Abstract
The aim of this study was to evaluate the percentage of Campylobacter (C. jejuni and C. coli) from samples collected at the slaughterhouse to describe the prevalence of resistance to selected antimicrobials, and to characterize the genetic determinants. In total, from 333 samples analyzed, 31% were positive for Campylobacter. More positive samples were detected before the chiller (46%) than after the chiller (16%). C. coli (59%) was more prevalent than C. jejuni (41%). Antimicrobial resistance differences between C. jejuni and C. coli were found (p < 0.001). Multidrug resistance was found in 72% of C. coli isolates and 69% of C. jejuni isolates (p < 0.001). Most C. jejuni isolates (57%) had the three genes of the cmeABC efflux pump. The tet(O) gene and resistance-associated point mutations within both the gyrA and 23S rRNA genes were detected in 100% of C. coli isolates. On the other hand, C. jejuni only had more prevalence of the blaOXA-61 gene than C. coli (p < 0.001), and most of the C. jejuni isolates (70–80%) had the tet(O) and gyrA point mutation. These results could contribute to knowledge about the status of thermotolerant Campylobacter resistant to antimicrobials isolated from food animals in Argentina and to develop an antimicrobial resistance surveillance system.
Keywords: Antimicrobial resistance, Mechanisms of resistance, Campylobacter, Slaughterhouse
Graphical abstract
Highlights
-
•
From samples analyzed, 31% were positive for Campylobacter.
-
•
More positive samples were detected before the chiller (46%) than after it (16%).
-
•
Multidrug resistance was found in 72% of C. coli isolates and 69% of C. jejuni isolates.
-
•
The tet(O) gene was detected in 100% of the tetracycline-, resistant C. coli isolates.
-
•
C.jejuni only had more prevalence of the blaOXA-61 gene than C. coli (p < 0.001).
1. Introduction
The World Health Organization considered that thermotolerant Campylobacter is one of the main causes of enteric infection due to food consumption in developed and developing countries (World Health Organization, 2017; Natsos et al., 2016; Pedersen et al., 2018). In developing countries like Argentina, information on food-borne disease is scant due to the inadequate data provided by the surveillance systems. Additionally, outbreak information is frequently unsubstantial because health authorities lack the capabilities or resources for detection of diarrheal diseases (Zaidi et al., 2008).
C. jejuni and C. coli are the most important species of thermotolerant Campylobacter and they are enteric commensal bacteria of poultry (Kaakoush et al., 2015). Broilers are the main reservoir of Campylobacter spp., and colonization in broiler ceca can reach 109 cfu/g of cecal content (Stern et al., 2008; EFSA and ECDC, 2016).
In Argentina, the production of chicken meat has grown substantially during the last years, reaching a total of over 757,9 million chickens processed in 2020 and producing 1,779,000 tonnes of poultry meat, which is approximately 0,13% higher than the figures for 2019. The apparent per capita consumption of chicken meat has increased by 1,2% in the last year, reaching 45.9 kg/inhabitant/year in 2020 (MAGYP, 2020).
Poultry production in Argentina is concentrated within 3800 farms and there are 54 slaughterhouses in the country. During the slaughter process, broiler carcasses may be contaminated with thermotolerant Campylobacter. As a common inhabitant of the gastrointestinal tract of warm-blooded animals, Campylobacter can be expected to contaminate meat during slaughter and evisceration as a result of fecal contamination (García Sanchez et al., 2018; Osimani et al., 2017). The improper handling or consumption of raw or undercooked meat and meat products are the main risk factors associated with campylobacteriosis in humans (Damjanova et al., 2011).
The increase of global antimicrobial resistance threatens human and animal health. Although human campylobacteriosis is self-limiting, ciprofloxacin (fluoroquinolone) and erythromycin (macrolide) are being used as the first line antimicrobial therapy to treat this disease (World Health Organization, 2011; Wieczorek et al., 2013). However, the increase of Campylobacter antimicrobial resistance, mainly against fluoroquinolones, has been demonstrated by numerous studies (Wieczorek et al., 2013; EFSA and ECDC, 2016). In this sense, the use of antimicrobial agents in veterinary medicine as a growth promotor, preventive treatment or as clinical therapy was related to the increase in antimicrobial resistance in microorganisms (Radostits and Rubinstein, 2002; Ventola 2015). Therefore, thermotolerant Campylobacter resistant isolates could spread throughout the food chain, posing a risk to public health (CDC, 2014; World Health Organization, 2017).
The emergence of multidrug resistance may reflect the acquisition of different resistance determinants, and the mechanisms of genetic resistance might be chromosomal or plasmid-borne, representing a combination of endogenous and acquired genes (Whyte et al., 2011; Nguyen et al., 2016). Different genes related to antimicrobial resistance in Campylobacter were described (Tang et al., 2017; Iovine 2013). These mechanisms include restricting antimicrobial access to their targets (efflux pumps), antimicrobial target modification or antimicrobial inactivation. Also, these mechanisms may act together in resisting different classes of antimicrobials (Tang et al., 2017).
In Argentina, only a few studies have evaluated antimicrobial resistance in Campylobacter strains (Pantozzi et al., 2010; Tamborini et al., 2012; Zbrun et al., 2015). Additionally, no epidemiological studies in Argentina have assessed the prevalence of Campylobacter resistant to antimicrobials throughout the poultry meat chain in general and at slaughterhouses in particular. This information is essential to establish a public health program to control the disease and it is fundamental for the creation of a surveillance program to monitor resistance. Because of the importance of Campylobacter as regards food safety and public health, the aim of this study was to evaluate the percentage of Campylobacter (C. jejuni and C. coli) from samples collected at the slaughterhouse, to describe the prevalence of resistance to selected antimicrobials, and to characterize the genetic determinants.
2. Materials and methods
2.1. Sample collection and Campylobacter isolation
Samples were taken from different areas of the slaughterhouse. The slaughterhouse belongs to a company with 35 chicken farms and 70 retail markets in Argentina. In the slaughterhouse, 600.000 chickens were slaughtered per month. Sampling was performed in nine visits, one per month during 2015 (from April to December). The slaughterhouse was divided in two areas, before and after the chiller. The samples before the chiller were taken from: cecum (n = 90), evisceration knives (n = 27), processing line surfaces (n = 18), workers' hands (n = 27); and after the chiller: processing line surfaces (n = 27), workers' hands in the packing area (n = 27), packing area surfaces (n = 27), and carcasses (n = 90). Campylobacter spp. were isolated using selective media Bolton Broth and Modified Charcoal Cefoperazone Deoxycholate (mCCDA) agar plates (ISO 10272–1). The cecum and carcasses were processed as described previously by Zbrun et al. (2017). The knives and surfaces were sampled using sterile cotton swabs. Each cotton was immersed in 5 mL Bolton Broth and incubated for 24 h at 42 °C under microaerobic conditions (5% O2, 10% CO2 and 85% H2). Then, the same procedure described by Zbrun et al. (2017) was used for Campylobacter isolation. The workers’ hands were washed with 200 ml of sterile PBS 1X, and the buffer was collected in a sterile screw flask. Then, the procedure employed was the same as for carcasses (Zbrun et al., 2017).
2.2. Identification of Campylobacter species
Preliminary identification of thermotolerant Campylobacter isolates was based on colony morphology, microscopic appearance (curved Gram-negative bacilli with typical motility), and the following phenotypic characteristics: oxidase and catalase production. All presumptive Campylobacter spp. isolates were identified to the species level (C. jejuni and C. coli) by multiplex PCR, as proposed by Vandamme et al. (1997). DNA was extracted using a Wizard genomic DNA purification kit (Promega®), and PCR products were analyzed on 1.5% agarose gels and stained with GelRed (Biotium®). Positive isolates were sub-cultured on Columbia blood agar and stored in glycerol broth (15% glycerol and 85% serum broth) at −80°C.
