Abstract
Prenatal exposure to alcohol has adverse effects on offspring neuroendocrine and behavioural functions. Alcohol readily crosses the placenta, thus directly affecting developing foetal endocrine organs. In addition, alcohol-induced changes in maternal endocrine function can disrupt the normal hormonal interactions between the pregnant female and foetal systems, altering the normal hormone balance and, indirectly, affecting the development of foetal metabolic, physiological and endocrine functions. The present review focuses on the adverse effects of prenatal alcohol exposure on offspring neuroendocrine function, with particular emphasis on the hypothalamic-pituitary-adrenal (HPA) axis, a key player in the stress response. The HPA axis is highly susceptible to programming during foetal and neonatal development. Here, we review data demonstrating that alcohol exposure in utero programmes the foetal HPA axis such that HPA tone is increased throughout life. Importantly, we show that, although alterations in HPA responsiveness and regulation are robust phenomena, occurring in both male and female offspring, sexually dimorphic effects of alcohol are frequently observed. We present updated findings on possible mechanisms underlying differential effects of alcohol on male and female offspring, with special emphasis on effects at different levels of the HPA axis, and on modulatory influences of the hypothalamic–pituitary–gonadal hormones and serotonin. Finally, possible mechanisms underlying foetal programming of the HPA axis, and the long-term implications of increased exposure to endogenous glucocorticoids for offspring vulnerability to illnesses or disorders later in life are discussed.
Keywords: prenatal ethanol, foetal alcohol spectrum disorder (FASD), sex differences, stress, hypothalamic–pituitary–adrenal axis, hypothalamic–pituitary–gonadal axis, foetal programming
Alcohol (ethanol) consumption in adulthood results in clinical abnormalities of endocrine function and neuroendocrine regulation (1–3). Ethanol acts both directly and indirectly on many hormone systems, including the adrenal, gonadal, and thyroid axes, as well as on aldosterone, growth hormone, parathyroid hormone, calcitonin, insulin, and glucagon. Changes in peripheral hormone metabolism and hormone binding have been reported with ethanol consumption, and withdrawal from ethanol may also impact endocrine function. Furthermore, secondary complications such as liver disease, malnutrition and other medical conditions often present in alcoholics may, in themselves, have endocrine consequences and can potentially exacerbate the adverse effects of ethanol (1).
Exposure to alcohol or other drugs during the prenatal or early postnatal periods constitutes an early insult to the organism that may significantly alter normal growth and development. Whether ethanol-induced endocrine imbalances contribute to the etiology of the foetal alcohol spectrum disorders (FASD) is unknown, but it is certainly a possibility (4). The effects of ethanol on interactions between the pregnant female and foetus are complex (5–8), resulting in both direct and indirect effects on foetal development. Ethanol readily crosses the placenta, thus directly affecting developing foetal cells and tissues, including those related to endocrine function. In addition, ethanol-induced changes in endocrine function can disrupt the normal hormonal interactions between the pregnant female and foetal systems, altering the normal hormone balance and, indirectly, affecting the development of foetal metabolic, physiological and endocrine functions. Ethanol-induced changes in metabolic and/or endocrine function can also affect a female’s ability to maintain a successful pregnancy, resulting in miscarriage or, if the foetus is carried to term, possible congenital defects. Of particular relevance to the present review is that disturbances of the reciprocal interconnections between the pregnant female and foetus, or the maternal and neonatal hypothalamic-pituitary-adrenal (HPA) axes, may provide a common pathway for foetal programming (9). Foetal/early programming refers to the concept that early environmental or nongenetic factors, including pre- or perinatal exposure to drugs or other toxic agents, can permanently organise or imprint physiological and behavioural systems and increase vulnerability to illnesses or disorders later in life (10–13).
The present review focuses on the adverse effects of prenatal alcohol exposure (PAE) on offspring neuroendocrine function, with particular emphasis on sex differences and the HPA axis. The HPA axis is highly susceptible to programming during foetal and neonatal development (11–13). Data indicate that prenatal or early postnatal exposure to ethanol is an early environmental insult that can programme the offspring HPA axis such that HPA tone is increased throughout life. Here, we review data demonstrating that ethanol exposure in utero alters HPA function in the offspring. Importantly, we show that, although alterations in HPA responsiveness and regulation are robust phenomena, occurring in both male and female offspring, sexually dimorphic effects of ethanol are frequently observed. Studies investigating the mechanisms underlying differential effects of ethanol on male and female offspring are discussed, with special emphasis on altered modulatory influences of the hypothalamic-pituitary-gonadal (HPG) hormones and serotonin on offspring HPA function. Finally, possible mechanisms underlying foetal programming of the HPA axis, and the long-term implications of increased exposure to endogenous glucocorticoids for offspring vulnerability to illnesses or disorders later in life, are discussed. This review is based, in part, on previously published reviews (14, 15), and on published and ongoing studies. However, this review presents both a new perspective and new data on sex differences following PAE, and their potential implications for later life outcomes. We present updated findings on the regulation of the HPG and HPA axes in both males and females, including recent novel data on the regulation of the HPG axis in PAE females by kisspeptin. In addition, we discuss the possible significance of PAE on the development of depressive/anxiety-like behaviours in adulthood.
Foetal alcohol spectrum disorder: description in human and animal studies
FASD in humans
FASD is an umbrella term that describes the range of adverse effects that can occur in children born to women who drink alcohol during pregnancy (16, 17). At the most severe end of the spectrum is foetal alcohol syndrome (FAS), which can occur with chronic consumption of high doses of alcohol (18, 19). The diagnostic criteria for FAS include pre and postnatal growth retardation, a characteristic facial dysmorphology, and central nervous system (CNS) involvement, including neurological abnormalities, developmental delays, and intellectual impairment (20). Exposure to alcohol at levels that do not produce the full syndrome can result in partial FAS, where only some of the diagnostic features occur, or in numerous alcohol-related effects that are either primarily physical (alcohol-related birth defects) or primarily neurobehavioural (alcohol-related neurodevelopmental disorder) (20). Interestingly, regardless of level of prenatal alcohol exposure, children may exhibit similar cognitive, neuropsychological, and behavioural problems (e.g. hyperactivity, poor attention span, impaired habituation, cognitive and perceptual problems, impulsivity, lack of inhibition, and poor sensitivity to social cues) (21–23). In addition, secondary disabilities are frequently associated with FASD in both children and adults. These include a high incidence of mental health problems, with depression and anxiety constituting a large proportion of those problems (24–27). Furthermore, the link between prenatal alcohol and depression occurs not only in those with low IQ, but also in children (28, 29) and adults (30) with normal intelligence.
Animal models of PAE
Features similar to those observed in human FASD are observed in rodent models of prenatal ethanol exposure (e.g. retarded pre and postnatal growth and development, physical malformations, and CNS abnormalities) (7, 31–36). Studies have also reported physiological abnormalities such as altered hormonal and immune function (8, 14, 15), as well as a wide range of cognitive and behavioural changes, including hyperactivity, hyper-responsivity to stressors, deficits in response inhibition, and deficits in appropriate use of environmental cues (37–47). These findings are a correlate of the cognitive and behavioural abnormalities observed in alcohol-exposed children. Such deficits can impair the organism’s ability to function in its environment (e.g. to recognise and act upon significant stimuli, to inhibit responses to irrelevant stimuli, and to adapt to new and changing environmental conditions). Importantly, these findings may have implications for the secondary disabilities observed in children with FASD, such as mental health problems, disrupted school experience, and trouble with the law. As will be discussed later in the section ‘Future directions’, foetal programming of HPA activity by prenatal ethanol exposure may play a role in the vulnerability to neurobehavioural alterations and, in particular, the increased vulnerability to mental health problems such as depression and anxiety, observed in children and adults with FASD (25, 26, 48).
A major question in animal models of PAE relates to the issue of appropriate control groups. Ethanol-consuming animals typically reduce their food intake, and therefore their nutrient intake, below the levels that they would consume if given the same diet without ethanol. Therefore, it is common practice to include a control group that is ‘yoked’ or ‘pair-fed’ (PF) to the alcohol group. Each PF animal receives a control diet, typically with a carbohydrate such as maltose-dextrin isocalorically substituted for alcohol, in the amount (g/kg body weight) consumed by its ‘yoked’ alcohol partner on the same day of gestation. However, pair-feeding is an imperfect control procedure. For example, although pair-feeding controls for the reduced intake of the ethanol-consuming animals, it can never control for the effects of ethanol on absorption and utilisation of nutrients. Indeed, we must accept that ethanol intake always produces secondary nutritional effects that cannot be controlled and that are part of its effects on the body. In addition, pair-feeding is, in itself, a type of treatment beyond its effects on caloric intake (49–52). Despite the fact that most experimental alcohol diets are formulated to provide optimal nutrition during pregnancy, and despite our finding (53) that the nutrient intake of PF dams in our model meets the published requirements for pregnant laboratory rats (54), these animals are underfed compared to their ad lib-fed counterparts. As a result, they are hungry, and typically consume their entire daily food ration within a few hours of presentation, remaining food deprived for the rest of the day. We have suggested (51, 55) that pair-feeding is thus a mild prenatal stressor, and that its effects on offspring behavioural and physiological responsiveness represent, at least partially, an effect of stress above and beyond the nutritional aspect of receiving a reduced food ration. Moreover, in some cases, pair-feeding in itself can have effects that differ from those seen in PAE animals. For example, we found (49) that following adrenalectomy, PF animals showed adrenocorticotrophin (ACTH) and corticotrophin-releasing hormone (CRH) increases similar to those seen in PAE animals, and greater than those of their control counterparts, which would typically be interpreted as indicating mediation by the nutritional effects of ethanol. However, specific effects of pair-feeding were also observed. Corticosterone replacement following adrenalectomy failed to decrease CRH mRNA levels in PF females and arginine vasopressin (AVP) mRNA levels in PF males and females as it did in PAE animals, suggesting differential central regulation of HPA activity in PAE compared to control animals. There are many other examples in the literature where pair-feeding in itself has effects different from those of ethanol (50, 51, 56, 57). Thus, even if behavioural or hormonal responsiveness is similar in PAE and PF animals, examination of the brain or of the unique effects of pair-feeding suggests that mechanisms underlying the changes observed may differ in PAE and PF animals rather than occurring along a continuum of effects on the same pathway. Moreover, one must consider that food restriction in itself can programme physiological systems and have long-term effects on outcome measures in adulthood (58–61). Further research will continue to elucidate possible differential mechanisms underlying ethanol and pair-feeding effects.
Gestational ethanol exposure and HPA activity
Human studies
Compared to the fairly large literature on the effects of ethanol consumption on the HPA axis of adults following acute or chronic ethanol intake, only a few human studies have examined the effects of drinking during pregnancy on the HPA axis of the developing child. In case studies by Root et al. (62), plasma cortisol concentrations were within the normal range in children with FAS. On the other hand, data from Jacobson et al. (63) showed that heavy drinking at conception and during pregnancy is associated with higher basal and post-stress (blood draw) cortisol concentrations in 13-month-old infants. Basal cortisol levels were also higher in 2-month-old infants exposed in utero to ethanol or cigarettes (64). Recently, Haley et al. (65) examined cortisol, heart rate and negative affect in 5–7-month-old infants during a modified ‘still face’ procedure, a standardised developmental paradigm used to study emotion and stress regulation. They found that greater prenatal ethanol exposure was associated with greater cortisol reactivity, negative affect, and elevated heart rate. Of relevance to the present review, these effects differed in boys and girls, with girls showing greater changes in heart rate and negative affect than boys, and boys showing greater changes in cortisol than girls, indicating that sex differences can occur even prior to puberty (see ‘Organisational and activational effects of sex hormones’).
Animal studies
Animal studies strongly support these findings of marked ethanol effects on the offspring HPA axis. Data from both rodent and primate models have shown that, in adulthood, animals prenatally exposed to ethanol are typically hyper-responsive to stressors and to drugs such as ethanol and morphine (6, 7, 14, 15, 66–68). However, the timing of alcohol administration has a significant influence on the effects observed. Work by Rivier et al. (69) indicates that alcohol exposure during the second, but not the first or third week of gestation enhances ACTH secretion and increases CRH biosynthesis and expression in weanling rats. Interestingly, the second week of gestation represents the time of maximal development of the foetal HPA axis (70). To put these data into perspective in relation to human development, we can divide human gestation into the period of the ovum (first 2 weeks), the embryonic period (weeks 3–8, the major period of organogenesis), and the foetal period (weeks 9–38, when organogenesis and differentiation are completed, and marked growth occurs), with the brain growth spurt occurring primarily during the third trimester (weeks 25–38). For the rat, the embryonic period is approximately the first 7–10 days, and the foetal period from day 11–21. Importantly, the first 10 postnatal days represent the third trimester equivalent, and comprise the period of the brain growth spurt. Development of the human adrenal gland begins during gestation weeks 6–8, and continues with differentiation and growth during the foetal period, becoming functional prior to birth. Similarly, the rat adrenal gland begins to develop during the first week of gestation, can secrete corticosterone by day 13–14, and then continues to differentiate and grow, becoming fully functional during approximately the last 4 days of gestation.
Sex differences in ethanol effects
Importantly, while hyper-responsiveness is a robust phenomenon, occurring in both male and female offspring, and in a variety of test paradigms, sexually dimorphic effects on offspring HPA activity are often observed, and are dependent on the nature of the stressor, and the time course and hormonal endpoints measured (71–75).
Early studies on female PAE offspring consistently demonstrate enhanced responses to stressors, such as intermittent footshock, cardiac puncture, noise and shake, and ether, and to drugs such as ethanol and morphine (74, 76–78). Furthermore, studies comparing responses of male and female offspring to acute or short duration stressors often show greater changes in females than in males. For example, in response to acute restraint or swim stress, or acute ethanol or morphine challenge, corticosterone and/or ACTH levels are greater in PAE females compared to their control counterparts, whereas prenatal treatment had no significant effects in males (68, 71, 79–81). Similarly, PAE females but not males show deficits in their ability to use or respond to environmental cues (47, 71). By contrast, in studies using stressors of different intensity or duration, HPA hyper-responsiveness is observed in PAE males or in both male and female offspring. For example, PAE males but not females show HPA hyperactivity in response to prolonged restraint or cold stress (72, 82). By contrast, both PAE males and females show HPA hyper-responsiveness following repeated restraint stress, but their responses differ depending on the experimental conditions. Following five or ten exposures to restraint, PAE females show greater plasma levels of corticosterone and ACTH but not β-endorphin compared to controls, whereas PAE males show greater β-endorphin responses following five or ten exposures to restraint, but greater corticosterone and ACTH responses following 16 exposures to restraint compared to controls (83). Furthermore, both male and female offspring typically exhibit increased corticosterone and/or ACTH responses to immune challenges such as interleukin-1β or lipopolysaccharide (LPS; an endotoxin used to mimic infection or inflammation) compared to controls (6, 66, 68, 75, 84, 85). Furthermore, following the combination of moderate prenatal alcohol exposure and noise stress, both male and female rhesus monkeys show increased HPA responses to the stress of maternal separation, with intercorrelated basal and stress ACTH levels that predict behaviour during separation (67, 86).
Possible mechanisms mediating ethanol-induced HPA hyper-responsiveness
The mechanisms underlying HPA hyper-responsiveness in PAE offspring are beginning to be elucidated, and appear to involve changes at several levels of the axis. Furthermore, the sexually dimorphic effects of PAE on the HPA axis suggest that the gonadal hormones or altered gonadal–adrenal interactions probably play a significant role in mediating the effects of prenatal ethanol on HPA activity. Here, we discuss possible mechanisms related to ethanol-induced alterations at the levels of the hypothalamus, pituitary, adrenal, and hippocampus, as well as altered interactions between the HPA and HPG axes, and the HPA axis and the serotonergic system.
PAE induces HPA alterations at multiple levels of the axis
Hypothalamus
Stimulatory inputs or drive to the paraventricular nucleus (PVN) of the hypothalamus are enhanced by PAE. For example, weanling PAE offspring have increased basal concentrations of CRH mRNA in the PVN (69), and adult PAE male but not female offspring exhibit increased basal concentrations of hypothalamic CRH and glucocorticoid receptor (GR) mRNA (87, 88). However, Kim et al. (82) report no differences in basal CRH or AVP mRNA concentrations between adult PAE and control animals, and Lee et al. (85) found no differences in basal CRH heteronuclear (hn)RNA, or in basal CRH and AVP median eminence protein concentrations in PAE compared to control rats. Importantly, however, the latter investigators showed that, in response to both footshock and LPS challenge, PAE males and females both exhibit enhanced hypothalamic neuronal activity compared to controls, as reflected in increased mRNA concentrations of the immediate early genes c-fos and NGFI-B, as well as significantly increased CRH hnRNA content. These changes were accompanied by higher CRH but not AVP hnRNA levels in the PVN and a marginal increase in responsiveness to AVP among PAE females only. These data provide important evidence for a selective stress- and LPS-induced increase in the activity of hypothalamic CRH neurones in PAE animals, indicating a potential hypothalamic mechanism through which ethanol may upregulate the HPA axis.
