Abstract
Introduction:
Gestational diabetes mellitus (GDM) and hypertensive disorders of pregnancy (HDP) increase the risk for future adverse health outcomes in the pregnant woman and baby, and disparities exist in rates of GDM and HDP by race/ethnicity. The objective of this study is to identify differences in GDM and HDP rates by maternal place of birth within race/ethnicity groups.
Methods:
In women aged 15–44 years at first live singleton birth in U.S. surveillance data between 2014 and 2019, age-standardized rates of GDM and HDP and rate ratios (RRs) of GDM and HDP in women born outside versus in the U.S. were evaluated stratified by race/ethnicity. Analyses were conducted in 2021.
Results:
Of 8,574,264 included women, 6,827,198 were born in the U.S. (mean age=26.2 [SD 5.7] years) and 1,747,066 were born outside the U.S. (mean age=28.2 [SD=5.8] years). Overall, the GDM rate was higher in women born outside compared with in the U.S. (70.3, 95% CI=69.9, 70.7 vs 53.2, 95% CI=53.0, 53.4 per 1,000 live births; RR=1.32, 95% CI=1.31, 1.33), a pattern observed in most race/ethnic groups. By contrast, the overall HDP rate was lower in those born outside versus in the U.S. (52.5, 95% CI=52.2, 52.9 vs 90.1, 95% CI=89.9, 90.3 per 1,000 live births; RR=0.58, 95% CI=0.58, 0.59), a pattern observed in most race/ethnic groups.
Conclusions:
In the U.S., GDM rates were higher and HDP rates were lower in women born outside the U.S. compared with those born in the U.S. in most race/ethnicity groups.
INTRODUCTION
Gestational diabetes mellitus (GDM) and hypertensive disorders of pregnancy (HDP; gestational hypertension and pre-eclampsia) are common adverse pregnancy outcomes (APOs) and incidence of both have increased in the U.S. over the last decade.1,2 GDM and HDP are both associated with adverse maternal health outcomes.2–4 Significant differences in GDM and HDP rates across race/ethnicity subgroups are recognized in the U.S., with highest GDM burden among Asian American women and highest HDP burden among non-Hispanic Black women.1,5 Social determinants are important contributors to racial disparities in cardiometabolic outcomes.6 Place of birth, and by extension immigration, may influence environmental exposures, behavior patterns, economic opportunity, social integration, and healthcare access.7 APO rates may differ among foreign-born compared with U.S.-born women, but these data are frequently self-reported and in aggregated race/ethnicity categories.8–10 Therefore, the objective of this study is to quantify contemporary relative rates and trends of GDM and HDP experienced by nulliparous women born in versus outside the U.S. between 2014 and 2019.
METHODS
Study Sample
Data from the National Center for Health Statistics (NCHS) birth registration records were used to calculate rates of GDM and HDP between 2014 and 2019. The NCHS records capture all first live births to women within the U.S. Only records using the 2003 revised U.S. Standard Certificate of Live Birth were eligible as prior versions did not distinguish GDM from pre-gestational diabetes. In this analysis, all records using the revised birth certificate from U.S. states that adopted this version were included. Records from individuals aged <15 years or >44 years, and records from those who were not U.S. residents (i.e., who listed a state of residence outside the 50 U.S. states and District of Columbia, irrespective of citizenship or visa status) at time of delivery were ineligible (Figure 1). Also, records from non-singleton births (e.g., twins) were ineligible to avoid duplication of the outcome, as records are de-identified and cannot be linked. From the remaining eligible sample, records missing data on maternal place of birth, GDM, or HDP were excluded. Collection of maternal data for birth certificate records is recommended to be completed by the attendant clinician at delivery (e.g., midwife or physician). Data are compiled from maternal self-report, prenatal records, labor and delivery triage records, admission history and physical, or delivery record based on a standard NCHS protocol. This study was deemed to be exempt from IRB review because data are de-identified from a publicly available vital statistics data set.
