Abstract
Aging across the Primate Order is poorly understood because ages of individuals are often unknown, there is a dearth of aged animals available for study, and because aging is best characterized by longitudinal studies which are difficult to carry out in long-lived species. The human population is aging rapidly, and advanced age is a primary risk factor for several chronic diseases and conditions that impact healthspan. As lifespan has increased, diseases and disorders of the central nervous system (CNS) have become more prevalent, and Alzheimer’s disease and related dementias have become epidemic. Non-human primate (NHP) models are key to understanding the aging primate CNS. This Special Issue presents a review of current knowledge about NHP CNS aging across the Primate Order. Similarities and differences to human aging, and their implications for the validity of NHP models of aging are considered. Topics include aging-related brain structure and function, neuropathologies, cognitive performance, social behavior and social network characteristics, and physical, sensory, and motor function. Challenges to primate CNS aging research are discussed. Together, this collection of articles demonstrates the value of studying aging in a breadth of NHP models to advance our understanding of human and nonhuman primate aging and healthspan.
Keywords: aging, Alzheimer’s disease, cognitive decline, gait speed, nonhuman primate
1 |. INTRODUCTION
The human population is aging. By 2050, the United Nations Department of Economic and Social Affairs predicts that 1 in 6 people will be over the age of 65 (United Nations, 2019). Aging is accompanied by a suite of physiologic changes, not all of which are detrimental to health. However, advancing age represents a key risk factor for several chronic diseases and conditions, including neurodegenerative disorders such as Alzheimer’s disease (AD) (Hou et al, 2019). The purpose of this Special Issue is to present a review of current knowledge about nonhuman primate (NHP) aging, with a focus on the central nervous system (CNS), and to examine similarities and differences to human aging and their implications for the validity of NHP models of human aging. This Special Issue examines aging-related changes across the Primate Order, from prosimians to hominids, in multiple domains including cognitive performance, brain structure and function, neuropathologies, social behavior and social network characteristics, and physical, sensory, and motor function.
1.1 |. Animal models
Animal models of human health are important for several reasons. Human studies enroll individuals that vary in many ways that can affect outcome variables, and many of these variables cannot be controlled or accounted for in statistical analyses. For example, differences in social status in humans are accompanied by disparities in education, health care, living conditions, access to food, clean water, and green spaces, all confounding factors that are known to affect a myriad of health outcomes. Long-term clinical trials also suffer from selection bias (Abdelnour et al, 2020) or cohort effects (Dodge et al, 2014), reducing the reproducibility of results. In contrast to human studies, captive animals can be housed under controlled conditions in which light/dark cycles, diet composition, water availability, physical and social characteristics of housing, and health care are uniform across all individuals in a study. Studies of free-ranging animals may provide opportunities to study age-related processes independent of the effects of modern sociocultural practices. Longer term human studies also rely largely on self-report of characteristics that may impact health outcomes such as diet, use of alcohol or other drugs, or exposure to psychological stressors. The accuracy of self-report of many of these variables has been shown to be poor. Such variables can either be excluded, controlled or accurately measured in animal studies. Further, many outcomes cannot be directly measured in humans for logistic or ethical reasons; in these cases, studies rely on indirect measures of biomarkers, which less accurately reflect experimental effects. For all of these reasons determination of causal relationships relies on animal models (Verdier et al, 2015).
1.2 |. Nonhuman primate models of aging
NHPs have played a critical role in medical science and health because of their close phylogenetic relatedness and similarity to humans in structure and function of multiple systems (Phillips et al., 2014). NHPs are useful models for investigations involving the reproductive system, bioenergetics, diet, obesity, diabetes, cardiovascular health, the musculoskeletal system, CNS structure and function, cognitive and social behavior, and diseases of aging. Studies on prospective (birth-to-death) NHP wild populations have provided unique opportunities to assess variables which drive maturation, rates of aging, and lifespan (Bjork et al., 2019; Campos et al., 2021; Sapolsky & Altmann, 1991; Snyder-Mackler et al., 2020). NHPs also have been critical models to understand women’s health, in particular the role sex hormones in disease susceptibility and resistance (Shively & Clarkson, 2009). NHP research was instrumental in stemming the rate and impact of HIV infection (Friedman et al., 2017; Veazey & Lackner, 2017), and NHPs provided the foundation for the rapid development of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) vaccines (Lu et al., 2020). The Office of Research Infrastructure Programs of the National Institutes of Health (ORIP, NIH) listed well over 150 coronavirus SARS-CoV-2 studies and reviews using NHPs just 9 months after the COVID-19 epidemic was first identified (https://orip.nih.gov/Nonhuman-primate-models).
NHPs are particularly valuable to understanding human aging because they appear to age like humans (Verdier et al., 2015). Humans experience a relatively long, slow degradation of motor, sensory, and cognitive function and accumulate chronic diseases of aging such as sarcopenia, arthritis, hypertension, chronic kidney disease, diabetes, cardiovascular disease, and AD (Jaul & Barron, 2017). This is due in part to the aging of a population supported by good health care and nutrition (Chetty et al., 2016; Hao et al., 2020). However, with advancing age has come an increase in aging-related diseases. A primary goal of geroscience is to understand how aging enables diseases and use that knowledge to slow the rate and progression of disease and disability, thus extending the healthspan (Olshansky, 2018; Seals et al., 2016; Sierra, 2016). NHPs also experience aging-related declines in sensory, motor, and cognitive function, and social interaction (Verdier et al., 2015), accumulate many similar disabilities and diseases with age, and thus may be used to understand the basic biology of aging and how aging enables disease (Mattison & Vaughan, 2017).
This Special Issue explores aging in detail in seven NHP species. Table 1 presents approximate developmental time points and approximate lifespan parameters drawn from captive data for these NHP species, and these are graphically compared in Figure 1. Table 1 also shows that all seven NHP species naturally develop neuropathology similar to two major types found in human neurodegenerative disorders: β-amyloid (Aβ) and tau-related pathologies. Likewise, Table 1 identifies the NHP species in which Aβ and tau-pathologies have been experimentally induced to manipulate and study disease progression, a valuable approach that is not possible in human participants. Freire-Cobo et al. (2021) in this issue provides a comprehensive review of age-related brain changes in NHPs and details on exact neuropathological findings, beyond the seven species summarized here.
TABLE 1.
Approximate developmental milestones and neuropathology of nonhuman primates across the Primate Order
| Species (Scientific Name) | Adult Weight (kg) | Age of maturity (years) | Lifespan (years) | Age (years) of menopause | Age assumed elderly (years) | Neuropathology | ||
|---|---|---|---|---|---|---|---|---|
| Typical | Maximum | Naturala | Induced | |||||
| Gray mouse lemur (Microcebus murinus) | 0.06–0.12b | 1c | 5–8**d,e,f | 18f | Absentd | 6b | Aβ, Tau | Aβg, Taug |
| Common marmoset (Callithrix jacchus) | 0.25–0.60i | 1–1.5h | 5–8*,i | 22f,j | Absentk | 7–8k | Aβ, Tau | Aβl,m,n |
| Vervet/African green monkey (Chlorocebus aethiops sabaeus) | 3–9o | 4–6p,q | 22*o | 30q,r | 20p | 16–18s,t | Aβ, Tau | Not studied |
| Macaque species | ||||||||
| Rhesus macaque (Macaco mulatto) | 5–10u,v | 3–5u,v | 25–27*,w,x | 44y | 25z | 20x | Aβ, Tau | Aβaa, Taubb |
| Cynomolgus macaque (M. fascicularis) | 3–8cc | 3_5dd, ee | 25–30**,ee | 37g | 29ff | 20gg | Aβ, Tau | Aβhh |
| Barbary macaque (M. sylvanus) | 8–18ii | 4–5jj | 25kk | 29f,jj | 25jj | 20kk | Aβ, Tau | Not studied |
| Chimpanzee (Pan troglodytes) | 35–60ll | 10mm | 35–50**,nn | 62oo | 50oo | 37pp | Aβ, Tau | Not studied |
| Human (Homo sapiens) | 60–80qq | 12–16rr | yg***,SS | 122tt | 50–53uu | 65vv | Aβ, Tau | Not studied |
Note:
Approximate median lifespan;
Approximate mean lifespan;
Life expectancy. Absent: does not exist; not studied: no published reports.
References:
(Perret, 1997),
(Weigl, 2005),
(Ross, 2019),
(Jorgensen, 2021) & Jorgensen pers. comm.,
(Plant, 2005),
(Walker, 1995),
(Fa, 1989),
(Fooden, 2007),
(Robine & Allard, 1999),
(Gold, 2011),
(Shoven, 2007).
