Abstract
The purine nucleoside analogue NBMPR {nitrobenzylthioinosine or 6-[(4-nitrobenzyl)thio]-9-β-d-ribofuranosylpurine} was selectively phosphorylated to its nucleoside 5′-monophosphate by Toxoplasma gondii but not mammalian adenosine kinase (EC 2.7.1.20). NBMPR was also cleaved in toxoplasma to its nucleobase, nitrobenzylmercaptopurine. However, nitrobenzylmercaptopurine was not a substrate for either adenosine kinase or hypoxanthine-guanine-xanthine phosphoribosyltransferase (EC 2.4.2.8). Because of this unique and previously unknown metabolism of NBMPR by the parasite, the effect of NBMPR as an antitoxoplasmic agent was tested. NBMPR killed T. gondii grown in human fibroblasts in a dose-dependent manner, with a 50% inhibitory concentration of approximately 10 μM and without apparent toxicity to host cells. Doses of up to 100 μM had no significant toxic effect on uninfected host cells. The promising antitoxoplasmic effect of NBMPR led to the testing of other 6-substituted 9-β-d-ribofuranosylpurines, which were shown to be good ligands of the parasite adenosine kinase (M. H. Iltzsch, S. S. Uber, K. O. Tankersley, and M. H. el Kouni, Biochem. Pharmacol. 49:1501–1512, 1995), as antitoxoplasmic agents. Among the analogues tested, 6-benzylthioinosine, p-nitrobenzyl-6-selenopurine riboside, N6-(p-azidobenzyl)adenosine, and N6-(p-nitrobenzyl)adenosine, like NBMPR, were selectively toxic to parasite-infected cells. Thus, it appears that the unique characteristics of purine metabolism in T. gondii render certain 6-substituted 9-β-d-ribofuranosylpurines promising antitoxoplasmic drugs.
The parasitic protozoon Toxoplasma gondii is the etiologic agent of toxoplasmosis, a parasitic disease widespread among various warm-blooded animals, including humans. Infection with T. gondii is quite common in humans (as much as 60% of the population in the United States is seropositive) but is asymptomatic in the general population. In contrast, the disease represents a major health problem for immunocompromised individuals, such as AIDS patients, and the unborn children of infected mothers (15, 22, 25). Toxoplasmic encephalitis has become the most common cause of intracerebral mass lesions in AIDS patients and possibly the most commonly recognized opportunistic infection of the central nervous system (15, 22). The incidence of congenital toxoplasmosis is as high as 1 in 1,000 live births, and this disease causes blindness, psychomotor or mental retardation, severe brain damage, or even death of infected children (22). In spite of the tragic consequences of toxoplasmosis, the therapy of the disease has not changed in the last 20 years. The efficacy of the current therapy for toxoplasmosis (a combination of pyrimethamine and sulfadiazine) is limited, primarily by serious host toxicity and ineffectiveness against tissue cysts (15, 22, 25, 27). Furthermore, as many as 50% of patients do not respond to therapy (15). Other therapies, e.g., clindamycin or atovaquone, have met with limited success, particularly in the long-term management of these patients. Hence, there is a continuing need to develop new and effective drugs for the treatment and long-term management of toxoplasmosis.
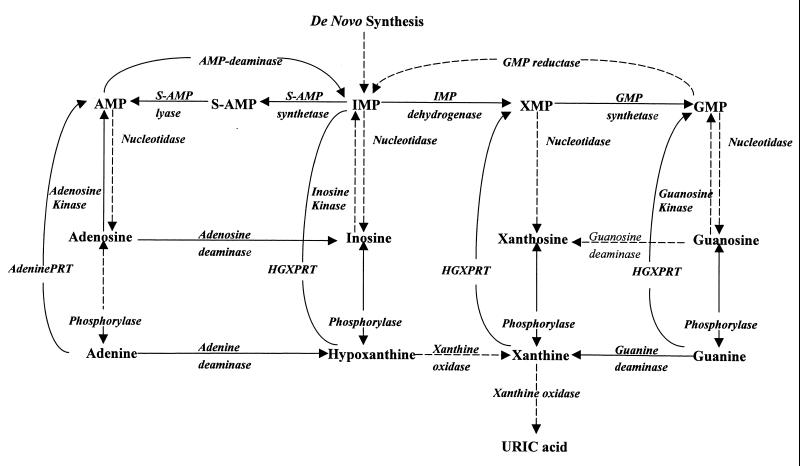
The rational design of a drug is usually based on biochemical and physiological differences between pathogens and the host. Some of the most striking differences between toxoplasmas and their mammalian host are the characteristics of the enzyme adenosine kinase (EC 2.7.1.20) and its role in the salvage of adenosine in these parasites. The adenosine kinase reaction (Fig. 1) is the main route of adenosine metabolism in T. gondii (14, 20). It has also been reported that adenosine kinase activity is 10-fold higher than that of hypoxanthine-guanine-xanthine phosphoribosyltransferase (HGXPRT) (EC 2.4.2.8), the next most active enzyme in the purine salvage pathways in toxoplasmas (14). These differences result in the preferential incorporation of adenosine into adenine nucleotides by a rate at least 10-fold higher than that of any other purine precursor tested (14). This situation contrasts sharply with that in most mammalian cells, where adenosine is predominantly deaminated by adenosine deaminase (EC 3.5.4.4) to inosine, which is then cleaved to hypoxanthine by purine nucleoside phosphorylase (EC 2.4.2.1) (Fig. 1). Neither of these two enzymes is significantly active in toxoplasmas (14). The high level of activity of adenosine kinase along with the unique metabolism of adenosine in toxoplasmas make adenosine kinase an excellent target for chemotherapy. However, deficiency of adenosine kinase is not lethal to the parasites, indicating that inhibition of the enzyme will not lead to toxicity in toxoplasmas (20). Therefore, we have recently carried out a search to identify adenosine analogues that can be used as “subversive” substrates and activated to toxic nucleotides selectively by T. gondii but not host adenosine kinase (12). Preferential metabolism of such substrates to toxic nucleotides in the parasites should lead to selective toxicity against toxoplasmas.
