Abstract
On September 11th, 2001 the World Trade Center (WTC) in New York was attacked by terrorists, causing the collapse of multiple buildings including the iconic 110 story “Twin Towers” and a nearby 47 story office building. Thousands died that day from the collapse, fires, jumping from the buildings, falling debris, or other accidents due to rapid deceleration trauma incurred by jumping from the windows of upper floors of the WTC. Survivors of the attacks, those who worked in search and rescue during and after the buildings collapsed, and those working in recovery and clean-up operations were exposed to severe stressors. Concurrently, these “WTC-affected” individuals breathed and ingested a mix of organic and particulate neurotoxins and pro-inflammogens that resulted from the attack and building collapse. Nearly 20 years later, researchers have documented increasing risk of neurocognitive and motor dysfunction resembling typical features of neurodegenerative diseases, as well as the presence of cortical atrophy, among WTC-affected individuals with cognitive impairment at midlife. This risk has been associated with both physical exposures at the WTC and chronic post-traumatic stress disorder including regularly re-experiencing their traumatic memories of the events. Despite these advances, little is understood about long-term effects of these physical and mental exposures on the brain health of WTC-affected individuals, and the potential for organic neurological disease. The goal of this report was to educate policymakers and elucidate risks that were previously nascent but are now emerging in this population, while providing a rationale for monitoring neurological health of WTC-affected individuals.
INTRODUCTION
On September 11th, 2001 (hereafter “9/11”), terrorists flew jetliners into each of the 110 story Twin Towers located at the World Trade Center (WTC) in New York. The collapse of the WTC and surrounding infrastructures culminated in a dust plume that engulfed the surrounding area (“Ground Zero”) and parts of downtown New York City. At Ground Zero fires fed by jet fuel burned noxious compounds burned for months, emitting clouds of smoke and fumes that permeated the New York City air and burned until December 2002. Nearly 3,000 individuals died because of the 9/11 attacks and hundreds of thousands more were injured. First responders (i.e, law enforcement, emergency medical and firefighting personnel), were onsite within minutes of the first attack and began evacuating survivors and escorting others to safety. Surviving office employees, area residents, first responders, and volunteers who engaged in search and recovery and clean-up operations (all hereafter “WTC-affected individuals”), were exposed to severe psychological stressors, smoke, dust, and volatile organics. The thousands who remained on-site for search and recovery efforts were exposed to a toxic mix of contaminants and aerosolized particulate matter (PM) expelled from the burning buildings and pulverized during the collapse and during clean-up and rebuilding operations thereafter1.
When efforts shifted from rescue to recovery and clean-up, the types of activities and exposures to inhaled PM also shifted2. In the days that followed, while lower Manhattan remained engulfed in dust, rescue and recovery workers dug through an inordinate amount of wreckage and rubble in the six story ‘pile’, searching for human remains, victim belongings, and genetic markers for DNA matching and person identification3. Thousands of individuals who remained on site for prolonged periods of time were exposed to industrial solvents and environmental toxins, many of which have been implicated in the pathogenesis of neurodegeneration and/or neuroinflammation. The physical and psychological challenges endured by these individuals were extraordinary4. Since then, researchers have reported that as many as 23% of rescue and recovery workers continue to endorse WTC-related posttraumatic stress disorder (PTSD)5,6.
WTC-affected individuals are getting older, but at present, there is little understanding about how WTC exposures might have promoted neuroinflammatory and neurodegenerative processes that influence brain aging. Now, 20 years since the 9/11 attacks on the WTC there are no guidelines in place for research programs seeking to monitor WTC-affected individuals across the life course for signs and symptoms that may be indicative of emerging neurological diseases. This review was written to help update policymakers, WTC-affected individuals, their friends and family, clinicians, and researchers with strategies outlining the need for an effective cognitive monitoring program, facilitated by ongoing research with WTC-affected individuals.
Methods
The WTC Health Program was established by Congress with the passage of the James Zadroga 9/11 Health and Compensation Act of 20107 (Zadroga Act). Administered by the National Institute for Occupational Safety and Health (NIOSH), the program operates a survey-based registry and provides medical monitoring and treatment benefits to WTC-affected individuals with conditions having emerged from exposures to toxins, trauma, or adverse conditions resulting from the 9/11 terrorist attacks. To date, the NIOSH-funded clinical monitoring programs have enrolled 80,146 WTC responders along with and 28,349 WTC survivors8. Twenty percent of responders were exposed to the dust cloud, and 45% of responders spent ≥60 days working on-site at the WTC, with ≥10% of the population have been diagnosed with WTC-related PTSD. Additionally, the NYC WTC Health Registry enrolled 71,437 individuals who self-identified at enrollment in 2003–2004 as having been exposed to the WTC sites based on occupational or residential exposures9.
In October 2019, NIOSH convened a meeting to discuss the concerns and interpretations of recent studies that demonstrate evidence for cognitive impairment among WTC-affected individuals, and to offer considerations for potential strategies to design a cognitive screening program for WTC-affected individuals. The meeting was focused on questions about aging-related cognitive performance among individuals exposed to significant PM exposures in addition to carrying long-term psychiatric implications, experts across the realm of Alzheimer’s disease and related dementias (ADRD), aging, environmental exposures, and PTSD were invited to create these recommendations. Research and support staff from the National Institute on Aging and National Institute on Occupational Safety and Health (NIOSH) also attended. The meeting was supported by funding from NIOSH, with no sponsorship from individuals or pharmaceutical companies.
A comprehensive literature review was completed in advance of the meeting using a database of papers published about WTC exposures and outcomes maintained by the NIOSH program. Speakers were invited by two WTC researchers and asked to provide two representative papers of their own work to be briefly discussed during the meeting that could help to educate audience members about their research. In preparation for this review, a scoping literature search was also conducted using PubMed including articles published in the areas of cognition, aging, neurobiology, neuroimaging, or neurodegeneration in survivor and responder populations affected by “World Trade Center” attacks from 9/11/2001–5/1/2021 (search terms: “World Trade Center” AND (cogniti* OR aging OR neurobiology OR neuroimage* OR neurodegener*). The search resulted in 70 non-overlapping citations; relevant studies were retained and cited in this review and studies specific to the topics of interest in this review were highlighted. Attendees were also asked to discuss the relevance of MCI in this cohort and highlight important aspects that merited further investigation. The meeting was recorded and transcribed. Each presenter was asked to review transcripts for errors and for clarity, and all presenters were asked to review this manuscript for accuracy and to ensure final agreement with these materials. A writing team was tasked with capturing meeting materials and relevant background in this paper, and all authors were provided with opportunities to edit, modify, and update sections in the final manuscript.
NEUROLOGICALLY ACTIVE WTC EXPOSURES
Understanding the long-term consequences of a severe exposure event such as 9/11, likely requires several years or even decades to be accurately identified and to then reach a consensus with relevant experts. This process must also account for the heterogeneity in brain resilience and cognitive reserve among each WTC-affected individual, which may modify their risk for cognitive decline10. Yet, the long-term impacts of trauma can often become “hidden” as time passes on, which is demonstrated by the reduced willingness to talk about one’s traumatic experiences and having those memories recede11. Furthermore, research determining the neurotoxic effects from inhalations of various mixtures of toxins may be pose challenging, as the types of fine or ultrafine PM that was present both in the air and the dust at the WTC sites, may have been invisible to the people that were exposed.
Airborne Pollutants
Mounting evidence suggests that exposure to air pollutants may be detrimental to brain health and might cause cognitive impairment and dementia12. Three potential pathways link inhaled airborne PM to neurodegenerative disease (Figure 1). Exposure to coarse PM (>10nM in aerosolized diameter - PM10) was present in the dust cloud, and fine PM (<2.5mm in aerosolized diameter – PM2.5) in settled dust throughout the response period, with most bulk dust including coarse PM with a small percentage (1–2%) being PM2.513. Dust exposures also included significant levels of polycyclic aromatic hydrocarbons (PAHs) that could be detected at several different WTC sites13, and included detectable levels of possible neurotoxic metals14. PM2.5 may be inhaled into the nasal passages and while coarser particles are likely to remain trapped in the lung, finer particles may enter the brain by direct translocation through the cribriform plate or olfactory bulb15,16. The ‘lung-to-brain’ axis17 could also include direct passage of PM2.5 into the brain but could also involve modulation of systemic inflammatory responses such as the SOD3R213G pathway. Indeed, studies in humans have reported that exposures to various airborne contaminants is associated with cognitive dysfunction in children, adults, and the elderly 18,19. As PAHs are hydrophobic and can remain in the body for weeks, they could accumulate to a critical concentration in the periphery potentially allowing them to permeate slowly across the lipophilic blood-brain-barrier (BBB) and contribute to a variety of brain pathologies20.
Figure 1.
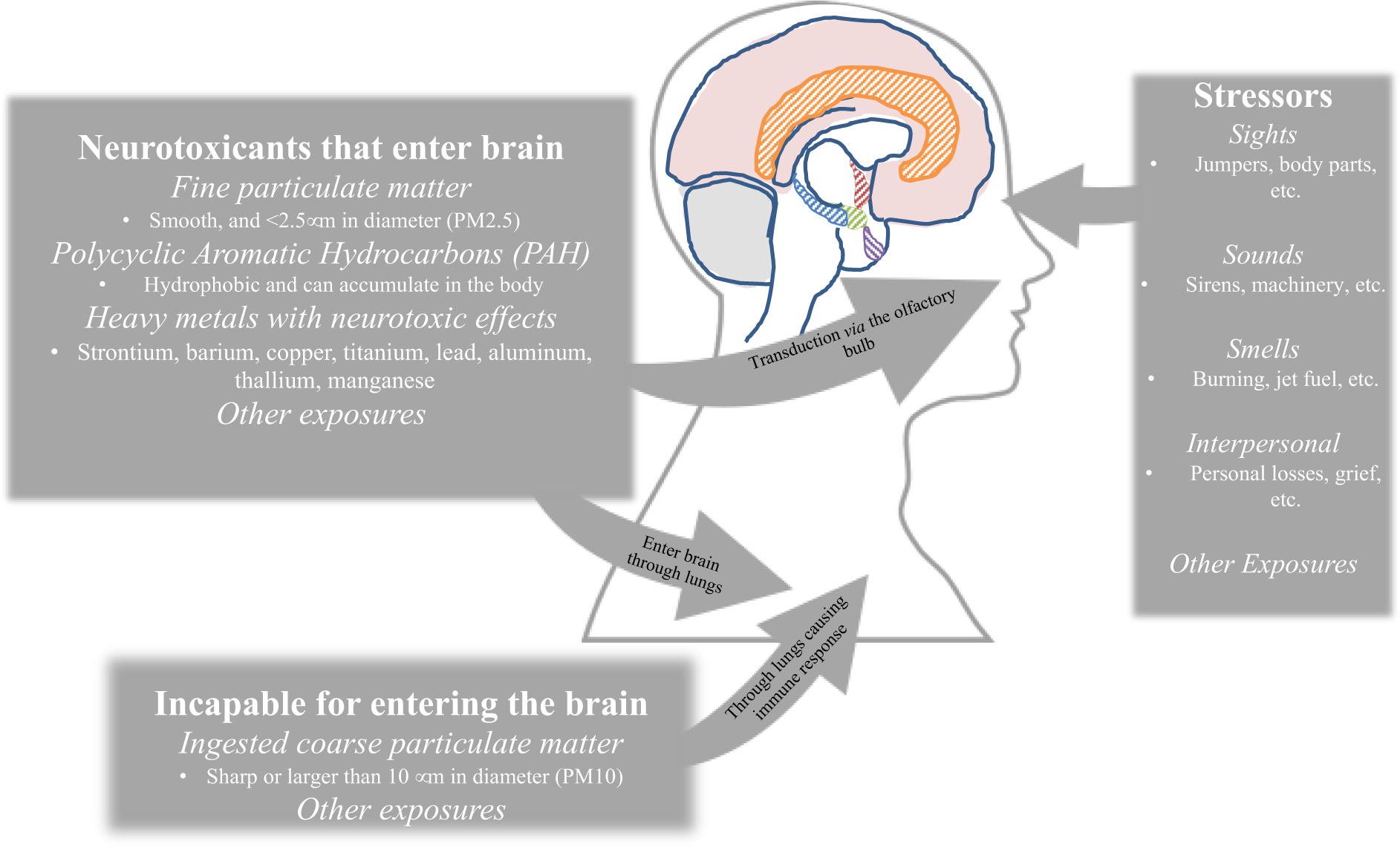
Exposures at the World Trade Center separated by potential pathways to neurogenerative disease
Post-Traumatic Stress Disorder
A secondary result of the events related to 9/11/2001 is elevated rates of posttraumatic stress disorder (PTSD)21 resulting from a larger array of stressful experiences (Figure 1). PTSD is a relatively common outcome of stressful exposures and can persist chronically and is heterogeneous in symptom presentation and is characterized by the presence of distinct symptom clusters including avoidance, hyperarousal, emotional numbing, and chronic re-experiencing of exposure-related memories5. These changes have been long been shown to cause functioning changes including changes in nutritional intake and illicit substance usage, often coupled with sedentary behavior22. Although one-third of trauma exposed individuals are resilient in the aftermath of exposure and maintain a stable trajectory of mental health following exposure to a potentially traumatic event23, an important subset of individuals experience chronic PTSD or report subsyndromal levels of symptoms that fail to meet diagnostic criteria but can nevertheless still cause distress and be refractory to management. The neurobiological underpinnings of risk for PTSD and determinants of resilience in the aftermath of exposure are complex and multifactorial, however considerable studies demonstrate the important association between elevated stress and impairment in brain health.