Figure 1.
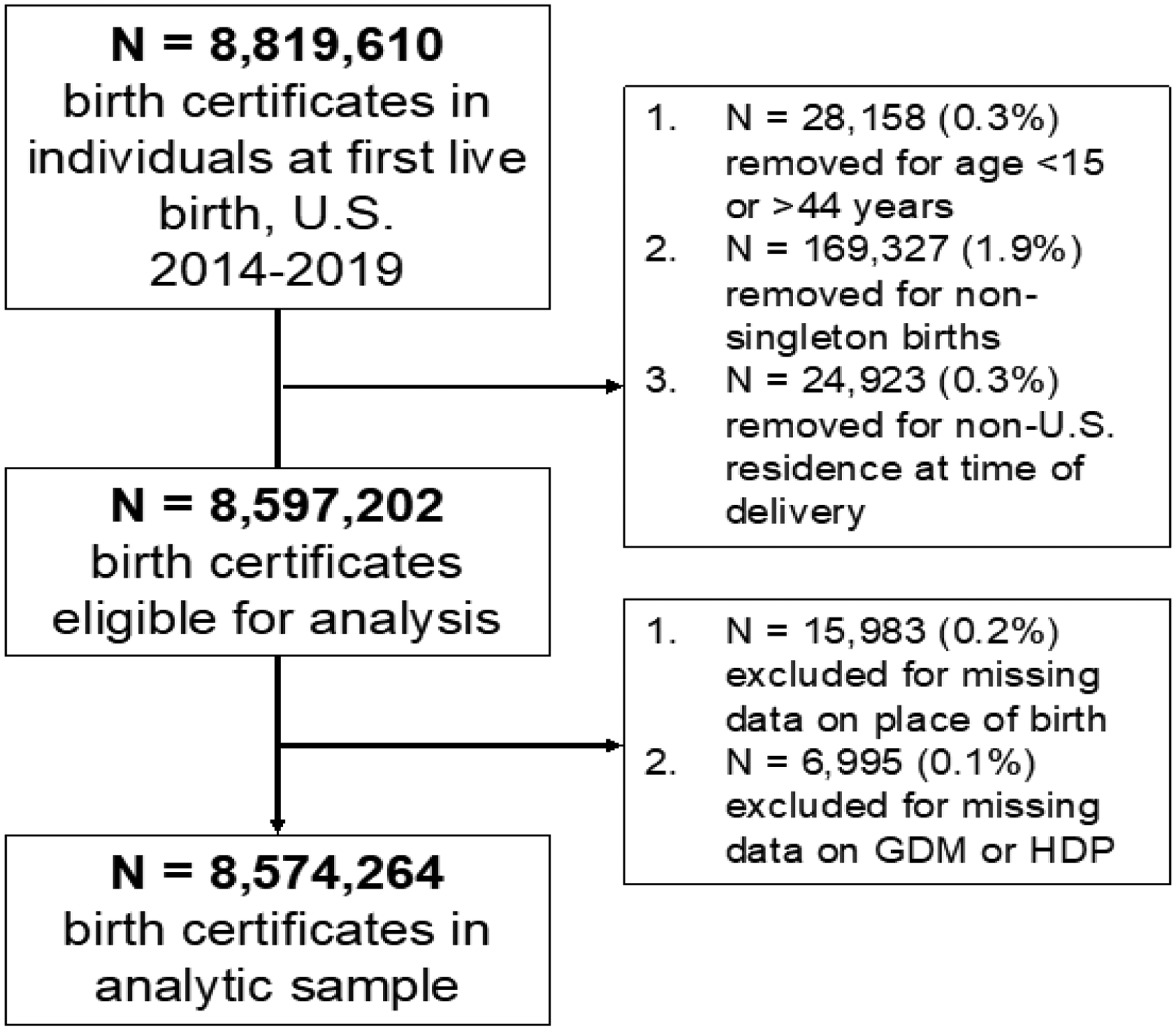
Diagram of birth records included for primary analysis, U.S. 2014‒2019. GDM, gestational diabetes mellitus; HDP, hypertensive disorders of pregnancy.
Measures
The outcomes of interest utilized the standard definition outlined by the National Vital Statistics System on the U.S. 2003 Revised Certificate of Live Birth. This includes GDM, defined as diabetes diagnosed during pregnancy, and HDP, defined as pregnancy-induced hypertension or pre-eclampsia (excluding pre-pregnancy [chronic] hypertension), with or without eclampsia. This documentation is completed by the professional attendant at birth (e.g., physician, midwife) and is based on self-report and health records. Maternal place of birth was defined as: born inside the U.S. (1 of the 50 U.S. states or the District of Columbia), or outside the U.S. (not in 1 of the 50 U.S. states or District of Columbia, including U.S. territories). Age, maternal race/ethnicity, education, insurance (primary source of payment for delivery: private, Medicaid, self-pay, or other), pre-gestational BMI, and receipt of prenatal care are also collected. Race/ethnicity was identified by maternal self-report from fixed categories, and was evaluated given known differences in APOs across race/ethnic groups. Women identifying as Hispanic or Latina ethnicity, and among Hispanic subgroups, were categorized separately. Non-Hispanic women were categorized according to race. Because of inconsistencies across states in how multiple-race identification data were collected, NCHS recommends use of bridged-race categories in analysis of overall race groups. For overall race categorization, the White, Black, and Asian or Pacific Islander race categories were used, which allow for calculation and comparison of population-level rates.11 Single-race categories, which are collected on the 2003 revised birth certificate, identified Asian ethnicity subgroups. The American Indian or Alaskan Native bridged-race category or the Native Hawaiian single-race category were not evaluated, as these women would largely be categorized as born inside the U.S., or the Pacific Islander single-race category as these women would largely be categorized as born outside the U.S. No records were missing race data. Approximately 0.8% of records were missing ethnicity, so these records are captured in the overall population but not in race/ethnic group and subgroup analyses.
Statistical Analysis
The GDM and HDP rates were calculated overall (all race/ethnicity groups combined), in 4 primary race/ethnic groups (non-Hispanic White, non-Hispanic Black, Hispanic/Latina, and non-Hispanic Asian/Pacific Islander), in Hispanic/Latina subgroups (Mexican, Puerto Rican, Cuban, and Central/South American), and in non-Hispanic Asian subgroups (Asian Indian, Chinese, Filipina, Japanese, Korean, and Vietnamese). Age-standardized rates of GDM and HDP were calculated per 1,000 live births overall, in race/ethnic groups, and in Hispanic/Latina and non-Hispanic Asian subgroups. Rates were age-standardized to the age distribution of U.S. women who gave birth in 2011 to allow for direct comparisons with previously published rates of GDM from 2011–2019.1 GDM and HDP rates per 1,000 live births were calculated from pooled 2014–2019 data. Within each race/ethnic group, the pooled 2014–2019 GDM and HDP rates in women born outside the U.S. were compared relative to women born inside the U.S. Age-standardized rate ratios (RRs) and 95% CIs were calculated with modified F intervals, with women born inside the U.S. as the reference category for each race/ethnic group. Calculation of age-standardized rates and RRs were conducted with Stata, version 15.1.
In a secondary analysis, GDM and HDP rates were calculated in each individual year from 2014 to 2019. Joinpoint Regression statistical software, version 4.7.0.0 was used to calculate mean annual percentage change (APC) of age-standardized GDM and HDP rates from 2014 to 2019, across all strata of race/ethnicity and place of birth.12 For all analyses, 2-sided p-values <0.05 indicated statistical significance.
