Summary
(1) In contrast to mammals and birds, reptiles have been considered as indeterminate growers, whose growth reflects differential allocation of resources to growth versus other energetically demanding processes such as reproduction. (2) We monitored the growth and activity of bone growth plates, hormonal profiles, and reproductive activity in males and females of the male-larger gecko Paroedura picta. We show that growth plates fuse in this species in a sex-specific manner. The more abrupt epiphyseal closure and more pronounced growth deceleration in females coincide with the increased activity of their reproductive organs. (3) We conclude that at least some lizards are determinate growers whose sexual size dimorphism is potentially driven by ovarian hormones. The major difference in growth between endothermic and ectothermic amniotes appears to be in the magnitude of growth before and after the first reproduction, not in the mechanistic processes such as senescence of growth plate cells
Subject areas: Zoology, Ichthyology, Evolutionary biology, Evolutionary developmental biology
Graphical abstract
Highlights
-
•
We monitored activity of bone growth plates in a male-larger gecko
-
•
Growth plates fused in a sex-specific manner
-
•
At least some lizards are determinate growers
-
•
Their sexual size dimorphism seems to be driven by ovarian hormones
Zoology; Ichthyology; Evolutionary biology; Evolutionary developmental biology
Introduction
Body size is a key determinant of physiological and life-history traits of an organism (Peters, 1983). Despite its crucial importance, animals differ in the way they attain maximal body size during their life. It used to be generally accepted (for a long time as textbook knowledge) that ectothermic vertebrates as fish, amphibians, and non-avian reptiles possess indeterminate growth, i.e., that they grow significantly after sexual maturity and continue in growth throughout their whole life. On the other hand, endotherms (birds and mammals) are believed to possess determinate growth, i.e., to stop growing prior or around sexual maturation (Ricklefs, 1968; Charnov et al., 2001; Vitt and Caldwell, 2014), although the ability of some mammals to grow even after sexual maturity has been observed (Twigg, 1965; Bryden, 1968; Shohoji and Sasaki, 1987; Geiger et al., 2014).
The question of whether animals have determinate or indeterminate growth is crucial for animal energetics, life history evolution, and development of sexual dimorphism in body size. Several influential models attempting to explain the ontogenetic growth trajectories from the energetical perspective assumed that ontogenetic growth is very phenotypically plastic and that changes in growth are directly affected by energy allocation to other traits, mainly to reproduction (von Bertalanffy, 1957; West et al., 2001; Martin et al., 2019; Sibly and Brown, 2020). These models are aimed as “a mechanistic understanding of why growth slows progressively as mature body size is approached” (Sibly and Brown, 2020). They explained the deceleration of growth at a higher age in indeterminate vertebrate growers, such as reptiles, by increasing the allocation to other resource-demanding traits. For example, the general model by West et al. (2001) suggests that acquired energy related to metabolic rate is split into three components: the maintenance of existing tissue, the replacement of cells, and the formation of new tissue. According to this model, a substantial portion of energy is later in ontogeny allocated to reproduction, which is accompanied directly, due to energy limitations, with a reduction in growth (West et al., 2001). The same reasoning was also applied to explain the ontogeny of sexual size dimorphism (SSD), the differences in size between sexes. The so-called “reproductive cost” hypothesis states that the allocation to growth should be smaller in the sex with higher reproductive cost (Cox, 2006), i.e., that the amount of energy allocated to reproduction is directly traded-off with the allocation to growth (recently reviewed in Meter et al., 2020).
Although the sharp contrasts between ectotherms and endotherms in growth and the models explaining deceleration of growth with age in ectothermic vertebrates based on direct allocation of energy to other processes are intuitively appealing, evidence against them is accumulating. Whenever studied in detail, growth even in many non-avian reptiles shows signs of determinacy. The fusion of epiphyseal growth plates in femur, a marker of determinate growth, was confirmed in older animals by X-ray, histology, or micro-computed tomography (micro-CT) in monitor lizards (de Buffrénil et al., 2005, Frýdlová et al., 2017). Later, using micro-CT, a similar pattern was found among the majority of examined species of squamates (Frýdlová et al., 2019; 2020). In the tuatara, the lineage sister to squamates, the determinate growth was confirmed using skeletal cyclic growth marks as well (Castanet et al., 1988). The same method confirmed that growth is determinate in the American alligator (Klein et al., 2009; Woodward et al., 2011) and in some dinosaurs (de Ricqlès et al., 2003; Myhrvold, 2013). However, a study focusing on the ontogeny and the dynamics of growth via growth plate activity in ectothermic reptiles in a systematic way has been still missing.
Furthermore, it remains to be determined how growth cessation differs between sexes in reptiles and, when different, what proximate determinants of the ontogeny of sexual size dimorphism are responsible. As vertebrates are generally monomorphic at early ontogenetic stages, sexual dimorphism in them can be principally reached either by sex-specific heterochrony in sexual maturation connected to growth cessation (i.e., growth duration), by sex-specific growth rates, or a combination of these mechanisms controlled by sex-specific growth regulators (Stamps, 1993; Badayev, 2002). In squamate reptiles, monomorphic, male-larger, and female-larger species can be found even among closely related species (Cox et al., 2009). In this important vertebrate group, it is often accepted that SSD occurs due to the sex-specific allocation of resources to growth versus reproduction or other traits enhancing fitness (i.e. activity connected with territory defense), and the sex investing proportionally less into the growth and more into traits connected with reproduction is smaller (e.g. Cox and John-Alder, 2005; Cox and Calsbeek, 2010; Frynta et al., 2010). However, the reproductive cost hypothesis was not supported in experimental manipulations in Madagascar ground geckos (Paroedura picta) where females grew at the same rate and to the same final snout-vent length (SVL) regardless of the investment in fat reserves and egg production (Kubička and Kratochvíl, 2009; Starostová et al., 2013; Kubička et al., 2017; reviewed in Meter et al., 2020). Based on these results, Starostová et al. (2013) and Kubička et al. (2017) suggested that the earlier experimental support for the reproductive cost hypothesis in squamates based on comparison of ovariectomized and reproducing females (Cox and Calsbeek, 2010) was biased by the experimental protocol removing not only costs of reproduction but also gonads, an important source of hormones, potential growth regulators (Cutler, 1997; Weise et al., 2001; Nilsson et al., 2005, 2014).
