Evidence for uterine cancer prevention, diagnosis, and special issues from the Uterine Cancer Evidence Review Conference is summarized.
Abstract
The Centers for Disease Control and Prevention recognized the need for educational materials for clinicians on the prevention and early diagnosis of gynecologic cancers. The American College of Obstetricians and Gynecologists convened a panel of experts in evidence review from the Society for Academic Specialists in General Obstetrics and Gynecology and content experts from the Society of Gynecologic Oncology to review relevant literature, best practices, and existing practice guidelines as a first step toward developing evidence-based educational materials for women's health care clinicians about uterine cancer. Panel members conducted structured literature reviews, which were then reviewed by other panel members and discussed at a virtual meeting of stakeholder professional and patient advocacy organizations in January 2021. This article is the evidence summary of the relevant literature and existing recommendations to guide clinicians in the prevention, early diagnosis, and special considerations of uterine cancer. Substantive knowledge gaps are noted and summarized to provide guidance for future research.
The Centers for Disease Control and Prevention previously launched a campaign in collaboration with the American College of Obstetricians and Gynecologists (ACOG) to increase patient and clinician awareness of early-onset breast cancer.1 The Centers for Disease Control and Prevention expanded the project with ACOG to include educational material for clinicians for early diagnosis and prevention of gynecologic cancers. Uterine cancer is the most common gynecologic cancer. There were 65,620 new cases (3.6% of all new cancer cases) and 12,590 deaths (2.1% of all cancer deaths) projected for 2020. Rates rose on average 1.3% per year from 2007 to 2016.2 Because of its effect on so many women, uterine cancer was chosen as the first gynecologic cancer for educational material development. To ensure these materials were based on the most current literature and guidelines, an extensive literature review was conducted. This article is the evidence summary, which is presented in detail in the Appendices 2–8, available online at http://links.lww.com/AOG/C620. The health care professional educational material is available online at acog.org.
METHODS
Methods for the evidence review and educational material development closely followed the process for the early-onset breast cancer project,1 although virtual meetings replaced in-person meetings because of the coronavirus disease 2019 (COVID-19) pandemic. The American College of Obstetricians and Gynecologists convened an expert panel to identify the best evidence and practices from the literature and existing relevant guidelines. The panel was recruited from the Society for Academic Specialists in General Obstetrics and Gynecology to review and summarize the evidence. The panel was supplemented by representatives from the Society of Gynecologic Oncology (SGO). Panel members were selected based on expertise in evidence review and synthesis. The panel developed research questions and used the PICO criteria (P=patient, problem, or population; I=intervention; C=comparison, control, or comparator; O=outcome[s]) to frame the literature review.
Experts in literature searches from the ACOG Resource Center searched the Cochrane Library, MEDLINE (through Ovid), and PubMed (for references not indexed through MEDLINE) for articles published between January 2000 and October 2020. Literature was organized by level of evidence. Published guidelines were categorized separately from studies. A primary reviewer was assigned to each topic to review titles and abstracts, and then the entire manuscript when appropriate. Reference lists from relevant articles found in the search were also reviewed. Reviewers did additional searches as necessary, including extending the search range. Internet searches were performed using standard search engines to seek guidelines, recommendations, and tools that might not have been published in peer-reviewed publications. Relevant information was evaluated and compiled into an evidence summary template by a primary reviewer. Completed templates were then reviewed by a secondary reviewer. The primary and secondary reviewer worked together to revise the evidence summary in response to the secondary reviewer's comments.
The American College of Obstetricians and Gynecologists convened the Uterine Cancer Evidence Review Conference virtually on January 13, 2021, bringing together expert panel members and representatives from stakeholder professional and patient advocacy organizations (Appendix 1, http://links.lww.com/AOG/C620). The panel members who served as primary reviewers for each of the research topics prerecorded their presentations, which were viewed in advance by meeting participants, including the stakeholder representatives. At the meeting, expert panel members presented a brief summary of their evidence review findings, which was followed by an open comment and discussion period with conference attendees. Comments were integrated into the evidence review summary by the primary reviewer. The revised summaries were sent to the secondary reviewer for final review, and final revisions were made by the primary reviewer (see Appendices 2–8, http://links.lww.com/AOG/C620). The final evidence review summaries were used to develop the educational material (available online at acog.org).
In performing the review, there was significant overlap between the results of the literature searches for the research questions about risk factors and risk reduction. The appendices for these two topics present the full evidence summary for each (Appendices 3 and 4, http://links.lww.com/AOG/C620). For this executive summary, epidemiologic and retrospective studies from both searches are combined in the Risk Factors section, and the Risk Reduction section contains summaries of intervention trials and recommendations. Major society guidelines cited in the evidence reviews were replaced with the most current versions during the executive summary preparation.
When reporting results of individual studies, we used the terminology describing gender, race, and ethnicity from the source article. Studies almost uniformly used the term “women” or “females” to refer to the gender of those affected by uterine cancer. Although we acknowledge that uterine cancer can affect individuals of different genders who possess a uterus, we used the term “women” or “females” in this review to reflect the cited literature. In keeping with the most common categories of race and ethnicity used in national data collection, when we had a choice of terminology, we used “Black” to refer to non-Hispanic Black or African American individuals and “White” to refer to non-Hispanic White individuals. We used the term “Hispanic,” and not “Latinx,” because “Latinx” was rarely used in any of the articles reviewed. Although some studies restricted their analysis to Hispanic White individuals, others included Hispanic individuals of any race. Given the lack of consistency in the literature, we used the term “Hispanic,” without reference to race.
EPIDEMIOLOGY AND CLASSIFICATION
Uterine cancer is the fourth most common cancer in the United States, accounting for 7% of cancers affecting women.2,3 Most cases are confined to the uterus at diagnosis, with local or regional disease in 21% of cases and distant disease in only 8%. The prognosis is typically good, with uterine cancer accounting for only 4% of female cancer-related deaths.2 Uterine cancers can be divided into endometrial cancers affecting the epithelial lining and much less common mesenchymal malignancies, which represent only 3% of uterine cancers.3
Endometrial Cancer
Although endometrial cancer can affect women of all ages, it is most commonly diagnosed between ages 55 and 64 years, with a median age of 63 years.4 Both the incidence and mortality rates have been increasing since 2007, with the mortality rate increasing faster.2 Aggressive high-risk subtypes have become more frequent, which may explain why the mortality rate has increased more than the incidence rate. Endometrial cancer rates are also increasing significantly in Hispanic women younger than age 50 years.5
Traditionally, endometrial cancers have been classified into type 1 and type 2 cancers. Type 1 cancers are estrogen-driven, low-grade (grade 1–2) endometrioid tumors and account for approximately 65–80% of cases. These low-grade endometrial cancers are usually diagnosed at an early stage, with 80% limited to the uterus at diagnosis. The prognosis is usually good, with 5-year survival rates of 80–90% for patients with stage I disease. Type 2 cancers are more common in older women and are typically characterized by more aggressive behavior and worse prognoses. Extrauterine disease is more common at diagnosis, and the risk of recurrence of both local and distant disease is higher. Subtypes of type 2 cancers include serous carcinoma, clear cell carcinoma, carcinosarcoma, most grade 3 endometrioid carcinomas, and undifferentiated or dedifferentiated cancers. Although there are important nuances between these histologic subtypes, they are often grouped together because of their aggressive behavior and relatively poor prognoses compared with type I cancers.3 (See Appendix 2 [http://links.lww.com/AOG/C620] for further details about the histologic subtypes.) Rates of type 1 cancer are highest in White women in Western populations; type 2 cancers disproportionately affect non-Hispanic Black women.6
Uterine serous carcinoma is the most common high-risk histology, accounting for 10% of endometrial cancer cases, but up to 40% of deaths.7 These cancers demonstrate the loss of function of p53. Human epidermal growth factor receptor 2 overexpression is present in up to 62% of cases; it has been used as a therapeutic target, resulting in improved outcomes.7
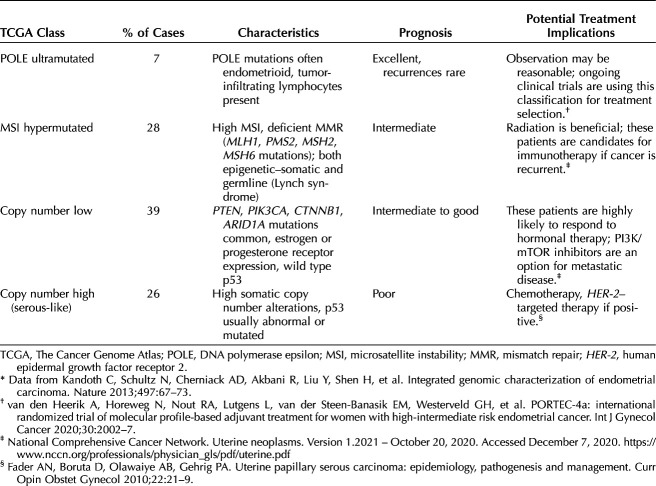
Although the type 1 and 2 classification has been a useful framework for categorizing endometrial cancer, recent trends have shifted toward molecular-based risk stratification systems.8,9 The Cancer Genome Atlas landmark trial performed an integrated analysis of the genome, transcriptome, and proteome of 373 endometrial carcinoma samples and identified four molecular subtypes that naturally clustered based on four distinct profiling patterns with associated survival differences (Table 1).10 Molecular-based classification of endometrial cancer is likely the future of endometrial cancer care and is increasingly being used to inform treatment decisions.11 Use of this classification approach may eventually replace decision making based on histology alone, and is being evaluated in an ongoing prospective trial.12
Table 1.
The Cancer Genome Atlas Endometrial Cancer Classification*
Uterine Sarcomas
Uterine leiomyosarcoma, endometrial stromal sarcoma, and undifferentiated uterine sarcoma are the three main types of uterine mesenchymal tumors (sarcomas). Leiomyosarcoma is the most common, with an incidence of 0.36 per 100,000 female life-years. Leiomyosarcomas make up 1% of all uterine cancers and 70% of uterine sarcomas. They are twice as common in Black women than White women.13 Uterine leiomyosarcoma is an aggressive uterine malignancy. Patients with uterine leiomyosarcoma usually present at older than age 40 years, and the incidence increases significantly after age 50 years.13–15 Leiomyosarcoma typically spreads hematogenously, and metastatic disease is frequently present at the time of initial resection.13 The 5-year survival rate for stage I disease is only 55% and falls to 21.7% for stage IV disease.16 Other rare stromal malignancies include adenosarcoma, rhabdomyosarcoma, and malignant perivascular epithelioid tumors. (See Appendix 2 [http://links.lww.com/AOG/C620] for complete evidence summary.)
RISK FACTORS
Age and Menopause
In the United States, endometrial cancer is significantly more common in women after age 50 years.4 One study showed that more than 75% of women diagnosed with endometrial cancer before age 25 years are obese (body mass index [BMI, calculated as weight in kilograms divided by height in meters squared] 30 or higher).17 Additional risk factors for endometrial cancer before age 50 years include not using combined hormonal contraceptives and first birth after age 30 years.18,19 Uterine sarcomas occur most commonly during perimenopause and early menopause. Leiomyosarcomas have the highest incidence between ages 45 and 59 years. Endometrial stromal sarcoma incidence rates are steady starting at age 45 years.20
Lifestyle
Two large meta-analyses have shown progressively increasing endometrial cancer risk with increasing BMI. Women who are overweight (BMI 25–29) have a relative risk (RR) of about 1.3, and women who are obese (BMI 30 or higher) have an RR of about 2.5 compared with normal-weight women.21,22 In a meta-analysis of 19 studies, a 5-unit increase in BMI was associated with a 1.59 (95% CI 1.50–1.68) increase in RR of endometrial cancer.23 Obesity increases risk for uterine sarcoma as well (odds ratio [OR] 1.73, 95% CI 1.22–2.46).24
Physical activity is associated with a decreased risk of endometrial cancer.25 Multiple prospective studies and meta-analyses have confirmed this protective role, regardless of BMI (most vs least physical activity: pooled RR 0.78, 95% CI 0.63–0.95).26,27
High glycemic index diets,28–30 diets high in saturated fats,31 and pro-inflammatory diets32 are possibly associated with increased risk of endometrial cancer. Retrospective studies have shown increased endometrial cancer risk with high meat consumption and decreased risk with high fruit and vegetable intake.25,33,34 A meta-analysis of 13 retrospective studies suggested an inverse association between isoflavone consumption and endometrial cancer (OR 0.81, 95% CI 0.74–0.89), but this was not shown in a randomized clinical trial.35,36 Dietary fiber may be inversely associated with endometrial cancer. A meta-analysis noted an RR of 0.82 (95% CI 0.75–0.90) for each 5 g/1,000 kcal of dietary fiber intake.37 Antiinflammatory diets that include fish or other sources of omega-fatty acids,38 coffee,39 and healthy dietary patterns (high in vegetables, fruit, whole grains, olive oil, fish, poultry, and low-fat dairy)40,41 have been associated with decreased endometrial cancer risk.