Neurobiological mechanisms linking WTC exposures with cognitive decline
Overall, as outlined in Figure 2 growing evidence links PM exposure with cognitive decline12, resulting in the recent inclusion of inhaled airborne PM in a list of avoidable dementia risk factors24. Large studies of older Americans identified higher prevalence of working memory deficits and disorientation with increased PM2.5 exposures and with PAH exposures25,26. The possibility of detrimental cognitive effects of chronic PM2.5 inhalation received early and detailed attention from researchers who demonstrated associations with white matter glial apoptosis, blood-brain barrier (BBB) disruption (perhaps as mediated by age or by the presence of APOLIPOPROTEIN-ε4), oxidative stress, neuro-vascularization and neuroinflammation, along with evidence of accumulation of oligomeric and fibrillar forms of β-amyloid (Aβ) and tauopathy, as well as demonstrable evidence of neurodegeneration in canines, children, and young adults27. Studies have reported that lifetime exposure to PM2.5 is associated with both brain atrophy and disruption in white matter connectivity28,29. PAHs have been shown to modulate Aβ aggregation through the possible formation of micelle-like Aβ-PAH co-aggregates that may induce pro-aggregation pathways30. The presence of PM in the central nervous system has been demonstrated in individuals with ADRD31, along with evidence of cerebrovascular injury as measured in CSF32. By whatever route, rodent models show that the brain’s response to PM is neuroinflammatory with mechanisms pointing to increased glial activation33 and accumulation of Aβ peptides34 in a feed-forward cycle.
Figure 2.
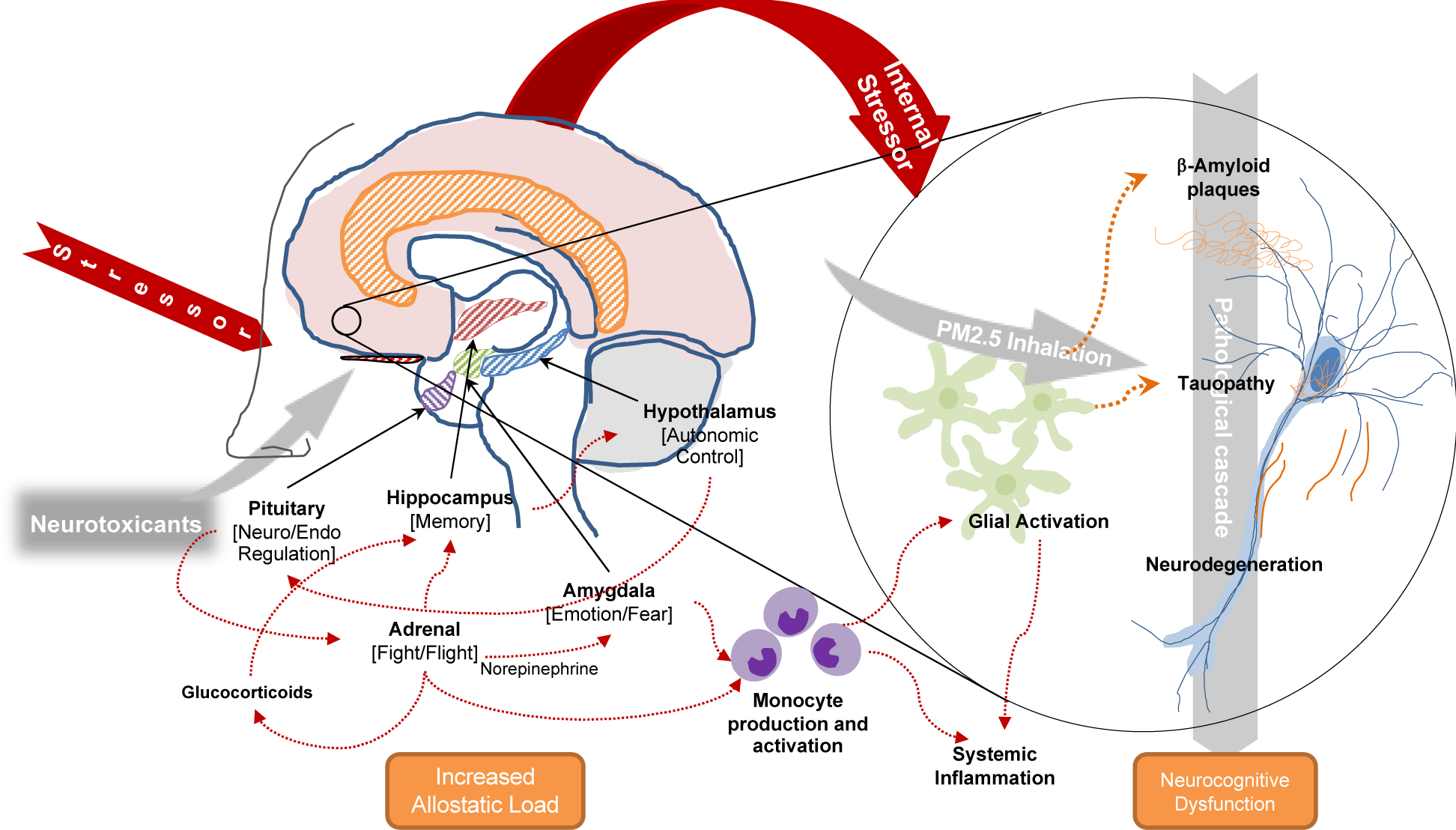
Theoretical mechanisms linking exposure to World Trade Center activities and inclusion of Alzheimer’s disease pathological cascade indicative of potential pathways between proteinopathy, and cognitive symptoms identified in responders
Chronic PTSD concomitant with WTC-related neurotoxic exposures may amplify the effects of neurotoxic exposures and initiate a cycle of proteinopathy and neuroinflammation (Figure 2), with markers indicative of AD neuropathology but in regions or topographies that may be rarely involved by typical AD pathology35. Repeated exposure to severe stressors in animal models has been shown to increase Aβ levels with short-term and chronic exposures36, through overactivation of microglia37. Chronic PTSD has also been associated with immunologic changes including neuroinflammation38, neuro-immunologic dysregulation39, and differential gene expression and activation in microglia and monocytes40,41. Converging evidence suggests that chronic PTSD may cause changes to four key brain regions also implicated in AD resulting in hippocampal atrophy42, changes in the amygdala structure43 alongside dysregulation of rhythmicity in neuroendocrine and circadian systems resulting in abnormal sleep hygiene44,45. Chronic PTSD may additionally activate the “fight or flight response”46 thereby diminishing the brain’s ability to cope with other exposures35, while sustaining the chronic stress response resulting in increased Aβ aggregation and accumulation and hastening tauopathy hyperphosphorylation47 and propagation48.
Findings in WTC-affected individuals
To investigate the hypothesis that WTC exposures caused increased risk of neurodegenerative disease, we began monitoring cognition in WTC responders in their mid-50s, who had no history of traumatic brain injury and who participated in rescue and recovery work following the terrorist attacks on 9/11. The WTC responder cohorts used in this research are unusual in several crucial ways including that their high levels of education in comparison to the general population and other WTC-affected populations (>95% have high school diplomas, and >60% have college degrees) and are relatively young (mean age = 38 on 9/11/2001, and 54 when research on cognitive aging in WTC-affected individuals began). Nevertheless, work has suggested that WTC responders were at high risk of being cognitively impaired at initial assessment with initial results suggesting that common correlates of cognitive impairment including the possession of the APOLIPOPROTEIN-ε4 allele was associated with the presence of possible dementia 49. In longitudinal follow-ups, responders who were cognitively unimpaired at baseline were at high risk of fulfilling criteria for mild cognitive impairment (MCI)50. In that study of WTC responders at midlife, 17.8% of responders were determined to be cognitively impaired at baseline while an additional 14.2% developed MCI over the course of 2.5 years. Responders with re-experiencing symptoms consistent with PTSD and those who were APOLIPOPROTEIN-ε4 carriers who were exposed to the WTC for lengthy periods of time were at increased risk of developing MCI. The incidence rate in this population was determined to be more than two-fold higher than estimates in population databases aged >70 years on average. Detailed neuropsychological information revealed that cognitive impairment was associated with changes to memory as a locus of cognitive dysfunction51, a result that was upheld in a study of individuals with possible dementia52. When detailed neuropsychological data were longitudinally followed, WTC responders determined by a deep learning protocol to be at high risk of cortical atrophy were determined to have had higher WTC exposures, more severe PTSD symptomatology, and were at higher risk of MCI, dementia, and death53. Critically, there was a moderation effect found whereby responders possessing the APOLIPOPROTEIN-ε4 allele who were also more severely exposed to WTC events were at greater risk of developing MCI. A large number of responders self-reported functional limitations54–56 that appeared to be indicative of psychiatric comorbidities55.
Examining the potential for physical functional limitations, WTC research detailed associations between WTC exposures and reduced grip strength57 and physical functional limitations58. Physical functional limitations including slowed walking, slowed chair-rise speed, and lower hand grip strength are indicators of body strength that are associated with increased disability and mortality59–61. Physical functional limitations have been associated with depressive symptoms62,63 and better cognitive performance64,65. Therefore, combined with the finding that PTSD was associated with poorer physical functioning in WTC responders in addition to cognitive impairment66, physical functional limitations might serve as an important clinical biomarker for non-cognitive dysfunction in WTC-affected individuals.
In vivo neuroimaging work also reported that responders with PTSD had heightened amygdala reactivity67, and evidence of amygdala and hippocampal atrophy68. Furthering this work, a recent study of white matter health showed evidence of changes to connectivity in responders with diagnosis of MCI69. Research using a two-by-two design to examine differences between PTSD and dementia in WTC responders found reduced cortical thickness when compared to cognitively unimpaired responders across 21 of 34 regions clustered over the frontal, temporal, and parietal lobes (Figure 3A). Further analyses in this study clarified that differences in hippocampal atrophy were evident in WTC responders with dementia across six hippocampal subfields with focal changes in the presubiculum70, but this work clarified that hippocampal and cortical atrophy were only evident in PTSD when accompanied by cognitive impairment. Comparisons to normal controls were troubling and revealed (Figure 3B) that while cognitively unimpaired individuals (orange bars) had cortices that were generally larger than the normative data, both cognitively impaired and unimpaired responders had evidence of atrophy in the entorhinal cortices, the fusiform gyri, and throughout the temporal lobes52. Follow-up analyses using deep learning revealed that cortical atrophy was able to accurately classify 90% of responders as cognitively impaired71. Overall, these changes suggest evidence consistent with AD (Table 1), though other diseases may cause similar changes.
Figure 3.
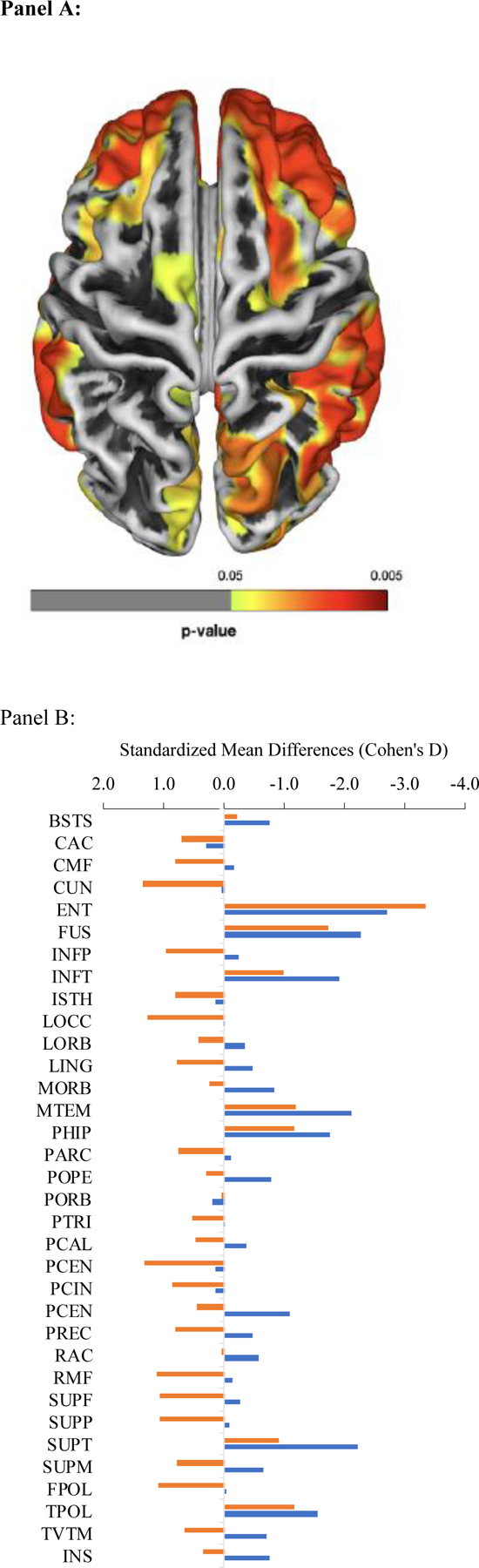
Differences in cortical atrophy comparing (Panel A) cognitively impaired WTC responders compared to cognitively normal responders and (Panel B) cognitively unimpaired WTC responders to normative data. Regions where cortical atrophy was evident are highlighted with colors showing areas of increasingly severe cortical loss including regions in the frontal, temporal, and parietal lobes among others. Source: 52
Table 1.
