Abstract
Background
Current guidelines recommend empirical antifungal therapy in patients with sepsis with high risk of invasive Candida infection. However, many different risk factors have been derived from multiple studies. These risk factors lack specificity, and broad application would render most ICU patients eligible for empirical antifungal therapy.
Research Question
What risk factors for invasive Candida infection can be identified by a systematic review and meta-analysis?
Study Design and Methods
We searched PubMed, Web of Science, ScienceDirect, Biomed Central, and Cochrane and extracted the raw and adjusted OR for each risk factor associated with invasive Candida infection. We calculated pooled ORs for risk factors present in more than one study.
Results
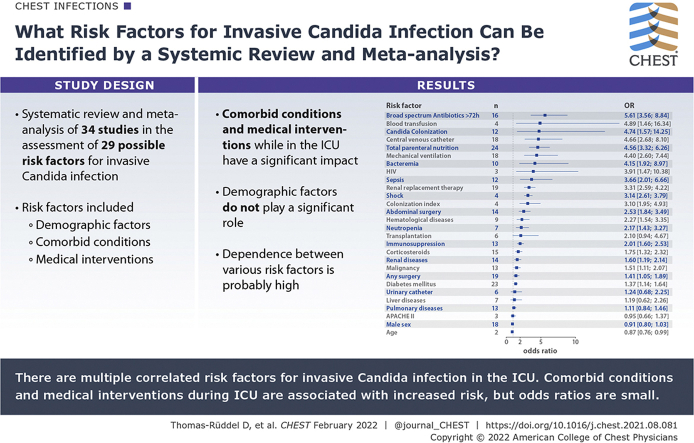
We included 34 studies in our meta-analysis resulting in the assessment of 29 possible risk factors. Risk factors for invasive Candida infection included demographic factors, comorbid conditions, and medical interventions. Although demographic factors do not play a role for the development of invasive Candida infection, comorbid conditions (eg, HIV, Candida colonization) and medical interventions have a significant impact. The risk factors associated with the highest risk for invasive Candida infection were broad-spectrum antibiotics (OR, 5.6; 95% CI, 3.6-8.8), blood transfusion (OR, 4.9; 95% CI, 1.5-16.3), Candida colonization (OR, 4.7; 95% CI, 1.6-14.3), central venous catheter (OR, 4.7; 95% CI, 2.7-8.1), and total parenteral nutrition (OR, 4.6; 95% CI, 3.3-6.3). However, dependence between the various risk factors is probably high.
Interpretation
Our systematic review and meta-analysis identified patient- and treatment-related factors that were associated with the risk for the development of invasive Candida infection in the ICU. Most of the factors identified were either related to medical interventions during intensive care or to comorbid conditions.
Key Words: Candida; candidiasis, invasive/epidemiology; candidiasis, invasive; critical care; hospital infections; risk factors
Abbreviations: ICI, invasive Candida infection
Graphical Abstract
Take-home Points.
Study Question: What risk factors for invasive Candida infection can be identified by a systematic review and meta-analysis?
Results: We identified 29 risk factors from 34 studies mostly related to medical interventions during intensive care or to comorbid conditions, but most ORs were small.
Interpretation: There are multiple correlated risk factors for invasive Candida infection in the intensive care setting.
The burden of invasive fungal infections on ICUs is increasing,1 and Candida species cause approximately 80% of those infections.2 Presence of invasive Candida infection (ICI) is associated with a high risk of death with an attributable mortality of 49%,3 but may increase up to 98% in patients with septic shock with delayed antifungal therapy.4 However, early identification of ICI is difficult. Therefore, current guidelines recommend empirical antifungal therapy in patients with sepsis with high risk of ICI.5,6 However, many conditions are known as possible risk factors for ICI.7, 8, 9 These risk factors lack specificity and broad application would render almost every ICU patient eligible for empirical antifungal therapy. The risk factors are mostly derived from retrospective studies reflecting a wide variety of study populations and very different investigated conditions. A systematic review found 13 publications published before 2009 addressing this issue, but heterogeneity of the selected studies was too large to allow a meta-analysis.10 Since then, multiple additional studies on this topic have been published. The goal of this meta-analysis is to systematically review the literature on potential risk factors for the development of ICIs in critically ill adult patients and to calculate common ORs for identification of the most important risk factors.
Methods
The reporting of this study follows the recommendations for Meta-analysis Of Observational Studies in Epidemiology,11 and the reporting checklist is provided in e-Table 1. The study protocol was stored locally but not registered prospectively.
Study Identification
An internet search of relevant publications was performed on five databases (PubMed, Web of Science, ScienceDirect, Biomed Central, and Cochrane) on June 23, 2014. A search algorithm was applied which has been modified from a previous systematic review.10 The algorithm contained the following three main criteria: fungal disease, patient population, and risk factors. Each of the three criteria consists of several key words where at least one of the key words in each of the main criteria had to match. The full algorithm is provided in e-Table 2. The PubMed search was updated regularly until December 5, 2018. We reviewed personal files and reference lists of review articles and of articles fulfilling our inclusion criteria for additional relevant publications.
Eligibility Criteria
We included cohort and case-control studies on adult patients (≥ 18 years of age) admitted to an ICU, which assessed risk factors for the occurrence of ICI either retrospectively or which prospectively followed patients for the development of ICI. The study-specific definition of ICI could be either bloodstream infection (candidemia) or using the European Organization of the Research and Treatment of Cancer/Mycoses Study Group criteria12 or using study-specific similar criteria. Studies including the growth of Candida species from urine or tracheal aspirates in their case definition were excluded because this mainly reflects colonization and not invasive infection. Control groups had to come from the same ICU population as the cases. Abstracts were included if they contained analyzable results, and no full paper with those data was available. Only publications in English, German, or French were included. If two or more publications were based on the same patient cohort, only one publication was included in the analyses, but all papers were used to extract all available information.
Study Selection and Data Extraction
Initially, all duplicates from the searches were removed. All titles and in a second step all remaining abstracts were screened by one author (D. T.-R.) for possible eligibility. The full texts of all potentially eligible publications were then rescreened by two authors (F. B. and D. T.-R.); conflicting opinions about eligibility could be resolved in all cases by discussion. Potentially eligible publications identified by the updated PubMed search, personal files, or reference lists were screened the same way.
