Abstract
Diabetic mellitus (DM) is a common metabolic disorder prevailing throughout the world. It may affect a child to an older person depending upon the physiology and the factors influencing the internal metabolic system of the body. Several treatments are available in the market ranges from synthetic drugs, insulin therapy, herbal drugs, and transdermal patches. Interestingly, the development of technologies and digital health have proving very helpful in improving the lifestyle of diabetic patients. All treatment approaches have their own advantages and disadvantages in the form of effectiveness and side effects. Medicinal plants have a long history of traditional application in the treatment of diabetes and even the use of plants are growing day-by-day due to the significant results against diseases and fewer side effects as compared to other treatment therapies. The intention behind writing this review is to gather all information and discussed them exhaustively in an article. The novel Coronavirus 2019 (COVID-19) pandemic has affected my lives including diabetic patients. The antidiabetic treatment strategies during this period has also discussed. In this article, we highlighted the molecular mechanism and herbal phytoconstituents that are responsible for lowering blood glucose level. The factors responsible for the progression of metabolic disorders can be controlled with the use of phytoconstituents present in herbal plants to maintain β-cells performance and restore blood glucose level. It can be concluded that medicinal plants are effective and affordable with lesser side effects for treating DM.
Keywords: Diabetes mellitus, Plants, Pancreas, Insulin, Glucose
1. Introduction
Diabetes mellitus (DM) has become a serious metabolic disorder and threatening public health all-over-the world. DM is among the top 10 deadly disorder in adults and estimated to cause four million deaths every year globally (Saeedi et al., 2019). According to the results of the International Diabetes Federation 2019, the global diabetes prevalence in 2019 was 463 million people. It is expected to rise up to 578 million by 2030 and 700 million by 2045 (Saeedi et al., 2019). DM is a condition of carbohydrate and lipid metabolism imbalance that increases blood glucose levels. Mechanistically, the body cannot regulate the amount of glucose in the blood during DM. It leads to hyperglycemia due to rise in carbohydrate contents (Peixoto Araujo et al., 2021). The two main types of diabetes are type 1 diabetes mellitus (T1DM), and type 2 diabetes mellitus (T2DM). T1DM is insulin-dependent DM generally occurs in children and youngsters; in this condition pancreas does not produce sufficient insulin. On the other hand, T2DM is non-insulin-dependent DM that occurs when the body cannot effectively use the amiable insulin. T2DM has reached to epidemic proportions due to sedentary lifestyle and over-nutrition. It is very common in adults. It is considered as a complex disease that requires continuous medical care and strategies to reduce blood glucose level. Several biochemical and chemical agents are known that helps in controlling diabetes but no cure is yet available to get complete recover from the disorder (Verma et al., 2018). Education and support are very critical in controlling acute phases of diabetes and reducing risk of long-term complications (ADA, 2021). The treatment of diabetes has been improving day-by-day due to new research and technology that improving the well-being and health of diabetic patients. The field of diabetes care has been changing rapidly due to new research, technology, and treatments. These can improve the health and well-being of people with diabetes. The conventional treatments are no doubt effective but associated with certain complications or side effects. The complications are thus promoting researchers to develop some safe, affordable and efficient treatments against diabetes.
Interestingly, traditional herbal medicines have been found effective against diabetes (Kumar et al., 2017; Rathore et al., 2014). The phytoconstituents from extract have always been a rich source for controlling disorder and other complications arises due to it (Kumar et al., 2016). Medicinal plants have been used in the treatment of diabetes since ancient times (Singh et al., 2016). The World Health Organization (WHO) has found that 21,000 medicinal plants have been used for the treatment of different diseases all over the world, in which 2500 species of plants is found in India. The selection of herbs against diabetes depends on various factors such as stage of diabetes progression, availability, types of co-morbidities in patients, toxicities and safety profile of the herbs (Jain et al., 2014, 2015, 2016; Shrawan et al., 2015, 2016). The objective of the present review was to describe the utility of medicinal plants in the treatment of diabetes mechanistically. Thus, this article may help researchers in conducting future studies for the development of antidiabetic drugs and other therapies in the management of diabetes based on phytoconstituents and extracts from plants.
2. Factors causing diabetes
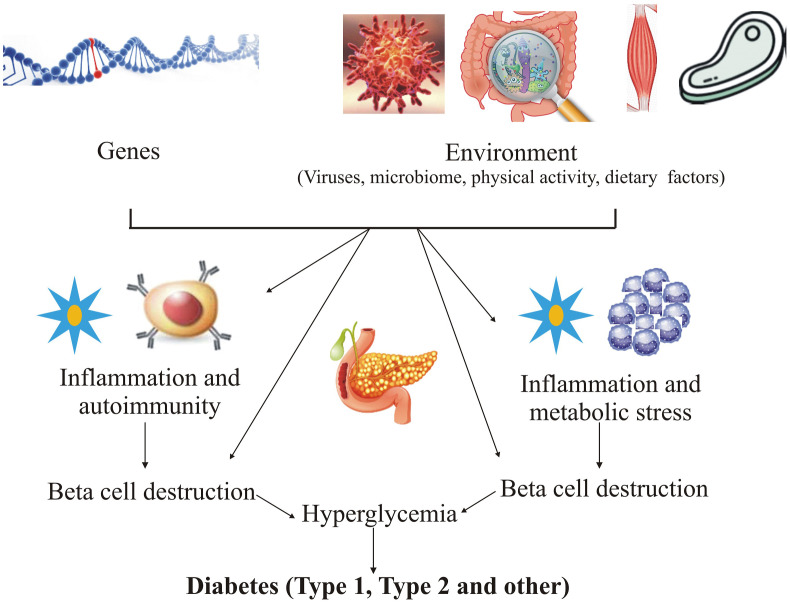
Diabetes is more prevailing in developing or low-income countries as compared to high-income nations, it is due to lifestyle factors and urbanization (IDF, 2017). The modifiable risk factors are obesity, hyperlipidemia, consumption of alcohol and tobacco (Barik et al., 2016). The vulnerable factors responsible for development of diabetes are biological risk factors including sex, age, marriage status, race, stress, and family history of diabetes; financial factors; severity of disease including poor disease control, complications, and co-morbidities; lack of education or low literacy; distrust of primary health services; occupational restriction; limited daily life behaviors; lifestyle factors including consumption of unhealthy food, lack of exercises and low-quality sleep; and mental condition including lack of friends and family and community environment (Chen et al., 2019; Bosun-Arije et al., 2019). Fig. 1 showing several genetic and environmental factors causing loss of β-cell mass and that leads to hyperglycemia. In this state, insulin do not respond to its demand in the body that causes increase in the level of blood glucose sufficient to diagnose diabetes (Skyler et al., 2017).
Fig. 1.
Genetic and environmental factors causing hyperglycemia.
3. Pharmacologic therapy for treating diabetes
3.1. Treatment for type 1 diabetes mellitus (T1DM)
T1DM is a condition of serious decrease in the level on endogenous insulin and its release from β-cells of pancreas (Oram et al., 2019). The precise pathological mechanism and etiology are still not clear. It usually develops in child or young ones. People with type 1 diabetes are treated with multiple injection of prandial and basal insulin. The rapid acting insulin analogues are also used for reducing hypoglycemic risk. The rapid-acting prandial analogues i.e. lispro, aspart and glulisine are preferred over regular human insulin. However, aspart as faster-acting insulin is a new option due to advantage of better postprandial glucose coverage (Janež et al., 2020). The diabetic patients are being aware about prandial insulin dose to pre-meal blood glucose, carbohydrate intake and anticipated physical activity. The surgical treatment of type 1 diabetes such as islet and pancreas transplantation can also normalize the glucose levels and mitigate the complication of the disease (Meloche et al., 2007). However, patients availing this treatment need to take life long immunosuppressant drugs to prevent graft rejection of islet and pancreas.
3.2. Treatment for type 2 diabetes mellitus (T2DM)
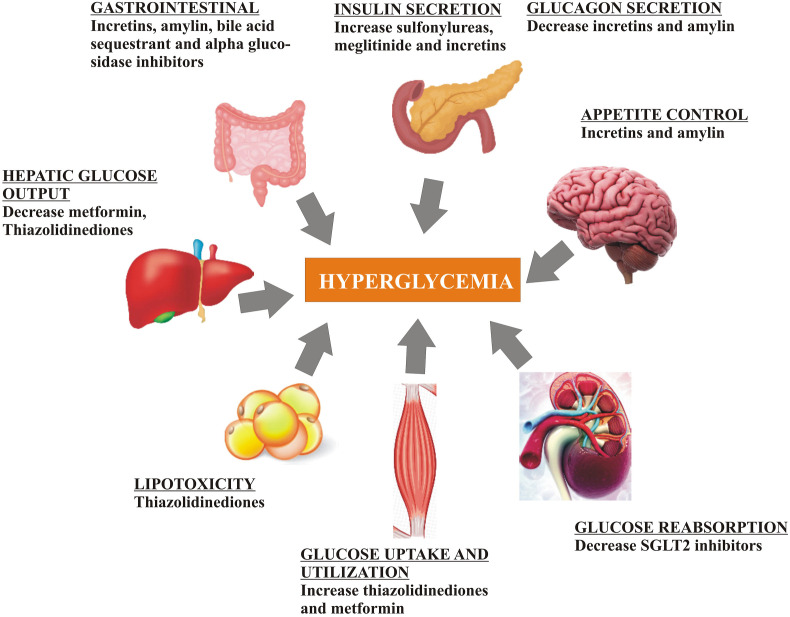
T2DM is chronic metabolic disorder that develops due to defective insulin secretion and action. The primary cause of fasting hyperglycemia is the excessive production of glucose in the presence of hyperinsulinemia (ADA, 2013). However, after meals the impaired suppression of glucose production by insulin leading to decrease in the uptake of glucose by muscles and tissues. Metformin being the most preferred oral antidiabetic drug for the treatment of type 2 diabetes. It should be continued for life-long and not contraindicated with insulin. The second generation sulfonylureas include glyburide, glipizide and glimepiride; meglitinides include repaglinide, and nateglinide; thiazolidinediones include pioglitazone and rosiglitazone; alpha-glucosidase inhibitors include acarbose and miglitol; depeptidyl peptidase-4 inhibitors include sitagliptin, saxagliptin, linagliptin and alogliptin; colesevelam as bile acid sequestrant; bromocriptin as dopamine-2 agonist; pramlintide as amylin mimetic; sodium glucose cotransporter - 2 inhibitors include canagliflozin, dapagliflozin, and empaglifozin; and glucagon-like peptide-1 receptor agonists include exenatide, liraglutide albiglutide and dulaglutide (Chamberlain et al., 2017). Table 1 showing the list of antidiabetic drugs with their mechanism and side effects. The injection of insulin is also considered during evidence of ongoing weight loss, symptoms of hyperglycemia (≧ 300 mg/dL). Fig. 2 showing pharmacological treatment for diabetes.
Table 1.