Smoking is associated with decreased endometrial cancer risk (RR 0.81, 95% CI 0.74–0.88), with larger decreases observed among smokers who are obese 42 and postmenopausal smokers taking hormone therapy (HT).43 Alcohol appears to have no significant effect on endometrial cancer risk.44
Hormonal Risk Factors
Endogenous Hormones
Increased length of exposure to endogenous hormones has been associated with increased endometrial cancer risk. Early menarche45 and later menopause46 are both associated with increased risk. Late menarche is inversely associated with uterine sarcoma risk.24 Pregnancy appears to be protective. Parous women have an RR of endometrial cancer of 0.69 (95% CI 0.65–0.74) compared with nulliparous women.47 Risk decreases with increasing parity. Women with late first birth (age 35–39 years) had an RR of 9.14 (95% CI 3.33–25.05) for endometrial cancer.48 Giving birth to one's last child after age 40 years decreased risk by 44% compared with giving birth to one's last child before age 25 years.49
Menopausal Hormone Therapy
A recent systematic review reported endometrial cancer risk was elevated in five out of six observational studies of unopposed estrogen therapy, with risk persisting more than 10 years after the end of treatment.50 An older meta-analysis calculated a summary RR of 2.3 for estrogen therapy users compared with nonusers, with an RR of 9.5 in women with more than 10 years of use.51 Estrogen-only replacement is not recommended in patients who still have their uterus, for whom combined HT with at least 7–10 days of progestin per month is typically recommended. In the WHI (Women's Health Initiative) study, patients taking combined HT had fewer endometrial cancers (hazard ratio [HR] 0.65, 95% CI 0.48–0.89) than women taking placebo.52 Women with high BMIs using combined HT have reduced risk of endometrial cancer compared with nonusers. A meta-analysis found that women who are extremely obese (BMI 42) who had never used HT had an RR of endometrial cancer of 20.70 (95% CI 8.28–51.84) compared with combined HT users.53 The WHI cohort study reported no statistically different levels of endometrial cancer in vaginal estrogen users compared with nonusers.54 Testosterone therapy can cause atrophic or proliferative endometrium.55
Progestin Contraceptives
The Norwegian Women and Cancer Study demonstrated a profound reduction in endometrial cancer risk with the levonorgestrel intrauterine device ([IUD], ever use vs never use: RR 0.22, 95% CI 0.13–0.40), even after adjusting for BMI and other risk factors.56 No studies evaluating progestin IUDs in women with obesity or a genetic predisposition to uterine cancer were identified. A Cochrane review showed reduced risk of endometrial polyps and endometrial hyperplasia among women using the levonorgestrel IUD who were taking tamoxifen, but the included studies were not powered to evaluate endometrial cancer risk.57
Combined Oral Contraceptives
Use of combined oral contraceptives was associated with a decreased risk of endometrial cancer in two meta-analyses, a population-based study, and a large prospective study in U.S. women.58–61 In the most recent, the Danish Sex Hormone Register Study, current or recent combined oral contraceptive use significantly reduced endometrial cancer risk (RR 0.57, 95% CI 0.43–0.75). Risk reduction increased with longer duration of use and persisted for more than 10 years after discontinuation.60 In the Black Women's Health Study, similar results were noted in a cohort of 47,555 women, with an incident rate ratio of 0.45 (95% CI 0.24–0.74) for women with more than 10 years of oral contraceptive use compared with never users.62 Use of combined oral contraceptives was associated with decreased endometrial cancer risk in a cohort of women with Lynch syndrome (HR 0.39, 95% CI 0.23–0.64 for 1 year or more of contraceptive use vs less than 1 year).63
Tubal Ligation
Two large population-based cohort studies demonstrated a decreased risk of endometrial cancer among women who underwent tubal sterilization (standardized incidence ratio 0.66, 95% CI 0.5–1.0, and HR 0.73, 95% CI 0.65–0.83).64,65 The mechanism of risk reduction is unclear.
Family History and Genetic Syndromes
Several genetic cancer syndromes increase the risk of uterine cancer. These syndromes are caused by pathogenic mutations or variants in tumor suppressor genes, each with different lifetime risks (see Appendix 3 [http://links.lww.com/AOG/C620]).66 The lifetime risk of endometrial cancer in patients with Lynch syndrome ranges from 13% to 57%.66 Cowden syndrome (PTEN mutations) and Peutz-Jeghers syndrome (STK11 mutations) increase endometrial cancer risk 5% to 10%. Hereditary retinoblastoma (RB1 mutation) increases the risk of leiomyosarcoma. A number of studies examined the risk of serous-type uterine cancer among individuals with the BRCA1 mutation, some of which showed a small association.8,67–71 The National Comprehensive Cancer Network states that this association may reflect tamoxifen use rather than genetic predisposition.68 A family history of endometrial cancer without known familial gene mutations is associated with an increased risk in first-degree relatives (RR 1.82, 95% CI 1.65–1.98).72
Medications
Though type 2 diabetes mellitus, obesity, and polycystic ovarian syndrome (PCOS) are associated with endometrial cancer, a 2018 meta-analysis did not show a decreased risk of endometrial cancer with metformin use (OR 1.05, 95% CI 0.82–1.35).73 Aspirin use has been associated with a decreased risk of endometrial cancer among women with obesity (pooled RR 0.83, 95% CI 0.69–0.99) and of type I endometrial cancer (pooled RR 0.89, 95% CI 0.82–0.96).74 Bisphosphonate use has been associated with a reduction in endometrial cancer risk (ever use vs never use: RR 0.73, 95% CI 0.58–0.93). Risk reduction is greatest in postmenopausal women and with longer duration of use.75
Prior Health History
Multiple meta-analyses have shown an increased risk of endometrial cancer with diabetes, with the most recent reporting an RR of 1.72 (95% CI 1.48–2.01).76 Diabetes also increases risk of uterine sarcoma (OR 2.33, 95% CI 1.41–3.83).24 A systematic review of 25 studies found hypertension to be an independent risk factor for endometrial cancer (RR 1.61, 95% CI, 1.41–1.85).77 A meta-analysis of studies of metabolic syndrome found too much heterogeneity between studies and in metabolic syndrome diagnostic criteria to calculate a pooled RR but noted increased prevalence of metabolic syndrome among patients with endometrial cancer compared with a control group in the individual studies.78 Infertility treatment does not appear to be associated with uterine cancer risk, although there appears to be increased incidence in some subgroups of women with infertility, including those with ovulatory dysfunction, progesterone deficiency, and obesity.79 Polycystic ovarian syndrome is associated with a 2.89 OR (95% CI 1.52–5.48) of endometrial cancer.80 Breast and ovarian cancer do not appear to increase uterine cancer risk, although related treatments such as tamoxifen and pelvic radiation do increase uterine cancer risk.
Some preexisting histopathologic abnormalities increase risk. A meta-analysis reported that 32.6% of women with complex endometrial hyperplasia had an occult endometrial cancer (95% CI 24.1–42.4%).81 Using newer endometrial intraepithelial neoplasia criteria, patients with endometrial intraepithelial neoplasia had a pooled RR of 19.37 (95% CI 5.86–64.01) for progression to cancer.82 A meta-analysis found a 1.12% and 4.93% risk of endometrial polyps containing malignancy in premenopausal and postmenopausal women, respectively.83 (See Appendices 3 and 4 [http://links.lww.com/AOG/C620] for complete evidence summaries.)
RISK REDUCTION
Lifestyle Modifications
The large, prospective National Institutes of Health-AARP Diet and Health cohort study and the WHI Dietary Modification randomized controlled trial did not show a protective effect from dietary modifications.84,85 We did not find any interventional trials specifically looking at risk reduction with physical activity. Given the many other health benefits of healthy diet and physical activity, patients should be counseled to follow national recommendations.86,87
Weight Reduction
We found no specific intervention trials of weight reduction for endometrial cancer risk reduction. One retrospective study of self-reported intentional weight loss showed risk reduction in a number of cancers, but not endometrial cancer.88 Patients in the WHI observation study who lost weight had a significant reduction in endometrial cancer risk, with an HR of 0.71 (95% CI 0.54–0.95) overall and an HR of 0.44 (95% CI 0.25–0.78) in women who are obese.89 Bariatric surgery was associated with a decreased risk of endometrial cancer in a meta-analysis of retrospective studies (pooled RR 0.43, 95% CI 0.26–0.72).90 Prospective studies demonstrate decreased endometrial proliferation on biopsy and a reduction in circulating endometrial cancer biomarkers after bariatric surgery.91,92 Patients with BMIs 30 or higher should receive behavioral counseling given the many other benefits of weight reduction.93
Progestins and Oral Contraceptives
We found no specific interventional trials or recommendations for progestins or combined oral contraceptives for endometrial cancer prevention. Based on the weight of the epidemiologic evidence, patients using these methods for clinical indications are likely experiencing endometrial cancer risk reduction as an ancillary benefit.
Other Medications
We found no interventional trials or recommendations for use of aspirin, metformin, or bisphosphonates for risk reduction.
Special Populations
Lynch Syndrome
Based on expert opinion, hysterectomy should be considered for risk reduction for women with Lynch syndrome who have completed childbearing.66,69,94,95 Hysterectomy significantly reduces endometrial cancer incidence but not mortality in women with Lynch syndrome.66,96 The National Comprehensive Cancer Network recommends considering risk-reducing hysterectomy for Lynch syndrome patients, with timing, “individualized based on whether childbearing is complete, comorbidities, family history, and [Lynch syndrome] gene, as risks for endometrial cancer vary by pathogenic variant.”66 The American College of Obstetricians and Gynecologists recommends discussing hysterectomy by a patient's early to mid-40s.94
Individuals With the BRCA Mutation
The National Comprehensive Cancer Network states the practitioner may discuss the risks and benefits of concurrent hysterectomy at the time of risk-reducing bilateral salpingo-oophorectomy for individuals with the BRCA1 mutation, but also notes that further clarification of the magnitude of risk of serous uterine cancer is needed.68
Other Genetic Syndromes
We found no studies evaluating risk-reducing hysterectomy among women with Cowden syndrome, PTEN hamartoma tumor syndrome, or Peutz-Jeghers syndrome (pathogenic STK11 variants). For patients with Cowden syndrome or PTEN hamartoma tumor syndrome, the National Comprehensive Cancer Network recommends that clinicians, “discuss option of hysterectomy upon completion of childbearing and counsel regarding the degree of protection, extent of cancer risk, and reproductive desires.”68
SCREENING
Asymptomatic Average-Risk Patients
Limitations to effective screening for endometrial cancer in asymptomatic individuals include the low prevalence of disease and the most common symptom, abnormal uterine bleeding (AUB), usually arising at an early disease stage when high cure rates are possible. Our review found no study or major society recommendation supporting endometrial cancer screening in asymptomatic women at usual risk.
Studies of ultrasonography in women with postmenopausal bleeding have demonstrated low positive predictive value, varying between 0 and 0.2.97 Screening tests would be expected to demonstrate even poorer performance in asymptomatic women, given the even lower disease prevalence. The literature review did not find studies assessing the use of endometrial sampling, hysteroscopy, or saline-infusion ultrasonography in asymptomatic women at usual risk. Endometrial cytology has been used in some countries as a screening modality, but its use has not demonstrated improvement in surgical stage at diagnosis or survival.98 No studies were found supporting cervical cytology for screening for endometrial cancer. Three retrospective studies of different detection methods found no survival difference between cancers diagnosed in asymptomatic individuals and those diagnosed after postmenopausal bleeding.98–100 These studies also suggest there is no difference in stage at diagnosis, although there was some variation in this outcome. The American Cancer Society first concluded that there was no indication for screening women without identified risk factors in 2001 and continues to maintain this conclusion.101,102
High-Risk Populations
Several professional societies recommend screening patients with Lynch syndrome (Table 2). These recommendations are largely based on expert opinion, and ACOG, the National Comprehensive Cancer Network, and the American Cancer Society all state that their recommendations have not been validated.66,94,101 Although other genetic conditions, such as Cowden syndrome, Peutz-Jeghers syndrome, and Li-Fraumeni syndrome, confer an elevated risk of uterine cancer, we found no guidelines or studies to support screening in these populations. We found no guidelines or studies supporting endometrial cancer screening in individuals with the BRCA mutation.