Abbreviation: Aβ, amyloid-beta.
FIGURE 1.
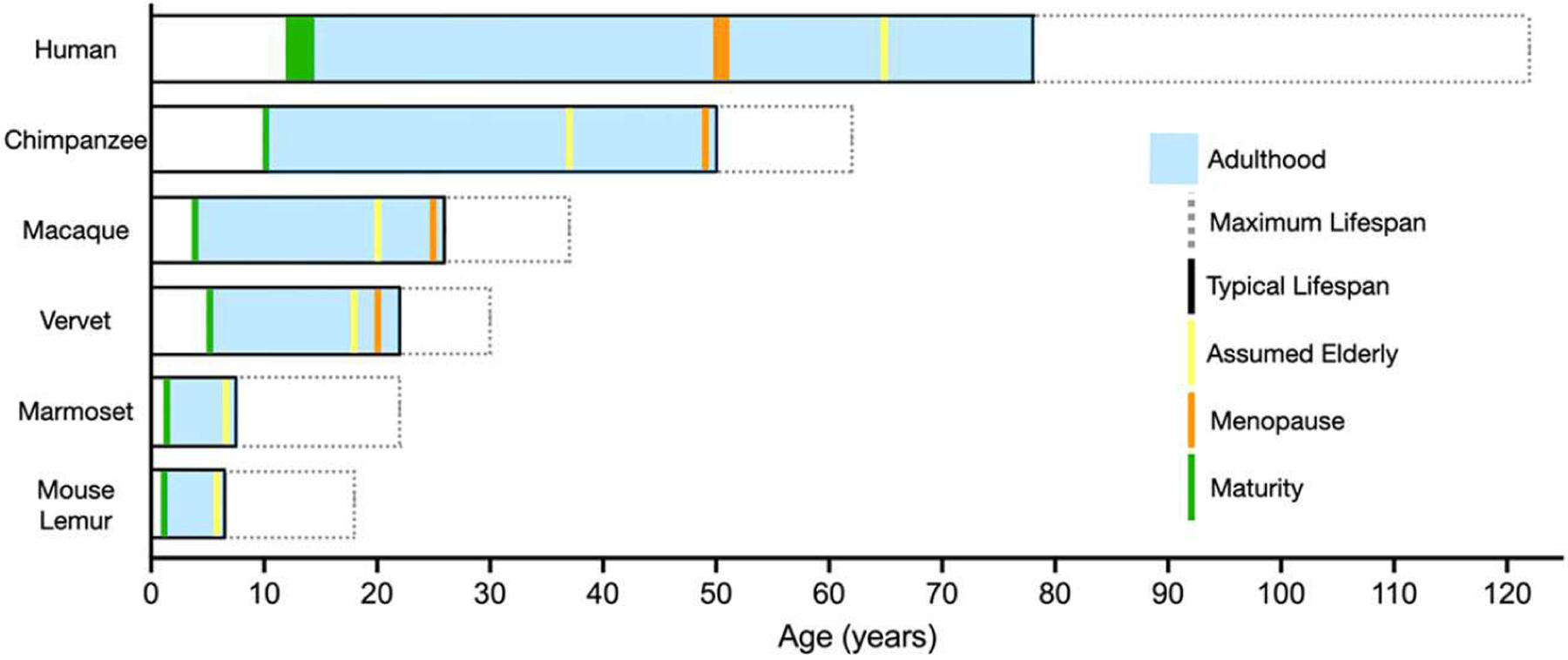
Comparison of major developmental time points and approximate lifespan in species discussed in this Special Issue, drawn from captive NHP data. Definitions: (1) Maturity. Age of known sexual maturity or first successful conception; (2) Adulthood. Span between maturity and end of lifespan; (3) Assumed Elderly. Ages used to categorize old-age; (4) Menopause. Age at cessation of menstrual cycling for females; (5) Typical Lifespan. Typical age at death for captive individuals in this species, drawn from life expectancy and mean or median lifespan values; (6) Maximum Lifespan. Oldest documented age for an individual of this species. Reference values are drawn from Table 1. Vervet: aka African green monkey
1.3 |. Alzheimer’s disease
Neurocognitive decline, and AD and related dementias (ADRD) have become epidemic in the US and around the world causing a public health crisis. Ninety-five percent of cases are diagnosed after 65 years of age, and are referred to as late-onset AD. AD is the sixth leading cause of death, and the only one in the top 10 with no known treatment or cure (“2020 Alzheimer’s Disease Facts and Figures,” 2020). AD also is one of the most expensive disease in the world, costing societies trillions of dollars in the forms of direct medical, social, and informal care (Wimo et al., 2017). In the US alone, over five million adults have AD, and that figure is expected to grow to 13.8 million by 2050 (“2020 Alzheimer’s Disease Facts and Figures,” 2020). Moreover, AD disproportionately affects women and people of color; two-thirds of cases are women, and Hispanics and African-Americans have 1.5 and 2 times higher rates of AD than do the rest of the population, respectively (https://www.cdc.gov/aging/aginginfo/pdfs/Module1-Alzheimers-Disease-Public-Health-Crisis.pdf).
Longitudinal studies of cognitive function, neuroimaging (positron emission tomography [PET] and magnetic resonance imaging [MRI], and cerebrospinal fluid (CSF) biomarkers have characterized a long preclinical phase of accumulating pathophysiology that precedes the onset of clinical AD symptoms by 1–2 decades (Sperling et al., 2011; Vermunt et al., 2019) (Figure 2). The course of disease is characterized by early (β-amyloid (Aβ) accumulation in the precuneus and other cortical regions, followed by neuroinflammation indicated by microglia and astrocyte activation (PET) (Jack et al., 2019); (Gordon et al., 2016; Gordon et al., 2018). Cortical hypometabolism manifests after Aβ accumulation, indicating the onset of synaptic dysfunction (fluorodeoxyglucose PET) (Mosconi et al., 2008). Synaptic dysfunction gives way to tau phosphorylation, detected via changes in the CSF (Karikari et al., 2020), neurofibrillary tangle tau pathology (PET), hippocampal atrophy (structural MRI), and finally cognitive impairment (Bateman et al., 2012; T. Fagan et al., 2006; Gordon et al., 2016); (A. M. Fagan et al., 2007; A. M. Fagan et al., 2014; Gordon et al., 2018; Hanseeuw et al., 2019; Jack & Holtzman, 2013; Long & Holtzman, 2019; Morris et al., 2009; Vos et al., 2013).
FIGURE 2.
