of psychiatric mechanisms, and have yielded several helpful treatments. To provide an example from the eating disorders field, in the traditional cognitive-behavioral model of eating disorders, a facet of body image concern (i.e., overvaluation of weight and shape) is considered to be the central mechanism and focus of treatment [14]. Overvaluation of weight and shape has face validity as a factor impacting eating disorder behaviors; individuals with eating disorders typically find weight-related stimuli more salient than unaffected individuals [15•]. Further, cognitive behavioral therapy (CBT) for eating disorders, which has been developed out of this descriptive model, has a wealth of evidence supporting its efficacy [16].
However, there are also potential limitations associated with this approach. Most critically, this process often depends upon clinical judgment to determine what might be perpetuating a disorder based on its outward presentation, which can lead to unintentional biases or oversights, and limit treatment options [17]. For instance, although many individuals with eating disorders report overvaluation of weight and shape, this experience is not endorsed by all people with eating disorders [18] and is often not predictive of eating disorder risk or maintenance [19]. This may explain limitations in the ability for CBT to yield positive outcomes for all individuals with eating disorders. Approximately 25% of eating disorder patients withdraw from CBT [20] and over half do not fully respond to treatment [21]. Further, many attempts to develop treatments for AN based on descriptive validity have been met with limited success. Although AN shares significant symptom overlap with bulimia nervosa (BN) and patients frequently transition between these diagnoses [22], treatments that work reasonably well for BN (e.g., CBT, fluoxetine) have not been as successful in treating AN [5, 23].
Thus, the descriptive approach may be limited in the ability to translate treatments from disorders that have outward similarities because in some cases they may be promoted through distinct mechanistic processes. Recognizing the limitations of the descriptive approach, other psychiatric subdisciplines have moved away from treatments targeting surface qualities. In addictive disorders, for example, research has advanced through focusing on targeting treatments based on distinct decision-making concerns (e.g., valuation impairments, impulsivity, and discounting of the future) rather than specific drug of choice (e.g., alcohol versus opioid addiction) [24•, 25–27]. However, it should be noted that the addictions field similarly struggles to identify treatments with long-lasting efficacy and there are not yet sufficient data to determine whether targeting such decision-making mechanisms may enhance treatment for these disorders [28].
The Deficit Perspective of Psychiatry
A related psychiatric viewpoint is the deficit perspective. Mental health research overwhelmingly focuses on identifying under-functioning in various cognitive abilities (i.e., deficits) as contributing to a disorder. Psychiatric disorders are associated with lower functioning across life domains [29–31]. In line with descriptive psychopathology models, there is a tendency to assume that poor life outcomes arise from poor cognitive abilities. It is clear that there is much validity in this perspective. At a population level, elevated executive functioning is associated with better mental health outcomes [32]. Further, many psychiatric disorders are characterized by deficiencies in a range of higher-level decision-making abilities necessary to pursue long-term goals (e.g., planning, delay and effort tolerance, impulse inhibition) [33•, 34, 35]. As just a few examples, deficits in higher order executive functions are reflected in habitual drug-taking despite negative consequences [36], persistent avoidance in safe contexts in post-traumatic stress disorder [37, 38], and rigid negative cognitions in depression [39]. Binge-eating disorder [BED] and BN frequently have been linked with impairments in the ability to inhibit impulses in order to access future rewards [33•, 40, 41]. Under-functioning of cognitive control circuitry, including fronto-parietal, frontostriatal, and cingulate-directed circuits, has been identified across many disorders, including BN and BED, potentially mediating these impairments [42–44]. Thus, compromised functioning in cognitive domains needed for goal pursuit underlies many psychiatric disorders.
However, this perspective may lead to blind spots in examining psychiatric mechanisms. Because there is a tendency to search for executive functioning deficits, it is less common to look for areas in which individuals with psychiatric disorders show exaggerations or excesses in decision-making abilities typically considered positive for pursuing long-term goals. It is possible that the decision-making abilities that are typically encouraged to promote mental health can be taken too far, to the point at which they begin to impede functioning. As we highlight below, there are several lines of evidence suggesting that this process may drive the hallmark symptomatology (e.g., extreme pursuit of weight loss) of AN.
Excesses in Decision-Making and Goal Pursuit in AN
Much evidence suggests that excess goal pursuit may reflect an overarching phenotype, rather than a disorder-specific process in AN. Extensive self-report data from personality measures have identified a heightened tendency towards persistence in AN [45]. In addition to over-pursuing weight loss, individuals with AN show elevated drive towards other typically encouraged activities, such as academics [46–49] and athletics [50, 51]. It remains unclear how individuals with such a severe psychiatric disorder can engage in behaviors that require such intensive cognitive and physical resources. It may be that executive functioning excesses promote heightened goal pursuit in AN across multiple domains.
The best-known example of an exaggeration of healthy decision-making in AN pertains to delay discounting. Delay discounting paradigms require individuals to choose between smaller amounts of money available earlier (smaller-sooner choices) and larger amounts of money available later (larger-later), with the assumption that selecting more larger-later selections (i.e., shallower discounting) reflects better self-control in inhibiting immediate impulses in service of long-term gain [52]. Across several studies (although not all [53, 54]), individuals with AN demonstrate shallower discounting rates, reflecting greater selection of larger-later rewards (and, thereby, greater self-control) compared to individuals without an eating disorder [55–57] or with BN or BED [58, 59].
There is also emerging evidence that effort tolerance may be heightened in AN. Effort, the intensification of physical or mental activity towards a goal, is typically considered aversive and avoided by most organisms [60••]. However, effort persistence is also necessary for achieving long-term goals (e.g., cognitive effort is needed for learning new skills). Therefore, effort endurance is typically considered to be desirable. Most psychiatric disorders are characterized by an over-discounting of mental and physical effort (i.e., a low tolerance to endure effort), at least for rewards that are unrelated to their disorder (e.g., money) [35, 61, 62]. However, one study found that eating pathology was associated with less mental effort avoidance [63]. This finding corresponds with extensive laboratory evidence demonstrating that individuals with AN and other restrictive eating pathology will engage in extreme motor effort to access disorder-relevant rewards, such as restrictive eating and exercise [64–69].
Some research has found similarly enhanced decision-making performance in AN on other facets of executive functioning, such as planning, goal persistence and attainment, and set-shifting [70–73]. Additionally, although the neuroimaging literature is highly mixed [15•], several studies have found evidence of over-engagement of cognitive control circuitry, such as the frontoparietal circuit, in AN during decision-making [74] and symptom provocation [75, 76]. These findings may reflect a disposition for individuals with AN to over-engage in executive functioning to sustain heightened goal pursuit, even while experiencing negative physical and psychiatric symptoms [1, 2].
Reconciling Contrary Evidence on Decision-Making in AN
In contrast to the above hypothesis, other research has found individuals with AN to show deficits in decision-making similar to those found in other psychiatric disorders. Meta-analyses have identified a moderate negative relationship between a range of executive functioning abilities and AN [77, 78]. There are several ways to account for these discrepancies. First, the profound effects of starvation upon cognitive functioning are likely responsible for many of the executive functioning deficits detected in AN. Older age and lower BMI are associated with poorer executive functioning in AN [77, 78], suggesting that prolonged or severe starvation may fundamentally alter decision-making. Several studies have identified fewer executive functioning deficits in adolescent versus adult AN and in weight-restored versus acutely underweight AN [56, 79–81]. Thus, decisional processes contributing to AN may vary according to illness stage and severity, with heightened goal pursuit characterizing earlier illness stages.