Gonadal hormones, namely steroids, were considered to be the major sex-specific growth regulators in lizards. Particularly, male-typical circulating levels of testicular androgens were suggested to be the main regulator of sex-specific growth (Cox et al. 2005, 2009; Cox and John-Alder, 2005; Cox, 2006; Duncan et al., 2020). According to these studies, male androgens, namely testosterone (T), are responsible for male-typical growth in both male- and female-larger species through their bipotential (either stimulating or suppressive) effect on growth. However, our long-term studies did not support the main role of male gonadal androgens in the development of SSD in squamates: castrated males attained the same final body size in chameleons and both male-larger and female-larger gecko species (Starostová et al., 2013; Kubička et al., 2013, 2015; Bauerová et al., 2020). Surprisingly, exogenous T affects growth in females (Starostová et al., 2013 and citations therein; Kubička et al., 2013; Cox et al., 2014; Bauerová et al., 2020). However, T in this case may not have the direct masculinization effect but can cause growth defeminization by interfering with normal levels of female gonadal hormones (Starostová et al., 2013; Kubička et al., 2017). We suggested that ovarian hormones, particularly estrogens, play a significant role in the ontogeny of SSD in squamate reptiles by feminization of the growth in females (Kubička et al., 2017). In support, the application of exogenous estradiol (E2) reduced the growth of ovariectomized females in Madagascar ground gecko (Kubička et al., 2017). The feminization effect of ovarian hormones, namely E2, on female growth was also supported in mammals, where it seems to be manifested through its direct effect on the activity of growth plates (Cutler, 1997; Weise et al., 2001; Nilsson et al., 2005, 2014). Cutler (1997) showed that relatively low levels of E2 stimulate the growth of prepubertal girls and boys. However, in peripubertal girls, higher levels of E2, appearing due to maturation of the female gonadal axis, stimulate growth plate maturation leading to earlier termination of growth compared to boys. In rabbits, estrogens directly accelerate senescence of growth plates, leading to their rapid fusion after exhaustion of its proliferative zone (Weise et al., 2001; Nilsson et al., 2014). The effect of gonadal hormones on SSD can be manifested through their receptors on the cartilage surface (Chagin et al., 2004).
Here, we report the result of a long-term growth experiment in the female-larger gecko P. picta. We monitored the growth plate activity during the lifetime growth of this species to elucidate whether the growth in this squamate species is determinate or not and whether the activity changes during ontogeny in a sex-specific manner. In addition, we explored ontogeny in the circulating levels of androgens and estrogens and tested whether their ontogenetic changes correlate with the departure of male and female growth trajectories and the activity of growth plates. Previous experimental manipulations suggested that male gonadal androgens do not, while female gonadal hormones do represent sex-specific growth regulators in this species as well as in other reptiles (Starostová et al., 2013; Kubička et al., 2017; Bauerová et al., 2020). However, the hormonal manipulations often have important side effects (e.g. cytotoxicity of experimentally induced hormone levels) and/or represent an unnatural situation (e.g. low circulating hormone levels in castrated and ovariectomized animals, non-fluctuating levels of E2). Therefore, our aim was to test whether the conclusions from hormonal manipulations agree with the results in intact animals.
Results
Onset of sexually dimorphic growth
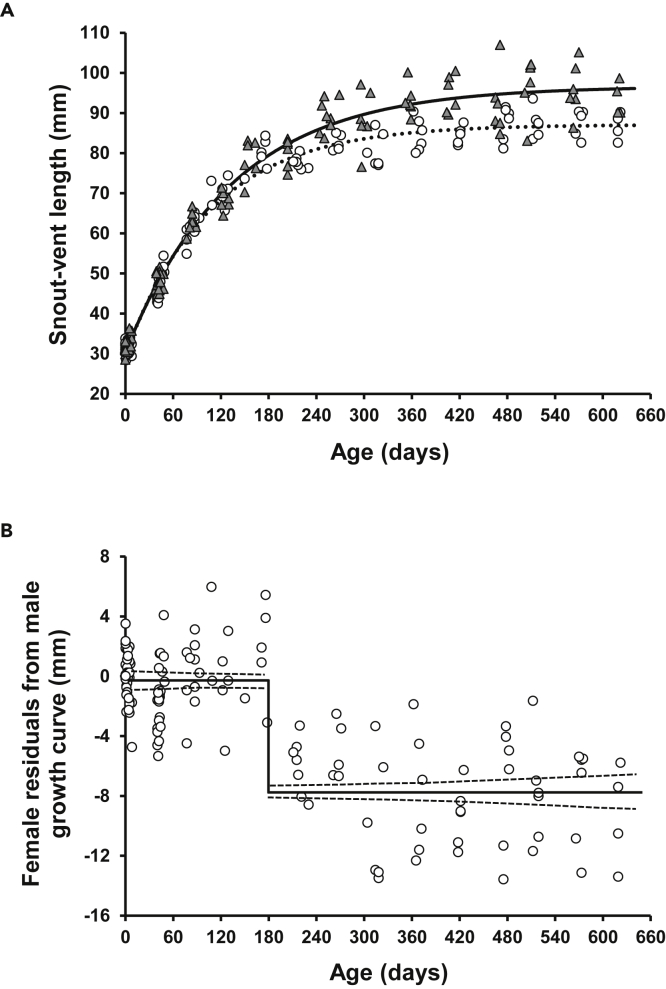
Von Bertalanffy growth models explained 97.4% of the variance in males and 98.3% in females. The male asymptotic SVL was more than 10% larger than the female asymptotic SVL (Figure 1A). The best-fitting regression model of the female residuals from male growth curve on age was the piecewise regression with two horizontal segments with different intercepts. According to this model, the breakpoint indicating the onset of sexually dimorphic growth occurred at 180.4 (±0.53 SE) days of age. Until this age, the males and females grew in a similar way, which is supported by the mean of the female residuals (−0.29 mm ± 0.25 SE), which is not significantly different from zero (t-test: p = 0.26). After breakpoint, the females were significantly smaller than the males in a given age, as indicated by highly significantly negative residuals (mean = −7.75 mm ± 0.47 SE, t-test: p << 0.001; Figure 1B).
Figure 1.
Ontogeny of sexual size dimorphism in Paroedura picta
(A) Growth in experimental males (solid line, gray triangles) and females (dotted line, open circles). Sex-specific growth curves are estimated using the von Bertalanffy model. Each point represents a single individual.
(B) Female raw residuals from the male growth curve. The best-fitting piecewise regression model revealed the breakpoint between male and female growth trajectories at the age of around 180 days.
