Abstract
Osteoarthritis (OA) is a prevalent and debilitating age-related joint disease characterized by articular cartilage degeneration, synovial membrane inflammation, osteophyte formation, as well as subchondral bone sclerosis. OA drugs at present are mainly palliative and do not halt or reverse disease progression. Currently, no disease-modifying OA drugs (DMOADs) are available and total joint arthroplasty remains a last resort. Therefore, there is an urgent need for the development of efficacious treatments for OA management. Among all novel pharmaco-therapeutical options, exosome-based therapeutic strategies are highly promising. Exosome cargoes, which include proteins, lipids, cytokines, and various RNA subtypes, are potentially capable of regulating intercellular communications and gene expression in target cells and tissues involved in OA development. With extensive research in recent years, exosomes in OA studies are no longer limited to classic, mesenchymal stem cell (MSC)-derived vesicles. New origins, structures, and functions of exosomes are constantly being discovered and investigated. This review systematically summarizes the non-classic origins, biosynthesis, and extraction of exosomes, describes modification and delivery techniques, explores their role in OA pathogenesis and progression, and discusses their therapeutic potential and hurdles to overcome in OA treatment.
Keywords: osteoarthritis, exosome, extracellular vesicle, regenerative medicine, chondrocyte, cartilage injury
1. Introduction
Osteoarthritis (OA) is the most common form of arthritis, causing chronic joint pain, decline in joint function, physical disability, and impaired quality of life in the affected population [1]. According to data from the National Health Interview Survey (NHIS), doctor-diagnosed OA and other forms of arthritis affected 52.5 million American adults during 2011–2012, and by 2040, this number is expected to be increased by 49% [2], creating a considerable socioeconomic burden [3]. During OA progression, pathological changes have been reported to affect the whole joint, including cartilage degradation, osteophyte formation, abnormal subchondral bone remodeling, synovitis, meniscus and ligament degeneration, hypertrophy of the joint capsule, and increased vascularization, inflammatory infiltration, and fibrosis in the infrapatellar fat pad (IPFP) [4,5]. Risk factors of OA, including age, gender, genetic predisposition, obesity, inflammation, and excessive mechanical loading, increases the probability of OA occurrence and development [6]. With the combined effects of aging, obesity, and an increasing number of joint injuries in the global population, this burdensome syndrome is expected to become more prevalent [7].
Treatment strategies of OA are limited due to the lack of knowledge about OA pathogenesis. At present, no disease-modifying osteoarthritis drugs (DMOADs) are available to reverse or halt OA progression [8]. Pharmacological approaches, such as the use of non-steroidal anti-inflammatory drugs (NSAIDs), analgesics, and surgical interventions are current options to offer symptomatic relief [9]. However, these options are ineffective in repairing damaged articular cartilage, and are also challenged by relatively small effect sizes and uncertainty about their long-term efficacy and safety. These limitations hinder their clinical applications [10]. Considering that OA is a multifactorial disease with complex comorbid conditions, personalized treatment is essential to optimize outcomes [11]. To achieve this, researchers focus on developing personalized in situ intra-articular (IA) therapeutic options. IA drug delivery is superior to systemic administration with higher levels of efficacy and a lower risk of side effects. Different drug delivery systems have emerged to improve the local delivery of small molecules to joints [12]. Among them, exosomes, as a novel bio-cargo, have attracted significant attention in recent years.
Exosomes are a type of extracellular vehicles (EVs) with a diameter ranging between 30 and 150 nm, and a density of 1.13–1.19 g/mL [13]. These extracellular membrane-bound vesicles are able to work as cell-specific cargoes, which contain complex signaling molecules such as lipids, proteins, metabolites, nucleic acids, and cytosolic and cell-surface proteins [13]. Exosomes functions to mediate intercellular communications, and can be released into the extracellular environment by almost all types of cells through fusing plasma membrane and multivesicular bodies (MVBs) [14]. The biomedical applications of exosomes have been rapidly expanding in recent years because of their active roles in the function and pathophysiology of various body systems and potential in clinical therapeutics and diagnosis [15]. Diverse therapeutic payloads, such as DNAs, RNAs, antisense oligonucleotides, metabolites, chemotherapeutic agents, cytokines, and immune modulators, can be delivered to a target by engineered exosomes [16]. In OA related research, exosomes from multiple origins in the joint, such as tissue-specific mesenchymal stem cells (MSCs), chondrocytes, synovial fibroblasts (SFBs), osteoblasts, tenocytes, IPFP adipocytes, and platelet-rich plasma (PRP), have been detected and change with OA progression [17,18,19] (Figure 1). Herein we discuss the biosynthesis, origins, and contents of exosomes, and review their roles in OA pathogenesis, progression, and treatment.
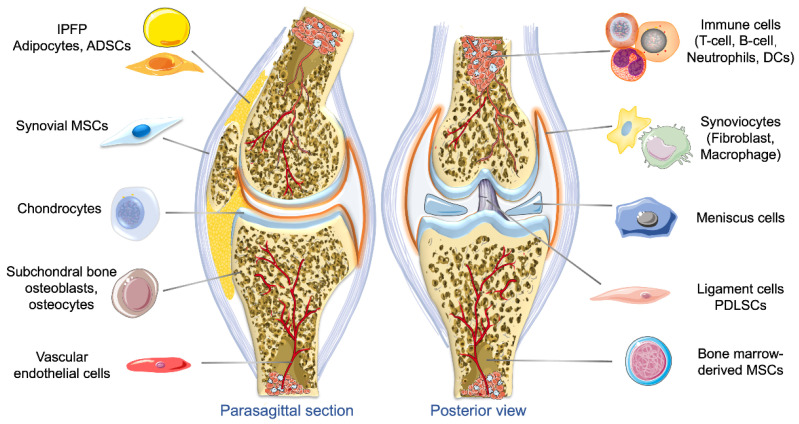
Figure 1.
Tissue sources of exosomes in the knee joint. Exosomes are secreted by multiple types of cells of the joint, including adipocytes, adipose-derived stem cells (ADSCs), synovium-derived mesenchymal stem cells (MSCs), synovial fibroblasts and macrophages, chondrocytes, osteoblasts and osteocytes in the subchondral bone, vascular endothelial cells, immune cells such as T cells, B cells, and dendritic cells (DCs) meniscus cells, periodontal ligament cells, tenocytes, tendon stem cells, and bone marrow-derived MSCs. These exosomes are involved in the regulation of joint homeostasis, cell–cell communications, and the initiation and progression of OA.
2. Formation and Origin of Exosomes
The concept of ‘exosomes’ was first proposed in 1981 by Trams et al. [20]. In 1983, the currently defined exosomes were first identified in sheep reticulocytes and named by Johnstone et al. [21]. However, the widespread clinical applications were limited by the low yield for the production method used and unexpected therapeutic effects [22]. Besides, the function of exosomes is dependent on both the type and condition of the cells that they are released from, and thus varies a lot. To optimize the application, a comprehensive understanding of the generation, origins, and contents of exosomes is required.
2.1. Biogenesis of Exosomes
The detailed biological synthesis process of exosomes is shown in Figure 2. The cellular biogenesis process of exosomes begins with double invagination of the plasma membrane [23]. This is followed by the accumulation of bioactive substances in the early sorting endosomes (ESEs), such as lipids, proteins, small molecules, ions, and metabolites present in the extracellular space. The ESEs subsequently mature into late sorting endosomes (LSEs), a process regulated by endosomal sorting complex required for transport (ESCRT) proteins and others. After that, invagination of the limiting membrane of LSEs results in the formation of MVBs (also referred to as multivesicular endosomes) [16]. The MVBs can be degraded by fusing with autophagosomes or lysosomes; alternatively, MVBs fuse with the plasma membrane and release exosomes—vesicles containing the intra-endosome substances—to the extracellular space [23].
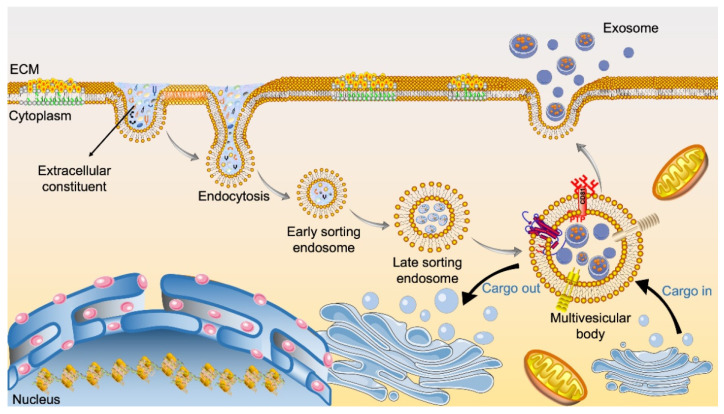
Figure 2.
Scheme of the biogenesis of exosomes. Endocytosis and plasma membrane invagination facilitate the entry of cell surface proteins and extracellular components such as lipids, proteins, metabolites, ions, and small molecules into cells, leading to the formation of early sorting exosomes (ESEs). The ESEs then fuse with the endoplasmic reticulum (ER) and/or trans-Golgi network (TGN) and result in late sorting exosome (LSE) formation. A second invagination in the LSEs leads to the generation of multivesicular bodies (MVBs). MVBs can then either fuse with lysosomes for degradation or be transported to the plasma membrane and undergo exocytosis—a process resulting in exosome release. Exosomes, filled with various cellular components such as proteins, mRNAs, miRNAs, lipids, enzymes, and carbohydrates, are released through exocytosis after MVBs fuse with the cell membrane. Released exosomes can be further taken up by adjacent or remote cells in various ways, including receptor-mediated endocytosis and fusion with the plasma membrane of cells.
A key component of OA treatment is the efficient delivery of therapeutic molecules to targeted cells, especially to chondrocytes embedded in a dense extracellular matrix (ECM), which requires the use of biocompatible molecular transport vehicles. Exosomes exhibit unique features, such as high serum stability and strong penetration across biological barriers, which make them ideal cargoes for drug delivery in OA treatment [24]. However, pristine exosomes can experience fast clearance in the body and have weak cell-targeting abilities, resulting in unsatisfactory treatment outcomes. Therefore, bioengineered exosome-mediated delivery strategies, such as drug loading and surface modifications, have been explored to improve the cell-targeting property of exosomes [23]. For example, genetic engineering methods have been utilized to introduce specific proteins, such as ligands for receptors or antibodies against target cells, to the surface of exosomes to achieve precise delivery [25].
2.2. Origins of Exosomes and Their Roles in OA
Osteoarthritis is a whole-joint disease with pathological changes observed in all joint components [26]. Exosomes secreted by cells in joint tissues or from IA-injected therapeutic agents exhibit complex regulatory effects on the progression of OA [27]. MSCs, derived from tissues within the joint (e.g., subchondral bone, IPFP, and synovium) and elsewhere, represent the most widely studied sources of exosome production. In addition, exosomes have also been obtained from non-classic sources including, but not limited to, articular chondrocytes, adipocytes, osteoblasts, osteocytes, vascular endothelial cells, and PRP [28]. Exosomes derived from different origins exhibit varying effects. Some exosomes showed chondroprotective effects, while others, such as vascular endothelial cell (VEC) and OA chondrocyte-derived exomes, promoted OA progression. Detailed information and potential regulatory mechanisms of exosomes generated by different cells are listed in Table 1. In this section, the different exosome sources are discussed, with an emphasis on joint-related tissues and cells, followed by a description of their roles in OA.
Table 1.
Summary of major findings of OA-related studies involving the use of exosomes.