All reported risk factors and their definition were extracted from the selected publications and thematically grouped by one author (D. T.-R.). Two authors (F. B. and D. T.-R.) decided to collapse identical or nearly identical risk factors into one category and to omit risk factors only assessed by single publications and without reasonable association with ICI from further data extraction. Zero counts in a two-by-two table were replaced by 0.5 to avoid infinite ORs (Haldane-Anscombe-correction). Univariate and, if reported, multivariable ORs and 95% CIs of the applicable risk factors for developing ICI were independently extracted by two authors (F. B. and D. T.-R.) onto standardized data extraction sheets. ORs and CIs were independently calculated by both authors if only frequency data were reported. If a publication reported several cohorts, only data from the cohorts fulfilling inclusion criteria were extracted. Discrepancies were resolved by repeat extraction by both authors, discussion between the data extractors, and if still unresolved discussion with a third author (M. P. and O. K.). The corresponding author was contacted for all articles where data of interest were found to be missing.
Risk of Bias Assessment
Two authors (F. B. and D. T.-R.) independently assessed the risk of bias for each included study by adapting the Scottish Intercollegiate Guidelines Network quality checklists for cohort studies and case-control studies. The checklists provided measures for assessing internal validity (selection of subjects, assessment of exposure, confounding, and statistical analysis) and overall study quality. Because of the specifics of epidemiologic research in the ICU setting, not all items were applicable, and the checklists were accordingly modified. The modification of the Scottish Intercollegiate Guidelines Network checklist resulted in seven checklist items each for the case-control studies and the cohort studies (e-Table 3). One point was given for each checklist item fulfilled. No points were given if a checklist item was not fulfilled, not applicable, or sufficient information for assessment was not available. In addition, risk of bias was assessed in both study types on a scale of 0 to 2, resulting in a maximum attainable score of 9 in both study types. Discrepancies were resolved by discussion between the data extractors and if still unresolved discussion with a third author (M. P. and O. K.). Both study types were deemed high quality when the score was at 9 points, acceptable quality when the score was 6 to 8 points, and low quality when the score was ≤ 5 points.
Data Synthesis
Pooled-adjusted univariate and multivariable ORs for each risk factor were calculated using a general inverse variance method with a random effects model. Heterogeneity between studies was investigated using I2 and the heterogeneity variance τ2. Calculations were performed with R (R Foundation for Statistical Computing) and the metagen-function from the meta-package.13 SE of the OR was calculated from the OR, the upper limit of the 95% CI, and the probability density of the 97.5% percentile from the normal distribution. Risk of publication bias was assessed by visual inspection of funnel plots for all risk factors reported in at least seven studies and was tested by Egger regression test if at least 10 studies reported a risk factor. In addition, we performed a cumulative meta-analysis for all risk factors with studies ranked starting from the lowest SE for all risk factors. Additional details are provided in e-Figures 1-4.
Results
Study Identification
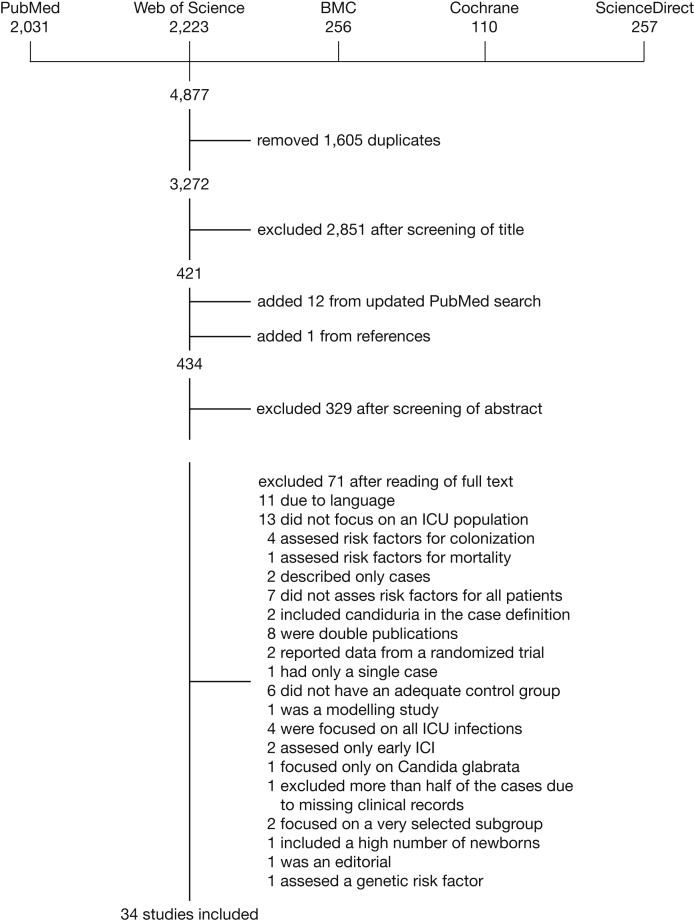
Through the literature search in MEDLINE and Embase, we identified 4,877 references. Duplicate references were identified and removed (n = 1,605), resulting in 3,272 articles. After screening of titles and abstracts and search updates, 104 articles were selected for full-text review, resulting in 34 studies included in this meta-analysis (Fig 1).
Figure 1.
Article flow through different stages of the review. BMC = Biomed Central.
Study Characteristics
A list of the 34 included studies with their characteristics is presented in Table 1.14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49, 50, 51 Of these studies,14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47 12 were prospective cohort studies in design. The remaining 22 studies were either retrospective cohort studies (n = 11) or case-control studies (n = 11). About one-half of the studies (n = 19, 55.9%) recruited patients during the years 2000 and 2010, six studies started recruitment between 1993 and 1999, and nine studies finished recruitment between 2011 and 2015. Median study duration was 2 years (interquartile range, 1-5 years). Most of the studies were monocenter studies (n = 27, 79.4%), whereas only five of the prospective and two of the retrospective cohorts were multicentric in design. In total, cohort studies encompassed 962 cases with ICI out of 86,603 patients and case-control studies encompassed 690 cases and 2,188 patients without ICI. The largest study was the Fungal Infection Risk Evaluation (FIRE) study by the National Institute of Health Research with 16,405 patients observed in 96 UK adult general critical care units.25 Table 1 presents the characteristics for each study. Five studies (14.7%) were judged to be of high quality, 18 (52.9%) of acceptable quality, and 11 (32.4%) of low quality (e-Figs 5, 6; e-Table 4). Three datasets were only published as congress abstracts. Two datasets were analyzed in several papers,27,30,48, 49, 50, 51 sometimes with variations in the included patients or outcomes.
Table 1.
