Abstract
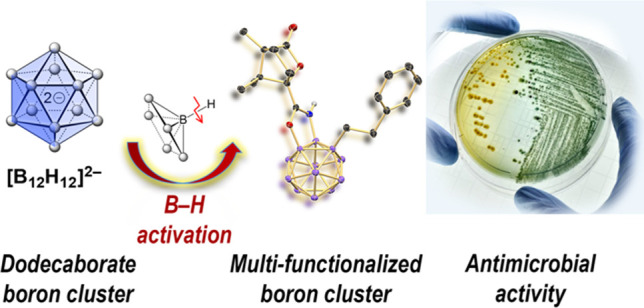
The identification of an alternative chemical space in order to address the global challenge posed by emerging antimicrobial resistance is very much needed for the discovery of novel antimicrobial lead compounds. Boron clusters are currently being explored in drug discovery due to their unique steric and electronic properties. However, the challenges associated with the synthesis and derivatization techniques of these compounds have limited their utility in the rapid construction of a library of molecules for screening against various biological targets as an alternative molecular platform. Herein, we report a transition-metal-catalyzed regioselective direct B–H alkylation–annulation of the closo-dodecaborate anion with natural products such as menthol and camphor as the directing groups. This method allowed the rapid construction of a library of 1,2,3-trisubstituted clusters, which were evaluated in terms of their antibacterial activity against WHO priority pathogens. Several of the synthesized dodecaborate derivatives displayed medium- to high-level bactericidal activity against Gram-positive and Gram-negative bacteria.
Short abstract
Multidrug-resistant bacteria pose a global challenge and necessitate the development of new antimicrobial agents. Because many traditional organic scaffolds have already been evaluated to that end, the identification of novel chemical space is crucial. We report on the stereoselective synthesis of a new class of boron clusters, which was prepared by natural-product-directed, catalytic B−H activation. The resulting 2D/3D organic−inorganic hybrid compounds showed medium- to high-level bactericidal activity against Gram-positive and Gram-negative bacteria.
Introduction
The discovery of novel bioactive molecules is essential to overcome the impending challenges posed by emerging infectious diseases caused by multidrug-resistant pathogens worldwide.1−4 The availability of antibiotics without prescription and their prophylactic use have spurred resistance, and bacteria of concern are, among others, Staphylococcus aureus, Escherichia coli, Salmonella spp. and Neisseria gonorrhoeae.5 The Center of Disease Control antibiotic resistance threat report 2019 disclosed more than 2.8 million cases of antibiotic-resistant infections with more than 35000 fatalities in the US every year.6 Research toward the discovery of antimicrobial agents is not attractive to pharmaceutical companies due to low profits and the limited lifespan associated with antibiotics, resulting in drying up of the corresponding pipeline and the risk of returning to the preantibiotic era.7 In addition, studies of resistance mechanisms suggest high chances of mutations, leading to the ineffectiveness of well-established compounds.8 Strategies to address this challenge involve the chemical modification of natural products as well as existing drugs.9 Historically, screening of secondary metabolites obtained from microorganisms has been a primary source of bioactive molecules that prevent the growth of pathogens.10 Studies on the biosynthesis of metabolites, probes of unexplored strains of microorganisms, and the availability of genome mining tools to activate silent gene clusters have yielded numerous antibacterial compounds.11−13 Currently marketed drugs involve aminoglycosides, β-lactams, glycopeptides, polymyxins, and the corresponding semisynthetic derivatives.14−16 Additionally, molecules bearing oxazolidinone, pyrimidine, quinolone, and sulfa functionalities have provided antibacterial candidates.17,18
The chemical space of natural products primarily comprises chiral compounds, whereas synthetic libraries often consist of flat aromatic molecules.19 Icosahedral boron-rich clusters exhibit a spherelike distribution of electron density and can be compared to classical arenes.20−27 Combining natural products with boron clusters can therefore enable access to a unique chemical space for the discovery of novel bioactive molecules. The closo-dodecaborate dianion [B12H12]2– is a highly symmetrical molecule, and the installation of three different substituents leads to Rcage-1 and Scage-1 stereoisomers (Figure 1a). The chirality due to such cage substitution has the potential for applications in designing molecules for medicinal chemistry, asymmetric synthesis, and materials science. However, there are limited reports on the enantioselective synthesis of such chiral compounds, and their utility has not been explored.28−31
Figure 1.
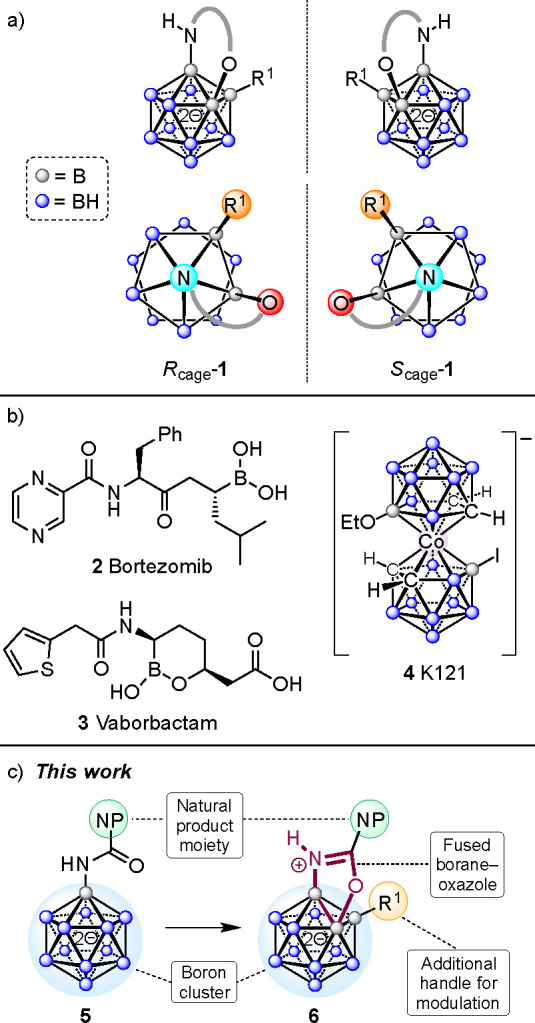
(a) Cage chirality of 1,2,3-trisubstituted closo-dodecaborates. (b) Boron-containing bioactive compounds. (c) Design of functionalized dodecaborates in this study. Color code: gray spheres, B; blue spheres, B–H.