Other factors may influence these discrepancies within the literature. It may be that the expectation of deficits influences publication patterns or influences interpretation. The most recent meta-analysis of executive functioning in AN found evidence of publication bias [77], indicating that papers finding decision-making deficits in AN may be more likely to be published than those with null or discrepant results. Interpretation bias may also affect the framing of decision-making results in AN. Within the same meta-analysis [77], higher education was associated with poorer set-shifting (difficulty changing focus from one task to another). Thus, it is possible that the same quality could be perceived as a deficit (poor set-shifting) or enhancement (elevated goal focus) depending on the perspective. Indeed, one review found that the characteristics encouraged in “good athletes” (e.g., asceticism, pursuit of excellence) were the same as those perpetuating AN [51]. Further, not all decision-making abilities function similarly within an individual and across contexts. It is possible to demonstrate decision-making deficits in some situations and excesses in others. For instance, in AN set-shifting deficits have been commonly identified [82], but higher order executive functioning processes (e.g., problem-solving) appear intact or enhanced [77, 78]. A strategy also could be adaptive or maladaptive depending on the problem an individual is trying to solve (Table 1). The alternative lens offered by computational psychiatry offers promising tools for addressing these issues.
Table 1.
Representative examples of the relative advantages and disadvantages of different decision-making strategies
| Decision-making strategy | Advantages | Disadvantages |
|---|---|---|
| Model-based decision-making | Deliberative; precise; adaptable to changing environments | Slow; inefficient |
| Model-free decision-making | Rapid; efficient; useful for conserving resources when stimulus–response relations are well established | Insensitive to the broader reward structure of the world; imprecise; rigid |
| Delay discounting | Permits access to immediate rewards under scarcity; more sensitive to immediate reward value | Narrow-focused on current goals; discourages planning for future goals |
| Effort discounting | Conserves resources when reward-seeking is not vital; avoids aversive mental and physical sensations | Reduces ability to achieve goals requiring persistence; encourages inaction |
| Sunk costs | Encourages final actions towards a long-term goal, even under aversive conditions; less sensitive to momentary affective state | Over-weights the value of past actions over the future; Insensitive to diminishing returns |
| Rule-following | Simple rubric for goal-directed action; Permits rapid learning without direct experience of the consequences of certain actions | Rigid; success dependent upon the rule accuracy; vulnerable to over-generalization |
Computational Psychiatry Offers a Novel Framework for Identifying Mechanisms
Computational psychiatry, which integrates theory and methods from translational and computational neuroscience into psychiatric models, offers one promising new paradigm for enhancing mechanistic and intervention science for AN [83••]. The premise of the computational approach is that psychological symptoms arise from imbalanced or inappropriate mental calculations performed by neurally-separable decision systems, which may be shared or unique across diagnostic entities [84]. Contrasting descriptive models, computational models do not assume mechanistic function by outward presentation. Instead, computational psychiatry posits that different algorithmic processes can lead to the same cognitive or behavioral manifestation. A classic example of this principle from behavioral neuroscience relates to how rats trained to run from the south to the west arm of a plus maze might use two different computational processes to obtain food from the west arm [85]. One process involves deriving a cognitive map and identifying the food location on that map, allowing the rat to plan a path from the south to the west arm. The other process involves using the simple rubric of turning left from the starting location. Both processes yield the same outcome in this starting scenario, but different outcomes when the environment changes (i.e., when starting from the north arm). Importantly, each process has advantages and disadvantages and is best suited for different situations. Deliberative, map-based planning may be well-suited to goal-pursuit within a complex and changing environment, but is unable to respond quickly when rapid action is needed (e.g., if being chased by a predator).
These concepts can extend to understanding the type of computational problems that yield psychiatric symptoms. For instance, the low mood and anhedonia that characterize depression have been associated with several different computational dysfunctions [86], including poorer learning from rewards [87, 88], enhanced sensitivity to punishing experiences [87], inability to learn from interoceptive body signals [89], and effort aversion [86, 90]. Each of these computational breakdowns likely characterizes a subset of individuals with depression. Each unique process would warrant a distinct treatment aimed at the level of decisional dysfunction, as opposed to outward symptoms. For instance, depressive symptoms associated with interoceptive imprecision would be likely to improve with interoceptive exposure [91], while symptoms resulting from low effort tolerance would not.
Computational approaches also circumvent the potential oversights that can result from the deficit perspective. From the framework of computational psychiatry, each decision strategy is presumed to serve an important function; problems only occur when these strategies are engaged inappropriately (Table 1) [83••]. For instance, many research groups have investigated the balance of model-based and model-free learning in the maintenance of psychopathology [92•]. Model-based decision-making involves acting towards an imagined future based on an internal model of the environment and the simulated consequences of different actions [93]. This is a flexible and precise decision process, but it is typically time consuming. Model-free decision-making, on the other hand, entails learning specific, arbitrary action-chains to release in certain situations, informed by past consequences. Often, under-use of the model-based system or over-use of the model-free system has been identified as a suboptimal decision strategy contributing to psychopathology [90]. Many psychiatric disorders, including the full spectrum of eating disorders, demonstrate model-based learning impairments [94–96]. However, from a computational perspective, model-free learning is not inherently negative; healthy non-human animals and humans regularly effectively engage in this strategy in situations in which the consequences of certain actions are well established (i.e., stopping at a crosswalk maintains safety) and decisional efficiency is vital (i.e., cars are speeding down the road) [92•, 97]. Extending the same logic, heightened engagement of executive functioning could at times be harmful if this strategy is a functional mismatch with the environmental demands (e.g., over-attending to future consequences when meeting immediate needs is more critical).
Towards a Computational Account of Excess Goal Pursuit in AN
We propose that psychiatric disorders may arise from computational problems resulting in “too much” in addition to “too little” goal pursuit. Highlighted in Fig. 1, we suggest that both low and high pursuit of desirable goals, supported by algorithmic misalignment between the environmental requirements and decisional approach, can lead to negative psychiatric outcomes. This misalignment may occur in over-use of several different decision processes in AN, necessitating investigation into varied computational models of weight loss in this group.
Fig. 1.
