Abstract
Colletotrichum is regarded as one of the 10 most important genera of plant pathogens in the world. It causes diseases in a wide range of economically important plants, including peaches. China is the largest producer of peaches in the world but little is known about the Colletotrichum spp. affecting the crop. In 2017 and 2018, a total of 286 Colletotrichum isolates were isolated from symptomatic fruit and leaves in 11 peach production provinces of China. Based on multilocus phylogenetic analyses (ITS, ACT, CAL, CHS-1, GAPDH, TUB2, and HIS3) and morphological characterization, the isolates were identified to be C. nymphaeae, C. fioriniae, and C. godetiae of the C. acutatum species complex, C. fructicola and C. siamense of the C. gloeosporioides species complex, C. karsti of the C. boninense species complex, and one newly identified species, C. folicola sp. nov. This study is the first report of C. karsti and C. godetiae in peaches, and the first report of C. nymphaeae, C. fioriniae, C. fructicola, and C. siamense in peaches in China. C. nymphaeae is the most prevalent species of Colletotrichum in peaches in China, which may be the result of fungicide selection. Pathogenicity tests revealed that all species found in this study were pathogenic on both the leaves and fruit of peaches, except for C. folicola, which only infected the leaves. The present study substantially improves our understanding of the causal agents of anthracnose on peaches in China.
Keywords: Colletotrichum, peach anthracnose, multilocus phylogeny, pathogenicity, taxonomy
1. Introduction
The peach (Prunus persica (L.) Batsch) originated in China [1] and has been grown in many temperate climates around the world. China is the largest peach producer in the world, accounting for 55.28% of the total peach acreage in the world and 61.12% of global peach production [2]. The country produced 15,016,103 metric tons on 779,893 ha in 2020 [2].
When the temperature and humidity are favorable, Colletotrichum spp. can infect peaches and other fruits and cause massive economic losses [3]. Colletotrichum spp. pathogenic on peaches mainly infect the fruit but may also cause leaf or twig lesions. Fruit lesions appear as firm, brown, sunken (Figure 1a,c,d) areas often displaying concentric rings (Figure 1e) of small orange acervuli (Figure 1b,c,f). The acervuli produce conidia that are primarily spread by rainfall and splashing [4]. If a conidium lands on susceptible host plant tissue, it can cause secondary infection. Gumming can be observed when Colletotrichum spp. infect fruitlets (Figure 1a). Infected fruitlets do not reach maturity (Figure 1i), display atrophy, and eventually shrink from water loss (Figure 1i,j). Several lesions on green or mature fruit may coalesce (Figure 1a,f). Colletotrichum can also infect leaves with brown lesions (Figure 1g,h) and orange acervuli (Figure 1h). Severe twig infections can lead to twig dieback (Figure 1j). Colletotrichum species overwinter in fruit mummies and affected twigs, and form conidia in early spring [5]. In addition to asexual reproduction, they may also produce ascospores in perithecia, which were observed on apples in dead wood and on pears in leaves [6,7,8].
Figure 1.
Symptoms of peach anthracnose on fruit and leaves. (a–f) Various symptoms on fruit of Prunus persica (a–c,f) and P. persica var. nucipersica (d,e): (a,c–e) lesions on fruitlets and (b,f) lesions on mature peach fruit; (g,h) anthracnose symptoms on leaves; (i) mumified young fruit; (j) infected twig.
In the past, the taxonomy of the genus Colletotrichum mainly relied on host range and morphological characteristics [9]. However, these characteristics are not suitable for species-level identification since they are dependent on environmental conditions, many Colletotrichum species are polyphagous, and multiple species can infect the same host plant [10,11,12,13]. Molecular identification based on multilocus phylogenetic analyses or specific gene sequencing has been used for the classification and description of species concepts [3]. To date, 15 Colletotrichum species complexes and 22 individual species have been identified [14,15,16].
The causal agents of peach anthracnose were first reported as Colletotrichum acutatum and Colletotrichum gloeosporioides [17,18,19,20]. However, the use of molecular tools for the classification of anthracnose pathogens revealed that peach anthracnose in the USA was mostly caused by Colletotrichum nymphaeae and Colletotrichum fioriniae of the C. acutatum species complex [21], and Colletotrichum siamense and Colletotrichum fructicola of the C. gloeosporioides species complex [22]. C. nymphaeae was also reported in Brazil on peaches [23], and C. fioriniae, C. fructicola, and C. siamense were identified in South Korea on peaches [24]. Peach infections by Colletotrichum truncatum and Colletotrichum acutatum are rare [25,26].
The objective of this study was to systematically identify Colletotrichum spp. associated with peach fruit and leaf anthracnose in China using morphological characterization and multilocus phylogenetic analyses.
2. Materials and Methods
2.1. Isolation of Colletotrichum spp. from Peach Samples
During 2017 and 2018, the fruit and leaves of peaches with anthracnose symptoms were collected from 14 commercial peach orchards and two nurseries (Wuhan, Hubei and Fuzhou, Fujian) in 11 provinces of China, which were dry-farmed and sprayed with fungicides for anthracnose control. Conidia on diseased tissues were dipped in a cotton swab and spread on a potato dextrose agar (PDA, 20% potato infusion, 2% glucose, and 1.5% agar, and distilled water) medium and picked up with a glass needle under a professional single spore separation microscope (Wuhan Heipu Science and Technology Ltd., Wuhan, China). If no conidia were present, leaf and fruit pieces (5 × 5 mm) at the intersection of healthy and diseased tissues were surface sterilized with a sodium hypochlorite solution (1%) for 30 s and washed three times in sterilized water, followed by 75% ethanol for 30 s, then washed three times in sterilized water again. After the tissue pieces were dried, they were placed on PDA and incubated at 25 °C with a 12 h/12 h fluorescent light/dark cycle for about seven days to produce spores. Cultures were transferred to 15% diluted oatmeal agar (0.9% oatmeal, 1.5% agar, and distilled water) plates if there was no sporulation on PDA [27]. The ex-type living culture of novel species in this study was deposited in the China Center for Type Culture Collection (CCTCC), Wuhan, China.
2.2. Morphological Characterization
Mycelial plugs (5 mm) were transferred from the edge of actively growing cultures to fresh PDA plates and incubated at 25 °C in the dark. Colony diameters were measured after three days to calculate the mycelial growth rates (mm/d). The shape and color of colonies were investigated on the sixth day. Sexual morphs of some species were produced after four weeks. The characteristics of conidiomata were observed using fluorescence stereo microscope (Leica M205 FA, Leica Microsystem Ltd., Wetzlar, Germany). Moreover, the shape and color of conidia, conidiophores, appressoria, ascomata, asci, ascospores, and setae were recorded using a light microscope (Nikon Eclipse E400, Nikon Instruments Inc., San Francisco, CA, USA), and the length and width of 30 randomly selected conidia and 30 appressoria were measured for each representative isolate. Appressoria were induced by dropping 50 μL conidial suspension (105 conidia/mL) on a microscope slide, which was placed inside a plate containing moistened filter papers with distilled water, and incubated at 25 °C in the dark for 24 to 48 h [28].
2.3. DNA Extraction, PCR Amplification, and Sequencing
From the 286 obtained isolates, 51 were selected for further multilocus phylogenetic analyses. They represented each geographical population, colony type, conidia morphology, and host tissue.
Fungal DNA was extracted as described previously [29]. The 5.8S nuclear ribosomal gene with the two flanking internal transcribed spacers (ITS), partial sequences of the glyceraldehyde-3-phosphate dehydrogenase gene (GAPDH), chitin synthase 1 gene (CHS-1), actin gene (ACT), beta-tubulin gene (TUB2), histone3 gene (HIS3), and calmodulin gene (CAL) were amplified and sequenced using the primer pairs described in Table S1. The PCR conditions were 4 min at 95 °C, followed by 35 cycles of 95 °C for 30 s, annealing for 30 s at different temperatures for different genes/loci (Table S1), and 72 °C for 45 s, with a final extension at 72 °C for 7 min. DNA sequencing was performed at Tianyi Huiyuan Biotechnology Co., Ltd. (Wuhan, China) with an ABI 3730XL sequencer from Thermo Fisher Scientific (China) Co., Ltd. (Shanghai, China). The consensus sequences were assembled from forward and reverse sequences with MEGA v. 7.0 [30]. All sequences of 51 representative Colletotrichum isolates in this study were submitted to GenBank and the accession numbers are listed in Table S2.
2.4. Phylogenetic Analyses
Isolates were divided into four groups based on multilocus phylogenetic analyses, and type isolates of each species were selected and included in the analyses (Table 1). Multilocus phylogenetic analyses with concatenated ITS, GAPDH, CHS-1, HIS3, ACT, and TUB2 sequences were conducted for the C. acutatum species complex [31]; ACT, CAL, CHS-1, GAPDH, ITS, and TUB2 sequences were concatenated for the analysis of the C. gloeosporioides species complex [32]; the combined ITS, GAPDH, CHS-1, HIS3, ACT, TUB2, and CAL sequences were used to analyze the C. boninense species complex [33]; and the ITS, GAPDH, CHS-1, ACT, and TUB2 sequences were applied for remaining species [34]. Multiple sequences were aligned and combined using MAFFT v.7 [35] and MEGA v.7.0 [30].
Table 1.
Strains used for the phylogenetic analysis of Colletotrichum spp. and other species with details about host, location, and GenBank accession numbers.
| Species | Culture a | Host | Location | GenBank Accession Number | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| ITS | GAPDH | CHS-1 | ACT | HIS3 | TUB2 | CAL | ||||
| C. acerbum | CBS 128530 * | Malus domestica | New Zealand | JQ948459 | JQ948790 | JQ949120 | JQ949780 | JQ949450 | JQ950110 | - |
| C. acutatum | CBS 112996 * | Carica papaya | Australia | JQ005776 | JQ948677 | JQ005797 | JQ005839 | JQ005818 | JQ005860 | - |
| C-1 | Prunus persica | China | KX611163 | KY049983 | - | KY049982 | - | KY049984 | - | |
| C. aenigma | ICMP 18608 * | Persea americana | Israel | JX010244 | JX010044 | JX009774 | JX009443 | - | JX010389 | JX009683 |
| C. aeschynomenes | ICMP 17673 * | Aeschynomene virginica | USA | JX010176 | JX009930 | JX009799 | JX009483 | - | JX010392 | JX009721 |
| C. agaves | CBS 118190 | Agave striate | Mexico | DQ286221 | - | - | - | - | - | - |
| C. alatae | ICMP 17919 * | Dioscorea alata | India | JX010190 | JX009990 | JX009837 | JX009471 | - | JX010383 | JX009738 |
| C. alienum | ICMP 12071 * | Malus domestica | New Zealand | JX010251 | JX010028 | JX009882 | JX009572 | - | JX010411 | JX009654 |
| C. annellatum | CBS 129826 * | Hevea brasiliensis | Colombia | JQ005222 | JQ005309 | JQ005396 | JQ005570 | JQ005483 | JQ005656 | JQ005743 |
| C. aotearoa | ICMP 18537 * | Coprosma sp. | New Zealand | JX010205 | JX010005 | JX009853 | JX009564 | - | JX010420 | JX009611 |
| C. arecicola | CGMCC 3.19667 * | Areca catechu | China | MK914635 | MK935455 | MK935541 | MK935374 | - | MK935498 | - |
| C. artocarpicola | MFLUCC 18-1167 * | Artocarpus heterophyllus | Thailand | MN415991 | MN435568 | MN435569 | MN435570 | - | MN435567 | - |
| C. arxii | CBS 132511 * | Paphiopedilum sp. | Germany | KF687716 | KF687843 | KF687780 | KF687802 | - | KF687881 | - |
| C. asianum | ICMP 18580 * | Coffea arabica | Thailand | FJ972612 | JX010053 | JX009867 | JX009584 | - | JX010406 | FJ917506 |
| C. australe | CBS 116478 * | Trachycarpus fortunei | South Africa | JQ948455 | JQ948786 | JQ949116 | JQ949776 | JQ949446 | JQ950106 | - |
| C. bambusicola | CFCC 54250 * | Phyllostachys edulis | China | MT199632 | MT192844 | MT192871 | MT188638 | - | MT192817 | - |
| C. beeveri | CBS 128527 * | Brachyglottis repanda | New Zealand | JQ005171 | JQ005258 | JQ005345 | JQ005519 | JQ005432 | JQ005605 | JQ005692 |
| C. boninense | CBS 123755 * | Crinum asiaticum var. sinicum | Japan | JQ005153 | JQ005240 | JQ005327 | JQ005501 | JQ005414 | JQ005588 | JQ005674 |
| C. brasiliense | CBS 128501 * | Passiflora edulis | Brazil | JQ005235 | JQ005322 | JQ005409 | JQ005583 | JQ005496 | JQ005669 | JQ005756 |
| C. brassicicola | CBS 101059 * | Brassica oleracea var. gemmifera | New Zealand | JQ005172 | JQ005259 | JQ005346 | JQ005520 | JQ005433 | JQ005606 | JQ005693 |
| C. brisbanense | CBS 292.67 * | Capsicum annuum | Australia | JQ948291 | JQ948621 | JQ948952 | JQ949612 | JQ949282 | JQ949942 | - |
| C. cairnsense | CBS 140847 * | Capsicum annuum | Australia | KU923672 | KU923704 | KU923710 | KU923716 | KU923722 | KU923688 | - |
| C. camelliae-japonicae | CGMCC 3.18118 * | Camellia japonica | Japan | KX853165 | KX893584 | - | KX893576 | - | KX893580 | - |
| C. chlorophyti | IMI 103806 * | Chlorophytum sp. | India | GU227894 | GU228286 | GU228384 | GU227992 | - | GU228188 | - |
| C. chrysanthemi | IMI 364540 | Chrysanthemum coronarium | China | JQ948273 | JQ948603 | JQ948934 | JQ949594 | JQ949264 | JQ949924 | - |
| C. ciggaro | ICMP 18539 * | Olea europaea | Australia | JX010230 | JX009966 | JX009800 | JX009523 | - | JX010434 | JX009635 |
| CBS 237.49 * | Hypericum perforatum | Germany | JX010238 | JX010042 | JX009840 | JX009450 | - | JX010432 | JX009636 | |
| C. citricola | CBS 134228 * | Citrus unshiu | China | KC293576 | KC293736 | - | KC293616 | - | KC293656 | KC293696 |
| C. citrus-medicae | HGUP 1554 *, GUCC 1554 | Citrus medica | China | MN959910 | MT006331 | MT006328 | MT006325 | MT006334 | - | - |
| GUCC 1555 | Citrus medica | China | MN959911 | MT006332 | MT006329 | MT006326 | MT006335 | - | - | |
| GUCC 1556 | Citrus medica | China | MN959912 | MT006333 | MT006330 | MT006327 | MT006336 | - | - | |
| C. clidemiae | ICMP 18658 * | Clidemia hirta | USA | JX010265 | JX009989 | JX009877 | JX009537 | - | JX010438 | JX009645 |
| C. colombiense | CBS 129818 * | Passiflora edulis | Colombia | JQ005174 | JQ005261 | JQ005348 | JQ005522 | JQ005435 | JQ005608 | JQ005695 |