The incorporation of boron as a part of bioactive compounds has recently gained much interest.32−35 Several boron-containing compounds are in clinical use, such as bortezomib (2), a proteasome inhibitor, and vaborbactam (3), an antibiotic (Figure 1b).36−38 Boron clusters are relatively nontoxic pharmacophores with steric and electronic properties that set them apart from organic building blocks.39−43 Studies on their medicinal applications have focused on boron neutron capture therapy (BNCT) and on the inhibition of enzymes. On the other hand, their antimicrobial properties have been investigated only to a limited degree.44 In an early review article on the potential applications of boron clusters, Plešek postulated that derivatives resembling known antibiotics may be promising drug analogues that cannot easily be degraded by pathogens.45 Examples where polyhedral boron moieties seem to play a crucial role in antibacterial activity are the metallacarboranes: e.g., the bis(dicarbollide) K121 (4; Figure 1b).46 Recently, the groups of Šicha and Viñas have probed related compounds to that end.47−50 In 2020, Spokoyny reported on the synthesis and properties of the borane–saccharide hybrid [B12(OCH2C6H4-1-thio-d-galactose)12]2–, which exhibits strong binding affinity to the B subunit of Shiga toxin 1.51 Our own group has found that fused 2D/3D heterocycles based on the [B12H12]2– framework possess antimicrobial properties.52 We therefore wondered whether closo-dodecaborates comprising a fused N,O-heterocycle and an additional group at a boron vertex would show similar effects. Amides 5 were anticipated to serve as starting materials for the target compounds 6, which can be viewed as 3D analogues of benzoxazoles with an organic handle R1 (Figure 1c). This strategy requires double B–H activation, including B–C bond formation and B–O annulation. The synthesis of functionalized polyhedral boranes and carboranes by B–H activation has emerged as a powerful tool,53−58 but derivatization of anionic {CB11} and {B12} clusters has only been accomplished in recent years.59−64 For dodecaborates, ureido and amide functionalities can serve as directing groups to achieve B–C and concomitant B–O bond formation.61,64
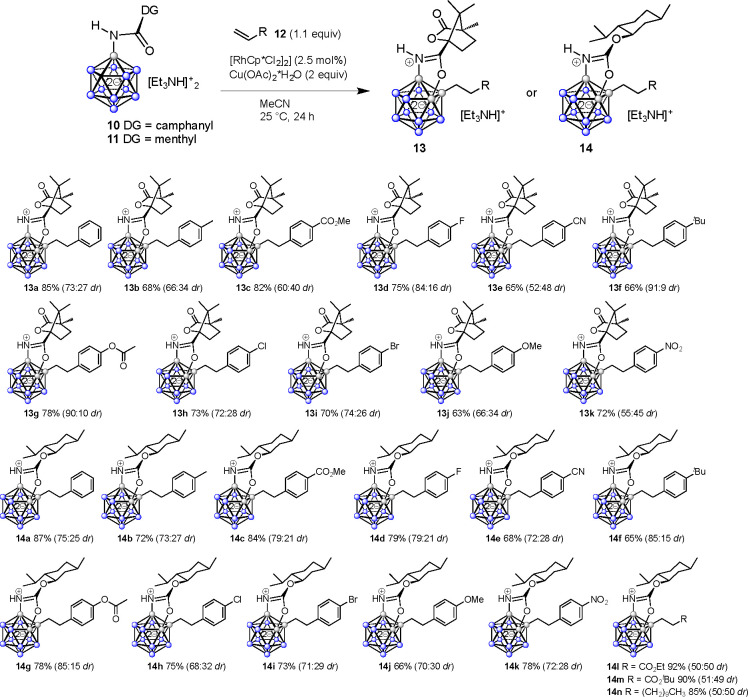
A major challenge for the transformation 5 → 6 was the choice of a suitable directing group. Our aim was to use a motif that provides the possibility to explore cage chirality as well as antimicrobial properties. Scage/Rcage stereoinduction required a substituent with saturated stereogenic centers close to the transition metal and boron vertices in the relevant transition state(s). However, aliphatic amides have not been explored in dodecaborate B–H activation. We decided to focus on directing groups involving (−)-menthol and (−)-camphanic acid on the basis of their rigid alicyclic structure, commercial availability, and reported bioactivities.65,66 We herein present a transition-metal-catalyzed, fully regioselective alkylation–annulation reaction for the construction of fused diboraoxazoles of the closo-dodecaborate cluster by using the aforementioned directing groups and alkene coupling partners. The method enabled the synthesis of a library of diversity-oriented boron clusters 13 and 14 under mild conditions in good yields and moderate to high stereoselectivity. Antibacterial properties were observed for several molecules of the series 13 and 14, thus suggesting that multiply functionalized fused closo-dodecaborates represent a feasible alternative chemical space to traditional frameworks of organic antibiotics. Notably, one of the compounds, 14k, was found to be active with a minimum inhibitory concentration (MIC) of up to 4 μM against Gram-positive S. aureus and Enterococcus faecalis and an MIC of up to 2 μM against Gram-negative N. gonorrhoeae.
Results and Discussion
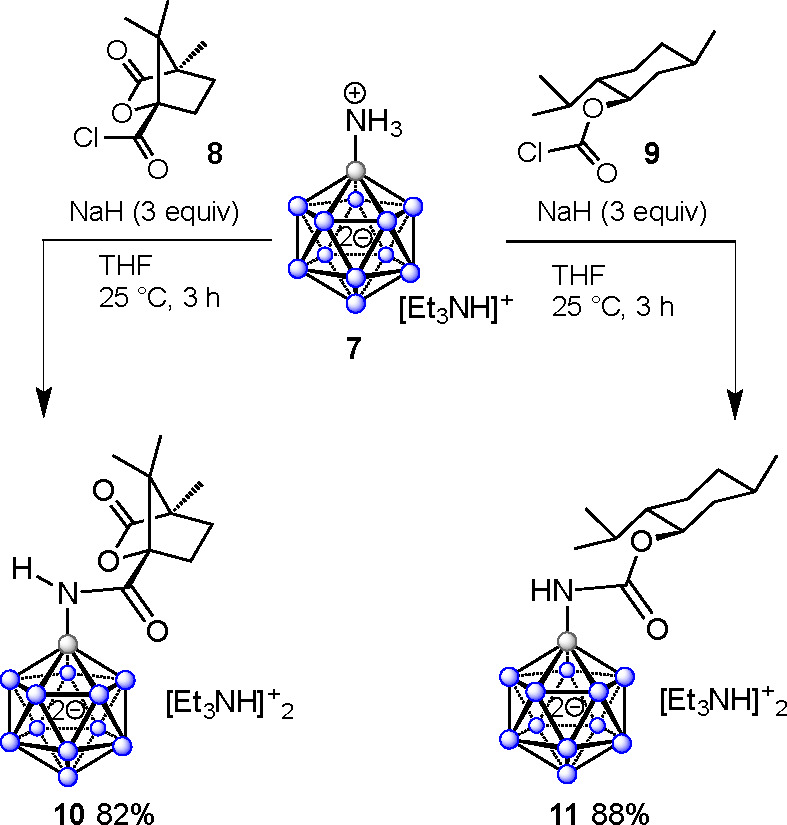
The design of target compounds 6 required directing groups that allow for stereoselective B–H activation of the dodecaborate cage and also possess bioactivity on their own. We selected amides of (−)-camphanic acid and (−)-menthyl carbonate as directing groups, which fulfill the following desirable criteria: (1) they possess a rigid structure with nonracemizing stereogenic centers, (2) they contain a functional group with the ability to coordinate to a transition metal to initiate B–H activation, (3) they can be easily installed on the boron cluster, and (4) they do not undergo side reactions. We anticipated [B12H12(NH3)]− to serve as a convenient cluster starting material enabling facile amide bond formation.52 Thus, [B12H12(NH3)]− was treated with 3 equiv of NaH in THF for complete deprotonation of the NH3 moiety, followed by combination with 1.1 equiv of (−)-camphanic acid chloride (8) or (−)-menthyl chlorocarbonate (9) (Scheme 1). The reactions were carried out at 25 °C for 2 h under anhydrous conditions and subsequently quenched with a saturated aqueous solution of [Et3NH]Cl. The amidations provided products 10 and 11 in 82% and 88% yields, respectively, after purification by column chromatography. Compounds 10 and 11 were fully characterized by multinuclear NMR spectroscopy and mass spectrometry.
Scheme 1. Acylation of [B12H11NH3]− Providing Camphanyl Amide 10 and Menthyl Amide 11.
Single crystals of the composition [10]2[Et3NH]4·(acetone) suitable for X-ray diffraction were obtained from acetone–hexane at room temperature. The solid-state structure revealed the distances (Å) B1–N1 1.525(4), N1–C1 1.316(4), C1–O1 1.239(3), and C1–C2 1.516(3) (Figure 2). The structural features are similar to those of typical organic amides; in particular, the coordination geometry around C1 is trigonal planar with a sum of angles of 360.1(2)° around this atom. The oxygen atoms O1 and O2 adopt a transoid geometry with respect to the C1–C2 axis, as indicated by the torsion angle of −178.4(2)° for O1–C1–C2–O2. Overall, the structure is similar to that of the closely related dodecaborate amide [B12H11(NH)(CO)(thiophen-2-yl)]−.52
Figure 2.
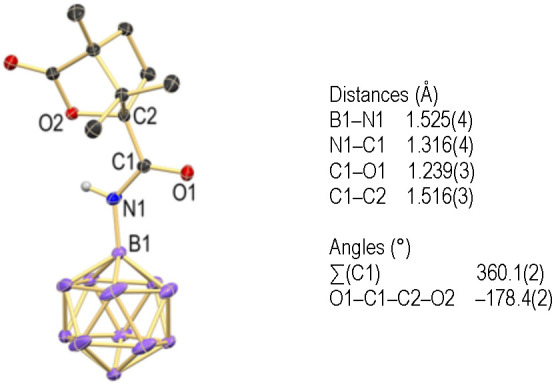
X-ray crystal structure of [10]2[Et3NH]4·(acetone) (only one of the two anions in the asymmetric unit is shown; 25% displacement ellipsoids; cations, acetone solvent molecule, and hydrogen atoms except for N–H are omitted for clarity).