Table 2.
Recommendations for Endometrial Cancer Screening in People With Lynch Syndrome

Patients Using Tamoxifen
Tamoxifen users are at increased risk of developing benign polyps and endometrial cancer, with risk increasing with duration of use.103 Tamoxifen use is associated with endometrial thickening, and there are no established thresholds for ultrasonographic measurement of endometrial thickness in this population. Two studies found that transvaginal ultrasonography of asymptomatic premenopausal and postmenopausal women taking tamoxifen yields a high false-positive rate, leading to unnecessary intervention.104,105 The American College of Obstetricians and Gynecologists, the National Cancer Institute, and the American Cancer Society recommend against routine screening.101,106,107 Because emerging evidence suggests tamoxifen users with baseline endometrial polyps are more likely to develop atypical hyperplasia, ACOG states there may be a role for pretreatment screening before initiation of tamoxifen therapy.106 (See Appendix 5 [http://links.lww.com/AOG/C620] for complete evidence summary.)
EARLY DIAGNOSIS
Postmenopausal bleeding is the presenting symptom in 91% of women with endometrial cancer.108,109 Ten percent of women have bleeding in the first year after menopause.110 The pooled risk of endometrial cancer in women with postmenopausal bleeding is 9%.108 In a U.K. study, 41 of 85 women with endometrial cancer reported that they were not aware that bleeding was a sign of possible cancer.111
Techniques for Evaluation
Transvaginal Ultrasonography
Transvaginal ultrasonography is commonly used for first-line evaluation in patients with postmenopausal bleeding. In a meta-analysis, the sensitivity and specificity for endometrial cancer detection with an endometrial thickness threshold of 5 mm were 90% and 54%, and with a threshold of 3 mm, they were 98% and 35%, respectively.112 For premenopausal women with AUB, multiple studies have shown saline-infusion ultrasonography to be superior to transvaginal ultrasonography in the detection of intracavitary pathology.113–115 One study compared endometrial thickness to surgical or sonohysterogram findings in premenopausal patients and found endometrial thickness to be inadequate for excluding abnormalities.116 The American College of Obstetricians and Gynecologists states that ultrasonographic measurement of endometrial thickness in premenopausal women has no diagnostic value and should not be performed.117
Office Endometrial Sampling
Office-based endometrial sampling is minimally invasive and cost-effective. The Pipelle catheter is an accurate method of endometrial sampling, with detection rates for endometrial cancer of 99.6% in postmenopausal women and 91% in premenopausal women.118 The pooled sensitivity of office endometrial sampling for the diagnosis of endometrial cancer was 100% in studies using dilation and curettage (D&C) and 90% in studies using hysteroscopy with biopsy or curettage as the reference standard. Specificity for both ranged from 99% to 100%.119 The accuracy of office endometrial biopsy is more limited with an inadequate specimen or if the endometrial pathology is not global.120 In a meta-analysis of 11 studies, the posttest probability of endometrial cancer with a negative biopsy was 0.9%.121 The SGO and ACOG both recommend that persistent AUB should be further evaluated, with the SGO specifying use of hysteroscopic-guided biopsy.8,120
Diagnostic Hysteroscopy
A meta-analysis of studies of hysteroscopic visualization in premenopausal and postmenopausal women showed a positive hysteroscopy has a high likelihood ratio (LR) for presence of endometrial cancer, but a negative hysteroscopy has only a moderate LR for ruling out cancer. Diagnostic hysteroscopy is highly accurate for diagnosing endometrial cancer among premenopausal and postmenopausal women with AUB when there is adequate visualization of the uterine cavity (LR for positive result 60.9, 95% CI 52.1–72.5). A negative hysteroscopy alone is less accurate for the exclusion of endometrial cancer (LR for negative result 0.15, 95% CI 0.13–0.18), and diagnosis of endometrial hyperplasia is also more limited (positive result LR 10.4, 95% CI 9.7–11.1; negative result LR 0.24, 95% CI, 0.22–0.25).122 The meta-analysis did not look at hysteroscopic visualization in combination with endometrial sampling, as is typically practiced. In one study, office hysteroscopy had a positive predictive value of 96% and negative predictive value of 98% when compared with histology at the time of hysterectomy.123 Office-based hysteroscopy with guided biopsies can also be used.120
Evaluation of Postmenopausal Bleeding
The American College of Obstetricians and Gynecologists and the SGO recommend initial evaluation with either transvaginal ultrasonography or endometrial sampling.8,124 In the setting of insufficient tissue on endometrial sampling, transvaginal ultrasonography can be used. If endometrial sampling is negative and the bleeding persists or recurs, hysteroscopy with D&C is recommended. The American College of Obstetricians and Gynecologists recommends using an endometrial thickness of greater than 4 mm to prompt endometrial sampling if starting evaluation with transvaginal ultrasonography.124 Endometrial sampling in patients with postmenopausal bleeding using the Pipelle catheter is as effective and less costly than D&C, even after accounting for sampling failure, further supporting initial office-based evaluation.125 In a cost-effectiveness analysis from the United Kingdom, a strategy using transvaginal ultrasonography with a threshold of 5 mm was the least expensive. Transvaginal ultrasonography using a threshold of 4 mm for endometrial sampling was similar in cost-effectiveness to endometrial sampling for initial evaluation.126
Detecting Endometrial Cancer in Premenopausal Patients
The American College of Obstetricians and Gynecologists recommends endometrial sampling in patients with AUB who are older than 45 years as a first-line test. The American College of Obstetricians and Gynecologists also recommends sampling in patients younger than age 45 years with risk factors, including history of unopposed estrogen exposure (such as seen in obesity or PCOS), failed medical management, and persistent AUB.120 The International Federation of Gynecology and Obstetrics states, “for those at increased risk, endometrial biopsy is probably warranted.”127 The National Institute for Health and Care Excellence recommends hysteroscopy for patients with heavy and abnormal bleeding, with consideration of biopsy at the time of hysteroscopy for patients at high risk for endometrial pathology.128 The SGO recommends endometrial sampling if the patient has risk factors or the workup of the bleeding is negative.8
Evaluation of Incidental Findings in Asymptomatic Patients
Among asymptomatic postmenopausal women, an increased endometrial thickness of 4 mm or more on transvaginal ultrasonography has poor accuracy for the diagnosis of endometrial cancer.97,129 In a decision analysis, an endometrial thickness of more than 11 mm in asymptomatic postmenopausal women conferred similar risk for endometrial cancer (6.7%) as a patient with postmenopausal bleeding and an endometrial thickness of more than 5 mm (7.3%).130 In a meta-analysis, the prevalence of malignancy in endometrial polyps was 2.73% overall (1.12% for premenopausal women and 4.73% for postmenopausal women). Asymptomatic women had a lower risk of malignancy (1.89%) compared with symptomatic women (5.14%, P<.001).83 The American College of Obstetricians and Gynecologists states that management of endometrial polyps can be expectant or surgical depending on patient symptoms and risk factors for malignancy. Abnormal uterine bleeding is an indication for polypectomy.131 We found no guidelines regarding evaluation or treatment of asymptomatic postmenopausal women with incidental endometrial polyps.
Cervical Cytology Findings Prompting Uterine Cancer Evaluation
The 2014 Bethesda System recommends reporting benign-appearing endometrial cells for women aged 45 years and older, and endometrial assessment is recommended for postmenopausal women.132,133 In a systematic review of 22 studies, the prevalence of normal endometrial cells on cervical cytology was 0.7% (95% CI 0.4–1.4%) in women aged 40 years and older. Seven percent (95% CI 4–10%) of patients with normal endometrial cells on cytology had endometrial hyperplasia or cancer.134 The 2019 ASCCP Risk-Based Management Consensus Guidelines recommend endometrial sampling for postmenopausal women with endometrial cells on cytology. They do not recommend evaluating asymptomatic premenopausal women with benign appearing endometrial cells. They recommend endometrial sampling in conjunction with colposcopy and endocervical sampling in nonpregnant patients 35 years or older with all categories of atypical glandular cells or adenocarcinoma in situ on cytology. They also recommend endometrial sampling for nonpregnant patients younger than age 35 years with these findings and risk factors for endometrial neoplasia. For patients with atypical endometrial cells, preferred management is endometrial and endocervical sampling alone, but colposcopy is acceptable as part of the initial evaluation.133 (See Appendix 6 [http://links.lww.com/AOG/C620] for complete evidence summary.)
HEALTH DISPARITIES
Significant uterine cancer health disparities were noted in the evidence review, including higher mortality and poorer survival for Black women than for any other racial or ethnic group. Black women were also much less likely than White women to receive evidence-based care. These differences were important enough that panel members and stakeholder representatives agreed that the topic merits its own summary. Please see the companion health disparities summary, “Health Disparities in Uterine Cancer: Report From the Uterine Cancer Evidence Review Conference.”135 Our review found no articles meeting inclusion criteria on uterine cancer risk among individuals who do not identify as cisgender or females.
DIAGNOSIS AND CARE COORDINATION
History
Medical history is important to detect endometrial cancer, as well as to assess patients with a new diagnosis. Relevant risk factors include age, obesity, use of unopposed estrogen, medical comorbidities (including PCOS and type 2 diabetes mellitus), atypical glandular cells on cervical cytology, and family history of gynecologic malignancy.117,124 Postmenopausal bleeding is the most common symptom of endometrial cancer and warrants evaluation.117,136,137 Our review did not identify studies establishing an age after which continued menstrual bleeding is abnormal. A systematic review did not find any studies about counseling or education interventions, but counseling postmenopausal women about the significance of bleeding may be important given this unmet need.138
Family History and Genetics
Endometrial cancer may be the presenting cancer in approximately 50% of patients with Lynch syndrome.139,140 Any patient with a known family history of Lynch syndrome should be referred to a genetic counselor or tested for Lynch syndrome. Patients with a personal history of colorectal cancer or endometrial cancer who are diagnosed younger than age 50 years, who have another Lynch-related cancer, who have any relative with a Lynch-related cancer younger than age 50 years, or who have two or more relatives with a Lynch-related cancer at any age should also be evaluated for Lynch syndrome. The National Comprehensive Cancer Network recommends universal testing of endometrial carcinomas for mismatch repair proteins or microsatellite instability. Patients with abnormalities on genetic testing of their tumor and those with a significant family history of endometrial or colorectal cancer should be referred for genetic counseling and testing. Genetic testing results can also help inform choice of treatment options.3
Evaluation
The pelvic examination is important to evaluate and confirm the source of bleeding; assess the size, contour, and mobility of the uterus for surgical planning; assess for disease spread; and evaluate for other synchronous problems. Appropriate tissue sampling or transvaginal ultrasonography should be performed based on history and examination findings.117 Ultrasonography is often performed in the initial evaluation of abnormal bleeding and can be useful for surgical planning when uterine size cannot be appreciated on examination because of body habitus or other factors. Magnetic resonance imaging is used for determining the primary cancer site (uterus vs cervix) and for assessing for cervical involvement or parametrial extension.3 Because high-risk histologies, including uterine serous carcinoma, clear cell carcinoma, carcinosarcoma, and high-grade or undifferentiated carcinomas, often have extrauterine disease at time of diagnosis, computed tomography of the chest, abdomen, and pelvis and CA 125 evaluation may be considered as part of the initial evaluation.3,141 For low-grade disease without other risk factors, chest X-ray is often sufficient. Chest X-ray evaluates for the presence of distant disease. Lung metastasis is rare in this setting but, if present, would significantly change the treatment plan. Additional information about the role of ovarian conservation and treatment alternatives in elderly or frail patients can be found in Appendix 7 (http://links.lww.com/AOG/C620).
Indications for Referral
Approximately 20% of patients with presumed early-stage disease are found to have metastatic disease at the time of surgery. The American College of Obstetricians and Gynecologists recommends, “Physicians with advanced training and expertise in the treatment of women with endometrial cancer, such as gynecologic oncologists, understand the nuances of uterine cancer management, including the selection and sequencing of treatment modalities likely to benefit the individual patient. Patient outcomes are improved when high-volume surgeons in high-volume institutions render care, and this outcomes model typically is reproduced by standard gynecologic oncology practice.”117
The World Health Organization endometrial precancer nomenclature has two categories: benign endometrial hyperplasia (nonneoplastic) and endometrial intraepithelial neoplasia (atypical hyperplasia, premalignant disease).142 Endometrial intraepithelial neoplasia is the precursor to type 1 endometrial carcinoma. Approximately 42.6–62.5% of patients with a presumed diagnosis of endometrial intraepithelial neoplasia will have concurrent carcinoma, with an 11% risk of deep myometrial invasion.143,144 The risk of having lymph node involvement with atypical hyperplasia was 3.3% in one study, and lymph node assessment influenced clinical decision making in approximately 28% of patients in another study.145,146 This review did not identify recommendations regarding which patients with endometrial intraepithelial neoplasia should be referred to a gynecologic oncologist, the role of imaging in the evaluation of endometrial intraepithelial neoplasia, or the need for hysteroscopy or D&C before hysterectomy. If invasive cancer is found at the time of surgery, referral to a gynecologic oncologist and a second surgery may be indicated, with additional morbidity and risk. Additional information regarding the management of endometrial cancer, including the role of and approaches to lymph node assessment, can be found in Appendix 7 (http://links.lww.com/AOG/C620).
