Abstract
Background
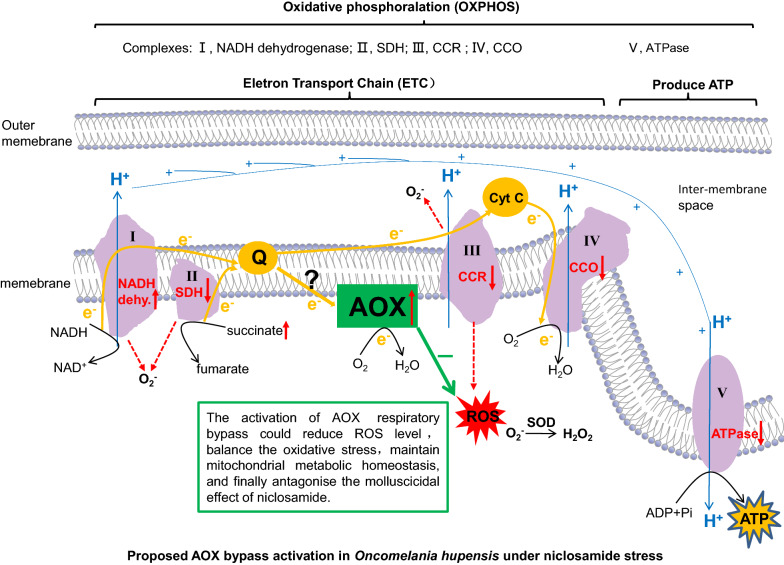
Snail intermediate hosts are mandatory for the transmission of schistosomiasis, which has to date infected more than 200 million people worldwide. Our previous studies showed that niclosamide treatment caused the inhibition of aerobic respiration and oxidative phosphorylation, and the disruption of energy supply, in one of the intermediate hosts of schistosomiasis, Oncomelania hupensis, which eventually led to the death of the snails. Meanwhile, the terminal oxidase in the mitochondrial respiratory chain, alternative oxidase (AOX), was significantly up-regulated, which was thought to counterbalance the oxidative stress and maintain metabolic homeostasis in the snails. The aims of the present study are to identify the AOXs in several species of snails and investigate the potential activation of O. hupensis AOX (OhAOX) under niclosamide-induced stress, leading to enhanced survival of the snail when exposed to this molluscicide.
Methods
The complete complementary DNA was amplified from the AOXs of O. hupensis and three species of Biomphalaria; the sequence characteristics were analysed and the phylogenetics investigated. The dynamic expression and localisation of the AOX gene and protein in O. hupensis under niclosamide-induced stress were examined. In addition, the expression pattern of genes in the mitochondrial respiratory complex was determined and the production of reactive oxygen species (ROS) calculated. Finally, the molluscicidal effect of niclosamide was compared between snails with and without inhibition of AOX activity.
Results
AOXs containing the invertebrate AOX-specific motif NP-[YF]-XPG-[KQE] were identified from four species of snail, which phylogenetically clustered together into Gastropoda AOXs and further into Mollusca AOXs. After niclosamide treatment, the levels of OhAOX messenger RNA (mRNA) and OhAOX protein in the whole snail were 14.8 and 2.6 times those in untreated snails, respectively, but varied widely among tissues. Meanwhile, the level of cytochrome C reductase mRNA showed a significant decrease in the whole snail, and ROS production showed a significant decrease in the liver plus gonad (liver-gonad) of the snails. At 24 h post-treatment, the mortality of snails treated with 0.06–0.1 mg/L niclosamide and AOX inhibitor was 56.31–76.12% higher than that of snails treated with 0.1 mg/L niclosamide alone.
Conclusions
AOX was found in all the snail intermediate hosts of Schistosoma examined here. AOX was significantly activated in O. hupensis under niclosamide-induced stress, which led to a reduction in oxidative stress in the snail. The inhibition of AOX activity in snails can dramatically enhance the molluscicidal effect of niclosamide. A potential target for the development of an environmentally safe snail control method, which acts by inhibiting the activity of AOX, was identified in this study.
Graphical abstract
Supplementary Information
The online version contains supplementary material available at 10.1186/s13071-022-05227-5.
Keywords: Alternative oxidase, Niclosamide, Oncomelania hupensis, Intermediate host, Schistosoma, Mitochondrial respiratory chain
Background
Schistosomiasis is a widespread zoonotic disease in tropical and subtropical regions, and the second most prevalent parasitic disease in the world [1]. Seven species of Schistosoma can parasitise humans, and all of them are transmitted through contact between the skin and their cercaria, which are released from the specific intermediate snail host [2]. Since snails are obligatory intermediate hosts in the life cycle of Schistosoma, snail control is one of the most important measures used to block schistosomiasis transmission, including in areas where schistosomiasis elimination is the aim, such as in areas endemic for Schistosomiasis japonicum in China. Although the infection rates in humans and livestock in China remain low, high-risk factors for schistosomiasis transmission still exist, such as an increasing number of imported cases of schistosomiasis [3], the spread of Biomphalaria straminea (the intermediate host of Schistosoma mansoni in South America) in south China [4], wild animals as sources of infection [5], and the large geographical distribution of intermediate host snails of the genus Oncomelania [6]. Oncomelania hupensis is the only intermediate host of S. japonicum. In the past 20 years, the distribution of O. hupensis in China has not significantly reduced, and the population has remained at approximately 3.52–3.86 billion m2 [7]. In addition, the distribution of the snail in some areas endemic for schistosomiasis has shown an increasing trend as a consequence of ecological and environmental changes [6, 8].
Among the various approaches for snail control, chemical molluscicides are widely used due to their high potential coverage, the simplicity of their application, and their fast action [9]. Niclosamide is the only chemical molluscicide recommended by the World Health Organization, and has been used in areas of China endemic for schistosomiasis for nearly 30 years [10]. Some other chemicals and plant extracts, such as niclosamidate and luo-wei, were reported to have considerable molluscicidal effects with limited toxicity to aquatic animals [11, 12]. However, these compounds are not as effective as niclosamide, and logistic difficulties may arise during their application in the field [11, 12]. Niclosamide, therefore, has remained the main molluscicide for snail control. Although snails of the genus Oncomelania have not shown apparent resistance to niclosamide, there is a potential risk of resistance due to the long-term and extensive use of this single molluscicide [13]. In addition, niclosamide is highly toxic to fish and other aquatic animals. Thus, a deeper understanding of the response of Oncomelania to niclosamide is required to enable the development of an environmentally safe method for the highly efficient and specific control of these snails.
A previous study [14] showed that niclosamide can damage the liver and gonad (liver-gonad), ganglion, and muscle tissues of O. hupensis, with systems such as those of the mitochondrion, endoplasmic reticulum, and nucleus being the most affected. The mitochondrion is an organelle central to the production of chemical energy; it is involved in carbohydrate, amino acid and fatty acid metabolism, redox homeostasis, and cell signal transduction pathways, and is responsible for more than 90% of the adenosine triphosphate (ATP) produced in the cells of animals [15, 16]. Our previous studies on the enzymatic histochemistry and metabolic changes of O. hupensis in response to niclosamide revealed that the activities of cytochrome c oxidase (CCO), succinic dehydrogenase (SDH), and glucose-6-phosphatase in the respiratory chain of mitochondrial oxidative phosphorylation decreased after niclosamide treatment. In contrast, lactate dehydrogenase activity in the anaerobic glycolysis pathway increased [14, 17]. Niclosamide interfered with the metabolism of snails, damaged the structure of tissues, and led to the death of O. hupensis [14, 17]. In addition, the transcriptomic analysis of genes involved in mitochondrial oxidative phosphorylation revealed that nicotinamide adenine dinucleotide dehydrogenase subunit 3 was up-regulated and Na/K transporter ATPase subunit α was down-regulated after niclosamide treatment [18]. This indicated that niclosamide might induce oxidative stress by inhibiting the cytochrome c (Cyt c) pathway, i.e. aerobic respiration [14, 18]. Meanwhile, alternative oxidase (AOX), which is the terminal oxidase of a respiratory bypass of the mitochondrion [19], was significantly up-regulated [14, 18]. AOX is found in higher plants, algae, fungi, and protists, but not in vertebrates [19, 20]. It is involved in the generation of heat [19, 21], the optimisation of photosynthesis [22], the prevention of excessive reactive oxygen species (ROS) formation [23, 24], resistance to stress [24–26], and the maintenance of cellular energy homeostasis [26]. These functions of AOX were mostly found in plants. It is believed that the AOX bypass can accept electrons from ubiquinone, thereby reducing the membrane potential and preventing the over-reduction of ubiquinone in the respiratory chain from reducing ROS production [23, 24]. This occurs not only in plants but also in fungi and protozoa. Production of ROS increased in Aspergillus fumigatus after AOX was silenced [27]. H2O2 production in Acanthamoeba castellanii reduced significantly when the mitochondrial AOX respiratory pathway was activated, and this reduction was reversed when AOX was inhibited [28]. AOX in the molluscs Crassostrea virginica and Diplodon chilensis also played a role in resistance to stress [29, 30]. These findings led us to deduce that the activated AOX bypass could sustain metabolic homeostasis in O. hupensis under niclosamide-induced stress while the Cyt c pathway was inhibited [14, 17, 18], reduce cell damage caused by ROS accumulation in the mitochondrion [14, 17, 18], and weaken the molluscicidal effect of niclosamide.
First, this study proved the existence of AOX in the intermediate snail hosts of different Schistosoma species, including O. hupensis and three species of Biomphalaria. The biological characteristics of the AOXs of these four snail species and the phylogenetic evolution of the mollusc AOXs based on their sequences were then investigated. After niclosamide treatment, the dynamic expression of O. hupensis AOX (OhAOX) and tissue localisation of OhAOX were checked by measuring mRNA and protein levels. Also, the dynamic expression of genes involved in oxidative phosphorylation was investigated, and the ROS level was evaluated. Finally, snail mortality was determined in a molluscicidal assay with or without OhAOX inhibition to verify the resistance of OhAOX to niclosamide-induced stress and evaluate the possibility that OhAOX can be used as a molluscicidal enhancer target.
Methods
Snail collection
Oncomelania hupensis snails were collected on several occasions from habitats with different environmental conditions in a location (30.0011°N, 111.9716°E) in Gong’an county, Hubei Province, China, under the permission and assistance of the local snail control organisation, the Institute of Schistosomiasis Control of Gong’an County. No infected snail has been found in the area for years, and no molluscicide has been used there. The snails were brought back to the laboratory and rinsed with dechlorinated tap water. Snails with higher crawling activity that ranged in shell height from 6 to 8 mm were selected to ensure uniformity. These were maintained on wet rough filter paper with antibiotics in Petri dishes covered with plastic mesh, and then placed in an incubator at 25 °C with fresh humidified air. The snails were fed and the filter papers changed every 3 days for a month to allow them to adapt to the laboratory conditions.
Biomphalaria glabrata snails that have been kept and bred for a period of 10 years in Dr Yousheng Liang’s laboratory at Jiangsu Provincial Institute of Schistosomiasis Control and Prevention, were kindly provided for this study. Biomphalaria alexandrina snails were collected from a location (30.0802°N, 31.0962°E) in Teraat Al Mansoureya, Abu Rawash, Giza, Egypt, with the assistance of the Department of Medical Malacology, Theodor Bilharz Research Institute. Biomphalaria straminea snails were collected from a location (22.5794°N, 113.9504°E) in Shenzhen city, Guangdong Province, China, under the permission and assistance of the Shenzhen Center for Disease Control and Prevention. The snails were brought back to the laboratory, rinsed with dechlorinated tap water, and prepared for the experiments.
Amplification, identification, and phylogenetic analysis of snail AOXs
BLAST analysis and selection of AOX fragments
Based on our previous work [18], a BLAST database was constructed using local sequence alignment of the O. hupensis transcriptomic unigene (NCBI SRA accession no. SRP041729) using ncbi-blast-2.8.1+ (ftp://ftp.ncbi.nlm.nih.gov/blast/executables/blast+). Each AOX-related unigene filtered out in a Swiss-Prot database analysis with an E-value of < 10–9 was checked, and 23 meaningful fragments were then obtained from the local BLAST analysis. Finally, the longest sequence was selected as the reference sequence for subsequent OhAOX complementary DNA (cDNA) amplification. The predicted B. glabrata AOX mRNA (GenBank accession no. XM_013230806) computed from a B. glabrata genomic sequence (NW_013418361.1) was chosen as the reference sequence for subsequent cDNA amplification of B. glabrata AOX (BgAOX), B. alexandrina AOX (BaAOX), and B. straminea AOX (BsAOX).
RNA extraction and AOX cDNA amplification
Live snails were washed in sterilised water, and then their shells were cleaned with 70% ethanol and removed. Whole snails were dissected individually on glass slides under a microscope, and those confirmed to have no helminthic infection were used for further study. For each species of snail, the whole soft body of each snail without the intestinal tract was carefully washed three times with ice cold RNAase-free 0.3% NaCl and then pooled and stored immediately in liquid nitrogen.
The total RNA from each sample was extracted using TRIzol Reagent (Ambion, Carlsbad, CA), in accordance with the manufacturer's instructions. The integrity and size distribution of the RNA was evaluated using agarose gel electrophoresis, and the RNA concentration determined using a NanoDrop 2000 spectrophotometer. Finally, single-stranded cDNAs for each sample were synthesised using a PrimeScript RT Reagent Kit with gDNA Eraser (Takara, Tokyo, Japan).
