Abstract
Simple Summary
Aromia bungii (Faldermann) (Coleoptera: Cerambycidae) is a serious wood borer of stone fruit trees. Native to parts of Asia and Russia, this beetle recently invaded Germany, Italy, and Japan, causing enormous economic losses. Repellents are effective and sustainable control methods of insect pests. In this study, we identified the A. bungii female-repellency ingredients from Mentha spicata: myrcene, (S)-(+)-carvone, (E)-β-caryophyllene, and borneol, as well as their recommended quantities for use. These results contribute to research on repellents that prevent infestation and damage caused by A. bungii.
Abstract
Aromia bungii (Coleoptera: Cerambycidae) is an economically important wood-boring insect pest of stone fruit trees, particularly Prunus persica, in China. It has entered Japan and several European countries as an invasive species in recent years. It is difficult to control because of the cryptic feeding behaviour of larvae beneath the bark. Identification of repellent constituents from non-host plants has potential for use in management strategies against this beetle. Mentha spicata is cultivated extensively in Hebei Province (China) as a medicinal plant. Firstly, antennal responses of female A. bungii to M. spicata volatiles were evaluated by coupled gas chromatography-electroantennograms (GC-EAD), and then the EAD-active components were tested in semi-field trials. The results showed that A. bungii females were significantly repelled by myrcene, (S)-(+)-carvone, (E)-β-caryophyllene, and borneol compared with the control. The presence of myrcene (100 µL; 90% purity), (S)-(+)-carvone (200 µL; 96% purity), (E)-β-caryophyllene (500 µL; 98.5% purity), and borneol (800 µL; 80% purity) significantly reduced the perching rates of A. bungii females on both peach logs and leaves. Considering cost and commercial availability, we suggest that myrcene, (S)-(+)-carvone, and (E)-β-caryophyllene could be promising repellents against A. bungii females in the field.
Keywords: Aromia bungii, Mentha spicata, behavioural responses, repellent, wood borer
1. Introduction
The red-necked longhorn beetle Aromia bungii (Faldermann) (Coleoptera: Cerambycidae: Cerambycinae) is a destructive wood-boring pest of trees in the genus Prunus, which includes a number of economically important stone fruit species including peaches, cherries, plums, and apricots [1,2]. This beetle is widely distributed in China, Korea, Mongolia, and eastern Russia [3]. It has invaded Japan, Germany, and Italy, and has been intercepted in cargoes entering the UK and the USA [1]. In 2014, A. bungii was added to the EPPO A1 list of pests recommended for regulation as quarantine pests [4,5]. A very recent datasheet from CABI (Center for Agriculture and Bioscience International) stated that A. bungii “presents a significant risk to all stone fruit-growing countries in Europe and neighbouring countries” [6].
Aromia bungii adults lay eggs in cracks and crevices in the bark of host trees. Developing larvae bore galleries in the phloem and xylem beneath the bark; the complete gallery can reach 50–60 cm in length [7]. Beetles overwinter as larvae and then emerge as adults between June and August. The life cycle lasts 2 to 4 years, depending on the latitude and the climate [8]. The ratio of males to females is about 1:1 [9] and adults can live for over 40 days in the laboratory [10]. Females can mate and oviposit multiple times on the tree trunk, laying an average of >300 eggs [11]. Due to the cryptic feeding damage of larvae and the high fecundity of adults, it has already caused heavy economic losses to Prunus persica orchards in China. Since the adult stage coincides with the maturing and harvesting period of peach fruit, it is not recommended to control adults using insecticides. To date, some biological control studies have been done [12,13], and pheromone-based monitoring and control techniques have been developed [14,15,16,17,18,19]. However, A. bungii remains a big problem in China.
Plant derivatives or botanical repellents have been used against arthropods for at least two millennia in ancient China, Egypt and India [20,21,22,23,24,25]. For example, Mentha spicata (spearmint) has long been used as a medicinal and aromatic plant for its distinctive smell, which makes it very popular as a flavouring and calming agent [26]. Extracts of M. spicata stems and leaves are repellent to adult Plutella xylostella (Lepidoptera: Plutellidae) [27]; the mosquito, Anopheles stephensi (Diptera: Anophelinae) [28]; the carmine spider mite, Tetranychus cinnabarinus (Acarina: Tetranychidae) [29,30] and Frankliniella occidentalis (Thysanoptera: Thripidae) [31,32]. Since A. bungii adults mainly oviposit on the lower sections of peach tree trunks in the orchard, prevention of oviposition could be an operable and effective method for reducing the population density of the next generation of A. bungii.
To our knowledge, there is little information concerning the behavioural effect of M. spicata on long-horned beetles. We hypothesise that extracts of M. spicata or M. spicata itself could influence the behaviour of A. bungii adults. Firstly, we used gas chromatography-electroantennogram detection (GC-EAD) to screen for active compounds in M. spicata volatiles. Secondly, semi-field cage bioassays were conducted to explore what kind of adult behavioural response was aroused by different quantities of EAD-active components. Finally, we used semi-field cage bioassays to check whether A. bungii females were repelled by the identified active components from M. spicata when in the presence of P. persica leaves and stems. We hypothesised that compounds from M. spicata that repelled A. bungii females could be useful in integrated management strategies.
