Abstract
As a kind of nature-derived bioactive materials, polyphenol-based hydrogels possess many unique and outstanding properties such as adhesion, toughness, and self-healing due to their specific crosslinking structures, which have been widely used in biomedical fields including wound healing, antitumor, treatment of motor system injury, digestive system disease, oculopathy, and bioelectronics. In this review, starting with the classification of common polyphenol-based hydrogels, the pyramid evolution process of polyphenol-based hydrogels from crosslinking structures to derived properties and then to biomedical applications is elaborated, as well as the efficient reverse design considerations of polyphenol-based hydrogel systems are proposed. Finally, the existing problems and development prospects of these hydrogel materials are discussed. It is hoped that the unique perspective of the review can promote further innovation and breakthroughs of polyphenol-based hydrogels in the future.
Keywords: Biomaterials, Adhesive hydrogels, Polyphenol, Disease treatment, Reverse design
Graphical abstract
Highlights
-
•
Polyphenol-based hydrogels combine advantages of polyphenols with common hydrogels.
-
•
Cognition of such hydrogels underwent from structures to properties to applications.
-
•
Various crosslinked structures of such hydrogels can derive outstanding properties.
-
•
Such hydrogels can be widely used in biomedicine due to the outstanding properties.
-
•
Reverse design thought from applications to properties to structures is promising.
Abbreviations
- DOPA
3, 4-dihydroxyphenylalanine
- PEG
Poly(ethylene glycol)
- PDA
Polydopamine
- TA
Tannic acid
- EGCG
Epigallocatechin gallate
- PAM
Polyacrylamide
- Mefps
Mytilus edulis foot proteins
- NIR
Near-infrared
- ROS
Reactive oxygen species
- AD
Arginine derivative
- GG
Guar gum
- BTZ
Bortezomib
- OA
Osteoarthritis
- PAL
Postoperative anastomotic leakage
- RPE
Retinal pigment epithelium
- e-skin
Electronic biomimetic skin
1. Introduction
Hydrogel, as a kind of polymeric semisolid material with high water content and good biocompatibility, has been studied in the field of biomedicine for more than 40 years [[1], [2], [3], [4]]. Due to their physical similarities to human tissue, hydrogels were initially used as cell culture substrates and wound dressings [5,6]. With the rise and development of tissue engineering, it was further used in the repair and regeneration of various tissues and organs [7,8]. In recent years, with the intersection and fusion of biomedicine and material science, a variety of responsive hydrogel systems have been born [9], which are used for drug delivery [10,11], biosensing [12], and bioimaging [13] in response to the complex environment of the human body, so as to carry out accurate diagnosis and treatment for a variety of diseases such as cancers [14,15], infections [16], diabetes [17], and rheumatoid arthritis [18]. In this process of development, hydrogel materials have realized the transformation from traditional to intelligent, and it also plays an increasingly important role in the field of biomedicine.
Polyphenols are a kind of compound with a chemical structure of phenyl and combined with two or more phenolic hydroxyl, widely distributed in various plants and marine organisms [19,20]. So far, polyphenols have been proved to have good effects in antioxidant, antibacterial, anti-tumor, immune regulation, anti-radiation, and other aspects [[21], [22], [23], [24]]. Meanwhile, they have high safety for the human body, so they have been widely used in medicine, food, and cosmetics in recent years [25,26]. Polyphenol compounds contain groups such as catechol and pyrogallol, which can interact with many molecules by forming a variety of non-covalent (hydrogen bonding, π-π interactions, cation-π interactions, etc.) or covalent interactions (Michael addition/Schiff-base reaction, polyphenol–metal coordination, etc.) [27,28], and then reflect excellent properties such as adhesion and self-healing [[29], [30], [31]], which are also urgently needed for modern intelligent biomedical hydrogels.
Since 2002, Lee et al. modified 3, 4-dihydroxyphenylalanine (DOPA) onto linear or branched poly(ethylene glycol) (PEG) and prepared highly adhesive hydrogels by oxidative crosslinking [32], a class of hydrogels based on polyphenols were born. Polyphenol-based hydrogels are a kind of hydrogel materials whose structures and properties are significantly improved by introducing polyphenols into the hydrogel systems. These advanced materials highly combine the advantages of hydrogels and polyphenols and show great vitality in the field of biomedicine. For example, polyphenol introductions bring strong adhesion and self-healing properties, so the hydrogels can easily cope with the complex environment in vivo [33].
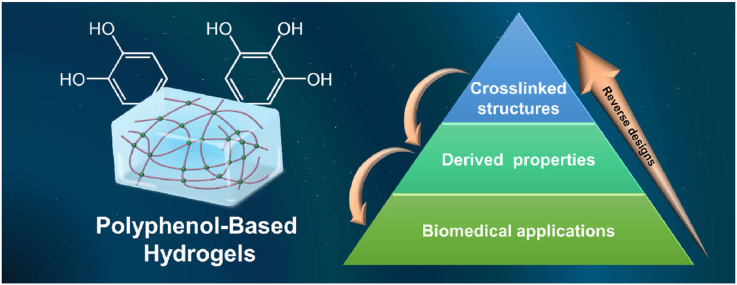
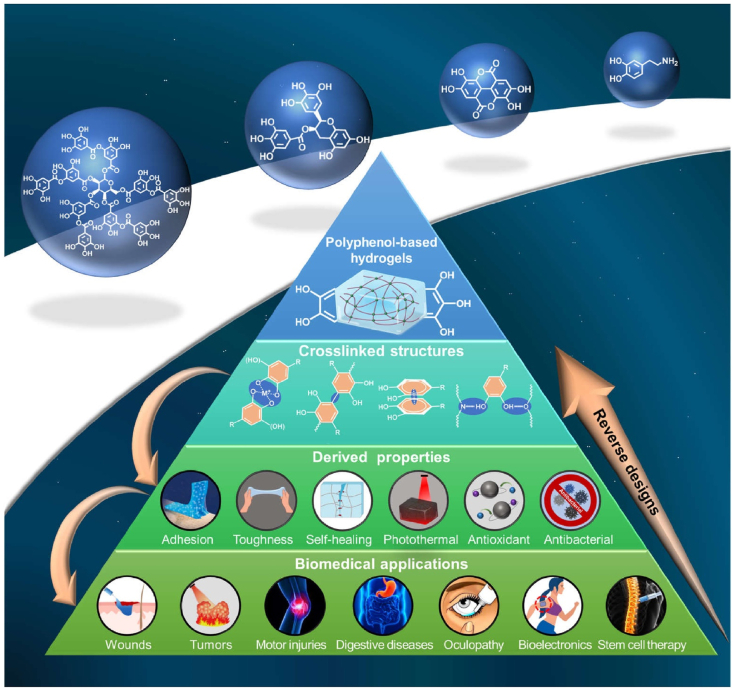
Up to now, polyphenol-based hydrogels have undergone recognition from crosslinking structures to derived properties, and their applications cover many aspects in the field of biomedicine. However, there are still few reviews focusing on polyphenol-based hydrogels, and the existing reviews still have some problems such as incomplete summary and lack of recent research progress. Therefore, based on the recent research progress of polyphenol-based hydrogels, we dissected the pyramid evolution process of polyphenol-based hydrogels from several crosslinking structures to numerous derived properties and then to a wide range of biomedical applications in detail. Meanwhile, the efficient reverse design consideration of such research projects was proposed. The framework and train of thought of the review are shown in Fig. 1.
Fig. 1.
Schematic illustration of pyramid evolution and reverse design processes of polyphenol-based hydrogels.
2. Classification of polyphenol-based hydrogels
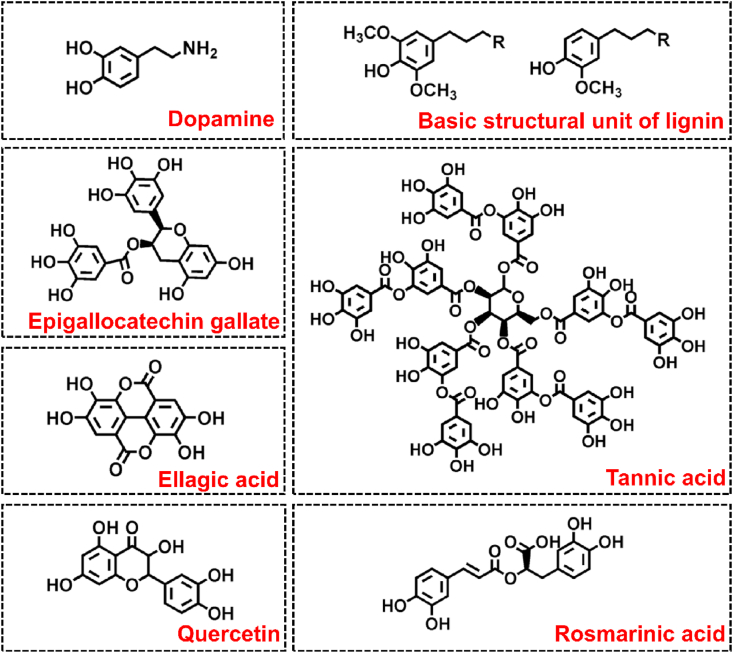
So far, more than 8000 polyphenol molecules have been identified [26], and several of them from natural sources have been directly used or modified for the construction of polyphenol-based hydrogel systems (Fig. 2). This section will classify polyphenol-based hydrogels according to the types of polyphenols.
Fig. 2.
Typical polyphenol-based hydrogel components.
2.1. Dopamine and its derivatives-based hydrogels
Dopamine is the most abundant catecholamine neurotransmitter in the human brain, which is involved in the regulation of various functions including movement, cognition, emotion, and so on [34]. As a small molecular polyphenol compound with a very simple structure, dopamine is often used as a building block to construct more complex polyphenol hydrogel platforms. Dopamine can self-polymerize into polydopamine (PDA) with outstanding adhesivity, modifiability, and biocompatibility under weak alkaline conditions, which may self-assemble into PDA nanoparticles or attach to the surface of other nanomaterials to form PDA coatings [25,30]. The introduction of these PDA-based nanomaterials into hydrogels can increase the adhesion, toughness, and strength of the system. Meanwhile, these nanomaterials can also be used for drug delivery and controlled release as well as photothermal therapy, so they have a wide range of biomedical applications and development prospects [[35], [36], [37], [38]]. In addition, considering that dopamine and its derivatives have typical catechol groups without extra groups, they can be connected to classical hydrogel materials such as hyaluronic acid and chitosan through simple substitution reaction, which can improve the adhesion and strengthen hydrogel systems through polymerization [[39], [40], [41], [42]]. Up to now, dopamine and its derivatives-based hydrogels are the most widely studied and applied polyphenol hydrogels.
2.2. Tannic acid (TA)-based hydrogels
Tannic acid (TA) is a naturally plant-derived polyphenol compound that provides the protection of plants from insects, pathogens, and ultraviolet [43]. The research on functional hydrogels based on TA has also been one of the research hot areas of polyphenol-based hydrogels for several years. Different from small molecular dopamine, a TA molecule owns ten polyphenol groups with a much larger molecular weight, so it can directly participate in the crosslinking of hydrogels as a component without further polymerization or connection with other polymer materials. Hydrogen bond interaction is the most common crosslinking method in the existing TA-based hydrogels [[44], [45], [46]], and on this basis, there were also studies on further introducing TA-metal ion coordination interactions and TA-boronate interaction to improve the properties of self-healing and mechanical toughness [47,48]. Besides, because TA also has plenty of pharmacological activities such as antioxidant, antibacterial and anti-inflammatory, the related hydrogels may possess a very outstanding performance in the applications of wound healing [49,50].
2.3. Epigallocatechin gallate (EGCG)-based hydrogels
Studies have shown that regular drinking of green tea has many benefits such as cancer prevention, blood lipid reduction, and immunity promotion, which is mainly due to tea polyphenols [51,52]. More than 30 polyphenols with a high level of contents are contained in green tea, with epigallocatechin gallate (EGCG) at the top of the list [53]. Considering the multiple biological activities and admirable biocompatibility, EGCG has also been used to participate in the construction of many hydrogel platforms [[54], [55], [56]]. In terms of crosslinking mechanism, EGCG-based hydrogels are mainly formed by covalent crosslinking, because the low molecular weight of EGCG makes it difficult to form a three-dimensional (3D) network structure only by non-covalent crosslinking [57].
2.4. Lignin-based hydrogels
Lignin is the second richest renewable organic resource on earth, which is widely found in the xylem of plants to maintain rigidity [58,59]. As a class of very complex 3D polymers, natural lignin possesses a polyphenol-like structure containing a large number of aromatic rings with phenolic hydroxyl and methoxyl groups. However, an aromatic unit usually contains only one phenolic hydroxyl group, which determines that natural lignin does not have obvious polyphenol properties. Therefore, abundant polyphenol units can be obtained by further demethylating to produce a series of polyphenol-related properties [60,61]. Through the redox reaction between lignin and high valence-state metal ions, ammonium persulfate can be induced to generate free radicals, which can lead to the polymerization of monomers such as acrylic acid and hydroxyethyl acrylamide, so as to prepare hydrogels without initiator [60,62,63]. Besides, given the abundant source and low cost of lignin, hydrogels prepared from lignin have a good prospect of large-scale production and application.
2.5. Other polyphenol-based hydrogels
In addition to the common polyphenol-based hydrogels mentioned above, ellagic acid, quercetin, rosmarinic acid, proanthocyanin, carminic acid, rutin trihydrate, and other natural sources of polyphenols are also used in the manufacture of hydrogels [[64], [65], [66]]. These polyphenol-based hydrogels generally have biological activities such as antibacterial, antioxidant, and immunomodulatory with good biocompatibility and have been optimized in mechanical properties, which owns a high research value and bright prospect for biomedical applications.
3. Crosslinking structures of polyphenol-based hydrogels
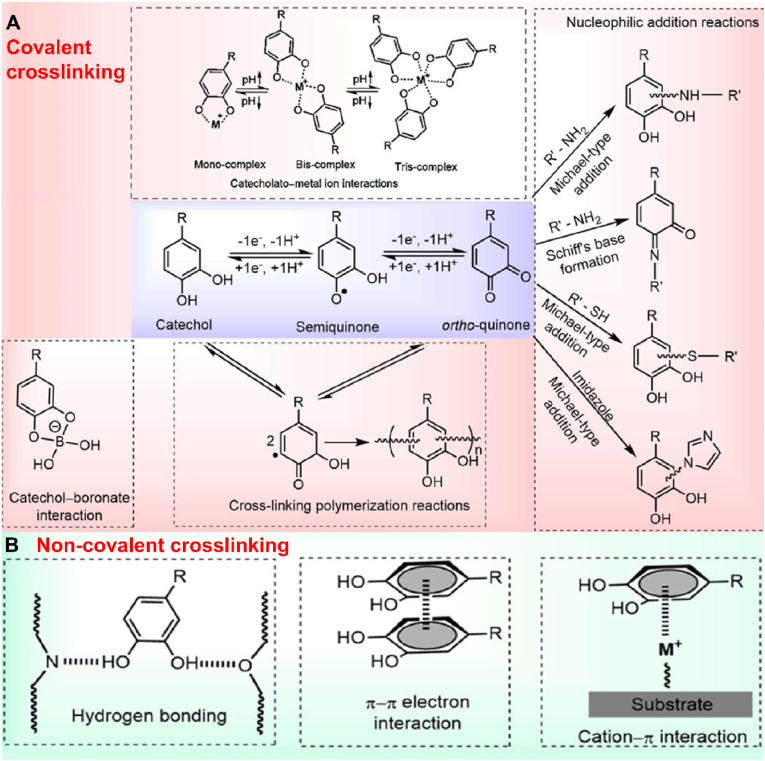
One of the most vital parts in the preparation of hydrogels is the formation of 3D network structures through crosslinking, which is also the source of the properties of hydrogels. Due to the diverse interactions that polyphenols can produce, polyphenol-based hydrogels with 3D structures can be formed through physical and/or chemical crosslinking in various forms, and the representative crosslinking mechanisms of catechol-based hydrogels are summarized in Fig. 3 [67]. Such various crosslinking ways produce plentiful structures of polyphenol-based hydrogels, and at the same time provide more possibilities of composition combination for the construction of polyphenol-based hydrogel platforms, as well as further expand the properties and application ranges. In this section, different crosslinking methods and network structures of polyphenol-based hydrogels are discussed in detail.
Fig. 3.
(A) Covalent and (B) non-covalent crosslinking mechanisms of catechol-based hydrogels. Reproduced with permission from Ref. [27]. Copyright 2018, Elsevier.
3.1. Covalent crosslinking
3.1.1. Polymerization reactions
Catechol can be oxidized to o-quinone in the presence of oxidants, which can form dimers by self-coupling or a reaction with other catechol, as well as gradually polymerize into oligomers or/and polymers [[68], [69], [70]]. Similarly, pyrogallol also can be oxidized and further crosslinked [19,67]. As a very common crosslinking method, the polymerization of catechol provides a stable and durable foundation for many catechol-based hydrogels. For example, Han et al. constructed a dynamic DNA hydrogel that has permanent and temporary dual networks to respond to different solvents, while ensuring structural integrity which is due to the firm covalent crosslinking among dopamine units [71]. A heart hydrogel patch developed by Liang et al. also has a more stable and firm crosslinking network due to the coupling between dopamine in the system, which can cope with the complex environment in vivo [72].
3.1.2. Nucleophilic addition reactions
After the catechol group is oxidized to quinone, the electrophilic conjugated system is easy to be attacked by molecules containing nucleophilic functional groups (such as amino and thiol), and then generate substituted benzoquinone compounds via Michael addition reactions [36,73]. In addition, it may also occur that the nitrogen atoms of a nucleophile amine compound attack the carbonyl carbon atoms and then dehydrate to form Schiff bases [25,74,75]. The conditions of the reactions are mild and easy to operate, so it has been widely used for further modification of polyphenols, such as surface modification of PDA-based nanomedicines [[76], [77], [78]]. Gradually, Michael addition/Schiff-base reactions began to be introduced into the crosslinking of polyphenol-based hydrogel system, which increased the strength of hydrogels and enabled the crosslinking of polyphenol with more kinds of molecules, greatly broadening the design ideas. Han et al. synthesized a polyacrylamide-polydopamine (PAM-PDA) hydrogel by crosslinking PAM and PDA via Michael addition/Schiff-base reactions, which could prevent overoxidation of the catechol groups to achieve durable adhesion [79]. Wang et al. prepared a hydrogel by crosslinking PDA nanoparticles with thiol-terminated four-arm poly(ethylene glycol), which could be performed by simple Michael addition without special crosslinking agents [80]. In addition, in other studies, materials such as poloxamers [81], polyethyleneimine [82], poly(ethylene glycol) diacrylate [83], and bioactive proteins [84] were also introduced into polyphenol-based hydrogels through Michael addition/Schiff-base reactions and endowed the hydrogel systems with diverse excellent properties such as temperature sensitivity.
3.1.3. Polyphenol-metal ion interactions
Polyphenols can form an oxygen center with high electron density after deprotonation that can coordinate with metal cations [85]. At the same time, because polyphenols are a kind of polydentate ligands, they can produce a much stronger coordination effect than monodentate ligands [86]. When a large number of metal ions are involved in the coordination with polyphenols, a polyphenol network with metal ions as the connection point can be formed named metal-phenolic network (MPN). Due to the good adhesion, simple preparation process, and ease to further modify, MPN coating has been widely used in the surface modification of various substances [87]. In many polyphenol-based hydrogel platforms, polyphenol-metal ion coordination is also used in the preparation of hydrogels as a kind of crosslinking mode, and the introduction of metal ions can also bring special properties to the hydrogel system. For example, Zhou et al. prepared a conductive hydrogel using TA and pyrrole (Py) as raw materials, which added Fe3+ as an oxidation initiator to promote the polymerization of Py, and to coordinate with polyphenol groups of TA to participate in the crosslinking of the hydrogel [48]. Xiang et al. fabricated a smart injectable hydrogel from folic acid, dopamine, and Zn2+. Zn2+ can coordinate with both catechol groups of dopamine and the carboxyl groups of folic acid to participate in crosslinking, and introduce antibacterial and tissue repair functions to the hydrogel platform [88].
3.1.4. Polyphenol-boronate interaction
Boronic acid can form dynamic cyclic ester species with diol-based molecules and therefore can form complexation with polyphenol [89,90]. Polyphenol-boronate complexation is capable of forming at pH above the pKa of polyphenol and breaking below its pKa, thus possessing a pH-responsive property [57]. Many studies have used polyphenol-boronate interaction for responsive delivery of the antitumor drug bortezomib considering its pH-responsive property [91,92]. In the field of hydrogel research, polyphenol-boronate interaction is also used to participate in the formation of hydrogel crosslinking [47,93,94]. In addition, through dynamic polyphenol-boronate interaction, polyphenol groups can be selectively hidden or exposed under different pH conditions, thus achieving pH-dependent reversible adhesion of hydrogels, which has a very broad application prospect [95,96].
