Abstract
Background:
Metabolic divergence of macrophages polarized into different phenotypes represents a mechanistically relevant target for non-invasive characterization of atherosclerotic plaques using positron emission tomography (PET). Carbon-11 (11C)-labeled acetate is a clinically available tracer which accumulates in atherosclerotic plaques, but its biological and clinical correlates in atherosclerosis are undefined.
Methods and Results:
Histological correlates of 14C-acetate uptake were determined in brachiocephalic arteries of western diet fed apoE−/− mice. The effect of polarizing stimuli on 14C-acetate uptake was determined by pro-inflammatory (interferon-γ+lipopolysaccharide) vs. inflammation-resolving (interleukin-4) stimulation of murine macrophages and human carotid endarterectomy specimens over two days. 14C-acetate accumulated in atherosclerotic regions of arteries. CD68-positive monocytes/macrophages vs. smooth muscle actin-positive smooth muscle cells were the dominant cells in regions with high vs. low 14C-acetate uptake. 14C-acetate uptake progressively decreased in proinflammatory macrophages to 25.9±4.5% of baseline (P<0.001). A delayed increase in 14C-acetate uptake was induced in inflammation-resolving macrophages, reaching to 164.1±21.4% (P<0.01) of baseline. Consistently, stimulation of endarterectomy specimens with interferon-γ+lipopolysaccharide decreased 14C-acetate uptake to 66.5±14.5%, while interleukin-4 increased 14C-acetate uptake to 151.5±25.8% compared to non-stimulated plaques (P<0.05).
Conclusions:
Acetate uptake by macrophages diverges upon pro-inflammatory and inflammation-resolving stimulations, which may be exploited for immuno-metabolic characterization of atherosclerosis.
Keywords: Acetate, Atherosclerosis, Macrophage polarization, Metabolism, Imaging
Introduction
Immuno-metabolism, i.e., the cross-talk of intra-cellular metabolic pathways and immune cell function, plays a pivotal role in the pathogenesis of inflammatory diseases, including atherosclerosis1–3. For example, hypoxia, a major trigger of enhanced glycolysis in atherosclerotic plaques, promotes the production of pro-inflammatory cytokines, e.g., interleukin-1β, by macrophages4–6, and expansion of the necrotic core7. Moreover, pro-inflammatory stimulation of macrophages by lipopolysaccharide (LPS) enhances glucose uptake and glycolysis8–10, which in turn accentuates the production of interleukin-1β through succinate-mediated stabilization of hypoxia-inducible factor-1α and development of a pseudo-hypoxic state8. The unprecedented recognition of the vessel wall immuno-metabolic heterogeneity and its mechanistic contribution to atherogenesis provides the opportunity to explore the potential applications of metabolic imaging in cardiovascular diseases, analogous to similar efforts in oncology11, 12.
Imaging of glucose utilization by 18F-fluorodeoxyglucose (18F-FDG) positron emission tomography (PET) has been instrumental in elucidating the in vivo metabolic heterogeneity of the vessel wall, supplementing the information obtained by ex vivo metabolomics techniques1. However, the non-specificity of 18F-FDG uptake, which targets a nearly ubiquitous metabolic process upregulated in both pro-inflammatory and inflammation-resolving (reparative) states, makes it suboptimal for characterization of the inflammatory response as a stand-alone imaging marker9, 10, 13–15. Additionally, myocardial uptake of 18F-FDG has been a major challenge for its utilization in coronary artery disease15, 16. These limitations have prompted investigators to exploit the utility of metabolic substrates other than glucose, e.g., glutamine9 and acetate17, 18, in non-invasive characterization of plaque immuno-metabolism. Notably, the safety of several metabolic substrates has already been established in oncological or cardiac imaging studies17, 19, which facilitates their applications in imaging atherosclerosis.
Carbon-11 (11C)-labeled acetate (11C-acetate) has been extensively studied to detect cancer-associated lipogenesis and myocardial blood flow and oxidative metabolism20, 21. More recently, pre-clinical18 and pilot clinical17 studies have demonstrated the feasibility of imaging 11C-acetate uptake in atherosclerosis. However, the biological correlates of acetate uptake in plaques and its clinical implications have not been addressed. The purpose of this study was to determine the histological correlates of acetate uptake in atherosclerotic plaques and its link to inflammatory states of macrophages, using 14C-acetate where the long half-lived β-emitting C-14 allows ex vivo imaging via autoradiography. We hypothesized that acetate uptake in macrophage-rich plaques differentiates their pro-inflammatory vs. inflammation-resolving polarization states. Histological correlates of acetate uptake were determined by combined immuno-histology and high-resolution 14C-acetate autoradiography in murine atherosclerotic brachiocephalic arteries. The effect of pro-inflammatory vs. inflammation-resolving stimuli on acetate uptake was assessed in murine macrophage and human carotid endarterectomy specimens.
Materials and Methods
Animals
Wild-type (N=12) and apoE−/− (N=3) mice on a C57BL/6J background were purchased from Jackson Laboratories. Atherosclerosis was induced by feeding with a western diet (21% anhydrous fat, TD88137, Envigo, 33 weeks) in apoE−/− mice. Experiments were conducted in accordance with a protocol approved by Institutional Animal Care and Use Committee.
Cell Culture
Commercial experimental reagents are listed in Supplemental Table 1. Elicited peritoneal cells were harvested from wild-type mice through lavage, three days after intra-peritoneal injection of 1.5 mL of 3% thioglycolate22. Adherent macrophages were cultured for two days in RPMI-1640 supplemented with 10% fetal bovine serum, glucose (5.5 mM), sodium pyruvate (1 mM), glutamine (2 mM), non-essential amino acids (1X), HEPES buffer (20 mM), penicillin (50 U/mL) and streptomycin (50 μg/mL), as described9, 10, 13. Macrophages which were not activated by the addition of polarizing stimuli were considered as non-activated (Mφ0). Polarization was induced by addition of the conventional pro-inflammatory (M1) or inflammation-resolving (M2) stimuli: i) MφIFNγ+LPS: recombinant murine interferon-γ (50 ng/mL) plus LPS (10 ng/mL); and ii) MφIL4: recombinant murine IL-4 (10 ng/mL).
Primary aortic vascular smooth muscle cells (VSMCs) obtained from C57BL/6J mice23 were cultured for two days in DMEM/F12 supplemented with 10% fetal bovine serum, L-glutamine (2 mM), HEPES buffer (10 mM), penicillin (100 U/mL) and streptomycin (100 μg/mL). Polarization was induced by addition of the conventional pro-inflammatory or inflammation-resolving stimuli: i) VSMCIFNγ+LPS: recombinant murine interferon-γ (50 ng/mL) plus LPS (10 ng/mL); and ii) VSMCIL4: recombinant murine IL-4 (10 ng/mL).
Culture of Human Endarterectomy Specimens
Anonymized atherosclerotic specimens were collected from three patients who underwent carotid endarterectomy as the standard-of-care, under a “No Human Subject involvement” classification by Institutional Review Board. Freshly collected specimens were cut into ~3-mm thick slices and cultured ex vivo in complete RPMI-1640 medium24, 25. Plaques were incubated for two days in the absence (control) or presence of i) recombinant human interferon-γ (50 ng/mL) plus LPS (10 ng/mL) vs. ii) recombinant human IL-4 (10 ng/mL).
Uptake of 14C-Acetate by Macrophages and VSMCs
14C-acetate uptake was measured in macrophages and VSMCs after 6, 24 or 48 hours of polarization. Immediately prior to the assay, cells were washed with phosphate-buffered saline and were incubated with an uptake buffer (NaCl: 140 mM, KCl: 5.4 mM, CaCl2: 1.8 mM, MgSO4: 0.8 mM, D-glucose: 5.5 mM, HEPES: 25 mM) supplemented with 2 μCi/mL (74 kBq/mL) 14C-acetate at 37°C for 2 hours. Subsequently, cells were washed and lysed with 0.1 mM NaOH. 14C-acetate uptake was quantified by a liquid scintillation counter (Beckman Coulter LS6500), normalized to the DNA content of the cell lysates (PicoGreen, Invitrogen), and expressed relative to the uptake values of unstimulated MФ0 or VSMCs9, 10, 13.