Supportive indicators for common neurodegenerative diseases
| Phenotype | Cerebrovascular Disease | Alzheimer’s Disease | Limbic Age-related TAR DNA-protein-43 Encephalopathy | Primary Age-Related Tauopathy | Chronic Traumatic Encephalopathy | Parkinson’s Disease | Dementia with Lewy Bodies | Frontotemporal Lobular Degeneration | WTC- Cognitive Impairment [Evidence To Date] | |
|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||
| Symptoms | Episodic Memory | Present | Present | Present | Present | Present | Present | Present | ||
| Executive Function | Present | Present | Present | Present | Present | Present | ||||
| Visuo-spatial Function | Present | Present | Present | Present | Present | Present | ||||
| Aphasia | Present | Unknown | ||||||||
| Resting Tremor | Present | Unknown | ||||||||
| Physical Functioning | Present | Present | Present | |||||||
| Behavioral Changes | Present | Present | Present | Present | Present | Unknown | ||||
| Disorientation | Present | Present | Present | Present | Present | Present | Present | |||
|
| ||||||||||
| Bioindicators | White Matter Changes | Present | Present | Unknown | ||||||
| Amyloid Deposition | Present | Absent | Absent | [Plasma] | ||||||
| Tauopathy | Present | Present | Present | Present | Unknown | |||||
| Cortical Atrophy | Present | Present | Present | Present | ||||||
| Hippocampal Atrophy | Present | Present | Present | |||||||
| a-Synuclein | Absent | Absent | Absent | Absent | Absent | Present | Present | Absent | Unknown | |
Note: We note when characteristics are either necessary for the condition or are absent, but we do not note when symptoms or bioindicators are common comorbidities. For example, patients diagnosed with Dementia with Lewy Bodies often also have evidence of amyloid positivity. Additionally, while evidence of the presence of atrophy may be listed as present, in many cases including in the analysis of cortical atrophy along with amyloid deposition and tauopathy, the specific pattern of biomarker changes may help to distinguish between diseases.
Differences in responder characteristics have not reliably shown that female responders, or responders from minority backgrounds are at greater risk of meeting diagnostic criteria for MCI. Consistent with cognitive reserve theory72, protective factors in WTC responders have shown that individuals with higher education have lower risk of meeting criteria for MCI as compared to those with lower education. However, associations between education and incidence of diagnosis of MCI were sometimes explained by differences in exposures50. Other studies in WTC-affected populations has identified changes in cellular mechanisms consistent with more rapid aging including, for example, decays in pulmonary functioning comparing pre- versus post-exposure data in the WTC responders73. Thre are also trends among WTC-affected populations in elevated C-reactive protein in survivors with PTSD when compared to those without PTSD74, increased allostatic load in WTC responders75, and increased numbers of responders with shortened telomere length that was also associate with poorer pulmonary functioning 76, as well as changes in DNA methylation and gene expression40. Further interrogating biological systems, PTSD in WTC responders was associated with changes in gene expression including in the FKBP5 gene77 that may result in transcriptome-wide changes in pathways linked to monocyte gene expression in single-cell analyses40. Researchers also identified changes in proteins including neurocan and brevican that were associated with both PTSD and a diagnosis of MCI, and PTSD-related MCI has been reported to represent a more severe form of PTSD in terms of proteomic markers of systemic dysfunction78.
A COMPREHENSIVE RESEARCH AGENDA
Aging-related cognitively impairing diseases are often characterized by an insidious onset period, which is often decades long. The most recent NIA-AA Alzheimer’s disease (AD) Research Framework now considers AD a diagnosis based solely on biomarkers of pathology, with MCI and AD dementia being clinical consequences which may be heterogeneous in symptoms and may not always be present79. This terminology replaces the prior one which included AD, MCI due to AD, or prodromal and preclinical AD80,81, and therefore forms the basis for the overall proposed nosological structure (Table 1). AD and a number of related dementias (ADRD) are the most common causes of ageing-related cognitive impairment worldwide82. Cerebrovascular disease is the second most common cause in most aging populations, and often results in symptoms that mimic AD-like dementia and cause instead vascular cognitive impairment and dementia (VCID), making the differential diagnosis of cognitive impairment difficult in the absence of neuroimaging-based and biofluid-based biomarkers. Other diseases causing neurodegenerative changes and dementia are also increasingly well described. Yet, both AD and VCID are at least partly contributory to dementia seen in other aging-related neurodegenerative conditions including, for example, Parkinson’s disease (PD)83. AD can be preceded or accompanied by neuropsychiatric symptoms depressive symptoms and/or mood disorders84, in addition to change in gait, motor, and other physical functions85,86. At the same time, there is an increasing recognition that some rarer conditions may be less rare in their milder forms than originally believed. For example, dementia with Lewy bodies (DLB) appears to cause symptoms that may be consistent with AD, likely because it appears to emerge alongside the typical features of AD-related biomarkers including amyloidosis and tauopathy87. Other entities include conditions such as chronic traumatic encephalopathy that has emerged as a clinical phenotype among individuals with repeated head injuries and a number of novel disease states including, for example, limbic age-related TAR DNA-binding-protein-43 encephalopathy (LATE) and the presence of tauopathy and neurodegeneration lacking amyloid now called primary age-related tauopathy (PART)88, and suspected non-AD pathophysiology (SNAP)89. While these comparisons are useful, neurodegenerative diseases are often mixed in etiology and the presence of one may hasten the onset of others90.
Next steps in research with WTC-affected individuals
To improve the capacity to identify outcomes more proximally in time to their relevant exposures, prior clinical work has focused on the identification of MCI, preclinical, or prodromal AD. These prodromal disorders indicate possible neurodegenerative disease representing early clinical symptoms emerging during cognitively impairing diseases91. However, these disorders are different. For example, while preclinical AD is now defined as a biological disorder characterized by the presence of AD biomarkers92 as often found in asymptomatic individuals93, MCI is a clinical syndrome that, while originally focusing on individuals with isolated memory impairment in particular94, subsequent studies have demonstrated that MCI could present as impairment isolated to other cognitive domains95. MCI is often a prodrome for more severe diseases, and individuals with MCI may begin to exhibit non-cognitive symptoms including worsening depressive symptoms and changes in mood and anxiety alongside changes in physical functioning and appearance of biomarkers and changes in cognitive performance.
Though the WTC health program is a comprehensive clinic-based monitoring program, it does not actively monitor cognitive performance or decline systematically across populations, outside of assessments done in research studies. There is a growing consensus that the neurocognitive findings in several research studies are identifying a syndrome that raises concerns about the level of risk in WTC-affected individuals, and that there is a need for improved characterization of cognitive decline and identification of neuropathology explaining the presentation of these effects. It is therefore important to understand how and why we observe cognitive impairment at midlife in WTC-affected individuals and whether it will progress to dementia. Nevertheless, it is also important to note that since we have not elucidated the underlying neurobiological etiology for the observed early-onset cognitive impairments in WTC-affected individuals, have not examined neuropathology in the brains of WTC-affected individuals, our use of language and nomenclature remain in the tentative and exploratory but not confirmatory stages.
There is a lack of consensus as to whether individuals with normal risk should be routinely cognitively monitored for clinical purposes, in part because costs to individuals and their family from receiving a cognitive diagnosis may be high and to date few benefits are expected from such diagnoses96. Yet, research presented above suggests that some WTC-affected individuals may be considered at high-risk for developing MCI and dementia because of their experiences at the WTC. Noting that though treatment may be still in nascent stages, a central benefit of cognitive monitoring may be the ability for individuals and their family to anticipate changes that can occur and act preventatively where possible97, while a second benefit may be that it helps patients and their family to understand cognitive and behavioral changes as they emerge. Therefore, the following research suggestions proposed a multitiered approach focused on relying on cognitive and biomarker tools could be employed in research to characterize and understand the WTC phenotype (see Box 1 for recommendations).
Box 1. Recommendations for further research in WTC-affected individuals.
Monitoring Cognitive and Non-cognitive Symptoms
Goal:
To clarify cognitive and non-cognitive changes including measures of emotional, social, and physical function to identify hallmarks of cognitive impairment in WTC-affected individuals.
-
1.1.
Further research utilizing regular neurocognitive screening to establish incidence rates and risk factors for dementia in WTC-affected individuals at high risk of cognitive impairment.
-
1.2.
A secondary tiered research effort incorporating more comprehensive cognitive evaluations alongside non-cognitive functional measures to monitor changes across domain-specific functional changes alongside and in addition to brief screening efforts may be useful.
-
1.3.
Memory loss is a key symptom of cognitive impairment in WTC responders; however, more research is needed to understand when changes are accompanied by other cognitive and non-cognitive changes.
-
1.4.
Associations between multidomain cognitive decline with concomitant motor and behavioral changes may be unusual features that require further evaluation to elucidate whether they indicate the presence of a WTC-related phenotype.
Needed Neuropathological Information
Goal:
To examine markers of neuropathology to explain the etiology of cognitive impairment to improve diagnosis and monitoring of changes in neuronal proteinopathy in WTC-affected individuals.
-
2.1.
Biofluids- and neuroimaging-based markers of neurodegeneration including cortical/hippocampal atrophy and proteinopathy evident in vivo or postmortem in blood, CSF, or the brain may be investigated to help with characterization of neuropathological biomarkers among WTC-affected individuals.
-
2.2.
Determination of the range and quantity of neurodegenerative proteinopathy is crucial to improving our understanding of etiology of cognitive impairment WTC-affected individuals and to help in determining correspondence with existing diseases.
-
2.3.
Determination of cerebrovascular disease burden is vital to improve our understanding of the etiology of cognitive impairment in WTC-affected individuals, and in determining correspondence with existing diagnostic entities.
Research in Treatment Safety and Efficacy
Goal:
To examine safety, feasibility, and efficacy of interventions to improve cognition and ameliorate neurocognitive effects of posttraumatic stress disorder in WTC-affected individuals.
-
3.1.
Little is known about the safety or feasibility of any proposed interventions in WTC-affected individuals with additional physical and psychological comorbidities.
-
3.2.
The use of interventions specifically targeted at reducing risk of dementia including active efforts to aggressively reduce hypertension via pharmaceutical or targeted exercise and nutritional interventions in older WTC-affected individuals is a critical point of intervention for this work.
-
3.2.
Given the large burden of cognitive impairment in responders reporting re-experiencing symptoms, efforts to reduce symptoms in this group, including by cognitive behavioral therapies that are effective at addressing active PTSD symptoms may provide secondary benefits for cognitive performance.
-
3.3.
Treatments, such as cognition-oriented training, for improvement of cognitive performance that focus on everyday functioning facilitate activity may retain cognition in those with mild impairment manifesting as slowed processing speed or attentional difficulties.
-
1
Assessing cognitive function and associated symptoms (Box 1.1)
Comprehensive neuropsychological assessments are frequently expensive, time-consuming, burdensome to participants and can be complicated to analyze, but an increasing number of testing alternatives are available including online versions of many cognitive screening tools, mobile phone-based cognitive assessments98. To characterize the extent to which cognitive dysfunction may manifest clinically, a brief cognitive tool (e.g., Montreal Cognitive Assessment [MoCA]) may be useful if it collects information from a variety of cognitive domains including attention, concentration, executive function, memory, and visuospatial skills. The MoCA was used in responder research because it has a high reported sensitivity/specificity for MCI and dementia (for example, specificity/sensitivity=95/96% to identify dementia)99. However, there are reports showing that the MoCA is less specific in lower educated and more disadvantaged populations99. Recent work has relied on a validated auditory version of the MoCA that can be delivered via videoconferencing services100. However, while global measures including the MoCA are excellent to screen individuals efficiently and effectively but is insufficient to confirm a clinical diagnosis of MCI. As is typically employed in the neurological or cognitive health care setting, a more specialized and detailed instrument is necessary to survey all the domains of cognitive function that may become impaired in WTC-affected individuals. In the WTC responder efforts, one research team relied on the Cogstate platform to provide cognitive scores in a time and cost-efficient manner. These measures utilize a computer-administered approach that uniformly measures fluid cognition using data and metadata during game-like tasks that measures multiple cognitive domains but have minimal practice effects in repeat administrations on these tests101,102. Recently, a Cogstate profile was found to be highly effective at differentiating WTC responders with or without cortical atrophy53.