Upon attaching the desired directing groups to the closo-dodecaborate cage, we evaluated transition-metal-catalyzed coupling to explore the feasibility of formation of compounds 6. Initially, we investigated the B–H activation of 10 with styrene (12a) in the presence of Rh or Ir catalysts. A reaction with [Cp*RhCl2]2 or [Cp*IrCl2]2 (10 mol %) at 25 or 60 °C for 24 h indicated only a trace of the desired product by ESI-MS and mostly unchanged starting material (Table 1). Therefore, we tried addition of Cu(OAc)2 and AgOAc. The reaction of 10 in the presence of [Cp*RhCl2]2 or [Cp*IrCl2]2 and Cu(OAc)2·H2O (2 equiv) at 60 °C suggested more than 50% conversion along with a mixture of other compounds by MS, whereas using AgOAc as an additive was not found to be helpful. Thus, we lowered the temperature as well as catalyst loading. The reaction of 10 in the presence of [Cp*RhCl2]2 or [Cp*IrCl2]2 and Cu(OAc)2·H2O (2 equiv) at 40 °C significantly improved the yield of the desired product to up to 65–75%. Further lowering of the catalyst loading and temperature to 2.5 mol % at 25 °C furnished the desired product in 85% yield upon isolation by column chromatography. The reaction in other solvents such as acetone, THF, and DCE gave yields of 56% or less. EtOH or MeOH afforded primarily unchanged starting materials.
Table 1. Optimization of the Alkylation–Annulation Reactiona.
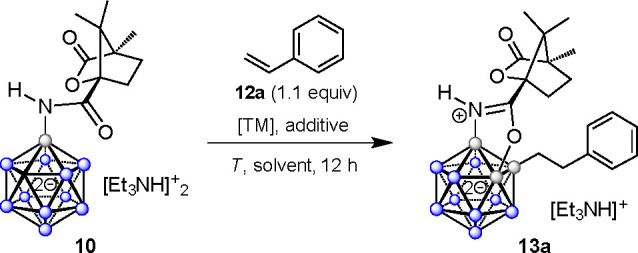
| no. | [TM]b (amt, mol %) | additive (amt, equiv)c | T (°C) | solvent | yield (%) |
|---|---|---|---|---|---|
| 1 | [Rh] (10) | 60 | MeCN | ||
| 2 | [Ir] (10) | 60 | MeCN | ||
| 3 | [Rh] (10) | [Cu] (2) | 60 | MeCN | 50 |
| 4 | [Rh] (10) | [Ag] (1) | 60 | MeCN | |
| 5 | [Ir] (10) | [Cu] (2) | 60 | MeCN | 50 |
| 6 | [Ir] (10) | [Ag] (1) | 60 | MeCN | |
| 7 | [Ir] (10) | [Cu] (2) | 40 | MeCN | 65 |
| 8 | [Rh] (10) | [Cu] (2) | 40 | MeCN | 75 |
| 9 | [Rh] (5) | [Cu] (2) | 25 | MeCN | 82 |
| 10 | [Ir] (5) | [Cu] (2) | 25 | MeCN | 72 |
| 11 | [Rh] (2.5) | [Cu] (2) | 25 | MeCN | 85 |
| 12 | [Rh] (2.5) | [Cu] (2) | 25 | EtOH | |
| 13 | [Rh] (2.5) | [Cu] (2) | 25 | Acetone | 52 |
| 14 | [Rh] (2.5) | [Cu] (2) | 25 | MeOH | |
| 15 | [Rh] (2.5) | [Cu] (2) | 25 | THF | 56 |
| 16 | [Rh] (2.5) | [Cu] (2) | 25 | DCE | 48 |
Reactions were conducted on a 20 mg scale in 1 mL of the solvent in a glass vial sealed with a screw cap.
Definitions: [Rh], [RhCp*Cl2]2; [Ir] = [IrCp*Cl2]2.
Definitions: [Cu], Cu(OAc)2·H2O; [Ag] = AgOAc.
From these screening experiments, entry 11 of Table 1 was used as the basis for transformations on a larger scale and an exploration of the substrate scope. Under these conditions, we performed the reaction of 10 with 12a on a 200 mg scale to give the corresponding product 13a in 85% yield after purification by column chromatography. 1H NMR spectroscopy and mass spectrometry suggested reductive coupling of 10 with 12a, leading to a B–(CH2)2–Ph moiety. 11B and 11B{1H} NMR spectra showed desymmetrization of the cage as well as characteristic, distinct resonances at 6.6, −4.5, and −10.2 ppm corresponding to B–O, B–N, and B–C vertices, respectively (Figure 3). This peak pattern was in full agreement with that observed for related dodecaborates in earlier studies.61,64
Figure 3.
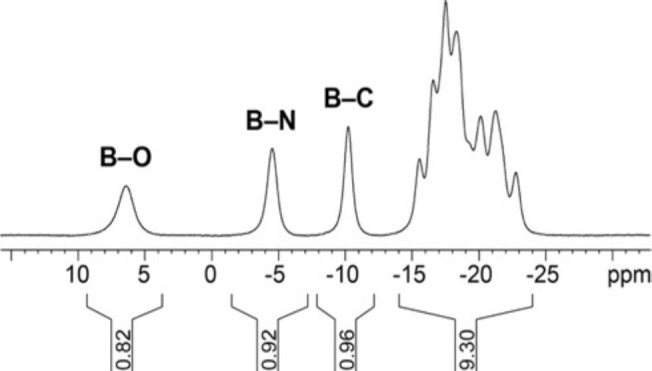
11B NMR spectrum of [Et3NH][13a] (128 MHz, acetone-d6, 23 °C).
Single crystals of 13a were obtained from a H2O/EtOH solution by slow evaporation of most of the EtOH over 21 days at room temperature. An X-ray diffraction analysis revealed the composition [13a]4[Et3NH]4·5H2O with four anions in the asymmetric unit (Figure 4). The cage showed the anticipated 1,2,3-trisubstitution caused by B–C coupling and heterocycle generation upon B–O bond formation. All of the anions exhibited an Rcage configuration and similar structural features. Therefore, only one of them is described in detail in the following. The B1–B3 distance is 1.730(6) Å, slightly contracted in comparison to other B–B distances, indicative of electron delocalization within the diboraoxazole ring. Although this effect is not very strong, it is consistent with reports on similar compounds and all other distances within the ring.52,61,64 The coordination around C1 is trigonal planar with an internal angle of O1–C1–N1 of 118.2(4)° and a sum of angles of 360.0(4)°. The C11–C12 distance of 1.522(7) Å confirmed reductive coupling with 12a, resulting in a CH2–CH2 single bond.
Figure 4.
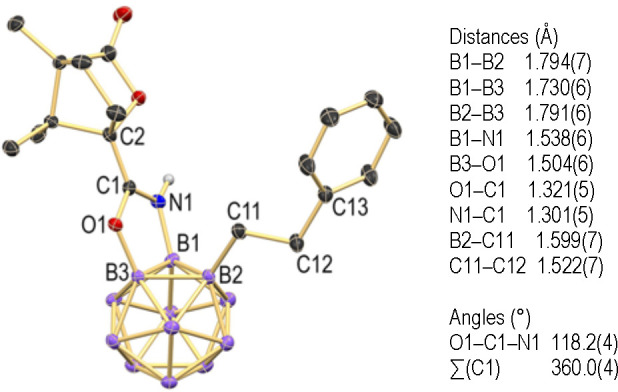
X-ray crystal structure of [13a]4[Et3NH]4·5H2O (only one of the four anions in the asymmetric unit is shown; 25% displacement ellipsoids; cations, H2O solvent molecules, and hydrogen atoms except for N–H are omitted for clarity).
Using the established protocol, we evaluated the generality of the reaction of 10 and 11 with various substituted styrenes as well as other olefins to generate a library of compounds. In general, the coupling–cyclization consistently provided access to products 13 and 14 in moderate to high yields under ambient conditions (Table 2). For all of the compounds, two sets of signals were observed in the 1H and 13C{1H} NMR spectra (but not in the 11B NMR spectra due to the naturally broadened signals), consistent with the formation of diastereomers featuring an unchanged absolute configuration of the directing group and Rcage/Scage configuration at the cage. For 13a and 14a, 1D and 2D NMR experiments were performed to assign all 1H and 13C resonances. On the basis of this analysis, diagnostic signals were used to determine the diastereomeric ratios dr (see the Supporting Information for details). Although in each of the series 13 and 14 one diastereoisomer consistently dominated, at present we are unable to state whether this corresponds to the Rcage or the Scage configuration.67
Table 2. Synthesis of Fused closo-Dodecaborate–Oxazolesa.
Reactions were performed on a 100 mg scale in MeCN (5 mL) in a 20 mL glass vial with a screw cap. The yields noted are isolated yields after purification by chromatography. dr values were determined by NMR. See the Supporting Information for details.