| C. constrictum | CBS 128504 * | Citrus limon | New Zealand | JQ005238 | JQ005325 | JQ005412 | JQ005586 | JQ005499 | JQ005672 | JQ005759 |
| C. cordylinicola | ICMP 18579 * | Cordyline fruticosa | Thailand | JX010226 | JX009975 | JX009864 | HM470235 | - | JX010440 | HM470238 |
| C. curcumae | IMI 288937 * | Curcuma longa | India | GU227893 | GU228285 | GU228383 | GU227991 | - | GU228187 | - |
| C. cuscutae | IMI 304802 * | Cuscuta sp. | Dominica | JQ948195 | JQ948525 | JQ948856 | JQ949516 | JQ949186 | JQ949846 | - |
| C. cymbidiicola | IMI 347923 * | Cymbidium sp. | Australia | JQ005166 | JQ005253 | JQ005340 | JQ005514 | JQ005427 | JQ005600 | JQ005687 |
| C. dacrycarpi | CBS 130241 * | Dacrycarpus dacrydioides | New Zealand | JQ005236 | JQ005323 | JQ005410 | JQ005584 | JQ005497 | JQ005670 | JQ005757 |
| C. dracaenophilum | CBS 118199 * | Dracaena sp. | China | JX519222 | JX546707 | JX519230 | JX519238 | - | JX519247 | - |
| C. eriobotryae | BCRC FU31138 * | Eriobotrya japonica | China | MF772487 | MF795423 | MN191653 | MN191648 | MN19168 | MF795428 | - |
| C. euphorbiae | CBS 134725 * | Euphorbia sp. | South Africa | KF777146 | KF777131 | KF777128 | KF777125 | KF777247 | - | |
| C. fioriniae | CBS 128517 * | Fiorinia externa | USA | JQ948292 | JQ948622 | JQ948953 | JQ949613 | JQ949283 | JQ949943 | - |
| IMI 324996 | Malus pumila | USA | JQ948301 | JQ948631 | JQ948962 | JQ949622 | JQ949292 | JQ949952 | - | |
| CBS 126526 | Primula sp. | Netherlands | JQ948323 | JQ948653 | JQ948984 | JQ949644 | JQ949314 | JQ949974 | - | |
| CBS 124958 | Pyrus sp. | USA | JQ948306 | JQ948636 | JQ948967 | JQ949627 | JQ949297 | JQ949957 | - | |
| CBS 119292 | Vaccinium sp. | New Zealand | JQ948313 | JQ948643 | JQ948974 | JQ949634 | JQ949304 | JQ949964 | - | |
| ICKb31 | Prunus persica | South Korea | LC516639 | LC516653 | LC516660 | - | - | LC516646 | - | |
| ICKb36 | Prunus persica | South Korea | LC516640 | LC516654 | LC516661 | - | - | LC516647 | - | |
| ICKb47 | Prunus persica | South Korea | LC516641 | LC516655 | LC516662 | - | - | LC516648 | - | |
| C.2.4.2 | Prunus persica | USA | KX066091 | KX066094 | - | - | - | KX066088 | - | |
| CaEY12_1 | Prunus persica | USA | KX066093 | KX066096 | - | - | - | KX066090 | - | |
| C. fructicola | ICMP 18581 * | Coffea arabica | Thailand | JX010165 | JX010033 | JX009866 | FJ907426 | - | JX010405 | - |
| ICMP 18613 | Limonium sinuatum | Israel | JX010167 | JX009998 | JX009772 | JX009491 | - | JX010388 | JX009675 | |
| ICMP 18581 * | Coffea arabica | Thailand | JX010165 | JX010033 | JX009866 | FJ907426 | - | JX010405 | FJ917508 | |
| ICMP 18727 | Fragaria × ananassa | USA | JX010179 | JX010035 | JX009812 | JX009565 | - | JX010394 | JX009682 | |
| CBS 125397 * | Tetragastris panamensis | Panama | JX010173 | JX010032 | JX009874 | JX009581 | - | JX010409 | JX009674 | |
| CBS 238.49 * | Ficus edulis | Germany | JX010181 | JX009923 | JX009839 | JX009495 | - | JX010400 | JX009671 | |
| ICKb18 | Prunus persica | South Korea | LC516635 | LC516649 | LC516656 | - | - | LC516642 | LC516663 | |
| ICKb132 | Prunus persica | South Korea | LC516636 | LC516650 | LC516657 | - | - | LC516643 | LC516664 | |
| RR12-3 | Prunus persica | USA | - | KJ769247 | - | - | - | KM245092 | KJ769239 | |
| SE12-1 | Prunus persica | USA | - | KJ769248 | - | - | - | - | KJ769237 | |
| C. fusiforme | MFLUCC 12– 0437 * | unknown | Thailand | KT290266 | KT290255 | KT290253 | KT290251 | - | KT290256 | - |
| C. gigasporum | CBS 133266 * | Centella asiatica | Madagascar | KF687715 | KF687822 | KF687761 | - | - | KF687866 | - |
| C. gloeosporioides | CBS 112999 * | Citrus sinensis | Italy | JQ005152 | JQ005239 | JQ005326 | JQ005500 | JQ005413 | JQ005587 | JQ005673 |
| ICMP 17821 * | Citrus sinensis | Italy | JX010152 | JX010056 | JX009818 | JX009531 | - | JX010445 | JX009731 | |
| C. godetiae | CBS 796.72 | Aeschynomene virginica | USA | JQ948407 | JQ948738 | JQ949068 | JQ949728 | JQ949398 | JQ950058 | - |
| CBS 133.44 * | Clarkia hybrida | Denmark | JQ948402 | JQ948733 | JQ949063 | JQ949723 | JQ949393 | JQ950053 | - | |
| IMI 351248 | Ceanothus sp. | UK | JQ948433 | JQ948764 | JQ949094 | JQ949754 | JQ949424 | JQ950084 | - | |
| C. guangxiense | CFCC 54251 * | Phyllostachys edulis | China | MT199633 | MT192834 | MT192861 | MT188628 | - | MT192805 | - |
| C. hippeastri | CBS 125376 * | Hippeastrum vittatum | China | JQ005231 | JQ005318 | JQ005405 | JQ005579 | JQ005492 | JQ005665 | JQ005752 |
| C. horii | ICMP 10492 * | Diospyros kaki | Japan | GQ329690 | GQ329681 | JX009752 | JX009438 | JX010450 | JX009604 | |
| C. indonesiense | CBS 127551 * | Eucalyptus sp. | Indonesia | JQ948288 | JQ948618 | JQ948949 | JQ949609 | JQ949279 | JQ949939 | - |
| C. javanense | CBS 144963 * | Capsicum annuum | Indonesia | MH846576 | MH846572 | MH846573 | MH846575 | - | MH846574 | - |
| C. jishouense | GZU_HJ2_G2 | Nothapodytes pittosporoides | China | MH482931 | MH681657 | - | MH708134 | - | MH727472 | - |
| C. johnstonii | CBS 128532 * | Solanum lycopersicum | New Zealand | JQ948444 | JQ948775 | JQ949105 | JQ949765 | JQ949435 | JQ950095 | - |
| C. kahawae | IMI 319418 * | Coffea arabica | Kenya | JX010231 | JX010012 | JX009813 | JX009452 | - | JX010444 | - |
| C. karsti | CBS 128524 | Citrullus lanatus | New Zealand | JQ005195 | JQ005282 | JQ005369 | JQ005543 | JQ005456 | JQ005629 | JQ005716 |
| CBS 129824 | Musa AAA | Colombia | JQ005215 | JQ005302 | JQ005389 | JQ005563 | JQ005476 | JQ005649 | JQ005736 | |
| CBS 128552 | Synsepalum dulcificum | Taiwan | JQ005188 | JQ005275 | JQ005362 | JQ005536 | JQ005449 | JQ005622 | JQ005709 | |
| C. laticiphilum | CBS 112989 * | Hevea brasiliensis | India | JQ948289 | JQ948619 | JQ948950 | JQ949610 | JQ949280 | JQ949940 | - |
| C. ledebouriae | CBS 141284 * | Ledebouria floridunda | South Africa | KX228254 | - | - | KX228357 | - | - | - |
| C. liaoningense | CGMCC 3.17616 * | Capsicum sp. | China | KP890104 | KP890135 | KP890127 | KP890097 | - | KP890111 | - |
| C. limetticola | CBS 114.14 * | Citrus aurantifolia | USA | JQ948193 | JQ948523 | JQ948854 | JQ949514 | JQ949184 | JQ949844 | - |
| C. lindemuthianum | CBS 144.31 * | Phaseolus vulgaris | Germany | JQ005779 | JX546712 | JQ005800 | JQ005842 | - | JQ005863 | - |
| C. magnisporum | CBS 398.84 * | unknown | unknown | KF687718 | KF687842 | KF687782 | KF687803 | - | KF687882 | - |
| C. magnum | CBS 519.97 * | Citrullus lanatus | USA | MG600769 | MG600829 | MG600875 | MG600973 | - | MG601036 | - |
| C. makassarense | CBS 143664 * | Capsicum annuum | Indonesia | MH728812 | MH728820 | MH805850 | MH781480 | - | MH846563 | - |
| C. musae | CBS 116870 * | Musa sp. | USA | JX010146 | JX010050 | JX009896 | JX009433 | - | HQ596280 | JX009742 |
| C. neosansevieriae | CBS 139918 * | Sansevieria trifasciata | South Africa | KR476747 | KR476791 | - | KR476790 | - | KR476797 | - |
| C. novae-zelandiae | CBS 128505 * | Capsicum annuum | New Zealand | JQ005228 | JQ005315 | JQ005402 | JQ005576 | JQ005489 | JQ005662 | JQ005749 |
| C. nupharicola | ICMP 18187 * | Nuphar lutea subsp.polysepala | USA | JX010187 | JX009972 | JX009835 | JX009437 | - | JX010398 | JX009663 |
| C. nymphaeae | CBS 515.78 * | Nymphaea alba | Netherlands | JQ948197 | JQ948527 | JQ948858 | JQ949518 | JQ949188 | JQ949848 | - |
| CBS 130.80 | Anemone sp. | Italy | JQ948226 | JQ948556 | JQ948887 | JQ949547 | JQ949217 | JQ949877 | - | |
| IMI 360386 | Pelargonium graveolens | India | JQ948206 | JQ948536 | JQ948867 | JQ949527 | JQ949197 | JQ949857 | - | |
| CBS 125973 | Fragaria × ananassa | UK | JQ948232 | JQ948562 | JQ948893 | JQ949553 | JQ949223 | JQ949883 | - | |
| CaC04_42 | Prunus persica | USA | KX066092 | KX066095 | - | - | - | KX066089 | - | |
| PrpCnSC13–01 | Prunus persica | Brazil | MK761066 | MK770424 | MK770421 | - | - | MK770427 | - | |
| PrpCnSC13–02 | Prunus persica | Brazil | MK765508 | MK770425 | MK770422 | - | - | MK770428 | - | |
| PrpCnSC13–10 | Prunus persica | Brazil | MK765507 | MK770426 | MK770423 | - | - | MK770429 | - | |
| C. oncidii | CBS 129828 * | Oncidium sp. | Germany | JQ005169 | JQ005256 | JQ005343 | JQ005517 | JQ005430 | JQ005603 | JQ005690 |
| C. orbiculare | CBS 570.97 * | Cucumis sativus | Europe | KF178466 | KF178490 | KF178515 | KF178563 | - | KF178587 | - |
| C. orchidearum | CBS 135131 * | Dendrobium nobile | Netherlands | MG600738 | MG600800 | MG600855 | MG600944 | - | MG601005 | - |
| C. orchidophilum | CBS 632.80 * | Dendrobium sp. | USA | JQ948151 | JQ948481 | JQ948812 | JQ949472 | JQ949142 | JQ949802 | - |
| C. parsonsiae | CBS 128525 * | Parsonsia capsularis | New Zealand | JQ005233 | JQ005320 | JQ005407 | JQ005581 | JQ005494 | JQ005667 | JQ005754 |
| C. paxtonii | IMI 165753 * | Musa sp. | Saint Lucia | JQ948285 | JQ948615 | JQ948946 | JQ949606 | JQ949276 | JQ949936 | - |
| C. petchii | CBS 378.94 * | Dracaena marginata | Italy | JQ005223 | JQ005310 | JQ005397 | JQ005571 | JQ005484 | JQ005657 | JQ005744 |
| C. phormii | CBS 118194 * | Phormium sp. | Germany | JQ948446 | JQ948777 | JQ949107 | JQ949767 | JQ949437 | JQ950097 | - |
| C. phyllanthi | CBS 175.67 * | Phyllanthus acidus | India | JQ005221 | JQ005308 | JQ005395 | JQ005569 | JQ005482 | JQ005655 | JQ005742 |
| C. piperis | IMI 71397 * | Piper nigrum | Malaysia | MG600760 | MG600820 | MG600867 | MG600964 | - | MG601027 | - |
| C. pseudomajus | CBS 571.88 * | Camellia sinensis | China | KF687722 | KF687826 | KF687779 | KF687801 | - | KF687883 | - |
| C. psidii | CBS 145.29 * | Psidium sp. | Italy | JX010219 | JX009967 | JX009901 | JX009515 | - | JX010443 | JX009743 |
| C. pyricola | CBS 128531 * | Pyrus communis | New Zealand | JQ948445 | JQ948776 | JQ949106 | JQ949766 | JQ949436 | JQ950096 | - |
| C. pyrifoliae | CGMCC 3.18902 * | Pyrus pyrifolia | China | MG748078 | MG747996 | MG747914 | MG747768 | - | MG748158 | - |
| C. queenslandicum | ICMP 1778 * | Carica papaya | Australia | JX010276 | JX009934 | JX009899 | JX009447 | - | JX010414 | JX009691 |
| C. radicis | CBS 529.93 * | unknown | Costa Rica | KF687719 | KF687825 | KF687762 | KF687785 | - | KF687869 | - |
| C. salicis | CBS 607.94 * | Salix sp. | Netherlands | JQ948460 | JQ948791 | JQ949121 | JQ949781 | JQ949451 | JQ950111 | - |
| C. salsolae | ICMP 19051 * | Salsola tragus | Hungary | JX010242 | JX009916 | JX009863 | JX009562 | - | JX010403 | JX009696 |
| C. sansevieriae | MAFF 239721 * | Sansevieria trifasciata | Japan | AB212991 | - | - | - | - | - | - |
| C. scovillei | CBS 1265299 * | Capsicum sp. | Indonesia | JQ948267 | JQ948597 | JQ948928 | JQ949588 | JQ949258 | JQ949918 | - |
| C. siamense | ICMP 18578 *, MFLU 090230 | Coffea arabica | Thailand | JX010171 | JX009924 | JX009865 | FJ907423 | - | JX010404 | FJ917505 |
| C. siamense (syn. C. hymenocallidis) | CBS 125378 * | Hymenocallis americana | China | JX010278 | JX010019 | GQ856730 | GQ856775 | - | JX010410 | JX009709 |
| C. siamense (syn. C. jasmini-sambac) | CBS 130420 * | Jasminum sambac | Vietnam | HM131511 | HM131497 | JX009895 | HM131507 | - | JX010415 | JX009713 |
| ICKb21 | Prunus persica | South Korea | LC516637 | LC516651 | LC516658 | - | - | LC516644 | LC516665 | |
| ICKb23 | Prunus persica | South Korea | LC516638 | LC516652 | LC516659 | - | - | LC516645 | LC516666 | |
| OD12-1 | Prunus persica | USA | - | KJ769240 | - | - | - | KM245089 | KJ769234 | |
| EY12-1 | Prunus persica | USA | - | KJ769246 | - | - | - | KM245086 | KJ769236 | |
| C. simmondsii | CBS 122122 * | Carica papaya | Australia | JQ948276 | JQ948606 | JQ948937 | JQ949597 | JQ949267 | JQ949927 | - |
| C. sloanei | IMI 364297 * | Theobroma cacao | Malaysia | JQ948287 | JQ948617 | JQ948948 | JQ949608 | JQ949278 | JQ949938 | - |
| C. sojae | ATCC 62257 * | Glycine max | USA | MG600749 | MG600810 | MG600860 | MG600954 | - | MG601016 | - |
| C. sydowii | CBS 135819 | Sambucus sp. | China | KY263783 | KY263785 | KY263787 | KY263791 | - | KY263793 | - |
| C. tainanense | CBS 143666 * | Capsicum annuum | Taiwan | MH728818 | MH728823 | MH805845 | MH781475 | - | MH846558 | - |
| C. theobromicola | CBS 124945 * | Theobroma cacao | Panama | JX010294 | JX010006 | JX009869 | JX009444 | - | JX010447 | JX009591 |
| C. ti | ICMP 4832 * | Cordyline sp. | New Zealand | JX010269 | JX009952 | JX009898 | JX009520 | - | JX010442 | JX009649 |
| C. tongrenense | GZU_TRJ1-37 | Nothapodytes pittosporoides | China | MH482933 | MH705332 | - | MH717074 | - | MH729805 | - |
| C. torulosum | CBS 128544 * | Solanum melongena | New Zealand | JQ005164 | JQ005251 | JQ005338 | JQ005512 | JQ005425 | JQ005598 | JQ005685 |
| C. trichellum | CBS 217.64 * | Hedera helix | UK | GU227812 | GU228204 | GU228302 | GU227910 | - | GU228106 | - |
| C. tropicale | CBS 124949 * | Theobroma cacao | Panama | JX010264 | JX010007 | JX009870 | JX009489 | - | JX010407 | JX009719 |
| C. truncatum | CBS 151.35 * | Phaseolus lunatus | USA | GU227862 | GU228254 | GU228352 | GU227960 | - | GU228156 | - |
| C. vietnamense | CBS 125478 * | Coffea sp. | Vietnam | KF687721 | KF687832 | KF687769 | KF687792 | - | KF687877 | - |
| C. walleri | CBS 125472 * | Coffea sp. | Vietnam | JQ948275 | JQ948605 | JQ948936 | JQ949596 | JQ949266 | JQ949926 | - |
| C. wanningense | CGMCC 3.18936 * | Hevea brasiliensis | China | MG830462 | MG830318 | MG830302 | MG830270 | - | MG830286 | - |
| C. wuxiense | CGMCC 3.17894 * | Camellia sinensis | China | KU251591 | KU252045 | KU251939 | KU251672 | - | KU252200 | KU251833 |
| C. xanthorrhoeae | ICMP 17903 * | Xanthorrhoea preissii | Australia | JX010261 | JX009927 | JX009823 | JX009478 | - | JX010448 | JX009653 |
| C. yunnanense | CBS 132135 * | Buxus sp. | China | JX546804 | JX546706 | JX519231 | JX519239 | - | JX519248 | - |
| Monilochaetes infuscans | CBS 869.96 * | Ipomoea batatas | South Africa | JQ005780 | JX546612 | JQ005801 | JQ005843 | - | JQ005864 | - |
a CBS: Culture collection of the Centraalbureau voor Schimmelcultures; ICMP: International Collection of Microorganisms from Plants, Auckland, New Zealand; CGMCC: China General Microbiological Culture Collection; MFLUCC: Mae Fah Luang University Culture Collection, Chiang Rai, Thailand; IMI: Culture collection of CABI Europe UK Centre, Egham, UK; BCRC: Bioresource Collection and Research Center, Hsinchu, Taiwan; MFLU: Herbarium of Mae Fah Luang University, Chiang Rai, Thailand; MAFF: MAFF Genebank Project, Ministry of Agriculture, Forestry and Fisheries, Tsukuba, Japan; ATCC: American Type Culture Collection. * = Ex-holotype or ex-epitype cultures.