Motor dysfunction may also be monitored, as patients often experience changes in movements prior to onset of manifest diagnosable neurocognitive disease85. Long-term and serial screening exams will be critical to successful identification and characterization of patterns of decline across a range of objectively assessed functional symptoms, either independently or as a part of clinical measures such as the Short Physical Performance Battery (SPPB), which measures walking speed, chair-rise capabilities, and balance limitations, as well as grip strength testing103. Indeed, recent work has suggested that mild motor dysfunction, including reduced grip strength and slowed chair-rise speed, are aging-related functional indicators that may precede PTSD-related cognitive impairment, but may not be implicated in other, non PTSD-related forms of WTC-cognitive impairment 104. Further work is needed, however, that assess fine motor skills.
Changes to behaviors and, in part due to changes in persona, impaired social functioning and social cognition are important parameters studied in dementia research. Very little is known about the nature, severity, and natural history of symptoms of dementia in WTC-affected individuals. However, the onset of neuropsychiatric symptoms such as increased anxiety and depression are common in dementia105. They are extremely important to friends and family and can be identified by family members, since they begin causing caregiver stress among individuals with cognitive impairment106 and account for emotional difficulties107 and reduced quality of life108 among patients with cognitive impairment and their caregivers. Concurrently, cognitive impairment often results in increased difficulties navigating physical spaces109, leading to impaired abilities to operate motor vehicles110 or finances111. Together, functional limitations such as these can fundamentally change the experience of aging, thereby warranting close clinical follow-up.
-
2
Potential bioindicators in WTC-affected individuals (Box 1.2)
WTC-affected individuals have not been thoroughly profiled in this way and no neuropathological information exists to determine if WTC dust might be present in responders with cognitive dysfunction. A plethora of post-translational modifications have been associated with the tauopathy of various neurodegenerative dementias. How these might differ across WTC-exposed individuals remains to be characterized. Recent studies have suggested an increased reliance on biomarkers as outcomes of interest, in part because they can help to understand the reasons for differences in cognitive outcomes. Though ultimately critical to improving our understanding of the impact of exposures on WTC-affected individuals, in the age range of the WTC-affected individuals postmortem examination remains rare enough that brains from WTC-affected individuals have not yet been studied. Although many of the following sections focus on features of AD pathology in the WTC population, it is crucial to note that we do not know whether WTC-related cognitive impairment and/or dementia follow a pathogenesis resembling that of typical sporadic AD, while also recognizing that neurodegenerative conditions are often comorbid with one-another112.
The predominant neuropathology necessary to determine whether WTC-affected individuals with MCI are experiencing early AD/ADRD are discussed in greater depth below. An increasing body of research suggests that neuropathology may be effectively characterized by the presence of AD-related proteinopathy in the brain, notably including the formation of amyloid plaques made up predominantly of Aβ proteins with intraneuronal propagation of tauopathy throughout the brain113. To Aβ is a central characteristic in the pathogenesis of AD. APOLIPOPROTEIN-ε4 is the most potent and prevalent risk factor for late onset sporadic AD, with APOLIPOPROTEIN-ε4 homozygosity increases AD risk by more than tenfold, which may be particularly relevant to WTC-affected individuals because of its influence on BBB permeability114. Cerebrospinal fluid (CSF) sampling for quantification of Aβ levels is slightly more sensitive than amyloid PET imaging; however, amyloid PET images can be completed in vivo and judged positive or negative visually and can also be reliably quantified. An advantage of amyloid PET scans over CSF markers of Aβ levels is that amyloid PET provides information about regional distribution of deposition.
The microtubule associated protein tau undergoes aggregation and a range of post-translational modifications including “hyperphosphorylation” in response to TBI and in AD. On tauopathy PET images, SUVR images normalized to cerebellar gray matter can be used to determine the extent of 3/4R Tauopathy in specific brain regions, including the entorhinal cortex – regions involved both in aging and in AD. To detect early changes, studies suggest relying on location and spread of tau in AD 115. Tau can also be measured in CSF and is small enough (48–67kDA depending on isoform) to pass through the BBB and, therefore, to be found in plasma. Early work suggests that plasma total tau (tTAU) is elevated in clinically diagnosed AD116, acute traumatic brain injury (TBI)117, and other neuropathologies118–120. Tau protein phosphorylated at residues 181, 213, or 217 (pTAU) appear to be more specific biomarkers for AD-related neurodegeneration121–124. Plasma markers may be used to identify cognitive impairment in WTC-affected individuals125. Additionally, glial activation and changes to cholinergic synapses may facilitate the usage of PET imaging in the pathophysiology of brain disorders.
Neurodegeneration:
Early AD is often marked by hippocampal and cortical atrophy; researchers have proposed using cortical thinning in the differential diagnosis of patients with cognitive impairment and AD126,127. Similar results were recently reported in WTC responders with possible mild dementia52. Diffusion tensor MR imaging is a useful MRI technique to detect abnormalities in white matter and to assess structural connectivity of different brain regions128. Brain glucose hypometabolism, an early indicator of neurodegeneration, can inform us about brain regional integrity and functional states129. Fluorodeoxyglucose PET is a standard marker for neurodegeneration due to decreased metabolic rate in degenerated brain regions (hypometabolism)129,130. Neurofilament-Light chain protein is a small (70kDA) protein that is a critical component providing structural support to the microtubule array that passes nutrients and maintains neuronal integrity. It is small enough to pass through the BBB and is detectable in plasma and CSF as a non-specific marker of neurodegeneration and the two mediums are highly correlated131. While elevated plasma neurofilament-light indicates systemic changes consistent with neuronal damage as well as loss of neocortical tissue.
While the AD research framework is a compelling method for characterizing disease using amyloid, tau, and neurodegeneration, a large autopsy study consisting of individuals over the age of 50 reported that only one-third of all-cause dementia cases were attributed to “pristine” pathological AD as indicated by Aβ amyloid plaques and neurofibrillary tangles, while up to seven other neuropathologies including Lewy bodies and cerebrovascular disease explained much of the remaining disease132. Indeed, examining the residual for cognitive reserve, which may prevent the expression of clinical symptoms despite significant pathology, neuronal characteristics modified in development133, and the presence of protective proteins134 may help to predict the rate of cognitive decline. These results suggest that some individuals may vary in vulnerability to old-age neuropathology but that a small number of proteins can cause a substantial amount of cognitive decline that may be managed by those who are not burdened by multiple neuropathologies.
-
3
Potential treatment targets in WTC-affected individuals (Box 1.3)
Plassman, et al. 135 showed that variation in old-age cognitive performance could be attributed to early life exposures that were carried through to adulthood, findings which have been replicated 136–139. However, in older age other factors appear to become increasingly damaging especially when they are chronically activated. For example, research tracking cognitive trajectories from age 43–69 elucidating the emergence of age-graded effects of APOLIPOPROTEIN-ε4 carrier status on the rapidity of cognitive decline140. These efforts have supported a recent review of intervention studies providing guidelines to help prevent AD141. Such factors included prevention and aggressive treatment of hypertension, and less obesity at midlife as well as smoking cessation, physical activity, socialization, and treatment of diabetes. While aging is the time axis along which events unfold, exogenous insults accumulate over time to affect the rapidity of aging and contribute to risk for ADRD142. WTC-affected individuals were mostly exposed after childhood investments in educational attainment were completed, and many are now aging despite relatively low smoking rates and high levels of access to healthcare. It is therefore critical that treatment efforts seek to maximize therapeutic interventions for cognitive impairment by implementing them while sufficient cortical substrate allow interventions to have maximal benefit. Among recent advances in interventions to improve outcomes among individuals with mild cognitive impairment includes proactive treatment of hypertension143, while studies focused at AD have made steps toward improving amyloid clearance144, and reducing risk of symptom progression in dementia145. However, the unique WTC exposures may provide exposure-relevant treatment targets. As noted below improved physical activity, treatments for increased re-experiencing symptoms, and cognitive remediation all provide some potential to mitigate health outcomes long-term.
Physical activity to help improve brain health
A meta-analysis of randomized controlled trials of physical exercise interventions in persons age 50+ found that some characteristics were associated with beneficial outcomes146. Specifically, cognitive benefits were seen for aerobic, resistance, multimodal, and tai-chi interventions; for 45–60-minute interventions but not those shorter or longer; for interventions of moderate or high intensity; regardless of frequency or length of the exercise intervention. Benefits were seen for attention, executive function, memory, and working memory, but interestingly not for global cognitive measures. Benefits were observed for people with MCI and those with normal cognition. While these meta-analysis findings would seem to support exercise programs for physically able individuals, it should be noted that most of the 36 studies included were quite small, and unmeasured confounding cannot be ruled out. To further examine reasons inconsistency across studies, a randomized clinical trial (N=132) followed participants for 24 weeks and found significant improvement in executive functioning in groups trained in improving aerobic capacity147. A second study examined inflammation and found that exercise was associated with increased levels of serologic interleukin-6 and tumor necrosis factor-α148. Exercise training, however, is a challenging activity in which to engage reliable adherence.
Pharmacological and Neurobehavioral treatments for PTSD
Chronic PTSD is a debilitating condition with significant societal costs due to loss of productivity and treatment efforts to date have been largely ineffective149,150. Paroxetine and Sertraline are the only FDA approved selective serotonin reuptake inhibitors ameliorating symptoms in 60 and 30% respectively of treated individuals; yet fewer than 20% achieve full remission151. However, more promising evidence for the use of off label pharmacotherapies has been reported152,153. Considering the potential for counteracting the effects of central noradrenergic hyperactivity in PTSD, six of seven positive randomized control trials tested the α1-adrenoreceptor antagonist, Prazosin as a therapeutic intervention154,155. One of the largest studies examined Prazosin among 67 active-duty soldiers (mean age 29, mean rank E5 - sergeant) with combat-related PTSD nightmares and sleep disturbance randomized to prazosin or placebo156. They found that prazosin improved sleep quality and Clinician Administered PTSD Scale (CAPS) nightmare scores and improved the CAPS distressing dreams item, the Pittsburgh Sleep Quality Index, and total CAPS score157, though a follow-up study of predominantly Vietnam veterans with stable chronic PTSD suggested that benefits may be concentrated among those with evidence of central noradrenergic hyperactivity157. While Prazosin may be uniquely targeted at reducing re-experiencing symptoms, Cognitive Behavioral Therapy (CBT) and Cognitive Processing Therapy are strongly recommended clinical practice guidelines for interventions when treating patients with PTSD. CBT emphasizes treatment to understanding the associations between thoughts, feeling, and behaviors associated with trauma, and specifically focuses on changing behavioral patterns and though processes158 by using structured sessions to help patients reconceptualize traumatic events in a way that diminishes ongoing negative effects in daily life159–161. Cognitive Therapy, derived from CBT, aims to additionally interrupt disturbing behavioral and thought patterns by altering negative memories of trauma162,163. Finally, Prolonged Exposure is a treatment regimen with a modified CBT approach to teach patients to combat avoidance in approaching trauma-specific memories, feelings and situations, so as to not associate them with danger, ultimately leading to the cessation of avoidance164.
Cognitive Remediation
Cognition-oriented training is a type of non-invasive set of standardized training tasks that are arranged to train individuals with mild or severe dementia, on distinct cognitive processes and abilities such as attention, memory, information processing and problem solving165,166. The goal is to retain or improve functioning within a specific cognitive domain that leads to improved performance in associated tasks in the same cognitive domain, mainly through mechanisms of restorative neuroplasticity (reviewed in 167). Indeed, studies employing cognitive training have demonstrated associations between structured training programs and neural activation in key brain regions among healthy older adults168 and people with MCI169,170. Useful Field of View (UFOV) is designed to improve speed of information processing through practice of visual attention tasks. Results from multiple clinical trials suggest that UFOV training significantly improved instrumental activities of daily living, and improved well-being as measured by reduced depressive and anxiety symptoms relative to controls171. Edwards, et al. 172 demonstrated that UFOV cognitive training reduced the risk of dementia over a ten-year follow-up. Participants who completed higher amounts of UFOV training were up to 48% less likely to develop dementia over the ten-year follow-up period. However, there is a need to be aware of the potential for psychiatric symptoms to increase in individuals with neurodegenerative disease and to monitor and treat these symptoms accordingly, as they tend to account for an outsized role in patient and caregiver distress.
SUMMARY
The research performed thus far has advanced our level of knowledge of a range of neurological issues in WTC-affected individuals. However, there are crucial gaps that suggest next steps that are required to move this research program forward (Box 1). First, because WTC-affected individuals with cognitive impairment appear to have broad reductions across domains of cognitive performance (including episodic memory and processing speed) there is a need to more clearly articulate exposure-related data versus PTSD-related neurocognitive symptoms to determine if and how WTC-related neurocognitive impairment fits a known clinical phenotype. Second, neuroimaging, neuropathological, genetic, and multi-omic data are needed to elucidate the etiologies of the underlying cognitive and behavioral symptoms that are central features of clinically diagnosed MCI in WTC-affected populations. Third, we need to examine relevant biofluid- and neuroimaging-based evidence of proteinopathy and neurodegeneration in WTC-affected individuals to develop the appropriate diagnostic, prognostic, and mechanistic paradigms for WTC-related exposures and their clinic-biomarker correlations. Finally, we need to nominate, investigate, and validate any potentially prophylactic interventions actions that are safe and effective for improving functioning in WTC-affected individuals with PTSD and those who are at risk of developing clinical cognitive impairment.