3.2. Non-covalent crosslinking
3.2.1. Hydrogen bonding
The biggest characteristic of polyphenols is that they contain abundant phenolic hydroxyl groups, which determines that they are very easy to form a large number of hydrogen bonds with each other and other molecules containing hydroxyl and amino groups [97]. Therefore, hydrogen bonding is the most common crosslinking mode in most polyphenol-based hydrogel systems. Hydrogen bonding is weaker than covalent bonds but stronger than van der Waals's force. Considering that it can break and form reversibly, it is often associated with the self-healing and some mechanical properties of hydrogels [98,99]. In addition, the hydrogen bonding formed between polyphenols and water molecules can increase the hydrophilicity of hydrogels, which is conducive to improving the biocompatibility of polyphenol-based hydrogels [69].
3.2.2. π-π stacking interaction
π-π stacking interaction is a special spatial arrangement between π-electron-rich aromatic compounds, and polyphenols contain a large number of aromatic ring structures, so they can form a wide range of π-π interaction [100]. In polyphenol-based hydrogel systems, π-π stacking is as important as hydrogen bonding in recoverable non-covalent interactions. As sacrificial bonds, they can dissipate energy and contribute significantly to maintaining the elasticity and self-healing properties of hydrogels [79].
3.2.3. Cation-π bonding
Cation-π bonding can be formed through electrostatic and polarization interactions between cations and π-electron-rich aromatic compounds [101]. These interactions exist widely in biological systems and are involved in stabilizing protein structures, protein-nucleic acid interactions, and so on. In polyphenol-based hydrogels, the strength of cationic-π bonding is similar to that of hydrogen bonding, which plays a vital role in promoting the adhesion and cohesion of hydrogels [99]. Many biomimetic adhesive materials based on catechol and amino moieties have been designed using the principle and have shown stronger adhesion than catechol groups alone [[102], [103], [104]].
4. Derived properties of polyphenol-based hydrogels
Based on the mutual crosslinking structures of polyphenol-based hydrogels, some common properties are produced, and the combination of these properties laid a solid foundation for their significantly wide applications in the biomedical field. In this section, the outstanding derived properties of polyphenol-based hydrogels are elaborated.
4.1. Adhesion and bioaffinity
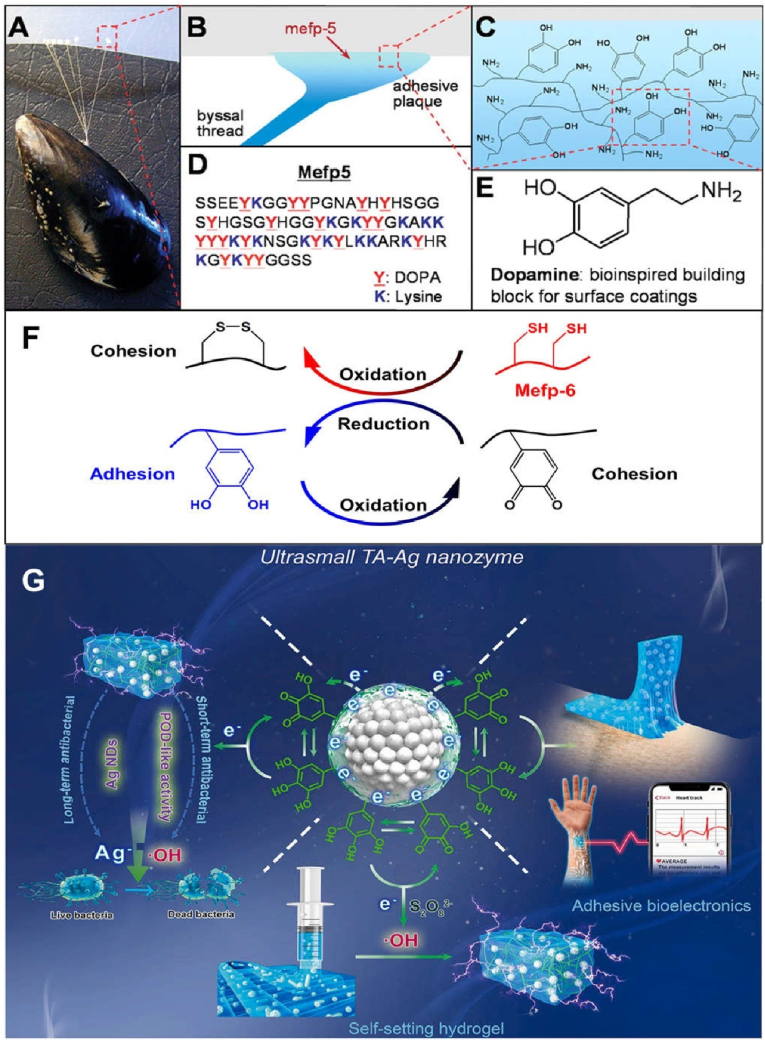
The most prominent property of polyphenol hydrogels is the high undifferentiated adhesion to different surfaces of substances, and the adhesion is also embodied as a high affinity with tissues and cells when reflected in the microscopic level of biomedicine [33]. The adhesion was originally inspired by marine mussels. Mussels can secrete Mytilus edulis foot proteins (Mefps) that can stick firmly to objects such as rocks and hulls in wet environments (Fig. 4A–E) [74,105,106]. Mefps were divided into Mefp-2, Mefp-3, Mefp-4, Mefp-5, and Mefp-6, among which Mefp-3 and Mefp-5 mainly contributed to high adhesion and interfacial binding strength due to a large number of DOPA units [[107], [108], [109]]. DOPA is a kind of polyphenol that contains a catechol group. Polyphenols can bind to surfaces of different materials through multiple interactions such as coordination, hydrogen bonding, cation-π interaction, etc [110]. In addition, catechol/pyrogallol are highly reactive to various natural nucleophiles (such as thiols and amines) of tissue surface proteins, so polyphenol-based hydrogels can produce excellent bioadhesion and bioaffinity [111,112]. Using these principles, Jung et al. prepared a PAM-PDA hydrogel system and embedded extra-large pore mesoporous silica nanoparticles with a hydrophilic surface to enhance hydrogen bonding, thus fabricating hydrogel patches with super adhesion for drug transdermal delivery [113].
Fig. 4.
(A) Photograph of a mussel adhesion, (B) Position of Mepf-5 on the adhesive interface, (C) A simplified molecular representation of catechol and amino groups in Mepf-5, (D) The amino acid sequence of Mepf-5, (E) Dopamine can be used as a building block due to its similar local structure to Mepf, Reproduced with permission from Ref. [74]. Copyright 2007, American Association for the Advancement of Science. (F) The dynamic redox equilibrium exists in Mepf to maintain the persistent adhesion of mussels, (G) The mussel-inspired TA-Ag redox system endowed the hydrogel with long-term adhesion. Reproduced with permission from Ref. [117]. Copyright 2021, KeAi.
Nevertheless, adhesion produced by polyphenol groups presents a problem of persistence. Catechol/pyrogallol are the structural basis of all adhesion-related properties, but they are very easy to be oxidized to quinone when exposed to air, and thus lose adhesion [114]. Hence some studies have integrated materials such as clay nanosheets and PAM into the polyphenol-based hydrogel systems to isolate oxygen and protect the polyphenol groups, so as to achieve more lasting adhesion [79,115]. In nature, mussels are usually able to produce a very persistent adhesion effect due to the redox balance in their bodies. After the adhesive-producing polyphenol groups are oxidized to quinone, mussels are able to continuously secrete reducing proteins (such as Mepf-6 with cysteine thiols) for regenerating phenolic hydroxyl groups, resulting in long-term adhesion (Fig. 4F) [116]. By constructing redox systems to simulate the long-term adhesion process of mussels, several durable polyphenol-based hydrogel platforms have been developed. Jia et al. prepared a TA-Ag nanozyme to catalyze the self-setting of a hydrogel, and the dynamic redox balance between polyphenol groups in TA and Ag nanoparticles promoted the long-term and repeatable adhesion of the hydrogel (Fig. 4G) [117]. Similar Ag-lignin redox systems have also been used to enhance the adhesion persistence of polyphenol-base hydrogels [60,118,119].
At the same time, due to the bioadhesion of polyphenol-based hydrogels, they also show high affinity to tissues and cells, which has been reflected in many studies. For example, Xu et al. observed significant enhancement of cell migration and recruitment after introducing PDA-modified nanomedicines into a hydrogel system [35]. Such bioaffinity not only ensures the biosafety of polyphenol-based hydrogels but also is very suitable for promoting healing and recovery in diseases such as skin trauma and bone injury.
The adhesion and bioaffinity of polyphenol-based hydrogels lay a solid foundation for their application in biomedicine. With the development of the research, the polyphenol-based adhesion should be further refined, and be more complementary to other excellent properties through the modification of hydrogels, so as to obtain more extensive application spaces.
4.2. Toughness and strength
The body's muscles, tendons, ligaments, and other soft tissue usually have the characteristics of high toughness and strength, while hydrogels are usually used to simulate and replace these soft tissues in the biomedical field [120]. Therefore, hydrogels are also required to have high toughness and strength. Polyphenol-based hydrogel systems usually have more than one way of crosslinking. The firm covalent crosslinking formed by chemical reactions such as polymerization and Michael addition usually have high bond energy, which endows hydrogels with high strength [121]. Meanwhile, the weak crosslinking formed by hydrogen bonding, π-π stacking, and other dynamic interactions can dissipate energy under external forces, thus providing high toughness for hydrogels [122]. Lin et al. produced a chitin-TA multi-crosslinked hydrogel through a polyphenol-mediated self-assembly strategy [123]. The hydrogel's compressive stress, Young's modulus, and fracture energy can reach 8.18 MPa, 0.36 MPa, and 0.97 MJ m−3, respectively, which combines the characteristics of high strength and toughness [123]. Apart from that, the introduction of nanomaterials into polymer networks of hydrogels is also a common method to reinforce the hydrogel systems. Inorganic nanomaterials, such as mesoporous silica nanoparticles and black phosphorus nanoparticles, have high mechanical strength and can absorb energy in hydrogel systems to prevent crack propagation during deformation, thus effectively improving the strength and toughness of the hydrogels [124,125]. Furthermore, if polyphenol-modified nanomaterials are introduced into hydrogel systems, their large specific surface area may produce a broader interaction between polyphenols and polymer crosslinking networks, which can combine the advantages of the two strategies above, thus raising the strength and toughness of hydrogels to a new level [115].
4.3. Self-healing
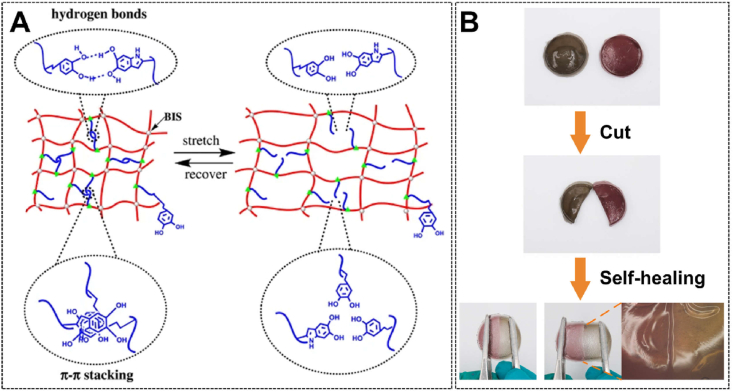
In the human body, soft tissues such as skins have a good self-repair ability within a certain extent of injury, to maintain a healthy life. Inspired by this, self-healing hydrogels, a class of smart materials that are structurally capable of repairing damage caused by long-term mechanical use, were born [126]. If the strength and toughness of hydrogels are improved to reduce the damage caused by external forces, then the self-healing property is a remedial measure after structural damage occurs. Such a "double insurance" strategy can greatly prolong the life of hydrogels, thus expanding their applications in the biomedical field. Polyphenol-based hydrogels generally have great self-healing ability due to the existence of multiple dynamic bonds in the crosslinking system, which can achieve repair after fracture under mild conditions [79]. According to the different types of dynamic bonds in polyphenol-based hydrogels, self-healing can be divided into physical and chemical self-healing [127]. Physical self-healing is mediated by dynamic non-covalent interactions such as hydrogen bonding and π-π stacking (Fig. 5A), while chemical self-healing is mediated by dynamic imine bonds, boronate-diol bonds, and other dynamic covalent bonds [79,127]. A large number of polyphenol-based hydrogel platforms with excellent self-healing properties have been developed through physical and/or chemical self-healing mechanisms. Yuan et al. constructed a double-crosslinked hydrogel system containing reversible imine bonds and Fe3+-catechol coordination, and the two-part hydrogels could self-heal into a state without cracks within 15 min (Fig. 5B) [128]. Wei et al. fabricated a polyacrylamide (PAAm)-based conductive hydrogel system introducing TA-decorated cellulose nanofibrils (TA@CNF) and MXene nanosheets. Due to the dynamic catechol-borate bonds in the TA@CNF network and the repairable supramolecular interactions among TA@CNF, MXene, and PAAm, the healed hydrogel reemerged with conductivity as well as high mechanical strength and stretch strain [129].
Fig. 5.
(A) The mechanism of reversible non-covalent interaction-mediated self-healing. Reproduced with permission from Ref. [79]. Copyright 2017, Nature Portfolio. (B) Macro and micro-processes of dynamic covalent bond-mediated self-healing. Reproduced with permission from Ref. [128]. Copyright 2021, Elsevier.
4.4. Photothermal effect
Melanin is a kind of biological macromolecular pigment that has a wide range of optical absorption and can convert the absorbed photon energy into heat, so it can protect organisms from ultraviolet damage [130,131]. PDA, as a kind of melanin-like polyphenol, has similar optical absorption and photothermal properties. Particularly, PDA has high absorption in the near-infrared region (650–900 nm) with a high photothermal conversion efficiency of ∼40%, so it has been widely used in antitumor and antibacterial photothermal therapy researches [25]. In the research field of polyphenol-based hydrogels, PDA-based nanocomposite hydrogels or the polymer networks generated by the polymerization of dopamine and its derivatives also have good photothermal performance [132,133]. In addition, the polyphenol-metal ion coordination can produce a photothermal effect due to a strong charge transfer from phenol hydroxyl to metal [[134], [135], [136]], and this strategy can be further applied to introduce or enhance the photothermal effect of polyphenol-based hydrogel systems.
4.5. Antioxidant and free radical scavenging ability
Free radicals are a kind of strong oxidizing compounds produced in the oxidative reaction of our body, and excessive free radicals will cause damage to tissues and cells, which may lead to a variety of inflammatory reactions. More and more studies show that Alzheimer's disease, Parkinson's disease, diabetes, tumor, the aging process, and so on are closely related to the body's free radicals [137,138]. Nevertheless, as a class of antioxidants, polyphenols can scavenge free radicals by transferring hydrogen atoms of phenolic hydroxyl groups or donating transfer electrons to free radicals [19,139]. Given this, the polyphenol hydrogel systems contain abundant polyphenol groups, so they always have excellent antioxidant effects, naturally. For instance, Yang et al. designed a TA-based hydrogel band-aid to address the inflammatory response and oxidant stress during diabetic wound healing and utilized its antioxidant properties to effectively promote skin incision recovery in diabetic mice [49].
4.6. Antibacterial activity
Many naturally-derived polyphenols, such as TA, EGEG, and lignin, have been proven to have antibacterial activities, and their antibacterial mechanisms are mainly related to the damage of bacterial cell membranes, the binding with peptidoglycan on bacterial cell walls, and the inhibition of biosynthesis of the cell constituents [58,[140], [141], [142]]. Therefore, hydrogel platforms constructed based on these polyphenols also have certain antibacterial activities. For example, an EGCG-based hydrogel system constructed by Zhao et al. showed excellent antibacterial ability against both E. coli and S. aureus [54].
5. Biomedical applications
Considering that the properties of polyphenol-based hydrogels are highly compatible with many biomedical applications such as wound healing promotion and cancer therapy, more and more studies have been conducted to solve the problems in the biomedical field by constructing multi-functional polyphenol-based hydrogel platforms. Therefore, in this section, the applications of polyphenol-based hydrogels in diverse biomedical fields are comprehensively summarized.
5.1. Wound healing
During the wound healing process, the complex wound microenvironment will cause extremely serious consequences if they are not correctly treated. Generally, hemostasis, inflammation, proliferation, and remodeling are regarded as four distinct stages of the whole process in wound healing [143]. Therefore, with the properties discussed above, the polyphenol-based hydrogel can be treated as promising candidates for wound healing, since they satisfy the requirement of hydrogels designed for wound healing, such as adhesion, bioaffinity, biocompatibility, antioxidant and antibacterial activities. In general, wound dressings can be divided into two typical kinds based on the shape of the wounds: hydrogel patches adhering directly to the wound site and injectable hydrogels for filling irregularly shaped wounds [57], with the latter promising wider application because of the dramatic adaptability in all kinds of wounds and no need for highly invasive surgical procedures [144].
The mechanism of wound healing polyphenol-based hydrogels can be divided into hemostasis, antibacterial and anti-inflammatory. It is worth noting that some hydrogels used for wound repair can perform a combination of these functions.
5.1.1. Hemostasis
Hydrogel sealants are treated as candidates for controlling bleeding for a long time [46], however, the traditional hydrogels cannot be treated as ideal hemostatic agents for many reasons, such as poor adhesion, bad mechanical properties, poor biodegradability, low elasticity, toxicity, immunogenicity, and so on [145,146]. For example, with favorable cell adhesion and higher hemostatic capacity, gelatin-based hydrogels are considered as the alternative to collagen for controlling bleeding [147,148]. However, there are still many reasons largely limiting their applications in the clinic, including bad mechanical properties, sensitivity to water, and poor adhesion [50,149]. Chemically active synthetic crosslinkers were added in gelatin-based hydrogels for improving mechanical properties and stability [150]. However, these additives may increase costs and the risk of introducing toxicity [50,151], hindering its further biomedical applications. Moreover, poor biodegradability of hemostatic agents may cause thrombosis [152], and low elasticity will further hinder the application of traditional hydrogels in deep wounds [45].
In general, ideal hemostatic agents should be endowed with many properties discussed above [115], and the design of new hemostatic agents is the trade-off of them. But in most cases, the traditional hydrogel can only have a few required properties. Polyphenol-based hydrogels always show robust wet tissue adhesion ability, which is useful in the presence of continuous blood flow and can be used under the situation of arterial and visceral wounds [45,153]. Besides, Self-healing and high elasticity properties of polyphenol-based hydrogels are other factors that ensure excellent hemostatic performance even with deep wounds after adhering to the wound site [154].
Wang et al. prepared a powerful wound dressing based on the polyphenol-based hydrogel system inspired by mussel adhesive protein [155]. Because of the biomimetic catechol-Lys residue distribution in the system, it showed the ability to facilely and intimately integrate with biological tissue, which resulted in the great potential in hemostatic properties and wound healing. The mouse liver bleeding experiment and in vivo experiment confirmed its hemostatic properties and feasibility of wound repair. Han et al. developed a novel dual bionic adhesive hydrogel, and dopamine was introduced for adhesion. In vitro adhesive test and in vivo hemostatic test both certificated the excellent potential in wound healing of this system (Fig. 6A) [42].
Fig. 6.
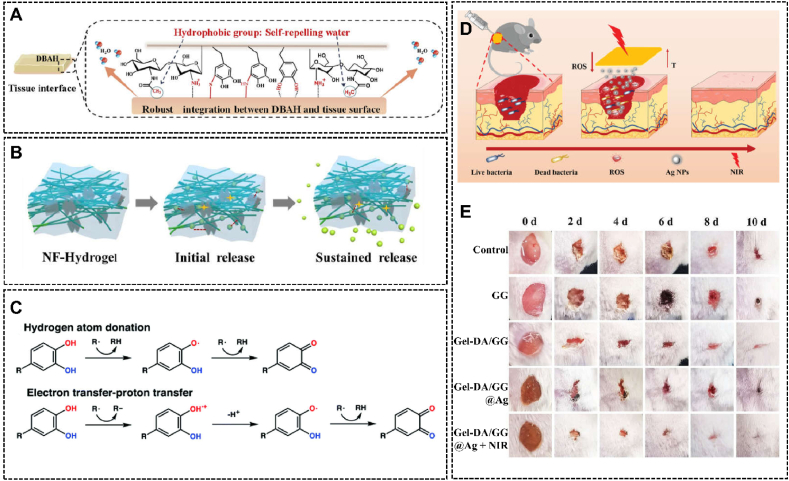
(A) Strong adhesion for hemostasis caused by the interactions between the polyphenol-based hydrogel and tissues. Reproduced with permission from Ref. [42]. Copyright 2020, KeAi. (B) Sustained release of tetracycline hydrochloride for long-lasting anti-bacteria. Reproduced with permission from Ref. [169]. Copyright 2020, Elsevier. (C) The mechanisms of polyphenol-based hydrogels to scavenge free radicals. Reproduced with permission from Ref. [19]. Copyright 2020, The Royal Society of Chemistry. (D) The schematic diagram of multifunctional Gel-DA/GG@Ag hydrogel for wound healing, (E) The healing processes of S. aureus-infected wounds under different treatments. Reproduced with permission from Ref. [175]. Copyright 2021, Wiley-VCH.