We have undertaken studies aiming to resolve the issue of whether changes in central HPA regulation occur in PAE animals under basal or nonstressed conditions, and the extent to which sex differences in outcome are observed. Two studies utilised adrenalectomy, with or without corticosterone replacement, as a probe to investigate steady-state HPA function and the role of corticosterone in mediating changes in HPA regulation. We found that PAE males, but not females, showed a greater ACTH response to adrenalectomy, whereas hypothalamic CRH mRNA concentrations were significantly higher in both PAE males and females compared to controls (49, 89). Increased steady-state activity of hypothalamic CRH neurones could increase HPA tone and thus play a role in mediating alterations in stress-related HPA activity.
Pituitary
Although the hypothalamus appears to represent a major site of prenatal ethanol effects (85), some studies suggest a possible role for the pituitary in mediating effects of PAE on HPA activity, and similar to studies on the hypothalamus, indicate a marked sexual dimorphism in the effects of PAE. For example, studies report increased pituitary pro-opiomelanocortin (POMC) mRNA in PAE males but not females (88), and a reversal of this effect by maternal adrenalectomy. It was suggested that these findings indicate feedback changes at the level of the pituitary, although we cannot rule out the possibility that changes also may have occurred at higher levels of the axis. In relation to this, we have shown that PAE males but not females have lower pituitary CRH-R1 mRNA expression under both intact and adrenalectomised conditions (Fig. 1), but no change in POMC mRNA levels compared to controls (49). By contrast, we report altered pituitary responsiveness to secretagogues and to stressors in both PAE males and females. If pre-treated with dexamethasone (DEX) to block endogenous HPA activity, PAE animals exhibit increased ACTH responses to CRH (90) and greater corticosterone and ACTH elevations following ether stress (91) compared to their respective controls. Interestingly, the effects were particularly robust when testing occurred in the late afternoon during the circadian peak, when HPA feedback regulation is less efficient (90, 91). The finding by Lee et al. (85) that CRH infusion without prior DEX treatment does not differentially alter ACTH responses in PAE compared to control animals suggests that blocking endogenous HPA activity may help to unmask HPA abnormalities in PAE animals. On the other hand, following the stress of chronic alcohol exposure in adulthood (74), PAE males show decreased pituitary POMC and increased pituitary GR mRNA levels, whereas PAE females show no change in pituitary POMC but decreased pituitary GR mRNA levels. These data suggest that, in contrast to the response to ether or restraint stress, there is a specific HPA-related vulnerability in males to the deleterious effects of ethanol consumption in adulthood. Interestingly, results from in vitro studies have shown both direct effects of alcohol on pituitary corticotrophs (92), as well as indirect activation through alcohol’s action on CRH and AVP (93). Further studies are needed to understand fully the possible role of the pituitary in mediating HPA hyper-responsiveness in PAE animals.
Fig. 1.
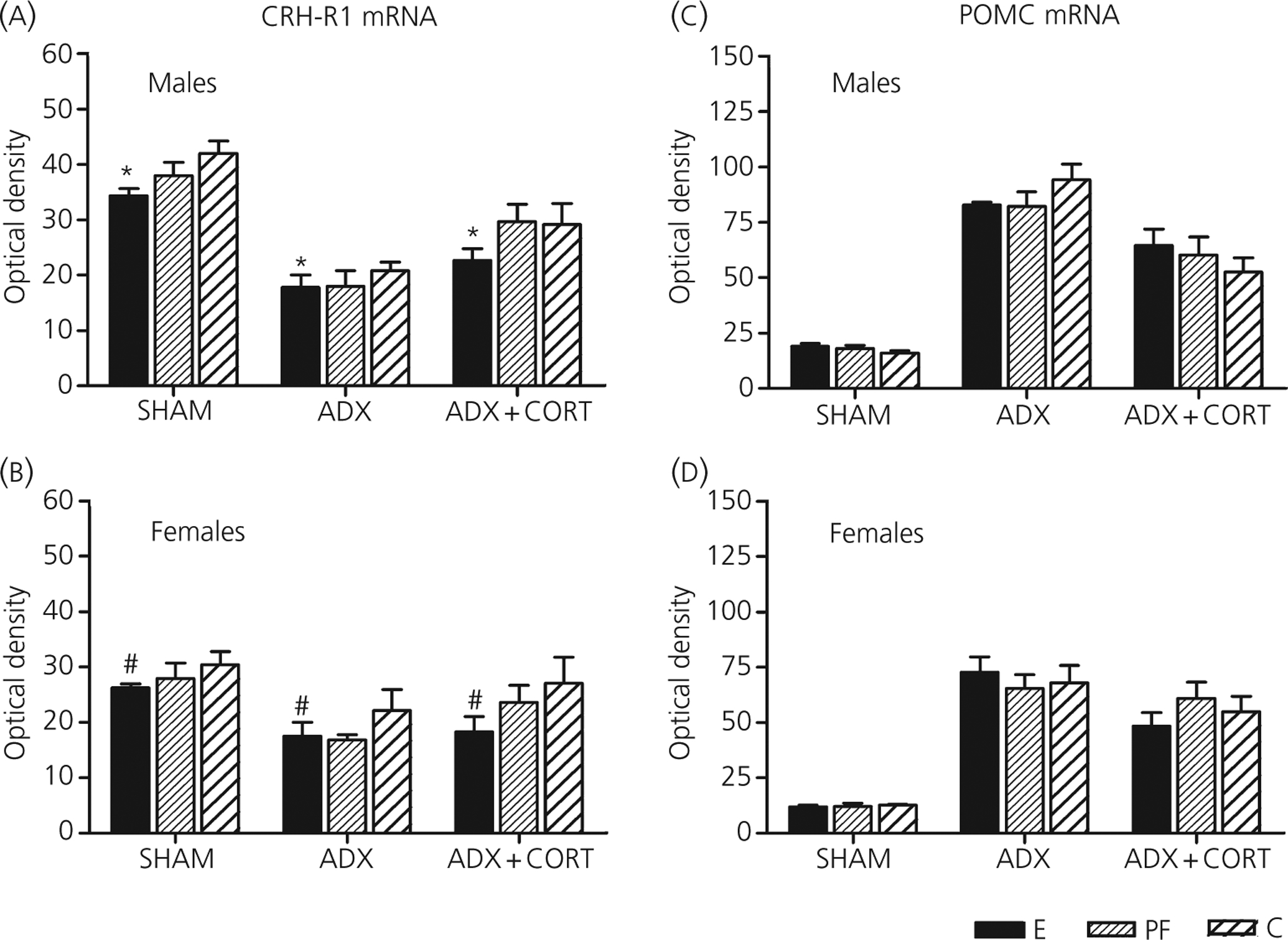
Anterior pituitary corticotrophin-releasing hormone (CRH)-R1 mRNA and pro-opiomelanocortin (POMC) mRNA levels in male and female rats from prenatal alcohol exposure (E), pair-fed (PF) and control (C) groups, 7 days following sham surgery or adrenolectomy (ADX) without or with corticosterone replacement. Values represent the mean ± SEM of 5–6 rats per group. Main effect of prenatal treatment (*E < C, P < 0.05; #E < C, P = 0.065). Reprinted with permission [49].
Adrenal
There is also a body of evidence indicating a role for the maternal HPA axis in mediating ethanol’s effects on PAE offspring. Studies by Taylor et al. (94) reveal that maternal adrenalectomy but not adrenal demedullation reverses the alcohol-induced decrease in birth weight among PAE pups. By contrast, Redei et al. (95) found that maternal adrenalectomy leads to decreased birth weight and increased foetal corticosterone, and that these changes are reversed by administration of corticosterone to the adrenalectomised dams. Methodological differences may account for the conflicting findings obtained. Interestingly, there is evidence from in vitro studies that alcohol may have direct stimulatory effects on the adrenal cortex (96). That is, addition of ethanol to the medium of isolated rat adrenal glands resulted in secretion of corticosterone at levels similar to those observed when ACTH was added. Whether the effects of PAE on offspring HPA activity are mediated to any extent at the level of the adrenal gland remains to be determined.
Hippocampus
We are the only group that has explored changes in hippocampal mineralocorticoid receptor (MR) and GR mRNA concentrations to assess feedback regulation in PAE animals at the level of the hippocampus (49). We found that PAE females showed a greater MR response and PAE males a greater GR response to adrenalectomy, and that corticosterone replacement was less effective in normalising MR mRNA levels in PAE compared to control males (Fig. 2). The alterations in MR and GR mRNA concentrations are noteworthy, as previous studies found no ethanol-induced differences in MR and GR receptor concentrations or binding affinity in the hippocampus or other brain regions between PAE and control animals (97, 98). Moreover, we have shown that MR and GR regulation are differentially altered in PAE compared to control females across the oestrous cycle (see further discussion in ‘Interactions between the HPA axis and the serotonergic system in PAE females’). Together, these data suggest that PAE may alter both HPA drive and feedback regulation and/or the balance between drive and feedback in a sex- and hormone-specific manner.
Fig. 2.
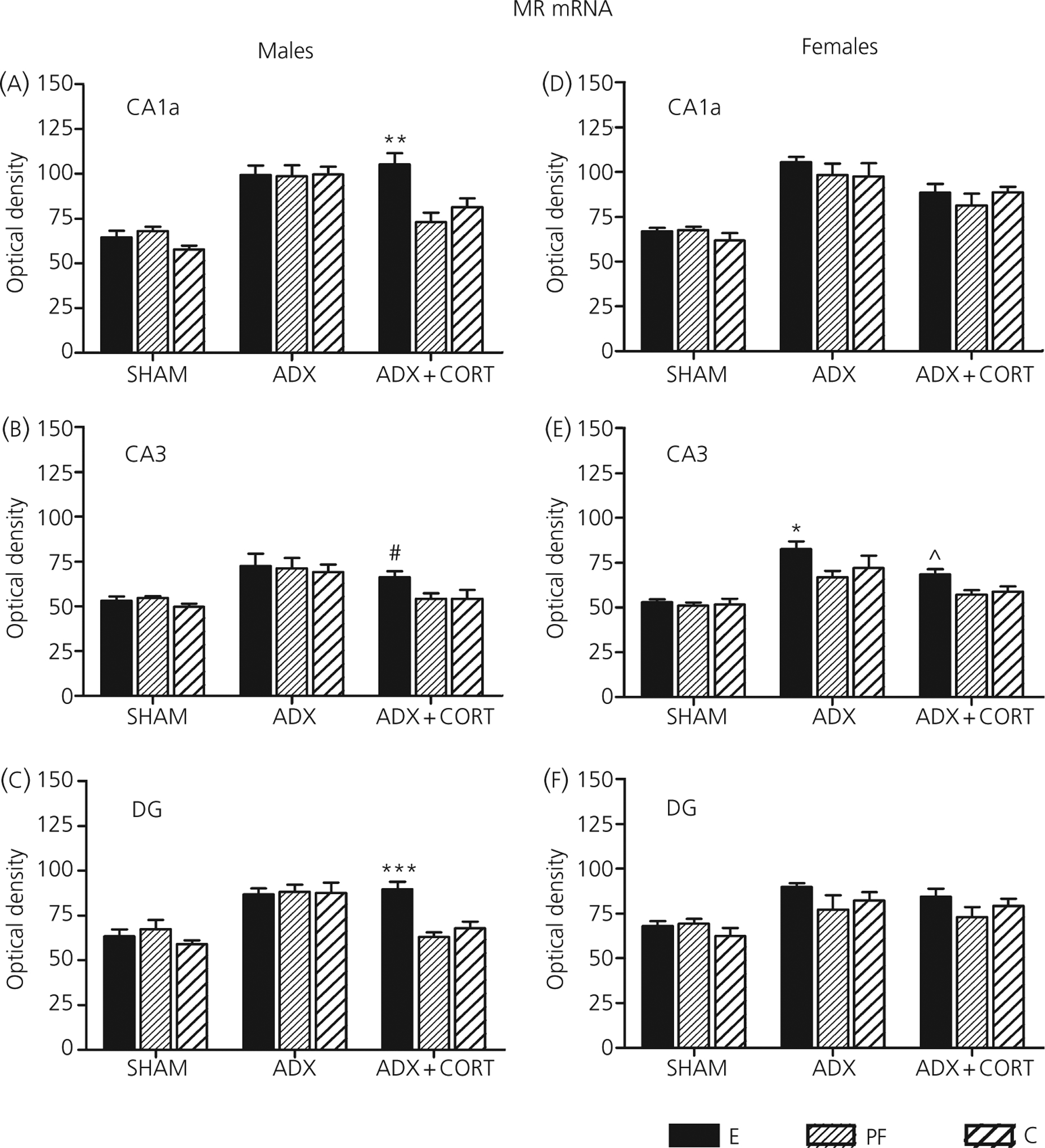
Mineralocorticoid receptor (MR) mRNA in prenatal alcohol exposure (E), pair-fed (PF) and control (C) males (a–c) and females (d–f) in dorsal hippocampal subfields CA1a, CA3 and the dentate gyrus (DG), 7 days following sham surgery or adrenolectomy (ADX) without or with corticotrophin (CORT) replacement. Values represent the mean ± SEM of semi-quantitative densitometry of slides hybridized with a 35S-dUTP-labeled riboprobe for MR (n = 4–6 rats per group). *P < 0.05; **P < 0.005; ***P < 0.001 compared to PF and control; #P = 0.07 compared to PF; ^P < 0.07 compared to PF and control. Reprinted with permission [49].
Summary of HPA changes
In summary, the data presented above demonstrate HPA dysregulation in PAE animals following stressor, drug and immune (LPS) challenges. Moreover, we have shown that HPA dysregulation also occurs under basal conditions, even in the face of similar basal hormone levels, and that differences are further unmasked following perturbations of the system by adrenalectomy. Dysregulation is evident at multiple levels of the axis, and appears to reflect changes in both HPA drive and feedback regulation and/or in the balance between drive and feedback. The altered CRH-R1 mRNA responses and the lack of differences in POMC mRNA concentrations in PAE animals suggest possible compensatory mechanisms, probably at the level of the pituitary, such that the enhanced activation seen at the hypothalamus does not translate directly into enhanced pituitary activity. This may be protective for ethanol-exposed animals, minimising enhanced basal pituitary activity in the face of enhanced hypothalamic drive. Finally, the sexually dimorphic effects of PAE suggest a role for the gonadal steroids or possibly an alteration in gonadal–adrenal interactions in mediating the effects of ethanol on HPA activity and regulation.
HPA changes induced by PAE are not mediated by the same mechanisms as those induced by prenatal stress
Like prenatal alcohol exposure, prenatal stress can have long-term effects on offspring HPA activity and regulation (99) and some studies have questioned whether prenatal alcohol exposure is really just a prenatal stressor. However, studies have shown that this is not the case. Prenatal stress effects are most pronounced if stress occurs during the third week of gestation (100, 101), whereas prenatal alcohol effects are mediated primarily by alcohol exposure during the second week of gestation (69). In addition, a number of studies demonstrate a significant role for the maternal glucocorticoids in mediating prenatal stress effects on the foetus (99, 102). By contrast, as noted, the effects of ethanol on the foetal HPA axis are complex (5–8), involving both direct effects of ethanol and indirect effects of elevated maternal glucocorticoids. An elegant study by Lee and Rivier (103) provides strong evidence that the HPA changes in offspring prenatally exposed to high levels of corticosterone are not the same as those produced by PAE. Adrenalectomised dams were given corticosterone at levels that mimicked those previously measured in intact dams exposed to ethanol during days 8–14 of gestation. Importantly, weanling pups exposed to corticosterone prenatally showed no increase in basal or stress ACTH levels or in hypothalamic CRH mRNA levels compared to controls. Moreover, corticosterone responses to shock were actually lower, and not higher, in offspring exposed prenatally to corticosterone compared to controls. Thus, elevated maternal corticosterone levels do not appear to be the primary mediator of prenatal ethanol effects.
