Key Points
Question
Does antiplatelet therapy administered to critically ill patients with COVID-19 improve organ support–free days (a composite end point of in-hospital mortality and duration of intensive care unit–based respiratory or cardiovascular support) up to day 21?
Findings
In this bayesian randomized clinical trial that included 1557 patients, antiplatelet therapy with either aspirin or a P2Y12 inhibitor, compared with no antiplatelet therapy, resulted in a 95.7% posterior probability of futility with regard to the odds of improvement in organ support–free days within 21 days.
Meaning
Among critically ill patients with COVID-19, there was a low likelihood that treatment with an antiplatelet agent provided improvement in organ support–free days within 21 days.
Abstract
Importance
The efficacy of antiplatelet therapy in critically ill patients with COVID-19 is uncertain.
Objective
To determine whether antiplatelet therapy improves outcomes for critically ill adults with COVID-19.
Design, Setting, and Participants
In an ongoing adaptive platform trial (REMAP-CAP) testing multiple interventions within multiple therapeutic domains, 1557 critically ill adult patients with COVID-19 were enrolled between October 30, 2020, and June 23, 2021, from 105 sites in 8 countries and followed up for 90 days (final follow-up date: July 26, 2021).
Interventions
Patients were randomized to receive either open-label aspirin (n = 565), a P2Y12 inhibitor (n = 455), or no antiplatelet therapy (control; n = 529). Interventions were continued in the hospital for a maximum of 14 days and were in addition to anticoagulation thromboprophylaxis.
Main Outcomes and Measures
The primary end point was organ support–free days (days alive and free of intensive care unit–based respiratory or cardiovascular organ support) within 21 days, ranging from −1 for any death in hospital (censored at 90 days) to 22 for survivors with no organ support. There were 13 secondary outcomes, including survival to discharge and major bleeding to 14 days. The primary analysis was a bayesian cumulative logistic model. An odds ratio (OR) greater than 1 represented improved survival, more organ support–free days, or both. Efficacy was defined as greater than 99% posterior probability of an OR greater than 1. Futility was defined as greater than 95% posterior probability of an OR less than 1.2 vs control. Intervention equivalence was defined as greater than 90% probability that the OR (compared with each other) was between 1/1.2 and 1.2 for 2 noncontrol interventions.
Results
The aspirin and P2Y12 inhibitor groups met the predefined criteria for equivalence at an adaptive analysis and were statistically pooled for further analysis. Enrollment was discontinued after the prespecified criterion for futility was met for the pooled antiplatelet group compared with control. Among the 1557 critically ill patients randomized, 8 patients withdrew consent and 1549 completed the trial (median age, 57 years; 521 [33.6%] female). The median for organ support–free days was 7 (IQR, −1 to 16) in both the antiplatelet and control groups (median-adjusted OR, 1.02 [95% credible interval {CrI}, 0.86-1.23]; 95.7% posterior probability of futility). The proportions of patients surviving to hospital discharge were 71.5% (723/1011) and 67.9% (354/521) in the antiplatelet and control groups, respectively (median-adjusted OR, 1.27 [95% CrI, 0.99-1.62]; adjusted absolute difference, 5% [95% CrI, −0.2% to 9.5%]; 97% posterior probability of efficacy). Among survivors, the median for organ support–free days was 14 in both groups. Major bleeding occurred in 2.1% and 0.4% of patients in the antiplatelet and control groups (adjusted OR, 2.97 [95% CrI, 1.23-8.28]; adjusted absolute risk increase, 0.8% [95% CrI, 0.1%-2.7%]; 99.4% probability of harm).
Conclusions and Relevance
Among critically ill patients with COVID-19, treatment with an antiplatelet agent, compared with no antiplatelet agent, had a low likelihood of providing improvement in the number of organ support–free days within 21 days.
Trial Registration
ClinicalTrials.gov Identifier: NCT02735707
This randomized clinical trial assesses the effect of treatment with an antiplatelet agent vs no antiplatelet therapy on days alive and free of intensive care unit respiratory or cardiovascular organ support within 21 days among patients critically ill with COVID-19.
Introduction
Thrombotic events are common in patients hospitalized with COVID-19 and occur in spite of standard thromboprophylaxis, with critically ill patients being at highest risk.1,2,3,4,5,6,7 Thrombotic events have been reported in the venous, arterial, and microvascular circulations and are independently associated with poor outcomes.4 Vascular endothelial injury and inflammation activate intravascular coagulation through diverse mediators such as elevated fibrinogen, factor VIII, von Willebrand factor, platelet activation, impaired fibrinolysis, and reduced antithrombin.8
In a collaborative multiplatform trial that included the Randomized, Embedded, Multifactorial Adaptive Platform Trial for Community-Acquired Pneumonia (REMAP-CAP), therapeutic-dose heparin was found to improve organ support–free days in non–critically ill patients,9 but not in critically ill patients,10 hospitalized for COVID-19. Accordingly, despite a high occurrence of thrombosis, optimal antithrombotic strategies in critically ill patients remain unknown. Platelet activation has been implicated in the COVID-19 inflammatory response,11,12 and autopsies have shown microvascular thrombi with megakaryocyte and platelet-fibrin deposition in the setting of organ failure.13,14,15 In this trial, the effect of antiplatelet therapy (aspirin or P2Y12 inhibitor) on the composite of hospital survival and organ support provision for up to 21 days was evaluated in patients hospitalized with COVID-19.
Methods
Trial Design and Oversight
REMAP-CAP is an international, adaptive platform trial designed to iteratively determine best treatment strategies for patients with severe pneumonia in both pandemic and nonpandemic settings, and has reported on corticosteroids, anticoagulants, antivirals, interleukin 6 receptor antagonists, and convalescent plasma in patients with COVID-19.9,10,16,17,18,19 Patients eligible for the platform are assessed for eligibility and potentially randomized to 1 or more interventions across multiple domains. Domains encompass therapeutic areas and contain 2 or more interventions (including control). Details of the trial design have been reported previously20 and are available in the trial protocol and statistical analysis plan (Supplement 1). The trial was approved by relevant regional ethics committees and conducted in accordance with Good Clinical Practice guidelines and the principles of the Declaration of Helsinki.21 Written or oral informed consent, in accordance with regional legislation, was obtained from all patients or their surrogates. To account for the observed racial and ethnic differences in outcomes during the pandemic, this trial collected self-reported race and ethnicity data from either the participants or their surrogates via fixed categories appropriate to their region.
Participants
Patients admitted to the hospital, aged 18 years or older, with clinically suspected or microbiologically confirmed COVID-19 were eligible for enrollment. Patients admitted to an intensive care unit (ICU) and receiving respiratory or cardiovascular organ support were classified as critically ill and all others as non–critically ill. Respiratory organ support was defined as invasive or noninvasive mechanical ventilation including via high-flow nasal cannula if the flow rate was at least 30 L/min and the fraction of inspired oxygen was at least 0.4. Cardiovascular organ support was defined as receipt of vasopressors or inotropes. Exclusion criteria included presumption that death was imminent with lack of commitment to full support, clinical or laboratory-based bleeding risk sufficient to contraindicate antiplatelet therapy, creatinine clearance less than 30 mL/min or receipt of kidney replacement therapy, enrollment in an external trial of anticoagulation or antiplatelet therapy, or enrollment in the anticoagulation domain of the trial platform for participants older than 75 years. Patients were also excluded if they were already receiving antiplatelet therapy or nonsteroidal anti-inflammatory drugs (NSAIDs), if a clinical decision had been made to commence antiplatelet or NSAID therapy, or if a treating clinician believed that participation in the domain would not be in the best interests of a patient. Critically ill patients had to be enrolled within 48 hours of admission to an ICU. Patients were enrolled from 105 sites in 8 countries (Canada, France, Germany, India, Italy, Nepal, the Netherlands, and the United Kingdom). Additional platform and antiplatelet domain–specific exclusion criteria are listed in eAppendix 1 in Supplement 2.
