Abstract
Objectives
Sexually transmitted infections (STIs) cause significant morbidity among women with HIV and increase HIV transmission. We estimated the prevalence of four STIs among women with HIV in sub-Saharan Africa (SSA) and compared prevalence among women with and without HIV.
Design
Systematic review and meta-analysis.
Methods
We searched for studies published January 1, 1999-December 19, 2019 reporting prevalence of gonorrhea, chlamydia, trichomoniasis, or Mycoplasma genitalium among women with HIV in SSA. We excluded studies conducted in high-risk groups (e.g, female sex workers). We extracted data on laboratory-confirmed STIs among women with HIV and, when included, among women without HIV. We estimated pooled prevalence for each STI among women with HIV using inverse variance heterogeneity meta-analysis, compared prevalence to women without HIV, and examined the influences of region, clinical setting, and pregnancy status in subgroup analyses.
Results
We identified 3756 unique records; 67 studies were included in the meta-analysis. Prevalence of gonorrhea, chlamydia, trichomoniasis, and Mycoplasma genitalium was 3.5%, 4.0%, 15.6%, and 10.2%, respectively. Chlamydia prevalence was lower in Eastern (2.8%) than in Southern (12.5%) and West/Central (19.1%) Africa combined. Prevalence of chlamydia and trichomoniasis was higher among pregnant (8.1%, 17.6%) than nonpregnant (1.7%, 12.3%) women. All STIs were more prevalent among women with than without HIV (relative risks ranging 1.54-1.89).
Conclusion
STIs are common among women with HIV in SSA, and more common among women with than without HIV. Integrated STI and HIV care could substantially impact STI burden among women with HIV, with potential downstream impacts on HIV transmission.
Keywords: Sexually transmitted infections, HIV, chlamydia, gonorrhea, trichomoniasis, Mycoplasma genitalium, sub-Saharan Africa
Introduction
Management of common, curable sexually transmitted infections (STIs) is an important component of multimodal approaches to prevent HIV. STIs that cause genital tract inflammation increase HIV transmission and acquisition ([1,2]). For women with HIV (WWH), particularly those who have not achieved virologic suppression, STIs increase risks of both HIV transmission to sexual partners and perinatal HIV transmission to children [3–7]. Recognizing the potential impact of STI management on HIV incidence, the World Health Organization (WHO) recommends STI screening as an essential component of comprehensive care for people with HIV [8] and has identified greater integration of HIV and sexual and reproductive health (SRH) services as a strategy to improve clinical outcomes [9].
Improved STI management for prevention of HIV transmission and acquisition could be particularly impactful in sub-Saharan Africa (SSA), which shoulders 40% of the global burden of STIs, with approximately 60 million new infections with gonorrhea, chlamydia, and trichomoniasis annually [9]. Women in SSA, as elsewhere, disproportionately bear STI-related morbidity [10], as untreated infections can lead to pelvic inflammatory disease, ectopic pregnancy, infertility, and adverse pregnancy outcomes [11,12]. Women with HIV, therefore, are a priority population for improved STI management in SSA.
Among women with urethral or vaginal discharge, the 2021 WHO STI Guidelines recommend pathogen-specific testing for chlamydia, gonorrhea, and trichomoniasis, as well as Mycoplasma genitalium if symptoms recur or persist[13]. Despite the individual and public health consequences of these STIs if untreated, routine molecular-based testing has not been widely implemented for WWH in SSA [14–17], due in part to a lack of accessible, accurate, and affordable diagnostic tests for curable STIs other than syphilis [16,18]. Instead, the standard of care in most of SSA remains syndromic management (i.e., empiric symptom-based treatment), which fails to address the approximately 70% of curable STIs that are asymptomatic in women, while exposing a majority of treated women to inappropriate antibiotics, potentially increasing the risk of antibiotic resistance and adverse drug effects [19–24].
Recent advances in rapid molecular-based diagnostic tests have the potential to increase access to screening for several curable STIs, including gonorrhea, chlamydia, trichomoniasis, and M. genitalium [25], enabling improved management of these non-ulcerating STIs among women with HIV. Understanding the epidemiology of STI and HIV co-infection is critical to inform the implementation of STI screening programs within integrated HIV and SRH services for WWH, but estimates of STI prevalence among WWH in SSA are lacking [26]. Therefore, we sought to estimate the prevalence of laboratory-confirmed gonorrhea, chlamydia, trichomoniasis, and Mycoplasma genitalium among WWH in SSA using existing data from the literature.
Methods
Search strategy and selection criteria
Database searches were performed by a medical librarian (LLP) in PubMed, Embase, African Index Medicus via Global Index Medicus, and Web of Science. Keywords relevant to the three concepts of sub-Saharan Africa, HIV/AIDS, and STIs were combined (Table S1). We searched databases for journal articles and conference abstracts published between January 1, 1999 and December 19, 2019. We manually searched bibliographies of related systematic reviews for additional eligible references. Search results were uploaded to Covidence Systematic Review Software (Melbourne, Australia) for deduplication and screening.
Two reviewers among JJ, LRP, MRC, KB, and CMD independently screened each abstract. Two reviewers among JJ, LRP, MRC, and CMD then independently reviewed each eligible full text reference for inclusion using pre-specified criteria. Conflicts were adjudicated by consensus among the review team.
Observational studies and clinical trials were included if they reported the results of cross-sectional testing for prevalent infection with at least one of the STIs of interest (i.e., gonorrhea, chlamydia, trichomoniasis, or Mycoplasma genitalium) among women with HIV in SSA aged ≥13 years. We included studies reporting STI prevalence among adolescent WWH as this age group comprises a notable proportion of WWH in sub-Saharan Africa [27]. Women with clinically-confirmed or self-reported HIV were included, irrespective of HIV treatment status. Based on changes in STI epidemiology and diagnostic modalities, data collected prior to 1999 were not thought to be representative of current STI prevalence and were excluded. Studies were included only if STI testing was performed by: nucleic acid amplification testing (NAAT)/polymerase chain reaction (PCR) or bacterial culture for gonorrhea; NAAT/PCR or antigen enzyme immune assay (EIA) for chlamydia; NAAT/PCR, culture, antigen testing, or microscopy for trichomoniasis; or NAAT/PCR for Mycoplasma genitalium. Facility-based and population-based studies were included. Other reasons for study exclusion included: reporting STI incidence only; enrolling only participants with unknown or negative HIV status; reporting on fewer than 10 participants with HIV; or presenting STI data not disaggregated by sex or HIV status. To obtain prevalence estimates representative of the general population of women with HIV, we excluded studies enrolling only participants from populations known to be at higher risk for STIs (i.e., self-identifying as a female sex worker, person who injects drugs, prisoner, or victim of sexual assault). When multiple references reported data from overlapping clinical cohorts, we included only the one with the more comprehensive sample.
Data extraction and quality assessment
Two reviewers among JJ, LP, MC, and CMD independently extracted data from each study using a pre-specified template. We recorded data on geographic and clinical setting of participant recruitment, years of data collection, cohort characteristics, STI test modality, number of WWH tested for each STI, and number of positive results for each STI. As the clinical setting of participant recruitment was hypothesized to contribute to between-study heterogeneity, we recorded clinical setting for each study in mutually exclusive categories: general care (i.e., general HIV care, primary care), antenatal care, STI clinic, community-based recruitment, or other. When able, we stratified the numbers of women tested and number of positive results by the presence or absence of symptoms and pregnancy status. For studies also enrolling women without HIV from the same target population, we recorded their cohort characteristics, number of women without HIV tested, and number of women with positive results for each STI.
