Abstract
Objective
To understand the influence of drug manufacturers on the prescribing patterns of medical oncologists and urologists, we examined the relationship between promotional payments from the manufacturers of abiraterone and enzalutamide and prescriptions for either drug by medical oncologists and urologists.
Methods
Promotional payments for abiraterone or enzalutamide made to medical oncologists and urologists between January 2014 and December 2017 reported through the Open Payments Program were categorized as $0, $1–$999, and $1000 or more. Prescriptions filled between January 2013 and December 2017 were identified in the Medicare Part D File. Associations between promotional payments and prescribing were assessed using generalized linear models.
Results
From 2013 through 2017, the number of medical oncologists and urologists prescribing abiraterone or enzalutamide increased by 38% and 298%, respectively. The odds of prescribing among medical oncologists receiving $1–$999 and those receiving $1,000 or more were 1.69 (95%CI:1.59–1.79) and 2.61 (95% CI: 2.14–3.18) times that of medical oncologists receiving no payments. Among urologists receiving $1–$999 and those receiving $1,000 or more, the odds of prescribing were 4.04 (95%CI: 3.59–4.54) and 13.57 (95%CI: 9.69–19.0) times that of urologists receiving no payments.
Conclusions
Increasing promotional payments were associated with prescribing among medical oncologists and urologists, with a stronger relationship evident for urologists. Prescribing patterns for abiraterone and enzalutamide, particularly among urologists, may be influenced by payments from drug manufacturers.
Keywords: prostate cancer, industry payments, financial incentives
Introduction
An estimated 34,000 men die from prostate cancer annually, making it the second leading cause of cancer death among men in the United States.1 The most advanced form of prostate cancer, castration-resistant prostate cancer, was traditionally managed with intravenous chemotherapy. After approvals in 2011 and 2012,2–5 abiraterone and enzalutamide became the most widely prescribed drugs for treatment of men with castration-resistant prostate cancer,6 providing better tolerated alternatives to cytotoxic chemotherapy.7,8 In 2019, abiraterone and enzalutamide accounted for $2.7 billion in Medicare Part D spending.9 Unlike intravenous chemotherapy which is prescribed by medical oncologists, abiraterone and enzalutamide are oral agents that can be prescribed by any physician, including urologists. This paradigm shift now enables urologists to manage patients with prostate cancer throughout the disease continuum, in some instances independent of medical oncologists.
Some worry that the rapid adoption of abiraterone and enzalutamide, by urologists in particular,10 may be influenced by financial incentives afforded through industry payments, which are associated with increased utilization across a range of clinical contexts.10–14 The potential benefits of increased urologist involvement in the medical management of men with advanced prostate cancer include enhanced continuity of care, afforded by the longstanding patient-urologist relationship. Nonetheless, rapid entry of urologists into this space may outpace the development of clinical skills needed to manage the metabolic and cardiovascular side effects of abiraterone and enzalutamide.15,16 As indications for abiraterone and enzalutamide expand to earlier stages of the disease,17–21 an increasing number of men will be prescribed these beneficial but expensive drugs for longer periods of time, expanding their time at risk for side effects and underscoring the critical need to examine the factors associated with prescribing patterns.
Linking public disclosures of industry payments with national Medicare data, we examined the relationship between payments made for the promotion of abiraterone or enzalutamide by the respective drug manufacturers and prescribing patterns of these drugs by medical oncologists and urologists. Understanding the extent to which drug manufacturers may influence prescribing patterns will provide important insights for all stakeholders.
Methods
All medical oncologists and urologists providing care to Medicare beneficiaries from 2013 to 2017 were identified using Medicare Data on Physician Practice and Specialty.22 Publicly available data from the Centers for Medicare & Medicaid Services Open Payments Program23 was used to characterize payments for abiraterone or enzalutamide made to medical oncologists and urologists by the drug manufacturers (Janssen Biotech and Astellas Pharma, respectively). The Open Payments Program, created under the Physician Payment Sunshine Act, requires manufacturers and group purchasing organizations to report payments and other transfers of value made to physicians and teaching hospitals beginning August 2013. Payments are categorized as general payments, research payments, or ownership and investment interests. General payments are further classified as education, food and beverages, honoraria, travel and lodging, consulting fee, and services other than consulting (i.e., speaking, training, and education engagements that are not for continuing education). To focus on the effects of industry marketing, we limited our analysis to promotional payments that were made specifically for abiraterone or enzalutamide, as reported by the manufactures to the Open Payments Program (hereafter referred to as “promotional payments”, as in prior literature)10,24,25. Due to partial-year reporting of the Open Payments data in 2013, we limited our payment-related analyses to 2014 and beyond. To facilitate comparisons, we elected a priori to sort yearly payments into three groups ($0, $1–$999, and $1000 or more). We used a 20% national sample of the Medicare Part D Event file26 from 2013 to 2017 to identify prescriptions for abiraterone or enzalutamide and the associated prescribing physician.
