Abstract
Context
Adrenocortical carcinoma (ACC) is a rare endocrine malignancy that affects patients across the age spectrum. Although the overall survival in patients with ACC is poor, there is significant heterogeneity in terms of outcomes, presentation, and underlying genetic drivers.
Evidence Acquisition
This review is based on the evidence collected from primary research studies, expert reviews, and published guidelines. The studies were identified through PubMed search with key words “adrenocortical carcinoma,” “prognosis,” “pathology,” and “genetics.” The PubMed search was complemented by authors’ expertise, research, and clinical experience in the field of ACC.
Evidence Synthesis
Identification of biomarkers has been critical to gain better insight into tumor behavior and to guide therapeutic approach to patients. Tumor stage, resection status, and Ki67 are pathological tumor characteristics that have been identified as prognosticators in patients with ACC. Cortisol excess also correlates with worse prognosis. Clinical and histopathological characteristics help stratify patient outcomes, yet still up to 25% of patients have a different outcome than predicted. To bridge this gap, comprehensive genomic profiling studies have characterized additional profiles that correlate with clinical outcomes. In addition, studies of clinically applicable molecular markers are under way to further stratify outcomes in patients with ACC tumors.
Conclusions
Clinical predictors in combination with pathological markers play a critical role in the approach to patients with ACC. Recent advances in genetic prognosticators will help extend the stratification of these tumors and contribute to a personalized therapeutic approach to patients with ACC.
Keywords: pathology, genetics adrenocortical carcinoma
Clinical Case
The patient is 20-year-old white female who presented with rapid onset of weight gain, acne, and excess hair on the face and abdomen. She developed irregular menses in the past 3 to 6 months and was referred to obstetrics and gynecology for evaluation of possible polycystic ovarian syndrome. On physical examination, her weight has increased about 5 kg (11 lb) over the past 3 months. Her face was round with plethora and cystic acne. Terminal dark hairs were noted on the chin and abdomen, above and below the umbilicus. Patient had violaceous purple stria on her abdomen that she noted about 3 months ago, but no excess bruising or proximal muscle weakness. She was not taking any medication and had no significant medical history. Family history was unknown as she was adopted in infancy. Initial laboratory evaluation showed suppressed LH and FSH, with total testosterone of 420 ng/dL (8-60) and SHBG of 40 nmol/mL (16.8-125.2). Further evaluation included early afternoon dehydroepiandrosterone sulfate of 1218 mcg/dL (9.7-159), 11-deoxycortisol of 314 ng/dL (10-79 ng/dL), cortisol of 26.5 μg/dL (3.44-22.45), ACTH < 5 pg/mL (5-65), TSH of 3.4 U/mL (0.55-4.78),hemoglobin A1c of 6.0% (<6.5). The significant elevation of dehydroepiandrosterone sulfate and 11-deoxycortisol prompted abdominal computed tomography imaging which showed a large 12 × 10 × 15 cm heterogenous right adrenal mass. There was a significant mass effect on the liver and right kidney with invasion of the posterior hepatic lobe. A thrombus was noted in the inferior vena cava (IVC). Subsequently, additional laboratory tests were notable for normal plasma metanephrines, low aldosterone, suppressed renin, elevated 24-hour urine free cortisol of 2540 μg/d (<60), and elevated morning cortisol of 35.2 μg/dL (<1.8 μg/dL) following 1 mg dexamethasone the night before. Taken together, this made the conclusive diagnosis of adrenocortical carcinoma (ACC) causing Cushing syndrome and hyperandrogenism. The patient was referred to an expert center with a multidisciplinary team for care of patients with ACC and underwent right adrenalectomy, partial hepatectomy, right nephrectomy, and IVC thrombectomy. Pathologic review of the resected specimen showed high-grade (>20/50 high power fields [HPFs]), high Ki67 (53%) ACC with tumor extension involving the liver, renal vein, and IVC, with extensive viable tumor thrombus within vessel wall of the IVC. Atypical mitosis as well as necrosis was noted. Zero of 1 lymph nodes showed carcinoma. The modified Weiss criteria score was 7. Pathologic stage classification was pT4 pN0 pM0. The surgery was considered an R0 resection and the patient returned to clinic to discuss the approach and her prognosis.
Adrenocortical Carcinoma: Incidence and Distribution
In adults, ACC is a very uncommon, highly aggressive malignancy. In contrast to adrenal lesions incidentally diagnosed on up to 5% of high-resolution abdominal imaging examinations (1, 2), ACC has been reported with a US-wide annual incidence of 1 to 2 per million (3-7). This number is probably an underestimate (3-7). ACC, in adults, is more common in females (8) and can occur at any age with a peak in the fourth and fifth decades of life (9). Nevertheless, Surveillance, Epidemiology, and End Results data report a median age at diagnosis of 55 years with more than 60% of the cases diagnosed between 40 and 70 years of age (7). In adults, ACC has a heterogeneous clinical presentation, with 40% to 60% of patients presenting with signs and symptoms of adrenal steroid hormone excess (virilization, Cushing syndrome) or local mass effect (3).
ACC in children is also a very rare diagnosis. The incidence of pediatric ACC is 0.2 to 0.3 cases per million children per year in Surveillance, Epidemiology, and End Results data and represents 1.3% of all carcinomas in this age group (7, 10) compared with 0.02% to 0.2% of adult cancers, confirming a relatively high incidence early in life. Although rare, there is a remarkably high incidence of pediatric ACC in South and Southeast Brazil, ~15 times higher than that observed in the United States (11, 12). Overall, the incidence of pediatric adrenocortical tumors (ACTs; either adenomas or carcinomas) peaks between 0 and 4 years of age, although there is a second, smaller peak during adolescence (10). An increased incidence of ACTs in females, particularly among children younger than 3 years and older than 13 years, but not between 4 and 12 years, is consistently observed (10). At presentation, most children show signs and symptoms of virilization, with or without the presence of other adrenocortical hormone excess (13). Histological features are used to classify pediatric ACTs as adrenocortical adenomas or ACC; however, the histopathologic distinction between these 2 subtypes is often difficult (13, 14). Pediatric and adult ACTs are distinct in clinical manifestations, molecular alterations, and prognosis, suggesting that tumorigenesis is different in pediatric and adult patients and potentially dependent on cell of origin (15-19).
