Significance
We studied goose bones from Tianluoshan—a 7,000-y-old rice cultivation village in the lower Yangtze River valley, China—using histological, geochemical, biochemical, and morphological approaches. Our analyses reveal an early stage of goose domestication at Tianluoshan. The goose population seemed to have been maintained for several generations without the introduction of individuals from other populations and might have been fed cultivated paddy rice. These findings indicate that goose domestication dates back 7,000 y, making geese the oldest domesticated poultry species in history.
Keywords: poultry farming, domestication, Middle Neolithic, stable isotope composition
Abstract
Poultry are farmed globally, with chicken (Gallus gallus domesticus) being the leading domesticated species. Although domestic chicken bones have been reported from some Early Holocene sites, their origin is controversial and there is no reliable domestic chicken bone older than the Middle Holocene. Here, we studied goose bones from Tianluoshan—a 7,000-y-old rice cultivation village in the lower Yangtze River valley, China—using histological, geochemical, biochemical, and morphological approaches. Histological analysis revealed that one of the bones was derived from a locally bred chick, although no wild goose species breed in southern China. The analysis of oxygen-stable isotope composition supported this observation and further revealed that some of the mature bones were also derived from locally bred individuals. The nitrogen-stable isotope composition showed that locally bred mature birds fed on foods different from those eaten by migrant individuals. Morphological analysis revealed that the locally bred mature birds were homogenous in size, whereas radiocarbon dating clearly demonstrated that the samples from locally bred individuals were ∼7,000 y old. The histological, geochemical, biochemical, morphological, and contextual evidence suggest that geese at Tianluoshan village were at an early stage of domestication. The goose population appears to have been maintained for several generations without the introduction of individuals from other populations and may have been fed cultivated paddy rice. These findings indicate that goose domestication dates back 7,000 y, making geese the oldest domesticated poultry species in history.
The domestication of plants and animals is one of the most important human innovations (1–3). Therefore, the investigation of when, where, and how domestication took place has fascinated researchers from many different disciplines, including the physical, biological, and social sciences (1–3). Domesticated birds raised for eggs, meat, and feathers are referred to as poultry. The term “poultry” covers a wide range of birds, including indigenous and commercial breeds of chickens, geese, ducks, turkeys, guinea fowl, and pigeons. Currently, poultry are raised globally, with chicken (Gallus gallus domesticus) being the most commonly farmed species. In 2019, chickens accounted for ∼92.9% of the world’s poultry population, followed by ducks (4.2%), turkeys (1.5%), and geese and guinea fowl (1.3% in total) (4). The total number of poultry individuals is five times higher than that of mammalian livestock (4). However, the history of poultry domestication has received less attention than that of mammalian livestock (5). With respect to the earliest evidence of domestication, chickens have been observed between the third and second millennium BC; geese, turkeys, and peafowl from the second millennium BC; and ducks and pigeons from the first millennium BC (1, 2, 5). Although studies have found domestic chicken bones dating to the ninth millennium BC and, thus, reported a much longer history of chicken domestication (6–8), the reliability of these studies is questionable (5, 9–14). In this study, we analyzed goose bones from Tianluoshan in southern China to investigate if the history of the domesticated goose—currently a minor poultry species—dates back to the fifth millennium BC, thereby making geese the oldest domesticated poultry species in history.
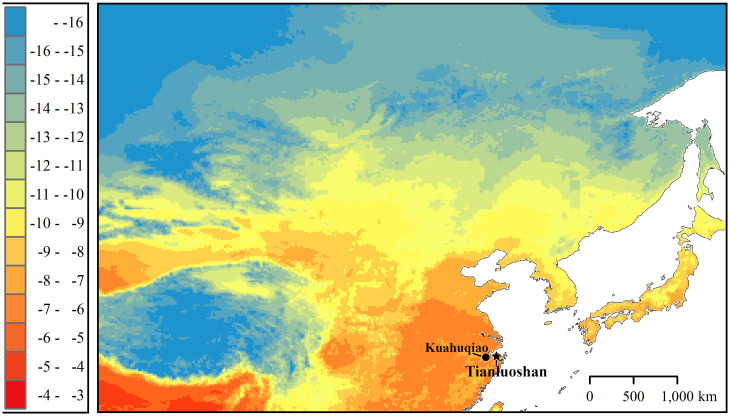
The Tianluoshan site contains the remains of a Middle Neolithic early rice cultivation village (7000 to 5500 cal BP) of the Hemudu Culture, and is located in the lower Yangtze River valley, China (121° 22′ 46″ E, 30° 01′ 27″ N) (Fig. 1) (15). The village was situated in the center of a basin, ∼1 km2 in size, adjacent to a freshwater subwetland, and was nearly 10 km from the East China Sea coastline (15, 16). Archaeological surveys revealed that the village included raised-floor–style wooden buildings, wooden fences, storage and refuse pits, and rice paddy fields. Although people cultivated rice and possibly kept pigs, they mainly hunted, fished, and gathered food from terrestrial and water environments (17–19). In an earlier analysis of bird remains from the site, we found that ducks (Anatinae), rails (Rallidae), and geese (Anserinae) dominated in every stratigraphic layer, and concluded that the primary avifaunal taxa exploited at the site were birds wintering in inland waters (20). Moreover, we also identified immature goose bones. The lower Yangtze River and adjacent regions currently host six species of migratory goose species that winter in the region (21). There are no breeding goose species in the lower Yangtze River valley, and it is unlikely that any goose species were breeding near the site during the warmest period of the Holocene when the site was active. If these immature goose bones were from individuals shown to have originated near the site, they may have belonged to a captive bred population (20). The medullary bone—a secondary woven bone tissue in the marrow cavities of breeding female birds (22)—disappears before the birds arrive at their wintering areas, and is therefore considered a reliable indicator of domestic geese in their wintering areas (23). Demonstrating that the bones of adult geese found at the wintering sites were derived from nonmigratory resident individuals would suggest human influence. The existence of adult domestic geese would also indicate successful attempts at raising domestic geese.
Fig. 1.
Distribution map for oxygen isotope compositions of annual precipitation (‰) across Northeast Asia (28, 42) and excavation locations for bone samples used in this study.
Ecological studies frequently reconstruct bird migration by analyzing the oxygen isotope composition (δ18O) (24, 25). The δ18O of precipitation varies geographically and is correlated with latitude, altitude, and distance from oceans (26–28). This is due to isotope effects associated with the evaporation and condensation processes of meteoric water. As the oxygen in animal body tissues is derived mainly from drinking water and food (29, 30), the δ18O of animal body tissues is correlated with the δ18O of the local meteoric water and precipitation (25, 31, 32). Therefore, it is possible to estimate the habitat area of a target animal using the δ18O values of its tissues. These values trace the migrant bird’s place of origin, which may be different from the area in which the individual was captured. A few ecological studies on modern birds have used feathers for measurement (33, 34), whereas archeological studies typically use bone or teeth samples to analyze carbonates (35) and phosphates (36).
In this study, we conducted morphological, histological, biochemical, and geochemical analyses to reveal goose domestication in a 7,000-y-old rice cultivation village in the lower Yangtze River valley. We analyzed immature goose bones from museum collections to determine the age (in weeks) of immature goose bones from the Tianluoshan site. We also measured the δ18O of phosphates (δ18OP) in the archeological bones of adult and immature geese to identify whether they were resident birds inhabiting the lower Yangtze River all year round. Furthermore, we conducted radiocarbon dating to confirm that the bones were not derived from more recent contaminants. Based on these multiple sources of evidence, we discuss the possibility that domestic geese were present at Tianluoshan and the subsequent significance of this for understanding the history of poultry domestication.