We assessed study quality using a risk of bias tool for prevalence studies adapted from Hoy et al. (Table S2) [28]. Key measures of study quality included representativeness of the study population, method of participant selection, and thoroughness of outcome reporting. Summary risk of bias scores ranged 0-10 for each study; scores of 0-3, 4-6, and 7-10 were considered low, medium, and high risk, respectively.
Statistical analysis
To determine STI prevalence, we pooled proportions and calculated 95% confidence intervals (CIs) using an inverse variance heterogeneity model [29][30] with double arcsine transformation. In secondary analyses, we compared the prevalence of each STI among women with HIV to women without HIV, including only studies that tested a cohort of HIV-negative women recruited from the same population for the same STI using the same diagnostic test. We calculated study-specific relative risks and 95% CIs for each STI among women with versus without HIV, and again used an inverse variance heterogeneity model to calculate overall relative risk.
In both the pooled proportion and comparative analyses, we evaluated heterogeneity across studies using the I2 statistic, with a value above 50% indicating significant heterogeneity. We performed pre-specified subgroup analyses to assess differences in STI prevalence by SSA region, clinical setting, pregnancy status, diagnostic test, year of study start, presence of STI symptoms, and study quality. Finally, we performed meta-regression using inverse variance heterogeneity weighting to assess for sources of between-study heterogeneity. For each STI, when differences were observed in univariate analyses (overall p<0.05), we conducted reductive comparisons between categories to isolate contributors to the differences observed, and adjusted P-values using a Bonferroni correction. For clinical setting we performed a pre-specified pairwise comparison between STI clinics and general care settings. Meta-regression was performed in Stata (version 16.1); all other analyses were performed using MetaXL v5.3. This study is registered with PROSPERO, CRD42020167328.
Role of the funding source
The funders of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report. The corresponding author had full access to all data in the study and had final responsibility for the decision to submit for publication.
Results
Our search identified 6,886 total records, of which 67 studies were eligible and were included in the meta-analysis (Fig. 1). Sixty-six studies provided non-overlapping data for pooled prevalence analyses among WWH. Forty-two of the included studies tested a comparable cohort of women without HIV for the same STIs, contributing data to the comparative analysis. The included studies encompass 15 different countries and include 18,255 WWH tested for gonorrhea, 17,486 tested for chlamydia, 21,364 tested for trichomoniasis, and 3,685 tested for Mycoplasma genitalium (Table 1, Table S3).
Figure 1. PRISMA diagram.
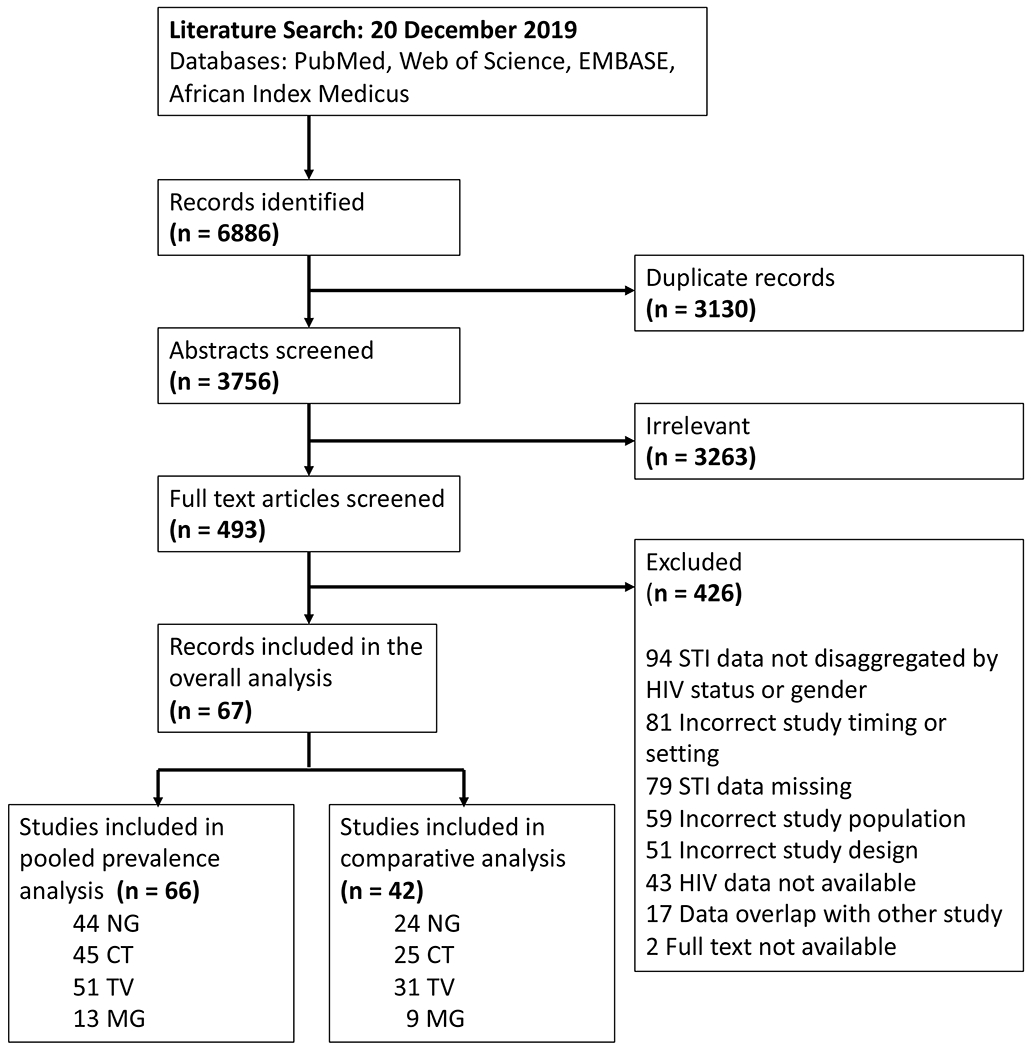
The pooled prevalence analysis includes only women with HIV. The comparative analysis includes women with and without HIV. STI, sexually transmitted infection; NG, Neisseria gonorrhoeae; CT, Chlamydia trachomatis; TV, Trichomonas vaginalis; MG, Mycoplasma genitalium.
Table 1.
Summary characteristics of included studies
| STI | Gonorrhea | Chlamydia | Trichomoniasis | Mycoplasma genitalium | Total |
|---|---|---|---|---|---|
| Included in pooled prevalence analysis | 44 | 45 | 51 | 13 | 66 |
| Included in comparative analysis | 24 | 25 | 31 | 9 | 42 |
|
| |||||
| Region | |||||
|
| |||||
| Eastern Africa | 24 | 25 | 23 | 2 | 31 |
| Southern Africa | 14 | 14 | 17 | 8 | 22 |
| West/Central Africa | 5 | 5 | 9 | 3 | 12 |
| Multiple regions | 1 | 1 | 2 | 0 | 2 |
|
| |||||
| Clinical setting a | |||||
|
| |||||
| General care | 16 | 19 | 22 | 6 | 27 |
| STI clinic | 4 | 5 | 5 | 3 | 6 |
| Antenatal care | 12 | 9 | 14 | 1 | 17 |
| Community-based | 5 | 6 | 4 | 1 | 6 |
| Otherb | 7 | 7 | 6 | 2 | 9 |
|
| |||||
| Pregnancy | |||||
|
| |||||
| Pregnant | 14 | 11 | 15 | 2 | 19 |
| Nonpregnant | 8 | 9 | 10 | 4 | 13 |
|
| |||||
| Symptoms | |||||
|
| |||||
| Symptomatic | 4 | 5 | 7 | 6 | 11 |
| Asymptomatic | 2 | 2 | 2 | 2 | 3 |
|
| |||||
| Study start year c | |||||
|
| |||||
| Before 2010 | 24 | 25 | 29 | 5 | 37 |
| 2010 or Later | 15 | 15 | 16 | 6 | 22 |
|
| |||||
| Risk of bias category | |||||
|
| |||||
| Low | 17 | 18 | 19 | 6 | 26 |
| Medium | 27 | 27 | 32 | 7 | 41 |
| High | 0 | 0 | 0 | 0 | 0 |
|
| |||||
| Test type a | |||||
|
| |||||
| NAAT/PCR | 33 | 36 | 22 | 13 | 46 |
| Culture | 10 | 7 | 16 | ||
| EIA | 9 | 9 | |||
| Microscopy | 19 | 19 | |||
| Otherd | 2 | 3 | 5 | ||
Totals may be higher than total number of studies because different enrollment sites within one study were conducted in different clinical settings and/or used a different testing modality for the same STI.
