Abstract
(−)-β-2′,3′-Dideoxy-3′-thiacytidine (lamivudine [3TC]) is a nucleoside analog which effectively interferes with the replication of hepatitis B virus (HBV) DNA in vitro and in vivo. We have investigated the antiviral properties of 3TC in vitro in HepG2 cells infected with recombinant HBV baculovirus. Different types of information can be obtained with the HBV baculovirus-HepG2 system because (i) experiments can be carried out at various levels of HBV replication including levels significantly higher than those that can be obtained from conventional HBV-expressing cell lines, (ii) cultures can be manipulated and/or treated prior to or during the initiation of HBV expression, and (iii) high levels of HBV replication allow the rapid detection of HBV products including covalently closed circular (CCC) HBV DNA from low numbers of HepG2 cells. The treatment of HBV baculovirus-infected HepG2 cells with 3TC resulted in an inhibition of HBV replication, evidenced by reductions in the levels of both extracellular HBV DNA and intracellular replicative intermediates. The effect of 3TC on HBV replication was both dose and time dependent, and the reductions in extracellular HBV DNA that we observed agreed well with the previously reported efficacy of 3TC in vitro. As expected, levels of HBV transcripts and extracellular hepatitis B surface antigen and e antigen were not affected by 3TC. Importantly, the HBV baculovirus-HepG2 system made it possible to observe for the first time that CCC HBV DNA levels are lower in cells treated with 3TC than in control cells. We also observed that the treatment of HepG2 cells prior to HBV baculovirus infection resulted in a slight increase in the efficacy of 3TC compared to treatments starting 24 h postinfection. The treatment of HepG2 cells with the highest concentration of 3TC tested in this study (2 μM) prior to the initiation of HBV replication markedly inhibited the accumulation of CCC DNA, whereas treatment with the same concentration of 3TC at a time when CCC HBV DNA pools were established within the cells was considerably less effective. In addition, our results suggest that in HepG2 cells, non-protein-associated relaxed circular HBV DNA and particularly CCC HBV DNA are considerably more resistant to 3TC treatment than other forms of HBV DNA, including replicative intermediates and extracellular DNA. We conclude from these studies that the HBV baculovirus-HepG2 system has specific advantages for drug studies and can be used to complement other in vitro model systems currently used for testing antiviral compounds.
Hepatitis B virus (HBV) is a hepatotropic DNA virus capable of causing both acute and chronic hepatitis in man. The World Health Organization estimates that over 350 million people are chronically infected with HBV worldwide. Those persistently infected with HBV serve as a reservoir for the horizontal and vertical transmission of the virus and are also at increased risk of developing further liver disease (2). Approximately one of every four HBV carriers will eventually succumb to chronic active hepatitis, cirrhosis, or hepatocellular carcinoma. HBV is believed to cause between 60 and 80% of the world’s primary liver cancer (37). Although effective vaccines against HBV exist (17), vaccination is expensive and not readily available in all parts of the world and not all individuals develop immunity following vaccination. Therefore, research must also focus on developing effective treatments for the millions of people who remain persistently infected, as well as the population who will become infected despite the existence of vaccines. Currently, the most commonly used treatment for chronic HBV infection is the cytokine alpha interferon (IFN-α). Long-term studies on IFN-α therapy indicate that treatment can lead to the loss of circulating HBV antigens and improved survival rates but only in about 30% of patients receiving treatment (13, 26, 36). IFN also must be administered by injection and can have undesirable side effects which limit dosage. Alternative treatment options which are effective alone or in combination with IFN-α must be explored.
(−)-β-2′,3′-Dideoxy-3′-thiacytidine (lamivudine [3TC]) is a nucleoside analog originally described as an agent capable of inhibiting the replication of human immunodeficiency virus type 1 and type 2 (8). It was subsequently reported that 3TC was also effective at inhibiting HBV replication in vitro (12, 20, 22) and at reducing the level of HBV DNA in vivo in the sera of some animal models (33). The use of 3TC to treat chronic HBV infection has recently been approved by the Federal Drug Administration. Treatment with 3TC appears to be well tolerated and effective at reducing or clearing HBV DNA from the sera of patients (11, 16, 23, 25). A major concern with 3TC therapy is that cessation of drug treatment results in the rapid reappearance of HBV DNA in serum, and the level and rapidity of rebound depends upon the length of 3TC treatment (11, 23, 25). The reason for this rebound is postulated to be the persistence of a covalently closed circular (CCC) form of the HBV genome which resides in the nuclei of infected hepatocytes (32). Although replicative forms of HBV DNA can be prematurely terminated by the incorporation of 3TC, there is little or no evidence to suggest that existing CCC DNA pools can be affected by treatment with 3TC or other nucleoside analogs. However, Moraleda et al. (24) have reported that the treatment of primary woodchuck hepatocytes with 3TC prior to infection with woodchuck hepatitis virus (WHV) can inhibit the formation of WHV CCC DNA in vitro. Recently, 3TC has also been used as a prophylactic agent during orthotopic liver transplants, when HBV reinfection is a risk (1, 3, 14). Short-term results from these trials indicate that 3TC suppresses HBV DNA production by the donor liver in some patients; however, the prevention of reinfection and long-term efficacy have yet to be determined. It is unknown if preemptive treatment with nucleosides such as 3TC prior to a transplant would be sufficient to prevent the accumulation of CCC HBV DNA in new liver tissue and potential reactivation of the virus.
We have recently developed a novel in vitro system for the study of HBV (10). This system is based on the use of the baculovirus Autographa californica as a vector for the efficient delivery of a replication-competent HBV genome into HepG2 cells. The advantages of the HBV baculovirus-HepG2 system include (i) the ability to initiate extremely high levels of HBV expression, (ii) a reproducible and precise control over the level of HBV expression, and (iii) the ability to rapidly detect HBV antigens, RNA, and both intracellular and extracellular DNA from low numbers of HepG2 cells. The HBV baculovirus-HepG2 cell system also allows the detection of CCC HBV DNA, which can be difficult to detect or undetectable in stably transfected HBV-expressing cell lines such as HepG2 2.2.15 and HB611 (28, 31). Unlike stable cell lines, the time of infection can also be controlled, allowing the manipulation or treatment of cells prior to or during the initiation of HBV expression. The primary goals of the studies described here were (i) to evaluate the utility of the HBV baculovirus-HepG2 system as a tool for antiviral research using 3TC, an established inhibitor of HBV replication, (ii) to provide further data on the in vitro efficacy of 3TC by investigating its effects on various levels of HBV replication, and (iii) to examine the effect of administering 3TC prior to the initiation of HBV expression, shortly after the initiation of HBV replication, and after the establishment of intracellular CCC HBV DNA pools in HepG2 cells.
MATERIALS AND METHODS
Cell culture.
The HepG2 cell line was maintained at 37°C in humidified incubators at 5% CO2 (18). HepG2 cells were fed minimal essential medium (Gibco BRL, Gaithersburg, Md.) supplemented with 10% heat-inactivated fetal bovine serum.
HBV baculovirus production and infection of HepG2 cells.
The generation, amplification, and purification of the HBV baculovirus encoding a 1.3-unit length replication-competent HBV genome has been previously described (10). The mechanism of baculovirus uptake by mammalian cells is currently unknown. Here, we use the term “infection” to describe the exposure and uptake of baculovirus particles by HepG2 cells. This process is not the same as a true viral infection but rather is a mechanism for the transduction of HBV DNA into the cell. The infection procedure for HepG2 cells has been previously described (10).
3TC treatment.
3TC was a gift from BioChem Therapeutic Inc. (Laval, Quebec, Canada). 3TC was resuspended in sterile water, aliquoted, and frozen at −20°C to avoid repeated freezing and thawing of the drug. Medium containing 3TC was prepared daily as needed with fresh aliquots of 3TC. In experiments in which 3TC treatment was initiated after viral infection, HepG2 cells were exposed to the indicated concentration of 3TC 24 h postinfection (p.i.) or 4 days p.i. In experiments utilizing pretreatment with 3TC, cells were fed a medium containing 3TC 16 h prior to HBV baculovirus infection, HBV baculovirus infection was carried out in a medium containing 3TC, and cells were refed a fresh medium containing 3TC immediately after completion of the infection and washing procedures.
