Abstract
Aims
Multi‐organ dysfunction was recently reported to be a common condition in patients with heart failure (HF). The Model for End‐stage Liver Disease eXcluding International normalized ratio (MELD‐XI) score reflects liver and kidney function. The prognostic relevance of this score has been reported in patients with a variety of cardiovascular diseases who are undergoing interventional therapies. However, the relationship between the severity of hepatorenal dysfunction assessed by the MELD‐XI score and the long‐term clinical outcomes of HF patients receiving cardiac resynchronization therapy (CRT) has not been evaluated.
Methods and results
Clinical records of 283 patients who underwent CRT implantation between March 2003 and October 2020 were retrospectively evaluated (mean age 67 ± 12, 22.6% female). Blood samples were collected before CRT implantation. Patients were divided into three groups based on tertiles of the MELD‐XI score: first tertile (MELD‐XI = 9.44, n = 95), second tertile (9.44 < MELD‐XI < 13.4, n = 94), and third tertile (MELD‐XI ≥ 13.4, n = 94). The primary endpoint was all‐cause mortality. Compared with the other groups, the third tertile group exhibited significantly older age, higher prevalence of diabetes mellitus and hypertension, lower haemoglobin level, and higher N‐terminal pro‐brain natriuretic peptide level (all P < 0.05). The functional CRT response rate was also significantly lower in the third tertile group (P = 0.011). During a median follow‐up of 30 months (inter‐quartile range, 9–67), 105 patients (37.1%) died. Kaplan–Meier analysis revealed that patients with a higher MELD‐XI score had a greater risk of all‐cause mortality (log‐rank test: P < 0.001). Even after adjustment for clinically relevant factors and a conventional risk score, the MELD‐XI score was still associated with mortality (adjusted hazard ratio: 1.04, 95% confidence interval: 1.00–1.07, P = 0.014, and adjusted hazard ratio: 1.04, 95% confidence interval: 1.01–1.09, P = 0.005, respectively). A higher MELD‐XI score was associated with a greater risk of all‐cause mortality than a lower MELD‐XI score regardless of whether a pacemaker or defibrillator was implanted (log‐rank test: P = 0.010 and P < 0.001, respectively).
Conclusions
Impaired hepatorenal function assessed by the MELD‐XI score was associated with older age, higher prevalence of multiple co‐morbidities, severity of HF, lower CRT response rates, and subsequent all‐cause mortality in HF patients undergoing CRT implantation. These results suggest that the MELD‐XI score can provide additional prognostic information and may be useful for improving risk stratification in this population.
Keywords: Heart failure, Arrhythmia, Pacemaker implantation, Multi‐organ dysfunction
Introduction
Cardiac resynchronization therapy (CRT) is an established treatment for patients who have advanced‐stage heart failure (HF) with a reduced left ventricular ejection fraction (LVEF) and wide QRS complex. 1 , 2 However, individual outcomes in CRT recipients vary significantly, and long‐term death rates remain high. 3 Certain patients, such as those with ischaemic cardiomyopathy, severely dilated ventricles, or right ventricular (RV) dysfunction, have been reported to derive less survival benefit than expected from CRT. 4 , 5 Therefore, risk stratification of potential CRT candidates on the basis of pre‐implantation assessment remains important.
Low cardiac output and systemic venous congestion due to advanced HF are known to cause multiple organ dysfunction or tissue damage, which leads to disease progression and adverse outcomes. 6 , 7 Traditionally, organ dysfunction is evaluated in isolation, meanwhile in clinical settings, several organs may be caused, which can be a marker of more severe HF. 8
The Model for End‐stage Liver Disease (MELD) score, which is based on bilirubin, creatinine, and the international normalized ratio, reflects liver and kidney function. 9 This score was originally developed for prognostic assessment in patients with advanced liver disease. 10 The MELD‐XI score is one of the several modified MELD scores, and unlike the standard MELD score, it excludes the international normalized ratio value. 11 Recently, several studies reported the prognostic relevance of the MELD‐XI score in a variety of patients, including those with advanced HF undergoing left ventricular (LV) assist device implantation, those with severe mitral regurgitation (MR) undergoing percutaneous mitral valve repair, and those with severe aortic valve stenosis undergoing transcatheter aortic valve implantation. 12 , 13 , 14 However, the relationship between the severity of hepatorenal dysfunction assessed by the MELD‐XI score and the long‐term clinical outcomes of HF patients receiving CRT has not been evaluated.
In this real‐world, observational study, we examine the potential use of the MELD‐XI score as a risk assessment tool for all‐cause mortality in HF patients receiving CRT. In addition, we investigated whether the predictive value of the MELD‐XI score differed between patients receiving CRT with a pacemaker (CRT‐P) or an implantable cardioverter–defibrillator (CRT‐D).
Methods
Patients and study protocol
This was a single‐centre, retrospective, observational cohort study. We screened 285 consecutive patients who underwent CRT implantation at Nihon University Itabashi Hospital between March 2004 and October 2020. Two patients were excluded because of lack of data for one or both of the components of the MELD‐XI score (total bilirubin or creatinine), and the remaining 283 patients were investigated. This study was approved by the ethics committee of Nihon University Itabashi Hospital (RK‐210209‐8). The investigation conformed to the principles outlined in the Declaration of Helsinki.
Laboratory tests and the MELD‐XI score
The MELD‐XI score was determined based on total bilirubin and creatinine levels obtained before CRT implantation. The MELD‐XI score was calculated as previously reported: 11.76 × ln (creatinine [mg/dL]) + 5.11 × ln (total bilirubin [mg/dL]) + 9.44. 11 If a patient had a creatinine or total bilirubin level lower than 1.0 mg/dL, a value of 1.0 mg/dL was used to prevent negative logarithmic values in the formula. 15 Patients were divided into three groups based on the tertile of the MELD‐XI score.
Echocardiographic measurement
Echocardiography was performed by experienced technicians according to the guidelines of the American Society of Echocardiography. 16 End‐systolic and end‐diastolic LV volumes were measured in the apical four‐chamber and two‐chamber views. LVEF was measured by the modified Simpson's method. The RV end‐diastolic diameter (RVDd) was measured at the basal ventricular level of the RV in end‐diastole. The RV fractional area change (RVFAC) was obtained by tracing the RV end‐diastolic area (RVEDA) and end‐systolic area (RVESA) in the apical four‐chamber view using the following formula: (RVEDA − RVESA)∕RVEDA × 100. MR and tricuspid regurgitation (TR) were graded on a 4‐point scale based on colour‐flow Doppler images. The TR pressure gradient (TRPG) was measured using continuous‐wave Doppler imaging. From the subcostal view, the diameter of the inferior vena cava (IVC) in its long axis was measured within 3 cm of the IVC–right atrium junction during passive respiration.