List of existing anti-diabetic drugs with their mechanisms and side effects.
| S. No. | Class | Mechanism of action | Side effects | Complication/Toxicities |
|---|---|---|---|---|
| 1 | Biguanide (Metformin) | Increases the effect of insulin | Reduced vitamin B12 absorption, weight loss, lactic acidosis, diar rhea, abdominal cramps | Liver failure, chronic kidney disease |
| 2 | Sulfonylureas (glyburid e, glimepiride) | Enhances insulin secr etion from pancreatic beta-cel ls | Hemolysis, agranulo cytosis, weight gain | Obesity, cardiovascular comorbidities, allergy |
| 3 | Meglitinides (nateglinide, rep aglinide) | Increase insulin secr etion from pancreatic beta-cel ls | Weight gain, risk of hypoglycemia | Liver and renal failure |
| 4 | DPP-4 inhibitors (saxagliptin, sitagli ptin) | Inhibit glucagon-like peptide-1 degra dation and promotes glucose-de pendent insulin secretion | Pancreatitis, headache, gastrointestinal complaints, arthralgia, dizziness | Liver and renal failure |
| 5 | GLP-1 agonists (exenatide, liraglutide, albiglutide) | Direct stimulation of glucagon-like peptide-1 receptor | Pancreatitis, pancreati c cancer, nausea | Gastrointestinal motility disorders |
| 6 | SGLT-2 inhibitors (canagliflozin, dapagliflozin, empagliflozin) | Increased glucosuria by inhibiting SGLT-2 in the kidney | Urinary tract infections, polyuria, dehydration, yeast infections, diabetic ketoacidosis | Urinary tract infections, chronic kidney disease |
| 7 | Alpha-glucosidase inhibitors (acarbose) | Reduce intestinal glucose absorption | Flatulence, diarrhea | Renal failure, inflammatory bowel disease |
| 8 | Thiazolidinediones (pioglitazone) | Reduce insulin resistance through the stimulation of PPARs | Cardiac failure, weight gain, osteoporosis, edema | Liver disease, congestive heart failure |
| 9 | Amylin analogues (pramlintide) | Reduce glucagon release | Risk of hypoglycemia, nausea | Gastroparesis |
DPP-4, Dipeptidyl peptidase-4; SGLT-2, Sodium-glucose co-transporter-2; GLP-1, Glucagon-like peptide-1; PPARs, Peroxisome proliferator-activated receptors.
Fig. 2.
Pharmacological treatment approaches for T1DM
SGLT-2, Sodium-glucose co-transporter-2.
4. Medicinal plants used against diabetes traditionally
Medicinal plants have been used in the treatment of diseases since ancient times (McGaw et al., 2019). The importance of plants as raw material for pharmaceutical industries cannot be overlooked. The use of medical plants is increasing worldwide due to its better long-term results with fewer side effects. They have been used to maintain and augment mental, spiritual and physical health along with treating diseased condition and ailments. Plants possess several active constituents present in their secondary metabolites such as alkaloids, phenol, glycosides, tannins, flavonoids, and terpenoids that are responsible for varieties of biological effects (Akinyemi et al., 2018). Thus, medicinal plants have an immense role to play in sustainable human health. Table 2 showing the list of medicinal plants having reported antidiabetic property.
Table 2.
List of medicinal plants with reported antidiabetic activity.
| S.No. | Plant | Family | Chemical constituents | Antidiabetic action | References |
|---|---|---|---|---|---|
| 1 | Abies pindrow | Pinaceae | Globulol, maltol, borneol, linalool | Stimulation of insulin secretion | Sinha et al., 2019 |
| 2 | Acacia arabica | Fabaceae | Mallic acid, chlorogenic acid, catechin, epicatechin, chiorogenic acid, ellagic acid, corosolic acid, | Increasing the release of insulin |
Hegazy et al. (2013), Bharti et al. (2018) |
| 3 | Achyranthes rubrofusca | Amaranthaceae | Betulinic acid, momordin Ib, zingibroside R1, Achyranthoside IV | Reduction of blood glucose level and restore pancreatic enzymatic activity | Geetha et al. (2011); He et al. (2017) |
| 4 | Agrimony eupatoria | Rosaceae | 4-caffeoylquinic acid, 3,5-dicaffeoyl quinic acid and luteolin-7-O-glucoside | Insulin-like and insulin releasing activity | Kuczmannová et al. (2016) |
| 5 | Albizzia lebbeck | Fabaceae | Albizziahexoside, lupeol, docosanoic acid, oleanolic acid, beta- sitosterol | α-amylase and α-glucosidase inhibitor activity | Verma et al. (2013); Ahmed et al. (2014) |
| 6 | Aloe vera | Asphodelaceae | lophenol, and cycloartanol | Inhibition of α-amylase and α-glucosidase enzymes | Tanaka et al. (2006); Muñiz-Ramirez et al. (2020) |
| 7 | Amaranthus tricolor | Amaranthaceae | Betacyanins, betaxanthins, rutin, isoquercetin, ferulic,ellagic, p-coumaric | Anti-α-amylase and anti-α-glucosidase activity | Yang et al. (2020); Peter and Gandhi (2017) |
| 8 | Anacardium occidentale | Anacardiaceae | Anacardic acid, cardanol, cardol | α-glucosidase inhibitor | Jaiswal et al. (2016); Palheta and Ferreira (2018) |
| 9 | Annona squamosa | Annonaceae | Acetogenin, annonacin, annonastatin samaquasine, squamone, acetogenin | Increase level of plasma insulin | Shirwaikar et al. (2004); Zahid et al. (2018) |
| 10 | Averrhoa bilimbi | Oxalidaceae | Cyanidin-3-o-h-glucoside, citric acids, amino acids | Increase level of plasma insulin | Kurup and S, 2017; Mathew et al. (2017) |
| 11 | Azadirachta indica | Meliaceae | Nimbidin | Normalizing altered insulin signaling molecules | Satyanarayana et al. (2015) |
| 12 | Barleria prionitis | Acanthaceae | Balarenone, pipataline, prioniside B, lupeol | Decrease blood glucose and increase serum insulin level | Dheer and Bhatnagar (2010); Shukla and Gunjegaonkar (2018); Banerjee et al. (2012) |
| 13 | Bauhinia thoningii | Fabaceae | Alepterolic acid, methyl-ent-3β-hydroxylabd-8(17)-en-15-oate, 2β-methoxyclovan-9α-ol (1) | Insulin mimetic activity | Salehi et al. (2019) |
| 14 | Bixa orellana | Bixaceae | Cryptoxanthin, phytoene, lutein, zeaxanthin | Promote binding of insulin on receptor and increase plasm insulin level | Vilar et al. (2014) |
| 15 | Boerhaavia diffusa | Nyctaginaceae | β-Sitosterol, arachidic acid, Ecdysone, urosilic acid, palmitic acid, hexacosonoic, β-triacontanol, hentriacontane |
Increase level of plasma insulin | Alam et al. (2018) |
| 16 | Caesalpinia ferrea | Fabaceae | Galactomannan, kaempferol, galloylorientin, heptacosan | α-Amylase enzyme inhibitory action |
Hassan et al. (2015); Macêdo et al. (2020) |
| 17 | Camellia sinensis | Theaceae | Catechins, theaflavins, and caffeine | α-Amylase and α-Glucosidase inhibitory activity | Fu et al. (2017); Ardiana et al. (2017) |
| 18 | Capsicum frutescens | Solanaceae | Octadecadienal (Z), tetracosane, 3-carene, 5-eicosene, docosane | Stimulation of insulin secretion | Dougnon and Gbeassor (2016); Gurnani et al. (2016) |
| 19 | Casearia esculenta | Flacourtiaceae | 3-hydroxymethyl xylitol | Insulin mimetic action | Govindasamy et al. (2011); Prakasam et al. (2005) |
| 20 | Cassia fistula | Fabaceae | β-sitosterol, lupeol, kaempferol, fistulin, rhein, leucopelargonidin | Increase insulin-stimulated glucose uptake | Einstein et al. (2013); Rahmani et al., 2015 |
| 21 | Cassia grandis | Fabaceae | Linalool | α-glycosidase inhibitory activity | Lodha et al. (2010); Prada et al. (2018) |
| 22 | Catharanthus roseus | Apocynaceae | Limonene, dotriacontane, geraniol, citral, phytol | Increased secretion of insulin from β-cells pancreas | Rasineni et al. (2010); Lawal et al. (2015) |
| 23 | Cecropia pachystachya | Urticaceae | C-glycosyl flavonoids, proanthocyanidins | Increase secretion of insulin | Rivera-Mondragón et al. (2017); Costa, 2011 |
| 24 | Ceriops decandra | Rhizophoraceae | Ceriopsin, lupeol, oleanolic acid, α-amyrin, ursolic acid, and catechin | Regeneration of β cells | Mahmud et al. (2018); Nabeel et al., 2010; Arora et al. (2014) |
| 25 | Chiliadenus iphionoides | Asteraceae | 1,8-cineole, camphor, α-terpineol, terpin-4-ol, and bornyl formate | Increase secretion of insulin | Salehi et al. (2019); Abdelhalim et al., 2020 |
| 26 | Cinnamomum cassia | Lauraceae | Cinnamaldehyde | Insulin mimetic action | Elumalai et al. (2011); Yan et al. (2015) |
| 27 | Citrullus colocynthis | Cucurbitaceae | Cucurbitacin L, isovitexin, khekadaengoside E |
Restoration of pancreatic β-cells | Zheng et al. (2020); Dhakad et al. (2017); Shi et al. (2014) |
| 28 | Clausena anisata | Rutaceae | β-pinene, germacrene-D, sabinene, linalool, estragole | Stimulation of insulin secretion | Yakoob et al. (2016); Govindarajan, 2010 |
| 30 | Coscinium fenestratum | Menispermaceae | Hentriacontan, palmitic acid, β-sitosterol, oleic acid | Decrease gluconeogenesis and increase enzymatic activity | Nayak et al. (2012); Malarvili et al. (2011) |
| 31 | Eucalyptus citriodora | Myrtaceae | Citronellol acetate, cis-geraniol, dihydrocarveol acetate, β-bisabolene, 3-hexen-1-ol, and pregn-5-en-20-one,3,17-dihydroxy-3-acetate | Increase glucose transporter 4 (GLUT-4) translocation | Wang et al. (2014); Dey and Mitra (2013) |
| 32 | Eucalyptus globulus | Myrtaceae | 1,8-cineol, α-pinene | Enhance release of insulin from clonal pancreatic beta line | Chakraborty et al. (2018); Sebei et al. (2015) |
| 33 | Ficus religiosa | Moraceae | n-octacosanol, β-sitosteryl-D-glucoside, stigmasterol, lanosterol, methyl oleanolate, lupen-3-one |
Stimulation of insulin secretion | Pandit et al. (2010); Chandrasekar et al. (2010) |
| 34 | Gymnema sylvestre | Apocynaceae | Gymnemic acid, gurmarin, tartaric acid, glucose, calcium oxalate, betaine, stigmasterol, and choline | Prevents absorption of glucose by the intestine to reduce blood sugar level | Kanetkar et al. (2007); Khan et al. (2019); Tiwari et al. (2014) |
| 35 | Heinsia crinata | Rubiaceae | Sapogenin, neochlorogenin and diosgenin | Insulin level elevating effect | Okokon et al. (2009); Yobe et al. (2017) |
| 36 | Helicteres isora | Sterculiaceae | Kaempferol 7-O-coumaroylhexoside,rosmarinic acid and kaempferol 7-O-rhamnosylhexosides | Increase uptake of glucose | Olivas-Quintero et al. (2017) |
| 37 | Hibiscus rosa | Malvaceae | Beta-carotene, anthocyanin, Beta-sitosterol, arabinogalactans, gossypetin, l-ascorbic acid, citric acid | Stimulation of insulin secretion from beta pancreatic cells | Moqbel et al. (2011) |
| 38 | Ipomoea batata | Convolvulaceae | Caffeic acid, chlorogenic acid, rutin, quercetin | Decrease insulin resistance | Akhtar et al. (2018); Zengin et al. (2017) |
| 39 | Juniperus communis | Pinaceae | Longifolene, totarol, transcommunic acid | Stimulation of insulin secretion and increase glucose consumption | Raina et al. (2019) |
| 40 | Momordica charantia | Cucurbitaceae | Charantin, cucurbitacins, karounidiols, multiflorenol and nerolidol | Regulate glucose absorption by the gut and stimulate its uptake into muscles | Joseph and Jini (2013); Singh et al. (2011) |
| 41 | Moringa oleifera | Moringaceae | Oleic acid, ascorbic acid, 9-octadecenoic acid and 9- octadecenamide | α-glucosidase and pancreatic lipase inhibitory activity | Chen et al. (2020); Aja et al. (2014) |
| 42 | Murraya koenigii | Rutaceae | Linalool, geranyl acetate, elemol, allo-ocimene, myrcene, α-terpinene and neryl acetate | Increased the secretion of insulin and glycogenesis process | Rajendran et al. (2014); Sk et al. (2017) |
| 43 | Olea europia | Oleaceae | Oleuropeoside | Increase uptake of glucose and release of insulin | Paramanick and Sharma (2017) |