Uterine Sarcoma
Endometrial biopsy or curettage may detect uterine leiomyosarcoma in a subset of patients, but a negative test does not exclude malignancy, and there is no preoperative diagnostic test to reliably diagnose uterine sarcoma. Magnetic resonance imaging can be used to characterize uterine masses concerning for sarcoma.13 Given the high risk of metastatic disease to distant sites, computed tomography of the chest, abdomen, and pelvis is recommended at the time of diagnosis.3 Referral to a gynecologic oncologist should be considered if sarcoma is suspected based on an enlarging mass in a postmenopausal patient, evidence of metastases, or suspicious imaging findings. (See Appendix 7 [http://links.lww.com/AOG/C620] for complete evidence summary.)
SPECIAL CONSIDERATIONS
Survivorship is essential to the care of patients with uterine cancer. Women may have questions or concerns about future fertility or sexual health. Although chemotherapy, radiation, and surgery may prove lifesaving, their negative effects should be discussed with the patient and balanced against potential benefits in conjunction with patient priorities. These issues may need to be revisited throughout the evaluation and treatment course, as new relationships, understandings, information, and side effects may become relevant.
Fertility Preservation
An increasing number of women of reproductive age are being diagnosed with endometrial cancer and may want to preserve their fertility.4 Consultation should be obtained from a gynecologic oncologist to ensure adequate risk assessment. Counseling should cover the risks and benefits of fertility preservation, treatments and side effects, chance of recurrence, relevant assisted reproductive techniques and their cost, and chances of pregnancy complications and successful live birth. Endometrial cancers in this age group are often early-stage, well-differentiated endometrioid type adenocarcinomas, with rare cases of myometrial invasion or lymph node metastasis.147 The American Society for Reproductive Medicine recommends that patients of reproductive age undergoing potentially gonadotoxic therapies be informed by their health care professional about fertility preservation and future reproduction before treatment initiation. A collaborative, multidisciplinary, team-based approach may be helpful.148
Although fertility-sparing techniques limit complete surgical staging, the SGO and the National Comprehensive Cancer Network identify candidates for fertility-sparing treatment as those who strongly desire fertility, have a grade 1 tumor limited to the endometrium, have no contraindications for medical management, and are willing to accept the risks of nonstandard treatment.3,9 Dilation and curettage is the optimal method to confirm grading and histologic differentiation of the endometrial cancer, in combination with contrast-enhanced magnetic resonance imaging to assess cervical, myometrial, adnexal, lymph node, or peritoneal involvement.3,9,148
Although hysterectomy is the definitive treatment, progestin treatment can be considered for patients with stage 1A, grade 1, minimally invasive endometrial cancer who desire fertility-sparing therapy. Continuous-dose oral therapy with medroxyprogesterone acetate or megestrol acetate is frequently used.9 Response rates to oral progestin therapy range from 48.2% to 76.2% of patients, with recurrence rates of 35.4–40.6% and a 28% live birth rate.149,150 Several studies support the use of progestin-releasing IUDs.151–154 Patients desiring fertility preservation should be placed on a continuous progestin-based therapy with megestrol, medroxyprogesterone, or the levonorgestrel IUD. Patients undergoing fertility-sparing progestin therapy should have endometrial sampling by office biopsy or D&C every 3–6 months, with a complete response expected by 6–12 months. None of the recommendations include serial imaging as part of surveillance. Treatment is typically continued for 6–12 months. Hysterectomy is recommended once childbearing has been completed.3,9,154
Sexual Health
Sexuality is complex and has many different components that may negatively or positively influence sexual health. Having a sexual partner, age, relationship issues, psychological and physical components, other aspects of overall physical health, endometrial cancer diagnosis, surgery that removes organs that may help define sexuality, chemotherapy, radiation, and a desire to please a sexual partner are some of the important contributors that affect the sexual health of patients with endometrial cancer.155,156 In a cohort of patients with endometrial cancer, 40.5% were sexually active. Reasons for not being active include lack of a partner, lack of interest, physical problems, and fatigue.157,158 Multiple studies have compared the effects of surgery on sexual dysfunction, and results vary, with no conclusive findings.159–161 Studies of radiotherapy also found no consistent effect on sexual function for patients with endometrial cancer.162–165
We found very few studies on interventions to improve sexual health. A brief mindfulness-based cognitive behavioral therapy intervention showed improvement in a small group of survivors of cervical and endometrial cancer compared with a control group.166 A study with no control group looked at vaginal dilator use in a cohort of women undergoing radiation for endometrial cancer. Although sexual activity increased over the course of therapy and for a year afterward, sexual enjoyment decreased, and vaginal dilator use did not prevent sexual problems or vaginal stenosis.167 (See Appendix 8 [http://links.lww.com/AOG/C620] for complete evidence summary.)
RESEARCH GAPS AND OPPORTUNITIES
The evidence review and stakeholder discussion identified many research gaps and opportunities for uterine cancer, the highest priority of which are listed here. (See Appendices 2–8 [http://links.lww.com/AOG/C620] for a more thorough analysis of research gaps and opportunities for each topic.)
Strategies to eliminate disparities in uterine cancer outcomes and to ensure equity in diagnosis and treatment of uterine cancer between racial and ethnic groups, regardless of socioeconomic and insurance type
Obtaining data on risk factors, incidence rate, mortality rate, and survival of endometrial cancer among individuals who do not identify as cisgender females
Strategies to mitigate rising mortality, including improving understanding of why high-risk histologic subtypes are increasing
Better definition of patients at high risk for endometrial cancer, with evidence-based recommendations for surveillance and risk reduction
Development of recommendations for the prevention of endometrial cancer in average-risk women, particularly diet and lifestyle modifications and progestin use
Identification of risk factors and preventive measures and development of effective strategies for the early diagnosis of leiomyosarcomas and endometrial stromal sarcomas
Comparative effectiveness studies of diagnostic algorithms for postmenopausal bleeding
Optimal management of incidentally detected endometrial polyps
Development of guidelines for referral of patients with endometrial intraepithelial neoplasia
Evidence-based interventions for sexual health in patients with and survivors of uterine cancer
Improved strategies and educational materials for patients and practitioners about warning signs of endometrial cancer, including at what point in the menopausal spectrum and age continuum continued bleeding is concerning
Development of data-driven guidelines regarding the endometrial evaluation of patients taking tamoxifen
Development of noninvasive diagnostic tests for endometrial cancer
Footnotes
Supported by the Centers for Disease Control and Prevention (CDC) of the U.S. Department of Health and Human Services (HHS) under cooperative agreement number 6 NU38OT000287-03-03, which was awarded to the American College of Obstetricians and Gynecologists (ACOG).
Financial Disclosure David Chelmow is the president of ASCCP and immediate past president of the Council of University Chairs of Obstetrics and Gynecology, receives a stipend as the editor-in-chief of the Medscape Obstetrics and Gynecology Clinical Reference Book, and was on the American Board of Obstetrics and Gynecology Board of Directors during the evidence review and manuscript writing period. David Chelmow is a member of the United States Preventive Services Task Force (USPSTF). This article does not necessarily represent the views and policies of the USPSTF. Rebecca Brooks is a member of an AstraZeneca speakers' bureau related to ovarian cancer, a past advisory board member and consultant for GSK and Merck & Co., Inc., and a past non-branded speaker for Clinical Care Options. Sangini Sheth is a past consultant for Merck & Co., Inc., and a research study grant recipient (drug only: HPV vaccine) from Merck & Co., Inc. She was paid costs of travel and meeting registration (paid directly) and received honoraria payment for an education course from the ASCCP. She also has research grants from the NIH and CDC for studies unrelated to the submitted work. Brett Worly is an unpaid speaker for AbbVie, Inc. All authors received a one-time payment from ACOG for their participation in the development of uterine cancer educational materials.
The authors thank Jean Riedlinger, MSLS, AHIP, Yvonnada McNeil, MSLS, Elizabeth York, MPH, and Jessica Butler, MPH, for their assistance with the database searches; Dana Trevas, Nancy O'Reilly, MHS, PMP, and Apurvi Shah, MPH, for facilitating the management of the review and editing process; and the individuals who attended the January 2021 Uterine Cancer Evidence Review Conference, listed in Appendix 1 online at http://links.lww.com/AOG/C620, for their discussion, input, and comments.
Participation in this project as an attendee of the Evidence Review Conference does not constitute organizational or individual endorsement of the conclusions. Information in this article should not be construed as the official position or policy of, or should any endorsements be inferred by CDC, HHS, or the U.S. Government.
Each author has confirmed compliance with the journal's requirements for authorship.
Peer reviews and author correspondence are available at http://links.lww.com/AOG/C621.