Genetic Predisposition to Adrenocortical Carcinoma
ACC has been reported as part of several familial cancer predisposition syndromes (Table 1). The relative increase in incidence in childhood ACC is mainly explained by germline TP53 variants, which are present in approximately 50% of children with ACC (15, 20, 21). TP53 germline pathogenic variants lead to Li Fraumeni syndrome (LFS). LFS is a rare autosomal dominant cancer predisposition syndrome, initially described in 1969 (22). Description of the original families showed an increased risk for breast cancer, soft-tissue sarcomas, ACC, and multiple neoplasms diagnosed early in life, supporting an underlying familial cancer predisposition syndrome. Twenty-one years after the initial clinical recognition of the syndrome, germline TP53 alterations were identified via a candidate gene approach investigating genes inactivated in sporadic forms of the associated cancers (23). Germline testing for TP53 is indicated for individuals with either some core cancers associated with LFS or a suggestive family history (24). Interestingly, an increasing number of rare or novel TP53 variants have been found through routine panel gene testing for cancer susceptibility even in apparently isolated single cancers. Most of these TP53 variants are hypomorphic, retaining significant wild-type activity in p53 protein function (12, 21) consistent with family histories of cancers associated less with the classic LFS and more with Li-Fraumeni-like syndrome criteria (12). Some of these novel variants are not yet proven to be pathogenic. Given the diversity of clinical presentations associated with germline TP53 variants and the limitation of determining pathogenicity for all variants, expansion of LFS concept to a wider cancer predisposition syndrome named heritable TP53-related cancer (hTP53rc) syndrome (25, 26) has been proposed to accommodate the rare and hypomorphic TP53 variants.
Table 1.
Genetic predisposition syndromes associated with adrenocortical carcinoma
| Syndrome | Mutated gene | Prevalence in ACC patients | Associated tumors and phenotype |
|---|---|---|---|
| Li-Fraumeni syndrome, Li-Fraumeni-like syndrome, and heritable TP53-related cancer. Founder TP53 haplotype |
TP53
TP53 p.R337H |
~50% in children ~5% in adults ~90% in children in Southern Brazil |
Early breast cancer, sarcoma, brain cancer, leukemia, choroid plexus carcinoma, lung cancer Choroid plexus carcinoma (children) Early breast cancer, sarcoma, lung cancer, stomach cancer (adults) |
| Lynch syndrome | MSH2, MSH6, MLH1, PMS2 | ~4% in adults | Colorectal cancer, endometrial cancer, ovarian cancer, pancreatic cancer, prostate cancer, urethral cancer, sebaceous gland cancer |
| Multiple endocrine neoplasia type 1 | MEN1 | ~1% in adults | Pituitary tumors, parathyroid adenomas, gastrointestinal and pancreatic neuroendocrine tumors, collagenoma, angiofibroma, benign adrenocortical tumors |
| Beckwith-Wiedemann syndrome | IGF2, CDKN1C, H19, dysregulation ofchromosome11p15 | Rare | Wilms’ tumor, hepatoblastoma, neuroblastoma, rhabdomyosarcoma, macroglossia, omphalocele, adrenocortical cytomegaly |
| Familial adenomatous polyposis | APC | Rare | Colorectal cancer and polyps, duodenal cancer and adenomas, thyroid cancer, osteoma, epidermoid cysts, adrenocortical adenomas |
The high incidence of pediatric ACC observed in Southeast Brazil is explained by the presence of the founder variant TP53 p.R337H in this population (11, 12, 27). In the largest population-based screening study, it was estimated that approximately 1 in every 300 individuals in Southeast Brazil are carriers of the founder p.R337H variant (28). In addition to ACC, pediatric choroid plexus carcinoma is commonly seen. In adult carriers, this variant is associated with early onset of breast cancer, sarcomas, lung cancer, stomach cancer, and a wide range of tumor types (29). Interestingly, though, cancer risk in TP53 p.R337H carriers includes a range from individuals and families with no history of cancer over a lifetime to others showing an apparently sporadic pattern of cancer presentation, and to those with a more aggressive cancer phenotype and positive family history (29). This represents a remarkable example of a reduced-penetrance allele. Recently, it was determined that approximately 70% of carriers of the TP53 p.R337H variant also harbor a nonsense variant in the tumor suppressor XAF1 (p.E134*) on the same chromosome (30). The constitutive haplotype harboring both mutant alleles likely contributes to a more aggressive cancer phenotype comparable to carriers of only the TP53 p.R337H variant (30). In this study, patients with ACC developed a second primary tumor associated with LFS only if they carried both mutant alleles in TP53 and XAF1, supporting the idea that XAF1 is a genetic modifier of the TP53 p.R337H variant (30).
The contribution of germline TP53 variants to ACC in adults is lower, yet still important. A German ACC registry with 103 adult patients reveals 4 patients with germline TP53 variants (31). In these 4 patients, the family history of cancer was not remarkable. There was an excess of adrenal malignancies in 1 family where the proband was a carrier of 2 variants in TP53 (31). Another proband was diagnosed at age 71 during ultrasound examination as part of colon cancer surveillance and had the p.R337H variant, not thought to be related to the founder allele observed in Brazil (31). A second study included 114 patients with ACC from the United States who were offered TP53 testing, independent of age at diagnosis or family history (32). Of 53 patients that completed genetic testing, 4 were positive (3 adults) for a germline TP53 variant (32). This case series illustrates the complexity to determine cancer risk in the presence of likely pathogenic TP53 variants because none of the families met classic LFS criteria.
In children, the risk for ACTs is also associated with Beckwith-Wiedemann syndrome (BWS) spectrum disorders (33). The underlying mechanism of BWS includes genetic and epigenetic alterations on chromosome 11p15 affecting the genes H19, IGF2, and CDKN1C. Dysregulation of 11p15 cell-cycle proteins and growth factors seem to be strongly associated with embryonal tumorigenesis. Pediatric patients with BWS have a low incidence of ACC (34). Interestingly, many patients with BWS have baseline histological changes in the adrenal cortex that are seen in ACC but without the presence of malignancy (34). In addition, 8% to 10% of nonsyndromic pediatric patients with ACTs, who have wild-type germline TP53, exhibit abnormalities in the germline chromosome 11p15, mostly uniparental paternal disomy (20), supporting consideration of genetic testing for chromosome 11p15 for pediatric patients negative for TP53.
ACC is also associated with other hereditary cancer syndromes. Lynch syndrome is caused by germline pathogenic variants in mismatch repair (MMR) genes (MSH2, MSH6, MLH1, PMS2) and is associated with hereditary colorectal cancer as the main malignancy, and ACC can be seen (35, 36). Lynch-associated ACC will often show loss of MMR protein staining and some degree of microsatellite instability, albeit less pronounced than in Lynch-associated colorectal cancer (35, 37). Multiple endocrine neoplasia type 1 (MEN1) predisposes to benign adrenal lesions (24%) (38), and ACC can be seen in up to 13.8% of MEN1-associated adrenal tumors in some cohorts (39). Familial adenomatous polyposis (FAP) (caused by germline APC variants) has been described as one of the hereditary syndromes predisposing to ACC based on a limited number of case reports (40, 41). More commonly adrenal lesions in patients with FAP are benign adenomas (16%) (42).
Cases of ACC have been potentially associated with other cancer predisposition syndromes. Recently, ACC was observed in 4 patients with germline pathogenic variants in succinate dehydrogenase subunit genes, which cause hereditary paraganglioma-pheochromocytoma syndrome (43). ACC also has been observed in patients with Carney complex (44, 45), hereditary breast and ovarian cancer from BRCA2 pathogenic variants (46), and Birt Hogg Dube syndrome (47). Whether these represent true causal associations or chance events remains uncertain. For example, some syndromes (such as Carney complex) are extremely rare and therefore conclusive numbers are difficult to derive. In other cases of common germline predisposition genes (e.g., BRCA2), proving a true association would require large numbers of ACCs in this population, and this epidemiological evidence is lacking, making this likely a chance event. Even molecular support, such as loss of heterozygosity, needs to be interpreted carefully because a subset of ACC tumors lose half their genome and undergo genome duplication (as described in The Cancer Genome Atlas study) (17).