Bayesian inference (BI) was used to construct phylogenetic trees in MrBayes v.3.2.2 [36]. Best-fit models of nucleotide substitution were selected using MrModeltest v.2.3 [37] based on the corrected Akaike information criterion (AIC) (Table 2, Table 3, Table 4 and Table 5). BI analyses were launched with two MCMC chains that were run for 1 × 106 generations (C. acutatum species complex and C. boninense species complex) [31,33], and trees sampled every 100 generations; or run 1 × 107 generations (C. gloeosporioides species complex, and remaining species) [8,34], and trees sampled every 1000 generations. The calculation of BI analyses was stopped when the average standard deviation of split frequencies fell below 0.01. On this basis, the first 25% of generations were discarded as burn-in. Maximum parsimony (MP) analyses were implemented by using Phylogenetic Analysis Using Parsimony (PAUP*) v.4.0b10 [38]. Goodness of fit values including tree length (TL), consistency index (CI), retention index (RI), rescaled consistency index (RC), and homoplasy index (HI) were calculated for the bootstrap analyses (Table 2, Table 3, Table 4 and Table 5). Phylogenetic trees were generated using the heuristic search option with Tree Bisection Reconnection (TBR) branch swapping and 1000 random sequence additions, with all characters equally weighted and alignment gaps treated as missing data. Maximum likelihood (ML) analyses were carried out by using the CIPRES Science Gateway v.3.3 (www.phylo.org, accessed on 29 December 2021), while RAxML-HPC BlackBox was selected with default parameters. Phylogenetic trees were visualized in FigTree v.1.4.2 [39]. TreeBASE was used to store the concatenated multilocus alignments (submission number: 29227).
Table 2.
Comparison of alignment properties in parsimony analyses of gene/locus and nucleotide substitution models used in phylogenetic analyses of C. acutatum species complex.
| Gene/Locus | ITS | GAPDH | CHS-1 | HIS3 | ACT | TUB2 | Combined |
|---|---|---|---|---|---|---|---|
| No. of taxa | 72 | 72 | 68 | 60 | 63 | 72 | 72 |
| Aligned length (with gaps) | 546 | 265 | 282 | 387 | 248 | 492 | 2240 |
| Invariable characters | 501 | 152 | 244 | 289 | 170 | 374 | 1750 |
| Uninformative variable characters | 26 | 56 | 13 | 32 | 30 | 60 | 217 |
| Phylogenetically informative characters | 19 | 57 | 25 | 66 | 48 | 58 | 273 |
| Tree length (TL) | 59 | 176 | 64 | 190 | 117 | 165 | 827 |
| Consistency index (CI) | 0.85 | 0.80 | 0.73 | 0.66 | 0.75 | 0.79 | 0.71 |
| Retention index (RI) | 0.97 | 0.95 | 0.94 | 0.93 | 0.94 | 0.94 | 0.93 |
| Rescaled consistency index (RC) | 0.82 | 0.76 | 0.69 | 0.61 | 0.71 | 0.75 | 0.65 |
| Homoplasy index (HI) | 0.15 | 0.20 | 0.27 | 0.34 | 0.25 | 0.21 | 0.30 |
| Nucleotide substitution model | HKY + I | HKY + G | K80 + I | GTR + I + G | GTR + G | GTR + G | GTR + I + G |
Table 3.
Comparison of alignment properties in parsimony analyses of gene/locus and nucleotide substitution models used in phylogenetic analyses of C. gloeosporioides species complex.
| Gene/Locus | ACT | CAL | CHS-1 | GAPDH | ITS | TUB2 | Combined |
|---|---|---|---|---|---|---|---|
| No. of taxa | 54 | 58 | 58 | 62 | 58 | 61 | 62 |
| Aligned length (with gaps) | 314 | 744 | 300 | 307 | 614 | 735 | 3034 |
| Invariable characters | 232 | 520 | 239 | 154 | 555 | 489 | 2209 |
| Uninformative variable characters | 54 | 139 | 22 | 77 | 36 | 156 | 484 |
| Phylogenetically informative characters | 28 | 85 | 39 | 76 | 23 | 90 | 341 |
| Tree length (TL) | 115 | 324 | 102 | 264 | 78 | 349 | 1303 |
| Consistency index (CI) | 0.84 | 0.83 | 0.69 | 0.75 | 0.81 | 0.83 | 0.76 |
| Retention index (RI) | 0.85 | 0.92 | 0.84 | 0.84 | 0.87 | 0.87 | 0.84 |
| Rescaled consistency index (RC) | 0.71 | 0.76 | 0.58 | 0.63 | 0.70 | 0.72 | 0.63 |
| Homoplasy index (HI) | 0.17 | 0.17 | 0.31 | 0.25 | 0.19 | 0.17 | 0.24 |
| Nucleotide substitution model | HKY + G | GTR + G | K80 + G | HKY + I | SYM + I + G | HKY + I | GTR + I + G |
Table 4.
Comparison of alignment properties in parsimony analyses of gene/locus and nucleotide substitution models used in phylogenetic analyses of C. boninense species complex.
| Gene/Locus | ITS | GAPDH | CHS-1 | HIS3 | ACT | TUB2 | CAL | Combined |
|---|---|---|---|---|---|---|---|---|
| No. of taxa | 25 | 25 | 23 | 23 | 25 | 25 | 24 | 25 |
| Aligned length (with gaps) | 553 | 286 | 280 | 393 | 276 | 502 | 449 | 2763 |
| Invariable characters | 489 | 120 | 224 | 295 | 174 | 348 | 259 | 1932 |
| Uninformative variable characters | 40 | 82 | 25 | 28 | 53 | 75 | 103 | 408 |
| Phylogenetically informative characters | 24 | 84 | 31 | 70 | 49 | 79 | 87 | 423 |
| Tree length (TL) | 87 | 286 | 89 | 210 | 164 | 237 | 300 | 1404 |
| Consistency index (CI) | 0.86 | 0.80 | 0.76 | 0.66 | 0.82 | 0.75 | 0.80 | 0.76 |
| Retention index (RI) | 0.88 | 0.79 | 0.79 | 0.79 | 0.83 | 0.75 | 0.85 | 0.79 |
| Rescaled consistency index (RC) | 0.75 | 0.64 | 0.60 | 0.52 | 0.68 | 0.56 | 0.70 | 0.60 |
| Homoplasy index (HI) | 0.14 | 0.20 | 0.24 | 0.34 | 0.18 | 0.25 | 0.18 | 0.24 |
| Nucleotide substitution model | SYM + I + G | HKY + I | K80 + G | GTR + I + G | GTR + G | HKY + I | HKY + G | GTR + I + G |
Table 5.
Comparison of alignment properties in parsimony analyses of gene/locus and nucleotide substitution models used in phylogenetic analyses of C. folicola and other taxa.
| Gene/Locus | ITS | GAPDH | CHS-1 | ACT | TUB2 | combined |
|---|---|---|---|---|---|---|
| No. of taxa | 50 | 47 | 44 | 47 | 44 | 50 |
| Aligned length (with gaps) | 571 | 321 | 265 | 279 | 529 | 1981 |
| Invariable characters | 367 | 63 | 163 | 102 | 223 | 934 |
| Uninformative variable characters | 53 | 21 | 20 | 39 | 50 | 183 |
| Phylogenetically informative characters | 151 | 237 | 82 | 138 | 256 | 864 |
| Tree length (TL) | 630 | 1312 | 389 | 671 | 1300 | 4405 |
| Consistency index (CI) | 0.51 | 0.44 | 0.41 | 0.48 | 0.44 | 0.44 |
| Retention index (RI) | 0.76 | 0.68 | 0.66 | 0.71 | 0.67 | 0.68 |
| Rescaled consistency index (RC) | 0.39 | 0.30 | 0.27 | 0.34 | 0.30 | 0.30 |
| Homoplasy index (HI) | 0.49 | 0.56 | 0.59 | 0.53 | 0.56 | 0.56 |
| Nucleotide substitution model | GTR + I + G | HKY + I + G | GTR + I + G | HKY + I + G | HKY + I + G | GTR + I + G |
New species and their most closely related neighbors were analyzed using the Genealogical Concordance Phylogenetic Species Recognition (GCPSR) model by performing a pairwise homoplasy index (PHI) test [40]. The PHI test was carried out on SplitsTree v.4.14.6 [41,42] using concatenated sequences (ITS, GAPDH, CHS-1, ACT, and HIS3). The result of pairwise homoplasy index below a 0.05 threshold (Φw < 0.05) indicated the presence of significant recombination in the dataset. The relationship between closely related species was visualized by constructing a splits graph. In addition, the results of relationships between closely related species were visualized by constructing EqualAngle splits graphs, using both LogDet character transformation and split decomposition distances options.
2.5. Pathogenicity Test
Two to five isolates of each Colletotrichum sp. were used in pathogenicity tests on detached fruit and leaves. The experimental varieties for fruit and leaf inoculations were “Xiaohong” and “Xiahui No. 5”, respectively. Commercially mature fruit (still firm but with no green background color) and asymptomatic, fully developed leaves with short twigs (1–2 cm) were washed with soap and water, and surface sterilized in 1% sodium hypochlorite for 2 min and 30 s, respectively, then rinsed with sterile water and air-dried on sterile paper. Fruit was stabbed with sterilized toothpicks to produce wounds of about 5 mm deep, while leaves were punctured with sterile, medical needles. For inoculation, a 10-μL droplet of conidia suspension (1.0−2.0 × 105 conidia/mL) was dropped on each wounded site, and control fruit or leaves received sterile water without conidia. Each fruit and leaf had two inoculation sites. Three fruits and three leaves were used for each isolate. Inoculated fruit and leaves were placed in a plastic tray onto 30 mm diameter plastic rings for stability. The bottom of the tray (65 cm × 40 cm × 15 cm, 24 peaches or leaves per tray) contained wet paper towels and the top was sealed with plastic film to maintain humidity. Peaches and leaves were incubated at 25 °C for six days. Pathogenicity was evaluated by the infection rates and lesion diameters. The infection rates were calculated by the formula (%) = (infected inoculation sites/all inoculation sites) × 100%. The lesion size was determined as the mean of two perpendicular diameters. The experiment was performed twice.
The fungus was re-isolated from the resulting lesions and identified as described above, thus fulfilling Koch’s postulates.
3. Results
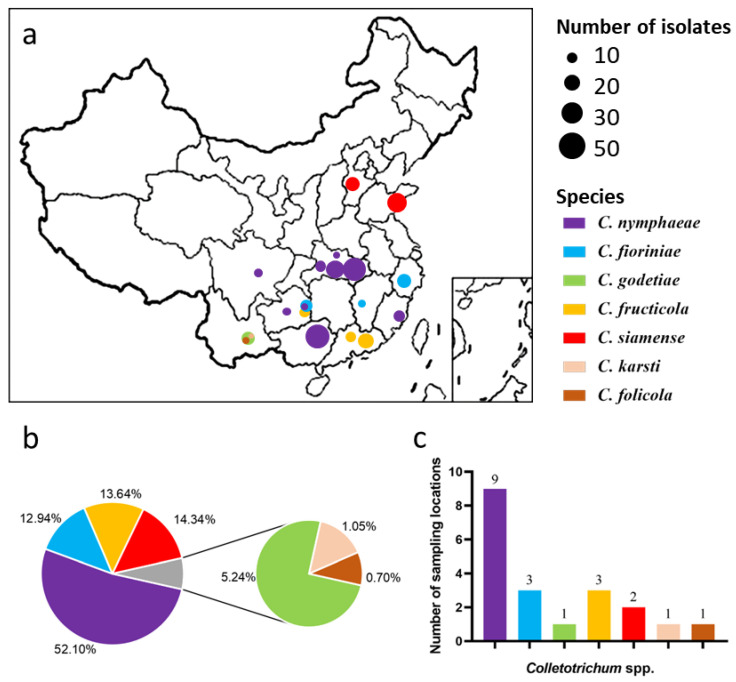
From 2017 to 2018, a total of 286 Colletotrichum isolates were obtained from 11 provinces in China (Table 6; Figure 2a); 33 isolates were from leaves and 253 isolates were from fruit (Table 6). Although we tried to collect samples in Gansu and Shanxi provinces in northern China, no symptomatic leaves or fruit were found. C. nymphaeae was the most widespread and most prevalent species (Figure 2b,c), with presence in Hubei, Guizhou, Guangxi, Fujian, and Sichuan provinces. C. fioriniae was found in three centrally located provinces (Zhejiang, Guizhou, and Jiangxi). C. siamense was only found in the northernmost orchards of the collection area in Shandong and Hebei provinces, while C. fructicola was only found in the southernmost provinces of the collection area of Guangdong and Guizhou provinces. C. folicola, C. godetiae, and C. karsti were only found in Yunnan province in the westernmost border of the collection area (Table 6; Figure 2a).
Table 6.