Altered HPG–HPA interactions
The interaction between the HPG and HPA axes is bidirectional. Stress inhibits reproductive function, and in turn, the gonadal steroids modulate HPA activity at all levels of the axis (104–108). Sex differences in basal and stress-related HPA activity as well as in central regulation have been reported (104). Females have higher basal corticosterone, as well as a faster onset of corticosterone secretion, and greater corticosterone and ACTH responses to stress compared to males (108, 109). Females also have higher levels of corticosterone binding globulin, which buffer stress-induced corticosterone increases. Females are most sensitive to stress during early pro-oestrous (110, 111), when oestradiol levels are high. Oestradiol facilitates ACTH release during stress, whereas progesterone inhibits the facilitatory effects of oestradiol (111). Oestrogen regulation of HPA activity appears to occur at sites upstream from the PVN, as few PVN CRH neurones contain oestrogen receptor mRNA (112). Importantly, the stimulatory effects of oestrogen occur only against a background of low progesterone levels (112). Both oestrogen and progesterone appear to have antagonistic effects on glucocorticoid feedback, acting primarily at GR (108, 113). Conversely, males typically have lower ACTH and corticosterone responses under both basal and stress conditions, as well as lower CRH and CRH mRNA levels in the PVN than females, mediated, at least in part, by the inhibitory effects of testosterone (106). Consistent with the data in females, testosterone effects on HPA regulation occur upstream from the PVN (107), at the level of the amygdala and bed nucleus of the stria terminalis (114), as there is little overlap in androgen receptor and CRH mRNA in the PVN (113). Testosterone also modulates corticosterone feedback effects on MR and GR (104, 115–118). Hormone responses to stressors as well as CRH mRNA levels are increased by gonadectomy, and normalised by treatment with testosterone, an effect mediated by androgen receptors (106, 119, 120).
Organisational and activational effects of sex hormones
Mature males and females have sexually distinct reproductive capabilities and behaviours. This sexual dimorphism is the result of several interacting forces. These include genetic influences, which control gonad determination during foetal development, organisational effects, which are the permanent structural changes arising from the actions of perinatal sex hormones, and activational effects, which are the direct and temporary influences of circulating gonadal hormones on function and behaviour following puberty and throughout adulthood (121). Activational effects of sex hormones arise at puberty, and post-pubertal circulating sex hormones interact with sexually differentiated brain structures to promote sex differences in behaviour (121) and in numerous physiological functions. Thus, sex differences can be observed in young organisms, prior to puberty, through organisational effects, and in post-pubertal organisms through a combination of organisational and activational effects of the HPG hormones.
Prenatal ethanol exposure has marked effects on development and maturation of the HPG axis of both male and female offspring
Adverse effects of ethanol on the foetal testis have been reported in PAE males, including decreased numbers of Leydig cells, the presence of vacuoles in the seminiferous tubules, and insensitivity to luteinising hormone (LH) (122). Both the pre- and postnatal testosterone surges are also suppressed in PAE foetuses/neonates (122–124). At birth, PAE males exhibit decreased brain and plasma testosterone, and deficits in testicular steroidogenic enzyme activity compared with control pups (125, 126). In addition, PAE neonates exhibit a decreased anogenital distance compared to controls, suggesting feminisation (127–130). In adulthood, PAE males may show reduced weights of testes, prostate and seminal vesicles (131), decreased serum testosterone and LH levels, and altered neurotransmitter responses to testosterone (132), suggesting central dysregulation of HPG activity. Deficits in sexual behaviour (131), as well as feminisation of nonsexual sexually dimorphic behaviours, have also been reported in PAE males (128, 133–136). These findings are supported by neuroanatomical data indicating reduced volume of the sexually dimorphic nucleus of the preoptic area in PAE compared to control males (130, 137).
Among PAE females, sexual maturation (vaginal opening) (138–140), onset of follicle stimulating hormone (FSH) secretion (141), and onset of puberty (142) may be delayed. Altered developmental patterns of prolactin and LH secretion have also been reported (139). Furthermore, alterations in LH and FSH activity and responsiveness following gonadectomy and hormone replacement indicate defects in both pituitary and central regulation (143–145). The volume of the PVN is also smaller in PAE than PF females (135). Also, PAE females exhibit no LHβ-mRNA response to gonadotrophin-releasing hormone (GnRH) and decreased LHβ-mRNA responses to oestradiol in vitro (146), as well as an earlier incidence of acyclicity (premature reproductive ageing) (147). Parameters of sexual and maternal behaviour are also adversely affected by prenatal ethanol (133, 148).
Previous studies provide support for the hypothesis that the HPG hormones may differentially modulate HPA activity in PAE and control offspring. Aird et al. (149) studied the effects of ethanol-induced elevations in maternal glucocorticoids on the ontogeny of steady-state levels of pituitary POMC and hypothalamic CRH in male and female offspring. In control neonates, the developmental increase in CRH expression typically occurs faster in females than in males, and prenatal alcohol exposure delayed and exaggerated the rise of CRH expression. By contrast, anterior pituitary POMC mRNA levels were suppressed in PAE male but not female offspring at day 21. These studies demonstrate a sex-dependent sensitivity of the foetus to prenatal ethanol exposure and changes in maternal glucocorticoids, and suggest that, since these sexually dimorphic changes in gene expression occur before the presence of adult levels of sex steroids, the HPG hormones have an organisational effect on developing HPA function. These findings were extended in work by Lee and Rivier (75) who demonstrated that, as with the response to stressors, PAE animals also show increased responsiveness to immune challenges including interleukin-1β, LPS and turpentine. Interestingly, removal of circulating ovarian steroids prior to puberty attenuated the differences in ACTH released by PAE and control females. The authors concluded that these findings suggest the presence of a functional relationship between the pathways influenced by prenatal ethanol and those influenced by female sex steroids, both of which are important in regulating HPA activity.
Recent work in our laboratory has investigated specific modulatory influences of the HPG hormones on the differential HPA responsiveness of PAE males and females, compared to each other and to their control counterparts. Here, we present updated findings on the regulation of the HPG and HPA axes in both males and female, under basal conditions and following stress. As well, we discuss recent novel data on the regulation of the HPG axis in PAE females by kisspeptin.
Effects of prenatal ethanol exposure on HPG–HPA interactions in male offspring
We examined the effects of gonadectomy in unmasking the extent to which prenatal ethanol influences both HPG and HPA activity and regulation (150). In terms of HPA function, we found that, under intact conditions, PAE males showed significantly higher ACTH levels than controls following 30 min of restraint stress, suggesting increased responsiveness to stress at the level of the pituitary. There were no differences among prenatal groups in CRH mRNA levels, but PAE males had lower AVP mRNA levels than PF and control males 90 min post-stress. AVP is a weak ACTH secretagogue on its own, but acts synergistically with CRH and plays an important role in sustaining pituitary responsiveness during chronic stress (151, 152) or to a novel heterotypic stressor following repeated stress (153). Moreover, CRH biosynthesis in the PVN is corticosterone-dependent, whereas AVP biosynthesis is testosterone-dependent (106). Thus, these data suggest that intact PF and control males have a greater propensity to utilise AVP than intact PAE males. In terms of HPG function, we found that intact PAE males had blunted testosterone responses to restraint stress compared to PF and control males, and that only control males showed a stress-induced increase in LH levels.
Importantly, gonadectomy unmasked differential HPG influences on HPA activity, as well as differential responsiveness of central components of the HPG axis to androgens in PAE compared to control males. Gonadectomy selectively increased the ACTH stress response in controls such that the differences between intact PAE and control males were eliminated, and also eliminated the differences in AVP mRNA levels among prenatal groups. In addition, following gonadectomy, basal GnRH mRNA levels were significantly higher in PAE than control males, and gonadectomised PAE males had higher LH levels than PF males 90 min post-stress. These data suggest a potential shift in how testosterone regulates GnRH transcription (154), as well as stress-induced LH responses in PAE compared to control males. Together, these findings demonstrate ethanol-induced alterations in regulation of both the HPA and HPG axes, and suggest that the normal testicular influences on HPA function are markedly reduced in PAE males.
We then examined whether the differential HPA responsiveness in PAE and control males depends on testosterone. We explored dose-related effects of testosterone on HPA regulation and responsiveness to determine whether testosterone has a decreased capacity to regulate HPA activity (155–157). Adult PAE, PF and control males were subjected to: (i) Sham gonadectomy (INTACT); (ii) gonadectomy; (iii) gonadectomy and implantation of silastic capsules that provided circulating testosterone concentrations approximating low (1 ng/ml) or (iv) high (4–6 ng/ml) physiological levels.
HPA activity in PAE males showed a number of alterations that relate specifically to testosterone status. Sensitivity of the adrenal to circulating testosterone was reduced in PAE rats, and gonadectomy increased CORT levels for PF and control but not PAE males, indicating that PAE males were less sensitive to the effect of androgen removal than controls. In addition, low testosterone replacement restored corticosterone levels for gonadectomised PF and control males, whereas high testosterone levels were needed to restore corticosterone levels for gonadectomised PAE males. The finding that a negative correlation exists between pre-stress testosterone and post-stress corticosterone levels in control but not in PAE and PF males further demonstrates the reduced adrenal sensitivity to testosterone. Importantly, prenatal ethanol altered the capacity of testosterone to regulate central CRH and AVP pathways (Fig. 3). Basal CRH mRNA levels in the medial parvocellular dorsal part of the PVN were increased by gonadectomy in PF and control but not PAE males. Intact PAE also had lower CRH mRNA levels in the fusiform nucleus of the anterior bed nucleus of the stria terminalis (BNST) than Intact control males, but higher CRH mRNA levels under high testosterone replacement conditions in the central amygdala (CeA). CRH mRNA levels in the fusiform nucleus of the anterior BNST were reduced following gonadectomy, indicating that testosterone exerts a stimulatory effect on CRH mRNA expression in this region (107). On the other hand, CRH mRNA levels in the CeA are known to vary negatively with testosterone, but only in the presence of corticosterone (107). Thus, lower CRH mRNA levels in the fusiform nucleus of the anterior BNST and higher CRH mRNA levels in the CeA in PAE rats might reflect a reduced influence of testosterone on CRH mRNA expression in these regions. Taken together, these findings suggest reduced sensitivity of central CRH pathways to testosterone in PAE males. By contrast, testosterone appears to have greater effects on central AVP pathways in PAE and/or PF compared to control males. Both PAE and PF males showed higher AVP mRNA levels in the posterior BNST following high testosterone replacement compared to either intact or low testosterone replacement conditions, respectively, and PAE males had higher AVP mRNA levels under intact conditions in the MeA compared to controls. Furthermore, although testosterone had an inhibitory role in both MR and GR mRNA levels in the hippocampus across all prenatal groups, prenatal ethanol exposure appeared to differentially regulate basal hippocampal GR expression, with PAE males showing GR upregulation compared to PF and/or control males.
Fig. 3.
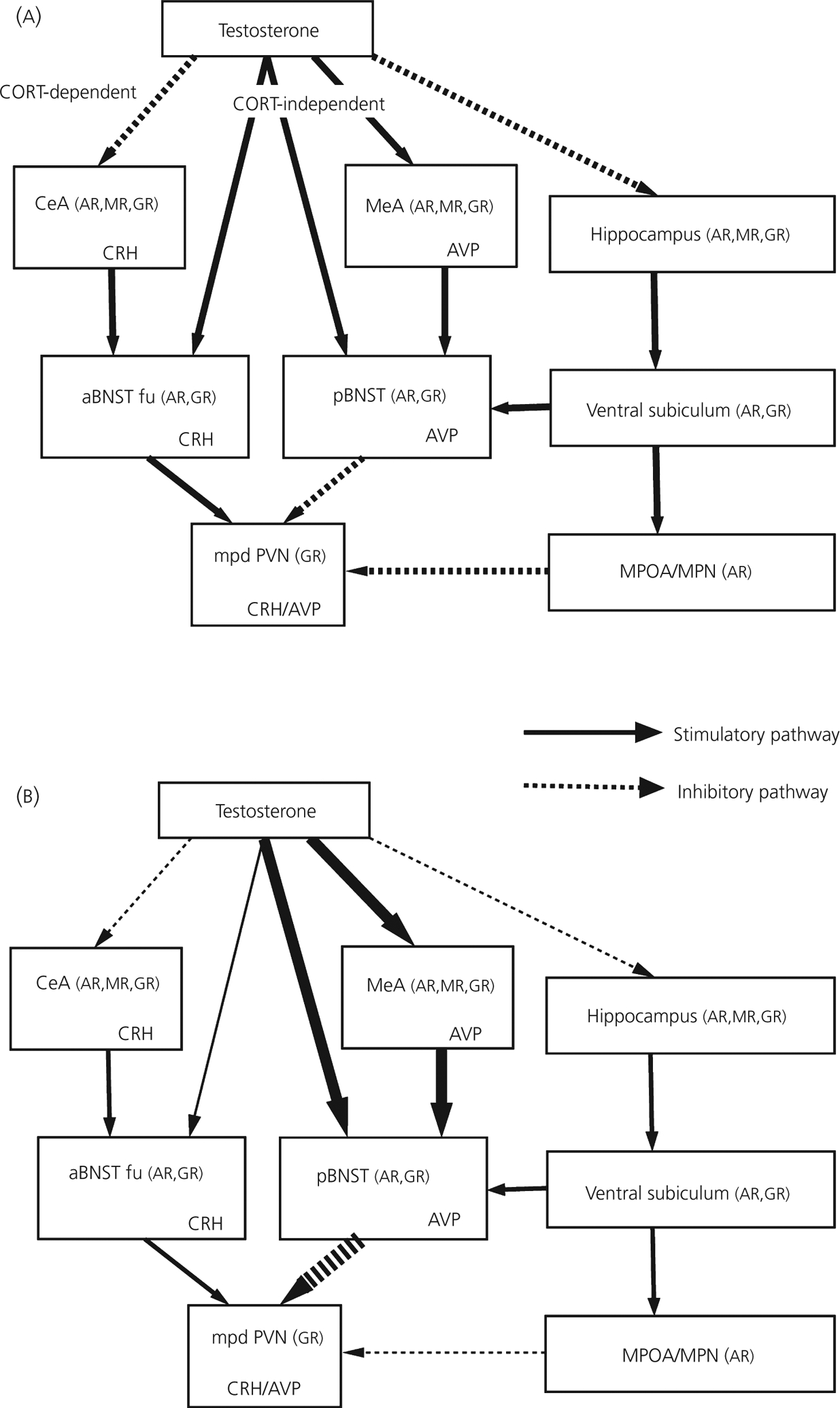
Working models to illustrate possible effects of testosterone on neuropeptide and steroid receptor expression within the limbic forebrain circuits that project to the mpd PVN in normal (a) and prenatal alcohol exposed (PAE) (b) adult male rats. Findings suggest a reduced capacity for testosterone to regulate corticotrophin-releasing hormone (CRH) mRNA expression in central CRH pathways, but enhanced effects of testosterone on central arginine vasopressin (AVP) pathways in PAE and/or pair-fed (PF) compared to control males. PAE males had lower CRH mRNA levels in the fusiform nucleus of the anterior division of the bed nucleus of stria terminalis (aBNST) and higher CRH mRNA levels in the central nucleus of amygdala (CeA). In addition, basal CRH mRNA levels were increased by GDX in PF and control but not PAE males in the medial parvocellular dorsal part of the paraventricular nucleus (mpd PVN). By contrast, both PAE and PF males showed higher AVP mRNA levels in the posterior division of the BNST (pBNST) following high testosterone replacement compared to either intact or low testosterone replacement conditions, and intact PAE had higher AVP mRNA levels in the medial nucleus of amygdala (MeA) than intact control males. See text for further discussion. CORT, corticosterone; aBNST fu, Fusiform nucleus of the anterior division of the bed nucleus of stria terminalis; MPOA: medial preoptic area; MPN: medial preoptic nucleus; AR: androgen receptor; MR: mineralocorticoid receptor; GR: glucocorticoid receptor; NTS: nucleus of the solitary tract [155].
In terms of specific HPG effects of PAE, we found that testosterone had less of an inhibitory effect on stress-induced LH increases in PAE than in PF and control males. In addition, AR mRNA levels in the median preoptic nucleus and the principal nucleus of the posterior BNST were lower in PAE and PF compared to control males under intact conditions. If decreased AR mRNA levels in key brain areas reflects downregulation of AR levels, this may counteract the increased inhibitory AVP signals upstream from the PVN, and thus contribute to the HPA hyper-responsiveness observed in PAE males.
