Abstract
Human mesenchymal stem cells (MSCs), also known as mesenchymal stromal cells or medicinal signaling cells, are important adult stem cells for regenerative medicine, largely due to their regenerative characteristics such as self-renewal, secretion of trophic factors, and the capability of inducing mesenchymal cell lineages. MSCs also possess homing and trophic properties modulating immune system, influencing microenvironment around damaged tissues and enhancing tissue repair, thus offering a broad perspective in cell-based therapies. Therefore, it is not surprising that MSCs have been the broadly used adult stem cells in clinical trials. To gain better insights into the current applications of MSCs in clinical applications, we perform a comprehensive review of reported data of MSCs clinical trials conducted globally. We summarize the biological effects and mechanisms of action of MSCs, elucidating recent clinical trials phases and findings, highlighting therapeutic effects of MSCs in several representative diseases, including neurological, musculoskeletal diseases and most recent Coronavirus infectious disease. Finally, we also highlight the challenges faced by many clinical trials and propose potential solutions to streamline the use of MSCs in routine clinical applications and regenerative medicine.
Graphical abstract
Keywords: Stem Cell Therapy, Precision Medicine, Regenerative Medicine, Personalized Medicine, Gene and Stem Cell Therapy, iPSC
Introduction
Stem cells have promising features such as self-renewal, and the capability of giving rise to cells of various lineages. Thus, they have been broadly explored in the therapies to treat various human diseases such as type 1 diabetes, neurodegenerative diseases, e.g., Parkinson's disease (PD), Alzheimer's disease (AD), spinal cord injury, and cancers. There are three major groups of stem cells that have been mainly used for such therapeutic purposes, including embryonic stem cells (ESCs), induced pluripotent stem cells (iPSC), and adult stem cells such as mesenchymal stem cells (MSCs). ESCs are pluripotent cells derived from the inner cell mass of blastocytes and have the potential to induce cell types of all three-germ layers. However, the use of ESCs in clinical practice has a major limitation related to ethical concerns as the generation of ESCs is linked to the use of germ cells and destruction of human embryos [1]. The iPSCs technology was first described in 2006 in mouse cells [2] and later successfully reported for human cells in 2007 [3]. The characteristics of the iPSCs are generally similar to those of the ESCs and have been shown to have great potentials for cell therapy (see reviews [4–6]). However, the use of iPSCs for cell therapy also has concerns and challenges. The potential of tumorigenicity caused by the non-complete differentiation, accumulation of genetic mutations, epigenetic abnormalities, expression of the reprogramming factors (if the iPSCs were generated by integrated plasmids or viral vectors) are considered as the major challenges. Apart from this, immunogenicity caused by long-term in vitro culture and heterogeneity of within and between different iPSC clones greatly hamper the use of iPSCs in clinical applications [6–13]. Adult stem cells are multipotent stem cells existing in partial or fully differentiated prenatal, fetal, adult tissues, such as mesenchymal stem cells (MSCs) (found in placenta, umbilical cord, umbilical cord blood, adipose tissue, bone marrow, dental pulp, and other tissues), haematopoietic stem cells (HSCs), muscle stem cells (MuSCs, also known as satellite cells). In this review, we focus on MSCs, which have been reported to have been derived from different tissue sources and are broadly applied. For the latest advances, applications, and challenges in using HSCs and MuSCs for cell therapies, we refer readers to the recent reviews on the topics [14–18]. MSCs can be derived from a broad range of tissues such as adipose tissue [19], amniotic fluid [20], bone marrow [21], dental pulp [22], peripheral blood [23], umbilical cord [24], and umbilical cord blood [25]. In addition to tissue-derived MSCs, we previously developed a simple method for differentiating ESCs and iPSCs into functional MSCs [26, 27]. This method is based on switching ESCs/iPSCs from stem cell medium to MSCs medium and cultured for 2–3 weeks. We demonstrated that the iPSC-derived MSCs are capable of osteogenesis and chondrogenesis efficiently, lack of tumorigenic and adipogenic capacities [26, 28]. We also showed that these iPSC-derived MSCs (iPS-MSCs) can be used with biodegradable 3D materials and nanomaterials for tissue engineering purposes [28]. This broad availability of MSCs (including iPS-MSCs) makes them a promising and attractive cell source for regenerative medicine.
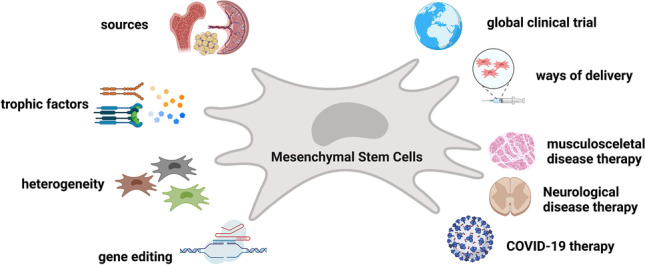
However, the use of MSCs is not without limitations. An increasing number of studies reveal that MSCs are highly heterogeneous. They are a heterogeneous cell population containing cells of different multipotential properties, progenitors, and cell states. Taking this into account, the International Society for Cellular Therapy (ISCT) established unique criteria that apply for all MSCs isolated from different sources [29]. Based on the ISCT criteria for MSCs, authentic human MSC-like cells must express certain MSC positive surface markers 5′-nucleotidase (CD73), Thy-1 (CD90), and Endoglin (CD105) and they must lack the expression of macrophage marker CD14, HSC marker CD34, lymphocyte marker CD45, B cell marker CD19, B-cell antigen receptor complex-associated protein alpha chain CD79a and MHC class II cell surface receptor HLA-DR. In addition to the expression fingerprint, the cells must also show capacities of differentiating into adipogenic, osteogenic, and chondrogenic lineages in vitro [29]. In addition to these characteristics, MSCs secrete bioactive factors that favor tissue remodeling and repair, as well as immunoregulatory properties. These regenerative characteristics of MSCs collectively made them the most broadly tested adult stem cells in clinical trials (summarized in Fig. 1). According to researcher-initiated registered data from the US National Institutes of Health (https://clinicaltrials.gov/), there has been a rapid development of cellular therapy during the last decade using MSCs in clinical trials. The number of MSC-based clinical trials has doubled over the last five years. As of July 14th, 2021, 1014 MSCs-based clinical trials have been registered in ClinicalTrials.gov database either as completed or in process.
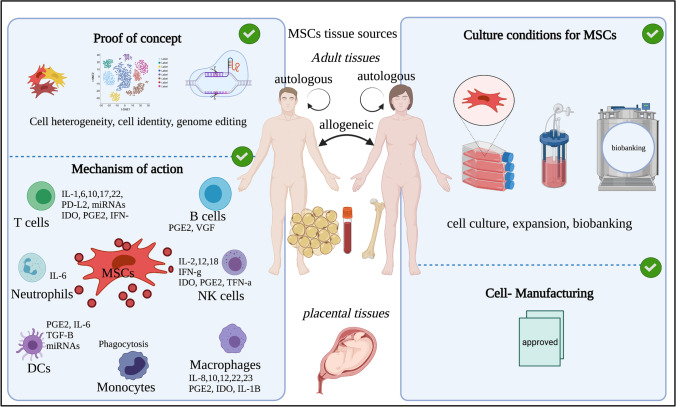
Fig. 1.
Schematic illustration of MSC sources and four focusing areas. Along with immune cells, MSCs isolated from various tissues show immune modulating functions. MSCs heterogeneity, application of single cell RNA sequencing, and gene-editing are three proof of concepts used as advanced technologies in deciphering MSC uniqueness (central and right). Cell culture, expansion and biobanking are standard procedures to establish good laboratory practices for further use in clinical practice (left). The figure was prepared with BioRender
In this review, we summarize the biological and regenerative properties of MSCs, their therapeutic effects that reached the clinical investigational phases on a broad scale. We provide a global overview and trends in MSC-based therapy. As examples, we highlight a few studies conducting clinical trials for therapy of musculoskeletal, neurological and pneumological (COVID-19) diseases. We discuss the great potential on revisiting the MSC heterogeneity using single cell RNA sequencing. We also highlight the potentials and limitations of genetically engineered MSCs derived from iPSCs for the development of personalized regenerative medicine.
The Biological and Regenerative Properties of MSCs
A Brief History of MSC Nomenclature
There are several nomenclatures for MSCs all with identical meaning. Each one indicates a subtype of multipotent mesodermal cells capable of giving rise to stromal cell lineages. These nomenclatures include the Mesodermal Stem Cells closely related to the embryonic chick limb bud mesodermal cells (ECLBMCs) by Arnold Caplan in 1970s [30], Marrow Stromal Cells defined by Owen in 1988 [31], Mesenchymal stem cells (MSCs) defined by Arnold Caplan in 1991 [32], Multipotent Stromal Cells in 2001 [33], Mesenchymal Stromal Cells defined by a group of scientists at ISCT in 2005 [34], and Medicinal Signaling Cells defined by Arnold Caplan in 2017 [35]. The call to change the name of Mesenchymal Stem Cells (MSCs) to Medicinal Signaling Cells results from a better understanding of the mechanisms of action. At the embryonic and fetus stages, MSCs are derived from the mesoderm and can gives rise to skeletal tissues such as cartilage, bone, tendon, ligament, stroma cells in the bone marrow, and connective tissues. In adult tissues, the regenerative capacity of tissue-resident MSCs were confirmed by a significant number of studies demonstrating that these cells could be further differentiated in vitro into stromal cells such as adipocytes, chondrocytes, myoblasts and osteoblasts [36–39]. However, in most MSC-based cell therapies, MSCs contribute to tissue repair and regeneration through the secretion of trophic factors rather than the differentiation into new cell types. The call for changing the name of MSCs to Medicinal Signaling Cells by Caplan was supported by Daniel B F Saris and colleagues [40]. Despite all the efforts in refining the nomenclature of MSCs, the scientific community is still largely using Mesenchymal Stem Cells for MSCs. Between 2017 and 2021 (according to PubMed, November 2021), there are over 27,000 scientific publications published using the name of “mesenchymal stem cells”, while there are only 4,000 published with “mesenchymal stromal cells” and 29 published with “medicinal signaling cells”. Since nearly all registered clinical trials are based on “mesenchymal stromal cells” or “mesenchymal stem cells”, the summarize of MSC-based applications are referred to with these two terms.
Sources of MSCs
One major advantage of MSCs is its broad availability. MSCs have been reported to be isolated from embryonic, prenatal, fetal and adult tissues and can give rise to multiple lineages under defined culture conditions. The main sources of MSCs isolated after the birth are cells from umbilical cord and placental tissues. Sources of MCSs from adult tissues are mainly bone marrow [21] and adipose tissue [19] (Fig. 1). Bone marrow-derived MSCs (BM-MSCs) were applied as the most common source 20 years ago, however, umbilical cord-derived MSCs (UC-MSC) and adipose tissue-derived MSCs (AT-MSCs) have also been widely explored. According to registered studies in ClinicalTrials.gov, all these three types of MSCs are broadly tested (Fig. 2, right). Compared to UC-MSCs, it is relatively easier to collect BM-MSCs and AT-MSCs autologously from the patients. However, important elements such as the health status of the patient, age, gender, somatic mutations, and the consequences of the relatively invasive procedure of isolating BM-MSCs and AT-MSCs, must be considered. The cryopreservation of UC-MSCs, AT-MSCs, MSCs from dental pulp and from other sources are commercially exploited in developed countries (Fig. 1, right). MSCs isolated from different sources exhibit distinct characteristics, known as tissue sources-associated heterogeneity. For instance, compared to BM-MSCs and AT-MSCs, UC-MSCs show higher proliferation ability, lifespan, and differentiation ability [41–43]. However, the therapeutic effects of UC-MSCs-derived trophic factors and vesicles are currently under extensive investigations. It seems that UC-MSCs are rich in angiogenic factors that makes it attractive alternatives to BM-MSCs secretome [44]. In addition to tissue-derived MSCs, we previously described methods and protocols for generating functional MSCs from pluripotent stem cells (ESCs and iPSCs) [26, 28, 45]. A great advantage of iPS-MSCs or ES-MSCs is that a large number of cells can be generated using the in vitro 2D or 3D culture systems, which is an important criterion for cell therapy. However, it should be noted that we consistently observed that these iPS-MSCs or ES-MSCs are less potent with regard to adipogenesis [28].
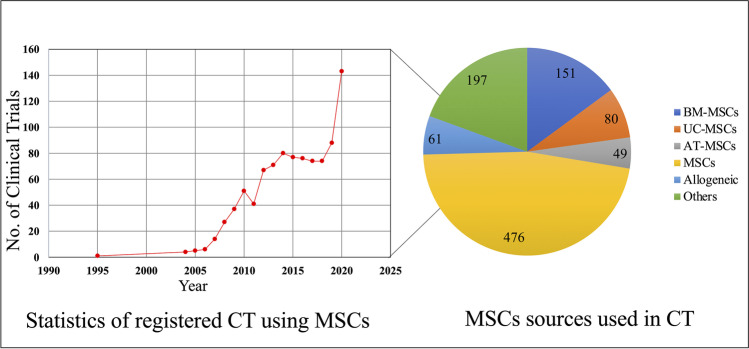
Fig. 2.
Overview of registered MSC based clinical trial growth and cell source. A Line plot yearly registered mesenchymal stem cells-based clinicals trials at ClinicalTrials.gov since the first use in 1995 up to 2020. B Pie chart of number of clinical trials based on main sources used: BM-MSCs, UC-MSCs, AT-MSCs. 476 clinical trials did not disclose the sources of the MSCs, or the cells source is named as allogenic (61). Others refers to cells derived from tissue such as placenta, dental pulp, amniotic mesenchymal stem cells and other
Mechanism of Action
Initially, it was believed that therapeutic effects of MSCs were attributed to their homing ability to migrate and graft in target tissues. However, a large number of studies later showed that MSCs possess biological and regenerative effects mostly due to their secreted trophic (regenerative) factors that mediate cell-to-cell communications, regulate cell proliferation/differentiation, and anti-inflammatory properties [46–51]. By producing extracellular vesicles (EVs), cytokines, and growth factors, MSCs have demonstrated excellent potential to modulate both adaptive and innate immune system responses. These findings are supported by in vitro, and in vivo experiments, as well as by clinical data that shows a complex network of interactions between immune cells and MSCs (Fig. 1, left).