2. Materials and Methods
2.1. Insect Collection
Prunus persica trees heavily infested with A. bungii larvae were felled and logs transferred from peach orchards in Shunping County, Hebei province, China, to our laboratory in early April 2019. The logs varied in length from 80–120 cm and in diameter from 20–30 cm. Cut edges were sealed with paraffin wax and a plastic film to avoid desiccation, and placed in steel gauze mesh cages (20 mesh sieve) in the laboratory at 25 ± 4 °C, RH 60 ± 10%. Adult A. bungii were collected as soon as they emerged in late May to July 2019. Each adult was kept individually in a plastic chamber (PE, 18 cm × 11 cm × 8 cm) and fed with commercial P. persica jelly. Adults used for GC-EAD experiments were all more than 3 days old and unmated. Since our main aim was to find the oviposition repellent, only females were tested in our experiments. Females used in the semi-field cage bioassays were captured from peach orchards in Shunping County, Hebei province, China.
2.2. Coupled Gas Chromatography-Electroantennogram Detection (GC-EAD)
2.2.1. Collection of Volatiles
Potted M. spicata were purchased from the flower market. Volatiles were collected from 2.7 g M. spicata leaves (cut into 1–2 cm pieces: in order to get a high concentration of volatiles) using Tenax-TA adsorbent (60–80 mesh, 100 mg, Sigma-Aldrich, Saint Louis, MO, USA) held in a glass tube (12 cm long with an inner diameter of 0.5 cm), with the Teflon tube connected to the clean air (filtered with colour changing silicone and activated carbon) outlet. Leaf volatiles were collected for 2 h at 400 mL/min flow rate during daylight at 26 °C. Extracted volatiles were eluted with re-steaming chromatographic grade hexane (400 µL), and kept at −20 °C prior to analyses. In total, ten aeration extracts were prepared from M. spicata leaves.
2.2.2. GC-EAD Analysis of Volatiles
The Mentha spicata aeration extract was analyzed by GC-EAD on an Agilent 7890A GC (Agilent, Santa Clara, CA, USA) fitted with a HP-5 capillary column (30 m × 0.32 mm × 0.25 µm). Nitrogen (99.999% purity) was used as the carrier gas. The injector temperature was 250 °C, and injections were made in splitless mode. The GC oven was programmed from 40 °C for 1 min, then run to 120 °C (held for 1 min) at 10 °C/min, and run to 280 °C (held for 2 min) at 20 °C/min. Column effluent was split equally between the flame ionization detector (FID) and the electroantennogram detector (EAD) with a press-fit Y splitter (Agilent, part number: 5181-3398).
The two terminal flagella of an antenna from an A. bungii female were gently cut, then approximately 1 mm from the antennal tip was removed. The prepared antenna was centered in the effluent air stream from the GC. The antennal signals were amplified and filtered using an IDAC-4 data acquisition controller (Syntech, Hilversum, The Netherlands). Data were recorded in parallel with the FID signal and analyzed by GC-EAD 2000 software. In total, analyses were replicated with antennae from 10 females, and 1 antenna was used per female, with each antennal preparation being reused in 2–3 analyses.
2.3. GC-MS Analysis of Volatiles
GC-MS was performed with an Agilent Technologies 7890B GC coupled with an Agilent Technologies 5977A mass spectrometer. Samples were analyzed on a HP-5MS UI column (30 m × 0.25 mm × 0.25 µm) (Agilent) to obtain the molecular ion signals, and compared with the NIST 14.0 library. The GC oven conditions were the same as the conditions of the GC-EAD. Helium (99.999% purity) was used as the carrier gas at a rate of 1.2 mL/min. The mass spectrometer was operated in electron-impact (EI) mode (70 eV). The scan range was m/z 50–550. The temperatures at ionization source and interface were 230 °C and 285 °C, respectively. Authentic compounds were purchased to compare the retention time (RT) and molecular ion signals with the different chemicals in the volatiles.
2.4. Olfactory Response of A. bungii Females to Four Synthetic Chemicals
2.4.1. Compounds Used
Myrcene (90%) and (E)-β-caryophyllene (98.5%) were purchased from Sigma-Aldrich (Saint Louis, MO, USA). Borneol (1000 µg/mL in methanol, including 200 µg/mL isoborneol) was purchased from Tokyo Chemical Industry Co., Ltd. (Tokyo, Japan). (S)-(+)-Carvone (96%) was purchased from J&K Scientific Ltd. (Beijing, China). Synthetic chemicals used in bioassays were standard reagents with no dilution.
2.4.2. Semi-Field Cage Bioassays of A. bungii Females’ Responses to Synthetic Chemicals
To be closer to the natural environment, two semi-field experiments were conducted in cages (120 cm × 50 cm × 50 cm, Nylon mesh with 60 mesh) under the shadow of trees in the peach orchard in Shunping County, Hebei province, China, in June 2020.
First, different quantities of the four tested synthetic chemicals and distilled water were placed in the two opposite corners of the cage, respectively. For the treatment compounds, the starting quantity was 200 µL, followed by lower quantities (100 µL, 50 µL) or higher quantities (500 µL, 800 µL) to determine the quantities needed to achieve biological activity.
To further test the repellent effects of synthetic chemicals, a second experiment was done using particular chemicals in the presence of the host plant. Host plants were placed in the two opposite corners of each cage and the synthetic chemicals under evaluation placed on one of those host plants. Two different host plant treatments were used: either leaves (15 g) or logs (40 cm in height, 18 cm in diameter) of P. persica and the cut edges were sealed with paraffin wax. The synthetic chemicals were presented in polythene-sealed bags (3 cm × 3 cm) containing a particular quantity and were hung on the P. persica leaves or logs.
In all tests, one female A. bungii was introduced at the central point of each cage. During the bioassay, if the beetle moved to within 30 cm of an odour source in 10 min, it was considered to have made a choice. The position of the two treatments was reversed after every five beetles tested. Each beetle was used only once, and at least 30 responsive adults were required for each choice test. All bioassays were conducted from 8:30 to 10:00 AM beneath the natural light at 23 ± 2 °C, and the RH was 70 ± 5%.