Characteristics of Selected Studies
| Study | Inclusion Period | Design | Patients | Control Patients | Cases | Quality Indicator |
|---|---|---|---|---|---|---|
| Adiguzel et al14 | 2006 | Retrospective monocenter cohort | 163 | … | 26 | 4 |
| Agvald-Ohman et al15 | 2004-2005 | Prospective monocenter cohort | 59 | … | 10 | 6 |
| Ahmed et al16 | 2013-2014 | Prospective monocenter cohort | 198 | … | 17 | 7 |
| Arslan et al17 | Not reported | 1:1 case control | … | 140 | 139 | 6 |
| Blumberg et al18 | 1993-1995 | Prospective multicenter (n = 6) cohort | 4,276 | … | 42 | 9 |
| Burghi et al19,a | 2005-2010 | Retrospective monocenter cohort | 86 | … | 7 | 2 |
| Chander et al20 | 2009 | Retrospective monocenter cohort | 205 | … | 24 | 6 |
| Chow et al21 | 1995-2005 | 1:5 case-control | … | 780 | 146 | 8 |
| Eneh et al22,a | 2007-2009 | Retrospective monocenter cohort | 260 | … | Not reported | 0 |
| Hall et al23 | 2003-2011 | Retrospective monocenter cohort | 101 | … | 18 | 5 |
| Han et al24 | 2000-2006 | 1:3 case-control | … | 147 | 49 | 7 |
| Harrison et al25 | 2008-2010 | Prospective multicenter (n = 96) cohort | 16,405 | … | 85 | 8 |
| Hermsen et al26 | 2003-2008 | 1:3 case control | … | 264 | 88 | 9 |
| Jorda-Marcos et al,27 cohort also analyzed in two other publications48,49 | 1998-1999 | Prospective multicenter (n = 70) cohort | 1,765 | … | 63 | 9 |
| Kautzky et al28 | 2010-2011 | Prospective monocenter cohort | 65 | … | 5 | 5 |
| Kontopoulou et al29,a | 2010-2013 | Prospective monocenter cohort | 588 | … | 30 | 1 |
| Lau et al,30 cohort seems to overlap with two other publications50,51,b | 2007-2012 | Prospective multicenter (n = 7) cohort | 6,015 | … | 73 | 7 |
| Leleu et al31 | 1995-1997 | 1:1 case control | 49,063 | … | 149 | 4 |
| Leon et al32 | 2006-2007 | Prospective multicenter (n = 36) cohort | 1,107 | … | 58 | 6 |
| Liao et al33 | 2008-2011 | Retrospective monocenter cohort | 1,253 | … | 89 | 8 |
| Manolakaki et al34 | 2002-2007 | Retrospective monocenter cohort | 374 | … | 23 | 5 |
| Michalopoulos et al35 | 1997-1999 | 1:4 case control | … | 120 | 30 | 9 |
| Ortiz Ruiz et al36 | 2008-2012 | 1:2 case control | … | 162 | 81 | 9 |
| Ostrosky-Zeichner et al38 | 2000-2002 | Retrospective multicenter (n = 12) cohort | 2,890 | … | 88 | 7 |
| Ostrosky-Zeichner et al37 | 2005 | Retrospective multicenter (n = 6) cohort | 597 | … | 22 | 5 |
| Papadimitriou-Olivgeris et al39 | 2012-2015 | 1:7 case control | … | 371 | 53 | 8 |
| Paphitou et al40 | 2000 | Retrospective monocenter cohort | 327 | … | 36 | 6 |
| Pasero et al41 | 2005-2007 | Prospective monocenter cohort | 349 | … | 26 | 8 |
| Peres-Bota et al42 | 1999-2000 | Retrospective monocenter cohort | 280 | … | 31 | 6 |
| Posteraro et al43 | 2010 | Prospective monocenter cohort | 95 | … | 16 | 6 |
| Pratikaki et al44 | 2004-2006 | 1:4 case control | … | 132 | 33 | 6 |
| Presterl et al45 | 2004 | Prospective monocenter cohort | 82 | … | 24 | 6 |
| Tukenmez et al46 | 2011-2013 | 1:1 case control | … | 37 | 36 | 4 |
| Vardakas et al47 | 2001-2007 | 1:1 case control | … | 35 | 35 | 5 |
Data only reported as abstracts.
Authors were contacted for clarification several times but did not provide any additional information.
Unadjusted Risk Factors
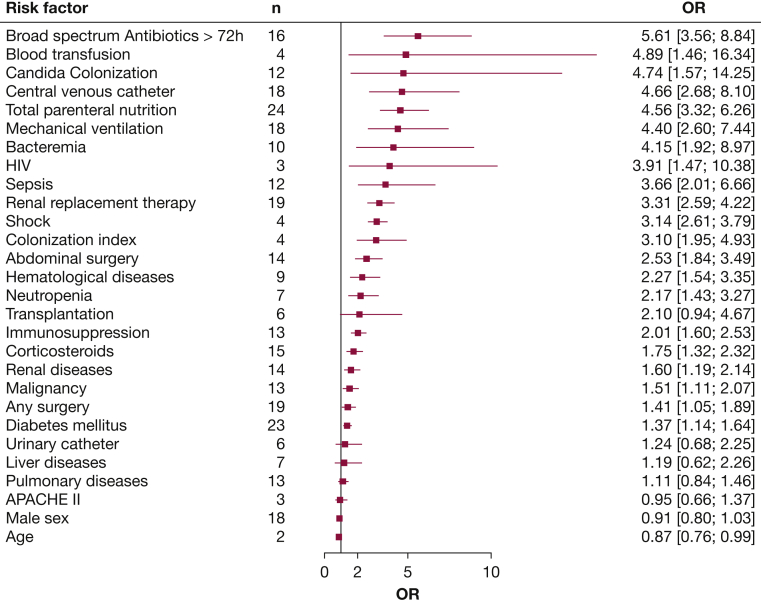
Twenty-nine risk factors were extracted from the selected publications. The results for all risk factors except ICU length of stay (e-Fig 7) are presented in Figure 2 (e-Fig 8). The risk factors associated with the highest risk for ICI were broad-spectrum antibiotics (OR, 5.6; 95% CI, 3.6-8.8), blood transfusion (OR, 4.9; 95% CI, 1.5-16.3), Candida colonization (OR, 4.7; 95% CI, 1.6-14.3), central venous catheter (OR, 4.7; 95% CI, 2.7-8.1), and total parenteral nutrition (OR, 4.6; 95% CI, 3.3-6.3). ICU length of stay, assessed by four studies, was an outlier with an extremely high risk (OR, 17.3; 95% CI, 4.1-73.0). Meta-analyses, differing definitions, funnel plots, and cumulative meta-analyses for individual risk factors are presented in e-Figures 9-85.