Micro-Autoradiography of 14C-Acetate Uptake
Excised murine brachiocephalic arteries or human endarterectomy specimens were incubated with the uptake buffer (described above) supplemented with 2 μCi/mL (74 kBq/mL) 14C-acetate at 37°C for 2 hours. Specimens were then washed with a cold buffer and embedded in optimal cutting temperature (OCT) compound for cryosectioning (Leica CM1860) at 10-μm-thickness. Tissues, along with known amounts of 14C-acetate blotted on papers as standards, were exposed to high-resolution phosphor screens (BAS-IP SR2025 Super Resolution, GE Healthcare). Phosphor screens were scanned by a Sapphire Biomolecular Imager (Azure Biosystems) at 10-μm resolution. Autoradiography images were adjusted using the Fire Look-Up Table and a smoothing algorithm (Image J). The average uptake of 14C-acetate by endarterectomy specimens stimulated with pro-inflammatory and inflammation-resolving stimuli was quantified (ImageJ) by drawing regions of interests around the tissues (2–3 sections per specimen at 250 μm intervals). Uptake values are expressed relative to control (non-stimulated) plaques.
Histology
Histological analysis of the brachiocephalic arteries was performed after completion of the autoradiography by Movat’s pentachrome staining per standard protocols. Smooth muscle cells and macrophages were identified by immunostaining with anti-mouse α-smooth muscle actin (αSMA) and anti-mouse CD68, respectively (Supplemental Table 1). Immunostaining for inducible nitric oxide synthase (iNOS) and CD206 was performed as conventional markers of M1 and M2 polarization, respectively. Slides were mounted using ProLong™ Gold Antifade with 4′,6-diamidino-2-phenylindole (DAPI) for nuclear counter-staining. Tissues were photographed using an AxioVert Microscope (Zeiss) equipped with a digital camera, controlled by ZEN software (Zeiss).
High-Resolution Quantification of 14C-Acetate Uptake
AzureSpot software was used to place a 5×5 μm grid on top of overlayed autoradiography and immunostaining (both CD68 and αSMA) (Supplemental Figure 1). 14C-aceate intensity data were transferred to FlowJo 10.5.3 software. Each 5×5 μm box was ranked from the lowest to the highest based on its 14C-acetate uptake and assigned a quartile (Q1 = lowest, Q4 = highest). CD68+ and αSMA+ percent areas were determined in each quartile of 14C-acetate uptake (averaged from 2–3 tissues sections per mouse).
Gene Expression Analysis
Messenger RNA (mRNA) extraction and reverse transcription were performed on cultured mouse macrophages and endarterectomy specimens two days after stimulation using TRIzol® reagent and QuantiTect® Reverse Transcription Kit, per standard protocols9, 10, 13. Quantitative PCR (qPCR) was performed using a QuantStudio-3 Real-Time PCR System (ThermoFisher) and TaqMan® gene expression assays (Supplemental Table 2). Transcript amplification data were analyzed using QuantStudio v1.4.3 software (Applied Biosystems) and were normalized to the expression level of 18S ribosomal RNA (Rn18s), as the housekeeping gene. mRNA expression levels are expressed as relative to the level of expression in MФ0 macrophages and summarized as a heat map. Transcript levels of macrophage polarization markers in endarterectomies are expressed relative to the expression of CD68 to account for variations in the macrophage contents of different specimens.
Statistical Analysis
Data were expressed as mean ± standard error of the mean. Statistical analyses were performed with Prism-8 software (GraphPad). Student t test or analysis of variance (followed by Fisher’s Exact post hoc test) were used to test the differences between two or multiple groups, respectively. P < 0.05 was considered statistically significant.
Results
Uptake of 14C-Acetate by Macrophage-Rich Murine Brachiocephalic Artery Plaques
Micro-autoradiography of murine brachiocephalic arteries from western diet fed apoE−/− mice demonstrated focal areas of increased 14C-acetate uptake in the vessel wall, corresponding to atherosclerotic plaques, as confirmed by overlaid histological (Movat’s pentachrome staining) and autoradiography images (Figure 1A). Consistent with the visual assessment, quantitative assessment of the brachiocephalic arteries trended towards higher 14C-acetate uptake in plaques compared to plaque-free regions (Figure 1B). To determine the cellular composition of plaque regions with focal 14C-acetate uptake, we performed immunofluorescent staining of brachiocephalic arteries using CD68 and αSMA antibodies, delineating macrophages and smooth muscle cells, as the two most abundant cells within the plaques. As demonstrated in representative images of combined autoradiography and immunostaining (Figure 2A–D), CD68+ macrophages were highly abundant in plaque regions with high 14C-acetate uptake. Quantification of CD68+ and αSMA+ areas (Figure 2E) confirmed that macrophages were the predominant cells in regions with the highest quartile (Q4) of 14C-acetate uptake (CD68+ macrophages: 41.2 ± 0.8% vs. αSMA+ smooth muscle cells: 18.1 ± 3.5%, P < 0.01). On the other hand, vessel wall regions with the lowest quartile (Q1) of 14C-acetate uptake were predominantly comprised of αSMA+ smooth muscle cells (43.3 ± 1.3%) compared with CD68+ macrophages (30.5 ± 2.1%) (Figure 2E, P < 0.01). Of note, 14C-acetate uptake within the macrophage-rich regions of plaques was heterogeneous, and CD68+ regions consisted of areas with high (marked by the red oval shape in Figure 2A and 2D) and low (marked by the red box in Figure 2A and 2D) 14C-acetate uptake. On the other hand, smooth muscle cells both within the plaques (blue arrows in Figure 2B and 2D) and normal regions of arteries (blue arrowheads in Figure 2B and 2D) had relatively low 14C-acetate uptake.
Figure 1: Focal uptake of 14C-acetate by murine atherosclerotic plaques.
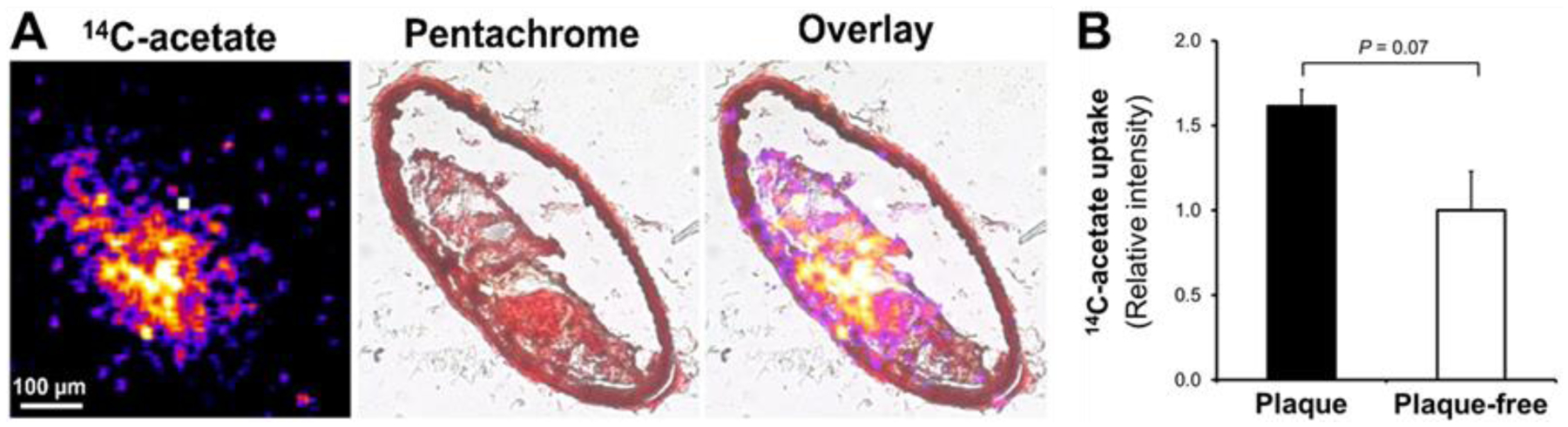
Micro-autoradiography (A, left), Movat’s pentachrome (A, middle) and their superimposed (A, right) images demonstrate that vessel wall 14C-acetate uptake colocalizes with brachiocephalic artery atherosclerotic plaques of apoE−/− mice fed with a western diet. Plaque uptake of 14C-acetate (B) trended higher in arterial regions with plaques rather than plaque-free regions.