Substituted styrenes with electron-withdrawing and electron-donating functionalities furnished products 13a–k and 14a–n with very high regioselectivity and control over the degree of substitution as well as moderate to good diastereoselectivity. Minor undesired compounds were dialkylated species and trace amounts of unchanged starting material. Purification by chromatography afforded isolated yields of 63–92%. Typically, diastereomeric ratios were in the range of 60:40 to 80:20. Notably, higher values of up to 91:9 were observed for styrenes with 4-tBu and 4-OC(O)Me substitution (13f,g and 14f,g). Coupling of 11 with the nonaromatic alkenes CH2–CH–R (R = CO2Me, CO2tBu, (CH2)9CH3) proceeded in high yields of 85–92% (14l–n). However, in these cases the dr was 1:1, suggesting that the nature of the alkene coupling partner plays a decisive role in the diastereodiscriminating step.
Our previous studies suggested that the alkylation–annulation cascade occurs via B–C coupling followed by B–O bond formation as the essential steps.61,63 Both of these events require B–H activation, and several intermediates with B–H–Rh agostic-like and B–Rh direct interactions are likely to be involved. A proposed mechanism is displayed and discussed in pages S9 and S10 in the Supporting Information. Stereoinduction occurs in the second B–H activation step (affording the intermediates Rcage-V and Scage-V in Scheme S2) and is governed by the absolute stereochemistry of the natural product moiety. Subsequently, B–O bond formation–cyclization generates Rcage-13/Rcage-14 and Scage-13/Scage-14 diastereomers. We intend to investigate the mechanistic manifold and the question as to which stereochemical outcome is preferred by the chiral directing groups with the assistance of calculations in a separate study.
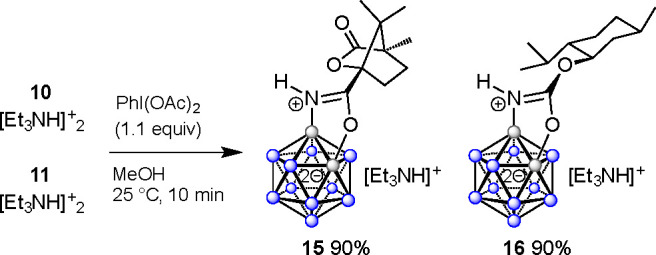
To probe and compare the bioactivity of the trisubstituted boron clusters with that of disubstituted boron clusters, we carried out further transformations of 10 and 11. Treatment with 1.1 equiv of the iodine(III) reagent (diacetoxyiodo)benzene in MeOH gave the cyclized products 15 and 16 cleanly in 90% yield after silica gel chromatography (Scheme 2). The reaction proceeded under mild conditions in MeOH in air within 10 min, and no side reactions such as cage overoxidation and formation of Bcage–iodonium species were observed.
Scheme 2. Synthesis of Cyclized Compounds 15 and 16.
Antibacterial Activity
The antimicrobial activity of all synthesized compounds was evaluated against commonly encountered “problem germs”, Gram-positive and Gram-negative antimicrobial-resistant bacteria that are defined in the WHO priority list (for the complete table of all tested strains, see the Supporting Information).68 The minimum inhibitory concentrations (MICs) of our compounds and the antibiotics ceftriaxone, azithromycin, and ciprofloxacin were determined against international reference strains N. gonorrhoeae ATCC 49226, S. aureus ATCC 25923, E. faecalis ATCC 29212, Acinetobacter baumannii ATCC 19606, Klebsiella pneumonia ATCC 700603, Pseudomonas aeruginosa ATCC 27853, E. coli ATCC 25922, Enterobacter cloacae ATCC 700323, Stenotrophomonas maltophilia ATCC 17666, Listeria monocytogenes EGDe, and Shigella sonnei SD10053 using the agar dilution method (see Table S1 in the Supporting Information). All compounds of the series 13 showed strong antimicrobial activity against the Gram-negative species N. gonorrhoeae, with compounds 13h,I displaying the best activity at an MIC of 4 μM (Table 3). Most of the series 13 compounds furthermore displayed activity against the Gram-positive species S. aureus and E. faecalis, with the best activities being observed for compounds 13f,h,I, which displayed MICs of 8–16 μM against S. aureus and 16–32 μM against E. faecalis. None of the series 13 compounds displayed activity against any of the other tested bacterial species (see Table S1 in the Supporting Information). Similarly, all of the series 14 compounds showed strong activity against N. gonorrhoeae, with the best activity being observed for compound 14k at an MIC of 2 μM (Table 3). Most of the series 14 compounds also displayed strong activity against S. aureus, E. faecalis, and L. monocytogenes, with the most consistent activity against all three species being observed for compound 14i at an MIC of 4 μM. Antimicrobial activity was also observed against the Gram-negative species S. maltophilia, although to a lesser degree, with compound 14h being most active with an MIC of 16 μM. No activity for the series 14 compounds was observed against the other tested bacterial species (see Table S1 in the Supporting Information). As a general trend, compounds containing the −tBu group, halides, or polar functionalities such as −OMe and −NO2 within the aryl moiety feature higher effectivity. Importantly, the antimicrobial activity of the series 13 compounds was dependent on the additional arylethyl group of these trisubstituted compounds, since the disubstituted control compound 15 did not display any antimicrobial activity. In contrast, for the series 14 compounds the additional arylethyl group did not appear to be essential for activity against N. gonorrhoeae, S. aureus, and E. faecalis, albeit the activity of the disubstituted control compound 16 was lower than that observed for the trisubstituted compounds that contained the additional handle. Therefore, it appears that addition of the menthyl moiety, but not the camphanic acid moiety, was beneficial for antimicrobial activity, which might explain the overall better activity observed for the series 14 compounds. In the case of noncyclized amides 10 and 11, no significant activity was detected. Similarly, the MIC values of the building blocks [B12H11-NH3]−, (−)-menthol, and (−)-camphanic acid were all >256 μM. This comparison highlights the effect of the combination of the cluster–oxazole fusion with the additional B–C derivatization of the adjacent boron vertex position.
Table 3. MIC Data (μM) for Our Compounds against Selected Gram-Positive and Gram-Negative Bacteria.
| Gram
negative |
Gram
positive |
||||
|---|---|---|---|---|---|
| compound | Neisseria gonorrhoeae ATCC 49226 | Stenotrophomonas maltophilia ATCC 17666 | Staphylococcus aureus ATCC 25923 | Enterococcus faecalis ATCC 29212 | Listeria monocytogenes EGDe |
| 10 | >256 | >256 | >256 | >256 | >256 |
| 13a | 16 | >256 | 128 | 128 | >256 |
| 13b | 16 | >256 | 32 | 64 | >256 |
| 13c | 16 | >256 | 64 | >256 | >256 |
| 13d | 8 | >256 | 64 | >256 | >256 |
| 13e | 16 | >256 | 64 | 64 | >256 |
| 13f | 8 | >256 | 8 | 16 | >256 |
| 13g | 64 | >256 | 256 | 256 | >256 |
| 13h | 4 | >256 | 16 | 32 | >256 |
| 13i | 4 | 256 | 16 | 16 | >256 |
| 13j | 32 | >256 | 64 | 64 | >256 |
| 13k | 8 | >256 | 64 | 64 | >256 |
| 15 | >256 | >256 | >256 | >256 | >256 |
| 11 | 128 | >256 | >256 | >256 | >256 |
| 14a | 4 | 64 | 4 | 4 | 8 |
| 14b | 8 | 64 | 8 | 8 | 16 |
| 14c | 16 | 256 | 8 | 16 | 16 |
| 14d | 8 | 32 | 4 | 8 | 8 |
| 14e | 4 | 128 | 4 | 8 | 16 |
| 14f | 8 | 256 | 16 | 16 | 16 |
| 14g | 4 | >256 | 4 | 32 | 32 |
| 14h | 4 | 16 | 4 | 4 | 8 |
| 14i | 4 | 32 | 4 | 4 | 4 |
| 14j | 4 | 64 | 4 | 8 | 8 |
| 14k | 2 | >256 | 4 | 4 | 32 |
| 14l | 4 | 128 | 8 | 16 | >256 |
| 14m | 4 | 128 | 8 | 8 | >256 |
| 14n | 16 | >256 | >256 | 64 | >256 |
| 16 | 16 | >256 | 64 | 128 | >256 |
| (−)-menthol | >256 | >256 | >256 | >256 | >256 |
| (−)-camphanic acid | >256 | >256 | >256 | >256 | >256 |
| [Et3NH][B12H11-NH3] | >256 | >256 | >256 | >256 | >256 |
Compounds of series 13 and series 14 both showed particularly strong activity against the N. gonorrhoeae reference strain. N. gonorrhoeae has developed resistance against all of the previously and currently used antimicrobials, and due to the continued emergence of multidrug-resistant strains, infections with N. gonorrhoeae have become increasingly difficult or even impossible to treat successfully.69,70 Resistance against the previously recommended antimicrobials ciprofloxacin and azithromycin is widespread, and susceptibility to the currently last available first-line therapy ceftriaxone is rapidly waning.71−75 Therefore, it is of utmost importance to develop novel antimicrobials for this multidrug-resistant bacterial pathogen. Importantly, compounds of the series 13 and 14 also showed strong activity against two recent multidrug-resistant clinical isolates, with the susceptibility being almost identical with the susceptibility displayed by the reference strain (Table 4), indicating that the molecular target of these compounds is distinct from that of previously or currently used antimicrobials.