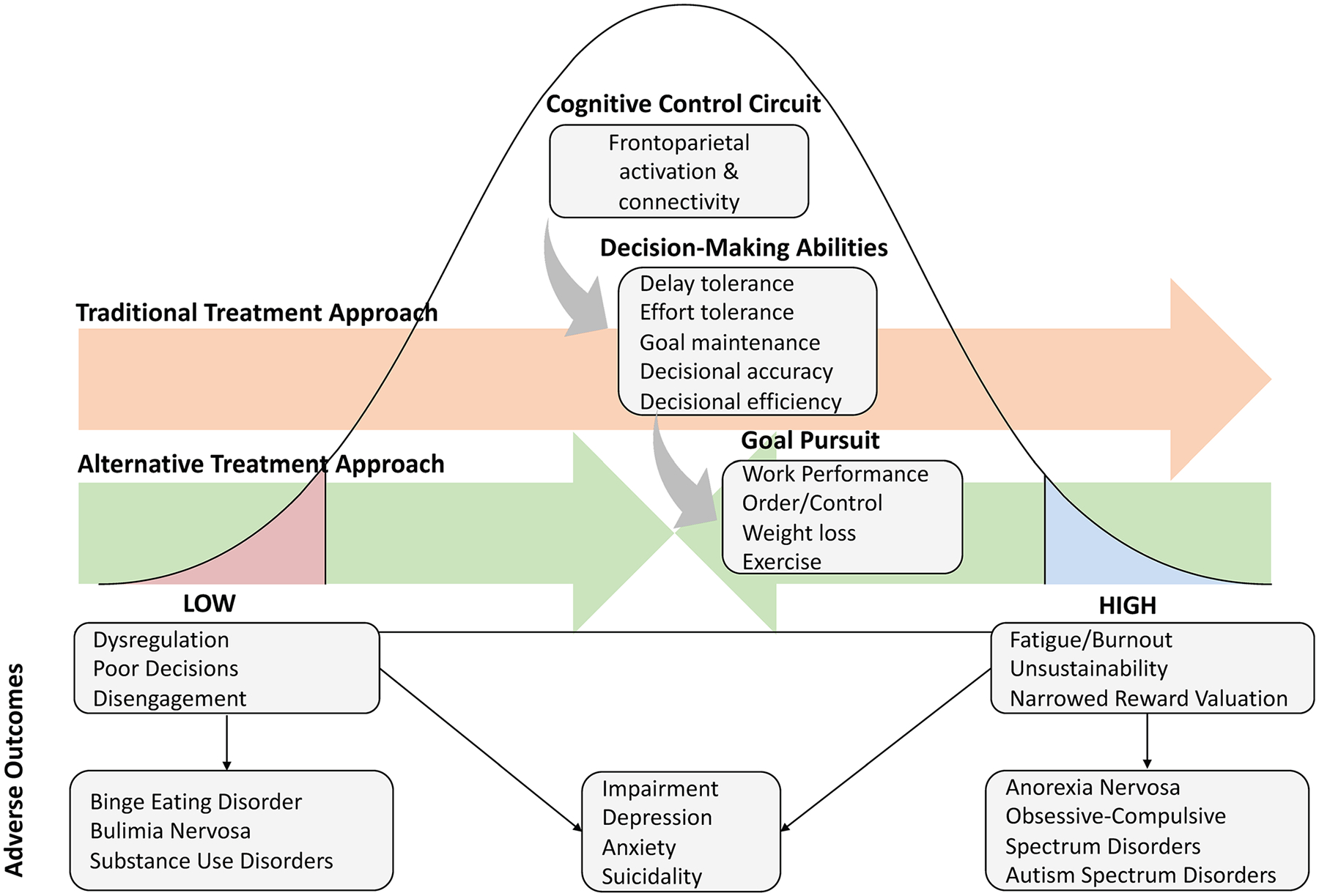
The goal pursuit paradox: too little and too much can lead to negative outcomes. Note: Orange line represents the typical psychiatric approach to addressing goal pursuit and decision-making, which typically aims to increase these qualities to support enhanced mental health. Green line represents an alternate approach emerging from computational psychiatry in which decision-making strategy and goal pursuit are modulated in the appropriate direction for optimal health, dependent upon the context demands
Model-Based Versus Model-free Learning Models
Noted above, one of the most common models of goal pursuit distinguishes between model-based and model-free decision processes, typically measured using a two-stage Markov task [98•]. Long-term goal pursuit is typically considered to depend on model-based processes, since these decision patterns incorporate precise information about how to best reach desired outcomes [99, 100••]. Yet, AN has frequently been hypothesized as demonstrating model-based decision-making deficits [101••]. The neural correlates of model-free decision-making (i.e., dorsal striatum activity, dorsal striatal-dorsolateral prefrontal cortex connectivity) have been implicated in restrictive food choice [102–104]; however, while some research has found evidence for generalized deficits in model-based learning across AN [95], other research has not [105].
However, a recent study identified two different computational subgroups with AN [106], one of which was characterized by greater use of model-free decision-making and the other greater model-based decision-making, supported by distinct patterns of frontostriatal engagement. Another study utilizing self-report data found greater habit strength (a measure of the outcome of model-free learning) related to restrictive eating to be associated with longer duration of illness in AN [107]. Thus, it is possible that heightened model-based decision-making may contribute to excess goal pursuit in the earlier stages of AN, but that, over time, restrictive behavior may become “habitized” and transferred to the model-free system [7••]. More research is needed to investigate how these computational processes vary according to illness stage.
Foraging Models
Foraging paradigms offer another opportunity to assess decision-making processes that may lead to excess goal pursuit. These models, drawn originally from animal behavior in foraging for food rewards, allow researchers to determine how organisms decide to pursue or relinquish outcomes when other potential alternatives are available and time and resources are scarce [108]. Foraging paradigms require individuals to decide whether to accept a current reward or not without knowing the other rewards available, similar to decision-making in the real world (e.g., when someone selects a job or a partner, there are many other possibilities unknown to them). Thus, these models operationalize the current reward against a threshold representing the opportunity cost of selecting the current reward. Foraging models may be particularly useful for examining goal pursuit in AN, given the tendency of this group to overvalue future outcomes [33•], perhaps leading to suboptimal decisions when immediate needs (e.g., nourishment) are more critical. Our collaborative group has developed one such foraging paradigm that can be translated from rodents to humans [109•] and has found distinct behavioral patterns within animal models [97], which may provide insights to the decision process that allows individuals with AN to over-focus on future goals even when most individuals would attend to immediate desires.
Effort-Based Decision-Making Models
Computational models of effort-based decision-making have been derived to determine the degree to which the decision to expend cognitive or physical effort is impacted by perceptions of the relative value versus cost of effort [86, 110]. These models fit the elasticity of effort-based decision-making (i.e., how much response decreases with increasing effort cost [111]) by incorporating data on the magnitude of the reward that may be achieved from effort (e.g., amount of money) and the effort magnitude (e.g., amount of work needed to achieve the reward) alongside parameters that determine the steepness and shape of the effort discounting function. Although one study examined the association between mental effort-based decision-making and eating disorder symptoms [63], models of effort-based decision-making have not been applied specifically to AN [112]. Given the excess goal pursuit in this group, it would be expected that individuals with AN may under-discount effort. For instance, individuals with AN may exhibit lower sensitivity to effort costs and greater sensitivity to the value of the rewards that can be gained through effort. Alternatively, in AN, it is possible that effort has been conditioned to acquire secondary properties of reward itself [113]. In this case, AN participants would be expected to demonstrate less sensitivity to both effort costs and external rewards (because the effort itself would be the reward). Findings in line with this hypothesis have been identified in effort modeling for autism-spectrum disorders (ASD), which are also characterized by excess focus on a narrow set of goals [114]. Thus, further investigation of effort-based decision-making models in AN is warranted.