Cardiac resynchronization therapy
All patients underwent device implantation under local anaesthesia. As previously described, atrioventricular delay was optimized automatically by each device, but if the QRS duration did not narrow sufficiently, the atrioventricular and interventricular delays were optimized manually based on the QRS duration observed on the electrocardiogram. 17 Thereafter, patients were followed up in dedicated device therapy clinics at regular 3–6 month intervals. We evaluated two definitions of CRT response: functional and echocardiographic. 17 The functional CRT response was defined as the combination of improvement by at least one New York Heart Association (NYHA) functional class and the absence of death or hospitalization due to HF at 6 months after CRT implantation. 17 , 18 The echocardiographic CRT response was defined as an improvement in the LVEF of at least 5% or a reduction in the LV end‐systolic volume (LVESV) of at least 15% at 6 months after CRT implantation. 17
Follow‐up and endpoint
The primary endpoint was all‐cause mortality, and the secondary endpoint was the incidence of cardiac death. Patients were followed from the date of device implantation to December 2020 or until the endpoint. Follow‐up data were collected in a blinded fashion via review of all available medical records.
Statistical analysis
Continuous variables are presented as medians (inter‐quartile range) and categorical variables as numbers (percentage). Statistical differences between continuous variables were compared using one‐way analysis of variance followed by the post hoc Tukey–Kramer test, or the Kruskal–Wallis test followed by the Steel–Dwass test. Categorical variables were compared by the χ 2 test with Bonferroni correction. Correlations between variables were tested by Pearson's correlation coefficient. The Kaplan–Meier method was used to analyse patient survival, and the log‐rank test was used to compare group differences.
The associations between pre‐CRT implantation characteristics and all‐cause mortality were assessed with a Cox proportional hazards regression analysis. Hazard ratios with 95% confidence intervals were calculated. To satisfy the model assumptions, data of N‐terminal pro‐brain natriuretic peptide (NT‐proBNP) were subjected to natural log transformation (ln). Until January 2010, we measured BNP levels instead of NT‐proBNP levels, and all BNP data were converted to NT‐proBNP data using the following formula: NT‐proBNP = BNP1.341 − 15. 19
Multivariate Cox proportional hazards regression analysis was used to evaluate the impact of the MELD‐XI score. We constructed multivariate models to adjust for the effect of established confounders such as the following: age, sex, diabetes mellitus (DM), ischaemic myopathy, atrial fibrillation, QRS duration >150 ms, LVESV, and moderate or severe MR (Model 1); the effect of a conventional risk score (the VALID‐CRT risk score, Model 2); and the effect of echocardiographic parameters related to the severity of right HF (RVDd, RVFAC, TRPG, moderate or severe TR, and maximal IVC diameter) (Model 3). The VALID‐CRT risk score was constructed and validated using the following variables: age, sex, implantable cardioverter defibrillator backup, atrial fibrillation, presence or absence of atrioventricular junction ablation in the case of atrial fibrillation, ischaemic aetiology, DM, NYHA class, and LVEF. 20 Furthermore, to assess whether the accuracy of predicting all‐cause mortality would improve after adding the MELD‐XI score to a baseline model consisting of the VALID‐CRT risk score, the C‐statistics, net reclassification improvement, and integrated discrimination improvement were calculated.
In the sensitive analysis, we classified patients into three groups based on the lowest 20%, middle 60%, and the highest 20% of the MELD‐XI score. We then compared the clinical characteristics and clinical outcomes among three groups.
For all analyses, P < 0.05 was considered statistically significant. All statistical analyses were performed using JMP 13.0 (SAS Institute, Cary, NC, USA) and the R Statistics Version 3.5.3 (R Foundation for Statistical Computing, Vienna, Austria).
Results
Study population
The distribution of MELD‐XI scores among the study patients is shown in Figure 1 . The median (inter‐quartile range) MELD‐XI score was 10.5 (9.4–14.6). The cut‐off values used to define the MELD‐XI score tertiles were determined to be 9.44 and 13.3, and patients were stratified into three groups accordingly: first tertile (MELD‐XI = 9.44, n = 95), second tertile (9.44 < MELD‐XI < 13.4, n = 94), and third tertile (MELD‐XI ≥ 13.4, n = 94). The baseline clinical characteristics for each group are shown in Table 1 . Compared with the other two groups, the third tertile group exhibited the following significant differences: older age; higher prevalence of men; higher prevalence of diabetes mellitus and hypertension; lower administration rates of angiotensin‐converting enzyme inhibitor and angiotensin receptor blocker; lower haemoglobin level; and higher levels of total bilirubin, blood urea nitrogen, creatinine, and NT‐proBNP (all P < 0.05). The third tertile group also exhibited a higher prevalence of moderate or severe TR, as well as higher TRPG (all P < 0.05). Echocardiographic parameters related to left HF (LV end‐diastolic volume, LVESV, LVEF, and MR severity) did not differ significantly between the three groups.
Figure 1.
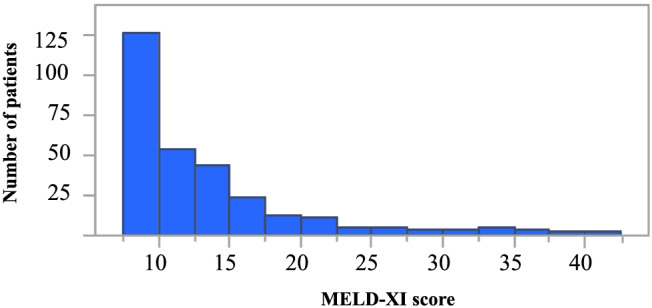
Distribution of MELD‐XI scores.
Table 1.