Germline Genetic Testing for Patients With ACC
Clinical genetic testing for patients with ACC should be considered for 3 main reasons: (1) future surveillance for other syndrome associated cancers; (2) basis for family cascade testing; and (3) impact on treatment decisions. Overall, about 10% of ACC arises in patients with a hereditary predisposition (48). Given this, all patients with ACC are recommended to be counseled for clinical genetic testing per the American College of Medical Genetics/Genomics (49). A thorough review of personal medical history and family history for any syndrome-associated manifestation can be used as a basis for prioritization of specific genes, but the current practice is to use next-generation sequencing for multigene panels. Some panels are tailored to the particular cancer and others are broader. Based on the known predisposition syndromes for ACC, the panel should at least include TP53, MMR genes (MSH2, MSH6, MLH1, PMS2), MEN1, and APC. This panel might be reduced depending on the clinical history (e.g., a 60-year-old patient with MEN1 or FAP would have likely either developed primary hyperparathyroidism or colon polyps, respectively, earlier in life). On the other hand, the testing panel might be extended depending on clinical history (e.g., Carney complex) or family history, and might even include testing for other cancer predisposition syndromes, which do not necessarily predispose to ACC, based on family history.
Traditionally, the major impact for clinical genetic testing was to identify the at-risk individuals (proband and family members) for other cancers more commonly associated with the respective hereditary syndromes (e.g., annual colonoscopies for Lynch syndrome). More recently, however, there also has been a growing impact of clinical genetic testing on treatment decisions. For example, radiation therapy in patients harboring germline TP53 variants is associated with a high risk of secondary cancer development. Therefore, radiation therapy is to be avoided, if possible, in this population if not definitively necessary for tumor control. Another example is Lynch syndrome-associated cancers (MMR gene defects) that have become a tissue agnostic indication for immunotherapy, including ACC.
It is important to mention, that, because ACC is rare, even in those with the aforementioned cancer predisposition syndromes, there is no recommended routine screening for ACC except for those with germline variants in TP53 (50). Nevertheless, it is imperative to be aware of the associations if/when adrenal tumors are seen on imaging for other reasons.
Pathological Evaluation
The histopathologic classification of adrenocortical neoplasia is one of the most challenging areas in diagnostic surgical pathology. Part of the difficulty lies in their rarity, with only 1 to 2 patients diagnosed with ACC yearly per 1 million people (51). On the other hand, adrenocortical adenomas are relatively common in the general population (2, 52). It is this disparity that leads to a general lack of expertise in differentiating benign and malignant lesions. The pathologist also must differentiate these tumors from their histologic mimics, including other primary adrenal tumors and metastases to the adrenal gland (53). These lesions are relatively rare and may not be clinically apparent using standard laboratory and radiographic techniques.
Another hurdle to overcome pertains to the assessment of histopathologic variables. Assigning a risk category to an ACT (i.e., determining the risk of malignancy) relies on using a variety of complex and multiparametric scoring algorithms (54). Further complicating matters, the spectrum of pertinent findings differs depending upon the age of the patient. As previously discussed, ACC has a bimodal age distribution, with a peak in children around the third year and the second peak in adults in the fifth and sixth decades (55). In general, pediatric ACTs tend to display a greater range of atypical histologic features, and in combination with their more favorable prognosis, this results often in overclassification of ACTs as ACC, thus necessitating different means of stratification (56).
For adult ACTs, the first comprehensive guide for establishing a diagnosis of malignancy were the initial Weiss criteria, published in 1984 (57). Although a significant step forward in objectifying the process of risk stratification, some of Weiss’ original 9 risk assessment features suffered from poor reproducibility (58). This led to a trimmed version of the criteria used to estimate a Modified Weiss Score published by Aubert et al. in 2002 (59) (Table 2). Although not included as a specific parameter, Ki67 was first introduced in this publication to have utility in diagnosing ACC (59). The Modified Weiss Risk Assessment Model represents the current standard of care in adult disease (50).
Table 2.
Risk prediction models in pediatric and adult adrenocortical neoplasia
| Modified Weiss criteria (adult) | |
| Criteria | Points |
| > 5 mitoses/50 high power fields | 2 |
| Presence of < 25% clear tumor cells | 2 |
| Abnormal mitoses | 1 |
| Necrosis | 1 |
| Capsular invasion | 1 |
| Score (up to 7) | ≥ 3 associated with malignancy |
| Wieneke criteria (pediatric) | |
| Criteria | Points |
| Tumor weight > 400 g | 1 |
| Tumor size > 10.5 cm | 1 |
| Extension into the periadrenal soft tissue or adjacent organs | 1 |
| Invasion into vena cava | 1 |
| Venous invasion | 1 |
| Capsular invasion | 1 |
| Presence of tumor necrosis | 1 |
| > 15 mitoses/20 high power fields | 1 |
| Atypical mitoses | 1 |
| Score (up to 9) | ≥ 4 associated with malignancy |
In pediatric disease, the Wieneke criteria are the most commonly used for risk assessment (14) (Table 2). Although similar to the original Weiss criteria, the Wieneke model underscores some features to account for differences in pediatric cases. The Wieneke criteria include gross examination of tumor size and weight (14). These are particularly relevant in the setting of children with small/early-stage tumors because they tend to do exceptionally well, and these cases represent a scenario in which overclassification and overtreatment are a significant concern (60). The Wieneke model also uses a slightly different point allocation system, intentionally putting less weight on microscopic variables when compared with the Modified Weiss model given the variability seen in pediatric adrenal cortex features. The criteria of the Wieneke model have now been thoroughly vetted and are considered user friendly by general surgical pathologists (61).
Immunohistochemical staining has greatly aided in the classification of adrenal neoplasia, and ACTs have a reliable immunoprofile consisting of a combination of neuroendocrine and stromal markers (Table 3, Fig. 1). Recent advancements in antigens specific to steroidogenic tissue such as SF1 (62) have simplified the diagnostic process. SF1 is a transcription factor involved in adrenal development and is positive in 90% to 98% of ACTs (62, 63). Once a diagnosis of carcinoma is determined, there is evidence that expression of SF1 might correlate with overall survival in a stage-independent manner (62). Future studies are needed to validate this finding.
Table 3.
Immunohistochemical staining characteristics of adrenocortical neoplasia
| Universal utility | Pertinent negatives |
| Ki67 | Chromogranin |
| Recommended | HMB-45 |
| SF-1 | EMA |
| Synaptophysin | PAX8 |
| Cytokeratin cam 5.2 | |
| Inhibin | |
| Melan A | |
| Situational utility | |
| Beta-catenin | |
| p53 | |
| ATRX | |
| Mismatch repair (MLH1, MSH2, MSH6, PMS2) |
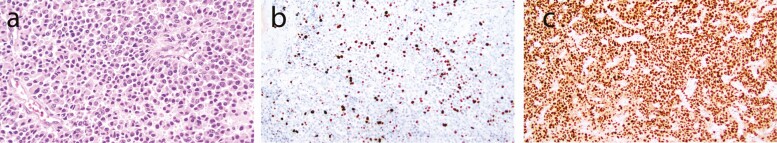
Figure 1.