2.5. Statistical Analyses
Data for behavioural responses in the semi-field cage tests were dependent and did not have a normal distribution, so the binomial test (Nonparametric tests) was used to identify significant differences in the responses of A. bungii adults to different odours in the semi-field cage tests. There were at least 30 adults that made choices in each experiment. All analyses were conducted by SPSS software (Version, 22.0).
3. Results
3.1. Identification of Active Chemicals from M. spicata Volatiles
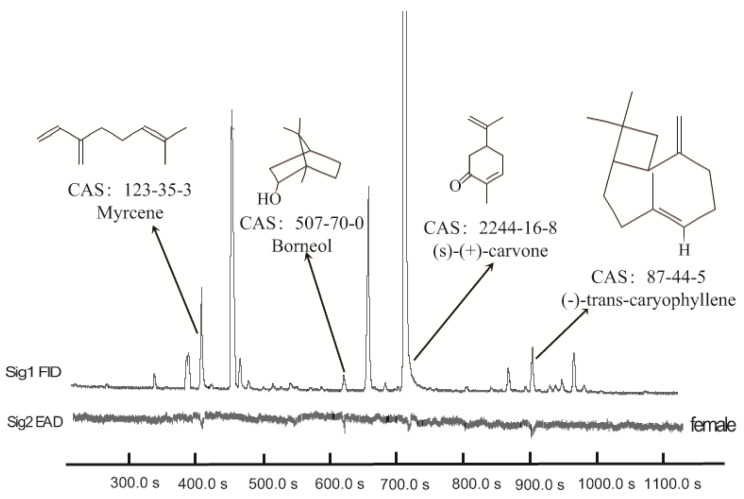
In GC-EAD analysis of M. spicata volatiles, antennae of female A. bungii responded to four components (Figure 1), which were then identified by GC-MS as myrcene (m/z 136, m/z 93, m/z 79, m/z 69), borneol (m/z 154, m/z 110, m/z 95, m/z 67), (S)-(+)-carvone (m/z 150, m/z 108, m/z 82, m/z 54), and (E)-β-caryophyllene (m/z 204, m/z 133, m/z 93, m/z 79), respectively. They were confirmed again with those of authentic myrcene, borneol, (S)-(+)-carvone, and (E)-β-caryophyllene.
Figure 1.
Coupled gas chromatography-electroantennogram detection of M. spicata volatiles. The upper trace is the GC chromatogram; the lower inverted trace is the antennal response of a female A. bungii; CAS number, chemical names, and structural formulae of antennal-active compounds are indicated.
3.2. Semi-Field Cage Bioassay of A. bungii Females to Different Quantities of Four Synthetic Chemicals
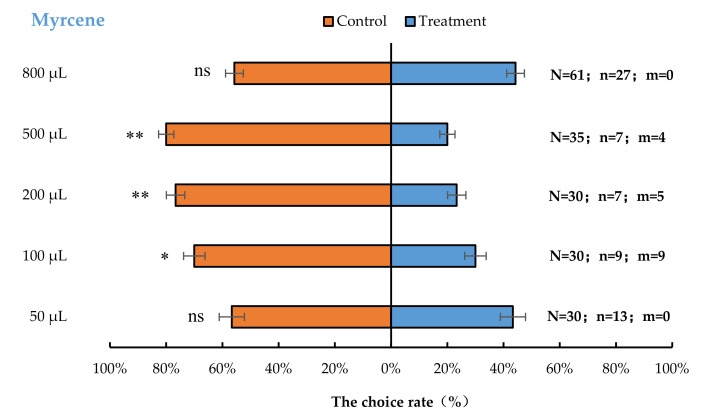
The result of the cage bioassays showed that A. bungii females exhibited significant behavioural repulsion to 100 µL myrcene (p < 0.05), 200 µL myrcene (p < 0.01), and 500 µL myrcene (p < 0.01), but no significant behavioural response to 50 µL (p = 0.59) or 800 µL (p = 0.37) myrcene compared with the control (Figure 2).
Figure 2.
Behavioural responses of A. bungii females to different quantities of myrcene. Note: N = number of responsive A. bungii; n = number of A. bungii females choosing the treatment; m = number of A. bungii that made no choice. ns = no significant difference; * = significant difference at the 0.05 level. ** = significant difference at the 0.01 level (the binomial test).
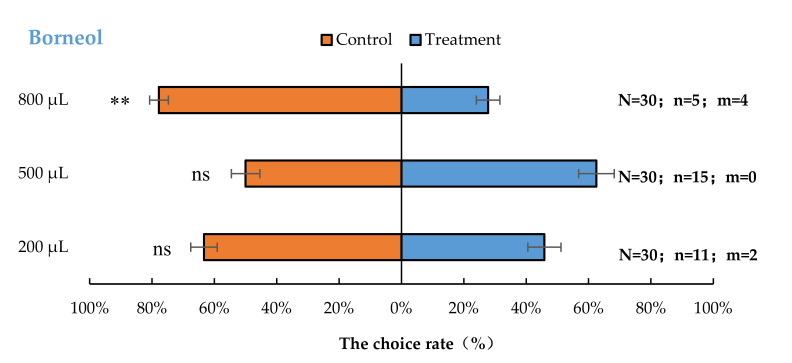
Aromia bungii females exhibited significant behavioural repulsion to 800 µL borneol (p < 0.01), but no significant behavioural response to 200 µL (p = 0.20) and 500 µL (p = 1.00) borneol compared with the control (Figure 3).
Figure 3.
Behavioural responses of A. bungii females to different quantities of borneol. Note: N = number of responsive A. bungii; n = number of A. bungii females choosing the treatment; m = number of A. bungii that made no choice. ns = no significant difference; ** = significant difference at the 0.01 level (the binomial test).