Figure 2.
Pooled ORs with 95% CIs for each risk factor. ICU length of stay as an extreme outlier was omitted and is presented in the Supplement (e-Fig 7). Meta-analyses for individual risk factors together with heterogeneity and sequential analysis are presented in the Supplement (e-Figs 9-85). APACHE = Acute Physiology And Chronic Health Evaluation.
Age17,28,34,39,42,47 (e-Fig 9), APACHE II17,39,42,47 (e-Fig 14), ICU length of stay46,47 (e-Fig 16), colonization index16 (e-Fig 70), and days of mechanical ventilation and renal replacement therapy42 (e-Figs 18, 21) were presented as continuous variables without ORs in several studies. The associations (and lack of associations) of the continuous variables with the risk of ICI were consistent with the findings from the pooled ORs. Heterogeneity measured by I2 showed a wide range (0%-96%), resulting in significant heterogeneity in 12 of 29 of the observed risk factors. Egger test showed a significant asymmetry of funnel plots only for central venous catheter (e-Fig 63). Cumulative meta-analyses revealed a relevant influence of less accurate studies only for bacteremia (e-Fig 55).
Restricting the analysis to cohort studies or studies of at least acceptable quality did not change results substantially (e-Figs 86-89). Restricting the analysis to the five highest-quality studies resulted in only 18 risk factors reported in median by 3 studies (interquartile range, 2-4 studies) (e-Fig 90), comprising 6,786 patients with 304 cases with ICI. Therefore, risk factor estimates supported by high-quality studies are limited.
Adjusted Risk Factors
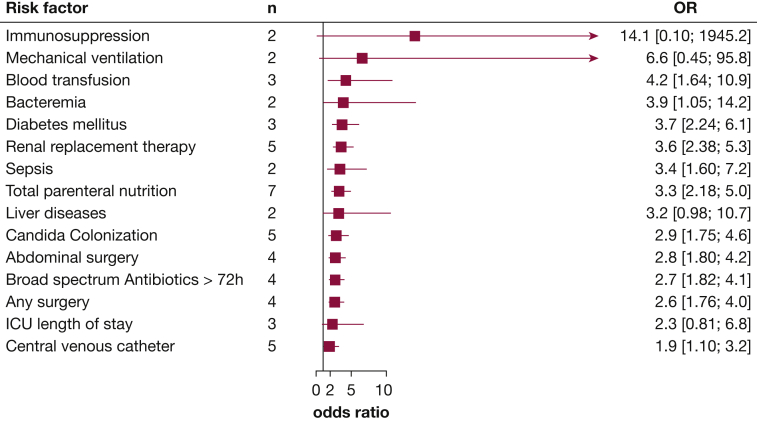
Multivariable analysis results were reported by 17 studies15,18,19,21,23, 24, 25,27,29,33, 34, 35, 36,39, 40, 41,44 (e-Table 5). All but one study23 used stepwise selection in their regression analysis and reported only the final model. Therefore, risk factors not added to the final model were unavailable for meta-analysis. Authors from two studies21,24 were able to provide regression coefficients for all predictors analyzed in their model on request. All others did either not respond, had no access to the modeling data anymore, or had used a forward selection approach.
Pooled ORs for 15 risk factors derived from multivariable analysis in at least two studies are presented in Figure 3 (e-Fig 91). ICU length of stay, which was associated with the highest OR in unadjusted analysis, was associated with no significant risk for ICI in the pooled adjusted analyses. Meta-analyses for individual risk factors including information on risk factors omitted from the final models are presented in e-Figures 92-131. Nine risk factors were only reported in one study and were not included in this meta-analysis (e-Figs 92, 93, 100-102, 105, 108, 121, 125). Funnel plot for parenteral nutrition gave no indication of publication bias (e-Fig 123).
Figure 3.
Pooled ORs with 95% CIs for each risk factor from multivariable analyses. Meta-analyses for individual risk factors from multivariable analyses are presented in the Supplement (e-Figs 92-131).
Discussion
We identified 34 articles that investigated risk factors for ICI in critically ill adults. Apart from confirming the widely recognized risk factors (eg, total parenteral nutrition, Candida colonization, [abdominal] surgery, broad-spectrum antibiotics, sepsis), our meta-analysis also identified renal replacement therapy, mechanical ventilation, blood transfusion, and diabetes as important risk factors.
There is biological plausibility for almost all risk factors significantly associated with ICI acquired in the ICU. In health, the immune system is capable in avoiding invasive fungal infections despite presence of these pathogens in primary unsterile body fluids or surfaces. Therefore, immunosuppression, including patients after transplantation or with HIV infections, solid and hematologic malignancies,52 neutropenia, and corticosteroid therapy, is an obvious risk factor for developing ICI. Liver cirrhosis,53 renal disease,54 diabetes,55 and blood transfusions56 are also associated with immune dysfunction. Candida albicans is the most common fungal pathogen that can form biofilms on host-associated abiotic surfaces, including implanted medical devices (eg, central venous, dialysis, or urinary catheters). Therefore, implanted medical devices or Candida colonization per se obviously provide a potential source for ICI. Surgery or chemotherapy52 might impair the natural body barrier. Especially, translocation from the GI tract may be an important issue because the gut is colonized with Candida species.57 Broad-spectrum antibiotic therapy increases Candida colonization58 and might severely affect bacterial and fungal microbiome interaction resulting in a higher pathogenic potential of Candida species.59 Similar effects have been postulated for oncological chemotherapy.52 Candida species are capable of multiplying in several parenteral nutrition solutions even in preparations where bacteria cannot grow.60,61 Parenteral nutrition solutions may therefore be contaminated with C albicans.62 Lipid-containing solutions propagate biofilm formation and germination of Candida species.63 Similarly, high glucose serum concentrations, also present in diabetes, can increase Candida biofilm formation and pathogenicity.55 Critically ill patients with renal failure are at risk for ICI because of immune dysfunction54 and renal replacement therapy via a vascular catheter instead of a shunt.64 Other factors (eg, contamination of the dialysate, colonization of the dialysis machines) have been described for the intermittent dialysis setting65,66 but have not been assessed for the continuous or slow extended daily dialysis on the ICU. Patients with sepsis or septic shock exhibit several risks for developing ICU including antibiotic therapy, invasive therapeutic strategies (implanted medical devices, surgery for source control), gut barrier dysfunction,67 and sepsis-induced immunosuppression.68
Length of stay in the ICU is the risk factor with the highest OR in the unadjusted analyses. However, there was significant heterogeneity between the studies, likely caused by different cutoffs, and we could not observe an e-table OR in the cumulative meta-analysis. ICU length of stay was extensively analyzed in a recent meta-analyses using wider inclusion criteria than ours but reaching similar results.69 This risk factor reflects time at risk and time under observation. A long ICU stay selects patients with severe disease and associated invasive therapies. Therefore, ICU length of stay as a risk factor for ICI is probably highly correlated with other risk factors. Indeed, the effect of ICU length of stay disappears in a study of patients with pancreatitis with 18 ICI cases after adjustment for Candida colonization.23 In our meta-analysis, ICU length of stay was not associated with the development of ICI in the meta-analysis of multivariable models. However, only three studies included this variable in their model.