Together, these data suggest that altered HPA activity in PAE males represents a complex balance between reduced sensitivity of central CRH pathways and increased sensitivity of central AVP pathways to the inhibitory effects of testosterone, with some effects possibly mediated by the nutritional effects of ethanol.
Effects of prenatal ethanol exposure on HPG–HPA interactions in female offspring
A number of novel findings demonstrate effects of PAE on both peripheral and central measures of HPG and HPA function (158). Under our conditions, PAE females showed relatively normal oestrous cyclicity but delayed sexual maturation. Importantly, prenatal ethanol effects on HPA function varied according to oestrous cycle stage. PAE females had lower basal corticosterone levels than control females in oestrous, but elevated basal and stress corticosterone levels and elevated stress ACTH levels compared to PF and/or control females during pro-oestrous. Moreover, positive correlations between basal oestradiol and corticosterone were found in PAE and PF but not control females. In addition, both PAE and PF females had higher basal and stress oestradiol levels in pro-oestrous compared to other phases of the cycle, as well as higher stress oestradiol levels than control females during pro-oestrous. PAE and PF females also showed downregulation of GnRH mRNA compared to control females in dioestrous, when oestradiol levels are low. However, PAE females showed greater variation in LH levels than PF and control females across the cycle, and in pro-oestrous, showed a significant LH increase following stress (158). Together, these data support the possibility that ethanol-induced changes in HPA activity may reflect differential regulation by ovarian steroids in PAE compared to control females, with increased HPA sensitivity to oestradiol. Once again, nutritional effects of ethanol may also play some role in the changes that were observed, although different mechanisms may underlie the changes in PAE and PF females.
Recently, we have investigated the role of the neuropeptide kisspeptin in modulating HPG function, as well as the role of oestrogen in regulation of kisspeptin expression in PAE compared to control female rats (159). The family of kisspeptin neuropeptides is derived from the 145-amino-acid precursor peptide coded for by the KISS-1 gene; post-translational processing results in C-terminal peptide fragments (kisppeptin-54, −14, −13, and −10) that activate the G-protein coupled receptor 54 (GPR54) (160–162). In the brain, kisspeptin neurones are most numerous in the arcuate nucleus (ARC), and two major populations are also found in the periventricular and anteroventral periventricular (AVPV) nuclei (163, 164). Interestingly, there are sex differences in the expression of kisspeptin in the AVPV, with female rats having a 10-fold greater KISS-1 expression than males (165).
Studies have shown that central and peripheral administration of kisspeptin result in a dose-dependent increase in secretion of GnRH and gonadotrophins (163, 166–169), mediated by binding to GPR54 (170). In mice, oestradiol is stimulatory to kisspeptin neurones in the AVPV, but inhibitory to kisspeptin neurones in the ARC, which is mediated by oestrogen receptor α (164). Thus, kisspeptin neurones in the ARC appear to be interneurones mediating the negative feedback effect of the gonadal steroids on the HPG axis (171), while kisspeptin neurones in the AVPV may mediate the GnRH/LH surge induced by the positive feedback action of oestrogen (172).
To determine whether the expression of kisspeptin is altered in adult female PAE rats, offspring from PAE, PF and control conditions were ovariectomised, with or without oestradiol replacement. We found that, regardless prenatal treatment, oestradiol replacement decreased the number of kisspeptin-ir cells in the ARC compared to that in ovariectomised animals. Importantly, the number of kisspeptin-ir cells was decreased more in PAE than in control females following oestradiol replacement (159) (Fig. 4). To our knowledge, this is the first report of changes in kisspeptin expression in adult PAE females. In view of the role of kisspeptin neurones in the ARC in oestradiol negative feedback (164, 173), the present data suggest that prenatal ethanol exposure may potentiate the inhibitory actions of oestradiol on kisspeptin neurones in the ARC. Indeed, it is possible that ethanol-induced changes in kisspeptin expression play an important role in mediating the HPG axis dysregulation observed in PAE animals, and thus are a major factor in mediating the altered HPG-HPA interactions and the HPA hyper-responsiveness in these animals.
Fig. 4.
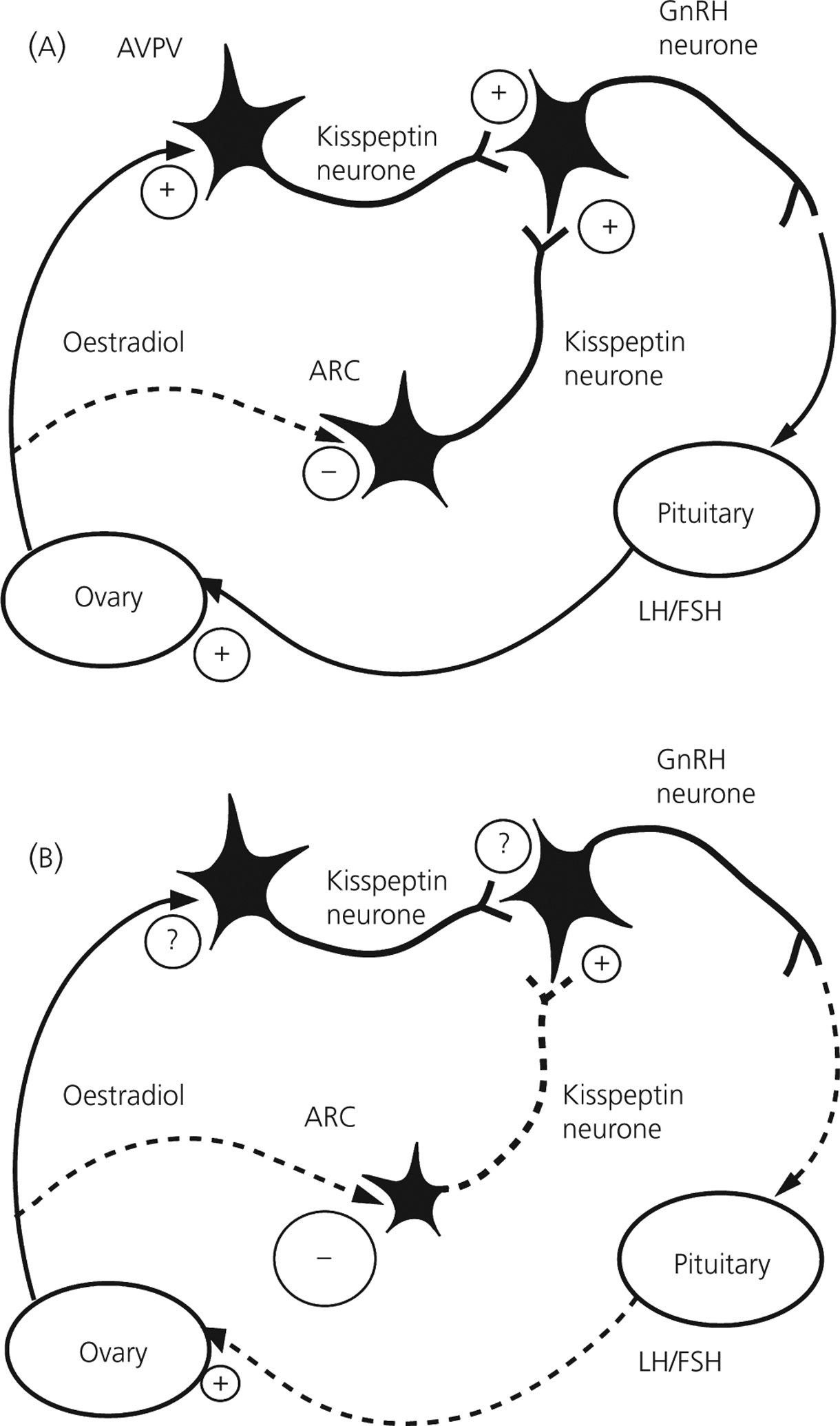
Working model illustrating kisspeptin regulation of the hypothalamic-pituitary-gonadal axis in normal (a) and prenatal alcohol exposure (PAE) (b) adult female rats. Kisspeptin released by neurons in the anteroventral periventricular (AVPV) and arcuate nucleus (ARC) stimulates gonadotrophin-releasing hormone (GnRH) release, which induces the release of luteinising hormone (LH) and follicle-stimualting hormone (FSH). The ovaries respond to gonadotrophins by secreting oestradiol, which feeds back to regulate the activity of kisspeptin neurons, inhibiting kisspeptin expression in the ARC and inducing the expression in the AVPV (modified from Dungan et al., 2006). Adult PAE females had a decreased number of kisspeptin-immunoreactive neurons in the ARC, which would result in less GnRH and LH/FSH release, and decreased stimulation of the ovary. We speculate that PAE females show increased sensitivity to oestradiol, which leads to downregulation of kisspeptin-immunoreactive neurones in the ARC. The role of kisspeptin regulation at the level of AVPV is currently under investigation. See text for further discussion. +, Stimulation; − and − − − −, inhibition; ?, unknown action.
Interactions between the HPA axis and the serotonergic system in PAE females
Serotonin, or 5-hydroxytryptamine (5-HT) is implicated in the control of numerous behavioural and physiological functions, including mood, emotion, sleep and appetite. In addition, 5-HT plays a crucial role in modulating the HPA axis (174). The effects of prenatal ethanol exposure on the 5-HT system have received special attention because 5-HT also acts as a neurotrophic factor, and early insult to this system may affect its own development as well as that of other neurotransmitter systems. Of relevance to this review, PAE decreases the concentrations of 5-HT or its metabolites during foetal life and in weanling rats (175, 176), and results in long-lasting 5-HT deficits in mice, possibly through selective apoptosis (177). Foetal ethanol exposure also increases the number of binding sites for the 5-HT transporter in some areas of the developing rat brain, but decreases transporter binding site numbers in other areas (178). Importantly, prenatal administration of buspirone, or ipsapirone, partial 5-HT1A agonists, in conjunction with ethanol, ameliorates some of these ethanol-induced changes (179). On the other hand, postnatal ethanol exposure increases hypothalamic 5-HT content in adult rats of both sexes, with females showing greater overall concentrations than males (180), whereas exposure during both pre- and postnatal life does not differentially alter hypothalamic 5-HT concentrations (181). Together, these studies indicate that ethanol exposure in utero permanently alters 5-HT levels and 5-HT transporter binding sites in the developing brain, with the effects being dependent on the period of exposure and the brain region examined.
We found that animals prenatally exposed to ethanol exhibit physiological and behavioural abnormalities consistent with altered 5-HT function. For example, PAE animals exhibit altered hypothermic responses to the 5-HT1A receptor agonist 8-OH-DPAT [8-hydroxy-2–2(di-n-propylamino)tetralin], and PAE females, but not males, show an increased rate of ‘wet dog shakes’ in response to the 5-HT2A/C receptor agonist DOI [1-(2,5-dimethoxy-4-iodophenyl)-2-aminopropane hydrochloride], suggesting ethanol-induced alterations in 5-HT receptor function (55). Furthermore, PAE females but not males exhibit blunted ACTH responses to 8-OH-DPAT, but increased ACTH responses to DOI compared to controls, suggesting an altered interaction between the HPA axis and the 5-HT system in PAE animals (182).
In view of the above findings, we examined the modulatory effects of the gonadal hormones and the 5-HT system on HPA regulation in adult PAE females tested across the oestrous cycle under basal conditions (183). We hypothesised that an MR/GR imbalance may underlie the HPA hyper-responsiveness observed in PAE females, manifested differentially as a function of the oestrous cycle. Our data demonstrate, for the first time, long-lasting consequences of prenatal ethanol exposure on basal levels of hippocampal MR, GR and 5-HT1A mRNA as a function of oestrous cycle stage, supporting a role for the gonadal hormones in mediating these effects. Hippocampal MR mRNA levels were decreased in PAE compared to PF and control females, with the greatest effects in pro-oestrous, and PAE, but not PF or control females also had higher hippocampal GR mRNA levels in pro-oestrous than in oestrous and dioestrous, suggesting a possible shift in the MR/GR balance in PAE females. In addition, 5-HT1A receptor mRNA levels were increased in PAE compared to PF and control females in dioestrous (Table 1). Taken together, these data suggest that altered interactions between the serotonergic and HPA systems may play a role in mediating the HPA hyper-responsivity reported in PAE animals following stress. In addition, these data may have important implications for understanding the increased incidence of secondary disabilities, and in particular, the increased rate of depression, reported in children with FASD (25, 26, 48).
Table 1.
Summary of Changes in Basal Hypothalamic-Pituitary-Adrenal (HPA) and Hypothalamic-Pituitary-Gonadal (HPG) Hormone Levels and Hippocampal Mineralocorticoid Receptor, Glucocorticoid Receptor and 5-HT1A Receptor mRNA Levels in Adult PAE Female Rats Across the Oestrus Cycle.
| Outcome measured | Result |
|---|---|
| HPA hormones | |
| Corticotrophin | ↑ (Proestrous) |
| Adrenocorticotrophin | - |
| HPG hormones | |
| Oestradiol | - |
| Progesterone | - |
| Hippocampal receptors | |
| Mineralocorticoid receptor mRNA | ↓ (Proestrous) |
| Glucocorticoid receptor mRNA | Proestrous > oestrous = dioestrous |
| 5-HT1A mRNA | ↑ (Dioestrous) |
↑, increased; ↓, decreased, -, no change; ( ), stage(s) of the oestrus cycle where changes were the most prominent. PAE, prenatal alcohol exposed.
Dysfunction of the serotonergic system is observed in a wide range of conditions or disorders including alcoholism, anxiety, depression, suicide and insomnia (184–188). Similarly, psychopathologies including anxiety and depression are hypothesised to involve persistent changes in HPA activity (189, 190) and expression of hippocampal corticosteroid receptors (191). Moreover, a growing literature suggests that adverse experiences during early life (e.g. early stressful or traumatic experiences) increase the risk for development of depression and anxiety disorders in adulthood (192–194). In light of these findings, we suggest that prenatal alcohol exposure is one type of early adverse experience that can increase the risk for depression and anxiety disorders in adulthood.
Prenatal ethanol exposure reprogrammes foetal HPA function
The concept of programming refers to the associations between environmental events (internal and external to the organism) and stable alterations in phenotype (195). According to this concept, maternal adversities (e.g. stress, alcohol, tobacco, and/or protein deprivation) predict foetal growth retardation and increased HPA activity, which in turn, leads to lower offspring body weight and increased HPA responses to stressors later in life. Greater HPA reactivity confers increased risk for metabolic disorders in adulthood, such as hypolipidemia, hyperglycemia and hypertension (195). Importantly, although these outcomes are deleterious, and may appear maladaptive, the biological ‘purpose’ of programming is probably adaptive, preparing the offspring to face postnatal life under adverse conditions (196). By the same token, programming during foetal and early neonatal life can have positive effects. Supportive and nurturant environments for the mother and the neonate can enhance foetal growth and development, modulate HPA reactivity, reduce the risk for diseases or disorders in adulthood, and increase resiliency throughout life (195).
The routes by which the foetal and early neonatal environment can programme adult HPA function and behaviour are described eloquently by Matthews et al. (12), Welberg and Seckl (13) and Meaney et al. (195), amongst others. The developing limbic system, primarily the hippocampus, hypothalamus, and anterior pituitary, synthesises high amounts of GRs and is highly sensitive to glucocorticoids. Exposure to high concentrations of glucocorticoids during early life can alter the development and subsequent function of both the limbic system and the HPA axis. The limbic system regulates HPA activity and, in turn, endogenous glucocorticoids modify numerous limbic system functions. Mechanisms underlying these mutual effects involve modification of developing neurotransmitter systems, the development of MR and GR expression in the hippocampus, and the development and responsiveness of the hypothalamic PVN which regulates glucocorticoid secretion. Furthermore, it is likely that the perinatal environmental and genetic factors mutually influence each other in determining the development of HPA activity and regulation. The overall effect of early developmental programming is altered exposure to endogenous glucocorticoids throughout life (12, 197) which, in turn, can modify behaviour, cognition, learning, memory and emotion, and either predispose the individual to cardiovascular, metabolic, immune and mental/cognitive disorders, or promote resilience to later life challenges. Moreover, though the effects of programming are often long-lasting, postnatal and later environmental events can modulate prenatal programming effects.