Results
Histological Analysis.
Following naked-eye observation of the bones, 4 of the 232 goose bones found in Tianluoshan were identified as immature geese (Fig. 2). Two were found in the stratigraphic layer (Layer) 6, and one each in Layers 7 and 8. Medullary bone was not observed. Upon comparison with those of age-known immature goose specimens (SI Appendix, Table S1), the femur, tibiotarsus, and ulna were determined to be from 4- to 16-wk-old geese based on their porous texture. The tarsometatarsus was determined to be from a 4- to 8-wk-old individual, based on an unfused proximal tarsal bone.
Fig. 2.
Immature and local bred goose bones found at Tianluoshan. 1, ulna; 2, femur; 3, tibiotarsus; 4, tarsometatarsus; 5, humerus; 6, carpometacarpus; 7, tibiotarsus; and 8, tarsometatarsus. Note that 1 to 4 are immature, whereas 5 to 8 are mature.
Oxygen Isotope Analysis of Bone Phosphate.
The silver phosphate was purified and the δ18OP values were measured from 25 goose and 11 mammal bones from Tianluoshan and 5 goose bones from Kuahuqiao (8200 to 7000 cal BP). The δ18OP values (mean ± SD [σ]) were 13.9 ± 2.2 ‰ (geese, Tianluoshan) (SI Appendix, Fig. S1 and Table S1), 11.5 ± 3.0 ‰ (geese, Kuahuqiao) (SI Appendix, Table S2), and 17.3 ± 0.7 ‰ (mammals, Tianluoshan) (SI Appendix, Table S2). After correcting the δ18OP values in relation to the δ18O in drinking water (δ18OW), the δ18OW values were −8.6 ± 2.5 ‰ (geese, Tianluoshan), −11.3 ± 3.4 ‰ (geese, Kuahuqiao), and −6.3 ± 0.8 ‰ (mammals, Tianluoshan). Using the mean ± σ of δ18OW in mammalian bones from the Tianluoshan site as an indicator of the local δ18OW values, four (two each from Layers 6 and 8) goose bones from Tianluoshan and none from Kuahuqiao were assigned to local individuals. One of the three immature goose bones from Tianluoshan was assigned to a local individual; however, the others had considerably higher δ18OW values than those of the local mammals.
Carbon and Nitrogen Isotope Analyses of Bone Collagen.
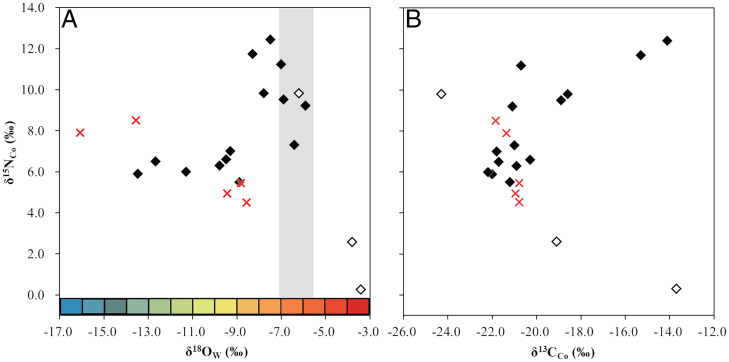
Nineteen of the 25 goose bones from Tianluoshan and all goose bones from Kuahuqiao were used to measure δ13CCo and δ15NCo (Fig. 3 and SI Appendix, Tables S2 and S3). The δ13CCo and δ15NCo values in the geese bones from Tianluoshan were −19.8 ± 3.0 ‰ and 7.5 ± 3.0 ‰, respectively. The δ13CCo and δ15NCo values in the Kuahuqiao bones were −21.1 ± 0.5 ‰ and 6.3 ± 1.8 ‰, respectively.
Fig. 3.
Isotope compositions of oxygen, nitrogen, and carbon in goose bones. (A) δ15NCo and δ18OW, and (B) δ15NCo and δ13CCo in goose bones found at the Tianluoshan site (adult: closed diamonds, immature: open diamonds) and the Kuahuqiao site (adult: red cross symbols). The gray area indicates the range in δ18OW values (mean ± σ) in mammal bones from Tianluoshan. Note that the samples represented by the five symbols in the gray area are presumably from local individuals. The colored bars that represent δ18OW values correspond to the same colors used in Fig. 1 for the oxygen isotope composition of annual precipitation.
Radiocarbon Dating of Bone Collagen.
The 14C ages of the bones of five geese, including three immatures and two locally bred mature geese assigned by oxygen isotopes, were determined between 5885 ± 35 BP and 6085 ± 40 BP. After calibration, these ages were calculated as 7150 to 6670 cal BP (SI Appendix, Fig. S2 and Table S1).
Morphological Analysis.
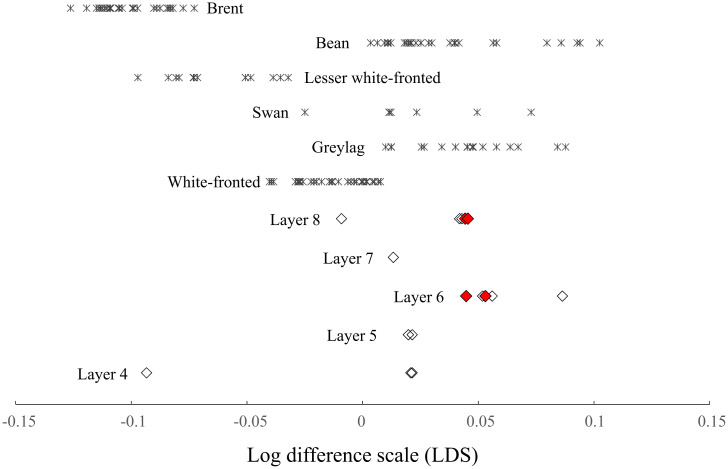
At least one data point was obtained from 16 of the 22 mature goose bones. Log difference scale (LDS) values were then calculated (range, −0.093 to 0.086; mean, 0.029) (Fig. 4 and SI Appendix, Table S2). Compared to those of the six extant goose species, the archeological samples ranged from larger species (swan goose [Anser cygnoides], bean goose [Anser fabalis], and greylag goose [Anser anser]) to smaller species (lesser white-fronted goose [Anser erythropus] and brant goose [Branta bernicla]). The four bones assigned to locally bred individuals (LDS range, 0.044 to 0.053; mean, 0.047) corresponded to the larger species. In the randomization test, the observed LDS variance of the four bones was significantly lower than that of the random sampling of the analyzed bones (P < 0.01). For F-test with Bonferroni correction, the variance for the locally bred individuals was significantly lower than that for the modern swan goose (F = 49.360; df = 7, 3; P < 0.05) and lesser white-fronted goose (F = 26.467; df = 12, 3; P < 0.05).
Fig. 4.