Our wide meta-analysis approach has several limitations. Most of the included studies have only a limited number of ICI cases while assessing a large number of risk factors. Such an approach may result in chance findings. On the other hand, the large number of small studies published together with funnel plot inspection makes relevant publication bias unlikely. The assessed patient populations are very heterogeneous. Inclusion and exclusion criteria of the studies, their definitions of ICI, and their matching algorithms for case-control studies differ among the included studies. Some results from special populations might not be generalizable to less selected populations and vice versa. In addition, risk factor definitions or cutoffs differ between studies; most studies do not report definitions at all. Sometimes the wording for a risk factor changes within the same article. Because of these imprecisions in the underlying studies, we might in some cases have calculated common ORs of incongruous risk factors. To help readers with interpretation, we transparently report cutoffs and definitions in the supplement. Many of the assessed risk factors are highly correlated with each other. Therefore, multivariable analyses are of great importance to elucidate the real independent importance of each risk factor. However, only about one-half of the studies performed such an analysis, and the limited number of studies reporting each risk factor limits interpretability. Calculation of reliable common ORs was further hampered by the fact that most studies only reported statistically significant variables in their multivariable analysis. Model building for multivariable analysis including variable selection differed considerably between the studies. Ideally, all studies on such a topic should be consistent in their definitions and report complete multivariable models. A wider use of data repositories for epidemiologic studies in the ICU setting would enable individual patient data meta-analyses. Some studies were excluded because of language restrictions. They were published in six different languages not available in the study team and tended to be smaller studies. Because cumulative meta-analysis showed e-Table ORs for most risk factors, we do not think their inclusion would substantially change our findings. Quality assessment was difficult because all tools found by us were more focused on epidemiologic research in the population and not in a hospitalized cohort and a lot of the checked items were not reported in detail by most studies. Therefore, quality assessment might be less precise than in meta-analyses of randomized trials.
Our central finding is that a large number of risk factors are associated with ICU-acquired ICI, but the risk increase by each factor is relatively small or moderate at best. Future very large epidemiologic studies or individual patient data meta-analyses could only result in more precise estimators for this multitude of risk factors. The success of a risk-driven antifungal therapy5,6 is limited by these low ORs. It would require treating a large number of patients with only moderately elevated risk of ICI. This would result in overtreatment for a large proportion, or it would require a complicated risk assessment incorporating multiple factors to treat only the highest risk patients. Strategies using biomarkers in addition to clinical risk factors70, 71, 72 could be a solution for this dilemma and should be further assessed in large high-quality studies.
Interpretation
Our systematic review and meta-analysis identified acute and chronic factors that were associated with the risk for the development of ICI in the ICU. Most of the factors identified were either related to medical interventions during intensive care or to comorbid conditions. However, dependence between the various risk factors is probably high. The underlying studies do not sufficiently allow to identify those risk factors independently associated with ICI.
Acknowledgments
Author contributions: D. T.-R. and F. B. take responsibility for (are the guarantors of) the content of the manuscript, including the data and analysis. F. B. and D. T.-R. designed the study with important advice from M. P. and O. K. P. S. designed the meta-analysis approach for risk factor synthesis. F. B. did the literature search and D. T.-R. and F. B. did the study selection, data extraction, and risk of bias assessment. F. B. did the final analysis with advice from P. S. D. T.-R. drafted the manuscript, and all others contributed to the interpretation of results and the final manuscript. All authors read and approved the final manuscript for publication. F. B. and D. T.-R. had full access to and verified the underlying data.
Financial/nonfinancial disclosures: None declared.
Role of sponsors: The funding bodies had no role in the design of the study, in the collection, analysis, and interpretation of the data, or in writing the manuscript.
Additional information: The e-Figures and e-Tables can be found in the Supplemental Materials section of the online article.
Footnotes
FUNDING/SUPPORT: The study was partly funded by the Federal Ministry of Education and Research [Grant 01EO1502] via the Center for Sepsis Control and Care. The German National Reference Center NRZMyk is funded by the Robert Koch Institute from funds provided by the German Ministry of Health [Grant 1369-240].