FIG. 1.
Purine salvage pathways in T. gondii. Solid lines indicate reactions that have been established. Broken lines indicate reactions which could not be detected. PRT, phosphoribosyltransferase.
NBMPR {nitrobenzylthioinosine or 6-[(4-nitrobenzyl)thio]-9-β-d-ribofuranosylpurine} and various 6-substituted 9-β-d-ribofuranosylpurines were found to be among the best ligands for binding to T. gondii adenosine kinase (12). This finding was quite unusual, since the compounds are not known to be active ligands of adenosine kinase from other species. This unexpected finding prompted us to study the metabolic fate of NBMPR. We report here that, unlike their mammalian host, T. gondii can phosphorylate NBMPR to the nucleotide level via adenosine kinase and are also capable of cleaving this nucleoside to its nucleobase, nitrobenzylmercaptopurine (NBMP). Because of this unique and previously unknown metabolism of NBMPR, NBMPR and certain related 6-substituted 9-β-d-ribofuranosylpurines were tested and showed selective toxicity against T. gondii grown in cultures.
(A preliminary report of this work has been presented previously [9].)
MATERIALS AND METHODS
Chemicals.
[G-3H]NBMPR (36 Ci/mmol), [5,6-3H]uracil (38 Ci/mol), [8-14C]adenosine (55 Ci/mol), and [8-14C]hypoxanthine (55 Ci/mol) were purchased from Moravek Biochemicals, Brea, Calif. 5-Iodotubercidin was purchased from Research Biochemicals, Natick, Mass. NBMPR 5′-monophosphate (NBMPR-P), N6-benzyladenosine, and N6-(p-azidobenzyl)adenosine were generously provided by A. R. P. Paterson, University of Alberta, Edmonton, Alberta, Canada. Silica Gel G and cellulose thin-layer chromatography (TLC) Polygram plates were obtained from Fisher Scientific (Pittsburgh, Pa.). Bovine gamma globulin and dye reagent for protein assays were obtained from Bio-Rad Laboratories (Richmond, Calif.). All other chemicals and compounds were obtained from the Drug Synthesis and Chemistry Branch, Developmental Therapeutic Program, National Cancer Institute; Sigma Chemical Co., St. Louis, Mo.; or Fisher Scientific.
Sources of T. gondii and tissues.
Wild-type strain RH is maintained in our laboratory. The adenosine kinase-deficient mutant Ara-AR (20) and the HGXPRT-deficient mutant ThxR-1 (21) of T. gondii were provided by courtesy of E. R. Pfefferkorn, Dartmouth Medical School, Hanover, N.H. Murine tissues were obtained from female CD-1 mice (Charles River Laboratories), and human liver tissue was obtained from the Tissue Procurement Facility, Comprehensive Cancer Center, University of Alabama at Birmingham.
Maintenance of T. gondii.
The three strains of T. gondii were propagated by intraperitoneal passage in female CD-1 mice (20 to 25 g). Mice were injected intraperitoneally with an inoculum of 106 cells of T. gondii contained in 0.2 ml of sterile phosphate-buffered saline (PBS) (pH 7.2) and were sacrificed after 2 to 3 days by inhalation of ether. The parasites were harvested from the peritoneal cavity by injection, aspiration, and reinjection of 3 to 5 ml of PBS (two or three times). The peritoneal fluid was examined microscopically to determine the concentration of T. gondii and to ascertain the extent of contamination by host cells. Two-day transfers generally produce parasite preparations that contain very little contamination and have a viability of >95%.
When T. gondii was used for in vitro incorporation studies, the procedure was performed aseptically, and the parasites were washed and resuspended in Dulbecco’s modified Eagle medium (GIBCO BRL) containing 100 U of penicillin G per ml, 100 μg of streptomycin sulfate per ml, and 3% fetal bovine serum (FBS) (HyClone Laboratories, Logan, Utah). For the purpose of enzyme studies, to ascertain the purity of T. gondii for enzyme isolation and purification, the parasites were purified from host cells and debris by coagulating the host cells by the addition of 50 μl of phytohemagglutinin P (Difco) to a suspension of parasites in 5 ml of medium, shaking slowly for 15 min, and filtering slowly through a 5-μm-pore-size Nuclepore polycarbonate filter (Costar, Cambridge, Mass.). Using the trypan blue exclusion method, and counting cells with a hemacytometer, these procedures produced parasite preparations that were >99% pure. The parasites were pelleted by centrifugation, washed twice with PBS, and resuspended in 1 ml of the homogenization buffer described below.
Preparation of enzyme extracts.
Enzyme extracts from parasites or tissues were prepared by homogenizing the parasites or tissues in 3 volumes of ice-cold 50 mM Tris-Cl (pH 7.4) by use of a Brinkmann Instruments Polytron homogenizer fitted with a microprobe. The homogenate was centrifuged at 105,000 × g for 1 h, and the supernatant was collected and used as the source of enzyme.
Enzyme assays.
Assays were run under conditions where activity was linear with time and enzyme concentration. Adenosine kinase activity was determined by monitoring the formation of radiolabeled nucleotides and nucleobases from their respective nucleosides (adenosine or NBMPR). The assay mixtures contained 50 mM Tris-Cl (pH 7.4), 2.5 mM ATP, 5 mM MgCl2, 5 mM creatine phosphate, creatine kinase, 50 μM [G-3H]NBMPR (1.95 Ci/mol) or 5 μM [8-14C]adenosine (55 Ci/mol), and 50 μl of enzyme extract in a final volume of 100 μl. When adenosine was used as a substrate, 5 μM deoxycoformycin, a specific inhibitor of adenosine deaminase, was added to inhibit any adenosine deaminase present. Incubation was carried out at 37°C and terminated by boiling in a water bath for 2 min followed by freezing for at least 20 min. Precipitated proteins were removed by centrifugation, and 10 μl of the supernatant was spotted on silica gel TLC plates.