Histologic and immunohistochemical studies in ACC. (A) Hematoxylin and eosin-stained section highlights a diffuse proliferation of rounded cells with abundant cytoplasm, compatible with adrenocortical neoplasia. (B) Ki67 (RRID:AB_2335949; https://antibodyregistry.org/search.php?q=AB_2335949) staining shows increased expression, > 10%, with regional heterogeneity. (C) SF1 (RRID: AB_2894906; https://antibodyregistry.org/search.php?q=AB_2894906) staining shows diffuse and strong nuclear positivity.
Determining Ki67 using immunohistochemistry has become a staple in clinical models for ACTs (50). Estimating Ki67 can be accomplished via various mechanisms, including pathologist estimation, formal nuclear counting, and more recently via digital image analysis (64). In general, these methods suffer from poor reproducibility, nonuniform access (digital analysis software), and significant interobserver variability (65). It is also notable that clinically relevant thresholds are not widely agreed upon. The most extensive study to date has provided support for significant thresholds of < 10%, 10% to 19%, and ≥ 20% (66). In general, pathologists should estimate Ki67 by whichever system they are most familiar with using and it is important to gain local experience with resulting numbers.
Pathological and Clinical Prognostic Factors and Stratification
The prognosis for patients with ACC varies. Although the overall 5-year survival is approximately 37% to 47% (51, 67), some long-term survivors, even with the presence of metastatic disease, have been reported, with approximately 5% of patients surviving more than 10 years after initial diagnosis (48, 68). Tumor stage is one of the most important prognostic factors. American Joint Committee on Cancer and European Network for the Study of Adrenal Tumors staging classification have been widely adopted and used in clinical practice (50, 69, 70). Table 4 shows survival data of patients with ACC correlating to disease stage at initial diagnosis. When a localized ACC is resected early, stage 1, the 5-year survival is 82%; however, because of rapid growth and metastatic potential, most ACC tumors are identified in advanced stages (50, 69). The limitation of the survival data includes relatively short mean follow-up, potential beneficial effect of new treatments because these studies were published, and a study bias that tends to retrospectively include all patients, hence producing a bias toward “noncured” patients presenting to specialized centers with disease recurrence (71) (Table 4).
Table 4.
Adrenocortical cancer stage and 5-y survival according AJCC and ENSAT
| Stage | AJCC/ENSAT staging | 5-y survivala | ||
|---|---|---|---|---|
| 1 | T1 | N0 | M0 | 82% (95% CI, 69-99) |
| 2 | T2 | N0 | M0 | 61% (95% CI, 51-69) |
| 3 | T1-2 | N1 | M0 | 50% (95% CI, 39-61) |
| T3-4 | N0-1 | M0 | ||
| 4 | T1-4 | N0-1 | M1 | 13% (95% CI, 5-21) |
AJCC, American Joint Committee on Cancer; ENSAT, European Network for the Study of Adrenal Tumors.
a Lughezzani G et al (69).
In addition to stage, presence of metastatic disease, resection status, tumor grade (assessed as mitoses per HPF and Ki67), and hormone production have been established as prognostic factors. Presence of metastatic disease and the number of distant organs involved at initial diagnosis strongly correlate with worse prognosis (48, 72). Regarding surgical outcome, surgeon expertise plays an important role on long-term prognosis as shown in several studies (73, 74). One study from the Netherlands showed significantly improved 5-year survival in adrenal centers of excellence (63%) compared with centers without ACC expertise (42%) (73). Even with surgical expertise, the local recurrence rate is still high at 20% to 35% even with an R0 resection (48). Another study examined a large cohort of almost 4000 patients and demonstrated that 9% of ACC patients undergoing surgery had microscopic disease identified and an additional 10% had evidence of macroscopic positive margins on pathological examination (51). Resection status correlated with worse 5-year survival of 21% after R1 and 10% after R2 resection compared with 49% after R0 resection (51).
Tumor grade as characterized by Ki67 is a major pathological prognostic factor in patients with ACC, with higher Ki67 correlating to worse outcomes. Although this relationship is a continuum and exact cutoffs are not well-defined, clinical guidelines consensus is that ACC tumors with Ki67 < 10% are considered low grade, whereas those with Ki67 > 10% are high-grade tumors (50, 66, 75). Ki67 often is variable across an individual tumor and is subject to observer bias as discussed previously. Although Ki67 can be evaluated either by manual counts or using image analysis software across the entire tumor, it is important that the Ki67 is reported as the area of the highest percent labeling (50, 65). When Ki67 analysis is not available, mitotic count can alternatively be a helpful prognosticator with > 20 mitoses per 50 HPFs indicative of high-grade ACC tumor (50).
Several other factors have been investigated to help predict prognosis. ACC tumors associated with hypercortisolism correlate with worse outcomes (76-78). Age at diagnosis also has been evaluated as potential prognosticator with advanced age associated with shorter survival in several studies (72, 79), although other studies did not find this correlation (80-82).
Recent ACC guidelines, which stratify patients based on these clinical and pathological prognostic factors, proposed 2 risk classes for localized disease: low/moderate-risk ACC that includes stage 1 or 2, Ki67 < 10%, and R0 resection, and high-risk ACC that includes stage 3, Ki67 > 10%, and R1 resection (50). Patients who present with stage 4 disease, have R2 resection, have high tumor burden, and high Ki67 usually have a worse prognosis (50). Furthermore, any disease recurrence within 6 months following the initial resection correlates with very poor prognosis (50). In addition to prognostic value, the 2-class ACC tumor stratification is used to guide treatment decision to assess the potential benefit of adjuvant chemotherapeutic treatment with mitotane following initial resection, with benefit in high-risk ACC and low or no benefit for patients with low-risk disease (50, 83).
In an effort to establish a useful clinical prognosticator tool, Libe and colleagues developed the Grade, Resection status, Age and Symptoms score parameters (GRAS) for patients with stage 3 and 4 ACC (75). They showed that the GRAS score adds additional stratification of patients even with advanced disease (75). Two recent retrospective studies examined the prognostic utility of the GRAS scoring system in stages 1 through 3 or 4 and showed that GRAS score stratified patients by overall survival and disease-free survival following the initial surgical resection (84, 85); however, the utility of the GRAS score compared with stage alone was not evaluated because even the larger study was underpowered for that analysis (85).
