Abstract
Hydrogels are of interest in cartilage tissue engineering due to their ability to support the encapsulation and chondrogenesis of mesenchymal stromal cells (MSCs). However, features such as hydrogel crosslink density, which can influence nutrient transport, nascent matrix distribution, and the stability of constructs during and after implantation must be considered in hydrogel design. Here, we first demonstrate that more loosely crosslinked (i.e., softer, ~2 kPa) norbornene-modified hyaluronic acid (NorHA) hydrogels support enhanced cartilage formation and maturation when compared to more densely crosslinked (i.e., stiffer, ~6–60 kPa) hydrogels, with a >100-fold increase in compressive modulus after 56 days of culture. While soft NorHA hydrogels mature into neocartilage suitable for the repair of articular cartilage, their initial moduli are too low for handling and they do not exhibit the requisite stability needed to withstand the loading environments of articulating joints. To address this, we reinforced NorHA hydrogels with polycaprolactone (PCL) microfibers produced via melt-electrowriting (MEW). Importantly, composites fabricated with MEW meshes of 400 μm spacing increased the moduli of soft NorHA hydrogels by ~50-fold while preserving the chondrogenic potential of the hydrogels. There were minimal differences in chondrogenic gene expression and biochemical content (e.g., DNA, GAG, collagen) between hydrogels alone and composites, whereas the composites increased in compressive modulus to ~350 kPa after 56 days of culture. Lastly, integration of composites with native tissue was assessed ex vivo; MSC-laden composites implanted after 28 days of pre-culture exhibited increased integration strengths and contact areas compared to acellular composites. This approach has great potential towards the design of cell-laden implants that possess both initial mechanical integrity and the ability to support neocartilage formation and integration for cartilage repair.
Keywords: melt-electrowriting, hydrogel, cartilage, interfacial strength, tissue integration
1. Introduction
Articular cartilage damage is a pervasive problem that significantly inhibits quality of life and joint mobility in afflicted patients [1]. Focal defects on the articulating surface of joints may form in patients due to trauma, sports injuries, or daily activities associated with joint function [2]. Native cartilage unfortunately does not possess significant regenerative capacity [3], and these defects may further progress if left untreated, resulting in significant pain and dysfunction [4]. To this end, a number of clinical approaches have been developed for cartilage defect repair, including microfracture, mosaicplasty, and matrix-assisted chondrocyte implantation (MACI) [5]. However, despite their promise, these surgical procedures often result in repair cartilage with inferior composition and mechanical properties when compared to healthy hyaline cartilage [1,6,7]. Thus, there is a continued and significant clinical need for the development of new approaches that support the restoration of functional cartilage.
Hydrogels have emerged as a promising approach for the encapsulation of cells that then synthesize and organize nascent cartilagenous extracellular matrix. A range of materials have been used for the formation of neocartilage from cell-laden hydrogels [8], and advancements in both hydrogel processing and our ability to incorporate physiochemical cues within hydrogels (e.g., patterning of singaling ligands, controlled release of biochemical signals) have improved the quality of engineered cartilage in vitro [9]. Towards translating these hydrogels into the clinic, biofabrication approaches have enabled the fabrication of cell-laden hydrogels with patient-specific geometries and high porosity. For instance, the biopen is a handheld device that permits extrusion of bioinks into focal cartilage defects intraoperatively, such that cartilage repair can occur in situ within defects [10,11]. Other extrusion-based bioprinting techniques have facilitated the expansion of candidate bioinks for cartilage tissue engineering [12], while lithographic and new tomographic bioprinting approaches have drastically improved the resolution and throughput with which cell-laden implants can be engineered [13,14]. Despite these recent advances in bioprinting, one of the persistent challenges associated with engineering hydrogels for cartilage tissue engineering is the balance of two, opposing design criteria. Specifically, hydrogels with large mesh sizes are promising candidates given their ability to maintain cell viability and to promote the distribution of deposited matrix, but these hydrogels have much lower initial mechanical properties [15,16].
Hydrogels with tunable degradability have been engineered to address this challenge, such that higher initial mechanical properties can be achieved while cell-mediated enzymatic degradation ensures that the mesh size increases over time, permitting matrix distribution and cartilage maturation [17]. Similarly, hydrolytically degradable polyethylene glycol (PEG) and hyaluronic acid (HA) hydrogels were designed to improve matrix production and distribution by encapsulated chondrocytes and mesenchymal stromal cells (MSCs), respectively, when compared to non-degradable hydrogel controls [18,19]. However, these approaches are generally still limited with regards to initial hydrogel mechanics due to cell viability concerns and they also require that the rate of hydrogel degradation be carefully balanced with the rate of tissue formation and maturation to maintain mechanical properties [20].
Alternatively, a range of strategies have been employed to enhance the mechanical properties of hydrogels for cartilage repair. Interpenetrating network (IPN) hydrogels, which are composed of multiple interdigitating networks, are one approach to engineering hydrogels with high toughness. By tuning the properties of combined brittle and ductile networks at the molecular scale, non-additive increases in hydrogel moduli can be achieved [21]. As an alternative, extruded polycaprolactone (PCL) may be incorporated within 3D printed hydrogels (e.g., fibrin-collagen, alginate, agarose, PEG) containing encapsulated chondrocytes or MSCs for cartilage formation [22–26]. PCL is a well-established biomaterial with extended degradation profiles and significantly higher moduli than traditional hydrogels, such that its combination with hydrogels results in improved mechanical integrity. To this end, electrospun nanofibrous PCL scaffolds have also been incorporated into bioprinted hydrogels to improve both the shape fidelity and mechanical properties of fabricated constructs [27]. In another approach, IPNs composed of alginate and methacryloyl-modified gelatin (GelMA) were reinforced with 3D printed PCL templates towards recapitulating the tension-compression non-linearity of native cartilage [28,29]. A multi-head printing setup enabled fabrication of these composites with encapsulated MSCs and chondrocytes toward the formation of hyaline cartilage [28]. However, while IPNs or composite scaffolds containing PCL may improve the mechanical properties of cell-laden hydrogels, these approaches can also reduce the relative volume available for the formation of new tissue by embedded cells [30].
In response to this design limitation, reinforcement of printed GELMA hydrogels with PCL microfibers has been achieved via melt-electrowriting (MEW) [31,32]. MEW is a biofabrication process that allows for the controlled deposition of electrically charged polymer melt fibers in a layer-by-layer manner [33]. Similar to conventional electrospinning, a voltage source is applied to a polymer to extract the material from a spinneret onto a collector. However, unlike electrospinning, where large distances between the spinneret and collector typically lead to whipping instabilities and unpredictable flow behaviors, MEW permits control over a stable polymer jet. The high viscosity of the polymer melt, along with a reduced spinneret-to-collector distance and the applied voltage source helps to stabilize the flow of polymer melt so that it may be predictably and directly written onto a computer-controlled collector. After controlled deposition, the rapid cooling of the polymer melt gives rise to a stable, fiber structure. Thus, the advantage of MEW over electrospinning is its ability to finely control the organization of polymer melt fibers to fabricate user-defined geometries. Moreover, highly porous, microfiber meshes can be printed via MEW at even submicron resolutions that are not possible via traditional extrusion 3D printing [34].
Hyaluronic acid (HA)-based hydrogels are a specific class of hydrogels that have been shown to support the chondrogenesis of MSCs, but exhibit the aforementioned limitations with significantly inferior mechanical properties when compared to native cartilage [35]. In consideration of advances in the biofabrication field, the overall aim of this study was to introduce MEW reinforcement into engineered HA hydrogels to meet desired design criteria for cartilage repair. To do this, we first screened formulations of norbornene-modified HA (NorHA) across varied crosslinking densities to identify a hydrogel formulation that would be most permissive to the formation of neocartilage. Next, MEW meshes were introduced into NorHA hydrogels to increase the initial mechanical properties and stability of these soft hydrogels [31]. Last, composites of NorHA and MEW meshes were assessed for their integration potential with native cartilage rings. Acellular composites, cell-laden composites, and pre-cultured cell-laden composites were press-fit into cartilage rings, and their integration within rings was compared to autologous cartilage controls. These studies collectively demonstrate that NorHA-MEW composites maximize the chondrogenesis of encapsulated MSCs while increasing the mechanical properties of hydrogels, both initially and over extended culture periods, suggesting that composites may improve in vivo integration and cartilage formation in future studies.
2. Materials and Methods
2.1. Materials
Sodium HA was purchased from Lifecore Biomedical (Chaska, MN) and lithium phenyl-2,4,6-trimethylbenzoylphosphinate (LAP) was purchased from Colorado Photopolymer Solutions (Boulder, CO). Unless otherwise specified, all other reagents and materials were purchased from Sigma-Aldrich (St. Louis, MO).
2.2. Hydrogel Fabrication and Characterization
2.2.1. NorHA Synthesis
NorHA was synthesized as previously reported [36]. Briefly, sodium HA was first converted into its tetrabutylammonium salt form (HA-TBA) and then modified with norbornene functional groups via benzotriazole-1-yl-oxy-tris-(dimethylamino)-phosphonium hexafluorophosphate (BOP) coupling. After dissolving sodium HA in distilled water, Dowex 50Wx200 resin was added to the solution in a 3:1 mass ratio. The solution was then mixed for 30 minutes, and Dowex resin was subsequently removed via vacuum filtration. Thereafter, the filtrate was titrated with tetrabutylammonium hydroxide solution to a pH of 7.02–7.05, frozen, and lyophilized. The resulting lyophilized HA-TBA and 5-norbornene-2-methylamine were then dissolved in anhydrous dimethyl sulfoxide (DMSO) under inert nitrogen. BOP was then added to the reaction solution via cannulation and the reaction proceeded for 2 hours at room temperature. The reaction was quenched with the addition of cold distilled water and subsequently dialyzed for 5 days. Any precipitates within the crude product solution were then removed via filtration and the solution was dialyzed for an additional 3–5 days. After freezing and lyophilizing the synthesized NorHA, the extent of norbornene modification was determined via 1H-NMR to be ~22% of the disaccharide repeat units of HA (figure S1).