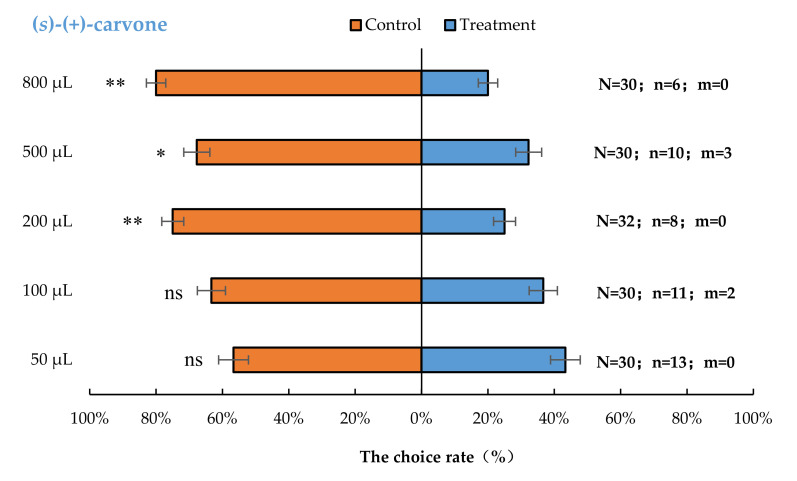
Aromia bungii females exhibited significant behavioural repulsion to 500 µL (S)-(+)-carvone (p < 0.05), 200 µL (S)-(+)-carvone (p < 0.01), and 800 µL (S)-(+)-carvone (p < 0.01), but no significant behavioural response to 50 µL (p = 0.59) and 100 µL (p = 0.20) (S)-(+)-carvone compared with the control (Figure 4).
Figure 4.
Behavioural responses of A. bungii females to different quantities of (S)-(+)-carvone. Note: N = number of responsive A. bungii; n = number of A. bungii females choosing the treatment; m = number of A. bungii that made no choice. ns = no significant difference; * = significant difference at the 0.05 level. ** = significant difference at the 0.01 level (the binomial test).
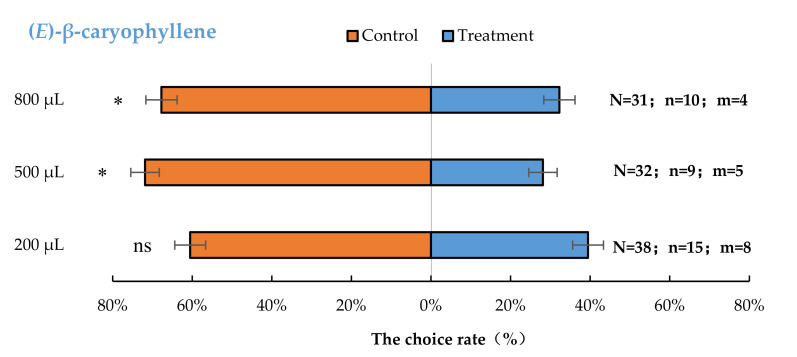
Aromia bungii females exhibited significant behavioural repulsion to 500 µL (E)-β-caryophyllene (p < 0.05) and 800 µL (E)-β-caryophyllene (p < 0.05), but no significant behavioural response to 200 µL (p = 0.26) (E)-β-caryophyllene compared with the control (Figure 5).
Figure 5.
Behavioural responses of A. bungii females to different quantities of (E)-β-caryophyllene. Note: N = number of responsive A. bungii; n = number of A. bungii females choosing the treatment; m = number of A. bungii that made no choice. ns = no significant difference; * = significant difference at the 0.05 level.
3.3. Semi-Field Cage Bioassay of A. bungii Females’ Responses to Host Plant in the Presence of Synthetic Chemicals
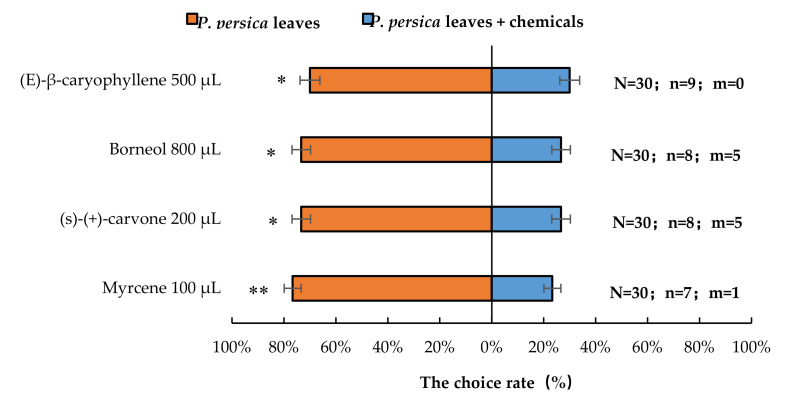
The results showed that the presence of 200 µL (S)-(+)-carvone (p < 0.05), 500 µL (E)-β-caryophyllene (p < 0.05), 100 µL myrcene (p < 0.01), or 800 µL borneol (p < 0.05) significantly reduced the number of A. bungii females choosing host plant P. persica leaves (Figure 6).
Figure 6.
Behavioural responses of A. bungii females to P. persica leaves in the presence of synthetic chemicals. Note: N = number of responsive A. bungii; n = number of A. bungii females choosing the treatment; m = number of A. bungii that made no choice. * = significant difference at the 0.05 level. ** = significant difference at the 0.01 level (the binomial test).