Both males and females possess smaller reproductive organs before than after the breakpoint of 180 days of age (Mann-Whitney U test; testicle mass: U = 68.5, p << 0.001; ovary mass: U = 203.0, p < 0.001; oviduct mass: U = 203.0, p < 0.001; Table 1). Females had lower levels of T and E2 before the breakpoint (Mann-Whitney U test; T: U = 160.0, p = 0.040; E2: U = 155.0, p = 0.031; Table 1); however, the circulating levels of both these hormones did not differ significantly in males before and after the breakpoint (Mann-Whitney U test; T: U = 258.0, p = 1.0; E2: U = 181.0, p = 0.13; Table 1). Males had higher levels of T than females before and after the breakpoint (Mann-Whitney U test, U ≤ 8.0, p << 0.001 in both cases). Both sexes did not differ in E2 levels prior to the breakpoint (Mann-Whitney U test, U = 71.0, p = 1.0), but females had much higher levels than males after the breakpoint (Mann-Whitney U test, U = 354.0, p << 0.001).
Table 1.
Reproductive organ mass and plasma hormone levels before and after the breakpoint in sexually dimorphic growth of age 180 days in males and females of gecko Paroedura picta
| Prior the breakpoint |
After the breakpoint |
|
|---|---|---|
| median, range (N) | median, range (N) | |
| Testicle mass (g) | 0.047, 0.012–0.124 (36) | 0.147, 0.086–0.20 (54)∗ |
| Ovary mass (g) | 0.054, 0.010–0.215 (17) | 0.097, 0.029–0.417 (52)∗ |
| Oviduct mass (g) | 0.023, 0.006–0.916 (18) | 0.468, 0.044–1.136 (52)∗ |
| Testosterone levels in females (ng mL−1) | 0.010, 0.010–0.172 (13) | 0.010, 0.010–5.308 (40)∗ |
| Estradiol levels in females (ng mL−1) | 0.179, 0.055–0.359 (13) | 0.256, 0.010–1.154 (40)∗ |
| Testosterone levels in males (ng mL−1) | 15.753, 0.016–100.0 (11) | 21.603, 1.862–100.0 (47) |
| Estradiol levels in males (ng mL−1) | 0.148, 0.056–0.326 (11) | 0.136, 0.047–0.423 (47) |
Asterisks indicate significant differences.
Growth plate’s closure
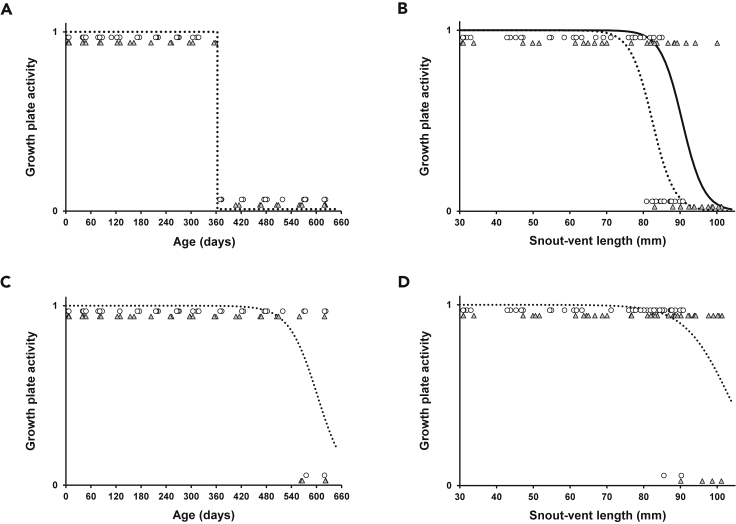
The growth plates of the proximal femur were already closed in the oldest animals, specifically in 18 females and 14 males (minimal age: females: 369 days, males: 407 days; Figures S1A and S1B). The open and closed growth plates of the proximal femur were perfectly separated by the age of the animal (Figure 2A). Therefore, the logistic regression model could not be applied in this case. All animals younger than 360 had all open growth plates, while all animals older than 368 days had degenerated growth plates. Females at the age of 369 had already closed growth plates, while males had them open, suggesting that there may be sexual differences at the age of growth plate closure, but our sampling was not so dense to allow rigorous testing of these differences. Females closed growth plates of the proximal femur at significantly smaller body size (SVL) than males (Table S1; Figure 2B). The growth plates of the distal femur remained open much longer as they were degenerated only in six of the oldest and the largest animals, two females and four males (minimal age: females: 575 days, males: 563 days; Figures S1C and S1D). Probably, due to the small sample size of these oldest animals, we did not detect a significant effect of sex, only age, and SVL on the closure of growth plates in the distal femur (Table S1; Figures 2C and 2D). The growth plate cessation of the vertebrae occurs much earlier than in the femur. A male and female of age 40 and 48 days, i.e., well before the onset of sexually dimorphic growth, possesed active vertebral growth plates. The vertebrae growth plates were already closed in three examined males and three females after the breakpoint (age ranging from 204 to 575 days, Figures S1E and S1F).
Figure 2.
Growth plate activity in the femur throughout development in Paroedura picta
(A) Open and closed growth plates of the proximal femur are perfectly separated by the age of the animal.
(B) Females close the growth plates of the proximal femur at significantly smaller snout-vent length than males (logistic regression: males: solid line, females: dotted line). (C and D) The growth plate closure in the distal femur is associated with age (C) and snout-vent length (D). Open growth plates are coded as 1, closed as 0; females: open circles, males: gray triangles.
Discussion
This study is the first report describing the long-term systematic monitoring of growth plate activity in a lizard. The results corroborate the current paradigm shift that lizards are not generally indeterminate growers, but that their growth plates fuse and thus the elongation of bones terminates, often in relatively early age (Frýdlová et al., 2019; 2020). We documented in the Madagascar ground gecko that individual anatomical sites can have different timings of growth cessation, which is similar to mammals (Weise et al., 2001). We can conclude that, as birds and most mammals, at least some reptiles also have determinate growth (Figures 1 and 2). For these lineages, a mechanistic basis of why bone growth slows progressively as mature body size is approached and at the end stops completely is the deceleration of the activity of growth plates ending with epiphyseal closure (Figure 3, and Figure S1).