LDS for mature goose bones: modern species (cross symbols) and archaeological bones from Tianluoshan (squares). Red (closed) and open diamonds indicate the samples assigned to local and migrant individuals, respectively (based on the stable isotope composition of oxygen). Some goose bones are stored at the Bavarian State Collection for Anthropology and Paleoanatomy, Munich (Germany), Historic England (United Kingdom) Hokkaido University Museum (Japan), Nara National Research Institute for Cultural Properties (Japan), the National Science Museum, Tokyo (Japan), the Natural History Museum at Tring (United Kingdom), the Smithsonian National Museum of Natural History (United States), Southampton University (United Kingdom), the University of Copenhagen Zoological Museum (Denmark), Yamashina Institute for Ornithology (Japan), and few are the personal collections of Kazuto Kawakami (Forestry and Forest Products Research Institute, Tsukuba, Japan) and Toyohiro Nishimoto (National Museum of Japanese History, Sakura, Japan).
Discussion
Multiple Sources of Evidence for the Early Stage of Goose Domestication at Tianluoshan.
Histological evidence.
Of the 232 goose bones found in Tianluoshan, 3 were estimated to be 4 to 16 wk old, and 1 (no. 539) (SI Appendix, Table S2) was estimated to be 4 to 8 wk old. Recently, goslings have been observed traveling long distances, arriving at wintering grounds at the age of 16 wk, despite being present at breeding sites at the age of 8 wk (37). Therefore, the three archeological bones could have belonged to birds that migrated from breeding areas to Tianluoshan, where they were hunted. However, the latter bone likely belonged to a bird that originated in the region, as 8-wk-old birds are too young to migrate to wintering areas (37). This suggests that at least the tarsometatarsus (no. 539) (SI Appendix, Table S2) was from a locally bred individual. Although the presence of bones, including medullary bone, was considered to be a reliable indicator of domestic geese in their wintering areas (23), no goose bones, including medullary bone, were found in Tianluoshan. The absence of medullary bone could be explained by the limited formation periods (approximately 1 or 2 mo in a year) (38) of this secondary bone structure and exploitation strategies that did not involve the consumption of breeding female birds.
Geochemical evidence.
As a result of its densely packed crystalline structure, tooth enamel is usually considered to be a reliable material for oxygen isotope analyses of archaeological samples (39, 40). However, geese, the target animal of this study, have no tooth enamel, so we measured the δ18OP in bone apatite. To evaluate the diagenetic effects of phosphate in bird bones, we measured the δ18OP in tooth enamel, tooth dentine, and bone apatite from each water buffalo from Tianluoshan (SI Appendix, S1 Preservation Status and Diagenetic Alternation for Bioapatite). The difference between the δ18OP in tooth enamel, and bone (−0.1 ‰) and dentine (−0.1 ‰) was smaller than the SD among the δ18OP in local mammal bones (0.7 ‰). This suggested that bone apatite was preserved to the same standard as tooth enamel. Therefore, it was expected that diagenetic δ18OP shifts would also not have occurred in the goose bones that were exposed to the same depositional environment in Tianluoshan, nor would it have affected the habitat estimates in this study.
The δ18OW in the Tianluoshan mammals was −6.3 ± 0.8 ‰, which is comparable to the δ18OW in precipitation based on the current annual mean in Zhejiang Province (−6.8 ‰) (41), where the Tianluoshan site is located. This suggests that the δ18OW in precipitation has not changed significantly, and that it is a useful metric to discriminate resident animals from migrants. Assuming that the δ18OW (mean ± σ) in mammalian bones from Tianluoshan was indicative of the local values, 4 of the 22 mature goose bones from Tianluoshan were considered to be from local individuals. The δ18OW in precipitation is lower in the subarctic region (where wild geese breed) than in the lower Yangtze River (28, 42); therefore, the δ18OW in wild geese migrating between breeding grounds and the lower Yangtze River is expected to be at an intermediate level between the δ18OW values of the two places. Therefore, 18 mature goose samples with δ18OW values lower than those of the mammals were considered to be migrant individuals.
Notably, the δ18OW values of all three immature geese were as high as—or higher than—those of the local mammals: one ulna (no. 54) had a δ18OW value of −6.2 ‰ and was assigned to a local individual, whereas a tarsometatarsus (no. 539) and a femur (no. 504) had δ18OW values of −3.8 and −3.4 ‰, respectively. If immature birds died soon after migrating from the breeding grounds, the influence of the water in the wintering ground would be lower on immature birds than on adults, and their δ18OW values would be much lower than in the precipitation in the lower Yangtze River valley. Therefore, two immature birds found at Tianluoshan were not considered to have been born in the subarctic region. This result was concordant with the histological analysis in which the individual that the tarsometatarsus (no. 539) belonged to was considered to be too young to migrate from the breeding area. In the Egyptian goose (Alopochen aegyptiaca), the oxygen isotope composition of water in the egg albumin is known to be 2.1 ± 0.4 ‰ higher than that in the drinking water of parent birds (43). Therefore, the higher δ18OW observed in the two immature birds may have been retained from the egg stage.
Biochemical evidence.
Combining δ18OW and δ15NCo, 14 mature goose samples were divided into 2 groups: 7 samples with higher δ18OW and δ15NCo values and 7 samples with lower δ18OW and δ15NCo values. As δ15N reflects ingested foods—especially the trophic level (44)—results suggest that the samples came from two different food groups. All four samples assigned to local individuals were included in the higher δ18OW and δ15NCo group, whereas the other group consisted of samples assigned to migrant individuals. This suggested that local individuals consumed different foods compared to migrant individuals. The nitrogen isotope composition of paddy rice cultivated in an anaerobic environment increases because of denitrification in the soil (45, 46). Therefore, the increase in δ15NCo observed in local geese may have been due to paddy rice consumption. In contrast, δ15N decreases with decreasing annual temperature (47). It is possible that migrant geese have lower δ15N values than local geese in low latitudes because they forage in high latitudes with lower temperatures. Alternatively, other mechanisms, such as animal dung input as manure (48) and denitrification caused by frequent seasonal flooding (46, 49), can lead to soil 15N enrichment. However, although it is thought that pigs were domesticated at Tianluoshan, the animal bones are more likely to have been dominated by the process of hunting (50, 51). Based on low levels of animal management at the site, we believe that manuring with animal dung was rarely practiced in Tianluoshan.
Three samples assigned to migrant individuals were included in the group with higher δ15NCo values. It is possible that the threshold for local individuals was too narrow and that these three individuals were also derived from a local population. However, in terms of the δ13CCo, two of the three samples had much higher values (greater than −16.0 ‰) than the others (less than −18.0 ‰). As δ13C reflects ingested foods—especially the derived food chain (52)—results suggest that the samples mainly differed because of different food chain items. A δ13CCo range of −18.0 ‰ to −22.0 ‰ was proposed for C3 plant-eating animals in northwestern China (53, 54). The δ13CCo range for individuals with lower values (−18.6 ‰ to −22.0 ‰) was consistent with the proposed range, whereas the δ13CCo values of the two individuals with higher values were outside this range. Besides, a water buffalo from Tianluoshan had high δ13CCo and δ15NCo values (SI Appendix, Table S3), which suggests they exploited wild C4 plants with high δ15NCo values. Although the use of cultivated C4 plants—such as millet and foxtail millet—has not been found from the analysis of plant remains (18) or the δ13CCo and δ15NCo in human bones from Tianluoshan (55), wild C4 sedges such as Cyperaceae may have grown in wetlands and on riverbanks (56). In addition, because δ15N in plants from waterlogged environments is higher than that of plants from well-drained environments (57), the high δ15NCo in the water buffalo and the two geese with high δ13CCo may be attributed primarily to the consumption of C4 plants in the wetlands around Tianluoshan. Alternatively, high δ13CCo and high δ15NCo values could be associated with feeding in the marine ecosystem. For example, brant geese prefer to feed on sea grasses and green algae with high δ13C (−11.2 ‰ to −14.1 ‰) values (58) and winter in the coastal areas of the lower Yangtze River (21), suggesting that the two samples with high δ13CCo and δ15NCo values may have been derived from wild geese that fed in the marine ecosystem.