Treatment Allocation
The antiplatelet domain included 3 groups to which patients could be assigned: aspirin, P2Y12 inhibitor, and no antiplatelet therapy (control). Each site’s clinical investigator team chose a priori at least 2 intervention groups, one of which had to be control, to which patients could be randomized. Sites that chose the P2Y12 inhibitor intervention further selected which P2Y12 inhibitor would be administered at their site (clopidogrel, prasugrel, or ticagrelor) according to availability and local preference. Patients were randomized via centralized computer program with allocation ratios dependent on the number of interventions at each site. Patients were initially randomized equally across the interventions available at each site. The domain also permitted variation in allocation ratios based on regular adaptive analysis. Randomization started on October 30, 2020. Response-adaptive randomization was applied on April 21, 2021 (see eFigure 1 in Supplement 2 for recruitment rates over time). Patients could be randomized to additional interventions within other domains, depending on domains active at the site, patient eligibility, and consent (see http://www.remapcap.org). Other aspects of care were provided per each site’s standard care.
Interventions
All antiplatelet interventions were administered enterally until study day 14 or hospital discharge, whichever occurred first. After 14 days, decisions regarding antiplatelet therapy were at the discretion of treating clinicians. Antiplatelet dosing was as follows: aspirin, 75 to 100 mg once daily; clopidogrel, 75 mg once daily without a loading dose; ticagrelor, 60 mg twice daily without a loading dose; prasugrel, a 60-mg loading dose followed by 10 mg daily (if aged <75 years and weight ≥60 kg) or 5 mg daily (if aged ≥75 years or weight <60 kg). Gastric acid suppression was recommended for patients receiving antiplatelet therapy through co-administration of either proton pump inhibitor or H2 receptor antagonist. Antiplatelet therapy could be discontinued if there was an adverse event or commenced in the control group if clinically warranted for a standard indication other than COVID-19. Patients received concurrent anticoagulation thromboprophylaxis according to standard care if not randomized in the anticoagulation domain of the trial.
Outcome Measures
The primary outcome was respiratory and cardiovascular organ support–free days to day 21. In this composite ordinal outcome, all deaths occurring during the index hospitalization were assigned the worst possible outcome (–1). Among survivors, respiratory and cardiovascular organ support–free days were calculated up to day 21 (survivors with no organ support were assigned a score of 22). Secondary outcomes were survival to day 90, progression to invasive mechanical ventilation, extracorporeal membrane oxygenation or death among those not receiving that support at baseline, vasopressor-/inotrope-free days, respiratory support–free days, duration of ICU stay, duration of hospital stay, serious adverse events, World Health Organization ordinal score for clinical improvement (ranging from 0 [no evidence of infection] to 8 [death]), major bleeding up to day 14 defined according to International Society of Hemostasis and Thrombosis criteria (see eAppendix 1 in Supplement 2 for details) including fatal and intracranial bleeding, venous thromboembolism (deep vein thrombosis, pulmonary embolism, and other venous thromboembolism), arterial thrombosis (cerebrovascular event, myocardial infarction, and other arterial thrombotic event), as well as a composite of thrombosis or death. Individual components of the above mentioned composite outcomes were also prespecified as secondary outcomes but are not analyzed individually in this report. Thrombotic outcomes and major bleeding events were centrally adjudicated in a blinded manner.
Sample Size Calculation
The trial was designed with no maximum sample size given the uncertainty of the pandemic. Sample size calculations for the primary outcome were performed using trial simulations of the adaptive design rules (see eFigure 2 in Supplement 2). The domain had at least 90% power to demonstrate superiority of an antiplatelet therapy to no antiplatelet therapy with 900 patients enrolled assuming an odds ratio effect size of 1.5. The cumulative type I error rate up to 3000 patients was less than 5%.
Statistical Analysis
The primary analysis was a bayesian cumulative logistic model, which calculated posterior probability distributions of organ support–free days (primary outcome) based on evidence accumulated in the trial and prior information. Prior distributions for treatment effects in critically and non–critically ill patients were nested in a hierarchical prior distribution centered on an overall intervention effect estimated with a neutral prior assuming no treatment effect (standard normal prior on the log odds ratio; see eFigure 2 in Supplement 2). The primary model estimated treatment effects for each intervention within each domain and prespecified treatment-by-treatment interactions. The primary model also adjusted for location (site nested within country), age (categorized into 6 groups), sex, and time period (2-week epochs). The model was fit using a Markov chain Monte Carlo algorithm that calculated the posterior distribution of the proportional odds ratios, including medians and 95% credible intervals (CrIs). The predefined statistical triggers for trial conclusions were (1) a superiority conclusion if there was greater than 99% posterior probability that an intervention was optimal compared with all other interventions; (2) an inferiority conclusion if there was less than 1% posterior probability that an intervention was optimal; (3) intervention efficacy if there was greater than 99% posterior probability that the odds ratio was greater than 1 compared with control; (4) intervention futility if there was greater than 95% posterior probability that the odds ratio was less than 1.2 compared with control; or (5) intervention equivalence if there was greater than 90% probability that the odds ratio (compared with each other) was between 1/1.2 and 1.2 for 2 noncontrol interventions.
On March 22, 2021, the equivalence trigger was reached for the primary outcome in critically ill patients for the aspirin and P2Y12 inhibitor groups. Randomization continued for these 2 antiplatelet groups, but for critically ill patients, the groups were statistically pooled and a single treatment effect relative to control was estimated for subsequent primary analysis. The 2 antiplatelet groups were not pooled for non–critically ill patients as no equivalence threshold had been reached. A prespecified interaction was modeled between antiplatelet therapy and therapeutic-dose heparin in the anticoagulation domain of the trial.
The primary analysis was conducted by an independent statistical analysis committee including all patients with COVID-19 randomized to any domain up to June 23, 2021 (and with complete follow-up for the primary outcome). Patients were analyzed in the groups to which they were originally randomized. There was no imputation of missing data for primary or secondary outcomes. Recruitment of non–critically ill patients was stopped due to slow recruitment and external evidence even though no statistical threshold for the primary outcome had been reached. The analysis of the results for non–critically ill patients is presented in eTables 2-4 in Supplement 2 for completeness.
Not all patients enrolled in the platform were eligible for all domains or interventions (dependent on active domains/interventions at the site, eligibility criteria, and patient/surrogate consent). Therefore, the analytical model included covariate terms reflecting randomization to each domain and the site, so that treatment effects were estimated only from patients who were concurrently randomized within the domain and directly comparing specific interventions available at each site. Patients enrolled outside the antiplatelet domain did not contribute to estimates of antiplatelet treatment effect but did contribute to the estimates of the covariate effects, providing the most robust estimation of covariate effects.16,20
Sensitivity and secondary analyses were performed by investigators blinded to ongoing interventions, so these analyses were restricted to data from patients enrolled in domains that were unblinded at the time of analysis with no adjustment for assignment in the ongoing domains. Treatment effects were also analyzed for aspirin and P2Y12 inhibitors compared with control separately. Prespecified sensitivity analyses included removing time and site effects from the model, as well as independent priors for the 2 antiplatelet treatments and alternative priors for interactions with other interventions. Secondary dichotomous outcomes were analyzed with bayesian logistic regression models. The secondary time-to-event outcomes (mortality and length of stay) were analyzed using a piecewise exponential bayesian model to estimate hazard ratio effects. No formal hypothesis tests were performed on secondary outcomes, and summaries of the posterior distributions and probabilities are provided for descriptive purposes only. Prespecified subgroup analyses included baseline mechanical ventilation status, age category (<50, 50-70, or ≥70 years), and baseline anticoagulation dose (defined in eTable 1 in Supplement 2). In a post hoc analysis, the baseline anticoagulation categories were collapsed into 2 categories, therapeutic dose and less than therapeutic dose. If the dose was not recorded, it was included in an unknown-dose category. Further details of all analyses are provided in eAppendix 1 in Supplement 2 and in the statistical analysis plan (Supplement 1). Data management and summaries were created using R version 3.6.0; the primary analysis was computed in R version 4.0.0 using the rstan package version 2.21.1. Additional data management and analyses were performed in SQL 2016, SPSS version 26, and Stata version 14.2.