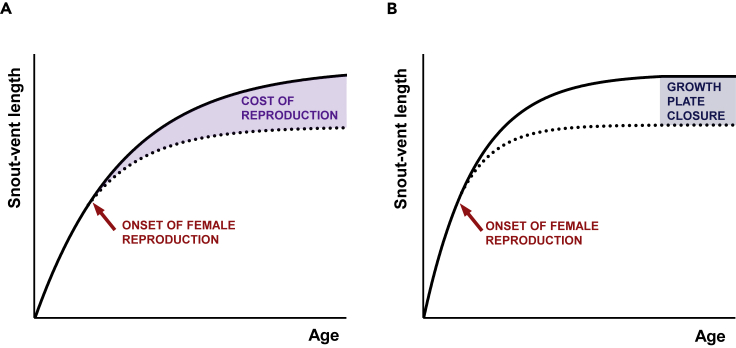
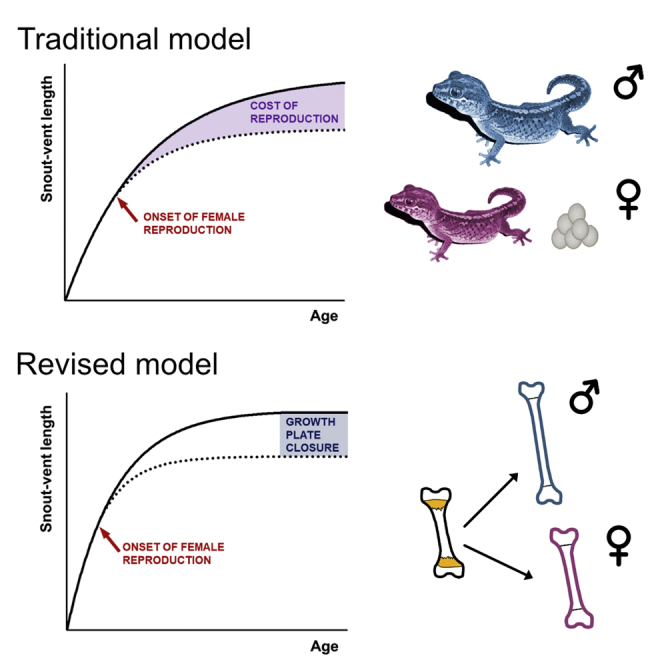
Figure 3.
Traditional and revised models of growth and ontogeny of sexual size dimorphism in lizards
(A and B) In reptiles, the traditional model (A) assumes indeterminate growth and sexual size dimorphism directly reflecting the sex-specific costs of reproduction. The revised model (B) postulates reptiles as determinate growers whose bone growth plate activity is as in mammals influenced by sex-specific modifiers, particularly ovarian hormones.
We compared the ontogenetic dynamics of epiphyseal growth plate fusion in the femur of male and female P. picta and found that it occurs pronouncedly at least in its proximal part at approximately the same age in both sexes (Figure 1A). In reptiles, several alternatives for the proximate mechanism controlling sexual size dimorphism were suggested. For male-larger species, more intense growth in males was suggested to be driven by high levels of circulating gonadal androgens (reviewed by Cox et al., 2009). However, in P. picta, the levels of T in males are highly increased much before the emergence of sexually dimorphic growth (Table 1), which does not support the role of male gonadal androgens in the ontogeny of sexual size dimorphism (see also e.g. Taylor and Denardo, 2005 for similar results in an experiment monitoring growth and ontogeny of T levels in a snake). These results agree with the earlier manipulative experiments in this and other species of lizards, where castration with and without T supplementation does not largely influence the final body length in males (Starostová et al., 2013; Kubička et al., 2013, 2015; Bauerová et al., 2020). On the other hand, the start of the notable fluctuation in E2 levels in females and the increase in ovary and oviduct size coincide well with the deceleration of their growth, which supports the experimental evidence that sexual dimorphism in lizards is controlled by ovarian hormones (Kubička et al., 2017). We suggest that, as in mammals, high levels of estrogens increase the senescence of growth plates in bones (Cutler, 1997; Weise et al., 2001; Nilsson et al., 2005, 2014), which relates to growth cessation. As this mechanism seems to be shared between mammals and reptiles, it may be highly evolutionarily conserved, but more research should be done in this direction before a credible conclusion can be reached. We should keep in mind that many squamate species are female-larger (including some members of the genus Paroedura; Starostová et al., 2010); it will be very interesting to investigate the role of ovarian hormones in such lineages. In mammals, it was suggested that low levels of estrogens promote, while high levels of estrogens decrease growth (Cutler, 1997), but to our knowledge, no evidence of the contribution of the development of stimulative and inhibiting effect of estrogens is available in female-larger species of amniotes.
The existence of determinate growth has important practical consequences for comparative and experimental studies. Many studies in squamates compared size between sexes or among populations and species (Meiri, 2008). It was argued that this procedure is not very substantiated in indeterminate growers and that knowledge of growth curves is necessary for any such comparisons (Stamps, 1993). While we fully agree that knowing the growth curves is essential, determinate growth in at least some lizards such as geckos justifies using measures such as upper percentiles of the distribution of body length (Kratochvíl and Frynta, 2002) as an estimation of final size in the given sample adequate for comparative studies. The final size should be considered as the reliable measure of structural size in lizards with determinate growth. Importantly, for experimental studies on growth in lizards, in species with determinate growth, the short-term effects of experimental manipulations on growth rate should not be interpreted as evidence for the effect of the manipulation on body size, especially in animals of unknown age. The final body size should be taken as a measure of body size for a given species or population and sex (see also Kubička et al., 2015).
Traditionally, the growth of reptiles and other poikilothermic vertebrates was expected to be directly influenced by energy availability (von Bertalanffy, 1957; West et al., 2001; Martin et al., 2019; Sibly and Brown, 2020). Under these models, the results of growth experiments demonstrating comparable structural growth in controls and female lizards with experimentally largely decreased allocation to reproduction (Kubička et al., 2017), in lizards under different feeding regimes, or in lizards with and without tail regeneration seem paradoxical (reviewed in Meter et al., 2020). The endogenous control of structural growth can explain these observations. The finding of determinate growth in lizards also revises the hypotheses about the thermal dependence of body size (Atkinson, 1994). It seems that instead of the energetics of the whole animal, the major driver of the dependence of final body size on temperature (documented in P. picta by Starostová et al., 2010) is the thermal dependence of closure of bone growth plates. Nevertheless, we stress that some lizard species in the wild may not live long enough to reach their final body size and they can still naturally function as indeterminate growers.
