Abstract
Sixty-six (66) Staphylococcus bacterial isolates were withdrawn from separate clinical samples of hospitalized patients with various clinical infections. Conventional bacteriological tests identified the species of all isolates, and standard microbiological techniques differentiated them into CoPS or CoNS. Their biofilm development was followed by an analysis via the MTP (microtiter tissue culture plates) technique, and we then investigated the presence/absence of icaA and icaB, which were qualified in the top-30 potent biofilm-forming isolates. Thirteen isolates (46.7%) showed the presence of one gene, six (20%) isolates exhibited the two genes, while ten (33.3%) had neither of them. The formation of staphylococci biofilms in the absence of ica genes may be related to the presence of other biofilm formation ica-independent mechanisms. CoPS was the most abundant species among the total population. S. aureus was the sole representative of CoPS, while S. epidermidis was the most abundant form of CoNS. Antibiotic resistance was developing against the most frequently used antimicrobial drugs, while vancomycin was the least-resisted drug. The totality of the strong and medium-strength film-forming isolates represented the majority proportion (80%) of the total investigated clinical samples. The biochemical pattern CoPS is associated with antibiotic resistance and biofilm formation and can be an alarming indicator of potential antibiotic resistance.
Keywords: Staphylococcus aureus, biofilm, icaA, icaB, microtiter plate assay, multidrug resistance
1. Introduction
Generally, the Gram-positive staphylococci are the leading cause of device-related infections (DRIs). Among the staphylococci, Staphylococcus aureus is the focus of most clinical concerns since its infections are commonly more severe and aggressive than those caused by other Staphylococci spp. due to its vast and diverse arsenal of aggressive toxins and virulence factors. Next to S. aureus, Staphylococcus epidermidis, a less aggressive skin commensal, has drawn attention as a frequent cause of biofilm-associated infection from medical devices and associated complications, including bloodstream infections [1]. The species of Staphylococci were distinguished into two groups based on their ability to coagulate blood: coagulase-positive Staphylococci (CoPS), where Staphylococcus aureus is the most important, and coagulase-negative staphylococci (CoNS), which comprises the majority of other species, including S. epidermidis. The species S. aureus is a dangerous human pathogen, causing opportunistic infections and severe life-threatening diseases, such as severe sepsis, pneumonia, toxic shock syndrome and endocarditis. Additionally, some pathogenic strains of S. aureus are resistant to the antibiotic methicillin (methicillin-resistant S. aureus (MRSA)), engendering a critical issue in hospitals [2].
The production of biofilms is a conservative process, enabling bacterial survival under challenging environmental conditions. However, unwanted biofilm accumulation may lead to many undesirable impacts, such as the biofouling of heat alteration systems and pelagic structures, microbial deterioration of metal sheeting, dental decay and home contamination of food and medications, as well as short- and long-term biomedical implants and devices [3]. Staphylococci are the most frequent source of biofilm-related infections, being a common pathogen of human skin and mucosal surfaces and a variety of other animals, more evidently and distinctly than other biofilm-associated pathogens. In addition, when implanted during an operation, staphylococci may represent a potentially infectious agent contaminating the surgical equipment that access these regions [4]. The importance of biofilm production in the pathogenesis of S. aureus and the development of multidrug-resistant bacterial strains (MDR strains) has been documented [5].
A polysaccharide intercellular adhesin (PIA) encoded within ica operon was concluded to be an essential factor for staphylococcal biofilm formation, among other adhesive factors [6]. The locus of intercellular adhesion of ica consists of a four-gene operon (ica ABCD), encoding the essential proteins necessary for PIA production. The first two genes, icaA and icaB, play primary roles in exopolysaccharide synthesis [7]. The icaA gene encodes N-acetylglucosaminyl-transferase, while icaB encodes PIA de-acetylase [7,8].
The purpose of this study was to delineate the biofilm-forming ability of some pathogenic isolates of staphylococci within a random population of medical samples, using a quantitative microtiter plate assay. Simultaneously, the presence or absence of the icaA and icaB genes was assessed via PCR in strong biofilm-forming staphylococci, confirmed using the MTP method, to establish the potential association between the two. Establishing this association may suggest that these genes can be used as biomarkers in screening Staphylococci for their potential biofilm formation and potential antibiotic resistance.
2. Materials and Methods
2.1. Bacterial Isolates
This study was conducted within the Mansoura Faculty of Medicine Ethical Committee (ethical approval code: R.20. 10.15). A prospective cross-sectional study was performed in Mansoura University Hospital, Egypt, from February 2019 to February 2020. A total of sixty-six (66) bacterial isolates of staphylococci were withdrawn from separate clinical samples of hospitalized patients with various clinical infections, collected from different medical departments, i.e., Allergy and Immunity, Blood Diseases, Cardiology, Contagious Diseases and Malnutrition, Endocrine Glands and Sugar, External Section, Heart disease, ICU, Nephrology, Neurology, Surgery Care and The Care of Novices.
Conventional bacteriological tests identified all isolates. All the samples were inoculated into blood agar (BA) and MacConkey agar (MA), and the isolates were identified as CoPS or CoNS using standard microbiological techniques, including biochemical, slide and tube coagulase tests [9]. The bacterial isolates were maintained at −70 °C until being investigated.