“Other” includes studies recruiting in multiple settings, family planning clinics, infertility clinics, labor& delivery ward, or unspecified settings.
Studies that did not report dates of data collection are not included.
Includes ligase chain reaction for gonorrhea; OSOM antigen test, rapid immunochromatographic assay, and cytology for trichomoniasis; and multiple test types.
STI, sexually transmitted infection; NAAT/PCR, nucleic acid amplification testing/polymerase chain reaction; EIA, enzyme immunoassay.
Of the 67 studies included in the meta-analysis, 31 (46%) were conducted in Eastern Africa, 22 (33%) in Southern Africa, 12 (18%) in West/Central Africa, and two (3%) in multiple SSA regions (Fig. S1, Table 1).[31] The most common clinical setting overall and for each individual STI was general care (sites of routine HIV or primary care; 27 studies, 40%), followed by antenatal care settings (17 studies, 25%), STI clinics (six studies, 9%), and community-based settings (six studies, 9%). In most included studies, STI testing was performed as part of a research study or pilot program in contexts in which syndromic management, rather than pathogen-specific testing, was local standard of care. NAAT/PCR was the most common testing modality for all four STIs. In addition to the six studies conducted in STI clinics and thus enrolling symptomatic women, seven other studies provided data on the presence or absence of STI symptoms. Only one study enrolled both pregnant and non-pregnant women and reported STI prevalence for the two groups separately, while 18 studies enrolled pregnant women exclusively – 17 in antenatal care and one in a labor & delivery ward.
