Abstract
Background:
Nephrotic syndrome is a common complication of pig-to-baboon kidney xenotransplantation (KXTx) that adversely affects outcomes. We have reported that upregulation of CD80 and down-regulation of SMPDL-3b in glomeruli have an important role in the development of proteinuria following pig-to-baboon KXTx. Recently we found induced expression of human CD47 (hCD47) on endothelial cells and podocytes isolated from hCD47 transgenic (Tg) swine markedly reduced phagocytosis by baboon and human macrophages. These observations led us to hypothesize that transplanting hCD47 Tg porcine kidneys could overcome the incompatibility of the porcine CD47-baboon SIRPα interspecies ligand-receptor interaction and prevent the development of proteinuria following KXTx.
Methods:
Ten baboons received pig kidneys with vascularized thymic grafts (n = 8) or intra-bone bone marrow transplants (n = 2). Baboons were divided into three groups (A, B, and C) based on the transgenic expression of hCD47 in GalT-KO pigs. Baboons in Group A received kidney grafts with expression of hCD47 restricted to glomerular cells (n = 2). Baboons in Group B received kidney grafts with high expression of hCD47 on both glomerular and tubular cells of the kidneys (n = 4). Baboons in Group C received kidney grafts with low/no glomerular expression of hCD47, and high expression of hCD47 on renal tubular cells (n = 4).
Results:
Consistent with this hypothesis, GalT-KO/hCD47 kidney grafts with high expression of hCD47 on glomerular cells developed minimal proteinuria. However, high hCD47 expression in all renal cells including renal tubular cells induced an apparent destructive inflammatory response associated with upregulated thrombospondin-1. This response could be avoided by a short course of weekly anti-IL6R antibody administration, resulting in prolonged survival without proteinuria (mean 170.5 days from 47.8 days).
Conclusion:
Data showed that transgenic expression of hCD47 on glomerular cells in the GalT-KO donor kidneys can prevent xenograft nephropathy, a significant barrier for therapeutic applications of xenotransplantation. The ability to prevent nephrotic syndrome following KXTx overcomes a critical barrier for future clinical applications of KXTx.
Keywords: CD47, kidney, podocyte, preclinical model, proteinuria, xenotransplantation
1 |. INTRODUCTION
Xenotransplantation (XTx) could provide a limitless supply of organs that would address the current worldwide shortage, including life-saving organs for recipients previously unable to receive a transplant due to allo presensitization.1–3 Induction of tolerance could reduce the need for the extensive immunosuppression now required to prevent rejection of pig-to-primate xenografts.4,5 Thymic transplantation (Tx) has proven to be a powerful strategy to induce T cell tolerance across both allogeneic and xenogeneic barriers in pig-to-pig, pig-to-mouse, pig-to-humanized mouse models.6–10 In the pig-to-pig model, we found that donor thymic tissue should be transplanted as a vascularized graft, either as a thymokidney (TK)11 or as a vascularized thymic lobe graft (VTL-Tx).12 The vascularized thymus (VT) transplantation strategy can induce tolerance across fully allogeneic barriers.4,6–8 Our initial study of kidney plus VT (K+VT) XTx from α–1,3-galactosyltransferase gene knockout (GalT-KO) pigs with the administration of an anti-CD40 ligand (CD40L) monoclonal antibody (mAb) induced donor-specific unresponsiveness and significantly extended xenograft survival time, from 29 days up to 83 days in baboons.13,14 While our regimen that included the VT-Tx prevented sensitization of recipient baboons to the xenograft, all baboon recipients developed proteinuria, occurring as early as postoperative day (POD) 2. Interestingly, renal function was preserved, and the renal histology showed only focal changes with minimal or absent anti-pig antibody (Ab) deposits by immunofluorescence and with electron microscopy showing diffuse effacement of podocyte foot processes.15,16 This histologic pattern of “xenograft nephropathy” has a similarity with the clinical entities of minimal change disease (MCD) and early focal segmental glomerulosclerosis.15,17 Consistent with this type of disease pattern, baboons also developed severe nephrotic syndrome with anasarca, ascites, and pleural effusions, as well as infections and thrombosis, increasing the risk of graft loss, and mortality.
The nephrotic syndrome in our model resembles a steroid-resistant type of MCD, and mechanistically involves down-regulation of sphingomyelin phosphodiesterase acid-like 3b (SMPDL-3b) and upregulation of CD80 on pig endothelial cells and podocytes (Figure S1).16,17 We previously reported that Rituximab (anti-CD20 antibody) binds and stabilizes porcine podocyte SMPDL-3b16 and delayed the onset of proteinuria in baboons receiving pig xenograft kidneys.15 Nevertheless, this effect lasted only 2–3 weeks. Therefore, there is a need for additional strategies to both prevent and treat xenograft nephropathy if we are to improve the long-term survival of baboon recipients.
CD47 is an important ligand that binds SIRPα on macrophages and other immune cells where it helps maintain the macrophages in a nonactivated state. However, porcine CD47 is not able to induce SIRPα tyrosine phosphorylation in human macrophage-like cell lines consistent with incompatibility, while soluble human CD47-Fc fusion protein was able to inhibit the phagocytic activity of human macrophages toward porcine cells.18 Consistent with this observation, we reported that phagocytosis of porcine ECs by baboon macrophages was significantly reduced when porcine ECs and podocytes expressed hCD47/hCD55 but not hCD46/hCD55.19 This observation led us to hypothesize that an incompatibility between pig CD47 and baboon SIRPα could play a role in initiating the nephrotic syndrome in this xenograft model.
Here, we report a new treatment strategy that appears effective at preventing proteinuria and prolonging survival in baboon recipients of porcine kidney grafts co-transplanted with VTs. We found that porcine kidneys expressing hCD47 on glomeruli developed minimal proteinuria post-KXTx in baboons. We also found high hCD47 expression in all renal cells including renal tubular cells induced anapparent destructive inflammatory response associated with upregulated thrombospondin-1 (TSP-1).20 This response could be avoided by a short course of weekly anti-IL6R antibody administration, resulting in prolonged survival without proteinuria (mean 170.5 days).
2 |. METHODS
2.1 |. Animals
2.1.1 |. Baboons
Papio hamadryas were purchased from the Mannheimer Foundation (Home- stead, FL). We used baboons that had less than 30% cytotoxicity of preformed nAb against GalT-KO pig PBMC (after % killing of negative control was subtracted) at 1:4 ratio of effector to targets for xenotransplantation in this study. The method of the screening assays using GalT-KO pig PBMCs is described below. Baboons that were euthanized in 30 days due to early postoperative complications associated with posttransplant technical failure or acute cytomegalovirus (CMV) infection were excluded.
2.1.2 |. Pigs
Pig donors were provided by Accuro Farms, Inc. (Southbridge, MA, USA) and Revivicor Inc. (Blacksburg, VA, USA).21–24 Details of the use of pigs are described in the Groups and immunosuppression sections. All organ donors were porcine CMV (PCMV) negative.25
All animal work was conducted in accordance with NIH and USDA guidelines and with approval from the Columbia University Institutional Animal Care and Use Committee.
