Abstract
The COVID-19 pandemic caused by SARS-CoV-2 infection has been of unprecedented clinical and socio-economic worldwide relevance. The case fatality rate for COVID-19 grows exponentially with age and the presence of comorbidities. In the older patients, COVID-19 manifests predominantly as a systemic disease associated with immunological, inflammatory, and procoagulant responses. Timely diagnosis and risk stratification are crucial steps to define appropriate therapies and reduce mortality, especially in the older patients. Chronically and systemically activated innate immune responses and impaired antiviral responses have been recognized as the results of a progressive remodeling of the immune system during aging, which can be described by the words ‘immunosenescence’ and ‘inflammaging’. These age-related features of the immune system were highlighted in patients affected by COVID-19 with the poorest clinical outcomes, suggesting that the mechanisms underpinning immunosenescence and inflammaging could be relevant for COVID-19 pathogenesis and progression. Increasing evidence suggests that senescent myeloid and endothelial cells are characterized by the acquisition of a senescence-associated pro-inflammatory phenotype (SASP), which is considered as the main culprit of both immunosenescence and inflammaging. Here, we reviewed this evidence and highlighted several circulating biomarkers of inflammaging that could provide additional prognostic information to stratify COVID-19 patients based on the risk of severe outcomes.
Keywords: SARS-CoV-2, COVID-19, Inflammaging, Biomarkers, Neutrophils
1. Introduction
The aging process is associated with a chronic, systemic, and low-grade proinflammatory condition previously termed as “inflammaging” (Franceschi et al., 2000). During human aging, the accumulation of macromolecular damage leads to the stochastic build-up of senescent cells that fail to accomplish their normal tasks and acquire a senescence-associated pro-inflammatory phenotype (SASP) (Coppe et al., 2008). Current thinking is that the short-term presence of senescent cells in a younger milieu can promote the plasticity of neighboring cells, whereas their progressive accumulation during aging can contribute to fuel inflammaging and to spread senescence at paracrine and systemic levels (Hernandez-Segura et al., 2018, Prata et al., 2018). Notably, a complex cross-link exists between inflammaging and cellular senescence, since a chronic proinflammatory status can contribute to increase the amount of senescent cells and amplify a vicious circle that increases the risk to develop the most common age-related diseases (Fulop et al., 2017, Lagnado et al., 2021).
Human aging is accompanied by a complex remodeling of the immune system characterized on one hand by an increased innate immune activity (Cunha et al., 2020), and on the other hand by a gradual decline in adaptive functions (Lee et al., 2016, Salminen et al., 2018a). Immune cells can themselves become senescent and acquire the SASP so that we previously coined the term ‘macroph-aging’ to describe the contribution of the senescent macrophages to inflammaging (Prattichizzo et al., 2016). Mechanistically, both inflammaging and immunosenescence can be promoted not only by the individual repeated exposures to different pathogens during life but also by the age-related increased burden of senescent cells (Humphreys et al., 2020).
Mounting evidence showed that the clinical severity of Coronavirus disease 19 (COVID-19) can be attributed to an inadequate antiviral immune function and excessive systemic self-damaging inflammation, to the point that level of immunosenescence associated with the rate of inflammaging can be overall the main risk factor for the development of COVID-19 severe outcomes (Hazeldine and Lord, 2020). In this framework, the comprehension of the age-related modulation of the immune system responses during aging appears essential to better characterize COVID-19 progression and widen the spectrum of therapeutic options.
Here, we will briefly review the age-associated remodeling of the innate and adaptive immune systems and discuss features of inflammaging and immunosenescence that are found in COVID-19. Finally, circulating biomarkers that recapitulate these features and can therefore be used in the clinical practice for the risk assessment of COVID-19 patients will be presented.
2. Age-related remodeling of the immune system
One of the main pillars contributing to inflammaging is the accumulation of senescent cells in different organs and tissues. In this framework, immune cells are responsible for the clearance of senescent cells and disposal of the extracellular debris, that is, damage-associated molecular patterns (DAMPs), which are released from cells undergoing necrosis (Ferrucci and Fabbri, 2018). During aging, the accumulation of senescent immune cells impairs clearance of non-immune senescent cells in solid organs, thus accelerating the rate of systemic aging. Indeed, administration of aged splenocytes to young mice induced organ infiltration by senescent immune cells, resulting in increased serum levels of a number of SASP components, including interleukin 1 beta (IL-1β), monocyte chemoattractant protein 1 (MCP-1), and tumor necrosis factor alpha (TNF-α), and biomarkers of liver, pancreas, and kidney damage. Overall, loss of tissue homeostasis driven by an aged immune system was accompanied by a significant lifespan reduction (Yousefzadeh et al., 2021).
2.1. Innate immunity
Age-related remodeling of innate immunity involves all myeloid-derived cells, such as monocytes/macrophages, neutrophils, and natural killer (NK) cells (Cunha et al., 2020). Myeloid cells, especially macrophages, were identified as the main culprits of pro-inflammatory shifting of the innate immune response that accompanies human aging (Franceschi et al., 2000, Prattichizzo et al., 2016). However, during the last years, inflammaging was reconceptualized evolving from a macrophage-centered concept towards a multilevel process (Fulop et al., 2021), in which macrophages (as well as other immune cells) are recruited in peripheral organs, especially visceral fat, promote pro-inflammatory phenotypic changes of parenchymal cells and sustain systemic inflammation (Frasca and Blomberg, 2017). Several studies in humans and animals have reported the most relevant age‐related changes of monocytes, such as an increased absolute number (Della Bella et al., 2007), significant changes in their polarization (Costantini et al., 2018), impaired responses against bacteria and viruses (Metcalf et al., 2017, Molony et al., 2017), and increased secretory ability (Lu et al., 2021).
Other myeloid cells, i.e. polymorphonuclear neutrophilic cells, were also investigated for their contribution to this scenario (Mantovani et al., 2011). Neutrophils, the most abundant immune cells in human blood, play a protective role during bacterial and fungal infections and participate in antiviral defenses by triggering an efficient killing mechanism, characterized by the release of mitochondrial and nuclear chromatin through a regulated process that results in the appearance of the so-called neutrophil extracellular traps (NETs) (Brinkmann et al., 2004). NETs comprise a matrix of DNA and histones which are decorated with antimicrobial proteins, such as myeloperoxidase (MPO), neutrophil elastase (NE), gelatinases, and other proteases (Naumenko et al., 2018). Two main mechanisms resulting in NET formation have been described, i.e., vital and lytic (also known as suicidal) NETosis (Boeltz et al., 2019), with differences according to the nature of the stimuli and the timing of NET release (Castanheira and Kubes, 2019, Fuchs et al., 2007, Yipp and Kubes, 2013). While their role in entrapping and killing large microbes is well established (Fuchs et al., 2007), their antiviral activity is more controversial and has been demonstrated only for some viral strands, such as human immunodeficiency virus-1 (HIV-1) (Saitoh et al., 2012) and respiratory syncytial virus (RSV) (Souza et al., 2018). Viruses can induce NET release both by directly engaging neutrophil pattern recognition receptors (PRRs) or indirectly through the release of pro-inflammatory cytokines by the virus-infected non-immune cells (Schonrich and Raftery, 2016). Of note, the excessive NET formation has been associated with the progression of acute lung injury (ALI) in both infectious, e.g. H1N1 influenza (Narasaraju et al., 2011), and non-communicable, e.g. transfusion-related ALI (Caudrillier et al., 2012), diseases.
Importantly, senescent neutrophils, which are characterized by the up-regulation of CXCR4, adhesion molecules (e.g., Mac-1, ICAM-1), and toll-like receptor (TLR)4 expression (Zhang et al., 2015), display an enhanced release of NETs, suggestive of a shift toward a proinflammatory phenotype (Ortmann and Kolaczkowska, 2018). Formation of NETs can occur also at the intravascular level, especially in the presence of senescent vascular cells (Binet et al., 2020), and histones can be recognized by TLR4 on platelets, thus promoting the phenomenon of immunothrombosis (Engelmann and Massberg, 2013). Excessive activation of immunothrombosis can contribute not only to pulmonary failure but can also result in a systemic prothrombotic condition (Creel-Bulos et al., 2020). In this framework, senescent neutrophils, which are more prone to form NETs, can increase the prothrombotic risk.
Paradoxically, recent studies revealed that the aging process is associated with the activation of an immunosuppressive network, including myeloid-derived suppressor cells (MDSCs) that are the enhancers of other immunosuppressive cells, e.g., regulatory T cells (T regs) and B cells (B regs) (Pawelec et al., 2021, Salminen et al., 2018b). Remarkably, MDSCs comprise a heterogeneous group of myeloid cells originating from the common myeloid progenitor, including monocytic (M-MDSC) and polymorphonuclear (PMN-MDSC) myeloid-derived cells which are immature myeloid cells induced by inflammatory mediators, i.e., IL-6 (Lee et al., 2016, O'Connor et al., 2017). During systemic and chronic inflammation immature neutrophils and monocytes can be recruited from the bone marrow (Drifte et al., 2013) and this set of immature cells could play a central role in the immune paralysis observed after severe inflammation (Mortaz et al., 2018).
The suppressive activity of M-MDSC can be reversed by treatment with TLR7/8 agonists, which induce human M-MDSC to differentiate into M1-like proinflammatory macrophages (Wang et al., 2015). Notably, M-MDSC cultured in presence of the pro-inflammatory cytokine interferon (IFN)γ or the combination of TNFα plus IL-6 differentiate into proinflammatory M1 more efficiently than those treated with TLR7/8 agonists (Bayik et al., 2018). TLR7/8 are involved in the recognition of single-stranded RNA leading to the production of NF-κB-mediated cytokines and type I IFNs (Hornung et al., 2008). Overall, we can argue that MDSC expansion and/or differentiation observed during viral infections in presence of a proinflammatory milieu, could promote M1 macrophage and neutrophil differentiation rather than restraining inflammation through a suppressor activity. These conditions could appear more likely in aged and comorbid patients, rather than in younger healthy subjects.
NK cells can show some relevant dysfunction during aging, exhibiting impaired perforin release upon stimulation and granule exocytosis (Hazeldine and Lord, 2020). Since NK cells are involved in the elimination of senescent cells through interaction between the NKG2D receptor and its ligands expressed on senescent cells (Kale et al., 2020), the age-related remodeling of NK cells could reduce their ability to clear senescent cells (Sagiv et al., 2013). Notably, aging reduces the abundance of an NK subset involved in the resolution of inflammation and elimination of effectors (Almeida-Oliveira et al., 2011).
2.2. Adaptive immunity
Regarding adaptive immunity, reduced number of peripheral blood naïve T and B cells with a relative increase in the frequency of memory cells are considered as hallmarks of immunosenescence, even if the complex reshape of adaptive immune system function during aging is currently under investigation (Fulop et al., 2017, Pawelec et al., 2020). The senescence of T cells, as well as of other cell types, can be triggered by two major cellular mechanisms: replicative and premature senescence. Replicative senescence occurs after several rounds of proliferation leading to a shortening of telomeric ends, whereas premature senescence is telomere-independent, and it is induced by outside factors such as cellular stress (van Deursen, 2014). An extensive proliferation can lead to the emergence of dysfunctional T CD4 + cells with features resembling senescent cells and therefore called senescence-associated T (SA-T) cells, that secrete abundant pro-inflammatory factors reminiscent of SASP acquisition (Fukushima et al., 2018). Data obtained in mouse models indicated that SA-T cells are involved in systemic autoimmunity as well as chronic tissue inflammation following tissue stresses (reviewed in Minato et al., 2020). The progressive loss of immunological expansion of memory cells during aging, especially of CD8 + memory T cells, correlates with reduced proliferative capacity and shortened telomeres of T cells, a phenotype that can be recapitulated during chronic viral infection (Moro-Garcia et al., 2012). Notably, the dysfunctional memory T cells lack telomerase, the enzyme capable of extending and stabilizing chromosome ends, thus promoting replicative senescence (Bellon and Nicot, 2017). Moreover, peripheral blood mononuclear cells (PBMCs) from the elderly contain decreased percentages of naïve CD8 + cells and increased amounts of T effector memory cell re-expressing CD45RA (TEMRA) cells (Koch et al., 2008). These cells show some features of senescent cells, including short telomeres, lack of proliferation, mitochondrial dysfunction, and the release of pro-inflammatory mediators. However, these cells retain their immunosurveillance function against cytomegalovirus (CMV) and, in contrast to senescent cells, can reacquire a proliferating phenotype upon stimulation (Pawelec, 2019b).
Similarly, a remarkable decrease in circulating B lymphocytes occurs during aging as a result of reduced bone marrow output and lack of circulating factors, such as BAFF and APRIL, that promote mature B cell survival (Bulati et al., 2017). Moreover, aging is accompanied by an adaptive increase of IgG-producing memory B cells compared to IgM-producing naïve B cells (Pawelec, 2019a). This change is also reflected by a relative increase of serum IgG levels and a proportional reduction of IgM in aged individuals, where no difference in total immunoglobulin levels was observed (Listi et al., 2006). Interestingly, late memory B cells, which are expanded during aging, express high levels of SASP components, such as TNF-α, IL-6, and IL-8, thus negatively affecting the function of other immune cells (Frasca, 2018). This cell population has been implicated in autoimmune disease (Anolik et al., 2007), impaired response to influenza vaccine (Frasca et al., 2017), and, more recently, in COVID-19, where its expansion was associated with severe disease and poorer outcomes (Woodruff et al., 2020).
Overall, during aging, an imbalance of the anti-inflammatory responses occurs, with a derailment of all the immunity cell types towards a pro-inflammatory activation.
3. Inflammaging and COVID-19
Increasing evidence strongly suggested that SARS-CoV-2 infection stimulates the release of a plethora of pro-inflammatory cytokines and chemokines, which can promote not only local tissue inflammation but also a systemic dangerous inflammatory response called “cytokine storm” (Jafarzadeh et al., 2020). The cytokine storm plays a fundamental role in the development of COVID-19-related complications, such as acute respiratory distress syndrome (ARDS), which is the main cause of death in COVID-19 patients (Wang et al., 2021d). Peripheral blood immune cell profile and related-biomarkers observed in COVID-19 patients confirmed a myeloid cell-driven hyper-inflammation and highlighted the contribution of macrophages and neutrophils to the exacerbation of this inflammatory response.
Regarding macrophages in COVID-19 patients, the acquisition of a macrophage-specific transcriptional program characterized by the upregulation of M2-type molecules was reported in monocytes and monocyte-derived macrophages isolated from the blood of COVID-19 patients (Boumaza et al., 2021). Notably, M2 macrophages are alternatively activated by exposure to the anti-inflammatory IL-10 cytokine (Roszer, 2015), strongly suggesting that SARS-CoV-2 can drive monocytes and macrophages towards host immune paralysis (Boumaza et al., 2021). Several studies confirmed that the hyperinflammatory response to COVID-19 is accompanied by a simultaneous anti-inflammatory response, which is associated with poor outcomes and may increase the risk of bacterial superinfections (Henry et al., 2021).
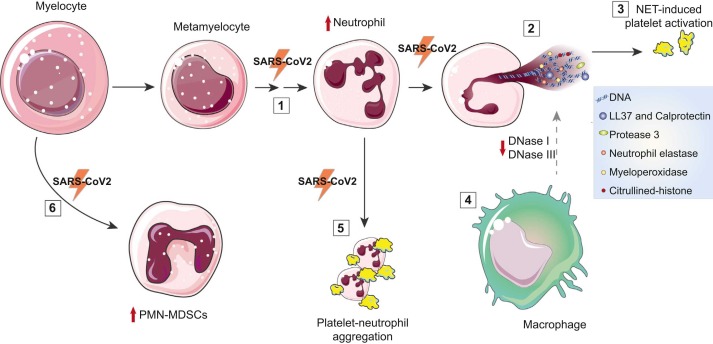
Regarding the contribution of neutrophils to COVID-19 severe outcomes, hyper-NETosis or ineffective clearance of NETotic cells could likely increase the risk of pro-inflammatory and/or pro-coagulant complications, such as thrombosis, through activation of platelets and the coagulation cascade, auto-antibody generation against NETs components, and increased spreading of pro-inflammatory cytokines (Holmes et al., 2019). In this complex framework, NETs interconnect immunity, inflammation, and thrombosis, thus promoting immunothrombosis associated with poorer COVID-19 prognosis (Arroyo et al., 2021a, Cavalcante-Silva et al., 2021) ( Fig. 1 ).
Fig. 1.
Neutrophils in COVID-19 induce a pro-inflammatory and prothrombotic status. (1) Increased neutrophil count is associated with poorer prognosis in COVID-19 patients, which exhibit a left shift of the WBC count with accumulation of immature granulocytes. (2) Direct interaction of SARS-CoV-2 with neutrophils results in increased release of NETs in COVID-19 patients. During NETosis, the enzyme PAD4 catalyzes the conversion of histone arginine to citrulline leading to decondensed chromatin release by neutrophils. Circulating markers of NETosis in COVID-19 patients are citrullinated-histone (Cit-H3), Myeloperoxidase (MPO), proteases – neutrophil elastase (NE) and Protease 3 (PR3) – cell-free DNA, antimicrobial peptides (LL-37), and calprotectin. (3) NETotic cells induce platelet activation, thus promoting coagulation cascade. (4) COVID-19 patients show an ineffective clearance of NETs. NETs are usually degraded by plasma DNases (DNase I and DNase III and are then eliminated by macrophages. DNase I levels are decreased in COVID-19 patients. Auto-antibodies anti-NETs impaired clearance of NETs by DNases. (5) In COVID-19 patients showing signs of acute respiratory distress syndrome (ARDS), the activation of type I IFN signaling in neutrophils is associated with degranulation and formation of circulating neutrophil-platelet aggregates. (6) Myeloid-derived suppressor cell (MDSC) are precursors of monocytes and PMN, including neutrophils. The polymorphonuclear (PMN)-MDSC population is expanded during COVID-19, in particular in patients requiring intensive care treatments (Rowlands et al., 2021). Parts of the figure were provided by Servier Medical Art (https://smart.servier.com).
Several reports have demonstrated that a considerable fraction of the nucleic acids contained in NETs have a mitochondrial origin (Wang et al., 2015). Compared with nuclear DNA, which is protected by histones, mitochondrial DNA (mtDNA) is more vulnerable to injury, including oxidation, and contains unmethylated CpG DNA repeats which act as highly potent TLR9 ligands (Fernandes-Alnemri et al., 2009, Gaidt et al., 2017). While cell-free extracellular DNA can be detected by TLR9, cytosolic DNA induces the intracellular sensors cGAS/STING and AIM2 (Hornung et al., 2008, Ishikawa and Barber, 2008). Since extracellular DNA can be internalized and detected by the cytosolic DNA receptors (Chamilos et al., 2012, Gehrke et al., 2013, Lood et al., 2016, Paludan, 2016), the hyper-oxidation of NET mtDNA can increase its IFN-inducing capacity via the activation of the cGAS-STING axis (Lood et al., 2016). Notably, infection of epithelial cells with SARS-CoV-2 triggered a cGAS-STING mediated activation of the NF-κB pathway, which was not coupled with transcription of IFN and interferon-stimulated genes (ISGs), suggesting that infected epithelial cells contribute to the hyper-inflammatory response observed in severe COVID-19. Interestingly, activation of NF-κB was mediated by the binding of cytosolic DNA released from stressed nuclei and mitochondria rather than by the viral RNA recognizing sensor (Neufeldt et al., 2022).
Since macrophages and dendritic cells (DCs) act as antigen-presenting cells (APCs), the infection of these cells by SARS-CoV-2 can contribute to impairing the adaptive immune responses against the virus. Mononuclear phagocytes, including monocytes, macrophages, and dendritic cells, play substantial roles in fueling systemic inflammation and end-organ malfunctions. Macrophage viral exposure led to robust pro-inflammatory cytokine and chemokine expression but attenuated type I IFN activity (Abdelmoaty et al., 2021).
