Abstract
Objective:
Consumption of a Western diet during adolescence results in hippocampus (HPC)-dependent memory impairments and gut microbiome dysbiosis. Whether these adverse outcomes persist in adulthood following intervention with a healthy diet is unknown. Here we assessed the short- and long-term effects of adolescent consumption of a Western diet enriched with either sugar alone, or sugar and fat on metabolic outcomes, HPC-dependent memory, and gut microbiota.
Methods:
Adolescent female rats (PN 26) were fed a healthy standard chow diet (CHOW), a chow diet with access to 11% sugar solution (SUG), or a junk food cafeteria-style diet (CAF) containing a variety of foods high in fat and/or sugar. During adulthood (PN 65+), metabolic outcomes, HPC-dependent memory, and gut microbial populations were evaluated. In a subsequent experiment, the same outcomes were evaluated following a 5-week dietary intervention (PN 100+) where the CAF and SUG groups were maintained on water and standard chow.
Results:
When examined after the adolescent diet treatments, both CAF and SUG groups demonstrated impaired HPC-dependent memory, increased adiposity, and altered gut microbial populations relative to the CHOW group. However, impaired peripheral glucose regulation was only observed in the SUG group. In another experiment, when examined following dietary intervention, metabolic dysfunction was not observed in either the CAF or SUG groups, whereas HPC-dependent memory impairments were observed in the CAF group but not the SUG group. The composition of the gut microbiota was distinct from chow-fed rats in both groups after the healthy dietary intervention.
Conclusions:
While the metabolic impairments associated with adolescent cafeteria diet consumption are not present in adulthood following dietary intervention, the HPC-dependent memory impairments and the gut microbiome dysbiosis persist.
Keywords: Obesity, hippocampus, microbiota, diet, high-fat, sugars
1. Introduction
Globalization has brought technological advances in food processing, shelf-life, marketing, and distribution practices have increased the availability of low-cost palatable foods that have a high percentage of calories derived from saturated fat and sugar. The increased prevalence and accessibility of high fat, high sugar foods, herein referred to as a Western diet (WD), has directly impacted the diet quality of U.S. citizens, particularly children, as U.S. children on average exceed the recommended guidelines for consumption of sugars and fats before they reach school age (Wang et al., 2018). Furthermore, in U.S. children aged 2 to 19 years, ultra-processed foods rich in lipids and added sugars contribute to ~65% of total energy intake (Neri et al., 2019). Indeed, the declining quality in the WD is likely contributing to the increasing rates of childhood obesity as consumption of ultra-processed foods in children is positively associated with body fat (Wang et al., 2015; Costa et al., 2018; Gallagher et al., 2020) and increased risk for metabolic syndrome (Ambrosini et al., 2010; Mager et al., 2013).
In addition to adverse metabolic outcomes, WD exposure during early life periods of development adversely impacts neurocognitive development (Francis and Stevenson, 2013; Noble and Kanoski, 2016; Reichelt, 2016; Tsan et al., 2021). For example, habitual added sugar consumption in children is associated with altered hippocampus (HPC) volume and HPC-cortical connectivity (Clark et al., 2020), whereas habitual saturated fatty acid intake is negatively associated with HPC-dependent relational memory (Baym et al., 2014). Rodent studies also reveal impaired HPC-dependent learning and memory associated with early life consumption of either a WD or excessive sugar consumption without elevated fat intake, even in cases where the WD (or sugar) consumption is not accompanied by metabolic dysfunction and/or obesity (Kendig et al., 2013; Hsu et al., 2015; Ferreira et al., 2018; Noble et al., 2019). The overwhelming majority of these rodent model studies tested memory performance during adulthood while the animals were still being maintained on the WD or the high sugar diet. While memory impairments associated with WD consumption in male rats can be reversed with intervention, such as exercise (Noble et al., 2014), very little is understood about whether these early life WD-induced memory impairments can be remediated with healthy dietary intervention during adulthood, with one study finding that removing access to a sugar-sweetened beverage solution for 4 months in adulthood after prolonged access in adolescence with an otherwise standard rodent diet did not reverse sugar-sweetened beverage-induced HPC-dependent memory impairment in male rats (Noble et al., 2019). Additionally, few studies have specifically investigated the effects of healthy dietary intervention on diet-induced cognitive dysfunction in females.
Emerging evidence suggests that the gut microbiome influences cognitive function (Noble et al., 2017b; Provensi et al., 2019; Leigh et al., 2020; Darch et al., 2021; Noble et al., 2021) and that this relationship may be dependent on early life nutrition (Cerdó et al., 2019). For example, excessive early life sugar consumption in male rats yields HPC-dependent memory impairments (Hsu et al., 2015), an outcome that was recently connected functionally to robust changes in the microbiome relative to chow-fed controls (Noble et al., 2021). In another study, transplanting the microbiota of male mice that received an early life high fat diet to chow-fed mice conferred HPC-dependent learning and memory deficits, suggesting a possible functional connection between microbiota composition and HPC-dependent learning and memory (Yang et al., 2019). However, as is the case with WD-associated memory impairments, it is poorly understood whether microbiome outcomes linked to early life WD consumption can be reversed with healthy dietary intervention. In humans, after the first 3 years of life an estimated 60-70% of the microbiota composition remains relatively stable, yet ~30-40% may be more susceptible to changes induced by diet or other factors (Kashtanova et al., 2016). The extent to which microbiome changes induced by dietary factors during early life developmental periods are long-lasting vs. reversible when the habitual diet changes during adulthood is unknown.
In the present study, we evaluate, neurocognitive (HPC-dependent memory, anxiety-like behavior), metabolic (glucose tolerance, body weight, adiposity ratio, caloric intake), and gut microbiome outcomes in female rats maintained on either a standard chow diet (CHOW; free access to water and a healthy standard low-fat rat chow) or one of two different WD models during the entire adolescent period of development: 1) a junk food cafeteria-style diet (CAF) group, with free choice access to water, a rodent high-fat diet, an 11% carbohydrate weight-by-volume (w/v) high fructose corn syrup (HFCS) solution, potato chips, and chocolate peanut butter cups, or 2) a group with access to water, standard low-fat rat chow, and the 11% w/v HFCS solution (SUG). In order to explore possible links between gut microbiota and memory outcomes, linear regression analyses were conducted to determine the relationship between bacterial taxa abundancies and HPC-dependent memory performance.
2. Materials and methods
2.1. Subjects and Diets
Adolescent (postnatal day [PN] 26) female Sprague Dawley rats (n=101, Envigo; 50-70g) were housed individually in a climate-controlled (22–24 °C) environment with a 12:12 reverse light/dark cycle and maintained on standard chow (Lab Diet 5001; PMI Nutrition International, Brentwood, MO, USA; 29.8 % kcal from protein, 13.4% kcal from fat, 56.7% kcal from carbohydrate) and water unless otherwise stated. All experiments were approved by the Animal Care and Use Committee at the University of Southern California and performed in accordance with the National Research Council Guide for the Care and Use of Laboratory Animals.
2.2. Experiment 1 Design (Junk Food-Style Cafeteria Diet)
At PN 26, rats were randomized to one of two groups (matched for body weight) and received ad libitum access to either [1] standard chow and water (chow-fed [CHOW] group, n=10), or [2] a cafeteria (CAF) diet, with free access to potato chips (Ruffles), peanut butter cups (Reese’s minis), 45% kcal high fat/sucrose chow (Research Diets D12451, New Brunswick, NJ, USA), a bottle of 11% weight/volume (w/v) high fructose corn syrup (HFCS)-55 solution (Best Flavors, Orange, CA, USA), and water (CAF group, n=10). The concentration of sugar (11% w/v) was selected to model the amount of sugar present in sugar-sweetened beverages commonly consumed by humans, and is also based on our prior studies in male rats (Hsu et al., 2015; Noble et al., 2019). Three food hoppers were used in each cage for precise measure of individual solid food types (in CAF group) or for consistency across experimental groups (in CHOW group, all hoppers filled with standard chow). Papers were placed underneath the cages to enable the collection and recording of food spillage. Body weights and food intake were measured 3x/week throughout the study.
Experiment 1A:
At PN 61 (young adulthood), testing began for the Novel Object in Context (NOIC) task, which measures hippocampal-dependent episodic/contextual memory (Martínez et al., 2014). Anxiety-like behavior was assessed using a Zero Maze at PN 67. Fecal samples for analyses of gut microbiota were collected at PN 70 (Timepoint A, n=10/group). Body composition was measured using nuclear magnetic resonance (NMR) spectroscopy at PN 74 and an intraperitoneal glucose tolerance test (IPGTT) was conducted at PN 78. At PN 79, animals in CAF were switched to a standard chow diet with water access for the remainder of the study as a dietary intervention. Body composition was reevaluated at PN 115.
Experiment 1B:
A second cohort of female rats (n=31, 50-70g) was treated as described above in order to collect behavioral data following the dietary intervention. These rats were tested on NOIC after 5 weeks of dietary intervention (chow and water only) at PN 101 and Zero Maze at PN 108 (Figure 1B), with the interventional timeframe being similarly adapted from other studies that evaluated the metabolic and neurocognitive effects of WD consumption after dietary intervention (Gomez-Smith et al., 2016; Kruse et al., 2019). Fecal samples for microbiota analyses were collected after the dietary intervention at PN 106 (n=15 CHOW group, n=16 CAF group). Caloric intake and body weight were recorded 3x/week and IPGTT conducted while still on the diet (PN 66).
Figure 1: Timeline of experiments.
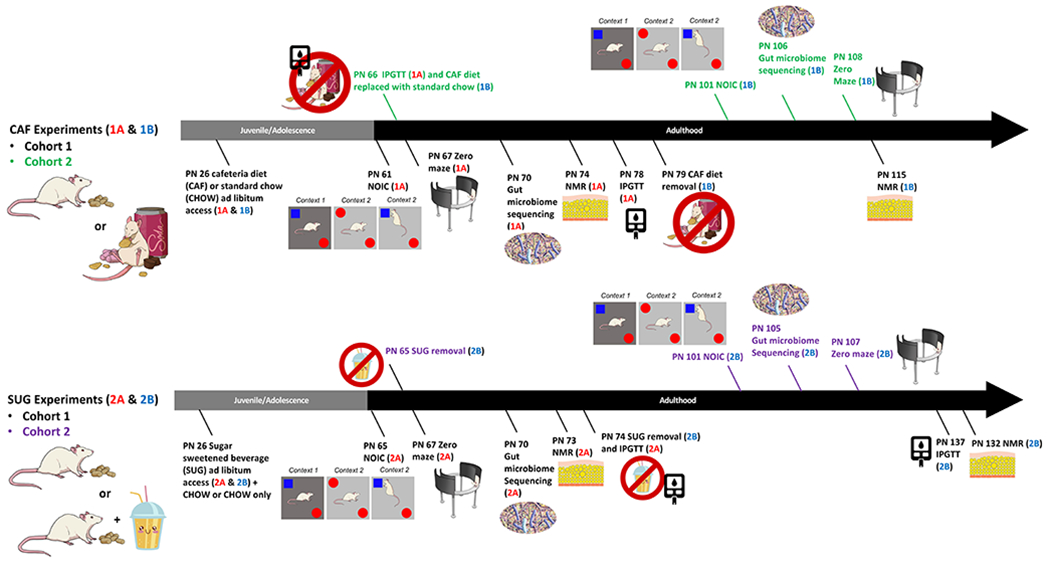
During the adolescent period of development, rats were exposed to either a junk food-style cafeteria diet (top) or an otherwise healthy diet with access to a sugar-sweetened beverage (bottom). Metabolic, cognitive, and gut microbiome outcomes were evaluated either before (Experiments 1A and 2A) or after (Experiments 1B and 2B) a healthy dietary intervention (access to standard chow and water only) during early adulthood. PN: postnatal day, CAF: cafeteria diet, SUG: sugar diet, NOIC: novel object in context, NMR: nuclear magnetic resonance imaging spectroscopy, IPGTT: intraperitoneal glucose tolerance test
2.3. Experiment 2 Design (Sugar-Sweetened Beverage)
At PN 26, rats were randomized to two groups matched for body weight and were given ad libitum access to standard chow and either: [1] 11% weight-by-volume (w/v) sugar-sweetened beverage solution (high fructose corn syrup type 55; Best Flavors, Orange, CA, USA) diluted in water (SUG; n=16) or [2] a second bottle of water (CHOW; n=16).
