ABSTRACT
Diet is a modifiable, noninvasive, inexpensive behavior that is crucial in shaping the intestinal microbiome. A microbiome “imbalance” or dysbiosis in inflammatory bowel disease (IBD) is linked to inflammation. Here, we aim to define the impact of specific foods on bacterial species commonly depleted in patients with IBD to better inform dietary treatment. We performed a single-arm, pre-post intervention trial. After a baseline period, a dietary intervention with the IBD-Anti-Inflammatory Diet (IBD-AID) was initiated. We collected stool and blood samples and assessed dietary intake throughout the study. We applied advanced computational approaches to define and model complex interactions between the foods reported and the microbiome. A dense dataset comprising 553 dietary records and 340 stool samples was obtained from 22 participants. Consumption of prebiotics, probiotics, and beneficial foods correlated with increased abundance of Clostridia and Bacteroides, commonly depleted in IBD cohorts. We further show that specific foods categorized as prebiotics or adverse foods are correlated to levels of cytokines in serum (i.e., GM-CSF, IL-6, IL-8, TNF-alpha) that play a central role in IBD pathogenesis. By using robust predictive analytics, this study represents the first steps to detangle diet-microbiome and diet-immune interactions to inform personalized nutrition for patients suffering from dysbiosis-related IBD.
KEYWORDS: Microbiome, IBD, diet, dysbiosis
Introduction
The etiology of inflammatory bowel disease (IBD) is thought to be linked to an inappropriate immune response to an altered or dysbiotic gut microbiome in genetically susceptible individuals. Dysbiosis in IBD patients is characterized by depletion of Clostridia and Bacteroides.1–6 These bacterial species are known to maintain gut homeostasis via the production of short-chain fatty acids (SCFAs).7–11 Dietary interventions represent an ideal strategy to revert gut dysbiosis in IBD patients as diet change is often more embraced by individuals than medication.12 Also, diet is safe, does not require FDA approval 12 , and has been proven to rapidly change the microbiome.13
Recent trials have demonstrated that dietary therapy is effective for pediatric patients with Crohn’s disease (CD). The diets tested as a therapy for pediatric patients included the Specific Carbohydrate Diet (SCD), the modified SCD (mSCD, which includes oats), the Crohn’s disease exclusion diet with partial enteral nutrition (CDED+PEN), and the exclusive enteral nutrition (EEN) diet.14–22 A recent randomized trial comparing treatment with either SCD, the mSCD, and whole foods found that 100% of children completing 12 weeks on either treatment achieved remission.14 The latest multicenter randomized trial comparing treatment of children with CDED+PEN or EEN showed that either treatment resulted in 63% and 67% remission rates after 3 and 6 weeks of treatment, respectively.15 Diet treatment favored increased abundance of Clostridia species, including Faecalibacterium prausnitzii, Roseburia hominis, and Eubacterium eligens.14–16
In adults with CD, a recent randomized trial that included interventions with either the SCD or the Mediterranean diet has also demonstrated a remarkable effect of diet in inducing remission.23 Specifically, after only 6 weeks on either diet half of the patients in the trial achieved symptomatic remission with ≥30% showing reduction of fecal calprotectin levels.23 For ulcerative colitis (UC), a catered nutritious low-fat/high-fiber diet has been shown to improve the overall quality of life, lower inflammatory markers, decreased dysbiosis, and specifically favor Faecalibacterium prausnitzi.24 We created the IBD-Anti-Inflammatory Diet or IBD-AID.25,26 The IBD-AID has been designed to revert dysbiosis in patients with IBD, through increased consumption of prebiotics and probiotics foods,26 beneficial foods necessary for human nutrition27, and avoidance of foods known to trigger intestinal symptoms and dysbiosis.25,26,28–34 In a retrospective study, we reported that adult patients, both CD or UC patients, adopting the IBD-AID experienced reduction of disease activity and lowered their medication intake only after 4 weeks on the diet.26
In this current work, our primary outcome is to rigorously establish whether the IBD-AID can revert dysbiosis by favoring SCFA-producing bacteria that are depleted in patients with IBD. To achieve this primary outcome we took into account the fact that there is highly interpersonal variability of microbiome,35 also individual-specific response to diet,36 and the fact that consuming a placebo diet is not an option for a control group.37 Thus, we conducted a prospective, single-arm, pre-post intervention trial, where participants were used as their own control, as previously done.13,38 We leveraged our robust and validated predictive analytic and mathematical modeling 39–41 to perform fine-scale analysis focused only on bacterial species favored by specific foods during an 8-week dietary intervention with the IBD-AID.
Results
Demographics of the participants of the study
We enrolled 25 subjects with CD or UC to complete an 8-week IBD-AID dietary intervention (Figure 1). A total of 22 participants completed the baseline period (age average = 40.5 ± 12.8. Table 1). Nineteen subjects continued to complete the intervention period (12 CD and 7 UC). The average body mass index (BMI) for participants in the study was 27.9 ± 5.8 (overweight and obese), which is comparable to the average BMI among Americans.42 Only 1 UC participant was underweight (BMI = 17.9). Except for 2 CD participants reporting no IBD-related medications, participants were using biologics (31.8%), aminoacylates (27.2%), steroids (22.7%), and immunomodulators (13.60%).
Figure 1.
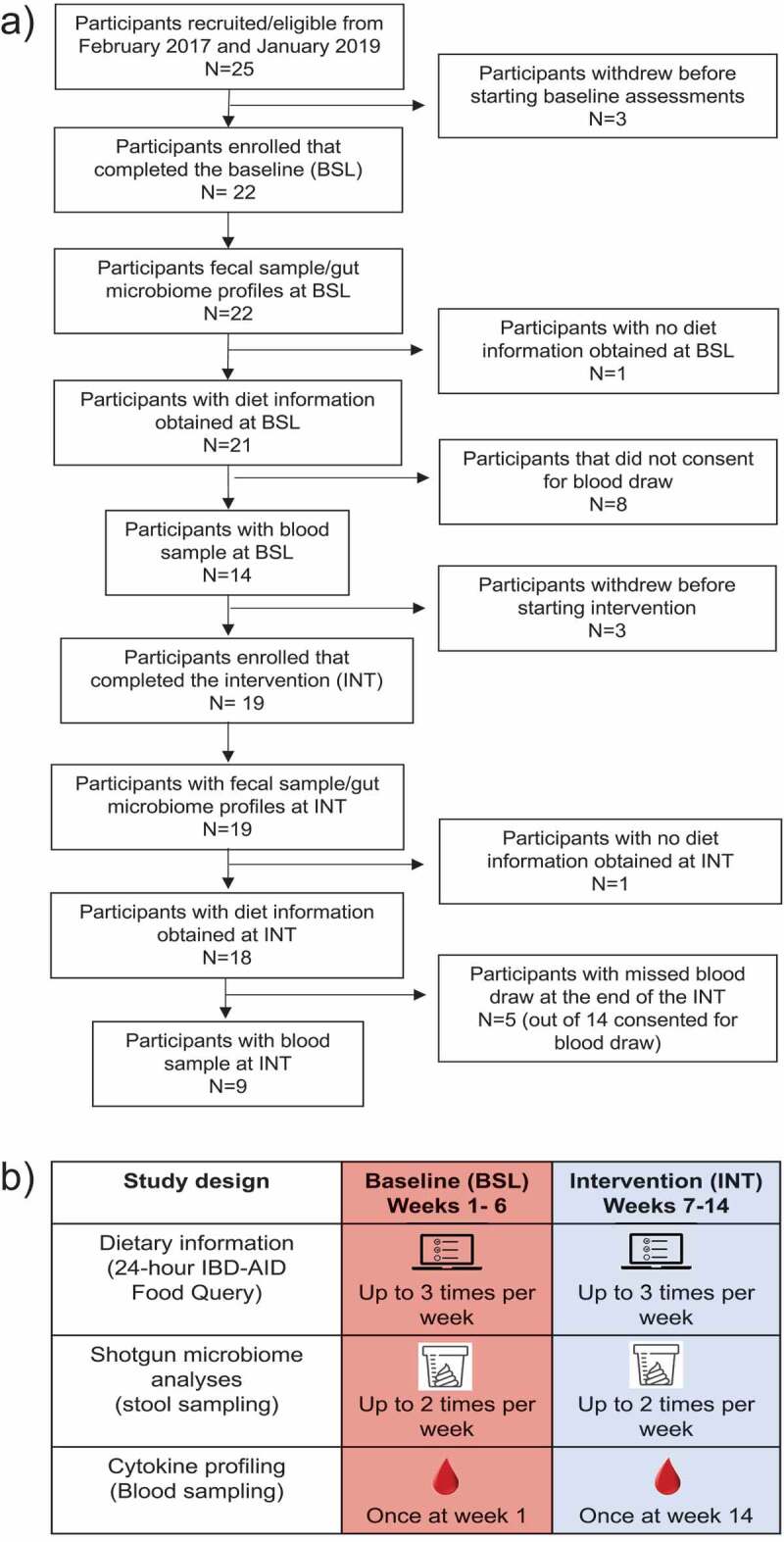
A) Participant inclusion and exclusion during the study duration. B) A schematic representation of the study design which involved bi-weekly stool samples collection and completion of 24-hour IBD-AID Food Querys up to three times a week throughout the study. At the beginning of the baseline and the end of the intervention, blood samples were collected.
Table 1.
Demographic description of all the participants recruited for the study between February 2017 and January 2019
| Patient information | Participants included in analyses (n = 22) |
Enrolled participants (n = 25) |
||
|---|---|---|---|---|
| Crohn’s disease (n = 15) | Ulcerative colitis (n = 7) | Crohn’s disease (n = 16) | Ulcerative colitis (n = 9) | |
| Demographics | ||||
| Average age (years) | 41.7 ± 13.3 | 37.8 ± 11.5 | 41.4 ± 12.9 | 39.6 ± 11.5 |
| Average weight (lbs) | 173.6 ± 31.4 | 190.1 ± 57.0 | 171.5 ± 31.3 | 190.1 ± 57.0 |
| Average BMI | 29.3 ± 5.4 | 25.4 ± 6.7 | 29.5 ± 5.2 | 25.4 ± 6.7 |
| Female sex (%) | 11 (73.3%) | 2 (28.5%) | 12 (75%) | 3 (33%) |
| White race (%) | 14 (93.3%) | 6 (85.7%) | 14 (87.5%) | 8 (88.8%) |
| IBD Medications | ||||
| Current use of Amisosalicylates | 3 (20% | 3 (42.8%) | 3 (18.7) | 4 (44.4%) |
| Current use of Biologics | 6 (40%) | 1 (14.2%) | 6 (37.5%) | 1 (11.1%) |
| Current use of Immnomodulators | 1 (6.6%) | 2 (28.5%) | 1 (6.2%) | 2 (2.22%) |
| Current use of Steroids | 2 (20%) | 2 (28.5%) | 4 (25%) | 3 (33%) |
| Other Medications | ||||
| Current use of Antihistamine | 4 (26.6%) | 0 | 5 (31.2%) | 0 |
| Current use of SSRI | 4 (26.6%) | 2 (28.5%) | 4 (25%) | 3 (33%) |
| Current use of Diuretic | 4 (26.6%) | 0 | 5 (31.2%) | |
| Current use of Vitamin D supplement | 4 (26.6%) | 2 (28.5%) | 4 (25%) | 4 (44.4%) |
Subjects profoundly changed their diet during the intervention
At baseline, we obtained 134 and 89 unique 24-hour IBD-AID Food Querys from 14 CD and 7 UC participants, respectively. We observed that all the participants reported similar diets at baseline (Mann–Whitney test, p-value >0.5. Supplementary Table S2), except for intakes of lean animal protein (included in beneficial foods on the IBD-AID), which was higher in UC patients. As expected, participants reported a low intake of fruits and vegetables comparable to an average American (<2 servings).43
At the intervention, we obtained 218 and 112 unique 24-hour IBD-AID Food Querys from 11 CD and 7 UC participants, respectively. We observed that overall, participants profoundly changed their diet reporting an average of 1.8-fold increase in prebiotics consumption, a 1.5-fold increase in probiotics consumption, a 1.6-fold increase in beneficial foods consumption, and a 3.7-fold reduction in adverse foods consumption (Figure 2. Table 2). More detailed analyses showed that participants significantly increased their intake of all foods contained in the prebiotic category, fermented dairy products within probiotic foods, and omega 3 fatty acids from the beneficial foods category. In contrast, participants significantly reduced consumption of most of the foods included in the adverse food category with exception of artificial sweeteners (Figure 2). We also observed that changes in food intake occurred within the first weeks of the intervention (Figure 2), suggesting rapid adaptation to the diet.