Patients affected by COVID-19 have a peculiar circulating cell profile, characterized by decreased numbers of circulating T, B, and NK cells and by the presence of CD8 + T cells with a senescent phenotype (Varchetta et al., 2021). Importantly, functional exhaustion and other alterations of NK and T cells were observed in COVID-19 patients with poorer prognoses (Li et al., 2020b). Reduced antiviral cytokine production (Mazzoni et al., 2020) and impaired cytotoxicity (Westmeier et al., 2020) of T lymphocytes and NK cells, in association with expansion of atypical memory B cells (Oliviero et al., 2020), were identified as prominent features of elderly COVID-19 patients. When the impact of age on viral replication, inflammation, innate, and adaptive cellular immune responses was investigated in hospitalized COVID-19 patients, those above 65 years had significantly higher viral load and pro-inflammatory marker levels, in association with inadequate mobilization and activation of monocytes, dendritic cells, NK, and CD8 + T cells compared to patients below 65 years (Cham et al., 2021).
The landscape of peripheral blood immune cell types of patients affected by COVID-19 mirrors the peculiarity of the immune profile of old subjects, characterized by T cell polarization from naive to memory cells, and by exhausted effector and regulatory cells, in association with increased pro-inflammatory monocytes and dendritic cells (Zheng et al., 2020). Overall, these data suggest that the response to COVID-19 mirrors the immune cell polarization remodeling observed during aging. The synergistic effect of diminished responses to pathogens and enhanced responses to self-involving T cells was hypothesized to impact on the age-related clinical severity of COVID-19 (Wang et al., 2021d).
From an immunological perspective, the peculiarities of the immune system of older individuals may explain both the deficiency of effector mechanisms essential to fighting viral pathogens and the exacerbated inflammatory response, which can accelerate and intensify lung damage. Increasing evidence highlighted these relevant correlations between immunosenescence, particularly the increased production of inflammatory cytokines resulting from inflammaging, and the susceptibility of aged individuals to SARS-CoV-2 infection and severe outcomes (Bonafè et al., 2020, Pietrobon et al., 2020).
In this framework, functional biomarkers of inflammaging could contribute to improving the risk stratification of COVID-19 patients in order to identify those groups of patients at higher risk of developing adverse outcomes. Biomarkers of inflammaging could also pave the way for new therapeutic strategies to contain the spread of COVID-19 and to curtail its most severe complications.
4. Circulating biomarkers of inflammaging with potential relevance in COVID-19
With the aim of highlighting the biomarkers with the highest chances to be translated into clinical routine, we will focus our analysis on circulating, and therefore minimally invasive biomarkers of inflammaging, with potential clinical relevance in the context of COVID-19 diagnosis/prognosis. These biomarkers, summarized in Table 1, include variables that already have an established role in the core diagnostic laboratory, e.g. complete blood count and coagulation assays, multivariable parameters that can be assessed by adaptations of already available diagnostic pipelines, e.g. surface biomarkers of immune cells, and biomarkers that are currently limited to the research setting due to their complexity and lack of standardization, i.e. circulating miRNAs and extracellular vesicles. This wide range of complexity allows the construction of minimal sets of circulating biomarkers with rapid turnaround time, high accuracy, and ease of interpretation, which can be incrementally expanded based on the available facilities and expertise.
Table 1.
Summary of the circulating biomarkers with a predictive value for COVID-19 adverse outcomes.
4.1. Circulating biomarkers of inflammation
4.1.1. Neutrophil count and phenotype
The diffuse alveolar damage observed in COVID-19 patients with poorer outcomes does not directly associate with the viral load, supporting the concept of aberrant host immune responses as drivers of tissue injury and pulmonary disease progression (Dorward et al., 2021). A disordered myeloid response was evidenced in circulating cells of patients with mild and severe COVID-19 (Schulte-Schrepping et al., 2020) characterized by increased circulating neutrophil counts (Lai et al., 2020, Li et al., 2020c). It was hypothesized that the elevated neutrophil count in COVID-19 patients and its significant correlation with disease severity could indicate the importance of neutrophils in the progression of COVID-19 complications (Tomar et al., 2020).
Some reports highlighted that the neutrophil-to-lymphocyte ratio is significantly elevated in patients with severe COVID-19, suggesting that this ratio can serve as a clinical marker for predicting fatal complications in patients affected by COVID-19 (Lagunas-Rangel, 2020).
Notably, recent reports suggested that COVID-19 patients do not exhibit neutrophilia, but a shifting of the neutrophil compartment toward an immature profile, a feature suggestive of an emergency myelopoiesis (Linssen et al., 2020, Silvin et al., 2020, Spijkerman et al., 2021). A signature characterized by lung accumulation of naïve lymphoid cells associated with a systemic expansion and activation of myeloid cells with immunosuppressive activity was recently observed in patients with severe COVID-19 outcomes (Bost et al., 2021). A higher frequency of PMN-MDSC was observed in peripheral blood from COVID-19 patients admitted to ICU compared to non-ICU COVID-19 patients and healthy individuals, with a decreasing trend over time from infection (Falck-Jones et al., 2021). PMN-MDSCs from COVID-19 patients expressed markers associated with immune suppressive functions, such as ArgI, TGF-β, and iNOS, and were shown to inhibit T cell proliferation in vitro, thus confirming their central role in the decline of antiviral T-cell responses observed in severe COVID-19 (Sacchi et al., 2020). Taken together, these observations support the need for a more comprehensive evaluation of the circulating blood cell phenotype, which can be achieved also in the routine laboratory setting through the analysis of research parameters provided by hematology analyzers and the use of standardized flow cytometry protocols.
On the functional side, a metabolic rewiring of neutrophils in response to type I IFN resulting in increased degranulation and formation of neutrophil-platelet aggregates in the blood was recently observed in COVID-19 patients with ARDS, confirming the link between dysfunctional neutrophils and the COVID-19 related immunothrombosis (Reyes et al., 2021) (Fig. 1). A recent interesting work showed that a low expression of adhesion molecules together with a high expression of inhibitory receptors in neutrophils from children with COVID-19 might prevent tissue infiltration by neutrophils thus preserving lung function (Seery et al., 2021).
4.1.2. NET related circulating biomarkers
The pathogenic role of NETs has been described for many human communicable and non-communicable diseases (Lee et al., 2017, Zucoloto and Jenne, 2019). Regarding viral infections, a complex cross-regulatory talk exists between IFNs and neutrophils for the activation of appropriate antiviral immune responses and minimization of tissue damages (Stegelmeier et al., 2021). NETs are able to cause lung damage not only by expanding in the pulmonary alveoli but also by directly inducing epithelial and endothelial cell death. NET formation has been reported in several pulmonary diseases, including asthma, chronic obstructive pulmonary disease, influenza, and bacterial pneumonia, among others (Porto and Stein, 2016). Notably, NET release can be induced by the direct interaction of the SARS-CoV-2 virus with neutrophils (Veras et al., 2020).
Significant deregulation of NET markers, such as citrullinated histone H3, cell-free DNA (cfDNA), MPO, and NE was observed in COVID-19 patients and associated with clinical outcomes, suggesting a potential role of circulating NET markers in clinical decision-making (Huckriede et al., 2021). A recent work analyzing protein-protein interaction in naso-oropharyngeal swab samples of COVID − 19 patients and healthy subjects confirmed that the pathways primarily altered in SARS-CoV-2 infected patients are related to neutrophil degranulation (Akgun et al., 2020).
A few recent studies have highlighted the correlation between NET markers, with some relevant markers of in vivo coagulation, fibrinolysis, and endothelial damage (Middleton et al., 2020, Veras et al., 2020, Zuo et al., 2021a, Zuo et al., 2021d). These evidence reinforce the hypothesis of neutrophils at the crossroad between inflammation, coagulation, and endothelial dysfunction that characterize COVID-19 patients with severe outcomes, especially among the older patients.
4.1.3. Citrullinated nucleosomes
NET activation is associated with increased histone citrullination, an epigenetic post-translational modification that converts histone arginine to citrulline, leading to a reduction in hydrogen bonding and a looser chromatin structure. This epigenetic mechanism is catalyzed by a family of enzymes called peptidyl arginine deiminases (PADIs), including PAD4, that in humans has been shown to play a crucial role in chromatin decondensation (Leshner et al., 2012, Thiam et al., 2020). Interestingly, PAD4 relies on proteolysis by calpain to achieve efficient nuclear lamina breakdown and chromatin decondensation (Gosswein et al., 2019).
Citrullination is dispensable for NETosis but potentiates histone-mediated signaling, probably by activating TLR4 signaling (Tsourouktsoglou et al., 2020).
The phenomenon of NET associated with PAD4-dependent hypercitrullination of histones was described for some viral infections, including rhinovirus-induced type-2 allergic asthma (Toussaint et al., 2017) and influenza A virus (IAV) (Hsieh et al., 2021). Recently, elevated citrullination of circulating nucleosomes was observed in patients infected by SARS-CoV-2 (Veras et al., 2020), especially in those with severe diseases (Cavalier et al., 2021).
4.1.4. Myeloperoxidase and neutrophil elastase
MPO catalyzes the production of hypochlorous acid (HClO) from hydrogen peroxide (H2O2) and chloride anion (Cl-). NE is a serine protease playing proinflammatory activity and stimulating mucus secretion. Both MPO and NE can be stored in azurophilic granules and can be released upon degranulation, NET formation, and NETosis (Voglis et al., 2009).
MPO and NE were recently analyzed in COVID-19 patients showing that MPO+ CIt-H3 + cells were significantly higher in COVID-19 patients (Godement et al., 2021) (Fig. 1). Importantly, the lack of decrease of NET blood level between day-1 and day-3 from admission was able to discriminate patients who died within day-28 (Godement et al., 2021).
A recent multicenter study of markers of neutrophil innate immunity in COVID-19 patients and healthy controls showed that blood levels of NE, histone-DNA, MPO-DNA, and free double-stranded DNA (dsDNA) were dramatically increased in COVID-19 patients compared with healthy controls (Gueant et al., 2021). Target neutrophils NET, i.e., through the administration of NE inhibitors, was proposed as an innovative strategy to prevent COVID-19 severe outcomes (Chiang et al., 2020, Mohamed et al., 2020, Sahebnasagh et al., 2020). Aerosol- or nebulizer-dosed of NE inhibitors could significantly improve their efficacy and lower the incidence of adverse effects (Barth et al., 2020).
Several others serine proteases different from NE, such as protease 3 (PR3), cathepsin G (CatG), and neutrophil serine protease 4 (NSP4), can be released by activated neutrophils swarming around the place of pathogen invasion to provoke an immune response. However, proteases can be hijacked by several viruses to prime virus-derived surface proteins and evade immune detection by entering the host cell. NE and PR3, but not CatG, hydrolyze the scissile peptide bond adjacent to the polybasic amino acid sequence of the S1/S2 interface of SARS-CoV-2 (Mustafa et al., 2021).
4.1.5. Cell-free (Cf)-DNA
Elevated levels of cell-free DNA were observed in COVID-19 patients, as well as highly specific markers of NETosis, i.e. MPO-DNA and histone-DNA (his-DNA), along with other typical markers, such as C-reactive protein, D-dimer, and neutrophil count, among others (Middleton et al., 2020, Zuo et al., 2020). Increasing evidence showed that cell-free his-DNA and MPO-DNA complexes are increased in plasma of COVID-19 patients and MPO-DNA levels are correlated with disease severity (Krauel and Duerschmied, 2021, Teluguakula, 2021, Veras et al., 2020).
Notably, the endogenous DNase I levels are persistently decreased in septic patients (Sohrabipour et al., 2021) as well as in COVID-19 patients (Gueant et al., 2021).
4.1.6. Anti-NET antibodies
NET components, on one hand, can directly stimulate both neutrophils and macrophages, which acquire a phenotype of activated antigen-presenting cells, and on the other hand, can also be recognized by autoantibodies. High levels of anti-NET antibodies were observed in patients hospitalized for COVID-19, in association with impaired NET clearance and increased SARS-CoV-2 mediated thromboinflammation (Ding et al., 2021, Zuo et al., 2021c). Mechanistically, anti-NET antibodies of the IgG isotype can impair the ability of DNases to degrade NETs (Zuo et al., 2021c).
When antinuclear antibodies (ANA), anti-neutrophil cytoplasmic antibodies (ANCA) and the degradation capacity of NETs were addressed in serum of COVID-19 patients, a deficient degradation capacity of NETs and increased levels of anti-NET antibodies were observed (Torres-Ruiz et al., 2021). A recent review on the mechanisms by which a viral infection could be exacerbated by, and even trigger, autoimmunity, highlighted that 10–15% of patients with critical COVID-19 pneumonia exhibited autoantibodies against type I INF, and that functional autoantibodies promoting thrombosis or antagonizing cytokine signaling can be detected after SARS-CoV-2 infection (Knight et al., 2021). Interestingly, it was suggested that these autoantibodies may arise from a predominantly extrafollicular B cell response that is more prone to generating autoantibody-secreting B cells.
NETs are composed not only by decondensed chromatin and enzymes but also by molecules such as the Cathelicidin LL-37, Calprotectin (also known as S100A8/S100A9) and high-mobility group protein 1 (HMGB1), which have been linked to inflammation (Lande et al., 2011, Ulas et al., 2017, Urban et al., 2009, Urbonaviciute et al., 2008, Vogl et al., 2007, Zheng et al., 2007).
4.1.7. Cathelicidin LL-37
LL-37 is the only cathelicidin-derived antimicrobial peptide found in humans (Chieosilapatham et al., 2018). It has been found to play additional defensive roles, such as the modulation of the inflammatory response and the chemo-attraction of adaptive immune system cells, to promote re-epithelialization and wound closure. LL-37 is expressed in different cell types, such as epithelial cells and leukocytes, including monocytes, neutrophils, NK, T, and B cells, in inflamed or infected tissue. Recently, it was reported that LL‐37 is mainly expressed by neutrophils and it can promote peripheral neutrophils to form NETs in a dose‐dependent manner (Ribon et al., 2019).
Importantly, LL-37 also functions as an antiviral-activity enhancer. Peripheral neutrophils from chronic rhinosinusitis with nasal polyps (CRSwNP) patients were more susceptible to LL‐37–mediated NET formation, compared with neutrophils derived from control subjects (Cao et al., 2019). It was suggested that LL-37 could be a therapeutic agent for the treatment of viral infections in inflammatory pulmonary diseases (Li et al., 2020a). In the framework of COVID-19, LL-37 is characterized by a high structural similarity to the N-terminal helix of the receptor for SARS-CoV-2, Angiotensin-Converting Enzyme 2, with which the virus interacts (Lokhande et al., 2021). Cell and mouse experiments assessing the antiviral properties of LL-37 against SARS-CoV-2 pseudovirions showed that this antimicrobial peptide can simultaneously block SARS-CoV-2 spike 1 (S1) protein by binding to its receptor-binding domain (RBD) and cloak the ligand-binding domain of host angiotensin-converting enzyme-2 (ACE2). This dual effect, which reduces S1 adherence to ACE2 and further recruitment of this receptor, has been proposed as a potential tool for COVID-19 prevention (Wang et al., 2021a).
Unexpectedly, in psoriatic patients, PMNs mount a fulminant and self-propagating NET and cytokine response, independently of DNA but dependently from RNA that in complex with LL-37 can trigger TLR8/TLR13-mediated cytokines in vitro and in vivo (Herster et al., 2020). Notably, anti-LL-37 antibodies are present in psoriatic arthritis patients (Frasca et al., 2018).
Vitamin D influences the expression of many genes with well-established relevance to airway infections including the transcription of cathelicidin, which is cleaved to generate LL-37. Recently, vitamin D deficiency has been recognized as a risk factor for the incidence and severity of COVID-19 (Vyas et al., 2021). In this regard, the weakened antimicrobial response against SARS-CoV-2 in subjects with reduced levels of vitamin D may be related to reduced LL-37 levels (Crane-Godreau et al., 2020).
4.1.8. Calprotectin
Calprotectin (S100A8/S100A9) is a heterodimer involved in neutrophil-related inflammatory processes. In COVID-19 patients, calprotectin levels were reported to be associated with reduced survival time, especially in patients with severe pulmonary disease (Mahler et al., 2021, Shi et al., 2021). Massive amounts of calprotectin were observed in severe cases of COVID-19 (Silvin et al., 2020). Notably, S100A8/A9 levels were linked to immature neutrophils and non-classical monocyte phenotype, which were able to discriminate mild from severe COVID-19 (Silvin et al., 2020). This evidence suggests an S100A8/A9-driven expansion of myeloid cells in COVID-19 patients with poorer outcomes.
It was therefore hypothesized that S100A8/A9 can be a key agent, leading to potent activation of NF-κB (nuclear factor κ-light-chain-enhancer of activated B cells) through interaction with the receptor for advanced glycation end-products (RAGE) and TLR4, and it could play a role in the expansion of inflammatory monocytes, neutrophils, and platelets that, in turn, can contribute to the hypercoagulable state observed in COVID-19 patients with severe outcomes (Hanssen et al., 2021). This evidence suggests also that the S100A8/A9–RAGE interaction could be increased in diabetic patients affected by COVID-19, increasing the risk of immunothrombosis, pulmonary embolism, and ischemic strokes (Hanssen et al., 2021). Notably, it was recently suggested that S100A8/A9 could serve as a clinically available hematologic index for differential diagnosis of COVID-19 and influenza (Huang et al., 2021).
4.1.9. Monocyte count and phenotype
A study in high-dimensional flow cytometry and RNA-seq of peripheral blood cells from patients affected by COVID-19 revealed that SARS-CoV-2 infection induces changes in the relative abundance of monocyte and neutrophil subsets, with loss of non-classical CD14lowCD16high monocytes and a significant reduction of the HLA-DR expression on classical monocytes (Silvin et al., 2020). These features are characteristics of immunosuppressive M-MDSC, which can be induced by massive exposure of myeloid progenitors to bacterial or viral products. Moreover, as highlighted above, M-MDSC cultured in presence of pro-inflammatory cytokines can differentiate into proinflammatory M1 cells (Bayik et al., 2018).
The immunosuppressive network characterized by an increase in the numbers of MDSC, regulatory T cells (Treg), and macrophages (Mreg/M2c) shows an age-related activation trend (Salminen, 2021). This age-related immunosuppression should prevent the potential inflammatory damages even if it can promote the tissue degeneration associated with aging and age-related diseases. However, when the immunosuppressive network is rapidly induced in a proinflammatory milieu, the immunosuppression can be lost.
Of note, COVID-19 patients had a higher proportion of CD14highCD16 + monocytes than SARS-CoV-2–negative patients (Rebillard et al., 2021). This monocyte subset shows robust reactive oxygen species (ROS) and TNF production and strongly promotes proliferation and antigen presentation to T cells. Interestingly, CD14+CD16+ monocytes in COVID-19 patients showed the features of mixed M1/M2 macrophage polarization with higher expression of CD80 + and CD206 + and secretion of higher levels of the proinflammatory IL-6 and TNF-α cytokines concomitantly with the anti-inflammatory IL-10, when compared with the normal controls (Zhang et al., 2020c).
Recently, CD14 +HLA-DR low monocytes were observed to be expanded in severe COVID-19 patients, a likely consequence of the pervasive emergency myelopoiesis triggered by SARS-CoV-2 infection. Based on gene expression profiles, these cell subsets were deemed to have immune-suppressive features (Bost et al., 2021). Notably, a significant correlation was observed between arginase 1 (ARG1) expression and the immunosuppressive activity of monocytes, with a minor contribution of PD-L1. Overall, the authors suggested that the loss of function in myeloid cells mirrors an immune pathological status progressing from immune paralysis to “immune silence” associated with higher susceptibility to death events (Bost et al., 2021).