A partial cDNA fragment of OhAOX was amplified from O. hupensis cDNA using the following specific primers: OhAOX-F, 5′-TGAACTCCAAGCAGAGCGAAT -3′; OhAOX-R, 5′-TGCTGGTTGGGATGAAAACTG-3′. These were designed based on the AOX reference sequence inferred from transcriptomics. Partial cDNA fragments of BgAOX, BaAOX, and BsAOX were amplified from the corresponding snail cDNA with the following specific primers: Bg/Ba/BsAOX-F, 5′-ATGAACAGGGTTTCAATTATTCGAG-3′; Bg/Ba/BsAOX-R, 5′-TTAATGACCAGGTTTGTAAGG-3′. These were designed based on the predicted B. glabrata AOX (XM_013230806.1). Each polymerase chain reaction (PCR) was performed with 25 µL of 2× Phanta Max buffer (Vazyme Biotech, Nanjing, China), 2 µL cDNA template, 1 µL dNTP mix (10 mM each), 1 µL of each primer (10 μM), 1 µL Phanta Max Super-Fidelity DNA polymerase (Vazyme Biotech), and water to a final volume of 100 µl. Amplification was performed under the following conditions: 95 °C for 3 min; followed by 15 cycles of 30 s at 95 °C, 30 s at 62 °C (0.5 °C decrease per cycle), and 3 min at 72 °C; 15 cycles of 30 s at 95 °C, 30 s at 55 °C, and 3 min at 72 °C; and a final extension step at 72 °C for 10 min. Reactions were then checked by running 5 µL product on 1% agarose gel. The products of the successful amplifications were purified from the gel with AxyPrep DNA gel extraction kit (Axygen Biosciences, Union City, CA) and sent to Sangon Biotech (Shanghai, China) for sequencing.
The partial sequences of OhAOX, BgAOX, BaAOX, and BsAOX were blasted with the reference sequences and other mollusc AOXs (https://blast.ncbi.nlm.nih.gov/Blast.cgi?PROGRAM=blastn&PAGE_TYPE=BlastSearch&LINK_LOC=blasthome). Based on the verified 2,909 base pair (bp) partial cDNA of OhAOX and 1,023 bp partial cDNA of Bg/Ba/BsAOX, gene-specific primers in RACE-PCR and specific primers (SP) in genome walking were designed (for details about the primers, see Additional file 1: Table S1). The 5′- and 3′-RACE-Ready cDNAs were synthesised from the total RNA of the corresponding snail by using a Simple Modular Architecture Research Tool (SMART) RACE cDNA Amplification Kit (Clontech, Mountain View, CA). The RACE procedure was performed with touchdown PCR following the manufacturer’s instructions, and amplified products were purified from gels and sequenced. Geneious V8.1.8 [31] was used to complete and identify the combined cDNA sequences based on each partial fragment. BLAST analysis was used to check each combined sequence to ensure complete and unique.
Sequence features, protein structure, and phylogenetic analysis
BLASTP (https://blast.ncbi.nlm.nih.gov/Blast.cgi?PROGRAM=blastp&PAGE_TYPE=BlastSearch&LINK_LOC=blasthome
) was used to confirm the high similarity (37.62–75.42%) between OhAOX/BgAOX/BsAOX/BaAOX and other AOXs from 63 species, including dicotyledonous plants, monocotyledonous plants, fungi, protists, and molluscs (for species, see Additional file 2: Table S2). After alignment in ClustalW (https://www.genome.jp/tools-bin/clustalw) with protein sequences, the .aln file was uploaded to ESPrit3.0 (https://espript.ibcp.fr/ESPript/cgi-bin/ESPript.cgi), to predict the secondary structure of OhAOX, BgAOX, BaAOX, and BsAOX using the Trypanosoma brucei AOX (TbAOX) protein structure as a template. Also, the conserved region analysis, amino acid residue analysis, and post-translational regulation prediction of OhAOX, BgAOX, BaAOX, and BsAOX were conducted by aligning the proteins with that of TbAOX. The best model for the tertiary structure of OhAOX, BgAOX, BaAOX, and BsAOX was evaluated in SWISS-MODEL (Expert Protein Analysis System; https://swissmodel.expasy.org/) with Global Model Quality Estimation and Qualitative Model Energy Analysis.
The complete protein sequences of OhAOX, BgAOX, BaAOX, and BsAOX were aligned using ClustalW, together with those of another 65 AOXs (Additional file 2: Table S2). Thirty of these AOXs were from animals: six species of the phylum Mollusca, eight species of the phylum Cnidaria, four species of the phylum Echinodermata, four species of the phylum Euglenozoa [32–34], one species of the phylum Chordata, two species of the phylum Apicomplexa, one species of the phylum Ciliophora [35], one species of the phylum Choanozoa, and one species of the phylum Brachiopoda. Twenty were from plants: 17 species of the phylum Streptophyta [36–42], and three of the phylum Chlorophyta. Twelve of the AOXs were from fungi: five species of the phylum Basidiomycota and seven of the phylum Ascomycota. Three of the AOXs were from bacterial species of the phylum Proteobacteria. The protein sequences of ribonucleotide reductase from B. glabrata (XP_013082032.1) and O. hupensis (obtained from previous transcriptomic data [18]) were set as outgroups. The phylogenetic trees were then constructed using the maximum likelihood method in Molecular Evolutionary Genetics Analysis (MEGA)X [43] with 1000 bootstrap replicates. The LG + G evolutionary model was the model with the best fit for protein sequence evolution for AOXs, with the default settings based on the Bayesian information criterion.
Niclosamide treatment and collection of specific tissues from O. hupensis
In accordance with previous molluscicidal assays [14, 17, 18], niclosamide was used at a final concentration of 0.1 mg/L. The solution was prepared with 50% wettable powder of niclosamide (WPN) (production license HNP 32280-N0013, product standard no. Q/320623 PAJ 004-2019; Luosen Chemical, Jiangsu, China). Twenty active, mature O. hupensis snails were individually exposed to 80 mL WPN solution, or H2O, in 100-mL flasks. After being separately immersed for 6, 12, 24, and 48 h, the snails were washed with dechlorinated water, and mortality assessed by the mobility of the head and foot muscle (head–foot) under the microscope [44]. All the treatments were performed in parallel with from four to six replicates at each time point, and repeated three times. Mortality data were statistically analysed using ANOVA (SPSS 20.0).
The live snails of each group were dissected individually under a microscope. For RNA and protein extraction, the whole soft bodies were cleaned and stored in liquid nitrogen. In addition, some snails were dissected for the separate collection of the head-foot region and liver-gonad. For histochemical observations, the whole soft body, head-foot region, and liver-gonad of each group were separately fixed in 4% polyformaldehyde and embedded in paraffin. Some of these parts were individually embedded with optimum cutting temperature compound (Sakura, Torrance, USA), snap-frozen in liquid nitrogen, and stored at − 80 °C.
Dynamic quantification and tissue localisation of OhAOX mRNA, and protein levels after WPN treatment
Expression profile of OhAOX in different tissues determined by quantitative real-time PCR
At time points from 0 to 48 h after WPN treatment, the single-stranded cDNAs from the different tissues, including the whole soft body, head-foot region, and liver-gonad, were subjected to random-hexamer priming and synthesised from the corresponding extracted RNA using a PrimeScript RT Reagent Kit with gDNA Eraser (Takara). Before the quantitative real-time PCR (qPCR) was carried out, semi-quantitative PCR was performed to confirm the presence of the OhAOX mRNA in the tissue and the specificity of the primers in the amplification (for primers, see Additional file 3: Table S3). Specific primers were synthesised based on the verified OhAOX sequence. At the same time, a partial sequence of the 18S ribosomal RNA gene of O. hupensis (AF367667) was also used to monitor the transcription as an internal control for normalisation. For each sample, qPCR was performed in triplicate in a 20-μL reaction volume, using TB Green Premix Ex Taq II (Tli RNaseH Plus; Takara) and a Bio-Rad CFX96 thermal cycler, with the following protocol: 94 °C for 40 s, followed by 40 cycles of 94 °C for 5 s, 60 °C for 40 s, and 72 °C for 20 s, and melt curves analysis at 95 °C for 15 s, 65 °C for 10 s, and 95 °C for 10 s. The expression profiles of OhAOX in the different tissues after WPN treatment were normalised to the expression of the 18S gene using the comparative delta-delta Ct method [45] after the amplification efficiency was adjusted to 1.87–2.26. The results were statistically analysed for at least six samples per group using one-way ANOVA (among groups) or independent t-test analysis (between groups) in SPSS 20.0.
Localisation of OhAOX in tissues by in situ hybridisation
The head-foot and liver-gonad sections embedded and frozen with optimum cutting temperature compound were collected at 0–48 h post-treatment and cut into 5- to 8-μm-thick slices for in situ hybridisation with the 5'-digoxigenin-labelled RNA probes (5′-ACATACCAGATGCTGAACAGGGTCACAAACACAC-3′), to observe the localisation of OhAOX. Each slide was fixed in RNAase-free 4% polyformaldehyde for 10 min, followed by three washes with phosphate-buffered saline (PBS) for 5 min, and then incubated in 20 µg/mL proteinase K for 10 min at 37 °C for antigen retrieval. After incubation in pre-hybridisation solution at 37 °C for 1 h, the diluted probes (200 ng/mL) were added to the slides for hybridisation at 42 ℃ overnight, followed by stringent washing with saline sodium citrate and blocking with 8% rabbit serum (Roche, Basel, Switzerland) at room temperature for 30 min. The blocking buffer was then drained off, the diluted (1:400) anti-digoxigenin-AP (Jackson, Lancaster, USA) was added, and the samples incubated at 37 ℃ for 50 min. After applying Tris-buffered saline (TBS) washes, 5-bromo-4-chloro-3-indolyl phosphate/nitro blue tetrazolium was added to each slide; the slides were incubated for an optimal time to develop a purple-blue colour, then rinsed in distilled water, air dried, and sealed. The slides were observed under an Olympus CX431 microscope. The entire area of tissue on each slide was photographed under 100× magnification. The integrated optical density (IOD) and area were calculated for each photo with Image Pro-Plus [46] to quantify the OhAOX mRNA in the different tissues. Total IOD/total area was calculated for each slide, and the staining data statistically assessed using one-way ANOVA (among groups) or independent t-test analysis (between groups) in SPSS 20.0.
Expression of recombinant OhAOX protein and specificity of anti-OhAOX serum
A pair of primers that included SalI and NotI restriction sites and protective bases were designed based on the verified complete cDNA sequence of OhAOX, the forward primer 5′-GCGTCGACTCATGTCGTTGTGTTTTAGTGCGT-3′ and the reverse primer 5′-ATAAGAATGCGGCCGCCTGTCCAGGCTTGTAAGGGTTA-3′. PCR was performed to amplify the complete open reading frame (ORF) of OhAOX from O. hupensis cDNA, and the purified PCR product was digested and ligated into pGEX-4 T-1 plasmid DNA. The resulting pGEX-4 T-1-OhAOX plasmid was then transformed into BL21 (DE3) competent cells, and positive clones were selected by PCR and sequencing. The expression of the recombinant protein was induced by adding isopropyl-β-thiogalactopyranoside to the culture medium to a final concentration of 0.2 mM and the expressed protein was purified using glutathione agarose beads (Smart-Lifesciences, Changzhou, China). After successful cleavage with thrombin, affinity GST tags were separated from the recombinant protein, and recombinant OhAOX protein was further purified with sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE), gel extraction and dialysis recycling. The concentration of extracted purified OhAOX protein was calculated by the bicinchoninic acid protein assay.
An antiserum to the recombinant OhAOX protein was generated by subcutaneous injection of five laboratory Kunming mice with 30 µg purified OhAOX protein per mouse in the back and abdomen, followed by three boosts with the same dose of protein at 2-week intervals. The mice were sacrificed, and sera were collected after the antiserum titer was confirmed by enzyme-linked immunosorbent assay.
The total protein extracted from the whole soft body of O. hupensis by an SDS lysis method (Beyotime, Shanghai, China) was screened by western blot to evaluate the specificity of the anti-OhAOX serum. Briefly, 60 µg of snail crude extract was loaded and separated by SDS-PAGE, and 0.2 µg purified recombinant OhAOX protein was loaded at the same time as the positive control. The separated proteins in the gel were then electrophoretically transferred to a polyvinylidene fluoride membrane at 200 mA for 2 h at 4 °C, and then blocked with 5% skim milk in Tris-buffered saline containing 0.05% TWEEN 20 for 2 h. Next, the membrane was probed with 1:300 diluted anti-OhAOX serum or PBS immune serum (primary antibody) at 4 °C overnight. After washing three times with PBS containing 0.05% Tween 20, the membrane was probed with 1:10,000 diluted horseradish peroxidase (HRP)-conjugated anti-mouse immunoglobulin G (IgG) (ABclonal, Wuhan, China) (secondary antibody) in PBS containing 0.05% Tween 20 for 2 h. The membrane was then washed three times and visualised using an enhanced chemiluminescence kit (Epizyme, Shanghai, China). Immunoblot images were acquired with the G:Box Chemi Imaging System analyser (Labgene, Châtel-Saint Denis, Switzerland).