2.2.2. Hydrogel Fabrication
Lyophilized NorHA macromer was dissolved in phosphate buffered saline (PBS) and LAP photoinitiator was added to a final concentration of 0.05%. DL-dithiothreitol (DTT) was subsequently added at concentrations of 0.54 mM, 2.17 mM, 5.71 mM, or 13.58 mM (to obtain compressive moduli of approximately 2, 6, 20, and 60 kPa, respectively). After all precursor materials were thoroughly mixed, hydrogels were cast into molds (diameter ~4 mm, thickness ~1 mm) and irradiated with blue light (400–500 nm, Omnicure lamp with an affixed collimator, I=10 mW/cm2) for 5 minutes.
2.2.3. Compression Testing
To evaluate the compressive properties of hydrogels, samples were subjected to unconfined, uniaxial compressive testing with a constant loading rate of 0.2 N/min (Q800 DMA, TA Instruments). The compressive modulus was then quantified as the slope of the stress-strain curves between 10–20% strain.
2.3. Cell Culture and Characterization of MSC-laden Constructs
2.3.1. Cell/Tissue Isolation and Culture
Juvenile bovine knee joints were obtained (Research 87, Boylston, MA) and dissected under sterile conditions as previously described [16]. Femoral bone marrow was extracted and MSCs were isolated via plastic adherence during culture in Dulbecco’s modified eagle medium (DMEM) supplemented with 10% fetal bovine serum (FBS) and 1% penicillin/streptomycin (P/S). After expansion, MSCs were washed, trypsinized (0.05%), centrifuged, and resuspended in PBS for use. NorHA macromer solution with sterile filtered LAP and DTT was prepared as described above prior to the suspension and encapsulation of MSCs (P1, 20 × 106 cells/mL) with blue light exposure. Constructs (~15 μL gel volume, ~3×105 cells per construct) were subsequently cultured in chondrogenic media (1% ITS+; 2.50 μg/mL amphotericin B; 1 × 10−3 M sodium pyruvate; 50 μg/mL ascorbic acid 2-phosphate; 40 μg/mL L-proline; 1 × 10−7 M dexamethasone; 10 ng/mL TGF-β3) for up to 56 days.
2.3.2. Cell Viability
To evaluate the cytocompatibility of constructs, hydrogels were stained with calcein AM and ethidium homodimer (0, 3, 7 days) in accordance with manufacturer’s instructions (Invitrogen). Cell viability was quantified via analysis of confocal images (Leica SP5) using Image J software. Viability was calculated as the number of live cells per total cells within an image (n ≥ 3 hydrogels, 9 images per sample).
2.3.3. Gene Expression Analysis and Biochemical Assays
Each sample was immediately placed in 1 mL ice-cold TRIzol (Invitrogen) and stored at −80°C for later RNA isolation. Pre-processing of samples was performed by first homogenizing samples in TRIzol on ice, subsequently adding 0.2 mL of chloroform, vigorously shaking by hand for 15 seconds, and centrifuging for 15 minutes at 4°C. RNA was then isolated by collecting and mixing the aqueous layer with equal-parts 70% ethanol via pipetting and proceeding with the RNeasy Mini kit (QIAGEN) per manufacturer’s instructions; isolated RNA concentrations were then quantified (NanoDrop 1000). RNA was processed with DNase to remove any DNA impurities and then reverse-transcribed into cDNA using the High Capacity cDNA Reverse Transcription kit (Applied Biosystems). qRT-PCR reactions were performed with 10ng cDNA and Taqman probes (Life Technologies, table S1); type I collagen (Col1a1), type II collagen (Col2a1), aggrecan (ACAN), and SOX9 were selected as targets, with glyceraldehyde 3-phosphate dehydrogenase (GAPDH) used as a housekeeping gene. Relative gene expression of experimental samples was determined using the ΔΔCT method and MSCs expanded on tissue culture plastic as the control [37].
To quantify the biochemical content of cell-laden constructs, samples were minced and digested via overnight incubation at 60°C in solution containing papain and hyaluronidase (0.56 U/mL papain and 750–3000 U/mL hyaluronidase were dissolved in buffer containing 0.1 M sodium acetate, 10 M cysteine hydrochloric acid, and 0.05 M ethylenediaminetetraacetic acid). The dimethylmethylene blue assay was utilized to quantify the sulfated glycosaminoglycan (sGAG) content, the hydroxyproline (OHP) assay was performed to determine collagen content (Abcam Hydroxyproline Assay Kit, ab222941), and the Picogreen dsDNA assay was performed to measure total DNA content within cultured constructs [38].
2.3.4. Histology and Immunohistochemistry
After culture, constructs were fixed in 10% formalin for two hours at room temperature and then washed in PBS. Samples were then dehydrated, embedded in paraffin, and sectioned (5 μm) prior to staining. Sulfated glycosaminoglycan (sGAG) deposition by embedded cells was visualized via Alcian blue staining (1%, pH 1.0, Newcomer Supply), while deposition of type I and type II collagen were visualized via labeling with anti-collagen type I (COL I, mouse monoclonal anti-collagen type 1, Millipore Sigma) and anti-collagen type II (COL II, mouse monoclonal anti-collagen type II, Developmental Studies Hybridoma Bank) antibodies and staining with DAB chromogen (Millipore Sigma). To quantify staining intensity, acquired images were converted to 8-bit and then inverted [39]. For each sample section, the mean intensities for three separate and randomly selected frames were measured in Image J.
2.4. Composite Fabrication and Characterization
2.4.1. Melt-electrowriting of PCL Meshes
Box-structured meshes (4 × 4 cm2) composed of polycaprolactone (Purasorb PC 12, Corbion Inc., Gorinchem, Netherlands) were fabricated with 70 layers (1 mm height) of overlaying fibers (layered in orthogonal directions) as previously described [40]. A custom-built MEW device equipped with an electrical heating system (TR 400, HKEtec, Germany; heating temperature = 90 °C) was used to feed PCL polymer melt (feed pressure = 3 bar) through a 23G spinneret charged by a high voltage power supply (LNC 10000–5 pos, Heinzinger Electronic GmbH, Rosenheim, Germany). Processed PCL fibers (diameter ~20 μm) were then collected on a computer-controlled collector plate (acceleration voltage=5.5 kV, spinning gap= 3.3 mm, E = 1.3 kV/mm). Each mesh was fabricated with a 90° lay-down pattern and the spacing between deposited fibers was 200μm, 400 μm or 800 μm. Disc-shaped mesh constructs were obtained from printed 1 mm thick MEW meshes using a 4 mm biopsy punch.
2.4.2. Composite Fabrication
To create composites combining NorHA hydrogels and PCL meshes, lyophilized NorHA macromer and meshes (4 mm diameter, 1 mm height) were first sterilized via irradiation with a germicidal lamp in a laminar flow hood. Thereafter, NorHA (matching the formulation for 2 kPa hydrogels from above) was dissolved in PBS along with sterile filtered LAP and DTT. Juvenile bovine MSCs were then trypsinized (0.05%), counted, and suspended in the macromer solution (P1, 20 × 106 cells/mL). This solution (100 μL) was then carefully pipetted on top of MEW meshes and allowed to fill into the interstitial spaces of the box-structured scaffolds [41]. Meshes were then flipped, so that additional macromer could be pipetted on the other side. Finally, macromer was crosslinked within the meshes via photocrosslinking with visible light irradiation as described above (~15 μL final gel volume, ~2.8×105 cells per construct). Since the MEW meshes incorporated within composites account for ~6% volume fraction, fabricated composites contained slightly fewer cells than hydrogels alone [40]; however, the overall cell density within both composites and hydrogels was conserved.
Cells and meshes within composites were visualized using CellTracker Red CMTPX dye (Invitrogen) and fluorescein isothiocyanate-bovine serum albumin (FITC-BSA, adsorbed onto PCL filaments prior to composite fabrication), respectively, and were imaged via confocal microscopy. For visualization of hydrogel within composites, FITC-BSA was encapsulated within the NorHA hydrogels during photocrosslinking. The density of cells within the top 100 μm and bottom 100 μm of composites was calculated by counting the total number of cells within randomly placed 600 × 600 μm2 image frames (n ≥ 3 hydrogels, 9 images per group). Composites were cultured in chondrogenic media for up to 56 days and characterized for cell viability, gene expression, biochemical content, histology/immunohistochemistry, and biomechanics as described above and compared to hydrogels alone.
2.5. Assessment of Ex Vivo Integration Capacity
2.5.1. Fabrication of Press-Fit Constructs in Cartilage Ring Explants
Juvenile bovine joints were dissected in a similar fashion as previously described and osteochondral plugs were biopsied from the trochlear groove to obtain cartilage explants for ex vivo integration studies. After conditioning osteochondral plugs in serum-free expansion media for 1–2 days (DMEM; 1% P/S; 10mM HEPES, 0.1 mM non-essential amino acids; 2.50 μg/mL amphotericin B; 1 × 10−3 M sodium pyruvate; 50 μg/mL ascorbic acid 2-phosphate; 40 μg/mL L-proline) [42], cartilage rings were isolated and prepared (8 mm outer diameter, 4 mm inner diameter, 1 mm thickness) such that acellular composites, cell-laden composites (i.e., composites immediately after MSC encapsulation), or cell-laden composites that were pre-cultured for 28 days in chondrogenic media (cell-laden+PC, where “PC” refers to the pre-culture period of 28 days) could be press-fit into the inner cores of cartilage rings. As a control, biopsied autologous cartilage was press-fit back into the inner cores of rings. Each of these four different press-fit constructs (i.e., autologous cartilage control, acellular, cell-laden, cell-laden+PC) were then cultured within cartilage rings in chondrogenic media for 28 days
2.5.2. Push-out Testing
The integration strength (i.e., failure stress) of press-fit constructs cultured within explanted cartilage rings was determined via push-out testing as previously described [43]. Briefly, an indenter (3.8 mm) was affixed to an Instron 5848 testing device and used to push out the central core of the cartilage constructs (0.2 mm/s). The failure stress was calculated by dividing the load at failure by the lateral surface area of press-fit constructs (i.e., interfacial area).