Analysis of RNA.
Total RNA was isolated from HepG2 cells by the single-step acid guanidium method (6). Northern blot analysis was performed with 20 μg of total RNA as described previously (9). Nucleic acid hybridization was performed as described previously (27). A full-length double-stranded (DS) HBV genome was used as a template to generate 32P-radiolabeled probes by using a Boehringer Mannheim (Indianapolis, Ind.) random prime DNA labeling kit.
Analysis of intracellular replicative intermediates.
Cytoplasmic preparations containing HBV core particles were isolated from HepG2 cells as described previously (15). Unprotected DNA was removed by adjusting cytoplasmic preparations so that they contained 10 mM MgCl2 and 500 μg of DNase I (Boehringer Mannheim) per ml followed by a 1-h incubation at 37°C. Replicative intermediates were then isolated by proteinase K digestion, sequential phenol and chloroform extractions, and isopropanol precipitation as described previously (10). Precipitated nucleic acids were resuspended in a small volume of TE (10 mM Tris, 1 mM EDTA), normalized by measurement of optical density at 260 nm, and digested with 100 μg of RNase (Boehringer Mannheim) for 1 h at 37°C. Replicative intermediates were then analyzed by electrophoresis in 1% agarose gels followed by Southern blotting as described previously (27). Nucleic acid hybridization was performed as described above for Northern blotting. A model 100A laser densitometer (Molecular Dynamics, Sunnyvale, Calif.) equipped with Quantity One software (Protein Databases Inc., Huntington Station, N.Y.) was used to analyze suitable exposures of Southern blots.
Detection of extracellular HBV DNA.
Conditioned medium was collected from HepG2 cells, centrifuged at 10,000 × g for 10 min, and transferred to clean tubes to remove cellular debris. HBV particles were precipitated from medium samples with polyethylene glycol 8000 (Sigma Chemical Co., St. Louis, Mo.) as described previously (35). Viral pellets were resuspended in phosphate-buffered saline, and DNA was extracted by proteinase K digestion, sequential phenol and chloroform extractions, and isopropanol precipitation as described above. Ten micrograms of tRNA (Boehringer Mannheim) was added as a carrier during precipitation. Nucleic acid pellets were resuspended in a small volume of TE and digested with 500 μg of RNase per ml prior to analysis by electrophoresis and Southern blotting.
Detection of CCC HBV DNA.
Non-protein-associated circular HBV DNA was extracted from HepG2 cells essentially as described previously (30). Extracted nucleic acids were resuspended in water and normalized by measurement of optical density at 260 nm prior to digestion with 100 μg of RNase per ml and 30 U of Plasmid-Safe ATP-dependent DNase (Epicenter Technologies, Madison, Wis.) for 3 h at 37°C. Samples were analyzed by electrophoresis and Southern blotting.
Analysis of secreted HBV antigens.
The detection of hepatitis B surface antigen (HBsAg) was carried out by using a radioimmunoassay kit according to the manufacturer’s instructions (Abbott Laboratories, Abbott Park, Ill.). Hepatitis B e antigen (HBeAg) was also detected with a radioimmunoassay kit according to the manufacturer’s instructions (Sorin Biomedica, Saluggia, Italy). Medium samples collected from HepG2 cells were centrifuged at 6,000 × g to remove cellular debris, transferred to clean tubes, and stored at −20°C until analyzed.
RESULTS
Effect of 3TC on extracellular HBV DNA.
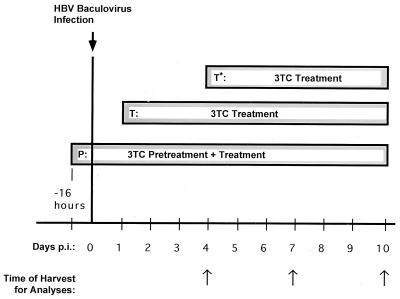
Previous results have indicated that 3TC concentrations of approximately 0.2 μM are effective at downregulating HBV replication and/or virion secretion by 50 to 90% in vitro in HBV-expressing cell lines (12, 20, 22). We therefore chose to examine the effects of 3TC on HBV expression and replication mediated by HBV baculovirus by using 0.02, 0.2, and 2.0 μM concentrations of 3TC. HepG2 cells were infected with 50 PFU of HBV baculovirus/cell because our previous findings have indicated that cells infected at a multiplicity of infection (MOI) of 50 maintain high levels of HBV replication for more than 10 days (10). For each concentration of 3TC tested in initial studies, drug treatment starting 16 h prior to baculovirus infection (pretreatment) and a treatment starting 24 h after baculovirus infection were used (Fig. 1). It is important to note that 24 h p.i. (50 PFU of HBV baculovirus/cell) HBV replication has begun but the predominant forms of DNA in cytoplasmic nucleocapsids are single-stranded (SS) HBV DNA molecules (10). Little mature DS HBV DNA appears in core particles and no extracellular HBV DNA or CCC HBV DNA can be detected at this time. At 48 h p.i., DS replicative intermediates as well as extracellular HBV DNA and non-protein-associated relaxed circular (RC) and CCC HBV DNA are readily detectable. Therefore, pretreatment with 3TC introduces the drug to the cell prior to the presence of a replication-competent HBV genome, while treatments starting 24 h p.i. introduce the drug when both SS and DS HBV DNA are actively being synthesized.
FIG. 1.
Treatment schedules of HBV baculovirus-infected HepG2 cells with 3TC prior to HBV RNA and DNA analyses. HepG2 cells were infected with HBV baculovirus and treated with 3TC according to three treatment schedules. For the first schedule (P), 3TC treatment was initiated 16 h prior to HBV baculovirus infection (pretreatment); in this group 3TC treatment was continued during and after infection. For the second schedule (T), 3TC treatment was initiated 24 h p.i. For the third treatment schedule (T*), 3TC was initiated 4 days after HBV baculovirus infection. On the indicated days (arrows), HBV replication in HepG2 cultures was assessed by examining intracellular and extracellular HBV DNA and HBV RNA by Southern and Northern blotting, respectively.
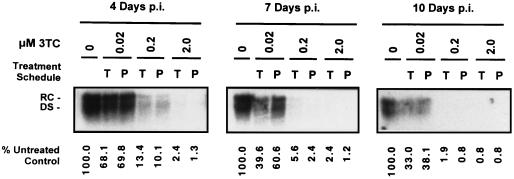
Previous analyses of HBV DNA present in the medium of HBV baculovirus-infected HepG2 cells suggest that HBV DNA is present predominantly in the form of Dane particles (10). The effects of 3TC on HBV virion secretion were therefore assayed by Southern analysis of DNA extracted from the media of cultures treated with the drug for various time periods (Fig. 2). Results indicated that 3TC had a profound dose-dependent inhibitory effect on the levels of extracellular HBV DNA in the media of treated cells. When HepG2 cells were treated 24 h p.i. with 0.02, 0.2, and 2.0 μM concentrations of 3TC, the extracellular HBV DNA levels were 68.1, 13.4, and 2.4% of control levels, respectively, after 3 days of treatment. Decreases in extracellular HBV DNA levels were dependent not only on the dose but also on the length of 3TC treatment; levels of extracellular HBV DNA were progressively inhibited to 33.0, 1.9, and <1% of control levels in the cultures treated 24 h p.i. with 0.02, 0.2, and 2.0 μM concentrations of 3TC, respectively, after an additional 6 days (10 days p.i.). Pretreatment 16 h prior to HBV baculovirus infection with 0.2 and 2.0 μM 3TC appeared to have a small but consistent ability to downregulate virion secretion to a greater extent than was observed when treatment was initiated 24 h p.i.
FIG. 2.
Analysis of HBV DNA secreted by HepG2 cells infected with 50 PFU of HBV baculovirus/cell and treated with increasing concentrations of 3TC over a 10-day period. Treatments with 0, 0.02, 0.2, and 2.0 μM concentrations of 3TC were initiated either 16 h prior to HBV baculovirus infection (P) or 24 h p.i. (T). At 4, 7, and 10 days p.i., DNA was extracted from the medium of each culture and analyzed by Southern blotting. RC and DS forms of HBV DNA are indicated. The percentages of HBV DNA present in treated cultures relative to untreated controls are indicated.
