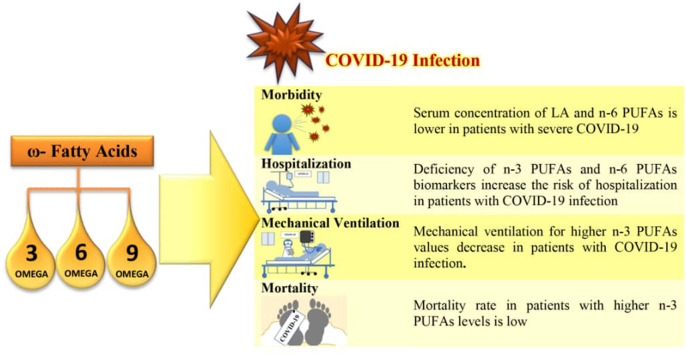
Abstract
Background
Covid-19 mortality is largely associated with a severe increase in inflammatory cytokines and polyunsaturated fatty acids (PUFAs) play an important role in modulating immune pathways and inflammatory responses; so this study was done to evaluate the effect of polyunsaturated fatty acids on the prognosis of Covid-19 disease.
Methods and materials
A comprehensive search was conducted in PubMed, Scopus and Web of Science. For systematic identification, the search was performed based on the following keywords COVID-19, SARS-CoV-2, COVID, Coronavirus Disease 19, SARS COV- 2 Infection, SARS-CoV-2, COVID19, Coronavirus Disease, Fatty Acids, Omega-3, Omega-3 Fatty Acid, Omega-6, n 3 Fatty and Omega-9 in the mentioned databases, using OR, and AND. All searched articles were included in the study and retrieved, and End-Note X7 software was used to manage the studies.
Results
Findings on the relationship between omega-3 and omega-6 fatty acids and the risk of Covid-19 are various, but omega-3 supplements have been found to be 12 to 21% effective in reducing the risk of Covid-19. Most studies emphasized the increasing severity of the disease and the need for mechanical ventilation and hospitalization due to polyunsaturated fatty acid deficiency. It is also demonstrated that omega-3 fatty acid deficiency increased mortality in patients with Covid-19. However, there is also a warning that in critical cases, elevated levels of fatty acids in patients' lungs and a cytokine storm are the main reasons for mortality in Covid-19 patients.
Conclusion
Polyunsaturated fatty acids can reduce the risk of covid-19 which could be considered as a preventative, inexpensive and safe method. However, the risk of taking high-dose omega-3 supplements before or during SARS-COV-2 infection needs to be investigated.
Keywords: Omega, Fatty acids, Covid-19, Systematic review, Mortality, Severity
Graphical abstract
1. Introduction
Covid-19 is an acute respiratory syndrome disease caused by SARS-COV-2, which was introduced as a pandemic in early 2020 and has been diagnosed in more than 230 million people worldwide by September 2021 [1], [2]. The disease worsens with age (especially over 60 years), male sex, and underlying diseases and its mortality is largely associated with rapidly increasing inflammatory cytokines, including interleukin-6 (IL-6) [3]. In SARS-CoV-2 infection, excessive and uncontrolled production of pro-inflammatory cytokines by innate immune cells, intensify secretion of other pro-inflammatory chemical agents such as vascular endothelial growth factor (VEGF), MCP-1, interleukin-8 (IL-8) while reduce the expression of E-cadherin in endothelial cells [4]. VEGF and decreased E-cadherin expression contribute to permeability and vascular leakage, leading to pulmonary dysfunction (ALI), acute respiratory syndrome (ARDS) and ultimately systemic inflammation and multiple organ failure in individuals infected with covid-19 [4], [5], [6]. Thus, cytokine storms are considered as a key factor in disease control, which can exacerbate COVID-19 and even cause mortality. Accordingly, a prophylactic approach to prevent the covid-19 infection is to minimize the release of inflammatory cytokines [3]. Poly unsaturated fatty acids (PUFAs) are an integral component of cell membrane and play an important role in the structural integrity and fluidity of membrane phospholipids. In addition to PUFAs antioxidant function, they play an important role in modulating immune pathways and inflammatory responses which can be helpful in the treatment of viral diseases with a tendency to increase inflammatory cytokines [7], [8], [9].
PUFAs include omega-3 PUFA and omega-6 PUFA which the former acids originate from natural sources and include alpha linoleic acid (ALA), eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) [2], [3] and the latter one, mainly contain linoleic acid (LA) and arachidonic acid (AA) [10].
Up to 25% of the fatty acids in the phospholipids of skeletal muscles, brain, liver, platelets, and immune cells can be attributed to AA [11]. The interaction of AA with molecular oxygen produces mediators known as eicosanoids, which include prostaglandins (PGs), thromboxanes (TXs), and leukotrienes (LTs) [12], [13]. In the presence of certain stimuli such as inflammatory stimuli, enough AA is released to cause significant increases in eicosanoid production. In this situation eicosanoids such as PGD2 and E2, as well as 4-series LTs, are produced in greater quantities and serve as mediators and regulators of the inflammatory response [12].
Recent studies has revealed that omega-3 PUFAs are significant mediators of inflammation that can amplify anti-inflammatory responses, block hyper inflammatory reactions, reduce the incidence of systemic inflammatory response syndrome (SIRS), and complications of infection [14], [15]. One of the anti-inflammatory effects of EPA and DHA is reduced activation of the NF-κB pro-inflammatory transcription factor in response to inflammatory stimuli. This effect has been linked to EPA and DHA's membrane-mediated actions, which block the early phases of inflammatory signaling [16], [17].
However, it appears that EPA and DHA can reduce inflammatory responses by acting directly on inflammatory cells via membrane receptors. For instance binding long-chain fatty acids, especially DHA to GPR120 receptor in macrophages reduce NF_κB activation and decrease the production of inflammatory cytokines. This mechanism of action suggests that EPA and DHA can have anti-inflammatory effects without having to be incorporated into cell membranes or affecting lipid mediator production [18]. Also, Omega-3 fatty acids are thought to give rise to mediators referred to as specialized pro-resolving mediators (SPMs). SPMs including resolvins, protectins and maresins activate the resolution of inflammation in various diseases [9], [19], [20], [21], [22]. In addition, omga-3 fatty acids are involved in regulating the activation of immune cells, including macrophages, neutrophils, basophils, eosinophils, T and B cells. Studies have shown that omega-3 fatty acids are present in neutrophil cell membrane phospholipids, and by secreting cytokines and chemokines improve macrophage function and increase phagocytic ability, thereby enhancing immune function [23]. These findings show that omega-3 fatty acids might be effective as a pharmaconutrient in lowering the impact of inflammation produced by COVID-19 [24].
Given the public health concerns the current covid-19 epidemic and its mortality, it is necessary to investigate modifiable risk factors for severe complications of inflammatory storm. One of the possible preventives and relatively cost-effective methods for high-risk patients can be the use of diets and supplements rich in unsaturated fatty acids, especially omega-3 PUFA. According to the contradictory results obtained from various studies, the present study has been reviewed the studies to find out the possible role of polyunsaturated fatty acids in the severity of Covid-19 disease.
2. Methods and materials
2.1. Search strategy
This systematic review was based on Preferred Reporting Items for Systematic Reviews (PRISMA). A comprehensive search was conducted in PubMed, Scopus and Web of Science. For systematic identification, the search was performed based on the following keywords COVID-19, SARS-CoV-2, COVID, Coronavirus Disease 19, SARS COV-2 Infection, SARS-CoV-2, COVID19, Coronavirus Disease, Fatty Acids, Omega-3, Omega-3 Fatty Acid, Omega-6, n 3 Fatty and Omega-9 in the mentioned databases, using OR, AND. The search was conducted in Aug 17th, 2021; all searched articles were included in the study and retrieved, and End-Note X7 software was used to manage the studies. Also, to increase the validity of the search, the list of references used in all final articles selected for meta-analysis was manually.
2.2. Inclusion and exclusion criteria
Inclusion criteria included the following: evaluation the effect of polyunsaturated fatty acids on mortality, severity, admission to ICU and hospital admission among COVID Patients, articles in English and all original research articles.
Exclusion criteria were as follows: articles written in languages other than English, case report articles, reviews and letters to the editor.