In addition to adhesion and seal for hemostasis, polyphenol-based materials can also interact with blood proteins to form a complex as a physical barrier for hemostasis, which is expected to become a promising research direction of polyphenol-based hemostatic hydrogels [156].
For further enhancing the hemostatic effect, coagulants can be introduced into polyphenol-based hydrogel systems for synergistic hemostasis. For example, a TA and gallic acid-containing hydrogel system with a coagulant polyphosphate loading was designed by Cao et al. for wound healing, which showed accelerating blood coagulation in the hemostatic test in vivo [46].
5.1.2. Antibacterial
Bacterial infection is the "natural enemy" of wound healing. Improper treatment will not only cause a prolonged healing process, but also delay collagen synthesis, and additional tissue damage may even occur [157]. Based on the serious consequences of bacterial infection, hydrogels with antibacterial properties can also show excellent potential in wound healing. As a result, in addition to antihemorrhagic properties, antibacterial performances are another reason for polyphenol-based hydrogels showing great ability in wound healing.
The antibacterial performances of polyphenol-based hydrogels can be attributed to many factors [158,159], including the inherent antibacterial activity of polyphenols [160,161], photothermal property of polyphenols [162,163], and the introduction of antimicrobial substances such as cationic polymers and antibiotics [155,[164], [165], [166]].
The antibacterial activity of natural polyphenols is the most common source of the antibacterial performances of these hydrogels. Taking TA for example, as a typical representative of these natural polyphenols, it is usually employed as a major building block in antibacterial hydrogels [167]. Ma et al. fabricated an intriguing injectable and antibacterial hydrogel with TA [160]. The long-lasting antibacterial activity enabled the system to show an accelerating wound healing rate in the in vivo full-thickness cutaneous defect model, certificating its potential in wound healing with antibacterial properties. Ahmadian et al. developed a novel gelatin-TA hydrogel, showing great potential in wound healing [50]. In the agar diffusion test, the antibacterial effect of this hydrogel was tested, and because of the introduction of TA, this system showed excellent antibacterial effect and exhibited great wound healing ability in the further experiment with rat full-thickness skin wound model.
Photothermal property is another advantage for antibacterial polyphenol-based hydrogels in wound healing, especially the PDA-introducing hydrogels. By introducing PDA-coated reduction graphene oxide into the hydrogel, Li et al. prepared a platform with the enhanced antibacterial ability through photothermal effect, which was also reflected in the killing effects against E. coli and S. aureus [168]. Besides, Deng et al. designed an agarose-based hydrogel containing TA-Fe3+ composites and showed outstanding photothermal conversion capability [162]. The outcome of the in vivo test showed effective antibacterial effects under near-infrared (NIR) irradiation.
The introduction of antimicrobial substances in hydrogel systems is a straight way to kill bacterial. There are a large number of substances that show both excellent antibacterial properties and biocompatibility, allowing them to become the candidates for antibacterial hydrogel in wound healing. Cationic polymers are one of the antibacterial substances used in hydrogel for wound healing. In Yang et al.'s study, cationic polyelectrolyte brushes grafted from bacterial cellulose were added in the hydrogel, providing high-efficiency and long-lasting antibacterial performance [165]. Through contact sterilization experiments, the gorgeous antibacterial performance of this system was certificated. Ag nanoparticles (Ag NPs) are another widely used antibacterial agent. Hu et al. fabricated an Ag NPs-containing hydrogel for infected diabetic wound treatments [166]. The polyphenol-based hydrogel system showed enhanced antibacterial properties, which was proved by agar plate counting experiments. Besides, based on the multi-interactional sites with drugs, polyphenols exhibit a strong binding affinity with lots of antibiotics. For example, in Chen et al.'s research, tetracycline hydrochloride-loaded electrospun nanofibers were inserted into the hydrogel, endowing the system with sustained antibacterial performances (Fig. 6B) [169].
5.1.3. Anti-inflammatory
For wound healing, another adverse factor is the excessive generation of reactive oxygen species (ROS) [170]. Because of the superfluous ROS, the redox balance will be disrupted, which may further cause the peroxidation of lipid, inactivation of enzymes, and damage of DNA [171,172]. It has been proved that antioxidants can make a positive impact on reducing inflammation and thus promoting wound healing [173,174]. As a kind of good antioxidant material, polyphenol-based hydrogels can scavenge free radicals through different pathways (Fig. 6C) [19], so that making outstanding contributions in fighting inflammation. For instance, Zhang et al. fabricated a dopamine-modified arginine derivative (AD)-containing hydrogel system, which showed enhanced wound healing and tissue regeneration effects due to the antioxidant and anti-inflammatory abilities derived from AD and catechol groups [170].
5.1.4. Comprehensive wound healing
A combination of the mechanisms discussed above may show more excellent antibacterial properties and prevent bacterial drug resistance, which is gradually being the pursuit and tendency in this field. For example, Zhang et al. synthesized dopamine-modified gelatin (Gel-DA) @Ag nanoparticles and introduced them into guar gum (GG)-based hydrogels by forming dynamic boronate-diol bonds. The prepared Gel-DA/GG@Ag hydrogel system with superior photothermal, Ag NPs synergistic bactericidal and anti-inflammatory effects showed promising application in bacteria-derived wound infection (Fig. 6D and E) [175]. Liang et al. developed a wound dressing hydrogel based on hyaluronic acid-graft-dopamine and reduced graphene oxide with a series of wound healing-promoting properties including tissue adhesiveness, antioxidant, hemostatic and photothermal properties [154]. The hydrogel platform showed the best wound healing effect in a full-thickness skin defect mode.
Due to the early start of research on wound healing materials, and the natures of polyphenol-based hydrogels are highly compatible with the needs of wound healing, at present, this direction has become the most popular biomedical application field of polyphenol-based hydrogels. More research outputs on wound healing and the details of polyphenol-based hydrogels involved are presented in Table 1.
Table 1.
Summary of recent studies of polyphenol-based hydrogels for would healing.
| Introduced polyphenols | Main crosslinking methods | Key properties | Applicable wounds | Mechanisms for wound healing | References |
|---|---|---|---|---|---|
| TA | Hydrogen bonding | Adhesion; Antioxidant; Antibacterial | Full-thickness skin wounds | Hemostatic; Antibacterial; Anti-inflammatory |
[50] |
| TA | Hydrogen bonding; Polymerization |
Antioxidant; Antibacterial; Photothermal effect | Multi-drug resistant infected wounds | Hemostatic; Antibacterial; Anti-inflammatory |
[46] |
| TA | Hydrogen bonding; π-π stacking | Adhesion; Toughness; Self-healing; Antioxidant; Antibacterial; | Diabetic wounds | Hemostatic; Antibacterial; Anti-inflammatory |
[49] |
| TA | Hydrogen bonding; Boronate ester bonding | Self-healing; Adhesion; Conductivity; Antioxidant; Antibacterial | Deep wounds; Diabetic wounds |
Hemostatic; Antibacterial; Anti-inflammatory |
[176] |
| Dopamine | Hydrogen bonding; Michael addition/Schiff base reaction; Boronate ester bonding | Self-healing; Antioxidant; Antibacterial; Photothermal effect | Bacteria-derived infected wound with overexpression of reactive oxygen species | Antibacterial; Anti-inflammatory | [175] |
| Dopamine | Polymerization; Hydrogen bonding; Van der Waal's force; Electrostatic interaction | Adhesion; Toughness; Antibacterial | Skin wounds | Antibacterial | [165] |
| Dopamine | Hydrogen bonding; π-π stacking; Michael addition/Schiff base reaction | Self-healing; Adhesion; Toughness; Antibacterial; pH-response | Diabetic wounds | Antibacterial | [166] |
| Dopamine | Hydrogen bonding; π-π stacking; Electrostatic interaction | Self-healing; Adhesion; Toughness; Antibacterial; Sustained drug release | / | Antibacterial | [169] |
| Dopamine | Michael addition/Schiff base reaction; Dopamine-Fe coordination | Self-healing; Adhesion; Shape adaptability Toughness; Antibacterial; | Burn wounds | Hemostatic; Antibacterial | [128] |
| Dopamine | Polymerization | Adhesion; Toughness; Antibacterial; | Viscera wounds with haemorrhage and skin wounds | Hemostatic; Antibacterial | [42] |
| Dopamine | Polymerization; Electrostatic interaction | Adhesion; Toughness; Antioxidant | Skin wounds | Anti-inflammatory | [170] |
| Dopamine | Hydrogen bonding; π-π stacking; Electrostatic interaction |
Adhesion; Toughness; Antioxidant; Antibacterial; | Infected wounds | Antibacterial; Anti-inflammatory | [177] |
| Dopamine | Michael addition/Schiff base reaction; Dopamine-Fe coordination; Hydrogen bonding; | Adhesion; Zn2+ response | Skin wounds | / | [83] |
| Dopamine | Michael addition/Schiff base reaction; Hydrogen bonding; π-π stacking | Adhesion; Toughness; Antibacterial; Photothermal effect | Bacteria-infected wounds | Antibacterial | [178] |
| Dopamine | Michael addition/Schiff base reaction; Hydrogen bonding; π-π stacking | Self-healing; Adhesion; Toughness | Full-thickness skin wound healing | / | [179] |
| Dopamine | Hydrogen bonding; π-π stacking | Self-healing; Adhesion; Toughness | Cutaneous wounds | Hemostatic; Anti-inflammatory |
[180] |
| Dopamine | Polymerization; Hydrogen bonding; π-π stacking |
Self-healing; Conductivity; Adhesion; Antibacterial; Antioxidant; Photothermal effect; Sustained drug release | Full-thickness skin traumas | Hemostatic; Antibacterial; | [154] |
| PDA | Michael addition/Schiff base reaction | Self-healing; Antibacterial; Photothermal effect | Multi-drug resistant infected wounds | Antibacterial | [132] |
| PDA | Polymerization; Hydrogen bonding; Dopamine-Zn coordination | Antibacterial; Photothermal effect; | Bacteria-infected exposed wounds | Antibacterial | [88] |
| PDA | Hydrogen bonding | Self-healing; Conductivity; Thermosensitivity Antioxidant; Antibacterial; | Diabetic wounds | Antibacterial; Anti-inflammatory |
[44] |
| PDA | Polymerization | Toughness; Antioxidant; | Skin wounds | Anti-inflammatory | [181] |
| Lignin | Polymerization; Hydrogen bonding; | Adhesion; Toughness; Antibacterial; | Surgical operation | Antibacterial | [60] |
| Lignin | Polymerization; Electrostatic interaction | Self-healing; Toughness; Antibacterial; Antioxidant | Skin wounds | Antibacterial; Anti-inflammatory | [182] |
| Catechin | Polymerization; Electrostatic interaction | Antibacterial; Antioxidant; | Infected severe burn wounds | Antibacterial; Anti-inflammatory | [183] |
| EGCG | Polymerization | Adhesion; Toughness; Antioxidant; | Skin wounds | Hemostatic; Anti-inflammatory | [55] |
| EGCG | Polymerization; Boronate ester bonding | Self-healing; Toughness; Adhesion; Antibacterial; Antioxidant; | Diabetic chronic wounds | Hemostatic; Antibacterial; Anti-inflammatory | [54] |
5.2. Cancer therapy
Compared to intravenous anticancer drugs or nanomedicines, local tumor therapy can significantly reduce the risk of systemic toxicity [184,185]. At present, tumor resection and local radiotherapy are the most common local treatment methods, but the persistence of a single treatment cannot be guaranteed, which is easy to cause recurrence problems. Therefore, in contrast, local long-term implant therapy through bioactive materials such as hydrogels can exhibit unique advantages [186]. Polyphenol-based hydrogels with injectability, adhesion, toughness, biocompatibility anticancer agents loading and release capacity are very suitable for local implantation therapy of tumors [187], and the combination with traditional treatment methods such as surgical resection and local radiotherapy can often produce great therapeutic effects. In this section, according to the different functions of load contents of polyphenol-based hydrogels, the anticancer hydrogels are divided into chemotherapy, photothermal therapy, and immunotherapy hydrogels to elaborate and summarize (Table 2).
Table 2.
Summary of studies of polyphenol-based hydrogels for cancer therapy.
| Cancer treatment | Introduced polyphenols | Key properties | References |
|---|---|---|---|
| Chemotherapy | Dopamine | Injectability; Self-healing; Local sustained release | [185] |
| Chemotherapy | PDA | Adhesion; High drug loading efficiency; Tumor environment-sensitive degradability | [188] |
| Chemotherapy | PDA | Injectability; pH-sensitive drug release; Biodegradability | [189] |
| Photothermal-mediated therapy | PDA | Injectability; Self-healing | [132] |
| Photothermal-mediated therapy | PDA | NIR-sensitive thrombin release | [194] |
| Immunotherapy | Dopamine | Adhesion; Injectability; Thermosensitivity | [196] |
| Chemo/photothermal therapy | Dopamine | On-demand drug release | [197] |
5.2.1. Chemotherapy
Polyphenol-based hydrogels are good chemotherapeutic reservoirs that can be directly loaded with chemotherapy drugs or through the introduction of drug-loaded nanoparticles to achieve long-lasting local chemotherapy. Lee et al. fabricated a hydrogel platform based on multiple interactions between dopamine and phenylboronic acid-modified hyaluronic acids for erlotinib delivery [185]. The polyphenol-based hydrogel system displayed good injectable and self-healing properties, which could be peritumorally injected with prolonged retention, resulting in superior tumor-suppressive efficiencies on A549 tumor-implanted mice. In another work, Jiang et al. used PDA-hybridized zeolitic imidazolate framework-8 loaded with cisplatin and bone morphogenetic protein-2, which can be assembled onto the surface of 3D-printed gelatin hydrogel scaffolds via a layer-by-layer assembly strategy for sustained suppression of bone tumor recurrence and repair of bone defects after tumor resection [188]. In addition, bortezomib (BTZ) was also loaded into polyphenol-based hydrogels through boronate ester bonds between BTZ and catechol for subcutaneous and sustained drug delivery [189].
5.2.2. Photothermal-mediated therapy
Similar to the antibacterial applications, the photothermal properties of polyphenol-based hydrogels can also be used to mediate temperature rise for inducing tumor ablation or triggering the subsequent treatment processes, which have a broader research basis [[190], [191], [192], [193]]. For instance, Zhou et al. prepared a hydrogel system containing PDA-functionalized bioactive glass nanoparticles with excellent photothermal performances for skin-tumor therapy, which showed a tumor growth inhibition rate up to 94% in a subcutaneous skin-tumor model [132]. Moreover, in the study of Wang et al., a PDA-crosslinked collagen/silk fibroin composite hydrogel system was fabricated, and the photothermal properties of the system were used to trigger the release of thrombin for blocking blood vessels [194]. The in vivo test proved that the system was a potent candidate for the therapy of recurrence and metastasis in triple-negative breast cancer.
5.2.3. Immunotherapy
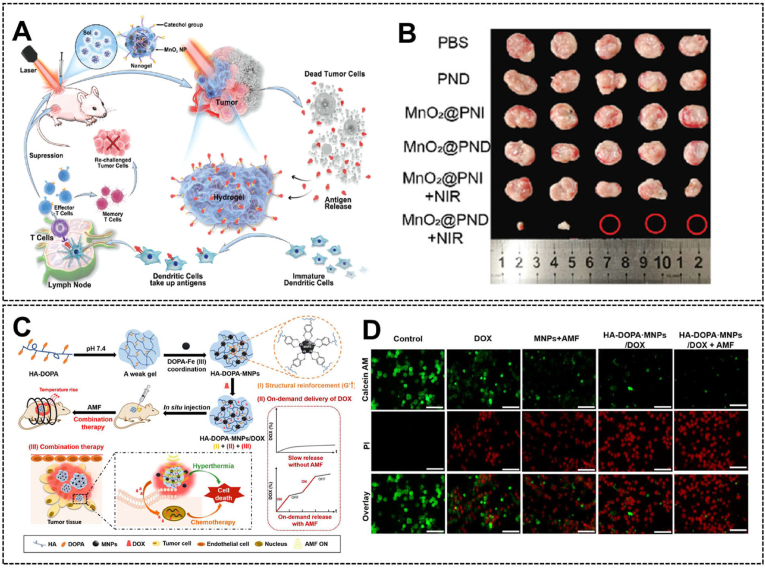
In recent years, cancer immunotherapy had attracted more people's attention compared with traditional therapy and gradually become a promising treatment strategy [195]. There are also hydrogel systems designed for cancer immunotherapy. For example, Fan et al. developed an injectable polyphenol-based hydrogel system loading with MnO2 nanoparticles, and a large number of autologous tumor-derived protein antigens could be released under NIR and captured by the adhesive hydrogel, resulting in a long-term immune-memory anti-tumor effect (Fig. 7A and B) [196].
Fig. 7.
(A) The schematic diagram of the catechol-modified adhesive hydrogel acted as an “antigen reservoir” for long-term immune-memory anti-tumor therapy, and (B) the antitumor effects in vivo. Reproduced with permission from Ref. [196]. Copyright 2021, Wiley-VCH. (C) The schematic diagram of the dopamine-functionalized hydrogel platform with iron oxide magnetic nanoparticles doping and doxorubicin loading for chemo/photothermal therapy, and (D) the killing effects on A375 tumor cells. Reproduced with permission from Ref. [197]. Copyright 2021, Elsevier.
5.2.4. Comprehensive cancer therapy
The comprehensive therapy with multi-mechanism may exhibit synergistic effects in cancer treatment, thus significantly improving the treatment efficiency. In recent years, comprehensive anticancer hydrogel systems attract much attention and show excellent potential in cancer therapy. For example, Dai et al. developed an iron oxide magnetic nanoparticles-doped, doxorubicin-loaded, and dopamine-functionalized hydrogel platform (Fig. 7C and D) [197]. The combination of chemo/photothermal therapy displayed improved antitumor effects in vivo.
5.3. Motor system injury repair
As the significant part that supports various movements, the injury of the motor system severely influences the quality of life. Polyphenol-based hydrogel systems, with inherent tissue-like properties of hydrogels and additional useful properties such as adhesion derived from polyphenol substances, are increasingly used for the repair of motor system injury [163,188,198,199]. According to the composition of the motor system, polyphenol-based hydrogels are mainly divided into the applications of bone injury and articular cartilage injury repair to elaborate and summarize (Table 3).
Table 3.
Summary of studies of polyphenol-based hydrogels for motor system injury repair.
| Repair types | Introduced polyphenols | Key properties | References |
|---|---|---|---|
| Bone repair | TA | Adhesion; Toughness; Biocompatibility | [200] |
| Bone repair | PDA | Adhesion; Photothermal; property Toughness | [163] |
| Bone repair | TA | Adhesion; Toughness; In-situ injectability; Thermosensitivity |
[205] |
| Bone repair | Dopamine | Adhesion; Toughness; Biodegradability | [206] |
| Articular cartilage repair | Dopamine | Adhesion; Toughness; | [198] |
| Articular cartilage repair | EGCG | Anti-inflammatory; Injectability | [199] |
5.3.1. Bone repair
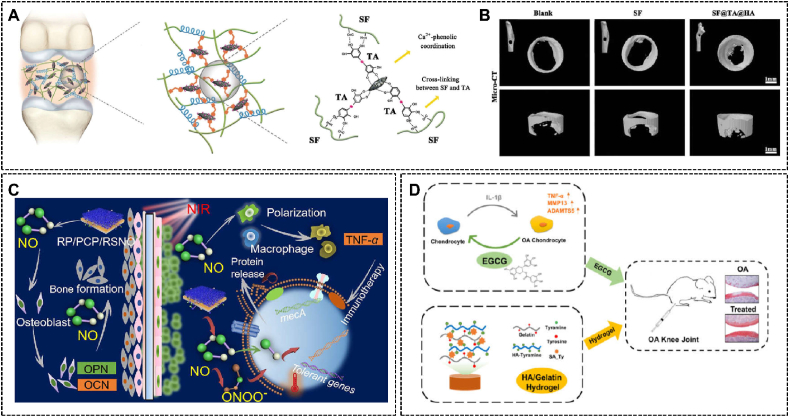
In general, bone is a dynamic tissue with the function of self-repair [200]. Lots of factors like skeletal stem cells can promote the self-healing of injured bones [201]. However, under certain conditions that cause severe destruction of bone, such as traumatic injury, bone tumor resection, and osteitis, the self-healing property may not compensate for the corresponding damage, resulting in serious bone injury, one of the most critical public health issues [19,202]. Therefore, there is an urgent need to develop an effective treatment that can fix ruptured osseous tissues and facilitate healing [202,203]. Polyphenol-based hydrogels are one of the potential candidates, which improve the possibility of a combination of many characteristics, such as adhesion, appropriate mechanical strength, and biocompatibility [204]. For example, Bai et al. developed a robust hydrogel system with strong bone adhesion and appropriate mechanical strength used tannic acid as a phenolic glue molecule to interact with silk fibroin and hydroxyapatite (Fig. 8A) [200]. The bone regenerative capacity was tested in vivo, and the result showed that after the 8-week implantation of this system, the formation of bone inside the original defect area was observed (Fig. 8B).
