Abstract
Postural Tachycardia Syndrome (POTS) is a chronic disorder characterized by symptoms of orthostatic intolerance such as fatigue, lightheadedness, dizziness, palpitations, dyspnea, chest discomfort and remarkable tachycardia upon standing.
Non-invasive transdermal vagal stimulators have been applied for the treatment of epilepsy, anxiety, depression, headache, and chronic pain syndromes. Anti-inflammatory and immunomodulating effects after transdermal vagal stimulation raised interest for applications in other diseases. Patients with sympathetic overactivity, reduced cardiac vagal drive and presence of systemic inflammation like POTS may benefit from tVNS.
This article will address crucial methodological aspects of tVNS and provide preliminary results of its acute and chronic use in POTS, with regards to its potential effectiveness on autonomic symptoms reduction and heart rate modulation.
Introduction
Postural Tachycardia Syndrome
Postural Tachycardia Syndrome (POTS) is a chronic disorder characterized by symptoms of orthostatic intolerance such as fatigue, lightheadedness, dizziness, palpitations, dyspnea, chest discomfort and remarkable tachycardia upon standing. Positional symptoms occur without concomitant orthostatic hypotension (Freeman et al., 2011; Furlan et al., 1998; Jacob et al., 2000; Mar and Raj, 2020; Raj, 2013; Robertson, 1999). Patients affected by POTS have pronounced orthostatic distress which affects their daily living, eventually resulting in poor quality of life (Dipaola et al., 2020) and reduced work ability (Barbic et al., 2020).
Notably, a subtype of POTS patients, defined as hyperadrenergic, may display a sympathetic over-activity and a concomitant vagal impairment when supine (Furlan et al., 1998). This results in the presence of excessive catecholamine plasma titers and related signs and symptoms, such as palpitations, dyspnea, chest discomfort and tremors mostly upon standing. Such a hyperadrenergic profile along with low parasympathetic activity, leads to the hypothesis that any intervention resulting in an enhancement of the parasympathetic modulation and/or a decrease of the cardiovascular sympathetic modulation, might diminish symptom intensity. Additionally, it is likely that an increase in parasympathetic activity or modulation might also promote an anti-inflammatory effect in POTS. In this syndrome, chronic and inappropriate sympathetic activation was associated with elevated plasma titers of the systemic inflammatory marker Interleukin- 6 (IL-6) compared to healthy individuals, although a causal relationship between inflammation and POTS symptoms has not yet been elucidated (Furlan et al., 2000; Okamoto et al., 2015).
Vagal Stimulation
The idea of applying controlled electrical currents to specific human body locations with therapeutic aims has fascinated physicians and researchers since the first report of surgically implantable devices enabling the stimulation of the vagus nerves in canines (Zabara, 1992). Early clinical trials investigating the use of implanted Vagus Nerve Stimulation (iVNS) devices in pharmaco-resistant epilepsy (Penry and Dean, 1990; Uthman et al., 1993) showed the efficacy of that methodology in humans, as assessed by a remarkable reduction in seizure frequency. However, the invasiveness of that technique, clinical side effects related to direct electrical nerve stimulation, late complications due to surgery and significant costs associated with the implantable stimulator restricted its application to otherwise untreatable Central Nervous System disorders. More recently, a non-invasive technique based on transdermal electrical vagus nerve stimulation (tVNS) was developed (Yuan and Silberstein, 2016). Briefly, tVNS treatment involves the application of low-voltage electrical currents to readily accessible anatomical regions innervated by vagus nerve afferents, i.e., outer-ear cymba conchae (Figure 1) or the neck, resulting in their activation.
Figure 1.
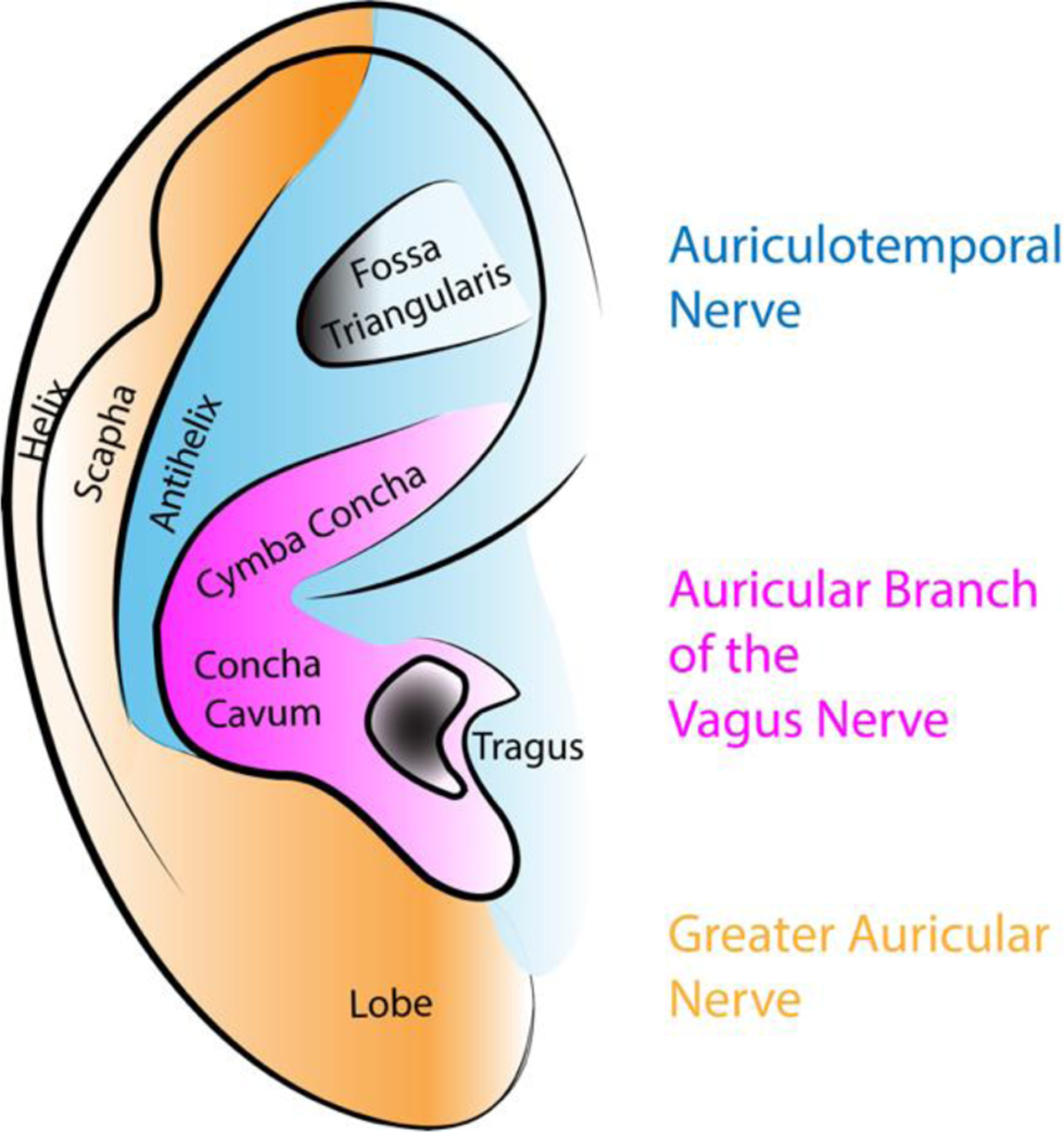
Simplified schematic of the innervation of the ear. The Cymba Concha and Concha Cavum are innervated by the Auricular Branch of the Vagus Nerve and therefore preferred anatomical regions for transdermal vagal stimulation (tVNS). The tragus and fossa triangularis have also been proposed as sites for tVNS.
Several devices were approved by American and European Regulatory Agencies for the treatment of various conditions, including epilepsy (Ardesch et al., 2007), anxiety and depression (Hein et al., 2013), primary chronic headache (Garcia et al., 2017) and chronic pain syndromes (Kirchner et al., 2000).
Recently, anti-inflammatory and immunomodulating properties of tVNS have raised interest for its possible applications in autoimmune diseases, such as Rheumatoid Arthritis and Crohn’s disease (Drewes et al., 2021; Koopman et al., 2016; Tracey, 2007). In this context, the cholinergic anti-inflammatory reflex model was hypothesized based on evidence that a potent anti-inflammatory action of acetylcholine exists and it depends on 1) activation of central cholinergic projections 2) integrity of the vagus nerve 3) effective peripheral nicotinic receptors functions (Bernik et al., 2002; Borovikova et al., 2000; Pavlov et al., 2006) 4) and intact spleen (Huston et al., 2006). The unifying model suggested that vagal activation can decrease proinflammatory cytokine production by activating nicotinic receptors present on splenic macrophages. However, consensus regarding the precise mechanism is far from being reached as evidence of vagal splenic innervation is scarce (Rosas-Ballina et al., 2008), and the hypothesis of di-synaptic connections involving vagal efferent fibers and postganglionic sympathetic fibers located in the celiac ganglion has been challenged by Bratton (Bratton et al., 2012) and colleagues’ experiments. The same research group postulated that the efferent arm of the anti-inflammatory reflex might be composed by the sympathetic nervous system alone (Martelli et al., 2019, 2014), challenging the traditional view of a direct correlation between sympathetic activity and inflammation (Cervi et al., 2014; Furlan et al., 2006). In light of these considerations, the effects of directly stimulating the vagus (iVNS) or stimulating vagal afferents (tVNS) may result in profound differences in anti-inflammatory effects (Figure 2).
Figure 2.
