Abstract
Introduction
Atopic dermatitis (AD) can affect multiple body regions and is especially burdensome when involving exposed skin areas. Rapid, effective treatment of AD across body regions remains an unmet need, particularly for difficult-to-treat areas such as the head and neck area. We investigated the temporal and regional patterns of clinical improvement in AD with the use of abrocitinib, an orally available Janus kinase 1 selective inhibitor under development for the treatment of moderate-to-severe AD.
Methods
We performed a post hoc analysis of data from JADE COMPARE, a phase 3, multicenter, randomized, double-blind, double-dummy trial that evaluated the efficacy and safety of abrocitinib 200 mg once daily, abrocitinib 100 mg once daily, dupilumab 300 mg subcutaneous injection every 2 weeks, and placebo in adult patients with moderate-to-severe AD who were concomitantly receiving medicated topical therapy. Assessments included the Eczema Area and Severity Index (EASI) and SCORing Atopic Dermatitis (SCORAD) index.
Results
With abrocitinib 200 mg, time to ≥ 75% improvement in EASI (EASI-75) occurred at a median of 29 days across body regions, including the head and neck region. With abrocitinib 100 mg, EASI-75 response was achieved at a median of 30–32 days for the trunk and lower limbs, and at 56–57 days for the head and neck region and upper limbs. With dupilumab, EASI-75 response was achieved at a median of 43 days for the trunk and 57 days for other regions. EASI body region scores significantly improved with abrocitinib 200 mg and 100 mg versus placebo at week 2 (p < 0.0001 for all comparisons). Improvements with abrocitinib were maintained up to week 16.
Conclusions
Rapid and persistent improvement in AD across all body regions was observed with abrocitinib treatment. Abrocitinib may be useful in patients with AD that affects difficult-to-treat anatomical areas or who require a rapid response.
Trial registration
Clinicaltrials.gov identifier: NCT03720470.
Graphical abstract
Supplementary Information
The online version contains supplementary material available at 10.1007/s13555-022-00694-1.
Keywords: Abrocitinib, Atopic dermatitis, EASI, Regional improvement, SCORAD, Temporal improvement
Plain Language Summary
Atopic dermatitis (AD) is the most common form of eczema. It is a long-lasting skin disease that often affects multiple body regions. AD may decrease a person’s quality of life, especially when it involves skin areas that are visible to others. Quick, effective treatment for AD across multiple body regions is needed, especially in areas that are difficult to treat and/or cosmetically important, such as the head and neck area. Abrocitinib is a medicine taken orally that is being developed to treat moderate-to-severe AD. Researchers analyzed data from a clinical study called JADE COMPARE (Clinicaltrials.gov identifier: NCT03720470). It evaluated the effectiveness and safety of abrocitinib 200 mg taken once daily, abrocitinib 100 mg taken once daily, and placebo (no study medication) plus medicated skin cream in adults with moderate-to-severe AD. Abrocitinib 200 mg and 100 mg significantly improved the extent and severity of AD as assessed by a measure called the Eczema Area and Severity Index (EASI) compared to placebo at week 2. Improvements were maintained for up to 16 weeks. With abrocitinib 200 mg, the time to ≥ 75% improvement in EASI (EASI-75) was approximately 29 days across body regions, including the head and neck region. With abrocitinib 100 mg, this EASI-75 response occurred at approximately 30 to 32 days for the trunk and legs and at 56 to 57 days for the head and neck region and arms. Abrocitinib may be effective in people with AD that affects difficult-to-treat parts of the body or in those who need a fast response.
Supplementary Information
The online version contains supplementary material available at 10.1007/s13555-022-00694-1.
Key Summary Points
| Why carry out this study? |
| Atopic dermatitis (AD) often affects multiple body regions and is associated with a high burden for patients when exposed skin areas are involved. |
| Rapid and effective treatment of AD across multiple body regions is an unmet need, especially in body areas that are difficult to treat, such as the head and neck area. |
| This post hoc analysis investigated the temporal and regional patterns of clinical improvement in AD with abrocitinib in JADE COMPARE, a phase 3, multicenter, randomized, double-blind, double-dummy trial that evaluated the efficacy and safety of abrocitinib 200 mg once daily, abrocitinib 100 mg once daily, dupilumab 300 mg subcutaneous injection every 2 weeks, and placebo, combined with background medicated topical therapy in adult patients with moderate-to-severe AD. |
| What was learned from the study? |
| Eczema Area and Severity Index body region scores significantly improved with abrocitinib 200 mg and 100 mg versus placebo at week 2 and were maintained up to 16 weeks. |
| Abrocitinib may be useful in patients with AD that affects difficult-to-treat anatomical areas or who require a rapid response. |
Digital Features
This article is published with digital features, including an infographic, to facilitate understanding of the article. To view digital features for this article go to 10.6084/m9.figshare.19153649
Introduction
Atopic dermatitis (AD) is a chronic inflammatory skin condition associated with pruritic, painful lesions that can affect multiple body regions [1]. In exposed regions, AD may cause additional patient burden [2–8]. AD in the facial area is associated with increased AD pain intensity [9]. Furthermore, AD in the facial area and/or head/neck region is associated with lower quality of life than AD affecting other regions [4, 8, 10]. AD on the hands poses a large burden due to functional impairment in work and other activities [8, 11].
Consistent with this increased burden, patients regard AD involvement of exposed body sites as one of the most important factors when judging treatment response [12] and place high importance on treatments that can provide almost complete or complete skin clearance of the face or neck [10, 13]. However, AD afflicting some areas of the body, including the head and neck, is difficult to treat [14]. Exposed skin areas may be more susceptible to environmental exposures that are common contributors to loss of disease control [15]. Post-inflammatory pigment alterations, which are common among patients with skin of color, may add further burden to the disease and can be more stigmatizing when exposed areas of the skin are involved [16].
Current treatments for the head and neck area have limitations. Topical medicated therapies can cause a stinging or burning sensation at the application site [9, 15]. Patients are discouraged from using medium- or high-potency topical corticosteroids on the face and neck area [15] because their prolonged use can cause facial skin atrophy, periorificial dermatitis (mouth, nose, eyes), corticosteroid-induced rosacea (forehead, cheeks, nose), rebound dermatitis (including facial “hot flashes” and papules), and hypopigmentation [16–18].
Limitations to the use of systemic treatments include the persistence of lesions that may not respond as well to dupilumab as AD lesions in other body regions or occurrence of facial rash in association with dupilumab, which may represent new-onset dermatitis (i.e., a treatment side effect) [19–23]. Thus, the ability to treat AD quickly across all body regions, including the head and neck area, remains an unmet need.