Statistical analysis
We first characterized physicians (i.e., specialty, age, gender, and region of practice) and payments (i.e., median payments per physician, nature) according to the magnitude of promotional payments in each year. Next, we summarized total payments and prescribing patterns (number of prescribers, cumulative growth in prescribers benchmarked to 2013, number of prescriptions filled, cumulative growth in prescriptions filled benchmarked to 2013) by specialty. To contrast specialty prescribing, we measured the percentages of medical oncologists or urologists prescribing abiraterone or enzalutamide by the magnitude of promotional payments received ($0, $1–$999, or $1000 or more) in each year.
Next, we fit a generalized linear model to examine the relationship between promotional payments and prescribing patterns. The exposure was the promotional payments tied to abiraterone or enzalutamide made to a physician in a year and the outcome was a filled prescription for either drug, measured at the patient level. To determine whether the relationship between promotional payments and a prescription fill varied by specialty, we tested for an interaction between promotional payments and specialty. Since the interaction term was significant, we stratified the analysis by specialty. The model was adjusted for year and physician characteristics (i.e., age, gender, and region of practice). To isolate the influence of promotional payments on subsequent prescribing, we performed a secondary analysis in which we excluded physicians prescribing either drug in the 12-month period prior to receiving their first payment from either company. Finally, using the models, we estimated the adjusted percentage of prescribers in both specialties by the magnitude of promotional payments received.
All analyses were performed using SAS software (version 9.4; SAS Institute, Cary, NC). This study used de-identified administrative claims and publicly available data and was therefore deemed exempt by the institutional review board.
Results
Physician and payment characteristics for all medical oncologists and urologists treating Medicare beneficiaries from 2014 to 2017 according to the magnitude of promotional payments for abiraterone or enzalutamide are shown in Table 1. A decreasing percentage of medical oncologists and an increasing percentage of urologists received promotional payments from the two drug manufacturers, regardless of whether they prescribed the drugs. In 2017, approximately one-quarter of all medical oncologists received promotional payments (23.7% and 0.9% received $1–$999 and $1000 or more, respectively), while more than half of all urologists received promotional payments (52.0% and 1.0% received $1–$999 and $1000 or more, respectively). Virtually all faculty, speaker, and consulting payments were valued $1000 or more per payment, whereas payments for education, and food and beverages were valued between $1–$999.
Table 1a.
Physician and payment characteristics for all medical oncologists and urologists treating Medicare beneficiaries, by year
| 2014 | 2015 | |||||
|---|---|---|---|---|---|---|
| $0 | $1–$999 | ≥$1000 | $0 | $1–$999 | ≥$1000 | |
| Specialty, n (%) | ||||||
| Medical oncology | 8,532 (68.3) | 3,859 (30.9) | 107 (0.9) | 9,163 (72.6) | 3,329 (26.4) | 123 (1.0) |
| Urology | 5,232 (54.7) | 4,222 (44.1) | 112 (1.2) | 5,065 (52.9) | 4,356 (45.5) | 157 (1.6) |
| Age, mean (SD) | 51.4 (12.2) | 52.4 (10.9) | 51.4 (9.3) | 51.5 (12.1) | 52.7 (11.0) | 52.7 (9.7) |
| Female, n (%) | 3,463 (74.5) | 1,178 (25.3) | 10 (0.2) | 3,777 (77.5) | 1,087 (22.3) | 12 (0.3) |
| Region of practice, n (%) | ||||||
| South | 4,083 (53.7) | 3,440 (45.3) | 80 (1.1) | 4,238 (55.4) | 3,307 (43.2) | 104 (1.4) |
| Northeast | 3,423 (66.6) | 1,660 (32.3) | 56 (1.1) | 3,556 (68.9) | 1,549 (30.0) | 57 (1.1) |
| Midwest | 3,259 (68.7) | 1,447 (30.5) | 39 (0.8) | 3,301 (69.2) | 1,413 (29.6) | 54 (1.1) |
| West | 2,887 (65.7) | 1,461 (33.3) | 44 (1.0) | 3,005 (67.9) | 1,357 (30.7) | 64 (1.5) |
| Median payment per physician, (IQR) | - | $66 ($26–$124) | $8,635 ($4,000–$20,091) | - | $69 ($28–$133) | $5,896 ($3,442–$16,862) |
| Medical oncology | - | $68 ($28–$120) | $8,517 ($4,491–$19,215) | - | $57 ($24–$110) | $5,162 ($3,935–$12,617) |
| Urology | - | $65 ($25–$127) | $8,676 ($3,680–$21,472) | - | $81 ($33–$154) | $7,141 ($3,381–$20,653) |
| Nature of payment, n (%) | ||||||
| Faculty, speaker | - | 0 (0.0) | 121 (100.0) | - | 0 (0.0) | 129 (100.0) |
| Consulting | - | 20 (18.5) | 88 (81.5) | - | 2 (1.3) | 148 (98.7) |
| Education | - | 1,446 (100.0) | 0 (0.0) | - | 966 (100.0) | 0 (0.0) |
| Food and beverage | - | 6,610 (100.0) | 0 (0.0) | - | 6,716 (100.0) | 0 (0.0) |
| Honoraria | - | 0 (0.0) | 0 (0.0) | - | 0 (0.0) | 0 (0.0) |
| Travel and lodging | - | 5 (33.3) | 10 (66.7) | - | 1 (25.0) | 3 (75.0) |
Abbreviations: SD, standard deviation; IQR, interquartile range.
