Abstract
Fatigue is the most commonly encountered symptom in patients with chronic liver disease (CLD). The resulting decrease in quality of life contributes markedly to the societal costs of fatigue. Moreover, fatigue is associated with social dysfunction, increased daytime somnolence, impaired working ability, and increased risk of mortality. Fatigue is not related to the severity of the underlying liver fibrosis or dysfunction. In CLD patients, fatigue manifests with both central symptoms, characterised by cognitive impairment, sleep disturbance, apathy, and autonomic dysfunction, and peripheral symptoms, characterised by decreased exercise tolerance and reduced physical activity levels. The pathogenesis of fatigue in CLD is multifactorial and involves changes in the brain–liver axis resulting from changes in inflammatory cytokines or the gut microbiome. Numerous interventions have attempted to alleviate fatigue in CLD by improving its central and peripheral manifestations or the underlying liver disease. Currently, however, there are no widely accepted or effective treatments for fatigue in CLD patients. In this review, we highlight the problem of fatigue in CLD, the current theories regarding its pathogenesis, and current approaches to its treatment.
Keywords: fatigue, liver diseases
Introduction
Chronic liver disease (CLD) affects over 1.5 billion people worldwide [1], and the prevalence of CLD is rapidly rising, in particular due the increasing number of individuals with nonalcoholic fatty liver disease (NAFLD). CLD can have different aetiologies, including viral hepatitis, autoimmune liver diseases (autoimmune hepatitis (AIH), primary biliary cholangitis (PBC), primary sclerosing cholangitis (PSC)), alcoholic liver disease (ALD), and metabolic and genetic conditions (Wilson’s disease, haemochromatosis) [1, 2].
The management of CLD is commonly focused on the prevention of progression to cirrhosis through the effective treatment of the underlying liver disease, the treatment of the complications of cirrhosis in the setting of end-stage liver disease and, where necessary, liver transplantation. The management of symptoms has previously been of secondary importance [3]. Now, however, it is being increasingly recognised that CLD can impair quality of life (QoL). Related changes include increased fatigue, non-encephalopathic cognitive impairment, autonomic dysfunction, a loss of appetite, or mood alternation (anxiety, depression) [4, 5]. These symptoms can occur at any stage of liver disease and may not be alleviated with treatment of the underlying process. The most common symptom reported by patients with CLD is fatigue [4–6]. Fatigue is common and is experienced by everyone during their lives, but it is a complex symptom that includes lethargy, exhaustion, and malaise. It can present as a specific clinical problem or as an occult problem not linked to liver disease; this makes it easy to miss in clinical assessment. The management of extrahepatic symptoms and their impact on QOL is an important aspect of treatment for CLD patients.
The aim of this review is to highlight the problem of fatigue in CLD, the current theories on its pathogenesis, and, perhaps most importantly to patients, current treatment approaches.
Prevalence of fatigue in CLD patients
The term “fatigue” can be used to describe a subjective sense of weakness in terms of difficulty initiating any activity, readily becoming tired during activities, or a mental tiredness involving difficulties with concentration, memory, and emotional stability [7]. Careful history examination has revealed that patients often describe fatigue as sleepiness or an uncontrollable need to sleep. Acute fatigue is defined as fatigue lasting for one month or less, subacute fatigue as lasting between 1 and 6 months, and chronic fatigue as lasting for over 6 months [8].
Most studies on fatigue in CLD patients separate it into 2 types: peripheral and central. Peripheral fatigue is manifested by muscle weakness and is associated with neuromuscular dysfunction at the peripheral nervous system and muscular levels [5, 9]. Recent studies [10] have described muscle metabolism changes in patients with CLD, but, in general, peripheral fatigue is not a major problem in the early stages of CLD. On the other hand, it causes the main burden in the setting of cirrhosis and liver failure, where it is associated with sarcopaenia and increased muscle catabolism [5].
Central fatigue, which results from changes in neurotransmission within the brain, is characterised by a lack of self-motivation to initiate or sustain attentional tasks. Because of its central origin, it can overlap with other neuropsychiatric symptoms such as depression and anxiety [5, 9, 11].
