Key Point
allo-HSCT improves outcome of patients aged >60 years with ELN IR and UR AML in CR1.
Visual Abstract
Abstract
The benefit of allogeneic hematopoietic stem cell transplantation (allo-HSCT) for patients with acute myeloid leukemia (AML) aged >60 years remains a matter of debate, notably when performed in first complete remission (CR1). To clarify this issue, the French Innovative Leukemia Organization (FILO) performed a 10-year real-world time-dependent analysis. The study enrolled patients between 60 and 70 years of age with AML in CR1 after intensive chemotherapy with intermediate (IR) or unfavorable (UR) risk according to the European LeukemiaNet (ELN) 2010 classification. The impact of allo-HSCT was analyzed through three models: (1) time-dependent Cox; (2) multistate for dynamic prediction; and (3) super landmark. The study enrolled 369 (73%) IR and 138 (27%) UR patients with AML, 203 of whom received an allo-HSCT. Classical multivariate analysis showed that allo-HSCT significantly improved relapse-free survival (RFS; hazard ratio [HR] [95% confidence interval (CI)], 0.47 [0.35-0.62]; P < .001) and overall survival (OS; HR [95% CI], 0.56 [0.42-0.76]; P < .001), independently of the ELN risk group. With the multistate model, the predicted 5-year probability for IR and UR patients to remain in CR1 without allo-HSCT was 8% and 1%, respectively. Dynamic predictions confirmed that patients without allo-HSCT continue to relapse over time. Finally, the super landmark model showed that allo-HSCT significantly improved RFS (HR [95% CI], 0.47 [0.36-0.62]; P < .001) and OS (HR [95% CI], 0.54 [0.40-0.72]; P < .001). allo-HSCT in CR1 is reported here as significantly improving the outcome of fit older patients with AML. Long-term RFS without allo-HSCT is very low (<10%), supporting allo-HSCT as being the best curative option for these patients.
Introduction
Allogeneic hematopoietic stem cell transplantation (allo-HSCT) is a curative option for patients with acute myeloid leukemia (AML) but is limited by treatment-related toxicity and donor availability, notably in older patients. However, since the early 2000s, major improvements in transplantation procedures (eg, conditioning regimens, graft-versus-host disease prophylaxis, alternative donors) have remarkably extended the feasibility of allo-HSCT. As a result, this procedure is now an option for older patients up to 70 years or more.1-4 Concomitantly, pretransplantation therapy has also improved over the years, extending the use of intensive treatment to older patients fit enough to receive such a regimen.5-8 Thus, AML patients aged >60 years are now routinely referred to allo-HSCT in first complete remission (CR1) based on indications defined in the setting of younger patients (ie, in the absence of a favorable risk profile).9-12 However, prospective studies evaluating the efficacy of allo-HSCT in this age group are lacking, and its benefits overall and in specific cytogenetic/molecular subgroups remain a matter of debate.
A large retrospective study was thus conducted, and is reported here, of patients with AML aged >60 years treated in 7 centers of the French Innovative Leukemia Organization (PHYLLO) who were in CR1 after intensive chemotherapy. Several multistate models were used to compare outcomes, based on whether the patients received an allo-ASCT.
Patients and methods
Selection criteria
Patients were retrospectively enrolled according to the following selection criteria: (1) age between 60 and 70 years old; (2) diagnosis of AML between 2007 and 2017; (3) CR1 after 1 or 2 courses of intensive chemotherapy; and (4) intermediate or unfavorable risk according to the European LeukemiaNet (ELN) 2010 classification.13 These are consensual indications to consider allo-HSCT across PHYLLO centers for patients aged <60 years. For older patients, the PHYLLO group recommended either to apply allo-HSCT indications from younger patients or to provide consolidation therapy solely based on nonintensive chemotherapy, according to physician decision. Patients diagnosed before the publication of the ELN 2010 classification were retrospectively classified according to cytogenetic and molecular data obtained at the time of diagnosis. Patients with missing cytogenetic and/or mutational data precluding their stratification according to the ELN 2010 classification were not included. Patients with primary induction failure (ie, failure after 2 cycles of chemotherapy) were not included. The study was conducted in accordance with the Declaration of Helsinki.
Treatments
Induction chemotherapy was prescribed according to PHYLLO recommendations for patients aged >60 years.5 Briefly, they received anthracycline-based (daunorubicin or idarubicin) and cytarabine-based (100 or 200 mg/m2 per d for 7 days) regimens, with or without lomustine. Consolidation chemotherapy was based on anthracycline and low-dose subcutaneous cytarabine (50 mg/m2 per 12 hours for 5 days). allo-HSCT modalities were based on local institutional guidelines.
Statistical analysis
All time-to-event analyses were calculated from the time of CR1. Relapses or deaths from any cause were considered events for relapse-free survival (RFS), whereas only death was considered for overall survival (OS). AML relapse and nonrelapse mortality (NRM) were considered as competing events. Patients without event were censored at last contact. Follow-up was computed by using the reverse Kaplan-Meier method. To investigate the impact of allo-HSCT on outcome after CR1, three statistical methods were used to deal with the guarantee-time issue (ie, survival time from CR1 to allo-HSCT favoring time-to-event duration of patients who actually underwent allo-HSCT).
The first method was a time-dependent analysis considering allo-HSCT as a categorical time-dependent variable switching at the time of allo-HSCT.14 RFS and OS curves were plotted by using Simon-Makuch plots.15 Univariate and multivariate comparisons were performed by using a time-dependent Cox model, also producing cause-specific hazard ratios (HRs) for calculation of the risk of relapse and of NRM that were considered competing events.16,17 A multivariate time-dependent Cox model was performed to adjust HRs of allo-HSCT according to age (as continuous variable), ELN risk group (categorical, intermediate vs unfavorable), treatment period (categorical, 2007-2010 vs 2011-2013 vs 2014-2017), and numbers of induction courses to achieve CR1 (categorical, one vs two).