2.2. Species Identification of Clinical Isolates
Staphylococci isolates were identified utilizing Siemens Healthcare Diagnostics’ MicroScan WalkAway-96 Systeme International d’Unites (Pos ID Type 2) panels. This system was developed for in vitro diagnostic analysis to identify the species level of aerobic and facultative Gram-positive Staphylococci.
2.3. Antibiotic Susceptibility Assessment
Antibiotics susceptibility was conducted using a disc diffusion method complying with the Clinical and Laboratory Standards Institute guidelines (CLSI) [10]. The discs used for antibiotic susceptibility were vancomycin (VA) 30 μg, amoxicillin–clavulanate (Aug) 25 μg, erythromycin 20 μg, trimethoprim–sulfamethoxazole (SXT) 25 μg, imipenem (IPM) 10 μg, oxacillin (OX) 1 μg, ceftriaxone (CAX) 30 μg and clindamycin (CLI).
2.4. Studying Biofilm Formation via Microtiter Plate (MTP)
A crystal violet staining assay determined all Staphylococcus isolates’ capacity to form biofilms [11]. An uninoculated medium was used as a negative control to determine the background OD. The typical OD values were calculated for all tested strains and negative controls, and therefore the cut-off OD value (ODc) was established. For interpreting the results, strains were divided into the following groups:
(I) OD ≤ ODc = no biofilm producer, (II), ODc < OD ≤ (2 × ODc) = weak biofilm producer, (III) (2 × ODc) < OD ≤ (4 × ODc) = moderate biofilm producer, (IV) (4 × ODc) < OD = strong biofilm producer [12].
2.5. Detection of icaA and icaB Genes
2.5.1. Genomic DNA Extraction
For extracting the genomic DNA of an isolate, several colonies were mixed and suspended in an aliquot of lysis buffer (20 μL) containing 0.25% SDS, 0.05 N NaOH. The suspension was heated at 95 °C for 7 min, cooled on an ice bath and centrifuged briefly at 16,000× g for 2 min. The supernatant was diluted by adding 180 μL of distilled water and centrifuging for 5 min at 16,000× g to remove cell debris. The final supernatant was used as the source template for DNA amplification.
2.5.2. PCR
Strong biofilm-forming Staphylococcus species were selected for molecular screening for icaA and icaB using PCR. The genes icaA and icaB were amplified using PCR in staphylococci strains to identify amplicons of 188 bp and 190 bp utilizing reference primers [13,14]. An Eppendorf master cycler®, MA thermocycler (Hamburg, Germany) was used for DNA amplification. The running program consisted of 30 denatured cycles at 94 °C for 60 s, 55 °C for 60 s of annealing time and 72 °C for 60 s of expansion, with the final 10 min at 72 °C. The icaB amplification included five minutes of initial denaturation at 94 °C, 30 cycles of denaturation at 94 °C over 60 s, 30 s of annealing at 52 °C and 10 min of final extension at 72 °C over 90 sec. [13]. After electrophoresis on a 1% agarose gel, a UV transilluminator (Bio-Rad, Watford, United Kingdom, WD17) visualized the PCR products.
2.6. Statistical Analysis
All statistical studies utilized SPSS statistical analysis software (SPSS, Inc., Chicago, IL, USA) version 22. The significance of the obtained results was judged at the 0.05 level. The Chi-square test was used for comparing two or more groups.
3. Results
3.1. Bacterial Isolates and Relative Distribution
This study included 66 Staphylococcus isolates extracted from various biological materials, such as blood (72.2%, 48/66), urine (12.1%, 8/12.1), pus (9.1%, 6/66), tube (3%, 2/66), drain (1.5%, 1/66) and abscess (1.5%, 1/66). Table 1 represents the distribution of Staphylococcus spp. isolated from patients. The information indicates that CoPS, represented by Staphylococcus aureus, was isolated from 46 patients accounting for about 70% of the total, i.e., the majority. On the other hand, CoNS isolates were collected from 20 patients, representing about 30% of the total. The species S. epidermidis was the dominant species in the isolates of CoNS, representing about 9% of the total (Table 1). Finally, S. aureus was the most dominant of all detected Staphylococcus spp. among the studied isolates (70%) and the sole CoPS that was represented, while S. epidermidis was the second most dominant one (9%), followed by S. hyicus (6%), within the CoNS group.
Table 1.
Relative distribution of Staphylococcus spp. isolates collected from patients.
| Bacterial Type | Staphylococcus spp. | No. | % |
|---|---|---|---|
| CoPS | 46 | 69.7 | |
| S. aureus | 46 | 69.7 | |
| CoNS | 20 | 30.3 | |
| S. epidermidis | 6 | 9.1 | |
| S. hyicus | 4 | 6.1 | |
| S. hominis | 3 | 4.5 | |
| S. xylosus | 3 | 4.5 | |
| S. haemolyticus | 2 | 3.0 | |
| S. auricularies | 1 | 1.5 | |
| S. sciuri | 1 | 1.5 | |
| Total | 66 | 100 |
CoPS: Coagulase-positive staphylococci, represented only by S. aureus. CoNS: Coagulase-negative staphylococci, represented by 7 species of staphylococci.
3.2. Antibiotic Resistance Distribution
The studied isolates (66) were divided into two groups based on their sensitivity to different antibiotics and classified into CoPS or CoNS isolate biochemical patterns on the basis of standard microbiological techniques, including biochemical, slide and tube coagulase tests [9].