Figure 2: Colocalization of 14C-acetate uptake and macrophage-rich regions of atherosclerotic plaques.
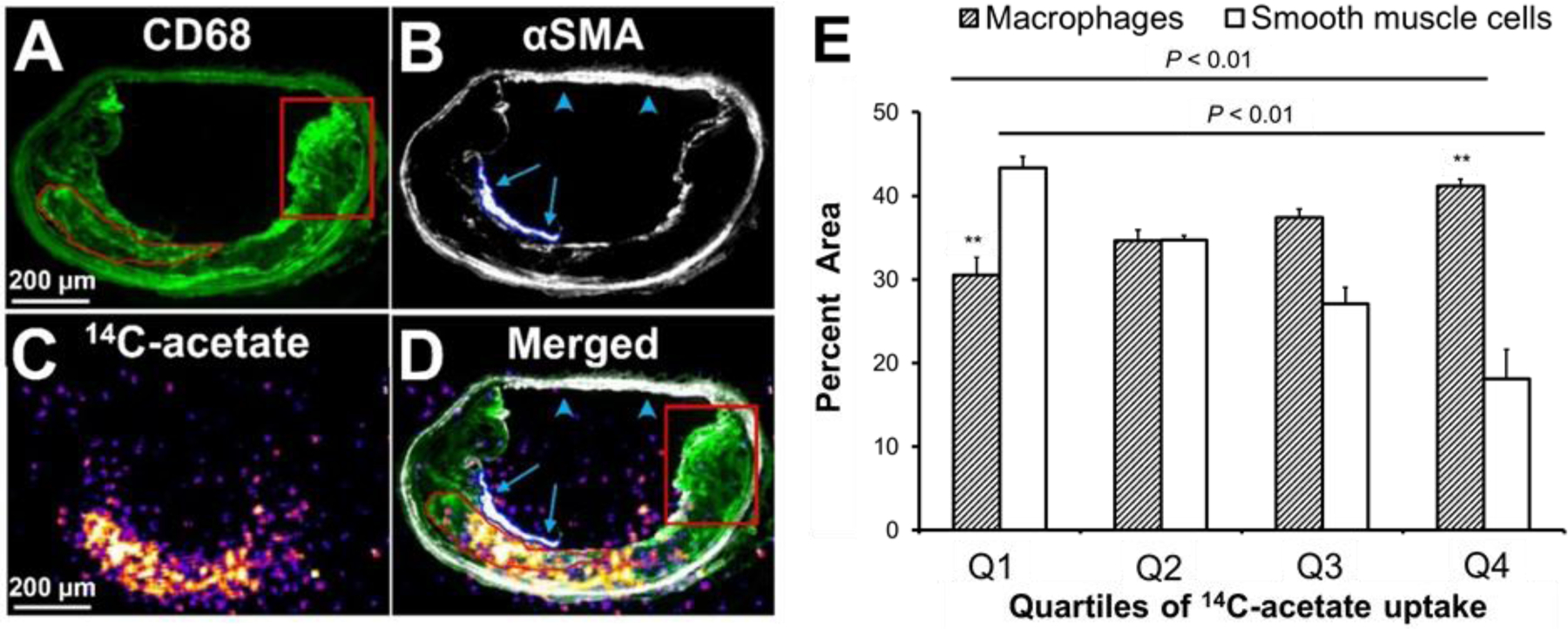
Combine micro-autoradiography and immunofluorescent staining for CD68 (macrophage marker) and αSMA (smooth muscle cell marker) (A–D) demonstrates that areas of focal uptake of 14C-acetate primarily correspond to macrophage-rich regions of murine brachiocephalic artery atherosclerotic plaques. Of note, 14C-acetate uptake within the macrophage-rich regions of plaques was heterogeneous. Some macrophage-rich regions demonstrated high 14C-acetate uptake (marked by the red oval shape in A and D), while other regions had low uptake (marked by the red box in A and D). Smooth muscle cells both within the plaque (blue arrows in B and D) and normal regions of artery (blue arrowheads in B and D) had relatively low 14C-acetate uptake. Quantification of CD68+ and αSMA+ areas in vessel wall regions with increasing quartiles of 14C-acetate uptake (E) confirms that macrophages are the most abundant cells in regions with higher 14C-acetate uptake, while smooth muscle cells comprise the most abundant cells in regions with lower 14C-acetate uptake (N = 3 mice, 2–3 tissue sections per each mouse). ** indicates P < 0.01 for within quartile comparison of abundance of macrophages and VSMCs; P values above the brackets indicates between quartiles comparisons of the abundance of macrophages and VSMCs.
Divergence of 14C-Acetate Uptake upon M1 and M2 Polarization of Macrophages
The striking heterogeneity of 14C-acetate uptake in CD68+ regions of plaques led us to examine the association between acetate uptake and different polarization states of macrophages. We determined through immunofluorescence staining that both M1-like (CD68+/iNOS+) and M2-like (CD68+/CD206+) macrophages were present in murine brachiocephalic plaque (Supplemental Figure 2).
We utilized a conventional approach to induce pro-inflammatory (M1) and inflammation-resolving (M2) polarization states in murine macrophages using IFN-γ plus LPS (MφIFNγ+LPS) and IL-4 (MφIL4), respectively, over a 2-day period. Successful conversion of macrophages into different polarization states was confirmed by markedly different transcript levels of a panel of conventional M1 (Arg2, Cxcl9, Cxcl10, Il1b, Nos2 and Tnf) and M2 (Arg1, Cd36, Chil3, Mrc1, Tfrc, Tgfb) markers (Figure 3).
Figure 3: Distinct inflammatory profiles of Mφ0, MφIFNγ+LPS and MφIL4.
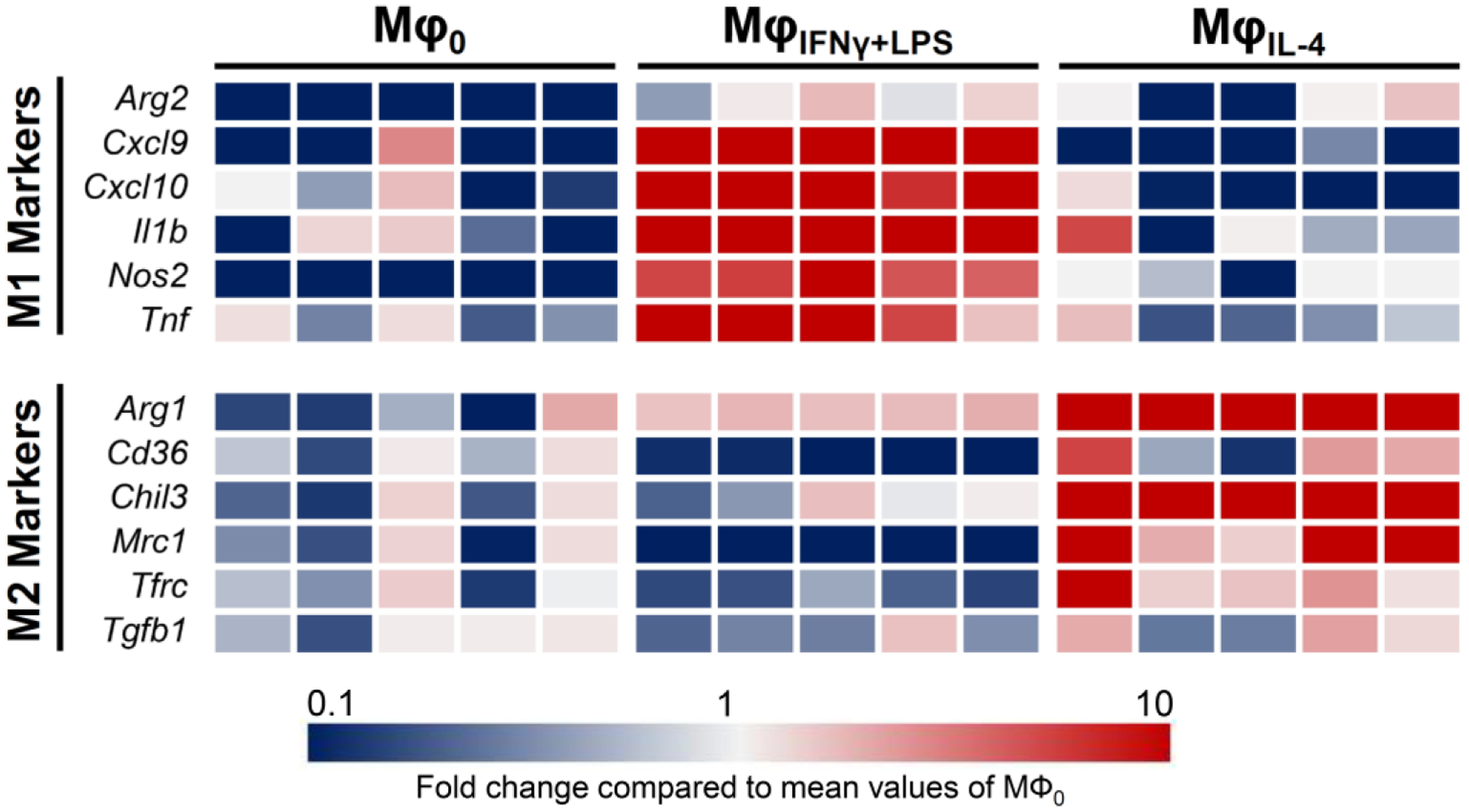
Heat map illustration of macrophage polarization transcript levels (relative to MΦ0) confirms the development of distinct polarization phenotypes upon 2-day culture of murine macrophages with by IFN-γ + LPS vs. IL-4. (N = 5)
The uptake of 14C-acetate by macrophages were determined at 6, 24, and 48 hours after the induction of polarization. As demonstrated in Figure 4A, there was a steady decline in 14C-acetate uptake in MφIFNγ+LPS, which was noticeable as early as 6 hours after stimulation (59.5 ± 3.7% of the baseline level, P < 0.001) and reached to its minimum at 48 hours (25.9 ± 4.5% of the baseline level, P < 0.001). On the other hand, MφIL4 demonstrated a significant, but delayed, increase in 14C-acetate uptake, reaching to 164.1 ± 21.4% of the baseline level at 48 hours after stimulation (P < 0.01). The 14C-acetate uptake by VSMCs, however, was essentially unchanged upon stimulation by either IFNγ + LPS or IL-4 (Supplemental Figure 3).