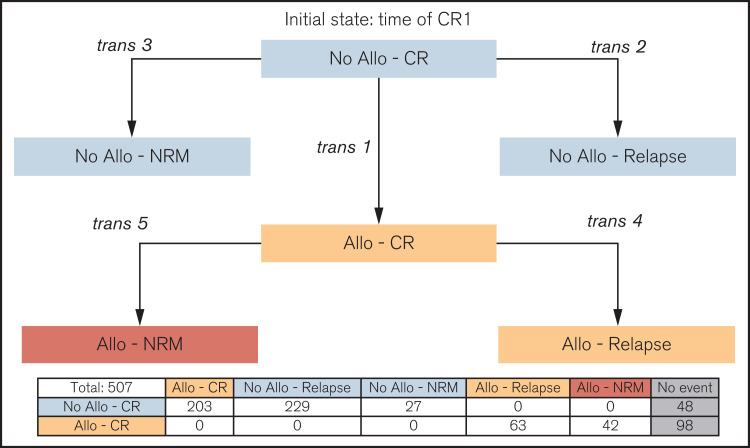
The second approach was a multistate model18 in which the initial state for all patients was “No allo-CR” (ie, not transplanted, still in CR1) with time 0 at the time of CR1 (description in Figure 1). From this initial state, patients can move (transition 1) to the “allo-CR” state (ie, transplanted, still in CR1) at the time of allo-HSCT, or to either the “No allo-relapse” (ie, relapse without previous allo-HSCT, transition 2) or the “No allo-NRM” (ie, NRM without allo-HSCT, transition 3) states. Similarly, once transplanted (ie, from the “allo-CR” state on), patients can move to the “allo-relapse” (ie, relapse after allo-HSCT, transition 4) or “allo-NRM” (ie, NRM after allo-HSCT, transition 5) states at the time of post–allo-HSCT relapse or NRM, respectively. The risks of transition between different states were computed by using a stratified Cox model including age and ELN risk as covariates. This model was used to calculate the predicted probabilities of being in a specific state at a certain time after CR1. In addition, dynamic predictions19 were obtained by considering different landmark starting times (0, 3, 6, 9, and 12 months after CR1). For that specific analysis, predicted probabilities were given separately for patients in the “No allo-CR” and “allo-CR” states at the specific landmark starting time (except for landmark time 0, at which point all patients were in the “No allo-CR” state). The mstate package20 of the R-project software was used for these predictions.
Figure 1.
Description of states and transitions in the multistate model. All patients start at the time of CR1 in the initial state “No allo-CR.” From that initial state, transition 1 occurs at the time of allo-SCT to the “allo-CR” state. Alternatively, transition 2 (to “No allo-Relapse” state) and transition 3 (to “No allo-NRM”) occur at the time of relapse or NRM without transplantation, respectively. Once transplanted (ie, in “allo-CR” state), transitions 4 and 5 to “allo-relapse” and “allo-NRM” absorbing states, respectively, occur at the time of relapse or NRM. Numbers of patients in the state transition matrix are provided below the transition diagram.
Finally, a dynamic landmarking analysis21,22 was performed. For each landmark time (one per month between the time of CR1 and 5 years’ post-CR1), data sets for landmark analyses were generated and pooled together as a super landmark data set. Then, a Cox model stratified on the landmark time was used to assess the impact on post-CR1 outcome of the same covariates as in the first model.
Results
Patient characteristics and transplantation rates
A total of 507 consecutive patients, with a median age of 65 years (range, 60-70 years) met selection criteria (Table 1). allo-HSCT was performed for 203 (40%) of these patients: 135 (36%) of 369 and 68 (49%) of 138 in the ELN intermediate (intermediate-1, 86 of 233 [37%]; intermediate-2, 49 of 136 [36%]) and unfavorable risk groups, respectively. Of note, transplantation rates increased over time for patients aged >65 years (before 2011, 11%; 2011-2013, 26%; after 2013, 35%) while they remained stable in younger patients (before 2011, 51%; 2011-2013, 52%; after 2013, 62%). Patients undergoing transplant were significantly younger (median age, 63 vs 66 years; P < .001), more frequently needed 2 induction treatments to achieve CR1 (12% vs 4%; P < .001), and were more frequently of ELN unfavorable cytogenetic risk (33% vs 23%; P = .013). Among the 203 patients undergoing transplant, 25 (12%), 153 (75%), and 25 (12%), respectively, received non-myeloablative (based on 2-Gy total body irradiation), reduced-intensity (based on 2-day equivalent busulfan dose), and myeloablative (based on 3- or 4-day equivalent busulfan dose) conditioning regimens.
Table 1.
Patient characteristics
| Characteristics | All patients (N = 507) | allo-HSCT (n = 203) | No allo-HSCT (n = 304) | P |
|---|---|---|---|---|
| Age, median [range], y | 65 [60-70] | 63 [60-70] | 66 [60-70] | <.001 |
| ELN 2010 classification | ||||
| Intermediate risk | 369 (73%) | 135 (67%) | 234 (77%) | .013 |
| Abnormal karyotype | 136 (27%) | 49 (24%) | 136 (45%) | |
| Normal karyotype with NPM1-mut and FLT3-ITD | 60 (12%) | 19 (9%) | 60 (20%) | |
| Normal karyotype with NPM1-wt and FLT3-wt | 156 (31%) | 55 (27%) | 156 (51%) | |
| Normal karyotype with NPM1-wt and FLT3-ITD | 17 (3%) | 12 (6%) | 17 (6%) | |
| Adverse risk | 138 (27%) | 68 (33%) | 70 (23%) | |
| Induction therapy | ||||
| 1 course | 472 (93%) | 179 (88%) | 293 (96%) | <.001 |
| 2 courses | 35 (7%) | 24 (12%) | 11 (4%) | |
| HCT-CI | ||||
| <3 | 102 (56%) | |||
| ≥3 | 80 (44%) | |||
| Missing | 21 | |||
| Conditioning regimen | ||||
| NMAC | 25 (12%) | |||
| RIC | 153 (75%) | |||
| MAC | 25 (12%) | |||
| Donor type | ||||
| Matched sibling | 58 (29%) | |||
| Unrelated donor | 113 (56%) | |||
| Cord blood | 9 (4%) | |||
| Haploidentical | 23 (11%) | |||
| Follow-up from CR1, median [95% CI], mo | 52 [45-59] | 51 [45-62] | 54 [45-61] | .900 |
HCT-CI, hematopoietic stem cell comorbidity index; FLT3-ITD, FMS-like tyrosine kinase 3–internal tandem duplication; MAC, myeloablative conditioning; NMAC, non-myeloablative conditioning; RIC, reduced intensity conditioning.