Southern analysis of HBV replicative intermediates.
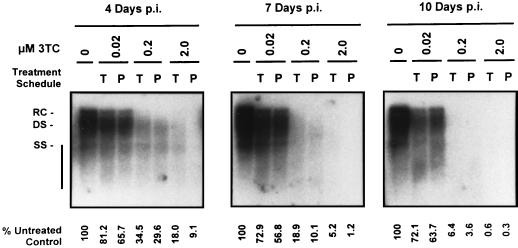
Replicative intermediates were extracted from intracellular HBV core particles isolated from treated and control cultures. Southern analysis indicated that 3TC had an inhibitory effect on replicative intermediates, which was similar to what was observed for extracellular HBV DNA (Fig. 3). Levels of replicative intermediates were progressively downregulated by both increasing 3TC concentrations and increasing the length of treatment. Maximal levels of inhibition were observed after 9 days of 3TC treatment; at this time, levels of replicative intermediates were 72.1, 6.4, and <1% of control levels in cultures treated starting 24 h p.i. with 0.02, 0.2, and 2.0 μM concentrations of 3TC, respectively. Replicative intermediates were not reduced as extensively as extracellular HBV DNA at equal dose and treatment lengths with the exception of the highest dose (2.0 μM) at the longest treatment time. Analysis of replicative intermediates also revealed that completed forms of the HBV genome (RC and full-length DS DNA) appeared to be more sensitive to 3TC treatment than smaller DNA species including incomplete DS DNA and SS DNA.
FIG. 3.
Analysis of HBV replicative intermediates in HepG2 cells infected with 50 PFU of HBV baculovirus/cell and treated with increasing concentrations of 3TC over a 10-day period. Treatments with 0, 0.02, 0.2, and 2.0 μM concentrations of 3TC were initiated either 16 h prior to HBV baculovirus infection (P) or 24 h p.i. (T). At 4, 7, and 10 days p.i., replicative intermediates were extracted from cytoplasmic core particles isolated from each culture. Replicative intermediates were analyzed by Southern blotting. RC, DS, and SS forms of HBV genomic DNA are indicated. The percentages of HBV DNA present in treated cultures relative to untreated control cultures are indicated.
No marked differences in the levels of replicative intermediates were observed when cultures were pretreated with 0.02 μM 3TC prior to infection with 50 PFU of HBV baculovirus/cell, compared to those treated 24 h p.i. Pretreatment with 0.2 and 2.0 μM 3TC resulted in a greater reduction in levels of replicative intermediates at 4 days p.i. than in cultures treated 24 h p.i.; this effect was most obvious with 2.0 μM 3TC at the earliest time analyzed (4 days p.i.).
Southern analysis of CCC HBV DNA.
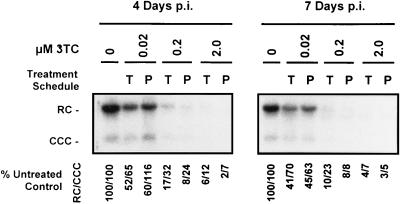
The HBV baculovirus-HepG2 system allows the detection of circular forms of the HBV genome which accumulate in the nuclei of infected cells during hepadnavirus replication (32). The effects of 3TC on non-protein-associated RC and CCC HBV DNA in vitro have not been reported previously. Therefore, we examined the effects of treating and pretreating HepG2 cultures with increasing concentrations of 3TC on the levels of RC and CCC HBV DNA produced after HBV baculovirus infection. HepG2 cultures were harvested and analyzed for non-protein-associated RC and CCC HBV DNA after 3 and 6 days of 3TC treatment. Increasing doses of 3TC resulted in the progressive inhibition in the accumulation of RC and CCC HBV DNA present within cells (Fig. 4); this was similar to the effect of 3TC on replicative intermediates and extracellular HBV DNA. Treatment with 2.0 μM 3TC resulted in a greater than 90% inhibition of accumulation of both RC and CCC HBV DNA after 6 days of treatment. The level of reduction of RC and CCC HBV DNA was not as marked as that observed for extracellular DNA. Pretreatment with 0.2 and 2.0 μM concentrations of 3TC resulted in a further inhibition in the amount of RC and CCC HBV DNA, compared to treatments initiated after infection. These results indicate that treatment with 3TC prior to and during the initiation of HBV expression can inhibit the formation and accumulation of RC and CCC HBV DNA in HepG2 cells.
FIG. 4.
Analysis of CCC HBV DNA produced in HepG2 cells infected with 50 PFU of HBV baculovirus/cell and treated with increasing concentrations of 3TC over a 7-day period. Treatments with 0, 0.02, 0.2, and 2.0 μM concentrations of 3TC were initiated either 16 h prior to HBV baculovirus infection (P) or 24 h p.i. (T). At 4 and 7 days p.i., non-protein-associated DNA was extracted from each culture and analyzed by Southern blotting. RC and CCC forms of HBV DNA are indicated. The percentages of HBV RC DNA and the percentages of CCC HBV DNA present in treated cultures relative to untreated control cultures are indicated.
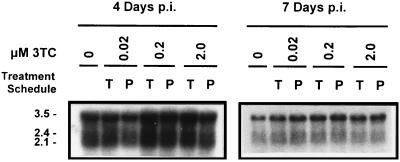
Northern analysis of HBV transcripts.
Total RNA was harvested from 3TC-treated and -pretreated cultures as well as untreated cultures on days 4 and 7 p.i. and was analyzed by Northern blotting. The 3.5-, 2.4-, and 2.1-kb HBV transcripts were detectable in RNA samples from HBV baculovirus-infected cells at both time points (Fig. 5). As expected from previous results (10), the abundance of HBV transcripts produced in HBV baculovirus-infected HepG2 cells decreased with time in culture. Levels of all three major classes of HBV transcripts were present in approximately equal abundance in treated, pretreated, and untreated cultures analyzed at both time points; no correlation between transcript level and either the 3TC concentration or the time of 3TC addition was evident. These results indicate that 3TC has no effect on the transcription of HBV genes from recombinant HBV baculovirus DNA in infected cells.
FIG. 5.
Analysis of HBV transcripts in HepG2 cells infected with 50 PFU of HBV baculovirus/cell and treated with increasing concentrations of 3TC over a 7-day period. Treatments with 0, 0.02, 0.2, and 2.0 μM concentrations of 3TC were initiated either 16 h prior to HBV baculovirus infection (P) or 24 h p.i. (T). At 4 and 7 days p.i., total RNA was harvested from cultures and 20 μg of total RNA was analyzed by Northern blotting. The 3.5-, 2.4-, and 2.1-kb HBV transcripts are indicated.
Analysis of secreted HBV antigens following 3TC treatment.
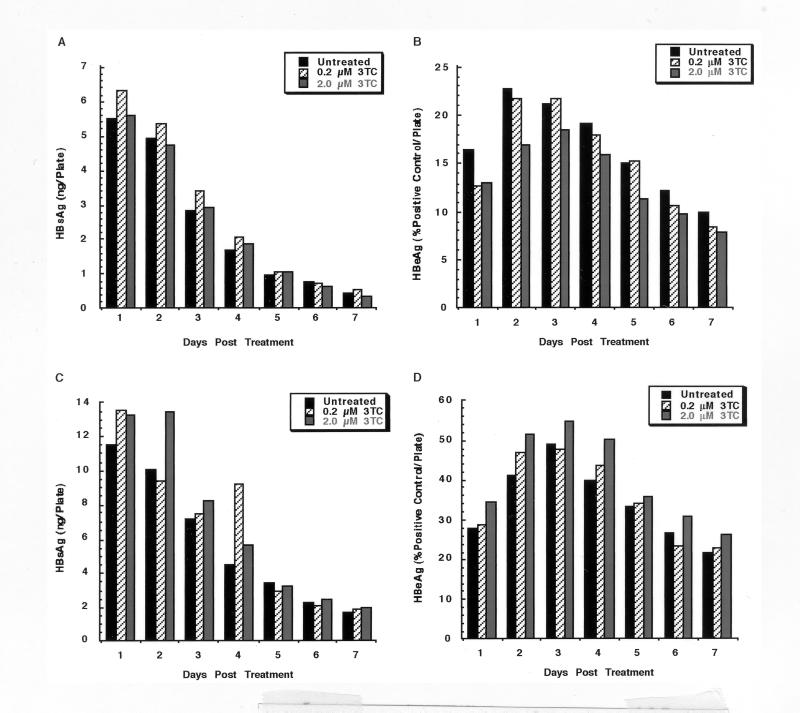
Analysis of HBV RNA produced in 3TC-treated cells indicated that the transcription of HBV genes was unaffected by 3TC. This finding also suggests that 3TC treatment would not alter the capacity of the cells to produce HBV proteins. To test whether concentrations of 3TC which inhibited HBV replication have an effect on the secretion of HBV antigens, we analyzed media from HepG2 cells infected with HBV baculovirus and treated with a 0.2 or 2.0 μM concentration of 3TC. HepG2 cells were infected with either 25 or 50 PFU of HBV baculovirus/cell, and 3TC treatment was initiated 24 h p.i. Cells were fed daily for 1 week, and conditioned medium was collected for the analysis of HBsAg and HBeAg (Fig. 6). In contrast to the production of replicative intermediates and extracellular virions, the production and secretion of HBsAg and HBeAg by HepG2 cells infected at an MOI of either 25 (Fig. 6A and B) or 50 (Fig. 6C and D) PFU/cell were unaffected by 3TC during the 1-week time course. The results of HBsAg and HBeAg analyses support the results of Northern analysis and also demonstrate that the extensive downregulation of HBV replication and virion secretion does not have an inhibitory effect on the trafficking and secretion of HBsAg and HBeAg from HepG2 cells.