2.3. Screening and selection of studies
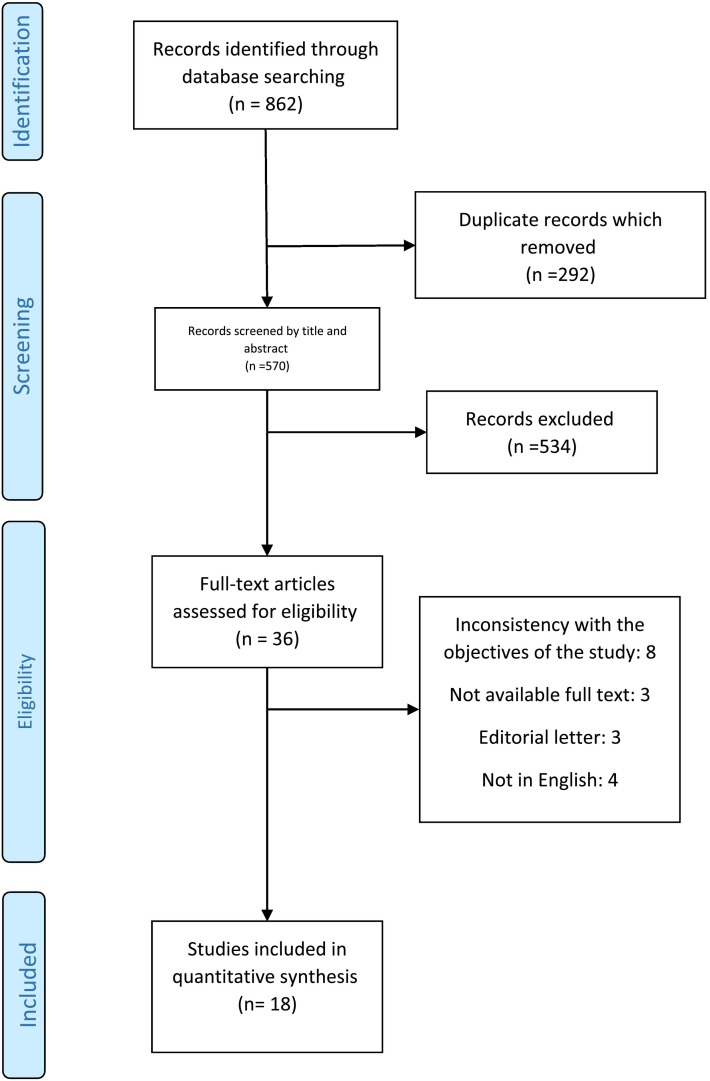
After completing the search and entering articles in Endnote software 7, duplicate articles were found by EndNote and removed, then all articles were evaluated by title and abstract, by reading the abstracts, articles related to the effect of polyunsaturated fatty acids on COVID-19 were entered in this review. PRISMA flow diagram was used for study selection (Fig. 1 ).
Fig. 1.
PRISMA flowchart of information through the various phases of the systematic review.
2.4. Data extraction
To extract the data, the prepared checklist was used and the following information was extracted from each study: surname of the first author, year of publication, country of study, type of study, sample size, age and sex of the target group, period of evaluation, and the main findings.
2.5. Quality assessment
“Adapted Newcastle–Ottawa Quality Assessment Scales” checklist was used to evaluate the quality of the articles in this review [25]. This tool consists of 3 separate sections: selection, comparison, and conclusion. Studies were scored based on overall scores and divided into 3 categories: good, fair, and poor.
3. Results
3.1. Selection of studies
The search result in the databases based on the intended keywords included 862 articles. After removing duplicates, the articles (570) were screened based on the title and abstract information and 36 articles were assessed for eligibility. Afterward, a thorough review of the remaining articles was performed; then, 18 other articles were excluded due to publication in a language other than English [4 articles], a letter to the editor [3 articles], etc. Subsequently, the full text of the articles was reviewed and 3 articles were deleted due to lack of access to the full text or inconsistency with the objectives of the study. Finally, 18 articles were analyzed in this systematic review (Fig. 1).
3.2. Characteristics of studies and quality assessment
According to the goals of this study, included articles was divided in 4 groups; PUFAs and risk of Covid-19 [26], [27], [28], [29], [30], [31], PUFAs and severity of Covid-19 [27], [32], [33], [34], [35], [36], [37], PUFAs and Risk of Death Due to Covid-19 [3], [26], [29], [32], [38], [39], [40] and Covid-19 and receiving PUFAs [26], [41].
Based on the results of “Adapted Newcastle–Ottawa Quality Assessment Scales” checklist, 13 articles/studies were of good quality, and 4 articles/studies were of fair quality and 1 articles/studies was of poor quality. The results were summarized in Table 1 . The completed checklist was presented in supplementary table.
Table 1.
The characteristics of articles included in a systematic review of omega-3 and omega-6 fatty acids and COVID-19.
| First Author; (year) | Place (Country) | Sample size | Type of study | Age | Review period or Comparison date | Quality Assessment | Examined indicators |
|---|---|---|---|---|---|---|---|
| Zapata B et al. (2021) [32] | Chile | 74: Male: 39 Female: 35 (74 patients (39 m and 35 f) with severe COVID-19 and 10 healthy quality-control) |
Cross sectional | Patients: 21–82 (59.68 ± 13.6) | November 2020 and April 2021 | Good | - Omega-3 Index in patients with severe COVID-19: 4.15% ± 0.69% - Risk of mechanical ventilation for the lowest O3I quartile (<3.57%) compared to higher quartiles: OR = 1.348, 95%CI: 0.925–1.964; P = 0.183 - Risk of death for the lowest O3I quartile (<3.57%) compared to higher quartiles: OR = 3.111, 95%CI:1.261–7.676; P = 0.032 - Reduction in the risk of mechanical ventilation for the highest O3I quartile (>4.51%) compared to the lo west quartile: OR = 0.257, 95%CI: 0.083–0.791; P = 0.026 - Reduction in the risk of death for the highest O3I quartile of (>4.51%) compared to the lowest quartile: OR = 0.195 95%CI: 0.024–1.605; P = 0.165 |
| Archambault et al. (2021) [35] | Canada | 25 healthy subjects and 33 COVID-19 patients | – | healthy subjects:26 ± 1 COVID-19 patients: 58 ± 3 |
between May and June 2020, before COVID-19 |
Poor | - Higher in bronchoalveolar lavage of COVID-19 patients compared with healthy subjects Mean ± SD of: - arachidonic acid, 89.3 ± 6.4 vs. 16 ± 9 nmol/ml - docosahexaenoic acid, 290 ± 35 vs. 35 ± 20 nmol/ml - eicosapentaenoic acid, 8.9 ± 0.9 vs. 8.6 ± 0 nmol/ml |
| Asher et al. (2021) [3] | USA | 100: 59 male, 41 female (86 alive, 14 dead) |
pilot study | 72.5 (16.5; 25,100) | from March 1, 2020 onwards | Good | - patients with an O3 index at 5.7% or greater: 75% lower risk for death compared with those below that value (p = 0.071) - Q4: O3I ≥ 5.7% omega-3 index with death adjusted for age and sex: 32.0% (8/25); OR = 0.25, 95% CI: 0.03–1.11; p = 0.071 - Omega-3 Index and death: Q3 (4.7 < O3I < 5.7%) vs other quartiles: OR = 3.13, 95% CI: 0.82–14.30; p = 0.1 |
| Doaei et al. (2021) [38] | Iran | 101 patients infected with COVID-19: 28 fortified formula with n3-PUFA and 73 controls; 60 male, 41 female. Interventions: 15 m, 13 f; controls: 45 m, 28 f |
A double-blind, randomized clinical trial | between 35 and 85 years (Interventions: 66 (14.58); Controls:64 (14.25)) |
from May to July 2020 | Good | Effects of omega-3 supplementation(one capsule of 1000 mg omega-3 daily (Vita Pharmed, Switzerland) containing 400 mg EPAs and 200 mg DHAs for 14 days) in intervention group vs. control group: - On 1-month survival rate: significantly higher, 21% (n = 6) vs. 3% (n = 2); P = 0.003 - On kidney function: levels of BUN (35.17 vs 43.19, F = 4.76, P = 0.03) and Cr (1.29 vs 1.68, F = 5.90, P = 0.02), significantly lower and the amount of urine excreted (2101 vs 1877.02, F = 12.26, p = 0.01), significantly higher. - On arterial blood gas (ABG) parameters: levels of arterial pH (7.30 vs 7.26, F = 19.11, P = 0.01), HCO3 (22.00 vs 18.17, F = 10.83, P = 0.01), and Be (−4.97 vs −3.59, F = 23.01, P = 0.01), significantly higher. - On the mean of Glasgow coma scale (GCS): at admission time8.37 vs. 7.90, P > 0.05, significantly lower; after 14 days 7.90 vs 7.49, F = 6.07, P = 0.05. No significant difference in APACHE II score (15.54 ± 1.73 vs 15.42 ± 1.92, P = 0.78). - On serum electrolytes: The level of K, significantly reduced (4.00 vs 4.14, F = 10.15, P = 0.01) after 14 days. No significant differences between the levels of serum electrolytes including Na, Ca, and P. - On blood clotting function and cell blood count (CBC): The lymphocyte count increased, marginally significant (11.59 vs 11.80, F = 4.08, P = 0.05). no significant differences in levels of PTT, hematocrit, neutrophil, monocyte, hemoglobin, and Plt - On the other blood factors: No significant differences in blood glucose, albumin, MAP, and O2 sat. |
| Hamulka et al. (2021) [26] | Worldwide and Poland | First wave: 2296 Second wave: 978 |
Online cross-sectional | ≥18 | (1) in April and May 2020 (2) in November 2020 during the second wave |
Good | Spearman rank's coefficients Omega-3 fatty acids and Worldwide: COVID-19 cases: 0.06; Deaths: 0.06 Coronavirus relative search value (RSV): −0.74; p ≤ 0.01 Poland: COVID-19 cases: 0.21; Deaths: 0.21, coronavirus relative search value (RSV): −0.26 - Omega-3 fatty acids supplement consumption: increase from 2.8% to 8.2% |
| Jontez et al. (2021) [41] | Slovenia | 38 (14 m , 24 f) |
web survey |
36.3 ± 10.1 | December 2019 | Fair | Mean ± SD fatty acids intake ratio (PUFA+MUFA)/SFA) in healthy Adults: Baseline 1.98 ± 1.34, During Lockdown 1.77 ± 1.20 and Post-Lockdown 1.54 ± 0.78 |
| Julkunen et al. (2021) [42] | UK |
Pneumonia participants: n = 105,142; 102,639 controls, 2507 severe incident cases COVID-19 Participants: n = 92,725; 92,073 control, 653 severe incident cases |