Future Direction: Molecular Pathology and Genetic Stratification Toward Personalized Treatment
Although staging and clinical and pathological factors have contributed to prognostic stratification of ACC patients, improved tools are still needed for a more personalized approach to prognosis and therapy. Initial transcriptome analysis of ACC identified activation of cell-cycle pathways in more aggressive tumors (86), and identified dysregulation of BUB1 mitotic kinase checkpoint serine/threonine kinase B (BUB1B) and PTEN induced kinase 1 (PINK1) as predictors of poor outcome (87, 88). Subsequently, 2 comprehensive pan-genomic studies in adult ACC showed 2 distinct cohorts associated with different outcomes (16, 17). ACCs associated with numerous mutations and widespread methylation at CpG sites within gene promoter and CpG island regions showed worse outcome compared with those with very little CpG island methylation, a pattern observed in normal tissue (16, 17). As a surrogate to the genomewide methylation signature, hypermethylation and consequent silencing of a single gene G0/G1 Switch 2 (G0S2) was recently reported as a potentially useful clinical tool to identify very aggressive ACC tumors in adult patients (89), with prospective studies under way.
Although there is not enough evidence to support generalized tumor testing for somatic changes to predict prognosis or treatment, it has become common practice at many institutions (90, 91). It is most often done either through in-house pipelines or commercial laboratories and often does not use matched patient germline DNA. Therefore, variants found through this analysis may reflect either somatic or germline variants, and patients with tumors identifying variants in known susceptibility genes should be referred for clinical genetic testing to identify potential pathogenic germline variants. The main value of somatic tumor testing is in potentially identifying tumor-specific targetable variants. For example, 2% to 7% of ACC have variants in cyclin dependent kinase 4 (CDK4) and up to 14% variants in cyclin dependent kinase inhibitor 2A, both offering therapeutic intervention with CDK4 inhibitors (90, 91). In addition, a recent study showed that up to 14% of ACC has an alteration in MMR genes, which implies that a significant number of patients would qualify for immunotherapy (91). Studies suggest that an average 59% of ACC contains potentially targetable variants (90, 91). Future studies will reveal how successful this individualized approach is and whether targeted somatic profiling combined with clinical and pathological predictors will improve prognostication capability in patients with ACC. For example, there are also ongoing studies exploring control of hypercortisolism and therefore glucocorticoid-induced immunosuppression, as an enhancing factor for response to immunotherapy (NCT04373265).
Back to the Patient
Based on clinical stage, pathological predictors, and hormonal activity, this patient was high risk for disease recurrence; therefore, adjuvant mitotane was recommended. The patient was counselled on nonendocrine (fatigue, diarrhea, vomiting, transaminitis) and endocrine (adrenal insufficiency, central hypothyroidism, hypogonadism, hypocholesteremia) adverse reactions to mitotane (92). The potential for a hereditary predisposing syndrome was discussed because the patient’s risk may be higher given her young age. The patient consented to a have commercial genetic testing for a hereditary cancer panel, which ultimately identified a pathogenic variant in the MSH2 consistent with Lynch syndrome. The patient was then screened per guidelines for other Lynch-associated tumors. Because she was adopted and did not have any children, there were no known blood relatives identified for cascade genetic testing. Given the known MSH2 variant, if the ACC was to recur in the future, she could be offered treatment with immunotherapy. While receiving mitotane, surveillance is recommended for at least 5 years to include laboratory evaluation and cross-sectional imaging of the chest, abdomen, and pelvis every 3 months for 2 years and if no recurrence, the interval can be expanded to every 12 months.
Conclusion
In the era of precision medicine, recent advances in histopathological and genetic classifications have significantly contributed to a more personalized approach to patients with ACC. Tumor stage and grade, as well as resection status, are the most currently used prognostic stratification tools. There are several emerging tools including tumor specific targeted next-generation sequencing and altered methylated gene targets that may offer new opportunities for improved tumor stratification for prognostic and therapeutic approaches. The studies are under way to develop clinically practical classification systems incorporating both histopathologic and molecular data to help stratify and improve outcomes in patients with ACC.
Glossary
Abbreviations
- ACC
adrenocortical cancer
- ACT
pediatric adrenocortical tumor
- BWS
Beckwith-Wiedemann syndrome
- FAP
familial adenomatous polyposis
- GRAS
Grade, Resection status, Age and Symptoms score
- HPF
high power field
- MEN-1
multiple endocrine neoplasia type 1
- MMR
mismatch repair.
Acknowledgments
Financial Support: This work was supported by NIH NCI K08CA222620 (to K.K.V.)
Additional Information
Disclosure Summary: The authors declare no potential conflicts of interest.
Data Availability
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
References
- 1. Mansmann G, Lau J, Balk E, Rothberg M, Miyachi Y, Bornstein SR. The clinically inapparent adrenal mass: update in diagnosis and management. Endocr Rev. 2004;25(2):309-340. [DOI] [PubMed] [Google Scholar]
- 2. Song JH, Chaudhry FS, Mayo-Smith WW. The incidental adrenal mass on CT: prevalence of adrenal disease in 1,049 consecutive adrenal masses in patients with no known malignancy. AJR Am J Roentgenol. 2008;190(5):1163-1168. [DOI] [PubMed] [Google Scholar]
- 3. Allolio B, Fassnacht M. Clinical review: adrenocortical carcinoma: clinical update. J Clin Endocrinol Metab. 2006;91(6):2027-2037. [DOI] [PubMed] [Google Scholar]
- 4. Fassnacht M, Allolio B. Clinical management of adrenocortical carcinoma. Best Pract Res Clin Endocrinol Metab. 2009;23(2):273-289. [DOI] [PubMed] [Google Scholar]
- 5. Kebebew E, Reiff E, Duh QY, Clark OH, McMillan A. Extent of disease at presentation and outcome for adrenocortical carcinoma: have we made progress? World J Surg. 2006;30(5):872-878. [DOI] [PubMed] [Google Scholar]