| Cells | Source | Extraction | Dose | Delivery Method | Target Cells | Results | Ref |
|---|---|---|---|---|---|---|---|
| VECs | Conditioned medium | Ultrafiltration | 100 μg | Co-incubation for 24 h | Primary chondrocytes | Promoted OA progression by inhibiting chondrocyte autophagy, downregulating p21 expression, and increasing ROS production and apoptosis. | [29] |
| OA chondrocytes | Culture supernatant | Ultracentrifugation | 1 × 106/mL | Co-incubation | Synovial macrophages | Promoted OA progression by stimulating inflammasome activation and upregulating mature IL-1β production in synovial macrophages | [30] |
| Primary chondrocytes | Conditioned medium | Ultracentrifugation | 200 μg/mL | Co-incubation for 48 h Intra-articular injection |
Chondrocytes | Prevented OA via the restoration of mitochondrial function and macrophage polarization toward the M2 phenotype | [31] |
| OA osteoblasts | Conditioned medium | Ultracentrifugation | 20 μg/mL | Co-incubation for 14 d | Chondrocytes | Promoted OA progression by suppressing oxygen consumption by chondrocytes via miR-210-5p. | [32] |
| BM-MSCs | Conditioned medium | Ultracentrifugation | 10 μg/mL | Co-incubation for 24 h | Chondrocytes | Promoted proliferation and inhibited apoptosis of chondrocyte via miR-206/GIT1 axis | [33,34] |
| BM-MSCs | Conditioned medium | Ultracentrifugation | 250 ng | Intra-articular injection | Chondrocytes | Prevented OA development by inhibiting the degradation of cartilage and the formation of osteophyte | [35] |
| BM-MSCs | Conditioned medium | Ultracentrifugation | 200 μg/mL | 3D printed ECM/GelMA/exosome scaffolds | Osteochondral defect rabbit model | Prevented OA development by facilitating cartilage regeneration and restoring chondrocyte mitochondrial function | [36] |
| SMSCs | Conditioned medium | Ultracentrifugation | 5 μg | Co-incubation for 12 h | Chondrocytes | Prevented the development of OA by facilitating migration, proliferation and ECM secretion and suppressing chondrocyte apoptosis | [37] |
| SMSCs | Conditioned medium | Ultracentrifugation | 1010 particles | Intra-articular injection | DMM mice model | Prevented OA development by enhancing cartilage tissue regeneration via miR-140-5p upregulation of Wnt and YAP | [38] |
| ESC-MSCs | Conditioned medium | Ultrafiltration | 5 μg/mL 100 μg |
Co-incubation for 48 h Intra-articular injection |
TMJ condylar chondrocytes | Prevented OA development via inflammation attenuation and matrix homeostasis restoration | [39] |
| ESC-MSCs | Conditioned medium | Ultracentrifugation | 881 ng | Intra-articular injection | DMM OA model | Prevented OA development by balancing cartilage ECM synthesis and degradation | [40] |
| iPSC-MSCs | Conditioned medium | Ultracentrifugation | 8 μL 1010/mL |
Intra-articular injection | Collagenase-induced OA model | Prevented OA development by promoting migration and proliferation of chondrocytes | [41] |
| UC-MSCs | Conditioned medium | Ultracentrifugation | 10 μg/mL 100 μg |
Co-incubation for 72 h Intra-articular injection |
Rat cartilage defect model | Mechanical stimulation increased the expression level of LncRNA H19 in exosomes, which promoted chondrocyte proliferation, matrix synthesis, and inhibited apoptosis | [42] |
| ADSCs | Conditioned medium | Ultracentrifugation | 400 µg/mL | Co-incubation for 48 h | Chondrocytes | Prevented OA development by promoting chondrogenesis and suppressing inflammation via upregulating miR-221 and miR-145 | [43] |
| ADSCs | Conditioned medium | Ultracentrifugation | 108 particles | Intra-articular injection | DMM and MIA induced OA model | Prevented OA development by inhibiting proteoglycan degradation and cartilage destruction and ameliorating gait abnormality | [44,45] |
| AFSC | Conditioned medium | Precipitation | 30 μg 100 μg |
Co-incubation for 72 h Intra-articular injection |
MIA-induced OA mice model | Prevent the development of OA by promoting chondrocyte proliferation, cartilage matrix synthesis, and polarizing macrophages to M2 phenotype | [46] |
| Engineered CAP-Lamp2b exosomes | Conditioned medium | Ultracentrifugation | 10 μg 100 μg |
Co-incubation for 3 h Intra-articular injection |
Chondrocytes DMM OA rat model |
Prevented OA development by delivering miR-140 to deep cartilage regions and inhibiting cartilage-degrading proteases | [47] |
| CPCs | Conditioned medium | Ultracentrifugation | 108/mL 8 × 107 particle |
Co-incubation for 3 h Intra-articular injection |
Chondrocytes | Enhanced articular cartilage repair by stimulating chondrocyte proliferation and migration via upregulating miRNA 221-3p | [48] |
| Synoviocytes | Conditioned medium | Ultracentrifugation | 20 μg/mL | Co-incubation for 24 h | Chondrocytes | Promoted OA progression by inducing apoptosis and cartilage matrix degradation via upregulating miR-142-5p/RUNX2 | [49] |
| Synovial fibroblasts | Patient synovial fluid | Ultracentrifugation | 2 × 109/mL 20 μg |
Co-incubation for 48 h Intra-articular injection |
ACLT + MMx OA rat model | Prevented OA development by suppressing chondrocyte apoptosis, constraining inflammation, and cartilage degeneration | [50] |
| PRP | PRP | exoEasy Maxi Kit | 50 μg/mL 100 μg/mL |
Co-incubation for 24 h Intra-articular injection |
Chondrocytes | Prevented OA development by facilitating proliferation and reducing apoptosis of chondrocyte via Wnt/β-catenin | [17] |
| CPRP | Whole blood | Ultracentrifugation | 1.42 × 109 particles | Co-incubation for 48 h | OA chondrocytes | Prevented OA development by inducing chondrogenic gene expression changes and preventing proinflammatory cytokine release | [51] |
| IPFP | IPFP | Ultracentrifugation | 10 μL 1010/mL |
Intra-articular injection | DMM mice model | Prevented OA development by alleviating articular cartilage damage via miR-100-5p downregulation of mTOR | [44] |
| Tenocyte | Conditioned medium | Ultracentrifugation | 486.3 μg/mL | Co-incubation for 48 h | Tendon stem cells | Promoted tendon healing by regulating tendon ECM metabolism and inducing the tenogenic differentiation of MSCs via upregulating transforming growth factor-beta | [52,53] |
| Periodontal ligament cells | PureExo® exosome isolation kit | Precipitation | 5 μg/mL | Co-incubation for 48 h | Macrophage | Regulated macrophage function and maintained inflammation homeostasis by suppressing IL-1β via inhibiting NF-κB signaling pathway | [54] |
| LPS-pretreated PDLFs | Conditioned medium | Ultracentrifugation | 100 μg/mL | Co-incubation for 48 h | Osteoblast | Prevented bone remodeling by inducing inflammation and inhibiting osteogenic activity of osteoblasts, promoting macrophage polarization toward M1 via YAP | [55,56] |
VECs: vascular endothelial cell; BM-MSCs: bone marrow mesenchymal stem cells; ESC-MSCs: embryonic stem cell-derived MSCs; iPSC-MSCs: induced pluripotent stem cells-derived MSCs; UC-MSCs: umbilical cord mesenchymal stem cells; CPCs: chondrogenic progenitor cells; DMM: destabilization of the medial meniscus; ACLT + MMx: anterior cruciate ligament and resecting the medial menisci; PRP: platelet-rich plasma; CPRP: citrate-anticoagulated platelet-rich plasma; SMSCs: synovial mesenchymal stem cells; IPFP: infrapatellar fat pad; AFSC: amniotic fluid stem cells; ADSCs: adipose-derived stem cells; MIA: monosodium iodoacetate; PDLSCs: periodontal ligament-derived stem cells; PDLFs: periodontal ligament fibroblasts.
2.2.1. Exosomes Derived from Different Types of MSCs
MSCs possess multilineage differentiation potential and have been applied in IA injection therapies for OA treatment [57]. However, MSCs also face several limitations, including heterogeneity, inconsistent stemness, variable differentiation capacity, limited homing ability, and potential adverse effects, such as immune incompatibility, tumorigenicity, and chromosomal aberrations [58,59]. A growing body of evidence suggests that MSC-secreted exosomes should be credited for many of the previously reported regenerative properties of MSCs [60]. It was found that bone marrow MSC (BMSC)-derived exosomes can be endocytosed by chondrocytes. These exosomes showed capability in restoring the proliferation of chondrocytes, promoting ECM synthesis, and relieving knee OA pain [34]. Using MSC-derived exosomes to deliver mitochondrial-related proteins was reported to alleviate oxidative stress-induced damage and reverse mitochondrial dysfunction in degenerative OA cartilage [36]. MSC-derived exosomes containing a novel lncRNA KLF3-AS1 (KLF3 Antisense RNA 1; Ensembl: ENST00000440181) reversed the suppressive effects of miR-206 on the expression of G-protein-coupled receptor kinase interacting protein-1 (GIT1). It has been reported that GIT1 could promote the proliferation and inhibit the apoptosis of chondrocytes [61]. Thus, the lncRNA-KLF3-AS1/miR-206/GIT1 axis is possibly responsible for the chondroprotective effects of MSC-derived exosomes in OA [33].
In addition to BMSC, embryonic stem cell-derived MSCs (ESC-MSCs) [40], synovial MSCs (SMSC) [37,38], adipose-derived MSCs (ADSC) [21,62], umbilical cord mesenchymal stem cells (UC-MSCs) [42], periodontal ligament-derived stem cells (PDLSCs) [63], amniotic fluid stem cells (AFSCs) [46], and IPFP-MSCs [44,64,65] are other important origins of MSC-derived exosomes in OA treatment [38]. IA injection of ESC-MSC-derived exosomes facilitated the repair of osteochondral defects, maintained the chondrocyte phenotype, promoted cartilage formation, reduced matrix degradation, and impeded cartilage destruction both in vitro and in the destabilization of medial meniscus (DMM)-induced OA model in mice [40,66]. Mechanistically, these effects were achieved by promoting chondrocyte proliferation and migration, increasing collagen type II synthesis, and decreasing ADAMTS5 (A disintegrin and metalloproteinase with thrombospondin motifs 5) expression [40]. Exosomes obtained from miR-155-5p-overexpressing SMSC were used to treat OA chondrocytes and promoted their migration and proliferation, suppressed apoptosis, and enhanced the secretion of ECM; such exosomes also effectively prevented OA from occurring in mice undergoing cold water stimulation for 4 h/day over 20 days (which induced OA in the mice without exosome treatment) [37]. Recently, it was found that exosomes released by induced pluripotent stem cell (iPSC)-derived MSCs have a greater therapeutic effect compared with those from SMSCs [41]. IA injection of ADSC-derived exosomes showed an inhibitive effect on M1 macrophage infiltration into the synovium, significantly attenuating OA progression and preventing cartilage degeneration in both surgically induced (through DMM) mouse OA models and monosodium iodoacetate (MIA)-insulted rat joints [45]. Besides, ADSC-derived exosomes decreased the activity of senescence-related β-galactosidase in OA osteoblasts and the accumulation of γ H2AX foci, which were probably attributed to the protective effects on mitochondria [62]. Exosomes derived from UC-MSCs, which contained a high level of LncRNA H19, promoted chondrocyte proliferation and inhibited apoptosis in vitro; the exosomes also improved macroscopic assessment and relieved pain levels in a rat model of cartilage defect [42]. Furthermore, exosomes extracted from the conditioned medium of PDLSCs showed anti-inflammatory effects on chondrocytes, synoviocytes, and meniscus cells, mediating the inflammatory processes in various tissues in the joint [63]. AFSC-derived exosomes were found to increase pain tolerance and induce the restoration of hyaline cartilage with good surface regularity in an MIA-induced OA model [46]. An in vivo study showed that miR-100-5p-abundant IPFP-MSC-derived exosomes (IPFP-Exos) had chondroprotective effects and ameliorated gait abnormalities via inhibiting mTOR-autophagy pathway in an OA mouse model [44]. In vitro studies suggested that IPFP-Exos promoted chondrogenesis in periosteal cells via upregulating the expression of miR-221 and miR-145 and suppressing the production of proinflammatory cytokines [43]. Exosomes derived from kartogenin-pretreated IPFP-MSCs showed a stronger ability to induce stem cell chondrogenesis and promoted the proliferation of chondrocytes and the repair of articular cartilage defects both in vivo and in vitro [64]. Detailed information is summarized in Table 1.
2.2.2. Exosomes Derived from Chondrocytes and Chondrogenic Progenitor Cells
Exosomes released by chondrocytes participate in the pathologic mineralization of OA cartilage and cell–cell communication [67,68], affecting cartilage maintenance and OA pathogenesis [19]. These exosomes have dual roles in OA, depending on the cell condition and cell types. Exosomes from healthy chondrocytes had high bioactivity in the elimination of mitochondrial dysfunction and restoration of immune reaction by regulating M2 macrophage penetration, thus delaying OA progression [31]. These chondrocyte-derived exosomes contained miR-8485, which inhibited the expression of glycogen synthase kinase (GSK)-3β and activated the Wnt/β-catenin pathway, promoting the chondrogenic differentiation of BMSCs [69]. On the contrary, exosomes derived from OA chondrocytes enhanced chondrocyte apoptosis, inhibited cell proliferation, stimulated the activation of inflammasome, and upregulated the production of mature interleukin (IL)-1β in macrophages via promoting miR-449a-5p/ATG4B-mediated autophagy [30].
The chondrogenic progenitor cells (CPCs) are a type of resident cells in cartilage with high chondrogenic differentiation potential and a strong ability to self-renew and possess a regenerative ability in both diseased and healthy articular cartilage tissues [70]. CPC-derived exosomes enhanced the proliferation and migration of chondrocytes and alleviated OA in a DMM mouse model, probably via upregulating miRNA 221-3p [48].
2.2.3. Exosomes Derived from SFBs and Macrophages
Synoviocytes generally refer to SFBs and synovial macrophages, and SFBs are the major cell type in synovium [71]. Except for SMSC, SFBs and synovial macrophages also secret exosomes that regulate cartilage homeostasis and osteophyte formation [72]. Exosomes released by IL-1β treated SFB induced OA-like changes in articular chondrocytes by increasing the expression of catabolic genes, such as ADAMTS-5 and matrix metalloproteinases (MMP)-13, and downregulating the expression of anabolic genes, such as collagen type II (COL2) and aggrecan (ACAN) [71]. Exosomes from other synovial cells, including immune cells (e.g., macrophages, lymphocytes, and T cells) and endothelial cells, though not widely studied, are also believed to participate in the regulation of OA development [73]. Zeng et al. found that long non-coding RNA (lncRNA) prostate cancer gene expression marker 1 (PCGEM1) was overexpressed in exosomes from OA fibroblast-like synoviocytes (FLSs). FLS-derived exosomal PCGEM1 aggravated IL-1β-caused apoptosis and cartilage matrix degeneration in chondrocytes by sponging miR-142-5p and upregulating RUNX2 [49]. FLS-derived exosomal lncRNA H19 enhanced cell migration and proliferation, inhibited matrix degradation as well as alleviated OA progression by suppressing the miR-106b-5p/TIMP2 axis [74]. Though cytokines produced by macrophages and the imbalance between M1 and M2 macrophages are critical in OA pathogenesis, the effects of macrophage-derived exosomes on OA have been rarely studied thus far [75].
2.2.4. Exosomes Derived from Osteoblasts and Osteocytes
The remodeling of subchondral bone is a critical feature of OA and strongly associated with disease severity and joint pain in clinical OA patients [76]. Altered crosstalk between articular cartilage and the subchondral bone, which can be modulated by exosomes in OA progression, has attracted much attention but not been well studied. Wu et al. found that exosomes produced by osteoblasts in osteoarthritic, sclerotic subchondral bone contained a high level of miR-210-5p, which decreased the rate of oxygen consumption by chondrocytes, altered their bioenergetic state, and accelerated the progression of cartilage degeneration [32]. Exosome-like EVs have been extracted from osteoblasts harvested from OA subchondral bones. The OA osteoblast-derived exosomes were found to have upregulated expression of five miRNAs—hsa-miR-885-3p, hsamiR-4717-5p, hsamiR-210-5p, hsa-miR-135a-3p, and hsa-miR-1225-5p—than those obtained from the healthy controls; the physiological and pathological roles of these molecules still remain unclear [19].
