Abstract
Purpose
The midline catheter (MC) is a peripheral venous access device with the catheter tip located in the axilla and available for mid-term intravenous (IV) therapy. This study evaluated the efficacy and clinical outcomes associated with the placement of MCs with an integrated wire-accelerated Seldinger technique for IV access.
Methods
Materials and A retrospective review was conducted at a single center in South Korea between March 2020 and July 2020. Consecutive patients in whom MC insertions were performed by vascular surgeons were enrolled. The outcomes included catheter indwelling time and incidence of catheter-related adverse events.
Results
Ninety-five patients (117 catheters) were included in the study. The total indwelling time was 1,964 days, with a median of 16.7 days (range, 0-76). The complication-free catheter rates at 5 and 28 days were 92.9% and 65.5%, respectively. Overall, 32 (27.4%) catheters were removed due to complications; however, major complications, such as symptomatic deep venous thrombosis and catheter-induced bloodstream infections, were confirmed in only 3 (2.6%) catheters. A common reason for premature catheter removal is inadvertent removal owing to patient inattention. A high body mass index and female sex were identified as risk factors for short indwelling times and complicated premature catheter removal.
Conclusion
MC insertion is a simple and operator-friendly procedure with a low rate of major complication. It enables mid-term IV treatment through a single procedure if there are no specific complications, thereby improving quality of life of patients during hospital stay.
Keywords: Peripheral venous catheterization, Intravenous infusions, Indwelling catheters, Vascular access devices
INTRODUCTION
During hospital admission, most patients require intravenous (IV) access for the administration of medications, fluids, blood products, and nutritional support. There are two types of IV access catheters: peripheral venous catheters (PVCs) and central venous catheters (CVCs). The oldest types of PVCs are short catheters commonly placed on the forearm or dorsal aspect of the hand, which are used in patients with fewer complications and easier establishment of access [1]. However, repetitive catheter insertions are usually required every 72 to 96 hours, causing pain and discomfort from repeated venipuncture in patients who require long-term fluid therapy [2]. This discomfort is exacerbated in patients lacking a usable vein for the establishment of peripheral access.
Recent guidelines [1,2] recommend a short PVC for patients who require short-term IV fluid therapy ≤4 days. They also recommended using other types of vascular access if long-term treatment is required. A midline catheter (MC), as defined by the Infusion Nurses Society, is a vascular access device for placement in the peripheral vein of the upper arm, including the basilic, cephalic, and brachial veins, with the internal tip positioned at or near the level of the axilla [1]. Standard MCs can range from 10 to 20 cm in length and have a single or double lumen. They are intended to provide lasting IV access over a duration of 1 to 4 weeks for patients requiring fluid therapy, in whom establishment of IV access is otherwise difficult [1]. Currently, MCs are gaining attention as a satisfactory replacement for PVC in patients who require mid-term fluid therapy and have poor IV access. This can reduce the repetition of venipuncture and enable repeated blood sampling [3].
The Seldinger- and modified Seldinger-type MCs consist of individual catheters, puncture needles, and wires. Therefore, each step, including needle puncture, wire insertion, and catheter insertion, was performed separately, resulting in some bleeding between steps. However, the newly designed and recently launched next-generation MC device uses the accelerated Seldinger technique (AST) [4]. These integrated wire-AST-MCs are configured with a needle, guidewire, dilator, and sheath in one package. The needle device was inserted into the target vein under ultrasound guidance and a flash was subsequently observed. The internal guidewire was then advanced into the vein and snapped in the needle hub. The dilator collar is turned, and the dilator and sheath are advanced. Finally, the dilator hub was disengaged from the needle hub, and the guidewire, dilator, and needle were removed as a single unit. All of these processes are accomplished in a single step. Hence, bleeding rarely occurs in this method unlike conventional Seldinger-type MC.
Herein, we report the initial experience using a new device in a single institution. The main purpose of this study was to evaluate the clinical outcomes of AST-MC placement, in terms of efficacy and safety.
MATERIALS AND METHODS
1) Study design
This retrospective study was conducted at a single center in South Korea. Records of patients who underwent AST-MC insertion between March 2020 and July 2020 were reviewed. Study data, including patient demographics, diagnoses, number of catheter insertion attempts, reasons for MC insertion, catheterized vein, vein size, and complications, were obtained. Catheter indwelling time (CIT) and occurrence of adverse events were analyzed to determine the safety and efficacy of the catheter.