3 |. EXPERIMENTAL GROUPS AND IMMUNOSUPPRESSION
A total of 10 baboons were divided into three groups (Table 1). Eight baboons received kidneys and vascularized thymic grafts (K+VT) from the same donors,7,14,26 and two received a kidney 1 month after intra-bone bone marrow transplantation (IBBMTx followed by delayed KTx).27 Baboons were divided into three groups (A, B, and C) based on the transgenic expression of hCD47 in GalT-KO pigs (Table 1). Glomerular expression of hCD47 was assessed quantitatively by mean fluorescence value (MFV) using ImageJ software version 1.51k (https://imagej.nih.gov/ij/). All animals, except animals in Group A, received anti-CD40 mAb instead of anti-CD40L mAh following K+VT XTx. CTLA4-Ig at 10 mg/kg was given once a week as “rescue CTLA4-Ig therapy” after 2+ proteinuria developed in the first postoperative month.17 Anti-IL6R mAb (ACTEMRA [tocilizumab], Roche) at 10 mg/kg/day was given weekly from POD 15 in three animals (Two in Group B and one in Group C). Details are described in supplemental document.
TABLE 1.
human CD47 expression, other transgenes (Tg), immunosuppression, urine protein and survival of recipients after GalT-KO+Tg K+VT XTx
| hCD47 in graft kidney (High, Low or No) |
Other Tgs |
Immunosuppression |
U-proteinuria |
Survival |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Group | Distribution of hCD47 Expression in the donor kidneys | Baboon ID | Glomeruli | Tubules | Human CRP | Other Tg | Anti-CD40L mab or anti-CD40 mab | Anti-IL6R Ab | CTLA4-lg Rescue therapy After 2+ proteinuria | 1+ or less throughout | 2+ | 3+ | Reverse to 1+ | POD | Avg | Cause of euthanasia and sCre at the end point |
| A | HIGH/LOW Glomerular &NO Tubular expression of hCD47 | 12P86 | High | NO | CD55 | NO | Anti-CD40L | NO | NO | POD 1-128 | NO | 128 | 128 | Graft growth, Cr 1 mg/dl | ||
|
| ||||||||||||||||
| 14P9 | Low | NO | NO | POD 47-68 | NO | 68 | 68 | Pleural effusion, Cr 2.8 mg/dl | ||||||||
|
| ||||||||||||||||
| B | HIGH Glomerular & HIGH Tubular expression of hCD47 | 1615 | High | High | CD55 | NO | Anti-CD40 | NO | NO | POD 1-48 | POD 49-50 | NO | 50 | 51.5 | Tracheal edema Cr 1.6 mg/dl | |
|
| ||||||||||||||||
| 2415 | High | High | POD 1-53 | NO | 53 | Tracheal edema Cr 2.1 mg/dl | ||||||||||
|
| ||||||||||||||||
| 14P18 | High | High | CD46, CD55 | CMAHKO, B4KO, EPCR, TBM, HO-1 | Anti-CD40 | YES | NO | POD 1-154 | NO | 154 | 170.5 | Graft growth Cr 2.8 mg/dl | ||||
|
| ||||||||||||||||
| 14P66 | High | High | POD 1-185 | POD 186-187 | NO | 187 | MMF crystallization in SVC, Cr 0.9 mg/dl | |||||||||
|
| ||||||||||||||||
| C | LOW/NO Glomerular & HIGH Tubular expression of hCD47 (Rescue CTLA4-lg) | 13P60 | NO | High | CD46 | EPCR | Anti-CD40 | NO | POD 16 | NO | POD 15-42 | NO | 42* | 56* | Kidney graft excised from this animal on POD 42 for studies unrelated to this project Cre 2.0mg/dl | |
|
| ||||||||||||||||
| 13P79** | NO | High | TFPI | POD 22 | NO | POD 21-36 | NO | POD 37-70 | 70 | **IBBMTx delayed KTx, Pneumonia, Cr 0.8 mg/dl | ||||||
|
| ||||||||||||||||
| 14P56** | NO | High | TFPI | POD 24 | NO | POD 17-34 | NO | POD 35-41 | 41 | **IBBMTx delayed KTx. Pneumonia, Cr 0.9 mg/dl | ||||||
|
| ||||||||||||||||
| 14P65 | Low | High | CD46 | CMAHKO, TBM, EPCR | Anti-CD40 | YES | POD 17 | NO | POD 15-80 | NO | NO | 80 | 80 | Baboon CMV.Cr 0.8 mg/dl | ||
Excluded 13P60 from the average because the kidney graft was removed for studies unrelated to this project.
Baboons that received IBBMTx one month prior to kidney Tx. No VT grafts were co-transplanted.
4 |. SURGERY AND ASSESSMENT OF KIDNEY GRAFTS FOLLOWING TRANSPLANTATION
All recipients of K+VT grafts were thymectomized 1 week before XTx. Simultaneous K+VT XTx were performed as previously reported.7,14,26,28 Host thymectomy was performed prior to Tx.14 Central venous lines were placed for drug administration and blood sampling 1 week prior to Tx. Bilateral native kidney nephrectomies and splenectomy were performed at the time of Tx. Kidney graft function was assessed daily by sCre levels. Pathology of excised kidneys and biopsy samples were extensively evaluated using H&E, PAS, and immunofluorescence for IgM, IgG, and complement (C) deposits. In order to assess proteinuria daily, urine dipsticks (Multistix, Siemens AG, Munich, Germany) that qualitatively measures urinary albumin concentration with the following range (1+; 30 mg/dl, 2+; 100 mg/dl, 3+; 500 mg/dl, 4+; > 500 mg/dl) were used. Urine samples were taken from a 24-hour urine collection container. We minimized the risk of protein contamination of urine from food or feces by rapid drainage of urine from the cage pan into a urine collection container.
5 |. STATISTICS
Statistical analysis was performed by two-sided Student’s t-test and Log rank test with EZR version 1.50),29 which is for R (version 4.0.2). p < 0.05 was considered to be significant.
6 |. RESULTS
6.1 |. Effects of human CD47 expression in porcine kidney grafts following K+VT Tx in baboons
6.1.1 |. Variable hCD47 expression in kidneys of hCD47 Tg pigs
Baboons were divided into three groups (A, B, and C) based on the transgenic expression of hCD47 in GalT-KO pigs. Although multiple passages of the transgene through the germline was reported to stabilize the expression of the transgene,22 animals used in this study were all recently derived by nuclear transfer, and the transgene expression may not yet have stabilized. Details of the Tg and KO production have been published.21–24 Table 1 shows glomerular and tubular hCD47 expression patterns of the donor kidneys, type of induction therapy, duration of survival, the cause of euthanasia, and the sCre levels at the endpoint of each baboon recipients in Groups A-C.
Baboons (12P86 and 14P9) in Group A received kidney grafts with expression of hCD47 restricted to glomerular cells with weak expression on endothelial cells and without tubular expression of hCD47. Baboon 12P86 received a kidney with high glomerular expression of hCD47 with peritubular capillary hCD47 expression (MFV of 22.3 for glomerular structures, Figure 1A–a), while the other baboon 14P9 received a kidney with low glomerular expression of hCD47 (MFV 14.2, Figure 1A–b). The MFV of a GalT-KO kidney as the control was 6.7 (Figure 1A–c).