A list of all Colletotrichum isolates collected from peaches in China based on preliminary identification.
| Species | Location | Host | Number of Isolates |
Date | Daily Mean Temperature (°C) a |
|---|---|---|---|---|---|
| C. fioriniae | Lishui, Zhejiang | Juicy peach, Yanhong, fruit | 17 | 14 September 2017 | 29 |
| Tongren, Guizhou | Juicy peach, fruit | 14 | 8 August 2018 | 29 | |
| Jian, Jiangxi | Yellow peach, fruit | 6 | 21 August 2018 | 31 | |
| C. folicola | Honghe, Yunnan | Winter peach, Hongxue, leaf | 2 | 17 August 2017 | 26 |
| C. fructicola | Heyuan, Guangdong | Juicy peach, fruit | 19 | 28 June 2017 | 29 |
| Shaoguan, Guangdong | Juicy peach, Yingzui, fruit | 10 | 3 August 2018 | 30 | |
| Tongren, Guizhou | Juicy peach, fruit | 10 | 8 August 2018 | 29 | |
| C. godetiae | Honghe, Yunnan | Winter peach, Hongxue, leaf | 15 | 17 August 2017 | 26 |
| C. karstii | Honghe, Yunnan | Winter peach, Hongxue, leaf | 3 | 17 August 2017 | 26 |
| C. nymphaeae | Yichang, Hubei | Yellow peach, NJC83, fruit | 11 | 30 April 2017 | 19 |
| Jingmen, Hubei | Yellow peach, NJC83, fruit | 14 | 25 April 2017 | 18 | |
| Jingmen, Hubei | Juicy peach, Chunmi, fruit | 11 | 25 April 2017 | 18 | |
| Wuhan, Hubei | Juicy peach, Zaoxianhong, fruit | 17 | 18 April 2017 | 20 | |
| Wuhan, Hubei | Flat peach, Zaoyoupan, fruit | 12 | 18 April 2017 | 20 | |
| Wuhan, Hubei | Juicy peach, leaft | 9 | 14 June 2017 | 25 | |
| Xiaogan, Hubei | Juicy peach, Chunmei, fruit | 4 | 10 May 2017 | 20 | |
| Qingzhen, Guizhou | Juicy peach, Yingqing, fruit | 8 | 21 August 2017 | 24 | |
| Tongren, Guizhou | Juicy peach, fruit | 2 | 08 August 2018 | 29 | |
| Guilin, Guangxi | Juicy peach, Chunmi, fruit | 38 | 18 May 2018 | 25 | |
| Guilin, Guangxi | Juicy peach, Chunmi, leaf | 4 | 18 May 2018 | 25 | |
| Fuzhou, Fujian | Yellow peach, huangjinmi, fruit | 12 | 27 July 2018 | 31 | |
| Chengdu, Sichuan | Yellow peach, Zhongtaojinmi, fruit | 7 | 28 June 2018 | 26 | |
| C. siamense | Qingdao, Shandong | Juicy peach, Yangjiaomi, fruit | 27 | 22 August 2017 | 27 |
| Shijiazhuang, Hebei | Juicy peach, Dajiubao, fruit | 14 | 3 August 2018 | 30 | |
| Total | 286 |
a The average of the daily mean temperatures on the sampling day and the previous six days.
Figure 2.
Prevalence of Colletotrichum spp. associated with peaches in China. (a) Map of the distribution of Colletotrichum spp. on peaches in China. Each color represents one Colletotrichum species, and the size of the circle indicates the number of isolates collected from that location. (b) Overall isolation rate (%) of Colletotrichum species; (c) number of sampling locations for each Colletotrichum species.
3.1. Phylogenetic Analyses
Phylogenetic trees were constructed based on the concatenated gene/locus sequences. MP and ML trees are not shown because the topologies were similar to the displayed BI tree (Figure 3, Figure 4, Figure 5 and Figure 6). The number of taxa, aligned length (with gaps), invariable characters, uninformative variable characters, and phylogenetically informative characters of each gene/locus and combined sequences are listed in Table 2, Table 3, Table 4 and Table 5
Figure 3.
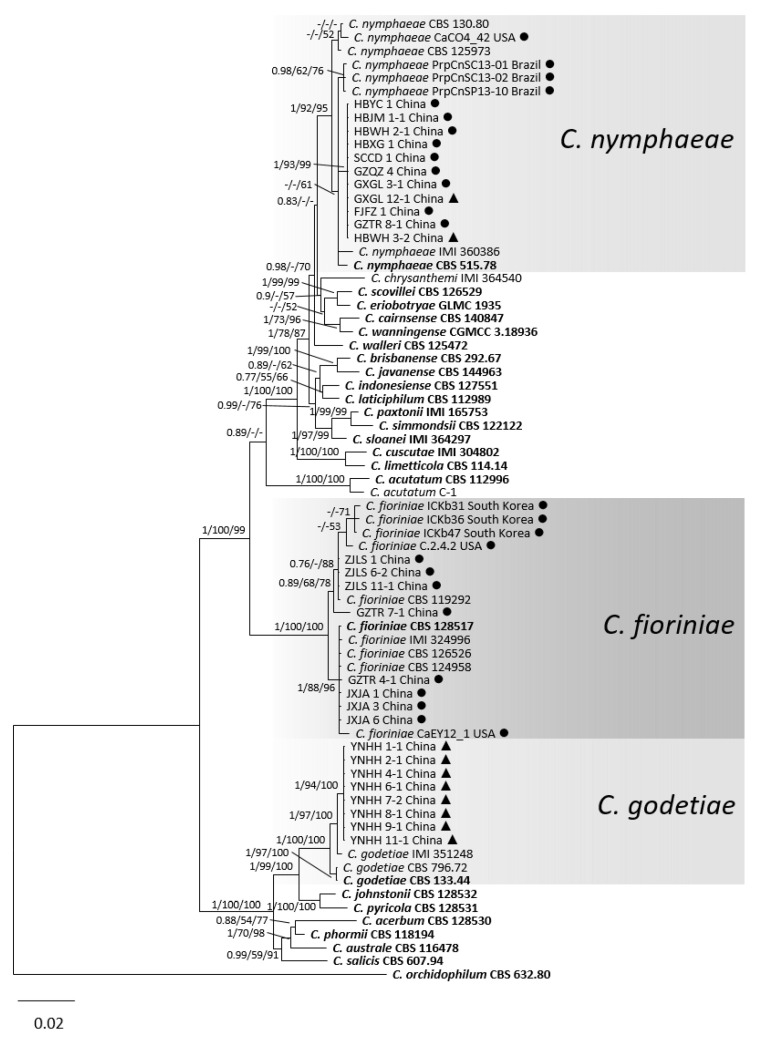
A Bayesian inference phylogenetic tree of 71 isolates in the C. acutatum species complex. C. orchidophilum (CBS 632.80) was used as the outgroup. The tree was built using combined sequences of the ITS, GAPDH, CHS-1, HIS3, ACT, and TUB2. BI posterior probability values (BI ≥ 0.70), MP bootstrap support values (MP ≥ 50%), and RAxML bootstrap support values (ML ≥ 50%) were shown at the nodes (BI/MP/ML). Tree length = 827, CI = 0.71, RI = 0.93, RC = 0.65, HI = 0.30. Ex-type isolates are in bold. Circles indicate isolates from fruits, and triangles indicate isolates from leaves.
Figure 4.
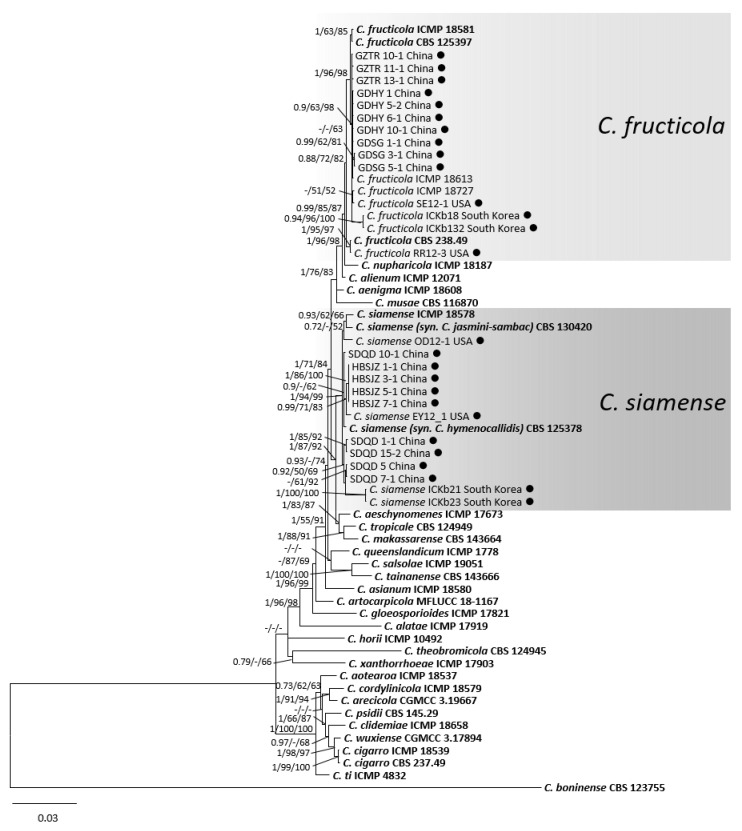
A Bayesian inference phylogenetic tree of 61 isolates in the C. gloeosporioides species complex. C. boninense (CBS 123755) was used as the outgroup. The tree was built using combined sequences of the ACT, CAL, CHS-1, GAPDH, ITS, and TUB2. BI posterior probability values (BI ≥ 0.70), MP bootstrap support values (MP ≥ 50%), and RAxML bootstrap support values (ML ≥ 50%) were shown at the nodes (BI/MP/ML). Tree length = 1303, CI = 0.76, RI = 0.84, RC = 0.63, HI = 0.24. Ex-type strains are in bold. Circles indicate isolates from fruits, and triangles indicate isolates from leaves.
Figure 5.
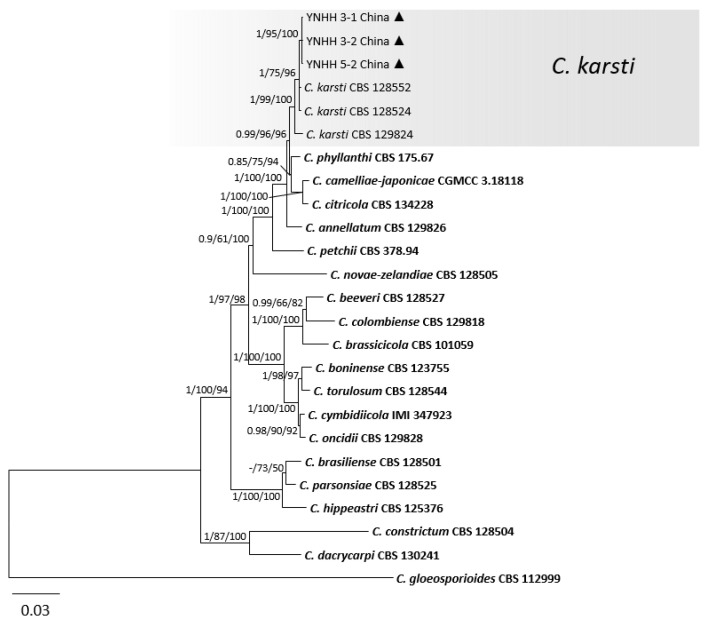
A Bayesian inference phylogenetic tree of 24 isolates in the C. boninense species complex. C. gloeosporioides (CBS 112999) was used as the outgroup. The tree was built using combined sequences of the ITS, GAPDH, CHS-1, HIS3, ACT, TUB2 and CAL. BI posterior probability values (BI ≥ 0.70), MP bootstrap support values (MP ≥ 50%), and RAxML bootstrap support values (ML ≥ 50%) were shown at the nodes (BI/MP/ML). Tree length = 1404, CI = 0.76, RI = 0.79, RC = 0.60, HI = 0.24. Ex-type strains are in bold. Circles indicate isolates from fruits, and triangles indicate isolates from leaves.
Figure 6.
A Bayesian inference phylogenetic tree of 49 isolates of Colletotrichum spp. and outgroup. Monilochaetes infuscans (CBS 869.96) was used as the outgroup. The tree was built using combined sequences of the ITS, GAPDH, CHS-1, ACT, and TUB2. BI posterior probability values (BI ≥ 0.70), MP bootstrap support values (MP ≥ 50%), and RAxML bootstrap support values (ML ≥ 50%) were shown at the nodes (BI/MP/ML). Tree length = 4405, CI = 0.44, RI = 0.68, RC = 0.30, HI = 0.56. Ex-type strains are in bold. Circles indicate isolates from fruits, and triangles indicate isolates from leaves.
For the C. acutatum species complex, in the multilocus sequence analyses (gene/locus boundaries in the alignment: ITS: 1–546, GAPDH: 551–815, CHS-1: 820–1101, HIS3: 1106–1492, ACT: 1497–1744, TUB2: 1749–2240) of 27 isolates from peaches in this study, 44 reference strains of C. acutatum species complex and one Colletotrichum species (C. orchidophilum strains CBS 632.80) as the outgroup, 2240 characters including the alignment gaps were processed. For the Bayesian analysis, a HKY + I model was selected for ITS, a HKY + G model for GAPDH, a K80 + I model for CHS-1, a GTR + I + G model for HIS3, and a GTR + G model for ACT and TUB2, and all were incorporated in the analysis (Table 2). As the phylogenetic tree shows in Figure 3, the 27 isolates of the C. acutatum species complex were clustered in three groups: 11 with C. nymphaeae, eight with C. fioriniae, and eight with C. godetiae. Although in the same general cluster, C. nymphaeae from China were genetically distinct from C. nymphaeae isolates from the USA and Brazil.
For the C. gloeosporioides species complex, DNA sequences of six genes/loci were obtained from 19 isolates from peaches in this study, with 42 reference isolates from the C. gloeosporioides species complex and the outgroup C. boninense CBS 123755. The gene/locus boundaries of the aligned 3034 characters (with gaps) were: ACT: 1–314, CAL: 319–1062, CHS-1: 1067–1366, GAPDH: 1371–1677, ITS: 1682–2295, TUB2: 2300–3034. For the Bayesian analysis, a HKY + G model was selected for ACT, a GTR + G model for CAL, a K80 + G model for CHS-1, a HKY + I model for GAPDH and TUB2, and a SYM + I + G model for ITS, and they were all incorporated in the analysis (Table 3). In the phylogenetic tree of the C. gloeosporioides species complex, 10 isolates clustered with C. fructicola and nine isolates clustered with C. siamense (Figure 4). They clustered together with isolates from South Korea and the USA.
Regarding the C. boninense species complex, in the multilocus analyses (gene/locus boundaries of ITS: 1–553, GAPDH: 558–843, CHS-1: 848–1127, HIS3: 1132–1524, ACT: 1529–1804, TUB2: 1809–2310, CAL: 2315–2763) of three isolates from peaches in this study, from 21 reference isolates of C. boninense species complex and one outgroup strain C. gloeosporioides CBS 112999, 2763 characters including the alignment gaps were processed. For the Bayesian analysis, a SYM + I + G model was selected for ITS, HKY + I for GAPDH and TUB2, K80 + G for CHS-1, GTR + I + G for HIS3, GTR + G for ACT, and HKY + G for CAL, and they were all incorporated in the analysis (Table 4). In Figure 5, three Chinese isolates clustered with C. karsti in the C. boninense species complex.