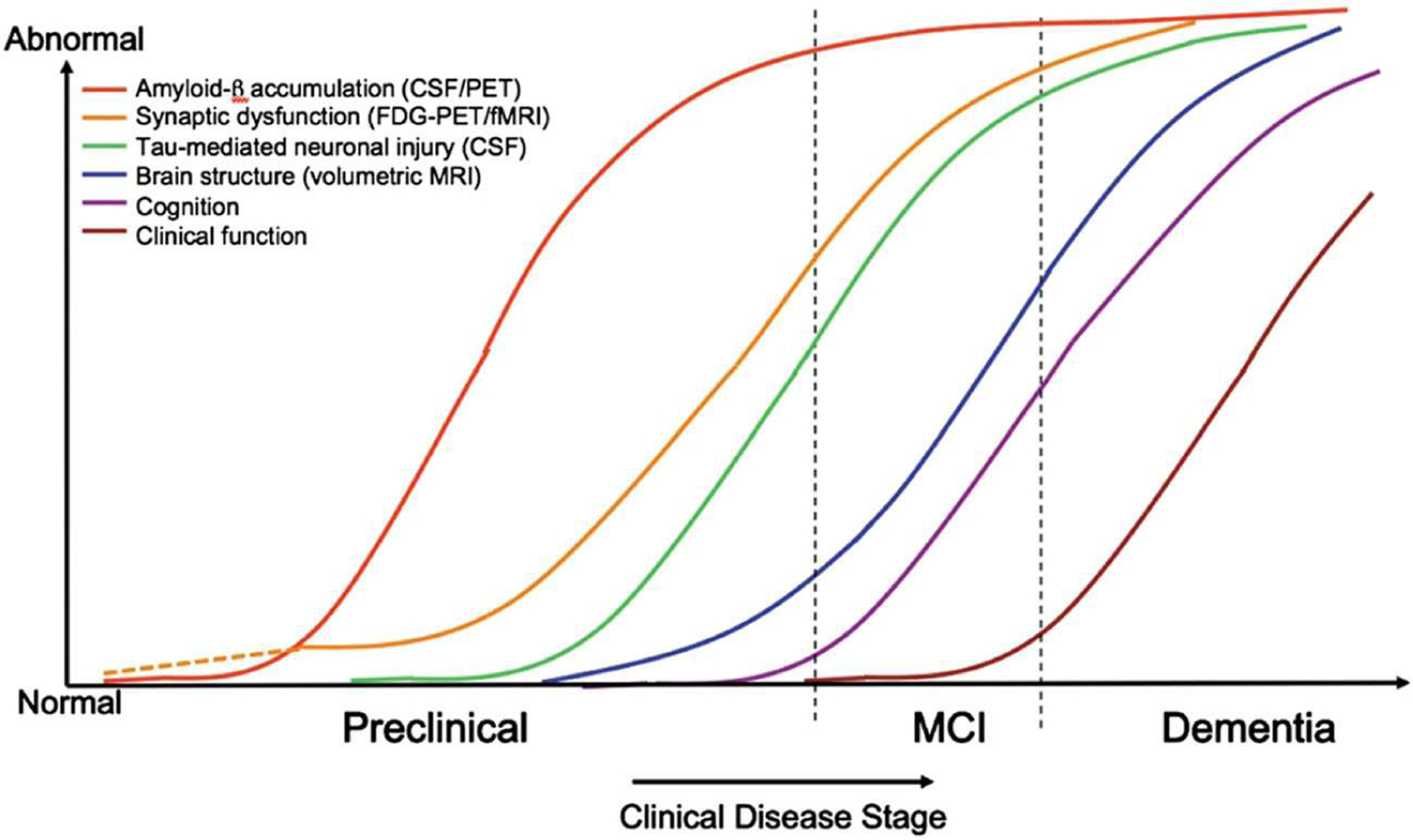
Hypothetical model of accumulating late-onset Alzheimer’s disease (AD) pathophysiology, including Aβ deposition, synaptic dysfunction, tauopathy-associated neuronal injury, volumetric reductions, and cognitive and functional declines. Dashed lines indicate that synaptic dysfunction may be detectable before Aβ accumulation in carriers of the ε4 allele of the apolipoprotein E. Figure is reprinted with permission from Sperling et al., 2011
The accumulation of Aβ results in extracellular amyloid plaque deposition which impairs intercellular communication (Hughes et al., 2020). Amyloid may also be deposited in the cerebrovasculature resulting in cerebral amyloid angiopathy (Greenberg et al., 2020). The ensuing vascular dysfunction reduces perivascular amyloid clearance, thereby promoting amyloid deposition in the brain (Greenberg et al., 2020). The lack of clearance of amyloid is thought to promote tau phosphorylation, and accumulation of these pathological tau proteins disrupts intracellular function, and results in intracellular neurofibrillary tangles that interrupt processes associated with intracellular transport (Long & Holtzman, 2019). Thus, the available data strongly support the central role of pathologic Aβ accumulation in mediating AD pathogenesis and that aggregated, hyperphosphorylated forms of tau may be a primary driver of neurodegeneration and cognitive decline (Hanseeuw et al., 2019; Long & Holtzman, 2019).
The earliest cognitive changes typically include impairments in learning, memory, attention, and executive function (Guarino et al., 2019, 2020; Kirova et al., 2015; McKhann et al., 2011). Impairments in hearing, vision, olfaction, physical function, and sleep may precede or accompany cognitive decline (Brzecka et al., 2018; Bubu et al., 2017; Chen et al., 2016; Ju et al., 2013; Lim et al., 2013; Lloret et al., 2020; Murphy, 2019; Spira et al., 2012, 2014; Vanderheyden et al., 2018). Importantly, heterogeneity is characteristic of the underlying neuropathology and clinical presentation. AD overlaps clinical presentation and neuropathology with a number of other dementias which together are considered ADRD (https://www.ninds.nih.gov/Current-Research/Focus-Disorders/Alzheimers-Related-Dementias).
Given the long preclinical period and heterogeneous presentation of pathologies, and role(s) of comorbidities, the study of AD etiology faces considerable challenges. Moreover, by the time of diagnosis, neuropathology is extensive, defying intervention (Long & Holtzman, 2019). While late-onset AD may have a heritable component (Gispert et al., 2017), risk factors amenable to modification have been identified and include many of the most common chronic diseases and disorders of aging mentioned above, as well as lifestyle factors (Askarova et al., 2020; Edwards et al., 2019). Notably, many of these are also cardiovascular risk factors including obesity, hypertension, diabetes, hypercholesterolemia, depression, psychological stress, social isolation, physical inactivity, and poor nutrition (Serrano-Pozo & Growdon, 2019).
1.4 |. Aging-related neurocognitive decline and neuropathology in NHP models
NHPs have been valuable models for the study of most of these known risk factors for AD (Phillips et al., 2014). The current understanding of the basic biology of AD is largely dependent on rodent models, however, to date, the translation of novel therapeutics from rodent to humans has had little success (Drummond & Wisniewski, 2017). Rodents and humans diverged much earlier than humans and NHPs, and this may have led to fundamental differences in their aging processes (Messaoudi & Ingram, 2012). NHPs are particularly valuable to understanding aging effects on the CNS because of their long lifespan, and similarity to humans in the accrual of decrements in sensory, motor, social and cognitive function (Mattison & Vaughan, 2017). Unlike the rodent brain, many aging-related gene expression changes in the brain are conserved from NHPs to humans (Loerch et al., 2008). Thus, NHPs may fill the gap between rodent models and humans (Verdier et al., 2015).
Several NHP species develop aging-related neuropathologies that are reminiscent of preclinical and early changes observed in human AD (see (Bateman et al., 2012; Long & Holtzman, 2019; Sperling et al., 2011) for reviews). Table 1 summarizes naturally occurring age-related and induced neuropathologies of the species that are the focus of articles in this special issue, and a broader overview of NHP neuropathology is provided by Freire-Cobo et al. (2021). Taken together, these data demonstrate that several aging NHPs naturally display human AD-like amyloid plaques and aggregated hyperphosphorylated tau protein, but a relative lack of tangles.
1.4.1 |. Induced models
The use of induced NHP models of AD complements studies of naturally occurring NHP models, thereby advancing our mechanistic understanding of the transitions from normal aging to AD. For example, induced cynomolgus macaque (Macaca fascicularis) models, in which Aβ oligomers were injected directly into the brain, re-capitulated several signatures of human AD, including Aβ accumulation, tau hyperphosphorylation, microglial and astrocyte activation, cognitive impairment, and the formation of neurofibrillary tangles (Forny-Germano et al., 2014). Recent work in rhesus macaques (M. mulatto) supports these findings, showing that Aβ injection leads to dendritic spine loss reminiscent of normal aging, induces neuroinflammation, and increases AD biomarkers (Beckman et al., 2019b). Induced models of tau propagation (Beckman et al., 2021) may be critically important given that NHPs do not appear to develop the extensive neurofibrillary tangles due to tauopathies characteristic of human AD. Taken together with the ongoing work in naturally occurring NHP models of aging and NHPs, studies using induced models will support the development of therapeutic interventions to slow or even reverse AD progression.
1.5 |. Current limitations of NHP models of aging and AD
There is a need to better understand CNS aging and factors that influence it across the primate Order. The following limit the understanding of aging in NHPs: (1) Currently, the field lacks critical data regarding the physiologic changes that accompany normal aging for many species of NHPs. These data are necessary to distinguish healthy from pathologic aging phenotypes. (2) There is limited availability of aged animals. Relatively long lifespans (compared to rodents) as well as the costs associated with maintaining aged populations of NHPs in captivity are primary factors contributing to the low availability of age-appropriate subjects (NIH, 2018). In addition, high demands for a particular species (e.g., rhesus monkeys for vaccine testing, marmosets for neuroscience studies), and lack of commercially available resources severely restrict the numbers of animals available for aging studies (Servick, 2018). Within the populations of NHPs that are available for study, the sex ratios of older-aged animals often are skewed. This situation arises because husbandry practices may aim to recapitulate the normal social structures of wild animals, in which there is a low male: female ratio. Thus, the availability of older-aged male monkeys is limited for many species of interest to aging and AD. Finally, due to the NIH chimpanzee breeding moratorium, retirement of NIH-owned animals to sanctuaries, and restrictions on the type of research that can be conducted, the population of chimpanzees available for aging research has been drastically reduced (Collins, 2015). (3) It is difficult to establish stable long-term funding for long-lived animals. While efforts to increase the availability of aged NHPs are currently underway (e.g., “Brain Initiative” NIH), many funding mechanisms are less than five years, and multiple successful renewals are necessary to reach natural end of life. As such, ongoing longitudinal studies of NHP aging may end prematurely due to financial constraints. These challenges emphasize the need for increased support for long-term studies of aging NHP cohorts.