This study was approved by the institutional review board of Presbyterian Medical Center (no. 2020-04-018). The requirement for informed consent was waived due to the retrospective nature of the study and the method of analysis using anonymized clinical data.
2) Catheter insertion procedure
Each MC insertion was performed at the patient’s bedside (Fig. 1, 2). Materials such as 2% lidocaine, saline, syringes, and the one-handed sliding accelerated Seldinger MC (PowerGlide Pro midline catheter; BD, Franklin Lakes, NJ, USA) were prepared accordingly. Three different ultrasound machines with linear probes (GE Healthcare, Boston, MA, USA; Philips, Andover, MA, USA; Samsung Medison, Seoul, Korea) were used in this study. All MCs were inserted by two vascular surgeons with >5 years of experience in ultrasound-guided vascular access procedures. First, a suitable vein was identified in the non-dominant arm under ultrasound guidance. When selecting the target vein, the physician measured the vein diameter in the transverse ultrasound view, accounting for each length and width, under tourniquet application. An axial vein without an accessory branch was selected for this procedure. If the selection of a vein with an accessory branch was inevitable, the location was set such that the accessory vein was not punctured. The largest vein with the lowest number of accessory veins was selected among the brachial, basilic, and cephalic veins. In patients with chronic kidney disease requiring dialysis, the basilic and cephalic veins were excluded to preserve these for arteriovenous fistula creation.
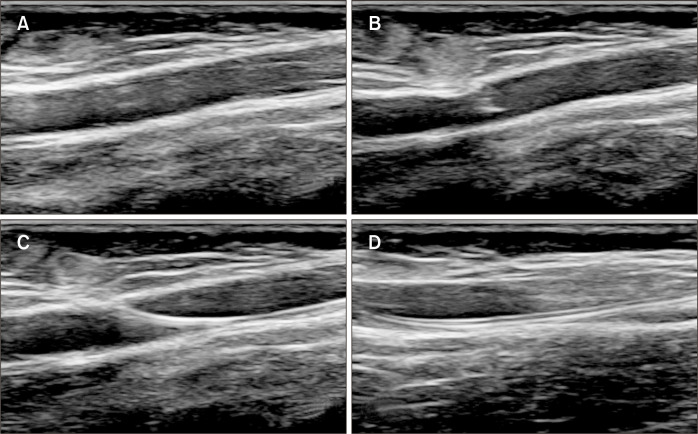
Fig. 1.
Ultrasonographic images showed the following findings during the catheter insertion. (A) Checking the target vein. (B) Venipuncture. (C) Wire insertion. (D) Catheter insertion and checking the tip location.
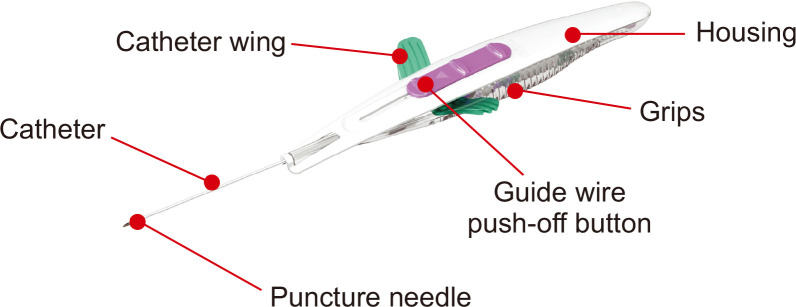
Fig. 2.
Structure of integrated wire accelerated Seldinger technique midline catheters is shown. Image from BD (https://static.bd.com/assets/product/Documents/Peripheral%20Intervention%20(PI)/PF10364-0747882_powerglide_pro_ifu_web.pdf), with original copyright holder’s permission.
After vein selection, the most appropriate insertion site was determined and a topical anesthetic agent (2% lidocaine) was administered at the puncture site. Subsequently, the targeted vein was punctured using the MC needle tip. After confirming cannulation using ultrasonography (US), a guidewire was advanced into the main branch of the vein. Finally, the wing of the introducer was advanced into the vein, and the position of the catheter tip was confirmed using ultrasound (Fig. 3). The target location of the catheter tip was the axillary area; however, the AST-MCs were sometimes shorter, and the tip was located slightly distal to the axilla at a distance <5 cm.
Fig. 3.