In quality assessment, 26 studies were assessed as having low, 41 as having medium, and zero studies as having high risk of bias (Table S3). Common sources of bias included poor representativeness of the study target population and sampling frame, as well as convenience methods of participant recruitment, non-participation, and incomplete outcome reporting.
Meta-analysis of the included studies demonstrated an overall pooled prevalence of 3.5% for gonorrhea (95% CI, 1.3 – 6.1), 4.0% for chlamydia (0.9 – 7.8), 15.6% for trichomoniasis (11.4 – 20.0), and 10.2% for Mycoplasma genitalium (6.5 – 14.2) across SSA (Fig. 2a–2d). The prevalence estimates had significant heterogeneity, with an I2 >80% for all four STIs. The high heterogeneity between studies was partially explained by observed differences in pre-specified subgroup analyses (Tables 2 and S4).
Figure 2a-d: Forest plots of pooled prevalence estimate for each STI, by region and overall.
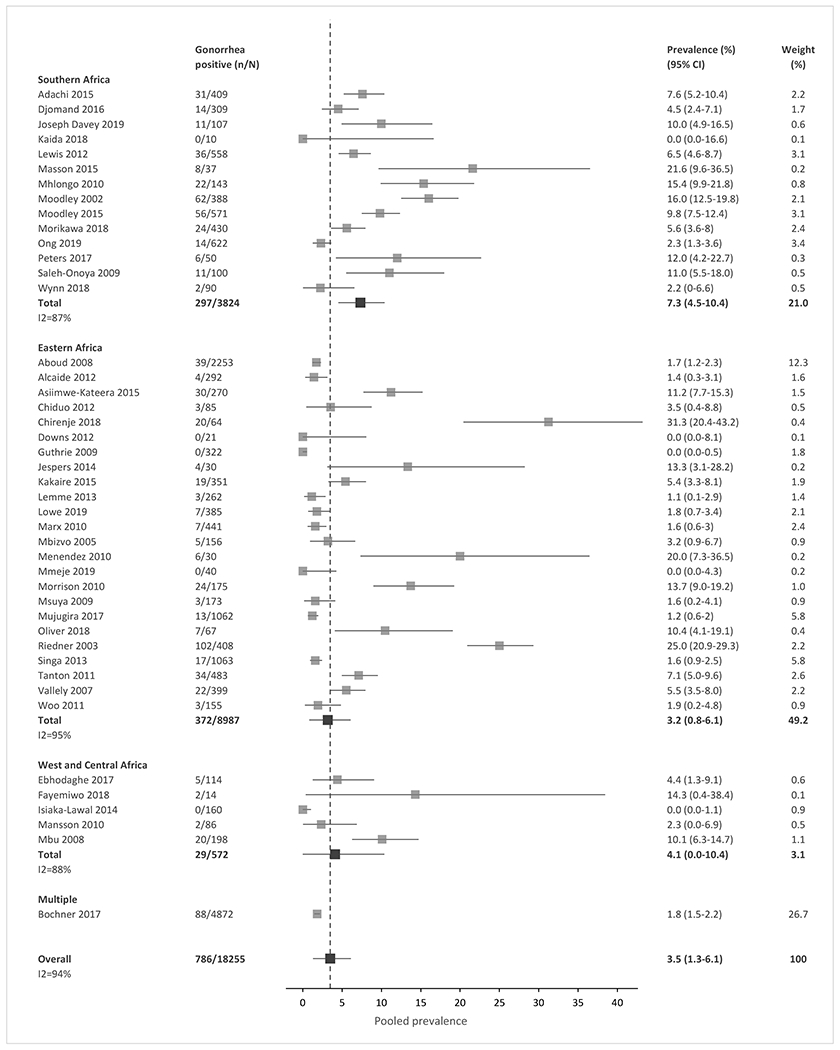
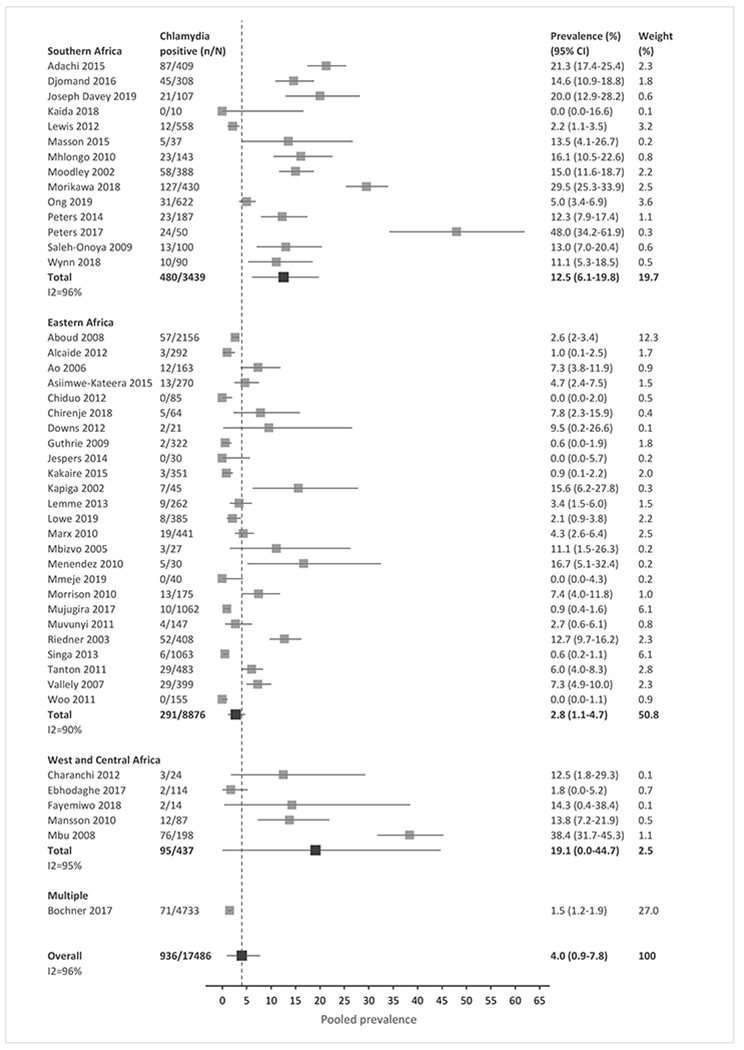
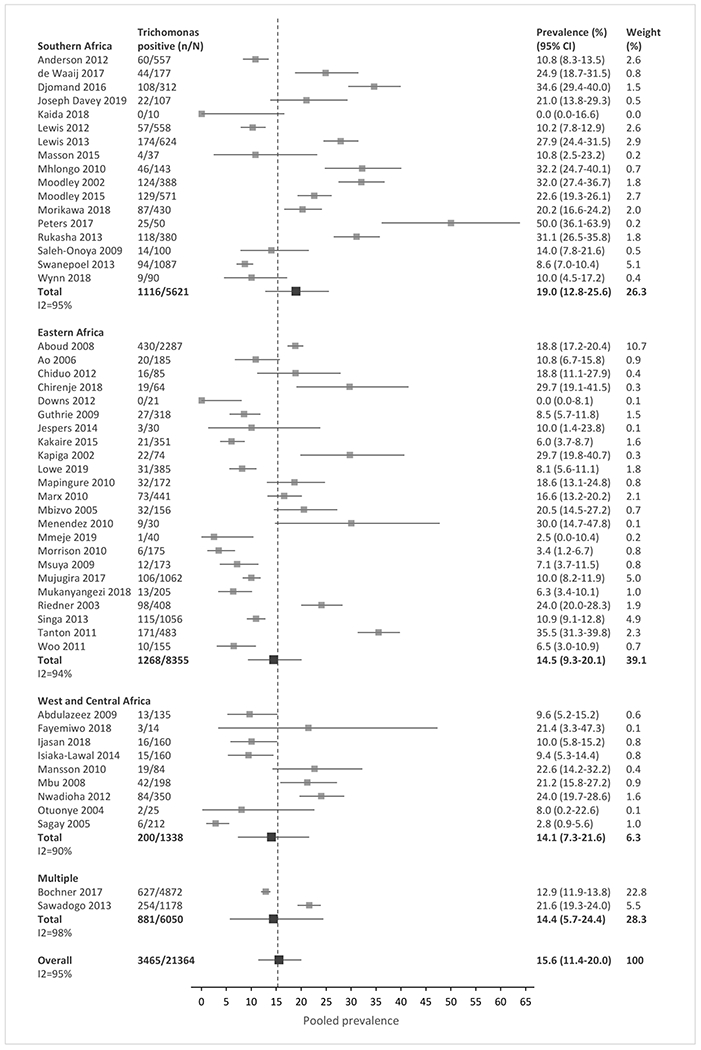
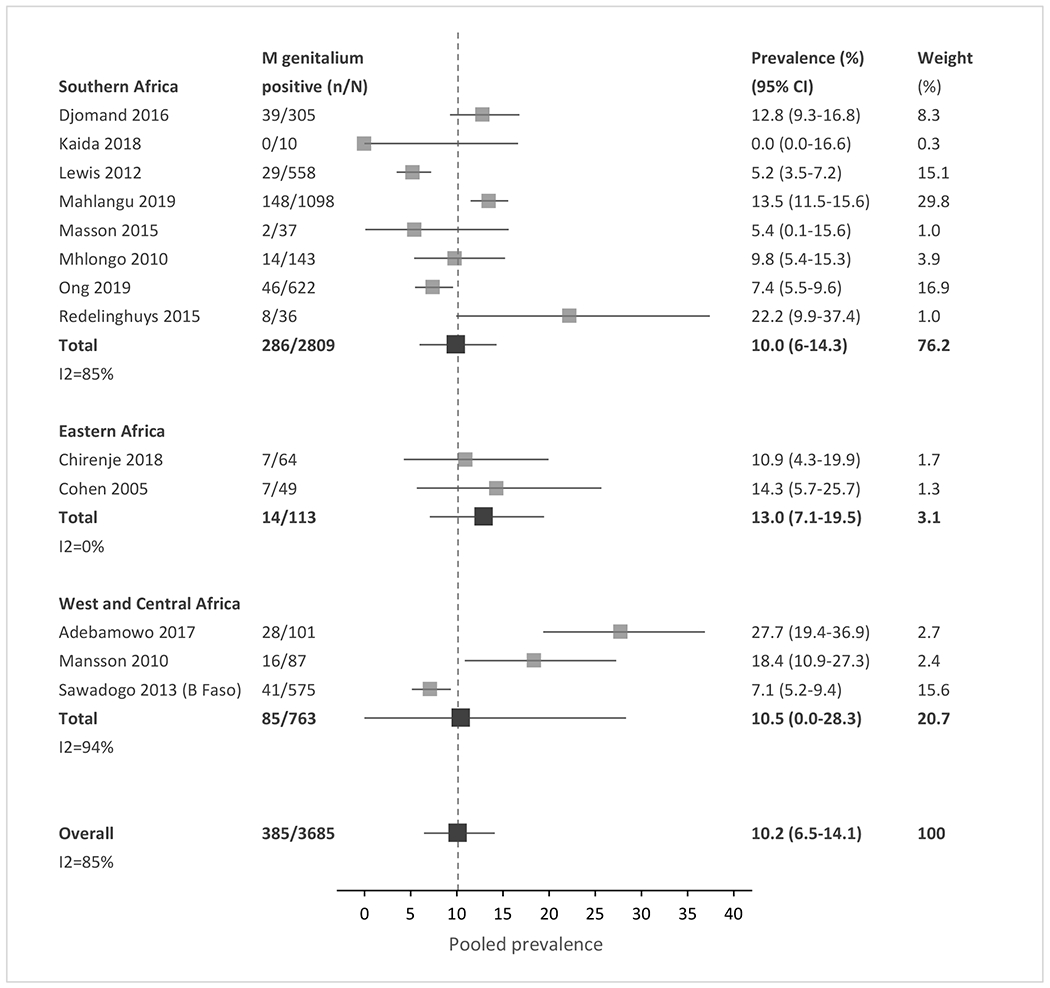
(A) Gonorrhea. (B) Chlamydia. (C) Trichomoniasis. (D) Mycoplasma genitalium. n, number of cases; N, number of women with HIV tested; CI, confidence interval. Sub-Saharan African regions were defined using the 2019 United Nations Statistical Division geographic region compositions.[31]
Table 2.