| 44 | Opuntia ficus-indica | Cactaceae | Phytol, palmitate palmitic acid, and vitamin E | Increases glucose uptake through activation of AMPK/p38 MAPK pathway | Halmi et al. (2012); Luo et al. (2010); Leem et al. (2016) |
| 45 | Origanum vulgare | Lamiaceae | Amburoside A, apigenin 7-O-glucuronide, luteolin 7-O-glucuronide, lithospermic acid, rosmarinic acid, and demethylbenzolignanoid | α-glucosidase inhibitory activity | Yu et al. (2021); |
| 46 | Passiflora nitida | Passifloraceae | Luteolin, apigenin, kaempferol and quercetin | α-glucosidase inhibitory activity | Casierra-Posada and Jarma-Orozco (2016) |
| 47 | Paspalum scrobiculatum | Poaceae | Vanillic acid, syringic acid, cis- ferulic acid, p-hydroxy benzoic acid and melilotic acid | Increase glycogen synthesis and decrease in glycated haemoglobin levels | Kiran et al. (2014); Jain et al. (2009) |
| 48 | Persea americana | Lauraceae | Peptone, glycosylated abscisic acid, cellulose, b-galactoside, polyuronoids, and polygalacto urease | Inhibition of insulinase activity | Yasir et al. (2010); Ezejiofor et al. (2013) |
| 49 | Phoenix dactylifera | Arecaceae | β-carotene, ascorbic acid, α-tocopherols, selenium | Glucose lowering effect | Abdelaziz et al. (2015) |
| 50 | Phyllanthus niruri | Euphorbiaceae | Phyllanthin, coumarins, chlorogenic acids, and anthocyanins | Inhibition of glucose absorption and enhancement of glucose storage | Sibiya et al. (2020); |
| 51 | Phyllanthus simplex | Euphorbiaceae | Corilagin, gallic acid, phyllanthin, geraniin and niranthin | Glucose lowering effect | Mao et al. (2016) |
| 52 | Picralima nitida | Magnoliopsida | Akuammine, akuammidine, akuammicine, pseudo-akuammigine | Regeneration of β cells | Teugwa et al. (2013); |
| 53 | Piper longum | Piperaceae | Piplartine and piperine | Glucose lowering effect | Singh and Navneet, 2018; Nabi et al. (2013) |
| 54 | Scoparia dulcis | Scrophulariaceae | scoparic acid D | Secretagogue activity of insulin | Latha et al. (2009) |
| 55 | Sonchus oleraceus | Asteraceae | Apigenin, luteolin, 1-cerotol, germanicyl acetate, and oleanolic acid | α-amylase and α-glucosidase inhibitory activity | Xu and Liang (2005); Li and Yang (2018) |
| 56 | Swertia chirayata | Gentianaceae | Amarogentin, gentianine, ursolic acid, isobellidifolin, sweroside, magniferin | Stimulation of insulin secretion from pancreatic islets | Dey et al. (2020) |
| 57 | Syzygium jambolana | Myrtaceae | Glucoside, anthocyanins, isoquercetin, kaemferol, ellagic acid and myrecetin | Stimulates insulin secretion | Ayyanar and Subash-Babu (2012) |
| 58 | Tamarindus indica | Fabaceae | Citric acid, tartaric acid, malic acid, acetic acid, formic acid, and succinic acid | α-amylase and α-glucosidase inhibitory activity | Bhadoriya et al. (2011); Krishna et al. (2020) |
| 59 | Terminalia chebula | Combretaceae | Chebulic acid, neo-chebulic acid mannitol, chebulagic acid, corilagin | Insulin mimetic action | Senthilkumar et al. (2006); Chang and Lin (2012) |
| 60 | Terminalia catappa | Combretaceae | Asiatic acid, vitexin, ursolic acid, isovitexin, gallic acid, tergallagin, tercatain, punicalagin, chebulagic acid, punicalin, terflavins A and B, and geranin | α-glucosidase and α-amylase inhibitory activity | Mininel et al. (2014); Behl and Kotwani (2017) |
| 61 | Tinospora crispa | Menispermaceae | apeginin, diosmetin, genkwanin, cycloeucalenol, cycloeucalenone | Stimulation of insulin secretion from pancreatic islets | Klangjareonchai et al. (2012) |
| 62 | Trigonella foenum-graecum | Fabaceae | Diosgenin, trigonelline, gentianine, carpaine, butanoic acid, and isovaleric acid | Restoration of pancreatic β-cells | Wani and Kumar, 2018; Geberemeskel et al. (2019) |
| 63 | Urtifca dioica | Urticaceae | Histamine, acetylcholine, 5-hydroxytryptamine | Stimulation of insulin secretion | El Haouari et al. (2019); Joshi et al. (2019) |
| 64 | Vaccinium arctostaphylos | Ericaceae | Linalool, α-Pinene, Safranal and Sandaracopimaradiene | Insulin level elevating effect | Salehi et al. (2019); Teimouri et al. (2015); Kianbakht and Hajiaghaee (2013) |
| 65 | Vernonia amygdalina | Asteraceae | Vernomygdin, Vernoniosides Vernodalol Vernodalin, Epivernodalol | Suppression of gluconeogenesis | Atangwho et al. (2009); Atangwho et al. (2014) |
| 66 | Vinca rosea | Apocynaceae | Vincristine, vinblastine | Regeneration and rejuvenation of beta cells | Ahmed et al. (2010) |
| 67 | Zaleya decandra | Aizoaceae | 6-octadecenoic acid, n-hexadecanoic acid | Stimulation of insulin secretion | Meenakshi et al. (2010) |
| 68 | Zingiber officinale | Zingiberaceae | Gingerols, paradols, shogaols, gingerdiones, zingerones, gingerdiols | Stimulation of insulin secretion and decrease glucose level | Obih et al. (2017) |
| 69 | Zizyphus mauritiana | Rhamnaceae | Palmitic acid, ethyl stearate and α-linolenic acid | Restoration of blood glucose level | Ashraf et al. (2015); Niamat et al. (2012) |
GLUT-4, Glucose transporter-4; AMPK, AMP-activated protein kinase; MAPK, Mitogen-activated protein kinase.
4.1. Acacia arabica
Acacia arabica has been used traditionally in the treatment of diabetes, kidney and liver problems, healing wounds, and preventing of microbial attack. The mechanism of its antidiabetic action is due to presence of polyphenols in its bark. Its gives hypoglycemic effect by improving insulin sensitivity and reduction of fatty acid synthesis. Fisetinidol and robinetinidol are the two phytoconstituents responsible for antidiabetic action (Ikarashi et al., 2011).
4.2. Bambusa arundinasia
Bambusa arundinasia has been used traditionally for the treatment of diabetes, dysentery, peptic ulcer and aphthous. Mechanistically, it shows, ⍺-glucosidase and ⍺-amylase enzyme inhibitor action. Additionally, showing regeneration of pancreatic tissue and improvement in serum insulin level. It has also shown reduction of HbA1c (glycated hemoglobin), fructose-1-6-biphophatase, glucose-6-phosphatase, triglycerides and total cholesterol. Stigmasterol and β-sitosterol are the two major compounds responsible for antidiabetic activity (Nazreen et al., 2011).
4.3. Boswellia carterii
Boswellia carterii is helpful in diabetes due to presence of oleo-gum resin. It shows antidiabetic activity by increasing liver glycogen, serum insulin and prevents degenerative change in β-cells of pancreas. Boswellia carterii shows hypoglycemic activity due to suppression of proinflammatory cytokine, apoptosis of cells, and lymphocytes penetration into pancreatic islets. The boswellic acid is the major activity phytoconstituent that showing inhibitor activity on diabetic complication following polyol aldose reductase pathway (Rao et al., 2013).
4.4. Chrysanthemum indicum
Chrysanthemum indicum has been used since a long term ago in the treatment of hypertension, inflammation and respiratory disorders in various countries. The phytoconstituents present in plant are luteolin and linarin that are responsible for anti-inflammatory effect on prostatitis and chronic pelvic inflammation. It is due to reduced activation of tissue necrosis factor - alpha (TNF-α) and interleukin-1beta (IL-1β) (Dasgupta, 2019). Chrysanthemum morifolium Ramat. a species of Chrysanthemum used for antidiabetic activity (Yamamoto et al., 2015). It is a leading flowering plant with applied value worldwide (Su et al., 2019).
4.5. Coriandrum sativum
Coriandrum sativum is mainly used for its carminative property. However, its fruits have shown antidiabetic effect via enhancement of insulin release from β-cells along with increasing glucose uptake, glycogenesis and glucose oxidation. C. sativum improves cardio-protective indices and atherosclerotic index (Parsaeyan, 2012).
4.6. Glycyrrhiza glabra
The antidiabetic activity of G. glabra is due to presence of glycyrrhizic acid present in roots of the plant. It increases expression of lipoprotein lipase in body tissues and improves sensitivity of insulin. It reduces total cholesterol, fatty acids in serum, and lipid deposition (Eu et al., 2010).
4.7. Lactuca sativa
The seeds of L. sativa has been used for its antidiabetic property by Persians since ancient times. It possesses alpha-glucosidase and alpha-amylase enzyme inhibitory activity. The mechanism of glucose lowering activity is based on elevation of serum insulin level, reduction of fructose-1-6- biphophatase and glucose-6-phosphatase (Ahmed et al., 2013).
4.8. Myrtus communis
The leaves of M. communis possesses antidiabetic action by enhancing antioxidant function in liver of animals. The alpha-glucosidase enzyme inhibitory activity is the mechanism of its antidiabetic activity (Sepici-Dincel et al., 2007).
4.9. Portulaca oleracea
The seeds of P. oleracea shows hypoglycemic activity by reducing fating and post-prandial blood glucose level. The mechanism of its antidiabetic activity is based on inhibition of alpha-glucosidase and alpha-amylase enzymes (Heidarzadeh et al., 2013).
4.10. Punica granatum
The flowers of P. granatum has been used for antidiabetic action, it is due to its alpha-glucosidase enzyme inhibitory activity and improvement of insulin sensitivity. It also enhances GLUT4 (Glucose Transporter 4) and PPAR- (Peroxisome Proliferator-Activated Receptor–) (Huang et al., 2005).
4.11. Rosa damascena
The fruits and flowers of R. damascena possess glucose lowering and alpha-glucosidase enzyme inhibitory activity.
4.12. Vitis vinifera
V. vinifera shows antidiabetic activity due to presence of procyanidins in seeds. It also shows insulinomimetic activity via stimulation of insulin pathway mediator and upgrading glucose uptake. The pharmacological mechanism of plant against chronic diabetes include suppression of oxidative stress, reduction in advanced end glycation products (Li et al., 2008).
5. Highly valuable drug therapies and medicinal plants in 2020–21 for treating diabetes
The coronavirus disease 2019 (COVID-19) has imposed a global economy loss at various levels. During coronavirus disease 2019 (COVID-19) pandemic the diabetic patients were at increased risk of complication like Adult Respiratory Distress Syndrome and multi-organ failure. The following drugs were found more effective and having metabolically interfering effects against COVID-19 patients with diabetes: Metformin; Sodium-glucose-co-transporter-2 inhibitor including dapagliflozin, canagliflozin, and empagliflozin; Glucagon-like peptide-1 receptor agonists including dulaglutide, albiglutide, liraglutide, semaglutide, and lixisenatide; Dipeptidyl peptidase-4-inhibitors including linagliptin, alogliptin, sitagliptin, and saxagliptin; and Insulin (Bornstein et al., 2020). Vaccination should be prioritized in these patients. However, routine vaccination against Hepatitis B, influenza and pneumococcal pneumonia is recommended in diabetic patients (Pal et al., 2021). The current trends prevailing in the market under numerous segments as alternatives to injectable diabetes care. Based on product types, it include insulin pumps, smart glucose meter, tethered insulin pumps, implantable insulin pumps, insulin patches and artificial pancreas. Based on device types include wearable and hand held.