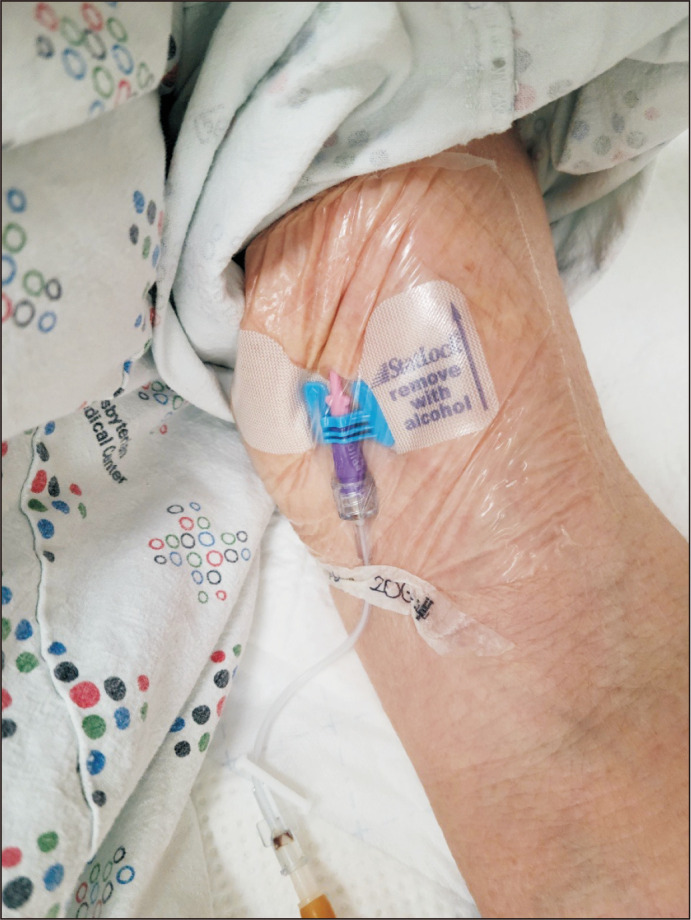
This picture shows a midline catheter inserted into the patient’s left upper arm basilic vein.
3) Outcomes
The clinical outcomes were analyzed accordingly. Efficacy outcomes included CIT, and safety outcomes included the incidence of catheter-related adverse events and premature removal of MCs. Adverse events included vein thrombosis, systemic or local infections, and catheter dysfunction. Catheter removal upon completion of treatment was considered a planned removal, whereas a premature catheter removal was categorized into complicated and non-complicated cases. Accidental catheter removal was regarded as non-complicated catheter removal, whereas removal for any other reason was regarded as a complicated removal. Major complications were defined as adverse events requiring further therapy, including symptomatic upper arm deep vein thrombosis (DVT) and catheter-associated bloodstream infections (CA-BSI). Minor complications were defined as those that required premature catheter removal but did not require further complication-specific therapies. These complications included minor thrombosis, local infection, and catheter dysfunction, including catheter kinking or partial catheter removal. Catheter extrusion, due to poor fixation, was defined as a partial catheter removal, whereas a catheter kinking was defined as folding of the catheter body. Minor thrombotic complications were defined as symptomatic focal segmental thrombi detected on US. Minor infections, including exit-site, tunnel, and pocket infections, showed tenderness, erythema, induration, and clinical signs of subcutaneous pocket infection, without bloodstream infection. CA-BSI was defined as growth of the same pathogen upon culture of the blood and catheter tip, or as signs of infection that resolved following catheter removal.
4) Statistical analysis
Statistical analysis was performed using the SPSS software, version 28.0 (IBM Corp., Armonk, NY, USA). The characteristics of all patients were analyzed and compared. Indwelling time (in days) was calculated as the date of removal minus the date of insertion. Complications were described using a composite rate that included the number of complications per 1,000 catheter days to allow a standard measure that could be compared with other studies. The correlation between indwelling time and patient factors was analyzed using multiple linear regression. The correlation between patient factors and complicated catheter removal was analyzed using multivariate analysis of the Cox proportional hazard model. Statistical significance was considered at P<0.05.