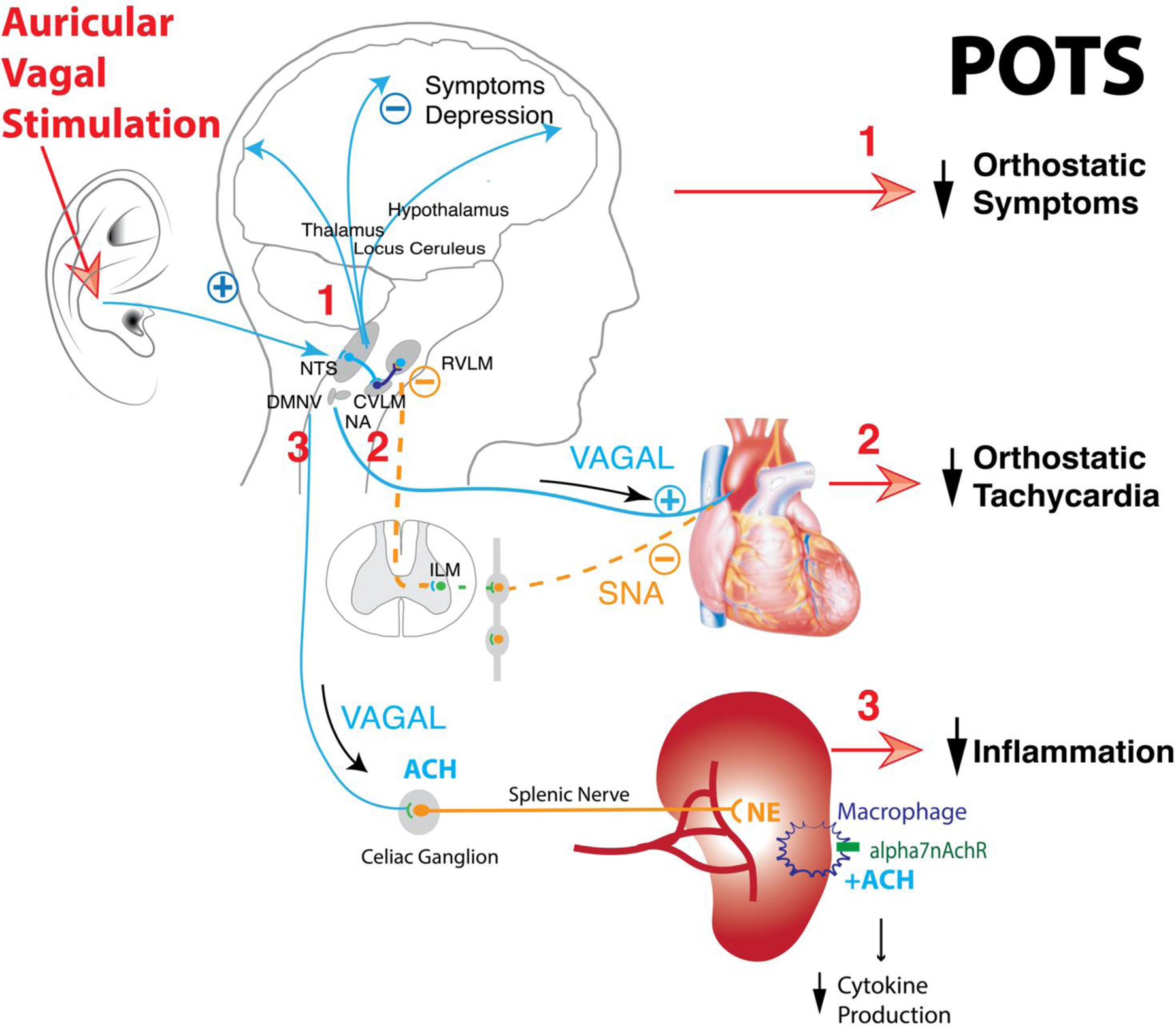
Transdermal stimulation of the auricular branch of the vagal nerve of the ear modulates vagal afferent inputs which activate regions of the brain such as the locus coeruleus, the thalamus, the prefrontal cortex, the postcentral gyrus, the posterior cingulate gyrus and the insula cortex. This can modulate perception and mood (1). Projection via nucleus tractus solitary (NTS) increases activity of neurons in the caudal ventrolateral medulla (CVLM), increases inhibition of sympathetic pacemaker neurons in the rostral ventrolateral medulla (RVLM), and increases vagal activity in the nucleus ambiguous (NA). Increased vagal activity and reduced sympathetic neural activity (SNA) to the sinus atrial node reduces heart rate (2). Vagal stimulation mobilizes the cholinergic anti-inflammatory pathway through sympathetic axons supplying the spleen. It increases norepinephrine (NE) release in the spleen. NE activates beta2 receptors expressed in splenic macrophages and attenuates cytokine production when signaling through nicotinic acetylcholine receptor 7 subunit (alpha7nAchR) is present (3). All these effects are beneficial for patients with postural tachycardia syndrome (POTS).
An additional effect of vagal stimulation might be the modulation of persistent cardiac sympathetic overactivity. Previous studies documented that a persistent prevalence of cardiac sympathetic modulation is a relevant maladaptive mechanism resulting in increased cardiovascular morbidity and mortality (Barretto et al., 2009; De Ferrari et al., 2011; Malliani and Montano, 2004; Zoccali et al., 2002). Indeed, well-known and documented associations exist between excessive cardiovascular sympathetic tone and essential hypertension (Mancia and Grassi, 2014), persistent atrial fibrillation (Chen et al., 2014), ischemic heart disease (Malliani and Montano, 2004) and heart failure (Florea and Cohn, 2014). In this context, disorders potentially characterized by sympathetic overactivity, reduced cardiac vagal drive and presence of systemic inflammation, including POTS (Furlan et al., 2006; Okamoto et al., 2015), may theoretically benefit from tVNS as illustrated in Figure 2.
Many detailed reviews about the mechanism and application of vagal stimulation in patients with epilepsy, chronic pain, and other diseases are available (Beekwilder and Beems, 2010; Ellrich, 2011; Farmer et al., 2021; He et al., 2012; Johnson and Wilson, 2018; Kaniusas et al., 2019b, 2019a; Yap et al., 2020). In this article, we will address crucial methodological aspects of tVNS and provide preliminary results of its acute and chronic use in POTS, with regards to its potential effectiveness on autonomic symptoms reduction and heart rate modulation.
Meta Research
We reviewed current literature on the topic using the main scientific research engines: PubMed, UpToDate, Google Scholar, CiteSeer, GetCITED, Microsoft Academic Research, Bioline International, Directory of Open Access Journals, PLOS ONE, BioOne, Science and Technology of Advanced Material.
The following keywords were used: vagal nerve stimulation (VNS), VNS effects on cardiovascular system, VNS effects on heart rate and VNS effects on blood pressure. The initial search revealed 1103 papers.
After reviewing the abstracts, we used exclusion parameters such as duplicate articles, review articles, abstract only papers, animal model studies and non-English language published studies, to reduce the number of articles to 169.
During our analysis of those 169 original works, we ultimately focused on those presenting data on heart rate, blood pressure, heart rate variability, vagal, sympathetic, and inflammatory markers. These papers concerning the effects of VNS (26 in total) contained information on both tVNS and invasive VNS (iVNS) delivery. Results are summarized in tables 1 (iVNS) and table 2 (tVNS).
Table 1.
Reported effects of implanted vagal stimulators on autonomic cardiovascular parameters, inflammation, and symptoms in humans.
| Author | Sample | Site & parameters | Main outcomes |
||||||
|---|---|---|---|---|---|---|---|---|---|
| HR | Vagal indices | LF/HF | BP | MSNA | Infl | Symp | |||
| Nearing et al. (2021) | Heart Failure N= 21 |
Right vs left cervical impl. 10–50 Hz, 250 µs pw, 1.5–3 mA |
↓ | ↓ | = | • | • | • | • |
| Bonaz et al. (2016) | Chron disease N = 7 |
Left cervical impl. 10 Hz, 500 µs pw, 0,25 mA |
• | ↑ | = | • | • | ↓ | ↓ |
| Koopman et al. (2016) | Rheumatoid arthritis N= 17 |
Left cervical impl. 20 Hz, 500 µs pw, 0,25–2,0 mA |
• | • | • | • | • | ↓ | ↓ |
| De Ferrari et al. (2014) | Heart failure N= 32 |
Right cervical impl. 1 pulse/hb, 5.5 mA |
↓ | ↑ | • | = | • | • | ↓ |
| Sperling et al. (2010) | Depression N = 9 |
Left cervical impl. | ↓ | ↑ | • | • | • | • | = |
| Barone et al. (2007) | Epilepsy N= 8 |
Left cervical impl. 30 Hz, 500 ms pw, 0.75–1.75 mA |
= | = | = | • | • | = | • |
| Ronkainen et al. (2006) | Epilepsy vs healthy N= 14 vs 28 |
Impl. left cervical 30 Hz, 500 µs pw, |
= | = | = | • | • | • | ↓ |
| Setty et al. (1998) | Epilepsy N= 10 |
Impl. left cervical 30 Hz, 750 µs pw, |
= | = | = | • | • | • | • |
| Kamath et al. (1992) | Epilepsy N = 8 |
Impl. left cervical 30 Hz, 500 µs pw VS 2 Hz, 130 µs pw |
= | ↑ | ↓ | • | • | • | • |
HR, heart rate; LF/HF, ratio between the low and high frequency components of heart rate variability; BP, blood pressure; MSNA, muscle sympathetic nerve activity; Infl, inflammation; Symp, symptoms; pw, pulse width; Impl, implant; ↑ increased; = unchanged; ↓ decreased.
Table 2.