Table 4. MIC Data (μM) for Selected Compounds and Marketed Antibiotics against Multidrug-Resistant Clinical Isolates of Neisseria gonorrhoeae.
| compound |
||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 13d | 13f | 13h | 13i | 13k | 14a | 14b | 14d | 14e | 14f | 14g | 14h | 14i | 14j | 14k | 14l | 14m | ceftriaxone | azithromycin | ciprofloxacin | |
| N. gonorrhoeae ATCC 49226 | 8 | 8 | 4 | 4 | 8 | 4 | 8 | 8 | 4 | 8 | 4 | 4 | 4 | 4 | 2 | 4 | 4 | 0.008 | 0.016 | 0.03 |
| N. gonorrhoeae ZJXSH 89 | 8 | 8 | 4 | 4 | 8 | 4 | 8 | 8 | 4 | 16 | 4 | 4 | 4 | 4 | 2 | 4 | 4 | 0.016 | 2048 | 48 |
| N. gonorrhoeae ZJXSH 86 | 8 | 8 | 4 | 4 | 8 | 4 | 8 | 8 | 4 | 8 | 4 | 4 | 4 | 4 | 2 | 4 | 4 | 0.008 | 0.016 | 48 |
Generally, the activity of antimicrobials can be divided into bacteriostatic compounds, which inhibit growth but do not kill, and bactericidal compounds that are able to directly kill the bacteria. This distinction is clinically relevant, since bacteriostatic compounds are dependent on an active host immune response to clear the infection, which might be problematic in immunocompromised individuals or not rapid enough for infections of the central nervous system or the heart.76,77 Therefore, we selected compound 14k, which features a phenyl-nitro moiety that showed the lowest MIC values, and tested its mode of activity against N. gonorrhoeae and S. aureus in time-kill analyses. As controls, we included the currently recommended first-line bactericidal antibiotics ceftriaxone69,70 and vancomycin78 for comparison. Compound 14k displayed rapid bactericidal activity against both N. gonorrhoeae and S. aureus (Figure 5). Exposure of N. gonorrhoeae to compound 14k at 4 × MIC resulted in >100000-fold reduction in CFU counts within the first hour, whereas ceftriaxone required 8 h to achieve similar inactivation. Of note, the MIC of ceftriaxone against N. gonorrhoeae is 250-fold lower in comparison with compound 14k, while the time-kill analyses were performed on the basis of relative × MIC values. In the case of S. aureus, exposure to compound 14k at 4 × MIC caused a >100000-fold inactivation within the first 2 h, whereas vancomycin could not effectively achieve inactivation, even after 8 h of exposure. The time-kill assays, corroborated by the results of bacterial live/dead staining (Figure 5e), thus accentuate the strong potential of 14k as a lead for novel antimicrobial compounds to treat bacterial infections caused by N. gonorrhoeae and S. aureus. For a compound to become a useful new antibiotic, it must exhibit not only high activity against pathogens but also low toxicity to the host. We are currently evaluating the fused borane–oxazoles in terms on their effects on eukaryotic cells and mice.
Figure 5.
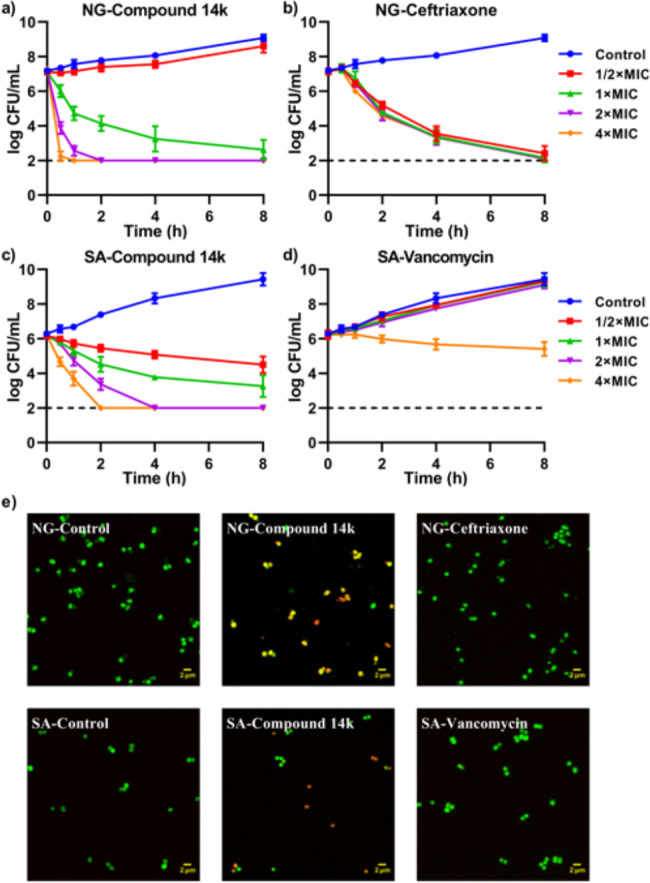
Bactericidal activity of compound 14k against Neisseria gonorrhoeae and Staphylococcus aureus. Bacterial suspensions of N. gonorrhoeae strain ATCC 49226 (NG) and S. aureus strain ATCC 25923 (SA) in GC broth supplemented with 1% Vitox were incubated with compound 14k or the control antimicrobials ceftriaxone (NG) and vancomycin (SA) at 4×, 2×, 1×, and 1/2× the minimum inhibitory concentration (MIC) or the vehicle control. Samples were taken in a time series for CFU determination or live/dead staining. (a) Survival curves of N. gonorrhoeae after exposure to compound 14k (1 × MIC: 2 μM). (b) Survival curves of N. gonorrhoeae after exposure to ceftriaxone (1 × MIC: 0.008 μM). (c) Survival curves of S. aureus after exposure to compound 14k (1 × MIC: 4 μM). (d) Survival curves of S. aureus after exposure to vancomycin (1 × MIC: 7 μM). Survival curves represent the mean and SD of three biological independent repeats. (e) Live/dead staining of N. gonorrhoeae and S. aureus after exposure to the vehicle control, compound 14k, or control antibiotics ceftriaxone and vancomycin at 1× MIC for 1 h. Viable bacteria are stained with SYTO 9 (green), whereas dead bacteria are stained with propidium iodide (red) or with both SYTO 9 and propidium iodide (yellow).
Conclusion
An efficient synthetic protocol has been developed for the stereoselective synthesis of highly functionalized fused dodecaborate–oxazoles. The protocol is mild and allows for the rapid construction of a library of compounds in moderate to high yields and stereoselectivities. This method provides access to a previously unexplored chemical space involving the natural products menthol and camphanic acid hybridized with the [B12H12]2– cage. The evaluation of antimicrobial activity against various pathogens has resulted in the identification of several active compounds. Particularly, product 14k exhibits potential for further drug development, as evidenced by its MIC values, time-kill assays showing bactericidal activity, and live/dead staining. These results lay the foundation for the further exploration of dodecaborate cage chirality as well as the study and improvement of antimicrobial properties of fused 2D/3D organic/inorganic heterocycle hybrid molecules.
Acknowledgments
Financial support by the National Natural Science Foundation of China (Nos. 21871231 and 21850410451) and the Special Funds for Basic Scientific Research of Zhejiang University (Nos. K20210335, 2019QNA3010, and 2018QNA3011) is gratefully acknowledged. The structures of 10a and 13a have been deposited with the Cambridge Crystallographic Data Centre (deposition numbers CCDC 2085680 and CCDC 2085681, respectively).
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acscentsci.1c01132.
Author Present Address
⊥ Glenmark Life Sciences, MIDC Industrial Area, Mahape, Navi Mumbai, Maharashtra 400709, India
Author Present Address
∇ Neuland Laboratories Ltd., Veerabhadraswamy Temple Road, Bonthapally Village, Gummadidala Mandal, Sangareddy District 502313, Telangana, India
Author Present Address
Δ Department of Biological Sciences, Carnegie Mellon University, 4400 Fifth Avenue, Pittsburgh, PA 15213, United States
Author Contributions
∥ R.V., F.Y., and R.D. contributed equally to this paper.