RESULTS
During the study period, 97 patients were referred to the Department of Vascular Surgery for an MC insertion. Two patients with prior failed attempts at catheter insertion were excluded. The remaining 95 patients (117 catheters) were included in this study. The characteristics of the patients and MCs they received are shown in Table 1. The mean age was 75.2±12.4 years (range, 31-92), and 58% were female. The mean body mass index (BMI) was 23.0±4.8 kg/m2 (range, 12.5-35.5). At the time of admission, 30 (31.6%) patients were treated for infectious diseases in parts of the body other than the lung, 19 (20.0%) had malignant disease, 14 (14.7%) patients had fractures, 17 (17.9%) had pneumonia, and the remaining 15 (15.8%) were treated for other diseases.
Table 1.
Patient demographics and catheter characteristics
| Characteristic | Number |
|---|---|
| Patients (n=95) | |
| Age (y) | 75.2±12.4 (31-92) |
| Sex, female | 55 (57.9) |
| Body mass index (kg/m2) | 23.0±4.8 (12.5-35.5) |
| Diagnosis | |
| Infection | 30 (31.6) |
| Malignancy | 19 (20.0) |
| Pneumonia | 17 (17.9) |
| Bone fracture | 14 (14.7) |
| Others | 15 (15.8) |
| Number of catheter insertions | |
| 1 | 77 (81.1) |
| 2 | 16 (16.8) |
| 3 | 1 (1.1) |
| 5 | 1 (1.1) |
| Catheters (n=117) | |
| Reasons for catheter insertion | |
| Intravenous antibiotics | 68 (58.1) |
| Difficult venous access | 36 (30.8) |
| Total parenteral nutrition | 10 (8.5) |
| Vasopressor infusions | 3 (2.6) |
| Catheterized vein | |
| Brachial vein | 73 (62.4) |
| Basilic vein | 43 (36.8) |
| Cephalic vein | 1 (0.9) |
| Left-sided catheter | 62 (53.0) |
| Vein size (mm) | 4.5±0.8 (3.2-7.7) |
| Catheter indwell time (d) | 16.8±13.5 (0-76) |
Values are presented as mean±standard deviation (range) or number (%).
Seventy-seven (81.1%) patients underwent only one MC insertion, 16 (16.8%) underwent two insertions, and three and five insertions were performed for one patient each. Of the 16 patients who underwent two insertions, six were due to accidental removal and the other six were due to catheter-related complications. Three insertions in one patient were due to accidental catheter removal. Five insertions in one patient were due to accidental removal and complications. The most common indication for MC insertion was antibiotic therapy in 68 (58.1%) catheters, followed by difficult venous access for scheduled procedures (e.g., surgeries) in 30.8%, parenteral nutrition in 8.5%, and vasopressor infusion in 2.6%.
The most common location for MC placement was the brachial vein in 73 (62.4%), followed by the basilic vein in 43 (36.8%), and cephalic vein in one. The mean vein diameter was 4.5±0.8 mm (range, 3.2-7.7). Catheter insertion was performed in the non-dominant arm in 62 patients (53.0%). The average procedure time from patient preparation to dressing was 11.3 min (range, 5-40). The mean CIT was 16.8±13.5 days (range, 0-76), and the 5-day complication-free catheter survival rate was 92.9%. Conversely, the complication-free catheter survival rate at 28 days was 65.5%. Twenty-two MCs had CIT <5 days, including seven MCs with adverse events and 15 cases of accidental removal or otherwise early termination of treatment that required mid-term peripheral access.
Table 2 shows the causes of the catheter removal. Premature removal occurred in 51 (43.6%) MC cases, including 32 complicated and 19 non-complicated accidental removal cases. The most common cause of accidental catheter removal is a lack of patient awareness of the need to maintain IV access, especially in dementia.
Table 2.
Catheter removal issues (n=117)
| Cause of catheter removal | Number (%) |
|---|---|
| Planned removal: finished IV treatment | 66 (56.4) |
| Premature removal | 51 (43.6) |
| Complicated | 32 (27.4) |
| Thrombosisa | 18 (15.4) |
| Dysfunction | 9 (7.7) |
| Infectionb | 5 (4.3) |
| Non-complicated: accidental removal | 19 (16.2) |
aOne patient had a major complication - deep vein thrombosis in the upper arm. bTwo patients had major complications - catheter-associated bloodstream infection.
The total CIT was 1,964 days, and complicated catheter removal occurred in 16.2 per 1,000 catheter days. Thrombotic complications developed in 18 MCs, including 17 minor and one major adverse event. The major complication was DVT in the upper arm, with swelling and pain, which required further anticoagulation therapy. Minor complications were defined as focal thrombi without swelling or pain. The focal thrombi resolved naturally after catheter removal. Nine cases of catheter dysfunction, such as catheter kinking and partial removal, occurred in 4.6 per 1,000 catheter days. The skin exit site of the catheter was the most common site of kinking, and in the case of partial catheter removal, the kink developed in the middle catheter body.