FIGURE 1.
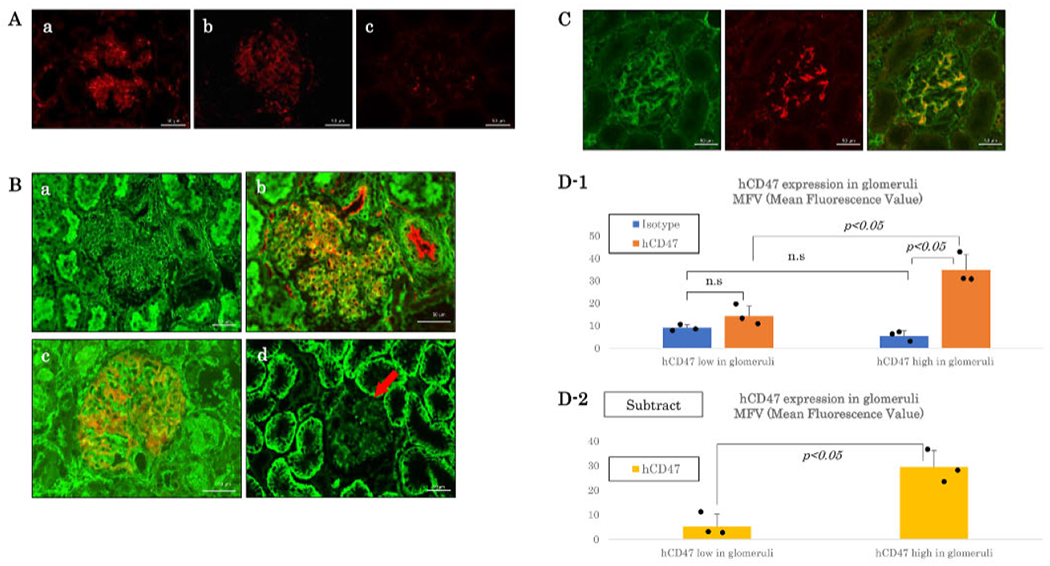
Glomerular and/or Tubular hCD47 expression pattern in hCD47Tg GalT-KO kidneys in Groups A, B and C. (A) Immunofluorescence staining of hCD47 (red) in hCD47Tg GalT-KO kidneys in Group A. (a) High hCD47 expression on glomeruli and peritubular capillaries (red) of Baboon # 12P86’s donor kidney, (b) Weak expression of hCD47 only in glomeruli (red) of Baboon # 14P9’s donor kidney (c) hCD47 expression in a GalT-KO pig kidney as the control. Bar: 50 μm. (B) Immunofluorescence staining of hCD47 (green) in GalT-KO/hCD47 kidneys in Groups B and C. (a) hCD47 expression was high in both tubular and glomerular cells of # 2415’s (Group B). Donor kidney, (b) Double staining of hCD47 (green) and CD31 (red) in # 2415’s (Group B) donor kidney, (c) Double staining of hCD47 (green) and Nephrin (red) (contra lateral kidney from the # 2415’s). (d) hCD47 expression was high in renal tubular cells with weak/no hCD47 expression on glomerular cells (red arrow) (# 13P60’s donor kidney. Group C). (C) hCD47 (green), Nephrin (red), and merged images of naive baboon kidneys showing high expression hCD47 in the podocytes of the glomerular cells with a feeble expression on renal tubular cells. Bar: 50 μ m. (D) Quantitative analysis of hCD47 in glomeruli of donor kidneys. D1: Average ± SD of MFVs of isotype controls and hCD47 in glomeruli (n = 3, each). D2: MFVs of isotype controls were subtracted from MFVs of hCD47. The dots in the figure D1 and D2 indicate each value. The two-sided Student’s t-test was used
Baboons in Group B received kidney grafts with high expression of hCD47 on both glomerular and tubular cells of the kidney (Figure 1B–a, b, and c). Figure 1B–b shows hCD47 expression on glomerular capillaries by double immunofluorescence (hCD47–green and CD31–red). Figure 1B–c shows hCD47 is expressed on the glomerular podocytes (hCD47–green and Nephrin, podocyte marker–red). Baboons in Group C received kidney grafts with low/no glomerular expression of hCD47 (red arrows in Figure 1B–d), and high expression of hCD47 on renal tubular cells (green in Figure 1B–d). Figure 1C showed representative findings of hCD47 (green), Nephrin (red), and merged images of naive baboon kidneys showing high expression hCD47 in the podocytes of the glomerular cells with minimal expression on renal tubular cells.
In order to assess glomerular expression of hCD47 was also assessed semi-quantitatively using tmageJ software version 1.51k (https://imagej.nih.gov/ij/), paraffin-embedded samples containing at least 10 glomeruli were obtained from contra-lateral kidneys of donors in Groups B and C. Unfortunately, no samples were available to restain renal graft tissue with FITC from baboons in Group A. Scoring of high versus low hCD47 expression shown in Table 1 correlated with the results of quantitative data of MFV assessed using Image J version 1.51k (Figure 1D). Average MFV of glomerular hCD47 was 34.86 ± 6.89 in those with high glomerular expression (Group B) whereas it was 14.36 ± 4.40 in kidneys considered to have a low glomerular expression (Group C) (Figure 1D–1). No statistical difference in isotype staining was found (MFV 9.09 ± 1.27 vs. 5.36 ± 2.33. p > 0.05). MFVs of hCD47 after isotype-subtraction between hCD47 low and high groups also reached statistical differences (5.27 ± 5.04 vs. 29.50 ± 6.61. p < 0.05) (Figure 1D–2). One sample, each from 2415 (Group B) and 13P79 donor (Group C), was excluded from this analysis because of high background isotype staining (MFV > average+2SD) due to sample degradation (long-term storage in formalin).
6.1.2 |. High expression of hCD47 on renal glomerular cells in pig kidney grafts prevents the development of proteinuria in baboon recipients
Baboon 12P86 in Group A and all baboon recipients in Group B received kidney grafts with high expression of hCD47 on glomerular cells (Figure 1A–a, 1B–a, b, and c). These baboons maintained proteinuria of less than 1+ throughout the experimental period except for two baboons (1615 and 14P66), which developed 2+ proteinuria in their last two days (Table 1). None of these recipients received prophylactic CTLA4-lg. In contrast, a baboon recipient (14P9) in Group A that received a kidney graft with weak expression of hCD47 (Figure 1A–b) on the glomerular cells developed proteinuria at POD 47 and was euthanized due to pleural effusions and respiratory distress at POD 68. Similarly, all baboons in Group C that obtained kidneys with “low or no expression of hCD47 on the glomerular cells” and “high hCD47 expression on the renal tubular cells” (Figure 1B–d) also developed 2+ proteinuria by POD 21. Although CTLA4-lg rescue therapy was partially effective to reverse proteinuria to 1+ in two animals, they were euthanized at POD 41, 70, and 80. One recipient (13P60) had its graft excised on POD 42 for studies unrelated to this project.