Figure 4: Divergence of 14C-acetate uptake in MφIFNγ+LPS and MφIL4.
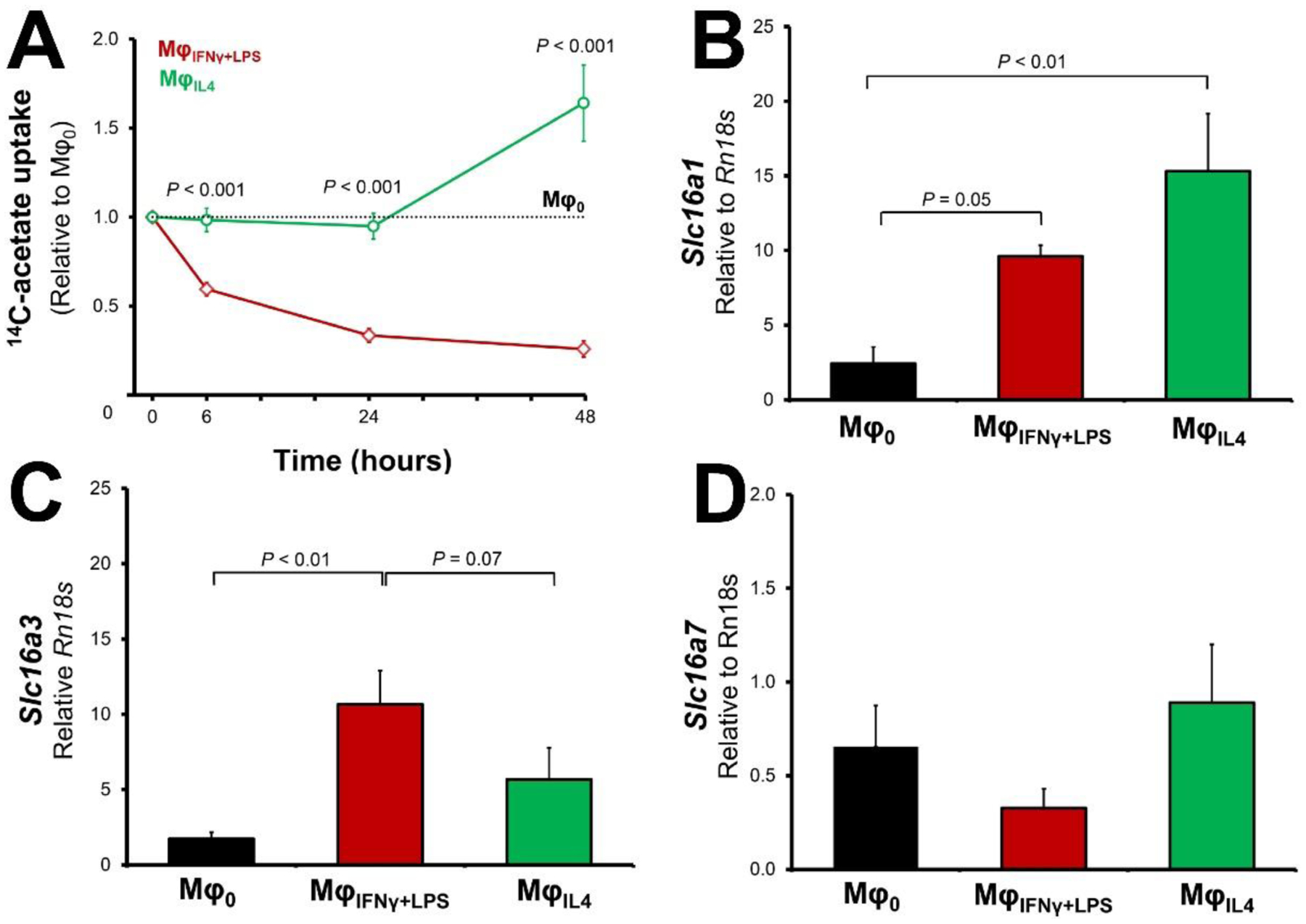
Stimulation of murine macrophages with IFN-γ + LPS vs. IL-4 resulted in the divergence of 14C-acetate (A), characterized by a progressive decline by ~4 folds in MφIFNγ+LPS and a delayed ~64% increase in MφIL4. mRNA level of Slc16a1 (B) is increased in both MφIFNγ+LPS (3.9-fold) and MφIL4 (6.3-fold). Expression of Slc16a3 transcript (C) was also increased in MφIFNγ+LPS (6.1-fold); but the apparent overexpression of Slc16a3 in MφIL4 (3.3-fold) did not reach statistical significance (P = 0.14). The expression of Slc16a7 (D) was not significantly changed by the polarization state of macrophages (N = 7 for uptake assays and = 5 for gene expression assays).
The transport of acetate through cell membrane occurs through members of the monocarboxylate transporter family26. Therefore, we determined the mRNA expression of Slc16a1, Slc6a3, and Slc16a7, encoding monocarboxylic acid transporter −1, −3, and −2, respectively (Figure 4B–D). The expression of Slc16a1, representing the most abundant of the three transporters26, 27, was 3.9 ± 0.3 (P = 0.05) and 6.3 ± 1.6 (P < 0.01) fold higher in MφIFNγ+LPS and MφIL4 compared to Mφ0, respectively (P < 0.001). The expression of Slc16a3 was also 6.1 ± 1.3 (P < 0.01) and 3.3 ± 1.2 (P = 0.14) fold higher in MφIFNγ+LPS and MφIL4 compared to Mφ0, respectively. The expression of Slc16a7, the least abundant transporter, was comparable among the different polarization states of macrophages.