Clinical characteristics of patients stratified into three groups according to tertiles of the MELD‐XI score
| Item |
First tertile MELD‐XI = 9.44 (n = 95) |
Second tertile 9.44 < MELD‐XI < 13.4 (n = 94) |
Third tertile MELD‐XI ≥ 13.4 (n = 94) |
P value |
|---|---|---|---|---|
| Baseline clinical data | ||||
| Age (years) | 69 (60–74) | 67 (57–76) | 72 (64–79)* , † | 0.019 |
| Male, n (%) | 64 (67.3) | 79 (84.0)* | 76 (80.8)* | 0.016 |
| Body mass index (kg/m2) | 21.4 (18.8–24.3) | 22.4 (20.4–25.3) | 22.3 (19.9–24.8) | 0.096 |
| NYHA IV, n (%) | 9 (9.4) | 11 (11.7) | 20 (21.8) | 0.052 |
| Diabetes mellitus, n (%) | 25 (26.3) | 36 (38.3) | 44 (46.8)* | 0.012 |
| Hypertension, n (%) | 38 (40.0) | 49 (52.1) | 56 (59.5)* | 0.024 |
| Ischaemic cardiomyopathy, n (%) | 25 (26.3) | 36 (38.3) | 35 (37.2) | 0.15 |
| Atrial fibrillation, n (%) | 17 (17.8) | 23 (24.4) | 27 (28.7) | 0.21 |
| QRS duration (ms) | 152 (128–172) | 150 (123–174) | 150 (130–168) | 0.89 |
| VALID‐CRT risk score | 0.80 (−0.08–1.23) | 0.76 (0.19–1.35) | 0.89 (0.27–1.51) | 0.066 |
| Medications | ||||
| ACE‐I or ARB, n (%) | 65 (68.4) | 70 (75.2) | 52 (55.3) † | 0.013 |
| Beta‐blocker, n (%) | 85 (89.4) | 89 (95.7) | 83 (88.3) | 0.16 |
| Diuretic, n (%) | 86 (90.5) | 87 (93.5) | 78 (82.9) | 0.058 |
| Laboratory data | ||||
| Haemoglobin (g/dL) | 12.7 (11.7–13.8) | 13.4 (11.2–14.3) | 11.7 (10.4–13.0)* , † | <0.001 |
| Platelet count (× 103/μL) | 201 (169–253) | 188 (157–221) | 190 (146–220) | 0.033 |
| Total bilirubin (mg/dL) | 0.6 (0.4–0.8) | 0.8 (0.5–1.1)* | 0.6 (0.3–1.3) | 0.002 |
| AST (U/L) | 22 (19–31) | 25 (19–34) | 23 (17–31) | 0.37 |
| ALT (U/L) | 18 (13–30) | 19 (15–28) | 17 (12–28) | 0.42 |
| GGT (U/L) | 41 (22–84) | 49 (27–97) | 43 (24–72) | 0.58 |
| Sodium (mEq/L) | 140 (138–142) | 139 (137–141) | 139 (136–141) | 0.15 |
| BUN (mg/dL) | 18 (13–22) | 21 (16–26)* | 33 (25–47)* , † | <0.001 |
| Cr (mg/dL) | 0.8 (0.7–0.9) | 1.0 (1.0–1.1)* | 1.7 (1.4–2.5)* , † | <0.001 |
| NT‐proBNP (pg/mL) | 2310 (1018–5689) | 2743 (1417–8029) | 7119 (2319–15,461)* , † | <0.001 |
| Echocardiographic data | ||||
| LVEDV (mL) | 199 (162–259) | 215 (163–263) | 197 (144–255) | 0.46 |
| LVESV (mL) | 147 (105–191) | 153 (101–201) | 134 (90–189) | 0.36 |
| LVEF (%) | 30 (21–38) | 27 (21–36) | 30 (23–38) | 0.35 |
| Moderate or severe MR, n (%) | 17 (17.8) | 10 (10.6) | 19 (20.2) | 0.17 |
| RVDd (mm) | 31 (25–35) | 34 (30–38) | 32 (29–38) | 0.13 |
| RVFAC (%) | 45 (34–52) | 44 (32–50) | 42 (35–52) | 0.41 |
| Moderate or severe TR, n (%) | 11 (14.3) | 22 (29.7) | 26 (34.2)* | 0.009 |
| TRPG (mmHg) | 18 (5–30) | 22 (5–31) | 27 (15–39)* , † | 0.019 |
| Maximal IVC diameter (mm) | 14 (11–17) | 16 (11–19) | 15 (13–19) | 0.17 |
ACE‐I, angiotensin‐converting enzyme inhibitor; ALT, alanine aminotransferase; ARB, angiotensin receptor blocker; AST, aspartate aminotransferase; BUN, blood urea nitrogen; Cr, creatinine; CRT, cardiac resynchronization therapy; GGT, γ‐glutamyl transferase; IVC, inferior vena cava; LVEDV, left ventricular end‐diastolic volume; LVEF, left ventricular ejection fraction; LVESV, left ventricular end‐systolic volume; MELD‐XI, Model for End‐stage Liver Disease excluding the International normalized ratio; MR, mitral regurgitation; NT‐proBNP, N‐terminal pro‐brain natriuretic peptide; NYHA, New York Heart Association; RVDd, right ventricular end‐diastolic diameter; RVFAC, right ventricular fractional area change; TR, tricuspid regurgitation; TRPG, tricuspid regurgitation pressure gradient.
Values are shown as the median (inter‐quartile range) or number (%). For multiple comparisons, the ANOVA test was used for symmetrical continuous variables, the Kruskal–Wallis test was used for non‐symmetrical continuous variables, and the χ 2 test was used for categorical variables. All pair comparisons were performed based on the Tukey–Kramer test for symmetrical continuous variables, the Steel–Dwass test for non‐symmetrical continuous variables, and the χ 2 test with Bonferroni correction for categorical variables.
P < 0.05 vs. first tertile.
P < 0.05 vs. second tertile.
Hepatorenal function and cardiac resynchronization therapy response
In this study population, 277 patients underwent 6 months of follow‐up. Of these patients, 210 (75.8%) were categorized as functional CRT responders. The functional CRT response rates were 79.3% in the first tertile group, 82.9% in the second tertile group, and 64.8% in the third tertile group. The functional CRT response rate was significantly lower in the third tertile group than in the other two groups (P = 0.011). Of 263 patients who underwent follow‐up echocardiography 6 months after CRT implantation, 190 (72.2%) were categorized as echocardiographic CRT responders. The echocardiographic CRT response rates were 78.6% in the first tertile group, 73.3% in the second tertile group, and 64.2% in the third tertile group (P = 0.10). The MELD‐XI score before CRT implantation was not significantly correlated with the rate of LVEF change from before to after CRT implantation (r = −0.08, P = 0.18).