The δ13CCo and δ15NCo values in the three immature birds were highly variable. The values of one immature assigned as a local individual were similar to those of mature local birds, suggesting that the immature birds shared similar food sources with local matures birds. In contrast, two immatures with higher δ18OW values than the range for local mammals showed lower δ15NCo and higher δ13CCo values than local mature individuals. A study of wild geese and other bird species reported that in embryos synthesized from parental foods, down feathers have isotope discrimination factors that are higher for δ13C and lower for δ15N than adult feathers (59). The differences in δ13C and δ15N values between chick feathers in the embryo and adult feathers shown in the aforementioned study were smaller than those observed in this study; however, the immatures with lower δ15NCo and higher δ13CCo values were considered very young individuals, who were still strongly influenced by their egg stages.
Morphological evidence.
The LDS values of the four mature goose bones assigned to local individuals were 0.044 to 0.053 and corresponded to larger species, such as swan geese, bean geese, and greylag geese. The observed LDS variance of the bones was significantly lower than that observed from a random sampling of the analyzed bones and that of the modern monomorphic goose species. This suggests that the local population was maintained for several generations without the influx of individuals from outside populations, and that their body size may have been homogenized.
Contextual evidence.
Radiocarbon dating clearly showed that the five geese—including three immatures and two locally bred matures, assigned by oxygen isotope analysis—dated to 7150 to 6670 cal BP (SI Appendix, Fig. S2). There was some mixing in the sample, as the oldest age was observed for an immature femur from Layer 6 and the youngest was observed for a mature humerus from Layer 8. However, all of these samples belonged to the Hemudu Culture period (7000 to 5300 cal BP) (60) and were not intrusive. In contrast to those from Tianluoshan, all five goose samples from Kuahuqiao were assigned to migrant individuals based on δ18OW values, and none were assigned to locally bred individuals. The carbon- and nitrogen-stable isotope compositions of goose bones from Kuahuqiao were also consistent with the previously proposed range for C3 plant-eating animals in northwestern China (53, 54), suggesting that these geese mainly fed on terrestrial C3 plants.
Currently, six goose species (greater white-fronted goose, swan goose, bean goose, lesser white-fronted goose, greylag goose, and brant goose) winter in the lower Yangtze River and adjacent regions but none of them breed in the region (21, 37). Concerning the large-sized species: swan geese breed in the southern regions of the Altai Mountains from Mongolia to coastal northern China; greylag geese breed across Europe and Asia, including central and southern Russia, Mongolia, and northern China; and bean geese breed in northern Eurasia north of the borders of Russia, Mongolia, and China (SI Appendix, Fig. S1) (21, 37). Therefore, the lower Yangtze River is at least 1,500 km south from the current southern limit of the breeding range for these three large-sized goose species. In the region that encompasses the lower Yangtze River, the warmest and wettest period is thought to have been around 7,000 to 6,000 y ago, and, thereafter, both temperature and humidity have gradually decreased (61, 62). It is unlikely that any goose species were breeding in the lower Yangtze River during a warmer and more humid period than the present day. Furthermore, there were no records of immature goose bones (or bones containing medullary bone) in Kuahuqiao, Liangzhu (5300 to 2500 cal BP), and other Neolithic sites in the lower Yangtze River and adjacent regions (63–66), suggesting that wild geese did not breed in these regions in the Early and Middle Holocene periods.
The histological, geochemical, biochemical, morphological, and contextual evidence suggest that large-sized geese were domesticated or at least were at the early stage of domestication in Tianluoshan, a 7000-y-old rice cultivation village in the lower Yangtze River valley. The locally bred goose population appears to have been maintained for several generations without the introduction of individuals from other populations. Locally bred geese likely fed on different food sources—including cultivated paddy rice—compared to wild migrant geese. In contrast, we did not find any evidence of goose domestication in the Kuahuqiao Cultural period, suggesting that goose domestication may have started during the Kuahuqiao Culture (Early Neolithic) and the Hemudu Culture periods (Middle Neolithic).
In Tianluoshan, geese accounted for 7.4% of the identified specimens and were the third most exploited avian taxa. Ducks dominated in this respect (61.4%), followed by rails (24.6%) (20). The presence of many individuals with low δ18OW values revealed that the hunting of wild migrant geese continued to be an essential activity even after keeping locally bred geese. Geese—and almost all ducks—winter in the lower Yangtze River (21). The rails that inhabit this area include summer, winter, and resident birds (21), indicating that avifaunal resources were mainly acquired during winter, with some species of rails being exploited during other seasons (20). It is possible that geese were bred to compensate for the reduced number of available birds from spring to autumn. Butchering and manufacturing marks were found on the goose bones, suggesting that both locally bred and wild geese provided meat and raw materials for bone tools, such as awls, needle holders, and other instruments (67). The demand for locally bred geese could have included some ceremonial activities, such as sacrifices, during spring and autumn.
Significance of the Tianluoshan Locally Bred Goose in the History of Poultry.
The pathways to animal domestication were highly variable but grouped into three general scenarios: the commensal pathway, prey pathway, and directed pathway (68). In poultry, chicken, Muscovy duck, and turkey were assigned to the commensal pathway, whereas emu and ostrich were assigned to the directed pathway (68). The candidate species for the locally bred large-sized goose (i.e., graylag, swan, and bean goose) are essentially herbivores and prefer to consume a wide variety of crop plants, including fallen paddy, in wintering areas (37, 69). Paddy fields at that time were created by clearing and leveling natural wetlands and surrounding them with soil mounds (70). In addition, archaeobotanical data suggest that the growth of nonshattering domesticated rice increased in the later period but approximately half of all rice grown was the wild shattering type (18). Therefore, paddy fields built at the Tianluoshan village are thought to have become a good feeding ground for wild wintering geese. Although geese are attracted to anthropogenic rice fields, it is difficult to maintain the two-way partnership required for the commensal pathway because of the migratory nature of the wild goose. Without human intervention, such as excessive feeding, enclosure, and removal of wing feathers, the geese of that time would have migrated toward their breeding grounds in the spring, just like wild geese in modern times. Goose domestication could have been initiated by the anthropogenic need to enhance the yield or predictability of the resources provided by geese (i.e., the prey pathway).
Two lineages of domestic geese—Chinese and European domestic geese, derived from swan and greylag geese, respectively—are currently farmed, although some admixture of these two domestic lineages are recognized (71). To the best of our knowledge, there are no records of domestic goose bones from Neolithic sites in China, and domestic geese are not mentioned in a review of domestic animals in prehistoric China (72, 73). The earliest accounts of the domestication of greylag geese are based on Egyptian tomb paintings from the 18th Dynasty (midsecond millennium BC), which include depictions of geese of various colors as well as immature individuals (5, 74). Additionally, large goose bones (thought to belong to domesticated geese) have been found to correspond to the Late Dynastic period of Egypt (midfirst millennium BC) (5, 74). However, this study suggested three things: goose domestication in the lower Yangtze River dates back ∼7,000 y, the history of goose domestication is much longer than previously thought, and southern China was at least one of the origins of the domestic goose. Intriguingly, a recent genome study estimated that European domestic and wild graylag geese split ∼14,000 y ago, which is earlier than any animal domestication, except that of dogs (71). As the most likely reason for this early estimation, the authors suggested that the potential modern wild populations of the greylag progenitor were not sampled in their study. Greylags in northern China were not included in the study and the locally bred geese at Tianluoshan may, indeed, be ancestors of today’s European domestic geese.