Abrocitinib is a Janus kinase (JAK)-1 selective inhibitor [24] that is under clinical investigation for treatment of moderate-to-severe AD. The efficacy and safety of abrocitinib 200 mg and 100 mg once-daily (QD) monotherapy versus placebo have been demonstrated in the phase 3 JADE MONO-1 and JADE MONO-2 trials [25, 26]. The phase 3 JADE COMPARE trial, in which all patients were to apply concomitant topical anti-inflammatory agents to active lesions, demonstrated the efficacy and safety of abrocitinib 200 mg and 100 mg QD versus placebo and dupilumab [27]. The COMPARE trial was powered for direct comparison to dupilumab at a single endpoint, namely, itch response at 2 weeks.
Treatments for AD are desired that act rapidly and consistently across all body regions and do not exacerbate or cause redness or rash in exposed areas that are more difficult to treat. There are only a limited number of reports in the literature describing the efficacy of abrocitinib according to body region [28]. The objective of this post hoc analysis of data from the phase 3 JADE COMPARE trial was to investigate the temporal and regional patterns of clinical improvement in AD with abrocitinib.
Methods
Study Design and Patient Eligibility
The phase 3, multicenter, randomized, double-blind, double-dummy, placebo-controlled JADE COMPARE trial (Clinicaltrials.gov identifier: NCT03720470) evaluated the efficacy and safety of abrocitinib in adults with moderate-to-severe AD who were receiving background medicated topical therapy [27]. This study also included dupilumab as an active treatment arm. The study enrolled patients from 29 October 2018 to 5 August 2019, in North and South America, Australia, Europe, and Asia. Full inclusion and exclusion criteria have been published previously. Eligible patients were aged ≥ 18 years, had moderate-to-severe AD at baseline (Investigator’s Global Assessment ≥ 3; Eczema Area and Severity Index [EASI] ≥ 16; body surface area involvement ≥ 10%; Peak Pruritus Numerical Rating Scale [PP-NRS] ≥ 4), and had a documented history of inadequate response to treatment with medicated topical therapy given for ≥ 4 weeks or required systemic therapy to control AD. Patients who previously used a systemic JAK inhibitor or dupilumab were ineligible to participate.
Treatment
Patients were randomly assigned in a 2:2:2:1 ratio to 16 weeks of treatment with oral abrocitinib 200 mg QD (plus placebo subcutaneous injection every other week), oral abrocitinib 100 mg QD (plus placebo subcutaneous injection every other week), subcutaneous dupilumab 300 mg every other week following a 600-mg loading dose (plus oral placebo once daily), or placebo. Background medicated topical therapy with low- or medium-potency topical corticosteroids, topical calcineurin inhibitors, or topical phosphodiesterase-4 inhibitors was to be applied once daily to active lesions throughout the study. Non-medicated topical emollient was also required to be applied at least twice daily to all body areas affected with AD for the 7 days prior to day 1 and throughout the study.
The study protocol and informed consent documents were reviewed and approved by the Institutional Review Board and/or Independent Ethics Committee at each of the investigational sites. The study was conducted in accordance with the ethical principles of the Declaration of Helsinki, the International Council for Harmonization Good Clinical Practice guidelines, and all local regulatory requirements.
Assessments
The EASI [15] was assessed at weeks 2, 4, 8, 12, and 16. AD lesions were scored by body region for the head and neck, trunk, upper limbs, and lower limbs. In JADE COMPARE, the EASI assessments excluded the scalp, palms, and soles. Body region scores were calculated based on severity of erythema, induration/papulation, excoriation and lichenification, and area of involvement.
The SCORing Atopic Dermatitis (SCORAD) [15] score was assessed at weeks 2, 4, 8, 12, and 16. In the extent component of SCORAD, AD lesions were scored by body region for the head and neck, left upper limbs, right upper limbs, left lower limbs, right lower limbs, anterior trunk, back, and genitals. In the intensity component of SCORAD, AD lesions were scored for erythema, edema/papulation, oozing/crusting, excoriation, lichenification, and dryness. SCORAD assessment also included subjective symptoms of pruritus and sleep loss scores.
Statistical Analyses
Efficacy was analyzed in the full analysis set, which was defined as all patients randomly assigned to treatment who received ≥ 1 dose of study medication. Least squares mean (LSM) percentage change from baseline on EASI body region scores and subscores and SCORAD extent, intensity, pruritus, and sleep loss scores were analyzed using mixed-model repeated measures with fixed factors of treatment, visit, treatment-by-visit interaction, baseline disease severity, baseline value, and an unstructured covariate matrix.
Distribution of time to ≥ 75% and ≥ 90% improvement in EASI regional subscores (EASI-75 and EASI-90, respectively) was estimated based on the Kaplan–Meier method. Confidence intervals were based on the Brookmeyer and Crowley method, and the log-rank test comparison of abrocitinib versus placebo was controlled for baseline disease severity.
No familywise type 1 error–controlled statistical comparisons were made for abrocitinib 200 mg, abrocitinib 100 mg, or placebo versus dupilumab in these analyses. JADE COMPARE included only a familywise type 1 error–controlled comparison between abrocitinib and dupilumab at a single endpoint (4-point improvement in PP-NRS at week 2). Nominal p values are presented for abrocitinib versus placebo without multiplicity adjustment.
Results
Baseline Demographics and Disease Characteristics
Demographics and baseline disease characteristics were balanced across groups, including the baseline EASI total score and scores by body region (Tables 1, 2). Most patients had EASI involvement of the head and neck area at baseline, including 96.9% in the abrocitinib 200 mg group, 97.5% in the abrocitinib 100 mg group, 96.3% in the dupilumab group, and 97.7% in the placebo group. Baseline SCORAD extent, intensity, pruritus, and sleep loss scores also were similar across treatment arms (Electronic Supplementary Material [ESM] Table S1). The genital region had very low involvement (mean 0.1 across groups, on a scale of 0.1–1.0).
Table 1.
Patient demographics
| Patient demographics | Placebo (N = 131) | Abrocitinib 100 mg QD (N = 238) | Abrocitinib 200 mg QD (N = 226) | Dupilumab 300 mg Q2W (N = 242) |
|---|---|---|---|---|
| Age, years, mean (SD) | 37.4 (15.2) | 37.3 (14.8) | 38.8 (14.5) | 37.1 (14.6) |
| Female, n (%) | 54 (41.2) | 118 (49.6) | 122 (54.0) | 134 (55.4) |
| Race, n (%) | ||||
| White | 87 (66.4) | 182 (76.5) | 161 (71.2) | 176 (72.7) |
| Black | 6 (4.6) | 6 (2.5) | 9 (4.0) | 14 (5.8) |
| Asian | 31 (23.7) | 48 (20.2) | 53 (23.5) | 46 (19.0) |
| Other | 7 (5.4) | 2 (0.8) | 3 (1.3) | 6 (2.4) |
Q2W Every 2 weeks, QD once daily, SD standard deviation
Table 2.