2.3. Antimicrobial susceptibility testing and determination of MICs
The antimicrobial sensitivity of Campylobacter isolates was tested by agar dilution assay as recommended by the Clinical and Laboratory Standards Institute in the standard M100-S23 (CLSI, 2013). C. jejuni and C. coli isolates were tested with eight antimicrobial agents: erythromycin (ERY), ciprofloxacin (CIP), gentamicin (GEN), streptomycin (STR), tetracycline (TET), enrofloxacin (ENR), chloramphenicol (CLO), and ampicillin (AMP) (Table 1). The strains were removed from the freezer and streaked onto Columbia blood agar and then incubated for 48 h at 42 °C under microaerobic conditions. Several colonies were transferred to a tube with 5 ml of Mueller-Hinton broth to reach a standard inoculum adjusted to 0.5 McFarland. Approximately 104 cfu of these suspensions was inoculated onto Mueller-Hinton agar containing a two-fold dilution series of antimicrobials and supplemented with 5% defibrinated sheep blood using a multipoint inoculator (a Steers replicator system) with 1-mm pins. The plates were incubated for 24 h at 42 °C under microaerobic conditions. C. jejuni ATCC 33560 was used as a reference strain. The inhibition was evaluated according to the standards of the Clinical and Laboratory Standards Institute (CLSI, 2010). If an isolate was resistant to three or more antimicrobial classes, it was considered to be a multi-resistant profile.
Table 1.
MIC QC ranges and breakpoints used for antimicrobial susceptibility testing by agar dilution.
| Antimicrobial groups | Antimicrobial agent | aMIC QC range (mg/mL) |
bMIC breakpoint (mg/mL) |
||
|---|---|---|---|---|---|
| S | I | R | |||
| Fluoroquinolone | Ciprofloxacin | 0.06–0.5 | ≤1 | 2 | ≥4 |
| Enrofloxacin | N/A | ≤0.5 | 1–2 | ≥4 | |
| Macrolide | Erythromycin | 1–8 | ≤8 | 16 | ≥32 |
| Amphenicols | Chloramphenicol | 1–4 | ≤8 | 16 | ≥32 |
| Aminoglycoside | Gentamicin | 0.4–4 | ≤2 | 4 | ≥8 |
| Streptomycin | 1–4 | – | – | c4 | |
| Tetracycline | Tetracycline | 0.25–1 | ≤4 | 8 | ≥16 |
| β-lactam | Ampicillin | N/A | ≤8 | 16 | ≥32 |
The QC ranges of C. jejuni ATCC 33560 were directly adopted from CLSI (2010). Due to the lack of QC ranges of C. jejuni ATCC 33560 for enrofloxacin, we used E. coli ATCC 25922 as QC strain for these two antimicrobial agents (CLSI, 2010).
MIC breakpoints for ciprofloxacin, erythromycin, tetracycline, and gentamicin are those recommended by the CLSI (2010). Since standardized MIC breakpoints for enrofloxacin and chloramphenicol are not available for Campylobacter spp., we used the breakpoints of Enterobacteriaceae for these four antimicrobial agents, as recommended by CLSI (2010).
Cut off values used for the interpretation of MIC results were in accordance with EUCAST (www.eucast.org).
2.4. Detection of antimicrobial resistance determinants
The efflux pump was evaluated by PCR using different protocols for each component of the pump: cmeA (Koolman et al., 2015), cmeB (Lin et al., 2002), and cmeC (Fakhr and Logue, 2007). Mutation at position 2075 in domain V of the 23S rRNA gene, associated with high-level erythromycin resistance, was detected by the mismatch amplification mutation assay PCR (MAMA-PCR) (Alonso et al., 2005). For tetracycline resistance, tet(O) was detected by PCR assay as described previously by Gibreel et al. (2004). Mutations in the quinolone resistance determining region of gyrA, resulting in resistance-associated T86I substitutions, were identified by MAMA-PCR as reported by Zirnstein et al. (2000) and Zirnstein et al. (1999). β-lactamase gene blaOXA-61 was detected as described by Obeng et al. (2012). PCR primers are described in Table 2. PCR products were visualized by electrophoresis in 1.5% agarose gels, stained with GelRed® and viewed under UV light.
Table 2.
List of primers and primer sequences used for detection of antimicrobial resistance genes.
| Antimicrobial | Gene | Primer | Sequence (5′-3′) | Amplicon length (bp) | Reference |
|---|---|---|---|---|---|
| Multiple antimicrobials (Efflux pump) | cmeA | cmeA F | TGTGCATCAGCTCCTGTGTAA | 957 | Koolman et al. (2015) |
| cmeA R | ACGGACAAGCTTTGATGGCT | ||||
| cmeB | cmeB F | GGTACAGATCCTGATCAAGCC | 820 | Lin et al. (2002) | |
| cmeB R | AGGAATAAGTGTTGCACGGAAATT | ||||
| cmeC | cmeC F | AGATGAAGCTTTTGTAAATT | 500 | Fakhr and Logue (2007) | |
|
cmeC R |
TATAAGCAATTTTATCATTT |
||||
| Tetracycline | tet(O) | tet(O) F | GGCGTTTTGTTTATGTGCG | 559 | Gibreel et al. 2004 |
|
tet(O) R |
ATGGACAACCCGACAGAAGC |
||||
| Ampicillin | blaOXA-61 | blaOXA-61 F | AGAGTATAATACAAGCG | 372 | Obeng et al. (2012) |
|
blaOXA-61 R |
TAGTGAGTTGTCAAGCC |
||||
| Ciprofloxacin | gyrA C. jejuni | gyrA F | CAACTGGTTCTAGCCTTTTG | 1083 | Wang et al., 2016 |
| gyrA R | AATTTCACTCATAGCCTCACG | ||||
| gyrA C. coli | gyrA F | TATGAGCGTTATTATCGGTC | 505 | Zirnstein et al. (2000) | |
| gyrA R | GTCCATCTACAAGCTCGTTA | ||||
| Mutation Thr-86-Ile C. jejuni | gyrA F | CAACTGGTTCTAGCCTTTTG | 410 | Wang et al., 2016 | |
| MAMAgyrA-R | CAAAGCATCATAAACTGCAA | Zirnstein et al., 1999 | |||
| Mutation Thr-86-Ile C. coli | gyrA F | TATGAGCGTTATTATCGGTC | 192 | Zirnstein et al. (2000) | |
| MAMAgyrA-R |
TAAGCCATCGTAAACAGCCA |
||||
| Erythromycin | ARNr23S | ARNr23 S F | GTAAACGGCGGCCGTAACTA | 699 | Jensen and Aarestrup (2001) |
| ARNr23 S R | GACCGAACTGTCTCACGACG | ||||
| Mutation A2075-G ARNr23S | ARNr23 S F | GTAAACGGCGGCCGTAACTA | 184 | Jensen and Aarestrup (2001) | |
| MAMAARNr 23S- R | TAGTAAAGGTCCACGGGGTCGC | Alonso et al. (2005) |
2.5. Statistical analysis
The resistant frequencies for each class of antimicrobial agents and multidrug resistance (MDR) in C. jejuni and C. coli isolates, the association between the resistance of each antimicrobial and the presence of genes related to the mechanism of resistance were compared with the chi-square test and Fisher Exact Test using Infostat (Universidad Nacional de Córdoba). Differences were considered significant at p < 0.05.