Dynamic expression profile of OhAOX protein by western blot
The dynamic expression profiles of OhAOX protein in O. hupensis snails were evaluated by a semi-quantified western blot. Briefly, the frozen soft bodies of the snails at 0–48 h post-treatment were homogenised separately in SDS lysis buffer (Beyotime, Shanghai, China), and 100 µg of extracted protein of each sample was electrophoresed and transferred to a polyvinylidene fluoride membrane. The membrane was blocked with 5% skim milk, incubated with 1:500 diluted mice serum, then washed and incubated with 1:10,000 diluted HRP-conjugated anti-mouse IgG. α-Tubulin was set as the control by using 1:10,000 diluted anti-α-tubulin monoclonal antibody (Proteintech, Manchester, UK) as the first antibody. After visualisation using the enhanced chemiluminescence method, the grey values of OhAOX-positive and α-tubulin-positive bands were analysed in Image J. The relative quantification of OhAOX protein was calculated using the grey values, which were calibrated using the α-tubulin grey value as the internal control. The statistical analysis of the quantity of OhAOX protein among the different groups was performed using one-way ANOVA in SPSS20.0
Immunohistochemical localisation of OhAOX
The tissue localisation of OhAOX protein was performed using immunohistochemical staining. Paraffin sections (3 µm) of head-foot and liver-gonad were treated with xylene (two steps of 100% for 5 min for each) and rehydrated in an alcohol gradient (100%, 100%, 95%, 80%, and 70%) and PBS. After heat-induced epitope retrieval and blocking with 10% sheep serum (Roche, Basel, Switzerland) for 1 h at 37 ℃, the slides were incubated with 1:50 diluted mouse anti-OhAOX serum, PBS immune serum, and blocking sheep serum, respectively, at 4 ℃ overnight. The slides were washed three times for 5 min per wash in PBS and incubated for 1 h at 37 ℃ with 1:100 diluted HRP-conjugated goat anti-mouse IgG (ABclonal, Wuhan, China). Immunoreactivity was developed using 3,3′-diaminobenzidine. The slides were washed in tap water and counterstained with haematoxylin, then dehydrated in ethanol and hyalinised in xylene, air dried, and sealed. The sections were examined and photographed using an Olympus light microscope, and the brown-yellow regions were identified as OhAOX positive. Five visually different areas were randomly selected for each slide at a magnification of 400×, and the IOD and area of each image were measured using Image Pro-Plus [46]. Total IOD (sum)/total area (sum) was used for the quantitative analysis of OhAOX protein among groups, and the statistical analysis was performed using one-way ANOVA in SPSS 20.0.
In addition, the same sections as used above were also stained using the conventional hematoxylin–eosin method as the reference for the immunohistochemical staining to observe the positive sites and cell types.
Expression profile of genes in the mitochondrial respiratory complex, and the production of ROS in snails after WPN treatment
Expression profile of genes in the mitochondrial respiratory complex determined by qPCR
The single-stranded cDNAs from the whole soft body at 0–48 h post-treatment were prepared from the same sample used for OhAOX qPCR. To assess the response of other genes in the mitochondrial respiratory pathway, the expression profiles of several genes, including those encoding nicotinamide adenine dinucleotide (NADH) dehydrogenase, SDH, cytochrome c reductase (CCR), CCO, and ATP synthase, were investigated by qPCR using the same procedure and system as used for OhAOX. Primers were designed based on previously determined transcriptomic sequences [18] (for primers, see Additional file 3: Table S3). The expression profile of each target gene was normalised to the expression of the 18S gene using the comparative delta-delta Ct method [45] after the amplification efficiency of the genes was adjusted to 1.87–2.38. The results were statistically analysed using one-way ANOVA (among groups) or independent t-test analysis (between groups) in SPSS 20.0 software.
ROS production
The fresh soft body, head-foot region, and liver-gonad of the snails at 0–48 h post-treatment were collected individually, five biological replicates were prepared for the same tissue at each time point and the experiment repeated three times. To each sample was added 150 µL PBS, followed by phenylmethylsulfonyl fluoride to a final concentration of 0.5 mM; the mixture was then ground on ice, and centrifuged at 12,000 g at 4 ℃ for 20 min. The supernatant was collected, and the amount of extracted protein was calibrated using bovine serum albumin (BSA).
The 10–15 µL volume of supernatant per individual sample (10 µL for a liver-gonad sample and 15 µL for a soft body or head-foot sample) and 60–75 µL PBS (60 µL for a soft body sample, 65 µL for a liver-gonad sample, and 75 µL for a head-foot sample) were added to each well of the black 96-well plate. The 1:50 diluted 2',7'-dichlorodihydrofluorescein diacetate (DCFH-DA) in PBS (10 µL for a head-foot sample and 25 µL for a soft body or liver-gonad sample) was added to each well to a final concentration of 25 µM, followed by incubation at 37 ℃ for 30 min in the dark. The intensity of DCF fluorescence was evaluated at 488 nm and 525 nm to indicate oxidative stress. The ROS in each sample was calibrated using the amount the individual extracted protein and statistically analysed among the different groups using one-way ANOVA.
Molluscicidal assay with niclosamide and AOX inhibitor
The qPCR showed that the quantity of OhAOX in the snails had increased significantly by as early as 6 h after WPN treatment. In this assay, salicylhydroxamic acid (SHAM) (CAS, 89-73-6; product no. S817724; Shanghai Macklin Biochemical, China), a specific inhibitor of AOX enzyme activity, was added together with WPN at 0 or at 6 h to observe the inhibition of the molluscicidal effect of niclosamide by AOX. Using the same experimental design as described above for the niclosamide treatment, the snails were randomly divided into the following groups: WPN group, H2O group, methanol group (control solvent for SHAM), SHAM 0 h group (SHAM was added at 0 h), SHAM 6 h group (SHAM was added at 6 h), WPN + SHAM 0 h group (SHAM was added at 0 h post-WPN treatment), and WPN + SHAM 6 h group (SHAM was added at 6 h post-WPN treatment). The final concentrations of WPN and SHAM were 0.1 mg/L and 0.4 mM, respectively, and the concentration of methanol (in the methanol group and the SHAM-containing groups) was 0.08%. Three biological replicates were set up in parallel for each group. All treatments were performed in triplicate. Snail mortality was calculated as described above, at 0, 6, 12, 24, and 48 h after treatment, and the data statistically analysed with the chi-square test and the chi-square test for Trend in SPSS 20.0.
The results of this assay suggested that SHAM could significantly enhance the molluscicidal effect of niclosamide. The dosage of WPN was reduced in the following experiment to investigate the potential ability of AOX to diminish the molluscicidal effect of niclosamide. With the same experimental design as described above for the niclosamide treatment, the snails were randomly divided into 11 groups: H2O group, SHAM group, methanol group, 0.1 mg/L WPN group, 0.08 mg/L WPN group, 0.06 mg/L WPN group, 0.04 mg/L WPN group, 0.1 mg/L WPN + SHAM group, 0.08 mg/L WPN + SHAM group, 0.06 mg/L WPN + SHAM group, 0.04 mg/L WPN + SHAM group. The final concentration of SHAM in all relevant group was 0.4 mM, and the solutions were added in each group at 0 h. All the treatments were performed in triplicate. Snail mortality was calculated for each group at 0, 6, 12, 24, and 48 h after treatment and the data statistically analysed with the chi-square test and a chi-square test for Trend in SPSS 20.0.
Results
Identification and sequence characteristics of snail AOXs
OhAOX cDNA was found to comprise 4412 bp through cDNA amplification and sequencing, with a 1266-bp complete ORF, but varied with respect to a six base (CTAAAC) deletion encoding 419 or 421 amino acids (aa). The identified cDNAs of BgAOX, BaAOX and BsAOX were 2003 bp, 1974 bp, and 1238 bp in length, with complete ORFs of 1023 bp, 1029 bp, and 1029 bp encoding 340 aa, 342 aa, and 342 aa, respectively. These confirmed AOXs were deposited in GenBank under accession numbers MZ436164-MZ436167. The OhAOX cDNA had the highest similarity, 74.65%, with the predicted Branchiostoma belcheri AOX mRNA (XM_019758882.1). BgAOX, BaAOX, and BsAOX had the highest similarity, 94.69–97.41%, with the predicted B. glabrata AOX transcript variant X1 (XM_013230806.1), derived from the B. glabrata genomic sequence. Identity between the predicted OhAOX protein sequence and those of BgAOX, BaAOX, and BsAOX was 63.12%, 57.35%, and 57.65%, respectively.
All four AOXs were identified as members of the ferritin-like superfamily through Conserved Domain Search and SMART analysis, with conserved motifs located at 194–408 aa for OhAOX, 113–327 aa for BgAOX, and 115–329 aa for BaAOX and BsAOX (Additional file 4: Fig. S1). OhAOX contained two transmembrane helixes (TMhelix) located at 248–270 aa and 306–328 aa, while none of the three Biomphalaria AOXs had a TMhelix (Additional file 4: Fig. S1). The four AOXs had no signal peptide and were deduced to be unstable proteins with a high aliphatic index (77.60–80.67). Ten α-helices and six conserved diiron-binding motifs were identified in each AOX, as in TbAOX (Additional file 5: Fig. S2). The NP-[YF]-XPG-[KQE] motif, an animal AOX-specific motif, was identified in the C-terminal of the four snail AOXs (Additional file 5: Fig. S2).
The tertiary structure of TbAOX was used as the template to build the homology model, in which OhAOX, BgAOX, BaAOX, and BsAOX showed sequence identity of 49.58%, 49.57%, 49.36%, and 48.94%, respectively, with Global Model Quality Estimation values of 0.41, 0.50, 0.50, and 0.50, and Qualitative Model Energy Analysis values of − 3.89, − 2.62, − 2.67, and − 2.61, respectively. Similar to TbAOX, each snail AOX was identified as a homodimer, with a diiron core in each monomer surrounded by a four-helix bundle and ligated by four glutamic acid and two histidine residues (Additional file 6: Fig. S3), which were considered the active sites of the AOX.
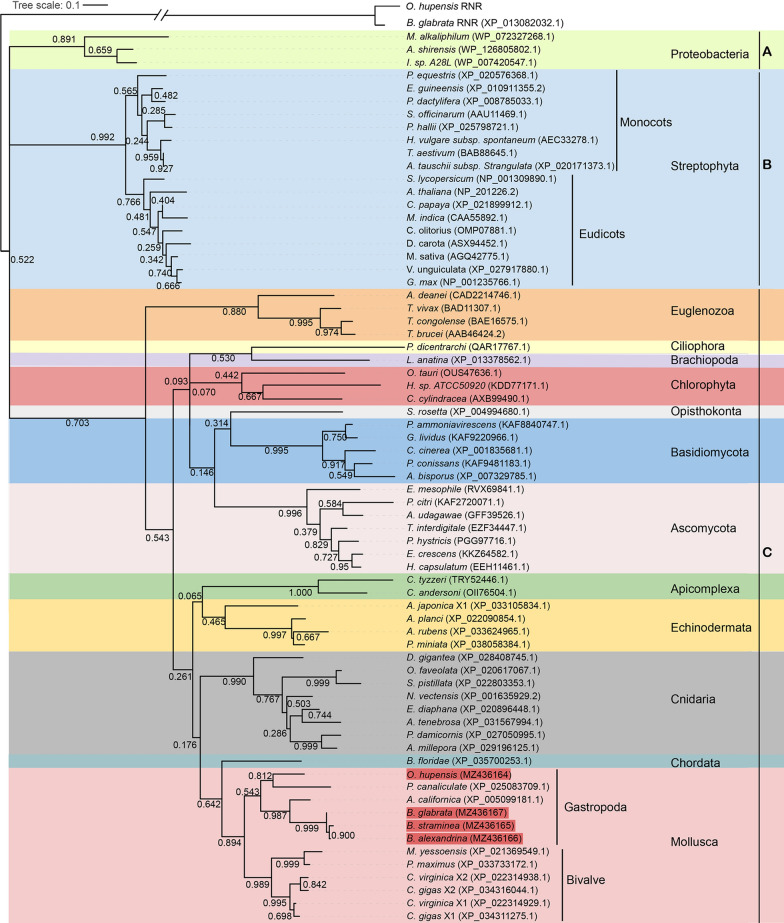
Phylogenetic analysis of snail AOXs
Sixty-nine AOXs were separated into three main clades (Fig. 1). Clade A was a bacterial AOX group consisting of AOXs from three species in the phylum Proteobacteria. Clade B was a Viridiplantae group containing all AOXs from 17 species of Streptophyta, with a high supporting rate but not including the Chlorophyta AOX. Clade B was further separated into monocot and eudicot groups (Fig. 1). The other 49 AOXs from metazoans, protists, fungae, and chlorophytes were clustered into the giant clade C. All AOXs in clade C clustered together well at the level of phylum, with high supporting rates (Fig. 1). The Mollusca group diverged distinctly into the Gastropoda and Bivalvia AOX branches. The AOX of O. hupensis, which is in the order Littorinimorpha, clustered closely together with the AOX of P. canaliculate, which is in the Architaenioglossa, while the three AOXs of the Biomphalaria, which are in the order Basommatophora, clustered closely together with the AOX of Aplysia californica, a species in the family Aplysiidae. Interestingly, the Mollusca AOX group first clustered with the AOX of B. floridae, which is in the phylum Chordata, and then with the Apicomplexa AOX to form one of the main clades in the Metazoa. It is worth mentioning that the AOX of Lingula anatina, of the metazoan phylum Brachiopoda, did not cluster together with other metazoan AOXs but clustered closely with the AOX of Philasterides dicentrarchi, which is in the phylum Ciliophora, kingdom Protista, and then with the AOX of Chlorophyta, which is in the kingdom Viridiplantae. In addition, the AOX of the protist Salpingoeca rosetta, which is in the phylum Choanozoa, clustered with the AOXs of the fungal phylum Basidiomycota at a low support rate, and then with the AOXs of Ascomycota to form the main fungal group.