The manufacturers of abiraterone and enzalutamide consistently paid urologists more in total value of promotional payments than medical oncologists, despite medical oncologists being responsible for the majority of prescriptions (Table 2). Between 2013 and 2017, the number of medical oncologists and urologists prescribing abiraterone or enzalutamide increased by 38% and 298%, respectively, and the number of patients filling prescriptions from medical oncologists and urologists increased by 62% and 476%, respectively. The percentages of prescribing and non-prescribing medical oncologists and urologists according to the magnitude of promotional payments received are illustrated in Supplementary Figure 1.
Table 2.
Specialty-level total payment, prescribers, and prescriptions.
| Total payment | No. of prescribers (%) | No. of prescriptions | |||
|---|---|---|---|---|---|
| Medical oncology | |||||
| 2014 | $2,007,817 | 2,694 (21.6) | 119,155 | ||
| 2015 | $1,847,384 | 2,912 (23.1) | 140,320 | ||
| 2016 | $1,705,784 | 2,923 (22.7) | 138,285 | ||
| 2017 | $2,194,336 | 2,971 (22.6) | 129,240 | ||
| Urology | |||||
| 2014 | $2,671,404 | 335 (3.5) | 12,840 | ||
| 2015 | $3,336,518 | 572 (6.0) | 24,950 | ||
| 2016 | $3,194,405 | 692 (7.2) | 31,465 | ||
| 2017 | $2,917,381 | 660 (6.8) | 28,840 | ||
relative to 2013
Total payment was derived by summing all promotional payments for abiraterone or enzalutamide made by the manufacturers of the two drugs to all medical oncologists or urologists serving Medicare beneficiaries. Percentage of prescribers was derived by dividing the number of medical oncologists or urologists who prescribed abiraterone or enzalutamide over all medical oncologists or urologists serving Medicare beneficiaries. Growth in the numbers of prescribers and prescriptions were calculated relative to numbers of prescribers and prescriptions filled in 2013.
Table 3 demonstrates the independent association between promotional payments and prescribing. Relative to medical oncologists receiving no promotional payments, those receiving between $1–$999 had 1.69 (95% confidence interval [CI]: 1.59–1.79) times the odds of prescribing abiraterone or enzalutamide and those receiving $1000 or more had 2.61 (95% CI: 2.14–3.18) times the odds of prescribing (overall p<0.01). Relative to urologists receiving no promotional payments, those receiving between $1–$999 had 4.04 (95% CI: 3.59–4.54) times the odds of prescribing abiraterone or enzalutamide and those receiving $1000 or more had 13.57 (95% CI: 9.69–19.0) times the odds of prescribing (overall p<0.01).
Table 3.
Multivariable model examining factors associated with prescription fills for abiraterone or enzalutamide by specialty
| Medical Oncology | Urology | |||
|---|---|---|---|---|
| Odds Ratio (95% CI) | p-value | Odds Ratio (95% CI) | p-value | |
| Payment | ||||
| $0 | Ref | <0.01 | Ref | 0.01 |
| $1–$999 | 1.69 (1.59–1.79) | 4.04 (3.59–4.54) | ||
| ≥$1000 | 2.61 (2.14–3.18) | 13.57 (9.69–19.0) | ||
| Year of payment | ||||
| 2014 | Ref | <0.01 | Ref | <0.01 |
| 2015 | 1.14 (1.10–1.19) | 1.79 (1.59–2.03) | ||
| 2016 | 1.15 (1.10–1.21) | 2.18 (1.91–2.50) | ||
| 2017 | 1.17 (1.10–1.23) | 2.14 (1.85–2.47) | ||
| Age | 1.00 (1.00–1.00) | 0.81 | 1.00 (1.00–1.01) | 0.10 |
| Gender | ||||
| Male | Ref | <0.01 | Ref | <0.01 |
| Female | 0.48 (0.44–0.51) | 0.14 (0.08–0.27) | ||
| Region | ||||
| South | Ref | <0.01 | Ref | 0.09 |
| Northeast | 0.73 (0.66–0.79) | 0.95 (0.79–1.13) | ||
| Midwest | 1.03 (0.94–1.12) | 1.18 (0.99–1.41) | ||
| West | 1.18 (1.08–1.28) | 0.90 (0.75–1.09) | ||
Abbreviations: CI, confidence interval; Ref, reference.
Results shown are odds radios with 95% confidence intervals of prescribing abiraterone or enzalutamide relative to not prescribing either drug.
