Abstract
Simple Summary
Non-Hodgkin lymphoma is one of the most frequently occurring hematologic diseases in the world. Current drugs and therapies have improved outcomes for patients with lymphoma, but there is still a need to identify novel medications for treatment-resistant cases. The aim of this review is to gather the latest findings on non-Hodgkin lymphoma in children, including genetic approaches, the application of therapy, the available treatment options, and resistance to medications.
Abstract
One of the most common cancer malignancies is non-Hodgkin lymphoma, whose incidence is nearly 3% of all 36 cancers combined. It is the fourth highest cancer occurrence in children and accounts for 7% of cancers in patients under 20 years of age. Today, the survivability of individuals diagnosed with non-Hodgkin lymphoma varies by about 70%. Chemotherapy, radiation, stem cell transplantation, and immunotherapy have been the main methods of treatment, which have improved outcomes for many oncological patients. However, there is still the need for creation of novel medications for those who are treatment resistant. Additionally, more effective drugs are necessary. This review gathers the latest findings on non-Hodgkin lymphoma treatment options for pediatric patients. Attention will be focused on the most prominent therapies such as monoclonal antibodies, antibody–drug conjugates, chimeric antigen receptor T cell therapy and others.
Keywords: non-Hodgkin lymphoma, children and adolescents, aggressive non-Hodgkin lymphoma
1. Introduction
Non-Hodgkin lymphoma (NHL) is one of the most frequently occurring hematologic disease in the world [1]. In children, NHL comprises four wide categories: lymphoblastic lymphoma, Burkitt lymphoma (BL), diffuse large B-cell lymphoma (DLBCL), and anaplastic large cell lymphoma (Table 1). Among the rarer subtypes are pediatric-type follicular lymphoma (FL) and pediatric marginal zone lymphoma (MZL) [2]. ALK-positive (ALK+) anaplastic large cell lymphoma (ALCL) is the most common subtype of T-NHL in children and represents about 30% of all pediatric lymphomas [3]. The prognosis for NHL patients depends on site of involvement and the pathologic the subtype and stage of the disease; nonetheless, pediatric cases have better outcomes than adults, mainly due to tumor biology [4,5]. Additionally, it is thought that the higher toleration of intensive treatment or distinct pathogenetic mechanisms contribute to better curability in children [4]. Adolescent age or age > 10 years, respectively, has been reported to be associated with inferior outcomes in pediatric trials in patients with mature B-NHL, mainly Burkitt’s lymphoma and DLBCL [6]. The treatment for children diagnosed with NHL includes multiagent systemic chemotherapy with intrathecal administration, surgical intervention mainly used for diagnosing the tumors, while radiation therapy is only for central nervous system involvement, superior mediastinal syndrome, or paraplegias [5].
Table 1.
| NHL Type | Frequency of Occurrence in Pediatric NHLs | NHL Subtypes | Clinical Features | Genetic Rearrangements and Prognosis | |
|---|---|---|---|---|---|
| B-NHL | 86% | DLBCL BL BLL PMBCL pediatric type FLpediatric nodal MZL | generally localized lesionsabdominal tumor nasopharyngeal tumor jaw bone tumor solid tumor syndrome | DLBCL | |
| MYC—8q25 rearrangements | poor prognosis | ||||
| t(14;18)(q32;q21) IGH::BCL2 | poor prognosis | ||||
| BCL6—3q27 rearrangements | poor prognosis | ||||
| t(6;14)(p25;q32) IGH::IRF4 | favorable outcomes | ||||
| translocations MYC/BCL2, MYC/BCL6, MYC/BCL2/BCL6 | poor prognosis | ||||
| BL | |||||
| c-MYC translocations: t(8;14)(q24;q32) IGH::MYC, t(8;22)(q24.1;q11.2) IGL::MYC, t(2;8)(p12;q24.1) IGK::MYC | poor prognosis | ||||
| del(13q14.3) or del(13q34) | poor prognosis | ||||
| ID3-TCF3-CCND3 pathway mutations | no correlation to the outcome | ||||
| BLL | |||||
| 11q aberration with proximal gains and telomeric losses | favorable outcomes | ||||
| FL | |||||
| In pediatric FL, there rarely occurs t(14;18), which is typical for FL; lack of this translocation correlates with excellent outcomes in pediatric FL | |||||
| LBL-T/B | 1–4 years old 40% 15–19 years old 20% | LBL-T (75%) LBL-B | mediastinal tumor pleural effusion respiratory failure not likely to involve the CNS at diagnosis or relapse relapse into the marrow | LBL-T | |
| Chromosomal abnormalities including TCR genes, e.g., translocations in TAL1, LMO2, LYL1, HOXA9, TLX1, TLX3 | unknown | ||||
| t(7;14)(p15;q32) HOXA::TCL1A | unknown | ||||
| Notch1 mutations +/− FBXW7 mutations | favorable outcomes | ||||
| LOH6q16 | poor prognosis | ||||
| ABD | poor prognosis | ||||
| PTEN mutations | poor prognosis, unless presence of notch1 or absence of LOH6q | ||||
| PHF6 mutations | favorable outcomes | ||||
| NRAS/KRAS mutations | no correlation to the outcome | ||||
| ALCL | Median around 16 years −10% | ALCL extra-nodal NK/T cell lymphoma T cell hepatosplenic lymphoma subcutaneous panniculitis like T cell lymphoma | mediastinal tumor | t(2;5)(p23;q35) NPM1::ALK | unknown; although t(2;5) is found in aggressive high grade tumors, a 80% 5-yr survival seems to be associated with this anomaly |
| tumors in the digestive tract | |||||
| peripheral, mediastinal, or abdominal lymphadenopathy | |||||
| hepatosplenomegaly | |||||
| skin changes | |||||
| changes in the lung parenchyma | |||||
| extra-nodal lesions (brain, marrow, bones, liver, spleen) | |||||
| associated hemophagocytic lymphohistocytosis | |||||
NHL, non-Hodgkin lymphoma; BL, Burkitt lymphoma; DLBCL, diffuse large B-cell lymphoma; PMBCL, primary mediastinal B-cell lymphoma; FL, follicular lymphoma; MZL, marginal zone lymphoma; LBL-T, T cell lymphoblastic lymphoma; LBL-B, B-cell lymphoblastic lymphoma; CNS, central nervous system; ALCL, anaplastic large cell lymphoma; ID3, inhibitor of DNA binding 3; BLL, Burkitt-like lymphoma; TCF3, transcription factor 3; ALK, anaplastic lymphoma kinase gene; NPM, nucleophosmin gene; ABD, absence of biallelic deletion of the T cell Receptor Gamma(TRG) locus.
Overall survival (OS) rates in children, adolescents and young adults diagnosed with NHL increased to 80–90% during the last 30 years, giving the opportunity to investigate the long-term effects of prior chemo- and radiotherapy (RT). Children and adolescent NHL survivors are at significant risk of late mortality from secondary neoplasms, recurrent/progressive disease and chronic health conditions (cardiomyopathy, pneumonia and defects in neurocognitive function), and late morbidity of multiple organ systems and poor health-related quality of life. These risks are similar to other long-term risks of childhood and adolescent acute lymphoblastic leukaemia (ALL), Wilms tumor and Hodgkin lymphoma (HL) survivors [20]. Increasing the dosage of chemotherapeutic or radiation dose not improve the therapeutic response but contributes to the acceleration of side effects development and resistance to therapy [21]. Additionally, 10–30% of pediatric and adolescent patients will relapse; therefore, more effective drugs are necessary [22].
Novel approaches are required to reduce the burden of late morbidity and mortality in childhood and adolescent NHL survivors, and obtain methods to identify at-risk patients who are at significantly increased risk of these complications. Nowadays, we can distinguish several therapeutic substances that work in various ways. These include immunomodulatory drugs, monoclonal antibodies (mAbs), immune checkpoint inhibitors (ICI), antibody–drug conjugates (ADCs) and genetically modified chimeric T cell receptor antigens (CAR) [23]. In addition, research is currently underway on a number of further classes of drugs, the action of which may be based on cross-linking of deoxyribonucleic acid (DNA), inhibition of DNA synthesis, inhibition of the signaling pathway of B-cell receptors, inhibition of proteins regulating apoptosis, or epigenetic modulation [24,25].
More detailed treatment of the different types of NHL in pediatric patients is summarized in Table 2. This review gathers the latest findings on NHL treatment options for pediatric patients.
Table 2.
| NHL Type | Classical Treatment | Treatment after Lack of Response to Classical Treatment or Relapse | Novel Treatment Options |
|---|---|---|---|
| B-NHL | rituximab prednisone vincristine methotrexate doxorubicin arabinoside cyclophosphamide etoposide |
ibrutinib mega chemotherapy + allo-HSCT |
mAbs (obinutuzumab) ADCs (inotuzumab) CAR-T cell therapy ICIs (pembrolizumab) pathway inhibitors (buparlisib, ibrutinib) |
| LBL-T/B | multidrug chemotherapy | chemotherapy with nelarabine, cyclophosphamide and etoposide mega chemotherapy + auto/allo-HSCT |
ruxolitinib tyrosine-serotonin kinase inhibitors gamma secretase inhibitors |
| ALCL | methotrexate combination of cyclophosphamide, doxorubicin, vincristine, corticosteroids, ifosfamide and etoposide tumor removal surgery |
allo-HSCT vinblastine re-induction salvage chemotherapy + auto-SCT re-induction salvage chemotherapy + alloSCT |
mAbs Bv kinase inhibitors (ceretynib)ICIs (nivolumab) signaling pathway inhibitors (ruxolitinib) anaplastic lymphoma kinase inhibitors (crizotinib, alectinib, ceritinib) |
| ALK+ALCL | doxorubicin-containing polychemotherapy, typically CHOP 3-week induction therapy (vincristine, prednisone, cyclophosphamide, daunomycin, asparaginase) followed by a 3-week consolidation period (vincristine, prednisone, etoposide, 6-thioguanine, cytarabine, asparaginase, methotrexate), subsequently 6 courses of maintenance chemotherapy (cyclophosphamide, 6-thioguanine, vincristine, prednisone, asparaginase, methotrexate, etoposide, cytarabine) at 7-week intervals HDC/ASCT |
HDC/ASCT allo-SCT |
crizotinib crizotinib + multiagent chemotherapy ceritinib Bv |
NHL, non-Hodgkin lymphoma; allo-HSCT, allogenic hematopoietic stem-cell transplantation; mAbs, monoclonal antibodies; ADCs, antibody–drug conjugates; CAR-T, chimeric antigen receptor T; ICIs, immune checkpoint inhibitors; LBL-T/B, T/B-cell lymphoblastic lymphoma; ALCL, anaplastic large cell lymphoma; auto-SCT, autologous stem-cell transplantation; all-SCT, allogenic stem cell transplantation; Bv, brentuximab vedotin; CHOP, cyclophosphamide, doxorubicin, vincristine, prednisone; ALK+, anaplastic lymphoma kinase positive; HDC/ASCT, high-dose chemotherapy supported by autologous stem cell transplantation.
2. New Approaches in Treatment
2.1. Monoclonal Antibodies (mAbs) Therapy
Monoclonal antibodies target specific markers on cancer cells by activating the patient’s immune system. In this way, the therapy avoids widespread non-specific cytotoxic effects [31]. Molecular mechanisms that allow mAbs for the destruction of cancer cells are direct cytotoxic actions, immunomodulatory effects and inhibitory effects on promitogenic signaling pathways [32]. There are 50 different mAbs approved by the Food and Drug Administration (FDA). Those directed against antigens expressed on B lymphocytes are ibritumomab tiuxetan, obinutuzumab, ofatumumab, rituximab, brentuximab vedotin (Bv) and alemtuzumab (Table 3) [32]. Rituximab conjugated with chemotherapy is successfully used in most intermediate- and high-risk pediatric mature B-NHL [2]. Additionally, it can also act as a single agent. In this case, studies show that it is effective in high-grade, high-risk, mature B-NHL in children [33].
Table 3.
| mAbs name | Generation | Origin | Target | Antigen |
|---|---|---|---|---|
| rituximab | I | chimeric | B-cells | anti-CD20 |
| obinutzumab | II | humanized | ||
| ofatumumab | I | human | ||
| ublituximab | I | chimeric | ||
| tafasitamab | II | humanized | anti-CD19 | |
| inebilizumab | next generation | humanized | ||
| epratuzumab | next generation | humanized | anti-CD22 | |
| blinatumomab | next generation | mouse | T cells | anti-CD19 CD3 |
| mosunetuzumab | next generation | humanized | anti-CD20 CD3 |
|
| glofitamab | next generation | humanized | ||
| odronextamab | next generation | humanized | ||
| epcoritamab | next generation | humanized |
Some mAbs, such as obinutuzumab and ofatumumab, did not improve outcomes in NHL patients [2]. Nevertheless, obinutuzumab conjugated with chemotherapy was approved for the treatment of FL patients and they can benefit from this therapy [34]. Unfortunately, usage of mAbs for immunotherapy is tied with the appearance of a wide range of side effects, such as anaphylaxis, gastrointestinal symptoms, transient rashes, autoimmunity, cytopenias, toxidermias and pulmonary/cardiac/hepatic/kidney/neurological/embryo–fetal toxicities. Therefore, identification of biomarkers that help with the prediction of a patient’s response is essential for selection of the patients, who are most likely to benefit from mAbs treatment. For example, individuals with positive HER2 gene amplification are classified for trastuzumab therapy or patients with a mutation in codon 12 of the KRAS are unlikely to respond to cetuximab [32]. It is known that the anti-CD19 mAb named B43 conjugated with genistein has entered clinical trials in children with NHL [35].
The following generation of mAbs are bispecific antibodies (BiAbs), which are derived from mAbs. They consist of two single-chain variable fragments (scFv) that target tumor-associated antigens. BiAbs engage the cells of immune system to attack indicated tumor cells [37]. Blinatumomab application leads to long-term remission and improved OS rate for patients with relapsed or refractory (R/R) B-NHL. It is approved for treatment both in children and adults [38,39].