4.1.10. Monocyte-related biomarkers
An increased amount of soluble (s)CD14 and (s)CD163, biomarkers of M1 and M2 macrophages polarization, were observed in COVID-19 patients in comparison with healthy subjects (Gomez-Rial et al., 2020). Significant correlations were observed between sCD14 levels and other inflammatory markers, particularly IL-6. Notably, treatment with corticoids showed interference with sCD14 levels.
CD163 is a scavenger receptor that can bind to haptoglobin-hemoglobin complexes, mediating their endocytosis, and its soluble form (sCD163) can be released by activated cells and it is correlated with infective disease severity. In the context of COVID-19, sCD163 levels showed a positive correlation with the presence of ARDs (Gomez-Rial et al., 2020).
These data were confirmed also by an independent study, showing a significant increase in sCD163 and sCD14 plasmatic levels in COVID-19 patients at hospital admission, especially in those who developed ARDS (Zingaropoli et al., 2021). The correlations of these monocyte/macrophage activation markers with typical inflammatory markers of COVID-19 pneumonia, underline their potential use to assess the risk of progression of the disease.
4.1.11. Lymphocyte count and phenotype
An elevated neutrophil count has been observed in hospitalized COVID-19 patients in association with reduced peripheral blood absolute lymphocyte count. In a recent meta-analysis, the reduction of lymphocyte subset counts in COVID-19 patients was investigated across 20 peer-reviewed studies showing that CD4 + T cell, CD8 + T cell, B cell, NK cell, and total lymphocyte cell counts all showed a statistically significant reduction in patients with severe/critical COVID-19 disease compared to mild/moderate disease (Huang et al., 2020).
Interestingly, alterations of the T cell immunophenotype observed in COVID-19 patients share some similarities with the age-related remodeling of this cell population. An increased expression of programmed cell death protein 1 (PD-1) and Tim-3, two markers commonly associated with T cell exhaustion (Sakuishi et al., 2010), was observed in hospitalized COVID-19 patients (Bobcakova et al., 2021, Diao et al., 2020). Moreover, higher frequencies of IFN-γ-expressing CD8 + effector memory cells and CCR7 − CD45RA+ TEMRA cells were associated with severe COVID-19 (Kreutmair et al., 2021).
4.1.12. Circulating Leukocyte Telomere length
The incidence of severe manifestations of COVID-19 increases with age with older patients showing the highest mortality, suggesting that molecular pathways underlying aging contribute to the severity of COVID-19. One of the most well-characterized biomarkers of aging is the progressive shortening of telomeres, which are protective structures at chromosome ends (Mensa et al., 2019). Critically short telomeres impair the regenerative capacity of tissues and trigger loss of tissue homeostasis and disease. Leukocyte telomere length (TL) is associated with natural life span limit in humans (Arbeev et al., 2020).
The SARS-CoV-2 virus infects many different cell types, forcing cell turn-over and regeneration to maintain tissue homeostasis. It was hypothesized that the presence of short telomeres in older patients limits the tissue response to SARS-CoV-2 infection (Aviv, 2020, Tsilingiris et al., 2020).
It was recently proposed that compromised TL-dependent T-cell proliferative response, driven by short telomere in the TL distribution, can contribute to COVID-19 lymphopenia among old adults, inferring that infection with SARS-CoV-2 uncovers the limits of the TL reserves of older persons (Benetos et al., 2021).
TL has been measured in peripheral blood lymphocytes of COVID-19 patients with ages between 29 and 85 years old, showing that shorter telomeres are associated with increased severity of the disease. Individuals within the lower percentiles of TL have a higher risk of developing severe COVID-19 outcomes (Sanchez-Vazquez et al., 2021). A few studies confirmed that shorter leukocyte TL was associated with higher risk of adverse COVID-19 outcomes, independent of several major risk factors for COVID-19 including age (Dos Santos et al., 2021, Wang et al., 2021c).
In a prospective study, TL was measured by Flow-FISH in 70 hospitalized COVID-19 patients and compared to the TL distribution with a reference cohort of 491 healthy volunteers (Froidure et al., 2020).
A significantly higher proportion of patients with short telomeres (<10th percentile) was observed in COVID-19 patients as compared to the reference cohort. Short telomeres were significantly associated with higher risk of critical disease, defined as admission to ICU or death. TL was also negatively correlated with C-reactive protein and neutrophil-to-lymphocyte ratio, and lung tissue from patients with very short telomeres exhibit signs of senescence in structural and immune cells, suggesting that TL influences the severity of the disease (Froidure et al., 2020).
4.1.13. Circulating cytokines: interleukin-6 (IL-6)
The cytokine storm, described in COVID-19 patients, especially in those affected by ARDS and multiorgan dysfunction, is currently recognized as the leading cause of death in COVID-19 critically ill patients (Liu et al., 2020a). Therefore, it is not surprising the increasing studies on IL-6, a pleiotropic cytokine with complex interactions with multiple signalling cascades, at times producing either proinflammatory or anti-inflammatory effects (Velazquez-Salinas et al., 2019). IL-6 exerts its activity mainly through the binding to the cell membrane IL-6 receptor (IL-6R), and to the soluble form of IL-6R (sIL-6R), which is cleaved from the cell membrane (Tanaka and Kishimoto, 2014). The paradigm of IL-6 signal transduction via the membrane-bound IL-6R is called “classic signalling”, whereas when IL-6 signal is activated by the link with sIL-6R, it is referred to as “trans-signalling” (Garbers et al., 2012). Notably, the regenerative or anti-inflammatory activities of IL-6 are mediated by classic signalling whereas pro-inflammatory responses of IL-6 are rather mediated by trans-signalling (Rabe et al., 2008, Scheller et al., 2011). A number of studies highlighted the relevance of IL-6 serum level as a biomarker of healthy/unhealthy aging, demonstrating that IL-6 blood levels increase with advancing age, and high IL-6 circulating levels are associated with increased mortality risk (Ershler et al., 1993, Ferrucci et al., 2005, Giovannini et al., 2011, Wei et al., 1992). On the contrary, low IL-6 serum levels were associated with successful aging (Akbaraly et al., 2013). Overall, these results were confirmed by the Cardiovascular Health Study, by the InCHIANTI study (Michaud et al., 2013, Varadhan et al., 2014) and by the PolSenior study, showing that both IL-6 and CRP levels were good predictors of physical and cognitive performance and the risk of mortality in both the entire elderly population and in successfully aging individuals (Puzianowska-Kuznicka et al., 2016).
Recently, increasing interest was devoted to the anti–IL-6 therapy as a treatment option for COVID-19 patients (Xu et al., 2020). Several randomized control trials investigated the efficacy of anti–IL-6 therapy in COVID-19 patients, i.e. Tocilizumab (TCZ), an interleukin-6 receptor antibody, suggesting that subgroups of patients may benefit from IL-6 blockade treatment (Merad and Martin, 2020), especially when the administration of TCZ starts in an early stage of cytokine storm (IL-6 level < 100 pg/mL) (Li et al., 2021b). Hospitalized COVID-19 patients with hypoxia and systemic inflammation (Recovery Collaborative Group, 2021), or patients with a baseline concentration of IL-6 > 100 pg/mL, particularly if they need oxygen supplementation owing to the lower value of SpO2 ≤ 90% (Flisiak et al., 2021), seems to be the most responsive COVID-19 settings of patients. The most recent review on this issue highlighted high certainty evidence that TCZ reduces the risk of mechanical ventilation in hospitalized patients with severe COVID-19, and moderate certainty evidence that TCZ reduces the risk of poor outcome and the risk of secondary infections in hospitalized COVID-19 patients (Tleyjeh et al., 2021).
Overall, even if the theoretical framework on IL-6 supports a crucial role of cytokine storm and its prototypical cytokine IL-6 in severe COVID-19 outcomes, it remains unknown whether elevated IL-6 in viral infections represents a therapeutic target or part of a functioning adaptive immune response.
4.1.14. Circulating microRNAs modulating inflammatory response: inflamma-miRNAs
A specific group of microRNAs, short non-coding RNA involved in the modulation of gene expression, able to modulate inflammatory processes and therefore defined as inflamma-miRs (Olivieri et al., 2013), was recently investigated in COVID-19 patients in relationship with the clinical response to TCZ (Sabbatinelli et al., 2021). We analyzed miR-146a-5p, miR-21–5p, and miR-126–3p, in 30 serum samples from patients enrolled in a clinical trial assessing the clinical response to a single-dose intravenous infusion of TCZ in COVID-19 patients with multifocal interstitial pneumonia. We showed that COVID-19 patients who did not respond to TCZ had lower serum levels of miR-146a-5p after the treatment and among non-responders, those with the lowest serum levels of miR-146a-5p experienced the most adverse outcome (Sabbatinelli et al., 2021). Indeed, albeit not directly targeting the mRNAs of IL-6 or its receptor, miR-146a-5p acts as a negative regulator of NF-κB, which is a widely acknowledged transcription factor of the IL-6 gene (Su et al., 2020). At the same time, transcription of miR-146a-5p is controlled by NF-κB, even if the two molecules – IL-6 and miR-146a-5p – play opposite roles in the inflammatory process. Our data pointed out towards an imbalance of the IL-6/miR-146a-5p axis in COVID-19 patients with the worst prognosis and showed that a blood-based biomarker, such as miR-146a-5p, can provide clues about the molecular link between inflammaging and COVID-19 clinical course, thus allowing a better understanding of the use of biologic drug armory against this worldwide health threat (Sabbatinelli et al., 2021).
An independent study on miRNAs expression in PBMCs of COVID-19 patients confirmed that miR-146a-3p in association with miR-29a-3p and miR-155–5p may serve as novel biomarker for COVID-19 diagnosis with high specificity and sensitivity (Donyavi et al., 2021).
Importantly, miR-146a is crucially involved in linking inflammation, thrombosis, and NETosis (Arroyo et al., 2021b). Downregulation of microRNA-146a observed in patients with diabetes, obesity and hypertension could also contribute to severe COVID-19 outcomes (Roganovic, 2021).
Other works confirmed the reduction of circulating miR-21–5p and miR-146b-5p levels, in association with other miRNAs, in COVID-19 patients (Li et al., 2021a). Remarkably, miR-16–2–3p was the most upregulated miRNA, and miR-627–5p was the most downregulated miRNA (Li et al., 2021a). From patient transcriptomic data miR-2392 emerged as the circulating miRNA that is directly involved with SARS-CoV-2 machinery during host infection, driving downstream suppression of mitochondrial gene expression, increasing inflammation, glycolysis, and hypoxia as well as promoting many symptoms associated with COVID-19 infection (McDonald et al., 2021).
Overall, increasing evidence support the hypothesis that circulating microRNAs could be innovative tools for COVID-19 patient management, but the analysis of the circulating miRNA signature and its translation to clinical practice requires strict control of a wide array of methodological details (Pinilla et al., 2021).
4.1.15. MiRNAs and cell-free DNA loaded into extracellular vesicles
Both cfDNA and microRNAs present in the bloodstream have been found inside extracellular vesicles (EVs) (Drula et al., 2020). EVs are endogenous nanosized subcellular structures, released in all biological fluids, that can transfer signals among tissues and body compartments (Mensà et al., 2020). It was recently hypothesized that EVs may contribute to the spread SARS-CoV-2 infection by transferring some receptors, such as CD9 and ACE2, which make recipient cells susceptible to virus docking (Hassanpour et al., 2020). Upon entry, SARS-CoV-2 may be directed into the exosomal pathway, and its components are packaged into exosomes for secretion (Hassanpour et al., 2020). Evidence from cellular models demonstrated that SARS-CoV-2 gene products, i.e., Spike, are able to modify the host exosomal cargo by inducing the enrichment of specific miRNAs, which could exert detrimental effects on recipient cells (Mishra and Banerjea, 2021). These preliminary observations suggest that viral infection could modulate exosomal cargoes and explain some of the effects of COVID-19 on non-infected cells and organs.
EV-based therapies characterized by the ability to target multiple pathways and enhance tissue regeneration can hold great potential for COVID-19 related lung injuries. EVs may provide targeted delivery of protease inhibitors, with fewer systemic side effects, are eligible for major aseptic processing, and can be upscaled for mass production (Kumar et al., 2020). However, these therapeutic approaches are not standardized, and dosage and administration routes should be further investigated (Khalaj et al., 2020). Overall, understanding the molecular mechanisms behind EVs related COVID-19 virus infection may provide us with innovative tools to overcome its adverse effects.
4.2. Circulating Biomarkers of coagulation dysfunction
A number of meta-analyses confirmed that venous thromboembolism (VTE) represents a frequent complication in hospitalized COVID-19 patients, especially in the ICU setting (Boonyawat et al., 2020, Jimenez et al., 2021, Lu et al., 2020, Porfidia et al., 2020). The review of the available evidence regarding the anticoagulation approach to prevent VTE among COVID-19 patients admitted to ICU showed a high prevalence of thromboprophylaxis failure among COVID-19 patients, pointing out the need for individualized therapeutic protocols (Hasan et al., 2020). Recent findings suggest that therapeutic doses might be associated with better survival compared to prophylactic doses (Kamel et al., 2021).
Evidence available so far suggested that COVID-19 associated coagulopathy should be regarded as a low-grade disseminated intravascular coagulation (DIC) with features of pulmonary microangiopathy (Asakura and Ogawa, 2021). Autopsy analyses have revealed fibrin-rich thrombi containing neutrophils in the alveolar capillaries and increased lung megakaryocytes producing young platelets which are more thrombogenic (Mitchell, 2020).
A healthy vascular endothelium represents a protective barrier with anti-thrombotic and anti-inflammatory properties. Therefore, it is reasonable to assume that endothelial cell (EC) dysfunction can contribute to COVID-19-associated vascular inflammation, particularly endotheliitis, in the lung, heart, and kidney, as well as COVID-19-associated coagulopathy, specifically pulmonary fibrinous microthrombi in the alveolar capillaries (Zhang et al., 2020a). The EC dysfunction-induced hypercoagulation state can be caused by alterations in the levels of different factors such as plasminogen activator inhibitor 1 (PAI-1), von Willebrand factor (vWF) antigen, soluble thrombomodulin, tissue factor, and tissue factor pathway inhibitor (TFPI) (Norooznezhad and Mansouri, 2021).
4.2.1. von Willebrand factor
von Willebrand factor is a large multimeric protein produced mainly by endothelial cells and stored into Weibel-Palade bodies, which are released upon endothelial activation, such as in the case of systemic inflammation. vWF mediates platelet aggregation and adhesion by binding their GPIX-Ib receptor. In COVID-19, vWF antigen levels have been observed to progressively increase according to disease severity. Notably, in critical patients the ratio between high and low molecular weight vWF multimers is skewed towards the former, suggesting that the activity of the endogenous metalloproteinase ADAMTS13, which physiologically cleaves vWF into smaller and less active multimers, is overwhelmed by the massive release from endothelial cells (Philippe et al., 2021). On the other hand, at the later stages of COVID-19, when most of the endothelial damage has occurred, vWF levels are reduced, suggesting a progressive depletion which is directly related to the magnitude of the cytokine storm and associated with poorer prognosis (Grobler et al., 2020).
4.2.2. Tissue factor (TF) and endothelial anticoagulant systems
The expression of TF, i.e. the main activator of the coagulation cascade, can be enhanced in endothelial cells, platelets, and monocytes upon a plethora of pro-inflammatory stimuli. The involvement of TF in the onset of COVID-19 related thrombotic complications has been demonstrated at varying levels. A higher TF expression was reported in lung specimens from COVID-19 patients with ARDS compared to non-COVID-19 related ARDS samples. Notably, bronchoalveolar areas with the highest TF expression were characterized by the extensive presence of fibrin and PF4 in neighboring blood vessels (Subrahmanian et al., 2021). As a transmembrane protein, TF can also be mounted onto EVs released into the bloodstream by TF-producing cells (Gardiner et al., 2015). This phenomenon gained remarkable importance in the attempt to fully explain the hypercoagulable state associated with many systemic disorders, including cancer, but the precise determination of the EV-associated TF contribution to thrombotic risk is still hampered by many analytical challenges (Hisada and Mackman, 2019). A recent report showed that COVID-19 patients had higher levels of circulating EV-TF activity and that this parameter is able to discriminate patient survival and degree of required respiratory support (Rosell et al., 2021). These findings were confirmed by another study using a different method to assess the formation of antithrombin/Factor VIIa as an indirect measure of blood exposure to TF. Interestingly, complement-mediated stimulation of neutrophils during COVID-19 results in the release of NETs particularly enriched in TF which can enhance the expression of TF by the endothelium and be themselves mediators of immunothrombosis (Skendros et al., 2020).
Not surprisingly, COVID-19 is accompanied by a progressive increase in the levels of endothelial surface proteins participating in the major endogenous anticoagulant systems, including TFPI, soluble EPCR, and thrombomodulin, which on one side further support the evidence of a high degree of endothelial activation and, on the other hand, poses some difference between COVID-19 coagulopathy and overt DIC, where coagulation inhibitors are gradually exhausted upon the persistence of the procoagulant stimuli (Francischetti et al., 2021).
In this framework, assessment of circulating TF activity and levels of the endogenous endothelial anticoagulant systems could enhance the predictive value of the routinely assessed coagulation biomarkers and offer more in-depth mechanistic insights into COVID-19 related coagulopathy.
4.2.3. Abnormalities of the fibrinolytic system
Multiple evidence showed that fibrinolysis is impaired in COVID-19 patients. The global activity of the fibrinolytic system is the result of the balance between pro-fibrinolytic, including t-PA and u-PA, and anti-fibrinolytic factors, such as PAI-1, TAFI, and alfa2-antiplasmin. PAI-1 is released by many cellular types, including endothelial cells, following damage to the vascular bed, presence of thrombin, stimulation by inflammatory cytokines, and angiotensin II (Morrow et al., 2021). For this reason, PAI-1 has been proposed as a bridge between inflammation and coagulation, and its circulating levels are generally high in patients with systemic inflammatory conditions. During COVID-19, upregulation of both t-PA and PAI-1 was observed, so that it has been hypothesized that PAI-1 overwhelms the effects of t-PA resulting in a net prothrombotic state. Interestingly, both PAI-1 and t-PA levels were positively related to the neutrophil count and degree of activation, assessed by the means of circulating calprotectin. The elevation of PAI-1 levels has been confirmed by multiple reports and related to the extent of hypofibrinolysis evaluated ex vivo through thromboelastographic tests (Blasi et al., 2020). Similarly, levels of thrombin-activatable fibrinolysis inhibitor (TAFI), which prevents the degradation of fibrin by plasmin, were found to be higher in COVID-19 patients admitted to ICU compared to non-ICU patients, independently of pre-existing conditions and thromboprophylaxis (Nougier et al., 2020). These results suggest that circulating biomarkers of fibrinolysis should be considered in addition to the assessment of the hypercoagulability state in patients presenting with severe COVID-19.
4.3. Circulating Biomarkers of hyperglycemia-induced damage
4.3.1. Soluble receptors for Advanced-glycation end-products (sRAGE)
Obesity and type 2 diabetes are major factors in COVID-19 causing a progression to excessive morbidity and mortality. An important characteristic of these conditions is poor glycemic control leading to inappropriate chemical reactions and the production of Advanced Glycation End-products (AGEs) (Birts and Wilton, 2021). RAGE plays a central role in the inflammatory response activating NF-κB, and the subsequent production of inflammatory cytokines. During the COVID-19 pandemic, many clinical reports have pointed out that diabetes (DM) increases the risk of COVID-19 complications, hospitalization requirements, as well as the overall severe acute case-fatality rate. It was suggested that the basal activation state of the RAGE axis in common preexisting conditions in DM patients such as endothelial dysfunction and hyperglycemia-related prothrombotic phenotype, as well as the contribution of RAGE signaling in lung inflammation, may then lead to the increased mortality risk of COVID-19 in these patients (Rojas et al., 2021). Since RAGE is mainly expressed by type 2 epithelial cells in the alveolar sac, which has a critical role in SARS-CoV-2-associated hyper inflammation and lung injury, it was suggested that AGEs, through their interaction with RAGE amongst other molecules, could modulate COVID-19 pathogenesis and related comorbidities, especially in the elderly (Sellegounder et al., 2021). Recent data strongly support this hypothesis, confirming that serum sRAGE concentrations are positively associated with COVID-19 disease severity, and sRAGE levels could be considered as an interesting biomarker for predicting mortality in COVID-19 patients (Lim et al., 2021).