Osteocytes release miRNA-containing exosomes, which deliver their components via blood circulation to the recipient cells to regulate biological processes [77]. In addition, osteocytes are sensitive to mechanical strains. Cultured under cyclic stretch of 8% shape variable at a frequency of 0.1 Hz for 30 min, osteocytes produce exosomes containing differentially expressed miRNAs compared with those from non-loading groups. These exosomes promoted the proliferation and osteogenesis of human PDLSCs by activating the miR-181b-5p/PTEN/AKT signaling pathway [78]. Myostatin, a myokine secreted by muscles, suppressed the expression of miR-218 in osteocyte-derived exosomes. Treated with these exosomes, osteoblasts showed decreased osteoblastic differentiation and down-regulated activity of the Wnt signaling pathway [79]. Osteocyte exosomes were also found to accelerate benign prostatic hyperplasia development by promoting cell proliferation [80].
2.2.5. Exosomes Derived from Adipose Tissue
IPFP is intraarticular adipose tissue that functions to reduce mechanical loading and absorb shock, and act as an abundant source of cytokines, lipid mediators as well as regenerative cells for cartilage repair [81]. IPFP is primarily comprised of adipocytes, and other cell types, including IPFP-derived MSCs and immune cells, are also found. As discussed earlier, intense interest has been spurred in IPFP-derived MSCs and IPFP-Exos [65].
Given the regulatory roles of adipose tissue in immune and nonimmune functions, compositional and functional analyses of adipocyte-derived exosomes can provide valuable information on the communications between adipocytes and other cells, such as immune cells, in the joint. A proteomic analysis of exosomes from obese diabetic and obese non-diabetic rats has been conducted. Among the 509 proteins identified, 200 of them were differentially expressed [82]. Sano et al. characterized the proteomic profiles of exosomes obtained from differentiated 3T3-L1 adipocytes and found that hypoxic culture upregulated the total protein amount in the exosomes and enriched the enzymes related to de novo lipogenesis [83]. According to Kita et al., adipose-derived exosomes can function as signaling packages and waste disposal bags [84]. Several lines of evidence support the role of adipose-derived exosomes in modulating macrophage polarization and hence inflammation [85,86,87]. Considering that obesity is a major risk factor for OA, investigations into adipose-derived exosomes may shed light onto molecular mechanisms underlying OA pathogenesis and the concurrent crosstalk between joint tissues.
2.2.6. Exosomes Derived from PRP
Blood-derived products, including plasma- and serum-based whole blood derivatives, have been applied to OA treatment via IA injection for years [88]. IA injection of PRP has been reported to promote the proliferation and differentiation of chondrocytes and facilitate matrix synthesis [89]. Three types of platelet granules have been defined: dense granules, α-granules, and lysosomes, and they differ in size, content, biomarker, synthesis process, and function [90]. Extracting exosomes from other types of granules is mainly based on size and specific membrane proteins [91]. Previous studies showed that exosomes originating from platelets were sufficient to enhance anabolic marker expression and prevent the release of proinflammatory cytokines in chondrocytes derived from OA patients, showing the same regulatory effects as the full blood product [51]. In addition, the therapeutic effects of PRP-derived exosomes in inhibiting apoptosis and promoting proliferation of chondrocytes were achieved by activating the Wnt/β-catenin signaling pathway [17]. The PRP-derived exosomes are relatively easy to prepare, do not require cell culture, and have minimal risks of disease transmission, making PRP-derived exosomes highly promising in OA treatment.
2.2.7. Exosomes Derived from Other Cells
Exosomes derived from vascular endothelial cells (EC-Exos) were found to promote the progression of OA; EC-Exos increased the susceptibility of mouse chondrocytes to anoxidative stress by inhibiting p21 expression and autophagy, leading to more apoptotic chondrocytes in the mouse OA model [29]. The serum of OA patients was found to have elevated levels of T cell-derived, CD3- and CD4-positive exosomes, and platelet-derived EVs positive for annexin V and CD61+ and negative for CD45, as compared to that of healthy controls [92,93]. Exosomes from immune cells, such as B cells, T cells, and dendritic cells, caused the production of several cartilage-degrading enzymes (including MMP-1, MMP-3, MMP-9, and MMP-13) and inflammatory cytokines and chemokines (including IL-6, IL-8, monocyte chemoattractant protein (MCP)-1, and MCP-2) in SFBs from OA patients [94,95,96,97]. Exosomes from tenocytes were found to facilitate the tenogenic differentiation of MSCs, promoting the healing of injured tendons and increasing the maximum loading and ultimate stress in tendons [53]. Cyclic stretch force-induced PDL cells secreted exosomes that suppressed the production of IL-1β by inhibiting the NF-κB pathway in macrophages [54]. Human PDL fibroblast (hPDLFs)-derived exosomes induced inflammation and inhibited osteogenesis by osteoblasts [55]. Static compressive force stimulated the production of exosomes in PDLFs. These exosomes, containing a high level of the Yes-associated protein (YAP), promoted macrophage polarization toward the M1 phenotype [56]. Research on exosomes derived from these cells is just the beginning. More research is needed on their roles in OA pathogenesis and treatment.
3. Extraction, Bioengineering Modification, and Delivery of Exosomes
EVs are heterogeneous, cell-secreted membranous structures, which can be classified into exosomes, microvesicles, and apoptotic bodies based on biogenesis, size, and release pathways [98]. Depending on the intrinsic functions and conditions of source cells, unique protein profiles are exhibited by exosomes derived from different cells [99]. Due to the similarities between different kinds of EVs, it is vital to isolate and identify high-purity exosomes to understand their biological functions and elucidate their mechanisms of action. In addition, naturally occurring exosomes have several drawbacks, such as insufficient targeting ability and efficacy. Therefore, bioengineering processes are required to overcome these limitations. Figure 3 depicts the general steps of cargo loading, isolation, and delivery strategies for engineered exosomes, which are discussed thoroughly in the following sections.
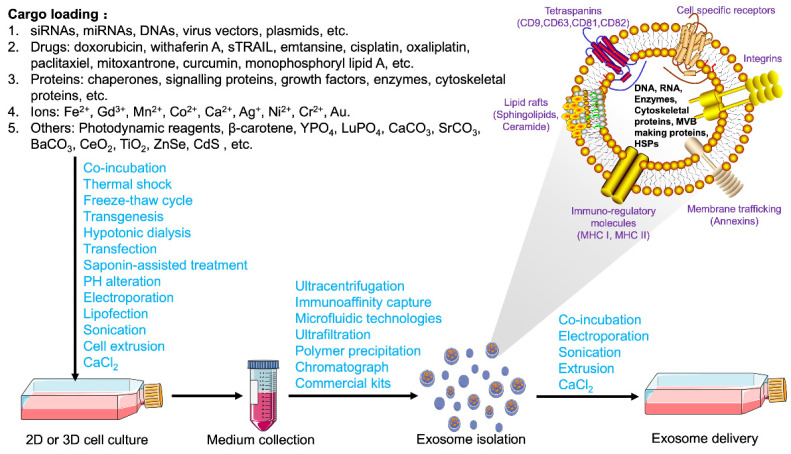
Figure 3.
Cargo loading, isolation, and delivery strategies for engineered exosomes. Bioactive molecules, such as nucleic acids, vectors, plasmids, drugs, ions, and other compounds were added in the cell culture medium. Exogenous cargo can be loaded into exosomes by several methods, such as electroporation, lipofection, sonication, and CaCl2 treatment. Cells loaded with exogenous cargo secreted exosomes containing these bioactive molecules into cell culture medium. Cells expressing target peptides by plasmid transfection produce exosomes that can target specific cell populations. These engineered exosomes were isolated and purified from the culture medium via different methods. Through co-incubation or other strategies, exosomes loaded with endogenous and/or exogenous cargo can be taken up by recipient cells for the regulation of gene expression and cell function.
3.1. Extraction, Identification, and Storage of Exosomes
Conditioned cell culture media are the most common source for exosome collection. Different methods based on the physical, chemical, and biological properties of exosomes have been developed to optimize the extraction, but standard operation procedures have not been established. Ultracentrifugation, immunoaffinity capture, ultrafiltration, size-exclusion chromatograph, charge neutralization-based polymer precipitation, and microfluidics-based techniques are commonly used methods for exosome extraction [100]; various precipitation- and column-based exosome isolation kits have also been developed (Figure 3) [101]. Whether a certain method or a combination of different methods should be selected depends on sample properties and research objectives. Whichever methods are applied, the goal for extraction remains the same, i.e., to maximize yield and purity while minimizing changes in protein content, size distribution, and surface charge during extraction. An in-depth discussion of different collection methods is beyond the scope of this article. Detailed extraction processes have been elaborated thoroughly in the published literature [102,103]. Several publications discussed the strengths and weaknesses of different methods to extract, characterize, and purify exosomes, and the selection of the most appropriate method(s) depends on the application and origin of exosomes [23,100,103,104].
There are two major types of exosome characterization methods: external characterization and inclusion characterization [105]. External characterization refers to the examination of morphology and particle size. Transmission electron microscopy (TEM) and scanning electron microscopy (SEM) are common methods for observing exosome morphology. SEM reveals the exosome surface microstructure, while TEM shows the internal structure and morphology of exosomes [106]. Nanoparticle tracking analysis (NTA) technology is applied for measuring the concentration and size of exosomes. Inclusion characterization is generally employed to detect membrane proteins, lipid rafts, and phospholipids present in the lipid bilayer, which can be detected by dynamic light scattering (DLS), flow cytometry, and western blotting [105]. Exosomes exhibit unique protein and lipid profiles that reflect the nature of donor cells and could be used as biomarkers for exosome identification. Common protein components include cytoskeletal proteins (e.g., actin), heat shock proteins (e.g., Hsp70 and Hsp90), bioactive proteins (e.g., GTPases, annexins, and flotillin), cytosolic proteins (e.g., GAPDH), antigen presentation proteins (major histocompatibility complex (MHC)-I, -II), tetraspanin membrane proteins (e.g., CD9, CD63, CD81, and CD82), proteins involved in multivesicular body biogenesis (e.g., Alix and TSG101), and vesicle trafficking (e.g., Tsg101) [19]. Membrane lipids vary in different exosomes and include cholesterol, ceramide, sphingolipids, phosphoglycerides, glycolipid GM3, and glycerophospholipids with saturated fatty-acyl chains [107]. Besides the methods described above, DLS, tunable resistive pulse sensing, and atomic force microscopy can also be employed to identify exosomes [108].
The preservation technologies of exosomes mainly include cryopreservation, freeze-drying, and spray-drying. Low temperature helps to maintain the quantity and contents of exosomes. It is recommended by the International Society of Extracellular Vesicles that exosomes be suspended in phosphate buffered saline and stored at −80 °C [109]. The addition of permeable and non-permeable antifreeze protects exosomes from ice crystal formation inside the vesicles and the imbalanced osmosis in the freezing process [110]. Freeze-drying, as a widely used method for preserving heat-sensitive materials, can dehydrate and dry exosomes at low temperatures under vacuum conditions [111]. Lyophilized exosomes can be stored at room temperature and conveniently reconstituted without affecting their pharmacokinetics [112]. Unlike lyophilization, which requires three continuous stages, spray drying is a single-step process. It is more economical but brings the risk of changing exosomal morphology [113]. Compared with cell-based therapies, the storage conditions of exosomes are generally less strict. Besides, frozen cells require recovery and function restoration prior to their clinical application, making them less convenient and more time consuming to handle compared to exosomes [114,115].
3.2. Contents and Loading Strategies for Exosomes
The constituent molecules of exosomes, including nucleic acids, lipids, proteins, and metabolites, differ in different exosomes, depending on the biogenesis mechanism, the cellular origin, developmental phase, environment, and epigenetic modification [116]. In vitro intracellular exosome loading during exosome biogenesis can be achieved by changing the culture condition and gene expression of the origin cells. For example, physical factors, such as low intensity pulsed ultrasound, moderate mechanical stress, and hypoxia have been found to convert exosomal contents to a chondroprotective mode [117,118,119]. Pretreatment with pharmacological agents, such as curcumin and kartogenin, enhances the exosomes’ ability to induce chondrogenesis by stem cells, promotes chondrocyte proliferation, and facilitates the repair of articular cartilage defects [64,120,121]. Biological factor-treated exosomes, such as those pretreated with transforming growth factor (TGF)-β1 or modified with activated transcription factor 4 mRNA, protect cartilage and alleviate OA progression by promoting the M2 polarization of synovial macrophages and inducing autophagy, respectively [122,123].
Genetic alteration is another widely used method to change exosomal content and function. miRNAs incorporated in exosomes participate in the intercellular communication in osteoarthritic joints [124]. The SF of OA patients was found to have upregulated miRNAs such as miR-155-3p, miR-16-2-3p, miR-504-3p, and miR-210-5p, and downregulated ones including miR-6878-3p, miR-146a-5p, and miR-26a-5p [125]. Available techniques to load therapeutic RNA mimics into exosomes include co-transfecting producer cells with plasmids, viruses, or bicistronic vectors, electroporating cells to facilitate the migration of small RNAs, and transient transfection with commercially available transfection reagents [126]. By using genetically modified parent cells, therapeutic agents were integrated into the corresponding exosomes [127]. Usually, non-coding RNA such as microRNA and lncRNA are induced to overexpress in the parent cells. These cells, usually MSCs, secrete exosomes containing high levels of desired RNAs that perform different roles in OA progression according to the gene properties [128]. Thus far, genetically modified cells have been employed to generate exosomes that are chondroprotective, anti-inflammatory, anti-apoptosis, and promote chondrocyte proliferation and migration [33,129,130].
The widely applied ex vivo extracellular exosome loading strategy refers to directly co-incubating exosomes with therapeutic agents and mixing them under appropriate conditions (Figure 3). For example, doxorubicin was successfully loaded onto pancreatic stellate cell-, pancreatic cancer cell-, and macrophage-derived exosomes via co-incubation [131]. Mixed with milk-derived exosomes, paclitaxel was loaded on the vesicles and showed significant therapeutic effects with low systemic and immunologic toxicities [132]. Co-incubation requires no special equipment, possesses high reproducibility, and does not compromise the integrity of exosome membrane structure. However, because of the relatively low loading efficiency, a large quantity of therapeutic agents is required [23]. To improve the drug loading efficiency on exosomes, electroporation, thermal shock, ultrasonic treatment, freeze-thaw cycles, transgenesis, pH gradient method, extrusion, hypotonic dialysis, transfection, and saponin-assisted treatments are applied to the synthesis process and show promising results (Figure 3) [133,134,135,136].