RESULTS
From 8,819,610 birth records representing a live birth among women in the U.S. from 2014 to 2019, records removed and excluded are detailed in Figure 1. Among the remaining 8,574,264 women with a singleton first live birth in the primary analytic sample, 6,827,198 were born in the U.S. (Table 1). Among U.S.-born women, mean age at delivery was 26.2 (SD=5.7) years; 66% were non-Hispanic White, 15% non-Hispanic Black, 2% Asian/Pacific Islander, and 16% Hispanic; 90% were at least a high school graduate; 57% had private insurance and 37% had Medicaid; and 81% received prenatal care starting in the first trimester. Median pre-pregnancy BMI was 24.9 kg/m2 (IQR=21.8–30.0), 2% had pre-pregnancy hypertension, and 1% had pre-pregnancy diabetes. In the 1,727,066 women born outside the U.S. with a singleton first live birth, the mean age at delivery was 28.2 (SD=5.8) years; 18% were non-Hispanic White, 10% non-Hispanic Black, 31% Asian/Pacific Islander, and 41% Hispanic; 86% were at least a high school graduate; 47% had private insurance and 39% had Medicaid; and 74% received prenatal care starting in the first trimester. Median pre-pregnancy BMI was 23.4 kg/m2 (IQR=20.9–26.8), 1% had pre-pregnancy hypertension, and 1% had pre-pregnancy diabetes. Characteristics of women in race/ethnicity groups, non-Hispanic Asian subgroups, and Hispanic subgroups are shown in Appendix Tables 1‒3.
Table 1.
Characteristics of Women by Maternal Place of Birth, 2014‒2019
| Maternal place of birth | U.S. N=6,827,198 | Outside the U.S. N=1,747,066 |
|---|---|---|
| Age, years, mean (SD) | 26.2 (5.7) | 28.2 (5.8) |
| Maternal race/ethnicity | ||
| Non-Hispanic White | 4,431,830 (66.1) | 312,835 (18.1) |
| Non-Hispanic Black | 1,034,029 (15.4) | 173,451 (10.0) |
| Non-Hispanic Asian/Pacific Islander | 151,127 (2.3) | 539,177 (31.2) |
| Asian Indian | 19,752 (2.1) | 178,262 (16.7) |
| Chinese | 22,387 (2.3) | 142,664 (13.3) |
| Filipina | 20,760 (2.2) | 51,037 (4.8) |
| Japanese | 4,897 (0.5) | 12,596 (1.2) |
| Korean | 9,630 (1.0) | 30,677 (2.9) |
| Vietnamese | 11,777 (1.2) | 39,337 (3.7) |
| Hispanic/Latina | 1,090,071 (16.3) | 702,605 (40.7) |
| Mexican | 657,738 (68.8) | 328,659 (30.7) |
| Puerto Rican | 114,300 (12.0) | 35,088 (3.3) |
| Cuban | 25,938 (2.7) | 34,856 (3.3) |
| Central/South American | 68,673 (7.2) | 215,846 (20.2) |
| Education | ||
| Less than high school | 651,513 (9.6) | 244,367 (14.3) |
| High school graduate | 1,683,645 (24.9) | 364,368 (21.3) |
| Any college | 4,429,278 (65.5) | 1,102,068 (64.4) |
| Insurance | ||
| Medicaid | 2,511,233 (37.0) | 679,021 (39.1) |
| Private insurance | 3,888,818 (57.3) | 817,111 (47.1) |
| Self-pay | 118,546 (1.7) | 168,462 (9.7) |
| Other | 265,850 (3.9) | 70,565 (4.1) |
| Receipt of prenatal care | ||
| Starting 1st trimester | 5,350,382 (80.5) | 1,247,258 (73.7) |
| Starting 2nd trimester | 982,344 (14.8) | 292,975 (17.3) |
| Starting 3rd trimester | 235,180 (3.5) | 122,675 (7.2) |
| No prenatal care | 82,172 (1.2) | 29,859 (1.8) |
| Pre-pregnancy BMI (kg/m2), median (IQR) | 24.9 (21.8, 30.0) | 23.4 (20.9, 26.8) |
| Pre-pregnancy hypertension | 123,176 (1.8) | 16,160 (0.9) |
| Pre-pregnancy DM | 54,421 (0.8) | 11,700 (0.7) |
Notes: Data presented are N (%) unless otherwise specified. DM, diabetes mellitus.
DM, diabetes mellitus.
Overall, 1% of women experienced both GDM and HDP, 5% experienced GDM without HDP, and 7% experienced HDP without GDM (Appendix Table 4). In women born in the U.S., 1% experienced both GDM and HDP, 4% experienced GDM without HDP, and 8% experienced HDP without GDM. Among those born outside the U.S., 1% experienced both GDM and HDP, 7% experienced GDM without HDP, and 5% experienced HDP without GDM. Characteristics of women who experienced GDM and HDP by place of birth are shown in Appendix Table 5.
Pooled (2014–2019) age-standardized rates of GDM are shown by race/ethnicity groups and subgroups in Table 2. Overall, the GDM rate was 70.3 (95% CI=69.9, 70.7) per 1,000 live births in women born outside the U.S., and 53.2 (95% CI=53.0, 53.4) per 1,000 in women born in the U.S. RRs for GDM among race/ethnic subgroups in women born outside the U.S. relative to women born in the U.S. are shown in Figure 2. Compared with women born in the U.S., GDM rates were higher in those born outside the U.S. overall (RR=1.32, 95% CI=1.31, 1.33), in non-Hispanic Black women (RR=1.06, 95% CI=1.04, 1.09), and in non-Hispanic Asian/Pacific Islander women (RR=1.32, 95% CI=1.29, 1.35).
Table 2.