The data in Table 2 revealed that the antibiotic resistance developed more pronouncedly among the CoNS isolates, exhibiting more than 90% resistance against four antibiotics, i.e., amoxicillin–clavulanate, imipenem, oxacillin and ceftriaxone. A similar magnitude of resistance (more than 90%) was noticed within CoPS against only one antibiotic (oxacillin). Therefore, the resistance developed against oxacillin is highly spread among all the studied isolates, whether CoPS or CoNS. Within the CoPS group, the highest resistance was developed against oxacillin (93.5%), followed by clindamycin (76.1%), then ceftriaxone and erythromycin (60.9%). On the other hand, the least resistance to antibiotics is noticed against vancomycin, recording only 24% and 15% resistance in isolates among CoPS and CoNS, respectively. Generally, antibiotic resistance developed against most of the frequently used antimicrobial drugs, while vancomycin was the least-resisted antibiotic, where the recorded resistance was about 24 and 15% within the CoPS and CoNS isolates, respectively.
Table 2.
Mapping resistance extent (%) within the Staphylococcus isolates collected from patients.
| Type of Antibiotics | CoPS | CoNS | p Value | |||
|---|---|---|---|---|---|---|
| Code | No | % | No | % | % | |
| Vancomycin | 1 | 11 | 23.9 | 3 | 15.0 | χ2 = 0.663 p = 0.416 |
| Amoxicillin–clavulanate | 2 | 20 | 43.5 | 19 | 95.0 | χ2 = 15.31 p < 0.001 * |
| Erythromycin | 3 | 28 | 60.9 | 15 | 75.0 | χ2 = 1.23 p = 0.268 |
| Trimethoprim–sulfamethoxazole | 4 | 21 | 45.7 | 8 | 40.0 | χ2 = 0.181 p = 0.671 |
| Imipenem | 5 | 15 | 32.6 | 18 | 90.0 | χ2 = 0.181 p < 0.001 * |
| Oxacillin | 6 | 43 | 93.5 | 18 | 90.0 | χ2 = 0.241 p = 0.624 |
| Ceftriaxone | 7 | 28 | 60.9 | 19 | 95.0 | χ2 = 7.92 p = 0.005 * |
| Clindamycin | 8 | 35 | 76.1 | 10 | 50.0 | χ2 = 4.37 p = 0.037 * |
* Statistically significant (at p < 0.05) using Chi-square test.
3.3. Detection of the Biofilm Formation via Microtiter Tissue Culture Plates
The microtiter plates assay (MTP) outcomes (Table 3) indicate that each staphylococci isolate of the tested samples exhibited attachment at different extents. Thirty isolates out of the total tested isolates (66 isolates), representing 45.5% of the total, strongly produced biofilm on MTP. These strong biofilm-forming isolates comprised 19 CoPS isolates and 11 CoNS isolates.
Table 3.
Biofilm formation, estimated via microtiter tissue culture plates, amongst 66 bacterial isolates collected from patients and classified into 46 CoPS and 20 CoNS isolates.
| Biofilm Formation | CoPS | CoNS | p-Value | ||
|---|---|---|---|---|---|
| No. | % | No. | % | ||
| Strong | 19 | 41.3 | 11 | 55 | 0.304 |
| Moderate | 15 | 32.6 | 8 | 40 | 0.562 |
| Weak | 12 | 26.1 | 1 | 5 | 0.047 * |
| Total | 46 | 100 | 20 | 100 | |
Chi-square test; * statistically significant (at p < 0.05). CoPS is represented only by S. aureus, while CoNS is represented by 7 species of staphylococci.
Strong and moderate film formation was more evident in CoNS than CoPS, occurring in about 55 and 40% (totaling 95%) of the investigated CoNS isolates against 41.3 and 32.6% (totaling 74%) of the CoPS isolates. There was only one weak biofilm-forming isolate within the CoNS group against twelve isolates in the CoPS group, representing 5 and 26% of the respective total isolates in each group.
Generally, the total number of strong and medium biofilm-forming isolates represented the majority, amounting to about 80% of the total investigated clinical samples with relatively more frequency in the CoNS than the CoPS type.
3.4. Detection of icaA and icaB Genes
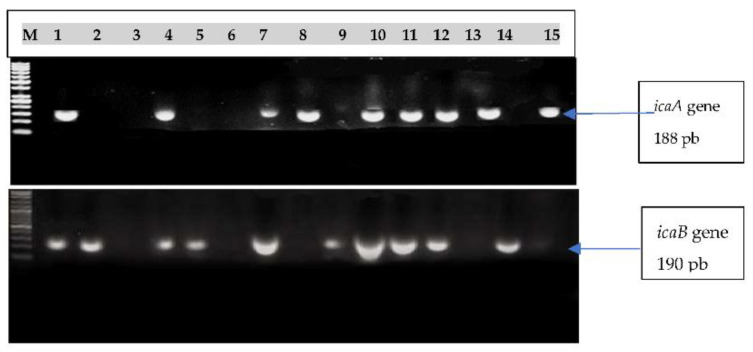
The 30 strong biofilm-forming isolates were tested for the presence of icaA or icaB genes via molecular screening using PCR amplification, and the visualized amplicons are shown in Figure 1. The icaA gene appeared in 9 isolates, while icaB appeared in 10 out of 15 investigated isolates.
Figure 1.