Baseline disease characteristics
| Placebo (N = 131) | Abrocitinib 100 mg QD (N = 238) | Abrocitinib 200 mg QD (N = 226) | Dupilumab 300 mg Q2W (N = 242) | |
|---|---|---|---|---|
| Duration of AD, years, mean (SD) | 21.4 (14.4) | 22.7 (16.3) | 23.4 (15.6) | 22.8 (14.8) |
| IGAa, n (%) | ||||
| Moderate | 88 (67.2) | 153 (64.3) | 138 (61.1) | 162 (66.9) |
| Severe | 43 (32.8) | 85 (35.7) | 88 (38.9) | 80 (33.1) |
| %BSA affected, mean (SD) | 48.9 (24.9) | 48.1 (23.1) | 50.8 (23.0) | 46.5 (22.1) |
| EASIb, mean (SD) | 31.0 (12.6) | 30.3 (13.5) | 32.1 (13.1) | 30.4 (12.0) |
| Head and neck (max 7.2) | 3.0 (1.6) | 2.8 (1.7) | 2.9 (1.6) | 2.9 (1.6) |
| Trunk (max 21.6) | 8.9 (4.6) | 8.4 (4.5) | 9.1 (4.7) | 8.5 (4.9) |
| Upper limbs (max 14.4) | 7.1 (3.0) | 6.8 (3.3) | 7.3 (3.3) | 6.8 (2.9) |
| Lower limbs (max 28.8) | 11.8 (6.8) | 12.3 (7.2) | 12.7 (6.6) | 12.0 (6.1) |
| SCORADc, mean (SD) | 67.9 (12.0) | 66.8 (13.8) | 69.3 (12.7) | 67.9 (11.4) |
AD Atopic dermatitis, BSA body surface area, EASI Eczema Area and Severity Index, IGA Investigator’s Global Assessment, max maximum, SCORAD SCORing Atopic Dermatitis
aIGA ranges from clear (0) to severe (4)
bEASI total score ranges from 0 to 72, with higher scores indicating more severe disease. The scalp, palms, and soles were not assessed in this study
cSCORAD total score ranges from 0 to 103, with higher scores representing more severe disease
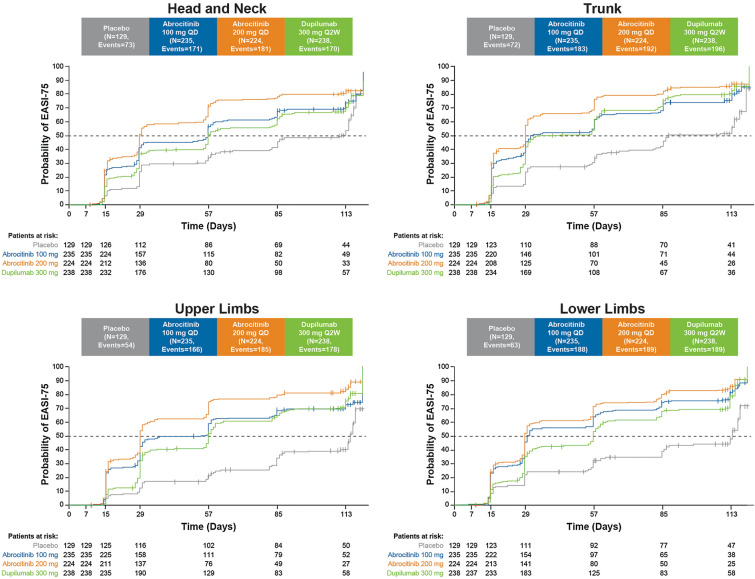
Time to Achieve EASI-75 and EASI-90 Responses by Body Region
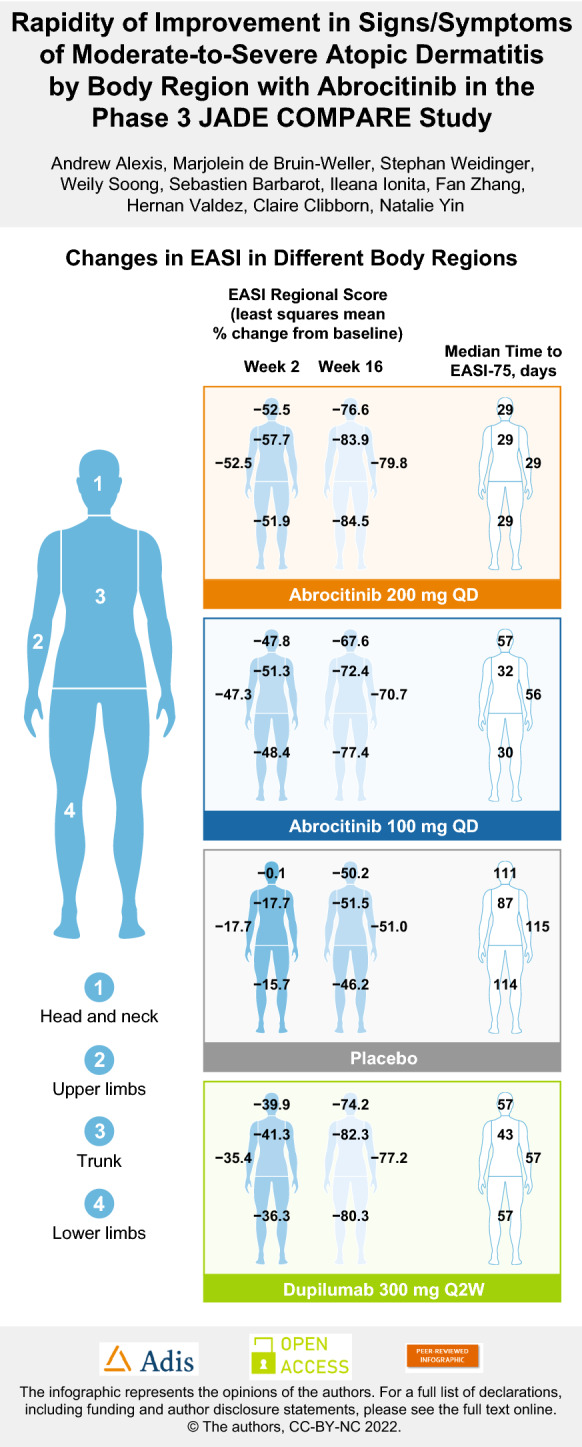
With abrocitinib 200 mg, EASI-75 response was achieved at a median of 29 days across body regions, including the head and neck area (Fig. 1; Table 3). With abrocitinib 100 mg, EASI-75 response was achieved at a median of 30–32 days for the trunk and lower limbs, and at 57 days for the head and neck area and 56 days for the upper limbs. With dupilumab, EASI-75 response was achieved at a median of 57 days for the head and neck area, upper limbs, and lower limbs, and at 43 days for the trunk.