2.2. Antibody–Drug Conjugates (ADCs)
ADCs comprise of a mAb connected to a small cytotoxic molecule. When attached to the cell-surface antigen of cancer cells, the ADC is internalized; next, the cytotoxin is released, causing cell cycle termination and cell apoptosis. The drug can also kill adjacent cells by “bystander killing” (Table 4) [40]. The design of ADCs depends on the type of antigen, which is present on tumor subtype. Starting with ALCL, whose cells express the CD30 antigen, the Bv is currently under investigation, with its therapeutic potential already proven in adults [41]. CD30 is a member of the tumor necrosis factor receptor superfamily and plays a role in T cell response regulation [42]. Brentuximab consists of an anti-CD30 antibody and anti-microtubule agent monomethyl auristatin E (MMAE), which disrupt mitosis and lead to cell death [43]. Sekimizu et al. involved in their Phase I studies children from 2 to 17 years old with R/R Hodgkin lymphoma or systemic ALCL. Their studies will estimate the safety profile among Japanese children [44]. Meanwhile, results from an ANHL12P1 trial, conducted by the Children Oncology Group (COG) on pediatric patients with ALK+ ALCL, confirmed that Bv combined with chemotherapy outbalanced standard chemotherapy. In these studies, patients below the age of 22 were enrolled (median 12 years old) and achieved a two-year event free survival (EFS) and a two-year OS of 79.1% and 97%, respectively [45]. Another study evaluated Bv alone in the treatment of R/R Hodgkin lymphoma (19 patients) and systemic ALCL (17 patients) in children. In the ALCL group, patients were 2 to 17 years old and 12 of them were ALK+. The safety dosage was estimated at 1.8 mg/kg for phase 2, in which nine patients (53%) of systemic ALCL achieved overall response (seven complete responses (CR) and two partial responses (PR)); for patients with a first relapse of ALCL, six (60%) of them achieved overall responses (four CR and two PR). The adverse effects of this treatment were pyrexia (16/36 patients) and nausea (13/36). Moreover, four patients developed neutropenia [46]. Future perspectives of ADCs usage in pediatric NHLs are sought, for example, for inotuzumab ozogamicin (IO). CD22 is expressed on B-cells, from the pro-b-cell phase to immunoblasts. This antigen plays a great role in the process of proliferation and differentiation of B-cells. CD20 is also present on B-NHL cells [47]. Thus, novel drugs aim at this antigen. IO activates scission of DNA strands in tumor cells with CD20 antigen on their surface [48]. Currently, COG conducts research on treatment with IO in patients diagnosed with B-lymphoblastic lymphoma, or R/R CD22 + B acute lymphoblastic leukemia (NCT02981628). The patients involved in this study are 21 years old or younger [49].
Table 4.
Comparison of antibodies used in the treatment of pediatric NHLs [2,33,37,38,39,40,41,45,49,52,53,54,55].
| Antibody Type | Mechanism of Action | Approved Abs | Abs in Preclinical/Clinical Research |
|---|---|---|---|
| mAbs | activation of apoptosis binding of membrane receptors on the surface of the tumor cell causing inhibition of signal transduction pathway antibody-dependent cellular cytotoxicity complement-dependent cytotoxicities |
rituximab (as single-agent therapy or conjugated with chemotherapy)—B-NHL, FL obinutuzumab + chemotherapy—FL |
B43 + genistein—NHL epratuzumab galiximab tafasitamab—R/R NHL, FL, DLBCL, MCL MEDI-551—R/R FL, DLBCL |
| BiAbs | engaging the cells of the immune system to attack malignant cells by targeting the tumor-associated antigen binding to the CD3 antigen on T cells to induce T cell activation and proliferation in an MHC-independent manner |
blinatumomab—R/R B-NHL | mosunetuzumab—DLBCL, FL, MCL odronextamab—DLBCL, FL, MCL epcoritamab—DLBCL, FL, MCL plamotamab—DLBCL glofitamab—DLBCL, FL, MCL |
| ADCs | preferential release of a potent cytotoxic agent at the tumor region, which is caused by proteases or alterations in pH bystander killing |
Bv—R/R ALCL polatuzumab vedotin-piiq—DLBCL loncastuximab tesirine-lpyl—DLBCL |
Bv—DLBCL Bv + chemotherapy—ALK+ ALCL IO JBH492 Pinatuzumab vedotin—R/R DLBCL, FL Vorsetuzumab mafodotin—R/R NHL Coltuximab Ravtansine (SAR3419) IMGN529 (CD37 ADC) |
mAbs, monoclonal antibodies; NHL, non-Hodgkin lymphoma; FL, follicular lymphoma; R/R, relapsed or refractory; DLBCL, diffuse large B-cell lymphoma; MCL, mantle cell lymphoma; BiAbs, bispecific antibodies; ADCs, antibody–drug conjugates; Bv, brentuximab vedotin; ALCL, anaplastic large cell lymphoma; ALK+, anaplastic lymphoma kinase positive; IO, inotuzumab ozogamicin.
Polatuzumab vedotin, whose clinical trial (NCT02257567) ended in FDA approval for treatment of R/R DLBCL in adults, is the combination of the CD79b antibody and MMAE [50]. Polatuzumab vedotin acts as a microtubule inhibitor and is currently under trial among patients 12–70 years old, suffering from B-NHL or Hodgkin Lymphoma, with previous autologous stem-cell transplantation (auto-SCT) (NCT04491370) [51]. The therapies involving ADCs develop rapidly, and therefore there is a consistent need to seek novel antigens to target, such as JBH492 aiming at CCR7, which is currently under clinical investigation (NCT04240704) [52].
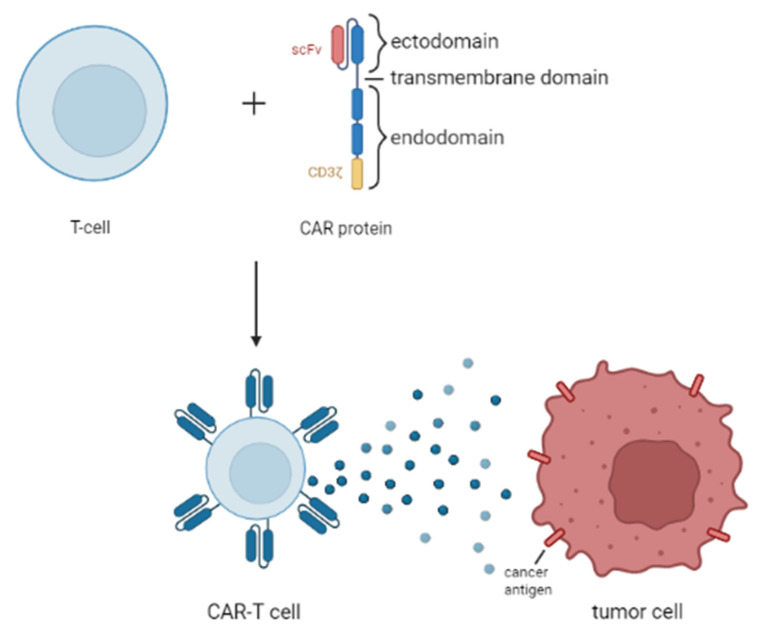
2.3. Chimeric Antigen Receptor T Cell (CAR-T Cell) Therapy
CAR-T cell therapy uses T lymphocytes, which are engineered with synthetic chimeric antigen receptors (CAR). The CAR-T cell recognizes and then eliminates specific cancer cells, independently of major histocompatibility complex molecules [56]. The creation and mechanism of action of CAR-T cell therapy is shown in Figure 1. The targets of this therapy, among others, are B-cell markers CD19, CD20, and CD22, which are highly expressed in many B-cell malignancies. Unfortunately, such action leads to the elimination of healthy B-cells. For that reason, B-cell maturation antigen (BCMA) is used as an alternative target for CAR-T cell therapy. BCMA is expressed by the plasma cells and the light chain κ/λ of malignant B-cells [53]. The application of second-generation CAR-T cells targeting CD19 with stimulatory domains of CD28/4-1BB has shown significant positive outcomes in the treatment of B-cell lymphomas (FL, PMBCL, DLBCL, mantle cell lymphoma (MCL), and splenic MZL). Furthermore, after using dual specific CD19/CD22-targeted CAR-T cells on a patient with acute B-cell lymphoblastic lymphoma and Li-Fraumeni syndrome, a complete relief of the tumor and negative minimal residual disease (MRD) took place. CAR-T cell therapy is being examined to treat MCL. Application of third-generation CD20-directed CAR-T cells was well tolerated in patients. Additionally, CD20 CAR-T cell treatment resulted in the achievement of CR in BL [53]. Tisagenlecleucel is a second-generation CD19 CAR-T, improving cytokine production, proliferation and CAR-T persistence. It is highly active in children. In a phase II trial, tisagenlecleucel achieved CR for 40% of DLBCL patients. It was estimated that 65% of patients will experience a two-year relapse-free survival [2].
Figure 1.
Structure of CAR-T cells and their antitumor function.
In a recent study of Juan Du, an eight-year-old child suffering from R/R BL showed no clear response after being treated with CD19-specific CAR-T cells. After the attempt to treat the malignancy with CD22-specific CAR-T cells, the disease reoccurred. Subsequently, CD20 CAR-T cell treatment was applied, and that action resulted in the achievement of CR. What is more, CAR-T cell therapy targeting CD23 and the tyrosine kinase-like orphan receptor brought about promising results in the improvement of R/R NHL treatment in children [57]. One of the favorable qualities of using CAR-T for pediatric NHL is that the infusions require minimal additional chemotherapy before administration [58].
2.4. DNA Methyltransferase (DNMT) Inhibitors
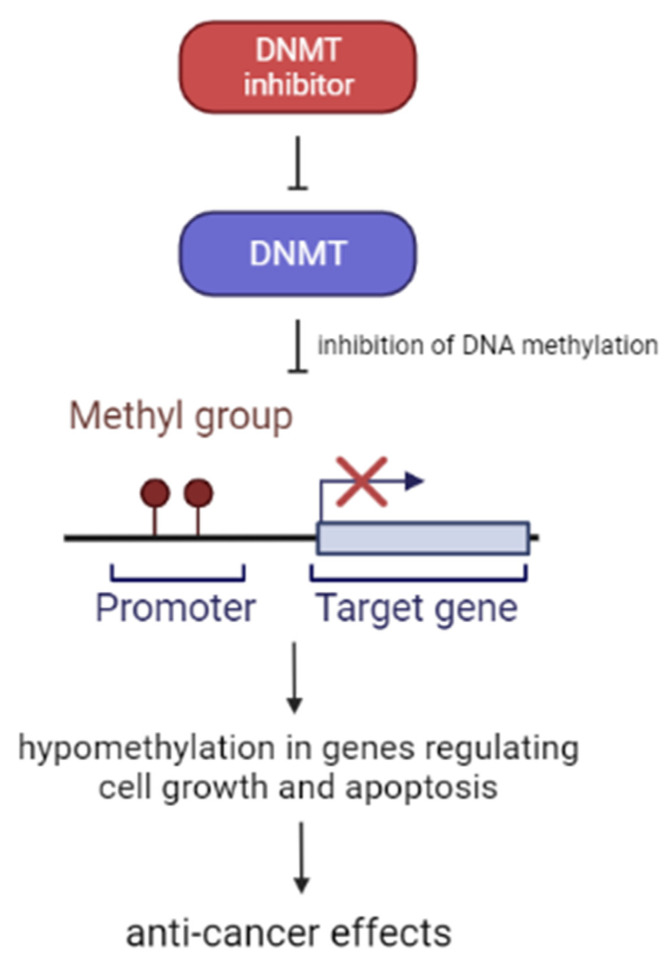
DNMT inhibitors belong to a family of enzymes that catalyze the methylation of DNA [59]. There are three major types of DNMT in mammals: DNMT1, DNMT3a, and DNMT3b [60]. DNMTs have a huge impact on gene regulation process and they are a potential predictive biomarker of genetic disorders and diseases [59]. Methylation is a post-translational modification, whose biological role is to preserve DNA transcriptionally on hold. Such action results in gene silencing. DNMT inhibitors cause cell cycle and growth arrest, differentiation and apoptosis. The molecular mechanisms by which DNMT inhibitors induce anti-cancer effects are partially mediated by the hypomethylation of DNA, which at higher concentrations may lead to cytotoxic effects (Figure 2) [21].
Figure 2.
Effect of DMNT inhibitors application.
The conclusions of Han Weidong’s study on children under 16 years of age inform us that decitabine-primed CAR-T cells can recognize and kill the CD19 negative malignant cells, which leads to death of lymphoma tumor cells. Decitabine increases tumor antigens and human leukocyte antigen expression, enhances antigen processing, promotes T cell infiltration and boosts effector T cell function; therefore, it can be used in DLBCL, high grade B-cell lymphoma and other aggressive B-cell lymphomas in pediatric patients [61]. Decitabine is also thought to be effective in R/R T-lymphoblastic lymphoma treatment in people who are over 14 years old [62].
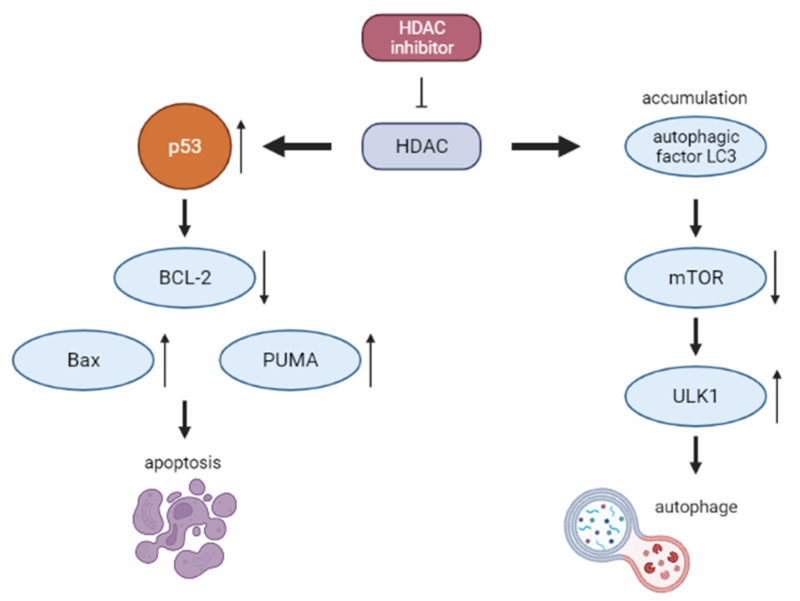
2.5. Histone Deacetylase Inhibitors (HDACIs)
For many years, the modulation of epigenetic mechanisms was the subject of trials and studies [63]. The HDACIs influence the degree of acetylation in non-histone proteins, which results in alteration of certain proteins functions, thereby affecting the cell cycle [64,65]. The role of HDACIs in lymphoma treatment, as well as their detailed mechanism of action, was extensively described in a recent review by Chen et al. [66]. Simplified mechanism of action is presented on the Figure 3.
Figure 3.
Mechanism of action HDACI.
At the time of this article’s publication, there are two ongoing pediatric clinical trials involving vorinostat in NHL. They cover combination with chemotherapy before donor stem cell transplant (NCT04220008) and a potential graft-versus-host disease (GVHD) incidence reducing drug (NCT03842696) [67,68]. Promising results were brought by van Tilburg et al., who managed to estimate a safe dosage of vorinostat in several types of pediatric malignancies [69].
In the case of panobinostat, the results of only one clinical trial (NCT01321346) were published by Goldberg et al. Among 22 pediatric patients, only one was diagnosed with NHL. The researchers observed several adverse effects, predominantly regarding the gastrointestinal tract. Moreover, the clinical activity of panobinostat was unsatisfactory, and now more efficient therapies are available [70].
The following examples of HDACIs were registered in trials in patients at least 16 years old. With a positive outcome of these studies, we may assume the age of enrollment to these trials will be lowered in the future.
Chidamide is being tested in R/R peripheral T cell lymphoma with encouraging results and a satisfying safety profile [71]. Moreover, the NCT03151876 brought the results of a favorable combination of chidamide with cladribine, gemcitabine and busulfan. Adults patients with a high risk or R/R NHL reached the OS of 86.1% [72]. Moreover, Wang et al. describe treatment with chidamide combined with various chemotherapy protocols. Their patients were diagnosed with hepatosplenic T cell lymphoma. Due to limited data, the conclusions are not clear. However, 7 out of 14 patients responded to therapy, with three patients achieving CR [73].