- 6. Sharma E, Dahal S, Sharma P, et al. The characteristics and trends in adrenocortical carcinoma: a United States population based study. J Clin Med Res. 2018;10(8):636-640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. McAteer JP, Huaco JA, Gow KW. Predictors of survival in pediatric adrenocortical carcinoma: a Surveillance, Epidemiology, and End Results (SEER) program study. J Pediatr Surg. 2013;48(5):1025-1031. [DOI] [PubMed] [Google Scholar]
- 8. Audenet F, Méjean A, Chartier-Kastler E, Rouprêt M. Adrenal tumours are more predominant in females regardless of their histological subtype: a review. World J Urol. 2013;31(5):1037-1043. [DOI] [PubMed] [Google Scholar]
- 9. Ng L, Libertino JM. Adrenocortical carcinoma: diagnosis, evaluation and treatment. J Urol. 2003;169(1):5-11. [DOI] [PubMed] [Google Scholar]
- 10. Michalkiewicz E, Sandrini R, Figueiredo B, et al. Clinical and outcome characteristics of children with adrenocortical tumors: a report from the International Pediatric Adrenocortical Tumor Registry. J Clin Oncol. 2004;22(5):838-845. [DOI] [PubMed] [Google Scholar]
- 11. Ribeiro RC, Sandrini F, Figueiredo B, et al. An inherited p53 mutation that contributes in a tissue-specific manner to pediatric adrenal cortical carcinoma. Proc Natl Acad Sci U S A. 2001;98(16):9330-9335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Latronico AC, Pinto EM, Domenice S, et al. An inherited mutation outside the highly conserved DNA-binding domain of the p53 tumor suppressor protein in children and adults with sporadic adrenocortical tumors. J Clin Endocrinol Metab. 2001;86(10):4970-4973. [DOI] [PubMed] [Google Scholar]
- 13. Pinto EM, Zambetti GP, Rodriguez-Galindo C. Pediatric adrenocortical tumours. Best Pract Res Clin Endocrinol Metab. 2020;34(3):101448. [DOI] [PubMed] [Google Scholar]
- 14. Wieneke JA, Thompson LD, Heffess CS. Adrenal cortical neoplasms in the pediatric population: a clinicopathologic and immunophenotypic analysis of 83 patients. Am J Surg Pathol. 2003;27(7):867-881. [DOI] [PubMed] [Google Scholar]
- 15. Pinto EM, Chen X, Easton J, et al. Genomic landscape of paediatric adrenocortical tumours. Nat Commun. 2015;6:6302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Assié G, Letouzé E, Fassnacht M, et al. Integrated genomic characterization of adrenocortical carcinoma. Nat Genet. 2014;46(6):607-612. [DOI] [PubMed] [Google Scholar]
- 17. Zheng S, Cherniack AD, Dewal N, et al. ; Cancer Genome Atlas Research Network . Comprehensive pan-genomic characterization of adrenocortical carcinoma. Cancer Cell. 2016;29(5):723-736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lalli E, Figueiredo BC. Pediatric adrenocortical tumors: what they can tell us on adrenal development and comparison with adult adrenal tumors. Front Endocrinol (Lausanne). 2015;6:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hammer GD, Parker KL, Schimmer BP. Minireview: transcriptional regulation of adrenocortical development. Endocrinology. 2005;146(3):1018-1024. [DOI] [PubMed] [Google Scholar]
- 20. Pinto EM, Rodriguez-Galindo C, Pounds SB, et al. Identification of clinical and biologic correlates associated with outcome in children with adrenocortical tumors without germline TP53 mutations: a St Jude Adrenocortical Tumor Registry and Children’s Oncology Group Study. J Clin Oncol. 2017;35(35):3956-3963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Wasserman JD, Novokmet A, Eichler-Jonsson C, et al. Prevalence and functional consequence of TP53 mutations in pediatric adrenocortical carcinoma: a Children’s Oncology Group study. J Clin Oncol. 2015;33(6):602-609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Li FP, Fraumeni JF Jr. Rhabdomyosarcoma in children: epidemiologic study and identification of a familial cancer syndrome. J Natl Cancer Inst. 1969;43(6):1365-1373. [PubMed] [Google Scholar]
- 23. Malkin D, Li FP, Strong LC, et al. Germ line p53 mutations in a familial syndrome of breast cancer, sarcomas, and other neoplasms. Science. 1990;250(4985):1233-1238. [DOI] [PubMed] [Google Scholar]
- 24. McBride KA, Ballinger ML, Killick E, et al. Li-Fraumeni syndrome: cancer risk assessment and clinical management. Nat Rev Clin Oncol. 2014;11(5):260-271. [DOI] [PubMed] [Google Scholar]
- 25. Evans DG, Woodward ER. New surveillance guidelines for Li-Fraumeni and hereditary TP53 related cancer syndrome: implications for germline TP53 testing in breast cancer. Fam Cancer. 2021;20(1):1-7. [DOI] [PubMed] [Google Scholar]
- 26. Frebourg T, Bajalica Lagercrantz S, Oliveira C, Magenheim R, Evans DG; European Reference Network GENTURIS . Guidelines for the Li-Fraumeni and heritable TP53-related cancer syndromes. Eur J Hum Genet. 2020;28(10):1379-1386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Pinto EM, Billerbeck AE, Villares MC, Domenice S, Mendonça BB, Latronico AC. Founder effect for the highly prevalent R337H mutation of tumor suppressor p53 in Brazilian patients with adrenocortical tumors. Arq Bras Endocrinol Metabol. 2004;48(5):647-650. [DOI] [PubMed] [Google Scholar]
- 28. Custódio G, Parise GA, Kiesel Filho N, et al. Impact of neonatal screening and surveillance for the TP53 R337H mutation on early detection of childhood adrenocortical tumors. J Clin Oncol. 2013;31(20):2619-2626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Pinto EM, Zambetti GP. What 20 years of research has taught us about the TP53 p.R337H mutation. Cancer. 2020;126(21):4678-4686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Pinto EM, Figueiredo BC, Chen W, et al. XAF1 as a modifier of p53 function and cancer susceptibility. Sci Adv. 2020;6(26):eaba3231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Herrmann LJ, Heinze B, Fassnacht M, et al. TP53 germline mutations in adult patients with adrenocortical carcinoma. J Clin Endocrinol Metab. 2012;97(3):E476-E485. [DOI] [PubMed] [Google Scholar]
- 32. Raymond VM, Else T, Everett JN, Long JM, Gruber SB, Hammer GD. Prevalence of germline TP53 mutations in a prospective series of unselected patients with adrenocortical carcinoma. J Clin Endocrinol Metab. 2013;98(1):E119-E125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Brioude F, Kalish JM, Mussa A, et al. Expert consensus document: clinical and molecular diagnosis, screening and management of Beckwith-Wiedemann syndrome: an international consensus statement. Nat Rev Endocrinol. 2018;14(4):229-249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. MacFarland SP, Mostoufi-Moab S, Zelley K, et al. Management of adrenal masses in patients with Beckwith-Wiedemann syndrome. Pediatr Blood Cancer. 2017;64(8):e26432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Raymond VM, Everett JN, Furtado LV, et al. Adrenocortical carcinoma is a Lynch syndrome-associated cancer. J Clin Oncol. 2013;31(24):3012-3018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Challis BG, Kandasamy N, Powlson AS, et al. Familial adrenocortical carcinoma in association with Lynch syndrome. J Clin Endocrinol Metab. 2016;101(6):2269-2272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Hampel H, Frankel WL, Martin E, et al. Screening for the Lynch syndrome (hereditary nonpolyposis colorectal cancer). N Engl J Med. 2005;352(18):1851-1860. [DOI] [PubMed] [Google Scholar]