Special points of consideration in WTC-affected populations
An array of limitations has been noted when characterizing occupational cohorts that may help to contextualize recommendations. There is a healthy responder effect because not every person is eligible to be a police officer or firefighter, and others chose not to volunteer. First responders receive substantial training and preparation that may have helped them to maintain cognitive reserve72, whereas other WTC-affected individuals may have been exposed lacked physical and psychological preparation. With respect to prior military experience that is common in a small number of responders and survivors, there are many dimensions to warzone deployment that may be crucial to lifelong differences in cognitive performance173.
There are limitations to relying solely on research in WTC responders, suggesting an increasing need to develop research with non-responding survivors and with non-WTC-exposed controls. Comparing WTC responders to internal controls assumes that cognitively unimpaired WTC responders are a good comparison group yet, several studies reported that this may not be true thereby highlighting the need for a normal comparison population. The potential for unimpaired responders to have early signs of cortical atrophy, for example, points to the need for future work to improve characterization of the earliest indicators of the disease to provide responders with access to treatment as early as possible to potentially avoid poorer long-term outcomes. Additionally, while there is a growing body of work in the responder population, studies in other WTC-affected populations have been limited to examining only subjective indicators of cognitive functioning. Area residents and other community members encompassed a wide range of ages from newborn to elderly, covered a wide spectrum of occupations and socioeconomic statuses, and came from disparate backgrounds. To date, studies have not been published examining indicators of cognitive impairment in WTC survivors or, using objective information, in Fire Department of New York employees. Relying on WTC responders may therefore bias results relating to race/ethnicity and sex/gender since responders are a uniformly advantaged group with relatively high socioeconomic standing. A limited focus on Fire Department of New York employees may also be concerning since they were more severely exposed than other WTC-affected populations.
CONCLUSION
The Zadroga Act authorizes ongoing research activities and manages care for covered WTC conditions, programs to date have focused on WTC conditions including, for example, respiratory conditions, gastrointestinal disease, PTSD, depression, and cancer but have not monitored neurological symptoms or diseases in WTC-affected individuals. An increasing number of studies suggest the potential impact of WTC exposures on neurological outcomes, specifically, in WTC-affected individuals. Crucially, those who endured and responded to the attacks on the WTC on 9/11/2001 have highlighted the potential for a novel emerging neurological condition of unknown etiology or that may be a new manifestation of a known disorder, such as AD. While the U.S. preventive services task force has recently suggested that cognitive screening may not always be beneficial in situations where there are not actionable. The consensus developed among experts attending this meeting was that WTC-affected populations may be at heightened risk as compared to the general population, most likely because of their experiences on-site and concluded that more research was needed in a number of highlighted areas. This statement therefore provided consensus recommendations to help researchers and, if supported, policymakers to consider next steps for monitoring WTC responders as they age.
Key points.
The 9/11/2001 terrorist attacks on the World Trade Center resulted in thousands of deaths and injuries and significantly impacted the health of responders, volunteers, survivors, and area residents.
Twenty years later, emerging studies have identified higher than expected levels of mild cognitive impairment (MCI) in WTC-affected individuals compared to normative data in individual studies and meta-analyses of general population samples.
MCI has consistently been identified among individuals with the most severe WTC exposures and chronic symptoms of post-traumatic stress disorder (PTSD).
This review provides a rationale for the inclusion of screening for cognitive function among WTC-affected individuals to facilitate an improved understanding of the potential for WTC-related cognitive decline.
Future work will be required to characterize the scope, determinants, and rate of cognitive decline among WTC-affected individuals as well as the neuropathological and clinico-biomarker correlates of this decline.
Acknowledgements:
We would like to acknowledge Robert D. Daniels, Ph.D., C.H.P.; Travis Kubale, Ph.D.; and Rear Admiral Dori Reismann, M.D., M.P.H. for their assistance in planning and participating in the meeting that germinated this review.
Highlighted References:
Kuan, P. F. et al. Single-cell transcriptomics analysis of mild cognitive impairment in World Trade Center disaster responders. Alzheimers Dement (Amst) 13, e12154, doi:10.1002/dad2.12154 (2021).
This study used a cutting-edge single cell sequencing to identify 226 genes that were differentially expressed in monocytes and to propose that monocytic changes as a key cell type to target in blood-based biomarker work in WTC responders at risk of MCI.
Kuan, P.-F. et al. Molecular linkage between post-traumatic stress disorder and cognitive impairment: a targeted proteomics study of World Trade Center responders. Translational psychiatry 10, 1–15 (2020).
This study used a targeted proteomics approach (276 proteins) to identify 50 proteins that were dysregulated in PTSD and/or MCI (Neurocan and Brevican were dysregulated in MCI and PTSD with MCI) and to develop a multi-protein score for use in differentiating these disease states (area under the receiver-operating curve of 0.77–0.85).
Deri Y., et al. Selective hippocampal subfield volume reductions in World Trade Center responders with cognitive impairment. Alzheimer’s & Dementia: Diagnosis, Assessment & Disease Monitoring 13: e12165, doi:10.1002/dad2.12165 (2021).
This study has shown that in individuals with dementia (N=99), hippocampal subfield volumes were reduced in possible dementia as measured on structural magnetic resonance imaging.
Clouston, S. A. P. et al. Incidence of mild cognitive impairment in World Trade Center responders: Long-term consequences of re-experiencing the events on 9/11/2001. Alzheimers Dement (Amst) 11, 628–636, doi:10.1016/j.dadm.2019.07.006 (2019).
This study has found that in a large population (N=1,800) of WTC responders determined to be cognitively unimpaired at baseline, the incidence of mild cognitive impairment at follow-up was higher than expected and was concentrated in those with the longest exposures and those who experienced symptoms consistent with post-traumatic stress disorder.
Clouston, S. A. P. et al. Traumatic exposures, posttraumatic stress disorder, and cognitive functioning in World Trade Center responders. Alzheimers Dement (N Y) 3, 593–602, doi:10.1016/j.trci.2017.09.001 (2017).
This cross-sectional study of cognitive functioning across six domains has revealed that five of six domains of functioning—including visual memory and processing speed, but not attention—are associated with WTC exposures and symptoms consistent with post-traumatic stress disorder.
Clouston, S. A. P. et al. Reduced cortical thickness in World Trade Center responders with cognitive impairment. Alzheimer’s & Dementia: Diagnosis, Assessment, & Disease Monitoring (2020).
This study has shown that in individuals with dementia (N=99), episodic memory is the most strongly affected domain of cognition, and individuals with possible dementia have evidence of cortical atrophy as measured using structural magnetic resonance imaging.
Clouston, S. A. P., Guralnik, J. M., Kotov, R., Bromet, E. J. & Luft, B. J. Functional Limitations Among Responders to the World Trade Center Attacks 14 Years After the Disaster: Implications of Chronic Posttraumatic Stress Disorder. J Trauma Stress 30, 443–452, doi:10.1002/jts.22219 (2017).
This study has revealed that the relationship between PTSD is not limited to cognitive features but is also associated with changes in motor functioning indicative of early onset functional limitations.
Kritikos, M. et al. Pathway Analysis for Plasma β-Amyloid, Tau and Neurofilament Light (ATN) in World Trade Center Responders at Midlife. Neurology and Therapy, doi:10.1007/s40120-020-00189-1 (2020).
This study using a multiplex array of plasma-based neuropathological biomarkers measured through single molecule analysis (Simoa) has shown that cognitive impairment in WTC responders is associated with changes in plasma biomarkers consistent with involvement of β-amyloid1–42 and the total-TAU burden.
Mukherjee, S., Clouston, S., Kotov, R., Bromet, E. & Luft, B. Handgrip Strength of World Trade Center (WTC) Responders: The Role of Re-Experiencing Posttraumatic Stress Disorder (PTSD) Symptoms. Int J Environ Res Public Health 16, 1128, doi:10.3390/ijerph16071128 (2019).
This study in a large cross-sectional group of WTC responders has found strong associations between PTSD and measures of muscle weakness consistent with frailty seen in older individuals and those with neurodegenerative disease.
Contributor Information
Sean A. P. Clouston, Population, and Preventive Medicine, Program in Public Health, Renaissance School of Medicine at Stony Brook University, Stony Brook, NY, USA.
Charles B. Hall, Department of Epidemiology and Population Health, and Saul R. Korey Department of Neurology, Albert Einstein College of Medicine, Bronx, NY, USA.
Minos Kritikos, Renaissance School of Medicine at Stony Brook University, Stony Brook, NY, USA.
David Bennett, Rush Alzheimer’s Disease Center and Robert C. Borwell Professor of Neurological Science, Department of Neurological Sciences, Rush Medical College at Rush University, Chicago, IL, USA.
Steven DeKosky, Department of Neurology and Neuroscience, Director of the Evelyn F. and William L. McKnight Brain Institute and Florida Alzheimer’s Disease Research Center, University of Florida, Gainesville, FL, USA.
Jerri Edwards, Morsani College of Medicine at the University of South Florida, Tampa, FL, USA.
Caleb Finch, USC Leonard Davis School of Gerontology, University of Southern California, Los Angeles, CA, USA.
William Kreisl, Taub Institute for Research on Alzheimer’s Disease and the Aging Brain, Columbia University, New York, NY, USA.
Michelle Mielke, Mayo Clinic, Director, Specialized Center of Research Excellence on Sex Differences, Rochester, MN, USA.
Elaine R. Peskind, Education, and Clinical Center, VA Puget Sound Health Care System; Friends of Alzheimer’s Research Professor of Psychiatry, University of Washington School of Medicine, Seattle, WA, USA.
Murray Raskind, VA VISN 20 Northwest Mental Illness Research, Education, and Clinical Center; VA Puget Sound Health Care System Professor of Psychiatry and Behavioral Sciences, University of Washington School of Medicine, Seattle, WA, USA.
Marcus Richards, MRC Unit for Lifelong Health and Ageing, University College London, London, UK.
Richard P. Sloan, Department of Psychiatry, Columbia University Irving Medical Center, New York, NY, USA.
Avron Spiro, III, Boston University School of Public Health; Associate Professor of Health Policy and Health Services research, Boston University Goldman School of Dental Medicine; and Senior Research Career Scientist, Veteran’s Affairs, Boston Healthcare System, Boston, MA, USA.
Neil Vasdev, CAMH Resarch Imaging Center Director, Azrrieli Centre for Neuro-Radiochemistry, CAMH Tier 1 Canada Research Chair in Radiochemistry and Nuclear Medicine & Endowed Professor of Psychiatry, University of Toronto, Toronto, ON, Canada.
Robert Brackbill, World Trade Center Health Registry, New York Department of Health and Mental Hygiene, New York, NY.
Mark Farfel, World Trade Center Health Registry, New York Department of Health and Mental Hygiene, New York, NY.
Megan Horton, Department of Environmental Medicine and Public Health, Icahn School of Medicine at Mount Sinai, New York, NY, USA.
Sandra Lowe, The World Trade Center Mental Health Program, Icahn School of Medicine at Mount Sinai, New York, NY.
Roberto G. Lucchini, Robert Stempel College of Public Health and Social Work, Florida International University, Miami, FL, USA.
David Prezant, Office of Medical Affairs, Fire Department of New York, New York, NY, USA; Division of Pulmonary Medicine, Department of Medicine, Albert Einstein College of Medicine and Montefiore Medical Center, Bronx, NY, USA..
Joan Reibman, New York University Langone Health, New York, NY, USA.
Rebecca Rosen, Department of Psychiatry, Mental Health Director at the World Trade Center Environmental Health Center, New York University, New York, NY, USA.
Kacie Seil, World Trade Center Health Registry, New York Department of Health and Mental Hygiene, New York, NY.
Rachel Zeig-Owens, Fire Department of New York, New York, NY, USA; and Research Assistant Professor, Department of Epidemiology and Population Health, Albert Einstein College of Medicine, Bronx, NY, USA.
Yael Deri, Renaissance School of Medicine at Stony Brook University, Stony Brook, NY, USA.
Erica D. Diminich, Renaissance School of Medicine at Stony Brook University, Stony Brook, NY, USA.
Bernadette A. Fausto, Aging & Brain Health Alliance, Center for Molecular & Behavioral Neuroscience, Rutgers, The State University of New Jersey–Newark campus, Newark, NJ, USA.
Sam Gandy, Associate Director of the Mount Sinai Alzheimer’s Disease Research Center, Professor of Psychiatry and of Neurology, James J. Peters Department of Veteran’s Affairs Medical Center, Icahn School of Medicine at Mount Sinai, New York, NY, USA.
Mary Sano, Professor of Psychiatry, Icahn School of Medicine at Mount Sinai, New York, NY, USA.