Fig. 1.
Kaplan–Meier curve of time to first achieve ≥ 75% improvement from baseline in EASI body region score. EASI Eczema Area and Severity Index, EASI-75 ≥ 75% improvement in EASI from baseline, Q2W every 2 weeks, QD once daily
Table 3.
Kaplan–Meier median time to EASI-75 and EASI-90 by body region
| Placebo | Abrocitinib 100 mg QD | Abrocitinib 200 mg QD | Dupilumab 300 mg Q2W | |
|---|---|---|---|---|
| Median time to EASI-75 in days (95% CI) | ||||
| Head and neck | 111 (83–114) | 57 (31–58) | 29 (29–32) | 57 (56–83) |
| p = 0.0002 vs. placebo | p < 0.0001 vs. placebo | |||
| Trunk | 87 (84–114) | 32 (29–57) | 29 (NE-NE) | 43 (30–57) |
| p < 0.0001 vs. placebo | p < 0.0001 vs. placebo | |||
| Upper limbs | 115 (114-NE) | 56 (30–57) | 29 (29–30) | 57 (56–59) |
| p < 0.0001 vs. placebo | p < 0.0001 vs. placebo | |||
| Lower limbs | 114 (87–117) | 30 (29–54) | 29 (29–30) | 57 (55–59) |
| p < 0.0001 vs. placebo | p < 0.0001 vs. placebo | |||
| Median time to EASI-90 in days (95% CI) | ||||
| Head and neck | NE (113-NE) | 110 (85–114) | 57 (57–84) | 112 (85–114) |
| p = 0.0056 vs. placebo | p < 0.0001 vs. placebo | |||
| Trunk | NE (114-NE) | 85 (66–112) | 57 (32–58) | 85 (63–112) |
| p < 0.0001 vs. placebo | p < 0.0001 vs. placebo | |||
| Upper limbs | 117 (117-NE) | 113 (85-NE) | 58 (57–85) | 114 (112–115) |
| p < 0.0001 vs. placebo | p < 0.0001 vs. placebo | |||
| Lower limbs | NE (NE-NE) | 83 (58–88) | 57 (57–60) | 113 (86–115) |
| p < 0.0001 vs. placebo | p < 0.0001 vs. placebo | |||
CI Confidence interval, EASI-75 ≥ 75% improvement in EASI from baseline, EASI-90 ≥ 90% improvement in EASI from baseline, NE not evaluable as too few events were observed
With abrocitinib 200 mg, EASI-90 response was achieved at a median of 57–58 days across all assessed body regions (ESM Fig. S1; Table 3). With abrocitinib 100 mg, EASI-90 response was achieved at a median of 83–85 days for the trunk and lower limbs, and at 110−113 days for the head and neck area and upper limbs. With dupilumab, EASI-90 response was achieved at a median of 112–114 days for the head and neck area, upper limbs, and lower limbs, and at 85 days for the trunk.
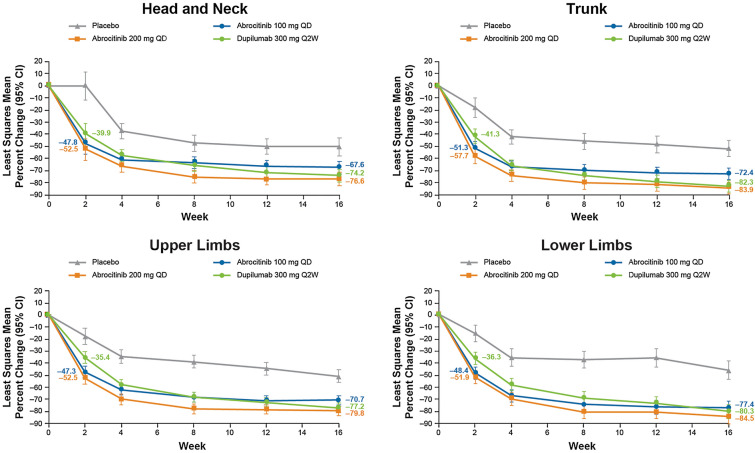
Change from Baseline in EASI Score and Subscores
Significant improvement (reduction) in EASI body region scores was demonstrated with abrocitinib 200 mg and 100 mg versus placebo at week 2 (p < 0.0001 for all comparisons) and at all subsequent time points (p ≤ 0.0002 for all comparisons). At week 2, LSM percentage change from baseline in EASI body region scores ranged from − 51.9% to − 57.7% for abrocitinib 200 mg, − 47.3% to − 51.3% for abrocitinib 100 mg, − 35.4% to − 41.3% for dupilumab, and − 0.1% to − 17.7% for placebo (Fig. 2). At week 16, LSM percentage change from baseline in EASI body region scores ranged from − 76.6% to − 84.5% for abrocitinib 200 mg, − 67.6% to − 77.4% for abrocitinib 100 mg, − 74.2% to − 82.3% for dupilumab, and − 46.2% to − 51.5% for placebo.
Fig. 2.
