Abstract
Unmedicated individuals with major depressive disorder (MDD) show abnormal interoception, but it is unclear whether antidepressant treatment via serotonergic medication alters this relationship. The current cross-sectional study examined associations between neural and behavioral indices of interoceptive processing and chronic serotonergic medication administration in MDD. 47 selective serotonin reuptake inhibitor (SSRI)-medicated MDD (MDD-SSRI) individuals were propensity-matched with 48 unmedicated current MDD (MDD-UnMed) and 41 healthy comparison (HC) participants on demographics including age, sex, body mass index, education, as well as on dimensional scales of symptom severity including depression and anxiety. All participants completed an interoceptive attention task during functional magnetic resonance imaging, and a behavioral heartbeat tapping task under three conditions: Guessing, No Guessing, and Breath Hold. Relative to HC, both MDD groups: (1) exhibited lower mid-insula, amygdala, putamen, and caudate activation during interoceptive versus exteroceptive attention; and (2) showed poorer heartbeat tapping performance during the Breath Hold condition. However, the MDD-SSRI group reported higher intensity ratings of heartbeat and stomach sensations than MDD-UnMed and HC during the interoceptive attention task. These findings suggest that the attenuated patterns of neural activation observed in depressed individuals during interoceptive attention are not ameliorated by the chronic administration of serotonergic medications. However, amplified interoceptive sensation ratings suggest a potential impact of chronic serotonergic medication on conscious experiences of internal body states. Future investigations will need to determine the extent to which serotonergic medications acutely influence interoceptive processing, and whether such changes play a role in therapeutic responses during treatment initiation.
Keywords: fMRI, major depressive disorder, interoception, insula, selective serotonin reuptake inhibitor
1. Introduction
Mood disorders are the most prevalent and disabling mental health conditions worldwide (Rehm and Shield, 2019), and there are limited psychobiological explanatory (Kendler, 2008) or predictive (Dinga et al., 2018) models currently available. Interoception is a multi-level process describing how the nervous system senses, interprets, and integrates internal bodily signals (Berntson and Khalsa, 2021) and offers one potentially important mechanism for understanding the pathophysiology of depression (Khalsa et al., 2018). Studies have revealed that individuals with major depressive disorder (MDD) show impairments related to the sensation, interpretation, and integration of internal bodily signals (Barrett et al., 2016; Eggart et al., 2019; Harshaw, 2015; Khalsa et al., 2018; Khalsa and Lapidus, 2016; Paulus and Stein, 2010). Individuals with MDD may also have difficulty predicting the body’s future metabolic demands that may result in over- or under-estimating resources needed to maintain a steady equilibrium (Barrett et al., 2016; Stephan et al., 2016). The insular cortex is suggested to integrate interoceptive signals with emotionally salient information during a process that generates interoceptive prediction errors, defined as the difference between anticipated versus experienced bodily states (Paulus and Stein, 2006; Seth and Critchley, 2013; Seth and Friston, 2016). Researchers have proposed that aberrant body prediction errors affect motivated behavior in depressed individuals (Barrett and Simmons, 2015; Paulus and Stein, 2010) and that a disconnect between these two processes also contributes to somatic symptoms and alexithymia (Harshaw, 2015).
Empirical data on the relationship between depression and interoceptive processing has grown considerably over the past decade. First, functional magnetic resonance imaging (fMRI) studies demonstrate that unmedicated current MDD patients show reduced insula activation relative to healthy comparisons (HC) while: (a) attending to heart, stomach, and bladder sensations (Avery et al., 2014); (b) recalling neutral stimuli previously paired with an aversive breathing load manipulation (DeVille et al., 2018); (c) experiencing shifts in cold versus hot skin stimulation (Strigo et al., 2010); and (d) viewing appetitive food images (Simmons et al., 2016). Second, MDD has been linked to abnormal behavioral performance on heartbeat counting tasks (Eggart et al., 2019; Furman et al., 2013; Terhaar et al., 2012) (but see (Ainley et al., 2020; Corneille et al., 2020; Ring and Brener, 2018) for a debate regarding the validity of behavioral scores on this measure). Third, MDD individuals exhibit lower scores than HC on the Multidimensional Assessment of Interoceptive Awareness (MAIA) scale (Mehling et al., 2012), potentially reflecting a greater difficulty in attending to and regulating bodily signals (Flasinski et al., 2020). Fourth, lower insula signals during interoceptive attention are associated with greater somatic symptoms in MDD (Avery et al., 2014). Even though antidepressant medication is a commonly used approach to the treatment of depression, none of the aforementioned studies investigated the effect of medication treatment (i.e., whether participants were medicated or unmedicated at the time of assessment) on interoceptive processing in MDD.
Pharmacotherapy is recommended as a preferred initial treatment option for MDD, with selective serotonin reuptake inhibitors (SSRIs) being the most popular medication class based on a tolerable side effect profile (APA., 2010) and as the first step in pharmacotherapy (Rush et al., 2006). Although such medications can be effective, they may not alter potential interoceptive impairments in MDD. There is some evidence that current but not remitted MDD individuals show lower insula activity during heartbeat counting than HC (Wiebking et al., 2015), which suggests that medication status and symptom severity may both be important moderators of insular function during interoceptive attention. Taken together, although there is converging evidence of interoceptive attention and awareness dysfunction in depression, the influence of medication status and the moderation by symptom severity on interoceptive processing in MDD requires clarification.
Using a secondary analysis of cross-sectional data, this investigation evaluated the influence of antidepressant pharmacotherapy on neural activity during interoceptive attention and on behavioral measures of cardiac interoceptive awareness, by examining group differences between HC and unmedicated versus medicated current MDD patients who reported taking SSRIs. We also aimed to replicate previously reported patterns of interoceptive processing dysfunctions at the neural and behavioral levels between unmedicated current MDD patients and HC (Avery et al., 2014; Flasinski et al., 2020). Participants performed the visceral interoceptive awareness (VIA) task during fMRI scanning (Avery et al., 2014), a behavioral heartbeat tapping task outside of the scanner (Smith et al., 2021), and a self-report measure of interoception (the Multidimensional Assessment of Interoceptive Awareness scale; MAIA) (Mehling et al., 2012). The VIA task examines interoceptive attention by asking individuals to focus on and rate the intensity of interoceptive (heart, stomach) and exteroceptive (visual) sensations during fMRI scanning. The heartbeat tapping task evaluated interoceptive accuracy using our previously developed ‘Beat-to-Tap’ consistency measure (Smith et al., 2021). Based on previous studies, we hypothesized that relative to HC, both unmedicated and SSRI-medicated MDD patients would: (1) exhibit lower insular cortex activation during interoceptive vs. exteroceptive VIA conditions, reflecting reduced neural capacity for interoceptive attention; (2) exhibit lower Beat-to-Tap consistency scores on the heartbeat tapping task, reflecting reduced perceptual accuracy of their heartbeat sensations; and (3) report lower MAIA scores, reflecting diminished self-reported interoceptive awareness. Although neural differences within interoceptive cortices were not predicted between SSRI medicated and non-medicated individuals with MDD, additional exploratory analysis were planned for other interoceptive variables including interoceptive awareness.
2. Materials and Methods
2.1. Participants
Participants were selected from the first 500 individuals who completed numerous baseline assessments as part of the Tulsa 1000 study, a naturalistic longitudinal study of 1000 individuals including HC and treatment-seeking individuals with mood, anxiety, substance use and eating disorders (Victor et al., 2018). Individuals between the ages of 18 and 55 were recruited via flyers, newspaper, radio and other media advertisements from the Laureate Psychiatric Clinic and Hospital, other local mental health providers, and the general community in Tulsa and the surrounding regions of Oklahoma. The study was approved by the Western Institutional Review Board and carried out in accordance with The Code of Ethics of the World Medical Association. Participants provided written informed consent and received compensation. See Victor et al. (2018) for full protocol and inclusion/exclusion details.
All participants were evaluated in person with structured clinical interview for Diagnostic and Statistical Manual of Mental Disorders (DSM)–IV or DSM-5 diagnoses determined by the Mini International Neuropsychiatric Inventory (MINI) (Sheehan et al., 1998) with trained clinical interviewers. To address the study aims most effectively, only HC without any psychiatric diagnoses on the MINI and individuals meeting criteria for current MDD without comorbid illicit drug use or eating disorders were included in this analysis (comorbid anxiety and alcohol use disorders were permitted). HC or MDD participants were excluded if they had poor quality or missing VIA fMRI data; those with poor quality or missing data on heartbeat tapping were excluded from the heartbeat tapping analysis.