HDACIs are undeniably under rapid development. Certainly, we will observe more clinical trials of either HDACIs as single agents or in combined therapies with novel drugs [74].
2.6. Immune Checkpoint Inhibitors (ICIs)
ICIs have become a great milestone as a cancer cure. Pediatric malignancies, such as leukemias and solid tumors, also benefit from ICIs, with their expansion in our sight [75,76]. Despite limited data, there are a few clinical trials involving NHL treatment in children.
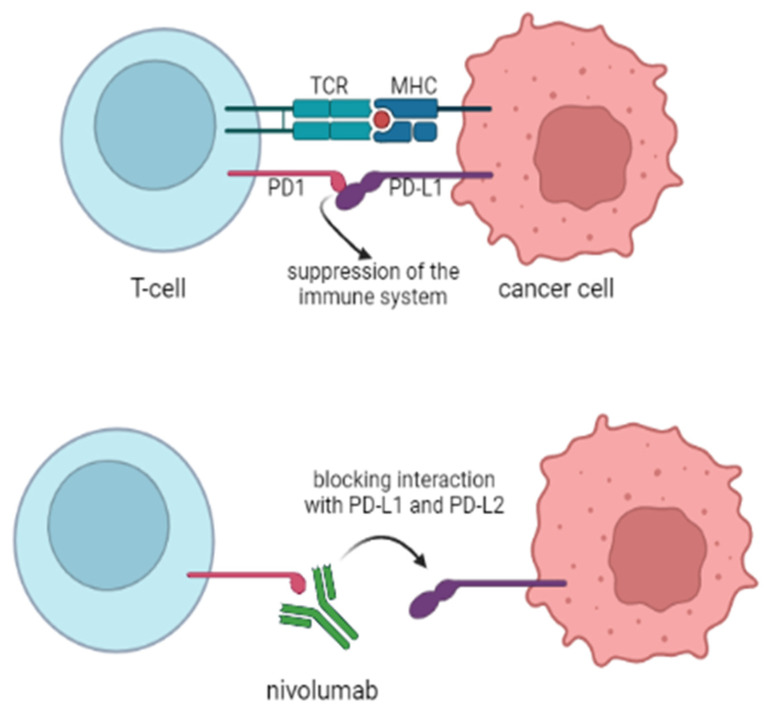
As for nivolumab, which is a fully human IgG4 mAb targeting the PD-1 receptor (Figure 4), there are very limited data in the field of this review [77]. The first results of the NCT02304458 clinical trials were collected with the conclusions of safety and tolerance of nivolumab in a group of pediatric patients [78]. NHL constituted only 10 of the 72 patients of “Group B”, with a median age of 15. However, only eight NHL cases were tested for PD1 expression, of whom seven were positive. Despite this fact, only one patient responded to PD-1 blocking therapy, which resulted in the termination of testing nivolumab as a single agent for this group [79]. However, there may be a subgroup of patients that can benefit from anti-PD-1 receptor therapy. A case of a 17-year-old patient, with ALK+ ALCL, received nivolumab as the third line therapy, obtaining a CR [80]. With regard to the patient’s age, we are aware that further studies are necessary for pediatric patients; nonetheless, with the promising results in the adults’ group, we hope to achieve a positive outcome with PD-1 blockade [81,82].
Figure 4.
Construction of the CAR-T cell.
In terms of other checkpoint inhibitors, the fully human IgG1 PD-1L antibody, atezolizumab, has become the object of clinical trials [83]. However, the study (NCT02541604) was terminated, and in NHL, only three patients were enrolled (3% of all participants), achieving poor results with one partial response and one progression of the disease. Nevertheless, atezolizumab was proved to be safe in the pediatric group of patients. Due to limited data, the results of the treatment were highly reduced.
There are several active clinical trials, which will determine the usage of the mentioned ICIs, along with others, such as anti-CTLA-4. The National Cancer Institute is conducting studies (NCT02304458) on combined nivolumab with ipilimumab (which is the anti-CTLA-4 antibody) in patients with R/R NHL [84].
2.7. Enhancer of Zeste Homolog 2 (EZH2) Inhibitors
EZH2 is a histone methyltransferase, which comes from the gene family containing epigenetic regulators that inhibit transcription [85]. EZH2 alters gene expression by trimethylation of Lys-27 in histone 3 (H3K27me3) [86,87]. Thus, EZH2 can act as a regulator of cell cycle progression, and its mutations will lead to abnormal histone methylation profiles, resulting in oncogenic transformation [88]. In B-cells, EZH2 is highly expressed in the germinal center (GC), where maturing B-cells undergo somatic mutations to form a series of sublines. Due to the fact that EZH2 is involved in the mechanisms of checkpoints, it influences the maintenance of an orderly process in the GC, thus controlling the transition of lymphocytes from pro-B to pre-B [89].
When EZH2 mutation occurs, the suppression of GC output genes and checkpoints persists, which in turn leads to hyperplasia [90]. Until now, EZH2 somatic mutations associated with the acquisition of function were most often detected in DLBCL and in GC-derived FL [91,92]. In this context, efforts to find inhibitors of EZH2 have become understandable.
Since 2012, several specific EZH2 inhibitors have been investigated. Their task was to inhibit H3K27 methylation, reactivate silenced PRC2 target genes, or inhibit the survival of B-cell lymphoma cells (GC-derived and containing the EZH2 activating mutation) [93]. These selective compounds included: El1, UNC1999, OR-S1 and OR-S2 inhibitors, EPZ0005687, and GSK126, CPI-360, CPI-169, DS-3201 (Walemetostat), and EPZ-6438 (tazemetostat) [94,95,96,97,98]. The last of them, tazemetostat, as a compound with high affinity to mutant forms of EZH2, is currently being evaluated in clinical trials in the treatment of pediatric patients with R/R NHL [99,100,101,102].
EZH2 inhibitors have the potential to be useful in the treatment of lymphomas, but there is still insufficient research to draw specific conclusions. It is possible that in the future it will be possible to use compounds from this group in combination therapy, e.g., with chemotherapy, which would result in improved patient outcomes. However, for this to happen, a lot of research has yet to be conducted.
2.8. Isocitrate Dehydrogenase (IDH) Inhibitors
IDH is an enzyme that catalyzes the conversion of isocitrate to α-ketoglutarate (αKG) [103]. Recently, more and more has been said about mutations related to these dehydrogenases, as they are detected in many cancers. IDH1/2 mutations may involve both loss of enzyme function and an increase in its activity. They lead to the disturbance of the oxidative decarboxylation of isocitrate to α-KG, additionally giving new enzymatic activity, resulting in the reduction of α-KG to the oncometabolite D-2-hydroxyglutarate (D-2HG) [104,105]. There are reports of IDH mutations in angioimmunoblastic lymphomas, classified as NHLs [104]. Due to the prevalence of mutant IDH in tumors, efforts were made to develop inhibitors of this dehydrogenase. Currently, the group of drugs that are IDH inhibitors includes enasidenib, ivosidenib (AG-120), AG-881, FT2102, and IDH-305 [103].
Enasidenib and ivosidenib are orally selective inhibitors of mutant IDH, with enasidenib blocking IDH2 and ivosidenib blocking IDH1. Both of these drugs have already been approved by the FDA for the treatment of acute myeloid leukemia (AML) [106,107,108].
Further clinical trials are currently underway to test these compounds in other diseases as well. One of these studies, on ivosidenib, is currently being conducted in the age group 12 months to 21 years. It focuses on patients with R/R NHL, advanced solid tumors, or histiocytic disorders that have IDH1 genetic alterations [109].
2.9. The B2 Cell Lymphoma Protein (BCL-2) Inhibitors
Expression of anti-apoptotic proteins from the BCL-2 family can be disrupted by several different mechanisms, including gene amplification, chromosomal translocation, increased gene transcription, or altered post-translational processing [105]. In the case of FL and DLBCL, it has been shown that increased BCL-2 expression may result from the t(14; 18) translocation of this gene [110,111,112]. The BCL-2 gene is then inserted proximate to the immunoglobulin heavy chain (IgH) gene enhancer [113]. The consequence of this is activated transcription of the BCL-2 gene and, thus, inhibited cell apoptosis. It is worth mentioning that in NHL subtypes, there may also be changes leading to overexpression of BCL-2, regardless of t(14;18). This can occur, for example, by the aforementioned amplification of this gene [114]. From a therapeutic point of view, it therefore appears beneficial to target regulators of apoptosis in cancer patients with mutations within the BCL-2. This could ultimately enhance the apoptotic response and thus translate into improved clinical outcomes.
Currently, several compounds that are inhibitors of BCL-2 can be distinguished. One of them, ABT-263 (navitoclax), initially showed good efficacy. However, its use has been limited due to its main toxicity: thrombocytopenia. Despite this, research can still be found in the context of NHL and navatoclax. In one study, although each patient had at least one treatment-related adverse event, the safety profile of navitoclax in R/R FL was assessed as acceptable [115]. In turn, ABT-199 (venetoclax/GDC-0199) shows a high binding affinity to BCL-2, where it exerts its clinical effect without the simultaneous thrombocytopenia [116,117]. Recent studies suggest that venetoclax has good clinical outcomes. An example may be the study by Davids et al., who decided to evaluate the effect of venetoclax on a group of patients with R/R NHL. In their studies (although the results differed by NHL subtype), they achieved overall responses rates (ORRs) in 75% of patients with MCL and 38% with FL. Although only 5/29 FL patients and 6/28 MCL patients achieved CR, all of them had a median duration of response greater than 30 months [118,119]. For this reason, many studies on its effectiveness are currently underway, including several involving a group of children with NHL [120,121]. These studies focus not only on the safety assessment and pharmacokinetics of venetoclax in children with R/R malignancies (including NHL), but also investigate the effect of combination therapy (venetoclax + chemotherapy) on the treatment of R/R lymphoblastic lymphoma (LL) [122]. It is worth mentioning that a study evaluating venetoclax + navitoclax + chemotherapy in children and adults with R/R acute lymphoblastic leukemia or LL has recently ended. In this study, the CR rate was 60%, and 28% of patients crossed over to either transplantation or CAR-T therapy [123].
In the context of the future, VOB560 (65487) and MIK665 (S64315) may also be significant. Both of these compounds were designed to potently and selectively block BCL-2 and MCL-1, respectively. Their combination in preclinical studies has shown strong anti-cancer properties [124].
Undoubtedly, the discovery of inhibitors of the BCL-2 family opened a new path in the targeted therapy of many cancers. There is the need for new clinical trials comparing the effectiveness of these substances in similar and the same diseases. Their toxicity profile is also extremely important. As already proven, the side effect of individual BCL-2 inhibitors is not only the abovementioned thrombocytopenia. In addition, with venetoclax, diarrhea, upper respiratory tract infections, neutropenia and tumor lysis syndrome may occur [125,126,127]. However, for the other substances mentioned, we have to wait for the completion of ongoing studies [116]. To be able to efficiently operate these agents, we must also be aware of the potential resistance of cancer cells to BCL-2 inhibitors; this has already been observed in the case of navitoclax. Although samples of CLL patients initially responded to treatment, there was a 1000-fold increase in resistance to navitoclax over a longer period of time [128]. A similar situation occurred with the use of venetoclax [129,130]. A solution to this situation may be the synergism of BCL-2 and MCL-1 inhibitors described above.
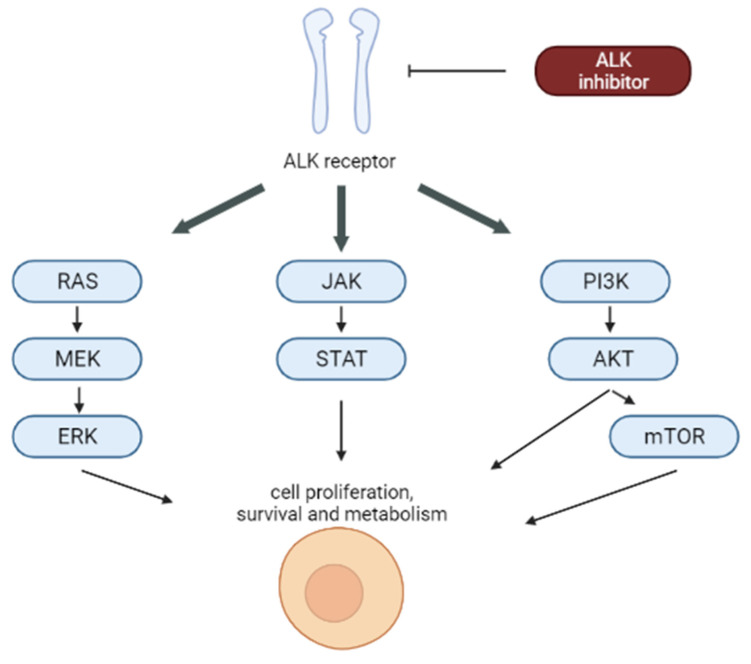
2.10. Anaplastic Lymphoma Kinase (ALK) Inhibitors
Approximately 90% of pediatric ALK+ ALCL is due to t(2;5) (p23;q35) chromosomal translocation, which entails the emergence of oncogenic fusion protein nucleophosmin activating several proliferation and survival pathways [131]. The reason why ALK is one of the targets of personalized therapies is that ALK-transformed cells are dependent on ALK tyrosine kinase activity in terms of survival and proliferation. Because ALK expression is limited in non-tumoral cells, its blockage is catastrophic for cancer cells but irrelevant for normal tissues (Figure 5) [132].
Figure 5.
Signalling pathways blocked by ALK inhibitors.
Crizotinib is a first-generation ALK inhibitor [133]. Based on the tolerability, toxicity and activity of single agent crizotinib, it was demonstrated that the discussed molecule synergizes with chemotherapy [134]. Crizotinib in combination with multiagent chemotherapy is being evaluated in an ongoing randomized NCT01979536 trial in childhood ALCL [27]. In an NCT02034981 trial investigating crizotinib in pediatric ALK+ ALCL patients, 1/3 of the examined children progressed in the first three months of treatment [135]. On the other hand, the secondary outcomes of NCT00939770 trial showed that 70% of pediatric patients with R/R ALK+ ALCL treated with crizotinib achieved CR or PR within eight years [136]. Recently, crizotinib was approved by the FDA for R/R ALK+ ALCL in treatment for patients aged over one year, as it was well tolerated and led to a CR in 67–83% of children with R/R ALCL [28].
Ceritinib is a second-generation ALK inhibitor that binds to ALK with a higher affinity than crizotinib [133], but its use is associated with significant toxicities, limiting its application to children and adolescents [137]. Still, in a phase I NCT01742286 trial, where single-agent ceritinib in pediatric patients with ALK+ malignancies was examined, two out of two children with ALK+ ALCL achieved CR [29].
2.11. Ibrutinib/Bruton’s Tyrosine Kinase (BTK) Inhibitor
BTK is a kinase with a protein structure involved in the regulation of B-cell signaling [138]. As one of five members, it belongs to the TEC family of non-receptor tyrosine kinases [139]. BTK is expressed in B-cells, bone marrow cells, mast cells and platelets [140]. This kinase, as a key component of the B-cell antigen receptor (BCR) signaling cascade, is involved in all aspects of B-cell development [141]. However, if its action is unregulated, it leads to uncontrolled proliferation, differentiation, consequent survival of B-cells and, hence, the development of cancer [138]. So far, BTK expression has been described in B-cell leukemias and lymphomas. Its involvement in the regulation of FcγR signaling and in mast cell degranulation after FcεR activation has been observed. These features make it an attractive target for the treatment of both B-cell cancers and autoimmune diseases, leading to the development of BTK inhibitors [140].