5. Conclusion and future perspectives
Altogether, the findings that we reviewed strongly suggest that even if SARS-CoV-2 infection can affect subjects of different ages, including children. However, the most severe COVID-19 outcomes are expected in elderly subjects, and age, age-related diseases risk factors and age-related diseases are, overall, the main risk factors for COVID-19 mortality (Santesmasses et al., 2020). This observation is not trivial since it suggests that COVID-19, and deadly respiratory diseases in general, may be targeted, in addition to antiviral approaches, by approaches that target or delay the aging process and, on the other hand, that selected biomarkers of inflammaging and immunosenescence should be implemented into the routine clinical practice for predicting COVID-19 prognosis.
Funding
This work was supported by grants from Ministero della Salute, Italy (Ricerca Corrente to IRCCS INRCA) and Università Politecnica delle Marche (RSA grant) to FO.
Declarations of interest
none.
Data Availability
No data was used for the research described in the article.
References
- Abdelmoaty M.M., Yeapuri P., Machhi J., Olson K.E., Shahjin F., Kumar V., Zhou Y., Liang J., Pandey K., Acharya A., Byrareddy S.N., Mosley R.L., Gendelman H.E. Defining the innate immune responses for SARS-CoV-2-Human Macrophage Interactions. Front. Immunol. 2021;12 doi: 10.3389/fimmu.2021.741502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akbaraly T.N., Hamer M., Ferrie J.E., Lowe G., Batty G.D., Hagger-Johnson G., Singh-Manoux A., Shipley M.J., Kivimaki M. Chronic inflammation as a determinant of future aging phenotypes. CMAJ. 2013;185:E763–E770. doi: 10.1503/cmaj.122072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akgun E., Tuzuner M.B., Sahin B., Kilercik M., Kulah C., Cakiroglu H.N., Serteser M., Unsal I., Baykal A.T. Proteins associated with neutrophil degranulation are upregulated in nasopharyngeal swabs from SARS-CoV-2 patients. PLoS One. 2020;15 doi: 10.1371/journal.pone.0240012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almeida-Oliveira A., Smith-Carvalho M., Porto L.C., Cardoso-Oliveira J., Ribeiro Ados S., Falcao R.R., Abdelhay E., Bouzas L.F., Thuler L.C., Ornellas M.H., Diamond H.R. Age-related changes in natural killer cell receptors from childhood through old age. Hum. Immunol. 2011;72:319–329. doi: 10.1016/j.humimm.2011.01.009. [DOI] [PubMed] [Google Scholar]
- Anolik J.H., Barnard J., Owen T., Zheng B., Kemshetti S., Looney R.J., Sanz I. Delayed memory B cell recovery in peripheral blood and lymphoid tissue in systemic lupus erythematosus after B cell depletion therapy. Arthritis Rheum. 2007;56:3044–3056. doi: 10.1002/art.22810. [DOI] [PubMed] [Google Scholar]
- Arbeev K.G., Verhulst S., Steenstrup T., Kark J.D., Bagley O., Kooperberg C., Reiner A.P., Hwang S.J., Levy D., Fitzpatrick A.L., Christensen K., Yashin A.I., Aviv A. Association of leukocyte telomere length with mortality among adult participants in 3 longitudinal studies. JAMA Netw. Open. 2020;3 doi: 10.1001/jamanetworkopen.2020.0023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arroyo A.B., Aguila S., Fernandez-Perez M.P., Reyes-Garcia A.M.L., Reguilon-Gallego L., Zapata-Martinez L., Vicente V., Martinez C., Gonzalez-Conejero R. miR-146a in cardiovascular diseases and sepsis: an additional burden in the inflammatory balance? Thromb. Haemost. 2021;121:1138–1150. doi: 10.1055/a-1342-3648. [DOI] [PubMed] [Google Scholar]
- Arroyo A.B., Fernandez-Perez M.P., Del Monte A., Aguila S., Mendez R., Hernandez-Antolin R., Garcia-Barber N., de Los Reyes-Garcia A.M., Gonzalez-Jimenez P., Arcas M.I., Vicente V., Menendez R., Andres V., Gonzalez-Conejero R., Martinez C. miR-146a is a pivotal regulator of neutrophil extracellular trap formation promoting thrombosis. Haematologica. 2021;106:1636–1646. doi: 10.3324/haematol.2019.240226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asakura H., Ogawa H. COVID-19-associated coagulopathy and disseminated intravascular coagulation. Int. J. Hematol. 2021;113:45–57. doi: 10.1007/s12185-020-03029-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aviv A. Telomeres and COVID-19. FASEB J. 2020;34:7247–7252. doi: 10.1096/fj.202001025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barth P., Bruijnzeel P., Wach A., Sellier Kessler O., Hooftman L., Zimmermann J., Naue N., Huber B., Heimbeck I., Kappeler D., Timmer W., Chevalier E. Single dose escalation studies with inhaled POL6014, a potent novel selective reversible inhibitor of human neutrophil elastase, in healthy volunteers and subjects with cystic fibrosis. J. Cyst. Fibros. 2020;19:299–304. doi: 10.1016/j.jcf.2019.08.020. [DOI] [PubMed] [Google Scholar]
- Bayik D., Tross D., Klinman D.M. Factors influencing the differentiation of human monocytic myeloid-derived suppressor cells into inflammatory macrophages. Front. Immunol. 2018;9:608. doi: 10.3389/fimmu.2018.00608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellon M., Nicot C. Telomere dynamics in immune senescence and exhaustion triggered by chronic viral infection. Viruses. 2017:9. doi: 10.3390/v9100289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benetos A., Lai T.P., Toupance S., Labat C., Verhulst S., Gautier S., Ungeheuer M.N., Perret-Guillaume C., Levy D., Susser E., Aviv A. The nexus between telomere length and lymphocyte count in seniors hospitalized with COVID-19. J. Gerontol. A Biol. Sci. Med. Sci. 2021;76:e97–e101. doi: 10.1093/gerona/glab026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binet F., Cagnone G., Crespo-Garcia S., Hata M., Neault M., Dejda A., Wilson A.M., Buscarlet M., Mawambo G.T., Howard J.P., Diaz-Marin R., Parinot C., Guber V., Pilon F., Juneau R., Laflamme R., Sawchyn C., Boulay K., Leclerc S., Abu-Thuraia A., Cote J.F., Andelfinger G., Rezende F.A., Sennlaub F., Joyal J.S., Mallette F.A., Sapieha P. Neutrophil extracellular traps target senescent vasculature for tissue remodeling in retinopathy. Science. 2020:369. doi: 10.1126/science.aay5356. [DOI] [PubMed] [Google Scholar]
- Birts C.N., Wilton D.C. Age, obesity and hyperglycaemia: Activation of innate immunity initiates a series of molecular interactions involving anionic surfaces leading to COVID-19 morbidity and mortality. Med. Hypotheses. 2021;155 doi: 10.1016/j.mehy.2021.110646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blasi A., von Meijenfeldt F.A., Adelmeijer J., Calvo A., Ibanez C., Perdomo J., Reverter J.C., Lisman T. In vitro hypercoagulability and ongoing in vivo activation of coagulation and fibrinolysis in COVID-19 patients on anticoagulation. J. Thromb. Haemost. 2020;18:2646–2653. doi: 10.1111/jth.15043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bobcakova A., Petriskova J., Vysehradsky R., Kocan I., Kapustova L., Barnova M., Diamant Z., Jesenak M. Immune profile in patients with COVID-19: lymphocytes exhaustion markers in relationship to clinical outcome. Front. Cell. Infect. Microbiol. 2021;11 doi: 10.3389/fcimb.2021.646688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boeltz S., Amini P., Anders H.J., Andrade F., Bilyy R., Chatfield S., Cichon I., Clancy D.M., Desai J., Dumych T., Dwivedi N., Gordon R.A., Hahn J., Hidalgo A., Hoffmann M.H., Kaplan M.J., Knight J.S., Kolaczkowska E., Kubes P., Leppkes M., Manfredi A.A., Martin S.J., Maueroder C., Maugeri N., Mitroulis I., Munoz L.E., Nakazawa D., Neeli I., Nizet V., Pieterse E., Radic M.Z., Reinwald C., Ritis K., Rovere-Querini P., Santocki M., Schauer C., Schett G., Shlomchik M.J., Simon H.U., Skendros P., Stojkov D., Vandenabeele P., Berghe T.V., van der Vlag J., Vitkov L., von Kockritz-Blickwede M., Yousefi S., Zarbock A., Herrmann M. To NET or not to NET:current opinions and state of the science regarding the formation of neutrophil extracellular traps. Cell Death Differ. 2019;26:395–408. doi: 10.1038/s41418-018-0261-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonafè M., Prattichizzo F., Giuliani A., Storci G., Sabbatinelli J., Olivieri F. Inflamm-aging: why older men are the most susceptible to SARS-CoV-2 complicated outcomes. Cytokine Growth Factor Rev. 2020;53:33–37. doi: 10.1016/j.cytogfr.2020.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boonyawat K., Chantrathammachart P., Numthavaj P., Nanthatanti N., Phusanti S., Phuphuakrat A., Niparuck P., Angchaisuksiri P. Incidence of thromboembolism in patients with COVID-19: a systematic review and meta-analysis. Thromb. J. 2020;18:34. doi: 10.1186/s12959-020-00248-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bost P., De Sanctis F., Cane S., Ugel S., Donadello K., Castellucci M., Eyal D., Fiore A., Anselmi C., Barouni R.M., Trovato R., Caligola S., Lamolinara A., Iezzi M., Facciotti F., Mazzariol A., Gibellini D., De Nardo P., Tacconelli E., Gottin L., Polati E., Schwikowski B., Amit I., Bronte V. Deciphering the state of immune silence in fatal COVID-19 patients. Nat. Commun. 2021;12:1428. doi: 10.1038/s41467-021-21702-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boumaza A., Gay L., Mezouar S., Bestion E., Diallo A.B., Michel M., Desnues B., Raoult D., La Scola B., Halfon P., Vitte J., Olive D., Mege J.L. Monocytes and macrophages, targets of severe acute respiratory syndrome coronavirus 2: the clue for coronavirus disease 2019 immunoparalysis. J. Infect. Dis. 2021;224:395–406. doi: 10.1093/infdis/jiab044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brinkmann V., Reichard U., Goosmann C., Fauler B., Uhlemann Y., Weiss D.S., Weinrauch Y., Zychlinsky A. Neutrophil extracellular traps kill bacteria. Science. 2004;303:1532–1535. doi: 10.1126/science.1092385. [DOI] [PubMed] [Google Scholar]
- Bulati M., Caruso C., Colonna-Romano G. From lymphopoiesis to plasma cells differentiation, the age-related modifications of B cell compartment are influenced by “inflamm-ageing”. Ageing Res. Rev. 2017;36:125–136. doi: 10.1016/j.arr.2017.04.001. [DOI] [PubMed] [Google Scholar]
- Cai J., Li H., Zhang C., Chen Z., Liu H., Lei F., Qin J.J., Liu Y.M., Zhou F., Song X., Zhou J., Zhao Y.C., Wu B., He M., Yang H., Zhu L., Zhang P., Ji Y.X., Zhao G.N., Lu Z., Liu L., Mao W., Liao X., Lu H., Wang D., Xia X., Huang X., Wei X., Xia J., Zhang B.H., Yuan Y., She Z.G., Xu Q., Ma X., Wang Y., Yang J., Zhang X., Zhang X.J., Li H. The neutrophil-to-lymphocyte ratio determines clinical efficacy of corticosteroid therapy in patients with COVID-19. Cell Metab. 2021;33 doi: 10.1016/j.cmet.2021.01.002. 258-269 e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao Y., Chen F., Sun Y., Hong H., Wen Y., Lai Y., Xu Z., Luo X., Chen Y., Shi J., Li H. LL-37 promotes neutrophil extracellular trap formation in chronic rhinosinusitis with nasal polyps. Clin. Exp. Allergy. 2019;49:990–999. doi: 10.1111/cea.13408. [DOI] [PubMed] [Google Scholar]
- Castanheira F.V.S., Kubes P. Neutrophils and NETs in modulating acute and chronic inflammation. Blood. 2019;133:2178–2185. doi: 10.1182/blood-2018-11-844530. [DOI] [PubMed] [Google Scholar]
- Caudrillier A., Kessenbrock K., Gilliss B.M., Nguyen J.X., Marques M.B., Monestier M., Toy P., Werb Z., Looney M.R. Platelets induce neutrophil extracellular traps in transfusion-related acute lung injury. J. Clin. Invest. 2012;122:2661–2671. doi: 10.1172/JCI61303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavalcante-Silva L.H.A., Carvalho D.C.M., Lima E.A., Galvao J., da Silva J.S.F., Sales-Neto J.M., Rodrigues-Mascarenhas S. Neutrophils and COVID-19: the road so far. Int. Immunopharmacol. 2021;90 doi: 10.1016/j.intimp.2020.107233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavalier E., Guiot J., Lechner K., Dutsch A., Eccleston M., Herzog M., Bygott T., Schomburg A., Kelly T., Holdenrieder S. Circulating nucleosomes as potential markers to monitor COVID-19 disease progression. Front. Mol. Biosci. 2021;8 doi: 10.3389/fmolb.2021.600881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cham L.B., Pahus M.H., Gronhoj K., Olesen R., Ngo H., Monrad I., Kjolby M., Tolstrup M., Gunst J.D., Sogaard O.S. Effect of age on innate and adaptive immunity in hospitalized COVID-19 patients. J. Clin. Med. 2021:10. doi: 10.3390/jcm10204798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chamilos G., Gregorio J., Meller S., Lande R., Kontoyiannis D.P., Modlin R.L., Gilliet M. Cytosolic sensing of extracellular self-DNA transported into monocytes by the antimicrobial peptide LL37. Blood. 2012;120:3699–3707. doi: 10.1182/blood-2012-01-401364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiang C.C., Korinek M., Cheng W.J., Hwang T.L. Targeting neutrophils to treat acute respiratory distress syndrome in coronavirus disease. Front. Pharm. 2020;11 doi: 10.3389/fphar.2020.572009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chieosilapatham P., Ikeda S., Ogawa H., Niyonsaba F. Tissue-specific regulation of innate immune responses by human cathelicidin LL-37. Curr. Pharm. Des. 2018;24:1079–1091. doi: 10.2174/1381612824666180327113418. [DOI] [PubMed] [Google Scholar]
- Coppe J.P., Patil C.K., Rodier F., Sun Y., Munoz D.P., Goldstein J., Nelson P.S., Desprez P.Y., Campisi J. Senescence-associated secretory phenotypes reveal cell-nonautonomous functions of oncogenic RAS and the p53 tumor suppressor. PLoS Biol. 2008;6:2853–2868. doi: 10.1371/journal.pbio.0060301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costantini A., Viola N., Berretta A., Galeazzi R., Matacchione G., Sabbatinelli J., Storci G., De Matteis S., Butini L., Rippo M.R., Procopio A.D., Caraceni D., Antonicelli R., Olivieri F., Bonafè M. Age-related M1/M2 phenotype changes in circulating monocytes from healthy/unhealthy individuals. Aging. 2018;10:1268–1280. doi: 10.18632/aging.101465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotter A.H., Yang S.T., Shafi H., Cotter T.M., Palmer-Toy D.E. Elevated von Willebrand factor antigen is an early predictor of mortality and prolonged length of stay for coronavirus disease 2019 (COVID-19) inpatients. Arch. Pathol. Lab. Med. 2021 doi: 10.5858/arpa.2021-0255-SA. [DOI] [PubMed] [Google Scholar]
- Crane-Godreau M.A., Clem K.J., Payne P., Fiering S. Vitamin D deficiency and air pollution exacerbate COVID-19 through suppression of antiviral peptide LL37. Front. Public Health. 2020;8:232. doi: 10.3389/fpubh.2020.00232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creel-Bulos C., Liu M., Auld S.C., Gaddh M., Kempton C.L., Sharifpour M., Sniecinski R.M., Maier C.L., Nahab F.B., Rangaraju S. Trends and diagnostic value of D-dimer levels in patients hospitalized with coronavirus disease 2019. Medicine. 2020;99 doi: 10.1097/MD.0000000000023186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunha L.L., Perazzio S.F., Azzi J., Cravedi P., Riella L.V. Remodeling of the immune response with aging: immunosenescence and its potential impact on COVID-19 immune response. Front. Immunol. 2020;11:1748. doi: 10.3389/fimmu.2020.01748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Gonzalo-Calvo D., Benitez I.D., Pinilla L., Carratala A., Moncusi-Moix A., Gort-Paniello C., Molinero M., Gonzalez J., Torres G., Bernal M., Pico S., Almansa R., Jorge N., Ortega A., Bustamante-Munguira E., Gomez J.M., Gonzalez-Rivera M., Micheloud D., Ryan P., Martinez A., Tamayo L., Aldecoa C., Ferrer R., Ceccato A., Fernandez-Barat L., Motos A., Riera J., Menendez R., Garcia-Gasulla D., Penuelas O., Torres A., Bermejo-Martin J.F., Barbe F., Project C. Circulating microRNA profiles predict the severity of COVID-19 in hospitalized patients. Transl. Res. 2021;236:147–159. doi: 10.1016/j.trsl.2021.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de la Morena-Barrio M.E., Bravo-Perez C., Minano A., de la Morena-Barrio B., Fernandez-Perez M.P., Bernal E., Gomez-Verdu J.M., Herranz M.T., Vicente V., Corral J., Lozano M.L. Prognostic value of thrombin generation parameters in hospitalized COVID-19 patients. Sci. Rep. 2021;11:7792. doi: 10.1038/s41598-021-85906-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Della Bella S., Bierti L., Presicce P., Arienti R., Valenti M., Saresella M., Vergani C., Villa M.L. Peripheral blood dendritic cells and monocytes are differently regulated in the elderly. Clin. Immunol. 2007;122:220–228. doi: 10.1016/j.clim.2006.09.012. [DOI] [PubMed] [Google Scholar]
- Dhar S.K., K., V., Damodar S., Gujar S., Das M. IL-6 and IL-10 as predictors of disease severity in COVID-19 patients: results from meta-analysis and regression. Heliyon. 2021;7 doi: 10.1016/j.heliyon.2021.e06155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diao B., Wang C., Tan Y., Chen X., Liu Y., Ning L., Chen L., Li M., Liu Y., Wang G., Yuan Z., Feng Z., Zhang Y., Wu Y., Chen Y. Reduction and functional exhaustion of T cells in patients with coronavirus disease 2019 (COVID-19) Front. Immunol. 2020;11:827. doi: 10.3389/fimmu.2020.00827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding J., Hostallero D.E., El Khili M.R., Fonseca G.J., Milette S., Noorah N., Guay-Belzile M., Spicer J., Daneshtalab N., Sirois M., Tremblay K., Emad A., Rousseau S. A network-informed analysis of SARS-CoV-2 and hemophagocytic lymphohistiocytosis genes’ interactions points to Neutrophil extracellular traps as mediators of thrombosis in COVID-19. PLoS Comput. Biol. 2021;17 doi: 10.1371/journal.pcbi.1008810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donyavi T., Bokharaei-Salim F., Baghi H.B., Khanaliha K., Alaei Janat-Makan M., Karimi B., Sadri Nahand J., Mirzaei H., Khatami A., Garshasbi S., Khoshmirsafa M., Jalal Kiani S. Acute and post-acute phase of COVID-19: analyzing expression patterns of miRNA-29a-3p, 146a-3p, 155-5p, and let-7b-3p in PBMC. Int. Immunopharmacol. 2021;97 doi: 10.1016/j.intimp.2021.107641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorward D.A., Russell C.D., Um I.H., Elshani M., Armstrong S.D., Penrice-Randal R., Millar T., Lerpiniere C.E.B., Tagliavini G., Hartley C.S., Randle N.P., Gachanja N.N., Potey P.M.D., Dong X., Anderson A.M., Campbell V.L., Duguid A.J., Al Qsous W., BouHaidar R., Baillie J.K., Dhaliwal K., Wallace W.A., Bellamy C.O.C., Prost S., Smith C., Hiscox J.A., Harrison D.J., Lucas C.D. Tissue-specific immunopathology in fatal COVID-19. Am. J. Respir. Crit. Care Med. 2021;203:192–201. doi: 10.1164/rccm.202008-3265OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dos Santos G.A., Pimenta R., Viana N.I., Guimaraes V.R., Romao P., Candido P., de Camargo J.A., Hatanaka D.M., Queiroz P.G., Teruya A., Leite K.R.M., Srougi V., Srougi M., Reis S.T. Shorter leukocyte telomere length is associated with severity of COVID-19 infection. Biochem. Biophys. Rep. 2021;27 doi: 10.1016/j.bbrep.2021.101056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drifte G., Dunn-Siegrist I., Tissieres P., Pugin J. Innate immune functions of immature neutrophils in patients with sepsis and severe systemic inflammatory response syndrome. Crit. Care Med. 2013;41:820–832. doi: 10.1097/CCM.0b013e318274647d. [DOI] [PubMed] [Google Scholar]