2.2. Surveys completed during the baseline visit
During the screening visit, participants completed the following surveys: (1) Patient Health Questionnaire (PHQ) (Kroenke et al., 2001) to index depression symptoms; (2) Overall Anxiety Severity and Impairment Scale (OASIS) (Norman et al., 2006) to assess severity and impairment associated with any anxiety disorder or multiple anxiety disorders; (3) Drug Use Questionnaire (DAST) (Skinner, 1982) for a quick index of drug abuse problems; and (4) Sick Control One Fat Food Questionnaire (SCOFF) (Morgan et al., 1999) to screen for eating disorders. During the clinical interview session, participants completed the following demographic information and clinical ratings: (1) age, sex, body mass index (BMI), education, and employment status; (2) International Physical Activity Questionnaire (IPAQ) developed by the World Health Organization (WHO) and the Center for Disease Control and Prevention (CDC) in United States to assess physical activity; (3) Patient-Reported Outcomes Measurement Information System (PROMIS) (Cella et al., 2010) to collect data on alcohol use, nicotine dependence, depression and anxiety; (4) State-Trait Anxiety Inventory (STAI) (Spielberger et al., 1983) to measure state and trait anxiety; (5) Anxiety Sensitivity Index (ASI) (Taylor et al., 2007) to assess fear of anxiety sensations; (6) MAIA (Mehling et al., 2012) to evaluate self-reported experiences of interoceptive sensations including subscales of Attention Regulation, Body Listening, Emotional Awareness, Not-Distracting, Noticing, Not-Worrying, Self-Regulation, and Trusting; (7) Toronto Alexithymia Scale (TAS) (Bagby et al., 1994) to measure alexithymia; (8) Ruminative Responses Scale (RRS) (Nolen-Hoeksema and Morrow, 1991) to assess rumination that is related to, but not confounded by depression; and (9) Customary Drinking And Drug Use Record (CDDR) (Brown et al., 1998) to quantify lifetime alcohol use.
2.3. Subgroup identification and propensity matching
Three subgroups were identified based on their diagnostic and psychiatric medication status: (1) current MDD (a) taking SSRIs for at least 6 weeks with no change in the dose (MDD-SSRI, n = 56), (b) taking selective norepinephrine reuptake inhibitors, or SNRI (n = 14), or (c) taking various other antidepressants (n = 30); (2) current MDD with no psychiatric medication use ≤ 6 weeks prior to study enrollment (MDD-UnMed, n = 49); and (3) HC with no psychiatric disorders (n = 46). To address the study aims, only unmedicated current MDD (MDD-UnMed), medicated current MDD taking SSRIs (MDD-SSRI) and HC participants were included in the present analysis (i.e., the latter two depression subgroups were excluded from analysis due to the small subgroup sample size). Participants were also excluded if they were taking medications that could affect heart and/or stomach or other bodily sensations such as beta-blockers (3 MDD-SSRI) and opioids (6 MDD-SSRI, 1 MDD-UnMed). HC were subsequently selected to match the remaining MDD-UnMed and MDD-SSRI participants on age, sex, BMI, education, employment status, PHQ, OASIS, SCOFF and DAST scores. After group propensity matching, 47 MDD-SSRI (11M, 36F), 48 MDD-UnMed (15M, 33F) and 41 HC (18M, 23F) participants remained for data analysis (see Table 1 for demographic information). Within the MDD-SSRI group, the average daily dose of SSRI medication was 121mg (sertraline), 42mg (fluoxetine), 19mg (escitalopram), 23mg (citalopram), and 20mg (paroxetine), respectively.
Table 1.
Sample Demographics and Clinical Characteristics
| Group | HC (n=41) | MDD-SSRI (n=47) | MDD-UnMed (n=48) | p-value | |
|---|---|---|---|---|---|
| Mean (sd) | Mean (sd) | Mean (sd) | 3 groups | MDD-Med vs. UnMed | |
|
| |||||
| Age | 31.49 (10.54) | 35.87 (11.21) | 32.31 (11.28) | .14a | .13c |
| Body Mass Index | 27.42 (5.16) | 29.32 (5.39) | 27.10 (5.60) | .11a | .06c |
| Sex = Male (%) | 18 (43.9) | 11 (23.4) | 15 (31.2) | .12b | .53b |
| Education | 6.39 (1.45) | 6.72 (1.17) | 6.12 (1.70) | .14a | .05c |
| Employed = Yes (%) | 30 (76.9) | 27 (58.7) | 33 (71.7) | .17b | .27b |
| IPAQ minutes per week | 4659.21 (3678.56) | 3849.07 (4279.15) | 3616.51 (4388.08) | .51a | .80c |
| IPAQ Category (%) | .30b | .81b | |||
| HEPA Active | 21 (58.3) | 17 (37.8) | 18 (40.9) | ||
| Inactive | 6 (16.7) | 14 (31.1) | 15 (34.1) | ||
| Minimally Active | 9 (25.0) | 14 (31.1) | 11 (25.0) | ||
| OASIS | 1.12 (1.35) | 10.32 (3.50) | 9.29 (3.40) | <.01a | .15c |
| PHQ | 0.78 (1.17) | 14.52 (5.41) | 14.88 (4.13) | <.01a | .72c |
| SCOFF | 0.05 (0.22) | 1.30 (1.27) | 1.33 (1.29) | <.01a | .89c |
| DAST | 0.07 (0.26) | 0.51 (0.88) | 0.50 (1.22) | .04a | .96c |
| PROMIS Alcohol Use Score | 43.96 (6.86) | 49.37 (5.92) | 50.02 (7.36) | <.01a | .64c |
| PROMIS Nicotine Dependence Score | 25.54 (7.36) | 27.28 (10.54) | 27.51 (10.39) | .59a | .91c |
| PROMIS Depression Score | 42.95 (6.26) | 63.07 (6.39) | 63.63 (6.73) | <.01a | .68c |
| PROMIS Anxiety Score | 45.50 (7.61) | 63.93 (6.47) | 63.34 (6.96) | <.01a | .67c |
| STAI Trait | 27.71 (7.14) | 55.96 (9.55) | 57.85 (10.88) | <.01a | .36c |
| STAI State | 26.10 (6.45) | 47.62 (11.08) | 50.42 (10.73) | <.01a | .21c |
| ASI Total Score | 5.59 (6.99) | 22.68 (14.20) | 26.83 (15.31) | <.01a | .17c |
| MAIA Attention Regulation Score | 3.54 (0.93) | 2.88 (0.97) | 2.81 (1.00) | <.01a | .73c |
| MAIA Body Listening Score | 2.64 (1.39) | 1.89 (1.17) | 1.86 (1.36) | <.01a | .91c |
| MAIA Emotional Awareness Score | 3.44 (1.07) | 3.54 (1.08) | 3.22 (1.22) | .37a | .18c |
| MAIA Not-Distracting Score | 2.12 (1.08) | 1.67 (0.90) | 1.68 (0.88) | <.05a | .97c |
| MAIA Noticing Score | 3.41 (1.08) | 3.54 (1.09) | 3.44 (1.20) | .83a | .64c |
| MAIA Not-Worrying Score | 3.43 (0.98) | 3.00 (0.83) | 2.47 (1.20) | <.01a | .01c |
| MAIA Self-Regulation Score | 3.59 (1.04) | 2.57 (0.88) | 2.12 (1.11) | <.01a | .03c |
| MAIA Trusting Score | 3.95 (1.04) | 2.71 (1.12) | 2.45 (1.10) | <.01a | .25c |
| TAS-20 Difficulty Describing Feelings | 12.29 (3.66) | 15.09 (3.47) | 15.96 (3.03) | <.01a | .20c |
| TAS-20 Difficulty Identifying Feelings | 10.68 (4.27) | 18.04 (6.20) | 19.51 (6.38) | <.01a | .26c |
| TAS-20 Externally Oriented Thinking | 26.10 (3.58) | 26.02 (3.27) | 25.43 (2.64) | .54a | .33c |
| TAS-20 Total Score | 49.07 (8.89) | 59.15 (9.15) | 60.89 (9.02) | <.01a | .35c |
| RRS Score | 28.65 (7.14) | 56.94 (10.84) | 58.54 (10.43) | <.01a | .46c |
| Current Depression = Yes (%) | NA | 47 (100.0) | 48 (100.0) | NA | NA |
| Recurrent Depression = Yes (%) | NA | 45 (95.7) | 36 (75.0) | NA | .04b |
| Comorbid Anxiety Disorder = Yes (%) | NA | 33 (70.2) | 31 (64.6) | NA | .94b |
| Comorbid Alcohol Use Disorder = Yes (%) | NA | 4 (8.5) | 8 (16.7) | NA | .33b |
| CDDR Lifetime Alcohol Use d | 496.97 (1370.00) | 623.98 (1397.15) | 977.46 (4408.74.75) | .70a | .59c |
Note. HC = healthy control. MDD = major depressive disorder. SSRI = Use of selective serotonin reuptake inhibitors. UnMed = unmedicated. IPAQ, International Physical Activity Questionnaire; OASIS, Overall Anxiety Severity and Impairment Scale; PHQ, Patient Health Questionnaire; SCOFF, Sick Control One Fat Food Questionnaire; DAST, Drug Use Questionnaire; PROMIS, Patient-Reported Outcomes Measurement Information System; STAI, State-Trait Anxiety Inventory; ASI, Anxiety Sensitivity Index; MAIA, Multidimensional Assessment of Interoceptive Awareness; TAS-20, Toronto Alexithymia Scale; RRS, Ruminative Responses Scale; CDDR, Customary Drinking And Drug Use Record.
One-way ANOVA test
X2 test.
Two Sample t-test.
One outlier from the MDD-SSRI group was excluded.