Extensions of Excess Goal Pursuit to Other Psychiatric Disorders
Over-engagement of what are normally considered adaptive decision-making processes may extend to other psychiatric concerns. There are other psychiatric disorders beyond AN that over-engage towards goals that are typically considered positive. Individuals with ASD and OCD often have elevated educational achievement relative to healthy individuals [46, 115]. Obsessive–compulsive personality disorder (OCPD), a condition of excessive concern with order and control, is associated with excess focus on a number of activities typically considered desirable, including work, saving money, and pursuit of moral good [116]. These same populations have also shown enhancements in certain decision-making abilities. Individuals with ASD and OCD demonstrate evidence of heightened effort tolerance [63, 114]. OCPD is associated with shallow delay discounting (i.e., heightened delay tolerance [33•]) and excess functional engagement of frontoparietal cognitive control circuitry [117]. In some models of anxiety, over-engagement of planning systems is considered a strategy for patients to reduce the distress associated with the unpredictability of the world [118].
Further, some individuals without one of the above diagnoses over-pursue other activities (e.g., work, exercise) to the exclusion of important life domains [119, 120]. These patterns are not currently classified as psychiatric disorders, but are associated with a range of psychiatric and physical ailments [119, 121]. As such, this model could also partially explain the elevated suicide rates in high functioning professions associated with heightened cognitive abilities (e.g., healthcare) [122]. Investigation into the computational processes maintaining AN may yield useful information about maintenance mechanisms promoting these other psychiatric concerns. Excess goal pursuit may also extend to subgroups within psychiatric disorders traditionally associated with decision-making deficits. For instance, some subsets of individuals with depression demonstrate shallow delay discounting [33•]. Thus, excess goal pursuit may represent an under-investigated subset of individuals with psychiatric disorders. Revealing the computational processes promoting AN will provide a first step to evaluating whether these other concerns are maintained through the same, or different, mechanistic processes.
Conclusion: Clinical Implications
If this excess goal pursuit hypothesis is correct, some portion of psychiatric problems may require novel treatment approaches. Individuals with excess goal pursuit may present for treatment of a secondary problem that may constitute an outcome of excess goal pursuit (e.g., depression, anxiety), rather than the core mechanism [123]. Informed by the deficit perspective, many common psychiatric interventions aim to enhance cognitive control circuitry and associated decision-making abilities to guide goal pursuit [124, 125]. However, in this psychiatric subset, it is possible that improving cognitive control could enhance the processes that led to symptom development. This could render these interventions less effective for this subset, or, in the worst-case scenario, could increase the potential for future mental health concerns (see Fig. 1, orange arrow). However, it should be emphasized that this proposition remains hypothetical; further research will be needed to both determine the populations impacted by excess goal pursuit, and the treatment implications for these individuals.
However, this process could explain why many treatments that work for disorders more commonly characterized by goal pursuit deficits (e.g., BN) have had limited success in AN [5, 23]: many existing treatments for AN may be targeted at an incorrect computational process. Some have hypothesized, for instance, that the highly structured, rule-bound settings in which individuals with AN are treated may ultimately perpetuate the rigid and perfectionist tendencies characteristic of this group [126•]. However, it is also worth noting that these highly structured treatment settings have also demonstrated the best efficacy for interrupting acute symptoms [127]. Addressing the computational processes promoting goal pursuit in AN can allow for adaptations of existing treatments or development of novel treatments that may be targeted more precisely to the underlying dysfunction.
Ultimately, treatment implications will depend upon the identified computational misalignments. However, in accordance with this approach, patients should be taught to identify when and how under- and over-use of certain decisional strategies lead to dysfunction, and alter their actions in a less extreme direction (Fig. 1, green arrows) rather than discouraging or encouraging use of a strategy altogether (e.g., always encouraging delaying gratification). In these ways, the novel field of computational psychiatry holds significant promise for developing more sensitive treatments for AN and related disorders designed to target the problems that arise when any decision strategy, whether classically considered good or bad, is extended too far.
Acknowledgements
Authors would like to acknowledge the Neuro-plasticity in Support of Mental Health (NeuroPRSMH) interdisciplinary workgroup who provided feedback on and refinement of the theoretical areas outlined in this manuscript and Jesse W. Dzmobak who assisted with our figure.
Funding
This work was supported in part by the National Institutes of Health (K23MH112867, K23MH123910; T32MH096679; P50MH119569; UH3NS100548, R01MH119384, R21MH120785), the Hilda and Preston Davis Foundation, the MnDRIVE Brain Conditions Initiative, and the Medical Discovery Team—Addictions at the University of Minnesota.
Footnotes
Competing Interests A.S. Widge reports consulting income from Circuit Therapeutics and Dandelion Science and multiple unlicensed patents in the area of neurostimulation. Other authors have no relevant financial or non-financial interests to disclose.
Publisher's Disclaimer: Disclaimer These funding agencies did not influence the design or writing of the manuscript.
References
Papers of particular interest, published recently, have been highlighted as:
• Of importance
•• Of major importance
- 1.Mitchell JE, Crow S. Medical complications of anorexia nervosa and bulimia nervosa. Curr Opin Psychiatry. 2006;19:438–43. [DOI] [PubMed] [Google Scholar]
- 2.O’Brien KM, Vincent NK. Psychiatric comorbidity in anorexia and bulimia nervosa: nature, prevalence, and causal relationships. Clin Psychol Rev. 2003;23:57–74. [DOI] [PubMed] [Google Scholar]
- 3.Crow SJ, Peterson CB, Swanson SA, Raymond NC, Specker S, Eckert ED, et al. Increased mortality in bulimia nervosa and other eating disorders. Am J Psychiatry. 2009;166:1342–6. [DOI] [PubMed] [Google Scholar]
- 4.•.Pennesi J-L, Wade TD. A systematic review of the existing models of disordered eating: Do they inform the development of effective interventions? Clin Psychol Rev. 2016;43:175–92. [DOI] [PubMed] [Google Scholar]; (This manuscript conducted a systematic review of existing theoretical models of eating disorders and the degree to which these progressed to mechanistically informed interventions. The findings highlight how rarely theoretical models in eating disorders have been incorporated into treatment development.)
- 5.van den Berg E, Houtzager L, de Vos J, Daemen I, Katsaragaki G, Karyotaki E, et al. Meta-analysis on the efficacy of psychological treatments for anorexia nervosa. Eur Eat Disord Rev J Eat Disord Assoc. 2019;27:331–51. [DOI] [PubMed] [Google Scholar]
- 6.Hay PJ, Claudino AM, Touyz S, Abd Elbaky G. Individual psychological therapy in the outpatient treatment of adults with anorexia nervosa. Cochrane Database Syst Rev. 2015;CD003909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.••.Walsh BT. The enigmatic persistence of anorexia nervosa. Am J Psychiatry. 2013;170:477–84. [DOI] [PMC free article] [PubMed] [Google Scholar]; (This was the first article to outline the habit learning hypothesis of AN, suggesting that eating disorder behaviors develop from rewarded behaviors into over-trained habits in the development of this disorder.)