Fatigue is a common complaint among CLD patients, with a prevalence of between 50% and 85% [12] (Table I). It mostly interferes with physical activity, family life, and the ability to work [13]. Fatigue has been best characterised in the context of PBC [14, 15], along with the development of special tools and diagnostic criteria [16, 17], but it is worth mentioning that it may also affect everyday activities in a significant proportion of patients with NAFLD and ALD [18]. Moreover, it may play an important role in the course of drug-induced liver injury (DILI) [19]. Fatigue is a significant problem for patients who have acute presentation of viral and autoimmune hepatitis, and it often corresponds with hepatic inflammation. It usually subsides while antiviral or immunosuppressive treatment is being received [11, 20, 21]. On the other hand, it is well documented that fatigue reduces the QoL in patients with chronic hepatitis C (HCV) because it can impair cognitive abilities and reduce work performance and is associated with a higher prevalence of depression in this group of patients [11, 20]. Inversely, there is less evidence for the impact of fatigue on chronic hepatitis B (HBV) patients who have been shown, in some studies, to have QoL scores similar to those of healthy controls [11, 22]. In summary, the severity of fatigue is not related to the severity of CLD, so CLD management will not necessarily ameliorate fatigue symptoms.
Table I.
Prevalence of fatigue in different liver disorders
| Condition | NAFLD | ALD | AIH | PSC | PBC | DILI | HBV | HCV |
|---|---|---|---|---|---|---|---|---|
| Prevalence of fatigue | 70% [18] | 75% [18] | 50% [55] | 35–50% [56] | 68–85% [39] 45% [15] |
40% [19] | 90% [57] | 50% [58] |
Chronically fatigued CLD patients should be under special supervision, which is almost impossible to achieve in the real world, but fatigue may negatively impact patients’ survival. Follow-up studies conducted in a geographically defined cohort of PBC patients showed that higher fatigue scores at baseline are associated with significantly reduced survival rates [23]. The mechanism associated with this relationship is yet to be determined.
Pathophysiology of fatigue in CLD
The pathogenesis of fatigue in CLD remains unclear. The most popular concept assumes the existence of peripheral pathways between the liver and brain which, when activated, can lead to neurotransmission changes within the brain and the development of sickness-associated behaviours, including fatigue (Figure 1) [6, 24].
Figure 1.
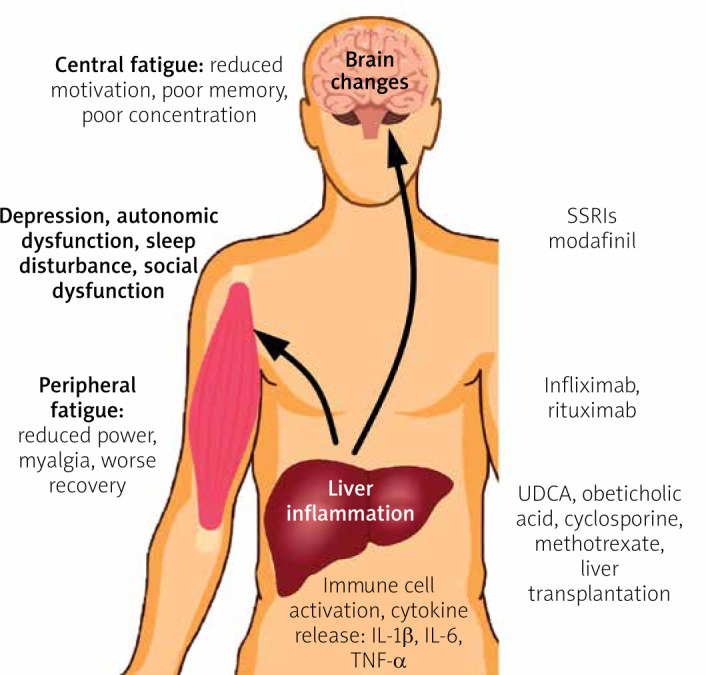
Pathogenesis and potential treatment of fatigue in CLD
The inflammation of the liver involves the production of increased amounts of inflammatory cytokines, such as interleukin (IL) 1β (IL-1β), IL-6, and tumor necrosis factor α (TNF-α), which can affect brain neurotransmission via neural routes through the activation of vagal nerve afferents, or via a humoral route when carried to the brain within the blood circulation. Both of these routes can directly or indirectly modify brain neurotransmission through the stimulation of cerebral endothelial cells and microglia. On the other hand, liver inflammation is associated with the activation of immune cells that are carried within the circulation and can enter brain parenchyma. Activated immune cells can initiate inflammation within the brain through local cytokine release, which leads to neurotransmission changes. The pathogenesis of central fatigue is linked to alterations in neural pathways associated with the regulation of motivational and reward behaviours. The occurrence of CLD can contribute to changes in the basal ganglia [6, 11].