To isolate the potential association of receipt of payment on subsequent prescribing patterns, we performed a secondary analysis that excluded 449 medical oncologists (2.8% of all medical oncologists in study population) and 79 urologists (0.7% of all urologists in study population) who had previously prescribed abiraterone or enzalutamide in the 12-month period before receiving their first promotional payment. After establishing temporal precedence of payment, we found that payment magnitude remained strongly associated with prescribing patterns for physicians of both specialties (Supplementary Table 1).
The percentages of physicians prescribing abiraterone or enzalutamide based on the three models—unadjusted analysis, adjusted main analysis, and secondary analysis with exclusion of physicians with prescriptions in the 12-months prior to receiving their first payment—are demonstrated in Figure 1. Across the three models, the percentages of physicians prescribing abiraterone or enzalutamide increased with increasing promotional payments for both specialties, with a notably steeper gradient among urologists.
Figure 1.
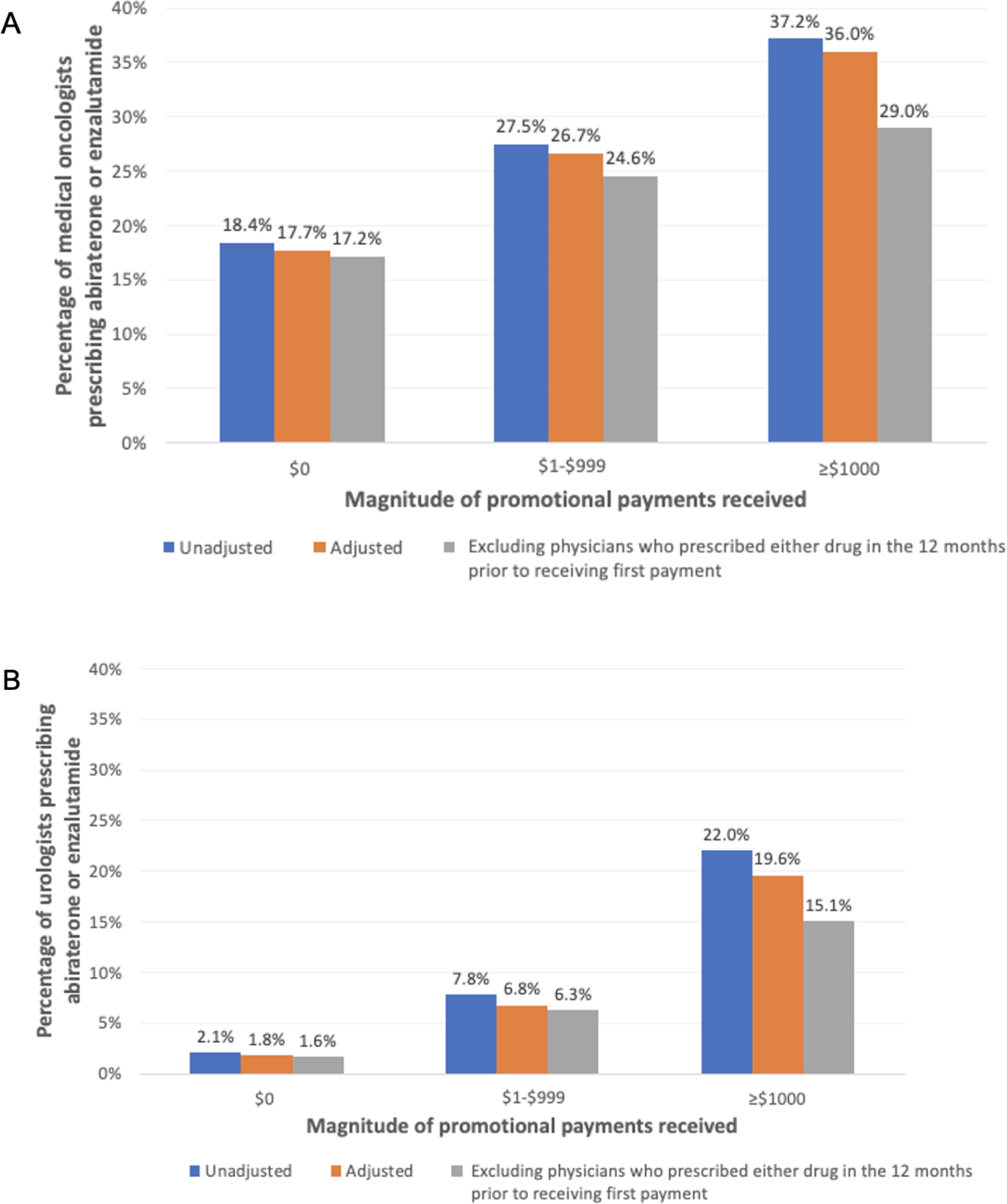
Model-derived percentages of physicians prescribing abiraterone or enzalutamide according to the magnitude of promotional payments received among A. medical oncologists and B. urologists
Source: Authors’ analysis of data from the Medicare Part D Event File, Open Payments Program, and Medicare Data on Physician Practice and Specialty.