Electrophoretic pattern of Multiplex PCR amplicons of icaA gene and icaB gene on agarose gel in 15 samples of the 30 investigated staphylococci isolates (Table 4). Lane M was a 50 bp DNA ladder.
The results in Table 4 and Table 5 indicate that, among the selected thirty strong-film forming isolates, six isolates, representing 20% of the total, contained both of the two ica genes (icaA and icaB) and 14 isolates had one of the ica genes (icaA or icaB). Conclusively, 20 out of 30 isolates, i.e., 66.6% of the total, were positive for either genes or both. On the other hand, 10 out of 30 isolates (33.3% of the total), were negative for both genes (icaA or icaB). Alternatively, the icaA gene was positive in nine (47%) CoPS isolates while only in one CoNS isolate (9%). In total, this gene was present in 10 isolates, representing about 33% of selected staphylococci species. Consequently, icaA was absent in most of the tested isolates, i.e., 10 CoPS and 10 CoNS, representing together about 52.6 and 90.9% of the total, respectively.
Table 4.
Distribution of (icaA) and (icaB) genes in the strong biofilm-forming isolates within the tested Staphylococcus species isolated from patients, classified as strong biofilm-forming isolates.
| No. of Isolates | Tested Isolates | icaA Gene | icaB Gene |
|---|---|---|---|
| 1 | S. aureus | + | + |
| 2 | S. aureus | − | + |
| 4 | S. aureus | − | − |
| 6 | S. aureus | + | + |
| 7 | S. aureus | − | + |
| 8 | S. aureus | − | − |
| 9 | S. aureus | + | + |
| 11 | S. aureus | + | − |
| 12 | S. aureus | − | + |
| 16 | S. aureus | + | + |
| 18 | S. aureus | + | + |
| 21 | S. aureus | + | + |
| 25 | S. aureus | + | − |
| 41 | S. aureus | − | + |
| 42 | S. aureus | + | − |
| 43 | S. aureus | − | − |
| 44 | S. aureus | − | − |
| 45 | S. aureus | − | − |
| 46 | S. aureus | − | − |
| 47 | S. sciuri | − | + |
| 48 | S. hominis | − | + |
| 52 | S. haemolyticus | + | − |
| 54 | S. hominis | − | − |
| 55 | S. xylosus | − | + |
| 56 | S. hyicus | − | + |
| 57 | S. hyicus | − | + |
| 58 | S. hyicus | − | − |
| 59 | S. xylosus | − | + |
| 60 | S. epidermidis | − | − |
| 63 | S. auricularies | − | − |
Table 5.
Relative detectability of icaA and icaB genes in PCR results within the tested staphylococci species isolated from patients.
| Category | Isolate Code | Isolate Quantity | |||||
|---|---|---|---|---|---|---|---|
| No. | % | ||||||
| Two ica gene | 1, 6, 9, 16, 18, 21. | 6 | 20 | ||||
| One ica gene | 2, 7, 11, 12, 25, 41, 42, 47, 48,52, 55, 56, 57, 59. | 14 | 46.7 | ||||
| No ica gene | 4, 8, 43, 44, 45, 46, 54, 58, 60, 63. | 10 | 33.3 | ||||
| CoPS (n = 19) | CoNS (n = 11) | Total (n = 30) | p Value | ||||
| icaA | No. | % | No. | % | No. | % | |
| Positive | 9 | 47.4 | 1 | 9.1 | 10 | 33.3 |
χ2 = 4.59
p = 0.032 * |
| Negative | 10 | 52.6 | 10 | 90.9 | 20 | 66.7 | |
| icaB |
χ2 = 0.01
p = 0.919 |
||||||
| Positive | 10 | 52.60% | 6 | 54.50% | 16 | 53.30% | |
| Negative | 9 | 47.40% | 5 | 45.50% | 14 | 46.70% | |
Used test: Chi-square test; * statistically significant (at p < 0.05). CoPS is represented only by S. aureus, while CoNS is represented by 7 species of staphylococci.
The second gene (icaB) was relatively more detected in both CoPS and CoNS isolates, i.e., 10 and 6 isolates, respectively, representing 53.3% of the total. As a result, the absence of this gene was noticed in nine and five isolates of CoPS and CoNS, respectively. These represented about 47.4 and 45.5% of the total, respectively. Furthermore, the amplified icaA and icaB regions were detected on agarose gel electrophoresis at 188 and 190 bp, respectively (Figure 1). Finally, the majority (66.7%) of the studied strong biofilm-forming isolates indicated the presence of both icaA or icaB genes or one of them, while a still considerable portion (33.3%) of isolates did not contain any of these genes. Additionally, icaB gene could be noticed as more associated with strong biofilm formation in the two groups of staphylococci species (CoPS and CoNS).
3.5. The Association between Antibiotic Resistance, Biofilm Formation and the Biochemical Pattern
The biochemical pattern (CoPS or CoNS) was analyzed in the investigated antibiotic-resistant isolates and the biofilm-forming ones (Table 6). It can be observed that a CoPS pattern was prevalent in most antibiotic-resistant isolates. It reached more than 65% of the isolates resistant to most studied antibiotics (six out of eight studied antibiotics). The maximum prevalence (79%) of CoPS pattern was witnessed in the vancomycin-resistant isolates. The isolates resistant to amoxicillin–clavulanate and imipenem exhibited less presence of CoPS pattern (51 and 45%, respectively). The relatively higher prevalence of CoPS in the antibiotic-resistant isolates was also observed in all biofilm-forming isolates, with either strong, medium or weak biofilm forming, indicating a high association between the two phenomena, irrespective of the degree of biofilm formation.