Reported effects of transdermal vagal stimulation on autonomic cardiovascular parameters, inflammation, and symptoms in humans.
| Author | Sample | Site & parameters | Main outcomes |
||||||
|---|---|---|---|---|---|---|---|---|---|
| HR | Vagal indices | LF/HF | BP | MSNA | Infl | Symp | |||
| Aranow et al. (2021) | SLE N= 18 |
Left concha/posterior ear 30 Hz, 300 µs pw. max tolerated |
• | • | • | • | • | = | ↓ |
| Paleczny et al. (2021) | Healthy controls N= 12 |
25 Hz, 1000 µs/phase, 30 µs interphase interval. 10 µA, 80% pain threshold | ↓ | = | = | = | • | • | • |
| Drewes et al. (2021) | Rheumatoid arthritis N = 36 |
Cervical vagus nerve 1 ms, 5 sine waves, 200us; 1burst/40 ms, 25 Hz, 60 mA, 24 V |
• | ↓ | • | • | • | ↓ | ↓ |
| Gauthey et al. (2020) | Healthy males N = 23 |
Right cymba 5–20 Hz, 200 µs pw, sensory perception Right Lobe (sham): 5 Hz, 200 µs pw, sensory perception |
↓ | = | ↑ | = | ↓ | • | • |
| Borges et al. (2019) | Healthy controls N = 61 |
Left cymba conchae 25 Hz, 200–300 µs, 0.1–1.0 mA, on-off 30s cycle, different intensities |
• | ↑ | • | • | • | • | • |
| Tobaklini et al. (2019) | Healthy controls N = 13 |
Left cymba conchae 25 Hz, 200 µs pw, 1–6 mA |
↓ | = | = | = | • | • | • |
| Badran et al. (2018) | Healthy controls N= 15 |
Left tragus 1/10/25 Hz; 100/200/500 µs; 60s; 200% pain threshold |
↓ | • | • | • | • | • | • |
| Colzato et al. (2018) | Healthy males N = 32 |
Left cymba conchae 0.5 mA 200–300 µs at 25 Hz, 30 min |
↓ | • | • | • | • | • | • |
| Antonino et al. (2017) | Healthy males N = 13 |
Left tragus 30 Hz, 200 µs pw 10–50 mA |
↓ | = | ↓ | = | • | • | • |
| De Couck et al. (2017) | Healthy controls N = 30 |
Left cymba conchae vs right cymba conchae 25 Hz, 250 µs pw, ±1 mA 30s; 50% pain threshold |
• | ↑ | ↑ | • | • | • | • |
| Wang et al. (2015) | Myocardial Infarction N = 42 |
Left tragus vs right tragus 30 Hz, 200 µs pw, 10–50 mA |
• | • | ↓ | • | • | • | • |
| Stavrakis et al. (2015) | Atrial Fibrillation N = 42 |
Right tragus 20 Hz; 250–200 ms/cyde |
↓ | • | • | • | • | ↓ | • |
| Clancy et al. (2014) | Healthy controls N = 4S |
Tragus 30 Hz, 200 µs pw, 10–50 mA | ↓ | = | ↓ | • | ↓ | • | • |
| Popov et al. (2013) | Coronary artery disease N = 43 |
Bilateral cymba concha 3 Hz, 0.2– 1.5 mA, 1.5 ms, |
↓ | • | • | • | • | • | ↓ |
| Rong et al. (2012) | Depression N= 49 |
Cymba concha 20 Hz, 1 mA | • | • | ↓ | • | • | • | • |
| Zamotrinsky et al. (2001) | Preoperative coronary artery disease N = 38 |
Bilateral cymba concha 0.2–1.5 mA, 1.5 ms, 3 Hz |
↓ | • | • | ↓ | • | • | ↓ |
| Zamotrinsky et al. (2001) | Preoperative coronary artery disease N = 20 |
Bilateral cymba concha 0.2–1.5 mA, 1.5 ms, 3 Hz |
• | • | • | • | • | • | ↓ |
HR, heart rate; LF/HF, ratio between the low and high frequency components of heart rate variability; BP, blood pressure; MSNA, muscle sympathetic nerve activity; lnflam, inflammation; Symp, symptoms; pw, pulse width; ↑ increased; — unchanged; ↓ decrease.
Invasive Vagus Nerve Stimulation
Among the 9 articles exploring the iVNS technique, only 7 focused on the effects on heart rate (Barone et al., 2007; De Ferrari, 2014; Kamath et al., 1992; Nearing et al., 2021; Ronkainen et al., 2006; Setty et al., 1998; Sperling et al., 2010). Out of these, 3 articles successfully provided evidence of mean heart rate decrease after stimulation (De Ferrari, 2014; Nearing et al., 2021; Sperling et al., 2010) while the remaining 4 studies described no changes in mean heart rate after iVNS (Barone et al., 2007; Kamath et al., 1992; Ronkainen et al., 2006; Setty et al., 1998). It is important to emphasize that all three studies with evidence of decreased heart rate during stimulation included patients with heart failure or depression, whereas the negative studies dealt with subjects affected by epilepsy. Moreover, only one article investigated the effect of VNS on blood pressure (De Ferrari, 2014): no changes in blood pressure following iVNS were observed. We could not find studies on the effects of iVNS on muscle sympathetic nerve activity (MSNA).
The effects of iVNS on heart rate variability were addressed in 6 articles (Barone et al., 2007; Bonaz et al., 2016; Kamath et al., 1992; Nearing et al., 2021; Ronkainen et al., 2006; Setty et al., 1998). Only one showed changes in heart rate variability parameters, namely a decrease in ratio of low and high frequency power (LF/HF) of heart rate variability after iVNS (Kamath et al., 1992).
The possible effect of iVNS on inflammation has been investigated in 3 studies (Barone et al., 2007; Bonaz et al., 2016; Koopman et al., 2016). Two of them showed a decrease in the inflammatory markers (Bonaz et al., 2016; Koopman et al., 2016) while in the remaining one no effects were observed (Barone et al., 2007).
From the clinical standpoint, 5 articles focused on potential symptoms changes after iVNS (Barone et al., 2007; Bonaz et al., 2016; De Ferrari, 2014; Koopman et al., 2016; Sperling et al., 2010). Out of those, 4 studies showed a decrease in symptoms intensity (Barone et al., 2007; Bonaz et al., 2016; De Ferrari, 2014; Koopman et al., 2016), whereas one investigation showed no change in disease-related symptoms (Sperling et al., 2010). Importantly, symptoms changes refer to the different diseases taken into account in each single study.
In two case reports patients with POTS underwent implantation of a vagal stimulator for the treatment of epilepsy (Early and Stankovic, 2018; von Wrede et al., 2019). Results of iVNS concerning the chronic effects of vagal stimulation are provided below.
Transdermal Vagus Nerve Stimulation
Review of the literature about the effects of tVNS on heart rate showed a decrease in heart rate in 10 articles (Antonino et al., 2017; Badran et al., 2018; Clancy et al., 2014; Colzato et al., 2018; Gauthey et al., 2020; Paleczny et al., 2021; Stavrakis et al., 2015; Tobaldini et al., 2019; Zamotrinsky et al., 2001). In studies demonstrating a reduction in mean heart rate after tVNS, the stimulation frequency was set around 25–30Hz. Only 5 papers presented data dealing with potential effects of tVNS on blood pressure (Antonino et al., 2017; Gauthey et al., 2020; Paleczny et al., 2021; Tobaldini et al., 2019; Zamotrinsky et al., 2001). Out of them, four studies demonstrated a clear effect of tVNS on blood pressure (Antonino et al., 2017; Gauthey et al., 2020; Paleczny et al., 2021; Tobaldini et al., 2019) while one showed a decrease in blood pressure (Zamotrinsky et al., 2001).
The effects of tVNS on heart rate variability were studied in 8 different investigations (Antonino et al., 2017; Clancy et al., 2014; De Couck et al., 2017; Gauthey et al., 2020; Paleczny et al., 2021; Rong et al., 2012; Tobaldini et al., 2019; Wang et al., 2014). Out of these, two studies showed no change under electrical stimulation (Paleczny et al., 2021; Tobaldini et al., 2019), two studies showed an increase in parameters reflecting cardiac vagal modulation (De Couck et al., 2017; Gauthey et al., 2020) whereas the remaining four studies showed a decrease in the same vagal related indices (Antonino et al., 2017; Clancy et al., 2014; Rong et al., 2012; Wang et al., 2014). Furthermore, tVNS was associated with a decrease in the LF/HF ratio in two different studies when the electrical stimulation was applied on the tragus (Antonino et al., 2017; Clancy et al., 2014), while two studies showed an increase in the LF/HF ratio when the electrical stimulus was delivered at the cymba site (De Couck et al., 2017; Gauthey et al., 2020).
tVNS and direct recordings of muscle sympathetic nerve activity (MSNA) were investigated in two studies only. Both found a decrease in MSNA (Clancy et al., 2014; Gauthey et al., 2020).
The effects of tVNS on inflammatory marker plasma titers were addressed in three studies. One study observed no effects (Aranow et al., 2021). The other two investigation showed a decrease of inflammatory markers (Drewes et al., 202; Stravrakis et al., 2015)
Regarding the potential changes induced by tVNS on pain and related symptoms intensity, five studies found a significant decrease of symptomatology after tVNS (Zaotrinsky et al., 1997; Zamotrinsky, Kondratiev and de Jong, 2001; Aranow et al., 2021; Drewes et al., 2021).
In summary, our meta research suggests that vagal stimulation may have beneficial effects on symptom intensity and may reduce mean heart rate and plasma titers of systemic inflammation biochemical markers in different disorders. Presently, these aspects have never been considered in a unitary and systematic manner in POTS.
Methodology of Transdermal Vagus Nerve Stimulation
Site of stimulation
Although cardiac parasympathetic innervation seems to be equally distributed between the right and left vagus nerves, experiments performed on conscious dogs (Ardell and Randall, 1986) showed that low-voltage electrical stimulation of the right vagus nerve produced a greater degree of bradycardia (Randall et al., 1986).
These findings were later supported by human studies reporting that 1) neuro-cardiovascular sympathetic interactions are predominantly modulated by the right cerebral hemisphere and 2) the expression of peripheral vascular sympathetic activity exhibits right predominance, at least in right-handed healthy individuals (Diedrich et al., 2009). Evidence suggesting right-sided lateralization of autonomic functions is crucial, as the choice of a specific stimulation site may be necessary to achieve specific therapeutic effects.
In addition, the finding of diverging changes in the indices of autonomic activity observed in previous studies may highlight the importance of the site of stimulation, for example the cymba conchae versus the tragus (Antonino et al., 2017; Clancy et al., 2014; De Couck et al., 2017; Gauthey et al., 2020). This suggests that site specificity must be taken into account when planning the stimulation procedure. However, a systematic methodological study of this issue is still lacking.