Summary results of subgroup analyses, by STI
| Number of studies | # women with STI / # tested | Pooled prevalence, % (95% CI) | I2, %‡ | p-value, meta-regression | |
|---|---|---|---|---|---|
| Gonorrhea | |||||
|
| |||||
| Region | 0.0504 | ||||
| Eastern Africa | 24 | 372/8987 | 3.18 (0.82-6.08) | 95 | |
| Southern Africa | 14 | 297/3824 | 7.34 (4.53-10.42) | 87 | |
| West/Central Africa | 5 | 29/572 | 4.10 (0-10.38) | 88 | |
|
| |||||
| Clinical setting | <0.001 | ||||
| General care | 16 | 196/4865 | 3.43 (1.41 – 5.79) | 91 | |
| STI clinic | 4 | 106/681 | 15.01 (4.62 – 27.20) | 90 | |
| Antenatal care | 12 | 175/4672 | 3.26 (0.39 – 7.00) | 91 | |
| Community-based | 5 | 161/1562 | 8.86 (0.83 – 19.33) | 97 | |
| Other* | 7 | 147/6475 | 2.08 (0 – 5.98) | 91 | |
|
| |||||
| Pregnancy | 0.17 | ||||
| Pregnant | 14 | 211/5159 | 3.58 (0.78 – 7.09) | 91 | |
| Not pregnant | 8 | 129/5918 | 2.10 (0 – 5.13) | 77 | |
|
| |||||
| Test type | 0.82 | ||||
| NAAT/PCR | 33 | 639/14 598 | 3.57 (0.99 – 6.71) | 95 | |
| Culture | 10 | 141/3530 | 3.18 (0 – 10.52) | 94 | |
| Other | 2 | 6/127 | 4.95 (0-17.41%) | 59 | |
|
| |||||
| Chlamydia | |||||
|
| |||||
| Region | 0.0015 | ||||
| Eastern Africa | 25 | 291/8876 | 2.76 (1.14 – 4.66) | 90 | |
| Southern Africa | 14 | 480/3439 | 12.54 (6.11-19.76) | 96 | |
| West/Central Africa | 5 | 95/437 | 19.11 (0-44.73) | 95 | |
|
| |||||
| Clinical setting | <0.001 | ||||
| General care | 19 | 192/5085 | 2.95 (0.93 – 5.38) | 92 | |
| STI clinic | 5 | 101/706 | 14.51 (11.96 – 17.15) | 0 | |
| Antenatal care | 9 | 336/3671 | 6.79 (0 – 24.50) | 98 | |
| Community-based | 6 | 126/1607 | 7.64 (4.19 – 11.48) | 81 | |
| Other* | 7 | 181/6416 | 2.12 (0 – 10.45) | 97 | |
|
| |||||
| Pregnancy | 0.049 | ||||
| Pregnant | 11 | 436/4150 | 8.07 (0 – 23.71) | 98 | |
| Not pregnant | 9 | 110/5915 | 1.74 (0 – 5.04) | 82 | |
|
| |||||
| Test type | 0.51 | ||||
| NAAT/PCR | 36 | 716/14 356 | 3.65 (0.47 – 7.78) | 96 | |
| EIA (antigen) | 9 | 220/3129 | 5.59 (0 – 21.99) | 97 | |
|
| |||||
| Trichomoniasis | |||||
|
| |||||
| Region | 0.62 | ||||
| Eastern Africa | 23 | 1268/8355 | 14.51 (9.34-20.09) | 94 | |
| Southern Africa | 17 | 1116/5621 | 18.97 (12.77-25.58) | 95 | |
| West/Central Africa | 9 | 200/1338 | 14.07 (7.29-21.60) | 90 | |
| Multiple | 2 | 881/6050 | 14.43 (5.69-24.43) | 98 | |
|
| |||||
| Clinical setting | <0.001 | ||||
| General care | 22 | 1036/7237 | 13.46 (8.93 – 18.32) | 95 | |
| STI clinic | 5 | 210/704 | 29.82 (22.56 – 37.33) | 62 | |
| Antenatal care | 14 | 915/5136 | 17.45 (11.25 – 24.12) | 90 | |
| Community-based | 4 | 291/975 | 29.70 (18.92 – 41.06) | 86 | |
| Other* | 6 | 758/6134 | 12.32 (7.86 – 17.16) | 76 | |
|
| |||||
| Pregnancy | 0.0012 | ||||
| Pregnant | 15 | 937/5214 | 17.60 (11.43 – 24.23) | 90 | |
| Not pregnant | 10 | 839/6683 | 12.31 (4.01 – 22.06) | 92 | |
|
| |||||
| Test type | 0.001 | ||||
| NAAT/PCR | 22 | 1412/8043 | 16.77 (11.77 – 22.08) | 95 | |
| Culture | 7 | 567/2235 | 24.96 (17.71 – 32.58) | 93 | |
| Microscopy | 19 | 705/4571 | 14.83 (7.24 – 23.32) | 90 | |
| Other | 3 | 781/6516 | 11.95 (8.28 – 15.87) | 88 | |
|
| |||||
| Mycoplasma genitalium † | |||||
|
| |||||
| Region | 0.48 | ||||
|
| |||||
| Eastern Africa | 2 | 14/113 | 12.97 (7.10-19.48) | 0 | |
|
| |||||
| Southern Africa | 8 | 286/2809 | 9.95 (5.98-14.32) | 85 | |
|
| |||||
| West/Central Africa | 3 | 85/763 | 10.47 (0-28.34) | 94 | |
|
| |||||
| Clinical setting | 0.49 | ||||
| General care | 6 | 292/2721 | 10.37 (5.30 – 16.07) | 92 | |
| STI clinic | 3 | 37/294 | 12.73 (7.73 – 18.20) | 43 | |
|
| |||||
| Pregnancy | n/a¶ | ||||
| Pregnant | 2 | 19/113 | 16.94 (10.12 – 24.94) | 9 | |
| Not pregnant | 4 | 61/373 | 15.96 (5.83 – 27.80) | 78 | |
Only subgroups with 2 or more studies were included. STI, sexually transmitted infection. CI, confidence interval. NAAT/PCR, nucleic acid amplification testing/polymerase chain reaction; EIA, enzyme immunoassay.
I2 >50% reflects significant between-study heterogeneity.
“Other” includes studies recruiting in multiple settings, family planning clinics, infertility clinics, labor& delivery ward, or unspecified settings.
Subgroup analysis on test type was not performed as all studies used NAAT/PCR to test for M genitalium.
Meta-regression not performed due to small number of studies per subgroup.
In meta-regression analyses, chlamydia prevalence in Southern Africa and West/Central Africa was similar (12.5% vs. 19.1%, p=0.5409), but the prevalence was lower in Eastern Africa (2.8%) than in the other two regions combined (p=0.015). The prevalence of other STIs was similar across regions (Fig. 2a–2d, Table 2). Prevalence was higher in STI clinics than in general care settings for gonorrhea (15.0% vs. 3.4%), chlamydia (14.5% vs. 3.0%), and trichomoniasis (29.8% vs. 13.5%, all p-values <0.0002). Mycoplasma genitalium prevalence did not differ significantly by clinical setting. Prevalence was higher among pregnant than non-pregnant women for chlamydia (8.1% vs. 1.7%, p=0.049) and trichomoniasis (17.6% vs. 12.3%, p=0.0012); gonorrhea and Mycoplasma genitalium prevalence did not differ by pregnancy status. For trichomoniasis, prevalence was higher when diagnosed by culture methods (25.0%) than by NAAT/PCR, microscopy, or other methods combined (p=0.028); prevalence did not differ by test type for the other STIs (Table 2). There was no significant difference in the prevalence of all four STIs over time (Fig. S2), or when the analysis compared studies with low risk of bias to studies with medium or high risk of bias (Table S4).