FIG. 6.
Analysis of HBV antigens secreted by HBV baculovirus-infected HepG2 cells after a 1-week treatment with 3TC. HepG2 cells were infected with either 25 (A and B) or 50 (C and D) PFU of HBV baculovirus/cell and were left untreated or were treated daily with a 0.2 or 2.0 μM concentration of 3TC starting 24 h p.i. Conditioned medium was collected from each culture for 1 week and analyzed for HBsAg (A and C) or HBeAg (B and D) content by radioimmunoassay.
The effect of 3TC treatment on HBV DNA content of media from HepG2 cells exhibiting a broad range of HBV expression.
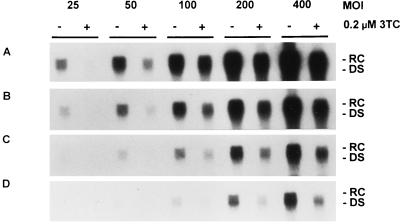
Previous studies on the efficacy of 3TC in vitro have been conducted by using the stably transfected cell lines HepG2 2.2.15 and HB611 (12, 20, 22). Since HBV expression and replication in these cell lines is generally considered modest, we investigated the efficacy of 3TC over a range of HBV expression levels by using the HBV baculovirus-HepG2 system. HepG2 cells were infected with HBV baculovirus at MOIs of 25, 50, 100, 200, and 400 PFU/cell, and treatment with 0.2 μM 3TC was initiated 24 h after HBV baculovirus infection. After 3 days of drug treatment, media exposed to cells for 24 h were collected from both treated and untreated cultures and analyzed for HBV DNA content by Southern blotting (Fig. 7).
FIG. 7.
Effect of 0.2 μM 3TC on the HBV DNA content of media from HepG2 cells expressing increasing levels of HBV. HepG2 cultures were infected with 25, 50, 100, 200, or 400 PFU of HBV baculovirus/cell and were treated with 0.2 μM 3TC starting 24 h p.i. Cultures were fed a fresh 3TC-supplemented medium daily. After 3 days of treatment (4 days p.i.), DNA was extracted from the media of treated and untreated control cultures and analyzed by Southern blotting. The autoradiograms shown indicate different lengths of time of exposure of the blot on film: 8 h (A), 4 h (B), 2 h (C), and 1 h (D). RC and DS forms of HBV DNA are indicated.
In HBV baculovirus-infected cells which were not treated with 3TC, extracellular HBV DNA was present in an MOI-dependent manner, as expected on the basis of previous results (10). The amount of HBV DNA secreted by untreated cells 3 days p.i. was measured by using appropriate HBV DNA standards and determined to be approximately 1,070 pg of HBV DNA/60-mm-diameter plate/day for cultures infected with 200 PFU/cell, 530 pg of HBV DNA/60-mm-diameter plate/day for cultures infected with 100 PFU/cell, and 210 pg of HBV DNA/60-mm-diameter plate/day for cultures infected with 50 PFU/cell. Values could not be determined for cultures infected with HBV baculovirus at 25 or 400 PFU/cell because the levels did not fall within the range of the HBV DNA standards used.
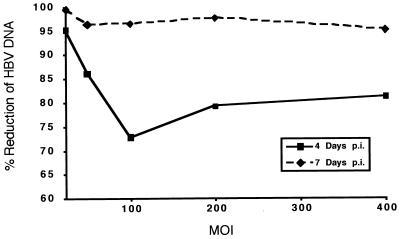
Cultures treated with 3TC showed a considerable inhibition of the production of extracellular HBV DNA. Densitometric analysis of suitable exposures of the Southern blot indicated that after 3 days of treatment the most effective downregulation was observed in cells infected at an MOI of 25 PFU/cell (Fig. 8). A 95.2% inhibition of extracellular HBV DNA was observed. At higher MOIs, the inhibition of extracellular HBV DNA was approximately 80%. Levels of extracellular HBV DNA were also determined after an additional 3 days of drug treatment or a total of 6 days of treatment (Fig. 8). The inhibition of extracellular HBV DNA in each treated culture was more extensive after 6 days of treatment; these results were consistent with our previous observations that the efficacy of 3TC increased with time. The largest inhibition was again exhibited by the culture infected with 25 PFU of HBV baculovirus/cell; a greater than 99% reduction in extracellular HBV DNA was observed in this culture. At 50 PFU/cell and higher, the average inhibition of extracellular HBV DNA was greater than 96%. We conclude from these data that at MOIs ranging from 50 to 400 PFU/cell, treatment with 3TC inhibits the level of HBV produced by essentially the same magnitude. These results also demonstrate that the HBV baculovirus system (because of its ability to be manipulated so that HBV can be expressed over a wide range) can be used to evaluate the effect of a specific concentration of an antiviral compound on different levels of HBV production.
FIG. 8.
Quantitative analysis of the effect of 0.2 μM 3TC on the HBV DNA content of media from HepG2 cells expressing increasing levels of HBV. HepG2 cultures were infected with 25, 50, 100, 200, and 400 PFU of HBV baculovirus/cell and were treated with 0.2 μM 3TC starting 24 h p.i. Cultures were fed a fresh 3TC-supplemented medium daily. After 3 and 6 days of treatment (4 and 7 days p.i.), DNA was extracted from the media of treated and untreated control cultures and analyzed by Southern blotting. The percent reduction of HBV DNA in the treated cultures was determined by laser densitometry of suitable exposures of the Southern blot. The percent reduction of HBV DNA after 4 and 7 days p.i. is plotted against the MOI. Data shown for 4 days p.i. represent a quantitative evaluation of the data shown in Fig. 7.
The effect of 3TC treatment on CCC HBV DNA accumulation in HepG2 cells with respect to the time of treatment initiation.