The major difference between birds, most of the mammals, and at least some reptiles is likely the timing of the first reproduction with respect to the structural growth cessation, but not that mammals and birds would be generally determinate and reptiles indeterminate growers. Reptiles continue to grow after their first reproduction. For illustration, in this study, males and females of P. picta began the reproduction at SVL and mass around 65 mm and 7 g, while their maximal final size was 107 mm and 40 g in males and 94 mm and 27 g in females. From the perspective of the evolution of life histories, it seems that mammals and birds postpone their reproduction until after reaching optimal body size for a given species and sex. Reptiles with much smaller growth rates (Case, 1978) and thus high mortality risk before reaching final, optimal body size (due to extended time needed for attaining it) are forced to start reproduction at smaller, suboptimal body size at the expense of smaller egg size and, in the case of lineages with variable clutch size, also number of eggs per clutch (Kratochvíl and Kubička, 2007). The final, assumingly optimal size is reached only later in the ontogeny, when the growth of bones stops. As hormones produced by active ovaries can influence growth trajectory, the timing of the first reproduction should be under selection for the final body size in female reptiles.
In conclusion, we demonstrate that bone elongation is terminated by the fusion of the growth plates of the femur and presacral vertebrae in a lizard, the growth of which is then necessarily determinate. We show that the cessation of closure is quite abrupt in both sexes. It occurs at a smaller body size in females, where it correlates with the beginning of a profound cycling in E2 levels and the activity of the reproductive organs. It seems that the basic growth processes of bones and their regulators in at least some lizards are much more similar to the situation in mammals than was generally assumed.
Limitations of the study
The study following growth in relatively slow growing animals such as reptiles is demanding. It requires a lot of time and effort related to animal care etc. Therefore, we have not been able to perform analogous experiments in more species yet. We caution against overgeneralization of our single-species study and welcome its replication in another species, particularly in the representatives of other squamate lineages than geckos. Also, we infer the conclusions of our study regarding the role of testicular and ovarian steroids in the sex-specific growth regulation based on a correlation of the ontogeny of SSD with timing of the development of hormonal profiles and reproductive organs. Manipulative studies in P. picta using hormonal manipulations are in agreement with the conclusions presented here (Starostová et al., 2013; Kubička et al., 2015, 2017). Nevertheless, much more experimental work is needed for a functional proof of the candidate sex-specific growth modifiers.
Ethics approval and consent to participate
The experiment was conducted in the accredited animal care facility with the approval of the Ethical Committee of Charles University and the Central Commission for Animal Welfare and the Environment of the Czech Republic (permit number 35484/2015-11).
STAR★Methods
Key resources table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Biological samples | ||
| Femur and vertebra tissue | experimental animals euthanized for this study | experimental animals euthanized for this study |
| Blood plasma | experimental animals euthanized for this study | experimental animals euthanized for this study |
| Chemicals, peptides, and recombinant proteins | ||
| Formaldehyde | SIGMA | Cat# 15512 |
| Formic acid | SIGMA | Cat# F0507 |
| Hematoxylin | SIGMA | Cat# H3136 |
| Eosin | SIGMA | Cat# 861006 |
| Canada balsam | SIGMA | Cat# C1795 |
| Alcian blue | SIGMA | Cat# A3157 |
| Critical commercial assays | ||
| Estradiol radioimmunoassay kit ESTR-US-CT | Cisbio Bioassays; used as commercial kit at the Institute of Endocrinology (commercial lab for hormonal analyses) | Cisbio Bioassays, Codolet, France |
| Testosterone levels assessment via radioimmunoassay | Method of assessment after Hampl (1994) | Used as an inhouse method at the Institute of Endocrinology (commercial lab for hormonal analyses) |
| Deposited data | ||
| Raw and analyzed data | This paper, Mendeley database | https://doi.org/10.17632/fxcdd6j4sh.1 |
| Experimental models: Organisms/strains | ||
| Madagascar ground gecko Paroedura picta (Peters 1854) | own outbreed population of descendants of wild-caught animals | own outbreed population of descendants of wild-caught animals |
| Software and algorithms | ||
| Statistica | StatSoft (2011) | StatSoft, Tulsa, USA |
| R | R Core Team (2021) | https://www.R-project.org/ |
| SegReg | Oosterbaan (2011) | https://www.waterlog.info/segreg.htm |
Resource availability
Lead contact
Further information and requests for resources and reagents should be directed to the lead contact: Lukáš Kratochvíl (lukas.kratochvil@natur.cuni.cz).
Materials availability
This study did not generate new unique reagents or material.
Experimental model and subject details
Animals and rearing conditions
We followed 256 individuals of P. picta; all were progeny of wild-caught animals or of the first generation in captivity. The experimental individuals were incubated and held individually after hatching at constant temperature 30°C (±0.2) and 12: 12 day night: light regime in climatic chambers during the entire growth experiment. This temperature ensures low embryonic mortality, rapid development, and growth (Starostová et al., 2010, Starostová et al., 2012). The animals were fed twice a week ad libitum with crickets (Gryllus assimilis) dusted with vitamin powder (Roboran H, UNIVIT, Czech Republic). The cages were equipped with a sandy substrate, a shelter, and a water dish. Water was available ad libitum and supplemented with calcium continuously except its replacement by vitamins A, D3 and E (Combinal E and Combinal A + D3; IVAX Pharmaceuticals, Czech Republic) once every two weeks. An extra dish with a calcium powder was provided for the egg-laying females.
Method details
Growth cohorts
Based on our previous studies, body size in geckos older than 20 months is very close to asymptotic body size (Starostová et al., 2013; Kubička et al., 2017). Therefore, in this experiment, we followed growth since hatching untill the age of c. 22 months (Figure 1A). When an experimental female reached four months of age or seven grams body mass, she was mated with an unrelated experimental male of the same age. This procedure allows us to maintain typical physiological processes, including hormonal cycles, in females. To track ontogenetic changes in bone growth and hormonal cycles, we established 14 age cohorts in c. six-week intervals. Each cohort consists of a minimum of eight animals, four males and four females, in the oldest cohort (average age of 620 days) to a maximum of 67 individuals in the youngest cohort (average age of three days). At the time the animals reached the age required for a particular cohort (ranging between 0 and 620 days), they were sacrificed by rapid decapitation, blood plasma was collected and used for hormone measurements. The sex of each individual was confirmed by direct gonadal inspection. The mass of testicles, ovaries, and oviducts was taken by a digital balance to the nearest 0.001 g. If a gonad was too small to be weighed (i.e. below the balance detectability), its mass was not taken.