Several plants have been used for treating diabetes and counting is continue to get most beneficial agents with lesser side effects. Interestingly, few plants are very common in different countries that are frequently used in diabetes due to their effectiveness, economic profile and easy availability. The passed year was very difficult due to COVID pandemic throughout the world; peoples stayed at their homes and protecting themselves. However, medicines were accessible to patients even though the diabetic patients have been consuming herbs to remain healthy and maintain normal blood glucose levels. Famous medicinal plants used for antidiabetic purposes in the year 2020–21 are as follows:
5.1. Aloe vera
Aloe vera L. has been used traditionally in the treatment of various diseases in many countries since a long term ago. The major chemical constituents present in the plant are anthraquinones, phytosterols, lignins, salicylic acid and polysaccharides. It is very efficient in reducing blood glucose level of diabetic patients by improving the body response towards insulin. A. vera is also effective in reducing lipid level in the body due to presence of phytosterols such as lupeol, campesterol and sitosterol that are structurally similar to cholesterol and reduce its absorption by competition with it (Joseph et al., 2018; Choudhary et al., 2014).
5.2. Cinnamon
Cinnamomum cassia is commonly known as cinnamon. The bark of cinnamon is sweet in taste and most widely used as flavoring agents in the food and beverages. It is well recognized due to its medicinal properties and traditionally used in menstrual irregularities, diarrhea and arthritis. Cinnamon is a well known plant, its bark is used for antidiabetic action. Around 250 species of Cinnamon has reported in which C. cassia and C. zeylanicum are popular ones (Medagama, 2015).
5.3. Ginger
Ginger (Zingiber officinale) is a safe and non-toxic spice with lesser side effects as considered by the food and drug administration (Huang et al., 2019). Its origin is from Southeast Asia and has a long traditional use due to its medicinal properties. Ginger is used as an antidiabetic herb due to its action on inhibiting the enzymes α-glucosidase and α-amylase. It is due to the presence of shagol and gingerol as phytoconstituents (Lindstedt, 2018).
5.4. Garlic
Garlic (Allium sativum) belongs to family Liliaceae. A. sativum is used exhaustively due to its medicinal and nutritional properties. It is native to central Asia and being used for culinary purposes due to its health benefits since ancient times. The phytoconstituents present in plant parts are Diallyl disulfide, Allicin, S-allyl cysteine, Allyl mercaptan and Alliin. A sativum is effective in reducing blood glucose level and insulin resistance in the body. The other well established uses of garlic include anticoagulant, antiatherosclerotic, anticancer, antimicrobial, immunomodulatory, antidote and hypolipidemic activities (Shabani et al., 2019).
5.5. Bitter gourd
Bitter gourd is Momordica charantia Linn. belongs to family Cucurbitaceae. It is also known as karela and balsam pear. The fruits of M. charantia has been used for several decades to treat diabetes to the indigenous people of Asia, East Africa and South America. The hypoglycemic mechanism of M. charantia is due to presence of phytoconstituents that possess AMP-activated protein kinase activity. The phytoconstituents present in the plant are charantin, cucurbitins, karounidiols, momordicinin, stigmasterol, zeaxanthin and pipecolic acid (Joseph and Jini, 2013; Mahmoud et al., 2017).
5.6. Sweet potato
Sweet potato (Ipomoea batatas) is used as an edible product in various countries including India, Tanzania, Japan, Peru and New Zealand. The shoots and leaves have been employed traditionally as medicine in the treatment of diabetes. It belongs to the family Convolvulaceae. The roots of I. batatas are rich in mineral salts and vitamin C. It is used to alleviate vitamin A deficiency in Eastern India and East Africa due to high quantity of beta-carotene in roots (Gunn et al., 2013; Kusano and Abe, 2000).
5.7. Black seed
Black seed (Nigella sativa) or black cumin is dicotyledon plant belongs to family Ranunculaceae. It has been used as an important culinary herb containing medicinal properties. The phytoconstituents present in the seeds are p-cymene, nigellone, flavonoids, carvone and thymoquinone in which thymoquinone is considered the chief active compound. The seeds are used as good antidiabetic agents that can lower increased glucose level of the body and also restore lipid profile. It is also used for neuroprotective, nephroprotective, anticonvulsant, anticancer and antimutagenic properties (Heshmati and Namazi, 2015; Bamosa et al., 2010; Gunn et al., 2013).
5.8. Chirayita
Chirayita (Swertia chirayita) is a popular indigenous herb of Himalayas belongs to family Gentianaceae. It has been traditionally used in the treatment of several ailment including diabetes, malaria and liver disorders. It is a rich sources of flavonoids, xanthones, terpenoids, irridoid and alkaloids. S. chirayita is known for its bitter taste, it is due to the presence of phytoconstituents such as amarogentin, swertiamarin, and swerchirin (Alam et al., 2011; Kumar and Van Staden, 2016; Dey et al., 2020).
6. Advancement in the treatment of diabetes
-
•
An artificial pancreatic system is a big addition in the treatment of diabetes. It is critical to quantify blood glucose level non-enzymatically, accurately and in stable manner (Wang et al., 2019).
-
•
Teplizumab, an immune stimulant drug is under phase 3 trial that delayed type 1 diabetes. Anti-thymocyte globulin (ATG) is good alternative for people with newly diagnosed type 1 diabetes. It can preserve the function of beta cells and decreases blood sugar levels.
-
•
A new protein, Hybrid Insulin Peptides (HIPs) has found on beta-cells of pancreas with type 1 diabetes and this are recognized as foreign body by the immune cells of patients (Baker et al., 2019). Thus, HIPs can be an important target for type 1 diabetic patients.
-
•
Bisphenol A (BPA), a synthetic chemical present in our food has identified recently its association with increased risk for developing type 2 diabetes. Therefore, it should be administered in a controlled manner to humans due to its direct effect on glucose and insulin levels (Hagobian et al., 2019).
-
•
The digital diabetes care market has gone beyond the earlier diabetes management. The digital solutions for diabetes such as applications, connected devices and services along with healthcare professionals and payers have change the earlier scenario of the disease (Deepa et al., 2020). Digital health is proving very helpful in improving the lifestyle of diabetic patients (Kaufman and Khurana, 2016). It is a complementary tool in diabetes-intervention studies.
7. Scope and prospects
Diabetes is a common metabolic disorder that can affect people of any group in developed as well as developing countries (Zaid et al., 2016). The rise in global diabetic patients has been linked with several factors like poor diet, obesity and sedentary lifestyles. However, older people have share a significant portion of diabetic patient worldwide. Several conventional and pharmacological treatments are available to restore normal blood glucose level. However, the conventional therapies and drugs treatments are associated with certain side effects. The advancement in the technologies like mobile phones and personal digital assistants are very helpful to tell the current status of health of an individual. The most frequently used digital services for healthcare wellness including mobile telemedicine, health call centers and emergency toll-free telephone services according to the second global survey on electronic health (WHO, 2011). However, a long-term study is desired to determine their sustainability, efficacy, cost-effectiveness and patient satisfaction. The traditional herbal medicines have been used in the treatment of several disorders (Jain et al., 2015; Rao et al., 2014, 2015; Rathore et al., 2014). The bioactive compounds are very effective in controlling bacteria, pathogens, viruses, and other foreigns agents (Jain et al., 2019 a; Jain et al., 2018; Jain et al., 2020 a; Jain et al., 2020 b; Jain et al., 2020 c; Rao et al., 2016; Jain et al., 2020 d; Jain et al., 2020 e). The medicinal plants have been used in the treatment of diabetes and they are getting recognition after their standardization and proper clinical trials (Jain et al., 2019 b). The herbs have several modes of antidiabetic action including insulin mimetic, alteration of glucose metabolizing enzymes, inhibition of intestinal absorption of glucose, increase uptake of glucose peripherally, regeneration of pancreatic cells, promotes insulin release and ameliorating oxidative stress (Mwiti and Jide, 2015). Several herbs have been used traditionally in the treatment of diabetes since ancient times in many countries. It is due to their effective role in medicinal field. The herbal drugs are recognizing worldwide because of their safety profile improved pharmacokinetic, pharmacological and clinical status (Choudhury et al., 2017).
8. Conclusion
Medicinal plants have been used in the treatment of several disorders. They can lower the blood glucose level through combination of more than one mechanism. These plants have similar mode of action and mechanism as conventional drugs for treating diabetes but advantageously they have lesser side effects. There is a need to standardize all the traditional herbs used for medicinal purposes and determine their molecular mechanism for alleviating diabetes. The standardized, validated and characterized herbal drugs with their identified phytoconstituents can enter the world of clinical trials and further reach to their ultimate market. Thus, traditional knowledge for the use of medicinal herbs against diabetes with molecular mechanism may be a useful tool for new drug development.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgement
The authors are thankful for providing all facilities required for preparing the review article. We are showing our explicitly gratitude towards Probecell: Scientific Writing Services for proofreading and editing of this article.