- Drula R., Ott L.F., Berindan-Neagoe I., Pantel K., Calin G.A. MicroRNAs from liquid biopsy derived extracellular vesicles: recent advances in detection and characterization methods. Cancers. 2020:12. doi: 10.3390/cancers12082009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engelmann B., Massberg S. Thrombosis as an intravascular effector of innate immunity. Nat. Rev. Immunol. 2013;13:34–45. doi: 10.1038/nri3345. [DOI] [PubMed] [Google Scholar]
- Ershler W.B., Sun W.H., Binkley N., Gravenstein S., Volk M.J., Kamoske G., Klopp R.G., Roecker E.B., Daynes R.A., Weindruch R. Interleukin-6 and aging: blood levels and mononuclear cell production increase with advancing age and in vitro production is modifiable by dietary restriction. Lymphokine Cytokine Res. 1993;12:225–230. [PubMed] [Google Scholar]
- Falck-Jones S., Vangeti S., Yu M., Falck-Jones R., Cagigi A., Badolati I., Osterberg B., Lautenbach M.J., Ahlberg E., Lin A., Lepzien R., Szurgot I., Lenart K., Hellgren F., Maecker H., Salde J., Albert J., Johansson N., Bell M., Lore K., Farnert A., Smed-Sorensen A. Functional monocytic myeloid-derived suppressor cells increase in blood but not airways and predict COVID-19 severity. J. Clin. Investig. 2021:131. doi: 10.1172/JCI144734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandes-Alnemri T., Yu J.W., Datta P., Wu J., Alnemri E.S. AIM2 activates the inflammasome and cell death in response to cytoplasmic DNA. Nature. 2009;458:509–513. doi: 10.1038/nature07710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrucci L., Corsi A., Lauretani F., Bandinelli S., Bartali B., Taub D.D., Guralnik J.M., Longo D.L. The origins of age-related proinflammatory state. Blood. 2005;105:2294–2299. doi: 10.1182/blood-2004-07-2599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrucci L., Fabbri E. Inflammageing: chronic inflammation in ageing, cardiovascular disease, and frailty. Nat. Rev. Cardiol. 2018;15:505–522. doi: 10.1038/s41569-018-0064-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flisiak R., Jaroszewicz J., Rogalska M., Lapinski T., Berkan-Kawinska A., Bolewska B., Tudrujek-Zdunek M., Kozielewicz D., Rorat M., Leszczynski P., Klos K., Kowalska J., Pabjan P., Piekarska A., Mozer-Lisewska I., Tomasiewicz K., Pawlowska M., Simon K., Polanska J., Zarebska-Michaluk D. Tocilizumab improves the prognosis of COVID-19 in patients with high IL-6. J. Clin. Med. 2021:10. doi: 10.3390/jcm10081583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franceschi C., Bonafe M., Valensin S., Olivieri F., De Luca M., Ottaviani E., De Benedictis G. Inflamm-aging. An evolutionary perspective on immunosenescence. Ann. N. Y Acad. Sci. 2000;908:244–254. doi: 10.1111/j.1749-6632.2000.tb06651.x. [DOI] [PubMed] [Google Scholar]
- Francischetti I.M.B., Toomer K., Zhang Y., Jani J., Siddiqui Z., Brotman D.J., Hooper J.E., Kickler T.S. Upregulation of pulmonary tissue factor, loss of thrombomodulin and immunothrombosis in SARS-CoV-2 infection. EClinicalMedicine. 2021;39 doi: 10.1016/j.eclinm.2021.101069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frasca D. Senescent B cells in aging and age-related diseases: their role in the regulation of antibody responses. Exp. Gerontol. 2018;107:55–58. doi: 10.1016/j.exger.2017.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frasca D., Blomberg B.B. Adipose tissue inflammation induces B cell inflammation and decreases B cell function in aging. Front. Immunol. 2017;8:1003. doi: 10.3389/fimmu.2017.01003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frasca D., Diaz A., Romero M., Blomberg B.B. Human peripheral late/exhausted memory B cells express a senescent-associated secretory phenotype and preferentially utilize metabolic signaling pathways. Exp. Gerontol. 2017;87:113–120. doi: 10.1016/j.exger.2016.12.001. [DOI] [PubMed] [Google Scholar]
- Frasca L., Palazzo R., Chimenti M.S., Alivernini S., Tolusso B., Bui L., Botti E., Giunta A., Bianchi L., Petricca L., Auteri S.E., Spadaro F., Fonti G.L., Falchi M., Evangelista A., Marinari B., Pietraforte I., Spinelli F.R., Colasanti T., Alessandri C., Conti F., Gremese E., Costanzo A., Valesini G., Perricone R., Lande R. Anti-LL37 antibodies are present in psoriatic arthritis (PsA) patients: new biomarkers in PsA. Front. Immunol. 2018;9:1936. doi: 10.3389/fimmu.2018.01936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Froidure A., Mahieu M., Hoton D., Laterre P.F., Yombi J.C., Koenig S., Ghaye B., Defour J.P., Decottignies A. Short telomeres increase the risk of severe COVID-19. Aging. 2020;12:19911–19922. doi: 10.18632/aging.104097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs T.A., Abed U., Goosmann C., Hurwitz R., Schulze I., Wahn V., Weinrauch Y., Brinkmann V., Zychlinsky A. Novel cell death program leads to neutrophil extracellular traps. J. Cell Biol. 2007;176 doi: 10.1083/jcb.200606027. 231-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukushima Y., Minato N., Hattori M. The impact of senescence-associated T cells on immunosenescence and age-related disorders. Inflamm. Regen. 2018;38:24. doi: 10.1186/s41232-018-0082-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fulop T., Larbi A., Dupuis G., Le Page A., Frost E.H., Cohen A.A., Witkowski J.M., Franceschi C. Immunosenescence and inflamm-aging as two sides of the same coin: friends or foes? Front. Immunol. 2017;8:1960. doi: 10.3389/fimmu.2017.01960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fulop T., Larbi A., Pawelec G., Khalil A., Cohen A.A., Hirokawa K., Witkowski J.M., Franceschi C. Immunology of Aging: the Birth of Inflammaging. Clin. Rev. Allergy Immunol. 2021 doi: 10.1007/s12016-021-08899-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaidt M.M., Ebert T.S., Chauhan D., Ramshorn K., Pinci F., Zuber S., O’Duill F., Schmid-Burgk J.L., Hoss F., Buhmann R., Wittmann G., Latz E., Subklewe M., Hornung V. The DNA Inflammasome in Human Myeloid Cells Is Initiated by a STING-Cell Death Program Upstream of NLRP3. Cell. 2017;171 doi: 10.1016/j.cell.2017.09.039. 1110-1124 e18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garbers C., Hermanns H.M., Schaper F., Muller-Newen G., Grotzinger J., Rose-John S., Scheller J. Plasticity and cross-talk of interleukin 6-type cytokines. Cytokine Growth Factor Rev. 2012;23:85–97. doi: 10.1016/j.cytogfr.2012.04.001. [DOI] [PubMed] [Google Scholar]
- Gardiner C., Harrison P., Belting M., Boing A., Campello E., Carter B.S., Collier M.E., Coumans F., Ettelaie C., van Es N., Hochberg F.H., Mackman N., Rennert R.C., Thaler J., Rak J., Nieuwland R. Extracellular vesicles, tissue factor, cancer and thrombosis - discussion themes of the ISEV 2014 educational day. J. Extra Vesicles. 2015;4:26901. doi: 10.3402/jev.v4.26901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gehrke T.H., Lischke U., Gasteiger K.L., Schneider S., Arnold S., Muller H.C., Stephenson D.S., Zipse H., Carell T. Unexpected non-Hoogsteen-based mutagenicity mechanism of FaPy-DNA lesions. Nat. Chem. Biol. 2013;9:455–461. doi: 10.1038/nchembio.1254. [DOI] [PubMed] [Google Scholar]
- Giovannini S., Onder G., Liperoti R., Russo A., Carter C., Capoluongo E., Pahor M., Bernabei R., Landi F. Interleukin-6, C-reactive protein, and tumor necrosis factor-alpha as predictors of mortality in frail, community-living elderly individuals. J. Am. Geriatr. Soc. 2011;59:1679–1685. doi: 10.1111/j.1532-5415.2011.03570.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godement M., Zhu J., Cerf C., Vieillard-Baron A., Maillon A., Zuber B., Bardet V., Geri G. Neutrophil extracellular traps in SARS-CoV2 related pneumonia in ICU patients: the NETCOV2 study. Front. Med. 2021;8 doi: 10.3389/fmed.2021.615984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez-Rial J., Curras-Tuala M.J., Rivero-Calle I., Gomez-Carballa A., Cebey-Lopez M., Rodriguez-Tenreiro C., Dacosta-Urbieta A., Rivero-Velasco C., Rodriguez-Nunez N., Trastoy-Pena R., Rodriguez-Garcia J., Salas A., Martinon-Torres F. Increased Serum Levels of sCD14 and sCD163 Indicate a Preponderant Role for Monocytes in COVID-19 Immunopathology. Front. Immunol. 2020;11 doi: 10.3389/fimmu.2020.560381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gosswein S., Lindemann A., Mahajan A., Maueroder C., Martini E., Patankar J., Schett G., Becker C., Wirtz S., Naumann-Bartsch N., Bianchi M.E., Greer P.A., Lochnit G., Herrmann M., Neurath M.F., Leppkes M. Citrullination licenses calpain to decondense nuclei in neutrophil extracellular trap formation. Front. Immunol. 2019;10:2481. doi: 10.3389/fimmu.2019.02481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grobler C., Maphumulo S.C., Grobbelaar L.M., Bredenkamp J.C., Laubscher G.J., Lourens P.J., Steenkamp J., Kell D.B., Pretorius E. Covid-19: the rollercoaster of fibrin(Ogen), D-Dimer, Von Willebrand Factor, P-selectin and their interactions with endothelial cells, platelets and erythrocytes. Int. J. Mol. Sci. 2020:21. doi: 10.3390/ijms21145168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Group R.C. Tocilizumab in patients admitted to hospital with COVID-19 (RECOVERY): a randomised, controlled, open-label, platform trial. Lancet. 2021;397:1637–1645. doi: 10.1016/S0140-6736(21)00676-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gueant J.L., Gueant-Rodriguez R.M., Fromonot J., Oussalah A., Louis H., Chery C., Gette M., Gleye S., Callet J., Raso J., Blanchecotte F., Lacolley P., Guieu R., Regnault V. Elastase and exacerbation of neutrophil innate immunity are involved in multi-visceral manifestations of COVID-19. Allergy. 2021;76:1846–1858. doi: 10.1111/all.14746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guervilly C., Bonifay A., Burtey S., Sabatier F., Cauchois R., Abdili E., Arnaud L., Lano G., Pietri L., Robert T., Velier M., Papazian L., Albanese J., Kaplanski G., Dignat-George F., Lacroix R. Dissemination of extreme levels of extracellular vesicles: tissue factor activity in patients with severe COVID-19. Blood Adv. 2021;5:628–634. doi: 10.1182/bloodadvances.2020003308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanssen N.M.J., Spaetgens B., Nagareddy P.R., Murphy A.J. DAMPening mortality in COVID-19: therapeutic insights from basic cardiometabolic studies on S100A8/A9. Circulation. 2021;143:971–973. doi: 10.1161/CIRCULATIONAHA.120.053025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasan S.S., Radford S., Kow C.S., Zaidi S.T.R. Venous thromboembolism in critically ill COVID-19 patients receiving prophylactic or therapeutic anticoagulation: a systematic review and meta-analysis. J. Thromb. Thrombolysis. 2020;50:814–821. doi: 10.1007/s11239-020-02235-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassanpour M., Rezaie J., Nouri M., Panahi Y. The role of extracellular vesicles in COVID-19 virus infection. Infect. Genet. Evol. 2020;85 doi: 10.1016/j.meegid.2020.104422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hazeldine J., Lord J.M. Immunesenescence: a predisposing risk factor for the development of COVID-19? Front. Immunol. 2020;11 doi: 10.3389/fimmu.2020.573662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry B.M., Benoit S.W., Vikse J., Berger B.A., Pulvino C., Hoehn J., Rose J., Santos de Oliveira M.H., Lippi G., Benoit J.L. The anti-inflammatory cytokine response characterized by elevated interleukin-10 is a stronger predictor of severe disease and poor outcomes than the pro-inflammatory cytokine response in coronavirus disease 2019 (COVID-19) Clin. Chem. Lab. Med. 2021;59:599–607. doi: 10.1515/cclm-2020-1284. [DOI] [PubMed] [Google Scholar]
- Hernandez-Segura A., Nehme J., Demaria M. Hallmarks of cellular senescence. Trends Cell Biol. 2018;28:436–453. doi: 10.1016/j.tcb.2018.02.001. [DOI] [PubMed] [Google Scholar]
- Herster F., Bittner Z., Archer N.K., Dickhofer S., Eisel D., Eigenbrod T., Knorpp T., Schneiderhan-Marra N., Loffler M.W., Kalbacher H., Vierbuchen T., Heine H., Miller L.S., Hartl D., Freund L., Schakel K., Heister M., Ghoreschi K., Weber A.N.R. Neutrophil extracellular trap-associated RNA and LL37 enable self-amplifying inflammation in psoriasis. Nat. Commun. 2020;11:105. doi: 10.1038/s41467-019-13756-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hisada Y., Mackman N. Measurement of tissue factor activity in extracellular vesicles from human plasma samples. Res. Pract. Thromb. Haemost. 2019;3:44–48. doi: 10.1002/rth2.12165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes C.L., Shim D., Kernien J., Johnson C.J., Nett J.E., Shelef M.A. Insight into neutrophil extracellular traps through systematic evaluation of citrullination and peptidylarginine deiminases. J. Immunol. Res. 2019;2019 doi: 10.1155/2019/2160192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hornung V., Barchet W., Schlee M., Hartmann G. RNA recognition via TLR7 and TLR8. Handb. Exp. Pharm. 2008:71–86. doi: 10.1007/978-3-540-72167-3_4. [DOI] [PubMed] [Google Scholar]
- Hsieh I.N., White M., Hoeksema M., Deluna X., Hartshorn K. Histone H4 potentiates neutrophil inflammatory responses to influenza A virus: down-modulation by H4 binding to C-reactive protein and Surfactant protein D. PLoS One. 2021;16 doi: 10.1371/journal.pone.0247605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang W., Berube J., McNamara M., Saksena S., Hartman M., Arshad T., Bornheimer S.J., O’Gorman M. Lymphocyte subset counts in COVID-19 patients: a meta-analysis. Cytometry A. 2020;97:772–776. doi: 10.1002/cyto.a.24172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang W., Li M., Luo G., Wu X., Su B., Zhao L., Zhang S., Chen X., Jia M., Zhu J., Su W., Zhang D. The inflammatory factors associated with disease severity to predict COVID-19 progression. J. Immunol. 2021;206:1597–1608. doi: 10.4049/jimmunol.2001327. [DOI] [PubMed] [Google Scholar]
- Huckriede J., de Vries F., Hultstrom M., Wichapong K., Reutelingsperger C., Lipcsey M., Garcia de Frutos P., Frithiof R., Nicolaes G.A.F. Histone H3 cleavage in severe COVID-19 ICU patients. Front. Cell. Infect. Microbiol. 2021;11 doi: 10.3389/fcimb.2021.694186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphreys D., ElGhazaly M., Frisan T. Senescence and host-pathogen interactions. Cells. 2020:9. doi: 10.3390/cells9071747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishikawa H., Barber G.N. STING is an endoplasmic reticulum adaptor that facilitates innate immune signalling. Nature. 2008;455:674–678. doi: 10.1038/nature07317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jafarzadeh A., Chauhan P., Saha B., Jafarzadeh S., Nemati M. Contribution of monocytes and macrophages to the local tissue inflammation and cytokine storm in COVID-19: lessons from SARS and MERS, and potential therapeutic interventions. Life Sci. 2020;257 doi: 10.1016/j.lfs.2020.118102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jimenez D., Garcia-Sanchez A., Rali P., Muriel A., Bikdeli B., Ruiz-Artacho P., Le Mao R., Rodriguez C., Hunt B.J., Monreal M. Incidence of VTE and bleeding among hospitalized patients with coronavirus disease 2019: a systematic review and meta-analysis. Chest. 2021;159:1182–1196. doi: 10.1016/j.chest.2020.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kale A., Sharma A., Stolzing A., Desprez P.Y., Campisi J. Role of immune cells in the removal of deleterious senescent cells. Immun. Ageing. 2020;17:16. doi: 10.1186/s12979-020-00187-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamel A.M., Sobhy M., Magdy N., Sabry N., Farid S. Anticoagulation outcomes in hospitalized Covid-19 patients: a systematic review and meta-analysis of case-control and cohort studies. Rev. Med. Virol. 2021;31 doi: 10.1002/rmv.2180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khalaj K., Figueira R.L., Antounians L., Lauriti G., Zani A. Systematic review of extracellular vesicle-based treatments for lung injury: are EVs a potential therapy for COVID-19? J. Extra Vesicles. 2020;9 doi: 10.1080/20013078.2020.1795365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight J.S., Caricchio R., Casanova J.L., Combes A.J., Diamond B., Fox S.E., Hanauer D.A., James J.A., Kanthi Y., Ladd V., Mehta P., Ring A.M., Sanz I., Selmi C., Tracy R.P., Utz P.J., Wagner C.A., Wang J.Y., McCune W.J. The intersection of COVID-19 and autoimmunity. J. Clin. Investig. 2021 doi: 10.1172/JCI154886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch S., Larbi A., Derhovanessian E., Ozcelik D., Naumova E., Pawelec G. Multiparameter flow cytometric analysis of CD4 and CD8 T cell subsets in young and old people. Immun. Ageing. 