Results
Enrollment and Participant Characteristics
The first patient was enrolled into the antiplatelet domain on October 30, 2020. On March 22, 2021, the prespecified equivalence trigger for the aspirin and P2Y12 inhibitor groups (compared with each other) was reached in critically ill patients with 1016 patients enrolled with complete data (P2Y12 inhibitor to aspirin odds ratio, 1.00 [95% CrI, 0.80-1.23]; 90.1% posterior probability of equivalence). These groups continued to enroll separately but were subsequently statistically pooled into a combined antiplatelet group for all further adaptive analyses. On June 24, 2021, enrollment was discontinued after an adaptive analysis demonstrated that the prespecified stopping criterion for futility had been reached in critically ill patients, and patient follow-up continued until July 26, 2021. At that time, 1557 critically ill and 267 non–critically ill patients had been enrolled and randomized (Figure 1). Of these, 8 critically ill patients and 1 non–critically ill patient withdrew consent, and outcome data were not available for 17 critically ill and 3 non–critically ill patients. For non–critically ill patients, based on slow enrollment rates and external data,22 the international trial steering committee decided to simultaneously stop enrollment. Results for non–critically ill patients are shown in eAppendix 2 and eTables 2-4 in Supplement 2.
Figure 1. Flow of Participants in the COVID-19 Antiplatelet Domain of the REMAP-CAP Randomized Clinical Trial.
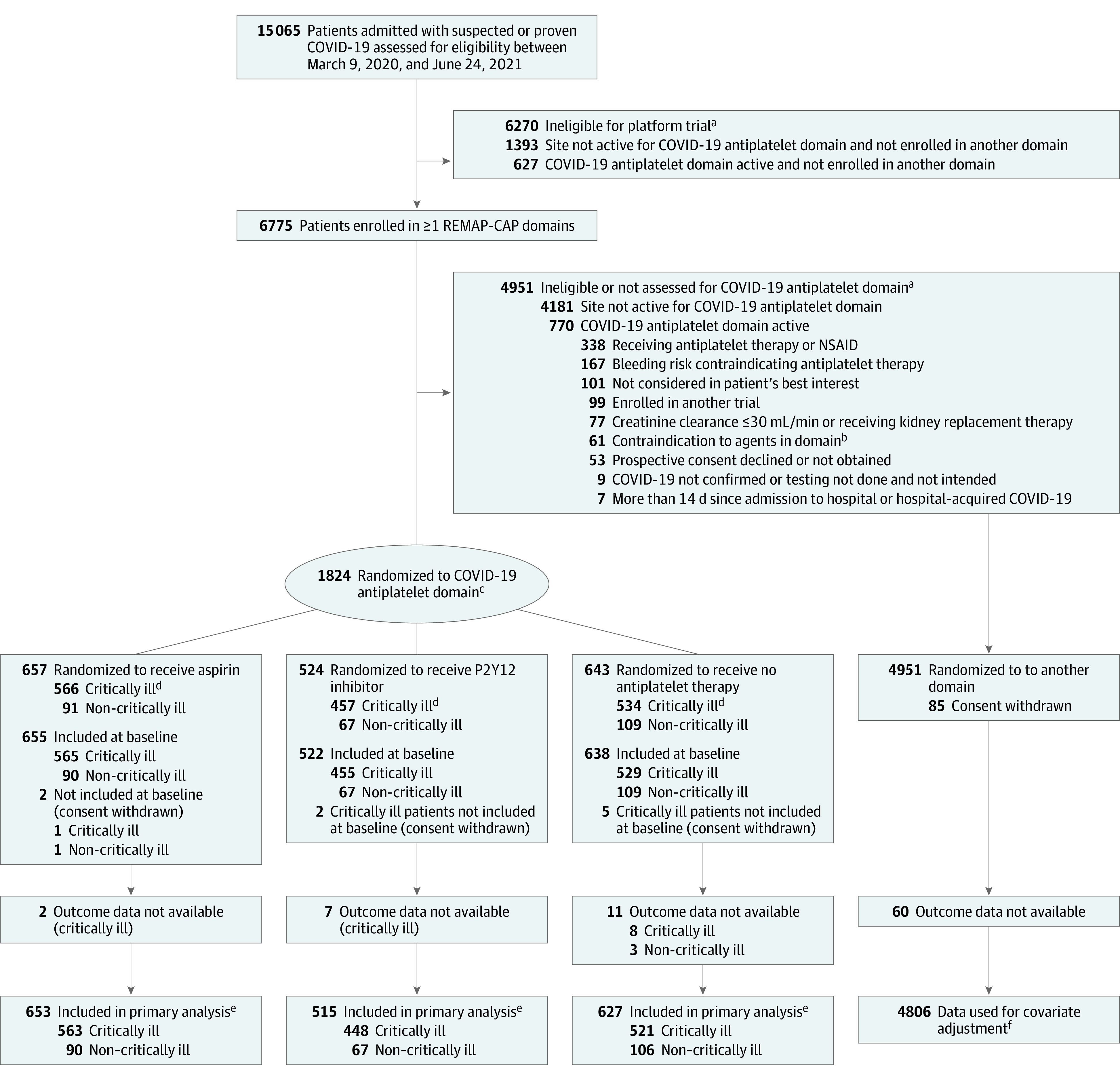
NSAID indicates nonsteroidal anti-inflammatory drug; REMAP-CAP, Randomized, Embedded, Multifactorial Adaptive Platform Trial for Community-Acquired Pneumonia. As a platform trial with a single master protocol (Supplement 1) and multiple treatments evaluated simultaneously, this trial applied eligibility criteria at the platform level and at the domain level. Patients had to be eligible for both platform and domain to be randomized. With an adaptive platform design, it allowed treatments to be stopped for futility, to declare 1 or more treatments to be superior, or to add new treatments or whole therapeutic areas during the course of the trial. A domain refers to a common therapeutic area (eg, antiviral therapy or immunoglobulin therapy) within which several interventions or intervention dosing strategies could be randomly assigned.
aPatients could meet more than 1 ineligibility criterion.
bContraindications to antiplatelet agents, including hypersensitivity to an antiplatelet agent specified as an intervention, excluded patients from receiving that antiplatelet intervention; known or suspected pregnancy excluded patients from the P2Y12 inhibitor intervention; administration or intention to administer lopinavir/ritonavir excluded patients from receiving a P2Y12 inhibitor at sites that were using clopidogrel and ticagrelor.
cParticipants were randomized via a centralized computer program to each intervention starting with balanced assignment and then adapted with preferential assignment to interventions that appeared most favorable until predefined statistical triggers of superiority or futility were met.
dCritically ill patients were required to have at least 1 of high-flow nasal cannula oxygenation, invasive or noninvasive mechanical ventilation, or vasopressor or inotropic infusion.
eResults for non–critically ill patients were used for borrowing within the primary model, meaning that results for non–critically ill patients were partially pooled with critically ill patients in the primary analysis. This partial pooling provides a more precise estimate of the treatment effect of antiplatelet therapy in critically ill patients if the observed data in the 2 groups are similar.
fThe primary analysis of alternative interventions within the antiplatelet domain is estimated from a model that adjusts for patient factors and for assignment to other interventions; all patients enrolled in the COVID-19 cohort for whom consent was obtained and follow-up data were available are included. The final estimate of an antiplatelet domain intervention’s effectiveness relative to any other within that domain is generated from patients who might have been randomized to either.
Baseline characteristics were comparable between the intervention groups (Table 1; eTable 2 in Supplement 2).
Table 1. Baseline Characteristics of Critically Ill Participantsa.