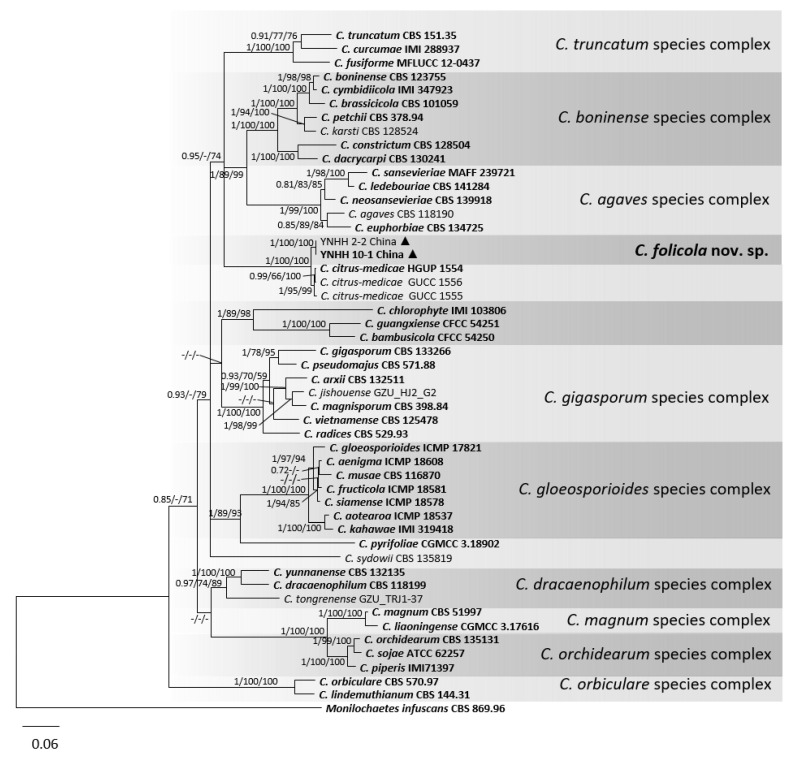
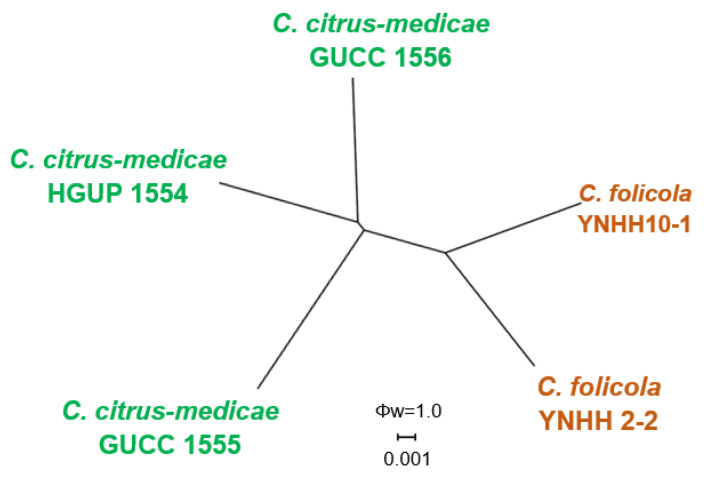
For the remaining phylogenetic analyses, the alignment of combined DNA sequences was obtained from 50 taxa, including two isolates from peaches in this study, 47 reference isolates of Colletotrichum species, and one outgroup strain Monilochaetes infuscans CBS 869.96. The gene/locus boundaries of the aligned 1981 characters (with gaps) were: ITS: 1–571, GAPDH: 576–896, CHS-1: 901–1165, ACT: 1170–1448, TUB2: 1453–1981. For the Bayesian analysis, a GTR + I + G model was selected for ITS and CHS-1, and HKY + I + G for GAPDH, ACT, and TUB2, and they were incorporated in the analysis (Table 5). In the phylogenetic tree, two isolates (YNHH2-2 and YNHH10-1 (CCTCC M 2020345)) clustered distantly from all known Colletotrichum species and are described herein as a new species, C. folicola (Figure 6). The PHI test result (Φw = 1) of C. folicola and its related species C. citrus-medicae ruled out the possibility of gene recombination interfering with the species delimitation (Figure 7). This is further evidence that C. folicola is a new species.
Figure 7.
PHI test of C. folicola and phylogenetically related species using both LogDet transformation and splits decomposition. PHI test value (Φw) < 0.05 indicate significant recombination within the datasets.
3.2. Taxonomy
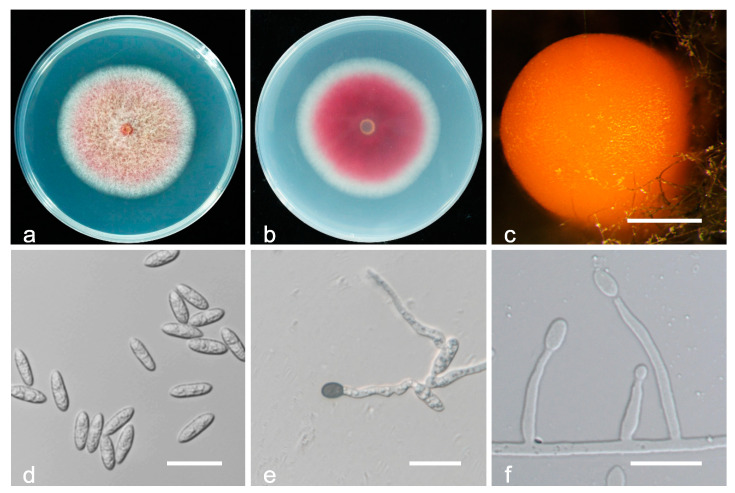
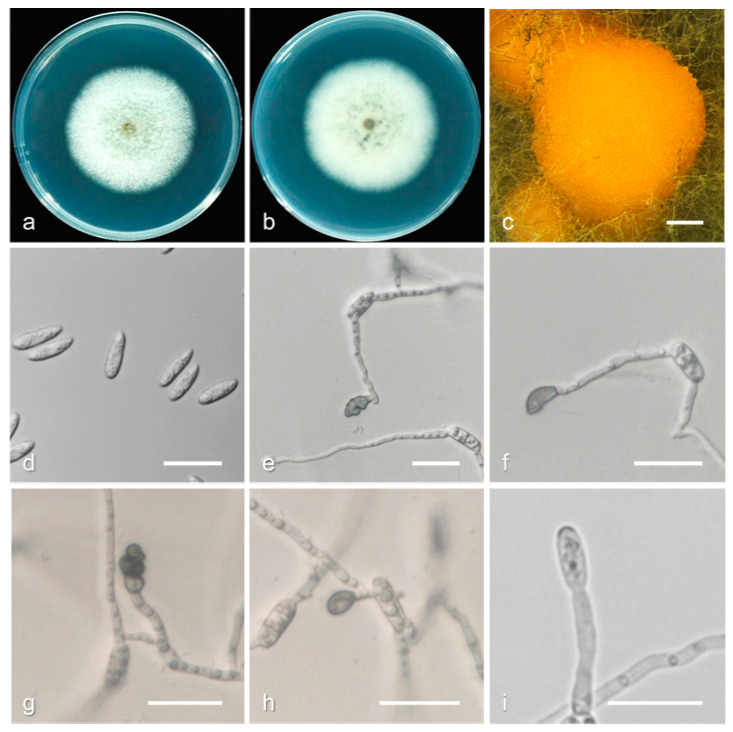
Colletotrichum nymphaeae H.A. van der Aa, Netherlands Journal of Plant Pathology. 84: 110. (1978) (Figure 8).
Figure 8.
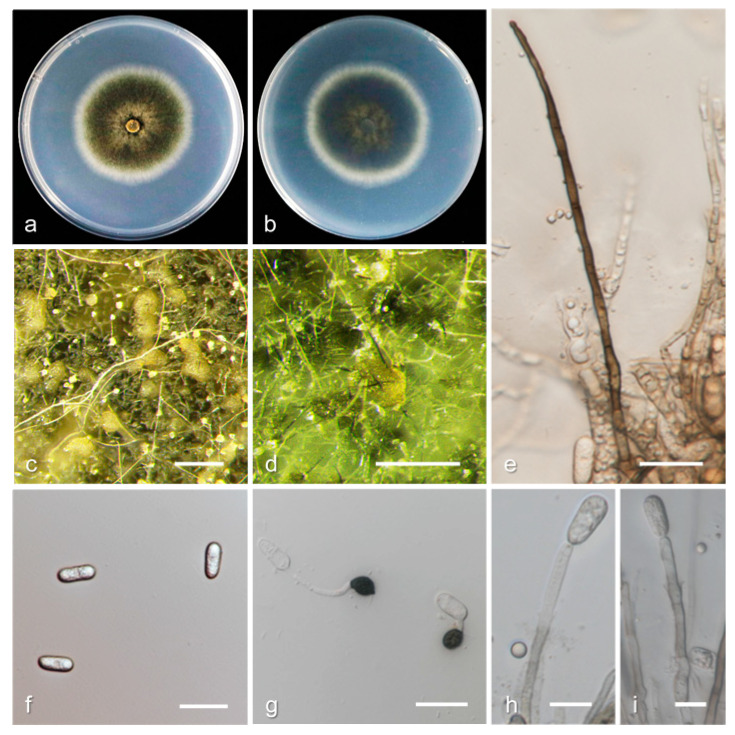
Biological characteristics of Colletotrichum nymphaeae. (a,b) Front and back view of six-day-old PDA culture; (c) conidiomata; (d) conidia; (e) appressoria; (f) conidiophores ((a–e) isolate HBYC 1; (f) isolate SCCD 1). Scale bars: (c) = 200 μm; (d–f) = 20 μm.
Description and illustration—Damm et al. [31].
Materials examined: China, Hubei province, Yichang city, on fruit of P. persica cv. NJC83, April 2017, Q. Tan, living culture HBYC1; Sichuan province, Chengdu city, on fruit of P. persica cv. Zhongtaojinmi, June 2018, Q. Tan, living culture SCCD 1; Fujian province, Fuzhou city, on fruit of P. persica cv. Huangjinmi, July 2018, Q. Tan, living culture FJFZ 1; Guangxi province, Guilin city, on leaves of P. persica cv. Chunmei, May 2018, Q. Tan, living culture GXGL 13-1; Guizhou province, Tongren city, on fruit of P. persica, June 2018, Q. Tan living culture GZTR 8-1; Hubei province, Jingmen city, on fruit of P. persica cv. NJC83, April 2018, Q. Tan, living culture HBJM 1-1; Hubei province, Wuhan city, on fruit of P. persica var. nucipersica cv. Zhongtaojinmi, April 2017, Q. Tan, living culture HBWH 2-1; ibid, on leaves of P. persica, June 2017, L.F. Yin, living culture HBWH 3-2; Hubei province, Xiaogan city, on fruit of P. persica cv. Chunmei, May 2017, Q. Tan, living culture HBXG 1.
Notes: Colletotrichum nymphaeae was first described on leaves of Nymphaea alba in Kortenhoef by Van der Aa [43]. C. nymphaeae is well separated from other species with TUB2, but all other genes have very high intraspecific variability [31]. Consistently, C. nymphaeae isolates collected in this study are different from ex-type strain CBS 515.78 in ITS (2 bp), GAPDH (1 bp), CHS-1 (3 bp), ACT (1 bp), HIS3 (3 bp), but with 100% identity in TUB2.
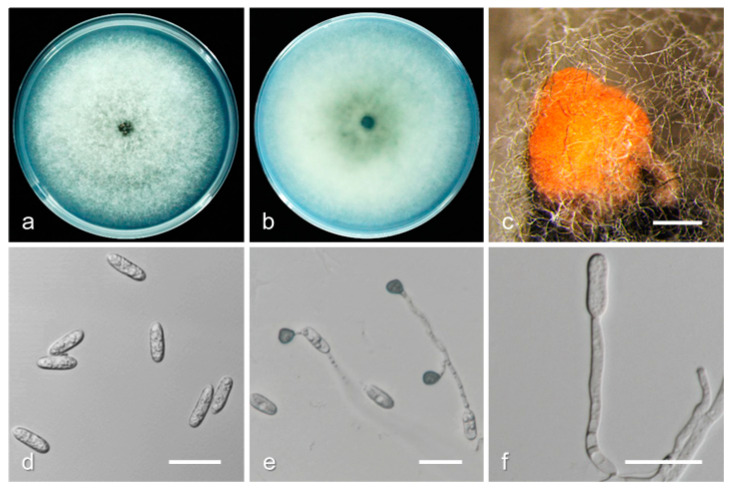
Colletotrichum fioriniae (Marcelino and Gouli) R.G. Shivas and Y.P. Tan, Fungal Diversity 39: 117. (2009) (Figure 9).
Figure 9.
Biological characteristics of Colletotrichum fioriniae. (a,b) Front and back view of six-day-old PDA culture; (c) conidiomata; (d) conidia; (e) appressoria; (f) conidiophores ((a–e) isolate JXJA 6; (f) isolate JXJA 1). Scale bars: (c) = 200 μm; (d–f) = 20 μm.
Description and illustration—Damm et al. [31].
Materials examined: China, Jiangxi province, Jian city, on fruit of P. persica, August 2018, Q. Tan, living cultures JXJA 1, JXJA 6; Zhejiang province, Lishui city, on fruit of P. persica, September 2017, Q. Tan, living cultures ZJLS 1, ZJLS 11-1; Guizhou province, Tongren city, on fruit of P. persica, August 2018, Q. Tan, living culture GZTR 7-1.
Notes: Colletotrichum acutatum var. fioriniae was first isolated from Fiorinia externa [44] and host plants of the scale insect as an endophyte [45] in New York, USA. In 2009, Shivas and Tan identified it from Acacia acuminate, Persea americana, and Mangifera indica in Australia as a separate species and named it Colletotrichum fioriniae [46]. C. fioriniae was mainly isolated from wide host plants and fruits in the temperate zones [3,31]. In this study, the C. fioriniae isolates clustered in two subclades, which is consistent with the results of Damm’s study [31].
Colletotrichum godetiae P. Neergaard, Friesia 4: 72. (1950) (Figure 10).
Figure 10.
Biological characteristics of Colletotrichum godetiae. (a,b) Front and back view of six-day-old PDA culture; (c) conidiomata; (d) conidia; (e–h) appressoria; (i) conidiophores ((a–f,i) isolate YNHH 1-1, (g,h) YNHH 9-1). Scale bars: (c) = 200 μm; (d–i) = 20 μm.
Description and illustration—Damm et al. [31].
Materials examined: China, Yunnan Province, Honghe City, on leaves of P. persica cv. Hongxue, August 2017, Q. Tan, living cultures YNHH 1-1, YNHH 4-1, YNHH 6-1, YNHH 8-2 and YNHH 9-1.
Notes: Colletotrichum godetiae was first reported on the seeds of Godetia hybrid in Denmark by Neergaard in 1943 [47], and given detailed identification seven years later [48]. C. godetiae was also recovered from fruits of Fragaria × ananassa, Prunus cerasus, Solanum betaceum, Citrus aurantium, and Olea europaea [49]; leaves of Laurus nobilis and Mahonia aquifolium; twigs of Ugni molinae; and canes of Rubus idaeus [31]. In this study, the isolates were obtained from peach leaves and could infect both the peach fruit and leaf.
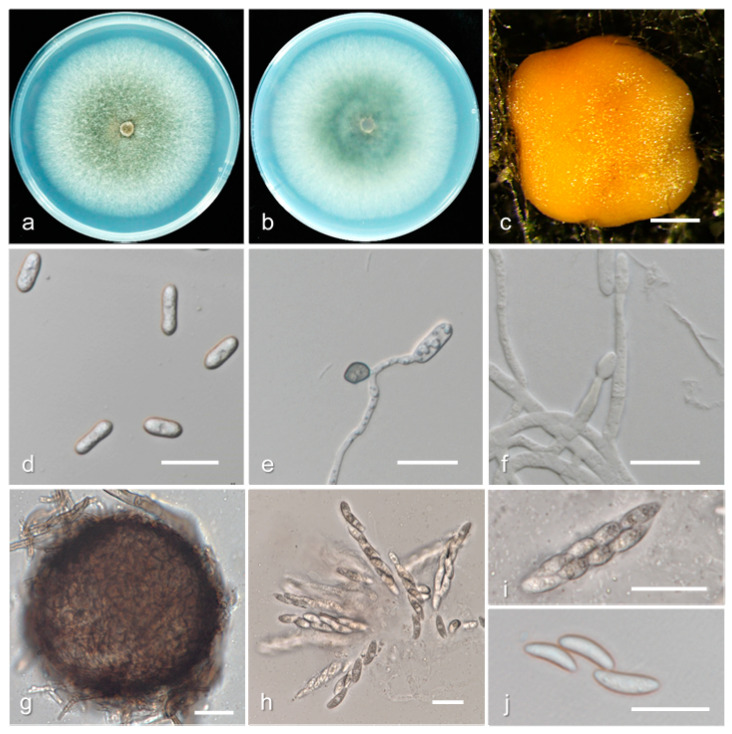
Colletotrichum fructicola H. Prihastuti et al., Fungal Diversity 39: 96. (2009) (Figure 11).
Figure 11.