Hepatorenal function and clinical outcomes
The median (inter‐quartile range) follow‐up period was 30 (9–67) months, and 105 patients died (58 cardiac deaths and 47 non‐cardiac deaths). Kaplan–Meier curves revealed that patients with a higher MELD‐XI score had a greater risk of all‐cause mortality than those with lower MELD‐XI scores (log‐rank test: P < 0.001, Figure 2 ). Furthermore, the rate of cardiac deaths was significantly higher in patients with a higher MELD‐XI score (log‐rank test: P = 0.002, Figure 3 ). Univariate Cox proportional hazards regression analysis revealed that a higher MELD‐XI score was significantly associated with all‐cause mortality, along with lower body mass index, higher NYHA functional class, atrial fibrillation, QRS duration, higher VALID‐CRT risk score, lower haemoglobin and sodium levels, and higher blood urea nitrogen and NT‐proBNP levels (all P < 0.05, Table 2 ). Regarding echocardiographic parameters, lower RVFAC, moderate or severe TR, and higher TRPG were significantly associated with all‐cause mortality in univariate Cox proportional hazards regression analysis (all P < 0.05, Table 2 ). Total bilirubin and creatine levels did not separately show a significant association with all‐cause mortality. A higher MELD‐XI score was significantly associated with all‐cause mortality after adjusting for the VALID‐CRT risk score, other previously reported clinically relevant factors (age, sex, DM, ischaemic myopathy, atrial fibrillation, QRS duration >150 ms, LVESV, and moderate or severe MR), and echocardiographic parameters related to right HF (RVDd, RVFAC, moderate or severe TR, TRPG, and maximal IVC diameter) (Table 3 ). Furthermore, adding the MELD‐XI score to a baseline model consisting of the VALID‐CRT risk score significantly increased the net reclassification improvement and integrated discrimination improvement for predicting all‐cause mortality (Table 4 ).
Figure 2.
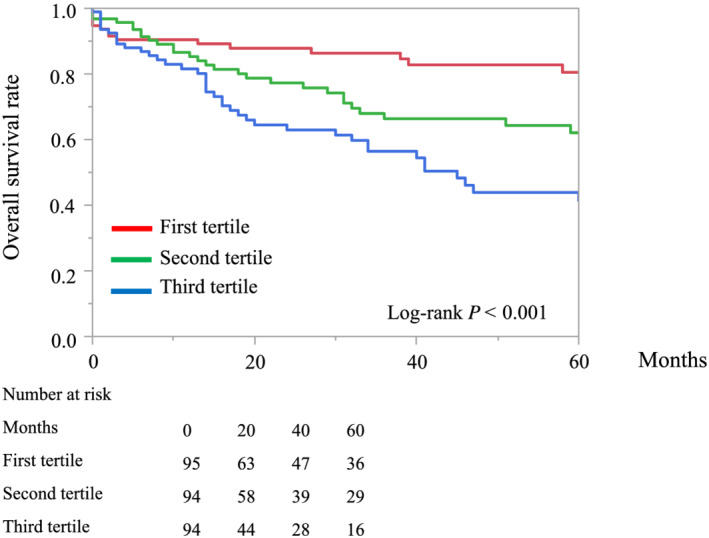
Kaplan–Meier curves of overall survival for patient groups defined according to the first, second, and third tertiles of the MELD‐XI score.
Figure 3.
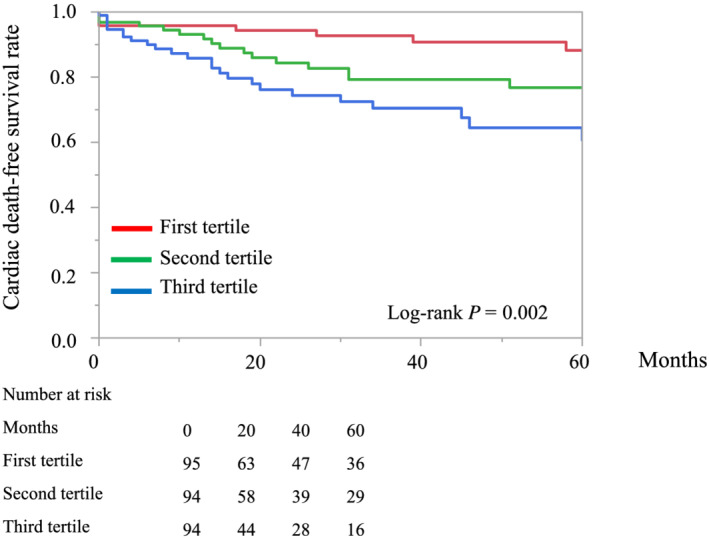
Kaplan–Meier curves of event (cardiac death)‐free survival for patient groups defined according to the first, second, and third tertiles of the MELD‐XI score.
Table 2.
Univariate Cox proportional hazards analysis for risk of all‐cause mortality
| Item | Hazard ratio | 95% CI | P |
|---|---|---|---|
| Age (per 1 year increase) | 1.00 | 0.98–1.01 | 0.69 |
| Male | 1.16 | 0.73–1.94 | 0.53 |
| Body mass index (per 1 kg/m2 increase) | 0.9 | 0.87–0.936 | <0.001 |
| NYHA IV (vs. NYHA II or III) | 9.31 | 5.73–14.9 | <0.001 |
| Diabetes mellitus | 1.22 | 0.81–1.80 | 0.32 |
| Ischaemic cardiomyopathy | 1.22 | 0.81–1.81 | 0.33 |
| Atrial fibrillation | 1.74 | 1.14–2.61 | 0.011 |
| QRS duration >150 ms | 0.55 | 0.37–0.82 | 0.003 |
| VALID‐CRT risk score (per 1 increase) | 1.40 | 1.12–1.76 | 0.002 |
| Haemoglobin (per 1 g/dL increase) | 0.80 | 0.73–0.88 | <0.001 |
| Platelet count (per 1 × 103/μL increase) | 0.99 | 0.99–1.00 | 0.24 |
| Total bilirubin (per 0.1 mg/dL increase) | 1.00 | 0.66–1.45 | 0.98 |
| AST (per 10 U/L increase) | 0.95 | 0.81–1.00 | 0.37 |
| ALT (per 10 U/L increase) | 0.96 | 0.85–1.00 | 0.37 |
| GGT (per 10 U/L increase) | 1.00 | 0.98–1.02 | 0.40 |
| Sodium (per 1 mmol/L increase) | 0.91 | 0.86–0.96 | <0.001 |
| BUN (per 1 mg/dL increase) | 1.02 | 1.00–1.02 | <0.001 |
| Cr (per 0.1 mg/dL increase) | 1.01 | 0.99–1.01 | 0.052 |
| ln [NT‐proBNP] (per 1 increase) | 1.61 | 1.38–1.89 | <0.001 |
| MELD‐XI score (per 1 increase) | 1.04 | 1.01–1.07 | 0.002 |
| LVEDV (per 10 mL increase) | 1.00 | 0.97–1.02 | 0.96 |
| LVESV (per 10 mL increase) | 1.00 | 0.97–1.03 | 0.67 |
| LVEF (per 1% increase) | 0.98 | 0.96–1.00 | 0.066 |
| Moderate or severe MR | 1.26 | 0.78–1.96 | 0.32 |
| RVDd (per 1 mm increase) | 0.99 | 0.97–1.02 | 0.99 |
| RVFAC (per 1% increase) | 0.98 | 0.96–0.99 | 0.046 |
| Moderate or severe TR | 1.74 | 1.04–2.84 | 0.032 |
| TRPG (per 1 mmHg increase) | 1.02 | 1.01–1.04 | <0.001 |
| Maximal IVC diameter (per 1 mm increase) | 1.02 | 0.98–1.06 | 0.19 |
CI, confidence interval. Other abbreviations as in Table 1 .