Donors were HLA-matched relatives for 58 patients (29%), matched unrelated for 113 (56%), cord blood for 9 (4%), and haploidentical for 23 (11%). The hematopoietic stem cell comorbidity index was ≥3 in 44% of the patients. Among the 304 patients who did not receive allo-HSCT in CR1, the majority were not referred for allo-HSCT by physician choice (n = 142 [47%]), whereas contraindication for allo-HSCT (n = 57 [19%]), the absence of donor (n = 50 [16%]), early relapse (n = 39 [13%]), and patient decision (n = 13 [4%]) were the other causes for not proceeding to allo-HSCT (missing data, n = 3 [1%]). Supplemental Figure 1 shows how transplantation rates and causes for not undergoing allo-HSCT evolved over years.
allo-HSCT as a time-dependent variable: univariate and multivariate analyses
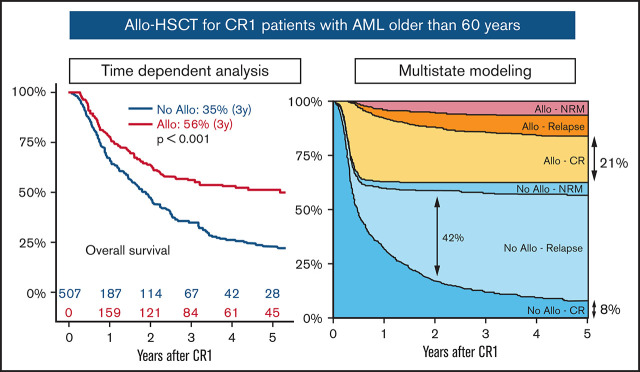
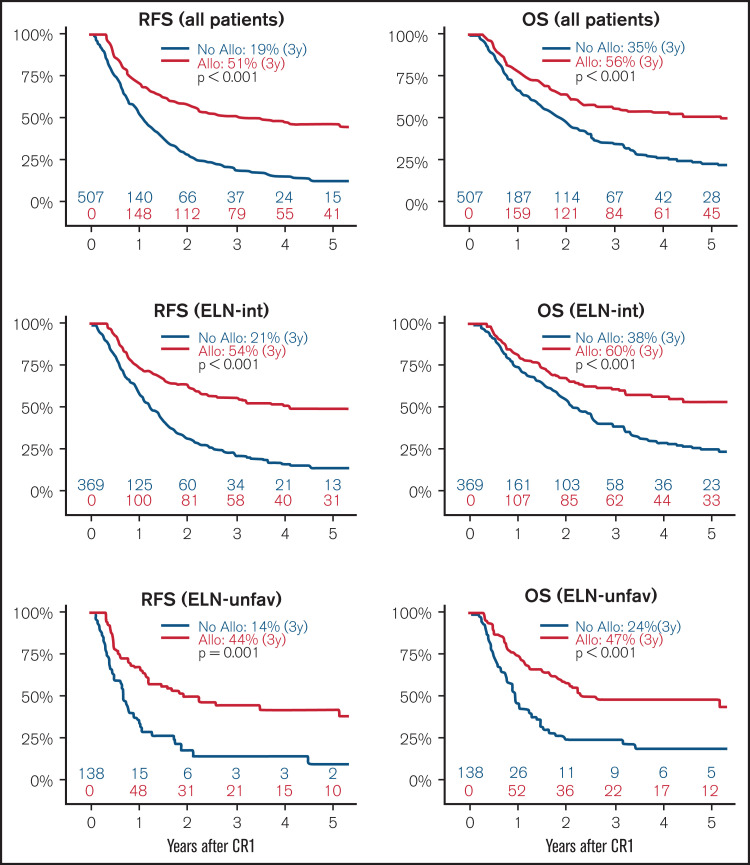
For the whole cohort, allo-HSCT as a time-dependent variable was significantly associated with better 3-year RFS (No allo vs allo, 19% vs 51%; P < .001) and OS (No allo vs allo, 35% vs 56%; P < .001) (Table 2; Figure 2). This was observed both in intermediate and unfavorable ELN risk subgroups as well as in both age groups (ie, aged ≤65 years or >65 years). There was no significant difference in 3-year RFS according to patients with ELN intermediate-1 and intermediate-2 risk (33% vs 33%; P = .864), and allo-HSCT had a similar impact across these 2 groups (intermediate-1: No allo vs allo, 22% vs 53% [P < .001]; intermediate-2: No allo vs allo, 19% vs 58% [P < .001]) (supplemental Figure 2).
Table 2.
Impact of allo-HSCT on RFS and OS in univariate analyses for all patients and across ELN risk and age subgroups
| Subgroup analyses | N | allo-HSCT | RFS | OS | ||||
|---|---|---|---|---|---|---|---|---|
| 3-y % | 95% CI | P * | 3-y % | 95% CI | P * | |||
| All patients | 507 | No | 19 | (15-25) | <.001 | 35 | (29-41) | <.001 |
| Yes | 51 | (44-58) | 56 | (49-64) | ||||
| ELN subgroup | ||||||||
| Intermediate | 369 | No | 21 | (16-27) | <.001 | 38 | (32-46) | <.001 |
| Yes | 54 | (46-64) | 60 | (52-70) | ||||
| Unfavorable | 138 | No | 14 | (6-30) | .001 | 24 | (15-38) | <.001 |
| Yes | 44 | (33-58) | 47 | (37-62) | ||||
| Age subgroup | ||||||||
| 60-64 years old | 234 | No | 19 | (12-30) | <.001 | 32 | (24-44) | .001 |
| Yes | 51 | (43-61) | 57 | (49-66) | ||||
| 65-70 years old | 273 | No | 19 | (14-26) | <.001 | 36 | (30-44) | .002 |
| Yes | 50 | (39-63) | 54 | (43-68) | ||||
3-y %, survival probability at 3 years.