The immune modulating functions of MSCs are via secreted paracrine factors such as the beta fibroblast growth factor (bFGF), the insulin-like growth factor 1 (IGF-1), the vascular endothelial growth factor (VEGF), the epidermal growth factor (EGF), the tissue inhibitor of metalloproteinase-1 (TIMP-1), progranulin, and the brain-derived neurotrophic factor (BDNF) (also see review [52]), or by direct cell-to-cell communications with various immune cells such as T cells, B cells, natural killer (NK) cells, macrophages, monocytes, dendritic cells (DCs), and neutrophils, stimulating or suppressing the immune responses [53–57]. MSCs also have a specific function known as “sensor and switcher of the immune system”. This function controls the precise switch to promote inflammation when the immune system is underactivated or hold inflammation when immune system is overactivated [53]. The constant interaction between MSCs and the immune system is key to balancing inflammatory responses and maintaining tissue homeostasis. Several types of synergistic communication between MSCs and immune cells play important roles in clinical applications of MSCs. There is increasing evidence that MSCs play an important role in both innate and adaptive immunity. For instance, MSCs can inhibit CD4+ (helper) and CD8+ (cytotoxic) T cell [58], affect B cell functions through cell-to-cell contact [59], suppress the proliferation and cytotoxicity of NK cells, as well as increase regulatory T cells (Tregs) generation via cell communication and soluble factors both in vitro and in vivo [60–62]. MSCs also express several surface adhesion molecules including integrins, vascular cell adhesion molecule 1 (VCAM-1), intercellular adhesion molecules 1 and 2 (ICAM-1, ICAM2), CD72, and CD58 (LFA-3), which have high binding affinity to T cells and they play important roles in immune suppression [63].
Recently, a number of preclinical and clinical trials show that although MSCs and regulatory T cells (Tregs) are two distinguish cell types, they share common properties such as suppression or modulation of harmful immune responses, with the result that these two synergistic cell types are recently focused in translational research especially in inflammatory diseases. Tregs are suppressor T cells regulating immune response, preventing inflammation or inflammatory reaction [64]. By investigating the effects of BM-MSCs infusion on collagen antibody-induced arthritis in mice, Nam et al. found that MSCs can migrate to inflamed tissues and promote the differentiation of CD4 + T cells to Tregs [65]. Similar findings were reported by Reux and colleagues, which showed that functional CD4 + FOXP3 + Tregs were induced when co-cultured with human induced pluripotent stem cell-derived MSCs (hu-iPS-MSCs) in vitro. These findings were confirmed in vivo when hu-iPS-MSCs were administered in a humanized mouse model [66]. Several clinical studies have also reported that Tregs were increased after administration of either autologous or allogeneic MSCs in liver transplantation [67], osteoarthritis [68], kidney transplantation [69], Crohn’s disease [70], systemic lupus erythematosus [71], Type 2 diabetes [72], and chronic graft versus host disease [73]. Wang and colleagues treated intravenously thirty systemic lupus erythematosus patients with allogenic UC-MSCs and reported significant elevation in the percentage of Tregs [71]. It is important to highlight that although there are clinical trials targeting MSCs and Tregs, mechanisms of MSCs/Treg cross talk are still not well understood. More randomized, placebo-controlled clinical studies are needed to determine the conditions under which MSCs administration can induce Treg differentiation and expansion.
MSC Secretome (Trophic Factors)
MSCs can secrete the biologically active molecules such as growth factors, chemokines, cytokines, cellular adhesion molecules, interleukins, hormones, extracellular vesicles, lipids, proteins, microRNAs and different DNA materials to remodel tissue microenvironment. These trophic factors have pro-regenerative effects through modulating immune system, inhibiting cell death and fibrosis, stimulating vascularization, promoting tissue remodeling and repair, promoting wound healing [52]. The trophic factors that are usually released are transforming growth factor-b-1 (TGF- b-1), tumor necrosis factor-α (TNF-α), PGE2, IFN-γ, hepatocyte growth factor (HGF), fibroblast growth factor (FGF), indoleamine-pyrrole 2,3-dioxygenase (IDO), vascular endothelial growth factor A (VEGF-A), and nitric oxide [74]. The paracrine factors are released in encapsulated lipid bilayers called extracellular vesicles (EVs) and based on their size or cell of origin are divided to exosomes, microvesicles and apoptotic bodies [75–77]. Although EVs display immunoregulatory functions similar to MSCs [78], their paracrine actions differ by the source of the MSCs, the microenvironment surrounding the cells and the target cells [79]. In addition, EVs have better safety profiles than MSCs as ECs are cell-free, more immunocompatible, and capable of bypassing the blood–brain-barrier (BBB) providing a delivery vehicle to supply essential bio-components to the central nervous system [80].
An increasing number of studies show that many of the therapeutic effects of MSCs may be the result of the paracrine factors secretion of EVs, rather than cellular engraftment and response to the site of injury [81, 82]. MSC-EVs cannot self-replicate, thereby becoming attractive cell-free and safer therapeutic sources because uncontrolled cell division and risks of contamination with other cells are prevented [83]. MSC-EVs have been tested in several clinical trials for the repair and regeneration of lung (clinical trials numbers: NCT04313647, NCT04276987, NCT03857841), cartilage (NCT04223622), brain (NCT03384433), skin (NCT04173650), Type-1-diabetes (NCT02138331), refractory molecular holes (NCT03437759), acute ischemic stroke (NCT03384433), and kidney injuries (NCT04700631). Although there is evidence of the beneficial effects of EVs, long-term toxicity and immunogenicity on the human body, investigation should be considered to determine if EVs might trigger immune responses or toxic reactions. Gene editing technology with big-data analysis of transcriptome and proteome analysis is essential prior to undertaking clinical applications, in order to produce safe, reproducible, and cost-effective products that must be the goal for both standardized and optimized products.
MSC Heterogeneity
The existence of MSC heterogeneity is well acknowledged, as the MSCs from different tissues express tissue-specific genes and exhibit various differentiation potentials [84–86]. However, it is important to look closely into single-cell identity concept such as phenotype, lineage and state that might provide new angles for understanding and manipulating cell fate and thus enable use of MSCs in clinical studies more accurately. Bulk RNA sequencing analysis of heterogeneous cell populations has been used for decades. Mixing signals from different sub-populations, and thus masking identities of new or rare cell populations, limiting us to precisely identify phenotype of each individual cells. With development of single cell RNA-sequencing (scRNA-seq)[87], it is now possible to bridge the gap between studies on individual cells and bulk cells and reveal the transcriptome of each analyzed cells. scRNA-seq can profile the transcriptome of millions of individual cells in each tissue. With the development of computational tools, it becomes possible to reduce the complexity, and cluster the cells based on their genome-wide gene expression similarity [88]. Initially, this technique was mostly used to identify the heterogeneous cell populations in cancers, brain, liver, and other tissues, but recently it has become a useful tool for stem cell research enabling us to distinguish the transcriptional difference at the single-cell level for further deciphering the heterogeneity of stem cells and progenitor cells [89–91].
In vitro cultured MSCs and MSCs derived from different tissue sources are known to be heterogenous, however, the understanding of their heterogeneity is very limited. By single cell RNA sequencing of cultured UC-MSCs and BM-MSCs, Barret and colleagues identified 436 significantly differentially expressed genes involved in immunomodulation and other biological processes between UC-MSCs and BM-MSCs. Most importantly, UC-MSCs contained more than ten different cell clusters which showed expression variations in the genes that encode extracellular matrix proteins, protein expressions or cell cycle-regulations [92]. Most of the cell subtypes in cultured MSCs are characterized by different cell-cycle states. Unlike cultured MSCs, single cell RNA sequencing of freshly isolated UC-MSCs only depicted two different subgroups, suggesting that UC-MSCs give rise to more subpopulations while expanded in vitro. In addition, these cells in a 2D culture microenvironment might lose their original gene expression patterns and activities [93]. Phenotypic expressions might increase in 2D culture due to uses of various naturally derived or engineered biocompatible extracellular matrices that can determine MSCs fate and lineage specification through diverse signal transduction present in such microenvironment. MSCs adhesion molecules such as integrins play important role in mechanotransduction which triggers a specific intracellular signaling response and might be responsible for broaden heterogeneity in the 2D cell culture system [94].
In addition to these studies, it is both necessary and of great interest to compare the therapeutic effects of these different clusters of MSCs. It is of substantial value and clinically relevant to explore the heterogeneity of MSCs, which is determined by multiple factors including donors and tissue sources, health status of the donors, cell heterogeneity, protocols, as well as reagents used for isolation, culturing, cryopreservation, and thawing. In addition to tissue sources, MSCs isolated from different individuals might have distinct characteristics due to differences in gender, age, genetics, and health conditions [95–98]. As mentioned, cells derived from different sources such as adipose, umbilical or bone marrow tissues might vary in terms of cell purity, sub-populations, differentiation capacity and even the expression level of MSC markers. In addition, MSC heterogeneity exhibit both inter-clonally and intra-clonally variations that influence gene expressions depending on the cell-to-cell communications and locations. For example, cells found in the dense inner location express more extracellular matrix genes such as (VCAM-1) and outer cells on the border have higher expression of genes related to proliferation (MKI67 and PODXL) [99]. Depending on the heterogeneity, the MSC morphology and differentiation capacity can be remarkable different. Choosing proper culture conditions to maintain multipotency is an important step and the majority of the MSCs subpopulations have distinct surface markers, therefore fully characterize biological functions and biomarkers are essential for the application of MSCS in clinical therapies.
Genetically Engineered MSCs
While naturally isolated, MSCs have already proven to be an attractive source of cells for clinical therapies, due to the great heterogeneity of MSCs. Genetically engineered MSCs with enhanced regenerative capacity have also been regarded as attractive cell sources for disease therapy [100]. Freshly isolated MSCs from adult tissues cannot be propagated by too many passages in order to preserve their multipotent and trophic properties. We reason that one promising solution is to introduce desired genetic modifications in human iPSCs using i.e., the CRISPR-Cas9 gene editing technology, followed by differentiating the genetically edited iPSCs into MSCs [26]. Indeed, CRISPR/Cas9 has been used and proved to be effective in several MSC-based applications [101–106]. In addition, it has also been described that high passage numbers can negatively affect the MSCs self-renewal and multipotent activities [107]. To develop cell therapeutics with gene-edited stem cell lines, it is vital to develop and maintain safety standards with criteria that include precise off-target checks, high efficiency, and reproducibility of data. Pioneering clinical trials have used genetically modified MSCs in treatment of gastrointestinal adenocarcinoma and Kabuki syndrome. TREAT-ME1, an uncontrolled, single-arm phase I/II study was the first reported in human clinical study using genetically modified BM-MSCs for advanced, recurrent, or metastatic gastrointestinal or hepato-pancreato-biliary adenocarcinoma. The study was designed to evaluate the safety, tolerability, and efficacy of the GM-MSCs [108]. Another study, registered under the ClinicalTrials.gov, number NCT03855631, used patient fibroblasts that are initially reprogramed into MSCs followed by gene editing with CRISPR/Cas9 for the treatment of Kabuki syndrome. Mutations in either KMT2D gene (in up to 80% cases) or KDM6A gene can cause the rare Kabuki disease [109]. The Epi-KAB therapy has been developed for the disease caused by the KMT2D mutation to restore MLL4 activity. The plan of this clinical trial is to enroll 8 patients in a Phase I study.
Although a large number of therapeutic benefits have been described for MSCs, isolation of MSCs from autologous tissue sources might be limited due to the patient age, health status, invasion methods, or tissue availability at the moment of the treatment. To overcome this problem, we previously described a simple and efficient method for generating personalized and functional MSCs [26]. This approach is based on reprogramming patient-specific fibroblasts into iPSCs, followed by differentiating iPSCs into MSCs with a simplified protocol. This method offers a promising approach for large-scale expansion, cell banking, and compatibility of comprehensive gene editing [26, 28, 45, 110]. Unlike cell replacement therapies, most MSC-based therapies are centered on the MSC-derived products, such as exosomes. The potential off-target effects caused by CRISPR/Cas9 are not as fragile as other CRISPR-mediated direct gene therapy applications. To date, several studies have been reported to have achieved enhanced therapeutic effects using CRISPR/Cas9-edited MSCs. For example, by disrupting the oxidative and electrophilic stress gene Keap1, enhanced anti-oxidative ability and viability after grafting were achieved in AT-MSCs [111]. Since the mechanism of action of MSCs are largely due to their release of trophic factors, genetically engineered MSCs with overexpression of BMP-4 [112] and BMP-9 [113] have been generated to enhance the osteogenic potential of MSCs and in bone regeneration.