Experiment 2A:
Episodic memory was tested during young adulthood at PN 65 using the hippocampal-dependent NOIC task. At PN 67, rats were then tested for anxiety-like behavior using the Zero Maze. Fecal samples for microbiota analyses following sugar-sweetened beverage exposure (n=16/group) were collected at PN 70. At PN 73, body composition using NMR was assessed. At PN 74, rodents underwent an IPGTT, and the dietary intervention where sugar solutions were removed was initiated. Post-dietary intervention body composition was measured at PN 132 and IPGTT was conducted at PN 134 (n=8/group; Figure 1C).
Experiment 2B:
A second cohort of juvenile (PN 25) female Sprague Dawley rats (n=18, 50-70g) were treated as described above for post dietary intervention behavioral testing. These rats were tested on NOIC after dietary intervention after 5 weeks of chow maintenance at PN 101 and Zero Maze at PN 107 (Figure 1D). Fecal samples for microbiome analysis were collected after dietary intervention at PN 105 (n=9/group). Caloric intakes and body weights were recorded in this group as described above.
General Procedures
2.4. Novel Object in Context
The Novel Object in Context (NOIC) task, which measures contextual episodic memory, was adapted from previous reports (Balderas et al., 2008; Martínez et al., 2014). Rats are habituated on consecutive days to both Context 1, a semi-transparent box (15in W × 24in L × 12in H) with yellow stripes, and Context 2, a grey opaque box (17in W × 17in L × 16in H) with the order counterbalanced between groups. Following the two habituation days, on the next day (Day 1 of NOIC), each animal is placed in Context 1 and allowed to explore two different objects (Object A and Object B) placed on diagonal, equidistant markings with ample space for the rat to circle the objects. The markings on which each object is placed are counterbalanced within each group. Objects used were either: a magic eight ball paired with a pyramid-shaped Lego object or a 12 oz. soda can (Coca-Cola®) paired with an upside-down stemless transparent wine glass. On the following day (NOIC day 2), rats are placed in Context 2 with Object A and a duplicate of Object A, one on each marking. On the subsequent day (test day; NOIC day 3), rats are placed again in Context 2 with Object A and Object B (which is not a novel object per se, but its presence in Context 2 is novel). Sessions are 5 minutes long and conducted under diffuse lighting conditions and are video recorded using an overhead camera (Digital USB 2.0 CMOS Camera with Vari-focal, 2.8-12mm lens; Stöelting) connected to a computer licensed with Any-Maze software (Stöelting, Wood Dale, IL, USA), which objectively tracks the time spent investigating each object based on a programmed template. The discrimination index for the novel object is calculated as follows: (Time spent exploring Object B/ [Time spent exploring Object A + Time spent exploring Object B]). The shift from baseline exploration of Object B is then calculated by subtracting the discrimination indices of NOIC day 1 (baseline) from NOIC day 3 (test day) and then multiplying by 100 to calculate percent shift from baseline. Intact rats typically preferentially explore Object B on NOIC day 3 due to its novelty in that particular context, an effect that is disrupted with hippocampal inactivation (Martínez et al., 2014), or by early life sugar consumption in male rats (Noble et al., 2019).
2.5. Zero Maze
The Zero Maze test was utilized to measure anxiety-like behavior (Shepherd et al., 1994). The maze consists of an elevated circular platform (63.5 cm height, 116.8 cm external diameter) with two closed zones and two open zones, all of which are equal in length. The closed zones are enclosed with 17.5 cm high walls whereas the open zones have only 3 cm high curbs. Animals are placed in the maze for a single 5-min session, and the apparatus is cleaned with 10% ethanol in between animals. Any-Maze software scripts (Stoelting Co., Wood Dale, IL, USA) are used to record videos and analyze the following parameters: time spent in the open zones and number of entries into the open zones.
2.4. Body Composition using NMR
Rats are food restricted for one hour prior to being weighed and scanned for body composition as previously described (Noble et al., 2017a) using the Bruker NMR Minispec LF 90II (Bruker Daltonics, Inc., Billerica, MA, USA), which is based on Time Domain NMR signals from all protons and has the benefit of being non-invasive and requiring no anesthesia. Percent body fat is calculated as [fat mass (g)/body weight (g)] x 100.
2.5. Intraperitoneal Glucose Tolerance Test
An intraperitoneal glucose tolerance test (IPGTT) was administered to estimate peripheral insulin sensitivity. Rats are food restricted for 22 hours prior to IPGTT. Baseline blood glucose readings are collected from blood sampled from the tip of the tail and measured using a glucometer (One touch Ultra2, LifeScan Inc., Milpitas, CA, USA). Each rat receives an intraperitoneal injection of a 50% dextrose solution (0.5g/kg body weight) and blood glucose readings are obtained from the tail snip at the 0 (immediately before injection), 30, 60, 90, and 120 min timepoints following injection.
2.6. Fecal Sample Collection and 16s Ribosomal RNA (rRNA) Gene Sequencing
Rats were placed in a sterile cage with no bedding and were mildly restrained until defecation occurred. Two fecal samples were collected per animal. Samples were weighed under sterile conditions and then placed into a DNA/RNA free 2 mL cryogenic vial embedded in dry ice. Samples were then stored in a −80°C freezer until processing. The cages and all materials used to collect samples were cleaned with 70% ethanol in between rats. Bacterial genomic DNA was extracted from rat fecal samples using Laragen’s validated in-house fecal DNA extraction protocol. Quantification of 16S rRNA gene loads was performed by qPCR using the SYBR Green master mix (Roche, Basel, Switzerland), universal 16S rRNA gene primers55 and the QuantStudio 5 thermocycler (cycling parameters: 2 min at 50 °C, 10 min at 95 °C, 40 cycles of 10 s at 95 °C, and 45 s at 62 °C). Sequencing libraries were generated using methods previously described (Caporaso et al., 2011). The V4 regions of the 16S rRNA gene were PCR amplified using individually barcoded universal primers and 3 ng of the extracted genomic DNA. The PCR reaction was set up in a single reaction, and the PCR product was purified using Laragen’s validated in-house bead-based purification. Two hundred and fifty nanograms of purified PCR product from each sample was pooled and sequenced by Laragen, Inc. using the Illumina MiSeq platform and 2 × 150 bp reagent kit for paired-end sequencing.
2.7. Taxonomic Classification of 16S rRNA Gene Sequences
The sequencing reads were analyzed with QIIME2 and DADA2 following the developers’ instructions for quality control, de-noising and chimera removal (Callahan et al., 2016; Bolyen et al., 2019). The forward sequencing reads were truncated to 110 bp and denoised to 2067 amplicon sequence variants (ASVs) using DADA2. The chimeras were identified and removed using the DADA2 ‘consensus’ method. The ASVs were classified using the QIIME2 feature classifier classify-sklearn based on the SILVA database (release 132). The taxonomic abundance tables were normalized to correct for the different sequencing depth as previously described (Jones et al., 2018).
2.8. Statistics
Data, presented as means ± SEM, were analyzed and graphed using Prism software (GraphPad Inc., version 8.4.2), with significance set as p < 0.05. Body weights, caloric intake, and the IPGTT were analyzed using a Two-way mixed ANOVA with time as a within-subjects factor and diet as a between-subjects factor. Data were corrected for multiple comparisons using Sidak’s multiple comparison test. For the IPGTT, area under the curve (AUC) was measured using Prism software. NOIC and Zero maze results were analyzed by two-tailed t-test. The statistical analyses and visualization for microbiome outcomes were primarily performed using R statistical software (Version 3.6.3). The principal coordinates analysis (PCoA) of the rat gut microbiomes were calculated based on the Bray-Curtis dissimilarity at the genus level and visualized with functions in the R package ‘vegan’. The associations between gut microbiome and treatment were analyzed with the PERMANOVA test with 999 permutations using function ‘adonis’ in R package ‘vegan’. Shannon index was used for analyzing the alpha-diversity of gut microbiomes. The associations of individual taxa and treatment were analyzed with Wilcoxon test. The P-values were adjusted with the Benjamini-Hochberg method for multiple hypotheses testing. The correlations between each taxa and NOIC performance, defined by the rat’s % shift from baseline investigation of the object novel to the context, calculated from time (s) spent investigating the objects in each context (see NOIC methods for more details), were analyzed separately for each experiment with Spearman’s correlations and the P-values were adjusted with the Benjamini-Hochberg method.
3. Results
3.1. The increased adiposity associated with early life CAF diet consumption is not observed after healthy dietary intervention
In Experiment 1A, free access to the CAF diet throughout the juvenile and adolescence period did not result in significant differences in body weight (F(1, 18) = 0.2917; P = 0.5958) or total caloric intake (F(1, 18) = 2.065; P = 0.1679) (Fig. 2A–B). A significant time x diet interaction, however, was observed for total calories consumed (F(33, 589) = 4.229; P < 0.0001). Surprisingly, this interaction was driven by reduced caloric consumption in the CAF group vs. chow-fed group following a healthy dietary intervention, as confirmed by a significant group main effect when analyzed separately over the post-intervention period (F(1, 18) = 12.68; P = 0.0022) and the lack of significant group differences when analyzed separately over the CAF diet maintenance period before the intervention (F(1, 18) = 0.1916; P = 0.6668). However, in Experiment 1A NMR-based body composition analyses revealed that CAF rats had elevated fat mass relative to chow-fed rats at PN 74 after consuming a CAF during the juvenile and adolescence period (P = 0.0059). Results from an IPGTT conducted before the CAF group was switched to chow maintenance (Experiment 1A) revealed no significant group differences in area under the curve (AUC) (P = 0.3711) (Fig 2D), suggesting that glucose tolerance was not affected by CAF consumption. IPGTT results were comparable in the 2nd cohort (Supplemental Figure 1). Importantly, the elevated adiposity in CAF vs. chow-fed groups was not present at PN 115 after 41 days of standard chow maintenance (Experiment 1B; Fig. 2E).
Figure 2: Energy balance and metabolic outcomes following adolescent cafeteria diet consumption.
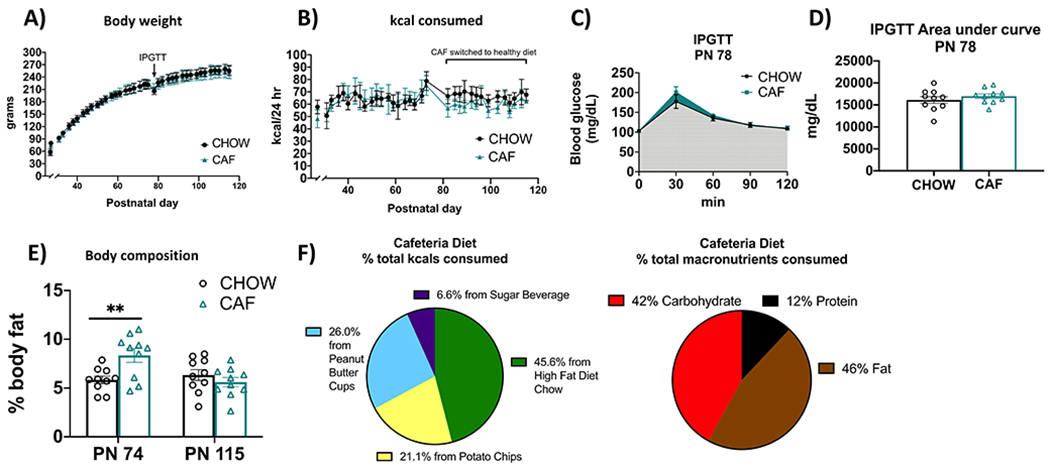
There were no overall significant group differences in body weight (A), total caloric intake (B), or glucose tolerance in the IPGTT test (C and D). Rats in the CAF group consumed significantly fewer calories following the healthy dietary intervention (B). CAF-exposed rats had significantly greater adiposity than CHOW rats (E). Percent total calories from each food item in the CAF diet as well as % macronutrient composition of total calories consumed in Cohort 1 are depicted in (F). Data are means ± SEM; n = 10/group, **P < 0.01. CHOW: chow-fed; CAF: cafeteria diet; kcal: kilocalories; IPGTT: intraperitoneal glucose tolerance test
Rats in the CAF group derived approximately 45.6% of their total calories from the high-fat-diet chow, 21.1% from potato chips, 26.0% from peanut butter cups, and 6.6% from the sugar beverage (Fig. 2F). The percentage of total kcals consumed from each macronutrient, calculated from the nutritional information and kcals consumed from each dietary component, was 12% from protein, 46% from fat, and 42% from carbohydrates. All caloric intake and body weight data were comparable in the 2nd cohort (Supplemental Figure 1).