2.5.3. MicroCT and Interfacial Contact Area
To visualize the integration between press-fit composites (or autologous cartilage) and the cartilage rings after 28 days of culture in chondrogenic media, samples were incubated in Lugol’s solution overnight at room temperature and imaged using a Scanco MicroCT 35 system (Scanco Medical, Southeastern, PA, USA; exposure: 300 ms, voltage: 55 kVp, isotropic voxel size: 6 μm). MicroCT reconstructions were then created using DragonFly software (Version 2021.1 for Windows; Object Research Systems (ORS) Inc, Montreal, Canada, 2021; software available at http://www.theobjects.com/dragonfly). MicroCT reconstructions were utilized to quantify the interfacial contact area between press-fit composites (or autologous cartilage) and the cartilage rings. To measure interfacial contact area, three cross-sections were first identified within every sample, such that the middle-cross section and two cross-sections 1 mm away in each orthogonal direction revealed the interface. The contact lengths at each of these cross-sections was measured, and the interfacial contact area was then calculated by assuming that the area could be approximated as the lateral surface area of an oblique cylinder.
2.6. Statistical Analysis
All statistical analyses were performed using GraphPad Prism 9 software, data are reported as mean ± standard deviation, and significance for all performed analyses was determined at p<0.05. Two-way ANOVAs were performed with construct formulation and culture time set as independent variables, and multiple comparisons were performed with α=0.05 and Tukey’s honestly significant difference (HSD) post-hoc test. Comparisons between just two groups were made via student t-tests with two-tailed criteria. For comparisons between more than two groups, one-way ANOVAs were performed, with Tukey’s HSD post-hoc test; Kruskal–Wallis tests were performed for non-parametric comparisons (normality assessed via Shapiro-Wilk test, α=0.05), with multiple comparisons performed via Dunn’s multiple comparison test.
3. Results and Discussion
3.1. Influence of Crosslink Density on Cartilage Formation in NorHA Hydrogels
When designing hydrogels for cartilage tissue engineering, consideration must be given to the choice of material used as well as the crosslinking chemistry selected. Here we chose HA, due to its native presence in cartilage and roles in development, wound healing, and natural extracellular matrix (ECM) organization and maintenance [44]. HA possesses innate bioactivity, can be readily degraded by hyaluronidases and oxidative species, and can be easily modified with pendant functional groups for crosslinking, all of which supports its use in tissue engineering applications [45]. In this work, we modified HA with norbornene groups for crosslinking via thiol-ene photocrosslinking (figure 1(a)), which enables the crosslink density to be easily modulated by the crosslinker concentration used during the step-growth crosslinking reaction [46]. Although other modifications are possible (e.g., methacrylation or MeHA), it is challenging to modify crosslinking due to the uncontrolled radical polymerization used for gelation [16]. Further, the use of NorHA not only allows for more modular control of hydrogel crosslinking, but also enables photopatterning with signaling ligands (i.e., peptides) of interest [36].
Figure 1: NorHA hydrogels with varied crosslink densities.
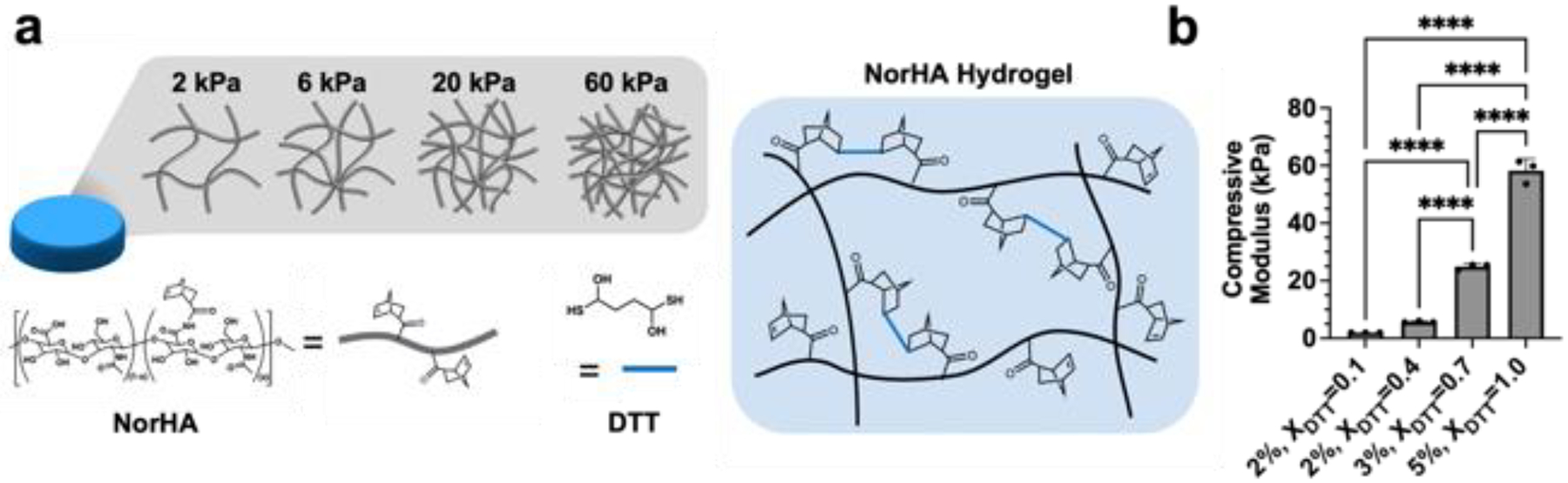
(a) Hyaluronic acid modified with norbornene (NorHA) groups undergoes thiol-ene crosslinking in the presence of a dithiol crosslinker (DL-dithiothreitol, DTT), LAP photoinitiator, and visible light. (b) The crosslink density and compressive moduli of NorHA hydrogels are tuned (i.e., 2–60 kPa) via the polymer concentration (w/v%) and the extent of macromer crosslinking (thiol-to-norbornene ratio: XDTT). ****p<0.0001, n=3.
By changing both the macromer concentration and crosslinker concentration, NorHA hydrogels ranging from ~2 to 60 kPa (figure 1(b)) were fabricated and are hereafter referred to by their approximate initial compressive moduli (i.e., 2 kPa, 6 kPa, 20 kPa, 60 kPa). Since the crosslink density of hydrogels has been previously shown to influence both encapsulated cell viability and matrix distribution by encapsulated cells [15], we first aimed to identify which hydrogel formulation best supported the viability and chondrogenesis of encapsulated MSCs. While softer, more loosely crosslinked hydrogels (i.e., 2 kPa, 6 kPa) exhibited high cell viability after 7 days of culture (~90%), more densely crosslinked hydrogels (i.e., 20 kPa, 60 kPa) resulted in significant loss in cell viability over time (figure S2). Past fluorescent recovery after photobleaching (FRAP) studies in NorHA hydrogels suggest that the relative diffusivity of macromolecules within these networks decreases with increasing crosslink density, which may explain the observed differences in cell viability in these hydrogels [47].
To assess the ability of these hydrogel formulations to support MSC chondrogenesis and cartilage formation, cell-laden hydrogels were cultured for up to 56 days in chondrogenic media and characterized for gene expression, mechanical properties, and biochemical content. All hydrogels exhibited increased expression of aggrecan and type II collagen over time, both of which are hallmark ECM components of hyaline cartilage and suggest that embedded MSCs underwent chondrogenesis (figure 2(a)). Generally, expression of each of these genes increased the most within the first week of culture. Importantly, encapsulated MSCs also expressed SOX9, a marker of chondrogenesis [48], at early culture times, and type I collagen expression was low and decreased over culture time for 2 kPa hydrogels (figure S3).
Figure 2: Influence of NorHA crosslink density on MSC chondrogenesis and neocartilage formation.