References
- Abdelaziz D.H.A., Ali S.A., Mostafa M.M.A. Phoenix dactylifera seeds ameliorate early diabetic complications in streptozotocin-induced diabetic rats. Pharm. Biol. 2015;53(6):792–799. doi: 10.3109/13880209.2014.942790. [DOI] [PubMed] [Google Scholar]
- Abdelhalim A., Al-Munawarah A.-M. Pharmacological properties and chemical constituents of chiliadenus iphionoides (syn. Varthemia iphionoides): a review. Eur. J. Med. Plants. 2020;31:84–97. [Google Scholar]
- ADA. American Diabetes Association Diagnosis and classification of diabetes mellitus. Diabetes Care. 2013;36(1):67–74. [Google Scholar]
- ADA American diabetes association. Standards of medical care in diabetes—2021. Diabetes Care. 2021;44(1):1–2. [Google Scholar]
- Ahmed D., Kumar V., Sharma M., Verma A. Target guided isolation, in-vitro antidiabetic, antioxidant activity and molecular docking studies of some flavonoids from Albizzia Lebbeck Benth. bark. BMC Compl. Alternative Med. 2014;14(1):155. doi: 10.1186/1472-6882-14-155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed D., Sharma M., Mukerjee A., Ramteke P.W., Kumar V. Improved glycemic control, pancreas protective and hepatopro- tective effect by traditional poly-herbal formulation “Qurs Tabasheer” in streptozotocin induced diabetic rats. BMC Comple- ment Altern Med. 2013;13:10. doi: 10.1186/1472-6882-13-10. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Ahmed M.F., Kazim S.M., Ghori S.S., Mehjabeen S.S., Ahmed S.R., Ali S.M., et al. Antidiabetic activity of Vinca rosea extracts in alloxan-induced diabetic rats. Dandona P.K., editor. Internet J. Endocrinol. 2010;2010:841090. doi: 10.1155/2010/841090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aja P.M., Nwachukwu N., Ibiam U.A., Igwenyi I.O., Offor C.E., Orji U.O. Chemical constituents of moringa oleifera leaves and seeds from Abakaliki , Nigeria. AJPCT. 2014;2(3):310–321. [Google Scholar]
- Akhtar N., Akram M., Daniyal M., Ahmad S. Evaluation of antidiabetic activity of Ipomoea batatas L. extract in alloxan-induced diabetic rats. Int. J. Immunopathol. Pharmacol. 2018;32 doi: 10.1177/2058738418814678. 2058738418814678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akinyemi O., So O., Ka J. Medicinal plants and sustainable human health : a review. 2018;2(4):194–195. [Google Scholar]
- Alam K., Ali S., Mahjabeen S., Hassan R., Rahman M.F., Chowdhury R. Potential hypoglycemic effect of Swertia chirayita—an Indian subcontinent herb with important medicinal value. Pharmacologyonline. 2011;2:642–647. [Google Scholar]
- Alam P., Shahzad N., Gupta A.K., Mahfoz A.M., Bamagous G.A., Al-Ghamdi S.S., et al. Anti-diabetic effect of boerhavia diffusa L. Root extract via free radical scavenging and antioxidant mechanism. Toxicol Environ Health Sci. 2018;10(3):220–227. [Google Scholar]
- Ardiana L., Sauriasari R., Elya B. Antidiabetic activity studies of white tea (camellia sinensis (L.) O. Kuntze) ethanolic extracts in streptozotocin-nicotinamide induced diabetic rats. Pharmacogn J. 2017;10:186–189. [Google Scholar]
- Arora K., Nagpal M., Jain U., Jat R.C., Jain S. Mangroves : a novel gregarious phyto medicine for diabetes. Int. J. Res. Dev. Pharm. Life Sci. 2014;3(6):1231–1244. [Google Scholar]
- Ashraf A., Sarfraz R.A., Anwar F., Shahid S.A.L.I., Alkharfy K.M. Chemical composition and biological activities of leaves of ziziphus mauritiana l . native to Pakistan. Pakistan J. Bot. 2015;47(1):367–376. [Google Scholar]
- Atangwho I., Ebong P., Eyong E., Williams I., Egbung E. Comparative chemical composition of leaves of some antidiabetic medicinal plants: Azadirachta indica, Vernonia amygdalina and Gongronema latifolium. Afr. J. Biotechnol. 2009;8 [Google Scholar]
- Atangwho I.J., Yin K.B., Umar M.I., Ahmad M., Asmawi M.Z. Vernonia amygdalina simultaneously suppresses gluconeogenesis and potentiates glucose oxidation via the pentose phosphate pathway in streptozotocin-induced diabetic rats. BMC Compl. Alternative Med. 2014;14:426. doi: 10.1186/1472-6882-14-426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayyanar M., Subash-Babu P. Syzygium cumini (L.) Skeels: a review of its phytochemical constituents and traditional uses. Asian Pac J Trop Biomed. 2012;2(3):240–246. doi: 10.1016/S2221-1691(12)60050-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker R.L., Rihanek M., Hohenstein A.C., Nakayama M., Michels A., Gottlieb P.A., Haskins K., Delong T. Hybrid insulin peptides are autoantigens in type 1 diabetes. Diabetes. 2019;68(9):1830–1840. doi: 10.2337/db19-0128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bamosa A.O., Kaatabi H., Lebdaa F.M., Elq A.M., Al-Sultanb A. Effect of Nigella sativa seeds on the glycemic control of patients with type 2 diabetes mellitus. Indian J. Physiol. Pharmacol. 2010;54(4):344–354. [PubMed] [Google Scholar]
- Banerjee D., Maji A.K., Mahapatra S., Banerji P. Barleria prionitis linn.: a review of its traditional uses, phytochemistry, pharmacology and toxicity. Res. J. Phytochem. 2012;6:31–41. [Google Scholar]
- Barik A., Mazumdar S., Chowdhury A., Rai R.K. Physiological and behavioral risk factors of type 2 diabetes mellitus in rural India. BMJ Open Diabetes Res & Care. 2016;4(1) doi: 10.1136/bmjdrc-2016-000255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behl T., Kotwani A. Proposed mechanisms of Terminalia catappa in hyperglycaemia and associated diabetic complications. J. Pharm. Pharmacol. 2017;69(2):123–134. doi: 10.1111/jphp.12676. [DOI] [PubMed] [Google Scholar]
- Bhadoriya S.S., Ganeshpurkar A., Narwaria J., Rai G., Jain A.P. Tamarindus indica: extent of explored potential. Pharmacogn Rev. 2011;5(9):73–81. doi: 10.4103/0973-7847.79102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bharti S.K., Krishnan S., Kumar A., Kumar A. Antidiabetic phytoconstituents and their mode of action on metabolic pathways. Ther Adv Endocrinol Metab. 2018;9(3):81–100. doi: 10.1177/2042018818755019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bornstein S.R., Rubino F., Khunti K., Mingrone G., Hopkins D., Birkenfeld A.L., et al. Practical recommendations for the management of diabetes in patients with COVID-19. Lancet Diabetes Endocrinol. 2020;8(6):546–550. doi: 10.1016/S2213-8587(20)30152-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosun-Arije F.S., Ling J., Graham Y., Hayes C. A systematic review of factors influencing Type 2 Diabetes Mellitus management in Nigerian public hospitals. Int J Africa Nurs Sci [Internet] 2019;11:100151. [Google Scholar]
- Casierra-Posada F., Jarma-Orozco A. 2016. Nutritional composition of passiflora species; pp. 517–534. [Google Scholar]
- Chakraborty M., Bagchi B., Das S., Basu R., Nandy P. A dose Dependent hepatoprotective and nephroprotective activity of eucalyptus oil on Streptozotocin induced diabetic mice model. Clin Phytoscience. 2018;4(1):10. [Google Scholar]
- Chamberlain J.J., Herman W.H., Leal S., Rhinehart A.S., Shubrook J.H., Skolnik N., et al. Pharmacologic therapy for type 2 diabetes: synopsis of the 2017 American diabetes association standards of medical care in diabetes. Ann. Intern. Med. 2017;166(8):572–578. doi: 10.7326/M16-2937. [DOI] [PubMed] [Google Scholar]
- Chandrasekar S.B., Bhanumathy M., Pawar A.T., Somasundaram T. Phytopharmacology of Ficus religiosa. Pharmacogn Rev. 2010;4(8):195–199. doi: 10.4103/0973-7847.70918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang C.L., Lin C.S. In: Evidence-Based Complement Altern Med. Zhang B., editor. 2012. Phytochemical composition, antioxidant activity, and neuroprotective effect of Terminalia chebula retzius extracts; p. 125247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen G.L., Xu Y.B., Wu J.L., Li N., Guo M.Q. Hypoglycemic and hypolipidemic effects of Moringa oleifera leaves and their functional chemical constituents. Food Chem. 2020;333:127478. doi: 10.1016/j.foodchem.2020.127478. [DOI] [PubMed] [Google Scholar]
- Chen J., Jing X., Liu X., Volkmann A.-M., Chen Y., Liu Y., et al. Assessment of factors affecting diabetes management in the City Changing Diabetes (CCD) study in Tianjin. PloS One. 2019;14(2) doi: 10.1371/journal.pone.0209222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choudhary M., Kochhar A., Sangha J. Hypoglycemic and hypolipidemic effect of Aloe vera L. in non-insulin dependent diabetics. J. Food Sci. Technol. 2014;51(1):90–96. doi: 10.1007/s13197-011-0459-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choudhury H., Pandey M., Hua C.K., Mun C.S., Jing J.K., Kong L., et al. An update on natural compounds in the remedy of diabetes mellitus: a systematic review. J Tradit Complement Med. 2017;8(3):361–376. doi: 10.1016/j.jtcme.2017.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deepa M., Shruti M., Mohan V. Diabetes Digital Health. INC; 2020. Reducing the global burden of diabetes using mobile health; pp. 1–24. [Google Scholar]
- Dey B., Mitra A. Chemo-profiling of eucalyptus and study of its hypoglycemic potential. World J. Diabetes. 2013;4(5):170–176. doi: 10.4239/wjd.v4.i5.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dey P., Singh J., Suluvoy J.K., Dilip K.J., Nayak J. Utilization of swertia chirayita plant extracts for management of diabetes and associated disorders: present status, future prospects and limitations. Nat Products Bioprospect. 2020;10(6):431–443. doi: 10.1007/s13659-020-00277-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhakad P.K., Sharma P.K., Kumar S. A review on phytochemical studies and biological potential of Citrullus colocynthis ( L .) schrad . ( cucurbitaceae ) 2017;5(4):55–64. [Google Scholar]
- Dheer R., Bhatnagar P. A study of the antidiabetic activity of Barleria prionitis Linn. Indian J. Pharmacol. 2010;42:70–73. doi: 10.4103/0253-7613.64493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dougnon T.J., Gbeassor M. Evaluation of the effects of the powder of Capsicum frutescens on glycemia in growing rabbits. Vet. World. 2016;9(3):281–286. doi: 10.14202/vetworld.2016.281-286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Einstein J.W., Mohd Rais M., Mohd M.A. Comparative evaluation of the antidiabetic effects of different parts of Cassia fistula linn, a Southeast asian plant. Valentao P., editor. J. Chem. 2013;2013:714063. [Google Scholar]
- El Haouari M., Rosado J.A. Phytochemical, anti-diabetic and cardiovascular properties of urtica dioica L. (urticaceae): a review. Mini Rev. Med. Chem. 2019;19(1):63–71. doi: 10.2174/1389557518666180924121528. [DOI] [PubMed] [Google Scholar]
- Elumalai S., Kesavan R., Selvarajan R., Murugasen R., Elumalai E. Isolation, purification and identification of the anti- diabetic components from Cinnamomum zeylanicum and Cinnamomum cassia bark oil extracts. Curr. Bot. 2011;2 [Google Scholar]
- Eu C.H., Lim W.Y., Ton S.H., et al. Glycyrrhizic acid improved lipoprotein lipase expression, insulin sensitivity, serum lipid and lipid deposition in high-fat diet-induced obese rats. Lipids Health Dis. 2010;9:81. doi: 10.1186/1476-511X-9-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ezejiofor A.N., Okorie A., Orisakwe O.E. Hypoglycaemic and tissue-protective effects of the aqueous extract of persea americana seeds on alloxan-induced albino rats. Malays. J. Med. Sci. 2013;20(5):31–39. [PMC free article] [PubMed] [Google Scholar]