| Characteristics | Aspirin (n = 565) | P2Y12 inhibitors (n = 455) | Control (n = 529)b |
|---|---|---|---|
| Age, median (IQR), y | 57.0 (48.0-64.0) | 57.0 (49.0-65.0) | 57.0 (48.0-63.0) |
| Sex, No. (%) | |||
| Female | 199 (35.2) | 139 (30.5) | 183 (34.6) |
| Male | 366 (64.8) | 316 (69.5) | 346 (65.4) |
| Race and ethnicity, No./total (%)c | |||
| Asian | 46/460 (10.0) | 36/372 (9.7) | 58/419 (13.8) |
| Black | 16/460 (3.5) | 9/372 (2.4) | 16/419 (3.8) |
| Multiracial | 19/460 (4.1) | 5/372 (1.3) | 10/419 (2.4) |
| White | 352/460 (76.5) | 307/372 (82.5) | 309/419 (73.7) |
| Other | 27/460 (5.9) | 15/372 (4.0) | 26/419 (6.2) |
| Body mass index, median (IQR)d | 31.7 (27.4-37.6) | 31.3 (26.9-37.2) | 31.1 (27.0-35.9) |
| APACHE II score, median (IQR)e | 12.0 (8.0-17.0) | 12.0 (8.0-18.0) | 12.0 (8.0-17.0) |
| Confirmed SARS-CoV-2 infection, No./total (%)f | 505/519 (97.3) | 400/415 (96.4) | 465/477 (97.5) |
| Preexisting condition, No./total (%)g | |||
| Diabetes | 134/562 (23.8) | 93/449 (20.7) | 112/521 (21.5) |
| Respiratory disease | 113/562 (20.1) | 88/449 (19.6) | 97/522 (18.6) |
| Kidney disease | 16/528 (3.0) | 18/416 (4.3) | 18/485 (3.7) |
| Severe cardiovascular disease | 18/552 (3.3) | 23/437 (5.3) | 26/517 (5.0) |
| Any immunosuppressive condition | 23/562 (4.1) | 19/449 (4.2) | 23/522 (4.4) |
| Time to enrollment, median (IQR) | |||
| From hospital admission, d | 1.5 (0.9-3.0) | 1.8 (1.0-3.5) | 1.8 (0.9-3.7) |
| From intensive care unit admission, h | 17.2 (9.8-22.4) | 17.9 (12.0-23.5) | 18.2 (10.7-23.9) |
| Acute respiratory support, No. (%) | |||
| Invasive mechanical ventilation | 213 (37.7) | 161 (35.4) | 194 (36.7) |
| Noninvasive ventilation only | 227 (40.2) | 173 (38.0) | 202 (38.2) |
| High-flow nasal cannula | 125 (22.1) | 121 (26.6) | 132 (25.0) |
| None/supplemental oxygen | 0 | 0 | 1 (0.2) |
| Pao2/Fio2, median (IQR) | 115 (86-148) [n = 537] | 118 (90-163) [n = 430] | 113 (89-147) [n = 495] |
| Vasopressor support, No. (%) | 121 (21.4) | 72 (15.8) | 88 (16.6) |
| Laboratory values, median (IQR)h | |||
| C-reactive protein, μg/mL | 118 (62-180) [n = 486] | 109 (59-179) [n = 411] | 113 (60-180) [n = 452] |
| D-dimer, μg/mL | 972 (500-2599) [n = 334] | 850 (386-2297) [n = 279] | 898 (490-2705) [n = 307] |
| D-dimer ratioi | 2.5 (1.3-7.6) [n = 213] | 2.1 (1.3-5.1) [n = 60] | 2.2 (1.3-6.6) [n = 234] |
| Platelet count, ×109/L | 251 (194-313) [n = 547] | 239 (184-312) [n = 444] | 253 (196-327) [n = 513] |
| Concomitant therapies within 48 h of randomization, No./total (%) | |||
| Steroids | 552/562 (98.2) | 433/448 (96.7) | 511/521 (98.1) |
| Remdesivir | 113/562 (20.1) | 87/448 (19.4) | 126/521 (24.2) |
| Tocilizumab | 248/562 (44.1) | 201/448 (44.9) | 217/521 (41.7) |
| Sarilumab | 66/562 (11.7) | 45/448 (10.0) | 54/521 (10.4) |
| Concurrent anticoagulant type, No./total (%) | |||
| Low-molecular-weight heparin | 512/522 (98.1) | 395/408 (96.8) | 480/489 (98.2) |
| Unfractionated heparin | 8/522 (1.5) | 8/408 (2.0) | 8/489 (1.6) |
| Direct oral anticoagulants | 2/522 (0.4) | 5/408 (1.2) | 1/489 (0.2) |
| Anticoagulant dose, No. (%) | |||
| Low prophylactic | 112 (19.8) | 87 (19.1) | 75 (14.2) |
| Intermediate prophylactic | 312 (55.2) | 214 (47.0) | 312 (59.0) |
| Subtherapeutic | 22 (3.9) | 21 (4.6) | 24 (4.5) |
| Therapeutic | 54 (9.6) | 65 (14.3) | 56 (10.6) |
| Unknown | 65 (11.5) | 68 (14.9) | 62 (11.7) |
Abbreviation: Pao2/Fio2, ratio of partial pressure of arterial oxygen to fraction of inspired oxygen.
Percentages may not sum to 100 because of rounding.
The control group include all patients randomized to control who were also eligible to be randomized to an antiplatelet agent.
Data collection was not approved in Asia, Canada, and continental Europe. “Other” includes Māori, Aboriginal, Pacific Islander, and “other ethnic group.” Participants (or their surrogates) self-reported their race and ethnicity via fixed categories appropriate to their region. A patient may decline to provide their race or ethnicity at the time of registration, and the person performing the registration may decline to ask the patient to clarify race or ethnicity at the time of registration.
Body mass index is calculated as weight in kilograms divided by the square of height in meters.
The Acute Physiology and Chronic Health Evaluation (APACHE) II measures severity of illness based on age, medical history, and physiologic variables. The score ranges from 0 to 71, with higher scores indicating more severe disease and a higher risk of death; eg, an APACHE II score of 12 indicates a 15% probability of mortality in a medical patient admitted for a respiratory condition (outside of COVID-19). The median score of 12 is typical for COVID-19 patients admitted to intensive care units in England, Wales, and Northern Ireland. However, the hospital mortality rate for the 261 patients in this study’s current data with an APACHE II score of 12 is 30.1% (95% CI, 24.0%-36.2%).
SARS-CoV-2 infection was confirmed by respiratory tract polymerase chain reaction test.
Kidney disease was determined from the most recent serum creatinine level prior to this hospital admission, except in patients who were receiving dialysis. Abnormal kidney function was defined as a creatinine level of 130 μmol/L (1.5 mg/dL) or greater for men or 100 μmol/L (1.1 mg/dL) or greater for women not previously receiving dialysis. Cardiovascular disease was defined as New York Heart Association class IV symptoms. Immunosuppression was defined by receipt of recent chemotherapy, radiation, high-dose or long-term steroid treatment, or presence of immunosuppressive disease.
Laboratory results were available when captured for clinical care.
Given variability in clinical assays, relative D-dimer is reported, calculated as the ratio of the measured D-dimer to the local site’s upper limit of normal.
The median duration of antiplatelet therapy for critically ill patients randomized to receive aspirin was 12 (IQR, 7-14) days (data available for 560/565), and for those receiving a P2Y12 inhibitor the median duration was 11 (IQR, 6-14) days (data available for 433/455). Among 455 participants allocated to receive a P2Y12 inhibitor, 403 (88.5%) received clopidogrel, 6 (1.3%) received ticagrelor, 6 (1.3%) received prasugrel, and in 40 (8.8%) the P2Y12 inhibitor administered was unknown (for these remaining patients, site choice was clopidogrel for 13, ticagrelor for 17, prasugrel for 4, and unknown for 6). All patients with data available (n = 1419) received concurrent thromboprophylaxis according to usual care at the site or were concomitantly enrolled in the platform anticoagulation study. The most frequent concurrent anticoagulant at baseline was low-molecular-weight heparin (97.7%), and the most frequent dose was an intermediate dose (59%) (see eTable 1 in Supplement 2 for anticoagulation dose classification).
Primary Outcome
Among critically ill participants, the median number of organ support–free days was 7 (IQR, –1 to 16) in both the pooled antiplatelet and control groups. The median adjusted odds ratio for the effect of antiplatelet therapy compared with control was 1.02 (95% CrI, 0.86-1.23), yielding a posterior probability of futility of 95.7% (Table 2 and Figure 2). The proportions of patients surviving to hospital discharge were 71.5% (723/1011) and 67.9% (354/521) in the antiplatelet and control groups, respectively, yielding a median adjusted odds ratio for hospital survival of 1.27 (95% CrI, 0.99-1.62), with an adjusted absolute difference of 5% (95% CrI, −0.2% to 9.5%) and a posterior probability of efficacy of 97.0% for antiplatelet therapy compared with control. The median number of organ support–free days in survivors was 14 days in both groups (IQRs, 4-17 days in the antiplatelet group and 6.25-18 days in the control group). Patients with missing data for organ support–free days were excluded from the primary analysis (8/529 [1.5%] in the control group and 9/1020 [0.9%] in the pooled antiplatelet group). The secondary analysis of separate treatment effects of aspirin and P2Y12 inhibitors on the primary outcome are shown in Table 2.