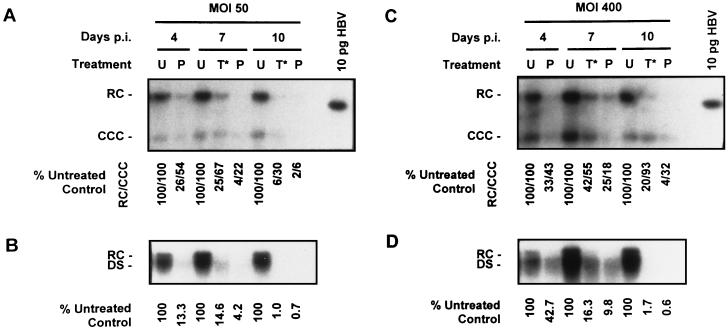
To investigate the effect of 3TC treatment on existing CCC DNA pools, we conducted experiments in which treatment was initiated several days after HBV baculovirus infection when CCC DNA had already accumulated (10). Cultures of HepG2 cells were infected with HBV baculovirus at an MOI of either 50 or 400 PFU/cell and treated with 0.2 μM 3TC starting 4 days p.i. CCC HBV DNA was extracted 4, 7, and 10 days p.i., and the levels of non-protein-associated RC and CCC DNA in cells treated 4 days p.i. were compared to those in untreated control cultures and cultures pretreated with 3TC starting 16 h prior to baculovirus infection (pretreatment) (Fig. 9A and C). In cultures pretreated with 0.2 μM 3TC, a time-dependent inhibition in the accumulation of non-protein-associated RC and CCC DNA was observed; this was true for cultures infected at 50 PFU of HBV baculovirus/cell and those infected at a higher MOI (400 PFU/cell). The examination of cultures which were treated starting on day 4 indicated that treating HepG2 cells with 3TC after CCC DNA pools were established resulted in an inhibition in the amount of CCC DNA found in the cells at later time points. At both MOIs tested, non-protein-associated RC HBV DNA appeared to be more sensitive to 3TC treatment than CCC DNA. At an MOI of 50 PFU/cell, CCC DNA was inhibited to 67% of control levels after 3 days of treatment and 30% of control levels after 6 days of treatment. At a higher MOI (400 PFU/cell) CCC DNA appeared to be more stable than control cultures; CCC DNA was inhibited to 55% of control levels after 3 days of drug treatment; however, after 6 days of treatment CCC DNA levels were comparable (93%) to those found in untreated cultures. We also observed that after equal treatment lengths, 0.2 μM 3TC treatment was more effective in cultures which were pretreated than in cultures which were treated after CCC HBV DNA formation.
FIG. 9.
Effect of 0.2 μM 3TC treatment on CCC HBV DNA accumulation in HepG2 cells with respect to the time of initiation of treatment. Cultures of HepG2 cells were infected with HBV baculovirus at MOIs of either 50 (A and B) or 400 (C and D) PFU of HBV baculovirus/cell and were treated with 0.2 μM 3TC. 3TC treatment was initiated either 16 h prior to HBV baculovirus infection (P) or 4 days p.i. (T*); untreated HepG2 cells (U) were also examined for comparative purposes. CCC HBV DNA was extracted from each treatment group and analyzed by Southern blotting at 4, 7, and 10 days p.i. (A and C). RC and CCC forms of HBV DNA are indicated. The percentages of HBV RC DNA and the percentages of CCC HBV DNA present in treated cultures relative to untreated control cultures are indicated. Extracellular HBV DNA was also extracted from the medium exposed to each culture for 24 h on days 4, 7, and 10 days p.i. and analyzed by Southern analysis (B and D). RC and DS forms of HBV DNA are indicated. The percentages of extracellular HBV DNA produced by treated cultures relative to untreated controls are indicated.
Medium samples exposed to each culture for 24 h prior to CCC DNA extraction were also analyzed for extracellular HBV DNA content at 4, 7, and 10 days p.i. (Fig. 9B and D). As with previous results (Fig. 2 and 7) we observed a time-dependent inhibition in the secretion of HBV DNA from treated cultures. We also observed that the inhibition of extracellular HBV DNA production by 3TC was greater than the inhibition observed in intracellular non-protein-associated RC and CCC HBV DNA.
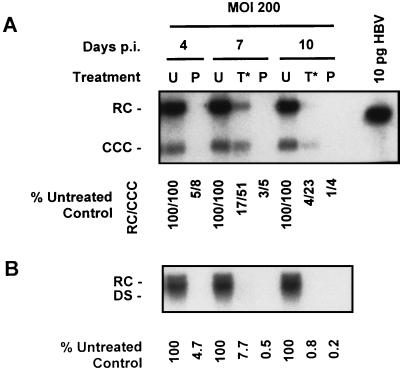
To further study the sensitivity of existing CCC HBV DNA pools to 3TC, we performed an additional experiment with a higher concentration of 3TC. HepG2 cells were infected with 200 PFU of HBV baculovirus/cell and treated with 2.0 μM 3TC starting 4 days p.i. (Fig. 1, T*). CCC HBV DNA was extracted 4, 7, and 10 days p.i., and the levels of CCC HBV DNA in cells treated 4 days p.i. were compared to those in untreated control cultures and cultures which had been treated with 2.0 μM 3TC 16 h prior to HBV baculovirus infection (Fig. 10A). Similar to our previous results (Fig. 4), pretreatment with 2.0 μM 3TC resulted in an almost complete inhibition of non-protein-associated RC and CCC HBV DNA formation. In contrast, treatment with 2.0 μM 3TC initiated after CCC DNA had already accumulated was considerably less effective; the CCC DNA levels were 51% of control levels after 3 days of treatment and 23% of control levels after 6 days of 3TC treatment. Pretreatment of HepG2 cells with 2.0 μM 3TC was clearly more effective than an equal treatment length of HepG2 cells which contained established CCC DNA pools. As with the previous experiment (Fig. 9A and C), the non-protein-associated RC form of HBV DNA appeared to be more sensitive to 3TC treatment than the CCC DNA form.
FIG. 10.
Effect of 2.0 μM 3TC treatment on CCC HBV DNA accumulation in HepG2 cells with respect to the time of initiation of treatment. Cultures of HepG2 cells were infected with HBV baculovirus at an MOI of 200 PFU of HBV baculovirus/cell and were treated with 2.0 μM 3TC. 3TC treatment was initiated either 16 h prior to HBV baculovirus infection (P) or 4 days p.i. (T*); untreated HepG2 cells (U) were also examined for comparative purposes. CCC HBV DNA was extracted from each treatment group and analyzed by Southern blotting at 4, 7, and 10 days p.i. (A). RC and CCC forms of HBV DNA are indicated. The percentages of HBV RC DNA and the percentages of CCC HBV DNA present in treated cultures relative to untreated control cultures are indicated. Extracellular HBV DNA was also extracted from the medium exposed to each culture for 24 h on days 4, 7, and 10 days p.i. and analyzed by Southern analysis (B). RC and DS forms of HBV DNA are indicated. The percentages of extracellular HBV DNA produced by treated cultures relative to untreated controls are indicated.
Analysis of the 24-h medium samples collected prior to CCC DNA extraction again revealed a time-dependent reduction in extracellular HBV DNA in response to 3TC (Fig. 10B) as observed in previous experiments (Fig. 2 and 7). These results also produced strong evidence that the secretion of HBV DNA into a culture medium is more sensitive to inhibition by 3TC than are the levels of non-protein-associated RC and CCC HBV DNA. In cultures in which 3TC treatment was initiated 4 days p.i., extracellular HBV DNA was inhibited to 8% of control levels (compared to 51% for CCC DNA) after 3 days of drug treatment and less than 1% of control levels (compared to 23% for CCC DNA) after 6 days of 3TC treatment.
Together, these results suggest that in HepG2 cells, non-protein-associated RC HBV DNA and particularly CCC HBV DNA are considerably more resistant to 3TC treatment than other forms of HBV DNA including replicative intermediates and extracellular DNA. Importantly, these results also suggest that the intracellular levels of CCC DNA and especially non-protein-associated RC DNA found in HBV baculovirus-infected HepG2 cells can be depleted with time by blocking the formation of other replicative intermediates which presumably serve as precursors for CCC HBV DNA formation.
DISCUSSION
Currently the best model systems available for in vitro study of HBV include stably transfected hepatocyte-derived cell lines such as HepG2 2.2.15 (28, 29), derived from the human hepatoblastoma cell line HepG2, and HB611, derived from the hepatoma cell line Huh-6 (31, 34). Alternatively, plasmid DNA containing the HBV genome can be transiently transfected into cell lines which support HBV replication such as HepG2 or the hepatoma cell line Huh-7 (5, 38). Stably transfected cell lines, in particular, the 2.2.15 cell line, have been used frequently as in vitro culture systems for studying the efficacy of antiviral agents (21, 22, 34). In contrast to stable cell lines whose expression is restricted to the specific copy number of integrated HBV genomes in that cell line, the HBV baculovirus-HepG2 system developed recently in our laboratory allows the manipulation of HBV expression levels over a wide range (10). Infection with HBV baculovirus at moderate MOIs also results in overall levels of viral gene expression and replication far higher than those observed in 2.2.15 cells. Because higher levels of HBV replication can be obtained after HBV baculovirus infection, the rapid detection of intracellular DNA, in particular, non-protein-associated RC and CCC HBV DNA, is possible. The purpose of the study reported here was twofold: to explore the use of the HBV baculovirus-HepG2 system as a model for in vitro testing of antivirals and to further characterize the antiviral properties of the cytosine analog 3TC.