- 38. Schaefer S, Shipotko M, Meyer S, et al. Natural course of small adrenal lesions in multiple endocrine neoplasia type 1: an endoscopic ultrasound imaging study. Eur J Endocrinol. 2008;158(5):699-704. [DOI] [PubMed] [Google Scholar]
- 39. Gatta-Cherifi B, Chabre O, Murat A, et al. Adrenal involvement in MEN1. Analysis of 715 cases from the Groupe d’etude des Tumeurs Endocrines database. Eur J Endocrinol. 2012;166(2):269-279. [DOI] [PubMed] [Google Scholar]
- 40. Seki M, Tanaka K, Kikuchi-Yanoshita R, et al. Loss of normal allele of the APC gene in an adrenocortical carcinoma from a patient with familial adenomatous polyposis. Hum Genet. 1992;89(3):298-300. [DOI] [PubMed] [Google Scholar]
- 41. Wakatsuki S, Sasano H, Matsui T, Nagashima K, Toyota T, Horii A. Adrenocortical tumor in a patient with familial adenomatous polyposis: a case associated with a complete inactivating mutation of the APC gene and unusual histological features. Hum Pathol. 1998;29(3):302-306. [DOI] [PubMed] [Google Scholar]
- 42. Shiroky JS, Lerner-Ellis JP, Govindarajan A, Urbach DR, Devon KM. Characteristics of adrenal masses in familial adenomatous polyposis. Dis Colon Rectum. 2018;61(6):679-685. [DOI] [PubMed] [Google Scholar]
- 43. Else T, Lerario AM, Everett J, et al. Adrenocortical carcinoma and succinate dehydrogenase gene mutations: an observational case series. Eur J Endocrinol. 2017;177(5):439-444. [DOI] [PubMed] [Google Scholar]
- 44. Anselmo J, Medeiros S, Carneiro V, et al. A large family with Carney complex caused by the S147G PRKAR1A mutation shows a unique spectrum of disease including adrenocortical cancer. J Clin Endocrinol Metab. 2012;97(2):351-359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Morin E, Mete O, Wasserman JD, Joshua AM, Asa SL, Ezzat S. Carney complex with adrenal cortical carcinoma. J Clin Endocrinol Metab. 2012;97(2):E202-E206. [DOI] [PubMed] [Google Scholar]
- 46. El Ghorayeb N, Grunenwald S, Nolet S, et al. First case report of an adrenocortical carcinoma caused by a BRCA2 mutation. Medicine (Baltimore). 2016;95(36):e4756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Raymond VM, Long JM, Everett JN, et al. An oncocytic adrenal tumour in a patient with Birt-Hogg-Dubé syndrome. Clin Endocrinol (Oxf). 2014;80(6):925-927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Else T, Kim AC, Sabolch A, et al. Adrenocortical carcinoma. Endocr Rev. 2014;35(2):282-326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Hampel H, Bennett RL, Buchanan A, Pearlman R, Wiesner GL; Guideline Development Group, American College of Medical Genetics and Genomics Professional Practice and Guidelines Committee and National Society of Genetic Counselors Practice Guidelines Committee . A practice guideline from the American College of Medical Genetics and Genomics and the National Society of Genetic Counselors: referral indications for cancer predisposition assessment. Genet Med. 2015;17(1):70-87. [DOI] [PubMed] [Google Scholar]
- 50. Fassnacht M, Dekkers OM, Else T, et al. European Society of Endocrinology Clinical Practice Guidelines on the management of adrenocortical carcinoma in adults, in collaboration with the European Network for the Study of Adrenal Tumors. Eur J Endocrinol. 2018;179(4):G1-G46. [DOI] [PubMed] [Google Scholar]
- 51. Bilimoria KY, Shen WT, Elaraj D, et al. Adrenocortical carcinoma in the United States: treatment utilization and prognostic factors. Cancer. 2008;113(11):3130-3136. [DOI] [PubMed] [Google Scholar]
- 52. Fassnacht M, Arlt W, Bancos I, et al. Management of adrenal incidentalomas: European Society of Endocrinology Clinical Practice Guideline in collaboration with the European Network for the Study of Adrenal Tumors. Eur J Endocrinol. 2016;175(2):G1-G34. [DOI] [PubMed] [Google Scholar]
- 53. Spartalis E, Drikos I, Ioannidis A, et al. Metastatic carcinomas of the adrenal glands: from diagnosis to treatment. Anticancer Res. 2019;39(6):2699-2710. [DOI] [PubMed] [Google Scholar]
- 54. Erickson LA. Challenges in surgical pathology of adrenocortical tumours. Histopathology. 2018;72(1):82-96. [DOI] [PubMed] [Google Scholar]
- 55. Lodish M. Genetics of adrenocortical development and tumors. Endocrinol Metab Clin North Am. 2017;46(2):419-433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Das S, Sengupta M, Islam N, et al. Weineke criteria, Ki-67 index and p53 status to study pediatric adrenocortical tumors: is there a correlation? J Pediatr Surg. 2016;51(11):1795-1800. [DOI] [PubMed] [Google Scholar]
- 57. Weiss LM. Comparative histologic study of 43 metastasizing and nonmetastasizing adrenocortical tumors. Am J Surg Pathol. 1984;8(3):163-169. [DOI] [PubMed] [Google Scholar]
- 58. Papotti M, Libè R, Duregon E, Volante M, Bertherat J, Tissier F. The Weiss score and beyond–histopathology for adrenocortical carcinoma. Horm Cancer. 2011;2(6):333-340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Aubert S, Wacrenier A, Leroy X, et al. Weiss system revisited: a clinicopathologic and immunohistochemical study of 49 adrenocortical tumors. Am J Surg Pathol. 2002;26(12):1612-1619. [DOI] [PubMed] [Google Scholar]
- 60. Zambaiti E, Duci M, De Corti F, et al. Clinical prognostic factors in pediatric adrenocortical tumors: a meta-analysis. Pediatr Blood Cancer. 2021;68(3):e28836. [DOI] [PubMed] [Google Scholar]
- 61. Chatterjee G, DasGupta S, Mukherjee G, et al. Usefulness of Wieneke criteria in assessing morphologic characteristics of adrenocortical tumors in children. Pediatr Surg Int. 2015;31(6):563-571. [DOI] [PubMed] [Google Scholar]
- 62. Sbiera S, Schmull S, Assie G, et al. High diagnostic and prognostic value of steroidogenic factor-1 expression in adrenal tumors. J Clin Endocrinol Metab. 2010;95(10):E161-E171. [DOI] [PubMed] [Google Scholar]
- 63. Enriquez ML, Lal P, Ziober A, Wang L, Tomaszewski JE, Bing Z. The use of immunohistochemical expression of SF-1 and EMA in distinguishing adrenocortical tumors from renal neoplasms. Appl Immunohistochem Mol Morphol. 2012;20(2):141-145. [DOI] [PubMed] [Google Scholar]
- 64. Papathomas TG, Pucci E, Giordano TJ, et al. An international Ki67 reproducibility study in adrenal cortical carcinoma. Am J Surg Pathol. 2016;40(4):569-576. [DOI] [PubMed] [Google Scholar]
- 65. Yamazaki Y, Nakamura Y, Shibahara Y, et al. Comparison of the methods for measuring the Ki-67 labeling index in adrenocortical carcinoma: manual versus digital image analysis. Hum Pathol. 2016;53:41-50. [DOI] [PubMed] [Google Scholar]