Note: Percentages of physicians prescribing abiraterone or enzalutamide according to the magnitude of promotional payments received ($0 vs $1–$999 vs ≥$1000) were derived from three models: unadjusted (blue bar), adjusted (orange bar), and adjusted with exclusion of physicians prescribing abiraterone or enzalutamide in the 12 months prior to receiving their first promotional payment (gray bar). In the two adjusted models, year, physician age, physician gender, and region of practice were included as covariates. Across all three models, the percentages of physicians prescribing abiraterone or enzalutamide increased with higher payments for both specialties, with a notably steeper gradient of increase among urologists.
Discussion
In this national study of the association between promotional payments and prescriptions filled for abiraterone or enzalutamide, we found that medical oncologists and urologists receiving increasing promotional payments for abiraterone or enzalutamide had higher odds of prescribing either drug, with a stronger relationship observed among urologists. Importantly, these relationships were similar in direction and magnitude after excluding physicians who prescribed the drugs prior to receiving their first payment. Collectively, these findings suggest that prescribing behavior, and perhaps entry into this space, may be influenced by compensation from drug manufacturers, particularly among urologists.
Our study adds to recent literature highlighting the association between payments from drug manufacturers and prescriptions for the promoted drugs evident across a range of clinical contexts.10–14,27 We build on prior studies by juxtaposing the association of promotional payments on prescribing patterns of two groups of physicians with different footholds in the space of medical management of men with advanced prostate cancer. Given that the care of men with advanced prostate cancer was traditionally under the purview of medical oncologists, it is conceivable that drug manufacturers recognized unbalanced barriers to drug adoption and strategically invested more in promotional payments to urologists—up to $1.5 million annually—as a means to nudge them into entering this space. We found large numbers of physicians receiving $1000 or more did not prescribe abiraterone or enzalutamide in the same year of receiving promotional payment. One possible explanation is that drug manufacturers did not target their payments to medical oncologists and urologists who managed men with prostate cancer, such that physicians who did not have the opportunity to prescribe abiraterone or enzalutamide were also receiving large sums of promotional payment. It is also possible that some of these physicians were acting as key opinion leaders,28 promoting the use of these drugs rather than spending time in clinic treating men with advanced prostate cancer with either drug. Since the approval of abiraterone and enzalutamide predated the establishment of the Open Payments Program, precluding analyses on the extent to which promotional payments may have contributed to the early dissemination of the two drugs, our study captures a partial picture of the influence of drug manufacturers on prescribing patterns.
Our study finding of an association between promotional payments and prescribing patterns are in contrast to early important work in this area.29 In prior work, authors found no correlation between payment amount and prescription count for abiraterone prescribers and only a weak correlation for enzalutamide prescribers. However, there are several important differences between that study and ours. First, our study focused on general payments only rather than aggregating all forms of payments (including research payments) from the manufacturers of abiraterone and enzalutamide. Second, that study used the Medicare Part D Prescriber Public Use File and thus only included physicians who dispensed more than 10 prescriptions, whereas our study included the research identifiable Medicare Part D Event File, which was not limited by that factor. Third, we were able to investigate several years of data and our methodology allowed us to establish temporal precedence for payment and prescribing, whereas the previous study linked payments made in 2014 to prescriptions dispensed in 2013 – evaluating payments that came after prescribing was established – and with data limited to 2013 when prescribing of abiraterone and enzalutamide among urologists was extremely rare. Finally, we examined the odds of prescribing as a function of receipt of open payments among all medical oncologists and urologists caring for Medicare beneficiaries; the previous study’s primary analyses were limited to those who were already prescribing abiraterone or enzalutamide.
Financial incentives may be a double-edged sword. On the one hand, financial incentives can accelerate adoption of effective therapies and be leveraged to address underuse and to improve treatment access. On the other hand, financial incentives may foster over-utilization in circumstances of clinical uncertainty, if payments are left unchecked over time. While there is currently no evidence of inappropriate prescribing, stakeholders should be mindful of the influence of the manufacturers of these therapies that are costly, heavily promoted, and not without harms, especially as abiraterone and enzalutamide are now used in earlier phases of the disease trajectory, and prescribed to increasing numbers of men for longer periods of time.
This study has several limitations. First, despite establishing temporal precedence in the secondary analysis, we cannot infer a causal relationship from cross-sectional findings. Second, by including all medical oncologists and urologists treating Medicare beneficiaries in our study, our denominator might have included physicians who do not currently provide care for men with prostate cancer. However, this approach allowed us to investigate the potential to become abiraterone or enzalutamide prescribers among all physicians who care for Medicare beneficiaries. Third, as indications for these drugs have evolved, patients included in the study were likely at various stages in the disease trajectory. As claims lack information on cancer severity, we could not determine the appropriateness of prescribing for either drug. Nonetheless, differences in severity at the patient level were unlikely to be associated with promotional payments made to the prescribing physician or to explain the magnitude of increase in odds of prescribing after receiving payment. Fourth, our outcome measure was based on prescriptions written for and filled by Medicare beneficiaries, precluding the generalizability of our findings to younger and privately insured men with prostate cancer. However, we do not expect insurance status to change the influence of promotional payments on prescribing patterns. Lastly, because Open Payments data do not include payments made to advanced practice providers, who often work in tandem with medical oncologists and urologists to prescribe these drugs, we are only highlighting one sphere of influence of the drug manufacturers. With the expansion of the Open Payments Program to cover non-physician prescribers starting in 2022,30 future studies should examine the extent of the influence of drug manufacturers on prescribing patterns among all providers serving patients with advanced prostate cancer.