Although it remains controversial as to when or where chickens were domesticated, chickens are widely recognized as the oldest poultry in the world (1, 2, 5). Domestic chicken bones have been reported from Early Holocene sites in Nanzhuangtou (northern China, ∼10,000 cal BP) (6) and Hotnista (Bulgaria, ∼7000 cal BP) (7). However, these discoveries are limited by the scarcity of candidate chicken bones from Neolithic and Bronze age archaeological sites in China (9), and contrast with various sources: the findings of a review of Holocene paleoclimate and archaeofaunal archives (10), the results of ecological niche modeling of extant red junglefowl—the main wild progenitor of domestic chicken— (11), the mitochondrial DNA diversity of modern domestic chicken in northern China (12), and records in assigned strata and species identification (13). To the best of our knowledge, there are no domestic chicken bones older than those found in the Middle Holocene that are reliable in terms of identification and age (14). Although Wang et al. (75) found that the common ancestor of chickens and their wild ancestor (Gallus gallus spadiceus) diverged at ∼9500 ± 3300 cal BP, this does not necessarily correlate with the beginning of the domestication process. Our findings suggest that geese were the first poultry species to be domesticated. At present, chickens account for 92.9% of the world’s poultry population, with domestic geese accounting for less than 1.3% (4). However, the most abundant poultry today is not necessarily the oldest. It would be surprising if this migrating bird was domesticated in its wintering region (East Asia), prior to soybean, ramie, melon, silkworm, horse, yak, and Bactrian camel, and as early as rice and pig (1). Even today, China is still the world’s largest producer of goose meat (76). Further studies using ancient DNA analysis are required to investigate which species were bred to become local geese populations. It is also necessary to investigate—by extensive analyses using histological, geochemical, biochemical, morphological, and contextual approaches—how goose husbandry spread (or became extinct) to the lower Yangtze River and adjacent regions, and whether it has influenced current Chinese domestic geese farming.
Materials and Methods
Sampling Methods.
We studied 232 goose remains from the Tianluoshan site in Zhejiang Province, China. Target Anserinae bone samples were determined using naked-eye taxonomic identification by one of the authors (M.E.), and comparisons with osteological specimens at Hokkaido University Museum and the personal collections of K. Kawakami (Forestry and Forest Products Research Institute, Ibaraki, Japan) and one of the authors (M.E.). The naked-eye observations of 188 samples are detailed in our previous studies (20, 77, 78). Of the 232 bones, 25 relatively well-preserved bones were selected to represent each of the six sampled soil layers (Layers 3 to 8) that contained bone samples. The habitats were reconstructed using oxygen isotope analysis of bone phosphate (δ18OP), and the diets were reconstructed using carbon and nitrogen isotope analysis of bone collagen (δ13CCo and δ15NCo). Five goose bones, including three immature bones and bones from two locally bred mature birds (as assigned by oxygen isotope composition analysis) were subjected to radiocarbon dating of bone collagen. As an indicator of the δ18O values of the study location, we measured the δ18OP in 11 mammal bones—5 buffalos, 2 Père David’s deer, 3 sambars, and 1 sika deer—from the Tianluoshan site. We could not measure oxygen isotopes in specific resident bird species because species level identification of bird bones is very challenging. It was, therefore, impossible to obtain reliable species data for the study location. For comparison with bones from Tianluoshan, we analyzed the δ18OP, δ13CCo, and δ15NCo values in five goose bones from Kuahuqiao (79): an Early Neolithic site neighboring Tianluoshan and inhabited by the Kuahuqiao Culture (60). The studied bones from Tianluoshan and Kuahuqiao are stored at the Hemudu Culture Research Center (Zhejiang Institute of Cultural Relics and Archaeology) and the Kuahuqiao Site Museum, respectively.
Histological Analysis.
During taxonomic identification, information regarding whether the bones were from immature individuals and whether the samples included the medullary bone was recorded. To determine the age in weeks of immature goose bones, five modern osteological specimens of immature geese were used as references.
Oxygen Isotope Analysis of Bone Phosphate.
To measure the oxygen isotope composition of bone phosphate, we used the silver phosphate method (80, 81) with slight improvements. Bone powder was treated overnight with sodium hypochlorite (2.5%) to eliminate organic matter, and subsequently immersed for 4 h in acetic acid buffer solution (0.1 M, pH 4.4) to eliminate diagenetic contaminants. Nitric acid (60%, 0.2 mL) and hydrogen fluoride solution (3 M, 1.8 mL) were added to precipitate calcium as CaF2. Ammonia (25%, 2 mL) and silver nitrate solutions (1 M, 1 mL) were added to the supernatant, and the mixture concentrated at 70 °C overnight to precipitate silver phosphate. The δ18O in the silver phosphate was measured using a thermal conversion elemental analyzer–isotope ratio mass spectrometer system (TC/EA–IRMS; TC/EA coupled to a Delta V Advantage IRMS, Thermo Fisher Scientific) at the University Museum of the University of Tokyo. The δ18O values in this study were standardized using Vienna standard mean ocean water. The δ18OP values were corrected to the δ18O value of drinking water to obtain δ18OW values for geese according to Amiot et al. (32), and for mammals according to Kohn and Cerling (82).
Carbon and Nitrogen Isotope Analyses of Bone Collagen.
Collagen samples were extracted from the bones by gelatinization using methods improved from previous studies (83, 84). The bone fragments were soaked in 0.4 M HCl for 48 h at 4 °C to remove hydroxyapatite, after which they were soaked in 0.1 M NaOH to remove humic material. Finally, the remains were heated in aqueous HCl (pH 4) at 90 °C for 48 h. They were then filtered and freeze-dried to produce collagen. The stable carbon and nitrogen isotope compositions of the collagen were determined using an EA–IRMS system (Flash 2000 EA coupled to a Delta V Advantage IRMS, Thermo Fisher Scientific) at the University Museum of the University of Tokyo. The atomic C/N ratio of collagen was expected to be in the range of 2.9 to 3.6 (85), and data for samples outside this range were excluded from the analysis.
Radiocarbon Dating of Bone Collagen.
To measure 14C, collagen was prepared and graphitized using the methods described by Omori et al. (86). An elemental analyzer (vario ISOTOPE select, Elementar Analysensysteme GmbH) was used to combust the samples and isolate pure CO2 from the combusted gas (86). Graphite was then produced by the catalytic reduction of the sample CO2 with H2 gas and Fe powder. The radiocarbon content of the graphite was measured using an accelerator mass spectrometer at the University Museum of the University of Tokyo. The radiocarbon dates were calibrated using OxCal (87) and IntCal20 (88).
Morphological Analysis.