One of the potential factors modulating the brain–liver axis is the gut microbiome. Recent data suggest that it plays roles in mood disturbances and behavioural changes in the setting of CLD [25]. A study conducted in an animal model of cholestatic liver disease showed a reduction in sickness-associated behaviours after administration of the probiotic mixture VSL#3 [26]. Because of the increased gut permeability and interruption of the detoxication process, increased levels of endotoxin are found in patients with CLD. Endotoxin is a TLR4 ligand, and TLR4 was recently identified as a key regulator in signalling between activated immune cells and the brain [27, 28]. This suggests that manipulation of the gut microbiome using pro- or pre-biotics may be a promising option for the treatment of extrahepatic symptoms, including fatigue, in CLD patients.
Studies on inflammatory diseases, such as rheumatoid arthritis (RA) and inflammatory bowel disease (IBD), have confirmed a link between elevated levels of the circulating proinflammatory cytokine TNF-α and the development of fatigue [29]. In the setting of CLD, similar data have emerged from animal model studies [4]. A study of over 2000 PBC patients confirmed the presence of an association between fatigue and autonomic vasomotor dysfunction, which leads to postural hypotension and secondary tachycardia [15]. Interestingly, in the DIANA study, elevated TNF-α levels were linked to autonomic dysfunction in arthritis patients [30]. This suggests that the inhibition of TNF-α production may be the next approach for treating fatigue and associated autonomic dysfunction in CLD patients.
Advanced imaging techniques allow us to describe changes within the central neural systems of fatigued patients. In a study of PBC patients, changes were described in the basal ganglia [31]. The basal ganglia contain subcortical nuclei, such as the subthalamic nucleus, globus pallidus, substantia nigra, and striatum, which, respectively, contain the nucleus accumbens, putamen, and caudate nucleus. They regulate the motor functions and play an important role in processes like learning, motivation, and reward-guided behaviour. They are connected to the brain cortex through the striatal–cortical pathway. One hypothesis postulates that changes in the basal ganglia can lead to the development of central fatigue by interrupting the scheme of motivational behaviour and the effort–reward balance [32]. These findings have also been observed in patients with structural changes within the basal ganglia during the course of neurologic diseases such as multiple sclerosis and Parkinson’s disease, who also suffered from fatigue [32, 33]. Structural changes in the basal ganglia can be observed in cirrhotic patients, but in PBC they can occur at the early stages of the disease, even within 6 months of diagnosis [34]. The pathomechanism of these changes is still unclear and needs further investigation.
Normal neural activity results from correct interactions between various neurotransmitter systems. There is no specific pathway that regulates fatigue, but dopaminergic and serotonergic neurotransmission seems to be a key factor. Dopamine is a central neurotransmitter involved in the regulation of reward and motivational behaviour, which is responsible for signalling within the basal ganglia. Any imbalance in the dopaminergic system due to either central (e.g. multiple sclerosis, Parkinson’s disease) or peripheral (RA, liver disease) inflammation leads to decreased motivation and fatigue [35, 36]. On the other hand, mood alternations and anxiety are commonly associated with serotonin. Serotonergic neural pathways can regulate dopamine release in the basal ganglia through stimulation of its receptors, such as 5-HT1A and 5-HT3. In the clinical setting, the administration of the 5-HT3 antagonist ondansetron has been associated with the amelioration of fatigue in HCV patients [37]. Interestingly, there was no improvement in patients with PBC [38]. This can probably be explained by the occurrence of serotonergic neurotransmission alternations in HCV patients. Because of the close relationship between the dopaminergic and serotonergic neural pathways, central fatigue can be complicated by other neuropsychiatric symptoms such as depression and anxiety. In a study of PBC patients, 44.8% met the criteria for the diagnosis of depression based on the Beck Depression Inventory (BDI). This study also identified a significant correlation between the level of fatigue and the presence of depression, which suggests that severe fatigue may induce secondary depression [39]. Moreover, it is difficult to distinguish between fatigue and depression using standard questionnaires. A study on depression in PSC and PBC revealed that 42% of patients had depressive symptoms based on the self-report BDI scale, but when interviewed using a structured psychiatric interview, only 3.7% met the DSM-IV criteria for depressive syndrome [40]. The authors suggested that fatigue in patients with PSC and PBC cannot be explained by depression. This also underlines that most depression questionnaires include questions about fatigue-related symptoms.