Overview of MSC-Based Clinical Trials
Global Trends
Analysis of clinical trials using MSCs in the past three decades offers a comprehensive overview of the actual number and content of clinical trials involving this promising cell type. According to the ClinicalTrials.gov, 1014 studies were registered using predefined criteria “mesenchymal stem cells” as of our search date: July 14th, 2021. There has been a significant increase in registered clinical trials since the first one reported in 1995. In the first decade (from 1995 to 2005), there were only 9 registered clinical trials. From 2006 to 2015 clinical trials increased 500 worldwide, and almost same number of new entries were reported between 2016–2022, of which 53 trials are registered to begin in 2021 and 2 in 2022 (Fig. 2, left). It is worth mentioning that rapid increase in clinical trials was observed in 2020. Notably, with more precise analysis, most MSC-based clinical trials (n = 65) were registered for the treatment of COVID-19. Although vaccinations have started already in many countries around the globe, effective therapy to treat or significantly reduce the severity of COVID-19 is still needed. Treatments that could reduce the fatality rate and improve recovery of critically ill patients are in high demand. MSCs offer a promising solution for COVID-19 therapy due their multifunctional mode of actions, including release of various growth factors such as keratinocyte growth factor (KGF), prostaglandin E2 (PGE2), granulocyte–macrophage colony-stimulating factor (GM-CSF), IL-6, and IL-13 that ease the phagocytosis, activate alveolar macrophages, might alter the cytokine secretion profile of dendritic cell subsets, and decrease the release of interferon γ from natural killer cells [114].
We next explored the global distribution of clinical trials using MSCs shown in Table 1. Overall, 294 clinical trials were conducted in China, 216 in the USA, 78 in South Korea, 70 in Spain, 39 in Iran, 29 in France, Indonesia 21, Jordan 20 and a few in other countries registered in clinical trials database. Many of these clinical trials are carried out between different centers in different countries, emphasizing the importance of international collaboration and the inclusion of more diverse populations. When focusing on interventional studies, there are 289 studies registered by research groups in China, followed by USA (192), South Korea (70), Spain (69), Iran (39), and other countries as shown in Table 1. For observational studies 11 are from USA, 8 from South Korea, 5 from China, 3 from Italy, 2 from Germany, and 1 from Belarus, Canada, Columbia, Egypt, France, Spain, Turkey, and Thailand. Most of the studies performed in China are interventional which indicates that use of the MSCs cells in clinical practice is larger than the other cell products such as iPSCs which had far fewer clinical studies and were mostly observational [115].
Table 1.
Number of interventional and observational clinical trials based on countries. Data collected by July 14th, 2021
| Country | All clinical study | Percentage | Interventional | Observational |
|---|---|---|---|---|
| China | 294 | 28.50% | 289 | 5 |
| United States | 216 | 20.90% | 192 | 11 |
| South Korea | 78 | 7.60% | 70 | 8 |
| Spain | 70 | 6.80% | 69 | 1 |
| Iran | 39 | 3.80% | 39 | 0 |
| France | 29 | 2.80% | 28 | 1 |
| Indonesia | 21 | 2.00% | 21 | 0 |
| Jordan | 20 | 1.90% | 20 | 0 |
| Brazil | 18 | 1.70% | 18 | 0 |
| Italy | 16 | 1.50% | 13 | 3 |
| India | 16 | 1.50% | 16 | 0 |
| Germany | 15 | 1.50% | 13 | 2 |
| Egypt | 15 | 1.50% | 14 | 1 |
| Turkey | 14 | 1.40% | 13 | 1 |
| Poland | 13 | 1.30% | 13 | 0 |
| Belarus | 13 | 1.30% | 12 | 1 |
| Russia | 12 | 1.20% | 12 | 0 |
| Netherlands | 11 | 1.10% | 11 | 0 |
| Denmark | 11 | 1.10% | 11 | 0 |
| Canada | 10 | 1.00% | 9 | 1 |
| United Kingdom | 9 | 0.90% | 9 | 0 |
| Malaysia | 9 | 0.90% | 9 | 0 |
| Pakistan | 8 | 0.80% | 8 | 0 |
| Israel | 8 | 0.80% | 8 | 0 |
| Vietnam | 8 | 0.80% | 8 | 0 |
| Czech Rep | 5 | 0.50% | 5 | 0 |
| Colombia | 5 | 0.50% | 4 | 1 |
| Australia | 4 | 0.40% | 4 | 0 |
| Norway | 4 | 0.40% | 4 | 0 |
| Chile | 4 | 0.40% | 4 | 0 |
| Greece | 4 | 0.40% | 4 | 0 |
| Panama | 4 | 0.40% | 4 | 0 |
| Mexico | 4 | 0.40% | 4 | 0 |
| Japan | 4 | 0.40% | 4 | 0 |
| Austria | 3 | 0.30% | 3 | 0 |
| Switzerland | 3 | 0.30% | 3 | 0 |
| Tobago | 2 | 0.20% | 2 | 0 |
| Kazakhstan | 2 | 0.20% | 2 | 0 |
| Thailand | 2 | 0.20% | 1 | 1 |
| United Arab Emirates | 1 | 0.10% | 1 | 0 |
| New Zealand | 1 | 0.10% | 1 | 0 |
| Andorra | 1 | 0.10% | 1 | 0 |
| Ecuador | 1 | 0.10% | 1 | 0 |
| Lebanon | 1 | 0.10% | 1 | 0 |
| Bangladesh | 1 | 0.10% | 1 | 0 |
| Singapore | 1 | 0.10% | 1 | 0 |
| Ireland | 1 | 0.10% | 1 | 0 |
| Slovenia | 1 | 0.10% | 1 | 0 |
| Cayman Islands | 1 | 0.10% | 1 | 0 |
| Total | 1033 | 100 | 983 | 37 |
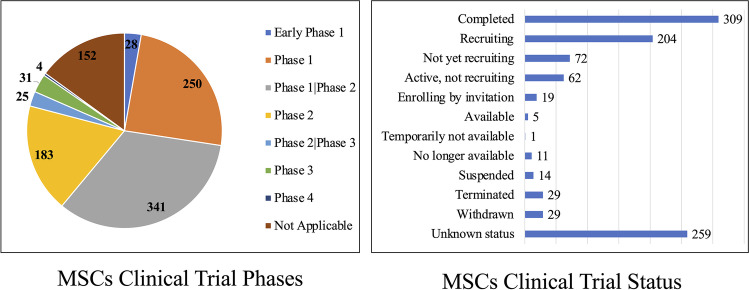
Among all the registered clinical trials, 28 were registered as early Phase I, 250 as Phase I, 341 studies as Phase I/Phase II, 183 studies as Phase II, 255 studies as Phase II/III, 31 studies as Phase III, and 4 studies as phase IV clinical trials (Fig. 3, left). Among the 31 clinical trials in phase III, there is a double-blind study (NCT01541579) conducted at nearly 50 hospitals in Europe and Israel, which investigated the treatment of 212 Crohn’s disease and perianal fistula patients with allogeneic adipose-derived stem cells (Cx601). After one year, the follow up study, showed that Cx601 was safe and effective compared to a placebo. Results from this clinical trial were published in 2018. More post clinical studies observations are needed several years after the treatment [116]. Another study on retinitis pigmentosa treated by Wharton's jelly-derived mesenchymal stem cells, clinical study no. NCT04224207. One-year after the treatment. The study confirmed that transplantation of Wharton’s Jelly MSCs was safe and effective on slowing or stopping the disease progression [117]. Regarding clinical trial status, more than 300 clinical trials were completed following 259 studies with unknown status and 204 recruiting (Fig. 3, right). There is also a notable number of studies terminated (29) or suspended (14) with no explanation given on the reasons for these decisions. In all these registered clinical trials, there were 55,733 registered participants, 18,215 were recruited by clinical trials conducted in China, followed by USA (15,411), South Korea (2,518), Spain (2,348) and other countries (Fig. 4).
Fig. 3.
Overview of registered MSC-based clinical trial phase and status. A. Pie chart distribution of clinical trials according to investigation phases. B. Bar blot of clinical trials according to the study status. Notably, a large number of clinical trials are lacking updates on the progressing status. All data was obtained by July 14th, 2021
Fig. 4.
Overview of the number of registered participants for MSC-based clinical trials according to countries. Data was collected by July 14th, 2021
MSC Delivery
Several administrative routes have been used in MSC clinical trials (Fig. 5A). Intravenous, subcutaneous, and intraperitoneal injections are systemic routes, which are applicable to diseases occurring in the whole body. Other administrative routes are local delivery for more specific disease conditions. For example, surface application is mainly used in dermatology area where the risks are the lowest. Intracardial injection are mostly for cardiovascular diseases, while intra-articular injection is likely for knee injuries and osteoarthritis. These two administration methods require precision and great care. For most trials, MSCs was dosed at the lesion site except when systemic methods were employed. Dense tissue such as bone or cartilage requires more sophisticated procedures. We found that hundreds of clinical trials used intravenous infusion delivery [118]. One systematic review provided by Lalu and colleagues gave comprehensive meta-analysis overview of more than 1000 patients that confirmed no biological link between the MSCs administration and development of acute inflammatory related toxicity, organ system complications and failure, infection, malignancy, or death confirming that intravenous delivery is safe and reliable [119]. In a later study of more than 1400 patients involved in 70 clinical trials, adipose-derived MSC therapies were proven safe and only few side effects had been reported with a follow up of 3 years after the first administration [120]. Severe case of thromboembolism in the lungs was reported in one case after 4 weeks of intramyocardial stromal vascular fraction injection in a patient with class II heart failure based on the New York Heart Association Functional Classification [121].
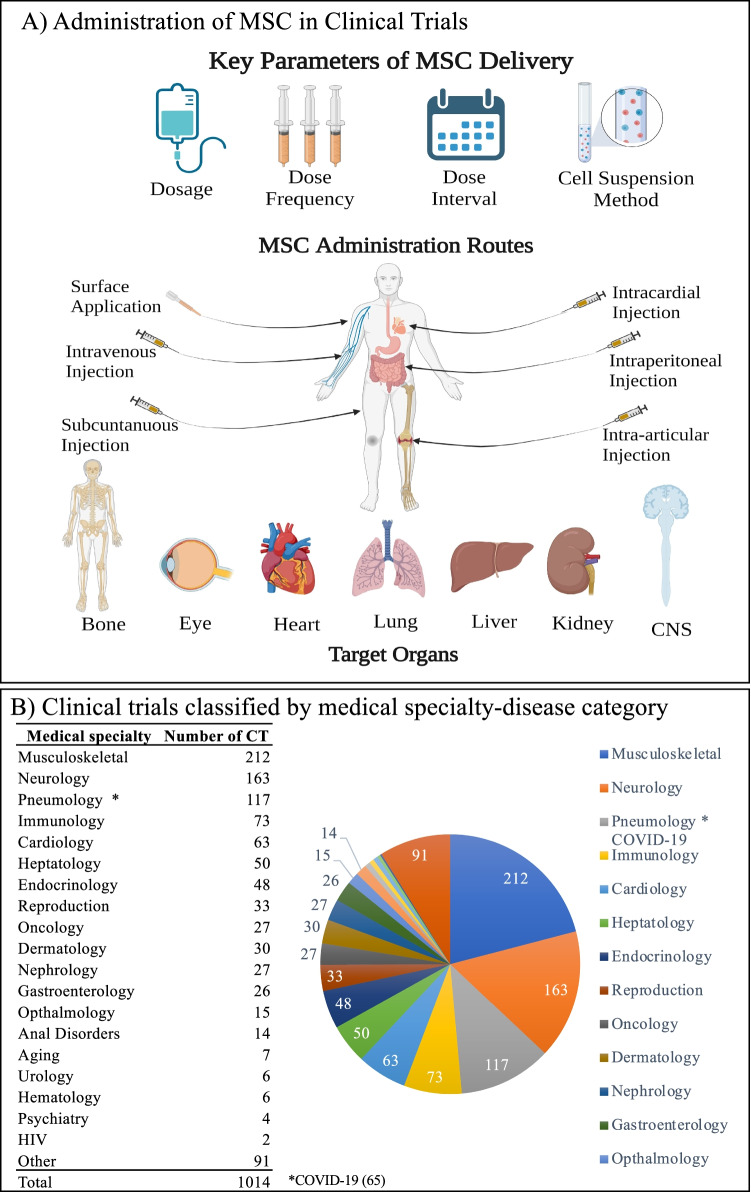
Fig. 5.
Overview of the administration and targeted diseases for MSC-based clinical trials. A. Four key administration (delivery) decision-determining are dosage, dose frequency, interval, and suspension method. Six administration routs and organs targeted by these clinical trials are highlighted. B. Overview of the types of diseases targeted by these registered clinical trials. Data collected by July 14th,2021. For Pneumological disease, approximal half of the clinical trials are registered for treatment of COVID-19. Pie chart was shown to the right. Part A) was prepared using by BioRender
There are also several techniques for delivering MSCs directly into specific area of organs and tissues. For example delivery of MSCs has been shown to be achievable through direct syringe injection to ventricle under visual control that is usually guided by specific catheter delivery NOGA system, which is shown to be minimally invasive [122].
Dosage, dose frequency, intervals and suspensions are important parameters for MSC administration. The dosages reported in many clinical trials are heterogeneous depending on the route of injections [123]. The MSC dosages are usually decided by the patient’s condition and therapeutic properties. In MSC clinical trials, dose frequency, interval and dosage vary significantly. The dosages are typically described in cells/kg body weight (0.5–12 × 106 cells/kg as a single dose). Allogeneic MSCs doses might go up to 1 × 108 and in follow-up studies performed after a year after administration, there are no serious adverse contraindication found in the patients with sepsis, ischemic heart disease, perianal fistula, spinocerebellar ataxia, rheumatoid arthritis, or severe osteoarthritis [116, 124–128]. There are also several reports that include more than 2 × 108 MSCs, but at this moment, we cannot define exact criteria since there are so many different diseases and conditions that required a specific and precise approach and related decisions for each disease.