1.6 |. Contributions of the articles in this Special Issue
Within the comparative perspective of this Special Issue, the authors have addressed various aspects of the aging phenotype in well-characterized and emerging NHP models, ranging from prosimians to hominids, including: gray mouse lemurs (Chaudron et al. 2021), common marmosets (Rothwell et al., 2021), vervet/African green monkeys (Frye et al, 2021), three macaque species: rhesus (Arnsten et al, 2021; Beckman & Morrison, 2021; Upright & Baxter, 2021), cynomolgus (Darusman et al, 2021), and Barbary (Rathke & Fischer, 2021); and chimpanzees (Mulholland et al, 2021). Four main themes emerge from this collection.
One theme highlights the diversity of NHP models suitable for aging research. The review by Chaudron et al. (2021) provides a detailed description of the aging phenotype in a well-characterized prosimian, the gray mouse lemur. The authors review the cognitive and psychomotor changes that develop with age in this species and summarize age-related cerebral, metabolic and cellular alterations that correlate with cognitive decline. They also discuss the effectiveness of nutritional interventions such as caloric restriction and diet supplementation in affecting aging trajectories in this species. Similarly, Frye et al. (2021) provide a comprehensive characterization of the aging phenotype in vervet/African green monkeys. Their review emphasizes the many similarities between humans and vervets in age-related decline in cognitive, physical, metabolic, and cerebral function. Rothwell et al. (2021) comment on the crucial importance of longitudinal assessments for understanding neurocognitive aging and discuss the value of the common marmoset, an NHP with a relatively short lifespan, to assess age-related change in cognition, behavior, and brain function. They point out that the heterogeneity of cognitive profiles identified in aging marmosets provides an opportunity to capture trajectories of healthy versus pathological aging and to identify predictors of cognitive decline. While the papers above highlight the advantages and limitations of NHP models of natural AD pathology, Beckman & Morrison (2021) underline the usefulness of an induced model of early AD, based on cerebral injection of Aβ soluble oligomers. Their paper summarizes the suite of neural alterations observed in Aβ-treated female rhesus monkeys and argue for the development of this model for a better understanding of sex-specific manifestations of AD.
The second theme of this volume addresses one aspect of aging that has received little attention until recently, social aging. Rathke & Fischer (2021) use social network analysis to assess age-related differences in social activity in semi-free barbary macaques. Interestingly, age-related reductions in social effort were found, and social aging was independent of sex. This work provides much needed comparative insights into patterns typifying social aging in both male and female primates.
The third theme highlighted in the Special Issue is brain aging and AD neuropathology. Based on cytoarchitectonie and structural similarities between the human and macaque prefrontal cortex, Upright & Baxter (2021) emphasize the value of cross-species comparisons and review the prefrontal-dependent cognitive changes observed in healthy aging in the rhesus monkey. Also focusing on healthy aging, Mulholland et al. (2021) illustrate how neuroimaging and cognitive data can be used in concert to characterize neurocognitive aging. Using Volumetric Based Morphometry in chimpanzees who were cognitively characterized, they show that successful agers have greater gray matter volume in many brain regions compared to apes who underperform for their age, and that these differences concern regions that typically decline with age. Frye et al. (2021) also provide evidence that vervet monkeys recapitulate many of the features of early AD pathophysiology. Finally, the review by Freire-Cobo et al. (2021) offers a much-needed inventory of the age-related cellular changes that occur with normal and pathological aging across primate species.
The last theme of this volume focuses on potential mechanisms for age-related cognitive decline. Focusing on molecular mechanisms, Darusman et al. (2021) use qPCR to examine the expression of 6 APP-pathway related genes in blood samples from cognitively impaired versus unimpaired cynomolgus macaques. One gene (GAPDPH) involved in amyloidogenesis was upregulated in cognitively impaired monkeys versus control monkeys and correlated with the magnitude of cognitive impairment, providing a potential target for treatment. Based on extensive data collected in rhesus monkeys and other primate comparisons, Arnsten et al. (2021) propose that dysregulation of calcium signaling due to aging or inflammation is a key factor in initiating tau pathology. Framing this hypothesis in an evolutionary framework, the authors argue that the expansion of association cortices in the course of primate evolution led to an increase in synapses critical to calcium signaling, making the brain of humans and closely related species more vulnerable to calcium toxicity and tau pathology. This compelling hypothesis may lead to identifying key cortical circuits for early intervention.
2 |. CONCLUSION
This Special Issue provides a comprehensive description of phenotypes that accompany aging in several species of NHPs that are valuable models for advancing our understanding of human aging. In addition to providing important comparative insights across the Primate Order, this body of work delivers important translational lessons about aging trajectories that may culminate in chronic diseases, including neurodegenerative disorders, such as Alzheimer’s disease. Characterizing these patterns of aging in primates will promote understanding of the degree to which aging is malleable and identification of interventions that extend healthspan.
ACKNOWLEDGMENTS
This study complies with the Journal’s policies on ethical research and treatment of Nonhuman primates and this study adhered to the American Society of Primatologists (ASP) Principles for the Ethical Treatment of Nonhuman Primates. This study was supported by National Institutes of Health (NIH) RF1AG058829 (CAS & SC), P30 AG049638 (SC), ROI AG046266 (AL), F32 AG064925 (ESR), P30 AG21332 (SK), P40 OD010965 (MJ), UL1TR001420 (Wake Forest Clinical and Translational Science Institute) T32 AG033534 (SK), Wake Forest School of Medicine Department of Pathology Intramural Grant (CAS).
Funding information
National Center for Advancing Translational Sciences, Grant/Award Number: UL1TR001420; National Institute on Aging, Grant/Award Numbers: ROI AG046266, P30 AG21332, F32 AG064925, P30 AG049638, RF1 AG058829, T32 AG033534; Wake Forest School of Medicine Department of Pathology Intramural Grant; NIH Office of the Director, Grant/Award Number: P40 OD010965
Footnotes
CONFLICT OF INTERESTS
The authors declare that there are no conflict of interests.
DATA AVAILABILITY STATEMENT
This is an Introduction and thus exempt. Data sharing is not applicable to this article as no new data were created or analyzed in this study.