Figure 2.
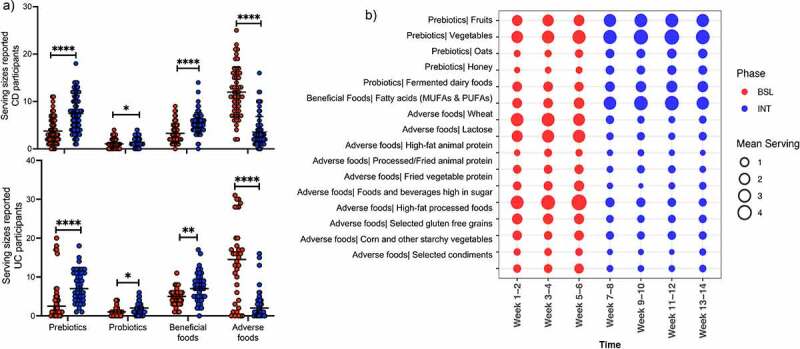
Participants adhere to the IBD-AID. A) Boxplot of the serving sizes reported for each food category consumed by CD and UC participants at baseline (BSL) and intervention (INT). B) Reported servings per week of foods with increased consumption during the intervention (Multiple T-test, p-value < 0.05). The mean servings per study period: BSL (in red) and INT (in blue), was calculated on the average intake per food category per week. Each circle represents the mean intake per food category grouped in 2 weeks intervals.
Table 2.
Mean servings reported on the 24-hour IBD-AID Food Query at baseline and intervention
| Food categories | Mean servings/d reported at BSL | Mean servings/d reported at INT | Difference between means (BSL – INT) ± SEM |
|---|---|---|---|
| Prebiotics | 4.08 | 7.51 | 3.44 ± 0.58 |
| Probiotics | 1.09 | 1.59 | 0.50 ± 0.17 |
| Beneficial foods | 3.88 | 6.13 | 2.26 ± 0.37 |
| Adverse foods | 12.64 | 3.42 | −9.23 ± 0.86 |
Separating by disease phenotype, we observed that CD and UC participants reported a similar increase in intake of foods encouraged during the intervention, except for oats and vegetable protein, which were only significantly increased during the intervention in UC or CD participants, respectively. Intakes of processed fried animal protein, corn, and starchy vegetables were only significantly decreased during the intervention on CD participants; and selected avoided condiments (i.e., wheat-based soy sauce, condiments high in fructose corn sugar, containing carrageenan, maltodextrin or/and emulsifiers 44–47) only decreased in UC participants (Supplementary Tables S3 and S4) during the intervention. CD participants reported no consumption of artificial sweeteners in all the study periods while UC participants did consume this food item in both baseline and intervention. This might explain the lack of differences described above.
Lastly, during the intervention alcohol consumption was reported higher for CD participants (Mann–Whitney test, p-value <0.01. Supplementary Table S3). However, there were no differences in alcohol consumption between study periods. In UC participants, there was a trend of decreasing alcohol consumption during the intervention (Mann–Whitney test, p-value = 0.1. Supplementary Table S4), which might explain the differences in alcohol intakes between the CD and UC participants.
In sum, we observed that overall participants can rapidly adopt the IBD-AID.
The IBD-AID favors SCFA-producing bacterial species
We collected a total of 340 stool samples: 143 at baseline and 197 during the intervention. The average number of stool samples per participant was 6.5 ± 2.1 at baseline (n = 22) and 10.3 ± 5.1 at intervention (n = 19). At baseline, we observe high microbiome inter-personal variability among participants with no differences by disease phenotype (CD vs UC) in alpha and beta diversity (Supplementary Figure S1) nor in microbiota representation (BH p-value >0.05, data not shown).
We then investigated the impact of the IBD-AID intervention on the gut microbiome. First, we did not find differences in alpha and beta diversity between samples collected at baseline vs. intervention (Supplementary Figure S2). However, compared to baseline, we found specific bacterial species have a reduced or increased abundance during the intervention window (BH-adjusted p-value <0.05). The top 10 bacteria with increased abundance in both CD and UC participants during intervention are SCFA-producing bacteria mostly belonging to the Clostridia class (Figure 3). Overall, the increased abundance of Roseburia hominis distinguished the highest likelihood of samples being collected during the intervention. Conversely, reduced abundance of members of the Bacteroidia, Coriobacteriia, Clostridia, and Negativicutes classes predicted the highest likelihood of samples being collected during the intervention (Figure 3).
Figure 3.
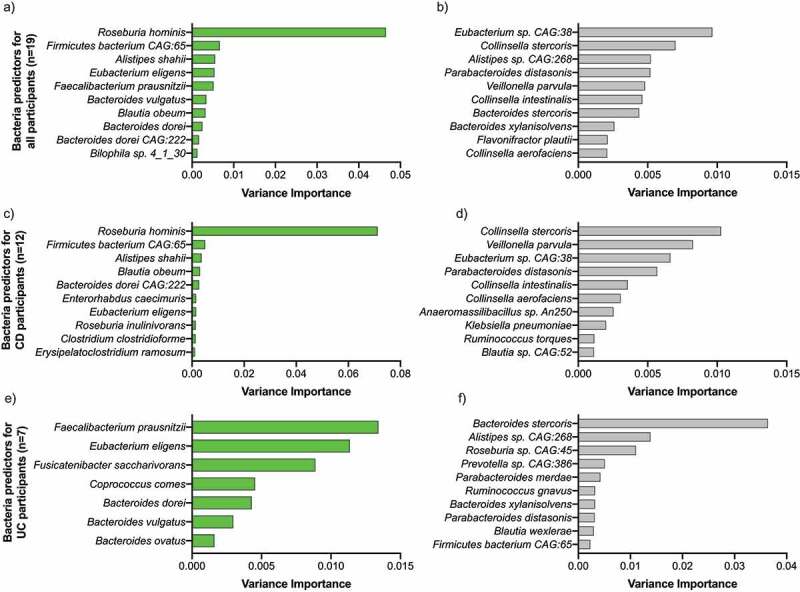
Mixed effect random forest classification analysis identified microbes affected by the intervention. Bar plots show the variance of the importance of bacterial species found to be enriched (in green) or depleted (in gray) during the intervention in all (A and B), CD (C and D), and UC (E and F) subjects completing the intervention (BH p-value >0.05).
We then investigated whether different species could be enriched by disease phenotype during the intervention (Supplementary Table S5). In patients with CD, the top bacteria with significantly increased abundance during the intervention were mostly species members of the Clostridia, Bacteroidia, and Coriobacteriia classes, and two Firmicutes species (Figure 3). Bacterial species significantly reduced during the intervention belonged not only to Gammaproteobacteria and Negativicutes classes, but also to Clostridia, Bacteroidia, and Coriobacteriia classes (Figure 3). Despite the overlap of bacterial classes as being positively or negatively affected during the IBD-AID intervention, there were specific species within those classes that seemed to be directionally altered by the intervention. These results suggest that specific foods affect the abundance of bacteria at the species level and are consistent with previous studies.33,48,49
In subjects with UC, similar results were observed. The abundance of specific Clostridia and Bacteroides species known to be depleted in UC patients (i.e., Eubacterium eligens, Faecalibacterium prausnitzii, Fusicatenibacter saccharivorans, Bacteroides dorei, Bacteroides ovatus, and Bacteroides vulgatus) were significantly increased during the intervention. Conversely, other Clostridia and Bacteroides were significantly decreased during the intervention (Figure 3).
Taken together, these findings show an overall shift of the microbiome during the intervention that differs by disease phenotype and is specie specific. The top bacteria favored by the IBD-AID intervention were two major butyrate producers Roseburia hominis and Faecalibacterium prautnizii 50 in CD and UC subjects, respectively (BH p-value >0.05). Two acetate producers species, Eubacterium eligens and Bacteroides dorei, 51 were enriched during intervention in both CD and UC (BH p-value >0.05); while Parabacteroides distasonis was consistently decreased in all participants regardless of disease phenotype during the intervention (BH p-value >0.05).
The IBD-AID favors a microbiome with anti-inflammatory capacity
We next evaluated the functional capacity of the microbiome during the intervention. At baseline, we found that the metagenomic capacity varied greatly by participant, with most samples clustering by participant (data not shown). However, we observe that during the intervention the microbiome exhibited an increased genetic capacity for 1) biosynthesis of several key amino acids (i.e., histidine, lysine, threonine, methionine, serine, glycine, isoleucine, and arginine); 2) degradation of mannan (a dietary fiber); and 3) β-oxidation for fatty acid degradation (Figure 4). Roseburia sp. and Faecalibacterium sp. – both favored during the IBD-AID intervention are main degraders of dietary mannan ultimately producing SCFA.52,53 Mannans are found in the endospermic tissue of nuts (homopolymeric mannan), barley, oats (β-glucans or mannoproteins), coffee beans, coconut palm, tomato, and legume seeds (galactomannan).54 Similarly, increased microbiome gene capacity for oxidation of fatty acids during the intervention also suggests increased availability of SCFAs. Thus, we further investigated the impact of IBD-AID on the pool of microbial genes involved in SCFA production during the intervention.
Figure 4.
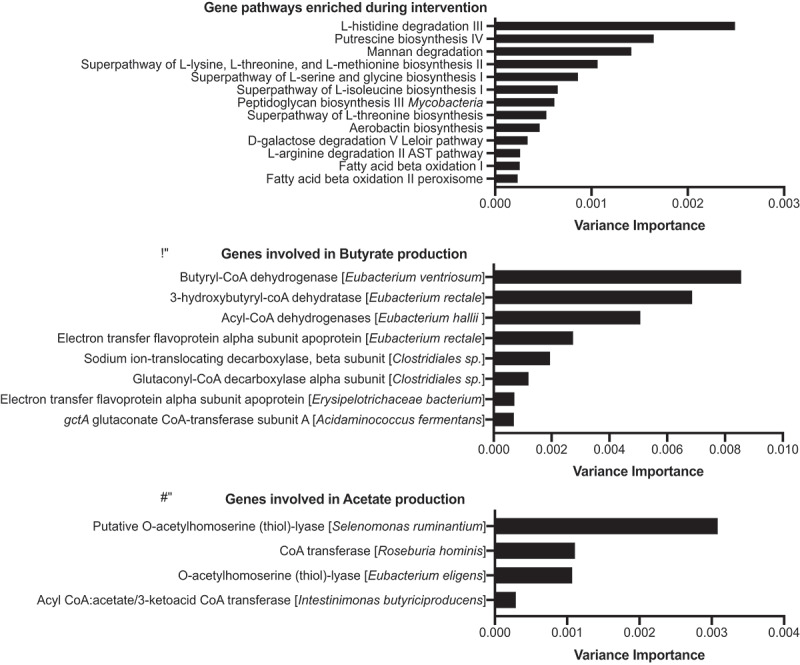
The IBD-AID increases the microbiome capacity for SCFA production. Bar plots represent the variance importance of the: A) gene pathways, B) genes involved in butyrate production, or C) genes involved in acetate production; that were enriched during the intervention (BH p-value >0.05).