Several generations can be distinguished among these drugs. The first-generation BTK inhibitors include ibrutinib, which is administered orally. It binds irreversibly to a cysteine residue (C481) in the active site of BTK, thereby inhibiting B-cell receptor signaling (through decreased activation of extracellular signal regulated kinase, PLCγ2, and the NF-κB signaling pathway) [142,143]. As a result, it reduces cell growth, proliferation, survival, adhesion, and migration [141,144].
Studies to date have shown that ibrutinib is well tolerated in many B-cell tumors, including DLBCL [145,146]. However, most of them still relate to adults. As for children, a study evaluating the safety and efficacy of ibrutinib in a group of patients from 1 to 30 years with R/R NHL was completed at the end of last year. However, its results have yet to be published (NCT02703272) [147,148]. Nevertheless, we already know that it is not a drug without its drawbacks. This is due to the fact that it can cause common side effects such as diarrhea, nausea, and shortness of breath. Moreover, there have also been cases of thrombocytopenia, bleeding, and atrial fibrillation [149]. For this reason, the second-generation of BTK inhibitors has been developed, including acalabrutinib (ACP-196), zanubrutinib (BGB-3111), and tirabrutinib (ONO/GS-4059) [150,151,152,153,154]. These inhibitors, although similar to ibrutinib, bind covalently to the C481 residue in BTK, exhibiting higher target selectivity. It is possible that this could reduce the frequency of side effects, including the cardiological ones. Currently, acalabrutinib and zanubrutinib have already received FDA approval for treatment [153,155,156,157,158].
It is worth mentioning that, as in the case of other drugs, the possibility of combining BTK inhibitors with, e.g., BCL2 inhibitors, is being considered. In one such study, Constantine et al. proved that the combination of ibrutinib + venetoclax was highly effective in patients with MCL. They received an OS rate of 79% at 12 months and 74% at 18 months [159]. In turn, in the study by Amos Burke et al. evaluating children with R/R mature B-NHL, 57.1% of patients who received ≥ 1 dose of ibrutinib responded. In contrast, 72.7% of patients who received ibrutinib with modified RICE (rituximab plus ifosfamide, carboplatin, and etoposide) responded. This proves the validity of further research of this type in the future [160].
2.12. Bortezomib, Inhibitor of Proteasome
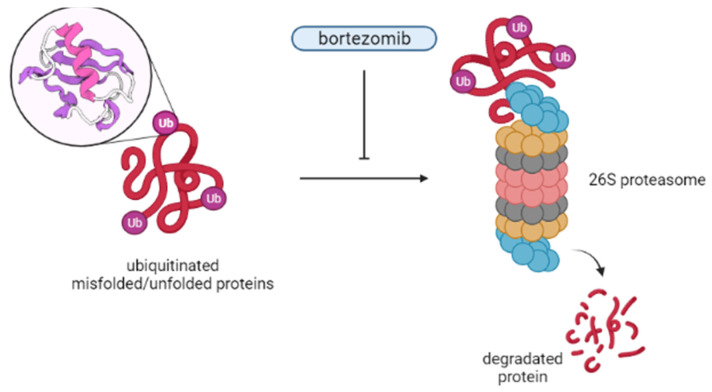
Bortezomib (PS-341) is a peptide aldehyde derivative. It is the first potent, selective, and reversible inhibitor of the 26S proteasome in its class (Figure 6) [161].
Figure 6.
Bortezomib mechanism of action.
Its role is to inhibits the ubiquitin–proteasome pathway by binding directly to the active sites of the proteasome, which in turn disrupts targeted protein proteolysis [161,162]. The consequence of this is the deregulation of homeostatic mechanisms at the cellular level, leading to apoptosis and inhibition of cell cycle progression, angiogenesis, cell adhesion, and proliferation. It is worth mentioning that bortezomib has also been shown to induce apoptosis in cells overexpressing BCL2 [161,163]. Initially, its development was to be used in inflammation and cachexia. However, this changed in the late 1990s, when its anti-cancer activity was established [162,164,165]. Now, it is approved by the FDA for the treatment of MCL. This decision was based on a phase III study, in which a higher CR rate was obtained in the group of patients with the discussed drug [166,167]. Bortezomib has been found to be effective in other NHLs as well. In the study by Ruan et al., combining this drug with R-CHOP in the DLBCL cohort resulted in a CR/CR unconfirmed (CRu) percentage of 86%. In addition, the study authors concluded that such treatment was safe and could improve outcomes, especially for patients with non-germinal center DLBCL [168,169]. In the case of bortezomib, there are also several side effects that it can cause. The most important of these is peripheral neuropathy, which is usually sensory, distal, and symmetrical, and affects the feet more often than the hands. In most cases it is reversible on stopping treatment [170]. In addition, its risk has been shown to be lower with subcutaneous bortezomib compared to intravenous administration [161]. Other side effects that may be mentioned are gastrointestinal toxicity (such as diarrhea or constipation), fatigue, neutropenia, and thrombocytopenia. Shingles may also reactivate during bortezomib treatment, and antiviral prophylaxis is usually recommended [170]. Of note is isolated cutaneous vasculitis caused by bortezomib, which does not always lead to treatment discontinuation. It has been shown that it may herald a better clinical response in some patients with B-NHL [171].
Although most studies on bortezomib in NHL patients concern adults, for several years there have also been studies in which the target group are children. One of them was conducted by Horton et al. and related to the use of combination therapy of bortezomib with ifosfamide/vinorelbine (IVB) in pediatric NHL patients. Although few patients achieved the primary goal (which was complete anatomical response after two IVB cycles), the CR after two cycles was 83% [172,173]. Recently, a study evaluating the effect of bortezomib on combination chemotherapy in patients with R/R NHL has been completed. The BICE group (bortezomib, ifosfamide, carboplatin, and etoposide) achieved a slightly higher OR rate (70%) compared to the group without bortezomib (60%). However, to be able to talk about the effectiveness of bortezomib in the treatment of children with NHL, much more research is needed [174].
2.13. Temsirolimus
Temsirolimus is one of the mammalian targets of the rapamycin (mTOR) inhibitor, which, as a derivative of the sirolimus ester, exhibits antibacterial, immunosuppressive, and antitumor properties [122,175]. The main function of the mTOR protein kinase is the regulation of growth and proliferation, as well as the translation and transcription processes [176,177].
Currently, temsirolimus is approved in the European Union for the treatment of R/R MCL, but not in the USA [122,178,179]. The discrepancy in the approval of temsirolimus in the NHL is likely due to the fact that although it has been shown to be effective, the response rate is usually well below 50%. In addition, the median disease-free survival is often a few months or less [180,181,182].
For this reason, combinations of temsirolimus with other agents, both cytotoxic drugs and other targeted inhibitors, are currently at the forefront of NHL treatment [180]. One such study was conducted by Fenske et al. and related to the combination of temsirolimus with bortezomib in R/R B-NHL patients. As the treatment did not cause any unexpected toxic effects in any of the patients, this combination was assessed as safe. The OR rate was 31% and the median progression-free survival (PFS), although different by the B-NHL subtypes, in patients with MCL was 16.5 months. Thus, the observed results justify further research in this direction [122]. In turn, the study by Hess et al. evaluating the combination of bendamustine + rituximab + temsirolimus (BeRT) in R/R MCL and FL also achieved promising efficacy with an acceptable toxicity [183]. As it turns out, temsirolimus can be effectively used in other NHL subtypes. A study by Witzens-Harig et al. confirmed that it can be safely added to the rituximab regimen in combination with dexamethasone, cytarabine, and cisplatinum (R-DHAP). The ORR in this study was 66%, while the two-year overall PFS and OS were 53% and 59% [184]. As can be seen, many studies involving temsirolimus in NHL have already been conducted in adults, and many more are still in clinical trials. In the case of children there are fewer of them, but we can also distinguish a few. These include studies evaluating the side effects and the best effective dose when combining temsirolimus with dexamethasone, mitoxantrone hydrochloride, vincristine sulfate, and pegaspargase in young NHL patients, as well as studies evaluating the combination of temsirolimus with etoposide [185,186].
As can be seen, despite the approval of temsirolimus for treatment in MCL, there is still a space for improvement in its effectiveness in this and other types of NHL. It is possible to achieve this by developing better biomarkers, which would allow for better stratification of the patient prior to drug administration. Moreover, such biomarkers could help to elucidate the mechanisms of resistance and to develop new therapeutic combinations, which would certainly improve the effectiveness of treatment [180].
3. Conclusions
Our knowledge of the refractory NHL’s treatment has grown significantly. Novel drugs trial results give hope to those who suffer from the mentioned hematologic dis-eases and make up a large percentage of people.
There are many approved drugs for NHL therapy, e.g., temsirolimus for MCL or bilinatumomab for R/R B-NHL in children and adults. The application of second-generation CD19 CAR-T cells has shown significant positive outcomes in the treatment of FL, PMBCL, DLBCL, MCL, and splenic MZL. Moreover, CD20 CAR-T cell treatment resulted in CR in BL.
EZH2 inhibitors in combination with chemotherapy will probably improve patients’ outcomes, if examined more closely. When the effective doses have been improved and the side effects suppressed, BCL-2 inhibitors will be a promising class of drugs to fight NHL. Additionally, combination of ibrutinib and venetoclax is considered highly effective in treating MCL. Lastly, chidamide indicated encouraging results in its effectiveness and safety profile in T cell lymphoma treatment; for that reason, we can expect many clinical trials validating this method.
There is a necessity to develop better biomarkers, which could help with elucidation of resistance mechanisms and stratification of the patient before administrating the drug.
It is likely that the majority of the compounds described in this review will be used in widespread NHL therapies; however, much more research needs to be conducted and many years have to pass to establish personalized treatment options. As always, future pediatric treatment regimens will arrive after clinical trials take place in adults. Thus, we expect rapid progress in this branch of medicine. All figures presented in this article have been created with Biorender.com.
Abbreviations
| Abbreviations | Definitions |
| NHL | Non-Hodgkin lymphoma |
| DLBCL | Diffuse large B-cell lymphoma |
| BL | Burkitt lymphoma |
| PMBCL | Primary mediastinal B-cell lymphoma |
| FL | Follicular lymphoma |
| MZL | Marginal zone lymphoma |
| ALK+ | ALK-positive |
| ALCL | Anaplastic large cell lymphoma |
| LBL-T | T cell lymphoblastic lymphoma |
| LBL-B | B-cell lymphoblastic lymphoma |
| ID3 | Inhibitor of DNA binding 3 |
| BLL | Burkitt-like lymphoma |
| TCF3 | Transcription Factor 3 |
| ALK | Anaplastic lymphoma kinase gene |
| NPM | Nucleophosmin gene |
| ABDTRG | Absence of biallelic deletion of the TRG locusT cell receptor gamma |
| CNS | Central nervous system |
| RT | Radiotherapy |
| B-NHL | B-cell non-Hodgkin lymphoma |
| FDA | Food and Drug Administration |
| mAbs | Monoclonal antibodies |
| ICIs | Immune checkpoint inhibitors |
| ADCs | Antibody–drug conjugates |
| CAR | Chimeric antigen receptor |
| HDAC | Histone deacetylase |
| DNMT | DNA methyltransferase |
| IDH | Isocitrate dehydrogenase |
| Allo-HSCT | Allogenic hematopoietic stem-cell transplantation |
| Auto-ASCT | Autologous stem cell transplantation |
| CHOP | Cyclophosphamide, doxorubicin, vincristine, prednisone |
| allo-SCT | Allogenic stem cell transplantation |
| HDC/ASCT | High-dose chemotherapy supported by autologous stem cell transplantation |
| Bv | Brentuximab vedotin |
| BiAbs | Bispecific antibodies |
| scFv | Single-chain variable fragments |
| OS | Overall survival |
| R/R | Relapsed or refractory |
| MMAE | Monomethyl auristatin E |
| COG | Children Oncology Group |
| EFS | Event free survival |
| CR | Complete Response |
| PR | Partial response |
| IO | Inotuzumab ozogamicin |
| BCMA | B-cell maturation antigen |
| MCL | Mantle cell lymphoma |
| MRD | Minimal residual disease |
| HDACIs | Histone deacetylase inhibitors |
| GVHD | Graft-versus-host disease |
| EZH2 | Enhancer of Zeste Homolog 2 |
| GC | Germinal center |
| αKG | α-ketoglutarate |
| D-2HG | D-2-hydroxyglutarate |
| AML | Acute myeloid leukemia |
| BCL-2 | B2 cell lymphoma protein |
| ORRs | Overall responses rates |
| LL | Lymphoblastic lymphoma |
| ALK | Anaplastic lymphoma kinase |
| BTK | Bruton’s tyrosine kinase |
| BCR | B-cell antigen receptor |
| RICE | Rituximab plus ifosfamide, carboplatin, and etoposide |
| CRu | Complete response unconfirmed |
| IVB | Bortezomib with ifosfamide/vinorelbine |
| BICE | Bortezomib, ifosfamide, carboplatin, and etoposide |
| OR | Overall response |
| mTOR | Mammalian targets of the rapamycin |
| PI3K | Protein-3-phosphoinositide kinase B |
| PFS | Progression-free survival |
| BeRT | Bendamustine + rituximab + temsirolimus |
| DNA | Deoxyribonucleic acid |
Author Contributions
Conceptualization, J.D. and M.L.; writing—original draft preparation, J.D., K.P. and M.M., writing—review and editing, M.L. and J.Z.; visualization, J.D.; supervision, M.L. and J.Z.; All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Moubadder L., McCullough L.E., Flowers C.R., Koff J.L. Linking Environmental Exposures to Molecular Pathogenesis in Non-Hodgkin Lymphoma Subtypes. Cancer Epidemiol. Biomark. Prev. 2020;29:1844–1855. doi: 10.1158/1055-9965.EPI-20-0228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Harker-Murray P.D., Pommert L., Barth M.J. Novel Therapies Potentially Available for Pediatric B-Cell Non-Hodgkin Lymphoma. J. Natl. Compr. Cancer Netw. 2020;18:1125–1134. doi: 10.6004/jnccn.2020.7608. [DOI] [PubMed] [Google Scholar]