It has been also documented that the dosage would start with a lowest dose and gradually increased if there were no severe adverse side effects [129]. Many early-phase MSCs trials only administered one dose to achieve simplicity as well as to reduce the cost. On the other hand, some chronic indications such as osteoarthritis and chronic liver failure require multiple doses in order to ensure an effective treatment. For example, there are several studies confirming that dose administration and intervals have to be carefully designed. For example, in one study (NCT02223897), 66 patients, suffering from Ischemic-type Biliary Lesions, received UC-MSCs once per week for the first month and once per month for 6 months (9 times in total), at a dose of 1 × 106 cells/kg body weight. Another study, registered with ClinicalTrials.gov, number NCT01933802, used BM-MSCs derived neural progenitor cells in 20 patients with very progressive Multiple Sclerosis (MS) in 3 separate doses with up to 1 × 107 cells/dose in 3 months. Alcoholic liver cirrhosis (NCT01875081) study used injections of MSCs twice a year, and demonstrated that MSCs could be used as a potential supplementary therapeutic tool, however the precise mechanism for fibrosis reduction and improvement of liver function over a year was not completely elucidated. Further studies including phase 3 are needed to confirm effectiveness of the cells treatment in improving survival and outcome of the patients [130].
Administration of single cell suspension is another important aspect that must be considered. Henry and colleagues performed intramyocardial adipose-derived MSCs injections in 17 chronic ischemic cardiomyopathy patients, and 2 patients suffered heart failure, however the cause was not directly associated with the procedures [131]. The study did not provide evidence if the cells were filtered before administration to ensure that the injected cells were in single cell suspensions. Thrombosis and embolism complications might occur when injected cells are not in single cell suspensions, especially if they form cell clumps that can be particularly dangerous when intramyocardially injected.
Intravenous injection (i.v.) is the most commonly used route in clinical therapies. It is essential to carefully assess and choose the optimal method for cell transplantation depending on the injured area, injured organ, and networks of local vasculature system. It is essential to peruse preclinical data with the greatest care as there are not yet sufficient clinical trials that compare the efficacy and safety of different routes. Studies on animal models have already shown that cells transplanted via intravenous injection will accumulate in the lung and since it encountered pulmonary impasse could not later move to other organs [132–135]. For example, Eggenhofer and colleagues show that in the first few hours, 60% of MSCs injected intravenously accumulated in the mouse lungs and did not subsequently move to the liver. These studies show that cells were probably eliminated by immune cells [134]. Another study performed by Higashimoto et al. [135] on ConA induced hepatitis mice model showed that intravenously injected GFP-labeled MSCs were accumulated only in the lung but not in the liver. In addition, a further study led by Pang et al. show that intravenous administrated MSCs rapidly undergo apoptosis in the lungs of the mice, demonstrating that dying MCSs have therapeutic effects [136].
In conclusion, it is essential to evaluate in preclinical studies and thoroughly evaluate the condition of patients, progression of diseases, treatment regimens and the potential route risks before choosing the optimal MSC transplantation dose and administration method.
Quality Control
MSCs of high quality are essential for assuring the safety and efficacy in clinical trials. Understanding the molecular and cellular mechanisms of each derived MSC line with the appropriate and complete clinical trials documentation as well as all important factors including careful selection of the patient, treatment protocols and procedures, time, dose, routes and if decided multiple administrations of the selected cells. It is also essential to note that MSCs derived from autologous bone marrow are highly heterogenous and vary from patient to patient due to age, gender, and general health status. This was confirmed by study from Brazil, where researchers reported that BM-MSCs isolated from multiple sclerosis patients, have distinct gene expressions profiles and express immunomodulatory and immunosuppressive activity defects [137]. Yet another study showed that MSCs isolated from hepatitis B patients in a culture environment proliferated very slowly and aged rapidly [138]. Thus, the quality control system needs to include evaluation guidance system and standard based on which would enable investigators to distinguish and select the MSCs from autologous or allogenic sources for further up-scaling and manufacturing. Xie et al., previously reported a quality evaluation system for human UC-MSCs that was tested on 225 patients during a one-year follow-up investigation process. The research group introduced a comprehensive evaluation of MSCs including environmental monitoring, reagent QC, donor screening, cell QC and biological effects for their procedure [139]. Similar standardization and qualification assessment approaches have also reported for AT-MSCs and BM-MSCs [140, 141]. Despite these efforts, there is still a great need for international cooperation to establish a more streamlined and standardized guideline to characterize and accommodate MSCs from all sources [142].
Regulations
Before delivery of the cell products for a clinical use, MSCs need to be processed from bench to bedside according to Good Manufacturing Practice (GMP) which is mainly called biomanufacturing or biobanking, as well as producing Advanced Therapy Medicinal Products (ATMP). The processes include cell culture, expansion and biobanking using variety of protocols that has to be successfully registered to the primary regulatory bodies which has to contain approved regulatory outlines for the use of cell-based medicinal products in a clinical practice. The regulation includes aspects from pharmacological, immunological, and metabolic activity of MSCs products; how cells will be used in regards of their functions in the recipients, whether the MSCs require an in vitro expansion to reach the optimal dosage and how to consider minimal possible manipulation of the cells before the administration. The development of cell-based products is almost the same as drug administered products which involves steps from nonclinical to clinical studies with marketing authorization procedures regulated by different authorized bodies. For a comprehensive overview of the international regulatory frameworks for MSC-based therapy and ATMPs, we refer readers to recent reviews on this topic [143, 144].
Examples of MSC-Based Cell Therapy
MSC-based cell therapy is widely applied in clinical trials of a variety of diseases (Fig. 5B), mostly in the area where current therapies could not produce a complete cure. As shown in Fig. 5B, the number of MSC clinical trials have increased rapidly during the past two years. Most registered MSC clinical trials are for the treatment of COVID-19. Although the minority of these trials have provided published results, it is certain that MSC therapy is a safe and effective therapeutic approach [145]. As shown in Fig. 5B, the health problem which has the largest number of MSC therapeutic clinical trials is musculoskeletal diseases, containing 212 trials around the world. Followed by neurology and pneumology, the numbers of clinical trials are respectively 163 and 117. Other medical specialties involving in MSC clinical trials include immunology, cardiology, hepatology. There are 91 clinical trials could not be categorized in a particular medical specialty, such as sweat gland disease and sepsis. Lately MSC therapy has been a hotspot in the medical area, especially in regenerative medicine. The regenerative and anti-inflammatory functions are the benefits for the patients. Here we describe several successful MSCs clinical outcomes for musculoskeletal, neurological, and pneumological (COVID-19) diseases.
Musculosceletal Diseases
A global disease burden study shows that in 2020 more than 1.71 billion people around the world are suffering from musculoskeletal diseases [146]. MSC-based cell therapy can provide bone and cartilage regeneration for the elder or injured patients. Moreover, the immunomodulation ability of MSCs can ease inflammation symptoms associated with bone degeneration [147]. The most common musculoskeletal diseases treated by MSC trials is osteoarthritis. Osteoarthritis is a degenerative joint disease accompanied with inflammation and pain. Currently, no significantly effective medicine has been invented to restore the structure and function of the joint. Common treatments for osteoarthritis are often limited to symptom and pain control, rather than long-term cure [148]. As a result, osteoarthritis treatment is one of the most focused musculoskeletal diseases in MSC-based clinical trials.
CARTISTEM is an MSC product that composes of allogeneic umbilical cord blood derived MSCs and 4% hyaluronate hydrogel (NCT01041001, NCT01626677). As a promising treatment for knee cartilage defects in patients with osteoarthritis, it was approved for market entry by the regulatory authority of Republic of Korea MFDS in 2012. CARTISTEM was applied to the lesion sites for the restoration of full-thickness cartilage defects by surgical implantation. Lim and colleagues described the result of CARTISTEM phase III multicenter randomized 48-week Clinical Trial and extended 5-Year Clinical Follow-up in 2021 [149]. This clinical trial enrolled 103 participants who had a ICRS (International Cartilage Repair Society) grade 4—single, symptomatic, large, full-thickness femoral condyle or trochlear cartilage defects in the knee. Microfracture was carefully chosen as the active control since previous reported clinical advantages in similar conditions [150, 151]. The participants were randomly divided into two groups at a ratio of 1:1, including the UCB-MSC-HA group (50 participants) and the microfracture group (53 participants). The primary outcome was the proportion of participants who achieved by at least 1 grade on the ICRS assessment at 48-week arthroscopy. 97.7% of the UCB-MSC-HA group reached the primary outcome, while 71.7% of the microfracture group did. Secondary outcomes included multiple parameters, such as histologic assessment, changes in pain visual analog scale (VAS) score, Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC), and adverse events. UCB-MSC-HA group scored higher in histologic assessment in biopsies analysis. Both groups had significantly improved VAS and WOMAC scored at 48 weeks after the treatment. However, no obvious difference was seen between these two groups. During the 5 years extended follow-up study, the previous positive effects of ICRS, VAS and WOMAS in the UCB-MSC-HA group maintained. Whereas in the microfracture group, these scores were deteriorated. However, during the past 5-year follow-up observation, 2 total knee replacements and 1 osteotomy were done in patients in UCB-MSC-HA group. While 3 total knee replacements, 1 osteotomy and 1 meniscectomy were done in microfracture group. The one-time CARTISTEM treatment could not cure the osteoarthritis in some patients. Safety issues were determined by several examinations including physical examinations, laboratory tests, adverse event monitoring and 24-week mixed lymphocyte reaction. There were no differences between the groups in adverse events.
Neurological Diseases
The mammalian neurons have limited regeneration potential. Once they are damaged or degenerated, it is very difficult to regain their functions [152]. There are a huge variety of neurological disorders, mainly nerve injuries and neurodegenerative diseases. The traumatic nerve injuries in the brain and spinal cord have high disability and mortality rates and may induce symptoms in cognition and behavior [153]. It was previously reported that BM-MSCs can cross the blood–brain barrier without any damage [154], migrate into the injury site and improve the neuron regeneration by producing neurotrophic factors. UC-MSCs were also proven to be safe and beneficial of the sensory and motor function recovery and ease the neuropathic pain combing with multiple biomolecule scaffolds [155, 156]. Some neurological disorders including Alzheimer's disease (AD), Parkinson's disease (PD), multiple sclerosis (MS) and amyotrophic lateral sclerosis (ALS) are more common in the elderly, and their prevalence is increasing with higher life expectancies [157]. Pre-clinical and clinical trial studies use various sources of MSCs to treat AD [158, 159], PD, MS, and AML. The pathophysiology of these diseases is still under investigation, but it is hypothesized that these diseases are caused by abnormal proteins aggregation in neurons as a result of aging [160, 161].
163 clinical trials were registered in the ClinicalTrials.gov database using MSCs for the treatment of neurological diseases. Although, many clinical trials have been registered for the use of MSCs or MSCs-EVs products in patients with AD (NCT01297218, NCT01696591, NCT02054208, NCT03172117, NCT03117738, NCT04228666, NCT02600130, NCT04855955), there are very few that have been officially completed and have published results such as NCT01297218 [159]. There are many challenges in using MSCs that need to be addressed including improvements in both pre- and clinical trials such as establishment of the effective animal model and the precise number of injections and delivery routes optimization [162]. With regard to MSCs-EVs administrations, their heterogeneity, isolation and production techniques need to be well established before use in human trials [163]. In addition, it might be necessary to further confirm the safety usage of the MSCs and MSCs-EVs products on a long-term basis.
There are more than 10 clinical trials registered for the treatment of the PD (to mentioned only few: NCT02611167 NCT03684122, NCT04506073, NCT03550183) and 34 for the treatment of MS (NCT01377870, NCT01854957 NCT01730547 NCT03778333, etc.). But similar to AD, many results are not published. PD treatment is not a fully understood process, which requires additional studies and a longer follow-up period. One study, which is a double-blind, placebo-compared phase I/II clinical trial with autologous BM-MSCs in MS involved patients from 8 different locations. In this trial, safety and efficacy of one single dosage of intravenous injection of autologous BM-MSCs for multiple sclerosis was assesed [164]. Only a few patients participated in this trial for both MS and ALS. Thus, it did not provide any comprehensive conclusion on whether preclinical findings on animal models are fully relevant to human beings [164, 165]. Although, preclinical studies on animal model in MS were efficient, the mechanism of action seems to have involved a combination of modulation of the peripheral immune system and promoting tissue protection. In contrast, there have been several trials using different types of MSCs that show moderate and beneficial effect of cells applications. For instance, a phase 1b study using human placental MSCs show that intravenous injections into 16 patients was safe, and most patients were stable or improved after the treatment [166]. Another study by Fernandez injected different doses of AT-MSCs into 19 MS patients and 11 controls, showing that procedure was safe and indicated beneficial clinical and radiological effects [167]. Riordan and colleagues injected UC-MSCs into 20 patients intravenously using a multiple infusions protocol [168]. The study suggested that the procedure was safe and resulted in some clinical improvements. Another study conducted by Harris et al. injected MSCs-derived neural progenitors (MSC-NP) intrathecally (IT) in three doses spaced three months apart. No serious adverse events were observed and there were indications of some clinical benefits [169]. In two-year follow-up study, the same group showed that safety and efficacy of repeated IT-MSCs-NP treatment was sustained for 2 years, however, the degree of disability reversal was not sustained in a subset of patients [170].
Studies on spinal cord injury (SCI) function have also shown that traditional use of MSCs cellular therapy does not have a substantially beneficial effect and may result in various complications. In preclinical and clinical studies, researchers have taken alternative strategies to improve treatment results using combination of biocompatible biomaterial scaffolds with MSCs to provide proof of the beneficial concept [171–174]. Scaffolds appear to be able to increase the survival chances for stem cells and even promote neural reconnection. Yousefifard and colleagues performed meta-analysis to compare research data for a combination of scaffolds with different sources of MSCs. Their results showed that combination of scaffolds and MSCs are superior to scaffolds and MSCs applied alone in improving SCI in animal models. The use of BM-MSCs and UC-MSCs in combination with scaffolds is more effective than scaffolds alone. However, it remains inconclusive whether the combination of AT-MSCs with scaffolds is superior to scaffolds alone in SCI treatment [175].