Phosphoribosyltransferase activity was measured by monitoring the conversion of [8-14C]hypoxanthine (55 Ci/mol) or [G-3H]NBMP (1.95 Ci/mol) to their respective nucleoside 5′-monophosphates (and nucleosides formed by phosphohydrolase activity) as previously described (17). The standard reaction mixtures contained 50 mM Tris-Cl (pH 7.4), 4 μM [8-14C]hypoxanthine (55 Ci/mol) or 10 μM [G-3H]NBMP (1.95 Ci/mol), 4 mM 5-phosphoribosyl-1-pyrophosphate (PRPP), 20 mM MgCl2, 50 mM KCl, and 50 μl of enzyme extract (4 to 8 μg of protein) in a final volume of 100 μl. Reaction mixtures were incubated at 37°C, and reactions were terminated by boiling in a water bath for 2 min. Precipitated proteins were removed by centrifugation, and a 10-μl aliquot of the resulting supernatant was spotted on cellulose or silica gel TLC plates for the hypoxanthine or NBMP reaction, respectively.
When adenosine was used as a substrate, the TLC plates were developed with a mixture of chloroform-methanol-acetic acid (102:12:6 [vol/vol/vol]). The Rf values were as follows: adenosine, 0.27; adenine, 0.36; and AMP, 0.17. When NBMPR or NBMP was used as a substrate, the TLC plates were developed with n-butanol–ethyl acetate–NH3OH–methanol (49:28:28:21 [vol/vol/vol/vol]). The Rf values were as follows: NBMPR, 0.76; NBMPR-P, 0.50; and NBMP, 0.89. When hypoxanthine was used as a substrate, the TLC plates were developed with 5% dibasic potassium phosphate. The Rf values were as follows: hypoxanthine, 0.49; inosine, 0.65; and IMP, 0.77. The amounts of radioactivity in the substrate and product(s) were calculated on a percentage basis with computerized Berthold LB-284 Automatic TLC-Linear Analyzers (Wallac Inc., Gaithersburg, Md.).
Protein determinations.
Protein concentrations were determined by the method of Bradford (2) with a Bio-Rad protein assay kit and bovine gamma globulin as a standard.
Enzymatic synthesis and isolation of [G-3H]NBMP.
[G-3H]NBMP (36 Ci/mol) was synthesized enzymatically from NBMPR (36 Ci/mmol) by using T. gondii cytosolic extract. The reaction mixture contained 50 mM Tris-Cl (pH 7.4), 27.9 μM [G-3H]NBMPR (36 Ci/mmol), and 500 μl of enzyme extract in a final volume of 750 μl. Incubation was carried out overnight at 37°C and terminated by boiling in a water bath for 2 min followed by freezing for at least 20 min. The assay mixture was deproteinized, and [G-3H]NBMP was isolated from the supernatant by high-pressure liquid chromatography (HPLC) as described below.
Preparation of samples for HPLC analysis.
When the enzyme assay mixtures for measuring NBMPR metabolism were analyzed by HPLC, they were deproteinized with 2 volumes of 15% trichloroacetic acid. After centrifugation (16,000 × g, 4°C) for 5 min, the acid-soluble supernatant material was neutralized by extraction with a 1:2 mixture of tri-n-octylamine in Freon (1,1,2-trichlorotrifluoroethane). The neutralized supernatant was filtered through a Gelman Acrodisc LC 13 0.2-μm-pore-size polyvinylidene difluoride filter prior to HPLC analysis.
HPLC analysis.
The HPLC analysis was performed with two Hypersil C18 reverse-phase columns (25 by 0.4 cm, octyldecyl silane, 5 μm) (Jones Chromatography, Littleton, Colo.) in tandem on a computer-controlled Hewlett-Packard 1050 HPLC system with an autosampler, a quaternary pump, and a multiple-wavelength diode-array-based triple-channel UV monitor. Elution was performed stepwise with two mobile phases: 50 mM ammonium acetate in 0.5% acetonitrile (pH 4.8) (buffer A) and 50 mM ammonium acetate in 60% acetonitrile (pH 4.8) (buffer B). A 25-μl sample was injected. Elution was carried out with buffer A for 10 min, followed by a 120-min linear gradient to 60% buffer B and then a 10-min isocratic elution with 40% buffer A–60% buffer B. The flow rate was 1 ml/min, except that from 5 to 10 min and 120 to 132 min it was 0.5 ml/min. The eluent was monitored at 254 nm and λmax of NBMPR (289 nm). Under these conditions, NBMPR-P eluted at 86 to 87 min, NBMPR eluted at 102 to 103 min, and NBMP eluted at 106 to 107 min. The authenticity of NBMPR-P was verified by coelution with an authentic sample and by the retention time. The authenticity of NBMP was deduced from studies performed on an API III triple-quadrupole Perkin-Elmer SCIEX LC/MS/MS mass spectrometer.
Mass spectrometry analysis.
NBMP was further purified and separated by reverse-phase HPLC on an Aquapore C8 column (100 by 2.1 mm) with a 0 to 50% acetonitrile gradient in aqueous 10 mM ammonium acetate at a flow rate of 0.2 ml/min (5% per min). The eluent was split 1:1, with 100 μl/min going to the electrospray interface. Positive- and negative-ion mass spectra were recorded in this mode. The LC/MS/MS analysis of NBMP produced a molecular weight of 287, the proper molecular weight of NBMP.
Incorporation of radiolabeled uracil.