Gestational Diabetes and Hypertensive Disorders of Pregnancy in Race/Ethnic Subgroups by Maternal Place of Birth
| Place of birth | Gestational diabetes mellitus | Hypertensive disorders of pregnancy | ||
|---|---|---|---|---|
| Pooled rate (2014–2019) | Average APC (2014 to 2019) | Pooled rate (2014–2019) | Average APC (2014 to 2019) | |
| Overall | ||||
| Outside U.S. | 70.3 (69.9, 70.7) | 4.6 (3.4, 5.9) | 52.5 (52.2, 52.9) | 9.4 (7.7, 11.2) |
| U.S. | 53.2 (53.0, 53.4) | 4.4 (3.6, 5.2) | 90.1 (89.9, 90.3) | 8.2 (7.5, 9.0) |
| Non-Hispanic White | ||||
| Outside U.S. | 52.1 (51.2, 52.9) | 5.0 (2.3, 7.8) | 46.8 (46.0, 47.7) | 8.0 (6.1, 10.0) |
| U.S. | 51.8 (51.6, 52.0) | 4.5 (3.4, 5.6) | 91.5 (91.2, 91.8) | 8.5 (7.9, 9.1) |
| Non-Hispanic Black | ||||
| Outside U.S. | 55.3 (54.2, 56.4) | 2.4 (1.4, 3.4) | 71.3 (70.0, 72.6) | 8.7 (5.6, 11.9) |
| U.S. | 52.0 (51.4, 52.6) | 1.6 (0.5, 2.8) | 105.4 (104.6, 106.2) | 7.4 (6.0, 8.9) |
| Hispanic/Latina | ||||
| Outside U.S. | 61.0 (60.4, 61.7) | 3.5 (1.4, 5.7) | 60.6 (60.0, 61.2) | 9.7 (8.5, 10.8) |
| U.S. | 61.2 (60.6, 61.9) | 5.0 (4.5, 5.0) | 78.4 (77.7, 79.0) | 7.7 (6.7, 8.9) |
| Mexican | ||||
| Outside U.S. | 70.2 (69.2, 71.3) | 3.5 (0.9, 6.3) | 60.9 (60.0, 61.8) | 9.7 (6.8, 12.6) |
| U.S. | 64.6 (63.7, 65.5) | 4.4 (3.3, 5.5) | 79.0 (78.1, 79.9) | 7.6 (6.1, 9.1) |
| Puerto Rican | ||||
| Outside U.S. | 71.2 (68.0, 74.6) | 19.6 (0, 42.9) | 83.0 (79.6, 86.5) | 13.7 (9.6, 18.0) |
| U.S. | 66.2 (64.3, 68.2) | 5.3 (2.2, 8.5) | 81.8 (79.9, 83.9) | 9.3 (2.1, 17.0) |
| Cuban | ||||
| Outside U.S. | 56.9 (54.2, 59.6) | 9.5 (4.6, 14.6) | 66.1 (63.4, 69.0) | 11.4 (7.0, 16.1) |
| U.S. | 46.0 (43.4, 48.8) | 11.6 (6.4, 17.0) | 75.0 (71.6, 78.5) | 10.3 (7.1, 13.5) |
| C/S American | ||||
| Outside U.S. | 50.4 (49.4, 51.4) | 4.2 (0.9, 7.6) | 53.5 (52.5, 54.5) | 9.5 (8.1, 10.9) |
| U.S. | 48.8 (46.9, 50.8) | 8.3 (1.5, 15.5) | 67.5 (65.4, 69.7) | 8.1 (6.3, 9.9) |
| Non-Hispanic Asian/Pacific Islander | ||||
| Outside U.S. | 97.1 (96.2, 98.0) | 5.5 (4.6, 6.5) | 39.0 (38.3, 39.7) | 9.7 (5.4, 14.1) |
| U.S. | 73.5 (72.1, 74.9) | 5.3 (4.5, 6.2) | 59.7 (58.4, 61.0) | 9.5 (6.9, 12.1) |
| Asian Indian | ||||
| Outside U.S. | 122.7 (120.6, 124.9) | 5.0 (2.0, 8.1) | 44.3 (42.7, 46.0) | 9.7 (5.3, 14.2) |
| U.S. | 75.5 (71.2, 80.1) | 6.5 (4.9, 8.2) | 50.5 (46.7, 54.5) | 10.0 (3.5, 16.9) |
| Chinese | ||||
| Outside U.S. | 79.7 (77.6, 81.8) | 5.5 (2.5, 8.6) | 19.9 (18.5, 21.5) | 11.4 (−0.8, 25.0) |
| U.S. | 68.4 (63.1, 74.2) | 7.7 (−1.2, 17.4) | 39.5 (35.5, 44.0) | 11.0 (5.5, 16.8) |
| Filipina | ||||
| Outside U.S. | 96.8 (94.1, 99.7) | 5.7 (3.9, 7.6) | 68.6 (66.1, 71.1) | 9.8 (6.1, 13.7) |
| U.S. | 82.8 (79.0, 86.8) | 5.4 (0, 11.2) | 80.7 (76.7, 84.8) | 7.5 (3.0, 12.2) |
| Japanese | ||||
| Outside U.S. | 37.5 (32.4, 45.7) | 9.7 (3.5, 16.2) | 24.1 (18.6, 33.0) | 9.0 (−21.2, 50.6) |
| U.S. | 61.8 (53.5, 71.6) | 5.6 (−7.6, 20.8) | 57.7 (46.9, 70.5) | 16.0 (−3.5, 39.4) |
| Korean | ||||
| Outside U.S. | 61.5 (56.8, 66.6) | 8.6 (5.2, 12.2) | 36.2 (31.1, 42.0) | 1.0 (−4.0, 6,3) |
| U.S. | 62.3 (55.8, 69.5) | 4.2 (−4.9, 14.0) | 45.8 (38.0, 54.7) | 18.6 (−0.9, 41.8) |
| Vietnamese | ||||
| Outside U.S. | 100.1 (96.7, 103.6) | 5.5 (1.2, 9.9) | 25.0 (23.0, 27.1) | 9.7 (2.7, 17.1) |
| U.S. | 77.1 (72.0, 82.4) | 2.7 (−4.1, 10.0) | 48.6 (44.3, 53.2) | 6.4 (−5.3, 19.5) |
Notes: Rates per 1,000 live births. Boldface indicates statistical significance (p<0.05).