3.2. The impaired peripheral glucose regulation induced by excessive early life sugar consumption is not observed after healthy dietary intervention
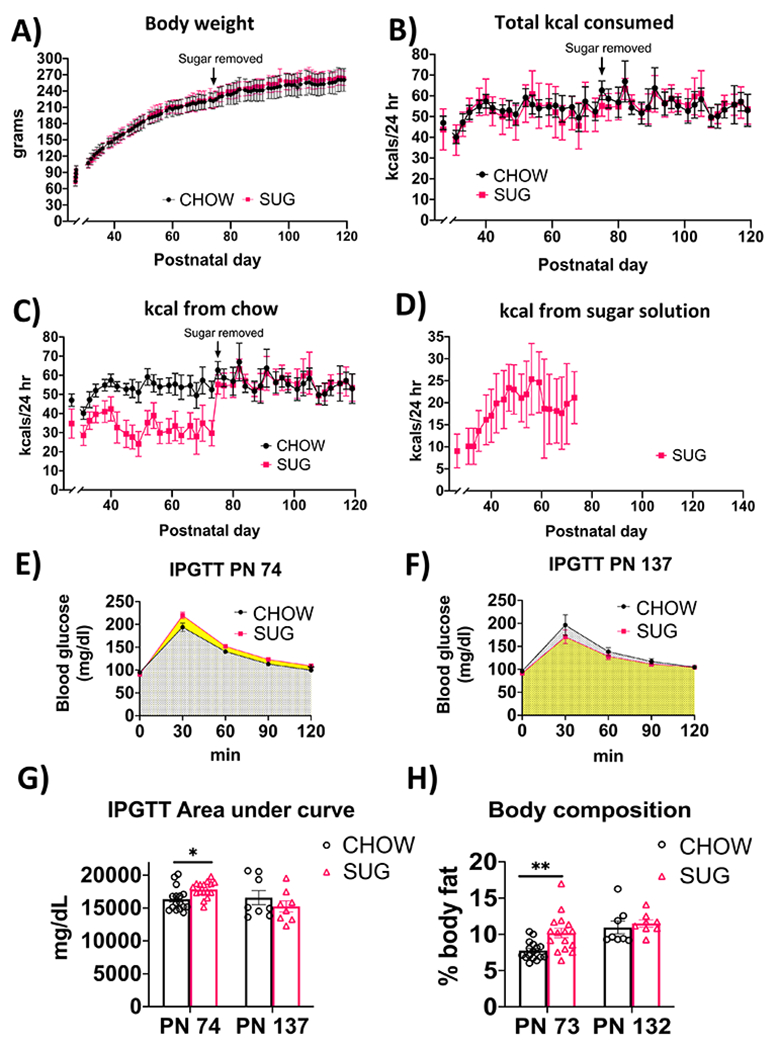
In Experiment 2, body weights and total caloric intake were comparable in the SUG and chow-fed groups throughout the experiment from PN 26-137 despite ad libitum access to a sugar beverage from PN 26-74 in the SUG group (Fig. 3A and 3B). However, there was a significant interaction (time x diet) for calories consumed from standard chow (F(39, 850) = 17.58; P < 0.0001) with a main effect of time (F(7.808, 170.2) = 41.55; P < 0.0001) and diet (F(1, 30) = 94.52; P < 0.0001). Post hoc analyses showed that from PN 28-73, SUG rats consumed fewer calories from chow (Fig. 3C), suggesting that the female SUG rats were reducing their chow intake to compensate for the kcals consumed from the sugar solution (Fig. 3D). Rats in the SUG group derived approximately 36.0% of their total calories from the sugar beverage (data not shown). Caloric intake and body weights within and between groups were comparable in the 2nd cohort (Supplemental Figure 2), who also derived around 33.6% of their total calories from the sugar beverage (data not shown). Similarly, in Experiment 2A adiposity measured by body composition using NMR was higher in the SUG group before removal of the sugar at PN 73 (P = 0.0021), but not at PN 132 after a dietary intervention (Experiment 2B; Fig. 3H).
Figure 3. Energy balance and metabolic outcomes following adolescent sugar diet consumption.
There were no overall significant group differences in body weight (A) or total caloric intake (B), although rats in the SUG group consumed less calories from chow (C) when consuming the sugar solution (D). The SUG group had significantly higher blood glucose levels (E and G) and adiposity (H) relative to CHOW rats, which normalized after healthy dietary intervention (F, G and H). Data are means ± SEM; n=16/group before sugar removal (PN 74), n=8/group after sugar removal (PN 75+) *P < 0.05, **P < 0.01. CHOW: chow-fed; SUG: sugar; kcal: kilocalories; IPGTT: intraperitoneal glucose tolerance test
In Experiment 2A, analyses of IPGTT results at PN 74 for the SUG and chow-fed rats revealed a significant interaction (time x diet) for blood glucose levels (F(4, 120) = 3.196, P = 0.0156) with a main effect of both time (F(1.681, 50.42) = 231.8, P < 0.0001) and diet (F(1, 30) = 7.642, P = 0.0097). Post hoc analyses revealed that the SUG group had higher glucose levels at the 120-minute timepoint after glucose administration (Fig. 3E). The IPGTT AUC analyses revealed that the SUG rats had significantly higher glucose levels than CHOW rats (P = 0.0106), further supporting that the SUG animals had impaired glucose tolerance at PN 74 (Fig. 3G). However, when tested at PN 137 after having removed the sugar for ~1.5 months as a healthy dietary intervention (Experiment 2B), SUG rats did not exhibit significantly impaired glucose tolerance relative to chow-fed rats (Fig. 3F).
3.3. Hippocampal-dependent memory impairments associated with early life junk food diet consumption are observed despite healthy dietary intervention
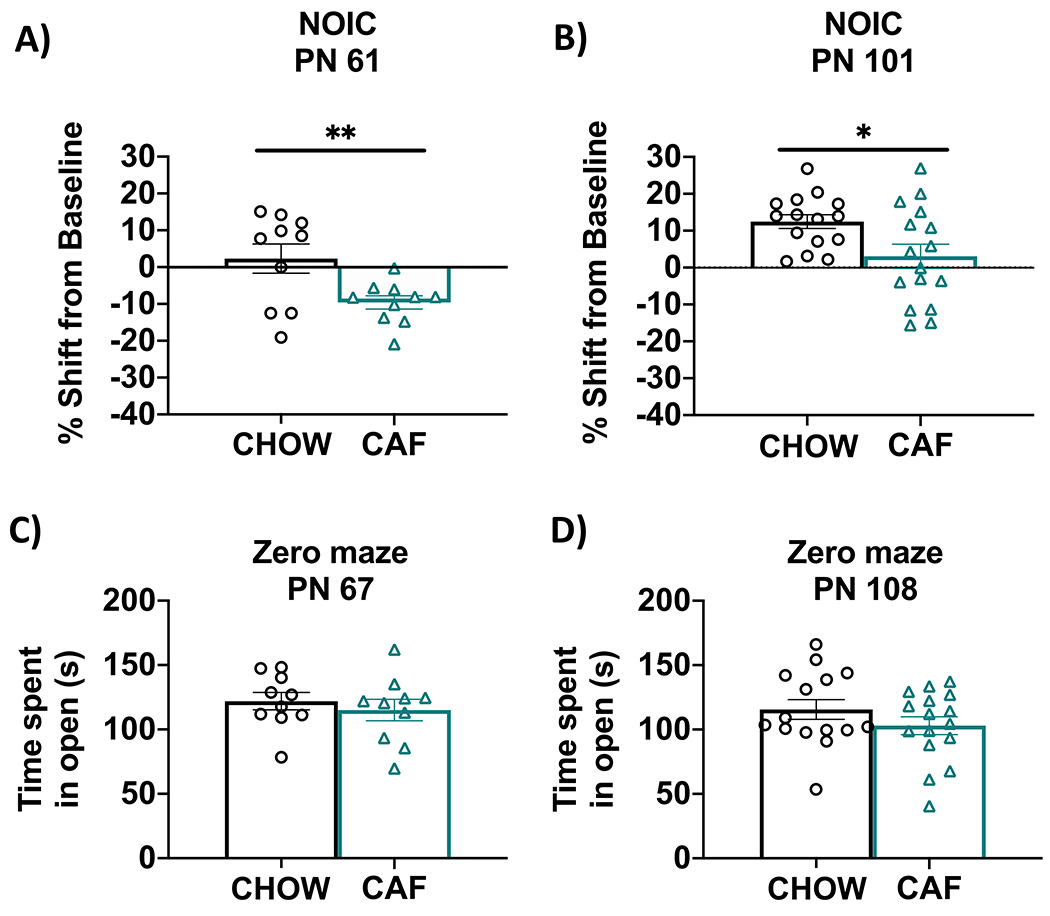
In Experiment 1A, results from the NOIC task revealed that CAF rats had deficits in hippocampal-dependent episodic contextual memory relative to chow-fed rats, which was supported by a lower shift from baseline discrimination index for the novel object (P = 0.008; Fig. 4A). After switching the CAF animals to standard chow as a healthy dietary intervention (Experiment 1B), rats that had been exposed to the CAF diet during early life displayed a significantly reduced shift from baseline discrimination of the novel object (P = 0.02; Fig. 4B). This suggests that the memory impairment associated with ~1.5 months of CAF diet exposure during adolescence may persist even after 5 weeks of healthy dietary intervention. There were no differences in anxiety-like behavior in the Zero Maze test before switching to a low-fat diet at PN 67 (Experiment 1A) or after the dietary intervention at PN 108 (Experiment 1B; Fig. 4C–D).
Figure 4: Hippocampal-dependent memory following adolescent cafeteria diet consumption.
CAF-exposed rats were impaired in the NOIC memory task (calculated as shift from baseline discrimination index on test day) when tested either before (A) or after (B) a healthy dietary intervention. There were no significant group differences in anxiety-like behavior in the Zero Maze when tested either before (C) or after (D) a dietary intervention. Data are means ± SEM; A/C: n = 10/group, B/D: n = 15/CHOW group, n = 16/CAF group, *P < 0.05, **P < 0.01. CHOW: chow-fed; CAF: cafeteria diet; NOIC = novel object in context
In Experiment 2A, SUG rats were impaired in the hippocampal-dependent NOIC task relative to chow-fed rats when tested at PN 65, supported by a significantly lower shift from baseline discrimination index for the novel object relative to chow-fed rats (P = 0.0309; Fig. 5A). After removing the SUG beverages as a dietary intervention in Experiment 2B, rats that had been previously exposed to the SUG diet during adolescence had a similar shift from baseline discrimination of the novel object as the CHOW group (Fig. 5B). This suggests that the memory impairments associated with early life SUG consumption in female rats may benefit from healthy dietary intervention. There were no differences in anxiety-like behavior in the Zero Maze test either when tested at PN 67 (Experiment 2A) or PN 107 following a dietary intervention (Experiment 2B; Fig. 5C).