2008;5:6. doi: 10.1186/1742-4933-5-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krauel K., Duerschmied D. ICODE: the international COVID-19 thrombosis biomarkers colloquium-novel soluble biomarkers: circulating cell-free nucleic acids and other molecules. J. Thromb. Thrombolysis. 2021 doi: 10.1007/s11239-021-02468-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreutmair S., Unger S., Nunez N.G., Ingelfinger F., Alberti C., De Feo D., Krishnarajah S., Kauffmann M., Friebel E., Babaei S., Gaborit B., Lutz M., Jurado N.P., Malek N.P., Goepel S., Rosenberger P., Haberle H.A., Ayoub I., Al-Hajj S., Nilsson J., Claassen M., Liblau R., Martin-Blondel G., Bitzer M., Roquilly A., Becher B. Distinct immunological signatures discriminate severe COVID-19 from non-SARS-CoV-2-driven critical pneumonia. Immunity. 2021;54 doi: 10.1016/j.immuni.2021.05.002. 1578-1593 e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuluozturk M., Deveci F., Turgut T., Oner O. The Glasgow Prognostic Score and fibrinogen to albumin ratio as prognostic factors in hospitalized patients with COVID-19. Expert Rev. Respir. Med. 2021;15:1061–1068. doi: 10.1080/17476348.2021.1923483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar S., Zhi K., Mukherji A., Gerth K. Repurposing antiviral protease inhibitors using extracellular vesicles for potential therapy of COVID-19. Viruses. 2020:12. doi: 10.3390/v12050486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagnado A., Leslie J., Ruchaud-Sparagano M.H., Victorelli S., Hirsova P., Ogrodnik M., Collins A.L., Vizioli M.G., Habiballa L., Saretzki G., Evans S.A., Salmonowicz H., Hruby A., Geh D., Pavelko K.D., Dolan D., Reeves H.L., Grellscheid S., Wilson C.H., Pandanaboyana S., Doolittle M., von Zglinicki T., Oakley F., Gallage S., Wilson C.L., Birch J., Carroll B., Chapman J., Heikenwalder M., Neretti N., Khosla S., Masuda C.A., Tchkonia T., Kirkland J.L., Jurk D., Mann D.A., Passos J.F. Neutrophils induce paracrine telomere dysfunction and senescence in ROS-dependent manner. EMBO J. 2021;40 doi: 10.15252/embj.2020106048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagunas-Rangel F.A. Neutrophil-to-lymphocyte ratio and lymphocyte-to-C-reactive protein ratio in patients with severe coronavirus disease 2019 (COVID-19): a meta-analysis. J. Med. Virol. 2020;92:1733–1734. doi: 10.1002/jmv.25819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai J., Ma S., Wang Y., Cai Z., Hu J., Wei N., Wu J., Du H., Chen T., Li R., Tan H., Kang L., Yao L., Huang M., Wang H., Wang G., Liu Z., Hu S. Factors associated with mental health outcomes among health care workers exposed to coronavirus disease 2019. JAMA Netw. Open. 2020;3 doi: 10.1001/jamanetworkopen.2020.3976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lande R., Ganguly D., Facchinetti V., Frasca L., Conrad C., Gregorio J., Meller S., Chamilos G., Sebasigari R., Riccieri V., Bassett R., Amuro H., Fukuhara S., Ito T., Liu Y.J., Gilliet M. Neutrophils activate plasmacytoid dendritic cells by releasing self-DNA-peptide complexes in systemic lupus erythematosus. Sci. Transl. Med. 2011;3 doi: 10.1126/scitranslmed.3001180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee B.R., Kwon B.E., Hong E.H., Shim A., Song J.H., Kim H.M., Chang S.Y., Kim Y.J., Kweon M.N., Youn J.I., Ko H.J. Interleukin-10 attenuates tumour growth by inhibiting interleukin-6/signal transducer and activator of transcription 3 signalling in myeloid-derived suppressor cells. Cancer Lett. 2016;381:156–164. doi: 10.1016/j.canlet.2016.07.012. [DOI] [PubMed] [Google Scholar]
- Lee D.S., Ma S., Chu A., Wang C.X., Wang X., Austin P.C., McAlister F.A., Kalmady S.V., Kapral M.K., Kaul P., Ko D.T., Rochon P.A., Schull M.J., Rubin B.B., Wang B., Collaboration C. Predictors of mortality among long-term care residents with SARS-CoV-2 infection. J. Am. Geriatr. Soc. 2021 doi: 10.1111/jgs.17425. [DOI] [PubMed] [Google Scholar]
- Lee K.H., Kronbichler A., Park D.D., Park Y., Moon H., Kim H., Choi J.H., Choi Y., Shim S., Lyu I.S., Yun B.H., Han Y., Lee D., Lee S.Y., Yoo B.H., Lee K.H., Kim T.L., Kim H., Shim J.S., Nam W., So H., Choi S., Lee S., Shin J.I. Neutrophil extracellular traps (NETs) in autoimmune diseases: a comprehensive review. Autoimmun. Rev. 2017;16:1160–1173. doi: 10.1016/j.autrev.2017.09.012. [DOI] [PubMed] [Google Scholar]
- Leshner M., Wang S., Lewis C., Zheng H., Chen X.A., Santy L., Wang Y. PAD4 mediated histone hypercitrullination induces heterochromatin decondensation and chromatin unfolding to form neutrophil extracellular trap-like structures. Front. Immunol. 2012;3:307. doi: 10.3389/fimmu.2012.00307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C.X., Chen J., Lv S.K., Li J.H., Li L.L., Hu X. Whole-transcriptome RNA sequencing reveals significant differentially expressed mRNAs, miRNAs, and lncRNAs and related regulating biological pathways in the peripheral blood of COVID-19 patients. Mediat. Inflamm. 2021;2021 doi: 10.1155/2021/6635925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li K., Tao N., Zheng L., Sun T. LL-37 restored glucocorticoid sensitivity impaired by virus dsRNA in lung. Int. Immunopharmacol. 2020;79 doi: 10.1016/j.intimp.2019.106057. [DOI] [PubMed] [Google Scholar]
- Li M., Guo W., Dong Y., Wang X., Dai D., Liu X., Wu Y., Li M., Zhang W., Zhou H., Zhang Z., Lin L., Kang Z., Yu T., Tian C., Qin R., Gui Y., Jiang F., Fan H., Heissmeyer V., Sarapultsev A., Wang L., Luo S., Hu D. Elevated exhaustion levels of NK and CD8(+) T cells as indicators for progression and prognosis of COVID-19 disease. Front. Immunol. 2020;11 doi: 10.3389/fimmu.2020.580237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li P., Lu Z., Li Q., Wang Z., Guo Y., Cai C., Wang S., Liu P., Su X., Huang Y., Dong Y., Qiu W., Ling Y., Yarmus L., Luo F., Zeng L., Bai C., Zhang W. Administration timing and efficacy of tocilizumab in patients with COVID-19 and elevated IL-6. Front Mol. Biosci. 2021;8 doi: 10.3389/fmolb.2021.651662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X., Xu S., Yu M., Wang K., Tao Y., Zhou Y., Shi J., Zhou M., Wu B., Yang Z., Zhang C., Yue J., Zhang Z., Renz H., Liu X., Xie J., Xie M., Zhao J. Risk factors for severity and mortality in adult COVID-19 inpatients in Wuhan. J. Allergy Clin. Immunol. 2020;146:110–118. doi: 10.1016/j.jaci.2020.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim A., Radujkovic A., Weigand M.A., Merle U. Soluble receptor for advanced glycation end products (sRAGE) as a biomarker of COVID-19 disease severity and indicator of the need for mechanical ventilation, ARDS and mortality. Ann. Intensive Care. 2021;11:50. doi: 10.1186/s13613-021-00836-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linssen J., Ermens A., Berrevoets M., Seghezzi M., Previtali G., van der Sar-van der Brugge S., Russcher H., Verbon A., Gillis J., Riedl J., de Jongh E., Saker J., Munster M., Munnix I.C., Dofferhof A., Scharnhorst V., Ammerlaan H., Deiteren K., Bakker S.J., Van Pelt L.J., Kluiters-de Hingh Y., Leers M.P., van der Ven A.J. A novel haemocytometric COVID-19 prognostic score developed and validated in an observational multicentre European hospital-based study. Elife. 2020:9. doi: 10.7554/eLife.63195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Listi F., Candore G., Modica M.A., Russo M., Di Lorenzo G., Esposito-Pellitteri M., Colonna-Romano G., Aquino A., Bulati M., Lio D., Franceschi C., Caruso C. A study of serum immunoglobulin levels in elderly persons that provides new insights into B cell immunosenescence. Ann. N. Y Acad. Sci. 2006;1089:487–495. doi: 10.1196/annals.1386.013. [DOI] [PubMed] [Google Scholar]
- Liu B., Li M., Zhou Z., Guan X., Xiang Y. Can we use interleukin-6 (IL-6) blockade for coronavirus disease 2019 (COVID-19)-induced cytokine release syndrome (CRS)? J. Autoimmun. 2020;111 doi: 10.1016/j.jaut.2020.102452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z., Li J., Chen D., Gao R., Zeng W., Chen S., Huang Y., Huang J., Long W., Li M., Guo L., Wang X., Wu X. Dynamic interleukin-6 level changes as a prognostic indicator in patients with COVID-19. Front. Pharm. 2020;11:1093. doi: 10.3389/fphar.2020.01093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lokhande K.B., Banerjee T., Swamy K.V., Ghosh P., Deshpande M. An in silico scientific basis for LL-37 as a therapeutic for Covid-19. Proteins. 2021 doi: 10.1002/prot.26198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long H., Nie L., Xiang X., Li H., Zhang X., Fu X., Ren H., Liu W., Wang Q., Wu Q. D-dimer and prothrombin time are the significant indicators of severe COVID-19 and poor prognosis. Biomed. Res. Int. 2020;2020 doi: 10.1155/2020/6159720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lood C., Blanco L.P., Purmalek M.M., Carmona-Rivera C., De Ravin S.S., Smith C.K., Malech H.L., Ledbetter J.A., Elkon K.B., Kaplan M.J. Neutrophil extracellular traps enriched in oxidized mitochondrial DNA are interferogenic and contribute to lupus-like disease. Nat. Med. 2016;22:146–153. doi: 10.1038/nm.4027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Castaneda S., Garcia-Larragoiti N., Cano-Mendez A., Blancas-Ayala K., Damian-Vazquez G., Perez-Medina A.I., Chora-Hernandez L.D., Arean-Martinez C., Viveros-Sandoval M.E. Inflammatory and prothrombotic biomarkers associated with the severity of COVID-19 infection. Clin. Appl. Thromb. Hemost. 2021;27 doi: 10.1177/1076029621999099. 1076029621999099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu R.J., Wang E.K., Benayoun B.A. Functional genomics of inflamm-aging and immunosenescence. Brief. Funct. Genom. 2021 doi: 10.1093/bfgp/elab009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Y.F., Pan L.Y., Zhang W.W., Cheng F., Hu S.S., Zhang X., Jiang H.Y. A meta-analysis of the incidence of venous thromboembolic events and impact of anticoagulation on mortality in patients with COVID-19. Int J. Infect. Dis. 2020;100:34–41. doi: 10.1016/j.ijid.2020.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahler M., Meroni P.L., Infantino M., Buhler K.A., Fritzler M.J. Circulating calprotectin as a biomarker of COVID-19 severity. Expert Rev. Clin. Immunol. 2021;17:431–443. doi: 10.1080/1744666X.2021.1905526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantovani A., Cassatella M.A., Costantini C., Jaillon S. Neutrophils in the activation and regulation of innate and adaptive immunity. Nat. Rev. Immunol. 2011;11:519–531. doi: 10.1038/nri3024. [DOI] [PubMed] [Google Scholar]
- Mazzoni A., Salvati L., Maggi L., Capone M., Vanni A., Spinicci M., Mencarini J., Caporale R., Peruzzi B., Antonelli A., Trotta M., Zammarchi L., Ciani L., Gori L., Lazzeri C., Matucci A., Vultaggio A., Rossi O., Almerigogna F., Parronchi P., Fontanari P., Lavorini F., Peris A., Rossolini G.M., Bartoloni A., Romagnani S., Liotta F., Annunziato F., Cosmi L. Impaired immune cell cytotoxicity in severe COVID-19 is IL-6 dependent. J. Clin. Investig. 2020;130:4694–4703. doi: 10.1172/JCI138554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald, J.T., Enguita, F.J., Taylor, D., Griffin, R.J., Priebe, W., Emmett, M.R., McGrath, M., Sajadi, M.M., Harris, A.D., Clement, J., Dybas, J.M., Aykin-Burns, N., Guarnieri, J.W., Singh, L.N., Grabham, P., Baylin, S.B., Yousey, A., Pearson, A.N., Corry, P.M., Saravia-Butler, A., Aunins, T.R., Nagpal, P., Meydan, C., Foox, J., Mozsary, C., Cerqueira, B., Zaksas, V., Singh, U., Wurtele, E.S., Costes, S.V., Galeano, D., Paccanaro, A., Meinig, S.L., Hagan, R.S., Bowman, N.M., Consortium, U.C.-P., Wolfgang, M.C., Altinok, S., Sapoval, N., Treangen, T.J., Frieman, M., Vanderburg, C., Wallace, D.C., Schisler, J., Mason, C.E., Chatterjee, A., Meller, R. and Beheshti, A., 2021. The Great Deceiver: miR-2392's Hidden Role in Driving SARS-CoV-2 Infection. bioRxiv.
- Mennuni M.G., Rolla R., Grisafi L., Spinoni E.G., Rognoni A., Lio V., Castello L.M., Sainaghi P.P., Pirisi M., Avanzi G.C., Krengli M., Bellan M., Ferrante D., Aimaretti G., Dianzani U., Patti G. Interaction between thrombin potential and age on early clinical outcome in patients hospitalized for COVID-19. J. Thromb. Thrombolysis. 2021;52:746–753. doi: 10.1007/s11239-021-02497-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mensà E., Guescini M., Giuliani A., Bacalini M.G., Ramini D., Corleone G., Ferracin M., Fulgenzi G., Graciotti L., Prattichizzo F., Sorci L., Battistelli M., Monsurrò V., Bonfigli A.R., Cardelli M., Recchioni R., Marcheselli F., Latini S., Maggio S., Fanelli M., Amatori S., Storci G., Ceriello A., Stocchi V., De Luca M., Magnani L., Rippo M.R., Procopio A.D., Sala C., Budimir I., Bassi C., Negrini M., Garagnani P., Franceschi C., Sabbatinelli J., Bonafè M., Olivieri F. Small extracellular vesicles deliver miR-21 and miR-217 as pro-senescence effectors to endothelial cells. J. Extra Vesicles. 2020;9 doi: 10.1080/20013078.2020.1725285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mensa E., Latini S., Ramini D., Storci G., Bonafe M., Olivieri F. The telomere world and aging: Analytical challenges and future perspectives. Ageing Res. Rev. 2019;50:27–42. doi: 10.1016/j.arr.2019.01.004. [DOI] [PubMed] [Google Scholar]
- Merad M., Martin J.C. Pathological inflammation in patients with COVID-19: a key role for monocytes and macrophages. Nat. Rev. Immunol. 2020;20:448. doi: 10.1038/s41577-020-0331-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metcalf T.U., Wilkinson P.A., Cameron M.J., Ghneim K., Chiang C., Wertheimer A.M., Hiscott J.B., Nikolich-Zugich J., Haddad E.K. Human monocyte subsets are transcriptionally and functionally altered in aging in response to pattern recognition receptor agonists. J. Immunol. 2017;199:1405–1417. doi: 10.4049/jimmunol.1700148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michaud M., Balardy L., Moulis G., Gaudin C., Peyrot C., Vellas B., Cesari M., Nourhashemi F. Proinflammatory cytokines, aging, and age-related diseases. J. Am. Med Dir. Assoc. 2013;14:877–882. doi: 10.1016/j.jamda.2013.05.009. [DOI] [PubMed] [Google Scholar]
- Middleton E.A., He X.Y., Denorme F., Campbell R.A., Ng D., Salvatore S.P., Mostyka M., Baxter-Stoltzfus A., Borczuk A.C., Loda M., Cody M.J., Manne B.K., Portier I., Harris E.S., Petrey A.C., Beswick E.J., Caulin A.F., Iovino A., Abegglen L.M., Weyrich A.S., Rondina M.T., Egeblad M., Schiffman J.D., Yost C.C. Neutrophil extracellular traps contribute to immunothrombosis in COVID-19 acute respiratory distress syndrome. Blood. 2020;136:1169–1179. doi: 10.1182/blood.2020007008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minato N., Hattori M., Hamazaki Y. Physiology and pathology of T-cell aging. Int. Immunol. 2020;32:223–231. doi: 10.1093/intimm/dxaa006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishra R., Banerjea A.C. SARS-CoV-2 spike targets USP33-IRF9 axis via exosomal miR-148a to activate human microglia. Front. Immunol. 2021;12 doi: 10.3389/fimmu.2021.656700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell W.B. Thromboinflammation in COVID-19 acute lung injury. Paediatr. Respir. Rev. 2020;35:20–24. doi: 10.1016/j.prrv.2020.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohamed M.M.A., El-Shimy I.A., Hadi M.A. Neutrophil elastase inhibitors: a potential prophylactic treatment option for SARS-CoV-2-induced respiratory complications? Crit. Care. 2020;24:311. doi: 10.1186/s13054-020-03023-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molony R.D., Nguyen J.T., Kong Y., Montgomery R.R., Shaw A.C., Iwasaki A. Aging impairs both primary and secondary RIG-I signaling for interferon induction in human monocytes. Sci. Signal. 2017:10. doi: 10.1126/scisignal.aan2392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moro-Garcia M.A., Alonso-Arias R., Lopez-Larrea C. Molecular mechanisms involved in the aging of the T-cell immune response. Curr. Genom. 2012;13:589–602. doi: 10.2174/138920212803759749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrow G.B., Whyte C.S., Mutch N.J. A serpin with a finger in many PAIs: PAI-1’s central function in thromboinflammation and cardiovascular disease. Front. Cardiovasc. Med. 2021;8 doi: 10.3389/fcvm.2021.653655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mortaz E., Alipoor S.D., Adcock I.M., Mumby S., Koenderman L. Update on neutrophil function in severe inflammation. Front. Immunol. 2018;9:2171. doi: 10.3389/fimmu.2018.02171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mustafa Z., Zhanapiya A., Kalbacher H., Burster T. Neutrophil elastase and proteinase 3 cleavage sites are adjacent to the polybasic sequence within the proteolytic sensitive activation loop of the SARS-CoV-2 spike protein. ACS Omega. 2021;6:7181–7185. doi: 10.1021/acsomega.1c00363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narasaraju T., Yang E., Samy R.P., Ng H.H., Poh W.P., Liew A.A., Phoon M.C., van Rooijen N., Chow V.T. Excessive neutrophils and neutrophil extracellular traps contribute to acute lung injury of influenza pneumonitis. Am. J. Pathol. 2011;179:199–210. doi: 10.1016/j.ajpath.2011.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naumenko V., Turk M., Jenne C.N., Kim S.J. Neutrophils in viral infection. Cell Tissue Res. 2018;371:505–516. doi: 10.1007/s00441-017-2763-0. [DOI] [PubMed] [Google Scholar]