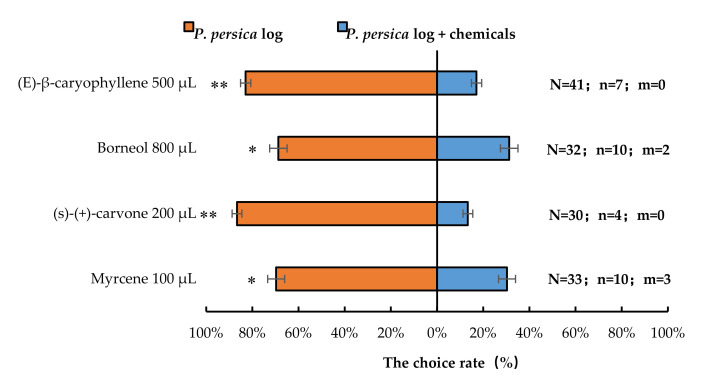
Similarly, the presence of 100 µL myrcene (p < 0.05), 800 µL borneol (p < 0.05), 200 µL (S)-(+)-carvone (p < 0.01), or 500 µL (E)-β-caryophyllene (p < 0.01) significantly reduced the number of A. bungii females choosing host P. persica logs. Therefore, compared with P. persica leaves or logs alone, P. persica leaves or logs in presence with synthetic chemicals significantly repelled A. bungii females, respectively (Figure 7).
Figure 7.
Behavioural responses of A. bungii females to P. persica logs in the presence of synthetic chemicals. Note: N = number of responsive A. bungii; n = number of A. bungii females choosing the treatment; m = number of A. bungii that made no choice. * = significant difference at the 0.05 level. ** = significant difference at the 0.01 level (the binomial test).
4. Discussion
Some compounds derived from M. spicata essential oils and plant extracts have exhibited insecticidal and insect-repellent activity for mosquitoes and stored products pests [33]. Therefore, we extracted the volatiles from M. spicata in order to identify the active components with respect to A. bungii adults, and four EAD-active components were obtained.
Some studies have shown that myrcene is an aggregation synergist [34] for Monochamus alternatus (Coleoptera: Cerambycidae) [35] and Megalurothrips sjostedti (Thysanoptera: Thripidae) [36,37]. However, A. bungii females showed significant repulsion to 100 µL, 200 µL, and 500 µL myrcene in the semi-field cage bioassays, and 100 µL myrcene significantly reduced the number of A. bungii females choosing the host plant. The olfactory organ of A. bungii might have become desensitized in the high quantity of myrcene (800 µL) treatment in cages. Whether myrcene has a synergistic effect on other repellent chemicals will be tested in a future study.
The aroma and flavour of M. spicata is mainly due to the presence of carvone [38]. Carvone is a major contributor to the fumigant activity, contact toxicity and antifeedant activity of M. spicata against Blattella germanica (Blattodea: Blmtdlidae) [39], Deroceras reticulatum (Gastropoda: Pulmonata: Stylommatophora) [40], Meloidogyne incognita (Tylenchida: Meloidogynidae), and Hylobius abieties (Coleoptera: Curculionidae) [41,42,43,44]. As we expected, 200 µL, 500 µL, and 800 µL (S)-(+)-carvone all showed a clear repellent effect on A. bungii females in our study.
(E)-β-caryophyllene is a chemical component of many plants, and has been reported as an insect repellant [45]. For instance, it was repellent to Reticulitermes flaviceps (Isoptera: Rhinotermitidae) [46], Aphis gossypii Glover (Homoptera: Aphididae) [47], Aedes aegypti (Diptera: Culicidae) [48], and Agrilus zanthoxylumi (Coleoptera: Buprestidae) [49]. In our study, it was shown to have a repellent effect on A. bungii females (500 µL and 800 µL). (E)-β-caryophyllene is also attractive to Coccinella septempunctata (Coleoptera: Coccinellidae) at a quantity of 20 µg [50], and lower quantities of (E)-β-caryophyllene (0.01 µg to 10 µg) are attractive to both virgin and mated Drosophila suzukii (Diptera: Drosophilidae) females (0.01 µg), though higher quantities repelled them [51]. Therefore, the influence of (E)-β-caryophyllene on insect behaviour might be related to the quantities present.
Borneol is known to attract Cyzenis albicans (Diptera: Tachinidae), a tachinid parasitoid of the winter moth, Operophtera brumata (Lepidoptera: Geometridae) [52]. However, borneol was significantly repellent to A. bungii females at the 800 µL quantity in our study. If commercial costs are considered, then we would not recommend borneol as an A. bungii repellent for use in the field.
Deterrence of A. bungii oviposition could reduce populations and be useful in management strategies. Myrcene, borneol, (S)-(+)-carvone, and (E)-β-caryophyllene were all repellent to A. bungii females in this study. However, several issues such as customer approval, formulation, and non-target toxicity must be addressed before they can be used in practice [53,54]. They also have potential to be used together with aggregation sex pheromones in the ‘push and pull strategy’ for control of A. bungii.
5. Conclusions
Our results show that myrcene, borneol, (S)-(+)-carvone, and (E)-β-caryophyllene from M. spicata were all repellent to A. bungii females, and the effective quantities were 100 µL, 800 µL, 200 µL, and 500 µL, respectively. Taking commercial costs and quantity into consideration, myrcene, (S)-(+)-carvone, (E)-β-caryophyllene, or a three-component blend have greatest potential as repellents for management of A. bungii females.
Acknowledgments
We thank Jiancheng Li (Plant Protection Institute, Hebei Academy of Agriculture and Forest Sciences, Baoding, China) and Lei Wang (Hebei Research Center for Geoanalysis, Baoding, China) for their contributions to this research project.