COVID-19
COVID-19 is caused by severe acute respiratory syndrome-coronavirus-2 (SARS-CoV-2) infection, which spread around the world in last two years [176, 177]. Severe COVID-19 cases suffered from respiratory failure, sepsis, and abnormal blood coagulation. Many critically ill patients in ICU needed innovative approach such as MSC therapy [178, 179]. MSCs treatments had been effective in acute respiratory distress symptoms as shown in clinical trial I phase [180]. So far, several clinical trials of MSC therapies on COVID-19 syndrome have shown safety assurance and early-phase positive results NCT04252118 [181], NCT04269525 [182], NCT04355728 [183], NCT02097641 [184]. Due to limited patient number and lack of control group in the urgent situation, further studies are still necessary to allow valid conclusions [185].
One clinical trial NCT04355728 described a double-blind, phase 1/2a, randomized controlled trial using umbilical cord-derived MSCs (UC-MSCs) for COVID-19 acute respiratory distress syndrome (ARDS) [183]. The objectives of this clinical trial were to acquire early safety and efficacy data of MSC therapy on COVID-19 ARDS treatments. 24 patients were enrolled and assigned randomly and evenly into the treatment group and the control group. The primary outcomes were both safety-related, including adverse events within 6 h and cardiac arrest or death within 24 h after the infusion. No serious adverse events, nor death were observed related to UC-MSCs infusions. Between the control and treatment groups, no difference was observed. Intravenous delivery of UC-MSCs was considered to be safe in COVID-19 ARDS. The secondary endpoints included patient survival 28 days after the treatment and time for recovery. 91% subjects in the treatment survived which was significantly improved compared with control group (42%). Moreover, on day 6, blood analysis showed that inflammatory cytokines were significantly decreased in treatment subjects. Time for recovery was also significantly shorter in the UC-MSCs treatment group. This study indicated that UC-MSCs therapy is safe and has potential in treating patients with COVID-19 ARDS. However, the relatively small number of patients enrolled in the clinical trial should be taken into consideration when drawing any conclusion.
Another clinical trial (NCT04288102) also illustrated positive effects of UC-MSCs on lung damage in severe COVID-19 patients. 101 severe COVID-19 patients with lung damage were recruited and randomly divided at a 2:1 ratio. 66 patients received three doses of 4 × 107 cells UC-MSCs intravenously on Day 0, 3, and 6, while 35 patients received placebo infusions respectively. Compared with the placebo group, UC-MSCs treatment resulted in significant improvement in whole lung lesion volume with the median difference was -13.31%. Furthermore, patients in the UC-MSCs group demonstrated significantly reduced the proportions of solid component lesion volume with median difference of -15.45%. The six-minute walking distance was also 27 m longer in the treatment group. No difference of the adverse events was observed in two groups. These results indicated that UC-MSCs treatment is safe in the short-term observation study and possibly effective therapeutic treatment for COVID-19 patients with lung damage [186].
The current approaches and results utilizing MSCs in COVID-19 treatment, will be vital to efficiently, accurately and quickly respond on the possible further pandemic crises, pulmonary and other infective disease outbreaks that might quickly spread across the globe. Manufacturing and logistic bases should be equally geographically distributed to efficiently supply sufficient doses of high-quality products in a reasonable time and reproducible manner.
Concluding Remarks
In conclusion, this review provides a concise analysis of the current MSC-based clinical applications. Globally, there are increasing interests in using MSCs or MSC-derived ATMPs for disease-modifying and/or disease-curing therapies. With over 5 decades of accumulated investigations and understandings, MSCs are one the most studied and applied adult stem cells for regenerative medicine. Methods for isolation, propagation, maintenance, up-scale production, and functional characterization and qualification of MSCs have been well-established. However, while as discussed in this review, MSC heterogeneity is well recognized by the scientific society, it still requires to be fully revisited and to unravel the MSC heterogeneity in humans using i.e., single cell RNA sequencing technologies.
In this review, we focused on the use of MSCs or MSC-based ATMPs per se in clinical applications. However, another important and largely explored application of MSCs in regenerative medicine is their integration with regenerative biomaterials (see reviews [187–190]). Biomaterial scaffolds provide several important physical properties i.e., creation of a three-dimensional biomimetic milieu, preservation of tissue structure, formation of extracellular matrix, which can enhance MSCs growth, migration, immunomodulation, generation of tropic factors [190, 191]. In return, MSCs also facilitate the integration of biomaterial scaffolds into the damaged tissues/organs through their immune modulating, releasing of trophic factors, tissue remodeling and regenerative capacities.
A few years ago, we highlighted one great potential of generating genetically engineered iPS-MSCs as a promising cell source for ATMPs and personalized regenerative therapy [110]. With the enormous development in CRISPR gene editing technology over the last few years [192, 193], we still see this strategy as an attractive approach for generating more personalize and off-the-shelf ATMPs for disease-modifying and/or disease-curing therapies. Compared to freshly isolated MSCs from adult tissues, genetically engineered iPS-MSCs (GE-iPS-MSCs) have several advantages. First, GE-iPS-MSCs are not limited by tissue types, since iPSCs can be generated from many types of somatic cells, including PBMCs. Second, GE-iPS-MSCs are less invasive as compared to BM-MSCs or AT-MSCs. Third, GE-iPS-MSCs can be generated in a such a large scale that no adult tissue-MSCs can reach. Fourth, which is also one important feature, GE-iPS-MSCs can be made with disease-causing mutation corrected or even with therapeutic enhancing properties. Last but not least, GE-iPS-MSCs have great advantage for the production of ATMPs. GE-iPS-MSCs also have their limitations such as the introduction of potential off-targets by CRISPR gene editing, transgene integration and over expression, accumulation of somatic mutations. Despite these few shortcomings, GE-iPS-MSCs are attractive cell sources in industrial productions. The current base of MSCs investigations, for sure, may provide critical guidance in translational process, and new technologies in life, computational sciences, and manufacturing advances can assist in-depth studies to prove effective treatment options for variety of serious conditions in a future.
Acknowledgements
We thank Prof. Fred Dubee to his critical comments on the manuscript. The stem cell project was partially supported by the Qingdao-Europe Advanced Institute for Life Sciences.
Author Contributions
D.J. Y.L. and F.X. conceived the idea. D.J. Y.Y. and Y.L. prepared figures and tables. All authors are involved in drafting the manuscript, providing critical comments, reviewing and approving the final manuscript.
Funding
The stem cell project was partially supported by the Qingdao-Europe Advanced Institute for Life Sciences.
Availability of Data and Material
Not applicable.
Declarations
Ethics Approval and Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Conflict of Interest
Authors declare no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.de Wert G, Mummery C. Human embryonic stem cells: Research, ethics and policy. Human Reproduction. 2003;18:672–682. doi: 10.1093/humrep/deg143. [DOI] [PubMed] [Google Scholar]
- 2.Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 3.Takahashi K, et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861–872. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- 4.Doss, M.X. & Sachinidis, A. Current Challenges of iPSC-Based Disease Modeling and Therapeutic Implications. Cells8 (2019). [DOI] [PMC free article] [PubMed]
- 5.Sharkis, S.J., Jones, R.J., Civin, C. & Jang, Y.Y. Pluripotent stem cell-based cancer therapy: promise and challenges. Sci Transl Med4, 127ps129 (2012). [DOI] [PMC free article] [PubMed]
- 6.Yamanaka S. Pluripotent Stem Cell-Based Cell Therapy-Promise and Challenges. Cell Stem Cell. 2020;27:523–531. doi: 10.1016/j.stem.2020.09.014. [DOI] [PubMed] [Google Scholar]
- 7.Fu X, Xu Y. Challenges to the clinical application of pluripotent stem cells: Towards genomic and functional stability. Genome Med. 2012;4:55. doi: 10.1186/gm354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Quinlan AR, et al. Genome sequencing of mouse induced pluripotent stem cells reveals retroelement stability and infrequent DNA rearrangement during reprogramming. Cell Stem Cell. 2011;9:366–373. doi: 10.1016/j.stem.2011.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Laurent LC, et al. Dynamic changes in the copy number of pluripotency and cell proliferation genes in human ESCs and iPSCs during reprogramming and time in culture. Cell Stem Cell. 2011;8:106–118. doi: 10.1016/j.stem.2010.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu X, Li W, Fu X, Xu Y. The Immunogenicity and Immune Tolerance of Pluripotent Stem Cell Derivatives. Frontiers in Immunology. 2017;8:645. doi: 10.3389/fimmu.2017.00645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yoshihara M, Oguchi A, Murakawa Y. Genomic Instability of iPSCs and Challenges in Their Clinical Applications. Advances in Experimental Medicine and Biology. 2019;1201:23–47. doi: 10.1007/978-3-030-31206-0_2. [DOI] [PubMed] [Google Scholar]
- 12.Zhao T, Zhang ZN, Rong Z, Xu Y. Immunogenicity of induced pluripotent stem cells. Nature. 2011;474:212–215. doi: 10.1038/nature10135. [DOI] [PubMed] [Google Scholar]
- 13.Ben-David U, Benvenisty N. The tumorigenicity of human embryonic and induced pluripotent stem cells. Nature Reviews Cancer. 2011;11:268–277. doi: 10.1038/nrc3034. [DOI] [PubMed] [Google Scholar]
- 14.Ferrari G, Thrasher AJ, Aiuti A. Gene therapy using haematopoietic stem and progenitor cells. Nature Reviews Genetics. 2021;22:216–234. doi: 10.1038/s41576-020-00298-5. [DOI] [PubMed] [Google Scholar]
- 15.Wilkinson AC, Igarashi KJ, Nakauchi H. Haematopoietic stem cell self-renewal in vivo and ex vivo. Nature Reviews Genetics. 2020;21:541–554. doi: 10.1038/s41576-020-0241-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Laurenti E, Gottgens B. From haematopoietic stem cells to complex differentiation landscapes. Nature. 2018;553:418–426. doi: 10.1038/nature25022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Feige P, Brun CE, Ritso M, Rudnicki MA. Orienting Muscle Stem Cells for Regeneration in Homeostasis, Aging, and Disease. Cell Stem Cell. 2018;23:653–664. doi: 10.1016/j.stem.2018.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Judson RN, Rossi FMV. Towards stem cell therapies for skeletal muscle repair. NPJ Regen Med. 2020;5:10. doi: 10.1038/s41536-020-0094-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zuk PA, et al. Multilineage cells from human adipose tissue: Implications for cell-based therapies. Tissue Engineering. 2001;7:211–228. doi: 10.1089/107632701300062859. [DOI] [PubMed] [Google Scholar]
- 20.Fei X, et al. Isolation, culture, and identification of amniotic fluid-derived mesenchymal stem cells. Cell Biochemistry and Biophysics. 2013;67:689–694. doi: 10.1007/s12013-013-9558-z. [DOI] [PubMed] [Google Scholar]
- 21.Gartner S, Kaplan HS. Long-term culture of human bone marrow cells. Proc Natl Acad Sci U S A. 1980;77:4756–4759. doi: 10.1073/pnas.77.8.4756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Peng Y, et al. Donor-derived mesenchymal stem cells combined with low-dose tacrolimus prevent acute rejection after renal transplantation: A clinical pilot study. Transplantation. 2013;95:161–168. doi: 10.1097/TP.0b013e3182754c53. [DOI] [PubMed] [Google Scholar]
- 23.Zvaifler NJ, et al. Mesenchymal precursor cells in the blood of normal individuals. Arthritis Research. 2000;2:477–488. doi: 10.1186/ar130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Romanov YA, Svintsitskaya VA, Smirnov VN. Searching for alternative sources of postnatal human mesenchymal stem cells: Candidate MSC-like cells from umbilical cord. Stem Cells. 2003;21:105–110. doi: 10.1634/stemcells.21-1-105. [DOI] [PubMed] [Google Scholar]
- 25.Erices A, Conget P, Minguell JJ. Mesenchymal progenitor cells in human umbilical cord blood. British Journal of Haematology. 2000;109:235–242. doi: 10.1046/j.1365-2141.2000.01986.x. [DOI] [PubMed] [Google Scholar]
- 26.Zou L, et al. A simple method for deriving functional MSCs and applied for osteogenesis in 3D scaffolds. Science and Reports. 2013;3:2243. doi: 10.1038/srep02243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Feng, L. et al. Cell-Based Therapy for Canavan Disease Using Human iPSC-Derived NPCs and OPCs. Adv Sci (Weinh)7, 2002155 (2020). [DOI] [PMC free article] [PubMed]
- 28.Kang R, et al. Mesenchymal stem cells derived from human induced pluripotent stem cells retain adequate osteogenicity and chondrogenicity but less adipogenicity. Stem Cell Research & Therapy. 2015;6:144. doi: 10.1186/s13287-015-0137-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dominici, M. et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy8, 315–317 (2006). [DOI] [PubMed]