Table 2. Primary and Selected Secondary Outcomes of Critically Ill Participants.
| Outcomes | Pooled antiplatelets (n = 1020) | Aspirin (n = 565)a | P2Y12 inhibitors (n = 455)a | Control (n = 529) |
|---|---|---|---|---|
| Organ support–free days to day 21b | ||||
| No. of patients with known outcome | 1011 | 563 | 448 | 521 |
| Median (IQR), d | 7 (–1 to 16) | 8 (–1 to 16) | 7 (–1 to 16) | 7 (–1 to 16) |
| Adjusted proportional odds ratio (95% CrI) | 1.02 (0.86-1.23) | 1.05 (0.85-1.30) | 1.00 (0.80-1.27) | 1 [Reference] |
| Probability of futility, % | 95.7 | 88.6 | 93.4 | |
| Probability of efficacy, % | 58.0 | 66.5 | 51.8 | |
| Survival to hospital discharge | ||||
| No./total (%) | 723/1011 (71.5) | 402/563 (71.4) | 321/448 (71.7) | 354/521 (67.9) |
| Adjusted odds ratio (95% CrI) | 1.27 (0.99-1.62) | 1.30 (0.97-1.72) | 1.18 (0.86-1.62) | 1 [Reference] |
| Adjusted absolute risk difference, % (95% CrI) | 5.0 (–0.2 to 9.5) | 5.4 (–0.7 to 10.5) | 3.5 (–3.4 to 9.5) | |
| Probability of efficacy, % | 97.0 | 96.0 | 85.8 | |
| Thrombotic events or deathc,d | ||||
| No./total (%) with | ||||
| Venous thrombotic event | 87/998 (8.7) | 54/563 (9.6) | 33/448 (7.4) | 56/516 (10.9) |
| Arterial thrombotic event | 37/996 (3.7) | 22/556 (4.0) | 15/440 (3.4) | 12/513 (2.3) |
| Any thrombotic event | 112/996 (11.2) | 69/556 (12.4) | 43/440 (9.8) | 65/513 (12.7) |
| Death in hospital | 288/1011 (28.5) | 161/563 (28.6) | 127/448 (28.4) | 167/521 (32.1) |
| Thrombotic events or death | 355/1011 (35.1) | 204/563 (37.0) | 151/448 (36.2) | 212/521 (40.7) |
| Adjusted odds ratio for composite of death and/or thrombosis (95% CrI) | 0.70 (0.54-0.90) | 0.69 (0.52-0.93) | 0.73 (0.53-0.99) | 1 [Reference] |
| Adjusted absolute risk difference, % (95% CrI) | –8.2 (–13.7 to –2.5) | –8.6 (–14.4 to –1.7) | –7.3 (–14.0 to –0.2) | |
| Probability of efficacy, % | 99.7 | 99.3 | 98.0 | |
| Major bleedingc,e | ||||
| No./total (%) | 21/1002 (2.1) | 11/559 (2.0) | 10/443 (2.3) | 2/517 (0.4) |
| Adjusted odds ratio (95% CrI) | 2.97 (1.23-8.28)f | 2.34 (0.93-5.93) | 2.50 (0.95-6.56) | 1 [Reference] |
| Adjusted absolute risk difference, % (95% CrI) | 0.8 (0.1-2.7)f | 0.5 (0.0-1.9) | 0.6 (0.0-2.1) | |
| Probability of harm, % | 99.4f | 96.5 | 96.9 |
Abbreviation: CrI, credible interval.
The analysis of individual treatment effects was a secondary analysis conducted in the unblinded patient population (ie, excluding covariate adjustments for ongoing interventions).
Composite ordinal scale consisting of survival to hospital discharge and days free of organ support to day 21. An odds ratio greater than 1 indicates a benefit of treatment. Probabilities of efficacy (proportional odds ratio >1), harm (proportional odds ratio <1), and futility (proportional odds ratio <1.2) are computed from the posterior distribution. Dynamic borrowing was used across illness severity (ie, critically and non–critically ill patients), whereby like treatment effects are shrunk together based on their degree of similarity. Accordingly, observations about treatment effect are shared between groups.
An odds ratio greater than 1 indicates a harm of treatment. Probabilities of benefit (odds ratio <1) and harm (odds ratio >1) are computed from the posterior distribution.
Thrombotic events include pulmonary embolism, myocardial infarction, ischemic cerebrovascular event, systemic arterial thromboembolism, and deep venous thrombosis.
Major bleeding (according to International Society of Hemostasis and Thrombosis definition) is defined as fatal bleeding, symptomatic or clinically manifest bleeding in a critical area or organ (such as intracranial, intraspinal, intraocular, retroperitoneal, intra-articular, pericardial, or intramuscular with compartment syndrome), or bleeding causing a decrease in hemoglobin of 2 g/dL or greater or leading to transfusion of 2 or more whole blood or red blood cell units.
Summaries are based on the post hoc analysis of major bleeding estimating a pooled antiplatelet treatment effect.
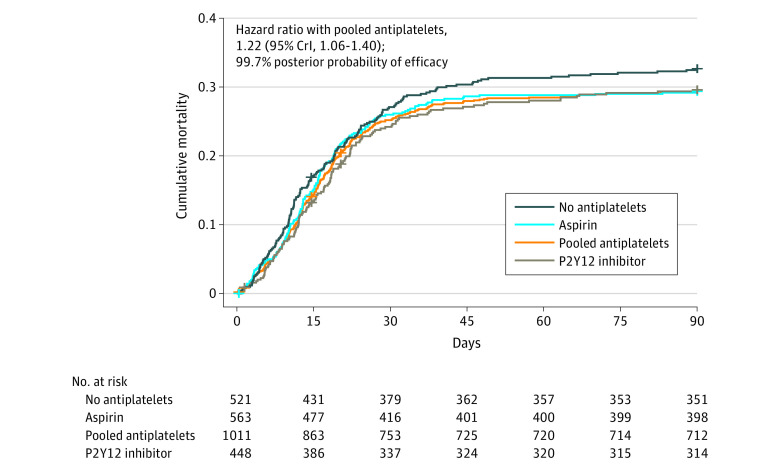
Figure 2. Primary Outcome: Organ Support–Free Days Up to Day 21 in Critically Ill Patients.

A, Distributions of organ support–free days (days alive and free of intensive care unit–based organ support) up to day 21 in critically ill patients. The ordinal scale includes in-hospital death (the worst possible outcome) and the numbers of days alive without organ support. The curves represent the cumulative proportion (y-axis) for each group by day (x-axis), and the bars represent the proportion (y-axis) for each group by day (x-axis). Curves that increase more slowly are more favorable. The difference in the height of the 2 curves at any point represents the difference in the cumulative probability of having a value for days without organ support of less than or equal to that point on the x-axis. B, Organ support–free days as horizontally stacked proportions by intervention group. Red represents worse values and blue represents better values; the deepest red is death and deepest blue is 21 organ support–free days. The primary analysis compared organ support–free days in the control (no antiplatelet therapy) group with the pooled aspirin and P2Y12 inhibitor groups. Accordingly, the distribution of organ support–free days is shown for the pooled antiplatelet group, separate aspirin and P2Y12 inhibitor groups, and control (no antiplatelets) group.
Secondary Outcomes
Select secondary outcomes are shown in Table 2 and others are shown in eAppendix 2 and eTable 5 in Supplement 2. The effect of antiplatelet therapy on survival over 90 days is shown in Figure 3, with a median adjusted hazard ratio of 1.22 (95% CrI, 1.06-1.40) and 99.7% posterior probability of improved survival of the pooled antiplatelet group compared with control. Five patients were censored before 90 days (1 in the control group, 1 in the aspirin group, and 3 in the P2Y12 inhibitor group). The estimated mortality rate at 90 days for the control group was 32.7% (95% CI, 28.5%-36.6%) and for the pooled antiplatelet group was 29.5% (95% CI, 26.6%-32.2%) (Figure 3). Thrombotic event frequencies alongside mortality and major bleeding data are provided in Table 2, and other secondary outcomes are shown in eTable 5 in Supplement 2. Major bleeding occurred in 21 of 1002 participants (2.1%) in the pooled antiplatelet group and in 2 of 517 participants (0.4%) in the control group. An analysis of major bleeding comparing the pooled antiplatelet group with control showed an adjusted odds ratio of 2.97 (95% CrI, 1.23-8.28) and an adjusted absolute risk difference of 0.8% (95% CrI, 0.1%-2.7%), with a posterior probability of harm of 99.4%. The separate treatment effects of aspirin and P2Y12 inhibitors on thrombotic and bleeding rates are presented in Table 2.