2.4. fMRI VIA task and data preprocessing
Participants completed two runs of the VIA task, wherein they were presented with three conditions cued by a visually presented word (10 s duration): (1) “heart” cued internal attention toward heartbeat sensations; (2) “stomach” cued internal attention toward stomach sensations; and (3) “target” cued external attention toward word color changes at varying intensities. The VIA task has been previously shown to be effective at mapping the neural signal associated with interoceptive attention in a variety of clinical and non-clinical populations (Avery et al., 2017; Avery et al., 2014; DeVille et al., 2020; Kerr et al., 2016; Simmons et al., 2013; Stewart et al., 2020). Participants were asked to provide ratings of stimulus intensity (0 = ‘no sensation’ to 6 = ‘extreme sensation’) after 50% of trials, which also helped to ensure they remained awake and were attending to the task. Each run included 6 trials per condition (intertrial interval range 2.5–12.5 s). MRI data were acquired on two identical GE Discovery MR750 3T scanners operating identical pulse sequences for functional [repetition time (TR)/echo time (TE) = 2000/27 ms, field of view (FOV)/slice = 240/2.9 mm, 128 × 128 matrix, 39 axial slices, 180 TRs] and structural scans [magnetization prepared rapid acquisition gradient recalled echo (MP-RAGE) TR/TE = 5/2.012 ms, FOV/slice=240 × 192/0.9 mm, 186 axial slices].
Single-subject preprocessing was completed using Analysis of Functional NeuroImages (AFNI) software (Cox, 1996). The first three TRs were discarded, followed by despiking, slice-timing correction, co-registration to anatomical volumes, motion correction, transformation to Montreal Neurological Institute space via an affine transformation, application of a 4mm Gaussian full-width at half-max smoothing kernel, and a voxelwise general linear model analysis. Block regressors were convolved with a canonical hemodynamic response function and used to model blood oxygen level dependent (BOLD) response for heart, stomach, and target conditions. Six motion parameters (three translations and three rotations) were included as nuisance regressors. Censoring was done at the regression step by removing volumes with either a Euclidean norm of the derivatives of the six motion parameters greater than 0.3 mm or greater than 10% outlier voxels, determined by 3dToutcount. Percent signal change during each condition was defined as the estimated beta coefficient from single-subject analysis, which was relative to the implicit baseline during unmodeled fixation and scaled by the zeroth order regressor to convert to percent signal change.
2.5. Heartbeat tapping task and data preprocessing
Participants completed a heartbeat detection task with simultaneous electrocardiogram recording as an objective measure of interoceptive awareness. This task included four 60-second trials with varied instructions. In each trial, participants were instructed to keep their eyes closed and press a key in synchrony with an event. The first (Guessing) trial included instructions to press a key in line with their heartbeat and to make their best guess if they were uncertain. The second (Tone) trial was an exteroceptive control, with participants pressing in synchrony with a tone presented at approximately 80 tones per minute. The third (No Guessing) trial tasked participants to press the key in synchrony with their heartbeat, but only when they were certain they had felt a heartbeat. The final (Breath Hold) trial had the same instructions as the No Guessing trial, except that participants also began the trial with a maximal inspiratory breath hold.
Interoceptive accuracy was quantified using the Beat-to-Tap consistency metric (or Tone-to-Tap consistency, for Tone trials). This measure is described fully in (Smith et al., 2021), but, briefly, it quantifies how consistently a person taps with an event (heartbeat or tone), compared to what would be expected if they tapped randomly. This is done by comparing the standard deviation of the delay between each tap and the temporally closest event to an empirically derived distribution based on the actual train of events for that trial and a person randomly tapping the same number of times as the participant responded. Beat-to-Tap consistency is a Z-score, with larger values indicating less randomness or more precision.
2.6. Statistical analysis
For demographics (age, BMI, education, IPAQ minutes per week) and clinical characteristics (OASIS, PHQ, SCOFF, DAST, PROMIS, STAI, ASI, MAIA, TAS, RRS and CDDR lifetime alcohol use), one-way analysis of variance (ANOVA) was used to test differences between MDD-SSRI, MDD-UnMed and HC groups. For significant ANOVA group results, a two-sample t-test was then used to measure differences between MDD-Med and MDD-UnMed. Chi-square tests evaluated group differences in sex, employment status, IPAQ exercise category, current or recurrent depression, anxiety disorder comorbidity, and alcohol use disorder comorbidity.
Group differences on interoceptive attention were tested in a whole-brain voxel-wise analysis. Percent fMRI signal change from the contrast of the average of heart and stomach interoception versus the target exteroceptive condition (INT-EXT) was extracted for each subject and included in AFNI’s group analysis program (Chen et al., 2014) using multivariate modeling (‘3dMVM’). Because of previously stated concerns about false positive rates in fMRI cluster thresholding (Eklund et al., 2016), the resulting group statistical map was corrected for multiple comparisons (p < 0.05) using a traditionally conservative approach. Specifically, a non-Gaussian spatial autocorrelation function (acf) was used in the AFNI programs ‘3dFWHMx’ and ‘3dClustSim’, which estimate intrinsic smoothness and the probability of false positives, respectively. This approach has been shown to address many of the previously existing issues regarding false positives (Cox et al., 2017). Clusters with significant group effects were extracted for post-hoc analyses. Intensity ratings on INT-EXT were calculated using the average of VIA heart and stomach ratings versus target ratings. One-way ANOVAs were used to assess group differences on INT-EXT for: (a) percent fMRI signal change, and (b) intensity ratings, followed by Tukey’s Honest Significant Difference post-hoc tests if the overall test was significant. Two-way mixed ANOVAs with group as the between subjects variable and condition (heart, stomach, and target) as the within-subjects variable were used to follow-up on the one-way ANOVA contrasts for the INT-EXT intensity ratings and fMRI percent signal change to determine whether results were driven by differences in one or both of the interoception conditions (heart, stomach) or the exteroception condition (target).
In addition to the whole brain analysis, a region of interest (ROI) analysis was performed focusing on insular subregions. Specifically, three insular subregions defined from the Brainnetome atlas (Fan et al., 2016) were selected for the analysis: dorsal agranular (anterior), dorsal granular (posterior) and dorsal dysgranular (mid) insula (Supplemental Figure S1). One-way ANOVAs were used to assess group differences on INT-EXT within the dorsal anterior, posterior, and mid-insula ROIs for percent fMRI change in BOLD signal, followed by Tukey’s Honest Significant Difference method if the overall test was significant. In regions with significant group effects, we further quantified the evidence for pairwise differences using Bayes Factors (BF).
Kruskal-Wallis non-parametric tests, followed by Mann-Whitney-Wilcoxon non-parametric post-hoc tests were used to test group differences in Beat-to-Tap consistency scores for each active condition (Guessing, Tone, No Guessing and Breath Hold).
Pearson’s correlations explored potential relationships between interoceptive awareness, insular function, and depressive symptom severity within MDD participants. Specifically, we correlated MAIA subscale scores that were significantly lower in MDD than HC with: (1) bilateral anterior and left mid-insula BOLD signal for the INT-EXT contrast; (2) self-reported intensity ratings for the INT-EXT contrast; and (3) PROMIS depression scores.
3. Results
3.1. Demographic and clinical assessments
Table 1 lists descriptive statistics and group tests for demographic and clinical measures. All three groups did not differ on age, BMI, sex, education, employment status or physical activity. Although the two MDD groups did not differ on substance use, depression or anxiety symptoms, or comorbid disorders, both MDD-SSRI and MDD-UnMed reported higher PROMIS depression, PROMIS anxiety, TAS, and RRS symptoms than HC. Moreover, both MDD groups endorsed lower ratings on the MAIA Attention Regulation, Body Listening, Not Distracting, Not Worrying, Self-Regulation, and Trusting subscales than HC. In contrast, group ratings did not differ for the MAIA Emotional Awareness or Noticing subscales. Finally, the MDD-SSRI group exhibited a higher rate of recurrent depression compared to the MDD-UnMed group.
3.2. Neuroimaging assessments of interoception
Whole brain results in Figure 1 demonstrate that INT-EXT group differences emerged for: the left dorsal mid-insula (F2,133 = 7.09, p = .001), bilateral dorsolateral putamen (F2,133 = 7.45, p < .001), right amygdala (F2,133 = 6.89, p = .001) and bilateral dorsal caudate (F2,133 = 5.32, p = .006). Table 2 illustrates that compared to HC: (a) MDD-SSRI exhibited lower INT-EXT percent fMRI signal change within the left dorsal mid-insula (p < .01, d = .80, BF = 73.5), bilateral dorsolateral putamen (p < .01, d = .65, BF = 12.3), bilateral dorsal caudate (p = .02, d = .54, BF = 3.7), and right amygdala (p = .02, d = .59, BF = 5.7); and (b) MDD-UnMed also showed lower INT-EXT percent fMRI signal change within the left dorsal mid-insula (p = .03, d = .49, BF = 2.2), dorsal putamen (p < .01, d = .73, BF = 30.4), dorsal caudate (p < .01, d = .62, BF = 8.6), and right amygdala (p < .01, d = .76, BF = 45.2). See Table 3 for details of coordinates and volumes for these regions.
Figure 1.