Figure 5. Hippocampal-dependent memory following adolescent sugar diet consumption.
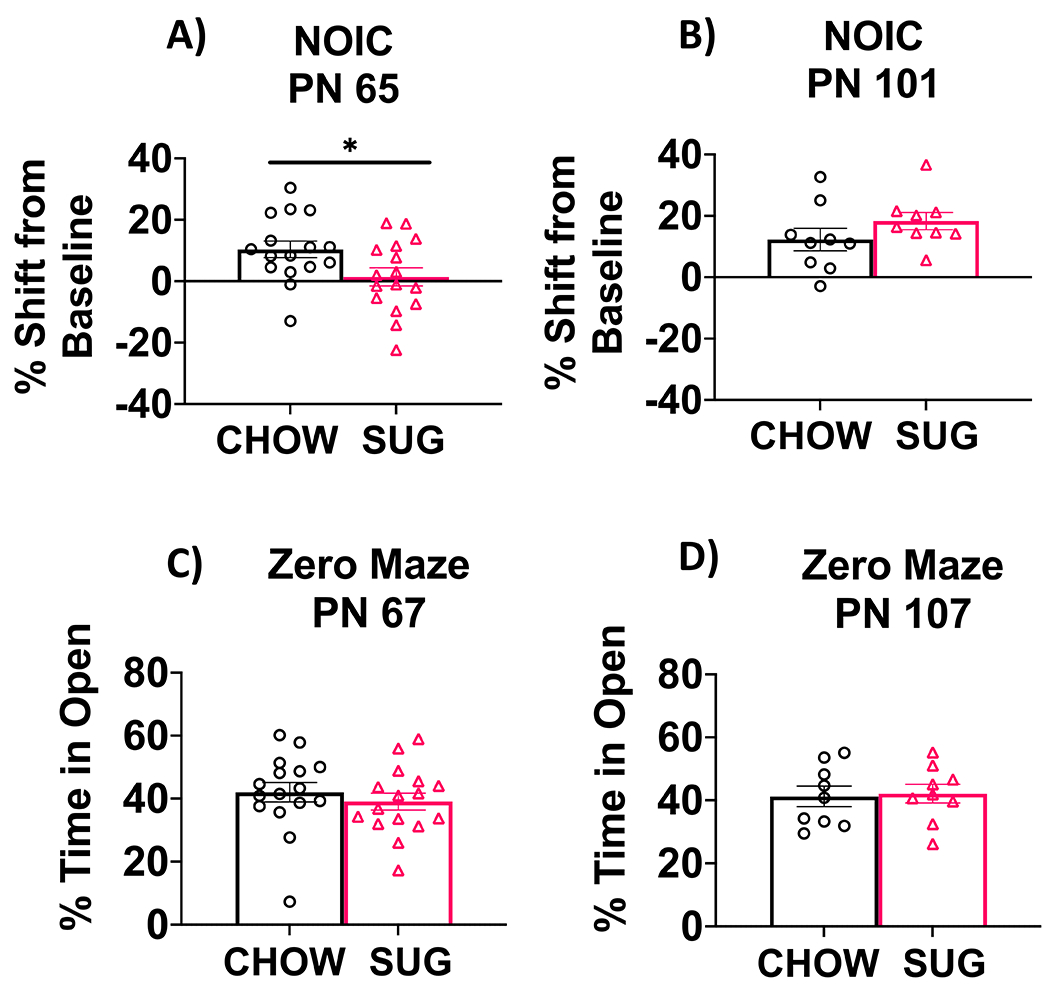
SUG-exposed rats were impaired in the NOIC memory task (calculated as shift from baseline discrimination index on test day) when tested before (A), but not after (B) a healthy dietary intervention. There were no significant group differences in anxiety-like behavior in the Zero Maze when tested either before (C) or after (D) the dietary intervention. Data are means ± SEM; A/C: n=16/group, B/D: n=9/group *P < 0.05. CHOW: chow-fed; SUG: sugar; NOIC: novel object in context
3.4. Gut microbiome changes associated with early life Western diet consumption (junk food diet or excessive sugar consumption) are observed despite healthy dietary intervention.
We characterized the rat gut microbiome to estimate the impact of early life Western diet consumption. The CAF and CHOW groups’ gut microbiomes are separated in the PCoA plots for Experiment 1A (PN 70, 44 days after the start of the WD diet) and for Experiment 1B (27 days after healthy dietary intervention), but the separation for Experiment 1B is more distinct (Fig. 6A and B). The alpha diversity of CAF rats for Experiment 1B was significantly reduced compared to other groups (Fig. 6C).
Figure 6. Gut microbiome following adolescent cafeteria diet consumption.
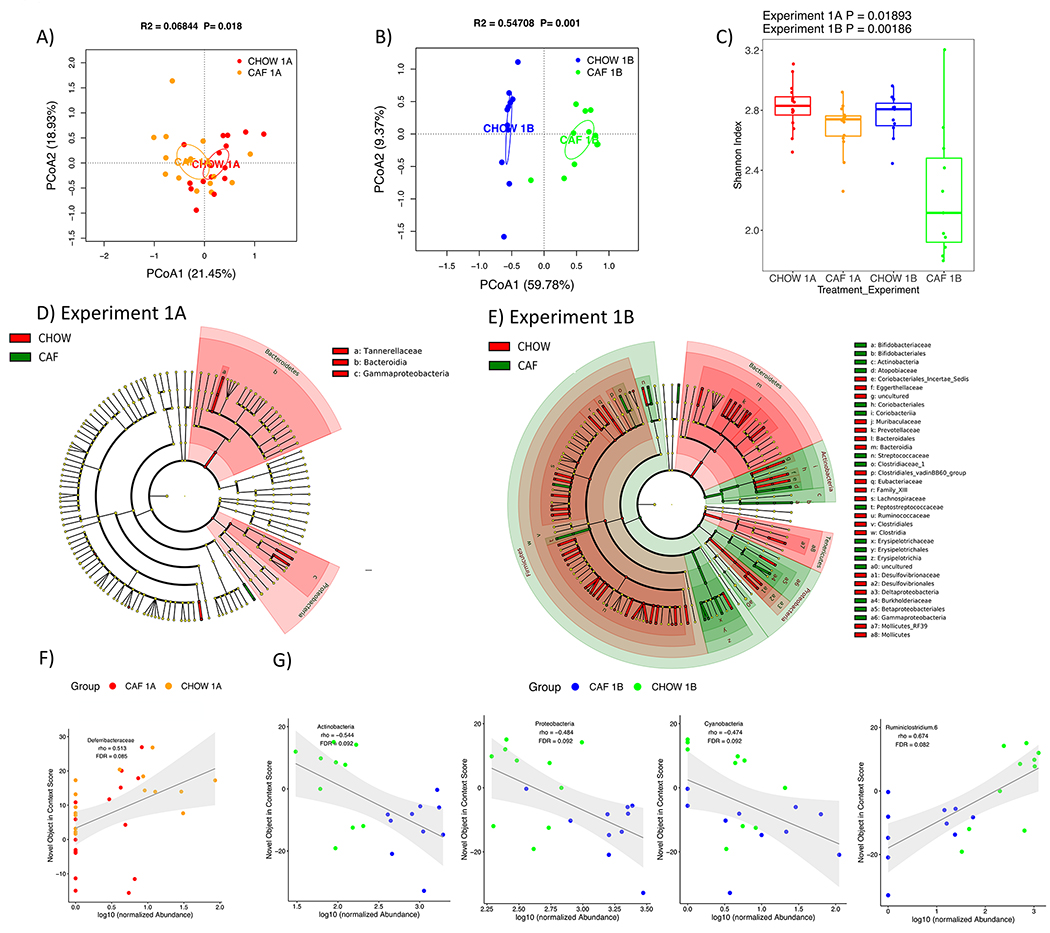
(A and B) PCoA plots of CAF-exposed rats gut microbiomes when analyzed either in Experiment 1A (no healthy dietary intervention) or in Experiment 1B (after healthy dietary intervention). Ellipses indicate 95% confidence limits. R2 and P are from PERMANOVA tests. (C) Shannon index of CAF-exposed rats gut microbiomes either before or after healthy dietary intervention. The differences were tested with Wilcoxon tests. (D and E) Significant differential taxa between treatment and chow-fed rats at phylum to species level (Wilcoxon test, FDR<0.1) are highlighted on the phylogenetic trees of all taxa identified in this study. An FDR cutoff of 0.1 was used here for visualization. (F) The correlation plots of Deferribacteraceae in Experiment 1A, Proteobacteria, Actinobacteria (phylum), Cyanobacteria and Ruminiclostridium in Experiment 1B with NOIC performance (% shift from baseline discrimination index on test day) across CAF and CHOW samples. Spearman’s correlation was used for the analysis and P-values were corrected for multiple hypotheses testing with the Benjamini-Hochberg method. CHOW: chow-fed; CAF: cafeteria diet; PCoA: Principal Coordinate Analysis; FDR: false discovery rate; rho: Spearman’s ρ
In order to generate a more detailed picture of changes to the microbiome, we next analyzed the differential abundance of individual taxa between CAF and CHOW rats for both experiments. Consistent with the view given by the PCoA plots, there are more taxa with significant group differences for Experiment 1B compared to Experiment 1A (148 vs 22 taxa at phylum to species level, Wilcoxon test, FDR<0.1; Fig. S3). The differential taxa for Experiment 1 are broadly distributed across various taxonomic groups, as depicted in the cladograms (Fig. 6D and E).
Similar to Experiment 1, Experiment 2 PCoA plots also showed clear separation of the SUG and CHOW rats’ gut microbiomes in both Experiments 2A (27 days after healthy dietary intervention) and 2B (27 days after healthy dietary intervention), with a stronger separation observed in Experiment 2B (Fig. 7A and B). Unlike the CAF data, however, the alpha diversity of SUG and CHOW rats was not significantly different for either Experiment 2A or 2B (Fig. 7C). Statistical tests of individual taxa at phylum to species level revealed 69 and 60 significant taxa between SUG and CHOW rats for Experiments 2A and 2B, respectively (Wilcoxon test, FDR<0.1) (Fig. S5). The differential taxa for Experiment 2 are broadly distributed across various taxonomic groups, as depicted in the cladograms (Fig. 7D and E).
Figure 7. Gut microbiome following adolescent sugar diet consumption.