In comparative analyses, women with HIV had a higher prevalence of gonorrhea, chlamydia, trichomoniasis, and Mycoplasma genitalium than women without HIV. The relative risk of STI between women with and without HIV was 1.89 (95% CI, 1.48 – 2.41) for gonorrhea, 1.83 (1.13-2.95) for chlamydia, 1.54 (1.35 –1.75), for trichomoniasis, and 1.71 (1.05 – 2.78) for Mycoplasma genitalium (Table 3). Heterogeneity remained generally high with I2 values between 44% and 84%. Meta-regression analyses revealed that relative risks for gonorrhea and chlamydia among WWH compared to women without HIV were similar in Eastern Africa and Southern Africa (p=0.30 and p=0.97, respectively), but the relative risk was higher for both STIs in West/Central Africa than in Eastern and Southern Africa combined (p≤0.001, Table S5). Relative risks of STI among women with and without HIV did not differ by region for trichomoniasis or Mycoplasma genitalium. Relative risks for gonorrhea among WWH compared to women without HIV were similar in STI clinics, ANC, community, and other settings (p=0.0516), but higher in general care settings than in the other four settings combined (p=0.001). For trichomoniasis, the relative risk was higher in antenatal care than in the other four settings combined (p<0.001). Relative risk of trichomoniasis among women with HIV increased over time compared to women without HIV (p=0.03, Table S5).
Table 3.
Comparative analyses – relative risk of STIs among women with versus without HIV
| STI | Studies (n) | # women with STI/# tested (%) | Relative risk (95% CI) | I2, %‡ | |
|---|---|---|---|---|---|
| HIV+* | HIV− | ||||
| Gonorrhea | 24 | 448/8777 (5.10) | 390/19 334 (2.02) | 1.89 (1.48 – 2.41) | 44 |
| Chlamydia | 25 | 437/8106 (5.39) | 793/16 889 (4.70) | 1.83 (1.13 – 2.95) | 84 |
| Trichomoniasis | 31 | 2059/12 205 (16.87) | 1666/17 604 (9.46) | 1.54 (1.35 – 1.75) | 47 |
| Mycoplasma genitalium | 9 | 230/1625 (14.15) | 197/2660 (7.41) | 1.71 (1.05 – 2.78) | 57 |
These estimates include only prevalence from studies that provided data on a comparable cohort of women without HIV and therefore differ from pooled prevalence values.
I2 >50% reflects significant between-study heterogeneity.
Discussion
In this systematic review and meta-analysis, we found a substantial burden of STIs among women with HIV in SSA with an estimated prevalence of 3.5% for gonorrhea, 4.0% for chlamydia, 15.6% for trichomoniasis, and 10.2% for Mycoplasma genitalium. We additionally found that the prevalence of gonorrhea, chlamydia, trichomoniasis, and Mycoplasma genitalium was consistently higher among women with HIV than women without HIV. These findings highlight the need to include WWH as a priority group for epidemiologic surveillance and to integrate STI testing with HIV care for women disproportionately affected by these syndemic infections [13].
Compared to WHO 2016 African Region estimates of STI prevalence in the general population, we found a similar prevalence of chlamydia among WWH (4.0% vs. 5.0%), but a higher prevalence of gonorrhea (3.5% vs. 1.9%) and trichomoniasis (15.6% vs. 11.7%) [32]. These differences are consistent with our finding of moderately higher STI prevalence among WWH than women without HIV. Our prevalence estimates for gonorrhea and chlamydia are similar to those in a meta-analysis of STI prevalence among women aged 15-24 enrolled in HIV prevention trials in SSA [33] and suggests a significant burden of treatable infections among WWH in this region.
Subgroup analyses did not fully explain the between-study heterogeneity noted in our results, and prevalence estimates did not differ by study risk of bias. However, we did identify several patterns of STI prevalence among different settings and populations. Chlamydia was significantly less common in Eastern than in Southern and West/Central Africa, mirroring the results of a recent meta-analysis of STI prevalence among women at risk for HIV in SSA [33]. Contributors to this regional difference in STI prevalence are not clear based on the data we collected. Data on sexual risk behavior were scarce in the included studies, though previous studies have postulated that regional differences in rates of marriage or cohabitation may play a role [33,34]. Despite its substantial geographic size and population, we found that data on STI prevalence from West/Central Africa were scant, highlighting the need for further epidemiologic studies to better define STI prevalence in this region.
STI prevalence estimates further differed by clinical setting. Gonorrhea, chlamydia, and trichomoniasis were all more prevalent among women tested at STI clinics than women tested in general care. While not unexpected, this finding highlights the importance of screening for STI symptoms as part of routine HIV care for WWH, who are already engaged with the healthcare system and used to providing laboratory samples for monitoring. Similarly, diagnostic testing for WWH presenting with STI symptoms could have substantial impacts on clinical management. The current approach to STI treatment in most of SSA consists of syndromic management, which lacks sensitivity and specificity [19] and may contribute to rising antibiotic resistance to gonorrhea worldwide [35]. With prevalence estimates for the four STIs ranging from 12% to 28% among symptomatic women, diagnostic testing could yield actionable results among a substantial proportion and allow pathogen-specific management rather than a broad, empiric approach. While prevalence was higher in STI clinics than in general care settings for most of the STIs studied, the substantial prevalence of all four STIs among women recruited at general care sites also underscores the need for increased access to STI screening among asymptomatic women and within routine HIV care settings.
STI screening of asymptomatic WWH may be particularly high-yield in antenatal care settings. Chlamydia and trichomoniasis were more common among pregnant than non-pregnant women, and prevalence of all four STIs was high among women tested in antenatal care, ranging from 3.3% for gonorrhea to 17.5% for trichomoniasis. This finding is of particular clinical importance given the additional risks of untreated STIs in pregnancy, including adverse birth outcomes (e.g., stillbirth, pre-term delivery, and small for gestational age) [11,12], congenital infection, and increased risk of vertical transmission of HIV [4,5]. Empiric symptom-driven treatment also risks exposing the fetus to antibiotics unnecessarily. Thus, our study highlights antenatal care as an important opportunity for STI screening and pathogen-targeted treatment. Pregnancy is a time during which women are often more likely to seek care and when the consequences of both under- and over-treatment of STIs are greater.
In this analysis, we found that women with HIV had a 1.54 to 1.89-fold higher prevalence of STIs than comparable cohorts of women without HIV. There are many potential contributors to this association. Untreated STIs are known to increase the risk of HIV acquisition [2,3]. Additionally, HIV and other STIs share multiple behavioral and socioeconomic risk factors [3,36], including poverty and gender inequity. It remains unclear whether biological factors such as STI-related perturbations of the vaginal microbiome [37,38] could also contribute to increased HIV acquisition. This finding supports the WHO recommendation for STI services as an essential component of HIV care [8,39] and the stance that women with HIV are among priority populations for STI management [9]. Our study suggests that the yield of testing is likely to be higher in this population, and the beneficial effects of treatment-both to reduce morbidity and decrease risk of HIV transmission-could be more pronounced.
Overall, the high prevalence of STIs among WWH in SSA underscores the potential impact of integration of HIV care and sexual and reproductive health services as advised by the WHO [9,40]. A recent meta-analysis revealed a scarcity of data on clinical outcomes for such integration in resource-limited settings [41], however, a qualitative assessment in Kenya demonstrated interest among healthcare providers in delivering STI management within HIV care [42]. Expansion of STI screening in general care settings for WWH will need to address structural and societal barriers beyond the need for accessible diagnostic tests. STI screening will require additional clinic space and staff time for specimen collection and processing and may prolong clinic visits. The decentralized nature of HIV care means that laboratory services may not be available at sites of HIV care; increasing access to novel, rapid, point-of-care STI tests is crucial to successful HIV and sexual and reproductive health service integration. STI testing may also introduce additional stigma, compounding existing HIV-related stigma, and leading to reluctance to test or access services. However, given the potential public health benefits of STI screening for this population with high STI prevalence, additional investment is warranted to overcome these barriers and address this important health problem.