When testing viral mRNA synthesis and HBV antigen secretion by HBV baculovirus-infected HepG2 cells, we found no differences between treated and untreated cultures. This finding has also been reported by other investigators and is not unexpected, based on the mechanism by which 3TC acts. 3TC is phosphorylated inside cells and is subsequently incorporated into nascent viral DNA by the HBV polymerase during replication (4). 3TC incorporation results in the termination of DNA elongation by virtue of its lack of a 3′ hydroxyl group. Therefore, the expected result would be that 3TC would not directly affect the transcription or translation of HBV gene products from nuclear DNA because it acts downstream of these events. It is interesting to note that an almost complete inhibition of the presence of extracellular HBV DNA did not result in any discernible alteration in the trafficking or secretion of HBeAg or HBsAg in HepG2 cells. This effect is also observed in patients, the majority of whom do not clear either HBeAg or HBsAg after long-term treatment with 3TC, even though their serum HBV DNA levels are markedly reduced (3, 25).
When the effects of increasing 3TC concentration and treatment time on a single level of HBV replication (at an MOI of 50 PFU/cell) were measured, we found that both HBV DNA synthesis and the secretion of virions into the medium were highly sensitive to 3TC. The production of extracellular HBV DNA was inhibited by more than 99% after 9 days of treatment with 2.0 μM 3TC. Intracellular replicative forms of the HBV genome were only slightly less sensitive to inhibition than extracellular DNA at equal 3TC concentrations and treatment times. It should be noted that many of the intracellular HBV DNA molecules detected in 3TC-treated cultures were partially double-stranded and single-stranded species; this might suggest that many replicating genomes had incorporated 3TC and were blocked from fully elongating into mature DS and RC genomes. One might expect to observe an increasing accumulation of chain-terminated SS species in 3TC-treated cells with time. We have never observed this phenomenon in the HBV baculovirus-HepG2 system. Since the amount of SS DNA present in treated cells is a function of (i) its rate of formation and (ii) its rate of removal (either by completion to DS DNA and export from the cell or by the degradation of intracellular capsids) and taking into account that extracellular HBV DNA does not increase with time in 3TC-treated cells, this data could suggest that capsids containing SS chain-terminated HBV genomes may have a relatively short intracellular half-life in HepG2 cells and thus would not continually accumulate in the cells.
The pretreatment of cells with 2.0 μM 3TC 16 h before HBV baculovirus infection resulted in a consistently greater inhibition of replicative intermediates and extracellular HBV DNA than that observed when 3TC treatment was initiated 24 h p.i. The effects of pretreatment were generally most evident at the earliest time points tested. These findings were not surprising and may simply indicate that cells which were pretreated with the drug were exposed to the drug for a slightly longer period of time by 4 days p.i. or instead may reflect an actual difference between adding 3TC prior to infection and adding it once the HBV DNA replication cycle had been initiated.
The data presented here agree well with published studies on the efficacy of 3TC in vitro. After treating HepG2 2.2.15 cells for 12 days, Doong et al. (12) and Kruining et al. (22) reported a 50% reduction of extracellular HBV DNA at concentrations of 0.05 and 0.02 μM 3TC, respectively. We found that a 9-day treatment with 0.02 μM 3TC resulted in a 64% reduction of extracellular HBV DNA produced by HepG2 cells infected at an MOI of 50 PFU of HBV baculovirus/cell. Analysis of results from earlier time points indicated that the decrease in extracellular HBV DNA was dependent on time; after only 3 days of treatment HBV DNA in the medium was reduced by only about 30%. Korba (20) reported that treatment with a 0.222 μM concentration of 3TC resulted in a 90% decrease in virion DNA produced by HepG2 2.2.15 after 9 days of treatment. Similarly, we found approximately a 98% reduction in extracellular DNA after a 9-day treatment of HBV baculovirus-infected HepG2 cells with 0.2 μM 3TC. Two important distinctions must be made between the 2.2.15 cell line used by other investigators and the HBV baculovirus-HepG2 system used here. First, at an MOI of 50 PFU of HBV baculovirus/cell, HepG2 cells exhibit much higher levels of HBV expression and replication than 2.2.15 cells. Second, with the exception of the appearance of CCC DNA, the copy number of HBV transcriptional templates per culture does not increase with time after HBV baculovirus infection. This is in contrast to cell lines containing integrated HBV genomes which double the number of transcriptional templates per culture each time the cells divide. Bearing these differences in mind, the results obtained by using stable cell lines and the HBV baculovirus-HepG2 system are remarkably similar.
During hepadnaviral replication, CCC HBV DNA can be produced by two pathways: (i) the entry of exogenous Dane particles into host cells and subsequent migration of HBV cores to nuclei and (ii) the cycling of newly synthesized progeny core particles from the cytoplasms of infected cells back to the nuclei. Once a core particle reaches the nucleus by either pathway, the HBV genome gains access to the nucleus by an unknown mechanism, is repaired to form RC DNA, and is subsequently supercoiled into CCC DNA. The effects of 3TC treatment on the first pathway cannot be evaluated by using the HBV baculovirus-HepG2 system or any cell lines because HBV does not directly infect cultured hepatic cell lines. However, the effects of 3TC on the second pathway were addressed by the experiments carried out in this study. Analysis of non-protein-associated RC and CCC forms of the HBV genome revealed that in addition to replicative intermediates and extracellular HBV DNA, the amplification of CCC DNA also can be inhibited by 3TC in a dose-dependent manner. Here, we report a greater than 90% inhibition of non-protein-associated RC and CCC HBV DNA production by HepG2 cells treated with 2.0 μM 3TC. These data were similar to those reported previously (24), showing that treatment of primary woodchuck hepatocytes with 3TC prior to infection with WHV caused an 80% inhibition of CCC WHV DNA amplification.
Our findings are consistent with the prediction that the cycling of newly synthesized HBV genomes back to the nucleus for CCC DNA amplification appears to require the completion of second-strand synthesis. The ability of 3TC to interfere with the synthesis of viral DNA effectively reduces the pool of mature core particles available to become enveloped virions or to cycle back to the nucleus to form RC and CCC DNA. It is also possible that 3TC could interfere with the nuclear repair of mature DS HBV genomes into CCC DNA. However, previous studies (19) have suggested that this repair is most likely carried out by host polymerases which are not sensitive to the concentrations of 3TC used in our experiments. RC and particularly CCC HBV DNA were not reduced to the same extent as extracellular HBV DNA when the same 3TC protocols were used. These findings could suggest that when very few mature HBV cores are present in the cytoplasm, there is a tendency for those cores to enter the CCC amplification pathway instead of acquiring an envelope and exiting the cell. Indeed, the finding in this study that extracellular HBV DNA levels were suppressed to a greater extent than intracellular replicative intermediates provides support for this hypothesis. This finding in 3TC-treated cells would not be unlike the natural early stages of hepadnaviral replication when the initial cores produced after infection are believed to cycle back to the nucleus to allow an amplification of CCC DNA before the secretion of virions takes place (32).
While studying the effects of initiating 3TC treatment on HBV baculovirus-infected HepG2 cells which had already accumulated CCC DNA, we made several interesting observations. The treatment of cultures which had already accumulated CCC DNA with 3TC did result in a reduction in the levels of CCC DNA. We believe that this reduction is occurring as a result of the block of HBV replication in the cytoplasm, which limits the number of mature genomes potentially available for cycling back to the nucleus to replenish CCC DNA pools. Not surprisingly, treating cultures with existing CCC DNA was not as effective as treating HepG2 cells prior to the initiation of HBV replication. Using the highest 3TC concentration that we tested (0.2 μM 3TC), we observed that CCC DNA was at 51% of control levels after 3 days of treatment and 23% of control levels after 6 days of treatment. The data we have obtained suggest that, at least under the conditions used, the half-life of CCC HBV DNA in HepG2 cells is roughly 3 days. This number would agree well with the previously reported 3- to 5-day half-life of duck hepatitis virus CCC in primary duck hepatocytes (7). However, it is necessary to take into consideration that the half-life of CCC DNA in an intact liver in which the CCC HBV DNA resides in nonreplicating hepatocytes may differ substantially from that in the in vitro HBV baculovirus-HepG2 system. It is also important to note that the production of extracellular HBV DNA was consistently more sensitive to equal 3TC concentrations and treatment lengths than those of replicative intermediates and particularly CCC HBV DNA.