APC, annual percent change; C/S, Central/South.
Figure 2.
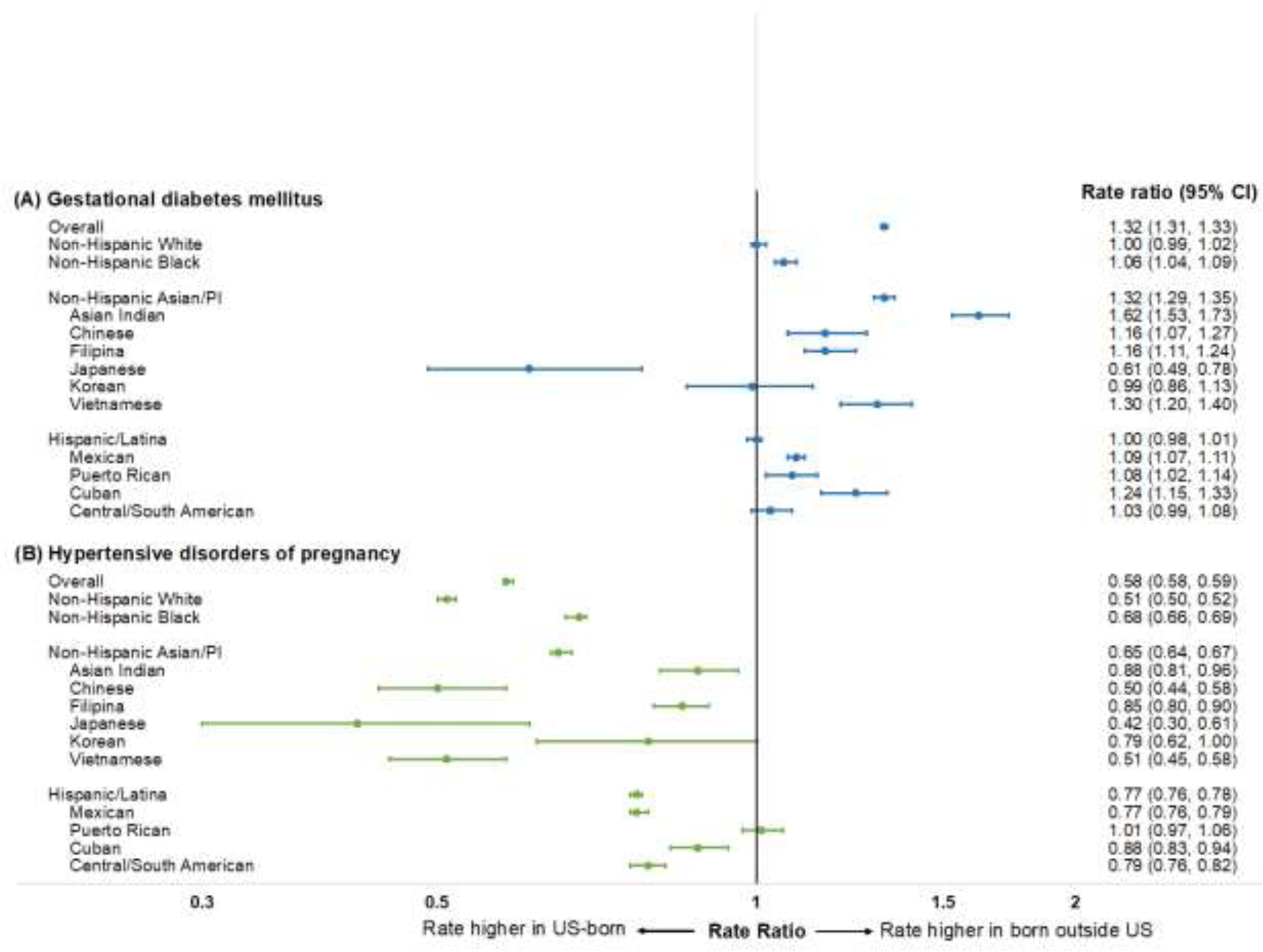
Standardized rate ratios for gestational diabetes mellitus and hypertensive disorders of pregnancy by maternal nativity among race/ethnic subgroups in the U.S., 2014‒2019. Notes: Rate ratios (95% CIs) within each race/ethnic subgroup represent rates in women born outside the U.S., relative to women born in the U.S.
Among non-Hispanic Asian/Pacific Islander subgroups, GDM rates were higher in those born outside the U.S. (versus inside the U.S.) in Asian Indian (RR=1.62, 95% CI=1.53, 1.73), Chinese (RR=1.16, 95% CI=1.07, 1.27), Filipina (RR=1.16, 95% CI=1.11, 1.24), and Vietnamese (RR=1.30, 95% CI=1.20, 1.40) women, whereas GDM rates were lower in those born outside the U.S. (versus inside the U.S.) in Japanese women (RR=0.61, 95% CI=0.49, 0.78). Among Hispanic/Latina subgroups, GDM rates were higher in those born outside the U.S. (versus inside the U.S.) in Mexican (RR=1.09, 95% CI=1.07, 1.11), Puerto Rican (RR=1.08, 95% CI=1.02, 1.14), and Cuban (RR=1.24, 95% CI=1.15, 1.33) women.