Management of fatigue in CLD
Pharmacological treatment
Because of the impact of fatigue on the QoL of patients with PBC, most interventions used to alleviate this symptom have been performed on this specific group of patients. These interventions include 2 types of strategy: PBC-specific therapies and fatigue-directed therapies. The first group includes the use of ursodeoxycholic acid (UDCA), obeticholic acid (OCA), fibrates, and liver transplantation (LT). UDCA is commonly known to be effective for the treatment of PBC, and it reduces the risk of death and liver transplantation. However, a recent meta-analysis showed no improvement regarding the relative risk of fatigue after treatment with UDCA [41]. Similarly, the only study on fatigue outcomes after the use of OCA demonstrated no improvement [41]. Based on a recent meta-analysis, LT reduced fatigue in PBC patients, but PBC patients with fatigue remain more fatigued after LT than controls [42]. These arguments confirm that effective treatment of the underlying liver disease does not reduce fatigue nor indicate the extrahepatic cause of fatigue. Fatigue-directed therapies involving the use of the selective serotonin reuptake inhibitors (SSRIs). Fluvoxamine [43] and fluoxetine [44] have also been shown to be ineffective in patients with PBC with no concomitant depression. There are no data on the effectiveness of this type of therapy for treating fatigue in patients with co-existing depression. Initial studies on modafinil, an eugeroic, showed a reduction in daytime sleepiness and a decrease in fatigue scores, bringing some promising data into the field [45, 46]. However, a long-term study and randomised controlled trial did not demonstrate the efficacy of modafinil in the treatment of fatigue in PBC patients [47].
Observations from immune-mediated inflammatory diseases (RA, IBD) indicate that the use of anti-TNF-α agents is associated with a significant reduction in fatigue [48]. Anti-TNF-α agents are effectively used for the treatment of refractory autoimmune hepatitis [49]. Despite the high cost and risk of severe side effects, this kind of therapy could be considered for the treatment of severe fatigue in CLD patients in the future. On the other hand, the inhibition of TNF-α production is one of the mechanisms of action of other promising agents, like pentoxifylline (PTX) and S-adenosylmethionine (SAMe). PTX has been shown to reduce fatigue and the clinical course of NASH [50]. Therefore, it may be considered in future studies on CLD. Supplementation with SAMe, the most important methyl donor in humans, was observed to have beneficial effects on liver biochemistry and extrahepatic symptoms, including reduced fatigue in patients with cholestatic liver disease [51, 52]. However, although this agent is in use in many centres worldwide, there are still a lack of solid data to implement this treatment in everyday practice.
Nonpharmacological strategies
As specific pharmacological interventions for fatigue in CLD patients are limited, supportive management is currently recommended. An example of a structured approach that was initially developed for the management of fatigue in PBC is TrACE (Treat, Ameliorate, Cope, Empathise) (Table II) [53]. It can be applied to all extrahepatic symptoms in all CLD patient groups. Direct management should be focussed specifically on identifying and treating the underlying liver disease and non-disease-specific causes of fatigue (e.g. hypothyroidism in PBC or type 2 diabetes in NAFLD) (Table III). This intervention represents the “treat” element of the approach. The “ameliorate” element of the algorithm involves the identification and modification of factors that can worsen fatigue, e.g. sleep disturbances, autonomic dysfunction, itching, etc. At this point, there are some specific interventions that can be used, such as advising the patient about sleep hygiene, increasing fluid intake, or taking care when standing from a lying position in patients with autonomic dysfunction. It is worth mentioning modafinil because it can be useful to treat daytime somnolence in PBC patients. Moreover, where possible, it is suggested that drugs that can exacerbate fatigue are discontinued (e.g. β-blockers). It is crucial for patients to retain ownership of the problem and for clinicians to be there to help to create strategies to “cope” together. This element consists of lifestyle changes, which should be openly discussed with patients. These changes may include a reduction in workload, a gradual increase in physical activity, or the provision of individual or group therapy. Cognitive behavioural therapy may play a beneficial role in managing fatigue for some patients [54]. The final point of this concept is to “empathise”. Clinicians need to be understanding and aware of their abilities and barriers in the management of fatigue in CLD patients.