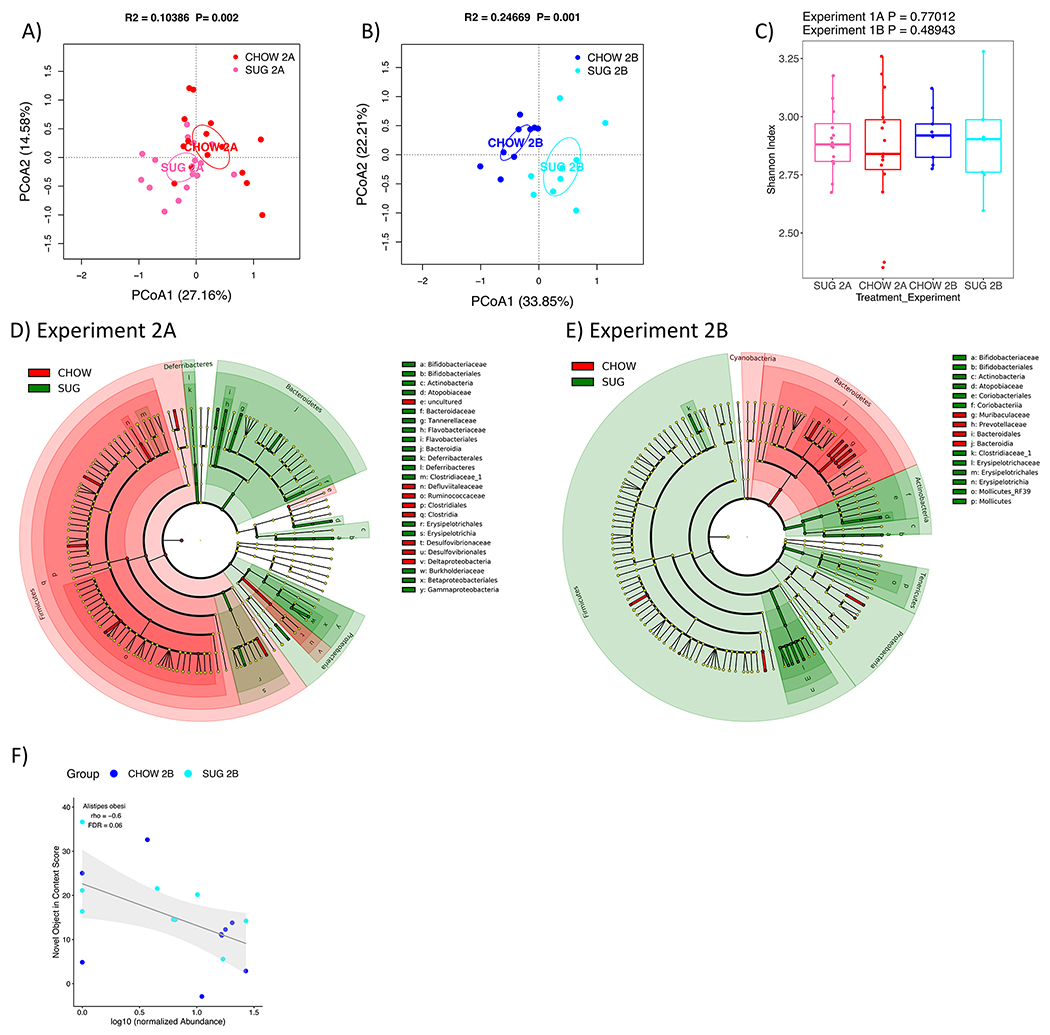
(A and B) PCoA plots of SUG-exposed rats gut microbiomes when analyzed either before (A) or after (B) a healthy dietary intervention. Ellipses indicate 95% confidence limits. R2 and P are from PERMANOVA tests. (C) Shannon index of SUG-exposed rats gut microbiomes when analyzed either before or after a healthy dietary intervention. The differences were tested with Wilcoxon tests. (D and E) Significant differential taxa between treatment and CHOW at phylum to species level (Wilcoxon test, FDR<0.1) are highlighted on the phylogenetic trees of all taxa identified in this study. An FDR cutoff of 0.1 was used here for visualization. (F) The correlations plots of Alistipes obesi with NOIC performance (% shift from baseline discrimination index on test day) in Experiment 2B. Spearman’s correlation was used for the analysis and P-values were corrected for multiple hypotheses testing with the Benjamini-Hochberg method. CHOW: chow-fed; SUG: sugar; PCoA: Principal Coordinate Analysis; FDR: false discovery rate; rho: Spearman’s ρ
3.5. Microbiome changes are associated with changes in NOIC
We next analyzed the correlations between individual taxa and NOIC to explore the potential microbiome signature associated with memory performance. In the CAF diet study (Experiment 1), we found several taxa significantly correlated with NOIC (Spearman’s correlation, FDR<0.1). This included a significant positive correlation with memory performance for Deferribacteraceae (family) for Experiment 1A (Fig. 6F), and significant negative correlations for Actinobacteria (phylum), Proteobacteria (phylum), and Cyanobacteria (phylum), and a significant positive correlation for Ruminiclostridium 6 (genus) for Experiment 1B (Fig. 6G). In the SUG experiment (Experiment 2), only Alistipes obesi (species) was significantly (negatively) associated with NOIC memory performance in Experiment 2A (Fig. 7F).
4. Discussion
Our results reveal that female rats who were maintained on a CAF diet throughout adolescence demonstrate impaired HPC-dependent episodic memory, altered abundances of gut microbiome bacterial taxa, and increased adiposity relative to chow-fed rats maintained on a healthy standard rodent diet. The CAF diet had no effect, however, on total caloric intake, body weight, or glucose tolerance relative to standard chow-fed rats. Similar to previous results in male rats (Hsu et al., 2015; Noble et al., 2019), our results further reveal that adolescent access to healthy chow, water, and an 11% carbohydrate w/v sugar solution (SUG) in female rats impaired HPC-dependent episodic memory, altered the gut microbiome, increased adiposity, and impaired glucose tolerance without affecting body weight or total caloric intake relative to chow-fed rats. Additional results revealed that some, but not all of these early life WD-associated outcomes may benefit from a 5-week healthy diet intervention in which all animals were maintained on a standard chow rodent diet beginning at early adulthood. CAF rats that received a dietary intervention also had episodic memory deficits and significant separation in gut microbial taxa, whereas the increased adiposity relative to chow-fed rats normalized. For SUG rats that received a dietary intervention, impaired metabolic and cognitive outcomes were not observed. However, similar to the CAF rats, the gut microbiome from SUG rats was distinct from chow-fed rats despite a 5-week dietary intervention. Together, these results show that some, but not all, negative outcomes associated with early life Western diet (WD) consumption in female rats may be remediated by switching to a healthy diet.
Consistent with the present results, previous studies conducted in male rodents revealed that dietary intervention ameliorates metabolic deficits associated with WD consumption (high fat or high fat, high sugar diets) (Guo et al., 2009; South et al., 2012; Kowalski et al., 2016; Hatzidis et al., 2017; Crisóstomo et al., 2019; Noble et al., 2019). However, some of the present results from female rats differ from studies conducted in male rodents. For instance, unlike the female CAF rats in the present study, switching to a low fat diet following adolescent high fat diet exposure in male rats resulted in marginal improvements in HPC-dependent memory (Boitard et al., 2016) and cognitive flexibility (McNeilly et al., 2016). In another study, male rats that received an adolescent CAF diet were not impaired in a spatial memory task despite showing symptoms of metabolic syndrome, which were reversible by switching to standard chow (Gomez-Smith et al., 2016). Moreover, studies using a diet manipulation similar to our SUG model revealed that a high sugar diet during adolescence led to impairments in both HPC-dependent episodic (Noble et al., 2019) and spatial memory (Fierros-Campuzano et al., 2020) in male rats that were not reversible by dietary intervention (i.e., removing the sugar access). Compared to these findings in males, the present findings suggest that female rats may benefit more from dietary intervention for neurocognitive impairments associated with early life excessive sugar consumption, despite consuming more of their total calories from the sugar beverage than males (here, the females consumed around ~33-36% of their daily energy intake from the sugar beverage whereas Noble et al. found that males on the same diet consumed on average ~24% of their daily energy intake from the sugar beverage) (Noble et al., 2019). There is also evidence to suggest that even metabolic and place-recognition memory impairments due to excessive sugar consumption in adult female rats can be reversed with dietary intervention (switching to saccharin or water) (Kendig et al., 2018). However, female rats may be more susceptible to sustained detrimental effects on memory when the diet is high in both sugar and fat (e.g., the CAF diet). Given that estradiol levels can affect food intake, body weight, and learning and memory function in female rodents (Butera et al., 2010; Frick et al., 2015), and estrogen and sex hormones have been shown to influence gut bacteria as well (Jašarević et al., 2016; Chen et al., 2018; Kaliannan et al., 2018), one limitation of the present study is that we did not evaluate the estrous stage at the time of behavioral testing or tissue harvest. Future studies that evaluate both sexes directly and carefully track estrous in females are needed to disentangle the role of sex and sex hormones on the enduring neurocognitive outcomes associated with early life WD consumption.
Given that increased anxiety-like behavior may develop following early life WD exposure in rodents (Tsan et al., 2021), we tested the rats in the Zero Maze test but found no effects of CAF exposure on anxiety-like behavior either before or after the dietary intervention. Similar to our CAF rats, an anxiety-like phenotype was not seen in the SUG cohorts, which is consistent with our previous findings in male rats using the Zero Maze test (Hsu et al., 2015). However, other studies have reported an anxiety-like phenotype in the open field test in male rodents after switching to standard chow for 1 week following an adolescent CAF diet (Lalanza et al., 2014) and after adolescent SUG exposure even after removing the sugar access for 25 days in young adulthood (Kruse et al., 2019). Thus, it is possible that the detection of anxiety-like behavior after WD exposure may be more or less sensitive depending on the type of behavioral test used.
Our data show that gut microbial richness, as measured with the Shannon Index, is significantly reduced following early life CAF, but not SUG, diet, and that this effect was actually greater in animals that underwent the healthy dietary intervention compared to those that did not. This outcome is consistent with other studies in which microbial richness was reduced after the removal of high-fat, high-sugar diets (Fülling et al., 2020; McNamara et al., 2021) or reduced adiposity following lifestyle modifications (eating breakfast, avoiding sugar-sweetened beverages, decreasing processed foods rich in animal fat or lengthening meal duration, and implementing more exercise) (Cho, 2021). Composition of the gut microbiota was significantly distinct from chow-fed rats immediately after either the adolescent CAF or SUG exposure period analyzed with PERMANOVA tests. Surprisingly, microbial separation between CAF and chow-fed rats and between SUG and chow-fed rats was greater after a 5-week healthy dietary intervention, suggesting that diet-induced shifts in the gut microbiome that occur during early life periods of development may be long-lasting independent of dietary patterns during adulthood. One possible explanation for the increased microbial divergence from chow-fed rats in the experimental groups that underwent dietary intervention is that both of these groups (CAF and SUG) were switched to a higher fiber diet (all kcal from standard chow) for the intervention. Further, cellulose contained within the high fat chow is the main dietary fiber of the CAF diet, whereas the standard chow diet contains a diverse set of dietary fibers from the ground corn, beet pulp, ground oats, alfalfa meal, and wheat middlings ingredients of this diet (e.g., arabinoxylan [corn, wheat], beta glucan [oat], cellulose [corn, alfalfa, wheat, beet], hemicellulose [corn, alfalfa, wheat, beet], pectin [beet]) (Sugawara et al., 1994; King et al., 2010; Huang et al., 2020; Yang et al., 2021). These different dietary fibers have been shown to serve as nutrient sources for a variety of gut microbial taxa, including the genus Bifidobacterium (cellulose, pectin, oat, arabinoxylan) (Connolly et al., 2010; Paesani et al., 2019; Nguyen et al., 2020; Nsor-Atindana et al., 2020; Elshahed et al., 2021), the genus Parasutterella (pectin) (Elshahed et al., 2021), the phylum Actinobacteria (pectin) (Elshahed et al., 2021), and the genera Bacteroides-Prevotella (oat, arabinoxylan) (Connolly et al., 2010), among others. The present study was not designed to pinpoint the effects of specific dietary fibers (or lack thereof) on cognitive function or the microbiome, but this is an important area of future work. Another factor to consider with regards to observed group differences in gut microbial richness is that our procedures for handling animals and food were not conducted in a completely sterile environment, and thus microbial cross-contamination between experimental groups was not completely prevented. Thus, it is likely that the dietary treatments may have led to even greater differences in the Shannon Index compared to chow-fed rats had all procedures been conducted under pure sterility.