Pooled (2014–2019) age-standardized rates of HDP are shown by race/ethnicity including subgroups in Table 2. The overall HDP rate was 52.5 (95% CI=52.2, 52.9) per 1,000 live births in women born outside the U.S., and 90.1 (95% CI=89.9, 90.3) per 1,000 in women born in the U.S. Standardized RRs for HDP among race/ethnic subgroups in women born outside the U.S. relative to women born in the U.S. are shown in Figure 2. Compared with women born in the U.S., HDP rates were lower in those born outside the U.S. overall (RR=0.58, 95% CI=0.58, 0.59), in non-Hispanic Black women (RR=0.51, 95% CI=0.50, 0.52), in non-Hispanic Asian/Pacific Islander women (RR=0.65, 95% CI=0.64, 0.67), and in Hispanic/Latina women (RR=0.77, 95% CI=0.76, 0.78), but were not significantly different in non-Hispanic White women (RR=1.00, 95% CI=0.99, 1.02).
Among non-Hispanic Asian/Pacific Islander subgroups, HDP rates were lower in those born outside the U.S. (versus inside the U.S.) in Asian Indian (RR=0.88, 95% CI=0.81, 0.96), Chinese (RR=0.50, 95% CI=0.44, 0.58), Filipina (RR=0.85, 95% CI=0.80, 0.90), Japanese (RR=0.42, 95% CI=0.30, 0.61), and Vietnamese (RR=0.51, 95% CI=0.45, 0.58) women. Among Hispanic/Latina subgroups, HDP rates were lower in those born outside the U.S. (versus inside the U.S.) in Mexican (RR=0.77, 95% CI=0.76, 0.79), Cuban (RR=0.88, 95% CI=0.83, 0.94), and Central/South American (RR=0.79, 95% CI=0.76, 0.82) women.
Trends in GDM and HDP rates by nativity status between 2014 and 2019, represented by the average APC in rates, are shown in Table 2. Sample characteristics for women in 2014 and 2019 are shown in Appendix Table 6, and age-standardized rates of GDM in 2014 and 2019 are shown in Appendix Table 7. For GDM, rates increased from 2014 to 2019 in the overall population among those born outside the U.S. (APC=4.6% per year, 95% CI=3.4, 5.9) and inside the U.S. (APC=4.4% per year, 95% CI=3.6, 5.2). GDM rates significantly increased among both women born outside the U.S. and inside the U.S. in non-Hispanic White, non-Hispanic Black, Hispanic/Latina, and non-Hispanic Asian/Pacific Islander women. In Hispanic/Latina subgroups, GDM rates increased in both those born outside the U.S. and inside the U.S. in Mexican, Puerto Rican, Cuban, and Central/South American women. In non-Hispanic Asian subgroups, GDM rates increased among those born outside the U.S. in all subgroups, and increased in Asian Indian women born inside the U.S. (Table 2).
Age-standardized rates of HDP in 2014 and 2019 are shown in Appendix Table 8. For HDP, rates increased from 2014 to 2019 in the overall population among those born outside the U.S. (APC=9.4% per year, 95% CI=7.7, 11.2) and inside the U.S. (APC=8.2% per year, 95% CI=7.5, 9.0). HDP rates increased significantly among both women born outside the U.S. and inside the U.S. in non-Hispanic White, non-Hispanic Black, Hispanic/Latina, and non-Hispanic Asian/Pacific Islander women. In Hispanic/Latina subgroups, HDP rates increased in both those born outside the U.S. and inside the U.S. in Mexican, Puerto Rican, Cuban, and Central/South American women. In non-Hispanic Asian subgroups, HDP rates increased among those born outside the U.S. in Asian Indian, Filipina, and Vietnamese women. HDP rates increased among those born in the U.S. in Asian Indian, Chinese, and Filipina women (Table 2).
DISCUSSION
Among pregnant women with a singleton first live birth, GDM rates were predominantly higher in women born outside the U.S. compared with those born in the U.S. By contrast, HDP rates were predominantly lower in women born outside the U.S. compared with those born in the U.S. The GDM rate was highest in Asian Indian women born outside the U.S., and the HDP rate was highest in non-Hispanic Black women born inside the U.S. GDM and HDP rates increased in most race/ethnicity groups in women born either outside or in the U.S.
These findings extend prior studies documenting the contrasting association of nativity with HDP and GDM in smaller cohort or population-based samples,9,13,14 and similar patterns in several studies from populations in Europe.15,16 Although the “healthy immigrant paradox”—which hypothesizes selective immigration to the U.S. of women who are healthier at baseline and therefore with better health outcomes—may contribute to the difference in HDP by maternal nativity,17 a similar explanation is inconsistent with the observation of higher GDM rates among most women born outside the U.S.
Despite overlapping risk factors such as older age and higher BMI, and prior identification of GDM as a risk factor for hypertension in pregnancy, concomitant GDM and HDP occurred infrequently.18 Further, the generally opposing directions of differences in GDM and HDP rates by place of birth among the same group of women likely indicate that factors beyond those identified in these data contribute to differences by place of birth. For instance, women with GDM born outside the U.S. have a higher average maternal age, lower educational attainment, and higher prevalence of Medicaid or no insurance compared with their U.S.-born counterparts. These same women born outside the U.S. had lower HDP rates on average than women born in the U.S., suggesting that other factors may contribute to differences in HDP by place of birth.
There are likely complex interactions between place of birth, immigration, and race/ethnicity in influencing the divergent risks for GDM and HDP. These factors compose a wide range of health determinants, including cultural behaviors, SES, and neighborhood-level characteristics such as racial segregation and built environment.19,20 Early-life dietary patterns, which vary by region and race/ethnicity, may particularly influence GDM.21 Women born outside the U.S. may have increasing risk of GDM after immigration as they are exposed to highly processed foods more readily accessible and at lower cost in the U.S.22,23 Systemic factors likely also contribute, including access to and affordability of care, and experiences of discrimination and racism. The relative contribution of these factors may vary across race/ethnicity groups.7
Related to immigration, acculturation (i.e., retaining, adopting, or integrating beliefs, values, and behaviors between 2 cultures) and duration of residence in the U.S. may contribute to risk for APOs,24 which may in part reflect changes in dietary patterns related to exposures to unhealthful food environments and a sedentary lifestyle.25,26 One contrasting finding is in Japanese American women, who have higher GDM rates in U.S.-born women. Lifestyle differences (e.g., dietary pattern) may contribute to this observation, but further investigation specifically in this population is warranted. Differences across countries of origin, and potential heterogeneity within the level of country of origin, also warrant further study. Ultimately, clinical and public health prevention efforts focusing on optimizing lifestyle behaviors (i.e., healthful diet, exercise) to reduce GDM and HDP incidence may help mitigate their burden, in the U.S. and abroad.