- Fu Q.Y., Li Q.S., Lin X.M., Qiao R.Y., Yang R., Li X.M., et al. Antidiabetic effects of tea. Molecules. 2017;22(5):849. doi: 10.3390/molecules22050849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geberemeskel G.A., Debebe Y.G., Nguse N.A. Antidiabetic effect of fenugreek seed powder solution (trigonella foenum-graecum L.) on hyperlipidemia in diabetic patients. Portha B., editor. J Diabetes Res. 2019;2019:8507453. doi: 10.1155/2019/8507453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geetha G., Kalavalarasariel Gopinathapillai P., Sankar V. Anti diabetic effect of Achyranthes rubrofusca leaf extracts on alloxan induced diabetic rats. Pak. J. Pharm. Sci. 2011;24(2):193–199. [PubMed] [Google Scholar]
- Govindarajan M. Chemical composition and larvicidal activity of leaf essential oil from Clausena anisata (Willd.) Hook. f. ex Benth (Rutaceae) against three mosquito species. Asian Pac J Trop Med. 2010;3(11):874–877. [Google Scholar]
- Govindasamy C., Al-Numair K.S., Alsaif M.A., Viswanathan K.P. Influence of 3-hydroxymethyl xylitol, a novel antidiabetic compound isolated from Casearia esculenta (Roxb.) root, on glycoprotein components in streptozotocin-diabetic rats. J. Asian Nat. Prod. Res. 2011;13(8):700–706. doi: 10.1080/10286020.2011.585157. [DOI] [PubMed] [Google Scholar]
- Gunn J., Che C.-T., Farnsworth N. Watson RR, Preedy VRBT-BF as DI for D. Academic Press; San Diego: 2013. Chapter 33 - diabetes and natural products; pp. 381–394. [Google Scholar]
- Gurnani N., Gupta M., Mehta D., Mehta B.K. Chemical composition, total phenolic and flavonoid contents, and in vitro antimicrobial and antioxidant activities of crude extracts from red chilli seeds (Capsicum frutescens L.) J Taibah Univ Sci. 2016;10(4):462–470. [Google Scholar]
- Hagobian T.A., Bird A., Stanelle S., Williams D., Schaffner A., Phelan S. Pilot study on the effect of orally administered Bisphenol A on glucose and insulin response in nonobese adults. Journal of the Endocrine Society. 2019;3(3):643–654. doi: 10.1210/js.2018-00322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halmi S., Benlakssira B., Bechtarzi K., Djerrou Z., Djeaalab H., Riachi F., et al. Antihyperglycemic activity of prickly pear (Opuntia ficus-indica) aqueous extract. Int. J. Med. Aromatic Plants. 2012;2:2249–4340. [Google Scholar]
- Hassan S., El-Sammad N., Moussa A., Mohammed M., Farrag A.R., Hashim A., et al. 2015. Hypoglycemic and Antioxidant Activities of Caesalpinia Ferrea Martius Leaf Extract in Streptozotocin-Induced Diabetic Rats. [Google Scholar]
- He X., Wang X., Fang J., Chang Y., Ning N., Guo H., et al. The genus Achyranthes: a review on traditional uses, phytochemistry, and pharmacological activities. J. Ethnopharmacol. 2017;203:260–278. doi: 10.1016/j.jep.2017.03.035. [DOI] [PubMed] [Google Scholar]
- Hegazy G.A., Alnoury A.M., Gad H.G. The role of Acacia Arabica extract as an antidiabetic, antihyperlipidemic, and antioxidant in streptozotocin-induced diabetic rats. Saudi Med. J. 2013;34(7):727–733. [PubMed] [Google Scholar]
- Heidarzadeh S., Farzanegi P., Azarbayjani M.A., et al. Purslane effect on GLP-1 and GLP-1 receptor in type 2 diabetes. Electron. Physician. 2013;5:582–587. doi: 10.14661/2013.582-587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heshmati J., Namazi N. Effects of black seed (Nigella sativa) on metabolic parameters in diabetes mellitus: a systematic review. Compl. Ther. Med. 2015;23(2):275–282. doi: 10.1016/j.ctim.2015.01.013. [DOI] [PubMed] [Google Scholar]
- Huang F., Deng T., Meng L., Ma X. Dietary ginger as a traditional therapy for blood sugar control in patients with type 2 diabetes mellitus: a systematic review and meta-analysis. Medicine (Baltim.) 2019;98(13) doi: 10.1097/MD.0000000000015054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang T.H.W., Peng G., Kota B.P., et al. Anti-diabetic action of Punica granatum flower extract: activation of PPAR-g and iden- tification of an active component. Toxicol. Appl. Pharmacol. 2005;207:160–169. doi: 10.1016/j.taap.2004.12.009. [DOI] [PubMed] [Google Scholar]
- IDF. International Diabetes Federation . eighth ed. IDF; 2017. Diabetes Atlas. [Google Scholar]
- Ikarashi N., Toda T., Okaniwa T., Ito K., Ochiai W., Sugiyama K. Anti-obesity and anti-diabetic effects of acacia polyphenol in obese diabetic KKAy mice fed high-fat diet. Evide Based Complement Alternat Med. 2011;2011:952031. doi: 10.1093/ecam/nep241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain A., Jain P., Parihar D.K. Comparative study of in-vitro antidiabetic and antibacterial activity of non-conventional curcuma species. JBAPN. 2019 b;9(6):457–464. [Google Scholar]
- Jain A., Parihar D.K. Antibacterial, biofilm dispersal and antibiofilm potential of alkaloids and flavonoids of Curcuma. Biocatal Agric Biotechnol. 2018;16:677–682. [Google Scholar]
- Jain A., Parihar D.K. Molecular marker based genetic diversity study of wild, cultivated and endangered species of Curcuma from Chhattisgarh region for in situ conservation. Biocatal Agric Biotechnol. 2019;18:101033. [Google Scholar]
- Jain P., Pandey R., Shukla S.S. Acute and subacute toxicity studies of polyherbal formulation talisadya churna in experimental animal model. MJPMS. 2015;1(1):7–10. [Google Scholar]
- Jain P., Pandey R., Shukla S.S. Reproductive and developmental toxicity study of talisadya churna: an ancient polyherbal formulation. IAJPR. 2016;6(5):5641–5653. [Google Scholar]
- Jain P., Rao S.P., Singh V., Pandey R., Shukla S.S. Acute and sub-acute toxicity studies of an ancient ayurvedic formulation: agnimukha churna. Columbia Journal of Pharmaceutical Sciences. 2014;(1):18–22. [Google Scholar]
- Jain P., Satapathy T., Pandey R.K. A mini review of methods to control ticks population infesting cattle in Chhattisgarh with special emphasis on herbal acaricides. IJNPR. 2020 e;11(12):217–223. [Google Scholar]
- Jain P., Satapathy T., Pandey R.K. Efficacy of arecoline hydrobromide against cattle tick Rhipicephalus (Boophilus) microplus. Int. J. Acarol. 2020 c;46(4):268–275. [Google Scholar]
- Jain P., Satapathy T., Pandey R.K. First report on ticks (Acari: ixodidae) controlling activity of cottonseed oil (Gossypium Sp.) Int. J. Acarol. 2020 d;46(4):263–267. [Google Scholar]
- Jain P., Satapathy T., Pandey R.K. Rhipicephalus microplus (acari: ixodidae): clinical safety and potential control by topical application of cottonseed oil (Gossypium sp.) on cattle. Exp. Parasitol. 2020 a doi: 10.1016/j.exppara.2020.108017. [DOI] [PubMed] [Google Scholar]
- Jain P., Satapathy T., Pandey R.K. Rhipicephalus microplus: a parasite threatening cattle health and consequences of herbal acaricides for upliftment of livelihood of cattle rearing communities in Chhattisgarh. Biocatal Agric Biotechnol. 2020 b:101611. [Google Scholar]
- Jain S., Bhatia G., Barik R., Kumar P., Jain A., Dixit V. Antidiabetic activity of Paspalum scrobiculatum Linn. in alloxan induced diabetic rats. J. Ethnopharmacol. 2009;127:325–328. doi: 10.1016/j.jep.2009.10.038. [DOI] [PubMed] [Google Scholar]
- Jaiswal Y.S., Tatke P.A., Gabhe S.Y., Vaidya A.B. Antidiabetic activity of extracts of Anacardium occidentale Linn. leaves on n-streptozotocin diabetic rats. J Tradit Complement Med. 2016;7(4):421–427. doi: 10.1016/j.jtcme.2016.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janež A., Guja C., Mitrakou A., Lalic N., Tankova T., Czupryniak L., et al. Insulin therapy in adults with type 1 diabetes mellitus: a narrative review. Diabetes Ther. 2020;11(2):387–409. doi: 10.1007/s13300-019-00743-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joseph B., Jini D. Antidiabetic effects of Momordica charantia (bitter melon) and its medicinal potency. Asian Pacific J Trop Dis. 2013;3(2):93–102. [Google Scholar]
- Joseph J., Joseph M.G., Latha S. Effect of aloe vera juice on glucose level among diabetics in a selected old age home at mangalore. 2018;8(9):135–141. [Google Scholar]
- Joshi B.C., Mukhija M., Kalia A.N. Phytochemistry and pharmacologic properties of Urtica. Int. J. Green Pharm. 2019:201–209. [Google Scholar]
- Kanetkar P., Singhal R., Kamat M. Gymnema sylvestre: a memoir. J. Clin. Biochem. Nutr. 2007;41(2):77–81. doi: 10.3164/jcbn.2007010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman N., Khurana I. Using digital health technology to prevent and treat diabetes. Diabetes Technol. Therapeut. 2016;18:56–68. doi: 10.1089/dia.2016.2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan F., Sarker M.M.R., Ming L.C., Mohamed I.N., Zhao C., Sheikh B.Y., et al. Comprehensive review on phytochemicals, pharmacological and clinical potentials of gymnema sylvestre. Front. Pharmacol. 2019;10:1223. doi: 10.3389/fphar.2019.01223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kianbakht S., Hajiaghaee R. Anti-hyperglycemic effects of vaccinium arctostaphylos L. fruit and leaf extracts in alloxan-induced diabetic rats. J Med Plants. 2013;12:43–50. [Google Scholar]
- Kiran P., Denni M., Daniel M., Laboratories D., Farm O.H. vols. 13–5. 2014. (Antidiabetic Principles , Phospholipids and Fixed Oil of Kodo Millet ( Paspalum Scrobiculatum Linn .)). [Google Scholar]
- Klangjareonchai T., Roongpisuthipong C. The effect of tinospora crispa on serum glucose and insulin levels in patients with type 2 diabetes mellitus. Shibata M.-A., editor. J. Biomed. Biotechnol. 2012;2012:808762. doi: 10.1155/2012/808762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishna R.N., Anitha R., Ezhilarasan D. Aqueous extract of Tamarindus indica fruit pulp exhibits antihyperglycaemic activity. Avicenna J phytomedicine. 2020;10(5):440–447. [PMC free article] [PubMed] [Google Scholar]
- Kuczmannová A., Balažová A., Račanská E., Kameníková M., Fialová S., Majerník J., Nagy M., Gál P., Mučaji P. Agrimonia eupatoria L. And cynara cardunculus L. Water infusions: comparison of anti-diabetic activities. Molecules. 2016;21(5):564. doi: 10.3390/molecules21050564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar V., Jain P., Rathore K., Ahmed Z. Biological evaluation of pupalia lappacea for antidiabetic, antiadipogenic, and hypolipidemic activity both in vitro and in vivo. Sci. Tech. Rep. 2016;9 doi: 10.1155/2016/1062430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar V., Rathore K., Jain P., Ahmed Z. Biological activity of bauhinia racemose against diabetes and interlinked disorders like obesity and hyperlipidemia. Clin. Phytoscience. 2017;3:7. [Google Scholar]
- Kumar V., Van Staden J. A review of swertia chirayita (gentianaceae) as a traditional medicinal plant. Front. Pharmacol. 2016;6:308. doi: 10.3389/fphar.2015.00308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurup Sb S.M. Protective potential of Averrhoa bilimbi fruits in ameliorating the hepatic key enzymes in streptozotocin-induced diabetic rats. Biomed. Pharmacother. 2017;85:725–732. doi: 10.1016/j.biopha.2016.11.088. [DOI] [PubMed] [Google Scholar]