Early experiences can result in long term consequences from the behavioural to the molecular level through epigenetic processes (198). In the model of Weaver et al. (198), naturally occurring variations in maternal behaviour are associated with the development of individual differences in behavioural and HPA responses to stressors. Thus, offspring of mothers showing high levels of maternal behaviour (licking, grooming and arched back nursing) are less fearful and show better modulated HPA responses to stressors than offspring of mothers showing low levels of maternal behaviour. This outcome is not due to a change in the underlying genetic profile, as they have shown that if pups are cross-fostered from ‘low behaviour’ to ‘high behaviour’ mothers at birth, the offspring profile is associated with the adoptive, rather than the biological mother. Therefore, it appears that variations in maternal behaviour can serve as a mechanism for nongenomic transmission of individual differences in stress reactivity across generations. At least two major epigenomic mechanisms are thought to be involved: demethylation of one key site in the NGFI-A binding sequence of the first exon of the GR gene, and increased acetylation of the histones surrounding the GR gene (198). These two alterations result in increased expression of GRs and increased access of transcription factors to GRs, thus increasing HPA feedback regulation and altering the physiological and behavioural profiles of the offspring.
In our model, prenatal ethanol exposure is the adverse early life environmental variable that programmes the foetal HPA axis, resulting in long-term alterations in the physiological and behavioural profiles of the offspring. We have reviewed evidence demonstrating long-term alterations in HPA regulation and responsiveness under both basal and stress conditions, reflecting increased HPA tone throughout life. In view of the evidence (199, 200) that foetal programming of the HPA axis, at least in part, underlies the connection between the early environment and adult stress-related and behavioural disorders in humans, further studies are essential to elucidate mechanisms underlying PAE effects on HPA programming.
A new collaborative programme of research (collaborators: S. Innis and A. Devlin, University of British Columbia) is investigating possible epigenetic mechanisms underlying foetal programming of HPA activity by prenatal ethanol exposure. We are exploring the hypothesis that prenatal ethanol exposure disrupts the methionine cycle in both the dam and the foetus, and thus provides the basis for alterations in DNA methylation. Methionine is the precursor to S-adenosylmethionine, the primary methyl donor in numerous biological reactions, including the methylation of DNA and histones (201). Alterations in the methionine cycle are associated with changes in tissue methylation capacity and may affect DNA and histone methylation, thereby altering epigenentic regulation of gene expression and causing long-term phenotypic changes. Our preliminary results suggest that maternal ethanol consumption increases homocysteine levels in the dams, and results in increased methionine levels, decreased activity of the choline cycle, and decreased methionine adenosyltransferase levels in both PAE dams and foetuses compared to their control counterparts (202). It remains to be determined if these marked alterations in methionine metabolism during prenatal exposure to ethanol result in long-term changes in tissue methylation capacity and epigenetic modifications, thereby contributing to the HPA programming observed in PAE animals.
Summary and conclusions
The present review discusses the adverse effects of PAE on offspring neuroendocrine function, with particular emphasis on the HPA axis and sex differences in outcome. We have shown that prenatal ethanol exposure programmes the foetal HPA axis such that HPA tone is increased throughout life, with alterations in HPA regulation under both basal and stress conditions. Ethanol-induced disturbances of the reciprocal interconnections between the pregnant female and the foetus may provide a common pathway for foetal programming by early life events. The finding that ethanol causes alterations in the methylation state within cells (201), together with our preliminary data showing foetal ethanol-induced changes in methionine metabolism, provides support for the possibility that epigenetic mechanisms similar to those described by Weaver et al. (198) could mediate the changes induced by ethanol exposure in utero. Data from such investigations may have important implications for the development of therapeutic interventions focused at reversing the long-term adverse effects of prenatal alcohol exposure.
Future directions
Currently, we are investigating some of the functional outcomes of PAE. Specifically, one line of investigation is testing the hypothesis that foetal programming of HPA activity by PAE sensitises neuroadaptive mechanisms that underlie the stress response, increases reactivity to subsequent stressful life events, and mediates the link between PAE and increased vulnerability to depression.
Mental disorders are common secondary disabilities among FASD populations (26, 29, 30, 48, 203), and HPA dysregulation is often observed in major depression (204–206). Consistent with these findings, brain areas implicated in depression overlap with areas that mediate the stress response, with the HPA axis a key player in both (205, 207–209). Studies suggest that pre-existing HPA abnormalities may be a major contributory factor in the genesis of some forms of depression (206, 210). In particular, a large literature points to a relationship between depression in adulthood and adverse early life events (211, 212). We propose that prenatal exposure to ethanol can be considered an adverse early life event that results in HPA abnormalities similar to those seen in depression, and which could underlie an increased vulnerability to depression in adulthood. Indeed, work from our laboratory and others has demonstrated that PAE results in HPA hyperactivity and dysregulation in a manner parallel to that seen in depression. As noted, PAE animals are typically hyper-responsiveness to stressors (6, 66, 68, 71–73, 83–85), and show increased HPA drive (49, 69, 75, 87, 89) and deficits in HPA feedback regulation (50, 91, 214). Furthermore, our data (214) suggest an alteration in MR-mediated corticosterone signalling, and perhaps an altered MR/GR balance, which is proposed to underlie neurobiological alterations in depression (191). Finally, PAE animals exhibit altered neurotransmitter regulation of HPA activity, particularly in the serotonergic system (55, 182, 215, 216), suggesting altered serotonergic influences on HPA activity similar to those seen in depression.
We are currently examining the links among PAE, stress system abnormalities, the gonadal and serotonergic systems and depressive/anxiety-like behaviours in adulthood. We have developed a multidimensional battery of behavioural tests selected from categories measuring ‘behavioural despair’ (Porsolt forced swim test, 217), anhedonia (sucrose contrast test), social interaction and exploratory behaviour (open field), and anxiety (elevated plus maze; EPM). This latter is noteworthy, as depression and anxiety are comorbid in a large percentage of individuals diagnosed either with depression or an anxiety disorder (218–220). Further, to test our hypothesis that PAE would increase vulnerability to the effects of later life stressors on depressive symptomatology, we have developed a modified chronic mild stress (CMS) protocol (221), designed to model milder “everyday life” stressors. Preliminary data suggest that PAE offspring subjected to CMS in adulthood show increased anxiety and depressive-like behaviours, with changes occurring in a sex-dependent manner. PAE males were more anxious on the elevated plus maze, showed anhedonic behaviour in the sucrose contrast test, and showed decreased affiliative and non-affiliative behaviours in the social interaction test. By contrast, PAE females were more immobile in the forced swim test, and had significantly greater elevations of corticosterone and progesterone, but lower oestradiol levels following acute stress compared to PF and control females. These data suggest that the behavioural effects of CMS on anxiety/depressive-like behaviour are dissociable by sex in animals prenatally exposed to ethanol. Furthermore, these data support the hypothesis that prenatal exposure to ethanol reprogrammes the HPA axis, sensitising the organism to subsequent stressful experiences, and thereby increasing the propensity to develop depression in adulthood. Currently, studies in our laboratory are exploring the possibility that anti-depressants that specifically target the HPA axis may ameliorate or reverse the anxiety- and depressive-like behaviours as well as the increased HPA activity observed among PAE animals.
Acknowledgements
The research reported in this review is supported by NIAAA grants AA007789 and AA016683, and grants from the BC Ministry of Children and Family Development (through the UBC Human Early Learning Partnership) and the Canadian Institute for Advanced Research to JW, Fellowships from IMPART (CIHR Strategic Training Initiative in Health Research) to JHS and KGCH, an NSERC Canada Graduate Scholarships to NL, and a Fellowship from the Michael Smith Foundation for Health Research to KGCH. The authors are grateful to Linda Ellis and Wayne Yu for expert assistance in all aspects of the research, and to numerous graduate and undergraduate students who have participated actively in this work.
References
- 1.Morgan MY. Alcohol and nutrition. Br Med Bull 1982; 38: 21–29. [DOI] [PubMed] [Google Scholar]
- 2.Adler RA. Clinical review 33: clinically important effects of alcohol on endocrine function. J Clin Endocrinol Metab 1992; 74: 957–960. [DOI] [PubMed] [Google Scholar]
- 3.Glavas MM, Weinberg J. Stress alcohol and the hypothalamic–pituitary–adrenal axis. In: Yehuda S, Mostofsky D, eds. Stress, Nutrition and Medical Disorders. Totowa, NJ: Humana Press Inc., 2005: 165–184. [Google Scholar]
- 4.Anderson RA Jr. Endocrine balance as a factor in the etiology of the fetal alcohol syndrome. Neurobehav Toxicol Teratol 1981; 3: 89–104. [PubMed] [Google Scholar]
- 5.Rudeen PK, Taylor JA. Fetal alcohol neuroendocrinopathies. In: Watson RR, ed. Alcohol and Neurobiology: Brain Development and Hormone Regulation. Boca Raton, FL: CRC Press, 1992: 109–138. [Google Scholar]
- 6.Weinberg J Neuroendocrine effects of prenatal alcohol exposure. Ann NY Acad Sci 1993; 697: 86–96. [DOI] [PubMed] [Google Scholar]
- 7.Weinberg J Prenatal alcohol exposure: endocrine function of offspring. In: Zakhari S, ed. Alcohol and the Endocrine System. Research Monograph No. 23, Bethesda, MD: NIH (NIAAA), 1993: 23. [Google Scholar]
- 8.Weinberg J, Bezio S. Alcohol-induced changes in pituitary–adrenal activity during pregnancy. Alcohol Clin Exp Res 1987; 11: 274–280. [DOI] [PubMed] [Google Scholar]
- 9.Angelucci L, Patacchioli FR, Scaccianoce S, Di Sciullo A, Cardillo A, Maccari S. A model for later-life effects of perinatal drug exposure: maternal hormone mediation. Neurobehav Toxicol Teratol 1985; 7: 511–517. [PubMed] [Google Scholar]
- 10.Bakker JM, van Bel F, Heijnen CJ. Neonatal glucocorticoids and the developing brain: short-term treatment with life-long consequences? Trends Neurosci 2001; 24: 649–653. [DOI] [PubMed] [Google Scholar]
- 11.Matthews SG. Antenatal glucocorticoids and programming of the developing CNS. Pediatr Res 2000; 47: 291–300. [DOI] [PubMed] [Google Scholar]
- 12.Matthews SG, Owen D, Banjanin S, Andrews MH. Glucocorticoids, hypothalamo–pituitary–adrenal (HPA) development, and life after birth. Endocr Res 2002; 28: 709–718. [DOI] [PubMed] [Google Scholar]
- 13.Welberg LA, Seckl JR. Prenatal stress, glucocorticoids and the programming of the brain. J Neuroendocrinol 2001; 13: 113–128. [DOI] [PubMed] [Google Scholar]
- 14.Sliwowska JH, Zhang X, Weinberg J. Prenatal ethanol exposure and fetal programming: Implications for endocrine and immune development and long-term health. In: Miller MW, ed. Brain Development: Normal Processes and the Effects of Alcohol and Nicotine. New York, NY: Oxford University Press, 2006: 153–181. [Google Scholar]
- 15.Zhang X, Sliwowska JH, Weinberg J. Prenatal alcohol exposure and fetal programming: effects on neuroendocrine and immune function. Exp Biol Med (Maywood) 2005; 230: 376–388. [DOI] [PubMed] [Google Scholar]
- 16.Riley EP, McGee CL. Fetal alcohol spectrum disorders: an overview with emphasis on changes in brain and behavior. Exp Biol Med (Maywood) 2005; 230: 357–365. [DOI] [PubMed] [Google Scholar]
- 17.Manning MA, Eugene Hoyme H. Fetal alcohol spectrum disorders: a practical clinical approach to diagnosis. Neurosci Biobehav Rev 2007; 31: 230–238. [DOI] [PubMed] [Google Scholar]
- 18.Jones KL, Smith DW. Recognition of the fetal alcohol syndrome in early infancy. Lancet 1973; 2: 999–1001. [DOI] [PubMed] [Google Scholar]
- 19.Jones KL, Smith DW, Ulleland CN, Streissguth P. Pattern of malformation in offspring of chronic alcoholic mothers. Lancet 1973; 1: 1267–1271. [DOI] [PubMed] [Google Scholar]
- 20.Stratton K, Howe C, Battaglia F. Fetal Alcohol Syndrome – Diagnosis, Epidemiology, Prevention and Treatment. Washington: National Academy Press, 1996. [Google Scholar]
- 21.Shaywitz SE, Cohen DJ, Shaywitz BA. Behavior and learning difficulties in children of normal intelligence born to alcoholic mothers. J Pediatr 1980; 96: 978–982. [DOI] [PubMed] [Google Scholar]
- 22.Streissguth AP. The behavioral teratology of alcohol: performance, behavioral and intellectual deficits in prenatally exposed children. In: West JR, ed. Alcohol and Brain Development. New York, NY: Oxford University Press, 1986: 3–44. [Google Scholar]
- 23.Mattson SN, Schoenfeld AM, Riley EP. Teratogenic effects of alcohol on brain and behavior. Alcohol Res Health 2001; 25: 185–191. [PMC free article] [PubMed] [Google Scholar]
- 24.Kodituwakku PW. Defining the behavioral phenotype in children with fetal alcohol spectrum disorders: a review. Neurosci Biobehav Rev 2007; 31: 192–201. [DOI] [PubMed] [Google Scholar]
- 25.Streissguth AP, Bookstein FL, Barr HM, Sampson PD, O’Malley K, Young JK. Risk factors for adverse life outcomes in fetal alcohol syndrome and fetal alcohol effects. J Dev Behav Pediatr 2004; 25: 228–238. [DOI] [PubMed] [Google Scholar]