14C-Acetate Uptake by Human Carotid Plaques Stimulated by IFN-γ + LPS vs. IL-4 Mirrors the Changes in Polarized Macrophages
In order to determine the effect of different polarizing agents on acetate uptake in the vessel wall micro-environment, we utilized a previously validated technique of ex vivo culture of human carotid endarterectomy specimens24, 25. Induction of different polarization states in endarterectomy specimens were confirmed by the increased expression of M1 polarization markers, interleukin-1β (IL-1β) and nitric oxide synthase-2 (NOS2), by IFN-γ + LPS (Figure 5A) vs. M2 polarization markers, CD163 and mannose receptor C-type 1 (MRC1), by IL-4 (Figure 5B). Consistent with our observation in polarized murine macrophages, stimulation of human endarterectomy specimens with IFN-γ + LPS led to reduction of 14C-acetate uptake to 66.5 ±14.5% of non-stimulated plaques over a 2-day period (Figure 5C–D). On the other hand, stimulation by IL-4 increased 14C-acetate uptake to 151.5 ± 25.8% (Figure 5C–D) (P<0.05).
Figure 5: Divergence of 14C-acetate uptake by human atherosclerotic plaques upon pro-inflammatory vs. inflammation-resolving stimulation.
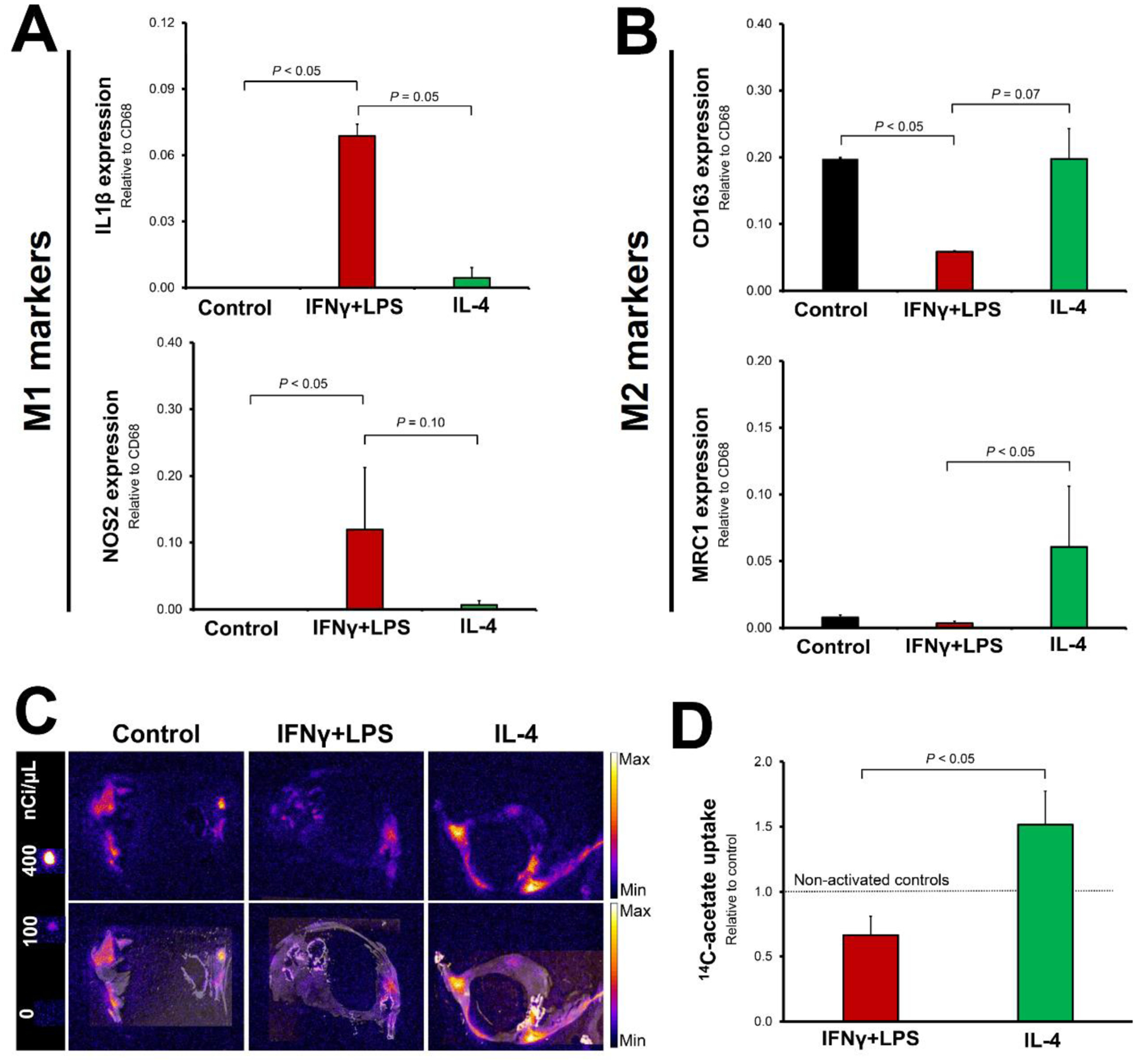
Induction of distinct polarization states in human carotid endarterectomy specimens is confirmed by quantification of M1 polarization markers (interleukin-1β (IL-1β) and nitric oxide synthase-2 (NOS2), panel A) vs. M2 polarization markers (CD163 and mannose receptor C-type 1 (MRC1), panel B). Quantitative autoradiography demonstrates that stimulation of plaques with IFN-γ + LPS leads to a significant decline in 14C-acetate uptake, while IL-4 stimulation increases 14C-acetate uptake (C–D). N = 3 endarterectomy specimens (2–3 tissue sections per each specimen quantified).
Discussion
As summarized in schematic Figure 6, our study demonstrated that uptake of acetate is primarily localized to macrophage-rich regions of atherosclerotic plaques. Additionally, we showed a divergence in acetate uptake by macrophages and endarterectomy specimens stimulated with pro-inflammatory (IFNγ + LPS) vs. inflammation-resolving (IL-4) stimuli, suggesting the potential of 11C-acetate PET in characterizing the inflammatory state of the vessel wall.
Figure 6: Schematic summary of the study.
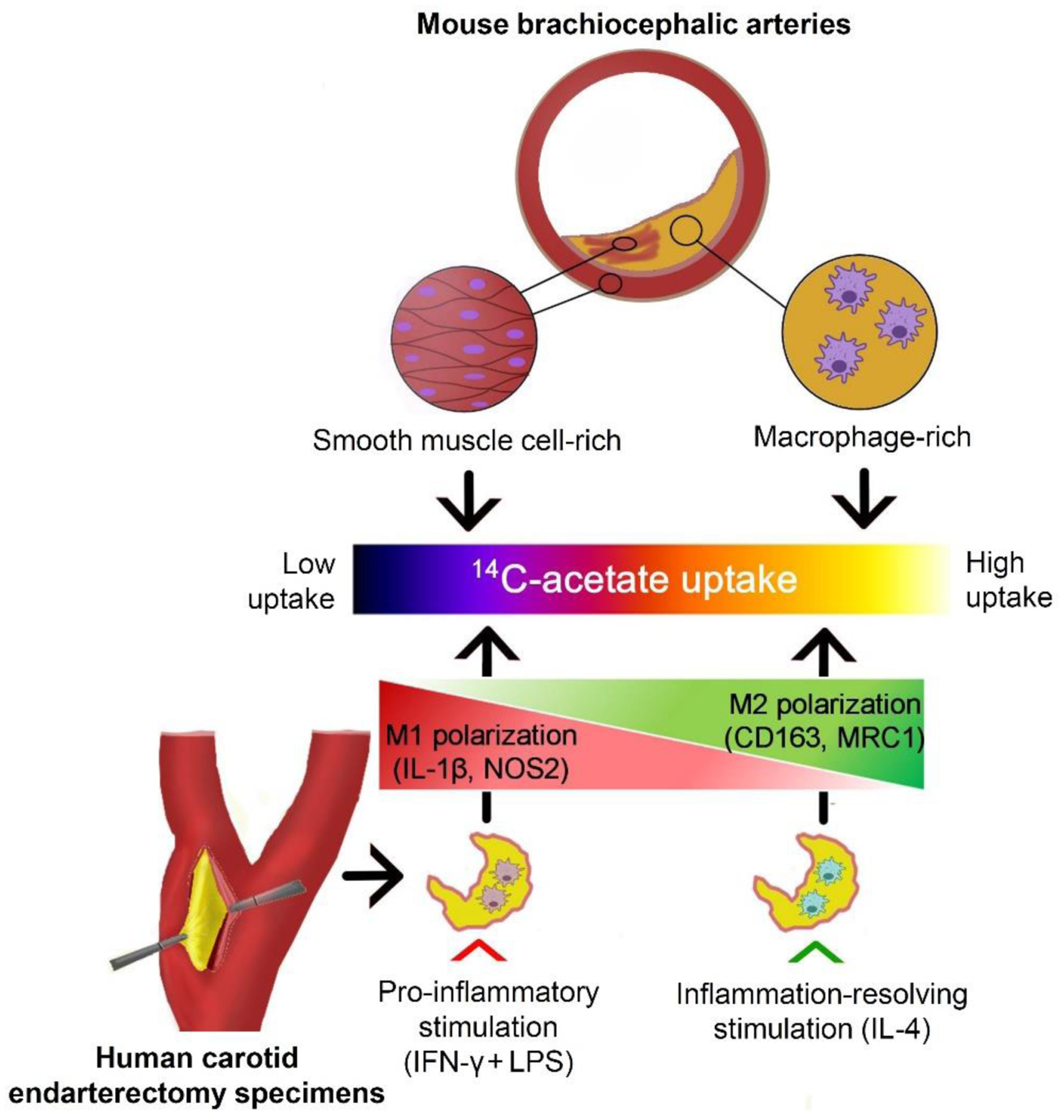
14C-acetate uptake is primarily localized within the macrophage-rich regions of atherosclerotic plaques in brachiocephalic arteries of apoE−/− mice. Ex vivo polarization of murine peritoneal macrophages and human carotid endarterectomy specimens into pro-inflammatory and inflammation-resolving states, by IFN-γ + LPS vs. IL-4, respectively, leads to a marked divergence in their uptake of 14C-acetate, suggesting a potential venue for immuno-metabolic characterization of atherosclerotic plaques by PET.