- 3.Tsuyama N., Sakamoto K., Sakata S., Dobashi A., Takeuchi K. Anaplastic Large Cell Lymphoma: Pathology, Genetics, and Clinical Aspects. J. Clin. Exp. Hematop. 2017;57:120–142. doi: 10.3960/jslrt.17023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Reedijk A.M.J., Beishuizen A., Coebergh J.W., Hoeben B.A.W., Kremer L.C.M., Hebeda K.M., Pieters R., Loeffen J.L.C., Karim-Kos H.E. Progress against Non-Hodgkin′s Lymphoma in Children and Young Adolescents in the Netherlands since 1990: Stable Incidence, Improved Survival and Lower Mortality. Eur. J. Cancer. 2022;163:140–151. doi: 10.1016/j.ejca.2021.12.010. [DOI] [PubMed] [Google Scholar]
- 5.Sandlund J.T., Martin M.G. Non-Hodgkin lymphoma across the pediatric and adolescent and Young Adult Age Spectrum. Hematol. Am. Soc. Hematol. Educ. Progr. 2016;2016:589–597. doi: 10.1182/asheducation-2016.1.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Burkhardt B., Oschlies I., Klapper W., Zimmermann M., Woessmann W., Meinhardt A., Landmann E., Attarbaschi A., Niggli F., Schrappe M., et al. Non-Hodgkin′s lymphoma in adolescents: Experiences in 378 adolescent NHL patients treated according to pediatric NHL-BFM Protocols. Leukemia. 2010;25:153–160. doi: 10.1038/leu.2010.245. [DOI] [PubMed] [Google Scholar]
- 7.Chybicka A., Sawicz-Birkowska K., Kazanowska B., Adamkiewicz-Drożyńska E.M. Onkologia I Hematologia Dziecięca. PZWL Wydawnictwo Lekarskie; Warszaw, Poland: 2021. [Google Scholar]
- 8.Teachey D.T., O’Connor D. How I treat newly diagnosed T-cell acute lymphoblastic leukemia and T-cell lymphoblastic lymphoma in children. Blood. 2020;135:159–166. doi: 10.1182/blood.2019001557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wei J., Liu Y., Wang C., Zhang Y., Tong C., Dai G., Wang W., Rasko J.E., Melenhorst J.J., Qian W., et al. The model of cytokine release syndrome in car T-cell treatment for B-cell non-Hodgkin Lymphoma. Signal Transduct. Target. Ther. 2020;5:134. doi: 10.1038/s41392-020-00256-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Eyre T.A., Khan D., Hall G.W., Collins G.P. Anaplastic lymphoma kinase-positive anaplastic large cell lymphoma: Current and future perspectives in Adult and Paediatric Disease. Eur. J. Haematol. 2014;93:455–468. doi: 10.1111/ejh.12360. [DOI] [PubMed] [Google Scholar]
- 11.Yunis J.J., Frizzera G., Oken M.M., McKenna J., Theologides A., Arnesen M. Multiple recurrent genomic defects in follicular lymphoma. N. Engl. J. Med. 1987;316:79–84. doi: 10.1056/NEJM198701083160204. [DOI] [PubMed] [Google Scholar]
- 12.Barrans S.L., O′Connor S.J., Evans P.A., Davies F.E., Owen R.G., Haynes A.P., Morgan G.J., Jack A.S. Rearrangement of the BCL6 locus at 3q27 is an independent poor prognostic factor in nodal diffuse large B-cell lymphoma. Br. J. Haematol. 2002;117:322–332. doi: 10.1046/j.1365-2141.2002.03435.x. [DOI] [PubMed] [Google Scholar]
- 13.Ramis-Zaldivar J.E., Gonzalez-Farré B., Balagué O., Celis V., Nadeu F., Salmerón-Villalobos J., Andrés M., Martin-Guerrero I., Garrido-Pontnou M., Gaafar A., et al. Distinct molecular profile of IRF4-rearranged large B-cell lymphoma. Blood. 2020;135:274–286. doi: 10.1182/blood.2019002699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zimonjic D.B., Keck-Waggoner C., Popescu N.C. Novel genomic imbalances and chromosome translocations involving c-myc gene in Burkitt′s lymphoma. Leukemia. 2001;15:1582–1588. doi: 10.1038/sj.leu.2402281. [DOI] [PubMed] [Google Scholar]
- 15.Angi M., Kamath V., Yuvarani S., Meena J., Sitaram U., Manipadam M.T., Nair S., Ganapule A., Fouzia N.A., Abraham A., et al. The T(8;14)(q24.1;Q32) and its variant translocations: A study of 34 cases. Hematol. Oncol. Stem Cell Ther. 2017;10:126–134. doi: 10.1016/j.hemonc.2017.03.002. [DOI] [PubMed] [Google Scholar]
- 16.Nelson M., Perkins S.L., Dave B.J., Coccia P.F., Bridge J.A., Lyden E.R., Heerema N.A., Lones M.A., Harrison L., Cairo M.S., et al. An increased frequency of 13Q deletions detected by Fluorescencein situhybridization and its impact on survival in children and adolescents with Burkitt lymphoma: Results from the Children’s Oncology Group Study CCG-5961. Br. J. Haematol. 2010;148:600–610. doi: 10.1111/j.1365-2141.2009.07967.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rohde M., Bonn B.R., Zimmermann M., Lange J., Möricke A., Klapper W., Oschlies I., Szczepanowski M., Nagel I., Schrappe M., et al. Relevance of id3-TCF3-CCND3 pathway mutations in pediatric aggressive B-cell lymphoma treated according to the non-hodgkin lymphoma Berlin-frankfurt-münster protocols. Haematologica. 2017;102:1091–1098. doi: 10.3324/haematol.2016.156885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gonzalez-Farre B., Ramis-Zaldivar J.E., Salmeron-Villalobos J., Balagué O., Celis V., Verdu-Amoros J., Nadeu F., Sábado C., Ferrández A., Garrido M., et al. Burkitt-like lymphoma with 11Q aberration: A germinal center-derived lymphoma genetically unrelated to Burkitt lymphoma. Haematologica. 2019;104:1822–1829. doi: 10.3324/haematol.2018.207928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Abla O., Attarbaschi A. Non-Hodgkin’s Lymphoma in Childhood and Adolescence. Springer Nature; Cham, Switzerland: 2019. pp. 52–53. [Google Scholar]
- 20.Cairo M.S., Beishuizen A. Childhood, adolescent and young adult non-Hodgkin lymphoma: Current perspectives. Br. J. Haematol. 2019;185:1021–1042. doi: 10.1111/bjh.15764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gravina G.L., Festuccia C., Marampon F., Popov V.M., Pestell R.G., Zani B.M., Tombolini V. Biological rationale for the use of DNA methyltransferase inhibitors as new strategy for modulation of tumor response to chemotherapy and radiation. Mol. Cancer. 2010;9:305. doi: 10.1186/1476-4598-9-305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shiramizu B., Mussolin L., Woessmann W., Klapper W. Paediatric non-Hodgkin Lymphoma-Perspectives in translational biology. Br. J. Haematol. 2016;173:617–624. doi: 10.1111/bjh.14009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Neelapu S.S., Adkins S., Ansell S.M., Brody J., Cairo M.S., Friedberg J.W., Kline J.P., Levy R., Porter D.L., van Besien K., et al. Society for immunotherapy of cancer (SITC) clinical practice guideline on immunotherapy for the treatment of lymphoma. J. Immunother. Cancer. 2020;8:e001235. doi: 10.1136/jitc-2020-001235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ribeiro M.L., Reyes-Garau D., Armengol M., Fernández-Serrano M., Roué G. Recent Advances in the Targeting of Epigenetic Regulators in B-Cell Non-Hodgkin Lymphoma. Front. Genet. 2019;10:986. doi: 10.3389/fgene.2019.00986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.van Bruggen J.A., Martens A.W., Tonino S.H., Kater A.P. Overcoming the hurdles of autologous T-cell-based therapies in B-cell Non-Hodgkin Lymphoma. Cancers. 2020;12:3837. doi: 10.3390/cancers12123837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brugieres L., Afify Z., Lowe E. ALK inhibitors for Alk-altered paediatric malignancies. Lancet Oncol. 2021;22:1646–1648. doi: 10.1016/S1470-2045(21)00608-2. [DOI] [PubMed] [Google Scholar]
- 27.Brentuximab Vedotin or Crizotinib and Combination Chemotherapy in Treating Patients with Newly Diagnosed Stage II–IV Anaplastic Large Cell Lymphoma. [(accessed on 12 March 2021)]; Available online: https://clinicaltrials.gov/ct2/show/NCT01979536.
- 28.Hare L., Burke G.A., Turner S.D. Resistance to targeted agents used to treat paediatric Alk-positive ALCL. Cancers. 2021;13:6003. doi: 10.3390/cancers13236003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Phase I Study of LDK378 in Pediatric, Malignancies with a Genetic Alteration in Anaplastic Lymphoma Kinase (ALK) [(accessed on 12 March 2021)]; Available online: https://clinicaltrials.gov/ct2/show/NCT01742286.
- 30.Ferreri A.J.M., Govi S., Pileri S.A., Savage K.J. Anaplastic large cell lymphoma, Alk-positive. Crit. Rev. Oncol. Hematol. 2012;83:293–302. doi: 10.1016/j.critrevonc.2012.02.005. [DOI] [PubMed] [Google Scholar]
- 31.Willard P., McKay J., Yazbeck V. Role of antibody-based therapy in indolent non-Hodgkin′s lymphoma. Leuk. Res. Rep. 2021;16:100275. doi: 10.1016/j.lrr.2021.100275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Baldo B.A. Monoclonal antibodies approved for cancer therapy. Saf. Biol. Ther. 2016:57–140. [Google Scholar]
- 33.Minard-Colin V., Aupérin A., Pillon M., Burke G.A.A., Barkauskas D.A., Wheatley K., Delgado R.F., Alexander S., Uyttebroeck A., Bollard C.M., et al. Rituximab for high-risk, mature B-cell Non-Hodgkin’s lymphoma in children. N. Engl. J. Med. 2020;382:2207–2219. doi: 10.1056/NEJMoa1915315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pytlik R., Polgarova K., Karolova J., Klener P. Current immunotherapy approaches in Non-Hodgkin lymphomas. Vaccines. 2020;8:708. doi: 10.3390/vaccines8040708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kyriakidis I., Vasileiou E., Rossig C., Roilides E., Groll A.H., Tragiannidis A. Invasive fungal diseases in children with hematological malignancies treated with therapies that target cell surface antigens: Monoclonal antibodies, immune checkpoint inhibitors and car T-cell therapies. J. Fungi. 2021;7:186. doi: 10.3390/jof7030186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Salvaris R., Ong J., Gregory G.P. Bispecific antibodies: A review of development, clinical efficacy and toxicity in B-cell lymphomas. J. Pers. Med. 2021;11:355. doi: 10.3390/jpm11050355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Castaneda-Puglianini O., Chavez J.C. Bispecific antibodies for non-Hodgkin’s lymphomas and multiple myeloma. Drugs Context. 2021;10:1–12. doi: 10.7573/dic.2021-2-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Clements J.D., Zhu M., Kuchimanchi M., Terminello B., Doshi S. Population pharmacokinetics of Blinatumomab in pediatric and adult patients with hematological malignancies. Clin. Pharmacokinet. 2019;59:463–474. doi: 10.1007/s40262-019-00823-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dufner V., Sayehli C.M., Chatterjee M., Hummel H.D., Gelbrich G., Bargou R.C., Goebeler M.-E. Long-term outcome of patients with relapsed/refractory B-cell non-hodgkin lymphoma treated with blinatumomab. Blood Adv. 2019;3:2491–2498. doi: 10.1182/bloodadvances.2019000025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chu Y., Zhou X., Wang X. Antibody-drug conjugates for the treatment of lymphoma: Clinical advances and latest progress. J. Hematol. Oncol. 2021;14:88. doi: 10.1186/s13045-021-01097-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pro B., Advani R., Brice P., Bartlett N.L., Rosenblatt J.D., Illidge T., Matous J., Ramchandren R., Fanale M., Connors J.M., et al. Five-year results of brentuximab vedotin in patients with relapsed or refractory systemic anaplastic large cell lymphoma. Blood. 2017;130:2709–2717. doi: 10.1182/blood-2017-05-780049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.van der Weyden C.A., Pileri S.A., Feldman A.L., Whisstock J., Prince H.M. Understanding CD30 biology and therapeutic targeting: A historical perspective providing insight into future directions. Blood Cancer J. 2017;7:e603. doi: 10.1038/bcj.2017.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Okeley N.M., Miyamoto J.B., Zhang X., Sanderson R.J., Benjamin D.R., Sievers E.L., Senter P.D., Alley S.C. Intracellular activation of SGN-35, a potent anti-cd30 antibody-drug conjugate. Clin. Cancer Res. 2010;16:888–897. doi: 10.1158/1078-0432.CCR-09-2069. [DOI] [PubMed] [Google Scholar]
- 44.Sekimizu M., Iguchi A., Mori T., Koga Y., Kada A., Saito A.M., Horibe K. Phase I clinical study of Brentuximab Vedotin (SGN-35) involving children with recurrent or refractory CD30-positive hodgkin’s lymphoma or systemic anaplastic large cell lymphoma: Rationale, design and methods of BV-HLALCL study: Study protocol. BMC Cancer. 2018;18:122. doi: 10.1186/s12885-018-4042-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lowe E.J., Reilly A.F., Lim M.S., Gross T.G., Saguilig L., Barkauskas D.A., Wu R., Alexander S., Bollard C.M. Brentuximab Vedotin in combination with chemotherapy for pediatric patients with ALK+ ALCL: Results of cog trial ANHL12P1. Blood. 2021;137:3595–3603. doi: 10.1182/blood.2020009806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Locatelli F., Mauz-Koerholz C., Neville K., Llort A., Beishuizen A., Daw S., Pillon M., Aladjidi N., Klingebiel T., Landman-Parker J., et al. Brentuximab Vedotin for paediatric relapsed or refractory Hodgkin’s lymphoma and anaplastic large-cell lymphoma: A Multicentre, open-label, phase 1/2 study. Lancet Haematol. 2018;5:450–461. doi: 10.1016/S2352-3026(18)30153-4. [DOI] [PubMed] [Google Scholar]
- 47.Solimando A.G., Ribatti D., Vacca A., Einsele H. Targeting B-cell non Hodgkin Lymphoma: New and old tricks. Leuk. Res. 2016;42:93–104. doi: 10.1016/j.leukres.2015.11.001. [DOI] [PubMed] [Google Scholar]
- 48.Yurkiewicz I.R., Muffly L., Liedtke M. Inotuzumab Ozogamicin: A CD22 mab–drug conjugate for adult relapsed or refractory B-cell precursor Acute lymphoblastic leukemia. Drug Des. Devel. Ther. 2018;12:2293–2300. doi: 10.2147/DDDT.S150317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Inotuzumab Ozogamicin in Treating Younger Patients with B-Lymphoblastic Lymphoma or Relapsed or Refractory CD22 Positive B Acute Lymphoblastic Leukemia. [(accessed on 13 January 2022)]; Available online: https://clinicaltrials.gov/ct2/show/NCT02981628.
- 50.Sehn L.H., Herrera A.F., Flowers C.R., Kamdar M.K., McMillan A., Hertzberg M., Assouline S., Kim T.M., Kim W.S., Ozcan M., et al. Polatuzumab vedotin in relapsed or refractory diffuse large B-cell lymphoma. J. Clin. Oncol. 2020;38:155–165. doi: 10.1200/JCO.19.00172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Autologous Stem Cell Transplant Followed by Polatuzumab Vedotin in Patients with B-Cell Non-Hodgkin and Hodgkin Lymphoma. [(accessed on 13 January 2022)]; Available online: https://clinicaltrials.gov/ct2/show/NCT04491370.