- 26.O’Connor MJ, Kasari C. Prenatal alcohol exposure and depressive features in children. Alcohol Clin Exp Res 2000; 24: 1084–1092. [PubMed] [Google Scholar]
- 27.O’Connor MJ, Paley B. The relationship of prenatal alcohol exposure and the postnatal environment to child depressive symptoms. J Pediatr Psychol 2006; 31: 50–64. [DOI] [PubMed] [Google Scholar]
- 28.O’Connor TM, O’Halloran DJ, Shanahan F. The stress response and the hypothalamic–pituitary–adrenal axis: from molecule to melancholia. QJM 2000; 93: 323–333. [DOI] [PubMed] [Google Scholar]
- 29.O’Connor MJ, Shah B, Whaley S, Cronin P, Gunderson B, Graham J. Psychiatric illness in a clinical sample of children with prenatal alcohol exposure. Am J Drug Alcohol Abuse 2002; 28: 743–754. [DOI] [PubMed] [Google Scholar]
- 30.Famy C, Streissguth AP, Unis AS. Mental illness in adults with fetal alcohol syndrome or fetal alcohol effects. Am J Psychiatry 1998; 155: 552–554. [DOI] [PubMed] [Google Scholar]
- 31.Abel EL, Dintcheff BA. Effects of prenatal alcohol exposure on growth and development in rats. J Pharmacol Exp Ther 1978; 207: 916–921. [PubMed] [Google Scholar]
- 32.Chernoff GF. The fetal alcohol syndrome in mice: an animal model. Teratology 1977; 15: 223–229. [DOI] [PubMed] [Google Scholar]
- 33.Druse MJ. Neurotransmitter function: changes associated with in utero alcohol exposure. In: Abel EL, ed. Fetal Alcohol Syndrome: From Mechanisms to Prevention. New York: CRC Press, 1996: 171–189. [Google Scholar]
- 34.Leichter J, Lee M. Effect of maternal ethanol administration on physical growth of the offspring in rats. Growth 1979; 43: 288–293. [PubMed] [Google Scholar]
- 35.Randall CL, Taylor J, Walker DW. Ethanol-induced malformations in mice. Alcohol Clin Exp Res 1977; 1: 219–224. [DOI] [PubMed] [Google Scholar]
- 36.Weinberg J, Gallo PV. Prenatal ethanol exposure: pituitary–adrenal activity in pregnant dams and offspring. Neurobehav Toxicol Teratol 1982; 4: 515–520. [PubMed] [Google Scholar]
- 37.Abel EL. Prenatal effects of alcohol on adult learning in rats. Pharmacol Biochem Behav 1979; 10: 239–243. [DOI] [PubMed] [Google Scholar]
- 38.Anandam N, Felegi W, Stern JM. In utero alcohol heightens juvenile reactivity. Pharmacol Biochem Behav 1980; 13: 531–535. [DOI] [PubMed] [Google Scholar]
- 39.Bond NW, Di Giusto EL. Effects of prenatal alcohol consumption on open-field behaviour and alcohol preference in rats. Psychopharmacologia 1976; 46: 163–165. [DOI] [PubMed] [Google Scholar]
- 40.Bond NW, Digiusto EL. Effects of prenatal alcohol consumption on shock avoidance learning in rats. Psychol Rep 1977; 41: 1269–1270. [DOI] [PubMed] [Google Scholar]
- 41.Gallo PV, Weinberg J. Neuromotor development and response inhibition following prenatal ethanol exposure. Neurobehav Toxicol Teratol 1982; 4: 505–513. [PubMed] [Google Scholar]
- 42.Kim CK, Kalynchuk LE, Kornecook TJ, Mumby DG, Dadgar NA, Pinel JP, Weinberg J. Object-recognition and spatial learning and memory in rats prenatally exposed to ethanol. Behav Neurosci 1997; 111: 985–995. [DOI] [PubMed] [Google Scholar]
- 43.Lochry EA, Riley EP. Retention of passive avoidance and T-maze escape in rats exposed to alcohol prenatally. Neurobehav Toxicol 1980; 2: 107–115.7290306 [Google Scholar]
- 44.Martin JC, Martin DC, Sigman G, Radow B. Maternal ethanol consumption and hyperactivity in cross-fostered offspring. Physiol Psychol 1978; 6: 362–365. [Google Scholar]
- 45.Riley EP, Baron S, Hannigan JH. Response inhibition deficits following prenatal alcohol exposure: a comparison to the effects of hippocampal lesions in rats. In: West JR, ed. Alcohol and Brain Development. New York, NY: Oxford University Press, 1986: 7–102. [Google Scholar]
- 46.Riley EP, Lochry EA, Shapiro NR. Lack of response inhibition in rats prenatally exposed to alcohol. Psychopharmacology (Berl) 1979; 62: 47–52. [DOI] [PubMed] [Google Scholar]
- 47.Weinberg J Prenatal ethanol exposure alters adrenocortical response to predictable and unpredictable stressors. Alcohol 1992; 9: 427–432. [DOI] [PubMed] [Google Scholar]
- 48.Streissguth AP, O’Malley K. Neuropsychiatric implications and long-term consequences of fetal alcohol spectrum disorders. Semin Clin Neuropsychiatry 2000; 5: 177–190. [DOI] [PubMed] [Google Scholar]
- 49.Glavas MM, Ellis L, Yu WK, Weinberg J. Effects of prenatal ethanol exposure on basal limbic–hypothalamic–pituitary–adrenal regulation: role of corticosterone. Alcohol Clin Exp Res 2007; 31: 1598–1610. [DOI] [PubMed] [Google Scholar]
- 50.Hofmann C, Glavas M, Yu W, Weinberg J. Glucocorticoid fast feedback is not altered in rats prenatally exposed to ethanol. Alcohol Clin Exp Res 1999; 23: 891–900. [PubMed] [Google Scholar]
- 51.Redila VA, Olson AK, Swann SE, Mohades G, Webber AJ, Weinberg J, Christie BR. Hippocampal cell proliferation is reduced following prenatal ethanol exposure but can be rescued with voluntary exercise. Hippocampus 2006; 16: 305–311. [DOI] [PubMed] [Google Scholar]
- 52.Weinberg J Nutritional issues in perinatal alcohol exposure. Neurobehav Toxicol Teratol 1984; 6: 261–269. [PubMed] [Google Scholar]
- 53.Weinberg J Effects of ethanol and maternal nutritional status on fetal development. Alcohol Clin Exp Res 1985; 9: 49–55. [DOI] [PubMed] [Google Scholar]
- 54.Subcommittee on Laboratory Animal Nutrition, Committee on Animal Nutrition, Board on Agriculture and National Research Council. Nutrient Requirements of Laboratory Animals, 4th revised edn. Washington, DC: Anonymous National Academy, 1995 [Google Scholar]
- 55.Hofmann CE, Simms W, Yu WK, Weinberg J. Prenatal ethanol exposure in rats alters serotonergic-mediated behavioral and physiological function. Psychopharmacology (Berl) 2002; 161: 379–386. [DOI] [PubMed] [Google Scholar]
- 56.Yirmiya R, Chiappelli F, Tio DL, Tritt SH, Taylor AN. Effects of prenatal alcohol and pair feeding on lipopolysaccharide-induced secretion of TNF-alpha and corticosterone. Alcohol 1998; 15: 327–335. [DOI] [PubMed] [Google Scholar]
- 57.Taylor AN, Branch BJ, Nelson LR, Lane LA, Poland RE. Prenatal ethanol and ontogeny of pituitary–adrenal responses to ethanol and morphine. Alcohol 1986; 3: 255–259. [DOI] [PubMed] [Google Scholar]
- 58.Vieau D, Sebaai N, Leonhardt M, Dutriez-Casteloot I, Molendi-Coste O, Laborie C, Breton C, Deloof S, Lesage J. HPA axis programming by maternal undernutrition in the male rat offspring. Psychoneuroendocrinology 2007; 32 (Suppl. 1): S16–S20. [DOI] [PubMed] [Google Scholar]
- 59.Symonds ME, Stephenson T, Gardner DS, Budge H. Long-term effects of nutritional programming of the embryo and fetus: mechanisms and critical windows. Reprod Fertil Dev 2007; 19: 53–63. [DOI] [PubMed] [Google Scholar]
- 60.Burdge GC, Slater-Jefferies J, Torrens C, Phillips ES, Hanson MA, Lillycrop KA. Dietary protein restriction of pregnant rats in the F0 generation induces altered methylation of hepatic gene promoters in the adult male offspring in the F1 and F2 generations. Br J Nutr 2007; 97: 435–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Fowden AL, Giussani DA, Forhead AJ. Intrauterine programming of physiological systems: causes and consequences. Physiology (Bethesda) 2006; 21: 29–37. [DOI] [PubMed] [Google Scholar]
- 62.Root AW, Reiter EO, Andriola M, Duckett G. Hypothalamic–pituitary function in the fetal alcohol syndrome. J Pediatr 1975; 87: 585–588. [DOI] [PubMed] [Google Scholar]
- 63.Jacobson SW, Bihun JT, Chiodo LM. Effects of prenatal alcohol and cocaine exposure on infant cortisol levels. Dev Psychopathol 1999; 11: 195–208. [DOI] [PubMed] [Google Scholar]
- 64.Ramsay DS, Bendersky MI, Lewis M. Effect of prenatal alcohol and cigarette exposure on two- and six-month-old infants’ adrenocortical reactivity to stress. J Pediatr Psychol 1996; 21: 833–840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Haley DW, Handmaker NS, Lowe J. Infant stress reactivity and prenatal alcohol exposure. Alcohol Clin Exp Res 2006; 30: 2055–2064. [DOI] [PubMed] [Google Scholar]
- 66.Kim CK, Osborn JA, Weinberg J. Stress reactivity in fetal alcohol syndrome. In: Abel E, ed. Fetal Alcohol Syndrome: From Mechanism to Behavior. New York: CRC Press, 1996: 215–236. [Google Scholar]
- 67.Schneider ML, Moore CF, Kraemer GW, Roberts AD, DeJesus OT. The impact of prenatal stress, fetal alcohol exposure, or both on development: perspectives from a primate model. Psychoneuroendocrinology 2002; 27: 285–298. [DOI] [PubMed] [Google Scholar]
- 68.Taylor AN, Branch BJ, van Zuylen JE, Redei E. Maternal alcohol consumption and stress responsiveness in offspring. In: Chrousos GP, Loriaux DL, Gold PW, eds. Mechanisms of Physical and Emotional Stress. Advances in Experimental Medicine and Biology New York, NY: Plenum Press, 1988: 311–317. [DOI] [PubMed] [Google Scholar]
- 69.Lee S, Imaki T, Vale W, Rivier C. Effect of prenatal exposure to ethanol on the activity of the hypothalamic–pituitary–adrenal axis of the offspring: importance of the time of exposure to ethanol and possible modulating mechanisms. Mol Cell Neurosci 1990; 1: 168–177. [DOI] [PubMed] [Google Scholar]
- 70.Eguchi Y Interrelationships between the fetal and maternal hypophyseal-adrenal axes in rats and mice. In: Bajusz E, ed. Physiology and Pathology of Adaptation Mechanism. New York, NY: Pergamon Press, 1996: 3–27. [Google Scholar]
- 71.Weinberg J Hyperresponsiveness to stress: differential effects of prenatal ethanol on males and females. Alcohol Clin Exp Res 1988; 12: 647–652. [DOI] [PubMed] [Google Scholar]
- 72.Weinberg J Prenatal ethanol effects: sex differences in offspring stress responsiveness. Alcohol 1992; 9: 219–223. [DOI] [PubMed] [Google Scholar]
- 73.Weinberg J In utero alcohol exposure and hypothalamic–pituitary–adrenal activity: Gender differences in outcome. In: Zakhari S, Hunt WA, eds. Stress, Gender and Alcohol-Seeking Behavior, Research Monograph 29. Bethesda, MD: National Institute on Alcohol Abuse and Alcoholism, 1995: 29. [Google Scholar]
- 74.Halasz I, Aird F, Li L, Prystowsky MB, Redei E. Sexually dimorphic effects of alcohol exposure in utero on neuroendocrine and immune functions in chronic alcohol-exposed adult rats. Mol Cell Neurosci 1993; 4: 343–353. [DOI] [PubMed] [Google Scholar]
- 75.Lee S, Rivier C. Gender differences in the effect of prenatal alcohol exposure on the hypothalamic–pituitary–adrenal axis response to immune signals. Psychoneuroendocrinology 1996; 21: 145–155. [DOI] [PubMed] [Google Scholar]
- 76.Nelson LR, Taylor AN, Lewis JW, Poland RE, Redei E, Branch BJ. Pituitary–adrenal responses to morphine and footshock stress are enhanced following prenatal alcohol exposure. Alcohol Clin Exp Res 1986; 10: 397–402. [DOI] [PubMed] [Google Scholar]
- 77.Taylor AN, Branch BJ, Liu SH, Kokka N. Long-term effects of fetal ethanol exposure on pituitary–adrenal response to stress. Pharmacol Biochem Behav 1982; 16: 585–589. [DOI] [PubMed] [Google Scholar]
- 78.Taylor AN, Branch BJ, Liu SH, Wiechmann AF, Hill MA, Kokka N. Fetal exposure to ethanol enhances pituitary–adrenal and temperature responses to ethanol in adult rats. Alcohol Clin Exp Res 1981; 5: 237–246. [DOI] [PubMed] [Google Scholar]
- 79.Kelly SJ, Mahoney JC, Randich A, West JR. Indices of stress in rats: effects of sex, perinatal alcohol and artificial rearing. Physiol Behav 1991; 49: 751–756. [DOI] [PubMed] [Google Scholar]
- 80.Taylor AN, Branch BJ, Cooley-Matthews B, Poland RE. Effects of maternal ethanol consumption in rats on basal and rhythmic pituitary–adrenal function in neonatal offspring. Psychoneuroendocrinology 1982; 7: 49–58. [DOI] [PubMed] [Google Scholar]
- 81.Weinberg J, Nelson LR, Taylor AN. Hormonal effects of fetal alcohol exposure. In: West JR, ed. Alcohol and Brain Development. New York, NY: Oxford University Press, 1986: 310–342. [Google Scholar]
- 82.Kim CK, Giberson PK, Yu W, Zoeller RT, Weinberg J. Effects of prenatal ethanol exposure on hypothalamic–pituitary–adrenal responses to chronic cold stress in rats. Alcohol Clin Exp Res 1999; 23: 301–310. [PubMed] [Google Scholar]
- 83.Weinberg J, Taylor AN, Gianoulakis C. Fetal ethanol exposure: hypothalamic–pituitary–adrenal and beta-endorphin responses to repeated stress. Alcohol Clin Exp Res 1996; 20: 122–131. [DOI] [PubMed] [Google Scholar]
- 84.Kim CK, Turnbull AV, Lee SY, Rivier CL. Effects of prenatal exposure to alcohol on the release of adenocorticotropic hormone, corticosterone, and proinflammatory cytokines. Alcohol Clin Exp Res 1999; 23: 52–59. [PubMed] [Google Scholar]
- 85.Lee S, Schmidt D, Tilders F, Rivier C. Increased activity of the hypothalamic–pituitary–adrenal axis of rats exposed to alcohol in utero: role of altered pituitary and hypothalamic function. Mol Cell Neurosci 2000; 16: 515–528. [DOI] [PubMed] [Google Scholar]
- 86.Schneider ML, Moore CF, Kraemer GW. Moderate level alcohol during pregnancy, prenatal stress, or both and limbic–hypothalamic–pituitary–adrenocortical axis response to stress in rhesus monkeys. Child Dev 2004; 75: 96–109. [DOI] [PubMed] [Google Scholar]
- 87.Gabriel KI, Glavas MM, Ellis LI, Weinberg J. Postnatal handling does not normalize corticotropin releasing factor mRNA levels in animals prenatally exposed to ethanol. Devel Brain Res 2005; 157: 74–82. [DOI] [PubMed] [Google Scholar]
- 88.Redei E, Halasz I, Li LF, Prystowsky MB, Aird F. Maternal adrenalectomy alters the immune and endocrine functions of fetal alcohol-exposed male offspring. Endocrinology 1993; 133: 452–460. [DOI] [PubMed] [Google Scholar]
- 89.Glavas MM, Hofmann CE, Yu WK, Weinberg J. Effects of prenatal ethanol exposure on hypothalamic–pituitary–adrenal regulation after adrenalectomy and corticosterone replacement. Alcohol Clin Exp Res 2001; 25: 890–897. [PubMed] [Google Scholar]
- 90.Osborn JA, Yu C, Stelzl GE, Weinberg J. Effects of fetal ethanol exposure on pituitary–adrenal sensitivity to secretagogues. Alcohol Clin Exp Res 2000; 24: 1110–1119. [PubMed] [Google Scholar]
- 91.Osborn JA, Kim CK, Yu W, Herbert L, Weinberg J. Fetal ethanol exposure alters pituitary–adrenal sensitivity to dexamethasone suppression. Psychoneuroendocrinology 1996; 21: 127–143. [DOI] [PubMed] [Google Scholar]
- 92.Redei E, Branch BJ, Taylor AN. Direct effect of ethanol on adrenocorticotropin (ACTH) release in vitro. J Pharmacol Exp Ther 1986; 237: 59–64. [PubMed] [Google Scholar]