Supplementary Data
References
- 1.Goemaere B., Becker P., Van Wijngaerden E., et al. Increasing candidaemia incidence from 2004 to 2015 with a shift in epidemiology in patients preexposed to antifungals. Mycoses. 2018;61(2):127–133. doi: 10.1111/myc.12714. [DOI] [PubMed] [Google Scholar]
- 2.Bassetti M., Garnacho-Montero J., Calandra T., et al. Intensive care medicine research agenda on invasive fungal infection in critically ill patients. Intensive Care Med. 2017;43(9):1225–1238. doi: 10.1007/s00134-017-4731-2. [DOI] [PubMed] [Google Scholar]
- 3.Zaoutis T.E., Argon J., Chu J., Berlin J.A., Walsh T.J., Feudtner C. The epidemiology and attributable outcomes of candidemia in adults and children hospitalized in the United States: a propensity analysis. Clin Infect Dis. 2005;41(9):1232–1239. doi: 10.1086/496922. [DOI] [PubMed] [Google Scholar]
- 4.Kollef M., Micek S., Hampton N., Doherty J.A., Kumar A. Septic shock attributed to Candida infection: importance of empiric therapy and source control. Clin Infect Dis. 2012;54(12):1739–1746. doi: 10.1093/cid/cis305. [DOI] [PubMed] [Google Scholar]
- 5.Pappas P.G., Kauffman C.A., Andes D.R., et al. Clinical Practice Guideline for the Management of Candidiasis: 2016 Update by the Infectious Diseases Society of America. Clin Infect Dis. 2016;62(4):e1–e50. doi: 10.1093/cid/civ933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rhodes A., Evans L.E., Alhazzani W., et al. Surviving Sepsis Campaign: International Guidelines for Management of Sepsis and Septic Shock: 2016. Intensive Care Med. 2017;43(3):304–377. doi: 10.1007/s00134-017-4683-6. [DOI] [PubMed] [Google Scholar]
- 7.Delaloye J., Calandra T. Invasive candidiasis as a cause of sepsis in the critically ill patient. Virulence. 2014;5(1):161–169. doi: 10.4161/viru.26187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ostrosky-Zeichner L. New approaches to the risk of Candida in the intensive care unit. Curr Opin Infect Dis. 2003;16(6):533–537. doi: 10.1097/00001432-200312000-00004. [DOI] [PubMed] [Google Scholar]
- 9.Eggimann P., Garbino J., Pittet D. Epidemiology of Candida species infections in critically ill non-immunosuppressed patients. Lancet Infect Dis. 2003;3(11):685–702. doi: 10.1016/s1473-3099(03)00801-6. [DOI] [PubMed] [Google Scholar]
- 10.Muskett H., Shahin J., Eyres G., Harvey S., Rowan K., Harrison D. Risk factors for invasive fungal disease in critically ill adult patients: a systematic review. Crit Care. 2011;15(6):R287. doi: 10.1186/cc10574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stroup D.F., Berlin J.A., Morton S.C., et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA. 2000;283(15):2008–2012. doi: 10.1001/jama.283.15.2008. [DOI] [PubMed] [Google Scholar]
- 12.De Pauw B., Walsh T.J., Donnelly J.P., et al. Revised definitions of invasive fungal disease from the European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group and the National Institute of Allergy and Infectious Diseases Mycoses Study Group (EORTC/MSG) Consensus Group. Clin Infect Dis. 2008;46(12):1813–1821. doi: 10.1086/588660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schwarzer G. meta: An R package for meta-analysis. R News. 2007;7(3):40–45. [Google Scholar]
- 14.Adiguzel N., Karakurt Z., Gungor G., et al. Mortality rates and risk factors associated with nosocomial Candida infection in a respiratory intensive care unit. Tuberkuloz ve toraks. 2010;58(1):35–43. [PubMed] [Google Scholar]
- 15.Agvald-Ohman C., Klingspor L., Hjelmqvist H., Edlund C. Invasive candidiasis in long-term patients at a multidisciplinary intensive care unit: Candida colonization index, risk factors, treatment and outcome. Scand J Infect Dis. 2008;40(2):145–153. doi: 10.1080/00365540701534509. [DOI] [PubMed] [Google Scholar]
- 16.Ahmed A., Baronia A.K., Azim A., et al. External validation of risk prediction scores for invasive candidiasis in a medical/surgical intensive care unit: an observational study. Indian J Critical Care Med. 2017;21(8):514–520. doi: 10.4103/ijccm.IJCCM_33_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Arslan F., Caskurlu H., Sari S., et al. Risk factors for noncatheter-related Candida bloodstream infections in intensive care units: a multicenter case-control study. Med Mycol. 2018;57(6):668–674. doi: 10.1093/mmy/myy127. [DOI] [PubMed] [Google Scholar]
- 18.Blumberg H.M., Jarvis W.R., Soucie J.M., et al. Risk factors for candidal bloodstream infections in surgical intensive care unit patients: the NEMIS prospective multicenter study. The National Epidemiology of Mycosis Survey. Clin Infect Dis. 2001;33(2):177–186. doi: 10.1086/321811. [DOI] [PubMed] [Google Scholar]
- 19.Burghi G., Ortiz G., Bagnulo H. Blood transfusions: an independent risk factor for the development of Candida infections in critically ill surgical patients. Crit Care. 2011;15(suppl 1):P237. [Google Scholar]
- 20.Chander J., Singla N., Sidhu S.K., Gombar S. Epidemiology of Candida blood stream infections: experience of a tertiary care centre in North India. J Infect Dev Ctries. 2013;7(9):670–675. doi: 10.3855/jidc.2623. [DOI] [PubMed] [Google Scholar]
- 21.Chow J.K., Golan Y., Ruthazer R., et al. Risk factors for albicans and non-albicans candidemia in the intensive care unit. Crit Care Med. 2008;36(7):1993–1998. doi: 10.1097/CCM.0b013e31816fc4cd. [DOI] [PubMed] [Google Scholar]
- 22.Eneh K., Zahir M., Patolia S., et al. Risk of candidemia in colonized non neutropenic critically ill patients: a retrospective analysis in an inner city hospital. Am J Respir Crit Care Med. 2010;181:A6135. [Google Scholar]
- 23.Hall A.M., Poole L.A.L., Renton B., et al. Prediction of invasive candidal infection in critically ill patients with severe acute pancreatitis. Crit Care. 2013;17(2):R49. doi: 10.1186/cc12569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Han S.-S., Yim J.-J., Yoo C.-G., et al. Clinical characteristics and risk factors for nosocomial candidemia in medical intensive care units: experience in a single hospital in Korea for 6.6 years. J Korean Med Sci. 2010;25(5):671–676. doi: 10.3346/jkms.2010.25.5.671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Harrison D., Muskett H., Harvey S., et al. Development and validation of a risk model for identification of non-neutropenic, critically ill adult patients at high risk of invasive Candida infection: the Fungal Infection Risk Evaluation (FIRE) Study. Health Technol Assess. 2013;17(3):1–156. doi: 10.3310/hta17030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hermsen E., Zapapas M., Maiefski M., Rupp M., Freifeld A., Kalil A. Validation and comparison of clinical prediction rules for invasive candidiasis in intensive care unit patients: a matched case-control study. Crit Care. 2011;15(4):R198. doi: 10.1186/cc10366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jorda-Marcos R., Alvarez-Lerma F., Jurado M., et al. Risk factors for candidaemia in critically ill patients: a prospective surveillance study. Mycoses. 2007;50(4):302–310. doi: 10.1111/j.1439-0507.2007.01366.x. [DOI] [PubMed] [Google Scholar]