Table II.
The TrACE approach to the management of fatigue in CLD patients
| Treat the treatable | Underlying liver disease: autoimmune, viral, metabolic, etc. |
| Hypothyroidism | |
| Arthritis | |
| Coeliac disease | |
| Type 2 diabetes mellitus | |
| Anaemia | |
| Ameliorate the ameliorable | Sleep disturbance (especially daytime somnolence) |
| Autonomic dysfunction | |
| Depression | |
| Itch | |
| Avoid or discontinue benzodiazepines, antidepressants, muscle relaxants, first-generation antihistamines, β-blockers, opioids | |
| Cope | “Ownership of the problem” |
| Help patients to develop coping strategies | |
| Physical activity and diet | |
| Psychological help | |
| Social support | |
| Empathise | Try to understand the impact of fatigue on the patient |
| Be optimistic: “don’t fail before you start” |
Table III.
Differential diagnoses of causes of subacute and chronic fatigue
| Conditions | |
|---|---|
| Cardiopulmonary | Congestive heart failure Chronic obstructive pulmonary disease Sleep apnoea |
| Endocrinologic/metabolic | Hypothyroidism Hyperthyroidism Chronic renal disease Adrenal insufficiency Electrolyte abnormalities: Hyponatraemia Hypercalcaemia |
| Haematologic/neoplastic | Anaemia Occult malignancy |
| Infectious diseases | Mononucleosis syndrome Viral hepatitis HIV infection Subacute bacterial endocarditis Tuberculosis |
| Rheumatologic | Fibromyalgia Polymyalgia rheumatica Rheumatoid arthritis |
| Neurological | Multiple sclerosis Parkinson’s disease |
| Psychological | Depression Anxiety disorder Somatisation disorder |
| Medication toxicity | Benzodiazepines, antidepressants, muscle relaxants, first-generation antihistamines, β-blockers, opioids |
| Substance use | Alcohol, marijuana, opioids, cocaine/other stimulants |
Conclusions
Fatigue continues to represent a major, but commonly unrecognised, debilitating symptom in patients with chronic liver diseases. Therefore, there is a need to increase the awareness of the impact of extrahepatic symptoms during the course of CLD. Much effort has been expended to explain the potential drivers of fatigue in CLD, but our knowledge in this area remains incomplete. A better understanding of the underlying pathophysiological mechanisms may provide new therapeutic options, which will bring relief from this debilitating symptom. Despite the lack of targeted therapies, fatigue should be managed through a supportive approach.
Conflict of interest
The authors declare no conflict of interest.