- 93.Lee S, Selvage D, Hansen K, Rivier C. Site of action of acute alcohol administration in stimulating the rat hypothalamic–pituitary–adrenal axis: comparison between the effect of systemic and intracerebroventricular injection of this drug on pituitary and hypothalamic responses. Endocrinology 2004; 145: 4470–4479. [DOI] [PubMed] [Google Scholar]
- 94.Tritt SH, Tio DL, Brammer GL, Taylor AN. Adrenalectomy but not adrenal demedullation during pregnancy prevents the growth-retarding effects of fetal alcohol exposure. Alcohol Clin Exp Res 1993; 17: 1281–1289. [DOI] [PubMed] [Google Scholar]
- 95.Slone-Wilcoxon J, Redei EE. Maternal-fetal glucocorticoid milieu programs hypothalamic–pituitary–thyroid function of adult offspring. Endocrinology 2004; 145: 4068–4072. [DOI] [PubMed] [Google Scholar]
- 96.Cobb CF, Van Thiel DH, Gavaler JS, Lester R. Effects of ethanol and acetaldehyde on the rat adrenal. Metabolism 1981; 30: 537–543. [DOI] [PubMed] [Google Scholar]
- 97.Weinberg J, Petersen TD. Effects of prenatal ethanol exposure on glucocorticoid receptors in rat hippocampus. Alcohol Clin Exp Res 1991; 15: 711–716. [DOI] [PubMed] [Google Scholar]
- 98.Kim CK, Yu W, Edin G, Ellis L, Osborn JA, Weinberg J. Chronic intermittent stress does not differentially alter brain corticosteroid receptor densities in rats prenatally exposed to ethanol. Psychoneuroendocrinology 1999; 24: 585–611. [DOI] [PubMed] [Google Scholar]
- 99.Weinstock M Gender differences in the effects of prenatal stress on brain development and behaviour. Neurochem Res 2007; 32: 1730–1740. [DOI] [PubMed] [Google Scholar]
- 100.Maccari S, Darnaudery M, Morley-Fletcher S, Zuena AR, Cinque C, Van Reeth O. Prenatal stress and long-term consequences: implications of glucocorticoid hormones. Neurosci Biobehav Rev 2003; 27: 119–127. [DOI] [PubMed] [Google Scholar]
- 101.Weinstock M The potential influence of maternal stress hormones on development and mental health of the offspring. Brain Behav Immun 2005; 19: 296–308. [DOI] [PubMed] [Google Scholar]
- 102.Zagron G, Weinstock M. Maternal adrenal hormone secretion mediates behavioural alterations induced by prenatal stress in male and female rats. Behav Brain Res 2006; 175: 323–328. [DOI] [PubMed] [Google Scholar]
- 103.Lee S, Rivier C. Administration of corticosterone to pregnant adrenalectomized dams does not alter the hypothalamic–pituitary–adrenal axis’ acitivity of the offspring. Mol Cell Neurosci 1992; 3: 118–123. [DOI] [PubMed] [Google Scholar]
- 104.Patchev VK, Hayashi S, Orikasa C, Almeida OF. Implications of estrogen-dependent brain organization for gender differences in hypothalamo–pituitary–adrenal regulation. FASEB J 1995; 9: 419–423. [DOI] [PubMed] [Google Scholar]
- 105.Rivier C, Rivest S. Effect of stress on the activity of the hypothalamic–pituitary–gonadal axis: peripheral and central mechanisms. Biol Reprod 1991; 45: 523–532. [DOI] [PubMed] [Google Scholar]
- 106.Viau V Functional cross-talk between the hypothalamic-pituitary-gonadal and -adrenal axes. J Neuroendocrinol 2002; 14: 506–513. [DOI] [PubMed] [Google Scholar]
- 107.Viau V, Soriano L, Dallman MF. Androgens alter corticotropin releasing hormone and arginine vasopressin mRNA within forebrain sites known to regulate activity in the hypothalamic–pituitary–adrenal axis. J Neuroendocrinol 2001; 13: 442–452. [DOI] [PubMed] [Google Scholar]
- 108.Young EA. The role of gonadal steroids in hypothalamic–pituitary–adrenal axis regulation. Crit Rev Neurobiol 1995; 9: 371–381. [PubMed] [Google Scholar]
- 109.Young EA. Sex differences in response to exogenous corticosterone: a rat model of hypercortisolemia. Mol Psychiatry 1996; 1: 313–319. [PubMed] [Google Scholar]
- 110.Carey MP, Deterd CH, de Koning J, Helmerhorst F, de Kloet ER. The influence of ovarian steroids on hypothalamic–pituitary–adrenal regulation in the female rat. J Endocrinol 1995; 144: 311–321. [DOI] [PubMed] [Google Scholar]
- 111.Viau V, Meaney MJ. Variations in the hypothalamic–pituitary–adrenal response to stress during the estrous cycle in the rat. Endocrinology 1991; 129: 2503–2511. [DOI] [PubMed] [Google Scholar]
- 112.Dallman MF, Viau V, Bhatnagar S, Gomez F, Laugero K, Bell ME. Corticotropin-releasing factor (CRF), corticosteroids, and sugar: energy balance, the brain and behavior. In: Pffaf DW, Arnold AP, Etgen AM, Fahrback SE, Rubin RT, eds. Mammalian hormone systems. San Diego: Academic Press, 2002: 571–632. [Google Scholar]
- 113.Handa RJ, Burgess LH, Kerr JE, O’Keefe JA. Gonadal steroid hormone receptors and sex differences in the hypothalamo–pituitary–adrenal axis. Horm Behav 1994; 28: 464–476. [DOI] [PubMed] [Google Scholar]
- 114.Williamson M, Bingham B, Viau V. Central organization of androgen-sensitive pathways to the hypothalamic–pituitary–adrenal axis: implications for individual differences in responses to homeostatic threat and predisposition to disease. Prog Neuropsychopharmacol Biol Psychiatry 2005; 29: 1239–1248. [DOI] [PubMed] [Google Scholar]
- 115.Karandrea D, Kittas C, Kitraki E. Contribution of sex and cellular context in the regulation of brain corticosteroid receptors following restraint stress. Neuroendocrinology 2000; 71: 343–353. [DOI] [PubMed] [Google Scholar]
- 116.Kerr JE, Beck SG, Handa RJ. Androgens modulate glucocorticoid receptor mRNA, but not mineralocorticoid receptor mRNA levels, in the rat hippocampus. J Neuroendocrinol 1996; 8: 439–447. [DOI] [PubMed] [Google Scholar]
- 117.Kerr JE, Allore RJ, Beck SG, Handa RJ. Distribution and hormonal regulation of androgen receptor (AR) and AR messenger ribonucleic acid in the rat hippocampus. Endocrinology 1995; 136: 3213–3221. [DOI] [PubMed] [Google Scholar]
- 118.Viau V, Meaney MJ. The inhibitory effect of testosterone on hypothalamic–pituitary–adrenal responses to stress is mediated by the medial preoptic area. J Neurosci 1996; 16: 1866–1876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Bingaman EW, Magnuson DJ, Gray TS, Handa RJ. Androgen inhibits the increases in hypothalamic corticotropin-releasing hormone (CRH) and CRH-immunoreactivity following gonadectomy. Neuroendocrinology 1994; 59: 228–234. [DOI] [PubMed] [Google Scholar]
- 120.Handa RJ, Nunley KM, Lorens SA, Louie JP, McGivern RF, Bollnow MR. Androgen regulation of adrenocorticotropin and corticosterone secretion in the male rat following novelty and foot shock stressors. Physiol Behav 1994; 55: 117–124. [DOI] [PubMed] [Google Scholar]
- 121.Kelly SJ, Ostrowski NL, Wilson MA. Gender differences in brain and behavior: hormonal and neural bases. Pharmacol Biochem Behav 1999; 64: 655–664. [DOI] [PubMed] [Google Scholar]
- 122.McGivern RF, Raum WJ, Salido E, Redei E. Lack of prenatal testosterone surge in fetal rats exposed to alcohol: alterations in testicular morphology and physiology. Alcohol Clin Exp Res 1988; 12: 243–247. [DOI] [PubMed] [Google Scholar]
- 123.McGivern RF, Handa RJ, Raum WJ. Ethanol exposure during the last week of gestation in the rat: inhibition of the prenatal testosterone surge in males without long-term alterations in sex behavior. Neurotoxicol Teratol 1998; 20: 483–490. [DOI] [PubMed] [Google Scholar]
- 124.McGivern RF, Handa RJ, Redei E. Decreased postnatal testosterone surge in male rats exposed to ethanol during the last week of gestation. Alcohol Clin Exp Res 1993; 17: 1215–1222. [DOI] [PubMed] [Google Scholar]
- 125.Kakihana R, Butte JC, Moore JA. Endocrine effects of meternal alcoholization: plasma and brain testosterone, dihydrotestosterone, estradiol, and corticosterone. Alcohol Clin Exp Res 1980; 4: 57–61. [DOI] [PubMed] [Google Scholar]
- 126.Kelce WR, Rudeen PK, Ganjam VK. Prenatal ethanol exposure alters steroidogenic enzyme activity in newborn rat testes. Alcohol Clin Exp Res 1989; 13: 617–621. [DOI] [PubMed] [Google Scholar]
- 127.Chen JJ, Smith ER. Effects of perinatal alcohol on sexual differentiation and open-field behavior in rats. Horm Behav 1979; 13: 219–231. [DOI] [PubMed] [Google Scholar]
- 128.McGivern RF. Influence of prenatal exposure to cimetidine and alcohol on selected morphological parameters of sexual differentiation: a preliminary report. Neurotoxicol Teratol 1987; 9: 23–26. [DOI] [PubMed] [Google Scholar]
- 129.Parker S, Udani M, Gavaler JS, Van Thiel DH. Adverse effects of ethanol upon the adult sexual behavior of male rats exposed in utero. Neurobehav.Toxicol.Teratol 1984; 6: 289–293. [PubMed] [Google Scholar]
- 130.Rudeen PK. Reduction of the volume of the sexually dimorphic nucleus of the preoptic area by in utero ethanol exposure in male rats. Neurosci Lett 1986; 72: 363–368. [DOI] [PubMed] [Google Scholar]
- 131.Udani M, Parker S, Gavaler J, Van Thiel DH. Effects of in utero exposure to alcohol upon male rats. Alcohol Clin Exp Res 1985; 9: 355–359. [DOI] [PubMed] [Google Scholar]
- 132.Jungkuntz-Burgett L, Paredez S, Rudeen PK. Reduced sensitivity of hypothalamic-preoptic area norepinephrine and dopamine to testosterone feedback in adult fetal ethanol-exposed male rats. Alcohol 1990; 7: 513–516. [DOI] [PubMed] [Google Scholar]
- 133.Hard E, Dahlgren IL, Engel J, Larsson K, Lindh AS, Musi B. Impairment of reproductive behavior in prenatally ethanol-exposed rats. In: Rydberg U, ed. Alcohol and the Developing Brain. New York, NY: Raven Press, 1985: 93–108. [Google Scholar]
- 134.McGivern RF, Clancy AN, Hill MA, Noble EP. Prenatal alcohol exposure alters adult expression of sexually dimorphic behavior in the rat. Science 1984; 224: 896–898. [DOI] [PubMed] [Google Scholar]
- 135.McGivern RF, Ervin MG, McGeary J, Somes C, Handa RJ. Prenatal ethanol exposure induces a sexually dimorphic effect on daily water consumption in prepubertal and adult rats. Alcohol Clin Exp Res 1998; 22: 868–875. [PubMed] [Google Scholar]
- 136.Meyer LS, Riley EP. Social play in juvenile rats prenatally exposed to alcohol. Teratology 1986; 34: 1–7. [DOI] [PubMed] [Google Scholar]
- 137.Barron S, Tieman SB, Riley EP. Effects of prenatal alcohol exposure on the sexually dimorphic nucleus of the preoptic area of the hypothalamus in male and female rats. Alcohol Clin Exp Res 1988; 12: 59–64. [DOI] [PubMed] [Google Scholar]
- 138.Boggan WO, Randall CL, Dodds HM. Delayed sexual maturation in female C57BL/6J mice prenatally exposed to alcohol. Res Commun Chem Pathol Pharmacol 1979; 23: 117–125. [PubMed] [Google Scholar]
- 139.Esquifino AI, Sanchis R, Guerri C. Effect of prenatal alcohol exposure on sexual maturation of female rat offspring. Neuroendocrinology 1986; 44: 483–487. [DOI] [PubMed] [Google Scholar]
- 140.Farry K, Tittmar HG. Alcohol as a teratogen: effects of maternal administration in rats on sexual development in female offspring. Irc Med Sci Reprod Obstet Gynecol 1975; 3: 620. [Google Scholar]
- 141.Wilson ME, Handa RJ. Gonadotropin secretion in infantile rats exposed to ethanol in utero. Alcohol 1997; 14: 497–501. [DOI] [PubMed] [Google Scholar]
- 142.McGivern RF, Yellon SM. Delayed onset of puberty and subtle alterations in GnRH neuronal morphology in female rats exposed prenatally to ethanol. Alcohol 1992; 9: 335–340. [DOI] [PubMed] [Google Scholar]
- 143.Handa RJ, McGivern RF, Noble ES, Gorski RA. Exposure to alcohol in utero alters the adult patterns of luteinizing hormone secretion in male and female rats. Life Sci 1985; 37: 1683–1690. [DOI] [PubMed] [Google Scholar]
- 144.Li Y, McGivern RF, Nagahara AH, Handa RJ. Alterations in the estrogen sensitivity of hypothalamic proenkephalin mRNA expression with age and prenatal exposure to alcohol. Brain Res Mol Brain Res 1997; 47: 215–222. [DOI] [PubMed] [Google Scholar]
- 145.Wilson ME, Marshall MT, Bollnow MR, McGivern RF, Handa RJ. Gonadotropin-releasing hormone mRNA and gonadotropin beta-subunit mRNA expression in the adult female rat exposed to ethanol in utero. Alcohol Clin Exp Res 1995; 19: 1211–1218. [DOI] [PubMed] [Google Scholar]
- 146.Creighton-Taylor JA, Rudeen PK. Fetal alcohol exposure and effects of LHRH and PMA on LH beta-mRNA expression in the female rat. Alcohol Clin Exp Res 1991; 15: 1031–1035. [DOI] [PubMed] [Google Scholar]
- 147.McGivern RF, McGeary J, Robeck S, Cohen S, Handa RJ. Loss of reproductive competence at an earlier age in female rats exposed prenatally to ethanol. Alcohol Clin Exp Res 1995; 19: 427–433. [DOI] [PubMed] [Google Scholar]
- 148.Barron S, Riley EP. Pup-induced maternal behavior in adult and juvenile rats exposed to alcohol prenatally. Alcohol Clin Exp Res 1985; 9: 360–365. [DOI] [PubMed] [Google Scholar]
- 149.Aird F, Halasz I, Redei E. Ontogeny of hypothalamic corticotropin-releasing factor and anterior pituitary pro-opiomelanocortin expression in male and female offspring of alcohol-exposed and adrenalectomized dams. Alcohol Clin Exp Res 1997; 21: 1560–1566. [PubMed] [Google Scholar]
- 150.Lan N, Yamashita F, Halpert AG, Ellis L, Yu WK, Viau V, Weinberg J. Prenatal ethanol exposure alters the effects of gonadectomy on hypothalamic–pituitary–adrenal activity in male rats. J Neuroendocrinol 2006; 18: 672–684. [DOI] [PubMed] [Google Scholar]
- 151.Aguilera G Regulation of pituitary ACTH secretion during chronic stress. Front Neuroendocrinol 1994; 15: 321–350. [DOI] [PubMed] [Google Scholar]
- 152.Gillies GE, Linton EA, Lowry PJ. Corticotropin releasing activity of the new CRF is potentiated several times by vasopressin. Nature 1982; 299: 355–357. [DOI] [PubMed] [Google Scholar]
- 153.Ma XM, Lightman SL, Aguilera G. Vasopressin and corticotropin-releasing hormone gene responses to novel stress in rats adapted to repeated restraint. Endocrinology 1999; 140: 3623–3632. [DOI] [PubMed] [Google Scholar]
- 154.Kalra PS, Simpkins JW, Kalra SP. Testosterone raises LHRH levels exclusively in the median eminence of castrated rats. Neuroendocrinology 1984; 39: 45–48. [DOI] [PubMed] [Google Scholar]
- 155.Lan N, Hellemans KGC, Ellis LI, Viau V, Weinberg J. Role of testosterone in mediating prenatal ethanol effects on hypothalamic–pituitary–adrenal activity in male rats. Psychoneuroendocrinology submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Lan N, Bach P, Westendorp S, Ellis L, Yu WK, Viau V, Weinberg J. Role of testosterone in hippocampal mineralocorticoid (MR) and glucocorticoid (GR) receptor mRNA levels in male rats prenatally exposed to ethanol. Endocrine Soc Abstr 2007: 3–57. [Google Scholar]
- 157.Lan N, Westendorp S, Bach P, Ellis L, O’Neill R, O’Rourke D, Yu WK, Vogl W, Weinberg J. Prenatal ethanol exposure delays testes seminiferous tubule development. Alcohol Clin Exp Res 2007; 31: 100A.17207107 [Google Scholar]