Least squares mean percentage change from baseline in EASI body region score at weeks 2, 4, 8, 12, and 16. CI Confidence interval
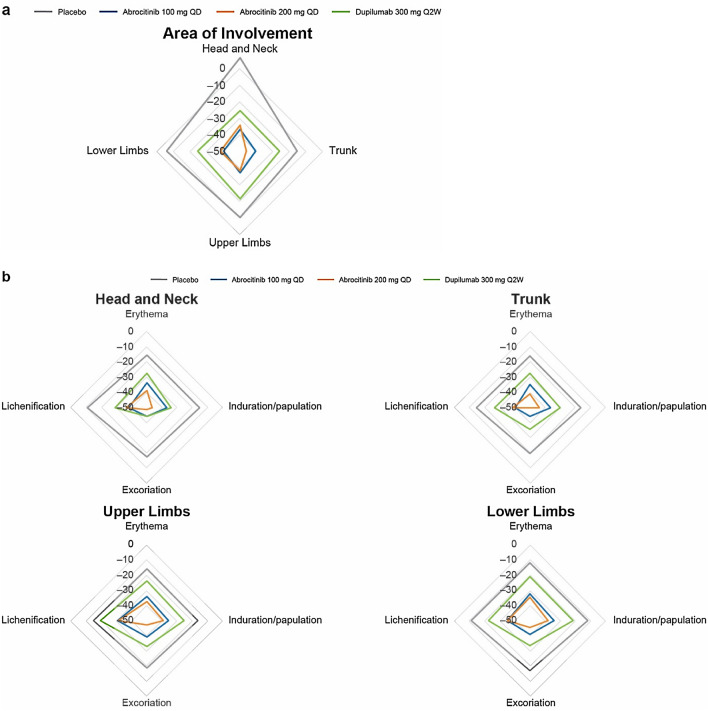
Significant improvement (reduction) in EASI body region subscores for area of involvement was demonstrated with abrocitinib 200 mg and 100 mg versus placebo at week 2 (p < 0.0001 for all comparisons). At week 2, LSM percentage change from baseline in EASI body region subscores for area of involvement ranged from − 34.2% to − 46.1% for abrocitinib 200 mg, − 36.8% to − 40.6% for abrocitinib 100 mg, − 21.7% to − 25.9% for dupilumab, and − 5.7% to − 15.1% for placebo (Fig. 3a). At week 2, LSM percentage change from baseline in EASI body region subscores for erythema severity ranged from − 34.3% to − 41.0% for abrocitinib 200 mg, − 32.2% to − 34.7% for abrocitinib 100 mg, − 20.9% to − 27.3% for dupilumab, and − 11.5% to − 15.9% for placebo (Fig. 3b). At week 2, LSM percentage change from baseline in EASI body region subscores for induration/papulation severity ranged from − 38.4% to − 46.5% for abrocitinib 200 mg, − 34.4% to − 36.8% for abrocitinib 100 mg, − 21.4% to − 34.0% for dupilumab, and − 12.1% to − 16.4% for placebo (Fig. 3b). At week 2, LSM percentage change from baseline in EASI body region subscores for excoriation severity ranged from − 45.6% to − 50.0% for abrocitinib 200 mg, − 39.3% to − 44.2% for abrocitinib 100 mg, − 32.9% to − 44.1% for dupilumab, and − 17.1% to − 19.7% for placebo (Fig. 3b). At week 2, LSM percentage change from baseline in EASI body region subscores for lichenification severity ranged from − 31.3% to − 38.7% for abrocitinib 200 mg, − 31.0% to − 40.1% for abrocitinib 100 mg, − 19.5% to − 29.3% for dupilumab, and − 10.8% to − 14.9% for placebo (Fig. 3b).
Fig. 3.
Least squares mean percentage change from baseline in EASI body region subscores for area of involvement (a) and severity (erythema, induration/papulation, excoriation, and lichenification) (b) at week 2
SCORAD Change from Baseline at Week 2
At week 2, LSM percentage change from baseline in SCORAD extent scores (excluding the genital region that had low baseline involvement) ranged from − 34.0% to − 46.0% for abrocitinib 200 mg, − 35.0% to − 41.3% for abrocitinib 100 mg, − 20.4% to − 30.0% for dupilumab, and − 7.0% to − 21.8% for placebo (ESM Fig. S2a). Abrocitinib 200 mg and 100 mg had significantly larger improvements than placebo across body regions, including the head and neck area. The largest improvements in SCORAD extent at week 2 were observed in the anterior trunk and back.
At week 2, LSM percentage change from baseline in SCORAD intensity items ranged from − 30.1% to − 54.3% for abrocitinib 200 mg, − 23.5% to − 43.4% for abrocitinib 100 mg, − 14.1% to − 41.6% for dupilumab, and − 7.8% to − 25.7% for placebo (ESM Fig. S2b). Abrocitinib 200 mg and 100 mg were associated with significantly larger improvements than placebo across all intensity items, including dryness. The largest improvement at week 2 in all treatment arms was observed for oozing/crusting, followed by excoriation.
At week 2, LSM percentage change from baseline in SCORAD pruritus was − 49.2% for abrocitinib 200 mg, − 38.7% for abrocitinib 100 mg, − 30.8% for dupilumab, and − 20.0% for placebo (p < 0.0001 for both abrocitinib groups vs. placebo). At week 2, LSM percentage change from baseline in SCORAD sleep loss was − 51.1% for abrocitinib 200 mg, − 36.4% for abrocitinib 100 mg, − 32.0% for dupilumab, and − 17.0% for placebo (p < 0.0001 for abrocitinib 200 mg vs. placebo and p = 0.0008 for abrocitinib 100 mg vs. placebo).
Time to EASI-75 and EASI-90 Responses by Body Region in White and Asian Patients
Racial and ethnic group categories are shown in ESM Table S2. Time to EASI responses was assessed by race in two subpopulations, white and Asian, that included enough participants to make meaningful comparisons. Mean (standard deviation) baseline scores were similar across body regions for white and Asian subgroups (ESM Table S3). No clear differences were seen in median time to EASI-75 by body region identified between the overall population and the individual white and Asian subgroups (ESM Table S4). The median time to EASI-75 across body regions was 29–31 days with abrocitinib 200 mg for both white and Asian subgroups. No clear differences in median time to EASI-90 by body region were identified between the overall population and the individual white and Asian subgroups (ESM Table S5).
Time to EASI-75 and EASI-90 Responses by Body Region in Patients with High Baseline Involvement of the Head and Neck Region
The median time to first achieve EASI-75 and EASI-90 did not differ between individuals with high baseline involvement of the head and neck region (defined as a head and neck EASI score of ≥ 4) and the overall study population (ESM Table S6).
Discussion
This analysis of the JADE COMPARE trial demonstrated a rapid improvement in AD across all body regions, including the head and neck region, with abrocitinib, with responses persisting until at least week 16. With abrocitinib 200 mg QD, the median time to EASI-75 response and EASI-90 response was approximately 1 month and approximately 2 months, respectively, and was consistent across all body regions, including the head and neck area, trunk, upper limbs, and lower limbs. Treatment with abrocitinib 200 mg resulted in a 52.5% and 76.6% reduction in the head and neck area EASI score from baseline at week 2 and week 16, respectively, and significant reductions versus placebo across all head and neck region subscores for the area of involvement and severity of erythema, induration/papulation, excoriation, and lichenification. The data suggest that all anatomical regions are equally responsive to abrocitinib and that abrocitinib provides rapid effectiveness also in difficult-to-treat, exposed anatomical areas, such as the head and neck area [14]. No efficacy differences were observed between white and Asian patients. Subgroup analyses involving black and other populations were not possible due to small sample sizes.
The time required to achieve a treatment response is among the most important patient-reported attributes for AD therapies [29]. The rapidity of response demonstrated with abrocitinib in this analysis, with significant and clinically meaningful improvements observed as early as week 2, is anticipated to be desirable for patients.