Retrospective cohort | 49–84 | blood samples collected 2007–2010 | Good | - multi-biomarker score for fatty acids and susceptibility to severe COVID-19: odds ratio 2.9 [95%CI 2.1–3.8] for highest vs lowest quintile; p-value<0.001 |
| Mei et al. (2021) [39] | China | 223: 91 discharged and 132 deceased | multi-center study | ≥65 years old | Between January and March 2020 | Good | - Fatty acid: lower flux in the survivors vs. the deceased subgroup, AOR = 15.61 [95% CI: 6.66–36.6], p < 0.001. |
| Nguyen et al. (2021) [27] | France | 61: 34 non-COVID-19,27 COVID-19 |
prospective | non-COVID-19: 69 (± 12) COVID-19: 62 (± 11) |
– | Good |
In COVID-19 patients vs. non-COVID-19 patients: - Linoleic acid (C18:2 n-6): significantly increased 207 ± 109 vs. 113 ± 67 nmol/ml; p < 0.01. - Arachidonic acid (C20:4 n-6): significantly increased 16 ± 6 vs 12 ± 5 nmol/ml p < 0.01 - Relative proportion of linoleic acid: significantly higher 12.8 ± 3.6 vs. 8.3 ± 2.3%; p < 0.01 - Linoleic acid proportion and ventilator-free days: r = − 0.404, p = 0.001) |
| Perez-Torres et al. (2021) [28] | Mexico | COVID-19 patients n = 42: 31 m, 11 f (healthy subjects n = 22) |
– | over 18 years 62 ± 13 years |
– | Good | - Increased in COVID-19 patients: oleic (OA), p = 0.001; linoleic (LA), p = 0.03 and arachidonic acid (AA), p = 0.02. - Mean ± SE of Fatty acids in Healthy subjects vs. COVID-19 patients - Monounsaturated fatty acids (MUFA): 23.82 ± 0.70 vs. 32.09 ± 0.61; p = 0.001 - Omega 3 polyunsaturated fatty acids (PUFA (n-3)): 0.91 ± 0.11 vs. 0.31 ± 0.05; p = 0.001 - Omega 6 polyunsaturated fatty acids (PUFA (n-6)): 25.94 ± 0.53 vs. 28.19 ± 0.82; p = 0.02 |
| Vivar-Sierra et al. (2021) [29] | worldwide | – | Web based | – | – | Fair | - Eastern Mediterranean region: higher mean fatality rate (3.52%) and the lowest omega −3 intake from marine sources (45.14 mg/day) - South-East Asia: lowest fatality rate (1.01%) and the highest average consumption (634.00 mg/day) from marine sources - In nations with a consumption <250 mg/day from marine products, differences among regions were observed (chi2 = 59.361; p = 0.000), as well as a trend for higher fatality rates, >2.5 and 4% (chi2 = 10.432; p = 0.064) and (chi2 = 10.367; p = 0.066), - Omega −3 intake from plants and cumulative cases: rSpearman = 0.321; p < 0.001 - Omega −3 intake from plants and total cumulative cases per 1 million population: rSpearman = 0.329; p < 0.001 - Omega −3 intake from plants and fatality rates: rSpearman = 165; p > 0.05 |
| Bejan. (2021) [36] | USA | 7768 COVID-19 patients, 509 (6.55%) hospitalized, 82 (1.06%) admitted to ICU, 64 (0.82%) mechanical ventilation, and 90 (1.16%) died |
retrospective cohort | Median = 42 | Patient exposure to a drug during 1-year prior to the pandemic and COVID-19 diagnosis |
Good | - Hospitalized-mild, cumulative severity: supplement of Omega-3 fatty acids: Total exposed: 475 Total unexposed: 7293 Severity rate exposed: 10.7 Severity rate unexposed: 15.7 OR = 0.60, 95% CI: 0.39–0.94 - Hospitalized-mild, exclusive severity supplement of Omega-3 fatty acids: Total exposed: 456 Total unexposed: 7168 Severity rate exposed: 7.2 Severity rate unexposed: 11.5 OR = 0.56, 95% CI: 0.33–0.95(Lower risk for COVID-19 outcomes) |
| Hao et al. (2021) [33] | China | 89 asymptomatic COVID-19 patients and 178 healthy controls | – | 19 to 91 Mean ± SD: Asymptomatic: 45 ± 13; healthy controls: 45 ± 13 |
- |
Good | - FAs (including FA 18:1 and FA 20:0) decreased in asymptomatic COVID-19 patients. -Z-scored log 2-scaled peak area value for relative intensity of FA 18:1: 0.42, (95%CI: −0.31 to 1.09) in healthy controls and − 0.73, (95%CI: −1.1 to −0.14) in COVID-19 patients; adjusted p-value = 1.23e-12 - FA 18:1 (asymptomatic/healthy): 0.44 -Z-scored log 2-scaled peak area value for relative intensity of FA 20:0:0.068, (95%CI: −0.34 to 0.89) in healthy controls and − 0.50, (95%CI: −0.99 to −0.17) in COVID-19 patients; adjusted p-value = 8.74e-10 - FA 20:0 (asymptomatic/healthy): 0.65 |
| Louca et al. (2021) [30] | UK, USA, Sweden |
UK: n = 372,720: 39263 supplement users and 333,457 non-users. USA: n = 45,757, 8663 supplement users and 37,094 non-users Sweden: n = 27,373, 3039 supplement users and 24,334 non-users |
App-based community survey | aged 16–90 years | in the first waves of the pandemic up to 31 July 2020 |
Fair | - SARS-CoV-2 positive, n (%): UK: 10508 (6%) USA: 2002 (6.2%) Sweden: 1806 (13.5%) - UK cohort: users regularly supplementing their diet with omega-3 fatty acids had a lower risk of testing positive for SARS-CoV-2 by 12% (OR = 0.88, (95%CI: 0.84 to 0.92), p = 5.8 × 10−8) after adjusting for age, sex, BMI, sign-up health status and multiple testing - omega-3 supplement use was not associated with testing positive in Swedish females - Swedish men taking probiotics, omega-3 fatty acids had a decreased risk of infection - protective effect in omega-3 fatty acid supplements users with a 12% reduction in risk of testing positive for SARS-CoV-2 in the overall UK cohort, 21% in the US cohort and 16% in the SE cohort. - women taking multivitamins, omega-3 fatty acids have a slightly lower risk of SARS-CoV-2 infection in the UK, US and SE cohorts |
| El-Kurdi et al. (2020) [40] | 61 countries With >1000 COVID-19 death |
1,476,418 patients | Web survey | – | between 3/25/2020 and 04/08/2020 | Good | - %UFA intake was positively associated with mortality: Rate Ratio = 1.02, 95% CI: 1.01–1.03; (p < 0.001) - Multivariate analysis showed only %UFA as significantly associated with mortality (p < 0.0001). |
| Barberis et al. (2020) [37] | Italy |
Non-COVID-19 Patients:26 Healthy Control, 32 non-COVID-19 with symptom COVID-19 Patients:103 |
– | (Mean ± SD) Healthy Control: 50.1 ± 5.3 non-COVID-19 with symptoms: 68.6 ± 8.9 COVID-19 Patients: 67.3 ± 18.0 |
– | Good | - Free fatty acids, especially arachidonic acid (AUC = 0.99) and oleic acid (AUC = 0.98), were well correlated to the severity of the disease; p value <0.0001. - By using ROC curves, the quantification in the negative mode identified AUC values of 0.99 (SE: 93%, SP: 100%) for arachidonic acid (FA 20:4) and 0.98 (SE: 96%, SP: 88%) for oleic acid (FA 18:1). - Mean ± SD of oleic acid (FA 18:1) in covid-19 patients vs. controls: 2355 ± 1305 vs 0.567 ± 326 pmol/ml plasma - Mean ± SD of arachidonic acid (FA 20:4) in covid-19 patients vs. controls: 415 ± 237 vs. 49.5 ± 24.75.6 pmol/ml plasma - Oleic acid and arachidonic acid levels are directly correlated to the severity of the disease |
| Thomas et al. (2020) [31] | USA | COVID-19: n = 33 Controls: n = 16 |
– | (Mean ± SD) COVID-19: 56.5 ± 18.1 Controls: 37.8 ± 11.6 |
– | Fair | - Serum levels of free fatty acids (c18:0–3 and c 20:4–5) were significantly different when comparing COVID-19–positive patients and controls; P < 0.05 |
| Dierckx et al. (2020) [34] | Belgium | 581 samples from 480 patients in three different cohorts: UZL, n = 219 and JESSA, n = 164, subset of plasma samples |
retrospective | >18 years old | between March 2020 and September 2020 |
Good | - Increased poly-unsaturated FA (PUFA) content was associated with less severe disease - Increased mono-unsaturated FA (MUFA) content was associated to more severe disease - Linoleic acid (LA) and total Omega-6 FA: stronger and more consistent associations opposite associations with COVID-19 severity than Omega-3 FA. - Opposite associations with COVID-19 severity: - Linoleic acid (LA): OR = 0.55, percentile2.5 = 0.42, percentile97.5 = 0.71; p = 0.000 in UZL and OR = 0.72, percentile2.5 = 0.54, percentile97.5 = 0.96; p = 0.025 in Jessa - total Omega-3 FA: OR = 0.69, percentile2.5 = 0.54, percentile97.5 = 0.89; p = 0.003 in UZL and OR = 1.05, percentile2.5 = 0.75, percentile97.5 = 1.47; p = 0.77 in Jessa - Omega-6 fatty acids: OR = 0.59, percentile2.5 = 0.45, percentile97.5 = 0.75; p < 0.001 in UZL and OR = 0.66, percentile2.5 = 0.47, percentile97.5 = 0.92; p = 0.014 in Jessa - Docosahexaenoic acid (DHA) OR = 0.74, percentile2.5 = 0.57, percentile97.5 = 0.94; p = 0.015 in UZL, OR = 1.1, percentile2.5 = 0.84, percentile97.5 = 1.46; p = 0.48 in Jessa. |