The uptake and incorporation of radiolabeled uracil into nucleic acids of T. gondii were carried out, at least in triplicate, in tissue cultures of monolayers of human foreskin fibroblasts cultured for no more than 30 passages in Dulbecco’s modified Eagle medium and infected with T. gondii. Briefly, confluent cells (4 to 5 days of incubation) were cultured for 24 h in 24-well flat-bottom microtiter plates (∼5 × 105/ml/well) and incubated at 37°C in 5% CO2–95% air to allow the cells to attach. The medium was then removed, and the cells were infected with isolated T. gondii in medium containing 3% FBS (one parasite/cell). After 1 h of incubation, the cultures were washed with medium containing 10% FBS to remove extracellular parasites. FBS was maintained at a final concentration of 10%. The purine analogue under study was then added to cultures of the parasite-infected cells to yield a final concentration of 0, 5, 10, 25, or 50 μM. Drugs were dissolved in 50% ethanol to yield a final concentration of 2.5% ethanol when added to the wells. After an additional 18 h of incubation, the medium was replaced with 1 ml of drug-free medium containing [5,6-3H]uracil (2 μCi/ml); incubation was continued for another 6 h, after which the medium was removed. The fibroblasts were released from the wells by trypsinization with the addition of 200 μl of trypsin-EDTA (2.5×) to each well. After 10 min of incubation, 1 ml of ice-cold 10% trichloroacetic acid was added to each well. The plates were shaken to ensure the detachment of the cells. The suspended contents of each well were filtered through GF/A 2.4-cm glass microfiber filters (Whatman, Hillsboro, Oreg.) which were each prewashed with 1 ml of double-distilled H2O and dried. After filtration, the filters were washed with 10 ml of methanol, allowed to dry, and then placed in scintillation vials containing 5 ml of Econo-Safe scintillation fluor (Research Products International Corp., Mount Prospect, Ill.); radioactivity was counted with an LS5801 Beckman scintillation counter. When possible, the concentration of analogue required to inhibit parasite replication by 50% (IC50) was calculated from dose-response plots of percent inhibition versus the log of the analogue concentration.
Host cell toxicity.
The toxicity of the various analogues was determined at least in triplicate by use of a modification of the microculture tetrazolium test (MTT) in uninfected monolayers of human foreskin fibroblasts. Briefly, confluent cells were incubated for at least 24 h in 96-well flat-bottom microtiter plates (∼105/200 μl/well) at 37°C in 5% CO2–95% air to allow the cells to attach. The medium was then replaced with 200 μl of fresh medium. The appropriate concentration of the compounds was dissolved in 50 μl of medium, and the solution was added to each well to yield a final concentration of 0, 5, 10, 25, or 50 μM. The cultures were incubated for 48 h, after which 50 μl of sterile MTT solution (2 mg/ml of PBS) was added to each well. MTT solution was sterilized by filtration through 0.22-μm-pore-size filters (Costar). After 4 h of incubation, the medium was removed, 100 μl of dimethyl sulfoxide was added to each well, and the plates were shaken gently for 2 to 3 min to dissolve the formed formazan crystals. The absorbance was measured at 540 nm with a computerized microtiter plate reader (Themomax; Molecular Devices).
RESULTS AND DISCUSSION
Table 1 shows that the enzyme extract from wild-type T. gondii, unlike that from mouse spleen, is capable of converting NBMPR to its nucleoside 5′-monophosphate, NBMPR-P, and its nucleobase, NBMP. The formation of these metabolites increased with incubation time. The authenticity of the nucleotide and nucleobase formed was verified by HPLC and LC/MS/MS analyses as described in Materials and Methods.
TABLE 1.
Conversion of NBMPR to NBMPR-P and NBMP by enzyme extracts from T. gondii and mouse spleen
| Product | Enzyme extract source | % conversion after incubation fora:
|
||||
|---|---|---|---|---|---|---|
| 15 min | 30 min | 60 min | 120 min | 240 min | ||
| NBMPR-P | T. gondii | 0.5 ± 0.1 | 1.1 ± 0.1 | 3.7 ± 2.5 | 5.8 ± 0.7 | 11.8 ± 4.8 |
| Mouse | 0 | 0 | 0 | 0 | 0 | |
| NBMP | T. gondii | 0.9 ± 0.3 | 2.8 ± 3.8 | 4.0 ± 1.3 | 9.5 ± 1.3 | 14.3 ± 3.3 |
| Mouse | 0 | 0 | 0 | 0 | 0 | |
The data are means ± standard deviations.
The observed formation of NBMPR-P from NBMPR could be achieved by direct phosphorylation (i.e., NBMPR to NBMPR-P) via adenosine kinase (Fig. 1), which is the only purine nucleoside kinase in toxoplasmas (14). Alternatively, NBMPR-P could be formed indirectly via the formation of its nucleobase (i.e., NBMPR to NBMP to NBMPR-P) by the sequential activities of a phosphorylase and HGXPRT, respectively (Fig. 1). Therefore, to determine by which route NBMPR-P is synthesized, we performed enzyme studies with various alternative natural substrates as well as specific inhibitors of the key enzymes which could be involved in the metabolism of NBMPR (Fig. 1). The results shown in Table 2 indicate that the phosphorylation of NBMPR to NBMPR-P by T. gondii was inhibited by adenosine, the substrate of adenosine kinase. Inosine, a very poor substrate of adenosine kinase, had a minimal effect on the phosphorylation of NBMP, while adenine and hypoxanthine, substrates of the phosphoribosyltransferase reaction, had no effect on the phosphorylation of NBMPR, even at concentrations as high as 1 mM (data not shown). Furthermore, as discussed below, NBMPR was not phosphorylated to NBMPR-P when ATP (the cosubstrate of the kinase reaction) was replaced with PRPP (the cosubstrate of the phosphoribosyltransferase reaction). These results indicate that, under these experimental conditions, adenosine kinase is the enzyme responsible for the phosphorylation of NBMPR and that HGXPRT has a minimal effect or no effect on the formation of NBMPR-P from NBMPR. To further corroborate these results and examine the specificity of this reaction, the effects of NBMPR, iodotubercidin (a specific inhibitor of adenosine kinase), formycin A (a specific inhibitor of 5′-methylthioadenosine phosphorylase [EC 2.4.2.28]), and 8-aminoguanosine (a specific inhibitor of purine nucleoside phosphorylase) on the phosphorylation of adenosine were investigated with different enzyme sources.