Figure 3. Survival Through 90 Days in Critically Ill Patients.
Kaplan-Meier curve of 90-day all-cause mortality in critically ill patients. Patients who survived to 90 days were censored at day 90 with no event. The pooled antiplatelet group is the composite of patients in the aspirin and P2Y12 inhibitor groups. A hazard ratio greater than 1 represents improved survival. The hazard ratio for aspirin is 1.19 (95% credible interval [CrI], 1.00-1.42; 97.5% posterior probability of efficacy) and the hazard ratio for P2Y12 inhibitors is 1.23 (95% CrI, 1.02-1.49; 98.7% posterior probability of efficacy).
Adverse Events
Serious adverse events were reported in 5 of 565 (0.9%), 4 of 455 (0.9%), and 3 of 529 (0.6%) participants in the aspirin, P2Y12 inhibitor, and control groups, respectively (eTable 6 in Supplement 2).
Subgroup Analyses and Interactions
The prespecified subgroup analyses for critically ill patients by age, baseline use of mechanical ventilation, and anticoagulant dose are presented in eTable 7 in Supplement 2. In the prespecified interaction analysis of patients co-enrolled in the antiplatelet domain and the therapeutic anticoagulant domain (n = 122 critically ill patients), the odds ratio for the combination of antiplatelet therapy and therapeutic-dose heparin anticoagulation, compared with no antiplatelet therapy and standard thromboprophylaxis, was 0.73 (95% CrI, 0.44-1.21) for organ support–free days and 0.72 (95% CrI, 0.41-1.28) for hospital survival. The odds ratio for the interaction of antiplatelet therapy and therapeutic-dose heparin anticoagulation was 0.79 (95% CrI, 0.50-1.30) for organ support–free days and 0.64 (95% CrI, 0.39-1.05) for hospital survival (eTable 8 in Supplement 2).
Post Hoc Analysis
A post hoc subgroup analysis of hospital mortality according to baseline concomitant anticoagulation dose (randomized and usual care; n = 1360 with a known dose of anticoagulation therapy) demonstrated that for patients receiving therapeutic-dose anticoagulation (n = 179), the adjusted odds ratio for hospital survival of antiplatelet therapy compared with control was 0.63 (95% CrI, 0.31-1.28; 89.9% probability that antiplatelet therapy led to harm in this context). In contrast, for patients receiving anticoagulation doses lower than therapeutic (n = 1181), the adjusted odds ratio for hospital survival was 1.33 (95% CrI, 0.99-1.79; 97.1% probability that pooled antiplatelet therapy improved hospital survival in this context) (eTable 9 and eFigure 3 in Supplement 2). For patients missing data on a baseline concomitant anticoagulation dose (n = 162), the adjusted odds ratio of hospital survival for antiplatelet therapy compared with control was 1.09 (95% CrI, 0.53-2.17).
Discussion
Among critically ill patients with COVID-19, treatment with an antiplatelet agent, compared with no antiplatelet treatment, had a low likelihood of providing improvement in the number of organ support–free days within 21 days.
Thrombotic complications are common in patients admitted to the hospital with COVID-19 in spite of conventional thromboprophylaxis among critically ill patients at highest risk. Macrovascular thrombosis occurs within venous and arterial circulations and microvascular thrombi contribute to organ dysfunction, including acute respiratory distress syndrome. The pathogenesis of thrombosis in COVID-19 is intimately linked with the inflammatory response to the virus, endothelial infection, activation, and injury, as well as hypercoagulability.6,23,24,25,26,27,28,29,30,31,32,33 Recognition that thrombosis is a key contributor to clinical deterioration and death in COVID-19 has led to global interest in whether enhanced antithrombotic treatments or extended duration improves patient outcomes.34 It was recently reported that therapeutic-dose heparin improves organ support–free days in hospitalized non–critically ill patients.9 Results from 2 subsequent randomized clinical trials have also supported the role of therapeutic-dose heparin in this cohort.35,36 In contrast, in critically ill patients, therapeutic-dose heparin did not improve outcomes, with a high probability of harm.10 The INSPIRATION trial also failed to demonstrate benefit of intermediate-dose heparin compared with a conventional low dose in this critically ill patient group.37 The factor Xa inhibitor rivaroxaban at a therapeutic dose for an extended duration, including postdischarge (30 days postrandomization), was not beneficial in a mixed population of patients with mild, moderate, and severe COVID-19.38 Therefore, efficacy of antithrombotic agents may vary by mechanism of action, illness severity, dose, and duration.
Platelets are activated and hyperaggregable in patients with COVID-19.11,12 Activated platelets reciprocally upregulate systemic inflammation, and therefore, platelet inhibition may have antithrombotic and anti-inflammatory benefits.19,39 Observational data support an association between antiplatelet therapy and reduced lung injury, ICU requirement, and mortality, without increased bleeding.39,40 Accordingly, this trial evaluated antiplatelet treatments in patients hospitalized for COVID-19, stratified by baseline illness severity.
In this trial, antiplatelet therapy met the prespecified criterion for futility in critically ill patients based on very similar outcomes for organ support–free days compared with control. However, in critically ill patients, there was a 97% probability that antiplatelet therapy improved survival to hospital discharge, with an adjusted absolute reduction in mortality of 5% and a 99.7% probability that it improved survival over 90 days. As recruitment occurred in 8 countries and antiplatelet therapy is inexpensive, widely available, and easy to administer and dose, these results are expected to have global applicability. The reduction in mortality was counterbalanced by an increase in the number of patients receiving short durations of organ support (<6 days), resulting in an overall net neutral effect on the outcome of organ support–free days. It is possible that antiplatelet therapy may reduce fatal complications of COVID-19 in critically ill patients while potentially increasing the need for organ support, possibly through bleeding that may or may not be clinically evident, such as alveolar hemorrhage.41 Major bleeding occurred more frequently in patients randomized to antiplatelet therapy. It is also possible that the net neutral effect of antiplatelet therapy on organ support–free days may have been influenced by a harmful interaction between antiplatelet therapy and therapeutic-dose anticoagulation, whereby patients receiving the combination appeared to have worse outcomes. No substantive effects of antiplatelet therapy on organ support–free days were observed in non–critically ill patients.
In the RECOVERY trial, 28-day mortality was not different in patients allocated to receive aspirin compared with control (17% in both groups), although a slightly higher proportion of patients were discharged from the hospital alive within 28 days (75% vs 74%; rate ratio, 1.06; 95% CI, 1.02-1.10; P = .006).22 Compared with the current trial, the majority of patients recruited to the RECOVERY trial were non–critically ill, and among those receiving noninvasive or invasive ventilation, the relative risk for 28-day mortality was 0.95 (95% CI, 0.87-1.03) with aspirin treatment (mortality in the usual-care group, 29.9% [750/2505]; mortality in the aspirin group, 28.4% [685/2415]). Compared with RECOVERY, other differences in this trial included the recommendation for gastric protection, exclusion of patients at elevated risk of bleeding (including those with severe kidney failure), and lower aspirin dose.
The recently published ACTIV-4a trial of P2Y12 inhibition in non–critically ill patients with COVID-19 (n = 562) demonstrated that addition of P2Y12 inhibition to therapeutic-dose heparin did not increase the odds of improvement in days alive and free of cardiovascular or respiratory organ support within 21 days (adjusted odds ratio, 0.83; 96% posterior probability of futility) compared with usual-care therapeutic anticoagulation.42,43 That trial differed from the REMAP-CAP antiplatelet intervention trial in that the cohort was non–critically ill, P2Y12 inhibition was provided in combination with therapeutic-dose anticoagulation, and the predominant P2Y12 inhibitor was ticagrelor, a more potent agent than clopidogrel. The ACTIV-4a trial testing P2Y12 inhibitors alongside prophylactic-dose heparin for critically ill patients is ongoing (NCT04505774).