In some cases, the same bacterial taxa were altered by both CAF and SUG treatment, but in opposite directions, and differential by experimental design. For example, for Experiments 1A and 2A (analyses conducted before dietary intervention), the CAF and SUG group shared changes in the class Gammaproteobacteria, the order Betaproteobacteriales, the family Tannerellaceae, and the genera Parabacteroides and ASF356, yet all of these followed the same trend and differed by dietary treatment: the abundances of these taxa were lower in CAF rats vs. chow-fed rats and higher in SUG rats vs. chow-fed rats. For Experiments 1B and 2B (analyses conducted after dietary intervention), abundances in the class Gammaproteobacteria, which is more abundant in obese mice (Pindjakova et al., 2017) and increased after consumption of high-fructose syrup in honeybees (Wang et al., 2020), and the order Betaproteobacteriales were still significantly different in the CAF, but not SUG, group relative to their respective CHOW groups. However, the direction changed such that the values of these taxa were lower than chow-fed rats in the CAF group when analyzed without a dietary intervention, yet higher than chow-fed rats when analyzed after a dietary intervention. For Experiments 1B and 2B, the class Mollicutes and the order Mollicutes RF39 were the only taxa that were significantly different in both CAF and SUG treated groups, lower in the CAF group, but higher in the SUG group compared to respective chow-fed rats. Overall, it is clear that CAF and SUG diets both significantly altered the gut microbiomes, but the changes in microbiota were often divergent, which may be related to differences in dietary fiber composition or content in the diets. These dietary treatments had significant and distinct influences on the microbiomes that can persist even after the healthy dietary intervention.
There is evidence to suggest that gut dysbiosis occurs before metabolic and spatial memory impairment in rodents (Saiyasit et al., 2020) and that certain bacteria can improve memory performance (Romo-Araiza et al., 2018; Ishikawa et al., 2019) or have detrimental effects on HPC neurons (Li et al., 2019) and cognitive ability (Magnusson et al., 2015). Further, we recently showed that elevated levels of Parabacteroides in male rats given the SUG dietary treatment during adolescence were functionally connected to HPC-dependent memory impairments (Noble et al., 2021). This conclusion was based on findings that levels of Parabacteroides were negatively associated with memory performance, and targeted enrichment of Parabacteroides in rats that had never consumed sugar replicated the memory deficits. Similar to these findings, here we show in female rats that Parabacteroides levels were increased in the SUG group relative to chow-fed rats, and interestingly, that this elevation was not observed following the dietary intervention. Given that the HPC-dependent memory impairments were also not observed following dietary intervention in this group, this suggests that levels of Parabacteroides may be functionally connected to SUG-associated memory impairments in females as well. However, correlation analyses revealed that Parabacteroides abundance was not significantly correlated with memory performance in females after adjusting for multiple hypotheses testing, suggesting the associations between Parabacteroides and SUG-associated memory impairments may vary by the sex of rats. It is also possible that this association in female rats may require a larger sample size than male rats for adequate statistical power.
Our data identify multiple taxa that were significantly correlated with NOIC performance. Diet-induced changes in the abundance of some of these microbiome populations may be related to the impaired memory performance in CAF-fed animals that were tested after the dietary intervention. For example, in Experiment 1B the phyla Actinobacteria and Proteobacteria were both negatively correlated with NOIC performance and were significantly higher in abundance in CAF rats that received a dietary intervention relative to chow-fed rats. On the other hand, the genus Ruminiclostridium 6 was positively correlated with NOIC performance and was significantly lower in abundance in CAF rats that received dietary intervention relative to chow-fed rats. Although little is known about the association between Ruminiclostridium 6 and memory performance, Actinobacteria has been associated with memory performance in dogs, albeit in an opposing direction compared with the present study as reduced abundance in that study was associated with better performance in a memory task (Kubinyi et al., 2020). Consistent with the present results, however, greater abundance of Proteobacteria has been correlated with memory dysfunction in adult mice whose mothers were fed a high fat diet before and during pregnancy (Sanguinetti et al., 2019). Collectively, these data highlight various bacterial populations that may be associated with the poor memory performance associated with early life CAF exposure. However, one limitation of the present study is that correlations were analyzed with experimental and standard chow groups combined, as sample sizes were not sufficient to evaluate correlations within each diet group separately. Further, functional conclusions cannot be made from correlation alone, and thus future studies that directly target these bacterial populations will be required to determine the possible causal relationships between these bacteria and hippocampal-dependent memory.
In contrast to our results, some studies have reported that dietary interventions involving a switch to healthier diets following WD can alleviate gut dysbiosis in rodents (Zhang et al., 2012; Shang et al., 2017; Safari et al., 2019) and in humans (Haro et al., 2017; Qian et al., 2020). Our results, however, show an even greater divergence in the microbiome relative to chow-fed rats after switching from either a SUG or a CAF diet to a healthy standard chow diet for ~5 weeks, especially in the CAF animals. These discrepancies could be due to the timing of when WD was introduced (we exposed animals to the WD during a critical developmental period, whereas the studies cited above evaluated WD consumption in adulthood). Thus, the present results reinforce that diet composition during early life is critical to the composition of the gut microbiome in adulthood. Although further research needs to be conducted, this divergence despite healthy dietary intervention may be related to altered competition within the gut microbial community due to the long-term effects of an early life WD. Understanding the influence of early life diets on the competitive landscape of the gut microbiome will be key in helping to reverse WD-induced gut dysbiosis and its effect on cognitive ability. One possible therapy to explore further is the use of probiotics, especially since there is evidence to suggest that probiotics can help improve memory function after WD exposure in male rodents (Beilharz et al., 2018; Chunchai et al., 2018; Yang et al., 2019) and help improve WD-induced gut dysbiosis in female mice (Kong et al., 2019).
In conclusion, we found that consumption of either a high sugar diet or a cafeteria-style junk food diet during adolescence can lead to metabolic dysfunction, HPC-dependent episodic memory deficits, and gut microbiome dysbiosis in female rats. These negative metabolic outcomes were not observed following a 5-week healthy dietary intervention (maintained on chow and water only). On the other hand, HPC-dependent memory deficits associated with adolescent cafeteria-style junk food diet consumption were observed despite healthy dietary intervention, whereas memory deficits associated with early life sugar consumption were not observed after the intervention. The changes in the gut microbiome relative to chow-fed rats, however, are present either with or without dietary intervention, suggesting that dietary effects on gut bacterial populations during early life periods may have long-lasting implications for the microbiome during adulthood. However, more studies need to be conducted to evaluate Western diet-associated changes in the microbiome across time given that one limitation of the present study is that it was not longitudinal, but rather, compared experimental dietary groups to a standard chow-fed group under different conditions (either immediately following early life dietary treatment, or 5 weeks after a healthy dietary intervention). Cross study comparisons of present results with the literature identify the need to consider sex as a key variable in studying connections between WD, the microbiome, and neurocognitive outcomes.
Supplementary Material
Supplemental Figure 4 Abundance of the gut microbiota in the SUG vs CHOW groups when analyzed either before (2A) or after (2B) healthy dietary intervention. CHOW: chow-fed; SUG: sugar; FDR: false discovery rate
Supplemental Figure 3 Abundance of the gut microbiota in the CAF vs CHOW groups when analyzed either before (1A) or after (1B) healthy dietary intervention. CHOW: chow-fed; CAF: cafeteria diet; FDR: false discovery rate
Supplemental Figure 1 Energy balance and metabolic outcomes following adolescent cafeteria diet consumption in CAF cohort 2. There were no overall significant group differences in body weight (A), total caloric intake (B), or glucose tolerance in the IPGTT test (C and D), although rats in the CAF group consumed significantly fewer calories during the period following the onset of the healthy dietary intervention (B, right). Percent total calories from each food item in the CAF diet in cohort 2 as well as % macronutrient composition of the total calories consumed are depicted in (E). Data are means ± SEM; CHOW: chow-fed n=15; CAF: cafeteria diet n=16; kcal: kilocalories; IPGTT: intraperitoneal glucose tolerance test
Supplemental Figure 2 Energy balance and metabolic outcomes following adolescent sugar diet consumption in SUG cohort 2. There were no overall significant group differences in body weight (A) or total caloric intake (B), although rats in the SUG group consumed fewer calories from chow (C) when consuming the sugar solution (D). Data are means ± SEM; n=9/group CHOW: chow-fed; SUG: sugar; kcal: kilocalories
Acknowledgments
The authors would like to thank Logan Tierno Lauer for assisting in experiments and William J. Matloff for valuable suggestions to the latest version of this manuscript.
Funding
This work was supported by grant numbers DK123423 (Awarded to SK and AF) from the National Institute of Diabetes and Digestive and Kidney Diseases and also by the National Science Foundation Graduate Research Fellowship DGE-1842487 (Awarded to LT).
Biographies
Notes on contributors
Linda Tsan
Linda Tsan is a Ph.D. candidate in the Neuroscience Graduate Program at the University of Southern California, Los Angeles, California, USA.
Shan Sun
Shan Sun is a postdoctoral researcher at the Department of Bioinformatics and Genomics at the University of North Carolina at Charlotte, Charlotte, NC, USA.
Anna Hayes
Anna Hayes is a postdoctoral researcher in the Department of Human and Evolutionary Biology at the University of Southern California, Los Angeles, California, USA.
Lana Bridi
Lana Bridi is an undergraduate researcher at the University of Southern California, Los Angeles, California, USA.
Lekha S. Chirala
Lekha S. Chirala is a laboratory technician in the Department of Human and Evolutionary Biology at the University of Southern California, Los Angeles, California, USA.
Emily E. Noble
Emily E. Noble is an Assistant Professor at the Department of Foods and Nutrition at the University of Georgia, Athens, GA, USA.
Anthony A. Fodor
Anthony A. Fodor is a Professor at the Department of Bioinformatics and Genomics at the University of North Carolina at Charlotte, Charlotte, NC, USA.
Scott E. Kanoski
Scott E. Kanoski is an Associate Professor at the Department of Human and Evolutionary Biology at the University of Southern California, Los Angeles, California, USA.
Footnotes
Disclosure statement
The authors report no conflicts of interest.