Conclusion
Promotional payments for abiraterone and enzalutamide are strongly associated with prescribing patterns of abiraterone and enzalutamide among both medical oncologists and urologists. By examining the association of promotional payments on prescribing among two specialties with different footholds in the space of medical management of men with advanced prostate cancer, our study provided a novel lens into one strategy potentially used by drug manufacturers to expand growth into an emerging market. Future work examining prostate cancer care should take into consideration the potential external influence of drug manufacturers, who have substantial resources available for promotional marketing.
Supplementary Material
Supplementary Figure 1. Caption: Percentage of prescribers among all physicians categorized by the magnitude of promotional payments received between 2014 and 2017.
Source: Authors’ analysis of data from the Medicare Part D Event File, Open Payments Program, Medicare Data on Physician Practice and Specialty.
Note: All medical oncologists and urologists serving Medicare beneficiaries were first categorized based on the aggregate of promotional payments received in each given year ($0, $1–$999; ≥$1000). Then, the percentage of physicians who were prescribers of abiraterone or enzalutamide were calculated in each category. The percentage of physicians prescribing abiraterone or enzalutamide increased with higher promotional payments for both specialties, with a notably steeper gradient of increase among urologists. For instance, in 2017, 18.0% (n=1,777) of medical oncologists who received $0 (n=9,894) had prescribed abiraterone or enzalutamide, compared to 36.4% (n=1,135) of those who received $1–$999 (n=3,118) and 48.0% (n=59) of those who received $1000 or more (n=123). By contrast, 1.3% (n=58) of urologists who received $0 (n=4,535) had prescribed abiraterone or enzalutamide, compared to 10.9% (n=544) of those who received $1–$999 (n=5,012) and 60.4% (n=58) of those who received $1000 or more (n=96).
Table 1b.
Physician and payment characteristics for all medical oncologists and urologists treating Medicare beneficiaries, by year
| 2016 | 2017 | |||||
|---|---|---|---|---|---|---|
| $0 | $1–$999 | ≥$1000 | $0 | $1–$999 | ≥$1000 | |
| Specialty, n (%) | ||||||
| Medical oncology | 9,509 (74.0) | 3,249 (25.3) | 97 (0.8) | 9,894 (75.3) | 3,118 (23.7) | 123 (0.9) |
| Urology | 4,325 (45.2) | 5,110 (53.4) | 135 (1.4) | 4,535 (47.0) | 5,012 (52.0) | 96 (1.0) |
| Age, mean (SD) | 51.4 (12.1) | 52.7 (11.2) | 54.1 (9.7) | 51.3 (12.2) | 53.0 (11.4) | 52.7 (9.0) |
| Female, n (%) | 3,911 (76.8) | 1,171 (23.0) | 11 (0.2) | 4,193 (78.8) | 1,109 (20.8) | 22 (0.4) |
| Region of practice, n (%) | ||||||
| South | 4,024 (52.1) | 3,606 (46.7) | 89 (1.2) | 4,230 (53.7) | 3,576 (45.4) | 79 (1.0) |
| Northeast | 3,469 (67.3) | 1,633 (31.7) | 51 (1.0) | 3,619 (69.2) | 1,554 (29.7) | 57 (1.1) |
| Midwest | 3,249 (67.3) | 1,532 (31.7) | 47 (1.0) | 3,350 (68.9) | 1,476 (30.4) | 36 (0.7) |
| West | 2,969 (65.5) | 1,519 (33.5) | 44 (1.0) | 3,089 (66.9) | 1,484 (32.1) | 47 (1.0) |
| Median payment per physician, (IQR) | - | $75 ($32–$141) | $7,571 ($3,377–$18,350) | - | $83 ($35–$163) | $9,534 ($4,056–$21,663) |
| Medical oncology | - | $55 ($25–$108) | $6,842 ($4,100–$17,724) | - | $51 ($24–$100) | $7225 ($3,399–$20,405) |
| Urology | - | $90 ($41–$167) | $7,962 ($2,983–$19,483) | - | $114 ($48–$212) | $11,799 ($5,508–$25,080) |
| Nature of payment, n (%) | ||||||
| Faculty, speaker | - | 0 (0.0) | 99 (100.0) | - | 1 (0.7) | 153 (99.3) |
| Consulting | - | 4 (3.3) | 118 (96.7) | - | 1 (1.6) | 63 (98.4) |
| Education | - | 794 (100.0) | 0 (0.0) | - | 475 (100.0) | 0 (0.0) |
| Food and beverage | - | 7,559 (99.9) | 10 (0.1) | - | 7,651 (100.0) | 0 (0.0) |
| Honoraria | - | 0 (0.0) | 0 (0.0) | - | 0 (0.0) | 1 (100.0) |
| Travel and lodging | - | 2 (28.6) | 5 (71.4) | - | 2 (50.0) | 2 (50.0) |
Abbreviations: SD, standard deviation; IQR, interquartile range.