In critically ill patients, although major bleeding was more common in patients allocated to antiplatelet therapy (aspirin [2.0%] or P2Y12 inhibitor [2.1%]) compared with control (0.4%), these absolute frequencies appear lower than those reported in observational studies5,6 but are consistent with recently reported anticoagulation study results (in critically ill patients, major bleeding occurred in 2.3% allocated to standard-of-care thromboprophylaxis and 3.8% in those allocated to therapeutic-dose heparin).10 The relatively low major bleeding rates may reflect exclusion of patients at higher bleeding risk, underascertainment, or collection of data only for major bleeding events that met the International Society of Hemostasis and Thrombosis criteria.
Limitations
The trial has several limitations. First, it used an open-label design, although the primary outcome of survival and need for organ support was selected to minimize bias. Second, the use of a composite outcome has the potential to identify different effects of treatment on each component. Although each component is reported separately, there is limited power to give definitive answers about the effect of treatment on each component. Third, although there was an estimated effect of the combination of antiplatelet therapy with therapeutic-dose anticoagulation, the limited numbers of patients randomized simultaneously to both treatment domains and the wide 95% CrIs limit definitive conclusions. Fourth, results from 4 antiplatelet agents were pooled in the analyses (although very few patients received ticagrelor or prasugrel), and there was also substantial underlying heterogeneity in anticoagulation regimens used, limiting ability to draw firm conclusions for any given combination.
Conclusions
Among critically ill patients with COVID-19, treatment with an antiplatelet agent, compared with no antiplatelet agent, had a low likelihood of providing improvement in the number of organ support–free days within 21 days.
Trial Protocol and Statistical Analysis Plan
List of REMAP-CAP Investigators
eAppendix 1. Supplementary Methods
eTable 1. Categories of Anticoagulation Intensity According to Anticoagulant and Dose
eAppendix 2. Supplementary Results
eTable 2. Participant Characteristics at Baseline
eTable 3. Primary and Secondary Analyses of Non-Critically Ill Patients
eTable 4. Serious Adverse Events, Major Bleeding, and Thrombotic Events in Non-Critically Ill Patients
eTable 5. Other Secondary Outcomes for Critically Ill Patients
eTable 6. Serious Adverse Events, Major Bleeding, and Thrombotic Events in Critically Ill Patients
eTable 7. Organ Support Free Days (OSFD) Within 21 Days by Age, Mechanical Ventilation Status and Baseline Anticoagulation Dose in Critically Ill Patients
eTable 8. Treatment Effects on Organ Support-Free Days and Hospital Survival Among Critically Ill Patients Enrolled in the Antiplatelet Domain and/or Therapeutic Anticoagulation Domain
eTable 9. In-Hospital Survival and Organ Support by Differential Anticoagulant Dose Given (Randomized and Clinical Decision) in Critically Ill Patients
eFigure 1. Patient Enrolment by Intervention and Time-Period
eFigure 2. Distributions of OSFD Odds Ratio for Pooled Antiplatelet
eFigure 3. Interaction Between Antiplatelet Allocation and Anticoagulation Dose for OSFD
eReferences
Nonauthor Collaborators. REMAP-CAP Investigators
Data Sharing Statement
References
- 1.Bikdeli B, Madhavan MV, Jimenez D, et al. ; Global COVID-19 Thrombosis Collaborative Group . COVID-19 and thrombotic or thromboembolic disease: implications for prevention, antithrombotic therapy, and follow-up: JACC state-of-the-art review. J Am Coll Cardiol. 2020;75(23):2950-2973. doi: 10.1016/j.jacc.2020.04.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Helms J, Tacquard C, Severac F, et al. ; Clinical Research in Intensive Care and Sepsis Trial Group for Global Evaluation and Research in Sepsis . High risk of thrombosis in patients with severe SARS-CoV-2 infection: a multicenter prospective cohort study. Intensive Care Med. 2020;46(6):1089-1098. doi: 10.1007/s00134-020-06062-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Klok FA, Kruip MJHA, van der Meer NJM, et al. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thromb Res. 2020;191:145-147. doi: 10.1016/j.thromres.2020.04.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bilaloglu S, Aphinyanaphongs Y, Jones S, Iturrate E, Hochman J, Berger JS. Thrombosis in hospitalized patients with COVID-19 in a New York City health system. JAMA. 2020;324(8):799-801. doi: 10.1001/jama.2020.13372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Al-Samkari H, Karp Leaf RS, Dzik WH, et al. COVID-19 and coagulation: bleeding and thrombotic manifestations of SARS-CoV-2 infection. Blood. 2020;136(4):489-500. doi: 10.1182/blood.2020006520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shah A, Donovan K, McHugh A, et al. Thrombotic and haemorrhagic complications in critically ill patients with COVID-19: a multicentre observational study. Crit Care. 2020;24(1):561. doi: 10.1186/s13054-020-03260-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Middeldorp S, Coppens M, van Haaps TF, et al. Incidence of venous thromboembolism in hospitalized patients with COVID-19. J Thromb Haemost. 2020;18(8):1995-2002. doi: 10.1111/jth.14888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shaw RJ, Bradbury C, Abrams ST, Wang G, Toh CH. COVID-19 and immunothrombosis: emerging understanding and clinical management. Br J Haematol. 2021;194(3):518-529. doi: 10.1111/bjh.17664 [DOI] [PubMed] [Google Scholar]
- 9.Lawler PR, Goligher EC, Berger JS, et al. ; ATTACC Investigators; ACTIV-4a Investigators; REMAP-CAP Investigators . Therapeutic anticoagulation with heparin in noncritically ill patients with Covid-19. N Engl J Med. 2021;385(9):790-802. doi: 10.1056/NEJMoa2105911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Goligher EC, Bradbury CA, McVerry BJ, et al. ; REMAP-CAP Investigators; ACTIV-4a Investigators; ATTACC Investigators . Therapeutic anticoagulation with heparin in critically ill patients with Covid-19. N Engl J Med. 2021;385(9):777-789. doi: 10.1056/NEJMoa2103417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Manne BK, Denorme F, Middleton EA, et al. Platelet gene expression and function in patients with COVID-19. Blood. 2020;136(11):1317-1329. doi: 10.1182/blood.2020007214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zaid Y, Puhm F, Allaeys I, et al. Platelets can associate with SARS-CoV-2 RNA and are hyperactivated in COVID-19. Circ Res. 2020;127(11):1404-1418. doi: 10.1161/CIRCRESAHA.120.317703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wichmann D, Sperhake JP, Lütgehetmann M, et al. Autopsy findings and venous thromboembolism in patients with COVID-19: a prospective cohort study. Ann Intern Med. 2020;173(4):268-277. doi: 10.7326/M20-2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lax SF, Skok K, Zechner P, et al. Pulmonary arterial thrombosis in COVID-19 with fatal outcome: results from a prospective, single-center, clinicopathologic case series. Ann Intern Med. 2020;173(5):350-361. doi: 10.7326/M20-2566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rapkiewicz AV, Mai X, Carsons SE, et al. Megakaryocytes and platelet-fibrin thrombi characterize multi-organ thrombosis at autopsy in COVID-19: a case series. EClinicalMedicine. 2020;24:100434. doi: 10.1016/j.eclinm.2020.100434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Angus DC, Derde L, Al-Beidh F, et al. ; Writing Committee for the REMAP-CAP Investigators . Effect of hydrocortisone on mortality and organ support in patients with severe COVID-19: the REMAP-CAP COVID-19 corticosteroid domain randomized clinical trial. JAMA. 2020;324(13):1317-1329. doi: 10.1001/jama.2020.17022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Arabi YM, Gordon AC, Derde LPG, et al. ; REMAP-CAP Investigators . Lopinavir-ritonavir and hydroxychloroquine for critically ill patients with COVID-19: REMAP-CAP randomized controlled trial. Intensive Care Med. 2021;47(8):867-886. doi: 10.1007/s00134-021-06448-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gordon AC, Mouncey PR, Al-Beidh F, et al. ; REMAP-CAP Investigators . Interleukin-6 receptor antagonists in critically ill patients with Covid-19. N Engl J Med. 2021;384(16):1491-1502. doi: 10.1056/NEJMoa2100433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Estcourt LJ, Turgeon AF, McQuilten ZK, et al. ; Writing Committee for the REMAP-CAP Investigators . Effect of convalescent plasma on organ support–free days in critically ill patients with COVID-19: a randomized clinical trial. JAMA. 2021;326(17):1690-1702. doi: 10.1001/jama.2021.18178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Angus DC, Berry S, Lewis RJ, et al. The REMAP-CAP (Randomized Embedded Multifactorial Adaptive Platform for Community-Acquired Pneumonia) study: rationale and design. Ann Am Thorac Soc. 2020;17(7):879-891. doi: 10.1513/AnnalsATS.202003-192SD [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.World Medical Association . World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013;310(20):2191-2194. doi: 10.1001/jama.2013.281053 [DOI] [PubMed] [Google Scholar]