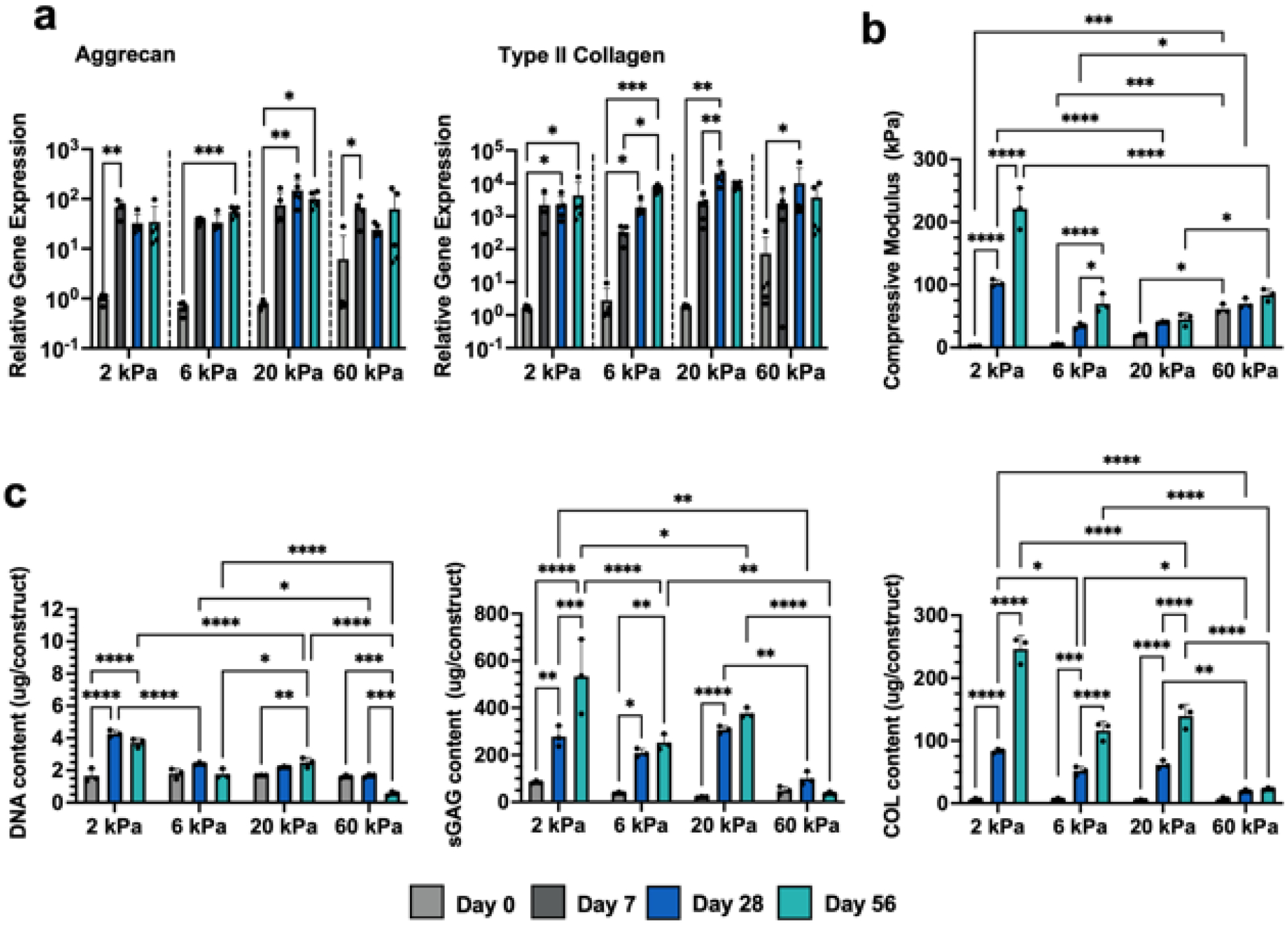
Mesenchymal stromal cell (MSC)-laden hydrogels are cultured in chondrogenic media for up to 56 days and assessed for (a) chondrogenic gene expression (Aggrecan, Type II Collagen), (b) compressive moduli, and (c) biochemical content (DNA, sulfated glycosaminoglycan (sGAG), and collagen (COL)) after 0 (light gray), 7 (dark gray), 28 (blue), and 56 (teal) days of culture in chondrogenic media. *p<0.05, **p<0.01, ***p<0.001, ****p<0.0001, n≥3, individual one-way ANOVAs (20 kPa) or Kruskal–Wallis tests (2,6,60 kPa) performed for each hydrogel formulation for qRT-PCR data.
The appearance of each hydrogel formulation noticeably changed over 56 days of culture. While more loosely crosslinked hydrogels turned opaque, suggesting the elaboration of neotissue by embedded cells, 60 kPa hydrogels remained relatively translucent (figure S4). All hydrogels also increased in compressive modulus with culture, although to varying extents based on initial crosslinking density (figure 2(b)). 2 kPa NorHA hydrogels resulted in the formation of cartilage with the highest compressive properties, reaching a compressive modulus of 102.6 ±5.4 kPa after 28 days and 221.4±33.0 kPa after 56 days. No other group reached values higher than 100 kPa, even after 56 days of culture, and the 60 kPa NorHA hydrogels barely increased in modulus with culture. These observed differences in compressive moduli were supported by the relative differences in biochemical content across each hydrogel formulation (figure 2(c)). 2 kPa hydrogels resulted in significant increases in DNA content with culture, likely due to some degree of cell proliferation, whereas the DNA content within 6 kPa hydrogels and higher were much more modest and did not significantly change throughout the duration of culture. 60 kPa hydrogels exhibited decreasing DNA content over time consistent with the observed reduction in cell viability (figure S2). With regards to biochemical content, 2 kPa hydrogels exhibited the largest increases in sulfated glycosaminoglycan (sGAG) and collagen (COL) contents with culture. 6 kPa and 20 kPa hydrogels similarly showed significant increases in both sGAG and COL content over the course of 56 days of culture, albeit with lower total amounts produced when compared to the 2 kPa group. Minimal changes in sGAG or COL content swere observed with the 60 kPa formulation.
These results indicate that softer NorHA hydrogels result in neocartilage with improved functional properties, and so we next aimed to elucidate the organization of nascent matrix within these hydrogels via histology for sGAG and immunohistochemistry for type I and type II collagen (figures 3, S5, S6). Alcian blue staining for sGAG revealed that 2 kPa hydrogels support increased sGAG deposition and dispersion, as indicated by significant increases in staining intensity between 28 and 56 days of culture (figure 3(a)). Moreover, 2 kPa hydrogels stained much more intensely and uniformly than the other investigated formulations, particularly at day 56. These results are consistent with past observations in MeHA hydrogels [16] and recent studies that demonstrated that the extent of nascent matrix dispersion decreases with increasing NorHA crosslink density [47]. We believe that these observed differences can be attributed to the hydrogel network being more permissive to matrix dispersion due to its increased mesh size [49], as well as the increased cell viability in less crosslinked formulations. Similar trends were observed for type II collagen staining, as 2 kPa hydrogels exhibited type II collagen that extended beyond the pericellular space of embedded cells and that was more homogenous (figure 3(b)). In contrast, dark staining localized around cells was observed in 6 kPa hydrogels after 56 days of culture, and both 20 kPa and 60 kPa hydrogels exhibited minimal type II collagen staining. Importantly, all hydrogels resulted in minimal type I collagen deposition over culture time, suggesting that hyaline-like cartilage formed within hydrogels as opposed to fibrocartilage, which is composed of more type I collagen (figure S6). Taken together, these results indicate that 2 kPa NorHA hydrogels support the formation of neocartilage in vitro, likely due to an increased mesh size that allows for increased matrix distribution and increased viability. The greater than 100-fold increase in compressive modulus achieved in these hydrogels over the culture period is particularly promising; however, the application of these soft hydrogels for tissue engineering is still limited by their initial mechanical properties, especially in terms of handling and stability.
Figure 3: Influence of NorHA crosslink density on matrix production and distribution.

Representative images and quantification of matrix distribution within NorHA hydrogels after 28 and 56 days of culture for (a) sulfated glycosaminoglycans (sGAG) via Alcian blue staining or (b) type II collagen (COLII) via immunohistochemistry. *p<0.05, **p<0.01, ***p<0.001, ****p<0.0001, n=45 images, 5 sections, 3 constructs.
3.2. Reinforcement of NorHA Hydrogels with MEW Meshes
To address the limitations of soft hydrogels, we reinforced the 2 kPa NorHA hydrogels with a secondary, microfiber mesh. Since MEW meshes can be readily incorporated within hydrogels to increase their compressive properties [31,50], we first demonstrated that composites composed of NorHA hydrogels and polycaprolactone (PCL) box-structured meshes could be formed by curing NorHA macromer within the interstitial spaces of MEW meshes (figure 4(a)). To permit comparisons between composites and hydrogels alone, the final dimensions of fabricated composites were matched to the initial dimensions of hydrogels alone (4 mm diameter, 1 mm thickness). The spacing between overlaying fibers within meshes was tuned between 200 μm and 800 μm to change the overall fiber density and porosity of the mesh (figure 4(b)). Interestingly, combinations of NorHA hydrogel with PCL meshes led to synergistic increases in compressive moduli, including an ~50-fold increase from the initial hydrogel modulus. The increase in mechanics is attributed to the ability of the hydrogel to mitigate MEW fiber buckling, which effectively increases the load-carrying capacity of MEW meshes since the PCL fibers can resist deformation in the transverse direction when loaded in compression [40]. Similarly, the presence of PCL fibers surrounding the NorHA hydrogel decreases the rate of water efflux from the hydrogel (i.e., syneresis) upon loading, further increasing the mechanical properties of the entire composite. The observed increases in compressive moduli are also consistent with similar composite systems that have leveraged MEW meshes to reinforce alternative hydrogels (i.e., gelatin, alginate, PEG, fibrin) [31,51,52]. The PCL fibers embedded within composites only account for ~6% of the composite’s volume fraction, such that constructs may be engineered largely with a cell-laden hydrogel conducive to neotissue formation [40]. As the interfiber spacing decreases, the total fiber density within composites increases, giving rise to elevated compressive moduli (figures 4(b), S7). However, decreasing the interfiber spacing also resulted in misalignment of overlaying fibers. As a result, composites composed of meshes with 400 μm spacing were selected and employed for all subsequent studies to maximize the compressive properties of formed composites while conserving mesh alignment for optimal filling of macromer within the interstitial spaces of the mesh. All subsequent studies were also performed with 2 kPa NorHA hydrogel formulations (i.e., 2% NorHA, XDTT=0.1).
Figure 4: PCL meshes reinforce soft NorHA hydrogels.