- 52.Safety and Preliminary Efficacy of JBH492 Monotherapy in Patients with Chronic Lymphocytic Leukemia (CLL) and Non-Hodgkin’s Lymphoma (NHL) [(accessed on 13 January 2022)]; Available online: https://clinicaltrials.gov/ct2/show/NCT04240704.
- 53.Marofi F., Rahman H.S., Achmad M.H., Sergeevna K.N., Suksatan W., Abdelbasset W.K., Mikhailova M.V., Shomali N., Yazdanifar M., Hassanzadeh A., et al. A deep insight into CAR-T cell therapy in Non-Hodgkin Lymphoma: Application, opportunities, and future directions. Front. Immunol. 2021;12:681984. doi: 10.3389/fimmu.2021.681984. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 54.Wysocki P.J. Monoclonal antibodies in medical oncology—Mechanism of action. Oncol. Clin. Pract. 2014;10:175–183. [Google Scholar]
- 55.Tong J.T., Harris P.W., Brimble M.A., Kavianinia I. An insight into FDA approved antibody-drug conjugates for cancer therapy. Molecules. 2021;26:5847. doi: 10.3390/molecules26195847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhao Z., Chen Y., Francisco N.M., Zhang Y., Wu M. The application of CAR-T cell therapy in hematological malignancies: Advantages and challenges. Acta Pharm. Sin. B. 2018;8:539–551. doi: 10.1016/j.apsb.2018.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Du J., Zhang Y. Sequential anti-cd19, 22, and 20 autologous chimeric antigen receptor T-cell (CAR-T) treatments of a child with relapsed refractory Burkitt lymphoma: A case report and literature review. J. Cancer Res. Clin. Oncol. 2020;146:1575–1582. doi: 10.1007/s00432-020-03198-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chu Y., Gardenswartz A., Termuhlen A.M., Cairo M.S. Advances in cellular and humoral immunotherapy—Implications for the treatment of poor risk childhood, adolescent, and Young Adult B-cell non-hodgkin lymphoma. Br. J. Haematol. 2019;185:1055–1070. doi: 10.1111/bjh.15753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Poh W.J., Wee C.P., Gao Z. DNA methyltransferase activity assays: Advances and challenges. Theranostics. 2016;6:369–391. doi: 10.7150/thno.13438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Nepali K., Liou J.-P. Recent developments in Epigenetic cancer therapeutics: Clinical advancement and emerging trends. J. Biomed. Sci. 2021;28:27. doi: 10.1186/s12929-021-00721-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Decitabine-Primed Tandem CD19/CD20 CAR T Cells Plus Epigenetic Agents in Aggressive r/r B-NHL with Huge Tumor Burden. [(accessed on 22 January 2022)]; Available online: https://clinicaltrials.gov/ct2/show/NCT04553393.
- 62.A Clinical Trial of Decitabine in Relapsed or Refractory T-Lymphoblastic Lymphoma. [(accessed on 22 January 2022)]; Available online: https://clinicaltrials.gov/ct2/show/NCT03558412.
- 63.Prince H.M., Bishton M.J., Harrison S.J. Clinical studies of histone deacetylase inhibitors. Clin. Cancer Res. 2009;15:3958–3969. doi: 10.1158/1078-0432.CCR-08-2785. [DOI] [PubMed] [Google Scholar]
- 64.Drummond D.C., Noble C.O., Kirpotin D.B., Guo Z., Scott G.K., Benz C.C. Clinical development of histone deacetylase inhibitors as anticancer agents. Annu. Rev. Pharmacol. Toxicol. 2005;45:495–528. doi: 10.1146/annurev.pharmtox.45.120403.095825. [DOI] [PubMed] [Google Scholar]
- 65.Yang X.-J., Seto E. HATs and hdacs: From structure, function and regulation to novel strategies for therapy and prevention. Oncogene. 2007;26:5310–5318. doi: 10.1038/sj.onc.1210599. [DOI] [PubMed] [Google Scholar]
- 66.Chen I.-C., Sethy B., Liou J.-P. Recent update of HDAC inhibitors in lymphoma. Front. Cell Dev. Biol. 2020;8:906. doi: 10.3389/fcell.2020.576391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Vorinostat and Combination Chemotherapy before Donor Stem Cell Transplantation for the Treatment of Relapsed Aggressive B-Cell or T-Cell Non-Hodgkin Lymphoma. [(accessed on 22 January 2022)]; Available online: https://clinicaltrials.gov/ct2/show/NCT04220008.
- 68.Vorinostat for Graft vs. Host Disease Prevention in Children, Adolescents and Young Adults Undergoing Allogeneic Blood and Marrow Transplantation. [(accessed on 22 January 2022)]; Available online: https://clinicaltrials.gov/ct2/show/NCT03842696.
- 69.van Tilburg C.M., Milde T., Witt R., Ecker J., Hielscher T., Seitz A., Schenk J.-P., Buhl J.L., Riehl D., Frühwald M.C., et al. Phase I/II intra-patient dose escalation study of Vorinostat in children with relapsed solid tumor, lymphoma, or leukemia. Clin. Epigenetics. 2019;11:188. doi: 10.1186/s13148-019-0775-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Goldberg J., Sulis M.L., Bender J., Jeha S., Gardner R., Pollard J., Aquino V., Laetsch T., Winick N., Fu C., et al. A phase I study of Panobinostat in children with relapsed and refractory hematologic malignancies. Pediatr. Hematol. Oncol. J. 2020;37:465–474. doi: 10.1080/08880018.2020.1752869. [DOI] [PubMed] [Google Scholar]
- 71.Shi Y., Jia B., Xu W., Li W., Liu T., Liu P., Zhao W., Zhang H., Sun X., Yang H., et al. Chidamide in relapsed or refractory peripheral T cell lymphoma: A multicenter real-world study in China. J. Hematol. Oncol. 2017;10:69. doi: 10.1186/s13045-017-0439-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ji J., Liu Z., Kuang P., Dong T., Chen X., Li J., Zhang C., Liu J., Zhang L., Shen K., et al. A new conditioning regimen with chidamide, cladribine, Gemcitabine and busulfan significantly improve the outcome of high-risk or relapsed/refractory non-Hodgkin′s lymphomas. Int. J. Cancer. 2021;149:2075–2082. doi: 10.1002/ijc.33761. [DOI] [PubMed] [Google Scholar]
- 73.Wang Q., Jiang Y., Zhu Q., Duan Y., Chen X., Xu T., Jin Z., Li C., Wu D., Huang H. Clinical features and treatment outcomes of 14 patients with hepatosplenic γ δ T-cell lymphoma. J. Cancer Res. Clin. Oncol. 2021;147:3441–3445. doi: 10.1007/s00432-021-03587-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Makena M.R., Nguyen T.H., Koneru B., Hindle A., Chen W.-H., Verlekar D.U., Kang M.H., Reynolds C.P. Vorinostat and FENRETINIDE synergize in preclinical models of T-cell lymphoid malignancies. Anti Cancer. Drugs. 2020;32:34–43. doi: 10.1097/CAD.0000000000001008. [DOI] [PubMed] [Google Scholar]
- 75.Panuciak K., Margas M., Makowska K., Lejman M. Insights into modern therapeutic approaches in pediatric acute leukemias. Cells. 2022;11:139. doi: 10.3390/cells11010139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kabir T.F., Chauhan A., Anthony L., Hildebrandt G.C. Immune checkpoint inhibitors in pediatric solid tumors: Status in 2018. Ochsner J. 2018;18:370–376. doi: 10.31486/toj.18.0055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Topalian S.L., Hodi F.S., Brahmer J.R., Gettinger S.N., Smith D.C., McDermott D.F., Powderly J.D., Carvajal R.D., Sosman J.A., Atkins M.B., et al. Safety, activity, and immune correlates of anti–PD-1 antibody in cancer. N. Engl. J. Med. 2012;366:2443–2454. doi: 10.1056/NEJMoa1200690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Nivolumab with or without Ipilimumab in Treating Younger Patients with Recurrent or Refractory Solid Tumors or Sarcomas. [(accessed on 22 January 2022)]; Available online: https://clinicaltrials.gov/ct2/show/NCT02304458.
- 79.Davis K.L., Fox E., Merchant M.S., Reid J.M., Kudgus R.A., Liu X., Minard C.G., Voss S., Berg S.L., Weigel B.J., et al. Nivolumab in children and young adults with relapsed or refractory solid tumours or lymphoma (ADVL1412): A Multicentre, open-label, single-arm, phase 1–2 trial. Lancet Oncol. 2020;21:541–550. doi: 10.1016/S1470-2045(20)30023-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Rigaud C., Abbou S., Minard-Colin V., Geoerger B., Scoazec J.Y., Vassal G., Jaff N., Heuberger L., Valteau-Couanet D., Brugieres L. Efficacy of nivolumab in a patient with systemic refractory ALK+ anaplastic large cell lymphoma. Pediatr. Blood Cancer. 2017;65:e26902. doi: 10.1002/pbc.26902. [DOI] [PubMed] [Google Scholar]
- 81.Lesokhin A.M., Ansell S.M., Armand P., Scott E.C., Halwani A., Gutierrez M., Millenson M.M., Cohen A.D., Schuster S.J., Lebovic D., et al. Nivolumab in patients with relapsed or refractory hematologic malignancy: Preliminary results of a phase IB study. J. Clin. Oncol. 2016;34:2698–2704. doi: 10.1200/JCO.2015.65.9789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Shen J., Li S., Medeiros L.J., Lin P., Wang S.A., Tang G., Yin C.C., You M.J., Khoury J.D., Iyer S.P., et al. PD-L1 expression is associated with ALK positivity and STAT3 activation, but not outcome in patients with systemic anaplastic large cell lymphoma. Mod. Pathol. 2019;33:324–333. doi: 10.1038/s41379-019-0336-3. [DOI] [PubMed] [Google Scholar]
- 83.Herbst R.S., Soria J.-C., Kowanetz M., Fine G.D., Hamid O., Gordon M.S., Sosman J.A., McDermott D.F., Powderly J.D., Gettinger S.N., et al. Predictive correlates of response to the anti-PD-L1 antibody MPDL3280A in cancer patients. Nature. 2014;515:563–567. doi: 10.1038/nature14011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Geoerger B., Zwaan C.M., Marshall L.V., Michon J., Bourdeaut F., Casanova M., Corradini N., Rossato G., Farid-Kapadia M., Shemesh C.S., et al. Atezolizumab for children and young adults with previously treated solid tumours, non-hodgkin lymphoma, and Hodgkin Lymphoma (imatrix): A multicentre phase 1–2 study. Lancet Oncol. 2020;21:134–144. doi: 10.1016/S1470-2045(19)30693-X. [DOI] [PubMed] [Google Scholar]
- 85.Pasini D., Di Croce L. Emerging roles for Polycomb proteins in cancer. Curr. Opin. Genet. Dev. 2016;36:50–58. doi: 10.1016/j.gde.2016.03.013. [DOI] [PubMed] [Google Scholar]
- 86.Simon J.A., Lange C.A. Roles of the EZH2 histone methyltransferase in cancer epigenetics. Mutat. Res. Mol. Mech. Mutagen. 2008;647:21–29. doi: 10.1016/j.mrfmmm.2008.07.010. [DOI] [PubMed] [Google Scholar]
- 87.Garapaty-Rao S., Nasveschuk C., Gagnon A., Chan E.Y., Sandy P., Busby J., Balasubramanian S., Campbell R., Zhao F., Bergeron L., et al. Identification of EZH2 and EZH1 small molecule inhibitors with selective impact on diffuse large B cell lymphoma cell growth. Chem. Biol. 2013;20:1329–1339. doi: 10.1016/j.chembiol.2013.09.013. [DOI] [PubMed] [Google Scholar]
- 88.Morin R.D., Johnson N.A., Severson T.M., Mungall A.J., An J., Goya R., Paul J.E., Boyle M., Woolcock B.W., Kuchenbauer F., et al. Somatic mutations altering EZH2 (TYR641) in follicular and diffuse large B-cell lymphomas of germinal-center origin. Nat. Genet. 2010;42:181–185. doi: 10.1038/ng.518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Su I., Basavaraj A., Krutchinsky A.N., Hobert O., Ullrich A., Chait B.T., Tarakhovsky A. EZH2 controls B cell development through histone H3 methylation and igh rearrangement. Nat. Immunol. 2002;4:124–131. doi: 10.1038/ni876. [DOI] [PubMed] [Google Scholar]
- 90.Béguelin W., Popovic R., Teater M., Jiang Y., Bunting K.L., Rosen M., Shen H., Yang S.N., Wang L., Ezponda T., et al. EZH2 is required for germinal center formation and somatic EZH2 mutations promote lymphoid transformation. Cancer Cell. 2013;23:677–692. doi: 10.1016/j.ccr.2013.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Lunning M.A., Green M.R. Mutation of chromatin modifiers; an emerging hallmark of germinal center B-cell lymphomas. Blood Cancer J. 2015;5:361. doi: 10.1038/bcj.2015.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Lue J.K., Amengual J.E. Emerging EZH2 inhibitors and their application in lymphoma. Curr. Hematol. Malig. Rep. 2018;13:369–382. doi: 10.1007/s11899-018-0466-6. [DOI] [PubMed] [Google Scholar]
- 93.Li B., Chng W.-J. EZH2 abnormalities in lymphoid malignancies: Underlying mechanisms and therapeutic implications. J. Hematol. Oncol. 2019;12:118. doi: 10.1186/s13045-019-0814-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Qi W., Chan H., Teng L., Li L., Chuai S., Zhang R., Zeng J., Li M., Fan H., Lin Y., et al. Selective inhibition of EZH2 by a small molecule inhibitor blocks tumor cells proliferation. Proc. Natl. Acad. Sci. USA. 2012;109:21360–21365. doi: 10.1073/pnas.1210371110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Konze K.D., Ma A., Li F., Barsyte-Lovejoy D., Parton T., MacNevin C.J., Liu F., Gao C., Huang X.-P., Kuznetsova E., et al. An orally bioavailable chemical probe of the lysine methyltransferases EZH2 and EZH1. ACS Chem. Biol. 2013;8:1324–1334. doi: 10.1021/cb400133j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Honma D., Kanno O., Watanabe J., Kinoshita J., Hirasawa M., Nosaka E., Shiroishi M., Takizawa T., Yasumatsu I., Horiuchi T., et al. Novel orally bioavailable ezh1/2 dual inhibitors with greater antitumor efficacy than an EZH2 selective inhibitor. Cancer Sci. 2017;108:2069–2078. doi: 10.1111/cas.13326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Vaswani R.G., Gehling V.S., Dakin L.A., Cook A.S., Nasveschuk C.G., Duplessis M., Iyer P., Balasubramanian S., Zhao F., Good A.C., et al. Identification of (R)-N-((4-methoxy-6-methyl-2-oxo-1,2-dihydropyridin-3-yl)methyl)-2-METHYL-1-(1-(1-(2,2,2-trifluoroethyl)piperidin-4-yl)ethyl)-1h-indole-3-carboxamide (CPI-1205), a potent and selective inhibitor of histone methyltransferase EZH2, suitable for phase I clinical trials for B-cell lymphomas. J. Med. Chem. 2016;59:9928–9941. doi: 10.1021/acs.jmedchem.6b01315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Maruyama D., Tobinai K., Makita S., Ishida T., Kusumoto S., Ishitsuka K., Yoshimitsu M., Imaizumi Y., Sawayama Y., Takeuchi S., et al. First-in-Human Study of the EZH1/2 Dual Inhibitor DS-3201b in Patients with Relapsed or Refractory Non-Hodgkin Lymphomas—Preliminary Results. Blood. 2017;130:4070. [Google Scholar]
- 99.Tazemetostat in Treating Patients with Relapsed or Refractory Advanced Solid Tumors, Non-Hodgkin Lymphoma, or Histiocytic Disorders with EZH2, SMARCB1, or SMARCA4 Gene Mutations (a Pediatric MATCH Treatment Trial) [(accessed on 6 January 2022)]; Available online: https://clinicaltrials.gov/ct2/show/NCT03213665.