References
- Ambrosini GL, Huang R-C, Mori TA, Hands BP, O’Sullivan TA, de Klerk NH, et al. (2010). Dietary patterns and markers for the metabolic syndrome in Australian adolescents. Nutrition, Metabolism and Cardiovascular Diseases 20, 274–283. doi: 10.1016/j.numecd.2009.03.024. [DOI] [PubMed] [Google Scholar]
- Balderas I, Rodriguez-Ortiz CJ, Salgado-Tonda P, Chavez-Hurtado J, McGaugh JL, and Bermudez-Rattoni F (2008). The consolidation of object and context recognition memory involve different regions of the temporal lobe. Learn. Mem 15, 618–624. doi: 10.1101/lm.1028008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baym CL, Khan NA, Monti JM, Raine LB, Drollette ES, Moore RD, et al. (2014). Dietary lipids are differentially associated with hippocampal-dependent relational memory in prepubescent children. Am J Clin Nutr 99, 1026–1032. doi: 10.3945/ajcn.113.079624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beilharz JE, Kaakoush NO, Maniam J, and Morris MJ (2018). Cafeteria diet and probiotic therapy: cross talk among memory, neuroplasticity, serotonin receptors and gut microbiota in the rat. Molecular Psychiatry 23, 351–361. doi: 10.1038/mp.2017.38. [DOI] [PubMed] [Google Scholar]
- Boitard C, Parkes SL, Cavaroc A, Tantot F, Castanon N, Layé S, et al. (2016). Switching Adolescent High-Fat Diet to Adult Control Diet Restores Neurocognitive Alterations. Front Behav Neurosci 10. doi: 10.3389/fnbeh.2016.00225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolyen E, Rideout JR, Dillon MR, Bokulich NA, Abnet CC, Al-Ghalith GA, et al. (2019). Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat Biotechnol 37, 852–857. doi: 10.1038/s41587-019-0209-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butera PC, Wojcik DM, and Clough SJ (2010). EFFECTS OF ESTRADIOL ON FOOD INTAKE AND MEAL PATTERNS FOR DIETS THAT DIFFER IN FLAVOR AND FAT CONTENT. Physiol Behav 99, 142–145. doi: 10.1016/j.physbeh.2009.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callahan BJ, McMurdie PJ, Rosen MJ, Han AW, Johnson AJA, and Holmes SP (2016). DADA2: High-resolution sample inference from Illumina amplicon data. Nature Methods 13, 581–583. doi: 10.1038/nmeth.3869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caporaso JG, Lauber CL, Walters WA, Berg-Lyons D, Lozupone CA, Turnbaugh PJ, et al. (2011). Global patterns of 16S rRNA diversity at a depth of millions of sequences per sample. Proc Natl Acad Sci U S A 108, 4516–4522. doi: 10.1073/pnas.1000080107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerdó T, Diéguez E, and Campoy C (2019). Early nutrition and gut microbiome: interrelationship between bacterial metabolism, immune system, brain structure, and neurodevelopment. American Journal of Physiology-Endocrinology and Metabolism 317, E617–E630. doi: 10.1152/ajpendo.00188.2019. [DOI] [PubMed] [Google Scholar]
- Chen KLA, Liu X, Zhao YC, Hieronymi K, Rossi G, Auvil LS, et al. (2018). Long-Term Administration of Conjugated Estrogen and Bazedoxifene Decreased Murine Fecal β-Glucuronidase Activity Without Impacting Overall Microbiome Community. Sci Rep 8, 8166. doi: 10.1038/s41598-018-26506-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho KY (2021). Lifestyle modifications result in alterations in the gut microbiota in obese children. BMC Microbiology 21, 10. doi: 10.1186/s12866-020-02002-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chunchai T, Thunapong W, Yasom S, Wanchai K, Eaimworawuthikul S, Metzler G, et al. (2018). Decreased microglial activation through gut-brain axis by prebiotics, probiotics, or synbiotics effectively restored cognitive function in obese-insulin resistant rats. J Neuroinflammation 15, 11. doi: 10.1186/s12974-018-1055-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark KA, Alves JM, Jones S, Yunker AG, Luo S, Cabeen RP, et al. (2020). Dietary Fructose Intake and Hippocampal Structure and Connectivity during Childhood. Nutrients 12, 909. doi: 10.3390/nu12040909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connolly ML, Lovegrove JA, and Tuohy KM (2010). In vitro evaluation of the microbiota modulation abilities of different sized whole oat grain flakes. Anaerobe 16, 483–488. doi: 10.1016/j.anaerobe.2010.07.001. [DOI] [PubMed] [Google Scholar]
- Costa CS, Del-Ponte B, Assunção MCF, and Santos IS (2018). Consumption of ultra-processed foods and body fat during childhood and adolescence: a systematic review. Public Health Nutrition 21, 148–159. doi: 10.1017/S1368980017001331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crisóstomo L, Rato L, Jarak I, Silva BM, Raposo JF, Batterham RL, et al. (2019). A switch from high-fat to normal diet does not restore sperm quality but prevents metabolic syndrome. Reproduction 158, 377–387. doi: 10.1530/REP-19-0259. [DOI] [PubMed] [Google Scholar]
- Darch HT, Collins MK, O’Riordan KJ, and Cryan JF (2021). Microbial memories: Sex-dependent impact of the gut microbiome on hippocampal plasticity. Eur J Neurosci. doi: 10.1111/ejn.15119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elshahed MS, Miron A, Aprotosoaie AC, and Farag MA (2021). Pectin in diet: Interactions with the human microbiome, role in gut homeostasis, and nutrient-drug interactions. Carbohydr Polym 255, 117388. doi: 10.1016/j.carbpol.2020.117388. [DOI] [PubMed] [Google Scholar]
- Ferreira A, Castro JP, Andrade JP, Dulce Madeira M, and Cardoso A (2018). Cafeteria-diet effects on cognitive functions, anxiety, fear response and neurogenesis in the juvenile rat. Neurobiol Learn Mem 155, 197–207. doi: 10.1016/j.nlm.2018.07.014. [DOI] [PubMed] [Google Scholar]
- Fierros-Campuzano J, Ballesteros-Zebadúa P, Manjarrez-Marmolejo J, Aguilera P, Méndez-Diaz M, Prospero-García O, et al. (2020). Irreversible hippocampal changes induced by high fructose diet in rats. Nutr Neurosci, 1–13. doi: 10.1080/1028415X.2020.1853418. [DOI] [PubMed] [Google Scholar]
- Francis H, and Stevenson R (2013). The longer-term impacts of Western diet on human cognition and the brain. Appetite 63, 119–128. doi: 10.1016/j.appet.2012.12.018. [DOI] [PubMed] [Google Scholar]
- Frick KM, Kim J, Tuscher JJ, and Fortress AM (2015). Sex steroid hormones matter for learning and memory: estrogenic regulation of hippocampal function in male and female rodents. Learn. Mem 22, 472–493. doi: 10.1101/lm.037267.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fülling C, Lach G, Bastiaanssen TFS, Fouhy F, O’Donovan AN, Ventura-Silva A-P, et al. (2020). Adolescent dietary manipulations differentially affect gut microbiota composition and amygdala neuroimmune gene expression in male mice in adulthood. Brain, Behavior, and Immunity. doi: 10.1016/j.bbi.2020.02.013. [DOI] [PubMed] [Google Scholar]
- Gallagher C, Moschonis G, Lambert KA, Karaglani E, Mavrogianni C, Gavrili S, et al. (2020). Sugar-sweetened beverage consumption is associated with visceral fat in children. Br J Nutr, 1–9. doi: 10.1017/S0007114520003256. [DOI] [PubMed] [Google Scholar]
- Gomez-Smith M, Karthikeyan S, Jeffers MS, Janik R, Thomason LA, Stefanovic B, et al. (2016). A physiological characterization of the Cafeteria diet model of metabolic syndrome in the rat. Physiol Behav 167, 382–391. doi: 10.1016/j.physbeh.2016.09.029. [DOI] [PubMed] [Google Scholar]
- Guo J, Jou W, Gavrilova O, and Hall KD (2009). Persistent Diet-Induced Obesity in Male C57BL/6 Mice Resulting from Temporary Obesigenic Diets. PLoS One 4. doi: 10.1371/journal.pone.0005370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haro C, García‐Carpintero S, Rangel‐Zúñiga OA, Alcalá‐Díaz JF, Landa BB, Clemente JC, et al. (2017). Consumption of Two Healthy Dietary Patterns Restored Microbiota Dysbiosis in Obese Patients with Metabolic Dysfunction. Molecular Nutrition & Food Research 61, 1700300. doi: 10.1002/mnfr.201700300. [DOI] [PubMed] [Google Scholar]
- Hatzidis A, Hicks JA, Gelineau RR, Arruda NL, De Pina IM, O’Connell KE, et al. (2017). Removal of a high-fat diet, but not voluntary exercise, reverses obesity and diabetic-like symptoms in male C57BL/6J mice. Hormones 16, 62–74. doi: 10.14310/horm.2002.1720. [DOI] [PubMed] [Google Scholar]
- Hsu TM, Konanur VR, Taing L, Usui R, Kayser BD, Goran MI, et al. (2015). Effects of sucrose and high fructose corn syrup consumption on spatial memory function and hippocampal neuroinflammation in adolescent rats. Hippocampus 25, 227–239. doi: 10.1002/hipo.22368. [DOI] [PubMed] [Google Scholar]
- Huang X, Yang Y, Liu Q, and He W-Q (2020). Effect of high pressure homogenization on sugar beet pulp: Physicochemical, thermal and structural properties. LWT 134, 110177. doi: 10.1016/j.lwt.2020.110177. [DOI] [Google Scholar]
- Ishikawa R, Fukushima H, Nakakita Y, Kado H, and Kida S (2019). Dietary heat-killed Lactobacillus brevis SBC8803 (SBL88TM) improves hippocampus-dependent memory performance and adult hippocampal neurogenesis. Neuropsychopharmacology Reports 39, 140–145. doi: 10.1002/npr2.12054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jašarević E, Morrison KE, and Bale TL (2016). Sex differences in the gut microbiome–brain axis across the lifespan. Philos Trans R Soc Lond B Biol Sci 371. doi: 10.1098/rstb.2015.0122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones RB, Zhu X, Moan E, Murff HJ, Ness RM, Seidner DL, et al. (2018). Inter-niche and inter-individual variation in gut microbial community assessment using stool, rectal swab, and mucosal samples. Sci Rep 8, 4139. doi: 10.1038/s41598-018-22408-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaliannan K, Robertson RC, Murphy K, Stanton C, Kang C, Wang B, et al. (2018). Estrogen-mediated gut microbiome alterations influence sexual dimorphism in metabolic syndrome in mice. Microbiome 6, 205. doi: 10.1186/s40168-018-0587-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kashtanova DA, Popenko AS, Tkacheva ON, Tyakht AB, Alexeev DG, and Boytsov SA (2016). Association between the gut microbiota and diet: Fetal life, early childhood, and further life. Nutrition 32, 620–627. doi: 10.1016/j.nut.2015.12.037. [DOI] [PubMed] [Google Scholar]
- Kendig MD, Boakes RA, Rooney KB, and Corbit LH (2013). Chronic restricted access to 10% sucrose solution in adolescent and young adult rats impairs spatial memory and alters sensitivity to outcome devaluation. Physiology & Behavior 120, 164–172. doi: 10.1016/j.physbeh.2013.08.012. [DOI] [PubMed] [Google Scholar]
- Kendig MD, Fu MX, Rehn S, Martire SI, Boakes RA, and Rooney KB (2018). Metabolic and cognitive improvement from switching to saccharin or water following chronic consumption by female rats of 10% sucrose solution. Physiology & Behavior 188, 162–172. doi: 10.1016/j.physbeh.2018.02.008. [DOI] [PubMed] [Google Scholar]
- King DL, Zeug R, and Pettit J (2010). “Appendix 1: Composition of grains and grain products,” in Cereal Grains Woodhead Publishing Series in Food Science, Technology and Nutrition., eds. Wrigley CW and Batey IL (Woodhead Publishing; ), 487–493. doi: 10.1533/9781845699529.5.487. [DOI] [Google Scholar]
- Kong C, Gao R, Yan X, Huang L, and Qin H (2019). Probiotics improve gut microbiota dysbiosis in obese mice fed a high-fat or high-sucrose diet. Nutrition 60, 175–184. doi: 10.1016/j.nut.2018.10.002. [DOI] [PubMed] [Google Scholar]
- Kowalski GM, Hamley S, Selathurai A, Kloehn J, De Souza DP, O’Callaghan S, et al. (2016). Reversing diet-induced metabolic dysregulation by diet switching leads to altered hepatic de novo lipogenesis and glycerolipid synthesis. Sci Rep 6. doi: 10.1038/srep27541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruse MS, Vadillo MJ, Miguelez Fernández AMM, Rey M, Zanutto BS, and Coirini H (2019). Sucrose exposure in juvenile rats produces long-term changes in fear memory and anxiety-like behavior. Psychoneuroendocrinology 104, 300–307. doi: 10.1016/j.psyneuen.2019.03.016. [DOI] [PubMed] [Google Scholar]