Evelyn J. Bromet, Department of Psychiatry, Renaissance School of Medicine at Stony Brook University, Stony Brook, NY, USA
Benjamin J. Luft, Medical Director of the World Trade Center Responder Health and Wellness Program, Department of Medicine, Renaissance School of Medicine at Stony Brook University, Stony Brook, NY, USA
REFERENCES
- 1.Dahlgren J, Cecchini M, Takhar H & Paepke O Persistent organic pollutants in 9/11 world trade center rescue workers: reduction following detoxification. Chemosphere 69, 1320–1325, doi: 10.1016/j.chemosphere.2006.05.127 (2007). [DOI] [PubMed] [Google Scholar]
- 2.Lippmann M, Cohen MD & Chen LC Health effects of World Trade Center (WTC) Dust: An unprecedented disaster’s inadequate risk management. Crit Rev Toxicol 45, 492–530, doi: 10.3109/10408444.2015.1044601 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Toom V Whose Body Is It? Technolegal Materialization of Victims’ Bodies and Remains after the World Trade Center Terrorist Attacks. Science, Technology, & Human Values 41, 686–708, doi: 10.1177/0162243915624145 (2015). [DOI] [Google Scholar]
- 4.Galea S et al. Psychological sequelae of the September 11 terrorist attacks in New York City. N Engl J Med 346, 982–987, doi: 10.1056/NEJMsa013404 (2002). [DOI] [PubMed] [Google Scholar]
- 5.Association, A. P. Diagnostic and statistical manual of mental disorders: DSM-5. 5th ed. 5th edn, (American Psychiatric Association, 2013). [Google Scholar]
- 6.Liu B, Tarigan LH, Bromet EJ & Kim H World Trade Center disaster exposure-related probable posttraumatic stress disorder among responders and civilians: a meta-analysis. PLoS One 9, e101491, doi: 10.1371/journal.pone.0101491 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vol. 847 (H.R, Washington, D.C.). [Google Scholar]
- 8.Centers for Disease Control and Prevention. World Trade Center Health Program At A Glance. Health NIOSH, ed. Atlanta, GA: Centers for Disease Control and Prevention; (2021). [Google Scholar]
- 9.Farfel M et al. An overview of 9/11 experiences and respiratory and mental health conditions among World Trade Center Health Registry enrollees. J Urban Health 85, 880–909, doi: 10.1007/s11524-008-9317-4 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stern Y et al. Whitepaper: Defining and investigating cognitive reserve, brain reserve, and brain maintenance. Alzheimer’s & Dementia, doi: 10.1016/j.jalz.2018.07.219 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Spiro A 3rd, Schnurr PP & Aldwin CM Combat-related posttraumatic stress disorder symptoms in older men. Psychol Aging 9, 17–26, doi: 10.1037//0882-7974.9.1.17 (1994). [DOI] [PubMed] [Google Scholar]
- 12.Haghani A, Morgan TE, Forman HJ & Finch CE Air Pollution Neurotoxicity in the Adult Brain: Emerging Concepts from Experimental Findings. Journal of Alzheimer’s Disease, 1–25. [DOI] [PubMed] [Google Scholar]
- 13.Lioy PJ et al. Characterization of the dust/smoke aerosol that settled east of the World Trade Center (WTC) in lower Manhattan after the collapse of the WTC 11 September 2001. Environ Health Perspect 110, 703–714, doi: 10.1289/ehp.02110703 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Landrigan PJ et al. Health and environmental consequences of the world trade center disaster. Environ Health Perspect 112, 731–739, doi: 10.1289/ehp.6702 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Forman HJ & Finch CE A critical review of assays for hazardous components of air pollution. Free Radical Biology and Medicine 117, 202–217 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Riediker M et al. Particle toxicology and health-where are we? Particle and fibre toxicology 16, 19 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mumaw CL et al. Microglial priming through the lung—brain axis: the role of air pollution-induced circulating factors. The FASEB Journal 30, 1880–1891 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lucchini RG et al. Neurofunctional dopaminergic impairment in elderly after lifetime exposure to manganese. Neurotoxicology 45, 309–317, doi: 10.1016/j.neuro.2014.05.006 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lucchini RG, Dorman DC, Elder A & Veronesi B Neurological impacts from inhalation of pollutants and the nose-brain connection. Neurotoxicology 33, 838–841, doi: 10.1016/j.neuro.2011.12.001 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zeliger HI in Interdiscip Toxicol Vol. 6 103–110 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bromet E et al. DSM-IV post-traumatic stress disorder among World Trade Center responders 11–13 years after the disaster of 11 September 2001 (9/11). Psychological medicine 46, 771–783, doi: 10.1017/S0033291715002184 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hall KS, Hoerster KD & Yancy WS Jr Post-traumatic stress disorder, physical activity, and eating behaviors. Epidemiologic reviews 37, 103–115 (2015). [DOI] [PubMed] [Google Scholar]
- 23.Bonanno GA Resilience in the face of potential trauma. Current directions in psychological science 14, 135–138 (2005). [Google Scholar]
- 24.Livingston G et al. Dementia prevention, intervention, and care: 2020 report of the Lancet Commission. The Lancet 396, 413–446, doi: 10.1016/S0140-6736(20)30367-6 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cho J et al. Association between exposure to polycyclic aromatic hydrocarbons and brain cortical thinning: The Environmental Pollution-Induced Neurological EFfects (EPINEF) study. Science of The Total Environment 737, 140097, doi: 10.1016/j.scitotenv.2020.140097 (2020). [DOI] [PubMed] [Google Scholar]
- 26.Best EA, Juarez-Colunga E, James K, LeBlanc WG & Serdar B in PLoS One Vol. 11 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gonzalez-Maciel A, Reynoso-Robles R, Torres-Jardon R, Mukherjee PS & Calderon-Garciduenas L Combustion-Derived Nanoparticles in Key Brain Target Cells and Organelles in Young Urbanites: Culprit Hidden in Plain Sight in Alzheimer’s Disease Development. J Alzheimers Dis 59, 189–208, doi: 10.3233/JAD-170012 (2017). [DOI] [PubMed] [Google Scholar]
- 28.Chen R et al. Parallel Comparative Studies on Mouse Toxicity of Oxide Nanoparticle- and Gadolinium-Based T1 MRI Contrast Agents. ACS Nano 9, 12425–12435, doi: 10.1021/acsnano.5b05783 (2015). [DOI] [PubMed] [Google Scholar]
- 29.Wilker EH et al. Long-term exposure to fine particulate matter, residential proximity to major roads and measures of brain structure. Stroke 46, 1161–1166, doi: 10.1161/STROKEAHA.114.008348 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wallin C et al. Alzheimer’s disease and cigarette smoke components: effects of nicotine, PAHs, and Cd(II), Cr(III), Pb(II), Pb(IV) ions on amyloid-beta peptide aggregation. Sci Rep 7, 14423, doi: 10.1038/s41598-017-13759-5 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ayton S, Diouf I & Bush AI Evidence that iron accelerates Alzheimer’s pathology: a CSF biomarker study. Journal of Neurology, Neurosurgery & Psychiatry 89, 456–460, doi: 10.1136/jnnp-2017-316551 (2018). [DOI] [PubMed] [Google Scholar]
- 32.Shaffer RM et al. Fine Particulate Matter Exposure and Cerebrospinal Fluid Markers of Vascular Injury. Journal of Alzheimer’s Disease 71, 1015–1025 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Campbell A et al. Particulate matter in polluted air may increase biomarkers of inflammation in mouse brain. Neurotoxicology 26, 133–140 (2005). [DOI] [PubMed] [Google Scholar]
- 34.Cacciottolo M et al. Traffic-related air pollutants (TRAP-PM) promote neuronal amyloidogenesis through oxidative damage to lipid rafts. Free Radical Biology and Medicine 147, 242–251 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gill JM, Saligan L, Woods S & Page G PTSD is associated with an excess of inflammatory immune activities. Perspect Psychiatr Care 45, 262–277, doi: 10.1111/j.1744-6163.2009.00229.x (2009). [DOI] [PubMed] [Google Scholar]
- 36.Justice NJ et al. Posttraumatic stress disorder-like induction elevates β-amyloid levels, which directly activates corticotropin-releasing factor neurons to exacerbate stress responses. Journal of Neuroscience 35, 2612–2623 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Filiano AJ, Gadani SP & Kipnis J Interactions of innate and adaptive immunity in brain development and function. Brain Res 1617, 18–27, doi: 10.1016/j.brainres.2014.07.050 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Andrews JA & Neises KD Cells, biomarkers, and post-traumatic stress disorder: evidence for peripheral involvement in a central disease. J Neurochem 120, 26–36, doi: 10.1111/j.1471-4159.2011.07545.x (2012). [DOI] [PubMed] [Google Scholar]
- 39.Glaser R & Kiecolt-Glaser JK Stress-induced immune dysfunction: implications for health. Nat Rev Immunol 5, 243–251, doi: 10.1038/nri1571 (2005). [DOI] [PubMed] [Google Scholar]
- 40.Kuan PF et al. Cell type-specific gene expression patterns associated with posttraumatic stress disorder in World Trade Center responders. Transl Psychiatry 9, 1, doi: 10.1038/s41398-018-0355-8 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Deslauriers J, Powell S & Risbrough VB Immune signaling mechanisms of PTSD risk and symptom development: insights from animal models. Curr Opin Behav Sci 14, 123–132, doi: 10.1016/j.cobeha.2017.01.005 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Felmingham K et al. Duration of posttraumatic stress disorder predicts hippocampal grey matter loss. Neuroreport 20, 1402–1406, doi: 10.1097/WNR.0b013e3283300fbc (2009). [DOI] [PubMed] [Google Scholar]
- 43.Ousdal OT et al. The association of PTSD symptom severity with amygdala nuclei volumes in traumatized youths. Translational Psychiatry 10, 1–10 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yehuda R Advances in understanding neuroendocrine alterations in PTSD and their therapeutic implications. Annals of the new York Academy of Sciences 1071, 137–166, doi: 10.1196/annals.1364.012 (2006). [DOI] [PubMed] [Google Scholar]
- 45.Wingenfeld K & Wolf OT HPA axis alterations in mental disorders: impact on memory and its relevance for therapeutic interventions. CNS neuroscience & therapeutics 17, 714–722, doi: 10.1111/j.1755-5949.2010.00207.x (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yehuda R Post-traumatic stress disorder. N Engl J Med 346, 108–114, doi: 10.1056/NEJMra012941 (2002). [DOI] [PubMed] [Google Scholar]
- 47.Akiyama H et al. Cell mediators of inflammation in the Alzheimer disease brain. Alzheimer Dis Assoc Disord 14 Suppl 1, S47–53, doi: 10.1097/00002093-200000001-00008 (2000). [DOI] [PubMed] [Google Scholar]
- 48.Leyns CEG & Holtzman DM Glial contributions to neurodegeneration in tauopathies. Mol Neurodegener 12, 50, doi: 10.1186/s13024-017-0192-x (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Clouston SA et al. Cognitive impairment among World Trade Center responders: Long-term implications of re-experiencing the 9/11 terrorist attacks. Alzheimers Dement (Amst) 4, 67–75, doi: 10.1016/j.dadm.2016.08.001 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Clouston SAP et al. Incidence of mild cognitive impairment in World Trade Center responders: Long-term consequences of re-experiencing the events on 9/11/2001. Alzheimers Dement (Amst) 11, 628–636, doi: 10.1016/j.dadm.2019.07.006 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Clouston S et al. Traumatic exposures, posttraumatic stress disorder, and cognitive functioning in World Trade Center responders. Alzheimers Dement (N Y) 3, 593–602, doi: 10.1016/j.trci.2017.09.001 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Clouston SAP et al. Reduced cortical thickness in World Trade Center responders with cognitive impairment. Alzheimers Dement (Amst) 12, e12059, doi: 10.1002/dad2.12059 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chen APF et al. A deep learning approach for monitoring parietal-dominant Alzheimer’s disease in World Trade Center responders at midlife. Brain Communications (In Press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Singh A et al. World Trade Center exposure, post-traumatic stress disorder, and subjective cognitive concerns in a cohort of rescue/recovery workers. Acta psychiatrica Scandinavica 141, 275–284 (2020). [DOI] [PubMed] [Google Scholar]