| (n = 198, from 97 patients) taken for the CONTAGIOUS observational clinical trial 69 in analyze |
prospective | >18 years old | Samples were taken at the time of admission (within maximum 48 h), at day 7, at the time of hospital discharge and 30 days after hospital discharge (if available). |
Good | - Ratio of omega-6 fatty acids to total fatty acids: median = 35.16; IQR = 34–36.6 in sv3: and median = 32.34; IQR = 30.2–34.7 in sv4; p = 0.002 - Ratio of polyunsaturated fatty acids to monounsaturated fatty acids(PUFA by MUFA): median = 1.4; IQR = 1.26–1.47 in sv3: and median = 1.27; IQR = 1.02–1.37 in sv4; p = 0.022 - Ratio of polyunsaturated fatty acids to total fatty acids (PUFA pct): median = 38.45; IQR = 36.9–40.2 in sv3: and median = 37.05; IQR = 34–38.6 in sv4; p = 0.008 - Higher relative PUFA content and PUFA to MUFA ratio were consistently associated with lower severity, in contrast to increased MUFA levels - Linoleic Acid concentration and total omega-6 fatty acid content (absolute concentration and relative to total FAcontent) were lower in severe COVID-19 cases. |
3.2.1. PUFAs and the risk of Covid-19
In terms of PUFAs and the risk of Covid-19, Hamulka et al. examined the trend of omega-3 PUFA intake worldwide and Covid-19 incidence and found that the correlation coefficient between Covid-19 incidence and omega-3 PUFA intake in the world was 0.06 and in Poland was 0.21 [26].
Nguyen et al. in France showed that the levels of Linoleic acid (C18: 2, n-6) and Arachidonic acid (C20: 4, n-6) increased in Covid-19 patients compared to healthy individuals (p < 0.01) [27].
In other study, Perez-Torreset al. revealed that the mean levels of oleic (OA), p = 0.001, linoleic (LA) (p = 0.03) and arachidonic acid (AA) (p = 0.02) increased in Covid-19 patients compared with healthy individuals. In addition, Omega-3 PUFA levels of polyunsaturated fatty acids (PUFA (n-3)) also decreased (0.91 ± 0.11 vs. 0.31 ± 0.05; p = 0.001) [28].
The results of an ecological study by Vivar-Sierraet al. showed that a positive correlation between omega-3 PUFA intake from plant sources and total accumulation (r = 0.321; p < 0.001) and total accumulation per 1 million populations (r = 0.329; p < 0.001) [29].
Furthermore, Louca et al. in a study that examined the status of omega-3 PUFA supplements and the risk of developing Covid-19 in the UK, USA and Sweden showed that using of omega-3 PUFA supplements reduced positive Covid-19 test to 12%, 21% and 16% in regular supplements users since the beginning of the pandemic compared with non-users, in the UK, the USA and Sweden respectively [30]. In Sweden, no relationship was found between omega-3 PUFA intake and positive covid-19 test in women [30].
In a study, Thomas et al. found that the mean serum levels of omega-3, omega-6 and omega-9 fatty acids were significantly different in healthy individuals and Covid-19 patients (p < 0.05) [31].
3.2.2. PUFAs and severity of Covid-19 disease
In terms of PUFAs and severity of Covid-19 disease, the mean omega-3 PUFA Index (consistent with insufficient fish and Omega-3 supplement consumption) was 4.15% ± 0.69% in patients with severe Covid-19 and markedly lower than the healthy control subjects (mean: 7.84%; range: 4.65–10.71%) [32].
The results of a study in China showed that the levels of omega-3 fatty acids, including oleic acid (FA 18: 1) and omega-3 PUFA (FA 20: 0) in patients with asymptomatic Covid-19 decreased. Also, the ratio of omega-3 fatty acids in asymptomatic Covid-19 patients decreased by 35% compared to healthy individuals [33].
A study conducted in Belgium found increased serum levels of PUFA with lower disease severity and increased serum levels of monounsaturated fatty acids (MUFA) were associated with higher disease severity [34]. This prospective study of a population showed that the median ratio of omega-6 fatty acids to total fatty acids as well as ratio of PUFA by MUFA fatty acids (P = 0.022) decreased in patients with severe Covid-19 and who dies compared to patients with lower severity of the disease (P = 0.002). It was also shown that the serum concentration of linoleic acid and omega-6 fatty acids was lower in patients with severe Covid-19 [34].
3.2.2.1. Requirement to mechanical ventilation
In terms of requirement to mechanical ventilation, Zapata et al. showed that the need for mechanical ventilation for low omega-3 PUFA values (lowest O3I quartile (<3.57%)) increased compared to higher values (OR = 1.348, 95% CI: 0.925–1.964; P = 0.183) and decreased for high levels of omega-3 PUFA (highest O3I quartile (> 4.51%) (OR = 3.111, 95% CI: 1.261–7.676; P = 0.032) [32].
Archambault et al. found that the mean arachidonic acid, docosahexaenoic acid, and eicosapentaenoic acid increased in the lungs of intubated Covid-19 patients requiring mechanical ventilation compared to healthy individuals [35].
The results of a study in France showed that the ratio of linoleic acid has a negative correlation with the number of days without ventilator (r = − 0.404, p = 0.001) [27].
3.2.2.2. Necessity to hospitalization
In terms of necessity to be hospitalized, in a study by Archambault et al., showed that deficiency of omega-3 PUFA and omega-6 PUFA biomarkers increased the risk of severe Covid-19 and hospitalization by 2.9 times (OR = 2.9, 95% CI 2.1–3.8for highest vs lowest quintile; p-value <0.00) [42].
Furthermore, the results of a study in the US revealed that taking omega-3 PUFA supplements reduced the risk of hospitalization and severe form of Covid-19 (OR = 0.60, 95% CI: 0.39–0.94) [36].
3.2.2.3. Necessity to ICU admission
Regarding necessity to admission to the ICU, Barberis et al. observed that fatty acids level, especially arachidonic acid and oleic acid, were directly correlated with the severity of Covid-19 disease and admission to the ICU (p-value <0.0001) and the mean amount of arachidonic acid in ICU patients was higher than hospitalized patients in other wards and healthy patients [37].
3.2.3. PUFAs and risk of death from Covid 19
Regarding PUFAs and risk of death due to Covid-19, in a study in Chile, the risk of death for patients with low omega-3 PUFA (lowest O3I quartile <3.57%) was more than tripled (OR = 3.111, 95% CI: 1.261–7.676; P = 0.032); While this risk decreased at high levels of omega-3 PUFA (OR = 0.195 95% CI: 0.024–1.605; P = 0.165) [32].
Asher et al. found that patients with higher omega-3 PUFA levels were less likely to die than patients with lower omega-3 PUFA levels (p = 0.071), as patients with omega-3 PUFA index higher than 5.7%, the risk of death was reduced by 75% (OR = 0.25, 95% CI: 0.03–1.11; p = 0.071) [3].
Doaei et al. in Iran showed that the one-month survival of patients with Covid-19 receiving omega-3 PUFA supplementation increased compared to the control group (21% (n = 6) vs. 3% (n = 2); p = 0.003) [38].
In Poland, a study of the trend of omega-3 PUFA consumption worldwide and death due to Covid-19 has shown that the correlation coefficient between Covid-19 incidence and omega-3 PUFA consumption in the world is 0.06 and in Poland is 0.21 [26].
In a study by Mei et al., In a study by Mei et al., the metabolic flux analysis showed that metabolic pathway of fatty acid (15.61 [95% CI 6.66–36.6], p < 0.001) showed a consistently lower flux in patients who improved compared to patients who died (AOR = 15.61,95% CI: 6.66–36.6, p < 0.001) [39].