3. Results
3.1. Campylobacter species prevalence in different areas of the slaughterhouse
In total, from 333 samples analyzed, 102 (31%) were positive for Campylobacter and were stored in freezer at −80 °C for subsequent studies. The highest Campylobacter prevalence was detected in cecum (63%), followed by the evisceration knives (26%), and workers’ hands before and after the chiller (26% and 22%). In addition, more positive samples were detected before the chiller (46%) than after the chiller (16%). C. coli (18%) was more prevalent than C. jejuni (13%) in all samples analyzed. Moreover, C. coli was the most prevalent species in cecum and before chilling, but after the chiller its prevalence decreased considerably (Table 3).
Table 3.
Prevalence of C. jejuni and C. coli at the slaughterhouse.
| Sampling location | n samples (% positive) | Isolates of |
||
|---|---|---|---|---|
| C. jejuni | C. coli | |||
| Before chiller | Cecum | 90 (63%) | 15 | 42 |
| Evisceration knives | 27 (26%) | 3 | 4 | |
| Line processing surfaces | 18 (17%) | 2 | 1 | |
| Workers' hands |
27 (26%) |
1 |
6 |
|
| Total before chiller |
162 (46%) |
21 (50%) |
53 (88%) |
|
| After chiller | Line processing surfaces | 27 (19%) | 4 | 1 |
| Workers' hands in packing area | 27 (22%) | 5 | 1 | |
| Packing area surfaces | 27 (7%) | 2 | 0 | |
| Carcasses | 90 (17%) | 10 | 5 | |
| Total after chiller |
171 (16%) |
21 (50%) |
7 (12%) |
|
| Total | 333 | 42 (41%) | 60 (59%) | |
3.2. Antimicrobial resistance and multidrug profiles of Campylobacter isolates
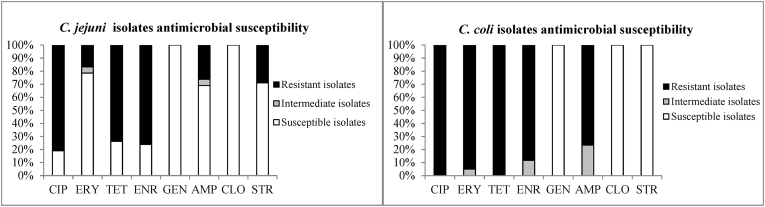
Antimicrobial resistance differences between C. jejuni and C. coli were found (p < 0.001). In general, C. coli isolates were more resistant against the antimicrobials tested than C. jejuni isolates (Fig. 1). The analysis of C. coli antimicrobial resistance shows isolates having higher resistance to ciprofloxacin (100%; 95%CI 94.1%–100.0%), tetracycline (100%; 95%CI 94.1%–100.0%), erythromycin (95%; 95%CI 86.3%–98.2%), enrofloxacin (88%; 95%CI 77.8%–94.2%), and ampicillin (77%; 95%CI 64.5%–85.5%) than C. jejuni. Moreover, several C. coli isolates were classified as “intermediate” when tested on media amended with erythromycin (5%; 95%CI 1.8%–13.7%), enrofloxacin (12%; 95%CI 5.8%–22.2%), or ampicillin (23%; 95%CI 14.5%–35.5%); thus, all C. coli isolates were classified as either “resistant” or “intermediate” with regards to these three antibiotics”. C. jejuni isolates showed high resistance to ciprofloxacin (81%, 95%CI 66.6%–90.0%), enrofloxacin (76%; 95%CI 61.4%–86.5%) tetracycline (74%; 95%CI 58.8%–84.7%), and a lower proportion of isolates were resistant to streptomycin (29%; 95%CI 17.2%–43.7%), ampicillin (26%; 95%CI 15.3%–41.2%), and erythromycin (17%; 95%CI 8.4%–30.7%). Both Campylobacter species were susceptible to gentamicin and chloramphenicol.
Fig. 1.
Thermotolerant Campylobacter antimicrobials susceptibility
Reference: ERY = erythromycin, CIP = ciprofloxacin, GEN = gentamicin, STR = streptomycin, TET = tetracycline, ENR = enrofloxacin, CLO = chloramphenicol, AMP = ampicillin.
Multidrug resistance to three or more classes of antimicrobials was found in 72% (n = 43) of C. coli isolates and 69% (n = 29) of C. jejuni isolates (p < 0.001) (Table 4). The only MDR profile in C. coli was quinolone ciprofloxacin and enrofloxacin, tetracycline, ampicillin and erythromycin. For C. jejuni, quinolone (ciprofloxacin and enrofloxacin), tetracycline and streptomycin was the more prevalent MDR profile (28%).
Table 4.
Multi-resistant profiles of thermotolerant Campylobacter.
| Multi-resistant resistant isolates | Antimicrobial resistance profile | No. of resistant isolates (%) |
|---|---|---|
|
C. coli (n = 60) |
CIP-TET-AMP-ENR-ERY |
43 (72%) |
| C. jejuni (n = 42) | CIP-TET-ENR-STR | 12 (28%) |
| CIP-TET-AMP-ENR | 10 (24%) | |
| CIP-TET-AMP-ENR-ERY | 7 (17%) |
3.3. Prevalence of resistant genes of Campylobacter isolates
The genes from three parts of the efflux pump were evaluated. The majority of C. jejuni isolates (57%) had the three genes of the efflux pump. In contrast, only one C. coli had the three components of the efflux pump. In addition, both species had more prevalence of cmeC than cmeB and cmeA. C. jejuni isolates had more prevalence of cmeA (43%) and cmeB (48%) of the efflux pump (P < 0.001) than C. coli isolates (cmeA 3%, cmeB 28%). Both species had a similar prevalence of cmeC (88.1% C. jejuni and 88.3% C. coli (P = 0.971).
The presence of the tet(O) gene, and resistance-associated point mutations within gyrA and the 23S rRNA were detected in 100% of the C. coli isolates. The blaOXA-61 gene was more prevalent in C. jejuni than in C. coli (p < 0.001), and most of the C. jejuni isolates (70–80%) possessed both tet(O) and the resistance-associated gyrA point mutation (Fig. 2).
Fig. 2.
Prevalence of genetic determinants to resistance of thermotolerant Campylobacter.
3.4. Antimicrobial resistance genes and resistance profile of Campylobacter isolates
The presence of the cmeA (P = 0.573), cmeB (P = 0.824), or cmeC (P = 0.343) genes was not associated with the prevalence of multidrug resistance in C. jejuni isolates. All ciprofloxacin resistant C. jejuni and C. coli had the resistance-associated point mutation in the gyrA gene. However, for enrofloxacin, 20% of susceptible isolates of C. jejuni and all C. coli with intermediate classification harbored the point mutation in the gyrA gene.
In addition, the C. jejuni and C. coli isolates which were phenotypically resistant to tetracycline harbored the tet(O) gene identified by PCR, and all isolates susceptible to tetracycline did not present this gene (Table 5).
Table 5.