Fig. 1.
Phylogenetic tree (maximum likelihood; ML) of alternative oxidases (AOXs) based on protein sequences. The number beside each node shows the ML support rate (bootstrap value). The four snail AOXs identified in this study are highlighted in red (in the Gastropoda branch)
Dynamic expression and localisation of OhAOX mRNA and OhAOX protein after WPN treatment
Up-regulation of OhAOX mRNA and OhAOX protein
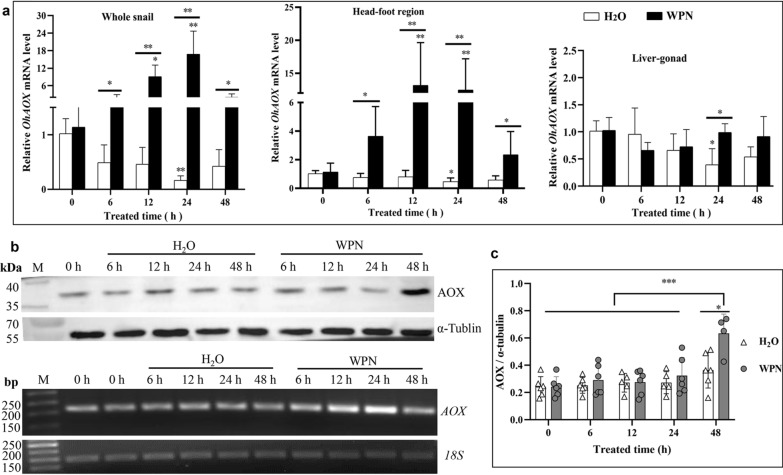
The quantity of OhAOX mRNA in the whole snail increased immediately and thence continuously after WPN treatment (Fig. 2a), reaching 8.04 times its value at 0 h after 12 h (P < 0.01). The highest increase was recorded at 24 h (14.75 times that at 0 h, P < 0.01), after which the level dropped, and at 48 h was almost the same as at 0 h (1.44 times that at 0 h, P > 0.05). Meanwhile, OhAOX mRNA expression decreased continuously from 0 h in H2O-treated snails, and was significantly lower at 24 h post-treatment (0.16 times that at 0 h, P < 0.05) (Fig. 2a). Moreover, the quantity of OhAOX mRNA in WPN-treated snails was significantly higher than that in H2O-treated snails at all time points, i.e. 102.39 times higher at 24 h (P < 0.01), at which point it had reached its highest level, and 3.89 times higher at 48 h (P < 0.05) (Fig. 2a).
Fig. 2a–c.
Dynamic expression profiles of Oncomelania hupensis AOX (OhAOX) messenger RNA (mRNA) and OhAOX protein in O. hupensis snails under wettable powder of niclosamide (WPN) stress. a Expression profiles of OhAOX in the whole snail, head and foot muscle (head-foot) region, and liver and gonad (liver-gonad) region after WPN treatment. Asterisks indicate significant difference (*P < 0.05, **P < 0.01) between time points and 0 h within the same treatment; underlined asterisks indicate significant difference between WPN- and H2O-treated snails at the same time point. b Expression of OhAOX protein (upper panel) in the whole snail 0–48 h after WPN and H2O treatment. The OhAOX mRNA profile calculated using quantitative real-time PCR (qPCR) is shown in the lower panel (and corresponds with the whole snail data in a). c Statistical analysis of OhAOX protein expression in the western blot (b); *P < 0.05, ***P < 0.001. bp Base pairs
The dynamic expression profiles of OhAOX mRNA in the different tissues, including those of the head-foot region and liver-gonad, were also calculated 0–48 h after WPN treatment. OhAOX mRNA in the head-foot region showed a similar trend to that of the whole snail. It increased after WPN treatment and reached its highest level at 12 h (11.52 times that at 0 h, P < 0.01), then decreased gradually until 48 h, at which point it had returned to a level closer to that considered normal (2.06 times that at 0 h, P > 0.05). For the snails that had been immersed in H2O, OhAOX mRNA in the head-foot region decreased slightly, showing a lower level at 24 h (0.46 times that at 0 h, P < 0.05) (Fig. 2a). In summary, OhAOX mRNA in the head-foot region was significantly higher after WPN treatment (4.86–26.87 times greater, P < 0.05 or 0.01) than after H2O treatment, at each time point (Fig. 2a). The expression of OhAOX mRNA in the liver-gonad of snails under WPN stress differed from that in the whole snail and the head-foot region, as no significant difference was observed from 0 to 48 h (P > 0.05) (Fig. 2a). The expression of OhAOX mRNA in the liver-gonad showed no significant difference between the WPN and H2O treatments at most time points, except at 24 h, when it was 2.52 times higher in the WPN-treated snails than in the H2O-treated snails (P < 0.05) (Fig. 2a).
After the recombinant OhAOX protein had been purified, cleaved, and analysed using SDS-PAGE (Additional file 7: Fig. S4a, b, c), its mass was verified to be the expected 47 kDa. The existence and specificity of the polyclonal antibody in mouse anti-OhAOX serum were proven by a single band that reacted with the recombinant OhAOX protein, or crude protein, of the whole snail, in the western blot analysis (Additional file 7: Fig. S4d). The natural OhAOX from the whole snail extract was smaller than the recombinant OhAOX protein (Additional file 7: Fig. S4d), which could be explained by the transmembrane transfer of natural OhAOX from the nucleus to the mitochondrion.
The dynamic expression of the OhAOX protein in the snails after WPN treatment was further investigated and quantified using α-tubulin as the internal control (Fig. 2b). The intensity of the specific band showed that the expression of OhAOX protein increased slowly after WPN treatment, and that the highest level of the protein was reached at 48 h (Fig. 2b), which was statistically significantly higher than that at 0 h (2.61 times that at 0 h, P < 0.05) (Fig. 2c), while no significant difference was observed for snails 0–48 h after H2O treatment (Fig. 2b, c). It was evident that, under WPN stress, the increase of OhAOX protein was hysteretic to that of OhAOX mRNA (Fig. 2a, b), which increased to its highest level at 24 h after WPN treatment.
Tissue expression of OhAOX mRNA and OhAOX protein
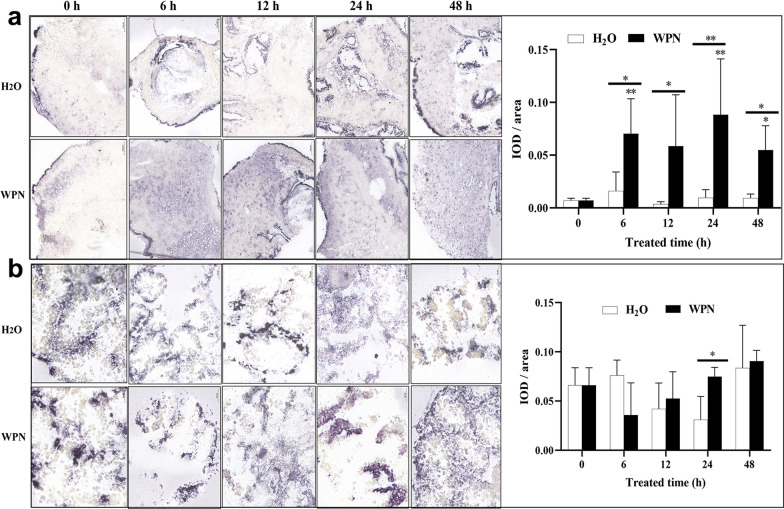
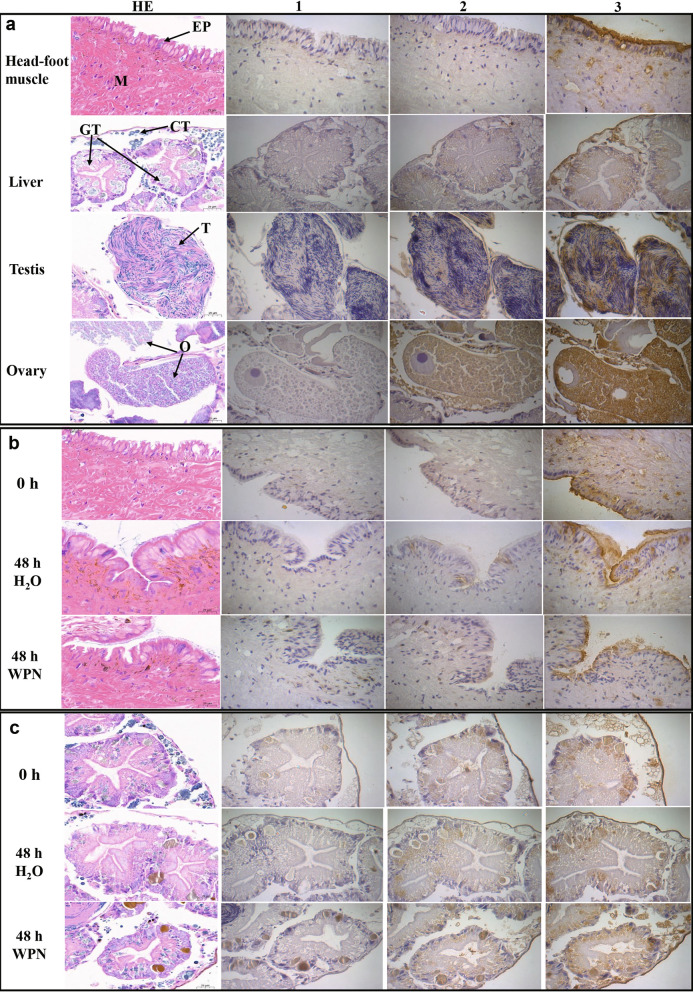
In untreated snails (0 h), the purple-blue colour indicating the presence of OhAOX mRNA was mainly distributed in the pellicle, liver, and the head-foot region, but was limited in the muscle (Additional file 8: Fig. S5); it also indicated positive expression in the testis. After WPN treatment, the head-foot muscle showed more distinct, positive signals at 6 h than at 0 h, and continued to show strong positive signals thereafter (Fig. 3a). The change in intensity of the positive signal showed a similar trend to the change in the expression profile of the protein in the qPCR, as it increased significantly 6 h after WPN treatment, when it was 9.99 times higher than that at 0 h, and was high from 12 to 48 h (7.69–13.33 times that at 0 h) (Fig. 3a). Meanwhile, in the H2O-treated snails, a positive signal only appeared sporadically in the head-foot region, and no significant difference was seen from 0 to 48 h (Fig. 3a). In summary, the positive signal of the head-foot under WPN stress was significantly stronger than that after H2O treatment at all time points from 6 to 48 h (Fig. 3a).
Fig. 3a, b.
Tissue expression of OhAOX mRNA in O. hupensis after WPN treatment following in situ hybridisation. a Tissue distribution and statistical evaluation of OhAOX mRNA in the muscle of the head-foot region. b Tissue distribution and statistical evaluation of OhAOX mRNA in the liver. The purple-blue area was identified as a positive signal of OhAOX mRNA. Asterisks indicate statistically significant difference (*P < 0.05, **P < 0.01) in the quantity of OhAOX mRNA between the given time point and 0 h under the same treatment. Underlined asterisks indicate the level of significant difference between WPN- and H2O-treated groups at the same time point. IOD Integrated optical density; for other abbreviations, see Fig. 2
The positive signal of OhAOX mRNA in the liver tissue showed no significant difference (P < 0.05) from 0 to 48 h after WPN or H2O treatment (Fig. 3b), except at 24 h, when it was 2.40 times stronger in the WPN-treated snails than in the H2O-treated snails (Fig. 3b).
The cellular localisation of OhAOX protein in different tissues was further investigated with an immunohistochemical assay. OhAOX protein was mainly located in the epithelium of the head-foot region, the reticular connective tissue in the liver-gonad, and the genital gland of untreated snails (0 h) (Fig. 4a). The positive signals in the gonads were mainly distributed in the testicular substrate and ovarian stroma, while there was no positive staining in the cytoplasm of immature and mature oocytes (Fig. 4a). In addition, a small amount of brown-yellow staining was observed in the muscle tissue of the head-foot region and the hepatic ducts (Fig. 4a). In accordance with the high level of OhAOX in snails at 48 h after WPN treatment in the western blot, the localisation of OhAOX protein at 48 h post-treatment was further investigated. The positive signals in the head-foot region were distributed mainly in the epithelium, with a few appearing in the muscle (Fig. 4b), but no significant difference (P > 0.05) was observed between 48 and 0 h in the H2O-treated or in the WPN-treated snails. Also, no significant difference was found in the head-foot region at 48 h between the H2O- and WPN-treated snails (Fig. 4b). In the liver, after WPN or H2O treatment, the positive signals were mainly observed in the same sites as those at 0 h, but the intensity increased significantly at 48 h after WPN treatment (P < 0.05) (Fig. 4c).
Fig. 4a, b.