REFERENCES
- 1.Chelmow D, Pearlman MD, Young A, Bozzuto L, Dayaratna S, Jeudy M, et al. Executive summary of the early-onset breast cancer review conference. Obstet Gynecol 2020;135:1457–78. doi: 10.1097/AOG.0000000000003889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Siegel R, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin 2020;70:7–30. doi: 10.3322/caac.21590 [DOI] [PubMed] [Google Scholar]
- 3.National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology. Uterine neoplasms version 1.2021. Accessed December 7, 2020. https://www.nccn.org/professionals/physician_gls/pdf/uterine.pdf
- 4.National Cancer Institute. Surveillance, Epidemiology, and End Results Program. Cancer stat facts: uterine cancer. Accessed December 12, 2020. https://seer.cancer.gov/statfacts/html/corp.html
- 5.Temkin SM, Kohn EC, Penberthy L, Cronin KA, Rubinsak L, Dickie LA, et al. Hysterectomy-corrected rates of endometrial cancer among women younger than age 50 in the United States. Cancer Causes Control 2018;29:427–33. doi: 10.1007/s10552-018-1018-z [DOI] [PubMed] [Google Scholar]
- 6.Clarke MA, Devesa SS, Harvey SV, Wentzensen N. Hysterectomy-corrected uterine corpus cancer incidence trends and differences in relative survival reveal racial disparities and rising rates of nonendometrioid cancers. J Clin Oncol 2019;37:1895–908. doi: 10.1200/JCO.19.00151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fader AN, Boruta D, Olawaiye AB, Gehrig PA. Uterine papillary serous carcinoma: epidemiology, pathogenesis and management. Curr Opin Obstet Gynecol 2010;22:21–9. doi: 10.1097/GCO.0b013e328334d8a3 [DOI] [PubMed] [Google Scholar]
- 8.Hamilton CA, Pothuri B, Arend RC, Backes FJ, Gehrig PA, Soliman PT, et al. Endometrial cancer: a Society of Gynecologic Oncology evidence-based review and recommendations. Gynecol Oncol 2021;160:817–26. doi: 10.1016/j.ygyno.2020.12.021 [DOI] [PubMed] [Google Scholar]
- 9.Hamilton CA, Pothuri B, Arend RC, Backes FJ, Gehrig PA, Soliman PT, et al. Endometrial cancer: a Society of Gynecologic Oncology evidence-based review and recommendations, part II. Gynecol Oncol 2021;160:827–34. doi: 10.1016/j.ygyno.2020.12.024 [DOI] [PubMed] [Google Scholar]
- 10.Cancer Genome Atlas Research Network; Kandoth C, Schultz N, Cherniack AD, Akbani R, Liu Y, Shen H, et al. Integrated genomic characterization of endometrial carcinoma. Nature 2013;497:67–73. doi: 10.1038/nature12113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Murali R, Delair DF, Bean SM, Abu-Rustum NR, Soslow RA. Evolving roles of histologic evaluation and molecular/genomic profiling in the management of endometrial cancer. J Natl Compr Canc Netw 2018;16:201–9. doi: 10.6004/jnccn.2017.7066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.van den Heerik ASVM, Horeweg N, Nout RA, Lutgens LCHW, van der Steen-Banasik EM, Westerveld GH, et al. PORTEC-4a: international randomized trial of molecular profile-based adjuvant treatment for women with high-intermediate risk endometrial cancer. Int J Gynecol Cancer 2020;30:2002–7. doi: 10.1136/ijgc-2020-001929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ricci S, Stone RL, Fader AN. Uterine leiomyosarcoma: epidemiology, contemporary treatment strategies and the impact of uterine morcellation. Gynecol Oncol 2017;145:208–16. doi: 10.1016/j.ygyno.2017.02.019 [DOI] [PubMed] [Google Scholar]
- 14.Cui RR, Wright JD. Risk of occult uterine sarcoma in presumed uterine fibroids. Clin Obstet Gynecol 2016;59:103–18. doi: 10.1097/GRF.0000000000000163 [DOI] [PubMed] [Google Scholar]
- 15.Hosh M, Antar S, Nazzal A, Warda M, Gibreel A, Refky B. Uterine sarcoma: analysis of 13,089 cases based on surveillance, epidemiology, and end results database. Int J Gynecol Cancer 2016;26:1098–104. doi: 10.1097/IGC.0000000000000720 [DOI] [PubMed] [Google Scholar]
- 16.Zivanovic O, Jacks LM, Iasonos A, Leitao MM, Jr, Soslow RA, Veras E, et al. A nomogram to predict postresection 5-year overall survival for patients with uterine leiomyosarcoma. Cancer 2012;118:660–9. doi: 10.1002/cncr.26333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rosen MW, Tasset J, Kobernik EK, Smith YR, Johnston C, Quint EH. Risk factors for endometrial cancer or hyperplasia in adolescents and women 25 years old or younger. J Pediatr Adolesc Gynecol 2019;32:546–49. doi: 10.1016/j.jpag.2019.06.004 [DOI] [PubMed] [Google Scholar]
- 18.Parslov M, Lidegaard O, Klintorp S, Pedersen B, Jonsson L, Eriksen PS, et al. Risk factors among young women with endometrial cancer: a Danish case-control study. Am J Obstet Gynecol 2000;182:23–9. doi: 10.1016/s0002-9378(00)70486-8 [DOI] [PubMed] [Google Scholar]
- 19.Soliman PT, Oh JC, Schmeler KM, Sun CC, Slomovitz BM, Gershenson DM, et al. Risk factors for young premenopausal women with endometrial cancer. Obstet Gynecol 2005;105:575–80. doi: 10.1097/01.AOG.0000154151.14516.f7 [DOI] [PubMed] [Google Scholar]
- 20.Koivisto-Korander R, Martinsen JI, Weiderpass E, Leminen A, Pukkala E. Incidence of uterine leiomyosarcoma and endometrial stromal sarcoma in Nordic countries: results from NORDCAN and NOCCA databases. Maturitas 2012;72:56–60. doi: 10.1016/j.maturitas.2012.01.021 [DOI] [PubMed] [Google Scholar]
- 21.Zhang Y, Liu H, Yang S, Zhang J, Qian L, Chen X. Overweight, obesity and endometrial cancer risk: results from a systematic review and meta-analysis. Int J Biol Markers 2014;29:e21–9. doi: 10.5301/jbm.5000047 [DOI] [PubMed] [Google Scholar]
- 22.Jenabi E, Poorolajal J. The effect of body mass index on endometrial cancer: a meta-analysis. Public Health 2015;129:872–80. doi: 10.1016/j.puhe.2015.04.017 [DOI] [PubMed] [Google Scholar]
- 23.Renehan AG, Tyson M, Egger M, Heller RF, Zwahlen M. Body-mass index and incidence of cancer: a systematic review and meta-analysis of prospective observational studies. Lancet 2008;371:569–78. doi: 10.1016/S0140-6736(08)60269-X [DOI] [PubMed] [Google Scholar]
- 24.Felix AS, Cook LS, Gaudet MM, Rohan TE, Schouten LJ, Setiawan VW, et al. The etiology of uterine sarcomas: a pooled analysis of the Epidemiology of Endometrial Cancer Consortium. Br J Cancer 2013;108:727–34. doi: 10.1038/bjc.2013.2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Linkov F, Edwards R, Balk J, Yurkovetsky Z, Stadterman B, Lokshin A, et al. Endometrial hyperplasia, endometrial cancer and prevention: gaps in existing research of modifiable risk factors. Eur J Cancer 2008;44:1632–44. doi: 10.1016/j.ejca.2008.05.001 [DOI] [PubMed] [Google Scholar]
- 26.Hidayat K, Zhou HJ, Shi BM. Influence of physical activity at a young age and lifetime physical activity on the risks of 3 obesity-related cancers: systematic review and meta-analysis of observational studies. Nutr Rev 2020;78:1–18. doi: 10.1093/nutrit/nuz024 [DOI] [PubMed] [Google Scholar]
- 27.Moore SC, Gierach GL, Schatzkin A, Matthews CE. Physical activity, sedentary behaviours, and the prevention of endometrial cancer. Br J Cancer 2010;103, 933–8. doi: 10.1038/sj.bjc.6605902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mulholland HG, Murray LJ, Cardwell CR, Cantwell MM. Dietary glycaemic index, glycaemic load and endometrial and ovarian cancer risk: a systematic review and meta-analysis. Br J Cancer 2008;99:434–41. doi: 10.1038/sj.bjc.6604496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Galeone C, Augustin LSA, Filomeno M, Malerba S, Zucchetto A, Pelucchi C, et al. Dietary glycemic index, glycemic load, and the risk of endometrial cancer: a case-control study and meta-analysis. Eur J Cancer Prev 2013;22:38–45. doi: 10.1097/CEJ.0b013e328354d378 [DOI] [PubMed] [Google Scholar]
- 30.Nagle CM, Olsen CM, Ibiebele TI, Spurdle AB, Webb PM;Australian National Endometrial Cancer Study Group, et al. Glycemic index, glycemic load and endometrial cancer risk: results from the Australian National Endometrial Cancer study and an updated systematic review and meta-analysis. Eur J Nutr 2013;52:705–15. doi: 10.1007/s00394-012-0376-7 [DOI] [PubMed] [Google Scholar]
- 31.Zhao J, Lyu C, Gao J, Du L, Shan B, Zhang H, et al. Dietary fat intake and endometrial cancer risk: a dose response meta-analysis. Medicine (Baltimore) 2016;95:e4121. doi: 10.1097/MD.0000000000004121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shivappa N, Hébert JR, Zucchetto A, Montella M, Serraino D, La Vecchia C, et al. Dietary inflammatory index and endometrial cancer risk in an Italian case-control study. Br J Nutr 2016;115:138–46. doi: 10.1017/S0007114515004171 [DOI] [PubMed] [Google Scholar]
- 33.Bandera EV, Kushi LH, Moore DF, Gifkins DM, McCullough ML. Consumption of animal foods and endometrial cancer risk: a systematic literature review and meta-analysis. Cancer Causes Control, 2007;18:967–88. doi: 10.1007/s10552-007-9038-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bandera EV, Kushi LH, Moore DF, Gifkins DM, McCullough ML. Fruits and vegetables and endometrial cancer risk: a systematic literature review and meta-analysis. Nutr Cancer 2007;58:6–21. doi: 10.1080/01635580701307929 [DOI] [PubMed] [Google Scholar]
- 35.Quaas AM, Kono N, Mack WJ, Hodis HN, Felix JC, Paulson RJ, et al. Effect of isoflavone soy protein supplementation on endometrial thickness, hyperplasia, and endometrial cancer risk in postmenopausal women: a randomized controlled trial. Menopause 2013;20:840–4. doi: 10.1097/gme.0b013e3182804353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhong XS, Ge J, Chen SW, Xiong YQ, Ma SJ, Chen Q. Association between dietary isoflavones in soy and legumes and endometrial cancer: a systematic review and meta-analysis. J Acad Nutr Diet 2018;118, 637–51. doi: 10.1016/j.jand.2016.09.036 [DOI] [PubMed] [Google Scholar]
- 37.Bandera EV, Kushi LH, Moore DF, Gifkins DM, McCullough ML. Association between dietary fiber and endometrial cancer: a dose-response meta-analysis. Am J Clin Nutr 2007;86:1730–37. doi: 10.1093/ajcn/86.5.1730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Terry P, Wolk A, Vainio H, Weiderpass E. Fatty fish consumption lowers the risk of endometrial cancer: a nationwide case-control study in Sweden. Cancer Epidemiol Biomarkers Prev 2002;11:143–45 [PubMed] [Google Scholar]
- 39.Lafranconi A, Micek A, Galvano F, Rossetti S, del Pup L, Berretta M, et al. Coffee decreases the risk of endometrial cancer: a dose-response meta-analysis of prospective cohort studies. Nutrients 2017;9:1223. doi: 10.3390/nu9111223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Si CJ, Shu L, Zheng PF, Zhang XY, Yu XL, Gao W, et al. Dietary patterns and endometrial cancer: a meta-analysis. Eur J Cancer Prev 2017;26:336–45. doi: 10.1097/CEJ.0000000000000266 [DOI] [PubMed] [Google Scholar]
- 41.Alizadeh S, Djafarian K, Alizadeh M, Shab-Bidar S. The relation of healthy and western dietary patterns to the risk of endometrial and ovarian cancers: a systematic review and meta-analysis. Int J Vitam Nutr Res 2020;90:365–75. doi: 10.1024/0300-9831/a00051 [DOI] [PubMed] [Google Scholar]
- 42.Polesel J, Serraino D, Zucchetto A, Lucenteforte E, Dal Maso L, Levi F, et al. Cigarette smoking and endometrial cancer risk: the modifying effect of obesity. Eur J Cancer Prev 2009;18:476–81. doi: 10.1097/CEJ.0b013e32832f9bc4 [DOI] [PubMed] [Google Scholar]
- 43.Zhou B, Yang L, Sun Q, Cong R, Gu H, Tang N, et al. Cigarette smoking and the risk of endometrial cancer: a meta-analysis. Am J Med 2008;121:501–8.e3. doi: 10.1016/j.amjmed.2008.01.044 [DOI] [PubMed] [Google Scholar]