The results of an ecological study showed that omega-3 PUFA intake through food sources varies with the mortality rate in Covid-19 patients in different regions; so that the countries of the Eastern Mediterranean region which have the lowest omega-3 PUFA intake from marine sources (45.14 mg/day) have the highest mortality in patients with Covid-19 (3.52%). While in Southeast Asian countries with the highest omega-3 PUFA intake (634.00 mg/day), the mortality in Covid-19 patients (1.01%) was the lowest in the world. Also, a positive correlation was observed between receiving omega-3 PUFA from plants and mortality rate (r = 165) (p > 0.05) [29].
In a study that evaluated the relationship between mortality from Covid-19 and the consumption of unsaturated fatty acids (UFA% intake) in 61 countries with more than 1000 deaths from Covid-19, positive correlation was observed between the intake of unsaturated fatty acids and mortality in patients with Covid-19 [40].
3.2.4. Covid-19 and receiving PUFAs
Regarding Covid-19 and receiving PUFAs, considering food intake and supplementation are important.
3.2.4.1. Food intake
The results of a study in Slovenia showed that the mean of fatty acids intake ratio (polyunsaturated fatty acids [PUFA] + monounsaturated fatty acids [MUFA])/saturated fatty acids [SFA]) in healthy adults decreased from 1.98 ± 1.34 before the corona crisis to 1.77 ± 1.20 during the crisis and 1.54 ± 0.78 in the post-limitation period (p = 0.026) [41].
3.2.4.2. Supplementation intake
In a study conducted in Poland, omega-3 PUFA supplements were found to increase from 2.8% in the pre-Covid-19 period to 8.2% in the epidemic period [26].
4. Discussion
The covid-19 pandemic has had devastating effects on mortality worldwide [43]. Although medications can help to reduce the side effects of infection, cost effective and preventative measures that moderate the consequences of the disease are much needed [38]. Therefore, a person's nutritional system plays an important role in protecting body against viral infections. Previous studies have shown that proper nutrition strengthens the immune system and nutritional deficiencies lead to oxidative stress in the body [44]. Omega polyunsaturated fatty acids modulate acquired immune responses. Furthermore, omega-3 PUFA interferes with various stages of viral infection, especially the entry and replication of the virus. Therefore, the nutritional status of omega polyunsaturated fatty acids (PUFAs) is important in inflammatory tissue status and overall immune response [45]. The therapeutic benefits of PUFA on pathological disorders such as infection or inflammation have been extensively researched [46], [47], [48]. Epidemiological, interventional and therapeutic studies have shown that omega polyunsaturated fatty acids, especially omega-3 PUFA, have several anti-inflammatory effects that could play a role in preventing cytokine storms by reducing the severity of inflammation [49], [50], [51], [52]. Given that COVID-19 is a viral infection that causes considerable inflammation, PUFA supplementation may be beneficial [9], [53], [54], [55].
The results of studies on the relationship between omega-3 fatty acids and disease severity in China showed that the proportion of omega-3 fatty acids in asymptomatic Covid-19 patients was 35% lower than in healthy individuals. A study in Belgium showed that in different parts of the country, increased serum levels of multiple PUFA related with lower disease severity and increased serum levels of monounsaturated fatty acids (MUFA) were related with higher disease severity [34]. Also, in this prospective study of a population showed that the median ratio of omega-6 fatty acids to total fatty acids decreased in patients with severe Covid-19 and patients who died compared to patients with less severity (P = 0.002) [34].
In addition, evidence has shown that omega polyunsaturated fatty acid deficiency increases the likelihood of hospitalization rate and ICU admission. Furthermore, the results of a study in the UK showed that a lack of omega-3 PUFA and omega-6 PUFA biomarkers increased the risk of hospitalization by 2.9 times [36], [37], [42]. Also, in a study conducted in Italy, it was observed that the average amount of arachidonic acid in patients admitted to the ICU was higher than patients admitted to other wards and healthy patients [37].
Numerous studies have also shown an increase in mortality with a decrease in omega polyunsaturated fatty acids [3], [26], [29], [32], [38], [39], [40]. As an illustration, Zapata et al. showed that the mortality rate of patients with covid-19 increased to 3 times in patients with low omega-3 PUFA levels [32].
4.1. PFUAs and lipid mediators role in inhibition of inflammation
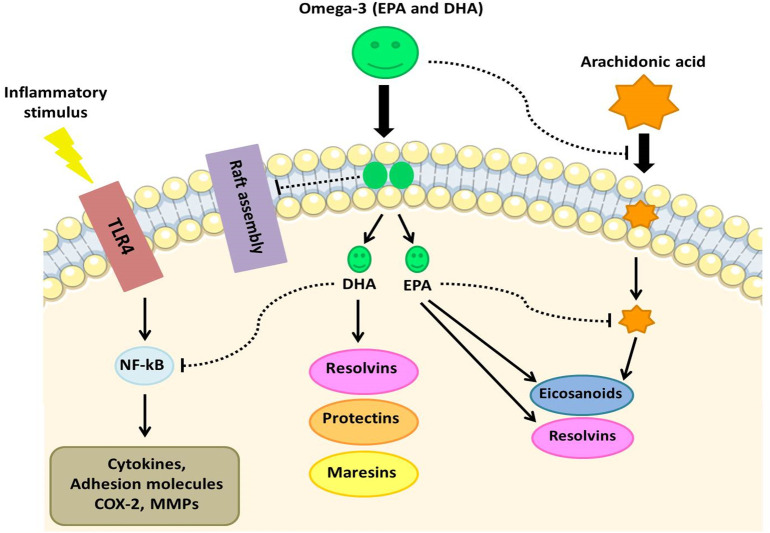
The synthesis of polyunsaturated fatty acids (PUFAs) and their metabolites (termed as bioactive lipid mediators) is one of the underappreciated mechanisms by which the human innate immune system may inactivate various microorganisms such as bacteria, fungi and enveloped viruses [56]. Specialized pro-resolving mediators (SPMs) play an important in the management of COVID-19 disease and effectively promote the resolution of infectious inflammation [57]. They are produced by the innate immune cells via the stereoselective enzymatic conversion of omega-3 fatty acids including eicosapentaenoic (EPA), docosapentaenoic (n-3 DPA), and docosahexaenoic (DHA) [57], [58], [59]. The most well characterised SPMs are grouped into four families, lipoxins (LXs), resolvins (RVs), protectins (PDs), and maresins (MaRs), which halt the progression of acute to chronic inflammation [60]. DHA and EPA lipid mediators named resolvins D and E, respectively; as well as EPA lipid mediators named protectins and maresins [61], [62]. These metabolites are synthesized by COX and LOX pathways in the presence and absence of aspirin [63], [64]. The anti-inflammatory effects of resolvins, protectins, and maresins are mediated by a number of mechanisms. This includes preventing neutrophil and monocyte migration across epithelial cells and promoting the removal of polymorphonuclear (PMN) leukocytes, debris from the inflammatory site and apoptotic cells [64]. Serhan et al., (2000) [65] showed DHA-derived RvD and EPA-derived RvE were discovered in inflammatory exudates during the phase of resolution of the inflammatory response. They act by preventing inflammatory cytokine production, activation macrophage autophagy, preventing entry of neutrophils to sites of inflammation by lowering the expression of surface adhesion receptors on neutrophils and removing inflammatory mediators [66], [67], [68]. Additionally, resolvins can reduce the production of reactive oxygen species (ROS) by neutrophils, induce neutrophil apoptosis and clearance by macrophages, and inhibit chemokine signaling [64], [69], [70]. Recently, Recchiuti A et al., (2020) [71] showed that RvD reduced SARS-CoV-2 induced inflammatory responses via reduction of inflammatory chemokines and cytokines. Lipidomic analysis has showed that higher levels of SPMs derived from omega-3 PUFA may be associated with mild COVID- 19 [72]. Furthermore, SARS-CoV-2 viral proteins can activate the resolvin biosynthetic pathways [71]. Hence the consumption of EPA and DHA supplementation has the potential to boost the production of these pro-resolving mediators. Randomized controlled studies (RCT) have supported increased levels of SPMs and a decreased inflammation after omega-3 PUFA supplementation [54], [73], [74], [75]. A recent meta-analysis of 12 RCTs (n = 1280 patients) in acute respiratory distress syndromepatients found that omega-3 PUFA supplementation was correlated with improved PaO2/FiO2 ratios, shorter ICU stays and shorter mechanical ventilation durations [76]. Maresins are sulfide conjugates synthesized by macrophages, which are involved in the resolution of acute inflammation and promotion tissue regeneration. Maresin-1 biosynthesis includes an active intermediate that promotes macrophage M1 (pro-inflammatory) to M2 (anti-inflammatory) phenotype conversion [64], [77]. Further, Protectins have also been shown to affect the inflammatory symptoms of respiratory viral diseases [78]. Importantly, pro-inflammatory cytokines, TNF-α and IL-6 inhibit the activity of desaturases, which are required for the production of AA, EPA, and DHA [64]. Hence, when there is a significant degree of inflammation caused by high levels of IL-6 and TNF- α, such as following COVID-19 infection, a deficiency of EPA and DHA can lead to a decrease in the production of resolvins, protectins, and maresins [79]. So, in COVID-19, treatment with PUFAs or their metabolites can decrease inappropriate IL-6 and TNF- production to resolve inflammation, improve recovery, and limit cytokine storm [80] (Fig. 2 ).