Biological characteristics of Colletotrichum fructicola. (a,b) Front and back view of six-day-old PDA culture; (c) conidiomata; (d) conidia; (e) appressoria; (f) conidiophores; (g) ascomata; (h,i) asci; (j) ascospores ((a–e) isolate GDHY 10-1; (f–j) isolate GDSG 1-1). Scale bars: (c) = 200 μm; (d–j) = 20 μm.
Description and illustration—Prihastuti et al. [50].
Materials examined: China, Guangdong province, Heyuan city, on fruit of P. persica, June 2017, Q. Tan, living culture GDHY 10-1; Guangdong province, Shaoguan city, on fruit of P. persica cv. Yingzuitao, August 2018, Q. Tan, living cultures GDSG 1-1, GDSG 5-1; Guizhou province, Tongren city, on fruit of P. persica, August 2018, Q. Tan, living culture GZTR 10-1.
Notes: Colletotrichum fructicola was first described from the berries of Coffea arabica in Chiang Mai Province, Thailand [50]. Subsequently, C. fructicola was reported on a wide range of hosts including Malus domestica, Fragaria × ananassa, Limonium sinuatum, Pyrus pyrifolia, Dioscorea alata, Theobroma cacao Vaccinium spp., Vitis vinifera, and Prunus persica [3,51]. In this study, the conidia and ascospores of C. fructicola isolates (9.3−18.9 × 3.4−8.2 µm, mean ± SD = 14.3 ± 1.7 × 5.6 ± 0.5 µm; 12.6−22.0 × 3.1–7.6 µm, mean ± SD = 17.3 ± 0.5 × 5.0 ± 0.5 µm) (Table S3) were larger than that of ex-type (MFLU 090228, ICMP 185819: 9.7−14 × 3−4.3 µm, mean ± SD = 11.53 ± 1.03 × 3.55 ± 0.32 µm; 9−14 × 3–4 µm, mean ± SD = 11.91 ± 1.38 × 3.32 ± 0.35 µm).
Colletotrichum siamense H. Prihastuti et al., Fungal Diversity 39: 98. (2009) (Figure 12).
Figure 12.
Biological characteristics of Colletotrichum siamense. (a,b) Front and back view of six-day-old PDA culture; (c) conidiomata; (d) conidia; (e) appressoria; (f) conidiophores ((a–e) isolate SDQD10-1; (f) isolate HBSJZ 1-1). Scale bars: (c) = 200 μm; (d–f) = 20 μm.
Description and illustration—Prihastuti et al. [50].
Materials examined: China, Shandong province, Qingdao city, on fruit of P. persica cv. Yangjiaomi, August 2017, Q. Tan, living cultures SDQD 1-1, SDQD 10-1; Hebei province, Shijiazhuang city, on fruit of P. persica cv. Dajiubao, August 2018, Q. Tan, living cultures HBSJZ 1-1, HBSJZ 3-1.
Notes: Colletotrichum siamense was first identified on the berries of Coffea arabica in Chiang Mai Province, Thailand [50] and reported to have a wide range of hosts across several tropical, subtropical, and temperate regions, including Persea americana and Carica papaya in South Africa; Fragaria × ananassa, Vitis vinifera, and Malus domestica in the USA; Hymenocallis americana and Pyrus pyrifolia in China; etc. [3,8,51]. In this study, we collected C. siamense isolates from the temperate zone in China; the conidia (13.2−18.3 × 4.6–6.3 µm, mean ± SD = 15.3 ± 0.4 × 5.4 ± 0.3 µm) (Table S3) were larger than those of the ex-holotype (MFLU 090230, ICMP 18578: 7–18.3 × 3–4.3 µm, mean ± SD = 10.18 ± 1.74 × 3.46 ± 0.36 µm).
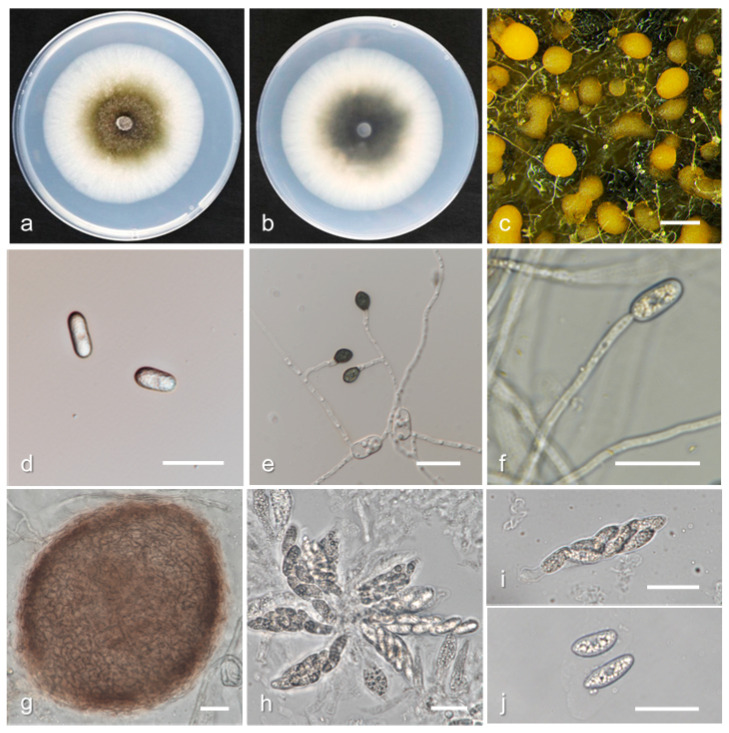
Colletotrichum karsti Y.L. Yang et al., Cryptogamie Mycologie. 32: 241. (2011) (Figure 13).
Figure 13.
Biological characteristics of Colletotrichum karsti. (a,b) Front and back view of six-day-old PDA culture; (c) conidiomata; (d) conidia; (e) appressoria; (f) conidiophores; (g) ascomata; (h,i) asci; (j) ascospores ((a–j) isolate YNHH 3-1). Scale bars: (c) = 200 μm; (d–j) = 20 μm.
Description and illustration—Yang et al. [52].
Materials examined: China, Yunnan province, Honghe city, on leaves of P. persica cv. Hongxue, August 2017, Q. Tan, living cultures YNHH 3-1, YNHH 3-2, and YNHH 5-2.
Notes: Colletotrichum karsti was first described from Vanda sp. (Orchidaceae) as a pathogen on diseased leaf and endophyte of roots in Guizhou province, China [52]. C. karsti is the most common and geographically diverse species in the C. boninense species complex, and occurs on wild hosts including Vitis vinifera, Capsicum spp., Lycopersicon esculentum, Coffea sp., Citrus spp., Musa banksia, Passiflora edulis, Solanum betaceum, Zamia obliqua, etc. [11,33,52,53]. In this study, the conidia of C. karsti isolates (10.6 − 14.9 × 5.8−7.4 µm, mean ± SD = 12.9 ± 0.3 × 6.7 ± 0.2 µm) (Table S3) were smaller than those of the ex-holotype (CGMCC3.14194: 12–19.5 × 5–7.5 µm, mean ± SD = 15.4 ± 1.3 × 6.5 ± 0.5 µm).
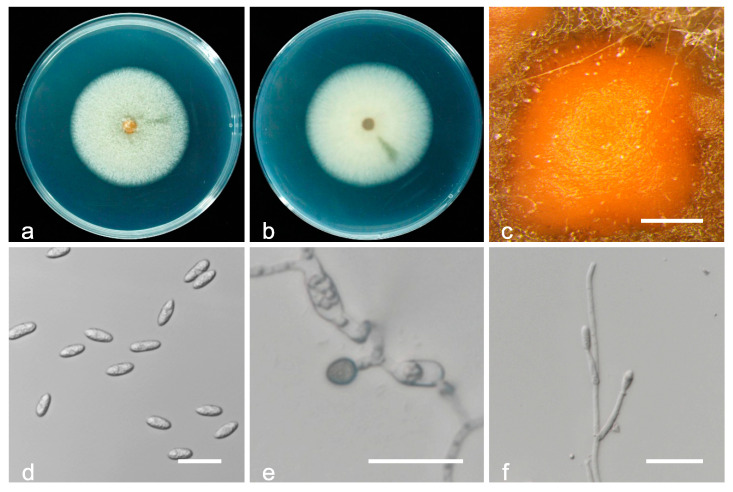
Colletotrichum folicola Q. Tan and C.X. Luo, sp. nov. (Figure 14).
Figure 14.
Biological characteristics of Colletotrichum folicola. (a,b) Front and back view of six-day-old PDA culture; (c,d) conidiomata; (e) setae; (f) conidia; (g) appressoria; (h) conidiophores ((a–h) isolate YNHH 10-1). Scale bars: (c,d) = 200 μm; (e–g) = 20 μm; (h,i) = 10 μm.
MycoBank Number: MB843363.
Etymology: Referring to the host organ from which the fungus was collected.
Type: China, Yunnan Province, Honghe City, on leaves of Prunus persica cv. Hongxue, August 2017, Q. Tan. Holotype YNHH 10-1, Ex-type culture CCTCC M 2020345.
Sexual morphs were not observed. Asexual morphs developed on PDA. Vegetative hyphae were hyaline, smooth-walled, septate, and branched. Chlamydospores were not observed. Conidiomata acervular, conidiophores, and setae formed on hyphae or brown to black stromata. Conidiomata color ranged from yellow to grayish-yellow to light brown. Setae were medium brown to dark brown, smooth-walled, 2–6 septa, 50–140 µm long, base cylindrical, 2.5–4.5 µm in diameter at the widest part, with tip acute. Conidiophores were hyaline to pale brown, smooth-walled, septate, and up to 55 µm long. Conidiogenous cells were hyaline, cylindrical, 12.3−14.5 × 4.4–6.3 µm, with an opening of 1.8–2.5 µm. Conidia were straight, hyaline, aseptate, cylindrical, and had a round end, 12.3−15.4 × 5.6–7.8 µm, mean ± SD = 13.6 ± 0.1 × 6.5 ± 0.3 µm, L/W ratio = 2.1. Appressoria were single, dark brown, elliptical to clavate, 5.6–13.7 × 4.0−8.2 µm, mean ± SD = 8.4 ± 0.5 × 5.9 ± 0.1 µm, L/W ratio = 1.4.
Culture characteristics: Colonies on PDA attained 16–21 mm diameter in three days at 25 °C and 7–10 mm diameter in three days at 30 °C; greenish-black, white at the margin, and aerial mycelium scarce.
Additional specimens examined: China, Yunnan Province, Honghe City, on leaves of Prunus persica cv. Hongxue, August 2017, Q. Tan, living culture YNHH 2-2.
Notes: Colletotrichum folicola is phylogenetically most closely related to C. citrus-medicae (Figure 6). The PHI test (Φw = 1) revealed no significant recombination between C. folicola and C. citrus-medicae (Figure 7), which was described from diseased leaves of Citrus medica in Kunming, Yunnan Province, China [54]. C. folicola is different from C. citrus-medicae holotype isolate HGUP 1554 in ITS (with 99.04% sequence identity), GAPDH (99.13%), CHS-1 (98.44%), and HIS3 (99.72%). The sequence data of ACT do not separate the two species. In terms of morphology, C. folicola differs from C. citrus-medicae by having setae, smaller conidia (12.3−15.4 × 5.6−7.8 µm vs. 13.5–17 × 5.5–9 µm), longer appressoria (5.6−13.7 × 4.0−8.2 µm vs. 6–9.5 × 5.5−8.5 µm), and colonies that are greenish-black rather than white and pale brownish as in C. citrus-medicae.
3.3. Pathogenicity Tests
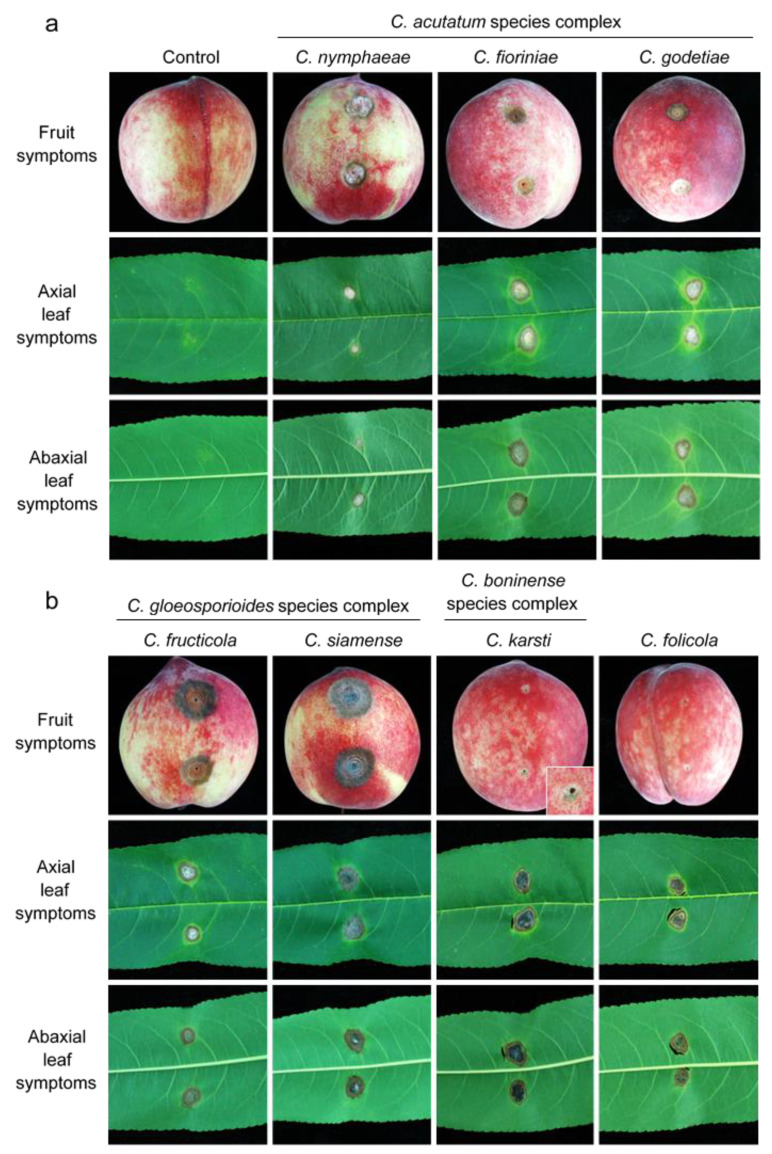
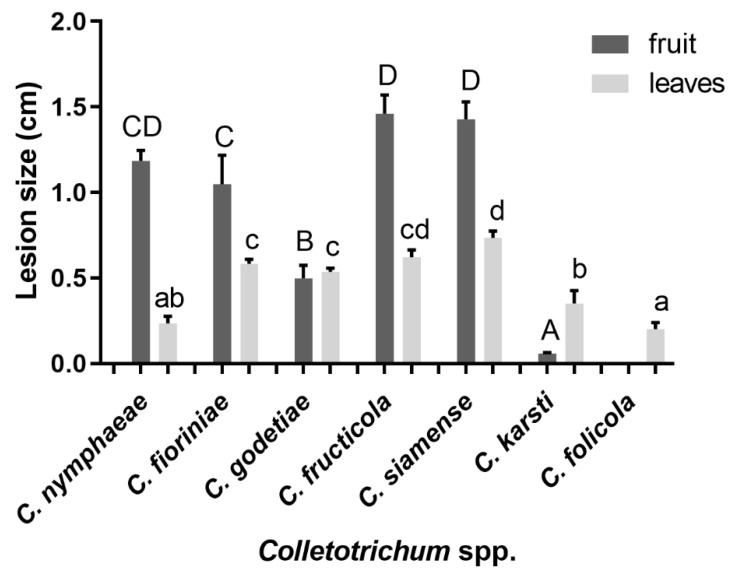
Pathogenicity tests were conducted to confirm Koch’s postulates on fruit and leaves for all species identified (Table S4; Figure 15 and Figure 16). Colletotrichum species collected in this study showed high diversity in virulence. C. nymphaeae, C. fioriniae, C. fructicola, and C. siamense, which were already reported to be pathogens of peaches, were pathogenic on both peach leaves and fruit. C. fructicola and C. siamense from the C. gloeosporioides species complex were more virulent compared to species from the C. acutatum species complex. Interestingly, C. folicola and C. karsti showed tissue-specific pathogenicity. Isolates of these two species were all collected from leaves, and mainly infected leaves in the pathogenicity test. C. folicola did not infect peach fruit at all, and the size of lesions on leaves was comparably small (0.20 ± 0.06 cm). C. karsti did infect peach fruit, but the infection rate was only around 20% (7/36 isolates) and the size of lesions was 0.06 ± 0.01 cm. In contrast, the infection rate on leaves was 63.9% (23/36 isolates) and the lesion size was 0.35 ± 0.13 cm. Isolates of C. godetiae collected from peach leaves in Yunnan province were virulent on both leaves and fruit, with the leaf and fruit infection rates and lesion diameters being 88.3% (53/60 isolates) and 0.54 ± 0.05 cm and 90% (54/60 isolates) and 0.50 ± 0.17 cm, respectively (Table S4; Figure 16).