Examination of growth plate activity
Growth plate activity was explored in the femurs of 95 selected individuals covering the entire growth duration. The femoral growth plates were examined in three to four individuals of each sex per each age cohort. The extracted femurs were stored in 4% formaldehyde. They were later rinsed in water, and either whole bone or, in the case of large animals, just both bone epiphyses were decalcified in the 10% formic acid solution. Based on bone size, this process lasted two to three weeks with solution replacement twice a week. This method provided the best results in the preliminary experiment. After decalcification, the samples were rinsed in water and embedded in paraffin. Bone sections seven micrometers thick were stained using the hematoxylin-eosin staining technique and mounted in Canada balsam. The images used for the monitoring of growth plate activity on both proximal and distal femur were taken by Leica DMLB microscope with MC170 HD camera (Leica Microsystems), stereo binocular Nikon Eclipse E400 and software QuickPhoto Camera 3.1. To strengthen our conclusions, we also examined the growth plate activity in the most caudal presacral vertebrae in four males and four females with known femoral growth plate activity but differing in age. The cartilage part was highlighted by alcian blue staining.
Quantification and statistical analysis
Hormonal analyses
The levels of circulating E2 and T were measured in the plasma samples using a radioimmunoassay (RIA) at the Institute of Endocrinology (Prague, Czech Republic). E2 was assayed using a commercial estradiol RIA kit ESTR-US-CT (Cisbio Bioassays, Codolet, France) with the declared detection limit of 0.001 ng per ml. The intra-assay and inter-assay coefficients of variation varied from 2.8% to 18.1%. T was assessed following the method of Hampl (1994). It consists of extracting plasma with diethyl-ether followed by radioimmunoassay using rabbit polyclonal antiserum to testosterone-3-(carboxymethyloxime) bovine serum albumin conjugate with homologous [125I]tyrosine methyl ester derivative as a tracer. The limit of detection of the assay was 0.01 ng per mL. The intra-assay and inter-assay coefficients of variation were 8.2% and 10.7%, respectively. At least in RIA for E2, the scope in coefficients of variation appears to be quite large with a tendency to be higher in samples possessing E2 levels closer to the lower limit of detection. However, its effect on data interpretation is rather negligible considering the high variability in detected hormonal levels and robust differences between compared experimental groups.
Hormone levels were measured in individual samples where possible. However, some animals, especially from the youngest cohorts, had very limited blood volume. In such cases, blood/plasma samples from individuals of a given sex within the cohort were pooled. The same volume was given to the pooled samples for each individual.
Statistical analyses
All statistical analyses were performed using Statistica version 10.0 (StatSoft, Tulsa, USA), R (R Core Team, 2021), and SegReg (Oosterbaan, 2011). The Shapiro-Wilk test was applied to test for a departure from the normal distribution. For all analyses, we use only the data collected just prior to the termination of each individual, i.e. each individual was used only once. When the null hypothesis of normal data distribution was rejected at α = 0.05, the non-parametric test was performed for the group comparison. Parametric tests were used for variables that did not significantly violate normality.
To describe growth curves, we used the asymptotic von Bertalanffy growth model applied separately in males and females where each data point represented a single individual:
where a is the asymptotic SVL (mm), e is the base of the natural logarithm, k is the rate of approach to the asymptotic SVL, t is the age (days) and t0 is the hypothetical time at length zero. This model is suitable for the description of lizard growth (St. Clair, 1998; Kratochvíl and Frynta, 2002; Kubička and Kratochvíl, 2009; Kubička et al., 2013, 2015, 2017).
For comparison of growth between sexes, we computed raw residuals from the estimated male asymptotic growth curve for each female. To estimate the age at which the male and female growth trajectories depart from each other, we applied piecewise regression of these residuals on age to detect a change in trends in the residual data. We tested seven different regression models using SegReg software (Oosterbaan, 2011): no relationship between residuals and age of the animals, a simple linear relationship with non-zero slope, a continuous relationship composed of two linear segments with non-zero slopes, a constant linear function followed by a line with non-zero slope, a line with non-zero slope followed by a constant function, two horizontal segments with different intercepts, and two segments with a non-zero slope in at least one of them. We selected the best model among them by ANOVA.
We also compared intrasexual differences in mean size of reproductive organs (testicles in males, ovaries and oviducts in females) and differences in T and E2 levels prior and after the onset of sexually dimorphic growth revealed by the break point estimated by the piecewise regression. To avoid pseudoreplication, the hormonal levels of the pooled plasma samples were used as a single data point.
We determined whether the growth plate in the proximal and distal epiphysis of the femur was open and active (containing the proliferative zone – column-shaped structures of proliferating chondrocytes), or closed (degenerated) based on histological slides. Next, we applied the logistic regression to test whether the closing of the growth plates is influenced by the sex (factor), age and SVL (continuous predictors, age and SVL were tested in separate models to prevent colinearity). The best models were selected based on the Akaike Information Criterion (AIC). When ΔAIC was <2, the models were considered equivalent and we selected the one with the least variables as the preferred model, while the more complex version was considered supported when ΔAIC >2 (Burnham et al., 2011; Symonds and Moussalli, 2010).
Acknowledgments
This project was supported by the Czech Science Foundation (GACR 19-19746S), participation of TK was supported by the project PROGRES Q25 of the Charles University. We would like to thank Jan Červenka for help with sample collection, Eliška Boučková for histological processing of samples and Barbora Straková for help with image processing.
Author contributions
LKr and LKu designed the study, AT and LKu collected the data on growth and hormones, TK contributed to the part on histology, LKr and LKu analyzed the data and drafted the manuscript. All authors commented and approved the final version.
Declaration of interests
Authors declare no competing interests.
Published: April 15, 2022
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.isci.2022.104041
Supplemental information
Data and code availability
Data reported in this paper are shared at Mendeley Data (https://doi.org/10.17632/fxcdd6j4sh.1). Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.