Univariate time-dependent Cox model considering allo-HSCT as a time-dependent variable.
Figure 2.
Simon-Makuch plots with allo-HSCT as a time-dependent variable. Survival curves for RFS (left panels) and OS (right panels) in the whole cohort (upper panels, N = 507), intermediate (ELN-int; middle panels, n = 369), and unfavorable (ELN-unfav; n = 138) ELN risk groups. P values are provided by an univariate time-dependent Cox model.
The multivariate time-dependent Cox model showed that allo-HSCT was associated with a significantly lower risk of relapse (73% risk reduction; HR [95% confidence interval (CI)], 0.27 [0.19-0.38]; P < .001) but an increased risk of NRM (threefold risk increase; HR [95% CI], 3.03 [1.57-5.84]; P < .001). This nevertheless translates into a significantly reduced risk of RFS (53% risk reduction; HR [95% CI], 0.47 [0.35-0.62]; P < .001) and OS (44% risk reduction; HR [95% CI], 0.56 [0.42-0.76]; P < .001) after allo-HSCT. The ELN risk group but not age was an independent adverse risk factor for relapse, translating into worse RFS and OS (Table 3).
Table 3.
Time-dependent Cox model
| Relapse | NRM | |||||
|---|---|---|---|---|---|---|
| Covariates | HR* | 95% CI | P | HR* | 95% CI | P |
| allo-HSCT† | ||||||
| No | 1 | |||||
| Yes | 0.27 | (0.19-0.38) | <.001 | 3.03 | (1.57-5.84) | .001 |
| Age | 1.04 | (0.99-1.08) | .098 | 1.00 | (0.91-1.09) | .988 |
| ELN risk | ||||||
| Intermediate | 1 | 1 | ||||
| Unfavorable | 1.78 | (1.36-2.34) | <.001 | 1.16 | (0.67-2.01) | .600 |
| No. of induction courses | ||||||
| One | 1 | 1 | ||||
| Two | 1.24 | (0.78-1.98) | .366 | 0.39 | (0.12-1.31) | .126 |
| Treatment period | ||||||
| 2007-2010 | 1 | 1 | ||||
| 2011-2013 | 1.10 | (0.81-1.48) | .552 | 0.64 | (0.32-1.29) | .214 |
| 2014-2017 | 0.90 | (0.67-1.22) | .502 | 1.05 | (0.56-1.98) | .883 |
| RFS | OS | |||||
| HR | 95% CI | P | HR | 95% CI | P | |
| allo-HSCT† | ||||||
| No | ||||||
| Yes | 0.47 | (0.35-0.62) | <.001 | 0.56 | (0.42-0.76) | <.001 |
| Age | 1.03 | (0.99-1.07) | .135 | 1.03 | (0.99-1.08) | .105 |
| ELN risk | ||||||
| Intermediate | 1 | 1 | ||||
| Unfavorable | 1.61 | (1.26-2.05) | <.001 | 1.59 | (1.23-2.06) | <.001 |
| No. of induction courses | ||||||
| One | 1 | 1 | ||||
| Two | 1.02 | (0.66-1.57) | .932 | 1.17 | (0.74-1.86) | .493 |
| Treatment period | ||||||
| 2007-2010 | 1 | 1 | ||||
| 2011-2013 | 0.99 | (0.75-1.30) | .918 | 1.00 | (0.74-1.35) | .989 |
| 2014-2017 | 0.94 | (0.71-1.23) | .629 | 0.98 | (0.73-1.32) | .899 |
Cause-specific HR.
allo-HSCT was included in the model as a time-dependent covariate.
Although a time-dependent model was used, the selection process before allo-HSCT results in an unavoidable selection bias. To deal in part with this issue, we performed 2 additional time-dependent analyses. First, after exclusion of patients who did not undergo transplant because of clinical contraindication or early relapse, we still observed a significantly higher 3-year RFS after allo-HSCT, independently of the ELN risk group (intermediate: No allo 22% vs allo 54% [P < .001]; unfavorable: No allo vs allo, 21% vs 44% [P = .038]) (supplemental Figure 3). The second approach was based on a landmark analysis. We chose a late landmark time (6 months’ post-CR1 achievement) to assess patients with early relapse and/or early death after CR1, which precludes performing allo-HSCT. Thus, 385 AML-free patients were included in this 6-month landmark analysis. Among them, 202 did not undergo transplant, whereas 183 patients underwent allo-HSCT (including 14 with late transplant [ie, after the landmark time of 6 months’ post-CR1]). For this cohort, the causes for not proceeding to allo-HSCT were as follows: not referred (52%), contraindication (17%), no donor (22%), early relapse (2%), and patient refusal (6%). Time-dependent analysis revealed that 3-year RFS (58% vs 25%; P < .001) and OS (63% vs 47%; P < .001) were significantly higher after allo-HSCT (supplemental Figure 4).