6.1.3 |. High expression of hCD47 in renal tubular cells in kidney grafts and porcine passenger thymocytes of VT grafts led to harmful effects associated with TSP-1 up-regulation
Baboons in Group B received K+VT grafts with ubiquitous and profuse expression hCD47 on all kidney cells, including renal tubular cells (Figure 1B–b) and thymocytes of VTL grafts. In our initial experiments with two baboons (1615 and 2415), we observed a limited survival of POD 50 and POD 53, respectively, similar to the historic controls (47.75 ± 8.84 days).15,17 Although neither of these baboons developed 2+ proteinuria during the first seven weeks (Figure 2A–1, 2), both baboons developed significant face and limb edema starting in the second week. These animals were euthanized on POD 50 and 53 because of the respiratory difficulty that developed with tracheal edema. Unlike other groups, the excised kidney graft of baboon 1615 had interstitial cell infiltrates and glomerular changes, despite the relatively low sCre (2.0 mg/dl) and proteinuria 1+ at POD 50 (Figure 2A–1, B). The other animal (2415), however, had mild cell infiltration (Figure 2C).
FIGURE 2.
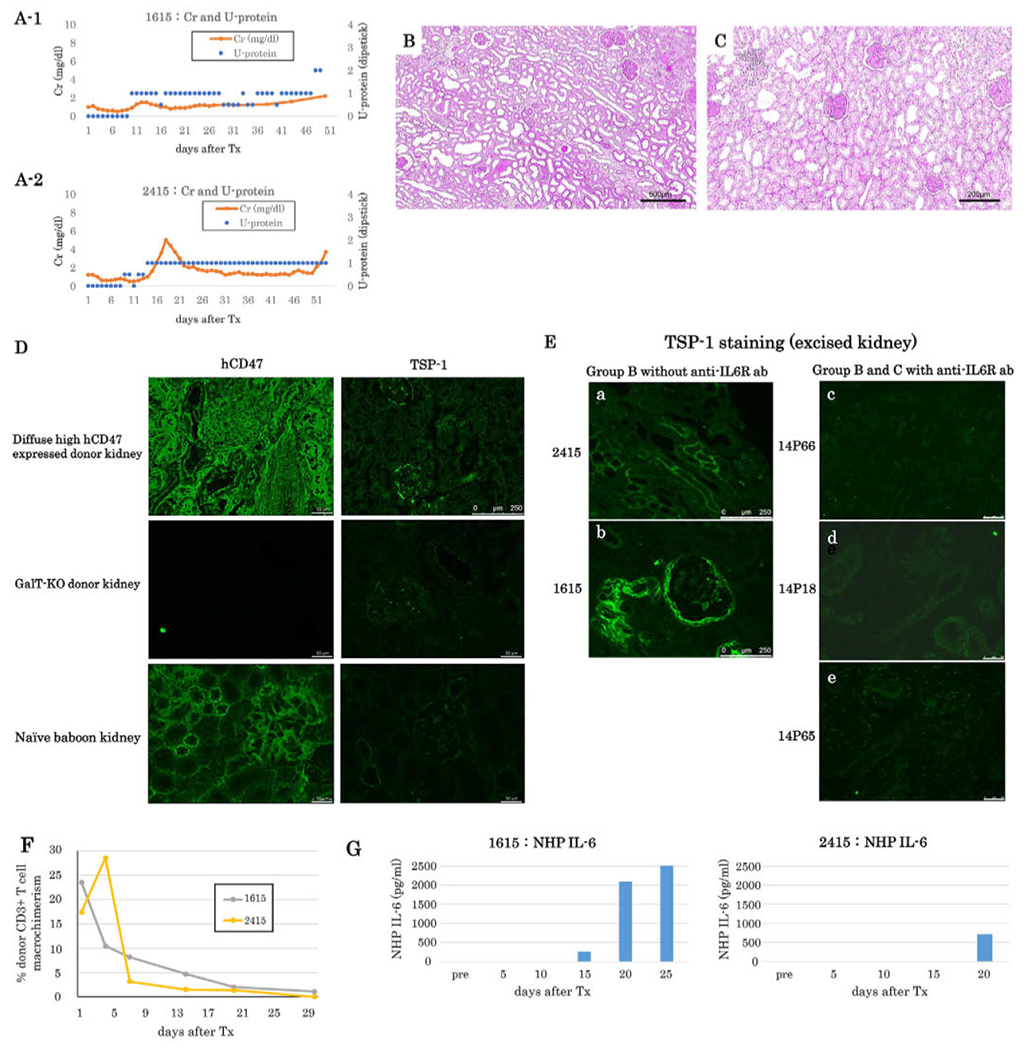
(A) sCr (orange lines) and urine protein (blue dots) of baboons following K+VT XTx in Group B (A-1: #1615, A-2: #2415). Proteinuria did not exceed 2+ except for the last two days in baboon #1615. (B) Excised graft kidney of #1615 showing interstitial cell infiltrates and glomerular changes (PAS, Bar: 500 μm). (C) Excised graft kidney of #2415 showing less interstitial cell infiltrates (PAS, Bar: 200μm. (D) hCD47 and TSP-1 expression under untreated normal condition in GalT-KO/hCD47 pig kidney (top left: hCD47 staining; top right: TSP-1 staining), GalT-KO without hCD47 Tg pig kidney (middle left: hCD47 staining; middle right: TSP-1 staining) and naïve baboon kidney (bottom left: hCD47 staining; bottom right: TSP-1 staining). TSP-1 was weakly positive in GalT-KO/hCD47 high pig kidney (Top right), while others did not show TSP+ cells. Bar: 50 μm. top right 250 μm. (E) TSP-1 expression in excised kidney graft tissue; a #2415 and b #1615 in Group B Without anti-IL6R Ab treatment (Bar: 250 μm), c #14P66 and d #14P18 in Group B with anti-IL6R ab treatment, and e; #14P65 in Group C with anti-IL6R Ab treatment. (Bar: 50μm). In Group B without anti-IL6R Ab treatment, both excised graft kidneys of #2415 and #1615 had upregulation of TSP-1 (a and b), while no TSP-1 upregulation was observed in kidney grafts of 14P66, 14P18, and 14P65 that were treated with anti-IL6R Ab. (F) The percentage of peripheral blood macrochimerism (pig CD3 positive T cells) of #1615 (gray line) and #2415 (yellow line) in Group B following K-VT XTx. (G) IL-6 concentration assessed by ELISA in the sera of Group B baboons (left: #1615, right; #2415). Both baboons had a high IL-6 concentration between POD 15 and 25
While CD47 binds to the SIRPα receptor and blocks its activation, CD47 also binds to TSP-1 (CD47-TSP-1 pathway),18,20 which inhibits nitric oxide signaling in vascular cells and activates the innate immune response.30 We, therefore, examined whether the expression of TSP-1 was upregulated in Group B baboon recipients that received kidney grafts with profuse and abundant expression of hCD47 across different cell types of the kidney. While no TSP-1 positive cells were observed in either naïve GalT-KO pig (without hCD47 Tg) or baboon kidneys, TSP-1 was weakly expressed fn the media of blood vessels of pig kidneys with an abundant and profuse expression of hCD47 among different cell types of the kidney (Figure 2D). The upregulation of TSP-1 in the excised kidney grafts was clearly seen in both baboons, 2415 and 1615) of Group B (Figure 2E–a, and b), while no upregulation of TSP-1 was observed in the kidney grafts in baboons that received anti-IL6R ab (Figure 2E–c, d, and e).