References
- 1.Moon AM, Singal AG, Tapper EB. Contemporary epidemiology of chronic liver disease and cirrhosis. Clin Gastroenterol Hepatol 2020; 18: 2650-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.An K, Jallo N, Menzies V, et al. Integrative review of co-occurring symptoms across etiologies of chronic liver disease and implications for symptom management research and practice. J Nurs Scholarsh 2015; 47: 310-7. [DOI] [PubMed] [Google Scholar]
- 3.Janik MK, Wunsch E, Raszeja-Wyszomirska J, et al. Autoimmune hepatitis exerts a profound, negative effect on health-related quality of life: a prospective, single-centre study. Liver Int 2019; 39: 215-21. [DOI] [PubMed] [Google Scholar]
- 4.D’Mello C, Swain MG. Liver-brain interactions in inflammatory liver diseases: implications for fatigue and mood disorders. Brain Behav Immun 2014; 35: 9-20. [DOI] [PubMed] [Google Scholar]
- 5.Swain MG. Fatigue in liver disease: pathophysiology and clinical management. Can J Gastroenterol 2006; 20: 181-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.D’Mello C, Swain MG. Liver-brain inflammation axis. Am J Physiol Gastrointest Liver Physiol 2011; 301: 749-61. [DOI] [PubMed] [Google Scholar]
- 7.Markowitz AJ, Rabow MW. Palliative management of fatigue at the close of life: “it feels like my body is just worn out”. JAMA 2007; 298: 217. [DOI] [PubMed] [Google Scholar]
- 8.Rosenthal TC, Majeroni BA, Pretorius R, et al. Fatigue: an overview. Am Fam Physician 2008; 78: 1173-9. [PubMed] [Google Scholar]
- 9.Austin PW, Gerber L, Karrar AK. Fatigue in chronic liver disease: exploring the role of the autonomic nervous system. Liver Int 2015; 35: 1489-91. [DOI] [PubMed] [Google Scholar]
- 10.De Bandt JP, Jegatheesan P, Tennoune-El-Hafaia N. Muscle loss in chronic liver diseases: the example of nonalcoholic liver disease. Nutrients 2018; 10: 1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Swain MG, Jones DEJ. Fatigue in chronic liver disease: new insights and therapeutic approaches. Liver Int 2019; 39: 6-19. [DOI] [PubMed] [Google Scholar]
- 12.Golabi P, Sayiner M, Bush H, et al. Patient-reported outcomes and fatigue in patients with chronic hepatitis C infection. Clin Liver Dis 2017; 21: 565-78. [DOI] [PubMed] [Google Scholar]
- 13.Cauch-Dudek K, Abbey S, Stewart DE, et al. Fatigue in primary biliary cirrhosis. Gut 1998; 43: 705-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Newton JL. Fatigue in primary biliary cirrhosis. Clin Liver Dis 2008; 12: 367-83. [DOI] [PubMed] [Google Scholar]
- 15.Mells GF, Pells G, Newton JL, et al. Impact of primary biliary cirrhosis on perceived quality of life: the UK-PBC national study. Hepatology 2013; 58: 273-83. [DOI] [PubMed] [Google Scholar]
- 16.Jacoby A, Rannard A, Buck D, et al. Development, validation, and evaluation of the PBC-40, a disease specific health related quality of life measure for primary biliary cirrhosis. Gut 2005; 54: 1622-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Raszeja-Wyszomirska J, Wunsch E, Krawczyk M, et al. Assessment of health related quality of life in Polish patients with primary biliary cirrhosis. Clin Res Hepatol Gastroenterol 2016; 40: 471-9. [DOI] [PubMed] [Google Scholar]
- 18.Elliott C, Frith J, Day CP, et al. Functional impairment in alcoholic liver disease and non-alcoholic fatty liver disease is significant and persists over 3 years of follow-up. Dig Dis Sci 2013; 58: 2383-91. [DOI] [PubMed] [Google Scholar]
- 19.Chen SS, Yu KK, Huang C, et al. The characteristics and clinical outcome of drug-induced liver injury in a Chinese hospital: a retrospective cohort study. Medicine 2016; 95: 4683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Adinolfi LE, Nevola R, Lus G, et al. Chronic hepatitis C virus infection and neurological and psychiatric disorders: an overview. World J Gastroenterol 2015; 21: 2269-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schramm C, Wahl I, Weiler-Normann C, et al. Health-related quality of life, depression, and anxiety in patients with autoimmune hepatitis. J Hepatol 2014; 60: 618-24. [DOI] [PubMed] [Google Scholar]