- 44.Turati F, Gallus S, Tavani A, Tramacere I, Polesel J, Talamini R, et al. Alcohol and endometrial cancer risk: a case-control study and a meta-analysis. Cancer Causes Control 2010;21:1285–96. doi: 10.1007/s10552-010-9556-z [DOI] [PubMed] [Google Scholar]
- 45.Gong TT, Wang YL, Ma XX. Age at menarche and endometrial cancer risk: a dose-response meta-analysis of prospective studies. Sci Rep 2015;5:14051. doi: 10.1038/srep14051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wu Y, Sun W, Liu H, Zhang D. Age at menopause and risk of developing endometrial cancer: a meta-analysis. Biomed Res Int 2019;2019:8584130. doi: 10.1155/2019/8584130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wu QJ, Li YY, Tu C, Zhu J, Qian KQ, Feng TB, et al. Parity and endometrial cancer risk: a meta-analysis of epidemiologic studies. Sci Rep 2015;5:14243. doi: 10.1038/srep14243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mogren I, Stenlund H, Högberg U. Long-term impact of reproductive factors on the risk of cervical, endometrial, ovarian and breast cancer. Acta Oncol 2001;40:849–54. doi: 10.1080/02841860152703481 [DOI] [PubMed] [Google Scholar]
- 49.Setiawan VW, Pike MC, Karageorgi S, Deming SL, Anderson K, Bernstein L, et al. Age at last birth in relation to risk of endometrial cancer: pooled analysis in the Epidemiology of Endometrial Cancer Consortium. Am J Epidemiol 2012;176:269–78. doi: 10.1093/aje/kws129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sjogren LL, Morch LS, Lokkegaard E. Hormone replacement therapy and the risk of endometrial cancer: a systematic review. Maturitas 2016;91:25–35. doi: 10.1016/j.maturitas.2016.05.013 [DOI] [PubMed] [Google Scholar]
- 51.Grady D, Gebretsadik T, Kerlikowske K, Ernster V, Petitti D. Hormone replacement therapy and endometrial cancer risk: a meta-analysis. Obstet Gynecol 1995;85:304–13. doi: 10.1016/0029-7844(94)00383-O [DOI] [PubMed] [Google Scholar]
- 52.Constantine GD, Kessler G, Graham S, Goldstein SR. Increased incidence of endometrial cancer following the Women's Health Initiative: an assessment of risk factors. J Womens Health (Larchmt) 2019;28:237–43. doi: 10.1089/jwh.2018.6956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Crosbie EJ, Zwahlen M, Kitchener HC, Egger M, Renehan AG. Body mass index, hormone replacement therapy, and endometrial cancer risk: a meta-analysis. Cancer Epidemiol Biomarkers Prev 2010;19:3119–30. doi: 10.1158/1055-9965.EPI-10-0832 [DOI] [PubMed] [Google Scholar]
- 54.Pinkerton JV, Kaunitz AM, Manson JE. Vaginal estrogen in the treatment of genitourinary syndrome of menopause and risk of endometrial cancer: an assessment of recent studies provides reassurance. Menopause 2017;24:1329–32. doi: 10.1097/GME.0000000000000996 [DOI] [PubMed] [Google Scholar]
- 55.Hawkins M, Deutsch MB, Obedin-Maliver J, Stark B, Grubman J, Jacoby A, et al. Endometrial findings among transgender and gender nonbinary people using testosterone at the time of gender-affirming hysterectomy. Fert Steril 2021;115:1312–7. doi: 10.1016/j.fertnstert.2020.11.008 [DOI] [PubMed] [Google Scholar]
- 56.Jareid M, Thalabard JC, Aarflot M, Bøvelstad HM, Lund E, Braaten T. Levonorgestrel-releasing intrauterine system use is associated with a decreased risk of ovarian and endometrial cancer, without increased risk of breast cancer: results from the NOWAC study. Gynecol Oncol 2018;149:127–32. doi: 10.1016/j.ygyno.2018.02.006 [DOI] [PubMed] [Google Scholar]
- 57.Dominick S, Hickey M, Chin J, Su HI. Levonorgestrel intrauterine system for endometrial protection in women with breast cancer on adjuvant tamoxifen. The Cochrane Database of Systematic Reviews 2015, Issue 12. Art. No.: CD007245. doi: 10.1002/14651858.CD007245.pub3 [DOI] [PMC free article] [PubMed]
- 58.Gierisch JM, Coeytaux RR, Urrutia RP, Havrilesky LJ, Moorman PG, Lowery WJ, et al. Oral contraceptive use and risk of breast, cervical, colorectal, and endometrial cancers: a systematic review. Cancer Epidemiol Biomarkers Prev 2013;22:1931–43. doi: 10.1158/1055-9965.EPI-13-0298 [DOI] [PubMed] [Google Scholar]
- 59.Mueck AO, Seeger H, Rabe T. Hormonal contraception and risk of endometrial cancer: a systematic review. Endocr Relat Cancer 2010;17:R263–71. doi: 10.1677/ERC-10-0076 [DOI] [PubMed] [Google Scholar]
- 60.Iversen L, Fielding S, Lidegaard Ø, Hannaford PC. Contemporary hormonal contraception and risk of endometrial cancer in women younger than age 50: a retrospective cohort study of Danish women. Contraception 2020;102:152–58. doi: 10.1016/j.contraception.2020.06.008 [DOI] [PubMed] [Google Scholar]
- 61.Michels KA, Pfeiffer RM, Brinton LA, Trabert B. Modification of the associations between duration of oral contraceptive use and ovarian, endometrial, breast, and colorectal cancers. JAMA Oncol 2018;4:516–21. doi: 10.1001/jamaoncol.2017.4942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sponholtz TR, Palmer JR, Rosenberg LA, Hatch EE, Adams-Campbell LL, Wise LA. Exogenous hormone use and endometrial cancer in U.S. Black women. Cancer Epidemiol Biomarkers Prev 2018;27:558–65. doi: 10.1158/1055-9965.EPI-17-0722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Dashti SG, Chau R, Ouakrim DA, Buchanan DD, Clendenning M, Young JP, et al. Female hormonal factors and the risk of endometrial cancer in Lynch syndrome. JAMA 2015;314:61–71. doi: 10.1001/jama.2015.6789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Falconer H, Yin L, Altman D. Association between tubal ligation and endometrial cancer risk: a Swedish population-based cohort study. Int J Cancer 2018;143:16–21. doi: 10.1002/ijc.31287 [DOI] [PubMed] [Google Scholar]
- 65.Kjaer SK, Mellemkjaer L, Brinton LA, Johansen C, Gridley G, Olsen JH. Tubal sterilization and risk of ovarian, endometrial and cervical cancer: a Danish population-based follow-up study of more than 65 000 sterilized women. Inter J Epidemiol 2004;33:596–602. doi: 10.1093/ije/dyh046 [DOI] [PubMed] [Google Scholar]
- 66.National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology. Genetic/familial high-risk assessment: colorectal. Version 1.2020. Accessed December 3, 2020. https://www.nccn.org/professionals/physician_gls/pdf/genetics_colon.pdf
- 67.Lee YC, Milne RL, Lheureux S, Friedlander M, McLachlan SA, Martin KL, et al. Risk of uterine cancer for BRCA1 and BRCA2 mutation carriers. Eur J Cancer 2017;84:114–20. doi: 10.1016/S0959-8049(17)31112-7 [DOI] [PubMed] [Google Scholar]
- 68.National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology. Genetic/familial high-risk assessment: breast, ovarian, and pancreatic. Version 2.2021. Accessed April 12, 2021. https://www.nccn.org/professionals/physician_gls/pdf/genetics_bop.pdf [DOI] [PubMed]
- 69.Piszczek C, Ma J, Gould CH, Tseng P. Cancer risk-reducing opportunities in gynecologic surgery. J Minim Invasive Gynecol 2018;25:1179–93. doi: 10.1016/j.jmig.2017.10.025 [DOI] [PubMed] [Google Scholar]
- 70.Shu CA, Pike MC, Jotwani AR, Friebel TM, Soslow RA, Levine DA, et al. Uterine cancer after risk-reducing salpingo-oophorectomy without hysterectomy in women with BRCA mutations. JAMA Oncol 2016;2:1434–40. doi: 10.1001/jamaoncol.2016.1820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Biron-Shental T, Drucker L, Altaras M, Bernheim J, Fishman A. High incidence of BRCA1-2 germline mutations, previous breast cancer and familial cancer history in Jewish patients with uterine serous papillary carcinoma. Eur J Surg Oncol 2006;32:1097–100. doi: 10.1016/j.ejso.2006.03.032 [DOI] [PubMed] [Google Scholar]
- 72.Win AK, Reece JC, Ryan S. Family history and risk of endometrial cancer: a systematic review and meta-analysis. Obstet Gynecol 2015;125:89–98. doi: 10.1097/AOG.0000000000000563 [DOI] [PubMed] [Google Scholar]
- 73.Chu D, Wu J, Wang K, Zhao M, Wang C, Li L, et al. Effect of metformin use on the risk and prognosis of endometrial cancer: a systematic review and meta-analysis. BMC Cancer 2018;18:438. doi: 10.1186/s12885-018-4334-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zhang D, Bai B, Xi Y, Zhao Y. Can aspirin reduce the risk of endometrial cancer? A systematic review and meta-analysis of observational studies. Int J Gynecol Cancer 2016;26:1111–20. doi: 10.1097/IGC.0000000000000731 [DOI] [PubMed] [Google Scholar]
- 75.Zhang XS, Zhang YM, Li B, Fan B, Zhao Y, Yang SJ. Risk reduction of endometrial and ovarian cancer after bisphosphonates use: a meta-analysis. Gynecol Oncol 2018;150:509–14. doi: 10.1016/j.ygyno.2018.06.012 [DOI] [PubMed] [Google Scholar]
- 76.Saed L, Varse F, Baradaran HR, Moradi Y, Khateri S, Friberg E, et al. The effect of diabetes on the risk of endometrial cancer: an updated a systematic review and meta-analysis. BMC Cancer 2019;19:527. doi: 10.1186/s12885-019-5748-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Aune D, Sen A, Vatten LJ. Corrigendum: hypertension and the risk of endometrial cancer: a systematic review and meta-analysis of case-control and cohort studies. Sci Rep 2018;8:46961. doi: 10.1038/srep46961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Adambekov S, Yi Y, Fabio A, Miljkovic I, Edwards RP, Lopa S, et al. Metabolic syndrome in endometrial cancer patients: systematic review. Metab Syndr Relat Disord 2019;17:241–9. doi: 10.1089/met.2018.0106 [DOI] [PubMed] [Google Scholar]
- 79.Practice Committee of the American Society for Reproductive Medicine. Fertility drugs and cancer: a guideline. Fertil Steril 2016;106:1617–26. doi: 10.1016/j.fertnstert.2016.08.035 [DOI] [PubMed] [Google Scholar]
- 80.Haoula Z, Salman M, Atiomo W. Evaluating the association between endometrial cancer and polycystic ovary syndrome. Hum Reprod 2012;27:1327–31. doi: 10.1093/humrep/des042 [DOI] [PubMed] [Google Scholar]
- 81.Doherty MT, Sanni OB, Coleman HG, Cardwell CR, McCluggage WG, Quinn D, et al. Concurrent and future risk of endometrial cancer in women with endometrial hyperplasia: a systematic review and meta-analysis. PLoS One 2020;15:e0232231. doi: 10.1371/journal.pone.0232231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Raffone A, Travaglino A, Saccone G, Insabato L, Mollo A, de Placido G, et al. Endometrial hyperplasia and progression to cancer: which classification system stratifies the risk better? A systematic review and meta-analysis. Arch Gynecol Obstet 2019;299:1233–42. doi: 10.1007/s00404-019-05103-1 [DOI] [PubMed] [Google Scholar]
- 83.Uglietti A, Buggio L, Farella M, Chiaffarino F, Dridi D, Vercellini P, et al. The risk of malignancy in uterine polyps: a systematic review and meta-analysis. Eur J Obstet Gynecol Reprod Biol 2019;237:48–56. doi: 10.1016/j.ejogrb.2019.04.009 [DOI] [PubMed] [Google Scholar]
- 84.Kabat GC, Park Y, Hollenbeck AR, Schatzkin A, Rohan TE. Intake of fruits and vegetables, and risk of endometrial cancer in the NIH-AARP Diet and Health Study. Cancer Epidemiol 2010;34:568–73. doi: 10.1016/j.canep.2010.06.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Prentice RL, Thomson CA, Caan B, Hubbell FA, Anderson GL, Beresford SAA, et al. Low-fat dietary pattern and cancer incidence in the Women's Health Initiative dietary modification randomized controlled trial. J Natl Cancer Inst 2007;99:1534–43. doi: 10.1093/jnci/djm159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.U.S. Department of Health and Human Services. Physical activity guidelines for Americans, 2nd ed. Washington, DC. Accessed February 15, 2022. https://health.gov/sites/default/files/2019-09/Physical_Activity_Guidelines_2nd_edition.pdf [Google Scholar]
- 87.U.S. Department of Health and Human Services, U.S. Department of Agriculture. 2015–2020 dietary guidelines for Americans. 8th ed. Accessed November 29, 2020. https://health.gov/our-work/food-nutrition/previous-dietary-guidelines/2015 [Google Scholar]