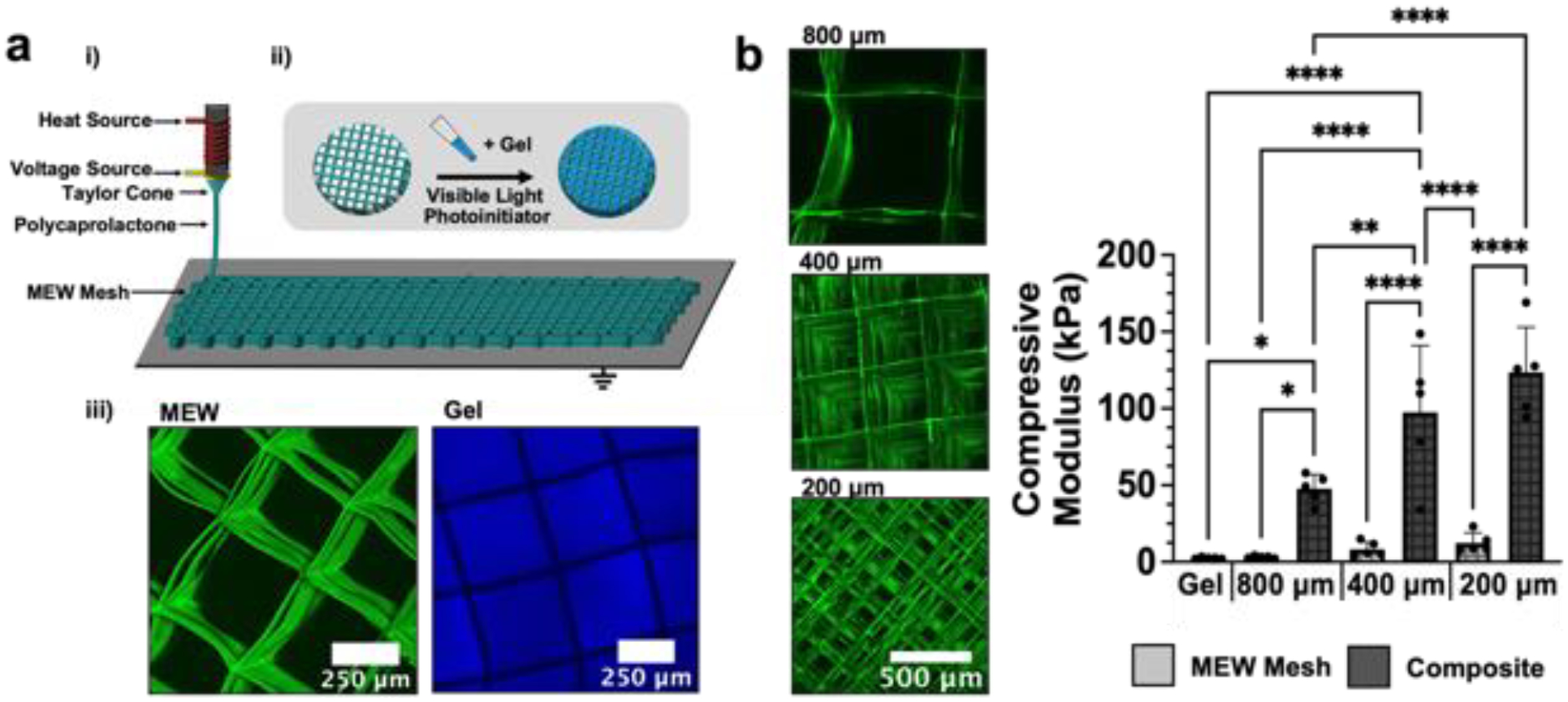
(a) (i) Schematic of the melt-electrowriting process (MEW) employed to fabricate fibrous PCL meshes. PCL is heated to form a polymer melt that can be readily extruded though a printhead with an attached voltage source to deposit PCL onto a grounded print bed. (ii) PCL meshes are then filled with NorHA macromer/crosslinker precursor (2 kPa NorHA hydrogel formulation) and exposed to visible light in the presence a photoinitiator to form composites. (iii) Images of composites containing PCL MEW meshes (green) and 2 kPa NorHA hydrogel (blue). (b) Representative images of MEW meshes of varied interfiber spacing (800 μm, 400 μm, 200 μm). Compressive moduli of NorHA hydrogel alone, PCL MEW meshes of varied interfiber spacing alone, and composites containing NorHA hydrogel infused into meshes with varied interfiber spacing. *p<0.05, **p<0.01, ****p<0.0001, n=5.
3.3. Neocartilage Formation in MEW-Reinforced NorHA Hydrogels
Although the incorporation of MEW meshes within NorHA hydrogels significantly improved their compressive properties, it remained unclear how the inclusion of PCL would impact embedded MSC chondrogenesis and their ability to synthesize and distribute ECM. Thus, chondrogenesis and cartilage formation was evaluated in hydrogels alone (2 kPa NorHA) and compared to cell-laden composites containing the same hydrogel within PCL meshes (figure 5(a)). Cell viability in composites was high (92.0 ± 2.7%) after one week of culture, and homogenous filling of the hydrogel within composites was observed, as indicated by comparable cell densities near the top (716 ± 130 cells/mm2) and bottom (638 ± 77 cells/mm2) of composites (figure S8). While local heterogeneity within cell-laden hydrogels may improve neocartilage formation [53], the observation of homogenous cell densities throughout constructs ensures that matrix deposition occurs throughout the full-thickness of composites. In addition, the presence of spaces between overlaying filaments throughout the entire thickness of composites ensures that both cells and deposited ECM can permeate through the walls of the PCL box structures over time (figures 4(a), 5(a)). As expected, MSCs exhibited significant increases in aggrecan and type II collagen expression over 56 days of culture, consistent with chondrogenesis and similar to their differentiation in hydrogels alone (figure 5(b)). Similarly, MSCs within both hydrogels alone and composites expressed SOX9 and decreasing amounts of type I collagen over culture time (figure S9).
Figure 5: Influence of MEW meshes on MSC chondrogenesis and neocartilage formation.

(a) Representative images of MSC-laden hydrogels and composites (2 kPa NorHA hydrogel formulation). Hydrogels and composites are cultured in chondrogenic media for up to 56 days and assessed for (b) chondrogenic gene expression (Aggrecan, Type II Collagen), (b) compressive moduli, and (c) biochemical content (DNA, sulfated glycosaminoglycan (sGAG), and collagen (COL)) after 0 (light gray), 7 (dark gray), 28 (blue), and 56 (teal) days of culture in chondrogenic media. *p<0.05, **p<0.01, ***p<0.001, ****p<0.0001, n.s. = not significant, n≥3, individual one-way ANOVAs (Aggrecan) or Kruskal–Wallis tests (Type II Collagen) performed for each formulation for qRT-PCR data.
Composites exhibited a higher compressive modulus than hydrogels alone initially and continued to increase in their mechanical properties over culture time, possessing a significantly higher modulus (367 ± 95 kPa) than hydrogels alone (239 ± 119 kPa) after 56 days of culture (figure 5(c)). Moreover, the compressive moduli of composites approached previously reported values for the Young’s modulus of native articular cartilage (0.1–1.6 MPa) [54,55]. The observed increases in mechanical properties can be attributed to the deposition of ECM by encapsulated MSCs, since acellular hydrogels and composites cultured for 56 days exhibited modest decreases in compressive properties over time due to degradation (figure S10). While all of the experimental groups exhibited increases in DNA content, no significant differences were observed across culture timepoints or between hydrogels and composites (figure 5(d)). The sGAG and COL contents for hydrogels and composites increased with culture time, with no significant differences between hydrogels or composites observed at the same culture times. Small differences in the absolute amount of sGAG or COL between composites and hydrogels alone may be attributed to the volume fraction of fibers, which slightly decreases the space available for matrix.
After 28 and 56 days of culture, dense and opaque tissue was macroscopically visible in both hydrogels alone and in composites, such that the two were indistinguishable upon qualitative observation (figure S11). The distribution of sGAG within both hydrogels and composites was comparable, with no significant differences observed in Alcian blue staining intensity (figure 6(a)). Similarly, both hydrogels and composites supported the deposition of homogenously distributed type II collagen, with no appreciable differences in staining intensity over culture time (figure 6(b)). In addition, MSCs in both hydrogels and composites deposited minimal amounts of type I collagen, consistent with a hyaline cartilage-like phenotype (figure S12). Although the staining intensity for type I collagen was significantly higher in composites at day 28 of culture, this may be attributed to the presence of additional surfaces along fibers, which may modulate gene expression and local mechanosensing of some cells [56,57]. However, no significant differences in type I collagen staining intensity between hydrogels and composites were observed after 56 days of culture. Importantly, the observed similarities in chondrogenic gene expression, biochemical content, and matrix staining between hydrogels and composites suggests that the inclusion of PCL meshes within cell-laden NorHA hydrogels does not attenuate the ability of cells to synthesize and distribute ECM. Thus, the higher initial mechanical properties and improved handling of the composites further motivates additional exploration of their use in cartilage repair.
Figure 6: Influence of MEW Meshes on matrix production and distribution.