- 22.Enescu A, Mitrut P, Balasoiu M, et al. Psychosocial issues in patients with chronic hepatitis B and C. Curr Health Sci J 2014; 40: 93-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jones DE, Al-Rifai A, Frith J, et al. The independent effects of fatigue and UDCA therapy on mortality in primary biliary cirrhosis: results of a 9 year follow-up. J Hepatol 2010; 53: 911-7. [DOI] [PubMed] [Google Scholar]
- 24.Jopson L, Dyson JK, Jones DE. Understanding and treating fatigue in primary biliary cirrhosis and primary sclerosing cholangitis. Clin Liver Dis 2016; 20: 131-42. [DOI] [PubMed] [Google Scholar]
- 25.D’Mello C, Swain MG. Immune-to-brain communication pathways in inflammation-associated sickness and depression. Curr Top Behav Neurosci 2017; 31: 73-94. [DOI] [PubMed] [Google Scholar]
- 26.D’Mello C, Ronaghan N, Zaheer R, et al. Probiotics improve inflammation-associated sickness behavior by altering communication between the peripheral immune system and the brain. J Neurosci 2015; 35: 10821-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.D’Mello C, Almishri W, Liu H, et al. Interactions between platelets and inflammatory monocytes affect sickness behavior in mice with liver inflammation. Gastroenterology 2017; 153: 1416-28. [DOI] [PubMed] [Google Scholar]
- 28.Woodhouse CA, Patel VC, Singanayagam A, et al. Review article: the gut microbiome as a therapeutic target in the pathogenesis and treatment of chronic liver disease. Aliment Pharmacol Ther 2018; 47: 192-202. [DOI] [PubMed] [Google Scholar]
- 29.Russell AS, Gulliver WP, Irvine EJ, et al. Quality of life in patients with immune-mediated inflammatory diseases. J Rheumatol Suppl 2011; 88: 7-19. [DOI] [PubMed] [Google Scholar]
- 30.Syngle A, Verma I, Krishan P, et al. Disease-modifying anti-rheumatic drugs improve autonomic neuropathy in arthritis: DIANA study. Clin Rheumatol 2015; 34: 1233-41. [DOI] [PubMed] [Google Scholar]
- 31.Forton DM, Patel N, Prince M, et al. Fatigue and primary biliary cirrhosis: association of globus pallidus magnetisation transfer ratio measurements with fatigue severity and blood manganese levels. Gut 2004; 53: 587-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dobryakova E, DeLuca J, Genova HM, et al. Neural correlates of cognitive fatigue: cortico-striatal circuitry and effort-reward imbalance. J Int Neuropsychol Soc 2013; 19: 849-53. [DOI] [PubMed] [Google Scholar]
- 33.Finke C, Schlichting J, Papazoglou S, et al. Altered basal ganglia functional connectivity in multiple sclerosis patients with fatigue. Mult Scler 2015; 21: 925-34. [DOI] [PubMed] [Google Scholar]
- 34.Grover VP, Southern L, Dyson JK, et al. Early primary biliary cholangitis is characterised by brain abnormalities on cerebral magnetic resonance imaging. Aliment Pharmacol Ther 2016; 44: 936-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chaudhuri A, Behan PO. Fatigue in neurological disorders. Lancet 2004; 363: 978-88. [DOI] [PubMed] [Google Scholar]
- 36.Felger JC, Treadway MT. Inflammation effects on motivation and motor activity: role of dopamine. Neuropsychopharmacology 2017; 42: 216-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Piche T, Vanbiervliet G, Cherikh F, et al. Effect of ondansetron, a 5-HT3 receptor antagonist, on fatigue in chronic hepatitis C: a randomised, double blind, placebo controlled study. Gut 2005; 54: 1169-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Theal JJ, Toosi MN, Girlan L, et al. A randomized, controlled crossover trial of ondansetron in patients with primary biliary cirrhosis and fatigue. Hepatology 2005; 41: 1305-12. [DOI] [PubMed] [Google Scholar]
- 39.Huet PM, Deslauriers J, Tran A, et al. Impact of fatigue on the quality of life of patients with primary biliary cirrhosis. Am J Gastroenterol 2000; 95: 760-7. [DOI] [PubMed] [Google Scholar]
- 40.van Os E, van den Broek WW, Mulder PG, et al. Depression in patients with primary biliary cirrhosis and primary sclerosing cholangitis. J Hepatol 2007; 46: 1099-103. [DOI] [PubMed] [Google Scholar]