Descriptive data for all medical oncologists and urologists serving Medicare beneficiaries and payment characteristics, according to the magnitude of promotional payments received each year.
Funding Support:
This work was supported by research funding from the AHRQ (R01 HS025707) to BKH and VBS. LYL is supported by the National Cancer Institute (T32 CA180984). TAS is supported by the National Cancer Institute (R01 CA242559 and R37 CA222885).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest: None to declare.
I have no conflicts of interest.
References
- 1.American Cancer Society. Key statistics for prostate cancer. Published 2021. Accessed March 14, 2021. https://www.cancer.org/cancer/prostate-cancer/about/key-statistics.html
- 2.Beer TM, Armstrong AJ, Rathkopf DE, et al. Enzalutamide in metastatic prostate cancer before chemotherapy. N Engl J Med. 2014;371(5):424–433. doi: 10.1056/NEJMoa1405095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ryan CJ, Smith MR, de Bono JS, et al. Abiraterone in metastatic prostate cancer without previous chemotherapy. N Engl J Med. 2013;368(2):138–148. doi: 10.1056/NEJMoa1209096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Scher HI, Fizazi K, Saad F, et al. Increased Survival with Enzalutamide in Prostate Cancer after Chemotherapy. N Engl J Med. 2012;367(13):1187–1197. doi: 10.1056/nejmoa1207506 [DOI] [PubMed] [Google Scholar]
- 5.de Bono JS, Logothetis CJ, Molina A, et al. Abiraterone and increased survival in metastatic prostate cancer. N Engl J Med. 2011;364(21):1995–2005. doi: 10.1056/NEJMoa1014618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Caram MEV, Estes JP, Griggs JJ, Lin P, Mukherjee B. Temporal and geographic variation in the systemic treatment of advanced prostate cancer. BMC Cancer. 2018;18(1):1–10. doi: 10.1186/s12885-018-4166-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tonyali S, Haberal HB, Sogutdelen E. Toxicity, Adverse Events, and Quality of Life Associated with the Treatment of Metastatic Castration-Resistant Prostate Cancer. Curr Urol. 2017;10(4):169–173. doi: 10.1159/000447176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang T, Zhu J, George DJ, Armstrong AJ. Enzalutamide versus abiraterone acetate for the treatment of men with metastatic castration-resistant prostate cancer. Expert Opin Pharmacother. 2015;16(4):473–485. doi: 10.1517/14656566.2015.995090 [DOI] [PubMed] [Google Scholar]
- 9.Centers for Medicare & Medicaid Services. Medicare Part D Drug Spending Dashboard & Data. Published 2020. Accessed March 15, 2021. https://www.cms.gov/Research-Statistics-Data-and-Systems/Statistics-Trends-and-Reports/Information-on-Prescription-Drugs/MedicarePartD
- 10.Hollenbeck BK, Oerline M, Kaufman SR, et al. Promotional payments made to urologists by the pharmaceutical industry and prescribing patterns for targeted therapies. Urology. Published online 2020. doi: 10.1016/j.urology.2020.08.080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Khan R, Nugent CM, Scaffidi MA, Gimpaya N, Grover SC. Association of Biologic Prescribing for Inflammatory Bowel Disease With Industry Payments to Physicians. JAMA Intern Med. 2019;179(10):1424. doi: 10.1001/jamainternmed.2019.0999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.DeJong C, Aguilar T, Tseng CW, Lin GA, Boscardin WJ, Dudley RA. Pharmaceutical industry-sponsored meals and physician prescribing patterns for medicare beneficiaries. JAMA Intern Med. 2016;176(8):1114–1122. doi: 10.1001/jamainternmed.2016.2765 [DOI] [PubMed] [Google Scholar]
- 13.Mitchell AP, Winn AN, Dusetzina SB. Pharmaceutical Industry Payments and Oncologists’ Selection of Targeted Cancer Therapies in Medicare Beneficiaries. JAMA Intern Med. 2018;178(6):854. doi: 10.1001/jamainternmed.2018.0776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Taylor SC, Huecker JB, Gordon MO, Vollman DE, Apte RS. Physician-Industry Interactions and Anti-Vascular Endothelial Growth Factor Use Among US Ophthalmologists. JAMA Ophthalmol. 2016;134(8):897–903. doi: 10.1001/jamaophthalmol.2016.1678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Garnick MB, Einstein DJ. Editorial Comment. Urology. 2019;131:182–183. doi: 10.1016/j.urology.2019.05.017 [DOI] [PubMed] [Google Scholar]
- 16.Dreicer R Editorial Comment. Urology. 2019;131:182. doi: 10.1016/j.urology.2019.05.016 [DOI] [PubMed] [Google Scholar]