As previously done by us,55 we created specific databases that included all the bacteria genes involved in the production of the main three SCFAs in the gut: butyrate, acetate, and propionate. We found that subjects completing the intervention displayed an increased abundance of specific genes involved in the production of butyrate, mostly from members of the Clostridia class (Figure 4); and acetate, specifically from Roseburia hominis and Eubacterium eligens species (Figure 4). Lastly, genes linked to propionate production were not enriched during the intervention. On the contrary, genes involved in propionate metabolism were augmented in baseline along with Ruminococcus torques, Flavonifractor plautii, and Parabacteroides distasonis (data not shown).
In sum, the diet-dependent changes of the microbiome were accompanied by increased microbial genomic capacity for butyrate and acetate metabolism during the intervention.
Foods responsible for the microbiome changes
We next sought to identify the foods reported on the 24-hour IBD-AID Food Querys that associate with specific bacteria abundances during the intervention. To do this, we first apply mixed effect random forest modeling to predict the abundance of each microbiome species as a function of the number of servings for each food category reported. To control for the effect of non-diet and other clinical covariates (i.e., age, gender, and BMI) we included them in the model as additional fixed effects. Similarly, to account for possible diagnosis-specific effects, we included in the model as additional fixed effects the interaction between every food category and the diagnosis. To determine the significance of the determined associations we run Permutated Importance (PIMP) analysis (see Methods). To determine the strength and direction of the association we then run Repeated Measure Correlations on the associations with a PIMP-associated p-value less than 0.05. We investigated the bacteria:food correlation of the top bacterial species enriched at either baseline or intervention in CD and UC participants. For these bacteria:food correlation analyses, we also included bacteria enriched in both CD and UC participants at intervention or baseline (i.e., B. dorei and P. distasonis, respectively. Figure 5). As expected, consumption of prebiotics, probiotics, and beneficial foods positively correlated with Clostridia and Bacteroides species both enriched during the intervention but negatively correlated with species enriched at baseline. Opposite correlations were observed with the consumption of adverse foods. A list with all the significant bacteria:food correlations are shown in Supplementary Table S6. Of interest, increased consumption of lean animal proteins (included in beneficial foods) during the intervention has a negative correlation with Roseburia hominis in UC but not in CD participants.
Figure 5.
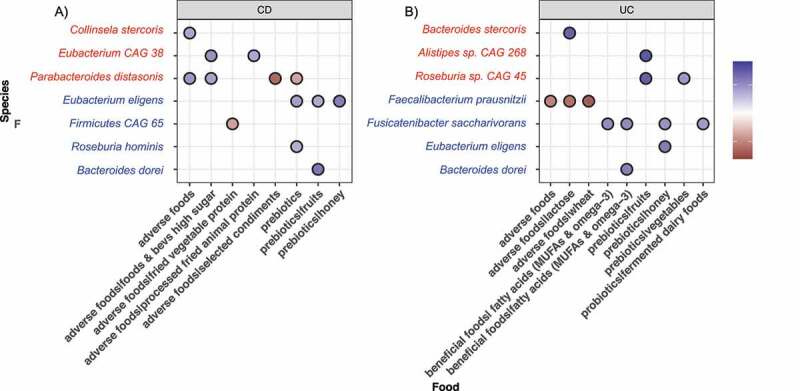
Significant correlations of foods with bacterial species enriched at baseline (red) or intervention (blue) in A) CD participants and B) UC participants.
Overall, our results show that increased consumption of prebiotics, probiotics, and beneficial foods during the intervention do favor Clostridia and Bacteroides species depleted in IBD patients. We observed that the effect of some foods on bacteria abundance is dependent on disease phenotype.
Immune modulation by food category
We obtained blood samples from nine patients before and after the intervention to measure circulating cytokines relevant to inflammation. Similar to previous report demonstrating correlation of specific foods with cytokine levels,38 we hypothesized that certain food categories encouraged (i.e., fruits) or discouraged (i.e., foods and beverages high in sugar) during the IBD-AID intervention could associate with levels of the serum cytokines regardless of study period. Thus, we determined the correlation of the reported food intakes during the study with the levels of cytokines (Supplementary Figure S3). Consistently, we observed that participants reporting high consumption of prebiotics and beneficial foods exhibit lower levels of inflammatory cytokines (i.e., IL-6 and IL-8) while higher levels of GM-CSF (Figure 6 A-E). Conversely, participants reporting high consumption of adverse foods exhibit higher levels of IL-8 and TNF-alpha (Figure 6F-J). In this subgroup of participants (n = 9), none of the 14 cytokines assessed changed from baseline to post-intervention (Supplementary Figure S3).
Figure 6.
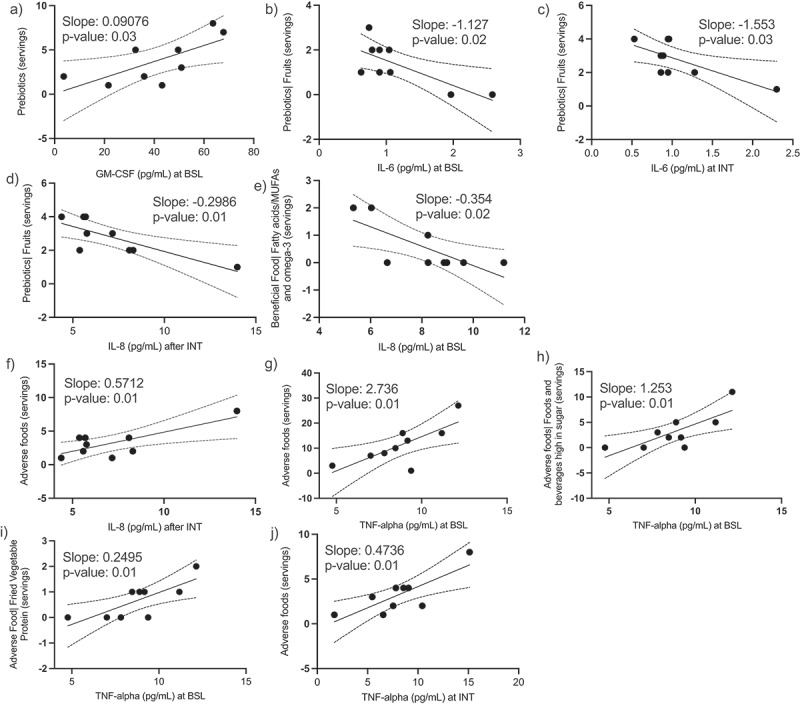
Levels of serum cytokines correlate with specific food categories. We obtained serum samples from 9 participants at baseline and at the end of the intervention. Consumption of prebiotics correlated with high levels of GM-CSF (A) and negatively correlated with IL-6 (B and C) and IL-8 (D). Consumption of fatty acids (MUFAs and omega-3) correlated with lower levels IL-8 (E). Consumption of adverse foods positively correlated with IL8 (F) and TNF-alpha (J-G). Simple linear regression, p-values <0.05. Dotted lines represent 95% confidence intervals.
In sum, we observed that higher consumption of foods encouraged on the IBD-AID (regardless of study period) do negatively correlate with pro-inflammatory cytokines and positively correlate with levels of colitis protective GM-CSF. Independent of study period, high consumption of foods discouraged on the IBD-AID consistently relates to higher levels of pro-inflammatory cytokines. We did not see an overall shift on circulatory cytokines after the IBD-AID intervention in the sub-group of participants included in this analysis.
Discussion
Here, we demonstrate that IBD patients can rapidly and dramatically change their diet and in doing so revert dysbiosis and modulate important cytokines driving IBD pathogenesis. Specifically, our results demonstrate that increased consumption of prebiotics (fiber-rich foods such as fruits, vegetables, oats, and honey), probiotics (fermented dairy products), and beneficial foods (lean animal protein and omega 3 fatty acids) can favor potent SCFA-producing Clostridia and Bacteroides species with known anti-inflammatory activity 9,11,39,50,56–64 and which are known to be reduced in numerous cohorts of IBD patients across the world.5,6,10,65–73
High-fiber diets are related to healthy-like microbiomes 74–76 and have received increasing attention to reducing IBD risk and symptoms.24,77–80 Here, increased consumption of fruits, vegetables, honey, and oats favored bacteria commonly depleted in IBD, namely: Roseburia hominis,F. praustnizii,E. eligens, F. saccharivorans, B. dorei, B. vulgatus. As with increased intakes of prebiotics vegetables and fruits, vegan and vegetarian diets have been also associated with increased microbiome capacity for biosynthesis of essential amino acids 81 that leads to the production of butyrate and acetate.82–87 Similarly, we observed that microbial gene pathways for biosynthesis of amino acids along with pathways involved in butyrate and acetate production were enriched along with participants’ increase in fruits and vegetable consumption.
Fermented foods have been shown to play an important role in microbiome diversity and concomitant immune tone on the host.38 In our cohort, there was a modest increase in the intake of fermented foods (average 0.5 servings per week), especially dairy products (i.e., yogurt, kefir). Despite the modest change in consumption, fermented dairy products in UC patients correlated with increased abundance of Fusicatenibacter saccharivorans, a bacteria known to be depleted on active UC patients.88
Within the beneficial foods, we found that increased intakes of MUFAs and omega-3 fatty acids also support potent SCFA-producing Clostridia and Bacteroides species. Omega-3 fatty acids have previously been found to reduce dysbiosis.89–94 Of interest, we observed that lean animal proteins, included in the beneficial foods for the IBD-AID, are negatively associated with Roseburia hominis in UC patients. In line with this observation, the International Organization for the Study of Inflammatory Bowel Diseases, recommends limiting animal protein intake for UC patients but not CD.95 Together, this highlights the importance of personalization of diet therapy based on the patient’s disease manifestation. Moreover, our results also emphasize the importance of a dietary approach for treating IBD that includes adding needed food components such as prebiotics, probiotics, and beneficial foods.
As expected, avoidance of foods also played an important role in shifting the microbiome during the intervention. We found that bacteria enriched at intervention (i.e., Faecalibacterium prausnitzii, Firmicutes CAG 65) negatively correlated with consumption of adverse foods. Conversely, bacteria species enriched at baseline (i.e, Collinsela stercoris, Parabacteroides distonis) were positively correlated with increased intakes of adverse foods. The abundance of Collinsella species has previously been associated with low-fiber diets 74,96 and processed foods.97 Moreover, Collinsella sp. isolated from IBD patients conferred significant susceptibility to colitis in germ-free mice.98 Thus, we speculate that the increase of prebiotics rich in fiber and reduction of adverse processed foods at intervention reduces Collinsella fitness;97 leading to an outgrowth of SCFA-producing bacteria as a result of “new” nutrient availability.99–101 Parabacteroides distonis, enriched at baseline in both CD and UC participants in this cohort, have been also found abundant in IBD patients 102 and it has been implicated in worsening DSS-induced colitis in mice.103
Comparable to a previous report,38 we also provide evidence of food-immune correlations. Namely, we observed that foods encouraged by the IBD-AID (i.e., prebiotics and beneficial foods) negative correlate with IL-6 and IL-8. Both cytokines have a major role in pro-inflammatory signaling, are elevated in patients with either CD or UC, and are correlated with mucosal inflammation.104–110 Recently, reduction of IL-6 was associated with clinical response to biological therapy.111 Prebiotics intakes was positively correlated with GM-CSF, a cytokine involved in myeloid cell development and maturation and dendritic cell differentiation. There is growing evidence that lower levels of GM-CSF are associated with the pathogenesis of CD.112–118 On the other hand, foods discouraged by the IBD-AID were positively correlated with IL-8 and TNF-alpha. TNF-alpha is a pro-inflammatory mediator that plays an integral role in the pathogenesis of IBD. Several available biological therapies for IBD are centered in blocking TNF-alpha. Altogether, this suggests that diet can be used as adjunctive therapy to treat IBD.