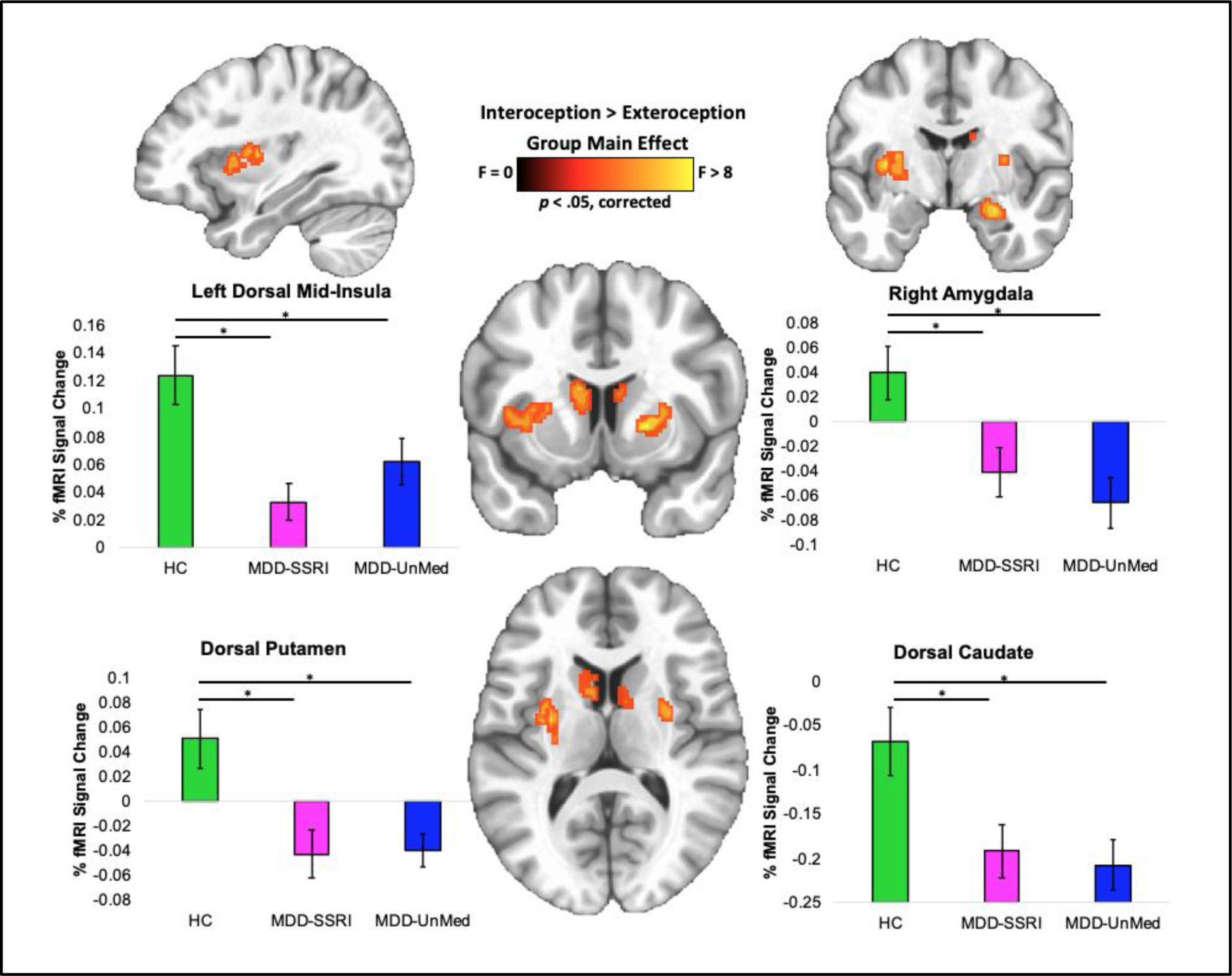
Interoception brain differences between HC, MDD-SSRI and MDD-UnMed during the visceral interoceptive awareness task. MDD = major depressive disorder. SSRI = Use of selective serotonin reuptake inhibitors. UnMed = unmedicated. Error bars reflect one standard error.
Table 2.
Percent fMRI signal change of interoception vs. exteroception as a function of group membership.
| Region | HC (n=41) | MDD-SSRI (n=47) | MDD-UnMed (n=48) | p-valuea | MDD-SSRI vs. HC | MDD-UnMed vs. HC | MDD-SSRI vs. MDD-UnMed | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean (sd) | Mean (sd) | Mean (sd) | p-value | d b | BF c | p-value | d b | BF c | p-value | d b | BF c | ||
|
| |||||||||||||
| Left Dorsal Mid-Insula | .12 (.14) | .03 (09) | .06 (.12) | <.01 | <.01 | .80 | 73.5 | .03 | .49 | 2.24 | .43 | .28 | 0.49 |
| Bilateral Dorsolateral Putamen | .05 (.15) | −.04 (.13) | −.04 (.09) | <.01 | <.01 | .65 | 12.3 | <.01 | .73 | 30.4 | .99 | .03 | 0.22 |
| Bilateral Dorsal Caudate | −.07 (.25) | −.19 (.20) | −.21 (.20) | <.01 | .02 | .54 | 3.7 | <.01 | .62 | 8.61 | .93 | .08 | 0.23 |
| Right Amygdala | .04 (.14) | −.04 (.13) | −.07 (.14) | <.01 | .02 | .59 | 5.7 | <.01 | .76 | 45.2 | .65 | .18 | 0.31 |
Note.
One way ANOVA followed by Tukey’s Honest Significant Difference method
Cohen’s d effect sizes computed with the effsize package in R. Statistically significant p-values are indicated in bold font. MNI = Montreal Neurological Institute. HC = healthy control. MDD = major depressive disorder.
Bayes factors computed with the using ttestBF I the BayesFactor packages in R. SSRI = Use of selective serotonin reuptake inhibitors. UnMed = unmedicated.
Table 3.
Regions exhibiting interoception vs. exteroception group differences in voxel-wise whole brain analysis.
| Cluster | Peak MNI Coordinates | Volume (mm3) | ||
|---|---|---|---|---|
| x | y | z | ||
|
| ||||
| Left Dorsal Mid-Insula and Left Dorsal Putamen | −27 | −15 | 5 | 1190 |
| Right Dorsal Putamen | 23 | 13 | −5 | 804 |
| Left Dorsal Caudate | −7 | 7 | 7 | 402 |
| Right Amygdala | 21 | −5 | −21 | 252 |
| Right Dorsal Caudate | 9 | 3 | 11 | 220 |
Note. MNI = Montreal Neurological Institute. All results corrected for multiple comparisons at p < 0.05. Coordinate order = LPI.
Figure 2 indicates that the left dorsal mid-insula and right amygdala differences appeared to be driven by MDD hypoactivation during heart and stomach attention, whereas the bilateral putamen and caudate differences appeared to be driven by MDD hyperactivation during visual (Target) attention. There was no statistically significant evidence for BOLD signal differences between the MDD-SSRI and MDD-UnMed groups on the VIA task, and Supplemental Figure S2 illustrates that any pattern of potential MDD group differences was absent in interoception-related regions. However, the MDD-SSRI group endorsed higher INT-EXT intensity ratings than MDD-UnMed (p < .01, d = .64) and HC (p = .02, d = .58; Figure 3A), reflecting heightened heart and stomach sensation intensities (Figure 3B).
Figure 2.
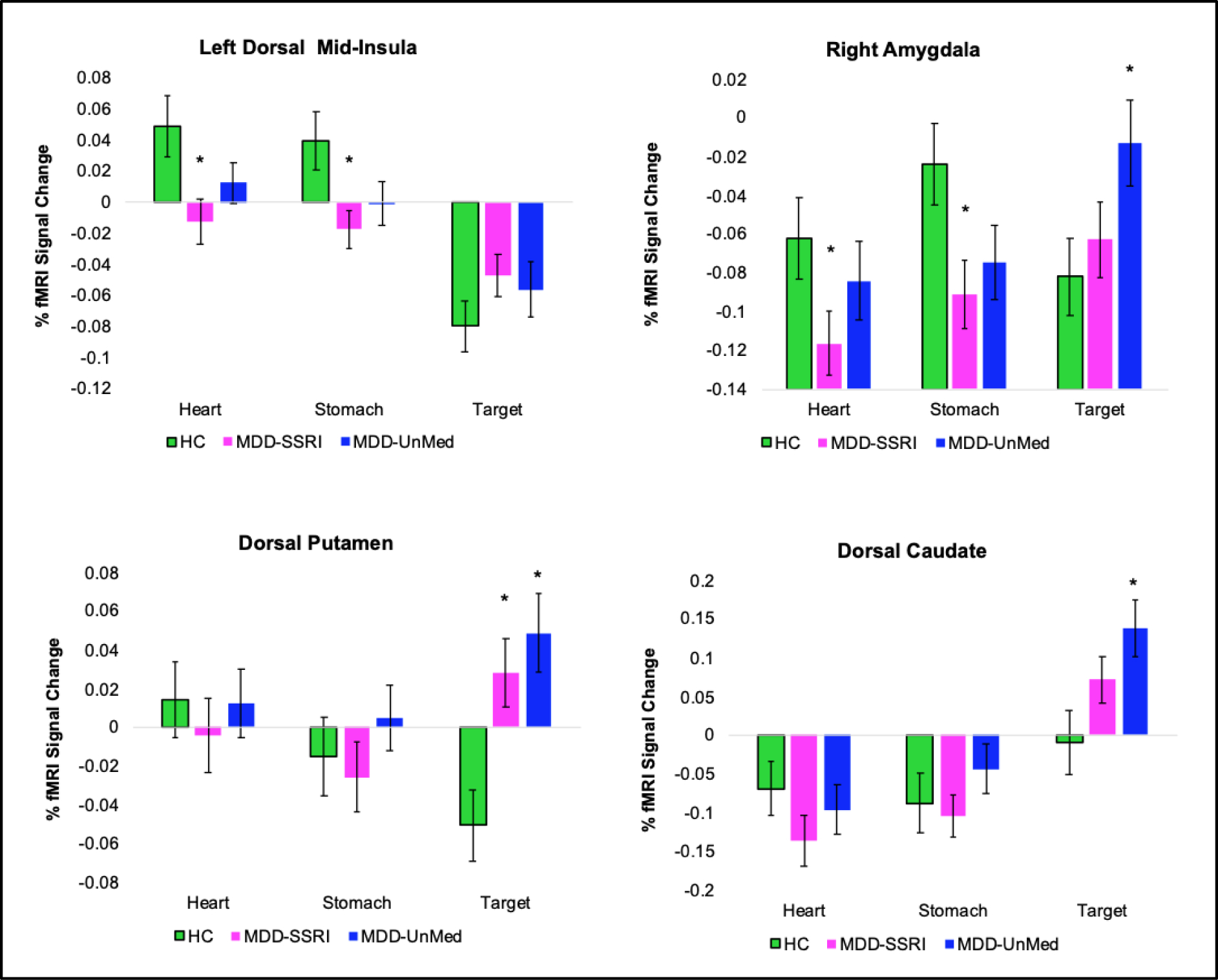
Group x condition interaction results for regions displayed in Figure 1. * Indicates a significant difference for the MDD-SSRI or MDD-UnMed vs. HC comparison. MDD = major depressive disorder. SSRI = Use of selective serotonin reuptake inhibitors. UnMed = unmedicated. Error bars reflect one standard error.