REFERENCES
- Abbott DH, Barnett DK, Colman RJ, Yamamoto ME, & Schultz-Darken NJ (2003). Aspects of common marmoset basic biology and life history important for biomedical research. Comparative Medicine, 53(4), 339–350. [PubMed] [Google Scholar]
- Abbott DH, & Hearn JP (1978). Physical, hormonal and behavioural aspects of sexual development in the marmoset monkey, Callithrix jacchus. J. Reprod. Fert, 53, 155–166. [DOI] [PubMed] [Google Scholar]
- Abdelnour C, Valero S, Rosende-Roca M, Lafuente A, Hernandez I, Tartari JP, … Boada M (2020). Gender and sex bias in clinical trial recruitment in Alzheimer’s disease: Findings from Fundadó ACE. Alzheimer’s & Dementia, 16(S10), 800. 10.1002/alz.041234 [DOI] [Google Scholar]
- Alzheimer Association. (2020). 2020 Alzheimer’s disease facts and figures. Alzheimer’s & Dementia, 16(1), 391. [Google Scholar]
- Arias E, Tejada-Vera B, & Ahmad F (2020). Provisional life expectancy estimates for January through June, 2020. Vital Statistics Rapid Release. Retrieved from https://stacks.cdc.gov/view/cdc/100392 [Google Scholar]
- Arnsten AFT, Datta D, & Preuss TM (2021). Studies of aging nonhuman primates illuminate the etiology of early-stage Alzheimer’s-like neuropathology: An evolutionary perspective. American Journal of Primatology, e23254. 10.1002/ajp.23254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Askarova S, Umbayev B, Masoud AR, Kaiyrlykyzy A, Safarova Y, Tsoy A, Olzhayev F, & Kushugulova A (2020). The links between the gut microbiome, aging, modern lifestyle and Alzheimer’s disease. Frontiers in Cellular and Infection Microbiology, 10, 104. 10.3389/fcimb.2020.00104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atkins HM, Willson CJ, Silverstein M, M. J, Floyd E, Kaplan JR, & Appt SE (2014). Characterization of ovarian aging and reproductive senescence in vervet monkeys (Chlorocebus aethiops abaeus). Comparative Medicine, 64(1), 55–62. [PMC free article] [PubMed] [Google Scholar]
- Bateman RJ, Xiong C, Benzinger TL, Fagan AM, Goate A, Fox NC, Marcus DS, Cairns NJ, Xie X, Blazey TM, Holtzman DM, Santacruz A, Buckles V, Oliver A, Moulder K, Aisen PS, Ghetti B, Klunk WE, McDade E, Dominantly Inherited Alzheimer N (2012). Clinical and biomarker changes in dominantly inherited Alzheimer’s disease. New England Journal of Medicine, 367(9), 795–804. 10.1056/NEJMoa1202753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckman D, Chakrabarty P, Ott S, Dao A, Zhou E, Janssen WG, Donis-Cox K, Muller S, Kordower JH, & Morrison JH (2021). A novel tau-based rhesus monkey model of Alzheimer’s pathogenesis. Alzheimer’s & Dementia: The Journal of the Alzheimer’s Association, 17, 933–945. 10.1002/alz.12318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckman D, & Morrison JH (2021). Towards developing a rhesus monkey model of early Alzheimer’s disease focusing on women’s health. American Journal of Primatology, e23289. 10.1002/ajp.23289 [DOI] [PubMed] [Google Scholar]
- Beckman D, Ott S, Donis-Cox K, Janssen WG, Bliss-Moreau E, Rudebeck PH, Baxter MG, & Morrison JH (2019a). Oligomeric Abeta in the monkey brain impacts synaptic integrity and induces accelerated cortical aging. Proceedings of the National Academy of Sciences of the United States of America, 10.1073/pnas.1902301116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckman D, Ott S, Donis-Cox K, Janssen WG, Bliss-Moreau E, Rudebeck PH, Baxter MG, & Morrison JH (2019b). Oligomeric Aβ in the monkey brain impacts synaptic integrity and induces accelerated cortical aging. Proceedings of the National Academy of Sciences of the United States of America, 116(52), 26239–26246. 10.1073/pnas.1902301116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjork JR, Dasari M, Grieneisen L, & Archie EA (2019). Primate microbiomes over time: Longitudinal answers to standing questions in microbiome research. American Journal of Primatology, 81(10–11), e22970. 10.1002/ajp.22970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brzecka A, Leszek J, Ashraf GM, Ejma M, Ávila-Rodriguez MF, Yarla NS, Tarasov VV, Chubarev VN, Samsonova AN, Barreto GE, & Aliev G (2018). Sleep disorders associated with Alzheimer’s disease: A perspective. Frontiers in Neuroscience, 12, 330. 10.3389/fnins.2018.00330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bubu OM, Brannick M, Mortimer J, Umasabor-Bubu O, Sebastião YV, Wen Y, Schwartz S, Borenstein AR, Wu Y, Morgan D, & Anderson WM (2017). Sleep, cognitive impairment, and Alzheimer’s disease: A systematic review and meta-analysis. Sleep, 40(1), 10.1093/sleep/zsw032 [DOI] [PubMed] [Google Scholar]
- Campos FA, Archie EA, Gesquiere LR, Tung J, Altmann J, & Alberts SC (2021). Glucocorticoid exposure predicts survival in female baboons. Science Advances, 7(17). 10.1126/sciadv.abf6759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudron Y, Pifferi F, & Aujard F (2021). Overview of age-related changes in psychomotor and cognitive functions in a prosimian primate, the grey mouse lemur (Microcebus murinus): Recent advances in risk factors and anti-aging interventions. American Journal of Primatology. [DOI] [PubMed] [Google Scholar]
- Chen JC, Espeland MA, Brunner RL, Lovato LC, Wallace RB, Leng X, Phillips LS, Robinson JG, Kotchen JM, Johnson KC, Manson JE, Stefanick ML, Sarto GE, & Mysiw WJ (2016). Sleep duration, cognitive decline, and dementia risk in older women. Alzheimer’s & Dementia: The Journal of the Alzheimer’s Association, 12(1), 21–33. 10.1016/j.jalz.2015.03.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chetty R, Stepner M, Abraham S, Lin S, Scuderi B, Turner N, Bergeron A, & Cutler D (2016). The association between income and life expectancy in the United States, 2001–2014. Journal of the American Medical Association, 315(16), 1750–1766. 10.1001/jama.2016.4226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins F (2015). NIH will no longer support biomedical research on chimpanzees. Retrieved from https://www.nih.gov/about-nih/who-we-are/nih-director/statements/nih-will-no-longer-support-biomedical-research-chimpanzees
- Cramer PE, Gentzel RC, Tanis KQ, Vardigan J, Wang Y, Connolly B, Manfre P, Lodge K, Renger JJ, Zerbinatti C, & Uslaner JM (2018). Aging African green monkeys manifest transcriptional, pathological, and cognitive hallmarks of human Alzheimer’s disease. Neurobiology of Aging, 64, 92–106. 10.1016/j.neurobiolaging.2017.12.011 [DOI] [PubMed] [Google Scholar]
- Darusman HS, Gjedde A, Sajuthi D, Schapiro SJ, Kalliokoski O, Kristianingrum YP, Handaryani E, & Hau J (2014). Amyloid beta 1–42 and phoshorylated tau threonin 231 in brains of aged cynomolgus monkeys (Macaca fascicularis). Frontiers in Aging Neuroscience, 313, 313. 10.3389/fnagi.2014.00313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darusman HS, Saepuloh U, Mariya SS, Sajuthi D, Schapiro SJ, & Hau J (2021). Increased expression of GAPDH in cynomolgus monkeys with spontaneous cognitive decline and amyloidopathy reminiscent of an Alzheimer’s-type disease is reflected in the circulation. American Journal of Primatology, e23296. 10.1002/ajp.23296 [DOI] [PubMed] [Google Scholar]
- Dodge HH, Zhu J, Lee CW, Chang CC, & Ganguli M (2014). Cohort effects in age-associated cognitive trajectories. The Journals of Gerontology. Series A, Biological Sciences and Medical Sciences, 69(6), 687–694. 10.1093/gerona/glt181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drummond E, & Wisniewski T (2017). Alzheimer’s disease: Experimental models and reality. Acta Neuropathologica, 133(2), 155–175. 10.1007/s00401-016-1662-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dyke B, Gage TB, Alford PL, Swenson B, & Williams-Blangero S (1995). Model life table for captive chimpanzees. American Journal of Primatology, 37(1), 25–37. 10.1002/ajp.1350370104 [DOI] [PubMed] [Google Scholar]
- Edwards III GA, Gamez N, Escobedo G Jr., Calderon O, & Moreno-Gonzalez I (2019). Modifiable risk factors for Alzheimer’s disease. Frontiers in Aging Neuroscience, 11, 146. 10.3389/fnagi.2019.00146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fa J (1989). The genus Macaca: A review of taxonomy and evolution. Mammal Review, 19(2), 458–481. [Google Scholar]
- Fagan AM, Roe CM, Xiong C, Mintun MA, Morris JC, & Holtzman DM (2007). Cerebrospinal fluid tau/beta-amyloid(42) ratio as a prediction of cognitive decline in nondemented older adults. Archives of Neurology, 64(3), 343–349. 10.1001/archneur.64.3.noc60123 [DOI] [PubMed] [Google Scholar]