A limitation of this study is the fact that we purposely enrolled patients with varying disease severity in a small cohort, therefore we do not intend to test the efficacy of the diet in symptomatic or clinical remission, nor we expected to see a large clinical effect. Other limitations include: the lack of a direct measure of butyrate and acetate production, which we speculate is increased due to our metagenomic results (i.e., increase of SCFA-producing bacteria and enrichment of microbial SCFA genes); and our online food journal: the ‘24-hour IBD-AID Food Query’, used for recording of food consumption, have not been rigorously validated. Overall, none of these limitations pertain to the primary outcome of the study which was to evaluate changes in the microbiome composition after IBD-AID intervention.
In conclusion, we demonstrate that the IBD-AID can favor bacteria commonly depleted in IBD patients which are key for maintaining immune tolerance and homeostasis in the gut via SCFA production. We demonstrated that foods encouraged/discouraged by the IBD-AID are linked to modulation of the immune tone. Moreover, the results provide evidence for further adjustments of the foods allowed on the IBD-AID according to the disease phenotype and immune target.
Methods
Power calculation
The primary outcome is to evaluate the effect of the IBD-AID in the increased abundance of SCFA-producing bacteria. Using Monte-Carlo simulations of empirical power and type-I-error for a Wilcoxon-signed rank test (paired; R package MKpower 119) we determined that 10 independent subjects (pre-post) will detect 0.005 ± 0.005 changes in the relative abundance of bacteria, with a power of 0.80. Our secondary outcome was associations between IBD-AID food categories and the microbiome. An unweighted Spearman correlation power analysis (R package genefu 120) determined that 14 independent samples will be sufficient to achieve a significance of 0.05 and a correlation coefficient of 0.1.
Participants
We recruited 25 subjects with an IBD diagnosis of either Crohn’s disease (CD) or ulcerative colitis (UC, Figure 1). Of the 19 subjects who completed the study, 7 CD and 2 UC subjects were in remission at enrollment. The remaining 10 subjects exhibited either mild, moderate, or active disease. Exclusion criteria included: use of the antibiotic within 3 months at the time of recruitment, presence of infection precipitating colitis (i.e., C. difficile), and pregnancy. For more inclusion and exclusion criteria see Supplementary Table S1. None of the participants reported antibiotic treatment during the study. The study was approved by the IRB at UMASS (Docket Number H00008033). ClinicalTrial.gov registry website: https://clinicaltrials.gov/ct2/show/NCT04757181
Trial number: NCT04757181.
The IBD-AID
As published elsewhere,25 the IBD-AID supports the avoidance of certain carbohydrates (sucrose and starches) from the original SCD.121 Before the mSCD, the IBD-AID was the first IBD diet to include oats as a source of fiber. The IBD-AID encourage the increased intakes of monounsaturated and polyunsaturated omega-3 and fatty acids while decreasing other saturated fats and eliminating trans-fatty acids.77,89,122–128 Moreover, the IBD-AID eliminates the consumption of processed and ultra-processed foods which have been associated with IBD risk.129 The IBD-AID includes prebiotics: foods rich in non-digestible fiber that serve as food for beneficial bacteria colonizing the colon.130,131 Epidemiological evidence 132–135 and results from a recent clinical trial study 38 support the role of fermented foods containing live active bacteria (probiotics) in health, microbiome diversity, and an anti-inflammatory immune status.38 Thus, the IBD-AID also encourages the consumption of probiotics. Finally, to avoid nutrient deficiencies that could be caused by restrictive diets, the IBD-AID also encourages the intake of nutritious foods recommended by the Dietary Guidelines for Americans;136 which includes a variety of foods rich in essential vitamins and minerals. The diet can be prepared at home and is designed to be healthful long term for the entire family.
Intervention
Due to the high interpersonal variability of the microbiome,35 the individual-specific response to diet,36 and the fact that consuming an ‘inert’ placebo diet is not an option for a control group,37 we conducted a prospective, single-arm, pre-post intervention trial, where participants were used as their own control, as previously done.13,38 After a baseline period of 6 weeks, the dietary intervention was initiated and continued for 8 weeks (Figure 1). To receive dietary instructions, subjects met in person with trained registered nutritionists at the beginning of the intervention and completed at least one counseling session per week throughout the 8-weeks intervention period.
Dietary assessment
Dietary intake is difficult to measure, and any single method cannot assess dietary exposure perfectly. We developed the 24-hour IBD-AID Food Query to address the main challenges of recording dietary intake by: collecting actual intake on specific days; reducing the burden of memory to only recall foods consumed in the past 24 hr, and we obtained repeated recordings per week to better estimate usual intake. The data obtained from this instrument was used to achieve our secondary outcome to determine associations between IBD-AID food categories and the microbiome. The 24-hour IBD-AID Food Query was programmed in REDCap and consists of 240 food items grouped in four main food categories: 1) prebiotic foods, including fruits, vegetables, legumes, oats, and honey; 2) probiotic foods: fermented dairy products, and fermented nondairy foods; 3) beneficial foods: fatty acids rich in monounsaturated and omega-3 polyunsaturated fatty acids, vegetable and lean animal proteins; and 4) adverse foods: wheat, corn, lactose, high fat animal and vegetable proteins, processed fried foods, artificial sweeteners, foods, and beverages high in sugar, high-fat processed foods, selected starchy vegetables, selected gluten-free grains, and certain condiments (i.e., wheat-based soy sauce, condiments high in fructose corn sugar, containing carrageenan, maltodextrin or/and emulsifiers, known to trigger gastrointestinal symptoms).[44–47] Alcohol consumption was accounted for in a separate category. A link to the electronic 24-hour IBD-AID Food Query was sent to the participants to be filled out 3 times per week (see Supplementary material for details on the 24-hour IBD-AID Food Query). The serving sizes recorded on each 24-hour IBD-AID Food Query were assigned to the individual food categories mentioned above and the serving sizes reported were averaged per week for analysis.
Sample collection
Subjects were provided materials and instructions for at-home self-collection using OMNIgene•GUT collection kits (#OM-200, DNA Genotek Inc., Ottawa, Canada). We also obtained blood samples at baseline and the end of the intervention. Once in the laboratory, samples were aliquoted and then stored at −80°C until processed.
DNA isolation and sequencing
DNA isolation was performed using the MagAttract PowerSoil DNA Kit (#27,100-4-EP, Qiagen, Germantown, MD, USA) on Eppendorf epMotion 5075 liquid handlers following the manufacturer’s instructions. Libraries for DNA sequencing were prepared using the Nextera XT DNA Library Preparation Kit (#FC-131-1096, Illumina, San Diego, CA, USA) and were sequenced on the Illumina NextSeq 500 platform using 150-nt paired-end reads. We obtained an average of 4,926,661 reads per sample. Read data were quality trimmed and filtered of host DNA using KneadData (version 0.7.2; https://bitbucket.org/biobakery/kneaddata/wiki/Home) against a prebuilt bowtie2 index for the human genome, hg19. All the filtered sequences generated were deposited in NCBI, BioProject: PRJNA642308.
Metagenomic profiling
We performed shotgun metagenomic sequencing of stool samples as previously described by us and others.55,137–139 Community composition was profiled using MetaPhlan2 (version 2.9.14; database mpa_v292_CHOCOPhlAn_201901).140 To assess the abundance of microbiota-encoded metabolic pathways we used HUMAnN2 (version 2.8).141 We used ShortBRED142 to profile metagenomics reads for the abundance of proteins involved in the production of SCFAs (e.g., butyrate, acetate, propionate) as we have previously described.55,143
Inflammatory markers
We used the Discovery Assay® Human High Sensitivity T-Cell Discovery Array 14-Plex (#HDHSTC14, Eve Technologies Corp, Calgary, Canada) to simultaneously quantified 14 cytokine/chemokine/growth involved in inflammation.
Mathematical modeling:
Microbiome associations with study periods: To determine the bacterial species impacted by the IBD-AID we applied mixed-effect random forest classification by adapting the MERF R engine.144 This framework enables to account for the repeated sampling nature of the dataset and is appropriately suited for this type of “large p, small n” multi-omics dataset common in clinical research.145 We classify a sample i from patient s as Intervention vs. Baseline (Yis = 1,0) as a function of microbiome abundance in that sample as a fixed effect (Xis) and controlling for the individual patient as a random effect (Z): . Compared to traditional linear mixed-effect modeling regression here f is a general function that is learned using a random forest model. The expectation-maximization algorithm runs via alternative optimization, in which, at the turn, one parameter is fitted while the other ones are fixed with the process running until convergence.144 This analysis was repeated using as predictors species abundances, metabolic pathways abundances, and SCFAs pathways, independently. Permutated importance (PIMP) analysis was used to estimate the significance of each microbiome feature in the classification analyses.137,146
Microbiome associations with food categories: We determined the effect of food categories on the microbiome by first applying mixed-effect random forest regression modeling while also controlling for other clinical and not-diet related covariates (i.e., age, gender, and BMI).144 To account for diagnosis (UC, CD)-dependent effects of food categories on the microbiome, we also consider the interactions between food-category (as number of servings, numerical) and the diagnosis (categorical) in the modeling. As above, PIMP analysis was used to estimate the significance of each model covariate in predicting the abundance of every modeled microbial feature 145,136. For the food covariates displaying a PIMP-associated p-value <0.05, we run repeated measure correlation for UC and CD individuals independently to determine the direction and significance of the identified association.
Statistical analysis
We used Prism 9 to perform the statistical analyses. We used the Mann–Whitney test with individual ranks computed per comparison of food intakes by study phase using the two-stage linear step-up procedure of Benjamini, Krieger, and Yekutieli correction. Wilcoxon matched-pairs signed-rank was used to evaluate differences in cytokine concentration in serum before and after the diet intervention; due to the low sample size (n = 9), p values of 0.1 are reported as trends. Simple linear regressions were calculated between the average of intakes of each food category and the levels of cytokines at each study period. We used the R package Phyloseq v1.19.1 147 to calculate the Shannon diversity index 148,149 and Bray-Curtis dissimilarity. Statistical significance of Bray-Curtis distances was assessed using PERMANOVA in R.150
Supplementary Material
Acknowledgments
We are grateful for the effort and willingness all the study participants demonstrated throughout the study. We thank Kanishka Bhattacharya for patient referral to the study. We appreciate Yurima Guillarte-Walker’s help in identifying patients for the study. The study was funded by the American Gastroenterological Association and partially by the Leona M. and Harry B. Helmsley Charitable Trust.
Funding Statement
This work was supported by the American Gastroenterological Association; Leona M. and Harry B. Helmsley Charitable Trust.
Abbreviations
Inflammatory bowel disease (IBD); Crohn’s disease (CD); Ulcerative colitis (UC); Inflammatory bowel disease Anti-Inflammatory Diet (IBD-AID); Short-chain fatty acids (SCFAs); Mediterranean diet (MD); Specific Carbohydrate Diet (SCD).
Disclosure statement
Beth A. McCormick (BAM) is a coinventor on a patent application (PGT/US 18/42116). She, along with her academic institution, stands to gain financially through potential commercialization outcomes resulting from activities associated with the licensing of that intellectual property. The remaining authors do not have any conflict of interest to disclose.
Authors contributions
BO, EO, DW, RP, BMC, and AMC – study conceptualization and methodology. BO, CC, RM, MMM, CM, DC, RP – investigation and data curation. BO, VB, EO, DW, DC, CF, SB, and AMC – formal analysis of data. BO, VB, DW, BMC, and AMC – writing – original draft, review & editing. AMC – funding acquisition. BO and AMC –supervision.