- Neufeldt C.J., Cerikan B., Cortese M., Frankish J., Lee J.Y., Plociennikowska A., Heigwer F., Prasad V., Joecks S., Burkart S.S., Zander D.Y., Subramanian B., Gimi R., Padmanabhan S., Iyer R., Gendarme M., El Debs B., Halama N., Merle U., Boutros M., Binder M., Bartenschlager R. SARS-CoV-2 infection induces a pro-inflammatory cytokine response through cGAS-STING and NF-kappaB. Commun. Biol. 2022;5:45. doi: 10.1038/s42003-021-02983-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norooznezhad A.H., Mansouri K. Endothelial cell dysfunction, coagulation, and angiogenesis in coronavirus disease 2019 (COVID-19) Micro Res. 2021;137 doi: 10.1016/j.mvr.2021.104188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nougier C., Benoit R., Simon M., Desmurs-Clavel H., Marcotte G., Argaud L., David J.S., Bonnet A., Negrier C., Dargaud Y. Hypofibrinolytic state and high thrombin generation may play a major role in SARS-COV2 associated thrombosis. J. Thromb. Haemost. 2020;18:2215–2219. doi: 10.1111/jth.15016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Connor M.A., Rastad J.L., Green W.R. The Role of Myeloid-Derived Suppressor Cells in Viral Infection. Viral Immunol. 2017;30:82–97. doi: 10.1089/vim.2016.0125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olivieri F., Rippo M.R., Procopio A.D., Fazioli F. Circulating inflamma-miRs in aging and age-related diseases. Front. Genet. 2013;4:121. doi: 10.3389/fgene.2013.00121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliviero B., Varchetta S., Mele D., Mantovani S., Cerino A., Perotti C.G., Ludovisi S., Mondelli M.U. Expansion of atypical memory B cells is a prominent feature of COVID-19. Cell Mol. Immunol. 2020;17:1101–1103. doi: 10.1038/s41423-020-00542-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortmann W., Kolaczkowska E. Age is the work of art? Impact of neutrophil and organism age on neutrophil extracellular trap formation. Cell Tissue Res. 2018;371:473–488. doi: 10.1007/s00441-017-2751-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paludan S.R. Innate antiviral defenses independent of inducible IFNalpha/beta production. Trends Immunol. 2016;37:588–596. doi: 10.1016/j.it.2016.06.003. [DOI] [PubMed] [Google Scholar]
- Pawelec G. Does patient age influence anti-cancer immunity? Semin. Immunopathol. 2019;41:125–131. doi: 10.1007/s00281-018-0697-6. [DOI] [PubMed] [Google Scholar]
- Pawelec G. Is there a positive side to T cell exhaustion? Front. Immunol. 2019;10:111. doi: 10.3389/fimmu.2019.00111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pawelec G., Bronikowski A., Cunnane S.C., Ferrucci L., Franceschi C., Fulop T., Gaudreau P., Gladyshev V.N., Gonos E.S., Gorbunova V., Kennedy B.K., Larbi A., Lemaitre J.F., Liu G.H., Maier A.B., Morais J.A., Nobrega O.T., Moskalev A., Rikkert M.O., Seluanov A., Senior A.M., Ukraintseva S., Vanhaelen Q., Witkowski J., Cohen A.A. The conundrum of human immune system “senescence”. Mech. Ageing Dev. 2020;192 doi: 10.1016/j.mad.2020.111357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pawelec G., Picard E., Bueno V., Verschoor C.P., Ostrand-Rosenberg S. MDSCs, ageing and inflammageing. Cell Immunol. 2021;362 doi: 10.1016/j.cellimm.2021.104297. [DOI] [PubMed] [Google Scholar]
- Philippe A., Chocron R., Gendron N., Bory O., Beauvais A., Peron N., Khider L., Guerin C.L., Goudot G., Levasseur F., Peronino C., Duchemin J., Brichet J., Sourdeau E., Desvard F., Bertil S., Pene F., Cheurfa C., Szwebel T.A., Planquette B., Rivet N., Jourdi G., Hauw-Berlemont C., Hermann B., Gaussem P., Mirault T., Terrier B., Sanchez O., Diehl J.L., Fontenay M., Smadja D.M. Circulating Von Willebrand factor and high molecular weight multimers as markers of endothelial injury predict COVID-19 in-hospital mortality. Angiogenesis. 2021;24:505–517. doi: 10.1007/s10456-020-09762-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pietrobon A.J., Teixeira F.M.E., Sato M.N. I mmunosenescence and inflammaging: risk factors of severe COVID-19 in older people. Front. Immunol. 2020;11 doi: 10.3389/fimmu.2020.579220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinilla L., Benitez I.D., Gonzalez J., Torres G., Barbe F., de Gonzalo-Calvo D. Peripheral blood microRNAs and the COVID-19 patient: methodological considerations, technical challenges and practice points. RNA Biol. 2021;18:688–695. doi: 10.1080/15476286.2021.1885188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porfidia A., Valeriani E., Pola R., Porreca E., Rutjes A.W.S., Di Nisio M. Venous thromboembolism in patients with COVID-19: systematic review and meta-analysis. Thromb. Res. 2020;196:67–74. doi: 10.1016/j.thromres.2020.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porto B.N., Stein R.T. Neutrophil extracellular traps in pulmonary diseases: too much of a good thing? Front. Immunol. 2016;7:311. doi: 10.3389/fimmu.2016.00311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prata L., Ovsyannikova I.G., Tchkonia T., Kirkland J.L. Senescent cell clearance by the immune system: Emerging therapeutic opportunities. Semin. Immunol. 2018;40 doi: 10.1016/j.smim.2019.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prattichizzo F., Bonafe M., Olivieri F., Franceschi C. Senescence associated macrophages and "macroph-aging": are they pieces of the same puzzle? Aging. 2016;8:3159–3160. doi: 10.18632/aging.101133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puzianowska-Kuznicka M., Owczarz M., Wieczorowska-Tobis K., Nadrowski P., Chudek J., Slusarczyk P., Skalska A., Jonas M., Franek E., Mossakowska M. Interleukin-6 and C-reactive protein, successful aging, and mortality: the PolSenior study. Immun. Ageing. 2016;13:21. doi: 10.1186/s12979-016-0076-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabe B., Chalaris A., May U., Waetzig G.H., Seegert D., Williams A.S., Jones S.A., Rose-John S., Scheller J. Transgenic blockade of interleukin 6 transsignaling abrogates inflammation. Blood. 2008;111:1021–1028. doi: 10.1182/blood-2007-07-102137. [DOI] [PubMed] [Google Scholar]
- Rebillard R.M., Charabati M., Grasmuck C., Filali-Mouhim A., Tastet O., Brassard N., Daigneault A., Bourbonniere L., Anand S.P., Balthazard R., Beaudoin-Bussieres G., Gasser R., Benlarbi M., Moratalla A.C., Solorio Y.C., Boutin M., Farzam-Kia N., Descoteaux-Dinelle J., Fournier A.P., Gowing E., Laumaea A., Jamann H., Lahav B., Goyette G., Lemaitre F., Mamane V.H., Prevost J., Richard J., Thai K., Cailhier J.F., Chomont N., Finzi A., Chasse M., Durand M., Arbour N., Kaufmann D.E., Prat A., Larochelle C. Identification of SARS-CoV-2-specific immune alterations in acutely ill patients. J. Clin. Investig. 2021:131. doi: 10.1172/JCI145853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reyes L., M., A.S.-G., Morrison T., Howden A.J.M., Watts E.R., Arienti S., Sadiku P., Coelho P., Mirchandani A.S., Zhang A., Hope D., Clark S.K., Singleton J., Johnston S., Grecian R., Poon A., McNamara S., Harper I., Fourman M.H., Brenes A.J., Pathak S., Lloyd A., Blanco G.R., von Kriegsheim A., Ghesquiere B., Vermaelen W., Cologna C.T., Dhaliwal K., Hirani N., Dockrell D.H., Whyte M.K.B., Griffith D., Cantrell D.A., Walmsley S.R. -------A type I IFN, prothrombotic hyperinflammatory neutrophil signature is distinct for COVID-19 ARDS. Wellcome Open Res. 2021;6:38. doi: 10.12688/wellcomeopenres.16584.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribon M., Seninet S., Mussard J., Sebbag M., Clavel C., Serre G., Boissier M.C., Semerano L., Decker P. Neutrophil extracellular traps exert both pro- and anti-inflammatory actions in rheumatoid arthritis that are modulated by C1q and LL-37. J. Autoimmun. 2019;98:122–131. doi: 10.1016/j.jaut.2019.01.003. [DOI] [PubMed] [Google Scholar]
- Roganovic J. Downregulation of microRNA-146a in diabetes, obesity and hypertension may contribute to severe COVID-19. Med. Hypotheses. 2021;146 doi: 10.1016/j.mehy.2020.110448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rojas A., Lindner C., Gonzalez I., Morales M.A. Advanced-glycation end-products axis: a contributor to the risk of severe illness from COVID-19 in diabetes patients. World J. Diabetes. 2021;12:590–602. doi: 10.4239/wjd.v12.i5.590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosell A., Havervall S., von Meijenfeldt F., Hisada Y., Aguilera K., Grover S.P., Lisman T., Mackman N., Thalin C. Patients with COVID-19 have elevated levels of circulating extracellular vesicle tissue factor activity that is associated with severity and mortality-brief report. Arterioscler. Thromb. Vasc. Biol. 2021;41:878–882. doi: 10.1161/ATVBAHA.120.315547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roszer T. Understanding the mysterious M2 macrophage through activation markers and effector mechanisms. Mediat. Inflamm. 2015;2015 doi: 10.1155/2015/816460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowlands M., Segal F., Hartl D. Myeloid-derived suppressor cells as a potential biomarker and therapeutic target in COVID-19. Front. Immunol. 2021;12 doi: 10.3389/fimmu.2021.697405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabbatinelli J., Giuliani A., Matacchione G., Latini S., Laprovitera N., Pomponio G., Ferrarini A., Svegliati Baroni S., Pavani M., Moretti M., Gabrielli A., Procopio A.D., Ferracin M., Bonafè M., Olivieri F. Decreased serum levels of the inflammaging marker miR-146a are associated with clinical non-response to tocilizumab in COVID-19 patients. Mech. Ageing Dev. 2021;193 doi: 10.1016/j.mad.2020.111413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacchi A., Grassi G., Bordoni V., Lorenzini P., Cimini E., Casetti R., Tartaglia E., Marchioni L., Petrosillo N., Palmieri F., D’Offizi G., Notari S., Tempestilli M., Capobianchi M.R., Nicastri E., Maeurer M., Zumla A., Locatelli F., Antinori A., Ippolito G., Agrati C. Early expansion of myeloid-derived suppressor cells inhibits SARS-CoV-2 specific T-cell response and may predict fatal COVID-19 outcome. Cell Death Dis. 2020;11:921. doi: 10.1038/s41419-020-03125-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sagiv A., Biran A., Yon M., Simon J., Lowe S.W., Krizhanovsky V. Granule exocytosis mediates immune surveillance of senescent cells. Oncogene. 2013;32:1971–1977. doi: 10.1038/onc.2012.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahebnasagh A., Saghafi F., Safdari M., Khataminia M., Sadremomtaz A., Talaei Z., Rezai Ghaleno H., Bagheri M., Habtemariam S., Avan R. Neutrophil elastase inhibitor (sivelestat) may be a promising therapeutic option for management of acute lung injury/acute respiratory distress syndrome or disseminated intravascular coagulation in COVID-19. J. Clin. Pharm. Ther. 2020;45:1515–1519. doi: 10.1111/jcpt.13251. [DOI] [PubMed] [Google Scholar]
- Saitoh T., Komano J., Saitoh Y., Misawa T., Takahama M., Kozaki T., Uehata T., Iwasaki H., Omori H., Yamaoka S., Yamamoto N., Akira S. Neutrophil extracellular traps mediate a host defense response to human immunodeficiency virus-1. Cell Host Microbe. 2012;12:109–116. doi: 10.1016/j.chom.2012.05.015. [DOI] [PubMed] [Google Scholar]
- Sakuishi K., Apetoh L., Sullivan J.M., Blazar B.R., Kuchroo V.K., Anderson A.C. Targeting Tim-3 and PD-1 pathways to reverse T cell exhaustion and restore anti-tumor immunity. J. Exp. Med. 2010;207:2187–2194. doi: 10.1084/jem.20100643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salminen A. Increased immunosuppression impairs tissue homeostasis with aging and age-related diseases. J. Mol. Med. 2021;99:1–20. doi: 10.1007/s00109-020-01988-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salminen A., Kaarniranta K., Kauppinen A. The role of myeloid-derived suppressor cells (MDSC) in the inflammaging process. Ageing Res. Rev. 2018;48:1–10. doi: 10.1016/j.arr.2018.09.001. [DOI] [PubMed] [Google Scholar]
- Salminen A., Kauppinen A., Kaarniranta K. Myeloid-derived suppressor cells (MDSC): an important partner in cellular/tissue senescence. Biogerontology. 2018;19:325–339. doi: 10.1007/s10522-018-9762-8. [DOI] [PubMed] [Google Scholar]
- Sanchez-Vazquez R., Guio-Carrion A., Zapatero-Gaviria A., Martinez P., Blasco M.A. Shorter telomere lengths in patients with severe COVID-19 disease. Aging. 2021;13:1–15. doi: 10.18632/aging.202463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santesmasses D., Castro J.P., Zenin A.A., Shindyapina A.V., Gerashchenko M.V., Zhang B., Kerepesi C., Yim S.H., Fedichev P.O., Gladyshev V.N. COVID-19 is an emergent disease of aging. Aging Cell. 2020;19 doi: 10.1111/acel.13230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheller J., Chalaris A., Schmidt-Arras D., Rose-John S. The pro- and anti-inflammatory properties of the cytokine interleukin-6. Biochim. Biophys. Acta. 2011;1813:878–888. doi: 10.1016/j.bbamcr.2011.01.034. [DOI] [PubMed] [Google Scholar]
- Schonrich G., Raftery M.J. Neutrophil extracellular traps go viral. Front. Immunol. 2016;7:366. doi: 10.3389/fimmu.2016.00366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulte-Schrepping J., Reusch N., Paclik D., Bassler K., Schlickeiser S., Zhang B., Kramer B., Krammer T., Brumhard S., Bonaguro L., De Domenico E., Wendisch D., Grasshoff M., Kapellos T.S., Beckstette M., Pecht T., Saglam A., Dietrich O., Mei H.E., Schulz A.R., Conrad C., Kunkel D., Vafadarnejad E., Xu C.J., Horne A., Herbert M., Drews A., Thibeault C., Pfeiffer M., Hippenstiel S., Hocke A., Muller-Redetzky H., Heim K.M., Machleidt F., Uhrig A., Bosquillon de Jarcy L., Jurgens L., Stegemann M., Glosenkamp C.R., Volk H.D., Goffinet C., Landthaler M., Wyler E., Georg P., Schneider M., Dang-Heine C., Neuwinger N., Kappert K., Tauber R., Corman V., Raabe J., Kaiser K.M., Vinh M.T., Rieke G., Meisel C., Ulas T., Becker M., Geffers R., Witzenrath M., Drosten C., Suttorp N., von Kalle C., Kurth F., Handler K., Schultze J.L., Aschenbrenner A.C., Li Y., Nattermann J., Sawitzki B., Saliba A.E., Sander L.E., Deutsche C.-O.I. Severe COVID-19 is marked by a dysregulated myeloid cell compartment. Cell. 2020;182 doi: 10.1016/j.cell.2020.08.001. 1419-1440 e23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seery V., Raiden S.C., Algieri S.C., Grisolia N.A., Filippo D., De Carli N., Di Lalla S., Cairoli H., Chiolo M.J., Meregalli C.N., Gimenez L.I., Gregorio G., Sarli M., Alcalde A.L., Davenport C., Bruera M.J., Simaz N., Perez M.F., Nivela V., Bayle C., Tuccillo P., Agosta M.T., Perez H., Villa Nova S., Suarez P., Takata E.M., Garcia M., Lattner J., Rolon M.J., Coll P., Sananez I., Holgado M.P., Ferrero F., Geffner J., Arruvito L., Workgroup ibotC.-N.P., in behalf of the Covid F.P.R.W. Blood neutrophils from children with COVID-19 exhibit both inflammatory and anti-inflammatory markers. EBioMedicine. 2021;67 doi: 10.1016/j.ebiom.2021.103357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sellegounder D., Zafari P., Rajabinejad M., Taghadosi M., Kapahi P. Advanced glycation end products (AGEs) and its receptor, RAGE, modulate age-dependent COVID-19 morbidity and mortality. A review and hypothesis. Int. Immunopharmacol. 2021;98 doi: 10.1016/j.intimp.2021.107806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi H., Zuo Y., Yalavarthi S., Gockman K., Zuo M., Madison J.A., Blair C., Woodward W., Lezak S.P., Lugogo N.L., Woods R.J., Lood C., Knight J.S., Kanthi Y. Neutrophil calprotectin identifies severe pulmonary disease in COVID-19. J. Leukoc. Biol. 2021;109:67–72. doi: 10.1002/JLB.3COVCRA0720-359R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silvin A., Chapuis N., Dunsmore G., Goubet A.G., Dubuisson A., Derosa L., Almire C., Henon C., Kosmider O., Droin N., Rameau P., Catelain C., Alfaro A., Dussiau C., Friedrich C., Sourdeau E., Marin N., Szwebel T.A., Cantin D., Mouthon L., Borderie D., Deloger M., Bredel D., Mouraud S., Drubay D., Andrieu M., Lhonneur A.S., Saada V., Stoclin A., Willekens C., Pommeret F., Griscelli F., Ng L.G., Zhang Z., Bost P., Amit I., Barlesi F., Marabelle A., Pene F., Gachot B., Andre F., Zitvogel L., Ginhoux F., Fontenay M., Solary E. Elevated calprotectin and abnormal myeloid cell subsets discriminate severe from mild COVID-19. Cell. 2020;182 doi: 10.1016/j.cell.2020.08.002. 1401-1418 e18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh Y., Singh A., Rudravaram S., Soni K.D., Aggarwal R., Patel N., Wig N., Trikha A. Neutrophil-to-lymphocyte ratio and platelet-to-lymphocyte ratio as markers for predicting the severity in COVID-19 patients: a prospective observational study. Indian J. Crit. Care Med. 2021;25:847–852. doi: 10.5005/jp-journals-10071-23906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skendros P., Mitsios A., Chrysanthopoulou A., Mastellos D.C., Metallidis S., Rafailidis P., Ntinopoulou M., Sertaridou E., Tsironidou V., Tsigalou C., Tektonidou M., Konstantinidis T., Papagoras C., Mitroulis I., Germanidis G., Lambris J.D., Ritis K. Complement and tissue factor-enriched neutrophil extracellular traps are key drivers in COVID-19 immunothrombosis. J. Clin. Investig. 2020;130:6151–6157. doi: 10.1172/JCI141374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohrabipour S., Muniz V.S., Sharma N., Dwivedi D.J., Liaw P.C. Mechanistic studies of DNase I activity: impact of heparin variants and PAD4. Shock. 2021;56:975–987. doi: 10.1097/SHK.0000000000001804. [DOI] [PubMed] [Google Scholar]
- Souza P.S.S., Barbosa L.V., Diniz L.F.A., da Silva G.S., Lopes B.R.P., Souza P.M.R., de Araujo G.C., Pessoa D., de Oliveira J., Souza F.P., Toledo K.A. Neutrophil extracellular traps possess anti-human respiratory syncytial virus activity: possible interaction with the viral F protein. Virus Res. 2018;251:68–77. doi: 10.1016/j.virusres.2018.04.001. [DOI] [PubMed] [Google Scholar]