Tissue distribution of OhAOX protein determined by immunohistochemistry. a Different tissues of the untreated snails. b Head-foot muscle at 48 h after WPN treatment. c Liver at 48 h after WPN treatment. 1 Blank controls, 2 phosphate-buffered saline (PBS) immune serum, 3 anti-OhAOX serum, HE hematoxylin–eosin staining, M muscle, EP epithelium, GT gland tissue, CT connective tissue, T testis, O ovary. The regions stained brown-yellow indicate the distribution of OhAOX protein
Expression profiles of genes involved in the mitochondrial respiratory complex, and ROS production after WPN treatment of snails
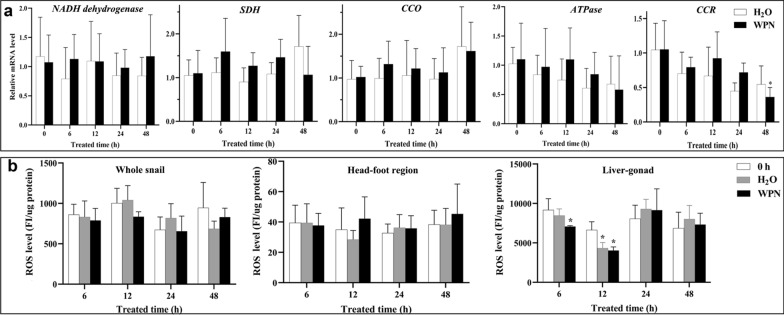
For most of the genes involved in the mitochondrial respiratory complex (MRC) investigated here, including those encoding NADH dehydrogenase, SDH, CCO, and ATPase, no significant difference in the quantity of mRNA was observed between WPN-treated and H2O-treated snails at any time point. Also, no significant difference was recorded at any time point from 0 to 48 h within the same treatment (Fig. 5a). The expression profile of CCR mRNA had decreased to its lowest level (0.34 times that at 0 h) at 48 h after WPN treatment, which was significantly lower than that at 0 h (P < 0.05) (Fig. 5a). However, the expression of CCR in H2O-treated snails showed no difference from 0 to 48 h (P > 0.05).
Fig. 5.
Expression profiles of genes involved in the mitochondrial respiratory complex (MRC), and reactive oxygen species (ROS) production in O. hupensis after WPN treatment. a Dynamic expression profile of genes coding for NADH dehydrogenase (NADH), succinic dehydrogenase (SDH), cytochrome C oxidase (CCO), ATPase, and cytochrome c reductase (CCR) in the whole snail determined using qPCR. b ROS production in the whole snail, head-foot region, and liver-gonad determined using the 2′,7′-dichlorodihydrofluorescein diacetate (DCFH-DA) method. Asterisk indicates statistically significant difference (*P < 0.05) when the level is compared with that at 0 h for the same tissue. For other abbreviations, see Fig. 2
The amount of ROS in the whole snail, head-foot region, and liver-gonad was calculated using the DCFH-DA assay. There was no significant difference in the level of ROS at the times points from 0 to 48 h (P > 0.05) in the whole snail and head-foot region after WPN or H2O treatment (Fig. 5b). Most of the ROS were produced in the liver-gonad (Fig. 5b), in which there was a significant decrease in levels at 6 h after WPN treatment (0.77 times that at 0 h, P < 0.05), and further decrease until ROS were at their lowest level at 12 h (0.61 times, P < 0.05). The levels had returned to normal at 24 h (1.07–1.14 times that at 0 h, P > 0.05) (Fig. 5b). The level of ROS in the liver-gonad fluctuated after H2O treatment, but no significant difference was shown for most time points (P > 0.05) when comparing the levels to that at 0 h, except at 12 h (0.65 times that at 0 h, P < 0.05) (Fig. 5b).
Inhibition of OhAOX activity significantly enhanced the molluscicidal effect of niclosamide
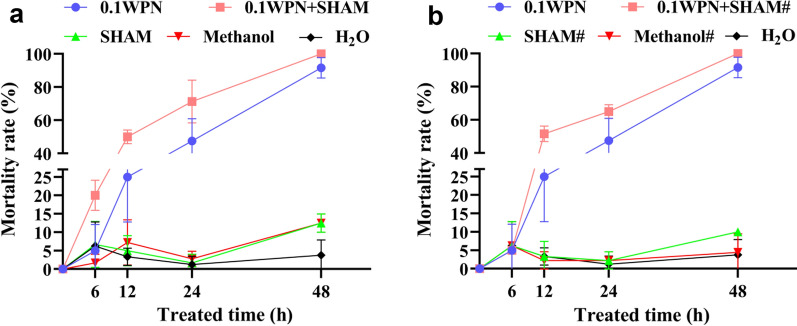
The chi-square test for Trend was used to compare the mortality rates of snails, treated with the same solution, at different time points. The mortality rate of snails in the H2O group, SHAM group, methanol group, SHAM# group (where the hash symbol indicates that the reagent was added at 6 h), and methanol# group showed no significant difference from 6 to 48 h (P > 0.05) (Additional file 9: Table S4) (Fig. 6). In contrast, the mortality rates of snails in the WPN group, WPN + SHAM group, and WPN + SHAM# groups showed highly significant differences (P < 0.001) at time points from 6 to 48 h (Additional file 9: Table S4). The molluscicidal effect gradually increased with prolonged exposure of the snails to WPN (Fig. 6). Snail mortality significantly increased at 6 h in the WPN + SHAM group (by 20.00%, P < 0.05) (Fig. 6a) and at 12 h in the WPN group (by 25.00%, P < 0.05), and in the WPN + SHAM# group (by 51.67%, P < 0.05) (Additional file 9: Table S4) (Fig. 6b) compared to their mortality at 0 h (0.00%). In addition, the WPN + SHAM group (snails exposed to SHAM and WPN starting from 0 h) showed a highly significant mortality rate compared to the WPN group at every time point from 6 to 48 h (Additional file 9: Table S4) (Fig. 6a). Similarly, the WPN + SHAM# group (snails exposed to SHAM at 6 h after exposure to WPN) showed a significantly higher mortality rate than the WPN group at every time point after 12 h (P < 0.05) (Additional file 9: Table S4) (Fig. 6b).
Fig. 6a, b.
Dynamic mortality rate of O. hupensis after WPN treatment with the addition of salicylhydroxamic acid (SHAM). a SHAM added together with WPN at 0 h. b SHAM added at 6 h after WPN treatment. 0.1WPN Niclosamide at 0.1 mg/L, SHAM SHAM at 0.4 mM, 0.1WPN–SHAM niclosamide at 0.1 mg/L and SHAM at 0.4 mM (added at the same time), Methanol methanol at 0.08% (control solvent for SHAM), hash symbol indicates that the corresponding reagent was added at 6 h
Niclosamide was also applied at several lower concentrations together with SHAM at 0 h to evaluate the impact of AOX inhibition on the molluscicidal effect of niclosamide. The mortality rates of the 0.04–0.1 mg/L WPN groups and of the 0.04–0.1 mg/L WPN + SHAM groups were examined by a chi-square test for Trend. This showed that the molluscicidal effect increased with niclosamide concentration at all time points from 6 to 48 h, except for the WPN groups at 6 h (Table 1; Fig. 7).
Table 1.
Mortality rate (%) of snails after wettable powder of niclosamide (WPN) treatment with or without the alternative oxidase (AOX) inhibitor salicylhydroxamic acid (SHAM)
| Group | 6 h | 12 h | 24 h | 48 h | χ2 | P |
|---|---|---|---|---|---|---|
| H2O | 5.00 ± 7.07 | 3.33 ± 2.36 | 1.67 ± 2.36 | 5.33 ± 4.11 | 0.247 | 0.684 |
| Methanol | 1.67 ± 2.36 | 7.22 ± 6.14 | 2.78 ± 2.08 | 12.50 ± 2.50 | 3.761 | 0.056 |
| SHAM | 6.67 ± 6.24 | 5.00 ± 4.08 | 1.67 ± 2.36 | 12.50 ± 2.50 | 0.751 | 0.415 |
| 0.04 mg/L WPN | 0.00 ± 0.00 | 0.00 ± 0.00 | 1.25 ± 2.17 | 10.89 ± 11.69 a, b | 14.656 | 0.000 |
| 0.06 mg/L WPN | 1.25 ± 2.17 | 1.25 ± 2.17 | 8.75 ± 4.15 | 32.50 ± 10.90* a, b, c | 40.815 | 0.000 |
| 0.08 mg/L WPN | 2.50 ± 2.50 | 2.50 ± 2.50 | 18.57 ± 7.49* a, b | 60.24 ± 13.12* a, b, c | 88.298 | 0.000 |
| 0.1 mg/L WPN | 5.24 ± 0.34 | 32.21 ± 12.66* a | 49.58 ± 9.60* a | 91.67 ± 8.03* a, b, c | 144.024 | 0.000 |
| 0.04 mg/L WPN + SHAM | 0.00 ± 0.00 | 3.67 ± 2.62 d | 34.90 ± 3.74* a, b | 91.72 ± 6.02* a, b, c | 216.473 | 0.000 |
| 0.06 mg/L WPN + SHAM | 2.50 ± 2.50 | 7.32 ± 4.02 d | 77.50 ± 5.59* a, b, d | 95.00 ± 3.54* a, b, c | 194.708 | 0.000 |
| 0.08 mg/L WPN + SHAM | 11.25 ± 4.15 | 34.29 ± 12.05* a | 82.50 ± 5.59* a, b, d | 100.00 ± 0.00* a, b, c, d | 161.253 | 0.000 |
| 0.1 mg/L WPN + SHAM | 19.29 ± 3.75* d | 46.68 ± 6.74* ad | 87.32 ± 9.17* a, b, d | 100.00 ± 0.00* a, b, c, d | 189.074 | 0.000 |
Lowercase letters indicate the following: a, mortality at that time point was significantly higher than that at 6 h for the same group (P < 0.05); b, mortality at that time point was significantly higher than that at 12 h for the same group (P < 0.05); c, mortality at that time point was significantly higher than that at 24 h, for the same group (P < 0.05); d, significant difference in mortality between the treatment group and the 0.1 WPN group at that time point (P < 0.05)
* P < 0.05 (significant difference in mortality between that treatment group and the H2O group at that time point)
Fig. 7.
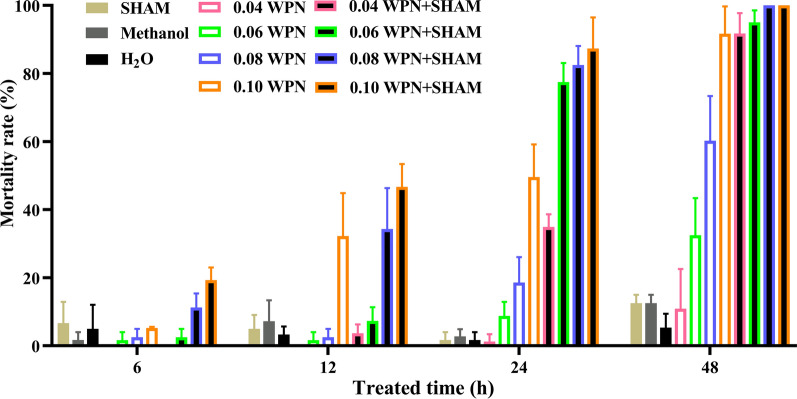
Mortality rate of O. hupensis treated with different concentrations of niclosamide with and without inhibition of OhAOX activity through the addition of SHAM. For abbreviations, see Figs. 2 and 6
Compared to the H2O group, the mortality rate was significantly higher in the 0.1 mg/L WPN + SHAM group at 6 h (P < 0.05) (Table 1), while none of the other groups showed a significant difference. The 0.1 mg/L WPN + SHAM group, 0.1 mg/L WPN group, and 0.08 mg/L WPN + SHAM group showed significantly higher mortalities than the H2O group at 12 h (P < 0.05) (Table 1). Furthermore, the 0.04–0.1 mg/L WPN + SHAM and 0.08–0.1 mg/L WPN groups showed significantly higher mortalities than that of the H2O group at 24 h (P < 0.05). All the groups showed higher mortality rates than the H2O group at 48 h (P < 0.05), except the 0.04 mg/L WPN group (Table 1). The mortality rates in the 0.04–0.1 mg/L WPN + SHAM groups were further compared with that in the 0.1 mg/L WPN group for each time point. No difference was observed in mortality between the 0.04 and 0.08 mg/L WPN + SHAM groups and the 0.1 WPN group at 6 h (P > 0.05), while the 0.1 mg/L WPN + SHAM group showed a significantly higher mortality rate than the 0.1 mg/L WPN group (P < 0.05) (Table 1; Fig. 7). At 12 h post-treatment, the 0.08–0.1 mg/L WPN + SHAM group and 0.1 mg/L WPN groups showed similar mortality rates (P > 0.05) (Table 1; Fig. 7). At 24 h post-treatment, the mortality rates in the 0.06–0.1 mg/L WPN + SHAM groups were significantly higher than that in the 0.1 mg/L WPN group (P < 0.05), with 56.31%, 66.40%, and 76.12% increases, respectively (Table 1; Fig. 7). At 48 h post-treatment, the mortality rates of the 0.08–0.1 mg/L WPN + SHAM groups were 100%, and significantly higher than that of 0.1 mg/L WPN group (P < 0.05).
In summary, 0.04 mg/L WPN had approximately the same molluscicidal effect as 0.1 WPN at 48 h. At 12–48 h, the molluscicidal effects of 0.06 and 0.08 mg/L WPN + SHAM were higher than that of 0.1 mg/L WPN; and at all time points, the molluscicidal effect of 0.1 mg/L WPN + SHAM was significantly higher than that of 0.1 mg/L WPN.