Data availability statement
The data that support the findings of this study are openly available in NCBI, BioProject: PRJNA642308 (https://www.ncbi.nlm.nih.gov/bioproject/?term=PRJNA642308)
Supplementary Material
Supplemental data for this article can be accessed on the publisher’s website.
References
- 1.Lee JY, Cevallos SA, Byndloss MX, Tiffany CR, Olsan EE, Butler BP, Young BM, Rogers AWL, Nguyen H, Kim K, et al. High-fat diet and antibiotics cooperatively impair mitochondrial bioenergetics to trigger dysbiosis that exacerbates pre-inflammatory bowel disease. Cell Host & Microbe. 2020;28:273–284.e6. doi: 10.1016/j.chom.2020.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rigottier-Gois L. Dysbiosis in inflammatory bowel diseases: the oxygen hypothesis. The ISME Journal. 2013;7:1256–21. doi: 10.1038/ismej.2013.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Michail S, Durbin M, Turner D, et al. Alterations in the gut microbiome of children with severe ulcerative colitis. Inflammatory Bowel Diseases. 2012;18:1799–1808. doi: 10.1002/ibd.22860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Frank DN, Robertson CE, Hamm CM, Kpadeh Z, Zhang T, Chen H, Zhu W, Sartor RB, Boedeker EC, Harpaz N, et al. Disease phenotype and genotype are associated with shifts in intestinal-associated microbiota in inflammatory bowel diseases. Inflammatory Bowel Diseases. 2011;17(1):179–184. doi: 10.1002/ibd.21339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gevers D, Kugathasan S, Denson LA, Vazquez-Baeza Y, Van Treuren W, Ren B, Schwager E, Knights D, Song S, Yassour M, et al. The treatment-naive microbiome in new-onset Crohn’s disease. Cell Host & Microbe. 2014;15(3):382–392. doi: 10.1016/j.chom.2014.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lloyd-Price J, Arze C, Ananthakrishnan AN, Schirmer M, Avila-Pacheco J, Poon TW, Andrews E, Ajami NJ, Bonham KS, Brislawn CJ, et al. Multi-omics of the gut microbial ecosystem in inflammatory bowel diseases. Nature. 2019;569(7758):655–662. doi: 10.1038/s41586-019-1237-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lopetuso LR, Scaldaferri F, Petito V, Gasbarrini A.. Commensal Clostridia: leading players in the maintenance of gut homeostasis. Gut Pathogens. 2013;5(1):23. doi: 10.1186/1757-4749-5-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rivera-Chavez F, Zhang LF, Faber F, Lopez CA, Byndloss MX, Olsan EE, Xu G, Velazquez E, Lebrilla C, Winter S, et al. Depletion of butyrate-producing clostridia from the gut microbiota drives an aerobic luminal expansion of salmonella. Cell Host & Microbe. 2016;19(4):443–454. doi: 10.1016/j.chom.2016.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Atarashi K, Tanoue T, Oshima K, Suda W, Nagano Y, Nishikawa H, Fukuda S, Saito T, Narushima S, Hase K, et al. Treg induction by a rationally selected mixture of Clostridia strains from the human microbiota. Nature. 2013;500(7461):232–236. doi: 10.1038/nature12331. [DOI] [PubMed] [Google Scholar]
- 10.Kumari R, Ahuja V, Paul J. Fluctuations in butyrate-producing bacteria in ulcerative colitis patients of North India. World Journal of Gastroenterology: WJG. 2013;19:3404–3414. doi: 10.3748/wjg.v19.i22.3404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Parada Venegas D, De la Fuente MK, Landskron G, Gonzalez MJ, Quera R, Dijkstra G, Harmsen HJM, Faber KN, Hermoso MA. Short Chain Fatty Acids (SCFAs)-mediated gut epithelial and immune regulation and its relevance for inflammatory bowel diseases. Front Immunol. 2019;10:277. doi: 10.3389/fimmu.2019.00277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hasler CM. Functional foods: benefits, concerns and challenges-a position paper from the American council on science and health. The Journal of Nutrition. 2002;132:3772–3781. doi: 10.1093/jn/132.12.3772. [DOI] [PubMed] [Google Scholar]
- 13.David LA, Maurice CF, Carmody RN, Gootenberg DB, Button JE, Wolfe BE. Diet rapidly and reproducibly alters the human gut microbiome. Nature. 2014;505:559–563. doi: 10.1038/nature12820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Suskind DL, Lee D, Kim YM, et al. The specific carbohydrate diet and diet modification as induction therapy for pediatric crohn’s disease: a randomized diet controlled trial. Nutrients. 2020;12(12). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sigall Boneh R, Van Limbergen J, Wine E, et al. Dietary therapies induce rapid response and remission in pediatric patients with active Crohn’s disease. Clinical Gastroenterology and Hepatology: The Official Clinical Practice Journal of the American Gastroenterological Association. 2021;19:752–759. doi: 10.1016/j.cgh.2020.04.006. [DOI] [PubMed] [Google Scholar]
- 16.Levine A, Wine E, Assa A, et al. Crohn’s Disease exclusion diet plus partial enteral nutrition induces sustained remission in a randomized controlled trial. Gastroenterology. 2019;157:440–50 e8. doi: 10.1053/j.gastro.2019.04.021. [DOI] [PubMed] [Google Scholar]
- 17.Miller T, Suskind DL. Exclusive enteral nutrition in pediatric inflammatory bowel disease. Curr Opin Pediatr. 2018;30:671–676. doi: 10.1097/MOP.0000000000000660. [DOI] [PubMed] [Google Scholar]
- 18.Borrelli O, Cordischi L, Cirulli M, et al. Polymeric diet alone versus corticosteroids in the treatment of active pediatric Crohn’s disease: a randomized controlled open-label trial. Clinical Gastroenterology and Hepatology: The Official Clinical Practice Journal of the American Gastroenterological Association. 2006;4:744–753. doi: 10.1016/j.cgh.2006.03.010. [DOI] [PubMed] [Google Scholar]
- 19.Berni Canani R, Terrin G, Borrelli O, et al. Short- and long-term therapeutic efficacy of nutritional therapy and corticosteroids in paediatric Crohn’s disease. Dig Liver Dis. 2006;38:381–387. doi: 10.1016/j.dld.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 20.Swaminath A, Feathers A, Ananthakrishnan AN, Falzon L, Li Ferry S. Systematic review with meta-analysis: enteral nutrition therapy for the induction of remission in paediatric Crohn’s disease. Alimentary Pharmacology & Therapeutics. 2017;46:645–656. doi: 10.1111/apt.14253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Grover Z, Burgess C, Muir R, Reilly C, Lewindon PJ. Early mucosal healing with exclusive enteral nutrition is associated with improved outcomes in newly diagnosed children with luminal Crohn’s disease. J Crohns Colitis. 2016;10:1159–1164. doi: 10.1093/ecco-jcc/jjw075. [DOI] [PubMed] [Google Scholar]
- 22.Yu Y, Chen KC, Chen J. Exclusive enteral nutrition versus corticosteroids for treatment of pediatric Crohn’s disease: a meta-analysis. World J Pediatr. 2019;15:26–36. doi: 10.1007/s12519-018-0204-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lewis JD, Sandler R, Brotherton C, et al. A Randomized trial comparing the specific carbohydrate diet to a Mediterranean diet in adults with Crohn’s disease. Gastroenterology. 2021. doi: 10.1053/j.gastro.2021.05.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fritsch J, Garces L, Quintero MA, et al. Low-fat, high-fiber diet reduces markers of inflammation and dysbiosis and improves quality of life in patients with ulcerative colitis. Clinical Gastroenterology and Hepatology: The Official Clinical Practice Journal of the American Gastroenterological Association. 2020;19(6): 1189–1199. doi: 10.1016/j.cgh.2020.05.026 . [DOI] [PubMed] [Google Scholar]
- 25.Peter I, Maldonado-Contreras A, Eisele C, et al. A dietary intervention to improve the microbiome composition of pregnant women with Crohn’s disease and their offspring: the MELODY (Modulating early life microbiome through dietary intervention in pregnancy) trial design. Contemp Clin Trials Commun. 2020;18:100573. doi: 10.1016/j.conctc.2020.100573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Olendzki BC, Silverstein TD, Persuitte GM, Ma Y, Baldwin KR, Cave D. An anti-inflammatory diet as treatment for inflammatory bowel disease: a case series report. Nutrition Journal. 2014;13:5. doi: 10.1186/1475-2891-13-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chiuve S E, Fung T T, Rimm E B, Hu F B, McCullough M L, Wang M, Stampfer M J and Willett W C. (2012). Alternative Dietary Indices Both Strongly Predict Risk of Chronic Disease. The Journal of Nutrition, 142(6), 1009–1018. 10.3945/jn.111.157222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pickert G, Wirtz S, Matzner J, et al. Wheat consumption aggravates colitis in mice via amylase trypsin inhibitor-mediated dysbiosis. Gastroenterology. 2020;159:257–72 e17. doi: 10.1053/j.gastro.2020.03.064. [DOI] [PubMed] [Google Scholar]
- 29.Zevallos VF, Raker V, Tenzer S, Jimenez-Calvente C, Ashfaq-Khan M, Russel N, et al. Nutritional wheat amylase-trypsin inhibitors promote intestinal inflammation via activation of myeloid cells. Gastroenterology. 2017;152:1100–13 e12. doi: 10.1053/j.gastro.2016.12.006. [DOI] [PubMed] [Google Scholar]
- 30.Caminero A, McCarville JL, Zevallos VF, Pigrau M, Yu XB, Jury J, et al. Lactobacilli degrade wheat amylase trypsin inhibitors to reduce intestinal dysfunction induced by immunogenic wheat proteins. Gastroenterology. 2019;156 8 :2266–2280. doi: 10.1053/j.gastro.2019.02.028. [DOI] [PubMed] [Google Scholar]
- 31.Green N, Miller T, Suskind D, Lee D. A review of dietary therapy for IBD and a vision for the future. Nutrients. 2019;11(5): 947. doi: 10.3390/nu11050947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.de Castro MM, Corona LP, Pascoal LB, Miyamoto JE, Ignacio-Souza LM, de Lourdes Setsuko Ayrizono M, et al. Dietary patterns associated to clinical aspects in Crohn’s disease patients. Sci Rep. 2020;10:7033. doi: 10.1038/s41598-020-64024-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Deehan EC, Yang C, Perez-Munoz ME, Nguyen NK, Cheng CC, Triador L, et al. Precision microbiome modulation with discrete dietary fiber structures directs short-chain fatty acid production. Cell Host & Microbe. 2020;27:389–404 e6. doi: 10.1016/j.chom.2020.01.006. [DOI] [PubMed] [Google Scholar]
- 34.Ang QY, Alexander M, Newman JC, Tian Y, Cai J, Upadhyay V, et al. Ketogenic diets alter the gut microbiome resulting in decreased intestinal Th17. Cell. 2020;181:1263–75 e16. doi: 10.1016/j.cell.2020.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ursell LK, Clemente JC, Rideout JR, Gevers D, Caporaso JG, Knight R. The interpersonal and intrapersonal diversity of human-associated microbiota in key body sites. J Allergy Clin Immunol. 2012;129(5):1204–1208. doi: 10.1016/j.jaci.2012.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Berry SE, Valdes AM, Drew DA, Asnicar F, Mazidi M, Wolf J, Capdevila J, Hadjigeorgiou G, Davies R, Al Khatib H, et al. Human postprandial responses to food and potential for precision nutrition. Nature Medicine. 2020;26(6):964–973. doi: 10.1038/s41591-020-0934-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Moayyedi P, Simren M, Bercik P. Evidence-based and mechanistic insights into exclusion diets for IBS. Nat Rev Gastroenterol Hepatol. 2020;17(7):406–413. doi: 10.1038/s41575-020-0270-3. [DOI] [PubMed] [Google Scholar]