References
- Atkinson D. Temperature and organism size: a biological law for ectotherms. Adv. Ecol. Res. 1994;25:1–58. [Google Scholar]
- Badyaev A.V. Growing apart: an ontogenetic perspective on the evolution of sexual size dimorphism. Trends Ecol. Evol. 2002;17:369–378. [Google Scholar]
- Bauerová A., Kratochvíl L., Kubička L. Little if any role of male gonadal androgens in ontogeny of sexual dimorphism in body size and cranial casque in chameleons. Sci. Rep. 2020;10:2673. doi: 10.1038/s41598-020-59501-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryden M.M. Control of growth in two populations of elephant seals. Nature. 1968;217:1106–1108. [Google Scholar]
- Burnham K.P., Anderson D.R., Huyvaert K.P. AIC model selection and multimodel inference in behavioral ecology: some background, observations, and comparisons. Behav. Ecol. Sociobiol. 2011;65:23–35. [Google Scholar]
- Case T.J. On the evolution and adaptive significance of postnatal growth rates in the terrestrial vertebrates. Q. Rev. Biol. 1978;53:243–282. doi: 10.1086/410622. [DOI] [PubMed] [Google Scholar]
- Castanet J., Newman D.G., Girons H.S. Skeletochronological data on the growth, age, and population structure of the tuatara, Sphenodon punctatus, on Stephens and Lady Alice Islands, New Zealand. Herpetologica. 1988;44:25–37. [Google Scholar]
- Chagin A.S., Lindberg M.K., Andersson N., Moverare S., Gustafsson J.A., Sävendahl L., Ohlsson C. Estrogen receptor-beta inhibits skeletal growth and has the capacity to mediate growth plate fusion in female mice. J. Bone Mineral Res. 2004;19:72–77. doi: 10.1359/JBMR.0301203. [DOI] [PubMed] [Google Scholar]
- Charnov E.L., Turner T.F., Winemiller K.O. Reproductive constraints and the evolution of life histories with indeterminate growth. Proc. Natl. Acad. Sci. U S A. 2001;98:9460–9464. doi: 10.1073/pnas.161294498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox R.M., Calsbeek R. Severe costs of reproduction persist in Anolis lizards despite the evolution of a single-egg clutch. Evolution. 2010;64:1321–1330. doi: 10.1111/j.1558-5646.2009.00906.x. [DOI] [PubMed] [Google Scholar]
- Cox R.M., John-Alder H.B. Testosterone has opposite effects on male growth in lizards (Sceloporus spp.) with opposite patterns of sexual size dimorphism. J. Exp. Biol. 2005;208:4679–4687. doi: 10.1242/jeb.01948. [DOI] [PubMed] [Google Scholar]
- Cox R.M., Skelly S.L., John-Alder H.B. Testosterone inhibits growth in juvenile male eastern fence lizards (Sceloporus undulatus): implications for energy allocation and sexual size dimorphism. Physiol. Biochem. Zool. 2005;78:531–545. doi: 10.1086/430226. [DOI] [PubMed] [Google Scholar]
- Cox R.M., Stenquist D.S., Calsbeek R. Testosterone, growth and the evolution of sexual size dimorphism. J. Evol. Biol. 2009;22:1586–1598. doi: 10.1111/j.1420-9101.2009.01772.x. [DOI] [PubMed] [Google Scholar]
- Cox R.M., Lovern M.B., Calsbeek R. Experimentally decoupling reproductive investment from energy storage to test the functional basis of a life-history trade-off. J. Anim. Ecol. 2014;83:888–898. doi: 10.1111/1365-2656.12228. [DOI] [PubMed] [Google Scholar]
- Cox R.M. A test of the reproductive cost hypothesis for sexual size dimorphism in Yarrow's spiny lizard Sceloporus jarrovii. J. Anim. Ecol. 2006;75:1361–1369. doi: 10.1111/j.1365-2656.2006.01160.x. [DOI] [PubMed] [Google Scholar]
- Cutler G.B. The role of estrogen in bone growth and maturation during childhood and adolescence. J. Steroid Biochem. Mol. Biol. 1997;61:141–144. [PubMed] [Google Scholar]
- de Buffrénil V., Ineich I., Böhme W. Comparative data on epiphyseal development in the family Varanidae. J. Herpetol. 2005;39:328–335. [Google Scholar]
- de Ricqlès A.J., Padian K., Horner J.R., Lamm E.T., Myhrvold N. Osteohistology of Confuciusornis sanctus (theropoda: aves) J. Vertebr. Paleontol. 2003;23:373–386. [Google Scholar]
- Duncan C.A., Cohick W.S., John-Alder H.B. Testosterone reduces growth and hepatic IGF-1 mRNA in a female-larger lizard, Sceloporus undulatus: evidence of an evolutionary reversal in growth regulation. Integr. Organismal. Biol. 2020;2:obaa036. doi: 10.1093/iob/obaa036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frýdlová P., Nutilová V., Dudák J., Žemlička J., Němec P., Velenský P., Jirásek T., Frynta D. Patterns of growth in monitor lizards (Varanidae) as revealed by computed tomography of femoral growth plates. Zoomorphology. 2017;136:95–106. [Google Scholar]
- Frýdlová P., Mrzílková J., Šeremeta M., Křemen J., Dudák J., Žemlička J., Němec P., Velenský P., Moravec J., Koleška D., et al. Universality of indeterminate growth in lizards rejected: the micro-CT reveals contrasting timing of growth cartilage persistence in iguanas, agamas, and chameleons. Sci. Rep. 2019;9:18913. doi: 10.1038/s41598-019-54573-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frýdlová P., Mrzílková J., Šeremeta M., Křemen J., Dudák J., Žemlička J., Minnich B., Kverková K., Němec P., Zach P., Frynta D. Determinate growth is predominant and likely ancestral in squamate reptiles. Proc. R. Soc. B Biol. Sci. 2020;287:20202737. doi: 10.1098/rspb.2020.2737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frynta D., Frýdlová P., Hnízdo J., Simková O., Cikánová V., Velenský P. Ontogeny of sexual size dimorphism in monitor lizards: males grow for a longer period, but not at a faster rate. Zool. Sci. 2010;27:917–923. doi: 10.2108/zsj.27.917. [DOI] [PubMed] [Google Scholar]
- Geiger M., Forasiepi A.M., Koyabu D., Sánchez-Villagra M.R. Heterochrony and post-natal growth in mammals – an examination of growth plates in limbs. J. Evol. Biol. 2014;27:98–115. doi: 10.1111/jeb.12279. [DOI] [PubMed] [Google Scholar]
- Hampl R. In: Advances in Steroid Analysis '93. Görög S., editor. Akadémiai Kiadó; 1994. Comparison of three immunoassays for testosterone determination; pp. 163–169. [Google Scholar]
- Klein N., Scheyer T., Tütken T. Skeletochronology and isotopic analysis of a captive individual of Alligator mississippiensis Daudin, 1802. Fossil Rec. 2009;12:121–131. [Google Scholar]
- Kratochvíl L., Frynta D. Body size, male combat and the evolution of sexual dimorphism in eublepharid geckos (Squamata: Eublepharidae) Biol. J. Linn. Soc. 2002;76:303–314. [Google Scholar]