- 88.Parker ED, Folsom AR. Intentional weight loss and incidence of obesity-related cancers: the IA Women's Health Study. Int J Obes Relat Metab Disord 2003;27:1447–52. doi: 10.1038/sj.ijo.0802437 [DOI] [PubMed] [Google Scholar]
- 89.Luo J, Chlebowski RT, Hendryx M, Rohan T, Wactawski-Wende J, Thomson CA, et al. Intentional weight loss and endometrial cancer risk. J Clin Oncol 2017;35:1189–93. doi: 10.1200/JCO.2016.70.5822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Upala S, Sanguankeo A. Bariatric surgery and risk of postoperative endometrial cancer: a systematic review and meta-analysis. Surg Obes Relat Dis 2015;11:949–55. doi: 10.1016/j.soard.2014.09.024 [DOI] [PubMed] [Google Scholar]
- 91.Linkov F, Goughnour SL, Ma T, Xu Z, Edwards RP, Lokshin AE, et al. Changes in inflammatory endometrial cancer risk biomarkers in individuals undergoing surgical weight loss. Gynecol Oncol 2017;147:133–38. doi: 10.1016/j.ygyno.2017.07.144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.MacKintosh ML, Derbyshire AE, McVey RJ, Bolton J, Nickkho-Amiry M, Higgins CL, et al. The impact of obesity and bariatric surgery on circulating and tissue biomarkers of endometrial cancer risk. Int J Cancer 2019;144:641–50. doi: 10.1002/ijc.31913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.U.S. Preventive Services Task Force; Curry SJ, Krist AH, Owens DK, Barry MJ, Caughey A, et al. Behavioral weight loss interventions to prevent obesity-related morbidity and mortality in adults: US Preventive Services Task Force Recommendation Statement. JAMA 2018;320:1163–71. doi: 10.1001/jama.2018 [DOI] [PubMed] [Google Scholar]
- 94.Lynch syndrome. Practice Bulletin No. 147. American College of Obstetricians and Gynecologists. Obstet Gynecol 2014;124:1042–54. doi: 10.1097/01/AOG/0000456325.50739/72 [DOI] [PubMed] [Google Scholar]
- 95.Chen LM, Blank SV, Burton E, Glass K, Penick E, Woodard T. Reproductive and hormonal considerations in women at increased risk for hereditary gynecologic cancers: Society of Gynecologic Oncology and American Society for Reproductive Medicine evidence-based review. Fertil Steril 2019;112:1034–42. doi: 10.1016/j.fertnstert.2019.07.1349 [DOI] [PubMed] [Google Scholar]
- 96.Schmeler KM, Lynch HT, Chen LM, Munsell MF, Soliman PT, Clark MB, et al. Prophylactic surgery to reduce the risk of gynecologic cancers in the Lynch syndrome. N Engl J Med 2006;354:261–9. doi: 10.1056/NEJMoa052627 [DOI] [PubMed] [Google Scholar]
- 97.Breijer MC, Peeters JAH, Opmeer BC, Clark TJ, Verheijen RHM, Mol BWJ, et al. Capacity of endometrial thickness measurement to diagnose endometrial carcinoma in asymptomatic postmenopausal women: a systematic review and meta-analysis. Ultrasound Obstet Gynecol 2012;40:621–29. doi: 10.1002/uog.12306 [DOI] [PubMed] [Google Scholar]
- 98.Jobo T, Arai T, Sato R, Kuramoto H. Clinicopathologic relevance of asymptomatic endometrial carcinoma. Acta Cytol 2003;47:611–5. doi: 10.1159/000326577 [DOI] [PubMed] [Google Scholar]
- 99.Gerber B, Krause A, Müller H, Reimer T, Külz T, Kundt G, et al. Ultrasonographic detection of asymptomatic endometrial cancer in postmenopausal patients offers no prognostic advantage over symptomatic disease discovered by uterine bleeding. Eur J Cancer 2001;37:64–71. doi: 10.1016/s0959-8049(00)00356-7 [DOI] [PubMed] [Google Scholar]
- 100.Gemer O, Segev Y, Helpman L, Hag-Yahia N, Eitan R, Raban O, et al. Is there a survival advantage in diagnosing endometrial cancer in asymptomatic postmenopausal patients? An Israeli Gynecology Oncology Group study. Am J Obstet Gynecol 2018;219:181.e1–6. doi: 10.1016/j.ajog.2018.05.013 [DOI] [PubMed] [Google Scholar]
- 101.Smith RA, von Eschenbach AC, Wender R, Levin B, Byers T, Rothenberger D, et al. American Cancer Society guidelines for the early detection of cancer: update of early detection guidelines for prostate, colorectal, and endometrial cancers. CA Cancer J Clin 2001;51:38–75. doi: 10.3322/canjclin.51.1.38 [DOI] [PubMed] [Google Scholar]
- 102.Smith RA, Andrews KS, Brooks D, Fedewa SA, Manassaram-Baptiste D, Saslow D, et al. Cancer screening in the United States, 2017: a review of current American Cancer Society guidelines and current issues in cancer screening. CA Cancer J Clin 2017;67:100–21. doi: 10.3322/caac.21392 [DOI] [PubMed] [Google Scholar]
- 103.Zarbo G, Caruso G, Zammitti M, Caruso S, Zarbo R. The effects of tamoxifen therapy on the endometrium. Eur J Gynaecol Oncol 2000;21:86–8 [PubMed] [Google Scholar]
- 104.Fung MFK, Reid A, Faught W, Le T, Chenier C, Verma S, et al. Prospective longitudinal study of ultrasound screening for endometrial abnormalities in women with breast cancer receiving tamoxifen. Gynecol Oncol 2003;91:154–9. doi: 10.1016/s0090-8258(03)00441-4 [DOI] [PubMed] [Google Scholar]
- 105.Kazerooni T, Ghaffarpasand F, Mosalaei A, Kazerooni Y. The value of transvaginal ultrasonography in the endometrial evaluation of breast cancer patients using tamoxifen. Med Princ Pract 2010;19:222–7. doi: 10.1159/000285296 [DOI] [PubMed] [Google Scholar]
- 106.Tamoxifen and uterine cancer. Committee Opinion No. 601. American College of Obstetricians and Gynecologists. Obstet Gynecol 2014;123:1394–7. doi: 10.1097/01.AOG.0000450757.18294.cf [DOI] [PubMed] [Google Scholar]
- 107.National Cancer Institute. Endometrial cancer prevention (PDQ®): health professional version. Accessed December 2, 2020. https://www.cancer.gov/types/uterine/hp/endometrial-prevention-pdq
- 108.Clarke MA, Long BJ, Del Mar Morillo A, Arbyn M, Bakkum-Gamez JN, Wentzensen N. Association of endometrial cancer risk with postmenopausal bleeding in women: a systematic review and meta-analysis. JAMA Intern Med 2018;178:1210–22. doi: 10.1001/jamainternmed.2018.2820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Boruta D, II, Gehrig PA, Fader AN, Olawaiye AB. Management of women with uterine papillary serous cancer: a Society of Gynecologic Oncology (SGO) review. Gynecol Oncol 2009;115:142–53. doi: 10.1016/j.ygyno.2009.06.011 [DOI] [PubMed] [Google Scholar]
- 110.Astrup K, Olivarius NDF. Frequency of spontaneously occurring postmenopausal bleeding in the general population. Acta Obstet Gynecol Scand 2004;83:203–07. doi: 10.1111/j.0001-6349.2004.00400.x [DOI] [PubMed] [Google Scholar]
- 111.Johnson N, Miles T, Bailey D, Tylko-Hill K, Das N, Ahson G, et al. Delays in treating endometrial cancer in the south west of England. Br J Cancer 2011;104:1836–9. doi: 10.1038/bjc.2011.173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Timmermans A, Opmeer BC, Khan KS, Bachmann LM, Epstein E, Clark TJ, et al. Endometrial thickness measurement for detecting endometrial cancer in women with postmenopausal bleeding: a systematic review and meta-analysis. Obstet Gynecol 2010;116:160–7. doi: 10.1097/AOG.0b013e3181e3e7e8 [DOI] [PubMed] [Google Scholar]
- 113.Alborzi S, Parsanezhad ME, Mahmoodian N, Alborzi S, Alborzi M. Sonohysterography versus transvaginal sonography for screening of patients with abnormal uterine bleeding. Int J Gynaecol Obstet 2007;96:20–3. doi: 10.1016/j.ijgo.2006.09.004 [DOI] [PubMed] [Google Scholar]
- 114.de Vries LD, Dijkhuizen FP, Mol BW, Brolmann HA, Moret E, Heintz AP. Comparison of transvaginal sonography, saline infusion sonography, and hysteroscopy in premenopausal women with abnormal uterine bleeding. J Clin Ultrasound 2000;28:217–23. doi: [DOI] [PubMed] [Google Scholar]
- 115.Guven MA, Bese T, Demirkiran F. Comparison of hydrosonography and transvaginal ultrasonography in the detection of intracavitary pathologies in women with abnormal uterine bleeding. Int J Gynecol Cancer 2004;14:57–63. doi: 10.1111/j.1048-891x.2004.14105.x [DOI] [PubMed] [Google Scholar]
- 116.Dueholm M, Jensen ML, Laursen H, Kracht P. Can the endometrial thickness as measured by trans-vaginal sonography be used to exclude polyps or hyperplasia in pre-menopausal patients with abnormal uterine bleeding? Acta Obstet Gynecol Scand 2001;80:645–51 [PubMed] [Google Scholar]
- 117.Endometrial cancer. Practice Bulletin No. 149. American College of Obstetricians and Gynecologists. Obstet Gynecol 2015;125:1006–26. doi: 10.1097/01.AOG.0000462977.61229.de [DOI] [PubMed] [Google Scholar]
- 118.Dijkhuizen FP, Mol BW, Brolmann HA, Heintz AP. The accuracy of endometrial sampling in the diagnosis of patients with endometrial carcinoma and hyperplasia: a meta-analysis. Cancer 2000;89:1765–72 [PubMed] [Google Scholar]
- 119.van Hanegem N, Prins MMC, Bongers MY, Opmeer BC, Sahota DS, Mol BWJ, et al. The accuracy of endometrial sampling in women with postmenopausal bleeding: a systematic review and meta-analysis. Eur J Obstet Gynecol Reprod Biol 2016;197:147–55. doi: 10.1016/j.ejogrb.2015.12.008 [DOI] [PubMed] [Google Scholar]
- 120.Diagnosis of abnormal uterine bleeding in reproductive-aged women. Practice Bulletin No. 128. American College of Obstetricians and Gynecologists. Obstet Gynecol 2012;120:197–206. doi: 10.1097/AOG.0b013e318262e320 [DOI] [PubMed] [Google Scholar]
- 121.Clark TJ, Mann CH, Shah N, Khan KS, Song F, Gupta JK. Accuracy of outpatient endometrial biopsy in the diagnosis of endometrial cancer: a systematic quantitative review. BJOG 2002;109:313–21. doi: 10.1111/j.1471-0528.2002.01088.x [DOI] [PubMed] [Google Scholar]
- 122.Clark TJ, Voit D, Gupta JK, Hyde C, Song F, Khan KS. Accuracy of hysteroscopy in the diagnosis of endometrial cancer and hyperplasia: a systematic quantitative review. JAMA 2002;288:1610–21. doi: 10.1001/jama.288.13.1610 [DOI] [PubMed] [Google Scholar]
- 123.Ceci O, Bettocchi S, Pellegrino A, Impedovo L, Di Venere R, Pansini N. Comparison of hysteroscopic and hysterectomy findings for assessing the diagnostic accuracy of office hysteroscopy. Fertil Steril 2002;78:628–31. doi: 10.1016/s0015-0282(02)03246-6 [DOI] [PubMed] [Google Scholar]
- 124.The role of transvaginal ultrasonography in evaluating the endometrium of women with postmenopausal bleeding. ACOG Committee Opinion No. 734. American College of Obstetricians and Gynecologists. Obstet Gynecol 2018;131:e124–9. doi: 10.1097/AOG.0000000000002631 [DOI] [PubMed] [Google Scholar]
- 125.Yi Y, Bryce CL, Adambekov S, Edwards RP, Goughnour SL, Linkov F. Cost-effectiveness analysis of biopsy strategies for endometrial cancer diagnosis in women with postmenopausal bleeding: Pipelle sampling curette versus dilatation & curettage. Gynecol Oncol 2018;150:112–8. doi: 10.1016/j.ygyno.2018.04.565 [DOI] [PubMed] [Google Scholar]
- 126.Clark TJ, Barton PM, Coomarasamy A, Gupta JK, Khan KS. Investigating postmenopausal bleeding for endometrial cancer: cost-effectiveness of initial diagnostic strategies. BJOG 2006;113:502–10. doi: 10.1111/j.1471-0528.2006.00914.x [DOI] [PubMed] [Google Scholar]
- 127.Munro MG, Critchley HOD, Broder MS, Fraser IS; FIGO Working Group on Menstrual Disorders. FIGO classification system (PALM-COEIN) for causes of abnormal uterine bleeding in nongravid women of reproductive age. Int J Gynecol Obstet 2011;113:3–13. doi: 10.1016/j.ijgo.2010.11.011 [DOI] [PubMed] [Google Scholar]
- 128.National Institute for Health and Care Excellence. Heavy menstrual bleeding: assessment and management. NICE Guideline [NG88]. Accessed May 25, 2021. https://www.nice.org.uk/guidance/ng88 [PubMed]
- 129.Yasa C, Dural O, Bastu E, Ugurlucan FG, Nehir A, Iyibozkurt AC. Evaluation of the diagnostic role of transvaginal ultrasound measurements of endometrial thickness to detect endometrial malignancy in asymptomatic postmenopausal women. Arch Gynecol Obstet 2016;294:311–6. doi: 10.1007/s00404-016-4054-5 [DOI] [PubMed] [Google Scholar]