- 38.Wastyk HC, Fragiadakis GK, Perelman D, Dahan D, Merrill BD, Yu FB, Topf M, Gonzalez CG, Van Treuren W, Han S, et al. Gut-microbiota-targeted diets modulate human immune status. Cell. 2021;184(16):4137–4153.e14. doi: 10.1016/j.cell.2021.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Stein RR, Tanoue T, Szabady RL, Bhattarai SK, Olle B, Norman JM, Suda W, Oshima K, Hattori M, Gerber GK, et al. Computer-guided design of optimal microbial consortia for immune system modulation. Elife. 2018;7. doi: 10.7554/eLife.30916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bucci V, Tzen B, Li N, Simmons M, Tanoue T, Bogart E, Deng L, Yeliseyev V, Delaney ML, Liu Q, et al. MDSINE: microbial dynamical systems inference engine for microbiome time-series analyses. Genome Biology. 2016;17(1):121. doi: 10.1186/s13059-016-0980-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bucci V, Xavier JB. Towards predictive models of the human gut microbiome. J Mol Biol. 2014;426(23):3907–3916. doi: 10.1016/j.jmb.2014.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fryar CD, Kruszon-Moran D, Gu Q, Ogden CL. Mean Body weight, height, waist circumference, and body mass index among adults: United States, 1999-2000 Through 2015-2016. Natl Health Stat Report. 2018;122: 1–16. [PubMed] [Google Scholar]
- 43.Stewart Hayden, Hyman, Jeffery . . In: Amber Waves: The Economics of Food, Farming, Natural Resources, and Rural America . Amber Waves; Americans Still Can Meet Fruit and Vegetable Dietary Guidelines for $2.10-$2.60 per Day. 2019. https://www.ers.usda.gov/amber-waves/2019/june/americans-still-can-meet-fruit-and-vegetable-dietary-guidelines-for-210-260-per-day [Google Scholar]
- 44.Martino JV, Van Limbergen J, Cahill LE. The role of carrageenan and carboxymethylcellulose in the development of intestinal inflammation. Front Pediatr. 2017;5:96. doi: 10.3389/fped.2017.00096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bancil AS, Sandall AM, Rossi M, Chassaing B, Lindsay JO, Whelan K. Food additive emulsifiers and their impact on gut microbiome, permeability, and inflammation: mechanistic insights in inflammatory bowel disease. J Crohns Colitis. 2021;15(6):1068–1079. doi: 10.1093/ecco-jcc/jjaa254. [DOI] [PubMed] [Google Scholar]
- 46.Laudisi F, Di Fusco D, Dinallo V, Stolfi C, Di Grazia A, Marafini I, Colantoni A, Ortenzi A, Alteri C, Guerrieri F, et al. The food additive maltodextrin promotes endoplasmic reticulum stress–driven mucus depletion and exacerbates intestinal inflammation. Cell Mol Gastroenterol Hepatol. 2019;7(2):457–473. doi: 10.1016/j.jcmgh.2018.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Montrose DC, Nishiguchi R, Basu S, Staab HA, Zhou XK, Wang H, Meng L, Johncilla M, Cubillos-Ruiz JR, Morales DK, et al. Dietary fructose alters the composition, localization, and metabolism of gut microbiota in association with worsening colitis. Cell Mol Gastroenterol Hepatol. 2021;11(2):525–550. doi: 10.1016/j.jcmgh.2020.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chung WS, Walker AW, Louis P, Parkhill J, Vermeiren J, Bosscher D, Duncan SH, Flint HJ. Modulation of the human gut microbiota by dietary fibres occurs at the species level. BMC Biol. 2016;14(1):3. doi: 10.1186/s12915-015-0224-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhao L, Zhang F, Ding X, Wu G, Lam YY, Wang X, Fu H, Xue X, Lu C, Ma J. Gut bacteria selectively promoted by dietary fibers alleviate type 2 diabetes. Science. 2018;359(6380):1151–1156. doi: 10.1126/science.aao5774. [DOI] [PubMed] [Google Scholar]
- 50.Machiels K, Joossens M, Sabino J, De Preter V, Arijs I, Eeckhaut V, Ballet V, Claes K, Van Immerseel F, Verbeke K, et al. A decrease of the butyrate-producing species Roseburia hominis and Faecalibacterium prausnitzii defines dysbiosis in patients with ulcerative colitis. Gut. 2014;63(8):1275–1283. doi: 10.1136/gutjnl-2013-304833. [DOI] [PubMed] [Google Scholar]
- 51.Gao G, Cao J, Mi L, Feng D, Deng Q, Sun X, Zhang H, Wang Q, Wang J. BdPUL12 depolymerizes β-mannan-like glycans into mannooligosaccharides and mannose, which serve as carbon sources for Bacteroides dorei and gut probiotics. Int J Biol Macromol. 2021;187:664–674. doi: 10.1016/j.ijbiomac.2021.07.172. [DOI] [PubMed] [Google Scholar]
- 52.La Rosa SL, Leth ML, Michalak L, Hansen ME, Pudlo NA, Glowacki R, Pereira G, Workman CT, Arntzen MØ, Pope PB. The human gut Firmicute Roseburia intestinalis is a primary degrader of dietary β-mannans. Nature Communications. 2019;10(1):905. doi: 10.1038/s41467-019-08812-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Leszek Michalak JCG, Lagos L, Leanti La Rosa S, Terrapon N, Lombard V, Henrissat B, Arntzen MØ, Heldal Hagen L, Dröge J, Øverland M, et al. Engineered fibre enables targeted activation of butyrate-producing microbiota in the distal gut. 2019. https://www.biorxiv.org/content/10.1101/799023v1.full.pdf doi: 10.1101/799023 [DOI]
- 54.Moreira LR, Filho EX. An overview of mannan structure and mannan-degrading enzyme systems. Appl Microbiol Biotechnol. 2008;79(2):165–178. doi: 10.1007/s00253-008-1423-4. [DOI] [PubMed] [Google Scholar]
- 55.Haran JP, Bhattarai SK, Foley SE, et al. Alzheimer’s Disease microbiome is associated with dysregulation of the anti-inflammatory P-Glycoprotein pathway. mBio. 2019;10(3) . doi: 10.1128/mBio.00632-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Riviere A, Selak M, Lantin D, Leroy F, De Vuyst L. Bifidobacteria and Butyrate-producing colon bacteria: importance and strategies for their stimulation in the human gut. Front Microbiol. 2016;7:979. doi: 10.3389/fmicb.2016.00979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Geirnaert A, Calatayud M, Grootaert C, Laukens D, Devriese S, Smagghe G, De Vos M, Boon N, Van de Wiele T. Butyrate-producing bacteria supplemented in vitro to Crohn’s disease patient microbiota increased butyrate production and enhanced intestinal epithelial barrier integrity. Sci Rep. 2017;7(1):11450. doi: 10.1038/s41598-017-11734-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chen L, Wilson JE, Koenigsknecht MJ, Chou W-C, Montgomery SA, Truax AD, Brickey WJ, Packey CD, Maharshak N, Matsushima GK. Erratum: corrigendum: NLRP12 attenuates colon inflammation by maintaining colonic microbial diversity and promoting protective commensal bacterial growth. Nature Immunology. 2017;18(11):1270. doi: 10.1038/ni1117-1270b. [DOI] [PubMed] [Google Scholar]
- 59.Atarashi K, Tanoue T, Shima T, Imaoka A, Kuwahara T, Momose Y, Cheng G, Yamasaki S, Saito T, Ohba Y, et al. Induction of colonic regulatory T cells by indigenous clostridium species. Science. 2011;331(6015):337–341. doi: 10.1126/science.1198469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sefik E, Geva-Zatorsky N, Oh S, Konnikova L, Zemmour D, McGuire AM, Burzyn D, Ortiz-Lopez A, Lobera M, Yang J, et al. Individual intestinal symbionts induce a distinct population of RORγ + regulatory T cells. Science. 2015;349(6251):993–997. doi: 10.1126/science.aaa9420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Geva-Zatorsky N, Sefik E, Kua L, Pasman L, Tan TG, Ortiz-Lopez A, Yanortsang TB, Yang L, Jupp R, Mathis D, et al. Mining the human gut microbiota for immunomodulatory organisms. Cell. 2017;168(5):928–43 e11. doi: 10.1016/j.cell.2017.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Geuking MB, McCoy KD, Macpherson AJ. Metabolites from intestinal microbes shape Treg. Cell Res. 2013;23(12):1339–1340. doi: 10.1038/cr.2013.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Arpaia N, Campbell C, Fan X, Dikiy S, van der Veeken J, deRoos P, Liu H, Cross JR, Pfeffer K, Coffer PJ, et al. Metabolites produced by commensal bacteria promote peripheral regulatory T-cell generation. Nature. 2013;504(7480):451–455. doi: 10.1038/nature12726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.An D, Oh SF, Olszak T, Neves JF, Avci FY, Erturk-Hasdemir D, Lu X, Zeissig S, Blumberg R, Kasper D, et al. Sphingolipids from a symbiotic microbe regulate homeostasis of host intestinal natural killer T cells. Cell. 2014;156(1–2):123–133. doi: 10.1016/j.cell.2013.11.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Joossens M, Huys G, Cnockaert M, De Preter V, Verbeke K, Rutgeerts P, Vandamme P, Vermeire S. Dysbiosis of the faecal microbiota in patients with Crohn’s disease and their unaffected relatives. Gut. 2011;60(5):631–637. doi: 10.1136/gut.2010.223263. [DOI] [PubMed] [Google Scholar]
- 66.Pascal V, Pozuelo M, Borruel N, Casellas F, Campos D, Santiago A, Martinez X, Varela E, Sarrabayrouse G, Machiels K. A microbial signature for Crohn’s disease. Gut. 2017;66(5):813–822. doi: 10.1136/gutjnl-2016-313235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lewis JD, Chen EZ, Baldassano RN, Otley AR, Griffiths AM, Lee D, Bittinger K, Bailey A, Friedman E, Hoffmann C, et al. Inflammation, Antibiotics, and diet as environmental stressors of the gut microbiome in pediatric Crohn’s disease. Cell Host & Microbe. 2015;18(4):489–500. doi: 10.1016/j.chom.2015.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Frank DN, St Amand AL, Feldman RA, Boedeker EC, Harpaz N, Pace NR. Molecular-phylogenetic characterization of microbial community imbalances in human inflammatory bowel diseases Proc Natl Acad Sci USA. 2007;104(34):13780–13785. doi: 10.1073/pnas.0706625104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Baumgart M, Dogan B, Rishniw M, Weitzman G, Bosworth B, Yantiss R, Orsi RH, Wiedmann M, McDonough P, Kim SG, et al. Culture independent analysis of ileal mucosa reveals a selective increase in invasive Escherichia coli of novel phylogeny relative to depletion of Clostridiales in Crohn’s disease involving the ileum. The ISME Journal. 2007;1(5):403–418. doi: 10.1038/ismej.2007.52. [DOI] [PubMed] [Google Scholar]
- 70.Lepage P, Hasler R, Spehlmann ME, Rehman A, Zvirbliene A, Begun A, Ott S, Kupcinskas L, Doré J, Raedler A, et al. Twin study indicates loss of interaction between microbiota and mucosa of patients with ulcerative colitis. Gastroenterology. 2011;141(1):227–236. doi: 10.1053/j.gastro.2011.04.011. [DOI] [PubMed] [Google Scholar]