Antimicrobial resistance genes and resistance profile of Campylobacter isolates.
| Campylobacter species | Antimicrobial agent | Agar dilution assay |
Gene presence |
|||||
|---|---|---|---|---|---|---|---|---|
| Susceptible isolates | Intermediate isolates | Resistant isolates | Gene | Susceptible isolates | Intermediate isolates | Resistant isolates | ||
| C. jejuni (n = 42) | Ciprofloxacin | 8 | 0 | 34 | Mut GyrA | 0 | 0 | 34 |
| Enrofloxacin | 10 | 0 | 32 | Mut GyrA | 2 | 0 | 32 | |
| Erythromycin | 33 | 2 | 7 | Mut 23SrRNA | 0 | 2 | 7 | |
| Tetracycline | 11 | 0 | 31 | tet(O) | 0 | 0 | 31 | |
| Ampicillin |
29 |
2 |
11 |
blaOXA-61 |
24 |
2 |
8 |
|
| C. coli (n = 60) | Ciprofloxacin | 0 | 0 | 60 | Mut GyrA | 0 | 0 | 60 |
| Enrofloxacin | 0 | 7 | 53 | Mut GyrA | 0 | 7 | 53 | |
| Erythromycin | 0 | 3 | 57 | Mut 23SrRNA | 0 | 3 | 57 | |
| Tetracycline | 0 | 0 | 60 | tet(O) | 0 | 0 | 60 | |
| Ampicillin | 0 | 14 | 46 | blaOXA-61 | 0 | 6 | 28 | |
The point mutation of the 23S rRNA gene was detected in all C. jejuni and C. coli isolates classified as resistant and intermediate to erythromycin. Additionally, none of the C. jejuni isolates susceptible to erythromycin presented the mutation (Table 5).
In some C. coli isolates that were resistant (61%) and intermediate (43%) to ampicillin, the blaOXA61 amplicon was detected. In most of the C. jejuni resistant isolates (73%) and in the intermediate resistant isolates, the blaOXA61 amplicon was detected. However, 83% of C. jejuni susceptible isolates harbored this amplicon (Table 5).
4. Discussion
In the present study, the presence in the slaughterhouse, microbial resistance and resistance mechanisms of thermotolerant Campylobacter were evaluated. Commercial chickens frequently carry high levels of Campylobacter spp. (primarily C. jejuni and C. coli) in their intestine as part of the normal microbiota (Newell and Wagenaar, 2000; Sahin et al., 2002). While C. jejuni is, in general, the most prevalent species of thermotolerant Campylobacter isolated at farm (Bull et al., 2006; Rossler et al., 2019), sometimes a predominant proportion of Campylobacter isolates from broilers are C. coli (Ma et al., 2014; Damjanova et al., 2011; Zbrun et al., 2013, Rossler et al., 2019).
Interestingly, in this study C. coli (42/90) was observed to be more prevalent than C. jejuni (15/90) in cecum samples. Similar results have been reported previously where C. coli was the predominant Campylobacter species in broiler intestinal tracts, which seems to depend on several factors as the geographical area evaluated (Hariharan et al., 2009; Henry et al., 2011; Ma et al., 2014), the age of the chicken and antibiotic selection pressure (Wang et al., 2016). However, in this study, the species proportion changed at the end of the slaughter line, where C. jejuni (10/90) was more prevalent than C. coli (5/90) in broiler carcasses. In this sense, some authors suggest that C. coli is less robust and might be more sensitive to the stress conditions found in poultry abattoirs (Peyrat et al., 2008).
Furthermore, thermotolerant Campylobacter were isolated from samples taken in the processing line surfaces, knives and workers' hands. Many studies have reported similar results, which can be explained by cross contamination with positive Campylobacter carcasses (Ono and Yamamoto, 1999; Chlebicz and Śliżewska, 2018; Zhang et al., 2018). In addition, biofilm formation may be another cause of Campylobacter presence on line processing surfaces. In this sense, previous research identified that some strains are able to form biofilms and can survive longer and resist inactivation (García-Sánchez et al., 2019; Lamas et al., 2018).
Regardless of the source of Campylobacter spp., a recent meta-analysis has shown that C. coli isolates presented a higher prevalence of antimicrobial resistance to most antimicrobials than C. jejuni (Signorini et al., 2018). In this study, more than 77% of C. coli isolates were resistant to five (CIP, ERY, TET, ENR and AMP) out of eight antimicrobials evaluated (Fig. 1). Also, 74% of the C. jejuni isolates showed resistance against three (CIP, TET and ENR) out of eight antimicrobials evaluated (Fig. 1). High resistance in thermotolerant Campylobacter was described previously, which may be linked to the use of antimicrobials in food-producing animals in each country (CDDEP, 2015; Lajhar et al., 2015, 255 Unicomb et al., 2006; Cha et al., 2017; Nelson et al., 2007). Antimicrobials used as growth promoters in animals, and the abuse or misuse of those antimicrobials, have many times affected the resistance profile of bacteria isolates (Ventola, 2015). Particularly in Argentina, different government institutions have begun to outline strategies to control the use of antimicrobials. However, nowadays there are no clear regulations for the use of antimicrobials in the different stages of the production of food of animal origin (Lazovski et al., 2017).
Furthermore, results have shown that the MDR rate in C. coli and C. jejuni isolates was high (Table 4). The main difference between the Campylobacter species were erythromycin and ampicillin resistance and might be due to C. coli having better adaptation and survivability under antimicrobial selection pressure (Wang et al., 2016), which allows it to develop resistance to these antimicrobials (Chen et al., 2010).
In different countries such as the United States (Tang et al., 2017; Ricotta et al., 2014), China (Li et al., 2017; Wang et al., 2016), Europa (European Food Safety Authority and European Centre for Disease Prevention and Control, 2019, European Food Safety Authority; European Center for Disease Prevention and Control, 2019), and Guatemala (Benoit et al., 2014); the most common resistance pattern was ciprofloxacin, nalidixic acid, and tetracycline, in concordance with our results.
An intermediate classification of different antimicrobials in thermotolerant Campylobacter isolates was detected in this study (Fig. 1). C. coli had more prevalence of isolates with an intermediate classification level of resistance; this is a serious public health concern because the “intermediate” category includes isolates which showed reduced susceptibility to antimicrobials in comparison with susceptible isolates (CLSI, 2010). In this sense, most of the C. coli isolates were susceptible to streptomycin. Interestingly, the prevalence of C. jejuni isolates resistant to streptomycin was higher than C. coli isolates. Many studies have found that C. coli had higher levels of resistance than C. jejuni to streptomycin (Wieczorek et al., 2013; Aarestrup et al., 1997), but a few studies have detected more prevalence in C. jejuni, as has been found in this study (Nguyen et al., 2016).
Molecular mechanisms of resistance were also evaluated, and PCR analysis was used to detect cmeABC genes. While the cmeABC efflux pump is widely distributed in Campylobacter and it is constitutively expressed (Lin et al., 2002; Payot et al., 2002), only 57% of the C. jejuni isolates tested in this study had the three parts of the cmeABC efflux pump. Similar results were found by Olah et al. (2006), and it can be explained by: a) the pump being inactive, having a non-functional role (Olah et al., 2006); or b) the efflux pump genes sequence variation (polymorphism) (Guo et al., 2004).
All isolates of this study which were resistant to ciprofloxacin and enrofloxacin carried a substitution of the amino acid 86 as consequence of a mutation in the gyrA gene, and this result was in agreement with previous reports (El-Adawy et al., 2015; Nguyen et al., 2016; Whitehouse et al., 2018). Interestingly, in two C. jejuni isolates, the gyrA point mutation was detected, but said isolates were not resistant to enrofloxacin. It could be suggested that the Thr-86-Ile substitution may not confer universal resistance to all quinolones as has been previously reported (Dionisi et al., 2004; Corcoran et al., 2005; Bolton et al.2013).
In Campylobacter, resistance to erythromycin is chromosomally encoded by a point mutation of the 23S rRNA gene. In this study, the mutation was detected by MAMA–PCR in resistant and intermediate isolates. Previously, this point mutation has been associated with high levels of erythromycin resistance (Corcoran et al., 2005; Payot et al., 2004; Taylor and Tracz, 2005).
The results of phenotypic and genetic analyses of resistance to tetracycline were fully concordant. All strains resistant to tetracycline were shown to carry the gene tet(O). A correlation study of susceptibility phenotypes and genotypes using WGS has shown that all tetracycline-resistant isolates (n = 108) carried tet(O), but none of the tetracycline-susceptible isolates had this gene (Zhao et al., 2001).