Predicted probabilities using a multistate model
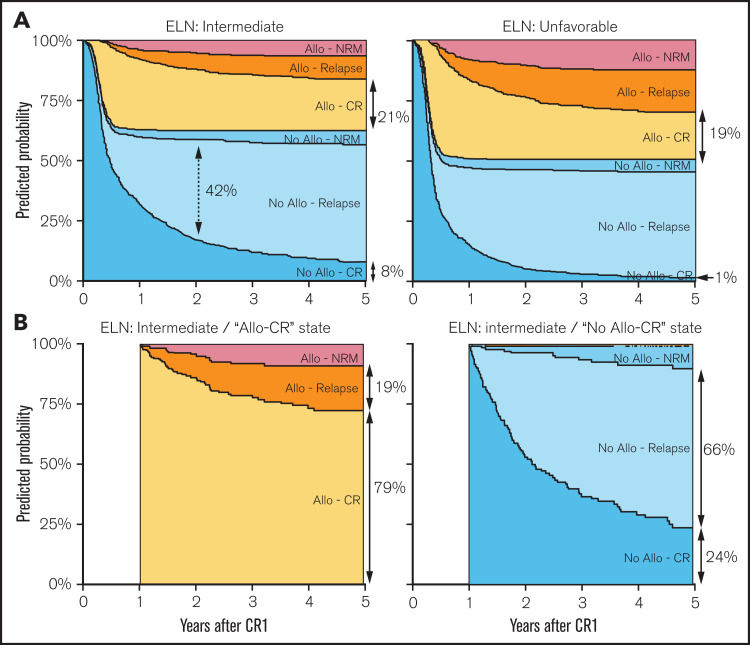
The multistate model and the number of patients entering the state transition matrix are described in Figure 1. Censoring distribution and predicted cumulative hazard plots are provided in supplemental Figures 5 and 6, respectively. In the intermediate ELN risk group, the 5-year predicted probabilities for being in the “No allo-CR,” “No allo-Relapse,” “No allo-NRM,” “allo-CR,” “allo-Relapse,” and “allo-NRM” groups were 8%, 49%, 6%, 21%, 10%, and 6%, respectively. For the unfavorable ELN risk group, these values were 1%, 44%, 5%, 19%, 18%, and 12% (supplemental Table 1). Thus, the predicted probabilities for being in CR1 (“No allo-CR” + “allo-CR”) at 5 years were 29% and 20% for the intermediate and unfavorable risk groups (Figure 3A).
Figure 3.
Predicted probabilities from the multistate model. Predicted probabilities to be in a specific state (according to area and color) over time. (A) Evolution over 5 years of state probabilities from the time of CR1 (ie, 100% of patients in the “No allo-CR” state) for a representative patient with AML 65 years of age with intermediate (left panel) or unfavorable (right panel) ELN risk. The length of arrows represent the predicted probabilities at specific time points according to the x-axis (eg, the dotted double arrow shows a predicted probability of 42% for being in the “No allo-relapse” state 2 years after CR1 for a patient with intermediate ELN risk). Full double arrows show the predicted probabilities to be in the “allo-CR” or “No allo-CR” state at 5 years’ post-CR1. (B) Evolution of state probabilities from a 1-year post-CR1 landmark time to 5 years’ post-CR1, in a virtual representative patient with AML aged 65 years with intermediate ELN risk according to transplantation status at the landmark time (ie, from the “allo-CR” [left panel] and “No allo-CR” [right panel] states regardless of transplant, respectively). Full predicted probabilities at 2, 3, 4, and 5 years’ post-CR1 from landmark times of 0, 3, 6, 9, and 12 months’ post-CR1 are provided in supplemental Table 1.
When considering event-free patients 1 year after CR1 (“allo-CR” or “No allo-CR”), the 5-year predicted probability of relapse after allo-HSCT was 19%, most patients (79%) still being in “allo-CR” (Figure 3B, left panel). In contrast, patients who did not receive a transplant had a 66% predicted probability to relapse within the next 4 years (Figure 3B, right panel). The detailed predicted probabilities of state occupancy at 2, 3, 4, and 5 years from CR1 and from different landmark times (3, 6, 9, and 12 months) are presented in supplemental Table 1 in relation to ELN risk groups.
Super landmark Cox model
As an alternative to the standard time-dependent Cox model, this super landmark Cox model evaluated the impact of allo-HSCT on outcomes every month for 60 months after CR1. For patients still at risk of event at the beginning of each interval, the allo-HSCT variable was set to “yes” or “no” depending on whether the patient had previously undergone transplant. Once stratified on landmark times, this model provides adjusted HRs for each included covariate over the whole 5-year period. The distribution of current values of the variable allo-HSCT is presented in supplemental Figure 7.
This model confirmed that allo-HSCT was significantly associated with a decreased risk of relapse (71% risk reduction: HR [95% CI], 0.29 [0.21-0.41]; P < .001) and an increased risk of NRM (2.36-fold; HR [95% CI], 2.36 [1.26-4.45]; P < .001). Finally, the risks of both RFS (53% risk reduction; HR [95% CI], 0.47 [0.36-0.62]; P < .001) and OS (46% risk reduction; HR [95% CI], 0.54 [0.40-0.72]; P < .001) were confirmed to be significantly decreased after allo-HSCT.
Discussion
This study used sophisticated time-dependent methodologies to analyze the impact of allo-HSCT on the outcomes of CR1 AML patients aged between 60 and 70 years. Previously, when the use of standard myeloablative regimens prevailed, patients aged >60 years were de facto ineligible for allo-HSCT because of an unacceptably high risk of NRM. Since the early 2000s, the feasibility of allo-HSCT for older patients has greatly improved because of the development of reduced-intensity regimens.1,2,23 However, the benefit of performing allo-HSCT in CR1 was not shown in patients aged >60 years. Indeed, although it is established that young patients with nonfavorable risk AML should undergo transplant in CR1,11 it can be argued that in older patients, the expected higher NRM after allo-HSCT may counterbalance its potential benefit, notably in the setting of intermediate-risk AML. To answer this question in the absence of prospective randomized trials, retrospective analyses might be methodologically challenging. Indeed, a retrospective front-to-front comparison of patients with or without allo-HSCT is not fair because of the presence of an immortality bias that highly favors patients who actually underwent allo-HSCT. Methods considering allo-HSCT as a time-dependent variable (Mantel-Byar calculation and Simon Makuch plots14,15) can be used to deal with this immortality bias. Using this type of strategy in 3 different models, we show here that allo-HSCT is indeed associated with a lower risk of relapse (HR, 0.27) and better RFS (HR, 0.47) and OS (HR, 0.56), although NRM (HR, 3.03) is significantly increased after allo-HSCT. Interestingly, this was true in both the intermediate and unfavorable ELN risk groups. These results confirm, with 3 different approaches, previous reports from several groups. Indeed, Versluis et al24 reported a retrospective time-dependent analysis showing that allo-HSCT significantly improved both RFS and OS in older patients with intermediate or unfavorable risk AML included in 4 prospective HOVON-SAKK (Dutch-Belgian Hemato-Oncology Cooperative Group and Swiss Group for Clinical Cancer Research) clinical trials. In a collaborative study (CIBMTR/Alliance/CALGB [Cancer and Leukemia Group B]/ECOG-ACRIN [Eastern Cooperative Oncology Group–American College of Radiology Imaging Network]/SWOG [Southwest Oncology Group]), Ustun et al25 showed that allo-HSCT significantly decreased the risk of relapse while increasing the risk of early death. Despite a worse OS early after allo-HSCT, long-term RFS and OS were significantly better in the allo-HSCT group, although with curves crossing at 12 months’ post-CR1.