Author Contributions
R.V., S.v.d.V., and S.D. designed the study and wrote the paper. R.V. and R.D. synthesized and characterized all new compounds. F.Y. and J.Z. evaluated the bioactivities of all compounds. J.L. and B.S. carried out the X-ray diffraction analyses. All authors have given approval to the final version of the manuscript.
The authors declare no competing financial interest.
Supplementary Material
References
- Spedding M. New directions for drug discovery. Dialogues Clin. Neurosci. 2006, 8, 295–301. 10.31887/DCNS.2006.8.3/mspedding. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morens D. M.; Folkers G. K.; Fauci A. S. The challenge of emerging and re-emerging infectious diseases. Nature 2004, 430, 242–249. 10.1038/nature02759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown E. D.; Wright G. D. Antibacterial drug discovery in the resistance era. Nature 2016, 529, 336–343. 10.1038/nature17042. [DOI] [PubMed] [Google Scholar]
- Walsh C. Molecular mechanisms that confer antibacterial drug resistance. Nature 2000, 406, 775–781. 10.1038/35021219. [DOI] [PubMed] [Google Scholar]
- Rossolini G. M.; Arena F.; Pecile P.; Pollini S. Update on the antibiotic resistance crisis. Curr. Opin. Pharmacol. 2014, 18, 56–60. 10.1016/j.coph.2014.09.006. [DOI] [PubMed] [Google Scholar]
- CDC’s Antibiotic Resistance Threats in the United States; CDC: 2019.
- Alanis A. J. Resistance to Antibiotics: Are We in the Post-Antibiotic Era?. Arch. Med. Res. 2005, 36, 697–705. 10.1016/j.arcmed.2005.06.009. [DOI] [PubMed] [Google Scholar]
- Blair J. M. A.; Webber M. A.; Baylay A. J.; Ogbolu D. O.; Piddock L. J. V. Molecular mechanisms of antibiotic resistance. Nat. Rev. Microbiol. 2015, 13, 42–51. 10.1038/nrmicro3380. [DOI] [PubMed] [Google Scholar]
- Butler M. S.; Blaskovich M. A. T.; Cooper M. A. Antibiotics in the clinical pipeline at the end of 2015. J. Antibiot. 2017, 70, 3–24. 10.1038/ja.2016.72. [DOI] [PubMed] [Google Scholar]
- Newman D. J.; Cragg G. M. Natural Products as Sources of New Drugs from 1981 to 2014. J. Nat. Prod. 2016, 79, 629–661. 10.1021/acs.jnatprod.5b01055. [DOI] [PubMed] [Google Scholar]
- Ling L. L.; Schneider T.; Peoples A. J.; Spoering A. L.; Engels I.; Conlon B. P.; Mueller A.; Schäberle T. F.; Hughes D. E.; Epstein S.; Jones M.; Lazarides L.; Steadman V. A.; Cohen D. R.; Felix C. R.; Fetterman K. A.; Millett W. P.; Nitti A. G.; Zullo A. M.; Chen C.; Lewis K. A new antibiotic kills pathogens without detectable resistance. Nature 2015, 517, 455–459. 10.1038/nature14098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutledge P. J.; Challis G. L. Discovery of microbial natural products by activation of silent biosynthetic gene clusters. Nat. Rev. Microbiol. 2015, 13, 509–523. 10.1038/nrmicro3496. [DOI] [PubMed] [Google Scholar]
- Wilson M. C.; Mori T.; Rückert C.; Uria A. R.; Helf M. J.; Takada K.; Gernert C.; Steffens U. A. E.; Heycke N.; Schmitt S.; Rinke C.; Helfrich E. J. N.; Brachmann A. O.; Gurgui C.; Wakimoto T.; Kracht M.; Crüsemann M.; Hentschel U.; Abe I.; Matsunaga S.; Kalinowski J.; Takeyama H.; Piel J. An environmental bacterial taxon with a large and distinct metabolic repertoire. Nature 2014, 506, 58–62. 10.1038/nature12959. [DOI] [PubMed] [Google Scholar]
- Ribeiro da Cunha B.; Fonseca L. P.; Calado C. R. C. Antibiotic Discovery: Where Have We Come from, Where Do We Go?. Antibiotics 2019, 8, 45–65. 10.3390/antibiotics8020045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aminov R. History of antimicrobial drug discovery: Major classes and health impact. Bioch. Pharmacol. 2017, 133, 4–19. 10.1016/j.bcp.2016.10.001. [DOI] [PubMed] [Google Scholar]
- Brown P.; Dawson M. J.; Lawton G.; Witty D. R. A Perspective on the Next Generation of Antibacterial Agents Derived by Manipulation of Natural Products. Progress in Medicinal Chemistry 2015, 54, 135–184. 10.1016/bs.pmch.2014.10.001. [DOI] [PubMed] [Google Scholar]
- Stokes J. M.; Yang K.; Swanson K.; Jin W.; Cubillos-Ruiz A.; Donghia N. M.; MacNair C. R.; French S.; Carfrae L. A.; Bloom-Ackerman Z.; Tran V. M.; Chiappino-Pepe A.; Badran A. H.; Andrews I. W.; Chory E. J.; Church G. M.; Brown E. D.; Jaakkola T. S.; Barzilay R.; Collins J. J. A Deep Learning Approach to Antibiotic Discovery. Cell 2020, 180, 688–702.e13. 10.1016/j.cell.2020.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fedorowicz J.; Saczewski J. Modifications of quinolones and fluoroquinolones: hybrid compounds and dual-action molecules. Monatsh. Chem. 2018, 149, 1199–1245. 10.1007/s00706-018-2215-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stratton C. F.; Newman D. J.; Tan D. S. Cheminformatic comparison of approved drugs from natural product versus synthetic origins. Bioorg. Med. Chem. Lett. 2015, 25, 4802–4807. 10.1016/j.bmcl.2015.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poater J.; Solà M.; Viñas C.; Teixidor F. Hückel’s Rule of Aromaticity Categorizes Aromatic closo-Boron Hydride Clusters. Chem.-Eur. J. 2016, 22, 7437–7443. 10.1002/chem.201600510. [DOI] [PubMed] [Google Scholar]
- King R. B. Three-Dimensional Aromaticity in Polyhedral Boranes and Related Molecules. Chem. Rev. 2001, 101, 1119–1152. 10.1021/cr000442t. [DOI] [PubMed] [Google Scholar]
- Handbook of Boron Science: With Applications in Organometallics Catalysis, Materials and Medicine; Hosmane N. S., Eagling R., Eds.; World Scientific: 2018. [Google Scholar]
- Axtell J. C.; Saleh L. M. A.; Qian E. A.; Wixtrom A. I.; Spokoyny A. M. Synthesis and Applications of Perfunctionalized Boron Clusters. Inorg. Chem. 2018, 57, 2333–2350. 10.1021/acs.inorgchem.7b02912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimes R. N.Carboranes, 3rd ed.; Elsevier: 2016. [Google Scholar]
- Hosmane N. S.Boron Science: New Technologies and Applications; Taylor & Francis Books/CRC: 2011. [Google Scholar]
- Sivaev I. B.; Bregadze V. I.; Sjöberg S. Chemistry of closo-dodecaborate anion [B12H12]2–: A Review. Collect. Czech. Chem. Commun. 2002, 67, 679–727. 10.1135/cccc20020679. [DOI] [Google Scholar]
- Contemporary Boron Chemistry; Davidson M., Hughes A. K., Marder T. B., Wade K., Eds.; Royal Society of Chemistry: 2000. [Google Scholar]
- Cheng R.; Li B.; Wu J.; Zhang J.; Qiu Z.; Tang W.; You S. L.; Tang Y.; Xie Z. Enantioselective Synthesis of Chiral-at-Cage o-Carboranes via Pd-Catalyzed Asymmetric B–H Substitution. J. Am. Chem. Soc. 2018, 140, 4508–4511. 10.1021/jacs.8b01754. [DOI] [PubMed] [Google Scholar]
- Levit G. L.; Krasnov V. P.; Gruzdev D. A.; Demin A. M.; Bazhov I. V.; Sadretdinova L. S.; Olshevskaya V. A.; Kalinin V. N.; Cheong C. S.; Chupakhin O. N.; Charushin V. N. Synthesis of N-[(3-amino-1,2-dicarba-closo-dodecaboran-1-yl)-acetyl] derivatives of alpha-amino acids. Collect. Czech. Chem. C 2007, 72, 1697–1706. 10.1135/cccc20071697. [DOI] [Google Scholar]