Table 6.
The association between antibiotic resistance, biofilm formation and biochemical pattern (CoPS or CoNS) in Staphylococci spp. isolates collected from medical samples.
| Antibiotic | Antibiotic Resistance |
Biofilm Formation | % CoPS * |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Strong | Medium | Weak | ||||||||||
| Quantity of Isolates | No. of Isolates | |||||||||||
| Total No |
% | Total No |
CoPS | CoNS | Total No |
CoPS | CoNS | Total No |
CoPS | CoNS | ||
| Vancomycin | 14 | 21 | 8 | 6 | 2 | 5 | 4 | 1 | 1 | 1 | 0 | 79 |
| Amox/clav | 39 | 59 | 19 | 8 | 11 | 14 | 7 | 7 | 6 | 5 | 1 | 51 |
| Erythpomycin | 43 | 65 | 17 | 11 | 6 | 17 | 9 | 8 | 9 | 8 | 1 | 65 |
| Trimeth/sulf | 29 | 44 | 10 | 6 | 4 | 12 | 8 | 4 | 7 | 7 | 0 | 72 |
| Imipenem | 33 | 50 | 17 | 7 | 10 | 14 | 7 | 7 | 2 | 1 | 1 | 45 |
| Oxacillin | 61 | 92 | 28 | 18 | 10 | 22 | 15 | 7 | 11 | 10 | 1 | 70 |
| Ceftriaxone | 47 | 71 | 20 | 13 | 5 | 18 | 15 | 4 | 9 | 8 | 0 | 77 |
| Clindamycin | 45 | 68 | 19 | 10 | 9 | 18 | 13 | 6 | 8 | 8 | 1 | 69 |
* Total no. of CoPS isolates in biofilm-forming isolates/total no. of antibiotic resistant isolates. The bold numbers indicate the prevalence of CoPS in comparison with CoNS.
4. Discussion
S. aureus was generally qualified as the most efficient bacterium in shielding itself against host antagonistic reactions through the production of biofilms. Biofilms can limit the bacterial clearance by antimicrobial agents and host immunological responses [15], and thus enables bacteria to invade host tissue, forming metastatic abscess growth leading to morbidity and mortality [16]. Sepsis caused by Staphylococcus aureus in cancer patients represents a substantial cause of morbidity and mortality in these patients [17].
The current analysis of 66 medical isolates revealed the presence of 46 isolates (69.7%) as CoPS, represented uniquely by S. aureus, and the presence of 20 medical isolates (30.3%) as CoNS, where S. epidermidis was the prevalent species. A similar outcome was noted by [18,19]. Hence, the spread of this type is complying with the global spreading pattern of this type (CoPS) and this species (S. aureus) without apparent impact from the geographical site.
A significant relationship between the biofilm type and the development of biofilms was reported in prior studies [20]. In the United States, a somewhat more significant proportion of biofilm-positive bacteria isolated from non-fluid locations, such as superficial/deep skin, bone, and the respiratory tract, than from host fluids, such as blood or urine, was seen in clinical trials [20]. However, in the current study, most of the investigated samples were fluids, i.e., 48 blood, 8 urine, 6 puss, 2 tubes and 1 drain. The biofilm formation phenomenon was prevalent and variably distributed among strong, intermediate and weak biofilm producers (data not shown). Most isolates belonged to the strong and intermediate biofilm producers; their sum reached 62 and 75% of blood and urine samples, respectively. The relatively higher proportion of strong and intermediate bio-film producers may agree with [21], who reported a higher incidence of urinary isolates in biofilms than in other clinical sample isolates.
A high degree of resistance against the frequently used antibiotics, e.g., oxacillin and ceftriaxone, clindamycin and erythromycin, has been noticed in the current study, indicating limited antimicrobial choices against staphylococci isolates. A lower degree of resistance was noticed against vancomycin. Comparable effects were also demonstrated by [22]. Although vancomycin was the least susceptible to microbial resistance, considerable proportions of developed resistance among CoPS and CoNS isolates amounting to about 24 and 15%, respectively, were recorded. Similar intermediate resistance of staphylococci isolates against vancomycin was previously reported [23]. This alarming report and the current findings should urgently motivate more research before we lose the race.
New proteins and peptides have been found to be effective antimicrobial agents [23,24,25] and can substitute the currently resisted antibiotics. Some new peptides and proteins were comparable or more effective than the current antibiotics in in vitro studies [26,27] and needed to be in vivo tested and validated. The relatively higher effectiveness of vancomycin against different pathogens may be partially due to its chemical nature as a glycopeptide, containing a carbohydrate moiety in its structure. Recently, a glycoprotein from catfish (CFG) exhibited considerable antibacterial activity. Combining CFG with specific antibiotics at equal ratios induced synergistic antibacterial actions [28]. Hence, these glycoproteins may be the target for generating new antibacterial agents capable of counteracting the developed antibiotic resistance.