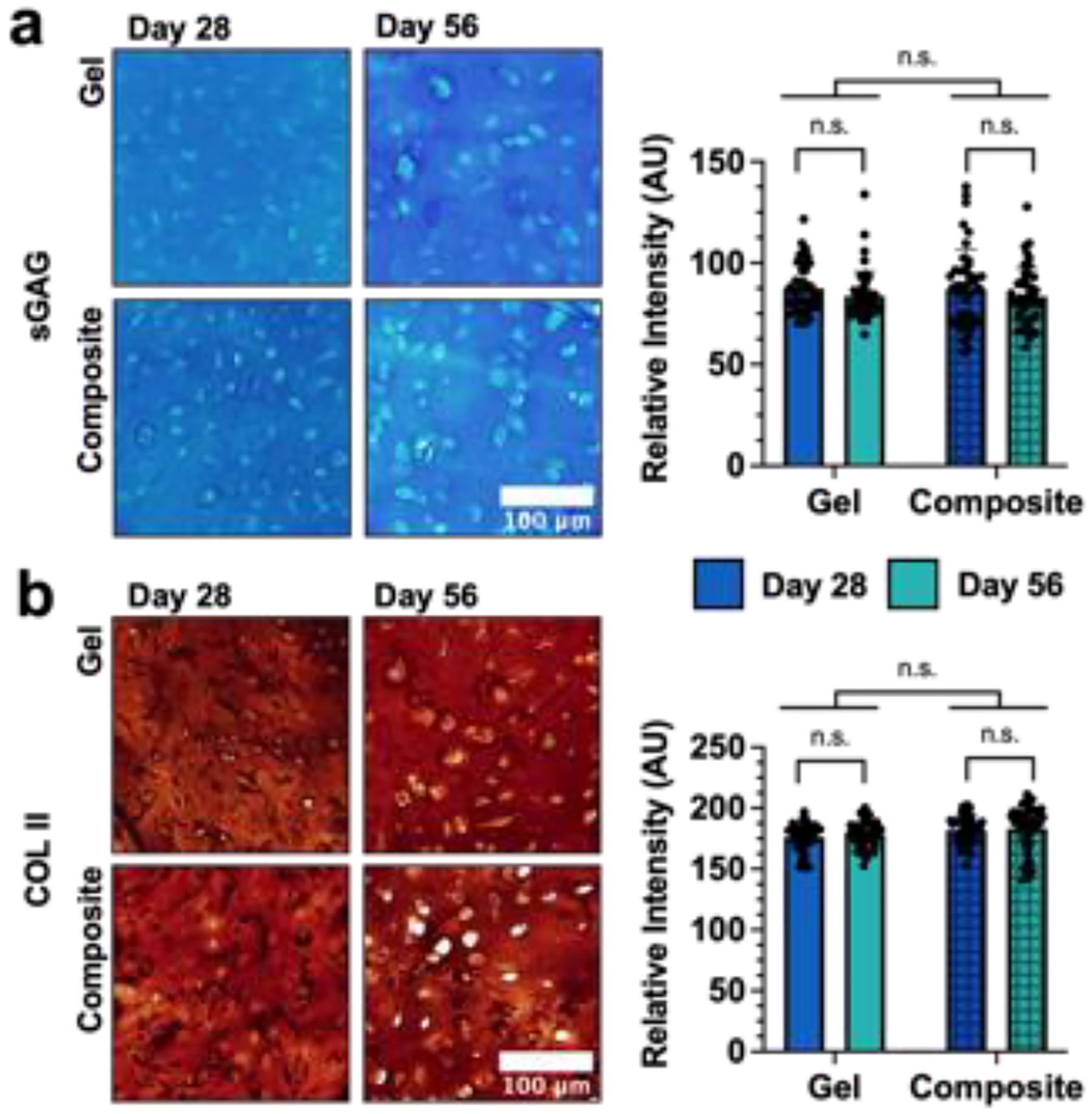
Representative images and quantification of matrix distribution within NorHA hydrogels and composites (2 kPa NorHA hydrogel formulation) after 28 and 56 days of culture for (a) sulfated glycosaminoglycans (sGAG) via Alcian blue staining or (b) type II collagen (COLII) via immunohistochemistry. n.s. = not significant, n=45 images, 5 sections, 3 constructs.
3.4. Integration of Composites Within Cartilage Explants
Towards translating the developed composites for the repair of focal cartilage defects, we assessed the ability of composites to integrate with explanted native cartilage ex vivo (figure 7(a)). After culture in chondrogenic media for 28 days, the formation of tissue resulted in changes in the opacity of press-fit cell-laden and cell-laden+PC composites (i.e., cell-laden composites that were pre-cultured for 28 days); specifically, the appearance of cell-laden+PC composites started to resemble the autologous cartilage controls (figure S13). The integration strength of press-fit constructs was then measured via push-out testing (figure S14). While acellular composites were easily displaced from the center of cartilage rings, cell-laden composites exhibited a much higher integration strength (113 ± 74 kPa; figure 7(b, c)). The addition of a pre-culture period and time for nascent matrix to form within composites further improved the integration strength of cell-laden+PC composites with surrounding cartilage (221 ± 115 kPa), which did not differ significantly from autologous tissue controls (272 ± 120 kPa) or previously reported integration strengths for autologous controls [43]. Uniaxial compressive testing was performed on central regions that were pushed out to confirm that culture within cartilage rings did not significantly impede cartilage formation and to compare the compressive moduli of composites with native cartilage controls (figure S15).
Figure 7: Integration of composites within explanted cartilage rings.

(a) Schematic of integration studies. (i) Osteochondral plugs are isolated from the trochlear groove of juvenile bovine knee joints and (ii) defects are created to produce cartilage rings with an outer diameter of 8mm and an inner diameter of 4mm. (iii) Autologous cartilage or composites (acellular, cell-laden, and cell-laden with 28 days of chondrogenic pre-culture (+PC); 2 kPa NorHA hydrogel formulation) are then press-fit into cartilage rings, cultured for 28 days, and then subjected to push-out testing. (b) Representative load-displacement curves generated during push-out testing. (c) Quantification of the integration strength of press-fit constructs with surrounding explanted tissue (red data points correspond to respective load-displacement curves in (b)). *p<0.05, **p<0.01, ****p<0.0001, n≥10. Created with BioRender.com.
In addition to push-out testing, microCT was performed on samples to assess the interfacial contact area between press-fit composites (or autologous cartilage) and explanted cartilage rings (figures S16, S17). The inclusion of cells within composites and the addition of a pre-culture period significantly improved composite integration with surrounding tissue (figures 8(a), S18). The contact area between samples and cartilage rings was quantified at three different cross sections (figures 8(b), S17) and then normalized to represent a fraction of the total possible contact area between each sample and the surrounding cartilage (figure 8(c)). While the normalized contact area was largest in control samples (0.85 ± 0.06), there were no significant differences from either of the cell-laden groups either without (0.66 ± 0.25) or with (0.78 ± 0.04) pre-culture. Notably, the normalized contact area was different between the acellular samples (0.33±0.17) and both the cell-laden+PC samples and the autologous cartilage control samples. It is likely that the lack of tissue formation in acellular composites over culture time resulted in attenuated interfacial strength, as reflected by the displacement of composites and gaps visible between composites and native cartilage in the microCT reconstructions (figures 8(a), S18). While these features are also pronounced in some cell-laden constructs, cell-laden+PC constructs generally showed intimate contact with the surrounding cartilage rings.
Figure 8: Characterization of composite-cartilage interfaces.
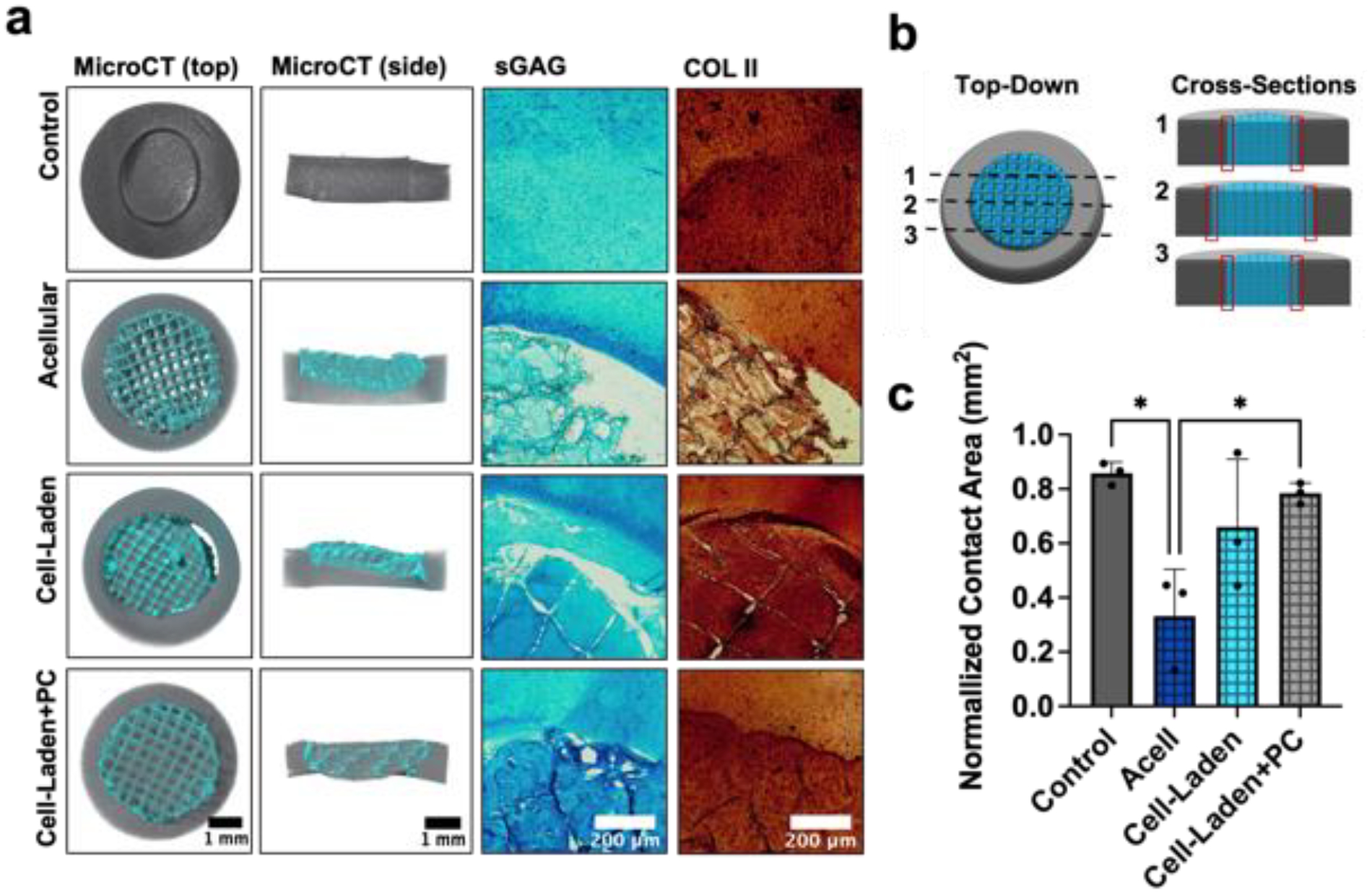
(a) Representative MicroCT reconstructions, Alcian blue staining, and type II collagen (COLII) immunohistochemistry for constructs press-fit and cultured within explanted cartilage rings (2 kPa NorHA hydrogel formulation used for all composites). (b) Schematic illustrating the three cross-sections (i.e., dashed lines; midplane, and planes 1 mm from the midplane in each respective direction) analyzed to determine the interfacial contact area (indicated by red boxes). (c) Quantification of normalized contact area between press-fit constructs and native cartilage at their interfaces. *p<0.05, n=3.