Five MCs (4.3%) were removed because of infection, including two major complications due to CA-BSI. The remaining three patients had minor complications, such as infection and phlebitis, characterized by redness at the catheter insertion site. Major complications developed in three MCs, including two CA-BSI and one symptomatic upper extremity DVT.
Multiple linear regression analysis was performed on patient factors related to CIT. CIT was significantly shorter in higher BMI (B=–0.872, P=0.001) and female patients (B=–6.481, P=0.015; Table 3). To identify the risk factors for complications during the study period, multivariable Cox regression analysis was conducted, with sex and BMI showing statistically significant differences. Females were 1.065 times more likely to develop complications than males (P=0.008), and a higher BMI was associated with an increased incidence of complications by as much as 0.147 times (P=0.006; Table 4).
Table 3.
Multiple linear regression of factors associated with midline catheter indwelling duration
| Risk factor | B | SE | β | t | P-value |
|---|---|---|---|---|---|
| Constant | 25.365 | 14.472 | - | 1.753 | 0.082 |
| Age | 0.163 | 0.097 | 0.155 | 1.681 | 0.096 |
| Sex, female* | –6.481 | 2.612 | –0.241 | –2.481 | 0.015 |
| Body mass index* | –0.872 | 0.267 | –0.306 | –3.263 | 0.001 |
| Vein size | 0.496 | 1.635 | 0.029 | 0.303 | 0.762 |
| Vein type | 2.763 | 2.561 | 0.104 | 1.079 | 0.283 |
| Reason for catheter insertion | –0.383 | 1.427 | –0.025 | –0.268 | 0.789 |
| Intensive care unit | 2.121 | 4.037 | 0.051 | 0.525 | 0.600 |
AdjR2=0.163, F=3.030 (P=0.006), Durbin–Watson=0.341.
B, unstandardized coefficients; SE, standard error; β, standardized coefficients.
*P<0.05.
Table 4.
Multivariate analysis, Cox proportional hazard model
| Risk factor | Relative risk | 95% Confidence interval | P-value |
|---|---|---|---|
| Age | –0.011 | 0.956-1.023 | 0.521 |
| Sex, female* | 1.065 | 1.319-6.384 | 0.008 |
| Body mass index* | 0.147 | 1.030-1.192 | 0.006 |
| Vein size | 0.320 | 0.852-2.228 | 0.192 |
| Catheterized vein | –0.413 | 0.282-1.550 | 0.341 |
| Diagnosis | –0.214 | 0.582-1.120 | 0.200 |
| Reason for catheter insertion | 0.278 | 0.890-1.960 | 0.168 |
| Intensive care unit | –0.544 | 0.175-1.927 | 0.374 |
*P<0.05.
DISCUSSION
This study is the first to evaluate the efficacy and outcomes of an integrated wire AST-MC in Korea. Currently, the role of MC appears to be for IV treatment in patients whose needs are not sufficiently covered by PVCs and CVCs. PVC is widely used because it is easy to access, inexpensive, and shows good results for short-term treatment. However, complications such as phlebitis, thrombophlebitis, infiltration, extravasation, and infection have been reported. The use of vascular irritants can cause severe tissue injury. Therefore, guidelines recommend that PVCs require close observation and should be changed every 72 to 96 hours [5], and CVCs should be considered for patients who need intermediate to long-term IV treatment or those who need to use medicines with vascular irritants [1]. However, it is necessary to consider the labor and effort of professional medical personnel during the procedure and the risk of mechanical and long-term complications when performing the CVC procedure. Contrarily, MCs can be used for up to 30 days, and infusion solutions or blood products with an osmolarity <600 mOsm and a pH from 5 to 9 can be administered. It can be used for power injection of contrast media for radiographic studies because of its high flow tolerability [5,6]. Despite some studies stating that MCs cannot be used for the administration of vesicant drugs, recent studies have shown that this is not the case [7]. Additionally, Moureau et al. [7] mentioned contraindications to MC use, but did not mention the administration of a vasopressor agent. Therefore, MC is a good option for patients in whom IV access is difficult to establish and who require mid- to long-term IV therapy. In addition, MCs may help avoid the complications of CVCs in patients in whom these devices are indicated. MCs may reduce the duration of CVC use by substitution with MC, although feasibility will depend on the patient’s condition.