Discussion
To the best of our knowledge, this is the first study in which the full-length cDNAs of mollusc AOXs have been amplified and confirmed experimentally. The protein sequences of the four AOXs—OhAOX, BgAOX, BaAOX, and BsAOX—were compared with those of 65 AOXs from 63 different species, and the secondary and tertiary structures of the four AOX proteins were predicted. The crystal structure of TbAOX indicates that the active site of AOX consists of a diiron core, four fully conserved glutamic acid residues, and two histidine residues [34]. Mutation of these six amino acid residues could seriously affect the activity of AOX [47]. In this study, the AOXs of all four species of snail contained the same six conserved diiron motifs as the AOX proteins of the other species, which strongly implied that AOX proteins are similar across different species, and correlation between various functions of these proteins.
Earlier studies suggested that AOX is limited to plants, some fungi, and protists; however, more recent studies confirmed that AOXs also exist in the animal kingdom, although they are not found in vertebrates [48]. The phylogenetic tree constructed in this study revealed an evolutionary relationship between the AOXs of different species. The AOX of land plants (monocots and eudicots) and the Euglenozoa formed a separate monophyletic clade with very high bootstrap values, which indicated that the AOXs of these species may have evolved from a homologous AOX carried by their common ancestors in each kingdom. This is consistent with the results of a phylogenetic analysis of AOXs from land plants and the phylum Euglenozoa carried out by Pennisi et al. [49]. Other kingdoms showed certain cross-relationships: metazoans in this study clustered into a large clade, except L. anatina in the phylum Brachiopoda. The AOX of L. anatina was close to the AOX of the protist phylum Ciliophora and also to the AOXs of the Chlorophyta in the Plantae. AOX has been found in various metazoan phyla [48], and evolved with the rapidly changing concentration of oxygen, hypoxia, and increased H2S concentration in the early atmosphere of the Earth [50–52]. AOX is not sensitive to sulphide, and can oxidise panthenol and reduce O2 to water when sulphide inhibits CCO, thus maintaining electron transport. When an organism is challenged with environmental stress, the inhibition of the Cyt c pathway often leads to the overproduction of ROS and dysfunction of the mitochondrial electron transport chain. At the same time, AOX can reduce the production of ROS by preventing overpotential of the mitochondrial membrane, thus alleviating oxidative stress [23, 30, 51, 53, 54]. Studies on some bivalve AOXs have shown that AOX transcripts and proteins can be up-regulated and further coordinated with the mitochondrial electron transport chain to support the survival of bivalves under stress conditions [30, 51]. In the present study, OhAOX was close to AOX of P. canaliculata, and the AOXs of the three species of Biomphalaria were close to the AOX of A. californica. Furthermore, all of these AOXs clustered together to form the Gastropoda group, which grouped together with the bivalve AOXs into the bigger clade of Mollusca, with high support values. This suggested that the AOXs of Oncomelania and Biomphalaria snails, which are in the Gastropoda, may have similar functions to bivalve AOXs in the mitochondrial respiratory chain.
Under WPN stress, the expression of OhAOX mRNA in the whole snail and the head-foot region showed a significant increase, and reached its highest levels at 24 h and 12 h. Moreover, western blot analysis revealed a significant increase of OhAOX protein in the whole snail after WPN treatment, although the highest level was observed at 48 h post-treatment. This hysteresis was explained well by the mathematical model used in our previous work [17]. Previous studies showed that the activities of the enzymes CCO and SDH in the Cyt c respiratory pathway decreased during periods of OhAOX increase [14, 55]. These results suggested that the up-regulated expression of OhAOX may be involved in regulating oxidative stress in snails under WPN stress, by responding to the inhibition of aerobic respiration caused by a decrease in CCO activity. During this response, AOX can replace CCO to play a role in electron transfer in oxidative phosphorylation, by scavenging electrons and free radicals that accumulate due to reduced CCO activity, and thus protect Oncomelania snails from oxidative damage [14, 56]. Although the expression of transcripts of several enzymes that play a role in oxidative phosphorylation in O. hupensis, such as NADH dehydrogenase, SDH, CCO, and ATPase, did not change after WPN treatment, that of CCR decreased significantly at 24 h post-treatment, which suggested the inhibition of aerobic respiration. The disagreement between the levels of enzyme transcripts in this study and those of enzymes such as NADH dehydrogenase subunit 3, CCO subunit 2 (COX2), and Na/K transporter ATPase subunit α in previous work on O. hupensis [14] could be explained by the variety of different subunits in each MRC. It also indicates that a comprehensive understanding of all MRC subunits in O. hupensis is necessary for further investigation of this species under WPN stress.
Tissue localisation showed that OhAOX mRNA significantly increased in the muscle of the head-foot after WPN treatment, to a level much higher than that in the other tissues, with a tendency to show first an increase then a decrease, as also shown by qPCR. This further confirmed that the response of OhAOX differed in different tissues of O. hupensis under WPN stress, and that the muscle of the head-foot was one of the critical effector tissues for the response of OhAOX to niclosamide. This tissue-specific response was also observed in other molluscs under stress. The up-regulation of AOX in the gills was much higher than that in the digestive glands when Crassostrea gigas was re-oxygenated after hypoxia [51], which suggested that AOX in the gills may be the first sensor in response to oxygen fluctuations [51]. Similarly, the transcript level of AOX in the gills of Diplodon chilensis increased fourfold after hypoxia treatment, but no significant difference was found in the mantle and head-foot [30]. In the present study, OhAOX in the head-foot region was up-regulated significantly more than in the liver-gonad. This may be related to the fact that the head-foot comes into contact with niclosamide first, as the liver-gonad and other organs are coiled in the shell. WPN may also enter an Oncomelania snail through the gill located in the gill pouch on the left of the snail’s neck, and the gill may act as a sensor similar to those in C. gigas. Although the increase of OhAOX in the liver-gonad was not significant after WPN treatment, it was significantly higher in the liver-gonads of WPN-treated snails than in the H2O-treated snails at 24 h. Also, at 48 h after treatment, the expression of OhAOX protein showed a significant increase in the liver-gonad, but not in the head-foot. These results implied that the effect of OhAOX in the liver-gonad is different from that in the head-foot. In addition, this inconsistency between the tissue expression of OhAOX mRNA and that of OhAOX protein may be related to the time periods at which they were measured and hysteresis.
The process of normal mitochondrial respiration is the primary source of ROS. ROS include H2O2, hydroxyl radicals, and superoxide anions, which affect cellular redox homeostasis [27, 57]. When the production rate of ROS exceeds the decomposition rate, oxidative stress occurs, resulting in changes in cell metabolism and oxidative damage to the cell structure [57, 58]. Xiong et al. [14] found that the superoxide dismutase (SOD) activity of O. hupensis was significantly higher at 3 h and 12 h after WPN treatment than after H2O treatment. This indicated that there was oxidative stress in Oncomelania snails after WPN treatment. In the present study, the dynamic change in ROS levels in snails after WPN treatment was estimated using the fluorescence probe DCFH-DA method. The level of ROS in the liver-gonad of snails declined significantly at 6 h after WPN treatment, reached its lowest value at 12 h, and then returned to its initial value. It is currently believed that AOX can reduce the production of ROS by preventing the excessive reduction of ubiquinone in the respiratory chain [59]. H2O2 production in Acanthamoeba castellanii was significantly reduced when purine nucleotides activated the mitochondrial AOX respiratory pathway. However, this effect was weakened when AOX activity was inhibited [28]. Therefore, it suggested that the decrease of ROS content in the liver-gonad of O. hupensis may have been related to AOX activation. In addition, cells in the digestive gland of snails contain a variety of antioxidant enzymes, such as SOD, catalase, and glutathione peroxidase, which decompose superoxide anion free radical and H2O2 [57]. Thus, the reduction of ROS in the liver-gonad suggests that certain antioxidant enzymes in O. hupensis were induced to resist oxidative stress in the early stages of responses to niclosamide, which is supported by the increase of SOD enzyme activity seen in snails in Xiong et al. [14]. Yan et al. [58] found that ROS rose to its highest level when Anadara subcrenata was exposed to the oxidative stress of Cd and phenylalanine for 5 days, and that it decreased to its normal level at 9 days when the antioxidant enzyme activity and ROS level reached equilibrium. Thus, the fact that the ROS level of the liver-gonad of O. hupensis showed no significant difference from 24 to 48 h after niclosamide treatment implies that ROS and the antioxidant system in the liver-gonad of O. hupensis might have reached equilibrium in the late stage of the treatment. In addition, no significant change in the ROS level was shown in the whole soft body and head-foot after WPN treatment. This may be explained by limited DCFH-DA, which mainly reacts with specific ROS like hydrogen peroxide and hydroxyl radicals. Other types of ROS, like superoxide, hypochlorite, and singlet oxygen, need to be further investigated in this context.
In the molluscicidal assay, the molluscicidal effect of niclosamide was significantly enhanced when the activity of AOX was inhibited by SHAM, and the combination of WPN and SHAM also benefited an earlier onset of the molluscicidal efficacy of WPN. The earlier AOX was inhibited, the more significant the molluscicidal effect of WPN. In addition, when AOX was inhibited by SHAM, enhanced molluscicidal efficacy was seen simultaneously with a reduced niclosamide dosage. Snails in the 0.08–0.1 mg/L WPN + SHAM groups showed higher mortality than those in the 0.1 WPN group at all time points, and snails in the 0.04–0.06 mg/L WPN + SHAM groups showed close to, or higher, mortality than those in the 0.1 mg/L WPN group after 24 h. More specifically, snail mortality in the 0.06, 0.08, and 0.1 mg/LWPN + SHAM groups increased by 56.31%, 66.40%, and 76.12% at 24 h, respectively, compared to that when 0.1 mg/L WPN was applied alone. This suggested that the mortality of O. hupensis could be strongly enhanced by inhibiting OhAOX activity, even when reducing the dosage of niclosamide by 50%.
At 48 h after WPN treatment, up-regulated OhAOX had decreased to its initial level. Meanwhile, the mortality rate of O. hupensis snails significantly increased to over 90%. This phenomenon was also reported in Crassostrea virginica, where up-regulated AOX decreased when the oysters were exposed to air for 8 days and faced death [29]. Our results confirmed that the response of OhAOX was no longer sufficient to protect Oncomelania snails when they faced a very high level of WPN stress, and implied that the most effective time point for AOX inhibition should be further examined.
In addition, the ovarian or testicular stroma of O. hupensis showed positive signals of OhAOX mRNA and OhAOX protein, as detected by histological localisation. AOX can alleviate developmental abnormalities in Drosophila by GeneSwitch, and is involved in cell apoptosis, differentiation, and development [60]. Work in our laboratory showed that the AOX of B. glabrata also plays an important role in reproductive development and egg production (data not shown). These results imply that OhAOX expression in the gonad might be involved in its early growth and development and in cell differentiation, all of which needs further investigation.
Conclusions
The AOX genes of O. hupensis and three species of Biomphalaria were identified in the current study. The typical molecular characteristics and phylogenetic relationships of the AOXs of these molluscs indicated that snail AOXs play a role in the mitochondrial respiratory chain. OhAOX mRNA and OhAOX protein significantly increased after WPN treatment, which weakened the molluscicidal effect of niclosamide and counterbalanced the oxidative stress in the snails. The main effect of OhAOX was in the head-foot tissues of snails. AOX in the liver-gonad played a role that may be different from that of AOX in the head-foot. Once the activity of OhAOX had been inhibited, the molluscicidal effect of niclosamide could be significantly enhanced even at lower concentrations of WPN. This study provides information for the development of an environmentally safe snail control method that targets AOX activity. The activity of the AOX pathway in plants under stress, or in thermogenic plants, has been shown to be mainly regulated at the post-translational level [61, 62]. In the present study, post-translation regulation was also implied for natural OhAOX, which was smaller than the recombinant OhAOX. In sum, it is necessary to analyse the activity of AOX in O. hupensis with a focus on the mechanism through which it is regulated and identification of the sites that affect it; there is already some indication of the latter from the protein structure deduced in this study.
Supplementary Information
Additional file 1: Table S1. The gene-specific primers used in the 5'- and 3-end RACE-PCR and specific primers used to amplify OhAOX, BgAOX, BaAOX, and BsAOX.
Additional file 2: Table S2. The species from which the AOX protein sequences were used for the multiple sequence alignments and phylogenetic analysis.
Additional file 3: Table S3. Gene-specific primers used in the qPCR for OhAOX and other genes related to the mitochondrial respiratory complex (MRC).
Additional file 4: Figure S1. The conserved and characteristic domains of OhAOX, BgAOX, BaAOX, and BsAOX with SMART searching.
Additional file 5: Figure S2. Secondary structure of the protein deduced from multiple AOX protein sequence alignments with ESPrit. The four snail AOXs in this study are highlighted in yellow on the left. α α-Helix, η 310-helix, hash symbol diiron binding motif. White characters on the red background indicate the complete identical protein sequences, blue frames indicate the homologous regions; the characters highlighted in blue represented the specific C-terminal motifs (NP-[YF]-XPG-[KQE]) in animal AOXs.
Additional file 6: Figure S3. The diiron core in the deduced tertiary structure of OhAOX, BgAOX, BaAOX, and BsAOX proteins. Each protein’s monomers are shown as chains 1 and 2. The diiron centre with two iron atoms and an -OH is coordinated with four glutamates (E) and two histidines (H).