The initiation of 3TC treatment in HBV-positive patients receiving orthotopic liver transplants prior to transplantation may have short-term benefits. First, administering 3TC before surgery should markedly lower the level of circulating virions capable of infecting new liver tissue. Second, in new hepatocytes which do become infected, a sufficient dose of 3TC may block or at least delay the onset of CCC DNA amplification by suppressing HBV replication. Depending on the stability of CCC DNA formed following infection, it is likely that some amplification will occur, albeit at a reduced rate, in 3TC-treated cells. Although it is unlikely that 3TC alone could prevent liver reinfection, it is possible that continual treatment with sufficient doses may result in a significant delay in the accumulation of CCC DNA within new tissue. Ultimately, a cure for HBV will likely require the elucidation of a method for eliminating episomal HBV DNA in the nuclei of infected cells. Whether this can be accomplished by an exogenous agent or by the induction of an existing cellular pathway remains to be seen. Although the initial formation of CCC HBV DNA due to viral entry may not be prevented by 3TC, the amplification of CCC DNA could potentially be blocked or delayed sufficiently to increase the efficacy of other antiviral agents.
One limitation of using stable cell lines, such as HepG2 2.2.15 cells, for evaluating the efficacy of an antiviral on HBV replication is that the magnitude of virus replication is at a static level predetermined by the number of integrated HBV genome copies. This limitation does not exist when HBV replication in HepG2 cells is mediated by recombinant HBV baculovirus, because it is possible to modulate the level of production of HBV virions over several magnitudes simply by altering the baculovirus MOI. In this study, we examined the effects of 3TC on HBV replication by using input MOIs of recombinant HBV baculovirus that varied over a 16-fold range. We found that the largest reduction of extracellular HBV DNA (>99% reduction after 6 days of treatment) occurred in cells infected with 25 PFU of baculovirus, the lowest MOI tested. Cultures infected at MOIs ranging from 50 PFU/cell to as high as 400 PFU/cell showed an average reduction of extracellular HBV DNA of greater than 96% after 6 days of 3TC treatment. This finding was somewhat unexpected and clearly indicated that 0.2 μM 3TC was highly effective at inhibiting HBV replication even in the presence of large amounts of the virus. However, it is also important to note that even a 96% reduction in HBV replication still allowed high levels of HBV virions to be secreted from 3TC-treated cells which were replicating very high levels of HBV (i.e., cells infected with HBV baculovirus at a high MOI). We estimated that cells infected with 200 PFU of HBV baculovirus/cell were secreting approximately 1,070 pg of HBV DNA/60-mm-diameter plate/day at 4 days p.i. Cultures infected with 200 PFU/cell and treated with 0.2 μM 3TC for 3 days exhibited a 79.3% reduction in extracellular HBV DNA; however, even after this reduction, the cells were still secreting approximately 220 pg of HBV DNA/60-mm-diameter plate/day at this time.
We conclude from these studies that the HBV baculovirus-HepG2 system has specific advantages for drug studies and can serve as a complement to other in vitro model systems currently used for testing antiviral compounds. The results presented here agree well with previous reports of the efficacy of 3TC in reducing levels of extracellular HBV DNA in vitro. Different types of information can be obtained by using the HBV baculovirus-HepG2 system because experiments can be carried out at various levels of HBV replication, including levels significantly higher than those that can be obtained from conventional HBV-expressing cell lines. The ability to manipulate and treat cells prior to HBV infection should also aid in studying the properties and potential efficacy of antivirals as prophylactic agents. Finally, the enhanced ability to detect CCC HBV DNA in the HBV baculovirus-HepG2 system facilitates the in vitro study of a crucial form of the HBV genome, which has to be evaluated in developing any treatment protocols for curing HBV infection.
ACKNOWLEDGMENTS
We thank Chris Tseng (NIH, NIAID Antiviral Research and Antimicrobial Chemistry Program) and Robert Rando of BioChem Therapeutic Inc. for providing the 3TC used in these studies. We also thank Tim Grierson for photographic assistance.
This work was supported in part by research grants from the National Institutes of Health (CA73045 and CA23931 to H.C.I.).
REFERENCES
- 1.Bartholomew M M, Jansen R W, Jeffers L J, Reddy K R, Johnson L C, Bunzendahl H, Condreay L D, Tzakis A G, Schiff E R, Brown N A. Hepatitis-B-virus resistance to lamivudine given for recurrent infection after orthotopic liver transplantation. Lancet. 1997;349:20–22. doi: 10.1016/S0140-6736(96)02266-0. . (Comment, 349:3–4.) [DOI] [PubMed] [Google Scholar]
- 2.Beasley R P, Hwang L Y, Lin C C, Chien C S. Hepatocellular carcinoma and hepatitis B virus. A prospective study of 22 707 men in Taiwan. Lancet. 1981;ii:1129–1133. doi: 10.1016/s0140-6736(81)90585-7. [DOI] [PubMed] [Google Scholar]
- 3.Ben-Ari Z, Shmueli D, Mor E, Shaharabani E, Bar-Nathan N, Shapira Z, Tur-Kaspa R. Beneficial effect of lamivudine pre- and post-liver transplantation for hepatitis B infection. Transplant Proc. 1997;29:2687–2688. doi: 10.1016/s0041-1345(97)00556-3. [DOI] [PubMed] [Google Scholar]
- 4.Cammack N, Rouse P, Marr C L, Reid P J, Boehme R E, Coates J A, Penn C R, Cameron J M. Cellular metabolism of (−) enantiomeric 2′-deoxy-3′-thiacytidine. Biochem Pharmacol. 1992;43:2059–2064. doi: 10.1016/0006-2952(92)90162-c. [DOI] [PubMed] [Google Scholar]
- 5.Chang C M, Jeng K S, Hu C P, Lo S J, Su T S, Ting L P, Chou C K, Han S H, Pfaff E, Salfeld J, et al. Production of hepatitis B virus in vitro by transient expression of cloned HBV DNA in a hepatoma cell line. EMBO J. 1987;6:675–680. doi: 10.1002/j.1460-2075.1987.tb04807.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987;162:156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- 7.Civitico G M, Locarnini S A. The half-life of duck hepatitis B virus supercoiled DNA in congenitally infected primary hepatocyte cultures. Virology. 1994;203:81–89. doi: 10.1006/viro.1994.1457. [DOI] [PubMed] [Google Scholar]
- 8.Coates J A V, Cammack N, Jenkinson H J, Jowett A J, Jowett M I, Pearson B A, Penn C R, Rouse P L, Viner K C, Cameron J M. (−)-2′-Deoxy-3′-thiacytidine is a potent, highly selective inhibitor of human immunodeficiency virus type 1 and type 2 replication in vitro. Antimicrob Agents Chemother. 1992;36:733–739. doi: 10.1128/aac.36.4.733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Davis L G, Dibner M D, Battey J F. Basic methods in molecular biology. New York, N.Y: Elsevier Science Publishing, Co., Inc.; 1986. Preparation and analysis of RNA from eukaryotic cells; pp. 129–156. [Google Scholar]
- 10.Delaney W E, IV, Isom H C. Hepatitis B virus replication in human HepG2 cells mediated by hepatitis B virus recombinant baculovirus. Hepatology. 1998;28:1134–1145. doi: 10.1002/hep.510280432. [DOI] [PubMed] [Google Scholar]