Author Contributions
D.C.: writing–original draft, funding acquisition, and project administration; J.L.: writing–editing, formal analysis, and data curation; Z.Z.: investigation, resources, and validation; X.Y.: investigation, resources, and methodology; W.W.: methodology and formal analysis; J.W.: writing–review, methodology, and validation. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the Youth Fund of Hebei Education Department (QN2017016); the Natural Science Foundation Youth project of Hebei Province (C2020201021); and the Key Research and Development Program of Hebei Province (20326511D).
Institutional Review Board Statement
Not applicable.
Data Availability Statement
Data can be provided on request from the lead author.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.European Union Commission Implementing Decision (EU) 2018/1503 of 8 October 2018 as regards measures to prevent the introduction into and the spread within the Union of Aromia bungii (Faldermann) Off. J. EU. 2018;L254:9–18. [Google Scholar]
- 2.Wang Q. Cerambycid pests in agricultural and horticultural crops. In: Wang Q., editor. Cerambycidae of the World: Biology and Pest Management. CRC Press; Boca Raton, FL, USA: 2017. pp. 409–562. [Google Scholar]
- 3.Germinara G.S., Pistillo M., Griffo R., Garonna A.P., Palma A.D. Electroantennographic responses of Aromia bungii (Faldermann 1835) (Coleoptera, Cerambycidae) to a range of volatile compounds. Insects. 2019;10:274. doi: 10.3390/insects10090274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cocquempot C. Aromia bungii. EPPO datasheet on pests recommended for regulation. EPPO Bull. 2015;45:4–8. [Google Scholar]
- 5.EPPO . Pest Risk Analysis for Aromia bungii. EPPO; Paris, France: 2014. [Google Scholar]
- 6.CABI . Invasive Species Compendium. CAB International; Wallingford, UK: 2019. Aromia bungii. [Google Scholar]
- 7.Peña E., Schrader G., Vos S. Pest Survey Card on Aromia bungii. European Food Safety Authority (EFSA); Parma, Italy: 2019. pp. 1–25. [Google Scholar]
- 8.Ma W.H., Sun L.Y., Yu L.G., Wang J.T., Chen J.Y. Study on the occurrence and life history in Aromia bungii (Faldermann) Acta Agric. Boreali Sin. 2007;22:247–249. (In Chinese with English Abstract) [Google Scholar]
- 9.Bai R.X., Wang Y.H., Ma Z.S., Jia Y.Y., Lv D.Z. Advance in research of Aromia bungii Faldermann. For. Pest Dis. 2017;36:5–9. (In Chinese with English Abstract) [Google Scholar]
- 10.Men J., Cao D.D., Zhao B., Wang W.C., Liu P.C., Wei J.R. Behavioral responses of adults of Dastarcus helophoroides (Coleoptera: Bothrideridae) populations originated from different hosts to larval frass of Aromia bungii (Coleoptera: Cerambycidae) and their control effect on A. bungii population. Acta Entomol. Sin. 2017;60:229–236. (In Chinese with English Abstract) [Google Scholar]
- 11.Zou Y., Hansen L., Xu T., Teale S.A., Hao D., Millar J.G. Optimizing pheromone-based lures for the invasive red-necked longhorn beetle, Aromia bungii. J. Pest Sci. 2019;92:1217–1225. doi: 10.1007/s10340-019-01108-6. [DOI] [Google Scholar]
- 12.Men J., Zhao B., Cao D.D., Wang W.C., Wei J.R. Evaluating host location in three native Sclerodermus species and their ability to cause mortality in the wood borer Aromia bungii (Coleoptera: Cerambycidae) in laboratory. Biol. Control. 2019;134:95–102. doi: 10.1016/j.biocontrol.2019.04.007. [DOI] [Google Scholar]
- 13.Wang J.X., Yan X.W., Cao D.D., Yang B.J., Zhao Z.P., Wei J.R. Study on biological control of Aromia bungii in the peach orchard by Dastarcus helophoroides. For. Pest Dis. 2021;40:16–21. (In Chinese with English Abstract) [Google Scholar]
- 14.Wei J.R., Liu X.B., Niu Y.L., Wang J.J. Identification of volatiles released from the living adult Aromia bungii Faldermann. For. Pest Dis. 2013;32:8–10. (In Chinese with English Abstract) [Google Scholar]
- 15.Xu T., Yasui H., Teale S.A., Fujiwara-Tsujii N., Wickham J.D., Fukaya M., Hansen L., Kiriyama S., Hao D., Nakano A., et al. Identification of a male-produced sex-aggregation pheromone for a highly invasive cerambycid beetle, Aromia bungii. Sci. Rep. 2017;7:7330. doi: 10.1038/s41598-017-07520-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fukaya M., Kiriyama S., Yasui H. Mate-location fight of the red-necked longicorn beetle, Aromia bungii (Coleoptera: Cerambycidae): An invasive pest lethal to Rosaceae trees. Appl. Entomol. Zool. 2017;52:559–565. doi: 10.1007/s13355-017-0509-9. [DOI] [Google Scholar]
- 17.Mori K. Pheromone synthesis. Part 263: Synthesis of the racemate and the enantiomers of (E)-cis-6,7-epoxy-2-nonenal, the male-produced pheromone of the red-necked longhorn beetle, Aromia bungii. Tetrahedron. 2018;74:1444–1448. doi: 10.1016/j.tet.2018.01.052. [DOI] [Google Scholar]