- 8.Haynos AF, Wall MM, Chen C, Wang SB, Loth K, Neumark-Sztainer D. Patterns of weight control behavior persisting beyond young adulthood: results from a 15-year longitudinal study. Int J Eat Disord. 2018;51:1090–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lampard AM, Maclehose RF, Eisenberg ME, Larson NI, Davison KK, Neumark-Sztainer D. Adolescents who engage exclusively in healthy weight control behaviors: who are they? Int J Behav Nutr Phys Act. 2016;13:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Haynos AF, Field AE, Wilfley DE, Tanofsky-Kraff M. A novel classification paradigm for understanding the positive and negative outcomes associated with dieting. Int J Eat Disord. 2015;48:362–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mayer LES, Schebendach J, Bodell LP, Shingleton RM, Walsh BT. Eating behavior in anorexia nervosa: before and after treatment. Int J Eat Disord. 2012;45:290–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fox KR, Wang SB, Boccagno C, Haynos AF, Kleiman E, Hooley JM. Comparing self-harming intentions underlying eating disordered behaviors and NSSI: evidence that distinctions are less clear than assumed. Int J Eat Disord. 2019;52:564–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.•.Lilienfeld SO, Treadway MT. Clashing diagnostic approaches: DSM-ICD versus RDoC. Annu Rev Clin Psychol. 2016;12:435–63. [DOI] [PMC free article] [PubMed] [Google Scholar]; (This theoretical paper describes the limitations of historical descriptive approaches to understanding psychopathology.)
- 14.Murphy R, Straebler S, Cooper Z, Fairburn CG. Cognitive behavioral therapy for eating disorders. Psychiatr Clin North Am. 2010;33:611–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.•.Haynos AF, Lavender JM, Nelson J, Crow SJ, Peterson CB. Moving towards specificity: a systematic review of cue features associated with reward and punishment in anorexia nervosa. Clin Psychol Rev. 2020;79:101872. [DOI] [PMC free article] [PubMed] [Google Scholar]; (This systematic review examines the reward literature parsed by stimulus/cue presentation to demonstrate that reward responsivity in AN varies considerable according to the type and context of a putative reward.)
- 16.Linardon J, Wade TD, de la Piedad GX, Brennan L. The efficacy of cognitive-behavioral therapy for eating disorders: a systematic review and meta-analysis. J Consult Clin Psychol. 2017;85:1080–94. [DOI] [PubMed] [Google Scholar]
- 17.Dawes RM, Faust D, Meehl PE. Clinical versus actuarial judgment. Science. 1989;243:1668–74. [DOI] [PubMed] [Google Scholar]
- 18.Becker AE, Thomas JJ, Pike KM. Should non-fat-phobic anorexia nervosa be included in DSM-V? Int J Eat Disord. 2009;42:620–35. [DOI] [PubMed] [Google Scholar]
- 19.Askew AJ, Peterson CB, Crow SJ, Mitchell JE, Halmi KA, Agras WS, et al. Not all body image constructs are created equal: Predicting eating disorder outcomes from preoccupation, dissatisfaction, and overvaluation. Int J Eat Disord. 2020;53:954–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Linardon J, Hindle A, Brennan L. Dropout from cognitive-behavioral therapy for eating disorders: a meta-analysis of randomized, controlled trials. Int J Eat Disord. 2018;51:381–91. [DOI] [PubMed] [Google Scholar]
- 21.Linardon J. Rates of abstinence following psychological or behavioral treatments for binge-eating disorder: Meta-analysis. Int J Eat Disord. 2018;51:785–97. [DOI] [PubMed] [Google Scholar]
- 22.Eddy KT, Dorer DJ, Franko DL, Tahilani K, Thompson-Brenner H, Herzog DB. Diagnostic crossover in anorexia nervosa and bulimia nervosa: implications for DSM-V. Am J Psychiatry. 2008;165:245–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Crow SJ. Pharmacologic treatment of eating disorders. Psychiatr Clin North Am. 2019;42:253–62. [DOI] [PubMed] [Google Scholar]
- 24.•.Redish AD, Jensen S, Johnson A. A unified framework for addiction: vulnerabilities in the decision process. Behav Brain Sci. 2008;31:415–37; discussion 437–487. [DOI] [PMC free article] [PubMed] [Google Scholar]; (This paper provides a framework for how computational psychiatry can be used to enhance classification and targeting of addictive disorders.)
- 25.Song S, Zilverstand A, Gui W, Pan X, Zhou X. Reducing craving and consumption in individuals with drug addiction, obesity or overeating through neuromodulation intervention: a systematic review and meta-analysis of its follow-up effects. Addict Abingdon Engl. 2021; [DOI] [PubMed] [Google Scholar]
- 26.Snider SE, LaConte SM, Bickel WK. Episodic future thinking: expansion of the temporal window in individuals with alcohol dependence. Alcohol Clin Exp Res. 2016;40:1558–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stein JS, Tegge AN, Turner JK, Bickel WK. Episodic future thinking reduces delay discounting and cigarette demand: an investigation of the good-subject effect. J Behav Med. 2018;41:269–76. [DOI] [PubMed] [Google Scholar]
- 28.Ostergren JE, Hammer RR, Dingel MJ, Koenig BA, McCormick JB. Challenges in translational research: the views of addiction scientists. PLoS One. 2014;9:e93482. 10.1371/journal.pone.0093482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ansell EB, Sanislow CA, McGlashan TH, Grilo CM. Psychosocial impairment and treatment utilization by patients with borderline personality disorder, other personality disorders, mood and anxiety disorders, and a healthy comparison group. Compr Psychiatry. 2007;48:329–36. [DOI] [PubMed] [Google Scholar]
- 30.Mehta S, Mittal PK, Swami MK. Psychosocial functioning in depressive patients: a comparative study between major depressive disorder and bipolar affective disorder. Depress Res Treat. Hindawi; 2014;2014:e302741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ruggero CJ, Chelminski I, Young D, Zimmerman M. Psychosocial impairment associated with bipolar II disorder. J Affect Disord. 2007;104:53–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Moffitt TE, Arseneault L, Belsky D, Dickson N, Hancox RJ, Harrington HL, Caspi A. A gradient of childhood self-control predicts health, wealth, and public safety. PNAS. 2011;108:2693–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.•.Lempert KM, Steinglass JE, Pinto A, Kable JW, Simpson HB. Can delay discounting deliver on the promise of RDoC? Psychol Med. 2018;1–10. [DOI] [PubMed] [Google Scholar]; (This review highlights the literature demonstrating extremes in self-control, as measured by delay discounting, within psychiatric disorders and discusses implications for conceptualization of these disorders.)