- Fagan AM, Xiong C, Jasielec MS, Bateman RJ, Goate AM, Benzinger TL, Ghetti B, Martins RN, Masters CL, Mayeux R, Ringman JM, Rossor MN, Salloway S, Schofield PR, Sperling RA, Marcus D, Cairns NJ, Buckles VD, Ladenson JH, … Dominantly Inherited Alzheimer N (2014). Longitudinal change in CSF biomarkers in autosomal-dominant Alzheimer’s disease. Science Translational Medicine, 6(226), 226ra230. 10.1126/scitranslmed.3007901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fagan T, Kagan B, Corbin D, Hwang W, Glabe C, Nault L, Lai R, Teplow D, Albensi B, & Sokolov Y (2006). Alzheimer research forum live discussion: Now you see them, now you don’t: The amyloid channel hypothesis. Journal of Alzheimer’s Disease, 9(2), 219–224. 10.3233/jad-2006-9213 [DOI] [PubMed] [Google Scholar]
- Fooden J (2007). Systematic review of the Barbary Macaque, Macaca Sylvanus (Linnaeus, 1758). Biochemical Pharmacology, 113, 1–60. 10.3158/0015 [DOI] [Google Scholar]
- Forny-Germano L, Lyra e Silva NM, Batista AF, Brito-Moreira J, Gralle M, Boehnke SE, Coe BC, Lablans A, Marques SA, Martinez AM, Klein WL, Houzel JC, Ferreira ST, Munoz DP, & De Felice FG (2014). Alzheimer’s disease-like pathology induced by amyloid-beta oligomers in nonhuman primates. Journal of Neuroscience, 34(41), 13629–13643. 10.1523/jneurosci.1353-14.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freire-Cobo C, Edler MK, Varghese M, Munger E, Laffey J, Raia S, In SS, Wicinski B, Medalla M, Perez SE, Mufson EJ, Erwin JM, Guevara EE, Sherwood CC, Luebke JI, Lacreuse A, Raghanti MA, & Hof PR (2021). Comparative primate neuropathology: A perspective. American Journal of Primatology, 23299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman H, Ator N, Haigwood N, Newsome W, Allan JS, Golos TG, Kordower JH, Shade RE, Goldberg ME, Bailey MR, & Bianchi P (2017). The critical role of nonhuman primates in medical research. Pathogens and Immunity, 2(3), 352–365. 10.20411/pai.v2i3.186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frye BM, Craft S, Latimer CS, Keene GD, Montine TJ, Register TC, Orr ME, Kavanagh K, Macauley SL, & Shively CA (2021). Aging-related Alzheimer’s disease-like neuropathology and functional decline in captive vervet monkeys (Chlorocebus aethiops sabaeus). American Journal of Primatology, e23260. 10.1002/ajp.23260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gary С, Lam S, Hérard AS, Koch JE, Petit F, Gipchtein P, Sawiak SJ, Caillierez R, Eddarkaoui S, Colin M, Aujard F, Deslys JP, French Neuropathology N, Brouillet E, Buée L, Comoy EE, Pifferi F, Picq JL, & Dhenain M (2019). Encephalopathy induced by Alzheimer brain inoculation in a nonhuman primate. Acta Neuropathologica Communications, 7(1), 126. 10.1186/s40478-019-0771-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gispert JD, Monté GС, Suárez-Calvet M, Falcon C, Tucholka A, Rojas S, Rami L, Sánchez-Valle R, Lladó A, Kleinberger G, Haass С, & Molinuevo JL (2017). The APOE epsilon4 genotype modulates CSF YKL-40 levels and their structural brain correlates in the continuum of Alzheimer’s disease but not those of sTREM2. Alzheimers Dement (Amst), 6, 50–59. 10.1016/j.dadm.2016.12.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold E (2011). The timing of the age at which natural menopause occurs. Obstetrics and Gynecology Clinics of North America, 38(3), 425–440. 10.1016/j.ogc.2011.05.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon BA, Blazey T, Su Y, Fagan AM, Holtzman DM, Morris JC, & Benzinger TL (2016). Longitudinal β-Amyloid deposition and Hippocampal volume in preclinical Alzheimer disease and suspected non-Alzheimer disease pathophysiology. JAMA Neurol, 73(10), 1192–1200. 10.1001/jamaneurol.2016.2642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon BA, Blazey TM, Su Y, Hari-Raj A, Dincer A, Flores S, Christensen J, McDade E, Wang G, Xiong C, Cairns NJ, Hassenstab J, Marcus DS, Fagan AM, Jack CR J.r, Hornbeck RC, Paumier KL, Anees ВМ, Berman SВ, … Benzinger T (2018). Spatial patterns of neuroimaging biomarker change in individuals from families with autosomal dominant Alzheimer’s disease: A longitudinal study. Lancet Neurology, 17(3), 241–250. 10.1016/s1474-4422(18)30028-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg SM, Bacskai BJ, Hernandez-Guillamon M, Pruzin J, Sperling R, & van Veluw SJ (2020). Cerebral amyloid angiopathy and Alzheimer disease—one peptide, two pathways. Nature Reviews Neurology, 16(1), 30–42. 10.1038/s41582-019-0281-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guarino A, Favieri F, Boncompagni I, Agostini F, Cantone M, & Casagrande M (2019). Executive functions in Alzheimer disease: A systematic review. Frontiers in Aging Neuroscience, 10, 437. 10.3389/fnagi.2018.00437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guarino A, Forte G, Giovannoli J, & Casagrande M (2020). Executive functions in the elderly with mild cognitive impairment: A systematic review on motor and cognitive inhibition, conflict control and cognitive flexibility. Aging & Mental Health, 24(7), 1028–1045. 10.1080/13607863.2019.1584785 [DOI] [PubMed] [Google Scholar]
- Hämäläinen A, Dammhahn M, Aujard F, Eberle M, Hardy I, Kappeler PM, Perret M, Schliehe-Diecks S, & Kraus C (2014). Senescence or selective disappearance? Age trajectories of body mass in wild and captive populations of a small-bodied primate. Proceedings of the Royal Society B: Biological Sciences, 281(1791), 20140830. 10.1098/rspb.2014.0830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanseeuw BJ, Betensky RA, Jacobs H, Schultz AP, Sepulcre J, Becker JA, Cosio D, Farrell M, Quiroz YT, Mormino EC, Buckley RF, Papp KV, Amariglio RA, Dewachter I, Ivanoiu A, Huijbers W, Hedden T, Marshall GA, Chhatwal JP, … Johnson K (2019). Association of Amyloid and Tau with cognition in preclinical Alzheimer disease: A longitudinal study. JAMA Neurol, 76(8), 915–924. 10.1001/jamaneurol.2019.1424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hao L, Xu X, Dupre ME, Guo A, Zhang X, Qiu L, Zhao Y, & Gu D (2020). Adequate access to healthcare and added life expectancy among older adults in China. BMC Geriatrics, 20(1), 129. 10.1186/s12877-020-01524-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hochberg ZE, & Konner M (2020). Emerging adulthood, a pre-adult life-history stage. Front Endocrinol (Lausanne), 10, 918. 10.3389/fendo.2019.00918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopkins WD, Mareno MC, Webb SJN, Schapiro SJ, Raghanti MA, & Sherwood CC (2020). Age-related changes in chimpanzee (Pan troglodytes) cognition: Cross-sectional and longitudinal analyses. bioRxiv. 10.1101/2020.04.27.064626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou Y, Dan X, Babbar M, Wei Y, Hasselbalch SG, Croteau DL, & Bohr VA (2019). Ageing as a risk factor for neurodegenerative disease. Nature Reviews Neurology, 15, 565–581. 10.1038/S41582-019-0244-7 [DOI] [PubMed] [Google Scholar]
- Hughes C, Choi ML, Yi JH, Kim SC, Drews A, George-Hyslop PS, Bryant C, Gandhi S, Cho K, & Klenerman D (2020). Beta amyloid aggregates induce sensitised TLR4 signalling causing long-term potentiation deficit and rat neuronal cell death. Commun Biol, 3(1), 79. 10.1038/s42003-020-0792-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack CR Jr., & Holtzman DM 2013Biomarker modeling of Alzheimer’s disease Neuron 80613471358 10.1016/j.neuron.2013.12.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack CR Jr., Holtzman DM, & Sperling R (2019). Dementia is not synonymous with Alzheimer’s disease. Science Translational Medicine, 11(522) 10.1126/scitranslmed.aav0511 [DOI] [PubMed] [Google Scholar]
- Jaul E, & Barron J (2017). Age-related diseases and clinical and public health implications for the 85 years old and over population. Frontiers in Public Health, 5, 335. 10.3389/fpubh.2017.00335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jorgensen MJ (2021). Behavioral biology of Vervets/African green monkeys. In Coleman K, & Schapiro SJ (Eds.), Behavioral biology of laboratory animals. CRC Press. [Google Scholar]