The growing appreciation of the contribution of plaques’ immuno-metabolic heterogeneity to the vessel wall biology1 underscores the potential role of metabolic imaging in non-invasive characterization of atherosclerosis, which may improve the risk stratification of patients and monitoring the response to preventive or therapeutic interventions15, 16. To further enhance the translational potential of this study, we used murine brachiocephalic plaques, rather than aortic plaques, as the former more accurately models the complexities of human plaque pathophysiology28–30.
Despite initial promising results indicating an association between 18F-FDG uptake and the pro-inflammatory state of vessel wall macrophages15, 16, 31, emerging evidence supports that glucose uptake, as a stand-alone marker, does not reveal sufficient immuno-metabolic information to allow discrimination of plaques macrophages from smooth muscle cells14, 32 or pro-inflammatory from inflammation-resolving macrophages5, 9, 10, 13.
A promising approach to overcome the non-specificity of 18F-FDG in elucidating vessel wall immuno-metabolism is a combined imaging approach to target other key metabolic pathways, which may aid in differentiation of different immune cell phenotypes. For example, combined imaging of glutamine and glucose uptake improves the identification of different polarization states of macrophages9. Considering the central roles of acetate in various immuno-metabolic pathways, including tricarboxylic acid cycle, lipogenesis and protein acetylation33, 11C-acetate PET represents a promising, but largely unexplored, approach in inflammatory diseases. Notably, 11C-acetate uptake has been reported in ~30% of atherosclerotic plaques17. However, biological/immuno-metabolic correlates and clinical implications of acetate uptake in atherosclerosis remained to be determined. In this study we showed that acetate uptake is primarily localized to macrophage-rich regions of atherosclerosis.
In order to identify the immuno-metabolic implications of acetate uptake, we determined the time-course of changes in cultured macrophages over a 2-day period of ex vivo polarization. We identified a progressive decline in acetate uptake by pro-inflammatory macrophages, but a delayed increase in acetate uptake in inflammation-resolving macrophages. The distinct temporal pattern of acetate uptake in polarized macrophages may reflect differences in IFN-γ + LPS vs. IL-4 induced cellular metabolic reprogramming, particularly in M2-like macrophages which rely on mitochondrial biogenesis for activation and subsequent upregulation of specific metabolic pathways (e.g., tricarboxylic acid cycle, β-oxidation, oxidative phosphorylation)10, 34, 35. By contrast, IFN-γ + LPS stimulation affects macrophage metabolism by more rapid regulation of flux through certain pathways, including enhanced glycolysis and suppression of the tricarboxylic acid cycle and oxidative phosphorylation cycle8. This divergence of acetate uptake in differentially polarized macrophages highlights the potential role of 11C-acetate PET in non-invasive discrimination of inflammatory states of atherosclerotic plaques. As a proof-of-principle ex vivo experiment, we confirmed that IFN-γ + LPS reduces, while IL-4 increases, the acetate uptake in human endarterectomy specimens.
Cellular transport of acetate is primarily through monocarboxylate transporters26. Consistent with prior reports, Slc16a1 and Slc16a3 were the most abundant transporters at the transcriptional level26, 27. Interestingly, Slc16a1 was ~6-fold over-expressed in MφIL4, consistent with their higher acetate uptake, compared to Mφ0. There was also ~4- and ~6-fold increase in the expression of Slc16a1 and Slc16a3 in MφIFNγ+LPS, despite their markedly reduced acetate uptake, which likely reflects their role in transport of other substrates, particularly lactate, which is required for glycolytic reprogramming of macrophages in response to LPS36.
Intra-cellular metabolic fate of various substrates, e.g., glucose and glutamine, plays crucial role in phenotypic alterations of macrophages in response to different stimuli8, 37–39. Through acetyl-CoA synthetase mediated conversion into acetyl-CoA and subsequent condensation with oxaloacetate to generate citrate, intra-cellular acetate is at the cross-road of metabolic pathways and may contributes to lipogenesis and tricarboxylic acid (TCA) cycle18. Analysis of rabbit atherosclerotic arteries administered with 14C-acetate has revealed that acetate contributes to both aqueous (e.g., glutamate) and fat-soluble (e.g., triglycerides and cholesterol esters) metabolites18, 40, 41. Our study demonstrated profound divergence of overall uptake of acetate by polarized murine macrophages and human atherosclerotic plaques. However, potential alterations in intra-cellular utilization of acetate by its differential fluxes through different pathways and the potential impact of these metabolic changes on macrophage biology and atherogenesis remained to be determined. Importantly, the dichotomy of IFNγ + LPS (i.e. M1-like) and IL-4 (i.e. M2-like) stimulated macrophages fails to capture the profound and nuanced complexity of macrophage phenotypes, and their unique metabolic and functional profiles42.
In this study, we took advantage of the higher spatial resolution of lower energy β− decay through 14C-acetate autoradiography, in contrast to high energy low-resolution β+ by 11C-acetate, to determine the histological distribution of acetate uptake within the vessel wall. The long half-life of 14C-acetate was also compatible with the lengthy tissue processing steps required for micro-autoradiography, which would have been challenging for the short half-life of 11C (~20 min). Although, the use of 14C-acetate eliminated the possibility of PET, the feasibility of in vivo detection of 11C-acetate uptake in atherosclerosis has been previously demonstrated17; hence, our primary purpose was to determine the histological correlates of acetate uptake to unravel its association with plaque biology and eventually its clinical implications. Future clinical studies are needed to correlate in vivo 11C-acetate uptake with molecular and histological markers of plaque vulnerability, like the macrophage burden, the abundance of different polarization states of macrophages, and pro/anti-inflammatory cytokine production, and disease outcome.
While acetate is a promising molecular imaging probe for atherosclerotic plaques17, certain limitations common to other immune-metabolic tracers, including low spatial resolution with respect to plaque size and low signal-to-background, remain roadblocks for translational application. Additionally, high 11C-acetate uptake by metabolically active organs, particularly heart and liver, may limit its usefulness in imaging coronary artery disease17, 43–45. 11C-acetate PET may therefore be more suited for assessment of atherosclerosis in larger arteries, particularly carotid arteries, to predict and monitor response to conventional, e.g., statin, or emerging immunomodulatory therapies.