To further elucidate the interface of composites and cartilage, we stained constructs for sGAG and COLII to visualize local ECM organization (figure 8(a)). Acellular composites failed to show sGAG or COLII along the entire perimeter of the interface, consistent with our microCT quantification. While cell-laden samples similarly possessed some gaps between composites and surrounding cartilage, cell-laden samples also showed increased sGAG and COLII staining when compared to acellular samples, suggesting that the presence of nascent ECM improved overall integration. While significant changes in composite volume were not observed over culture time, the formation of GAGs within composites might increase overall composite swelling, which may further improve composite integration within cartilage rings. Of the three composite groups, cell-laden+PC constructs contained interfaces with the most continuous sGAG and COLII staining and most closely resembled autologous cartilage controls. Taken together, these results highlight the importance of hydrogel stabilization with MEW composites, as well as composite pre-culture towards developing a nascent ECM template that improves tissue integration with cartilage ex vivo.
4. Conclusions
In this study we demonstrated that loosely crosslinked NorHA hydrogels support MSC chondrogenesis and neocartilage formation with greater properties after culture for 56 days when compared to more densely crosslinked hydrogels. Specifically, softer NorHA hydrogels provided embedded MSCs with a local microenvironment more conducive to the production and distribution of ECM consistent with hyaline-like cartilage (i.e., high sGAG and COLII contents). To address the low initial mechanical properties and stability of these hydrogels, we reinforced the NorHA hydrogels with melt-electrowritten PCL scaffolds and showed that this did not inhibit MSC chondrogenesis or neocartilage formation while simultaneously providing improved mechanics and handling characteristics. Finally, we demonstrated that the chondrogenic pre-culture of NorHA-MEW composites resulted in improved tissue integration within explanted cartilage rings relative to acellular controls, informing future approaches for the fixation and maturation of cartilage implants within cartilage defects in vivo. Future work will implement these NorHA-MEW composites in the repair of articular cartilage defects in vivo.
Supplementary Material
Acknowledgements
This work was supported by the AO Foundation, the National Science Foundation (graduate research fellowship to J.H.G.), the National Institutes of Health (R01AR077362 and T32 AR053461), the Department of Veterans Affairs (I01 RX003375 and IK6 RX003416), and the University of Pennsylvania Provost Postdoctoral Research Fellowship for Academic Diversity (to C.E.W.). The authors would like to thank Quentin Peiffer and Cody Fell for helpful discussions, as well as Dr. Jay Patel and Dr. Claudia Loebel for assistance with MSC isolation. The authors also thank the Penn Center for Musculoskeletal Disorders (PCMD, NIH/NIAMS P30 AR069619) for support with mechanical and microCT analyses.
References
- [1].Martín AR, Patel JM, Zlotnick HM, Carey JL and Mauck RL 2019. Emerging therapies for cartilage regeneration in currently excluded ‘ red knee ‘ populations npj Regen. Med 4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Widuchowski W, Widuchowski J and Trzaska T 2007. Articular cartilage defects: Study of 25,124 knee arthroscopies Knee 14 177–82 [DOI] [PubMed] [Google Scholar]
- [3].Sadtler K, Singh A, Wolf MT, Wang X, Pardoll DM and Elisseeff JH 2016. Design, clinical translation and immunological response of biomaterials in regenerative medicine Nat. Rev. Mater 1 16040 [Google Scholar]
- [4].Heir S, Nerhus TK, Rotterud JH, Loken S, Ekeland A, Engebretsen L and Aroen A 2010. Focal cartilage defects in the knee impair quality of life as much as severe osteoarthritis: a comparison of knee injury and osteoarthritis outcome score in 4 patient categories scheduled for knee surgery. Am. J. Sports Med 38 231–7 [DOI] [PubMed] [Google Scholar]
- [5].Kalson NS, Gikas PD and Briggs TWR 2010. Current strategies for knee cartilage repair Int. J. Clin. Pract 64 1444–52 [DOI] [PubMed] [Google Scholar]
- [6].Carey JL. Fibrocartilage Following Microfracture Is Not as Robust as Native Articular Cartilage. J. Bone Jt. Surg. Am. 2012;94:e80. doi: 10.2106/JBJS.L.00319. [DOI] [PubMed] [Google Scholar]
- [7].Devitt BM, Bell SW, Webster KE, Feller JA and Whitehead TS 2017. Surgical treatments of cartilage defects of the knee: Systematic review of randomised controlled trials Knee 24 508–17 [DOI] [PubMed] [Google Scholar]
- [8].Balakrishnan B and Banerjee R 2011. Biopolymer-based hydrogels for cartilage tissue engineering Chem. Rev 111 4453–74 [DOI] [PubMed] [Google Scholar]
- [9].Yang J, Shrike Zhang Y, Yue K and Khademhosseini A 2017. Cell-Laden Hydrogels for Osteochondral and Cartilage Tissue Engineering Acta Biomater. 57 1–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Di Bella C, Duchi S, O’Connell CD, Blanchard R, Augustine C, Yue Z, Thompson F, Richards C, Beirne S, Onofrillo C, Bauquier SH, Ryan SD, Pivonka P, Wallace GG and Choong PF 2018. In situ handheld three-dimensional bioprinting for cartilage regeneration J. Tissue Eng. Regen. Med 12 611–21 [DOI] [PubMed] [Google Scholar]
- [11].O’Connell CD, Di Bella C, Thompson F, Augustine C, Beirne S, Cornock R, Richards CJ, Chung J, Gambhir S, Yue Z, Bourke J, Zhang B, Taylor A, Quigley A, Kapsa R, Choong P and Wallace GG 2016. Development of the Biopen: A handheld device for surgical printing of adipose stem cells at a chondral wound site Biofabrication 8 015019. [DOI] [PubMed] [Google Scholar]
- [12].Levato R, Jungst T, Scheuring RG, Blunk T, Groll J and Malda J 2020. From Shape to Function: The Next Step in Bioprinting Adv. Mater 32 1906423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Lim KS, Levato R, Costa PF, Castilho MD, Alcala-Orozco CR, van Dorenmalen KMA, Melchels FPW, Gawlitta D, Hooper GJ, Malda J and Woodfield TBF 2018. Bio-resin for high resolution lithography-based biofabrication of complex cell-laden constructs Biofabrication 10 034101. [DOI] [PubMed] [Google Scholar]
- [14].Bernal PN, Delrot P, Loterie D, Li Y, Malda J, Moser C and Levato R 2019. Volumetric Bioprinting of Complex Living-Tissue Constructs within Seconds Adv. Mater 31 1904209. [DOI] [PubMed] [Google Scholar]
- [15].Bryant SJ and Anseth KS 2002. Hydrogel properties influence ECM production by chondrocytes photoencapsulated in poly(ethylene glycol) hydrogels J. Biomed. Mater. Res 59 63–72 [DOI] [PubMed] [Google Scholar]
- [16].Erickson IE, Huang AH, Sengupta S, Kestle S, Burdick JA and Mauck RL 2009. Macromer density influences mesenchymal stem cell chondrogenesis and maturation in photocrosslinked hyaluronic acid hydrogels Osteoarthr. Cartil 17 1639–48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Sridhar BV, Dailing EA, Brock JL, Stansbury JW, Randolph MA and Anseth KS 2015. A Biosynthetic Scaffold that Facilitates Chondrocyte-Mediated Degradation and Promotes Articular Cartilage Extracellular Matrix Deposition Regen. Eng. Transl. Med 1 11–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Roberts JJ, Nicodemus GD, Greenwald EC and Bryant SJ 2011. Degradation improves tissue formation in (Un)loaded chondrocyte-laden hydrogels Clin. Orthop. Relat. Res 469 2725–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Sahoo S, Chung C, Khetan S and Burdick JA 2008. Hydrolytically degradable hyaluronic acid hydrogels with controlled temporal structures Biomacromolecules 9 1088–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Chung C, Beecham M, Mauck RL and Burdick JA 2009. The influence of degradation characteristics of hyaluronic acid hydrogels on in vitro neocartilage formation by mesenchymal stem cells Biomaterials 30 4287–96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Xu C, Dai G and Hong Y 2019. Recent advances in high-strength and elastic hydrogels for 3D printing in biomedical applications Acta Biomater. 95 50–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Andrew CD, Susan EC, Emily MR and Daniel JK 2016. A comparison of different bioinks for 3D bioprinting of fibrocartilage and hyaline cartilage Biofabrication 8 45002. [DOI] [PubMed] [Google Scholar]