AD is associated with sleep disturbance, anxiety, depression, and diminished quality of life [30–34], and patients with AD affecting the facial area have increased AD pain intensity [9] and a lower quality of life than patients with lesions that occur in other body regions [4, 8, 10]. Patients place high importance on treatments that can provide complete or almost complete skin clearance of the face or neck area [10, 13]. Based on the findings of this study, abrocitinib may be particularly useful in patients with AD affecting difficult-to-treat, exposed areas [14]. Patients with high baseline involvement of the head and neck area experienced the same degree of improvement as the overall study population.
The significant benefit demonstrated with both abrocitinib 200 mg and 100 mg versus placebo in the SCORAD dryness intensity item at week 2 is a notable effect for a systemic treatment. Excessive dryness is commonly reported (following pruritus) as among the most burdensome symptoms of AD [34], and a drug’s effect on dry, flaky skin is considered by patients to be an important feature in assessing whether a treatment is working [12]. Patients in all treatment arms were required to use topical emollients throughout this study, in addition to medicated topical therapy for active lesions. As demonstrated here, abrocitinib had significant and clinically meaningful effects on SCORAD dryness at week 2, above any placebo effect observed from the use of topical emollients or medicated topical therapy. Evaluation of abrocitinib monotherapy may provide additional insights on the effect of abrocitinib on dryness. Skin barrier disruption, and the resulting exposure to allergens, is thought to be instrumental in development of AD [35]. In an animal model, a mutation causing JAK-1 hyperactivation has been demonstrated to cause skin barrier disruption and progressive pruritic dermatitis, with disease onset delayed by pharmacological JAK-1 inhibition [36]. Therefore JAK-1 inhibition by abrocitinib may directly improve skin barrier function and therefore dryness, as observed in this study.
A significant improvement in SCORAD pruritus, excoriation, and lichenification measures was also demonstrated at week 2 in this analysis. A significant improvement in the PP-NRS was previously demonstrated with abrocitinib 200 mg versus dupilumab at week 2, which was a key secondary endpoint of JADE COMPARE [27].
Published clinical trial data for dupilumab demonstrate an improvement in the overall head and neck EASI score [37], although case reports and retrospective studies have reported worsening or new onset dermatitis of the face and neck area [18–23, 38, 39].
This analysis has some limitations. First, the data are from a 16-week study, and thus assessed short-term treatment. Longer treatment periods need to be studied. Second, these analyses were post hoc; as such, these observations are considered to be hypothesis-generating rather than definitive. Statistical comparisons of abrocitinib versus dupilumab were not planned for these analyses, nor was JADE COMPARE powered or designed to make direct superiority comparisons between abrocitinib and dupilumab outside of the itch response at week 2. Direct comparisons between abrocitinib and dupilumab cannot be made. Third, there was no possibility to compare the impact of treatment on the scalp, palms, and soles. EASI assessments of the head and neck region excluded the scalp; upper limb assessments excluded the palms; and lower limb assessments excluded the soles. Future studies are required to specifically examine other exposed areas. Finally, disparities in enrollment have recently been highlighted for ethnic minorities in clinical trials of patients with AD [40]. Enrollment of a diverse patient population (including increased enrollment of black patients and other populations with skin of color) should be a priority for future studies.
Conclusions
The rapid and persistent improvement in AD across body regions that is observed with abrocitinib treatment is a desirable feature for patients suffering from moderate-to-severe AD. Abrocitinib may be particularly useful in patients with AD lesions that affect difficult-to-treat, exposed anatomical areas or who require a rapid response.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
Funding
This trial was sponsored by Pfizer Inc. The Rapid Service Fee for this article was funded by Pfizer Inc.
Medical Writing and Editorial Assistance
Editorial/medical writing support under the guidance of authors was provided by Renee Gordon, PhD, CMPP, and Mariana Ovnic, PhD, at ApotheCom, Philadelphia, PA, USA, and was funded by Pfizer Inc., New York, NY, USA, in accordance with Good Publication Practice (GPP3) guidelines (Ann Intern Med. 2015;163:461–464).
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Author Contributions
AA, MB, SB, SW, WS, and FZ contributed to data analysis and interpretation and revised the manuscript for important intellectual content. II contributed to collection and assembly of data and revised the manuscript for important intellectual content. HV and CC contributed to conception and design of the study and data analysis and interpretation, and revised the manuscript for important intellectual content. NY contributed to conception and design of the study and drafting of the work. All authors contributed to final approval of the manuscript and agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Disclosures
Andrew Alexis is an investigator (research grant to department) for LEO Pharma, Novartis, Almirall, Bristol Myers Squibb, Amgen, Menlo, Galderma, Valeant (Bausch Health), Cara, and Arcutis; an advisory board member for LEO Pharma, Galderma, Pfizer, Sanofi-Regeneron, Dermavant, Beiersdorf, Valeant, L’Oréal, Bristol Myers Squibb, Bausch Health, UCB, Vyne, Arcutis, Janssen, Allergan, Almirall, AbbVie, Sol–Gel, and Amgen; and a speaker for Regeneron, Sanofi-Genzyme, Pfizer, and Astra Zeneca. Marjolein de Bruin-Weller has been a consultant, advisory board member, and/or speaker for AbbVie, Almirall, Eli Lilly, Galderma, Janssen, LEO Pharma, Pfizer, Regeneron, Sanofi-Genzyme, and UCB. Stephan Weidinger has received institutional research grants from Sanofi Deutschland GmbH, LEO Pharma, and La Roche-Posay; has performed consultancies for Sanofi-Genzyme, Regeneron, LEO Pharma, AbbVie, Pfizer, Eli Lilly, Kymab, and Novartis; has lectured at educational events sponsored by Sanofi-Genzyme, Regeneron, LEO Pharma, AbbVie, Novartis, and Galderma; and is involved in performing clinical trials with many pharmaceutical industries that manufacture drugs used for the treatment of psoriasis and atopic dermatitis. Weily Soong has received research funding from 3M, Aimmune, AstraZeneca, Circassia, Genentech, Galderma, Glenmark, LEO Pharma, Menlo Therapeutics, Novartis, OptiNose, Pfizer, Regeneron Pharmaceuticals, Ralexar Therapeutics, Roche, Sanofi, Stallergenes, and Teva; has received speaking fees from AstraZeneca, Circassia, OptiNose, Roche-Genentech, GlaxoSmithKline, Sanofi, Regeneron Pharmaceuticals, and Teva; and has received consulting fees from AbbVie, ALK, AstraZeneca, Regeneron Pharmaceuticals, Stallergenes, and Teva. Sebastien Barbarot has served as a scientific adviser, consultant, and/or clinical study investigator for Pierre Fabre Laboratory, Bioderma, Laboratoire La Roche-Posay, Sanofi-Genzyme, AbbVie, Novartis, Janssen, LEO Pharma, Pfizer, Eli Lilly, and UCB Pharma. Natalie Yin is a former employee and shareholder of Pfizer. Natalie Yin currently works for US Dermatology Partners Practice, Denver, CO, USA. Ileana Ionita, Fan Zhang, Hernan Valdez, and Claire Clibborn are employees and shareholders of Pfizer, Inc.