In summary, our data demonstrate that acetate uptake within the vessel wall primarily correlates with macrophage-rich atherosclerotic plaques. Moreover, classical pro-inflammatory polarization of macrophages and atherosclerotic plaques significantly reduces their uptake of acetate, while alternative activation upregulates the uptake. Together, these data suggest a potential link between the enhanced uptake of acetate and the induction of an inflammation-resolving state in atherosclerotic plaques. Further in vivo imaging studies are required to determine if 11C-acetate PET, alone or in combination with other metabolic substrates, e.g., 18F-FDG and 18F-fluoroglutamine, may play a role in identifying vulnerable plaques and in monitoring the response to anti-inflammatory interventions, such as interleukin-1β blocking antibody, Canakinumab46.
Supplementary Material
New Knowledge Gained:
Our results demonstrate that arterial uptake of acetate is mostly localized to macrophage-rich regions of atherosclerotic plaques compared to regions enriched in smooth muscle cells. Additionally, we showed that a profound divergence in acetate uptake distinguishes the classical (pro-inflammatory) and alternative (inflammation-resolving) polarization states of macrophages, induced by interferon-γ + lipopolysaccharide vs. interleukin-4, respectively, in two different ex vivo models, i.e., cultured murine macrophages and human endarterectomy specimens. These data provide a new potential venue for in vivo immuno-metabolic characterization of plaques by non-invasive imaging.
Funding:
This study was supported by grants from National Institute of Health (NHLBI, K08 HL144911) and Radiological Society of North America (RSD-1820) as well as a Seed Fund from University of Pittsburgh/UPMC Departments of Radiology and Medicine to S.T.
Disclosures
This work was supported by Radiological Society of North America Seed Grant (RSD-1820), NIH NHLBI Mentored Clinical Scientist Research Career Development Award (1K08HL144911), and a Seed Fund from University of Pittsburgh/UPMC to S.T; as well as NIH NHLBI R01 HL146465 to DG. The authors have no conflicts of interest to disclose.
Abbreviations:
- 18F-FDG
18F-fluorodeoxyglucose
- PET
Positron emission tomography
- IL-1β
Interleukin-1β
- IL-4
Interleukin-4
- IFN-γ
Interferon-γ
- LPS
Lipopolysaccharide
- NOS2
Nitric oxide synthase-2
Footnotes
Disclosures of Conflicts of Interest
Selim Demirdelen: No conflicts to disclose
Philip Z. Mannes: No conflicts to disclose
Ali Mubin Aral: No conflicts to disclose
Joseph Haddad: No conflicts to disclose
Steven A. Leers: No conflicts to disclose
Delphine Gomez: No conflicts to disclose
Sina Tavakoli: No conflicts to disclose
References
- 1.Ketelhuth DFJ, Lutgens E, Back M, Binder CJ, Van den Bossche J, Daniel C, Dumitriu IE, Hoefer I, Libby P, O’Neill L, Weber C, Evans PC. Immunometabolism and atherosclerosis: Perspectives and clinical significance: A position paper from the working group on atherosclerosis and vascular biology of the european society of cardiology. Cardiovasc Res. 2019;115:1385–1392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Koelwyn GJ, Corr EM, Erbay E, Moore KJ. Regulation of macrophage immunometabolism in atherosclerosis. Nat Immunol. 2018;19:526–537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Russell DG, Huang L, VanderVen BC. Immunometabolism at the interface between macrophages and pathogens. Nat Rev Immunol. 2019;19:291–304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Folco EJ, Sukhova GK, Quillard T, Libby P. Moderate hypoxia potentiates interleukin-1beta production in activated human macrophages. Circ Res. 2014;115:875–883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Folco EJ, Sheikine Y, Rocha VZ, Christen T, Shvartz E, Sukhova GK, Di Carli MF, Libby P. Hypoxia but not inflammation augments glucose uptake in human macrophages: Implications for imaging atherosclerosis with 18fluorine-labeled 2-deoxy-d-glucose positron emission tomography. J Am Coll Cardiol. 2011;58:603–614 [DOI] [PubMed] [Google Scholar]
- 6.Marsch E, Sluimer JC, Daemen MJ. Hypoxia in atherosclerosis and inflammation. Curr Opin Lipidol. 2013;24:393–400 [DOI] [PubMed] [Google Scholar]
- 7.Marsch E, Theelen TL, Demandt JA, Jeurissen M, van Gink M, Verjans R, Janssen A, Cleutjens JP, Meex SJ, Donners MM, Haenen GR, Schalkwijk CG, Dubois LJ, Lambin P, Mallat Z, Gijbels MJ, Heemskerk JW, Fisher EA, Biessen EA, Janssen BJ, Daemen MJ, Sluimer JC. Reversal of hypoxia in murine atherosclerosis prevents necrotic core expansion by enhancing efferocytosis. Arterioscler Thromb Vasc Biol. 2014;34:2545–2553 [DOI] [PubMed] [Google Scholar]
- 8.Tannahill GM, Curtis AM, Adamik J, Palsson-McDermott EM, McGettrick AF, Goel G, Frezza C, Bernard NJ, Kelly B, Foley NH, Zheng L, Gardet A, Tong Z, Jany SS, Corr SC, Haneklaus M, Caffrey BE, Pierce K, Walmsley S, Beasley FC, Cummins E, Nizet V, Whyte M, Taylor CT, Lin H, Masters SL, Gottlieb E, Kelly VP, Clish C, Auron PE, Xavier RJ, O’Neill LA. Succinate is an inflammatory signal that induces il-1beta through hif-1alpha. Nature. 2013;496:238–242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tavakoli S, Downs K, Short JD, Nguyen HN, Lai Y, Jerabek PA, Goins B, Toczek J, Sadeghi MM, Asmis R. Characterization of macrophage polarization states using combined measurement of 2-deoxyglucose and glutamine accumulation: Implications for imaging of atherosclerosis. Arterioscler Thromb Vasc Biol. 2017;37:1840–1848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tavakoli S, Zamora D, Ullevig S, Asmis R. Bioenergetic profiles diverge during macrophage polarization: Implications for the interpretation of 18f-fdg pet imaging of atherosclerosis. J Nucl Med. 2013;54:1661–1667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ngoi NYL, Eu JQ, Hirpara J, Wang L, Lim JSJ, Lee SC, Lim YC, Pervaiz S, Goh BC, Wong ALA. Targeting cell metabolism as cancer therapy. Antioxid Redox Signal. 2020;32:285–308 [DOI] [PubMed] [Google Scholar]
- 12.Luengo A, Gui DY, Vander Heiden MG. Targeting metabolism for cancer therapy. Cell Chem Biol. 2017;24:1161–1180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tavakoli S, Short JD, Downs K, Nguyen HN, Lai Y, Zhang W, Jerabek P, Goins B, Sadeghi MM, Asmis R. Differential regulation of macrophage glucose metabolism by macrophage colony-stimulating factor and granulocyte-macrophage colony-stimulating factor: Implications for (18)f fdg pet imaging of vessel wall inflammation. Radiology. 2017;283:87–97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Al-Mashhadi RH, Tolbod LP, Bloch LO, Bjorklund MM, Nasr ZP, Al-Mashhadi Z, Winterdahl M, Frokiaer J, Falk E, Bentzon JF. (18)fluorodeoxyglucose accumulation in arterial tissues determined by pet signal analysis. J Am Coll Cardiol. 2019;74:1220–1232 [DOI] [PubMed] [Google Scholar]
- 15.Tavakoli S, Vashist A, Sadeghi MM. Molecular imaging of plaque vulnerability. J Nucl Cardiol. 2014;21:1112–1128; quiz 1129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tavakoli S Technical considerations for quantification of (18)f-fdg uptake in carotid atherosclerosis. J Nucl Cardiol. 2019;26:894–898 [DOI] [PubMed] [Google Scholar]
- 17.Derlin T, Habermann CR, Lengyel Z, Busch JD, Wisotzki C, Mester J, Pavics L. Feasibility of 11c-acetate pet/ct for imaging of fatty acid synthesis in the atherosclerotic vessel wall. J Nucl Med. 2011;52:1848–1854 [DOI] [PubMed] [Google Scholar]