- [23].Xu T, Binder KW, Albanna MZ, Dice D, Zhao W, Yoo JJ and Atala A 2013. Hybrid printing of mechanically and biologically improved constructs for cartilage tissue engineering applications Biofabrication 5 015001. [DOI] [PubMed] [Google Scholar]
- [24].Lee JS, Hong JM, Jung JW, Shim JH, Oh JH and Cho DW 2014. 3D printing of composite tissue with complex shape applied to ear regeneration Biofabrication 6 [DOI] [PubMed] [Google Scholar]
- [25].Kundu J, Shim J-H, Jang J, Kim S-W and Cho D-W 2015. An additive manufacturing-based PCL–alginate–chondrocyte bioprinted scaffold for cartilage tissue engineering J Tissue Eng Regen Med 9 1286–97 [DOI] [PubMed] [Google Scholar]
- [26].Critchley S, Sheehy EJ, Cunniffe G, Diaz-Payno P, Carroll SF, Jeon O, Alsberg E, Brama PAJ and Kelly DJ 2020. 3D printing of fibre-reinforced cartilaginous templates for the regeneration of osteochondral defects Acta Biomater. 113 130–43 [DOI] [PubMed] [Google Scholar]
- [27].Yoon Y, Kim CH, Lee JE, Yoon J, Lee NK, Kim TH and Park SH 2019. 3D bioprinted complex constructs reinforced by hybrid multilayers of electrospun nanofiber sheets Biofabrication 11 [DOI] [PubMed] [Google Scholar]
- [28].Schipani R, Scheurer S, Florentin R, Critchley SE and Kelly DJ 2020. Reinforcing interpenetrating network hydrogels with 3D printed polymer networks to engineer cartilage mimetic composites Biofabrication 12 [DOI] [PubMed] [Google Scholar]
- [29].Huang C-Y, Mow VC and Ateshian GA 2001. The Role of Flow-Independent Viscoelasticity in the Biphasic Tensile and Compressive Responses of Articular Cartilage J. Biomech. Eng 123 410–7 [DOI] [PubMed] [Google Scholar]
- [30].Moutos FT and Guilak F 2010. Functional properties of cell-seeded three-dimensionally woven poly(ε-Caprolactone) scaffolds for cartilage tissue engineering Tissue Eng. - Part A 16 1291–301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Visser J, Melchels FPW, Jeon JE, Van Bussel EM, Kimpton LS, Byrne HM, Dhert WJA, Dalton PD, Hutmacher DW and Malda J 2015. Reinforcement of hydrogels using three-dimensionally printed microfibres Nat. Commun 6 6933. [DOI] [PubMed] [Google Scholar]
- [32].de Ruijter M, Ribeiro A, Dokter I, Castilho M and Malda J 2019. Simultaneous Micropatterning of Fibrous Meshes and Bioinks for the Fabrication of Living Tissue Constructs Adv. Healthc. Mater 8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Robinson TM, Hutmacher DW and Dalton PD 2019. The Next Frontier in Melt Electrospinning : Taming the Jet Adv. Funct. Mater 29 1904664 [Google Scholar]
- [34].Hochleitner G, Jüngst T, Brown TD, Hahn K, Moseke C, Jakob F, Dalton PD and Groll J 2015. Additive manufacturing of scaffolds with sub-micron filaments via melt electrospinning writing Biofabrication 7 035002. [DOI] [PubMed] [Google Scholar]
- [35].Kim IL, Mauck RL and Burdick JA 2011. Hydrogel design for cartilage tissue engineering : A case study with hyaluronic acid Biomaterials 32 8771–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Vega SL, Kwon MY, Song KH, Wang C, Mauck RL, Han L and Burdick JA 2018. Combinatorial hydrogels with biochemical gradients for screening 3D cellular microenvironments Nat. Commun 9 614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Livak KJ and Schmittgen TD 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2-ΔΔCT method Methods 25 402–8 [DOI] [PubMed] [Google Scholar]
- [38].Kim M, Erickson IE, Choudhury M, Pleshko N and Mauck RL 2012. Transient exposure to TGF-β3 improves the functional chondrogenesis of MSC-laden hyaluronic acid hydrogels J. Mech. Behav. Biomed. Mater 11 92–101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Kwon MY, Vega SL, Gramlich WM, Kim M, Mauck RL and Burdick JA 2018. Dose and Timing of N-Cadherin Mimetic Peptides Regulate MSC Chondrogenesis within Hydrogels Adv. Healthc. Mater 7 1701199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Castilho M, Hochleitner G, Wilson W, Van Rietbergen B, Dalton PD, Groll J, Malda J and Ito K 2018. Mechanical behavior of a soft hydrogel reinforced with three-dimensional printed microfibre scaffolds Sci. Rep 8 1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Ragelle H, Tibbitt MW, Wu SY, Castillo MA, Cheng GZ, Gangadharan SP, Anderson DG, Cima MJ and Langer R 2018. Surface tension-assisted additive manufacturing Nat. Commun 9 1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Liebesny PH, Mroszczyk K, Zlotnick H, Hung HH, Frank E, Kurz B, Zanotto G, Frisbie D and Grodzinsky AJ 2019. Enzyme Pretreatment plus Locally Delivered HB-IGF-1 Stimulate Integrative Cartilage Repair In Vitro Tissue Eng. - Part A 25 1191–201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Erickson IE, Kestle SR, Zellars KH, Dodge GR, Burdick JA and Mauck RL 2012. Improved cartilage repair via in vitro pre-maturation of MSC-seeded hyaluronic acid hydrogels Biomed. Mater 7 024110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Dicker KT, Gurski LA, Pradhan-Bhatt S, Witt RL, Farach-Carson MC and Jia X 2014. Hyaluronan: A simple polysaccharide with diverse biological functions Acta Biomater. 10 1558–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Highley CB, Prestwich GD and Burdick JA 2016. Recent advances in hyaluronic acid hydrogels for biomedical applications Curr. Opin. Biotechnol 40 35–40 [DOI] [PubMed] [Google Scholar]
- [46].Lim KS, Galarraga JH, Cui X, Lindberg GCJ, Burdick JA and Woodfield TBF 2020. Fundamentals and Applications of Photo-Cross-Linking in Bioprinting Chem. Rev 120 10662–94 [DOI] [PubMed] [Google Scholar]
- [47].Loebel C, Kwon MY, Wang C, Han L, Mauck RL and Burdick JA 2020. Metabolic Labeling to Probe the Spatiotemporal Accumulation of Matrix at the Chondrocyte–Hydrogel Interface Adv. Funct. Mater 30 1909802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Akiyama H, Chaboissier MC, Martin JF, Schedl A and De Crombrugghe B 2002. The transcription factor Sox9 has essential roles in successive steps of the chondrocyte differentiation pathway and is required for expression of Sox5 and Sox6 Genes Dev. 16 2813–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Dhote V, Skaalure S, Akalp U, Roberts J, Bryant SJ and Vernerey FJ 2013. On the role of hydrogel structure and degradation in controlling the transport of cell-secreted matrix molecules for engineered cartilage J. Mech. Behav. Biomed. Mater 19 61–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Castilho M, Mouser V, Chen M, Malda J and Ito K 2019. Bi-layered micro-fibre reinforced hydrogels for articular cartilage regeneration Acta Biomater. 95 297–306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Bas O, De-Juan-Pardo EM, Meinert C, D’Angella D, Baldwin JG, Bray LJ, Wellard RM, Kollmannsberger S, Rank E, Werner C, Klein TJ, Catelas I and Hutmacher DW 2017. Biofabricated soft network composites for cartilage tissue engineering. Biofabrication 9 025014. [DOI] [PubMed] [Google Scholar]
- [52].Bas O, Lucarotti S, Angella DD, Castro NJ, Meinert C, Wunner FM, Rank E, Vozzi G, Klein TJ, Catelas I, De-Juan-Pardo EM and Hutmacher DW 2018. Rational design and fabrication of multiphasic soft network composites for tissue engineering articular cartilage: A numerical model-based approach Chem. Eng. J 340 15–23 [Google Scholar]
- [53].Vernerey FJ and Bryant S 2020. The role of percolation in hydrogel-based tissue engineering and bioprinting Curr. Opin. Biomed. Eng 15 68–74 [Google Scholar]
- [54].Wang CCB, Chahine NO, Hung CT and Ateshian GA 2003. Optical determination of anisotropic material properties of bovine articular cartilage in compression J. Biomech 36 339–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Töyräs J, Lyyra-Laitinen T, Niinimäki M, Lindgren R, Nieminen MT, Kiviranta I and Jurvelin JS 2001. Estimation of the Young’s modulus of articular cartilage using an arthroscopic indentation instrument and ultrasonic measurement of tissue thickness J. Biomech 34 251–6 [DOI] [PubMed] [Google Scholar]
- [56].Hogrebe NJ, Reinhardt JW and Gooch KJ 2017. Biomaterial microarchitecture: a potent regulator of individual cell behavior and multicellular organization J. Biomed. Mater. Res. - Part A 105 640–61 [DOI] [PubMed] [Google Scholar]
- [57].Eichholz KF and Hoey DA 2018. Mediating human stem cell behaviour via defined fibrous architectures by melt electrospinning writing Acta Biomater. 75 140–51 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