The MTP assay method is the most reliable, precise and reproducible approach for determining biofilm development by the clinical isolates from staphylococci. It has the benefit of being a quantitative approach to compare compliance with various strains [29]. The current study results indicated that strong and medium biofilm-forming isolates represented the majority and amounted to about 80% of the total investigated clinical samples with a relatively greater frequency of having a CoPS biochemical pattern [30], with a relatively lower frequency of biofilm formation in staphylococcal clinical isolates, amounting to 57.8%. There is a strong association between biofilm formation and the resistance developed against the frequently used antibiotics [31,32]. Hence, this high proportion of biofilm formation may explain considerable resistance against most frequently used antibiotics.
In our study, icaA and icaB were found in 33% and 53% of selected isolates, respectively, while Al-Mtory et al. (2016) demonstrated that the prevalence of icaA and icaB was 95.8% and 91.6%, respectively [33]. The study of Diemond-Hernández et al. (2010) detected icaA in 10.3% but did not detect the icaB gene [34]. Our study did not identify all tested ica operon genes in the tested strains, in agreement with previous reports [34,35].
The statistical test results did not indicate a link between icaA and icaB genes with the formation of biofilms in selected Staphylococci spp. These results may mean that in staphylococci, biofilm formation is determined not only by the expression of biofilm genes, i.e., the genes icaA and icaB, but also by other factors inherent in the medium composition, e.g., salt content, iron and anaerobic atmosphere may be influential [36]. Although most of the studied strong biofilm-forming isolates indicated the presence of both icaA and icaB genes or one of them, a still considerable portion (33.3%) of isolates did not contain any of these genes. This result may refer to the presence of additional biofilm formation mechanisms that are not necessarily ica-dependent. Therefore, the research for these potentially unknown mechanisms may open the way for discovering new appropriate drugs against these pathogens. However, further studies using different clinical samples and techniques may be needed to confirm the results that have so far been obtained.
The prevalence of the biochemical pattern CoPS in the investigated antibiotic-resistant isolates in most antibiotic-resistance strains may indicate that this pattern helps in establishing antibiotic resistance. The high prevalence (65%) of the isolates resistant to most studied antibiotics may indicate the importance of this biochemical activity in establishing antibiotic resistance. Furthermore, since the biochemical type CoPS was highly prevalent (79%) in biofilm-forming vancomycin-resistant isolates, it may also be involved in developing antibiotic resistance and can be employed as an alarming indicator of potential antibiotic resistance. Additionally, the prevalence of CoPS in all biofilm-forming isolates irrespective of their degree of film formation may refer to a high association between this biochemical pattern and biofilm formation.
5. Conclusions
CoPS are the most abundant species among the total population of the medical samples, where S. aureus was the sole representative of this group. In the second order comes S. epidermidis, which is the most abundant species of CoNS. Antibiotic resistance is developing against most frequently used antimicrobial drugs, while vancomycin was the least-resisted antibiotic. However, the relatively small resistance (15–24%) against vancomycin necessitates an urgent search for other effective antibiotics or antimicrobial agents. The totality of the strong and medium biofilm-forming isolates represented a majority proportion, amounting to about 80% of the total investigated clinical samples with relatively more frequency in the CoNS than the CoPS type. The majority (66.7%) of the studied strong biofilm-forming isolates indicated the presence of both icaA and icaB genes or one of them, while a still considerable portion (33.3%) of isolates did not contain any of them. Additionally, the icaB gene could be noticed as more associated with strong biofilm formation in the two groups of staphylococci species (CoPS and CoNS). This result may refer to the presence of additional biofilm formation mechanisms that are not necessarily ica-dependent. Therefore, research into these potentially unknown mechanisms may help us find the appropriate drugs for combatting these pathogens. The prevalence of biochemical pattern CoPS in antibiotic-resistant isolates may indicate some association between antibiotic resistance and biofilm formation. CoPS can serve as an alarming indicator of potential antibiotic resistance. Further studies using different clinical samples and different techniques may be needed to confirm the thus-far obtained results and conclusions.
Acknowledgments
The authors would like to thank the University of Zagazig and the University of Mansoura for their assistance in conducting the research. We also appreciate the assistance with Mansoura University Hospital samples and the County Council of Västerbotten (BS), Cancer Research Foundation in Northern Sweden and Lion’s Cancer Research Foundationin Northern Sweden (BS).