Fig. 2.
The anti-inflammatory effects of omega-3 PUFAs. COX, cyclooxygenase; DHA, docosahexaenoic acid; EPA, eicosapentaenoic acid; MMP, matrixmetalloproteinase; NF-κB, nuclear factor κB.
4.2. PFUAs role in inhibition of viral replication
According to new findings, the SARS-CoV-2 spike (S) glycoprotein of SARS-CoV-2 interacts with angiotensin-converting enzyme-2 (ACE2) and cellular protease transmembrane protease serine-2 (TMPRSS-2) as internalization receptors to enter host cells during the infection cycle. Downregulation of ACE2 by SARS-CoV-2 causes a reduction in ACE2 products such as Ang- [1], [2], [3], [4], [5], [6], [7], Ang [1], [2], [3], [4], [5], [6], [7], [8], [9], apelin [1], [2], [3], [4], [5], [6], [7], [8], [9], [10], [11], [12], and accumulation of substrates such as apelin [1], [2], [3], [4], [5], [6], [7], [8], [9], [10], [11], [12], [13] and Ang II [82]. ACE2 downregulation correlates with systemic RAS imbalance and facilitates the development of multiorgan damage from SARS-CoV-2 infections [83]. On the other hand, a decrease in the concentration of apelin 1–12 in the plasma of COVID-19 patients may increase endothelial cell damage [81], [82]. Interestingly, multiple studies have shown that omega-3 PUFAs can modulate the Renin-angiotensin aldosterone system by regulating the levels of both Ang II and ACE2 [64], [83].
Also omega-3 PUFAs indicate noticeable inhibitory effect on activity of host proteases TMPRSS2 and cathepsin L. PUFAs adopt an almost flat conformation or a spherical liposomal interface, which allows contact of hydroxyl groups with the aqueous environment acting via electrostatic forces as well. All this could interrupt the contact between the host membrane the viral envelope and subsequently inhibit SARS-CoV-2 attachment and entry upon FAs treatment [84].
Since PUFAs are components of membrane phospholipids, they can control membrane characteristics such as membrane fluidity and protein complex formation in lipid rafts. Entry gateway receptors for SARSCoV are mostly present in lipid rafts [61], [85]. The changes in membrane fluidity may disrupt the conformation of the host and be determining for the SARS-CoV-2 virus interaction. On the other hand, because PUFAs are lipophilic molecules, they could interfere with the viral envelope itself, changing its dynamics and inactivate viruses by disrupting their envelopes [84].
5. Conclusion
COVID-19 is contagious pathogenic viral infection which is involved respiratory system and different organs and cause a cytokine storm that is an indicator of disease severity. PUFAs, a cluster of significant fats, display biological activities at the molecular and cellular levels that can be the significant option to lessen the COVID-19 severity. In this review, the potential roles of omega polyunsaturated fatty acids as an adjunct therapy in mitigating inflammation and virus replication in patients with SAR-COV-2 were investigated. It is evident from literature the omega-3 PUFAs and their active metabolites have the potential to modulate and management of the COVID-19 disease complications and have a substantial role in the immunological defense against viral entry and replication to new copies. The evidence presented in this review supports the hypothesis that omega polyunsaturated fatty acids can reduce the risk of covid-19 disease and should considered as a preventative, cost effective and safe method. However, the risk of taking high-dose omega-3 PUFA supplements before or during SARS-COV-2 infection needs to be investigated.
CRediT authorship contribution statement
Afrooz Mazidimoradi: Conceptualization, Methodology, data extraction.
Esmat Alemzadeh: Data extraction, Writing- Original draft preparation.
Effat Alemzadeh: Visualization, Investigation, Data extraction, Writing- Original draft preparation.
Hamid Salehiniya: Conceptualization, searching, Validation, Writing- Reviewing and Editing.
Declaration of competing interest
There is no any conflict of interest.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.lfs.2022.120489.
Appendix A. Supplementary data
Supplementary table
Data availability
No data was used for the research described in the article.
References
- 1.Falah N.U., Hashmi S., Ahmed Z., Jaan A., Akhtar A., Khalid F., et al. Kawasaki disease-like features in 10 pediatric COVID-19 cases: a retrospective study. Cureus. 2020;12(10) doi: 10.7759/cureus.11035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Akram Z., et al. Role of OMEGA-3 fatty acid supplementation in COVID-19 patients: a narrative review. Arch. Intern. Med. Res. 2021;4(2):177–183. [Google Scholar]
- 3.Asher A., Tintle N.L., Myers M., Lockshon L., Bacareza H., Harris W.S. Blood omega-3 fatty acids and death from COVID-19: a pilot study. 2021;166 doi: 10.1016/j.plefa.2021.102250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Moore J.B., June C.H. Cytokine release syndrome in severe COVID-19. Science. 2020;368(6490):473–474. doi: 10.1126/science.abb8925. [DOI] [PubMed] [Google Scholar]
- 5.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y., et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. The Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kany S., Vollrath J.T., Relja B. Cytokines in inflammatory disease. Int. J. Mol. Sci. 2019;20(23) doi: 10.3390/ijms20236008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Narain S., Parmar M. StatPearls Publishing Copyright © 2021, StatPearls Publishing LLC Copyright © 2021, StatPearls Publishing LLC.; 2021.; Treasure Island (FL): 2021. Tolterodine. StatPearls. [Google Scholar]
- 8.Oppedisano F., Macrì R., Gliozzi M., Musolino V., Carresi C., Maiuolo J., et al. The anti-inflammatory and antioxidant properties of n-3 PUFAs: their role in cardiovascular protection. Biomedicines. 2020;8(9):306. doi: 10.3390/biomedicines8090306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Djuricic I., Calder P.C. Beneficial outcomes of omega-6 and omega-3 polyunsaturated fatty acids on human health: an update for 2021. Nutrients. 2021;13(7):2421. doi: 10.3390/nu13072421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Saini R.K., Keum Y.S. Omega-3 and omega-6 polyunsaturated fatty acids: dietary sources, metabolism, and significance - a review. Life Sci. 2018;203:255–267. doi: 10.1016/j.lfs.2018.04.049. [DOI] [PubMed] [Google Scholar]
- 11.Calder P.C. Dietary arachidonic acid: harmful, harmless or helpful? Br. J. Nutr. 2007;98(3):451–453. doi: 10.1017/S0007114507761779. [DOI] [PubMed] [Google Scholar]
- 12.Calder P.C. Eicosanoids. Essays Biochem. 2020;64(3):423–441. doi: 10.1042/EBC20190083. [DOI] [PubMed] [Google Scholar]
- 13.Christie W.W., Harwood J.L. Oxidation of polyunsaturated fatty acids to produce lipid mediators. Essays Biochem. 2020;64(3):401–421. doi: 10.1042/EBC20190082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bheliya V.K., Pathak A.K. clinical research and role of dietary supplement in the treatment of middle east respiratory syndrome current status. J. Pharm. Pharm. Sci. 2020;9(3):823–839. [Google Scholar]
- 15.Zhao Y., Wang C. Effect of ω-3 polyunsaturated fatty acid-supplemented parenteral nutrition on inflammatory and immune function in postoperative patients with gastrointestinal malignancy: a meta-analysis of randomized control trials in China. Medicine. 2018;97(16) doi: 10.1097/MD.0000000000010472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Weatherill A.R., Lee J.Y., Zhao L., Lemay D.G., Youn H.S., Hwang D.H. Saturated and polyunsaturated fatty acids reciprocally modulate dendritic cell functions mediated through TLR4. J. Immunol. 2005;174(9):5390–5397. doi: 10.4049/jimmunol.174.9.5390. [DOI] [PubMed] [Google Scholar]
- 17.Wong S.W., Kwon M.-J., Choi A.M., Kim H.-P., Nakahira K., Hwang D.H. Fatty acids modulate toll-like receptor 4 activation through regulation of receptor dimerization and recruitment into lipid rafts in a reactive oxygen species-dependent manner. J. Biol. Chem. 2009;284(40):27384–27392. doi: 10.1074/jbc.M109.044065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Talukdar S., Bae E.J., Imamura T., Morinaga H., Fan W., Li P., et al. GPR120 is an omega-3 fatty acid receptor mediating potent anti-inflammatory and insulin-sensitizing effects. Cell. 2010;142(5):687–698. doi: 10.1016/j.cell.2010.07.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Serhan C.N., Chiang N., Dalli J., editors. Seminars in Immunology. Elsevier; 2015. The resolution code of acute inflammation: Novel pro-resolving lipid mediators in resolution. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lopez-Vicario C., Rius B., Alcaraz-Quiles J., Garcia-Alonso V., Lopategi A., Titos E., et al. Pro-resolving mediators produced from EPA and DHA: overview of the pathways involved and their mechanisms in metabolic syndrome and related liver diseases. Eur. J. Pharmacol. 2016;785:133–143. doi: 10.1016/j.ejphar.2015.03.092. [DOI] [PubMed] [Google Scholar]