Figure 3.
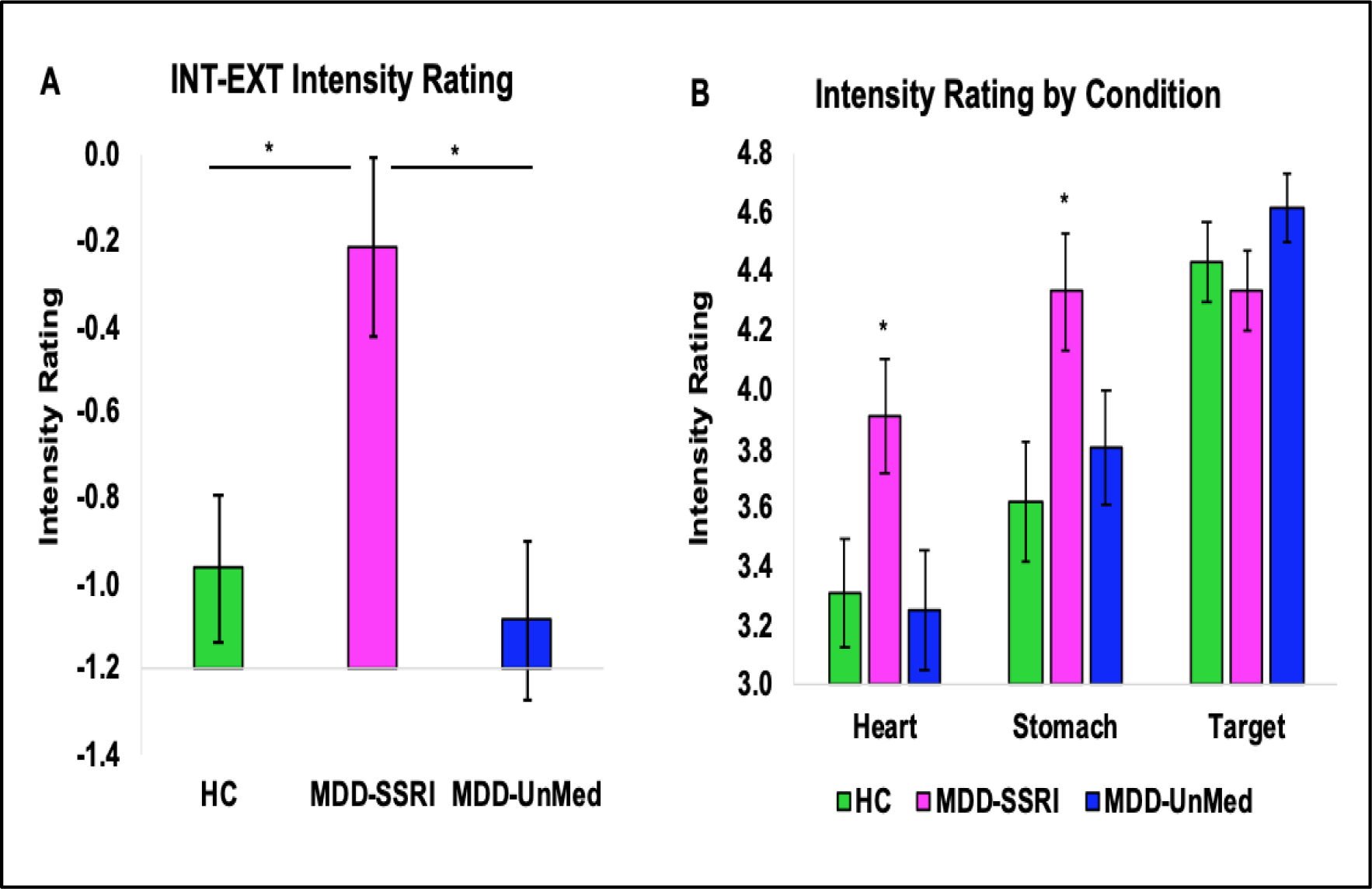
(A) Intensity ratings for the interoception versus exteroception contrast (INT-EXT) during the VIA task. * Indicates that the MDD-SSRI group endorsed significantly higher INT-EXT intensity ratings than MDD-UnMed and HC. (B) Group*condition interaction results for VIA intensity ratings. * Indicates that the MDD-SSRI group reported significant higher intensities of heart and stomach sensations than MDD-UnMed and HC. MDD = major depressive disorder. SSRI = Use of selective serotonin reuptake inhibitors. UnMed = unmedicated. Error bars reflect one standard error.
Insular ROI analysis showed that there was a significant group difference during INT-EXT for left (F2,130 = 3.73, p = .027) and right (F2,130 = 3.62, p = .029) dorsal anterior insula, as well as left dorsal mid-insula (F2,130 = 3.34, p = .038). Post-hoc tests demonstrated that MDD-SSRI exhibited lower BOLD signal during INT-EXT than HC for left (p = .037, d = .52, BF = 2.71) and right (p = .046, d = .50, BF = 2.19) dorsal anterior insula and left dorsal mid-insula (p = .034, d = .58, BF = 4.6) (Table 4). MDD-UnMed exhibited trending lower BOLD signal during INT-EXT than HC for left (p = .059, d = .48, BF = 1.98) and right (p = .056, d = .48, BF = 1.88) dorsal anterior insula. In each ROI, there was moderate evidence for no difference between MDD-SSRI and MDD-UnMed (BFs between 0.22 and 0.28).
Table 4.
Percent fMRI signal change for each group as a function of interoception-exteroception contrast and insula ROI.
| Region | HC (n=41) | MDD-SSRI (n=47) | MDD-UnMed (n=48) | p-valuea | MDD-SSRI vs. HC | UnMed vs. HC | MDD-SSRI vs. UnMed | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean (sd) | Mean (sd) | Mean (sd) | p | d b | BF c | p | d b | BF c | p | d b | BF c | ||
|
| |||||||||||||
| Left dorsal anterior insula | −0.23 (1.59) | −0.99 (1.33) | −0.93 (1.31) | .027 | 0.037 | 0.523 | 2.71 | 0.059 | 0.484 | 1.98 | 0.975 | 0.047 | 0.22 |
| Right dorsal anterior insula | −0.42 (1.87) | −1.23 (1.40) | −1.20 (1.41) | .029 | 0.046 | 0.500 | 2.19 | 0.056 | 0.478 | 1.88 | 0.994 | 0.025 | 0.22 |
| Left dorsal posterior insula | 0.55 (1.16) | 0.02 (0.94) | 0.38 (1.09) | .066 | |||||||||
| Right dorsal posterior insula | 0.69 (1.10) | 0.25 (0.98) | 0.40 (1.20) | .176 | |||||||||
| Left dorsal mid-insula | 1.79 (1.53) | 1.01 (1.18) | 1.22 (1.54) | .038 | 0.034 | 0.578 | 4.6 | 0.157 | 0.370 | 0.81 | 0.748 | 0.155 | 0.28 |
| Right dorsal mid-insula | 1.56 (1.46) | 1.02 (1.27) | 1.13 (1.52) | .192 | |||||||||
Note. HC = healthy control. MDD = major depressive disorder. SSRI = Use of selective serotonin reuptake inhibitors. UnMed = unmedicated.
One way ANOVA followed by Tukey’s Honest Significant Difference’ method
Cohen’s d effect sizes computed with the effsize package in R.
Bayes factors computed with the using ttestBF I the BayesFactor packages in R. Statistically significant p-values are indicated in bold font.
3.3. Behavioral assessments of interoception
The groups differed on Beat-to-Tap consistency for the Guessing (Kruskal-Wallis p < .01) and Breath Hold (Kruskal-Wallis p = .02) conditions (Figure 4). Specifically, for the Breath Hold condition both the MDD-SSRI (Wilcoxon p = .02, d = .60) and MDD-UnMed (Wilcoxon p < .01, d = .35) groups exhibited lower Beat-to-Tap consistency than HC; however, the two MDD groups did not differ from each other. The MDD-UnMed group also showed higher Beat-to-Tap consistency than HC (Wilcoxon p < .01, d = .82) and MDD-SSRI (Wilcoxon p = .01, d = .63) during the Guessing condition. All three groups did not differ in Beat-to-Tap consistency for the Tone or No Guessing conditions.
Figure 4. Heartbeat tapping task results.