Author Contributions
M.S., M.Z. and B.S.: design of methodologies, collections of the samples and examination of the laboratory, confirmation of evidence, writing and revising of manuscripts; the experimental design. H.E.-S., Y.E.-Z., B.S. and S.A.-S.: planning the report, funding acquisition, project management, personnel and evaluation. M.S.: revising results and the final manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
Zagazig University, Mansoura University and the County Council of Västerbotten (BS), Cancer Research Foundation in Northern Sweden and Lion’s Cancer Research Foundationin Northern Sweden (BS).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Zheng Y., He L., Asiamah T.K., Otto M. Colonization of Medical Devices by Staphylococci. Environ. Microbiol. 2018;20:3141–3153. doi: 10.1111/1462-2920.14129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Otto M. Staphylococcus Colonization of the Skin and Antimicrobial Peptides. Expert Rev. Dermatol. 2010;5:183–195. doi: 10.1586/edm.10.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tang L., Pillai S., Revsbech N.P., Schramm A., Bischoff C., Meyer R.L. Biofilm Retention on Surfaces with Variable Roughness and Hydrophobicity. Biofouling. 2011;27:111–121. doi: 10.1080/08927014.2010.544848. [DOI] [PubMed] [Google Scholar]
- 4.Khadije R.K., Aliyeh S., Mehdi H., Zahra S. Detection of Intercellular Adhesion Genes (icaA and icaD) in Staphylococcus Aureus Clinical Isolates in Zabol-Iran. Res. Mol. Med. 2017;5:40. [Google Scholar]
- 5.Namvar A.E., Asghari B., Ezzatifar F., Azizi G., Lari A.R. Detection of the Intercellular Adhesion Gene Cluster (ica) in Clinical Staphylococcus Aureus Isolates. GMS Hyg. Infect. Control. 2013;8:Doc03. doi: 10.3205/dgkh000203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kot B., Sytykiewicz H., Sprawka I., Witeska M. Effect of manuka honey on bioflm-associated genes expression during methicillin-resistant Staphylococcus aureus bioflm formation. Sci. Rep. 2020;10:13552. doi: 10.1038/s41598-020-70666-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Carla R.A., Davide C., Stefano R., Lucio M. Polysaccharide Intercellular Adhesinin Biofilm: Structural and Regulatory Aspects. Front Cell Infect. Microbiol. 2015;5:1. doi: 10.3389/fcimb.2015.00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cue D., Lei M.G., Lee C.Y. Genetic Regulation of the Intercellular Adhesion Locus in Staphylococci. Front. Cell. Infect. Microbiol. 2012;2:38. doi: 10.3389/fcimb.2012.00038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cheesbrough M. District Laboratory Practice in Tropical Countries. Cambridge University Press; Cambridge, UK: 2006. [Google Scholar]
- 10.Patel J.B. Performance Standards for Antimicrobial Susceptibility Testing. Clin. Lab. Stand. Inst. 2015;100:25. [Google Scholar]
- 11.Torlak E., Korkut E., Uncu A.T., Şener Y. Biofilm Formation by Staphylococcus Aureus Isolates from a Dental Clinic in Konya, Turkey. J. Infect. Public Health. 2017;10:809–813. doi: 10.1016/j.jiph.2017.01.004. [DOI] [PubMed] [Google Scholar]
- 12.Omidi M., Firoozeh F., Saffari M., Sedaghat H., Zibaei M., Khaledi A. Ability of Biofilm Production and Molecular Analysis of spa and ica Genes among Clinical Isolates of Methicillin-Resistant Staphylococcus Aureus. BMC Res. Notes. 2020;13:19. doi: 10.1186/s13104-020-4885-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mirzaee M., Peerayeh S.N., Ghasemian A. Detection of icaABCD Genes and Biofilm Formation in Clinical Isolates of Methicillin Resistant Staphylococcus Aureus. Iran. J. Pathol. 2014;9:257–262. [Google Scholar]
- 14.Deighton M.A., Capstick J., Domalewski E., Van Nguyen T. Methods for Studying Biofilms Produced by Staphylococcus Epidermidis. Methods Enzymol. 2001;336:177–195. doi: 10.1016/s0076-6879(01)36589-8. [DOI] [PubMed] [Google Scholar]
- 15.Kifaya A., Walaa Q., Ziad A. Screening of Genes Encoding Adhesion Factors and Biofilm Production in Methicillin Resistant Strains of Staphylococcus Aureus Isolated from Palestinian Patients. BMC Genom. 2019;20:578. doi: 10.1186/s12864-019-5929-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Edwards A.M., Bowden M.G., Brown E.L., Laabei M., Massey R.C. Staphylococcus Aureus Extracellular Adherence Protein Triggers TNF Alpha Release, Promoting Attachment to Endothelial Cells via Protein A. PLoS ONE. 2012;7:e43046. doi: 10.1371/journal.pone.0043046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bello-Chavolla O.Y., Bahena-Lopez J.P., Garciadiego-Fosass P., Volkow P., Garcia-Horton A., Velazquez-Acosta C., Vilar-Compte D. Bloodstream Infection Caused by S. Aureus in Patients with Cancer: A 10-Year Longitudinal Single-Center Study. Support Care Cancer. 2018;26:4057–4065. doi: 10.1007/s00520-018-4275-1. [DOI] [PubMed] [Google Scholar]
- 18.Rengaraj R., Velu P., Sekar U. Prevalence of MRSA and Biofilm Associated Gene among Clinical Isolates of Staphylococci. Int. J. Curr. Microbiol. App. Sci. 2018;7:745–752. [Google Scholar]