Surprisingly, 37% of premature catheter removals in this study were not because of complications but were accidental removals. This may be owing to the short length of the catheter, and the fixture consists of a simple sticker, allowing easy and inadvertent extrusion. The adverse events in this study accounted for 27.4% (16.2/1,000 catheter days), which was higher than the average complication rate of 10% (11-65.7/1,000 catheter days) reported [8-10]. This may be because the scope of adverse events was wider in this study. If the patient complained of slight pain or redness at the catheter site, the catheters were removed, and such instances were considered adverse events.
In this study, however, the rate of major complication such as CA-BSI and symptomatic DVT was low (2.6%). CA-BSIs are reported to occur at <0.2% to 2.0% or 0.1 to 0.2/1,000 catheter days [5,8-13], and the rate of CA-BSI in our study was 1.7% or 1/1,000 catheter days. Symptomatic upper arm DVT requiring additional medical treatment is rare and reported to occur at <4.5% or 2.1 to 3.3/1,000 catheter days [12-14], and symptomatic upper arm DVT in this study occurred at 1.7% or 1/1,000 catheter days, which was lower than that reported in other studies. Thrombotic occlusion in the MC was mostly focal without symptoms.
The AST was used for MC insertion. This new device allows unassisted, single-operator cannulation with a small operating radius. In addition, it allowed a shorter procedure time and almost no blood loss compared to other MCs using the Seldinger technique. In this study, the mean cannulation time was 11.3 min.
Previous studies reported an average CIT of 4 to 13 days (range, 0-84) [5,6,8-10,14]. However, these studies did not analyze the correlation between the CIT and patient factors. This study confirmed that male sex (20 vs. 13.9 days, B=–0.872, P=0.001) and lower BMI (B=–6.481, P=0.015) were associated with a longer CIT. Notably, obesity accounts for many problems encountered during vascular cannulation [15].
Real-time ultrasound guidance to achieve IV access can improve catheter outcomes [16]. In addition, the 2021 Infusion Nurses Society Standards of Practice recommends that in patients with difficult IV access tools that help with insertion, such as ultrasound and PVC, it is recommended to secure appropriate access to safely sustain the patient’s IV access during treatment [1].
This study has several limitations. First, it was a retrospective study with an inherent bias. Second, data on medications infused via MC and relevant patient history of anticoagulant use were not collected. Recent reviews have reported that medications, such as vancomycin, which can cause vascular irritation, predispose patients to catheter-related complications. Thus, it seems plausible to perform further studies to ascertain the safety of administration of each medication via MC. Despite the above limitations, this study is meaningful because it is the first to evaluate the efficacy of AST-MC devices in a single center with experienced vascular surgeons.
Further studies with larger sample sizes are necessary to compare the outcomes of different insertion sites and methods of vascular access with MCs. A prospective randomized study is necessary to clarify and confirm the efficacy and safety outcomes of AST-MCs compared with peripherally inserted central catheters or other types of MCs.
CONCLUSION
This study suggests that the integrated wire AST-MC devices are operator-friendly and have the advantage of minimal blood loss and short operation times. The complication rate was slightly higher; however, complications were mostly minor and did not require further medical treatment. The major complication rate in this study was lower than that reported in other studies. Subsequently, if the catheter’s proneness to accidental extrusion is resolved, next-generation MC may be more suitable as an alternative to achieve IV access in patients requiring moderate- to long-term administration of medication.
Footnotes
FUNDING
None.
CONFLICTS OF INTEREST
The authors have nothing to disclose.
AUTHOR CONTRIBUTIONS
Concept and design: MJK. Analysis and interpretation: MHJ, MJK. Data collection: MJK, KDH. Writing the article: MHJ, MJK. Critical revision of the article: MJK, CSK. Final approval of the article: all authors. Statistical analysis: MJK, KDH. Obtained funding: none. Overall responsibility: MJK, KDH, CSK.