- 30.Caplan AI, Rosenberg MJ. Interrelationship between poly (ADP-Rib) synthesis, intracellular NAD levels, and muscle or cartilage differentiation from mesodermal cells of embryonic chick limb. Proc Natl Acad Sci U S A. 1975;72:1852–1857. doi: 10.1073/pnas.72.5.1852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Owen M. Marrow stromal stem cells. Journal of Cell Science. Supplement. 1988;10:63–76. doi: 10.1242/jcs.1988.Supplement_10.5. [DOI] [PubMed] [Google Scholar]
- 32.Caplan AI. Mesenchymal stem cells. Journal of Orthopaedic Research. 1991;9:641–650. doi: 10.1002/jor.1100090504. [DOI] [PubMed] [Google Scholar]
- 33.Gronthos S, et al. Surface protein characterization of human adipose tissue-derived stromal cells. Journal of Cellular Physiology. 2001;189:54–63. doi: 10.1002/jcp.1138. [DOI] [PubMed] [Google Scholar]
- 34.Horwitz EM, et al. Clarification of the nomenclature for MSC: The International Society for Cellular Therapy position statement. Cytotherapy. 2005;7:393–395. doi: 10.1080/14653240500319234. [DOI] [PubMed] [Google Scholar]
- 35.Caplan AI. Mesenchymal Stem Cells: Time to Change the Name! Stem Cells Translational Medicine. 2017;6:1445–1451. doi: 10.1002/sctm.17-0051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bianco P, Robey PG, Simmons PJ. Mesenchymal stem cells: Revisiting history, concepts, and assays. Cell Stem Cell. 2008;2:313–319. doi: 10.1016/j.stem.2008.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pittenger MF, et al. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–147. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 38.Jaiswal N, Haynesworth SE, Caplan AI, Bruder SP. Osteogenic differentiation of purified, culture-expanded human mesenchymal stem cells in vitro. Journal of Cellular Biochemistry. 1997;64:295–312. doi: 10.1002/(SICI)1097-4644(199702)64:2<295::AID-JCB12>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 39.Johnstone B, Hering TM, Caplan AI, Goldberg VM, Yoo JU. In vitro chondrogenesis of bone marrow-derived mesenchymal progenitor cells. Experimental Cell Research. 1998;238:265–272. doi: 10.1006/excr.1997.3858. [DOI] [PubMed] [Google Scholar]
- 40.de Windt TS, Vonk LA, Saris DBF. Response to: Mesenchymal Stem Cells: Time to Change the Name! Stem Cells Translational Medicine. 2017;6:1747–1748. doi: 10.1002/sctm.17-0120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hass R, Kasper C, Bohm S, Jacobs R. Different populations and sources of human mesenchymal stem cells (MSC): A comparison of adult and neonatal tissue-derived MSC. Cell Communication and Signaling: CCS. 2011;9:12. doi: 10.1186/1478-811X-9-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jin HJ, et al. Comparative analysis of human mesenchymal stem cells from bone marrow, adipose tissue, and umbilical cord blood as sources of cell therapy. International Journal of Molecular Sciences. 2013;14:17986–18001. doi: 10.3390/ijms140917986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mastrolia I, et al. Challenges in Clinical Development of Mesenchymal Stromal/Stem Cells: Concise Review. Stem Cells Translational Medicine. 2019;8:1135–1148. doi: 10.1002/sctm.19-0044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hsieh, J.Y. et al. Mesenchymal stem cells from human umbilical cord express preferentially secreted factors related to neuroprotection, neurogenesis, and angiogenesis. PLoS One 8, e72604 (2013). [DOI] [PMC free article] [PubMed]
- 45.Luo, L. et al. Feeder-free generation and transcriptome characterization of functional mesenchymal stromal cells from human pluripotent stem cells. Stem Cell Res 48, 101990 (2020). [DOI] [PubMed]
- 46.Wagner J, Kean T, Young R, Dennis JE, Caplan AI. Optimizing mesenchymal stem cell-based therapeutics. Current Opinion in Biotechnology. 2009;20:531–536. doi: 10.1016/j.copbio.2009.08.009. [DOI] [PubMed] [Google Scholar]
- 47.Ha, D.H. et al. Mesenchymal Stem/Stromal Cell-Derived Exosomes for Immunomodulatory Therapeutics and Skin Regeneration. Cells 9 (2020). [DOI] [PMC free article] [PubMed]
- 48.Zhang L, et al. Exosomes from bone marrow mesenchymal stem cells enhance fracture healing through the promotion of osteogenesis and angiogenesis in a rat model of nonunion. Stem Cell Research & Therapy. 2020;11:38. doi: 10.1186/s13287-020-1562-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sun L, et al. Exosomes derived from human umbilical cord mesenchymal stem cells protect against cisplatin-induced ovarian granulosa cell stress and apoptosis in vitro. Science and Reports. 2017;7:2552. doi: 10.1038/s41598-017-02786-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Abbaszadeh H, Ghorbani F, Derakhshani M, Movassaghpour A, Yousefi M. Human umbilical cord mesenchymal stem cell-derived extracellular vesicles: A novel therapeutic paradigm. Journal of Cellular Physiology. 2020;235:706–717. doi: 10.1002/jcp.29004. [DOI] [PubMed] [Google Scholar]
- 51.Asgarpour K, et al. Exosomal microRNAs derived from mesenchymal stem cells: Cell-to-cell messages. Cell Communication and Signaling: CCS. 2020;18:149. doi: 10.1186/s12964-020-00650-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gnecchi M, Danieli P, Malpasso G, Ciuffreda MC. Paracrine Mechanisms of Mesenchymal Stem Cells in Tissue Repair. Methods in Molecular Biology. 2016;1416:123–146. doi: 10.1007/978-1-4939-3584-0_7. [DOI] [PubMed] [Google Scholar]
- 53.Aggarwal S, Pittenger MF. Human mesenchymal stem cells modulate allogeneic immune cell responses. Blood. 2005;105:1815–1822. doi: 10.1182/blood-2004-04-1559. [DOI] [PubMed] [Google Scholar]
- 54.Uccelli A, Moretta L, Pistoia V. Mesenchymal stem cells in health and disease. Nature Reviews Immunology. 2008;8:726–736. doi: 10.1038/nri2395. [DOI] [PubMed] [Google Scholar]
- 55.Klyushnenkova E, et al. T cell responses to allogeneic human mesenchymal stem cells: Immunogenicity, tolerance, and suppression. Journal of Biomedical Science. 2005;12:47–57. doi: 10.1007/s11373-004-8183-7. [DOI] [PubMed] [Google Scholar]
- 56.Nauta AJ, Fibbe WE. Immunomodulatory properties of mesenchymal stromal cells. Blood. 2007;110:3499–3506. doi: 10.1182/blood-2007-02-069716. [DOI] [PubMed] [Google Scholar]
- 57.Le Blanc K, Mougiakakos D. Multipotent mesenchymal stromal cells and the innate immune system. Nature Reviews Immunology. 2012;12:383–396. doi: 10.1038/nri3209. [DOI] [PubMed] [Google Scholar]
- 58.Krampera M, et al. Bone marrow mesenchymal stem cells inhibit the response of naive and memory antigen-specific T cells to their cognate peptide. Blood. 2003;101:3722–3729. doi: 10.1182/blood-2002-07-2104. [DOI] [PubMed] [Google Scholar]
- 59.Franquesa M, et al. Human adipose tissue-derived mesenchymal stem cells abrogate plasmablast formation and induce regulatory B cells independently of T helper cells. Stem Cells. 2015;33:880–891. doi: 10.1002/stem.1881. [DOI] [PubMed] [Google Scholar]
- 60.Yang, Z.X. et al. CD106 identifies a subpopulation of mesenchymal stem cells with unique immunomodulatory properties. PLoS One8, e59354 (2013). [DOI] [PMC free article] [PubMed]
- 61.Drago D, et al. Metabolic determinants of the immune modulatory function of neural stem cells. Journal of Neuroinflammation. 2016;13:232. doi: 10.1186/s12974-016-0667-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Volpe G, Bernstock JD, Peruzzotti-Jametti L, Pluchino S. Modulation of host immune responses following non-hematopoietic stem cell transplantation: Translational implications in progressive multiple sclerosis. Journal of Neuroimmunology. 2019;331:11–27. doi: 10.1016/j.jneuroim.2016.12.005. [DOI] [PubMed] [Google Scholar]
- 63.Ren G, et al. Inflammatory cytokine-induced intercellular adhesion molecule-1 and vascular cell adhesion molecule-1 in mesenchymal stem cells are critical for immunosuppression. The Journal of Immunology. 2010;184:2321–2328. doi: 10.4049/jimmunol.0902023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Vignali DA, Collison LW, Workman CJ. How regulatory T cells work. Nature Reviews Immunology. 2008;8:523–532. doi: 10.1038/nri2343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Nam, Y. et al. Intraperitoneal infusion of mesenchymal stem cell attenuates severity of collagen antibody induced arthritis. PLoS One13, e0198740 (2018). [DOI] [PMC free article] [PubMed]
- 66.Roux C, et al. Immunosuppressive Mesenchymal Stromal Cells Derived from Human-Induced Pluripotent Stem Cells Induce Human Regulatory T Cells In Vitro and In Vivo. Frontiers in Immunology. 2017;8:1991. doi: 10.3389/fimmu.2017.01991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Shi M, et al. A Pilot Study of Mesenchymal Stem Cell Therapy for Acute Liver Allograft Rejection. Stem Cells Translational Medicine. 2017;6:2053–2061. doi: 10.1002/sctm.17-0134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Pers YM, et al. Injection of Adipose-Derived Stromal Cells in the Knee of Patients with Severe Osteoarthritis has a Systemic Effect and Promotes an Anti-Inflammatory Phenotype of Circulating Immune Cells. Theranostics. 2018;8:5519–5528. doi: 10.7150/thno.27674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Erpicum P, et al. Infusion of third-party mesenchymal stromal cells after kidney transplantation: A phase I-II, open-label, clinical study. Kidney International. 2019;95:693–707. doi: 10.1016/j.kint.2018.08.046. [DOI] [PubMed] [Google Scholar]
- 70.Ciccocioppo R, et al. Autologous bone marrow-derived mesenchymal stromal cells in the treatment of fistulising Crohn's disease. Gut. 2011;60:788–798. doi: 10.1136/gut.2010.214841. [DOI] [PubMed] [Google Scholar]
- 71.Wang D, et al. The regulation of the Treg/Th17 balance by mesenchymal stem cells in human systemic lupus erythematosus. Cellular & Molecular Immunology. 2017;14:423–431. doi: 10.1038/cmi.2015.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kong D, et al. Umbilical cord mesenchymal stem cell transfusion ameliorated hyperglycemia in patients with type 2 diabetes mellitus. Clinical Laboratory. 2014;60:1969–1976. doi: 10.7754/Clin.Lab.2014.140305. [DOI] [PubMed] [Google Scholar]
- 73.Gao L, et al. Phase II Multicenter, Randomized, Double-Blind Controlled Study of Efficacy and Safety of Umbilical Cord-Derived Mesenchymal Stromal Cells in the Prophylaxis of Chronic Graft-Versus-Host Disease After HLA-Haploidentical Stem-Cell Transplantation. Journal of Clinical Oncology. 2016;34:2843–2850. doi: 10.1200/JCO.2015.65.3642. [DOI] [PubMed] [Google Scholar]
- 74.Kuraitis D, Giordano C, Ruel M, Musaro A, Suuronen EJ. Exploiting extracellular matrix-stem cell interactions: A review of natural materials for therapeutic muscle regeneration. Biomaterials. 2012;33:428–443. doi: 10.1016/j.biomaterials.2011.09.078. [DOI] [PubMed] [Google Scholar]
- 75.Raposo G, Stoorvogel W. Extracellular vesicles: Exosomes, microvesicles, and friends. Journal of Cell Biology. 2013;200:373–383. doi: 10.1083/jcb.201211138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Xu R, Greening DW, Zhu HJ, Takahashi N, Simpson RJ. Extracellular vesicle isolation and characterization: Toward clinical application. The Journal of Clinical Investigation. 2016;126:1152–1162. doi: 10.1172/JCI81129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Anderson JD, et al. Comprehensive Proteomic Analysis of Mesenchymal Stem Cell Exosomes Reveals Modulation of Angiogenesis via Nuclear Factor-KappaB Signaling. Stem Cells. 2016;34:601–613. doi: 10.1002/stem.2298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Mardpour S, et al. Interaction between mesenchymal stromal cell-derived extracellular vesicles and immune cells by distinct protein content. Journal of Cellular Physiology. 2019;234:8249–8258. doi: 10.1002/jcp.27669. [DOI] [PubMed] [Google Scholar]
- 79.Madrigal M, Rao KS, Riordan NH. A review of therapeutic effects of mesenchymal stem cell secretions and induction of secretory modification by different culture methods. Journal of Translational Medicine. 2014;12:260. doi: 10.1186/s12967-014-0260-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wang C, et al. Mesenchymal Stromal Cell-Derived Small Extracellular Vesicles Induce Ischemic Neuroprotection by Modulating Leukocytes and Specifically Neutrophils. Stroke. 2020;51:1825–1834. doi: 10.1161/STROKEAHA.119.028012. [DOI] [PubMed] [Google Scholar]
- 81.Han C, et al. Exosomes and Their Therapeutic Potentials of Stem Cells. Stem Cells Int. 2016;2016:7653489. doi: 10.1155/2016/7653489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Lai RC, Yeo RW, Lim SK. Mesenchymal stem cell exosomes. Seminars in Cell & Developmental Biology. 2015;40:82–88. doi: 10.1016/j.semcdb.2015.03.001. [DOI] [PubMed] [Google Scholar]
- 83.Togel F, et al. Vasculotropic, paracrine actions of infused mesenchymal stem cells are important to the recovery from acute kidney injury. American Journal of Physiology. Renal Physiology. 2007;292:F1626–1635. doi: 10.1152/ajprenal.00339.2006. [DOI] [PubMed] [Google Scholar]
- 84.Okamoto T, et al. Clonal heterogeneity in differentiation potential of immortalized human mesenchymal stem cells. Biochemical and Biophysical Research Communications. 2002;295:354–361. doi: 10.1016/S0006-291X(02)00661-7. [DOI] [PubMed] [Google Scholar]