- 100.Gulati N., Béguelin W., Giulino-Roth L. Enhancer of zeste homolog 2 (EZH2) inhibitors. Leuk. Lymphoma. 2018;59:1574–1585. doi: 10.1080/10428194.2018.1430795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Open-Label, Multicenter, Phase 1/2 Study of Tazemetostat (EZH2 Histone Methyl Transferase [HMT] Inhibitor) as a Single Agent in Subjects with Adv. Solid Tumors or with B-Cell Lymphomas and Tazemetostat in Combination with Prednisolone in Subjects with DLBC. [(accessed on 6 January 2022)]; Available online: https://clinicaltrials.gov/ct2/show/NCT01897571.
- 102.Targeted Therapy Directed by Genetic Testing in Treating Pediatric Patients with Relapsed or Refractory Advanced Solid Tumors, Non-Hodgkin Lymphomas, or Histiocytic Disorders (The Pediatric MATCH Screening Trial) [(accessed on 6 January 2022)]; Available online: https://clinicaltrials.gov/ct2/show/NCT03155620.
- 103.Lee S., Urman A., Desai P. Emerging drug profile: Krebs Cycle and cancer: IDH mutations and therapeutic implications. Leuk. Lymphoma. 2019;60:2635–2645. doi: 10.1080/10428194.2019.1602260. [DOI] [PubMed] [Google Scholar]
- 104.Churchill H., Naina H., Boriack R., Rakheja D., Chen W. Discordant intracellular and plasma D-2-hydroxyglutarate levels in a patient with IDH2 mutated angioimmunoblastic T-cell lymphoma. Int. J. Clin. Exp. Pathol. 2015;8:11753–11759. [PMC free article] [PubMed] [Google Scholar]
- 105.Lin A.-P., Abbas S., Kim S.-W., Ortega M., Bouamar H., Escobedo Y., Varadarajan P., Qin Y., Sudderth J., Schulz E., et al. D2HGDH regulates alpha-ketoglutarate levels and dioxygenase function by modulating IDH2. Nat. Commun. 2015;6:7768. doi: 10.1038/ncomms8768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.FDA Granted Regular Approval to Enasidenib for the Treatment of Relapsed or Refractory AML. [(accessed on 9 January 2022)]; Available online: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-granted-regular-approval-enasidenib-treatment-relapsed-or-refractory-aml.
- 107.FDA Approves Ivosidenib for Relapsed or Refractory Acute Myeloid Leukemia. [(accessed on 9 January 2022)]; Available online: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-ivosidenib-relapsed-or-refractory-acute-myeloid-leukemia.
- 108.FDA Approves Ivosidenib as First-Line Treatment for AML with IDH1 Mutation. [(accessed on 9 January 2022)]; Available online: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-ivosidenib-first-line-treatment-aml-idh1-mutation.
- 109.Ivosidenib in Treating Patients with Advanced Solid Tumors, Lymphoma, or Histiocytic Disorders with IDH1 Mutations (a Pediatric MATCH Treatment Trial) [(accessed on 9 January 2022)]; Available online: https://clinicaltrials.gov/ct2/show/NCT04195555.
- 110.Hata A.N., Engelman J.A., Faber A.C. The BCL2 family: Key mediators of the apoptotic response to targeted anticancer therapeutics. Cancer Discov. 2015;5:475–487. doi: 10.1158/2159-8290.CD-15-0011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Nann D., Ramis-Zaldivar J.E., Müller I., Gonzalez-Farre B., Schmidt J., Egan C., Salmeron-Villalobos J., Clot G., Mattern S., Otto F., et al. Follicular lymphoma t(14;18)-negative is genetically a heterogeneous disease. Blood Adv. 2020;4:5652–5665. doi: 10.1182/bloodadvances.2020002944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Galteland E., Sivertsen E.A., Svendsrud D.H., Smedshammer L., Kresse S.H., Meza-Zepeda L.A., Myklebost O., Suo Z., Mu D., DeAngelis P.M., et al. Translocation T(14;18) and gain of chromosome 18/BCL2: Effects on BCL2 expression and apoptosis in B-cell non-Hodgkin′s lymphomas. Leukemia. 2005;19:2313–2323. doi: 10.1038/sj.leu.2403954. [DOI] [PubMed] [Google Scholar]
- 113.Iqbal J., Sanger W.G., Horsman D.E., Rosenwald A., Pickering D.L., Dave B., Dave S., Xiao L., Cao K., Zhu Q., et al. BCL2 translocation defines a unique tumor subset within the Germinal Center B-cell-like diffuse large B-cell lymphoma. Am. J. Pathol. 2004;165:159–166. doi: 10.1016/S0002-9440(10)63284-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Monni O., Joensuu H., Franssila K., Klefstrom J., Alitalo K., Knuutila S. BCL2 overexpression associated with chromosomal amplification in diffuse large B-cell lymphoma. Blood. 1997;90:1168–1174. doi: 10.1182/blood.V90.3.1168. [DOI] [PubMed] [Google Scholar]
- 115.de Vos S., Leonard J.P., Friedberg J.W., Zain J., Dunleavy K., Humerickhouse R., Hayslip J., Pesko J., Wilson W.H. Safety and efficacy of navitoclax, a bcl-2 and Bcl-XL inhibitor, in patients with relapsed or refractory lymphoid malignancies: Results from a phase 2A study. Leuk. Lymphoma. 2020;62:810–818. doi: 10.1080/10428194.2020.1845332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Souers A.J., Leverson J.D., Boghaert E.R., Ackler S.L., Catron N.D., Chen J., Dayton B.D., Ding H., Enschede S.H., Fairbrother W.J., et al. ABT-199, a potent and selective bcl-2 inhibitor, achieves antitumor activity while sparing platelets. Nat. Med. 2013;19:202–208. doi: 10.1038/nm.3048. [DOI] [PubMed] [Google Scholar]
- 117.Knight T., Luedtke D., Edwards H., Taub J.W., Ge Y. A delicate balance-the bcl-2 family and its role in apoptosis, oncogenesis, and cancer therapeutics. Biochem. Pharmacol. 2019;162:250–261. doi: 10.1016/j.bcp.2019.01.015. [DOI] [PubMed] [Google Scholar]
- 118.Davids M.S., Roberts A.W., Seymour J.F., Pagel J.M., Kahl B.S., Wierda W.G., Puvvada S., Kipps T.J., Anderson M.A., Salem A.H., et al. Phase I first-in-human study of Venetoclax in patients with relapsed or refractory Non-Hodgkin lymphoma. J. Clin. Oncol. 2017;35:826–833. doi: 10.1200/JCO.2016.70.4320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Davids M.S., Roberts A.W., Kenkre V.P., Wierda W.G., Kumar A., Kipps T.J., Boyer M., Salem A.H., Pesko J.C., Arzt J.A., et al. Long-term follow-up of patients with relapsed or refractory non–hodgkin lymphoma treated with Venetoclax in a phase I, first-in-human study. Clin. Cancer Res. 2021;27:4690–4695. doi: 10.1158/1078-0432.CCR-20-4842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.An Extension Study of Venetoclax for Subjects Who Have Completed a Prior Venetoclax Clinical Trial. [(accessed on 9 January 2022)]; Available online: https://clinicaltrials.gov/ct2/show/NCT03844048.
- 121.Expanded Access to Venetoclax. [(accessed on 9 January 2022)]; Available online: https://clinicaltrials.gov/ct2/show/NCT03123029.
- 122.Fenske T.S., Shah N.M., Kim K.M., Saha S., Zhang C., Baim A.E., Farnen J.P., Onitilo A.A., Blank J.H., Ahuja H., et al. A phase 2 study of weekly temsirolimus and bortezomib for relapsed or refractory B-cell non-Hodgkin Lymphoma: A wisconsin oncology network study. Cancer. 2015;121:3465–3471. doi: 10.1002/cncr.29502. [DOI] [PubMed] [Google Scholar]
- 123.Pullarkat V.A., Lacayo N.J., Jabbour E., Rubnitz J.E., Bajel A., Laetsch T.W., Leonard J., Colace S.I., Khaw S.L., Fleming S.A., et al. Venetoclax and Navitoclax in combination with chemotherapy in patients with relapsed or refractory acute lymphoblastic leukemia and lymphoblastic lymphoma. Cancer Discov. 2021;11:1440–1453. doi: 10.1158/2159-8290.CD-20-1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Tiran A.L., Claperon A., Davidson J., Starck J.-B., Diguarher T.L., Chanrion M., Mistry P., Wang Y., Monceau E., Bernhardt F., et al. Abstract 1276: Identification of S65487/VOB560 as a potent and selective intravenous 2nd-generation BCL-2 inhibitor active in wild-type and clinical mutants resistant to Venetoclax. Cancer Res. 2021;81:1276. [Google Scholar]
- 125.Armand P., Chen Y.-B., Redd R.A., Joyce R.M., Bsat J., Jeter E., Merryman R.W., Coleman K.C., Dahi P.B., Nieto Y., et al. PD-1 blockade with pembrolizumab for classical hodgkin lymphoma after Autologous Stem Cell Transplantation. Blood. 2019;134:22–29. doi: 10.1182/blood.2019000215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Hughes M.E., Landsburg D.J., Rubin D.J., Schuster S.J., Svoboda J., Gerson J.N., Namoglu E., Nasta S.D. Treatment of patients with relapsed/refractory Non-Hodgkin lymphoma with Venetoclax: A single-center evaluation of off-label use. Clin. Lymphoma Myeloma Leuk. 2019;19:791–798. doi: 10.1016/j.clml.2019.09.612. [DOI] [PubMed] [Google Scholar]
- 127.Roberts A.W., Davids M.S., Pagel J.M., Kahl B.S., Puvvada S.D., Gerecitano J.F., Kipps T.J., Anderson M.A., Brown J.R., Gressick L., et al. Targeting BCL2 with Venetoclax in relapsed chronic lymphocytic leukemia. N. Engl. J. Med. 2016;374:311–322. doi: 10.1056/NEJMoa1513257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Vogler M., Butterworth M., Majid A., Walewska R.J., Sun X.-M., Dyer M.J., Cohen G.M. Concurrent up-regulation of Bcl-XL and BCL2A1 induces approximately 1000-fold resistance to ABT-737 in chronic lymphocytic leukemia. Blood. 2009;113:4403–4413. doi: 10.1182/blood-2008-08-173310. [DOI] [PubMed] [Google Scholar]
- 129.Tahir S.K., Smith M.L., Hessler P., Rapp L.R., Idler K.B., Park C.H., Leverson J.D., Lam L.T. Potential mechanisms of resistance to Venetoclax and strategies to circumvent it. BMC Cancer. 2017;17:399. doi: 10.1186/s12885-017-3383-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Kerkhofs M., Vervloessem T., Stopa K.B., Smith V.M., Vogler M., Bultynck G. DLBCL cells with acquired resistance to Venetoclax are not sensitized to bird-2 but can be resensitized to Venetoclax through bcl-XL inhibition. Biomolecules. 2020;10:1081. doi: 10.3390/biom10071081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Espinos E., Lai R., Giuriato S. The dual role of autophagy in crizotinib-treated Alk+ ALCL: From the lymphoma cells drug resistance to their demise. Cells. 2021;10:2517. doi: 10.3390/cells10102517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Sharma G., Mota I., Mologni L., Patrucco E., Gambacorti-Passerini C., Chiarle R. Tumor resistance against ALK targeted therapy-where it comes from and where it goes. Cancers. 2018;10:62. doi: 10.3390/cancers10030062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Subbiah V., Kuravi S., Ganguly S., Welch D.R., Vivian C.J., Mushtaq M.U., Hegde A., Iyer S., Behrang A., Ali S.M., et al. Precision therapy with anaplastic lymphoma kinase inhibitor ceritinib in Alk-rearranged anaplastic large cell lymphoma. ESMO Open. 2021;6:100172. doi: 10.1016/j.esmoop.2021.100172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Greengard E., Mosse Y.P., Liu X., Minard C.G., Reid J.M., Voss S., Wilner K., Fox E., Balis F., Blaney S.M., et al. Safety, tolerability and pharmacokinetics of crizotinib in combination with cytotoxic chemotherapy for pediatric patients with refractory solid tumors or anaplastic large cell lymphoma (ALCL): A Children’s Oncology Group Phase 1 Consortium Study (ADVL1212) Cancer Chemother. Pharmacol. 2020;86:829–840. doi: 10.1007/s00280-020-04171-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Prokoph N., Probst N.A., Lee L.C., Monahan J.M., Matthews J.D., Liang H.-C., Bahnsen K., Montes-Mojarro I.A., Karaca Atabay E., Sharma G.G., et al. IL10RA modulates crizotinib sensitivity in NPM1-alk-positive anaplastic large cell lymphoma. Blood. 2020;136:1657–1669. doi: 10.1182/blood.2019003793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Crizotinib in Treating Younger Patients with Relapsed or Refractory Solid Tumors or Anaplastic Large Cell Lymphoma. [(accessed on 12 March 2021)]; Available online: https://clinicaltrials.gov/ct2/show/NCT00939770.