The mechanisms of resistance to some β-lactams such as ampicillin and some of the expanded-spectrum cephalosporins are variable and not very clearly defined (Lachance et al., 1991; Reina et al., 1994; Tajada et al., 1996). The β-lactamase gene blaOXA-61 has spread widely in C. jejuni and C. coli, and the prevalence of the blaOXA-61 gene in ampicillin-resistant Campylobacter can reach up to 91% (Griggs et al., 2009). The C. coli isolates demonstrated to be resistant or intermediate to ampicillin; however, the blaOXA-61 gene was detected in only 57% of them. In C. jejuni, we detected the gene in 73% of resistant isolates. Other types of beta-lactamase were described (Lucain et al., 1985), which could explain these results. Lucain et al. (1985) described four enzymes based on their differing activity against eight B-lactams, relative rates of hydrolysis, molecular weight, immunological specificity, and isoelectric point (pI). However, the roles of beta-lactamases in the mechanism of resistance to ampicillin in campylobacters are not yet clear (Griggs et al., 2009).
However, 83% of C. jejuni susceptible isolates showed the presence of the blaOXA-61 gene. In this sense, Casagrande Proietti et al. (2020) hypothesize that the blaOXA-61 gene was poorly expressed in the ampicillin-sensitive isolates and, therefore, they produced less β-lactamase than resistant isolates.
In conclusion, although the size of the samples analyzed is limited and they come from a single slaughterhouse, this study has revealed that the slaughter process line and the carcasses are often contaminated with thermotolerant Campylobacter, suggesting a possible risk of infection to consumers by improper handling and preparation of poultry meat. Moreover, taking into account the limitations regarding the cut-off points of some of the ATMs used, resistance was detected in most of the antimicrobial agents tested, and many of the Campylobacter isolates showed resistance to three or more antimicrobial groups (MDR). Except for ampicillin, all the resistance molecular mechanisms evaluated were detected and correlated with phenotypic resistance. In Argentina, data on the prevalence of Campylobacter throughout the agry-food chain and the incidence of human campylobacteriosis are uncertain. In addition, only a few studies have evaluated antimicrobial resistance in Campylobacter strains. In 2015, an action plan to optimize the AMR surveillance in Argentina was launched by the National Commission for the Control of Antimicrobial Resistance (CoNaCra), coordinated by the National Directorate of Epidemiology. Thus, this information is essential to establish a public health program to control the disease, and it is fundamental for designing a surveillance program to monitor resistance. Therefore, coordinated actions are recommended to reduce or eliminate the risk of thermotolerant Campylobacter at different stages in the slaughterhouse.
CRediT authorship contribution statement
Mariana E. Schreyer: Investigation, Methodology, Writing – original draft. Carolina R. Olivero: Methodology. Eugenia Rossler: Investigation. Lorena P. Soto: Supervision. Laureano S. Frizzo: Validation, Data curation, Conceptualization. Jorge A. Zimmermann: Investigation. Marcelo L. Signorini: Writing – review & editing, Conceptualization, Formal analysis. Zbrun M. Virginia: Writing – original draft, Writing – review & editing, Conceptualization, Funding acquisition.
Declaration of competing interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests:Zbrun reports financial support was provided by Agencia Nacional de Promoción Científica y Tecnológica. Zbrun reports financial support was provided by National University of the Littoral.
Acknowledgments
The authors would like to thank Dr. Diego Diaz for his advice in order to improve our experimental work. This study was funded by PICT-Joven N°1491/2014 (Agencia Nacional de Promoción Científica y Tecnológica) and CAID-Joven 2016 (Código de proyecto: 50020150100061L, Universidad Nacional del Litoral).
References
- Aarestrup F.M., Nielsen E.M., Madsen M., Engberg J. Antimicrobial susceptibility patterns of thermophilic Campylobacter spp. from humans, pigs, cattle, and broilers in Denmark. Antimicrob. Agents Chemother. 1997;41(10):2244–2250. doi: 10.1128/aac.41.10.2244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alonso R., Mateo E., Churruca E., Martinez I., Girbau C., Fernández-Astorga A. MAMA-PCR assay for the detection of point mutations associated with high-level erythromycin resistance in Campylobacter jejuni and Campylobacter coli strains. J. Microbiol. Methods. 2005;63:99–103. doi: 10.1016/j.mimet.2005.03.013. [DOI] [PubMed] [Google Scholar]
- Benoit S.R., Lopez B., Arvelo W., Henao O., Parsons M.B., Reyes L., Moir J.C., Lindblade K. Burden of laboratory-confirmed Campylobacter infections in Guatemala 2008–2012: results from a facility-based surveillance system. J. Epidemiol. Glob. Health. 2014;4:51–59. doi: 10.1016/j.jegh.2013.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolton D., Patriarchi A., Fox A., Fanning S. A study of the molecular basis of quinolone and macrolide resistance in a selection of Campylobacter isolates from intensive poultry flocks. Food Control. 2013;30(1):222–226. [Google Scholar]
- Bull S.A., Allen V.M., Domingue G., Jorgensen F., Frost J.A., Ure R., Whyte R., Tinker D., Corry J.E., Gillard‐King J., Humphrey T.J. Sources of Campylobacter spp. colonizing housed broiler flocks during rearing. Appl. Environ. Microbiol. 2006;72:645–652. doi: 10.1128/AEM.72.1.645-652.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casagrande Proietti P., Guelfi G., Bellucci S., De Luca S., Di Gregorio S., Pieramati C., Franciosini M.P. Beta-lactam resistance in Campylobacter coli and Campylobacter jejuni chicken isolates and the association between blaOXA-61 gene expression and the action of β-lactamase inhibitors. Vet. Microbiol. 2020;241:4–8. doi: 10.1016/j.vetmic.2019.108553. [DOI] [PubMed] [Google Scholar]
- CDDEP, Center for Disease Dynamics, Economics and Policy . State of the World’s Antibiotics, 2015. CDDEP; Washington, D.C: 2015. [Google Scholar]
- Center for Disease Control and Prevention (CDC) U.S. Department of Health and Human Services, CDC; Atlanta, Georgia: 2014. Foodborne Diseases Active Surveillance Network (FoodNet): FoodNet Surveillance Report for 2014 (Final Report) [Google Scholar]
- Cha W., Mosci R.E., Wengert S.L., Venegas Vargas C., Rust S.R., Bartlett P.C., Grooms D.L., Manning S.D. Comparing the genetic diversity and antimicrobial resistance profiles of Campylobacter jejuni recovered from cattle and humans. Front. Microbiol. 2017;8:818. doi: 10.3389/fmicb.2017.00818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X., Naren G.-W., Wu C.-M., Wang Y., Dai L., Xia L.-N., Luo P.-J., Zhang Q., Shen J.-Z. Prevalence and antimicrobial resistance of Campylobacter isolates in broilers from China. Vet. Microbiol. 2010;144:133–139. doi: 10.1016/j.vetmic.2009.12.035. [DOI] [PubMed] [Google Scholar]