- Kusano S., Abe H. Antidiabetic activity of white skinned sweet potato (Ipomoea batatas L.) in obese Zucker fatty rats. Biol. Pharm. Bull. 2000;23(1):23–26. doi: 10.1248/bpb.23.23. [DOI] [PubMed] [Google Scholar]
- Latha M., Pari L., Ramkumar K.M., Rajaguru P., Suresh T., Dhanabal T., et al. Antidiabetic effects of scoparic acid D isolated from Scoparia dulcis in rats with streptozotocin-induced diabetes. Nat. Prod. Res. 2009;23(16):1528–1540. doi: 10.1080/14786410902726126. [DOI] [PubMed] [Google Scholar]
- Lawal O.A., Ogunwande I.A., Ibirogba A.E., Layode O.M., Opoku A.R. Chemical constituents of essential oils from catharanthus roseus (L.) G. Don grown in Nigeria. J Essent Oil Bear Plants. 2015;18(1):57–63. [Google Scholar]
- Leem K.-H., Kim M.-G., Hahm Y.-T., Kim H.K. Hypoglycemic effect of opuntia ficus-indica var. saboten is due to enhanced peripheral glucose uptake through activation of AMPK/p38 MAPK pathway. Nutrients. 2016;8(12):800. doi: 10.3390/nu8120800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li B., Cheng M., Gao H., et al. Back-regulation of six oxidative stress proteins with grape seed proanthocyanidin extracts in rat diabetic nephropathy. J. Cell. Biochem. 2008;104:668–679. doi: 10.1002/jcb.21658. [DOI] [PubMed] [Google Scholar]
- Li X., Yang P. Research progress of Sonchus species Research progress of Sonchus species. Int. J. Food Prop. 2018;21(1):147–157. [Google Scholar]
- Lindstedt I. Ginger and diabetes : a mini-review. Arch Gen Intern Med. 2018;2(2):29–33. [Google Scholar]
- Lodha S.R., Joshi S.V., Vyas B.A., Upadhye M.C., Kirve M.S., Salunke S.S., et al. Assessment of the antidiabetic potential of Cassia grandis using an in vivo model. "J. Adv. Pharm. Technol. Research"" (JAPTR)". 2010;1(3):330–333. doi: 10.4103/0110-5558.72429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo C., Zhang W., Sheng C., Zheng C., Yao J., Miao Z. Chemical composition and antidiabetic activity of opuntia milpa alta extracts. Chem. Biodivers. 2010;7:2869–2879. doi: 10.1002/cbdv.201000077. [DOI] [PubMed] [Google Scholar]
- Macêdo N.S., Silveira Z. de S., Bezerra A.H., Costa JGM da, Coutinho H.D.M., Romano B., et al. Caesalpinia ferrea C. Mart. (Fabaceae) phytochemistry, ethnobotany, and bioactivities: a review. Molecules. 2020;25(17) doi: 10.3390/molecules25173831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahmoud M.F., El Ashry F.E., El Maraghy N.N., Fahmy A. Studies on the antidiabetic activities of Momordica charantia fruit juice in streptozotocin-induced diabetic rats. Pharm. Biol. 2017;55(1):758–765. doi: 10.1080/13880209.2016.1275026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahmud I., Shahria N., Yeasmin S., Iqbal A., Mukul E.H., Gain S., Shilpi J.A., Islam M.K. Ethnomedicinal, phytochemical and pharmacological profile of a mangrove plant Ceriops Decandra GriffDin Hou. J. Compl. Integr. Med. 2018;16(1) doi: 10.1515/jcim-2017-0129. [DOI] [PubMed] [Google Scholar]
- Malarvili A., Selvaraja P., Ndyeabura A.W., Akowuah G., Okechukwu P. Antidiabetic activity of crude stem extracts of coscinium fenestratum on streptozotocin-induced type-2 diabetic rats. Asian J. Pharmaceut. Clin. Res. 2011;4:47–51. [Google Scholar]
- Mao X., Wu L.-F., Guo H.-L., Chen W.-J., Cui Y.-P., Qi Q., et al. Vol. 2016. Evid Based Complement Alternat Med; 2016. The genus Phyllanthus: an ethnopharmacological, phytochemical, and pharmacological review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathew J.J., Sajeshkumar N.K., Joy N. GRIN; Munich: 2017. Phytochemical Characterization of Averrhoa Bilimbi and in Vitro Analysis of Cholesterol Lowering Effect on Fatty Food Materials. [Google Scholar]
- McGaw L.J., Srivastava A.K., Lin C.H., Steenkamp V. 2019. Book Review: Medicinal Plants for Holistic Healing; p. 1053. 10. Frontiers in Pharmacology. [Google Scholar]
- Medagama A.B. The glycaemic outcomes of Cinnamon, a review of the experimental evidence and clinical trials. Nutr. J. 2015;14(1):108. doi: 10.1186/s12937-015-0098-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meenakshi P., Bhuvaneshwari R., Rathi M.A., Thirumoorthi L., Guravaiah D.C., Jiji M.J., et al. Antidiabetic activity of ethanolic extract of zaleya decandra in alloxan-induced diabetic rats. Appl. Biochem. Biotechnol. 2010;162(4):1153–1159. doi: 10.1007/s12010-009-8871-x. [DOI] [PubMed] [Google Scholar]
- Meloche R.M. Transplantation for the treatment of type 1 diabetes. World J. Gastroenterol. 2007;13(47):6347–6355. doi: 10.3748/wjg.v13.i47.6347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mininel F.J., Leonardo Junior C.S., Espanha L.G., Resende F.A., Varanda E.A., Leite C.Q.F., et al. In: van Vuuren S., editor. vol. 2014. 2014. Characterization and quantification of compounds in the hydroalcoholic extract of the leaves from Terminalia catappa linn. (Combretaceae) and their mutagenic activity; p. 676902. (Evidence-Based Complement Altern Med). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moqbel F.S., Naik P.R., Najma H.M., Selvaraj S. Antidiabetic properties of Hibiscus rosa sinensis L. leaf extract fractions on nonobese diabetic (NOD) mouse. Indian J. Exp. Biol. 2011;49(1):24–29. [PubMed] [Google Scholar]
- Muñiz-Ramirez A., Perez R.M., Garcia E., Garcia F.E. In: Sherman R., editor. vol. 2020. 2020. Antidiabetic activity of aloe vera leaves; p. 6371201. (Evidence-Based Complement Altern Med). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mwiti K.C., Jide A.A. Herbal therapy: a review of emerging pharmacological tools in the management of diabetes mellitus in Africa. 2015;11(44):258–274. doi: 10.4103/0973-1296.166046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nabi S.A., Kasetti R.B., Sirasanagandla S., Tilak T.K., Kumar M.V.J., Rao C.A. Antidiabetic and antihyperlipidemic activity of Piper longum root aqueous extract in STZ induced diabetic rats. BMC Compl. Alternative Med. 2013;13:37. doi: 10.1186/1472-6882-13-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nayak A., Sajini J., Padmavati D., Karthik R. Chemistry and medicinal properties of Coscinium fenestratum (Gaertn.) colebr (tree turmeric) Res. J. Pharm. Technol. 2012;5:198–202. [Google Scholar]
- Nazreen S., Kaur G., Alam M.M., et al. Hypoglycemic activity of Bambusa arundinacea leaf ethanolic extract in streptozotocin induced diabetic rats. Pharmacologyonline. 2011;1:964–972. [Google Scholar]
- Niamat R., Khan M.A., Khan kiran Y., Ahmed M., Mazari P., Ali B., et al. A review on zizyphus as antidiabetic. J. Appl. Pharmaceut. Sci. 2012;2:177–179. [Google Scholar]
- Obih P.O., Obih C.A., Brown A., Day X. In vitro investigations of the antidiabetic action of ginger (zingiber officinale) Faseb. J. 2017;31(1) 347-347. [Google Scholar]
- Okokon J., Umoh E., Etim E., Jackson C. Antiplasmodial and antidiabetic activities of ethanolic leaf extract of heinsia crinata. J. Med. Food. 2009;12:131–136. doi: 10.1089/jmf.2008.0116. [DOI] [PubMed] [Google Scholar]
- Olivas-Quintero S., López-Angulo G., Montes-Avila J., Díaz-Camacho S.P., Vega-Aviña R., López-Valenzuela J.Á., et al. Chemical composition and biological activities of Helicteres vegae and Heliopsis sinaloensis. Pharm. Biol. 2017;55(1):1473–1482. doi: 10.1080/13880209.2017.1306712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oram R.A., Sims E.K., Evans-Molina C. Beta cells in type 1 diabetes: mass and function; sleeping or dead? Diabetologia. 2019;62(4):567–577. doi: 10.1007/s00125-019-4822-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pal R., Bhadada S.K., Misra A. COVID-19 vaccination in patients with diabetes mellitus: current concepts, uncertainties and challenges. Diabetes Metab. Syndr. 2021;15(2):505–508. doi: 10.1016/j.dsx.2021.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palheta I.C., Ferreira L.R. Hypoglycemic potential of Anacardium occidentale L. J Anal Pharm Res. 2018;7(2):152–153. [Google Scholar]
- Pandit R., Phadke A., Jagtap A. Antidiabetic effect of Ficus religiosa extract in streptozotocin-induced diabetic rats. J. Ethnopharmacol. 2010;128(2):462–466. doi: 10.1016/j.jep.2010.01.025. Mar 24. [DOI] [PubMed] [Google Scholar]
- Paramanick D., Sharma N. A review on herbs which are used in diabetes mellitus. International Journal of Drug Development. 2017;9(3):12–17. [Google Scholar]
- Parsaeyan N. The effect of coriander seed powder consumption on atherosclerotic and cardioprotective indices of type 2 diabetic patients. Iran J Diabetes Obes. 2012;4:86–90. [Google Scholar]
- Peixoto Araujo N.M., Arruda H.S., de Paulo Farias D., Molina G., Pereira G.A., Pastore G.M. Plants from the genus Eugenia as promising therapeutic agents for the management of diabetes mellitus: a review. Food Res. Int. 2021;142:110182. doi: 10.1016/j.foodres.2021.110182. [DOI] [PubMed] [Google Scholar]
- Peter K., Gandhi P. Rediscovering the therapeutic potential of Amaranthus species: a review. Egypt J Basic Appl Sci. 2017;4(3):196–205. [Google Scholar]
- Prada A.L., Amado J.R.R., Keita H., Zapata E.P., Carvalho H., Lima E.S., et al. Cassia grandis fruit extract reduces the blood glucose level in alloxan-induced diabetic rats. Biomed. Pharmacother. 2018;103:421–428. doi: 10.1016/j.biopha.2018.04.059. [DOI] [PubMed] [Google Scholar]
- Prakasam A., Sethupathy S., Pugalendi K.V. Influence of Casearia esculenta root extract on glycoprotein components in streptozotocin diabetic rats. Pharmazie. 2005;60(3):229–232. [PubMed] [Google Scholar]
- Rahmani A.H. Cassia fistula Linn: potential candidate in the health management. Pharmacogn. Res. 2015;7(3):217–224. doi: 10.4103/0974-8490.157956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raina R., Verma P.K., Peshin R., Kour H. Potential of Juniperus communis L as a nutraceutical in human and veterinary medicine. Heliyon. 2019;5(8) doi: 10.1016/j.heliyon.2019.e02376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajendran M.P., Pallaiyan B.B., Selvaraj N. Chemical composition, antibacterial and antioxidant profile of essential oil from Murraya koenigii (L.) leaves. Avicenna J phytomedicine. 2014;4(3):200–214. [PMC free article] [PubMed] [Google Scholar]
- Rao A.R., Veeresham C., Asres K. In vitro and in vivo inhibi- tory activities of four Indian medicinal plant extracts and their major components on rat aldose reductase and generation of advanced glycation endproducts. Phytother Res. 2013;27:753–760. doi: 10.1002/ptr.4786. [DOI] [PubMed] [Google Scholar]
- Rao S.P., Amrit I., Jain P., Singh V. Antiulcer activity of agnimukha churna. Int. J. Ayur. Pharma Research. 2014;2(2):40–46. [Google Scholar]
- Rao S.P., Amrit I., Singh V., Jain P. Antiulcer activity of natural compounds: a review. Res. Rev. J. Pharmacogn. Phytochem. 2015;7(2):124–130. [Google Scholar]
- Rao S.P., Jain P., Rathore P., Singh V.K. Larvicidal and knockdown activity of Citrus limetta Risso oil against dengue virus vector. IJNPR. 2016;7(3):256–260. [Google Scholar]