- 11.Dienstag J L, Perrillo R P, Schiff E R, Bartholomew M, Vicary C, Rubin M. A preliminary trial of lamivudine for chronic hepatitis B infection. N Engl J Med. 1995;333:1657–1661. doi: 10.1056/NEJM199512213332501. . (Comment, 333:1704–1705.) [DOI] [PubMed] [Google Scholar]
- 12.Doong S L, Tsai C H, Schinazi R F, Liotta D C, Cheng Y C. Inhibition of the replication of hepatitis B virus in vitro by 2′,3′-dideoxy-3′-thiacytidine and related analogues. Proc Natl Acad Sci USA. 1991;88:8495–8499. doi: 10.1073/pnas.88.19.8495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Evans A A, Fine M, London W T. Spontaneous seroconversion in hepatitis B e antigen-positive chronic hepatitis B: implications for interferon therapy. J Infect Dis. 1997;176:845–850. doi: 10.1086/516538. [DOI] [PubMed] [Google Scholar]
- 14.Grellier L, Mutimer D, Ahmed M, Brown D, Burroughs A K, Rolles K, McMaster P, Beranek P, Kennedy F, Kibbler H, McPhillips P, Elias E, Dusheiko G. Lamivudine prophylaxis against reinfection in liver transplantation for hepatitis B cirrhosis. Lancet. 1996;348:1212–1215. doi: 10.1016/s0140-6736(96)04444-3. . (Erratum, 349:364, 1997.) [DOI] [PubMed] [Google Scholar]
- 15.Hirsch R, Colgrove R, Ganem D. Replication of duck hepatitis B virus in two differentiated human hepatoma cell lines after transfection with cloned viral DNA. Virology. 1988;167:136–142. doi: 10.1016/0042-6822(88)90062-1. [DOI] [PubMed] [Google Scholar]
- 16.Honkoop P, de Man R A, Niesters H G. Quantitative assessment of hepatitis B virus DNA during a 24-week course of lamivudine therapy. Ann Intern Med. 1998;128:697. doi: 10.7326/0003-4819-128-8-199804150-00028. . (Letter.) [DOI] [PubMed] [Google Scholar]
- 17.Katkov W N. Hepatitis vaccines. Med Clin North Am. 1996;80:1189–1200. doi: 10.1016/s0025-7125(05)70485-5. . (Review.) [DOI] [PubMed] [Google Scholar]
- 18.Knowles B B, Howe C C, Aden D P. Human hepatocellular carcinoma cell lines secrete the major plasma proteins and hepatitis B surface antigen. Science. 1980;209:497–499. doi: 10.1126/science.6248960. [DOI] [PubMed] [Google Scholar]
- 19.Kock J, Schlicht H J. Analysis of the earliest steps of hepadnavirus replication: genome repair after infectious entry into hepatocytes does not depend on viral polymerase activity. J Virol. 1993;67:4867–4874. doi: 10.1128/jvi.67.8.4867-4874.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Korba B E. In vitro evaluation of combination therapies against hepatitis B virus replication. Antivir Res. 1996;29:49–51. doi: 10.1016/0166-3542(95)00915-9. [DOI] [PubMed] [Google Scholar]
- 21.Korba B E, Gerin J L. Use of a standardized cell culture assay to assess activities of nucleoside analogs against hepatitis B virus replication. Antivir Res. 1992;19:55–70. doi: 10.1016/0166-3542(92)90056-b. [DOI] [PubMed] [Google Scholar]
- 22.Kruining J, Heijtink R A, Schalm S W. Antiviral agents in hepatitis B virus transfected cell lines: inhibitory and cytotoxic effect related to time of treatment. J Hepatol. 1995;22:263–267. doi: 10.1016/0168-8278(95)80277-0. [DOI] [PubMed] [Google Scholar]
- 23.Lai C L, Ching C K, Tung A K, Li E, Young J, Hill A, Wong B C, Dent J, Wu P C. Lamivudine is effective in suppressing hepatitis B virus DNA in Chinese hepatitis B surface antigen carriers: a placebo-controlled trial. Hepatology. 1997;25:241–244. doi: 10.1002/hep.510250144. [DOI] [PubMed] [Google Scholar]
- 24.Moraleda G, Saputelli J, Aldrich C E, Averett D, Condreay L, Mason W S. Lack of effect of antiviral therapy in nondividing hepatocyte cultures on the closed circular DNA of woodchuck hepatitis virus. J Virol. 1997;71:9392–9399. doi: 10.1128/jvi.71.12.9392-9399.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nevens F, Main J, Honkoop P, Tyrrell D L, Barber J, Sullivan M T, Fevery J, de Man R A, Thomas H C. Lamivudine therapy for chronic hepatitis B: a six-month randomized dose-ranging study. Gastroenterology. 1997;113:1258–1263. doi: 10.1053/gast.1997.v113.pm9322520. [DOI] [PubMed] [Google Scholar]
- 26.Niederau C, Heintges T, Lange S, Goldmann G, Niederau C M, Mohr L, Haussinger D. Long-term follow-up of HBeAg-positive patients treated with interferon alfa for chronic hepatitis B. N Engl J Med. 1996;334:1422–1427. doi: 10.1056/NEJM199605303342202. . (Comment, 334:1470–1471.) [DOI] [PubMed] [Google Scholar]
- 27.Sambrook J, Fritsch E F, Maniatis T. Analysis and cloning of eukaryotic genomic DNA. In: Ford N, Nolan C, Ferguson M, editors. Molecular cloning. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. pp. 9.1–9.62. [Google Scholar]
- 28.Sells M A, Chen M L, Acs G. Production of hepatitis B virus particles in Hep G2 cells transfected with cloned hepatitis B virus DNA. Proc Natl Acad Sci USA. 1987;84:1005–1009. doi: 10.1073/pnas.84.4.1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sells M A, Zelent A Z, Shvartsman M, Acs G. Replicative intermediates of hepatitis B virus in HepG2 cells that produce infectious virions. J Virol. 1988;62:2836–2844. doi: 10.1128/jvi.62.8.2836-2844.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Summers J, Smith P M, Horwich A L. Hepadnavirus envelope proteins regulate covalently closed circular DNA amplification. J Virol. 1990;64:2819–2824. doi: 10.1128/jvi.64.6.2819-2824.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tsurimoto T, Fujiyama A, Matsubara K. Stable expression and replication of hepatitis B virus genome in an integrated state in a human hepatoma cell line transfected with the cloned viral DNA. Proc Natl Acad Sci USA. 1987;84:444–448. doi: 10.1073/pnas.84.2.444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tuttleman J S, Pourcel C, Summers J. Formation of the pool of covalently closed circular viral DNA in hepadnavirus-infected cells. Cell. 1986;47:451–460. doi: 10.1016/0092-8674(86)90602-1. [DOI] [PubMed] [Google Scholar]
- 33.Tyrrell D L J, Fischer K, Savani K, Tan W, Jewell L. Treatment of chimpanzees and ducks with lamivudine, 2′-3′-dideoxy-3′thiacytidine, results in a rapid suppression of hepadnaviral DNA in sera. Clin Investig Med. 1993;16:B77. . (Abstract.) [Google Scholar]
- 34.Ueda K, Tsurimoto T, Nagahata T, Chisaka O, Matsubara K. An in vitro system for screening anti-hepatitis B virus drugs. Virology. 1989;169:213–216. doi: 10.1016/0042-6822(89)90057-3. [DOI] [PubMed] [Google Scholar]
- 35.Wei Y, Tavis J E, Ganem D. Relationship between viral DNA synthesis and virion envelopment in hepatitis B viruses. J Virol. 1996;70:6455–6458. doi: 10.1128/jvi.70.9.6455-6458.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wong D K, Cheung A M, O’Rourke K, Naylor C D, Detsky A S, Heathcote J. Effect of alpha-interferon treatment in patients with hepatitis B e antigen-positive chronic hepatitis B. A meta-analysis. Ann Intern Med. 1993;119:312–323. doi: 10.7326/0003-4819-119-4-199308150-00011. . (Comment, 120(Suppl. 1):12, 1994.) [DOI] [PubMed] [Google Scholar]
- 37.World Health Organization. Fighting disease, fostering development. World health report. (Executive summary.) Geneva, Switzerland: World Health Organization; 1996. [Google Scholar]
- 38.Yaginuma K, Shirakata Y, Kobayashi M, Koike K. Hepatitis B virus (HBV) particles are produced in a cell culture system by transient expression of transfected HBV DNA. Proc Natl Acad Sci USA. 1987;84:2678–2682. doi: 10.1073/pnas.84.9.2678. [DOI] [PMC free article] [PubMed] [Google Scholar]