- 19.Adeleye I.A., Akanmu A.S., Bamiro B.S., Obosi A.C., Unem A.V. Bacterial Bloodstream Infections in HIV—Infected Adults Attending a Lagos Teaching Hospital. J. Health Popul. Nutr. 2010;28:318–326. doi: 10.3329/jhpn.v28i4.6037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sánchez C.J., Mende K., Beckius M.L., Akers K.S., Romano D.R., Wenke J.C., Murray C.K. Biofilm Formation by Clinical Isolates and the Implications in Chronic Infections. BMC Infect. Dis. 2013;13:47. doi: 10.1186/1471-2334-13-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Solati S.M., Tajbakhsh E., Khamesipour F., Gugnani H.C. Prevalence of Virulence genes of Biofilm Producing Strains of Staphylococcus Epidermidis Isolated from Clinical Samples in Iran. AMB Express. 2015;5:47. doi: 10.1186/s13568-015-0134-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ghasemian A., Peerayeh S.N., Bakhshi B., Mirzaee M. Comparison of Biofilm Formation between Methicillin-Resistant and Methicillin-Susceptible Isolates of Staphylococcus Aureus. Iran. Biomed. J. 2016;20:175–181. doi: 10.7508/ibj.2016.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Osman A., El-Didamony G., Sitohy M., Khalifa M., Enan G. Soybean Glycinin Basic Subunit Inhibits Methicillin Resistant-Vancomycin Intermediate Staphylococcus Aureus (MRSA-VISA) in Vitro. Int. J. Appl. Res. Nat. Prod. 2016;9:17–26. [Google Scholar]
- 24.Al-Mohammadi A.R., Osman A., Enan G., Sitohy M., Taha M.A. Powerful Antibacterial Peptides from Egg Albumin Hydrolysates. Antibiotics. 2020;9:901. doi: 10.3390/antibiotics9120901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Saad A.M., Sitohy M.Z., Ahmed A.I., Soliman M.M., El-Saadony M.T. Biochemical and Functional Characterization of Kidney Bean Protein Alcalase-Hydrolysates and Their Preservative Action on Stored Chicken Meat. Molecules. 2021;26:4690. doi: 10.3390/molecules26154690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sitohy M., Mahgoub S., Osman A. Controlling Psychrotrophic Bacteria in Raw Buffalo Milk Preserved at 4 °C with Esterified Legume Proteins. LWT-Food Sci. Technol. 2011;44:1697–1702. doi: 10.1016/j.lwt.2011.03.008. [DOI] [Google Scholar]
- 27.Abdel-Shafi S., Osman A., Enan G., El-Nemer M., Sitohy M. Antibacterial Activity of Methylated Egg White Proteins against Pathogenic G+ and G− Bacteria Matching Antibiotics. SpringerPlus. 2016;5:983. doi: 10.1186/s40064-016-2625-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Abdel-Shafi S., Osman A., Al-Mohammadi A.-R., Kamal N., Sitohy M. Biochemical, Biological Characteristics and Antibacterial Activity of Glycoprotein Extracted from the Epidermal Mucus of African Catfish (Clarias Gariepinus) Int. J. Biol. Macromol. 2019;138:773–780. doi: 10.1016/j.ijbiomac.2019.07.150. [DOI] [PubMed] [Google Scholar]
- 29.Triveni A.G., Kumar M.S., Manjunath C., Shivnnavar C.T., Gaddad S.M. Biofilm Formation by Clinically Isolated Staphylococcus Aureus from India. J. Infect. Dev. Ctries. 2018;12:1062–1066. doi: 10.3855/jidc.10671. [DOI] [PubMed] [Google Scholar]
- 30.Gad G.F.M., El-Feky M.A., El-Rehewy M.S., Hassan M.A., Abolella H., Abd El-Baky R.M. Detection of icaA, icaD Genes and Biofilm Production by Staphylococcus Aureus and Staphylococcus Epidermidis Isolated from Urinary Tract Catheterized Patients. J. Infect. Dev. Ctries. 2009;3:342–351. doi: 10.3855/jidc.241. [DOI] [PubMed] [Google Scholar]
- 31.Nirwati H., Sinanjung K., Fahrunissa F., Wijaya F., Napitupulu S., Hati V.P., Nuryastuti T. Biofilm formation and antibiotic Resistance of Klebsiella Pneumoniae Isolated from Clinical Samples in a Tertiary Care Hospital, Klaten, Indonesia. BMC. 2019;13:20. doi: 10.1186/s12919-019-0176-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fauzia K.A., Miftahussurur M., Syam A.F., Waskito L.A., Doohan D., Rezkitha Y.A.A., Yamaoka Y. Biofilm Formation and Antibiotic Resistance Phenotype of Helicobacter Pylori Clinical Isolates. Toxins. 2020;12:473. doi: 10.3390/toxins12080473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Al-Mtory H.K.Y., Al-Shukri M.S., Al-Hassnawi H.H. Detection of Intracellular Adhesion (ica) Genes and Biofilm Formation in Staphylococcus spp. Isolated from Different Clinical Samples. Glob. J. Biochem. Biotechnol. 2016;5:505–511. [Google Scholar]
- 34.Diemond-Hernandez B., Solorzano- Santos F., Leanos-Miranda B., Peregrino-Bejarano L., Miranda- Novales G. Production of icaADBC-Encoded Polysaccharide Intercellular Adhesin and Therapeutic Failure in Pediatric Patients with Staphylococcal Device-Related Infections. BMC Infect. Dis. 2010;10:68. doi: 10.1186/1471-2334-10-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bazari P.A., Jahromy S.H., Karizi S.Z. Phenotypic and Genotypic Characterization of Biofilm Formation among Staphylococcus Aureus Isolates from Clinical Specimens, an Atomic Force Microscopic (AFM) Study, Phenotypic and Genotypic Characterization of Biofilm Formation among Staphylococcus Aureus Isolates from Clinical Specimens, an Atomic Force Microscopic (AFM) Study. Microb. Pathog. 2017;110:533–539. doi: 10.1016/j.micpath.2017.07.041. [DOI] [PubMed] [Google Scholar]
- 36.Arciola C.R., Campoccia D., Speziale P., Montanaro L., Costerton J.W. Biofilm Formation in Staphylococcus Implant Infections. A Review of Molecular Mechanisms and Implications for Biofilm-Resistant Materials. Biomaterials. 2012;33:5967–5982. doi: 10.1016/j.biomaterials.2012.05.031. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.