- 71.Haberman Y, Tickle TL, Dexheimer PJ, Kim M-O, Tang D, Karns R, Baldassano RN, Noe JD, Rosh J, Markowitz J, et al. Pediatric Crohn disease patients exhibit specific ileal transcriptome and microbiome signature. J Clin Invest. 2014;124(8):3617–3633. doi: 10.1172/JCI75436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Halfvarson J, Brislawn CJ, Lamendella R, Vazquez-Baeza Y, Walters WA, Bramer LM, D’Amato M, Bonfiglio F, McDonald D, Gonzalez A, et al. Dynamics of the human gut microbiome in inflammatory bowel disease. Nat Microbiol. 2017;2(5):17004. doi: 10.1038/nmicrobiol.2017.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Mondot S, Kang S, Furet JP, Aguirre de Carcer D, McSweeney C, Morrison M, Marteau P, Doré J, Leclerc M. Highlighting new phylogenetic specificities of Crohnʼs disease microbiota. Inflammatory Bowel Diseases. 2011;17(1):185–192. doi: 10.1002/ibd.21436. [DOI] [PubMed] [Google Scholar]
- 74.Gomez-Arango LF, Barrett HL, Wilkinson SA, Callaway LK, McIntyre HD, Morrison M, Dekker Nitert M. Low dietary fiber intake increases Collinsella abundance in the gut microbiota of overweight and obese pregnant women. Gut Microbes. 2018;9(3):189–201. doi: 10.1080/19490976.2017.1406584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wu GD, Chen J, Hoffmann C, Bittinger K, Chen -Y-Y, Keilbaugh SA, Bewtra M, Knights D, Walters WA, Knight R. Linking long-term dietary patterns with gut microbial enterotypes. Science. 2011;334(6052):105–108. doi: 10.1126/science.1208344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Sonnenburg ED, Smits SA, Tikhonov M, Higginbottom SK, Wingreen NS, Sonnenburg JL. Diet-induced extinctions in the gut microbiota compound over generations. Nature. 2016;529(7585):212–215. doi: 10.1038/nature16504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Marlow G, Ellett S, Ferguson IR, Zhu S, Karunasinghe N, Jesuthasan AC, Han DY, Fraser AG, Ferguson LR. Transcriptomics to study the effect of a Mediterranean-inspired diet on inflammation in Crohn’s disease patients. Human Genomics. 2013;7(1):24. doi: 10.1186/1479-7364-7-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ananthakrishnan AN, Khalili H, Konijeti GG, Higuchi LM, de Silva P, Korzenik JR, Fuchs CS, Willett WC, Richter JM, Chan AT. A Prospective study of long-term intake of dietary fiber and risk of Crohn’s disease and ulcerative colitis. Gastroenterology. 2013;145(5):970–977. doi: 10.1053/j.gastro.2013.07.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hung TV, Suzuki T. Dietary Fermentable fiber reduces intestinal barrier defects and inflammation in colitic mice. The Journal of Nutrition. 2016;146(10):1970–1979. doi: 10.3945/jn.116.232538. [DOI] [PubMed] [Google Scholar]
- 80.Wang H, Shi P, Zuo L, Dong J, Zhao J, Liu Q, Zhu W. Dietary Non-digestible polysaccharides ameliorate intestinal epithelial barrier dysfunction in IL-10 knockout mice. J Crohns Colitis. 2016;10(9):1076–1086. doi: 10.1093/ecco-jcc/jjw065. [DOI] [PubMed] [Google Scholar]
- 81.De Angelis M, Ferrocino I, Calabrese FM, De Filippis F, Cavallo N, Siragusa S, Rampelli S, Di Cagno R, Rantsiou K, Vannini L. Diet influences the functions of the human intestinal microbiome. Sci Rep. 2020;10(1):4247. doi: 10.1038/s41598-020-61192-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Dai Z-L, Wu G, Zhu WY. Amino acid metabolism in intestinal bacteria: links between gut ecology and host health. Front Biosci (Landmark Ed). 2011;16(1):1768–1786. doi: 10.2741/3820. [DOI] [PubMed] [Google Scholar]
- 83.Sridharan GV, Choi K, Klemashevich C, Wu C, Prabakaran D, Pan LB, Steinmeyer S, Mueller C, Yousofshahi M, Alaniz RC, et al. Prediction and quantification of bioactive microbiota metabolites in the mouse gut. Nature Communications. 2014;5(1):5492. doi: 10.1038/ncomms6492. [DOI] [PubMed] [Google Scholar]
- 84.Greenblum S, Turnbaugh PJ, Borenstein E. Metagenomic systems biology of the human gut microbiome reveals topological shifts associated with obesity and inflammatory bowel disease Proc Natl Acad Sci USA. 2012;109(2):594–599. doi: 10.1073/pnas.1116053109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Dai Z, Wu Z, Hang S, Zhu W, Wu G. Amino acid metabolism in intestinal bacteria and its potential implications for mammalian reproduction. Mol Hum Reprod. 2015;21(5):389–409. doi: 10.1093/molehr/gav003. [DOI] [PubMed] [Google Scholar]
- 86.Oliphant K, Allen-Vercoe E. Macronutrient metabolism by the human gut microbiome: major fermentation by-products and their impact on host health. Microbiome. 2019;7(1):91. doi: 10.1186/s40168-019-0704-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Barker HA. Amino acid degradation by anaerobic bacteria. Annu Rev Biochem. 1981;50(1):23–40. doi: 10.1146/annurev.bi.50.070181.000323. [DOI] [PubMed] [Google Scholar]
- 88.Takeshita K, Mizuno S, Mikami Y, Sujino T, Saigusa K, Matsuoka K, Naganuma M, Sato T, Takada T, Tsuji H, et al. A single species of clostridium subcluster XIVa decreased in ulcerative colitis patients. Inflammatory Bowel Diseases. 2016;22(12):2802–2810. doi: 10.1097/MIB.0000000000000972. [DOI] [PubMed] [Google Scholar]
- 89.Menni C, Zierer J, Pallister T, Jackson MA, Long T, Mohney RP, Steves CJ, Spector TD, Valdes AM. Omega-3 fatty acids correlate with gut microbiome diversity and production of N-carbamylglutamate in middle aged and elderly women. Sci Rep. 2017;7(1):11079. doi: 10.1038/s41598-017-10382-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kaliannan K, Wang B, Li X-Y, Kim K-J, Kang JX. A host-microbiome interaction mediates the opposing effects of omega-6 and omega-3 fatty acids on metabolic endotoxemia. Sci Rep. 2015;5(1):11276. doi: 10.1038/srep11276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Maldonado-Contreras A, Noel SE, Ward DV, Velez M, Mangano KM. Associations between diet, the gut microbiome, and short-chain fatty acid production among older Caribbean latino adults. J Acad Nutr Diet. 2020;120(12):2047–2060.e6. doi: 10.1016/j.jand.2020.04.018. [DOI] [PubMed] [Google Scholar]
- 92.Caesar R, Tremaroli V, Kovatcheva-Datchary P, Cani PD, Backhed F. Crosstalk between gut microbiota and dietary lipids aggravates WAT inflammation through TLR signaling. Cell Metab. 2015;22(4):658–668. doi: 10.1016/j.cmet.2015.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Watson H, Mitra S, Croden FC, Taylor M, Wood HM, Perry SL, Spencer JA, Quirke P, Toogood GJ, Lawton CL. A randomised trial of the effect of omega-3 polyunsaturated fatty acid supplements on the human intestinal microbiota. Gut. 2018;67(11):1974–1983. doi: 10.1136/gutjnl-2017-314968. [DOI] [PubMed] [Google Scholar]
- 94.Wolters M, Ahrens J, Romani-Perez M, Watkins C, Sanz Y, Benitez-Paez A, Stanton C, Günther K. Dietary fat, the gut microbiota, and metabolic health – a systematic review conducted within the MyNewGut project. Clinical Nutrition. 2019;38(6):2504–2520. doi: 10.1016/j.clnu.2018.12.024. [DOI] [PubMed] [Google Scholar]
- 95.Levine A, Rhodes JM, Lindsay JO, Abreu MT, Kamm MA, Gibson PR, Gasche C, Silverberg MS, Mahadevan U, Boneh RS. Dietary guidance from the international organization for the study of inflammatory bowel diseases. Clinical Gastroenterology and Hepatology: The Official Clinical Practice Journal of the American Gastroenterological Association. 2020;18(6):1381–1392. doi: 10.1016/j.cgh.2020.01.046. [DOI] [PubMed] [Google Scholar]
- 96.Candela M, Biagi E, Soverini M, Consolandi C, Quercia S, Severgnini M, Peano C, Turroni S, Rampelli S, Pozzilli P, et al. Modulation of gut microbiota dysbioses in type 2 diabetic patients by macrobiotic Ma-Pi 2 diet. The British Journal of Nutrition. 2016;116(1):80–93. doi: 10.1017/S0007114516001045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Wolf AR, Wesener DA, Cheng J, et al. Bioremediation of a common product of food processing by a human gut bacterium. Cell Host & Microbe. 2019;26(4):463–77 e8. doi: 10.1016/j.chom.2019.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Palm NW, de Zoete MR, Cullen TW, Barry NA, Stefanowski J, Hao L, Degnan P, Hu J, Peter I, Zhang W, et al. Immunoglobulin A coating identifies colitogenic bacteria in inflammatory bowel disease. Cell. 2014;158(5):1000–1010. doi: 10.1016/j.cell.2014.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Patnode ML, Beller ZW, Han ND, Cheng J, Peters SL, Terrapon N, Henrissat B, Le Gall S, Saulnier L, Hayashi DK, et al. Interspecies competition impacts targeted manipulation of human gut bacteria by fiber-derived glycans. Cell. 2019;179(1):59–73 e13. doi: 10.1016/j.cell.2019.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Cantu-Jungles TM, Hamaker BR. New view on dietary fiber selection for predictable shifts in gut microbiota. mBio. 2020;11(1) . doi: 10.1128/mBio.02179-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Scott KP, Martin JC, Duncan SH, Flint HJ. Prebiotic stimulation of human colonic butyrate-producing bacteria and bifidobacteria, in vitro. FEMS Microbiol Ecol. 2014;87:30–40. doi: 10.1111/1574-6941.12186. [DOI] [PubMed] [Google Scholar]
- 102.Lopetuso LR, Petito V, Graziani C, Schiavoni E, Paroni Sterbini F, Poscia A, Gaetani E, Franceschi F, Cammarota G, Sanguinetti M, et al. Gut microbiota in health, diverticular disease, irritable bowel syndrome, and inflammatory bowel diseases: time for microbial marker of gastrointestinal disorders. Dig Dis. 2018;36:56–65. doi: 10.1159/000477205. [DOI] [PubMed] [Google Scholar]
- 103.Dziarski R, Park SY, Kashyap DR, Dowd SE, Gupta D, Mizoguchi E. Pglyrp-regulated gut microflora prevotella falsenii, parabacteroides distasonis and bacteroides eggerthii enhance and alistipes finegoldii attenuates colitis in mice. PloS one. 2016;11:e0146162. doi: 10.1371/journal.pone.0146162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Mitsuyama K, Toyonaga A, Sasaki E, Watanabe K, Tateishi H, Nishiyama T, Saiki T, Ikeda H, Tsuruta O, Tanikawa K, et al. IL-8 as an important chemoattractant for neutrophils in ulcerative colitis and Crohn’s disease. Clin Exp Immunol. 1994;96:432–436. doi: 10.1111/j.1365-2249.1994.tb06047.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Daig R, Andus T, Aschenbrenner E, Falk W, Scholmerich J, Gross V. Increased interleukin 8 expression in the colon mucosa of patients with inflammatory bowel disease. Gut. 1996;38:216–222. doi: 10.1136/gut.38.2.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Mazzucchelli L, Hauser C, Zgraggen K, Wagner H, Hess M, Laissue JA, Mueller C. Expression of interleukin-8 gene in inflammatory bowel disease is related to the histological grade of active inflammation. Am J Pathol. 1994;144:997–1007. [PMC free article] [PubMed] [Google Scholar]
- 107.Bourgonje AR, von Martels JZH, Gabriels RY, Blokzijl T, Buist-Homan M, Heegsma J, Jansen BH, van Dullemen HM, Festen EAM, ter Steege RWF. A Combined set of four serum inflammatory biomarkers reliably predicts endoscopic disease activity in inflammatory bowel disease. Front Med (Lausanne). 2019;6:251. doi: 10.3389/fmed.2019.00251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Atreya R, Neurath MF. Involvement of IL-6 in the pathogenesis of inflammatory bowel disease and colon cancer. Clin Rev Allergy Immunol. 2005;28:187–196. doi: 10.1385/CRIAI:28:3:187. [DOI] [PubMed] [Google Scholar]