Table 3.
Multivariate Cox proportional hazards analysis for risk of all‐cause mortality
| Model | MELD‐XI (per 1 increase) | ||
|---|---|---|---|
| Hazard ratio | 95% CI | P | |
| Model 1 | 1.04 | 1.00–1.07 | 0.014 |
| Model 2 | 1.04 | 1.01–1.09 | 0.005 |
| Model 3 | 1.04 | 1.01–1.08 | 0.020 |
CI, confidence interval; MELD‐XI, Model for End‐stage Liver Disease excluding the International normalized ratio.
Model 1 = adjusted for age, sex, and clinically relevant factors (diabetes mellitus, ischaemic cardiomyopathy, atrial fibrillation, QRS duration >150 ms, left ventricular end‐systolic volume, and moderate or severe mitral regurgitation). Model 2 = adjusted for VALID–cardiac resynchronization therapy risk score. Model 3 = adjusted for age, sex, and factors related to right heart failure (right ventricular end‐diastolic diameter, right ventricular fractional area change, tricuspid regurgitation pressure gradient, moderate or severe tricuspid regurgitation, and maximal inferior vena cava diameter).
Table 4.
Use of the MELD‐XI score together with the VALID‐CRT risk score improves the prediction of all‐cause mortality
| Risk score | C‐statistics (95% CI) | P | NRI (95% CI) | P | IDI (95% CI) | P |
|---|---|---|---|---|---|---|
| VALID‐CRT risk score | 0.61 (0.54–0.67) | Ref. | ||||
| VALID‐CRT risk score + MELD‐XI score | 0.63 (0.57–0.70) | 0.16 | 0.31 (0.08–0.54) | 0.007 | 0.015 (<0.001–0.03) | 0.044 |
CI, confidence interval; IDI, integrated discrimination improvement; MELD‐XI, Model for End‐stage Liver Disease excluding the International normalized ratio; NRI, net reclassification improvement.
Prognostic value of MELD‐XI score in patients receiving cardiac resynchronization therapy with a pacemaker or implantable cardioverter–defibrillator
In our study population, 109 patients received CRT‐P and 174 patients received CRT‐D. In both groups, a higher MELD‐XI score was associated with a greater risk of all‐cause mortality than a lower MELD‐XI score [log‐rank test: P = 0.010 (CRT‐P) and P < 0.001 (CRT‐D), Figure 4 ].
Figure 4.
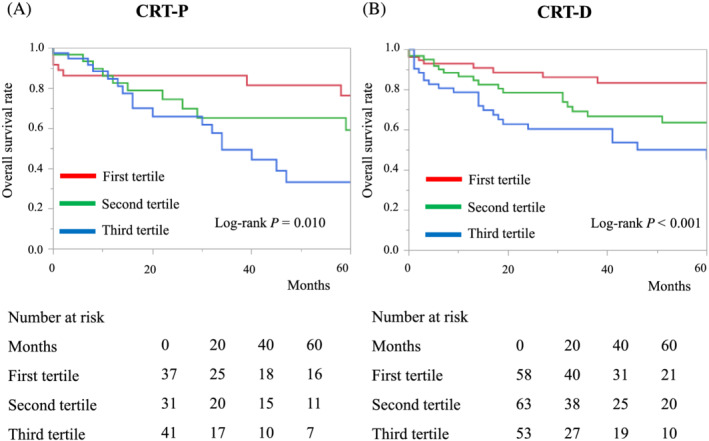
Kaplan–Meier curves of overall survival for patient groups defined according to the first, second, and third tertiles of the MELD‐XI score, divided according to cardiac resynchronization therapy device: (A) cardiac resynchronization therapy with a pacemaker (CRT‐P) and (B) cardiac resynchronization therapy with implantable cardioverter–defibrillator (CRT‐D).
Sensitive analysis
In the sensitive analysis, patients were stratified into three groups accordingly: lowest group (MELD‐XI = 9.44, n = 95), middle group (9.49 ≤ MELD‐XI < 15.4, n = 132), and highest group (MELD‐XI ≥ 15.4, n = 56). Trend in the baseline clinical characteristics for each group did not change in a sensitive analysis (Supporting Information, Table S1 ). The lowest group had significantly lower risk of all‐cause mortality (log‐rank test: P < 0.001) and cardiac deaths (log‐rank test: P = 0.024) compared with the other two groups (Supporting Information, Figures S1 and S2 ).
Discussion
This study was the first to investigate the correlation between hepatorenal dysfunction assessed by the MELD‐XI score and survival following CRT implantation. There were three key findings. First, a higher MELD‐XI score was associated with older age, a higher prevalence of men, anaemia, renal dysfunction, a higher prevalence of co‐morbidities (hypertension and DM), more severe HF, more severe pulmonary hypertension, a higher prevalence of severe TR, and a lower functional CRT response rate. Second, the MELD‐XI score was independently associated with all‐cause mortality following CRT implantation, even after adjusting for the conventional VALID‐CRT risk score. In addition, the use of both the MELD‐XI score and the conventional VALID‐CRT risk score resulted in an increased prognostic value relative to the VALID‐CRT risk score alone. Third, the MELD‐XI score was significantly associated with all‐cause mortality regardless of the type of CRT implant (CRT‐P or CRT‐D).