3.3. Bioengineered Modification and Delivery Strategies of Exosomes
Exosomes can be taken up by cells via endocytosis, direct membrane fusion, and pinocytosis [137]. The endocytic pathway is the main route by which exosomes enter the cell, release the contents, and exert their biological effects. However, direct delivery of exosomes, such as IA or subcutaneous injections, is associated with quick clearance in vivo and limited effective period [138]. Chondrocyte-targeted drug delivery is even more challenging due to the biological barrier formed by a dense matrix of proteoglycans, collagen, and highly negatively charged glycosaminoglycans in the cartilage [66], which requires more exosomes in a higher concentration. To improve yield, elongate retention time, and optimize treatment effects, several strategies have been proposed and studied, such as the development of exosome-mimetic nanovesicles (EMNVs), alteration of the culture condition, membrane surface modification, and controlled release with biomaterial platforms [103,139].
As mentioned above, appropriate cell culture conditions promote the production of exosomes. For example, UC-MSCs grown in 3D microcarrier-based scaffolds yielded 20-fold more exosomes than 2D cultures. If combined with tangential flow filtration (TFF) for exosome extraction, the production of exosomes could be further improved 7-fold more than 3D cultures [140]. A rotary cell culture system (RCCS) simultaneously provides shear stress, hydrostatic pressure, and buoyancy force, creating an environment of microgravity that benefits cell adhesion, proliferation, and aggregation; exosome secretion by UC-MSCs was significantly promoted at 36 rpm/min within 196 h [42]. EMNVs are another method to achieve a large-scale production of exosomes. The generation of EMNVs via serially extruding cells through micro-sized filters boosted the yield of exosomes by over 100 folds and kept the biological functions similar to naïve exosomes [141,142]. When applying EMNVs, attention should be paid to the changed lipid species as well as altered membrane compositions compared with naïve exosomes, as such changes may affect the PK/PD behavior of EMNVs in vivo [143].
Several strategies modifying exosomal surface structures have been put forward to improve the entry of exosomes to cells that might be applied in OA studies. For example, chondrocyte-targeting exosomes were prepared by fusing the lysosome-associated membrane glycoprotein 2b (Lamp2b) protein present on the exosome surface with the chondrocyte-affinity peptide (CAP). These exosomes effectively encapsulated miR-140 and specifically entered chondrocytes to deliver the cargoes in vitro [47]. Equipping exosomes with cell-penetrating peptides (CPPs), such as arginine-rich CPPs (e.g., octa-arginine peptides, oligoarginine peptides, and human immunodeficiency virus type 1 Tat (48–60) peptide), facilitated exosome entry into the cell by stimulating cell micropinocytosis [144]. Coating exosomes with the amphiphilic cationic CHP (cCHP) nanogel particles is a polymer-based surface engineering method to facilitate exosome content delivery and increase the encapsulation of large-size nucleic acids (e.g., plasmid) [145]. One issue concerning hybrid exosomes is their similar cytotoxicity as liposomes (Lipofectamine). Therefore, further investigation is required to develop liposomes with less toxicity [146]. Increasing the efficiency of fusion between exosomes and the targeted cells is another approach. Studies have shown that an increased fusion efficiency between recipient cells and exosomes was achieved by enhancing membrane rigidity by enriching cholesterol and sphingolipid [138]. Vascular stomatitis virus (VSV)-G protein, when harbored on the surface of fusogenic exosomes, facilitates the delivery of membrane proteins into the target cell membranes in vitro and in a mouse intramuscular injection model [147]. The integration of exosomes with connexin 43 also promotes direct cytoplasmic transfer of exosome payloads [148].
Biomaterials are applied for exosome encapsulation and sustained-delivery, to extend the half-life of exosomes and augment their therapeutic effects [149]. Human joints that may be affected by OA are enclosed in the joint capsule (Figure 1). Therefore, IA injection of exosomes is preferable, as it is safer than the systematic application and has a low risk of side effects. By virtue of their affinity and compatibility with cartilage, several kinds of bioengineered hydrogel scaffolds have been applied to optimize the delivery of exosomes to cartilage, such as photoinduced imine-crosslinking hydrogel glue [150], chitosan hydrogel [151], light triggerable hyaluronic acid hydrogel [152], alginate-based hydrogel [153], ECM/gelatin methacrylate composite scaffolds [36], and a highly adhesive hydrogel, the AD/CS/RSF/EXO hydrogel (alginate-dopamine, chondroitin sulfate, regenerated silk fibroin, and exosome hydrogel) [154].
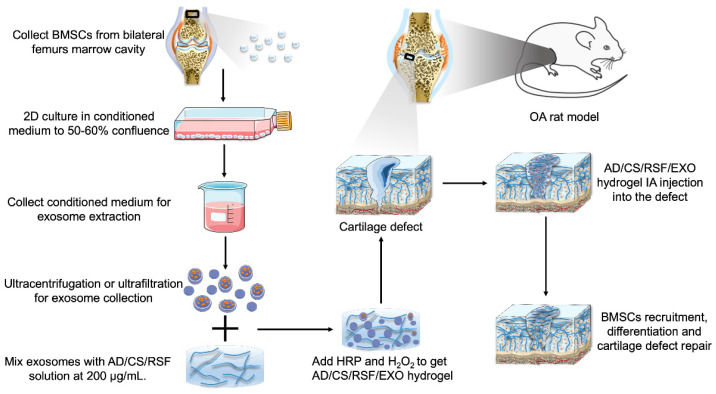
Processes for hydrogel-based scaffold preparation and delivery are similar among different kinds of hydrogels. Take the recently designed AD/CS/RSF/EXO hydrogel as an example [154]. As shown in Figure 4, exosomes extracted from the BMSCs-conditioned medium were mixed with the AD/CS/RSF pre-gel solution at 200 μg/mL. Then, horseradish peroxidase (HRP) and H2O2 were added to initiate crosslink formation and form a hydrogel. Subsequently, 500 μL AD/CS/RSF/EXO hydrogel containing 100 μg exosomes were injected into the cartilage defect of a rat knee joint via a syringe. The injected hydrogel quickly formed in situ and conformed to the defect shape within 3s. Covalent bonds formed between the amine and sulfhydryl groups on the surface of surrounding ECM and the chemical residues of the hydrogel (e.g., phenolic hydroxyl groups, N-hydroxysuccinimide, and catecholamine). As a result, the hydrogel generated adhesive binding with the surrounding native cartilage tissue due to the formation of covalently crosslinked networks. Besides, the loaded exosomes could be sustainedly released by the hydrogels, with around 87.51% of the encapsulated exosomes released into phosphate-buffered saline over 14 days. Exosomes released from hydrogels recruited BMSCs to scaffold implantation sites, promoted the proliferation and differentiation of MSCs, and accelerated ECM remodeling and cartilage defect regeneration. Hydrogel-based scaffolds are advantageous in controlled exosome release and operable for injection therapy under arthroscopy.
Figure 4.
Schematic of fabricating AD/CS/RSF/EXO hydrogels for cartilage defect repair in a rat OA model. BMSCs were aseptically isolated from the bilateral femur marrow cavities of male Sprague-Dawley (SD) rats. When the cells reached 50–60% confluency in 2D culture flasks, they were rinsed and incubated for 48 h in serum-free medium. The collected conditioned medium was ultracentrifuged and ultrafiltered to obtain exosomes. The exosomes were mixed with AD/CS/RSF pre-gel solution, and then H2O2 and HRP were added to induce gelation. Subsequently, the cartilage defect was filled with the exosome-containing adhesive hydrogel. The exosomes released by the hydrogels recruited BMSCs that migrated and infiltrated the hydrogel and promoted BMSC proliferation and differentiation into chondrocytes. By inducing ECM production and neo-cartilage formation, the hydrogel facilitated the regeneration of cartilage defect in situ.
4. In Vivo Efficacy of Exosomes for OA Treatment
Considerable advances in exosome-based therapies have been demonstrated in several disease models [16]. However, exosomes have not been utilized in OA-related studies until recent years. Therapeutic effects, such as pain relief [34], cartilage defect repair [155], subchondral bone protection [35], and synovitis amelioration [30], have been observed in OA research. The delivery method of exosomes for in vivo OA treatment reported thus far has only been intra-articular injection.
Exosomes derived from MSCs and other sources have been tested in vivo to evaluate their therapeutic effects in OA treatment. Used in an MIA-induced rat OA model, exosomes obtained from BM-MSCs effectively enhanced cartilage repair, ECM synthesis, and joint pain relief [34]. IPFP-MSC-derived exosomes also prevented cartilage damage and alleviated gait abnormality in a mouse OA model by maintaining cartilage homeostasis [44]. PRP-Exos decreased the apoptotic rate of OA chondrocytes and decreased the OARSI (Osteoarthritis Research Society International) score of cartilage samples from OA joints of rabbit models [17]. SFBs overexpressing miR-126-3p generated exosomes that suppressed cartilage degeneration and inflammation in an OA rat model [50]. CPC-derived, exosome-containing EVs enhanced the repair of articular cartilage in a surgically induced mouse OA model and stimulated chondrocyte migration and proliferation in vitro via upregulating miRNA 221-3p [48]. Such beneficial effects have been attributed to the role of exosomes in regulating different signaling pathways, such as mTOR, Wnt/β-catenin, YAP, and non-coding RNAs (Table 1). Besides, treatment of MSCs with engineered exosomes showed enhanced joint-protective effects in OA animal models. For example, by fusing the exosomal membrane protein, Lamp 2, with MSC-binding peptide E7, engineered exosomes (E7-Exo) could be employed in the targeted delivery of kartogenin, a small heterocyclic molecule, to synovial fluid-derived MSCs (SF-MSCs). E7-Exos induced in vitro and in vivo differentiation of SF-MSC into chondrocytes. Furthermore, co-intra-articular injection of SF-MSCs together with E7-Exo in the knee joints showed superior therapeutic effects compared to SF-MSC injection alone in a rat OA model [121].
5. Discussion
Mediating intercellular communications, exosomes have demonstrated therapeutic potential in the diagnosis and treatment of various diseases and can be harnessed in OA-related studies. Published research has confirmed that for OA patients, the production and contents of exosomes from chondrocytes, synovial fluid, and serum are largely changed [156]. Besides, the exosomes derived from aging chondrocytes were found to transmit senescence-associated characteristics to adjacent cells and hinder their chondrogenic abilities [157].
At present, disease-modifying therapeutic options for OA are rather limited, warranting future explorations and investigations into potential disease-modifying treatment regimens. Emerging as a trending research area, exosomal therapy has attracted much attention due to its good biocompatibility as well as unique regulatory roles in immunity, inflammation, senescence, tumorigenesis, etc. The pathogenesis of OA is closely related to inflammation and aging. Therefore, injecting bioengineered exosomes or modifying native cell-produced exosomes to regulate the joint microenvironment and related cell function is potentially beneficial for OA prevention and treatment.
Exosomes derived from different types of cells regulate and influence the functions of recipient cells in different ways. Previous studies on the beneficial effects of exosomes in OA treatment focused on exosomes derived from only one cell source. The observed beneficial or adverse effects and potential regulatory mechanism of exosomes from different origins have been illustrated. OA is a degenerative disease of the whole joint, and multiple types of cells and tissues are involved in OA initiation and progression. The intra-articular environment is particularly complex and dynamic. Therefore, using exosomes derived from different cell types to simultaneously target different cells and tissues of the joint could be a promising approach worth investigating in future studies. For example, exosomes isolated from several cell sources exhibited chondroprotective effects. The combined application of exosomes produced by BM-MSC, ADSC, and synovial fibroblasts can potentially show synergistic effects on OA treatment as they target different major cell types in the joint.
Although results from preclinical studies have confirmed the chondroprotective effects of bioengineered exosomes, investigations into the efficacy of exosomes for OA treatment are still in their early stages. To optimize and extend the application of exosomes in OA diagnosis and treatment, several issues should be taken into consideration in future studies. First, the average pore size in the articular cartilage ECM is estimated to be around 6.0 nm [158]. Only small cationic nanocarriers, usually with a diameter smaller than 15 nm, can overcome this biological barrier [159]. Considering that the diameter of exosomes ranges between 30–150 nm, it is important to increase the delivery efficiency of exosomal contents to chondrocytes. Besides, the thickness of cartilage considerably affects the delivery of exosomes. In vivo tests of exosomes conducted to date mostly employed small animals such as mice, rats, and rabbits. The cartilage thickness of these animal models is significantly lower than human cartilage (~50 µm in mice, 100–150 µm in rats, and 350–700 µm in rabbits compared to 1500–2000 µm in humans) [160]. In addition, most in vitro studies were conducted in cultured chondrocytes instead of full-thickness cartilage explants, limiting the applicability of the results to in vivo scenarios. Existing extraction methods are limited by the low exosome yield, posing a major challenge to the clinical applications of exosomes. Undesired RNAs (e.g., retroviral genomes) or proteins unintentionally incorporated in exosomes, as well as off-target delivery, are also issues that need to be carefully considered. In addition, although encapsulating exosomes within a scaffold is a feasible option to achieve controlled release of exosomes and reduce the number of injections needed [161], material pharmacokinetics and possible toxicity should be carefully evaluated. Due to a lack of effective methods to separate exosomes from the other two kinds of EVs, it remains a challenge to explicitly elucidate the functions and physiochemical properties of exosomes. Besides, extracting homotypic exosomes with consistent contents is critical for precision therapy and minimum side effects caused by unintended by-products. In addition, rational designs of exosome delivery tools require a further understanding of the mechanisms responsible for exosomes targeting recipient cells and the binding affinities. Lastly, it is unclear in some cases how or why exosomes derived from different cells have varying biological activities. Therefore, a future research avenue is to figure out the active factors in various exosomes and their potential mechanisms of action in OA treatment.
The quick turnover of synovial fluid in the joint and the rapidly decreased transport efficacy into cartilage with increasing thickness necessitate strategies for enhancing exosome uptake to maximize the therapeutic effects of exosomes on chondrocytes, which reside deep within the dense, anionic cartilage matrix [162]. Previous studies reported approaches to overcoming the biological barrier of cartilage and improving the delivery efficacy of drugs and biomolecules. For example, controlling the surface charge of exosomes to achieve desirable electrostatic interactions with ECM could be a promising strategy to enhance drug penetration and transport through the full thickness of cartilage [163]. Functionalizing polyamidoamine (PAMAM) dendrimer nanocarriers with poly(ethylene glycol) (PEG) improved the tissue binding ability, penetration depth, and residence time of PAMAM dendrimer [159]. It was found that this modified dendrimer, when conjugated with insulin-like growth factor 1 (IGF-1), penetrated bovine cartilage with comparable thickness to humans’ within 2 days and significantly enhanced the retention of therapeutic IGF-1 within rat knees [159]. Another method to deliver large-sized therapeutics is via cationic peptides and proteins [164,165,166]. These studies indicate that it is feasible, albeit difficult, to overcome the biological barrier formed by cartilage ECM for effective exosome delivery.