- 28.Kautzky S., Staudinger T., Presterl E. Invasive Candida infections in patients of a medical intensive care unit: attempt of improving diagnosis by quantifying the colonization. Wien Klin Wochenschr. 2015;127(3-4):132–142. doi: 10.1007/s00508-014-0644-z. [DOI] [PubMed] [Google Scholar]
- 29.Kontopoulou K., Antypa E., Sgouropoulos I., Voloudakis N., Chassou E., Antoniadou E. Risk factors of candidemia in postoperative ICU patients: a prospective study. Crit Care. 2014;18(suppl 1):P347. [Google Scholar]
- 30.Lau A.F., Kabir M., Chen S.C.A., et al. Candida colonization as a risk marker for invasive candidiasis in mixed medical-surgical intensive care units: development and evaluation of a simple, standard protocol. J Clin Microbiol. 2015;53(4):1324–1330. doi: 10.1128/JCM.03239-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Leleu G., Aegerter P., Guidet B. Systemic candidiasis in intensive care units: a multicenter, matched-cohort study. J Crit Care. 2002;17(3):168–175. doi: 10.1053/jcrc.2002.35815. [DOI] [PubMed] [Google Scholar]
- 32.Leon C., Ruiz-Santana S., Saavedra P., et al. Usefulness of the “Candida score” for discriminating between Candida colonization and invasive candidiasis in non-neutropenic critically ill patients: a prospective multicenter study. Crit Care Med. 2009;37(5):1624–1633. doi: 10.1097/CCM.0b013e31819daa14. [DOI] [PubMed] [Google Scholar]
- 33.Liao Y., Zhong M.-K., Xu H.-B., Li L. Development and validation of a risk score for predicting invasive fungal infectious in an intensive care. Pharmazie. 2013;68(6):459–464. [PubMed] [Google Scholar]
- 34.Manolakaki D., Velmahos G.C., Kourkoumpetis T., et al. Candida infection and colonization among trauma patients. Virulence. 2010;1(5):367–375. doi: 10.4161/viru.1.5.12796. [DOI] [PubMed] [Google Scholar]
- 35.Michalopoulos A.S., Geroulanos S., Mentzelopoulos S.D. Determinants of candidemia and candidemia-related death in cardiothoracic ICU patients. Chest. 2003;124(6):2244–2255. doi: 10.1378/chest.124.6.2244. [DOI] [PubMed] [Google Scholar]
- 36.Ortiz Ruiz G., Osorio J., Valderrama S., et al. Risk factors for candidemia in non-neutropenic critical patients in Colombia. Med Intensiva. 2016;40(3):139–144. doi: 10.1016/j.medin.2015.08.001. [DOI] [PubMed] [Google Scholar]
- 37.Ostrosky-Zeichner L., Pappas P.G., Shoham S., et al. Improvement of a clinical prediction rule for clinical trials on prophylaxis for invasive candidiasis in the intensive care unit. Mycoses. 2011;54(1):46–51. doi: 10.1111/j.1439-0507.2009.01756.x. [DOI] [PubMed] [Google Scholar]
- 38.Ostrosky-Zeichner L., Sable C., Sobel J., et al. Multicenter retrospective development and validation of a clinical prediction rule for nosocomial invasive candidiasis in the intensive care setting. Eur J Clin Microbiol Infect Dis. 2007;26(4):271–276. doi: 10.1007/s10096-007-0270-z. [DOI] [PubMed] [Google Scholar]
- 39.Papadimitriou-Olivgeris M., Spiliopoulou A., Fligou F., et al. Risk factors and predictors of mortality of candidaemia among critically ill patients: role of antifungal prophylaxis in its development and in selection of non-albicans species. Infection. 2017;45(5):651–657. doi: 10.1007/s15010-017-1050-z. [DOI] [PubMed] [Google Scholar]
- 40.Paphitou N.I., Ostrosky-Zeichner L., Rex J.H. Rules for identifying patients at increased risk for candidal infections in the surgical intensive care unit: approach to developing practical criteria for systematic use in antifungal prophylaxis trials. Med Mycology. 2005;43(3):235–243. doi: 10.1080/13693780410001731619. [DOI] [PubMed] [Google Scholar]
- 41.Pasero D., De Rosa F.G., Rana N.K., et al. Candidemia after cardiac surgery in the intensive care unit: an observational study. Interact Cardiovasc Thorac Surg. 2011;12(3):374–378. doi: 10.1510/icvts.2010.257931. [DOI] [PubMed] [Google Scholar]
- 42.Peres-Bota D., Rodriguez-Villalobos H., Dimopoulos G., Melot C., Vincent J.L. Potential risk factors for infection with Candida spp. in critically ill patients. Clin Microbiol Infect. 2004;10(6):550–555. doi: 10.1111/j.1469-0691.2004.00873.x. [DOI] [PubMed] [Google Scholar]
- 43.Posteraro B., De Pascale G., Tumbarello M., et al. Early diagnosis of candidemia in intensive care unit patients with sepsis: a prospective comparison of (1-->3)-beta-D-glucan assay, Candida score, and colonization index. Crit Care. 2011;15(5):R249. doi: 10.1186/cc10507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pratikaki M., Platsouka E., Sotiropoulou C., et al. Epidemiology, risk factors for and outcome of candidaemia among non-neutropenic patients in a Greek intensive care unit. Mycoses. 2011;54(2):154–161. doi: 10.1111/j.1439-0507.2009.01787.x. [DOI] [PubMed] [Google Scholar]
- 45.Presterl E., Parschalk B., Bauer E., Lassnigg A., Hajdu S., Graninger W. Invasive fungal infections and (1,3)-β-d-glucan serum concentrations in long-term intensive care patients. Int J Infect Dis. 2009;13(6):707–712. doi: 10.1016/j.ijid.2008.10.013. [DOI] [PubMed] [Google Scholar]
- 46.Tukenmez Tigen E., Bilgin H., Perk Gurun H., et al. Risk factors, characteristics, and outcomes of candidemia in an adult intensive care unit in Turkey. Am J Infect Control. 2017;45(6):e61–e63. doi: 10.1016/j.ajic.2017.02.022. [DOI] [PubMed] [Google Scholar]
- 47.Vardakas K.Z., Michalopoulos A., Kiriakidou K.G., Siampli E.P., Samonis G., Falagas M.E. Candidaemia: incidence, risk factors, characteristics and outcomes in immunocompetent critically ill patients. Clin Microbiol Infect. 2009;15(3):289–292. doi: 10.1111/j.1469-0691.2008.02653.x. [DOI] [PubMed] [Google Scholar]
- 48.Leon C., Ruiz-Santana S., Saavedra P., et al. A bedside scoring system (“Candida score’) for early antifungal treatment in nonneutropenic critically ill patients with Candida colonization. Crit Care Med. 2006;34(3):730–737. doi: 10.1097/01.CCM.0000202208.37364.7D. [DOI] [PubMed] [Google Scholar]