- Levit G. L.; Demin A. M.; Kodess M. I.; Ezhikova M. A.; Sadretdinova L. S.; Ol’shevskaya V. A.; Kalinin V. N.; Krasnov V. P.; Charushin V. N. Acidic hydrolysis of N-acyl-1-substituted 3-amino-1,2-dicarba-closo-dodecaboranes. J. Organomet. Chem. 2005, 690, 2783–2786. 10.1016/j.jorganchem.2005.01.043. [DOI] [Google Scholar]
- Krasnov V. P.; Levit G. L.; Charushin V. N.; Grishakov A. N.; Kodess M. I.; Kalinin V. N.; Ol’shevskaya V. A.; Chupakhin O. N. Enantiomers of 3-amino-1-methyl-1,2-dicarba-closo-dodecaborane. Tetrahedron: Asymmetry 2002, 13, 1833–1835. 10.1016/S0957-4166(02)00474-3. [DOI] [Google Scholar]
- Ali F.; Hosmane N. S.; Zhu Y. Boron Chemistry for Medical Applications. Molecules 2020, 25, 828–851. 10.3390/molecules25040828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva M. P.; Saraiva L.; Pinto M.; Sousa M. E. Boronic Acids and Their Derivatives in Medicinal Chemistry: Synthesis and Biological Applications. Molecules 2020, 25, 4323–4362. 10.3390/molecules25184323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viñas C.; Núñez R.; Bennour I.; Teixidor F. Periphery Decorated and Core Initiated Neutral and Polyanionic Borane Large Molecules: Forthcoming and Promising Properties for Medicinal Applications. Curr. Med. Chem. 2019, 26, 5036–5076. 10.2174/0929867326666190603123838. [DOI] [PubMed] [Google Scholar]
- Yinghuai Z.; Lin X.; Xie H.; Li J.; Hosmane N. S.; Zhang Y. The Current Status and Perspectives of Delivery Strategy for Boron-based Drugs. Curr. Med. Chem. 2019, 26, 5019–5035. 10.2174/0929867325666180904105212. [DOI] [PubMed] [Google Scholar]
- Trippier P. C.; McGuigan C. Boronic Acids in Medicinal Chemistry: Anticancer, Antibacterial and Antiviral Applications. Medchemcomm 2010, 1, 183–198. 10.1039/c0md00119h. [DOI] [Google Scholar]
- Richardson P. G.; Hideshima T.; Anderson K. C. Bortezomib (PS-341): a novel, first-in-class proteasome inhibitor for the treatment of multiple myeloma and other cancers. Cancer Control 2003, 10, 361–369. 10.1177/107327480301000502. [DOI] [PubMed] [Google Scholar]
- Hecker S. J.; Raja Reddy K.; Lomovskaya O.; Griffith D. C.; Rubio-Aparicio D.; Nelson K.; Tsivkovski R.; Sun D.; Sabet M.; Tarazi Z.; Parkinson J.; Totrov M.; Boyer S. H.; Glinka T. W.; Pemberton O. S.; Chen Y.; Dudley M. N. Discovery of Cyclic Boronic Acid QPX7728, an Ultrabroad-Spectrum Inhibitor of Serine and Metallo-β-lactamases. J. Med. Chem. 2020, 63, 7491–7507. 10.1021/acs.jmedchem.9b01976. [DOI] [PubMed] [Google Scholar]
- Leśnikowski Z. J. Challenges and Opportunities for the Application of Boron Clusters in Drug Design. J. Med. Chem. 2016, 59, 7738–7758. 10.1021/acs.jmedchem.5b01932. [DOI] [PubMed] [Google Scholar]
- Gabel D. Boron clusters in medicinal chemistry: perspectives and problems. Pure Appl. Chem. 2015, 87, 173–179. 10.1515/pac-2014-1007. [DOI] [Google Scholar]
- Scholz M.; Hey-Hawkins E. Carbaboranes as Pharmacophores: Properties, Synthesis, and Application Strategies. Chem. Rev. 2011, 111, 7035–7062. 10.1021/cr200038x. [DOI] [PubMed] [Google Scholar]
- Issa F.; Kassiou M.; Rendina L. M. Boron in Drug Discovery: Carboranes as Unique Pharmacophores in Biologically Active Compounds. Chem. Rev. 2011, 111, 5701–5722. 10.1021/cr2000866. [DOI] [PubMed] [Google Scholar]
- Valliant J. F.; Guenther K. J.; King A. S.; Morel P.; Schaffer P.; Sogbein O. O.; Stephenson K. A. The medicinal chemistry of carboranes. Coord. Chem. Rev. 2002, 232, 173–230. 10.1016/S0010-8545(02)00087-5. [DOI] [Google Scholar]
- Fink K.; Uchmann M. Boron cluster compounds as new chemical leads for antimicrobial therapy. Coord. Chem. Rev. 2021, 431, 213684–213693. 10.1016/j.ccr.2020.213684. [DOI] [Google Scholar]
- Plešek J. Potential Applications of the Boron Cluster Compounds. Chem. Rev. 1992, 92, 269–278. 10.1021/cr00010a005. [DOI] [Google Scholar]
- Zheng Y.; Liu W. W.; Chen Y.; Jiang H.; Yan H.; Kosenko I.; Chekulaeva L.; Sivaev I.; Bregadze V.; Wang X. M. A Highly potent antibacterial agent targeting methicillin-resistant staphylococcus aureus based on cobalt bis(1,2-dicarbollide) alkoxy derivative. Organometallics 2017, 36, 3484–3490. 10.1021/acs.organomet.7b00426. [DOI] [Google Scholar]
- Romero I.; Martinez-Medina M.; Camprubí-Font C.; Bennour I.; Moreno D.; Martínez-Martínez L.; Teixidor F.; Fox M. A.; Viñas C. Metallacarborane Assemblies as Effective Antimicrobial Agents, Including a Highly Potent Anti-MRSA Agent. Organometallics 2020, 39, 4253–4264. 10.1021/acs.organomet.0c00315. [DOI] [Google Scholar]
- Vaňková E.; Lokočová K.; Mat́átková O.; Krízǒvá I.; Masák J.; Grüner B.; Kaule P. Čermák, J.; Šícha, V. Cobaltbis-dicarbollide and its ammonium derivatives are effective antimicrobial and antibiofilm agents. J. Organomet. Chem. 2019, 899, 120891–120898. 10.1016/j.jorganchem.2019.120891. [DOI] [Google Scholar]
- Kvasničková E.; Masák J.; Čejka J.; Mat́átková O.; Šícha V. Preparation, characterization, and the selective antimicrobial activity of N-alkylammonium 8-diethyleneglycol cobalt bis-dicarbollide derivatives. J. Organomet. Chem. 2017, 827, 23–31. 10.1016/j.jorganchem.2016.10.037. [DOI] [Google Scholar]
- Popova T.; Zaulet A.; Teixidor F.; Alexandrova R.; Viñas C. Investigations on antimicrobial activity of cobaltabisdicarbollides. J. Organomet. Chem. 2013, 747, 229. 10.1016/j.jorganchem.2013.07.006. [DOI] [Google Scholar]
- Stauber J. M.; Qian E. A.; Han Y.; Rheingold A. L.; Král P.; Fujita D.; Spokoyny A. M. An Organometallic Strategy for Assembling Atomically Precise Hybrid Nanomaterials. J. Am. Chem. Soc. 2020, 142, 327–334. 10.1021/jacs.9b10770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y.; Zhang J.; Zhang Y.; Liu J.; van der Veen S.; Duttwyler S. The closo-Dodecaborate Dianion Fused with Oxazoles Provides 3D Diboraheterocycles with Selective Antimicrobial Activity. Chem.-Eur. J. 2018, 24, 10364–10371. 10.1002/chem.201801602. [DOI] [PubMed] [Google Scholar]
- Hamdaoui M.; Varkhedkar R.; Sun J.; Fan L.; Duttwyler S.. Recent advances in the selective functionalization of anionic icosahedral boranes and carboranes. In Synthetic Inorganic Chemistry; Hamilton E. J. M., Hosmane N. S., Eds.; Elsevier: 2021; pp 343–389. [Google Scholar]
- Quan Y.; Xie Z. Controlled functionalization of o-carborane via transition metal catalyzed B–H activation. Chem. Soc. Rev. 2019, 48, 3660–3673. 10.1039/C9CS00169G. [DOI] [PubMed] [Google Scholar]
- Zhang X.; Yan H. Transition metal-induced B–H functionalization of o-carborane. Coord. Chem. Rev. 2019, 378, 466–482. 10.1016/j.ccr.2017.11.006. [DOI] [Google Scholar]