- 55.Singh A et al. PTSD and depressive symptoms as potential mediators of the association between World Trade Center exposure and subjective cognitive concerns in rescue/recovery workers. International journal of environmental research and public health 17, 5683 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Seil K, Yu S & Alper H A Cognitive Reserve and Social Support-Focused Latent Class Analysis to Predict Self-Reported Confusion or Memory Loss among Middle-Aged World Trade Center Health Registry Enrollees. Int J Environ Res Public Health 16, doi: 10.3390/ijerph16081401 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mukherjee S, Clouston S, Kotov R, Bromet E & Luft B Handgrip Strength of World Trade Center (WTC) Responders: The Role of Re-Experiencing Posttraumatic Stress Disorder (PTSD) Symptoms. Int J Environ Res Public Health 16, 1128, doi: 10.3390/ijerph16071128 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Clouston SAP, Guralnik JM, Kotov R, Bromet EJ & Luft BJ Functional Limitations Among Responders to the World Trade Center Attacks 14 Years After the Disaster: Implications of Chronic Posttraumatic Stress Disorder. J Trauma Stress 30, 443–452, doi: 10.1002/jts.22219 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Massy-Westropp NM, Gill TK, Taylor AW, Bohannon RW & Hill CL Hand Grip Strength: age and gender stratified normative data in a population-based study. BMC Res Notes 4, 127, doi: 10.1186/1756-0500-4-127 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ling CH et al. Handgrip strength and mortality in the oldest old population: the Leiden 85-plus study. Cmaj 182, 429–435, doi: 10.1503/cmaj.091278 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Taekema DG, Gussekloo J, Maier AB, Westendorp RG & de Craen AJ Handgrip strength as a predictor of functional, psychological and social health. A prospective population-based study among the oldest old. Age Ageing 39, 331–337, doi: 10.1093/ageing/afq022 (2010). [DOI] [PubMed] [Google Scholar]
- 62.Fukumori N et al. Association between hand-grip strength and depressive symptoms: Locomotive Syndrome and Health Outcomes in Aizu Cohort Study (LOHAS). Age Ageing 44, 592–598, doi: 10.1093/ageing/afv013 (2015). [DOI] [PubMed] [Google Scholar]
- 63.Lino VT et al. Handgrip Strength and Factors Associated in Poor Elderly Assisted at a Primary Care Unit in Rio de Janeiro, Brazil. PLoS One 11, e0166373, doi: 10.1371/journal.pone.0166373 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Firth J et al. Association Between Muscular Strength and Cognition in People With Major Depression or Bipolar Disorder and Healthy Controls. JAMA Psychiatry 75, 740–746, doi: 10.1001/jamapsychiatry.2018.0503 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Firth J et al. Grip Strength Is Associated With Cognitive Performance in Schizophrenia and the General Population: A UK Biobank Study of 476559 Participants. Schizophrenia bulletin 44, 728–736, doi: 10.1093/schbul/sby034 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Diminich ED et al. Chronic Posttraumatic Stress Disorder and Comorbid Cognitive and Physical Impairments in World Trade Center Responders. J Trauma Stress, doi: 10.1002/jts.22631 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ganzel B, Casey B, Glover G, Voss HU & Temple E The aftermath of 9/11: Effect of intensity and recency of trauma on outcome. Emotion 7, 227 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ganzel BL, Kim P, Glover GH & Temple E Resilience after 9/11: Multimodal neuroimaging evidence for stress-related change in the healthy adult brain. NeuroImage 40, 788–795, doi: 10.1016/j.neuroimage.2007.12.010 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Huang C et al. White Matter Connectivity in Incident Mild Cognitive Impairment: A Diffusion Spectrum Imaging Study of World Trade Center Responders at Midlife. Journal of Alzheimer’s Disease, 1–11 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Deri Y et al. Selective hippocampal subfield volume reductions in World Trade Center responders with cognitive impairment. Alzheimer’s & Dementia: Diagnosis, Assessment, and Disease Monitoring 13, e12165 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Clouston SAP et al. A cortical thinning signature to identify World Trade Center responders with possible dementia. Intelligence-Based Medicine 5, 100032, doi: 10.1016/j.ibmed.2021.100032 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Stern Y et al. Whitepaper: Defining and investigating cognitive reserve, brain reserve, and brain maintenance. Alzheimers Dement 16, 1305–1311, doi: 10.1016/j.jalz.2018.07.219 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Aldrich TK et al. Lung function in rescue workers at the World Trade Center after 7 years. N Engl J Med 362, 1263–1272, doi: 10.1056/NEJMoa0910087 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Rosen RL et al. Elevated C-reactive protein and posttraumatic stress pathology among survivors of the 9/11 World Trade Center attacks. J Psychiatr Res 89, 14–21, doi: 10.1016/j.jpsychires.2017.01.007 (2017). [DOI] [PubMed] [Google Scholar]
- 75.Bello GA et al. Development of a Physiological Frailty Index for the World Trade Center General Responder Cohort. Curr Gerontol Geriatr Res 2018, 3725926, doi: 10.1155/2018/3725926 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Clouston SA, Edelman NH, Aviv A, Stewart C & Luft BJ Shortened leukocyte telomere length is associated with reduced pulmonary function and greater subsequent decline in function in a sample of World Trade Center responders. Scientific reports 9, 8148 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kuan PF et al. Gene expression associated with PTSD in World Trade Center responders: An RNA sequencing study. Transl Psychiatry 7, 1297, doi: 10.1038/s41398-017-0050-1 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kuan P-F et al. Molecular linkage between post-traumatic stress disorder and cognitive impairment: a targeted proteomics study of World Trade Center responders. Translational psychiatry 10, 1–15 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Jack CR Jr. et al. NIA-AA Research Framework: Toward a biological definition of Alzheimer’s disease. Alzheimers Dement 14, 535–562, doi: 10.1016/j.jalz.2018.02.018 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Albert MS et al. The diagnosis of mild cognitive impairment due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement 7, 270–279, doi: 10.1016/j.jalz.2011.03.008 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.McKhann GM et al. The diagnosis of dementia due to Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimer’s & dementia 7, 263–269 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Association, A. s. 2019 Alzheimer’s disease facts and figures. Alzheimer’s & dementia 15, 321–387 (2019). [Google Scholar]
- 83.Lim EW et al. Amyloid-β and Parkinson’s disease. Journal of neurology, 1–15 (2019). [Google Scholar]
- 84.Ismail Z et al. The Mild Behavioral Impairment Checklist (MBI-C): A Rating Scale for Neuropsychiatric Symptoms in Pre-Dementia Populations. J Alzheimers Dis 56, 929–938, doi: 10.3233/JAD-160979 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Zammit AR et al. A Coordinated Multi-study Analysis of the Longitudinal Association Between Handgrip Strength and Cognitive Function in Older Adults. J Gerontol B Psychol Sci Soc Sci 76, 229–241, doi: 10.1093/geronb/gbz072 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Duggan EC et al. A Multi-study Coordinated Meta-analysis of Pulmonary Function and Cognition in Aging. J Gerontol A Biol Sci Med Sci 74, 1793–1804, doi: 10.1093/gerona/glz057 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Chin KS, Yassi N, Churilov L, Masters CL & Watson R Prevalence and clinical associations of tau in Lewy body dementias: A systematic review and meta-analysis. Parkinsonism & Related Disorders 80, 184–193, doi: 10.1016/j.parkreldis.2020.09.030 (2020). [DOI] [PubMed] [Google Scholar]
- 88.Crary JF et al. Primary age-related tauopathy (PART): a common pathology associated with human aging. Acta Neuropathol 128, 755–766, doi: 10.1007/s00401-014-1349-0 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Jack CR Jr. et al. Suspected non-Alzheimer disease pathophysiology--concept and controversy. Nature reviews. Neurology 12, 117–124, doi: 10.1038/nrneurol.2015.251 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Power MC et al. Combined neuropathological pathways account for age-related risk of dementia. Annals of Neurology 84, 10–22, doi: 10.1002/ana.25246 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Jack CR Jr. et al. Tracking pathophysiological processes in Alzheimer’s disease: an updated hypothetical model of dynamic biomarkers. Lancet Neurol 12, 207–216, doi: 10.1016/S1474-4422(12)70291-0 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Bäckman L, Jones S, Berger A-K, Laukka EJ & Small BJ Cognitive impairment in preclinical Alzheimer’s disease: a meta-analysis. Neuropsychology 19, 520 (2005). [DOI] [PubMed] [Google Scholar]
- 93.Li G et al. Cerebrospinal fluid biomarkers for Alzheimer’s and vascular disease vary by age, gender, and APOE genotype in cognitively normal adults. Alzheimer’s research & therapy 9, 1–9 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Petersen R Mild cognitive impairment: transition between aging and Alzheimer’s disease. Neurologia 15, 93–101 (2000). [PubMed] [Google Scholar]
- 95.Bondi MW et al. Neuropsychological criteria for mild cognitive impairment improves diagnostic precision, biomarker associations, and progression rates. Journal of Alzheimer’s Disease 42, 275–289 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Force, U. P. S. T. Screening for Cognitive Impairment in Older Adults: US Preventive Services Task Force Recommendation Statement. JAMA 323, 757–763, doi: 10.1001/jama.2020.0435 (2020). [DOI] [PubMed] [Google Scholar]
- 97.Liss JL et al. Practical recommendations for timely, accurate diagnosis of symptomatic Alzheimer’s disease (MCI and dementia) in primary care: a review and synthesis. Journal of Internal Medicine n/a, doi: 10.1111/joim.13244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Sliwinski MJ et al. Reliability and validity of ambulatory cognitive assessments. Assessment 25, 14–30 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Luis CA, Keegan AP & Mullan M Cross validation of the Montreal Cognitive Assessment in community dwelling older adults residing in the Southeastern US. Int J Geriatr Psychiatry 24, 197–201, doi: 10.1002/gps.2101 (2009). [DOI] [PubMed] [Google Scholar]
- 100.Wittich W, Phillips N, Nasreddine ZS & Chertkow H Sensitivity and specificity of the Montreal Cognitive Assessment modified for individuals who are visually impaired. Journal of visual impairment & blindness 104, 360–368 (2010). [Google Scholar]
- 101.Hardy KK, Walsh KS, Harel BT, Hostetter SA, Packer RJ, & Acosta MT. Computerized Cognitive Training for Children with Neurofibromatosis Type 1 (NF1): A Pilot Study. Poster presented at the 40th Annual Meeting of the International Neuropsychological Society in Montreal, Quebec. (2012). [Google Scholar]
- 102.Stricker NH et al. Longitudinal Comparison of in Clinic and at Home Administration of the Cogstate Brief Battery and Demonstrated Practice Effects in the Mayo Clinic Study of Aging. J Prev Alzheimers Dis 7, 21–28, doi: 10.14283/jpad.2019.35 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Sanderson WC & Scherbov S Measuring the speed of aging across population subgroups. PLoS One 9, e96289, doi: 10.1371/journal.pone.0096289 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Clouston S et al. Posttraumatic stress-related cognitive and physical impairment: Clinical characterization of a novel disorder. Journal of Traumatic Stress (In Press). [Google Scholar]
- 105.Anor CJ et al. Neuropsychiatric symptoms in Alzheimer disease, vascular dementia, and mixed dementia. Neurodegenerative Diseases 17, 127–134 (2017). [DOI] [PubMed] [Google Scholar]
- 106.Mavounza C, Ouellet M-C & Hudon C Caregivers’ emotional distress due to neuropsychiatric symptoms of persons with amnestic mild cognitive impairment or Alzheimer’s disease. Aging & Mental Health 24, 423–430, doi: 10.1080/13607863.2018.1544208 (2020). [DOI] [PubMed] [Google Scholar]
- 107.Isik AT, Soysal P, Solmi M & Veronese N Bidirectional relationship between caregiver burden and neuropsychiatric symptoms in patients with Alzheimer’s disease: A narrative review. International Journal of Geriatric Psychiatry 34, 1326–1334, doi: 10.1002/gps.4965 (2019). [DOI] [PubMed] [Google Scholar]
- 108.Hongisto K et al. Quality of Life in relation to neuropsychiatric symptoms in Alzheimer’s disease: 5-year prospective ALSOVA cohort study. International Journal of Geriatric Psychiatry 33, 47–57, doi: 10.1002/gps.4666 (2018). [DOI] [PubMed] [Google Scholar]
- 109.Coughlan G, Laczó J, Hort J, Minihane A-M & Hornberger M Spatial navigation deficits—overlooked cognitive marker for preclinical Alzheimer disease? Nature Reviews Neurology 14, 496–506 (2018). [DOI] [PubMed] [Google Scholar]
- 110.Babulal GM et al. A Naturalistic Study of Driving Behavior in Older Adults and Preclinical Alzheimer Disease: A Pilot Study. Journal of Applied Gerontology 38, 277–289, doi: 10.1177/0733464817690679 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Lichtenberg PA Financial exploitation, financial capacity, and Alzheimer’s disease. Am Psychol 71, 312 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Richards M & Brayne C What do we mean by Alzheimer’s disease? BMJ 341, c4670 (2010). [DOI] [PubMed] [Google Scholar]
- 113.Pampuscenko K et al. Extracellular tau induces microglial phagocytosis of living neurons in cell cultures. J Neurochem 154, 316–329, doi: 10.1111/jnc.14940 (2020). [DOI] [PubMed] [Google Scholar]