- 85.Andrzejewska A, Lukomska B, Janowski M. Concise Review: Mesenchymal Stem Cells: From Roots to Boost. Stem Cells. 2019;37:855–864. doi: 10.1002/stem.3016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Wilson A, Webster A, Genever P. Nomenclature and heterogeneity: Consequences for the use of mesenchymal stem cells in regenerative medicine. Regenerative Medicine. 2019;14:595–611. doi: 10.2217/rme-2018-0145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Tang F, et al. mRNA-Seq whole-transcriptome analysis of a single cell. Nature Methods. 2009;6:377–382. doi: 10.1038/nmeth.1315. [DOI] [PubMed] [Google Scholar]
- 88.Satija R, Farrell JA, Gennert D, Schier AF, Regev A. Spatial reconstruction of single-cell gene expression data. Nature Biotechnology. 2015;33:495–502. doi: 10.1038/nbt.3192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kanazawa S, et al. Mesenchymal stromal cells in the bone marrow niche consist of multi-populations with distinct transcriptional and epigenetic properties. Science and Reports. 2021;11:15811. doi: 10.1038/s41598-021-94186-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Baccin C, et al. Combined single-cell and spatial transcriptomics reveal the molecular, cellular and spatial bone marrow niche organization. Nature Cell Biology. 2020;22:38–48. doi: 10.1038/s41556-019-0439-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Matsuzaki Y, Mabuchi Y, Okano H. Leptin receptor makes its mark on MSCs. Cell Stem Cell. 2014;15:112–114. doi: 10.1016/j.stem.2014.07.001. [DOI] [PubMed] [Google Scholar]
- 92.Barrett AN, et al. Human Wharton's Jelly Mesenchymal Stem Cells Show Unique Gene Expression Compared with Bone Marrow Mesenchymal Stem Cells Using Single-Cell RNA-Sequencing. Stem Cells Dev. 2019;28:196–211. doi: 10.1089/scd.2018.0132. [DOI] [PubMed] [Google Scholar]
- 93.Zhang S, Wang JY, Li B, Yin F, Liu H. Single-cell transcriptome analysis of uncultured human umbilical cord mesenchymal stem cells. Stem Cell Research & Therapy. 2021;12:25. doi: 10.1186/s13287-020-02055-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Wang L, et al. Integrins in the Regulation of Mesenchymal Stem Cell Differentiation by Mechanical Signals. Stem Cell Rev Rep. 2022;18:126–141. doi: 10.1007/s12015-021-10260-5. [DOI] [PubMed] [Google Scholar]
- 95.Siegel G, et al. Phenotype, donor age and gender affect function of human bone marrow-derived mesenchymal stromal cells. BMC Medicine. 2013;11:146. doi: 10.1186/1741-7015-11-146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Alt EU, et al. Aging alters tissue resident mesenchymal stem cell properties. Stem Cell Res. 2012;8:215–225. doi: 10.1016/j.scr.2011.11.002. [DOI] [PubMed] [Google Scholar]
- 97.Bruna F, et al. Regenerative Potential of Mesenchymal Stromal Cells: Age-Related Changes. Stem Cells Int. 2016;2016:1461648. doi: 10.1155/2016/1461648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Zupan, J. et al. Age-related alterations and senescence of mesenchymal stromal cells: Implications for regenerative treatments of bones and joints. Mech Ageing Dev198, 111539 (2021). [DOI] [PubMed]
- 99.Andrzejewska A, et al. Mesenchymal stem cells injected into carotid artery to target focal brain injury home to perivascular space. Theranostics. 2020;10:6615–6628. doi: 10.7150/thno.43169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Filho DM, et al. Enhancing the Therapeutic Potential of Mesenchymal Stem Cells with the CRISPR-Cas System. Stem Cell Rev Rep. 2019;15:463–473. doi: 10.1007/s12015-019-09897-0. [DOI] [PubMed] [Google Scholar]
- 101.Lee, S. et al. Enhancing the Therapeutic Potential of CCL2-Overexpressing Mesenchymal Stem Cells in Acute Stroke. Int J Mol Sci21 (2020). [DOI] [PMC free article] [PubMed]
- 102.Guo XR, et al. PTEN-mRNA engineered mesenchymal stem cell-mediated cytotoxic effects on U251 glioma cells. Oncology Letters. 2016;11:2733–2740. doi: 10.3892/ol.2016.4297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Meng X, et al. Transplantation of CRISPRa system engineered IL10-overexpressing bone marrow-derived mesenchymal stem cells for the treatment of myocardial infarction in diabetic mice. Journal of Biological Engineering. 2019;13:49. doi: 10.1186/s13036-019-0163-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Li SJ, Luo Y, Zhang LM, Yang W, Zhang GG. Targeted introduction and effective expression of hFIX at the AAVS1 locus in mesenchymal stem cells. Molecular Medicine Reports. 2017;15:1313–1318. doi: 10.3892/mmr.2017.6131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Lee MH, Wu X, Zhu Y. RNA-binding protein PUM2 regulates mesenchymal stem cell fate via repression of JAK2 and RUNX2 mRNAs. Journal of Cellular Physiology. 2020;235:3874–3885. doi: 10.1002/jcp.29281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Yin X, et al. PDGFB-expressing mesenchymal stem cells improve human hematopoietic stem cell engraftment in immunodeficient mice. Bone Marrow Transplantation. 2020;55:1029–1040. doi: 10.1038/s41409-019-0766-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Crisostomo PR, et al. High passage number of stem cells adversely affects stem cell activation and myocardial protection. Shock. 2006;26:575–580. doi: 10.1097/01.shk.0000235087.45798.93. [DOI] [PubMed] [Google Scholar]
- 108.Niess, H. et al. in BMC Cancer, Vol. 15 237 (2015). [DOI] [PMC free article] [PubMed]
- 109.Tsai IC, et al. Small molecule inhibition of RAS/MAPK signaling ameliorates developmental pathologies of Kabuki Syndrome. Science and Reports. 2018;8:10779. doi: 10.1038/s41598-018-28709-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Lin L, Bolund L, Luo Y. Towards Personalized Regenerative Cell Therapy: Mesenchymal Stem Cells Derived from Human Induced Pluripotent Stem Cells. Current Stem Cell Research & Therapy. 2016;11:122–130. doi: 10.2174/1574888X10666150723150236. [DOI] [PubMed] [Google Scholar]
- 111.Hu Y, Liu S, Zhu BM. CRISPR/Cas9-Induced Loss of Keap1 Enhances Anti-oxidation in Rat Adipose-Derived Mesenchymal Stem Cells. Frontiers in Neurology. 2019;10:1311. doi: 10.3389/fneur.2019.01311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Choi J, et al. CRISPR-Cpf1 Activation of Endogenous BMP4 Gene for Osteogenic Differentiation of Umbilical-Cord-Derived Mesenchymal Stem Cells. Mol Ther Methods Clin Dev. 2020;17:309–316. doi: 10.1016/j.omtm.2019.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Freitas, G.P. et al. Mesenchymal stem cells overexpressing BMP-9 by CRISPR-Cas9 present high in vitro osteogenic potential and enhance in vivo bone formation. Gene Ther (2021). [DOI] [PMC free article] [PubMed]
- 114.Jayaramayya K, et al. Immunomodulatory effect of mesenchymal stem cells and mesenchymal stem-cell-derived exosomes for COVID-19 treatment. BMB Reports. 2020;53:400–412. doi: 10.5483/BMBRep.2020.53.8.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Deinsberger J, Reisinger D, Weber B. Global trends in clinical trials involving pluripotent stem cells: A systematic multi-database analysis. NPJ Regen Med. 2020;5:15. doi: 10.1038/s41536-020-00100-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Panes J, et al. Expanded allogeneic adipose-derived mesenchymal stem cells (Cx601) for complex perianal fistulas in Crohn's disease: A phase 3 randomised, double-blind controlled trial. Lancet. 2016;388:1281–1290. doi: 10.1016/S0140-6736(16)31203-X. [DOI] [PubMed] [Google Scholar]
- 117.Ozmert E, Arslan U. Management of retinitis pigmentosa by Wharton's jelly-derived mesenchymal stem cells: Prospective analysis of 1-year results. Stem Cell Research & Therapy. 2020;11:353. doi: 10.1186/s13287-020-01870-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Caplan H, et al. Mesenchymal Stromal Cell Therapeutic Delivery: Translational Challenges to Clinical Application. Frontiers in Immunology. 2019;10:1645. doi: 10.3389/fimmu.2019.01645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Lalu, M.M. et al. Safety of cell therapy with mesenchymal stromal cells (SafeCell): a systematic review and meta-analysis of clinical trials. PLoS One7, e47559 (2012). [DOI] [PMC free article] [PubMed]
- 120.Toyserkani NM, et al. Concise Review: A Safety Assessment of Adipose-Derived Cell Therapy in Clinical Trials: A Systematic Review of Reported Adverse Events. Stem Cells Translational Medicine. 2017;6:1786–1794. doi: 10.1002/sctm.17-0031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Comella K, et al. Effects of the intramyocardial implantation of stromal vascular fraction in patients with chronic ischemic cardiomyopathy. Journal of Translational Medicine. 2016;14:158. doi: 10.1186/s12967-016-0918-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Litwinowicz R, Kapelak B, Sadowski J, Kedziora A, Bartus K. The use of stem cells in ischemic heart disease treatment. Kardiochir Torakochirurgia Pol. 2018;15:196–199. doi: 10.5114/kitp.2018.78446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Galipeau J, Sensebe L. Mesenchymal Stromal Cells: Clinical Challenges and Therapeutic Opportunities. Cell Stem Cell. 2018;22:824–833. doi: 10.1016/j.stem.2018.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Peeters CM, Leijs MJ, Reijman M, van Osch GJ, Bos PK. Safety of intra-articular cell-therapy with culture-expanded stem cells in humans: A systematic literature review. Osteoarthritis Cartilage. 2013;21:1465–1473. doi: 10.1016/j.joca.2013.06.025. [DOI] [PubMed] [Google Scholar]
- 125.Alvaro-Gracia JM, et al. Intravenous administration of expanded allogeneic adipose-derived mesenchymal stem cells in refractory rheumatoid arthritis (Cx611): Results of a multicentre, dose escalation, randomised, single-blind, placebo-controlled phase Ib/IIa clinical trial. Annals of the Rheumatic Diseases. 2017;76:196–202. doi: 10.1136/annrheumdis-2015-208918. [DOI] [PubMed] [Google Scholar]
- 126.Kastrup J, et al. Cryopreserved Off-the-Shelf Allogeneic Adipose-Derived Stromal Cells for Therapy in Patients with Ischemic Heart Disease and Heart Failure-A Safety Study. Stem Cells Translational Medicine. 2017;6:1963–1971. doi: 10.1002/sctm.17-0040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Kuah D, et al. Safety, tolerability and efficacy of intra-articular Progenza in knee osteoarthritis: A randomized double-blind placebo-controlled single ascending dose study. Journal of Translational Medicine. 2018;16:49. doi: 10.1186/s12967-018-1420-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Perlee D, et al. Intravenous Infusion of Human Adipose Mesenchymal Stem Cells Modifies the Host Response to Lipopolysaccharide in Humans: A Randomized, Single-Blind, Parallel Group, Placebo Controlled Trial. Stem Cells. 2018;36:1778–1788. doi: 10.1002/stem.2891. [DOI] [PubMed] [Google Scholar]
- 129.Petrou P, et al. Beneficial effects of autologous mesenchymal stem cell transplantation in active progressive multiple sclerosis. Brain. 2020;143:3574–3588. doi: 10.1093/brain/awaa333. [DOI] [PubMed] [Google Scholar]
- 130.Suk KT, et al. Transplantation with autologous bone marrow-derived mesenchymal stem cells for alcoholic cirrhosis: Phase 2 trial. Hepatology. 2016;64:2185–2197. doi: 10.1002/hep.28693. [DOI] [PubMed] [Google Scholar]
- 131.Henry TD, et al. The Athena trials: Autologous adipose-derived regenerative cells for refractory chronic myocardial ischemia with left ventricular dysfunction. Catheterization and Cardiovascular Interventions. 2017;89:169–177. doi: 10.1002/ccd.26601. [DOI] [PubMed] [Google Scholar]
- 132.Mathias LJ, et al. Alveolar macrophages are critical for the inhibition of allergic asthma by mesenchymal stromal cells. The Journal of Immunology. 2013;191:5914–5924. doi: 10.4049/jimmunol.1300667. [DOI] [PubMed] [Google Scholar]
- 133.Lee RH, et al. Intravenous hMSCs improve myocardial infarction in mice because cells embolized in lung are activated to secrete the anti-inflammatory protein TSG-6. Cell Stem Cell. 2009;5:54–63. doi: 10.1016/j.stem.2009.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Eggenhofer E, et al. Mesenchymal stem cells are short-lived and do not migrate beyond the lungs after intravenous infusion. Frontiers in Immunology. 2012;3:297. doi: 10.3389/fimmu.2012.00297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Higashimoto M, et al. Adipose tissue derived stromal stem cell therapy in murine ConA-derived hepatitis is dependent on myeloid-lineage and CD4+ T-cell suppression. European Journal of Immunology. 2013;43:2956–2968. doi: 10.1002/eji.201343531. [DOI] [PubMed] [Google Scholar]
- 136.Pang SHM, et al. Mesenchymal stromal cell apoptosis is required for their therapeutic function. Nature Communications. 2021;12:6495. doi: 10.1038/s41467-021-26834-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.de Oliveira GL, et al. Bone marrow mesenchymal stromal cells isolated from multiple sclerosis patients have distinct gene expression profile and decreased suppressive function compared with healthy counterparts. Cell Transplantation. 2015;24:151–165. doi: 10.3727/096368913X675142. [DOI] [PubMed] [Google Scholar]
- 138.Peng L, et al. Comparison of biological characteristics of marrow mesenchymal stem cells in hepatitis B patients and normal adults. World Journal of Gastroenterology. 2007;13:1743–1746. doi: 10.3748/wjg.v13.i11.1743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Xie Y, et al. The quality evaluation system establishment of mesenchymal stromal cells for cell-based therapy products. Stem Cell Research & Therapy. 2020;11:176. doi: 10.1186/s13287-020-01696-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Debnath T, Chelluri LK. Standardization and quality assessment for clinical grade mesenchymal stem cells from human adipose tissue. Hematol Transfus Cell Ther. 2019;41:7–16. doi: 10.1016/j.htct.2018.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Samsonraj RM, et al. Concise Review: Multifaceted Characterization of Human Mesenchymal Stem Cells for Use in Regenerative Medicine. Stem Cells Translational Medicine. 2017;6:2173–2185. doi: 10.1002/sctm.17-0129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Wright, A., Arthaud-Day, M.L. & Weiss, M.L. Therapeutic Use of Mesenchymal Stromal Cells: The Need for Inclusive Characterization Guidelines to Accommodate All Tissue Sources and Species. Front Cell Dev Biol9, 632717 (2021). [DOI] [PMC free article] [PubMed]