- 34.Lipszyc J, Schachar R. Inhibitory control and psychopathology: a meta-analysis of studies using the stop signal task. J Int Neuropsychol Soc JINS. 2010;16:1064–76. [DOI] [PubMed] [Google Scholar]
- 35.Treadway MT, Bossaller NA, Shelton RC, Zald DH. Effort-based decision-making in major depressive disorder: a translational model of motivational anhedonia. J Abnorm Psychol. 2012;121:553–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zilverstand A, Huang AS, Alia-Klein N, Goldstein RZ. Neuroimaging impaired response inhibition and salience attribution in human drug addiction: a systematic review. Neuron. 2018;98:886–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McTeague LM, Huemer J, Carreon DM, Jiang Y, Eickhoff SB, Etkin A. Identification of common neural circuit disruptions in cognitive control across psychiatric disorders. Am J Psychiatry. 2017;174:676–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McTeague LM, Goodkind MS, Etkin A. Transdiagnostic impairment of cognitive control in mental illness. J Psychiatr Res. 2016;83:37–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rodman AM, Jenness JL, Weissman DG, Pine DS, McLaughlin KA. Neurobiological markers of resilience to depression following childhood maltreatment: the role of neural circuits supporting the cognitive control of emotion. Biol Psychiatry. 2019;86:464–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lavagnino L, Arnone D, Cao B, Soares JC, Selvaraj S. Inhibitory control in obesity and binge eating disorder: a systematic review and meta-analysis of neurocognitive and neuroimaging studies. Neurosci Biobehav Rev. 2016;68:714–26. [DOI] [PubMed] [Google Scholar]
- 41.Wu M, Hartmann M, Skunde M, Herzog W, Friederich H-C. Inhibitory control in bulimic-type eating disorders: a systematic review and meta-analysis. PloS One. 2013;8:e83412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Janiri D, Moser DA, Doucet GE, Luber MJ, Rasgon A, Lee WH, et al. Shared neural phenotypes for mood and anxiety disorders: a meta-analysis of 226 task-related functional imaging studies. JAMA Psychiat. 2020;77:172–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nowrangi MA, Lyketsos C, Rao V, Munro CA. Systematic review of neuroimaging correlates of executive functioning: converging evidence from different clinical populations. J Neuropsychiatry Clin Neurosci. 2014;26:114–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Steward T, Menchón JM, Jiménez-Murcia S, Soriano-Mas C, Fernández-Aranda F. Neural network alterations across eating disorders: a narrative review of fmri studies. Curr Neuropharmacol. 2018;16:1150–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Atiye M, Miettunen J, Raevuori-Helkamaa A. A meta-analysis of temperament in eating disorders. Eur Eat Disord Rev J Eat Disord Assoc. 2015;23:89–99. [DOI] [PubMed] [Google Scholar]
- 46.Dalsgaard S, McGrath J, Østergaard SD, Wray NR, Pedersen CB, Mortensen PB, et al. Association of mental disorder in childhood and adolescence with subsequent educational achievement. JAMA Psychiat. 2020;77:797–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Duncan L, Yilmaz Z, Gaspar H, Walters R, Goldstein J, Anttila V, et al. Significant locus and metabolic genetic correlations revealed in genome-wide association study of anorexia nervosa. Am J Psychiatry. 2017;174:850–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sundquist J, Ohlsson H, Winkleby MA, Sundquist K, Crump C. School achievement and risk of eating disorders in a swedish national cohort. J Am Acad Child Adolesc Psychiatry. 2016;55:41–46.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Toro J, Nicolau R, Cervera M, Castro J, Blecua MJ, Zaragoza M, et al. A clinical and phenomenological study of 185 Spanish adolescents with anorexia nervosa. Eur Child Adolesc Psychiatry. 1995;4:165–74. [DOI] [PubMed] [Google Scholar]
- 50.Sundgot-Borgen J, Torstveit MK. Aspects of disordered eating continuum in elite high-intensity sports. Scand J Med Sci Sports. 2010;20(Suppl 2):112–21. [DOI] [PubMed] [Google Scholar]
- 51.Thompson RA, Sherman RT. “Good athlete” traits and characteristics of anorexia nervosa: are they similar? Eat Disord Rout-ledge. 1999;7:181–90. [Google Scholar]
- 52.Odum AL. Delay discounting: I’m a k, you’re a k. J Exp Anal Behav. 2011;96:427–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.King JA, Bernardoni F, Geisler D, Ritschel F, Doose A, Pauligk S, Pásztor K, Weidner K, Roessner V, Smolka MN, Ehrlich S. Intact value-based decision-making during intertemporal choice in women with remitted anorexia nervosa? An fMRI study J Psychiatry Neurosci. 2020;45:108–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ritschel F, King JA, Geisler D, Flohr L, Neidel F, Boehm I, Seidel M, Zwipp J, Ripke S, Smolka MN, Roessner V, Ehrlich S. Temporal delay discounting in acutely ill and weight-recovered patients with anorexia nervosa. Psychol Med. 2015;45:1229–39. [DOI] [PubMed] [Google Scholar]
- 55.Amlung M, Marsden E, Holshausen K, Morris V, Patel H, Vedelago L, et al. Delay discounting as a transdiagnostic process in psychiatric disorders: a meta-analysis. JAMA Psychiat. 2019;76:1176–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Decker JH, Figner B, Steinglass JE. On Weight and waiting: delay discounting in anorexia nervosa pretreatment and post-treatment. Biol Psychiatry. 2015;78:606–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Steinglass JE, Lempert KM, Choo T-H, Kimeldorf MB, Wall M, Walsh BT, et al. Temporal discounting across three psychiatric disorders: anorexia nervosa, obsessive compulsive disorder, and social anxiety disorder. Depress Anxiety. 2017;34:463–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bartholdy S, Rennalls S, Danby H, Jacques C, Campbell IC, Schmidt U, et al. Temporal discounting and the tendency to delay gratification across the eating disorder spectrum. Eur Eat Disord Rev J Eat Disord Assoc. 2017;25:344–50. [DOI] [PubMed] [Google Scholar]
- 59.Steward T, Mestre-Bach G, Vintró-Alcaraz C, Agüera Z, Jiménez-Murcia S, Granero R, et al. Delay discounting of reward and impulsivity in eating disorders: from anorexia nervosa to binge eating disorder. Eur Eat Disord Rev J Eat Disord Assoc. 2017;25:601–6. [DOI] [PubMed] [Google Scholar]
- 60.••.Inzlicht M, Shenhav A, Olivola CY. The effort paradox: effort is both costly and valued. Trends Cogn Sci. 2018;22:337–49. [DOI] [PMC free article] [PubMed] [Google Scholar]; (This paper reviews the research demonstrating that effort can be both modeled as a cost and a reward, and posit different models that incorporate these two perspectives on reward.)