Limitations
Strengths of this analysis include self-reported place of birth and race/ethnicity groups including disaggregated Hispanic/Latina and non-Hispanic Asian subgroups, which are frequently under-represented in research.27 However, there are several limitations. First, the potential for miscoding of GDM or HDP may exist; however, these items are reported by the professional birth attendant at the time of delivery using information from multiple sources, and miscoding would likely be random and lead to a greater chance of a null finding. The specificity of birth certificates for GDM is >98% and sensitivity is 46%–83% (median 65%)28 with substantial agreement of GDM diagnosis between birth certificates and medical records.29 The specificity of birth records for HDP is >96%, with sensitivities of 23%–99%.30 These data suggest birth certificates provide reasonable accuracy for identifying GDM and HDP. Notably, variation may exist in screening and diagnosis of GDM and HDP at the individual level or by region. Second, this analysis only included women with a singleton first live birth. The data set is de-identified so individuals with >1 delivery in the study period could not be separately identified and were thus excluded. Third, although GDM and HDP by maternal nativity were evaluated in Hispanic/Latina and non-Hispanic Asian subgroups, several other groups are not separately available on the 2003 birth certificate revision for self-identification, such as Middle Eastern, North African, or Southeast Asian populations. Fourth, these estimates do not account for women with fetal deaths, who were not included owing to state-level inconsistency in reporting. Though fetal death is associated with other APOs, these records represented <0.7% of pregnancy records during this study period. Fifth, vital statistics do not include data on covariates such as diet quality, physical activity, length of residence in the U.S., and other environmental, cultural, and socioeconomic factors, which limits assessment of the contribution of risk factors to nativity differences in GDM and HDP.
CONCLUSIONS
In the U.S., GDM rates were generally higher and HDP rates generally lower among women born outside the U.S. compared with those born in the U.S. across most race/ethnicity groups. Asian Indian women born outside the U.S. experienced the highest rate of GDM, while non-Hispanic Black women born in the U.S. experienced the highest rate of HDP.
Supplementary Material
ACKNOWLEDGMENTS
Nilay S. Shah and Sadiya S. Khan had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. No financial disclosures were reported by the authors of this paper. Research reported in this publication was supported, in part, by the National Heart, Lung, and Blood Institute grant number F32HL149187 (NSS) and NIH, grant numbers P30AG059988 and P30DK092939 (SSK). Research reported in this publication was also supported, in part, by the American Heart Association (#19TPA34890060) to SSK. The funding organizations were not involved in design or conduct of the study; collection, management, analysis, or interpretation of the data; preparation, review, or approval of the manuscript; or the decision to submit the manuscript for publication. The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the funding organizations.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Credit_statement
Nilay S. Shah: Conceptualization, Methodology, Formal analysis, Writing - Original Draft, Visualization, Supervision, Funding Acquisition; Michael C. Wang: Conceptualization, Methodology, Formal analysis, Writing – Review & Editing; Namratha R. Kandula: Conceptualization, Writing – Review & Editing; Mercedes R. Carnethon: Writing – Review & Editing; Erica P. Gunderson: Writing – Review & Editing; William A. Grobman: Writing – Review & Editing; Sadiya S. Khan: Conceptualization, Methodology, Writing – Review & Editing, Supervision, Funding Acquisition
REFERENCES
- 1.Shah NS, Wang MC, Freaney PM, et al. Trends in gestational diabetes at first live birth by race and ethnicity in the US, 2011‒2019. JAMA. 2021;326(7):660–669. 10.1001/jama.2021.7217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ying W, Catov JM, Ouyang P. Hypertensive disorders of pregnancy and future maternal cardiovascular risk. J Am Heart Assoc. 2018;7(17):e009382. 10.1161/jaha.118.009382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gunderson EP, Sun B, Catov JM, et al. Gestational diabetes history and glucose tolerance after pregnancy associated with coronary artery calcium in women during midlife: the CARDIA Study. Circulation. 2021;143(10):974–987. 10.1161/circulationaha.120.047320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Parikh NI, Gonzalez JM, Anderson CAM, et al. Adverse pregnancy outcomes and cardiovascular disease risk: unique opportunities for cardiovascular disease prevention in women: a scientific statement from the American Heart Association. Circulation. 2021;143(18):e902–e916. 10.1161/cir.0000000000000961. [DOI] [PubMed] [Google Scholar]
- 5.Ghosh G, Grewal J, Mannisto T, et al. Racial/ethnic differences in pregnancy-related hypertensive disease in nulliparous women. Ethn Dis. 2014;24(3):283–289. [PMC free article] [PubMed] [Google Scholar]
- 6.Havranek EP, Mujahid MS, Barr DA, et al. Social determinants of risk and outcomes for cardiovascular disease: a scientific statement from the American Heart Association. Circulation. 2015;132(9):873–898. 10.1161/cir.0000000000000228. [DOI] [PubMed] [Google Scholar]
- 7.Guadamuz JS, Kapoor K, Lazo M, et al. Understanding immigration as a social determinant of health: cardiovascular disease in Hispanics/Latinos and South Asians in the United States. Curr Atheroscler Rep. 2021;23(6):25. 10.1007/s11883-021-00920-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Singh GK, Yu SM. Adverse pregnancy outcomes: differences between US- and foreign-born women in major US racial and ethnic groups. Am J Public Health. 1996;86(6):837–843. 10.2105/ajph.86.6.837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Boakye E, Sharma G, Ogunwole SM, et al. Relationship of preeclampsia with maternal place of birth and duration of residence among non-Hispanic Black women in the United States. Circ Cardiovasc Qual Outcomes. 2021;14(2):e007546. 10.1161/circoutcomes.120.007546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Elo IT, Vang Z, Culhane JF. Variation in birth outcomes by mother’s country of birth among non-Hispanic Black women in the United States. Matern Child Health J. 2014;18(10):2371–2381. 10.1007/s10995-014-1477-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.User Guide to the 2014 Natality Public Use File. http://data.nber.org/natality/2014/natl2014.pdf. Published 2014. Accessed May 15, 2021.