Cardiorenal interaction in HF is well known, and pre‐implantation renal dysfunction has been reported to be associated with poor outcomes after CRT implantation. 21 , 22 Recently, cardiohepatic interaction in HF has attracted research interest. Severe HF is often accompanied by liver congestion as a result of elevated central venous pressure. Liver dysfunction caused by liver congestion is known as congestive hepatopathy, and it is associated with a poor prognosis. 23 Furthermore, biliary obstruction caused by elevated hepatic venous pressure leads to increased serum total bilirubin levels, 24 which were reported to correlate with elevated central venous pressure, severity of TR, and pulmonary artery wedge pressure. 25 , 26 These end‐organ dysfunctions often coexist, possibly because their underlying mechanisms have common pathways. 8 Hepatorenal dysfunction is a common co‐morbidity and is related to the severity of HF. 8
The MELD and MELD‐XI scores were developed to evaluate liver and kidney function and were originally used as prognostic markers in patients with advanced liver disease. 10 , 11 Several recent studies demonstrated that the MELD‐XI score had prognostic value in HF patients, 8 , 24 and it was also shown to be a prognostic marker in patients who had undergone LV assist device implantation, transcatheter aortic valve implantation, or cardiac surgery. 12 , 14 , 27 However, the relationship between hepatorenal dysfunction assessed by the MELD‐XI score and the prognosis of HF patients after CRT implantation has not been fully investigated.
In this study, a higher MELD‐XI score was associated with older age, multiple co‐morbidities, more severe HF, and a lower functional CRT response rate. Therefore, CRT recipients with high MELD‐XI scores may have more severe end‐organ impairment due to HF. Univariate Cox regression analysis demonstrated that neither total bilirubin nor creatine was individually associated with mortality in our population, while a higher MELD‐XI score was strongly associated with all‐cause mortality and cardiac death after CRT implantation. These results suggest that compared with markers of damage to individual organs, the MELD‐XI score more sensitively reflects multiple end‐organ dysfunction due to severe HF.
Risk stratification of patients using pre‐implantation assessments is clinically essential in the field of CRT. While several risk scores have been proposed, 28 , 29 the VALID‐CRT score is the most reliable. 30 Even in an unselected, real‐world population, the VALID‐CRT score was reported to reliably predict clinical outcome and CRT response after CRT implantation. 30 However, this conventional risk score does not consider the effects of co‐morbidities and end‐organ dysfunction that accompany HF. Our data demonstrated that the assessment of hepatorenal function using the MELD‐XI score identified a high‐risk population in patients undergoing CRT. Notably, the MELD‐XI score had good prognostic value regardless of whether patients received CRT‐P or CRT‐D.
In this study, we defined two types of CRT response: functional and echocardiographic. Several recent clinical studies investigated the relationship between renal dysfunction and LV remodelling after CRT. One study reported that CRT resulted in LV reverse remodelling across all stages of chronic kidney disease, but the degree of LVEF improvement was lower in patients with severe renal dysfunction. 31 Another study found that several electrocardiographic and echocardiographic parameters could predict LV reverse remodelling, while blood biomarkers such as creatine had no prognostic value. 32 Our results showed that the MELD‐XI score before CRT implantation was not significantly correlated with the rate of LVEF change from before to after CRT implantation, and the echocardiographic CRT response rates did not differ significantly between the three groups. However, the functional CRT response rate was significantly lower in the third tertile MELD‐XI score group than in the other two groups. Recent reports revealed that co‐morbidities of HF such as frailty or undernutrition were strongly associated with adverse clinical outcomes in CRT recipients. 33 , 34 Our data suggest that multiple end‐organ dysfunction, which is a severe co‐morbidity that may occur with HF, is strongly associated with all‐cause mortality and cardiac death after CRT, regardless of the degree of echocardiographic LV reverse remodelling.
Calculation of the MELD‐XI score is simple, rapid, objective, and repeatable and is based on parameters obtained by standard laboratory examination (total bilirubin and creatine). Furthermore, the speed with which the MELD‐XI score can be obtained enables it to be used in time‐sensitive emergency situations. Compared with ultrasound examination, this score provides objective information and does not depend on individual experience or skill. In terms of clinical application, the MELD‐XI score can be used for risk stratification following CRT implantation. Patients with high MELD‐XI scores may be at increased risk of an adverse clinical course and may require more intensive management or more aggressive therapy than those with low MELD‐XI scores.
This study has several limitations. First, it was a single‐centre, retrospective, observational study with a small sample size. Second, no comprehensive, ultrasonographic assessment of liver dysfunction was performed. Third, we converted BNP to NT‐proBNP in 65 patients who underwent CRT implantation before January 2010. Fourth, the MELD‐XI score was only determined before implantation; thus, the relationship between score changes and clinical outcomes was unclear. Further large‐scale, multicentre, prospective studies are needed to confirm our results.
Conclusion
In HF patients undergoing CRT implantation, a higher MELD‐XI score was associated with older age, a higher prevalence of multiple co‐morbidities, more severe HF, lower functional CRT response rates, and higher all‐cause mortality. These results suggest that the MELD‐XI score can provide additional prognostic information and may improve risk stratification in this population.
Conflict of interest
T.N. belongs to a department established by contributions from Abbot Medical, Biotronik Japan, Medtronic Japan, Japan Lifeline, and Boston Scientific. T.N. received lecture fees from Abbott Medical and Medtronic Japan. All other authors have no conflicts of interest to report.
Funding
This research received no external funding.
Supporting information
Figure S1. Kaplan–Meier curves of overall survival for patient groups defined according to the lowest 20%, middle 60% and the highest 20% of the MELD‐XI score.
Figure S2. Kaplan–Meier curves of event (cardiac death)–free survival for patient groups defined according to the lowest 20%, middle 60% and the highest 20% of the MELD‐XI score.
Table S1. Clinical characteristics of patients stratified into three groups based on the lowest 20%, middle 60% and the highest 20% of the MELD‐XI score.
Saito, Y. , Nakai, T. , Ikeya, Y. , Kogawa, R. , Otsuka, N. , Wakamatsu, Y. , Kurokawa, S. , Ohkubo, K. , Nagashima, K. , and Okumura, Y. (2022) Prognostic value of the MELD‐XI score in patients undergoing cardiac resynchronization therapy. ESC Heart Failure, 9: 1080–1089. 10.1002/ehf2.13776.