- 143.Lopez-Beas J, et al. An overview of international regulatory frameworks for mesenchymal stromal cell-based medicinal products: From laboratory to patient. Medicinal Research Reviews. 2020;40:1315–1334. doi: 10.1002/med.21659. [DOI] [PubMed] [Google Scholar]
- 144.Tanaka T, Lee SM, Mikami M, Yokota K, Takakura K. Gaps between Asian regulations for eligibility of human mesenchymal stromal cells as starting materials of cell therapy products and comparability of mesenchymal stromal cell-based products subject to changes in their manufacturing process. Regen Ther. 2020;15:265–273. doi: 10.1016/j.reth.2020.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Rodriguez-Fuentes DE, et al. Mesenchymal Stem Cells Current Clinical Applications: A Systematic Review. Archives of Medical Research. 2021;52:93–101. doi: 10.1016/j.arcmed.2020.08.006. [DOI] [PubMed] [Google Scholar]
- 146.Cieza A, et al. Global estimates of the need for rehabilitation based on the Global Burden of Disease study 2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet. 2021;396:2006–2017. doi: 10.1016/S0140-6736(20)32340-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Nagamura-Inoue T, He H. Umbilical cord-derived mesenchymal stem cells: Their advantages and potential clinical utility. World J Stem Cells. 2014;6:195–202. doi: 10.4252/wjsc.v6.i2.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Mobasheri A, Kalamegam G, Musumeci G, Batt ME. Chondrocyte and mesenchymal stem cell-based therapies for cartilage repair in osteoarthritis and related orthopaedic conditions. Maturitas. 2014;78:188–198. doi: 10.1016/j.maturitas.2014.04.017. [DOI] [PubMed] [Google Scholar]
- 149.Lim HC, et al. Allogeneic Umbilical Cord Blood-Derived Mesenchymal Stem Cell Implantation Versus Microfracture for Large, Full-Thickness Cartilage Defects in Older Patients: A Multicenter Randomized Clinical Trial and Extended 5-Year Clinical Follow-up. Orthopaedic Journal of Sports Medicine. 2021;9:2325967120973052. doi: 10.1177/2325967120973052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Bae DK, Yoon KH, Song SJ. Cartilage healing after microfracture in osteoarthritic knees. Arthroscopy. 2006;22:367–374. doi: 10.1016/j.arthro.2006.01.015. [DOI] [PubMed] [Google Scholar]
- 151.Yen YM, et al. Treatment of osteoarthritis of the knee with microfracture and rehabilitation. Medicine and Science in Sports and Exercise. 2008;40:200–205. doi: 10.1249/mss.0b013e31815cb212. [DOI] [PubMed] [Google Scholar]
- 152.Mahar M, Cavalli V. Intrinsic mechanisms of neuronal axon regeneration. Nature Reviews Neuroscience. 2018;19:323–337. doi: 10.1038/s41583-018-0001-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Bonilla, C. & Zurita, M. Cell-Based Therapies for Traumatic Brain Injury: Therapeutic Treatments and Clinical Trials. Biomedicines9 (2021). [DOI] [PMC free article] [PubMed]
- 154.Arciniegas DB, Held K, Wagner P. Cognitive Impairment Following Traumatic Brain Injury. Current Treatment Options in Neurology. 2002;4:43–57. doi: 10.1007/s11940-002-0004-6. [DOI] [PubMed] [Google Scholar]
- 155.Bae KS, et al. Neuron-like differentiation of bone marrow-derived mesenchymal stem cells. Yonsei Medical Journal. 2011;52:401–412. doi: 10.3349/ymj.2011.52.3.401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Yousefifard M, et al. Human bone marrow-derived and umbilical cord-derived mesenchymal stem cells for alleviating neuropathic pain in a spinal cord injury model. Stem Cell Research & Therapy. 2016;7:36. doi: 10.1186/s13287-016-0295-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Zhao Y, et al. Clinical Study of NeuroRegen Scaffold Combined With Human Mesenchymal Stem Cells for the Repair of Chronic Complete Spinal Cord Injury. Cell Transplantation. 2017;26:891–900. doi: 10.3727/096368917X695038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Lee NK, et al. Agouti Related Peptide Secreted Via Human Mesenchymal Stem Cells Upregulates Proteasome Activity in an Alzheimer's Disease Model. Science and Reports. 2017;7:39340. doi: 10.1038/srep39340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Kim HJ, et al. Stereotactic brain injection of human umbilical cord blood mesenchymal stem cells in patients with Alzheimer's disease dementia: A phase 1 clinical trial. Alzheimers Dement (N Y) 2015;1:95–102. doi: 10.1016/j.trci.2015.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Staff NP, Jones DT, Singer W. Mesenchymal Stromal Cell Therapies for Neurodegenerative Diseases. Mayo Clinic Proceedings. 2019;94:892–905. doi: 10.1016/j.mayocp.2019.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Araldi, R.P., D'Amelio, F., Vigerelli, H., de Melo, T.C. & Kerkis, I. Stem Cell-Derived Exosomes as Therapeutic Approach for Neurodegenerative Disorders: From Biology to Biotechnology. Cells9 (2020). [DOI] [PMC free article] [PubMed]
- 162.Hernandez AE, Garcia E. Mesenchymal Stem Cell Therapy for Alzheimer's Disease. Stem Cells Int. 2021;2021:7834421. doi: 10.1155/2021/7834421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Salem H, Colpo GD, Teixeira AL. Stem Cells in Alzheimer's Disease: Current Standing and Future Challenges. Advances in Experimental Medicine and Biology. 2018;1079:93–102. doi: 10.1007/5584_2018_214. [DOI] [PubMed] [Google Scholar]
- 164.Uccelli A, et al. MEsenchymal StEm cells for Multiple Sclerosis (MESEMS): A randomized, double blind, cross-over phase I/II clinical trial with autologous mesenchymal stem cells for the therapy of multiple sclerosis. Trials. 2019;20:263. doi: 10.1186/s13063-019-3346-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Karussis D, et al. Safety and immunological effects of mesenchymal stem cell transplantation in patients with multiple sclerosis and amyotrophic lateral sclerosis. Archives of Neurology. 2010;67:1187–1194. doi: 10.1001/archneurol.2010.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Lublin FD, et al. Human placenta-derived cells (PDA-001) for the treatment of adults with multiple sclerosis: A randomized, placebo-controlled, multiple-dose study. Mult Scler Relat Disord. 2014;3:696–704. doi: 10.1016/j.msard.2014.08.002. [DOI] [PubMed] [Google Scholar]
- 167.Fernandez, O. et al. Adipose-derived mesenchymal stem cells (AdMSC) for the treatment of secondary-progressive multiple sclerosis: A triple blinded, placebo controlled, randomized phase I/II safety and feasibility study. PLoS One13, e0195891 (2018). [DOI] [PMC free article] [PubMed]
- 168.Riordan NH, et al. Clinical feasibility of umbilical cord tissue-derived mesenchymal stem cells in the treatment of multiple sclerosis. Journal of Translational Medicine. 2018;16:57. doi: 10.1186/s12967-018-1433-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Harris VK, et al. Phase I Trial of Intrathecal Mesenchymal Stem Cell-derived Neural Progenitors in Progressive Multiple Sclerosis. eBioMedicine. 2018;29:23–30. doi: 10.1016/j.ebiom.2018.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Harris, V.K. et al. Mesenchymal stem cell-derived neural progenitors in progressive MS: Two-year follow-up of a phase I study. Neurol Neuroimmunol Neuroinflamm8 (2021). [DOI] [PMC free article] [PubMed]
- 171.Onuma-Ukegawa M, et al. Bone Marrow Stromal Cells Combined With a Honeycomb Collagen Sponge Facilitate Neurite Elongation In Vitro and Neural Restoration in the Hemisected Rat Spinal Cord. Cell Transplantation. 2015;24:1283–1297. doi: 10.3727/096368914X682134. [DOI] [PubMed] [Google Scholar]
- 172.Park SS, et al. Functional recovery after spinal cord injury in dogs treated with a combination of Matrigel and neural-induced adipose-derived mesenchymal Stem cells. Cytotherapy. 2012;14:584–597. doi: 10.3109/14653249.2012.658913. [DOI] [PubMed] [Google Scholar]
- 173.Wang N, et al. Collagen scaffold combined with human umbilical cord-derived mesenchymal stem cells promote functional recovery after scar resection in rats with chronic spinal cord injury. Journal of Tissue Engineering and Regenerative Medicine. 2018;12:e1154–e1163. doi: 10.1002/term.2450. [DOI] [PubMed] [Google Scholar]
- 174.Ropper AE, et al. Defining recovery neurobiology of injured spinal cord by synthetic matrix-assisted hMSC implantation. Proc Natl Acad Sci U S A. 2017;114:E820–E829. doi: 10.1073/pnas.1616340114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Yousefifard M, et al. A combination of mesenchymal stem cells and scaffolds promotes motor functional recovery in spinal cord injury: A systematic review and meta-analysis. Journal of Neurosurgery. Spine. 2019;32:269–284. doi: 10.3171/2019.8.SPINE19201. [DOI] [PubMed] [Google Scholar]
- 176.Grasselli G, Pesenti A, Cecconi M. Critical Care Utilization for the COVID-19 Outbreak in Lombardy, Italy: Early Experience and Forecast During an Emergency Response. JAMA. 2020;323:1545–1546. doi: 10.1001/jama.2020.4031. [DOI] [PubMed] [Google Scholar]
- 177.Fallucchi F, Faravelli M, Quercia S. Fair allocation of scarce medical resources in the time of COVID-19: What do people think? Journal of Medical Ethics. 2021;47:3–6. doi: 10.1136/medethics-2020-106524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Wu C, et al. Risk Factors Associated With Acute Respiratory Distress Syndrome and Death in Patients With Coronavirus Disease 2019 Pneumonia in Wuhan, China. JAMA Intern Med. 2020;180:934–943. doi: 10.1001/jamainternmed.2020.0994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Zhou F, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: A retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Wilson JG, et al. Mesenchymal stem (stromal) cells for treatment of ARDS: A phase 1 clinical trial. The Lancet Respiratory Medicine. 2015;3:24–32. doi: 10.1016/S2213-2600(14)70291-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Meng F, et al. Human umbilical cord-derived mesenchymal stem cell therapy in patients with COVID-19: A phase 1 clinical trial. Signal Transduction and Targeted Therapy. 2020;5:172. doi: 10.1038/s41392-020-00286-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Feng, Y. et al. Safety and feasibility of umbilical cord mesenchymal stem cells in patients with COVID-19 pneumonia: A pilot study. Cell Prolif53, e12947 (2020). [DOI] [PMC free article] [PubMed]
- 183.Lanzoni G, et al. Umbilical cord mesenchymal stem cells for COVID-19 acute respiratory distress syndrome: A double-blind, phase 1/2a, randomized controlled trial. Stem Cells Translational Medicine. 2021;10:660–673. doi: 10.1002/sctm.20-0472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Matthay MA, et al. Treatment with allogeneic mesenchymal stromal cells for moderate to severe acute respiratory distress syndrome (START study): A randomised phase 2a safety trial. The Lancet Respiratory Medicine. 2019;7:154–162. doi: 10.1016/S2213-2600(18)30418-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Moll G, et al. MSC Therapies for COVID-19: Importance of Patient Coagulopathy, Thromboprophylaxis, Cell Product Quality and Mode of Delivery for Treatment Safety and Efficacy. Frontiers in Immunology. 2020;11:1091. doi: 10.3389/fimmu.2020.01091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Shi L, et al. Effect of human umbilical cord-derived mesenchymal stem cells on lung damage in severe COVID-19 patients: A randomized, double-blind, placebo-controlled phase 2 trial. Signal Transduction and Targeted Therapy. 2021;6:58. doi: 10.1038/s41392-021-00488-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Wang YH, Wang DR, Guo YC, Liu JY, Pan J. The application of bone marrow mesenchymal stem cells and biomaterials in skeletal muscle regeneration. Regen Ther. 2020;15:285–294. doi: 10.1016/j.reth.2020.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Zippel N, Schulze M, Tobiasch E. Biomaterials and mesenchymal stem cells for regenerative medicine. Recent Patents on Biotechnology. 2010;4:1–22. doi: 10.2174/187220810790069497. [DOI] [PubMed] [Google Scholar]
- 189.Xu Y, Chen C, Hellwarth PB, Bao X. Biomaterials for stem cell engineering and biomanufacturing. Bioact Mater. 2019;4:366–379. doi: 10.1016/j.bioactmat.2019.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Chen Y, Shu Z, Qian K, Wang J, Zhu H. Harnessing the Properties of Biomaterial to Enhance the Immunomodulation of Mesenchymal Stem Cells. Tissue Engineering. Part B, Reviews. 2019;25:492–499. doi: 10.1089/ten.teb.2019.0131. [DOI] [PubMed] [Google Scholar]
- 191.Elashry MI, et al. Combined macromolecule biomaterials together with fluid shear stress promote the osteogenic differentiation capacity of equine adipose-derived mesenchymal stem cells. Stem Cell Research & Therapy. 2021;12:116. doi: 10.1186/s13287-021-02146-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Xiang X, et al. Enhancing CRISPR-Cas9 gRNA efficiency prediction by data integration and deep learning. Nature Communications. 2021;12:3238. doi: 10.1038/s41467-021-23576-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Anzalone AV, et al. Search-and-replace genome editing without double-strand breaks or donor DNA. Nature. 2019;576:149–157. doi: 10.1038/s41586-019-1711-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.