Figure 15.
Symptoms of peach fruits and leaves induced by inoculation of spore suspensions of seven Colletotrichum spp. after six days at 25 °C. (a) Symptoms resulting from H2O, isolates HBYC 1, JXJA 6, and YNHH 1-1 (left to right). (b) Symptoms resulting from isolates GDHY 10-1, SDQD 10-1, YNHH3-1, and YNHH10-1 (left to right).
Figure 16.
Lesion size on peach fruit and leaves of seven Colletotrichum spp. in the six days after inoculation. C. nymphaeae isolates FJFZ 1, HBJM 1-1, HBWH 3-2, HBYC 1, SCCD 1; C. fioriniae isolates GZTR 7-1, JXJA 1, JXJA 6, ZJLS 1, ZJLS 11-1; C. godetiae isolates YNHH 1-1, YNHH 2-1, YNHH 4-1, YNHH 7-2, YNHH 9-1; C. fructicola isolates GDHY 10-1, GDSG 1-1, GDSG 5-1, GZTR 10-1, GZTR 13-1; C. siamense isolates HBSJZ 1-1, HBSJZ 3-1, HBSJZ 5-1, HBSJZ 7-1, SDQD 10-1; C. karsti isolates YNHH 3-1, YNHH 3-2, YNHH 5-2; C. folicola isolates YNHH 2-2, YNHH 10-1. Letters over the error bars indicate a significant difference at the p = 0.05 level. Capital letters refer to fruit and lowercase letters to leaves.
4. Discussion
This study is the first large-scale investigation of Colletotrichum species causing anthracnose fruit and leaf diseases in peaches in China. The most common Colletotrichum species were C. nymphaeae and C. fioriniae of the C. acutatum species complex and C. fructicola and C. siamense of the C. gloeosporioides species complex. The same species were also identified in the southeastern USA [17,21,22], where a shift over time appeared to favor C. gloeosporioides species complex in South Carolina. The authors speculated that inherent resistance of C. acutatum to benzimidazole fungicides (MBCs) may have given this species complex a competitive advantage when MBCs were frequently used [22]. As MBCs were replaced by other fungicides (including quinone outside inhibitors and demethylation inhibitors), that competitive advantage may have disappeared and C. gloeosporioides species may have increased in prevalence [22,55]. In support of this hypothesis is previous research showing a higher virulence of C. gloeosporioides on peaches, pears, and apples compared to C. acutatum [8,56,57]. Also, this study and others show that the C. gloeosporioides species complex may be better adapted to the hot South Carolina climate compared to the C. acutatum species complex [3]. MBCs are still popular fungicides in Chinese peach production regions. Therefore, it is possible that the dominance of C. acutatum species complex, specifically C. nymphaeae is, at least in part, a result of fungicide selection.
The high prevalence of C. nymphaeae in Chinese peach orchards is consistent with other local studies reporting the same species affecting a wide variety of other fruit crops in China. For example, C. nymphaeae was reported in Sichuan province on blueberries and loquats [58,59], in Hubei province on strawberries and grapevines [60,61], and in Zhejiang province on pecans [62]. Internationally, it is one of the most common species affecting pome fruits, stone fruits, and small fruits [23,63,64].
C. godetiae, C. karsti, and C. folicola were reported on peaches for the first time. The three species were geographically isolated and only present in Yunnan province. Rare occurrences of Colletotrichum species have also been formerly observed on peaches, i.e., C. truncatum was only found in one of many orchards examined in South Carolina, USA [25]. C. godetiae and C. karsti are well-known pathogens of fruit crops. C. godetiae was reported to cause disease on apples, strawberries, and grapes [65,66,67,68], while C. karsti was reported to affect apples and blueberries [69,70]. It is, therefore, possible that these pathogens migrated from other hosts into Yunnan province peach orchards. The observed occurrence, however, does point to either a rather rare host transfer event or to environmental conditions that favor these species. Yunnan province is located in southwestern China and peach production is popular in the Yunnan–Guizhou high plateau, a region with low latitude and high altitude [71]. The complicated local topography and diverse climate lead to highly abundant biodiversity [72], which may explain the emergence of the new species C. folicola.
As mentioned above, regional differences in Colletotrichum species composition in commercial orchards may be influenced by fungicide selection pressure. For example, C. acutatum is less sensitive to benomyl, thiophanate-methyl, and other MBC fungicides compared with C. gloeosporioides [56,73,74]. Meanwhile, all C. nymphaeae strains in this study have been confirmed to be resistant to carbendazim (MBC) [75]. C. nymphaeae was reported to be less sensitive to demethylation inhibitor (DMIs) fungicides (flutriafol and fenbuconazole) compared with C. fioriniae, C. fructicola, and C. siamense [21] and C. gloeosporioides was reported to be inherently tolerant to fludioxonil [76,77]. Most of the peach farms in China are small and there is vast diversity in the approaches to managing diseases. However, MBC (i.e., carbendazim and thiophanate-methyl) fungicides are commonly used to control peach diseases, followed by DMIs (i.e., difenoconazole). Whether fungicide selection had an impact on the Colletotrichum species distribution is unknown, but the high prevalence of C. acutatum species complex and their resilience to MBCs (and, in the case of C. nymphaeae, to DMIs) would allow for such a hypothesis.
In conclusion, this study provides the morphological, molecular, and pathological characterization of seven Colletotrichum spp. occurring on peaches in China. This is of great significance for the prevention and control of anthracnose disease in different areas in China.
Acknowledgments
We sincerely thank the reviewers for their contributions during the revision process.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/jof8030313/s1, Table S1: Primers used in this study, with sequences, annealing temperature, and sources [51,78,79,80,81,82,83,84,85]; Table S2: Isolates of seven Colletotrichum species collected from peaches in China, with details about host tissue, location, and GenBank accession number; Table S3: The sizes of conidia, appresoria, ascospores, and mycelial growth rate of the representative isolates of Colletotrichum spp. obtained in this study; Table S4: Infection rates of seven Colletotrichum spp. inoculated on peach fruit and leaves.
Author Contributions
Conceptualization, Q.T., G.S. and C.-X.L.; methodology, Q.T., C.C., W.-X.Y., L.-F.Y. and C.-X.L.; software, Q.T. and C.C.; validation, Q.T. and C.-X.L.; formal analysis, Q.T. and G.S.; investigation, Q.T., L.-F.Y. and C.-X.L.; data curation, Q.T. and C.-X.L.; writing—original draft preparation, Q.T., G.S. and C.-X.L.; writing—review and editing, G.S., C.C. and C.-X.L.; visualization, Q.T. and C.C.; supervision, W.-X.Y. and C.-X.L.; project administration and funding acquisition, C.-X.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the China Agriculture Research System of the Ministry of Finance and the Ministry of Agriculture and Rural Affairs (CARS-30-3-03), and the Fundamental Research Funds for the Central Universities (No. 2662020ZKPY018).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Alignments generated during the current study are available from TreeBASE (http://treebase.org/treebase-web/home.html; study 29227). All sequence data are available in the NCBI GenBank, following the accession numbers in the manuscript.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Zheng Y., Crawford G.W., Chen X. Archaeological evidence for peach (Prunus persica) cultivation and domestication in China. PLoS ONE. 2014;9:e106595. doi: 10.1371/journal.pone.0106595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Food and Agricultural Organization of the United Nations (FAOSTAT) Website. 2020. [(accessed on 14 February 2022)]. Available online: https://www.fao.org/faostat/en/#data/QCL.
- 3.Dowling M., Peres N., Villani S., Schnabel G. Managing Colletotrichum on fruit crops: A “complex” challenge. Plant Dis. 2020;104:2301–2316. doi: 10.1094/PDIS-11-19-2378-FE. [DOI] [PubMed] [Google Scholar]
- 4.Dowling M., Schnabel G. Understanding plant diseases using art and technology. Int. J. Fruit Sci. 2020;20:959–966. doi: 10.1080/15538362.2020.1755767. [DOI] [Google Scholar]
- 5.Stensvand A., Borve J., Talgo V. Overwintering diseased plant parts and newly infected flowers and fruit as sources of inoculum for Colletotrichum acutatum in sour cherry. Plant Dis. 2017;101:1207–1213. doi: 10.1094/PDIS-11-16-1599-RE. [DOI] [PubMed] [Google Scholar]
- 6.Sutton T.B., Shane W.W. Epidemiology of the perfect stage of Glomerella cingulata on apples. Phytopathology. 1983;73:1179–1183. doi: 10.1094/Phyto-73-1179. [DOI] [Google Scholar]
- 7.De Silva D.D., Crous P.W., Ades P.K., Hyde K.D., Taylor P.W.J. Life styles of Colletotrichum species and implications for plant biosecurity. Fungal Biol. Rev. 2017;31:155–168. doi: 10.1016/j.fbr.2017.05.001. [DOI] [Google Scholar]
- 8.Fu M., Crous P.W., Bai Q., Zhang P.F., Xiang J., Guo Y.S., Zhao F.F., Yang M.M., Hong N., Xu W.X., et al. Colletotrichum species associated with anthracnose of Pyrus spp. in China. Persoonia. 2019;42:1–35. doi: 10.3767/persoonia.2019.42.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sutton B.C. The Coelomycetes. Fungi Imperfecti with Pycnidia, Acervuli and Stromata. Commonwealth Mycological Institute; Kew, UK: 1980. [Google Scholar]
- 10.Cacciola S.O., Gilardi G., Faedda R., Schena L., Pane A., Garibaldi A., Gullino M.L. Characterization of Colletotrichum ocimi population associated with black spot of sweet basil (Ocimum basilicum) in Northern Italy. Plants. 2020;9:654. doi: 10.3390/plants9050654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Riolo M., Aloi F., Pane A., Cara M., Cacciola S.O. Twig and shoot dieback of Citrus, a new disease caused by Colletotrichum species. Cells. 2021;10:449. doi: 10.3390/cells10020449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cai L., Hyde K.D., Taylor P.W.J., Weir B.S., Waller J.M., Abang M.M., Zhang J.Z., Yang Y.L., Phoulivong S., Liu Z.Y., et al. A polyphasic approach for studying Colletotrichum. Fungal Divers. 2009;39:183–204. [Google Scholar]
- 13.Liu F., Wang M., Damm U., Crous P.W., Cai L. Species boundaries in plant pathogenic fungi: A Colletotrichum case study. BMC Evol. Biol. 2016;16:14. doi: 10.1186/s12862-016-0649-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Talhinhas P., Baroncelli R. Colletotrichum species and complexes: Geographic distribution, host range and conservation status. Fungal Divers. 2021;110:109–198. doi: 10.1007/s13225-021-00491-9. [DOI] [Google Scholar]
- 15.Yu Z., Jiang X., Zheng H., Zhang H., Qiao M. Fourteen new species of foliar Colletotrichum associated with the invasive plant Ageratina adenophora and surrounding crops. J. Fungi. 2022;8:185. doi: 10.3390/jof8020185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zheng H., Yu Z., Jiang X., Fang L., Qiao M. Endophytic Colletotrichum species from aquatic plants in southwest China. J. Fungi. 2022;8:87. doi: 10.3390/jof8010087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bernstein B., Zehr E.I., Dean R.A., Shabi E. Characteristics of Colletotrichum from peach, apple, pecan, and other hosts. Plant Dis. 1995;79:478–482. doi: 10.1094/PD-79-0478. [DOI] [Google Scholar]
- 18.Adaskaveg J.E., Hartin R.J. Characterization of Colletotrichum acutatum isolates causing anthracnose of almond and peach in California. Phytopathology. 1997;87:979–987. doi: 10.1094/PHYTO.1997.87.9.979. [DOI] [PubMed] [Google Scholar]
- 19.Schnabel G., Chai W., Cox K.D. Identifying and characterizing summer diseases on ‘Babygold’ peach in South Carolina. Plant Health. Prog. 2006;7:30. doi: 10.1094/PHP-2006-0301-01-RS. [DOI] [Google Scholar]
- 20.Kim W.G., Hong S.K. Occurrence of anthracnose on peach tree caused by Colletotrichum species. Plant Pathol. J. 2008;24:80–83. doi: 10.5423/PPJ.2008.24.1.080. [DOI] [Google Scholar]
- 21.Chen S.N., Luo C.X., Hu M.J., Schnabel G. Sensitivity of Colletotrichum species, including C. fioriniae and C. nymphaeae, from peach to demethylation inhibitor fungicides. Plant Dis. 2016;100:2434–2441. doi: 10.1094/PDIS-04-16-0574-RE. [DOI] [PubMed] [Google Scholar]
- 22.Hu M.J., Grabke A., Schnabel G. Investigation of the Colletotrichum gloeosporioides species complex causing peach anthracnose in South Carolina. Plant Dis. 2015;99:797–805. doi: 10.1094/PDIS-10-14-1076-RE. [DOI] [PubMed] [Google Scholar]
- 23.Moreira R.R., Silva G.A., De Mio L.L.M. Colletotrichum acutatum complex causing anthracnose on peach in Brazil. Austral. Plant Pathol. 2020;49:179–189. doi: 10.1007/s13313-020-00690-z. [DOI] [Google Scholar]
- 24.Lee D.M., Hassan O., Chang T. Identification, characterization, and pathogenicity of Colletotrichum species causing anthracnose of peach in Korea. Mycobiology. 2020;48:210–218. doi: 10.1080/12298093.2020.1763116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Grabke A., Williamson M., Henderson G.W., Schnabel G. First report of anthracnose on peach fruit caused by Colletotrichum truncatum in South Carolina. Plant Dis. 2014;98:1154. doi: 10.1094/PDIS-12-13-1215-PDN. [DOI] [PubMed] [Google Scholar]
- 26.Du Y.X., Ruan H.C., Shi N.N., Gan L., Yang X.J., Dai Y.L., Chen F.R. First report of anthracnose on peach fruit caused by Colletotrichum acutatum in China. Plant Dis. 2017;101:1678. doi: 10.1094/PDIS-11-16-1568-PDN. [DOI] [Google Scholar]
- 27.Suzaki K. Improved method to induce sporulation of Colletotrichum gloeosporioides, causal fungus of grape ripe rot. J. Gen. Plant Pathol. 2011;77:81–84. doi: 10.1007/s10327-011-0296-z. [DOI] [Google Scholar]
- 28.Yang Y.L., Liu Z.Y., Cai L., Hyde K.D., Yu Z.N., McKenzie E.H.C. Colletotrichum anthracnose of Amaryllidaceae. Fungal Divers. 2009;39:123–146. [Google Scholar]
- 29.Chi M.H., Park S.Y., Lee Y.H. A quick and safe method for fungal DNA extraction. Plant Pathol. J. 2009;25:108–111. doi: 10.5423/PPJ.2009.25.1.108. [DOI] [Google Scholar]