The hCD47 expression on thymocytes of the VT grafts was highly positive in Group B. Potential pig thymic emigrants from the VT grafts may have contributed to the high pig CD3+ T cell macrochimerism (>10%) observed in the first five days following K+VT Tx (Figure 2F). Subsequently, the sera from these animals showed increased levels of IL-6 between POD 15, 20 and 25, and reaching >2000 pg/ml in baboon recipient 1615 (Figure 2G). The increase in IL-6 concentration in both baboons coincided with significant facial and tracheal edema without proteinuria.
6.1.4 |. Treatment with Anti-IL6R Ab prevented the detrimental effects of widespread hCD47 expression in kidneys and VT grafts and permitted long-term survival
Based on results in the first two animals in Group B, we administered anti-IL6R Ab from POD 15 once weekly and added low-dose steroids at 2 mg/kg in the first two weeks and then at 1 mg/kg until POD 60 to the baboon recipients in next two animals, 14P18 and 14P66, in Group B. Low-dose steroid was administered to decrease porcine thymocytes.31 Baboon 14P18 received anti-IL6R Ab throughout the experimental period; however, baboon 14P66 received only five doses until POD 42. Strikingly, both recipients suffered no complications from edema and survived for 154 and 187 days, respectively (Table 1). Both had no proteinuria except at the end point (Figure S2, Orange line). sCre levels were mostly stable except one had ureteral stent obstruction around POD 120 (Figure 3A–1). sCre decreased but was gradually increased thereafter due to graft growth induced compartment syndrome in baboon 14P18. Histology of the excised kidney graft had interstitial edema but no endothelialitis nor tubulitis was observed (Figure 3B–a, and b). The other (14P66) baboon had stable renal function with low sCre levels throughout the experimental period (Figure 3A–2). Unfortunately, this animal also was euthanized due to SVC syndrome from a large mass associated with MMF Intravenous infusion (Figure 3C–a). There was no rejection of kidney grafts grossly and histologically (Figure 3C–b, c, and d). These animals had lower % of donor CD3T cells chimerism (thymic emigrants) than animals in Group B (Figure 3D). Both baboons had only small increases in IL-6 in their sera following K+VT XTx (Figure 3E). Neither of the baboons in this group showed TSP-1 upregulation (Figure 2E–c, and d). We also used this anti-IL6R Ab with low dose steroid treatment in animal 14P65 that received K+VT Tx in Group C. The kidney graft had low expression of hCD47 on glomerular cells, and the animal developed proteinuria that required for the CTLA4-lg therapy. Anti-IL6R Ab therapy appeared to be effective to minimize development of facial edema, and no TSP upregulation was seen in the graft (Figure 2E–e). However, this anti-IL6R Ab and steroid therapy did not prevent the development of proteinuria, and the baboon was euthanized at POD 80 due to CMV colitis, suggesting the combined CTLA4-lg, anti-IL6R Ab and steroid therapy in baboon with 2+ proteinuria is toxic.
FIGURE 3.
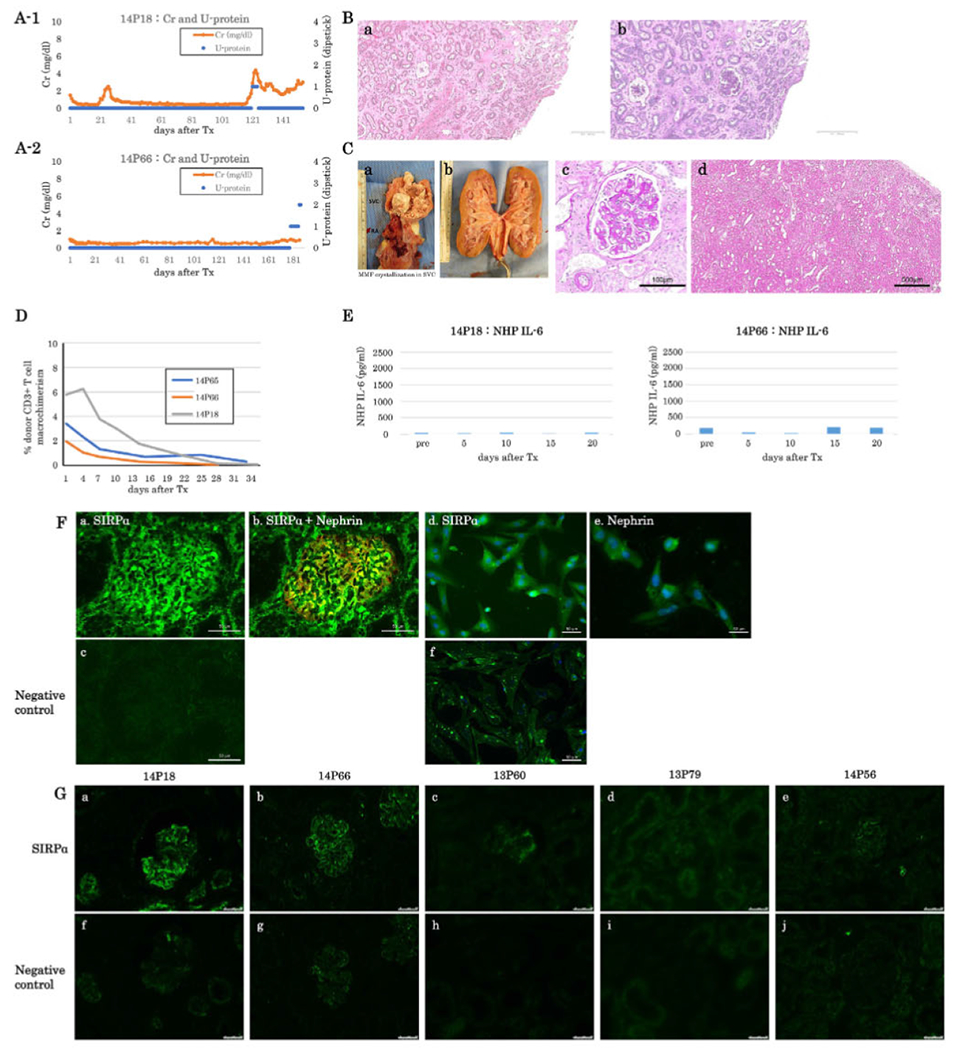
(A) sCre (orange lines) and urine protein (blue dots) of baboons following K+VT XTx in Group B with anti-IL6R Ab treatment (A-1: #14P18, A-2: #14P66). Both baboons survived over five months. Baboon #14P66 developed 2+ proteinuria only in the last two days (A-2). (B) Excised graft kidney of #14P18 on POD 154 (B-a: H&E and B-b PAS, low magnification, bar: 500 μm). (C) Photo of MMF crystallization in #14P66 SVC (C-a). Photo Of #14P66’s excised graft kidney (C-b). The color of the excised graft kidney was grossly normal. Excised graft kidney on POD 187 of #14P66 (C-c: PAS, high magnification, Bar: 100μm. C-d: HE, low magnification, Bar: 500 μm. No histologic evidence of rejection. (D) Percentage of peripheral blood macrochimerism (pig CD3 positive T cells) of #14P66 (orange line. Group B) and #14P18 (gray line, Group B) and #14P65 (blue line, Group C) following K-VTXTx. The macrochimerism of baboons in these baboons was lower than that 1615 and 2415 (F). (E) IL-6 concentration in sera of Group B (left: #14P18, right: #14P66). Either baboon showed no elevation of IL6 concentration. (F) SIRPα expression (green) in a naive pig kidney (a) and isolated podoeytes (d). (b) A merged image of SIRPα (green) and Nephrin (red) expression in the naive pig kidney, (e) Nephrin expression on isolated pig podoeytes. (c) and (f) negative controls. Bar: 50μm. (G) SIRPα expression in pig kidney grafts from long-term acceptors, 14P18 (a) and 14P66 (b) in Group B and from baboons 13P60 (c), 13P79 (d), and 14P56 (e) in Group C that developed > 2+ proteinuria, (f-j) are negative controls. Bar: 50μm
6.1.5 |. Glomerular SIRPα is downregulated in kidney grafts from baboons that developed proteinuria
We next examined the expression of SIRPα in glomeruli of naïve pig glomeruli and pig kidney grafts in baboons. SIRPα expression was observed in podocytes from naïve pig kidneys (Figure 3F–a, b, and d) and pig kidney grafts from long-term acceptors (14P18, 14P66) in Group B (Figure 3G–a, and b). In contrast, no SIRPα expression was detected in podocytes from pig kidney grafts in baboons that developed > 2+ proteinuria (13P60, 13P79, 14P56) in Group C (Figure 3G–c, d, and e). Interestingly, pig kidney grafts with preserved podocyte SIRPα expression had high hCD47 on glomerular cells whereas baboons with podocyte SIRPα loss had low hCD47 expression on glomerular cells.