- 49.Leon C., Alvarez-Lerma F., Ruiz-Santana S., et al. Fungal colonization and/or infection in non-neutropenic critically ill patients: results of the EPCAN observational study. Eur J Clin Microbiol Infect Dis. 2009;28(3):233–242. doi: 10.1007/s10096-008-0618-z. [DOI] [PubMed] [Google Scholar]
- 50.Playford E.G., Lipman J., Kabir M., et al. Assessment of clinical risk predictive rules for invasive candidiasis in a prospective multicentre cohort of ICU patients. Intensive Care Med. 2009;35(12):2141–2145. doi: 10.1007/s00134-009-1619-9. [DOI] [PubMed] [Google Scholar]
- 51.Playford E.G., Lipman J., Jones M., et al. Problematic dichotomization of risk for intensive care unit (ICU)-acquired invasive candidiasis: results using a risk-predictive model to categorize 3 levels of risk from a multicenter prospective cohort of Australian ICU patients. Clin Infect Dis. 2016;63(11):1463–1469. doi: 10.1093/cid/ciw610. [DOI] [PubMed] [Google Scholar]
- 52.Teoh F., Pavelka N. How chemotherapy increases the risk of systemic candidiasis in cancer patients: current paradigm and future directions. Pathogens. 2016;5(1):6. doi: 10.3390/pathogens5010006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Irvine K.M., Ratnasekera I., Powell E.E., Hume D.A. Causes and consequences of innate immune dysfunction in cirrhosis. Front Immunol. 2019;10:293. doi: 10.3389/fimmu.2019.00293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kato S., Chmielewski M., Honda H., et al. Aspects of immune dysfunction in end-stage renal disease. Clin J Am Soc Nephrol. 2008;3(5):1526–1533. doi: 10.2215/CJN.00950208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rodrigues C.F., Rodrigues M.E., Henriques M. Candida sp. infections in patients with diabetes mellitus. J Clin Med. 2019;8(1):76. doi: 10.3390/jcm8010076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Youssef L.A., Spitalnik S.L. Transfusion-related immunomodulation: a reappraisal. Curr Opin Hematol. 2017;24(6):551–557. doi: 10.1097/MOH.0000000000000376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kumamoto C.A. Inflammation and gastrointestinal Candida colonization. Curr Opin Microbiol. 2011;14(4):386–391. doi: 10.1016/j.mib.2011.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Guinan J., Wang S., Hazbun T.R., Yadav H., Thangamani S. Antibiotic-induced decreases in the levels of microbial-derived short-chain fatty acids correlate with increased gastrointestinal colonization of Candida albicans. Sci Rep. 2019;9(1):8872. doi: 10.1038/s41598-019-45467-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Seelbinder B., Chen J., Brunke S., et al. Antibiotics create a shift from mutualism to competition in human gut communities with a longer-lasting impact on fungi than bacteria. Microbiome. 2020;8(1):133. doi: 10.1186/s40168-020-00899-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kuwahara T., Shimono K., Kaneda S., Tamura T., Ichihara M., Nakashima Y. Growth of microorganisms in total parenteral nutrition solutions containing lipid. Int J Med Sci. 2010;7(3):101–109. doi: 10.7150/ijms.7.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kuwahara T., Kaneda S., Shimono K., Inoue Y. Growth of microorganisms in total parenteral nutrition solutions without lipid. Int J Med Sci. 2010;7(1):43–47. doi: 10.7150/ijms.7.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Guducuoglu H., Gultepe B., Otlu B., et al. Candida albicans outbreak associated with total parenteral nutrition in the neonatal unit. Indian J Med Microbiol. 2016;34(2):202–207. doi: 10.4103/0255-0857.180303. [DOI] [PubMed] [Google Scholar]
- 63.Swindell K., Lattif A.A., Chandra J., Mukherjee P.K., Ghannoum M.A. Parenteral lipid emulsion induces germination of Candida albicans and increases biofilm formation on medical catheter surfaces. J Infect Dis. 2009;200(3):473–480. doi: 10.1086/600106. [DOI] [PubMed] [Google Scholar]
- 64.Pyrgos V., Ratanavanich K., Donegan N., Veis J., Walsh T.J., Shoham S. Candida bloodstream infections in hemodialysis recipients. Med Mycol. 2009;47(5):463–467. doi: 10.1080/13693780802369332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Arvanitidou M., Spaia S., Velegraki A., et al. High level of recovery of fungi from water and dialysate in haemodialysis units. J Hosp Infect. 2000;45(3):225–230. doi: 10.1053/jhin.2000.0763. [DOI] [PubMed] [Google Scholar]
- 66.Oliveira L.T., Lopes L.G., Ramos S.B., Martins C.H.G., Jamur M.C., Pires R.H. Fungal biofilms in the hemodialysis environment. Microb Pathog. 2018;123:206–212. doi: 10.1016/j.micpath.2018.07.018. [DOI] [PubMed] [Google Scholar]
- 67.Meng M., Klingensmith N.J., Coopersmith C.M. New insights into the gut as the driver of critical illness and organ failure. Curr Opin Crit Care. 2017;23(2):143–148. doi: 10.1097/MCC.0000000000000386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hotchkiss R.S., Monneret G., Payen D. Sepsis-induced immunosuppression: from cellular dysfunctions to immunotherapy. Nat Rev Immunol. 2013;13(12):862–874. doi: 10.1038/nri3552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zhang Z., Zhu R., Luan Z., Ma X. Risk of invasive candidiasis with prolonged duration of ICU stay: a systematic review and meta-analysis. BMJ Open. 2020;10(7):e036452. doi: 10.1136/bmjopen-2019-036452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Rouze A., Loridant S., Poissy J., et al. Biomarker-based strategy for early discontinuation of empirical antifungal treatment in critically ill patients: a randomized controlled trial. Intensive Care Med. 2017;43(11):1668–1677. doi: 10.1007/s00134-017-4932-8. [DOI] [PubMed] [Google Scholar]
- 71.Bloos F., Held J., Schlattmann P., et al. (1,3)-beta-D-glucan-based diagnosis of invasive Candida infection versus culture-based diagnosis in patients with sepsis and with an increased risk of invasive Candida infection (CandiSep): study protocol for a randomized controlled trial. Trials. 2018;19(1):472. doi: 10.1186/s13063-018-2868-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.De Pascale G., Posteraro B., D’Arrigo S., et al. (1,3)-beta-D-Glucan-based empirical antifungal interruption in suspected invasive candidiasis: a randomized trial. Crit Care. 2020;24(1):550. doi: 10.1186/s13054-020-03265-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.