TABLE 2.
Inhibition by adenosine, adenine, inosine, and hypoxanthine of NBMPR-P synthesis from NBMPR and ATP by enzyme extracts from T. gondii
| Compound | % Inhibition
|
||||
|---|---|---|---|---|---|
| 10 μM | 50 μM | 75 μM | 100 μM | 150 μM | |
| Adenosine | 77 | 85 | 91 | 100 | 100 |
| Adenine | 0 | 0 | 0 | 0 | 0 |
| Inosine | 15 | NDa | 19 | ND | ND |
| Hypoxanthine | 0 | ND | 0 | ND | ND |
ND, not determined.
The results shown in Table 3 demonstrate that NBMPR reduced the phosphorylation of adenosine in T. gondii but not in human or mouse liver. Iodotubercidin, the adenosine kinase inhibitor, inhibited the phosphorylation of adenosine by extracts from all three tissues (Table 3) as well as the phosphorylation of NBMPR in T. gondii (Table 4). These results indicate that adenosine and NBMPR are substrates for the same enzyme, adenosine kinase. On the other hand, Tables 3 and 4 show that formycin A and 8-aminoguanosine, the inhibitors of 5′-methylthioadenosine phosphorylase and purine nucleoside phosphorylase, respectively, the only two known purine nucleoside phosphorylases which could be responsible for the phosphorolysis of NBMPR to its nucleobase NBMP, had no effect on the phosphorylation of adenosine or NBMPR by extracts from T. gondii, human liver, or mouse liver. These results indicate that the phosphorolysis pathway is insignificant in the synthesis of NBMPR-P from NBMPR. These results also indicate that T. gondii but not mammalian adenosine kinase is involved in the phosphorylation of NBMPR to NBMPR-P.
TABLE 3.
Effect of NBMPR and various enzyme inhibitors on adenosine phosphorylation by enzyme extracts from T. gondii, human liver, and mouse liver
| Compound and concn (μM) | % Inhibition:
|
||
|---|---|---|---|
| T. gondii | Human liver | Mouse liver | |
| NBMPR | |||
| 10 | 3.4 | 0 | 0 |
| 25 | 37.7 | 0 | 0 |
| 50 | 56.0 | 0 | 0 |
| Iodotubercidin | |||
| 10 | 82.9 | 95.9 | 97.2 |
| 25 | 85.6 | 100.0 | 97.5 |
| 50 | 100.0 | 100.0 | 97.1 |
| Formycin A | |||
| 100 | 0 | 0 | 0 |
| 250 | 0 | 0 | 0 |
| 500 | 0 | 0 | 0 |
| 8-Aminoguanosine | |||
| 25 | 0 | 0 | 0 |
| 50 | 0 | 0 | 0 |
| 100 | 0 | 0 | 0 |
TABLE 4.
Effect of various enzyme inhibitors on NBMPR phosphorylation by enzyme extracts from T. gondii
| Compound and concn (μM) | % Inhibition |
|---|---|
| Iodotubercidin | |
| 10 | 74.8 |
| 25 | 60.0 |
| 50 | 66.5 |
| Formycin A | |
| 100 | 0 |
| 250 | 0 |
| 500 | 0 |
| 8-Aminoguanosine | |
| 25 | 0 |
| 50 | 0 |
| 100 | 0 |
To further verify the conclusions drawn above, the phosphorylation of NBMPR, adenosine, hypoxanthine, and NBMP was tested with extracts from wild-type strain RH and from mutants deficient in adenosine kinase (Ara-AR) (20) and HGXPRT (ThxR-1) (21). Table 5 shows that only the wild-type (RH) and HGXPRT-deficient (ThxR-1) strains, which have adenosine kinase, can phosphorylate adenosine or NBMPR to their respective nucleoside 5′-monophosphates. Extracts from the adenosine kinase-deficient mutant (Ara-AR) had no activity with either substrate. Furthermore, the phosphorylation of adenosine or NBMPR can take place only in the presence of ATP, the cosubstrate of the adenosine kinase reaction, and not PRPP, the cosubstrate of the HGXPRT reaction. Furthermore, in spite of the fact that wild-type RH and adenosine kinase-deficient mutant Ara-AR contain HGXPRT, as evidenced by their ability to convert hypoxanthine in the presence of PRPP to IMP, neither was capable of converting NBMP to NBMPR-P. These results indicate further that adenosine kinase is a major route for the conversion of NBMPR to NBMPR-P in toxoplasmas.
TABLE 5.
Phosphorylation of adenosine, NBMPR, hypoxanthine, and NBMP by enzyme extracts from wild-type (RH), adenosine kinase-deficient (Ara-AR), and HGXPRT-deficient (ThxR-1) strains of T. gondii in the presence of ATP or PRPP
| Strain | Cosubstrate | Activity (pmol/min/mg of protein) with the following substratea:
|
|||
|---|---|---|---|---|---|
| Adenosine | NBMPR | Hypoxanthine | NBMP | ||
| RH | ATP | 41.8 ± 1.4 | 4.9 ± 0.5 | NDa | ND |
| PRPP | 0.0 | 0.0 | 10,400 ± 1,230 | 0.0 | |
| Ara-AR | ATP | 0.0 | 0.0 | ND | ND |
| PRPP | 0.0 | 0.0 | 11,970 ± 2,605 | 0.0 | |
| ThxR-1 | ATP | 51.3 ± 5.7 | 3.6 ± 1.3 | ND | ND |
| PRPP | 0.0 | 0.0 | 0.0 | 0.0 | |
Values are means ± standard deviations. ND, not determined.