- 22.Group RC, Horby PW, Pessoa-Amorim G, et al. ; RECOVERY Collaborative Group . Aspirin in patients admitted to hospital with COVID-19 (RECOVERY): a randomised, controlled, open-label, platform trial. Lancet. 2022;399(10320):143-151. doi: 10.1016/S0140-6736(21)01825-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Varga Z, Flammer AJ, Steiger P, et al. Endothelial cell infection and endotheliitis in COVID-19. Lancet. 2020;395(10234):1417-1418. doi: 10.1016/S0140-6736(20)30937-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Escher R, Breakey N, Lämmle B. Severe COVID-19 infection associated with endothelial activation. Thromb Res. 2020;190:62. doi: 10.1016/j.thromres.2020.04.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Goshua G, Pine AB, Meizlish ML, et al. Endotheliopathy in COVID-19-associated coagulopathy: evidence from a single-centre, cross-sectional study. Lancet Haematol. 2020;7(8):e575-e582. doi: 10.1016/S2352-3026(20)30216-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Middleton EA, He XY, Denorme F, et al. Neutrophil extracellular traps contribute to immunothrombosis in COVID-19 acute respiratory distress syndrome. Blood. 2020;136(10):1169-1179. doi: 10.1182/blood.2020007008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.O’Sullivan JM, Gonagle DM, Ward SE, Preston RJS, O’Donnell JS. Endothelial cells orchestrate COVID-19 coagulopathy. Lancet Haematol. 2020;7(8):e553-e555. doi: 10.1016/S2352-3026(20)30215-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Godoy LC, Goligher EC, Lawler PR, Slutsky AS, Zarychanski R. Anticipating and managing coagulopathy and thrombotic manifestations of severe COVID-19. CMAJ. 2020;192(40):E1156-E1161. doi: 10.1503/cmaj.201240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Panigada M, Bottino N, Tagliabue P, et al. Hypercoagulability of COVID-19 patients in intensive care unit: a report of thromboelastography findings and other parameters of hemostasis. J Thromb Haemost. 2020;18(7):1738-1742. doi: 10.1111/jth.14850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ranucci M, Ballotta A, Di Dedda U, et al. The procoagulant pattern of patients with COVID-19 acute respiratory distress syndrome. J Thromb Haemost. 2020;18(7):1747-1751. doi: 10.1111/jth.14854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tang N, Li D, Wang X, Sun Z. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J Thromb Haemost. 2020;18(4):844-847. doi: 10.1111/jth.14768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Morris G, Bortolasci CC, Puri BK, et al. Preventing the development of severe COVID-19 by modifying immunothrombosis. Life Sci. 2021;264:118617. doi: 10.1016/j.lfs.2020.118617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Frantzeskaki F, Armaganidis A, Orfanos SE. Immunothrombosis in acute respiratory distress syndrome: cross talks between inflammation and coagulation. Respiration. 2017;93(3):212-225. doi: 10.1159/000453002 [DOI] [PubMed] [Google Scholar]
- 34.Gomez K, Laffan M, Bradbury C. Debate: should the dose or duration of anticoagulants for the prevention of venous thrombosis be increased in patients with COVID-19 while we are awaiting the results of clinical trials? Br J Haematol. 2021;192(3):459-466. doi: 10.1111/bjh.17241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sholzberg M, Tang GH, Rahhal H, et al. ; RAPID Trial Investigators . Effectiveness of therapeutic heparin vs prophylactic heparin on death, mechanical ventilation, or intensive care unit admission in moderately ill patients with COVID-19 admitted to hospital: RAPID randomised clinical trial. BMJ. 2021;375(2400):n2400. doi: 10.1136/bmj.n2400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Spyropoulos AC, Goldin M, Giannis D, et al. Efficacy and safety of therapeutic-dose heparin vs standard prophylactic or intermediate-dose heparins for thromboprophylaxis in high-risk hospitalized patients with COVID-19: the HEP-COVID randomized clinical trial. JAMA Intern Med. 2021;181(12):1612-1620. doi: 10.1001/jamainternmed.2021.6203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sadeghipour P, Talasaz AH, Rashidi F, et al. ; INSPIRATION Investigators . Effect of intermediate-dose vs standard-dose prophylactic anticoagulation on thrombotic events, extracorporeal membrane oxygenation treatment, or mortality among patients with COVID-19 admitted to the intensive care unit: the INSPIRATION randomized clinical trial. JAMA. 2021;325(16):1620-1630. doi: 10.1001/jama.2021.4152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lopes RD, de Barros E Silva PGM, Furtado RHM, et al. ; ACTION Coalition COVID-19 Brazil IV Investigators . Therapeutic versus prophylactic anticoagulation for patients admitted to hospital with COVID-19 and elevated D-dimer concentration (ACTION): an open-label, multicentre, randomised, controlled trial. Lancet. 2021;397(10291):2253-2263. doi: 10.1016/S0140-6736(21)01203-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Akinosoglou K, Alexopoulos D. Use of antiplatelet agents in sepsis: a glimpse into the future. Thromb Res. 2014;133(2):131-138. doi: 10.1016/j.thromres.2013.07.002 [DOI] [PubMed] [Google Scholar]
- 40.Chow JH, Khanna AK, Kethireddy S, et al. Aspirin use is associated with decreased mechanical ventilation, intensive care unit admission, and in-hospital mortality in hospitalized patients with coronavirus disease 2019. Anesth Analg. 2021;132(4):930-941. doi: 10.1213/ANE.0000000000005292 [DOI] [PubMed] [Google Scholar]
- 41.Menter T, Haslbauer JD, Nienhold R, et al. Postmortem examination of COVID-19 patients reveals diffuse alveolar damage with severe capillary congestion and variegated findings in lungs and other organs suggesting vascular dysfunction. Histopathology. 2020;77(2):198-209. doi: 10.1111/his.14134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Berger JS, Kornblith LZ, Gong MN, et al. ; ACTIV-4a Investigators . Effect of P2Y12 inhibitors on survival free of organ support among non–critically ill hospitalized patients with COVID-19: a randomized clinical trial. JAMA. 2022;327(3):227-236. doi: 10.1001/jama.2021.23605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Spaetgens B, Nagy M, Ten Cate H. Antiplatelet therapy in patients with COVID-19—more is less? JAMA. 2022;327(3):223-224. doi: 10.1001/jama.2021.23866 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol and Statistical Analysis Plan
List of REMAP-CAP Investigators
eAppendix 1. Supplementary Methods
eTable 1. Categories of Anticoagulation Intensity According to Anticoagulant and Dose
eAppendix 2. Supplementary Results
eTable 2. Participant Characteristics at Baseline
eTable 3. Primary and Secondary Analyses of Non-Critically Ill Patients
eTable 4. Serious Adverse Events, Major Bleeding, and Thrombotic Events in Non-Critically Ill Patients
eTable 5. Other Secondary Outcomes for Critically Ill Patients
eTable 6. Serious Adverse Events, Major Bleeding, and Thrombotic Events in Critically Ill Patients
eTable 7. Organ Support Free Days (OSFD) Within 21 Days by Age, Mechanical Ventilation Status and Baseline Anticoagulation Dose in Critically Ill Patients
eTable 8. Treatment Effects on Organ Support-Free Days and Hospital Survival Among Critically Ill Patients Enrolled in the Antiplatelet Domain and/or Therapeutic Anticoagulation Domain
eTable 9. In-Hospital Survival and Organ Support by Differential Anticoagulant Dose Given (Randomized and Clinical Decision) in Critically Ill Patients
eFigure 1. Patient Enrolment by Intervention and Time-Period
eFigure 2. Distributions of OSFD Odds Ratio for Pooled Antiplatelet
eFigure 3. Interaction Between Antiplatelet Allocation and Anticoagulation Dose for OSFD
eReferences
Nonauthor Collaborators. REMAP-CAP Investigators
Data Sharing Statement