- Kubinyi E, Bel Rhali S, Sándor S, Szabó A, and Felföldi T (2020). Gut Microbiome Composition is Associated with Age and Memory Performance in Pet Dogs. Animals (Basel) 10, 1488. doi: 10.3390/ani10091488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lalanza JF, Caimari A, del Bas JM, Torregrosa D, Cigarroa I, Pallàs M, et al. (2014). Effects of a post-weaning cafeteria diet in young rats: metabolic syndrome, reduced activity and low anxiety-like behaviour. PLoS ONE 9, e85049. doi: 10.1371/journal.pone.0085049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leigh S-J, Kaakoush NO, Bertoldo MJ, Westbrook RF, and Morris MJ (2020). Intermittent cafeteria diet identifies fecal microbiome changes as a predictor of spatial recognition memory impairment in female rats. Translational Psychiatry 10, 1–12. doi: 10.1038/s41398-020-0734-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J-M, Yu R, Zhang L-P, Wen S-Y, Wang S-J, Zhang X-Y, et al. (2019). Dietary fructose-induced gut dysbiosis promotes mouse hippocampal neuroinflammation: a benefit of short-chain fatty acids. Microbiome 7, 98. doi: 10.1186/s40168-019-0713-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mager DR, Mazurak V, Rodriguez-Dimitrescu C, Vine D, Jetha M, Ball G, et al. (2013). A meal high in saturated fat evokes postprandial dyslipemia, hyperinsulinemia, and altered lipoprotein expression in obese children with and without nonalcoholic fatty liver disease. JPEN J Parenter Enteral Nutr 37, 517–528. doi: 10.1177/0148607112467820. [DOI] [PubMed] [Google Scholar]
- Martínez MC, Villar ME, Ballarini F, and Viola H (2014). Retroactive interference of object-in-context long-term memory: role of dorsal hippocampus and medial prefrontal cortex. Hippocampus 24, 1482–1492. doi: 10.1002/hipo.22328. [DOI] [PubMed] [Google Scholar]
- McNamara MP, Singleton JM, Cadney MD, Ruegger PM, Borneman J, and Garland T (2021). Early-life effects of juvenile Western diet and exercise on adult gut microbiome composition in mice. Journal of Experimental Biology. doi: 10.1242/jeb.239699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNeilly AD, Gao A, Hill AY, Gomersall T, Balfour DJK, Sutherland C, et al. (2016). The effect of dietary intervention on the metabolic and behavioural impairments generated by short term high fat feeding in the rat. Physiology & Behavior 167, 100–109. doi: 10.1016/j.physbeh.2016.08.035. [DOI] [PubMed] [Google Scholar]
- Neri D, Martinez-Steele E, Monteiro CA, and Levy RB (2019). Consumption of ultra-processed foods and its association with added sugar content in the diets of US children, NHANES 2009–2014. Pediatr Obes 14, e12563. doi: 10.1111/ijpo.12563. [DOI] [PubMed] [Google Scholar]
- Nguyen NK, Deehan EC, Zhang Z, Jin M, Baskota N, Perez-Muñoz ME, et al. (2020). Gut microbiota modulation with long-chain corn bran arabinoxylan in adults with overweight and obesity is linked to an individualized temporal increase in fecal propionate. Microbiome 8, 118. doi: 10.1186/s40168-020-00887-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noble EE, Hsu TM, Jones RB, Fodor AA, Goran MI, and Kanoski SE (2017a). Early-Life Sugar Consumption Affects the Rat Microbiome Independently of Obesity. J Nutr 147, 20–28. doi: 10.3945/jn.116.238816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noble EE, Hsu TM, and Kanoski SE (2017b). Gut to Brain Dysbiosis: Mechanisms Linking Western Diet Consumption, the Microbiome, and Cognitive Impairment. Front Behav Neurosci 11. doi: 10.3389/fnbeh.2017.00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noble EE, Hsu TM, Liang J, and Kanoski SE (2019). Early-life sugar consumption has long-term negative effects on memory function in male rats. Nutr Neurosci 22, 273–283. doi: 10.1080/1028415X.2017.1378851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noble EE, and Kanoski SE (2016). Early life exposure to obesogenic diets and learning and memory dysfunction. Curr Opin Behav Sci 9, 7–14. doi: 10.1016/j.cobeha.2015.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noble EE, Mavanji V, Little MR, Billington CJ, Kotz CM, and Wang C (2014). Exercise reduces diet-induced cognitive decline and increases hippocampal brain-derived neurotrophic factor in CA3 neurons. Neurobiol Learn Mem 114, 40–50. doi: 10.1016/j.nlm.2014.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noble EE, Olson CA, Davis E, Tsan L, Chen Y-W, Schade R, et al. (2021). Gut microbial taxa elevated by dietary sugar disrupt memory function. Translational Psychiatry. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nsor-Atindana J, Zhou YX, Saqib MN, Chen M, Douglas Goff H, Ma J, et al. (2020). Enhancing the prebiotic effect of cellulose biopolymer in the gut by physical structuring via particle size manipulation. Food Res Int 131, 108935. doi: 10.1016/j.foodres.2019.108935. [DOI] [PubMed] [Google Scholar]
- Paesani C, Salvucci E, Moiraghi M, Fernandez Canigia L, and Pérez GT (2019). Arabinoxylan from Argentinian whole wheat flour promote the growth of Lactobacillus reuteri and Bifidobacterium breve. Lett Appl Microbiol 68, 142–148. doi: 10.1111/lam.13097. [DOI] [PubMed] [Google Scholar]
- Pindjakova J, Sartini C, Lo Re O, Rappa F, Coupe B, Lelouvier B, et al. (2017). Gut Dysbiosis and Adaptive Immune Response in Diet-induced Obesity vs. Systemic Inflammation. Front Microbiol 8. doi: 10.3389/fmicb.2017.01157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Provensi G, Schmidt SD, Boehme M, Bastiaanssen TFS, Rani B, Costa A, et al. (2019). Preventing adolescent stress-induced cognitive and microbiome changes by diet. Proc. Natl. Acad. Sci. U.S.A 116, 9644–9651. doi: 10.1073/pnas.1820832116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian L, Huang J, and Qin H (2020). Probiotics and dietary intervention modulate the colonic mucosa-associated microbiota in high-fat diet populations. Turk J Gastroenterol 31, 295–304. doi: 10.5152/tjg.2020.19013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichelt AC (2016). Adolescent Maturational Transitions in the Prefrontal Cortex and Dopamine Signaling as a Risk Factor for the Development of Obesity and High Fat/High Sugar Diet Induced Cognitive Deficits. Front Behav Neurosci 10, 189. doi: 10.3389/fnbeh.2016.00189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romo-Araiza A, Gutiérrez-Salmeán G, Galván EJ, Hernández-Frausto M, Herrera-López G, Romo-Parra H, et al. (2018). Probiotics and Prebiotics as a Therapeutic Strategy to Improve Memory in a Model of Middle-Aged Rats. Front. Aging Neurosci 10. doi: 10.3389/fnagi.2018.00416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Safari Z, Monnoye M, Abuja PM, Mariadassou M, Kashofer K, Gérard P, et al. (2019). Steatosis and gut microbiota dysbiosis induced by high-fat diet are reversed by 1-week chow diet administration. Nutrition Research 71, 72–88. doi: 10.1016/j.nutres.2019.09.004. [DOI] [PubMed] [Google Scholar]
- Saiyasit N, Chunchai T, Prus D, Suparan K, Pittayapong P, Apaijai N, et al. (2020). Gut dysbiosis develops before metabolic disturbance and cognitive decline in high-fat diet–induced obese condition. Nutrition 69, 110576. doi: 10.1016/j.nut.2019.110576. [DOI] [PubMed] [Google Scholar]
- Sanguinetti E, Guzzardi MA, Tripodi M, Panetta D, Selma-Royo M, Zega A, et al. (2019). Microbiota signatures relating to reduced memory and exploratory behaviour in the offspring of overweight mothers in a murine model. Sci Rep 9, 12609. doi: 10.1038/s41598-019-48090-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shang Y, Khafipour E, Derakhshani H, Sarna LK, Woo CW, Siow YL, et al. (2017). Short Term High Fat Diet Induces Obesity-Enhancing Changes in Mouse Gut Microbiota That are Partially Reversed by Cessation of the High Fat Diet. Lipids 52, 499–511. doi: 10.1007/s11745-017-4253-2. [DOI] [PubMed] [Google Scholar]
- Shepherd JK, Grewal SS, Fletcher A, Bill DJ, and Dourish CT (1994). Behavioural and pharmacological characterisation of the elevated “zero-maze” as an animal model of anxiety. Psychopharmacology (Berl) 116, 56–64. doi: 10.1007/BF02244871. [DOI] [PubMed] [Google Scholar]
- South T, Westbrook F, and Morris MJ (2012). Neurological and stress related effects of shifting obese rats from a palatable diet to chow and lean rats from chow to a palatable diet. Physiology & Behavior 105, 1052–1057. doi: 10.1016/j.physbeh.2011.11.019. [DOI] [PubMed] [Google Scholar]
- Sugawara M, Suzuki T, Totsuka A, Takeuchi M, and Ueki K (1994). Composition of Corn Hull Dietary Fiber. Starch - Stärke 46, 335–337. doi: 10.1002/star.19940460904. [DOI] [Google Scholar]
- Tsan L, Décarie-Spain L, Noble EE, and Kanoski SE (2021). Western Diet Consumption During Development: Setting the Stage for Neurocognitive Dysfunction. Front. Neurosci 15. doi: 10.3389/fnins.2021.632312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Liu C, Liu Z, Wang Y, Ma L, and Xu B (2020). The different dietary sugars modulate the composition of the gut microbiota in honeybee during overwintering. BMC Microbiology 20, 61. doi: 10.1186/s12866-020-01726-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Shang L, Light K, O’Loughlin J, Paradis G, and Gray-Donald K (2015). Associations between added sugar (solid vs. liquid) intakes, diet quality, and adiposity indicators in Canadian children. Applied Physiology, Nutrition, and Metabolism. doi: 10.1139/apnm-2014-0447. [DOI] [PubMed] [Google Scholar]
- Wang Y, Guglielmo D, and Welsh JA (2018). Consumption of sugars, saturated fat, and sodium among US children from infancy through preschool age, NHANES 2009–2014. Am J Clin Nutr 108, 868–877. doi: 10.1093/ajcn/nqy168. [DOI] [PubMed] [Google Scholar]
- Yang C, Zhang F, Jiang X, Yang X, He F, Wang Z, et al. (2021). Identification of Genetic Loci Associated With Crude Protein Content and Fiber Composition in Alfalfa (Medicago sativa L.) Using QTL Mapping. Frontiers in Plant Science 12, 210. doi: 10.3389/fpls.2021.608940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Zhong Z, Wang B, Xia X, Yao W, Huang L, et al. (2019). Early-life high-fat diet-induced obesity programs hippocampal development and cognitive functions via regulation of gut commensal Akkermansia muciniphila. Neuropsychopharmacology 44, 2054–2064. doi: 10.1038/s41386-019-0437-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C, Zhang M, Pang X, Zhao Y, Wang L, and Zhao L (2012). Structural resilience of the gut microbiota in adult mice under high-fat dietary perturbations. ISME J 6, 1848–1857. doi: 10.1038/ismej.2012.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 4 Abundance of the gut microbiota in the SUG vs CHOW groups when analyzed either before (2A) or after (2B) healthy dietary intervention. CHOW: chow-fed; SUG: sugar; FDR: false discovery rate
Supplemental Figure 3 Abundance of the gut microbiota in the CAF vs CHOW groups when analyzed either before (1A) or after (1B) healthy dietary intervention. CHOW: chow-fed; CAF: cafeteria diet; FDR: false discovery rate
Supplemental Figure 1 Energy balance and metabolic outcomes following adolescent cafeteria diet consumption in CAF cohort 2. There were no overall significant group differences in body weight (A), total caloric intake (B), or glucose tolerance in the IPGTT test (C and D), although rats in the CAF group consumed significantly fewer calories during the period following the onset of the healthy dietary intervention (B, right). Percent total calories from each food item in the CAF diet in cohort 2 as well as % macronutrient composition of the total calories consumed are depicted in (E). Data are means ± SEM; CHOW: chow-fed n=15; CAF: cafeteria diet n=16; kcal: kilocalories; IPGTT: intraperitoneal glucose tolerance test
Supplemental Figure 2 Energy balance and metabolic outcomes following adolescent sugar diet consumption in SUG cohort 2. There were no overall significant group differences in body weight (A) or total caloric intake (B), although rats in the SUG group consumed fewer calories from chow (C) when consuming the sugar solution (D). Data are means ± SEM; n=9/group CHOW: chow-fed; SUG: sugar; kcal: kilocalories