Stimulation mode
Frequency and pulse width are important variables of vagal stimulation on brain activation. Mu et al. (Mu et al., 2004) found that a short pulse width of 130μs produced significantly less overall activation in the human brain than longer pulse widths of 250μs and 500μs. Several authors used transdermal stimulation at frequencies from 1 to 30Hz and pulse width from 250 to 1000μs. The strength of stimulation (current in mA) also plays an important role (Table 1 and 2). Stimulation can be delivered at subsensory level, sensory level, or maximal tolerance. Stimulation at 25Hz, 250μs pulse width, 30 seconds on/30 seconds off, for 4 hours a day are typically applied to reduce the number of seizures in patients with epilepsy (Hamer and Bauer, 2019). It is unknown what stimulation mode might be optimal for patients with POTS.
Optimal stimulation frequency to enhance cardiac vagal modulation
Diedrich et al. systematically studied the response to stimulation with different frequencies on heart rate variability using randomized transdermal electrical stimulation of the right auricular branch of the vagus nerve at subsensory levels in 14 patients with POTS (age 31±12 years, BMI 22.6±3.9 kg/m2) (Diedrich et al., 2018). The sensory threshold was determined by increasing the current intensity (starting from 0 up to 4.5mA) until the subject sensed a tingling sensation without pain and discomfort. This process was repeated to validate the threshold value. The sub-sensory threshold was determined by lowering the current until the stimulation was not felt by the subject. The threshold was verified when the subject could no longer distinguish between no stimulation (sham) and a sub-sensory stimulation. A protocol consisting of 5-minute recording blocks of subsensory stimulations at stimulation frequencies between 1 and 100Hz (rectangular waveform, pulse width 300µs, length 1ms) was performed in randomized order in the supine resting position. This approach allowed us to control for any placebo and time effects of stimulation. Figure 3 shows that the high frequency band (HF, 0.15–0.4Hz) of heart rate variability (HRV) obtained by power spectrum analysis increased with stimulation frequency. There was a non-significant prolongation of mean R-R interval. Blood pressure did not change. This pilot study showed that it is possible to increase cardiac vagal modulation, as assessed by the HF component of RR variability, during transdermal stimulation in POTS.
Figure 3.
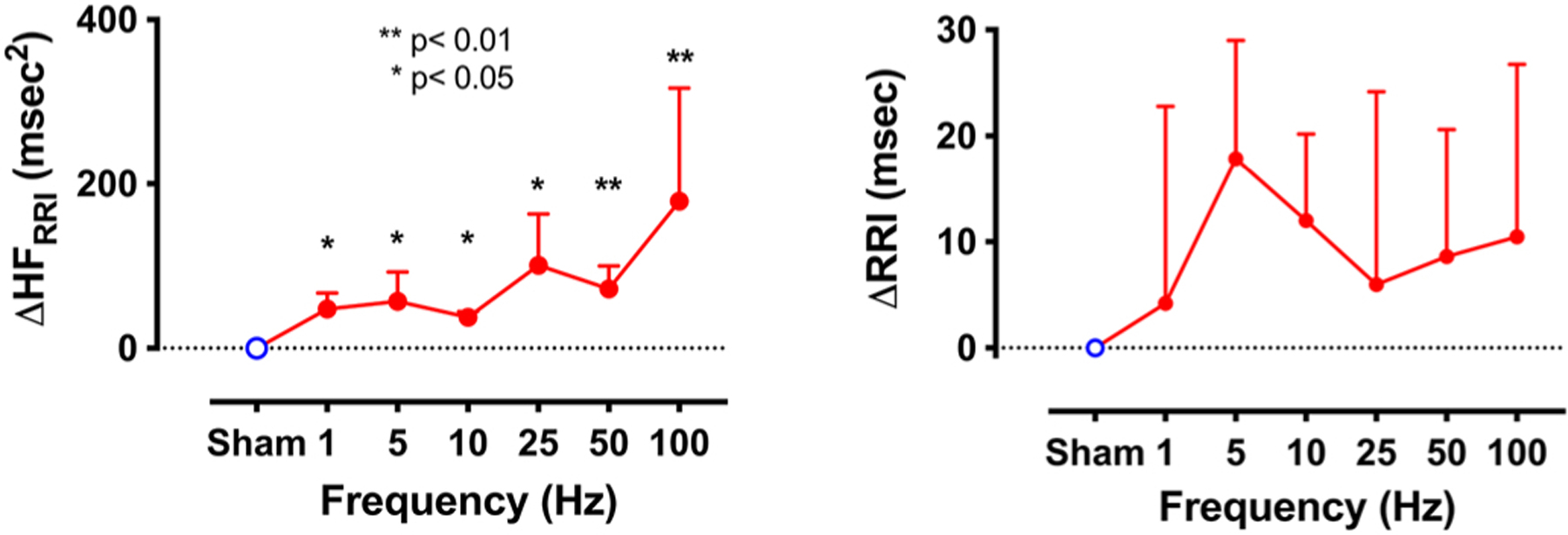
Frequency response of high frequency component of heart rate variability (HF RRI) and R-R intervals (RRI) to randomized transdermal stimulation of the right auricular branch of Vagus nerve at subsensory levels in patients with POTS and low resting HF component. Values are expressed as mean±SD.
Safety and adverse effects
Early et al. reported a case of a 58-year-old female patient who experienced sensorineural hearing loss (SNHL) after prolonged application of vagal transdermal stimulation. SNHL reversed to normal hearing after discontinuing the use of the vagal stimulator. This adverse effect could be caused by comorbidities of preexisting nonfluctuating SNHL. It was also discussed that SNHL was caused by a small current imbalance of the bipolar stimulation (Early and Stankovic, 2018). Other data obtained in patients without a history of cardiac disease and suffering from tinnitus suggest that long-term tVNS application of up to 6 months may be considered safe (Kreuzer et al., 2014).
Comparison between Invasive Vagal Stimulation and Transdermal Vagal Stimulation
Common invasive vagal nerve stimulation (iVNS) therapy requires the surgical implantation of electrodes, which seems safe and well tolerated. However, adverse events such as infection and dysrhythmias during the surgical procedure or dysrhythmias, voice alteration, paresthesia, cough, headache, dyspnea, pharyngitis and pain during stimulation have been reported (Beekwilder and Beems, 2010; Ben-Menachem, 2001; Ben-Menachem et al., 1994). Non-invasive transdermal vagal stimulation (tVNS) is an alternative delivery option that eliminates the need for surgical implantation and its associated risks. tVNS eliminates the adverse effects related to on−off stimulation cycles of implantable devices (Goadsby et al., 2014; Jürgens and Leone, 2013) and is a less expensive procedure. tVNS could be an alternative treatment in patients with comorbidities that exclude them from surgical procedures.
The vagal nerve consists of 80% afferent and 20% efferent fibers (Grimonprez et al., 2015). Therefore, iVNS has direct efferent effects on end organs (i.e.., heart) and direct afferent effects to the brain. Auricular tVNS has mainly direct afferent effects to the brain.
Efficacy cannot be compared between the two modalities of VNS stimulation at the current time as the delivery systems are in different stages of development. One common observation for implanted VNS is a consistent improvement over a period of about 18 months (Ben-Menachem, 2001; Morris et al., 2013; Nahas et al., 2005, 2005; Ryzí et al., 2013; Siddiqui et al., 2010). No long-term efficacy data are available for tVNS.
Clinical Studies with tVNS in POTS
Presently, the following three clinical trials exploiting tVNS in POTS have been registered at clincaltrials.gov.
Transdermal Vagal Stimulation for POTS (NCT02281097, PI Diedrich, Vanderbilt University, Nashville, TN). This is a pilot study. In a randomized order, tVNS and sham stimulations are acutely applied. (Diedrich et al., 2018). Outcomes: Heart rate, heart rate variability and symptom intensity changes during graded stepwise 15° head-up tilt after tVNS or sham stimulation.
Vagal Stimulation in POTS: The Autonomic Inflammatory Reflex (Pilot 3) (NCT03124355, PI Biaggioni, Vanderbilt University, Nashville, TN). This clinical trial applies tVNS with placebo or in combination with two medications (galantamine and pyridostigmine) acutely in POTS. Outcomes: The effect on orthostatic response on symptoms and inflammatory markers plasma titers are studied. Modification in the high frequency component of heart rate variability during head-up tilt, before and after intervention.
-
Long-term Effects of Transcutaneous Vagus Nerve Stimulation on Postural Orthostatic Tachycardia Syndrome (POTS-VAG) (NCT04632134 Study Director Raffaello Furlan, PI Dana Shiffer, Humanitas Research Hospital, Rozzano, Italy). This clinical trial aims to apply tVNS chronically, i.e. for 4 hours a day for 14 days, in POTS. The modifications induced by tVNS in the response of heart rate, blood pressure and MSNA to a gravitational challenge (15° stepwise head-up tilt, up to 75°) are under investigation.
Outcomes: changes in symptom intensity, in the hemodynamic and in the autonomic profile (i.e., in heart rate variability, plasma catecholamines titers and MSNA).
Acute Stimulation in POTS
While most researchers focus on the hyperadrenergic features of POTS, there is evidence of parasympathetic cardiovagal impairment in these patients. For instance, a diminished vagal marker of heart rate variability and abnormalities in the cardiovagal component of the Composite Autonomic Scoring Scale (CASS) have been previously reported (Diedrich et al., 2018; Jacob et al., 2019; Okamoto et al., 2015) A rational hypothesis would be that transdermal electrical stimulation of the auricular branch of the vagus nerve will enhance cardio-vagal modulation, reduce heart rate and upright symptoms, and improve orthostatic tolerance in POTS.