Fig. 8.
(A) The mechanisms of the considerably strong toughness of the TA-based hydrogel system, and (B) the X-ray micro-computed tomography images in the rat femoral defect model. Reproduced with permission from Ref. [200]. Copyright 2019, Wiley-VCH. (C) The mechanism of PDA-containing implant-coating hydrogel promoting bone formation. Reproduced with permission from Ref. [163] Copyright 2019, American Chemical Society. (D) The EGCG and hyaluronic acid-containing hydrogel with inflammation-modulatory and chondrogenic regenerative functions. Reproduced with permission from Ref. [199]. Copyright 2020, Elsevier.
Except for acting straightly on the defect area in bones, the polyphenol-based hydrogel system can also be used as a coating material of implants for bone injury therapy, which could solve the problems that seriously impede the application of implants in bone therapy, such as bacterial infection. Li et al. developed such an implant-coating hydrogel with strong adhesion, biocompatibility, and photothermal property for bacteria-killing and osteogenic differentiation promotion (Fig. 8C) [163]. Among the main components of the hydrogel, PDA was mainly used to produce adhesion and photothermal antibacterial effects, as well as S-nitrosuccinic acid was leveraged to produce NO for osteogenic differentiation by upregulating the expression of Opn and Ocn genes. The anti-infection and bone formation effects were certified through the experiments in vivo.
In addition, a polyphenol-based hydrogel system can also be treated as a delivery system in bone regeneration. Chen et al. fabricated a TA-containing composite hydrogel with adhesion and appropriate mechanical strength for an osteoinductive growth factor (engineered chimeric bone morphogenetic protein-2) delivery and spatio-temporal controlled release, and the experiment results exhibited that the hydrogel could effectively promote bone regeneration and accelerate new bone formation [205]. In another study, a dopamine-modified osteoconductive hydrogel was designed by Hasani-Sadrabadi et al. for the delivery of gingival mesenchymal stem cell and hydroxyapatite [206]. The research shows that the shortage of adhesion, retention, and mechanical properties in traditional hydrogels was avoided by the dopamine modification, and the bone regeneration ability was proved in a well-established peri-implantitis model, showing the great potential of craniofacial bone repair.
5.3.2. Articular cartilage repair
Articular cartilage is made up of dense connective tissue that can reduce bone-to-bone friction and cushion vibration caused by movement, which is an essential component of the motor system. Unfortunately, due to the absence of blood vessels, lymphangion, and nerves, articular cartilage owns poor regenerative and reparative abilities [207,208]. Furthermore, small amounts of local progenitor cells and low mobility also impede the regeneration of articular cartilage and increase the difficulty of treatments [209]. Based on the excellent properties such as adhesion and biocompatibility, polyphenol-based hydrogels have been introduced to assist articular cartilage repair and many research achievements have been achieved. For instance, Li et al. designed a double-crosslinking dopamine-modified polysaccharide hybrid hydrogel with, appropriate adhesion and mechanical strength for cartilage regeneration [198]. It can be seen from experiment results that accelerated proliferation, spreading, and cartilage matrix secretion of rabbit bone marrow stem cells was manifested, and expressions of related chondrogenic genes were promoted. Feng et al. developed a dopamine-modified microgel assembly to deliver bone marrow mesenchymal stem cells and sustained release kartogenin for articular cartilage repair [210]. The microgel assembly with the microenvironment of interconnected micropores and excellent adhesion ensured the high cell viability during the process of cell culture, preservation, and injection, which showed the promising application for cartilage repair and regeneration both in vitro and in vivo. In addition, wear of articular cartilage may gradually develop into osteoarthritis (OA), so the repair of articular cartilage damage also plays a very positive role in OA treatment. Base on this, an EGCG and hyaluronic acid-containing hydrogel constructed by Jin et al. could not only suppress inflammation but also promote chondrogenic regeneration, which had a good application prospect in OA treatment (Fig. 8D) [199].
5.4. Digestive system disease therapy
The digestive system consists of many organs from the oral cavity to the large intestine and is responsible for breaking down food, absorbing nutrients, and ridding waste products from the food. Including gastrohelcosis, colitis, and many oral diseases, the digestive system is an area with a high incidence of many diseases. Moreover, the complex environment with various food residues and digestive fluids determined by the physiological function of the digestive system puts forward high requirements for treatment methods. In existing studies, polyphenol-based hydrogels have also begun to be used to participate in the therapy process of digestive system-related diseases, which will be described in this section.
5.4.1. Dental disease therapy
In the oral cavity, teeth are an extremely important organ, which is responsible for the vital functions of cutting, biting, and chewing food. Nowadays, dental diseases have become one of the major global health problems [211]. Polyphenol-based hydrogels that have lots of gorgeous properties discussed above, show the potentials in the therapy in dental diseases. The mechanism of applications of polyphenol-based hydrogel in dental disease can be mainly be divided into two aspects: accelerating bone repair or regeneration and facilitating the regeneration of cells or tissues related to dental disease [212,213].
For the materials in the bone repair of teeth, biocompatibility, mechanical properties, stability, and long-term performance are the most crucial required properties [212,214]. In the study of Zhong et al., a dopamine-grafted hydrogel endowed with strong wet adhesion, antibacterial property, injectability, in situ formation property, was designed, and it could be treated as a bone repair material for periodontitis therapy [214]. In this system, the dopamine endowed hydrogel with strong wet adhesion and the amorphous calcium phosphate made this hydrogel to be antibacterial.
The regeneration of tooth-related cells or tissues, such as periodontium, gingival tissues, human dental pulp cells, gingival fibroblast cells, is another mechanism in the therapies for dental disease. Recently, many kinds of materials have been prepared in a form of tissue engineering scaffolds to promote the regeneration of tooth-related cells or tissues, and the polyphenol-based hydrogel is one of them [[215], [216], [217]]. Huerta et al. fabricated cellulose nanofiber hydrogels with lignin, which can be treated as scaffolds for gingival fibroblast cells proliferation [217]. The system was produced by high-intensity ultrasound technology, without the need for surface modification or crosslinkers, which showed high elasticity and excellent cells adhesion and viability for regenerative periodontal therapy.
5.4.2. Gastric ulcer therapy
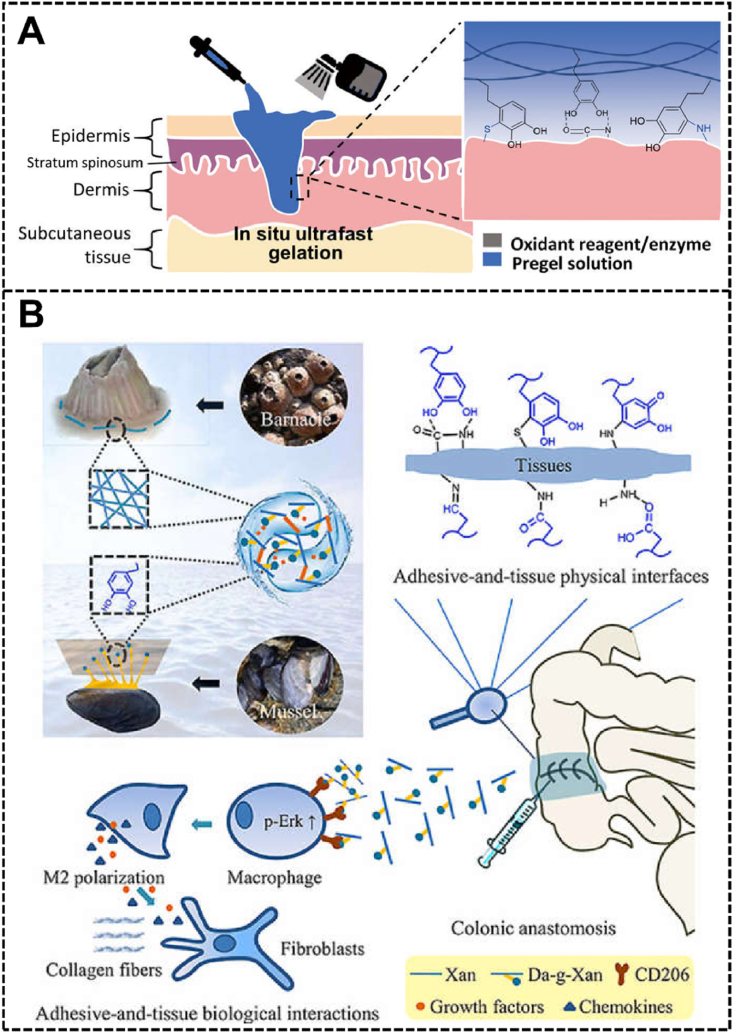
In the gastric ulcer therapy process, many properties of polyphenol-based hydrogels can be used to enhance gastric mucosa protection and promote recovery, including but not limited to adhesion, antibacterial activity, anti-inflammatory activity, and cell regeneration promotion ability [218]. For example, Xu et al. designed and prepare a hyaluronic acid and catechol-containing enduringly and strongly adhesive hydrogel with ultrafast and pH-independent gelation for ulcer surface protection (Fig. 9A) [219]. The experimental results showed that the hydrogel formed rapidly in the ulcer site could last for more than 48 h in a porcine model, providing a strong promotion for the treatment and rehabilitation of refractory gastric ulcers.
Fig. 9.
(A) The schematic diagram of the catechol-containing enduringly and strongly adhesive hydrogel formation by spraying oxidant in situ for ulcer surface protection. Reproduced with permission from Ref. [219]. Copyright 2020, American Association for the Advancement of Science. (B) The mechanisms of the dopamine-conjugated xanthan gum hydrogel for PAL treatment. Reproduced with permission from Ref. [220]. Copyright 2021, KeAi.
5.4.3. Other digestive diseases therapy
Postoperative anastomotic leakage (PAL) is a serious complication of digestive fluid leakage into the abdominal cavity or chest cavity after digestive tract reconstruction, which may cause local and systemic infections and even death if not treated appropriately. A hydrogel prepared with dopamine-conjugated xanthan gum was used by Huang et al. for PAL treatment, which not only showed protective effects by strong adhesion and significantly improving bursting pressure, but also induced type 2 macrophage polarization of macrophages to create an appropriate microenvironment for angiogenesis and fibroblast infiltration, and therefore promotes the recovery of PAL (Fig. 9B) [220].
5.5. Ophthalmic disease therapy
At present, the most common method for this disease is the eye drop, which is the treatment of choice for eye infection, inflammation, glaucoma, dry eye, and allergy [221]. However, resulting from the unique physiology and anatomy of the eye, many disadvantages of current therapy are still needed to be solved, such as limited bioavailability [221]. In addition, some ophthalmic diseases involve the damage of cells or tissue, requiring the delivery of cells or the ability to promote the regeneration of cells or tissue [222,223]. It is difficult to develop an eye drop with these functions, and therefore designing a novel therapy in ophthalmic disease is an urgent need.
As an important and effective system, because of the many excellent properties discussed above, polyphenol-based hydrogels have been applied in ophthalmic diseases. For example, a dopamine-functionalized hydrogel system designed by Lee et al. with biocompatibility, adhesion, and injectability was fabricated as a carrier for retinal pigment epithelium (RPE) cell delivery for countering degeneration of RPE cells and improving visual function [222]. The cell viability and cell-releasing ability were tested in vitro with expected results. In another example, Koivusalo et al. designed a dopamine-grafted and implantable tissued adhesive hydrogel that can deliver the therapeutic stem cells for simultaneous regeneration of corneal stroma and epithelium, which became a new therapy for the regeneration of a severely damaged cornea [223]. In this hydrogel, the introduction of dopamine played a considerable role, including imparting tissue adhesive function, augmenting the cell viability, and endowing hydrogel with appropriate mechanical properties. Efficient cell adhesion and better long-term cell viability by this hydrogel were observed in vitro, and the ability to deliver these undifferentiated cells to the corneal stroma was also proved, indicating its potential as an implantable tissue-engineered hydrogel for the severely damaged cornea.
5.6. Bioelectronics
Bioelectronics is an emerging subject field formed by the cross infiltration of biological and electronic information science and has gained great development in recent years [33]. However, the material difference between biological tissues and electronics hinders their interaction and restricts the further development of bioelectronics [224]. Polyphenol-based hydrogels with tissue-like properties and adhesion (bioaffinity) are expected to become a bridge between biological tissues and electronics and have a good application prospect in bioelectronics.
5.6.1. Flexible electronics
In general, flexible electronics involve biosensors, biomimetic skin, implantable bioelectrode, and so on, which have widespread applications, such as implantable bioelectronics for medical detection of electrocardiography and electromyography signals, wearable electronic devices for healthcare monitoring, electronic skins, and flexible supercapacitors.
The wearable flexible electronic device for healthcare monitoring is one of the major applications in flexible electronics. The pursue of wearable flexible electronic devices is the real-time detection of various body movements, which makes it necessary to have strong adhesion, biocompatibility, appropriate mechanical strength, detection stability, high sensitivity, conductive, and so on. Based on the advantages discussed above, a polyphenol-based hydrogel is a potent candidate. For example, Mo et al. developed a TA-based hydrogel system that satisfied most of the properties required in a wearable flexible electronic device and showed intriguing potential in human motion and healthcare monitoring [225]. TA played an indispensable role in this system: on the one hand, they acted as dynamic mediators, programing multiple dynamic interactions with the other components, so that ultra-stretchability, outstanding self-healing abilities were endowed, on the other hand, catechol group in tannic acid enable this system with outstanding adhesion. The strain sensor in monitoring various strain-related human activities was tested, and the ability to accurately detect the motion was certified in this research.
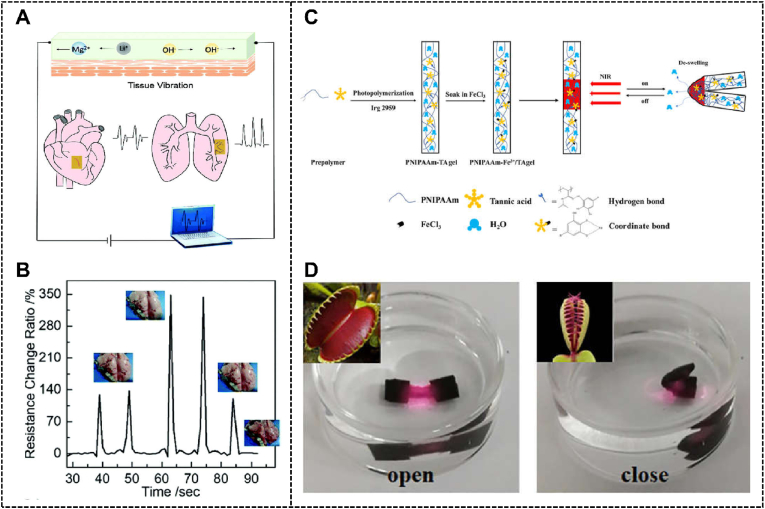
Implantable bioelectronics devices for monitoring the physiological status of patients and disease treatment are also the application directions in flexible electronics. The requirement for this application is similar to a wearable flexible electronic device, and therefore, polyphenol-based hydrogels also show unique advantages. Pei et al. fabricated a dopamine-containing hydrogel, which could be applied to remote monitoring of organ motions through wireless transmission (Fig. 10A and B) [226]. Because of the introduction of dopamine, this hydrogel had robust and reusable tissue adhesion, stretchability, self-healing ability, and biocompatibility.
Fig. 10.
(A) The schematic illustration of monitoring tissue vibration with the dopamine-containing hydrogel sensor, and (B) the real-time monitoring and wireless transmission in vitro. Reproduced with permission from Ref. [226]. Copyright 2020, The Royal Society of Chemistry. (C) Preparation and NIR-triggered actuation of the TA-based hydrogel soft actuator, (D) The simulation of the process of Venus flytrap's closing with the NIR-triggered hydrogel actuator. Reproduced with permission from Ref. [237]. Copyright 2021, American Chemical Society.
Electronic biomimetic skin (e-skin) is also important in the flexible electronics field [227]. Human skin is a highly sophisticated system, with flexibility, stretchability, self-healing ability, robust sensitivity, and resiliency to external environmental changes [228]. As the biomimetic counterpart, e-skin can be applied for artificial intelligence, human-machine interactions, and personal healthcare [229]. However, enormous efforts should be made to develop the e-skin, because integrating lots of required and excellent properties is difficult. Fortunately, the polyphenol-based hydrogel may bring this field with hope because of the properties that had been discussed above [230]. A dopamine-introduced, transparent, and conductive hydrogel was developed by Zhang et al. for biomimetic skin fabrication [231]. Dopamine endowed the system with many excellent performances, including but not limited to strong adhesion, robust elasticity, self-healing ability, and thermosensitivity. The prospect of the system to be used as strain, pressure, and temperature sensors was verified, which greatly contributed to the development of biomimetic skin.
5.6.2. Soft actuators
In bioelectronic applications of the polyphenol-based hydrogel, the construction of soft actuators is another application different from flexible electronics [232]. The requirements of this hydrogel are not only the ability to respond to the various environmental stimuli, including but not limited to temperature, pH, humidity, light, electricity, magnetic field, but also changing their shape and physical properties under these environmental stimuli [[232], [233], [234], [235]]. Hydrogels satisfying these requirements have lots of applications, such as actuators, artificial muscles, self-locomotion robotics, and prosthetic materials [233,236]. Zhang et al. developed a light-driven TA-based hydrogel soft actuator, which showed promising application in the fields of biomimetic devices, soft robotics, and so on (Fig. 10C and D) [237]. This system could display programmed deformation, with the properties of real-time spatiotemporal precision and remote control. TA in this system played a significant role in enhancing mechanical properties, stability, and adhesion, as well as complexing with Fe3+ for realizing a controllable photothermal effect. The super-soft and dynamic DNA/dopamine-grafted-dextran hydrogel designed by Han et al. can respond to volume rapidly and sensitively upon solvents with different polarities [71]. After changing the solvents, the volume of hydrogel would undergo a fast change, demonstrating the high sensitivity of the responsiveness. In this research, volume and shape-responsiveness, electric circuit switched by the water/petroleum ether switch pair, and a microbial metabolism process were tested in vitro, certifying its potential in soft robots, controlled electro-catalysis, and so on.
5.7. Sun block and skin care
With the increasing interest of people in beauty, more and more hydrogel systems have been developed for cosmetic applications. Hydrogels are favored by the cosmetics industry mainly because of their tissue-like properties such as high water content and biocompatibility [238]. Moreover, after the introduction of polyphenols, hydrogels will further produce adhesive, self-healing, antibacterial and antioxidant properties, which are more suitable for skin care. Wang et al. developed a TA-based hydrogel system with enhanced skin affinity, water resistance, and self-recovery [239]. The hydrogel can prevent the skin penetration of ultraviolet (UV) across broad UVA and UVB regions (360-275 nm) without irritation and sensitization and could be readily removed on demand. The long-term and stable UV protection functions were tested in vitro and in vivo, highlighting the potential of this hydrogel in sun block and skin care, even under sweaty and dynamic physiological conditions. In another study, Hong et al. designed a novel hydrogel as a soft tissue filler to reduce skin wrinkles [240]. Due to the crafting of dopamine, this hydrogel had numerous intriguing properties, including enhanced mechanical properties, tissue adhesion, and biocompatibility, which were certified both in vitro and vivo.
6. Efficient reverse design considerations
The cognition of most substances with natural origin will undergo a similar evolution process from cognizing structures to properties, and then considering uses. Polyphenol-based hydrogels are no exception. After nearly 20 years of evolution and development, it has formed a clear research vein from structures to properties to applications, which has been described in detail above. However, for polyphenol-based hydrogels, the ultimate value of their research lies in producing accurate and effective applications, thus contributing to human society. Therefore, after understanding the natural law of the evolution of polyphenol-based hydrogels, we need to design and make necessary modifications of polyphenol-based hydrogels artificially, to better meet the needs of different biomedical fields. Based on this purpose, in this section, we put forward a new kind of polyphenol-based hydrogel reverse design consideration. Specifically, starting from the application through a goal-directed way, designers can consider the required properties for that application, and then design the crosslinked structures which can produce these properties. Finally, the composition of the polyphenol-based hydrogel can be selected to complete the entire design process.