- Kratochvíl L., Kubička L. Why reduce clutch size to one or two eggs? Reproductive allometries reveal different evolutionary causes of invariant clutch size in lizards. Funct. Ecol. 2007;21:171–177. [Google Scholar]
- Kubička L., Kratochvíl L. First grow, then breed and finally get fat: hierarchical allocation to life-history traits in a lizard with invariant clutch size. Funct. Ecol. 2009;23:595–601. [Google Scholar]
- Kubička L., Golinski A., John-Alder H., Kratochvíl L. Ontogeny of pronounced female-biased sexual size dimorphism in the Malaysian cat gecko (Aeluroscalabotes felinus: squamata: Eublepharidae): a test of the role of testosterone in growth regulation. Gen. Comp. Endocrinol. 2013;188:183–188. doi: 10.1016/j.ygcen.2013.03.016. [DOI] [PubMed] [Google Scholar]
- Kubička L., Starostová Z., Kratochvíl L. Endogenous control of sexual size dimorphism: gonadal androgens have neither direct nor indirect effect on male growth in a Madagascar ground gecko (Paroedura picta) Gen. Comp. Endocrinol. 2015;224:273–277. doi: 10.1016/j.ygcen.2015.09.028. [DOI] [PubMed] [Google Scholar]
- Kubička L., Schořálková T., Červenka J., Kratochvíl L. Ovarian control of growth and sexual size dimorphism in a male-larger gecko. J. Exp. Biol. 2017;220:787–795. doi: 10.1242/jeb.146597. [DOI] [PubMed] [Google Scholar]
- Martin T., Thorbek P., Ashauer R. Common ground between growth models of rival theories: a useful illustration for beginners. Ecol. Model. 2019;407:108712. [Google Scholar]
- Meiri S. Evolution and ecology of lizard body sizes. Glob. Ecol. Biogeogr. 2008;17:724–734. [Google Scholar]
- Meter B., Starostová Z., Kubička L., Kratochvíl L. The limits of the energetical perspective: life-history decisions in lizard growth. Evol. Ecol. 2020;34:469–481. [Google Scholar]
- Myhrvold N.P. Revisiting the estimation of dinosaur growth rates. PLoS One. 2013;8:e81917. doi: 10.1371/journal.pone.0081917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsson O., Marino R., de Luca F., Phillip M., Baron J. Endocrine regulation of the growth plate. Horm. Res. 2005;64:157–165. doi: 10.1159/000088791. [DOI] [PubMed] [Google Scholar]
- Nilsson O., Weise M., Landman E.B., Meyers J.L., Barnes K.M., Baron J. Evidence that estrogen hastens epiphyseal fusion and cessation of longitudinal bone growth by irreversibly depleting the number of resting zone progenitor cells in female rabbits. Endocrinology. 2014;155:2892–2899. doi: 10.1210/en.2013-2175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oosterbaan R.J. SegReg software. 2011. https://www.waterlog.info/segreg.htm
- Peters R. Cambridge University Press; 1983. The Ecological Implications of Body Size (Cambridge Studies in Ecology) [Google Scholar]
- R Core Team . R Foundation for Statistical Computing; 2021. R: A Language and Environment for Statistical Computing.https://www.R-project.org/ [Google Scholar]
- Ricklefs R.E. Patterns of growth in birds. Ibis. 1968;110:419–451. [Google Scholar]
- Shohoji T., Sasaki H. An aspect of growth analysis of weight in savannah baboon. II. Gender comparison by adjusting age. Growth. 1987;51:425–431. [PubMed] [Google Scholar]
- Sibly R.M., Brown J.H. Toward a physiological explanation of juvenile growth curves. J. Zool. 2020;311:286–290. [Google Scholar]
- St Clair R.C. Patterns of growth and sexual size dimorphism in two species of box turtles with environmental sex determination. Oecologia. 1998;115:501–507. doi: 10.1007/s004420050547. [DOI] [PubMed] [Google Scholar]
- Stamps J.A. Sexual size dimorphism in species with asymptotic growth after maturity. Biol. J. Linn. Soc. 1993;50:123–145. [Google Scholar]
- Starostová Z., Kubička L., Kratochvíl L. Macroevolutionary pattern of sexual size dimorphism in geckos corresponds to intraspecific temperature-induced variation. J. Evol. Biol. 2010;23:670–677. doi: 10.1111/j.1420-9101.2010.01933.x. [DOI] [PubMed] [Google Scholar]
- Starostová Z., Angilletta M.J., Kubička L., Kratochvíl L. Thermal dependence of reproductive allocation in a tropical lizard. J. Therm. Biol. 2012;37:159–163. [Google Scholar]
- Starostová Z., Kubička L., Golinski A., Kratochvíl L. Neither male gonadal androgens nor female reproductive costs drive development of sexual size dimorphism in lizards. J. Exp. Biol. 2013;216:1872–1880. doi: 10.1242/jeb.079442. [DOI] [PubMed] [Google Scholar]
- StatSoft, Inc. (2011). STATISTICA (Data Analysis Software System), Version 10. http://www.statsoft.com.
- Symonds M.R.E., Moussalli A. A brief guide to model selection, multimodel inference and model averaging in behavioural ecology using Akaike’s information criterion. Behav. Ecol. Sociobiol. 2010;65:13–21. [Google Scholar]
- Taylor E.N., Denardo D.F. Sexual size dimorphism and growth plasticity in snakes: an experiment on the Western Diamond-backed Rattlesnake (Crotalus atrox) J. Exp. Zool. A Comp. Exp. Biol. 2005;303:598–607. doi: 10.1002/jez.a.189. [DOI] [PubMed] [Google Scholar]
- Twigg G.I. Studies on Holochilus sciureus berbicensis, a cricetine rodent from the coastal region of British Guiana. J. Zool. 1965;145:263–283. [Google Scholar]
- Vitt L.J., Caldwell J.P. Fourth edition. Academic Press; 2014. Herpetology: An Introductory Biology of Amphibians and Reptiles. [Google Scholar]
- von Bertalanffy L. Quantitative laws in metabolism and growth. Q. Rev. Biol. 1957;32:217–231. doi: 10.1086/401873. [DOI] [PubMed] [Google Scholar]
- Weise M., De-Levi S., Barnes K.M., Gafni R.I., Abad V., Baron J. Effects of estrogen on growth plate senescence and epiphyseal fusion. Proc. Natl. Acad. Sci. U S A. 2001;98:6871–6876. doi: 10.1073/pnas.121180498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West G.B., Brown J.H., Enquist B.J. A general model for ontogenetic growth. Nature. 2001;413:628–631. doi: 10.1038/35098076. [DOI] [PubMed] [Google Scholar]
- Woodward H.N., Horner J.R., Farlow J.O. Osteohistological evidence for determinate growth in the American alligator. J. Herpetol. 2011;45:339–342. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data reported in this paper are shared at Mendeley Data (https://doi.org/10.17632/fxcdd6j4sh.1). Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.