- 17.Hussain M, Fizazi K, Saad F, et al. Enzalutamide in Men with Nonmetastatic, Castration-Resistant Prostate Cancer. N Engl J Med. 2018;378(26):2465–2474. doi: 10.1056/nejmoa1800536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Davis ID, Martin AJ, Stockler MR, et al. Enzalutamide with Standard First-Line Therapy in Metastatic Prostate Cancer. N Engl J Med. 2019;381(2):121–131. doi: 10.1056/nejmoa1903835 [DOI] [PubMed] [Google Scholar]
- 19.Fizazi K, Tran NP, Fein L, et al. Abiraterone acetate plus prednisone in patients with newly diagnosed high-risk metastatic castration-sensitive prostate cancer (LATITUDE): final overall survival analysis of a randomised, double-blind, phase 3 trial. Lancet Oncol. 2019;20(5):686–700. doi: 10.1016/S1470-2045(19)30082-8 [DOI] [PubMed] [Google Scholar]
- 20.Armstrong AJ, Szmulewitz RZ, Petrylak DP, et al. Arches: A randomized, phase III study of androgen deprivation therapy with enzalutamide or placebo in men with metastatic hormone-sensitive prostate cancer. J Clin Oncol. 2019;37(32):2974–2986. doi: 10.1200/JCO.19.00799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.James ND, de Bono JS, Spears MR, et al. Abiraterone for Prostate Cancer Not Previously Treated with Hormone Therapy. N Engl J Med. 2017;377(4):338–351. doi: 10.1056/nejmoa1702900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Centers for Medicare & Medicaid Services. Medicare Data on Provider Practice and Specialty (MD-PPAS). Published 2017. Accessed December 23, 2020. https://www.resdac.org/cms-data/files/md-ppas
- 23.Centers for Medicare & Medicaid Services. Open Payments Data. Accessed December 23, 2020. https://openpaymentsdata.cms.gov/about
- 24.Hollands S Receipt of Promotional Payments at the Individual and Physician Network Level Associated with Higher Branded Antipsychotic Prescribing Rates. Adm Policy Ment Health. 2020;47(1):73–85. doi: 10.1007/s10488-019-00974-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nguyen T, Andraka-Christou B, Simon K, Bradford WD. Provider-directed marketing may increase prescribing of medications for opioid use disorder. J Subst Abuse Treat. 2019;104(January):104–115. doi: 10.1016/j.jsat.2019.06.014 [DOI] [PubMed] [Google Scholar]
- 26.ResDAC Research Data Assistance Center. Part D Event (PDE) File. Accessed March 14, 2021. https://www.resdac.org/cms-data/files/pde
- 27.Perlis RH, Perlis CS. Physician payments from industry are associated with greater medicare Part D prescribing costs. PLoS One. 2016;11(5):1–12. doi: 10.1371/journal.pone.0155474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Scher JU, Schett G. Key opinion leaders — a critical perspective. Nat Rev Rheumatol. 2021;17(2):119–124. doi: 10.1038/s41584-020-00539-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bandari J, Ayyash OM, Turner RM, Jacobs BL, Davies BJ. The lack of a relationship between physician payments from drug manufacturers and Medicare claims for abiraterone and enzalutamide. Cancer. 2017;123(22):4356–4362. doi: 10.1002/cncr.30914 [DOI] [PubMed] [Google Scholar]
- 30.Centers for Medicare & Medicaid Services. Law and Policy. Accessed January 29, 2021. https://www.cms.gov/OpenPayments/About/Law-and-Policy [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1. Caption: Percentage of prescribers among all physicians categorized by the magnitude of promotional payments received between 2014 and 2017.
Source: Authors’ analysis of data from the Medicare Part D Event File, Open Payments Program, Medicare Data on Physician Practice and Specialty.
Note: All medical oncologists and urologists serving Medicare beneficiaries were first categorized based on the aggregate of promotional payments received in each given year ($0, $1–$999; ≥$1000). Then, the percentage of physicians who were prescribers of abiraterone or enzalutamide were calculated in each category. The percentage of physicians prescribing abiraterone or enzalutamide increased with higher promotional payments for both specialties, with a notably steeper gradient of increase among urologists. For instance, in 2017, 18.0% (n=1,777) of medical oncologists who received $0 (n=9,894) had prescribed abiraterone or enzalutamide, compared to 36.4% (n=1,135) of those who received $1–$999 (n=3,118) and 48.0% (n=59) of those who received $1000 or more (n=123). By contrast, 1.3% (n=58) of urologists who received $0 (n=4,535) had prescribed abiraterone or enzalutamide, compared to 10.9% (n=544) of those who received $1–$999 (n=5,012) and 60.4% (n=58) of those who received $1000 or more (n=96).