- Chlebicz A., Śliżewska K. Campylobacteriosis, Salmonellosis, Yersiniosis, and Listeriosis as zoonotic foodborne diseases: a review. Int. J. Environ. Res. Publ. Health. 2018;15(5):863. doi: 10.3390/ijerph15050863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CLSI (Clinical and Laboratory Standards Institute) second ed. 2010. Methods for Antimicrobial Dilution and Disk Susceptibility Testing of Infrequently Isolated or Fastidious Bacteria; Approved Guideline. Wayne, PA. [Google Scholar]
- CLSI (Clinical and Laboratory Standards Institute) Twenty-Third Informational supplement; Wayne, PA: 2013. Performance Standards for Antimicrobial Susceptibility Testing. [Google Scholar]
- Corcoran D., Quinn T., Cotter L., O'Halloran F., Fanning S. Characterization of a cmeABC operon in a quinolone-resistant Campylobacter coli isolate of Irish origin. Microb. Drug Resist. 2005;11(4):303–308. doi: 10.1089/mdr.2005.11.303. [DOI] [PubMed] [Google Scholar]
- Damjanova I., Jakab M., Farkas T., Mészáros J., Galántai Z., Turcsányi I., Bistyák A., Juhász Á., Pászti J., Kiss I., Kardos G. From farm to fork follow-up of thermotolerant Campylobacters throughout the broiler production chain and in human cases in a Hungarian county during a ten-months period. Int. J. Food Microbiol. 2011;150:95–102. doi: 10.1016/j.ijfoodmicro.2011.07.011. [DOI] [PubMed] [Google Scholar]
- Dionisi A.M., Luzzi I., Carattoli A. Identification of ciprofloxacin-resistant Campylobacter jejuni and analysis of the gyrA gene by the LightCycler mutation assay. Mol. Cell. Probes. 2004;18(4):255–261. doi: 10.1016/j.mcp.2004.02.001. [DOI] [PubMed] [Google Scholar]
- European Food Safety Authority(EFSA) and European Centre for Disease Prevention and Control (ECDC) The European Union summary report on trends and sources of zoonoses, zoonotic agents and food-borne outbreaks in 2015. EFSA J. 2016;14(12):231. doi: 10.2903/j.efsa.2016.4634. 2016. 4634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- European Food Safety Authority(EFSA) and European Centre for Disease Prevention and Control (ECDC) The European union one health 2018 zoonoses report. EFSA J. 2019;17 doi: 10.2903/j.efsa.2019.5926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- European Food Safety Authority; European Center for Disease Prevention and Control The European Union summary report on antimicrobial resistance in zoonotic and indicator bacteria from humans, animals and food in 2017. EFSA J. 2019;17:5598. doi: 10.2903/j.efsa.2019.5598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Adawy H., Ahmed M.F., Hotzel H., Tomaso H., Tenhagen B.A., Hartung J., Neubauer H., Hafez H.M. Antimicrobial susceptibilities of Campylobacter jejuni and Campylobacter coli recovered from organic Turkey farms in Germany. Poultry Sci. 2015;94(11):2831–2837. doi: 10.3382/ps/pev259. [DOI] [PubMed] [Google Scholar]
- Fakhr M.K., Logue C.M. Sequence variation in the outer membrane protein-encoding gene cmeC conferring multidrug resistance among Campylobacter jejuni and Campylobacter coli strains isolated from different hosts. J. Food Clin. Microbiol. 2007;45:3381–3383. doi: 10.1128/JCM.01208-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- García-Sánchez L., Melero B., Rovira J. vol. 86. 2018. Campylobacter in the food chain; pp. 215–252. (Advances in Food and Nutrition Research). [DOI] [PubMed] [Google Scholar]
- García Sánchez L., Melero B., Jaime I., Rossi M., Ortega I., Rovira J. Biofilm formation, virulence and antimicrobial resistance of different Campylobacter jejuni isolates from a poultry slaughterhouse. Food Microbiol. 2019;83:193–199. doi: 10.1016/j.fm.2019.05.016. [DOI] [PubMed] [Google Scholar]
- Gibreel A., Tracz D.M., Nonaka L., Ngo T.M., Connell S.R., Taylor D.E. Incidence of antibiotic resistance in Campylobacter jejuni isolated in Alberta, Canada, from 1999 to 2002, with special reference to tet(O)-mediated tetracycline resistance. Antimicrob. Agents Chemother. 2004;48(9):3442–3450. doi: 10.1128/AAC.48.9.3442-3450.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griggs D.J., Peake L., Johnson M.M., Ghori S., Mott A., Piddock L.J.V. β-lactamase-mediated β-lactam resistance in Campylobacter species: prevalence of Cj0299 (blaOXA-61) and evidence for a novel β-lactamase in C. jejuni. Antimicrob. Agents Chemother. 2009;53:3357–3364. doi: 10.1128/AAC.01655-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo B., Lin J., Reynolds D.L., Zhang Q. Abstract and Presentation North Central Avian Diseases Con- ference; Ames, IA: 2004. Distribution, Genomic Organization, and Sequence Polymorphism of Multidrug Efflux Pump CmeABC Among Different Campylobacter Species. Oct 3-5. [Google Scholar]
- Hariharan H., Sharma S., Chikweto A., Matthew V., DeAllie C. Antimicrobial drug resistance as determined by the E-test in Campylobacter jejuni, C. coli, and C. lari isolates from the ceca of broiler and layer chickens in Grenada. Comp. Immunol. Microbiol. Infect. Dis. 2009;32(1):21–28. doi: 10.1016/j.cimid.2008.01.010. [DOI] [PubMed] [Google Scholar]
- Henry I., Reichardt J., Denis M., Cardinale E. Prevalence and risk factors for Campylobacter spp. in chicken broiler flocks in Reunion Island (Indian Ocean) Prev. Vet. Med. 2011;100:64–70. doi: 10.1016/j.prevetmed.2011.03.007. [DOI] [PubMed] [Google Scholar]
- Iovine N.M. Resistance mechanisms in Campylobacter jejuni. Virulence. 2013;4:230–240. doi: 10.4161/viru.23753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen L.B., Aarestrup F.M. Macrolide resistance in Campylobacter coli of animal origin in Denmark. Antimicrob. Agents Chemother. 2001;45:371–372. doi: 10.1128/AAC.45.1.371-372.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaakoush N.O., Castaño-Rodríguez N., Mitchell H.M., Man S.M. Global epidemiology of Campylobacter infection. Clin. Microbiol. Rev. 2015;28:687–720. doi: 10.1128/CMR.00006-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koolman L., Whyte P., Burgess C. vol. 12. 2015. pp. 424–432. (Distribution of Virulence-Associated Genes in a Selection of Campylobacter Isolates). [DOI] [PubMed] [Google Scholar]
- Lachance N., Gaudreau C., Lamothe F., Lariviere L.A. Role of the β-lactamase of Campylobacter jejuni in resistance to β-lactam agents. Antimicrob. Agents Chemother. 1991;35:813–818. doi: 10.1128/aac.35.5.813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lajhar S.A., Jennison A.V., Patel B., Duffy L.L. Comparison of epidemiologically linked Campylobacter jejuni isolated from human and poultry sources. Epidemiol. Infect. 2015;143(16):3498–3509. doi: 10.1017/S0950268815000886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamas A., Regal P., Vázquez B., Miranda J.M., Cepeda A., Franco C.M. Salmonella and Campylobacter biofilm formation: a comparative assessment from farm to fork. J. Sci. Food Agric. 2018;98(11):4014–4032. doi: 10.1002/jsfa.8945. [DOI] [PubMed] [Google Scholar]