There are several limitations to this systematic review and meta-analysis. First, we identified substantial gaps in current understanding of STI epidemiology in SSA, with a relative dearth of data from West/Central Africa. High-quality, population-level prevalence studies are needed to better define the burden of STIs among WWH in a wider range of SSA regions and contexts. Second, most references included in our analysis did not specify whether participants were symptomatic or asymptomatic. Thus, studies that recruited from symptomatic populations without indicating this history may have skewed the prevalence estimates higher than would be expected from screening the broader population of WWH. However, in our subgroup analyses, we found that STI prevalence remained high among WWH who were reported to be asymptomatic and who were tested at clinical sites outside of STI clinics. Similarly, some ‘average risk’ women with HIV may still engage in transactional sex without identifying as a female sex worker, and other disparities contributing to risk of HIV and STIs may be understated within the limitations of a meta-analysis. Further, most included studies were conducted in settings where syndromic management was the local standard of care, which may differ systematically from settings where pathogen-specific management is available. Lastly, we could not account for the influence of patient age on STI prevalence since few studies disaggregated data by age. It is worth noting, however, that previous studies of STI prevalence among women in SSA have found higher prevalence in younger women [33].
Our results demonstrate the high prevalence of gonorrhea, chlamydia, trichomoniasis, and Mycoplasma genitalium among women with HIV in SSA, revealing an enormous burden of STIs in this population. Women with HIV, particularly those who are symptomatic or pregnant, should be a priority population for STI testing due to increased prevalence and increased risk of complications in these groups. Effective integration of STI and HIV services could help to reduce morbidity among women with HIV and decrease HIV transmission.
Supplementary Material
Table S1. Pubmed Search strategy
Table S2. Risk of bias assessment tool
Table S3. Included studies of STI prevalence, by region
Table S4. Additional pooled prevalence subgroup analyses
Table S5. Comparative analyses by subgroup
Figure S1. Included studies by location and study size
Figure S2. STI prevalence by midyear of study
Figure S3a-d: Forest plots of comparative analyses, by STI
Acknowledgements:
We thank Alyssa Amick, Ogochukwu Ufio, Nicholas Errol Rahim, and Clare Flanagan for assistance with proofreading and data validation of the manuscript. We additionally thank Fatma Shebl for providing guidance on the use of MetaXL and associated meta-analytic methods.
Conflicts of Interest and Sources of Funding:
This work was supported by the National Institutes of Health through the National Institute of Allergy and Infectious Disease [T32AI007433 (JJ, LRP), K24AI141036 (IVB)], the Eunice Kennedy Shriver National Institute of Child Health and Human Development [R21HD100821 (CM, AM), R01HD079214 (ALC), K08HD101342 (CMD)], the National Institute of Mental Health R01MH114560 (MS), and the Harvard University Center for AIDS Research (CFAR), an NIH funded program [P30AI060354 (RAP)], which is supported by the following NIH Co-Funding and Participating Institutes and centers: NIAID, NCI, NICHD, NIDCR, NHLBI, NIDA, NIMH, NIA, NIDDK, NINR, NIMHD, FIC, and OAR. This work was also supported by the Bill & Melinda Gates Foundation [INV-022665 (MS)], the Wellcome Trust [082384/Z/07/Z (MS)], and the Weissman Family MGH Research Scholar Award (IVB). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or the other funders. All authors declare no conflicts of interest.
References
- 1.Johnson LF, Lewis DA. The effect of genital tract infections on HIV-1 shedding in the genital tract: a systematic review and meta-analysis. Sex Transm Dis 2008; 35:946–959. [DOI] [PubMed] [Google Scholar]
- 2.Sexton J, Garnett G, Rottingen J-A. Metaanalysis and metaregression in interpreting study variability in the impact of sexually transmitted diseases on susceptibility to HIV infection. Sex Transm Dis 2005; 32:351–357. [DOI] [PubMed] [Google Scholar]
- 3.Hayes R, Watson-Jones D, Celum C, van de Wijgert J, Wasserheit J. Treatment of sexually transmitted infections for HIV prevention: end of the road or new beginning? AIDS 2010; 24:S15–S26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Adachi K, Xu J, Yeganeh N, Camarca M, Morgado MG, Watts DH, et al. Combined evaluation of sexually transmitted infections in HIV-infected pregnant women and infant HIV transmission. PLoS ONE 2018; 13:e0189851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fawzi W a, Msamanga G e, Renjifo B d, Spiegelman D b, Urassa E f, Hashemi L a, et al. Predictors of intrauterine and intrapartum transmission of HIV-1 among Tanzanian women. AIDS 2001; 15:1157–1165. [DOI] [PubMed] [Google Scholar]
- 6.Venkatesh KK, van der Straten A, Cheng H, Montgomery ET, Lurie MN, Chipato T, et al. The relative contribution of viral and bacterial sexually transmitted infections on HIV acquisition in southern African women in the Methods for Improving Reproductive Health in Africa study. Int J STD AIDS 2011; 22:218–224. [DOI] [PubMed] [Google Scholar]
- 7.Wall KM, Karita E, Nyombayire J, Ingabire R, Mukamuyango J, Parker R, et al. Genital Abnormalities, Hormonal Contraception, and Human Immunodeficiency Virus Transmission Risk in Rwandan Serodifferent Couples. J Infect Dis 2021; 224:81–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.World Health Organization. Essential prevention and care interventions for adults and adolescents living with HIV in resource-limited settings. Geneva: World Health Organization; 2008. [Google Scholar]
- 9.World Health Organization. Global Health Sector Strategy on Sexually Transmitted Infections 2016-2021. Geneva, Switzerland: ; 2016. [Google Scholar]
- 10.World Health Organization. Report on global sexually transmitted infection surveillance, 2018. Geneva, Switzerland: ; 2018. https://apps.who.int/iris/bitstream/handle/10665/277258/9789241565691-eng.pdf?ua=1 (accessed 8 Dec2020). [Google Scholar]
- 11.Liu B, Roberts CL, Clarke M, Jorm L, Hunt J, Ward J. Chlamydia and gonorrhoea infections and the risk of adverse obstetric outcomes: a retrospective cohort study. Sex Transm Infect 2013; 89:672–678. [DOI] [PubMed] [Google Scholar]
- 12.Warr AJ, Pintye J, Kinuthia J, Drake AL, Unger JA, McClelland RS, et al. Sexually transmitted infections during pregnancy and subsequent risk of stillbirth and infant mortality in Kenya: a prospective study. Sex Transm Infect 2019; 95:60–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.World Health Organization. Guidelines for the management of symptomatic sexually transmitted infections. Geneva: World Health Organization; 2021. https://apps.who.int/iris/handle/10665/342523 (accessed 30 Oct2021). [PubMed] [Google Scholar]
- 14.Bekker L-G. Future STI & HIV Care in Africa: Best practices & future care of sexual health. 2018. http://programme.aids2018.org/Programme/Session/169 (accessed 19 Feb2021). [Google Scholar]
- 15.South Africa Department of Health. Sexually Transmitted Infections Management Guidelines 2018. Pretoria, South Africa: ; 2018. [Google Scholar]