- 61.McCarthy JM, Treadway MT, Bennett ME, Blanchard JJ. Inefficient effort allocation and negative symptoms in individuals with schizophrenia. Schizophr Res. 2016;170:278–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Treadway MT, Peterman JS, Zald DH, Park S. Impaired effort allocation in patients with schizophrenia. Schizophr Res. 2015;161:382–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Patzelt EH, Kool W, Millner AJ, Gershman SJ. The transdiagnostic structure of mental effort avoidance. Sci Rep. 2019;9:1689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gianini LM, Klein DA, Call C, Mayer L, Foltin RW, Walsh BT, et al. The reinforcing effect of exercise in anorexia nervosa: clinical correlates and relationship to outcome. Eat Disord. 2016;24:412–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Haynos AF, Hill B, Fruzzetti AE. Emotion regulation training to reduce problematic dietary restriction: an experimental analysis. Appetite. 2016;103:265–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Klein DA, Schebendach JE, Gershkovich M, Bodell LP, Foltinb RW, Walsh BT. Behavioral assessment of the reinforcing effect of exercise in women with anorexia nervosa: further paradigm development and data. Int J Eat Disord. 2010;43:611–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Schebendach J, Klein DA, Mayer LES, Attia E, Devlin MJ, Foltin RW, et al. Assessment of the motivation to use artificial sweetener among individuals with an eating disorder. Appetite. 2017;109:131–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Schebendach JE, Klein DA, Foltin RW, Devlin MJ, Walsh BT. Relative reinforcing value of exercise in inpatients with anorexia nervosa: model development and pilot data. Int J Eat Disord. 2007;40:446–53. [DOI] [PubMed] [Google Scholar]
- 69.O’Hara CB, Keyes A, Renwick B, Leyton M, Campbell IC, Schmidt U. The effects of acute dopamine precursor depletion on the reinforcing value of exercise in anorexia nervosa. PloS One. 2016;11:e0145894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.King JA, Korb FM, Vettermann R, Ritschel F, Egner T, Ehrlich S. Cognitive overcontrol as a trait marker in anorexia nervosa? Aberrant task- and response-set switching in remitted patients. J Abnorm Psychol. 2019;128:806–12. [DOI] [PubMed] [Google Scholar]
- 71.Lloyd S, Yiend J, Schmidt U, Tchanturia K. Perfectionism in anorexia nervosa: novel performance based evidence. PloS One. 2014;9:e111697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Pieters G, Hulstijn W, Vandereycken W, Maas Y, Probst M, Peuskens J, et al. Fast psychomotor functioning in anorexia nervosa: effect of weight restoration. J Clin Exp Neuropsychol. 2005;27:931–42. [DOI] [PubMed] [Google Scholar]
- 73.Pieters G, Hulstijn W, Maas Y, Vandereycken W, Peuskens J, Probst M, et al. Psychomotor performance and sequence planning in anorexia nervosa before and after weight restoration. Eat Weight Disord EWD. 2006;11:154–62. [DOI] [PubMed] [Google Scholar]
- 74.Ehrlich S, Geisler D, Ritschel F, King JA, Seidel M, Boehm I, et al. Elevated cognitive control over reward processing in recovered female patients with anorexia nervosa. J Psychiatry Neurosci JPN. 2015;40:307–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Boehm I, King JA, Bernardoni F, Geisler D, Seidel M, Ritschel F, et al. Subliminal and supraliminal processing of reward-related stimuli in anorexia nervosa. Psychol Med. 2018;48:790–800. [DOI] [PubMed] [Google Scholar]
- 76.Seidel M, King JA, Ritschel F, Boehm I, Geisler D, Bernardoni F, et al. Processing and regulation of negative emotions in anorexia nervosa: an fMRI study. NeuroImage Clin. 2018;18:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Stedal K, Broomfield C, Hay P, Touyz S, Scherer R. Neuropsychological functioning in adult anorexia nervosa: a meta-analysis. Neurosci Biobehav Rev. 2021;130:214–26. [DOI] [PubMed] [Google Scholar]
- 78.Zakzanis KK, Campbell Z, Polsinelli A. Quantitative evidence for distinct cognitive impairment in anorexia nervosa and bulimia nervosa. J Neuropsychol. 2010;4:89–106. [DOI] [PubMed] [Google Scholar]
- 79.Giannunzio V, Degortes D, Tenconi E, Collantoni E, Solmi M, Santonastaso P, et al. Decision-making impairment in anorexia nervosa: new insights into the role of age and decision-making style. Eur Eat Disord Rev J Eat Disord Assoc. 2018;26:302–14. [DOI] [PubMed] [Google Scholar]
- 80.Wu M, Brockmeyer T, Hartmann M, Skunde M, Herzog W, Friederich H-C. Reward-related decision making in eating and weight disorders: a systematic review and meta-analysis of the evidence from neuropsychological studies. Neurosci Biobehav Rev. 2016;61:177–96. [DOI] [PubMed] [Google Scholar]
- 81.Piccolo M, Milos GF, Bluemel S, Schumacher S, Mueller-Pfeiffer C, Fried M, et al. behavioral responses to uncertainty in weight-restored anorexia nervosa — preliminary results. Front Psychol. 2019;10:2492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Keegan E, Tchanturia K, Wade TD. Central coherence and set-shifting between nonunderweight eating disorders and anorexia nervosa: a systematic review and meta-analysis. Int J Eat Disord. 2021;54:229–43. [DOI] [PubMed] [Google Scholar]
- 83.••.Redish AD, Gordon JA, editors. Computational psychiatry: new perspectives on mental illness. Cambridge, MA, USA: MIT Press; 2016. [Google Scholar]; (This book outlines the theoretical underpinnings and postulates of computational psychiatry and provides examples of how this approach can be applied transdiagnostically to better characterize and understand psychiatric concerns.)
- 84.Friston KJ, Redish AD, Gordon JA. Computational nosology and precision psychiatry. Comput Psychiatry Camb Mass. 2017;1:2–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Redish AD. Beyond the cognitive map: from place cells to episodic memory. Cambridge, MA, USA: A Bradford Book; 1999. [Google Scholar]
- 86.Cooper JA, Arulpragasam AR, Treadway MT. Anhedonia in depression: biological mechanisms and computational models. Curr Opin Behav Sci. 2018;22:128–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Brown VM, Zhu L, Solway A, Wang JM, McCurry KL, King-Casas B, et al. Reinforcement learning disruptions in individuals with depression and sensitivity to symptom change following cognitive behavioral therapy. JAMA Psychiatry. 2021; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Chen C, Takahashi T, Nakagawa S, Inoue T, Kusumi I. Reinforcement learning in depression: a review of computational research. Neurosci Biobehav Rev. 2015;55:247–67. [DOI] [PubMed] [Google Scholar]
- 89.Smith R, Kuplicki R, Feinstein J, Forthman KL, Stewart JL, Paulus MP, et al. A Bayesian computational model reveals a failure to adapt interoceptive precision estimates across depression, anxiety, eating, and substance use disorders. PLoS Comput Biol. 2020;16:e1008484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Berwian IM, Wenzel JG, Collins AGE, Seifritz E, Stephan KE, Walter H, et al. Computational mechanisms of effort and reward decisions in patients with depression and their association with relapse after antidepressant discontinuation. JAMA Psychiat. 2020;77:513–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Boettcher H, Brake CA, Barlow DH. Origins and outlook of interoceptive exposure. J Behav Ther Exp Psychiatry. 2016;53:41–51. [DOI] [PubMed] [Google Scholar]
- 92.•.Voon V, Reiter A, Sebold M, Groman S. Model-based control in dimensional psychiatry. Biol Psychiatry. 2017;82:391–400. [DOI] [PubMed] [Google Scholar]; (This review outlines the differences between model-based and model-free learning and decision-making, as well as the literature suggesting that these distinctions may be useful for understanding the maintenance of psychopathology.)
- 93.Gläscher J, Daw N, Dayan P, O’Doherty JP. States versus rewards: dissociable neural prediction error signals underlying model-based and model-free reinforcement learning. Neuron. 2010;66:585–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Gillan CM, Kosinski M, Whelan R, Phelps EA, Daw ND. Characterizing a psychiatric symptom dimension related to deficits in goal-directed control. eLife. 2016;5:e11305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Foerde K, Daw ND, Rufin T, Walsh BT, Shohamy D, Steinglass JE. Deficient goal-directed control in a population characterized by extreme goal pursuit. J Cogn Neurosci. 2021;33:463–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Voon V, Derbyshire K, Rück C, Irvine MA, Worbe Y, Enander J, et al. Disorders of compulsivity: A common bias towards learning habits. Mol Psychiatry. 2015;20:345–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Hasz BM, Redish AD. Deliberation and procedural automation on a two-step task for rats. Front Integr Neurosci. 2018;12:30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.•.Daw ND, Gershman SJ, Seymour B, Dayan P, Dolan RJ. Model-based influences on humans’ choices and striatal prediction errors. Neuron. 2011;69:1204–15. [DOI] [PMC free article] [PubMed] [Google Scholar]; (This study distinguishes between model-based and model-free influences on human decision-making, demonstrating that people dysnamically switch between use of these different strategies.)