6.1. From biomedical applications to properties
So far, the application prospect of polyphenol-based hydrogels in biomedicine has been widely studied, but there is still a long way to go before large-scale application in the human body. Therefore, more researches will be carried out in the future to shorten the distance. In order to improve the efficiency of future research and production of polyphenol-based hydrogels, application orientation is a very operative consideration. In the biomedical field, many serious diseases with high incidences, such as severe wound infection and malignant tumor recurrence, are urgent need to introduce persistent and effective bioactive materials for adjuvant treatment, and considering the application directions with high patient demand during the research subject design of polyphenol-based hydrogels can also obtain more clinical and relevant research data supports so that more efficient research output can be well achieved and the requirements of more clinical patients can be well met.
Based on the requirements of biomedical applications and rich clinical experience, it is often possible to extract the special properties of polyphenol-based hydrogels required for the application direction, thus laying a foundation for the subsequent design of crosslinking structures. Taking the research of Han et al. for an example, considering the serious harm caused by clinical uncontrolled bleeding and infection, tissue-like materials with strong local hemostatic sealing and anti-infection properties were urgent to be developed, which requires the materials to possess strong adhesion and antibacterial properties in the wet environment with much interfacial water [42]. For another example, in the research of Wang et al., starting from the thorny clinical problem that triple-negative breast cancer is prone to in-situ recurrence and distal metastasis, the strategy of blocking blood vessels and inhibiting angiogenesis was considered for local treatment, and then the local implantation treatment system capable of the intelligent responsive release of thrombin needed to be designed [194]. Therefore, the system is required to have intelligent response units (such as photothermal substances) and sufficient strength, toughness, and local adhesion ability.
6.2. From properties to crosslinking structures
The properties and structure of polyphenol hydrogels are always closely related, and the interaction of microcosmic crosslinked structures creates the properties of polyphenol-based hydrogels at the macro level. After determining the direction of application and the necessary properties, the microcosmic crosslinked structures of polyphenol-based hydrogels can be reversely derived, such as the design of dynamic non-covalent interactions and covalent bonds for toughness and self-healing properties improvement. This part has been elaborated above on the common crosslinking structures of polyphenol-based hydrogels and their relationship with their unique properties. Further, when finishing the design of the internal structure of polyphenol-based hydrogels, suitable polyphenol substances can be selected, such as dopamine for polymerization crosslinking, as well as TA with rich aromatic rings and phenolic hydroxyl structures for π-π stacking and hydrogen bonding.
At this point, a complete design process of polyphenol-based hydrogel systems is completed, and the exploration of preparation can be started after evaluating the feasibility of experimental conditions.
7. Conclusions and perspectives
In conclusion, a class of amazing polyphenol-based hydrogel bioactive materials is born through the fusion of nature-derived or/and modified polyphenols with hydrogel materials. The 3D networks of hydrogel constructed by several covalent and non-covalent interactions among polyphenols and other substances produce various outstanding properties of adhesion, toughness, strength, self-healing, photothermal, antioxidant, and antibacterial, which can be widely used in the field of biomedicine such as wound healing, antitumor, treatment of motor system injury, digestive system disease, oculopathy, and bioelectronics. In this review, the pyramid evolution of polyphenol-based hydrogels from crosslinked structures to derivative properties and then to biomedical applications were described in detail, as well as the efficient reverse design considerations of these hydrogel systems were put forward, which was conducive to the further development of polyphenol-based hydrogel materials.
Although many scientific achievements have been produced in this field, there are still some challenges that need to be overcome in the future: (1) Most of the existing studies on polyphenol-based hydrogels spanned the whole process from a polyphenol selection to a biomedical application, so it is difficult to focus on a specific point for in-depth exploration. (2) More than 8000 polyphenols have been identified, but only a few have been used to construct polyphenol-based hydrogels. There is still a lot of works to be done for developing other natural or modified polyphenols as a hydrogel component for biomedical applications. (3) Polyphenol-based hydrogels have been applied in many biomedical fields, but the distribution of these researches is very uneven. For example, more than half of the researches have focused on wound healing. Therefore, it is necessary to invest more time and energy to explore more unexploited biomedical fields, to facilitate the balanced development of polyphenol-based hydrogel researches.
Moreover, in terms of clinical application, lots of commercial hydrogels have been used in biomedicine so far including drug delivery, wound dressings, tissue engineering, contact lenses, and fillers [238,241], but the ingredients of commercial hydrogels are confined to the substances with extensive sources, low costs, stable properties and thorough researches such as common natural polymers (alginate, collagen, etc.) and simple synthetic polymers (polyacrylic acid, polyvinyl alcohol, etc.). Compared with them, polyphenol-based hydrogels have unique advantages, such as owning the ability to meet the requirements of adhesion, toughness, strength, and self-healing simultaneously, but there are also obvious problems in further application in clinical practice. It is mainly embodied in the instability in storage and transportation process for the easily oxidized structure, the difficulties for some complex polyphenol-based systems to realize large-scale production, the increased costs due to scarcity of some polyphenol sources, and the incomplete toxicology researches, so there is still a certain distance to clinical translation. However, it is believed that with further research, when these problems can be greatly improved, the unique advantages of polyphenol-based hydrogels may enable them to quickly enter the discussion range of clinical application.
To sum up, the polyphenol-based hydrogel is a promising research direction, but it is still in the initial stage, and there is still plenty of works to be done. It is hoped that the summary of this review can give more inspiration to relevant researchers and further promote the development of polyphenol-based hydrogel materials.
Declaration of interest
The authors declare that there is no conflict of interest.
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
We are grateful for the financial support from the National Natural Science Foundation of China (32071342 and 82072513), Guangdong Special Support Program (2019TQ05Y209), the Natural Science Foundation of Guangdong Province (2021A1515010431), and the Science and Technology Program of Guangzhou (202102080182). Several picture materials from vecteezy.com are gratefully acknowledged.
Footnotes
Peer review under responsibility of KeAi Communications Co., Ltd.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.bioactmat.2022.01.038.
Contributor Information
Kebing Chen, Email: chkbing@mail.sysu.edu.cn.
Xiaowei Zeng, Email: zengxw23@mail.sysu.edu.cn.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Daly A.C., Riley L., Segura T., Burdick J.A. Hydrogel microparticles for biomedical applications. Nat. Rev. Mater. 2020;5(1):20–43. doi: 10.1038/s41578-019-0148-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nele V., Wojciechowski J.P., Armstrong J.P.K., Stevens M.M. Tailoring gelation mechanisms for advanced hydrogel applications. Adv. Funct. Mater. 2020;30(42):2002759. [Google Scholar]
- 3.Jiang Y., Krishnan N., Heo J., Fang R.H., Zhang L. Nanoparticle–hydrogel superstructures for biomedical applications. J. Contr. Release. 2020;324:505–521. doi: 10.1016/j.jconrel.2020.05.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen W., Tao W. Precise control of the structure of synthetic hydrogel networks for precision medicine applications. Matter. 2022;5(1):18–19. [Google Scholar]
- 5.Caliari S.R., Burdick J.A. A practical guide to hydrogels for cell culture. Nat. Methods. 2016;13(5):405–414. doi: 10.1038/nmeth.3839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ghobril C., Grinstaff M.W. The chemistry and engineering of polymeric hydrogel adhesives for wound closure: a tutorial. Chem. Soc. Rev. 2015;44(7):1820–1835. doi: 10.1039/c4cs00332b. [DOI] [PubMed] [Google Scholar]
- 7.Choi S.W., Guan W., Chung K. Basic principles of hydrogel-based tissue transformation technologies and their applications. Cell. 2021;184(16):4115–4136. doi: 10.1016/j.cell.2021.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tong Z., Jin L., Oliveira J.M., Reis R.L., Zhong Q., Mao Z., Gao C. Adaptable hydrogel with reversible linkages for regenerative medicine: dynamic mechanical microenvironment for cells. Bioactive Mater. 2021;6(5):1375–1387. doi: 10.1016/j.bioactmat.2020.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Abdollahiyan P., Baradaran B., de la Guardia M., Oroojalian F., Mokhtarzadeh A. Cutting-edge progress and challenges in stimuli responsive hydrogel microenvironment for success in tissue engineering today. J. Contr. Release. 2020;328:514–531. doi: 10.1016/j.jconrel.2020.09.030. [DOI] [PubMed] [Google Scholar]
- 10.Li J., Mooney D.J. Designing hydrogels for controlled drug delivery. Nat. Rev. Mater. 2016;1(12) doi: 10.1038/natrevmats.2016.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cooper R.C., Yang H. Hydrogel-based ocular drug delivery systems: emerging fabrication strategies, applications, and bench-to-bedside manufacturing considerations. J. Contr. Release. 2019;306:29–39. doi: 10.1016/j.jconrel.2019.05.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Khajouei S., Ravan H., Ebrahimi A. DNA hydrogel-empowered biosensing. Adv. Colloid Interface Sci. 2020;275:102060. doi: 10.1016/j.cis.2019.102060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li Z.Q., Hou Z.H., Fan H.X., Li H.R. Organic-Inorganic hierarchical self-assembly into robust luminescent supramolecular hydrogel. Adv. Funct. Mater. 2017;27(2):1604379. [Google Scholar]
- 14.Hu Q., Li H., Archibong E., Chen Q., Ruan H., Ahn S., Dukhovlinova E., Kang Y., Wen D., Dotti G., Gu Z. Nature Biomedical Engineering; 2021. Inhibition of Post-surgery Tumour Recurrence via a Hydrogel Releasing CAR-T Cells and Anti-PDL1-conjugated Platelets. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Feng C., Ouyang J., Tang Z., Kong N., Liu Y., Fu L., Ji X., Xie T., Farokhzad O.C., Tao W. Germanene-based theranostic materials for surgical adjuvant treatment: inhibiting tumor recurrence and wound infection. Matter. 2020;3(1):127–144. [Google Scholar]
- 16.Liu W., Wenbin O.-Y., Zhang C., Wang Q., Pan X., Huang P., Zhang C., Li Y., Kong D., Wang W. Synthetic polymeric antibacterial hydrogel for methicillin-resistant Staphylococcus aureus-infected wound healing: nanoantimicrobial self-assembly, drug- and cytokine-free strategy. ACS Nano. 2020;14(10):12905–12917. doi: 10.1021/acsnano.0c03855. [DOI] [PubMed] [Google Scholar]
- 17.Fuchs S., Ernst A.U., Wang L.-H., Shariati K., Wang X., Liu Q., Ma M. Hydrogels in emerging technologies for type 1 diabetes. Chem. Rev. 2021;121(18):11458–11526. doi: 10.1021/acs.chemrev.0c01062. [DOI] [PubMed] [Google Scholar]
- 18.Kim T., Suh J., Kim W.J. Polymeric aggregate-embodied hybrid nitric-oxide-scavenging and sequential drug-releasing hydrogel for combinatorial treatment of rheumatoid arthritis. Adv. Mater. 2021;33(34):2008793. doi: 10.1002/adma.202008793. [DOI] [PubMed] [Google Scholar]
- 19.Zhang X., Li Z., Yang P., Duan G., Liu X., Gu Z., Li Y. Polyphenol scaffolds in tissue engineering. Mater. Horiz. 2021;8(1):145–167. doi: 10.1039/d0mh01317j. [DOI] [PubMed] [Google Scholar]
- 20.Shavandi A., Bekhit A.E.-D.A., Saeedi P., Izadifar Z., Bekhit A.A., Khademhosseini A. Polyphenol uses in biomaterials engineering. Biomaterials. 2018;167:91–106. doi: 10.1016/j.biomaterials.2018.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Quideau S., Deffieux D., Douat-Casassus C., Pouysegu L. Plant polyphenols: chemical properties, biological activities, and synthesis. Angew. Chem. Int. Ed. 2011;50(3):586–621. doi: 10.1002/anie.201000044. [DOI] [PubMed] [Google Scholar]
- 22.Daglia M. Polyphenols as antimicrobial agents. Curr. Opin. Biotechnol. 2012;23(2):174–181. doi: 10.1016/j.copbio.2011.08.007. [DOI] [PubMed] [Google Scholar]
- 23.Yahfoufi N., Alsadi N., Jambi M., Matar C. The immunomodulatory and anti-inflammatory role of polyphenols. Nutrients. 2018;10(11):1618. doi: 10.3390/nu10111618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cory H., Passarelli S., Szeto J., Tamez M., Mattei J. The role of polyphenols in human health and food systems: a mini-review. Front. Nutr. 2018;5:87. doi: 10.3389/fnut.2018.00087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cheng W., Zeng X., Chen H., Li Z., Zeng W., Mei L., Zhao Y. Versatile polydopamine platforms: synthesis and promising applications for surface modification and advanced nanomedicine. ACS Nano. 2019;13(8):8537–8565. doi: 10.1021/acsnano.9b04436. [DOI] [PubMed] [Google Scholar]
- 26.Rahim M.A., Kristufek S.L., Pan S., Richardson J.J., Caruso F. Phenolic building blocks for the assembly of functional materials. Angew. Chem. Int. Ed. 2019;58(7):1904–1927. doi: 10.1002/anie.201807804. [DOI] [PubMed] [Google Scholar]
- 27.Patil N., Jerome C., Detrembleur C. Recent advances in the synthesis of catechol-derived (bio)polymers for applications in energy storage and environment. Prog. Polym. Sci. 2018;82:34–91. [Google Scholar]
- 28.Faure E., Falentin-Daudré C., Jérôme C., Lyskawa J., Fournier D., Woisel P., Detrembleur C. Catechols as versatile platforms in polymer chemistry. Prog. Polym. Sci. 2013;38(1):236–270. [Google Scholar]
- 29.Lee H., Lee B.P., Messersmith P.B. A reversible wet/dry adhesive inspired by mussels and geckos. Nature. 2007;448(7151):338–341. doi: 10.1038/nature05968. [DOI] [PubMed] [Google Scholar]
- 30.Liu Y., Ai K., Lu L. Polydopamine and its derivative materials: synthesis and promising applications in energy, environmental, and biomedical fields. Chem. Rev. 2014;114(9):5057–5115. doi: 10.1021/cr400407a. [DOI] [PubMed] [Google Scholar]
- 31.Wang W., Tang Z., Zhang Y., Wang Q., Liang Z., Zeng X. Mussel-inspired polydopamine: the bridge for targeting drug delivery system and synergistic cancer treatment. Macromol. Biosci. 2020;20(10):2000222. doi: 10.1002/mabi.202000222. [DOI] [PubMed] [Google Scholar]
- 32.Lee B.P., Dalsin J.L., Messersmith P.B. Synthesis and gelation of DOPA-modified poly(ethylene glycol) hydrogels. Biomacromolecules. 2002;3(5):1038–1047. doi: 10.1021/bm025546n. [DOI] [PubMed] [Google Scholar]
- 33.Xie C., Wang X., He H., Ding Y., Lu X. Mussel-inspired hydrogels for self-adhesive bioelectronics. Adv. Funct. Mater. 2020;30(25):1909954. [Google Scholar]
- 34.Liu C., Goel P., Kaeser P.S. Spatial and temporal scales of dopamine transmission. Nat. Rev. Neurosci. 2021;22(6):345–358. doi: 10.1038/s41583-021-00455-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Xu C., Xu Y., Yang M., Chang Y., Nie A., Liu Z., Wang J., Luo Z. Black-phosphorus-Incorporated hydrogel as a conductive and biodegradable platform for enhancement of the neural differentiation of mesenchymal stem cells. Adv. Funct. Mater. 2020;30(39):2000177. [Google Scholar]
- 36.Li Z., Yang Y., Wei H., Shan X., Wang X., Ou M., Liu Q., Gao N., Chen H., Mei L., Zeng X. Charge-reversal biodegradable MSNs for tumor synergetic chemo/photothermal and visualized therapy. J. Contr. Release. 2021;338:719–730. doi: 10.1016/j.jconrel.2021.09.005. [DOI] [PubMed] [Google Scholar]
- 37.Wu Y., Jiao L., Xu W., Gu W., Zhu C., Du D., Lin Y. Polydopamine-capped bimetallic AuPt hydrogels enable robust biosensor for organophosphorus pesticide detection. Small. 2019;15(17):1900632. doi: 10.1002/smll.201900632. [DOI] [PubMed] [Google Scholar]
- 38.Yang Y., Zeng W., Huang P., Zeng X., Mei L. Vol. 2. 2021. Smart materials for drug delivery and cancer therapy; p. 20200042. 2. [Google Scholar]
- 39.Yang Y., Liang Y., Chen J., Duan X., Guo B. Mussel-inspired adhesive antioxidant antibacterial hemostatic composite hydrogel wound dressing via photo-polymerization for infected skin wound healing. Bioactive Mater. 2022;8:341–354. doi: 10.1016/j.bioactmat.2021.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Xu C., Chang Y., Wu P., Liu K., Dong X., Nie A., Mu C., Liu Z., Dai H., Luo Z. Two-dimensional-germanium phosphide-reinforced conductive and biodegradable hydrogel scaffolds enhance spinal cord injury repair. Adv. Funct. Mater. 2021;31(41):2104440. [Google Scholar]
- 41.Yang M.Y., Lee S.Y., Kim S., Koo J.S., Seo J.H., Jeong D., Hwang C., Lee J., Cho H.J. Selenium and dopamine-crosslinked hyaluronic acid hydrogel for chemophotothermal cancer therapy. J. Contr. Release. 2020;324:750–764. doi: 10.1016/j.jconrel.2020.04.024. [DOI] [PubMed] [Google Scholar]