One limitation of time-dependent analyses is that although they provide unbiased HR (with respect to the immortality bias), they do not take into account the fact that some patients initially intended to receive allo-HSCT ultimately could not undergo transplant because of early relapse and/or poor medical condition. In addition, the clinical interpretation of outcomes in such groups as allo-HSCT and No allo-HSCT, which are not defined at time of origin and change over time, is difficult. To overcome this issue, we used a multistate model that allowed computation of predicted transition state probabilities. In this setting, the allo-HSCT and No allo-HSCT groups are not previously defined, and the probabilities of state transition and occupancy are alternatively provided. This model also allows inclusion of covariates for prediction adjustment and generate dynamic predictions by setting multiple landmark times.18,19 This latter point is of importance for evaluating how the risk of state transition evolves when the time origin moves over time. Using this model, it was possible to show that the probability of remaining in CR without allo-HSCT at 5 years after CR1 is very low (8% and 1% for the intermediate and unfavorable ELN risk groups, respectively). The predicted probabilities of state occupancy at 5 years also revealed that prolonged CR is mostly observed after allo-HSCT. In addition, the outcome of event-free patients at different landmark times after CR1 was analyzed, with the aim of avoiding the issue of early events (ie, death or relapse) making patients ineligible for allo-HSCT. By providing predicted probabilities of late events, it was shown that patients without allo-HSCT continued to relapse over time, 66% of them being likely to relapse within 4 years, although still in CR after 1 year. By contrast, 79% of transplanted patients event-free 1 year after CR1 will remain in CR during these 4 years. Similar results were obtained using other landmark times, suggesting that long-term cure without allo-HSCT is unlikely for elderly patients with intermediate ELN risk, and even virtually nonexistent for those with unfavorable ELN risk. These results are in line with a recent publication of the CALGB/Alliance groups showing that there are very few long-term AML survivors without allo-HSCT in CR1, notably in older patients (2.4%).26 However, one limitation of the current study is that, due to the enrollment period (2007-2017), it was not possible to categorize the patients according to the ELN 2017 classification. Indeed, Gardin et al,27 on behalf of the ALFA (Acute Leukemia French Association) group, recently showed that extensive mutational analysis could add prognostic value. In this study, elderly patients with intermediate ELN risk who harbored secondary AML-like gene mutations as defined by Lindsley et al28 benefited from allo-HSCT in CR1, both in terms of RFS and OS. Conversely, only RFS was favorably affected by allo-HSCT for intermediate-risk patients without such mutations.27 However, it is important to note that different statistical methods were used in this work compared with ours. The major difference is that the impact of allo-HSCT on OS was not calculated from the time of CR1 but at a 113-day landmark time, corresponding to the median duration between CR1 and allo-HSCT. In addition, as observed in the collaborative study of Ustun et al,25 the fact that OS curves are crossing possibly contradicts the proportional hazard assumption.29,30 A statistical test producing P values at specific time points is more appropriate for evaluations of the long-term benefit on OS, avoiding the issue of early mortality after allo-HSCT. The multistate and super landmark models that we used in fact precisely dealt with this issue, showing that patients without allo-HSCT continue to relapse even a long time after reaching CR1. Finally, in the ALFA study, only 9 patients without allo-HSCT were still alive at 4 years, and it is unknown whether they were still in CR. Taken together, although presented differently, the results from the ALFA group also show that there are few long-term survivors without allo-HSCT in this context of older patients with CR1 AML.
The results presented here support that allo-HSCT should be performed in CR1 for nonfavorable risk AML patients aged between 60 and 69 years, with long-term RFS being very unlikely without allo-HSCT. Strategies focusing on increasing the transplantation rate should thus improve the overall outcome of this group of patients. In this perspective, we and others previously reported that the absence of an HLA-matched donor should no longer be considered as an allo-HSCT contraindication for these patients, especially when a haploidentical donor is available.31-34 In addition, the recent development of new drugs has strongly modified the landscape of AML therapeutic strategies.35,36 It is not yet known whether these new therapies will increase the proportion of cured patients, but they significantly improve the rate, quality, and duration of response while potentially sparing patients from the toxicity of standard-induction high-dose chemotherapy.7,37 This is of importance because these improvements, such as venetoclax-based low-intensity regimens, may improve both the feasibility and the efficacy of allo-HSCT.6,38 Moreover, new drugs such as anti–FMS-like tyrosine kinase 339-41 or, more recently, anti–isocitrate dehydrogenase and epidrugs,42,43 also offer powerful tools for maintenance therapy after allo-HSCT, thus contributing to the overall improvement of survival in older patients with AML.
In conclusion, allo-HSCT for CR1 AML patients aged >60 years, known to be routinely feasible, significantly improves outcomes in both intermediate and unfavorable ELN risk groups. Less than 10% of patients display long-term RFS and OS without allo-HSCT, even in the intermediate-risk group, supporting the fact that allo-HSCT remains the first curative option for these patients.
Supplementary Material
The full-text version of this article contains a data supplement.