- Lazovski J., Corso A., Pasteran F., Monsalvo M., Frenkel J., Cornistein W., et al. Estrategia de control de la resistencia bacteriana a los antimicrobianos en Argentina. Rev. Panam. Salud Públic. 2017;41 doi: 10.26633/RPSP.2017.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li B., Ma L., Li Y., Jia H., Wei J., Shao D., Liu K., Shi Y., Qiu Y., Ma Z. Antimicrobial resistance of Campylobacter species isolated from broilers in live bird markets in Shanghai, China. Foodb. Pathog. Dis. 2017;14:96–102. doi: 10.1089/fpd.2016.2186. [DOI] [PubMed] [Google Scholar]
- Lin J., Michel L.O., Zhang Q. CmeABC functions as a multidrug efflux system in Campylobacter jejuni. Antimicrob. Agents Chemother. 2002;46:2124–2131. doi: 10.1128/AAC.46.7.2124-2131.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucain C., Goossens H., Pechere J.C. In: Campylobacter III. Pearson A.D., Skirrow M.B., Lior H., Rowe B., editors. Public Health Laboratory Service; London, United Kingdom: 1985. Beta-lactamases in Campylobacter jejuni, abstr. 005; pp. 36–37. [Google Scholar]
- Ma L., Wang Y., Shen J., Zhang Q., Wu C. Tracking Campylobacter contamination along a broiler chicken production chain from the farm level to retail in China. Int. J. Food Microbiol. 2014;181:77–84. doi: 10.1016/j.ijfoodmicro.2014.04.023. [DOI] [PubMed] [Google Scholar]
- MAGYP (Ministerio de Agricultura y pesca, Argentina) 2020. Anuario Avícola 2020 – Año XXV N° 83; p. 25. [Google Scholar]
- Natsos G., Koutoulis K.C., Sossidou E., Chemaly M., Mouttotou N.K. Campylobacter spp. infection in humans and poultry. J. Hell. Vet. Med. Soc. 2016;67(2):65–82. [Google Scholar]
- Nelson J.M., Chiller T.M., Powers J.H., Angulo F.J. Fluoroquinolone-resistant Campylobacter species and the withdrawal of fluoroquinolones from use in poultry: a public health success story. Clin. Infect. Dis.: Off. Publ. Infect. Dis. Soc. Am. 2007;44(7):977–980. doi: 10.1086/512369. [DOI] [PubMed] [Google Scholar]
- Newell D.G., Wagenaar J.A. In: Campylobacter. second ed. Nachamkin I., Blaser M.J., editors. ASM Press; Washington, DC: 2000. Poultry infections and their control at the farm level; pp. 497–509. [Google Scholar]
- Nguyen T.N.M., Hotzel H., El-Adawy H., Tran H.T., Le M.T.H., Tomaso H., Neubauer H., Hafez H.M. Genotyping and antibiotic resistance of thermophilic Campylobacter isolated from chicken and pig meat in Vietnam. Gut Pathog. 2016;8:19. doi: 10.1186/s13099-016-0100-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obeng A.S., Rickard H., Sexton M., Pang Y., Peng H., Barton M. Antimicrobial susceptibilities and resistance genes in Campylobacter strains isolated from poultry and pigs in Australia. J. Appl. Microbiol. 2012;113:294–307. doi: 10.1111/j.1365-2672.2012.05354.x. [DOI] [PubMed] [Google Scholar]
- Olah P.A., Doetkott C., Fakhr M.K., Logue C.M. Prevalence of the Campylobacter multi-drug efflux pump (CmeABC) in Campylobacter spp. Isolated from freshly processed Turkeys. Food Microbiol. 2006;23:453–460. doi: 10.1016/j.fm.2005.06.004. [DOI] [PubMed] [Google Scholar]
- Ono K., Yamamoto K. Contamination of meat with Campylobacter jejuni in Saitama, Japan. Int. J. Food Microbiol. 1999;47:211–219. doi: 10.1016/s0168-1605(99)00015-x. [DOI] [PubMed] [Google Scholar]
- Osimani A., Aquilanti L., Pasquini M., Clementi F. Prevalence and risk factors for thermotolerant species of Campylobacter in poultry meat at retail in Europe. Poultry Sci. 2017;96(9):3382–3391. doi: 10.3382/ps/pex143. [DOI] [PubMed] [Google Scholar]
- Pantozzi F.L., Moredo F.A., Vigo G.B., Giacoboni G.I. Resistencia a los antimicrobianos en bacterias indicadoras y zoonóticas aisladas de animales domésticos en Argentina. Rev. Argent. Microbiol. 2010;42:49–52. doi: 10.1590/S0325-75412010000100011. [DOI] [PubMed] [Google Scholar]
- Payot S., Cloeckaert A., Chaslus-dancla E. Selection and characterization of fluoroquinolone-resistant mutants of Campylobacter jejuni using enrofloxacin. Microb. Drug Resist. 2002;8:335–343. doi: 10.1089/10766290260469606. [DOI] [PubMed] [Google Scholar]
- Payot S., Avrain L., Magras C., Praud K., Cloeckaert A., Chaslus-Dancla E. Relative contribution of target gene mutation and efflux to fluo- roquinolone and erythromycin resistance, in French poultry and pig isolates of Campylobacter coli. Int. J. Antimicrob. Agents. 2004;23:468–472. doi: 10.1016/j.ijantimicag.2003.12.008. [DOI] [PubMed] [Google Scholar]
- Pedersen S.K., Wagenaar J.A., Vigre H., Roer L., Mikoleit M., Aidara-Kane A., Hendriksen R.S. Proficiency of WHO global foodborne infections network external quality assurance system participants in identification and susceptibility testing of thermotolerant Campylobacter spp. from 2003 to 2012. J. Clin. Microbiol. 2018;56(11) doi: 10.1128/JCM.01066-18. 01066-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peyrat M.B., Soumet C., Maris P., Sanders P. Recovery of Campylobacter jejuni from surfaces of poultry slaughterhouses after cleaning and disinfection procedures: analysis of a potential source of carcass contamination. Int. J. Food Microbiol. 2008;124(2):188–194. doi: 10.1016/j.ijfoodmicro.2008.03.030. [DOI] [PubMed] [Google Scholar]
- Radostits O.M., Rubinstein E. The therapeutic use of fluoroquinolones in poultry: the effect on Campylobacter and the potential human health consequences. Int. J. Infect. Dis. 2002;6(Suppl. 3):S49–S52. doi: 10.1016/s1201-9712(02)90184-0. [DOI] [PubMed] [Google Scholar]
- Reina J., Ros M.J., Serra A. Susceptibilities to 10 antimicrobial agents of 1,220 Campylobacter strains isolated from 1987 to 1993 from feces of pediatric patients. Antimicrob. Agents Chemother. 1994;38:2917–2920. doi: 10.1128/aac.38.12.2917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricotta E.E., Palmer A., Wymore K., Clogher P., Oosmanally N., Robinson T., Lathrop S., Karr J., Hatch J., Dunn J., et al. Epidemiology and antimicrobial resistance of international travel-associated Campylobacter infections in the United States, 2005–2011. Am. J. Publ. Health. 2014;104 doi: 10.2105/AJPH.2013.301867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossler E., Signorini M.L., Romero-Scharpen A., Soto L.P., Berisvil A., Zimmermann J.A., Fusari M.L., Olivero C., Zbrun M.V., Frizzo L.S. Meta-analysis of the prevalence of thermotolerant Campylobacter in food-producing animals worldwide. Zoonoses Publ. Health. 2019;66(4):359–369. doi: 10.1111/zph.12558. [DOI] [PubMed] [Google Scholar]
- Sahin O., Morishita T., Zhang Q. Campylobacter colonization in poultry: sources of infection and modes of transmission. Anim. Health Res. Rev. 2002;3:95–105. doi: 10.1079/ahrr200244. [DOI] [PubMed] [Google Scholar]