To investigate what effect such a unique metabolism of NBMPR would have on T. gondii, the effect of NBMPR on the survival of T. gondii grown in human fibroblasts was studied. Five days of incubation in the presence of 100 μM NBMPR decreased the number of toxoplasma plaques in human fibroblasts from 21 ± 3.5 to 3 ± 1.4. The effect of NBMPR on the growth of toxoplasmas was also studied by uracil uptake assays. Uracil uptake assays are highly specific for T. gondii, as mammalian cells do not incorporate uracil into their nucleoside and nucleotide pools or nucleic acids (19 and our own results). Therefore, an exponential increase in radiolabel incorporation closely correlates with the exponential growth of the parasites (19). Using this method, we found that the growth of toxoplasmas was inhibited by NBMPR and NBMPR-P and that the inhibition was dose dependent (Table 6). On the other hand, NBMPR and NBMPR-P had no significant toxic effect on the survival of uninfected host cells (Table 7). These results indicate that NBMPR and NBMPR-P are selectively toxic to T. gondii-infected cells and therefore are promising therapeutic agents against toxoplasmosis. Indeed, our preliminary results indicate that the administration of NBMPR-P increased the life span of mice infected with the virulent RH toxoplasma strain from 6 to 8 days. Refining the model (e.g., different doses, schedules, and appropriate toxoplasma strains other than the exceptionally virulent RH strain) may produce better results.
TABLE 6.
Effect and IC50s of NBMPR, various 6-substituted 9-β-d-ribofuranosylpurines, and other therapeutic compounds on the percentage of survival of T. gondii grown in human fibroblasts in culturesa
| Compound | % Survival of T. gondii
|
IC50 | ||||
|---|---|---|---|---|---|---|
| 0 μM | 5 μM | 10 μM | 25 μM | 50 μM | ||
| NBMPR | 100 | 85.9 | 35.8 | 13.9 | 6.0 | 10.2 |
| NBMPR-P | 100 | 70.5 | 39.9 | 31.4 | 21.4 | 9.9 |
| 6-Benzylthioinosine | 100 | 33.2 | 25.3 | 23.8 | 12.4 | 1.3 |
| p-Nitrobenzyl-6-selenopurine riboside | 100 | 35.5 | 7.7 | 2.2 | 0 | 3.3 |
| N6-Anisoyladenosine | 100 | 94.2 | 93.1 | 104.2 | 102.5 | ND |
| N6-Benzoyladenosine | 100 | 104.3 | 94.6 | 111.7 | 100.5 | ND |
| N6-Benzyladenosine | 100 | 67.4 | 46.5 | 52.9 | 28.0 | 14.3 |
| N6-(p-Aminobenzyl)adenosine | 100 | 100.0 | 95.6 | 108.3 | 119.9 | ND |
| N6-(p-Azidobenzyl)adenosine | 100 | 29.6 | 22.4 | 14.7 | 8.8 | 1.3 |
| N6-(p-Nitrobenzyl)adenosine | 100 | 36.3 | 22.3 | 12.8 | 8.4 | 2.3 |
| Sulfadiazine | 100 | 74.6 | 64.8 | 66.3 | 66.4 | ND |
| Pyrimethamine | 100 | 39.2 | 16.8 | 10.8 | 13.8 | 1.3 |
Survival was measured by the incorporation of [5,6-3H]uracil in at least two experiments with four replicates each. ND, not determined due to insufficient data.
TABLE 7.
Effect of NBMPR, various 6-substituted 9-β-d-ribofuranosylpurines, and other therapeutic compounds on the percentage of survival of uninfected human fibroblasts grown in culturesa
| Compound | % Survival of uninfected host cells
|
||||
|---|---|---|---|---|---|
| 0 μM | 5 μM | 10 μM | 25 μM | 50 μM | |
| NBMPR | 100 | 98.8 | 97.1 | 96.3 | 95.4 |
| NBMPR-P | 100 | 103.3 | 94.6 | 93.6 | 96.1 |
| 6-Benzylthioinosine | 100 | 92.2 | 89.5 | 79.6 | 80.7 |
| p-Nitrobenzyl-6-selenopurine riboside | 100 | 89.3 | 90.1 | 63.4 | 39.6 |
| N6-Anisoyladenosine | 100 | 92.9 | 92.6 | 99.2 | 97.4 |
| N6-Benzyladenosine | 100 | 43.3 | 21.8 | 32.4 | 29.6 |
| N6-Benzoyladenosine | 100 | 94.4 | 94.1 | 97.6 | 99.3 |
| N6-(p-Aminobenzyl)adenosine | 100 | 93.3 | 91.9 | 100.6 | 101.4 |
| N6-(p-Azidobenzyl)adenosine | 100 | 97.3 | 90.3 | 88.9 | 81.5 |
| N6-(p-Nitrobenzyl)adenosine | 100 | 104.1 | 102.1 | 92.3 | 106.8 |
| Sulfadiazine | 100 | 98.2 | 99.7 | 99.8 | 102.5 |
| Pyrimethamine | 100 | 103.6 | 98.8 | 108.2 | 118.4 |
Survival was measured by the absorbance at 540 nm of MTT-treated cells in at least two experiments with three replicates each.
Because of the promising potential of NBMPR in the treatment of toxoplasmosis, several other related 6-substituted 9-β-d-ribofuranosylpurines which were previously identified as good ligands of T. gondii adenosine kinase (12) were tested as antitoxoplasma agents and compared with the standard chemotherapeutic agents for the treatment of toxoplasmosis (sulfadiazine and pyrimethamine). Among the analogues tested, 6-benzylthioinosine, p-nitrobenzyl-6-selenopurine riboside, N6-benzyladenosine, N6-(p-azidobenzyl)adenosine, and N6-(p-nitrobenzyl)adenosine were promising (Tables 6 and 7). These five compounds were comparable or better agents than the prototype drug NBMPR and sulfadiazine or pyrimethamine, the standard chemotherapeutic agent for the treatment of toxoplasmosis (Table 6). However, N6-(benzyl)adenosine, unlike the other four compounds, was substantially toxic to uninfected host cells at therapeutic doses (Table 7). Therefore, N6-benzyladenosine is not suitable as an antitoxoplasmosis agent. Whether or not these active analogues are metabolized and exert their toxicity in the same manner as NBMPR remains to be determined. Previous experience dictates that similar analogues may not be metabolized in the same fashion in spite of the similarity in their structures (5).