Compliance with Ethics Guidelines
The study protocol and informed consent documents were reviewed and approved by the Institutional Review Board and/or Independent Ethics Committee at each of the investigational sites. The study was conducted in accordance with the ethical principles of the Declaration of Helsinki, the International Council for Harmonization Good Clinical Practice guidelines, and all local regulatory requirements.
Data Availability
Upon request, and subject to review, Pfizer will provide the data that support the findings of this study. Subject to certain criteria, conditions and exceptions, Pfizer may also provide access to the related individual de-identified participant data. See https://www.pfizer.com/science/clinical-trials/trial-data-and-results for more information.
References
- 1.Silverberg JI, Margolis DJ, Boguniewicz M, et al. Distribution of atopic dermatitis lesions in United States adults. J Eur Acad Dermatol Venereol. 2019;33(7):1341–1348. doi: 10.1111/jdv.15574. [DOI] [PubMed] [Google Scholar]
- 2.Ring J, Zink A, Arents BWM, et al. Atopic eczema: burden of disease and individual suffering—results from a large EU study in adults. J Eur Acad Dermatol Venereol. 2019;33(7):1331–1340. doi: 10.1111/jdv.15634. [DOI] [PubMed] [Google Scholar]
- 3.Holm EA, Esmann S, Jemec GB. Does visible atopic dermatitis affect quality of life more in women than in men? Gend Med. 2004;1(2):125–130. doi: 10.1016/S1550-8579(04)80017-2. [DOI] [PubMed] [Google Scholar]
- 4.Holm JG, Agner T, Clausen ML, Thomsen SF. Quality of life and disease severity in patients with atopic dermatitis. J Eur Acad Dermatol Venereol. 2016;30(10):1760–1767. doi: 10.1111/jdv.13689. [DOI] [PubMed] [Google Scholar]
- 5.Chernyshov PV. Stigmatization and self-perception in children with atopic dermatitis. Clin Cosmet Investig Dermatol. 2016;9:159–166. doi: 10.2147/CCID.S91263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Misery L, Seneschal J, Reguiai Z, et al. The impact of atopic dermatitis on sexual health. J Eur Acad Dermatol Venereol. 2019;33(2):428–432. doi: 10.1111/jdv.15223. [DOI] [PubMed] [Google Scholar]
- 7.Gupta MA, Pur DR, Vujcic B, Gupta AK. Suicidal behaviors in the dermatology patient. Clin Dermatol. 2017;35(3):302–311. doi: 10.1016/j.clindermatol.2017.01.006. [DOI] [PubMed] [Google Scholar]
- 8.Lio PA, Wollenberg A, Thyssen JP, et al. Impact of atopic dermatitis lesion location on quality of life in adult patients in a real-world study. J Drugs Dermatol. 2020;19(10):943–948. doi: 10.36849/JDD.2020.5422. [DOI] [PubMed] [Google Scholar]
- 9.Silverberg JI, Gelfand JM, Margolis DJ, et al. Pain is a common and burdensome symptom of atopic dermatitis in United States adults. J Allergy Clin Immunol Pract. 2019;7(8):2699–706 e7. doi: 10.1016/j.jaip.2019.05.055. [DOI] [PubMed] [Google Scholar]
- 10.Beikert FC, Langenbruch AK, Radtke MA, Kornek T, Purwins S, Augustin M. Willingness to pay and quality of life in patients with atopic dermatitis. Arch Dermatol Res. 2014;306(3):279–286. doi: 10.1007/s00403-013-1402-1. [DOI] [PubMed] [Google Scholar]
- 11.Sibbald C, Drucker AM. Patient burden of atopic dermatitis. Dermatol Clin. 2017;35(3):303–316. doi: 10.1016/j.det.2017.02.004. [DOI] [PubMed] [Google Scholar]
- 12.von Kobyletzki LB, Thomas KS, Schmitt J, et al. What factors are important to patients when assessing treatment response: an international cross-sectional survey. Acta Derm Venereol. 2017;97(1):86–90. doi: 10.2340/00015555-2480. [DOI] [PubMed] [Google Scholar]
- 13.Egeberg A, Thyssen JP. Factors associated with patient-reported importance of skin clearance among adults with psoriasis and atopic dermatitis. J Am Acad Dermatol. 2019;81(4):943–949. doi: 10.1016/j.jaad.2019.06.018. [DOI] [PubMed] [Google Scholar]
- 14.Wei W, Anderson P, Gadkari A, et al. Extent and consequences of inadequate disease control among adults with a history of moderate to severe atopic dermatitis. J Dermatol. 2018;45(2):150–157. doi: 10.1111/1346-8138.14116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Boguniewicz M, Fonacier L, Guttman-Yassky E, Ong PY, Silverberg J, Farrar JR. Atopic dermatitis yardstick: Practical recommendations for an evolving therapeutic landscape. Ann Allergy Asthma Immunol. 2018;120(1):10–22.e2. doi: 10.1016/j.anai.2017.10.039. [DOI] [PubMed] [Google Scholar]
- 16.Kaufman BP, Guttman-Yassky E, Alexis AF. Atopic dermatitis in diverse racial and ethnic groups—variations in epidemiology, genetics, clinical presentation and treatment. Exp Dermatol. 2018;27(4):340–357. doi: 10.1111/exd.13514. [DOI] [PubMed] [Google Scholar]
- 17.Maarouf M, Saberian C, Lio PA, Shi VY. Head-and-neck dermatitis: Diagnostic difficulties and management pearls. Pediatr Dermatol. 2018;35(6):748–753. doi: 10.1111/pde.13642. [DOI] [PubMed] [Google Scholar]
- 18.Jaros J, Hendricks AJ, Shi VY, Lio PA. A practical approach to recalcitrant face and neck dermatitis in atopic dermatitis. Dermatitis. 2020;31(3):169–177. doi: 10.1097/DER.0000000000000590. [DOI] [PubMed] [Google Scholar]
- 19.Jang DH, Heo SJ, Jung HJ, Park MY, Seo SJ, Ahn J. Retrospective study of dupilumab treatment for moderate to severe atopic dermatitis in Korea: efficacy and safety of dupilumab in real-world practice. J Clin Med. 2020;9(6):1982. doi: 10.3390/jcm9061982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Soria A, Du-Thanh A, Seneschal J, Jachiet M, Staumont-Sallé D, Barbarot S. Development or exacerbation of head and neck dermatitis in patients treated for atopic dermatitis with dupilumab. JAMA Dermatol. 2019;155(11):1312–1315. doi: 10.1001/jamadermatol.2019.2613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Waldman RA, DeWane ME, Sloan B, Grant-Kels JM. Characterizing dupilumab facial redness: a multi-institution retrospective medical record review. J Am Acad Dermatol. 2020;82(1):230–232. doi: 10.1016/j.jaad.2019.06.026. [DOI] [PubMed] [Google Scholar]