- 114.Montagne A et al. APOE4 leads to blood–brain barrier dysfunction predicting cognitive decline. Nature 581, 71–76 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Lowe VJ et al. Widespread brain tau and its association with ageing, Braak stage and Alzheimer’s dementia. Brain 141, 271–287, doi: 10.1093/brain/awx320 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Abe K et al. A New Serum Biomarker Set to Detect Mild Cognitive Impairment and Alzheimer’s Disease by Peptidome Technology. Journal of Alzheimer’s Disease, 1–11 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Bogoslovsky T et al. Increases of Plasma Levels of Glial Fibrillary Acidic Protein, Tau, and Amyloid beta up to 90 Days after Traumatic Brain Injury. J Neurotrauma 34, 66–73, doi: 10.1089/neu.2015.4333 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Fossati S et al. DIFFERENTIAL VALUE OF PLASMA TAU AS A BIOMARKER FOR ALZHEIMER’S DISEASE AND CHRONIC TRAUMATIC BRAIN INJURY. Alzheimer’s & Dementia: The Journal of the Alzheimer’s Association 13, P1307 (2017). [Google Scholar]
- 119.Mattsson N et al. Plasma tau in Alzheimer disease. Neurology 87, 1827–1835, doi: 10.1212/WNL.0000000000003246 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Pase M et al. (AAN Enterprises, 2018). [Google Scholar]
- 121.Thijssen EH et al. Diagnostic value of plasma phosphorylated tau181 in Alzheimer’s disease and frontotemporal lobar degeneration. Nature medicine 26, 387–397 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Janelidze S et al. Plasma P-tau181 in Alzheimer’s disease: relationship to other biomarkers, differential diagnosis, neuropathology and longitudinal progression to Alzheimer’s dementia. Nature Medicine 26, 379–386 (2020). [DOI] [PubMed] [Google Scholar]
- 123.Barthélemy NR, Horie K, Sato C & Bateman RJ Blood plasma phosphorylated-tau isoforms track CNS change in Alzheimer’s disease. Journal of Experimental Medicine 217, doi: 10.1084/jem.20200861 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Mielke MM et al. Plasma phospho-tau181 increases with Alzheimer’s disease clinical severity and is associated with tau- and amyloid-positron emission tomography. Alzheimers Dement 14, 989–997, doi: 10.1016/j.jalz.2018.02.013 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Kritikos M et al. Pathway Analysis for Plasma β-Amyloid, Tau and Neurofilament Light (ATN) in World Trade Center Responders at Midlife. Neurology and Therapy, doi: 10.1007/s40120-020-00189-1 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Savjani RR, Taylor BA, Acion L, Wilde EA & Jorge RE Accelerated Changes in Cortical Thickness Measurements with Age in Military Service Members with Traumatic Brain Injury. J Neurotrauma 34, 3107–3116, doi: 10.1089/neu.2017.5022 (2017). [DOI] [PubMed] [Google Scholar]
- 127.Whitwell JL et al. Patterns of atrophy differ among specific subtypes of mild cognitive impairment. Archives of Neurology 64, 1130–1138, doi:DOI 10.1001/archneur.64.8.1130 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.O’Donnell LJ & Westin CF An introduction to diffusion tensor image analysis. Neurosurg Clin N Am 22, 185–196, viii, doi: 10.1016/j.nec.2010.12.004 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Ciccarelli O et al. Pathogenesis of multiple sclerosis: insights from molecular and metabolic imaging. Lancet Neurol 13, 807–822, doi: 10.1016/S1474-4422(14)70101-2 (2014). [DOI] [PubMed] [Google Scholar]
- 130.Faria Dde P, Copray S, Buchpiguel C, Dierckx R & de Vries E PET imaging in multiple sclerosis. J Neuroimmune Pharmacol 9, 468–482, doi: 10.1007/s11481-014-9544-2 (2014). [DOI] [PubMed] [Google Scholar]
- 131.Mielke MM et al. Plasma and CSF neurofilament light. Relation to longitudinal neuroimaging and cognitive measures 93, e252–e260, doi: 10.1212/wnl.0000000000007767 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Boyle PA et al. Much of late life cognitive decline is not due to common neurodegenerative pathologies. Annals of neurology 74, 478–489 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Wilson RS et al. Neural reserve, neuronal density in the locus ceruleus, and cognitive decline. Neurology 80, 1202–1208 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Honer W et al. Cognitive reserve, presynaptic proteins and dementia in the elderly. Translational psychiatry 2, e114–e114 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Plassman BL et al. Intelligence and education as predictors of cognitive state in late life: a 50-year follow-up. Neurology 45, 1446–1450 (1995). [DOI] [PubMed] [Google Scholar]
- 136.Deary IJ, Whalley LJ, Lemmon H, Crawford JR & Starr JM The stability of individual differences in mental ability from childhood to old age: Follow-up of the 1932 Scottish Mental Survey. Intelligence 28, 49–55 (2000). [Google Scholar]
- 137.Snowdon DA et al. Linguistic ability in early life and cognitive function and Alzheimer’s disease in late life: Findings from the Nun Study. Jama 275, 528–532 (1996). [PubMed] [Google Scholar]
- 138.Clouston SAP et al. Benefits of educational attainment on adult fluid cognition: international evidence from three birth cohorts. Int J Epidemiol 41, 1729–1736, doi: 10.1093/ije/dys148 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Vňuková M, Richards M & Cadar D How do our decisions to smoke and drink in midlife affect our cognitive performance in later life? Findings from the 1946 British Birth Cohort. Journal of Aging and Geriatric Medicine (2017). [Google Scholar]
- 140.Rawle MJ et al. Apolipoprotein-E (Apoe) ε4 and cognitive decline over the adult life course. Translational psychiatry 8, 1–8 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Yu J-T et al. Evidence-based prevention of Alzheimer’s disease: systematic review and meta-analysis of 243 observational prospective studies and 153 randomised controlled trials. Journal of Neurology, Neurosurgery & Psychiatry (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Richards M et al. Identifying the lifetime cognitive and socioeconomic antecedents of cognitive state: seven decades of follow-up in a British birth cohort study. BMJ Open 9, e024404, doi: 10.1136/bmjopen-2018-024404 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.The SPRINT MIND Investigators for the SPRINT Research Group. Effect of Intensive vs Standard Blood Pressure Control on Probable Dementia: A Randomized Clinical TrialEffect of Intensive vs Standard Blood Pressure Control on Probable DementiaEffect of Intensive vs Standard Blood Pressure Control on Probable Dementia. JAMA 321, 553–561, doi: 10.1001/jama.2018.21442 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Mintun MA et al. Donanemab in early Alzheimer’s disease. New England Journal of Medicine (2021). [DOI] [PubMed] [Google Scholar]
- 145.Xu H et al. Long Term Effects of Cholinesterase Inhibitors on Cognitive Decline and Mortality. Neurology (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Northey JM, Cherbuin N, Pumpa KL, Smee DJ & Rattray B Exercise interventions for cognitive function in adults older than 50: a systematic review with meta-analysis. British journal of sports medicine 52, 154–160 (2018). [DOI] [PubMed] [Google Scholar]
- 147.Stern Y et al. Effect of aerobic exercise on cognition in younger adults: a randomized clinical trial. Neurology 92, e905–e916 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Sloan RP et al. Aerobic exercise training and inducible inflammation: Results of a randomized controlled trial in healthy, young adults. Journal of the American Heart Association 7, e010201 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Berg A et al. Treatment of PTSD: An assessment of the evidence. (Washington, DC: National Academies Press, 2007). [Google Scholar]
- 150.Hoskins M et al. Pharmacotherapy for post-traumatic stress disorder: systematic review and meta-analysis. The British Journal of Psychiatry 206, 93–100 (2015). [DOI] [PubMed] [Google Scholar]
- 151.Berger W et al. Pharmacologic alternatives to antidepressants in posttraumatic stress disorder: a systematic review. Progress in neuro-psychopharmacology and biological psychiatry 33, 169–180 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Feder A et al. Efficacy of intravenous ketamine for treatment of chronic posttraumatic stress disorder: a randomized clinical trial. JAMA psychiatry 71, 681–688 (2014). [DOI] [PubMed] [Google Scholar]
- 153.Kelmendi B et al. PTSD: from neurobiology to pharmacological treatments. European journal of psychotraumatology 7, 31858 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Raskind MA et al. A Parallel Group Placebo Controlled Study of Prazosin for Trauma Nightmares and Sleep Disturbance in Combat Veterans with Post-Traumatic Stress Disorder. Biological Psychiatry 61, 928–934, doi: 10.1016/j.biopsych.2006.06.032 (2007). [DOI] [PubMed] [Google Scholar]
- 155.Peskind ER, Bonner LT, Hoff DJ & Raskind MA Prazosin reduces trauma-related nightmares in older men with chronic posttraumatic stress disorder. Journal of geriatric psychiatry and neurology 16, 165–171 (2003). [DOI] [PubMed] [Google Scholar]
- 156.Raskind Murray A., M.D., et al. A Trial of Prazosin for Combat Trauma PTSD With Nightmares in Active-Duty Soldiers Returned From Iraq and Afghanistan. American Journal of Psychiatry 170, 1003–1010, doi: 10.1176/appi.ajp.2013.12081133 (2013). [DOI] [PubMed] [Google Scholar]
- 157.Raskind MA et al. Trial of Prazosin for Post-Traumatic Stress Disorder in Military Veterans. New England Journal of Medicine 378, 507–517, doi: 10.1056/NEJMoa1507598 (2018). [DOI] [PubMed] [Google Scholar]
- 158.Monson CM & Shnaider P Treating PTSD With Cognitive–Behavioral Therapies Interventions That Work. (American Psychological Association, 2014). [Google Scholar]
- 159.Difede J & Eskra D Cognitive Processing Therapy for PTSD in a Survivor of the World Trade Center Bombing. Journal of Trauma Practice 1, 155–165, doi: 10.1300/J189v01n03_09 (2002). [DOI] [Google Scholar]
- 160.Wachen JS et al. Implementing Cognitive Processing Therapy for Posttraumatic Stress Disorder With Active Duty U.S. Military Personnel: Special Considerations and Case Examples. Cognitive and Behavioral Practice 23, 133–147, doi: 10.1016/j.cbpra.2015.08.007 (2016). [DOI] [Google Scholar]
- 161.Waltman SH Functional Analysis in Differential Diagnosis: Using Cognitive Processing Therapy to Treat PTSD. Clinical Case Studies 14, 422–433, doi: 10.1177/1534650115571003 (2015). [DOI] [Google Scholar]
- 162.Ehlers A & Clark DM A cognitive model of posttraumatic stress disorder. Behaviour Research and Therapy 38, 319–345, doi: 10.1016/S0005-7967(99)00123-0 (2000). [DOI] [PubMed] [Google Scholar]
- 163.Ehlers Anke, et al. A Randomized Controlled Trial of 7-Day Intensive and Standard Weekly Cognitive Therapy for PTSD and Emotion-Focused Supportive Therapy. American Journal of Psychiatry 171, 294–304, doi: 10.1176/appi.ajp.2013.13040552 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Foa E, Hembree EA, Rothbaum BO & Rauch S Prolonged Exposure Therapy for PTSDEmotional Processing of Traumatic Experiences - Therapist Guide: Emotional Processing of Traumatic Experiences - Therapist Guide. (Oxford University Press, 2019). [Google Scholar]
- 165.Bahar-Fuchs A, Clare L & Woods B Cognitive training and cognitive rehabilitation for mild to moderate Alzheimer’s disease and vascular dementia. Cochrane Database Syst Rev 2013, Cd003260, doi: 10.1002/14651858.CD003260.pub2 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Mowszowski L, Batchelor J & Naismith SL Early intervention for cognitive decline: can cognitive training be used as a selective prevention technique? Int Psychogeriatr 22, 537–548, doi: 10.1017/s1041610209991748 (2010). [DOI] [PubMed] [Google Scholar]
- 167.Bahar-Fuchs A, Martyr A, Goh AM, Sabates J & Clare L Cognitive training for people with mild to moderate dementia. Cochrane Database Syst Rev 3, Cd013069, doi: 10.1002/14651858.CD013069.pub2 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Belleville S, Mellah S, de Boysson C, Demonet JF & Bier B The pattern and loci of training-induced brain changes in healthy older adults are predicted by the nature of the intervention. PLoS One 9, e102710, doi: 10.1371/journal.pone.0102710 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Belleville S et al. Training-related brain plasticity in subjects at risk of developing Alzheimer’s disease. Brain 134, 1623–1634, doi: 10.1093/brain/awr037 (2011). [DOI] [PubMed] [Google Scholar]
- 170.Hampstead BM et al. Activation and effective connectivity changes following explicit-memory training for face-name pairs in patients with mild cognitive impairment: a pilot study. Neurorehabil Neural Repair 25, 210–222, doi: 10.1177/1545968310382424 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Edwards JD, Fausto BA, Tetlow AM, Corona RT & Valdés EG Systematic review and meta-analyses of useful field of view cognitive training. Neuroscience & Biobehavioral Reviews 84, 72–91 (2018). [DOI] [PubMed] [Google Scholar]
- 172.Edwards JD et al. Speed of processing training results in lower risk of dementia. Alzheimer’s & Dementia: Translational Research & Clinical Interventions 3, 603–611 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Vasterling JJ, Verfaellie M & Sullivan KD Mild traumatic brain injury and posttraumatic stress disorder in returning veterans: perspectives from cognitive neuroscience. Clinical psychology review 29, 674–684 (2009). [DOI] [PubMed] [Google Scholar]