Diedrich et al. studied 14 patients with POTS (Diedrich et al., 2018). Sham or transdermal electrical vagal stimulation below perception threshold was applied in random order to the auricular branch in the right ear while supine and during a graded tilt. Patients with low vagal modulation (high frequency HFRR < 200ms2) responded to vagal stimulation (Kruskal Wallis p=0.01, n=7) with significant increase in HF power where the most consistent effect was found at 50Hz (Figure 2, stimulation at 50Hz, delta: +51±10 ms2, p=0.0032). Vagal stimulation during upright tilt tended to reduce orthostatic tachycardia and the overall orthostatic symptom score. tVNS improved tilt time significantly (delta: +5.3±2.6 min, p=0.0156) and there was a tendency toward blunted heart rate increase during standing (Figure 4). Patients with higher baseline vagal modulation (HF ≥ 200 ms2) did not respond to vagal stimulation (interaction p=0.41). This proof-of-concept study indicates that auricular transdermal vagal stimulation improves supine cardio-vagal function in POTS patients with low vagal modulation. Further research will determine if this approach can be used therapeutically, alone or in combination with other therapies.
Figure 4.
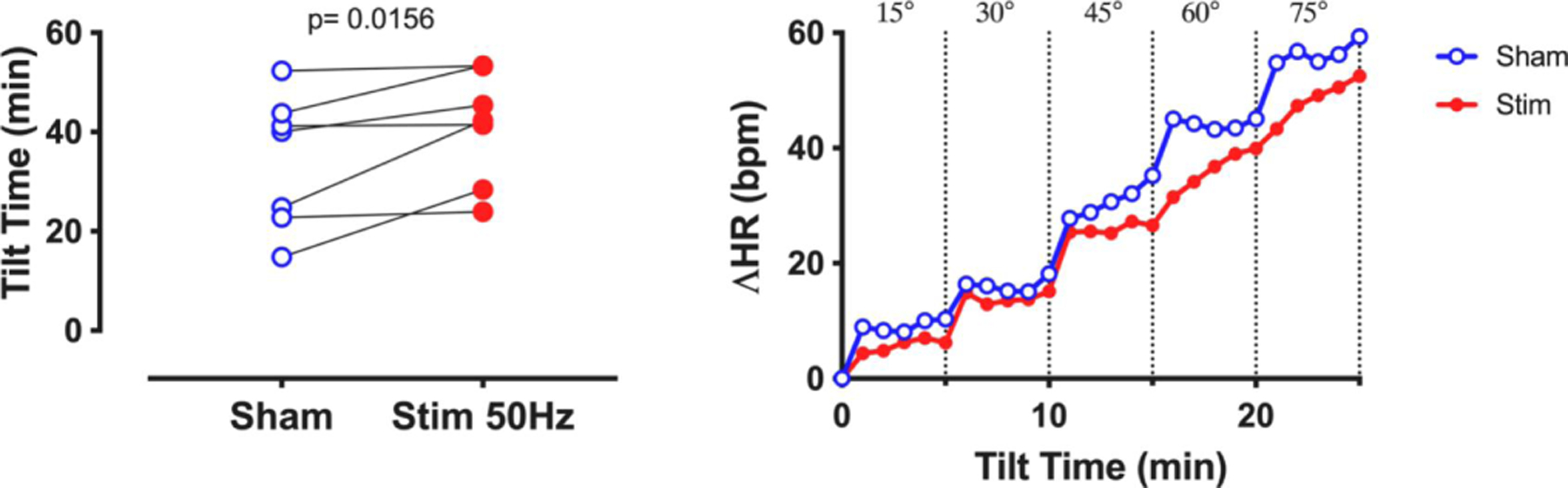
Improvement of orthostatic intolerance (Tilt Time, left panel) and tendency of reduction of heart rate response (ΔHR) after transdermal stimulation of the right auricular branch of the vagus nerve at subsensory levels in patients with POTS and low resting HF component.
Chronic Stimulation in POTS
Previous studies reported that acute stimulation could improve orthostatic tolerance in POTS (Diedrich et al., 2018; Shiffer et al., 2019). However, it is still unestablished whether such a positive outcome persists for a longer time period and if chronic application would be effective without the occurrence of major side effects. Initial attempts evaluating the clinical effectiveness of chronic stimulation in POTS were based on the use of implanted Vagus nerve stimulators. In this context, Lankford et al. (Lankford et al., 2015) reported the case of a nine-year-old female with history of intractable epilepsy, dysautonomia and developmental delay related to confirmed genetic abnormalities. The patient underwent implant of the vagus stimulator for treating epilepsy. An improvement in dysautonomia symptoms with the stimulator switched on was found. Importantly, these beneficial effects were abolished when the stimulator was turned off (Lankford et al., 2015). Gazde and colleagues (Petelin Gadze et al., 2018) reported the case of a patient with resistant epilepsy, who was referred for the iVNS procedure. As part of pre-operative workup, a head-up tilt was performed showing orthostatic symptoms and excessive tachycardia consistent with a possible POTS diagnosis. A second tilt test, performed after Vagus nerve stimulator implantation, showed disappearance of orthostatic intolerance symptoms. These case reports indicate the potential effectiveness of Vagus stimulation in improving orthostatic intolerance symptoms.
After evaluating the acute effects of tVNS on orthostatic tolerance and mean heart rate increment during orthostatic stress (Shiffer et al., 2019), Shiffer et al. expanded their study to evaluate the effects of a chronic right Vagus nerve stimulation in patients affected by the hyperadrenergic type of POTS. They studied 9 patients undergoing chronic electrical tVNS of the right cymba conchae by means of the Nemos© device (Cerbomed, Germany). Subjects were enrolled and evaluated at baseline (PRE) by continuously recording the ECG, non-invasive beat-by-beat blood pressure, respiratory activity both at rest and during a 75° head-up tilt test. Thereafter, patients underwent a stimulation protocol consisting of 1-hour stimulation blocks, 4 times a day, for 14 days. After completion, they came back for a follow-up evaluation (POST) identical to PRE.
Notably, stimulation was applied using a pulse width of 200μs and a squared impulse waveform with the stimulator switched on for 30 seconds and off for 30 seconds. The stimulation frequency was 25Hz and the electrical current was set to the highest intensity without causing patient discomfort (population mean 1.8mA±0.2).
A strength of this study is that the authors objectively quantified the whole spectrum of dysautonomia symptoms POST vs PRE using the validated Composite Autonomic Symptom Score (COMPASS-31) tool. Briefly, the COMPASS-31 scoring system yields individual autonomic domains symptoms burden and a total score, which is considered as a general summary index. Single domains and total score are expressed as a percentage from 0 to 100, the lowest indicating symptom absence and the highest indicating the greatest symptom intensity.
Analysis of POST vs PRE questionnaires revealed a statistically significant reduction (P<0.05) of orthostatic intolerance and gastrointestinal symptom domains percentages and of total COMPASS score, after chronic tVNS. Figure 5 shows individual and mean values of COMPASS-31 domains and total score before and after treatment. In addition, the effects of vagal stimulation on heart rate were assessed as mean heart rate difference between the 75° head-up tilt and the supine position. Of note, after 14 days stimulation, gastrointestinal, orthostatic and total scores were lower than those observed at baseline condition, suggesting the efficacy of chronic tVNS in decreasing symptoms intensity. Moreover, the magnitude of increase in heart rate induced by the upright posture tended to be blunted after chronic tVNS compared to baseline, although this finding was not statistically significant. These results are encouraging but will need to be validated by randomized controlled studies.
Fig. 5.
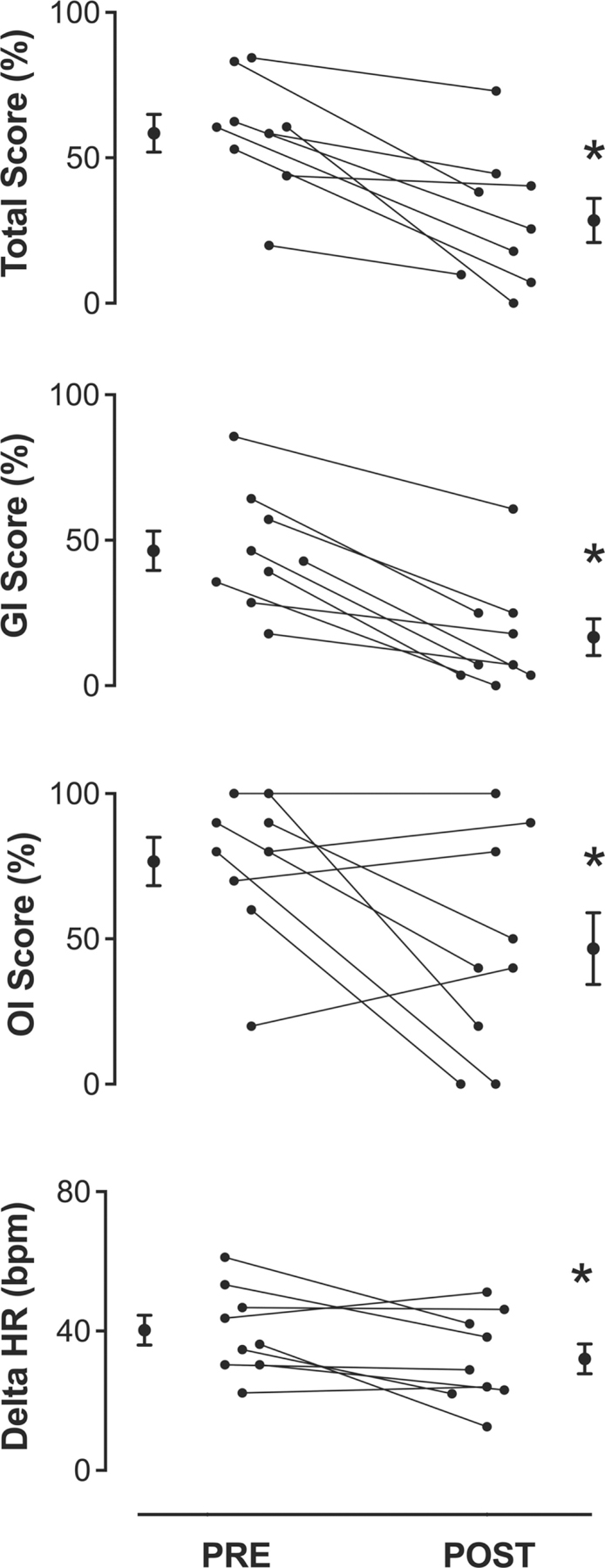
Dysautonomia symptoms and HR changes (Delta) after chronic tVNS. Individual values of COMPASS-31 domains and delta HR after 14-day tVNS are shown together with their mean ± SEM values. The reduction in dysautonomia symptom intensity is significant in Total Score and in Orthostatic Intolerance and Gastrointestinal symptom domains. The individual difference between supine and orthostatic heart rate was blunted, although not significantly, during POST evaluation. GI indicates gastrointestinal domain. OI, orthostatic intolerance. Delta HR, mean value of the individual differences between orthostatic and clinostatic heart rate. PRE, pre-treatment. POST, post-treatment.