To describe the size of archeological goose bones, the greatest length and width of the proximal (Bp) and distal epiphysis (Bd) of mature bones were measured according to the methods outlined by von den Driesch (89). Measurements were made at the 0.01-mm scale using a digital caliper. Compared with different mensural elements, we calculated the LDS (LDS = logeX − logeY; where X was the measurement from the excavated sample and Y was the measurement from the reference specimen) (90) for each measurement from each sample. When more than one measurement was obtained from a sample, the average LDS was used as the score for the sample. A greater white-fronted goose (Anser albifrons frontalis; USNM 432003) specimen was used as the reference sample. For comparison with the archeological specimens, the humerus greatest lengths were measured from 127 modern geese individuals from 6 species (greater white-fronted goose, swan goose, bean goose, lesser white-fronted goose, greylag goose, and brant goose) wintering in the lower Yangtze River and adjacent regions. The LDS was calculated for each sample. A randomization test was conducted to test whether the LDS variance of the candidate domestic individuals (assigned by oxygen-stable isotope analysis) differed from those of noncandidate domestic individuals. The LDS variance of candidate domestic individuals was also compared with recent monotypic species of the geese (i.e., the swan goose and lesser white-fronted goose) in the lower Yangtze River region, using the F-test with Bonferroni correction. All statistical tests were conducted using R v4.1.0 (91).
Supplementary Material
Acknowledgments
We thank Prof. Bin Liu for providing the opportunity to study bird bones from the Tianluoshan site; Dr. Kazuto Kawakami, Prof. Toyohiro Nishimoto, and the curators and collection managers in the Bavarian State Collection for Anthropology and Palaeoanatomy, Munich; Historic England; the Nara National Research Institute for Cultural Properties; the National Science Museum, Tokyo; the Natural History Museum at Tring; the Smithsonian National Museum of Natural History; Southampton University; the University of Copenhagen Zoological Museum; and the Yamashina Institute for Ornithology. The comments from the handling editor and two anonymous reviewers clarified the strengths and weaknesses of this study. This study received partial financial support from the Japan Society for the Promotion of Science KAKENHI, Grants JP15H05964, JP15H05969, JP18H04172, JP20H01345, JP20H01367, JP20H05821, and JP20H05819.
Footnotes
The authors declare no competing interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.2117064119/-/DCSupplemental.
Data Availability
All study data are included in the main text and SI Appendix.
References
- 1.Larson G., et al. , Current perspectives and the future of domestication studies. Proc. Natl. Acad. Sci. U.S.A. 111, 6139–6146 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McHugo G. P., Dover M. J., MacHugh D. E., Unlocking the origins and biology of domestic animals using ancient DNA and paleogenomics. BMC Biol. 17, 98–98 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zeder M. A., Core questions in domestication research. Proc. Natl. Acad. Sci. U.S.A. 112, 3191–3198 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Food and Agricultural Organization, Gateway to poultry production and products (2021). https://www.fao.org/poultry-production-products/en/. Accessed 19 September 2021.
- 5.Serjeantson D., Birds Cambridge Manual in Archaeology (Cambridge University Press, Cambridge, 2009). [Google Scholar]
- 6.Xiang H., et al. , Early Holocene chicken domestication in northern China. Proc. Natl. Acad. Sci. U.S.A. 111, 17564–17569 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Boev Z., Avian remains from the Late Chalcolithic settlement near Hotnitsa village (Veliko Tarnovo Region, CN Bulgaria). Acta Zool. Bulg. 61, 39–54 (2009). [Google Scholar]
- 8.Zhou B., Fauna remains of Cishan site, Wuan, Hebei. Acta Archaeologica Sinica 3, 339–347 (1981). [Google Scholar]
- 9.Eda M., et al. , Reevaluation of early Holocene chicken domestication in northern China. J. Archaeol. Sci. 67, 25–31 (2016). [Google Scholar]
- 10.Peters J., Lebrasseur O., Deng H., Larson G., Holocene cultural history of red junglefowl (Gallus gallus) and its domestic descendant in East Asia. Quat. Sci. Rev. 142, 102–119 (2016). [Google Scholar]
- 11.Pitt J., Gillingham P. K., Maltby M., Stewart J. R., New perspectives on the ecology of early domestic fowl: An interdisciplinary approach. J. Archaeol. Sci. 74, 1–10 (2016). [Google Scholar]
- 12.Huang X.-H., et al. , Was chicken domesticated in northern China? New evidence from mitochondrial genomes. Sci. Bull. (Beijing) 63, 743–746 (2018). [DOI] [PubMed] [Google Scholar]
- 13.Kyselý R., Review of the oldest evidence of domestic fowl Gallus gallus f. domestica from the Czech Republic in its European context. Acta Zool. Cracoviensia Ser. A, Vertebrata 53, 9–34 (2010). [Google Scholar]
- 14.Eda M., Origin of the domestic chicken from modern biological and zooarchaeological approaches. Anim. Front. 11, 52–61 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sun G., “A summary of the first excavation (2004-2008) at Tianluoshan site” in Interdisciplinary Research on Tianluoshan Site, Yuyao, Zhejiang, Nakamura S., Ed. (Laboratory of Field Study of Cultures, School of Humanities, Kanazawa University, Kanazawa, 2010), pp. 21–40. [Google Scholar]
- 16.Kanehara M., Zheng Y., “Diatom, pollen, and ova-parasite analyses from T103 west wall” in Interdisciplinary Research on Tianluoshan Site, Yuyao, Zhejiang, Nakamura S., Ed. (Laboratory of Field Study of Cultures, School of Humanities, Kanazawa University, Kanazawa, 2010), pp. 55–68. [Google Scholar]
- 17.Zhang Y., Yuan J. Y. H., Matsui A., Sun G., “A preliminary study of mammal remains from Tianluoshan site in fiscal year 2004” in Interdisciplinary Research on Tianluoshan Site, Yuyao, Zhejiang, Nakamura S., Ed. (Laboratory of Field Study of Cultures, School of Humanities, Kanazawa University, Kanazawa, 2010), pp. 79–124. [Google Scholar]
- 18.Fuller D. Q., et al. , “Archaeobotanical analysis at Tianluoshan: Evidence for wild-food gathering, rice cultivation and the process of the evolution of morphologically domesticated rice” in Interdisciplinary Research on Tianluoshan Site, Yuyao, Zhejiang, Nakamura S., Ed. (Laboratory of Field Study of Cultures, School of Humanities, Kanazawa University, Kanazawa, 2010), pp. 153–202. [Google Scholar]
- 19.Maruyama M., Kikuchi H., Sun G., Yu C., Zhang Y., Fish utilization during the Neolithic in the lower Yangtze river, China: A case study of Tianluoshan site. Japanese Journal of Zooarchaeology 38, 1–9 (2021). [Google Scholar]
- 20.Hsu K.-h., Eda M., Kikuchi H., Sun G., Neolithic avifaunal resource utilisation in the lower Yangtze River: A case study of the Tianluoshan site. J. Archaeol. Sci. Rep. 37, 102929 (2021). [Google Scholar]