It is worth noting that most in vivo tests of exosomes were conducted in small animals, including mouse, rat, and rabbit models. To date, no large animal studies or human clinical trials have been completed to evaluate exosomal treatment of OA. An ongoing clinical trial (ClinicalTrials.gov NCT04719793) evaluates the efficacy of umbilical cord-derived Wharton’s jelly (UC-WJ) for knee OA treatment. While exosomes are present in UC-WJ, it also contains various other components, such as hyaluronic acid, cytokines, growth factors, and other EVs [167,168]. The benefits of exosomes alone, therefore, will be unknown in this clinical trial. Few animal studies conducted thus far described the safety of exosomal treatment of OA, probably because unlike other pharmacological agents, exosomes are cell-secreted products and less likely to be toxic. Besides, exosomes are usually injected locally into the articular cavity, which is much safer than systematic administration. Therefore, a safety assessment of exosomal treatment is not as crucial as testing other OA drugs. Nevertheless, future studies are recommended to bridge this knowledge gap. Currently, insufficient evidence from preclinical research and clinical trials significantly hinders the translation of exosomal therapies from basic research to clinical applications. However, as the promising therapeutic effects of exosomes are being revealed in more basic research, an increasing number of large animal tests and clinical trials can be expected in the future. In conclusion, though faced with challenges, exosome-based therapies are promising in OA diagnosis and treatment and worthy of further investigations.
Author Contributions
Conceptualization, Y.H. and Z.L.; writing—original draft preparation, Y.F.; writing—review and editing, Y.H. and Z.L.; visualization, Z.L.; supervision, Y.H. and Z.L.; project administration, Y.H.; All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.He Y., Makarczyk M.J., Lin H. Role of mitochondria in mediating chondrocyte response to mechanical stimuli. Life Sci. 2020;263:118602. doi: 10.1016/j.lfs.2020.118602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.He Y., Li Z., Alexander P.G., Ocasio-Nieves B.D., Yocum L., Lin H., Tuan R.S. Pathogenesis of osteoarthritis: Risk factors, regulatory pathways in chondrocytes, and experimental models. Biol. 2020;9:194. doi: 10.3390/biology9080194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhao X., Shah D., Gandhi K., Wei W., Dwibedi N., Webster L., Sambamoorthi U. Clinical, humanistic, and economic burden of osteoarthritis among noninstitutionalized adults in the United States. Osteoarthr. Cartil. 2019;27:1618–1626. doi: 10.1016/j.joca.2019.07.002. [DOI] [PubMed] [Google Scholar]
- 4.Bosch M.H.V.D. Osteoarthritis year in review 2020: Biology. Osteoarthr. Cartil. 2021;29:143–150. doi: 10.1016/j.joca.2020.10.006. [DOI] [PubMed] [Google Scholar]
- 5.Favero M., El-Hadi H., Belluzzi E., Granzotto M., Porzionato A., Sarasin G., Rambaldo A., Iacobellis C., Cigolotti A., Fontanella C.G., et al. Infrapatellar fat pad features in osteoarthritis: A histopathological and molecular study. Rheumatology (Oxford) 2017;56:1784–1793. doi: 10.1093/rheumatology/kex287. [DOI] [PubMed] [Google Scholar]
- 6.Prieto-Alhambra D., Judge A., Javaid M., Cooper C., Diez-Perez A., Arden N.K. Incidence and risk factors for clinically diagnosed knee, hip and hand osteoarthritis: Influences of age, gender and osteoarthritis affecting other joints. Ann. Rheum. Dis. 2013;73:1659–1664. doi: 10.1136/annrheumdis-2013-203355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vina E., Kwoh C.K. Epidemiology of osteoarthritis: Literature update. Curr. Opin. Rheumatol. 2018;30:160–167. doi: 10.1097/BOR.0000000000000479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Van Spil W.E., Kubassova O., Boesen M., Bay-Jensen A.-C., Mobasheri A. Osteoarthritis phenotypes and novel therapeutic targets. Biochem. Pharmacol. 2019;165:41–48. doi: 10.1016/j.bcp.2019.02.037. [DOI] [PubMed] [Google Scholar]
- 9.Abramoff B., Caldera F.E. Osteoarthritis: Pathology, diagnosis, and treatment options. Med. Clin. N. Am. 2020;104:293–311. doi: 10.1016/j.mcna.2019.10.007. [DOI] [PubMed] [Google Scholar]
- 10.Latourte A., Kloppenburg M., Richette P. Emerging pharmaceutical therapies for osteoarthritis. Nat. Rev. Rheumatol. 2020;16:673–688. doi: 10.1038/s41584-020-00518-6. [DOI] [PubMed] [Google Scholar]
- 11.Hunter D.J., Bierma-Zeinstra S. Osteoarthritis. Lancet. 2019;393:1745–1759. doi: 10.1016/S0140-6736(19)30417-9. [DOI] [PubMed] [Google Scholar]
- 12.Salgado C., Jordan O., Allémann E. Osteoarthritis in vitro models: Applications and implications in development of intra-articular drug delivery systems. Pharmaceutics. 2021;13:60. doi: 10.3390/pharmaceutics13010060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Thery C., Amigorena S., Raposo G., Clayton A. Isolation and characterization of exosomes from cell culture supernatants and biological fluids. Curr. Protoc. Cell Biol. 2006;30:3.22.1–3.22.29. doi: 10.1002/0471143030.cb0322s30. [DOI] [PubMed] [Google Scholar]
- 14.Chen H., Wang L., Zeng X., Schwarz H., Nanda H.S., Peng X., Zhou Y. Exosomes, a new star for targeted delivery. Front. Cell Dev. Biol. 2021;9:751079. doi: 10.3389/fcell.2021.751079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gurunathan S., Kang M.-H., Kim J.-H. A comprehensive review on factors influences biogenesis, functions, therapeutic and clinical implications of exosomes. Int. J. Nanomed. 2021;16:1281–1312. doi: 10.2147/IJN.S291956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kalluri R., LeBleu V.S. The biology, function, and biomedical applications of exosomes. Science. 2020;367:eaau6977. doi: 10.1126/science.aau6977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu X., Wang L., Ma C., Wang G., Zhang Y., Sun S. Exosomes derived from platelet-rich plasma present a novel potential in alleviating knee osteoarthritis by promoting proliferation and inhibiting apoptosis of chondrocyte via Wnt/β-catenin signaling pathway. J. Orthop. Surg. Res. 2019;14:470. doi: 10.1186/s13018-019-1529-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Qiu B., Xu X., Yi P., Hao Y. Curcumin reinforces MSC-derived exosomes in attenuating osteoarthritis via modulating the miR-124/NF-kB and miR-143/ROCK1/TLR9 signalling pathways. J. Cell. Mol. Med. 2020;24:10855–10865. doi: 10.1111/jcmm.15714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ni Z., Zhou S., Li S., Kuang L., Chen H., Luo X., Ouyang J., He M., Du X., Chen L. Exosomes: Roles and therapeutic potential in osteoarthritis. Bone Res. 2020;8:25. doi: 10.1038/s41413-020-0100-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Trams E.G., Lauter C.J., Salem N., Jr., Heine U. Exfoliation of membrane ecto-enzymes in the form of micro-vesicles. Biochim. Biophys. Acta. 1981;645:63–70. doi: 10.1016/0005-2736(81)90512-5. [DOI] [PubMed] [Google Scholar]
- 21.Pan B.-T., Johnstone R.M. Fate of the transferrin receptor during maturation of sheep reticulocytes in vitro: Selective externalization of the receptor. Cell. 1983;33:967–978. doi: 10.1016/0092-8674(83)90040-5. [DOI] [PubMed] [Google Scholar]
- 22.Fan J., Lee C.-S., Kim S., Chen C., Aghaloo T., Lee M. Generation of small RNA-modulated exosome mimetics for bone regeneration. ACS Nano. 2020;14:11973–11984. doi: 10.1021/acsnano.0c05122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang Y., Bi J., Huang J., Tang Y., Du S., Li P. Exosome: A review of its classification, isolation techniques, storage, diagnostic and targeted therapy applications. Int. J. Nanomed. 2020;15:6917–6934. doi: 10.2147/IJN.S264498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liang G., Kan S., Zhu Y., Feng S., Feng W., Gao S. Engineered exosome-mediated delivery of functionally active miR-26a and its enhanced suppression effect in HepG2 cells. Int. J. Nanomed. 2018;13:585–599. doi: 10.2147/IJN.S154458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Morishita M., Takahashi Y., Matsumoto A., Nishikawa M., Takakura Y. Exosome-based tumor antigens–adjuvant co-delivery utilizing genetically engineered tumor cell-derived exosomes with immunostimulatory CpG DNA. Biomater. 2016;111:55–65. doi: 10.1016/j.biomaterials.2016.09.031. [DOI] [PubMed] [Google Scholar]
- 26.Loeser R.F., Goldring S.R., Scanzello C.R., Goldring M.B. Osteoarthritis: A disease of the joint as an organ. Arthritis Rheum. 2012;64:1697–1707. doi: 10.1002/art.34453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mustonen A.-M., Nieminen P. Extracellular vesicles and their potential significance in the pathogenesis and treatment of osteoarthritis. Pharmaceuticals. 2021;14:315. doi: 10.3390/ph14040315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li D., Gupta P., Sgaglione N., Grande D. Exosomes derived from non-classic sources for treatment of post-traumatic osteoarthritis and cartilage injury of the knee: In vivo review. J. Clin. Med. 2021;10:2001. doi: 10.3390/jcm10092001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yang R.-Z., Zheng H.-L., Xu W.-N., Zheng X.-F., Li B., Jiang L.-S., Jiang S.-D. Vascular endothelial cell-secreted exosomes facilitate osteoarthritis pathogenesis by promoting chondrocyte apoptosis. Aging (Albany NY) 2021;13:4647–4662. doi: 10.18632/aging.202506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ni Z., Kuang L., Chen H., Xie Y., Zhang B., Ouyang J., Wu J., Zhou S., Chen L., Su N., et al. The exosome-like vesicles from osteoarthritic chondrocyte enhanced mature IL-1β production of macrophages and aggravated synovitis in osteoarthritis. Cell Death Dis. 2019;10:1–16. doi: 10.1038/s41419-019-1739-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zheng L., Wang‡ Y., Qiu P., Xia C., Fang Y., Mei S., Fang C., Shi Y., Wu K., Chen Z., et al. Primary chondrocyte exosomes mediate osteoarthritis progression by regulating mitochondrion and immune reactivity. Nanomedicine (Lond) 2019;14:3193–3212. doi: 10.2217/nnm-2018-0498. [DOI] [PubMed] [Google Scholar]
- 32.Wu X., Crawford R., Xiao Y., Mao X., Prasadam I. Osteoarthritic subchondral bone release exosomes that promote cartilage degeneration. Cells. 2021;10:251. doi: 10.3390/cells10020251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu Y., Lin L., Zou R., Wen C., Wang Z., Lin F. MSC-derived exosomes promote proliferation and inhibit apoptosis of chondrocytes via lncRNA-KLF3-AS1/miR-206/GIT1 axis in osteoarthritis. Cell Cycle. 2018;17:2411–2422. doi: 10.1080/15384101.2018.1526603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.He L., He T., Xing J., Zhou Q., Fan L., Liu C., Chen Y., Wu D., Tian Z., Liu B., et al. Bone marrow mesenchymal stem cell-derived exosomes protect cartilage damage and relieve knee osteoarthritis pain in a rat model of osteoarthritis. Stem Cell Res. Ther. 2020;11:276. doi: 10.1186/s13287-020-01781-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cosenza S., Ruiz M., Toupet K., Jorgensen C., Noël D. Mesenchymal stem cells derived exosomes and microparticles protect cartilage and bone from degradation in osteoarthritis. Sci. Rep. 2017;7:1–12. doi: 10.1038/s41598-017-15376-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chen P., Zheng L., Wang Y., Tao M., Xie Z., Xia C., Gu C., Chen J., Qiu P., Mei S., et al. Desktop-stereolithography 3D printing of a radially oriented extracellular matrix/mesenchymal stem cell exosome bioink for osteochondral defect regeneration. Theranostics. 2019;9:2439–2459. doi: 10.7150/thno.31017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang Z., Yan K., Ge G., Zhang D., Bai J., Guo X., Zhou J., Xu T., Xu M., Long X., et al. Exosomes derived from miR-155-5p–overexpressing synovial mesenchymal stem cells prevent osteoarthritis via enhancing proliferation and migration, attenuating apoptosis, and modulating extracellular matrix secretion in chondrocytes. Cell Biol. Toxicol. 2021;37:85–96. doi: 10.1007/s10565-020-09559-9. [DOI] [PubMed] [Google Scholar]
- 38.Tao S.-C., Yuan T., Zhang Y.-L., Yin W.-J., Guo S.-C., Zhang C.-Q. Exosomes derived from miR-140-5p-overexpressing human synovial mesenchymal stem cells enhance cartilage tissue regeneration and prevent osteoarthritis of the knee in a rat model. Theranostics. 2017;7:180–195. doi: 10.7150/thno.17133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhang S., Teo K.Y.W., Chuah S.J., Lai R.C., Lim S.K., Toh W.S. MSC exosomes alleviate temporomandibular joint osteoarthritis by attenuating inflammation and restoring matrix homeostasis. Biomaterials. 2019;200:35–47. doi: 10.1016/j.biomaterials.2019.02.006. [DOI] [PubMed] [Google Scholar]