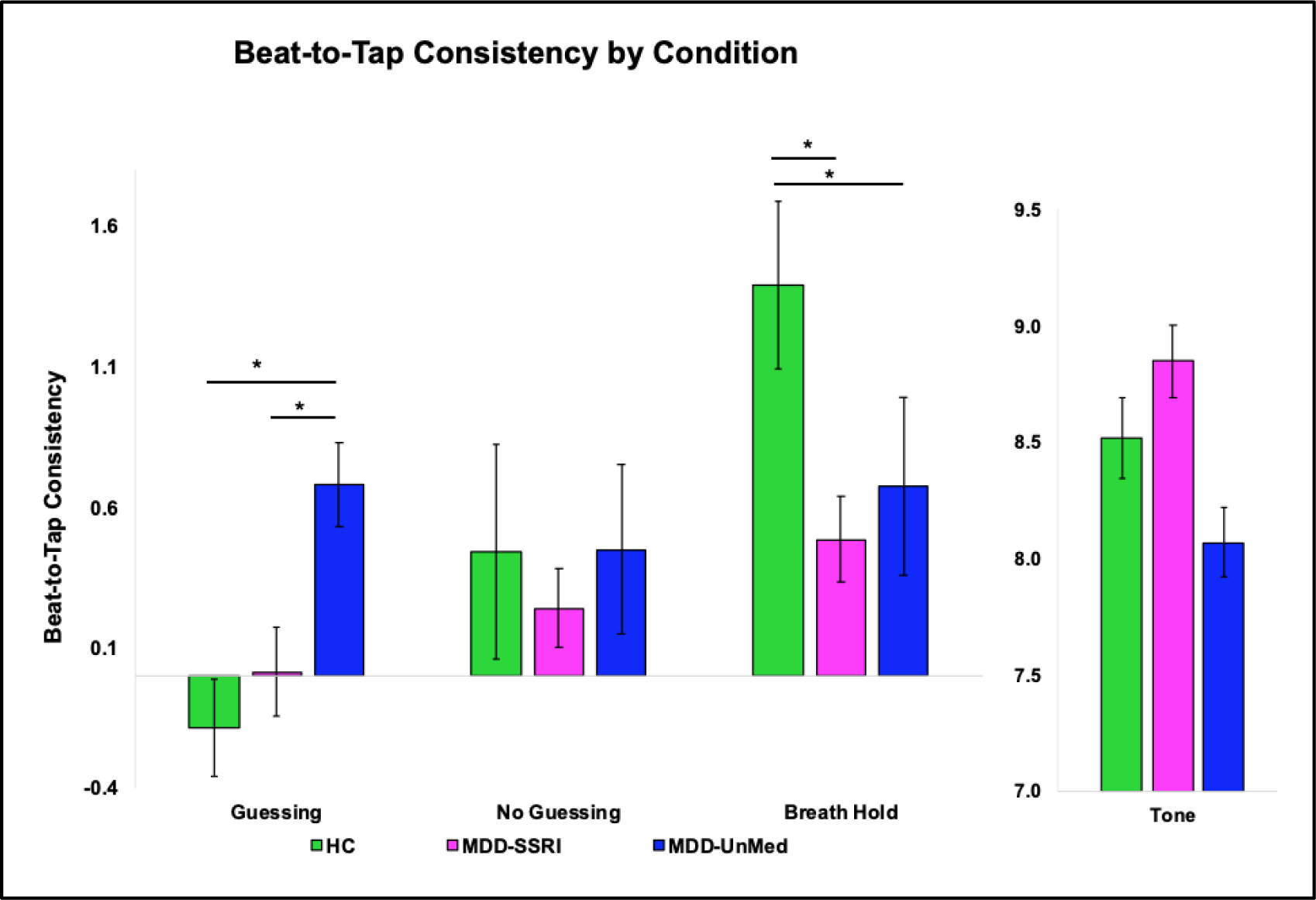
* Indicates significant group differences on Guessing or Breath Hold condition.
Error bars reflect one standard error.
3.4. Exploratory correlations within MDD
Lower ratings on two MAIA subscales were associated with lower left dorsal anterior insula BOLD signal for the INT-EXT contrast: (a) Emotional Awareness (r = 0.21, p = 0.04); (b) Self-Regulation (r = 0.24, p = 0.02). In contrast, lower ratings on four MAIA subscales were associated with higher PROMIS depression scores: (a) Not-Distracting (r = −0.24, p = 0.02); (b) Not-Worrying (r = −0.25, p = 0.01); (c) Self-Regulation (r = −0.40, p < 0.01); and (d) Trusting (r = −0.37, p < 0.01). No significant correlations between MAIA subscales emerged for: (1) left dorsal mid-insula or right dorsal anterior insula BOLD signal for the INT-EXT contrast; or (2) self-reported intensity ratings for the INT-EXT contrast.
4. Discussion
This cross-sectional study examined the effects of chronic serotonergic antidepressant medication – specifically SSRIs – on brain function during interoceptive attention, on behavioral measures of cardiac interoception, and on self-reported interoceptive awareness. We hypothesized that both SSRI-medicated and unmedicated MDD individuals would exhibit reduced brain activation during interoceptive attention and reduced interoceptive awareness relative to HC. Our hypotheses were supported by three out of four main findings.
First, both unmedicated and SSRI-medicated MDD exhibited lower left dorsal mid-insula, right amygdala, bilateral dorsolateral putamen, and bilateral dorsal caudate signals than HC to interoceptive versus exteroceptive VIA conditions, potentially reflecting reduced neural resource allocation during interoceptive attention. The dorsal mid-insula is robustly activated by the VIA task (Avery et al., 2014) and paradigms involving gustatory and cardiovascular perturbations (Avery et al., 2015; Hassanpour et al., 2018). This brain area may thus be important for the detection and initial processing of afferent interoceptive signals. A recent meta-analysis of neuroimaging studies across psychiatric disorders showed that left mid-insula activation was disrupted during interoceptive processing tasks (Nord et al., 2021), a converging finding that is generally consistent with the present result in depressed individuals. Moreover, our insular ROI results replicated whole brain findings demonstrating that medicated MDD participants exhibited significantly lower left mid-insula responses (as well as anterior insula attenuations) than HC to interoceptive versus exteroceptive signals; unmedicated MDD participants showed trend-level findings in the same direction as the medicated MDD group. There are several possible explanations for these brain imaging results. First, blunted interoceptive processing might be a trait marker for individuals at risk for MDD and is thus not influenced by medication. Second, this finding may be a consequence of being in a depressive episode regardless of treatment. Third, this finding may be transient and revert to non-blunted interoceptive salience processing as the acute episode remits – with or without medication. Thus, future studies will need to assess interoceptive processing as part of an active ongoing treatment study to disambiguate these possibilities.
Second, both SSRI medicated and unmedicated MDD individuals showed evidence of poorer heartbeat tapping performance than HC specifically during the Breath Hold condition. These findings align with prior work demonstrating that greater depressive symptoms are linked to more self-reported difficulty in maintaining mindfulness on breathing sensations (Burg and Michalak, 2011). Depressed individuals’ interoceptive focus may be particularly disrupted during a dual task involving respiration, given that respiration pattern variability is greater in MDD patients than healthy controls (Zamoscik et al., 2018). However, as depressed individuals tend to perform more poorly on tasks requiring split allocation of attentional resources than healthy individuals (Doumas et al., 2012; Levens et al., 2009; Rokke et al., 2002), poorer heartbeat tapping performance during breath holding may represent a more generalized difficulty with attention allocation irrespective of interoceptive versus exteroceptive task content. Additional research is warranted to clarify this issue.
Third, both SSRI-medicated and unmedicated MDD patients endorsed lower MAIA scores than HC on subscales reflecting difficulty focusing on and having confidence in their bodily signals, replicating prior work (Flasinski et al., 2020). Within MDD participants, difficulties in emotional awareness and self regulation measured by the MAIA were, in turn, linked to lower left anterior insula responses to interoceptive versus exteroceptive stimuli, findings consistent with research positing that anterior insula is a crucial region for facilitating emotional awareness (Gu et al., 2013). Fourth, in contrast to the brain activation results focusing on interoceptive versus exteroceptive signals, only the SSRI-medicated MDD group reported higher intensity ratings than MDD-UnMed and HC. Notably, this pattern of heightened interoceptive intensity to heart sensations, paired with lower dorsal mid-insula BOLD signal to heart and stomach sensations, in medicated MDD patients mirrors our previous VIA findings for individuals with stimulant (amphetamine and cocaine) use disorder, many of whom met criteria for a lifetime MDD diagnosis and were taking antidepressant and other types of medication (Stewart et al., 2020). Within stimulant users, greater heartbeat intensity was linked to more recent drug use, whereas a greater number of past-year drug uses related to higher anterior to dorsal mid-insula signal approximating that of HC (Stewart et al., 2020). It is important to note that individuals with a history of stimulant use disorder in Stewart et al. (2020) were taking medications for various health conditions, including hypertension, in addition to antidepressant drugs; similarly, a subset of our medicated MDD sample in the current analysis were taking multiple medications. Given the substantial drug heterogeneity within the medicated MDD group and the cross-sectional design of this project, we were unable to ascertain whether a particular substance was partially driving left dorsal-mid insula attenuation, but it is crucial to emphasize that the influence of medication was specifically focused on this region of cortex, as opposed to widespread attenuations across the brain.