- 21.Serhan C.N., Levy B.D. Resolvins in inflammation: emergence of the pro-resolving superfamily of mediators. J. Clin. Invest. 2018;128(7):2657–2669. doi: 10.1172/JCI97943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Serhan C.N. Discovery of specialized pro-resolving mediators marks the dawn of resolution physiology and pharmacology. Mol. Asp. Med. 2017;58:1–11. doi: 10.1016/j.mam.2017.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gutiérrez S., Svahn S.L., Johansson M.E. Effects of Omega-3 fatty acids on immune cells. Int. J. Mol. Sci. 2019;20(20):5028. doi: 10.3390/ijms20205028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ling V., Zabetakis I. The role of an anti-inflammatory diet in conjunction to COVID-19. Diseases. 2021;9(4):76. doi: 10.3390/diseases9040076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wells G.A., Shea B., O'Connell D., Peterson J., Welch V., Losos M., et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. 2021. http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp Available from:
- 26.Hamulka J., Jeruszka-Bielak M., Gornicka M., Drywien M.E., Zielinska-Pukos M.A. Dietary supplements during COVID-19 outbreak. Results of Google trends analysis supported by PLifeCOVID-19 online studies. Nutrients. 2021;13(1) doi: 10.3390/nu13010054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nguyen M., Bourredjem A., Piroth L., Bouhemad B., Jalil A., Pallot G., et al. High plasma concentration of non-esterified polyunsaturated fatty acids is a specific feature of severe COVID-19 pneumonia. Sci. Rep. 2021;11(1) doi: 10.1038/s41598-021-90362-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Perez-Torres I., Guarner-Lans V., Soria-Castro E., Manzano-Pech L., Palacios-Chavarria A., Valdez-Vazquez R.R., et al. Alteration in the lipid profile and the desaturases activity in patients with severe pneumonia by SARS-CoV-2. Front. Physiol. 2021;12 doi: 10.3389/fphys.2021.667024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vivar-Sierra A., Araiza-Macias M.J., Hernandez-Contreras J.P., Vergara-Castaneda A., Ramirez-Velez G., Pinto-Almazan R., et al. In silico study of polyunsaturated fatty acids as potential SARS-CoV-2 spike protein closed conformation stabilizers: epidemiological and computational approaches. Molecules. 2021;26(3) doi: 10.3390/molecules26030711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Louca P., Murray B., Klaser K., Graham M.S., Mazidi M., Leeming E.R., et al. Modest effects of dietary supplements during the COVID-19 pandemic: insights from 445 850 users of the COVID-19 symptom study app. BMJ Nutr. Prev. Health. 2021;4(1):149–157. doi: 10.1136/bmjnph-2021-000250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Thomas T., Stefanoni D., Reisz J.A., Nemkov T., Bertolone L., Francis R.O., et al. COVID-19 infection alters kynurenine and fatty acid metabolism, correlating with IL-6 levels and renal status. JciInsight. 2020;5(14) doi: 10.1172/jci.insight.140327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zapata B.R., Miguel Muller J., Enrique Vasquez J., Ravera F., Lago G., Canon E., et al. Omega-3 index and clinical outcomes of severe COVID-19: preliminary results of a cross-sectional study. Int. J. Environ. Res. Public Health. 2021;18(15) doi: 10.3390/ijerph18157722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hao Y., Zhang Z., Feng G., Chen M., Wan Q., Lin J., et al. Distinct Lipid Metabolic Dysregulation in Asymptomatic COVID-19. iScience. 2021:102974. doi: 10.1016/j.isci.2021.102974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dierckx T., van Elslande J., Salmela H., Decru B., Wauters E., Gunst J., et al. medRxiv; 2020. The Metabolic Fingerprint of COVID-19 Severity. 2020.11.09.20228221. [Google Scholar]
- 35.Archambault A.-S., Zaid Y., Rakotoarivelo V., Turcotte C., Dore E., Dubuc I., et al. High levels of eicosanoids and docosanoids in the lungs of intubated COVID-19 patients- CAN NOT EXTRACTED DATA. FASEB J. 2021;35(6) doi: 10.1096/fj.202100540R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bejan C.A. 2021. DrugWAS: Leveraging Drug-wide Association Studies to Facilitate Drug Repurposing for COVID-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Barberis E., Timo S., Amede E., Vanella V.V., Puricelli C., Cappellano G., et al. Large-scale plasma analysis revealed new mechanisms and molecules associated with the host response to SARS-CoV-2. Int. J. Mol. Sci. 2020;21(22) doi: 10.3390/ijms21228623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Doaei S., Gholami S., Rastgoo S., Gholamalizadeh M., Bourbour F., Bagheri S.E., et al. The effect of omega-3 fatty acid supplementation on clinical and biochemical parameters of critically ill patients with COVID-19: a randomized clinical trial. J. Transl. Med. 2021;19(1) doi: 10.1186/s12967-021-02795-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mei Q., Wang A.Y., Bryant A., Yang Y., Li M., Wang F., et al. Survival factors and metabolic pathogenesis in elderly patients (>= 65) with COVID-19: a multi-center study. Front. Med. 2021;7 doi: 10.3389/fmed.2020.595503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.El-Kurdi B., Khatua B., Rood C., Snozek C., Cartin-Ceba R., Singh V.P., et al. Mortality from coronavirus disease 2019 increases with unsaturated fat and may be reduced by early calcium and albumin supplementation. Gastroenterology. 2020;159(3):1015. doi: 10.1053/j.gastro.2020.05.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jontez N.B., Novak K., Kenig S., Petelin A., Praznikar Z.J., Mohorko N. The impact of COVID-19-related lockdown on diet and serum markers in healthy adults. Nutrients. 2021;13(4) doi: 10.3390/nu13041082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Julkunen H., Cichonska A., Slagboom P.E., Wurtz P., Nightingale Hlth U.K.B.I. Metabolic biomarker profiling for identification of susceptibility to severe pneumonia and COVID-19 in the general population. elife. 2021;10 doi: 10.7554/eLife.63033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Aburto J.M., Schöley J., Kashnitsky I., Zhang L., Rahal C., Missov T.I., et al. Quantifying impacts of the COVID-19 pandemic through life-expectancy losses: a population-level study of 29 countries. Int. J. Epidemiol. 2021;51(1):63–74. doi: 10.1093/ije/dyab207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Iddir M., Brito A., Dingeo G., Fernandez Del Campo S.S., Samouda H., La Frano M.R., et al. Strengthening the immune system and reducing inflammation and oxidative stress through diet and nutrition: considerations during the COVID-19 crisis. Nutrients. 2020;12(6):1562. doi: 10.3390/nu12061562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Weill P., Plissonneau C., Legrand P., Rioux V., Thibault R. May omega-3 fatty acid dietary supplementation help reduce severe complications in Covid-19 patients? Biochimie. 2020;179:275–280. doi: 10.1016/j.biochi.2020.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shahidi F., Ambigaipalan P. Omega-3 polyunsaturated fatty acids and their health benefits. Annu. Rev. Food Sci. Technol. 2018;9:345–381. doi: 10.1146/annurev-food-111317-095850. [DOI] [PubMed] [Google Scholar]
- 47.Li X., Bi X., Wang S., Zhang Z., Li F., Zhao A.Z. Therapeutic potential of ω-3 polyunsaturated fatty acids in human autoimmune diseases. Front. Immunol. 2019;2241 doi: 10.3389/fimmu.2019.02241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Michalak A., Mosińska P., Fichna J. Polyunsaturated fatty acids and their derivatives: therapeutic value for inflammatory, functional gastrointestinal disorders, and colorectal cancer. Front. Pharmacol. 2016;7:459. doi: 10.3389/fphar.2016.00459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.So J., Wu D., Lichtenstein A.H., Tai A.K., Matthan N.R., Maddipati K.R., et al. EPA and DHA differentially modulate monocyte inflammatory response in subjects with chronic inflammation in part via plasma specialized pro-resolving lipid mediators: a randomized, double-blind, crossover study. Atherosclerosis. 2021;316:90–98. doi: 10.1016/j.atherosclerosis.2020.11.018. [DOI] [PubMed] [Google Scholar]
- 50.Fontes J.D., Rahman F., Lacey S., Larson M.G., Vasan R.S., Benjamin E.J., et al. Red blood cell fatty acids and biomarkers of inflammation: a cross-sectional study in a community-based cohort. Atherosclerosis. 2015;240(2):431–436. doi: 10.1016/j.atherosclerosis.2015.03.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tan A., Sullenbarger B., Prakash R., McDaniel J.C. Supplementation with eicosapentaenoic acid and docosahexaenoic acid reduces high levels of circulating proinflammatory cytokines in aging adults: a randomized, controlled study. Prostaglandins Leukot. Essent. Fat. Acids. 2018;132:23–29. doi: 10.1016/j.plefa.2018.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.AbuMweis S., Jew S., Tayyem R., Agraib L. Eicosapentaenoic acid and docosahexaenoic acid containing supplements modulate risk factors for cardiovascular disease: a meta-analysis of randomised placebo-control human clinical trials. J. Hum. Nutr. Diet. 2018;31(1):67–84. doi: 10.1111/jhn.12493. [DOI] [PubMed] [Google Scholar]