- 41.Lee JY, Danford CJ, Trivedi HD, et al. Treatment of fatigue in primary biliary cholangitis: a systematic review and meta-analysis. Dig Dis Sci 2019; 64: 2338-50. [DOI] [PubMed] [Google Scholar]
- 42.Carbone M, Bufton S, Monaco A, et al. The effect of liver transplantation on fatigue in patients with primary biliary cirrhosis: a prospective study. J Hepatol 2013; 59: 490-4. [DOI] [PubMed] [Google Scholar]
- 43.ter Borg PC, van Os E, van den Broek WW, et al. Fluvoxamine for fatigue in primary biliary cirrhosis and primary sclerosing cholangitis: a randomised controlled trial. BMC Gastroenterol 2004; 4: 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Talwalkar JA, Donlinger JJ, Gossard AA, et al. Fluoxetine for the treatment of fatigue in primary biliary cirrhosis: a randomized, double-blind controlled trial. Dig Dis Sci 2006; 51: 1985-91. [DOI] [PubMed] [Google Scholar]
- 45.Jones DE, Newton JL. An open study of modafinil for the treatment of daytime somnolence and fatigue in primary biliary cirrhosis. Aliment Pharmacol Ther 2007; 25: 471-6. [DOI] [PubMed] [Google Scholar]
- 46.Ian Gan S, de Jongh M, Kaplan MM. Modafinil in the treatment of debilitating fatigue in primary biliary cirrhosis: a clinical experience. Dig Dis Sci 2009; 54: 2242-6. [DOI] [PubMed] [Google Scholar]
- 47.Silveira MG, Gossard AA, Stahler AC, et al. A randomized, placebo-controlled clinical trial of efficacy and safety: modafinil in the treatment of fatigue in patients with primary biliary cirrhosis. Am J Ther 2017; 24: 167-76. [DOI] [PubMed] [Google Scholar]
- 48.Almeida C, Choy EH, Hewlett S, et al. Biologic interventions for fatigue in rheumatoid arthritis. Cochrane Database Syst Rev 2016; 2016: Cd008334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Weiler-Normann C, Schramm C, Quaas A, et al. Infliximab as a rescue treatment in difficult-to-treat autoimmune hepatitis. J Hepatol 2013; 58: 529-34. [DOI] [PubMed] [Google Scholar]
- 50.Satapathy SK, Garg S, Chauhan R, et al. Beneficial effects of tumor necrosis factor-alpha inhibition by pentoxifylline on clinical, biochemical, and metabolic parameters of patients with nonalcoholic steatohepatitis. Am J Gastroenterol 2004; 99: 1946-52. [DOI] [PubMed] [Google Scholar]
- 51.Frezza M, Surrenti C, Manzillo G, et al. Oral S-adenosylmethionine in the symptomatic treatment of intrahepatic cholestasis. A double-blind, placebo-controlled study. Gastroenterology 1990; 99: 211-5. [DOI] [PubMed] [Google Scholar]
- 52.Wunsch E, Raszeja-Wyszomirska J, Barbier O, et al. Effect of S-adenosyl-L-methionine on liver biochemistry and quality of life in patients with primary biliary cholangitis treated with ursodeoxycholic acid. A prospective, open label pilot study. J Gastrointest Liver Dis 2018; 27: 273-9. [DOI] [PubMed] [Google Scholar]
- 53.Newton JL, Jones DE. Managing systemic symptoms in chronic liver disease. J Hepatol 2012; 56 Suppl 1: S46-55. [DOI] [PubMed] [Google Scholar]
- 54.Bernardy K, Klose P, Welsch P, et al. Efficacy, acceptability and safety of cognitive behavioural therapies in fibromyalgia syndrome–a systematic review and meta-analysis of randomized controlled trials. Eur J Pain 2018; 22: 242-60. [DOI] [PubMed] [Google Scholar]
- 55.van Gerven NM, Verwer BJ, Witte BI, et al. Epidemiology and clinical characteristics of autoimmune hepatitis in the Netherlands. Scand J Gastroenterol 2014; 49: 1245-54. [DOI] [PubMed] [Google Scholar]
- 56.Dyson JK, Elsharkawy AM, Lamb CA, et al. Fatigue in primary sclerosing cholangitis is associated with sympathetic over-activity and increased cardiac output. Liver Int 2015; 35: 1633-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hann HW, Han SH, Block TM, et al. Symptomatology and health attitudes of chronic hepatitis B patients in the USA. J Viral Hepat 2008; 15: 42-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Weissenborn K, Tryc AB, Heeren M, et al. Hepatitis C virus infection and the brain. Metab Brain Dis 2009; 24: 197-210. [DOI] [PubMed] [Google Scholar]