Additional file 7: Figure S4. Expression and identification of recombinant OhAOX protein in SDS-PAGE and the specificity of anti-OhAOX polyclonal antiserum in western blot. a Expression of recombinant proteins. M protein marker, lane 1 uninduced Escherichia coli with recombinant OhAOX–pGEX-4 T-1, lane 2 induced E. coli with pGEX-4 T-1, lane 3 precipitate of induced E. coli lysis with recombinant OhAOX–pGEX-4 T-1, lane 4 supernatant of induced E. coli lysis with recombinant OhAOX–pGEX-4T-1, lane 5 induced E. coli with OhAOX–pGEX-4T-1. b Purification of recombinant protein. Lane 1 Supernatant of induced E. coli lysis with recombinant OhAOX–pGEX-4 T-1, lane 2 flow-through after GST beads binding, lanes 3–5 washing solution, lanes 6–8 elution of recombinant protein. c Total protein of snail and purified recombinant OhAOX. Lane 1 Total protein extracted from whole O. hupensis snail, lane 2 purified recombinant OhAOX without GST tag. d Specificity of mouse anti-OhAOX serum by western blot. Lane 1 Total protein of O. hupensis snail, lane 2 PBS, lane 3 purified recombinant OhAOX protein, lane 1' control for lane reacted with mouse PBS immune serum, lane 3' control for lane 3 reacted with mouse PBS immune serum.
Additional file 8: Figure S5. Tissue distribution of OhAOX mRNA in untreated O. hupensis snail by in situ hybridisation. Upper image is from the whole snail, and the lower images are from the head-foot region (left) and liver-gonad. The stained purple-blue area was identified as positive for OhAOX mRNA. G Ganglia, HF head-foot, L liver, M muscle, P pellicle, T testis.
Additional file 9: Table S4. Mortality rate (%) of snails after WPN treatment, with the AOX inhibitor (SHAM) added at 0 h or 6 h.
Acknowledgements
Our most heartfelt thanks to Dr Yun-Hai Guo from the National Institute of Parasitic Diseases, China and Mr Qi-Jun Tang from Shenzhen CDC, for the Biomphalaria straminea snail collection, Shenzhen, China, and to Mr Zhenwen He and Zhaogang Xu and the group at the Institute of Schistosomiasis Control of Gong’an County, Hubei Province, China, for their assistance in collecting Oncomelania hupensis in the field.
Abbreviations
- AOX
Alternative oxidase
- ATP
Adenosine triphosphate
- BaAOX
Biomphalaria alexandrina alternative oxidase
- BgAOX
Biomphalaria glabrata alternative oxidase
- BsAOX
Biomphalaria straminea alternative oxidase
- CCO
Cytochrome c oxidase
- CCR
Cytochrome c reductase
- Cyt c
Cytochrome c
- DCF
Dichlorofluorescein
- DCFH-DA
2′,7′-Dichlorodihydrofluorescein diacetate
- HRP
Horseradish peroxidase
- IOD
Integrated optical density
- MRC
Mitochondrial respiratory complex
- NADH
Nicotinamide adenine dinucleotide
- OhAOX
Oncomelania hupensis alternative oxidase
- PBS
Phosphate-buffered saline
- PCR
Polymerase chain reaction
- qPCR
Quantitative real-time polymerase chain reaction
- ROS
Reactive oxygen species
- SDH
Succinic dehydrogenase
- SDS-PAGE
Sodium dodecyl sulfate–polyacrylamide gel electrophoresis
- SHAM
Salicylhydroxamic acid
- SMART
Simple Modular Architecture Research Tool
- SOD
Superoxide dismutase
- TbAOX
Trypanosoma brucei alternative oxidase
- WPN
Wettable powder of niclosamide
Authors' contributions
SZL and QZ conceived and designed the study. NJ, MRH, and TX collected the samples. NJ, YWQA, and SX performed the experiments. NJ and QPZ analysed the data and wrote the first draft of the manuscript. SZL, MRH, and HD reviewed the manuscript. All authors read and approved the final manuscript.
Funding
This work was supported by the Key Laboratory of Parasite and Vector Biology, Ministry of Health (WSBKFKT-201801), and the National Natural Science Foundation of China (32170506).
Availability of data and materials
The sequences of the four AOX genes identified in this study have been deposited in GenBank under accession numbers MZ436164-MZ436167. Most of the data generated or analysed during this study are included in this published article and its supporting files. Any related data are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
The procedures involving mice were carried out in accordance with the guidelines of the Association for Assessment and Accreditation of Laboratory Animal Care International and the Guidelines on How to Treat Experimental Animals issued by the Ministry of Science and Technology, China. The animal study protocol was approved by the Institutional Animal Care and Use Committee of Wuhan University (ethics review no. 2019188). The authors assert that all the procedures used in this work complied with the ethical standards of the relevant national and institutional guidelines on the care and use of laboratory animals.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Ni Jiang, Email: 1476484247@qq.com.
Shi-Zhu Li, Email: lisz@chinacdc.cn.
Yang-Wen-Qing Zhang, Email: 1209677617@qq.com.
Mohamed R. Habib, Email: m.habib@tbri.gov.eg
Tao Xiong, Email: xiongtao_28@126.com.
Sha Xu, Email: 519667348@qq.com.
Huifen Dong, Email: hfdong@whu.edu.cn.
Qin-Ping Zhao, Email: zhaoqinping@whu.edu.cn.
References
- 1.Gryseels B, Polman K, Clerinx J, Kestens L. Human schistosomiasis. Lancet. 2006;368:1106–1118. doi: 10.1016/S0140-6736(06)69440-3. [DOI] [PubMed] [Google Scholar]
- 2.Blanton RE. Population structure and dynamics of helminthic infection: schistosomiasis. Microbiol Spectr. 2019 doi: 10.1128/microbiolspec.AME-0009-2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang L, Wu X, Li X, Zheng X, Wang F, Qi Z, et al. Imported schistosomiasis: a new public health challenge for China. Front Med. 2020;7:553487. doi: 10.3389/fmed.2020.553487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Attwood S, Huo G, Qiu J. Update on the distribution and phylogenetics of Biomphalaria (Gastropoda: Planorbidae) populations in Guangdong Province, China. Acta Trop. 2014;141:258–270. doi: 10.1016/j.actatropica.2014.04.032. [DOI] [PubMed] [Google Scholar]
- 5.Zhou Y, Liang S, Jiang Q. Factors impacting on progress towards elimination of transmission of Schistosomiasis japonica in China. Parasites Vectors. 2012;5:275. doi: 10.1186/1756-3305-5-275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang LJ, Xu ZM, Dang H, Li YL, Lu S, Xu J, et al. Endemic status of schistosomiasis in People's Republic of China in 2019. Zhongguo Xue Xi Chong Bing Fang Zhi Za Zhi. 2020;32:551–558. doi: 10.16250/j.32.1374.2020263. [DOI] [PubMed] [Google Scholar]
- 7.Cao CL, Zhang LJ, Bao ZP, Dai SM, Lu S, Xu J, et al. Endemic situation of schistosomiasis in People's Republic of China from 2010 to 2017. Zhongguo Xue Xi Chong Bing Fang Zhi Za Zhi. 2019;31:519–521. doi: 10.16250/j.32.1374.2018232. [DOI] [PubMed] [Google Scholar]
- 8.Zhang LJ, Zhu HQ, Wang Q, Lu S, Xu J, Li SZ. Assessment of schistosomiasis transmission risk along the Yangtze River basin after the flood disaster in 2020. Zhongguo Xue Xi Chong Bing Fang Zhi Za Zhi. 2020;32:464–468. doi: 10.16250/j.32.1374.2020242. [DOI] [PubMed] [Google Scholar]
- 9.Yuan Y, Xu XJ, Dong HF, Jiang MS, Zhu HG. Transmission control of Schistosomiasis japonica: implementation and evaluation of different snail control interventions. Acta Trop. 2005;96:191–197. doi: 10.1016/j.actatropica.2005.07.014. [DOI] [PubMed] [Google Scholar]
- 10.Dai J, Li Y, Wang W, Xing Y, Qu G, Liang Y. Sensitivity of Oncomelania hupensis to niclosamide: a nation-wide survey in China. Int J Environ Res Public Health. 2014;11:3086–3095. doi: 10.3390/ijerph110303086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang W, Zhang X, Zhang H, Hu H, Li S, Liu X, et al. Field evaluation of a novel molluscicide (niclosamidate) against Oncomelania hupensis, intermediate host of Schistosoma japonicum. Parasitol Res. 2017;116:3423–3427. doi: 10.1007/s00436-017-5649-x. [DOI] [PubMed] [Google Scholar]
- 12.Jia TW, Wang W, Sun LP, Lv S, Yang K, Zhang NM, et al. Molluscicidal effectiveness of luo-wei, a novel plant-derived molluscicide, against Oncomelania hupensis, Biomphalaria alexandrina and Bulinus truncatus. Infect Dis Poverty. 2019;8:27. doi: 10.1186/s40249-019-0535-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dai JR, Li YZ, Wang W, Xing YT, Qu GL, Liang YS. Resistance to niclosamide in Oncomelania hupensis, the intermediate host of Schistosoma japonicum: should we be worried? Parasitology. 2014;142:332–340. doi: 10.1017/S0031182014000870. [DOI] [PubMed] [Google Scholar]
- 14.Xiong T, Zhao QP, Xu XJ, Liu R, Jiang MS, Dong HF. Morphological and enzymatical observations in Oncomelania hupensis after molluscicide treatment: implication for future molluscicide development. Parasitol Res. 2016;115:4139–4152. doi: 10.1007/s00436-016-5188-x. [DOI] [PubMed] [Google Scholar]
- 15.Saada A. Sea squirt alternative oxidase bypasses fatal mitochondrial heart disease. EMBO Mol Med. 2019;11:e9962. doi: 10.15252/emmm.201809962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sokolova I. Mitochondrial adaptations to variable environments and their role in animals’ stress tolerance. Integr Comp Biol. 2018;58:519–531. doi: 10.1093/icb/icy017. [DOI] [PubMed] [Google Scholar]
- 17.Xiong T, Jiang N, Xu S, Li SZ, Zhang Y, Xu XJ, et al. Metabolic profiles of Oncomelania hupensis after molluscicidal treatment: carbohydrate metabolism targeted and energy deficiency. Acta Trop. 2020;210:105580. doi: 10.1016/j.actatropica.2020.105580. [DOI] [PubMed] [Google Scholar]
- 18.Zhao QP, Xiong T, Xu XJ, Jiang MS, Dong HF. De Novo transcriptome analysis of Oncomelania hupensis after molluscicide treatment by next-generation sequencing: implications for biology and future snail interventions. PLoS ONE. 2015;10:e0118673. doi: 10.1371/journal.pone.0118673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vanlerberghe GC, McIntosh L. Alternative oxidase: from gene to function. Annu Rev Plant Phys. 1997;48:703–734. doi: 10.1146/annurev.arplant.48.1.703. [DOI] [PubMed] [Google Scholar]
- 20.Rogov AG, Sukhanova EI, Uralskaya LA, Aliverdieva DA, Zvyagilskaya RA. Alternative oxidase: distribution, induction, properties, structure, regulation, and functions. Biochemistry-Moscow. 2014;79:1615–1634. doi: 10.1134/S0006297914130112. [DOI] [PubMed] [Google Scholar]
- 21.Zhu Y, Lu J, Wang J, Chen F, Leng F, Li H. Regulation of thermogenesis in plants: the interaction of alternative oxidase and plant uncoupling mitochondrial protein. J Integr Plant Biol. 2011;53:7–13. doi: 10.1111/j.1744-7909.2010.01004.x. [DOI] [PubMed] [Google Scholar]
- 22.Dahal K, Wang J, Martyn GD, Rahimy F, Vanlerberghe GC. Mitochondrial alternative oxidase maintains respiration and preserves photosynthetic capacity during moderate drought in Nicotiana tabacum. Plant Physiol. 2014;166:1560–1574. doi: 10.1104/pp.114.247866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Weaver RJ. Hypothesised evolutionary consequences of the alternative oxidase (AOX) in animal mitochondria. Integr Comp Biol. 2019;59:994–1004. doi: 10.1093/icb/icz015. [DOI] [PubMed] [Google Scholar]
- 24.Hanqing F, Kun S, Mingquan L, Hongyu L, Xin L, Yan L, et al. The expression, function and regulation of mitochondrial alternative oxidase under biotic stresses. Mol Plant Pathol. 2010;11:429–440. doi: 10.1111/j.1364-3703.2010.00615.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jacoby RP, Li L, Huang SB, Lee C, Millar AH, Taylor NL. Mitochondrial composition, function and stress response in plants. J Integr Plant Biol. 2012;54:887–906. doi: 10.1111/j.1744-7909.2012.01177.x. [DOI] [PubMed] [Google Scholar]
- 26.Vanlerberghe GC. Alternative oxidase: a mitochondrial respiratory pathway to maintain metabolic and signaling homeostasis during abiotic and biotic stress in plants. Int J Mol Sci. 2013;14:6805–6847. doi: 10.3390/ijms14046805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Magnani T, Soriani FM, Martins Vde P, Policarpo AC, Sorgi CA, Faccioli LH, et al. Silencing of mitochondrial alternative oxidase gene of Aspergillus fumigatus enhances reactive oxygen species production and killing of the fungus by macrophages. J Bioenerg Biomembr. 2008;40:631–636. doi: 10.1007/s10863-008-9191-5. [DOI] [PubMed] [Google Scholar]
- 28.Czarna M, Jarmuszkiewicz W. Activation of alternative oxidase and uncoupling protein lowers hydrogen peroxide formation in amoeba Acanthamoeba castellanii mitochondria. FEBS Lett. 2005;579:3136–3140. doi: 10.1016/j.febslet.2005.04.081. [DOI] [PubMed] [Google Scholar]
- 29.Liu M, Guo X. A novel and stress adaptive alternative oxidase derived from alternative splicing of duplicated exon in oyster Crassostrea virginica. Sci Rep. 2017;7:10785. doi: 10.1038/s41598-017-10976-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yusseppone MS, Rocchetta I, Sabatini SE, Luquet CM, Rios de Molina MDC, Held C, et al. Inducing the alternative oxidase forms part of the molecular strategy of anoxic survival in freshwater bivalves. Front Physiol. 2018;9:100. doi: 10.3389/fphys.2018.00100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kearse M, Moir R, Wilson A, Stones-Havas S, Cheung M, Sturrock S, et al. Geneious basic: an integrated and extendable desktop software platform for the organisation and analysis of sequence data. Bioinformatics. 2012;28:1647–1649. doi: 10.1093/bioinformatics/bts199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Suzuki T, Nihei C, Yabu Y, Hashimoto T, Suzuki M, Yoshida A, et al. Molecular cloning and characterisation of Trypanosoma vivax alternative oxidase (AOX) gene, a target of the trypanocide ascofuranone. Parasitol Int. 2004;53:235–245. doi: 10.1016/j.parint.2004.02.001. [DOI] [PubMed] [Google Scholar]
- 33.Suzuki T, Hashimoto T, Yabu Y, Majiwa PAO, Ohshima S, Suzuki M, et al. Alternative oxidase (AOX) genes of African trypanosomes: phylogeny and evolution of AOX and plastid terminal oxidase families. J Eukaryot Microbiol. 2005;52:374–381. doi: 10.1111/j.1550-7408.2005.00050.x. [DOI] [PubMed] [Google Scholar]
- 34.Shiba T, Kido Y, Sakamoto K, Inaoka DK, Tsuge C, Tatsumi R, et al. Structure of the trypanosome cyanide-insensitive alternative oxidase. Proc Natl Acad Sci USA. 2013;110:4580. doi: 10.1073/pnas.1218386110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Folgueira I, Lamas J, Sueiro RA, Leiro JM. Molecular characterisation and gene expression modulation of the alternative oxidase in a scuticociliate parasite by hypoxia and mitochondrial respiration inhibitors. Sci Rep. 2020;10:11880. doi: 10.1038/s41598-020-68791-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Abu-Romman S, Shatnawi M, Hasan M, Qrunfleh I, Omar S, Salem N. cDNA cloning and expression analysis of a putative alternative oxidase HsAOX1 from wild barley (Hordeum spontaneum) Genes Genom. 2012;34:59–66. [Google Scholar]
- 37.Aoki K, Yano K, Suzuki A, Kawamura S, Sakurai N, Suda K, et al. Large-scale analysis of full-length cDNAs from the tomato (Solanum lycopersicum) cultivar Micro-Tom, a reference system for the Solanaceae genomics. BMC Genomics. 2010;11:210. doi: 10.1186/1471-2164-11-210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kazusa DNARI, Cold Spring Harbor and Washington University Sequencing Consortium, the European Union Arabidopsis Genome Sequencing Consortium, Institute of Plant Group, Crop Plant Research. Sequence and analysis of chromosome 5 of the plant Arabidopsis thaliana. Nature 2000; 408:823–26.