*P < 0.05 POST vs PRE; data are expressed as mean ± SEM.
SUMMARY
In this paper, we performed a systematic review that addressed the topic of non-invasive tVNS as an emerging tool targeting the vagal impairment and hyperadrenergic state occurring in POTS (Furlan et al., 2000; Okamoto et al., 2015). In addition, activation of the vagus nerve may theoretically decrease the concomitant chronic inflammatory state by activating the cholinergic anti-inflammatory pathway (Bonaz et al., 2016; Drewes et al., 2021; Furlan et al., 2006; Koopman et al., 2016; Tracey, 2007). We highlighted that several variables such as stimulation site, pulse width, frequency and amplitude of the electric current are believed to critically influence efferent parasympathetic activation and therefore tVNS efficacy. In addition, preliminary results from studies evaluating acute stimulation in POTS (Diedrich et al., 2018; Shiffer et al., 2019) suggest that tVNS may effectively increase cardiac vagal modulation in patients characterized by cardiovagal impairment. Of note, these findings are coupled with an increase in tilt tolerance time and an improvement in orthostatic intolerance symptoms.
Similarly, initial observations from an ongoing chronic tVNS study confirmed the cardiovascular modifications induced by acute tVNS. The study also showed that stimulation for 4 hours/day for 14 days was associated with a reduction of overall dysautonomia symptoms. Chronic tVNS was well tolerated and it was not associated with significant side effects.
These promising results suggest that tVNS might be a useful and safe treatment tool in POTS with reduced vagal activity. Additional studies are necessary to explore its combination with existing treatments and to define optimal stimulation parameters.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Antonino D, Teixeira AL, Maia-Lopes PM, Souza MC, Sabino-Carvalho JL, Murray AR, Deuchars J, Vianna LC, 2017. Non-invasive vagus nerve stimulation acutely improves spontaneous cardiac baroreflex sensitivity in healthy young men: A randomized placebo-controlled trial. Brain Stimulat 10, 875–881. [DOI] [PubMed] [Google Scholar]
- Aranow C, Atish-Fregoso Y, Lesser M, Mackay M, Anderson E, Chavan S, Zanos TP, Datta-Chaudhuri T, Bouton C, Tracey KJ, Diamond B, 2021. Transcutaneous auricular vagus nerve stimulation reduces pain and fatigue in patients with systemic lupus erythematosus: a randomised, double-blind, sham-controlled pilot trial. Ann. Rheum. Dis 80. [DOI] [PubMed] [Google Scholar]
- Ardell JL, Randall WC, 1986. Selective vagal innervation of sinoatrial and atrioventricular nodes in canine heart. Am. J. Physiol. - Heart Circ. Physiol 251. [DOI] [PubMed] [Google Scholar]
- Ardesch JJ, Buschman HPJ, Wagener-Schimmel LJJC, van der Aa HE, Hageman G, 2007. Vagus nerve stimulation for medically refractory epilepsy: A long-term follow-up study. Seizure 16. [DOI] [PubMed] [Google Scholar]
- Badran BW, Dowdle LT, Mithoefer OJ, LaBate NT, Coatsworth J, Brown JC, DeVries WH, Austelle CW, McTeague LM, George MS, 2018. Neurophysiologic effects of transcutaneous auricular vagus nerve stimulation (taVNS) via electrical stimulation of the tragus: A concurrent taVNS/fMRI study and review. Brain Stimulat 11, 492–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbic F, Minonzio M, Cairo B, Shiffer D, Zamuner AR, Cavalieri S, Dipaola F, Magnavita N, Porta A, Furlan R, 2020. Work ability assessment and its relationship with cardiovascular autonomic profile in postural orthostatic tachycardia syndrome. Int. J. Environ. Res. Public. Health 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barone L, Colicchio G, Policicchio D, Di Clemente F, Di Monaco A, Meglio M, Lanza GA, Crea F, 2007. Effect of vagal nerve stimulation on systemic inflammation and cardiac autonomic function in patients with refractory epilepsy. NeuroImmunoModulation 14. [DOI] [PubMed] [Google Scholar]
- Barretto ACP, Santos AC, Munhoz R, Rondon MUPB, Franco FG, Trombetta IC, Roveda F, de Matos LNJ, Braga AMW, Middlekauff HR, Negrão CE, 2009. Increased muscle sympathetic nerve activity predicts mortality in heart failure patients. Int. J. Cardiol 135. [DOI] [PubMed] [Google Scholar]
- Beekwilder JP, Beems T, 2010. Overview of the Clinical Applications of Vagus Nerve Stimulation. J. Clin. Neurophysiol 27, 130–138. [DOI] [PubMed] [Google Scholar]
- Ben-Menachem E, 2001. Vagus Nerve Stimulation, Side Effects, and Long-Term Safety: J. Clin. Neurophysiol 18, 415–418. [DOI] [PubMed] [Google Scholar]
- Ben-Menachem E, Manon-Espaillat R, Ristanovic R, Wilder BJ, Stefan H, Mirza W, Tarver WB, Wernicke JF, First International Vagus Nerve Stimulation Study Group, 1994. Vagus Nerve Stimulation for Treatment of Partial Seizures: 1. A Controlled Study of Effect on Seizures. Epilepsia 35, 616–626. [DOI] [PubMed] [Google Scholar]
- Bernik TR, Friedman SG, Ochani M, DiRaimo R, Ulloa L, Yang H, Sudan S, Czura CJ, Ivanova SM, Tracey KJ, 2002. Pharmacological Stimulation of the Cholinergic Antiinflammatory Pathway. J. Exp. Med 195, 781–788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonaz B, Sinniger V, Hoffmann D, Clarençon D, Mathieu N, Dantzer C, Vercueil L, Picq C, Trocmé C, Faure P, Cracowski JL, Pellissier S, 2016. Chronic vagus nerve stimulation in Crohn’s disease: A 6-month follow-up pilot study. Neurogastroenterol. Motil 28. [DOI] [PubMed] [Google Scholar]
- Borovikova LV, Ivanova S, Zhang M, Yang H, Botchkina GI, Watkins LR, Wang H, Abumrad N, Eaton JW, Tracey KJ, 2000. Vagus nerve stimulation attenuates the systemic inflammatory response to endotoxin. Nature 405, 458–462. [DOI] [PubMed] [Google Scholar]
- Bratton BO, Martelli D, McKinley MJ, Trevaks D, Anderson CR, McAllen RM, 2012. Neural regulation of inflammation: no neural connection from the vagus to splenic sympathetic neurons: Lack of vagal connection to splenic nerves. Exp. Physiol 97, 1180–1185. [DOI] [PubMed] [Google Scholar]
- Cervi AL, Lukewich MK, Lomax AE, 2014. Neural regulation of gastrointestinal inflammation: Role of the sympathetic nervous system. Auton. Neurosci 182, 83–88. [DOI] [PubMed] [Google Scholar]
- Chen PS, Chen LS, Fishbein MC, Lin SF, Nattel S, 2014. Role of the autonomic nervous system in atrial fibrillation: Pathophysiology and therapy. Circ. Res 114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clancy JA, Mary DA, Witte KK, Greenwood JP, Deuchars SA, Deuchars J, 2014. Non-invasive Vagus Nerve Stimulation in Healthy Humans Reduces Sympathetic Nerve Activity. Brain Stimulat 7, 871–877. [DOI] [PubMed] [Google Scholar]
- Colzato LS, Wolters G, Peifer C, 2018. Transcutaneous vagus nerve stimulation (tVNS) modulates flow experience. Exp. Brain Res 236, 253–257. [DOI] [PubMed] [Google Scholar]
- De Couck M, Cserjesi R, Caers R, Zijlstra WP, Widjaja D, Wolf N, Luminet O, Ellrich J, Gidron Y, 2017. Effects of short and prolonged transcutaneous vagus nerve stimulation on heart rate variability in healthy subjects. Auton. Neurosci 203, 88–96. [DOI] [PubMed] [Google Scholar]
- De Ferrari GM, 2014. Vagal stimulation in heart failure. J Cardiovasc. Transl. Res [DOI] [PubMed] [Google Scholar]
- De Ferrari GM, Crijns HJGM, Borggrefe M, Milasinovic G, Smid J, Zabel M, Gavazzi A, Sanzo A, Dennert R, Kuschyk J, Raspopovic S, Klein H, Swedberg K, Schwartz PJ, 2011. Chronic vagus nerve stimulation: A new and promising therapeutic approach for chronic heart failure. Eur. Heart J 32. [DOI] [PubMed] [Google Scholar]
- Diedrich A, Okamoto L, Black B, Hale MD, Biaggioni I, 2018. Sub-Perception Transdermal Vagal Stimulation in Postural Tachycardia Syndrome. Hypertension 72. [Google Scholar]
- Diedrich A, Porta A, Barbic F, Brychta RJ, Bonizzi P, Diedrich L, Cerutti S, Robertson D, Furlan R, 2009. Lateralization of expression of neural sympathetic activity to the vessels and effects of carotid baroreceptor stimulation. Am. J. Physiol.-Heart Circ. Physiol 296, H1758–H1765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dipaola F, Barberi C, Castelnuovo E, Minonzio M, Fornerone R, Shiffer D, Cairo B, Zamuner AR, Barbic F, Furlan R, 2020. Time course of autonomic symptoms in postural orthostatic tachycardia syndrome (Pots) patients: Two-year follow-up results. Int. J. Environ. Res. Public. Health 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drewes AM, Brock C, Rasmussen SE, Møller HJ, Brock B, Deleuran BW, Farmer AD, Pfeiffer-Jensen M, 2021. Short-term transcutaneous non-invasive vagus nerve stimulation may reduce disease activity and pro-inflammatory cytokines in rheumatoid arthritis: results of a pilot study. Scand. J. Rheumatol 50. [DOI] [PubMed] [Google Scholar]