In this study we found no evidence for differences in neural activity between the MDD-SSRI and MDD-UnMed groups on the VIA task. Importantly, both medicated and unmedicated groups showed consistent patterns of abnormal neural activity in regions believed to be important for interoception (i.e. insula, amygdala, caudate). Thus, it seems reasonable to expect that MDD patients who are stably medicated can be included in neural assessments of interoception without worrying that this will confound measurement of brain activity. At the same time, there was evidence of abnormal interoceptive awareness at the perceptual (i.e. higher intensity of cardiac and stomach sensations in the medicated group) and behavioral (i.e. lower Beat-to-Tap consistency during Guessing condition) levels for medicated versus unmedicated MDD. These findings suggest that additional work is needed to determine whether there is an acute versus chronic impact of serotonergic medications on perceptual mechanisms, particularly with respect to an increased focus on body sensations. One potential avenue forward would be to examine the impact of acute SSRI (or other serotonergic drug) administration on interoception using an experimental medicine approach (i.e., see (Livermore et al., 2021) for such an example as applied to healthy individuals). Indeed, autonomic symptoms such as flushing, heart palpitations, nausea, and constipation/diarrhea are commonly observed during the initial weeks of taking such medications in clinical settings (Trindade et al., 1998). However, these symptoms typically recede and would not be typically observed in the chronic administration setting as studied here. Whether such changes play a role in therapeutic responses during treatment initiation is also unknown, and await further study, perhaps via longitudinal sampling approaches.
Although this analysis replicates and extends work reporting abnormalities of interoception in MDD, several limitations related to our sample and study design are worth noting. First, the Tulsa 1000 study was comprised of a heterogeneous sample of treatment-seeking individuals. Although the present investigation excluded individuals with comorbid illicit drug use and eating disorders, approximately two-thirds of our MDD sample met criteria for at least one comorbid anxiety disorder; despite this comorbidity, it is important to note that SSRI-medicated and unmedicated MDD groups did not differ in anxiety symptom severity or other types of psychopathology (e.g., rumination, alexithymia, alcohol use disorder). As high comorbidity between MDD and anxiety disorders are the rule rather than the exception (Kessler et al., 2003), the findings from this study may be more generalizable to community samples than research focusing solely on pure MDD. Second, as two-thirds of the MDD groups were comprised of female participants, the results may be more applicable to depressed women than men. Ideally, future work would evaluate interoceptive function in MDD patients taking a more homogeneous antidepressant medication regimen. Third, this cross-sectional analysis cannot determine whether MDD brain patterns were present prior to medication onset or changed because of the initiation of medication. Longitudinal studies of MDD outpatients pre- and post-treatment with antidepressant medication are thus warranted, which would allow for a causal examination of the influence of specific drugs and degree of depression symptom remission on brain and self-report metrics of interoception.
5. Conclusions
Our findings suggest that the attenuated patterns of neural activation that are observed in depressed individuals during interoceptive attention are not ameliorated by the chronic administration of serotonergic medications. However, amplified interoceptive sensation ratings suggest a potential impact of chronic serotonergic medication on conscious experiences of internal body states.
Supplementary Material
Highlights.
MDD patients exhibited lower self-reported interoceptive awareness than HC.
MDD patients showed lower insula, amygdala, and striatum activation than HC.
Insula activation did not differ between unmedicated and SSRI-medicated MDD.
MDD-SSRI displayed greater interoceptive sensation ratings than MDD-UnMed and HC.
Acknowledgements
This research was supported by NIGMS grant number P20GM121312 (MPP), NIMH grant number K23MH112949 (SSK), NIDA grant number R01DA050677 (JLS, MPP) and The William K. Warren Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Ainley V, Tsakiris M, Pollatos O, et al. , 2020. Comment on “Zamariola et al. (2018), Interoceptive Accuracy Scores are Problematic: Evidence from Simple Bivariate Correlations”-The empirical data base, the conceptual reasoning and the analysis behind this statement are misconceived and do not support the authors’ conclusions. Biol Psychol 152, 107870. doi: 10.1016/j.biopsycho.2020.107870. [DOI] [PubMed] [Google Scholar]
- APA., 2010. PRACTICE GUIDELINE FOR THE Treatment of Patients With Major Depressive Disorder. American Psychiatric Association; Third Edition. [Google Scholar]
- Avery JA, Burrows K, Kerr KL, et al. , 2017. How the Brain Wants What the Body Needs: The Neural Basis of Positive Alliesthesia. Neuropsychopharmacology 42, 822–830. doi: 10.1038/npp.2016.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avery JA, Drevets WC, Moseman SE, et al. , 2014. Major depressive disorder is associated with abnormal interoceptive activity and functional connectivity in the insula. Biol Psychiatry 76, 258–266. doi: 10.1016/j.biopsych.2013.11.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avery JA, Kerr KL, Ingeholm JE, et al. , 2015. A common gustatory and interoceptive representation in the human mid-insula. Hum Brain Mapp 36, 2996–3006. doi: 10.1002/hbm.22823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagby RM, Taylor GJ, Parker JD, 1994. The Twenty-item Toronto Alexithymia Scale--II. Convergent, discriminant, and concurrent validity. J Psychosom Res 38, 33–40. doi: 10.1016/0022-3999(94)90006-x. [DOI] [PubMed] [Google Scholar]
- Barrett LF, Quigley KS, Hamilton P, 2016. An active inference theory of allostasis and interoception in depression. Philos Trans R Soc Lond B Biol Sci 371. doi: 10.1098/rstb.2016.0011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett LF, Simmons WK, 2015. Interoceptive predictions in the brain. Nat Rev Neurosci 16, 419–429. doi: 10.1038/nrn3950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berntson GG, Khalsa SS, 2021. Neural Circuits of Interoception. Trends Neurosci 44, 17–28. doi: 10.1016/j.tins.2020.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown SA, Myers MG, Lippke L, et al. , 1998. Psychometric evaluation of the Customary Drinking and Drug Use Record (CDDR): a measure of adolescent alcohol and drug involvement. J Stud Alcohol 59, 427–438. doi: 10.15288/jsa.1998.59.427. [DOI] [PubMed] [Google Scholar]
- Burg JM, Michalak J, 2011. The Healthy Quality of Mindful Breathing: Associations With Rumination and Depression. Cognitive Therapy and Research 35, 179–185. doi: 10.1007/s10608-010-9343-x. [DOI] [Google Scholar]
- Cella D, Riley W, Stone A, et al. , 2010. The Patient-Reported Outcomes Measurement Information System (PROMIS) developed and tested its first wave of adult self-reported health outcome item banks: 2005–2008. J Clin Epidemiol 63, 1179–1194. doi: 10.1016/j.jclinepi.2010.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen G, Adleman NE, Saad ZS, et al. , 2014. Applications of multivariate modeling to neuroimaging group analysis: a comprehensive alternative to univariate general linear model. Neuroimage 99, 571–588. doi: 10.1016/j.neuroimage.2014.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corneille O, Desmedt O, Zamariola G, et al. , 2020. A heartfelt response to Zimprich et al. (2020), and Ainley et al. (2020)’s commentaries: Acknowledging issues with the HCT would benefit interoception research. Biol Psychol 152, 107869. doi: 10.1016/j.biopsycho.2020.107869. [DOI] [PubMed] [Google Scholar]
- Cox RW, 1996. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res 29, 162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- Cox RW, Chen G, Glen DR, et al. , 2017. fMRI clustering and false-positive rates. Proc Natl Acad Sci U S A 114, E3370–E3371. doi: 10.1073/pnas.1614961114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeVille DC, Kerr KL, Avery JA, et al. , 2018. The Neural Bases of Interoceptive Encoding and Recall in Healthy Adults and Adults With Depression. Biol Psychiatry Cogn Neurosci Neuroimaging 3, 546–554. doi: 10.1016/j.bpsc.2018.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeVille DC, Kuplicki R, Stewart JL, et al. , 2020. Diminished responses to bodily threat and blunted interoception in suicide attempters. Elife 9. doi: 10.7554/eLife.51593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinga R, Marquand AF, Veltman DJ, et al. , 2018. Predicting the naturalistic course of depression from a wide range of clinical, psychological, and biological data: a machine learning approach. Transl Psychiatry 8, 241. doi: 10.1038/s41398-018-0289-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doumas M, Smolders C, Brunfaut E, et al. , 2012. Dual task performance of working memory and postural control in major depressive disorder. Neuropsychology 26, 110–118. doi: 10.1037/a0026181. [DOI] [PubMed] [Google Scholar]