- 40.Wang Y., Yu D., Liu Z., Zhou F., Dai J., Wu B., Zhou J., Heng B.C., Zou X.H., Ouyang H., et al. Exosomes from embryonic mesenchymal stem cells alleviate osteoarthritis through balancing synthesis and degradation of cartilage extracellular matrix. Stem Cell Res. Ther. 2017;8:1–13. doi: 10.1186/s13287-017-0632-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhu Y., Wang Y., Zhao B., Niu X., Hu B., Li Q., Zhang J., Ding J., Chen Y., Wang Y. Comparison of exosomes secreted by induced pluripotent stem cell-derived mesenchymal stem cells and synovial membrane-derived mesenchymal stem cells for the treatment of osteoarthritis. Stem Cell Res. Ther. 2017;8:64. doi: 10.1186/s13287-017-0510-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yan L., Liu G., Wu X. Exosomes derived from umbilical cord mesenchymal stem cells in mechanical environment show improved osteochondral activity via upregulation of LncRNA H19. J. Orthop. Transl. 2021;26:111–120. doi: 10.1016/j.jot.2020.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhao C., Chen J.Y., Peng W.M., Yuan B., Bi Q., Xu Y.J. Exosomes from adipose-derived stem cells promote chondrogenesis and suppress inflammation by upregulating miR-145 and miR-221. Mol. Med. Rep. 2020;21:1881–1889. doi: 10.3892/mmr.2020.10982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wu J., Kuang L., Chen C., Yang J., Zeng W.-N., Li T., Chen H., Huang S., Fu Z., Li J., et al. miR-100-5p-abundant exosomes derived from infrapatellar fat pad MSCs protect articular cartilage and ameliorate gait abnormalities via inhibition of mTOR in osteoarthritis. Biomaterials. 2019;206:87–100. doi: 10.1016/j.biomaterials.2019.03.022. [DOI] [PubMed] [Google Scholar]
- 45.Woo C.H., Kim H.K., Yang S., Park J.H., Jo D., Cho Y.W., Jung G.Y., Jung Y.J., Lee K.S., Yun Y.E., et al. Small extracellular vesicles from human adipose-derived stem cells attenuate cartilage degeneration. J. Extracell. Vesicles. 2020;9:1735249. doi: 10.1080/20013078.2020.1735249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zavatti M., Beretti F., Casciaro F., Bertucci E., Maraldi T. Comparison of the therapeutic effect of amniotic fluid stem cells and their exosomes on monoiodoacetate-induced animal model of osteoarthritis. BioFactors. 2020;46:106–117. doi: 10.1002/biof.1576. [DOI] [PubMed] [Google Scholar]
- 47.Liang Y., Xu X., Li X., Xiong J., Li B., Duan L., Wang D., Xia J. Chondrocyte-targeted microRNA delivery by engineered exosomes toward a cell-free osteoarthritis therapy. ACS Appl. Mater. Interfaces. 2020;12:36938–36947. doi: 10.1021/acsami.0c10458. [DOI] [PubMed] [Google Scholar]
- 48.Wang R., Jiang W., Zhang L., Xie S., Zhang S., Yuan S., Jin Y., Zhou G. Intra-articular delivery of extracellular vesicles secreted by chondrogenic progenitor cells from MRL/MpJ superhealer mice enhances articular cartilage repair in a mouse injury model. Stem Cell Res. Ther. 2020;11:93. doi: 10.1186/s13287-020-01594-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zeng G., Deng G., Xiao S., Li F. Fibroblast-like synoviocytes-derived exosomal PCGEM1 accelerates IL-1β-induced apoptosis and cartilage matrix degradation by miR-142-5p/RUNX2 in chondrocytes. Immunol. Investig. 2021;10:1–18. doi: 10.1080/08820139.2021.1936010. [DOI] [PubMed] [Google Scholar]
- 50.Zhou Y., Ming J., Li Y., Li B., Deng M., Ma Y., Chen Z., Zhang Y., Li J., Liu S. Exosomes derived from miR-126-3p-overexpressing synovial fibroblasts suppress chondrocyte inflammation and cartilage degradation in a rat model of osteoarthritis. Cell Death Discov. 2021;7:37. doi: 10.1038/s41420-021-00418-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Otahal A., Kramer K., Kuten-Pella O., Weiss R., Stotter C., Lacza Z., Weber V., Nehrer S., De Luna A. Characterization and chondroprotective effects of extracellular vesicles from plasma- and serum-based autologous blood-derived products for osteoarthritis therapy. Front. Bioeng. Biotechnol. 2020;8:584050. doi: 10.3389/fbioe.2020.584050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wang Y., He G., Guo Y., Tang H., Shi Y., Bian X., Zhu M., Kang X., Zhou M., Lyu J., et al. Exosomes from tendon stem cells promote injury tendon healing through balancing synthesis and degradation of the tendon extracellular matrix. J. Cell. Mol. Med. 2019;23:5475–5485. doi: 10.1111/jcmm.14430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Xu T., Xu M., Bai J., Lin J., Yu B., Liu Y., Guo X., Shen J., Sun H., Hao Y., et al. Tenocyte-derived exosomes induce the tenogenic differentiation of mesenchymal stem cells through TGF-β. Cytotechnology. 2019;71:57–65. doi: 10.1007/s10616-018-0264-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wang Z., Maruyama K., Sakisaka Y., Suzuki S., Tada H., Suto M., Saito M., Yamada S., Nemoto E. Cyclic stretch force induces periodontal ligament cells to secrete exosomes that suppress IL-1β production through the inhibition of the NF-κB signaling pathway in macrophages. Front. Immunol. 2019;10:1310. doi: 10.3389/fimmu.2019.01310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhao M., Dai W., Wang H., Xue C., Feng J., He Y., Wang P., Li S., Bai D., Shu R. Periodontal ligament fibroblasts regulate osteoblasts by exosome secretion induced by inflammatory stimuli. Arch. Oral Biol. 2019;105:27–34. doi: 10.1016/j.archoralbio.2019.06.002. [DOI] [PubMed] [Google Scholar]
- 56.Zhao M., Ma Q., Zhao Z., Guan X., Bai Y. Periodontal ligament fibroblast-derived exosomes induced by compressive force promote macrophage M1 polarization via Yes-associated protein. Arch. Oral Biol. 2021;132:105263. doi: 10.1016/j.archoralbio.2021.105263. [DOI] [PubMed] [Google Scholar]
- 57.Lopa S., Colombini A., Moretti M., De Girolamo L. Injective mesenchymal stem cell-based treatments for knee osteoarthritis: From mechanisms of action to current clinical evidences. Knee Surg. Sports Traumatol. Arthrosc. 2019;27:2003–2020. doi: 10.1007/s00167-018-5118-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lara-Barba E., Araya M.J., Hill C.N., Bustamante-Barrientos F.A., Ortloff A., García C., Galvez-Jiron F., Pradenas C., Luque-Campos N., Maita G., et al. Role of microRNA shuttled in small extracellular vesicles derived from mesenchymal stem/stromal cells for osteoarticular disease treatment. Front. Immunol. 2021;12:768771. doi: 10.3389/fimmu.2021.768771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhang S., Chuah S.J., Lai R.C., Hui J.H.P., Lim S.K., Toh W.S. MSC exosomes mediate cartilage repair by enhancing proliferation, attenuating apoptosis and modulating immune reactivity. Biomaterials. 2018;156:16–27. doi: 10.1016/j.biomaterials.2017.11.028. [DOI] [PubMed] [Google Scholar]
- 60.Basu J., Ludlow J.W. Exosomes for repair, regeneration and rejuvenation. Expert Opin. Biol. Ther. 2016;16:489–506. doi: 10.1517/14712598.2016.1131976. [DOI] [PubMed] [Google Scholar]
- 61.Zhang L.-Q., Zhao G.-Z., Xu X.-Y., Fang J., Chen J.-M., Li J.-W., Gao X.-J., Hao L.-J., Chen Y.-Z. Integrin-β1 regulates chondrocyte proliferation and apoptosis through the upregulation of GIT1 expression. Int. J. Mol. Med. 2015;35:1074–1080. doi: 10.3892/ijmm.2015.2114. [DOI] [PubMed] [Google Scholar]
- 62.Tofiño-Vian M., Guillén M.I., del Caz M.D.P., Castejón M.A., Alcaraz M.J. Extracellular vesicles from adipose-derived mesenchymal stem cells downregulate senescence features in osteoarthritic osteoblasts. Oxidative Med. Cell. Longev. 2017;2017:7197598. doi: 10.1155/2017/7197598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Huang C.-Y., Vesvoranan O., Yin X., Montoya A., Londono V., Sawatari Y., Garcia-Godoy F. Anti-inflammatory effects of conditioned medium of periodontal ligament-derived stem cells on chondrocytes, synoviocytes, and meniscus cells. Stem Cells Dev. 2021;30:537–547. doi: 10.1089/scd.2021.0010. [DOI] [PubMed] [Google Scholar]
- 64.Shao J., Zhu J., Chen Y., Fu Q., Li L., Ding Z., Wu J., Han Y., Li H., Qian Q., et al. Exosomes from kartogenin-pretreated infrapatellar fat pad mesenchymal stem cells enhance chondrocyte anabolism and articular cartilage regeneration. Stem Cells Int. 2021;2021:6624874. doi: 10.1155/2021/6624874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Huri P.Y., Hamsici S., Ergene E., Huri G., Doral A.M.N. Infrapatellar fat pad-derived stem cell-based regenerative strategies in orthopedic surgery. Knee Surg. Relat. Res. 2018;30:179–186. doi: 10.5792/ksrr.17.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lin J., Wang L., Lin J., Liu Q. The role of extracellular vesicles in the pathogenesis, diagnosis, and treatment of osteoarthritis. Molecules. 2021;26:4987. doi: 10.3390/molecules26164987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Shapiro I.M., Landis W.J., Risbud M.V. Matrix vesicles: Are they anchored exosomes? Bone. 2015;79:29–36. doi: 10.1016/j.bone.2015.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Mitton E., Gohr C.M., McNally M.T., Rosenthal A.K. Articular cartilage vesicles contain RNA. Biochem. Biophys. Res. Commun. 2009;388:533–538. doi: 10.1016/j.bbrc.2009.08.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Li Z., Wang Y., Xiang S., Zheng Z., Bian Y., Feng B., Weng X. Chondrocytes-derived exosomal miR-8485 regulated the Wnt/β-catenin pathways to promote chondrogenic differentiation of BMSCs. Biochem. Biophys. Res. Commun. 2020;523:506–513. doi: 10.1016/j.bbrc.2019.12.065. [DOI] [PubMed] [Google Scholar]
- 70.Koelling S., Kruegel J., Irmer M., Path J.R., Sadowski B., Miro X., Miosge N. Migratory chondrogenic progenitor cells from repair tissue during the later stages of human osteoarthritis. Cell Stem Cell. 2009;4:324–335. doi: 10.1016/j.stem.2009.01.015. [DOI] [PubMed] [Google Scholar]
- 71.Kato T., Miyaki S., Ishitobi H., Nakamura Y., Nakasa T., Lotz M.K., Ochi M. Exosomes from IL-1β stimulated synovial fibroblasts induce osteoarthritic changes in articular chondrocytes. Arthritis Res. Ther. 2014;16:R163. doi: 10.1186/ar4679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Mathiessen A., Conaghan P.G. Synovitis in osteoarthritis: Current understanding with therapeutic implications. Arthritis Res. Ther. 2017;19:1–9. doi: 10.1186/s13075-017-1229-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Tsuno H., Suematsu N., Sato T., Arito M., Matsui T., Iizuka N., Omoteyama K., Okamoto K., Tohma S., Kurokawa M.S., et al. Effects of methotrexate and salazosulfapyridine on protein profiles of exosomes derived from a human synovial sarcoma cell line of SW982. Proteom. Clin. Appl. 2015;10:164–171. doi: 10.1002/prca.201500064. [DOI] [PubMed] [Google Scholar]
- 74.Tan F., Wang D., Yuan Z. The fibroblast-like synoviocyte derived exosomal long non-coding RNA H19 alleviates osteoarthritis progression through the miR-106b-5p/TIMP2 axis. Inflammation. 2020;43:1498–1509. doi: 10.1007/s10753-020-01227-8. [DOI] [PubMed] [Google Scholar]
- 75.Chen Y., Jiang W., Yong H., He M., Yang Y., Deng Z., Li Y. Macrophages in osteoarthritis: Pathophysiology and therapeutics. Am J Transl Res. 2020;12:261–268. [PMC free article] [PubMed] [Google Scholar]
- 76.Barr A.J., Campbell T.M., Hopkinson D., Kingsbury S.R., Bowes M.A., Conaghan P.G. A systematic review of the relationship between subchondral bone features, pain and structural pathology in peripheral joint osteoarthritis. Arthritis Res. Ther. 2015;17:1–36. doi: 10.1186/s13075-015-0735-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Sato M., Suzuki T., Kawano M., Tamura M. Circulating osteocyte-derived exosomes contain miRNAs which are enriched in exosomes from MLO-Y4 cells. Biomed. Rep. 2017;6:223–231. doi: 10.3892/br.2016.824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lv P.-Y., Gao P.-F., Tian G.-J., Yang Y.-Y., Mo F.-F., Wang Z.-H., Sun L., Kuang M.-J., Wang Y.-L. Osteocyte-derived exosomes induced by mechanical strain promote human periodontal ligament stem cell proliferation and osteogenic differentiation via the miR-181b-5p/PTEN/AKT signaling pathway. Stem Cell Res. Ther. 2020;11:1–15. doi: 10.1186/s13287-020-01815-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Qin Y., Peng Y., Zhao W., Pan J., Ksiezak-Reding H., Cardozo C., Wu Y., Pajevic P.D., Bonewald L.F., Bauman W.A., et al. Myostatin inhibits osteoblastic differentiation by suppressing osteocyte-derived exosomal microRNA-218: A novel mechanism in muscle-bone communication. J. Biol. Chem. 2017;292:11021–11033. doi: 10.1074/jbc.M116.770941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wang Y.-Y., Xia K., Wang Z.-X., Xie H., Xu R. Osteocyte exosomes accelerate benign prostatic hyperplasia development. Mol. Cell. Endocrinol. 2021;531:111301. doi: 10.1016/j.mce.2021.111301. [DOI] [PubMed] [Google Scholar]