- 30.Kumar S., Stecher G., Tamura K. MEGA7: Molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol. Biol. Evol. 2016;33:1870–1874. doi: 10.1093/molbev/msw054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Damm U., Cannon P.F., Woudenberg J.H.C., Crous P.W. The Colletotrichum acutatum species complex. Stud. Mycol. 2012;73:37–113. doi: 10.3114/sim0010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Diao Y.Z., Zhang C., Liu F., Wang W.Z., Liu L., Cai L., Liu X.L. Colletotrichum species causing anthracnose disease of chili in China. Persoonia. 2017;38:20–37. doi: 10.3767/003158517X692788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Damm U., Cannon P.F., Woudenberg J.H.C., Johnston P.R., Weir B.S., Tan Y.P., Shivas R.G., Crous P.W. The Colletotrichum boninense species complex. Stud. Mycol. 2012;59:75–88. doi: 10.3114/sim0002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Marin-Felix Y., Groenewald J.Z., Cai L., Chen Q., Marincowitz S., Barnes I., Bensch K., Braun U., Camporesi E., Damm U., et al. Genera of phytopathogenic fungi: GOPHY 1. Stud. Mycol. 2017;86:99–216. doi: 10.1016/j.simyco.2017.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Katoh K., Standley D.M. MAFFT multiple sequence alignment software version 7: Improvements in performance and usability. Mol. Biol. Evol. 2013;30:772–780. doi: 10.1093/molbev/mst010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ronquist F., Teslenko M., van der Mark P., Ayres D.L., Darling A., Höhna S., Larget B., Liu L., Suchard M.A., Huelsenbeck J.P. MrBayes 3.2: Efficient Bayesian phylogenetic inference and model choice across a large model space. Syst. Biol. 2012;61:539–542. doi: 10.1093/sysbio/sys029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nylander J.A.A. MrModelTest v. 2. Evolutionary Biology Centre, Uppsala University; Uppsala, Sweden: 2004. [Google Scholar]
- 38.Swofford D. PAUP 4.0 b10: Phylogenetic Analysis Using Parsimony (*and Other Methods), 4.0 b10. Sinauer Associates; Sunderland, MA, USA: 2002. [Google Scholar]
- 39.Rambaut A. FigTree v. 1.4.2. Institute of Evolutionary Biology, University of Edinburgh; Edinburgh, Scotland: 2014. [(accessed on 2 January 2016)]. Available online: http://tree.bio.ed.ac.uk/software/figtree/ [Google Scholar]
- 40.Quaedvlieg W., Binder M., Groenewald J.Z., Summerell B.A., Carnegie A.J., Burgess T.I., Crous P.W. Introducing the consolidated species concept to resolve species in the Teratosphaeriaceae. Persoonia. 2014;33:1–40. doi: 10.3767/003158514X681981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Huson D.H. SplitsTree: Analyzing and visualizing evolutionary data. Bioinformatics. 1998;14:68–73. doi: 10.1093/bioinformatics/14.1.68. [DOI] [PubMed] [Google Scholar]
- 42.Huson D., Bryant D. Application of phylogenetic networks in evolutionary studies. Mol. Biol. Evol. 2006;23:254–267. doi: 10.1093/molbev/msj030. [DOI] [PubMed] [Google Scholar]
- 43.Van der Aa H.A. A leaf spot disease of Nymphaea alba in the Netherlands. Neth. J. Plant Pathol. 1978;84:109–115. [Google Scholar]
- 44.Marcelino J., Giordano R., Gouli S., Gouli V., Parker B.L., Skinner M., TeBeest D., Cesnik R. Colletotrichum acutatum var. fioriniae (teleomorph: Glomerella acutata var. fioriniae var. nov.) infection of a scale insect. Mycologia. 2008;100:353–374. doi: 10.3852/07-174R. [DOI] [PubMed] [Google Scholar]
- 45.Marcelino J.A.P., Gouli S., Parker B.L., Skinner M., Schwarzberg L., Giordano R. Host plant associations of an entomopathogenic variety of the fungus, Colletotrichum acutatum, recovered from the elongate hemlock scale, Fiorinia externa. J. Insect Sci. 2009;9:25. doi: 10.1673/031.009.2501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shivas R.G., Tan Y.P. A taxonomic re-assessment of Colletotrichum acutatum, introducing C. fioriniae comb. et stat. nov and C. simmondsii sp nov. Fungal Divers. 2009;39:111–122. [Google Scholar]
- 47.Pest Alerts. CABI; Kopenhagen, Danmark: 1943. Ohlsens Enkes plantepato logiske Laboratorium. 1 April 1942–31 March 1943; p. 21. [Google Scholar]
- 48.Neergaard P. Mycological notes. III. 7. Colletotrichum godetiae Neerg. 8. Phoma bellidis Neerg. 9. Zygosporium parasiticum (Grove) Bunting & Mason. 10. Peronospora di-anthicola Barthelet. Friesia. 1950;4:72–80. [Google Scholar]
- 49.Schena L., Abdelfattah A., Mosca S., Li Destri Nicosia M.G., Agosteo G.E., Cacciola S.O. Quantitative detection of Colletotrichum godetiae and C. acutatum sensu stricto in the phyllosphere and carposphere of olive during four phenological phases. Eur. J. Plant Pathol. 2017;149:337–347. doi: 10.1007/s10658-017-1185-x. [DOI] [Google Scholar]
- 50.Prihastuti H., Cai L., Chen H., McKenzie E.H.C., Hyde K.D. Characterization of Colletotrichum species associated with coffee berries in northern Thailand. Fungal Divers. 2009;39:89–109. [Google Scholar]
- 51.Weir B.S., Johnston P.R., Damm U. The Colletotrichum gloeosporioides species complex. Stud. Mycol. 2012;73:115–180. doi: 10.3114/sim0011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yang Y.L., Cai L., Yu Z.N., Liu Z.Y., Hyde K.D. Colletotrichum species on Orchidaceae in southwest China. Cryptogam. Mycol. 2011;32:229–253. [Google Scholar]
- 53.Wang W., de Silva D.D., Moslemi A., Edwards J., Ades P.K., Crous P.W., Taylor P.W.J. Colletotrichum species causing anthracnose of citrus in Australia. J. Fungi. 2021;7:47. doi: 10.3390/jof7010047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hyde K.D., Jeewon R., Chen Y.-J., Bhunjun C.S., Calabon M.S., Jiang H.-B., Lin C.-G., Norphanphoun C., Sysouphanthong P., Pem D., et al. The numbers of fungi: Is the descriptive curve flattening? Fungal Divers. 2020;103:219–271. doi: 10.1007/s13225-020-00458-2. [DOI] [Google Scholar]
- 55.Hu M.J., Grabke A., Dowling M.E., Holstein H.J., Schnabel G. Resistance in Colletotrichum siamense from peach and blueberry to thiophanate-methyl and azoxystrobin. Plant Dis. 2015;99:806–814. doi: 10.1094/PDIS-10-14-1077-RE. [DOI] [PubMed] [Google Scholar]
- 56.Munir M., Amsden B., Dixon E., Vaillancourt L., Gauthier N.A.W. Characterization of Colletotrichum species causing bitter rot of apple in Kentucky orchards. Plant Dis. 2016;100:2194–2203. doi: 10.1094/PDIS-10-15-1144-RE. [DOI] [PubMed] [Google Scholar]
- 57.Eaton M.J., Edwards S., Inocencio H.A., Machado F.J., Nuckles E.M., Farman M., Gauthier N.A., Vaillancourt L.J. Diversity and cross-infection potential of Colletotrichum causing fruit rots in mixed-fruit orchards in Kentucky. Plant Dis. 2021;105:1115–1128. doi: 10.1094/PDIS-06-20-1273-RE. [DOI] [PubMed] [Google Scholar]
- 58.Wu W.X., Liu Y., Huang X.Q., Zhang L. First report of anthracnose caused by Colletotrichum nymphaeae on loquat fruit in China. Plant Dis. 2018;102:243. doi: 10.1094/PDIS-06-17-0828-PDN. [DOI] [Google Scholar]
- 59.Liu X., Zheng X., Khaskheli M.I., Sun X., Chang X., Gong G. Identification of Colletotrichum species associated with blueberry anthracnose in Sichuan, China. Pathogens. 2020;9:718. doi: 10.3390/pathogens9090718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Han Y.C., Zeng X.G., Xiang F.Y., Ren L., Chen F.Y., Gu Y.C. Distribution and characteristics of Colletotrichum spp. associated with anthracnose of strawberry in Hubei, China. Plant Dis. 2016;100:996–1006. doi: 10.1094/PDIS-09-15-1016-RE. [DOI] [PubMed] [Google Scholar]
- 61.Liu M., Zhang W., Zhou Y., Liu Y., Yan J.Y., Li X.H., Jayawardena R.S., Hyde K.D. First report of twig anthracnose on grapevine caused by Colletotrichum nymphaeae in China. Plant Dis. 2016;100:2530. doi: 10.1094/PDIS-05-16-0632-PDN. [DOI] [Google Scholar]
- 62.Zhang Y.B., Meng K., Shu J.P., Zhang W., Wang H.J. First report of anthracnose on pecan (Carya illinoensis) caused by Colletotrichum nymphaeae in China. Plant Dis. 2019;103:1432–1433. doi: 10.1094/PDIS-11-18-1968-PDN. [DOI] [Google Scholar]
- 63.Braganca C.A.D., Damm U., Baroncelli R., Massola N.S., Crous P.W. Species of the Colletotrichum acutatum complex associated with anthracnose diseases of fruit in Brazil. Fungal Biol. 2016;120:547–561. doi: 10.1016/j.funbio.2016.01.011. [DOI] [PubMed] [Google Scholar]
- 64.Wang N.Y., Forcelini B.B., Peres N.A. Anthracnose fruit and root necrosis of strawberry are caused by a dominant species within the Colletotrichum acutatum species complex in the United States. Phytopathology. 2019;109:1293–1301. doi: 10.1094/PHYTO-12-18-0454-R. [DOI] [PubMed] [Google Scholar]
- 65.Baroncelli R., Sreenivasaprasad S., Thon M.R., Sukno S.A. First report of apple bitter rot caused by Colletotrichum godetiae in the United Kingdom. Plant Dis. 2014;98:1000–1001. doi: 10.1094/PDIS-11-13-1177-PDN. [DOI] [PubMed] [Google Scholar]
- 66.Munda A. First report of Colletotrichum fioriniae and C. godetiae causing apple bitter rot in Slovenia. Plant Dis. 2014;98:1282. doi: 10.1094/PDIS-04-14-0419-PDN. [DOI] [PubMed] [Google Scholar]
- 67.Zapparata A., Da Lio D., Sarrocco S., Vannacci G., Baroncelli R. First report of Colletotrichum godetiae causing grape (Vitis vinifera) berry rot in Italy. Plant Dis. 2017;101:1051–1052. doi: 10.1094/PDIS-12-16-1764-PDN. [DOI] [Google Scholar]
- 68.Karimi K., Arzanlou M., Pertot I. Weeds as potential inoculum reservoir for Colletotrichum nymphaeae causing strawberry anthracnose in Iran and rep-PCR fingerprinting as useful marker to differentiate C. acutatum complex on strawberry. Front. Microbiol. 2019;10:13. doi: 10.3389/fmicb.2019.00129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Rios J.A., Pinho D.B., Moreira W.R., Pereira O.L., Rodrigues F.A. First report of Colletotrichum karstii causing anthracnose on blueberry leaves in Brazil. Plant Dis. 2015;99:157–158. doi: 10.1094/PDIS-07-14-0717-PDN. [DOI] [PubMed] [Google Scholar]
- 70.Velho A.C., Stadnik M.J., Wallhead M. Unraveling Colletotrichum species associated with Glomerella leaf spot of apple. Trop. Plant Pathol. 2019;44:197–204. doi: 10.1007/s40858-018-0261-x. [DOI] [Google Scholar]
- 71.Yang W., Zhang X. A discussion on structural adjustment of fruit cultivation in Yunnan. Southest China J. Agric. Sci. 2003;16:103–106. [Google Scholar]
- 72.Yang Y.M., Tian K., Hao J.M., Pei S.J., Yang Y.X. Biodiversity and biodiversity conservation in Yunnan, China. Biodivers. Conserv. 2004;13:813–826. doi: 10.1023/B:BIOC.0000011728.46362.3c. [DOI] [Google Scholar]
- 73.Peres N.A.R., Souza N.L., Peever T.L., Timmer L.W. Benomyl sensitivity of isolates of Colletotrichum acutatum and C. gloeosporioides from citrus. Plant Dis. 2004;88:125–130. doi: 10.1094/PDIS.2004.88.2.125. [DOI] [PubMed] [Google Scholar]
- 74.Chung W.H., Ishii H., Nishimura K., Fukaya M., Yano K., Kajitani Y. Fungicide sensitivity and phylogenetic relationship of anthracnose fungi isolated from various fruit crops in Japan. Plant Dis. 2006;90:506–512. doi: 10.1094/PD-90-0506. [DOI] [PubMed] [Google Scholar]
- 75.Usman H.M., Tan Q., Fan F., Karim M.M., Yin W.X., Zhu F.X., Luo C.X. Sensitivity of Colletotrichum nymphaeae to six fungicides and characterization of fludioxonil resistant isolates in China. Plant Dis. 2021;106:165–173. doi: 10.1094/PDIS-05-21-0993-RE. [DOI] [PubMed] [Google Scholar]
- 76.Schnabel G., Tan Q., Schneider V., Ishii H. Inherent tolerance of Colletotrichum gloeosporioides to fludioxonil. Pest. Biochem. Physiol. 2021;172:6. doi: 10.1016/j.pestbp.2020.104767. [DOI] [PubMed] [Google Scholar]
- 77.Usman H.M., Tan Q., Karim M.M., Adnan M., Yin W.X., Zhu F.X., Luo C.X. Sensitivity of C. fructicola and C. siamense of peach in China to multiple classes of fungicides and characterization of pyraclostrobin-resistant isolates. Plant Dis. 2021;105:3459–3465. doi: 10.1094/PDIS-04-21-0693-RE. [DOI] [PubMed] [Google Scholar]
- 78.Gardes M., Bruns T.D. ITS primers with enhanced specificity for basidiomycetes-application to the identification of mycorrhizae and rusts. Mol. Ecol. 1993;2:113–118. doi: 10.1111/j.1365-294X.1993.tb00005.x. [DOI] [PubMed] [Google Scholar]
- 79.White T., Bruns T., Lee S., Taylor J., Innis M., Gelfand D., Sninsky J. PCR Protocols: A Guide to Methods and Applications. Academic Press; Cambridge, MA, USA: 1990. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics; pp. 315–322. [Google Scholar]
- 80.Guerber J.C., Liu B., Correll J.C., Johnston P.R. Characterization of diversity in Colletotrichum acutatum sensu lato by sequence analysis of two gene introns, mtDNA and intron RFLPs, and mating compatibility. Mycologia. 2003;95:872–895. doi: 10.2307/3762016. [DOI] [PubMed] [Google Scholar]
- 81.Carbone I., Kohn L.M. A method for designing primer sets for speciation studies in filamentous ascomycetes. Mycologia. 1999;91:553–556. doi: 10.2307/3761358. [DOI] [Google Scholar]
- 82.Woudenberg J.H.C., Aveskamp M.M., de Gruyter J., Spiers A.G., Crous P.W. Multiple Didymella teleomorphs are linked to the Phoma clematidina morphotype. Persoonia. 2009;22:56–62. doi: 10.3767/003158509X427808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.O’Donnell K., Cigelnik E. Two divergent intragenomic rDNA ITS2 types within a monophyletic lineage of the fungus Fusarium are nonorthologous. Mol. Phylogenet. Evol. 1997;7:103–116. doi: 10.1006/mpev.1996.0376. [DOI] [PubMed] [Google Scholar]
- 84.Glass N.L., Donaldson G.C. Development of primer sets designed for use with the PCR to amplify conserved genes from filamentous ascomycetes. Appl. Environ. Microbiol. 1995;61:1323–1330. doi: 10.1128/aem.61.4.1323-1330.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Crous P.W., Groenewald J.Z., Risede J.M., Simoneau P., Hywel-Jones N.L. Calonectria species and their Cylindrocladium anamorphs: Species with sphaeropedunculate vesicles. Stud. Mycol. 2004;50:415–430. doi: 10.3114/sim.55.1.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Alignments generated during the current study are available from TreeBASE (http://treebase.org/treebase-web/home.html; study 29227). All sequence data are available in the NCBI GenBank, following the accession numbers in the manuscript.