- Eggart M, Lange A, Binser MJ, et al. , 2019. Major Depressive Disorder Is Associated with Impaired Interoceptive Accuracy: A Systematic Review. Brain Sci 9. doi: 10.3390/brainsci9060131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eklund A, Nichols TE, Knutsson H, 2016. Cluster failure: Why fMRI inferences for spatial extent have inflated false-positive rates. Proc Natl Acad Sci U S A 113, 7900–7905. doi: 10.1073/pnas.1602413113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan L, Li H, Zhuo J, et al. , 2016. The Human Brainnetome Atlas: A New Brain Atlas Based on Connectional Architecture. Cereb Cortex 26, 3508–3526. doi: 10.1093/cercor/bhw157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flasinski T, Dierolf AM, Rost S, et al. , 2020. Altered Interoceptive Awareness in High Habitual Symptom Reporters and Patients With Somatoform Disorders. Front Psychol 11, 1859. doi: 10.3389/fpsyg.2020.01859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furman DJ, Waugh CE, Bhattacharjee K, et al. , 2013. Interoceptive awareness, positive affect, and decision making in major depressive disorder. J Affect Disord 151, 780–785. doi: 10.1016/j.jad.2013.06.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu X, Hof PR, Friston KJ, et al. , 2013. Anterior insular cortex and emotional awareness. J Comp Neurol 521, 3371–3388. doi: 10.1002/cne.23368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harshaw C, 2015. Interoceptive dysfunction: toward an integrated framework for understanding somatic and affective disturbance in depression. Psychol Bull 141, 311–363. doi: 10.1037/a0038101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassanpour MS, Simmons WK, Feinstein JS, et al. , 2018. The Insular Cortex Dynamically Maps Changes in Cardiorespiratory Interoception. Neuropsychopharmacology 43, 426–434. doi: 10.1038/npp.2017.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendler KS, 2008. Explanatory models for psychiatric illness. Am J Psychiatry 165, 695–702. doi: 10.1176/appi.ajp.2008.07071061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerr KL, Moseman SE, Avery JA, et al. , 2016. Altered Insula Activity during Visceral Interoception in Weight-Restored Patients with Anorexia Nervosa. Neuropsychopharmacology 41, 521–528. doi: 10.1038/npp.2015.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler RC, Berglund P, Demler O, et al. , 2003. The epidemiology of major depressive disorder: results from the National Comorbidity Survey Replication (NCS-R). JAMA 289, 3095–3105. doi: 10.1001/jama.289.23.3095. [DOI] [PubMed] [Google Scholar]
- Khalsa SS, Adolphs R, Cameron OG, et al. , 2018. Interoception and Mental Health: A Roadmap. Biol Psychiatry Cogn Neurosci Neuroimaging 3, 501–513. doi: 10.1016/j.bpsc.2017.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khalsa SS, Lapidus RC, 2016. Can Interoception Improve the Pragmatic Search for Biomarkers in Psychiatry? Front Psychiatry 7, 121. doi: 10.3389/fpsyt.2016.00121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroenke K, Spitzer RL, Williams JB, 2001. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med 16, 606–613. doi: 10.1046/j.1525-1497.2001.016009606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levens SM, Muhtadie L, Gotlib IH, 2009. Rumination and impaired resource allocation in depression. J Abnorm Psychol 118, 757–766. doi: 10.1037/a0017206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livermore JJA, Holmes CL, Moga G, et al. , 2021. Serotonergic Effects on Interoception. bioRxiv, 2020.2008.2028.262550. doi: 10.1101/2020.08.28.262550. [DOI] [Google Scholar]
- Mehling WE, Price C, Daubenmier JJ, et al. , 2012. The Multidimensional Assessment of Interoceptive Awareness (MAIA). PLoS One 7, e48230. doi: 10.1371/journal.pone.0048230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan JF, Reid F, Lacey JH, 1999. The SCOFF questionnaire: assessment of a new screening tool for eating disorders. BMJ 319, 1467–1468. doi: 10.1136/bmj.319.7223.1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nolen-Hoeksema S, Morrow J, 1991. A prospective study of depression and posttraumatic stress symptoms after a natural disaster: the 1989 Loma Prieta Earthquake. J Pers Soc Psychol 61, 115–121. doi: 10.1037//0022-3514.61.1.115. [DOI] [PubMed] [Google Scholar]
- Nord CL, Lawson RP, Dalgleish T, 2021. Disrupted Dorsal Mid-Insula Activation During Interoception Across Psychiatric Disorders. Am J Psychiatry 178, 761–770. doi: 10.1176/appi.ajp.2020.20091340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norman SB, Cissell SH, Means-Christensen AJ, et al. , 2006. Development and validation of an Overall Anxiety Severity And Impairment Scale (OASIS). Depress Anxiety 23, 245–249. doi: 10.1002/da.20182. [DOI] [PubMed] [Google Scholar]
- Paulus MP, Stein MB, 2006. An insular view of anxiety. Biol Psychiatry 60, 383–387. doi: 10.1016/j.biopsych.2006.03.042. [DOI] [PubMed] [Google Scholar]
- Paulus MP, Stein MB, 2010. Interoception in anxiety and depression. Brain Struct Funct 214, 451–463. doi: 10.1007/s00429-010-0258-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rehm J, Shield KD, 2019. Global Burden of Disease and the Impact of Mental and Addictive Disorders. Curr Psychiatry Rep 21, 10. doi: 10.1007/s11920-019-0997-0. [DOI] [PubMed] [Google Scholar]
- Ring C, Brener J, 2018. Heartbeat counting is unrelated to heartbeat detection: A comparison of methods to quantify interoception. Psychophysiology 55, e13084. doi: 10.1111/psyp.13084. [DOI] [PubMed] [Google Scholar]
- Rokke PD, Arnell KM, Koch MD, et al. , 2002. Dual-task attention deficits in dysphoric mood. J Abnorm Psychol 111, 370–379. [PubMed] [Google Scholar]
- Rush AJ, Trivedi MH, Wisniewski SR, et al. , 2006. Acute and Longer-Term Outcomes in Depressed Outpatients Requiring One or Several A STAR*D Report. Am J Psychiatry 163, 12. [DOI] [PubMed] [Google Scholar]
- Seth AK, Critchley HD, 2013. Extending predictive processing to the body: emotion as interoceptive inference. Behav Brain Sci 36, 227–228. doi: 10.1017/S0140525X12002270. [DOI] [PubMed] [Google Scholar]
- Seth AK, Friston KJ, 2016. Active interoceptive inference and the emotional brain. Philos Trans R Soc Lond B Biol Sci 371. doi: 10.1098/rstb.2016.0007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheehan DV, Lecrubier Y, Sheehan KH, et al. , 1998. The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry 59 Suppl 20, 22–33;quiz 34–57. [PubMed] [Google Scholar]
- Simmons WK, Avery JA, Barcalow JC, et al. , 2013. Keeping the body in mind: insula functional organization and functional connectivity integrate interoceptive, exteroceptive, and emotional awareness. Hum Brain Mapp 34, 2944–2958. doi: 10.1002/hbm.22113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmons WK, Burrows K, Avery JA, et al. , 2016. Depression-Related Increases and Decreases in Appetite: Dissociable Patterns of Aberrant Activity in Reward and Interoceptive Neurocircuitry. Am J Psychiatry 173, 418–428. doi: 10.1176/appi.ajp.2015.15020162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skinner HA, 1982. The drug abuse screening test. Addict Behav 7, 363–371. doi: 10.1016/0306-4603(82)90005-3. [DOI] [PubMed] [Google Scholar]
- Smith R, Feinstein JS, Kuplicki R, et al. , 2021. Perceptual insensitivity to the modulation of interoceptive signals in depression, anxiety, and substance use disorders. Sci Rep 11, 2108. doi: 10.1038/s41598-021-81307-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spielberger CD, Gorsuch RL, Lushene R, et al. , 1983. Manual for the State Trait Anxiety Inventory. (Form Y). Consulting Psychologists Press, Palo Alto. doi: 10.1037/t06496-000. [DOI] [Google Scholar]
- Stephan KE, Manjaly ZM, Mathys CD, et al. , 2016. Allostatic Self-efficacy: A Metacognitive Theory of Dyshomeostasis-Induced Fatigue and Depression. Front Hum Neurosci 10, 550. doi: 10.3389/fnhum.2016.00550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart JL, Khalsa SS, Kuplicki R, et al. , 2020. Interoceptive attention in opioid and stimulant use disorder. Addict Biol, e12831. doi: 10.1111/adb.12831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strigo IA, Matthews SC, Simmons AN, 2010. Right anterior insula hypoactivity during anticipation of homeostatic shifts in major depressive disorder. Psychosom Med 72, 316–323. doi: 10.1097/PSY.0b013e3181d07873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor S, Zvolensky MJ, Cox BJ, et al. , 2007. Robust dimensions of anxiety sensitivity: development and initial validation of the Anxiety Sensitivity Index-3. Psychol Assess 19, 176–188. doi: 10.1037/1040-3590.19.2.176. [DOI] [PubMed] [Google Scholar]
- Terhaar J, Viola FC, Bar KJ, et al. , 2012. Heartbeat evoked potentials mirror altered body perception in depressed patients. Clin Neurophysiol 123, 1950–1957. doi: 10.1016/j.clinph.2012.02.086. [DOI] [PubMed] [Google Scholar]
- Trindade E, Menon D, Topfer LA, et al. , 1998. Adverse effects associated with selective serotonin reuptake inhibitors and tricyclic antidepressants: a meta-analysis. CMAJ 159, 1245–1252. [PMC free article] [PubMed] [Google Scholar]
- Victor TA, Khalsa SS, Simmons WK, et al. , 2018. Tulsa 1000: a naturalistic study protocol for multilevel assessment and outcome prediction in a large psychiatric sample. BMJ Open 8, e016620. doi: 10.1136/bmjopen-2017-016620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiebking C, de Greck M, Duncan NW, et al. , 2015. Interoception in insula subregions as a possible state marker for depression-an exploratory fMRI study investigating healthy, depressed and remitted participants. Front Behav Neurosci 9, 82. doi: 10.3389/fnbeh.2015.00082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamoscik VE, Schmidt SNL, Gerchen MF, et al. , 2018. Respiration pattern variability and related default mode network connectivity are altered in remitted depression. Psychol Med 48, 2364–2374. doi: 10.1017/S0033291717003890. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