6.2 |. Statistical analysis of xenograft survival related to nephrotic syndrome
We compared groups that developed nephrotic syndrome (Nephrotic syndrome group; n = 5,) with non-nephrotic syndrome baboons (Non-nephrotic syndrome; n = 3), excluding Group B that were euthanized at PODs 50 and 53 due to hCD47-TSP-1 associated tracheal edema. The average survival ± SD was 60.2 ± 17.67 days and 156.33 ± 29.57 days, respectively. There was a statistically significant difference (p = 0.0136). Of note, all of animals in non-nephrotic syndrome received kidney grafts in which glomerular cells expressed hCD47 highly, while all in nephrotic syndrome animals had either no or low hCD47 expression in glomeruli (Table 1, Figure S2).
7 |. DISCUSSION
Nephrotic syndrome remains a major complication of pig-to-baboon KXTx. However, the pathogenesis of proteinuria in this model is not completely understood. In this study, we provided evidence to support that species incompatibility involving the SIRPα-CD47 pathway is responsible for the development of proteinuria following pig-to-baboon KXTx. Specifically, we showed that high expression of hCD47 on glomerular cells in GalT-KO pig kidney grafts prevented the development of proteinuria. In contrast, GalT-KO pig kidney grafts with low glomerular hCD47 expression exhibited significant proteinuria along with podocyte SIRPα loss. Therefore, disruption of the CD47-SIRPα pathway may impair podocyte integrity.
The mechanism by which high hCD47 expression may prevent proteinuria could relate to blocking SIRPα activation on circulating baboon monocyte-macrophage activation in response to hCD47 expression on porcine glomerular endothelial cells. By blocking the activation of the circulating inflammatory cells, it could potentially block the release of factors that could activate both glomerular endothelial cells and podocytes that could lead to podocyte shape change and proteinuria.32,33 In the absence of human CD47 in the glomerulus, the activation of the porcine glomerular endothelium due to CD47 incompatibility could lead to activation of glomerular endothelial cells and podocytes by CD80 expression which has been observed in both MCD34 and xenograft-associated nephrotic syndrome.17 In turn, endothelial cell activation could lead to release of mediators, including miRNA.35 that could theoretically activate podocytes and contribute to podocyte SIRPα loss36 leading to shape change and the development of proteinuria.37 In this scenario, the loss of SIRPα on the podocytes in proteinuric baboons could be a secondary phenomena to endothelial activation. It is also possible that the CD47 may preserve podocyte SIRPα activation, and this is important in prevention of proteinuria. Further studies will be needed to sort this out. While it is possible that other additional transgenes (EPCR, HLA-E, TFPI, thrombomodulin [TBM], and CMAH- and/or B4-KO) present in the kidney grafts of the Group B and C Baboons (Table 1) could have had protective role, the current data and our observations from the previous study22 suggests additional transgenes (EPCR, HLA-E, TFPI, TBM, and CMAH- and/or B4-KO) that were used in our studies may not necessarily have additional advantages inhibiting proteinuria or preventing xenograft rejection. In order to confirm it, the expression of proteins by these genetic modifications needs to be carefully assessed.
While the CD47-SIRPα interaction appears to reduce phagocytosis18,19 and prevent the development of proteinuria, the CD47-TSP-1 Interaction in the baboon may be associated with both systemic and intra-graft inflammatory changes.20,38,39 Recent reports have demonstrated that CD47 mAb blockade decreases ischemic reperfusion injury of DCD kidney grafts in allogeneic rat and swine model transplants.40,41 Using mouse allogeneic heart Tx models, a significant prolongation of WT allografts has been reported recently in recipient mice treated with antibodies against the CD47 ligand TSP-1 or with TSP-1 deficiency.40 These findings suggest that TSP-1-CD47 signaling may stimulate vascularized allograft rejection. In this study, two baboons (1615, 2415) in Group B that received K+VT grafts with an abundant and profuse expression of hCD47 expression on different cell types of kidneys developed systemic subcutaneous and tracheal edema, pleural effusion or ascites. We speculate that the systemic edema observed in Group B might have been triggered by graft versus host reaction (GVHR) in peripheral lymphoid tissue when pig thymic emigrants from pig hCD47+ VT graft started migrating into the peripheral blood/ lymph nodes during or after T cell chimerism. It remains unclear whether TSP-1 in blood vessels (Figure 2E) was upregulated by inflammatory responses related to a systemic GVHR or whether the intra-renal graft local inflammatory response was secondary to a direct interaction of TSP with high expression of CD47 in the tubulointerstitial compartment. The hCD47 expression in the grafts of the high expression group showed markedly higher and diffuse expression of hCD47 as compared to kidneys in naive baboons (Figure 2D). This interaction of TSP and CD47 has been shown to induce activation of T cells and innate responses42 in the grafts following transplantation. We plan to examine these mechanisms in the future using an in vitro culture system with isolated renal and vascular cells.
Uncontrolled graft growth witnessed in the present and earlier17,43,44 studies of kidney XTx in our pig-to-baboon models and the Munich group’s orthotopic heart XTx model45 was not reported in the pig-to-cynomolgus macaque’s K-XTx model, suggesting that xenogeneic species incompatibility may also be involved in this difference. Histology of the excised kidney grafts in baboons that were euthanized due to “graft growth” (indicated in Table 1) showed interstitial edema without tubulitis, vasculitis, or endothelialitis (consistent with a Page Kidney). We are currently studying the mechanisms of organ growth in our model.