- 66. Beuschlein F, Weigel J, Saeger W, et al. Major prognostic role of Ki67 in localized adrenocortical carcinoma after complete resection. J Clin Endocrinol Metab. 2015;100(3):841-849. [DOI] [PubMed] [Google Scholar]
- 67. Fassnacht M, Libé R, Kroiss M, Allolio B. Adrenocortical carcinoma: a clinician’s update. Nat Rev Endocrinol. 2011;7(6):323-335. [DOI] [PubMed] [Google Scholar]
- 68. Hermsen IG, Gelderblom H, Kievit J, Romijn JA, Haak HR. Extremely long survival in six patients despite recurrent and metastatic adrenal carcinoma. Eur J Endocrinol. 2008;158(6):911-919. [DOI] [PubMed] [Google Scholar]
- 69. Lughezzani G, Sun M, Perrotte P, et al. The European Network for the Study of Adrenal Tumors staging system is prognostically superior to the international union against cancer-staging system: a North American validation. Eur J Cancer. 2010;46(4):713-719. [DOI] [PubMed] [Google Scholar]
- 70. Kiseljak-Vassiliades K, Bancos I, Hamrahian A, et al. American Association of Clinical Endocrinology Disease state clinical review on the evaluation and management of adrenocortical carcinoma in an adult: a practical approach. Endocr Pract. 2020;26(11):1366-1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Fassnacht M, Johanssen S, Quinkler M, et al. ; German Adrenocortical Carcinoma Registry Group; European Network for the Study of Adrenal Tumors . Limited prognostic value of the 2004 International Union Against Cancer staging classification for adrenocortical carcinoma: proposal for a Revised TNM Classification. Cancer. 2009;115(2):243-250. [DOI] [PubMed] [Google Scholar]
- 72. Assié G, Antoni G, Tissier F, et al. Prognostic parameters of metastatic adrenocortical carcinoma. J Clin Endocrinol Metab. 2007;92(1):148-154. [DOI] [PubMed] [Google Scholar]
- 73. Kerkhofs TM, Verhoeven RH, Bonjer HJ, et al. ; Dutch Adrenal Network . Surgery for adrenocortical carcinoma in The Netherlands: analysis of the national cancer registry data. Eur J Endocrinol. 2013;169(1):83-89. [DOI] [PubMed] [Google Scholar]
- 74. Lombardi CP, Raffaelli M, Boniardi M, et al. Adrenocortical carcinoma: effect of hospital volume on patient outcome. Langenbecks Arch Surg. 2012;397(2):201-207. [DOI] [PubMed] [Google Scholar]
- 75. Libé R, Borget I, Ronchi CL, et al. ; ENSAT network . Prognostic factors in stage III-IV adrenocortical carcinomas (ACC): an European Network for the Study of Adrenal Tumor (ENSAT) study. Ann Oncol. 2015;26(10):2119-2125. [DOI] [PubMed] [Google Scholar]
- 76. Berruti A, Fassnacht M, Haak H, et al. Prognostic role of overt hypercortisolism in completely operated patients with adrenocortical cancer. Eur Urol. 2014;65(4):832-838. [DOI] [PubMed] [Google Scholar]
- 77. Vanbrabant T, Fassnacht M, Assie G, Dekkers OM. Influence of hormonal functional status on survival in adrenocortical carcinoma: systematic review and meta-analysis. Eur J Endocrinol. 2018;179(6):429-436. [DOI] [PubMed] [Google Scholar]
- 78. Abiven G, Coste J, Groussin L, et al. Clinical and biological features in the prognosis of adrenocortical cancer: poor outcome of cortisol-secreting tumors in a series of 202 consecutive patients. J Clin Endocrinol Metab. 2006;91(7):2650-2655. [DOI] [PubMed] [Google Scholar]
- 79. Luton JP, Cerdas S, Billaud L, et al. Clinical features of adrenocortical carcinoma, prognostic factors, and the effect of mitotane therapy. N Engl J Med. 1990;322(17):1195-1201. [DOI] [PubMed] [Google Scholar]
- 80. Crucitti F, Bellantone R, Ferrante A, Boscherini M, Crucitti P. The Italian Registry for Adrenal Cortical Carcinoma: analysis of a multiinstitutional series of 129 patients. The ACC Italian Registry Study Group. Surgery. 1996;119(2):161-170. [DOI] [PubMed] [Google Scholar]
- 81. Schulick RD, Brennan MF. Long-term survival after complete resection and repeat resection in patients with adrenocortical carcinoma. Ann Surg Oncol. 1999;6(8):719-726. [DOI] [PubMed] [Google Scholar]
- 82. Lee JE, Berger DH, el-Naggar AK, et al. Surgical management, DNA content, and patient survival in adrenal cortical carcinoma. Surgery. 1995;118(6):1090-1098. [DOI] [PubMed] [Google Scholar]
- 83. Terzolo M, Fassnacht M, Perotti P, et al. Results of the ADIUVO study, the first randomized trial on adjuvant mitotane in adrenocortical carcinoma patients. J Endocr Soc. 2021;5(Supplement_1):A166-A167. [Google Scholar]
- 84. Liang J, Liu Z, Zhou L, et al. The clinical utility of ‘GRAS’ parameters in stage I-III adrenocortical carcinomas: long-term data from a high-volume institution. Endocrine. 2020;67(2):449-456. [DOI] [PubMed] [Google Scholar]
- 85. Baechle JJ, Marincola Smith P, Solorzano CC, et al. Cumulative GRAS score as a predictor of survival after resection for adrenocortical carcinoma: analysis from the U.S. Adrenocortical Carcinoma Database. Ann Surg Oncol. 2021;28:6551-6561. [DOI] [PubMed] [Google Scholar]
- 86. Giordano TJ, Kuick R, Else T, et al. Molecular classification and prognostication of adrenocortical tumors by transcriptome profiling. Clin Cancer Res. 2009;15(2):668-676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. de Reyniès A, Assié G, Rickman DS, et al. Gene expression profiling reveals a new classification of adrenocortical tumors and identifies molecular predictors of malignancy and survival. J Clin Oncol. 2009;27(7):1108-1115. [DOI] [PubMed] [Google Scholar]
- 88. Fragoso MC, Almeida MQ, Mazzuco TL, et al. Combined expression of BUB1B, DLGAP5, and PINK1 as predictors of poor outcome in adrenocortical tumors: validation in a Brazilian cohort of adult and pediatric patients. Eur J Endocrinol. 2012;166(1):61-67. [DOI] [PubMed] [Google Scholar]
- 89. Mohan DR, Lerario AM, Else T, et al. Targeted assessment of G0S2 methylation identifies a rapidly recurrent, routinely fatal molecular subtype of adrenocortical carcinoma. Clin Cancer Res. 2019;25(11):3276-3288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Lippert J, Appenzeller S, Liang R, et al. Targeted molecular analysis in adrenocortical carcinomas: a strategy toward improved personalized prognostication. J Clin Endocrinol Metab. 2018;103(12):4511-4523. [DOI] [PubMed] [Google Scholar]
- 91. Pozdeyev N, Fishbein L, Gay LM, et al. Targeted genomic analysis of 364 adrenocortical carcinomas. Endocr Relat Cancer. 2021;28(10):671-681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Lysodren (mitotane) [package insert]. HRA Pharma; 1970. Updated 6.2021. ProMED-mail website. Accessed October 31, 2021. https://www.accessdata.fda.gov/spl/data/e28768f0-93bf-4517-9f9b-5bf128ac363d/e28768f0-93bf-4517-9f9b-5bf128ac363d.xml [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.