- Early S, Stankovic KM, 2018. Reversible Sensorineural Hearing Loss Associated with Off-Label Use of Transcutaneous Vagal Nerve Stimulator. Otolaryngol. - Head Neck Surg. U. S 159. [DOI] [PubMed] [Google Scholar]
- Ellrich J, 2011. Transcutaneous Vagus Nerve Stimulation. Eur. Neurol. Rev 6, 254. [Google Scholar]
- Farmer AD, Strzelczyk A, Finisguerra A, Gourine AV, Gharabaghi A, Hasan A, Burger AM, Jaramillo AM, Mertens A, Majid A, Verkuil B, Badran BW, Ventura-Bort C, Gaul C, Beste C, Warren CM, Quintana DS, Hämmerer D, Freri E, Frangos E, Tobaldini E, Kaniusas E, Rosenow F, Capone F, Panetsos F, Ackland GL, Kaithwas G, O’Leary GH, Genheimer H, Jacobs HIL, Van Diest I, Schoenen J, Redgrave J, Fang J, Deuchars J, Széles JC, Thayer JF, More K, Vonck K, Steenbergen L, Vianna LC, McTeague LM, Ludwig M, Veldhuizen MG, De Couck M, Casazza M, Keute M, Bikson M, Andreatta M, D’Agostini M, Weymar M, Betts M, Prigge M, Kaess M, Roden M, Thai M, Schuster NM, Montano N, Hansen N, Kroemer NB, Rong P, Fischer R, Howland RH, Sclocco R, Sellaro R, Garcia RG, Bauer S, Gancheva S, Stavrakis S, Kampusch S, Deuchars SA, Wehner S, Laborde S, Usichenko T, Polak T, Zaehle T, Borges U, Teckentrup V, Jandackova VK, Napadow V, Koenig J, 2021. International Consensus Based Review and Recommendations for Minimum Reporting Standards in Research on Transcutaneous Vagus Nerve Stimulation (Version 2020). Front. Hum. Neurosci 14, 568051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Florea VG, Cohn JN, 2014. The autonomic nervous system and heart failure. Circ. Res [DOI] [PubMed] [Google Scholar]
- Freeman R, Wieling W, Axelrod FB, Benditt DG, Benarroch E, Biaggioni I, Cheshire WP, Chelimsky T, Cortelli P, Gibbons CH, Goldstein DS, Hainsworth R, Hilz MJ, Jacob G, Kaufmann H, Jordan J, Lipsitz LA, Levine BD, Low PA, Mathias C, Raj SR, Robertson D, Sandroni P, Schatz I, Schondorff R, Stewart JM, Van Dijk JG, 2011. Consensus statement on the definition of orthostatic hypotension, neurally mediated syncope and the postural tachycardia syndrome. Clin. Auton. Res [DOI] [PubMed] [Google Scholar]
- Furlan R, Ardizzone S, Palazzolo L, Rimoldi A, Perego F, Barbic F, Bevilacqua M, Vago L, Bianchi Porro G, Malliani A, 2006. Sympathetic overactivity in active ulcerative colitis: effects of clonidine. Am. J. Physiol. Regul. Integr. Comp. Physiol 290, R224–232. [DOI] [PubMed] [Google Scholar]
- Furlan R, Jacob G, Snell M, Robertson D, Porta A, Harris P, Mosqueda-Garcia R, 1998. Chronic orthostatic intolerance: A disorder with discordant cardiac and vascular sympathetic control. Circulation [DOI] [PubMed] [Google Scholar]
- Furlan R, Porta A, Costa F, Tank J, Baker L, Schiavi R, Robertson D, Malliani A, Mosqueda-Garcia R, 2000. Oscillatory Patterns in Sympathetic Neural Discharge and Cardiovascular Variables During Orthostatic Stimulus. Circulation 101, 886–892. [DOI] [PubMed] [Google Scholar]
- Garcia RG, Lin RL, Lee J, Kim J, Barbieri R, Sclocco R, Wasan AD, Edwards RR, Rosen BR, Hadjikhani N, Napadow V, 2017. Modulation of brainstem activity and connectivity by respiratory-gated auricular vagal afferent nerve stimulation in migraine patients. Pain 158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gauthey A, Morra S, van de Borne P, Deriaz D, Maes N, le Polain de Waroux J-B., 2020. Sympathetic Effect of Auricular Transcutaneous Vagus Nerve Stimulation on Healthy Subjects: A Crossover Controlled Clinical Trial Comparing Vagally Mediated and Active Control Stimulation Using Microneurography. Front. Physiol 11, 599896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goadsby P, Grosberg B, Mauskop A, Cady R, Simmons K, 2014. Effect of noninvasive vagus nerve stimulation on acute migraine: An open-label pilot study. Cephalalgia 34, 986–993. [DOI] [PubMed] [Google Scholar]
- Grimonprez A, Raedt R, Baeken C, Boon P, Vonck K, 2015. The antidepressant mechanism of action of vagus nerve stimulation: Evidence from preclinical studies. Neurosci. Biobehav. Rev 56, 26–34. [DOI] [PubMed] [Google Scholar]
- Hamer HM, Bauer S, 2019. Lessons learned from transcutaneous vagus nerve stimulation (tVNS). Epilepsy Res 153, 83–84. [DOI] [PubMed] [Google Scholar]
- He W, Wang X, Shi H, Shang H, Li L, Jing X, Zhu B, 2012. Auricular Acupuncture and Vagal Regulation. Evid. Based Complement. Alternat. Med 2012, 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hein E, Nowak M, Kiess O, Biermann T, Bayerlein K, Kornhuber J, Kraus T, 2013. Auricular transcutaneous electrical nerve stimulation in depressed patients: A randomized controlled pilot study. J. Neural Transm [DOI] [PubMed] [Google Scholar]
- Huston JM, Ochani M, Rosas-Ballina M, Liao H, Ochani K, Pavlov VA, Gallowitsch-Puerta M, Ashok M, Czura CJ, Foxwell B, Tracey KJ, Ulloa L, 2006. Splenectomy inactivates the cholinergic antiinflammatory pathway during lethal endotoxemia and polymicrobial sepsis. J. Exp. Med 203, 1623–1628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacob G, Costa F, Shannon JR, Robertson RM, Wathen M, Stein M, Biaggioni I, Ertl A, Black B, Robertson D, 2000. The Neuropathic Postural Tachycardia Syndrome. N. Engl. J. Med [DOI] [PubMed] [Google Scholar]
- Jacob G, Diedrich L, Sato K, Brychta RJ, Raj SR, Robertson D, Biaggioni I, Diedrich A, 2019. Vagal and Sympathetic Function in Neuropathic Postural Tachycardia Syndrome. Hypertension 73, 1087–1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson RL, Wilson CG, 2018. A review of vagus nerve stimulation as a therapeutic intervention. J. Inflamm. Res Volume 11, 203–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jürgens TP, Leone M, 2013. Pearls and pitfalls: Neurostimulation in headache. Cephalalgia 33, 512–525. [DOI] [PubMed] [Google Scholar]
- Kamath MV, Upton ARM, Talalla A, Fallen EL, 1992. Effect of Vagal Nerve Electrostimulation on the Power Spectrum of Heart Rate Variability in Man. Pacing Clin. Electrophysiol 15, 235–243. [DOI] [PubMed] [Google Scholar]
- Kaniusas E, Kampusch S, Tittgemeyer M, Panetsos F, Gines RF, Papa M, Kiss A, Podesser B, Cassara AM, Tanghe E, Samoudi AM, Tarnaud T, Joseph W, Marozas V, Lukosevicius A, Ištuk N, Lechner S, Klonowski W, Varoneckas G, Széles JC, Šarolić A, 2019a. Current Directions in the Auricular Vagus Nerve Stimulation II – An Engineering Perspective. Front. Neurosci 13, 772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaniusas E, Kampusch S, Tittgemeyer M, Panetsos F, Gines RF, Papa M, Kiss A, Podesser B, Cassara AM, Tanghe E, Samoudi AM, Tarnaud T, Joseph W, Marozas V, Lukosevicius A, Ištuk N, Šarolić A, Lechner S, Klonowski W, Varoneckas G, Széles JC, 2019b. Current Directions in the Auricular Vagus Nerve Stimulation I – A Physiological Perspective. Front. Neurosci 13, 854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirchner A, Birklein F, Stefan H, Handwerker HO, 2000. Left vagus nerve stimulation suppresses experimentally induced pain. Neurology 55. [DOI] [PubMed] [Google Scholar]
- Koopman FA, Chavan SS, Miljko S, Grazio S, Sokolovic S, Schuurman PR, Mehta AD, Levine YA, Faltys M, Zitnik R, Tracey KJ, Tak PP, 2016. Vagus nerve stimulation inhibits cytokine production and attenuates disease severity in Rheumatoid arthritis. Proc. Natl. Acad. Sci. U. S. A 113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreuzer PM, Landgrebe M, Resch M, Husser O, Schecklmann M, Geisreiter F, Poeppl TB, Prasser SJ, Hajak G, Rupprecht R, Langguth B, 2014. Feasibility, safety and efficacy of transcutaneous vagus nerve stimulation in chronic tinnitus: An open pilot study. Brain Stimulat 7. [DOI] [PubMed] [Google Scholar]
- Lankford J, Numan M, Adejumo R, Martinez R, Von Allmen G, Koenig M, Butler I, 2015. Vagal Nerve Stimulation in Autonomic Dysfunction – A Case Study. Auton. Neurosci 192. [Google Scholar]
- Malliani A, Montano N, 2004. Sympathetic overactivity in ischaemic heart disease. Clin. Sci [DOI] [PubMed] [Google Scholar]