- Spijkerman R., Bongers S.H., Bindels B.J.J., Tinnevelt G.H., Giustarini G., Jorritsma N.K.N., Buitenwerf W., van Spengler D.E.J., Delemarre E.M., Nierkens S., van Goor H.M.R., Jansen J.J., Vrisekoop N., Hietbrink F., Leenen L.P.H., Kaasjager K.A.H., Koenderman L., group C.S. Flow cytometric evaluation of the neutrophil compartment in COVID-19 at hospital presentation: a normal response to an abnormal situation. J. Leukoc. Biol. 2021;109:99–114. doi: 10.1002/JLB.5COVA0820-520RRR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stegelmeier A.A., Darzianiazizi M., Hanada K., Sharif S., Wootton S.K., Bridle B.W., Karimi K. Type I interferon-mediated regulation of antiviral capabilities of neutrophils. Int. J. Mol. Sci. 2021:22. doi: 10.3390/ijms22094726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su Y.L., Wang X., Mann M., Adamus T.P., Wang D., Moreira D.F., Zhang Z., Ouyang C., He X., Zhang B., Swiderski P.M., Forman S.J., Baltimore D., Li L., Marcucci G., Boldin M.P., Kortylewski M. Myeloid cell-targeted miR-146a mimic inhibits NF-kappaB-driven inflammation and leukemia progression in vivo. Blood. 2020;135:167–180. doi: 10.1182/blood.2019002045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subrahmanian S., Borczuk A., Salvatore S., Fung K.M., Merrill J.T., Laurence J., Ahamed J. Tissue factor upregulation is associated with SARS-CoV-2 in the lungs of COVID-19 patients. J. Thromb. Haemost. 2021;19:2268–2274. doi: 10.1111/jth.15451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takano T., Matsumura T., Adachi Y., Terahara K., Moriyama S., Onodera T., Nishiyama A., Kawana-Tachikawa A., Miki S., Hosoya-Nakayama K., Nakamura-Hoshi M., Seki S., Tachikawa N., Yoshimura Y., Miyata N., Horiuchi H., Sasaki H., Miyazaki K., Kinoshita N., Sudo T., Akiyama Y., Sato R., Suzuki T., Matano T., Takahashi Y. Myeloid cell dynamics correlating with clinical outcomes of severe COVID-19 in Japan. Int. Immunol. 2021;33:241–247. doi: 10.1093/intimm/dxab005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka T., Kishimoto T. The biology and medical implications of interleukin-6. Cancer Immunol. Res. 2014;2:288–294. doi: 10.1158/2326-6066.CIR-14-0022. [DOI] [PubMed] [Google Scholar]
- Tang G., Huang M., Luo Y., Liu W., Lin Q., Mao L., Wu S., Xiong Z., Hou H., Sun Z., Wang F. The dynamic immunological parameter landscape in coronavirus disease 2019 patients with different outcomes. Front. Immunol. 2021;12 doi: 10.3389/fimmu.2021.697622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teluguakula N. Neutrophils set extracellular traps to injure lungs in coronavirus disease 2019. J. Infect. Dis. 2021;223:1503–1505. doi: 10.1093/infdis/jiab053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiam H.R., Wong S.L., Qiu R., Kittisopikul M., Vahabikashi A., Goldman A.E., Goldman R.D., Wagner D.D., Waterman C.M. NETosis proceeds by cytoskeleton and endomembrane disassembly and PAD4-mediated chromatin decondensation and nuclear envelope rupture. Proc. Natl. Acad. Sci. U.S.A. 2020;117:7326–7337. doi: 10.1073/pnas.1909546117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tleyjeh I.M., Kashour Z., Riaz M., Hassett L., Veiga V.C., Kashour T. Efficacy and safety of tocilizumab in COVID-19 patients: a living systematic review and meta-analysis, first update. Clin. Microbiol. Infect. 2021;27:1076–1082. doi: 10.1016/j.cmi.2021.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomar B., Anders H.J., Desai J., Mulay S.R. Neutrophils and neutrophil extracellular traps drive necroinflammation in COVID-19. Cells. 2020:9. doi: 10.3390/cells9061383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres-Ruiz J., Absalon-Aguilar A., Nunez-Aguirre M., Perez-Fragoso A., Carrillo-Vazquez D.A., Maravillas-Montero J.L., Mejia-Dominguez N.R., Llorente L., Alcala-Carmona B., Lira-Luna J., Nunez-Alvarez C., Juarez-Vega G., Meza-Sanchez D., Hernandez-Gilsoul T., Tapia-Rodriguez M., Gomez-Martin D. Neutrophil Extracellular traps contribute to COVID-19 hyperinflammation and humoral autoimmunity. Cells. 2021:10. doi: 10.3390/cells10102545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toussaint M., Jackson D.J., Swieboda D., Guedan A., Tsourouktsoglou T.D., Ching Y.M., Radermecker C., Makrinioti H., Aniscenko J., Edwards M.R., Solari R., Farnir F., Papayannopoulos V., Bureau F., Marichal T., Johnston S.L. Corrigendum: Host DNA released by NETosis promotes rhinovirus-induced type-2 allergic asthma exacerbation. Nat. Med. 2017;23:1384. doi: 10.1038/nm1117-1384a. [DOI] [PubMed] [Google Scholar]
- Tsilingiris D., Tentolouris A., Eleftheriadou I., Tentolouris N. Telomere length, epidemiology and pathogenesis of severe COVID-19. Eur. J. Clin. Investig. 2020;50 doi: 10.1111/eci.13376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsourouktsoglou T.D., Warnatsch A., Ioannou M., Hoving D., Wang Q., Papayannopoulos V. Histones, DNA, and citrullination promote neutrophil extracellular trap inflammation by regulating the localization and activation of TLR4. Cell Rep. 2020;31 doi: 10.1016/j.celrep.2020.107602. [DOI] [PubMed] [Google Scholar]
- Ulas T., Pirr S., Fehlhaber B., Bickes M.S., Loof T.G., Vogl T., Mellinger L., Heinemann A.S., Burgmann J., Schoning J., Schreek S., Pfeifer S., Reuner F., Vollger L., Stanulla M., von Kockritz-Blickwede M., Glander S., Barczyk-Kahlert K., von Kaisenberg C.S., Friesenhagen J., Fischer-Riepe L., Zenker S., Schultze J.L., Roth J., Viemann D. S100-alarmin-induced innate immune programming protects newborn infants from sepsis. Nat. Immunol. 2017;18:622–632. doi: 10.1038/ni.3745. [DOI] [PubMed] [Google Scholar]
- Urban C.F., Ermert D., Schmid M., Abu-Abed U., Goosmann C., Nacken W., Brinkmann V., Jungblut P.R., Zychlinsky A. Neutrophil extracellular traps contain calprotectin, a cytosolic protein complex involved in host defense against Candida albicans. PLoS Pathog. 2009;5 doi: 10.1371/journal.ppat.1000639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urbonaviciute V., Furnrohr B.G., Meister S., Munoz L., Heyder P., De Marchis F., Bianchi M.E., Kirschning C., Wagner H., Manfredi A.A., Kalden J.R., Schett G., Rovere-Querini P., Herrmann M., Voll R.E. Induction of inflammatory and immune responses by HMGB1-nucleosome complexes: implications for the pathogenesis of SLE. J. Exp. Med. 2008;205:3007–3018. doi: 10.1084/jem.20081165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Deursen J.M. The role of senescent cells in ageing. Nature. 2014;509:439–446. doi: 10.1038/nature13193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varadhan R., Yao W., Matteini A., Beamer B.A., Xue Q.L., Yang H., Manwani B., Reiner A., Jenny N., Parekh N., Fallin M.D., Newman A., Bandeen-Roche K., Tracy R., Ferrucci L., Walston J. Simple biologically informed inflammatory index of two serum cytokines predicts 10 year all-cause mortality in older adults. J. Gerontol. A Biol. Sci. Med Sci. 2014;69:165–173. doi: 10.1093/gerona/glt023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varchetta S., Mele D., Oliviero B., Mantovani S., Ludovisi S., Cerino A., Bruno R., Castelli A., Mosconi M., Vecchia M., Roda S., Sachs M., Klersy C., Mondelli M.U. Unique immunological profile in patients with COVID-19. Cell. Mol. Immunol. 2021;18:604–612. doi: 10.1038/s41423-020-00557-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velazquez-Salinas L., Verdugo-Rodriguez A., Rodriguez L.L., Borca M.V. The role of interleukin 6 during viral infections. Front. Microbiol. 2019;10:1057. doi: 10.3389/fmicb.2019.01057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veras F.P., Pontelli M.C., Silva C.M., Toller-Kawahisa J.E., de Lima M., Nascimento D.C., Schneider A.H., Caetite D., Tavares L.A., Paiva I.M., Rosales R., Colon D., Martins R., Castro I.A., Almeida G.M., Lopes M.I.F., Benatti M.N., Bonjorno L.P., Giannini M.C., Luppino-Assad R., Almeida S.L., Vilar F., Santana R., Bollela V.R., Auxiliadora-Martins M., Borges M., Miranda C.H., Pazin-Filho A., da Silva L.L.P., Cunha L.D., Zamboni D.S., Dal-Pizzol F., Leiria L.O., Siyuan L., Batah S., Fabro A., Mauad T., Dolhnikoff M., Duarte-Neto A., Saldiva P., Cunha T.M., Alves-Filho J.C., Arruda E., Louzada-Junior P., Oliveira R.D., Cunha F.Q. SARS-CoV-2-triggered neutrophil extracellular traps mediate COVID-19 pathology. J. Exp. Med. 2020:217. doi: 10.1084/jem.20201129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viana-Llamas M.C., Arroyo-Espliguero R., Silva-Obregon J.A., Uribe-Heredia G., Nunez-Gil I., Garcia-Magallon B., Toran-Martinez C.G., Castillo-Sandoval A., Diaz-Caraballo E., Rodriguez-Guinea I., Dominguez-Lopez J. Hypoalbuminemia on admission in COVID-19 infection: an early predictor of mortality and adverse events. A retrospective observational study. Med. Clin. 2021;156:428–436. doi: 10.1016/j.medcli.2020.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogl T., Tenbrock K., Ludwig S., Leukert N., Ehrhardt C., van Zoelen M.A., Nacken W., Foell D., van der Poll T., Sorg C., Roth J. Mrp8 and Mrp14 are endogenous activators of Toll-like receptor 4, promoting lethal, endotoxin-induced shock. Nat. Med. 2007;13:1042–1049. doi: 10.1038/nm1638. [DOI] [PubMed] [Google Scholar]
- Voglis S., Quinn K., Tullis E., Liu M., Henriques M., Zubrinich C., Penuelas O., Chan H., Silverman F., Cherepanov V., Orzech N., Khine A.A., Cantin A., Slutsky A.S., Downey G.P., Zhang H. Human neutrophil peptides and phagocytic deficiency in bronchiectatic lungs. Am. J. Respir. Crit. Care Med. 2009;180:159–166. doi: 10.1164/rccm.200808-1250OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vyas N., Kurian S.J., Bagchi D., Manu M.K., Saravu K., Unnikrishnan M.K., Mukhopadhyay C., Rao M., Miraj S.S. Vitamin D in prevention and treatment of COVID-19: current perspective and future prospects. J. Am. Coll. Nutr. 2021;40:632–645. doi: 10.1080/07315724.2020.1806758. [DOI] [PubMed] [Google Scholar]
- Wang C., Wang S., Li D., Chen P., Han S., Zhao G., Chen Y., Zhao J., Xiong J., Qiu J., Wei D.Q., Zhao J., Wang J. Human cathelicidin inhibits SARS-CoV-2 infection: killing two birds with one stone. ACS Infect. Dis. 2021;7:1545–1554. doi: 10.1021/acsinfecdis.1c00096. [DOI] [PubMed] [Google Scholar]
- Wang J., Shirota Y., Bayik D., Shirota H., Tross D., Gulley J.L., Wood L.V., Berzofsky J.A., Klinman D.M. Effect of TLR agonists on the differentiation and function of human monocytic myeloid-derived suppressor cells. J. Immunol. 2015;194:4215–4221. doi: 10.4049/jimmunol.1402004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L., Chen J., Zhao J., Li F., Lu S., Liu P., Liu X.H., Huang Q., Wang H., Xu Q.N., Liu X., Yu S., Liu L., Lu H. The predictive role of lymphocyte subsets and laboratory measurements in COVID-19 disease: a retrospective study. Ther. Adv. Respir. Dis. 2021;15 doi: 10.1177/17534666211049739. 17534666211049739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q., Codd V., Raisi-Estabragh Z., Musicha C., Bountziouka V., Kaptoge S., Allara E., Angelantonio E.D., Butterworth A.S., Wood A.M., Thompson J.R., Petersen S.E., Harvey N.C., Danesh J.N., Samani N.J., Nelson C.P. Shorter leukocyte telomere length is associated with adverse COVID-19 outcomes: a cohort study in UK biobank. EBioMedicine. 2021;70 doi: 10.1016/j.ebiom.2021.103485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W., Thomas R., Oh J., Su D.M. Thymic aging may be associated with COVID-19 pathophysiology in the elderly. Cells. 2021:10. doi: 10.3390/cells10030628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei J., Xu H., Davies J.L., Hemmings G.P. Increase of plasma IL-6 concentration with age in healthy subjects. Life Sci. 1992;51:1953–1956. doi: 10.1016/0024-3205(92)90112-3. [DOI] [PubMed] [Google Scholar]
- Westmeier J., Paniskaki K., Karakose Z., Werner T., Sutter K., Dolff S., Overbeck M., Limmer A., Liu J., Zheng X., Brenner T., Berger M.M., Witzke O., Trilling M., Lu M., Yang D., Babel N., Westhoff T., Dittmer U., Zelinskyy G. Impaired cytotoxic CD8(+) T cell response in elderly COVID-19 patients. mBio. 2020:11. doi: 10.1128/mBio.02243-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wibowo A., Pranata R., Lim M.A., Akbar M.R., Martha J.W. Endotheliopathy marked by high von Willebrand factor (vWF) antigen in COVID-19 is associated with poor outcome: a systematic review and meta-analysis. Int. J. Infect. Dis. 2021 doi: 10.1016/j.ijid.2021.06.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodruff M.C., Ramonell R.P., Nguyen D.C., Cashman K.S., Saini A.S., Haddad N.S., Ley A.M., Kyu S., Howell J.C., Ozturk T., Lee S., Suryadevara N., Case J.B., Bugrovsky R., Chen W., Estrada J., Morrison-Porter A., Derrico A., Anam F.A., Sharma M., Wu H.M., Le S.N., Jenks S.A., Tipton C.M., Staitieh B., Daiss J.L., Ghosn E., Diamond M.S., Carnahan R.H., Crowe J.E., Jr., Hu W.T., Lee F.E., Sanz I. Extrafollicular B cell responses correlate with neutralizing antibodies and morbidity in COVID-19. Nat. Immunol. 2020;21:1506–1516. doi: 10.1038/s41590-020-00814-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu D., Yang X.O. Dysregulation of pulmonary responses in severe COVID-19. Viruses. 2021:13. doi: 10.3390/v13060957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X., Han M., Li T., Sun W., Wang D., Fu B., Zhou Y., Zheng X., Yang Y., Li X., Zhang X., Pan A., Wei H. Effective treatment of severe COVID-19 patients with tocilizumab. Proc. Natl. Acad. Sci. U.S.A. 2020;117:10970–10975. doi: 10.1073/pnas.2005615117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yipp B.G., Kubes P. NETosis: how vital is it? Blood. 2013;122:2784–2794. doi: 10.1182/blood-2013-04-457671. [DOI] [PubMed] [Google Scholar]
- Yousefzadeh M.J., Flores R.R., Zhu Y., Schmiechen Z.C., Brooks R.W., Trussoni C.E., Cui Y., Angelini L., Lee K.A., McGowan S.J., Burrack A.L., Wang D., Dong Q., Lu A., Sano T., O’Kelly R.D., McGuckian C.A., Kato J.I., Bank M.P., Wade E.A., Pillai S.P.S., Klug J., Ladiges W.C., Burd C.E., Lewis S.E., LaRusso N.F., Vo N.V., Wang Y., Kelley E.E., Huard J., Stromnes I.M., Robbins P.D., Niedernhofer L.J. An aged immune system drives senescence and ageing of solid organs. Nature. 2021;594:100–105. doi: 10.1038/s41586-021-03547-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang D., Chen G., Manwani D., Mortha A., Xu C., Faith J.J., Burk R.D., Kunisaki Y., Jang J.E., Scheiermann C., Merad M., Frenette P.S. Neutrophil ageing is regulated by the microbiome. Nature. 2015;525:528–532. doi: 10.1038/nature15367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J., Tecson K.M., McCullough P.A. Endothelial dysfunction contributes to COVID-19-associated vascular inflammation and coagulopathy. Rev. Cardiovasc. Med. 2020;21:315–319. doi: 10.31083/j.rcm.2020.03.126. [DOI] [PubMed] [Google Scholar]
- Zhang P., Shi L., Xu J., Wang Y., Yang H. Elevated interleukin-6 and adverse outcomes in COVID-19 patients: a meta-analysis based on adjusted effect estimates. Immunogenetics. 2020;72:431–437. doi: 10.1007/s00251-020-01179-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., Qin L., Zhao Y., Zhang P., Xu B., Li K., Liang L., Zhang C., Dai Y., Feng Y., Sun J., Hu Z., Xiang H., Knight J.C., Dong T., Jin R. Interferon-induced transmembrane protein 3 genetic variant rs12252-C associated with disease severity in coronavirus disease 2019. J. Infect. Dis. 2020;222:34–37. doi: 10.1093/infdis/jiaa224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng Y., Liu X., Le W., Xie L., Li H., Wen W., Wang S., Ma S., Huang Z., Ye J., Shi W., Ye Y., Liu Z., Song M., Zhang W., Han J.J., Belmonte J.C.I., Xiao C., Qu J., Wang H., Liu G.H., Su W. A human circulating immune cell landscape in aging and COVID-19. Protein Cell. 2020;11:740–770. doi: 10.1007/s13238-020-00762-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng Y., Niyonsaba F., Ushio H., Nagaoka I., Ikeda S., Okumura K., Ogawa H. Cathelicidin LL-37 induces the generation of reactive oxygen species and release of human alpha-defensins from neutrophils. Br. J. Dermatol. 2007;157:1124–1131. doi: 10.1111/j.1365-2133.2007.08196.x. [DOI] [PubMed] [Google Scholar]
- Zingaropoli M.A., Nijhawan P., Carraro A., Pasculli P., Zuccala P., Perri V., Marocco R., Kertusha B., Siccardi G., Del Borgo C., Curtolo A., Ajassa C., Iannetta M., Ciardi M.R., Mastroianni C.M., Lichtner M. Increased sCD163 and sCD14 plasmatic levels and depletion of peripheral blood pro-inflammatory monocytes, myeloid and plasmacytoid dendritic cells in patients with severe COVID-19 pneumonia. Front. Immunol. 2021;12 doi: 10.3389/fimmu.2021.627548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zucoloto A.Z., Jenne C.N. Platelet-neutrophil interplay: insights into neutrophil extracellular trap (NET)-driven coagulation in infection. Front. Cardiovasc. Med. 2019;6:85. doi: 10.3389/fcvm.2019.00085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuo Y., Kanthi Y., Knight J.S., Kim A.H.J. The interplay between neutrophils, complement, and microthrombi in COVID-19. Best Pract. Res Clin. Rheumatol. 2021;35 doi: 10.1016/j.berh.2021.101661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuo Y., Warnock M., Harbaugh A., Yalavarthi S., Gockman K., Zuo M., Madison J.A., Knight J.S., Kanthi Y., Lawrence D.A. Plasma tissue plasminogen activator and plasminogen activator inhibitor-1 in hospitalized COVID-19 patients. Sci. Rep. 2021;11:1580. doi: 10.1038/s41598-020-80010-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuo, Y., Yalavarthi, S., Navaz, S., Hoy, C., Harbaugh, A., Gockman, K., Zuo, M., Madison, J.A., Shi, H., Kanthi, Y. and Knight, J.S., 2021c. Autoantibodies stabilize neutrophil extracellular traps in COVID-19. medRxiv. [DOI] [PMC free article] [PubMed]
- Zuo Y., Yalavarthi S., Navaz S.A., Hoy C.K., Harbaugh A., Gockman K., Zuo M., Madison J.A., Shi H., Kanthi Y., Knight J.S. Autoantibodies stabilize neutrophil extracellular traps in COVID-19. JCI Insight. 2021:6. doi: 10.1172/jci.insight.150111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuo Y., Yalavarthi S., Shi H., Gockman K., Zuo M., Madison J.A., Blair C., Weber A., Barnes B.J., Egeblad M., Woods R.J., Kanthi Y., Knight J.S. Neutrophil extracellular traps in COVID-19. JCI Insight. 2020:5. doi: 10.1172/jci.insight.138999. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No data was used for the research described in the article.