- 18.Yamasaki K, Yamashita A, Zhao Y, Shimizu Y, Nishii R, Kawai K, Tamaki N, Zhao S, Asada Y, Kuge Y. In vitro uptake and metabolism of [(14)c]acetate in rabbit atherosclerotic arteries: Biological basis for atherosclerosis imaging with [(11)c]acetate. Nucl Med Biol. 2018;56:21–25 [DOI] [PubMed] [Google Scholar]
- 19.Dunphy MPS, Harding JJ, Venneti S, Zhang H, Burnazi EM, Bromberg J, Omuro AM, Hsieh JJ, Mellinghoff IK, Staton K, Pressl C, Beattie BJ, Zanzonico PB, Gerecitano JF, Kelsen DP, Weber W, Lyashchenko SK, Kung HF, Lewis JS. In vivo pet assay of tumor glutamine flux and metabolism: In-human trial of (18)f-(2s,4r)-4-fluoroglutamine. Radiology. 2018;287:667–675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Karanikas G, Beheshti M. (1)(1)c-acetate pet/ct imaging: Physiologic uptake, variants, and pitfalls. PET Clin. 2014;9:339–344 [DOI] [PubMed] [Google Scholar]
- 21.Porenta G, Cherry S, Czernin J, Brunken R, Kuhle W, Hashimoto T, Schelbert HR. Noninvasive determination of myocardial blood flow, oxygen consumption and efficiency in normal humans by carbon-11 acetate positron emission tomography imaging. Eur J Nucl Med. 1999;26:1465–1474 [DOI] [PubMed] [Google Scholar]
- 22.Zhang X, Goncalves R, Mosser DM. The isolation and characterization of murine macrophages. Curr Protoc Immunol. 2008;Chapter 14:Unit 14 11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gomez D, Shankman LS, Nguyen AT, Owens GK. Detection of histone modifications at specific gene loci in single cells in histological sections. Nat Methods. 2013;10:171–177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Erbel C, Okuyucu D, Akhavanpoor M, Zhao L, Wangler S, Hakimi M, Doesch A, Dengler TJ, Katus HA, Gleissner CA. A human ex vivo atherosclerotic plaque model to study lesion biology. J Vis Exp. 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lebedeva A, Vorobyeva D, Vagida M, Ivanova O, Felker E, Fitzgerald W, Danilova N, Gontarenko V, Shpektor A, Vasilieva E, Margolis L. Ex vivo culture of human atherosclerotic plaques: A model to study immune cells in atherogenesis. Atherosclerosis. 2017;267:90–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Balmer ML, Ma EH, Bantug GR, Grahlert J, Pfister S, Glatter T, Jauch A, Dimeloe S, Slack E, Dehio P, Krzyzaniak MA, King CG, Burgener AV, Fischer M, Develioglu L, Belle R, Recher M, Bonilla WV, Macpherson AJ, Hapfelmeier S, Jones RG, Hess C. Memory cd8(+) t cells require increased concentrations of acetate induced by stress for optimal function. Immunity. 2016;44:1312–1324 [DOI] [PubMed] [Google Scholar]
- 27.Petersen C, Nielsen MD, Andersen ES, Basse AL, Isidor MS, Markussen LK, Viuff BM, Lambert IH, Hansen JB, Pedersen SF. Mct1 and mct4 expression and lactate flux activity increase during white and brown adipogenesis and impact adipocyte metabolism. Sci Rep. 2017;7:13101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jackson CL, Bennett MR, Biessen EA, Johnson JL, Krams R. Assessment of unstable atherosclerosis in mice. Arterioscler Thromb Vasc Biol. 2007;27:714–720 [DOI] [PubMed] [Google Scholar]
- 29.Rosenfeld ME, Polinsky P, Virmani R, Kauser K, Rubanyi G, Schwartz SM. Advanced atherosclerotic lesions in the innominate artery of the apoe knockout mouse. Arterioscler Thromb Vasc Biol. 2000;20:2587–2592 [DOI] [PubMed] [Google Scholar]
- 30.Johnson JL, Jackson CL. Atherosclerotic plaque rupture in the apolipoprotein e knockout mouse. Atherosclerosis. 2001;154:399–406 [DOI] [PubMed] [Google Scholar]
- 31.Sadeghi MM. (18)f-fdg pet and vascular inflammation: Time to refine the paradigm? J Nucl Cardiol. 2015;22:319–324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Guillermier C, Doherty SP, Whitney AG, Babaev VR, Linton MF, Steinhauser ML, Brown JD. Imaging mass spectrometry reveals heterogeneity of proliferation and metabolism in atherosclerosis. JCI Insight. 2019;4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bose S, Ramesh V, Locasale JW. Acetate metabolism in physiology, cancer, and beyond. Trends Cell Biol. 2019;29:695–703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vats D, Mukundan L, Odegaard JI, Zhang L, Smith KL, Morel CR, Wagner RA, Greaves DR, Murray PJ, Chawla A. Oxidative metabolism and pgc-1beta attenuate macrophage-mediated inflammation. Cell Metab. 2006;4:13–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tabas I, Bornfeldt KE. Intracellular and intercellular aspects of macrophage immunometabolism in atherosclerosis. Circ Res. 2020;126:1209–1227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tan Z, Xie N, Banerjee S, Cui H, Fu M, Thannickal VJ, Liu G. The monocarboxylate transporter 4 is required for glycolytic reprogramming and inflammatory response in macrophages. J Biol Chem. 2015;290:46–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Puchalska P, Huang X, Martin SE, Han X, Patti GJ, Crawford PA. Isotope tracing untargeted metabolomics reveals macrophage polarization-state-specific metabolic coordination across intracellular compartments. iScience. 2018;9:298–313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cumming BM, Addicott KW, Adamson JH, Steyn AJ. Mycobacterium tuberculosis induces decelerated bioenergetic metabolism in human macrophages. Elife. 2018;7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jha AK, Huang SC, Sergushichev A, Lampropoulou V, Ivanova Y, Loginicheva E, Chmielewski K, Stewart KM, Ashall J, Everts B, Pearce EJ, Driggers EM, Artyomov MN. Network integration of parallel metabolic and transcriptional data reveals metabolic modules that regulate macrophage polarization. Immunity. 2015;42:419–430 [DOI] [PubMed] [Google Scholar]
- 40.Howard CF Jr. Lipogenesis from glucose-2- 14C c and acetate-1- 14 c in aorta. J Lipid Res. 1971;12:725–730 [PubMed] [Google Scholar]
- 41.Day AJ, Wilkinson GK. Incorporation of 14-c-labeled acetate into lipid by isolated foam cells and by atherosclerotic arterial intima. Circ Res. 1967;21:593–600 [DOI] [PubMed] [Google Scholar]
- 42.Nahrendorf M, Swirski FK. Abandoning m1/m2 for a network model of macrophage function. Circ Res. 2016;119:414–417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Armbrecht JJ, Buxton DB, Schelbert HR. Validation of [1–11c]acetate as a tracer for noninvasive assessment of oxidative metabolism with positron emission tomography in normal, ischemic, postischemic, and hyperemic canine myocardium. Circulation. 1990;81:1594–1605 [DOI] [PubMed] [Google Scholar]
- 44.Sun KT, Chen K, Huang SC, Buxton DB, Hansen HW, Kim AS, Siegel S, Choi Y, Muller P, Phelps ME, Schelbert HR. Compartment model for measuring myocardial oxygen consumption using [1–11c]acetate. J Nucl Med. 1997;38:459–466 [PubMed] [Google Scholar]
- 45.Nesterov SV, Turta O, Han C, Maki M, Lisinen I, Tuunanen H, Knuuti J. C-11 acetate has excellent reproducibility for quantification of myocardial oxidative metabolism. Eur Heart J Cardiovasc Imaging. 2015;16:500–506 [DOI] [PubMed] [Google Scholar]
- 46.Ridker PM, Everett BM, Thuren T, MacFadyen JG, Chang WH, Ballantyne C, Fonseca F, Nicolau J, Koenig W, Anker SD, Kastelein JJP, Cornel JH, Pais P, Pella D, Genest J, Cifkova R, Lorenzatti A, Forster T, Kobalava Z, Vida-Simiti L, Flather M, Shimokawa H, Ogawa H, Dellborg M, Rossi PRF, Troquay RPT, Libby P, Glynn RJ, Group CT. Antiinflammatory therapy with canakinumab for atherosclerotic disease. N Engl J Med. 2017;377:1119–1131 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