Nephrotic syndrome has been a major complication of pig-to-baboon xenotransplantation and leads to shortened survival due to the development of anasarca, thrombosis, and infections. We demonstrate here that transgenic expression of hCD47 on glomerular cells in GalT-KO donor kidneys can prevent xenograft nephropathy. Our studies suggest that the inhibition of baboon macrophage activation to pig endothelial cells via the CD47-SIRPα pathway prevents the development of proteinuria. However, while the exogenous expression of hCD47 in glomeruli appeared to prevent proteinuria by overcoming the species incompatibility of the CD47-SIRPα pathway, hCD47 also appeared to be able to initiate an innate immune response by the CD47-TSP-1 pathway when it was expressed in tubules. The expression of human CD47 in tubules appeared to induce a TSP-1 mediated inflammatory response that could be managed by anti-IL6R Ab treatment in the induction period. It may be tempting to argue that the additional transgenes and/or deletion of the antigens could have contributed to the best survival of the recipient baboons in this study. Our observations from this study in Group B and C that included GalT-KO pigs with a variety of human transgenes, including EPCR, HLA-E, TFPI, and TBM offered no additional advantage over GalT-KO/hCD47 transgenic pigs for preventing xenograft proteinuria. Although the range of survival days is not wide in each group in this study, the number of cases in each group is small due to limitation of available pig donors. Our further studies with additional animals will provide firm conclusion about the role of effects of other transgene in preventing proteinuria. The identification of effective methods for preventing nephrotic syndrome following kidney xenotransplantation may remove one more barrier in our kidney xenotransplant project for patients suffering from end-stage kidney failure.
Supplementary Material
ACKNOWLEDGEMENTS
We would like to thank Dr. Masayuki Tasaki for his critical review of the manuscript. We thank Genzyme for providing rabbit ATG and Genentech for Rituximab. We also thank David Ayares, Harko Mulder, and Robert J Hawley for providing Tg pigs. We are grateful for the editorial and technical support of Michelle Santillan and Ermance Estime. This research was supported by NIH grant, P01AI45897, and sponsored research funding from Lung Biotechnology PBC. FCM analysis in this article was performed in the CCTI flow cytometry core, supported in part by the Office of the Director, NIH, under awards S10RR027050 and S10OD020056.
FUNDING INFORMATION
NIAID; Grant Number: P01AI045897
Abbreviations:
- CDC
complement-dependent cytotoxicity
- CVF
cobra venom factor
- EC
endothelial cell
- FCM
flow cytometric
- GalT-KO
α1,3 galactosyl transferase gene knockout
- KXTx
kidney xenotransplantation
- MCD
minimal change disease
- MFV
mean fluorescence value
- nAb
natural antibody
- PBMC
peripheral blood mononuclear cells
- POD
postoperative day
- sCre
serum creatinine
- SIRPα
signal-regulatory protein alpha
- SMPDL-3b
sphingomyelin phosphodiesterase acid-like 3b
- Tg
transgenic
- Tri-KO
triple knockout
- TSP-1
thrombospondin-1
- XTx
xenotransplantation
- K+VT
kidney plus vascularized thymus
- VT
Vascularized thymus
- VTL
vascularized thymic lobe
Footnotes
CONFLICT OF INTEREST
The authors declare no conflict of interest associated with this manuscript.
SUPPORTING INFORMATION
Additional supporting information may be found online in the Supporting Information section at the end of the article.
REFERENCES
- 1.Sautner T, Gnant M, Banhegyi C, et al. Risk factors for development of panel reactive antibodies and their impact on kidney transplantation outcome. Transpl Int. 1992;5(1):S116–S120. [DOI] [PubMed] [Google Scholar]
- 2.Ciurea SO, de Lima M, Cano P, et al. High risk of graft failure in patients with anti-HLA antibodies undergoing haploidentical stem-cell transplantation. Transplantation. 2009;88(8):1019–1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wong BS, Yamada K, Okumi M, et al. Allosensitization does not increase the risk of xenoreactivity to alpha1,3-galactosyltransferase gene-knockout miniature swine in patients on transplantation waiting lists. Transplantation. 2006;82(3):314–319. [DOI] [PubMed] [Google Scholar]
- 4.Yamada K, Sachs DH, DerSimonlan H. Human anti-porcine xenogeneic T cell response. Evidence for allelic specificity of mixed leukocyte reaction and for both direct and indirect pathways of recognition. J Immunol. 1995;155(11):5249–5256. [PubMed] [Google Scholar]
- 5.Le Bas-Bernardet S, Tillou X, Branchereau J, et al. Bortezomib, C1-inhibitor and plasma exchange do not prolong the survival of multi-transgenic GalT-KO pig kidney xenografts in baboons. Am J Transplant. 2015; 15(2):358–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nobori S, Samelson-Jones E, Shimizu A, et al. Long-term acceptance of fully allogeneic cardiac grafts by cotransplantation of vascularized thymus in miniature swine. Transplantation. 2006;81(1):26–35. [DOI] [PubMed] [Google Scholar]
- 7.Kamano C, Vagefi PA, Kumagai N, et al. Vascularized thymic lobe transplantation in miniature swine: thymopoiesis and tolerance induction across fully MHC-mismatched barriers. Proc Natl Acad Sci U S A 2004;101(11) :3827–3832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yamada K, Shimizu A, Utsugi R, et al. Thymic transplantation in miniature swine. II. Induction of tolerance by transplantation of composite thymokidneys to thymectomized recipients. J Immunol. 2000;164(6):3079–3086. [DOI] [PubMed] [Google Scholar]
- 9.Zhao Y, Swenson K, Sergio JJ, Arn JS, Sachs DH, Sykes M. Skin graft tolerance across a discordant xenogeneic barrier. Nat Med. 1996;2(11):1211–1216. [DOI] [PubMed] [Google Scholar]
- 10.Kalscheuer H, Onoe T, Dahmani A, et al. Xenograft tolerance and immune function of human T cells developing in pig thymus xenografts. J Immunol. 2014;192(7):3442–3450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yamada K, Shimizu A, lerino FL, et al. Thymic transplantation in miniature swine. I. Development and function of the “thymokidney”. Transplantation. 1999;68(11):1684–1692. [DOI] [PubMed] [Google Scholar]
- 12.LaMattina JC, Kumagai N, Barth RN, et al. Vascularized thymic lobe transplantation in miniature swine: i. Vascularized thymic lobe allografts support thymopoiesis. Transplantation. 2002;73(5):826–831. [DOI] [PubMed] [Google Scholar]
- 13.Barth RN, Yamamoto S, LaMattina JC, et al. Xenogeneic thymokidney and thymic tissue transplantation in a pig-to-baboon model: i. Evidence for pig-specific T-cell unresponsiveness. Transplantation. 2003;75(10):1615–1024. [DOI] [PubMed] [Google Scholar]
- 14.Yamada K, Yazawa K, Shimizu A, et al. Marked prolongation of porcine renal xenograft survival in baboons through the use of alpha1,3-galactosyltransferase gene-knockout donors and the cotransplantation of vascularized thymic tissue. Nat Med. 2005;11(1):32–34. [DOI] [PubMed] [Google Scholar]