- Duttwyler S. Recent advances in B–H functionalization of icosahedral carboranes and boranes by transition metal catalysis. Pure Appl. Chem. 2018, 90, 733–744. 10.1515/pac-2017-1202. [DOI] [Google Scholar]
- Quan Y.; Qiu Z.; Xie Z. Transition-Metal-Catalyzed Selective Cage B–H Functionalization of o-Carboranes. Chem.-Eur. J. 2018, 24, 2795–2805. 10.1002/chem.201704937. [DOI] [PubMed] [Google Scholar]
- Yu W.-B.; Cui P.-F.; Gao W.-X.; Jin G.-X. B–H activation of carboranes induced by late transition metals. Coord. Chem. Rev. 2017, 350, 300–319. 10.1016/j.ccr.2017.07.006. [DOI] [Google Scholar]
- Shen Y.; Zhang K.; Liang X.; Dontha R.; Duttwyler S. Highly selective palladium-catalyzed one-pot, five-fold B–H/C–H cross coupling of monocarboranes with alkenes. Chem. Sci. 2019, 10, 4177–4184. 10.1039/C9SC00078J. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin F.; Yu J.-Lu; Shen Y.; Zhang S.-Q.; Spingler B.; Liu J.; Hong X.; Duttwyler S. Palladium-Catalyzed Selective Five-Fold Cascade Arylation of the 12-Vertex Monocarborane Anion by B–H Activation. J. Am. Chem. Soc. 2018, 140, 13798–13807. 10.1021/jacs.8b07872. [DOI] [PubMed] [Google Scholar]
- Zhang Y.; Wang T.; Wang L.; Sun Y.; Lin F.; Liu J.; Duttwyler S. Rh(III)-Catalyzed Functionalization of closo-Dodecaborates by Selective B–H Activation: Bypassing Competitive C–H Activation. Chem.-Eur. J. 2018, 24, 15812–15817. 10.1002/chem.201803455. [DOI] [PubMed] [Google Scholar]
- Lin F.; Shen Y.; Zhang Y.; Sun Y.; Liu J.; Duttwyler S. Fusing Carborane Carboxylic Acids with Alkynes: 3D Analogues of Isocoumarins via Regioselective B–H Activation. Chem.-Eur. J. 2018, 24, 551–555. 10.1002/chem.201703802. [DOI] [PubMed] [Google Scholar]
- Shen Y.; Pan Y.; Zhang K.; Liang X.; Liu J.; Spingler B.; Duttwyler S. B–H functionalization of the monocarba-closo-dodecaborate anion by rhodium and iridium catalysis. Dalton Trans. 2017, 46, 3135–3140. 10.1039/C7DT00269F. [DOI] [PubMed] [Google Scholar]
- Zhang Y.; Sun Y.; Lin F.; Liu J.; Duttwyler S. Rhodium(III)-Catalyzed Alkenylation–Annulation of closo-Dodecaborate Anions through Double B–H Activation at Room Temperature. Angew. Chem., Int. Ed. 2016, 55, 15609–15614. 10.1002/anie.201607867. [DOI] [PubMed] [Google Scholar]
- Chen W.; Vermaak I.; Viljoen A. Camphor – a fumigant during the Black Death and a coveted fragrant wood in ancient Egypt and Babylon – a review. Molecules 2013, 18, 5434–5454. 10.3390/molecules18055434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- İşcan G.; Ki̇ri̇mer N.; Kürkcüoǧlu M. N.; Hüsnü Can B.; Demi̇rci̇ F. H. Antimicrobial Screening of Mentha piperita Essential Oils. J. Agr. Food Chem. 2002, 50, 3943–3946. 10.1021/jf011476k. [DOI] [PubMed] [Google Scholar]
- The amount of Rcage single crystals of 13a was not high enough for an NMR analysis, and crystallization of this and other compounds generally proved difficult. The chromatographic separation of diastereomers was also not successful.
- Tacconelli E.; Carrara E.; Savoldi A.; Harbarth S.; Mendelson M.; Monnet D. L.; Pulcini C.; Kahlmeter G.; Kluytmans J.; Carmeli Y.; Ouellette M.; Outterson K.; Patel J.; Cavaleri M.; Cox E. M.; Houchens C. R.; Grayson M. L.; Hansen P.; Singh N.; Theuretzbacher U.; Magrini N. WHO Pathogens Priority List Working Group. Discovery, research, and development of new antibiotics: the WHO priority list of antibiotic-resistant bacteria and tuberculosis. Lancet Infect. Dis. 2018, 18, 318–327. 10.1016/S1473-3099(17)30753-3. [DOI] [PubMed] [Google Scholar]
- Yang F.; Yan J.; van der Veen S. Antibiotic Resistance and Treatment Options for Multidrug-Resistant Gonorrhea. Infect. Microb. Dis. 2020, 2, 67–76. 10.1097/IM9.0000000000000024. [DOI] [Google Scholar]
- Unemo M.; Shafer W. M. Antimicrobial resistance in Neisseria gonorrhoeae in the 21st century: past, evolution, and future. Clin. Microbiol. Rev. 2014, 27, 587–613. 10.1128/CMR.00010-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gransden W. R.; Warren C. A.; Phillips I.; Hodges M.; Barlow D. Decreased susceptibility of Neisseria gonorrhoeae to ciprofloxacin. Lancet 1990, 335, 51. 10.1016/0140-6736(90)90177-7. [DOI] [PubMed] [Google Scholar]
- Starnino S.; Galarza P.; Carvallo M. E.; Benzaken A. S.; Ballesteros A. M.; Cruz O. M.; Hernandez A. L.; Carbajal J. L.; Borthagaray G.; Payares D.; Dillon J. A. Retrospective analysis of antimicrobial susceptibility trends (2000–2009) in Neisseria gonorrhoeae isolates from countries in Latin America and the Caribbean shows evolving resistance to ciprofloxacin, azithromycin and decreased susceptibility to ceftriaxone. Sex Transm. Dis. 2012, 39, 813–821. 10.1097/OLQ.0b013e3182631c9f. [DOI] [PubMed] [Google Scholar]
- Ni C.; Xue J.; Zhang C.; Zhou H.; van der Veen S. High prevalence of Neisseria gonorrhoeae with high-level resistance to azithromycin in Hangzhou, China. J. Antimicrob. Chemother. 2016, 71, 2355–2357. 10.1093/jac/dkw131. [DOI] [PubMed] [Google Scholar]
- Fifer H.; Hughes G.; Whiley D.; Lahra M. M. Lessons learnt from ceftriaxone-resistant gonorrhoea in the UK and Australia. Lancet Infect. Dis. 2020, 20, 276–278. 10.1016/S1473-3099(20)30055-4. [DOI] [PubMed] [Google Scholar]
- Yan J.; Chen Y.; Yang F.; Ling X.; Jiang S.; Zhao F.; Yu Y.; van der Veen S. High percentage of the ceftriaxone-resistant Neisseria gonorrhoeae FC428 clone among isolates from a single hospital in Hangzhou, China. J. Antimicrob. Chemother. 2021, 76, 936–939. 10.1093/jac/dkaa526. [DOI] [PubMed] [Google Scholar]
- a Pankey G. A.; Sabath L. D. Clinical relevance of bacteriostatic versus bactericidal mechanisms of action in the treatment of Gram-positive bacterial infections. Clin. Infect. Dis. 2004, 38, 864–870. 10.1086/381972. [DOI] [PubMed] [Google Scholar]
- Finberg R. W.; Moellering R. C.; Tally F. P.; Craig W. A.; Pankey G. A.; Dellinger E. P.; West M. A.; Joshi M.; Linden P. K.; Rolston K. V.; Rotschafer J. C.; Rybak M. J. The importance of bactericidal drugs: future directions in infectious disease. Clin. Infect. Dis. 2004, 39, 1314–1320. 10.1086/425009. [DOI] [PubMed] [Google Scholar]
- Liu C.; Bayer A.; Cosgrove S. E.; Daum R. S.; Fridkin S. K.; Gorwitz R. J.; Kaplan S. L.; Karchmer A. W.; Levine D. P.; Murray B. E.; Rybak M.; Talan D. A.; Chambers H. F. Infectious Diseases Society of America. Clinical practice guidelines by the infectious diseases society of America for the treatment of methicillin-resistant Staphylococcus aureus infections in adults and children. Clin. Infect. Dis. 2011, 52, e18–55. 10.1093/cid/ciq146. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.