- Ju YE, McLeland JS, Toedebusch CD, Xiong C, Fagan AM, Duntley SP, Morris JC, & Holtzman DM (2013). Sleep quality and preclinical Alzheimer disease. JAMA Neurol, 70(5), 587–593. 10.1001/jamaneurol.2013.2334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karikari TK, Emeršič A, Vrillon A, Lantero-Rodriguez J, Ashton NJ, Kramberger MG, Dumurgier J, Hourregue C, Čučnik S, Brinkmalm G, Rot U, Zetterberg H, Paquet С, & Blennow K (2020). Head-to-head comparison of clinical performance of CSF phospho-tau T181 and T217 biomarkers for Alzheimer’s disease diagnosis. Alzheimer’s & Dementia: The Journal of the Alzheimer’s Association, 17, 755–767. 10.1002/alz.12236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kavanagh K, Day SM, Pait MC, Mortiz WR, Newgard СB, Ilkayeva O, Mcdain DA, & Macauley SL (2019). Type-2-diabetes alters CSF but not plasma metabolomic and AD risk profiles in Vervet monkeys. Frontiers in Neuroscience, 13, 843. 10.3389/fnins.2019.00843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kavanagh K, Williams JK, & Wagner JD (2005). Naturally occurring menopause in cynomolgus monkeys: Changes in hormone, lipid, and carbohydrate measures with hormonal status. Journal of Medical Primatology, 34(4), 171–177. 10.1111/j.1600-0684.2005.00114.x [DOI] [PubMed] [Google Scholar]
- Kirova AM, Bays RB, & Lagalwar S (2015). Working memory and executive function decline across normal aging, mild cognitive impairment, and Alzheimer’s disease. BioMed Research International, 2015, 748212. 10.1155/2015/748212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacreuse A, Chennareddi L, Gould KG, Hawkes K, Wijayawardana SR, Chen J, Easley KA, & Herndon JG (2008). Menstrual cycles continue into advanced old age in the common chimpanzee(Pan troglodytes). Biology of Reproduction, 79(3), 407–412. 10.1095/biolreprod.108.068494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Languille S, Blanc S, Blin O, Canale СI, Dal-Pan A, Devau G, Dhenain M, Dorieux O, Epelbaum J, Gomez D, Hardy I, Henry PY, Irving EA, Marchal J, Mestre-Francés N, Perret M, Picq JL, Pifferi F, Rahman A, … Aujard F (2012). The grey mouse lemur: A non-human primate model for ageing studies. Ageing Research Reviews, 11(1), 150.-. 10.1016/j.arr.2011.07.001 [DOI] [PubMed] [Google Scholar]
- Leigh SR, & Shea BT (1995). Ontogeny and the evolution of adult body size dimorphism in apes. American Journal of Primatology, 36(1), 37–60. 10.1002/ajp.1350360104 [DOI] [PubMed] [Google Scholar]
- Lim AS, Kowgier M, Yu L, Buchman AS, & Bennett DA (2013). Sleep fragmentation and the risk of incident Alzheimer’s disease and cognitive decline in older persons. Sleep, 36(7), 1027–1032. 10.5665/sleep.2802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lloret MA, Cervera-Ferri A, Nepomuceno M, Monllor P, Esteve D, & Lloret A (2020). Is sleep disruption a cause or consequence of Alzheimer’s disease? Reviewing its possible role as a biomarker. International Journal of Molecular Sciences, 21(3). 10.3390/ijms21031168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loerch PM, Lu T, Dakin KA, Vann JM, Isaacs A, Geula C, Wang J, Pan Y, Gabuzda DH, Li C, Prolla TA, & Yankner BA (2008). Evolution of the aging brain transcriptome and synaptic regulation. PLOS One, 3(10), e3329. 10.1371/journal.pone.0003329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long JM, & Holtzman DM (2019). Alzheimer disease: An update on pathobiology and treatment strategies. Cell, 179(2), 312–339. 10.1016/j.cell.2019.09.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu S, Zhao Y, Yu W, Yang Y, Gao J, Wang J, Kuang D, Yang M, Yang J, Ma C, Xu J, Qian X, Li H, Zhao S, Li J, Wang H, Long H, Zhou J, Luo F … Peng X (2020). Comparison of nonhuman primates identified the suitable model for COVID-19. Signal Transduction and Targeted Therapy, 5(1), 157. 10.1038/S41392-020-00269-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luetjens CM, & Weinbauer GF (2012). Functional assessment of sexual maturity in male macaques (Macaca fascicularis). Regulatory Toxicology and Pharmacology, 63(3), 391–400. 10.1016/j.yrtph.2012.05.003 [DOI] [PubMed] [Google Scholar]
- Maclean CJ, Baker HF, Ridley RM, & Mori H (2000). Naturally occurring and experimentally induced-amyloid deposits in the brains of marmosets (Callithrix jacchus). International Journal of Neural Transmission, 107, 799–814. [DOI] [PubMed] [Google Scholar]
- Mattison JA, & Vaughan KL (2017). An overview of nonhuman primates in aging research. Experimental Gerontology, 94, 41–45. 10.1016/j.exger.2016.12.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKhann GM, Knopman DS, Chertkow H, Hyman BT, Jack CR J.r Jr., Kawas CH, Klunk WE, Koroshetz WJ, Manly JJ, Mayeux R, Mohs RC, Morris JC, Rossor MN, Scheltens P, Carrillo MG, Thies B, Weintraub S, Phelps GH (2011). The diagnosis of dementia due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimer’s & Dementia: The Journal of the Alzheimer’s Association, 7(3), 263–269. 10.1016/j.jalz.2011.03.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messaoudi I, & Ingram DK (2012). Overview of aging research using nonhuman primate models. Age (Dordr), 34(5), 1047–1049. 10.1007/s11357-011-9370-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris JC, Roe CM, Grant EA, Head D, Storandt M, Goate AM, Fagan AM, Holtzman DM, & Mintun MA (2009). Pittsburgh compound В imaging and prediction of progression from cognitive normality to symptomatic Alzheimer disease. Archives of Neurology, 66(12), 1469–1475. 10.1001/archneurol.2009.269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosconi L, Pupi A, & De Leon MJ (2008). Brain glucose hypometabolism and oxidative stress in preclinical Alzheimer’s disease. Annals of the New York Academy of Sciences, 1147,180–195. 10.1196/annals.1427.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller MN, Blurton Jones NG, Colchero F, Thompson ME, Enigk DK, Feldblum JT, & Pusey AE (2020). Sexual dimorphism in chimpanzee (Pan troglodytes schweinfurthii) and human age-specific fertility. Journal of Human Evolution, 144. 10.1016/j.jhevol.2020.102795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulholland MM, Sherwood CC, Schapiro SJ, Raghanti MA, & Hopkins WD (2021). Age- and cognition-related differences in the gray matter volume of the chimpanzee brain (Pan troglodytes): A voxel-based morphometry and conjunction analysis. American Journal of Primatology, e23264. 10.1002/ajp.23264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy C (2019). Olfactory and other sensory impairments in Alzheimer disease. Nature Reviews Neurology, 15(1), 11–24. 10.1038/S41582-018-0097-5 [DOI] [PubMed] [Google Scholar]
- NIH. (2018). Nonhuman Primate Evaluation and Analysis Part 2: Report of the Expert Panel Forum on Challenges in Assessing Nonhuman Primate Needs and Resources for Biomedical Research. Retrieved from https://orip.nih.gov/about-orip/research-highlights/nonhuman-primate-evaluation-and-analysis-part-2-report-expert-panel
- Nishijima K, Saitoh R, Tanaka S, Ohsato-Suzuki M, Ohno T, & Kitajima S (2012). Life span of common marmoset (Callithrix jacchus) at CLEA Japan breeding colony. Biogerontology, 13, 439–443. 10.1007/s10522-012-9388-l [DOI] [PubMed] [Google Scholar]
- Olshansky SJ (2018). From lifespan to healthspan. Journal of the American Medical Association, 320(13), 1323–1324. [DOI] [PubMed] [Google Scholar]
- Paul A, Kuester J, & Podzuweit D (1993). Reproductive senescence and terminal investment in female Barbary macaques (Macaca sylvanus) at Salem. International Journal of Primatology, 14(1), 105–124. 10.1007/BF02196506 [DOI] [Google Scholar]
- Perret M (1997). Change in photoperiodic cycle affects life span in a prosimian primate (Microcebus murinus). Journal of Biological Rhythms, 12(2), 136–145. 10.1177/074873049701200205 [DOI] [PubMed] [Google Scholar]
- Philippens IH, Ormel PR, Baarends G, Johansson M, Remarque EJ, & Doverskog M (2016). Acceleration of amyloidosis by inflammation in the amyloid-beta marmoset monkey model of Alzheimer’s disease. Journal of Alzheimer’s Disease, 55(1), 101–113. 10.3233/JAD-160673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips KA, Bales KL, Capitanio JP, Conley A, Czoty PW, t Hart BA, Voytko ML (2014). Why primate models matter. American Journal of Primatology, 76(9), 801–827. 10.1002/ajp.22281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plant TM (2005). Postnatal and pubertal development of the rhesus monkey (Macaca mulatto) testis. Annals of the New York Academy of Sciences, 1061(1), 149–162. 10.1196/annals.1336.016 [DOI] [PubMed] [Google Scholar]
- Rathke EM, & Fischer J (2021). Social aging in male and female Barbary macaques (Macaca sylvanus). American Journal of Primatology, 23272. [DOI] [PubMed] [Google Scholar]
- Ridley RM, Baker HF, Windle CP, & Cummings RM (2006). Very long term studies of the seeding of β-amyloidosis in primates. Journal of Neural Transmission, 113(9), 1243–1251. 10.1007/S00702-005-0385-2 [DOI] [PubMed] [Google Scholar]