- 158.Lan N, Yamashita F, Halpert AH, Sliwowska JH, Ellis L, Yu W, O’Rourke D, Viau V, Weinberg J. Prenatal ethanol effects on the hypothalmic–pituitary–adrenal response to stress across the estrus cycle. Alcohol Clin Exp Res 2006; 30: 880A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Sliwowska JH, Lee J, Ellis LI, Yu W, Weinberg J. The decrease of kisspeptin-ir cells in the arcuate nucleus of the hypothalamus in adult female rats prenatally exposed to ethanol is estradiol-dependent. Soc Neurosci 2007: (Abstract 194.8). [Google Scholar]
- 160.Kotani M, Detheux M, Vandenbogaerde A, Communi D, Vanderwinden JM, Le Poul E, Brezillon S, Tyldesley R, Suarez-Huerta N, Vandeput F, Blanpain C, Schiffmann SN, Vassart G, Parmentier M. The metastasis suppressor gene KiSS-1 encodes kisspeptins, the natural ligands of the orphan G protein-coupled receptor GPR54. J Biol Chem 2001; 276: 34631–34636. [DOI] [PubMed] [Google Scholar]
- 161.Muir AI, Chamberlain L, Elshourbagy NA, Michalovich D, Moore DJ, Calamari A, Szekeres PG, Sarau HM, Chambers JK, Murdock P, Steplewski K, Shabon U, Miller JE, Middleton SE, Darker JG, Larminie CG, Wilson S, Bergsma DJ, Emson P, Faull R, Philpott KL, Harrison DC. AXOR12, a novel human G protein-coupled receptor, activated by the peptide KiSS-1. J Biol Chem 2001; 276: 28969–28975. [DOI] [PubMed] [Google Scholar]
- 162.Ohtaki T, Shintani Y, Honda S, Matsumoto H, Hori A, Kanehashi K, Terao Y, Kumano S, Takatsu Y, Masuda Y, Ishibashi Y, Watanabe T, Asada M, Yamada T, Suenaga M, Kitada C, Usuki S, Kurokawa T, Onda H, Nishimura O, Fujino M. Metastasis suppressor gene KiSS-1 encodes peptide ligand of a G-protein-coupled receptor. Nature 2001; 411: 613–617. [DOI] [PubMed] [Google Scholar]
- 163.Gottsch ML, Cunningham MJ, Smith JT, Popa SM, Acohido BV, Crowley WF, Seminara S, Clifton DK, Steiner RA. A role for kisspeptins in the regulation of gonadotropin secretion in the mouse. Endocrinology 2004; 145: 4073–4077. [DOI] [PubMed] [Google Scholar]
- 164.Smith JT, Cunningham MJ, Rissman EF, Clifton DK, Steiner RA. Regulation of Kiss1 gene expression in the brain of the female mouse. Endocrinology 2005; 146: 3686–3692. [DOI] [PubMed] [Google Scholar]
- 165.Clarkson J, Herbison AE. Postnatal development of kisspeptin neurons in mouse hypothalamus; sexual dimorphism and projections to gonadotropin-releasing hormone neurons. Endocrinology 2006; 147: 5817–5825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Dhillo WS, Chaudhri OB, Patterson M, Thompson EL, Murphy KG, Badman MK, McGowan BM, Amber V, Patel S, Ghatei MA, Bloom SR. Kisspeptin-54 stimulates the hypothalamic–pituitary gonadal axis in human males. J Clin Endocrinol Metab 2005; 90: 6609–6615. [DOI] [PubMed] [Google Scholar]
- 167.Messager S, Chatzidaki EE, Ma D, Hendrick AG, Zahn D, Dixon J, Thresher RR, Malinge I, Lomet D, Carlton MB, Colledge WH, Caraty A, Aparicio SA. Kisspeptin directly stimulates gonadotropin-releasing hormone release via G protein-coupled receptor 54. Proc Natl Acad Sci USA 2005; 102: 1761–1766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Shahab M, Mastronardi C, Seminara SB, Crowley WF, Ojeda SR, Plant TM. Increased hypothalamic GPR54 signaling: a potential mechanism for initiation of puberty in primates. Proc Natl Acad Sci USA 2005; 102: 2129–2134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Thompson EL, Patterson M, Murphy KG, Smith KL, Dhillo WS, Todd JF, Ghatei MA, Bloom SR. Central and peripheral administration of kisspeptin-10 stimulates the hypothalamic–pituitary–gonadal axis. J Neuroendocrinol 2004; 16: 850–858. [DOI] [PubMed] [Google Scholar]
- 170.Kauffman AS, Clifton DK, Steiner RA. Emerging ideas about kisspeptin-GPR54 signaling in the neuroendocrine regulation of reproduction. Trends Neurosci 2007; 30: 504–511. [DOI] [PubMed] [Google Scholar]
- 171.Herbison AE, Pape JR. New evidence for estrogen receptors in gonadotropin-releasing hormone neurons. Front Neuroendocrinol 2001; 22: 292–308. [DOI] [PubMed] [Google Scholar]
- 172.Adachi S, Yamada S, Takatsu Y, Matsui H, Kinoshita M, Takase K, Sugiura H, Ohtaki T, Matsumoto H, Uenoyama Y, Tsukamura H, Inoue K, Maeda K. Involvement of anteroventral periventricular metastin/kisspeptin neurons in estrogen positive feedback action on luteinizing hormone release in female rats. J Reprod Dev 2007; 53: 367–378. [DOI] [PubMed] [Google Scholar]
- 173.Navarro VM, Castellano JM, Fernandez-Fernandez R, Tovar S, Roa J, Mayen A, Nogueiras R, Vazquez MJ, Barreiro ML, Magni P, Aguilar E, Dieguez C, Pinilla L, Tena-Sempere M. Characterization of the potent luteinizing hormone-releasing activity of KiSS-1 peptide, the natural ligand of GPR54. Endocrinology 2005; 146: 156–163. [DOI] [PubMed] [Google Scholar]
- 174.Lowry CA. Functional subsets of serotonergic neurones: implications for control of the hypothalamic–pituitary–adrenal axis. J Neuroendocrinol 2002; 14: 911–923. [DOI] [PubMed] [Google Scholar]
- 175.Druse MJ, Kuo A, Tajuddin N. Effects of in utero ethanol exposure on the developing serotonergic system. Alcohol Clin Exp Res 1991; 15: 678–684. [DOI] [PubMed] [Google Scholar]
- 176.Rathbun W, Druse MJ. Dopamine, serotonin, and acid metabolites in brain regions from the developing offspring of ethanol-treated rats. J Neurochem 1985; 44: 57–62. [DOI] [PubMed] [Google Scholar]
- 177.Sari Y, Zhou FC. Prenatal alcohol exposure causes long-term serotonin neuron deficit in mice. Alcohol Clin Exp Res 2004; 28: 941–948. [DOI] [PubMed] [Google Scholar]
- 178.Zafar H, Shelat SG, Redei E, Tejani-Butt S. Fetal alcohol exposure alters serotonin transporter sites in rat brain. Brain Res 2000; 856: 184–192. [DOI] [PubMed] [Google Scholar]
- 179.Tajuddin NF, Druse MJ. Treatment of pregnant alcohol-consuming rats with buspirone: effects on serotonin and 5-hydroxyindoleacetic acid content in offspring. Alcohol Clin Exp Res 1993; 17: 110–114. [DOI] [PubMed] [Google Scholar]
- 180.Kelly SJ. Alcohol exposure during development alters hypothalamic neurotransmitter concentrations. J Neural Transm 1996; 103: 55–67. [DOI] [PubMed] [Google Scholar]
- 181.Tran TD, Kelly SJ. Alterations in hippocampal and hypothalamic monoaminergic neurotransmitter systems after alcohol exposure during all three trimester equivalents in adult rats. J Neural Transm 1999; 106: 773–786. [DOI] [PubMed] [Google Scholar]
- 182.Hofmann CE, Ellis L, Yu WK, Weinberg J. Hypothalamic–pituitary–adrenal responses to 5-HT1A and 5-HT2A/C agonists are differentially altered in female and male rats prenatally exposed to ethanol. Alcohol Clin Exp Res 2007; 31: 345–355. [DOI] [PubMed] [Google Scholar]
- 183.Sliwowska JH, Lan N, Yamashita F, Halpert AG, Viau V, Weinberg J. Prenatal ethanol exposure induces long-term changes in hippocampal MR but not GR mRNA levels in adult female rats. Alcohol Clin Exp Res; 30: 119A.16433739 [Google Scholar]
- 184.Lopez JF, Chalmers DT, Little KY, Watson SJ. A.E. Bennett Research Award. Regulation of serotonin1A, glucocorticoid, and mineralocorticoid receptor in rat and human hippocampus: implications for the neurobiology of depression. Biol Psychiatry 1998; 43: 547–573. [DOI] [PubMed] [Google Scholar]
- 185.Lopez JF, Vazquez DM, Chalmers DT, Watson SJ. Regulation of 5-HT receptors and the hypothalamic–pituitary–adrenal axis. Implications for the neurobiology of suicide. Ann NY Acad Sci 1997; 836: 106–134. [DOI] [PubMed] [Google Scholar]
- 186.Heinz A, Mann K, Weinberger DR, Goldman D. Serotonergic dysfunction, negative mood states, and response to alcohol. Alcohol Clin Exp Res 2001; 25: 487–495. [PubMed] [Google Scholar]
- 187.Thase ME. Depression and sleep: pathophysiology and treatment. Dialogues Clin Neurosci 2006; 8: 217–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Tyrer P, Baldwin D. Generalised anxiety disorder. Lancet 2006; 368: 2156–2166. [DOI] [PubMed] [Google Scholar]
- 189.Pariante CM, Thomas SA, Lovestone S, Makoff A, Kerwin RW. Do antidepressants regulate how cortisol affects the brain? Psychoneuroendocrinology 2004; 29: 423–447. [DOI] [PubMed] [Google Scholar]
- 190.Swaab DF, Bao AM, Lucassen PJ. The stress system in the human brain in depression and neurodegeneration. Ageing Res Rev 2005; 4: 141–194. [DOI] [PubMed] [Google Scholar]
- 191.de Kloet ER, Vreugdenhil E, Oitzl MS, Joels M. Brain corticosteroid receptor balance in health and disease. Endocr Rev 1998; 19: 269–301. [DOI] [PubMed] [Google Scholar]
- 192.Gillespie CF, Nemeroff CB. Hypercortisolemia and depression. Psychosom Med 2005; 67 (Suppl. 1): S26–S28. [DOI] [PubMed] [Google Scholar]
- 193.Heim C, Plotsky PM, Nemeroff CB. Importance of studying the contributions of early adverse experience to neurobiological findings in depression. Neuropsychopharmacology 2004; 29: 641–648. [DOI] [PubMed] [Google Scholar]
- 194.Heim C, Nemeroff CB. The role of childhood trauma in the neurobiology of mood and anxiety disorders: preclinical and clinical studies. Biol Psychiatry 2001; 49: 1023–1039. [DOI] [PubMed] [Google Scholar]
- 195.Meaney MJ, Szyf M, Seckl JR. Epigenetic mechanisms of perinatal programming of hypothalamic–pituitary–adrenal function and health. Trends Mol Med 2007; 13: 269–277. [DOI] [PubMed] [Google Scholar]
- 196.Gluckman PD, Hanson MA. The developmental origins of the metabolic syndrome. Trends Endocrinol Metab 2004; 15: 183–187. [DOI] [PubMed] [Google Scholar]
- 197.Liu L, Li A, Matthews SG. Maternal glucocorticoid treatment programs HPA regulation in adult offspring: sex-specific effects. Am J Physiol Endocrinol Metab 2001; 280: E729–E739. [DOI] [PubMed] [Google Scholar]
- 198.Weaver IC, Cervoni N, Champagne FA, D’Alessio AC, Sharma S, Seckl JR, Dymov S, Szyf M, Meaney MJ. Epigenetic programming by maternal behavior. Nat Neurosci 2004; 7: 847–854. [DOI] [PubMed] [Google Scholar]
- 199.Phillips DI, Barker DJ, Fall CH, Seckl JR, Whorwood CB, Wood PJ, Walker BR. Elevated plasma cortisol concentrations: a link between low birth weight and the insulin resistance syndrome? J Clin Endocrinol Metab 1998; 83: 757–760. [DOI] [PubMed] [Google Scholar]
- 200.Phillips DI, Walker BR, Reynolds RM, Flanagan DE, Wood PJ, Osmond C, Barker DJ, Whorwood CB. Low birth weight predicts elevated plasma cortisol concentrations in adults from 3 populations. Hypertension 2000; 35: 1301–1306. [DOI] [PubMed] [Google Scholar]
- 201.Schalinske KL, Nieman KM. Disruption of methyl group metabolism by ethanol. Nutr Rev 2005; 63: 387–391. [DOI] [PubMed] [Google Scholar]
- 202.O’Neill R, Lan N, Innis S, Devlin A, Ellis L, Chan B, Weinberg J. Metabolic effects of prenatal ethanol exposure and epigenetic reprogramming of the HPA axis. Alcohol Clin Exp Res 2007; 31: 100A.17207107 [Google Scholar]
- 203.Streissguth AP, Barr HM, Kogan J, Bookstein FL. Understanding the Occurrence of Secondary Disabilities in Clients with Fetal Alcohol Syndrome (FAS) and Fetal Alcohol Effects (FAE). Final Report, CDCP Grant No. RO4/CCROO8515, Fetal Alcohol and Drug Unit, Seattle, 1996. [Google Scholar]
- 204.Bertoglio LJ, Joca SR, Guimaraes FS. Further evidence that anxiety and memory are regionally dissociated within the hippocampus. Behav Brain Res 2006; 175: 183–188. [DOI] [PubMed] [Google Scholar]
- 205.de Kloet ER, Joels M, Holsboer F. Stress and the brain: from adaptation to disease. Nat Rev Neurosci 2005; 6: 463–475. [DOI] [PubMed] [Google Scholar]
- 206.Heim C, Nemeroff CB. The impact of early adverse experiences on brain systems involved in the pathophysiology of anxiety and affective disorders. Biol Psychiatry 1999; 46: 1509–1522. [DOI] [PubMed] [Google Scholar]
- 207.Heim C, Newport DJ, Heit S, Graham YP, Wilcox M, Bonsall R, Miller AH, Nemeroff CB. Pituitary–adrenal and autonomic responses to stress in women after sexual and physical abuse in childhood. JAMA 2000; 284: 592–597. [DOI] [PubMed] [Google Scholar]
- 208.Hauger RL, Lorang M, Irwin M, Aguilera G. CRF receptor regulation and sensitization of ACTH responses to acute ether stress during chronic intermittent immobilization stress. Brain Res 1990; 532: 34–40. [DOI] [PubMed] [Google Scholar]
- 209.Bernet CZ, Stein MB. Relationship of childhood maltreatment to the onset and course of major depression in adulthood. Depress Anxiety 1999; 9: 169–174. [PubMed] [Google Scholar]
- 210.Matthews K, Christmas D, Swan J, Sorrell E. Animal models of depression: navigating through the clinical fog. Neurosci Biobehav Rev 2005; 29: 503–513. [DOI] [PubMed] [Google Scholar]
- 211.Gutman DA, Nemeroff CB. Persistent central nervous system effects of an adverse early environment: clinical and preclinical studies. Physiol Behav 2003; 79: 471–478. [DOI] [PubMed] [Google Scholar]
- 212.Nemeroff CB, Vale WW. The neurobiology of depression: inroads to treatment and new drug discovery. J Clin Psychiatry 2005; 66: 5–13. [PubMed] [Google Scholar]
- 213.Dungan HM, Clifton DK, Steiner RA. Minireview: KISS peptin neurons as central processors in the regulation of gonadotropin - releasing hormone secretion. Endocrinology 2006; 147: 1154–1158. [DOI] [PubMed] [Google Scholar]
- 214.Glavas MM, Yu WK, Weinberg J. Effects of mineralcorticoid and glucocorticoid receptor blockade on the hypothalmic–pituitary–adrenal function in rats prenatally exposed to ethanol. Alcohol Clin Exp Res 2006; 30: 1916–1924. [DOI] [PubMed] [Google Scholar]
- 215.Rudeen PK, Weinberg J. Prenatal ethanol exposure: changes in regional brain catecholamine content following stress. J Neurochem 1993; 61: 1907–1915. [DOI] [PubMed] [Google Scholar]
- 216.Hofmann CE, Patyk IA, Weinberg J. Prenatal ethanol exposure: sex differences in anxiety and anxiolytic response to a 5-HT1A agonist. Pharmacol Biochem Behav 2005; 82: 549–558. [DOI] [PubMed] [Google Scholar]
- 217.Porsolt RD. Animal model of depression. Biomedicine 1979; 30: 139–140. [PubMed] [Google Scholar]
- 218.Cameron OG, Abelson JL, Young EA. Anxious and depressive disorders and their comorbidity: effect on central nervous system noradrenergic function. Biol Psychiatry 2004; 56: 875–883. [DOI] [PubMed] [Google Scholar]
- 219.Gorman JM. Comorbid depression and anxiety spectrum disorders. Depress Anxiety 1996; 4: 160–168. [DOI] [PubMed] [Google Scholar]
- 220.Nemeroff CB. Comorbidity of mood and anxiety disorders: the rule, not the exception? Am J Psychiatry 2002; 159: 3–4. [DOI] [PubMed] [Google Scholar]
- 221.Willner P. Validity, reliability and utility of the chronic mild stress model of depression: a 10-year review and evaluation. Psychopharmacology (Berl) 1997; 134: 319–329. [DOI] [PubMed] [Google Scholar]