- 81.Chang J., Liao Z., Lu M., Meng T., Han W., Ding C. Systemic and local adipose tissue in knee osteoarthritis. Osteoarthr. Cartil. 2018;26:864–871. doi: 10.1016/j.joca.2018.03.004. [DOI] [PubMed] [Google Scholar]
- 82.Lee J.-E., Moon P.-G., Lee I.-K., Baek M.-C. Proteomic analysis of extracellular vesicles released by adipocytes of Otsuka Long-Evans Tokushima fatty (OLETF) rats. J. Protein Chem. 2015;34:220–235. doi: 10.1007/s10930-015-9616-z. [DOI] [PubMed] [Google Scholar]
- 83.Sano S., Izumi Y., Yamaguchi T., Yamazaki T., Tanaka M., Shiota M., Osada-Oka M., Nakamura Y., Wei M., Wanibuchi H., et al. Lipid synthesis is promoted by hypoxic adipocyte-derived exosomes in 3T3-L1 cells. Biochem. Biophys. Res. Commun. 2014;445:327–333. doi: 10.1016/j.bbrc.2014.01.183. [DOI] [PubMed] [Google Scholar]
- 84.Kita S., Maeda N., Shimomura I. Interorgan communication by exosomes, adipose tissue, and adiponectin in metabolic syndrome. J. Clin. Investig. 2019;129:4041–4049. doi: 10.1172/JCI129193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Xie Z., Wang X., Liu X., Du H., Sun C., Shao X., Tian J., Gu X., Wang H., Tian J., et al. Adipose-derived exosomes exert proatherogenic effects by regulating macrophage foam cell formation and polarization. J. Am. Hear. Assoc. 2018;7:e007442. doi: 10.1161/JAHA.117.007442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Deng Z.-B., Poliakov A., Hardy R.W., Clements R., Liu C., Liu Y., Wang J., Xiang X., Zhang S., Zhuang X., et al. Adipose tissue exosome-like vesicles mediate activation of macrophage-induced insulin resistance. Diabetes. 2009;58:2498–2505. doi: 10.2337/db09-0216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Zhao H., Shang Q., Pan Z., Bai Y., Li Z., Zhang H., Zhang Q., Guo C., Zhang L., Wang Q. Exosomes from adipose-derived stem cells attenuate adipose inflammation and obesity through polarizing M2 macrophages and beiging in white adipose tissue. Diabetes. 2018;67:235–247. doi: 10.2337/db17-0356. [DOI] [PubMed] [Google Scholar]
- 88.Fotouhi A., Maleki A., Dolati S., Aghebati-Maleki A., Aghebati-Maleki L. Platelet rich plasma, stromal vascular fraction and autologous conditioned serum in treatment of knee osteoarthritis. Biomed. Pharmacother. 2018;104:652–660. doi: 10.1016/j.biopha.2018.05.019. [DOI] [PubMed] [Google Scholar]
- 89.Shen L., Yuan T., Chen S., Xie X., Zhang C. The temporal effect of platelet-rich plasma on pain and physical function in the treatment of knee osteoarthritis: Systematic review and meta-analysis of randomized controlled trials. J. Orthop. Surg. Res. 2017;12:1–12. doi: 10.1186/s13018-017-0521-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Koseoglu S., Flaumenhaft R. Advances in platelet granule biology. Curr. Opin. Hematol. 2013;20:464–471. doi: 10.1097/MOH.0b013e3283632e6b. [DOI] [PubMed] [Google Scholar]
- 91.Ambrosio A.L., Di Pietro S.M. Mechanism of platelet α-granule biogenesis: Study of cargo transport and the VPS33B-VPS16B complex in a model system. Blood Adv. 2019;3:2617–2626. doi: 10.1182/bloodadvances.2018028969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Michael B.N.R., Kommoju V., Ganapathy C.K., Negi V.S. Characterization of cell-derived microparticles in synovial fluid and plasma of patients with rheumatoid arthritis. Rheumatol. Int. 2019;39:1377–1387. doi: 10.1007/s00296-019-04337-1. [DOI] [PubMed] [Google Scholar]
- 93.Oba R., Isomura M., Igarashi A., Nagata K. Circulating CD3+HLA-DR+extracellular vesicles as a marker for Th1/Tc1-Type immune responses. J. Immunol. Res. 2019;2019:1–13. doi: 10.1155/2019/6720819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Distler J.H.W., Jüngel A., Huber L.C., Seemayer C.A., Reich C.F., Gay R.E., Michel B.A., Fontana A., Gay S., Pisetsky D.S., et al. The induction of matrix metalloproteinase and cytokine expression in synovial fibroblasts stimulated with immune cell microparticles. Proc. Natl. Acad. Sci. USA. 2005;102:2892–2897. doi: 10.1073/pnas.0409781102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Störch H., Zimmermann B., Resch B., Tykocinski L.-O., Moradi B., Horn P., Kaya Z., Blank N., Rehart S., Thomsen M., et al. Activated human B cells induce inflammatory fibroblasts with cartilage-destructive properties and become functionally suppressed in return. Ann. Rheum. Dis. 2016;75:924–932. doi: 10.1136/annrheumdis-2014-206965. [DOI] [PubMed] [Google Scholar]
- 96.Takeuchi Y., Hirota K., Sakaguchi S. Synovial tissue inflammation mediated by autoimmune T cells. Front. Immunol. 2019;10:1989. doi: 10.3389/fimmu.2019.01989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Hu X.-X., Wu Y.-J., Zhang J., Wei W. T-cells interact with B cells, dendritic cells, and fibroblast-like synoviocytes as hub-like key cells in rheumatoid arthritis. Int. Immunopharmacol. 2019;70:428–434. doi: 10.1016/j.intimp.2019.03.008. [DOI] [PubMed] [Google Scholar]
- 98.Van Niel G., D’Angelo G., Raposo G. Shedding light on the cell biology of extracellular vesicles. Nat. Rev. Mol. Cell Biol. 2018;19:213–228. doi: 10.1038/nrm.2017.125. [DOI] [PubMed] [Google Scholar]
- 99.Asghar S., Litherland G.J., Lockhart J.C., Goodyear C.S., Crilly A. Exosomes in intercellular communication and implications for osteoarthritis. Rheumatology (Oxford) 2019;59:57–68. doi: 10.1093/rheumatology/kez462. [DOI] [PubMed] [Google Scholar]
- 100.Wu X., Showiheen S.A.A., Sun A.R., Crawford R., Xiao Y., Mao X., Prasadam I. Exosomes extraction and identification. Methods Mol. Biol. 2019;2054:81–91. doi: 10.1007/978-1-4939-9769-5_4. [DOI] [PubMed] [Google Scholar]
- 101.Macías M., Rebmann V., Mateos B., Varo N., Perez-Gracia J.L., Alegre E., González Á. Comparison of six commercial serum exosome isolation methods suitable for clinical laboratories. Effect in cytokine analysis. Clin. Chem. Lab. Med. (CCLM) 2019;57:1539–1545. doi: 10.1515/cclm-2018-1297. [DOI] [PubMed] [Google Scholar]
- 102.Greening D.W., Xu R., Ji H., Tauro B.J., Simpson R.J. A Protocol for exosome isolation and characterization: Evaluation of ultracentrifugation, density-gradient separation, and immunoaffinity capture methods. Methods Mol. Biol. 2015;1295:179–209. doi: 10.1007/978-1-4939-2550-6_15. [DOI] [PubMed] [Google Scholar]
- 103.Yang D., Zhang W., Zhang H., Zhang F., Chen L., Ma L., Larcher L.M., Chen S., Liu N., Zhao Q., et al. Progress, opportunity, and perspective on exosome isolation—efforts for efficient exosome-based theranostics. Theranostics. 2020;10:3684–3707. doi: 10.7150/thno.41580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Helwa I., Cai J., Drewry M.D., Zimmerman A., Dinkins M.B., Khaled M.L., Seremwe M., Dismuke W.M., Bieberich E., Stamer W.D., et al. A comparative study of serum exosome isolation using differential ultracentrifugation and three commercial reagents. PLoS ONE. 2017;12:e0170628. doi: 10.1371/journal.pone.0170628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Taghikhani A., Farzaneh F., Sharifzad F., Mardpour S., Ebrahimi M., Hassan Z.M. Engineered tumor-derived extracellular vesicles: Potentials in cancer immunotherapy. Front. Immunol. 2020;11:221. doi: 10.3389/fimmu.2020.00221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Wu Y., Deng W., Ii D.J.K. Exosomes: Improved methods to characterize their morphology, RNA content, and surface protein biomarkers. Analyst. 2015;140:6631–6642. doi: 10.1039/C5AN00688K. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Skotland T., Hessvik N.P., Sandvig K., Llorente A. Exosomal lipid composition and the role of ether lipids and phosphoinositides in exosome biology. J. Lipid Res. 2019;60:9–18. doi: 10.1194/jlr.R084343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Gurunathan S., Kang M.-H., Jeyaraj M., Qasim M., Kim J.-H. Review of the isolation, characterization, biological function, and multifarious therapeutic approaches of exosomes. Cells. 2019;8:307. doi: 10.3390/cells8040307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Witwer K.W., Buzás E.I., Bemis L.T., Bora A., Lässer C., Lötvall J., Nolte-’t Hoen E.N., Piper M.G., Sivaraman S., Skog J., et al. Standardization of sample collection, isolation and analysis methods in extracellular vesicle research. J. Extracell. Vesicles. 2013;2:20360. doi: 10.3402/jev.v2i0.20360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Bahr M.M., Amer M.S., Abo-El-Sooud K., Abdallah A.N., El-Tookhy O.S. Preservation techniques of stem cells extracellular vesicles: A gate for manufacturing of clinical grade therapeutic extracellular vesicles and long-term clinical trials. Int. J. Veter- Sci. Med. 2020;8:1–8. doi: 10.1080/23144599.2019.1704992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Yamashita T., Takahashi Y., Takakura Y. Possibility of exosome-based therapeutics and challenges in production of exosomes eligible for therapeutic application. Biol. Pharm. Bull. 2018;41:835–842. doi: 10.1248/bpb.b18-00133. [DOI] [PubMed] [Google Scholar]
- 112.Charoenviriyakul C., Takahashi Y., Nishikawa M., Takakura Y. Preservation of exosomes at room temperature using lyophilization. Int. J. Pharm. 2018;553:1–7. doi: 10.1016/j.ijpharm.2018.10.032. [DOI] [PubMed] [Google Scholar]
- 113.Kusuma G.D., Barabadi M., Tan J., Morton D., Frith J., Lim R. To protect and to preserve: Novel preservation strategies for extracellular vesicles. Front. Pharmacol. 2018;9:1199. doi: 10.3389/fphar.2018.01199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Harrell C.R., Markovic B.S., Fellabaum C., Arsenijevic A., Volarevic V. Mesenchymal stem cell-based therapy of osteoarthritis: Current knowledge and future perspectives. Biomed. Pharmacother. 2019;109:2318–2326. doi: 10.1016/j.biopha.2018.11.099. [DOI] [PubMed] [Google Scholar]
- 115.Zhang R., Ma J., Han J., Zhang W., Ma J. Mesenchymal stem cell related therapies for cartilage lesions and osteoarthritis. Am. J. Transl. Res. 2019;11:6275–6289. [PMC free article] [PubMed] [Google Scholar]
- 116.Mianehsaz E., Mirzaei H.R., Mahjoubin-Tehran M., Rezaee A., Sahebnasagh R., Pourhanifeh M.H., Mirzaei H., Hamblin M.R. Mesenchymal stem cell-derived exosomes: A new therapeutic approach to osteoarthritis? Stem Cell Res. Ther. 2019;10:1–13. doi: 10.1186/s13287-019-1445-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Xie F., Wen G., Sun W., Jiang K., Chen T., Chen S., Wen J. Mechanical stress promotes angiogenesis through fibroblast exosomes. Biochem. Biophys. Res. Commun. 2020;533:346–353. doi: 10.1016/j.bbrc.2020.04.159. [DOI] [PubMed] [Google Scholar]
- 118.Wang X., Lin Q., Zhang T., Wang X., Cheng K., Gao M., Xia P., Li X. Low-intensity pulsed ultrasound promotes chondrogenesis of mesenchymal stem cells via regulation of autophagy. Stem Cell Res. Ther. 2019;10:41. doi: 10.1186/s13287-019-1142-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Rong Y., Zhang J., Jiang D., Ji C., Liu W., Wang J., Ge X., Tang P., Yu S., Cui W., et al. Hypoxic pretreatment of small extracellular vesicles mediates cartilage repair in osteoarthritis by delivering miR-216a-5p. Acta Biomater. 2021;122:325–342. doi: 10.1016/j.actbio.2020.12.034. [DOI] [PubMed] [Google Scholar]
- 120.Li S., Stöckl S., Lukas C., Herrmann M., Brochhausen C., König M.A., Johnstone B., Grässel S. Curcumin-primed human BMSC-derived extracellular vesicles reverse IL-1β-induced catabolic responses of OA chondrocytes by upregulating miR-126-3p. Stem Cell Res. Ther. 2021;12:252. doi: 10.1186/s13287-021-02317-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Xu X., Liang Y., Li X., Ouyang K., Wang M., Cao T., Li W., Liu J., Xiong J., Li B., et al. Exosome-mediated delivery of kartogenin for chondrogenesis of synovial fluid-derived mesenchymal stem cells and cartilage regeneration. Biomaterials. 2021;269:120539. doi: 10.1016/j.biomaterials.2020.120539. [DOI] [PubMed] [Google Scholar]
- 122.Wang R., Xu B. TGF-β1-modified MSC-derived exosomal miR-135b attenuates cartilage injury via promoting M2 synovial macrophage polarization by targeting MAPK6. Cell Tissue Res. 2021;384:113–127. doi: 10.1007/s00441-020-03319-1. [DOI] [PubMed] [Google Scholar]
- 123.Wang Y., He S., Liang X., Zhang X., Li S., Li T. ATF4 -modified serum exosomes derived from osteoarthritic mice inhibit osteoarthritis by inducing autophagy. IUBMB Life. 2021;73:146–158. doi: 10.1002/iub.2414. [DOI] [PubMed] [Google Scholar]