- 12.Joinpoint Regression Program, Version 4.7.0.0 [computer program] National Cancer Institute; 2019. [Google Scholar]
- 13.Kieffer EC, Martin JA, Herman WH. Impact of maternal nativity on the prevalence of diabetes during pregnancy among U.S. ethnic groups. Diabetes Care. 1999;22(5):729–735. 10.2337/diacare.22.5.729. [DOI] [PubMed] [Google Scholar]
- 14.Singh GK, Siahpush M, Liu L, Allender M. Racial/ethnic, nativity, and sociodemographic disparities in maternal hypertension in the United States, 2014‒2015. Int J Hypertens. 2018;2018:7897189. 10.1155/2018/7897189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Strandberg RB, Iversen MM, Jenum AK, et al. Gestational diabetes mellitus by maternal country of birth and length of residence in immigrant women in Norway. Diabet Med. 2021;38(6):e14493. 10.1111/dme.14493. [DOI] [PubMed] [Google Scholar]
- 16.Seghieri G, Di Cianni G, Seghieri M, et al. Risk and adverse outcomes of gestational diabetes in migrants: a population cohort study. Diabetes Res Clin Pract. 2020;163:108128. 10.1016/j.diabres.2020.108128. [DOI] [PubMed] [Google Scholar]
- 17.Kennedy S, Kidd MP, McDonald JT, Biddle N. The healthy immigrant effect: patterns and evidence from four countries. J Int Migr Integr. 2015;16:317–332. 10.1007/s12134-014-0340-x. [DOI] [Google Scholar]
- 18.Bryson CL, Ioannou GN, Rulyak SJ, Critchlow C. Association between gestational diabetes and pregnancy-induced hypertension. Am J Epidemiol. 2003;158(12):1148–1153. 10.1093/aje/kwg273. [DOI] [PubMed] [Google Scholar]
- 19.Mayne SL, Yellayi D, Pool LR, Grobman WA, Kershaw KN. Racial residential segregation and hypertensive disorder of pregnancy among women in Chicago: analysis of electronic health record data. Am J Hypertens. 2018;31(11):1221–1227. 10.1093/ajh/hpy112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kershaw KN, Marsh DJ, Crenshaw EG, et al. Associations of the neighborhood built environment with physical activity across pregnancy. J Phys Act Health. 2021;18(5):541–547. 10.1123/jpah.2020-0510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schoenaker DA, Mishra GD, Callaway LK, Soedamah-Muthu SS. The role of energy, nutrients, foods, and dietary patterns in the development of gestational diabetes mellitus: a systematic review of observational studies. Diabetes Care. 2016;39(1):16–23. 10.2337/dc15-0540. [DOI] [PubMed] [Google Scholar]
- 22.Bao W, Tobias DK, Olsen SF, Zhang C. Pre-pregnancy fried food consumption and the risk of gestational diabetes mellitus: a prospective cohort study. Diabetologia. 2014;57(12):2485–2491. 10.1007/s00125-014-3382-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang X, Gong Y, Della Corte K, et al. Relevance of dietary glycemic index, glycemic load and fiber intake before and during pregnancy for the risk of gestational diabetes mellitus and maternal glucose homeostasis. Clin Nutr. 2021;40(5):2791–2799. 10.1016/j.clnu.2021.03.041. [DOI] [PubMed] [Google Scholar]
- 24.Premkumar A, Debbink MP, Silver RM, et al. Association of acculturation with adverse pregnancy outcomes. Obstet Gynecol. 2020;135(2):301–309. 10.1097/aog.0000000000003659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schoenaker DA, Soedamah-Muthu SS, Mishra GD. The association between dietary factors and gestational hypertension and pre-eclampsia: a systematic review and meta-analysis of observational studies. BMC Med. 2014;12:157. 10.1186/s12916-014-0157-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Spracklen CN, Ryckman KK, Triche EW, Saftlas AF. Physical activity during pregnancy and subsequent risk of preeclampsia and gestational hypertension: a case control study. Matern Child Health J. 2016;20(6):1193–1202. 10.1007/s10995-016-1919-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shah NS, Kandula NR. Addressing Asian American misrepresentation and underrepresentation in research. Ethn Dis. 2020;30(3):513–516. 10.18865/ed.30.3.513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Devlin HM, Desai J, Walaszek A. Reviewing performance of birth certificate and hospital discharge data to identify births complicated by maternal diabetes. Matern Child Health J. 2009;13(5):660–666. 10.1007/s10995-008-0390-9. [DOI] [PubMed] [Google Scholar]
- 29.Gregory ECW, Martin JA, Argov EL, Osterman MJK. Assessing the quality of medical and health data from the 2003 Birth Certificate Revision: results from New York City. Natl Vital Stat Rep. 2019;68(8):1–20. [PubMed] [Google Scholar]
- 30.Roberts CL, Bell JC, Ford JB, Hadfield RM, Algert CS, Morris JM. The accuracy of reporting of the hypertensive disorders of pregnancy in population health data. Hypertens Pregnancy. 2008;27(3):285–297. 10.1080/10641950701826695. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