Authorship
Contribution: R.D., A.H., N.V., and C.R. contributed to study design; and R.D., M.C.B., A.H., N.V., C.R., and A.P. wrote the manuscript. All authors provided clinical or biological data, and all authors reviewed and approved the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Raynier Devillier, Institut Paoli-Calmettes, Département d’Hématologie, 232 boulevard Sainte Marguerite, Marseille, France; e-mail: devillierr@ipc.unicancer.fr.
References
- 1.Gooley TA, Chien JW, Pergam SA, et al. Reduced mortality after allogeneic hematopoietic-cell transplantation. N Engl J Med. 2010;363(22):2091-2101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Blaise D, Devillier R, Lecoroller-Sorriano AG, et al. Low non-relapse mortality and long-term preserved quality of life in older patients undergoing matched related donor allogeneic stem cell transplantation: a prospective multicenter phase II trial. Haematologica. 2015;100(2):269-274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kasamon YL, Bolaños-Meade J, Prince GT, et al. Outcomes of nonmyeloablative HLA-haploidentical blood or marrow transplantation with high-dose post-transplantation cyclophosphamide in older adults. J Clin Oncol. 2015;33(28):3152-3161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Magliano G, Bacigalupo A. Allogeneic hematopoietic stem cell transplantation for acute myeloid leukemia of the elderly: review of literature and new perspectives. Mediterr J Hematol Infect Dis. 2020;12(1):e2020081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pigneux A, Béné MC, Salmi LR, et al. ; French Innovative Leukemia Organization . Improved survival by adding lomustine to conventional chemotherapy for elderly patients with AML without unfavorable cytogenetics: results of the LAM-SA 2007 FILO Trial. J Clin Oncol. 2018;36(32):3203-3210. [DOI] [PubMed] [Google Scholar]
- 6.Lancet JE, Uy GL, Cortes JE, et al. CPX-351 (cytarabine and daunorubicin) liposome for injection versus conventional cytarabine plus daunorubicin in older patients with newly diagnosed secondary acute myeloid leukemia. J Clin Oncol. 2018;36(26):2684-2692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chua CC, Roberts AW, Reynolds J, et al. Chemotherapy and Venetoclax in Elderly Acute Myeloid Leukemia Trial (CAVEAT): a Phase Ib dose-escalation study of venetoclax combined with modified intensive chemotherapy. J Clin Oncol. 2020;38(30):3506-3517. [DOI] [PubMed] [Google Scholar]
- 8.Castaigne S, Pautas C, Terré C, et al. ; Acute Leukemia French Association . Effect of gemtuzumab ozogamicin on survival of adult patients with de-novo acute myeloid leukaemia (ALFA-0701): a randomised, open-label, phase 3 study. Lancet. 2012;379(9825):1508-1516. [DOI] [PubMed] [Google Scholar]
- 9.Koreth J, Schlenk R, Kopecky KJ, et al. Allogeneic stem cell transplantation for acute myeloid leukemia in first complete remission: systematic review and meta-analysis of prospective clinical trials. JAMA. 2009;301(22):2349-2361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cornelissen JJ, Gratwohl A, Schlenk RF, et al. The European LeukemiaNet AML Working Party consensus statement on allogeneic HSCT for patients with AML in remission: an integrated-risk adapted approach. Nat Rev Clin Oncol. 2012;9(10):579-590. [DOI] [PubMed] [Google Scholar]
- 11.Döhner H, Estey E, Grimwade D, et al. Diagnosis and management of AML in adults: 2017 ELN recommendations from an international expert panel. Blood. 2017;129(4):424-447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tallman MS, Wang ES, Altman JK, et al. ; OCN . Acute Myeloid Leukemia, Version 3.2019, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2019;17(6):721-749. [DOI] [PubMed] [Google Scholar]
- 13.Döhner H, Estey EH, Amadori S, et al. ; European LeukemiaNet . Diagnosis and management of acute myeloid leukemia in adults: recommendations from an international expert panel, on behalf of the European LeukemiaNet. Blood. 2010;115(3):453-474. [DOI] [PubMed] [Google Scholar]
- 14.Mantel N, Byar DP. Evaluation of response-time data involving transient states: an illustration using heart-transplant data. J Am Stat Assoc. 1974;69(345):81-86. [Google Scholar]
- 15.Simon R, Makuch RW. A non-parametric graphical representation of the relationship between survival and the occurrence of an event: application to responder versus non-responder bias. Stat Med. 1984;3(1):35-44. [DOI] [PubMed] [Google Scholar]
- 16.Cox D. Regression models and life tables. J R Stat Soc B. 1972;34:187-220. [Google Scholar]
- 17.Prentice RL, Kalbfleisch JD, Peterson AV Jr, Flournoy N, Farewell VT, Breslow NE. The analysis of failure times in the presence of competing risks. Biometrics. 1978;34(4):541-554. [PubMed] [Google Scholar]
- 18.Putter H, Fiocco M, Geskus RB. Tutorial in biostatistics: competing risks and multi-state models. Stat Med. 2007;26(11):2389-2430. [DOI] [PubMed] [Google Scholar]
- 19.Nicolaie MA, van Houwelingen JC, de Witte TM, Putter H. Dynamic prediction by landmarking in competing risks. Stat Med. 2013;32(12):2031-2047. [DOI] [PubMed] [Google Scholar]
- 20.de Wreede LC, Fiocco M, Putter H. The mstate package for estimation and prediction in non- and semi-parametric multi-state and competing risks models. Comput Methods Programs Biomed. 2010;99(3):261-274. [DOI] [PubMed] [Google Scholar]
- 21.van Houwelingen HC, Putter H. Dynamic predicting by landmarking as an alternative for multi-state modeling: an application to acute lymphoid leukemia data. Lifetime Data Anal. 2008;14(4):447-463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.van Houwelingen HC. Dynamic prediction by landmarking in event history analysis. Scand J Stat. 2007;34(1):70-85. [Google Scholar]