- 42.Han W., Zhou B., Yang K., Xiong X., Luan S., Wang Y., Xu Z., Lei P., Luo Z., Gao J., Zhan Y., Chen G., Liang L., Wang R., Li S., Xu H. Biofilm-inspired adhesive and antibacterial hydrogel with tough tissue integration performance for sealing hemostasis and wound healing. Bioactive Mater. 2020;5(4):768–778. doi: 10.1016/j.bioactmat.2020.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Guo Z., Xie W., Lu J., Guo X., Xu J., Xu W., Chi Y., Takuya N., Wu H., Zhao L. Tannic acid-based metal phenolic networks for bio-applications: a review. J. Mater. Chem. B. 2021;9(20):4098–4110. doi: 10.1039/d1tb00383f. [DOI] [PubMed] [Google Scholar]
- 44.Wang Y., Li L., Ma Y., Tang Y., Zhao Y., Li Z., Pu W., Huang B., Wen X., Cao X., Chen J., Chen W., Zhou Y., Zhang J. Multifunctional supramolecular hydrogel for prevention of epidural adhesion after laminectomy. ACS Nano. 2020;14(7):8202–8219. doi: 10.1021/acsnano.0c01658. [DOI] [PubMed] [Google Scholar]
- 45.Qiao Z., Lv X., He S., Bai S., Liu X., Hou L., He J., Tong D., Ruan R., Zhang J., Ding J., Yang H. A mussel-inspired supramolecular hydrogel with robust tissue anchor for rapid hemostasis of arterial and visceral bleedings. Bioactive Mater. 2021;6(9):2829–2840. doi: 10.1016/j.bioactmat.2021.01.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cao C., Yang N., Zhao Y., Yang D., Hu Y., Yang D., Song X., Wang W., Dong X. Biodegradable hydrogel with thermo-response and hemostatic effect for photothermal enhanced anti-infective therapy. Nano Today. 2021;39:101165. [Google Scholar]
- 47.Lin F., Wang Z., Shen Y., Tang L., Zhang P., Wang Y., Chen Y., Huang B., Lu B. Natural skin-inspired versatile cellulose biomimetic hydrogels. J. Mater. Chem. 2019;7(46):26442–26455. [Google Scholar]
- 48.Zhou L., Fan L., Yi X., Zhou Z., Liu C., Fu R., Dai C., Wang Z., Chen X., Yu P., Chen D., Tan G., Wang Q., Ning C. Soft conducting polymer hydrogels cross-linked and doped by tannic acid for spinal cord injury repair. ACS Nano. 2018;12(11):10957–10967. doi: 10.1021/acsnano.8b04609. [DOI] [PubMed] [Google Scholar]
- 49.Yang Y., Zhao X., Yu J., Chen X., Wang R., Zhang M., Zhang Q., Zhang Y., Wang S., Cheng Y. Bioactive skin-mimicking hydrogel band-aids for diabetic wound healing and infectious skin incision treatment. Bioactive Mater. 2021;6(11):3962–3975. doi: 10.1016/j.bioactmat.2021.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ahmadian Z., Correia A., Hasany M., Figueiredo P., Dobakhti F., Eskandari M.R., Hosseini S.H., Abiri R., Khorshid S., Hirvonen J., Santos H.A., Shahbazi M.-A. A hydrogen-bonded extracellular matrix-mimicking bactericidal hydrogel with radical scavenging and hemostatic function for pH-responsive wound healing acceleration. Adv. Healthcare Mater. 2021;10(3):2001122. doi: 10.1002/adhm.202001122. [DOI] [PubMed] [Google Scholar]
- 51.Chen Z., Farag M.A., Zhong Z., Zhang C., Yang Y., Wang S., Wang Y. Multifaceted role of phyto-derived polyphenols in nanodrug delivery systems. Adv. Drug Deliv. Rev. 2021;176:113870. doi: 10.1016/j.addr.2021.113870. [DOI] [PubMed] [Google Scholar]
- 52.Wang S., Li Z., Ma Y., Liu Y., Lin C.-C., Li S., Zhan J., Ho C.-T. Immunomodulatory effects of green tea polyphenols. Molecules. 2021;26(12):3755. doi: 10.3390/molecules26123755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Li K., Xiao G., Richardson J.J., Tardy B.L., Ejima H., Huang W., Guo J., Liao X., Shi B. Targeted therapy against metastatic melanoma based on self-assembled metal-phenolic nanocomplexes comprised of green tea catechin. Adv. Sci. 2019;6(5):1801688. doi: 10.1002/advs.201801688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhao X., Pei D., Yang Y., Xu K., Yu J., Zhang Y., Zhang Q., He G., Zhang Y., Li A., Cheng Y., Chen X. Green tea derivative driven smart hydrogels with desired functions for chronic diabetic wound treatment. Adv. Funct. Mater. 2021;31(18):2009442. [Google Scholar]
- 55.Kim S.-H., Kim K., Kim B.S., An Y.-H., Lee U.-J., Lee S.-H., Kim S.L., Kim B.-G., Hwang N.S. Fabrication of polyphenol-incorporated anti-inflammatory hydrogel via high-affinity enzymatic crosslinking for wet tissue adhesion. Biomaterials. 2020;242:119905. doi: 10.1016/j.biomaterials.2020.119905. [DOI] [PubMed] [Google Scholar]
- 56.Hu B., Shen Y., Adamcik J., Fischer P., Schneider M., Loessner M.J., Mezzenga R. Polyphenol-binding amyloid fibrils self-assemble into reversible hydrogels with antibacterial activity. ACS Nano. 2018;12(4):3385–3396. doi: 10.1021/acsnano.7b08969. [DOI] [PubMed] [Google Scholar]
- 57.Zhang C., Wu B., Zhou Y., Zhou F., Liu W., Wang Z. Mussel-inspired hydrogels: from design principles to promising applications. Chem. Soc. Rev. 2020;49(11):3605–3637. doi: 10.1039/c9cs00849g. [DOI] [PubMed] [Google Scholar]
- 58.Sugiarto S., Leow Y., Tan C.L., Wang G., Kai D. How far is Lignin from being a biomedical material? Bioactive Mater. 2022;8:71–94. doi: 10.1016/j.bioactmat.2021.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Liu K., Du H., Zheng T., Liu W., Zhang M., Liu H., Zhang X., Si C. Lignin-containing cellulose nanomaterials: preparation and applications. Green Chem. 2021 [Google Scholar]
- 60.Gan D.L., Xing W.S., Jiang L.L., Fang J., Zhao C.C., Ren F.Z., Fang L.M., Wang K.F., Lu X. Plant-inspired adhesive and tough hydrogel based on Ag-Lignin nanoparticles-triggered dynamic redox catechol chemistry. Nat. Commun. 2019;10:1487. doi: 10.1038/s41467-019-09351-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Qian Y., Zhou Y., Lu M., Guo X., Yang D., Lou H., Qiu X., Guo C.F. Direct construction of catechol lignin for engineering long-acting conductive, adhesive, and UV-blocking hydrogel bioelectronics. Small Methods. 2021;5(5):2001311. doi: 10.1002/smtd.202001311. [DOI] [PubMed] [Google Scholar]
- 62.Sun D., Li N., Rao J., Jia S., Su Z., Hao X., Peng F. Ultrafast fabrication of organohydrogels with UV-blocking, anti-freezing, anti-drying, and skin epidermal sensing properties using lignin–Cu2+ plant catechol chemistry. J. Mater. Chem. 2021;9(25):14381–14391. [Google Scholar]
- 63.Wang J., Deng Y., Ma Z., Wang Y., Zhang S., Yan L. Lignin promoted the fast formation of a robust and highly conductive deep eutectic solvent ionic gel at room temperature for a flexible quasi-solid-state supercapacitor and strain sensors. Green Chem. 2021;23(14):5120–5128. [Google Scholar]
- 64.Huang Z., Delparastan P., Burch P., Cheng J., Cao Y., Messersmith P.B. Injectable dynamic covalent hydrogels of boronic acid polymers cross-linked by bioactive plant-derived polyphenols. Biomater. Sci. 2018;6(9):2487–2495. doi: 10.1039/c8bm00453f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Rizzo C., Marullo S., Dintcheva N.T., Gambarotti C., Billeci F., D'Anna F. Ionic liquid gels and antioxidant carbon nanotubes: hybrid soft materials with improved radical scavenging activity. J. Colloid Interface Sci. 2019;556:628–639. doi: 10.1016/j.jcis.2019.08.108. [DOI] [PubMed] [Google Scholar]
- 66.Pan X., Wang Q., He P., Liu K., Ni Y., Ouyang X., Chen L., Huang L., Wang H., Tan Y. Mussel-inspired nanocomposite hydrogel-based electrodes with reusable and injectable properties for human electrophysiological signals detection. ACS Sustain. Chem. Eng. 2019;7(8):7918–7925. [Google Scholar]
- 67.Cho J.H., Lee J.S., Shin J., Jeon E.J., An S., Choi Y.S., Cho S.-W. Ascidian-inspired fast-forming hydrogel system for versatile biomedical applications: pyrogallol chemistry for dual modes of crosslinking mechanism. Adv. Funct. Mater. 2018;28(6):1705244. [Google Scholar]
- 68.Lee H.A., Park E., Lee H. Polydopamine and its derivative surface chemistry in material science: a focused review for studies at KAIST. Adv. Mater. 2020;32(35):1907505. doi: 10.1002/adma.201907505. [DOI] [PubMed] [Google Scholar]
- 69.Hong S., Yang K., Kang B., Lee C., Song I.T., Byun E., Park K.I., Cho S.-W., Lee H. Hyaluronic acid catechol: a biopolymer exhibiting a pH-dependent adhesive or cohesive property for human neural stem cell engineering. Adv. Funct. Mater. 2013;23(14):1774–1780. [Google Scholar]
- 70.Yang J., Stuart M.A.C., Kamperman M. Jack of all trades: versatile catechol crosslinking mechanisms. Chem. Soc. Rev. 2014;43(24):8271–8298. doi: 10.1039/c4cs00185k. [DOI] [PubMed] [Google Scholar]
- 71.Han J., Cui Y., Han X., Liang C., Liu W., Luo D., Yang D. Super-soft DNA/Dopamine-Grafted-Dextran hydrogel as dynamic wire for electric circuits switched by a microbial metabolism process. Adv. Sci. 2020;7(13):2000684. doi: 10.1002/advs.202000684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Liang S., Zhang Y., Wang H., Xu Z., Chen J., Bao R., Tan B., Cui Y., Fan G., Wang W., Wang W., Liu W. Paintable and rapidly bondable conductive hydrogels as therapeutic cardiac patches. Adv. Mater. 2018;30(23):1704235. doi: 10.1002/adma.201704235. [DOI] [PubMed] [Google Scholar]
- 73.Tian Y., Cao Y., Wang Y., Yang W., Feng J. Realizing ultrahigh modulus and high strength of macroscopic graphene oxide papers through crosslinking of mussel-inspired polymers. Adv. Mater. 2013;25(21):2980–2983. doi: 10.1002/adma.201300118. [DOI] [PubMed] [Google Scholar]
- 74.Lee H., Dellatore S.M., Miller W.M., Messersmith P.B. Mussel-inspired surface chemistry for multifunctional coatings. Science. 2007;318(5849):426–430. doi: 10.1126/science.1147241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Cheng W., Nie J., Xu L., Liang C., Peng Y., Liu G., Wang T., Mei L., Huang L., Zeng X. pH-sensitive delivery vehicle based on folic acid-conjugated polydopamine-modified mesoporous silica nanoparticles for targeted cancer therapy. ACS Appl. Mater. Interfaces. 2017;9(22):18462–18473. doi: 10.1021/acsami.7b02457. [DOI] [PubMed] [Google Scholar]
- 76.Cheng W., Nie J., Gao N., Liu G., Tao W., Xiao X., Jiang L., Liu Z., Zeng X., Mei L. A multifunctional nanoplatform against multidrug resistant cancer: merging the best of targeted chemo/gene/photothermal therapy. Adv. Funct. Mater. 2017;27(45):1704135. [Google Scholar]
- 77.Li Z., Liu Q., Zhang Y., Yang Y., Zhou X., Peng W., Liang Z., Zeng X., Wang Q., Gao N. Charge-reversal nanomedicine based on black phosphorus for the development of A Novel photothermal therapy of oral cancer. Drug Deliv. 2021;28(1):700–708. doi: 10.1080/10717544.2021.1909176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Li Z., Shan X., Chen Z., Gao N., Zeng W., Zeng X., Mei L. Applications of surface modification technologies in nanomedicine for deep tumor penetration. Adv. Sci. 2021;8(1):2002589. doi: 10.1002/advs.202002589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Han L., Yan L., Wang K., Fang L., Zhang H., Tang Y., Ding Y., Weng L.-T., Xu J., Weng J., Liu Y., Ren F., Lu X. Tough, self-healable and tissue-adhesive hydrogel with tunable multifunctionality. NPG Asia Mater. 2017;9(4):e372. [Google Scholar]
- 80.Wang X., Wang C., Wang X., Wang Y., Zhang Q., Cheng Y. A polydopamine nanoparticle-knotted poly(ethylene glycol) hydrogel for on-demand drug delivery and chemo-photothermal therapy. Chem. Mater. 2017;29(3):1370–1376. [Google Scholar]
- 81.Ryu J.H., Lee Y., Kong W.H., Kim T.G., Park T.G., Lee H. Catechol-functionalized chitosan/pluronic hydrogels for tissue adhesives and hemostatic materials. Biomacromolecules. 2011;12(7):2653–2659. doi: 10.1021/bm200464x. [DOI] [PubMed] [Google Scholar]
- 82.Lee Y., Jun K., Lee K., Seo Y.C., Jeong C., Kim M., Oh I.-K., Lee H. Phenol-derived carbon sealant inspired by a coalification process. Angew. Chem. Int. Ed. 2020;59(10):3864–3870. doi: 10.1002/anie.201913181. [DOI] [PubMed] [Google Scholar]
- 83.Xie T., Ding J., Han X., Jia H., Yang Y., Liang S., Wang W., Liu W., Wang W. Wound dressing change facilitated by spraying zinc ions. Mater. Horiz. 2020;7(2):605–614. [Google Scholar]
- 84.Zhou Y., Yue Z., Chen Z., Wallace G. 3D coaxial printing tough and elastic hydrogels for tissue engineering using a catechol functionalized ink system. Adv. Healthcare Mater. 2020;9(24):2001342. doi: 10.1002/adhm.202001342. [DOI] [PubMed] [Google Scholar]
- 85.Yang Y., Gao P., Wang J., Tu Q., Bai L., Xiong K., Qiu H., Zhao X., Maitz M.F., Wang H., Li X., Zhao Q., Xiao Y., Huang N., Yang Z. Endothelium-Mimicking multifunctional coating modified cardiovascular stents via a stepwise metal-catechol-(amine) surface engineering strategy. Research. 2020;2020:9203906. doi: 10.34133/2020/9203906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Xu L.Q., Neoh K.-G., Kang E.-T. Natural polyphenols as versatile platforms for material engineering and surface functionalization. Prog. Polym. Sci. 2018;87:165–196. [Google Scholar]
- 87.Ejima H., Richardson J.J., Caruso F. Metal-phenolic networks as a versatile platform to engineer nanomaterials and biointerfaces. Nano Today. 2017;12:136–148. [Google Scholar]
- 88.Xiang Y., Mao C., Liu X., Cui Z., Jing D., Yang X., Liang Y., Li Z., Zhu S., Zheng Y., Yeung K.W.K., Zheng D., Wang X., Wu S. Rapid and superior bacteria killing of carbon quantum dots/ZnO decorated injectable folic acid-conjugated PDA hydrogel through dual-light triggered ROS and membrane permeability. Small. 2019;15(22):1900322. doi: 10.1002/smll.201900322. [DOI] [PubMed] [Google Scholar]
- 89.Cheng X., Li M., Wang H., Cheng Y. All-small-molecule dynamic covalent gels with antibacterial activity by boronate-tannic acid gelation. Chin. Chem. Lett. 2020;31(3):869–874. [Google Scholar]
- 90.Wang C., Sang H., Wang Y., Zhu F., Hu X., Wang X., Wang X., Li Y., Cheng Y. Foe to friend: supramolecular nanomedicines consisting of natural polyphenols and bortezomib. Nano Lett. 2018;18(11):7045–7051. doi: 10.1021/acs.nanolett.8b03015. [DOI] [PubMed] [Google Scholar]
- 91.Hu X., Chai Z., Lu L., Ruan H., Wang R., Zhan C., Xie C., Pan J., Liu M., Wang H., Lu W. Bortezomib dendrimer prodrug-based nanoparticle system. Adv. Funct. Mater. 2019;29(14):1807941. [Google Scholar]
- 92.Nie J., Cheng W., Peng Y., Liu G., Chen Y., Wang X., Liang C., Tao W., Wei Y., Zeng X., Mei L. Co-delivery of docetaxel and bortezomib based on a targeting nanoplatform for enhancing cancer chemotherapy effects. Drug Deliv. 2017;24(1):1124–1138. doi: 10.1080/10717544.2017.1362677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Shao C., Meng L., Wang M., Cui C., Wang B., Han C.-R., Xu F., Yang J. Mimicking dynamic adhesiveness and strain-stiffening behavior of biological tissues in tough and self-healable cellulose nanocomposite hydrogels. ACS Appl. Mater. Interfaces. 2019;11(6):5885–5895. doi: 10.1021/acsami.8b21588. [DOI] [PubMed] [Google Scholar]
- 94.Venkatesh V., Mishra N.K., Romero-Canelón I., Vernooij R.R., Shi H., Coverdale J.P.C., Habtemariam A., Verma S., Sadler P.J. Supramolecular photoactivatable Anticancer hydrogels. J. Am. Chem. Soc. 2017;139(16):5656–5659. doi: 10.1021/jacs.7b00186. [DOI] [PubMed] [Google Scholar]
- 95.Narkar A.R., Barker B., Clisch M., Jiang J., Lee B.P. pH responsive and oxidation resistant wet adhesive based on reversible catechol–boronate complexation. Chem. Mater. 2016;28(15):5432–5439. doi: 10.1021/acs.chemmater.6b01851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Narkar A.R., Lee B.P. Incorporation of anionic monomer to tune the reversible catechol–boronate complex for pH-responsive, reversible adhesion. Langmuir. 2018;34(32):9410–9417. doi: 10.1021/acs.langmuir.8b00373. [DOI] [PubMed] [Google Scholar]
- 97.Wang W., Zhang Y., Liu W. Bioinspired fabrication of high strength hydrogels from non-covalent interactions. Prog. Polym. Sci. 2017;71:1–25. [Google Scholar]
- 98.Zhang W., Wang R., Sun Z., Zhu X., Zhao Q., Zhang T., Cholewinski A., Yang F., Zhao B., Pinnaratip R., Forooshani P.K., Lee B.P. Catechol-functionalized hydrogels: biomimetic design, adhesion mechanism, and biomedical applications. Chem. Soc. Rev. 2020;49(2):433–464. doi: 10.1039/c9cs00285e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Hofman A.H., van Hees I.A., Yang J., Kamperman M. Bioinspired underwater adhesives by using the supramolecular toolbox. Adv. Mater. 2018;30(19):1704640. doi: 10.1002/adma.201704640. [DOI] [PubMed] [Google Scholar]
- 100.Grimme S. Do special noncovalent π–π stacking interactions really exist? Angew. Chem. Int. Ed. 2008;47(18):3430–3434. doi: 10.1002/anie.200705157. [DOI] [PubMed] [Google Scholar]
- 101.Chang G., Yang L., Yang J., Stoykovich M.P., Deng X., Cui J., Wang D. High-performance pH-switchable supramolecular thermosets via cation–π interactions. Adv. Mater. 2018;30(7):1704234. doi: 10.1002/adma.201704234. [DOI] [PubMed] [Google Scholar]
- 102.Maier G.P., Rapp M.V., Waite J.H., Israelachvili J.N., Butler A. Adaptive synergy between catechol and lysine promotes wet adhesion by surface salt displacement. Science. 2015;349(6248):628–632. doi: 10.1126/science.aab0556. [DOI] [PubMed] [Google Scholar]
- 103.Rapp M.V., Maier G.P., Dobbs H.A., Higdon N.J., Waite J.H., Butler A., Israelachvili J.N. Defining the catechol–cation synergy for enhanced wet adhesion to mineral surfaces. J. Am. Chem. Soc. 2016;138(29):9013–9016. doi: 10.1021/jacs.6b03453. [DOI] [PubMed] [Google Scholar]
- 104.Lim C., Huang J., Kim S., Lee H., Zeng H., Hwang D.S. Nanomechanics of poly(catecholamine) coatings in aqueous solutions. Angew. Chem. Int. Ed. 2016;55(10):3342–3346. doi: 10.1002/anie.201510319. [DOI] [PubMed] [Google Scholar]
- 105.Chen X., Gao Y., Wang Y., Pan G. Mussel-inspired peptide mimicking: an emerging strategy for surface bioengineering of medical implants. Smart Mater. Med. 2021;2:26–37. [Google Scholar]