- 99.Daw ND, Dayan P. The algorithmic anatomy of model-based evaluation. Philos Trans R Soc Lond B Biol Sci. 2014;369:20130478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.••.Redish AD. Vicarious trial and error. Nat Rev Neurosci. 2016;17:147–59. [DOI] [PMC free article] [PubMed] [Google Scholar]; (This paper describes the discovery of deliberation among non-human animals, and how this decision process translates to explain human cognition and behavior.)
- 101.••.Steinglass JE, Walsh BT. Neurobiological model of the persistence of anorexia nervosa. J Eat Disord. 2016;4:19. [DOI] [PMC free article] [PubMed] [Google Scholar]; (This manuscript reviews the state of the neurobiological evidence for the habit theory of AN.)
- 102.Foerde K, Steinglass JE, Shohamy D, Walsh BT. Neural mechanisms supporting maladaptive food choices in anorexia nervosa. Nat Neurosci. 2015;18:1571–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Foerde K, Walsh BT, Dalack M, Daw N, Shohamy D, Steinglass JE. Changes in brain and behavior during food-based decision-making following treatment of anorexia nervosa. J Eat Disord. 2021;9:48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Foerde K, Schebendach JE, Davis L, Daw N, Walsh BT, Shohamy D, et al. Restrictive eating across a spectrum from healthy to unhealthy: behavioral and neural mechanisms. Psychol Med. 2020;1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Godier LR, de Wit S, Pinto A, Steinglass JE, Greene AL, Scaife J, et al. An investigation of habit learning in anorexia nervosa. Psychiatry Res. 2016;244:214–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Steding J, Boehm I, King JA, Geisler D, Ritschel F, Seidel M, et al. Goal-directed vs. habitual instrumental behavior during reward processing in anorexia nervosa: an fMRI study. Sci Rep. 2019;9:13529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Davis L, Walsh BT, Schebendach J, Glasofer DR, Steinglass JE. Habits are stronger with longer duration of illness and greater severity in anorexia nervosa. Int J Eat Disord. 2020;53:413–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Constantino S, Daw ND. Learning the opportunity cost of time in a patch-foraging task. Cogn Affect Behav Neurosci. 2015;15:837–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.•.Abram SV, Breton Y-A, Schmidt B, Redish AD, MacDonald AW. The Web-Surf Task: a translational model of human decision-making. Cogn Affect Behav Neurosci. 2016;16:37–50. [DOI] [PMC free article] [PubMed] [Google Scholar]; (This study demonstrates that non-human animal foraging paradigms can be adapted for human samples, who demonstrate similar patterns of decision-making under foraging conditions.)
- 110.Shenhav A, Musslick S, Lieder F, Kool W, Griffiths TL, Cohen JD, et al. Toward a rational and mechanistic account of mental effort. Annu Rev Neurosci. 2017;40:99–124. [DOI] [PubMed] [Google Scholar]
- 111.Bickel WK, Johnson MW, Koffarnus MN, MacKillop J, Murphy JG. The behavioral economics of substance use disorders: reinforcement pathologies and their repair. Annu Rev Clin Psychol. 2014;10:641–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Brassard SL, Balodis IM. A review of effort-based decision-making in eating and weight disorders. Prog Neuropsychopharmacol Biol Psychiatry. 2021;110:110333. [DOI] [PubMed] [Google Scholar]
- 113.Eisenberger R Learned industriousness. Psychol Rev. 1992;99:248–67. [DOI] [PubMed] [Google Scholar]
- 114.Damiano CR, Aloi J, Treadway M, Bodfish JW, Dichter GS. Adults with autism spectrum disorders exhibit decreased sensitivity to reward parameters when making effort-based decisions. J Neurodev Disord. 2012;4:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Brainstorm Consortium, Anttila V, Bulik-Sullivan B, Finucane HK, Walters RK, Bras J, et al. Analysis of shared heritability in common disorders of the brain. Science. 2018;360:eaap8757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 5th Edition: DSM-5. 5th ed. Washington, D.C: American Psychiatric Publishing; 2013. [Google Scholar]
- 117.Lei H, Huang L, Li J, Liu W, Fan J, Zhang X, et al. Altered spontaneous brain activity in obsessive-compulsive personality disorder. Compr Psychiatry. 2020;96:152144. [DOI] [PubMed] [Google Scholar]
- 118.Cavanagh JF, Shackman AJ. Frontal midline theta reflects anxiety and cognitive control: meta-analytic evidence. J Physiol Paris. 2015;109:3–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Andreassen CS, Griffiths MD, Hetland J, Kravina L, Jensen F, Pallesen S. The prevalence of workaholism: a survey study in a nationally representative sample of norwegian employees. PloS One. Public Library of Science; 2014;9:e102446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Freimuth M, Moniz S, Kim SR. Clarifying exercise addiction: differential diagnosis, co-occurring disorders, and phases of addiction. Int J Environ Res Public Health. 2011;8:4069–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Egorov AY, Szabo A. The exercise paradox: an interactional model for a clearer conceptualization of exercise addiction. J Behav Addict. 2013;2:199–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Peterson C suicide rates by industry and occupation — national violent death reporting system, 32 states, 2016. MMWR Morb Mortal Wkly Rep [Internet]. 2020;69. Available from: https://www.cdc.gov/mmwr/volumes/69/wr/mm6903a1.htm [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Pinto A Treatment of obsessive-compulsive personality disorder. 2016. p. 415–29.
- 124.Yang Z, Oathes DJ, Linn KA, Bruce SE, Satterthwaite TD, Cook PA, et al. Cognitive behavioral therapy is associated with enhanced cognitive control network activity in major depression and posttraumatic stress disorder. Biol Psychiatry Cogn Neurosci Neuroimaging. 2018;3:311–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Hauer L, Sellner J, Brigo F, Trinka E, Sebastianelli L, Saltuari L, et al. Effects of repetitive transcranial magnetic stimulation over prefrontal cortex on attention in psychiatric disorders: a systematic review. J Clin Med. 2019;8:E416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.•.Treasure J, Crane A, McKnight R, Buchanan E, Wolfe M. First do no harm: iatrogenic maintaining factors in anorexia nervosa. Eur Eat Disord Rev J Eat Disord Assoc. 2011;19:296–302. [DOI] [PubMed] [Google Scholar]; (This manuscript hypothesizes ways in which common therapeutic practices could be harmful to individuals with AN if managed insensitively, highlighting the need to carefully consider treatment targets in this population.)
- 127.Halmi KA. Salient components of a comprehensive service for eating disorders. World Psychiatry. 2009;8:150–5. [DOI] [PMC free article] [PubMed] [Google Scholar]