- 21.del Hoyo J., Collar N. J., HBW and Birdlife International Illustrated Checklist of the Birds of the World. Volume 1: Non-passerines (Lynx Edicions, Barcelona, 2014). [Google Scholar]
- 22.Simkiss K., Calcium metabolism and avian reproduction. Biol. Rev. Camb. Philos. Soc. 36, 321–359 (1961). [Google Scholar]
- 23.Eda M., Yashima S., Inoué T., Medullary bone in goose remains: A reliable indicator of domestic individual in non‐breeding regions. Int. J. Osteoarchaeol. 25, 849–854 (2015). [Google Scholar]
- 24.Viljoen G. J., Luckins A. G., Naletoski I., Stable Isotopes to Trace Migratory Birds and to Identify Harmful Diseases (Springer Nature, Cham, Switzerland, 2016). [Google Scholar]
- 25.Wassenaar L. I., “Introduction to conducting stable isotope measurements for animal migration studies” in Tracking Animal Migration with Stable Isotopes, Hobson K. A., Wassenaar L. I., Eds. (Academic Press, San Diego, ed. 2, 2019), pp. 25–51. [Google Scholar]
- 26.Epstein S., Mayeda T., Variation of O18 content of waters from natural sources. Geochim. Cosmochim. Acta 4, 213–224 (1953). [Google Scholar]
- 27.Craig H., Isotopic variations in meteoric waters. Science 133, 1702–1703 (1961). [DOI] [PubMed] [Google Scholar]
- 28.Bowen G. J., Revenaugh J., Interpolating the isotopic composition of modern meteoric precipitation. Water Resour. Res. 39, 1299 (2003). [Google Scholar]
- 29.Yoshida N., Miyazaki N., Oxygen isotope correlation of cetacean bone phosphate with environmental water. J. Geophys. Res. Oceans 96 (C1), 815–820 (1991). [Google Scholar]
- 30.Kohn M. J., Schoeninger M. J., Valley J. W., Herbivore tooth oxygen isotope compositions: Effects of diet and physiology. Geochim. Cosmochim. Acta 60, 3889–3896 (1996). [Google Scholar]
- 31.Longinelli A., Oxygen isotopes in mammal bone phosphate: A new tool for paleohydrological and paleoclimatological research? Geochim. Cosmochim. Acta 48, 385–390 (1984). [Google Scholar]
- 32.Amiot R., et al. , Oxygen isotope fractionation between bird bone phosphate and drinking water. Naturwissenschaften 104, 47 (2017). [DOI] [PubMed] [Google Scholar]
- 33.Hobson K. A., Bowen G. J., Wassenaar L. I., Ferrand Y., Lormee H., Using stable hydrogen and oxygen isotope measurements of feathers to infer geographical origins of migrating European birds. Oecologia 141, 477–488 (2004). [DOI] [PubMed] [Google Scholar]
- 34.Wommack E. A., Marrack L. C., Mambelli S., Hull J. M., Dawson T. E., Using oxygen and hydrogen stable isotopes to track the migratory movement of Sharp-shinned Hawks (Accipiter striatus) along Western Flyways of North America. PLoS One 15, e0226318 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Somerville A. D., Nelson B. A., Knudson K. J., Isotopic investigation of pre-Hispanic macaw breeding in Northwest Mexico. J. Anthropol. Archaeol. 29, 125–135 (2010). [Google Scholar]
- 36.Linglin M., et al. , Isotopic systematics point to wild origin of mummified birds in Ancient Egypt. Sci. Rep. 10, 15463 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Johnsgard P. A., Ducks, Geese, and Swans of the World (University of Nebraska–Lincoln Libraries, Lincoln, Revised Edition, 2010). [Google Scholar]
- 38.Bloom M. A., Domm L. V., Nalbandov A. V., Bloom W., Medullary bone of laying chickens. Am. J. Anat. 102, 411–453 (1958). [DOI] [PubMed] [Google Scholar]
- 39.Blyth L., Oxygen isotope analysis and tooth enamel phosphate and its application to archaeology. University of Western Ontario Journal of Anthropology 9, 1–13 (2001). [Google Scholar]
- 40.France C. A. M., Owsley D. W., Stable carbon and oxygen isotope spacing between bone and tooth collagen and hydroxyapatite in human archaeological remains. Int. J. Osteoarchaeol. 25, 299–312 (2015). [Google Scholar]
- 41.Jin Z., et al. , Stable isotopes and chemical characteristics of precipitation in Hangzhou and Huzhou, East China. Environ. Sci. Pollut. Res. Int. 26, 23717–23729 (2019). [DOI] [PubMed] [Google Scholar]
- 42.Bowen G. J., Gridded Maps of the Isotopic Composition of Meteoric Waters (University of Utah, 2021). https://wateriso.utah.edu/waterisotopes/index.html. Accessed 18 June 2021. [Google Scholar]
- 43.Lazzerini N., et al. , Oxygen isotope fractionation between bird eggshell calcite and body water: Application to fossil eggs from Lanzarote (Canary Islands). Naturwissenschaften 103, 81 (2016). [DOI] [PubMed] [Google Scholar]
- 44.Deniro M. J., Epstein S., Influence of diet on the distribution of nitrogen isotopes in animals. Geochim. Cosmochim. Acta 45, 341–351 (1981). [Google Scholar]
- 45.Yoneyama T., Kouno K., Yazaki J., Variation of natural 15N abundance of crops and soils in Japan with special reference to the effect of soil conditions and Fertilizer application. Soil Sci. Plant Nutr. 36, 667–675 (1990). [Google Scholar]
- 46.Mariotti A., Leclerc A., Germon J. C., Nitrogen isotope fractionation associated with the NO2− → N2O step of denitrification in soils. Can. J. Soil Sci. 62, 227–241 (1982). [Google Scholar]
- 47.Amundson R., et al. , Global patterns of the isotopic composition of soil and plant nitrogen. Global Biogeochem. Cycles 17, 1031 (2003). [Google Scholar]
- 48.Wang X., et al. , Millet manuring as a driving force for the Late Neolithic agricultural expansion of north China. Sci. Rep. 8, 5552 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gao Y., et al. , Enhanced sediment denitrification for nitrogen removal by manipulating water level in the lakeshore zone. Water 13, 3323 (2021). [Google Scholar]
- 50.Zhang Y., “Exploring the wetland: Integrating the fish and plant remains into a case study from Tianluoshan, a middle Neolithic site in China” in Environmental Archaeology: Current Theoretical and Methodological Approaches, Pişkin E., Marciniak A., Bartkowiak M., Eds. (Springer International Publishing, Cham, Switzerland, 2018), pp. 199–227. [Google Scholar]
- 51.Yuan J., Flad R. K., Luo Y., Meat-acquisition patterns in the Neolithic Yangzi river valley, China. Antiquity 82, 351–366 (2008). [Google Scholar]
- 52.Deniro M. J., Epstein S., Influence of diet on distribution of carbon isotopes in animals. Geochim. Cosmochim. Acta 42, 495–506 (1978). [Google Scholar]
- 53.Barton L., et al. , The earliest farmers of northwest China exploited grain-fed pheasants not chickens. Sci. Rep. 10, 2556 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Barton L., et al. , Agricultural origins and the isotopic identity of domestication in northern China. Proc. Natl. Acad. Sci. U.S.A. 106, 5523–5528 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yoneda M., et al. , “Carbon and nitrogen isotope analyses on the Neolithic human and faunal remains from lower Yangtze River” in The Origin and Diffusion of Livestock and Poultry in Neolithic East Asia: New Zooarchaeological Evidence from China, Matsui A., Kikuchi H., Eds. (Meishin-sha, Nara, 2016), pp. 75–80. [Google Scholar]