- Signorini M.L., Rossler E., Díaz David D.C., Olivero C.R., Romero-Scharpen A., Soto L.P., Astesana D.M., Berisvil A.P., Zimmermann J.A., Fusari M.L., Frizzo L.S., Zbrun M.V. Antimicrobial resistance of thermotolerant Campylobacter species isolated from humans, food-producing animals, and products of animal origin: a worldwide meta-analysis. Microb. Drug Resist. 2018;24(8):1174–1190. doi: 10.1089/mdr.2017.0310. [DOI] [PubMed] [Google Scholar]
- Stern N.J., Eruslanov B.V., Pokhilenko V.D., Kovalev Y.N., Volodina L.L., Perelygin V.V., Mitsevich E.V., Mitsevich I.P., Borzenkov V.N., Levchuk V.P., Svetoch O.E., Stepanshin Y.G., Svetoch E.A. Bacteriocins reduce Campylobacter jejuni colonization while bacteria producing bacteriocins are ineffective. Microb. Ecol. Health Dis. 2008;20:74–79. [Google Scholar]
- Taylor D.E., Tracz D.M. In: Campylobacter: Molecular and Cellular Biology. Ketley J.M., Konkel M.E., editors. Horizon Bioscience; Norfolk, UK: 2005. Mechanisms of antimicrobial resistance in Campylobacter. 193e204. [Google Scholar]
- Tajada P., Gomez-Graces J.L., Alos J.I., Balas D., Cogollos R. Antimicrobial susceptibilities of Campylobacter jejuni and Campylobacter coli to 12 betalactam agents and combinations with beta-lactamase inhibitors. Antimicrob. Agents Chemother. 1996;40:1924–1925. doi: 10.1128/aac.40.8.1924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamborini A.I., Casabona L.M., Viñas M.R., Asato V., Hoffer A., Farace M.I., Lucero M.C., Corso A., Pichel M. Campylobacter spp.: prevalencia y caracterización feno-genotípica de aislamientos de pacientes con diarrea y de sus mascotas en la provincia de La Pampa, Argentina. Rev. Argent. Microbiol. 2012;44:266–271. [PubMed] [Google Scholar]
- Tang Y., Sahin O., Pavlovic N., Lejeune J., Carlson J., Wu Z. Rising fluoroquinolone resistance in Campylobacter isolated from feedlot cattle in the United States. Sci. Rep. 2017:1–8. doi: 10.1038/s41598-017-00584-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unicomb L.E., Ferguson J., Stafford R.J., Ashbolt R., Kirk M.D., Becker N.G., Patel M.S., Gilbert G.L., Valcanis M., Mickan L., Australian Campylobacter Subtyping Study Group Low-level fluoroquinolone resistance among Campylobacter jejuni isolates in Australia. Clin. Infect. Dis.: Off. Publ. Infect. Dis. Soc. Am. 2006;42(10):1368–1374. doi: 10.1086/503426. [DOI] [PubMed] [Google Scholar]
- Vandamme P., Van Doorn L.J., Al Rashid S.T., Quint W.G.V., Van Der Plas J., Chan V.L., On S.L. Campylobacter hyoilei Alderton et al. 1995 and Campylobacter coli Veron and Chatelain 1973 are subjective synonyms. Int. J. Syst. Evol. Microbiol. 1997;47(4):1055–1060. doi: 10.1099/00207713-47-4-1055. [DOI] [PubMed] [Google Scholar]
- Ventola C.L. The antibiotic resistance crisis: part 1: causes and threats. Pharmacol. Ther. 2015;40:277–283. [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Dong Y., Deng F., Liu D., Yao H., Zhang Q., Shen J., Liu Z., Gao Y., Wu C., Shen Z. Species shift and multidrug resistance of Campylobacter from chicken and swine, China, 2008–14. J. Antimicrob. Chemother. 2016;71:666–669. doi: 10.1093/jac/dkv382. [DOI] [PubMed] [Google Scholar]
- Whitehouse C.A., Zhao S., Tate H. Advances in Applied Microbiology. Elsevier Inc.; 2018. Antimicrobial resistance in Campylobacter species: mechanisms and genomic epidemiology; pp. 1–47. [DOI] [PubMed] [Google Scholar]
- World Health Organization (WHO) Tackling antibiotic resistance from a food safety perspective in Europe. World Heal. Organ. 2011;1–88 [Google Scholar]
- World Health Organization (WHO) Foodborne Disease Burden Epidemiology Reference Group 2007–2015. World Health Organization; Geneva, Switzerland: 2017. WHO estimates of the global burden of foodborne diseases. 2015. [Google Scholar]
- Whyte P., Fanning S., Brien S.O., Grady L.O., Solomon K. Elsevier; 2011. Tracing Pathogens in the Food Chain. [Google Scholar]
- Wieczorek K., Dykes G.a., Osek J., Duffy L.L. Antimicrobial resistance and genetic characterization of Campylobacter spp. from three countries. Food Control. 2013;34:84–91. [Google Scholar]
- Zaidi M.B., Calva J.J., Estrada-García M.T., Leon V., Vazquez G., Figueroa G., et al. Integrated food chain surveillance system for Salmonella spp. in Mexico. Emerg. Infect. Dis. 2008;14:429–435. doi: 10.3201/eid1403.071057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zbrun M.V., Olivero C., Romero-Scharpen A., Rossler E., Soto L.P., Astesana D.M., Blajman J.E., Berisvil A., Signorini M.L., Frizzo L.S. Antimicrobial resistance in thermotolerant Campylobacter isolated from different stages of the poultry meat supply chain in Argentina. Food Control. 2015;57:136–141. [Google Scholar]
- Zbrun M.V., Romero-Scharpen A., Olivero C., Rossler E., Soto L.P., Rosmini M.R., Sequeira G.J., Signorini M.L., Frizzo L.S. Occurrence of thermotolerant Campylobacter spp. at different stages of the poultry meat supply chain in Argentina. N. Z. Vet. J. 2013;61:337–343. doi: 10.1080/00480169.2013.817294. [DOI] [PubMed] [Google Scholar]
- Zbrun M.V., Romero-Scharpen A., Olivero C., Zimmermann J.A., Rossler E., Soto L.P., Astesana D.M., Blajman J.E., Berisvil A., Frizzo L.S., Signorini M.L. Genetic diversity of thermotolerant Campylobacter spp. isolates from different stages of the poultry meat supply chain in Argentina. Rev. Argent. Microbiol. 2017;49:235–241. doi: 10.1016/j.ram.2017.03.003. [DOI] [PubMed] [Google Scholar]
- Zhang Q., Al-Ghalith G.A., Kobayashi M., Segawa T., Maeda M., Okabe S., Knights D., Ishii S. High-throughput flaA Short variable region sequencing to assess Campylobacter diversity in fecal samples from birds. Front. Microbiol. 2018;9:2201. doi: 10.3389/fmicb.2018.02201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao C., Ge B., De Villena J., Sudler R., Yeh E., Zhao S., White D.G., Wagner D., Meng J. Prevalence of Campylobacter spp., Escherichia coli, and Salmonella serovars in retail chicken, Turkey, pork, and beef from the greater Washington, DC, area. Appl. Environ. Microbiol. 2001;67(12):5431–5436. doi: 10.1128/AEM.67.12.5431-5436.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zirnstein G., Helsel L., Li Y., Swaminathan B., Besser J. Characterization of gyrA mutations associated with fluoroquinolone resistance in Campylobacter coli by DNA sequence analysis and MAMA PCR. FEMS (Fed. Eur. Microbiol. Soc.) Microbiol. Lett. 2000;190:1–7. doi: 10.1111/j.1574-6968.2000.tb09253.x. [DOI] [PubMed] [Google Scholar]
- Zirnstein G., Li Y.U., Swaminathan B., Angulo F. Ciprofloxacin resistance in Campylobacter jejuni isolates: detection of gyrA resistance mutations by mismatch amplification mutation assay PCR and DNA sequence analysis. J. Clin. Microbiol. 1999;37:3276–3280. doi: 10.1128/jcm.37.10.3276-3280.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]