- 53.Lee C.H. Role of specialized pro-resolving lipid mediators and their receptors in virus infection: a promising therapeutic strategy for SARS-CoV-2 cytokine storm. Arch. Pharm. Res. 2021;1–15 doi: 10.1007/s12272-020-01299-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Doaei S., Gholami S., Rastgoo S., Gholamalizadeh M., Bourbour F., Bagheri S.E., et al. The effect of omega-3 fatty acid supplementation on clinical and biochemical parameters of critically ill patients with COVID-19: a randomized clinical trial. J. Transl. Med. 2021;19(1):1–9. doi: 10.1186/s12967-021-02795-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hathaway D., III, Pandav K., Patel M., Riva-Moscoso A., Singh B.M., Patel A., et al. Omega 3 fatty acids and COVID-19: a comprehensive review. Infect. Chemother. 2020;52(4):478. doi: 10.3947/ic.2020.52.4.478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Das U.N. Bioactive lipids-based therapeutic approach to COVID-19 and other similar infections. Arch. Med. Sci. 2021 doi: 10.5114/aoms/135703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Regidor P.-A., Santos F.G., Rizo J.M., Egea F.M. Pro resolving inflammatory effects of the lipid mediators of omega 3 fatty acids and its implication in SARS COVID-19. Med. Hypotheses. 2020;145 doi: 10.1016/j.mehy.2020.110340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Serhan C.N., Chiang N. Resolution phase lipid mediators of inflammation: agonists of resolution. Curr. Opin. Pharmacol. 2013;13(4):632–640. doi: 10.1016/j.coph.2013.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Serhan C.N. Treating inflammation and infection in the 21st century: new hints from decoding resolution mediators and mechanisms. FASEB J. 2017;31(4):1273–1288. doi: 10.1096/fj.201601222R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Serhan C.N. Resolution phase of inflammation: novel endogenous anti-inflammatory and proresolving lipid mediators and pathways. Annu. Rev. Immunol. 2007;25:101–137. doi: 10.1146/annurev.immunol.25.022106.141647. [DOI] [PubMed] [Google Scholar]
- 61.Baral P.K., Amin M.T., Rashid M., Or M., Hossain M.S. Assessment of polyunsaturated fatty acids on COVID-19-associated risk reduction. Rev. Bras. 2021:1–15. doi: 10.1007/s43450-021-00213-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Duvall M.G., Levy B.D. DHA-and EPA-derived resolvins, protectins, and maresins in airway inflammation. Eur. J. Pharmacol. 2016;785:144–155. doi: 10.1016/j.ejphar.2015.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Mas E., Croft K.D., Zahra P., Barden A., Mori T.A. Resolvins D1, D2, and other mediators of self-limited resolution of inflammation in human blood following n-3 fatty acid supplementation. Clin. Chem. 2012;58(10):1476–1484. doi: 10.1373/clinchem.2012.190199. [DOI] [PubMed] [Google Scholar]
- 64.Darwesh A.M., Bassiouni W., Sosnowski D.K., Seubert J.M. Can N-3 polyunsaturated fatty acids be considered a potential adjuvant therapy for COVID-19-associated cardiovascular complications? Pharmacol. Ther. 2021;219 doi: 10.1016/j.pharmthera.2020.107703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Serhan C.N., Clish C.B., Brannon J., Colgan S.P., Chiang N., Gronert K. Novel functional sets of lipid-derived mediators with antiinflammatory actions generated from omega-3 fatty acids via cyclooxygenase 2–nonsteroidal antiinflammatory drugs and transcellular processing. J. Exp. Med. 2000;192(8):1197–1204. doi: 10.1084/jem.192.8.1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Dalli J., Winkler J.W., Colas R.A., Arnardottir H., Cheng C.-Y.C., Chiang N., et al. Resolvin D3 and aspirin-triggered resolvin D3 are potent immunoresolvents. Chem. Biol. 2013;20(2):188–201. doi: 10.1016/j.chembiol.2012.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Winkler J.W., Orr S.K., Dalli J., Cheng C.-Y.C., Sanger J.M., Chiang N., et al. Resolvin D4 stereoassignment and its novel actions in host protection and bacterial clearance. Sci. Rep. 2016;6(1):1–11. doi: 10.1038/srep18972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Serhan C.N. Pro-resolving lipid mediators are leads for resolution physiology. Nature. 2014;510(7503):92–101. doi: 10.1038/nature13479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ariel A., Fredman G., Sun Y.-P., Kantarci A., Van Dyke T.E., Luster A.D., et al. Apoptotic neutrophils and T cells sequester chemokines during immune response resolution through modulation of CCR5 expression. Nat. Immunol. 2006;7(11):1209–1216. doi: 10.1038/ni1392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Schwab J.M., Chiang N., Arita M., Serhan C.N. Resolvin E1 and protectin D1 activate inflammation-resolution programmes. Nature. 2007;447(7146):869–874. doi: 10.1038/nature05877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Recchiuti A., Patruno S., Mattoscio D., Isopi E., Pomilio A., Lamolinara A., et al. bioRxiv; 2020. Resolvin D1 and D2 Reduce SARS-Cov-2-induced Inflammation in Cystic Fibrosis Macrophages. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Schwarz B., Sharma L., Roberts L., Peng X., Bermejo S., Leighton I., et al. MedRxiv; 2020. Severe SARS-CoV-2 Infection in Humans Is Defined by a Shift in the Serum Lipidome Resulting in Dysregulation of Eicosanoid Immune Mediators. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Arnardottir H., Pawelzik S.-C., Öhlund Wistbacka U., Artiach G., Hofmann R., Reinholdsson I., et al. Stimulating the resolution of inflammation through omega-3 polyunsaturated fatty acids in COVID-19: rationale for the COVID-omega-F trial. Front. Physiol. 2021;1748 doi: 10.3389/fphys.2020.624657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Elajami T.K., Colas R.A., Dalli J., Chiang N., Serhan C.N., Welty F.K. Specialized proresolving lipid mediators in patients with coronary artery disease and their potential for clot remodeling. FASEB J. 2016;30(8):2792–2801. doi: 10.1096/fj.201500155R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Mayer K., Gokorsch S., Fegbeutel C., Hattar K., Rosseau S., Walmrath D., et al. Parenteral nutrition with fish oil modulates cytokine response in patients with sepsis. Am. J. Respir. Crit. Care Med. 2003;167(10):1321–1328. doi: 10.1164/rccm.200207-674OC. [DOI] [PubMed] [Google Scholar]
- 76.Langlois P.L., D'Aragon F., Hardy G., Manzanares W. Omega-3 polyunsaturated fatty acids in critically ill patients with acute respiratory distress syndrome: a systematic review and meta-analysis. Nutrition. 2019;61:84–92. doi: 10.1016/j.nut.2018.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Serhan C.N., Yang R., Martinod K., Kasuga K., Pillai P.S., Porter T.F., et al. Maresins: novel macrophage mediators with potent antiinflammatory and proresolving actions. J. Exp. Med. 2009;206(1):15–23. doi: 10.1084/jem.20081880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Russell C.D., Schwarze J. The role of pro-resolution lipid mediators in infectious disease. Immunology. 2014;141(2):166–173. doi: 10.1111/imm.12206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Das U.N. Arachidonic acid and other unsaturated fatty acids and some of their metabolites function as endogenous antimicrobial molecules: a review. J. Adv. Res. 2018;11:57–66. doi: 10.1016/j.jare.2018.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Das U.N. Can bioactive lipids augment anti-cancer action of immunotherapy and prevent cytokine storm? Arch. Med. Res. 2019;50(6):342–349. doi: 10.1016/j.arcmed.2019.10.004. [DOI] [PubMed] [Google Scholar]
- 81.Letko M., Marzi A., Munster V. Functional assessment of cell entry and receptor usage for SARS-CoV-2 and other lineage B betacoronaviruses. Nat. Microbiol. 2020;5(4):562–569. doi: 10.1038/s41564-020-0688-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Yan R., Zhang Y., Li Y., Xia L., Guo Y., Zhou Q. Structural basis for the recognition of SARS-CoV-2 by full-length human ACE2. Science. 2020;367(6485):1444–1448. doi: 10.1126/science.abb2762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.South A.M., Diz D.I., Chappell M.C. COVID-19, ACE2, and the cardiovascular consequences. Am. J. Phys. Heart Circ. Phys. 2020;318(5):H1084–H1090. doi: 10.1152/ajpheart.00217.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Goc A., Niedzwiecki A., Rath M. Polyunsaturated ω-3 fatty acids inhibit ACE2-controlled SARS-CoV-2 binding and cellular entry. Sci. Rep. 2021;11(1):1–12. doi: 10.1038/s41598-021-84850-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ballout R.A., Sviridov D., Bukrinsky M.I., Remaley A.T. The lysosome: a potential juncture between SARS-CoV-2 infectivity and niemann-pick disease type C, with therapeutic implications. FASEB J. 2020;34(6):7253–7264. doi: 10.1096/fj.202000654R. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary table
Data Availability Statement
No data was used for the research described in the article.