- 56.Sage R., Wedin D., “The biogeography of C4 photosynthesis: Patterns and controlling factors” in C4 Plant Biology, Sage R. F., Monson R. K., Eds. (Academic Press, San Diego, 1999), vol. 161, pp. 313–373. [Google Scholar]
- 57.Hartman G., Danin A., Isotopic values of plants in relation to water availability in the Eastern Mediterranean region. Oecologia 162, 837–852 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Inger R., et al. , Temporal and intrapopulation variation in prey choice of wintering geese determined by stable isotope analysis. J. Anim. Ecol. 75, 1190–1200 (2006). [DOI] [PubMed] [Google Scholar]
- 59.Hahn S., Hoye B. J., Korthals H., Klaassen M., From food to offspring down: Tissue-specific discrimination and turn-over of stable isotopes in herbivorous waterbirds and other avian foraging guilds. PLoS One 7, e30242 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Liu L., Chen X., The Archaeology of China: From the Late Paleolithic to the Early Bronze Age (Cambridge University Press, Cambridge, 2012). [Google Scholar]
- 61.Mo D., et al. , “The environmental background of the Tianluoshan site and Hemudu culture” in Interdisciplinary Research on Tianluoshan Site, Yuyao, Zhejiang, Nakamura S., Ed. (Laboratory of Field Study of Cultures, School of Humanities, Kanazawa University, Kanazawa, 2010), pp. 41–53. [Google Scholar]
- 62.Libo Z., et al. , Holocene palaeoenvironment evolution and human activity of the Hemudu-Tianluoshan Sites in Yuyao of Zhejiang Province. Journal of Palaeogeography 18, 779–894 (2016). [Google Scholar]
- 63.Zhejiang Provincial Institute of Cultural Relics and Archaeology and Xiaoshan Museum, Kuahuquao (Cultural Relics Publishing House, Beijing, 2004). [Google Scholar]
- 64.Deng H., Yuan J., Song G., Wang C., Eda M., The reexamination of the domestic chicken in ancient China. Kaogu (Archaeology) 6, 83–96 (2013). [Google Scholar]
- 65.Zhang Y., “Zooarchaeological study of animal remains” in Bianjiashan, Zhejiang Provincial Institute of Cultural Relics and Archaeology, Ed. (Cultural Relics Press, Beijing, 2014), pp. 424–432. [Google Scholar]
- 66.Li F., Lv P., Eda M., Yuan J., Zhu Y., Bird remains of Tengjiagang site—The review and prospect of zooarchaeological researches on bird remains of China. Huaxia Archaeology 1, 34–40 (2015). [Google Scholar]
- 67.Li A., Guoping S., Tianluoshan: A New Window on the Hemudu Culture (Xiling Seal Society Publishing House, Hangzhou, 2009). [Google Scholar]
- 68.Zeder M., “Pathways to animal domestication” in Biodiversity in Agriculture: Domestication, Evolution, and Sustainability, Gepts P., et al., Eds. (Cambridge University Press, Cambridge, 2012), pp. 227–259. [Google Scholar]
- 69.Higuchi H., Morioka H., Yamagishi S., The Encyclopaedia of Animals in Japan 3. Bird I (Heibon Sha, Tokyo, 1996). [Google Scholar]
- 70.Sun G., “Retrospective and perspective of the investigation of Hemudu Culture” in Hemudu & Liangzhu Culture, Nakamura S., Liu B., Eds. (Yuzankaku, Tokyo, 2020), pp. 25–44. [Google Scholar]
- 71.Heikkinen M. E., et al. , Long-term reciprocal gene flow in wild and domestic geese reveals complex domestication history. G3 10, 3061–3070 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Liu L., Ma X., “The zooarchaeology of Neolithic China” in The Oxford Handbook of Zooarchaeology, Albarella U., Rizzetto M., Russ H., Vickers K., Viner-Daniels S., Eds. (Oxford University Press, New York, 2017), pp. 304–332. [Google Scholar]
- 73.Pearson R., Underhill A., The Chinese Neolithic: Recent trends in research. Am. Anthropol. 89, 807–822 (1987). [Google Scholar]
- 74.Bosseneck J., Riesige Hausgänse aus der Spätzeit des Alten Ägypten. Archiv für Gerflügelkunde 55, 105–110 (1991). [Google Scholar]
- 75.Wang M.-S., et al. , 863 genomes reveal the origin and domestication of chicken. Cell Res. 30, 693–701 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Pingel H., Waterfowl production for food security. Lohmann Information 46, 32–42 (2011). [Google Scholar]
- 77.Eda M., Matsui A., Sun G., “Bird remains from Tianluoshan Site, Zhejiang, China” in The Origin and Diffusion of Livestock and Poultry in Neolithic East Asia: New Zooarchaeological Evidence from China, Matsui A., Kikuchi H., Eds. (Meishin-sha, Nara, 2016), pp. 23–36. [Google Scholar]
- 78.Eda M., Kikuchi H., Sun G., Matsui A., Were chickens exploited in the Neolithic early rice cultivation society of the lower Yangtze River? Archaeol. Anthropol. Sci. 11, 6423–6430 (2019). [Google Scholar]
- 79.Pan Y., Zheng Y., Chen C., “Human ecology of the early Neolithic Kuahuqiao Culture in East Asia” in Handbook of East and Southeast Asian Archaeology, Habu J., Lape P. V., Olsen J. W., Eds. (Springer, New York, : 2017), pp. 347–377. [Google Scholar]
- 80.Daniel Bryant J., Koch P. L., Froelich P. N., Showers W. J., Genna B. J., Oxygen isotope partitioning between phosphate and carbonate in mammalian apatite. Geochim. Cosmochim. Acta 60, 5145–5148 (1996). [Google Scholar]
- 81.Vennemann T. W., Fricke H. C., Blake R. E., O’Neil J. R., Colman A., Oxygen isotope analysis of phosphates: A comparison of techniques for analysis of Ag3PO4. Chem. Geol. 185, 321–336 (2002). [Google Scholar]
- 82.Kohn M. J., Cerling T. E., Stable isotope compositions of biological apatite. Rev. Mineral. Geochem. 48, 455–488 (2002). [Google Scholar]
- 83.Longin R., New method of collagen extraction for radiocarbon dating. Nature 230, 241–242 (1971). [DOI] [PubMed] [Google Scholar]
- 84.Itahashi Y., et al. , Amino acid 15 N analysis reveals change in the importance of freshwater resources between the hunter-gatherer and farmer in the Neolithic upper Tigris. Am. J. Phys. Anthropol. 168, 676–686 (2019). [DOI] [PubMed] [Google Scholar]
- 85.DeNiro M. J., Postmortem preservation and alteration of invivo bone-collagen isotope ratios in relation to paleodietary reconstruction. Nature 317, 806–809 (1985). [Google Scholar]
- 86.Omori T., Yamazaki K., Itahashi Y., Ozaki H., Yoneda M., “Development of a simple automated graphitization system for radiocarbon dating at the University of Tokyo” 14th International Conference on Accelerator Mass Spectrometry (University of Ottawa, 2017). [Google Scholar]
- 87.Bronk Ramsey C., Bayesian analysis of radiocarbon dates. Radiocarbon 51, 337–360 (2009). [Google Scholar]
- 88.Reimer P. J., et al. , The IntCal20 Northern Hemisphere radiocarbon age calibration curve (0–55 cal kBP). Radiocarbon 62, 725–757 (2020). [Google Scholar]
- 89.von den Driesch A., A guide to the measurements of animal bones from archaeological sites. Peabody Museum Bull. 1, 1–136 (1976). [Google Scholar]
- 90.Simpson G. G., Large Pleistocene felines of North America. Am. Mus. Novit. 1136, 1–27 (1941). [Google Scholar]
- 91.R Core Team, R: A Language and Environment for Statistical Computing, 4.1.0 (R Foundation for Statistical Computing, Vienna, : 2021). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All study data are included in the main text and SI Appendix.