- 109.Liu G, Jin S, Jiang Q. Interleukin-6 receptor and inflammatory bowel disease: a Mendelian randomization study. Gastroenterology. 2019;156:823–824. doi: 10.1053/j.gastro.2018.09.059. [DOI] [PubMed] [Google Scholar]
- 110.Reimund JM, Wittersheim C, Dumont S, Muller CD, Baumann R, Poindron P, Duclos B. Mucosal inflammatory cytokine production by intestinal biopsies in patients with ulcerative colitis and Crohn’s disease. J Clin Immunol. 1996;16:144–150. doi: 10.1007/BF01540912. [DOI] [PubMed] [Google Scholar]
- 111.Caviglia GP, Rosso C, Stalla F, Rizzo M, Massano A, Abate ML. On-treatment decrease of serum interleukin-6 as a predictor of clinical response to biologic therapy in patients with inflammatory bowel diseases. J Clin Med. 2020;9:800. doi: 10.3390/jcm9030800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Rutella S, Pierelli L, Bonanno G, et al. Role for granulocyte colony-stimulating factor in the generation of human T regulatory type 1 cells. Blood. 2002;100:2562–2571. doi: 10.1182/blood-2001-12-0291. [DOI] [PubMed] [Google Scholar]
- 113.Morris ES, MacDonald KP, Rowe V, et al. Donor treatment with pegylated G-CSF augments the generation of IL-10-producing regulatory T cells and promotes transplantation tolerance. Blood. 2004;103:3573–3581. doi: 10.1182/blood-2003-08-2864. [DOI] [PubMed] [Google Scholar]
- 114.Bogunovic M, Ginhoux F, Helft J, et al. Origin of the lamina propria dendritic cell network. Immunity. 2009;31:513–525. doi: 10.1016/j.immuni.2009.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Rutella S, Bonanno G, Pierelli L, et al. Granulocyte colony-stimulating factor promotes the generation of regulatory DC through induction of IL-10 and IFN-alpha. Eur J Immunol. 2004;34:1291–1302. doi: 10.1002/eji.200324651. [DOI] [PubMed] [Google Scholar]
- 116.Lotfi N, Thome R, Rezaei N, et al. Roles of GM-CSF in the pathogenesis of autoimmune diseases: an update. Front Immunol. 2019;10:1265. doi: 10.3389/fimmu.2019.01265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Dabritz J. GM-CSF and the role of myeloid regulatory cells in the pathogenesis and treatment of Crohn’s disease. Mol Cell Pediatr. 2015;2:12. doi: 10.1186/s40348-015-0024-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Leal MC, Dabritz J. Immunoregulatory role of myeloid-derived cells in inflammatory bowel disease. Inflammatory Bowel Diseases. 2015;21:2936–2947. doi: 10.1097/MIB.0000000000000511. [DOI] [PubMed] [Google Scholar]
- 119.Kohl M, November. MKpower: power analysis and sample size calculation. https://cran.r-project.org/web/packages/MKpower/MKpower.pdf. 2020. (R project; ) [Google Scholar]
- 120.Gendoo DM, Ratanasirigulchai N, Schroder MS, et al. Genefu: an R/Bioconductor package for computation of gene expression-based signatures in breast cancer. Bioinformatics. 2016;32:1097–1099. doi: 10.1093/bioinformatics/btv693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Suskind DL, Wahbeh G, Gregory N, Vendettuoli H, Christie D. Nutritional therapy in pediatric Crohn disease: the specific carbohydrate diet. Journal of Pediatric Gastroenterology and Nutrition. 2014;58:87–91. doi: 10.1097/MPG.0000000000000103. [DOI] [PubMed] [Google Scholar]
- 122.Natto ZS, Yaghmoor W, Alshaeri HK, Van Dyke TE. Omega-3 fatty acids effects on inflammatory biomarkers and lipid profiles among diabetic and cardiovascular disease patients: a systematic review and meta-analysis. Sci Rep. 2019;9:18867. doi: 10.1038/s41598-019-54535-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.O’Mahoney LL, Matu J, Price OJ, et al. Omega-3 polyunsaturated fatty acids favourably modulate cardiometabolic biomarkers in type 2 diabetes: a meta-analysis and meta-regression of randomized controlled trials. Cardiovasc Diabetol. 2018;17:98. doi: 10.1186/s12933-018-0740-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Ebrahimi M, Ghayour-Mobarhan M, Rezaiean S, et al. Omega-3 fatty acid supplements improve the cardiovascular risk profile of subjects with metabolic syndrome, including markers of inflammation and auto-immunity. Acta Cardiol. 2009;64:321–327. doi: 10.2143/AC.64.3.2038016. [DOI] [PubMed] [Google Scholar]
- 125.Pisaniello AD, Psaltis PJ, King PM, et al. Omega-3 fatty acids ameliorate vascular inflammation: a rationale for their atheroprotective effects. Atherosclerosis. 2021;324:27–37. doi: 10.1016/j.atherosclerosis.2021.03.003. [DOI] [PubMed] [Google Scholar]
- 126.Finucane OM, Lyons CL, Murphy AM, et al. Monounsaturated fatty acid-enriched high-fat diets impede adipose NLRP3 inflammasome-mediated IL-1beta secretion and insulin resistance despite obesity. Diabetes. 2015;64:2116–2128. doi: 10.2337/db14-1098. [DOI] [PubMed] [Google Scholar]
- 127.Todoric J, Loffler M, Huber J, et al. Adipose tissue inflammation induced by high-fat diet in obese diabetic mice is prevented by n-3 polyunsaturated fatty acids. Diabetologia. 2006;49:2109–2119. doi: 10.1007/s00125-006-0300-x. [DOI] [PubMed] [Google Scholar]
- 128.Balfego M, Canivell S, Hanzu FA, et al. Effects of sardine-enriched diet on metabolic control, inflammation and gut microbiota in drug-naive patients with type 2 diabetes: a pilot randomized trial. Lipids Health Dis. 2016;15:78. doi: 10.1186/s12944-016-0245-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Narula N, Wong ECL, Dehghan M, et al. Association of ultra-processed food intake with risk of inflammatory bowel disease: prospective cohort study. BMJ. 2021;374. doi: 10.1136/bmj.n1554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Parnell JA, Reimer RA. Prebiotic fiber modulation of the gut microbiota improves risk factors for obesity and the metabolic syndrome. Gut Microbes. 2012;3:29–34. doi: 10.4161/gmic.19246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Makki K, Deehan EC, Walter J, Backhed F. The impact of dietary fiber on gut microbiota in host health and disease. Cell Host & Microbe. 2018;23:705–715. doi: 10.1016/j.chom.2018.05.012. [DOI] [PubMed] [Google Scholar]
- 132.Park S, Bae JH. Fermented food intake is associated with a reduced likelihood of atopic dermatitis in an adult population (Korean National Health and Nutrition Examination Survey 2012-2013). Nutr Res. 2016;36:125–133. doi: 10.1016/j.nutres.2015.11.011. [DOI] [PubMed] [Google Scholar]
- 133.Nozue M, Shimazu T, Sasazuki S, et al. Fermented Soy product intake is inversely associated with the development of high blood pressure: the Japan public health center-based prospective study. The Journal of Nutrition. 2017;147:1749–1756. [DOI] [PubMed] [Google Scholar]
- 134.Dimidi E, Cox SR, Rossi M, Whelan K. Fermented foods: definitions and characteristics, impact on the gut microbiota and effects on gastrointestinal health and disease. Nutrients. 2019;11(8) . doi: 10.3390/nu11081806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Staudacher HM, Nevin AN. Fermented foods: fad or favourable addition to the diet? Lancet Gastroenterol Hepatol. 2019;4:19. doi: 10.1016/S2468-1253(18)30392-3. [DOI] [PubMed] [Google Scholar]
- 136.U. S. D. o. H. a. H. S. a. U. S. D. o. Agriculture . 2015–2020. Dietary guidelines for Americans
- 137.Wipperman MF, Fitzgerald DW, Juste MAJ, et al. Antibiotic treatment for Tuberculosis induces a profound dysbiosis of the microbiome that persists long after therapy is completed. Sci Rep. 2017;7:10767. doi: 10.1038/s41598-017-10346-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Haran JP, Bucci V, Dutta P, Ward D, McCormick B. The nursing home elder microbiome stability and associations with age, frailty, nutrition and physical location. J Med Microbiol. 2018;67:40–51. doi: 10.1099/jmm.0.000640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Morgan XC, Tickle TL, Sokol H, et al. Dysfunction of the intestinal microbiome in inflammatory bowel disease and treatment. Genome Biology. 2012;13:R79. doi: 10.1186/gb-2012-13-9-r79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Truong DT, Franzosa EA, Tickle TL, et al. MetaPhlAn2 for enhanced metagenomic taxonomic profiling. Nat Methods. 2015;12:902–903. doi: 10.1038/nmeth.3589. [DOI] [PubMed] [Google Scholar]
- 141.Abubucker S, Segata N, Goll J, et al. Metabolic reconstruction for metagenomic data and its application to the human microbiome. PLoS Computational Biology. 2012;8:e1002358. doi: 10.1371/journal.pcbi.1002358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Kaminski J, Gibson MK, Franzosa EA, Segata N, Dantas G, Huttenhower C. High-specificity targeted functional profiling in microbial communities with ShortBRED. PLoS Computational Biology. 2015;11:e1004557. doi: 10.1371/journal.pcbi.1004557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Tanaka JS, Young RR, Heston SM, et al. Anaerobic antibiotics and the risk of graft-versus-host disease after allogeneic hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2020;26:2053–2060. doi: 10.1016/j.bbmt.2020.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Hajjem A, Bellavance F, Larocque D. Mixed-effects random forest for clustered data. Journal of Statistical Computation and Simulation. 2014;84:1313–1328. doi: 10.1080/00949655.2012.741599. [DOI] [Google Scholar]
- 145.Johnstone IM, Titterington DM. Statistical challenges of high-dimensional data. Philos Trans A Math Phys Eng Sci. 2009;367:4237–4253. doi: 10.1098/rsta.2009.0159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Altmann A, Tolosi L, Sander O, Lengauer T. Permutation importance: a corrected feature importance measure. Bioinformatics. 2010;26:1340–1347. doi: 10.1093/bioinformatics/btq134. [DOI] [PubMed] [Google Scholar]
- 147.McMurdie PJ, Holmes S. phyloseq: an R package for reproducible interactive analysis and graphics of microbiome census data. PloS one. 2013;8:e61217. doi: 10.1371/journal.pone.0061217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Shannon CE. The mathematical theory of communication. MD Comput 1997. 1963;14:306–317. [PubMed] [Google Scholar]
- 149.Bolyen E, Rideout JR, Dillon MR, et al. Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nature Biotechnology. 2019;37:852–857. doi: 10.1038/s41587-019-0209-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Anderson MJ, Walsh, D. PERMANOVA, ANOSIM and the Mantel test in the face of heterogeneous dispersions: What null hypothesis are you testing?. Ecological Monographs. 2013;83(4):557–57. doi: 10.1890/12-2010.1 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are openly available in NCBI, BioProject: PRJNA642308 (https://www.ncbi.nlm.nih.gov/bioproject/?term=PRJNA642308)