- 22.Zhu GA, Chen JK, Chiou A, Ko J, Honari G. Assessment of the development of new regional dermatoses in patients treated for atopic dermatitis with dupilumab. JAMA Dermatol. 2019;155(7):850–852. doi: 10.1001/jamadermatol.2019.0109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Muzumdar S, Zubkov M, Waldman R, DeWane ME, Wu R, Grant-Kels JM. Characterizing dupilumab facial redness in children and adolescents: a single-institution retrospective chart review. J Am Acad Dermatol. 2020;83(5):1520–1521. doi: 10.1016/j.jaad.2020.06.1003. [DOI] [PubMed] [Google Scholar]
- 24.Vazquez ML, Kaila N, Strohbach JW, et al. Identification of N-{cis-3-[Methyl(7H-pyrrolo[2,3-d]pyrimidin-4-yl)amino]cyclobutyl}propane-1-sulfo namide (PF-04965842): a selective JAK1 clinical candidate for the treatment of autoimmune diseases. J Med Chem. 2018;61(3):1130–1152. doi: 10.1021/acs.jmedchem.7b01598. [DOI] [PubMed] [Google Scholar]
- 25.Simpson EL, Sinclair R, Forman S, et al. Efficacy and safety of abrocitinib in adults and adolescents with moderate-to-severe atopic dermatitis (JADE MONO-1): a multicentre, double-blind, randomised, placebo-controlled, phase 3 trial. Lancet. 2020;396(10246):255–266. doi: 10.1016/S0140-6736(20)30732-7. [DOI] [PubMed] [Google Scholar]
- 26.Silverberg JI, Simpson EL, Thyssen JP, et al. Efficacy and safety of abrocitinib in patients with moderate-to-severe atopic dermatitis: a randomized clinical trial. JAMA Dermatol. 2020;156(8):863–873. doi: 10.1001/jamadermatol.2020.1406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bieber T, Simpson EL, Silverberg JI, et al. Abrocitinib versus placebo or dupilumab for atopic dermatitis. N Engl J Med. 2021;384(12):1101–1112. doi: 10.1056/NEJMoa2019380. [DOI] [PubMed] [Google Scholar]
- 28.Silverberg JI, Saeki H, Nosbaum A, et al. Efficacy of abrocitinib monotherapy by body location in patients with moderate-to-severe atopic dermatitis: pooled results from phase 2b/3 studies. In: 29th EADV congress; October 28-November 1, 2020. EADV Virtual2020.
- 29.Okubo Y, Ho KA, Fifer S, Fujita H, Oki Y, Taguchi Y. Patient and physician preferences for atopic dermatitis injection treatments in Japan. J Dermatolog Treat. 2020;31(8):821–30. [DOI] [PubMed]
- 30.Chiesa Fuxench ZC, Block JK, Boguniewicz M, et al. Atopic dermatitis in America study: a cross-sectional study examining the prevalence and disease burden of atopic dermatitis in the US adult population. J Invest Dermatol. 2019;139(3):583–590. doi: 10.1016/j.jid.2018.08.028. [DOI] [PubMed] [Google Scholar]
- 31.Simpson EL, Guttman-Yassky E, Margolis DJ, et al. Association of inadequately controlled disease and disease severity with patient-reported disease burden in adults with atopic dermatitis. JAMA Dermatol. 2018;154(8):903–912. doi: 10.1001/jamadermatol.2018.1572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.de Bruin-Weller M, Gadkari A, Auziere S, et al. The patient-reported disease burden in adults with atopic dermatitis: a cross-sectional study in Europe and Canada. J Eur Acad Dermatol Venereol. 2020;34(5):1026–1036. doi: 10.1111/jdv.16003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Silverberg JI, Gelfand JM, Margolis DJ, et al. Health utility scores of atopic dermatitis in US Adults. J Allergy Clin Immunol Pract. 2019;7(4):1246–52 e1. doi: 10.1016/j.jaip.2018.11.043. [DOI] [PubMed] [Google Scholar]
- 34.Silverberg JI, Gelfand JM, Margolis DJ, et al. Patient burden and quality of life in atopic dermatitis in US adults: a population-based cross-sectional study. Ann Allergy Asthma Immunol. 2018;121(3):340–347. doi: 10.1016/j.anai.2018.07.006. [DOI] [PubMed] [Google Scholar]
- 35.Howell MD, Kuo FI, Smith PA. Targeting the janus kinase family in autoimmune skin diseases. Front Immunol. 2019;10:2342. doi: 10.3389/fimmu.2019.02342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yasuda T, Fukada T, Nishida K, et al. Hyperactivation of JAK1 tyrosine kinase induces stepwise, progressive pruritic dermatitis. J Clin Invest. 2016;126(6):2064–2076. doi: 10.1172/JCI82887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Blauvelt A, Rosmarin D, Bieber T, et al. Improvement of atopic dermatitis with dupilumab occurs equally well across different anatomical regions: data from phase III clinical trials. Br J Dermatol. 2019;181(1):196–197. doi: 10.1111/bjd.17703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.de Wijs LEM, Nguyen NT, Kunkeler ACM, Nijsten T, Damman J, Hijnen DJ. Clinical and histopathological characterization of paradoxical head and neck erythema in patients with atopic dermatitis treated with dupilumab: a case series. Br J Dermatol. 2020;183(4):745–749. doi: 10.1111/bjd.18730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Seok SH, An JH, Shin JU, et al. Facial redness in atopic dermatitis patients treated with dupilumab: a case series. Allergy Asthma Immunol Res. 2020;12(6):1063–1065. doi: 10.4168/aair.2020.12.6.1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Price KN, Krase JM, Loh TY, Hsiao JL, Shi VY. Racial and ethnic disparities in global atopic dermatitis clinical trials. Br J Dermatol. 2020;183(2):378–380. doi: 10.1111/bjd.18938. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Upon request, and subject to review, Pfizer will provide the data that support the findings of this study. Subject to certain criteria, conditions and exceptions, Pfizer may also provide access to the related individual de-identified participant data. See https://www.pfizer.com/science/clinical-trials/trial-data-and-results for more information.