REFERENCES
- 1.Gorski LA, Hadaway L, Hagle ME, Broadhurst D, Clare S, Kleidon T, et al. Infusion therapy standards of practice, 8th Edition. J Infus Nurs. 2021;44(1S Suppl 1):S1–S224. doi: 10.1097/NAN.0000000000000396. [DOI] [PubMed] [Google Scholar]
- 2.O'Grady NP, Alexander M, Burns LA, Dellinger EP, Garland J, Heard SO, et al. Guidelines for the prevention of intravascular catheter-related infections. Clin Infect Dis. 2011;52:e162–e193. doi: 10.1093/cid/cir257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Penoyer D, Bennett M, Geddie PI, Nugent A, Volkerson T. Evaluation of processes, outcomes, and use of midline peripheral catheters for the purpose of blood collection. Br J Nurs. 2021;30:S24–S32. doi: 10.12968/bjon.2021.30.2.S24. [DOI] [PubMed] [Google Scholar]
- 4.Thaut L, Weymouth W, Hunsaker B, Reschke D. Evaluation of central venous access with accelerated Seldinger technique versus modified Seldinger technique. J Emerg Med. 2019;56:23–28. doi: 10.1016/j.jemermed.2018.10.021. [DOI] [PubMed] [Google Scholar]
- 5.Adams DZ, Little A, Vinsant C, Khandelwal S. The midline catheter: a clinical review. J Emerg Med. 2016;51:252–258. doi: 10.1016/j.jemermed.2016.05.029. [DOI] [PubMed] [Google Scholar]
- 6.Alexandrou E, Ramjan LM, Spencer T, Frost SA, Salamonson Y, Davidson PM, et al. The use of midline catheters in the adult acute care setting- clinical implications and recommendations for practice. J Assoc Vasc Access. 2011;16:35–38. 40–41. doi: 10.2309/java.16-1-5. [DOI] [Google Scholar]
- 7.Moureau N, Sigl G, Hill M. How to establish an effective midline program: a case study of 2 hospitals. J Assoc Vasc Access. 2015;20:179–188. doi: 10.1016/j.java.2015.05.001. [DOI] [Google Scholar]
- 8.Chopra V, Kaatz S, Swaminathan L, Boldenow T, Snyder A, Burris R, et al. Variation in use and outcomes related to midline catheters: results from a multicentre pilot study. BMJ Qual Saf. 2019;28:714–720. doi: 10.1136/bmjqs-2018-008554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Seo H, Altshuler D, Dubrovskaya Y, Nunnally ME, Nunn C, Ello N, et al. The safety of midline catheters for intravenous therapy at a large academic medical center. Ann Pharmacother. 2020;54:232–238. doi: 10.1177/1060028019878794. [DOI] [PubMed] [Google Scholar]
- 10.Nielsen EB, Antonsen L, Mensel C, Milandt N, Dalgaard LS, Illum BS, et al. The efficacy of midline catheters- a prospective, randomized, active-controlled study. Int J Infect Dis. 2021;102:220–225. doi: 10.1016/j.ijid.2020.10.053. [DOI] [PubMed] [Google Scholar]
- 11.Pathak R, Gangina S, Jairam F, Hinton K. A vascular access and midlines program can decrease hospital-acquired central line-associated bloodstream infections and cost to a community-based hospital. Ther Clin Risk Manag. 2018;14:1453–1456. doi: 10.2147/TCRM.S171748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lisova K, Hromadkova J, Pavelková K, Zauška V, Havlin J, Charvat J. The incidence of symptomatic upper limb venous thrombosis associated with midline catheter: prospective observation. J Vasc Access. 2018;19:492–495. doi: 10.1177/1129729818761276. [DOI] [PubMed] [Google Scholar]
- 13.Goetz AM, Miller J, Wagener MM, Muder RR. Complications related to intravenous midline catheter usage. A 2-year study. J Intraven Nurs. 1998;21:76–80. [PubMed] [Google Scholar]
- 14.Bundgaard Madsen E, Sloth E, Skov Illum B, Juhl-Olsen P. The clinical performance of midline catheters- an observational study. Acta Anaesthesiol Scand. 2020;64:394–399. doi: 10.1111/aas.13516. [DOI] [PubMed] [Google Scholar]
- 15.Nafiu OO, Burke C, Cowan A, Tutuo N, Maclean S, Tremper KK. Comparing peripheral venous access between obese and normal weight children. Paediatr Anaesth. 2010;20:172–176. doi: 10.1111/j.1460-9592.2009.03198.x. [DOI] [PubMed] [Google Scholar]
- 16.Houston PA. Obtaining vascular access in the obese patient population. J Infus Nurs. 2013;36:52–56. doi: 10.1097/NAN.0b013e31827989d8. [DOI] [PubMed] [Google Scholar]