- 23.Lioure B, Béné MC, Pigneux A, et al. ; GOELAMS . Early matched sibling hematopoietic cell transplantation for adult AML in first remission using an age-adapted strategy: long-term results of a prospective GOELAMS study. Blood. 2012;119(12):2943-2948. [DOI] [PubMed] [Google Scholar]
- 24.Versluis J, Hazenberg CL, Passweg JR, et al. ; HOVON and SAKK Leukemia Groups . Post-remission treatment with allogeneic stem cell transplantation in patients aged 60 years and older with acute myeloid leukaemia: a time-dependent analysis. Lancet Haematol. 2015;2(10):e427-e436. [DOI] [PubMed] [Google Scholar]
- 25.Ustun C, Le-Rademacher J, Wang HL, et al. Allogeneic hematopoietic cell transplantation compared to chemotherapy consolidation in older acute myeloid leukemia (AML) patients 60-75 years in first complete remission (CR1): an alliance (A151509), SWOG, ECOG-ACRIN, and CIBMTR study. Leukemia. 2019;33(11):2599-2609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vasu S, Kohlschmidt J, Mrózek K, et al. Ten-year outcome of patients with acute myeloid leukemia not treated with allogeneic transplantation in first complete remission. Blood Adv. 2018;2(13):1645-1650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gardin C, Pautas C, Fournier E, et al. Added prognostic value of secondary AML-like gene mutations in ELN intermediate-risk older AML: ALFA-1200 study results. Blood Adv. 2020;4(9):1942-1949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lindsley RC, Mar BG, Mazzola E, et al. Acute myeloid leukemia ontogeny is defined by distinct somatic mutations. Blood. 2015;125(9):1367-1376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Linden T, Gerss J, Jürgens H. The crux of the log rank test. Re: Locasciulli A, Oneto R, Bacigalupo A, Socie G, Korthof E, Bekassy A, Schrezenmeier H, Passweg J, Fuhrer M. Outcome of patients with acquired aplastic anemia given first line bone marrow transplantation or immunosuppressive treatment in the last decade: a report from the European Group for Blood and Marrow Transplantation. Haematologica 2007; 92:11-8. Haematologica. 2007;92(12):e122. [DOI] [PubMed] [Google Scholar]
- 30.Logan BR, Klein JP, Zhang M-J. Comparing treatments in the presence of crossing survival curves: an application to bone marrow transplantation. Biometrics. 2008;64(3):733-740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Devillier R, Legrand F, Rey J, et al. HLA-matched sibling versus unrelated versus haploidentical related donor allogeneic hematopoietic stem cell transplantation for patients aged over 60 years with acute myeloid leukemia: a single-center donor comparison. Biol Blood Marrow Transplant. 2018;24(7):1449-1454. [DOI] [PubMed] [Google Scholar]
- 32.Santoro N, Labopin M, Giannotti F, et al. Unmanipulated haploidentical in comparison with matched unrelated donor stem cell transplantation in patients 60 years and older with acute myeloid leukemia: a comparative study on behalf of the ALWP of the EBMT. J Hematol Oncol. 2018;11(1):55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Slade M, DiPersio JF, Westervelt P, Vij R, Schroeder MA, Romee R. Haploidentical hematopoietic cell transplant with post-transplant cyclophosphamide and peripheral blood stem cell grafts in older adults with acute myeloid leukemia or myelodysplastic syndrome. Biol Blood Marrow Transplant. 2017;23(10):1736-1743. [DOI] [PubMed] [Google Scholar]
- 34.Ciurea SO, Shah MV, Saliba RM, et al. Haploidentical transplantation for older patients with acute myeloid leukemia and myelodysplastic syndrome. Biol Blood Marrow Transplant. 2018;24(6):1232-1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wei AH, Tiong IS. Midostaurin, enasidenib, CPX-351, gemtuzumab ozogamicin, and venetoclax bring new hope to AML. Blood. 2017;130(23):2469-2474. [DOI] [PubMed] [Google Scholar]
- 36.Wei AH, Döhner H, Pocock C, et al. ; QUAZAR AML-001 Trial Investigators . Oral azacitidine maintenance therapy for acute myeloid leukemia in first remission. N Engl J Med. 2020;383(26):2526-2537. [DOI] [PubMed] [Google Scholar]
- 37.Guolo F, Fianchi L, Minetto P, et al. CPX-351 treatment in secondary acute myeloblastic leukemia is effective and improves the feasibility of allogeneic stem cell transplantation: results of the Italian compassionate use program. Blood Cancer J. 2020;10(10):96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.DiNardo CD, Jonas BA, Pullarkat V, et al. Azacitidine and venetoclax in previously untreated acute myeloid leukemia. N Engl J Med. 2020;383(7):617-629. [DOI] [PubMed] [Google Scholar]
- 39.Burchert A, Bug G, Fritz LV, et al. Sorafenib Maintenance After Allogeneic Hematopoietic Stem Cell Transplantation for Acute Myeloid Leukemia With FLT3-Internal Tandem Duplication Mutation (SORMAIN). J Clin Oncol. 2020;38(26):2993-3002. [DOI] [PubMed] [Google Scholar]
- 40.Maziarz RT, Levis M, Patnaik MM, et al. Midostaurin after allogeneic stem cell transplant in patients with FLT3-internal tandem duplication-positive acute myeloid leukemia. Bone Marrow Transplant. 2021;56(5):1180-1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ganguly S, Cortes JE, Krämer A, et al. Clinical outcomes in patients with FLT3–FLT3-ITD–mutated relapsed/refractory acute myelogenous leukemia undergoing hematopoietic stem cell transplantation after quizartinib or salvage chemotherapy in the Quantum-R Trial. Biol Blood Marrow Transplant. 2020;26(3):153-162. PMID: 33017662 [DOI] [PubMed] [Google Scholar]
- 42.de Lima M, Oran B, Champlin RE, et al. CC-486 maintenance after stem cell transplantation in patients with acute myeloid leukemia or myelodysplastic syndromes. Biol Blood Marrow Transplant. 2018;24(10):2017-2024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kalin B, van Norden Y, van Gelder M, et al. Panobinostat and decitabine prior to donor lymphocyte infusion in allogeneic stem cell transplantation. Blood Adv. 2020;4(18):4430-4437. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.