- 106.Xiao Y., Wang W.X., Tian X.H., Tan X., Yang T., Gao P., Xiong K.Q., Tu Q.F., Wang M., Maitz M.F., Huang N., Pan G.Q., Yang Z.L. A versatile surface bioengineering strategy based on mussel-inspired and bioclickable peptide mimic. Research. 2020;2020:7236946. doi: 10.34133/2020/7236946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Danner E.W., Kan Y., Hammer M.U., Israelachvili J.N., Waite J.H. Adhesion of mussel foot protein mefp-5 to mica: an underwater superglue. Biochemistry. 2012;51(33):6511–6518. doi: 10.1021/bi3002538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Harrington M.J., Masic A., Holten-Andersen N., Waite J.H., Fratzl P. Iron-clad fibers: a metal-based biological strategy for hard flexible coatings. Science. 2010;328(5975):216–220. doi: 10.1126/science.1181044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Yang Z., Zhao X., Hao R., Tu Q., Tian X., Xiao Y., Xiong K., Wang M., Feng Y., Huang N., Pan G. Bioclickable and mussel adhesive peptide mimics for engineering vascular stent surfaces. 2020;117(28):16127–16137. doi: 10.1073/pnas.2003732117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Waite J.H. Mussel adhesion – essential footwork. J. Exp. Biol. 2017;220(4):517–530. doi: 10.1242/jeb.134056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Cholewinski A., Yang F., Zhao B. Algae–mussel-inspired hydrogel composite glue for underwater bonding. Mater. Horiz. 2019;6(2):285–293. [Google Scholar]
- 112.Liu L., Tian X., Ma Y., Duan Y., Zhao X., Pan G. A versatile dynamic mussel-inspired biointerface: from specific cell behavior modulation to selective cell isolation. Angew. Chem. Int. Ed. 2018;57(26):7878–7882. doi: 10.1002/anie.201804802. [DOI] [PubMed] [Google Scholar]
- 113.Jung H., Kim M.K., Lee J.Y., Choi S.W., Kim J. Adhesive hydrogel patch with enhanced strength and adhesiveness to skin for transdermal drug delivery. Adv. Funct. Mater. 2020;30(42):2004407. [Google Scholar]
- 114.Yu J., Wei W., Danner E., Ashley R.K., Israelachvili J.N., Waite J.H. Mussel protein adhesion depends on interprotein thiol-mediated redox modulation. Nat. Chem. Biol. 2011;7(9):588–590. doi: 10.1038/nchembio.630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Han L., Lu X., Liu K., Wang K., Fang L., Weng L.-T., Zhang H., Tang Y., Ren F., Zhao C., Sun G., Liang R., Li Z. Mussel-inspired adhesive and tough hydrogel based on nanoclay confined dopamine polymerization. ACS Nano. 2017;11(3):2561–2574. doi: 10.1021/acsnano.6b05318. [DOI] [PubMed] [Google Scholar]
- 116.Wilker J.J. Redox and adhesion on the rocks. Nat. Chem. Biol. 2011;7(9):579–580. doi: 10.1038/nchembio.639. [DOI] [PubMed] [Google Scholar]
- 117.Jia Z., Lv X., Hou Y., Wang K., Ren F., Xu D., Wang Q., Fan K., Xie C., Lu X. Mussel-inspired nanozyme catalyzed conductive and self-setting hydrogel for adhesive and antibacterial bioelectronics. Bioactive Mater. 2021;6(9):2676–2687. doi: 10.1016/j.bioactmat.2021.01.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Afewerki S., Wang X., Ruiz-Esparza G.U., Tai C.-W., Kong X., Zhou S., Welch K., Huang P., Bengtsson R., Xu C., Strømme M. Combined catalysis for engineering bioinspired, lignin-based, long-lasting, adhesive, self-mending, antimicrobial hydrogels. ACS Nano. 2020;14(12):17004–17017. doi: 10.1021/acsnano.0c06346. [DOI] [PubMed] [Google Scholar]
- 119.He X., Li Z., Li J., Mishra D., Ren Y., Gates I., Hu J., Lu Q. 2021. Ultrastretchable, Adhesive, and Antibacterial Hydrogel with Robust Spinnability for Manufacturing Strong Hydrogel Micro/Nanofibers; p. 2103521. Small. [DOI] [PubMed] [Google Scholar]
- 120.Sun W., Xue B., Fan Q., Tao R., Wang C., Wang X., Li Y., Qin M., Wang W., Chen B., Cao Y. Molecular engineering of metal coordination interactions for strong, tough, and fast-recovery hydrogels. Sci. Adv. 2020;6(16) doi: 10.1126/sciadv.aaz9531. eaaz9531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Gong J.P., Katsuyama Y., Kurokawa T., Osada Y. Double-network hydrogels with extremely high mechanical strength. Adv. Mater. 2003;15(14):1155–1158. [Google Scholar]
- 122.Gong J.P. Why are double network hydrogels so tough? Soft Matter. 2010;6(12):2583–2590. [Google Scholar]
- 123.Lin X., Zhang L., Duan B. Polyphenol-mediated chitin self-assembly for constructing a fully naturally resourced hydrogel with high strength and toughness. Mater. Horiz. 2021;8(9):2503–2512. doi: 10.1039/d1mh00878a. [DOI] [PubMed] [Google Scholar]
- 124.Du R., Wu J., Chen L., Huang H., Zhang X., Zhang J. Hierarchical hydrogen bonds directed multi-functional carbon nanotube-based supramolecular hydrogels. Small. 2014;10(7):1387–1393. doi: 10.1002/smll.201302649. [DOI] [PubMed] [Google Scholar]
- 125.Liu R., Liang S., Tang X.-Z., Yan D., Li X., Yu Z.-Z. Tough and highly stretchable graphene oxide/polyacrylamide nanocomposite hydrogels. J. Mater. Chem. 2012;22(28):14160–14167. [Google Scholar]
- 126.Diesendruck C.E., Sottos N.R., Moore J.S., White S.R. Biomimetic self-healing. Angew. Chem. Int. Ed. 2015;54(36):10428–10447. doi: 10.1002/anie.201500484. [DOI] [PubMed] [Google Scholar]
- 127.Wang W., Narain R., Zeng H. Rational design of self-healing tough hydrogels. Min. Rev. 2018;6:497. doi: 10.3389/fchem.2018.00497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Yuan Y., Shen S., Fan D. A physicochemical double cross-linked multifunctional hydrogel for dynamic burn wound healing: shape adaptability, injectable self-healing property and enhanced adhesion. Biomaterials. 2021;276:120838. doi: 10.1016/j.biomaterials.2021.120838. [DOI] [PubMed] [Google Scholar]
- 129.Wei Y., Xiang L., Ou H., Li F., Zhang Y., Qian Y., Hao L., Diao J., Zhang M., Zhu P., Liu Y., Kuang Y., Chen G. MXene-based conductive organohydrogels with long-term environmental stability and multifunctionality. Adv. Funct. Mater. 2020;30(48):2005135. [Google Scholar]
- 130.Zou Y., Chen X., Yang P., Liang G., Yang Y., Gu Z., Li Y. Vol. 6. 2020. Regulating the absorption spectrum of polydopamine. 36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Premi S., Wallisch S., Mano Camila M., Weiner Adam B., Bacchiocchi A., Wakamatsu K., Bechara Etelvino J.H., Halaban R., Douki T., Brash Douglas E. Chemiexcitation of melanin derivatives induces DNA photoproducts long after UV exposure. Science. 2015;347(6224):842–847. doi: 10.1126/science.1256022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Zhou L., Xi Y., Xue Y., Wang M., Liu Y., Guo Y., Lei B. Injectable self-healing antibacterial bioactive polypeptide-based hybrid nanosystems for efficiently treating multidrug resistant infection, skin-tumor therapy, and enhancing wound healing. Adv. Funct. Mater. 2019;29(22):1806883. [Google Scholar]
- 133.Luo Y., Wei X., Wan Y., Lin X., Wang Z., Huang P. 3D printing of hydrogel scaffolds for future application in photothermal therapy of breast cancer and tissue repair. Acta Biomater. 2019;92:37–47. doi: 10.1016/j.actbio.2019.05.039. [DOI] [PubMed] [Google Scholar]
- 134.Zhao G., Wu H., Feng R., Wang D., Xu P., Jiang P., Yang K., Wang H., Guo Z., Chen Q. Novel metal polyphenol framework for MR imaging-guided photothermal therapy. ACS Appl. Mater. Interfaces. 2018;10(4):3295–3304. doi: 10.1021/acsami.7b16222. [DOI] [PubMed] [Google Scholar]
- 135.Liu T., Zhang M., Liu W., Zeng X., Song X., Yang X., Zhang X., Feng J. Metal ion/tannic acid assembly as a versatile photothermal platform in engineering multimodal nanotheranostics for advanced applications. ACS Nano. 2018;12(4):3917–3927. doi: 10.1021/acsnano.8b01456. [DOI] [PubMed] [Google Scholar]
- 136.Chen X., Yi Z., Chen G., Ma X., Su W., Cui X., Li X. DOX-assisted functionalization of green tea polyphenol nanoparticles for effective chemo-photothermal cancer therapy. J. Mater. Chem. B. 2019;7(25):4066–4078. [Google Scholar]
- 137.Zhang J., Fu Y., Yang P., Liu X., Li Y., Gu Z. ROS scavenging biopolymers for anti-inflammatory diseases: classification and formulation. Adv. Mater. Interfac. 2020;7(16):2000632. [Google Scholar]
- 138.Hu J., Yang L., Yang P., Jiang S., Liu X., Li Y. Polydopamine free radical scavengers. Biomater. Sci. 2020;8(18):4940–4950. doi: 10.1039/d0bm01070g. [DOI] [PubMed] [Google Scholar]
- 139.Liu H., Yang Y., Liu Y., Pan J., Wang J., Man F., Zhang W., Liu G. Melanin-like nanomaterials for advanced biomedical applications: a versatile platform with extraordinary promise. Adv. Sci. 2020;7(7):1903129. doi: 10.1002/advs.201903129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Guo J., Sun W., Kim J.P., Lu X., Li Q., Lin M., Mrowczynski O., Rizk E.B., Cheng J., Qian G., Yang J. Development of tannin-inspired antimicrobial bioadhesives. Acta Biomater. 2018;72:35–44. doi: 10.1016/j.actbio.2018.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Zhao W.-H., Hu Z.-Q., Hara Y., Shimamura T. Inhibition of penicillinase by epigallocatechin gallate resulting in restoration of antibacterial activity of penicillin against penicillinase-producing. Staphylococcus aureus. 2002;46(7):2266–2268. doi: 10.1128/AAC.46.7.2266-2268.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Nikoo M., Regenstein J.M., Ahmadi Gavlighi H. Antioxidant and antimicrobial activities of (-)-Epigallocatechin-3-gallate (EGCG) and its potential to preserve the quality and safety of foods. Compr. Rev. Food Sci. Food Saf. 2018;17(3):732–753. doi: 10.1111/1541-4337.12346. [DOI] [PubMed] [Google Scholar]
- 143.Stern D., Cui H. Crafting polymeric and peptidic hydrogels for improved wound healing. Adv. Healthcare Mater. 2019;8(9):1900104. doi: 10.1002/adhm.201900104. [DOI] [PubMed] [Google Scholar]
- 144.Dimatteo R., Darling N.J., Segura T. In situ forming injectable hydrogels for drug delivery and wound repair. Adv. Drug Deliv. Rev. 2018;127:167–184. doi: 10.1016/j.addr.2018.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Singh G., Nayal A., Malhotra S., Koul V. Dual functionalized chitosan based composite hydrogel for haemostatic efficacy and adhesive property. Carbohydr. Polym. 2020;247:116757. doi: 10.1016/j.carbpol.2020.116757. [DOI] [PubMed] [Google Scholar]
- 146.Shin J., Lee J.S., Lee C., Park H.-J., Yang K., Jin Y., Ryu J.H., Hong K.S., Moon S.-H., Chung H.-M., Yang H.S., Um S.H., Oh J.-W., Kim D.-I., Lee H., Cho S.-W. Tissue adhesive catechol-modified hyaluronic acid hydrogel for effective, minimally invasive cell therapy. Adv. Funct. Mater. 2015;25(25):3814–3824. [Google Scholar]
- 147.Zhao X., Guo B., Wu H., Liang Y., Ma P.X. Injectable antibacterial conductive nanocomposite cryogels with rapid shape recovery for noncompressible hemorrhage and wound healing. Nat. Commun. 2018;9(1):2784. doi: 10.1038/s41467-018-04998-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Dong Y., A S., Rodrigues M., Li X., Kwon S.H., Kosaric N., Khong S., Gao Y., Wang W., Gurtner G.C. Injectable and tunable gelatin hydrogels enhance stem cell retention and improve cutaneous wound healing. Adv. Funct. Mater. 2017;27(24):1606619. [Google Scholar]
- 149.Afewerki S., Sheikhi A., Kannan S., Ahadian S., Khademhosseini A. Gelatin-polysaccharide composite scaffolds for 3D cell culture and tissue engineering: towards natural therapeutics. Bioeng. Transl. Med. 2019;4(1):96–115. doi: 10.1002/btm2.10124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Yu L., Ding J. Injectable hydrogels as unique biomedical materials. Chem. Soc. Rev. 2008;37(8):1473–1481. doi: 10.1039/b713009k. [DOI] [PubMed] [Google Scholar]
- 151.Tavassoli-Kafrani E., Goli S.A.H., Fathi M. Fabrication and characterization of electrospun gelatin nanofibers crosslinked with oxidized phenolic compounds. Int. J. Biol. Macromol. 2017;103:1062–1068. doi: 10.1016/j.ijbiomac.2017.05.152. [DOI] [PubMed] [Google Scholar]
- 152.Cui C., Fan C., Wu Y., Xiao M., Wu T., Zhang D., Chen X., Liu B., Xu Z., Qu B., Liu W. Water-triggered hyperbranched polymer universal adhesives: from strong underwater adhesion to rapid sealing hemostasis. Adv. Mater. 2019;31(49):1905761. doi: 10.1002/adma.201905761. [DOI] [PubMed] [Google Scholar]
- 153.Bai S., Zhang X., Cai P., Huang X., Huang Y., Liu R., Zhang M., Song J., Chen X., Yang H. A silk-based sealant with tough adhesion for instant hemostasis of bleeding tissues. Nanoscale Horiz. 2019;4(6):1333–1341. [Google Scholar]
- 154.Liang Y., Zhao X., Hu T., Chen B., Yin Z., Ma P.X., Guo B. Adhesive hemostatic conducting injectable composite hydrogels with sustained drug release and photothermal antibacterial activity to promote full-thickness skin regeneration during wound healing. Small. 2019;15(12):1900046. doi: 10.1002/smll.201900046. [DOI] [PubMed] [Google Scholar]
- 155.Wang R., Li J., Chen W., Xu T., Yun S., Xu Z., Xu Z., Sato T., Chi B., Xu H. A biomimetic mussel-inspired ε-Poly-l-lysine hydrogel with robust tissue-anchor and anti-infection capacity. Adv. Funct. Mater. 2017;27(8):1604894. [Google Scholar]
- 156.Shin M., Ryu J.H., Kim K., Kim M.J., Jo S., Lee M.S., Lee D.Y., Lee H. Hemostatic swabs containing polydopamine-like catecholamine chitosan-catechol for normal and coagulopathic animal models. ACS Biomater. Sci. Eng. 2018;4(7):2314–2318. doi: 10.1021/acsbiomaterials.8b00451. [DOI] [PubMed] [Google Scholar]
- 157.Han L., Li P., Tang P., Wang X., Zhou T., Wang K., Ren F., Guo T., Lu X. Mussel-inspired cryogels for promoting wound regeneration through photobiostimulation, modulating inflammatory responses and suppressing bacterial invasion. Nanoscale. 2019;11(34):15846–15861. doi: 10.1039/c9nr03095f. [DOI] [PubMed] [Google Scholar]
- 158.Hu B., Owh C., Chee P.L., Leow W.R., Liu X., Wu Y.-L., Guo P., Loh X.J., Chen X. Supramolecular hydrogels for antimicrobial therapy. Chem. Soc. Rev. 2018;47(18):6917–6929. doi: 10.1039/c8cs00128f. [DOI] [PubMed] [Google Scholar]
- 159.Li S., Dong S., Xu W., Tu S., Yan L., Zhao C., Ding J., Chen X. Antibacterial hydrogels. Adv. Sci. 2018;5(5):1700527. doi: 10.1002/advs.201700527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Ma M., Zhong Y., Jiang X. Thermosensitive and pH-responsive tannin-containing hydroxypropyl chitin hydrogel with long-lasting antibacterial activity for wound healing. Carbohydr. Polym. 2020;236:116096. doi: 10.1016/j.carbpol.2020.116096. [DOI] [PubMed] [Google Scholar]
- 161.Ninan N., Forget A., Shastri V.P., Voelcker N.H., Blencowe A. Antibacterial and anti-inflammatory pH-responsive tannic acid-carboxylated agarose composite hydrogels for wound healing. ACS Appl. Mater. Interfaces. 2016;8(42):28511–28521. doi: 10.1021/acsami.6b10491. [DOI] [PubMed] [Google Scholar]
- 162.Deng H., Yu Z., Chen S., Fei L., Sha Q., Zhou N., Chen Z., Xu C. Facile and eco-friendly fabrication of polysaccharides-based nanocomposite hydrogel for photothermal treatment of wound infection. Carbohydr. Polym. 2020;230:115565. doi: 10.1016/j.carbpol.2019.115565. [DOI] [PubMed] [Google Scholar]
- 163.Li Y., Liu X., Li B., Zheng Y., Han Y., Chen D.-f., Yeung K.W.K., Cui Z., Liang Y., Li Z., Zhu S., Wang X., Wu S. Near-infrared light triggered phototherapy and immunotherapy for elimination of methicillin-resistant Staphylococcus aureus biofilm infection on bone implant. ACS Nano. 2020;14(7):8157–8170. doi: 10.1021/acsnano.0c01486. [DOI] [PubMed] [Google Scholar]
- 164.Gan D., Xu T., Xing W., Ge X., Fang L., Wang K., Ren F., Lu X. Mussel-inspired contact-active antibacterial hydrogel with high cell affinity, toughness, and recoverability. Adv. Funct. Mater. 2019;29(1):1805964. [Google Scholar]
- 165.Yang Z., Huang R., Zheng B., Guo W., Li C., He W., Wei Y., Du Y., Wang H., Wu D., Wang H. Highly stretchable, adhesive, biocompatible, and antibacterial hydrogel dressings for wound healing. Adv. Sci. 2021;8(8):2003627. doi: 10.1002/advs.202003627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Hu C., Long L., Cao J., Zhang S., Wang Y. Dual-crosslinked mussel-inspired smart hydrogels with enhanced antibacterial and angiogenic properties for chronic infected diabetic wound treatment via pH-responsive quick cargo release. Chem. Eng. J. 2021;411:128564. [Google Scholar]
- 167.Sahiner N., Sagbas S., Sahiner M., Silan C., Aktas N., Turk M. Biocompatible and biodegradable poly(Tannic Acid) hydrogel with antimicrobial and antioxidant properties. Int. J. Biol. Macromol. 2016;82:150–159. doi: 10.1016/j.ijbiomac.2015.10.057. [DOI] [PubMed] [Google Scholar]
- 168.Li M., Liang Y., He J., Zhang H., Guo B. Two-pronged strategy of biomechanically active and biochemically multifunctional hydrogel wound dressing to accelerate wound closure and wound healing. Chem. Mater. 2020;32(23):9937–9953. [Google Scholar]
- 169.Chen Y., Qiu Y., Wang Q., Li D., Hussain T., Ke H., Wei Q. Mussel-inspired sandwich-like nanofibers/hydrogel composite with super adhesive, sustained drug release and anti-infection capacity. Chem. Eng. J. 2020;399:125668. [Google Scholar]
- 170.Zhang S., Hou J., Yuan Q., Xin P., Cheng H., Gu Z., Wu J. Arginine derivatives assist dopamine-hyaluronic acid hybrid hydrogels to have enhanced antioxidant activity for wound healing. Chem. Eng. J. 2020;392:123775. [Google Scholar]
- 171.Chen G., Yu Y., Wu X., Wang G., Ren J., Zhao Y. Bioinspired multifunctional hybrid hydrogel promotes wound healing. Adv. Funct. Mater. 2018;28(33):1801386. [Google Scholar]
- 172.Gopinath D., Ahmed M.R., Gomathi K., Chitra K., Sehgal P.K., Jayakumar R. Dermal wound healing processes with curcumin incorporated collagen films. Biomaterials. 2004;25(10):1911–1917. doi: 10.1016/s0142-9612(03)00625-2. [DOI] [PubMed] [Google Scholar]
- 173.Li M., Chen J., Shi M., Zhang H., Ma P.X., Guo B. Electroactive anti-oxidant polyurethane elastomers with shape memory property as non-adherent wound dressing to enhance wound healing. Chem. Eng. J. 2019;375:121999. [Google Scholar]
- 174.Kant V., Gopal A., Pathak N.N., Kumar P., Tandan S.K., Kumar D. Antioxidant and anti-inflammatory potential of curcumin accelerated the cutaneous wound healing in streptozotocin-induced diabetic rats. Int. Immunopharm. 2014;20(2):322–330. doi: 10.1016/j.intimp.2014.03.009. [DOI] [PubMed] [Google Scholar]
- 175.Zhang H., Sun X., Wang J., Zhang Y., Dong M., Bu T., Li L., Liu Y., Wang L. Multifunctional injectable hydrogel dressings for effectively accelerating wound healing: enhancing biomineralization strategy. Adv. Funct. Mater. 2021;31(23):2100093. [Google Scholar]
- 176.Lei H., Fan D. Conductive, adaptive, multifunctional hydrogel combined with electrical stimulation for deep wound repair. Chem. Eng. J. 2021;421:129578. [Google Scholar]
- 177.Hussain M., Suo H., Xie Y., Wang K., Wang H., Hou Z., Gao Y., Zhang L., Tao J., Jiang H., Zhu J. Dopamine-substituted multidomain peptide hydrogel with inherent antimicrobial activity and antioxidant capability for infected wound healing. ACS Appl. Mater. Interfaces. 2021;13(25):29380–29391. doi: 10.1021/acsami.1c07656. [DOI] [PubMed] [Google Scholar]