References
- 1. Leyva F, Nisam S, Auricchio A. 20 years of cardiac resynchronization therapy. J Am Coll Cardiol 2014; 64: 1047–1058. [DOI] [PubMed] [Google Scholar]
- 2. Cleland JG, Daubert JC, Erdmann E, Freemantle N, Gras D, Kappenberger L, Tavazzi L. The effect of cardiac resynchronization on morbidity and mortality in heart failure. N Engl J Med 2005; 352: 1539–1549. [DOI] [PubMed] [Google Scholar]
- 3. Cleland J, Freemantle N, Ghio S, Fruhwald F, Shankar A, Marijanowski M, Verboven Y, Tavazzi L. Predicting the long‐term effects of cardiac resynchronization therapy on mortality from baseline variables and the early response a report from the CARE‐HF (Cardiac Resynchronization in Heart Failure) Trial. J Am Coll Cardiol 2008; 52: 438–445. [DOI] [PubMed] [Google Scholar]
- 4. De Boeck BW, Meine M, Leenders GE, Teske AJ, van Wessel H, Kirkels JH, Prinzen FW, Doevendans PA, Cramer MJ. Practical and conceptual limitations of tissue Doppler imaging to predict reverse remodelling in cardiac resynchronisation therapy. Eur J Heart Fail 2008; 10: 281–290. [DOI] [PubMed] [Google Scholar]
- 5. Leong DP, Höke U, Delgado V, Auger D, Witkowski T, Thijssen J, van Erven L, Bax JJ, Schalij MJ, Marsan NA. Right ventricular function and survival following cardiac resynchronisation therapy. Heart 2013; 99: 722–728. [DOI] [PubMed] [Google Scholar]
- 6. Ambrosy AP, Gheorghiade M, Bubenek S, Vinereanu D, Vaduganathan M, Macarie C, Chioncel O. The predictive value of transaminases at admission in patients hospitalized for heart failure: findings from the RO‐AHFS registry. Eur Heart J Acute Cardiovasc Care 2013; 2: 99–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Scholfield M, Schabath MB, Guglin M. Longitudinal trends, hemodynamic profiles, and prognostic value of abnormal liver function tests in patients with acute decompensated heart failure: an analysis of the ESCAPE trial. J Card Fail 2014; 20: 476–484. [DOI] [PubMed] [Google Scholar]
- 8. Biegus J, Zymliński R, Sokolski M, Siwołowski P, Gajewski P, Nawrocka‐Millward S, Poniewierka E, Jankowska EA, Banasiak W, Ponikowski P. Impaired hepato‐renal function defined by the MELD XI score as prognosticator in acute heart failure. Eur J Heart Fail 2016; 18: 1518–1521. [DOI] [PubMed] [Google Scholar]
- 9. Malinchoc M, Kamath PS, Gordon FD, Peine CJ, Rank J, ter Borg PC. A model to predict poor survival in patients undergoing transjugular intrahepatic portosystemic shunts. Hepatology 2000; 31: 864–871. [DOI] [PubMed] [Google Scholar]
- 10. Kamath PS, Wiesner RH, Malinchoc M, Kremers W, Therneau TM, Kosberg CL, D'Amico G, Dickson ER, Kim WR. A model to predict survival in patients with end‐stage liver disease. Hepatology 2001; 33: 464–470. [DOI] [PubMed] [Google Scholar]
- 11. Heuman DM, Mihas AA, Habib A, Gilles HS, Stravitz RT, Sanyal AJ, Fisher RA. MELD‐XI: a rational approach to “sickest first” liver transplantation in cirrhotic patients requiring anticoagulant therapy. Liver Transpl 2007; 13: 30–37. [DOI] [PubMed] [Google Scholar]
- 12. Critsinelis A, Kurihara C, Volkovicher N, Kawabori M, Sugiura T, Manon M 2nd, Wang S, Civitello AB, Morgan JA. Model of End‐Stage Liver Disease‐eXcluding International Normalized Ratio (MELD‐XI) scoring system to predict outcomes in patients who undergo left ventricular assist device implantation. Ann Thorac Surg 2018; 106: 513–519. [DOI] [PubMed] [Google Scholar]
- 13. Spieker M, Hellhammer K, Wiora J, Klose S, Zeus T, Jung C, Saeed D, Horn P, Kelm M, Westenfeld R. Prognostic value of impaired hepato‐renal function assessed by the MELD‐XI score in patients undergoing percutaneous mitral valve repair. Catheter Cardiovasc Interv 2019; 93: 699–706. [DOI] [PubMed] [Google Scholar]
- 14. Arai T, Yashima F, Yanagisawa R, Tanaka M, Shimizu H, Fukuda K, Watanabe Y, Naganuma T, Araki M, Tada N, Yamanaka F, Shirai S, Yamamoto M, Hayashida K. Prognostic value of liver dysfunction assessed by MELD‐XI scoring system in patients undergoing transcatheter aortic valve implantation. Int J Cardiol 2017; 228: 648–653. [DOI] [PubMed] [Google Scholar]
- 15. Kawahira M, Tamaki S, Yamada T, Watanabe T, Morita T, Furukawa Y, Kawasaki M, Kikuchi A, Kawai T, Seo M, Nakamura J, Kayama K, Kimura T, Ueda K, Sakamoto D, Kogame T, Ito S, Chang Y, Fukunami M. Prognostic value of impaired hepato‐renal function and liver fibrosis in patients admitted for acute heart failure. ESC Heart Fail 2021; 8: 1274–1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lang RM, Bierig M, Devereux RB, Flachskampf FA, Foster E, Pellikka PA, Picard MH, Roman MJ, Seward J, Shanewise JS, Solomon SD, Spencer KT, Sutton MS, Stewart WJ. Recommendations for chamber quantification: a report from the American Society of Echocardiography's Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr 2005; 18: 1440–1463. [DOI] [PubMed] [Google Scholar]
- 17. Nakai T, Ikeya Y, Kogawa R, Otsuka N, Wakamatsu Y, Kurokawa S, Ohkubo K, Nagashima K, Okumura Y. What are the expectations for cardiac resynchronization therapy? A validation of two response definitions. J Clin Med 2021; 10: 514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bleeker GB, Bax JJ, Fung JW, van der Wall EE, Zhang Q, Schalij MJ, Chan JY, Yu CM. Clinical versus echocardiographic parameters to assess response to cardiac resynchronization therapy. Am J Cardiol 2006; 97: 260–263. [DOI] [PubMed] [Google Scholar]