How NBMPR or NBMPR-P enters toxoplasma-infected cells to exert its selective toxicity remains to be determined. NBMPR and NBMPR-P have been extensively investigated as inhibitors of nucleoside transport in mammalian cells. Nevertheless, none of these studies, to our knowledge, has demonstrated that NBMPR or NBMPR-P enters the cytoplasm of or is metabolized by uninfected mammalian cells. The mammalian cell membrane has tightly restricted permeability. Nucleosides, but not nucleotides, are transported into mammalian cells by specific nucleoside transporters, for some of which NBMPR is an inhibitor. In infected cells, toxoplasmas reside within specialized membrane-surrounded vacuoles known as parasitophorous vacuoles. The parasitophorous vacuole membrane acts as a molecular sieve, allowing bidirectional equilibrated diffusion of small molecules (<1,300 daltons), including nucleosides and nucleotides, between the vacuolar space and the host cell cytoplasm (17, 23, and references therein). Infection was shown to alter the permeation and metabolism of purines as well as nucleoside transport specificity in host cells (10, 11, 26). Cells infected by other protozoa were reported to transport nucleosides which could not be transported before infection (26). Such alterations in nucleoside transport of infected cells could be due to the presence of “metabolic windows” or “ducts” by which intracellular parasites can obtain purines without any access via the host cell cytoplasm or membrane (26). Whatever the mechanism of altered permeability to nucleosides and nucleotides in T. gondii-infected cells, which is beyond the scope of the current study, the fact remains that preferential uptake and metabolism of toxic nucleosides selectively kill parasite-infected cells, while normal, uninfected cells are spared such toxicity, as observed in the present investigation.
In this regard, it should be noted that during our studies with NBMPR as an adjunct in the chemotherapy of schistosomiasis with purine analogues (3, 4, 6–8), we administered NBMPR at 25 mg/kg of body weight/day for up to 3 weeks without apparent host toxicity (3, 4, 6–8). Such doses of NBMPR were judged nontoxic to the animals by blood chemistry, hematological studies, and gross and histological examinations (7). No evidence for injury to the liver, kidneys, spleen, pancreas, mesentery, or peritoneal mesothelium was observed. Furthermore, the administration of NBMPR at the highest dose tested (100 mg/kg) showed no host cell toxicity (13). Therefore, it can be stated that the administration of NBMPR is safe at least up to the highest dose tested (100 mg/kg). Whether or not the other identified antitoxoplasmic purine analogues, 6-benzylthioinosine, p-nitrobenzyl-6-selenopurine riboside, N6-(p-azidobenzyl)adenosine, and N6-(p-nitrobenzyl)adenosine, are safe for in vivo administration, as was demonstrated in vitro, remains to be tested.
In conclusion, we discovered that NBMPR is selectively metabolized by T. gondii. The unique and previously unknown phosphorylation of this nucleoside analogue by T. gondii adenosine kinase appears to be a major reason for its selective toxicity against this parasite. Further studies on T. gondii adenosine kinase not only may contribute to the general knowledge of purine metabolism in this parasite but also may reveal potential drugs for the treatment of toxoplasmosis. Such studies may also benefit the development of drugs for other parasitic pathogens. Several purine analogues (e.g., pyrazolopyrimidines) were shown to be phosphorylated selectively by adenosine kinase from other animal-parasitic protozoa (16). Therefore, it is not unreasonable to assume that a similar situation may exist for other human pathogens.
ACKNOWLEDGMENTS
We thank Elmer R. Pfefferkorn for generously providing the mutant strains and for many helpful discussions, Brian Roberts for excellent technical assistance, and Marion C. Kirk for help with the LC/MS/MS mass spectrometer analysis.
This investigation was supported by grants AI-29849, AI-29950, and AI-42975 awarded by the NIAID. The mass spectrometer was purchased with grant S1ORR06487 and its operation was supported in part by grant CA-13148 awarded by the NCI, DHHS.
REFERENCES
- 1.Bermudes D, Peck K R, Afifi M A, Beckers C J M, Joiner K A. Tandemly repeated genes encode nucleoside triphosphate hydrolase isoforms secreted into the parasitophorous vacuole of Toxoplasma gondii. J Biol Chem. 1994;46:29252–29260. [PubMed] [Google Scholar]
- 2.Bradford M M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 3.el Kouni M H, Diop D, Cha S. Combination therapy of schistosomiasis by tubercidin and nitrobenzylthioinosine 5′-monophosphate. Proc Natl Acad Sci USA. 1983;80:6667–6670. doi: 10.1073/pnas.80.21.6667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.el Kouni M H, Knopf P M, Cha S. Combination therapy of Schistosoma japonicum by tubercidin and nitrobenzylthioinosine 5′-monophosphate. Biochem Pharmacol. 1985;34:3921–3923. doi: 10.1016/0006-2952(85)90445-9. [DOI] [PubMed] [Google Scholar]
- 5.el Kouni M H, Cha S. Metabolism of adenosine analogues by Schistosoma mansoni and the effect of nucleoside transport inhibitors. Biochem Pharmacol. 1987;36:1099–1106. doi: 10.1016/0006-2952(87)90420-5. [DOI] [PubMed] [Google Scholar]