- 124.Panagopoulos P., Lambrou G. The involvement of microRNAs in osteoarthritis and recent developments: A narrative review. Mediterr. J. Rheumatol. 2018;29:67–79. doi: 10.31138/mjr.29.2.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Kolhe R., Hunter M., Liu S., Jadeja R.N., Pundkar C., Mondal A.K., Mendhe B., Drewry M., Rojiani M.V., Liu Y., et al. Gender-specific differential expression of exosomal miRNA in synovial fluid of patients with osteoarthritis. Sci. Rep. 2017;7:1–14. doi: 10.1038/s41598-017-01905-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Barile L., Vassalli G. Exosomes: Therapy delivery tools and biomarkers of diseases. Pharmacol. Ther. 2017;174:63–78. doi: 10.1016/j.pharmthera.2017.02.020. [DOI] [PubMed] [Google Scholar]
- 127.Duan L., Xu L., Xu X., Qin Z., Zhou X., Xiao Y., Liang Y., Xia J. Exosome-mediated delivery of gene vectors for gene therapy. Nanoscale. 2020;13:1387–1397. doi: 10.1039/D0NR07622H. [DOI] [PubMed] [Google Scholar]
- 128.Wu X., Wang Y., Xiao Y., Crawford R., Mao X., Prasadam I. Extracellular vesicles: Potential role in osteoarthritis regenerative medicine. J. Orthop. Transl. 2020;21:73–80. doi: 10.1016/j.jot.2019.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Qiu M., Liu D., Fu Q. MiR-129-5p shuttled by human synovial mesenchymal stem cell-derived exosomes relieves IL-1β induced osteoarthritis via targeting HMGB1. Life Sci. 2021;269:118987. doi: 10.1016/j.lfs.2020.118987. [DOI] [PubMed] [Google Scholar]
- 130.Mao G., Xu Y., Long D., Sun H., Li H., Xin R., Zhang Z., Li Z., Yang Z., Kang Y. Exosome-transported circRNA_0001236 enhances chondrogenesis and suppress cartilage degradation via the miR-3677-3p/Sox9 axis. Stem Cell Res. Ther. 2021;12:1–14. doi: 10.1186/s13287-021-02431-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Kanchanapally R., Deshmukh S.K., Chavva S.R., Tyagi N., Srivastava S.K., Patel G.K., Singh A.P., Singh S. Drug-loaded exosomal preparations from different cell types exhibit distinctive loading capability, yield, and antitumor efficacies: A comparative analysis. Int. J. Nanomed. 2019;14:531–541. doi: 10.2147/IJN.S191313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Agrawal A., Aqil F., Jeyabalan J., Spencer W.A., Beck J., Gachuki B.W., Alhakeem S., Oben K., Munagala R., Bondada S., et al. Milk-derived exosomes for oral delivery of paclitaxel. Nanomedicine. 2017;13:1627–1636. doi: 10.1016/j.nano.2017.03.001. [DOI] [PubMed] [Google Scholar]
- 133.Familtseva A., Jeremic N., Tyagi S.C. Exosomes: Cell-created drug delivery systems. Mol. Cell. Biochem. 2019;459:1–6. doi: 10.1007/s11010-019-03545-4. [DOI] [PubMed] [Google Scholar]
- 134.Jafari D., Shajari S., Jafari R., Mardi N., Gomari H., Ganji F., Moghadam M.F., Samadikuchaksaraei A. Designer exosomes: A new platform for biotechnology therapeutics. BioDrugs. 2020;34:567–586. doi: 10.1007/s40259-020-00434-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Xi X.-M., Xia S.-J., Lu R. Drug loading techniques for exosome-based drug delivery systems. Pharmazie. 2021;76:61–67. doi: 10.1691/ph.2021.0128. [DOI] [PubMed] [Google Scholar]
- 136.Liu Y., Bai L., Guo K., Jia Y., Zhang K., Liu Q., Wang P., Wang X. Focused ultrasound-augmented targeting delivery of nanosonosensitizers from homogenous exosomes for enhanced sonodynamic cancer therapy. Theranostics. 2019;9:5261–5281. doi: 10.7150/thno.33183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Pegtel D.M., Gould S.J. Exosomes. Annu. Rev. Biochem. 2019;88:487–514. doi: 10.1146/annurev-biochem-013118-111902. [DOI] [PubMed] [Google Scholar]
- 138.Shao J., Zaro J., Shen Y. Advances in exosome-based drug delivery and tumor targeting: From tissue distribution to intracellular fate. Int. J. Nanomed. 2020;15:9355–9371. doi: 10.2147/IJN.S281890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Kenari A.N., Kastaniegaard K., Greening D., Shambrook M., Stensballe A., Cheng L., Hill A.F. Proteomic and post-translational modification profiling of exosome-mimetic nanovesicles compared to exosomes. Proteom. 2019;19:e1800161. doi: 10.1002/pmic.201800161. [DOI] [PubMed] [Google Scholar]
- 140.Haraszti R.A., Miller R., Stoppato M., Sere Y.Y., Coles A., Didiot M.-C., Wollacott R., Sapp E., Dubuke M.L., Li X., et al. Exosomes produced from 3D cultures of MSCs by tangential flow filtration show higher yield and improved activity. Mol. Ther. 2018;26:2838–2847. doi: 10.1016/j.ymthe.2018.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Wu J.-H., Ji A.-L., Wang Z.-X., Qiang G.-H., Qu Z., Jiang C.-P. Exosome-mimetic nanovesicles from hepatocytes promote hepatocyte proliferation in vitro and liver regeneration in vivo. Sci. Rep. 2018;8:1–11. doi: 10.1038/s41598-018-20505-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Kalimuthu S., Gangadaran P., Rajendran R.L., Zhu L., Oh J.M., Lee H.W., Gopal A., Baek S.H., Jeong S.Y., Lee S.-W., et al. A new approach for loading anticancer drugs into mesenchymal stem cell-derived exosome mimetics for cancer therapy. Front. Pharmacol. 2018;9:1116. doi: 10.3389/fphar.2018.01116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Liu X., Sun Y., Xu S., Gao X., Kong F., Xu K., Tang B. Homotypic cell membrane-cloaked biomimetic nanocarrier for the targeted chemotherapy of hepatocellular carcinoma. Theranostics. 2019;9:5828–5838. doi: 10.7150/thno.34837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Nakase I., Noguchi K., Fujii I., Futaki S. Vectorization of biomacromolecules into cells using extracellular vesicles with enhanced internalization induced by macropinocytosis. Sci. Rep. 2016;6:34937. doi: 10.1038/srep34937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Sawada S.-I., Sato Y.T., Kawasaki R., Yasuoka J.-I., Mizuta R., Sasaki Y., Akiyoshi K. Nanogel hybrid assembly for exosome intracellular delivery: Effects on endocytosis and fusion by exosome surface polymer engineering. Biomater. Sci. 2020;8:619–630. doi: 10.1039/C9BM01232J. [DOI] [PubMed] [Google Scholar]
- 146.Lin Y., Wu J., Gu W., Huang Y., Tong Z., Huang L., Tan J. Exosome-liposome hybrid nanoparticles deliver CRISPR/Cas9 system in MSCs. Adv. Sci. 2018;5:1700611. doi: 10.1002/advs.201700611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Yang Y., Hong Y., Nam G.-H., Chung J.H., Koh E., Kim I.-S. Virus-mimetic fusogenic exosomes for direct delivery of integral membrane proteins to target cell membranes. Adv. Mater. 2017;29:29. doi: 10.1002/adma.201605604. [DOI] [PubMed] [Google Scholar]
- 148.Lu M., Zhao X., Xing H., Liu H., Lang L., Yang T., Xun Z., Wang D., Ding P. Cell-free synthesis of connexin 43-integrated exosome-mimetic nanoparticles for siRNA delivery. Acta Biomater. 2019;96:517–536. doi: 10.1016/j.actbio.2019.07.006. [DOI] [PubMed] [Google Scholar]
- 149.Zhao X., Zhao Y., Sun X., Xing Y., Wang X., Yang Q. Immunomodulation of MSCs and MSC-derived extracellular vesicles in osteoarthritis. Front. Bioeng. Biotechnol. 2020;8:8. doi: 10.3389/fbioe.2020.575057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Liu X., Yang Y., Li Y., Niu X., Zhao B., Wang Y., Bao C., Xie Z., Lin Q., Zhu L. Integration of stem cell-derived exosomes with in situ hydrogel glue as a promising tissue patch for articular cartilage regeneration. Nanoscale. 2017;9:4430–4438. doi: 10.1039/C7NR00352H. [DOI] [PubMed] [Google Scholar]
- 151.Zhang K., Zhao X., Chen X., Wei Y., Du W., Wang Y., Liu L., Zhao W., Han Z., Kong D., et al. Enhanced therapeutic effects of mesenchymal stem cell-derived exosomes with an injectable hydrogel for hindlimb ischemia treatment. ACS Appl. Mater. Interfaces. 2018;10:30081–30091. doi: 10.1021/acsami.8b08449. [DOI] [PubMed] [Google Scholar]
- 152.Henriques-Antunes H., Cardoso R.M.S., Zonari A., Correia J., Leal E.C., Jiménez-Balsa A., Lino M.M., Barradas A., Kostic I., Gomes C., et al. The kinetics of small extracellular vesicle delivery impacts skin tissue regeneration. ACS Nano. 2019;13:8694–8707. doi: 10.1021/acsnano.9b00376. [DOI] [PubMed] [Google Scholar]
- 153.Lenzini S., Bargi R., Chung G., Shin J.-W. Matrix mechanics and water permeation regulate extracellular vesicle transport. Nat. Nanotechnol. 2020;15:217–223. doi: 10.1038/s41565-020-0636-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Zhang F.-X., Liu P., Ding W., Meng Q.-B., Su D.-H., Zhang Q.-C., Lian R.-X., Yu B.-Q., Zhao M.-D., Dong J., et al. Injectable Mussel-Inspired highly adhesive hydrogel with exosomes for endogenous cell recruitment and cartilage defect regeneration. Biomater. 2021;278:121169. doi: 10.1016/j.biomaterials.2021.121169. [DOI] [PubMed] [Google Scholar]
- 155.Kim Y.G., Choi J., Kim K. Mesenchymal stem cell-derived exosomes for effective cartilage tissue repair and treatment of osteoarthritis. Biotechnol. J. 2020;15:e2000082. doi: 10.1002/biot.202000082. [DOI] [PubMed] [Google Scholar]
- 156.Xiaomin W., Bian B., Lin Z., Wu C., Sun Y., Pan Y., Dai Y., Lui T.H., Zhuang T., Pan X. Identification of exosomal mRNA, lncRNA and circRNA signatures in an osteoarthritis synovial fluid-exosomal study. Exp. Cell Res. 2021;410:112881. doi: 10.1016/j.yexcr.2021.112881. [DOI] [PubMed] [Google Scholar]
- 157.Jeon O.H., Wilson D.R., Clement C.C., Rathod S., Cherry C., Powell B., Lee Z., Khalil A.M., Green J.J., Campisi J., et al. Senescence cell–associated extracellular vesicles serve as osteoarthritis disease and therapeutic markers. JCI Insight. 2019;4:4. doi: 10.1172/jci.insight.125019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Sophia Fox A.J., Bedi A., Rodeo S.A. The basic science of articular cartilage: Structure, composition, and function. Sports Health. 2009;1:461–468. doi: 10.1177/1941738109350438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Geiger B.C., Wang S., Padera R.F., Jr., Grodzinsky A.J., Hammond P.T. Cartilage-penetrating nanocarriers improve delivery and efficacy of growth factor treatment of osteoarthritis. Sci. Transl. Med. 2018;10:eaat8800. doi: 10.1126/scitranslmed.aat8800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Bajpayee A.G., Scheu M., Grodzinsky A.J., Porter R.M. A rabbit model demonstrates the influence of cartilage thickness on intra-articular drug delivery and retention within cartilage. J. Orthop. Res. 2015;33:660–667. doi: 10.1002/jor.22841. [DOI] [PubMed] [Google Scholar]
- 161.Ng C.Y., Chai J.Y., Foo J.B., Yahaya N.H.M., Yang Y., Ng M.H., Law J.X. Potential of exosomes as cell-free therapy in articular cartilage regeneration: A review. Int. J. Nanomed. 2021;16:6749–6781. doi: 10.2147/IJN.S327059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Bajpayee A.G., Wong C.R., Bawendi M.G., Frank E.H., Grodzinsky A.J. Avidin as a model for charge driven transport into cartilage and drug delivery for treating early stage post-traumatic osteoarthritis. Biomater. 2014;35:538–549. doi: 10.1016/j.biomaterials.2013.09.091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Bajpayee A.G., Grodzinsky A.J. Cartilage-targeting drug delivery: Can electrostatic interactions help? Nat. Rev. Rheumatol. 2017;13:183–193. doi: 10.1038/nrrheum.2016.210. [DOI] [PubMed] [Google Scholar]
- 164.Vedadghavami A., Zhang C., Bajpayee A.G. Overcoming negatively charged tissue barriers: Drug delivery using cationic peptides and proteins. Nano Today. 2020;34:100898. doi: 10.1016/j.nantod.2020.100898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Vedadghavami A., Wagner E., Mehta S., He T., Zhang C., Bajpayee A.G. Cartilage penetrating cationic peptide carriers for applications in drug delivery to avascular negatively charged tissues. Acta Biomater. 2019;93:258–269. doi: 10.1016/j.actbio.2018.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.He T., Zhang C., Vedadghavami A., Mehta S., Clark H.A., Porter R.M., Bajpayee A.G. Multi-arm Avidin nano-construct for intra-cartilage delivery of small molecule drugs. J. Control. Release. 2020;318:109–123. doi: 10.1016/j.jconrel.2019.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Gupta A., Maffulli N., Rodriguez H.C., Carson E.W., Bascharon R.A., Delfino K., Levy H.J., El-Amin S.F. Safety and efficacy of umbilical cord-derived Wharton’s jelly compared to hyaluronic acid and saline for knee osteoarthritis: Study protocol for a randomized, controlled, single-blind, multi-center trial. J. Orthop. Surg. Res. 2021;16:1–8. doi: 10.1186/s13018-021-02475-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Gupta A., Maffulli N., Rodriguez H.C., Lee C.E., Levy H.J., El-Amin S.F. Umbilical cord-derived Wharton’s jelly for treatment of knee osteoarthritis: Study protocol for a non-randomized, open-label, multi-center trial. J. Orthop. Surg. Res. 2021;16:1–7. doi: 10.1186/s13018-021-02300-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.