- 137.Prokoph N., Larose H., Lim M., Burke G., Turner S. Treatment options for paediatric anaplastic large cell lymphoma (ALCL): Current standard and beyond. Cancers. 2018;10:99. doi: 10.3390/cancers10040099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Lee D.H., Kim G.W., Kwon S.H. The hdac6-selective inhibitor is effective against non-hodgkin lymphoma and synergizes with ibrutinib in follicular lymphoma. Mol. Carcinog. 2019;58:944–956. doi: 10.1002/mc.22983. [DOI] [PubMed] [Google Scholar]
- 139.Bradshaw J.M. The SRC, Syk, and Tec family kinases: Distinct types of molecular switches. Cell. Signal. 2010;22:1175–1184. doi: 10.1016/j.cellsig.2010.03.001. [DOI] [PubMed] [Google Scholar]
- 140.Barf T., Covey T., Izumi R., van de Kar B., Gulrajani M., van Lith B., van Hoek M., de Zwart E., Mittag D., Demont D., et al. Acalabrutinib (ACP-196): A covalent Bruton tyrosine kinase inhibitor with a differentiated selectivity and in vivo potency profile. J. Pharmacol. Exp. Ther. 2017;363:240–252. doi: 10.1124/jpet.117.242909. [DOI] [PubMed] [Google Scholar]
- 141.Owen C., Berinstein N.L., Christofides A., Sehn L.H. Review of Bruton tyrosine kinase inhibitors for the treatment of relapsed or refractory mantle cell lymphoma. Curr. Oncol. 2019;26:233–240. doi: 10.3747/co.26.4345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Stephens D.M., Spurgeon S.E. Ibrutinib in mantle cell lymphoma patients: Glass Half Full? Evidence and opinion. Ther. Adv. Hematol. 2015;6:242–252. doi: 10.1177/2040620715592569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Herman S.E., Mustafa R.Z., Gyamfi J.A., Pittaluga S., Chang S., Chang B., Farooqui M., Wiestner A. Ibrutinib inhibits BCR and NF-ΚB signaling and reduces tumor proliferation in tissue-resident cells of patients with CLL. Blood. 2014;123:3286–3295. doi: 10.1182/blood-2014-02-548610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Dreyling M., Jurczak W., Jerkeman M., Silva R.S., Rusconi C., Trneny M., Offner F., Caballero D., Joao C., Witzens-Harig M., et al. Ibrutinib versus Temsirolimus in patients with relapsed or refractory mantle-cell lymphoma: An international, randomised, open-label, phase 3 study. Lancet. 2016;387:770–778. doi: 10.1016/S0140-6736(15)00667-4. [DOI] [PubMed] [Google Scholar]
- 145.Advani R.H., Buggy J.J., Sharman J.P., Smith S.M., Boyd T.E., Grant B., Kolibaba K.S., Furman R.R., Rodriguez S., Chang B.Y., et al. Bruton tyrosine kinase inhibitor Ibrutinib (PCI-32765) has significant activity in patients with relapsed/refractory B-cell malignancies. J. Clin. Oncol. 2013;31:88–94. doi: 10.1200/JCO.2012.42.7906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Hou K., Yu Z., Jia Y., Fang H., Shao S., Huang L., Feng Y. Efficacy and safety of ibrutinib in diffuse large B-cell lymphoma: A single-arm meta-analysis. Crit. Rev. Oncol. Hematol. 2020;152:103010. doi: 10.1016/j.critrevonc.2020.103010. [DOI] [PubMed] [Google Scholar]
- 147.Chu Y., Lee S., Shah T., Yin C., Barth M., Miles R.R., Ayello J., Morris E., Harrison L., Van de Ven C., et al. Ibrutinib significantly inhibited Bruton’s tyrosine kinase (BTK) phosphorylation, in-vitroproliferation and enhanced overall survival in a preclinical Burkitt lymphoma (BL) model. Oncoimmunology. 2018;8:1512455. doi: 10.1080/2162402X.2018.1512455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.A Safety and Efficacy Study of Ibrutinib in Pediatric and Young Adult Participants with Relapsed or Refractory Mature B-Cell Non-Hodgkin Lymphoma. [(accessed on 15 January 2022)]; Available online: https://clinicaltrials.gov/ct2/show/NCT02703272.
- 149.Pal Singh S., Dammeijer F., Hendriks R.W. Role of Bruton’s tyrosine kinase in B cells and malignancies. Mol. Cancer. 2018;17:57. doi: 10.1186/s12943-018-0779-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Wang M., Rule S., Zinzani P.L., Goy A., Casasnovas O., Smith S.D., Damaj G., Doorduijn J., Lamy T., Morschhauser F., et al. Acalabrutinib in relapsed or refractory mantle cell lymphoma (ace-ly-004): A single-arm, multicentre, phase 2 trial. Lancet. 2018;391:659–667. doi: 10.1016/S0140-6736(17)33108-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Tam C., Grigg A.P., Opat S., Ku M., Gilbertson M., Anderson M.A., Seymour J.F., Ritchie D.S., Dicorleto C., Dimovski B., et al. The BTK inhibitor, BGB-3111, is safe, tolerable, and highly active in patients with relapsed/ refractory B-cell malignancies: Initial report of a phase 1 first-in-human trial. Blood. 2015;126:832. doi: 10.1182/blood.V126.23.832.832. [DOI] [Google Scholar]
- 152.Walter H.S., Rule S.A., Dyer M.J., Karlin L., Jones C., Cazin B., Quittet P., Shah N., Hutchinson C.V., Honda H., et al. A phase 1 clinical trial of the selective BTK inhibitor Ono/GS-4059 in relapsed and refractory mature B-cell malignancies. Blood. 2016;127:411–419. doi: 10.1182/blood-2015-08-664086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Burger J.A. Bruton tyrosine kinase inhibitors. Cancer J. 2019;25:386–393. doi: 10.1097/PPO.0000000000000412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Wen T., Wang J., Shi Y., Qian H., Liu P. Inhibitors targeting Bruton’s tyrosine kinase in cancers: Drug development advances. Leukemia. 2020;35:312–332. doi: 10.1038/s41375-020-01072-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.FDA DISCO Burst Edition: FDA approvals of Brukinsa (Zanubrutinib), for Adult Patients with Relapsed or Refractory Marginal Zone Lymphoma, and Exkivity (Mobocertinib) for Adult Patients with Locally Advanced or Metastatic Non-Small Cell Lung Cancer with Epidermal Growth Factor Receptor Exon 20 Insertion Mutations. [(accessed on 15 January 2022)]; Available online: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-disco-burst-edition-fda-approvals-brukinsa-zanubrutinib-adult-patients-relapsed-or-refractory.
- 156.FDA Approves Zanubrutinib for Waldenström’s Macroglobulinemia. [(accessed on 15 January 2022)]; Available online: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-zanubrutinib-waldenstroms-macroglobulinemia.
- 157.Project Orbis: FDA Approves Acalabrutinib for CLL and SLL. [(accessed on 15 January 2022)]; Available online: https://www.fda.gov/drugs/resources-information-approved-drugs/project-orbis-fda-approves-acalabrutinib-cll-and-sll.
- 158.Paydas S. Management of adverse effects/toxicity of ibrutinib. Crit. Rev. Oncol. Hematol. 2019;136:56–63. doi: 10.1016/j.critrevonc.2019.02.001. [DOI] [PubMed] [Google Scholar]
- 159.Tam C.S., Anderson M.A., Pott C., Agarwal R., Handunnetti S., Hicks R.J., Burbury K., Turner G., Di Iulio J., Bressel M., et al. Ibrutinib plus venetoclax for the treatment of mantle-cell lymphoma. N. Engl. J. Med. 2018;378:1211–1223. doi: 10.1056/NEJMoa1715519. [DOI] [PubMed] [Google Scholar]
- 160.Burke G.A., Beishuizen A., Bhojwani D., Burkhardt B., Minard-Colin V., Norris R.E., Kabickova E., Pinarli F.G., Tacyildiz N., Howes A., et al. Ibrutinib plus CIT for R/R mature B-NHL in children (sparkle trial): Initial safety, pharmacokinetics, and efficacy. Leukemia. 2020;34:2271–2275. doi: 10.1038/s41375-020-0749-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Tan C.R., Abdul-Majeed S., Cael B., Barta S.K. Clinical pharmacokinetics and pharmacodynamics of Bortezomib. Clin. Pharmacokinet. 2018;58:157–168. doi: 10.1007/s40262-018-0679-9. [DOI] [PubMed] [Google Scholar]
- 162.O′Connor O.A., Czuczman M.S. Novel approaches for the treatment of NHL: Proteasome inhibition and immune modulation. Leuk. Lymphoma. 2008;49:59–66. doi: 10.1080/10428190802365033. [DOI] [PubMed] [Google Scholar]
- 163.Kunami N., Katsuya H., Nogami R., Ishitsuka K., Tamura K. Promise of combining a Bcl-2 family inhibitor with bortezomib or SAHA for adult T-cell leukemia/lymphoma. Anticancer Res. 2014;34:5287–5294. [PubMed] [Google Scholar]
- 164.Sánchez-Serrano I. Success in translational research: Lessons from the development of Bortezomib. Nat. Rev. Drug Discov. 2006;5:107–114. doi: 10.1038/nrd1959. [DOI] [PubMed] [Google Scholar]
- 165.Adams J., Palombella V.J., Sausville E.A., Johnson J., Destree A., Lazarus D.D., Maas J., Pien C.S., Prakash S., Elliott P.J. Proteasome inhibitors: A novel class of potent and effective antitumor agents. Cancer Res. 1999;59:2615–2622. [PubMed] [Google Scholar]
- 166.Raedler L. Velcade (Bortezomib) Receives 2 New FDA Indications: For Retreatment of Patients with Multiple Myeloma and for First-Line Treatment of Patients with Mantle-Cell Lymphoma. Am. Heal. Drug Benefits. 2015;8:135–140. [PMC free article] [PubMed] [Google Scholar]
- 167.FDA Approves VELCADE® (Bortezomib) for Injection for Previously Untreated Patients with Mantle Cell Lymphoma. [(accessed on 19 January 2022)]. Available online: https://www.takeda.com/newsroom/newsreleases/2014/fda-approves-velcade-bortezomib-for-injection-for-previously-untreated-patients-with-mantle-cell-lymphoma.
- 168.Ruan J., Martin P., Furman R.R., Lee S.M., Cheung K., Vose J.M., LaCasce A., Morrison J., Elstrom R., Ely S., et al. Bortezomib plus chop-rituximab for previously untreated diffuse large B-cell lymphoma and mantle cell lymphoma. J. Clin. Oncol. 2011;29:690–697. doi: 10.1200/JCO.2010.31.1142. [DOI] [PubMed] [Google Scholar]
- 169.Robak P., Robak T. Bortezomib for the treatment of hematologic malignancies: 15 years later. Drugs R. D. 2019;19:73–92. doi: 10.1007/s40268-019-0269-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Bose P., Batalo M.S., Holkova B., Grant S. Bortezomib for the treatment of non-Hodgkin’s lymphoma. Expert Opin. Pharmacother. 2014;15:2443–2459. doi: 10.1517/14656566.2014.965142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Gerecitano J., Goy A., Wright J., MacGregor-Cortelli B., Neylon E., Gonen M., Esseltine D., Boral A., Schenkein D., Busam K., et al. Drug-induced cutaneous vasculitis in patients with non-Hodgkin lymphoma treated with the novel proteasome inhibitor Bortezomib: A possible surrogate marker of response? Br. J. Haematol. 2006;134:391–398. doi: 10.1111/j.1365-2141.2006.06201.x. [DOI] [PubMed] [Google Scholar]
- 172.Horton T.M., Drachtman R.A., Chen L., Cole P.D., McCarten K., Voss S., Guillerman R.P., Buxton A., Howard S.C., Hogan S.M., et al. A phase 2 study of bortezomib in combination with ifosfamide/vinorelbine in paediatric patients and young adults with refractory/recurrent Hodgkin Lymphoma: A children’s oncology group study. Br. J. Haematol. 2015;170:118–122. doi: 10.1111/bjh.13388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Bortezomib, Ifosfamide, and Vinorelbine Tartrate in Treating Young Patients with Hodgkin’s Lymphoma That Is Recurrent or Did Not Respond to Previous Therapy. [(accessed on 19 January 2022)]; Available online: https://clinicaltrials.gov/ct2/show/study/NCT00381940.
- 174.Combination Chemotherapy with or without Bortezomib in Treating Patients with Classical Hodgkin Lymphoma That Has Returned or Does Not Respond to Prior Treatment. [(accessed on 19 January 2022)]; Available online: https://clinicaltrials.gov/ct2/show/study/NCT00967369.
- 175.Shokati T., Hartmann M., Davari B., Klawitter J., Klawitter J., Christians U. Temsirolimus Metabolic Pathways Revisited. Xenobiotica. 2019;50:640–653. doi: 10.1080/00498254.2019.1678793. [DOI] [PubMed] [Google Scholar]
- 176.Beevers C.S., Li F., Liu L., Huang S. Curcumin inhibits the mammalian target of rapamycin-mediated signaling pathways in cancer cells. Int. J. Cancer. 2006;119:757–764. doi: 10.1002/ijc.21932. [DOI] [PubMed] [Google Scholar]
- 177.Hay N., Sonenberg N. Upstream and downstream of mtor. Genes Dev. 2004;18:1926–1945. doi: 10.1101/gad.1212704. [DOI] [PubMed] [Google Scholar]
- 178.Robak T., Smolewski P., Robak P., Dreyling M. Mantle cell lymphoma: Therapeutic options in transplant-ineligible patients. Leuk. Lymphoma. 2019;60:2622–2634. doi: 10.1080/10428194.2019.1605511. [DOI] [PubMed] [Google Scholar]
- 179.Jurczak W., Ramanathan S., Giri P., Romano A., Mocikova H., Clancy J., Lechuga M., Casey M., Boni J., Giza A., et al. Comparison of two doses of intravenous temsirolimus in patients with relapsed/refractory mantle cell lymphoma. Leuk. Lymphoma. 2017;59:670–678. doi: 10.1080/10428194.2017.1357175. [DOI] [PubMed] [Google Scholar]
- 180.Schatz J.H. Targeting the PI3K/AKT/mtor pathway in Non-Hodgkin’s lymphoma: Results, biology, and development strategies. Curr. Oncol. Reports. 2011;13:398–406. doi: 10.1007/s11912-011-0187-7. [DOI] [PubMed] [Google Scholar]
- 181.Hess G., Herbrecht R., Romaguera J., Verhoef G., Crump M., Gisselbrecht C., Laurell A., Offner F., Strahs A., Berkenblit A., et al. Phase III study to evaluate Temsirolimus compared with investigator’s choice therapy for the treatment of relapsed or refractory mantle cell lymphoma. J. Clin. Oncol. 2009;27:3822–3829. doi: 10.1200/JCO.2008.20.7977. [DOI] [PubMed] [Google Scholar]
- 182.Fakhri B., Kahl B. Current and emerging treatment options for mantle cell lymphoma. Ther. Adv. Hematol. 2017;8:223–234. doi: 10.1177/2040620717719616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Hess G., Wagner K., Keller U., La Rosee P., Atta J., Hübel K., Lerchenmueller C., Schoendube D., Witzens-Harig M., Ruckes C., et al. Final results of a phase I/II trial of the combination bendamustine and Rituximab with Temsirolimus (BERT) in relapsed mantle cell lymphoma and follicular lymphoma. HemaSphere. 2020;4:398. doi: 10.1097/HS9.0000000000000398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Witzens-Harig M., Viardot A., Keller U., Wosniok J., Deuster O., Klemmer J., Geueke A.-M., Meißner J., Ho A.D., Atta J., et al. The mtor inhibitor temsirolimus added to rituximab combined with dexamethasone, Cytarabine, and CISPLATINUM (R-DHAP) for the treatment of patients with relapsed or refractory DLBCL—results from the phase-II storm trial. HemaSphere. 2021;5:636. doi: 10.1097/HS9.0000000000000636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Temsirolimus, Dexamethasone, Mitoxantrone Hydrochloride, Vincristine Sulfate, and Pegaspargase in Treating Young Patients with Relapsed Acute Lymphoblastic Leukemia or Non-Hodgkin Lymphoma. [(accessed on 22 January 2022)]; Available online: https://clinicaltrials.gov/ct2/show/study/NCT01403415.
- 186.A Trial of Temsirolimus with Etoposide and Cyclophosphamide in Children with Relapsed Acute Lymphoblastic Leukemia and Non-Hodgkins Lymphoma. [(accessed on 22 January 2022)]; Available online: https://clinicaltrials.gov/ct2/show/study/NCT01614197.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.