- 130.Smith-Bindman R, Weiss E, Feldstein V. How thick is too thick? When endometrial thickness should prompt biopsy in postmenopausal women without vaginal bleeding. Ultrasound Obstet Gynecol 2004;24:558–65. doi: 10.1002/uog.1704 [DOI] [PubMed] [Google Scholar]
- 131.The use of hysteroscopy for the diagnosis and treatment of intrauterine pathology. ACOG Committee Opinion No. 800. American College of Obstetricians and Gynecologists. Obstet Gynecol 2020;135:e138–48. doi: 10.1097/AOG.0000000000003712 [DOI] [PubMed] [Google Scholar]
- 132.Cibas ES, Chelmow D, Waxman AG, Moriarty AT. Endometrial cells: the how and when of reporting. In: Nayar R, Wilbur DC, editors. The Bethesda System for reporting cervical cytology: definitions, criteria, and explanatory notes. 3rd ed. Springer International Publishing; 2015. [Google Scholar]
- 133.Perkins RB, Guido RS, Castle PE, Chelmow D, Einstein MH, Garcia F, et al. ASCCP risk-based management consensus guidelines for abnormal cervical cancer screening tests and cancer precursors. J Low Genit Tract Dis 2020;24:102–31. doi: 10.1097/LGT.0000000000000525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Canfell K, Kang YJ, Clements M, Moa AM, Beral V. Normal endometrial cells in cervical cytology: systematic review of prevalence and relation to significant endometrial pathology. J Med Screen 2008;15:188–98. doi: 10.1258/jms.2008.008069 [DOI] [PubMed] [Google Scholar]
- 135.Whetstone S, Burke W, Sheth SS, Brooks R, Cavens A, Huber-Keener K, et al. Health disparities in uterine cancer: report from the Uterine Cancer Evidence Review Conference. Obstet Gynecol 2022;139:645–59. doi: 10.1097/AOG.0000000000004710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Burbos N, Musonda P, Giarenis I, Shiner AM, Giamougiannis P, Morris EP, et al. Predicting the risk of endometrial cancer in postmenopausal women presenting with vaginal bleeding: the Norwich DEFAB risk assessment tool. Br J Cancer 2010;102:1201–6. doi: 10.1038/sj.bjc.6605620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Begum J, Samal R. A clinicopathological evaluation of postmenopausal bleeding and its correlation with risk factors for developing endometrial hyperplasia and cancer: a hospital-based prospective study. J Midlife Health 2019;10:179–83. doi: 10.4103/jmh.JMH_136_18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Cheewakriangkrai C, Kietpeerakool C, Charoenkwan K, Pattanittum P, John D, Aue-Aungkul A, et al. Health education interventions to promote early presentation and referral for women with symptoms of endometrial cancer. The Cochrane Database of Systematic Reviews 2020, Issue 3. Art. No.: CD013253. doi: 10.1002/14651858.CD013253.pub2 [DOI] [PMC free article] [PubMed]
- 139.Aarnio M, Mecklin JP, Aaltonen LA, Nyström-Lahti M, Järvinen HJ. Life-time risk of different cancers in hereditary non-polyposis colorectal cancer (HNPCC) syndrome. Int J Cancer 1995;64:430–3. doi: 10.1002/ijc.2910640613 [DOI] [PubMed] [Google Scholar]
- 140.Dunlop MG, Farrington SM, Carothers AD, Wyllie AH, Sharp L, Burn J, et al. Cancer risk associated with germline DNA mismatch repair gene mutations. Hum Mol Genet 1997;6:105–10. doi: 10.1093/hmg/6.1.105 [DOI] [PubMed] [Google Scholar]
- 141.Oliwaiye AB, Leath CA, III. Contemporary management of uterine clear cell carcinoma: a Society of Gynecologic Oncology (SGO) review and recommendation. Gynecol Oncol 2019;155:365–73. doi: 10.1016/j.ygyno.2019.08.031 [DOI] [PubMed] [Google Scholar]
- 142.Endometrial intraepithelial neoplasia. Committee Opinion No. 631. American College of Obstetricians and Gynecologists. Obstet Gynecol 2015;125:1272–8. doi: 10.1097/01.AOG.0000465189.50026.20 [DOI] [PubMed] [Google Scholar]
- 143.Trimble CL, Kauderer J, Zaino R, Silverberg S, Lim PC, Burke JJ, II, et al. Concurrent endometrial carcinoma in women with a biopsy diagnosis of atypical endometrial hyperplasia: a Gynecologic Oncology Group study. Cancer 2006;106:812–9. doi: 10.1002/cncr.21650 [DOI] [PubMed] [Google Scholar]
- 144.Karamursel BS, Guven S, Tulunay G, Kucukali T, Ayhan A. Which surgical procedure for patients with atypical endometrial hyperplasia? Int J Gynecol Cancer 2005;15:127–31. doi: 10.1111/j.1048-891X.2005.15013.x [DOI] [PubMed] [Google Scholar]
- 145.Touhami O, Grégoire J, Renaud MC, Sebastianelli A, Grondin K, Plante M. The utility of sentinel lymph node mapping in the management of endometrial atypical hyperplasia. Gynecol Oncol 2018;148:485–90. doi: 10.1016/j.ygyno.2017.12.026 [DOI] [PubMed] [Google Scholar]
- 146.Whyte JS, Gurney EP, Curtin JP, Blank SV. Lymph node dissection in the surgical management of atypical endometrial hyperplasia. Am J Obstet Gynecol 2010;202:176.e1–4. doi: 10.1016/j.ajog.2009.10.855 [DOI] [PubMed] [Google Scholar]
- 147.Park JY, Nam JH. Progestins in the fertility-sparing treatment and retreatment of patients with primary and recurrent endometrial cancer. Oncologist 2015;20:270–8. doi: 10.1634/theoncologist.2013-0445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.American Society for Reproductive Medicine. Ethics Committee of the American Society for Reproductive Medicine. Fertility preservation in patients facing gonadotoxic therapies: an ethics committee opinion. Fertil Steril 2018;110:380–6. doi: 10.1016/j.fertnstert.2018.05.034 [DOI] [PubMed] [Google Scholar]
- 149.Gunderson CC, Faber AN, Carson KA, Bristow RE. Oncologic and reproductive outcomes with progestin therapy in women with endometrial hyperplasia and grade 1 adenocarcinoma: a systematic review. Gynecol Oncol 2012;125:477–82. doi: 10.1016/j.ygyno.2012.01.003 [DOI] [PubMed] [Google Scholar]
- 150.Gallos ID, Yap J, Rajkhowa M, Luesley DM, Coomarasamy A, Gupta JK. Regression, relapse, and live birth rates with fertility-sparing therapy for endometrial cancer and atypical complex endometrial hyperplasia: a systematic review and metaanalysis. Am J Obstet Gynecol 2012;207:266.e1–12. doi: 10.1016/j.ajog.2012.08.011 [DOI] [PubMed] [Google Scholar]
- 151.Kim MK, Seong SJ, Kim YS, Song T, Kim ML, Yoon BS, et al. Combined medroxyprogesterone acetate/levonorgestrel-intrauterine system treatment in young women with early-stage endometrial cancer. Am J Obstet Gynecol 2013;209:358.e1–4. doi: 10.1016/j.ajog.2013.06.031 [DOI] [PubMed] [Google Scholar]
- 152.Leone Roberti Maggiore U, Martinelli F, Dondi G, Bogani G, Chiappa V, Evangelista MT, et al. Efficacy and fertility outcomes of levonorgestrel-releasing intra-uterine system treatment for patients with atypical complex hyperplasia or endometrial cancer: a retrospective study. J Gynecol Oncol 2019;30:e57. doi: 10.3802/jgo.2019.30.e57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Kim MK, Seong SJ, Kang SB, Bae DS, Kim JW, Nam JH, et al. Six months response rate of combined oral medroxyprogesterone/levonorgestrel-intrauterine system for early-stage endometrial cancer in young women: a Korean Gynecologic-Oncology Group study. J Gynecol Oncol 2019;30:e47. doi: 10.3802/jgo.2019.30.e47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Rodolakis A, Biliatis I, Morice P, Reed N, Mangler M, Kesic V, et al. European Society of Gynecologic Oncology Task Force for Fertility Preservation: clinical recommendations for fertility-sparing management in young endometrial cancer patients. Int J Gynecol Cancer 2015;25:1258–65. doi: 10.1097/IGC.0000000000000493 [DOI] [PubMed] [Google Scholar]
- 155.Cianci S, Rosati A, Capozzi VA, Tarascio M, Uccella S, Palumbo MA, et al. Quality of life and sexual functioning of patient affected by endometrial cancer. Minerva Med 2021;112:81–95. doi: 10.23736/S0026-4806.20.07081-0 [DOI] [PubMed] [Google Scholar]
- 156.Ferrandina G, Petrillo M, Mantegna G, Fuoco G, Terzano S, Venditti L, et al. Evaluation of quality of life and emotional distress in endometrial cancer patients: a 2-year prospective, longitudinal study. Gynecol Oncol 2014;133:518–25. doi: 10.1016/j.ygyno.2014.03.015 [DOI] [PubMed] [Google Scholar]
- 157.Harter P, Schrof I, Karl LM, Hils R, Kullmann V, Traut A, et al. Sexual function, sexual activity and quality of life in women with ovarian and endometrial cancer. Geburtshilfe Frauenheilkd 2013;73:428–32. doi: 10.1055/s-0032-1328602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Armbruster SD Song J , Bradford A Carmack CL Lu KH Basen-Engquist KM. Sexual health of endometrial cancer survivors before and after a physical activity intervention: a retrospective cohort analysis. Gynecol Oncol 2016;143:589–95. doi: 10.1016/j.ygyno.2016.09.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Damast S, Alektiar KM, Goldfarb S, Eaton A, Patil S, Mosenkis J, et al. Sexual functioning among endometrial cancer patients treated with adjuvant high-dose-rate intra-vaginal radiation therapy. Int J Radiat Oncol Biol Phys 2012;84:e187–93. doi: 10.1016/j.ijrobp.2012.03.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Aerts L, Enzlin P, Verhaeghe J, Poppe W, Vergote I, Amant F. Sexual functioning in women after surgical treatment for endometrial cancer: a prospective controlled study. J Sex Med 2015;12:198–209. doi: 10.1111/jsm.12764 [DOI] [PubMed] [Google Scholar]
- 161.Buckingham L, Haggerty A, Graul A, Morgan M, Burger R, Ko E, et al. Sexual function following hysterectomy for endometrial cancer: a five-year follow up investigation. Gynecol Oncol 2019;152:139–44. doi: 10.1016/j.ygyno.2018.10.025 [DOI] [PubMed] [Google Scholar]
- 162.Damast S, Alektiar K, Eaton A, Gerber NK, Goldfarb S, Patil S, et al. Comparative patient-centered outcomes (health state and adverse sexual symptoms) between adjuvant brachytherapy versus no adjuvant brachytherapy in early stage endometrial cancer. Ann Surg Oncol 2014;21:2740–54. doi: 10.1245/s10434-014-3562-4 [DOI] [PubMed] [Google Scholar]
- 163.Nout RA, Putter H, Jurgenliemk-Schulz IM, Jobsen JJ, Lutgens LCHW, van der Steen-Banasik EM, et al. Quality of life after pelvic radiotherapy or vaginal brachytherapy for endometrial cancer: first results of the randomized PORTEC-2 trial. J Clin Oncol 2009;27:3547–56. doi: 10.1200/JCO.2008.20.2424 [DOI] [PubMed] [Google Scholar]
- 164.Foerster R, Schnetzke L, Bruckner T, Arians N, Rief H, Debus J, et al. Prognostic factors for long-term quality of life after adjuvant radiotherapy in women with endometrial cancer. Strahlenther Onkol 2016;192:895–904. doi: 10.1007/s00066-016-1037-1 [DOI] [PubMed] [Google Scholar]
- 165.de Boer SM, Nout RA, Jurgenliemk-Schulz IM, Jobsen JJ, Lutgens LC, van der Steen-Banasik EM, et al. Long-term impact of endometrial cancer diagnosis and treatment on health-related quality of life and cancer survivorship: results from the randomized PORTEC-2 trial. Int J Radiat Oncol Biol Phys 2015;93:797–809. doi: 10.1016/j.ijrobp.2015.08.023 [DOI] [PubMed] [Google Scholar]
- 166.Brotto LA, Erskine Y, Carey M, Ehlen T, Finlayson S, Heywood M, et al. A brief mindfulness-based cognitive behavioral intervention improves sexual functioning versus wait-list control in women treated for gynecologic cancer. Gynecol Oncol 2012;125:320–5. doi: 10.1016/j.ygyno.2012.01.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Akbaba S, Oelmann-Avendano JT, Krug D, Arians N, Bostel T, Hoerner-Rieber J, et al. The impact of vaginal dilator use on vaginal stenosis and sexual quality of life in women treated with adjuvant radiotherapy for endometrial cancer. Strahlenther Onkol 2019;195:902–12. doi: 10.1007/s00066-019-01466-1 [DOI] [PubMed] [Google Scholar]