- Rasineni K., Bellamkonda R., Singareddy S.R., Desireddy S. Antihyperglycemic activity of Catharanthus roseus leaf powder in streptozotocin-induced diabetic rats. Pharmacogn. Res. 2010;2(3):195–201. doi: 10.4103/0974-8490.65523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rathore K., Singh V., Jain P., Rao S.P., Ahmed Z., Thakur V.S. In- vitro and In-vivo antiadipogenic, antidiabetic and hypolipidemic activity of Diospyros melanoxylon (Roxb.) J. Ethnopharmacol. 2014;155:1171–1176. doi: 10.1016/j.jep.2014.06.050. [DOI] [PubMed] [Google Scholar]
- Rathore P., Rao S.P., Roy A., Satapathy T., Singh V., Jain P. Hepatoprotective activity of isolated herbal compounds. Res. J. Pharm. Technol. 2014;7(2) [Google Scholar]
- Rivera-Mondragón A., Ortíz O.O., Bijttebier S., Vlietinck A., Apers S., Pieters L., et al. Selection of chemical markers for the quality control of medicinal plants of the genus Cecropia. Pharm. Biol. 2017;55(1):1500–1512. doi: 10.1080/13880209.2017.1307421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saeedi P., Petersohn I., Salpea P., Malanda B., Karuranga S., Unwin N., et al. ninth ed. Vol. 157. Diabetes Res Clin Pract; 2019. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: results from the international diabetes federation diabetes atlas. [DOI] [PubMed] [Google Scholar]
- Salehi B., Ata A., Anil Kumar N V., Sharopov F., Ramírez-Alarcón K., Ruiz-Ortega A., et al. Antidiabetic potential of medicinal plants and their active components. Biomolecules. 2019;9(10) doi: 10.3390/biom9100551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satyanarayana K., Sravanthi K., Shaker I.A., Ponnulakshmi R. Molecular approach to identify antidiabetic potential of Azadirachta indica. J. Ayurveda Integr. Med. 2015;6(3):165–174. doi: 10.4103/0975-9476.157950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sebei K., Sakouhi F., Herchi W., Khouja M.L., Boukhchina S. Chemical composition and antibacterial activities of seven Eucalyptus species essential oils leaves. Biol. Res. 2015;48(1):7. doi: 10.1186/0717-6287-48-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senthilkumar G., Arulselvan P., Kumar D., Subramanian S. Anti-diabetic activity of fruits of Terminalia chebula on streptozotocin induced diabetic rats. J. Health Sci. 2006;52:283–291. [Google Scholar]
- Sepici-Dincel A., Acıkgoz S., Cevik C., et al. Effects of in vivo antioxidant enzyme activities of myrtle oil in normoglycaemic and alloxan diabetic rabbits. J. Ethnopharmacol. 2007;110:498–503. doi: 10.1016/j.jep.2006.10.015. [DOI] [PubMed] [Google Scholar]
- Shabani E., Sayemiri K., Mohammadpour M. The effect of garlic on lipid profile and glucose parameters in diabetic patients: a systematic review and meta-analysis. Prim Care Diabetes. 2019;13(1):28–42. doi: 10.1016/j.pcd.2018.07.007. [DOI] [PubMed] [Google Scholar]
- Sharwan G., Jain P., Pandey R., Shukla S.S. Toxicity and safety profiles of methanolic extract of pistacia integerrima J. L. Stewart ex brandis (PI) for wistar rats. J. Pharmacopuncture. 2016;19(3):253–258. doi: 10.3831/KPI.2016.19.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharwan G., Jain P., Pandey R., Shukla S.S. Toxicity profile of traditional herbal medicine. J Ayu Herb Med. 2015;1(3):81–90. [Google Scholar]
- Shi C., Karim S., Wang C., Zhao M., Murtaza G. A review on antidiabetic activity of Citrullus colocynthis Schrad. Acta Pol. Pharm. 2014;71(3):363–367. [PubMed] [Google Scholar]
- Shirwaikar A., Rajendran K., Dinesh Kumar C., Bodla R. Antidiabetic activity of aqueous leaf extract of Annona squamosa in streptozotocin–nicotinamide type 2 diabetic rats. J. Ethnopharmacol. 2004;91(1):171–175. doi: 10.1016/j.jep.2003.12.017. [DOI] [PubMed] [Google Scholar]
- Shukla S., Gunjegaonkar S.M. Pharmacognostical and pharmacological profiling of Barleria. J. Biol. Sci. Med. 2018;4:41–50. [Google Scholar]
- Sibiya A.M., Ramya A.K., Vaseeharan B. Bioactive molecules from Phyllanthus niruri and investigating their effects against diabetes. Recent Trends in Biochemistry. 2020;3:1–7. [Google Scholar]
- Singh A. Navneet. Critical review on various ethonomedicinal and pharmacological aspects of piper longum linn. (long pepper or pippali) IJIPSR. 2018;6(1):48–60. [Google Scholar]
- Singh J., Cumming E., Manoharan G., Kalasz H., Adeghate E. Medicinal chemistry of the anti-diabetic effects of momordica charantia: active constituents and modes of actions. Open Med Chem. 2011;5(2):70–77. doi: 10.2174/1874104501105010070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh P., Jain P., Pandey P., Shukla S.S. Phytotherapeutic review on diabetes. Spatula DD. 2016;6(2) [Google Scholar]
- Sinha D. Ethnobotanical and pharmacological importance of western himalayan fir abies pindrow ( royle ex D . Don ) Royle : A Review. 2019;31(6):1–14. [Google Scholar]
- Sk A., Chakrapani C., Alasyam N. Evaluation of antidiabetic effect of Murraya koenigii leaves chloroform extract (MKLCE) in alloxan induced diabetic albino rats. Pharma Innov. J. 2017;6:474–477. [Google Scholar]
- Skyler J.S., Bakris G.L., Bonifacio E., Darsow T., Eckel R.H., Groop L., et al. Differentiation of diabetes by pathophysiology, natural history, and prognosis. Diabetes. 2017;66(2):241. doi: 10.2337/db16-0806. LP – 255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su J., Jiang J., Zhang F., Liu Y., Ding L., Chen S., et al. Current achievements and future prospects in the genetic breeding of chrysanthemum: a review. Hortic. Res. 2019;6(1):109. doi: 10.1038/s41438-019-0193-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka M., Misawa E., Ito Y., Habara N., Nomaguchi K., Yamada M., et al. Identification of five phytosterols from aloe vera gel as antidiabetic compounds. Biol. Pharm. Bull. 2006;29:1418–1422. doi: 10.1248/bpb.29.1418. [DOI] [PubMed] [Google Scholar]
- Teimouri M. The chemical composition and antimicrobial activity of essential oils of vaccinium arctostaphylos L. Int. J. Adv. Biol. Biom. Res. 2015;3(3):270–274. [Google Scholar]
- Teugwa C.M., Mejiato P.C., Zofou D., Tchinda B.T., Boyom F.F. Antioxidant and antidiabetic profiles of two African medicinal plants: picralima nitida (Apocynaceae) and Sonchus oleraceus (Asteraceae) BMC Compl. Alternative Med. 2013;13:175. doi: 10.1186/1472-6882-13-175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiwari P., Mishra B.N., Sangwan N.S. Phytochemical and pharmacological properties of gymnema sylvestre: an important medicinal plant. Vincent J.B., editor. BioMed Res. Int. 2014;2014:830285. doi: 10.1155/2014/830285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verma D.S., Vashishth E., Singh R., Kumari A., Meena A., Pant P., et al. A review on parts of Albizia lebbeck (L.) Benth. used as Ayurvedic drugs. Res. J. Pharm. Technol. 2013;6:1307–1313. [Google Scholar]
- Verma S., Gupta M., Popli H., Aggarwal G. Diabetes Mellitus Treatment Using Herbal Drugs. 2018;10(1):1–10. [Google Scholar]
- Vilar D. de A., Vilar MS. de A., de Lima e Moura T.F.A., Raffin F.N., de Oliveira M.R., Franco CF. de O., et al. Traditional uses, chemical constituents, and biological activities of Bixa orellana L.: a review. ScientificWorldJournal. 2014;2014:857292. doi: 10.1155/2014/857292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang B., Chou K.H., Queenan B.N., Pennathur S., Bazan G.C. Molecular design of a new diboronic acid for the electrohydrodynamic monitoring of glucose. Angew. Chem. 2019;58(31):10612–10615. doi: 10.1002/anie.201904595. [DOI] [PubMed] [Google Scholar]
- Wang C., Yang J., Zhao P., Zhou Q., Mei Z., Yang G., et al. Chemical constituents from Eucalyptus citriodora Hook leaves and their glucose transporter 4 translocation activities. Bioorg. Med. Chem. Lett. 2014;24(14):3096–3099. doi: 10.1016/j.bmcl.2014.05.014. [DOI] [PubMed] [Google Scholar]
- Wani S.A., Fenugreek Kumar P. A review on its nutraceutical properties and utilization in various food products. J Saudi Soc Agric Sci. 2018;17(2):97–106. [Google Scholar]
- WHO. World Health Organization. mHealth . 2011. New Horizons for Health through Mobile Technolo- Gies: Second Global Survey on eHealth. [Google Scholar]
- Xu Y., Liang J.Y. Chemical constituents of Sonchus oleraceus L. J. China Pharm. Univ. 2005;36:411–413. [Google Scholar]
- Yakoob A.T., Tajuddin N.B., Hussain M.I.M., Mathew S., Govindaraju A., Qadri I. Antioxidant and hypoglycemic activities of clausena anisata (willd.) hook F. Ex benth. Root mediated synthesized silver nanoparticles. Pharmacogn J. 2016;8:579–586. [Google Scholar]
- Yamamoto J., Tadaishi M., Yamane T., Oishi Y., Shimizu M., Kobayashi-Hattori K. Hot water extracts of edible Chrysanthemum morifolium Ramat. exert antidiabetic effects in obese diabetic KK-Ay mice. Biosci. Biotechnol. Biochem. 2015;79(7):1147–1154. doi: 10.1080/09168451.2015.1008975. [DOI] [PubMed] [Google Scholar]
- Yan Y.M., Fang P., Yang M.T., Li N., Lu Q., Cheng Y.X. Anti-diabetic nephropathy compounds from Cinnamomum cassia. J. Ethnopharmacol. 2015;165:141–147. doi: 10.1016/j.jep.2015.01.049. [DOI] [PubMed] [Google Scholar]
- Yang Y.C., Mong M.C., Wu W.T., Wang Z.H., Yin M.C. Phytochemical profiles and anti-diabetic benefits of two edible Amaranthus species. CyTA - J. Food. 2020;18(1):94–101. [Google Scholar]
- Yasir M., Das S., Kharya M.D. The phytochemical and pharmacological profile of Persea americana Mill. Pharmacogn Rev. 2010;4(7):77–84. doi: 10.4103/0973-7847.65332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yobe M., Togenu F.B., Joyce O., Augustine U. Research article gas chromatographic characterization of aqueous saponin. Extract of Heinsia crinata Leaves. 2017;9(8):201–203. [Google Scholar]
- Yu H., Zhang P., Liu H., Sun X., Liang J., Sun L., et al. Hypoglycemic activity of Origanum vulgare L. and its main chemical constituents identified with HPLC-ESI-QTOF-MS. Food Funct. 2021;12:2580–2590. doi: 10.1039/d0fo03166f. [DOI] [PubMed] [Google Scholar]
- Zahid M., Mujahid M., Singh P.K., Farooqui S., Singh K., Parveen S., Arif M. Annona squamosa Linn. (custard apple): an aromatic medicinal plant fruit with immense nutraceutical and therapeutic potentials. Int. J. Pharma Sci. Res. 2018;9(5):1745–1759. [Google Scholar]
- Zaid H., Mahdi A.A., Tamrakar A.K., Saad B., Razzaque M.S., Dasgupta A. Natural active ingredients for diabetes and metabolism disorders treatment. Evidence-Based Complement Altern Med. 2016;2016 doi: 10.1155/2016/2965214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zengin G., Locatelli M., Stefanucci A., Macedonio G., Novellino E., Mirzaie S., et al. Chemical characterization, antioxidant properties, anti-inflammatory activity, and enzyme inhibition of Ipomoea batatas L. leaf extracts. Int. J. Food Prop. 2017;20(2):1907–1919. [Google Scholar]
- Zheng M.S., Liu Y.S., Yuan T., Liu L.Y., Li Z.Y., Huang X.L. [Research progress on chemical constituents of Citrullus colocynthis and their pharmacological effects] Zhongguo Zhongyao Zazhi. 2020;45(4):816–824. doi: 10.19540/j.cnki.cjcmm.20191104.201. [DOI] [PubMed] [Google Scholar]