- 18.Yasui H., Fujiwara-Tsujii N., Yasuda T., Fukaya M., Kiriyama S., Nakano A., Watanabe T., Mori K. Electroantennographic responses and field attraction of an emerging invader, the red-necked longicorn beetle Aromia bungii (Coleoptera: Cerambycidae), to the chiral and racemic forms of its male-produced aggregation-sex pheromone. Appl. Entomol. Zool. 2018;54:109–114. doi: 10.1007/s13355-018-0600-x. [DOI] [Google Scholar]
- 19.Wang W.C., Cao D.D., Men J., Wei J.R. (R)-(+)-citronellal identified as a female-produced sex pheromone of Aromia bungii Faldermann (Coleoptera: Cerambycidae) Egypt. J. Biol. Pest Control. 2018;28:77. doi: 10.1186/s41938-018-0083-7. [DOI] [Google Scholar]
- 20.Dardouri T., Gomez L., Schoeny A., Costagliola G., Gautier H. Behavioural response of green peach aphid Myzus persicae (Sulzer) to volatiles from different rosemary (Rosmarinus officinalis L.) Agric. For. Entomol. 2019;21:336–345. doi: 10.1111/afe.12336. [DOI] [Google Scholar]
- 21.Li X.H., Garvey M., Kaplan I., Li B.P., Carrillo J. Domestication of tomato has reduced the attraction of herbivore natural enemies to pest-damaged plants. Agric. For. Entomol. 2018;20:390–401. doi: 10.1111/afe.12271. [DOI] [Google Scholar]
- 22.Thacker J.R.M. An Introduction to Arthropod Pest Control. Cambridge University Press; New York, NY, USA: 2002. pp. 1–360. [Google Scholar]
- 23.Ware G.W., Whitacre D.M. The Pesticide Book. 6th ed. Meister Media Worldwide; Willoughby, OH, USA: 2004. 496p [Google Scholar]
- 24.Li X.W., Zhang Z.J., Hafeez M., Huang J., Zhang J.M., Wang L.K., Lu Y.B. Rosmarinus officinialis L. (Lamiales: Lamiaceae), a promising repellent plant for thrips management. J. Econ. Entomol. 2021;114:131–141. doi: 10.1093/jee/toaa288. [DOI] [PubMed] [Google Scholar]
- 25.Smith W.E.C., Shivaji R., Williams W.P., Luthe D.S., Sandoya G.V., Smith C.L., Sparks D.L., Brown A.E. A maize line resistant to herbivory constitutively releases (E)-β-Caryophyllene. J. Econ. Entomol. 2012;105:120–128. doi: 10.1603/EC11107. [DOI] [PubMed] [Google Scholar]
- 26.Lawrence B.M. Mint: The Genus Mentha. Volume 44. CRC Press; Boca Raton, FL, USA: 2006. p. 547. [Google Scholar]
- 27.Wu L.J., Li S., Xie J.X., Sun X.J., Yan X.Z., Hao C. Effects of stem and leaf ethanol extracts from Mentha spicata L. on antifeeding and antioviposition of diamondback moth Plutella xylostella. J. Plant Prot. 2016;43:1007–1013. (In Chinese with English Summary) [Google Scholar]
- 28.Tripathi A.K., Prajapati V., Ahmad A., Aggarwal K.K., Khanuja S.P.S. Piperitenone oxide as toxic, repellent, and reproduction retardant toward malarial vector Anopheles stephensi (Diptera: Anophelinae) J. Med. Entomol. 2004;41:691–698. doi: 10.1603/0022-2585-41.4.691. [DOI] [PubMed] [Google Scholar]
- 29.Sertkaya E., Kaya K., Soylu S. Acaricidal activities of the essential oils from several medicinal plants against the carmine spider mite Tetranychus cinnabarinus Boisd (Acarina: Tetranychidae) Ind. Crops Prod. 2010;31:107–112. doi: 10.1016/j.indcrop.2009.09.009. [DOI] [Google Scholar]
- 30.Kumar P., Mishra S., Malik A., Satya S. Insecticidal properties of Mentha species: A review. Ind. Crops Prod. 2011;34:802–817. doi: 10.1016/j.indcrop.2011.02.019. [DOI] [Google Scholar]
- 31.Stepanycheva E.A., Chermenskaya T.D., Pavela R., Petrova M.O. Effects of volatiles of essential oils on behavior of the western flower thrips Frankliniella occidentalis Perg (Thysanoptera, Thripidae) Entomol. Rev. 2018;98:801–806. doi: 10.1134/S0013873818070011. [DOI] [Google Scholar]
- 32.Bright L.Z., Handley M., Chien I., Curi S., Brownworth L.A., D’hers S., Bernier U.R., Gurman P., Elman N.M. Analytical models integrated with satellite images for optimized pest management. Precis. Agric. 2016;17:628–636. doi: 10.1007/s11119-016-9434-0. [DOI] [Google Scholar]
- 33.Singh P., Pandey A.K. Prospective of essential oils of the genus Mentha as biopesticides: A review. Front. Plant. Sci. 2018;9:1295. doi: 10.3389/fpls.2018.01295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhou P., Yu Y.X., Zhang J.H., Dou L.D., Ye B.H., Chen N.Z. Research and application of semiochemicals of Platypus spp. Plant Quar. 2015;29:22–25. (In Chinese with English Abstract) [Google Scholar]
- 35.Hao D.J., Ma L.J. Analysis of volatile components of Pinus teada L. by gas chromatography-mass spectrometry with solid-phase microextraction. J. Anal. Sci. 2008;24:88–90. (In Chinese with English Abstract) [Google Scholar]
- 36.Schröder R., Hilker M. The relevance of background odor in resource location by insects: A behavioral approach. Bioscience. 2008;58:308–316. doi: 10.1641/B580406. [DOI] [Google Scholar]