- 178.Huang S., Liu H., Liao K., Hu Q., Guo R., Deng K. Functionalized GO nanovehicles with nitric oxide release and photothermal activity-based hydrogels for bacteria-infected wound healing. ACS Appl. Mater. Interfaces. 2020;12(26):28952–28964. doi: 10.1021/acsami.0c04080. [DOI] [PubMed] [Google Scholar]
- 179.Yang B., Song J., Jiang Y., Li M., Wei J., Qin J., Peng W., Lasaosa F.L., He Y., Mao H., Yang J., Gu Z. Injectable Adhesive self-healing multicross-linked double-network hydrogel facilitates full-thickness skin wound healing. ACS Appl. Mater. Interfaces. 2020;12(52):57782–57797. doi: 10.1021/acsami.0c18948. [DOI] [PubMed] [Google Scholar]
- 180.Zhang D., Cai G., Mukherjee S., Sun Y., Wang C., Mai B., Liu K., Yang C., Chen Y. Elastic, persistently moisture-retentive, and wearable biomimetic film inspired by fetal starless repair for promoting skin wound healing. ACS Appl. Mater. Interfaces. 2020;12(5):5542–5556. doi: 10.1021/acsami.9b20185. [DOI] [PubMed] [Google Scholar]
- 181.Ou Q., Zhang S., Fu C., Yu L., Xin P., Gu Z., Cao Z., Wu J., Wang Y. More natural more better: triple natural anti-oxidant puerarin/ferulic acid/polydopamine incorporated hydrogel for wound healing. J. Nanobiotechnol. 2021;19(1):237. doi: 10.1186/s12951-021-00973-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Zhang Y., Yuan B., Zhang Y., Cao Q., Yang C., Li Y., Zhou J. Biomimetic lignin/poly(ionic liquids) composite hydrogel dressing with excellent mechanical strength, self-healing properties, and reusability. Chem. Eng. J. 2020;400:125984. [Google Scholar]
- 183.Kalirajan C., Palanisamy T. Bioengineered hybrid collagen scaffold tethered with silver-catechin nanocomposite modulates angiogenesis and TGF-β toward scarless healing in chronic deep second degree infected burns. Adv. Healthcare Mater. 2020;9(12):2000247. doi: 10.1002/adhm.202000247. [DOI] [PubMed] [Google Scholar]
- 184.Izci M., Maksoudian C., Manshian B.B., Soenen S.J. The use of alternative strategies for enhanced nanoparticle delivery to solid tumors. Chem. Rev. 2021;121(3):1746–1803. doi: 10.1021/acs.chemrev.0c00779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Lee S.Y., Yang M., Seo J.-H., Jeong D.I., Hwang C., Kim H.-J., Lee J., Lee K., Park J., Cho H.-J. Serially pH-modulated hydrogels based on boronate ester and polydopamine linkages for local cancer therapy. ACS Appl. Mater. Interfaces. 2021;13(2):2189–2203. doi: 10.1021/acsami.0c16199. [DOI] [PubMed] [Google Scholar]
- 186.Monteiro C.F., Santos S.C., Custódio C.A., Mano J.F. Human platelet lysates-based hydrogels: a novel personalized 3D platform for spheroid invasion assessment. Adv. Sci. 2020;7(7):1902398. doi: 10.1002/advs.201902398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.He G., Chen S., Xu Y., Miao Z., Ma Y., Qian H., Lu Y., Zha Z. Charge reversal induced colloidal hydrogel acts as a multi-stimuli responsive drug delivery platform for synergistic cancer therapy. Mater. Horiz. 2019;6(4):711–716. [Google Scholar]
- 188.Jiang Y., Pan X., Yao M., Han L., Zhang X., Jia Z., Weng J., Chen W., Fang L., Wang X., Zhang Y., Duan R., Ren F., Wang K., Chen X., Lu X. Bioinspired adhesive and tumor microenvironment responsive nanoMOFs assembled 3D-printed scaffold for anti-tumor therapy and bone regeneration. Nano Today. 2021;39:101182. [Google Scholar]
- 189.Lee A.L.Z., Voo Z.X., Chin W., Ono R.J., Yang C., Gao S., Hedrick J.L., Yang Y.Y. Injectable coacervate hydrogel for delivery of anticancer drug-loaded nanoparticles in vivo. ACS Appl. Mater. Interfaces. 2018;10(16):13274–13282. doi: 10.1021/acsami.7b14319. [DOI] [PubMed] [Google Scholar]
- 190.Xu C., Pu K. Second near-infrared photothermal materials for combinational nanotheranostics. Chem. Soc. Rev. 2021;50(2):1111–1137. doi: 10.1039/d0cs00664e. [DOI] [PubMed] [Google Scholar]
- 191.Li L., Han X., Wang M., Li C., Jia T., Zhao X. Recent advances in the development of near-infrared organic photothermal agents. Chem. Eng. J. 2021;417:128844. [Google Scholar]
- 192.Ding F., Gao X., Huang X., Ge H., Xie M., Qian J., Song J., Li Y., Zhu X., Zhang C. Polydopamine-coated nucleic acid nanogel for siRNA-mediated low-temperature photothermal therapy. Biomaterials. 2020;245:119976. doi: 10.1016/j.biomaterials.2020.119976. [DOI] [PubMed] [Google Scholar]
- 193.Li X., Lovell J.F., Yoon J., Chen X. Clinical development and potential of photothermal and photodynamic therapies for cancer. Nat. Rev. Clin. Oncol. 2020;17(11):657–674. doi: 10.1038/s41571-020-0410-2. [DOI] [PubMed] [Google Scholar]
- 194.Wang H., Jin Y., Tan Y., Zhu H., Huo W., Niu P., Li Z., Zhang J., Liang X.-j., Yang X. Photo-responsive hydrogel facilitates nutrition deprivation by an ambidextrous approach for preventing cancer recurrence and metastasis. Biomaterials. 2021;275:120992. doi: 10.1016/j.biomaterials.2021.120992. [DOI] [PubMed] [Google Scholar]
- 195.Waldman A.D., Fritz J.M., Lenardo M.J. A guide to cancer immunotherapy: from T cell basic science to clinical practice. Nat. Rev. Immunol. 2020;20(11):651–668. doi: 10.1038/s41577-020-0306-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Fan M., Jia L., Pang M., Yang X., Yang Y., Kamel Elyzayati S., Liao Y., Wang H., Zhu Y., Wang Q. Injectable Adhesive hydrogel as photothermal-derived antigen reservoir for enhanced anti-tumor immunity. Adv. Funct. Mater. 2021;31(20):2010587. [Google Scholar]
- 197.Dai G., Sun L., Xu J., Zhao G., Tan Z., Wang C., Sun X., Xu K., Zhong W. Catechol–metal coordination-mediated nanocomposite hydrogels for on-demand drug delivery and efficacious combination therapy. Acta Biomater. 2021;129:84–95. doi: 10.1016/j.actbio.2021.05.006. [DOI] [PubMed] [Google Scholar]
- 198.Li Z., Cao H., Xu Y., Li X., Han X., Fan Y., Jiang Q., Sun Y., Zhang X. Bioinspired polysaccharide hybrid hydrogel promoted recruitment and chondrogenic differentiation of bone marrow mesenchymal stem cells. Carbohydr. Polym. 2021;267:118224. doi: 10.1016/j.carbpol.2021.118224. [DOI] [PubMed] [Google Scholar]
- 199.Jin Y., Koh R.H., Kim S.-H., Kim K.M., Park G.K., Hwang N.S. Injectable anti-inflammatory hyaluronic acid hydrogel for osteoarthritic cartilage repair. Mater. Sci. Eng. C. 2020;115:111096. doi: 10.1016/j.msec.2020.111096. [DOI] [PubMed] [Google Scholar]
- 200.Bai S., Zhang X., Lv X., Zhang M., Huang X., Shi Y., Lu C., Song J., Yang H. Bioinspired mineral–organic bone adhesives for stable fracture fixation and accelerated bone regeneration. Adv. Funct. Mater. 2020;30(5):1908381. [Google Scholar]
- 201.Mizuhashi K., Ono W., Matsushita Y., Sakagami N., Takahashi A., Saunders T.L., Nagasawa T., Kronenberg H.M., Ono N. Resting zone of the growth plate houses a unique class of skeletal stem cells. Nature. 2018;563(7730):254–258. doi: 10.1038/s41586-018-0662-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Koons G.L., Diba M., Mikos A.G. Materials design for bone-tissue engineering. Nat. Rev. Mater. 2020;5(8):584–603. [Google Scholar]
- 203.Yan H., Li L., Wang Z., Wang Y., Guo M., Shi X., Yeh J.-M., Zhang P. Mussel-inspired conducting copolymer with aniline tetramer as intelligent biological adhesive for bone tissue engineering. ACS Biomater. Sci. Eng. 2020;6(1):634–646. doi: 10.1021/acsbiomaterials.9b01601. [DOI] [PubMed] [Google Scholar]
- 204.Filippidi E., Cristiani Thomas R., Eisenbach Claus D., Waite J.H., Israelachvili Jacob N., Ahn B.K., Valentine Megan T. Toughening elastomers using mussel-inspired iron-catechol complexes. Science. 2017;358(6362):502–505. doi: 10.1126/science.aao0350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Chen X., Tan B., Bao Z., Wang S., Tang R., Wang Z., Chen G., Chen S., Lu W.W., Yang D., Peng S. Enhanced bone regeneration via spatiotemporal and controlled delivery of a genetically engineered BMP-2 in a composite Hydrogel. Biomaterials. 2021;277:121117. doi: 10.1016/j.biomaterials.2021.121117. [DOI] [PubMed] [Google Scholar]
- 206.Hasani-Sadrabadi Mohammad M., Sarrion P., Pouraghaei S., Chau Y., Ansari S., Li S., Aghaloo T., Moshaverinia A. An engineered cell-laden adhesive hydrogel promotes craniofacial bone tissue regeneration in rats. Sci. Transl. Med. 2020;12(534) doi: 10.1126/scitranslmed.aay6853. [DOI] [PubMed] [Google Scholar]
- 207.Hong H., Seo Y.B., Kim D.Y., Lee J.S., Lee Y.J., Lee H., Ajiteru O., Sultan M.T., Lee O.J., Kim S.H., Park C.H. Digital light processing 3D printed silk fibroin hydrogel for cartilage tissue engineering. Biomaterials. 2020;232:119679. doi: 10.1016/j.biomaterials.2019.119679. [DOI] [PubMed] [Google Scholar]
- 208.Lee H.-p., Gu L., Mooney D.J., Levenston M.E., Chaudhuri O. Mechanical confinement regulates cartilage matrix formation by chondrocytes. Nat. Mater. 2017;16(12):1243–1251. doi: 10.1038/nmat4993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Zhang Y., Yu J., Ren K., Zuo J., Ding J., Chen X. Thermosensitive hydrogels as scaffolds for cartilage tissue engineering. Biomacromolecules. 2019;20(4):1478–1492. doi: 10.1021/acs.biomac.9b00043. [DOI] [PubMed] [Google Scholar]
- 210.Feng Q., Li D., Li Q., Li S., Huang H., Li H., Dong H., Cao X. Dynamic nanocomposite microgel assembly with microporosity, injectability, tissue-adhesion, and sustained drug release promotes articular cartilage repair and regeneration. Adv. Healthcare Mater. 2021:2102395. doi: 10.1002/adhm.202102395. n/a(n/a) [DOI] [PubMed] [Google Scholar]
- 211.Righolt A.J., Jevdjevic M., Marcenes W., Listl S. Global-, regional-, and country-level economic impacts of dental diseases in 2015. J. Dent. Res. 2018;97(5):501–507. doi: 10.1177/0022034517750572. [DOI] [PubMed] [Google Scholar]
- 212.Vaquette C., Mitchell J., Fernandez-Medina T., Kumar S., Ivanovski S. Resorbable additively manufactured scaffold imparts dimensional stability to extraskeletally regenerated bone. Biomaterials. 2021;269:120671. doi: 10.1016/j.biomaterials.2021.120671. [DOI] [PubMed] [Google Scholar]
- 213.Woo H.N., Cho Y.J., Tarafder S., Lee C.H. The recent advances in scaffolds for integrated periodontal regeneration. Bioactive Mater. 2021;6(10):3328–3342. doi: 10.1016/j.bioactmat.2021.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Zhong W., Xiong Y., Wang X., Yu T., Zhou C. Synthesis and characterization of multifunctional organic-inorganic composite hydrogel formed with tissue-adhesive property and inhibiting infection. Mater. Sci. Eng. C. 2021;118:111532. doi: 10.1016/j.msec.2020.111532. [DOI] [PubMed] [Google Scholar]
- 215.Dissanayaka W.L., Zhang C. Scaffold-based and scaffold-free strategies in dental pulp regeneration. J. Endod. 2020;46(9, Supplement):S81–S89. doi: 10.1016/j.joen.2020.06.022. [DOI] [PubMed] [Google Scholar]
- 216.Fakhri E., Eslami H., Maroufi P., Pakdel F., Taghizadeh S., Ganbarov K., Yousefi M., Tanomand A., Yousefi B., Mahmoudi S., Kafil H.S. Chitosan biomaterials application in dentistry. Int. J. Biol. Macromol. 2020;162:956–974. doi: 10.1016/j.ijbiomac.2020.06.211. [DOI] [PubMed] [Google Scholar]
- 217.Huerta R.R., Silva E.K., Ekaette I., El-Bialy T., Saldaña M.D.A. High-intensity ultrasound-assisted formation of cellulose nanofiber scaffold with low and high lignin content and their cytocompatibility with gingival fibroblast cells. Ultrason. Sonochem. 2020;64:104759. doi: 10.1016/j.ultsonch.2019.104759. [DOI] [PubMed] [Google Scholar]
- 218.R. Raman, T. Hua, D. Gwynne, J. Collins, S. Tamang, J. Zhou, T. Esfandiary, V. Soares, S. Pajovic, A. Hayward, R. Langer, G. Traverso, Light-degradable hydrogels as dynamic triggers for gastrointestinal applications, Sci. Adv. 6(3) eaay0065. [DOI] [PMC free article] [PubMed]
- 219.Xu X., Xia X., Zhang K., Rai A., Li Z., Zhao P., Wei K., Zou L., Yang B., Wong W.-K., Chiu Philip W.-Y., Bian L. Bioadhesive hydrogels demonstrating pH-independent and ultrafast gelation promote gastric ulcer healing in pigs. Sci. Transl. Med. 2020;12(558) doi: 10.1126/scitranslmed.aba8014. [DOI] [PubMed] [Google Scholar]
- 220.Huang J., Jiang Y., Liu Y., Ren Y., Xu Z., Li Z., Zhao Y., Wu X., Ren J. Marine-inspired molecular mimicry generates a drug-free, but immunogenic hydrogel adhesive protecting surgical anastomosis. Bioactive Mater. 2021;6(3):770–782. doi: 10.1016/j.bioactmat.2020.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Jumelle C., Gholizadeh S., Annabi N., Dana R. Advances and limitations of drug delivery systems formulated as eye drops. J. Contr. Release. 2020;321:1–22. doi: 10.1016/j.jconrel.2020.01.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Lee W., Choi J.H., Lee J., Youn J., Kim W., Jeon G., Lee S.W., Song J.E., Khang G. Dopamine-functionalized gellan gum hydrogel as a candidate biomaterial for a retinal pigment epithelium cell delivery system. ACS Appl. Bio. Mater. 2021;4(2):1771–1782. doi: 10.1021/acsabm.0c01516. [DOI] [PubMed] [Google Scholar]
- 223.Koivusalo L., Kauppila M., Samanta S., Parihar V.S., Ilmarinen T., Miettinen S., Oommen O.P., Skottman H. Tissue adhesive hyaluronic acid hydrogels for sutureless stem cell delivery and regeneration of corneal epithelium and stroma. Biomaterials. 2019;225:119516. doi: 10.1016/j.biomaterials.2019.119516. [DOI] [PubMed] [Google Scholar]
- 224.Ling Y., An T., Yap L.W., Zhu B., Gong S., Cheng W. Disruptive, soft, wearable sensors. Adv. Mater. 2020;32(18):1904664. doi: 10.1002/adma.201904664. [DOI] [PubMed] [Google Scholar]
- 225.Mo J., Dai Y., Zhang C., Zhou Y., Li W., Song Y., Wu C., Wang Z. Design of ultra-stretchable, highly adhesive and self-healable hydrogels via tannic acid-enabled dynamic interactions. Mater. Horiz. 2021 doi: 10.1039/d1mh01324f. [DOI] [PubMed] [Google Scholar]
- 226.Pei X., Zhang H., Zhou Y., Zhou L., Fu J. Stretchable, self-healing and tissue-adhesive zwitterionic hydrogels as strain sensors for wireless monitoring of organ motions. Mater. Horiz. 2020;7(7):1872–1882. [Google Scholar]
- 227.Guo J.H., Yu Y.R., Zhang D.G., Zhang H., Zhao Y.J. Morphological hydrogel microfibers with MXene encapsulation for electronic skin. Research. 2021;2021:7065907. doi: 10.34133/2021/7065907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Son D., Kang J., Vardoulis O., Kim Y., Matsuhisa N., Oh J.Y., To J.W.F., Mun J., Katsumata T., Liu Y., McGuire A.F., Krason M., Molina-Lopez F., Ham J., Kraft U., Lee Y., Yun Y., Tok J.B.H., Bao Z. An integrated self-healable electronic skin system fabricated via dynamic reconstruction of a nanostructured conducting network. Nat. Nanotechnol. 2018;13(11):1057–1065. doi: 10.1038/s41565-018-0244-6. [DOI] [PubMed] [Google Scholar]
- 229.Wang S., Xu J., Wang W., Wang G.-J.N., Rastak R., Molina-Lopez F., Chung J.W., Niu S., Feig V.R., Lopez J., Lei T., Kwon S.-K., Kim Y., Foudeh A.M., Ehrlich A., Gasperini A., Yun Y., Murmann B., Tok J.B.H., Bao Z. Skin electronics from scalable fabrication of an intrinsically stretchable transistor array. Nature. 2018;555(7694):83–88. doi: 10.1038/nature25494. [DOI] [PubMed] [Google Scholar]
- 230.Yang C., Suo Z. Hydrogel ionotronics. Nat. Rev. Mater. 2018;3(6):125–142. [Google Scholar]
- 231.Zhang C., Zhou Y., Han H., Zheng H., Xu W., Wang Z. Dopamine-triggered hydrogels with high transparency, self-adhesion, and thermoresponse as skinlike sensors. ACS Nano. 2021;15(1):1785–1794. doi: 10.1021/acsnano.0c09577. [DOI] [PubMed] [Google Scholar]
- 232.Chen Y., Zhao H., Mao J., Chirarattananon P., Helbling E.F., Hyun N.-s.P., Clarke D.R., Wood R.J. Controlled flight of a microrobot powered by soft artificial muscles. Nature. 2019;575(7782):324–329. doi: 10.1038/s41586-019-1737-7. [DOI] [PubMed] [Google Scholar]
- 233.Podstawczyk D., Nizioł M., Szymczyk-Ziółkowska P., Fiedot-Toboła M. Development of thermoinks for 4D direct printing of temperature-induced self-rolling hydrogel actuators. Adv. Funct. Mater. 2021;31(15):2009664. [Google Scholar]
- 234.Zhao Y., Lo C.-Y., Ruan L., Pi C.-H., Kim C., Alsaid Y., Frenkel I., Rico R., Tsao T.-C., He X. Somatosensory actuator based on stretchable conductive photothermally responsive hydrogel. Science Robotics. 2021;6(53) doi: 10.1126/scirobotics.abd5483. [DOI] [PubMed] [Google Scholar]
- 235.Jian Y., Wu B., Le X., Liang Y., Zhang Y., Zhang D., Zhang L., Lu W., Zhang J., Chen T. Antifreezing and stretchable organohydrogels as soft actuators. Research. 2019:2384347. doi: 10.34133/2019/2384347. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Li B., Song Z., Zhu K., Niu Q., Li Z., Li H. Multistimuli-responsive lanthanide-containing smart luminescent hydrogel actuator. ACS Appl. Mater. Interfaces. 2021;13(17):20633–20640. doi: 10.1021/acsami.1c02589. [DOI] [PubMed] [Google Scholar]
- 237.Zhang X., Chen L., Zhang C., Liao L. Robust near-infrared-responsive composite hydrogel actuator using Fe3+/tannic acid as the photothermal transducer. ACS Appl. Mater. Interfaces. 2021;13(15):18175–18183. doi: 10.1021/acsami.1c03999. [DOI] [PubMed] [Google Scholar]
- 238.Aswathy S.H., Narendrakumar U., Manjubala I. Commercial hydrogels for biomedical applications. Heliyon. 2020;6(4) doi: 10.1016/j.heliyon.2020.e03719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Wang R., Wang X., Zhan Y., Xu Z., Xu Z., Feng X., Li S., Xu H. A dual network hydrogel sunscreen based on poly-γ-glutamic acid/tannic acid demonstrates excellent anti-UV, self-recovery, and skin-integration capacities. ACS Appl. Mater. Interfaces. 2019;11(41):37502–37512. doi: 10.1021/acsami.9b14538. [DOI] [PubMed] [Google Scholar]
- 240.Hong B.M., Hong G.L., Gwak M.A., Kim K.H., Jeong J.E., Jung J.Y., Park S.A., Park W.H. Self-crosslinkable hyaluronate-based hydrogels as a soft tissue filler. Int. J. Biol. Macromol. 2021;185:98–110. doi: 10.1016/j.ijbiomac.2021.06.047. [DOI] [PubMed] [Google Scholar]
- 241.Cascone S., Lamberti G. Hydrogel-based commercial products for biomedical applications: a review. Int. J. Pharm. 2020;573:118803. doi: 10.1016/j.ijpharm.2019.118803. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.