- 19. Okumura Y, Yokoyama K, Matsumoto N, Tachibana E, Kuronuma K, Oiwa K, Matsumoto M, Kojima T, Hanada S, Nomoto K, Arima K, Takahashi F, Kotani T, Ikeya Y, Fukushima S, Itoh S, Kondo K, Chiku M, Ohno Y, Onikura M, Hirayama A, the Sakura Af Registry Investigators . Current use of direct oral anticoagulants for atrial fibrillation in Japan: findings from the SAKURA AF Registry. J Arrhythm. 2017; 33: 289–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Gasparini M, Klersy C, Leclercq C, Lunati M, Landolina M, Auricchio A, Santini M, Boriani G, Proclemer A, Leyva F. Validation of a simple risk stratification tool for patients implanted with cardiac resynchronization therapy: the VALID‐CRT risk score. Eur J Heart Fail 2015; 17: 717–724. [DOI] [PubMed] [Google Scholar]
- 21. Daly DD Jr, Maran A, Hyer JM, Funke F, Waring A, Cuoco FA, Sturdivant JL, Leman RB, Gold MR. The effect of chronic kidney disease on mortality with cardiac resynchronization therapy. Pacing Clin Electrophysiol 2016; 39: 863–869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Bazoukis G, Letsas KP, Korantzopoulos P, Thomopoulos C, Vlachos K, Georgopoulos S, Karamichalakis N, Saplaouras A, Efremidis M, Sideris A. Impact of baseline renal function on all‐cause mortality in patients who underwent cardiac resynchronization therapy: a systematic review and meta‐analysis. J Arrhythm 2017; 33: 417–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Poelzl G, Ess M, Mussner‐Seeber C, Pachinger O, Frick M, Ulmer H. Liver dysfunction in chronic heart failure: prevalence, characteristics and prognostic significance. Eur J Clin Invest 2012; 42: 153–163. [DOI] [PubMed] [Google Scholar]
- 24. Abe S, Yoshihisa A, Takiguchi M, Shimizu T, Nakamura Y, Yamauchi H, Iwaya S, Owada T, Miyata M, Sato T, Suzuki S, Oikawa M, Kobayashi A, Yamaki T, Sugimoto K, Kunii H, Nakazato K, Suzuki H, Saitoh S, Takeishi Y. Liver dysfunction assessed by model for end‐stage liver disease excluding INR (MELD‐XI) scoring system predicts adverse prognosis in heart failure. PLoS One 2014; 9: e100618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kubo SH, Walter BA, John DH, Clark M, Cody RJ. Liver function abnormalities in chronic heart failure. Influence of systemic hemodynamics. Arch Intern Med 1987; 147: 1227–1230. [PubMed] [Google Scholar]
- 26. Lau GT, Tan HC, Kritharides L. Type of liver dysfunction in heart failure and its relation to the severity of tricuspid regurgitation. Am J Cardiol 2002; 90: 1405–1409. [DOI] [PubMed] [Google Scholar]
- 27. Suman A, Barnes DS, Zein NN, Levinthal GN, Connor JT, Carey WD. Predicting outcome after cardiac surgery in patients with cirrhosis: a comparison of Child‐Pugh and MELD scores. Clin Gastroenterol Hepatol 2004; 2: 719–723. [DOI] [PubMed] [Google Scholar]
- 28. Leyva F, Foley PW, Stegemann B, Ward JA, Ng LL, Frenneaux MP, Regoli F, Smith RE, Auricchio A. Development and validation of a clinical index to predict survival after cardiac resynchronisation therapy. Heart 2009; 95: 1619–1625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Regoli F, Scopigni F, Leyva F, Landolina M, Ghio S, Tritto M, Calò L, Klersy C, Auricchio A. Validation of Seattle Heart Failure Model for mortality risk prediction in patients treated with cardiac resynchronization therapy. Eur J Heart Fail 2013; 15: 211–220. [DOI] [PubMed] [Google Scholar]
- 30. Bertaglia E, Arena G, Pecora D, Reggiani A, D'Onofrio A, Palmisano P, De Simone A, Caico SI, Marini M, Maglia G, Ferraro A, Solimene F, Cecchetto A, Malacrida M, Botto GL, Lunati M, Stabile G. The VALID‐CRT risk score reliably predicts response and outcome of cardiac resynchronization therapy in a real‐world population. Clin Cardiol 2019; 42: 919–924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ter Maaten JM, Martens P, L'Hoyes W, Maass AH, Damman K, Dupont M, Mullens W. Response to cardiac resynchronization therapy across chronic kidney disease stages. J Card Fail 2019; 25: 803–811. [DOI] [PubMed] [Google Scholar]
- 32. Maass AH, Vernooy K, Wijers SC, van't Sant J, Cramer MJ, Meine M, Allaart CP, De Lange FJ, Prinzen FW, Gerritse B, Erdtsieck E, Scheerder COS, Hill MRS, Scholten M, Kloosterman M, Ter Horst IAH, Voors AA, Vos MA, Rienstra M, Van Gelder IC. Refining success of cardiac resynchronization therapy using a simple score predicting the amount of reverse ventricular remodelling: results from the Markers and Response to CRT (MARC) study. Europace 2018; 20: e1–e10. [DOI] [PubMed] [Google Scholar]
- 33. Kubala M, Guédon‐Moreau L, Anselme F, Klug D, Bertaina G, Traullé S, Buiciuc O, Savouré A, Diouf M, Hermida JS. Utility of frailty assessment for elderly patients undergoing cardiac resynchronization therapy. JACC Clin Electrophysiol 2017; 3: 1523–1533. [DOI] [PubMed] [Google Scholar]
- 34. Boros AM, Széplaki G, Perge P, Jenei Z, Bagyura Z, Zima E, Molnár L, Apor A, Becker D, Gellér L, Prohászka Z, Merkely B. The ratio of the neutrophil leucocytes to the lymphocytes predicts the outcome after cardiac resynchronization therapy. Europace 2016; 18: 747–754. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Kaplan–Meier curves of overall survival for patient groups defined according to the lowest 20%, middle 60% and the highest 20% of the MELD‐XI score.
Figure S2. Kaplan–Meier curves of event (cardiac death)–free survival for patient groups defined according to the lowest 20%, middle 60% and the highest 20% of the MELD‐XI score.
Table S1. Clinical characteristics of patients stratified into three groups based on the lowest 20%, middle 60% and the highest 20% of the MELD‐XI score.