- 15.Griesemer AD, Hirakata A, Shimizu A, et al. Results of gal-knockout porcine thymokidney xenografts. Am J Transplant 2009;9(12):2669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tasaki M, Shimizu A, Hanekamp I, Torabi R, Villani V, Yamada K. Rituximab treatment prevents the early development of proteinuria following pig-to-baboon xeno-kidney transplantation. J Am Soc Nephrol. 2014;25(4):737–744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rivard CJ, Tanabe T, Lanaspa MA, et al. Upregulation of CD80 on glomerular podocytes plays an important role in development of proteinuria following pig-to-baboon xeno-renal transplantation - an experimental study. Transpl Int. 2018;31(10):1164–1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ide K, Wang H, Tabara H, et al. Role for CD47-SIRPalpha signaling in xenograft rejection by macrophages. Proc Natl Acad Sci U S A. 2007;104(12):5062–5066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nomura S, Ariyoshi Y, Watanabe H, et al. Transgenic expression of human CD47 reduces phagocytosis of porcine endothelial cells and podocytes by baboon and human macrophages. Xenotransplantation. 2020;27(1):e12549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hayat SMG, Bianconi V, Pirro M, Jaafari MR, Hatamipour M, Sahebkar A. CD47; role in the immune system and application to cancer therapy. Cell Oncol (Dordr). 2020;43(1):19–30. [DOI] [PubMed] [Google Scholar]
- 21.Tena A, Kurtz J, Leonard DA, et al. Transgenic expression of human CD47 markedly increases engraftment in a murine model of pig-to-human hematopoietic cell transplantation. Am J Transplant. 2014;14(12):2713–2722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Watanabe H, Sahara H, Nomura S, et al. GalT-KO pig lungs are highly susceptible to acute vascular rejection in baboons, which may be mitigated by transgenic expression of hCD47 on porcine blood vessels. Xenotransplantation. 2018;25(5):e12391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cooper DKC, Hara H, Iwase H, et al. Justification of specific genetic modifications in pigs for clinical organ xenotransplantation.Xenotransplantation. 2019;26(4):e12516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Watanabe H, Ariyoshi Y, Pomposelli T, et al. Intra-bone bone marrow transplantation from hCD47 transgenic pigs to baboons prolongs chimerism to >60 days and promotes increased porcine lung transplant survival. Xenotransplantation. 2020;27(1):e12552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yamada K, Tasaki M, Sekijima M, et al. Porcine cytomegalovirus infection is associated with early rejection of kidney grafts in a pig to baboon xenotransplantation model. Transplantation. 2014;98(4):411–418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yamamoto S, Lavelle JM, Vagefi PA. et al. Vascularized thymic lobe transplantation In a pig-to-baboon model: a novel strategy for xenogeneic tolerance induction and T-cell reconstitution. Transplantation. 2005;80(12):1783–1790. [DOI] [PubMed] [Google Scholar]
- 27.Tasaki M, Wamala I, Tena A, et al. High incidence of xenogenic bone marrow engraftment in pig-to-baboon intra-bone bone marrow transplantation. Am J Transplant. 2015;15(4):974–983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yamada K, Ariyoshi Y, Pomposelli T, Sekijima M. Co-transplantation of vascularized thymic graft with kidney in pig-to-nonhuman primates for the induction of tolerance across xenogeneic barriers. Methods Mol Biol. 2020;2110:151–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kanda Y Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transplant. 2013;48(3):452–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Seymour K, Han X, Sadowitz B, Maier KG, Gahtan V. Differential effect of nitric oxide on thrombospondin-1-, PDGF- and fibronectin-induced migration of vascular smooth muscle cells. Am J Surg. 2010;200(5):615–619. [DOI] [PubMed] [Google Scholar]
- 31.Nicoletti I, Migliorati G, Pagliacci MC, Grignani F, Riccardi C. A rapid and simple method for measuring thymocyte apoptosis by propidium iodide staining and flow cytometry. J Immunol Methods. 1991; 139(2):271–279. [DOI] [PubMed] [Google Scholar]
- 32.Ishimoto T, Cara-Fuentes G, Wang H, et al. Serum from minimal change patients in relapse increases CD80 expression in cultured podocytes. Pediatr Nephrol. 2013;28(9):1803–1812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shimada M, Ishimoto T, Lee PY, et al. Toll-like receptor 3 ligands induce CD80 expression in human podocytes via an NF-kappaB-dependent pathway. Nephrol Dial Transplant. 2012;27(1):81–89. [DOI] [PubMed] [Google Scholar]
- 34.Cara-Fuentes G, Venkatareddy M, Verma R, et al. Glomerular endothelial cells and podocytes can express CD80 in patients with minimal change disease during relapse. Pediatr Nephrol. 2020;35(10):1887–1896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Das S, Halushka MK. Extracellular vesicle microRNA transfer in cardiovascular disease. Cardiovasc Pathol. 2015;24(4):199–206. [DOI] [PubMed] [Google Scholar]
- 36.Zhu D, Pan C, Li L, et al. MicroRNA-17/20a/106a modulate macrophage inflammatory responses through targeting signal-regulatory protein alpha. J Allergy Clin Immunol. 2013;132(2):426–436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li L, Liu Y, Li S, et al. Signal regulatory protein alpha protects podocytes through promoting autophagic activity. JCI Insight. 2019;5:e124747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bige N, Shweke N, Benhassine S, et al. Thrombospondin-1 playsa profibrotic and pro-inflammatory role during ureteric obstruction. Kidney Int 2012;81(12):1226–1238. [DOI] [PubMed] [Google Scholar]
- 39.Thakar CV, Zahedi K, Revelo MP, et al. Identification of thrombospondin 1 (TSP-1) as a novel mediator of cell injury in kidney ischemia. J Clin Invest. 2005;115(12):3451–3459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chen M, Wang Y, Wang H, Sun L, Fu Y, Yang YG. Elimination of donor CD47 protects against vascularized allograft rejection in mice. Xenotransplantation. 2019;26(2) :e12459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Xu M, Wang X, Banan B, et al. Anti-CD47 monoclonal antibody therapy reduces ischemia-reperfusion injury of renal allografts in a porcine model of donation after cardiac death. Am J Transplant. 2018; 18(4):855–867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rogers NM, Sharifr-Sanjani M, Csányi G, Pagano PJ, Isenberg JS. Thrombospondin-1 and CD47 regulation of cardiac, pulmonary and vascular responses in health and disease. Matrix Biol. 2014;37:92–101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tanabe T, Watanabe H, Shah JA, et al. Role of intrinsic (graft) versus extrinsic (host) factors in the growth of transplanted organs following allogeneic and xenogeneic transplantation. Am J Transplant. 2017;17(7):1778–1790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shah JA, Tanabe T, Yamada K. Role of intrinsic factors in the growth of transplanted organs following transplantation. J Immunobiol. 2017;2(2):122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Längin M, Mayr T, Reichart B, et al. Consistent success in life-supporting porcine cardiac xenotransplantation. Nature. 2018;564(7736):430–433. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