- 16.Garrett NJ, McGrath N, Mindel A. Advancing STI care in low/middle-income countries: has STI syndromic management reached its use-by date? Sex Transm Infect 2017; 93:4–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chitneni P, Matthews LT. The Other U=U. Untested and Untreated genital tract inflammation in people living with and exposed to HIV. J Infect Dis 2021; 224:1–4. [DOI] [PubMed] [Google Scholar]
- 18.World Health Organization. WHO | Training modules for the syndromic management of sexually transmitted infections. WHO. 2007. https://apps.who.int/iris/bitstream/handle/10665/43275/9241593407_mod2_eng.pdf?sequence=3 (accessed 22 Feb2021). [Google Scholar]
- 19.Zemouri C, Wi TE, Kiarie J, Seuc A, Mogasale V, Latif A, et al. The performance of the vaginal discharge syndromic management in treating vaginal and cervical infection: a systematic review and meta-analysis. PLOS ONE 2016; 11:e0163365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Korenromp E, Sudaryo M, de Vlas S, Gray R, Sewankambo N, Serwadda D, et al. What proportion of episodes of gonorrhoea and chlamydia becomes symptomatic? Int J STD AIDS 2002; 13:91–101. [DOI] [PubMed] [Google Scholar]
- 21.Farley TA, Cohen DA, Elkins W. Asymptomatic sexually transmitted diseases: the case for screening. Prev Med 2003; 36:502–509. [DOI] [PubMed] [Google Scholar]
- 22.Otieno FO, Ndivo R, Oswago S, Ondiek J, Pals S, McLellan-Lemal E, et al. Evaluation of syndromic management of sexually transmitted infections within the Kisumu Incidence Cohort Study. Int J STD AIDS 2014; 25:851–859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mlisana K, Naicker N, Werner L, Roberts L, van Loggerenberg F, Baxter C, et al. Symptomatic vaginal discharge is a poor predictor of sexually transmitted infections and genital tract inflammation in high-risk women in South Africa. J Infect Dis 2012; 206:6–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Francis SC, Mthiyane TN, Baisley K, Mchunu SL, Ferguson JB, Smit T, et al. Prevalence of sexually transmitted infections among young people in South Africa: A nested survey in a health and demographic surveillance site. PLOS Med 2018; 15:e1002512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Murtagh M The Point-of-Care Diagnostic Landscape for Sexually Transmitted Infections (STIs). ; 2019. https://www.who.int/reproductivehealth/topics/rtis/Diagnostic-Landscape-for-STIs-2019.pdf (accessed 1 Mar2021).
- 26.Kalichman SC, Pellowski J, Turner C. Prevalence of sexually transmitted co-infections in people living with HIV/AIDS: systematic review with implications for using HIV treatments for prevention. Sex Transm Infect 2011; 87:183–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.UNAIDS. Global HIV & AIDS statistics — 2020 fact sheet. https://www.unaids.org/en/resources/fact-sheet (accessed 1 Nov2021).
- 28.Hoy D, Brooks P, Woolf A, Blyth F, March L, Bain C, et al. Assessing risk of bias in prevalence studies: modification of an existing tool and evidence of interrater agreement. J Clin Epidemiol 2012; 65:934–939. [DOI] [PubMed] [Google Scholar]
- 29.Doi SAR, Barendregt JJ, Khan S, Thalib L, Williams GM. Advances in the meta-analysis of heterogeneous clinical trials I: The inverse variance heterogeneity model. Contemp Clin Trials 2015; 45:130–138. [DOI] [PubMed] [Google Scholar]
- 30.Doi SAR, Barendregt JJ, Khan S, Thalib L, Williams GM. Simulation Comparison of the Quality Effects and Random Effects Methods of Meta-analysis. [Letter]. Epidemiology 2015; 26. doi: 10.1097/EDE.0000000000000289 [DOI] [PubMed] [Google Scholar]
- 31.United Nations Statistics Division. Methodology: Standard country or area codes for statiscical use (M49): Geographic Regions. https://unstats.un.org/unsd/methodology/m49/ (accessed 7 Dec2020).
- 32.Rowley J, Vander Hoorn S, Korenromp E, Low N, Unemo M, Abu-Raddad LJ, et al. Chlamydia, gonorrhoea, trichomoniasis and syphilis: global prevalence and incidence estimates, 2016. Bull World Health Organ 2019; 97:548–562P. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Torrone EA, Morrison CS, Chen P-L, Kwok C, Francis SC, Hayes RJ, et al. Prevalence of sexually transmitted infections and bacterial vaginosis among women in sub-Saharan Africa: An individual participant data meta-analysis of 18 HIV prevention studies. PLOS Med 2018; 15:e1002511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Marston M, Slaymaker E, Cremin I, Floyd S, McGrath N, Kasamba I, et al. Trends in marriage and time spent single in sub-Saharan Africa: a comparative analysis of six population-based cohort studies and nine Demographic and Health Surveys. Sex Transm Infect 2009; 85:i64–i71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.World Health Organization, World Health Organization, Reproductive Health and Research. Global action plan to control the spread and impact of antimicrobial resistance in Neisseria gonorrhoeae. Geneva, Switzerland: World Health Organization; 2012. http://apps.who.int/iris/bitstream/10665/44863/1/9789241503501_eng.pdf (accessed 22 Jan2021). [Google Scholar]
- 36.Rottingen J-A, William Cameron D, Garnett GP. A systematic review of the epidemiologic interactions between classic sexually transmitted diseases and HIV: how much really is known? Sex Transm Dis 2001; 28:579–597. [DOI] [PubMed] [Google Scholar]
- 37.Chehoud C, Stieh DJ, Bailey AG, Laughlin AL, Allen SA, McCotter KL, et al. Associations of the vaginal microbiota with HIV infection, bacterial vaginosis and demographic factors. AIDS Lond Engl 2017; 31:895–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Price JT, Vwalika B, Hobbs M, Nelson JAE, Stringer EM, Zou F, et al. Highly diverse anaerobe-predominant vaginal microbiota among HIV-infected pregnant women in Zambia. PLoS ONE 2019; 14:e0223128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Consolidated Guidelines on the Use of Antiretroviral Drugs for Treating and Preventing Hiv Infection: Recommendations for a Public Health Approach. ; 2016. https://www.deslibris.ca/ID/10089566 (accessed 19 Feb2021). [PubMed]
- 40.World Health Organization, Special Programme of Research D and Research Training in Human Reproduction (World Health Organization), Joint United Nations Programme on HIV/AIDS, United Nations Fund for Population Activities, United Nations, Office of the High Commissioner for Human Rights. Consolidated guideline on sexual and reproductive health and rights of women living with HIV. ; 2017. https://public.ebookcentral.proquest.com/choice/publicfullrecord.aspx?p=5910085 (accessed 9 Dec2020). [Google Scholar]
- 41.Kennedy CE, Haberlen SA, Narasimhan M. Integration of sexually transmitted infection (STI) services into HIV care and treatment services for women living with HIV: a systematic review. BMJ Open 2017; 7:e015310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chesang K, Hornston S, Muhenje O, Saliku T, Mirjahangir J, Viitanen A, et al. Healthcare provider perspectives on managing sexually transmitted infections in HIV care settings in Kenya: A qualitative thematic analysis. PLOS Med 2017; 14:e1002480. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Pubmed Search strategy
Table S2. Risk of bias assessment tool
Table S3. Included studies of STI prevalence, by region
Table S4. Additional pooled prevalence subgroup analyses
Table S5. Comparative analyses by subgroup
Figure S1. Included studies by location and study size
Figure S2. STI prevalence by midyear of study
Figure S3a-d: Forest plots of comparative analyses, by STI