- Mancia G, Grassi G, 2014. The autonomic nervous system and hypertension. Circ. Res [DOI] [PubMed] [Google Scholar]
- Mar PL, Raj SR, 2020. Postural Orthostatic Tachycardia Syndrome: Mechanisms and New Therapies. Annu. Rev. Med [DOI] [PubMed] [Google Scholar]
- Martelli D, Farmer DGS, McKinley MJ, Yao ST, McAllen RM, 2019. Anti-inflammatory reflex action of splanchnic sympathetic nerves is distributed across abdominal organs. Am. J. Physiol.-Regul. Integr. Comp. Physiol 316, R235–R242. [DOI] [PubMed] [Google Scholar]
- Martelli D, McKinley MJ, McAllen RM, 2014. The cholinergic anti-inflammatory pathway: A critical review. Auton. Neurosci 182, 65–69. [DOI] [PubMed] [Google Scholar]
- Morris GLIM, Gloss D, Buchhalter JM, Mack KJ, Nickels K, Harden C, 2013. Evidence-based guideline update: Vagus nerve stimulation for the treatment of epilepsy: Report of the Guideline Development Subcommittee of the American Academy of Neurology. Neurology 81, 1453–1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mu Q, Bohning DE, Nahas Z, Walker J, Anderson B, Johnson KA, Denslow S, Lomarev M, Moghadam P, Chae JH, George MS, 2004. Acute vagus nerve stimulation using different pulse widths produces varying brain effects. Biol. Psychiatry 55. [DOI] [PubMed] [Google Scholar]
- Nahas Z, Marangell LB, Husain MM, Rush AJ, Sackeim HA, Lisanby SH, Martinez JM, George MS, 2005. Two-Year Outcome of Vagus Nerve Stimulation (VNS) for Treatment of Major Depressive Episodes. J. Clin. Psychiatry 66, 1097–1104. [DOI] [PubMed] [Google Scholar]
- Nearing BD, Anand IS, Libbus I, Dicarlo LA, Kenknight BH, Verrier RL, 2021. Vagus Nerve Stimulation Provides Multiyear Improvements in Autonomic Function and Cardiac Electrical Stability in the ANTHEM-HF Study. J. Card. Fail 27. [DOI] [PubMed] [Google Scholar]
- Okamoto LE, Raj SR, Gamboa A, Shibao CA, Arnold AC, Garland EM, Black BK, Farley G, Diedrich A, Biaggioni I, 2015. Sympathetic activation is associated with increased IL-6, but not CRP in the absence of obesity: lessons from postural tachycardia syndrome and obesity. Am. J. Physiol. - Heart Circ. Physiol 309, H2098–H2107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paleczny B, Seredyński R, Ponikowska B, 2021. Inspiratory- and expiratory-gated transcutaneous vagus nerve stimulation have different effects on heart rate in healthy subjects: preliminary results. Clin. Auton. Res 31, 205–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavlov VA, Ochani M, Gallowitsch-Puerta M, Ochani K, Huston JM, Czura CJ, Al-Abed Y, Tracey KJ, 2006. Central muscarinic cholinergic regulation of the systemic inflammatory response during endotoxemia. Proc. Natl. Acad. Sci 103, 5219–5223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penry JK, Dean JC, 1990. Prevention of Intractable Partial Seizures by Intermittent Vagal Stimulation in Humans: Preliminary Results. Epilepsia 31. [DOI] [PubMed] [Google Scholar]
- Petelin Gadze Z, Bujan Kovac A, Adamec I, Milekic N, Sulentic V, 2018. Vagal nerve stimulation is beneficial in postural orthostatic tachycardia syndrome and epilepsy. Seizure [DOI] [PubMed] [Google Scholar]
- Raj SR, 2013. Postural tachycardia syndrome (POTS). Circulation 127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randall WC, Milosavljevic M, Wurster RD, Geis GS, Ardell JL, 1986. Selective vagal innervation of the heart. Ann. Clin. Lab. Sci 16. [PubMed] [Google Scholar]
- Robertson D, 1999. The epidemic of orthostatic tachycardia and orthostatic intolerance, in: American Journal of the Medical Sciences [DOI] [PubMed] [Google Scholar]
- Rong P-J, Fang J-L, Wang L-P, Meng H, Liu J, Ma Y, Ben H, Li L, Liu R-P, Huang Z-X, Zhao Y-F, Li X, Zhu B, Kong J, 2012. Transcutaneous vagus nerve stimulation for the treatment of depression: a study protocol for a double blinded randomized clinical trial. BMC Complement. Altern. Med 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronkainen E, Korpelainen JT, Heikkinen E, Myllylä VV, Huikuri HV, Isojärvi JIT, 2006. Cardiac autonomic control in patients with refractory epilepsy before and during vagus nerve stimulation treatment: A one-year follow-up study. Epilepsia 47. [DOI] [PubMed] [Google Scholar]
- Rosas-Ballina M, Ochani M, Parrish WR, Ochani K, Harris YT, Huston JM, Chavan S, Tracey KJ, 2008. Splenic nerve is required for cholinergic antiinflammatory pathway control of TNF in endotoxemia. Proc. Natl. Acad. Sci 105, 11008–11013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryzí M, Brázdil M, Novák Z, Chrastina J, Ošlejšková H, Rektor I, Kuba R, 2013. Long-term vagus nerve stimulation in children with focal epilepsy. Acta Neurol. Scand 127, 316–322. [DOI] [PubMed] [Google Scholar]
- Setty AB, Vaughn BV, Quint SR, Robertson KR, Messenheimer JA, 1998. Heart period variability during vagal nerve stimulation. Seizure 7. [DOI] [PubMed] [Google Scholar]
- Shiffer D, Furlan R, Minonzio M, Porta A, Montano N, Tobaldini E, Furlan L, Urechie V, Biaggioni I, Diedrich A, 2019. Effects of transcutaneous vagal nerve stimulation on orthostatic tolerance in patients with postural orthostatic tachycardia syndrome (POTS). Clin. Auton. Res 29, 527–528. [Google Scholar]
- Siddiqui F, Herial NA, Ali II, 2010. Cumulative effect of vagus nerve stimulators on intractable seizures observed over a period of 3years. Epilepsy Behav 18, 299–302. [DOI] [PubMed] [Google Scholar]
- Sperling W, Reulbach U, Bleich S, Padberg F, Kornhuber J, Mueck-Weymann M, 2010. Cardiac effects of vagus nerve stimulation in patients with major depression. Pharmacopsychiatry 43. [DOI] [PubMed] [Google Scholar]
- Stavrakis S, Humphrey MB, Scherlag BJ, Hu Y, Jackman WM, Nakagawa H, Lockwood D, Lazzara R, Po SS, 2015. Low-Level Transcutaneous Electrical Vagus Nerve Stimulation Suppresses Atrial Fibrillation. J. Am. Coll. Cardiol 65, 867–875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tobaldini E, Toschi-Dias E, Appratto de Souza L, Rabello Casali K, Vicenzi M, Sandrone G, Cogliati C, La Rovere MT, Pinna GD, Montano N, 2019. Cardiac and Peripheral Autonomic Responses to Orthostatic Stress During Transcutaneous Vagus Nerve Stimulation in Healthy Subjects. J. Clin. Med 8, 496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tracey KJ, 2007. Physiology and immunology of the cholinergic antiinflammatory pathway. J. Clin. Invest [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uthman BM, Wilder BJ, Penry JK, Dean C, Ramsay RE, Reid SA, Hammond EJ, Tarver WB, Wernicke JF, 1993. Treatment of epilepsy by stimulation of the vagus nerve. Neurology 43. [DOI] [PubMed] [Google Scholar]
- von Wrede R, Moskau-Hartmann S, Rüber T, Helmstaedter C, Surges R, 2019. Sustained seizure freedom with transcutaneous vagal nerve stimulation in drug-resistant epilepsy caused by subcortical band heterotopias. Seizure 70. [DOI] [PubMed] [Google Scholar]
- Wang Z, Yu L, Wang S, Huang B, Liao K, Saren G, Tan T, Jiang H, 2014. Chronic Intermittent Low-Level Transcutaneous Electrical Stimulation of Auricular Branch of Vagus Nerve Improves Left Ventricular Remodeling in Conscious Dogs With Healed Myocardial Infarction. Circ. Heart Fail 7, 1014–1021. [DOI] [PubMed] [Google Scholar]
- Yap JYY, Keatch C, Lambert E, Woods W, Stoddart PR, Kameneva T, 2020. Critical Review of Transcutaneous Vagus Nerve Stimulation: Challenges for Translation to Clinical Practice. Front. Neurosci 14, 284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan H, Silberstein SD, 2016. Vagus Nerve and Vagus Nerve Stimulation, a Comprehensive Review: Part II. Headache [DOI] [PubMed] [Google Scholar]
- Zabara J, 1992. Inhibition of Experimental Seizures in Canines by Repetitive Vagal Stimulation. Epilepsia 33. [DOI] [PubMed] [Google Scholar]
- Zamotrinsky AV, Kondratiev B, de Jong JW, 2001. Vagal neurostimulation in patients with coronary artery diseaseq 8. [DOI] [PubMed] [Google Scholar]
- Zoccali C, Mallamaci F, Parlongo S, Cutrupi S, Benedetto FA, Tripepi G, Bonanno G, Rapisarda F, Fatuzzo P, Seminara G, Cataliotti A, Stancanelli B, Malatino LS, 2002. Plasma norepinephrine predicts survival and incident cardiovascular events in patients with end-stage renal disease. Circulation 105. [DOI] [PubMed] [Google Scholar]