- Robine J-M Allard M, Herrmann FR, Jeune В (2019). The real facts supporting Jeanne Calment as the oldest ever human. The Journals of Gerontology: Series A, 74, (Supplement_1), S13–S20. 10.1093/gerona/glz198 [DOI] [PubMed] [Google Scholar]
- Ross CN (2019). Marmosets in aging research. The Common Marmoset in Captivity and Biomedical Research, 355–376. 10.1016/B978-0-12-811829-0.00021-2 [DOI] [Google Scholar]
- Rothwell ES, Freire-Cobo C, Varghese M, Edwards M, Janssen WGM, Hof PR, & Lacreuse A (2021). The marmoset as an important primate model for longitudinal studies of neurocognitive aging. American Journal of Primatology, e23271. 10.1002/ajp.23271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sapolsky RM, & Altmann J (1991). Incidence of hypercortisolism and dexamethasone resistance increases with age among wild baboons. Biological Psychiatry, 30(10), 1008–1016. 10.1016/0006-3223(91)90121-2 [DOI] [PubMed] [Google Scholar]
- Schmitt CA, Service SK, Jasinska AJ, Dyer TD, Jorgensen MJ, Cantor RM, Weinstock GM, Blangero J, Kaplan JR, & Freimer NB (2018). Obesity and obesogenic growth are both highly heritable and modified by diet in a nonhuman primate model, the African green monkey (Chlorocebus aethiops sabaeus). International Journal of Obesity (London), 42(4), 765–774. 10.1038/ijo.2017.301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seals DR, Justice JN, & LaRocca TJ (2016). Physiological geroscience: Targeting function to increase healthspan and achieve optimal longevity. Journal of Physiology, 594(8), 2001–2024. 10.1113/jphysiol.2014.282665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serrano-Pozo A, & Growdon JH (2019). Is Alzheimer’s disease risk modifiable? Journal of Alzheimer’s Disease, 67(3), 795–819. 10.3233/jad181028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Servick K (2018). US Labs clamour for marmosets: Shortage develops as new transgenic models for neurological diseases stroke interest. Science, 10.1126/science.aav8223 [DOI] [Google Scholar]
- Shively CA, & Clarkson TB (2009). The unique value of primate models in translational research. Nonhuman primate models of women’s health: introduction and overview. American Journal of Primatology, 71(9), 715–721. 10.1002/ajp.20720 [DOI] [PubMed] [Google Scholar]
- Shoven JB (2007). New age thinking: Alternative ways of measuring age, their relationship to labor force participation, government policies, and GDP. In. Cambridge, MA: National Beareau of Economic Reserach. [Google Scholar]
- Sierra F (2016). The Emergence of Geroscience as an Interdisciplinary Approach to the Enhancement of Health Span and Life Span. Cold Spring Harbor Perspectives in Medicine, 6(4), a025163. 10.1101/cshperspect.a025163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder-Mackler N, Burger JR, Gaydosh L, Belsky DW, Noppert GA, Campos FA, Bartolomucci A, Yang YC, Aiello AE, O’Rand A, Harris KM, Shively CA, Alberts SC, & Tung J (2020). Social determinants of health and survival in humans and other animals. Science, 368(6493) 10.1126/science.aax9553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sperling RA, Aisen PS, Beckett LA, Bennett DA, Craft S, Fagan AM, Iwatsubo T, Jack CR J.r, Kaye J, Montine TJ, Park DC, Reiman EM, Rowe CC, Siemers E, Stern Y, Yaffe K, Carrillo MC, Thies B, Morrison-Bogorad M, … Phelps CH (2011). Toward defining the preclinical stages of Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimer’s & Dementia: The Journal of the Alzheimer’s Association, 7(3), 280–292. 10.1016/j.jalz.2011.03.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spira AP, Covinsky K, Rebok GW, Punjabi NM, Stone KL, Hillier TA, Ensrud KE, & Yaffe K (2012). Poor sleep quality and functional decline in older women. Journal of the American Geriatrics Society, 60(6), 1092–1098. 10.1111/j.1532-5415.2012.03968.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spira AP, Kaufmann СN, Kasper JD, Ohayon ММ, Rebok GW, Skidmore E, Parisi JM, & Reynolds CF 3rd (2014). Association between insomnia symptoms and functional status in U.S. older adults. The Journals of Gerontology. Series B, Psychological Sciences and Social Sciences, 69(Suppl 1), S35–S41. 10.1093/geronb/gbu116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stonebarger GA, Urbanski HF, Woltjer RL, Vaughan KL, Ingram DK, Schultz PL, Calderazzo SM, Siedeman JA, Mattison JA, Rosene DL, & Kohama SG (2020). Amyloidosis increase is not attenuated by long-term calori restriction or related to neuronal density in the prefrontal cortex of extremely aged rhesus macaques. GeroScience, 42, 1733–1749. 10.1007/S11357-020-002-00259-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tigges J, Gordon TP, McClure HM, Hall EC, & Peters A (1988). Survival rate and life span of rhesus monkeys at the Yerkes regional primate research center. American Journal of Primatology, 15(3), 263–273. 10.1002/ajp.1350150308 [DOI] [PubMed] [Google Scholar]
- United Nation | Department of Economic and Social Affairs Population Dynamics. (2019). World population prospects 2019: Highlights. Retrieved from https://population.un.org/wpp
- Upright NA, & Baxter MG (2021). Prefrontal cortex and cognitive aging in macaque monkeys. American Journal of Primatology, e23250. 10.1002/ajp.23250 [DOI] [PubMed] [Google Scholar]
- Van Esch E, Cline JM, Buse E, Wood CE, De Rijk EPCT, & Weinbauer GF (2008). Summary comparison of female reproductive system in human and the cynomolgus monkey (Macaca fascicularis). Toxicologic Pathology, 36. [Google Scholar]
- van Wagenen G (1952). Age at menarche of the laboratory rhesus monkey. Anatomical Record, 112(2), 436. [Google Scholar]
- Vanderheyden WM, Lim MM, Musiek ES, & Gerstner JR (2018). Alzheimer’s disease and sleep-wake disturbances: Amyloid, astrocytes, and animal models. Journal of Neuroscience, 38(12), 2901–2910. 10.1523/jneurosci.1135-17.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veazey RS, & Lackner AA (2017). Nonhuman primate models and understanding the pathogenesis of HIV infection and AIDS. Institute for Laboratory Animal Research Journal, 58(2), 160–171. 10.1093/ilar/ilx032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verdier JM, Acquatella I, Lautier C, Devau G, Trouche S, Lasbleiz C, & Mestre-Francés N (2015). Lessons from the analysis of nonhuman primates for understanding human aging and neurodegenerative diseases. Frontiers in Neuroscience, 9, 64. 10.3389/fnins.2015.00064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vermunt L, Sikkes S, van den Hout A, Handels R, Bos I, van der Flier WM, Kern S, Ousset PJ, Maruff P, Skoog I, Verhey F, Freund-Levi Y, Tsolaki M, Wallin ÅK, Olde Rikkert M, Soininen H, Spiru L, Zetterberg H, Blennow K, … ICTUS/DSA study, g (2019). Duration of preclinical, prodromal, and dementia stages of Alzheimer’s disease in relation to age, sex, and APOE genotype. Alzheimer’s & Dementia: the journal of the Alzheimer’s Association, 15(7), 888–898. 10.1016/j.jalz.2019.04.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vos SJ, van Rossum IA, Verhey F, Knol DL, Soininen H, Wahlund LO, Hampel H, Tsolaki M, Minthon L, Frisoni GB, Froelich L, Nobili F, van der Flier W, Blennow K, Wolz R, Scheltens P, & Visser PJ (2013). Prediction of Alzheimer disease in subjects with amnestic and nonamnestic MCI. Neurology, 80(12), 1124–1132. 10.1212/WNL.0b013e318288690c [DOI] [PubMed] [Google Scholar]
- Walker ML (1995). Menopause in female rhesus monkeys. American Journal of Primatology, 35(1), 59–71. 10.1002/ajp.1350350106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walpole SC, Prieto-Merino D, Edwards P, Cleland J, Stevens G, & Roberts I (2012). The weight of nations: An estimation of adult human biomass. BMC Public Health, 12(1), 1–6. 10.1186/1471-2458-12-439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weigl R (2005). Longevity of mammals in captivity; from the Living Collections of the world: A list of mammalian longevity in captivity. Kleine Senckenberg-Reihe. [Google Scholar]
- Wimo A, Guerchet M, Ali GC, Wu YT, Prina AM, Winblad B, Jönsson L, Liu Z, & Prince M (2017). The worldwide costs of dementia 2015 and comparisons with 2010. Alzheimer’s & Dementia, 13(1), 1–7. 10.1016/j.jalz.2016.07.150 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
This is an Introduction and thus exempt. Data sharing is not applicable to this article as no new data were created or analyzed in this study.