- 37.Diabate S., Martin T., Murungi L.K., Fiaboe K.K.M., Subramanian S., Wesonga J., Deletre E. Repellent activity of Cymbopogon citratus and Tagetes minuta and their specific volatiles against Megalurothrips sjostedti. J. Appl. Entomol. 2019;143:855–866. doi: 10.1111/jen.12651. [DOI] [Google Scholar]
- 38.Buleandra M.A., Oprea E.B., Popa D.E.A., David I.G.A., Moldovan Z.A., Mihai I.A., Badea I.A.A. Comparative chemical analysis of Mentha piperita and M. spicata and a fast assessment of commercial peppermint teas. Nat. Prod. Commun. 2016;11:551–555. doi: 10.1177/1934578X1601100433. [DOI] [PubMed] [Google Scholar]
- 39.Yeom H.J., Kang J.S., Kim G.H., Park I.K. Insecticidal and acetylcholine esterase inhibition activity of Apiaceae plant essential oils and their constituents against adults of German cockroach (Blattella germanica) J. Agric. Food Chem. 2012;60:7194–7203. doi: 10.1021/jf302009w. [DOI] [PubMed] [Google Scholar]
- 40.Iglesias J., Ester A. Laboratory evaluation of potential molluscicides for the control of eggs of the pest slug Deroceras reticulatum (Pulmonata: Limacidae) Int. J. Pest Manag. 2002;48:19–23. doi: 10.1080/09670870110062553. [DOI] [Google Scholar]
- 41.Saxena D.B., Goswami B.K. Nematicidal activity of some essential oils against Meloidogyne incognita. India Perfum. 1987;31:150–154. [Google Scholar]
- 42.Bestmann C.B. Plant insecticidal VIII Synergistic activity of (−)-carvone and pyrethrine in the essential oil of Chrysanthemum balsamital. J. Appl. Entomol. 1998;106:144–149. doi: 10.1111/j.1439-0418.1988.tb00577.x. [DOI] [Google Scholar]
- 43.Watanabe F., Tadaki S., Takaoka M., Ishino M., Morimoto I. Killing activities of the volatiles emitted from essential oils for Dermatophagoides pteronyssinus, Dermatophagoides farine and Tyrophagus putrescentiae. Shoyakugaku Zasshi. 1989;43:163–168. [Google Scholar]
- 44.Frank T., Biert K., Speiser B. Feeding deterrent effect of carvone a compound from caraway seeds on the slug Arion lubitanicus. Ann. Appl. Biol. 2002;14:93–100. doi: 10.1111/j.1744-7348.2002.tb00200.x. [DOI] [Google Scholar]
- 45.Monzote L., Stamberg W., Staniek K., Gille L. Toxic effects of carvacrol, caryophyllene oxide, and ascaridole from essential oil of Chenopodium ambrosioides on mitochondria. Toxicol. Appl. Pharmacol. 2009;240:337–347. doi: 10.1016/j.taap.2009.08.001. [DOI] [PubMed] [Google Scholar]
- 46.Xu W.L., Luo L., Liu Z.L., Yuan Z.L. Biological activity of β-caryophyllene on Reticulitermes flaviceps (Isoptera: Rhinotermitidae) China Plant Prot. 2017;37:12–16. (In Chinese with English Abstract) [Google Scholar]
- 47.Liu Y.Q., Xue M., Zhang Q.C., Zhou F.Y., Wei J.Q. Toxicity of β-caryophyllene from Vitex negundo (Lamiales: Verbenaceae) to Aphis gossypii Glover (Homoptera: Aphididae) and its action mechanism. Acta Entomol. Sin. 2010;53:396–404. [Google Scholar]
- 48.Silva R.C.S., Milet-Pinheiro P., Silva P.C.B., Silva A.G., Silva M.V., Navarro D.M.A.F., Silva N.H. (E)-caryophyllene and α-humulene: Aedes aegypti oviposition deterrents elucidated by gas chromatography-electrophysiological assay of Commiphora leptophloeos leaf oil. PLoS ONE. 2015;10:e0144586. doi: 10.1371/journal.pone.0144586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yuan L.F. Master’s Thesis. Northwest Agriculture & Forest University; Xi’an, China: 2016. The preliminary study on mechanism of odor perception of Agrilus zanthoxylumi to volatiles from Zanthoxylum bungeanum. [Google Scholar]
- 50.Guo S.S., Yuan G.H., Chai X.L., Guo X.R., Teng X.H., Li W.Z. Interactive effect of (E)-β-farnesene and (−)-β-caryophyllene on chemical communication between Myzus persicae and Coccinella septempunctata. J. Henan Agric. Univ. 2017;51:42–47. (In Chinese with English Abstract) [Google Scholar]
- 51.Liu Y., Xie D.S., Hu C.H., Dong W.X., Zhang F., Zhang J.P., Xiao C. Influence of β-caryophyllene on the behaviors of female Drosophila suzukii. Environ. Entomol. 2018;40:684–689. [Google Scholar]
- 52.Roland J., Denford K.E., Jimenez L. Borneol as an attractant for Cyzenis albicans, a tachinid parasitoid of the winter moth, Operophtera brumata L. (Lepidoptera: Geometridae) Can. Entomol. 1995;127:413–421. doi: 10.4039/Ent127413-3. [DOI] [Google Scholar]
- 53.Katz T.M., Miller J.H., Hebert A.A. Insect repellents: Historical perspectives and new developments. J. Am. Acad. Dermatol. 2008;58:865–871. doi: 10.1016/j.jaad.2007.10.005. [DOI] [PubMed] [Google Scholar]
- 54.Nerio L.S., Olivero-Verbel J., Stashenko E. Repellent activity of essential oils: A review. Bioresour. Technol. 2010;101:372–378. doi: 10.1016/j.biortech.2009.07.048. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data can be provided on request from the lead author.