- 6.el Kouni M H, Messier N J, Cha S. Treatment of schistosomiasis by purine nucleoside analogues in combination with nucleoside transport inhibitors. Biochem Pharmacol. 1987;36:3815–3821. doi: 10.1016/0006-2952(87)90443-6. [DOI] [PubMed] [Google Scholar]
- 7.el Kouni M H, Diop D, O’Shea P, Carlisle R, Sommadossi J-P. Prevention of tubercidin host toxicity by nitrobenzylthioinosine 5′-monophosphate for the treatment of schistosomiasis. Antimicrob Agents Chemother. 1989;33:824–827. doi: 10.1128/aac.33.6.824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.el Kouni M H. Efficacy of combination therapy with tubercidin and nitrobenzylthioinosine 5′-monophosphate against chronic and advanced stages of schistosomiasis. Biochem Pharmacol. 1991;41:815–820. doi: 10.1016/0006-2952(91)90085-j. [DOI] [PubMed] [Google Scholar]
- 9.el Kouni M H, Guarcello V, Al Safarjalani O N, Naguib F N M. Metabolism and selective toxicity of 6-nitrobenzylthioinosine in Toxoplasma gondii. FASEB J. 1998;12:A126. doi: 10.1128/aac.43.10.2437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gati W P, Stoyke A F W, Gero A M, Paterson A R P. Nucleoside permeation in mouse erythrocytes infected with Plasmodium yoelii. Biochem Biophys Res Commun. 1987;145:1134–1141. doi: 10.1016/0006-291x(87)91555-5. [DOI] [PubMed] [Google Scholar]
- 11.Gero A M, Bugledich E M A, Paterson A R P, Jamieson G P. Stage-specific alteration of nucleoside membrane permeability and nitrobenzylthioinosine insensitivity in Plasmodium falciparum infected erythrocytes. Mol Biochem Parasitol. 1988;27:159–170. doi: 10.1016/0166-6851(88)90035-7. [DOI] [PubMed] [Google Scholar]
- 12.Iltzsch M H, Uber S S, Tankersley K O, el Kouni M H. Structure-activity relationship for the binding of nucleoside ligands to adenosine kinase from Toxoplasma gondii. Biochem Pharmacol. 1995;49:1501–1512. doi: 10.1016/0006-2952(95)00029-y. [DOI] [PubMed] [Google Scholar]
- 13.Kolassa N, Jakobs E S, Buzzell G R, Paterson A R P. Manipulation of toxicity and tissue distribution of tubercidin in mice by nitrobenzylthioinosine 5′-monophosphate. Biochem Pharmacol. 1982;31:1863–1874. doi: 10.1016/0006-2952(82)90489-0. [DOI] [PubMed] [Google Scholar]
- 14.Krug E C, Marr J J, Berens R L. Purine metabolism in Toxoplasma gondii. J Biol Chem. 1989;264:10601–10607. [PubMed] [Google Scholar]
- 15.Luft B J, Remington J S. AIDS commentary: toxoplasmic encephalitis in AIDS. Clin Infect Dis. 1992;15:211–222. doi: 10.1093/clinids/15.2.211. [DOI] [PubMed] [Google Scholar]
- 16.Miller R L, Adamczyk D L, Rideout J L, Krenitsky T A. Purification, characterization, substrate and inhibitor specificity of adenosine kinase from several Eimeria species. Mol Biochem Parasitol. 1982;6:209–223. doi: 10.1016/0166-6851(82)90055-x. [DOI] [PubMed] [Google Scholar]
- 17.Naguib F N M, Iltzsch M H, el Kouni M M, Panzica R P, el Kouni M H. Structure-activity relationships for the binding of ligands to xanthine or guanine phosphoribosyl-transferase from Toxoplasma gondii. Biochem Pharmacol. 1995;50:1685–1693. doi: 10.1016/0006-2952(95)02070-5. [DOI] [PubMed] [Google Scholar]
- 18.Perrotto J, Keister D B, Gelderman A H. Incorporation of precursors into Toxoplasma DNA. J Protozool. 1971;18:470–473. doi: 10.1111/j.1550-7408.1971.tb03356.x. [DOI] [PubMed] [Google Scholar]
- 19.Pfefferkorn E R, Pfefferkorn L C. Specific labeling of intracellular Toxoplasma gondii with uracil. J Protozool. 1977;24:449–453. doi: 10.1111/j.1550-7408.1977.tb04774.x. [DOI] [PubMed] [Google Scholar]
- 20.Pfefferkorn E R, Pfefferkorn L C. The biochemical basis for resistance to adenine arabinoside in a mutant of Toxoplasma gondii. J Parasitol. 1978;64:486–492. [PubMed] [Google Scholar]
- 21.Pfefferkorn E R, Borotz S E. Toxoplasma gondii: characterization of a mutant resistant to 6-thioxanthine. Exp Parasitol. 1994;79:374–382. doi: 10.1006/expr.1994.1099. [DOI] [PubMed] [Google Scholar]
- 22.Remington J S, Desmonts G. Toxoplasmosis. In: Remington J S, Klein J O, editors. Infectious diseases of the fetus and newborn infant. 4th ed. Philadelphia, Pa: The W. B. Saunders Co.; 1995. pp. 140–267. [Google Scholar]
- 23.Schwab J C, Beckers C J M, Joiner K A. The parasitophorous vacuole membrane surrounding intracellular Toxoplasma gondii as a membrane sieve. Proc Natl Acad Sci USA. 1994;91:509–513. doi: 10.1073/pnas.91.2.509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schwartzman J D, Pfefferkorn E R. Toxoplasma gondii: purine synthesis and salvage in mutant host cells and parasites. Exp Parasitol. 1982;53:77–86. doi: 10.1016/0014-4894(82)90094-7. [DOI] [PubMed] [Google Scholar]
- 25.Subauste C S, Remington J S. Immunity to Toxoplasma gondii. Curr Opin Immunol. 1993;5:532–537. doi: 10.1016/0952-7915(93)90034-p. [DOI] [PubMed] [Google Scholar]
- 26.Upston J M, Gero A M. Parasite induced permeation of nucleosides in Plasmodium falciparum malaria. Biochim Biophys Acta. 1995;1236:249–258. doi: 10.1016/0005-2736(95)00055-8. [DOI] [PubMed] [Google Scholar]
- 27.Wong S, Remington J S. Biology of Toxoplasma gondii. AIDS. 1993;7:299–316. doi: 10.1097/00002030-199303000-00001. [DOI] [PubMed] [Google Scholar]