- 39.Cruz-Hernández A, Gómez-Lim MA. Alternative oxidase from mango (Mangifera indica, L.) is differentially regulated during fruit ripening. Planta. 1995;197:569–576. doi: 10.1007/BF00191562. [DOI] [PubMed] [Google Scholar]
- 40.Considine MJ, Holtzapffel RC, Day DA, Whelan J, Millar AH. Molecular distinction between alternative oxidase from monocots and dicots. Plant Physiol. 2002;129:949–953. doi: 10.1104/pp.004150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cavalcanti JHF, Oliveira GM, Saraiva KDC, Torquato JPP, Maia IG, Fernandes de Melo D, et al. Identification of duplicated and stress-inducible Aox2b gene co-expressed with Aox1 in species of the Medicago genus reveals a regulation linked to gene rearrangement in leguminous genomes. J Plant Physiol. 2013;170:1609–1619. doi: 10.1016/j.jplph.2013.06.012. [DOI] [PubMed] [Google Scholar]
- 42.Takumi S, Tomioka M, Eto K, Naydenov N, Nakamura C. Characterization of two non-homologous nuclear genes encoding mitochondrial alternative oxidase in common wheat. Genes Genet Syst. 2002;77:81–88. doi: 10.1266/ggs.77.81. [DOI] [PubMed] [Google Scholar]
- 43.Kumar S, Stecher G, Li M, Knyaz C, Tamura K. MEGA X: molecular evolutionary genetics analysis across computing platforms. Mol Biol Evol. 2018;35:1547–1549. doi: 10.1093/molbev/msy096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.MOH . Handbook of schistosomiasis control (Ministry of Health) 3. Shanghai: Shanghai Science and Technology Literature Publishing House; 2000. [Google Scholar]
- 45.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 46.Wang CJ, Zhou ZG, Holmqvist A, Zhang H, Li Y, Adell G, et al. Survivin expression quantified by Image Pro-Plus compared with visual assessment. Appl Immunohistochem Mol Morphol. 2009;17:530–535. doi: 10.1097/PAI.0b013e3181a13bf2. [DOI] [PubMed] [Google Scholar]
- 47.Brew-Appiah RAT, Sanguinet KA. Considerations of AOX functionality revealed by critical motifs and unique domains. Int J Mol Sci. 2018;19:2972. doi: 10.3390/ijms19102972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.McDonald AE, Vanlerberghe GC, Staples JF. Alternative oxidase in animals: unique characteristics and taxonomic distribution. J Exp Biol. 2009;212:2627–2634. doi: 10.1242/jeb.032151. [DOI] [PubMed] [Google Scholar]
- 49.Pennisi R, Salvi D, Brandi V, Angelini R, Ascenzi P, Polticelli F. Molecular evolution of alternative oxidase proteins: a phylogenetic and structure modeling approach. J Mol Evol. 2016;82:207–218. doi: 10.1007/s00239-016-9738-8. [DOI] [PubMed] [Google Scholar]
- 50.Abele E, Philip E, Gonzalez PM, Puntarulo S. Marine invertebrate mitochondria and oxidative stress. Front Biosci. 2007;12:933–946. doi: 10.2741/2115. [DOI] [PubMed] [Google Scholar]
- 51.Sussarellu R, Dudognon T, Fabioux C, Soudant P, Moraga D, Kraffe E. Rapid mitochondrial adjustments in response to short-term hypoxia and re-oxygenation in the Pacific oyster, Crassostrea gigas. J Exp Biol. 2013;216:1561–1569. doi: 10.1242/jeb.075879. [DOI] [PubMed] [Google Scholar]
- 52.Turrens JF. Mitochondrial formation of reactive oxygen species. J Physiol. 2003;552:335–344. doi: 10.1113/jphysiol.2003.049478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Maxwell DP, Wang Y, McIntosh L. The alternative oxidase lowers mitochondrial reactive oxygen production in plant cells. Proc Natl Acad Sci USA. 1999;96:8271–8276. doi: 10.1073/pnas.96.14.8271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Woyda-Ploszczyca AM, Jarmuszkiewicz W. The conserved regulation of mitochondrial uncoupling proteins: from unicellular eukaryotes to mammals. Biochim Biophys Acta Bioenerg. 2017;1858:21–33. doi: 10.1016/j.bbabio.2016.10.003. [DOI] [PubMed] [Google Scholar]
- 55.Li H, Liang Y, Dai J, Xu M, Ru W, Xu Y. Enzyme-histochemical observation on influence of suspension concentrate of niclosamide in Oncomelania hupensis snails. Zhongguo Xue Xi Chong Bing Fang Zhi Za Zhi. 2006;18:427–430. [Google Scholar]
- 56.Vanlerberghe GC, McLntosh L. Signals regulating the expression of the nuclear gene encoding alternative oxidase of plant mitochondria. Plant Physiol. 1996;111:589–595. doi: 10.1104/pp.111.2.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Almeida EA, Bainy ACD, Dafre AL, Gomes OF, Medeiros MHG, Di Mascio P. Oxidative stress in digestive gland and gill of the brown mussel (Perna perna) exposed to air and re-submersed. J Exp Mar Biol Ecol. 2005;318:21–30. [Google Scholar]
- 58.Yan B, Liu X, Zhao X, Tian S. Single and joint oxidative stress of cadmium and phenanthrene on the bivalve Anadara subcrenata. J Environ Sci Health A Tox Hazard Subst Environ Eng. 2020;55:448–456. doi: 10.1080/10934529.2019.1707563. [DOI] [PubMed] [Google Scholar]
- 59.McDonald AE. Alternative oxidase: an inter-kingdom perspective on the function and regulation of this broadly distributed 'cyanide-resistant' terminal oxidase. Funct Plant Biol. 2008;35:535–552. doi: 10.1071/FP08025. [DOI] [PubMed] [Google Scholar]
- 60.Andjelković A, Kemppainen KK, Jacobs HT. Ligand-bound GeneSwitch causes developmental aberrations in Drosophila that are alleviated by the alternative oxidase. G3. 2016;6:2839–2846. doi: 10.1534/g3.116.030882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Del-Saz NF, Ribas-Carbo M, McDonald AE, Lambers H, Fernie AR, Florez-Sarasa I. An in vivo perspective of the role(s) of the alternative oxidase pathway. Trends Plant Sci. 2018;23:206–219. doi: 10.1016/j.tplants.2017.11.006. [DOI] [PubMed] [Google Scholar]
- 62.Stitt M, Gibon Y. Why measure enzyme activities in the era of systems biology? Trends Plant Sci. 2014;19:256–265. doi: 10.1016/j.tplants.2013.11.003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Table S1. The gene-specific primers used in the 5'- and 3-end RACE-PCR and specific primers used to amplify OhAOX, BgAOX, BaAOX, and BsAOX.
Additional file 2: Table S2. The species from which the AOX protein sequences were used for the multiple sequence alignments and phylogenetic analysis.
Additional file 3: Table S3. Gene-specific primers used in the qPCR for OhAOX and other genes related to the mitochondrial respiratory complex (MRC).
Additional file 4: Figure S1. The conserved and characteristic domains of OhAOX, BgAOX, BaAOX, and BsAOX with SMART searching.
Additional file 5: Figure S2. Secondary structure of the protein deduced from multiple AOX protein sequence alignments with ESPrit. The four snail AOXs in this study are highlighted in yellow on the left. α α-Helix, η 310-helix, hash symbol diiron binding motif. White characters on the red background indicate the complete identical protein sequences, blue frames indicate the homologous regions; the characters highlighted in blue represented the specific C-terminal motifs (NP-[YF]-XPG-[KQE]) in animal AOXs.
Additional file 6: Figure S3. The diiron core in the deduced tertiary structure of OhAOX, BgAOX, BaAOX, and BsAOX proteins. Each protein’s monomers are shown as chains 1 and 2. The diiron centre with two iron atoms and an -OH is coordinated with four glutamates (E) and two histidines (H).
Additional file 7: Figure S4. Expression and identification of recombinant OhAOX protein in SDS-PAGE and the specificity of anti-OhAOX polyclonal antiserum in western blot. a Expression of recombinant proteins. M protein marker, lane 1 uninduced Escherichia coli with recombinant OhAOX–pGEX-4 T-1, lane 2 induced E. coli with pGEX-4 T-1, lane 3 precipitate of induced E. coli lysis with recombinant OhAOX–pGEX-4 T-1, lane 4 supernatant of induced E. coli lysis with recombinant OhAOX–pGEX-4T-1, lane 5 induced E. coli with OhAOX–pGEX-4T-1. b Purification of recombinant protein. Lane 1 Supernatant of induced E. coli lysis with recombinant OhAOX–pGEX-4 T-1, lane 2 flow-through after GST beads binding, lanes 3–5 washing solution, lanes 6–8 elution of recombinant protein. c Total protein of snail and purified recombinant OhAOX. Lane 1 Total protein extracted from whole O. hupensis snail, lane 2 purified recombinant OhAOX without GST tag. d Specificity of mouse anti-OhAOX serum by western blot. Lane 1 Total protein of O. hupensis snail, lane 2 PBS, lane 3 purified recombinant OhAOX protein, lane 1' control for lane reacted with mouse PBS immune serum, lane 3' control for lane 3 reacted with mouse PBS immune serum.
Additional file 8: Figure S5. Tissue distribution of OhAOX mRNA in untreated O. hupensis snail by in situ hybridisation. Upper image is from the whole snail, and the lower images are from the head-foot region (left) and liver-gonad. The stained purple-blue area was identified as positive for OhAOX mRNA. G Ganglia, HF head-foot, L liver, M muscle, P pellicle, T testis.
Additional file 9: Table S4. Mortality rate (%) of snails after WPN treatment, with the AOX inhibitor (SHAM) added at 0 h or 6 h.
Data Availability Statement
The sequences of the four AOX genes identified in this study have been deposited in GenBank under accession numbers MZ436164-MZ436167. Most of the data generated or analysed during this study are included in this published article and its supporting files. Any related data are available from the corresponding author on reasonable request.